K+ doping-induced pillaring effect in Na3V2(PO4)3 for enhanced rate performance in sodium-ion batteries
Yingqi Wua,
Lijun Xua,
Jinhui Zhongab,
Xue Zhanga,
Xuejie Wanga,
Guoyu Tanga,
Bicheng Zhua and
Tao Liu *a
*a
aLaboratory of Solar Fuel, Faculty of Materials Science and Chemistry, China University of Geosciences, 68 Jincheng Street, Wuhan 430078, P. R. China. E-mail: liutao54@cug.edu.cn
bSchool of Mechanical Engineering, Jiangxi Polytechnic University, Jiujiang 332007, P. R. China
First published on 22nd July 2025
Abstract
K ions act as “molecular pillars” in NVP, propelling lattice expansion to create unobstructed Na+ migration channels and stabilize the structure against collapse during cycling. This pillaring effect also accelerates charge transfer kinetics and facilitates rapidly reversible phase transition via intermediate phase formation, enhancing overall battery performance.
Sodium-ion batteries (SIBs) have emerged as a prominent application due to their balanced energy storage capacity and environmental safety.1 The large-scale deployment of SIBs largely hinges on the energy density, coulomb efficiency, and safety of the cathode materials.2 This necessitates the development of positive electrode materials that exhibit high specific capacity, an appropriate voltage platform, and excellent cycling performance. Sodium vanadium phosphate (NVP) is regarded as a promising cathode material for SIBs, owing to its three-dimensional open framework and sodium superionic conductor structure.3
Nevertheless, the repetitive insertion and extraction of sodium ions during charge–discharge cycles induce structural distortions and potential lattice collapse, which impede ion migration and cause progressive capacity fading. Additionally, the inherently low electronic conductivity exacerbates sluggish phase transformations, particularly under high current densities, leading to rapid capacity decay and cyclic instability.4 Elemental doping has demonstrated remarkable efficacy. Non-metallic element doping, such as nitrogen, can generate oxygen vacancies, facilitating electron transport. For instance, Zhang et al. synthesized nitrogen-doped carbon-coated NVP using melamine as a nitrogen source via ball milling,5 exhibiting a specific capacity of 90 mAh g−1 even at 20C. Moreover, the substitution of metal atoms at the vanadium(V) sites in NVP holds particular significance for optimizing comprehensive properties. Metal cations, owing to their distinct ionic radii, oxidation states, and electronic configurations compared to V3+/V4+ ions, can induce profound structural and electronic modifications.6 When incorporated into the NASICON framework, these metal substitutions induce lattice distortion, thereby optimizing interlayer spacing and ion diffusion pathways. This distortion not only reduces the energy barrier for Na+ migration but also stabilizes the crystal structure against the volume changes associated with sodium-ion cycling. For example, Zhao et al. prepared Ca2+-doped Na3Ca0.05V1.95(PO4)3@C, creating broadened ion and electron transfer channels.7 Density functional theory (DFT) calculations confirmed that Ca2+ substitution enhanced lattice stability, allowing the electrode to deliver 93 mAh g−1 at 40C. In another study, Zhao et al. synthesized Na3.05V1.03Fe0.97(PO4)3 (NVFP), where Fe substitution regulated the crystal plane structure, significantly improving ion diffusion and minimizing volume changes.4a The NVFP half-cell achieved 92 mAh g−1 at 10C, outperforming pristine NVP by approximately 60 mAh g−1.
In this study, we developed a spray-drying-assisted calcination method to synthesize K-doped NVP nanospheres, which demonstrate a stable structure, high conductivity, and rapid ion diffusion rates. Notably, the emerging role of K doping, leveraging its “pillaring effect”, extends the lattice c-axis, widening Na+ transport channels and stabilizing the structure. The samples of Na3−xKxV2(PO4)3/C (x = 0, 0.05, 0.1, and 0.15) were designated as NVP, K0.05-NVP, K0.1-NVP, and K0.15-NVP, respectively. As a result, the optimized K0.1-NVP cathode achieves an impressive capacity of 106.3 mAh g−1 at 1C, and retains 96% of its initial capacity after 1000 cycles. Therefore, the strategy offers a viable solution for developing high-performance SIB cathode materials.
