A one-step strategy for synthesizing N-formanilide via benzamide activation and oxalic acid-driven reduction†
Heewon Lee,
Nithin Pootheri ,
Chang Woo Kim
,
Chang Woo Kim * and
Sunwoo Lee
* and
Sunwoo Lee *
*
Department of Chemistry, Chonnam National University, Gwangju, 61186 Republic of Korea. E-mail: sunwoo@chonnam.ac.kr; cwkim66@chonnam.ac.kr
First published on 10th July 2025
Abstract
A one-step synthetic strategy for directly converting benzamides into N-formanilide derivatives has been developed using oxalic acid as a hydride source. This strategy involves the selective cleavage of the amide C–N bond through a reaction with sodium azide, generating a benzyl azide intermediate. This intermediate subsequently undergoes Curtius rearrangement to form an isocyanate species, which is then reduced by thermally decomposed oxalic acid, yielding the final N-formylated product. This strategy demonstrates a broad substrate scope, including various substituted benzamides and heterocyclic amides, achieving yields of up to 95%.
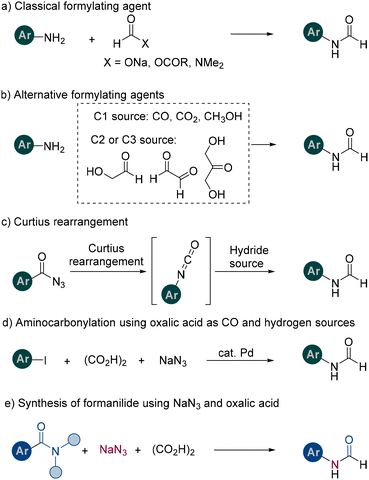
N-Formylaniline stands out as a pivotal structural motif in medicinal chemistry because of its potential to exhibit anti-inflammatory, antibacterial, anti-osteoporotic, and central nervous system activities when modified with suitable substituents (Fig. 1).1 Consequently, the development of efficient synthetic methods and modification strategies for N-formylaniline remains vital for producing novel bioactive agents and enhancing existing drugs.
Traditionally, N-formanilide has been synthesized by directly formylating aniline with formic acid or its derivatives. However, these reagents often raise concerns regarding stability and environmental impact (Scheme 1a).2 Consequently, alternative strategies that bypass the use of classical formylating agents have been developed by employing carbon monoxide (CO), carbon dioxide, or methanol as one-carbon (C1) sources—sometimes in tandem with the oxidation of C2 or C3 substrates (Scheme 1b).3 Although these strategies are both versatile and innovative, they often require highly specialized catalysts or have limitations in terms of broad applicability.
Another valuable method for preparing N-formanilide involves the Curtius rearrangement of acyl azides, which are frequently derived from a wide array of precursors (Scheme 1c).4 In this method, conventional organic hydrogen donors, including formaldehyde and formic acid, are routinely employed to facilitate the rearrangement and subsequent formation of N-formylated products.
In our laboratory, we recently developed an aminocarbonylation pathway that harnesses oxalic acid as both a CO source and a hydrogen donor under palladium catalysis, enabling the synthesis of N-formanilide in excellent yields (Scheme 1d).5 This pathway proceeds under comparatively mild reaction conditions, and its operational simplicity and scalability make it highly attractive for practical synthetic applications.
In recent years, numerous research groups have advanced methodologies that enable the selective cleavage of amide C–N bonds, thereby creating opportunities for introducing new functionalities or inducing rearrangements.6 Our laboratory has also contributed to this field by developing a range of acyl-substitution protocols for both activated and non-activated amides using various nitrogen-, carbon-, or oxygen-based nucleophiles.7 Additionally, we have demonstrated a route whereby amides react with NaN3 to generate acyl azides.8
Building on these findings, we now present a one-step procedure for synthesizing N-formanilide from amides via an acyl azide intermediate, with oxalic acid serving as an effective hydrogen donor. Although benzoyl chlorides are widely utilized and offer straightforward access to acyl derivatives, their handling can sometimes present challenges due to their sensitivity toward moisture and potential functional group compatibility issues. In contrast, our method employing activated amides offers a complementary synthetic route that addresses these concerns. Moreover, to the best of our knowledge, the direct transformation of activated amides into N-formanilide groups using oxalic acid has not been previously reported. We believe that this strategy not only broadens the synthetic toolbox but also provides organic chemists with a practical and versatile alternative for formyl group installation.
