Protein misfolding and amyloid nucleation through liquid–liquid phase separation
Semanti
Mukherjee
 a,
Manisha
Poudyal
a,
Manisha
Poudyal
 a,
Kritika
Dave
a,
Kritika
Dave
 b,
Pradeep
Kadu
b,
Pradeep
Kadu
 a and
Samir K.
Maji
a and
Samir K.
Maji
 *ab
*ab
aDepartment of Biosciences and Bioengineering, Indian Institute of Technology Bombay, Powai, Mumbai 400076, India. E-mail: samirmaji@iitb.ac.in
bSunita Sanghi Centre of Aging and Neurodegenerative Diseases, Indian Institute of Technology Bombay, Powai, Mumbai 400076, India
First published on 10th April 2024
Abstract
Liquid–liquid phase separation (LLPS) is an emerging phenomenon in cell physiology and diseases. The weak multivalent interaction prerequisite for LLPS is believed to be facilitated through intrinsically disordered regions, which are prevalent in neurodegenerative disease-associated proteins. These aggregation-prone proteins also exhibit an inherent property for phase separation, resulting in protein-rich liquid-like droplets. The very high local protein concentration in the water-deficient confined microenvironment not only drives the viscoelastic transition from the liquid to solid-like state but also most often nucleate amyloid fibril formation. Indeed, protein misfolding, oligomerization, and amyloid aggregation are observed to be initiated from the LLPS of various neurodegeneration-related proteins. Moreover, in these cases, neurodegeneration-promoting genetic and environmental factors play a direct role in amyloid aggregation preceded by the phase separation. These cumulative recent observations ignite the possibility of LLPS being a prominent nucleation mechanism associated with aberrant protein aggregation. The present review elaborates on the nucleation mechanism of the amyloid aggregation pathway and the possible early molecular events associated with amyloid-related protein phase separation. It also summarizes the recent advancement in understanding the aberrant phase transition of major proteins contributing to neurodegeneration focusing on the common disease-associated factors. Overall, this review proposes a generic LLPS-mediated multistep nucleation mechanism for amyloid aggregation and its implication in neurodegeneration.
1. Introduction
Neurodegenerative disorders refer to a wide variety of conditions characterized by the loss of a neuron's structure and functions due to glial and neuronal cell death.1,2 These disorders have been observed as some of the intractable problems in the aging population worldwide.3 The three most common neurodegenerative disorders are Alzheimer's disease (AD), Parkinson's disease (PD), and amyotrophic lateral sclerosis (ALS).4 Additionally, there are several other age-related chronic disorders, including multiple sclerosis (MS), frontotemporal dementia (FTD), Huntington's disease (HD), motor neuron diseases (MNDs), ataxia, multiple system atrophy (MSA), Creutzfeldt-Jakob disease (CJD) and various other dementias.5,6 Individuals suffering from these diseases may experience problems with movement, speech, memory, cognition, intelligence, and more.7 As life expectancies in many nations continue to rise, the prevalence and incidence of these disorders are likely to increase in the foreseeable future. Several molecular investigations show that the aberrant amyloid aggregates of misfolded proteins serve as a common pathological hallmark in all neurodegenerative diseases and play a vital role in disease onset as well as its progression.8–10 Amyloid fibrils are insoluble, highly ordered fibrillar aggregates, which are extremely stable under a wide variety of environmental conditions (Fig. 1a and b).11,12 The analyses of purified fibrils using X-ray diffraction indicated that the constituent proteins are rich in a highly ordered, cross-β-pleated sheet structure with 4.7–4.8 Å intra-strand distance and 6–12 Å inter-sheet distance, which vary depending on the amino acid side chain composition and their packing in the cross-β-sheet arrangement (Fig. 1b).13–15 Several unique properties such as tensile strength, insolubility in ionic detergents, and proteinase-K resistance are some distinct characteristics of amyloid that distinguish it from other disordered aggregates.16,17 The majorly studied misfolded amyloids are from neuronal proteins, including hyperphosphorylated tau (p-tau),18 the amyloid-β protein (Aβ),19 α-syn,20 the Huntingtin protein,21 and prion proteins,22 which are associated with an array of neurodegenerative diseases.23 Moreover, misfolding of the stress granule-associated RNA-binding proteins such as FUS,24 TDP-43,25 TIA1,26 and hnRNP family27 also form pathogenic inclusions, which are associated with several neurodegenerative diseases such as ALS and FTD.28,29 All these neurodegenerative diseases share some common features, such as the occurrence of brain lesions and an intracellular or extracellular accumulation of misfolded, ubiquitinated protein aggregates with amyloidogenic properties.10 For example, amyloid-β (Aβ) peptide accumulates as microscopic insoluble amyloid deposits, known as senile plaques, in AD.30 Similarly, in PD, lesions are known as Lewy bodies, which are present in the cytoplasm and are predominantly composed of the protein α-syn.31,32 Whereas in HD, lesions are observed as intranuclear and cytoplasmic inclusion bodies rich in Huntingtin protein.21 While the molecular pathology of FTD is still unknown, evidence suggests that FTD is caused by abnormal amyloid clusters, prevalently composed of TDP-43 and FUS, in frontal and temporal lobe neurons.33,34 Recent research has also pointed out that these amyloid deposits are not solely responsible for the neurotoxic effects associated with these disorders.35 Instead, soluble oligomers and protofibrils act as the proximate mediators of the diseases as they can easily transmit through the brain parenchyma and synaptic cleft affecting its structure and plasticity.36,37 Indeed, oligomers of Aβ protein,38 tau protein,39 α-syn,40 FUS,41 and Huntingtin42 are identified to play a crucial role in neurotoxicity as oligomers directly show concentration-dependent toxicity in neurons.43 Thus, the current focus of the research in the amyloid field has shifted to understanding the early aggregation mechanism, which is not easy to achieve due to the heterogeneity of oligomers and their transient nature. Since the clinical regime for neurodegeneration mostly relies on symptomatic treatment, understanding the nucleation and early events of amyloid aggregation is particularly important for designing small molecules/therapeutics against protein aggregation. Recently, neurodegenerative diseases-associated proteins have been shown to undergo liquid–liquid phase separation (LLPS) where protein-rich condensates often undergo liquid-to-solid-like transition with the formation of amyloid-like assemblies.44–46 Moreover, fibrillar species are shown to emerge out from the condensates upon aberrant amyloidogenic solidification suggesting that protein-rich condensates indeed have a direct role in the nucleation event for amyloid aggregation.47,48 Interestingly, neurodegenerative disease-promoting factors such as familial mutations, gene multiplication, post-translational modification, metal ions exposure, oxidative stress, DNA damage, and the presence of RNA also facilitate phase separation events and subsequent viscoelastic liquid-to-solid transition.49–55 Since the nucleation event is the rate-limiting step for amyloid aggregation,56 it is reasonable to hypothesize that these factors may modulate LLPS and subsequent amyloid nucleation from condensates. Indeed, the amyloid aggregation pathway preceded by LLPS is emerging as a common aggregation mechanism for various neurodegenerative disease-associated proteins apart from the traditional pathway of amyloid aggregation from the bulk solution.57–59 The present review focuses on the recent advancement in understanding amyloid aggregation from the aberrant phase separation. Specifically, the review addresses the molecular mechanism of amyloid nucleation and early-stage intermediate generation from the perspective of the emerging LLPS concept. The review also explains the underlying nucleation mechanism of protein self-assembly inside the liquid condensates, dissecting the molecular basis of viscoelastic liquid-to-solid transition, and proposes how metastable LLPS can serve as a misfolding/aggregation crucible for amyloid nucleation. Further, it also summarizes the effect of the common disease-associated factors on aberrant phase separation as well as subsequent amyloid aggregation. Overall, we propose that LLPS and the subsequent liquid-to-solid transition may serve as a generic mechanism for protein misfolding, aggregation and amyloid formation. | ||
| Fig. 1 Molecular mechanism of amyloid aggregation pathway: (a) the schematic representation showing sequential molecular events of amyloid growth kinetics comprising three distinct phases: (i) lag phase, (ii) exponential growth phase, and (iii) plateau phase. The lag phase indicates the initiation of monomer self-assembly into growth-competent oligomers. The addition of preformed fibrillar seeds shortens the lag phase and initiates an exponential growth phase producing higher-order self-assemblies, such as protofibrils and prefibrillar assemblies leading to amyloid fibril formation. The inset image indicates the atomic-resolution structure of a typical amyloid fibril from cryo-EM reconstruction. The atomic-resolution amyloid structure image is reproduced from ref. 80 with permission from Proceedings of the National Academy of Sciences. (b) Representative biophysical characterization of amyloid fibrils through TEM (upper left), X-ray fibril diffraction (upper right), circular dichroism spectroscopy (bottom left), and thioflavin-T fluorescence binding (bottom right). The classical fibrillar morphology of amyloid is evident from the TEM images and the fibre diffraction pattern (4.7 Å of meridional reflection and 10 Å of equatorial reflection). The secondary structural transition of monomeric α-syn protein (Mono) into cross-β-sheet rich fibrils (Fib) is observed from circular dichroism spectra. Representative thioflavin-T fluorescence spectra indicate the real-time monitoring of amyloid aggregation assay. The TEM image of α-syn fibril is reproduced from ref. 57 with permission from Elsevier Ltd, Copyright © 2022. The X-ray diffraction image is reproduced from ref. 81 with permission from MDPI, CC BY license. The thioflavin-T kinetic image is reproduced from ref. 47 with permission from Springer Nature, Copyright © 2020. | ||
2. Protein misfolding and amyloid aggregation: a mechanistic overview
The molecular mechanism of protein misfolding and amyloid aggregation from the native state is not straightforward, as amyloid aggregation is not a simple two-step aggregation process. Instead, multiple higher-ordered “on” and “off-pathway” species, which are sometimes difficult to track due to metastability, are associated with the aggregation pathway.60 Moreover, the same protein may follow multiple aggregation pathways depending on the genetic and environmental perturbation, protein concentration, and conformational plasticity.61,62 Amyloid aggregation from all the proteins typically follows a nucleation-dependent polymerization mechanism,63,64 when studied by the amyloid-specific thioflavin-T fluorescence enhancement, which exhibits sigmoidal growth kinetics with three consecutive steps: (1) a very slow nucleation or lag phase, (2) an exponential growth phase for fibril elongation, and (3) a stationary phase or dynamic equilibrium phase of all aggregated species with monomer (Fig. 1a and b).65 The secondary structural transition of protein from the native state to the cross-β-sheet rich amyloid state can also be observed from circular dichroism spectroscopy, which is often used for the biophysical characterization of amyloids (Fig. 1b). The nucleation phase is the kinetic bottleneck in the whole aggregation process due to the high interfacial energy barrier, which prevents amyloid nucleation from the native monomeric protein.66 However, once aggregation-competent nuclei are formed, the elongation phase is thermodynamically downhill with rapid monomer addition along with other secondary processes such as fragmentation, surface-catalyzed secondary nucleation, which produce oligomers, protofibril, and ordered fibrillar species (Fig. 1a).67 It is important to note that the formation of aggregation-competent critical clusters is not a single-step event as explained by the classical nucleation theory.68–70 Rather, amyloid nucleation typically follows a multistep non-classical nucleation pathway with minimal free energy change in each step instead of surpassing a high energy barrier in a single step.71–73 Thus, the metastable pre-nucleation precursors, which further convert into stably growing nuclei, play an important role during the multistep nucleation event.74 For example, folded proteins often undergo partial unfolding promoting non-specific intermolecular interaction to form micellar-like disordered oligomers, which serve as pre-nucleating clusters during amyloid nucleation.71,75 Indeed, partially folded pre-molten globule-like conformation on the lag phase of several neurodegenerative disease-related proteins (such as α-syn, tau, Aβ, and TDP-43) is recognized as a key intermediate, which quickly initiates nucleation due to the solvent-exposed hydrophobic patches.76–78 Interestingly, these partially folded states are reversible with the increase in the transient temperature but immediately induce oligomerization upon prolonged incubation at elevated temperature.79Moreover, the nucleation step can also be facilitated by the structural conversion of the early-stage oligomers into the self-catalyzing seeding-competent conformation, which promotes aggressive growth kinetics.82 Thus, the conformational conversion of oligomers may also act as a rate-limiting bottleneck in the whole aggregation process. For example, primarily disordered α-syn monomers get converted into helix-rich oligomers at the beginning of the elongation phase where the helix-rich conformation shows high aggregation propensity.83 However, during the elongation stage, a dramatic increase in the β-sheet content is observed leading to proto-fibril and fibril formation. Oligomer formation upon protein misfolding indeed plays an important role in the nucleation event of other disease-associated proteins, such as Sup35 protein,84 Aβ,82,85 tau,86 and Huntingtin protein.87 The added complexity of the aggregation-competent structural conversion in the nucleation step is considered in the nucleation–conversion–polymerization model of amyloid aggregation, which indeed supports the multistep nature of amyloid nucleation.84,88
Amyloid nucleation is the most crucial but poorly understood process due to the spatial and temporal resolution limit of instruments for detecting transient events. Primary nucleation is an important mechanism in the lag phase, which comprises the formation of the first aggregation-competent stable nuclei through monomer self-assembly (Fig. 2a).89 When the primary nucleation event starts from a homogeneous protein solution, the process is known as homogeneous primary nucleation.90 Since the monomer addition and dissolution rates are comparable in the homogeneous bulk solution, the primary nucleation becomes an exceedingly slow event. However, in the presence of a hydrophobic/hydrophilic interface or air–water interface, the primary nucleation kinetics often gets accelerated and the associated mechanism is termed heterogeneous primary nucleation (Fig. 2a).91 In the heterogeneous nucleation mechanism, the protein molecules attain a preferential conformation at the interface to maximize the overall hydrophilic and hydrophobic interactions, which promotes protein misfolding into nucleation-competent conformation due to surface adsorption.82,92 Thus, most in vitro aggregation assays require sample agitation, which is often performed by shaking, stirring, or rotating protein to introduce an air–water interface for faster nucleation.93 Moreover, nucleation-promoting surfaces, such as membranes,94 phospholipid vesicles,95 hydrophobic nanoparticles,96 and beads97 are also added externally to facilitate heterogeneous primary nucleation during the aggregation assays. If a protein follows only the primary nucleation mechanism, either it will never come out from the lag phase of aggregation or it will take an enormously long time to initiate the aggregation.64 The lag phase gets shortened when secondary nucleation starts to take place where oligomers and preformed fibrillar species, known as seeds, act as templates for the self-catalytic addition of other oligomers and/or monomers (Fig. 2a).98
 | ||
| Fig. 2 Molecular mechanism of primary nucleation and secondary nucleation: (a) a simplistic representation of primary and secondary nucleation in the amyloid aggregation pathway. Primary nucleation can be initiated directly from a homogeneous solution or can be surface-assisted forming growth-competent oligomers. Secondary nucleation comprises several molecular events, such as fibril elongation, surface-catalyzed secondary nucleation, and fragmentation, which aggressively generate amyloid fibrils. (b) Global fitting of amyloid-β42 aggregation kinetics at varying monomer concentrations demonstrating multiple ongoing nucleation mechanisms during fibril formation. (Top) Primary nucleation only with no secondary pathways, (middle) fragmentation along with primary nucleation, and (bottom) secondary nucleation in addition to primary nucleation acts as a predominant molecular mechanism during amyloid aggregation. The images are reproduced from ref. 99 with permission from Proceedings of the National Academy of Sciences. (c) Super-resolution microscopy images indicating direct experimental observation of seed size-dependent secondary nucleation mechanism of α-syn fibril. The left panel indicates surface-mediated secondary nucleation resulting branching of the daughter fibril (green) from a long fibrillar seed (red) whereas, the right panel indicates elongation as a prominent mechanism from shorter fibrillar seeds by monomer addition at both ends. Figure (c) is reproduced from ref. 100 with permission from the American Chemical Society, Copyright © 2022. | ||
Although fibril-catalyzed secondary nucleation and heterogeneous primary nucleation both follow a surface-mediated aggregation mechanism, the secondary nucleation process is much more aggressive due to multiple simultaneous processes such as fibril elongation, fragmentation, and autocatalytic aggregate formation (Fig. 2b), which are collectively exhibited as exponential growth kinetics of amyloid aggregation.56,101 The primary nucleation step of the amyloid aggregation can be entirely surpassed by the dose-dependent addition of seeds.89,102 Indeed the seeding nature of amyloid is observed for various neurodegenerative disease-associated proteins99,100,103 and plays an important role in disease propagation in amyloidosis.67 Interestingly, amyloid growth can follow distinct fibril amplification mechanisms depending on the seed size as experimentally observed in α-syn secondary nucleation. While the small seeds dominate the elongation mechanism through monomer addition, the large fibrillar seed facilitates surface-catalyzed secondary nucleation resulting in branching of the fibrils (Fig. 2c). Since fragmentation of fibrils can produce seeds with a range of size distribution, secondary nucleation is a complex heterogeneous process with variation in cellular uptake mechanism and cytotoxicity.
3. Oligomerization in the pathway of amyloid nucleation
Both the primary and secondary nucleation mechanisms result in the formation of early-stage oligomers, which play a crucial role in the multi-step nucleation mechanism of amyloid aggregation.72,75 Oligomer represents a vast umbrella term comprising diverse populations of intermediate protein aggregates ranging from dimer to a few hundred-mers with various secondary structures, morphology, solubility, and stability.104 Early-stage oligomers are metastable, highly soluble, and typically globular, annular, or linear in nature.105 Importantly, recent research has established that early-stage soluble oligomers are the proximate mediator of neurotoxicity for most neurodegenerative diseases104,106–112 and exhibit direct concentration-dependent toxicity in neurons.113,114 The toxicity of the early-stage, soluble oligomer arises due to their small size, high diffusivity, and surface hydrophobicity, which are responsible for an abnormal multitude of interactions with receptors, metabolites, nucleic acids, and cell membranes.107,115 An important aspect of the secondary nucleation process is the associated neurotoxicity due to the aggressive production of heterogeneous oligomeric species.116,117 Interestingly, some studies have pointed out that the ongoing process of amyloid aggregation with heterogeneous species populations shows more toxicity than the individual aggregated species.43 For example, it has been shown that the surface-catalyzed secondary nucleation and the formation of oligomers of Aβ protein are directly proportional to cell death.118 In this line, the Lashuel group has proposed that Aβ toxicity is associated with the process of oligomers transitioning into the fibrillar state that triggers the intracellular cascade leading to cell death.119 On the other hand, Linse and co-authors proposed that fibrillation alone does not drive toxicity, rather, the generation of oligomers through fibril-assisted secondary nucleation is the most toxic event.67 Indeed, the secondary nucleation mechanism has vast implications in neurotoxicity and is predominantly observed for many neurodegenerative disease-associated proteins, including Aβ,99 tau,120 α-syn,121 and prion protein.122 Although secondary nucleation aggressively generates toxic oligomers, small metastable oligomers can also be produced during the primary nucleation mechanism, which is enthalpically favored in the high monomer concentration.123 Often, molecular crowder has been shown to increase amyloid aggregation by enhancing the local protein concentration due to the excluded volume effect.90,124,125 In the diseased scenario, gene multiplication, compromised chaperone activity, and other disease-associated factors can elevate the local protein concentration above the physiological limit increasing the inherent threat of pathogenic oligomerization.126,127 Another recently explored physiological condition, which concentrates protein molecules in a confined volume relies on the phenomenon of liquid–liquid phase separation (LLPS), which has been shown to play a huge role in protein self-assembly, oligomerization, and aggregation process.46,128–131 The following section briefly describes the LLPS process, its potential role in the nucleation event of amyloid aggregation, and its implication in neurodegeneration based on the overwhelming number of recent publications.4. Liquid–liquid phase separation (LLPS): an emerging field in biology
Cells, the fundamental building blocks of life, spatiotemporally control thousands of complex biochemical reactions through compartmentalization, segregating distinct chemical microenvironments within cellular space.132 Compartmentalization through membrane-bound organelles such as the nucleus, mitochondria, Golgi body and endoplasmic reticulum are well-understood through decades where membranes impede permeation of biomolecules as well as regulate molecular transport with specificity and selectivity.133,134 However, cells also achieve compartmentalization through numerous membrane-less organelles such as nucleoli, Cajal bodies, PML bodies, paraspeckles in the nucleus; stress granules, Sec bodies, pyrenoid, germ granules, P Bodies in the cytoplasm; signaling clusters at the membrane (Fig. 3a).135 Since these micron-sized assemblies lack any physical barrier for separating them from the cellular milieu, a long-standing curious research question is how these open macromolecular assemblies maintain their structure and functional integrity. It is interesting to note that back in 1899, Edmund B. Wilson imagined a cell as a densely packed colloidal system and hypothesized protoplasm as a mixture of liquids, in the form of a fine emulsion.136 Moreover, in the early 1900s, Oparin and Haldane proposed that life originates within the membrane-less coacervate-like protocells from the Primordial Soup.137 However, the concept of coacervation in cell physiology has recently gained attention when P granules from Caenorhabditis elegans embryos are reported as spherical liquid droplet-like assemblies formed through the process of the liquid–liquid phase separation (LLPS).138 Over the last decade, LLPS has emerged as a wide-ranging generic phenomenon for membrane-deprived compartmentalization of biomolecules (proteins and nucleic acids), collectively referred to as “biomolecular condensates” due to their condensing property in the cellular milieu (Fig. 3a).139–142 The liquid-like condensates exhibit fast molecular diffusion, which can be tracked by the fluorescence recovery after the photobleaching (FRAP) experiment (Fig. 3b). Other classical features include droplet fusion, thermo-reversibility, and surface-wetting properties that are typically observed in a wide range of biomolecular condensates (Fig. 3c–e). These condensates spatiotemporally regulate a myriad of cellular functions including reversible stress response,143 cell signaling,144 vesicular trafficking,145 transcriptional regulation,146 genomic organization,147 molecular sequestration,148,149 and even cellular fitness.143 Interestingly, the phase-separated condensates can also serve as the reaction crucible for physiologically relevant nucleation reactions, which are observed in the case of mitotic spindle150 and heterochromatin formation.151 Mitotic spindles are majorly nucleated inside membrane-deprived organelles, named centrosomes.152 The spindle formation is initiated through microtubule polymerization where locally concentrated tubulin nucleates the polymerization reaction.153 Moreover, functional phase-separation of the microtubule-associated protein tau has been shown to drive the nucleation process of microtubule formation.154 Similarly, heterochromatin formation initiates from the formation of the nucleated foci from HP1α protein through the process of LLPS.155 | ||
| Fig. 3 LLPS in cell biology: (a) the schematic illustration of several membrane-less organelles in an eukaryotic cell at different locations (nucleus, cytoplasm, and membranes) that are formed through the process of LLPS. The inset shows the experimental observation of membrane-less organelles, such as signaling clusters, nucleolus, and P granules. The signalling cluster image is reproduced from ref. 156 with permission from The American Association for the Advancement of Science, Copyright © 2019. The image of the nucleolus is reproduced from ref. 157 with permission from Elsevier Inc., Copyright © 2016. The P-granules image is reproduced from ref. 138 with permission from The American Association for the Advancement of Science, Copyright © 2009. (b)–(e) Characterization of liquid-like biomolecular condensates: (b) representative FRAP images of α-syn condensate at day 0 (early time point) and day 20 (late time point). FRAP profiles show complete recovery at day 0, confirming liquid-like properties, which gradually solidify with time. The FRAP images and the corresponding profiles are reproduced from ref. 158 with permission from the American Chemical Society, Copyright © 2021. (c) Time-lapse differential interference contrast (DIC) images depicting the fusion event of two spherical nucleoli forming a larger nucleolus. The images are reproduced from ref. 159 with permission from Proceedings of the National Academy of Sciences. (d) Representative DIC images of α-syn condensates showing thermo-reversibility upon heating and cooling. The image is reproduced from ref. 47 with permission from Springer Nature, Copyright © 2020. (e) tau condensates showing glass surface wetting property, characteristics of liquid condensates. The image is reproduced from ref. 160 with permission from John Wiley and Sons, Copyright © 2018. (f) A schematic representation of critical molecular interactions including electrostatic, hydrophobic, hydrogen bonding, aromatic, and cation–π interaction that creates a hierarchy of weak multivalent interaction promoting biological LLPS. | ||
However, the physiological nucleation through biomolecular condensates is actively regulated by cellular homeostasis to prevent the inherent risk of aberrant nucleation of biomolecular aggregation. The intermolecular interaction strength of biomolecules is a key regulatory factor for physiological LLPS, which is often modulated by appropriate environmental conditions (such as pH, salt, and biomolecule concentration) in healthy cells.161,162
The primary driving force for biological LLPS is intermolecular interaction, which can be quantitatively evaluated based on Flory Huggins's theory of polymers.163 In this model, a polymer solution is considered as an infinite lattice where each lattice point is filled by either a polymer or a solvent molecule. So, in the lattice, three types of intermolecular interactions are possible. They are polymer–polymer, polymer–solvent, and solvent–solvent interaction. The empirical Flory interaction parameter (χ) measures the strength of intermolecular interaction, predicting the molecular arrangement in the lattice to minimize the free energy of the system. A negative value of χ (χ < 0) indicates a “good solvent” where heterotypic protein–solvent interactions dominate over homotypic interactions inhibiting phase separation. However, if the polymer–polymer interaction energy is very high in the scale of thermal energy, χ value will be positive (χ > 0), indicating a “poor solvent” condition, which favors phase separation of a polymeric system. Protein indeed can be considered as an associative biopolymer consisting of an array of amino acids where peptide dipole from polar amino acids gives rise to a net attraction between polypeptide backbones promoting phase separation.164–167 While Flory Huggins's theory oversimplifies the polymer nature considering them as a homopolymer; protein, on the other hand, is a complex heteropolymer with diverse amino acid sequences that give rise to multiple simultaneously acting unique intermolecular interactions.168 The molecular feature of protein and polypeptide driving LLPS is encoded in the primary amino acid sequence, where the cumulative effect of weak non-specific multivalent interactions such as ionic, hydrophobic, hydrogen bonding, cation–π interactions, and π–π interactions have been shown to dictate LLPS propensity169–173 (Fig. 3f). Multivalency is the governing force for phase separation, arising from different local structures of both folded and disordered proteins.141 Well-folded globular domains with predisposed associative interaction surfaces can act as “patchy colloids” driving phase separation.174–176 Often multiple repetitive folded domains (e.g. SH3 domains) interspersed by flexible linkers undergo phase separation through multivalency, which has been profoundly observed in signaling clusters.145 The recently developed sticker-spacer model of protein phase separation describes how multivalency arises from the combination of folded and flexible domains.177,178 However, the predominant contributor of multivalency comes from the intrinsically disordered region (IDR) of the proteins with conformational flexibility, which samples multiple weak non-specific interactions simultaneously.179,180 The IDR sequences are often enriched in charged residues (such as aspartic acid, glutamic acid, lysine, and arginine), aromatic residues (such as phenylalanine and tyrosine), and polar residues (such as serine, proline, and glutamine) resulting low-complexity domains (LCD) in disordered proteins. The LCD bias in protein sequence encodes the “fuzzy” interaction mode mediating a multitude of simultaneously showing multivalent interactions.181–183 On the other hand, prion-like domains are another specific class of IDR that is largely devoid of charged residues but enriched with polar moieties (such as serine, tyrosine, glutamine, and asparagine) and aromatic residues (such as phenylalanine and tyrosine) driving phase separation through weak, nonspecific cation–π and π–π interactions interaction.184 Apart from the multivalency, the specific patterning of amino acids in the polypeptide sequence such as a pair of high oppositely charged residues, also promotes intermolecular interaction and phase separation.171 Thus, it is essential to note that instead of a specific intermolecular interaction, different types of interactions based on protein sequence and their pattering contribute simultaneously to generate a hierarchical interplay of intermolecular interactions that results in LLPS of proteins and polypeptides.170,179,185,186 Interestingly, IDR, LCD, and PrLD, which are the predominant driving factors for LLPS are profoundly present in all neurodegeneration-disease-related proteins, making them suitable for undergoing LLPS.187–189 For example, TDP-43 and FUS contain large stretches of PrLD;190 whereas α-syn and tau are intrinsically disordered in nature with LCDs containing charged residues.191 Indeed, recent studies have shown that major neurodegenerative disease-associated proteins such as α-syn,47 tau,160 Prion protein,192 Huntingtin,193 FUS,194 TDP-43,48 and hnRNPA1,195 TIA1,196 C9orf72197 have an inherent property to undergo phase separation. However, the nature of the major intermolecular interaction driving LLPS of neurodegeneration-associated proteins may vary from protein to protein. While π–π and cation–π interactions in the LCD drive LLPS of FUS, TDP-43, and hnRNPA1,48,195,198 whereas tau protein undergoes LLPS mainly through electrostatic interaction;160,199 α-syn LLPS is mainly driven by the hydrophobic interaction from the exposed NAC domain.158 When these aggregation-prone proteins accumulate inside liquid droplets, the local concentration of the protein becomes enormously high and raises an innate threat for aggregation. Thus, understanding the time-dependent changes within biomolecular condensates and their consequence in protein aggregation has become a progressively important research aspect.