Field emission scanning electron microscopy (FESEM) images (Fig. S1) reveal that both materials exhibit a non-uniform microsphere structure, a characteristic of spray-dried products. From the perspective of morphological evolution, the inclusion of K+ ions does not induce significant alterations in the overall structure of the microspheres. Both the K+-doped and undoped microspheres retain a porous spherical shape, exhibiting no apparent close packing or agglomeration. Transmission electron microscopy (TEM) observations show a strong correspondence with the findings from FESEM. Notably, the interior of the microspheres is characterized by a multitude of pores, attributed to the thermal decomposition of sucrose during the calcination process (Fig. 1a). The presence of pores offers essential buffer space, effectively mitigating stress deformation of the material and thereby enhancing its structural stability. Furthermore, these pores facilitate the thorough penetration of the electrolyte, which is crucial for the efficient transfer of sodium ions, ultimately contributing to high rate performance.8 As illustrated in Fig. 1b, a thin and uniform carbon layer, with a thickness of ∼2.1 nm, is distinctly observable on the surface of the microspheres. It creates a continuous conductive network on the particle surface, thereby significantly enhancing the electronic conductivity and diminishing charge-transfer resistance during electrochemical processes.9 Moreover, it serves as a buffering layer, alleviating volumetric changes associated with sodium-ion insertion and extraction.10 This buffering effect is instrumental in preserving the structural integrity of the cathode material, preventing phenomena such as cracking and pulverization, which in turn improves long-term cycling stability. Fig. 1c reveals distinct lattice streaks on the crystal surface, exhibiting a lattice spacing of 0.37 nm, corresponding to the (113) crystal planes. Energy-dispersive X-ray (EDX) mapping (Fig. 1d and e) analysis was performed to elucidate the elemental distributions. The EDX mapping results unequivocally indicate that K is uniformly distributed throughout the K0.1-NVP/C composite, thus providing compelling evidence for the successful synthesis of uniformly K-doped NVP/C material.
The X-ray diffraction (XRD) patterns of all four samples precisely match the standard card (JCPDS no. 62-0345), demonstrating the formation of a single-phase NASICON structure without obvious heterophases (Fig. 2a). The high intensity of diffraction peaks indicates excellent crystallinity achieved through the sintering process, attributed to the thermally activated atomic diffusion that promotes ordered lattice arrangement. Notably, an amorphous carbon bulge around 24° was observed for all samples, originating from the (002) diffraction of turbostratic carbon coatings. In the structure of Na3V2(PO4)3, there are two distinct types of sodium ions: Na1 and Na2. To explore the potential doping sites for potassium ions, DFT calculations were performed (Fig. 2b). The results indicate that the system energy is reduced by 0.19 eV when a potassium ion replaces a Na1 ion, compared to when it substitutes a Na2 ion. This suggests that, from a thermodynamic standpoint, substituting a potassium ion at the Na1 site is more favorable. Rietveld refinement of the XRD data (Fig. 2c, Fig. S2 and Tables S1–S3) provided precise lattice parameters and atomic occupancies, revealing the structural impact of K+ doping. For K0.1-NVP/C, the c-axis extended from 21.788 to 21.799 nm, and the unit cell volume increased from 1434.78 to 1434.87 nm3. This expansion is attributed to the larger ionic radius of K+ (1.38 Å) compared to Na+ (1.02 Å), leading to lattice distortion upon substitution. The occupancy ratio at the Na1 site decreased from 0.936 to 0.905, directly evidencing K+ substitution at the Na1 site. K+ ions act as “pillar ions”, stabilizing the crystal structure by reinforcing the framework through stronger electrostatic interactions with surrounding oxygen atoms.11 This substitution mechanism mitigates volume changes during Na+ insertion/extraction, enhancing structural durability. The refined atomic positions further revealed that K+ ions occupy the same crystallographic sites as Na+ but with lower occupancy, forming a solid solution that maintains the NASICON framework. The negligible shift in other atomic positions indicates that K+ doping primarily affects the alkali metal sublattice, preserving the integrity of the NVP backbone.
