Discovery of a potent and selective PROTAC degrader for STAT3†
Kefeng Wang‡
 a,
Yuxin Zheng‡a,
Wenli Maoa,
Jing Xu
a,
Yuxin Zheng‡a,
Wenli Maoa,
Jing Xu *b and
Yukun Wang*a
*b and
Yukun Wang*a
aDepartment of Pharmacology, School of Medicine, Southern University of Science and Technology, Shenzhen, 518055, China. E-mail: wangyk@sustech.edu.cn
bDepartment of Chemistry and Shenzhen Grubbs Institute and Guangdong Provincial Key Laboratory of Catalysis and Shenzhen Key Laboratory of Small Molecule Drug Discovery and Synthesis, Southern University of Science and Technology, Shenzhen, 518055, China. E-mail: xuj@sustech.edu.cn
First published on 31st July 2025
Abstract
The signal transducer and activator of transcription 3 (STAT3) protein and the p53 protein play opposite roles in the regulation of cell pathways. Activation of STAT3 upregulates survival pathways, while activation of p53 triggers apoptosis pathways. Therefore, STAT3 inhibition of p53 expression may play a central role in tumor development, and targeting STAT3 represents a promising therapeutic method for p53 reactivation in many cancers. Here, we report the design of S3D5, a BP-1-102-based proteolysis targeting chimera (PROTAC) that induces time- and dose-dependent degradation of STAT3 in HepG2 cells without significant effects on other STAT proteins. Preliminary mechanism studies show that S3D5 degradation of the STAT3 protein is mediated by the ubiquitin–proteasome system (UPS). S3D5 exhibits good anti-hepatocellular carcinoma cell proliferation activity, which can be explained by activating the p53 pathway. These findings demonstrate the utility and importance of PROTACs as preliminary chemical tools to investigate the function of the STAT3 protein. Further, S3D5 may serve as a potential anti-hepatocellular carcinoma agent, laying a practical foundation for further development of potent STAT3-targeting PROTACs.
Introduction
Despite great progress in cancer treatment in the last few decades, cancer remains a major clinical problem.1 Two important reasons for tumor treatment failure are tumor heterogeneity and acquired treatment resistance.2 To solve these problems, it is critical to discover and exploit previously unrecognized molecular mechanisms involved in cell proliferation.1 The signal transducer and activator of the transcription 3 (STAT3) protein is often associated with the progression and poor prognosis of most cancers in humans.3 STAT3 not only regulates the expression of oncogenes in tumor cells, but also promotes the occurrence of human cancers through immunosuppression.4 Therefore, STAT3 has been widely recognized as an attractive target for the development of inhibitors for cancers as well as for other STAT3-related diseases.5The STAT3 protein is composed of 750–795 amino acids with a relative molecular weight of about 92 kDa and has four isoforms in cells, including the long form STAT3α, the truncated forms STAT3β and STAT3γ, and a putative novel form STAT3δ.6 STAT3 is an important member of the signal transducer and activator of transcription protein (STAT) family, and its overactivation can up-regulate the transcription and expression of multiple target genes.7 In recent years, inhibitors targeting STAT3 have become a hot research topic in the field of drug research and development. A large and diverse set of compounds have been discovered that directly inhibit STAT3 activity; however, very few of them have been approved for clinical practice,8 because most have inadequate efficacy or intolerable side effects.9
The emerging proteolysis targeting chimera (PROTAC) technology breaks through the mode of action of traditional small molecule inhibitors, which can directly induce the targeted protein degradation. PROTACs have become a hot research topic in the field of medicinal chemistry in recent years, providing a new therapeutic approach to solve diseases driven by abnormal expression of disease-related proteins.10 A PROTAC is a bifunctional molecule, in which a ligand of E3 ubiquitin ligase is linked with a small molecule inhibitor through a linker. In vivo, the inhibitor part of this bifunctional molecule can recognize a protein of interest (POI), and the ligand of E3 ubiquitin ligase can recognize the E3 ubiquitin ligase, so that the POI and the E3 ubiquitin ligase can be closer. The recruited E3 ligase then mediates the transfer of ubiquitin from an E2 enzyme to the POI, after which the ubiquitylated POI can be degraded through the ubiquitin-proteasome pathway.11 Therefore, designing PROTAC degraders of STAT3 to target STAT3 protein degradation may be another effective cancer treatment strategy.12