The reaction conditions were optimized to maximize the yield of 2a from the reaction of 1a, NaN3, and oxalic acid (Table 1). The optimal conditions were identified as using 2.0 equiv. of NaN3 and 6.0 equiv. of oxalic acid in NMP at 130 °C for 16 h, which afforded 2a in 89% yield (entry 1). Subsequently, the effect of varying reaction parameters was examined. Decreasing the NaN3 loading to 1.0 equiv. significantly lowered the yield to 32%, whereas increasing it to 1.5 equiv. provided an intermediate yield of 66% (entries 2 and 3). Further increasing the NaN3 loading to 2.5 equiv. did not significantly improve the yield, which remained at 81% (entry 4). Next, the influence of (CO2H)2 equivalents was studied. Reducing the (CO2H)2 loading to 2.0 equiv. completely suppressed product formation (entry 5). Using 4.0 equiv. led to a modest yield of 32%, and increasing the loading to 5.0 equiv. improved the yield to 64% (entries 6 and 7). Further increasing the loading to 7.0 equiv. afforded an 80% yield, which is comparable to the 89% yield obtained with 6.0 equiv. (entry 8). Solvent screening was then conducted. DMF provided a moderate yield of 61%, while DMAc and DMSO afforded significantly lower yields of 4% and 15%, respectively (entries 9–11). The use of toluene, diglyme, and monoglyme failed to produce any detectable product (entries 12–14). Lowering the reaction temperature to 110 °C reduced the yield to 42%, and further decreasing it to 90 °C resulted in no product formation (entries 15 and 16). Shortening the reaction time to 12 h resulted in a slightly reduced yield of 71%, whereas extending it to 24 h provided a 75% yield (entries 17 and 18).
| Entry | x equiv. | y equiv. | Solvent | Temperature (°C) | Time (h) | Yieldb (%) |
|---|---|---|---|---|---|---|
| a Reaction conditions: 1a (0.3 mmol), NaN3 (x equiv.), and (CO2H)2 (y equiv.) reacted in NMP.b Determined using gas chromatography with an internal standard (2-methoxynaphthalene). | ||||||
| 1 | 2.0 | 6.0 | NMP | 130 | 16 | 89 |
| 2 | 1.0 | 6.0 | NMP | 130 | 16 | 32 |
| 3 | 1.5 | 6.0 | NMP | 130 | 16 | 66 |
| 4 | 2.5 | 6.0 | NMP | 130 | 16 | 81 |
| 5 | 2.0 | 2.0 | NMP | 130 | 16 | 0 |
| 6 | 2.0 | 4.0 | NMP | 130 | 16 | 32 |
| 7 | 2.0 | 5.0 | NMP | 130 | 16 | 64 |
| 8 | 2.0 | 7.0 | NMP | 130 | 16 | 80 |
| 9 | 2.0 | 6.0 | DMF | 130 | 16 | 61 |
| 10 | 2.0 | 6.0 | DMAc | 130 | 16 | 4 |
| 11 | 2.0 | 6.0 | DMSO | 130 | 16 | 15 |
| 12 | 2.0 | 6.0 | Toluene | 130 | 16 | 0 |
| 13 | 2.0 | 6.0 | Diglyme | 130 | 16 | 0 |
| 14 | 2.0 | 6.0 | Monoglyme | 130 | 16 | 0 |
| 15 | 2.0 | 6.0 | NMP | 110 | 16 | 42 |
| 16 | 2.0 | 6.0 | NMP | 90 | 16 | 0 |
| 17 | 2.0 | 6.0 | NMP | 130 | 12 | 71 |
| 18 | 2.0 | 6.0 | NMP | 130 | 24 | 75 |
Under the optimized reaction conditions, various substituted N-phenyl-N-tosylbenzamide derivatives were investigated for their reactivity with NaN3 and oxalic acid (Scheme 2). As expected, N-phenyl-N-tosylbenzamide underwent the transformation smoothly, affording the desired product 2a in an isolated yield of 89%. The introduction of methyl substituents influenced the reaction efficiency, with the ortho-, meta-, and para-methyl derivatives producing 2b, 2c, and 2d in 38%, 69%, and 70% yields, respectively. Among the alkylated substrates, the para-tert-butyl-substituted derivative exhibited the highest efficiency, affording 2e in 91% yield. The presence of methoxy groups also affected the transformation, with the mono- and dimethoxy-substituted derivatives leading to the formation of 2f and 2g in 64% and 63% yields, respectively. The reaction was further extended to larger aromatic systems, such as biphenyl- and 1-naphthyl-based amides, which delivered 2h and 2i in 76% and 64% yields, respectively. Interestingly, fluorine substitution had a position-dependent effect on the reaction outcome. While the para-fluoro-substituted derivative efficiently produced 2j in an excellent 95% yield, its ortho-fluoro counterpart exhibited a significantly lower efficiency, producing 2k in only 34% yield. A similar trend was observed for halogen-substituted derivatives, where the para-chloro and para-bromo analogs provided 2l and 2m in 74% and 78% yields, respectively. However, the para-iodo derivative did not afford the expected product 2n; instead, the deiodinated compound 2a was isolated in 40% yield, indicating a competing reaction pathway. Electron-withdrawing groups exhibited varying effects on the transformation. The cyano-substituted amide