The full-range X-ray photoelectron spectroscopy (XPS) spectrum of K0.1-NVP/C (Fig. S4) showed distinctly resolved peaks corresponding to V 2p, O 1s, P 2s, K 2p, and C 1s, indicating the successful incorporation of K+ as pillar ions. The XPS spectra of V 2p (Fig. 2d) reveal two prominent peaks at 517.4 and 525.4 eV, corresponding to the V 2p3/2 and V 2p1/2 orbitals, respectively.12 These binding energies are consistent with the electronic configuration of V3+ in NVP. The introduction of K+ ions did not alter the valence state of vanadium, indicating that the doping process occurred without significant redox reactions. Instead, K+ ions likely substituted for Na+ ions in the crystal lattice through a simple ion-exchange mechanism, maintaining the overall charge neutrality of the crystal structure. For the K 2p XPS spectrum (Fig. 2e), two well-defined peaks at 295.8 and 293.1 eV were observed, corresponding to the K 2p3/2 and K 2p1/2 transitions.11a The unchanged binding energies of K 2p indicate that K+ ions retained their +1 oxidation state during the doping process. Raman spectroscopy (Fig. 2f) further characterized the carbon coating and phosphate framework. The vibration mode of PO43− ions appears in the 1000–1100 cm−1 range, corresponding to the symmetric stretching of P–O bonds, which validates the integrity of the phosphate tetrahedra in the NASICON structure. Both K0.1-NVP/C and NVP/C display broadened D (1350 cm−1) and G (1600 cm−1) bands of carbon. The D band originates from defect-induced vibrations in disordered carbon, while the G band represents graphitic sp2 carbon layers.13 The ID/IG ratios for K0.1-NVP/C and NVP/C were found to be 1.04 and 1.03, respectively.
Galvanostatic charge–discharge (GCD) and cyclic voltammetry (CV) analyses have been utilized to elucidate the impact of K+ doping on electrochemical performance. The GCD profiles (Fig. 3a and Fig. S5a) provide insightful information into the voltage-plateau behavior and initial capacity, which are linked to the redox processes and phase transitions within the cathode materials. Both the doped and undoped samples exhibit two primary voltage plateaus at approximately 3.3/3.4 V, corresponding to the V4+/V3+ redox couple.14 An additional minor plateau around 3.1 V suggests the occurrence of supplementary phase transitions, potentially involving the formation of intermediate sodium-rich phases or structural rearrangements within the NASICON framework. The persistent voltage plateau observed in the K-doped sample even after ten cycles can be attributed to the “pillar effect” of K+ ions. By occupying specific lattice sites, K+ ions reinforce the structural integrity, mitigating the volume changes associated with sodium ion insertion/extraction. The CV curves (Fig. S5b) display characteristic redox peaks corresponding to the V4+/V3+ transitions, with an additional anodic/cathodic feature around 3.1 V for K0.1-NVP/C, consistent with the GCD results. At various current densities ranging from 0.2 to 10C, K0.1-NVP/C delivers average specific discharge capacities of 110.2, 108.3, 106.1, 103.2, 98.2 and 91.1 mAh g−1, respectively, surpassing the NVP/C capacities of 85, 83, 82, 80, 78 and 74 mAh g−1 (Fig. 3b). Moreover, the discharge capacity of K0.1-NVP/C is slightly lower than that of K0.05-NVP/C at 2C, possibly due to excessive lattice distortion and decreased access to V3+/V4+ redox sites caused by higher K+ doping. At 1C, K0.1-NVP/C maintains an initial specific discharge capacity of 109 mAh g−1 with a remarkable 93% capacity retention after 500 cycles, outperforming the NVP/C electrode (Fig. 3c). Similarly, at 5C, K0.1-NVP/C achieves an initial discharge capacity of 103.6 mAh g−1 with 95% retention after 1000 cycles, compared to 79 mAh g−1 initial capacity and 90% retention of NVP/C (Fig. 3d).