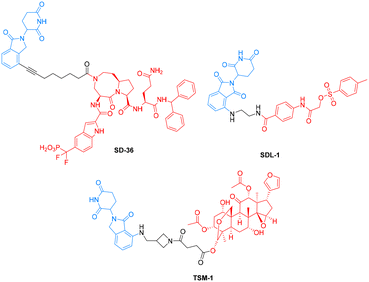
To date, several STAT3-targeting PROTACs have been reported. In 2019, Wang's research group optimized SI-109 from CJ-887, a previously reported peptidomimetic compound with high binding affinities for STAT3, and designed and synthesized SD-36 (Scheme 1). SD-36 induces rapid degradation of STAT3 at low nanomolar concentrations in leukemia and lymphoma cells and does not degrade other STAT proteins. In addition, it caused complete degradation of the STAT3 protein in the Molm-16 xenograft tumor model at well-tolerated dose-schedules.13 Subsequently, Guan's team made a series of STAT3 PROTACs based on S31-201 (Scheme 1). One of these PROTACs, SDL-1, achieves the degradation of the STAT3 protein in vitro and exhibits good anti-gastric cancer cell proliferation activity.14 Based on the triterpenoid toosendanin, Luan and colleagues designed several lenalidomide-conjugated PROTACs. Among them, TSM-1 (Scheme 1) presents the most potent antitumor effects in STAT3-dependent neck squamous cell carcinoma and colorectal cancer.15 Notably, another study developed a napabucasin-based STAT3 PROTAC XD2-149, which effectively inhibited STAT3 signaling in pancreatic cancer cell lines, but failed to induce proteasome-dependent STAT3 protein degradation, underscoring the ongoing challenges in developing therapeutics targeting STAT3 degradation.14
This study describes the synthesis and evaluation of a series of STAT3 PROTACs with cellular potency in multiple cell lines. Through investigation of the structure–activity relationship, we found an effective STAT3 degrader S3D5 with a DC50 = 110 nM in HepG2 cells and the ability to inhibit the proliferation, invasion and migration of cancer cells. In conclusion, the development of the STAT3 degrader suggests that it could be used as a potential anticancer drug and provides a new strategy for the treatment of cancers.
Results and discussion
In order to design and synthesize STAT3-degrading PROTACs, a well-established STAT3 inhibitor compound BP-1-102 (ref. 16) was selected and structurally modified to obtain compound 7a, which occupies an allosteric binding site as a STAT3-targeting ligand. The predicted binding modes of 7a with STAT3 (PDB code: 6QHD, Fig. 1B)17 indicate that the carboxyl part of compound 7a is exposed to the solvent surface. This offers a feasible site for introducing a linker to conjugate with an E3 ligase recruiting ligand for designing the corresponding PROTACs. We used pomalidomide as the E3 ligase ligand: according to the reported cocrystal structure of pomalidomide in complex with CRBN,18 its 3-amino-phthalic anhydride portion is exposed to the solvent, and so it can be utilized to tether drug-like small molecules for PROTAC formation. Thus, we designed and synthesized a set of STAT3 degraders by tethering 7a to pomalidomide through several linkers (Fig. 1A). | ||
| Fig. 1 Design of STAT3 degraders. (A) Design ideas of PROTACs. Using BP-1-102 analog and pomalidomide as ligands, a series of STAT3 degraders were designed by changing the linker. (B) Docking model of 7a binding to STAT3 (PDB code: 6QHD). | ||
The synthetic details of designed compounds are shown in Scheme 2. Commercially available sarcosine tert-butyl ester 1 and pentafluorobenzenesulfonyl chloride 2a were substituted and hydrolyzed to obtain N-methyl-N-((perfluorophenyl)sulfonyl)glycine 4a. After acid chlorination of 4a with thionyl chloride, and the nucleophilic substitution reaction by 4-aminobenzoic acid 6, the BP-1-102 analog 7a was afforded. Commercially available thalidomide 4-fluoride 8 and the primary PEG linkers 9a–9j with different lengths were subjected to nucleophilic substitution and deprotection to afford compounds 11a–11j. The end products S3D1–S3D10 were obtained by the amide condensation reaction between the above compound 7a and compounds 11a–11j.
To verify the enzymatic inhibitory activity of these degraders, we first treated hepatocellular carcinoma cells and non-small cell lung cancer cell lines with increasing concentrations of these PROTAC molecules (1–20 μM) and showed that they inhibited the viability of cancer cells (please see Table S1 in the ESI†). Next, we examined the ability of these degraders to induce STAT3 degradation in HepG2 cells (Fig. 2B). Based on both antiproliferative activity and degradation rates, S3D5 was selected for further evaluation.