5. Liquid-to-solid phase transition: implication in neurodegeneration
Most intracellular functional condensates are liquid-like and highly dynamic in nature with rapid molecular exchange and environment-responsive reversibility, which are critical for their functional purpose.200 The liquid-like dynamicity of condensates arises from short-range, weak, nonspecific interaction, which rapidly breaks and reforms within a pico-to nanosecond timescale.200 Moreover, the biological liquid is inherently active with persistent energy-consuming reactions and molecular flux, which maintains its extremely dynamic nature, quick reversibility, and molecular composition.142,201 In this regard, it is important to note that biomolecular condensate liquids are not simple Newtonian liquids (whose viscosity is independent of the rate of sheer stress).202 Rather, they behave as viscoelastic Maxwell fluid with a complex network-like arrangement where network strength dictates different material states of biomolecular condensates.203,204 Hence, biomolecular condensates are extremely metastable, where the protein-dense liquid phase can be transitioned into a range of solid-like condensed phases (such as gel, amorphous aggregates, glassy solid, kinetically-trapped assemblies, crystal, and amyloid fibrils) (Fig. 4). This solid-like transition is mainly assisted by strong long-range interactions that provide a long bond lifetime, structural specificity, less dynamicity, and more stability.205 The solid-like condensates serve important functionalities in cells; such as molecular sequestration (e.g., Balbiani Body, Amyloid-Bodies), organization hub (e.g., heterochromatin), reaction crucible (e.g., centrosomes), permeability (e.g., nucleopore complex), stress adaptation (e.g., stress granule), and polymerization (e.g., microtubule) (Fig. 4a). However, the active cellular machinery tightly regulates the condensate's material property, preventing the time-dependent aberrant solidification. Moreover, the active cellular processes also regulate the time-dependent disassembly of solid-like, non-dynamic condensates after serving their functionality.149 For instance, time-dependent viscoelastic transition has been observed as a stress survival strategy for yeast prion protein SUP35, which undergoes reversible gelation upon molecular sequestration (Fig. 4b).207 Even, functional amyloids formed through the liquid-to-solid transition of biomolecular condensates, such as Balbiani bodies and amyloid bodies, also undergo time-dependent dissolution after their functions, suggesting that cells indeed strategically utilize the diverse material states of condensates for their benefits (Fig. 4c).149 However, in the compromised cellular machinery, the unregulated viscoelastic transition is often irreversible in nature, which can be deleterious to cells.46,210 | ||
| Fig. 4 Liquid-to-solid phase transition in cell physiology: (a) (left) schematic representation showing the viscoelastic transition of biomolecular condensates with diverse material properties through different molecular mechanisms. While entanglement of the protein chain leads to amorphous glassy solid formation, network-like physical cross-linking results in vitrification or gelation, and cross-β arrangement produces amyloid fibrillation. (Right) Microscopy images of solid-like non-dynamic biomolecular condensates with diverse functionality. The image of Pab1 is reproduced from ref. 206 with permission from Elsevier Inc., Copyright © 2017. Sup35 image is reproduced from ref. 207 with permission from the American Association for the Advancement of Science, Copyright © 2018. The image of Balbiani body is reproduced from ref. 208 with permission from Elsevier Inc., Copyright © 2016. The amyloid body image is reproduced from ref. 209 with permission from Elsevier Inc., Copyright © 2016. The image of the centrosome is reproduced from ref. 150 with permission from Elsevier Ltd, Copyright © 2018. (b) Characterization of time-dependent liquid-to-gel transition of yeast prion protein SUP35 as observed from progressively low FRAP recovery. The images are reproduced from ref. 207 with permission from the American Association for the Advancement of Science, Copyright © 2018. (c) and (d) Balbiani body enriched with Xvelo protein forming functional amyloid through phase separation. Fluorescence images indicating thioflavin-T binding and TEM images showing fibrillar structure confirm the amyloid nature. Both (c) and (d) are reproduced from ref. 208 with permission from Elsevier Inc., Copyright © 2016. | ||
In fact, several human diseases, ranging from genetic disease,211 metabolic diseases,212 cardiovascular disease,213 viral infection,214,215 neurodegeneration,44,45,216–218 and cancer219,220 are reported to be preceded by aberrant liquid-to-solid phase transition of biomolecular condensates. During the aberrant liquid-to-solid transition, the physical cross-linking in the network-like liquid can enhance with time leading to vitrification or kinetically arrested states (e.g. gel or amorphous glassy solids) (Fig. 4a).221 It was suggested that the complex network-like microenvironment arises from the sequence-specific multivalent aromatic interaction, prevalently present in PrLD, which acts as stickers while spacer amino acids (such as glycine, serine, and glutamine) impart flexibility, reversibility, solubility and regulate the overall liquid-like material property of condensates.169 Above a threshold sticker concentration in protein, a system-spanning geometric transition exists, resulting in percolation or gelation due to multiple associative intermolecular interactions from sticker residues.222 Apart from the physical cross-linking, a second type of liquid-to-solid transition of protein condensates may occur due to the entanglement of polymeric chains around each other, resulting in amorphous solidification (Fig. 4a).223,224 A third type of liquid-to-solid transition can occur when the high local protein concentration within condensates either allowing the otherwise forbidden molecular interactions or triggering protein misfolding, resulting in the most stable cross-β-sheet rich amyloid aggregation (Fig. 4a).230 Indeed, several neurodegeneration disease-related protein condensates often lose the liquid-like dynamic properties (e.g., diffusion, coalescence, reversibility, etc.) with time and aberrantly produce amyloidogenic fibrous aggregates (Fig. 5).43,210,232,233 For example, transmission electron microscopy images clearly showed time-dependent morphology change of α-syn condensates where fibrils burst out from aged condensates (Fig. 5).47 Similarly, RNA-binding proteins FUS and hnRNPA1, also show liquid-like dynamic condensates at early time points, which rigidify with time (Fig. 5).195,227 Moreover, fibrils have been seen to protrude from the surface of the solid-like FUS condensates finally adopting a start-burst-like fibrous morphology upon condensate aging (Fig. 5).194 These solid-like condensates of neurodegenerative proteins, such as α-syn, tau, FUS, TDP-43, and Prion protein also bind to amyloid-specific dyes, like thioflavin-S and thioflavin-T, thus confirming their amyloid-like nature.47,160,234 Often the aberrant liquid-to-solid transition of the condensates from these neurodegenerative disease-related proteins results in irreversible mesh-like hydrogel formation, comprising amyloid aggregates, which has been reported for α-syn,47 tau,235 FUS,198 TDP-43,236 hnRNPA1,237 hnRNPA2.238 These porous hydrogel-entrapping solid condensates may act as a repository for toxic oligomers and other aggregated species. The hypothesis was strengthened based on our previous observation that α-syn hydrogels entrap helix-rich oligomers and short fibrils and exert neurotoxicity.239 Short-repeat-containing RNA, which is hugely deposited in the pathogenic inclusions such as in the case of HD, ALS, FTD, and muscular dystrophy, also plays a critical role in aberrant phase transition and sol–gel transition of these protein condensates.240 Consistent with the in vitro LLPS, phase separation of proteins associated with neurodegeneration is extensively observed in the relevant cellular microenvironment, which is converted into pathogenic inclusions by following various mechanisms.47,160,195,227,231 For example, disease-relevant conditions promote aberrant condensate formation leading to rapid transitions into pathogenic solid-like condensates (Fig. 6a).47,241
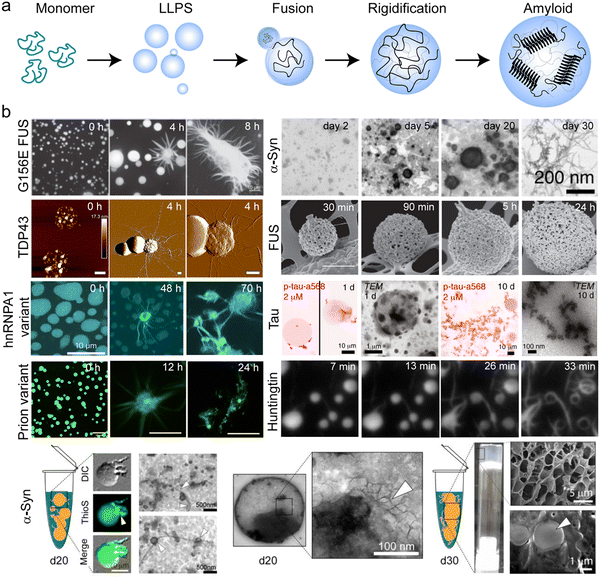 | ||
| Fig. 5 Aberrant liquid-to-solid transition of neurodegeneration-associated proteins: (a) schematic representation of the liquid-to-amyloid transition through LLPS. The monomeric protein undergoes self-assembly followed by LLPS, where liquid droplet grows in size through fusion. Protein-rich condensates eventually rigidify with the progressive generation of amyloid aggregates. (b) (Top) Time-dependent generation of amyloid fibrils due to solidification of biomolecular condensates by major neurodegeneration-related proteins, α-syn, tau, FUS, G156E mutant, TDP-43, Huntingtin, hnRNPA1 variant, and Prion variant Y145Stop. Images of α-syn are reproduced with permission from ref. 47 from Springer Nature, Copyright © 2020. Images of FUS are reproduced from ref. 225 with permission from Proceedings of the National Academy of Sciences. Images of tau are reproduced from ref. 160 with permission from John Wiley and Sons, Copyright © 2018. The images of the Huntingtin protein are reproduced from ref. 226 with permission from Cell Press, CC BY license. Images of G156E FUS are reproduced from ref. 227 with permission from Elsevier Inc., Copyright © 2015. Images of TDP-43 are reproduced from ref. 48 with permission from Elsevier, CC BY License. Images of the hnRNPA1 variant are reproduced with permission from ref. 228 from Springer Nature, Copyright © 2023. Images of the Prion variant are reproduced from ref. 229 with permission from Proceedings of the National Academy of Sciences. (Bottom) Emergence of thioflavin-S positive fibrillar structure from amyloidogenic α-syn condensates indicating amyloid fibril accumulation within condensates. Further, at a later time point, the LLPS solution transforms into fibrillar hydrogel containing solid condensates. The bottom panel of images are reproduced with permission from ref. 47 from Springer Nature, Copyright © 2020. | ||
 | ||
| Fig. 6 In-cell and in vivo aberrant phase separation of neurodegenerative disease-related proteins. (a) Schematic representation of aberrant phase separation leading to pathological solidification in a cellular model. Metal ions can trigger the formation of liquid-like biomolecular condensates, which can progressively solidify with time generating toxic aggregates. Further, environmental stress can promote stress-granule-like functional LLPS, which may turn into aberrant cytoplasmic aggregates upon prolonged stress. (b) Phase separation of neurodegeneration-associated proteins tau, TDP-43, Huntingtin, hnRNPA1, FUS and α-syn in the cellular microenvironment. The images of in-cell tau condensates are reproduced from ref. 160 with permission from John Wiley and Sons, Copyright © 2018. The images of TDP-43 condensates are reproduced with permission from ref. 231 from Elsevier Inc., Copyright © 2019. The images of Huntingtin condensates are reproduced from ref. 232 with permission from eLife, CC BY License. The images of hnRNPA1 condensates are reproduced from ref. 195 with permission from Elsevier Inc., Copyright © 2015. The images of FUS condensates are reproduced with permission from ref. 227 from Elsevier Inc., Copyright © 2015. The in-cell α-syn condensate images are reproduced from ref. 47 with permission from Springer Nature, Copyright © 2020. (c) PD-relevant Fe3+ exposure promoting α-syn condensate formation in HeLa cells leading to perinuclear aggresome formation as observed with aggresome-specific proteostat marker. Images are reproduced from ref. 47 with permission from Springer Nature, Copyright © 2020. (d) C. elegans in vivo model demonstrating liquid-like condensates in young population (day 7) while PD-relevant ubiquitinated inclusions in aged nematode population (day 15). Images are reproduced from ref. 233 with permission from Oxford University Press, Copyright © 2021. | ||
On the other hand, solid-like stress granule condensates, which were initially functional, can be converted into pathogenic solid-like condensates upon prolonged stress exposure242 (Fig. 6a). Indeed, all the major proteins associated with neurodegeneration, such as α-syn,47 tau,160 FUS,227 TDP-43,231 Huntingtin,232 and hnRNPA1195 have been experimentally observed to form aberrant condensates in a cellular milieu with potential pathological implications (Fig. 6b). For example, α-syn forms liquid-like pan cellular condensates in HeLa cell upon metal ion stress, which gradually covert into amyloidogenic solid assemblies and accumulates in the perinuclear region forming aggresome (Fig. 6c).47 The viscoelastic liquid-to-solid transition of α-syn condensates was also observed in the in vivo C. elegans model where the aged C. elegans accumulates amyloid-like inclusions, enriched with ubiquitin (Fig. 6d).233 The growing evidence on amyloid-like condensate maturation preceded by LLPS suggests that LLPS may play a key role as an early molecular event of pathogenic protein aggregation associated with neurodegeneration.
6. Molecular mechanism of amyloid aggregation from biomolecular condensates
6.1. Nucleation mechanism associated with aberrant phase separation
Amyloid aggregation from protein monomers follows an interconnected multistep nucleation process, which dictates the complexity of the nucleation landscape.243 The phenomenon of LLPS can potentially serve as an intermediate step where the nucleation and growth of biomolecular condensates make a suitable microenvironment to facilitate the nucleation toward amyloid aggregation further. Thus, to elucidate the molecular mechanism of amyloid aggregation from biomolecular condensates, one needs to focus on two overlapping nucleation events; the nucleation mechanism for LLPS along with the nucleation mechanism for liquid-to-amyloid transition. The biological phase separation may be initiated through a nucleation-dependent growth mechanism where intermolecular interaction dictates the formation of growth-competent critical nuclei above a threshold solute concentration, designated as the saturation concentration.244 Above the saturation concentration, the protein solution enters the metastable binodal regime, where thermal fluctuation initiates a nucleation event for phase separation. The nucleation event for physiological phase separation is an active process in the cellular microenvironment that regulates the formation, growth and dissolution of the biomolecular condensates.140,245 In this context, a recent study quantitatively investigated early molecular events of α-syn and Synphilin1 protein (α-syn interacting protein and a common tracer for the misfolded protein aggregates in LB), in the growth conditions of healthy mammalian cells.246 The study indicated that healthy mammalian cells contain heterogeneous size distribution of protein clusters, indicating a supersaturated protein solution associated with a nucleation energy barrier where clusters above a critical size are capable of further growth. Interestingly, the putative chaperone maintains tight proteostasis regulation, which preferentially disassembles large clusters, thus avoiding surpassing the nucleation energy barrier toward aberrant cluster growth.246 Important to note that, the nucleation and coalescence kinetics of biomolecular condensates of physiologically regulated phase separation are distinctly different from those of unregulated pathological phase separation, which shows altered size-distribution of condensates as well as their abnormal growth dynamics.247 Actively regulated native condensates show exponential size distribution with a single mean size as a result of discrete nucleation events whereas neurodegeneration-associated mutant Huntingtin protein yields broad power-law size distribution of condensates as a result of continuous nucleation events.247 Transient oligomerization of protein is also an important regulatory factor in modulating the nucleation energy barrier for LLPS.248–252 For example, TDP-43 can undergo phase separation without low complexity PrLD but the N-terminal oligomerization domain is essential for phase separation.253 Mutation and other structural modifications in the oligomerization domain can potentially alter the nucleation energy barrier of the phase separation with pathological implications. For example, UBQLN2, a protein quality control member of stress granule, undergoes LLPS through its proline-rich oligomerization domain.254 Interestingly, ALS-associated mutation on the proline-rich domain of UBQLN2 enhances its oligomerization propensity, decreasing the saturation concentration of LLPS and promoting an aberrant liquid-to-solid transition.255 Oligomer size can also vary with disease-associated mutation, as seen in tumor suppressor speckle-type POZ protein (SPOP), which is capable of altering the critical cluster formation rate, in other words, the nucleation rate.256 Transient oligomeric self-assembly may form nanoclusters below the saturation concentration, playing an important role in nucleating macroscopic phase separation, which indeed has been experimentally detected for several neurodegeneration-related proteins.249–252,257 For example, micron-sized condensate formation of hnRNPA1 low complexity domain follows a multi-step nucleation mechanism, which starts by forming low-affinity nanoclusters at the sub-saturated protein concentration.250 After the initial energetically uphill nanocluster formation, the monomer starts to be recruited with high affinity to form the observable condensates. A similar nanoscale cluster formation in the subsaturated protein concentration is observed for other disease-associated proteins, such as FUS, α-syn, and other RNA-binding proteins.249,252 As the protein concentration increases from sub-saturation concentration to saturation concentration, sequence-encoded molecular interaction drives nanoclusters toward macroscopic phase separation.252 Mutations, post-translational modification, and other molecular interactions can significantly alter the kinetics of these early molecular events, directly impacting the nucleation rate for aberrant protein aggregation.249,252 Indeed, Alzheimer's disease-associated familial mutations P301L and P301S of tau protein are shown to enhance the kinetics of pathogenic amyloid fibrillation from LLPS, initiated through nanocluster formation at subsaturated concentration.257 However, cluster formation at subsaturated concentration and macroscopic phase separation at supersaturated concentration may not always be coupled with each other as several mesoscopic clusters have been reported and many of them directly nucleate amyloid fibrils without forming macroscopic phase separation.258–261 Future research is necessary to explore the role of different mesoscale clusters in the complicated nucleation mechanism of LLPS. The regulation in the nucleation process of LLPS is indeed the key factor for preventing aberrant phase separation. The cellular regulation is even more challenging because most proteins are expressed close to their solubility limit as indicated by a proteome-wide study on Caenorhabditis elegans.262 This is advantageous for nucleating functional LLPS but alarming for cells against pathological nucleation. Moreover, the same study also showed that during aging, the total quantity of protein aggregation sharply increases and cellular machinery for maintaining protein solubility is gradually compromised.262 Since cellular protein concentration is on the verge of solubility, the nucleation event will likely act as a checkpoint for preventing aberrant phase separation in cells by active regulation.6.2. Amyloid nucleation from biomolecular condensates
Amyloid aggregates are the most stable thermodynamic states in the energy landscape of protein aggregation while biomolecular condensates are inherently metastable, especially for aggregation-prone proteins.263 The enhanced intermolecular interaction and reduced desolvation energy upon LLPS energetically favor the primary nucleation of amyloid aggregation, which is otherwise an extremely slow bottleneck process in the traditional aggregation pathway.264 Although the precise molecular mechanism of primary nucleation is not yet fully understood, it is reasonable to speculate that the high local protein concentration within the condensate can alter the native intermolecular interaction of protein, facilitating protein misfolding. Intriguingly, most of the neurodegeneration-associated intrinsically disordered proteins upon phase separation show conformational bias towards extended conformation due to stabilizing lateral intra-chain contacts, which might allow the otherwise inaccessible critical interaction for initiating amyloid nucleation.158,257,265–272 For example, the autoinhibitory conformation of α-syn due to the electrostatic interaction between the N-terminal and C-terminal protects the NAC domain from surface exposure.158 However, low pH or high salt conditions screen the surface charge of α-syn, disrupting the autoinhibitory conformation and leading to the exposure of the hydrophobic NAC domain.269 The alteration of intermolecular interaction due to conformational change is likely to reduce the nucleation barrier, manifesting as a faster liquid-to-amyloid transition at low protein concentration. Similarly, the autoinhibitory “paperclip” conformation of tau also gets extended upon LLPS exposing the microtubule-binding domain (MTBD).257,268 The aggregation-prone hexapeptide and regulatory KXGS motifs in the exposed MTBD domain attain a transient β-hairpin structure in the phase-separated condition leading to irreversible amyloid aggregation through phase separation.273 Full-length prion protein also undergoes conformational expansion during phase separation where exposed N-terminal proline and glycine-rich octapeptide (PHGGGWGQ) repeat rapidly to form a short cross-β motif, which drives amyloid aggregation within condensates.271 Protein misfolding due to conformational expansion can also be triggered at the condensate interface to maximize the protein–protein and protein–solvent interaction simultaneously.225,228,274,275 For example, the prion-like low complexity domain of protein hnRNPA1 shows reversible percolated crosslinking architecture in the condensate's interior while the interface shows the most-expanded protein conformation with a preferential perpendicular orientation toward the interface.270 Interestingly, the condensate interface possesses some unique molecular properties compared to the bulk interior, which may lead to a faster solidification.270 Indeed, the liquid-to-gel transition of FUS condensates is also experimentally observed to initiate from the interface, reminiscing a core–shell inhomogeneous architecture, where the fibrillar network gradually propagates towards the center.225 Moreover, amyloid fibrillation can preferentially occur only from the condensate interface, not from the bulk solution as observed for condensates of the low-complexity domain of hnRNPA1.228 Similarly, super-resolution single-molecule spectroscopy on FUS condensate revealed the formation of distinct nanoscale amyloid aggregates, which preferentially form on the condensate surface and restrict the local molecular diffusion generating a heterogeneous architecture of low-diffusive shell and diffusion-permitting condensate interior.275 Thus, the spatiotemporal heterogeneous microenvironment of condensate can effectively nucleate amyloid fibrillation, representing an emerging novel aggregation mechanism for disease-associated proteins.6.3. Oligomer toxicity and polymorphism associated with LLPS-mediated aggregation pathway
The conventional amyloid aggregation process often generates non-fibrillar off-pathway oligomers and pre-fibrillar on-pathway oligomeric species, which exert proximate cytotoxicity as compared to the matured amyloid fibrils.276–279 In fact, in some cases, the formation of the inclusion bodies has been proposed to be a neuroprotective response for sequestrating toxic oligomers and aggregated species.280 Interestingly, on-pathway and off-pathway toxic oligomers are also observed to accumulate within condensates, deciphering their pathological relevance in neurodegeneration. For example, prolonged incubation of tau condensate results in a time-dependent accumulation of soluble neurotoxic oligomers, which is further accelerated by FTD-associated mutation P301L.268 These oligomer-rich tau condensate does not significantly populate fibrillar aggregates with time suggesting that phase separation could be a mechanism to accumulate toxic “off-pathway” oligomers. Indeed, stress-granule protein TIA1 (T-cell intercellular antigen 1), which is a known driving factor for tau oligomerization and tau-dependent neurotoxicity, promotes the formation of toxic oligomers upon phase separation and inhibits the conversion of oligemic tau into fibrillar aggregates.129 Interestingly, small molecule-based inhibitors, such as C1 and Shikonin are designed to target LLPS-mediated oligomerization to alleviate cytotoxicity in neuroblastoma cells.281,282 A crucial factor in the toxicity mechanism is the membrane interaction where misfolded oligomers interact with membrane protein and disrupt biological membranes leading to mitochondrial dysfunction, calcium imbalance and reactive oxygen species generation.283 For example, soluble amyloid-β oligomers (AβOs) are known to trigger downstream neurotoxicity by binding cell surface receptors.284 A recent study showed that membrane-mimicking in vitro conditions can induce LLPS of AβOs where the protein attains membrane-binding toxic α-helical conformation.285 Prolonged incubation of AβOs condensates leads to amyloid fibril formation suggesting a possible on-pathway toxic oligomerization during LLPS. In this regard, another study has reported that amyloid-β oligomers isolated from AD autopsy brain lysate can co-phase separate with cellular prion protein at the plasma membrane leading to toxic hydrogel formation, which can trap neurotoxic co-receptor mGluR5.286 Similar to amyloid-β oligomers, our lab has previously observed that the structural transition of α-syn into cross-β amyloid accelerates through the formation of hydrophobic surface exposed helix-rich oligomers, which show cytotoxicity to SHSY-5Y cells.83 These cytotoxic α-helical oligomers have also been shown to accumulate in the hydrogel formed through the cross-β fibrillation.239 Interestingly, α-syn phase separation also generates a certain population of helix-rich oligomers within the condensates during the time course of condensate aging indicating a potential link with oligomer toxicity in the novel aggregation pathway.47 Indeed, a recent study has shown that a class of evolutionary conserved small EDRK-rich factors (SERF), which impede the accumulation of α-synuclein oligomers during the LLPS-mediated amyloid aggregation, exhibit reduced cellular toxicity.287 Thus, SERF might accelerate the conversion of highly toxic oligomers to less toxic amyloid aggregates to mitigate oligomer toxicity as a protective mechanism. Often, disease-associated mutation disrupts the physiological oligomerization and phase separation resulting not only in the loss of native functional role but also in gaining toxicity. For instance, TDP-43 self-oligomerization is a key step to trigger functional phase separation, which is hampered by ALS-associated mutations, A315T and M337V.288,289 These mutations not only hinder the native autoregulatory function of condensates but also disrupt the liquid property of condensates resulting in compromised protein homeostasis. Similarly, FTD-associated mutations (ΔK280, P301L, P301S, A152T) trigger the pathogenic oligomerization of tau during LLPS, which acts as a precursor for amyloid aggregation.160 However, further investigation is necessary to explore the mechanism of oligomerization from condensates and their structure–toxicity relationship in disease progression. Future research should also thoroughly investigate the role of membrane interaction in the toxicity mechanism of phase-separated oligomer-rich assemblies. Polymorphism is another classical feature of amyloid fibril at the molecular level, which generates multiple distinct fibrillar strains from the same protein with strain-specific diverse biological activity.81,290–293 The polymorphic nature of amyloid fibril results in clinical and pathological variation, which further complicates the toxicity mechanism in neurodegeneration.81,294,295 Interestingly, the LLPS-mediated aggregation pathway can produce distinct fibrillar polymorphs, as recently observed for α-syn.296 α-Syn aggregates preferentially attain an antiparallel β-sheet arrangement when initiated from a water-poor droplet microenvironment in contrast to the parallel β-sheet conformer commonly found when preceded at hydrophilic/hydrophobic interface.296 Interestingly, toxic oligomers of α-syn have been previously reported to generate aggregated species with antiparallel β-sheet conformation, which was speculated as “off-pathway” intermediates.297–299 The novel LLPS-mediated aggregation pathway may explain these intermediates as “on pathway” species and may also indicate a strong connection with toxicity. It is important to note that, high-resolution structural studies revealed that the in vitro amyloid fibrillar strain differs significantly from the patient-derived strain, as reported for several neurodegeneration-associated proteins.300–302 Future research should explore the high-resolution structural details of amyloids formed from the crowded protein-rich condensates, which may elucidate a new insight into fibril polymorphism and its implication in in vivo pathogenicity.6.4. Seeding-dependent amyloid aggregation within biomolecular condensates
Amyloid aggregation within biomolecular condensates can also follow autocatalytic amplification and seeded kinetics, which are the classical mechanisms for aggressive fibril production (Fig. 7a). Since liquid-like nascent condensates allow rapid molecular exchange, small preformed fibrillar seeds can be internalized rapidly within the condensates and can act as a template to initiate aberrant aggregation. Indeed, the addition of pre-formed fibrillar seed triggers dose-dependent rapid amyloid aggregation from α-syn condensates suggesting seeded-kinetics as a prominent molecular event in the novel aggregation pathway (Fig. 7b and c).303 Moreover, when exposed to preformed fibrillar seeds, α-syn condensates formed in HeLa cells and PD-relevant SH-SY5Y cells undergo a liquid-to-solid transition into phosphorylated amyloid aggregates, which may aid in prion-like cell-to-cell-transmission (Fig. 7d).304 Apart from the external addition of seeds, the amyloidogenic aggregates produced within condensates can also serve the seeding purpose for further auto-amplification of aggregates (Fig. 7a). For example, the liquid-to-solid transition of human prion protein shows the seeding behaviour producing self-replicating autocatalytic amyloid aggregates, a characteristic of the prion-like transmission mechanism.229 Sometimes, a small segment of a few amino acids within a large protein may have a high propensity for cross-β sheet structure formation, which may also act as an efficient endogenous seed in the concentrated microenvironment of phase separation to trigger the overall protein aggregation into amyloid. The phenomenon has been observed for the FUS low complexity domain where short amyloid-prone peptide segments show seeded aggregation kinetics on full-length FUS through a phase separation.305 Interestingly, in the phase-separated system, the presence of tiny seeds that are either endogenously formed (or added exogenously), can initiate simultaneous amyloid aggregation in the dense and dilute phases. This might lead to polymorphic amyloid formation from the same solution (Fig. 7a). For instance, the liquid-to-solid transition of TDP-43 condensates results in the accumulation of β-sheet-rich amyloid fibrillar structure within condensates. Interestingly, amyloid aggregation outside the condensate is also observed at a later time point indicating multiple simultaneously acting amyloid aggregation mechanisms in the LLPS solution.306 Intriguingly, the study further revealed that the amyloid fibrils formed within the condensate are distinct from the dilute phase fibril, which suggests that the heterogeneous microenvironment of the LLPS solution can generate structural polymorphs. Understanding the seed-dependent polymorphism in the LLPS-mediated aggregation pathway might open up a huge future research scope in neurodegeneration. Important to note that, the viscoelastic transition of biomolecular condensates can result in spatiotemporal heterogeneity in the condensate microenvironment. Indeed, during the liquid-to-solid transition, condensate from a single protein can progressively convert into multi-phasic architecture with spatial diversity in the molecular property within the condensates.307 The multi-phasic behavior can enormously affect the seeding mechanism of amyloid aggregation as small seeds can be spatially accumulated at different locations within a heterogeneous condensate microenvironment. An interesting observation in this regard is, that the hyperphosphorylated tau generates seeding-competent amyloid aggregates, which can initiate seeding inside and on top of the condensates during liquid-to-solid transition.160 The condensate interface is particularly efficient in preferentially accumulating fibrillar seeds and enhances amyloid aggregation.274,308 A curious question would be how the liquid-like condensates maintain spatial control for seed localization and aggregation. A recent theoretical model has explained that even the weak interaction within liquid condensates can effectively sequester fibrillar aggregates in high concentration and the sequestration efficiency increases with the condensate size.309 Moreover, partitioning seeds within condensates may suppress the seed availability in the dilute phase and effectively alter the aggregation kinetics outside the condensate. Future research can delineate the seeding mechanism in the aggregation pathway, which may decipher some intriguing insights into the relevance of secondary nucleation, oligomerization, amyloid polymorphism and toxicity in neurodegeneration. | ||
