Asymmetric synthesis and biological evaluation of ottensinin and its analogues†
Jun-Ting
Liang‡
,
Zichen
Xu‡
,
Yong-Bin
Xie
,
Wen-Tao
Chen
,
Yu-Tao
He
 * and
Ya-Jian
Hu
* and
Ya-Jian
Hu
 *
*
State Key Laboratory of Bioactive Molecules and Druggability Assessment, Institute for Advanced and Applied Chemical Synthesis, College of Pharmacy, Jinan University, Guangzhou 510632, China. E-mail: heyutao7@jnu.edu.cn; yajianhu@jnu.edu.cn
First published on 10th July 2025
Abstract
The asymmetric synthesis of ottensinin, a rearranged labdane diterpene with a broad range of favourable bioactivities, has been realized based on fragment coupling of 16a and 13 in eight steps from (+)-sclareolide. This approach would enable the diverse synthesis of ottensinin analogues to facilitate future drug development based on ottensinin.
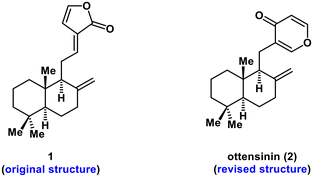
Rearranged labdane diterpenoids are a series of structurally intriguing natural products that are biosynthesized from labdane diterpenoid precursors through skeletal rearrangement.1 These natural products have attracted substantial interest from synthetic chemists owing to their diverse structures and broad range of biological activities.2 In 2006, Kikuzaki et al. isolated a new diterpene from rhizomes of Zingiber ottensii (Zingiberaceae) and its structure was incorrectly assigned as 1 with a γ-lactone ring based on extensive spectroscopic analysis (Fig. 1).3 In 2008, through synthesis and X-ray diffraction analysis of a synthetic sample, the structure of this diterpene was unambiguously revised by Boukouvalas et al. as 2, which contains a unique γ-pyrone moiety.4 The name ottensinin was assigned to this rearranged labdane diterpene by Boukouvalas and co-workers. Subsequent phytochemistry research5 resulted in the isolation of ottensinin (2) from several other plants of the Zingiberaceae family, especially in the genus of Amomum. Remarkably, biological studies revealed that 2 exhibited diverse important pharmacological properties, including antibacterial activity against Bacillus cereus (MIC = 0.25 μg μL−1),5a cytotoxicity against the SMMC-7721 cell line (human hepatoma, IC50 = 30.3 μg mL−1),5b inhibition of NF-κB (a potential target for the treatment of immunity and inflammation related disorders, IC50 = 7.99 μM),5c suppression of NO production (IC50 = 42.2 μM),5d and inhibitory effects on α-glucosidase (IC50 = 18.64 μg mL−1) and α-amylase (IC50 = 78.54 μg mL−1).5e Therefore, ottensinin (2) might serve as a promising lead compound for drug research and discovery.
In addition to its broad range of favourable bioactivities, ottensinin (2) was the proposed biosynthetic precursor of some other rearranged labdane diterpenes containing a 3-substituted γ-pyrone unit.5c,6 Nevertheless, the scarcity of ottensinin (2) from natural sources might impede in-depth evaluation of its biological activity as well as its significant role in synthetic biology. Hence, a general strategy to access 2 and its analogues is highly desirable. To date, two innovative approaches for synthesizing 2 have been reported (Scheme 1). In 2008, Boukouvalas and co-workers disclosed the first remarkable synthesis of 2, featuring an impressive 6-endo-dig cyclization of an enolate-ynone substrate 3 to assemble the 3-substituted γ-pyrone motif (Scheme 1A).4 Recently, Yue's group reported an elegant synthesis of 2, with a remarkable Ni-catalyzed C(sp2)–C(sp3) cross-electrophile coupling reaction of iodide 4 and 4-oxo-4H-pyran-3-yl triflate as a key step (Scheme 1B).7 Moreover, further in-depth pharmacological study from Yue's group indicated that ottensinin (2) was a promising anti-epileptic lead compound.7 Despite the above advances in ottensinin synthesis, development of a new approach capable of easily producing this biologically active molecule as well as its analogues remains desirable. As part of our continuing efforts toward the synthesis of bioactive natural products,8 we herein describe the asymmetric synthesis and biological evaluation of ottensinin and its analogues.