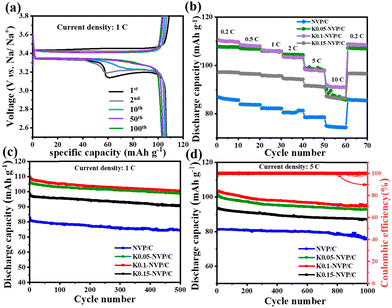 | ||
| Fig. 3 (a) GCD curves of K0.1-NVP/C. (b) Rate performances, and cycling performances of all samples at (c) 1C and (d) 5 C. | ||
In situ XRD measurements (Fig. 4a and b) present the structural evolutions of K0.1-NVP/C and NVP/C during the sodiation and desodiation processes. Both materials exhibit similar phase evolution patterns. This similarity is evident from the peaks appearing at the same positions and the comparable electrochemical behaviors, which follow a two-step three-phase redox reaction involving the transfer of two electrons. However, a closer examination reveals notable differences between the two materials. In the in situ XRD patterns of K0.1-NVP/C, the intermediate Na2V2(PO4)3 is detected earlier, with higher peak intensity, and persists for a longer time compared to that in NVP/C, where this phase almost disappears. The identification of the intermediate Na2V2(PO4)3 (with a space group of P21/c) and typical diffraction peaks at 24.3°, 29.2°, 32.5°, etc. During the charging process, for K0.1-NVP/C, the diffraction peaks at approximately 23.8°,28.8°, and 32.1°, corresponding to Na3V2(PO4)3, gradually diminish in the initial patterns. Simultaneously, the intermediate Na2V2(PO4)3 emerges, leading to a transformation of the space group from R3c to P21/c.15 As the charging continues, this intermediate is successfully converted into NaV2(PO4)3, as indicated by the appearance of typical peaks at 24.6°, 29.7°, and 32.9° in the subsequent patterns, accompanied by a space group transition from P21/c back to R3c. This evolution suggests that the presence of Na2V2(PO4)3 helps to reduce the lattice mismatch between Na3V2(PO4)3 and NaV2(PO4)3. Fig. S6 provides more detailed peak positions of the three phases, clearly showing distinct peak differences of the intermediate Na2V2(PO4)3. During the discharging process, the two steps of the three-phase reactions are completely reversed, with distinct peaks appearing at different stages of discharging. In K0.1-NVP/C, this intermediate exhibits a faster phase conversion rate, which can be attributed to the enhanced rates of ion and electron transfer. The influence of K+ doping on the diffusion behavior of electrodes was further explored using the galvanostatic intermittent titration technique (GITT).16 The GITT results (Fig. S7) indicate that the average diffusion coefficient of K0.1-NVP/C (1.77 × 10−11 cm2 s−1) is slightly higher than that of NVP/C (1.43 × 10−11 cm2 s−1). This suggests an enhanced diffusion efficiency in the K0.1-NVP/C electrode. The findings demonstrate that the introduction of K+ improves the transport rate of Na+ and enhances the electronic conductivity of the electrode material.
 | ||
| Fig. 4 In situ XRD patterns recorded in the first cycle at 0.2 C of (a) NVP/C and (b) K0.1-NVP/C. (c) Rate performance and (d) cycling performance of NVP/C‖HC and K0.1-NVP/C‖HC. | ||
The working diagram for the NVP/C and K0.1-NVP/C full batteries, assembled with hard carbon as anodes, is illustrated in Fig. S9a (K0.1-NVP/C‖HC and NVP/C‖HC). The GCD curve indicates that K0.1-NVP/C‖HC exhibits a larger discharge capacity while both configurations maintain a similar voltage platform (Fig. S9b). K0.1-NVP/C‖HC maintains discharge capacities of 109, 107, 105, 101 and 99 mAh g−1 at 0.2, 0.5, 1, 2 and 5C, respectively, outperforming NVP/C‖HC across each rate (Fig. 4c). While NVP/C‖HC shows a capacity decay of 12.5% at 5C, K0.1-NVP/C‖HC exhibits virtually no attenuation (Fig. 4d). This improvement may be attributed to K+ doping, which facilitates faster mesophase formation and enhances sodium ion migration rates.17 The more pronounced decay in NVP/C‖C is further supported by cycling performance analysis at 1C, where NVP/C‖HC shows a capacity retention rate of just 78%, significantly lower than the 97% retention seen in K0.1-NVP/C‖HC. This suggests that K+ doping plays a crucial role in accelerating charge and discharge processes, promoting rapid ion transfer, and enhancing cycle stability.
In summary, K-NVP/C composites were fabricated via spray drying. K+ doping induces lattice expansion to boost Na+ transport and creates a “pillaring effect” that reinforces structure, optimizes electrochemistry, and reduces volume changes. K0.1-NVP/C performs excellently, delivering 106.3 mA h g−1 at 1C with 96% capacity retention after 1000 cycles. Its scalable synthesis makes it a promising anode for next-gen SIBs.