| Fig. 7 Secondary nucleation and seed-mediated amplification of amyloid fibrils: (a) schematic representation illustrating the different possibilities of seed-dependent aggregation mechanism from biomolecular condensates. The amyloid fibrils formed within biomolecular condensates can act as a sink for monomer conversion into amyloid aggregates. The fibrils coming out from condensate can seed amyloid aggregation in the bulk solution. Further, seeds can nucleate new condensate promoting phase separation providing further possibility for accelerating solid transition. All these mechanisms can act simultaneously in the LLPS solution resulting in autocatalytic amplification of amyloid fibrils. (b) Fluorescence images indicating in vitro evidence of seeding mechanism within α-syn condensates when 2% preformed seed (green) is added during α-syn LLPS (red). (c) Global fitting of thioflavin-T kinetic profile showing dose-dependent seeding mechanism within α-syn biomolecular condensates suggesting secondary nucleation as the dominant aggregation mechanism in the presence of preformed fibrillar seed. Images for (b) and (c) are reproduced from ref. 303 with permission from Proceedings of the National Academy of Sciences. (d) In-cell seeding-dependent α-syn aggregation within condensate observed in HeLa cell. α-Syn condensates (green) turned into amyloidogenic solids in the presence of preformed fibril (red), which changes condensate morphology into irregularly shaped aggregates with time. Images are reproduced with permission from ref. 304 American Association for the Advancement of Science, CC BY Licence. | ||
7. Effect of neurodegeneration-associated factors on aberrant phase separation and amyloid aggregation
Neurons are extremely vulnerable to aberrant phase separation as the postmitotic cells cannot discard pathogenic self-assemblies through cell division and therefore, rely only on the protein-quality control machinery.310,311 Aging can potentially change the cellular proteostasis machinery with altered protein expression, localization, and degradation processes that hamper the tight regulation required for physiological LLPS.312–314 Moreover, aging can also affect protein quality control by compromising chaperone activity, receptor binding and ATP production, which in turn affects the condensate regulation.315 For example, inhibition of molecular chaperone HSP70 makes stress granule-associated protein (FUS, TDP-43, etc.) susceptible to aberrant liquid-to-solid transition by delaying the post-stress dissolution of stress granules.316 ATP is another important regulatory factor in preventing aberrant liquid-to-solid transition, which might act as a biological hydrotrope.317 Since neurons are extremely ATP-demanding due to very high metabolic activity, insufficient ATP supply upon aging can potentially hamper protein quality control in neurons, which in turn may promote neuronal protein for aberrant liquid-to-solid transition.318 Even though the mechanism for the loss of function and/or gain of toxic function of biomolecular condensates is yet elusive, altered cellular homeostasis in diseased conditions can initiate the formation of pathological phase separation and/or can convert physiological condensate to a pathological one.245,319,320 For example, the LLPS of FUS is quite critical for initiating DNA damage repair as demonstrated by the failure of the LLPS-deficient FUS variant to catalyze DNA repair at the site of the damage.321 This DNA damage response is impaired in the case of FUS mutation in the nuclear localization signals (NLS), which leads to aberrant liquid-to-solid phase transition and cytoplasmic accumulation of FUS aggregates.194,322–324 Similarly, disease-associated hyperphosphorylation of tau inhibits the native function of tubulin polymerization from biomolecular condensate and promotes amyloidogenic solidification of tau condensates.160,325 Although neurodegenerative diseases are sporadic in many cases, genetic and environmental factors play a huge role in the formation of pathogenic amyloid aggregates.326,327 Familial mutations,328–330 gene multiplications,126,127 deletion,331 and post-translational modifications332 are key factors associated with amino acid sequence modification, which significantly impact disease onset. Similarly, environmental changes due to metal ions, salt concentration, pH change, small organic molecules, pesticides, RNA, and lipids hugely alter the protein conformation and contribute to aggressive amyloid aggregation.333–338 Interestingly, the phase separation and aging properties of neurodegenerative disease-associated proteins are also very sensitive to these disease-associated genetic and environmental factors (Fig. 8).49,158,339,340 The following section will discuss how common neurodegeneration-associated factors aggravate the pathogenic phase separation leading to amyloid aggregation. | ||
| Fig. 8 Schematic representation of different effectors modulating LLPS and liquid-to-solid phase transition (LSPT) by various proteins. (a) Schematic representation showing LLPS and LSPT of proteins. (b) A table summarizing the effect of genetic mutation, post-transitional modification, and environmental factors in LLPS and LSPT for major neurodegeneration-related proteins. Upward arrow indicates increased LLPS propensity, while the downward arrow indicates reduced LLPS propensity, and the dash indicates no information available yet. (c)–(e) Different disease-associated conditions affecting LLPS and LSPT of α-syn leading to enhanced LLPS propensity and faster amyloid aggregation as observed from thioflavin-T fluorescence kinetics and slower FRAP recovery. (c)–(e) are reproduced from ref. 47 with permission from Springer Nature, Copyright © 2020. | ||
7.1. Genetic factors
All neurodegenerative diseases have shown a direct connection with genetic variations where familial mutations, truncation, and gene multiplication result in early disease progression. For example, early-onset familial mutations have a direct role in AD,341,342 PD,343–346 and other neurodegenerative diseases.347,348 Genetic modification can hugely alter protein surface charge and intermolecular interaction, which impacts liquid-to-solid phase transition probably due to accelerated misfolding of protein, leading to faster amyloid formation. For example, ALS-associated mutations (G156E and R244C) in the FUS proteins result in faster amyloid aggregation kinetics from a condensate, generating fibrillar outbursts from the condensates.227 Apart from affecting the aging kinetics, the disease-associated mutations can also promote LLPS by bringing down the saturation concentration within the physiological limit, which is otherwise a forbidden event to avoid the risk of protein aggregation. For example, early-onset familial mutants of α-syn such as E46K and A53T, which are known to undergo faster aggregation compared to wild-type, undergo LLPS at lower saturation concentrations and subsequently exhibit faster amyloid aggregation from condensates (Fig. 8c–e).47 Similarly, disease-associated point mutants of tau, such as P301L, P301S, ΔK280, and A152T show enhanced phase separation propensity compared to wild type protein, with an accelerated rigidity in droplet dynamics, followed by fibrillar aggregate formation upon prolonged incubation.160,268 However, another study pointed out that mutations such as P301L, ΔK280, and G272V show similar phase separation propensity as compared to wild-type but only mutants form fibrillar aggregates upon prolonged incubation.349 Thus, disease-associated mutations can specifically promote the amyloid nucleation mechanism without affecting the molecular mechanism associated with LLPS. A similar phenomenon is also observed for the hnRNPA1 protein where pathology-relevant D262V mutant undergoes LLPS with no significant difference as compared to the wild-type but with an enhanced propensity of the mutant to form fibrils.195,350 On a similar note, familial α-syn mutants A30P, H50Q, A53T, and A53E also exhibit the same LLPS propensity as compared to WT α-syn, except E46K, which significantly promotes LLPS propensity. Interestingly, all these mutants except A53E result in a faster liquid-to-solid transition leading to amyloid aggregation.351 Previously, we proposed that enhanced oligomerization propensity and/or their retention might be a shared toxicity mechanism of all α-syn familial mutations, which may contribute to the early onset of PD.345 However, based on these cumulative emerging observations, there is a possibility that faster liquid-to-solid transition might be a prevalently common phenomenon across several disease-relevant proteins, which can be closely associated with oligomerization, toxicity, and disease onset. Further systematic study should be carried out in this direction, which can enormously improve the understanding of pathogenic aggregation. Interestingly, disease-associated mutations of TDP-43, FUS, and hnRNPA1 protein can convert a reversible phase separation into an irreversible one with disease implications.255,323 Phase separation of these RNA binding proteins is regulated by π–π interactions arising from the regularly interspaced aromatic residues.169,352,353 This aromatic residue-rich amino acid sequence also forms “low-complexity aromatic-rich kinked segments” (LARKS) that might facilitate phase separation and functional reversible aggregation.354 Interestingly, mutations in the LARK domain reduce the reversible nature of aggregation resulting in irreversible protein aggregates.355 The high fluidity and less viscous nature of TDP-43 ribonucleoprotein (RNP) condensates is indeed essential for faster transport through axon.356 However, ALS-associated mutation rapidly rigidifies these RNP granules affecting the physiological transport in axon and motor neuron activity.356,357 Moreover, mutant TDP-43 undergoes faster oligomerization and aging kinetics with the emergence of amyloid-like aggregates from the aged condensate.289,358 Importantly, disease-associated mutations of stress granule-related proteins not only impact phase separation and liquid-to-solid phase transition but also can often impact the relative partitioning within cellular sub-compartments.359,360 It has been previously reported that ALS/FTD-associated mutation results in the defective nucleocytoplasmic shuttling of protein, which has a direct role in neurodegeneration.359 For example, in the case of the FUS protein, the majority of the ALS/FTD-causing mutations are located either in the PrLD or the protein–tyrosine-rich nuclear signal (PY-NLS) region.29,41,361,362 While mutations in the PrLD can accelerate aberrant phase transition and aggregation propensity of FUS, mutations in the PY-NLS region affect the physiological binding of FUS with nuclear transporter receptor, transportin-1 (also known as Karyopherin β2 or Kapβ2).41,194,363 The inability of Kapβ2 to bind to FUS PY-NLS leads to defective nucleocytoplasmic shuttling resulting in sustained accumulation of aberrant FUS solid-like condensates in the cytoplasm.360 Thus disease-associated mutations not only impede the native location and function of biomolecular condensate but also can initiate aberrant aggregation with implications in neurodegeneration.Apart from mutation, gene multiplication and overexpression of protein in the compromised proteostasis machinery often elevate the local protein concentration beyond the physiological limit, which is a crucial driving force for pathological phase separation.195,364 Interestingly, the concentration-dependent change in condensate material property is observed in poly-Q, FUS, and hnRNPA1 where a low concentration regime results in liquid-like dynamic condensates but with very high concentration leads to the formation of a hydrogel composed of amyloid fibrils.365,366 Misregulated translation and overexpression due to gene multiplication, such as repeat domain gene expansion, which is closely associated with neurodegeneration pathology.367 Interestingly, C9orf72 (chromosome 9 ORF 72) nucleotide repeat expansion, the most common reason for ALS and FTD, results in GR/PR dipeptide repeat expression. The gene multiplication promotes phase separation of hnRNPA1 and TIA1 proteins by decreasing the saturation concentration and also impairs the liquid-like dynamics of the stress granule.368 Similarly, the N-terminal poly-Q repeat expansion of Huntingtin protein can also trigger an aberrant phase transition from liquid-like condensates where fibrous solids are bursting out from aged condensates.226 Thus, disease-associated genetic variation is emerging as a common factor to promote pathological phase separation and aggregation with its potential role in neurodegeneration.
7.2. Post-translational modification (PTMs)
Post-translational modifications (PTMs) play a crucial role in modulating the protein structure and function in the cellular environment.369,370 The unregulated PTMs might change the protein's native structure, function, and localization, which often creates protein aggregation and cellular toxicity.371,372 Moreover, PTMs might alter the conformational stability of proteins, which might impact the LLPS propensity and the material property of the resulting condensates. For example, phosphorylation of tau is important for microtubule stabilization.373 However, hyperphosphorylation of tau results in not only disruption of the native function but also promotes pathogenic aggregation associated with AD.374 Similarly, 90% of the α-syn population in LB is majorly phosphorylated at Serine residues (S129 and S87), which is a classical hallmark of PD pathology.375 In this direction, it has been shown that phosphorylation of α-syn at S129 is the major form in the composition of LB of the PD brain.376,377 A phosphomimetic study on S129E demonstrates a decrease in the saturation concentration of α-syn phase separation with faster liquid-to-solid transition into amyloid-like aggregates.47 Moreover, intracellular α-syn condensates of the irregular shape, which are formed through LLPS have shown positive staining for S129 phosphorylation in HeLa cells, indicating a strong connection with PD pathology.378 Similar to α-syn, the addition of the negative charges due to hyperphosphorylation decreases the net positive charge of tau and hence enhances its LLPS propensity.160,268 Moreover, the phosphorylated tau (p-tau 441) droplet exhibits rapid loss in its dynamic behavior and binds to an amyloid cross-β specific dye, thioflavin-S.160 However, another antibody-specific study showed that in the absence of any cofactors, phosphorylated tau condensates consist of small oligomers, not matured fibrils, and these oligomers are more toxic than the fibrils, which may have direct implications in tauopathies.268 On the other hand, physiological phosphorylation of TDP-43 and FUS has been shown to disrupt the N-terminal oligomerization domain and to reduce LLPS propensity due to the additional negative charge.169,379 Methylation does not change the overall charge of protein like phosphorylation, rather it alters the charge distribution and steric interaction, which in turn affects the phase separation process.380 Arginine methylation shows a predominant effect on RNA binding proteins such as FUS and hnRNPs as they contain multiple RGG domains of RG-rich motifs.381 Arginine methylation suppresses the strength of cation–π interaction, hence performing a physiological role in preventing aberrant phase separation of FUS363 and hnRNPA2.382 Interestingly, hypomethylated FUS has been shown to form a large number of irregularly shaped condensates with a solid-like material property indicating a potential link with disease pathogenesis.383 Ubiquitination is another common PTM found in several pathogenic inclusions and is considered a classical biomarker to identify LB in PD pathology.384 Interestingly, ubiquitinated α-syn species have been reported in the condensates of the aged Caenorhabditis elegans population indicating a potential link with the disease mechanism (Fig. 6d).233 Ubiquitination has huge physiological importance in protein-quality control machinery by selective proteasomal degradation of misfolded proteins. An interesting study in this regard has shown that site-specific ubiquitination disfavors tau/heparin aberrant condensate formation; while non-specific ubiquitination stabilizes condensates towards aggregation playing a potential pathogenic role.385 N-Terminal acetylation is another physiologically occurring PTM, which prevents the protein from proteasomal degradation and reduces aggregation propensity.386 α-syn shows slow kinetics of phase separation as well as negligible binding with thioflavin-T in N-acetylated form indicating its increased solubility.158 Similarly, lysine acetylation also disfavors in vitro tau LLPS.387 However, TDP-43 acetylation in the nuclear localizing sequence increases cytoplasmic accumulation of TDP-43 condensates and the mis-localization drives pathogenic cytoplasmic inclusion from condensates.388 These cumulative observations indicate a strong influence of disease-associated PTM in aberrant phase separation and molecular aging.7.3. Environmental factors
Protein misfolding and aggregation are known to be influenced by various environmental factors.61,389–392 For example, pH changes, metal ion exposure, and the presence of glycosaminoglycans are known to promote amyloid aggregation of various proteins.334,390,393 Indeed, various environmental factors are reported as the causative factors associated with major neurological disorders, such as AD and PD.394–397 Unregulated environmental stress can also impact aberrant LLPS, their material property, and irreversibility. For example, pH change and metal ion binding can switch the native protein conformation to the misfolded states, which not only increases the propensity of LLPS by reducing the saturation concentration but also promotes the amyloid aggregation from condensates. On the other hand, polyanionic species, such as glycosaminoglycans and RNA might interact with positively charged proteins resulting in charge neutralization, which drives rapid phase separation and aggregation. Indeed, protein phase separation and subsequent amyloid aggregation have been shown to be modulated by these environmental factors.263,320,398 For example, the accumulation of several metal ions, such as Cu2+, Fe2+, Ca2+, Zn2+, Al3+, Ni2+, Co2+, and Cr2+ have been shown to reduce the saturation concentration of α-syn LLPS and further accelerate the amyloid aggregation kinetics from condensates (Fig. 8c–e).158,399,400 On the other hand, tau LLPS and solidification are specifically promoted by Zn2+ while other transition metal ions, such Mn2+, Fe2+, Co2+, Ni2+, and Cu2+ do not alter the propensity LLPS for tau.241,401 A recent study has shown that phase separation of Prion protein (PrP) is triggered by Cu2+ exposure both in vitro and in live cells across the cell surface, which might act as a protective sequestration mechanism to buffer excess Cu2+.402 However, oxidative stress induced by prolonged Cu2+ or H2O2 exposure results in the liquid-to-solid transition in vitro as well as in intracellular condensates into amyloid-rich PrP aggregates. Similarly, PD-relevant exposure to metal toxicants, Cu2+ and Fe3+ results in the faster transition of liquid-like condensates into amyloidogenic perinuclear solids.47 LLPS of α-syn is also enhanced by salts and low pH, which lowers the saturation concentration for phase separation.158 Charge screening by high salt and low pH between positively charged N-terminal and negatively charged C-terminal exposes the hydrophobic NAC region, which drives the self-assembly prerequisite for α-syn phase separation.158,403 On the other hand, tau LLPS is primarily driven by electrostatic interactions as the dissolution of the droplets takes place with the addition of 100 mM NaCl.404 However, the addition of very high salt concentration shows re-entrant behavior of tau LLPS with quick solidification where the residual hydrophobic interaction drives LLPS with faster droplet aging kinetics.405 Apart from pH, salt, and metal ions, glycosaminoglycan (GAGs) can also play a role in amyloid aggregation.406–410 Heparin, a glycosaminoglycan, is a well-known cofactor that affects tau oligomerization and fibril formation.409,411,412 This polyanionic cofactor induces molecular self-assembly by reducing the electrostatic repulsion of the positively charged tau. Heparin indeed promotes fibril-like structure formation inside tau phase-separated droplets.349,413 Moreover, phosphorylated tau in the presence of heparin not only shows accelerated LLPS kinetics but also forms amyloid-like fibrillar aggregates inside droplets.160 RNA is another important cofactor for regulating LLPS especially for RNA-binding proteins (RBPs) such as FUS, TDP-43, and hnRNPs, where concentration and length of RNA can potentially alter condensate stability, surface tension, and molecular transport properties.148,414–418 Interestingly, phase separation of RBP is favored at low RNA concentration while inhibited at high RNA concentration, which is in line with the hypothesis that high RNA abundance in the nucleus inhibits pathological LLPS while aberrant condensation typically occurs in low cytoplasmic RNA concentration.418 Moreover, condensate viscosity also increases with increasing length of RNA indicating a protein–RNA heterotypic interaction also actively regulates condensate aging.419 The competing protein–protein and protein–RNA interaction often results in stoichiometry-dependent multiphasic condensate formation recapitulating the complex architecture of in cellulo multicomponent condensates.420 Similar to RNA, ATP is another concentration-dependent regulatory environmental factor for the protein LLPS as its bivalent nature is predominant at low ATP concentrations while hydrophobicity predominates at high concentrations.421 At physiological concentration, ATP has been shown to prevent liquid-to-solid phase transition due to site-specific binding with TDP-43 while ALS-associated mutation D169G inhibits its ATP binding and promotes aberrant phase transition.422 Interestingly, α-syn condensate is also transitioned into a percolated mesh-like network structure at 1–5 mM ATP concentration, although the relevance of percolation in disease pathogenesis has to be evaluated in the near future.4237.4. Heterotypic protein phase separation and their impact on amyloid aggregation
Coaggregation of multiple neurodegenerative disease-associated proteins into mixed amyloid is a common pathological feature in the various neurodegenerative disease etiology.424,425 More than 50% of AD patients are reported to exhibit LB; similarly, PD cases are found to develop a high number of tau-containing neurofibrillary tangles (NFT).426,427 Moreover, α-syn and TDP-43 inclusions were found to co-exist in ALS, FTD, and PD.428,429 The amyloid deposits of α-syn have also been discovered in patients with CJD, which involves the misfolding of prion protein (PrP).430,431 Various emerging studies pointed out that α-syn, tau, TDP-43, FUS, and prion proteins can cross-talk and undergo heterotypic phase separation with distinct aging properties as compared to individual phase separation.428,432–435 The synergistic complex inter-protein interactions can often show phase separation at lower saturation concentrations than individual protein LLPS and they can lead to faster maturation into ordered solid amyloid species resembling pathological aggregates. For example, electrostatic interactions between the basic N-terminal of PrP and the acidic C-terminal of α-syn promote complex coacervation, which rapidly undergoes aggregation.433 The bypassing of the lag phase suggests that the synergistic interaction between PrP and α-syn in the complex biomolecular condensates may promote secondary nucleation resulting in a quick liquid-to-solid transition into amyloid-containing condensates. Prp can also form multiphasic condensate with tau driven by electrostatic interaction at a specific protein stoichiometry ratio, which gradually transitioned into amyloid-like co-aggregates upon aging.432 Similar to the tau-PrP system, α-syn can efficiently partition into RNA-induced tau condensate due to the direct binding of the C-terminal of α-syn to the proline-rich region of tau and forms amyloid fibrils upon aging.434 On the other hand, full-length α-syn and tau can also form complex coacervates, without RNA influence, only driven by opposite electrostatic net surface charge.435 Interestingly, the dynamics of small-sized α-syn-tau condensates are quickly arrested by gelation, while the large long-lived multicomponent condensates slowly co-aggregate into amyloid fibrils. This intriguing observation indicates that the intermolecular interaction for amyloid nucleation may not necessarily be the same with condensate formation and/or gelation. α-syn is also reported to modulate the phase behavior of TDP-43-RNA condensates in a multi-component system.436 TDP-43 co-incubated with α-syn showed increased thioflavin-T fluorescence with enhanced aggregation kinetics. Collectively these observations provide a mechanistic underpinning of the synergistic protein–protein interaction during multi-component phase separation, which has a potential connection with neurodegeneration. Although several studies have pointed out the synergistic amyloid aggregation in the multi-component system, few recent reports have shown that the preformed condensates from non-aggregating protein/peptide can accumulate amyloidogenic proteins and exert an inhibitory effect against amyloid aggregation.309,437,438 For example, the partitioning of the Aβ42 protein into a functional protein condensate has been shown to inhibit the nucleation for amyloid aggregation despite the increase in the local concentration of Aβ42 inside the condensate.437 The observation is very significant and it will open a new possibility of multi-component biomolecular condensate being a protective “safe reservoir” for amyloidogenic proteins. Interestingly, the heterotypic protein–protein interaction in the multicomponent systems can spatiotemporally modulate the aggregation propensity of the partitioning protein. An interesting observation highlights the spatiotemporal modulation of α-syn aggregation within a preformed condensate composed of nonaggregating protein where the condensate interface preferentially promotes its aggregation, but the interior of the condensate exerts a stabilizing effect against aggregation.308 The differential behavior is also dependent on the nature of the partitioning protein where full-length α-syn shows interface-mediated aggregation but the shortest variant comprising aggregation-prone NAC domain specifically accumulates in the condensate interior suppressing its aggregation. The preformed condensate interface can also deplete pathogenic aggregates from the dilute phase, as observed for LLPS of a chaperone protein, p62/SQSTM1, which accumulates Huntingtin diseases-associated polyQ aggregates on its interface for proteasomal degradation.274 Thus, strategically designed multicomponent phase separation can suppress amyloid aggregation for pathogenic proteins, which might open up a huge scope in the therapeutic development of neurodegenerative diseases.8. LLPS: a generic nucleation mechanism in the amyloid aggregation pathway
Protein misfolding can drive amyloid aggregation with very similar fibrillar properties from the diverse nature of the amino acid sequence, length, and native structures.439 Thus, the amyloid state is considered a highly organized generic state of protein apart from its functional native state.440,441 Interestingly, LLPS is also emerging as a proteome-wide common phenomenon guided by weak nonspecific multivalent interaction, which is prevalently present in all proteins irrespective of their structural classification.442–444 In this line, human proteome-wide amino acid sequence analysis revealed that most of the proteins have a propensity for LLPS, which could be accessed, at least transiently, under physiologically relevant cellular conditions.442 Moreover, we have recently reported that proteins and polypeptides with various sequences and structural diversity indeed undergo LLPS in the crowded microenvironment further proposing LLPS as a generic phenomenon of protein similar to amyloid state.443 However, there is a fundamental difference between LLPS and amyloid state in their thermodynamic stability as LLPS is highly metastable in nature, inclined towards viscoelastic transition, while the amyloid state represents the most stable state of protein aggregation.445 Interestingly, the relative propensity of a protein towards LLPS and amyloid aggregation is enhanced by the same molecular features, namely IDR, LCD, and PrLD. The prevalence of these regions in a protein makes it conformationally flexible facilitating transient multivalent interaction for phase separation and making the protein susceptible to misfolding for amyloid aggregation. Moreover, the molecular crowding within the phase-separated condensates can potentially drive protein misfolding for intrinsically amyloidogenic proteins if they are not under tight proteostasis regulation where chaperone and other cellular factors (e.g., functional PTMs, physiological pH, enzyme and metal binding, etc.) prevent the access to the misfolded pathogenic conformation. Indeed, protein misfolding towards expanded conformation is commonly observed in pathological phase separation of neurodegeneration-related proteins, which initiate the aberrant amyloid aggregation within condensates. Thus, a conceivable hypothesis connecting the dots may propose LLPS as a generic molecular mechanism to promote nucleation for pathogenic amyloid aggregation (Fig. 9). | ||
| Fig. 9 The generic amyloid aggregation pathway preceded by LLPS: (a) a schematic representation of the LLPS-mediated amyloid aggregation pathway. LLPS nucleation followed by fusion-dependent growth of biomolecular condensates makes the protein-rich condensates vulnerable to misfolding through the exposure of interacting regions. This structural conversion often leads to irreversible intermolecular contacts initiating the nucleation of amyloid aggregation. Disease-associated factors enhance amyloid aggregation kinetics from metastable condensates. Microscopy images (top) showing a representative example of α-syn LLPS that forms liquid-like condensates early point (day 2) but gradually transitions into an amyloid hydrogel at a later time point (day 30). Images are reproduced from ref. 47. with permission from Springer Nature, Copyright © 2020. (b) Schematic representation indicating time-dependent free energy change of protein during LLPS and liquid-to-solid phase transition (LSPT) interconversion. Both structured and intrinsically disordered proteins can undergo LLPS surpassing the nucleation energy barrier, designated as ΔG(I) and (ΔG(S)), respectively. The growth of the condensate by fusion might be associated with small energy changes (ΔG). The viscoelastic liquid-to-solid transition is associated with a high energy barrier, which is likely to be even higher for the most stable amyloid state (ΔG(A)) than amorphous solidification (ΔG(B)). Due to the high free energy barrier, amyloid aggregation is prevented in physiological conditions. However, disease-associated factors can potentially reduce the barrier leading to the rapid formation of pathogenic amyloids. Owing to the lowest energy states, amyloid formation is associated with irreversibility resulting in progressive accumulation of fibrillar content leading to pathogenicity. Note: to avoid complicacy, energy barriers are shown as a single-step transition for simplistic representation, which otherwise might comprise multistep energy changes in real scenarios. The relative energy states depicted in the schematics are likely to be context-dependent or protein-specific. | ||
The classical amyloid fibrils, associated with pathology, are typically irreversible where the stability comes through the steric zipper interaction from hydrophobic residues between the interlocking side chains of the two extended β-sheets.12,446 Interestingly, amyloid can also be associated with cellular function, represented as “functional amyloid”, which shows reversible dissolution under cellular regulation.447–450 For example, the low-complexity domain of FUS, TDP-43, and hnRNPA1, which are associated with pathogenic amyloid aggregation, are also capable of forming functional reversible amyloid-like cross-β arrangements, containing LARKS motifs, mainly driven by aromatic residues.354 The striking molecular difference between LARKS and the steric zipper is that the core of the reversible amyloid contains highly dense polar residues that can interact with water; whereas in steric zipper, hydrophobic interlocking amino acids show a dry interface, devoid of water.355 Protein misfolding can unleash the otherwise inaccessible critical hydrophobic interaction dictating the irreversible molecular arrangement with less protein–water interaction. Importantly, LLPS results in the water-poor microenvironment in protein-rich condensate, which is likely to enhance the hydrophobic interactions stabilizing the protein in the misfolded conformation. This is further supported by the observation that prolonged incubation of FUS condensate converts a reversible aggregate into irreversible pathological amyloids.323,451 Moreover, ALS-associated mutations in the LARKS domain of FUS and TDP-43 can also introduce irreversibility by favoring hydrophobic interaction.323 High temperature, high salt, and low pH are often reported to drive faster amyloid formation from condensates, which can be explained by the increase in the residual hydrophobic interaction in these altered conditions.158,442 Thus, a delicate balance between the solvation energy of hydrophilic and hydrophobic residues of a protein dictates the condensate's reversibility and its aging property towards irreversible amyloid aggregation.452 The molecular determinants that drive amyloid aggregation through the condensation pathway require three essential overlapping features: droplet-formation propensity, aggregation-promoting propensity, and multimodal interaction to make an amyloid-like ordered structure.59 While weak non-covalent interactions and electrostatic interactions are driving factors for the first two features, hydrophobic interaction is indeed mandatory for molecular ordering towards fibril formation, which is also experimentally supported for low-complexity FUS domain using a range of sophisticated optical techniques.453 Moreover, an intriguing theoretical study further supports the hydrophobicity-driven amyloid aggregation from the condensates for the Aβ42 protein, which has two extra hydrophobic amino acids towards the C-terminus compared to Aβ40 protein and comprises only a small population (∼10%) of the total Aβ content in pathogenic senile plaques.454 Based on a sticker-spacer-based model of unfolded Aβ, the authors hypothesized that the small Aβ42 population imparts sufficient non-specific hydrophobicity to drive amyloid-nucleation from Aβ condensates while increasing the population of Aβ40 drives LLPS towards lower saturation concentration. Thus, it is important to note that the molecular interaction prerequisite for LLPS and amyloid aggregation overlap but may not necessarily be the same. While weak multivalent interaction promotes the formation of protein-rich condensates through LLPS, the liquid-to-amyloid transition is mainly driven by enhanced hydrophobicity in the water-poor condensate interior, which might serve as a generic molecular interaction for protein misfolding and amyloid nucleation.