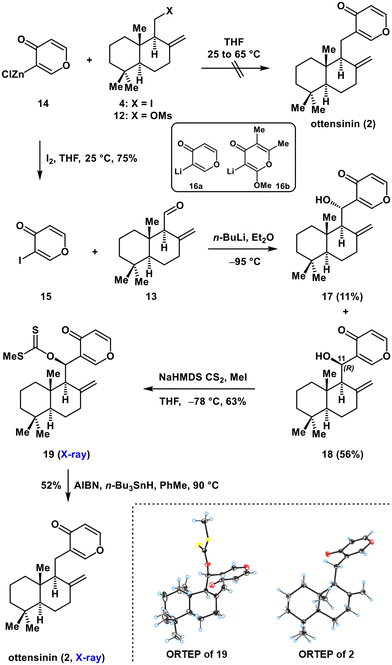
Our synthesis was based on a fragment coupling between a [6–6] fused electrophile 5 and γ-pyrone-containing nucleophile 6 (Scheme 2). This approach would allow the rapid formation of diverse ottensinin derivatives from analogues of 5 or 6 to facilitate further biological research. Thus, we began our synthesis with commercially available (+)-sclareolide (7) as a chiral pool9 starting material (Scheme 3), which was transformed into alcohol 11 by modification of a reported procedure.10 Treatment of 7 with methyllithium induced opening of the lactone moiety in 7 to give keto-alcohol 8. Subsequently, 8 underwent Baeyer–Villiger rearrangement (H2O2/Ac2O/maleic anhydride) to furnish compound 9. Regioselective elimination of the tertiary alcohol in 9 with pyridine/SOCl2 followed by treatment with mCPBA (to remove the small amount of impurity of endo products) gave exo olefin 10 in pure form. Deacetylation of 10 with K2CO3/MeOH afforded alcohol 11. An Appel reaction was used to convert 11 into iodide 4. Next, mesylation of the hydroxyl group in 11 generated mesylate 12. Swern oxidation of 11 delivered aldehyde 13.
With electrophiles 4, 12 and 13 in hand, we proceeded to investigate our proposed fragment coupling approach to synthesize ottensinin (Scheme 4). Initially, we attempted to employ an alkylation reaction of γ-pyrone zinc reagent 14, which was prepared by selective C3 zincation of 4H-pyran-4-one according to Knochel's protocol.11 Although 14 has been efficiently used to prepare some useful 3-substituted pyrones by reacting with various electrophiles (such as allyl bromide and pivaloyl chloride),11 unfortunately the alkylation of 14 with homoallylic iodide 4 or mesylate 12 did not proceed even at elevated temperature due to their poor reactivities, while quenching 14 with molecular iodine smoothly gave 3-iodopyran-4-one 15. Next, we focused our attention on a 1,2-addition reaction of the organolithium reagent 16a that was formed by treatment of 15 with n-BuLi to aldehyde 13. Notably, previously, the 1,2-addition reaction was fruitfully used in the synthesis of natural products bearing a fully substituted γ-pyrone, in which a fully substituted 3-lithio-γ-pyrone 16b was employed as a reactant.12 However, to our knowledge, there have been no reports on the application of mono-substituted 3-lithio-γ-pyrone 16a to form a C–C bond in natural product synthesis probably due to the relative instability of 16a. Therefore, this fragment coupling of 16a with other electrophiles (such as 13) might be challenging but would be useful for synthesizing the natural products with a mono 3-substituted γ-pyrone moiety. Actually, upon treatment of 16a (formed by Li–I exchange from 15 with n-BuLi) with 13 at the commonly used temperature (−78 °C), the desired coupling products 17 and 18 were not observed probably because of the instability of 16a at −78 °C. To our pleasure, after extensive trials, it was found that this reaction occurred well at a much lower temperature (−95 °C) to provide alcohol 18 (its 11(R)-OH configuration was confirmed by X-ray crystallographic analysis of its derivative 19) in a moderate yield of 56% coupled with a small amount of 17 (11% yield). Finally, a Barton–McCombie radical dehydroxylation reaction successfully transformed the alcohol 18 into ottensinin (2) through the methyl xanthate intermediate 19. The structures of 19 and 2 were unambiguously verified by X-ray crystallographic analysis. The analytical data for 2 were in agreement with those reported.4
Having successfully synthesized ottensinin (2), we extended this approach to synthesize its analogues using a similar route to enable further biological evaluations (Scheme 5). Thus, compound 20,13 an analogue of aldehyde 13 (see Scheme 4), was readily synthesized. Next, by employing a parallel route with aldehyde 20, the homolog of aldehyde 13 with an additional CH2 at the C9 side chain, as the coupling partner, alcohol 21 was produced in 89% yield. Finally, the secondary hydroxyl group in 21 was removed via a Barton–McCombie reaction to yield homoottensinin (22), whose structure was also determined by X-ray crystallographic analysis.