resulted in the formation of 2o in 58% yield, while the acetyl and methyl ester derivatives afforded 2p and 2q in 35% and 55% yields, respectively. In contrast, in the case of the ortho-methoxy-substituted substrate, the desired product 2r was not obtained. Analysis of the reaction mixture revealed that 2-methoxy-N-phenylbenzamide, formed via detosylation of the starting material, was the major product. Additionally, attempts to generate the desired products from 2-furanyl (2s) and alkyl amide derivatives (2t–2w) were unsuccessful. However, despite multiple attempts, heterocyclic substrates such as thiophene-, benzofuran-, pyrrole-, pyridine-, and quinoline-derived amides did not furnish the desired products. Optimization of reaction parameters, including temperature and concentration, did not lead to successful conversion in these cases.
To investigate the reactivity of benzamide derivatives bearing various substituents on their nitrogen atom, a series of substrates were examined under the optimized reaction conditions (Scheme 3). Among the activated benzamides, N-mesityl-N-phenylbenzamide and N-Boc-N-phenylbenzamide successfully underwent the transformation, affording 2a in 64% and 88% yields, respectively. Among deactivated benzamides, N-phenylbenzamide and benzamide did not produce the desired product, whereas N,N-dimethylbenzamide afforded 2a in 42% yield. N-Acyl-substituted activated amides, namely N-benzoyl succinimide, N-benzoyl glutarimide, and N-benzoyl pyrrolidin-2-one, produced 2a in 21%, 15%, and 17% yields, respectively. However, N-benzoyl saccharin did not transform to 2a.
To gain further insights into the reaction mechanism, a series of control experiments were conducted. To investigate the origin of the hydrogen atoms in the formamide product, a control experiment was conducted using deuterated oxalic acid. Under the standard reaction conditions, the desired product 2a-D was obtained in 85% yield. 1H NMR analysis of the crude reaction mixture revealed 51% deuterium incorporation9 at the formyl hydrogen and 3% incorporation at the N–H position of the aniline moiety. These results indicate that the hydrogen at the formyl position is partially derived from oxalic acid, supporting its role as a hydride source in the proposed mechanism (Scheme 4a). To examine the unique role of oxalic acid as a hydride source, we tested alternative reducing agents under the same reaction conditions. When sodium hydride (NaH) or triethylsilane was used in place of oxalic acid, no formation of the target N-formanilide product was observed. These control experiments underscore the essential and specific role of oxalic acid in facilitating the reduction step in this transformation (Scheme 4b). In one experiment, N-phenyl-N-tosylbenzamide and oxalic acid were placed in separate reaction vessels connected to one another. The oxalic acid-containing vessel was heated to 130 °C, while the N-phenyl-N-tosylbenzamide- and NaN3-containing vessel was maintained at 50 °C. Under these conditions, no detectable formation of 2a was observed. When NaN3 was introduced into the oxalic acid-containing vessel, only a trace amount of 2a was detected by gas chromatography. These findings indicate that hydrogen gas generated solely from the thermal decomposition of oxalic acid is insufficient to promote the formylation reaction (Scheme 4c). To further investigate the influence of electronic effects, a competitive experiment was performed using an electron-donating methyl-substituted amide (1d) and an electron-withdrawing fluoro-substituted amide (1j). When these substrates were reacted under identical conditions (130 °C) in the presence of NaN3 and oxalic acid, the corresponding N-formanilide products 2d and 2j were obtained in 25% and 86% yields, respectively.
A similar reaction was then conducted with a modified sequence, where NaN3 was first allowed to react at 25 °C for 6 h before oxalic acid was added. Under these conditions, 2d and 2j were obtained in yields nearly identical to those observed in the original experiment (Scheme 4d). These results suggest that the electronic properties of the substituents do not significantly influence the initial acyl substitution reaction with NaN3. However, at elevated temperatures, benzamides with electron-withdrawing groups exhibit enhanced reactivity, likely due to the more efficient formation and subsequent reaction of the isocyanate intermediate generated via the Curtius rearrangement.