Further, based on the observation that LLPS is commonly observed to initiate amyloid aggregation for several neurodegeneration-associated proteins, an obvious therapeutic strategy therefore could be to target LLPS where inhibition of LLPS and/or inhibition of liquid-to-solid state and fibril formation is possible.455 Interestingly, cellular proteostasis machinery utilizes several regulatory mechanisms to prevent amyloid nucleation from condensates, which can inspire the design of the therapeutics.456 For example, site-specific functional phosphorylation on an aggregation-prone FUS segment prevents its seeding potential, which can otherwise drive deleterious amyloid aggregation from condensates.305 Functional phosphorylation is also associated with triggering chaperone activity under the surveillance of proteostasis machinery.457 Stress-induced phosphorylation of canonical heat shock chaperone proteins Hsp27 leads to preferential enrichment of the chaperone within the FUS condensate inhibiting amyloid aggregation.458 Apart from Hsp27, other canonical heat shock chaperons, such as Hsp70 and HSPB1 actively regulate phase separation of stress granule-associated proteins for regulated disassembly with prevention against aberrant aggregation.242,459 Similarly, pathological phase separation of tau is also inhibited by several molecular chaperones; such as protein disulfide isomerase (PDI), and S100B protein; where disease-associated environmental stress abrogates their recruitment inside condensates.460,461 Furthermore, O-glycosylation is another emerging regulatory factor for synaptic LLPS and chaperone activity to prevent amyloid aggregation.462 These chaperones often bind to oligomers to prevent the nucleation for amyloid aggregation as observed for cellular protein Profilin, which binds to the phase-separated oligomers of Huntingtin N-terminal fragments (Htt-NTFs).463 These cumulative observations strongly suggest that enhancing molecular chaperone expression and/or designing chemical chaperones, chaperone homologs, small molecules, and peptides might inhibit pathological LLPS. Indeed, several chemical chaperones, peptides, and small molecule-based inhibitors that efficiently target the LLPS to prevent amyloid aggregation are reported.281,464–466 Recently, a theoretical model has been developed illustrating how to amplify inhibitor efficiency by tuning the condensate volume and partitioning coefficient of the inhibitor, which will increase its effective local concentration within condensates.467 Moreover, a remarkable study has revealed that the pharmacodynamic properties of a cancer therapeutic is modulated by the partitioning efficiency of small molecule selectively into the nuclear condensates.468 Similarly, small molecules are reported to selectively target a gain-of-function chemo-resistant p53 mutant condensate in the nucleus.469,470 Thus, designing therapeutics that specifically target nuclear condensates is appearing as a promising therapeutic approach against cancer.471 An intriguing observation in this regard indicates that the partitioning efficacy of a drug into biomolecular condensates is dictated by its physicochemical property but independent of the molecular target.468 The exciting observation implies that drug partitioning efficacy might be considered as an important optimization parameter in future drug design. Indeed, a novel therapeutic strategy, named condensate-modifying therapeutics (c-mods) has been proposed where drug candidates with distinct physicochemical properties target a specific biomolecular condensate and modulate its physical property, stability and/or function as a prevention strategy.472 Interestingly, some inhibitors are reported to prevent the aberrant liquid-to-solid transition without affecting the liquid condensate formation, which suggests that pathological fibrillar nucleation from condensates can be decoupled without hampering physiological phase separation by strategic therapeutic design.464–466 However, the underlying molecular mechanism for stabilizing condensate in a liquid state against amyloid aggregation remains elusive. Extensive future research is essential in this direction, which should include systematic library screening for inhibitor designing and elucidate the inhibitor mechanism at the molecular level.
Apart from neurodegeneration, the emerging amyloid aggregation mechanism preceded by LLPS may also aid in understanding another disease mechanism, such as cancer, which is recently linked with phase separation and amyloid aggregation.471,473–477 For example, malignant gain-of-function mutations on tumour suppressor protein p53 have been reported to undergo aberrant phase separation, acting as a precursor for pathogenic amyloid aggregation.469 Mis-regulation of physiological LLPS serving important cellular processes such as transcription regulation, signal transduction, oncogenic function, chromatic organization, DNA sensing, and damage response, nuclear organization can effectively contribute to tumorigenesis and cancer.220,478,479 To take an example, mutations in nucleoporin (NUP98 or NUP214) trigger an oncogenic transcription factor resulting in aberrant biomolecular condensates, which lead to abnormal chromatin structure and tumorigenesis.480 Moreover, cancer-associated loss-of-function mutation on another tumour suppressor protein SPOP (speckle-type POZ protein) disrupts the functionally active LLPS and ubiquitination, which might accumulate oncoprotein.481 Apart from cancer, lethal viral infections such as SARS-CoV-2 (the severe acute respiratory syndrome coronavirus 2) have also shown its potential connection with LLPS and amyloid aggregation as the nucleocapsid protein of the virus contains LCD promoting both events.482,483 Similarly, a metabolic disorder such as type II diabetes has also been shown to be affected by LLPS-mediated aberrant aggregation as insulin-derived peptides and islet amyloid polypeptide (IAPP) can promote the amyloidogenic pathway from phase separation.484,485 Since the LLPS phenomenon is ubiquitous in cell physiology, the underlying pathological risk of cellular mis-regulation is an unavoidable consequence leading to diverse disease pathogenesis. Hence elucidating a common pathogenic nucleation mechanism for aberrant protein aggregation might impart significant insight into the disease mechanism. Moreover, the novel amyloid aggregation pathway preceded by LLPS might also help in developing new functional material as the viscoelastic transition is associated with a range of material states. For example, functional amyloid hydrogel formed through LLPS can act as a potential drug delivery system where liquid-like droplets can efficiently entrap drugs while the hydrogel can modulate its release properties. Further, the knowledge from the LLPS-mediated amyloid aggregation pathway can be implemented to rationally design artificial condensates from synthetic polypeptides with interesting molecular features. For example, a very recent study designed an amphiphilic hybrid peptide that combines propensity amyloid formation and phase separation propensity in such a way that the complex condensate shows amyloid fibrillation while maintaining the liquid-like fusion property.486 Thus, a range of functional amyloids can be developed from condensates in the near future, which may impart valuable insights into the rational design of supramolecular material with diverse functionality. Moreover, unravelling the molecular mechanism connecting different states of protein self-assembly can open up a huge research scope in synthetic biology; such as enzyme modelling, artificial organelle designing, and biomimetic protocell development that can significantly improve our overall understanding of the origin of life.
Author contributions
Conceptualization – S. K. M.; literature survey – S. M., M. P., K. D. Writing draft; S. M., K. D. Drawings – P. K., M. P., review and editing – S. K. M., S. M, M. P., K. D., P. K. Project administration and supervision – S. K. M.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We acknowledge Shouvik Manna for providing circular dichroism spectroscopy of the α-syn protein. We acknowledge DST SERB-SUPRA (SPR/2021/000103), the Government of India for financial support. S. K. M and K. D. are thankful to Sunita Sanghi Centre for Aging and Neurodegenerative Diseases (SCAN), IIT Bombay, for the financial support.References
- Y. Hou, X. Dan, M. Babbar, Y. Wei, S. G. Hasselbalch and D. L. Croteau,
et al., Ageing as a risk factor for neurodegenerative disease, Nat. Rev. Neurol., 2019, 15(10), 565–581 CrossRef PubMed
.
- F. G. Jennekens, A short history of the notion of neurodegenerative disease, J. Hist. Neurosci., 2014, 23(1), 85–94 CrossRef PubMed
.
- V. L. Feigin, E. Nichols, T. Alam, M. S. Bannick, E. Beghi and N. Blake,
et al., Global, regional, and national burden of neurological disorders, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016, Lancet Neurol., 2019, 18(5), 459–480 CrossRef PubMed
.
- H. Checkoway, J. I. Lundin and S. N. Kelada, Neurodegenerative diseases, IARC Sci. Publ., 2011, 163, 407–419 Search PubMed
.
- S. Tabrizi, Neurodegenerative diseases neurobiology pathogenesis and therapeutics, J. Neurol., Neurosurg. Psychiatry, 2006, 77(2), 284, DOI:10.1136/jnnp.2005.072710
.
- A. J. Ehrenberg, A. Khatun, E. Coomans, M. J. Betts, F. Capraro and E. H. Thijssen,
et al., Relevance of biomarkers across different neurodegenerative diseases, Alzheimer's Res. Ther., 2020, 12(1), 56 CrossRef PubMed
.
- M. J. Millan, Y. Agid, M. Brüne, E. T. Bullmore, C. S. Carter and N. S. Clayton,
et al., Cognitive dysfunction in psychiatric disorders: characteristics, causes and the quest for improved therapy, Nat. Rev. Drug Discovery, 2012, 11(2), 141–168 CrossRef CAS PubMed
.
- C. Soto, Unfolding the role of protein misfolding in neurodegenerative diseases, Nat. Rev. Neurosci., 2003, 4(1), 49–60 CrossRef CAS PubMed
.
- D. Eisenberg and M. Jucker, The amyloid state of proteins in human diseases, Cell., 2012, 148(6), 1188–1203 CrossRef CAS PubMed
.
- F. Chiti and C. M. Dobson, Protein Misfolding, Amyloid Formation, and Human Disease: A Summary of Progress Over the Last Decade, Ann. Rev. Biochem., 2017, 86, 27–68 CrossRef CAS PubMed
.
- J.-C. Rochet and P. T. Lansbury Jr, Amyloid fibrillogenesis: themes and variations, Curr. Opin. Struct. Biol., 2000, 10(1), 60–68 CrossRef CAS PubMed
.
- R. Nelson, M. R. Sawaya, M. Balbirnie, A. Ø. Madsen, C. Riekel and R. Grothe,
et al., Structure of the cross-β spine of amyloid-like fibrils, Nature, 2005, 435(7043), 773–778 CrossRef CAS PubMed
.
-
M. Sunde and C. Blake, The Structure of Amyloid Fibrils by Electron Microscopy and X-Ray Diffraction, in Advances in Protein Chemistry, ed. F. M. Richards, D. S. Eisenberg and P. S. Kim, Academic Press, 1997, 50, pp. 123–59 Search PubMed
.
- M. Sunde, L. C. Serpell, M. Bartlam, P. E. Fraser, M. B. Pepys and C. C. F. Blake, Common core structure of amyloid fibrils by synchrotron X-ray diffraction 11Edited by F. E. Cohen, J. Mol. Biol., 1997, 273(3), 729–739 CrossRef CAS PubMed
.
- Y. Cao and R. Mezzenga, Food protein amyloid fibrils: origin, structure, formation, characterization, applications and health implications, Adv. Colloid Interface Sci., 2019, 269, 334–356 CrossRef CAS PubMed
.
- M. R. Krebs, E. H. Bromley and A. M. Donald, The binding of thioflavin-T to amyloid fibrils: localisation and implications, J. Struct. Biol., 2005, 149(1), 30–37 CrossRef CAS PubMed
.
- M. R. Nilsson, Techniques to study amyloid fibril formation in vitro, Methods., 2004, 34(1), 151–160 CrossRef CAS PubMed
.
- M. G. Spillantini and M. Goedert, Tau pathology and neurodegeneration, Lancet Neurol., 2013, 12(6), 609–622 CrossRef CAS PubMed
.
- V. H. Finder and R. Glockshuber, Amyloid-β aggregation, Neurodegener. Dis., 2007, 4(1), 13–27 CrossRef CAS PubMed
.
- M. G. Spillantini and M. Goedert, The alpha-synucleinopathies: Parkinson's disease, dementia with Lewy bodies, and multiple system atrophy, Ann. N. Y. Acad. Sci., 2000, 920, 16–27 CrossRef CAS PubMed
.
- J. M. Gil and A. C. Rego, Mechanisms of neurodegeneration in Huntington's disease, Eur. J. Neurosci., 2008, 27(11), 2803–2820 CrossRef PubMed
.
- S. B. Prusiner and S. J. Dearmond, Prion protein amyloid and neurodegeneration, Amyloid., 1995, 2(1), 39–65 CrossRef CAS
.
- J. P. Taylor, J. Hardy and K. H. Fischbeck, Toxic proteins in neurodegenerative disease, Science., 2002, 296(5575), 1991–1995 CrossRef CAS PubMed
.
- H. Deng, K. Gao and J. Jankovic, The role of FUS gene variants in neurodegenerative diseases, Nat. Rev. Neurol., 2014, 10(6), 337–348 CrossRef CAS PubMed
.
- H. Ling, E. Kara, R. Bandopadhyay, J. Hardy, J. Holton and G. Xiromerisiou,
et al., TDP-43 pathology in a patient carrying G2019S LRRK2ámutation and a novel p. Q124E MAPT, Neurobiol. Aging, 2013, 34(12), 2889.e5-e9 CrossRef PubMed
.
- T. Vanderweyde, D. J. Apicco, K. Youmans-Kidder, P. E. Ash, C. Cook and E. L. da Rocha,
et al., Interaction of tau with the RNA-binding protein TIA1 regulates tau pathophysiology and toxicity, Cell Rep., 2016, 15(7), 1455–1466 CrossRef CAS PubMed
.
- A. Bampton, L. M. Gittings, P. Fratta, T. Lashley and A. Gatt, The role of hnRNPs in frontotemporal dementia and amyotrophic lateral sclerosis, Acta Neuropathol., 2020, 140, 599–623 CrossRef CAS PubMed
.
- B. Maziuk, H. I. Ballance and B. Wolozin, Dysregulation of RNA binding protein aggregation in neurodegenerative disorders, Front. Mol. Neurosci., 2017, 10, 89 Search PubMed
.
- S.-C. Ling, M. Polymenidou and D. W. Cleveland, Converging mechanisms in ALS and FTD: disrupted RNA and protein homeostasis, Neuron., 2013, 79(3), 416–438 CrossRef CAS PubMed
.
- P. G. Vallet, R. Guntern, P. Hof, J. Golaz, A. Delacourte and N. Robakis,
et al., A comparative study of histological and immunohistochemical methods for neurofibrillary tangles and senile plaques in Alzheimer's disease, Acta Neuropathol., 1992, 83(2), 170–178 CrossRef CAS PubMed
.
- M. G. Spillantini, R. A. Crowther, R. Jakes, M. Hasegawa and M. Goedert, alpha-Synuclein in filamentous inclusions of Lewy bodies from Parkinson's disease and dementia with lewy bodies, Proc. Natl. Acad. Sci. U. S. A., 1998, 95(11), 6469–6473 CrossRef CAS PubMed
.
- S. Mehra, S. Sahay and S. K. Maji, α-Synuclein misfolding and aggregation: implications in Parkinson's disease pathogenesis, Biochim. Biophys. Acta, Proteins Proteomics, 2019, 1867(10), 890–908 CrossRef CAS PubMed
.
- I. R. Mackenzie, R. Rademakers and M. Neumann, TDP-43 and FUS in amyotrophic lateral sclerosis and frontotemporal dementia, Lancet Neurol., 2010, 9(10), 995–1007 CrossRef CAS PubMed
.
- R. Rademakers, M. Neumann and I. R. Mackenzie, Advances in understanding the molecular basis of frontotemporal dementia, Nat. Rev. Neurol., 2012, 8(8), 423–434 CrossRef CAS PubMed
.
- K. J. Wolfe and D. M. Cyr, Amyloid in neurodegenerative diseases: friend or foe?, Semin. Cell Dev. Biol., 2011, 22(5), 476–481 CrossRef CAS PubMed
.
- B. Winner, R. Jappelli, S. K. Maji, P. A. Desplats, L. Boyer and S. Aigner,
et al., In vivo demonstration that α-synuclein oligomers are toxic, Proc. Natl. Acad. Sci. U. S. A., 2011, 108(10), 4194–4199 CrossRef CAS PubMed
.
- C. Haass and D. J. Selkoe, Soluble protein oligomers in neurodegeneration: lessons from the Alzheimer's amyloid β-peptide, Nat. Rev. Mol. Cell Biol., 2007, 8(2), 101–112 CrossRef CAS PubMed
.
- K. Ono, M. M. Condron and D. B. Teplow, Structure–neurotoxicity relationships of amyloid β-protein oligomers, Proc. Natl. Acad. Sci. U. S. A., 2009, 106(35), 14745–14750 CrossRef CAS PubMed
.
- G. Farias, A. Cornejo, J. Jimenez, L. Guzman and B. Maccioni, R. Mechanisms of tau self-aggregation and neurotoxicity, Curr.
Alzheimer Res., 2011, 8(6), 608–614 CrossRef CAS PubMed
.
- N. Bengoa-Vergniory, R. F. Roberts, R. Wade-Martins and J. Alegre-Abarrategui, Alpha-synuclein oligomers: a new hope, Acta Neuropathol., 2017, 134(6), 819–838 CrossRef CAS PubMed
.
- Z. Sun, Z. Diaz, X. Fang, M. P. Hart, A. Chesi and J. Shorter,
et al., Molecular determinants and genetic modifiers of aggregation and toxicity for the ALS disease protein FUS/TLS, PLoS Biol., 2011, 9(4), e1000614 CrossRef CAS PubMed
.
- G. Hoffner and P. Djian, Monomeric, oligomeric and polymeric proteins in huntington disease and other diseases of polyglutamine expansion, Brain Sci., 2014, 4(1), 91–122 CrossRef PubMed
.
- C. Wells, S. Brennan, M. Keon and L. Ooi, The role of amyloid oligomers in neurodegenerative pathologies, Int. J. Biol. Macromol., 2021, 181, 582–604 CrossRef CAS PubMed
.
- A. Zbinden, M. Pérez-Berlanga, P. De Rossi and M. Polymenidou, Phase Separation and Neurodegenerative Diseases: A Disturbance in the Force, Dev. Cell, 2020, 55(1), 45–68 CrossRef CAS PubMed
.
- S. Elbaum-Garfinkle, Matter over mind: liquid phase separation and neurodegeneration, J. Biol. Chem., 2019, 294(18), 7160–7168 CrossRef CAS PubMed
.
- W. M. Babinchak and W. K. Surewicz, Liquid–Liquid Phase Separation and Its Mechanistic Role in Pathological Protein Aggregation, J. Mol. Biol., 2020, 432(7), 1910–1925 CrossRef CAS PubMed
.
- S. Ray, N. Singh, R. Kumar, K. Patel, S. Pandey and D. Datta,
et al., α-Synuclein aggregation nucleates through liquid–liquid phase separation, Nat. Chem., 2020, 12(8), 705–716 CrossRef CAS PubMed
.
- W. M. Babinchak, R. Haider, B. K. Dumm, P. Sarkar, K. Surewicz and J.-K. Choi,
et al., The role of liquid–liquid phase separation in aggregation of the TDP-43 low-complexity domain, J. Biol. Chem., 2019, 294(16), 6306–6317 CrossRef CAS PubMed
.
- B. Tsang, I. Pritišanac, S. W. Scherer, A. M. Moses and J. D. Forman-Kay, Phase separation as a missing mechanism for interpretation of disease mutations, Cell., 2020, 183(7), 1742–1756 CrossRef CAS PubMed
.
- A. C. Hall, L. A. Ostrowski and K. Mekhail, Phase separation as a melting pot for DNA repeats, Trends Genet., 2019, 35(8), 589–600 CrossRef CAS PubMed
.
- M. Hofweber and D. Dormann, Friend or foe-Post-translational modifications as regulators of phase separation and RNP granule dynamics, J. Biol. Chem., 2019, 294(18), 7137–7150 CrossRef CAS PubMed
.
- K. Sołtys, A. Tarczewska and D. Bystranowska, Modulation of biomolecular phase behavior by metal ions, Biochim. Biophys. Acta, Mol. Cell Res., 2023, 1870(8), 119567 CrossRef PubMed
.
- C. Liu and Y. Fang, New insights of poly(ADP-ribosylation) in neurodegenerative diseases: a focus on protein phase separation and pathologic aggregation, Biochem. Pharm., 2019, 167, 58–63 CrossRef CAS PubMed
.
- F. Pessina, U. Gioia, O. Brandi, S. Farina, M. Ceccon and S. Francia,
et al., DNA damage triggers a new phase in neurodegeneration, Trends Genet., 2021, 37(4), 337–354 CrossRef CAS PubMed
.
- M. Polymenidou, The RNA face of phase separation, Science, 2018, 360(6391), 859–860 CrossRef CAS PubMed
.
- M. Törnquist, T. C. Michaels, K. Sanagavarapu, X. Yang, G. Meisl and S. I. Cohen,
et al., Secondary nucleation in amyloid formation, Chem. Commun., 2018, 54(63), 8667–8684 RSC
.
- S. Mukherjee, A. Sakunthala, L. Gadhe, M. Poudyal, A. S. Sawner and P. Kadu,
et al., Liquid–liquid Phase Separation of alpha-Synuclein: A New Mechanistic Insight for alpha-Synuclein Aggregation Associated with Parkinson's Disease Pathogenesis, J. Mol. Biol., 2023, 435(1), 167713 CrossRef CAS PubMed
.
- A. Agarwal and S. Mukhopadhyay, Prion protein biology through the lens of liquid–liquid phase separation, J. Mol. Biol., 2022, 434(1), 167368 CrossRef CAS PubMed
.
- M. Vendruscolo and M. Fuxreiter, Sequence Determinants of the Aggregation of Proteins Within Condensates Generated by Liquid–liquid Phase Separation, J. Mol. Biol., 2022, 434(1), 167201 CrossRef CAS PubMed
.
- R. Pellarin, E. Guarnera and A. Caflisch, Pathways and intermediates of amyloid fibril formation, J. Mol. Biol., 2007, 374(4), 917–924 CrossRef CAS PubMed
.
- A. K. Buell, C. Galvagnion, R. Gaspar, E. Sparr, M. Vendruscolo and T. P. J. Knowles,
et al., Solution conditions determine the relative importance of nucleation and growth processes in α-synuclein aggregation, Proc. Natl. Acad. Sci. U. S. A., 2014, 111(21), 7671–7676 CrossRef CAS PubMed
.
- Z. L. Almeida and R. M. M. Brito, Structure and Aggregation Mechanisms in Amyloids, Molecules, 2020, 25(5), 1195 CrossRef CAS PubMed
.
- J. T. Jarrett and P. T. Lansbury Jr, Amyloid fibril formation requires a chemically discriminating nucleation event: studies of an amyloidogenic sequence from the bacterial protein OsmB, Biochemistry., 1992, 31(49), 12345–12352 CrossRef CAS PubMed
.
- C. Frieden, Protein aggregation processes: in search of the mechanism, Protein Sci., 2007, 16(11), 2334–2344 CrossRef CAS PubMed
.
- S. J. Wood, J. Wypych, S. Steavenson, J. C. Louis, M. Citron and A. L. Biere, alpha-synuclein fibrillogenesis is nucleation-dependent. Implications for the pathogenesis of Parkinson's disease, J. Biol. Chem., 1999, 274(28), 19509–19512 CrossRef CAS PubMed
.