With ottensinin (2) and its synthetic analogues in hand, we proceeded to carry out further pharmacological studies. The anti-cancer activity of compounds 2, 17–19, 21 and 22 against the SMMC-7721 (human hepatoma) cell line was evaluated and 5-fluorouracil was used as a positive control.5b,14 As shown in Table 1, all the tested compounds exhibited varying degrees of growth inhibitory activity against the tumour cell and 18 exhibited potent anti-cancer properties with an IC50 value of 18.68 ± 1.57 μg mL−1. These results therefore indicated that substituted ottensinins might serve as promising leads for more systematic anti-cancer drug research.
| Compounds | IC50 ± SD [μg mL−1] |
|---|---|
| Ottensinin (2) | 28.69 ± 3.24 |
| 17 | 26.69 ± 2.27 |
| 18 | 18.68 ± 1.57 |
| 19 | 28.15 ± 3.12 |
| 21 | 33.42 ± 1.71 |
| 22 | 21.22 ± 1.68 |
| 5-Fluorouracil | 13.59 ± 2.18 |
In conclusion, we have developed a new approach for the asymmetric synthesis of ottensinin (2) in eight steps from a chiral pool material (+)-sclareolide with a fragment coupling of 16a and 13 at a very low temperature (−95 °C) as a key step. This approach has also been applied in the synthesis of various ottensinin analogues with a mono 3-substituted pyrone moiety. Furthermore, biological evaluation indicated that the synthetic ottensinin analogue 18 exhibited a potent anti-cancer effect against the SMMC-7721 (human hepatoma) cell line. Since ottensinin (2) was a hypothetical precursor of other rearranged labdane diterpenes,5c this work would be beneficial for the investigation on the biosynthesis of natural products of this family. Moreover, given the diverse significant pharmacological activities of ottensinin,5 the method developed herein would enable the diverse synthesis of ottensinin analogues to facilitate future structural modifications and more systematic structure–activity relationship (SAR) studies of ottensinin. Such work is underway in our laboratory and will be reported in due course.
Author contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.Conflicts of interest
Jinan University filed a Chinese patent application based on part of this work.Data availability
The data supporting this article have been included as part of the ESI.†Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (22201104) and the Guangdong Basic and Applied Basic Research Foundation (2024A1515010884 and 2022A1515110367). The authors thank Dr Wei Xu from the Public Research Platform of the College of Pharmacy at Jinan University for X-ray crystallographic analysis.References
-
(a) H. Yin, J.-G. Luo, S.-M. Shan, X.-B. Wang, J. Luo, M.-H. Yang and L.-Y. Kong, Org. Lett., 2013, 15, 1572–1575 CrossRef CAS PubMed
; (b) X.-D. Wu, D. Luo, W.-C. Tu, Z.-T. Deng, X.-J. Chen, J. Su, X. Ji and Q.-S. Zhao, Org. Lett., 2016, 18, 6484–6487 CrossRef CAS PubMed
; (c) J. Zhou, J. Zhang, R. Li, J. Liu, P. Fan, Y. Li, M. Ji, Y. Dong, H. Yuan and H. Lou, Org. Lett., 2016, 18, 4274–4276 CrossRef CAS PubMed
; (d) Q. Zhao, L.-G. Xiao, L.-S. Bi, Y. Si, X.-M. Zhang, J.-H. Chen and H.-Y. Liu, Org. Lett., 2022, 24, 6936–6939 CrossRef CAS PubMed
; (e) M. Zhu, Y. Gao, Y. Li, F. Xie, J. Zhou, L. Xu, D. Lv, X. Zhang, Z. Xu, T. Dong, T. Shen, J. Zhang and H. Lou, J. Agric. Food Chem., 2023, 71, 19551–19567 CrossRef CAS PubMed
; (f) C. Zhang, Y. Li, Z. Chu, S. Yuan, Y. Qiao, J. Zhang, L. Li, Y. Zhang, R. Tian, Y. Tang and H. Lou, Chin. Chem. Lett., 2024, 35, 108206 CrossRef CAS
; (g) Z.-Y. Zhang, Y.-H. Chen, C.-Y. Zheng, Y. Gao, Y.-Y. Fan, J.-M. Yue and J.-X. Zhao, Org. Lett., 2025, 27, 2485–2491 CrossRef CAS PubMed
.