The ability of S3D5 to bind STAT3 was detected by SPR (KD = 4.35 μM). As shown in Fig. 3, STAT3 protein levels decreased in a dose-dependent manner with a DC50 (half-maximal degradation) value of 110 nM. Importantly, S3D5 could effectively degrade the STAT3 protein even at low concentrations, but had little effect at any concentration on STAT1, STAT2, STAT4, STAT5 and STAT6 proteins. In vitro, S3D5 demonstrated high intrinsic clearance in mouse and human liver microsomes, suggesting potential challenges related to metabolic stability. A time course experiment was also conducted, demonstrating that STAT3 degradation induced by S3D5 starts after 3 h of exposure. The amount of STAT3 protein kept decreasing and reached the maximum degradation at 48 h with the administration time prolonged.
The formation of the STAT3–PROTAC–E3 ternary complex is a prerequisite for the degradation of STAT3, and the ubiquitin–proteasome system is the main pathway for protein degradation by PROTACs. In order to elucidate the mechanism of STAT3 protein degradation induced by compound S3D5 in HepG2 cells, a series of rescue experiments were performed. Firstly, HepG2 cells were pretreated with the STAT3 inhibitor BP-1-102 or CRBN inhibitor pomalidomide to prevent the ternary complex formation. As shown in Fig. 3F, when either the STAT3 ligands or CRBN ligands were present, the degradation of the STAT3 protein by S3D5 was reduced. Subsequently, HepG2 cells were pretreated separately with proteasome inhibitor MG132 (ref. 19) and with ubiquitination inhibitor MLN4924.20 In both cases, the degradation of STAT3 induced by S3D5 was attenuated. These results suggest that S3D5-induced degradation of STAT3 requires the formation of the ternary complexes and that the STAT3 protein is likely to be degraded via the ubiquitin–proteasome system (UPS) pathway.
After confirming that the compound S3D5 can efficiently and selectively degrade the STAT3 protein, we used this PROTAC to verify the role of STAT3 in the proliferation, migration and invasion of HepG2 cells. The anti-proliferative activity of S3D5 against HepG2 cells were evaluated by CCK-8 assay. The CCK-8 results showed that S3D5 inhibited the growth of HepG2 cells in a dose-dependent manner (Fig. 4A). Furthermore, compared with the positive control compound BP-1-102, S3D5 demonstrated better anti-hepatoma activity. Subsequently, we evaluated the cytotoxicity of S3D5 in human embryonic kidney 293T cells. The CCK-8 results showed that 5 μM S3D5 inhibited the proliferation of 293T cells (Fig. 4A), suggesting that the degradation effect of S3D5 on STAT3 itself might have an impact on cells that are highly dependent on this pathway or have vigorous proliferation. The colony-formation assay was performed to investigate the colony formation capacity of S3D5. HepG2 cells treated with S3D5 formed significantly fewer colonies and had significantly smaller colonies than untreated control cells (Fig. 4B). To examine the effects of S3D5 on the migration and invasion of the HepG2 cell line, we performed a wound-healing assay and Transwell invasion assay, which showed that S3D5 inhibited the invasion and migration of HepG2 cells (Fig. 4C and D). These results suggest that the STAT3-directed PROTAC S3D5 can inhibit the proliferation, migration and invasion of HepG2 cells.
It has previously been found that STAT3 activation can negatively regulate the p53 signaling pathway and its related effects on apoptosis and growth inhibition.21 Therefore, we investigated the effect of treatment with STAT3-directed PROTAC S3D5 on STAT3-related signaling pathways. HepG2 cells were treated with S3D5, and p53 and other related proteins were detected by western blot. As shown in Fig. 5, S3D5 upregulated the level of p21 and p53 proteins in a concentration-dependent manner. It is well known that wild-type p53 (wtp53) upregulates p21 expression.22 Thus treatment with the STAT3-directed PROTAC S3D5 could positively regulate the p53 signaling pathway and its related effects on the cell growth inhibition.