- D. Hall, J. Kardos, H. Edskes, J. A. Carver and Y. Goto, A multi-pathway perspective on protein aggregation: implications for control of the rate and extent of amyloid formation, FEBS Lett., 2015, 589(6), 672–679 CrossRef CAS PubMed
.
- S. Linse, Monomer-dependent secondary nucleation in amyloid formation, Biophys. Rev., 2017, 9(4), 329–338 CrossRef CAS PubMed
.
- J. W. Gibbs, On the equilibrium of heterogeneous substances, Trans. Connecticut Acad. Arts Sci., 1876, 108–248 Search PubMed
.
-
D. Kashchiev, Nucleation, Elsevier, 2000 Search PubMed
.
-
F. F. Abraham, Homogeneous nucleation theory, Elsevier, 1974 Search PubMed
.
- C.-T. Lee and E. M. Terentjev, Mechanisms and rates of nucleation of amyloid fibrils, J. Chem. Phys., 2017, 147(10), 105103 CrossRef PubMed
.
- S. Auer, P. Ricchiuto and D. Kashchiev, Two-step nucleation of amyloid fibrils: omnipresent or not?, J. Mol. Biol., 2012, 422(5), 723–730 CrossRef CAS PubMed
.
- F. Castello, J. M. Paredes, M. J. Ruedas-Rama, M. Martin, M. Roldan and S. Casares,
et al., Two-Step Amyloid Aggregation: Sequential Lag Phase Intermediates, Sci. Rep., 2017, 7(1), 40065 CrossRef CAS PubMed
.
- L. Zhang and J. D. Schmit, Pseudo-one-dimensional nucleation in dilute polymer solutions, Phys. Rev. E, 2016, 93(6), 060401 CrossRef PubMed
.
- A. Šarić, Y. C. Chebaro, T. P. Knowles and D. Frenkel, Crucial role of nonspecific interactions in amyloid nucleation, Proc. Natl. Acad. Sci. U. S. A., 2014, 111(50), 17869–17874 CrossRef PubMed
.
- D. K. Eggers and J. S. Valentine, Crowding and hydration effects on protein conformation: a study with sol-gel encapsulated proteins 11Edited by P. E. Wright, J. Mol. Biol., 2001, 314(4), 911–922 CrossRef CAS PubMed
.
- M. Pillai and S. K. Jha, The folding and aggregation energy landscapes of tethered RRM domains of human TDP-43 are coupled via a metastable molten globule-like oligomer, Biochemistry., 2018, 58(6), 608–620 CrossRef PubMed
.
- V. N. Uversky and A. L. Fink, Conformational constraints for amyloid fibrillation: the importance of being unfolded, Biochim. Biophys. Acta, Proteins Proteomics, 2004, 1698(2), 131–153 CrossRef CAS PubMed
.
- V. N. Uversky, H.-J. Lee, J. Li, A. L. Fink and S.-J. Lee, Stabilization of Partially Folded Conformation during α-Synuclein Oligomerization in Both Purified and Cytosolic
Preparations, J. Biol. Chem., 2001, 276(47), 43495–43498 CrossRef CAS PubMed
.
- A. W. Fitzpatrick, G. T. Debelouchina, M. J. Bayro, D. K. Clare, M. A. Caporini and V. S. Bajaj,
et al., Atomic structure and hierarchical assembly of a cross-β amyloid fibril, Proc. Natl. Acad. Sci. U. S. A., 2013, 110(14), 5468–5473 CrossRef CAS PubMed
.
- S. Mehra, L. Gadhe, R. Bera, A. S. Sawner and S. K. Maji, Structural and Functional Insights into α-Synuclein Fibril Polymorphism, Biomolecules., 2021, 11(10), 1419 CrossRef CAS PubMed
.
- J. Lee, E. K. Culyba, E. T. Powers and J. W. Kelly, Amyloid-β forms fibrils by nucleated conformational conversion of oligomers, Nat. Chem. Biol., 2011, 7(9), 602–609 CrossRef CAS PubMed
.
- D. Ghosh, P. K. Singh, S. Sahay, N. N. Jha, R. S. Jacob and S. Sen,
et al., Structure based aggregation studies reveal the presence of helix-rich intermediate during α-Synuclein aggregation, Sci. Rep., 2015, 5, 9228 CrossRef CAS PubMed
.
- T. R. Serio, A. G. Cashikar, A. S. Kowal, G. J. Sawicki, J. J. Moslehi and L. Serpell,
et al., Nucleated conformational conversion and the replication of conformational information by a prion determinant, Science., 2000, 289(5483), 1317–1321 CrossRef CAS PubMed
.
- Z. Fu, D. Aucoin, J. Davis, W. E. Van Nostrand and S. O. Smith, Mechanism of nucleated conformational conversion of Aβ42, Biochemistry., 2015, 54(27), 4197–4207 CrossRef CAS PubMed
.
- B. Falcon, A. Cavallini, R. Angers, S. Glover, T. K. Murray and L. Barnham,
et al., Conformation determines the seeding potencies of native and recombinant Tau aggregates, J. Biol. Chem., 2015, 290(2), 1049–1065 CrossRef CAS PubMed
.
- A. S. Wagner, A. Z. Politi, A. Ast, K. Bravo-Rodriguez, K. Baum and A. Buntru,
et al., Self-assembly of mutant huntingtin exon-1 fragments into large complex fibrillar structures involves nucleated branching, J. Mol. Biol., 2018, 430(12), 1725–1744 CrossRef CAS PubMed
.
- G. A. Garcia, S. I. Cohen, C. M. Dobson and T. P. Knowles, Nucleation-conversion-polymerization reactions of biological macromolecules with prenucleation clusters, Phys. Rev. E: Stat., Nonlinear, Soft Matter Phys., 2014, 89(3), 032712 CrossRef PubMed
.
- P. Arosio, T. P. Knowles and S. Linse, On the lag phase in amyloid fibril formation, Phys. Chem. Chem. Phys., 2015, 17(12), 7606–7618 RSC
.
- J. D. Camino, P. Gracia and N. Cremades, The role of water in the primary nucleation of protein amyloid aggregation, Biophys. Chem., 2021, 269, 106520 CrossRef CAS PubMed
.
- A. K. Srivastava, J. M. Pittman, J. Zerweck, B. S. Venkata, P. C. Moore and J. R. Sachleben,
et al., β-Amyloid aggregation and heterogeneous nucleation, Protein Sci., 2019, 28(9), 1567–1581 CrossRef CAS PubMed
.
- C. Wang, N. Shah, G. Thakur, F. Zhou and R. M. Leblanc, α-Synuclein in α-helical conformation at air–water interface: implication of conformation and orientation changes during its accumulation/aggregation, Chem. Commun., 2010, 46(36), 6702–6704 RSC
.
- K. A. Conway, J. D. Harper and P. T. Lansbury, Accelerated in vitro fibril formation by a mutant alpha-synuclein linked to early-onset Parkinson disease, Nat. Med., 1998, 4(11), 1318–1320 CrossRef CAS PubMed
.
- M. Grey, S. Linse, H. Nilsson, P. Brundin and E. Sparr, Membrane interaction of α-synuclein in different aggregation states, J. Parkinsons Dis., 2011, 1(4), 359–371 CAS
.
- C. Galvagnion, J. W. P. Brown, M. M. Ouberai, P. Flagmeier, M. Vendruscolo and A. K. Buell,
et al., Chemical properties of lipids strongly affect the kinetics of the membrane-induced
aggregation of α-synuclein, Proc. Natl. Acad. Sci. U. S. A., 2016, 113(26), 7065–7070 CrossRef CAS PubMed
.
- R. Vácha, S. Linse and M. Lund, Surface effects on aggregation kinetics of amyloidogenic peptides, J. Am. Chem. Soc., 2014, 136(33), 11776–11782 CrossRef PubMed
.
- A. Abdolvahabi, Y. Shi, S. Rasouli, C. M. Croom, A. Chuprin and B. F. Shaw, How do gyrating beads accelerate amyloid fibrillization?, Biophys. J., 2017, 112(2), 250–264 CrossRef CAS PubMed
.
- S. I. Cohen, M. Vendruscolo, C. M. Dobson and T. P. Knowles, Nucleated polymerization with secondary pathways. II. Determination of self-consistent solutions to growth processes described by non-linear master equations, J. Chem. Phys., 2011, 135(6), 065106 CrossRef PubMed
.
- S. I. Cohen, S. Linse, L. M. Luheshi, E. Hellstrand, D. A. White and L. Rajah,
et al., Proliferation of amyloid-β42 aggregates occurs through a secondary nucleation mechanism, Proc. Natl. Acad. Sci. U. S. A., 2013, 110(24), 9758–9763 CrossRef CAS PubMed
.
- A. Sakunthala, D. Datta, A. Navalkar, L. Gadhe, P. Kadu and K. Patel,
et al., Direct Demonstration of Seed Size-Dependent α-Synuclein Amyloid Amplification, J. Phys. Chem. Lett., 2022, 13(28), 6427–6438 CrossRef CAS PubMed
.
- G. Ramachandran and J. B. Udgaonkar, Evidence for the existence of a secondary pathway for fibril growth during the aggregation of tau, J. Mol. Biol., 2012, 421(2–3), 296–314 CrossRef CAS PubMed
.
- J. D. Harper and P. T. Lansbury Jr, Models of amyloid seeding in Alzheimer's disease and scrapie: mechanistic truths and physiological consequences of the time-dependent solubility of amyloid proteins, Ann. Rev. Biochem., 1997, 66(1), 385–407 CrossRef CAS PubMed
.
- B. O'Nuallain, S. Shivaprasad, I. Kheterpal and R. Wetzel, Thermodynamics of Aβ (1–40) amyloid fibril elongation, Biochemistry., 2005, 44(38), 12709–12718 CrossRef PubMed
.
- C. Wells, S. Brennan, M. Keon and L. Ooi, The role of amyloid oligomers in neurodegenerative pathologies, Int. J. Biol. Macromol., 2021, 181, 582–604 CrossRef CAS PubMed
.
- L. Breydo and V. N. Uversky, Structural, morphological, and functional diversity of amyloid oligomers, FEBS Lett., 2015, 589(19, Part A), 2640–2648 CrossRef CAS PubMed
.
- L. Gadhe, A. Sakunthala, S. Mukherjee, N. Gahlot, R. Bera and A. S. Sawner,
et al., Intermediates of α-synuclein aggregation: implications in Parkinson's disease pathogenesis, Biophys. Chem., 2022, 281, 106736 CrossRef CAS PubMed
.
- M. Sakono and T. Zako, Amyloid oligomers: formation and toxicity of Aβ oligomers, FEBS J., 2010, 277(6), 1348–1358 CrossRef CAS PubMed
.
- C. A. Lasagna-Reeves, D. L. Castillo-Carranza, M. J. Guerrero-Muñoz, G. R. Jackson and R. Kayed, Preparation and characterization of neurotoxic tau oligomers, Biochemistry., 2010, 49(47), 10039–10041 CrossRef CAS PubMed
.
- M. Ingelsson, Alpha-synuclein oligomers—neurotoxic molecules in Parkinson's disease and other Lewy body disorders, Front. Neurosci., 2016, 10, 408 Search PubMed
.
- Y.-S. Fang, K.-J. Tsai, Y.-J. Chang, P. Kao, R. Woods and P.-H. Kuo,
et al., Full-length TDP-43 forms toxic amyloid oligomers that are present in frontotemporal lobar dementia-TDP patients, Nat. Commun., 2014, 5(1), 4824 CrossRef CAS PubMed
.
- L. G. Nucifora, K. A. Burke, X. Feng, N. Arbez, S. Zhu and J. Miller,
et al., Identification of novel potentially toxic oligomers formed in vitro from mammalian-derived expanded huntingtin exon-1 protein, J. Biol. Chem., 2012, 287(19), 16017–16028 CrossRef CAS PubMed
.
- S. Simoneau, H. Rezaei, N. Salès, G. Kaiser-Schulz, M. Lefebvre-Roque and C. Vidal,
et al., In vitro and in vivo neurotoxicity of prion protein oligomers, PLoS Pathog., 2007, 3(8), e125 CrossRef PubMed
.
- P. Cizas, R. Budvytyte, R. Morkuniene, R. Moldovan, M. Broccio and M. Lösche,
et al., Size-dependent neurotoxicity of β-amyloid oligomers, Archives Biochem. Biophys., 2010, 496(2), 84–92 CrossRef CAS PubMed
.
- P. J. Ebenezer, A. M. Weidner, H. LeVine III, W. R. Markesbery, M. P. Murphy and L. Zhang,
et al., Neuron specific toxicity of oligomeric amyloid-β: role for JUN-kinase and oxidative stress, Alzheimer's Res. Ther., 2010, 22(3), 839–848 CAS
.
- R. Kayed and C. A. Lasagna-Reeves, Molecular mechanisms of amyloid oligomers toxicity, Alzheimer's Res. Ther., 2013, 33(s1), S67–S78 Search PubMed
.
- R. Gaspar, G. Meisl, A. K. Buell, L. Young, C. F. Kaminski and T. P. Knowles,
et al., Secondary nucleation of monomers on fibril surface dominates α-synuclein aggregation and provides autocatalytic amyloid amplification, Q. Rev. Biophys., 2017, 50, e6 CrossRef PubMed
.
- M. Bucciantini, E. Giannoni, F. Chiti, F. Baroni, L. Formigli and J. Zurdo,
et al., Inherent toxicity of aggregates implies a common mechanism for protein misfolding diseases, Nature, 2002, 416(6880), 507–511 CrossRef CAS PubMed
.
- M. Wogulis, S. Wright, D. Cunningham, T. Chilcote, K. Powell and R. E. Rydel, Nucleation-dependent polymerization is an essential component of amyloid-mediated neuronal cell death, J. Neurosci., 2005, 25(5), 1071–1080 CrossRef CAS PubMed
.
- A. Jan, O. Adolfsson, I. Allaman, A.-L. Buccarello, P. J. Magistretti and A. Pfeifer,
et al., Aβ42 neurotoxicity is mediated by ongoing nucleated polymerization process rather than by discrete Aβ42 species, J. Biol. Chem., 2011, 286(10), 8585–8596 CrossRef CAS PubMed
.
- D. C. Rodriguez Camargo, E. Sileikis, S. Chia, E. Axell, K. Bernfur and R. L. Cataldi,
et al., Proliferation of tau 304–380 fragment aggregates through autocatalytic secondary nucleation, ACS Chem. Neurosci., 2021, 12(23), 4406–4415 CrossRef CAS PubMed
.
- P. Gracia, J. D. Camino, L. Volpicelli-Daley and N. Cremades, Multiplicity of α-Synuclein Aggregated Species and Their Possible Roles in Disease, Int. J. Mol. Sci., 2020, 21(21), 8043 CrossRef CAS PubMed
.
- L. E. Orgel, Prion replication and secondary nucleation, Chem. Biol., 1996, 3(6), 413–414 CrossRef CAS PubMed
.
- O. Press-Sandler and Y. Miller, Distinct primary nucleation of polymorphic aβ dimers yields to distinguished fibrillation pathways, ACS Chem. Neurosci., 2019, 10(10), 4407–4413 CrossRef CAS PubMed
.
- J. S. Schreck, J. Bridstrup and J.-M. Yuan, Investigating the Effects of Molecular Crowding on the Kinetics of Protein Aggregation, J. Phys. Chem. B, 2020, 124(44), 9829–9839 CrossRef CAS PubMed
.
- A. Magno, A. Caflisch and R. Pellarin, Crowding Effects on Amyloid Aggregation Kinetics, J. Phys. Chem. Lett., 2010, 1(20), 3027–3032 CrossRef CAS
.
- A. Singleton, M. Farrer, J. Johnson, A. Singleton, S. Hague and J. Kachergus,
et al., α-Synuclein locus triplication causes Parkinson's disease, science., 2003, 302(5646), 841 CrossRef CAS PubMed
.
- M.-C. Chartier-Harlin, J. Kachergus, C. Roumier, V. Mouroux, X. Douay and S. Lincoln,
et al., α-synuclein locus duplication as a cause of familial Parkinson's disease, The Lancet, 2004, 364(9440), 1167–1169 CrossRef CAS PubMed
.
- S. Alberti and D. Dormann, Liquid–Liquid Phase Separation in Disease, Ann. Rev. Genet., 2019, 53, 171–194 CrossRef CAS PubMed
.
- P. E. Ash, S. Lei, J. Shattuck, S. Boudeau, Y. Carlomagno and M. Medalla,
et al., TIA1 potentiates tau phase separation and promotes generation of toxic oligomeric tau, Proc. Natl. Acad. Sci. U. S. A., 2021, 118(9), e2014188118 CrossRef CAS PubMed
.
- T. C. Michaels, D. Qian, A. Šarić, M. Vendruscolo, S. Linse and T. P. Knowles, Amyloid formation as a protein phase transition, Nat. Rev. Phys., 2023, 5(7), 379–397 CrossRef CAS
.
- M. Poudyal, A. Sakunthala, S. Mukherjee, L. Gadhe and S. K. Maji, Phase separation and other forms of α-Synuclein self-assemblies, Essays Biochem., 2022, 66(7), 987–1000 CrossRef CAS PubMed
.
- I. Ellinger and A. Ellinger, Smallest unit of life: cell biology, Comp. Med., 2014, 19–33 Search PubMed
.
- C. P. Satori, M. M. Henderson, E. A. Krautkramer, V. Kostal, M. M. Distefano and E. A. Arriaga, Bioanalysis of eukaryotic organelles, Chem. Rev., 2013, 113(4), 2733–2811 CrossRef CAS PubMed
.
- M. P. Rout and M. C. Field, The evolution of organellar coat complexes and organization of the eukaryotic cell, Ann. Rev. Biochem., 2017, 86, 637–657 CrossRef CAS PubMed
.
- E. Gomes and J. Shorter, The molecular language of membraneless organelles, J. Biol. Chem., 2019, 294(18), 7115–7127 CrossRef CAS PubMed
.
- E. B. Wilson, The Structure of Protoplasm, Science., 1899, 10(237), 33–45 CrossRef CAS PubMed
.
- N. W. P, The Origin of Life, Nature, 1938, 142(3592), 412–413 CrossRef
.
- C. P. Brangwynne, C. R. Eckmann, D. S. Courson, A. Rybarska, C. Hoege and J. Gharakhani,
et al., Germline P granules are liquid droplets that localize by controlled dissolution/condensation, Science, 2009, 324(5935), 1729–1732 CrossRef CAS PubMed
.
- H. Ainani, N. Bouchmaa, R. Ben Mrid and R. El Fatimy, Liquid–liquid phase separation of protein tau: an emerging process in Alzheimer's disease pathogenesis, Neurobiol. Dis., 2023, 178, 106011 CrossRef CAS PubMed
.
- S. Alberti, Phase separation in biology, Curr. Biol., 2017, 27(20), R1097–r102 CrossRef CAS PubMed
.
- S. Boeynaems, S. Alberti, N. L. Fawzi, T. Mittag, M. Polymenidou and F. Rousseau,
et al., Protein Phase Separation: A New Phase in Cell Biology, Trends Cell Biol., 2018, 28(6), 420–435 CrossRef CAS PubMed
.
- S. F. Banani, H. O. Lee, A. A. Hyman and M. K. Rosen, Biomolecular condensates: organizers of cellular biochemistry, Nat. Rev. Mol. Cell Biol., 2017, 18(5), 285–298 CrossRef CAS PubMed
.
- T. M. Franzmann and S. Alberti, Protein Phase Separation as a Stress Survival Strategy, Cold Spring Harbor Perspect. Biol., 2019, 11(6), a034058 CrossRef CAS PubMed
.
- P. A. Chong and J. D. Forman-Kay, Liquid–liquid phase separation in cellular signaling systems, Curr. Opin. Struct. Biol., 2016, 41, 180–186 CrossRef CAS PubMed
.
- P. Li, S. Banjade, H.-C. Cheng, S. Kim, B. Chen and L. Guo,
et al., Phase transitions in the assembly of multivalent signalling proteins, Nature, 2012, 483(7389), 336–340 CrossRef CAS PubMed
.
- L. Peng, E.-M. Li and L.-Y. Xu, From start to end: phase separation and transcriptional regulation, Biochim. Biophys. Acta, Gene Regul. Mech., 2020, 1863(12), 194641 CrossRef CAS PubMed
.
- M. Feric and T. Misteli, Phase separation in genome organization across evolution, Trends Cell Biol., 2021, 31(8), 671–685 CrossRef CAS PubMed
.
- A. H. Fox, S. Nakagawa, T. Hirose and C. S. Bond, Paraspeckles: where long noncoding RNA meets phase separation, Trends Biochem. Sci., 2018, 43(2), 124–135 CrossRef CAS PubMed
.
- J. B. Woodruff, A. A. Hyman and E. Boke, Organization and function of non-dynamic biomolecular condensates, Trends Biochem. Sci., 2018, 43(2), 81–94 CrossRef CAS PubMed
.
- J. B. Woodruff, Assembly of Mitotic Structures through Phase Separation, J. Mol. Biol., 2018, 430(23), 4762–4772 CrossRef CAS PubMed
.
- A. R. Strom, A. V. Emelyanov, M. Mir, D. V. Fyodorov, X. Darzacq and G. H. Karpen, Phase separation drives heterochromatin domain formation, Nature, 2017, 547(7662), 241–245 CrossRef CAS PubMed
.
- S. Petry, Mechanisms of mitotic spindle assembly, Ann. Rev. Biochem., 2016, 85, 659–683 CrossRef CAS PubMed
.
- H. Hayashi, K. Kimura and A. Kimura, Localized accumulation of tubulin during semi-open mitosis in the Caenorhabditis elegans embryo, Mol. Biol. Cell, 2012, 23(9), 1688–1699 CrossRef CAS PubMed
.
- A. Hernández-Vega, M. Braun, L. Scharrel, M. Jahnel, S. Wegmann and B. T. Hyman,
et al., Local nucleation of microtubule bundles through tubulin concentration into a condensed tau phase, Cell Rep., 2017, 20(10), 2304–2312 CrossRef PubMed
.
- A. G. Larson, D. Elnatan, M. M. Keenen, M. J. Trnka, J. B. Johnston and A. L. Burlingame,
et al., Liquid droplet formation by HP1α suggests a role for phase separation in heterochromatin, Nature, 2017, 547(7662), 236–240 CrossRef CAS PubMed
.
- L. B. Case, X. Zhang, J. A. Ditlev and M. K. Rosen, Stoichiometry controls activity of phase-separated clusters of actin signaling proteins, Science., 2019, 363(6431), 1093–1097 CrossRef CAS PubMed
.
- M. Feric, N. Vaidya, T. S. Harmon, D. M. Mitrea, L. Zhu and T. M. Richardson,
et al., Coexisting Liquid Phases Underlie Nucleolar Subcompartments, Cell., 2016, 165(7), 1686–1697 CrossRef CAS PubMed
.
- A. S. Sawner, S. Ray, P. Yadav, S. Mukherjee, R. Panigrahi and M. Poudyal,
et al., Modulating α-Synuclein Liquid–Liquid Phase Separation, Biochemistry., 2021, 60(48), 3676–3696 CrossRef CAS PubMed
.
- C. P. Brangwynne, T. J. Mitchison and A. A. Hyman, Active liquid-like behavior of nucleoli determines their size and shape in Xenopus laevis oocytes, Proc. Natl. Acad. Sci. U. S. A., 2011, 108(11), 4334–4339 CrossRef CAS PubMed
.
- S. Wegmann, B. Eftekharzadeh, K. Tepper, K. M. Zoltowska, R. E. Bennett and S. Dujardin,
et al., Tau protein liquid–liquid phase separation can initiate tau aggregation, EMBO J., 2018, 37(7), e98049 CrossRef PubMed
.
- S. Alberti, Phase separation in biology, Curr. Biol., 2017, 27(20), R1097–R1102 CrossRef CAS PubMed
.
- Y. Shin and C. P. Brangwynne, Liquid phase condensation in cell physiology and disease, Science., 2017, 357(6357), eaaf4382 CrossRef PubMed
.
- P. J. Flory, Thermodynamics of high polymer solutions, J. Chem. Phys., 1942, 10(1), 51–61 CrossRef CAS
.
- J. Nott Timothy, E. Petsalaki, P. Farber, D. Jervis, E. Fussner and A. Plochowietz,
et al., Phase Transition of a Disordered Nuage Protein Generates Environmentally Responsive Membraneless Organelles, Mol. Cell, 2015, 57(5), 936–947 CrossRef CAS PubMed
.
- C. F. Lee, C. P. Brangwynne, J. Gharakhani, A. A. Hyman and F. Jülicher, Spatial organization of the cell cytoplasm by position-dependent phase separation, Phys. Rev. Lett., 2013, 111(8), 088101 CrossRef PubMed
.
- A. S. Holehouse, K. Garai, N. Lyle, A. Vitalis and R. V. Pappu, Quantitative assessments of the distinct contributions of polypeptide backbone amides versus side chain groups to chain expansion via chemical denaturation, J. Am. Chem. Soc., 2015, 137(8), 2984–2995 CrossRef CAS PubMed
.
- P. Brangwynne Clifford, P. Tompa and V. Pappu Rohit, Polymer physics of intracellular phase transitions, Nat. Phys., 2015, 11(11), 899–904 Search PubMed
.
- M.-T. Wei, S. Elbaum-Garfinkle, A. S. Holehouse, C. C.-H. Chen, M. Feric and C. B. Arnold,
et al., Phase behaviour of disordered proteins underlying low density and high permeability of liquid organelles, Nat. Chem., 2017, 9(11), 1118–1125 CrossRef CAS PubMed
.
- J. Wang, J.-M. Choi, A. S. Holehouse, H. O. Lee, X. Zhang and M. Jahnel,
et al., A Molecular Grammar Governing the Driving Forces for Phase Separation of Prion-like RNA Binding Proteins, Cell., 2018, 174(3), 688–699.e16 CrossRef CAS PubMed
.
- A. Bremer, M. Farag, W. M. Borcherds, I. Peran, E. W. Martin and R. V. Pappu,
et al., Deciphering how naturally occurring sequence features impact the phase behaviours of disordered prion-like domains, Nat. Chem., 2022, 14(2), 196–207 CrossRef CAS PubMed
.
- E. W. Martin, A. S. Holehouse, I. Peran, M. Farag, J. J. Incicco and A. Bremer,
et al., Valence and patterning of aromatic residues determine the phase behavior of prion-like domains, Science., 2020, 367(6478), 694–699 CrossRef CAS PubMed
.
- C. W. Pak, M. Kosno, A. S. Holehouse, S. B. Padrick, A. Mittal and R. Ali,
et al., Sequence Determinants of Intracellular Phase Separation by Complex Coacervation of a Disordered Protein, Mol. Cell, 2016, 63(1), 72–85 CrossRef CAS PubMed
.
- S. Das, Y.-H. Lin, R. M. Vernon, J. D. Forman-Kay and H. S. Chan, Comparative roles of charge, π, and hydrophobic interactions in sequence-dependent phase separation of intrinsically disordered proteins, Proc. Natl. Acad. Sci. U. S. A., 2020, 117(46), 28795–28805 CrossRef CAS PubMed
.
- J. J. McManus, P. Charbonneau, E. Zaccarelli and N. Asherie, The physics of protein self-assembly, Curr. Opin. Colloid Interface Sci., 2016, 22, 73–79 CrossRef CAS
.
- W. Li, B. R. A. Persson, M. Morin, M. A. Behrens, M. Lund and M. Zackrisson Oskolkova, Charge-induced patchy attractions between proteins, J. Phys. Chem. B, 2015, 119(2), 503–508 CrossRef CAS PubMed
.
- J. Cai and A. M. Sweeney, The proof is in the pidan: generalizing proteins as patchy particles, ACS Cent. Sci., 2018, 4(7), 840–853 CrossRef CAS PubMed
.