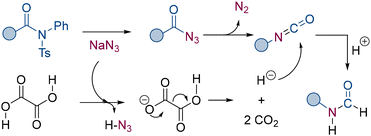
Based on our experimental findings and previous literature reports, a plausible reaction mechanism is proposed in Scheme 5. The reaction begins when the amide substrate reacts with NaN3, leading to the formation of an acyl azide intermediate. Upon heating, the acyl azide intermediate undergoes a Curtius rearrangement, generating an isocyanate intermediate. Concurrently, oxalic acid thermally decomposes or reacts with NaN3 to generate a hydride source. The isocyanate intermediate then reacts sequentially with the hydride source and a proton, ultimately yielding the final N-formylated product.
Conclusions
In summary, we report a one-step strategy to synthesize N-formanilide derivatives from activated amides using oxalic acid as a hydride source. The process involves sodium azide-mediated C–N bond cleavage, forming a benzyl azide intermediate, which undergoes a Curtius rearrangement to yield an isocyanate. This isocyanate is then reduced in situ by oxalic acid to produce the target N-formylated products. Substrate scope studies showed that electron-withdrawing groups enhance the reaction, while sterically hindered or alkyl amides show reduced reactivity. Optimization focused on reagent equivalents, temperature, and solvent. Control experiments confirmed that oxalic acid acts directly as a hydride donor, not via hydrogen gas. This method expands the utility of acyl azides and offers insight into amide bond activation. By using N-tosylated or N-Boc-protected amides, this approach provides a practical route to formylated anilines, even under mild conditions.Conflicts of interest
The authors declare no conflict of interest.Data availability
The data supporting this article have been included as part of the ESI.†Acknowledgements
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korean Government (MSIT) (RS-2023-00218219). Spectral data were obtained at the Korea Basic Science Institute, Gwangju Center. Mass data were acquired on a supercritical fluid chromatograph combined with a Xevo G2-XS QTOF mass spectrometer (Waters, Milford, MA, USA) at the Chiral Material Core Facility Center of Sungkyunkwan University.References
-
(a) R. Hett, Q. K. Fang, Y. Gao, S. A. Wald and C. H. Senanayake, Large-Scale Synthesis of Enantio- and Diastereomerically Pure (R,R)-Formoterol, Org. Process Res. Dev., 1998, 2, 96–99 CrossRef CAS
; (b) F. Campos, M. P. Bosch and A. Guerrero, An efficient enantioselective synthesis of (R,R)-formoterol, a potent bronchodilator, using lipases, Tetrahedron:Asymmetry, 2000, 11, 2705–2717 CrossRef CAS
; (c) D. Gschneidner, A. Leone-Bay, E. Wang, J. J. Freeman, Jr., D. O'Toole and L. E. Shields, Emisphere Technologies, Inc., WO 2001/44199, 2001
; (d) R. J. Booth, W. A. Denny, E. M. Dobrusin, A. J. Kraker, L. H. Mitchell, J. B. Smaill, A. M. Thompson, H. H. Lee, F. O. J. McCarthy and B. D. Palmer, Warner-Lambert Company LLC, WO 2003/091255, 2003
; (e) V. V. Haldavanekar, M. Prabhu, D. R. Rao and R. N. Kankan, Cipla Limited, WO 2009/147383, 2009
.
-
(a) F. F. Blicke and C.-J. Lu, Formylation of Amines with Chloral and Reduction of the N-Formyl Derivatives with Lithium Aluminum Hydride, J. Am. Chem. Soc., 1952, 74, 3933–3934 CrossRef
; (b) G. A. Olah, L. Ohannesian and M. Arvanaghi, Formylating Agents, Chem. Rev., 1987, 87, 671–686 CrossRef CAS
; (c) P. Strazzolini, A. G. Giumanini and S. Cauci, Acetic formic anhydride a review, Tetrahedron, 1989, 46, 1081–1118 CrossRef
; (d) G. Brahmachari and S. Laskar, A very simple and highly efficient procedure for N-formylation of primary and secondary amines at room temperature under solvent-free conditions, Tetrahedron Lett., 2010, 51, 2319–2322 CrossRef CAS
; (e) B. Kaboudin, H. Esfandiari, M. Kakavand, M. Sohrabi, E. Y. Amirkhiz, A. Neshat, T. Kawazoe, H. Fukaya and H. Yanai, Phosphite–imidazole catalyzed N-formylation and N-acylation of amines, Org. Biomol. Chem., 2023, 21, 8182–8189 RSC
; (f) W.-H. Lin and C.-F. Liang, Hexamethyldisilazane-Mediated Synthesis of N-Formamides, Benzimidazoles and Quinazolinones Using N,N-Dimethylformamide as a C1 Source, Asian J. Org. Chem., 2024, 13, e202300603 CrossRef CAS
.