 | ||
| Fig. 5 Effect of the STAT3-directed PROTAC on p53 and p21 in HepG2 cells. p53 and p21 levels were determined after cells were treated with the indicated concentrations of S3D5 for 48 hours. | ||
Conclusions
In this study, we first designed and synthesized the target protein ligands 7a and 7b based on the molecular structure of the STAT3 small molecule inhibitor BP-1-102 (please see Scheme S1 in the compound synthesis ESI†). To synthesize a series of BP-1-102-based STAT3 degraders, STAT3 protein ligands 7a or 7b were tethered to the cereblon E3 ligase ligand pomalidomide by a variety of linkers constructed by click reactions or amidation coupling reactions. After exploring the structure–activity relationship for STAT3 degradation, S3D5 was determined to have the best effect, potently inhibiting the proliferation of HepG2, H1299 and H1975 cell lines (please see Table S1 in the ESI†). STAT3 degradation induced by S3D5 is dose- and time-dependent with a high selectivity for STAT3 degradation. We also demonstrated that S3D5 significantly inhibited migration and invasion of HepG2 cells. Based on mechanism studies, we found that ternary complex formation and subsequent ubiquitination were crucial to S3D5-induced degradation of the STAT3 protein.Although p53 mutations have been reported to occur early and be involved in tumor initiation, it appears that mutations of p53 in certain cancers could develop late and play important roles in the advanced stages of tumorigenesis.23 In addition, loss of wtp53 function and accumulation of mtp53 can support STAT3-mediated tumor cell survival and expansion.24 Several inhibitors that target either STAT3 or p53 are in clinical trials, but their success has been limited by resistance to targeted cancer treatments.25 The use of PROTACs that trigger degradation of STAT3 proteins provides novel opportunities that might be particularly relevant to STAT3. Notably, after S3D5 treatment of HepG2 cells, STAT3 protein levels were downregulated, while p53 and p21 protein levels were upregulated, which could indicate that the degradation of STAT3 induced the cell growth inhibition via the regulation of the MDM2–P53–P21 signaling pathway.
In conclusion, we developed an effective STAT3-directed PROTAC S3D5, which can induce STAT3 protein degradation in vitro and inhibit hepatocellular carcinoma growth and metastasis. Overall, our study suggests that STAT3-directed PROTACs are novel modalities for STAT3-directed drug discovery in tumors and other STAT3-related diseases.
Data availability
Details about methods, experimental procedures, mechanistic studies, characterization data, and NMR spectra are available in the ESI.†Author contributions
J. X. and Y.-K. W. designed and supervised the project. K.-F. W. and Y.-X. Z. designed and performed the experiments; K.-F. W., W.-L. M. and Y.-X. Z. analysed all the results. J. X. and Y.-K. W. prepared the paper. All the authors discussed the results and commented on the paper.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We are grateful for the support of this work by the Shenzhen Science and Technology Plan Project (JCYJ20220530115203008).Notes and references
- Z. P. Xiao, S. S. Song, D. Chen, R. van Merkerk, P. E. van der Wouden, R. H. Cool, W. J. Quax, G. J. Poelarends, B. N. Melgert and F. J. Dekker, Angew. Chem., Int. Ed., 2021, 60, 17514–17521 CrossRef CAS PubMed
.
- I. Dagogo-Jack and A. T. Shaw, Nat. Rev. Clin. Oncol., 2018, 15, 81–94 CrossRef CAS PubMed
.
- P. S. Lai, D. A. Rosa, A. M. Ali, R. F. Gómez-Biagi, D. P. Ball, A. E. Shouksmith and P. T. Gunning, Expert Opin. Ther. Pat., 2015, 25, 1495 CrossRef CAS PubMed
.
- Y. Wang, Y. Shen, S. Wang, Q. Shen and X. Zhou, Cancer Lett., 2018, 415, 117–128 CrossRef CAS PubMed
.
- J. Dong, X.-D. Cheng, W.-D. Zhang and J.-J. Qin, J. Med. Chem., 2021, 64, 8884–8915 CrossRef CAS PubMed
.
- M. Benekli, M. R. Baer, H. Baumann and M. Wetzler, Blood, 2003, 101, 2940–2954 CrossRef CAS PubMed
.
- H. Yu, D. Pardoll and R. Jove, Nat. Rev. Cancer, 2009, 9, 798–809 CrossRef CAS PubMed
.
- Y. Jin, Y. H. Kim, J. Y. Park, Y.-J. Lee, H.-M. Oh, S.-K. Choi, D. C. Han and B.-M. Kwon, Bioorg. Med. Chem. Lett., 2018, 28, 853–857 CrossRef CAS PubMed
.