- A. N. Semenov and M. Rubinstein, Thermoreversible gelation in solutions of associative polymers. 1. Statics, Macromolecules., 1998, 31(4), 1373–1385 CrossRef CAS
.
- J.-M. Choi, A. S. Holehouse and R. V. Pappu, Physical principles underlying the complex biology of intracellular phase transitions, Ann. Rev. Biophys., 2020, 49, 107–133 CrossRef CAS PubMed
.
- W. Borcherds, A. Bremer, M. B. Borgia and T. Mittag, How do intrinsically disordered protein regions encode a driving force for liquid–liquid phase separation?, Curr. Opin. Struct. Biol., 2021, 67, 41–50 CrossRef CAS PubMed
.
- V. N. Uversky, Intrinsically disordered proteins in overcrowded milieu: membrane-less organelles, phase separation, and intrinsic disorder, Curr. Opin. Struct. Biol., 2017, 44, 18–30 CrossRef CAS PubMed
.
- M. Fuxreiter, Fuzzy protein theory for disordered proteins, Biochem. Soc. Trans., 2020, 48(6), 2557–2564 CrossRef CAS PubMed
.
- M. I. Freiberger, P. G. Wolynes, D. U. Ferreiro and M. Fuxreiter, Frustration in fuzzy protein complexes leads to interaction versatility, J. Phys. Chem. B, 2021, 125(10), 2513–2520 CrossRef CAS PubMed
.
- A. Hatos, A. M. Monzon, S. C. Tosatto, D. Piovesan and M. Fuxreiter, FuzDB: a new phase in understanding fuzzy interactions, Nucleic Acids Res., 2022, 50(D1), D509–D517 CrossRef CAS PubMed
.
- S. Alberti, R. Halfmann, O. King, A. Kapila and S. Lindquist, A systematic survey identifies prions and illuminates sequence features of prionogenic proteins, Cell., 2009, 137(1), 146–158 CrossRef CAS PubMed
.
-
A. E. Posey, A. S. Holehouse and R. V. Pappu, Phase separation of intrinsically disordered proteins, Methods Enzymology, Elsevier, 2018, 611, pp. 1–30 Search PubMed
.
- E. W. Martin and T. Mittag, Relationship of sequence and phase separation in protein low-complexity regions, Biochemistry., 2018, 57(17), 2478–2487 CrossRef CAS PubMed
.
- T. Matsumoto, K. Matsukawa, N. Watanabe, Y. Kishino, H. Kunugi and R. Ihara,
et al., Self-assembly of FUS through its low-complexity domain contributes to neurodegeneration, Human Mol. Genet., 2018, 27(8), 1353–1365 CrossRef CAS PubMed
.
- O. Coskuner-Weber, O. Mirzanli and V. N. Uversky, Intrinsically disordered proteins and proteins with intrinsically disordered regions in neurodegenerative diseases, Biophys. Rev., 2022, 14(3), 679–707 CrossRef CAS PubMed
.
- M. Polymenidou and D. W. Cleveland, Prion-like spread of protein aggregates in neurodegeneration, J. Exp. Med., 2012, 209(5), 889–893 CrossRef CAS PubMed
.
- A. D. Gitler and J. Shorter, RNA-binding proteins with prion-like domains in ALS and FTLD-U, Prion., 2011, 5(3), 179–187 CrossRef CAS PubMed
.
- K. Bhasne, S. Sebastian, N. Jain and S. Mukhopadhyay, Synergistic amyloid switch triggered by early heterotypic oligomerization of intrinsically disordered α-synuclein and tau, J. Mol. Biol., 2018, 430(16), 2508–2520 CrossRef CAS PubMed
.
- M. J. do Amaral, M. H. O. Freire, M. S. Almeida, A. S. Pinheiro and Y. Cordeiro, Phase separation of the mammalian prion protein: physiological and pathological perspectives, J. Neurochem., 2022, 58–75 Search PubMed
.
- F. Aktar, C. Burudpakdee, M. Polanco, S. Pei, T. C. Swayne and P. N. Lipke,
et al., The huntingtin inclusion is a dynamic phase-separated compartment, Life Sci. Alliance, 2019, 2(5), e201900489 CrossRef PubMed
.
- A. Patel, H. O. Lee, L. Jawerth, S. Maharana, M. Jahnel and M. Y. Hein,
et al., A Liquid-to-Solid Phase Transition of the ALS Protein FUS Accelerated by Disease Mutation, Cell., 2015, 162(5), 1066–1077 CrossRef CAS PubMed
.
- A. Molliex, J. Temirov, J. Lee, M. Coughlin, A. P. Kanagaraj and H. J. Kim,
et al., Phase separation by low complexity domains promotes stress granule assembly and drives pathological fibrillization, Cell., 2015, 163(1), 123–133 CrossRef CAS PubMed
.
- I. R. Mackenzie, A. M. Nicholson, M. Sarkar, J. Messing, M. D. Purice and C. Pottier,
et al., TIA1 mutations in amyotrophic lateral sclerosis and frontotemporal dementia promote phase separation and alter stress granule dynamics, Neuron., 2017, 95(4), 808–816.e9 CrossRef CAS PubMed
.
- K.-H. Lee, P. Zhang, H. J. Kim, D. M. Mitrea, M. Sarkar and B. D. Freibaum,
et al., C9orf72 dipeptide repeats impair the assembly, dynamics, and function of membrane-less organelles, Cell., 2016, 167(3), 774–788.e17 CrossRef CAS PubMed
.
- D. T. Murray, M. Kato, Y. Lin, K. R. Thurber, I. Hung and S. L. McKnight,
et al., Structure of FUS Protein Fibrils and Its Relevance to Self-Assembly and Phase Separation of Low-Complexity Domains, Cell., 2017, 171(3), 615–627.e16 CrossRef CAS PubMed
.
- S. Boyko, X. Qi, T.-H. Chen, K. Surewicz and W. K. Surewicz, Liquid–liquid phase separation of tau protein: the crucial role of electrostatic interactions, J. Biol. Chem., 2019, 294(29), 11054–11059 CrossRef CAS PubMed
.
- N. Galvanetto, M. T. Ivanović, A. Chowdhury, A. Sottini, M. F. Nüesch and D. Nettels,
et al., Extreme dynamics in a biomolecular condensate, Nature, 2023, 619(7971), 876–883 CrossRef CAS PubMed
.
- J. A. Ditlev, L. B. Case and M. K. Rosen, Who's In and Who's Out—Compositional Control of Biomolecular Condensates, J. Mol. Biol., 2018, 430(23), 4666–4684 CrossRef CAS PubMed
.
- L. Jawerth, E. Fischer-Friedrich, S. Saha, J. Wang, T. Franzmann and X. Zhang,
et al., Protein condensates as aging Maxwell fluids, Science., 2020, 370(6522), 1317–1323 CrossRef CAS PubMed
.
- N. Taylor, S. Elbaum-Garfinkle, N. Vaidya, H. Zhang, H. A. Stone and C. P. Brangwynne, Biophysical characterization of organelle-based RNA/protein liquid phases using microfluidics, Soft Matter, 2016, 12(45), 9142–9150 RSC
.
- I. Alshareedah, M. M. Moosa, M. Pham, D. A. Potoyan and P. R. Banerjee, Programmable viscoelasticity in protein–RNA condensates with disordered sticker-spacer polypeptides, Nat. Commun., 2021, 12(1), 6620 CrossRef CAS PubMed
.
- S. Alberti, The wisdom of crowds: regulating cell function through condensed states of living matter, J. Cell Sci., 2017, 130(17), 2789–2796 CAS
.
- J. A. Riback, C. D. Katanski, J. L. Kear-Scott, E. V. Pilipenko, A. E. Rojek and T. R. Sosnick,
et al., Stress-triggered phase separation is an adaptive, evolutionarily tuned response, Cell., 2017, 168(6), 1028–1040.e19 CrossRef CAS PubMed
.
- T. M. Franzmann, M. Jahnel, A. Pozniakovsky, J. Mahamid, A. S. Holehouse and E. Nüske,
et al., Phase separation of a yeast prion protein promotes cellular fitness, Science., 2018, 359(6371), eaao5654 CrossRef PubMed
.
- E. Boke, M. Ruer, M. Wühr, M. Coughlin, R. Lemaitre and S. P. Gygi,
et al., Amyloid-like Self-Assembly of a Cellular Compartment, Cell., 2016, 166(3), 637–650 CrossRef CAS PubMed
.
- E. Audas Timothy, E. Audas Danielle, D. Jacob Mathieu, J. J. D. Ho, M. Khacho and M. Wang,
et al., Adaptation to Stressors by Systemic Protein Amyloidogenesis, Dev. Cell, 2016, 39(2), 155–168 CrossRef CAS PubMed
.
- S. Alberti and D. Dormann, Liquid–liquid phase separation in disease, Ann. Rev. Genet., 2019, 53, 171–194 CrossRef CAS PubMed
.
- M. A. Mensah, H. Niskanen, A. P. Magalhaes, S. Basu, M. Kircher and H. L. Sczakiel,
et al., Aberrant phase separation and nucleolar dysfunction in rare genetic diseases, Nature, 2023, 614(7948), 564–571 CAS
.
- Z. Chen, Y. Huai, W. Mao, X. Wang, K. Ru and A. Qian,
et al., Liquid–Liquid Phase Separation of Biomacromolecules and Its Roles in Metabolic Diseases, Cells., 2022, 11(19), 3023 CrossRef CAS PubMed
.
- Y. Mo, Y. Feng, W. Huang, N. Tan, X. Li and M. Jie,
et al., Liquid–Liquid Phase Separation in Cardiovascular Diseases, Cells., 2022, 11(19), 3040 CrossRef CAS PubMed
.
- S. Wang, T. Dai, Z. Qin, T. Pan, F. Chu and L. Lou,
et al., Targeting liquid–liquid phase separation of SARS-CoV-2 nucleocapsid protein promotes innate antiviral immunity by elevating MAVS activity, Nat. Cell Biol., 2021, 23(7), 718–732 CrossRef CAS PubMed
.
- W. Wei, L. Bai, B. Yan, W. Meng, H. Wang and J. Zhai,
et al., When liquid–liquid phase separation meets viral infections, Front. Immunol., 2022, 13, 985622 CrossRef CAS PubMed
.
- A. Ahmad, V. N. Uversky and R. H. Khan, Aberrant liquid–liquid phase separation and amyloid aggregation of proteins related to neurodegenerative diseases, Int. J. Biol. Macromol., 2022, 220, 703–720 CrossRef CAS PubMed
.
- A. L. Darling and J. Shorter, Combating deleterious phase transitions in neurodegenerative disease, Biochim. Biophys. Acta, Mol. Cell Res., 2021, 1868(5), 118984 CrossRef CAS PubMed
.
- S. Spannl, M. Tereshchenko, G. J. Mastromarco, S. J. Ihn and H. O. Lee, Biomolecular condensates in neurodegeneration and cancer, Traffic., 2019, 20(12), 890–911 CrossRef CAS PubMed
.
- S. Alberti and A. A. Hyman, Biomolecular condensates at the nexus of cellular stress, protein aggregation disease and ageing, Nat. Rev. Mol. Cell Biol., 2021, 22(3), 196–213 CrossRef CAS PubMed
.
- S. Mehta and J. Zhang, Liquid–liquid phase separation drives cellular function and dysfunction in cancer, Nat. Rev. Cancer, 2022, 22(4), 239–252 CrossRef CAS PubMed
.
- R. Halfmann, A glass menagerie of low complexity sequences, Curr. Opin. Struct. Biol., 2016, 38, 18–25 CrossRef CAS PubMed
.
- R. V. Pappu, S. R. Cohen, F. Dar, M. Farag and M. Kar, Phase Transitions of Associative Biomacromolecules, Chem. Rev., 2023, 123(14), 8945–8987 CrossRef CAS PubMed
.
- H. Watanabe, Viscoelasticity and dynamics of entangled polymers, Prog. Polym. Sci., 1999, 24(9), 1253–1403 CrossRef CAS
.
- S. Kroschwald, S. Maharana, D. Mateju, L. Malinovska, E. Nüske and I. Poser,
et al., Promiscuous interactions and protein disaggregases determine the material state of stress-inducible RNP granules, eLife., 2015, 4, e06807 CrossRef PubMed
.
- Y. Shen, A. Chen, W. Wang, Y. Shen, F. S. Ruggeri and S. Aime,
et al., The liquid-to-solid transition of FUS is promoted by the condensate surface, Proc. Natl. Acad. Sci. U. S. A., 2023, 120(33), e2301366120 CrossRef CAS PubMed
.
- T. R. Peskett, F. Rau, J. O’Driscoll, R. Patani, A. R. Lowe and H. R. Saibil, A Liquid to Solid Phase Transition Underlying Pathological Huntingtin Exon1 Aggregation, Mol. Cell, 2018, 70(4), 588–601.e6 CrossRef CAS PubMed
.
- A. Patel, H. O. Lee, L. Jawerth, S. Maharana, M. Jahnel and M. Y. Hein,
et al., A liquid-to-solid phase transition of the ALS protein FUS accelerated by disease mutation, Cell., 2015, 162(5), 1066–1077 CrossRef CAS PubMed
.
- M. Linsenmeier, L. Faltova, C. Morelli, U. Capasso Palmiero, C. Seiffert and A. M. Küffner,
et al., The interface of condensates of the hnRNPA1 low-complexity domain promotes formation of amyloid fibrils, Nat. Chem., 2023, 1–10 Search PubMed
.
- A. Agarwal, S. K. Rai, A. Avni and S. Mukhopadhyay, An intrinsically disordered pathological prion variant Y145Stop converts into self-seeding amyloids via liquid–liquid phase separation, Proc. Natl. Acad. Sci. U. S. A., 2021, 118(45), e2100968118 CrossRef CAS PubMed
.
- C. Mathieu, R. V. Pappu and J. P. Taylor, Beyond aggregation: pathological phase transitions in neurodegenerative disease, Science., 2020, 370(6512), 56–60 CrossRef CAS PubMed
.
- J. R. Mann, A. M. Gleixner, J. C. Mauna, E. Gomes, M. R. DeChellis-Marks and P. G. Needham,
et al., RNA binding antagonizes neurotoxic phase transitions of TDP-43, Neuron., 2019, 102(2), 321–338.e8 CrossRef CAS PubMed
.
- L. Li, H. Liu, P. Dong, D. Li, W. R. Legant and J. B. Grimm,
et al., Real-time imaging of Huntingtin aggregates diverting target search and gene transcription, eLife., 2016, 5, e17056 CrossRef PubMed
.
- M. C. Hardenberg, T. Sinnige, S. Casford, S. T. Dada, C. Poudel and E. A. Robinson,
et al., Observation of an α-synuclein liquid droplet state and its maturation into Lewy body-like assemblies, J. Mol. Cell Biol., 2021, 13(4), 282–294 CAS
.
- Y. Xing, A. Nandakumar, A. Kakinen, Y. Sun, T. P. Davis and P. C. Ke,
et al., Amyloid aggregation under the lens of liquid–liquid phase separation, J. Phys. Chem. Lett., 2020, 12(1), 368–378 CrossRef PubMed
.
- K. Wang, J.-Q. Liu, T. Zhong, X.-L. Liu, Y. Zeng and X. Qiao,
et al., Phase separation and cytotoxicity of tau are modulated by protein disulfide isomerase and S-nitrosylation of this molecular chaperone, J. Mol. Biol., 2020, 432(7), 2141–2163 CrossRef CAS PubMed
.
- Y. Sun and A. Chakrabartty, Phase to Phase with TDP-43, Biochemistry., 2017, 56(6), 809–823 CrossRef CAS PubMed
.
- A. Molliex, J. Temirov, J. Lee, M. Coughlin, P. Kanagaraj Anderson and J. Kim Hong,
et al., Phase Separation by Low Complexity Domains Promotes Stress Granule Assembly and Drives Pathological Fibrillization, Cell., 2015, 163(1), 123–133 CrossRef CAS PubMed
.
- S. Xiang, M. Kato, C. Wu Leeju, Y. Lin, M. Ding and Y. Zhang,
et al., The LC Domain of hnRNPA2 Adopts Similar Conformations in Hydrogel Polymers, Liquid-like Droplets, and Nuclei, Cell., 2015, 163(4), 829–839 CrossRef CAS PubMed
.
- R. Kumar, S. Das, G. M. Mohite, S. K. Rout, S. Halder and N. N. Jha,
et al., Cytotoxic Oligomers and Fibrils Trapped in a Gel-like State of α-Synuclein Assemblies, Angew. Chem., Int. Ed., 2018, 57(19), 5262–5266 CrossRef CAS PubMed
.
- A. Jain and R. D. Vale, RNA phase transitions in repeat expansion disorders, Nature, 2017, 546(7657), 243–247 CrossRef CAS PubMed
.
- V. Singh, L. Xu, S. Boyko, K. Surewicz and W. K. Surewicz, Zinc promotes liquid–liquid phase separation of tau protein, J. Biol. Chem., 2020, 295(18), 5850–5856 CrossRef CAS PubMed
.
- D. Mateju, T. M. Franzmann, A. Patel, A. Kopach, E. E. Boczek and S. Maharana,
et al., An aberrant phase transition of stress granules triggered by misfolded protein and prevented by chaperone function, EMBO J., 2017, 36(12), 1669–1687 CrossRef CAS PubMed
.
- E. Chatani and N. Yamamoto, Recent progress on understanding the mechanisms of amyloid nucleation, Biophys. Rev., 2018, 10(2), 527–534 CrossRef CAS PubMed
.
- S. F. Shimobayashi, P. Ronceray, D. W. Sanders, M. P. Haataja and C. P. Brangwynne, Nucleation landscape of biomolecular condensates, Nature, 2021, 599(7885), 503–506 CrossRef CAS PubMed
.
- W. T. Snead and A. S. Gladfelter, The Control Centers of Biomolecular Phase Separation: How Membrane Surfaces, PTMs, and Active Processes Regulate Condensation, Mol. Cell, 2019, 76(2), 295–305 CrossRef CAS PubMed
.
- A. Narayanan, A. Meriin, J. O. Andrews, J.-H. Spille, M. Y. Sherman and I. I. Cisse, A first order phase transition mechanism underlies protein aggregation in mammalian cells, eLife., 2019, 8, e39695 CrossRef PubMed
.
- D. S. Lee, C.-H. Choi, D. W. Sanders, L. Beckers, J. A. Riback and C. P. Brangwynne,
et al., Size distributions of intracellular condensates reflect competition between coalescence and nucleation, Nat. Phys., 2023, 19(4), 586–596 Search PubMed
.
- I. Myrgorodska, I. Colomer and S. P. Fletcher, Oligomerization driven by phase separation, ChemSystemsChem, 2020, 2(5), e1900059 Search PubMed
.
- S. Ray, T. O. Mason, L. Boyens-Thiele, A. Farzadfard, J. A. Larsen and R. K. Norrild,
et al., Mass photometric detection and quantification of nanoscale α-synuclein phase separation, Nat. Chem., 2023, 1–11 Search PubMed
.
- E. W. Martin, T. S. Harmon, J. B. Hopkins, S. Chakravarthy, J. J. Incicco and P. Schuck,
et al., A multi-step nucleation process determines the kinetics of prion-like domain phase separation, Nat. Commun., 2021, 12(1), 4513 CrossRef CAS PubMed
.
- N. Sabri, M. J. Cuneo, M. R. Marzahn, J. Lee, J. J. Bouchard and S. Vaithiyalingam, et al., Reduction of oligomer size modulates the competition between cluster formation and phase separation of the tumor suppressor SPOP, bioRxiv, 2023 DOI:10.1101/2023.02.11.528154.
- M. Kar, F. Dar, T. J. Welsh, L. T. Vogel, R. Kühnemuth and A. Majumdar,
et al., Phase-separating RNA-binding proteins form heterogeneous distributions of clusters in subsaturated solutions, Proc. Natl. Acad. Sci. U. S. A., 2022, 119(28), e2202222119 CrossRef CAS PubMed
.
- G. C. Carter, C.-H. Hsiung, L. Simpson, H. Yang and X. Zhang, N-terminal Domain of TDP43 Enhances Liquid–Liquid Phase Separation of Globular Proteins, J. Mol. Biol., 2021, 433(10), 166948 CrossRef CAS PubMed
.
- T. P. Dao, R.-M. Kolaitis, H. J. Kim, K. O’Donovan, B. Martyniak and E. Colicino,
et al., Ubiquitin modulates liquid–liquid phase separation of UBQLN2 via disruption of multivalent interactions, Mol. Cell, 2018, 69(6), 965–978.e6 CrossRef CAS PubMed
.
- T. P. Dao, B. Martyniak, A. J. Canning, Y. Lei, E. G. Colicino and M. S. Cosgrove,
et al., ALS-linked mutations affect UBQLN2 oligomerization and phase separation in a position-and amino acid-dependent manner, Structure, 2019, 27(6), 937–951.e5 CrossRef CAS PubMed
.
- N. Sabri, M. J. Cuneo, M. R. Marzahn, J. Lee, J. J. Bouchard and Ö. Güllülü,
et al., Reduction of oligomer size modulates the competition between cluster formation and phase separation of the tumor suppressor SPOP, J. Biol. Chem., 2023, 105427 CrossRef CAS PubMed
.
- J. Wen, L. Hong, G. Krainer, Q.-Q. Yao, T. P. Knowles and S. Wu,
et al., Conformational expansion of tau in condensates promotes irreversible aggregation, J. Am. Chem. Soc., 2021, 143(33), 13056–13064 CrossRef CAS PubMed
.
- W. Pan, P. G. Vekilov and V. Lubchenko, Origin of anomalous mesoscopic phases in protein solutions, J. Phys. Chem. B, 2010, 114(22), 7620–7630 CrossRef CAS PubMed
.
- M. A. Vorontsova, H. Y. Chan, V. Lubchenko and P. G. Vekilov, Lack of dependence of the sizes of the mesoscopic protein clusters on electrostatics, Biophys. J., 2015, 109(9), 1959–1968 CrossRef CAS PubMed
.
- D. S. Yang, A. Saeedi, A. Davtyan, M. Fathi, M. B. Sherman and M. S. Safari,
et al., Mesoscopic protein-rich clusters host the nucleation of mutant p53 amyloid fibrils, Proc. Natl. Acad. Sci. U. S. A., 2021, 118(10), e2015618118 CrossRef CAS PubMed
.
- E. Bertrand, C. Demongin, I. Dobra, J. C. Rengifo-Gonzalez, A. S. Singatulina and M. V. Sukhanova,
et al., FUS fibrillation occurs through a nucleation-based process below the critical concentration required for liquid–liquid phase separation, Sci. Rep., 2023, 13(1), 7772 CrossRef CAS PubMed
.
- G. Vecchi, P. Sormanni, B. Mannini, A. Vandelli, G. G. Tartaglia and C. M. Dobson,
et al., Proteome-wide observation of the phenomenon of life on the edge of solubility, Proc. Natl. Acad. Sci. U. S. A., 2020, 117(2), 1015–1020 CrossRef CAS PubMed
.
- A. A. Hyman, C. A. Weber and F. Jülicher, Liquid–Liquid Phase Separation in Biology, Ann. Rev. Cell Dev. Biol., 2014, 30(1), 39–58 CrossRef CAS PubMed
.
- P. Arosio, T. P. Knowles and S. Linse, On the lag phase in amyloid fibril formation, Phys. Chem. Chem. Phys., 2015, 17(12), 7606–7618 RSC
.
- A. Garaizar, I. Sanchez-Burgos, R. Collepardo-Guevara and J. R. Espinosa, Expansion of Intrinsically Disordered Proteins Increases the Range of Stability of Liquid–Liquid Phase Separation, Molecules, 2020, 25(20), 4705 CrossRef CAS PubMed
.
- L. Li and Z. Hou, Crosslink-Induced Conformation Change of Intrinsically Disordered Proteins Have a Nontrivial Effect on Phase Separation Dynamics and Thermodynamics, J. Phys. Chem. B, 2023, 127(22), 5018–5026 CrossRef CAS PubMed
.
- A. Majumdar, P. Dogra, S. Maity and S. Mukhopadhyay, Liquid–Liquid Phase Separation Is Driven by Large-Scale Conformational Unwinding and Fluctuations of Intrinsically Disordered Protein Molecules, J. Phys. Chem. Lett., 2019, 10(14), 3929–3936 CrossRef CAS PubMed
.
- N. M. Kanaan, C. Hamel, T. Grabinski and B. Combs, Liquid–liquid phase separation induces pathogenic tau conformations in vitro, Nat. Commun., 2020, 11(1), 2809 CrossRef CAS PubMed
.
- D. Ubbiali, M. Fratini, L. Piersimoni, C. H. Ihling, M. Kipping and I. Heilmann,
et al., Direct Observation of “Elongated” Conformational States in α-Synuclein upon Liquid–Liquid Phase Separation, Angew. Chemie, 2022, 134(46), e202205726 CrossRef
.
- M. Farag, S. R. Cohen, W. M. Borcherds, A. Bremer, T. Mittag and R. V. Pappu, Condensates formed by prion-like low-complexity domains have small-world network structures and interfaces defined by expanded conformations, Nat. Commun., 2022, 13(1), 7722 CrossRef CAS PubMed
.
- H. Tange, D. Ishibashi, T. Nakagaki, Y. Taguchi, Y. O. Kamatari and H. Ozawa,
et al., Liquid–liquid phase separation of full-length prion protein initiates conformational conversion in vitro, J. Biol. Chem., 2021, 296 Search PubMed
.
- A. Joshi, A. Walimbe, A. Avni, S. K. Rai, L. Arora and S. Sarkar,
et al., Single-molecule FRET unmasks structural subpopulations and crucial molecular events during FUS low-complexity domain phase separation, Nat. Commun., 2023, 14(1), 7331 CrossRef CAS PubMed
.
- S. Ambadipudi, J. G. Reddy, J. Biernat, E. Mandelkow and M. Zweckstetter, Residue-specific identification of phase separation hot spots of Alzheimer's-related protein tau, Chem. Sci., 2019, 10(26), 6503–6507 RSC
.
- C.-H. Choi, D. S. Lee, D. W. Sanders and C. P. Brangwynne, Condensate interfaces can accelerate protein aggregation, Biophys. J., 2023 DOI:10.1016/j.bpj.2023.10.009
.
- C. He, C. Y. Wu, W. Li and K. Xu, Multidimensional super-resolution microscopy unveils nanoscale surface aggregates in the aging of FUS condensates, J. Am. Chem. Soc., 2023, 145(44), 24240–24248 CrossRef CAS PubMed
.
- M. Fändrich, Oligomeric intermediates in amyloid formation: structure determination and mechanisms of toxicity, J. Mol. Biol., 2012, 421(4–5), 427–440 CrossRef PubMed
.
- A. J. Dear, G. Meisl, A. Šarić, T. C. Michaels, M. Kjaergaard and S. Linse,
et al., Identification of on-and off-pathway oligomers in amyloid fibril formation, Chem. Sci., 2020, 11(24), 6236–6247 RSC
.
- V. N. Uversky, Mysterious oligomerization of the amyloidogenic proteins, FEBS J., 2010, 277(14), 2940–2953 CrossRef CAS PubMed
.
- B. Winner, R. Jappelli, S. K. Maji, P. A. Desplats, L. Boyer and S. Aigner,
et al., In vivo demonstration that α-synuclein oligomers are toxic, Proc. Natl. Acad. Sci. U. S. A., 2011, 108(10), 4194–4199 CrossRef CAS PubMed
.
- M. M. Tompkins and W. D. Hill, Contribution of somal Lewy bodies to neuronal death, Brain Res., 1997, 775(1), 24–29 CrossRef CAS PubMed
.
- A. Pradhan, S. Mishra, A. Surolia and D. Panda, C1 Inhibits Liquid–Liquid Phase Separation and Oligomerization of Tau and Protects Neuroblastoma Cells against Toxic Tau Oligomers, ACS Chem. Neurosci., 2021, 12(11), 1989–2002 CrossRef CAS PubMed
.