-
(a) T. Ishida and M. Haruta, N-Formylation, of Amines via the Aerobic Oxidation of Methanol over Supported Gold Nanoparticles, ChemSusChem, 2009, 2, 538–541 CrossRef CAS PubMed
; (b) L. Zhang, Z. Han, X. Zhao, Z. Wang and K. Ding, Highly Efficient Ruthenium-Catalyzed N-Formylation of Amines with H2 and CO2, Angew. Chem., Int. Ed., 2015, 54, 6186–6189 CrossRef CAS PubMed
; (c) P. Daw, S. Chakraborty, G. Leitus and Y. Diskin-Posner, Selective N-Formylation of Amines with H2 and CO2 Catalyzed by Cobalt Pincer Complexes, ACS Catal., 2017, 7, 2500–2504 CrossRef CAS
; (d) C. Zhang, Y. Zhang, Q. Liang, G. Zhang, W. Yang, N. Li, G. Qin and G. Zhang, Formamidation of a wide range of substituted and functionalized amines with CO and a base, Org. Chem. Front., 2022, 9, 6142–6148 RSC
; (e) M. T. Flynn, X. Liu, A. Dell'Acqua, J. Rabeah, A. Brückner, E. Baráth, S. Tin and J. G. de Vries, Glycolaldehyde as a Bio-Based C1 Building Block for Selective N-Formylation of Secondary Amines, ChemSusChem, 2022, 15, e202201264 CrossRef CAS PubMed
; (f) Z. Song, J. Liu, S. Xing, X. Shao, J. Li, J. Peng and Y. Bai, PNP-type ligands enabled copper-catalyzed N-formylation of amines with CO2 in the presence of silanes, Org. Biomol. Chem., 2023, 21, 832–837 RSC
; (g) X. Dai, X. Wang, C. Kreyenschulte, H. Yuan, A. Brückner, F. Shi and J. Rabeah, Cu-Catalysed sustainable synthesis of formamide with glycerol derivatives as a carbonyl source via a radical-relay mechanism, Org. Biomol. Chem., 2023, 21, 832–837 RSC
; (h) T. A. Gokhale, S. C. Gulhane and B. M. Bhanage, Highly Selective Catalyst-Free Oxidative Synthesis of N-Formamides from C2- and C3-Feedstocks, Eur. J. Org. Chem., 2023, e202200997 CrossRef CAS
.
-
(a) E. R. Barnum and C. S. Hamilton, 5-Amino- and 1-Aminobenzo(f)quinolines and Derivatives, J. Am. Chem. Soc., 1942, 64, 540–542 CrossRef CAS
; (b) A. Kumar, N. Kumar, R. Sharma, G. Bhargava and D. Mahajan, Direct Conversion of Carboxylic Acids to Various Nitrogen Containing Compounds in the One-Pot Exploiting Curtius Rearrangement, J. Org. Chem., 2019, 84, 11323–11334 CrossRef CAS PubMed
.
- N. Pootheri and S. Lee, Palladium-Catalyzed One-Pot Synthesis of N-Formylaniline Derivatives Using Oxalic Acid as a Dual Carbon Monoxide and Hydrogen Donor, Org. Lett., 2024, 26, 9407–9412 CrossRef CAS PubMed
.