-
(a) L. M. T. Frija, R. F. M. Frade and C. A. M. Afonso, Chem. Rev., 2011, 111, 4418–4452 CrossRef CAS PubMed
; (b) P. S. Grant and M. A. Brimble, Chem. – Eur. J., 2021, 27, 6367–6389 CrossRef CAS PubMed
.
- K. Akiyama, H. Kikuzaki, T. Aoki, A. Okuda, N. H. Lajis and N. Nakatani, J. Nat. Prod., 2006, 69, 1637–1640 CrossRef CAS PubMed
.
- J. Boukouvalas and J.-X. Wang, Org. Lett., 2008, 10, 3397–3399 CrossRef CAS PubMed
.
-
(a) Y. Sivasothy, H. Ibrahim, A. S. Paliany, S. A. Alias, N. R. Md. Nor and K. Awang, Planta Med., 2013, 79, 1775–1780 CrossRef CAS PubMed
; (b) J.-G. Luo, H. Yin, B.-Y. Fan and L.-Y. Kong, Helv. Chim. Acta, 2014, 97, 1140–1145 CrossRef CAS
; (c) K.-L. Ji, Y.-Y. Fan, Z.-P. Ge, L. Sheng, Y.-K. Xu, L.-S. Gan, J.-Y. Li and J.-M. Yue, J. Org. Chem., 2018, 84, 282–288 CrossRef PubMed
; (d) H. Yin, C. Guo and J.-M. Gao, J. Nat. Med., 2021, 75, 173–177 CrossRef CAS PubMed
; (e) C.-L. Lu, L.-N. Wang, Y.-J. Li, Q.-F. Fan, Q.-H. Huang and J.-J. Chen, Nat. Prod. Res., 2022, 36, 2570–2574 CrossRef CAS PubMed
.
- For a structurally related and intriguing bis-labdanic diterpene that was also isolated from the Zingiberaceae family, see: Y. Sivasothy, C. C. K. Zachariah, K. H. Leong, S. K. S. John, H. Ibrahim and K. Awang, Tetrahedron Lett., 2014, 55, 6163–6166 CrossRef CAS
.
- Q.-X. Li, L.-L. Wang, Y.-M. Zheng, Z.-B. Gao, J.-X. Zhao and J.-M. Yue, Org. Chem. Front., 2024, 11, 5160–5166 RSC
.
-
(a) Y.-J. Hu, C.-C. Gu, X.-F. Wang, L. Min and C.-C. Li, J. Am. Chem. Soc., 2021, 143, 17862–17870 CrossRef CAS PubMed
; (b) J.-H. Zhang, W.-C. Luo, W.-T. Chen, Y.-T. He and Y.-J. Hu, Org. Chem. Front., 2024, 11, 6333–6339 RSC
.
- Z. G. Brill, M. L. Condakes, C. P. Ting and T. J. Maimone, Chem. Rev., 2017, 117, 11753–11795 CrossRef CAS PubMed
.
- A. Skiredj, M. A. Beniddir, L. Evanno and E. Poupon, Eur. J. Org. Chem., 2016, 2954–2958 CrossRef CAS
.
- L. Klier, T. Bresser, T. A. Nigst, K. Karaghiosoff and P. Knochel, J. Am. Chem. Soc., 2012, 134, 13584–13587 CrossRef CAS PubMed
.
-
(a) K. Watanabe, K. Iwasaki, T. Abe, M. Inoue, K. Ohkubo, T. Suzuki and T. Katoh, Org. Lett., 2005, 7, 3745–3748 CrossRef CAS PubMed
; (b) T. Abe, K. Iwasaki, M. Inoue, T. Suzuki, K. Watanabe and T. Katoh, Tetrahedron Lett., 2006, 47, 3251–3255 CrossRef CAS
; (c) Z. Li and D. Yang, Tetrahedron Lett., 2015, 56, 3667–3669 CrossRef CAS
; (d) Y. Ji, Y. Liu, W. Guan, C. Guo, H. Jia, B. Hong and H. Li, J. Am. Chem. Soc., 2024, 146, 9395–9403 CrossRef CAS PubMed
.
- For the synthesis of aldehyde 20, see the ESI† for details.
- Y. Zhang, J. Wang, D. Wei, X. Wang, J. Luo, J. Luo and L. Kong, Phytochemistry, 2010, 71, 2199–2204 CrossRef CAS PubMed
.
Footnotes |
| † Electronic supplementary information (ESI) available: Experimental procedures, characterization data, X-ray crystallographic data, and NMR spectra. CCDC 2464862–2464864. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d5ob01028d |
| ‡ These authors contributed equally. |
| This journal is © The Royal Society of Chemistry 2025 |