- M. Hanafi, X. Chen and N. Neamati, J. Med. Chem., 2021, 64, 1626–1648 CrossRef CAS PubMed
.
- J. Salami and C. M. Crews, Science, 2017, 355, 1163–1167 CrossRef CAS PubMed
.
- A. C. Lai and C. M. Crews, Nat. Rev. Drug Discovery, 2017, 16, 101–114 CrossRef CAS PubMed
.
- L. Bai, H. Zhou, R. Xu, Y. Zhao, K. Chinnaswamy, D. McEachern, J. Chen, C.-Y. Yang, Z. Liu, M. Wang, L. Liu, H. Jiang, B. Wen, P. Kumar, J. L. Meagher, D. Sun, J. A. Stuckey and S. Wang, Cancer Cell, 2019, 36, 498–511 CrossRef CAS PubMed
.
- H. Zhou, L. Bai, R. Xu, Y. Zhao, J. Chen, D. McEachern, K. Chinnaswamy, B. Wen, L. Dai, P. Kumar, C.-Y. Yang, Z. Liu, M. Wang, L. Liu, J. L. Meagher, H. Yi, D. Sun, J. A. Stuckey and S. Wang, J. Med. Chem., 2019, 62, 11280–11300 CrossRef CAS PubMed
.
- H. Li, L. Wang, F. Cao, D. Yu, J. Yang, X. Yu, J. Dong, J.-J. Qin and X. Guan, Front. Pharmacol., 2022, 13, 944455 CrossRef CAS PubMed
.
- J. M. Jin, Y. P. Wu, Z. Zhao, Y. Wu, Y. D. Zhou, S. H. Liu, Q. Y. Sun, G. Z. Yang, J. Y. Lin, D. G. Nagle, J. J. Qin, Z. Y. Zhang, H. Z. Chen, W. D. Zhang, S. Y. Sun and X. Luan, JCI Insight, 2022, 7, e160606 CrossRef PubMed
.
- X. L. Zhang, P. B. Yue, B. D. G. Page, T. S. Li, W. Zhao, A. T. Namanja, D. Paladino, J. H. Zhao, Y. Chen, P. T. Gunning and J. Turkson, Proc. Natl. Acad. Sci. U. S. A., 2012, 109, 9623–9628 CrossRef CAS PubMed
.
- H. Chen, W. Zhou, A. Bian, Q. Zhang, Y. Miao, X. Yin, J. Ye, S. Xu, C. Ti, Z. Sun, J. Zheng, Y. Chen, M. Liu and Z. Yi, Clin. Cancer Res., 2023, 29, 815–830 CrossRef CAS PubMed
.
- Q. Li, Q. Guo, S. Wang, S. Wan, Z. Li, J. Zhang and X. Wu, Eur. J. Med. Chem., 2022, 238, 114455 CrossRef CAS PubMed
.
- N. Guo and Z. Peng, Asia Pac. J. Clin. Oncol., 2013, 9, 6–11 CrossRef PubMed
.
- Q. Zhou and Y. Sun, Mol. Cell. Oncol., 2019, 6, e1618174 CrossRef PubMed
.
- U. K. Mukhopadhyay, P. Mooney, L. Jia, R. Eves, L. Raptis and A. S. Mak, Mol. Cell. Biol., 2010, 30, 4980–4995 CrossRef CAS PubMed
.
- I. Beuvink, A. Boulay, S. Fumagalli, F. Zilbermann, S. Ruetz, T. O'Reilly, F. Natt, J. Hall, H. A. Lane and G. Thomas, Cell, 2005, 120, 747–759 CrossRef CAS PubMed
.
- N. Rivlin, R. Brosh, M. Oren and V. Rotter, Genes Cancer, 2011, 2, 466–474 CrossRef CAS PubMed
.
- R. Schulz-Heddergott, N. Stark, S. J. Edmunds, J. Li, L.-C. Conradi, H. Bohnenberger, F. Ceteci, F. R. Greten, M. Dobbelstein and U. M. Moll, Cancer Cell, 2018, 34, 298–314 CrossRef CAS PubMed
.
- M. J. Duffy, N. C. Synnott, S. O'Grady and J. Crown, Semin. Cancer Biol., 2022, 79, 58–67 CrossRef CAS PubMed
.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5md00260e |
| ‡ Co-first author with the equally contribution. |
| This journal is © The Royal Society of Chemistry 2025 |