- A. Venkatramani, S. Mukherjee, A. Kumari and D. Panda, Shikonin impedes phase separation and aggregation of tau and protects SH-SY5Y cells from the toxic effects of tau oligomers, Int. J. Biol. Macromol., 2022, 204, 19–33 CrossRef CAS PubMed
.
- M. Gonzalez-Garcia, G. Fusco and A. De Simone, Membrane interactions and toxicity by misfolded protein oligomers, Front. Cell Dev. Biol., 2021, 9, 642623 CrossRef PubMed
.
- L. N. Zhao, H. W. Long, Y. Mu and L. Y. Chew, The Toxicity of Amyloid β Oligomers, Int. J. Mol. Sci., 2012, 13(6), 7303–7327 CrossRef CAS PubMed
.
- X. Gui, S. Feng, Z. Li, Y. Li, B. Reif and B. Shi,
et al., Liquid–liquid phase separation of amyloid-β oligomers modulates amyloid fibrils formation, J. Biol.
Chem., 2023, 299(3), 102926 CrossRef CAS PubMed
.
- M. A. Kostylev, M. D. Tuttle, S. Lee, L. E. Klein, H. Takahashi and T. O. Cox,
et al., Liquid and Hydrogel Phases of PrP(C) Linked to Conformation Shifts and Triggered by Alzheimer's Amyloid-β Oligomers, Mol. Cell, 2018, 72(3), 426–443.e12 CrossRef CAS PubMed
.
- H.-N. Liu, T. Wang, J.-J. Hu, L. Chen, X. Shi and Y.-M. Li,
et al., The disordered protein SERF promotes α-Synuclein aggregation through liquid–liquid phase separation, J. Biol. Chem., 2024, 300(3) DOI:10.1016/j.jbc.2024.105667
.
- L. C. Koehler, Z. R. Grese, A. Bastos, L. D. Mamede, T. Heyduk and Y. M. Ayala, TDP-43 oligomerization and phase separation properties are necessary for autoregulation, Front. Neurosci., 2022, 16, 818655 CrossRef PubMed
.
- R. L. French, Z. R. Grese, H. Aligireddy, D. D. Dhavale, A. N. Reeb and N. Kedia,
et al., Detection of TAR DNA-binding protein 43 (TDP-43) oligomers as initial intermediate species during aggregate formation, J. Biol. Chem., 2019, 294(17), 6696–6709 CrossRef CAS PubMed
.
- M. Fändrich, S. Nyström, K. P. R. Nilsson, A. Böckmann, H. LeVine III and P. Hammarström, Amyloid fibril polymorphism: a challenge for molecular imaging and therapy, J. Int. Med., 2018, 283(3), 218–237 CrossRef PubMed
.
- W. Close, M. Neumann, A. Schmidt, M. Hora, K. Annamalai and M. Schmidt,
et al., Physical basis of amyloid fibril polymorphism, Nat. Commun., 2018, 9(1), 699 CrossRef PubMed
.
- R. Kodali and R. Wetzel, Polymorphism in the intermediates and products of amyloid assembly, Curr. Opin. Struct. Biol., 2007, 17(1), 48–57 CrossRef CAS PubMed
.
- S. Mehra, S. Ahlawat, H. Kumar, D. Datta, A. Navalkar and N. Singh,
et al., α-Synuclein Aggregation Intermediates form Fibril Polymorphs with Distinct Prion-like Properties, J. Mol. Biol., 2022, 434(19), 167761 CrossRef CAS PubMed
.
- M. Fändrich, J. Meinhardt and N. Grigorieff, Structural polymorphism of Alzheimer Aβ and other amyloid fibrils, Prion., 2009, 3(2), 89–93 CrossRef PubMed
.
- M. Stefani, Structural polymorphism of amyloid oligomers and fibrils underlies different fibrillization pathways: immunogenicity and cytotoxicity, Curr. Protein Pept. Sci., 2010, 11(5), 343–354 CrossRef CAS PubMed
.
- J. D. Camino, P. Gracia, S. W. Chen, J. Sot, I. de la Arada and V. Sebastián,
et al., The extent of protein hydration dictates the preference for heterogeneous or homogeneous nucleation generating either parallel or antiparallel β-sheet α-synuclein aggregates, Chem. Sci., 2020, 11(43), 11902–11914 RSC
.
- M. S. Celej, R. Sarroukh, E. Goormaghtigh, G. D. Fidelio, J. M. Ruysschaert and V. Raussens, Toxic prefibrillar α-synuclein amyloid oligomers adopt a distinctive antiparallel β-sheet structure, Biochem. J., 2012, 443(3), 719–726 CrossRef CAS PubMed
.
- E. Cerf, R. Sarroukh, S. Tamamizu-Kato, L. Breydo, S. Derclaye and Y. F. Dufrêne,
et al., Antiparallel beta-sheet: a signature structure of the oligomeric amyloid beta-peptide, Biochem. J., 2009, 421(3), 415–423 CrossRef CAS PubMed
.
- S. W. Chen, S. Drakulic, E. Deas, M. Ouberai, F. A. Aprile and R. Arranz,
et al., Structural characterization of toxic oligomers that are kinetically trapped during α-synuclein fibril formation, Proc. Natl. Acad. Sci. U. S. A., 2015, 112(16), E1994–E2003 CrossRef CAS PubMed
.
- M. Kollmer, W. Close, L. Funk, J. Rasmussen, A. Bsoul and A. Schierhorn,
et al., Cryo-EM structure and polymorphism of Aβ amyloid fibrils purified from Alzheimer's brain tissue, Nat. Commun., 2019, 10(1), 4760 CrossRef PubMed
.
- M. Schweighauser, Y. Shi, A. Tarutani, F. Kametani, A. G. Murzin and B. Ghetti,
et al., Structures of α-synuclein filaments from multiple system atrophy, Nature, 2020, 585(7825), 464–469 CrossRef CAS PubMed
.
- A. W. Fitzpatrick, B. Falcon, S. He, A. G. Murzin, G. Murshudov and H. J. Garringer,
et al., Cryo-EM structures of tau filaments from Alzheimer's disease, Nature, 2017, 547(7662), 185–190 CrossRef CAS PubMed
.
- S. T. Dada, M. C. Hardenberg, Z. Toprakcioglu, L. K. Mrugalla, M. P. Cali and M. O. McKeon,
et al., Spontaneous nucleation and fast aggregate-dependent proliferation of α-synuclein aggregates within liquid condensates at neutral pH, Proc. Natl. Acad. Sci. U. S. A., 2023, 120(9), e2208792120 CrossRef CAS PubMed
.
- L. Piroska, A. Fenyi, S. Thomas, M. A. Plamont, V. Redeker and R. Melki,
et al., α-Synuclein liquid condensates fuel fibrillar α-synuclein growth, Sci. Adv., 2023, 9(33), eadg5663 CrossRef CAS PubMed
.
- X. Ding, F. Sun, J. Chen, L. Chen, Y. Tobin-Miyaji and S. Xue,
et al., Amyloid-Forming Segment Induces Aggregation of FUS-LC Domain from Phase Separation Modulated by Site-Specific Phosphorylation, J. Mol. Biol., 2020, 432(2), 467–483 CrossRef CAS PubMed
.
- S. O. Shuster and J. C. Lee, Watching liquid droplets of TDP-43CTD age by Raman spectroscopy, J. Biol. Chem., 2022, 298(2), 101528 CrossRef CAS PubMed
.
- A. Garaizar, J. R. Espinosa, J. A. Joseph, G. Krainer, Y. Shen and T. P. Knowles,
et al., Aging can transform single-component protein condensates into multiphase architectures, Proc. Natl. Acad. Sci. U. S. A., 2022, 119(26), e2119800119 CrossRef CAS PubMed
.
- W. P. Lipiński, B. S. Visser, I. Robu, M. A. Fakhree, S. Lindhoud and M. M. Claessens,
et al., Biomolecular condensates can both accelerate and suppress aggregation of α-synuclein, Sci. Adv., 2022, 8(48), eabq6495 CrossRef PubMed
.
- C. Weber, T. Michaels and L. Mahadevan, Spatial control of irreversible protein aggregation, eLife., 2019, 8, e42315 CrossRef PubMed
.
- M. P. Mattson and T. Magnus, Ageing and neuronal vulnerability, Nat. Rev. Neurosci., 2006, 7(4), 278–294 CrossRef CAS PubMed
.
- Y. Hayashi, L. K. Ford, L. Fioriti, L. McGurk and M. Zhang, Liquid–liquid phase separation in physiology and pathophysiology of the nervous system, J. Neurosci., 2021, 41(5), 834–844 CrossRef CAS PubMed
.
- R. C. Taylor and A. Dillin, Aging as an event of proteostasis collapse, Cold Spring Harbor Perspect. Biol., 2011, 3(5), a004440 Search PubMed
.
- S. Kaushik and A. M. Cuervo, Proteostasis and aging, Nat. Med., 2015, 21(12), 1406–1415 CrossRef CAS PubMed
.
- C. L. Klaips, G. G. Jayaraj and F. U. Hartl, Pathways of cellular proteostasis in aging and disease, J. Cell Biol., 2018, 217(1), 51–63 CrossRef CAS PubMed
.
- A. S. Anisimova, A. I. Alexandrov, N. E. Makarova, V. N. Gladyshev and S. E. Dmitriev, Protein synthesis and quality control in aging, Aging, 2018, 10(12), 4269 CrossRef CAS PubMed
.
- M. Ganassi, D. Mateju, I. Bigi, L. Mediani, I. Poser and H. O. Lee,
et al., A Surveillance Function of the HSPB8-BAG3-HSP70 Chaperone Complex Ensures Stress Granule Integrity and Dynamism, Mol. Cell, 2016, 63(5), 796–810 CrossRef CAS PubMed
.
- A. Patel, L. Malinovska, S. Saha, J. Wang, S. Alberti and Y. Krishnan,
et al., ATP as a biological hydrotrope, Science., 2017, 356(6339), 753–756 CrossRef CAS PubMed
.
- E. A. Newman, Glial cell inhibition of neurons by release of ATP, J. Neurosci., 2003, 23(5), 1659–1666 CrossRef CAS PubMed
.
- A. M. Rice and M. K. Rosen, ATP controls the crowd, Science., 2017, 356(6339), 701–702 CrossRef CAS PubMed
.
- S. Alberti and A. A. Hyman, Are aberrant phase transitions a driver of cellular aging?, BioEssays., 2016, 38(10), 959–968 CrossRef CAS PubMed
.
- B. R. Levone, S. C. Lenzken, M. Antonaci, A. Maiser, A. Rapp and F. Conte,
et al., FUS-dependent liquid–liquid phase separation is important for DNA repair initiation, J. Cell Biol., 2021, 220(5), e202008030 CrossRef CAS PubMed
.
- M. Naumann, A. Pal, A. Goswami, X. Lojewski, J. Japtok and A. Vehlow,
et al., Impaired DNA damage response signaling by FUS-NLS mutations leads to neurodegeneration and FUS aggregate formation, Nat. Commun., 2018, 9(1), 335 CrossRef PubMed
.
- T. Murakami, S. Qamar, J. Q. Lin, G. S. K. Schierle, E. Rees and A. Miyashita,
et al., ALS/FTD Mutation-Induced Phase Transition of FUS Liquid Droplets and Reversible Hydrogels into Irreversible Hydrogels Impairs RNP Granule Function, Neuron., 2015, 88(4), 678–690 CrossRef CAS PubMed
.
- K. A. Burke, A. M. Janke, C. L. Rhine and N. L. Fawzi, Residue-by-residue view of in vitro FUS granules that bind the C-terminal domain of RNA polymerase II, Mol. Cell, 2015, 60(2), 231–241 CrossRef CAS PubMed
.
- A. Savastano, D. Flores, H. Kadavath, J. Biernat, E. Mandelkow and M. Zweckstetter, Disease-associated tau phosphorylation hinders tubulin assembly within tau condensates, Angew. Chem., Int. Ed., 2021, 60(2), 726–730 CrossRef CAS PubMed
.
- D. Ghosh, S. Mehra, S. Sahay, P. K. Singh and S. K. Maji, α-synuclein aggregation and its modulation, Int. J. Biol. Macromol., 2017, 100, 37–54 CrossRef CAS PubMed
.
- L. Migliore and F. Coppedè, Genetics, environmental factors and the emerging role of epigenetics in neurodegenerative diseases, Mutat. Res., 2009, 667(1–2), 82–97 CrossRef CAS PubMed
.
- S. Vucic and M. C. Kiernan, Pathophysiology of neurodegeneration in familial amyotrophic lateral sclerosis, Curr. Mol. Med., 2009, 9(3), 255–272 CrossRef CAS PubMed
.
- S. Sahay, D. Ghosh, K. Singh P and K. Maji S., Alteration of structure and aggregation of α-synuclein by familial Parkinson's disease associated mutations, Curr. Protein Pept. Sci., 2017, 18(7), 656–676 CrossRef CAS PubMed
.
- K. Sharma, S. Mehra, A. S. Sawner, P. S. Markam, R. Panigrahi and A. Navalkar,
et al., Effect of Disease-Associated P123H and V70M Mutations on β-Synuclein Fibrillation, ACS Chem. Neurosci., 2020, 11(18), 2836–2848 CrossRef CAS PubMed
.
- Z. A. Sorrentino and B. I. Giasson, The emerging role of α-synuclein truncation in aggregation and disease, J. Biol. Chem., 2020, 295(30), 10224–10244 CrossRef CAS PubMed
.
- A. Didonna and F. Benetti, Post-translational modifications in neurodegeneration, Aims Biophys., 2016, 3(1), 27–49 CAS
.
- S. S. Leal, H. M. Botelho and C. M. Gomes, Metal ions as modulators of protein conformation and misfolding in neurodegeneration, Coord. Chem. Rev., 2012, 256(19–20), 2253–2270 CrossRef CAS
.
- Y. Su and P.-T. Chang, Acidic pH promotes the formation of toxic fibrils from β-amyloid peptide, Brain Res., 2001, 893(1–2), 287–291 CrossRef CAS PubMed
.
- K. J. Barnham, C. L. Masters and A. I. Bush, Neurodegenerative diseases and oxidative stress, Nat. Rev. Drug Discovery, 2004, 3(3), 205–214 CrossRef CAS PubMed
.
- F. Sánchez-Santed, M. T. Colomina and E. H. Hernández, Organophosphate pesticide exposure and neurodegeneration, Cortex., 2016, 74, 417–426 CrossRef PubMed
.
- K. A. Burke, E. A. Yates and J. Legleiter, Biophysical
insights into how surfaces, including lipid membranes, modulate protein aggregation related to neurodegeneration, Front. Neurol., 2013, 4, 17 CAS
.
- J. Nahalka, The role of the protein–RNA recognition code in neurodegeneration, Cell. Mol. Life Sci., 2019, 76(11), 2043–2058 CrossRef CAS PubMed
.
- M. Hofweber and D. Dormann, Friend or foe—Post-translational modifications as regulators of phase separation and RNP granule dynamics, J. Biol. Chem., 2019, 294(18), 7137–7150 CrossRef CAS PubMed
.
- G. G. Moreira and C. M. Gomes, Tau liquid–liquid phase separation is modulated by the Ca2+-switched chaperone activity of the S100B protein, J. Neurochem., 2023, 166(1), 76–86 CrossRef CAS PubMed
.
- R. Cacace, K. Sleegers and C. Van Broeckhoven, Molecular genetics of early-onset Alzheimer's disease revisited, Alzheimer's Dementia, 2016, 12(6), 733–748 CrossRef PubMed
.
- L. Wu, P. Rosa-Neto, G.-Y. R. Hsiung, A. D. Sadovnick, M. Masellis and S. E. Black,
et al., Early-onset familial Alzheimer's disease (EOFAD), Can. J. Neurol. Sci., 2012, 39(4), 436–445 CrossRef PubMed
.
- G. M. Mohite, A. Navalkar, R. Kumar, S. Mehra, S. Das and L. G. Gadhe,
et al., The Familial α-Synuclein A53E Mutation Enhances Cell Death in Response to Environmental Toxins Due to a Larger Population of Oligomers, Biochemistry., 2018, 57(33), 5014–5028 CrossRef CAS PubMed
.
- A. Kapasi, J. R. Brosch, K. N. Nudelman, S. Agrawal, T. M. Foroud and J. A. Schneider, A novel SNCA E83Q mutation in a case of dementia with Lewy bodies and atypical frontotemporal lobar degeneration, Neuropathology, 2020, 40(6), 620–626 CrossRef CAS PubMed
.
- G. M. Mohite, S. Dwivedi, S. Das, R. Kumar, S. Paluri and S. Mehra,
et al., Parkinson's Disease Associated α-Synuclein Familial Mutants Promote Dopaminergic Neuronal Death in Drosophila melanogaster, ACS Chem. Neurosci., 2018, 9(11), 2628–2638 CrossRef CAS PubMed
.
- G. M. Mohite, R. Kumar, R. Panigrahi, A. Navalkar, N. Singh and D. Datta,
et al., Comparison of Kinetics, Toxicity, Oligomer Formation, and Membrane Binding Capacity of α-Synuclein Familial Mutations at the A53 Site, Including the Newly Discovered A53V Mutation, Biochemistry., 2018, 57(35), 5183–5187 CrossRef CAS PubMed
.
- N. Ticozzi, A. L. LeClerc, M. Van Blitterswijk, P. Keagle, D. M. McKenna-Yasek and P. C. Sapp,
et al., Mutational analysis of TARDBP in neurodegenerative diseases, Neurobiol. Aging, 2011, 32(11), 2096–2099 CrossRef CAS PubMed
.
- J.-C. García and R.-H. Bustos, The genetic diagnosis of neurodegenerative diseases and therapeutic perspectives, Brain Sci., 2018, 8(12), 222 CrossRef PubMed
.
- S. Boyko, K. Surewicz and W. K. Surewicz, Regulatory mechanisms of tau protein fibrillation under the conditions of liquid–liquid phase separation, Proc. Natl. Acad. Sci. U. S. A., 2020, 117(50), 31882–31890 CrossRef CAS PubMed
.
- Y. Lin, D. S. Protter, M. K. Rosen and R. Parker, Formation and maturation of phase-separated liquid droplets by RNA-binding proteins, Mol. Cell, 2015, 60(2), 208–219 CrossRef CAS PubMed
.
- B. Xu, F. Fan, Y. Liu, Y. Liu, L. Zhou and H. Yu, Distinct Effects of Familial Parkinson's Disease-Associated Mutations on α-Synuclein Phase Separation and Amyloid Aggregation, Biomolecules., 2023, 13(5), 726 CrossRef CAS PubMed
.
- R. M. Vernon, P. A. Chong, B. Tsang, T. H. Kim, A. Bah and P. Farber,
et al., Pi-Pi contacts are an overlooked protein feature relevant to phase separation, eLife., 2018, 7, e31486 CrossRef PubMed
.
- H.-R. Li, W.-C. Chiang, P.-C. Chou, W.-J. Wang and J.-R. Huang, TAR DNA-binding protein 43 (TDP-43) liquid–liquid phase separation is mediated by just a few aromatic residues, J. Biol. Chem., 2018, 293(16), 6090–6098 CrossRef CAS PubMed
.
- M. P. Hughes, M. R. Sawaya, D. R. Boyer, L. Goldschmidt, J. A. Rodriguez and D. Cascio,
et al., Atomic structures of low-complexity protein segments reveal kinked β sheets that assemble networks, Science., 2018, 359(6376), 698–701 CrossRef CAS PubMed
.
- E. L. Guenther, Q. Cao, H. Trinh, J. Lu, M. R. Sawaya and D. Cascio,
et al., Atomic structures of TDP-43 LCD segments and insights into reversible or pathogenic aggregation, Nat. Struct. Mol. Biol., 2018, 25(6), 463–471 CrossRef CAS PubMed
.
- P. P. Gopal, J. J. Nirschl, E. Klinman and E. L. Holzbaur, Amyotrophic lateral sclerosis-linked mutations increase the viscosity of liquid-like TDP-43 RNP granules in neurons, Proc. Natl. Acad. Sci. U. S. A., 2017, 114(12), E2466-e75 CrossRef PubMed
.
- N. H. Alami, R. B. Smith, M. A. Carrasco, L. A. Williams, C. S. Winborn and S. S. W. Han,
et al., Axonal transport of TDP-43 mRNA granules is impaired by ALS-causing mutations, Neuron., 2014, 81(3), 536–543 CrossRef CAS PubMed
.
- A. E. Conicella, G. H. Zerze, J. Mittal and N. L. Fawzi, ALS Mutations Disrupt Phase Separation Mediated by α-Helical Structure in the TDP-43 Low-Complexity C-Terminal Domain, Structure, 2016, 24(9), 1537–1549 CrossRef CAS PubMed
.
- N. Li and C. Lagier-Tourenne, Nuclear pores: the gate to neurodegeneration, Nat. Neurosci., 2018, 21(2), 156–158 CrossRef CAS PubMed
.
- W. Guo, Y. Chen, X. Zhou, A. Kar, P. Ray and X. Chen,
et al., An ALS-associated mutation affecting TDP-43 enhances protein aggregation, fibril formation and neurotoxicity, Nat. Struct. Mol. Biol., 2011, 18(7), 822–830 CrossRef CAS PubMed
.
- S. Ju, D. F. Tardiff, H. Han, K. Divya, Q. Zhong and L. E. Maquat,
et al., A yeast model of FUS/TLS-dependent cytotoxicity, PLoS Biol., 2011, 9(4), e1001052 CrossRef CAS PubMed
.
- D. Dormann, R. Rodde, D. Edbauer, E. Bentmann, I. Fischer and A. Hruscha,
et al., ALS-associated fused in sarcoma (FUS) mutations disrupt Transportin-mediated nuclear import. The, EMBO J., 2010, 29(16), 2841–2857 CrossRef CAS PubMed
.
- M. Hofweber, S. Hutten, B. Bourgeois, E. Spreitzer, A. Niedner-Boblenz and M. Schifferer,
et al., Phase separation of FUS is suppressed by its nuclear import receptor and arginine methylation, Cell., 2018, 173(3), 706–719.e13 CrossRef CAS PubMed
.
- F. Gasset-Rosa, S. Lu, H. Yu, C. Chen, Z. Melamed and L. Guo,
et al., Cytoplasmic TDP-43 de-mixing independent of stress granules drives inhibition of nuclear import, loss of nuclear TDP-43, and cell death, Neuron., 2019, 102(2), 339–357.e7 CrossRef CAS PubMed
.
- S. L. Crick, K. M. Ruff, K. Garai, C. Frieden and R. V. Pappu, Unmasking the roles of N-and C-terminal flanking sequences from exon 1 of huntingtin as modulators of polyglutamine aggregation, Proc. Natl. Acad. Sci. U. S. A., 2013, 110(50), 20075–20080 CrossRef CAS PubMed
.
- M. Kato, T. W. Han, S. Xie, K. Shi, X. Du and L. C. Wu,
et al., Cell-free formation of RNA granules: low complexity sequence domains form dynamic fibers within hydrogels, Cell., 2012, 149(4), 753–767 CrossRef CAS PubMed
.
- P. K. Todd and H. L. Paulson, RNA-mediated neurodegeneration in repeat expansion disorders, Ann. Neurol., 2010, 67(3), 291–300 CrossRef CAS PubMed
.
- S. Boeynaems, E. Bogaert, D. Kovacs, A. Konijnenberg, E. Timmerman and A. Volkov,
et al., Phase separation of C9orf72 dipeptide repeats perturbs stress granule dynamics, Mol. Cell, 2017, 65(6), 1044–1055.e5 CrossRef CAS PubMed
.
- T. M. Karve and A. K. Cheema, Small changes huge impact: the role of protein posttranslational modifications in cellular homeostasis and disease, J. Amino Acids, 2011, 2011, 207691 Search PubMed
.
- J.-W. Seo and K.-J. Lee, Post-translational modifications and their biological functions: proteomic analysis and systematic approaches, BMB Rep., 2004, 37(1), 35–44 CrossRef CAS PubMed
.
- L.-N. Schaffert and W. G. Carter, Do post-translational modifications influence protein aggregation in neurodegenerative diseases: a systematic review, Brain Sci., 2020, 10(4), 232 CrossRef CAS PubMed
.
- R.-J. Ren, E. B. Dammer, G. Wang, N. T. Seyfried and A. I. Levey, Proteomics of protein post-translational modifications implicated in neurodegeneration, Transl. Neurodegener., 2014, 3(1), 1–13 CrossRef PubMed
.
- Y. Wang and E. Mandelkow, Tau in physiology and pathology, Nat. Rev. Neurosci., 2016, 17(1), 22–35 CrossRef CAS PubMed
.
- T. Arendt, J. Stieler and M. Holzer, Brain hypometabolism triggers PHF-like phosphorylation of tau, a major hallmark of Alzheimer's disease pathology, J. Neural Transm., 2015, 122, 531–539 CrossRef CAS PubMed
.
- J. Kosten, A. Binolfi, M. Stuiver, S. Verzini, F.-X. Theillet and B. Bekei,
et al., Efficient modification of alpha-synuclein serine 129 by protein kinase CK1 requires phosphorylation of tyrosine 125 as a priming event, ACS Chem. Neurosci., 2014, 5(12), 1203–1208 CrossRef CAS PubMed
.
- J. P. Anderson, D. E. Walker, J. M. Goldstein, R. de Laat, K. Banducci and R. J. Caccavello,
et al., Phosphorylation of Ser-129 Is the Dominant Pathological Modification of α-Synuclein in Familial and Sporadic Lewy Body Disease, J. Biol. Chem., 2006, 281(40), 29739–29752 CrossRef CAS PubMed
.
- H. Fujiwara, M. Hasegawa, N. Dohmae, A. Kawashima, E. Masliah and M. S. Goldberg,
et al., alpha-Synuclein is phosphorylated in synucleinopathy lesions, Nat. Cell Biol., 2002, 4(2), 160–164 CrossRef CAS PubMed
.
- L. Piroska, A. Fenyi, S. Thomas, M.-A. Plamont, V. Redeker and R. Melki,
et al., α-Synuclein liquid condensates fuel fibrillar α-synuclein growth, Sci. Adv., 2023, 9(33), eadg5663 CrossRef CAS PubMed
.
- Z. Monahan, V. H. Ryan, A. M. Janke, K. A. Burke, S. N. Rhoads and G. H. Zerze,
et al., Phosphorylation of the FUS low-complexity domain disrupts phase separation, aggregation, and toxicity, EMBO J., 2017, 36(20), 2951–2967 CrossRef CAS PubMed
.
- M. Evich, E. Stroeva, Y. G. Zheng and M. W. Germann, Effect of methylation on the side-chain pKa value of arginine, Protein Sci., 2016, 25(2), 479–486 CrossRef CAS PubMed
.
- P. Thandapani, T. R. O’Connor, L. Bailey Timothy and S. Richard, Defining the RGG/RG Motif, Mol. Cell, 2013, 50(5), 613–623 CrossRef CAS PubMed
.
- V. H. Ryan, G. L. Dignon, G. H. Zerze, C. V. Chabata, R. Silva and A. E. Conicella,
et al., Mechanistic View of hnRNPA2 Low-Complexity Domain Structure, Interactions, and Phase Separation Altered by Mutation and Arginine Methylation, Mol. Cell, 2018, 69(3), 465–79.e7 CrossRef CAS PubMed
.
- S. Qamar, G. Wang, S. J. Randle, F. S. Ruggeri, J. A. Varela and J. Q. Lin,
et al., FUS Phase Separation Is Modulated by a Molecular Chaperone and Methylation of Arginine cation–π Interactions, Cell., 2018, 173(3), 720–734.e15 CrossRef CAS PubMed
.
- S. Engelender, Ubiquitination of α-synuclein and autophagy in Parkinson's disease, Autophagy., 2008, 4(3), 372–374 CrossRef CAS PubMed
.