-
(a) T. B. Nguyen, J. Sorres, M. Q. Tran, L. Emolenko and A. Al-Mourabit, Boric Acid: A Highly Efficient Catalyst for Transamidation of Carboxamides with Amines, Org. Lett., 2012, 14, 3202–3205 CrossRef CAS PubMed
; (b) J. E. Dander and N. K. Garg, Breaking Amides using Nickel Catalysis, ACS Catal., 2017, 7, 1413–1423 CrossRef CAS PubMed
; (c) G. Li and M. Szostak, Non-Classical Amide Bond Formation: Transamidation and Amidation of Activated Amides and Esters by Selective N–C/O–C Cleavage, Synthesis, 2020, 2579–2599 CAS
; (d) G. Li and M. Szostak, Transition-Metal-Free Activation of Amides by N-C Bond Cleavage, Chem. Rec., 2020, 20, 649–659 CrossRef CAS PubMed
; (e) K. Mashima, Y. Nishii and H. Nagae, Catalytic Cleavage of Amide C-N Bond: Scandium, Manganese, and Zinc Catalysts for Esterification of Amides, Chem. Rec., 2020, 20, 332–343 CrossRef CAS PubMed
; (f) P. Acosta-Guzmán, A. Mateus-Gómez and D. Gamba-Sánchez, Direct Transamidation Reactions: Mechanism and Recent Advances, Molecules, 2018, 23, 2382 CrossRef PubMed
; (g) S. Kalinin and A. Sapegin, Ring Expansion Reactions through Intramolecular Transamidation, Eur. J. Org. Chem., 2023, e202300754 CrossRef CAS
; (h) V. Kumar, S. Dhawan, R. Bala, P. S. Girase, P. Singh and R. Karpoormath, Recent advances in transamidation of unactivated amides, Chem. Pap., 2023, 77, 4057–4084 CrossRef CAS
; (i) K. Sakthivel, S. S. Ahmed, S. Niharan and F. V. Singh, Recent developments in metal-catalysed transamidation of quiescent amides, J. Organomet. Chem., 2024, 1018, 123290 CrossRef CAS
; (j) N. Sivaraj, T. Dohi and F. V. Singh, Advances in Metal-Free Transamidation: A Sustainable Approach to Amide Bond Formation, Chem. – Asian J., 2025, 20, e202500327 CrossRef CAS PubMed
; (k) K. R. Rajamanickam, R. P. S. Rajan, N. Pootheri, C.-F. Lee and S. Lee, Recent Advances in Transition Metal-Free Strategies for the Transformation of Amides into Carbonyl Compounds, Eur. J. Org. Chem., 2025, e202500210 CrossRef CAS
.
-
(a) S. Yu, T. Shin, M. Zhang, Y. Xia, H. Kim and S. Lee, Nickel/Briphos-Catalyzed Direct Transamidation of Unactivated Secondary Amides Using Trimethylsilyl Chloride, Org. Lett., 2018, 20, 7563–7566 CrossRef CAS PubMed
; (b) S. Yu, K. Song and S. Lee, Metal-Free Transamidation of Primary Amides using Trimethylsilyl Chloride, Asian J. Org. Chem., 2019, 8, 1613–1616 CrossRef CAS
; (c) M. Park and S. Lee, Transition-metal-catalyst-free reaction of amides and acetonitriles: synthesis of β-ketonitriles, Org. Chem. Front., 2022, 9, 5178–5184 RSC
; (d) H. Park and S. Lee, Palladium and Copper-Catalyzed Friedel–Crafts Acylation with Activated Amides, Adv. Synth. Catal., 2023, 365, 3167–3171 CrossRef CAS
; (e) H. Moon and S. Lee, Reductive cross-coupling of N-acyl pyrazole and nitroarene using tetrahydroxydiboron: synthesis of secondary amides, Org. Biomol. Chem., 2023, 21, 8329–8334 RSC
; (f) M. Park, K. R. Rajamanickam, K. Song and S. Lee, 1,3-Diketone-Mediated Synthesis of Symmetrical Aryl Imides from Activated Amides, Asian J. Org. Chem., 2023, 12, e202300469 CrossRef CAS
; (g) I. A. Bashir and S. Lee, Base-Mediated Synthesis of Anhydrides from Activated Amides, J. Org. Chem., 2023, 88, 6159–6167 CrossRef CAS PubMed
; (h) Y. An, J. Oh and S. Lee, Synthesis of N-Acyl-N′-Sulfonyl Hydrazides from Sulfonyl Hydrazides and Activated Amides, Synthesis, 2024, 3468–3474 CAS
; (i) M. Kim and S. Lee, Synthesis of Acyl Hydrazides and Hydrazones from Activated Amides, Synthesis, 2024, 2263–2269 CAS
.
- D. Joseph and S. Lee, Reaction of Amide and Sodium Azide for the Synthesis of Acyl Azide, Urea, and Iminophosphorane, Org. Lett., 2022, 24, 6186–6191 CrossRef CAS PubMed
.
- The formation of only 50% deuterated product is attributed to trace amounts of water present in the reaction system, despite our efforts to rigorously exclude moisture under carefully controlled conditions.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5ob00941c |
| This journal is © The Royal Society of Chemistry 2025 |