The work was supported by the National Natural Science Foundation of China (22478368), the Key Research and Development Program of Hubei Province (2023BAB113), the Natural Science Foundation of Hubei Province of China (2022CFA001 and 2023CFA088), and the Fundamental Research Funds for the Central Universities, China University of Geosciences (Wuhan) (CUG22061).
Conflicts of interest
The authors declare no competing financial interest.Data availability
The authors declare that the data supporting the findings of this study are available within the paper and its SI. Should any raw data files be needed in another format they are available from the corresponding author upon reasonable request. Source data are provided with this paper.Experimental section, FESEM, XRD, XPS and GCD. See DOI: https://doi.org/10.1039/d5cc03722k.
Notes and references
-
(a) F. Ding, et al., Nat. Energy, 2024, 9, 1529–1539 CrossRef CAS
; (b) X. Cai, et al., J. Am. Chem. Soc., 2025, 147, 5860–5870 CrossRef CAS PubMed
.
-
(a) Y. An, et al., Matter, 2024, 7, 1466–1502 CrossRef CAS
; (b) Z. Wu, et al., Chem. Commun., 2024, 60, 10029–10032 RSC
; (c) Y. An, et al., Sci. Adv., 2025, 11, eadv2007 CrossRef CAS PubMed
; (d) T. Liu, et al., Adv. Mater., 2023, 35, 2207752 CrossRef CAS PubMed
.
-
(a) K. Saravanan, et al., Adv. Energy Mater., 2013, 3, 444–450 CrossRef CAS
; (b) Y. Han, et al., ACS Appl. Mater. Interfaces, 2024, 16, 35114–35122 CrossRef CAS PubMed
; (c) R. Huang, et al., Adv. Energy Mater., 2024, 14, 2400595 CrossRef CAS
.
-
(a) X. X. Zhao, et al., Adv. Sci., 2023, 10, 2301308 CrossRef CAS PubMed
; (b) L. Xu, et al., Rare Met., 2025, 44, 185–194 CrossRef CAS
.
- H. Zhang, et al., Carbon, 2017, 124, 334–341 CrossRef CAS
.
- S. Wang, et al., Rare Met., 2024, 43, 4253–4262 CrossRef CAS
.
- L. Zhao, et al., J. Mater. Chem. A, 2019, 7, 9807–9814 RSC
.
-
(a) T. Liu, et al., Chem. Commun., 2023, 59, 14811–14814 RSC
; (b) T. Liu, et al., ACS Appl. Mater. Interfaces, 2023, 15, 43691–43701 CrossRef CAS PubMed
.
- T. Xu, et al., Acta Phys.-Chim. Sin., 2024, 40, 2303021 CrossRef
.
- Y. Tian, et al., Angew. Chem., Int. Ed., 2025, 64, e202423454 CrossRef CAS PubMed
.
-
(a) S.-J. Lim, et al., J. Mater. Chem. A, 2014, 2, 19623–19632 RSC
; (b) J. Zhou, et al., Electrochem. Commun., 2025, 173, 107883 CrossRef CAS
.
-
(a) W. Yan, et al., ACS Sustainable Chem. Eng., 2024, 12, 2394–2403 CrossRef CAS
; (b) L. Chen, et al., Rare Met., 2024, 43, 1836–1844 CrossRef CAS
; (c) S. D. Wansi Wendji, et al., Chem. Commun., 2025, 61, 10993–10996 RSC
.
- H. Lv, et al., Acta Phys.-Chim. Sin., 2023, 39, 2210014 Search PubMed
.
-
(a) S. Sarkar, et al., Chem. Commun., 2024, 60, 12868–12871 RSC
; (b) M. Pan, et al., Chem. Eng. J., 2024, 495, 153396 CrossRef CAS
.
-
(a) H. Zhang, et al., Adv. Sci., 2024, 11, 2306168 CrossRef CAS PubMed
; (b) X. Ding, et al., ACS Nano, 2024, 18, 35632–35643 CrossRef CAS
.
-
(a) M. T. Ahsan, et al., Rare Met., 2025, 44, 2328–2339 CrossRef CAS
; (b) X. Cai, et al., Adv. Funct. Mater., 2024, 34, 2409732 CrossRef CAS
.
- H. Yu, et al., ACS Nano, 2022, 16, 21174–21185 CrossRef CAS PubMed
.
| This journal is © The Royal Society of Chemistry 2025 |