- F. Parolini, R. Tira, C. G. Barracchia, F. Munari, S. Capaldi and M. D'Onofrio,
et al., Ubiquitination of Alzheimer's-related tau protein affects liquid–liquid phase separation in a site- and cofactor-dependent manner, Int. J. Biol. Macromol., 2022, 201, 173–181 CrossRef CAS PubMed
.
- S. Deng, B. Pan, L. Gottlieb, E. J. Petersson and R. Marmorstein, Molecular basis for N-terminal alpha-synuclein acetylation by human NatB, eLife., 2020, 9, e57491 CrossRef CAS PubMed
.
- J. C. Ferreon, A. Jain, K.-J. Choi, P. S. Tsoi, K. R. MacKenzie and S. Y. Jung,
et al., Acetylation disfavors tau phase separation, Int. J. Mol. Sci., 2018, 19(5), 1360 CrossRef PubMed
.
- J. Garcia Morato, F. Hans, F. von Zweydorf, R. Feederle, S. J. Elsässer and A. A. Skodras,
et al., Sirtuin-1 sensitive lysine-136 acetylation drives phase separation and pathological aggregation of TDP-43, Nat. Commun., 2022, 13(1), 1223 CrossRef CAS PubMed
.
- B. Morel, L. Varela, A. I. Azuaga and F. Conejero-Lara, Environmental conditions affect the kinetics of nucleation of amyloid fibrils and determine their morphology, Biophys. J., 2010, 99(11), 3801–3810 CrossRef CAS PubMed
.
- C. Ha, J. Ryu and C. B. Park, Metal ions differentially influence the aggregation and deposition of Alzheimer's β-amyloid on a solid template, Biochemistry., 2007, 46(20), 6118–6125 CrossRef CAS PubMed
.
- T. Antony, W. Hoyer, D. Cherny, G. Heim, T. M. Jovin and V. Subramaniam, Cellular polyamines promote the aggregation of α-synuclein, J. Biol. Chem., 2003, 278(5), 3235–3240 CrossRef CAS PubMed
.
- S. Jha, J. M. Snell, S. R. Sheftic, S. M. Patil, S. B. Daniels and F. W. Kolling,
et al., pH dependence of amylin fibrillization, Biochemistry., 2014, 53(2), 300–310 CrossRef CAS PubMed
.
- J. A. Cohlberg, J. Li, V. N. Uversky and A. L. Fink, Heparin and other glycosaminoglycans stimulate the formation of amyloid fibrils from α-synuclein in vitro, Biochemistry., 2002, 41(5), 1502–1511 CrossRef CAS PubMed
.
- K. Kieburtz and K. B. Wunderle, Parkinson's disease: evidence for environmental risk factors, Movement Disorders, 2013, 28(1), 8–13 CrossRef CAS PubMed
.
- A. Delamarre and W. G. Meissner, Epidemiology, environmental risk factors and genetics of Parkinson's disease, La Presse Médicale, 2017, 46(2), 175–181 CrossRef PubMed
.
- M. N. Wainaina, Z. Chen and C. Zhong, Environmental factors in the development and progression of late-onset Alzheimer's disease, Neurosci. Bull., 2014, 30, 253–270 CrossRef CAS PubMed
.
- M. Chin-Chan, J. Navarro-Yepes and B. Quintanilla-Vega, Environmental pollutants as risk factors for neurodegenerative disorders: Alzheimer and Parkinson diseases, Front. Cell. Neurosci., 2015, 9, 124 Search PubMed
.
- P. R. Prince, J. Hochmair, H. Brognaro, S. Gevorgyan, M. Franck and R. Schubert,
et al., Initiation and modulation of Tau protein phase separation by the drug suramin, Sci. Rep., 2023, 13(1), 3963 CrossRef CAS PubMed
.
- S. Huang, B. Xu and Y. Liu, Calcium promotes α-synuclein liquid–liquid phase separation to accelerate amyloid aggregation, Biochem. Biophys. Res. Commun., 2022, 603, 13–20 CrossRef CAS PubMed
.
- B. Xu, S. Huang, Y. Liu, C. Wan, Y. Gu and D. Wang,
et al., Manganese promotes α-synuclein amyloid aggregation through the induction of protein phase transition, J. Biol. Chem., 2022, 298(1), 101469 CrossRef CAS PubMed
.
- Y.-Y. Gao, T. Zhong, L.-Q. Wang, N. Zhang, Y. Zeng and J.-Y. Hu,
et al., Zinc enhances liquid–liquid phase separation of Tau protein and aggravates mitochondrial damages in cells, Int. J. Biol. Macromol., 2022, 209, 703–715 CrossRef CAS PubMed
.
- M. J. Do Amaral, S. Mohapatra, A. R. Passos, T. S. Lopes da Silva, R. S. Carvalho and M. da Silva Almeida,
et al., Copper drives prion protein phase separation and modulates aggregation, Sci. Adv., 2023, 9(44), eadi7347 CrossRef CAS PubMed
.
- D. Ubbiali, M. Fratini, L. Piersimoni, C. H. Ihling, M. Kipping and I. Heilmann, et al., First direct observation of ‘elongated’ conformational states in α-synuclein upon liquid–liquid phase separation, bioRxiv, 2022 DOI:10.1101/2022.04.20.488894.
- S. K. Rai, A. Savastano, P. Singh, S. Mukhopadhyay and M. Zweckstetter, Liquid–liquid phase separation of tau: from molecular biophysics to physiology and disease, Protein Sci., 2021, 30(7), 1294–1314 CrossRef CAS PubMed
.
- Y. Lin, Y. Fichou, A. P. Longhini, L. C. Llanes, P. Yin and G. C. Bazan,
et al., Liquid–Liquid Phase Separation of Tau Driven by Hydrophobic Interaction Facilitates Fibrillization of Tau, J. Mol. Biol., 2021, 433(2), 166731 CrossRef CAS PubMed
.
- C. Iannuzzi, G. Irace and I. Sirangelo, The effect of glycosaminoglycans (GAGs) on amyloid aggregation and toxicity, Molecules, 2015, 20(2), 2510–2528 CrossRef PubMed
.
- D. Papy-Garcia, M. Christophe, H. Minh Bao, S. Fernando, S. Ludmilla and S. Diaz Julia Elisa,
et al., Glycosaminoglycans, protein aggregation and neurodegeneration, Curr. Protein Pept. Sci., 2011, 12(3), 258–268 CrossRef CAS PubMed
.
- N. Quittot, M. Sebastiao and S. Bourgault, Modulation of amyloid assembly by glycosaminoglycans: from mechanism to biological significance, Biochem. Cell Biol., 2017, 95(3), 329–337 CrossRef CAS PubMed
.
- J. McLaurin, T. Franklin, X. Zhang, J. Deng and P. E. Fraser, Interactions of Alzheimer amyloid-β peptides with glycosaminoglycans: effects on fibril nucleation and growth, Eur. J. Biochem., 1999, 266(3), 1101–1110 CrossRef CAS PubMed
.
- S. Mehra, D. Ghosh, R. Kumar, M. Mondal, L. G. Gadhe and S. Das,
et al., Glycosaminoglycans have variable effects on α-synuclein aggregation and differentially affect the activities of the resulting amyloid fibrils, J. Biol. Chem., 2018, 293(34), 12975–12991 CrossRef CAS PubMed
.
- W. Zhang, B. Falcon, A. G. Murzin, J. Fan, R. A. Crowther and M. Goedert,
et al., Heparin-induced tau filaments are polymorphic and differ from those in Alzheimer's and Pick's diseases, eLife., 2019, 8, e43584 CrossRef PubMed
.
- M. Goedert, R. Jakes, M. Spillantini, M. Hasegawa, M. Smith and R. Crowther, Assembly of microtubule-associated protein tau into Alzheimer-like filaments induced by sulphated glycosaminoglycans, Nature, 1996, 383(6600), 550–553 CrossRef CAS PubMed
.
- Y. Lin, Y. Fichou, Z. Zeng, N. Y. Hu and S. Han, Electrostatically driven complex coacervation and amyloid aggregation of tau are independent processes with overlapping conditions, ACS Chem. Neurosci., 2020, 11(4), 615–627 CrossRef CAS PubMed
.
- M. M. Fay and P. J. Anderson, The role of RNA in biological phase separations, J. Mol. Biol., 2018, 430(23), 4685–4701 CrossRef CAS PubMed
.
- R. Onoguchi-Mizutani and N. Akimitsu, Long noncoding RNA and phase separation in cellular stress response, J. Biochem., 2022, 171(3), 269–276 CrossRef CAS PubMed
.
- A. G. Niaki, J. Sarkar, X. Cai, K. Rhine, V. Vidaurre and B. Guy,
et al., Loss of dynamic RNA interaction and aberrant phase separation induced by two distinct types of ALS/FTD-linked FUS mutations, Mol. Cell, 2020, 77(1), 82–94.e4 CrossRef CAS PubMed
.
- S. Maharana, J. Wang, D. K. Papadopoulos, D. Richter, A. Pozniakovsky and I. Poser,
et al., RNA buffers the phase separation behavior of prion-like RNA binding proteins, Science., 2018, 360(6391), 918–921 CrossRef CAS PubMed
.
- A. R. Tejedor, A. Garaizar, J. Ramírez and J. R. Espinosa, ‘RNA modulation of transport properties and stability in phase-separated condensates, Biophys. J., 2021, 120(23), 5169–5186 CrossRef CAS PubMed
.
- H. Zhang, S. Elbaum-Garfinkle, E. M. Langdon, N. Taylor, P. Occhipinti and A. Bridges Andrew,
et al., RNA Controls PolyQ Protein Phase Transitions, Mol. Cell, 2015, 60(2), 220–230 CrossRef CAS PubMed
.
- D. W. Sanders, N. Kedersha, D. S. Lee, A. R. Strom, V. Drake and J. A. Riback,
et al., Competing protein–RNA interaction networks control multiphase intracellular organization, Cell., 2020, 181(2), 306–324.e28 CrossRef CAS PubMed
.
- J. Kang, L. Lim and J. Song, ATP enhances at low concentrations but dissolves at high concentrations liquid–liquid phase separation (LLPS) of ALS/FTD-causing FUS, Biochem. Biophys. Res. Commun., 2018, 504(2), 545–551 CrossRef CAS PubMed
.
- M. Dang and J. Song, ALS-causing D169G mutation disrupts the ATP-binding capacity of TDP-43 RRM1 domain, Biochem. Biophys. Res. Commun., 2020, 524(2), 459–464 CrossRef CAS PubMed
.
- L. C. Rodríguez, N. N. Foressi and M. S. Celej, Modulation of α-synuclein phase separation by biomolecules, Biochim. Biophys. Acta, Proteins Proteomics, 2023, 1871(2), 140885 CrossRef PubMed
.
- T. L. Spires-Jones, J. Attems and D. R. Thal, Interactions of pathological proteins in neurodegenerative diseases, Acta Neuropathol., 2017, 134, 187–205 CrossRef CAS PubMed
.
- K. Murakami and K. Ono, Interactions of amyloid coaggregates with biomolecules and its relevance to neurodegeneration, FASEB J., 2022, 36(9), e22493 CrossRef CAS PubMed
.
- D. J. Irwin, V. M.-Y. Lee and J. Q. Trojanowski, Parkinson's disease dementia: convergence of α-synuclein, tau and amyloid-β pathologies, Nat. Rev. Neurosci., 2013, 14(9), 626–636 CrossRef CAS PubMed
.
- R. L. Nussbaum and C. E. Ellis, Alzheimer's disease and Parkinson's disease, N. Engl. J. Med., 2003, 348(14), 1356–1364 CrossRef CAS PubMed
.
- S. Dhakal, C. E. Wyant, H. E. George, S. E. Morgan and V. Rangachari, Prion-like C-terminal domain of TDP-43 and α-Synuclein interact synergistically to generate neurotoxic hybrid fibrils, J. Mol. Biol., 2021, 433(10), 166953 CrossRef CAS PubMed
.
- Y.-I. Takei, K. Oguchi, H. Koshihara, A. Hineno, A. Nakamura and S. Ohara, α-Synuclein coaggregation in familial amyotrophic lateral sclerosis with SOD1 gene mutation, Hum. Pathol., 2013, 44(6), 1171–1176 CrossRef PubMed
.
- T. Kasai, T. Tokuda, R. Ishii, N. Ishigami, Y. Tsuboi and M. Nakagawa,
et al., Increased α-synuclein levels in the cerebrospinal fluid of patients with Creutzfeldt–Jakob disease, J. Neurol., 2014, 261, 1203–1209 CrossRef CAS PubMed
.
- C. Soto and S. Pritzkow, Protein misfolding, aggregation, and conformational strains in neurodegenerative diseases, Nat. Neurosci., 2018, 21(10), 1332–1340 CrossRef CAS PubMed
.
- S. K. Rai, R. Khanna, A. Avni and S. Mukhopadhyay, Heterotypic electrostatic interactions control complex phase separation of tau and prion into multiphasic condensates and co-aggregates, Proc. Natl. Acad. Sci. U. S. A., 2023, 120(2), e2216338120 CrossRef CAS PubMed
.
- A. Agarwal, L. Arora, S. K. Rai, A. Avni and S. Mukhopadhyay, Spatiotemporal modulations in heterotypic condensates of prion and α-synuclein control phase transitions and amyloid conversion, Nat. Commun., 2022, 13(1), 1154 CrossRef CAS PubMed
.
- A. Siegert, M. Rankovic, F. Favretto, T. Ukmar-Godec, T. Strohäker and S. Becker,
et al., Interplay between tau and α-synuclein liquid–liquid phase separation, Protein Sci., 2021, 30(7), 1326–1336 CrossRef CAS PubMed
.
- P. Gracia, D. Polanco, J. Tarancón-Díez, I. Serra, M. Bracci and J. Oroz,
et al., Molecular mechanism for the synchronized electrostatic coacervation and co-aggregation of alpha-synuclein and tau, Nat. Commun., 2022, 13(1), 4586 CrossRef CAS PubMed
.
- S. Dhakal, C. E. Wyant, H. E. George, S. E. Morgan and V. Rangachari, Prion-like C-Terminal Domain of TDP-43 and α-Synuclein Interact Synergistically to Generate Neurotoxic Hybrid Fibrils, J. Mol. Biol., 2021, 433(10), 166953 CrossRef CAS PubMed
.
- A. M. Küffner, M. Linsenmeier, F. Grigolato, M. Prodan, R. Zuccarini and U. C. Palmiero,
et al., Sequestration within biomolecular condensates inhibits Aβ-42 amyloid formation, Chem. Sci., 2021, 12(12), 4373–4382 RSC
.
- M. Fukuyama, S. Nishinami, Y. Maruyama, T. Ozawa, S. Tomita and Y. Ohhashi,
et al., Detection of Fibril Nucleation in Micrometer-Sized Protein Condensates and Suppression of Sup35NM Fibril Nucleation by Liquid–Liquid Phase Separation, Anal. Chem., 2023, 95(26), 9855–9862 CrossRef CAS PubMed
.
- J. D. Sipe and A. S. Cohen, History of the amyloid fibril, J. Struct. Biol., 2000, 130(2–3), 88–98 CrossRef CAS PubMed
.
- M. Stefani and C. M. Dobson, Protein aggregation and aggregate toxicity: new insights into protein folding, misfolding diseases and biological evolution, J. Mol. Med., 2003, 81, 678–699 CrossRef CAS PubMed
.
- C. M. Dobson, Protein folding and misfolding, Nature, 2003, 426(6968), 884–890 CrossRef CAS PubMed
.
- M. Hardenberg, A. Horvath, V. Ambrus, M. Fuxreiter and M. Vendruscolo, Widespread occurrence of the droplet state of proteins in the human proteome, Proc. Natl. Acad. Sci. U. S. A., 2020, 117(52), 33254–33262 CrossRef CAS PubMed
.
- M. Poudyal, K. Patel, L. Gadhe, A. S. Sawner, P. Kadu and D. Datta,
et al., Intermolecular interactions underlie protein/peptide phase separation irrespective of sequence and structure at crowded milieu, Nat. Commun., 2023, 14(1), 6199 CrossRef CAS PubMed
.
- M. Fuxreiter and M. Vendruscolo, Generic nature of the condensed states of proteins, Nat. Cell Biol., 2021, 23(6), 587–594 CrossRef CAS PubMed
.
- A. J. Baldwin, T. P. Knowles, G. G. Tartaglia, A. W. Fitzpatrick, G. L. Devlin and S. L. Shammas,
et al., Metastability of native proteins and the phenomenon of amyloid formation, J. Am. Chem. Soc., 2011, 133(36), 14160–14163 CrossRef CAS PubMed
.
- M. R. Sawaya, S. Sambashivan, R. Nelson, M. I. Ivanova, S. A. Sievers and M. I. Apostol,
et al., Atomic structures of amyloid cross-β spines reveal varied steric zippers, Nature, 2007, 447(7143), 453–457 CrossRef CAS PubMed
.
- D. M. Fowler, A. V. Koulov, W. E. Balch and J. W. Kelly, Functional amyloid–from bacteria to humans, Trends Biochem. Sci., 2007, 32(5), 217–224 CrossRef CAS PubMed
.
- C. L. Pham, A. H. Kwan and M. Sunde, Functional amyloid: widespread in Nature, diverse in purpose, Essays Biochem., 2014, 56, 207–219 CrossRef PubMed
.
- C. P. J. Maury, The emerging concept of functional amyloid, J. Internal Med., 2009, 265(3), 329–334 CrossRef CAS PubMed
.
- S. K. Maji, M. H. Perrin, M. R. Sawaya, S. Jessberger, K. Vadodaria and R. A. Rissman,
et al., Functional amyloids as natural storage of peptide hormones in pituitary secretory granules, Science., 2009, 325(5938), 328–332 CrossRef CAS PubMed
.
- X. Gui, F. Luo, Y. Li, H. Zhou, Z. Qin and Z. Liu,
et al., Structural basis for reversible amyloids of hnRNPA1 elucidates their role in stress granule assembly, Nat. Commun., 2019, 10(1), 2006 CrossRef PubMed
.
- S. Ramos, J. Kamps, S. Pezzotti, K. F. Winklhofer, J. Tatzelt and M. Havenith, Hydration makes a difference! How to tune protein complexes between liquid–liquid and liquid–solid phase separation, Phys. Chem. Chem. Phys., 2023, 28063–28069 RSC
.
- D. Maltseva, S. Chatterjee, C.-C. Yu, M. Brzezinski, Y. Nagata and G. Gonella,
et al., Fibril formation and ordering of disordered FUS LC driven by hydrophobic interactions, Nat. Chem., 2023, 15(8), 1146–1154 CrossRef CAS PubMed
.
- J. P. Connor, S. D. Quinn and C. Schaefer, Sticker-and-spacer model for amyloid
beta condensation and fibrillation, Front. Mol. Neurosci., 2022, 15, 962526 CrossRef CAS PubMed
.
- R. J. Wheeler, Therapeutics—how to treat phase separation-associated diseases, Emerging Top. Life Sci., 2020, 4(3), 331–342 CrossRef CAS PubMed
.
- D. Sun, R. Wu, P. Li and L. Yu, Phase separation in regulation of aggrephagy, J. Mol. Biol., 2020, 432(1), 160–169 CrossRef CAS PubMed
.
- T. Rogalla, M. Ehrnsperger, X. Preville, A. Kotlyarov, G. Lutsch and C. Ducasse,
et al., Regulation of Hsp27 oligomerization, chaperone function, and protective activity against oxidative stress/tumor necrosis factor α by phosphorylation, J. Biol. Chem., 1999, 274(27), 18947–18956 CrossRef CAS PubMed
.
- Z. Liu, S. Zhang, J. Gu, Y. Tong, Y. Li and X. Gui,
et al., Hsp27 chaperones FUS phase separation under the modulation of stress-induced phosphorylation, Nat. Struct. Mol. Biol., 2020, 27(4), 363–372 CrossRef CAS PubMed
.
- S. Lu, J. Hu, O. A. Arogundade, A. Goginashvili, S. Vazquez-Sanchez and J. K. Diedrich,
et al., Heat-shock chaperone HSPB1 regulates cytoplasmic TDP-43 phase separation and liquid-to-gel transition, Nat. Cell Biol., 2022, 24(9), 1378–1393 CrossRef CAS PubMed
.
- K. Wang, J. Q. Liu, T. Zhong, X. L. Liu, Y. Zeng and X. Qiao,
et al., Phase Separation and Cytotoxicity of Tau are Modulated by Protein Disulfide Isomerase and S-nitrosylation of this Molecular Chaperone, J. Mol. Biol., 2020, 432(7), 2141–2163 CrossRef CAS PubMed
.
- G. G. Moreira and C. M. Gomes, Tau liquid–liquid phase separation is modulated by the Ca(2+) -switched chaperone activity of the S100B protein, J. Neurochem., 2023, 166(1), 76–86 CrossRef CAS PubMed
.
- X. Li, L. Pinou, Y. Du, X. Chen and C. Liu, Emerging roles of O-glycosylation in regulating protein aggregation, phase separation, and functions, Curr. Opin. Chem. Biol., 2023, 75, 102314 CrossRef CAS PubMed
.
- A. E. Posey, K. M. Ruff, T. S. Harmon, S. L. Crick, A. Li and M. I. Diamond,
et al., Profilin reduces aggregation and phase separation of huntingtin N-terminal fragments by preferentially binding to soluble monomers and oligomers, J. Biol. Chem., 2018, 293(10), 3734–3746 CrossRef CAS PubMed
.
- K. J. Choi, P. S. Tsoi, M. M. Moosa, A. Paulucci-Holthauzen, S. J. Liao and J. C. Ferreon,
et al., A Chemical Chaperone Decouples TDP-43 Disordered Domain Phase Separation from Fibrillation, Biochemistry., 2018, 57(50), 6822–6826 CrossRef CAS PubMed
.
- R. Oliva, S. K. Mukherjee, L. Ostermeier, L. A. Pazurek, S. Kriegler and V. Bader,
et al., Remodeling of the Fibrillation Pathway of α-Synuclein by Interaction with Antimicrobial Peptide LL-III, Chemistry, 2021, 27(46), 11845–11851 CrossRef CAS PubMed
.
- B. Xu, J. Chen and Y. Liu, Curcumin Interacts with α-Synuclein Condensates To Inhibit Amyloid Aggregation under Phase Separation, ACS Omega, 2022, 7(34), 30281–30290 CrossRef CAS PubMed
.
- T. C. Michaels, L. Mahadevan and C. A. Weber, Enhanced potency of aggregation inhibitors mediated by liquid condensates. Physical Review, Research., 2022, 4(4), 043173 CAS
.
- I. A. Klein, A. Boija, L. K. Afeyan, S. W. Hawken, M. Fan and A. Dall'Agnese,
et al., Partitioning of cancer therapeutics in nuclear condensates, Science., 2020, 368(6497), 1386–1392 CrossRef CAS PubMed
.
- E. C. Petronilho, M. M. Pedrote, M. A. Marques, Y. M. Passos, M. F. Mota and B. Jakobus,
et al., Phase separation of p53 precedes aggregation and is affected by oncogenic mutations and ligands, Chem. Sci., 2021, 12(21), 7334–7349 RSC
.
- C. Lemos, L. Schulze, J. Weiske, H. Meyer, N. Braeuer and N. Barak,
et al., Identification of small molecules that modulate mutant p53 condensation, iScience, 2020, 23(9), 101517 CrossRef CAS PubMed
.
- J. L. Silva, D. Foguel, V. F. Ferreira, T. C. Vieira, M. A. Marques and G. D. Ferretti,
et al., Targeting biomolecular condensation and protein aggregation against cancer, Chem. Rev., 2023, 123(14), 9094–9138 CrossRef CAS PubMed
.
- D. M. Mitrea, M. Mittasch, B. F. Gomes, I. A. Klein and M. A. Murcko, Modulating biomolecular condensates: a novel approach to drug discovery, Nat. Rev. Drug Discovery, 2022, 21(11), 841–862 CrossRef CAS PubMed
.
- S. Ghosh, S. Salot, S. Sengupta, A. Navalkar, D. Ghosh and R. Jacob,
et al., p53 amyloid formation leading to its loss of function: implications in cancer pathogenesis, Cell Death Differ., 2017, 24(10), 1784–1798 CrossRef CAS PubMed
.
- S. Sengupta, N. Singh, A. Paul, D. Datta, D. Chatterjee and S. Mukherjee,
et al., p53 amyloid pathology is correlated with higher cancer grade irrespective of the mutant or wild-type form, J Cell Sci., 2023, 136(17), jcs261017 CrossRef CAS PubMed
.
- D. Ishimaru, L. R. Andrade, L. S. P. Teixeira, P. A. Quesado, L. M. Maiolino and P. M. Lopez,
et al., Fibrillar Aggregates of the Tumor Suppressor p53 Core Domain, Biochemistry., 2003, 42(30), 9022–9027 CrossRef CAS PubMed
.
- C. Galea, P. Bowman and R. W. Kriwacki, Disruption of an intermonomer salt bridge in the p53 tetramerization domain results in an increased propensity to form amyloid fibrils, Protein Sci., 2005, 14(12), 2993–3003 CrossRef CAS PubMed
.
- Q. Xie, J. Cheng, W. Mei, D. Yang, P. Zhang and C. Zeng, Phase separation in cancer at a glance, J. Trans. Med., 2023, 21(1), 237 CrossRef CAS PubMed
.
- P.-H. Peng, K.-W. Hsu and K.-J. Wu, Liquid–liquid phase separation (LLPS) in cellular physiology and tumor biology, Am. J. Cancer Res., 2021, 11(8), 3766 CAS
.
- K. Taniue and N. Akimitsu, Aberrant phase separation and cancer, FEBS J., 2022, 289(1), 17–39 CrossRef CAS PubMed
.
- J. H. Ahn, E. S. Davis, T. A. Daugird, S. Zhao, I. Y. Quiroga and H. Uryu,
et al., Phase separation drives aberrant chromatin looping and cancer development, Nature, 2021, 595(7868), 591–595 CrossRef CAS PubMed
.
- J. J. Bouchard, J. H. Otero, D. C. Scott, E. Szulc, E. W. Martin and N. Sabri,
et al., Cancer mutations of the tumor suppressor SPOP disrupt the formation of active, phase-separated compartments, Mol. Cell, 2018, 72(1), 19–36.e8 CrossRef CAS PubMed
.
- E. Tayeb-Fligelman, J. T. Bowler, C. E. Tai, M. R. Sawaya, Y. X. Jiang and G. Garcia,
et al., Low complexity domains of the nucleocapsid protein of SARS-CoV-2 form amyloid fibrils, Nat. Commun., 2023, 14(1), 2379 CrossRef CAS PubMed
.
- H. Chen, Y. Cui, X. Han, W. Hu, M. Sun and Y. Zhang,
et al., Liquid–liquid phase separation by SARS-CoV-2 nucleocapsid protein and RNA, Cell Res., 2020, 30(12), 1143–1145 CrossRef CAS PubMed
.
- L. Pytowski, C. F. Lee, A. C. Foley, D. J. Vaux and L. Jean, Liquid–liquid phase separation of type II diabetes-associated IAPP initiates hydrogelation and aggregation, Proc. Natl. Acad. Sci. U. S. A., 2020, 117(22), 12050–12061 CrossRef CAS PubMed
.
- R. Dec, M. W. Jaworek, W. Dzwolak and R. Winter, Liquid-Droplet-Mediated ATP-Triggered Amyloidogenic Pathway of Insulin-Derived Chimeric Peptides: Unraveling the Microscopic and Molecular Processes, J. Am. Chem. Soc., 2023, 145(7), 4177–4186 CrossRef CAS PubMed
.
- A. Jain, S. Kassem, R. S. Fisher, B. Wang, T.-D. Li and T. Wang,
et al., Connected Peptide Modules Enable Controlled Co-Existence of Self-Assembled Fibers Inside Liquid Condensates, J. Am. Chem. Soc., 2022, 144(33), 15002–15007 CrossRef CAS PubMed
.
| This journal is © The Royal Society of Chemistry 2024 |





